
Dental Caries
123
Zhou Xuedong
Editor
Principles and
Management

Dental Caries

Zhou Xuedong
Editor
Dental Caries
Principles and Management
ISBN 978-3-662-47449-5 ISBN 978-3-662-47450-1 (eBook)
DOI 10.1007/978-3-662-47450-1
Library of Congress Control Number: 2015949119
Springer Berlin, Heidelberg Heidelberg New York Dordrecht London
© Springer-Verlag Berlin Heidelberg 2016
This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or
part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of
illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way,
and transmission or information storage and retrieval, electronic adaptation, computer software,
or by similar or dissimilar methodology now known or hereafter developed.
The use of general descriptive names, registered names, trademarks, service marks, etc. in this
publication does not imply, even in the absence of a specifi c statement, that such names are
exempt from the relevant protective laws and regulations and therefore free for general use.
The publisher, the authors and the editors are safe to assume that the advice and information in
this book are believed to be true and accurate at the date of publication. Neither the publisher nor
the authors or the editors give a warranty, express or implied, with respect to the material
contained herein or for any errors or omissions that may have been made.
Printed on acid-free paper
Springer-Verlag GmbH Berlin Heidelberg is part of Springer Science+Business Media
(www.springer.com)
Editor
Zhou Xuedong
State Key Laboratory of Oral Diseases
West China Hospital of Stomatology
Sichuan University
Chengdu
China

v
1 Tooth Development: Embryology
of the Craniofacial Tissues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Zheng Liwei , Wang Chenglin , and Ye Ling
2 Biofi lm and Dental Caries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Xu Xin , Zhou Yuan , Shi Wenyuan , Liu Yaling ,
and Zhou Xuedong
3 Saliva and Dental Caries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
Wang Renke
4 Demineralization and Remineralization . . . . . . . . . . . . . . . . . . 71
Cheng Lei , Li Jiyao , Xu Hockin H. K. ,
and Zhou Xuedong
5 The Diagnosis for Caries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 85
Yang Liu , Li Boer , Wang Shuang , Zhang Yaru ,
and Peng Li
6 Dental Caries: Disease Burden Versus Its Prevention . . . . . . . 91
Hong Xiao
7 Clinical Management of Dental Caries . . . . . . . . . . . . . . . . . . . 107
Li Jiyao
8 Dental Caries and Systemic Diseases . . . . . . . . . . . . . . . . . . . . . 129
Zou Ling and Hu Tao
9 Models in Caries Research . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 157
Huang Xuelian , Guo Qiang , Ren Biao , Li Yuqing ,
and Zhou Xuedong
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 175
Contents

vii
Ren Biao State Key Laboratory of Oral Diseases , Sichuan University ,
Chengdu , People’s Republic of China
Li Boer State Key Laboratory of Oral Diseases , West China Hospital of
Stomatology, Sichuan University , Chengdu , People’s Republic of China
Wang Chenglin State Key Laboratory of Oral Diseases, West China
Hospital of Stomatology, Sichuan University, Chengdu, China
Xu Hockin H. K. Biomaterials & Tissue Engineering Division,
Department of Endodontics, Prosthodontics and Operative Dentistry ,
University of Maryland Dental School , Baltimore , MD , USA
Li Jiyao State Key Laboratory of Oral Diseases , Sichuan University ,
Chengdu , People’s Republic of China
Department of Operative Dentistry and Endodontics , West China Hospital
of Stomatology, Sichuan University , Chengdu , People’s Republic of China
Cheng Lei State Key Laboratory of Oral Diseases , Sichuan University ,
Chengdu , People’s Republic of China
Department of Operative Dentistry and Endodontics , West China Hospital
of Stomatology, Sichuan University , Chengdu , People’s Republic of China
Peng Li State Key Laboratory of Oral Diseases , West China Hospital of
Stomatology, Sichuan University , Chengdu , People’s Republic of China
Ye Ling State Key Laboratory of Oral Diseases, West China Hospital
of Stomatology, Sichuan University, Chengdu, China
Zou Ling Department of Conservation Dentistry and Endodontics, West
China Hospital of Stomatology, Sichuan University , Chengdu , People’s
Republic of China
Yang Liu State Key Laboratory of Oral Diseases , West China Hospital of
Stomatology, Sichuan University , Chengdu , People’s Republic of China
Contributors

viii
Zheng Liwei State Key Laboratory of Oral Diseases, West China Hospital
of Stomatology, Sichuan University, Chengdu, China
Guo Qiang State Key Laboratory of Oral Diseases , Sichuan University ,
Chengdu , People’s Republic of China
Wang Renke West China Hospital of Stomatology, Sichuan University ,
Chengdu , People’s Republic of China
Wang Shuang State Key Laboratory of Oral Diseases , West China Hospital
of Stomatology, Sichuan University , Chengdu , People’s Republic of China
H u T a o Department of Preventive Dentistry, West China Hospital of
Stomatology, Sichuan University , Chengdu , People’s Republic of China
Shi Wenyuan School of Dentistry , University of California-Los Angeles ,
Los Angeles , CA , USA
Hong Xiao Department of Preventive Dentistry, West China Hospital of
Stomatology, Sichuan University, Chengdu, China
Xu Xin State Key Laboratory of Oral Diseases , West China Hospital of
Stomatology, Sichuan University , Chengdu , People’s Republic of China
Zhou Xuedong State Key Laboratory of Oral Diseases , West China
Hospital of Stomatology, Sichuan University , Chengdu , People’s Republic
of China
Department of Operative Dentistry and Endodontics , West China Hospital
of Stomatology, Sichuan University , Chengdu , People’s Republic of China
Huang Xuelian State Key Laboratory of Oral Diseases , Sichuan
University , Chengdu , People’s Republic of China
Department of Operative Dentistry and Endodontics , West China Hospital
of Stomatology, Sichuan University , Chengdu , China
Liu Yaling State Key Laboratory of Oral Diseases , West China Hospital of
Stomatology, Sichuan University , Chengdu , People’s Republic of China
Department of Oral Biology , College of Dentistry, University of Florida ,
Gainesville , FL , USA
Zhang Yaru State Key Laboratory of Oral Diseases , West China Hospital
of Stomatology, Sichuan University , Chengdu , People’s Republic of China
Zhou Yuan State Key Laboratory of Oral Diseases , West China Hospital of
Stomatology, Sichuan University , Chengdu , People’s Republic of China
Li Yuqing State Key Laboratory of Oral Diseases , Sichuan University ,
Chengdu , People’s Republic of China
Contributors

1
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1_1
Tooth Development: Embryology
of the Craniofacial Tissues
Zheng Liwei , Wang Chenglin , and Ye Ling
1.1 Embryology
of the Craniofacial Tissues
The development of craniofacial tissues is part of
human prenatal development. Generally, human
prenatal development goes through three stages:
the proliferative 2-week period, when cell divi-
sion is prevalent; the embryonic period, which
extends from the second to the eighth weeks; and
the fetal period, from eighth week to birth [ 1 ].
With normal accomplishment of the develop-
ment, human body forms stepwise.
1.1.1 Origin of Human Tissue
The origin of tissue begins with fertilization,
which is the fusion of spermatozoa and ova to
form a zygote. Then the zygote moves to the uter-
ine cavity where it will implant into the wall of
the uterus and, meanwhile, undergoes a series of
rapid divisions that lead to the formation of a
fl uid fi lled hollow ball, termed blastocyst, and
small inner cell mass. When this blastocyst
attaches to the sticky wall of the body of the
uterus, uterine endometrium is digested, allowing
blastocyst embedded in its surface and then
deeper penetration. Implantation takes place.
On either side of the inner cell mass, two small
cavities are formed. A small disk (the embryonic
disk) develops in the center, where they reach
each other. The embryonic disk becomes the
embryo, composed of two layers of cells. One
layer is lined with ectodermal cells, which will
form the outer body covering (epithelium), called
ectodermal layer. The cells on the ventral aspect
are endodermal cells, forming the endodermal
layer. This confi guration is completed in the fi rst 2
weeks, which is termed “proliferative period” [ 1 ].
During the third week, two-layered embryonic
disk is converted to a three-layered disk. Cells
that develop between the ectodermal and endo-
dermal layers become the mesodermal layer.
Next, major tissues and organs, including oral
maxillofacial tissue such as tooth and facial
bones, differentiate from these three layers [
2 ].
Key events are the development of the nervous
system, differentiation of neural crest tissue from
the ectoderm, and folding of the embryo.
1.1.2 The Neural Crest
The nervous system begins with a specifi cation of
the neural plate, which develops as a thickening
within the anterior ectodermal layer. Meanwhile,
the neural plate develops raised folds at its margins.
These folds in turn encompass and fuse so that neu-
ral tube forms and separates from the ectoderm.
Z. Liwei • W. Chenglin • Y. Ling (*)
State Key Laboratory of Oral Diseases , West China
Hospital of Stomatology, Sichuan University ,
Chengdu , 610041 Sichuan , China
e-mail:
1

2
Upon closure of the neural tube, a unique
population of cells known as neural crest cells
separate from the lateral aspect of the neural
plate. These cells have the capability of migration
and differentiation. This is especially obvious in
the head and neck region, and neural crest cells
have an important role in the head development.
They contribute to most of the embryonic connec-
tive tissue of facial region, which includes dental
tissues such as the pulp, dentin, and cementum.
Consequently, embryonic connective tissue in the
head is termed as ectomesenchyme, refl ecting its
origin from the ectoderm, whereas connective tis-
sue elsewhere is derived from the mesoderm and
is known as mesenchyme. Although the neural
crest tissues arise from neural ectoderm, they
exhibit properties of mesenchyme [ 2 , 3 ].
1.1.3 Head Formation
The head fold of the three-layered embryo is cru-
cial and produces the primitive stomatodeum or
oral cavity. When the stomatodeum fi rst forms, it
is surrounded by frontal prominence rostrally and
by the cardiac bulge caudally. And it is separated
from the foregut by buccopharyngeal membrane,
a bilaminar structure consisting of ectoderm and
endoderm, which breaks down soon so that the
stomatodeum communicates with the foregut.
Laterally the somatodeum becomes delimited by
the fi rst pair of pharyngeal arches [ 1 , 2 ].
1.2 Enamel Development
Fully mature enamel comprises 80–90 % (v/v)
carbonated calcium hydroxyapatite crystals,
which is in contrast to bone and dentin, both with
10 % and 13 % (v/v) carbonated calcium hydroxy-
apatite crystals, respectively. The mechanisms of
crystal initiation, crystal growth, as well as mor-
phology are related to amelogenesis. In develop-
ing teeth, sequential and reciprocal interactions
occur between the epithelium and the underlying
mesenchyme, which derive from the cranial neu-
ral crest. Enamel formation associates with the
differentiation of the tooth-specifi c cell types, the
epithelial ameloblasts. This chapter will provide a
brief overview in different aspects of tooth enamel
development with a particular emphasis on the
current knowledge of enamel morphogenesis, his-
togenesis, and cytodifferentiation.
1.2.1 Histogenesis
and Morphogenesis
The consecutive phases during tooth morphologic
changes, including lamina, bud, cap, and bell
stages, are characterized by epithelial histogene-
sis. The segmentation of the dental epithelium
occurs in the early tooth initiation, which indi-
cates that a local epithelial thickening corresponds
to the dental lamina. Experiment approaches sug-
gested that Wnt/Shh interactions may regulate the
delimitation between the dental epithelium and
the oral ectoderm. Nevertheless, the molecules
intervene in regulating epithelial cell apoptosis,
and survival or compartmentalization of different
elements is still poorly understood (Fig. 1.1 ).
1.2.1.1 Bud Stage
From the bud stage, the thickened presumptive
dental epithelium, which forms the basal epithe-
lium, can be distinguished from the round inter-
nal cells. Depending on the position, epithelial
cells are evident in changes of the expression of
different molecules, including γ-catenin, desmo-
glein, and P- and E-cadherins.
1.2.1.2 Cap Stage
During the cap stage, the dental epithelium
becomes the enamel organ which consists of four
different cell types: inner and outer dental epithe-
lia, the stellate reticulum, as well as transiently the
primary enamel knot. At this time, the inner
enamel epithelium becomes discernible from the
outer enamel epithelium. The histogenesis of the
inner dental epithelium is coordinated by a change
in the composition of the basement membrane.
The enamel knot is a dynamic transient struc-
ture and appears at the onset of mammalian tooth
shape development, which is in contact with sev-
eral cells, including peripheral cells, internal
round cells, and basement membrane cells.
Z. Liwei et al.

3
Studies indicated that the primary enamel knot
represents a signaling center in formatting cusps,
which may lead to unequal growth of the enamel
epithelium and induce the formation of second-
ary enamel knots.
It has been suggested that the structure and
organization of primary enamel knot rapidly
change during the time. At the beginning of the
cap stage, it appears as a long cylindrical shape
and the shape will extend along the mesial–distal
axis of the fi rst lower molar. Soon after, some of
internal cells begin apoptosis. While the fi rst
lower molar grows during cap formation, the pri-
mary enamel knot starts to extend in anterior and
posterior directions. It is suggested that in the
primary enamel knot, most cells do not divide and
its proliferation needs the recruitment of cells
within the enamel organ. However, the underlying
mechanism is still not known due to differences in
mouse strains or measuring stages of embryos.
1.2.1.3 Bell Stage
At the bell stage, the enamel organ delimitates the
dental papilla and starts to form cusps. At this
time, the secondary enamel knots form, which
only precede cusp formation by a few hours. The
secondary enamel knots are taken place at the tips
of the forming cusps at the bell stage. The rela-
tionship between primary and secondary enamel
knots has been suggested in several models. The
gene expression patterns elucidated the primary
enamel knot induces the secondary enamel knots
by a reaction–diffusion-related mechanism.
During the bell stage of tooth development,
the shape of the crown is determined. The growth
of crown results from cell division and reorgani-
zation of inner dental epithelium. Furthermore,
the programmed cell death is also accompanied
by the regulation of cell number in the inner den-
tal epithelium, suggesting its role in determining
the fi nal number of functional ameloblast cells.
1.2.2 Cytodifferentiation
From the lamina stage to the bell stage, changes
in reorganization of the epithelium compartment
can be distinguished. They not only regulate his-
togenesis but also determine the fi nal number and
specifi c positioning of functional ameloblasts.
Amelogenesis, or enamel formation, consists
of two main steps. The fi rst step creates partially
mineralized enamel (about 30 %). The second
step involves extreme infl ux of additional mineral
while removing organic material and water to
form more than 96 % mineral contents. The dif-
ferentiation of epithelial cells into functional ame-
loblast cells includes several morphologic changes
that occur in time and involve growth, elongation
of the cytoplasm, polarization, and secretion of
matrix protein. These epithelial cells exhibit a
unique character of progressively changed pheno-
type. Amelogenesis has been described in as
many as six stages but generally is divided into
three functional phases, known as presecretory,
secretory, and maturation stages (Fig.
1.2 ).
ab c d
Fig. 1.1 ( a – d ) Morphogenesis of the tooth development (from bud to bell stage)
1 Tooth Development: Embryology of the Craniofacial Tissues

4
1.2.2.1 Presecretory Stage
During the presecretory phase, the cells of the
inner enamel epithelium start to differentiate into
ameloblast cells. At the morphogenetic phase,
inner enamel epithelium cells are cuboidal or low
columnar, with large centrally located nuclei and
poorly formed organelles in the proximal portion
of the cells. During differentiation phase, once
stimulated, these cells elongate, their nuclei shift
distally toward the stratum intermedium, and the
Golgi elements increase and migrate distally.
Moreover, the cytoplasm becomes fi lled with
organelles which are needed for the synthesis and
secretion of enamel proteins. At this time, a sec-
ond junctional complex forms at the distal
extremity of the cell, compartmentalizing the
ameloblast cells into a body and a distal exten-
sion called Tomes’ process, against which enamel
develops.
Although the pre-ameloblasts have been
regarded as nonsecreting cells, more and more
researches demonstrate that the enamel protein
secretion starts much earlier, even before the sep-
aration between pre-ameloblasts and pre-
odontoblasts. Ameloblast cells are aligned closely
with each other due to the tight junctional com-
plex or attachment specializations [ 2 ]. These
junctional complexes greatly involved in amelo-
genesis determine what may pass between cells to
enter or leave the enamel at different times.
1.2.2.2 Secretory Stage
The newly formed ameloblasts near the dental
papilla are fl at and the matrix secreted is called
rodless enamel matrix. During the secretory
stage, the ameloblasts exhibit a tall columnar and
polarized morphology and secrete an extracellu-
lar protein-rich matrix. The fi ne structure of
secretory stage ameloblasts indicated their strong
synthetic and secretory activity. The Golgi com-
plex is intense and forms a cylindrical organelle
surrounded by many cisternae of rough endoplas-
mic reticulum. Ameloblast secretion is constitu-
tive, which means the secretion is successively,
and the secretory granules are not stored for pro-
longed periods of time.
When enamel formation begins, Tomes’ pro-
cess comprises only a proximal portion. The
secretory granules are released along the surface
of the process against the newly formed mantle
Fig. 1.2 Ameloblast
differentiation
Z. Liwei et al.

5
dentin to create an initial layer of the enamel
without enamel rods. The very fi rst hydroxyapatite
crystals formed interdigitate with the dentin crys-
tals. After forming the initial enamel layer, ame-
loblast cells migrate from the dentin surface and
form the distal portion of Tomes’ process as an
outgrowth of the proximal portion. The distal
portion extends into and interdigitates beyond the
initial layer of enamel, while the proximal por-
tion penetrates from the distal junctional com-
plex to the enamel layer surface [ 2 ].
It is believed that the distal portion of Tomes’
process progressively lengthens as the enamel
layer thickens and gradually turns to be thinner as
the rod developing in diameter presses it against
the wall of the interrod cavity. Eventually, the
process is squeezed out of existent, leaving a nar-
row area which is fi lled with organic materials
between the enamel rod and interrod enamel.
When the outer layer of enamel is being formed,
the distal portion of Tomes’ process is altered and
orientation also changed, leading to slightly dif-
ference of enamel rods in the outer third of layer
with a more rectilinear trajectory. Finally, the
ameloblasts become the same overall appearance
as when initial enamel was forming. Without the
distal portion of Tomes’ process, the fi nal enamel
has no rods. Notably, the initial, interrod, and
fi nal enamels are developed by the same secre-
tory surface and, indeed, form a continuum [ 2 ].
1.2.2.3 Maturation Stage
During the maturation stage, the ameloblasts aim
at resorbing much of the water and organic matrix
from the enamel in order to allow enough space
for the growing enamel crystals [ 2 ]. This change
results from the thickness and width growth of
preexisting crystals seeded during amelogenesis
formative stage, not due to additional crystal
accumulation.
It is believed that the stratum intermedium
cells are also related to secretory and absorptive
functions of amelogenesis and desmosomes facil-
itate their close contact with ameloblast cells. The
stratum intermedium cells appear less active as
enamel maturation is near completion [ 4 ].
After immature enamel has fully formed,
ameloblast cells undergo several morphologic
changes in preparing maturing the enamel. At
this time, a short transitional stage appears, dur-
ing which ameloblasts become shorter and their
volume and organelle content decrease. At the
maturation stage, some ameloblast cells undergo
programmed apoptosis; roughly about half of the
ameloblasts is reduced during amelogenesis.
In summary, ameloblasts arise from the inner
enamel epithelial cells and experience multiple
morphologic and functional changes. Following
the deposition of a layer of enamel, ameloblasts
deposit enamel in the form of rods or prisms that
become highly mineralized. The arrangement of
ameloblasts with their Tomes’ process plays a
critical role in the formation of enamel rods. The
process of amelogenesis is a series of successive
phases of proliferation, differentiation, secretion,
and maturation, eventually forming the enamel.
1.2.3 Microstructure of the Enamel
The enamel is a composite structure consisting
of mineral and organic phases. At the nanometer
scale, like most other naturally mineralized tis-
sues, dental enamel has hierarchical structures
and surface features [ 5 , 6 ]. On the microscale,
the enamel consists of highly organized architec-
tural units known as enamel prisms. On the
nanoscale, the enamel consists of highly crystal-
line nanorod- like calcium hydroxyl apatite crys-
tallites that are arranged roughly parallel to each
other [
7 ] (Fig. 1.3 ).
1.2.3.1 Enamel Rod
Using the scanning electron microscope and fol-
lowing a short etching part, enamel rods can be
observed in ground or fractured teeth. The enamel
rod represents the mineralized progress of amelo-
blasts and Tomes’ process. Enamel rods cross one
another and follow an undulating course as they
progress from the DEJ toward the enamel surface.
When the arcades connect to each other, enamel
rods have the features of keyholes or paddles, with
the convex surface of the arcades oriented in an
incisal or cuspal order. The enamel rods run nearly
perpendicular to all parts of tooth surface, stop-
ping at the fi nal layer of aprismatic enamel [ 4 ].
1 Tooth Development: Embryology of the Craniofacial Tissues

6
1.2.3.2 Enamel Spindle
Enamel spindles are generated during the differ-
entiation stage of amelogenesis. When the initial
enamel is formed, the enamel spindles become
terminal extensions of the primary dentinal
tubule into the enamel matrix. Spindles exhibit
bulbous structures at DEJ region in mature tooth
enamel.
1.2.3.3 Enamel Lamellae and Cracks
It is believed that enamel lamellae are the result
of local failure of the maturation process. Enamel
lamellae include thin sheets of organic materials
that extend throughout the enamel mineralization
and exhibit vertical orientation from incisal or
cuspal regions to cervical area. Cracks share
some similar characters with lamellae in ground
section and usually appear as artifacts during
teeth processing.
1.2.3.4 Enamel Tufts
Enamel tufts originate from the DEJ and extend
1/3 to 1/2 of the thickness of the enamel matrix.
They are formed during Tomes’ process develop-
ment and also during the elaboration of the initial
enamel of the enamel rod. They represent protein-
rich regions that failed to mature in the enamel
matrix.
1.2.3.5 Interpit Continuum
The secretory product is released from the ame-
loblast cells at two preferred sites. The compara-
tively superfi cial site forms the majority parts of
all developing enamel surfaces. This site is inter-
ameloblastic and the product determines pits,
naming interpit for this stage. In many circum-
stances, the interpit phase is continuous through-
out vast parts of the tissue [ 8 ]. The second
location at which the enamel matrix is released is
from the secretory pole of Tomes’ process proper,
which aims at fi lling in the pit. At these sites,
crystals may have orientations that merge with
those from the interpit stage, building open-sided
prism boundaries.
1.2.3.6 Functional Aspects of Enamel
Structure
The particular organization of the enamel serves
as enhancing hardness and wear resistance. The
parallel formation of crystals perpendicular to the
surface of the teeth brings about the best way for
dense packing of the crystals as well as obviates
the need to nucleate new crystals during enamel
maturation. It has a microporous structure, which
allows extra mineral fl ow in the crystals for fur-
ther growth while degrading matrix components
to be removed. Although single crystal is too
Fig. 1.3 Enamel structures
Z. Liwei et al.

7
fl exible, the perpendicular positions enable the
growth of long whisker-like crystals, allowing
the crystals form into larger domains to be stron-
ger and stiffer.
Enamel crystals are the largest crystals found
in the body. The primary structural unit of enamel
is the enamel rod, which is formed by the secre-
tory activity of ameloblasts. The orientation of
crystals and the distribution of organic matrix are
involved in maintaining structural properties of
enamel.
1.2.4 Enamel Matrix Proteins
Enamel formation requires a remarkable orches-
tration of diverse and essential enamel-secreted
proteins, including amelogenin, ameloblastin,
enamelin, amelotin, tuftelin, dentin sialoprotein,
and apin. Studies provide functional data show-
ing that the disruption of synthesizing, secreting,
and processing these genes can cause different
subtypes of amelogenesis imperfecta (AI), indi-
cating the indispensable role for enamel compo-
sition and maturation [ 9 ].
1.2.4.1 Enamelin
A number of studies have suggested that the fi rst
protein to be secreted by ameloblasts at the den-
tin–enamel junction (DEJ) region is enamelin
[ 10 , 11 ]. Enamelin is a novel acidic enamel pro-
tein that has been postulated to play an essential
role in enamel mineralization. By high-resolution
protein-A gold immunocytochemistry, the acidic
feature of enamelin proteins has been reported to
be in line with its capability to bind to enamel
mineral crystallite surfaces [
12 ]. Enamelin is rich
in aspartic acid and could be arranged in β-sheet
conformation that results in nucleation of the
mineral component.
The enamelin proteins initially secreted at the
very early phase of enamel formation are strictly
expressed by ameloblasts and persist throughout
enamel developing and maturing stages [ 10 ]. The
mutations of enamelin such as enamelin-null
phenotype are associated with aberrations of
enamel, causing AIH2. Several studies have
described the mutations of enamelin gene caus-
ing an autosomal-dominant AI phenotype [
13 ]. In
Enam
−/−
mice, the enamel layer is completely
absent. The crust over the dentin is thin, irregular,
and easily abraded [ 10 ]. These analyses indicate
that enamelin is essential for enamel matrix orga-
nization and mineralization.
1.2.4.2 Amelogenin
The amelogenin proteins of developing dental
enamel are tissue-specifi c components, rich in
leucine, histidine, proline, and glutamyl residues.
Among all the ameloblast-specifi c proteins, ame-
logenin is the most abundant extracellular pro-
tein. The initial enamel layer is dominated by
amelogenin protein secretion. In human, the
amelogenin gene has been shown to be located
on both X and Y chromosomes [ 14 ]. Human
amelogenin genes have 7 exons, with the princi-
pal variation of sequence homology occurring
within exon 6, which codes for most amelogenin
core and the C-terminus [ 15 ].
It has been shown that the amelogenin nano-
spheres, the supramolecular assembly of amelo-
genin, such as elastin, appear as a functional
structural protein during enamel formation [ 16 ].
Two human pedigrees with an X-linked AI (AIH1)
phenotype both share the same mutation in the
amino-terminal, tyrosine-rich amelogenin peptide
(TRAP) segment [ 17 , 18 ]. The recombinant pro-
teins of those two AIH1 point mutations have been
compared with wild-type amelogenin, exhibiting
altered nanosphere dimensions and amelogenin
assembly kinetics [
19 , 20 ]. During in vivo enamel
formation, the amelogenin nanosphere also can be
observed adjacent to HAP crystallites [ 21 ].
It has been found that human-inherited enamel
defect AI often associates with alterations in ame-
logenin X chromosome gene [ 22 ]. The mutations
in amelogenin are known to hypoplastic or hypo-
mineralized enamel [ 22 , 23 ]. Amelogenin knock-
out mice also display abnormal teeth with
chalky-white discoloration, broken tips of incisors
and molars, as well as disorganized hypoplastic
enamel, indicating amelogenin proteins play a
major role in the regulation of enamel thickness
and organization of crystal pattern [ 24 ] (Fig. 1.4 ).
1 Tooth Development: Embryology of the Craniofacial Tissues

8
1.2.4.3 Ameloblastin
Ameloblastin, a cell adhesion molecule, is one of
the unique tooth-specifi c proteins, expressed by
secretory ameloblasts, yet the expression decreases
during enamel maturation [ 25 ]. Shortly after den-
tal epithelium initial differentiation, the cells are
detached from the underlying matrix, resume pro-
liferation, and lose polarity, reversing to undiffer-
entiated one, indicating that ameloblastin
maintains the differentiation state of ameloblasts
at the secretory stage, by binding to ameloblasts
and by inhibiting their proliferation [ 26 ].
At secretory amelogenesis, ameloblastin distri-
bution following the ameloblast cell outline
appears to be a ‘fi shnet’-like partitioning [ 27 ]. The
ameloblastin null mice reveal severe enamel hypo-
plasia, and overexpression of ameloblastin in the
enamel organ infl uences enamel crystallite habit
and enamel rod morphology, resulting in a pheno-
type characteristic of AI. Undoubtedly, these data
all suggest that in the enamel matrix, either gain of
function or loss of function of ameloblastin can
cause enamel alterations. It has also demonstrated
that ameloblastin acts as a nucleator of crystalliza-
tion because it is expressed at mineralization initi-
ation sites within the enamel (Fig.
1.5 ).
1.2.4.4 Amelotin
Murine amelotin has been identifi ed recently,
which is the newest described enamel-specifi c
protein. In developing murine incisors and
molars, expression of amelotin mRNA was
restricted to maturation-stage ameloblasts [ 28 ].
Both murine and human amelotin genes contain
9 exons and 8 introns and are located on chromo-
somes 5 and 4q13.3, respectively, which are close
to the enamelin and ameloblastin genes. The
expression of amelotin mRNA is essentially lim-
ited in postsecretory ameloblasts, experiencing a
dramatic increase from secretory to maturation
phase ameloblasts and subsequently lessening
toward the zone of reduced ameloblast cells. Less
information is available describing whether or
not amelotin is a candidate gene for AI.
1.2.4.5 Tuftelin
Shortly after differentiation, tuftelin, an acidic pro-
tein, is synthesized and secreted. Tuftelin gene
localizes to chromosome 1q21 in human. In secre-
tory stage, the secretory pathway of amelogenin to
the extracellular space is from the Golgi complex
and then to Tomes’ processes [ 29 ]. However,
in vivo tuftelin accumulates in cytoplasmic area
other than the Golgi complex and secretes granules
in both mineralizing and nonmineralizing tissues.
1.2.4.6 Proteolytic Enzymes
There are two major proteinases secreted into the
enamel matrix, including matrix metalloprotein-
ase- 20 (MMP20, enamelysin) and kallikrein-4
(KLK4, enamel matrix serine proteinase-1, or ser-
ine proteinase 17).
Fig. 1.4 Immunostaining of amelogenin
Fig. 1.5 Immunostaining of ameloblastin
Z. Liwei et al.

9
Matrix Metalloproteinase-20 Human MMP20
gene consists of 10 exons and is part of the MMP
gene clusters. The human MMP20 is located on
chromosome 11q22.3, and an autosomal-
recessive form of AI was recently discovered in a
family that had a mutation in the intron 6 splice
acceptor [ 13 ]. In porcine teeth, both ameloblast
and odontoblast cells express MMP20. During
the early stage of enamel formation, MMP20
activity accounts for virtually all of the known
cleavage sites in amelogenin. The mutation of
MMP20 exhibits hypoplastic enamel with
improperly processed amelogenin and rod pat-
tern [
30 ]. In addition, the homozygous MMP20
mutation family reveals severely pigmented, brit-
tle, and soft enamel, which is characterized by
less radiodense.
Kallikrein-4 Human KLK4 gene is located on
chromosome 19q13.41. KLK4 was fi rst discov-
ered in the teeth, but it also expressed in other
tissues such as the prostate. In the teeth, KLK4 is
secreted by different cell types, including odon-
toblasts and late-secretory and maturation phase
ameloblasts [ 31 ]. KLK4 expression during
enamel maturation correlates with the degrada-
tion of enamel proteins, thus indicating it is nec-
essary for the enamel to achieve the high level of
mineralization. KLK4 mutation was found in a
family with autosomal recessive hypomaturation
AI, showing yellow-brown discolored teeth. The
enamel fractured from the teeth has normal thick-
ness but with a decreased mineral content.
Notably, the affected members are all females, so
it is not sure whether KLK4 has an effect on the
prostate. However, only the teeth were apparently
altered by the homozygous KLK4 mutation [
23 ] .
1.3 Pulpodentin Complex
In a mature tooth, dentin is a unique, avascular
mineralized connective tissue that forms the bulk
of the tooth, and dentin encloses a richly inner-
vated and highly vascularized soft connective tis-
sue, the dental pulp. Dentin and pulp are derived
from the dental papilla, whose cells migrate from
the cranial neural crest. The tissues remain
closely associated during development and
throughout the life of an adult tooth and are hence
most commonly referred to as the “pulpodentin
complex.”
During the process of tooth development, most
attentions are focused on the common themes
about odontoblast differentiation that have
emerged and what is known about the infl uence of
tooth-signaling molecules and transcription fac-
tors on the development and homeostasis of the
pulpodentin complex. In addition, the focus is the
theories about the general principles of dentin
matrix formation, particularly the synthesis and
secretion of extracellular matrix molecules and
their postulated roles in the biomineralization of
dentin, and the theories about the development
and homeostasis of differentiated and undifferen-
tiated or stem cell populations can be translated to
regenerative approaches targeted at restoring the
integrity of the adult pulpodentin complex.
1.3.1 Dentin
Fully mature dentin is composed of approximately
70 % inorganic material and 10 % water by weight.
The principal inorganic component consists of
Ca
10
(PO
4
)
6
(OH)
2
(hydroxyapatite). Organic matrix
accounts for 20 % of dentin. 91 % of organic matrix
is collagen, and most of the collagen is type I, with
a minor component of type V. Noncollagenous
matrix components include phosphoproteins, pro-
teoglycans, gamma-carboxyglutamate- containing
proteins (Gla- proteins), acidic glycoproteins,
growth factors, and lipids. By volume, inorganic
matter makes up 45 % of dentin, while organic
molecules and water 33 % and 22 %, respectively.
A characteristic of human dentin is the presence of
tubules that occupy from 20 to 30 % of the volume
of intact dentin. These tubules house the major cell
processes of odontoblasts. The elasticity of dentin
provides fl exibility for the overlying brittle enamel.
1.3.1.1 Structure of Dentin
Dentinal Tubules The characteristic of dentin
is the presence of tubules, which host the major
cell processes of odontoblasts. Tubules form
1 Tooth Development: Embryology of the Craniofacial Tissues

10
around the odontoblast processes and thus trans-
verse the entire width of the dentin from the DEJ
or DCJ to the pulp. They are slightly tapered in
the wider portion situated toward the pulp. This
tapering is the result of the progressive forma-
tion of peritubular dentin, which leads to a con-
tinuous decrease in the diameter of the tubules
toward the enamel.
In coronal dentin, the tubules have a gentle S
shape as they extend from the DEJ to the pulp.
The S-shaped curvature is presumably the result
of the crowding of odontoblasts as they migrate
toward the center of the pulp. As they approach
the pulp, the tubules converge because the sur-
face of the pulp chamber has a much smaller area
than the surface of dentin along the DEJ.
The number and diameter of the tubules are
different at various distances from the pulp, and
the mean number and diameter of tubules
decrease following the increased distance
(Fig. 1.6 ) [ 32 ]. Investigators found the number
and diameter of dentinal tubules to be similar in
rats, cats, dogs, monkeys, and humans, indicating
that mammalian orthodentin has evolved amaz-
ingly constantly [ 33 ].
Near the DEJ, the dentinal tubules ramify into
one or more terminal branches; this is due to the
fact that during the initial stage of dentinogene-
sis, the differentiating odontoblasts extended sev-
eral cytoplasmic processes toward the DEJ, but,
as the odontoblasts withdrew, their processes
converged into one major process (Fig. 1.7 ).
Peritubular Dentin Dentin lining the tubules is
termed peritubular dentin , whereas that between
the tubules is known as intertubular dentin
(Fig. 1.8 ). Presumably precursors of the dentin
matrix that is deposited around each odontoblast
process are synthesized by the odontoblast, trans-
ported in secretory vesicles out into the process,
and released by reverse pinocytosis. With the for-
mation of peritubular dentin, there is a correspond-
ing reduction in the diameter of the process.
Peritubular dentin represents a specialized
form of orthodentin not common to all mammals.
The matrix of peritubular dentin differs from that
of intertubular dentin in having relatively fewer
collagen fi brils and a higher proportion of sul-
fated proteoglycans. Because of its lower content
of collagen, peritubular dentin is more quickly
dissolved in acid than is intertubular dentin.
Peritubular dentin is more highly mineralized
and therefore harder than intertubular dentin. The
hardness of peritubular dentin may provide added
structural support for the intertubular dentin, thus
strengthening the tooth. By preferentially remov-
ing peritubular dentin, acid etching agents used
during dental restorative procedures enlarge the
openings of the dentinal tubules, thus making the
dentin more permeable.
Intertubular Dentin Intertubular dentin is
located between the rings of peritubular dentin
and constitutes the bulk of circumpulpal dentin.
Its organic matrix consists mainly of collagen
c
a
b
Fig. 1.6 Diagram illustrat-
ing the difference in size and
number of tubules on the
occlusal surface of coronal
dentin ( A and B ) and at the
cervical region of the root
surface ( C ). This combina-
tion is responsible for the
exponential increase in
dentin permeability with
depth (From Pashley [
32 ],
p. 106, fi gure 2)
Z. Liwei et al.

11
fi brils having diameters of 500–1000 Å. These
fi brils are oriented approximately at right angles
to the dentinal tubules.
Interglobular Dentin
The term interglobular
dentin refers to organic matrix that remains
unmineralized because the mineralizing globules
Odontoblast process
Rough endoplasmic reticulum
Golgi complex
Mitochondria
Cytoplasm
Cytomembrane
Nucleus
Nucleolus
Nerves
Capillary
Fibre
Fig. 1.7 Diagrammatic
representation of the
differentiated odontoblast
A
B
C
Fig. 1.8 The cross section
of dentinal tubules.
( A ) Peritubular dentin;
( B ) intertubular dentin;
( C ) dentinal tubule
1 Tooth Development: Embryology of the Craniofacial Tissues

12
fail to coalesce. This occurs most often in the
circumpulpal dentin just below the mantle dentin
where the pattern of mineralization is more likely
to be globular than appositional. In certain dental
anomalies (e.g., vitamin D-resistant rickets and
hypophosphatasia), large areas of interglobular
dentin are a characteristic feature.
1.3.1.2 Types of Dentin
Developmental dentin is that which forms during
tooth development. That formed physiologically
after the root is fully developed is referred to as
the secondary dentin . Developmental dentin is
classifi ed as orthodentin , the tubular form of den-
tin found in the teeth of all dentate mammals.
Mantle dentin is the fi rst formed dentin and is
situated immediately subjacent to the enamel or
cementum. It is typifi ed by its content of the thick
fan-shaped collagen fi bers deposited immedi-
ately subjacent to the basal lamina during the ini-
tial stages of dentinogenesis. Spaces between the
fi bers are occupied by smaller collagen fi brils
lying more or less parallel with DEJ or DCJ. The
width of mantle dentin in human teeth has been
estimated at 80–100 μm [ 34 ].
Circumpulpal dentin is formed after the layer
of mantle dentin has been deposited, and it con-
stitutes the major part of developmental dentin.
The organic matrix is composed mainly of colla-
gen fi brils, approximately 500 Å in diameter that
is oriented at right angles to the long axis of the
dentinal tubules. These fi brils are closely packed
together and form an interwoven network.
Predentin is the unmineralized organic matrix
of dentin situated between the layer of odonto-
blasts and the mineralized dentin. Its macromo-
lecular constituents include type I and type II
trimer collagens, and noncollagen elements con-
sist of several proteoglycans (dermatan sulfate,
heparan sulfate, hyaluronate, keratan sulfate,
chondroitin-4-sulfate, chondroitin-6-sulfate),
glycoproteins, glycosaminoglycans (GAGs),
Gla-proteins, dentin phosphoproteins (DPP), and
a tissue-specifi c molecule which is unique to the
odontoblast cell lineage. DPP is produced by the
odontoblast and transported to the mineralization
front, and it is thought to bind to calcium and
play a role in mineralization.
1.3.1.3 Mineralization of Dentin
Mineralization of dentin matrix commences with
the initial increment of mantle dentin. Calcium
phosphate crystals begin to accumulate in matrix
vesicles within the predentin [ 35 ]. Presumably
these vesicles bud off from the cytoplasmic pro-
cesses of odontoblasts. Although matrix vesicles
are distributed throughout the predentin, they are
most numerous near the basal lamina. The apatite
crystals grow rapidly within the vesicles, and in
time, the vesicles rupture. The crystals thus
released mix with crystals from adjoining vesi-
cles to form advancing crystal fronts that merge
to form small globules. As the globules expand,
they fuse with adjacent globules until the matrix
is completely mineralized.
Apparently matrix vesicles are involved only
in mineralization of initial layer of dentin. As the
process of mineralization progresses, the advanc-
ing front projects along the collagen fi brils of the
predentin matrix. Hydroxyapatite crystals appear
on the surface and within the fi brils and continue
to grow as mineralization progresses, resulting in
an increased mineral content of the dentin.
1.3.1.4 Dentinal Sclerosis
Partial or complete obturation of dentinal tubules
may occur as a result of aging or develop in
response to stimuli such as attrition of the tooth
surface or dental caries [ 36 ]. When tubules
become fi lled with mineral deposits, the dentin
becomes sclerotic. Dentinal sclerosis is easily
recognized in histologic ground sections because
of its translucency, which is due to the homoge-
neity of the dentin since both matrix and tubules
are mineralized. Studies using dyes, solvents, and
radioactive have shown that sclerosis results in
decreased permeability of dentin. By limiting the
diffusion of noxious substances through the den-
tin, dentinal sclerosis helps to shield the pulp
from irritation.
One form of dentinal sclerosis is thought to
represent an acceleration of peritubular dentin
formation. This form appears to be a physiologic
process, and in the apical third of the root, it
develops as a function of age [
36 ]. Dentinal
tubules can also become blocked by the precipi-
tation of hydroxyapatite and whitlockite crystals
Z. Liwei et al.

13
within the tubules. This type occurs in the trans-
lucent zone of carious dentin and in attrited den-
tin and has been termed “pathological sclerosis.”
1.3.1.5 Dentin Repair
Dentin that is produced in response to the injury
of primary odontoblasts has been known by sev-
eral different names: irregular secondary dentin,
irritation dentin, tertiary dentin, and reparative
dentin. The term most commonly applied to
irregularly formed dentin is reparative dentin,
presumably because it so frequently forms in
response to injury and appears to be a component
of the reparative process. It must be recognized,
however, that this type of dentin has also been
observed in the pulps of normal unerupted teeth
without any obvious injury [ 37 ]. The reasons of
this phenomenon and the difference between the
development and repair of irregular dentin are
still unclear.
It will be recalled that secondary dentin is
deposited circumpulpally at a very slow rate
throughout the life of the vital tooth. In contrast,
the formation of reparative dentin occurs at the
pulpal surface of primary of secondary dentin at
sites corresponding to areas of irritation. For
example, when a carious lesion has invaded den-
tin, the pulp usually responds by depositing a
layer of reparative dentin over the dentinal
tubules of the primary or secondary dentin which
communicate with the carious lesion. Similarly,
when occlusal wear removes the overlying
enamel and exposes the dentin to the oral envi-
ronment, reparative dentin is deposited over the
exposed tubules. In general, the amount of repar-
ative dentin formed in response to caries or attri-
tion of the tooth surface is proportional to the
amount of primary dentin that is destroyed. Thus,
the formation of reparative dentin allows the pulp
to retreat behind a barrier of mineralized tissue.
Compared to primary dentin, reparative dentin
is less tubular and the tubules tend to be more
irregular with larger lumina. In some cases, no
tubules are formed. The cells that form reparative
dentin are not as columnar as the primary odonto-
blasts of the coronal pulp and are often cuboidal.
The quality of reparative dentin (i.e., the extent to
which it resembles primary dentin) is quite vari-
able. If irritation to the pulp is relatively mild, as in
the case of a superfi cial carious lesion, the repara-
tive dentin formed may resemble primary dentin in
terms of tubularity and degree of mineralization.
On the other hand, reparative dentin deposited in
response to a deep carious lesion may be relatively
tubular and poorly mineralized with many areas of
interglobular dentin. The degree of irregularity of
reparative dentin is probably determined by fac-
tors such as the amount of infl ammation present,
the extent of cellular injury, and the state of dif-
ferentiation of the replacement odontoblasts.
The poorest quality of reparative dentin is usu-
ally observed in association with marked pulpal
infl ammation. In fact, the dentin may be so poorly
organized that areas of soft tissue are entrapped
within the dentinal matrix. In histologic sections,
these areas of soft tissue entrapment impart a
Swiss cheese appearance to the dentin. As the
entrapped soft tissue degenerates, products of tis-
sue degeneration further contribute to the infl am-
matory stimuli assailing the pulp.
1.3.2 Pulp
The pulp is a soft tissue of mesenchymal origin
residing within the pulp chamber and root canals
of the teeth. The primary role of the pulp is to
produce dentin, by specialized cells, the odonto-
blasts, arranged peripherally in direct contact
with dentin matrix. The close relationship
between odontoblasts and dentin is one of several
reasons why dentin and pulp should be consid-
ered as a functional entity, sometimes referred to
as the pulpodentin complex. Following tooth
development, the pulp retains its ability to form
dentin throughout life. This enables the vital pulp
to partially compensate for the loss of enamel or
dentin caused by mechanical trauma or disease.
How well it serves this function depends on many
factors, but the potential for regeneration and
repair is as much a reality in the pulp as in other
connective tissues of the body.
The dental pulp is in many ways similar to
other connective tissues of the body, but its special
characteristic deserves serious consideration. Even
the mature pulp bears a strong resemblance to
1 Tooth Development: Embryology of the Craniofacial Tissues

14
embryonic connective tissue. Certain peculiarities
are imposed on the pulp by the rigid mineralized
dentin in which it is enclosed. Thus it is situated
within a low-compliance environment that limits
its ability to increase in volume during episodes of
vasodilatation and increased vascular permeabil-
ity. The pulp houses a number of tissue elements,
including vascular tissues, nerves, connective tis-
sues, fi bers, ground substance, interstitial fl uid,
odontoblasts, fi broblasts, antigen-presenting cells,
and other minor cellular components.
1.3.2.1 Vascular Tissues
The pulp is actually a microcirculatory system
whose largest vascular components are arterioles
and venules. No true arteries or veins enter or
leave the pulp. Unlike most tissues, the pulp lacks
a true collateral system and is dependent upon the
relatively few arterioles entering through the root
foramina and occasional arteriole through a lat-
eral canal. Since with age there is a gradual reduc-
tion in the luminal diameters of these foramina,
the vascular system of pulp decreases progres-
sively. Since the pulp is relative incompressible,
the total volume of blood within the pulp chamber
cannot be greatly increased, although reciprocal
volume changes can occur between arterioles,
venules, lymphatics, and extravascular tissue. In
the pulp, therefore careful regulation of blood
fl ow is of critical importance.
Blood from the dental artery enters the tooth
via the arterioles, and these vessels pass through
the apical foramen or foramina in company with
nerve bundles. Smaller vessels may enter the
pulp via lateral or accessory canals. As the arteri-
oles pass into the coronal pulp, they fan out
toward the dentin, diminish in size, and give rise
to a capillary network in the subodontoblastic
region. This network provides the odontoblasts
with a rich source of metabolites.
Capillary blood fl ow in the coronal portion of
the pulp is nearly twice that in the root portion
[
13 ]. Moreover, blood fl ow in the region of the
pulp horns is greater than in other areas of the
pulp. In young teeth, capillaries commonly
extend into the odontoblast layer, thus assuring
an adequate supply of nutrients for the metaboli-
cally active odontoblasts. The subodontoblastic
capillaries are surrounded by a basement mem-
brane, and occasionally, fenestrations (pores) are
observed in capillary walls. These fenestrations
are thought to provide rapid transport of fl uid and
metabolites from the capillaries to the adjacent
odontoblasts.
1.3.2.2 Nerve Fibers
The pulp is a rather unique sensory organ capable
of transmitting information from its sensory recep-
tor to the central nervous system. Being encased in
a protective layer of dentin, which in turn is cov-
ered with enamel, it might be expected to be quite
unresponsive to stimulation; yet, despite the low
thermal conductivity of dentin, the pulp is undeni-
ably sensitive to thermal stimuli such an ice cream
and hot drinks. The innervation of the pulp
includes both afferent neurons, which conduct
sensory impulses, and autonomic fi bers, which
provide neurogenic modulation of the microcircu-
lation and perhaps regulate dentinogenesis.
Nerve fi bers are usually classifi ed according
to their diameter, conduction velocity, and func-
tion. In the pulp, there are two main types of sen-
sory nerve fi bers, myelinated A fi bers and
unmyelinated C fi bers. The A fi bers include both
A-8 and A-5 fi bers. A-8 fi bers may be slightly
more sensitive to stimulation than the A-5 fi bers,
but functionally these fi bers are grouped together.
Approximately 90 % of the A fi bers are A-8
fi bers, and A-8 fi ber terminals principally local in
region of dentin–pulp junction, and the stimula-
tion threshold of A fi bers is relatively low con-
trast with C fi bers which is probably distribute
throughout the pulp and feel the pain of burning,
aching.
In the human premolar, the number of unmy-
elinated axons entering the tooth at the apex
reached a maximum number shortly after tooth
eruption [
38 ]. At this stage, an average of 1800
unmyelinated axons and more than 400 myelin-
ated axons were observed, although in some teeth,
fewer than 100 myelinated axons were present.
The number of A fi bers gradually increased to
more than 700 fi ve years after eruption. The rela-
tively late appearance of A fi bers in the pulp may
help to explain why the electric pulp test tends to
be unreliable in young teeth.
Z. Liwei et al.

15
The nerve bundles pass upward through the
radicular pulp together with blood vessels. Once
they reach the coronal pulp, they fan out beneath
the cell-rich zone, branch into smaller bundles,
and fi nally ramify into a plexus of single-nerve
axons known as the plexus of Raschkow (Fig. 1.9 )
[ 39 ]. Full development of this plexus does not
occur until the fi nal stages of root formation.
One study showed that a reduction in pulpal
blood fl ow induced by stimulation of sympathetic
fi bers leading to the pulp results in depressed
excitability of pulpal A fi bers. The excitability of
C fi bers is less affected than that of A fi bers by a
reduction in blood fl ow. Pulpal nerve fi bers con-
tain neuropeptides such as neuropeptide Y, calci-
tonin gene-related peptide (CGRP), vasoactive
intestinal polypeptide (VIP), tyrosine hydroxy-
lase, and substance P. The release of these pep-
tides can be trigged by such things as tissue
injury, complement activation, antigen-antibody
reactions, or antidromic stimulation of the infe-
rior alveolar nerve.
Interestingly, pulpal nerve fi bers are relatively
resistant to pulp necrosis. This is apparently due
to the fact that nerve bundles in general are more
resistant to autolysis than other tissue elements.
Even in degenerating pulps, C fi bers might still
be able to respond to stimulation. Furthermore, it
may be that C fi bers remain excitable even after
blood fl ow has been compromised in the diseased
pulp, for C fi bers are better able to function in the
presence of hypoxia. This may offer an explana-
tion as to why instrumentation of the root canals
of apparently nonvital teeth sometimes elicits a
moderate level of pain.
1.3.2.3 Connective Tissue Fibers
Two types of structural proteins are found in the
pulp: collagen and elastin. Elastin fi bers are
confi ned to the walls of the arterioles and, unlike
collagen, are not a part of the ECM.
A single collagen molecule, referred to as tro-
pocollagen, consists of three polypeptide chains,
designated as either a-1 or a-2 depending on their
amino acid composition and sequence. The dif-
ferent combinations and linkages of chains mak-
ing up the tropocollagen molecule have allowed
collagen fi bers and fi brils to be classifi ed into a
number of types. Type I is found in the skin, ten-
don, bone, dentin, and pulp. Type II occurs in car-
tilage. Type III is found in most unmineralized
connective tissues, and it is a fetal form found in
the dental papilla and the mature pulp. Type IV
and VII collagens are components of basement
membranes. Type V collagen is a constituent of
interstitial tissues. Type I collagen is synthesized
by odontoblasts and osteoblasts, and fi broblasts
synthesize types I, III, V, and VII.
In collagen synthesis, the protein portion of
the molecule is formed by the polyribosomes of
the RER of connective tissue cells. The proline
and lysine residues of the polypeptide chains are
hydroxylated in the cisternae of the RER, and the
chains are assembled into a triple-helix confi gu-
ration in the smooth endoplasmic reticulum. The
product of this assembly is termed procollagen,
and it has a terminal unit of amino acids known
as the telopeptide of the procollagen molecule.
When these molecules reach the Golgi complex,
they are glycosylated and packaged in secretory
vesicles. The vesicles are transported to the
plasma membrane and secreted via exocytosis
into the extracellular matrix, thus releasing the
procollagen. Here the terminal telopeptide is
cleaved by a hydrolytic enzyme, and the tropo-
collagen molecules begin aggregating to form
collagen fi brils. It is believed that aggregation of
tropocollagen is somehow mediated by the
Fig. 1.9 Light microscopy showing the relationship of
parietal layer of nerves (plexus of Raschkow) below to the
cell-rich, cell-free zones, odontoblasts, and dentin at top
of the picture (From Avery [
39 ], p. 208, fi gure 8)
1 Tooth Development: Embryology of the Craniofacial Tissues

16
GAGs. The conversion of soluble collagen into
insoluble fi bers occurs as a result of cross-linking
of tropocollagen molecules.
In the young pulp, small collagen fi bers stain
black with silver impregnation stains and are thus
referred to as argyrophilic fi ber. They are very
similar, if not identical, to reticular fi bers in other
loose connective tissues in that they are not
arranged in bundles and tend to form delicate net-
works. The presence of collagen fi bers passing
from the dentin matrix between odontoblasts into
the dental pulp has been reported in fully erupted
teeth [ 40 ]; these fi bers are often referred to as von
Korff fi bers . Large collagen fi ber bundles are not
argyrophilic but can be demonstrated with special
histochemical methods such as the Masson tri-
chrome stain or Mallory’s triple connective tissue
stain. These fi bers are much more numerous in the
radicular pulp than in the coronal pulp. The high-
est concentration of these larger fi ber bundles is
usually found in the radicular pulp near the apex.
1.3.2.4 Ground Substance
Connective tissue is a system consisting of cells
and fi bers, both embedded in the pervading
ground substance. Cells that produce connective
tissue fi bers also synthesize the major constitu-
ents of ground substance. The term extracellular
matrix ECM is used to describe ground sub-
stance, regarding it as the material into which
fi bers are deposited. Because of its content of
polyelectric polysaccharides, the ECM is respon-
sible for the water-holding properties of connec-
tive tissues.
Nearly all proteins of the ECM are glycopro-
teins. Proteoglycans are an important subclass of
glycoproteins. These molecules support cells,
provide tissue turgor, and mediate a variety of
cell interactions. They have in common the pres-
ence of GAG chains and a protein core to which
the chains are linked. Except for heparan sulfate
and heparin, the chains are composed of disac-
charides. The primary function of GAG chains is
to act as adhesive molecules that can bond to cell
surfaces and other matrix molecules.
Fibronectin is a major surface glycoprotein
that, together with collagen, forms an integrated
fi brillary network that infl uences adhesion,
motility, growth, and differentiation of cells.
Laminin, an important component of basement
membranes, binds to type IV collagen and cell
surface receptors. Tenascin is another substrate
adhesion glycoprotein.
Degradation of ground substance can occur in
certain infl ammatory lesions in which there is a
high concentration of lysosomal enzymes.
Proteolytic enzymes, hyaluronidases, and chon-
droitin sulfatases of lysosomal and bacterial ori-
gin are examples of acid hydrolytic enzymes that
can attract components of the ground substance.
The pathways of infl ammation and infection are
infl uenced by the state of polymerization of the
ground substance components.
1.3.2.5 Lymphatics
The existence of lymphatics in the pulp has been
a matter of debate, since it is not easy to distin-
guish between venules and lymphatics by ordi-
nary light microscopic techniques, although
some studies utilizing light and electron micros-
copy have described lymphatic capillaries in
human and in cat dental pulps.
1.3.2.6 Accessory Canals
Occasionally during formation of the root sheath,
a break develops in the continuity of the sheath,
producing a small gap. When this occurs, den-
tinogenesis does not take place opposite to the
defect. The result is a small “accessory” canal
between the dental sac and the pulp. An acces-
sory canal can become established anywhere
along the root, thus creating a periodontal–end-
odontic pathway of communication and a possi-
ble portal of entry into the pulp if the periodontal
tissues lose their integrity. In periodontal disease,
the development of a periodontal pocket may
expose an accessory canal and thus allow micro-
organisms or their metabolic products to gain
access to the pulp.
1.3.2.7 Morphologic Zones of Pulp
Odontoblast Layer The outermost stratum of
cells of the healthy pulp is the odontoblast layer.
This layer is located immediately subjacent to the
predentin, with the odontoblast processes passing
on through the predentin into the dentin.
Consequently, the odontoblast layer is actually
Z. Liwei et al.

17
composed of the cell bodies of the odontoblasts.
Additionally, capillaries, nerve fi bers, and den-
dritic cells may be found among the odontoblasts.
The odontoblast layer in the coronal pulp con-
tains more cells per unit area than in the radicular
pulp. Whereas the odontoblasts of the mature
coronal pulp are usually columnar (Fig.
1.10 ),
those in the midportion of the radicular pulp are
more cuboidal. Near the apical foramen, the
odontoblasts appear as a fl attened cell layer.
Since there are fewer dentinal tubules per unit
area in the root than in the crown of the tooth, the
odontoblast cell bodies are less crowded and are
able to spread out laterally.
Cell-Poor Zone Immediately subjacent to the
odontoblast layer in the coronal pulp, there is
often a narrow zone approximately 40 μm in
width that is relatively free of cells. It is traversed
by blood capillaries, unmyelinated nerve fi bers,
and the slender cytoplasmic processes of fi bro-
blasts. The presence or absence of the cell-poor
zone depends on the functional status of the pulp.
It may not be apparent in young pulps where den-
tin forms rapidly or in older pulps where repara-
tive dentin is being produced.
Cell-Rich Zone Usually conspicuous in the
subodontoblastic area is a stratum containing a
relatively high proportion of fi broblasts compared
with the more central region in the pulp. It is
much more prominent in the coronal pulp than in
the radicular pulp. Besides fi broblasts, the cell-
rich zone may include a variable number of mac-
rophages and lymphocytes.
On this evidence obtained in rat molar teeth, it
has been suggested that the cell-rich zone forms
as a result of peripheral migration of cells popu-
lating the central regions of the pulp, commenc-
ing at about the time of tooth eruption. Although
cell division within the cell-rich zone is a rare
occurrence in normal pulps, death of odonto-
blasts causes a great increase in the rate of mito-
sis. Since irreversibly injured odontoblasts are
replaced by cells that migrate from the cell-rich
zone onto the inner surface of the dentin, this
motility ability is probably the fi rst step in the
formation of a new odontoblast.
Pulp Proper The pulp proper is the central mass
of the pulp. It contains the larger blood vessels
and nerves. The connective tissue cells in this
zone are fi broblasts or pulpal cells.
1.3.3 Cells in the Dental Pulp
1.3.3.1 Odontoblast
Because it is responsible for dentinogenesis both
during tooth development and in the mature
Fig. 1.10 Beside dentin ( 1 )
and predentin ( 2 ), there are
tall columnar odontoblasts ( 3 )
of the coronal pulp. Note the
presence of the cell-rich zone
( 5 ) and cell-poor zone ( 4 )
1 Tooth Development: Embryology of the Craniofacial Tissues

18
tooth, the odontoblast is the most characteristic
cell of the pulpodentin complex. During dentino-
genesis, the odontoblasts form the dentinal
tubules, and their presence within the tubules
makes dentin a living tissue [ 41 ].
Differentiation of epithelial and mesenchymal
cells into ameloblasts and odontoblasts, respec-
tively, occurs during the bell stage of tooth devel-
opment [ 42 ]. The preameloblasts differentiate at a
faster rate than the corresponding odontoblasts so
that at any given level, mature ameloblasts appear
before the odontoblasts have fully matured. In
spite of this difference in rate of maturation, den-
tin matrix is formed before enamel matrix. As the
ameloblasts undergo differentiation, changes are
happening across the basement membrane in the
adjacent dental papilla. Before differentiation of
odontoblasts, the dental papilla consists of
sparsely distributed polymorphic mesenchymal
cells with wide intercellular spaces. With the
onset of differentiation a single layer of cells, the
presumptive odontoblasts (preodontoblasts) align
themselves along the basement membrane sepa-
rating the inner enamel epithelium from the dental
papilla. These cells stop dividing and elongate
into short columnar cells with basally situated
nuclei (Fig. 1.7 ). Several cytoplasmic projections
from each of these cells extend toward the basal
lamina. At this stage, the preodontoblasts are still
relatively undifferentiated.
Dentinogenesis fi rst occurs in the developing
tooth at sites where the cusp tips or incisal edge
will be formed. It is in this region that odonto-
blasts reach full maturity and become tall colum-
nar cells. The production of the fi rst dentin matrix
involves the formation, organization, and matura-
tion of collagen fi brils and proteoglycans. As
more collagen fi brils accumulate subject to the
basal lamina, the lamina becomes discontinuous
and eventually disappears. This occurs as the col-
lagen fi bers become organized and extend into
the spaces between the ameloblast processes.
Concurrently the odontoblasts extend several
small processes toward the ameloblasts. Some of
these become interposed between the processes
of ameloblasts, resulting in the formation of
enamel spindles (dentinal tubules that extend into
the enamel). Membrane-bound vesicles bud off
from the odontoblast processes and become
interspersed among the collagen fi bers of the
dentin matrix. These vesicles subsequently play
an important role in the initiation of
mineralization. With the onset of dentinogenesis,
the dental papilla becomes the dental pulp.
As predentin matrix is formed, the odonto-
blasts commence to move away toward the central
pulp, depositing matrix at a rate of approximately
4–8 μm per day in their wake. Within this matrix,
a process from each odontoblast becomes accen-
tuated and remains to form the primary odonto-
blast process. It is around these processes that the
dentinal tubules are formed.
Dentinal tubule forms around each of the
major odontoblastic processes which occupy
space within the tubule and somehow mediates
the formation of peritubular dentin. Microtubules
and microfi laments are the principal ultrastruc-
tural components of the odontoblast process and
its lateral branches. Microtubules extend from
the cell body out into the process. These straight
structures follow a course that is parallel with the
long axis of the cell and impart the impression of
rigidity. Although their precise role is unknown,
theories as to their functional signifi cance sug-
gest that they may be involved in cytoplasmic
extension, transport of materials, or simply the
provision of a structural framework. The plasma
membrane of the odontoblast process closely
approximates the wall of the dentinal tubule.
Localized constrictions in the process occasion-
ally produce relatively large spaces between the
tubule wall and the process. Such spaces may
contain collagen fi brils and fi ne granular mate-
rial, which presumably represents ground sub-
stance. The peritubular dentin matrix lining the
tubule is circumscribed by an electron-dense lim-
iting membrane.
The odontoblast is considered to be fi xed post-
mitotic cell in that once it has fully differentiated,
it apparently cannot undergo further cell division.
If this is indeed the case, the life-span of the
odontoblast coincides with the life-span of the
viable pulp.
Apparently the odontoblast synthesizes mainly
type I collagen, although small amounts of type V
collagen have been found in the ECM. In addition
Z. Liwei et al.

19
to proteoglycans and collagen, the odontoblast
secretes phosphophoryn, a phosphoprotein involved
in extracellular mineralization. Phosphophoryn is
unique to dentin and is not found in any other mes-
enchymal cell lines. The odontoblast also secretes
alkaline phosphatase, an enzyme that is closely
linked to mineralization but whose precise role is
yet to be illuminated.
In contrast to the active odontoblast, the rest-
ing or injured odontoblast has a decreased num-
ber of organelles and may become progressively
shorter. These changes can begin with the com-
pletion of root development.
1.3.3.2 Pulp Fibroblast
The most numerous cells of the pulp tissues
appear to be tissue-specifi c cells capable of giv-
ing rise to cells that are committed to differentia-
tion as odontoblasts, given the proper signal.
These cells synthesize type I and III collagen as
well as proteoglycans and GAGs; since they
degrade collagen, they are also responsible for
collagen turnover in the pulp.
Although distributed throughout the pulp,
fi broblasts are particularly abundant in the cell-
rich zone. The early differentiating fi broblasts are
polygonal and appear to be widely separated and
evenly distributed within the ground substance.
Cell-to-cell contacts are established between the
multiple processes that extend out from each of
the cells. Many of these contacts take the form of
gap junctions, which provide for electronic cou-
pling of one cell to another. As they mature, the
cells become stellate in form and the Golgi com-
plex enlarges, the RER proliferates, secretory
vesicles appear, and the fi broblasts take on the
characteristic appearance of protein-secreting
cells. Along the outer surface of the cell body,
collagen fi brils commence to appear. With an
increase in the number of blood vessels, nerves,
and fi bers, there is a relative decrease in the num-
ber of fi broblasts in the pulp.
A colleague once remarked that the fi broblasts
of the pulp are very much like Peter Pan in that
they never grow up. There may be an element of
truth in this statement, for these cells do seem to
remain in a relatively undifferentiated modality as
compared to fi broblasts of most other connective
tissues. This perception has been fortifi ed by the
observation of large numbers of reticulin-like
fi bers in the pulp. However, it has been concluded
that because of distinct histochemical differences,
reticulin fi bers, such as most of gingiva and lym-
phoid organ, are not present in the pulp. In the
young pulp, the nonargyrophilic collagen fi bers
are sparse, but they progressively increase in
number as the pulp ages.
Many experimental models have been devel-
oped to study wound healing in the pulp, particu-
larly dentinal bridge formation following pulp
exposure or pulpotomy. One study demonstrated
that mitotic activity preceding the differentiation
of replacement odontoblasts appears to occur pri-
marily among fi broblasts. Thus, it appears that
pulpal fi broblasts can be regarded as odontopro-
genitor cells.
1.3.3.3 Macrophage
Tissue macrophage or histiocytes are monocytes
that have left the bloodstream, entered the tis-
sues, and differentiated into macrophages. They
are usually found in close proximity to blood ves-
sels. These cells are quite active in endocytosis
and phagocytosis. Because of their mobility and
phagocytic activity, they are able to act as scaven-
gers, removing extravasated red blood cells, dead
cells, and foreign bodies from the tissue ingested
material destroyed by the action of lysosomal
enzymes.
In addition, a proportion of macrophages,
when primed by cytokines, participate in immune
reactions by processing antigen and presenting it
to lymphocytes. The processed antigen is bound
to class II histocompatibility antigens on the
macrophage, where it can interact with specifi c
receptors present on immunocompetent T cells.
Such interaction is obligatory for the induction of
cell-mediated immunity. When activated by the
appropriate infl ammatory stimuli, macrophages
are capable of producing a large variety of solu-
ble factors including interleukin-1, tumor necro-
sis factor, growth factors, and other cytokines.
1.3.3.4 Dendritic Cell
Dendritic cells, like macrophages, are cells of the
immune system. Similar cells are found in the
1 Tooth Development: Embryology of the Craniofacial Tissues

20
epidermis and mucous membranes, where they are
called Langerhans cells . Dendritic cells are primar-
ily found in lymphoid tissues, but they are also
widely distributed in connective tissues, including
the pulp. These cells are termed antigen- presenting
cells and are characterized by dendritic cytoplasmic
processes and the presence of cell surface class II
antigens. Like macrophages, dendritic cells phago-
cytose and process antigens but are otherwise only
weakly phagocytic. Together with macrophages
and lymphocytes, dendritic cells are believed to par-
ticipate in immunosurveillance in the pulp.
1.3.3.5 Lymphocyte
T lymphocytes and B lymphocytes were found in
normal pulps from the human teeth. T
8
(suppres-
sor) lymphocytes were the predominant T lym-
phocyte subset present in pulps. Lymphocytes
have also been observed in the pulps of impacted
teeth. The presence of macrophages, dendritic
cells, and T lymphocytes indicates that the pulp is
well equipped with cells required for the initia-
tion of immune responses.
1.3.3.6 Mesenchymal Cell
Mesenchymal stem cells (MSCs) are found in
many adult tissues and organs. In tissues that
grow continuously or exhibit high rates of cell
turnover, MSCs provide a renewable source of
progenitor cells to differentiate and replace those
lost. In the majority of tissues, MSCs are found in
small numbers and remain quiescent until mobi-
lized in response to tissue damage. In pulp tissue,
some authors hold the opinion that primordial
mesenchymal cells persist in adult pulp tissues as
“undifferentiated” mesenchymal cells. However,
during wound healing, well-differentiated fi bro-
blasts undergo rapid serial division to give rise to
new fi broblasts. Similarly, replacement odonto-
blasts are derived from mature fi broblasts.
Consequently, there is no need to postulate that in
the pulp new mesenchymal cells arise from cells
other than pulpal fi broblasts. This controversy is
still unclear and needs more researches.
1.3.3.7 Mast Cell
Mast cells are widely distributed in the connec-
tive tissues, where they occur in small groups in
relation to blood vessels. This cell has been the
subject of considerable attention because of its
dramatic role in infl ammatory reactive. But mast
cells also present in the normal pulp tissue,
although they are routinely found in chronically
infl amed pulps.
1.4 Root Development
Root development is one of the important stages
in tooth development process, following the bell
stage. The interaction between dental epithelial
and neural crest-derived mesenchymal cells is
essential for tooth development.
1.4.1 The Initiation of Tooth Root
Development
After the crown formation is nearly complete, the
tooth root begins to develop. Then, the special
bilayered epithelial sheath is formed from the
outer and inner enamel epithelium at the neck
ring of the crown and grows in the apical direc-
tion, termed Hertwig’s epithelial root sheath
(HERS). HERS, dental papilla cells, and dental
follicle cells form the organ primordium of tooth
development together [ 43 ]
HERS is the morphogenesis signal of the ini-
tiation of tooth root development [ 44 ].
Morphologically, the epithelial root sheath is
located between the two regions of neural crest-
derived mesenchyme: the dental papilla and the
dental follicle. When the HERS grows apically,
the dental papilla cells adjacent to the inner epi-
thelial layer of the HERS and the epithelial base-
ment membrane induced to become odontoblasts
and later to form root dentin. After root dentin
formation, the epithelial root sheath enveloping
the root begins to be interrupted or perforated.
The formation of a mesh-like structure in the
HERS allows dental follicle cells to contact the
newly formed root dentin surface through the
epithelial root sheath. These dental follicle cells
differentiate into cementoblasts to form cemen-
tum. In addition, some of the HERS cells undergo
epithelial–mesenchymal transition to become
Z. Liwei et al.

21
cementoblasts and form cementum [ 13 ]. At the
same time, collagen fi bers secreted by dental fol-
licle cells are embedded into the new cementum
matrix and fi x the root in the jaw bone. Following
tooth root development and elongation, the tooth
erupts into the oral cavity to establish occlusal
contacts with the opposing teeth and performs its
physiological function.
Previous studies have shown that Hertwig’s
epithelial root sheath plays an important role in
root development, but the fate of HERS has
remained unknown. Until now, the fate of HERS
and its function are not clear. At least 6 possible
outcomes of HERS have been proposed: (1) epi-
thelial cell rests of Malassez, (2) apoptosis, (3)
incorporation into the advancing cementum front,
(4) epithelial–mesenchymal transformation, (5)
migration toward the periodontal ligament, and
(6) differentiation into cementoblasts [ 10 ].
1.4.1.1 Epithelial Cell Rests
of Malassez (ERM)
Epithelial cell rests of Malassez (ERM) are
involved in the maintenance and homeostasis of
the periodontal ligament. The ERM have a num-
ber of functions, such as to prevent root resorp-
tion, induce cementum formation, and maintain
the homeostasis of the PDL [ 45 ]. On the other
hand, ERM can be stimulated to proliferate in
response to injury in rats. When stimulated by
infl ammatory cytokines, ERM can proliferate
and differentiate into the lining epithelium of
periapical cysts.
Primary HERS/ERM cells had typical epithe-
lial cell morphology and characteristics, and they
maintained for more than fi ve passages. They
expressed epithelial stem cell-related genes:
ABCG2, ΔNp63, p75, EpCAM, and Bmi-1.
Moreover, the expression of embryonic stem cell
markers such as Oct-4, Nanog, and SSEA-4 was
detected [
46 ]
1.4.1.2 Induction of Differentiation
of Mesenchymal Stem Cells
HERS, morphologically, is a structural boundary
of two dental ectomesenchymal tissues: dental
papilla and dental follicle [ 47 ]. It breaks up into
epithelial rests and cords, allowing other cells to
come in contact with the outer dentin surface.
This sandwich structure plays at least two impor-
tant roles during root formation: biomineraliza-
tion (cementogenesis and dentin formation) and
induction of root organization.
HERS cells may be involved in regulating dif-
ferentiation of periodontal ligament stem cells
(PDLSCs) and forming cementum in vivo [
48 ].
Dental follicle cell sheets induced by HERS cells
are able to produce periodontal tissues through
epithelial–mesenchymal interactions.
HERS cells are detectable on the surface of
the root throughout root formation and do not
disappear. Most of the HERS cells are attached to
the surface of the cementum, and others separate
to become the epithelial rest of Malassez. HERS
cells secrete extracellular matrix components
onto the surface of the dentin before dental folli-
cle cells penetrate the HERS network to contact
dentin. HERS cells also participate in the cemen-
tum development and may differentiate into
cementocytes.
1.4.2 Related Signaling Pathway
of Root Morphogenesis
Recently, several genes were found to play cru-
cial role in the process of root development.
1.4.2.1 TGF-β/BMP Signaling
The TGF-β superfamily of cytokines is composed
of TGF-β, BMPs, activins, and related proteins.
TGF-β signaling plays an important role in devel-
opmental biology, disease, and regeneration [
49 ].
Smad4 is a central mediator of the canonical
TGF-β signaling pathway. Deletion of Smad4
results in the blockage of TGF-β/BMP signaling.
Ablation of Smad4 in the dental mesenchyme
(Osr2-IresCre:Smad4
fl /fl
mice) results in short
root formation and defects in odontoblast differ-
entiation and dentin formation. Moreover, ecto-
pic bone-like structures replaced normal dentin
in the teeth of Osr2-IresCre:Smad4
fl /fl
mutant
mice. Loss of Smad4 results in the upregulation
of canonical Wnt signaling and downregulation
of Dkk1 and Sfrp1, which are Wnt pathway
inhibitors.
1 Tooth Development: Embryology of the Craniofacial Tissues

22
Comparing different animal models provides
more detail about TGF-β signaling during root
dentin formation. In Osr2-IresCre:Smad4
fl /fl
mice, dental mesenchyme differentiation is
arrested at the late bell stage and secretory stage,
with no detectable expression of Dspp.
Odontoblast differentiation is delayed and Dspp
expression is eventually detectable in mice lack-
ing Tgfbr2 [ 50 ].
Bone morphogenetic protein 4 (BMP4) is
secreted by mesenchymal cells, acting on the dental
epithelium as a regulator of cell differentiation dur-
ing crown formation. Studies assessed the localiza-
tions of BMP4, BMP4 receptor (BMPR-1B), and
BMPR-2 during molar root formation in mouse
[ 51 ]. In K14-Noggin transgenic mice, molar epithe-
lial root sheath cell proliferation is abated, and root
development is retarded [ 52 ].
In the early development of mouse molar root,
mesenchymal progenitor cell markers such as
STRO-1 with BMP receptors are expressed in the
dental follicle, hence the hypothesis that mesen-
chymal STRO-1-positive cells are epithelial root
sheath BMP signaling pathways of target cells.
1.4.2.2 SHH Signaling
Shh, a member of the hedgehog signaling family,
is expressed in the dental epithelium and plays an
essential role during tooth development. During
root development, Shh is strongly expressed in
the HERS, which suggests a function in root for-
mation. Gli1, a transcript activated by Shh, is also
detectable in the root epithelium (HERS) and
mesenchyme [
53 ].
1.4.2.3 Wnt Signaling
The Wnt family of proteins plays an important
role in morphogenesis and cellular differentiation
in many tissues. The canonical Wnt signaling
pathway involves the stabilization and nuclear
accumulation of β-catenin, which activates the
expression of Wnt target genes. It is well known
that Wnt/β-catenin signaling plays multiple roles
in various stages of tooth morphogenesis [ 54 ].
During dentin formation, Wnt10a is expressed in
odontoblast-lineage cells, and Axin2 is also
expressed in developing odontoblasts and dental
pulp cells. In addition, Dkk1, an inhibitor of Wnt
signaling, is strongly expressed in pre-
odontoblasts but is decreased in secretory odon-
toblasts. Moreover, it was recently reported that
Lef-1 overexpression accelerates odontoblast dif-
ferentiation of dental pulp cells and constitutive
β-catenin stabilization in the dental mesenchyme
can lead to excessive dentin formation. β-catenin
has been shown to be strongly expressed in
odontoblast- lineage cells and is required for root
formation. Tissue-specifi c inactivation of
β-catenin in developing odontoblasts produced
molars lacking roots and aberrantly thin incisors.
These reports strongly suggest that modulation of
Wnt/β-catenin signaling may play an important
role in odontoblast differentiation.
1.4.2.4 Notch Signaling
The Notch pathway regulates the renewal and
fate decisions of stem cells in multiple tissues.
Notch1 and Notch2 as well as the Notch target
gene Hes1 are expressed in the putative stem
cells in the continuously growing mouse incisors.
Notch signaling is required for epithelial stem
cell survival and enamel formation in the contin-
uously growing mouse incisor [ 55 ].
At embryonic day 19, the molar and the inci-
sor of rat began differentially developing: the
molar formed double-layered cells of the root
sheath while the incisor formed a cervical loop.
By using the subtractive hybridization method, a
subtraction cDNA library of the rat molar and
incisor tissues was successfully constructed.
Differentially expressed gene clones were evalu-
ated by dot blot and sequencing. Sel1l, Nfi c,
Edar, GATA6, and some novel genes were found
differentially expressed, which were strongly
related to the tooth root patterning. The Notch
signaling contributes to the maintenance of cervi-
cal loop stem cell niches. SEL1L, the negative
regulating factor of notch signaling, was strongly
expressed in the molars but weakly expressed in
the incisors.
1.4.3 Tooth Eruption
Tooth eruption is the movement of the tooth from
the original developmental site to the functional
Z. Liwei et al.

23
position through the alveolar bone and gingiva in
the mouth. It is a continuous process, which can
be divided into 5 stages, respectively for pre-
eruptive movement, intraosseous eruption,
mucosal penetration, preocclusal eruption, and
post-occlusal eruption. Each step involves
intense reciprocal interactions between the tooth
and its surrounding tissues and is temporally and
spatially controlled to coordinate the growth of
the jaw and the position of other teeth. However,
the specifi c cellular, molecular, and genetic
mechanisms governing tooth eruption remain
unclear.
Until now, there are several theories explain-
ing how the tooth erupts [ 56 ]. One of the most
acceptable theories is that asymmetric bone
remodeling around the tooth is responsible for
the teeth moving into the oral cavity. When bone
resorbed on the coronal side, bone formed on the
basal side of the tooth. It is thought that the den-
tal follicle, which is a loose connective tissue sac
surrounding the enamel organ of each tooth,
plays a critical role in regulating osteoclasts and
osteoblasts during this process. In 1980s, it was
fi rstly shown that the removal of the dental folli-
cle from developing premolars of dogs prevented
tooth eruption. Meanwhile, if the tooth germ was
removed and replace with another artifi cial tooth,
which the dental follicle was kept intact in situ,
the artifi cial tooth still erupted on schedule.
Furthermore, removal of either the coronal or
basal halves of the dental follicle also prevented
tooth eruption, with the coronal part controlling
bone resorption and the eruption pathway, as
well as the basal part causing bone formation.
Recently, some important signaling molecules
were identifi ed at both these processes for tooth
eruption, such as RANKL, BMP2, and so on.
These studies provided convincing evidence that
the dental follicle is essential for tooth eruption
and also challenged the previously supposed
requirement of dental pulp, periodontal liga-
ments, and roots in the process of tooth
eruption.
For a long time, root development has been
considered as the main force responsible for the
tooth eruption. However, more and more studies
found that rootless tooth can still erupt into the
oral cavity in many species, including human,
dogs, monkeys, and rodents. In some patients
with special diseases, which may affect root
development, the rootless tooth crown did erupt
into the mouth. Developing premolar teeth with
the removal of roots in dogs can erupt into oral
cavity at normal speed, and the void created by
the absence of roots during eruption was fi lled
with the alveolar bone. All of these indicated that
root formation is not required for tooth eruption.
References
1. Sadler TW. Langman’s medical embryology. 12th ed.
Baltimore: Lippincott Williams & Wilkins, 2012.
2. Nanci A. Ten Cate’s oral histology: development,
structure, and function. St. Louis: Elsevier Health
Sciences; 2014.
3. Trainor PA, Melton KR, Manzanares M. Origins and
plasticity of neural crest cells and their roles in jaw
and craniofacial evolution. Int J Dev Biol. 2003;
47:541–53.
4. Avery JK, Steele PF, Avery N. Oral development and
histology. 3rd ed. New York: Thieme; 2002.
5. Khang D, Carpenter J, Chun YW, Pareta R, Webster
TJ. Nanotechnology for regenerative medicine.
Biomed Microdevices. 2010;12:575–87. doi:
10.1007/
s10544-008-9264-6
.
6. Cui FZ, Ge J. New observations of the hierarchical
structure of human enamel, from nanoscale to
microscale. J Tissue Eng Regen Med. 2007;1:185–91.
doi:
10.1002/term.21 .
7. Hannig M, Hannig C. Nanomaterials in preventive
dentistry. Nat Nanotechnol. 2010;5:565–9.
doi:
10.1038/nnano.2010.83 . nnano.2010.83 [pii].
8. Boyden PA, Frame LH, Hoffman BF. Activation map-
ping of reentry around an anatomic barrier in the
canine atrium. Observations during entrainment and
termination. Circulation. 1989;79:406–16.
9. Aldred MJ, Savarirayan R, Crawford PJ. Amelogenesis
imperfecta: a classifi cation and catalogue for the 21st
century. Oral Dis. 2003;9:19–23.
10. Huang X, Bringas Jr P, Slavkin HC, Chai Y. Fate of
HERS during tooth root development. Dev Biol.
2009;334:22–30.
11. Deutsch D. Structure and function of enamel gene
products. Anat Rec. 1989;224:189–210. doi:
10.1002/
ar.1092240209
.
12. Hayashi T. Recombinant study of mouse enamel
organ and dental papilla by renal transplantation:
effects of discrepancy in number of these two compo-
nents. Kokubyo Gakkai Zasshi. 1990;57:652–66.
13. Yamamoto H, et al. Developmental properties of the
Hertwig’s epithelial root sheath in mice. J Dent Res.
2004;83:688–92.
1 Tooth Development: Embryology of the Craniofacial Tissues

24
14. Lau EC, Mohandas TK, Shapiro LJ, Slavkin HC,
Snead ML. Human and mouse amelogenin gene loci
are on the sex chromosomes. Genomics. 1989;4:
162–8. doi:0888-7543(89)90295-4 [pii].
15. Salido EC, Yen PH, Koprivnikar K, Yu LC, Shapiro LJ.
The human enamel protein gene amelogenin is
expressed from both the X and the Y chromosomes.
Am J Hum Genet. 1992;50:303–16.
16. Du C, Falini G, Fermani S, Abbott C, Moradian-
Oldak J. Supramolecular assembly of amelogenin
nanospheres into birefringent microribbons. Science.
2005;307:1450–4. doi:
10.1126/science.1105675 ,
307/5714/1450 [pii].
17. Collier PM, Sauk JJ, Rosenbloom SJ, Yuan ZA, Gibson
CW. An amelogenin gene defect associated with human
X-linked amelogenesis imperfecta. Arch Oral Biol.
1997;42:235–42. doi:S0003996996000994 [pii].
18. Parry DA, et al. Mutations in CNNM4 cause Jalili
syndrome, consisting of autosomal-recessive
cone- rod dystrophy and amelogenesis imperfecta.
Am J Hum Genet. 2009;84:266–73. doi:
10.1016/j.
ajhg.2009.01.009
. S0002-9297(09)00016-0 [pii].
19. Paine ML, Lei YP, Dickerson K, Snead ML. Altered
amelogenin self-assembly based on mutations observed
in human X-linked amelogenesis imperfecta (AIH1).
J Biol Chem. 2002;277:17112–6. doi:
10.1074/jbc.
M110473200
. M110473200 [pii].
20. Moradian-Oldak J, Tan J, Fincham AG. Interaction of
amelogenin with hydroxyapatite crystals: an adherence
effect through amelogenin molecular self- association.
Biopolymers. 1998;46:225–38. doi:
10.1002/(SICI)1097-
0282(19981005)46:4<225::AID-BIP4>3.0.CO;2-R
.
10.1002/(SICI)1097- 0282(19981005)46:4<225::AID-
BIP4>3.0.CO;2-R [pii].
21. Fincham AG, Moradian-Oldak J. Recent advances in
amelogenin biochemistry. Connect Tissue Res. 1995;
32:119–24.
22. Wright JT, et al. Relationship of phenotype and geno-
type in X-linked amelogenesis imperfecta. Connect
Tissue Res. 2003;44 Suppl 1:72–8.
23. Hart PS, et al. Mutation in kallikrein 4 causes autoso-
mal recessive hypomaturation amelogenesis imper-
fecta. J Med Genet. 2004;41:545–9.
24. Gibson CW, et al. Amelogenin-defi cient mice display
an amelogenesis imperfecta phenotype. J Biol Chem.
2001;276:31871–5. doi:
10.1074/jbc.M104624200 .
M104624200 [pii].
25. Cerny R, Slaby I, Hammarstrom L, Wurtz T. A novel
gene expressed in rat ameloblasts codes for proteins
with cell binding domains. J Bone Miner Res. 1996;
11:883–91. doi:
10.1002/jbmr.5650110703 .
26. Fukumoto S, Yamada A, Nonaka K, Yamada Y.
Essential roles of ameloblastin in maintaining
ameloblast differentiation and enamel formation.
Cells Tissues Organs. 2005;181:189–95.
doi:
10.1159/000091380 . 91380 [pii].
27. Snead ML. Enamel biology logodaedaly: getting to
the root of the problem, or “who’s on fi rst…”. J Bone
Miner Res. 1996;11:899–904. doi:
10.1002/
jbmr.5650110705
.
28. Iwasaki K, et al. Amelotin – a novel secreted, ameloblast-
specifi c protein. J Dent Res. 2005;84:1127–32.
doi:84/12/1127 [pii].
29. Arana-Chavez VE, Nanci A. High-resolution immu-
nocytochemistry of noncollagenous matrix proteins in
rat mandibles processed with microwave irradiation. J
Histochem Cytochem. 2001;49:1099–109.
30. Bartlett JD, Beniash E, Lee DH, Smith CE. Decreased
mineral content in MMP-20 null mouse enamel is
prominent during the maturation stage. J Dent Res.
2004;83:909–13. doi:83/12/909 [pii].
31. Ryu O, et al. Porcine kallikrein-4 activation, glycosyl-
ation, activity, and expression in prokaryotic and
eukaryotic hosts. Eur J Oral Sci. 2002;110:358–65.
32. Pashley D. Dynamics of the pulpo-dentin complex.
Crit Rev Oral Biol Med. 1996;7(2):104–33.
33. Forssell-Ahlberg K, Brannström M, Edwall L. The
diameter and number of dentinal tubules in rat, cat,
dog and monkey: a comparative scanning electron
microscopic study. Acta Odontol. 1975;33:243–50.
34. Mjör IA, Pindborg JJ. Histology of the human tooth.
Copenhagen: Munksgaard; 1973.
35. Bonucci E. Matrix vesicles: their role in calcifi cation.
Dentin Dentinogen. 1984;1:135–54.
36. Stanley H, Pereira J, Spiegel E, Broom C, Schultz
M. The detection and prevalence of reactive and phys-
iologic sclerotic dentin, reparative dentin and dead
tracts beneath various types of dental lesions accord-
ing to tooth surface and age. J Oral Pathol Med.
1983;12:257–89.
37. Nitzan DW, Michaeli Y, Weinreb M, Azaz B. The
effect of aging on tooth morphology: a study on
impacted teeth. Oral Surg Oral Med Oral Pathol.
1986;61:54–60.
38. Johnsen D, Harshbarger J, Rymer H. Quantitative
assessment of neural development in human premo-
lars. Anat Rec. 1983;205:421–9.
39. Avery J. Repair potential of the pulp. J Endod. 1981;
7:205–12.
40. Bishop M, Malhotra M, Yoshida S. Interodontoblastic
collagen (von Korff fi bers) and circumpulpal dentin
formation: an ultrathin serial section study in the cat.
Am J Anat. 1991;191:67–73.
41. Couve E, Osorio R, Schmachtenberg O. The amazing
odontoblast activity, autophagy, and aging. J Dent
Res. 2013;92:765–72.
42. Ruch J, Lesot H, Begue-Kirn C. Odontoblast differen-
tiation. Int J Dev Biol. 1995;39:51–68.
43. Lee JH, et al. Dental follicle cells and cementoblasts
induce apoptosis of ameloblast-lineage and Hertwig’s
epithelial root sheath/epithelial rests of Malassez cells
through the Fas-Fas ligand pathway. Eur J Oral Sci.
2012;120:29–37.
44. Zeichner-David M, et al. Role of Hertwig’s epithelial
root sheath cells in tooth root development. Dev Dyn.
2003;228:651–63.
45. Wang Y, Lv L, Yu X, Zhang T, Li S. The characteris-
tics of epithelial cell rests of Malassez during tooth
eruption of development mice. J Mol Histol. 2014;45:
1–10.
Z. Liwei et al.

25
46. Nam H, et al. Expression profi le of the stem cell
markers in human Hertwig’s epithelial root sheath/
Epithelial rests of Malassez cells. Mol Cells. 2011;31:
355–60.
47. Sohn WJ, et al. Contribution of mesenchymal prolif-
eration in tooth root morphogenesis. J Dent Res.
2014;93:78–83.
48. Xiong J, Gronthos S, Bartold PM. Role of the
epithelial cell rests of Malassez in the development,
maintenance and regeneration of periodontal
ligament tissues. Periodontology. 2013;2000(63):
217–33.
49. Yamashiro T, Tummers M, Thesleff I. Expression of
bone morphogenetic proteins and Msx genes during
root formation. J Dent Res. 2003;82:172–6.
50. Oka S, et al. Cell autonomous requirement for TGF-
beta signaling during odontoblast differentiation and
dentin matrix formation. Mech Dev. 2007;124:
409–15.
51. Hosoya A, Kim JY, Cho SW, Jung HS. BMP4 signal-
ing regulates formation of Hertwig’s epithelial root
sheath during tooth root development. Cell Tissue
Res. 2008;333:503–9.
52. Plikus M, et al. Morpho-regulation of ectodermal
organs: integument pathology and phenotypic varia-
tions in K14-Noggin engineered mice through modu-
lation of bone morphogenic protein pathway. Am J
Pathol. 2004;164:1099–114.
53. Khan M, Seppala M, Zoupa M, Cobourne MT.
Hedgehog pathway gene expression during early
development of the molar tooth root in the mouse.
Gene Expr Patterns. 2007;7:239–43.
54. Chen J, Lan Y, Baek JA, Gao Y, Jiang R. Wnt/beta-
catenin signaling plays an essential role in activation
of odontogenic mesenchyme during early tooth devel-
opment. Dev Biol. 2009;334:174–85.
55. Felszeghy S, Suomalainen M, Thesleff I. Notch sig-
nalling is required for the survival of epithelial stem
cells in the continuously growing mouse incisor.
Differentiation. 2010;80:241–8.
56. Marks Jr SC, Schroeder HE. Tooth eruption: theories
and facts. Anat Rec. 1996;245:374–93.
1 Tooth Development: Embryology of the Craniofacial Tissues

27
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1_2
Biofi lm and Dental Caries
Xu Xin , Zhou Yuan , Shi Wenyuan , Liu Yaling , and
Zhou Xuedong
2.1 Dental Plaque and Microbial
Biofi lm
2.1.1 Bacterial Biofi lm:
An Advanced Mode of Life
Bacteria grow in two different ways: planktonic
and biofi lm forms. Decades ago, most microbiol-
ogists characterized and studied planktonic bacte-
ria in liquid culture in which bacteria were fl oating
free cells. In this period, plenty of fundamental
work in microbiology was accomplished [ 7 ].
However, it is estimated that only less than 0.1 %
of the microbial consortia in natural environment
grow in planktonic state [ 40 ]. Then how do the
most microbes live and grow? From the colorful
microbial mats in Yellowstone geothermal area to
the sophisticatedly structured microbial commu-
nity on tooth surfaces, we have the answer.
2.1.1.1 The Concept and Discovery
of Biofi lm
Biofi lm is defi ned as aggregates of bacterial cells
attached to a surface and embedded in a poly-
meric matrix that is self-produced and helps the
community to gain tolerance against antimicrobi-
als and host defenses. Usually biofi lm cells are
adhered to a surface, for example, catheter or
tooth surfaces. With the extensive biofi lm stud-
ies, the surface attachment feature is not indis-
pensable for biofi lm recognition now [ 15 ].
The fi rst observation of bacteria on surfaces
was made by Antonie van Leeuwenhoek in 1684
[
15 ]. He observed various types of the numerous
“animalcules” in the tartar taken from his own
teeth directly by microscope. He also found the
preliminary evidence of antimicrobial tolerance
of biofi lm from the fact that the surface-attached
cells on his teeth could survive after rinsing with
vinegar, while cells removed from teeth could
be killed by vinegar [ 146 ]. At that time, the
knowledge and techniques in microbiology
were too limited to understand the little com-
munities on surfaces. Recently with molecular
and bioinformatics techniques, the characteriza-
tion of microbial growth and development in
complex communities on surfaces have been
highly promoted [ 82 ].
X. Xin • Z. Yuan • Z. Xuedong (*)
State Key Laboratory of Oral Diseases ,
West China Hospital of Stomatology,
Sichuan University , No. 14, Section 3,
Renmin South Road , Chengdu 610041 ,
People’s Republic of China
e-mail:
S. Wenyuan
School of Dentistry ,
University of California-Los Angeles ,
Los Angeles , CA , USA
L. Yaling
Advanced Education in General Dentistry ,
Eastman Institute for Oral Health,
University of Rochester , Rochester , NY ,
USA
2

28
2.1.1.2 Extracellular Polymeric
Substances of Biofi lm
The structure of biofi lm is highly varied depend-
ing on the growth environment and component
cells. In addition to the bacterial aggregates,
another major component, the structural back-
bone of biofi lm, is the extracellular matrix com-
posed of self-secreted extracellular polymer
substances (EPS) [ 55 ]. EPS is a highly diverse
biopolymers mixture, including mainly polysac-
charides, proteins, lipids, nucleic acids, and other
substances from the environment or host
(Fig. 2.1 ) [ 62 ]. As the immediate microenviron-
ment surrounding cells, EPS serves various func-
tions through the entire life cycle of biofi lm. The
parameters of EPS include molecular concentra-
tion, charge condition, quantity, hydrated level,
components, and so on. These factors, varying
because of the embedded cells, stages of biofi lm,
nutrient sources, and local environment/host, are
essential for EPS functions.
Mechanically, EPS provides architectural sta-
bility for the biofi lm entity, helps bacterial cells
adhering to surfaces, immobilizes cells onto local
substratum, and fi nally forms a three-dimensional
network that supports the biofi lm cells.
Metabolically, at the extracellular retention space,
EPS helps the external substances such as oxygen
and carbon source to diffuse into the biofi lm, as
well as the internal metabolic waste moving out.
From the multicellular and polymicrobial features
of biofi lm, EPS is also an external metabolism
system containing free enzymes, metabolites,
extracellular DNA (eDNA), and other substances
from cell lysis. Therefore, within the matrix, gene
horizontal transfer through eDNA, mutualism or
antagonism interactions, and nutrient accumula-
tion are active [ 62 , 212 ]. By supporting vertical
extension of biofi lm, EPS also reduces the
biomass density to buffer impact from external
substances. Moreover, EPS can protect the
microbial consortia from multiple external chal-
lenges, such as antibiotics, and allow the long-
term existence of biofi lm community. EPS not
only provides a structural framework for biofi lm
but also keeps the homeostasis of microenviron-
ment. This relative stable and interactive space
offers chances for bacteria to survive and even
persist in cruel conditions.
2.1.1.3 Biofi lm Formation
The life cycle of surface-associated biofi lm is
well studied in in vitro models. The process is
promoted by all different members in the com-
munity, regulated by associated genetic networks,
and also infl uenced by the environment. Different
standpoints are taken in recent study models of
biofi lm development, such as developmental
genetic regulation network, environmental adap-
tation, and multispecies interactions.
The most accepted model describes the biofi lm
formation as a highly regulated spatiotemporal
process. The formation of biofi lm is generally
summarized into fi ve dynamic stages as (1) loose
attachment of planktonic cells, (2) irreversible
adherence, (3) microcolony formation, (4) mature
Fig. 2.1 The composition of
extracellular polymeric
substances of bacterial
biofi lms. Bacteria in the
mature biofi lm are embedded
in an EPS matrix, which
consists of polysaccharides,
proteins, extracellular DNA
(eDNA), amyloid fi bers and
bacteriophages, etc.
X. Xin et al.

29
macrocolony formation, and (5) dispersal. The
developmental model of biofi lm focuses on the
regulated transition through the cascade and
emphasizes genetic pathway involvement in the
group level development. Recent studies on the
proteomics and genetic network of gram- negative
bacteria Pseudomonas aeruginosa ( P. aeruginosa )
biofi lm have indicated that the developmental pro-
cess of biofi lm is sequentially regulated [ 181 ,
196 ]. P. aeruginosa biofi lm is one of the earliest
and most studied in medical microbiology fi eld
[ 114 ]. Stages 3 and 4 of its biofi lm formation
involve type 4 pili-mediated mechanism [ 109 ].
The general dynamic processes can be identifi ed
in most species, whereas distinct mechanisms
reside in different bacteria, even for strains from
the same species. For example, LapA from the
biofi lm-associated-protein (Bap) family is impor-
tant in adhesion and transition from reversible to
irreversible attachment in P. fl uorescens and P.
putida ; conversely, no evidence indicates the
involvement of Bap in the P. aeruginosa biofi lm
formation [ 57 , 159 ].
Environmental impact is crucial to biofi lm
formation. Therefore, environmental adaptation
model is employed to describe the biofi lm forma-
tion. The environmental adaptation model sug-
gests that the phenotypic or species heterogeneity
within the biofi lm is the response to the spatio-
temporal change in the ecological niche. For
instance, oxygen and carbon gradient is one of
the driving forces for biofi lm formation. Here the
developmental stages can be sketched as (1)
planktonic cells attachment; (2) initial seed cells
growth with suffi cient nutrients; (3) formation of
microcolonies where outer cell layers restrain the
nutrient availability of inner layers, thereafter
gradient and heterogeneity showing within each
colonies; (4) formation of macrocolonies, where
some cells express high level of EPS and facili-
tate the vertical growth of community, resulting
in the improvement of nutrient and oxygen distri-
bution; (5) biofi lm formation from mature mac-
rocolonies, where structures like pores and
channels form to further assist inner nutrient dif-
fusion [
159 ]. This model describes the changes in
population and structure from the perspective of
metabolism. The genes that regulate the
environmental adaptation should also be included
in the biofi lm-specifi c gene network as described
above.
In nature, a great portion of biofi lm is com-
posed of diverse species. This polymicrobial fea-
ture makes interspecies interaction an important
factor and variable in biofi lm formation. At the
early stages of biofi lm formation, distribution
and roles of different bacteria and/or fungi
depend on various factors, such as metabolic
requirement, surface structures, mutualism, and
quorum sensing regulatory system. For exam-
ple, metabolic cooperation exists between oral
commensal Streptococcus gordonii and patho-
gen Aggregatibacter actinomycetemcomitans .
Peroxide produced by S. gordonii can be utilized
by A. actinomycetemcomitans for further secre-
tion of complement-resistant protein ApiA that
can limit host complement-mediated killing
[
229 ].
So far, we still have a lot to ask about the
mechanisms of biofi lm formation, especially for
that of in vivo biofi lm. There is a long way to go
to fi nd out whether the discoveries from in vitro
models are consistent with in vivo phenotypes.
2.1.1.4 Survival Advantages of Biofi lm
The biofi lm form of growth with its surface asso-
ciation offers bacteria remarkable advantages to
survive and thrive in harsh environment compared
to the planktonic form. Several hypotheses on
how biofi lm gains benefi cial phenotypes and suc-
ceeds have been proposed. First, the underlying
surface offers a relatively stable space within the
environment and also a substratum for microbes
to grow on, facilitating further communities build-
ing, nutrient accumulation, and interaction of
cells in proximity. Second, microbial communi-
ties in biofi lm mode gain protection and higher
buffer ability against various challenges from the
environment, such as ultraviolet exposure [
56 ],
metal toxicity [ 201 ], fl uctuating pH [ 230 ], desic-
cation [ 231 ], phagocytosis [ 125 ], and antibiotics
and antimicrobial agents [ 69 , 137 , 195 ].
The biofi lm mode of survival has profound
importance for prokaryotes in both the natural
environment and human body, even being recog-
nized as the most successful forms of life on the
2 Biofi lm and Dental Caries

30
earth. In environmental microbiology, biofi lm
fossil records from as early as 3.2 billion years
ago were identifi ed [ 82 ]. From the standpoint of
evolution, we can speculate that biofi lm as an
advantageous form of growth offered space with
homeostasis for the primitive prokaryotes in the
rigid and ever-changing environment. Thus, now-
adays, biofi lms can be found almost everywhere
even in extreme conditions, such as hot spring,
deep-sea vents, and so on [ 15 ].
This survival advantage exists not only in the
nature but also on and in our body. With the
development of human microbiome project
(HMP), we have recognized that we are living in
close relationship with microbes, which are
termed “the microbial part of ourselves” [ 228 ].
Human microbiome has several major compo-
nents including the oral, skin, vaginal, gut, and
nasal/lung microbiome, affecting the homeosta-
sis of health and disease. Like microbes in nature,
human microfl ora usually exists as sessile aggre-
gates of cells with or without a surface. From
thousands of recent in vitro and in vivo biofi lm
studies, we can summarize that biofi lm is closely
associated with chronic and persistent infections.
Because bacterial biofi lm is almost 1000-fold
more tolerant to certain antibiotics compared to
the planktonic state, the persistence of biofi lm
infection is most likely resulted from this impor-
tant feature [ 15 , 58 ].
2.1.2 Dental Plaque as a Typical
Bacterial Biofi lm
In 1886, Black fi rst described the accumulated
bacteria on early carious lesions as plaque [ 18 ].
In oral cavity, tooth as mineralized hard tissue
provides non-shedding surfaces for oral microbes
to colonize. Attached bacteria, embedded in
polymeric matrix, form microcommunities called
dental plaque. In recent years, dental plaque as a
typical biofi lm has been studied extensively. As a
naturally formed biofi lm, dental plaque acts
actively as part of the host defense system against
colonization of exogenous pathogenic microfl ora
[ 145 ]. On the other hand, when the homeostasis
of dental plaque is disrupted, biofi lm-associated
diseases like caries and periodontal diseases
develop.
Dental plaque was the fi rst biofi lm being
investigated at both composition and antimicro-
bial sensitivity aspects. With the help of molecu-
lar and bioinformatics techniques, more than
800 phylotypes of bacteria in the oral microbi-
ome, including more than 300 species, have been
identifi ed [
193 ]. The diverse and numerous resi-
dents make dental plaque a highly complex and
varied community. Furthermore, a limited num-
ber of fungi and viruses are also found in oral
cavity. Notably, more than half of oral bacteria
cannot grow in pure culture in vitro, largely lim-
iting our investigation on their physiological
characteristics [ 111 ].
The tooth surfaces above gingival margin are
mainly enamel or dentin, where they directly face
the dynamic oral environment, whereas the sur-
faces below the gingival margin are mainly den-
tin or cementum, which are in close contact with
gingival tissue and relatively less fl uctuant.
According to the location of the attached sur-
faces, dental plaque can be roughly divided into
two categories: supragingival plaque and subgin-
gival plaque. Each type has its own special physi-
ological characteristics and relation to disparate
diseases.
Oral cavity is a unique ecological niche, which
is warm, moist and relatively opens to the outer
environment. Tooth surfaces as well as dental
plaque constantly encounter different challenges
from food intake, speech, oral hygiene proce-
dures, and so on. The oxygen and nutrient levels
are changing constantly at different sites. For
example, the oxygen level varies, from being
anaerobic in subgingival plaque and basal layers
of supragingival plaque to being almost aerobic
in outer layers of supragingival plaque [
142 ]. The
local microbial diversity and metabolism also
change enormously from health to disease. Apart
from some specifi c pathogen-induced diseases,
most oral diseases are caused by or associated
with long-term multispecies microbial activity
within dental plaque [ 111 ]. Here, we will take
supragingival biofi lm, for example, to demon-
strate the association between dental plaque and
caries.
X. Xin et al.

31
2.1.3 Composition of Dental Plaque
Like other biofi lms, dental plaque contains two
major components: microbes and the biopolymer
matrix. The bacterial species varies signifi cantly at
distinct surfaces, such as pits/fi ssures, smooth sur-
faces, approximal surfaces, and gingival crevice.
The habitats are selective for the preferable bacte-
ria that are able to grow, based on the different
anatomy and biological features in the local envi-
ronment [ 1 , 145 , 169 ]. The studies of microbial
composition of biofi lm have been shifting from
culture-based to molecular approaches. Through
16 s rRNA gene-based sequencing, a plethora of
new taxa have been discovered, although their
physiological characteristics are largely unknown.
In normal microfl ora on fi ssures and smooth
surfaces, the predominant species are mostly
gram-positive aerobic or facultative anaerobic
bacteria, mainly streptococci. Other common spe-
cies include Gemella , Granulicatella , Actinomyces ,
Rothia , Veillonella , Neisseria , Prevotella ,
Abiotrophia , Capnocytophaga , Fusobacterium ,
and so on [ 1 ]. In contrast, the subgingival species/
phylotypes are more diverse, from facultative
anaerobic to obligate anaerobic. In a locus with
features of both supra- and subgingival surfaces,
the approximal plaque had an intermediate micro-
bial constitution [ 146 ]. The dental microfl ora is
highly varied from person to person, depending on
the complex genetic and environmental variations
in population. Once established, the personal
microbial community stays stable and in a dynamic
balance (or homeostasis). This fl uctuant balance is
achieved by heterogeneous microfl ora from both
metabolic mutualism and antagonism within the
community. Staying in a relatively open niche, the
resident community is under constant challenges or
invasions. When the habitats change greatly and
affect local microbial activities, the homeostasis
will shift, and a new microbial composition may be
established to fi t new environmental conditions.
The biochemical constitution of dental plaque
varies for different hosts and bacterial composi-
tion. Dental plaque is highly hydrated and con-
tains about 80 % water. Changing with time
before and after food intake, the glucan takes
10–20 % of plaque dry weight, while fructan
takes 1–2 %. Protein comprises approximately
40 % of fry weight, which is usually from bacte-
rial secretion and saliva. In addition, lipid, nucleic
acid, and minerals like calcium, magnesium,
phosphate, and fl uoride are in varying amounts in
plaque [ 39 , 94 , 167 , 171 ].
The part of plaque without bacteria is the
extracellular polymeric matrix, which is an
essential component of biofi lm [ 18 , 221 ]. The
extracellular matrix, as that of other biofi lm, is a
biopolymeric mixture of various components
from multiple sources. The attached bacteria on
tooth surfaces secrete macromolecules by active
secretion or cell lysis. These molecules along
with salivary proteins, host cells, and intake sub-
stances develop into a complicated three-
dimensional network that surrounds cells and
further forms dental plaque. The structure and
elements of matrix are infl uenced by coresidents
and the local habitat. It can be considered as a
variable that refl ects plaque status.
This matrix aids bacteria to adhere to tooth
surface, promotes coaggregation, reserves nutri-
ents and enzymes, and protects the community
from external harms, such as diffusion of charged
or bioreactive molecules. Besides keeping the
local homeostasis, EPS contains high ratio of
polysaccharides and also acts as a virulence fac-
tor in the pathogenesis of dental caries. The poly-
saccharides in matrix are the resources that
acidogenic bacteria metabolize and generate
acids to initiate further caries progress. For
example, S. mutans and lactobacilli are able to
metabolize sucrose in matrix into glucan or fruc-
tan polymers which are either water soluble or
water insoluble (branching). The insoluble car-
bohydrate polymers produced by S. mutans offer
adhesion sites for S. mutans and thus are impor-
tant in the development and progression of dental
caries [
10 , 18 , 221 ]
2.1.4 Spatiotemporal Development
of Oral Biofi lms
When exposed to the oral environment, for exam-
ple, as soon as a few seconds after the tooth erup-
tion or after brushing, the tooth surfaces are
2 Biofi lm and Dental Caries

32
coated with a conditioning fi lm consisting of pro-
teins and glycoproteins. This fi lm is called
acquired pellicle, the sources of which are mainly
saliva and gingival crevicular fl uid (GCF) [ 100 ].
Selective salivary components absorb to the tooth
surface, including amylases, mucins, proline-rich
proteins, histatins, statherins, lysozymes, and so
on [ 150 , 190 ]. These molecules attach on tooth
surfaces and offer bioactive sites that facilitate
future colonizers to bind. Hence, acquired pelli-
cle is recognized as the sign of the initiation of
dental plaque formation [ 18 , 68 , 88 ].
Following acquired pellicle formation, a few
bacterial species, mostly Streptococcus spp., start
to absorb and attach loosely to the fi lm by a wide
range of nonspecifi c and relatively weak forces.
These initial colonizers (e.g., S. mitis and S. ora-
lis ) bear specifi c surface-anchored proteins
(adhesins) to form irreversible binding with host
receptors in acquired pellicle. Electron micros-
copy has shown that cocci can be detected on
enamel surfaces at as early as four hours after
cleaning in vivo [ 83 , 155 ]. As the initial attach-
ment and proliferation go on, the bacterial metab-
olism changes the microenvironment in favor of
future residents. As the biofi lm develops, more
early colonizers with surface adhesins or recep-
tors interact and attach to the initial colonized
bacteria, starting the cascade of biofi lm prolifera-
tion and biodiversity fl ourish. In this dynamic
process, coaggregation has been extensively
studied as one of the major mechanisms in early
biofi lm succession. This typical bacterial adhesin
and bacteria/host receptor interaction is often
based on protein-carbohydrate (lectin) reaction
and eventually leads to the construction of a more
stable and diverse network. S. oralis and
Actinomyces oris are one of the most studied pair
of coaggregation partners. The receptor polysac-
charides on the cell surface of S. oralis can inter-
act with type 2 fi mbriae on A. oris surface,
forming potent cell aggregates in vitro [
164 ].
With the involvement of more species, the com-
plexity of biofi lm composition and structure is
ever growing. In some cases, in a single site,
more than 50 different species can be found [ 1 ].
With community multiplication, inter- and
inner- species communications become active.
The quorum sensing regulatory pathway plays a
critical role during the development of dental
plaque community.
In supragingival plaque, oral bacteria are
mostly saccharolytic and able to utilize metabolic
substances from endogenous nutrients. Saliva, as
the major local source of amino acids, proteins,
and glycoproteins, is continuously available,
whereas diet intake has a minor role in nutrient
contribution due to its limited surface contact
time and unavailability of some highly complex
ingredients for bacteria. Salivary proteins and
glycoproteins are essential for initial biofi lm for-
mation and bacterial growth, whereas host food
intake and other activities enormously affect the
maturation and dynamic transition of biofi lm. As
previously mentioned, bacteria produce biopoly-
mers to build the matrix during biofi lm formation.
In health biofi lm, the metabolism with salivary
substrates induces minor change to the local
pH. In contrast, when diet change induces local
sucrose concentration increase, the acid produc-
tion will rise and further cause the homeostasis
shift, leading to the numerical predominance of S.
mutans and fi nally dental caries [
100 , 168 ].
Taken together, dental plaque as a typical
biofi lm possesses the social traits and benefi ts
from this community-based lifestyle. Microbial
cells colonize following their own nutrient and
interactive needs, and a gradient of nutrient, air,
pH, and partners gradually forms in the commu-
nity [
20 , 31 ]. The dental plaque as a microeco-
system can offer a wide range of habitats for
diverse microbes and integrate different resi-
dents into a highly organized and tolerant com-
munity. Within an immediate contact, oral
bacteria have better chances to conduct more
effi cient metabolism through mutualistic inter-
actions [ 170 ]. With the support from extracel-
lular matrix, bacteria in plaque are less sensitive
to inhibitory agents and host defenses [ 93 , 210 ,
213 ]. Driven by environmental cues, dental
plaque would shift to pathogenic biofi lm with
distinct microbial composition and metabolic
activity and thus initiates oral infectious dis-
eases such as dental caries.
X. Xin et al.

33
2.2 Microbial Etiology of Dental
Caries
2.2.1 Oral Microbiology at
Early Stage
There is a long history of understanding the etiol-
ogy of dental caries. A Sumerian text from 5000
BC describes a “tooth worm” as the cause of car-
ies. The “tooth worm” theory has also been
described in the literature of ancient China, India,
Egypt, and Japan. During the European Age of
Enlightenment, the “tooth worm” theory was
gradually rejected by the European medical com-
munity. Pierre Fauchard, known as the father of
modern dentistry, was one of the fi rst to oppose
the “tooth worm” theory and noted that sugar was
detrimental to the teeth and gingiva.
Around 1680, van Leeuwenhoek (1632–1723)
[ 67 ], a Dutch dry goods merchant, observed and
described fi rst microorganisms in the tartar from
his teeth with his primitive microscopes. In his
notebook, he recorded “I didn’t clean my teeth
for three days and then took the material that has
lodged in small amounts on the gums above my
front teeth…. I found a few living animalcules.”
The microbes sketched in his notebook are now
known as some of the most abundant bacteria
resided within oral cavity, including cocci, spiro-
chetes, and fusiform bacteria. These fascinating
observations at the birth of microbiology had
already signaled the complexity of the oral
microbial community [
91 ].
2.2.2 Dental Caries as an Infectious
Disease
W. D. Miller was arguably one of the most impor-
tant scientists who substantially advanced the
knowledge of dental caries and associated studies
in oral microbiology. As a practicing dentist with
a comprehensive training in natural sciences, he
identifi ed the “germs” responsible for tooth decay.
In his 1890 book titled Microorganisms of the
Human Mouth , he proposed a “chemoparasitic”
theory of dental caries, which suggested that oral
microorganisms can convert carbohydrate into
acid and eventually result in the demineralization
of teeth and development of dental caries in sus-
ceptible hosts. Miller’s chemoparasitic theory,
together with the description of “gelatinous
microbic plaques”, which are now commonly
known as “dental plaque,” by Black and Williams
[
16 , 219 ], has laid the foundation for our modern
knowledge of dental caries etiology.
Due to the limited bacterial isolation and cul-
tivation technique available in the nineteenth cen-
tury, Miller was unable to identify the causative
agent(s) of dental caries. In 1924, Clarke fi rst iso-
lated a bacterial species from human dental car-
ies site and named it S. mutans , which was
capable of fermenting several sugars and produc-
ing a pH of 4.2 in glucose broth [ 38 ].
Unfortunately, Clarke was unable to demonstrate
that this organism actually caused caries.
Since 1950, various experimental animal
models have been employed to better elucidate
the nature and etiology of oral diseases, including
dental caries [ 105 ]. In 1960, two important arti-
cles were published which were considered the
cornerstone of dental caries research. In the fi rst
paper, using hamster as animal model, Keyes
revealed the infectious and transmissible nature
of dental caries [ 108 ]. Then in another elegant
paper, Fitzgerald and Keyes successfully demon-
strated caries induction in an animal harboring a
“conventional” microfl ora, by a single type of
streptococcus that had been isolated by Clarke
more than 30 years ago [
61 ].
2.2.3 Dental Plaque as the Cause
of Dental Caries
One of the early achievements in oral microbiol-
ogy is to link dental plaques to dental and peri-
odontal diseases. Dental plaque was one of the
fi rst substances van Leeuwenhoek examined
under his microscope [ 67 ]. The living microor-
ganisms recorded by him revealed for the fi rst
time the complex nature of dental plaque in terms
of its microbial composition. Later on, both Erdi
and Ficinus described the presence of
2 Biofi lm and Dental Caries

34
microorganisms within the “membrane” on the
teeth [ 197 ]. However, the full implications of
dental plaque were not realized until the publica-
tion of [ 16 ] paper, in which he referred dental
plaque as “gelatinous microbic plaques,” a gela-
tin-like substance that carried microorganisms
[ 16 ]. Black believed that bacteria within dental
plaque generate acids that dissolve the dental
hard tissue. Black’s work, together with Miller’s
chemoparasitic theory, underscored the impor-
tant role of dental plaque in the etiology of dental
caries and has become one of the essential para-
digms of oral biology.
2.2.4 Association of Streptococcus
mutans with Dental Caries
Considering the involvement of S. mutans in the
pathogenesis of dental caries as demonstrated by
early in vitro and in vivo animal models, epide-
miologist tried to predict the development of den-
tal caries by the detected number of S. mutans
present on the teeth. Numerous studies suggested
a positive correlation, and S. mutans was recog-
nized as the chief culprit of dental caries. Hence,
efforts were gradually shifted to the exploration
of the cariogenic abilities of S. mutans .
Researchers in this fi eld proposed that the viru-
lence of S. mutans resides in three core attributes,
including the abilities (i) to metabolize dietary
sucrose to form insoluble polysaccharides that
mediate the persistent colonization of tooth sur-
faces, (ii) to produce large quantities of organic
acids (primarily lactic acid) from a wide range of
carbohydrates (acidogenicity), and (iii) to toler-
ate various environmental stresses, particularly
low pH which are toxic to most of the other bac-
terial species present in the mouth (aciduricity).
The complete genome sequence of S. mutans
published in 2002 further stimulated numerous
studies of this bacterium at genetic level. However,
the correlations between dental caries and S.
mutans were not defi nitive at the level of individu-
als. Today, it is clear that there are individuals and
population groups of high caries susceptibility
with low levels of S. mutans and vice versa. While
there is little doubt about the ability of S. mutans
to cause dental caries in humans or animal models,
its contribution to the pathogenesis and progres-
sion of dental caries is still unclear.
2.2.5 Nonspecifi c or Specifi c Plaque
Hypotheses?
Miller’s “chemicoparasitic theory” proposed a
“nonspecifi c plaque” hypothesis, in which den-
tal caries is the result of the overall interaction
of all the groups of bacteria within plaque.
However, the rat caries model and the positive
correlation of S. mutans with dental caries in
most cases suggested that this proposal might
not be absolutely right. Hence, the “nonspecifi c
plaque” hypothesis has been challenged for
decades. An alternative view is that among the
200–300 indigenous species identifi ed in the
oral cavity, only a fi nite number of them can be
recognized as dental pathogens. Thus, dental
caries can be considered as specifi c, treatable
infections. This proposal had the benefi t of
focusing studies on the control of specifi c
microbial targets. However, although Mutans
streptococci (including S. mutans , S. rattus , S.
sobrinus , and S. cricetus ) are strongly impli-
cated with caries, the association is not unique;
caries can occur in the apparent absence of
these species, while S. mutans can persist with-
out evidence of detectable demineralization.
Indeed, in such circumstances, some acido-
genic, non- S. mutans are implicated with dis-
ease. Due to the imperfection of both theories,
the debate is still ongoing up until today.
2.2.6 Ecological Plaque Hypothesis
Unlike many known medical pathogens that are
“foreign invaders with specifi c virulence factors,”
the oral “pathogens” such as S. mutans are part of
the normal fl ora. From the initial isolation of S.
mutans by J. K. Clarke in 1924 to the latest
metagenomic studies, over 700 bacterial species
have been identifi ed from the human oral cavity.
The oral microfl ora has been recognized as one
of the most complex microbial communities in
X. Xin et al.

35
the human body [ 122 ]. As proposed by Phil
Marsh in his “ecological plaque hypothesis,” it is
not merely the presence of a single organism in a
complex community that determines the proper-
ties of a biofi lm, but it is the interactions between
the biofi lm residents that are crucial. In dental
caries, there is a shift toward community domi-
nance by acidogenic/aciduric gram-positive bac-
teria (e.g., S. mutans and lactobacilli) at the
expense of the acid-sensitive species associated
with sound enamel [ 110 , 143 ].
The introduction of high-throughput metage-
nomic pyrosequencing has profoundly advanced
our understanding of the overall oral microbial
diversity and function. Data obtained from metage-
nomic level favorably support the ecological
plaque hypothesis. A recent metagenomic study
on microbiome in caries cavity showed that the
caries cavities are not dominated by S. mutans , but
are a complex community formed by tens of bacte-
rial species [ 77 ]. The data support the polymicro-
bial etiology nature of caries. Another investigation
on the oral microbiota of children with dental car-
ies revealed that genera Streptococcus , Veillonella ,
Actinomyces , Granulicatella , Leptotrichia , and
Thiomonas in plaques were signifi cantly associ-
ated with dental caries [ 42 ], further supporting the
idea that no one specifi c pathogen but rather patho-
genic populations in plaque correlate with dental
caries. In addition, a recent study on salivary
microbiome of caries-active population further
supports the ecological hypothesis that the shifts in
community structure, instead of the presence or
absence of specifi c groups of microbes, contribute
to the occurrence of dental caries [
232 ].
2.2.6.1 Microbial Ecology in the Oral
Cavity
It has been estimated that the human body is
made up of over 1 × 10
14
cells, of which 90 % are
the microorganisms that comprise the resident
microfl ora of the host. Thus, this resident micro-
fl ora has been currently proposed as a novel
organ in human body. The resident microfl ora
dynamically interacts with the human body, con-
tributing directly and indirectly to the normal
development of the physiology, nutrition, and
defense systems of the host [ 143 ].
Despite the continual shift of these microor-
ganisms, the composition of the resident
microfl ora is distinct in different habitats/
niches such as the oral cavity, gut, and vagina.
The different key ecological factors present in
these biohabitats have a great impact on the
community structure and metabolic function of
the resident microfl ora. Those ecological fac-
tors include appropriate receptors for attach-
ment, essential nutrients and cofactors for
growth, as well as an appropriate pH, redox
potential, and gaseous environment. Take the
oral cavity, for example, the tooth surfaces pro-
vide distinct binding factors for microorgan-
isms. Moreover, the mouth is continuously
bathed with saliva at a temperature of 35–36 °C
and a pH of 6.75–7.25. The nutritional condi-
tion of the oral cavity is often described as
“feast or famine”, further exerting far-reaching
infl uence on the composition of microfl ora.
Consistently, our recent study by sampling
microfl ora from various oral sites of different
age groups has demonstrated that the oral cav-
ity is a highly heterogeneous ecological system
containing distinct niches with signifi cantly
different microbial communities. More impor-
tantly, the phylogenetic microbial structure
varies with aging, and only a few taxa were
present across the whole populations [
231 ].
The fi rst stages of dental plaque formation
involve the attachment of bacteria to salivary pro-
teins and glycoproteins that are deposited as pel-
licle on the surfaces of teeth/dentures and other
oral tissues. Bacteria that fi rst attach to the sali-
vary pellicle are designated primary colonizers,
including S. oralis , S. mitis , S. sanguinis , S. para-
sanguinis , and S. gordonii . In addition,
Actinomyces , Veillonella , Gemella , Abiotrophia ,
and Granulicatella species are often detected.
These early colonizers proliferate and change
local environmental conditions, making the site
suitable for colonization by more fastidious spe-
cies (e.g., obligate anaerobes). These early colo-
nizers also form the base layers of complex dental
plaque biofi lms. Subsequently, new microbial
cells adhere via similar adhesin- receptor mecha-
nisms (a process termed coaggregation or coad-
hesion). Subsequent colonizers such as
2 Biofi lm and Dental Caries

36
Fusobacterium and P. gingivalis are especially
effective in attaching to earlier plaque colonizers
and eventually form complex, structured, multi-
species biofi lms. Once established, the microbial
composition of dental plaque remains relatively
stable over time, and this microbial homeostasis
is intricately maintained by the dynamic intermi-
crobial and host- microbial interactions [ 143 ].
2.2.6.2 Genetic and Environmental
Factors and Oral Microbial
Ecology
In order to fully understand the change of oral
microbial community during the occurrence and
development of oral diseases (e.g., dental caries),
it is necessary to completely characterize the
community structure of the oral microbiome and
its infl uencing factors. Previous studies in healthy
individuals have revealed signifi cant inter- and
intraindividual structure and function diversity in
the human oral microbiome. Nurture (environ-
ment) and nature factors (host genotype), such as
diet, hygiene, geography, cultural traditions, age,
gender, and human genotype, work together to
shape the oral microbiome [ 164 ].
Diet is one of the most important environment
factors that impose profound infl uence on the
structure and function of the human oral micro-
biome. Exogenous nutrients provided via the
diet exert strong selection on the composition of
the oral microbiota. Fermentable carbohydrates
are the class of nutrients that most affect the
microbial ecology of the mouth. They are catab-
olized to acids, inhibiting most of the species
while promoting the growth of aciduric organ-
isms. Frequent exposure to low pH can disrupt
microbial homeostasis and lead to the enrich-
ment of acidogenic/aciduric species within the
dental plaque. Other environment factors, such
as oral hygiene, medication, and geography, will
also infl uence the composition of oral microbi-
ota [
143 ].
Host immunity plays an essential role in
shaping oral microbiome. Saliva contains com-
ponents of innate (e.g., lysozyme, lactoferrin,
sialoperoxidase, antimicrobial peptides) and
adaptive immunity (e.g., sIgA), can directly
inhibit some exogenous microorganisms. Host
tissues surrounding the oral cavity may secrete
antimicrobial agents and immune modulators,
which have substantial infl uence on the proper-
ties of dental plaque. In addition to host immu-
nity, the tooth morphology is heavily determined
to be infl uenced by genetic factors also contrib-
utes to the community structure of human oral
microbiome [
143 ].
In fact, most factors involved in the diversity in
oral microbiome cannot be simply classifi ed into
nurtural or natural. Accumulating evidence from
twin-pair model favors the “environment domi-
nates” theory. By comparing the composition varia-
tion of salivary microbiome in a cohort of 27
monozygotic and 18 dizygotic twin pairs, Stahringer
et al. proposed the philosophy of shared environ-
ment serving as the main determinant of microbial
populations [ 193 ]. Nevertheless, a recent report by
the Human Microbiome Project Consortium has
shown that the ethnic or racial background associa-
tion is one of the most robust associations with
microbiome, indicating that the host genotype such
as race and ethnicity may also be one of the major
determinants for the human microbiome diversity
[ 205 ]. Moreover, several recent studies have sug-
gested that ethnicity tunes the oral microbiome at
more specifi c levels [ 147 , 239 ].
2.2.6.3 Interspecies Interactions
and Dental Caries
The viability of oral microbial community is
dependent not only on the host genetic and envi-
ronmental factors but also on interactions
between the microbial residents. The residents in
the complex oral microbial community interact
extensively, forming biofi lm structures, carrying
out physiological functions, and inducing micro-
bial pathogenesis. Therefore, “war and peace” is
usually used to describe the dynamic microbial
interactions within a biofi lm. In general, micro-
bial interactions include (i) competition between
bacteria for nutrients, (ii) synergistic interactions
for the growth or survival, (iii) antagonistic inter-
actions by secondary metabolites production, (iv)
neutralization of a virulence factor produced by
another resident, and (v) interference in the
growth-dependent signaling mechanisms of each
other [
122 ].
X. Xin et al.

37
S. mutans and other caries-associated organ-
isms such as lactobacilli and Actinomyces species
are capable of expressing certain pathogenic fac-
tors. A dynamic balance of both synergistic and
antagonistic interactions with the coresidents
plays an essential role in determining whether
these pathogenic factors cause damage or not.
Hence, in the case of dental caries, it is now gen-
erally recognized that this disease is not solely
the result of the presence of S. mutans or any
single organism in dental plaque. Rather, it is the
net result of the interaction of multiple acido-
genic/aciduric organisms such as S. mutans with
other commensals within the dental plaque.
Factors Involved in Interspecies Inter-
actions Metabolic interrelationship is one of
the most common interspecies interactions
within dental plaque. Nutrients could be avail-
able from the periodic intake of food, saliva, and
nutrients provided by other organisms as well as
polysaccharides present in dental plaque. S.
mutans metabolizes sucrose to generate acid
more effi ciently compared to other common oral
bacteria [ 83 ]. When sucrose is frequently con-
sumed, S. mutans may take advantage of the sub-
strate competition and acid selection to emerge
as a predominant resident in caries-associated
biofi lms. In addition, mutualism between two
organisms dependent on nutrients is also com-
mon. For example, lactate produced by strepto-
cocci is utilized directly by Veillonella for
growth. As lactate is removed from the immedi-
ate environment by Veillonella , so the fl ux of
glucose to lactate increases, thus in turn enhanc-
ing growth of streptococci [
155 ]. Another exam-
ple is the combined efforts of A. naeslundii and
S. oralis in metabolizing salivary components to
form extensive biofi lms on saliva- coated sur-
faces [ 168 ].
The secondary metabolites of one organism
also have effects on other coresidents within the
same biofi lms. One of the best examples is
between S. mutans and S. sanguinis . The ecologi-
cal antagonism between these two bacteria in the
oral cavity has been noted for decades. Early col-
onization and high levels of S. sanguinis in an
infant’s oral cavity correlate with signifi cantly
delayed colonization by S. mutans . Similarly,
high levels of S. mutans in the oral cavity corre-
late with low levels of S. sanguinis [
132 ]. Animal
study has also demonstrated a so-called competi-
tive exclusion between S. mutans and S. sanguinis
depending on the sequence of inoculation [ 156 ].
The lactic acid produced by S. mutans favors the
growth of itself relative to that of other less acidu-
ric oral streptococci including S. sanguinis . On
the other hand, S. sanguinis can produce antimi-
crobial hydrogen peroxide to antagonize the
growth of S. mutans , which lacks effective sys-
tems to detoxify hydrogen peroxide [ 117 , 119 ]. In
addition to antagonism, metabolic products of one
organism may promote the growth of other organ-
isms. For example, organisms which are able to
metabolize oxygen would favor the growth of
nearby anaerobic organisms [ 46 ].
Bacteriocin is another key factor involved in
the interspecies competition. Bacteriocins are
proteinaceous toxins produced by all major lin-
eages of bacteria. Unlike traditional antibiotics,
bacteriocins often have a narrow killing spectrum
and inhibit the growth of related organisms [ 178 ].
In oral cavity, streptococci and a variety of oral
bacteria including A. actinomycetemcomitans
produce bacteriocins that are lethal to other bac-
teria. S. mutans produces a number of distinct
bacteriocins (also known as mutacins) as an
“arsenal” against its competitors. At least fi ve
different bacteriocins (mutacins I to V) produced
by S. mutans have been identifi ed [ 36 , 174 , 175 ].
In addition, at least nine other putative
mutacin- encoding genes have been annotated in
the genome of S. mutans UA159, suggesting a
large repertoire that can be used against its com-
petitors. Some of these bacteriocins are able to
inhibit the growth of S. sanguinis and thus may
be responsible, in part, for the negative correla-
tion of the presence of S. sanguinis and S. mutans
in the dental plaque [
81 ]. Similarly, the expres-
sion of bacteriocins by some other biofi lm resi-
dents may determine which organisms are
coresidents in these structures.
Interactions mediated by signaling molecules
are also prevalent in the dental biofi lms. The
quorum- sensing regulatory molecule autoin-
ducer-2 (AI-2) is a potential signaling molecule
2 Biofi lm and Dental Caries

38
between heterogeneous bacteria within biofi lms.
One typical example is that the luxS mutants of P.
gingivalis and S. gordonii cannot form mixed
biofi lms, but a mutation in either strain alone
allows for such a biofi lm formation [ 153 ]. In
addition, AI-2 also mediates mutualistic biofi lm
formation by S. oralis and A. naeslundii strains
[ 177 ]. Hence, AI-2 functions as one of the pri-
mary mediators of interspecies interactions
within oral biofi lms. In addition to AI-2, many of
the oral streptococci, including S. mutans , S. gor-
donii , and S. sanguinis , signal by the competence
stimulating peptide (CSP). Unlike the AI-2, CSP
is highly species specifi c and is not likely to
interfere with the activity of another distinct CSP
molecule. Of note, the CSP still can be indirectly
involved in interspecies interactions. In the case
of S. gordonii vs S. mutans interactions, a prote-
ase (i.e., challisin) expressed by S. gordonii
inhibits CSP-dependent properties of S. mutans
[ 215 ]. In addition, S. mutans may acquire trans-
forming DNA from the coresidents through the
CSP-induced bacteriocin production [ 118 ].
Taken together, microbial homeostasis can only
be achieved when an equilibrium is established
among different species within the same biologi-
cal niche. The aforementioned factors play an
essential role in modulating interspecies interac-
tions within biofi lms, thus shaping the structure of
the oral microbial community. Changes of these
factors can perturb the established equilibrium,
leading to the emergence of newly predominant
bacteria more adaptive to the microenvironment
(niche). In dental caries, this reestablished micro-
bial community structure is characterized by a
numeric predominance of acidogenic/aciduric
bacteria (e.g., S. mutans ) at the expense of the
acid-sensitive commensal species (e.g., S. sangui-
nis ) associated with sound enamel [
110 , 143 ].
The Role of Interspecies Interactions in Dental
Caries The interspecies interactions within den-
tal biofi lms tune microbial composition, struc-
ture, and virulence factors of the oral microbial
community and thus are involved in the onset and
progression of dental caries [ 122 ].
Bacterial interactions can affect the growth of
individual organisms or groups of related
organisms, thus modulating the composition of
the oral microbial community. For example, S.
sanguinis gained competitive edge over mutacin-
generating S. mutans by producing cytotoxic
hydrogen peroxide under certain conditions. On
the other hand, gshAB is essential for the com-
petitiveness and prevalence of S. mutans by
detoxifying hydrogen peroxide produced by S.
sanguinis [
238 ]. The S. sanguinis / S. mutans ratio
resulting from the dynamic competition between
these two bacteria may have ecological signifi -
cance on the occurrence and incidence of dental
caries.
Bacterial interactions could also affect the
overall expression of bacterial virulence of the
biofi lm. Take S. mutans , for example, one of the
core cariogenic virulences of S. mutans is the
ability to produce large quantities of organic
acids by metabolizing carbohydrates. In this
regard, coresidents which are capable of neutral-
izing/depleting the acidic end products of S.
mutans tend to reduce the cariogenicity of the
biofi lm. In dental biofi lms, some commensal bac-
teria such as Veillonella are able to consume the
lactic acid. In addition, some coresidents such as
S. salivarius , S. gordonii , and S. sanguinis can
generate alkali to neutralize the acid, through the
metabolism of either urease by urease or arginine
by arginine deiminase system [ 26 ]. The quorum-
sensing systems also modulate the virulence
properties of oral residents. S. mutans utilizes
CSP-induced bacteriocin production to acquire
transforming DNA from other coresidents [
118 ],
thus achieving a great genomic diversity for bet-
ter environmental adaptation. The CSP-induced
bacteriocin production also modulates the growth
of related noncariogenic streptococci. In addi-
tion, the CSP-mediated quorum-sensing systems
are involved in the antimicrobial resistance of S.
mutans [ 149 ]. Therefore, biofi lm residents which
could affect the levels of the regulatory molecules
present in the biofi lm could indirectly exert pro-
found effects on the biofi lm virulence. Since non-
cariogenic S. gordonii can inactivate the CSP
produced by S. mutans , its presence could thus
modulate the virulence of these biofi lms.
In conclusion, the interaction with the dental
microbial community can not only affect the
X. Xin et al.

39
growth of certain species but also modulate the
virulence properties of oral residents, thus infl u-
encing the overall pathogenicity of the dental
plaque. The interspecies interactions have eco-
logical signifi cance in the occurrence and pro-
gression of dental caries.
2.3 Dental Caries-Associated
Bacteria
2.3.1 Carbohydrate Metabolism
and Acidogenic Bacteria
Dental caries is a multifactorial and chronic
infectious disease resulting in the localized
destruction of dental hard tissue. Dental caries is
one of the major disease burdens infl icting
humans throughout history. The pandemic of
dental caries has been linked to the two largest
dietary shifts in human evolution in terms of the
consumption of fermentable carbohydrates. With
the advent of Neolithic farming, the increased
consumption of domesticated grains positively
correlates with a marked prevalence of caries.
However, across the Neolithic and medieval
period, the degree of caries was mild and preva-
lence remained relatively stable until 1850. After
1850, a sudden expansion of caries lesion
occurred coinciding with the introduction of
refi ned fl our and sugar due to industrial revolu-
tion. Although the epidemiological history indi-
cates a correlation of sugar with dental caries, the
“culprit” of the dental caries remained enigmatic
until the late nineteenth century, when Miller evi-
denced that the acidic microbial metabolites from
dietary substrates contribute to the development
of dental caries [
157 , 158 ]. These observations
were further confi rmed in 1940s by Stephan, who
reported that microbial metabolism of carbohy-
drate in dental plaque could drive the local pH
values below 3.0 after continuous sugar exposure
[ 194 ]. Among those acidic microbial metabo-
lites, lactic acid has been identifi ed as the major
contributor to the pH decline in dental plaque.
Hence, the acidogenic bacteria are recognized as
the culprit for caries initiation and progression.
The accumulation of organic acids leads to con-
tinuous pH decline to the critical pH, below
which tooth hard tissue demineralization begins
and dental caries gradually occurs [
143 ].
2.3.2 Major Acidogenic Bacteria
2.3.2.1 Streptococcus mutans
S. mutans was fi rst described by J Kilian Clarke
in 1924 [ 38 ]. It is gram-positive facultative coc-
cus commonly arranged in chains. Oral strepto-
cocci are commensal bacterial but can
opportunistically initiate caries. Mutans strepto-
cocci are a group of most important bacteria
highly associated with caries, consisting of S.
mutans , S. sobrinus , S. rattus , S. cricetus , S.
ferus , S. downeii , and S. macaca . Their cario-
genic virulence mainly involves several attri-
butes, including (1) processing adhesins for
initial attachment to the saliva- coated tooth sur-
face; (2) the production of extracellular polysac-
charide, i.e., glucan, fructan, to facilitate retention
on tooth surface and plaque accumulation; and
(3) the production of organic acid to generate
acidic microenvironment and promote enrich-
ment of aciduric microfl ora.
2.3.2.2 Lactobacilli
Lactobacillus is a genus of gram-positive faculta-
tive anaerobe or microaerophilic rod-shaped bacte-
ria. Lactobacillus is able to convert lactose and
other sugars to lactic acid, mostly through
homofermentative metabolism. Lactobacillus count
has been used to assess caries activity for years.
Lactobacillus is acid tolerant and can carry out gly-
colysis at pH values as low as 3. However, lactoba-
cilli are poor colonizer of smooth tooth surface.
Therefore, lactobacilli are generally believed to
exacerbate the initial enamel lesion to deep dentine
lesion. After colonizing into the established dental
plaque, the lactobacilli can further acidify the
plaque and suppress the acid susceptible microor-
ganism, further enriching acidogenic and aciduric
bacteria.
2.3.2.3 Actinomyces
Actinomyces is a genus of gram-positive faculta-
tive or strict anaerobic pleomorphic rod-shaped
2 Biofi lm and Dental Caries

40
bacteria. As a saccharolytic and acidogenic bac-
terium, Actinomyces spp., especially A. naeslun-
dii , has been frequently isolated from both root
caries lesions and sound root surface, suggesting
their association with root caries. However, an in-
depth knowledge about the involvement of indi-
vidual Actinomyces spp. in root caries is still
sketchy.
2.3.3 Acid Tolerance of Acidogenic
Bacteria
The central pathogenesis of dental caries is the
production of organic acid and the resultant
decalcifi cation of dental hard tissue. The dental
plaque undergoes rapid, dynamic pH fl uctuations
ranging from pH 7.0 to 3.0 in less than 20 min
upon carbohydrate intake [ 102 ]. The cariogenic
bacteria emerge as numeric predominant species
during this acidifi cation process. Acid tolerance
or acidurity is the most important attribute for
those acidogenic bacteria to prevail in a cario-
genic biofi lm. Acidogenic bacteria such as S.
mutans can function better at pH 6 and can even
carry out glycolysis at pH below 4. Moderate
acidophile, especially Lactobacillus spp., can
also function better and carry out glycolysis at
low pH values of 3–4. To survive and proliferate
in acid conditions, the cariogenic bacteria have
developed a large repertoire of strategies to main-
tain intracellular homeostasis, which involves
both constitutive acid tolerance and acid-induced/
adaptive tolerance [
25 ]. Constitutive acid toler-
ance mainly relies on the F-ATPase proton pumps
in the cell membrane. Taking S. mutans , for
example, although the glycolytic pathway can
catabolize sugars at pH values as low as 4.0, the
S. mutans cell cannot grow at pH values lower
than 5.0. In this case, the F-ATPase will use the
ATP generated by glycolysis to pump out intra-
cellular protons and thus maintain neutral cyto-
solic pH favorable for normal enzymatic function
and cellular viability. In addition, the end metab-
olite, lactate, may also be extruded from cyto-
plasm by a membrane carrier for lactic acid, thus
maintaining a relative neutral cytosolic pH. The
adaptive acid tolerance response indicates the
induction of enhanced survival ability of bacteria
at a pH as low as pH 3.0 after exposure to a sub-
lethal pH of approximately 5.5. The adaptive acid
tolerance involves global cellular response,
including alteration of metabolism, regulation of
quorum sensing system, synthesis of chaperonin
protein for damaged protein, increased activity of
DNA protection/repair system, changes in mem-
brane fatty acid composition, etc. [
148 ]. Overall,
the acid tolerance mechanisms allow the cario-
genic bacteria to outcompete other acid-sensitive
coresidents under acid selection, which would
eventually lead to the enrichment of acidogenic/
aciduric bacteria and continual acidifi cation of
the dental plaque favorable for caries formation.
2.3.4 Base Generation and Caries
Protection
In addition to the aforementioned acid tolerance
mechanisms, alkali generation is widespread
among oral species. Alkali generation is particu-
larly important for the survival of acid-sensitive
commensal bacteria and thus plays an important
role in modulating the microbial ecology within
oral biofi lm. The primary source of alkali pro-
duction is through microbial metabolism of argi-
nine, agmatine, and urea (Fig. 2.2 ). Alkali
generated by these metabolism pathways might
neutralize the acid metabolites produced by car-
iogenic microfl ora and thus provides a promising
strategy for the development of ecological ther-
apy against caries (Fig.
2.3 ).
2.3.4.1 Urease
The physiological concentration of urea present
in saliva and crevicular fl uids ranges from 3 to
10 mmol/L, roughly equivalent to those in serum.
Some oral species, including S. salivarius , A.
naeslundii , and oral haemophili, can convert urea
by urease to ammonia and CO
2
, thus increasing
plaque pH [ 33 , 129 ]. Urease is a nickel ion-
dependent multisubunit metalloenzyme encoded
by at least seven genes arranged as operon in
most bacteria. ureC , ureA , and ureB encode the
urease apoenzyme, consisting of α, β, and γ sub-
units. These subunits assemble into (αβγ)
3
oligo-
X. Xin et al.

41
meric complex with six nickel ions incorporated
into the active site. ureE , ureF , ureG , and ureD
encode chaperone complex essential for the
incorporation of Ni
2+
and CO
2
into the metallo-
center. Other genes, such as ureM , ureQ , and
ureO , encode a Ni
2+
-specifi c ATP-binding cas-
sette transporter, and ureI encodes urea trans-
porter [ 32 ]. The expression of bacterial urease is
regulated by multiple environmental cues, includ-
ing low pH, the presence of urea, limited nitrogen
source, carbohydrate availability, and rate of
growth [ 32 ].
Urea + H
2
O
2NH
3
+ CO
2
L-lactate + CO
2
ATP
H
+
F
1
F
0
ATPase
L-malate
NH
3
+ CO
2
+ H
2
O
CO
2
Agmatine
Carbamoyl putrescine
NH
3
NH
3
Urease
ADP ATP
Arginine deiminase
Arginine decarboxylase
Agmatine deiminase
malolactic enzyme
Putrescine carbamoyl-
transferase
Pi
Ornithine
carbamoyl-
transferase
Arginine
Citrulline
Putrescine
Ornithine
Carbamoyphosphate
Carbamate kinase
Fig. 2.2 Summary of alkali-generating pathways in the oral cavity (Figure is reproduced from Liu et al. [ 131 ] )
Neutral pH
Low pH
Oral environmental
stresses
Oral alkali
generation
Disease
Caries
Acidogenic and aciduric bacteria
Oral commensals
Health
Enamel
Fig. 2.3 The role of alkali generation in caries preven-
tion. Dental biofi lms in a healthy host maintain a micro-
bial and pH homeostasis with a balanced demineralization/
remineralization of dental hard tissue. The environmental
cues, such as frequent sugar exposure, may enrich the
acidogenic/aciduric bacteria via acid selection and further
lead to plaque acidifi cation in favor of demineralization of
dental hard tissue. The continuous plaque acidifi cation
eventually results in the initiation and/or progression of
caries lesion. Alkali generation by biofi lm commensals
can directly neutralize plaque pH, ease the competitive
edge of acidogenic/aciduric bacteria, and restore a healthy
microbial equilibrium (Figure is reproduced under the
permission of Dr. Liu et al. [
131 ] )
2 Biofi lm and Dental Caries

42
2.3.4.2 Arginine Deiminase
System (ADS)
Arginine is abundant in salivary secretions as
polypeptides with average concentrations in duc-
tal saliva around 50 mmol/L [ 211 ]. Arginine is
primarily metabolized by the microbial ADS to
release ornithine, ammonia, and CO
2
. ADS has
been identifi ed in many commensal bacteria,
including S. sanguinis , S. gordonii , and S. para-
sanguis . Certain Lactobacillus and Actinomyces
species, other oral streptococci, and some oral
spirochetes have been also identifi ed as argino-
lytic [ 26 , 141 ]. Unlike urea hydrolysis by urease,
arginine catabolism by ADS generates ATP,
which could be further utilized to counter acid
stress through both constitutive and adaptive acid
tolerance pathways. Similar to urease, ADS-
encoding genes are commonly arranged in an
operon [ 47 , 136 ]. arcA gene encodes arginine
deiminase, which hydrolyzes arginine to generate
citrulline and ammonia. arcB gene encodes orni-
thine carbamoyltransferase, which converts citrul-
line to ornithine and carbamoylphosphate. arcC
gene encodes carbamate kinase that transfers a
phosphate group from carbamoylphosphate to
ADP to generate ATP, CO
2
, and ammonia. Many
organisms also possess an arginine/ornithine anti-
porter ( ArcD ) that is encoded in the same operon,
and arginine aminopeptidases and transcriptional
regulators are often encoded in ADS gene clusters
[ 235 ]. ADS expression is induced by arginine and
low pH. The operon is also sensitive to carbon
catabolite repression (CCR) and can be downreg-
ulated in response to elevated oxygen levels [
48 ].
In addition, ADS activity in S. gordonii has been
reported to be upregulated when it coaggregated
with A. naeslundii [ 99 ], indicating the ecological
involvement of ADS expression in multispecies
biofi lm.
2.3.4.3 Agmatine Deiminase System
(AgDS)
Agmatine present in the oral cavity can be
obtained from foods, such as rice, milk, and beer,
or be produced from arginine by bacterial argi-
nine decarboxylase enzymes. The physiological
concentration of agmatine is 0.75 mmol in dental
plaque and 0.2 mmol in saliva [
76 ]. Agmatine is
primarily metabolized by AgDS to putrescine,
ammonia, CO
2
, and ATP. AgDS is present in
many oral bacteria, including S. mutans , S. sobri-
nus , S. downeii , S. rattus , S. uberis , S. mitis , and
S. cricetus , as well Lactobacillus salivarius and
L. brevis [ 76 ]. AgDS is also encoded by genes
arranged in operon ( aguBDAC ) [ 74 ]. AguD
encodes agmatine-putrescine antiporter to allow
the entry of free agmatine into the cell. Agmatine
is then hydrolyzed to N-carbamoylputrescine and
ammonia by the agmatine deiminase enzyme
encoded by aguA . The putrescine carbamoyl-
transferase, encoded by aguB , further metabo-
lizes N-carbamoylputrescine to yield putrescine
and carbamoylphosphate. Finally, carbamate
kinase encoded by aguC gene transfers a phos-
phate group from carbamoylphosphate to ADP
to generate ATP, CO
2
, and NH
3
. The putrescine
generated can also be used in exchange of agma-
tine via the antiporter [ 74 ]. aguR gene, which is
located upstream of, and in the opposite orien-
tation to, the agu operon in S. mutans , encodes
a transcriptional activator of agu genes [ 75 ]. In
oral streptococci, AgD activity is generally lower
relative to that of arginine deiminase or urease
[ 74 ]. Therefore, AgDS may not be suffi cient to
counter the plaque acidifi cation. On the contrary,
accumulating evidence has indicated that AgDS
may actually favor the acid tolerance of S. mutans
by elevating the cytoplasmic pH and generating
extra ATP. In S. mutans , AgDS activity is growth
phase dependent, and it can also be induced by
the presence of agmatine and other environmen-
tal stresses, including low pH and heat shock
[
75 ]. Multiple two-component systems, includ-
ing CiaRH , ComDE , and VicRK , also involve in
the induction of AgDS genes under low pH or
thermal stress [ 130 ].
2.3.4.4 Alkali Production and Biofi lm
Ecology
Dental biofi lms are complex ecosystems with
hundreds of metabolically and physiologically
diverse species competing for nutrients. The abil-
ity of oral species to metabolize urea, arginine, or
agmatine at low pH favors the growth of these
X. Xin et al.

43
bacteria by cytoplasm alkalization. The genera-
tion of ATP could further enhance acid tolerance
by providing energy for proton extrusion, growth,
or maintenance [ 75 ]. The contributions of ADS,
urease, and AgDS to oral biofi lm pH homeostasis
and microbial ecology can be quite different. In
some oral commensals, such as S. salivarius and
S. gordonii , urease and ADS can protect the bac-
teria from excessive environmental acidifi cation
[ 33 ]. In the meantime, other less-aciduric coresi-
dents benefi t from the local alkalization by
ammonia generated from arginine or urea break-
down [ 48 ]. On the other hand, the caries patho-
gens S. mutans and S. sobrinus have no urease or
ADS but possess AgDS that is expressed at a
relatively lower level [ 76 ]. Consequently, AgDS
of S. mutans or S. sobrinus probably cannot sig-
nifi cantly alkalize the dental plaque, but instead,
it enhances the acid tolerance of these acidogenic
bacteria by neutralizing cytoplasm acid and gen-
erating extra ATP, thus promoting the prevalence
of these bacteria during the development of
caries.
2.3.4.5 Clinical Relevance of Alkali
Production
Alkali production holds the promise to be a
promising strategy for the management of dental
caries. Firstly, it directly increases the pH of den-
tal plaque in favor of dental hard tissue reminer-
alization. Secondly, alkali generation favors the
persistence of healthy commensals while pre-
venting the overgrowth of cariogenic bacteria
(e.g., S. mutans ) dependent on acid selection. The
activity of alkali generation of oral biofi lm has
shown negative correlation with the development
and progression of caries. A genetically modifi ed
strain of S. mutans expressing the urease genes
from S. salivarius showed potent anticaries
effects in a rat caries model [
37 ]. Chronic renal
failure patients with salivary urea levels signifi -
cantly higher than healthy population are more
resistant to caries, despite their high dietary car-
bohydrate intake [ 172 ]. Consistently, caries-
resistant subjects have higher ammonia
concentrations and resting pH in their plaque
compared with those of caries-free individuals
[ 211 ]. Signifi cantly higher levels of salivary ADS
activity and plaque urease activity have been
reported in caries-free population compared with
subjects with dental caries. Salivary arginine lev-
els are also positively correlated with caries resis-
tance [
163 , 188 ]. A longitudinal study on children
has further validated that ammonia generation by
plaque urease is correlated with dental caries
resistance [ 160 ]. The addition of arginine-
bicarbonate to mouth rinse effectively can raise
the plaque pH above the critical pH after a
sucrose challenge [ 216 ]. More importantly, an
increasing number of clinical trials have shown
that arginine-containing oral hygiene products
signifi cantly reduced the incidence of dental car-
ies [ 2 , 3 , 55 ]. Toothpaste containing 1.5 % argi-
nine and 1450 ppm fl uoride in a calcium base has
also been proven more effective in arresting and
reversing early carious lesions compared with
dentifrice containing 1450 ppm fl uoride alone
[ 116 , 192 , 234 ].
2.3.5 Other Caries-Associated
Bacteria
The oral cavity is inhabited by hundreds of bacterial
species, forming complex ecology system [ 45 ]. The
interplay between oral microfl ora and the host
orchestrates the status of oral health and disease.
The “specifi c pathogen hypothesis” has led to the
identifi cation of several other species including
Scardovia wiggsiae and Slackia exigua , and
Bifi dobacterium dentium as the potential cariogenic
pathogens [
199 ]. In addition, Atopobium , Olsenella ,
Propionibacterium , and Pseudoramibacter genera
are also indicated as bacteria associated with caries
progress [ 173 ]. In contrast, the “nonspecifi c plaque
hypothesis” supports the concept that caries is the
consequence of the overall acid production activity
of the total plaque microfl ora rather than a few spe-
cifi c bacteria. The “ecological plaque hypothesis”
further proposes that the bacterial consortium in the
dental plaque can interact in complex synergistic
and antagonistic fashion, and it is the structure and
function shift of the microbial community driven by
environmental cues (e.g., frequent exposure to car-
bohydrate) that eventually lead to the net pathogen-
esis of dental caries [ 144 ].
2 Biofi lm and Dental Caries

44
2.4 Antimicrobial Approaches
to the Management
of Dental Caries
Dental caries is a biofi lm-mediated disease. The
composition of the biofi lm associated with caries
initiation and progression is diverse. The man-
agement of dental caries is to target the dental
plaque, in particular to restore the microbial dis-
equilibrium within the oral biofi lm. This section
is to provide an overview of various antimicro-
bial strategies applied in dentistry and related
studies.
2.4.1 Chlorhexidine
Chlorhexidine is one of the most tested antiplaque
agents and represents a gold standard against
which the potency of other antiplaque agents is
compared [ 8 , 59 ]. Chlorhexidine molecules are
positively charged (cations). Chlorhexidine binds
strongly to most bacteria and surface structures in
the oral cavity, including tooth surfaces and
mucous membranes which are negatively charged
(anions). When chlorhexidine binds to microbial
cell walls, it destroys the surface structure, leading
to an osmotic imbalance with consequent
precipitation of cytoplasm causing cell death [ 59 ].
Chlorhexidine also possesses very good
substantive properties [ 139 ]. The antimicrobial
effect of chlorhexidine can be retained for up to
12 h or longer depending on the delivery dosage
and form [
4 ].
Chlorhexidine is a strong base, and it acts bac-
teriostatically when administered at low concen-
trations. It disrupts normal membrane functions
or causes leakage of cell constituents [ 95 ]. At
higher concentrations, chlorhexidine is bacteri-
cidal, inducing leakage of low molecular weight
cell constituents and precipitation of cell con-
tents. Chlorhexidine exhibits broad antibacterial
spectrum with gram-positive microorganisms
particularly sensitive than gram-negative
microorganisms.
The effi cacy of chlorhexidine mainly depends
on the concentration and the frequency of appli-
cation. Mouth rinses, sprays, gels and varnishes
are some of the most often used delivery forms of
chlorhexidine [
191 ]. The usually prescribed dos-
age for chlorhexidine mouthrinses has been
10 ml of a 0.2 % solution, with twice-daily
mouthrinses. By using 15 ml of a 0.12 %
chlorhexidine mouthrinse, a comparable effi cacy
can also achieved [ 59 ].
2.4.2 Fluoride
Fluoride has been applied in dentistry for more
than 70 years, and it is now recognized as the
major contributor to the dramatic decline in car-
ies prevalence worldwide [ 28 ]. Fluoride is a dual
functional anticaries agent, acting on both tooth
hard tissue and oral microbes [ 58 , 114 , 202 ].
Fluoride can disrupt enzyme activity and reduce
acid production by oral bacteria [ 84 ], thus sup-
pressing the enrichment of cariogenic bacteria
within dental plaque [ 20 , 21 ]. Amine fl uoride
[ 107 ] and stannous fl uoride [ 151 ] can be bacteri-
cidal at higher concentrations against oral bacte-
ria. Amine fl uoride in a gel formulation can
inhibit the growth of mixed bacterial populations
in subgingival plaque [ 11 , 24 ]. Amine fl uoride
and tin fl uorides can also inhibit the adhesion of
S. sanguinis to glass conditioned with either
saliva or bovine serum albumin in vitro [ 54 ].
Preincubation of hydroxyapatite with amine fl u-
oride can signifi cantly decrease the growth of S.
sobrinus in biofi lm in vitro [ 183 ]. Of note,
in vivo data reported by Weiger et al. [
218 ] have
shown that the amine/stannous fl uoride mouth-
rinse possesses a transient antibacterial effect
but no clear antiadhesive activity against oral
bacteria.
The methods of fl uoride delivery are either
systemic (water, supplements, milk and salt) or
topical (toothpaste, gels, varnishes, paint-on
applications and mouthrinses) [ 59 ]. Although the
role of fl uorides in caries prevention represents
one of the most successful stories in general pub-
lic health, excessive fl uoride intake during the
period of tooth development can cause dental
fl uorosis. In addition, fl uoride could nonselec-
tively suppress the growth of commensal bacteria
and thus disrupt the microbial equilibrium within
X. Xin et al.

45
the dental plaque. Therefore, cautions should be
taken in the application of fl uorides in order to
maximize the anticaries benefi ts while minimiz-
ing the risk.
2.4.3 Quaternary Ammonium
Compounds
Quaternary ammonium compounds (QACs) are
surface-active agents with a wide antimicrobial
spectrum against both bacteria and fungi [ 64 , 98 ,
123 ]. QACs deliver their antimicrobial activity
by binding to the cell membrane and causing
cytoplasmic leakage, similar to those polyca-
tionic agents.
Cetylpyridinium chloride (CPC) is a com-
monly used QAC in a variety of mouthwashes
over the past decades due to its antimicrobial
property. The typically used concentration (W/V)
of CPC is 0.05 %, although slightly higher con-
centrations (≥0.07) have also been used [ 44 , 80 ,
214 ]. The antiplaque effect of CPC was fi rst
reported by Schroeder and Hirzel [ 79 , 182 ].
Research has demonstrated that CPC mouthrinses
have antiplaque activity when used alone or in
conjunction with toothbrushing [ 79 , 140 , 222 ,
223 ]. Recently, a systematic review has further
validated the plaque- and gingivitis- inhibiting
effect of CPC-containing mouthrinses [ 87 ].
The CPC molecule allows ionic and hydro-
phobic interactions since it belongs to both
hydrophilic and hydrophobic groups. It interacts
with microorganisms via cationic binding, simi-
lar to chlorhexidine. Although CPC shows equal
or better antimicrobial activity against planktonic
culture compared to chlorhexidine, it exhibits an
inferior inhibitory effect against biofi lm [
59 ].
This divergence may be attributed to the monoca-
tionic nature of CPC [ 53 ]. Initial retention of
CPC is higher than that of chlorhexidine, but
CPC is cleared from the oral cavity more rapidly
[ 17 ]. CPC could be incorporated into dental
materials, such as orthodontic adhesives, to con-
trol caries lesion formation around orthodontic
brackets. Although CPC retains its antimicrobial
properties, the clinical effect remains to be
assessed [ 59 ].
Polymers containing QACs have also been
incorporated into dental materials [
34 , 35 , 236 ].
QACs are immobilized in the composite and not
released or lost over time by copolymerizing with
the resin through the formation of a covalent
bond with the polymer network [ 209 ]. Therefore,
the dental material has a durable and long-lasting
antibacterial capability without signifi cantly dis-
turbing the biologic balance in the oral cavity and
material’s mechanic properties [ 96 ]. This is also
due to the nonleaching properties of the material,
since leaching leads to increased water absorp-
tion and solubility and decreased mechanical
properties with time, decreasing the clinical lon-
gevity of these materials [ 227 ]. Previous study
has presented that adhesive system containing
QACs has similar antibiofi lm properties and last-
ing at least 6 months of water aging [ 237 ].
Quaternary ammonium polyethylenimine (QAS-
PEI) nanoparticles were incorporated in restor-
ative materials to increase antibacterial action
without further reduction on mechanical proper-
ties [ 12 ]. It has been demonstrated that the incor-
poration of 1 % QAC-PEI nanoparticles in dental
composite resin has a strong antibacterial effect
against S. mutans and can sustain over 1 month
without alteration of the original mechanical
properties [ 13 ]. QAC-PEI nanoparticles were
also reported to have antibacterial effect against
S. mutans and L. casei when incorporated in con-
ventional glass ionomer [ 14 ].
2.4.4 Triclosan
Triclosan is a nonionic antimicrobial agent being
used for more than 30 years as a preservative in
products such as deodorants, soaps, and body
powders. More recently, triclosan has been
applied to dentifrices and mouthrinses as a pro-
phylactic agent with the purpose of reducing den-
tal biofi lm formation and the development of
gingivitis [ 59 ].
Triclosan is active against both gram-positive
and gram-negative microorganisms and fungi.
Oral bacteria such as S. mutans , S. sanguinis , and
S. salivarius are susceptible to low concentra-
tions of triclosan in vitro. At low concentrations,
2 Biofi lm and Dental Caries

46
triclosan is bacteriostatic [ 59 ]. Triclosan func-
tions by specifi cally inhibiting bacterial lipid
synthesis which further impairs cell membrane
synthesis [ 152 ]. The antimicrobial/antiplaque
properties of triclosan have been demonstrated in
both in vitro and in vivo studies [ 78 , 101 , 176 ,
186 , 198 ]. Triclosan and amine fl uoride also
show combinatory antibacterial and plaque-
reducing effect in vivo [ 6 ].
The major concern of the widespread use of
triclosan-containing products is the possibility of
inducing antimicrobial resistance [ 124 , 233 ].
Although triclosan is bactericidal at high concen-
trations, triclosan-containing products such as
dentifrices leave residues that will dilute to sub-
lethal concentration. Bacteria could develop
reduced susceptibility to triclosan after repeated
exposure to sublethal concentrations of this
agent. A number of studies have verifi ed the
occurrence of triclosan resistance among dermal,
intestinal, and environmental microorganisms
[ 233 ]. More importantly, the widespread use of
triclosan may also lead to the development of
concomitant resistance to other clinically impor-
tant antimicrobials through cross-resistance or
coresistance mechanisms. Although studies
regarding the concomitant resistance to other
antimicrobials are still limited, cautions should
be taken on the widespread use of triclosan [ 59 ].
2.4.5 Xylitol
Xylitol is an effective tooth decay-preventive
pentitol accredited to its non-acidogenic prop-
erty. Xylitol inhibits the growth of several bacte-
rial species, among which S. mutans appear to be
the specifi c target [ 19 , 133 , 208 ]. The S. mutans
uptakes xylitol via a constitutive transport system
specifi c for fructose and enriches xylitol intracel-
lularly as a non- metabolizable metabolite. In this
way, xylitol inhibits the growth of S. mutans and
reduces the amount of plaque and the number of
S. mutans in both the plaque and saliva of xylitol
consumers [ 203 ]. Xylitol is able to disrupt the
bacterial metabolism and consequently halt the
pH drop in the dental plaque. In addition, xylitol
can suppress the critical cariogenic factor of S.
mutans by impairing their polysaccharide forma-
tion. Long-term consumption of xylitol also pro-
motes the selection of xylitol-resistant S. mutans ,
which are believed to be less virulent than xylitol-
sensitive strains [
207 ]. All the aforementioned
mechanisms suggest that xylitol is a promising
cariostatic agent in controlling dental caries.
Xylitol has been incorporated as an active
ingredient in dental hygiene products, such as
xylitol-containing dentifrices currently available
on the market. Anticaries effect of xylitol has
been reported in longitudinal xylitol dentifrice
studies [ 189 ]. Xylitol is an alternative sweetening
agent to substitute sucrose in chewing gums.
Besides the antimicrobial effect of xylitol, chew-
ing itself promotes secretion of saliva and thus
caries prevention. However, further well-
designed randomized clinical studies with proper
controls are needed to validate the caries-
prophylactic effects of xylitol or superiority
claims of xylitol over other polyols [ 59 ].
2.4.6 Phenolic Antiseptics
Phenols, either alone or in combination, have
been known to be bactericidal since Lister used it
as a surgical antiseptic in the 1860s [ 220 ].
Phenols are able to reduce plaque accumulation
when used at high concentrations [ 63 , 135 ].
Listerine, which is an essential oil/phenolic
mouthwash, has been demonstrated in a number
of clinical studies to possess moderate antimicro-
bial effect against dental plaque [
43 , 71 ].
However, Listerine has poor oral retention com-
pared to chlorhexidine and thus lacks profound
plaque inhibitory effect [ 53 ].
2.4.7 Natural Products
Natural products offer a wide variety of structur-
ally different substances with a broad range of
biological activities, which could be applied into
the development of alternative or adjunctive anti-
caries therapies [ 104 ].
X. Xin et al.

47
The antibacterial effect of Camellia sinensis
(i.e., tea) against caries-related bacteria has been
widely studied, using both in vitro and in vivo
models [ 165 , 166 , 184 , 185 , 228 – 230 ].
Accumulating evidence has indicated that the
bioactive components of green tea, polyphenolic
catechins, in particular (−)-epigallocatechin gal-
late (EGCg) and (−)-epicatechin gallate (ECg),
are able to inhibit the growth of the streptococci
[ 200 ]. Catechins inhibit the growth of S. mutans
and S. sobrinus with MICs ranging between 50
and 1000 μg/ml, which are within the concentra-
tions determined in brewed tea [ 106 , 180 ].
Catechins are also bactericidal at even higher
concentrations. In addition, many of the “fl avor
compounds” (e.g., nerolidol) in green tea,
although they are not antibacterial by themselves,
might act synergistically with the abundant cate-
chins to suppress bacterial growth [ 120 ]. Recent
studies by our group have also demonstrated that
tea catechins, particular EGCg, could not only
inhibit the growth of cariogenic S. mutans at high
concentrations but also suppress cariogenic fac-
tors involved in the acidogenicity, acidurity, and
extracellular polysaccharide synthesis at sub-
MIC levels [ 228 – 230 ]. The bioactivities of tea
catechins at sub-MIC levels further support the
translational application of tea extracts in vivo,
considering the substantivity of these active com-
ponents in the oral cavity after tea consumption.
Essential oils have also been extensively
investigated for their antimicrobial activity
against caries-related bacteria. Essential oils,
odorous and volatile products of plant secondary
metabolism, are typically a complex mixture of
approximately 20–60 compounds that are in
solution at various concentrations [
104 ]. The
main active component of essential oils is terpe-
noids, followed by aromatic and aliphatic con-
stituents [ 9 ]. Thymol and eugenol inhibit the
growth of a wide range of oral microorganisms
including S. mutans [ 184 , 185 ]. Essential oils
appear to act against bacterial viability by dis-
rupting the integrity of the bacterial membrane
and cause the rapid effl ux of intracellular bacte-
rial components [ 185 ].
Propolis is a natural composite balsam collected
by bees from tree buds and mixed with secreted
beeswax [
66 , 89 ]. Propolis not only inhibits the
growth of S. mutans and S. sobrinus but also sup-
presses the production of bacterial polysaccharides
[ 65 ]. The application of propolis extract on rat
molars also reduces the severity of carious lesions
[ 179 ]. Specifi c fl avanones (pinocembrin), dihydro-
fl avonols (pinobanksin- 3-acetate), and terpenoids
(tt- farnesol) are believed to be the bioactive compo-
nents of propolis [ 112 ]. tt-Farnesol is particularly
effective against the proliferation of both planktonic
and biofi lm-associated S. mutans cells [ 113 ]. The
antibacterial activity of tt-farnesol is likely associ-
ated with its lipophilic property and membrano-
tropic effect, which might destroy the integrity of
bacterial cell membrane [ 41 , 97 ] and increase pro-
ton permeability [ 103 ].
Sanguinarine is a benzophenanthridine alkaloid
originated from the alcoholic extraction of pow-
dered rhizomes of the blood-root plant,
Sanguinaria canadensis [ 73 ]. Sanguinarine at a
concentration of 16 μg/ml could completely inhibit
98 % of microbial isolates from human dental
plaque [ 49 ]. A sanguinarine mouthrinse and tooth-
paste regime has been shown to signifi cantly
reduce plaque by 57 % when given for 6 months
during orthodontic treatment [ 86 ]. Another study
of sanguinarine mouthrinse and toothpaste carried
out in 120 subjects showed 13–17 % lower plaque
scores compared with a placebo group after a
6-month treatment period [ 115 ].
There are many other reports available con-
cerning the anticaries properties of various other
plant extracts. Wu-Yuan et al. [
225 ] and Li et al.
[ 126 ] have identifi ed that gallotannins from
Melaphis chinensis and triterpenes (ceanothic
acid and ceanothetric acid) from Ceanothus
americanus possess antimicrobial activity
against S. mutans . Furthermore, a chemically
characterized extract of Galla chinensis (con-
taining gallic acid and methyl gallate) can
inhibit the growth of S. mutans and other caries-
related organisms, including L. rhamnosus and
A. naeslundii , within biofi lms [ 226 ].
Ginkgoneolic acid from Ginkgo biloba has also
been shown to be a potential natural anticario-
genic agent which exhibits antimicrobial activ-
ity against S. mutans and suppresses specifi c
virulence factors associated with its cariogenicity
2 Biofi lm and Dental Caries

48
[ 90 ]. 7-Epiclusianone, a prenylated benzophe-
none isolated from Rheedia gardneriana , and
some cranberry fl avonoids (e.g., myricetin, pro-
cyanidin A2, and A-type oligomers) have been
shown to inhibit the acid-sensitive intracellular
glycolytic enzymes by increasing the proton
permeability of S. mutans cells [ 72 , 161 ]. A
crude extract of Psidium cattleianum can also
inhibit the expression of proteins involved in
general metabolism, glycolysis, and lactic acid
production of S. mutans [ 23 ].
Despite the aforementioned efforts to identify
the natural substances potentially active against
cariogenic organisms, it is still challenging to
determine the precise mechanisms of action and
effi cacy due to the complexity of chemistry and
isolation procedures. Moreover, few studies have
actually been conducted in vivo (e.g., using
rodent models of dental caries) and even fewer
have been evaluated in clinical trials [ 104 ]. The
implementation of standardized randomly con-
trolled trials is needed to further validate the
application of natural products in the fi eld of car-
ies management.
2.5 Ongoing Direction of Oral
Dental Plaque Study
2.5.1 Metagenomics and Oral
Microbiome
The term “metagenomics” was fi rst invented by
Handelsman [ 85 ], and it is defi ned as “the appli-
cation of modern genomics techniques to the
study of communities of microbial organisms
directly in their natural environments, bypassing
the need for isolation and lab cultivation of indi-
vidual species.” The advances in the refi nements
of DNA amplifi cation, bioinformatics, and
enhanced computational power for analyzing
DNA sequences have enabled the adaptation of
shotgun sequencing, such as chip-based pyrose-
quencing, to metagenomic samples [ 22 , 52 ]. The
approach randomly shears DNA, sequences
many short sequences, and reconstructs them into
a consensus sequence [ 22 ].
By performing metabolic function analyses on
genes identifi ed via metagenomic approach,
researchers are able to retrieve information both
on which organisms are present and, more impor-
tantly, what functions or metabolic processes are
possible in that particular community [
70 ]. Using
comparative genetic studies coupled with expres-
sion experiments such as microarray and pro-
teomics, microbiologist will be able to piece
together a metabolic network that goes beyond
species boundaries and gain valuable insight into
the metabolism within the community. Recently, a
comparative metagenomic study has been initi-
ated, aiming to compare the microbial community
within dental plaque associated with healthy and
diseased sites. It is anticipated that such compari-
son will assist in identifying potential pathogenic
organisms which may not have been detected
using currently available technologies [ 121 ].
Our group has been working on the
comparative study of oral microbiome using
metagenomics- related techniques. By pyrose-
quencing the “hypervariable regions” of bacterial
16S rRNA of biological samples (i.e., saliva,
supragingival plaque, and mucosal plaque)
collected from healthy population at different age
groups, we have demonstrated that the oral cavity
is a highly heterogeneous ecological system con-
taining distinct niches with signifi cantly different
microbial communities. More importantly, the
phylogenetic microbial structure varies with
aging, and only a few taxa were present across
the whole populations [
231 ]. By comparing the
composition of supragingival plaque microbiota
of acute lymphoblastic leukemia (ALL) pediatric
patients with healthy controls, we have
demonstrated that ALL patients have a structural
imbalance of the oral microbiota, characterized
by reduced diversity and abundance alterations,
possibly involved in systemic infections, indicat-
ing the importance of immune status in shaping
the structure of oral microbiota [ 217 ]. By charac-
terizing the phylogenetic and functional gene
differences between periodontal and healthy indi-
viduals, we have shown that the phylogenetic and
functional gene structures of the oral microbiomes
are distinctly different between periodontal and
X. Xin et al.

49
healthy groups. Specifi cally, a variety of genes
involved in virulence factors, amino acid metabo-
lism, and glycosaminoglycan and pyrimidine
degradation are enriched in periodontitis, while
the genes involved in amino acid synthesis and
pyrimidine synthesis are suppressed in people
with periodontitis compared with healthy indi-
viduals [ 128 ]. Overall, metagenomics-related
work from our group and those from others have
provided new insights into our understanding of
phylogenetic and functional gene structures of
oral microbial communities interacting with the
host. More cohort studies are needed to further
elucidate the contribution of the comparative
microbial difference to the pathogenesis and
prognosis of oral infectious diseases and hence
provide comprehensive knowledge for the devel-
opment of novel approaches to a better control of
dental caries.
2.5.2 Evidence-Based Dental Caries
Diagnosis
Although the infectious nature of dental caries has
been proved for more than 100 years, instead of
treating it as infectious disease, traditional den-
tistry still focuses on treating the symptom (repair
the damaged tooth) via surgical approaches. The
recent advancement in caries pathogenesis allows
us to understand that a comprehensive analysis of
dental caries should be more than detecting tooth
demineralization sites and repairing damaged
teeth with surgical approaches. Instead, it should
include the detection of cariogenic bacteria and
plaque acidogenicity, followed by a comprehen-
sive treatment of dental caries that includes the
elimination of cariogenic bacteria, the reduction of
plaque acidogenicity, and the enhancement of
tooth remineralization [
204 ]. The combination of
accurate detection of oral bacteria and in situ mon-
itoring of plaque pH, such as the polyaniline-based
planer pH sensor developed by scientists at Jet
Propulsion Laboratory and combined NMR con-
focal microscopy from Pacifi c Northwest National
Laboratory which can monitor pH gradient within
dental plaque with high sensitivity and in real time
[ 138 ], is opening a new chapter for cariology.
Early detection and quantifi cation of cariogenic
bacteria in plaque or saliva samples can help clini-
cians take preventive measures to stop caries
development, much like early detection of cancer
markers before overt/detectable cancerous lesions
develop. New antibody- or nucleotide-based bac-
terial detection techniques have also been devel-
oped for the detection of cariogenic bacteria in
chair-side or laboratory settings [
187 ]. In conjunc-
tion with nanotechnology development, these tests
can be further developed into different forms of
nanochips for the detection of multiple pathogens
in the clinical settings [ 127 ].
2.5.3 Novel Antimicrobial Therapies
In addition to “traditional” antimicrobial agents
mentioned above, new approaches have been
developed to maintain oral biofi lm homeostasis.
Such strategies are aimed to inhibit plaque bio-
fi lm formation without disturbing the biological
equilibrium within the oral cavity.
2.5.3.1 Probiotics
Probiotics is defi ned by the World Health
Organization as live microorganisms which,
when applied in adequate amounts, will benefi t
the health of the host [ 5 ]. The use of probiotics,
which has been successful established in the
treatment of intestinal diseases, is now also con-
sidered for the treatment of oral diseases [ 162 ,
206 ]. Experimental studies and clinical trials
have recently demonstrated that certain gastroin-
testinal bacteria, including Lactobacillus and
Bifi dobacterium spp., may help in controlling the
proliferation of oral microorganisms, including
cariogenic streptococci. L. rhamnosus CG [ 162 ],
L. casei [ 27 ], L. reuteri [ 30 ], and Bifi dobacterium
DN-173 010 [ 29 ] have all been shown to have the
potential to alter colonization of cariogenic bac-
teria and thus contributed in the prevention of
dental caries. However, cautions should be taken
when using the lactobacillus species as they can
also produce acids [ 202 ]. Mechanisms of probi-
otic effect within the oral cavity are likely similar
to those proposed in gastrointestinal studies
[ 154 ]. The introduction of microorganisms as a
2 Biofi lm and Dental Caries

50
therapeutic tool for the management of dental
caries could possibly act through colonization
resistance and/or immune modulation within the
oral environment [ 5 ].
2.5.3.2 Salivary Antimicrobial
Substances
Antimicrobial substances in saliva and their syn-
thetic analogues are also promising in caries
control [ 92 ]. Whole saliva contains two peroxi-
dase that oxidize thiocyanate (SCN
−
) to hypo-
thiocyanite (OSCN
−
) in the presence of hydrogen
peroxide. Hypothiocyanite is antimicrobial and
inhibits some streptococci and lactobacilli
in vitro [ 134 ]. The effect of the salivary peroxi-
dase system is related to the availability of
hydrogen peroxide, which is produced by vari-
ous microorganisms as a metabolic end product
[ 134 ]. Glucose oxidase produces hydrogen per-
oxide from glucose provided by the enzyme
amyloglucosidase. The addition of these
enzymes to oral products is suggested to gener-
ate adequate hydrogen peroxide so as to control
the growth of microorganisms via enhanced per-
oxidase effect [ 59 ].
2.5.3.3 Specifi cally Targeted
Antimicrobial Peptides
(STAMPs)
The benefi ts of probiotics are mainly achieved by
modulating existing microbial fl ora associated
with the host, thus maintaining a balanced and
healthy microbe-host relationship. In addition to
the use of live organisms, microbiologists are
now developing novel techniques and products
that do not involve live bacteria, yet generate tar-
geted effects against pathogenic factors or organ-
isms and achieve similar probiotic effects [
91 ].
One good example is the targeted antimicrobial
therapy via a novel specifi cally targeted antimi-
crobial peptides technology [ 50 , 51 ]. A “STAMP”
is a fusion peptide with two moieties: a killing
moiety made of a nonspecifi c antimicrobial pep-
tide and a targeting moiety containing a species-
specifi c binding peptide. The targeting moiety
provides specifi c recognition of a selected patho-
gen and targeted delivery of an attached antimi-
crobial peptide. A pheromone produced by S.
mutans , namely, competence-stimulating peptide
(CSP), has been used as a STAMP targeting
domain to mediate S. mutans -specifi c delivery of
killing domain. Such STAMPs are potent against
S. mutans grown in liquid as well as in biofi lm
cultures [ 50 ]. Importantly, STAMPs are able to
eliminate S. mutans from multispecies biofi lms
without affecting other noncariogenic commen-
sal residents [ 50 , 51 ]. Hence, these molecules act
as “probiotic” antimicrobials that may selectively
eliminate pathogens while preserving the protec-
tive benefi ts of the normal fl ora. The success in
the development of S. mutans -specifi c STAMPs
may expand further to the design of other
STAMPs specifi cally targeting various pathogens
including periodontal pathogens within oral cav-
ity and provides an ecological approach to the
management of dental caries.
2.5.3.4 Light Active Killing
Light is more effective on bacteria in biofi lm
where chemical agent might encounter diffusion
limitations. Light therapy could be used to reduce
or modify the oral biofi lm and may offer substan-
tial health benefi ts. S. mutans biofi lm cells can be
killed by up to 3 log
10
folds when treated with
erythrosine and white light (500–650 nm) [ 224 ].
Exposure of S. mutans biofi lm to blue light had
an impeded effect on bacterial viability through-
out the biofi lm and a sustained antibacterial effect
on biofi lm newly formed by previously irradiated
bacteria [ 60 ]. CO
2
laser (wavelength of 10.6 μm)
under certain conditions is suggested as a poten-
tial novel preventive light therapy against bio-
fi lms. The activity of CO
2
laser irradiation on the
viability of S. mutans in biofi lm can reach in the
deep layers. However, CO
2
may potentially dam-
age the tooth surface, which limits its application
in plaque control [ 60 ] .
References
1. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE.
Defi ning the normal bacterial fl ora of the oral cavity.
J Clin Microbiol. 2005;43(11):5721–32.
2. Acevedo AM, Machado C, Rivera LE, Wolff M,
Kleinberg I. The inhibitory effect of an arginine
bicarbonate/calcium carbonate CaviStat-containing
X. Xin et al.

51
dentifrice on the development of dental caries in
Venezuelan school children. J Clin Dent. 2005;16(3):
63–70.
3. Acevedo AM, Montero M, Rojas-Sanchez F,
Machado C, Rivera LE, Wolff M, et al. Clinical eval-
uation of the ability of CaviStat in a mint confection
to inhibit the development of dental caries in chil-
dren. J Clin Dent. 2008;19(1):1–8.
4. Addy M, Jenkins S, Newcombe R. The effect of
some chlorhexidine-containing mouthrinses on sali-
vary bacterial counts. J Clin Periodontol. 1991;
18(2):90–3.
5. Allaker RP, Douglas CW. Novel anti-microbial thera-
pies for dental plaque-related diseases. Int J Antimicrob
Agents. 2009;33(1):8–13.
6. Arweiler NB, Henning G, Reich E, Netuschil L.
Effect of an amine‐fl uoride‐triclosan mouthrinse on
plaque regrowth and biofi lm vitality. J Clin
Periodontol. 2002;29(4):358–63.
7. Atlas RMBR, editor. Microbial ecology. Menlo Park:
Benjamin/Cummings Science Publishing; 1997.
8. Baehni PC, Takeuchi Y. Anti-plaque agents in the
prevention of biofi lm-associated oral diseases. Oral
Dis. 2003;9 Suppl 1:23–9.
9. Bakkali F, Averbeck S, Averbeck D, Idaomar M.
Biological effects of essential oils – a review. Food
Chem Toxicol. 2008;46(2):446–75.
10. Banas JA, Vickerman MM. Glucan-binding proteins
of the oral streptococci. Crit Rev Oral Biol Med.
2003;14(2):89–99.
11. Bansal G, Newman H, Wilson M. The survival of
subgingival plaque bacteria in an amine fl uoride‐con-
taining gel. J Clin Periodontol. 1990;17(7):414–8.
12. Beyth N, Yudovin-Farber I, Bahir R, Domb AJ,
Weiss EI. Antibacterial activity of dental composites
containing quaternary ammonium polyethylenimine
nanoparticles against Streptococcus mutans .
Biomaterials. 2006;27(21):3995–4002.
13. Beyth N, Yudovin-Fearber I, Domb AJ, Weiss EI.
Long-term antibacterial surface properties of compos-
ite resin incorporating polyethyleneimine nanoparti-
cles. Quintessence Int. 2009;41(10):827–35.
14. Beyth N, Pilo R, Weiss EI. Antibacterial activity of
dental cements containing quaternary ammonium
polyethylenimine nanoparticles. J Nanomater. 2012;
2012:58.
15. Bjarnsholt T, editor. Biofi lm infections. New York:
Springer Science + Business Media, LLC.; 2011.
16. Black GV. Dr. Blacks conclusions reviewed again.
Dent Cosmos. 1898;40:440.
17. Bonesvoll P, Gjermo P. A comparison between
chlorhexidine and some quaternary ammonium
compounds with regard to retention, salivary con-
centration and plaque-inhibiting effect in the human
mouth after mouth rinses. Arch Oral Biol. 1978;
23(4):289–94.
18. Bowen WH, Koo H. Biology of Streptococcus
mutans-derived glucosyltransferases: role in extra-
cellular matrix formation of cariogenic biofi lms.
Caries Res. 2011;45(1):69–86.
19. Bradshaw D, Marsh P. Effect of sugar alcohols on
the composition and metabolism of a mixed culture
of oral bacteria grown in a chemostat. Caries Res.
1994;28(4):251–6.
20. Bradshaw D, Marsh P. Analysis of pH–driven dis-
ruption of oral microbial communities in vitro.
Caries Res. 1998;32(6):456–62.
21. Bradshaw D, Marsh P, Hodgson R, Visser J. Effects
of glucose and fl uoride on competition and metabo-
lism within in vitro dental bacterial communities and
biofi lms. Caries Res. 2002;36(2):81–6.
22. Breitbart M, Salamon P, Andresen B, Mahaffy JM,
Segall AM, Mead D, et al. Genomic analysis of
uncultured marine viral communities. Proc Natl
Acad Sci U S A. 2002;99(22):14250–5.
23. Brighenti F, Luppens S, Delbem A, Deng D,
Hoogenkamp M, Dekker H, et al. Effect of Psidium
cattleianum leaf extract on Streptococcus mutans
viability, protein expression and acid production.
Caries Res. 2008;42(2):148–54.
24. Bullock S, Newman H, Wilson M. The in-vitro
effect of an amine fl uoride gel on subgingival plaque
bacteria. J Antimicrob Chemother. 1989;23(1):
59–67.
25. Burne RA. Oral streptococci… products of their
environment. J Dent Res. 1998;77(3):445–52.
26. Burne RA, Marquis RE. Alkali production by oral
bacteria and protection against dental caries. FEMS
Microbiol Lett. 2000;193(1):1–6.
27. Busscher H, Mulder A, Van der Mei H. In vitro adhe-
sion to enamel and in vivo colonization of tooth sur-
faces by lactobacilli from a Bio–Yoghurt. Caries
Res. 1999;33(5):403–4.
28. Buzalaf MA, Pessan JP, Honorio HM, ten Cate JM.
Mechanisms of action of fl
uoride for caries control.
Monogr Oral Sci. 2011;22:97–114.
29. Caglar E, Sandalli N, Twetman S, Kavaloglu S,
Ergeneli S, Selvi S. Effect of yogurt with
Bifi dobacterium DN-173 010 on salivary mutans
streptococci and lactobacilli in young adults. Acta
Odontol. 2005;63(6):317–20.
30. Çaglar E, Kavaloglu Cildir S, Ergeneli S, Sandalli N,
Twetman S. Salivary mutans streptococci and lacto-
bacilli levels after ingestion of the probiotic bacte-
rium Lactobacillus reuteri ATCC 55730 by straws or
tablets. Acta Odontol. 2006;64(5):314–8.
31. Chalmers NI, Palmer Jr RJ, Cisar JO, Kolenbrander
PE. Characterization of a Streptococcus sp.-Veillon-
ella sp. community micromanipulated from dental
plaque. J Bacteriol. 2008;190(24):8145–54.
32. Chen Y-YM, Burne RA. Identifi cation and charac-
terization of the nickel uptake system for urease bio-
genesis in Streptococcus salivarius 57. I. J Bacteriol.
2003;185(23):6773–9.
33. Chen YY, Weaver CA, Burne RA. Dual functions of
Streptococcus salivarius urease. J Bacteriol. 2000;
182(16):4667–9.
34. Cheng L, Weir MD, Xu HH, Antonucci JM,
Kraigsley AM, Lin NJ, et al. Antibacterial amor-
phous calcium phosphate nanocomposites with a
2 Biofi lm and Dental Caries

52
quaternary ammonium dimethacrylate and silver
nanoparticles. Dent Mater. 2012;28(5):561–72.
35. Cheng L, Zhang K, Melo MA, Weir MD, Zhou X,
Xu HH. Anti-biofi lm dentin primer with quaternary
ammonium and silver nanoparticles. J Dent Res.
2012;91(6):598–604.
36. Chikindas ML, Novak J, Driessen AJ, Konings WN,
Schilling KM, Caufi eld PW. Mutacin II, a bactericidal
antibiotic from Streptococcus mutans. Antimicrob
Agents Chemother. 1995;39(12):2656–60.
37. Clancy KA, Pearson S, Bowen WH, Burne RA.
Characterization of recombinant, ureolytic
Streptococcus mutans demonstrates an inverse rela-
tionship between dental plaque ureolytic capacity and
cariogenicity. Infect Immun. 2000;68(5):2621–9.
38. Clarke J. On the bacterial factor in the etiology of
dental caries. Br J Exp Pathol. 1924;5:141–7.
39. Cole MF, Bowen WH. Effect of sodium phytate on
the chemical and microbial composition of dental
plaque in the monkey (Macaca fascicularis). J Dent
Res. 1975;54(3):449–57.
40. Costerton JWSP. Bacterial biofi lms: a common cause
of persistent infections. Science. 1999;284(5418):
1318–22.
41. Cowan MM. Plant products as antimicrobial agents.
Clin Microbiol Rev. 1999;12(4):564–82.
42. Crielaard W, Zaura E, Schuller AA, Huse SM,
Montijn RC, Keijser BJ. Exploring the oral micro-
biota of children at various developmental stages of
their dentition in the relation to their oral health.
BMC Med Genom. 2011;4:22.
43. DePaola LG, Overholser CD, Meiller TF, Minah GE,
Niehaus C. Chemotherapeutic inhibition of supragin-
gival dental plaque and gingivitis development. J Clin
Periodontol. 1989;16(5):311–5.
44. DePaola LG, Spolarich AE. Safety and effi cacy of
antimicrobial mouthrinses in clinical practice. J Dent
Hyg. 2007;81(5):117.
45. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu
W-H, et al. The human oral microbiome. J Bacteriol.
2010;192(19):5002–17.
46. Diaz PI, Zilm PS, Rogers AH. Fusobacterium nuclea-
tum supports the growth of Porphyromonas gingivalis
in oxygenated and carbon-dioxide- depleted environ-
ments. Microbiology. 2002;148(Pt 2):467–72.
47. Dong Y, Chen Y-YM, Snyder JA, Burne RA. Isolation
and molecular analysis of the gene cluster for the argi-
nine deiminase system from Streptococcus gordonii
DL1. Appl Environ Microbiol. 2002;68(11):5549–53.
48. Dong Y, Chen Y-YM, Burne RA. Control of expres-
sion of the arginine deiminase operon of Streptococcus
gordonii by CcpA and Flp. J Bacteriol. 2004;
186(8):2511–4.
49. Dzink JL, Socransky SS. Comparative in vitro activ-
ity of sanguinarine against oral microbial isolates.
Antimicrob Agents Chemother. 1985;27(4):663–5.
50. Eckert R, He J, Yarbrough DK, Qi F, Anderson MH,
Shi W. Targeted killing of Streptococcus mutans by a
pheromone-guided “smart” antimicrobial peptide.
Antimicrob Agents Chemother. 2006;50(11):3651–7.
51. Eckert R, Sullivan R, Shi W. Targeted antimicrobial
treatment to re-establish a healthy microbial fl ora for
long-term protection. Adv Dent Res. 2012;24(2):
94–7.
52. Edwards RA, Rodriguez-Brito B, Wegley L, Haynes
M, Breitbart M, Peterson DM, et al. Using pyrose-
quencing to shed light on deep mine microbial ecol-
ogy. BMC Genomics. 2006;7:57.
53. Eley B. Periodontology: antibacterial agents in the
control of supragingival plaque—a review. Br Dent
J. 1999;186(6):286–96.
54. Embleton J, Newman H, Wilson M. Amine and tin
fl uoride inhibition of Streptococcus sanguis adhesion
under continuous fl ow. Oral Microbiol Immunol.
2001;16(3):182–4.
55. Epstein SR, Mandel I, Scopp IW. Salivary composi-
tion and calculus formation in patients undergoing
hemodialysis. J Periodontol. 1980;51(6):336–8.
56. Espeland EM, Wetzel RG. Complexation, stabiliza-
tion, and UV photolysis of extracellular and surface-
bound glucosidase and alkaline phosphatase:
implications for biofi lm microbiota. Microb Ecol.
2001;42(4):572–85.
57. Espinosa-Urgel M, Salido A, Ramos JL. Genetic
analysis of functions involved in adhesion of
Pseudomonas putida to seeds. J Bacteriol. 2000;182(9):
2363–9.
58. Featherstone JD. Remineralization, the natural car-
ies repair process–the need for new approaches. Adv
Dent Res. 2009;21(1):4–7.
59. Fejerskov O, Kidd E. Dental caries: the disease and
its clinical management. Wiley-Blackwell, Hoboken,
N.J.; 2008.
60. Feuerstein O. Light therapy complementary antibac-
terial treatment of oral biofi lm. Adv Dent Res.
2012;24(2):103–7.
61. Fitzgerald RJ, Keyes PH. Demonstration of the etio-
logic role of streptococci in experimental caries in
the hamster. J Am Dent Assoc. 1960;61:9–19.
62. Flemming HC, Wingender J. The biofi lm matrix.
Nat Rev Microbiol. 2010;8(9):623–33.
63. Fornell J, Sundin Y, Lindhe J. Effect of listerine on
dental plaque and gingivitis. Scand J Dent Res.
1975;83(1):18–25.
64. Göttler M. Über einige Additions-und Konden-
sationsprodukte der Halogenacetamide und ihrer
N-Methylolverbindungen und über die Einwirkung
von Formaldehyd und sekundären Basen auf Isatin:
Höfl ing; 1908.
65. Gebara E, Zardetto C, Mayer M. In vitro study of the
antimicrobial activity of natural substances against
S. mutans and S. sobrinus. Rev Odontol Univ Sao
Paulo. 1996;10:251–6.
66. Gebara EC, Lima LA, Mayer M. Propolis antimicro-
bial activity against periodontopathic bacteria. Braz
J Microbiol. 2002;33(4):365–9.
67. Gest H. The discovery of microorganisms by Robert
Hooke and Antoni Van Leeuwenhoek, fellows of the
Royal Society. Notes Rec R Soc Lond. 2004;58(2):
187–201.
X. Xin et al.

53
68. Gibbons RJ, Hay DI, Childs 3rd WC, Davis G. Role
of cryptic receptors (cryptitopes) in bacterial
adhesion to oral surfaces. Arch Oral Biol. 1990;
35(Suppl):107S–14.
69. Gilbert P, Allison DG, McBain AJ. Biofi lms in vitro
and in vivo: do singular mechanisms imply cross-
resistance? J Appl Microbiol. 2002;92(Suppl):98S–110.
70. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh
PJ, Samuel BS, et al. Metagenomic analysis of the
human distal gut microbiome. Science. 2006;
312(5778):1355–9.
71. Gordon J, Lamster I, Seiger M. Effi cacy of Listerine
antiseptic in inhibiting the development of plaque
and gingivitis. J Clin Periodontol. 1985;12(8):
697–704.
72. Gregoire S, Singh A, Vorsa N, Koo H. Infl uence of
cranberry phenolics on glucan synthesis by glucos-
yltransferases and Streptococcus mutans acidoge-
nicity. J Appl Microbiol. 2007;103(5):1960–8.
73. Grenby T. The use of sanguinarine in mouthwashes
and toothpaste compared with some other antimicro-
bial agents. Br Dent J. 1995;178(7):254–8.
74. Griswold AR, Chen Y-YM, Burne RA. Analysis of
an agmatine deiminase gene cluster in Streptococcus
mutans UA159. J Bacteriol. 2004;186(6):1902–4.
75. Griswold AR, Jameson-Lee M, Burne RA. Regulation
and physiologic signifi cance of the agmatine deimi-
nase system of Streptococcus mutans UA159. J
Bacteriol. 2006;188(3):834–41.
76. Griswold AR, Nascimento MM, Burne RA.
Distribution, regulation and role of the agmatine deim-
inase system in mutans streptococci. Oral Microbiol
Immunol. 2009;24(1):79–82.
77. Gross EL, Leys EJ, Gasparovich SR, Firestone ND,
Schwartzbaum JA, Janies DA, et al. Bacterial 16S
sequence analysis of severe caries in young perma-
nent teeth. J Clin Microbiol. 2010;48(11):4121–8.
78. Guggenheim B, Giertsen E, Schüpbach P, Shapiro
S. Validation of an in vitro biofi lm model of suprag-
ingival plaque. J Dent Res. 2001;80(1):363–70.
79. Gunsolley JC. A meta-analysis of six-month studies
of antiplaque and antigingivitis agents. J Am Dent
Assoc. 2006;137(12):1649–57.
80. Gunsolley JC. Clinical effi cacy of antimicrobial
mouthrinses. J Dent. 2010;38:S6–10.
81. Hale JD, Ting YT, Jack RW, Tagg JR, Heng NC.
Bacteriocin (mutacin) production by Streptococcus
mutans genome sequence reference strain UA159:
elucidation of the antimicrobial repertoire by genetic
dissection. Appl Environ Microbiol. 2005;71(11):
7613–7.
82. Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial
biofi lms: from the natural environment to infectious
diseases. Nat Rev Microbiol. 2004;2(2):95–108.
83. Hamada S, Slade HD. Biology, immunology, and
cariogenicity of Streptococcus mutans. Microbiol
Rev. 1980;44(2):331–84.
84. Hamilton I. Biochemical effects of fl uoride on oral
bacteria. J Dent Res. 1990;69:660–7; discussion
682–663.
85. Handelsman J, Rondon MR, Brady SF, Clardy J,
Goodman RM. Molecular biological access to the
chemistry of unknown soil microbes: a new frontier
for natural products. Chem Biol. 1998;5(10):R245–9.
86. Hannah J, Johnson J, Kuftinec M. Long-term clini-
cal evaluation of toothpaste and oral rinse containing
sanguinaria extract in controlling plaque, gingival
infl ammation, and sulcular bleeding during orth-
odontic treatment. Am J Orthod Dentofac Orthop.
1989;96(3):199–207.
87. Haps S, Slot D, Berchier C, Van der Weijden G. The
effect of cetylpyridinium chloride‐containing mouth
rinses as adjuncts to toothbrushing on plaque and
parameters of gingival infl ammation: a systematic
review. Int J Dent Hyg. 2008;6(4):290–303.
88. Hay DI, Gibbons RJ, Spinell DM. Characteristics of
some high molecular weight constituents with bacte-
rial aggregating activity from whole saliva and den-
tal plaque. Caries Res. 1971;5(2):111–23.
89. Hayacibara MF, Koo H, Rosalen PL, Duarte S,
Franco EM, Bowen WH, et al. In vitro and in vivo
effects of isolated fractions of Brazilian propolis on
caries development. J Ethnopharmacol. 2005;101(1):
110–5.
90. He J, Wang S, Wu T, Cao Y, Xu X, Zhou X. Effects
of ginkgoneolic acid on the growth, acidogenicity,
adherence, and biofi lm of Streptococcus mutans
in vitro. Folia Microbiol. 2013;58(2):147–53.
91. He XS, Shi WY. Oral microbiology: past, present
and future. Int J Oral Sci. 2009;1(2):47–58.
92. Helmerhorst EJ, Hodgson R, Van’t Hof W, Veerman
E, Allison C, Nieuw Amerongen A. The effects of
histatin-derived basic antimicrobial peptides on oral
biofi lms. J Dent Res. 1999;78(6):1245–50.
93. Herrera D, van Winkelhoff AJ, Dellemijn-Kippuw N,
Winkel EG, Sanz M. Beta-lactamase producing bacte-
ria in the subgingival microfl ora of adult patients with
periodontitis. A comparison between Spain and The
Netherlands. J Clin Periodontol. 2000;27(7):520–5.
94. Hotz P, Guggenheim B, Schmid R. Carbohydrates in
pooled dental plaque. Caries Res. 1972;6(2):103–21.
95. Hugo WB, Longworth AR. Some aspects of the
mode of action of chlorhexidine. J Pharm Pharmacol.
1964;16:655–62.
96. Imazato S, J-h C, Ma S, Izutani N, Li F. Antibacterial
resin monomers based on quaternary ammonium
and their benefi ts in restorative dentistry. Jap Dent
Sci Rev. 2012;48(2):115–25.
97. Inoue Y, Shiraishi A, Hada T, Hirose K, Hamashima
H, Shimada J. The antibacterial effects of terpene
alcohols on Staphylococcus aureus and their mode of
action. FEMS Microbiol Lett. 2004;237(2):325–31.
98. Jacobs WA. The bactericidal properties of the qua-
ternary salts of hexamethylenetetramine I. The prob-
lem of the chemotherapy of experimental bacterial
infections. J Exp Med. 1916;23(5):563–8.
99. Jakubovics NS, Gill SR, Iobst SE, Vickerman MM,
Kolenbrander PE. Regulation of gene expression in a
mixed-genus community: stabilized arginine biosynthesis
in Streptococcus gordonii by coaggregation with
2 Biofi lm and Dental Caries

54
Actinomyces naeslundii. J Bacteriol. 2008;190(10):
3646–57.
100. Jakubovics NS, Kolenbrander PE. The road to ruin:
the formation of disease-associated oral biofi lms.
Oral Dis. 2010;16(8):729–39.
101. Jenkins S, Addy M, Newcombe R. A comparison of
cetylpyridinium chloride, triclosan and chlorhexi-
dine mouthrinse formulations for effects on plaque
regrowth. J Clin Periodontol. 1994;21(6):441–4.
102. Jensen ME, Wefel JS. Human plaque pH responses
to meals and the effects of chewing gum. Br Dent
J. 1989;167(6):204–8.
103. Jeon J-G, Klein MI, Xiao J, Gregoire S, Rosalen PL,
Koo H. Infl uences of naturally occurring agents in
combination with fl uoride on gene expression and
structural organization of Streptococcus mutans in
biofi lms. BMC Microbiol. 2009;9(1):228.
104. Jeon JG, Rosalen PL, Falsetta ML, Koo H. Natural
products in caries research: current (limited) knowl-
edge, challenges and future perspective. Caries Res.
2011;45(3):243–63.
105. Jordan HV. Rodent model systems in periodontal
disease research. J Dent Res. 1971;50(2):236–42.
106. Kawamura J, Takeo T. Antibacterial activity of tea
catechin to Streptococcus mutans. J Jap Soc Food
Sci Technol. 1989;36(6):463–7.
107. Kay H, Wilson M. The in vitro effects of amine fl uo-
rides on plaque bacteria*. J Periodontol. 1988;59(4):
266–9.
108. Keyes PH. The infectious and transmissible nature
of experimental dental caries. Findings and implica-
tions. Arch Oral Biol. 1960;1:304–20.
109. Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-
Jorgensen A, Molin S, et al. Biofi lm formation by
Pseudomonas aeruginosa wild type, fl agella and type
IV pili mutants. Mol Microbiol. 2003;48(6):1511–24.
110. Kleinberg I. A mixed-bacteria ecological approach
to understanding the role of the oral bacteria in den-
tal caries causation: an alternative to Streptococcus
mutans and the specifi c-plaque hypothesis. Crit Rev
Oral Biol Med. 2002;13(2):108–25.
111. Kolenbrander PE, Jakubovics NS, Bachrach G. Oral
microbiology. In: Schaechter M, editor. Encyclopedia
of microbiology. Oxford, UK: Elsevier; 2009.
112. Koo H, Rosalen PL, Cury JA, Park YK, Bowen
WH. Effects of compounds found in propolis on
Streptococcus mutans growth and on glucosyltrans-
ferase activity. Antimicrob Agents Chemother. 2002;
46(5):1302–9.
113. Koo H, Hayacibara M, Schobel B, Cury J, Rosalen P,
Park Y, et al. Inhibition of Streptococcus mutans bio-
fi lm accumulation and polysaccharide production by
apigenin and tt-farnesol. J Antimicrob Chemother.
2003;52(5):782–9.
114. Koo H. Strategies to enhance the biological effects
of fl uoride on dental biofi lms. Adv Dent Res.
2008;20(1):17–21.
115. Kopczyk RA, Abrams H, Brown AT, Matheny JL,
Kaplan AL. Clinical and microbiological effects of a
sanguinaria-containing mouthrinse and dentifrice
with and without fl uoride during 6 months of use. J
Periodontol. 1991;62(10):617–22.
116. Kraivaphan P, Amornchat C, Triratana T, Mateo LR,
Ellwood R, Cummins D, et al. Two-year caries clinical
study of the effi cacy of novel dentifrices containing
1.5 % arginine, an insoluble calcium compound and
1,450 ppm fl uoride. Caries Res. 2013;47(6):582–90.
117. Kreth J, Merritt J, Shi W, Qi F. Competition and
coexistence between Streptococcus mutans and
Streptococcus sanguinis in the dental biofi lm.
J Bacteriol. 2005;187(21):7193–203.
118. Kreth J, Merritt J, Shi W, Qi F. Co-ordinated bacterio-
cin production and competence development: a possi-
ble mechanism for taking up DNA from neighbouring
species. Mol Microbiol. 2005;57(2):392–404.
119. Kreth J, Zhang Y, Herzberg MC. Streptococcal antago-
nism in oral biofi lms: Streptococcus sanguinis and
Streptococcus gordonii interference with Streptococcus
mutans. J Bacteriol. 2008;190(13):4632–40.
120. Kubo I, Muroi H, Himejima M. Antimicrobial activ-
ity of green tea fl avor components and their combina-
tion effects. J Agric Food Chem. 1992;40(2):245–8.
121. Kumar PS, Griffen AL, Barton JA, Paster BJ,
Moeschberger ML, Leys EJ. New bacterial species
associated with chronic periodontitis. J Dent Res.
2003;82(5):338–44.
122. Kuramitsu HK, He X, Lux R, Anderson MH, Shi W.
Interspecies interactions within oral microbial commu-
nities. Microbiol Mol Biol Rev. 2007;71(4):653–70.
123. Lawrence CA. Surface-active quaternary ammo-
nium germicides. New York: Academic; 1950.
124. Ledder R, Gilbert P, Willis C, McBain A. Effects of
chronic triclosan exposure upon the antimicrobial
susceptibility of 40 ex‐situ environmental and
human isolates. J Appl Microbiol. 2006;100(5):
1132–40.
125. Leid JG, Shirtliff ME, Costerton JW, Stoodley
P. Human leukocytes adhere to, penetrate, and
respond to Staphylococcus aureus biofi lms. Infect
Immun. 2002;70(11):6339–45.
126. Li X-C, Cai L, Wu CD. Antimicrobial compounds
from Ceanothus americanus against oral pathogens.
Phytochemistry. 1997;46(1):97–102.
127. Li Y, Denny P, Ho CM, Montemagno C, Shi W, Qi F,
et al. The oral fl uid MEMS/NEMS chip (OFMNC):
diagnostic and translational applications. Adv Dent
Res. 2005;18(1):3–5.
128. Li Y, He J, He Z, Zhou Y, Yuan M, Xu X, et al.
Phylogenetic and functional gene structure shifts of
the oral microbiomes in periodontitis patients. ISME
J. 2014;8(9):1879–91.
129. Liu Y, Hu T, Zhang J, Zhou X. Characterization of
the Actinomyces naeslundii ureolysis and its role
in bacterial aciduricity and capacity to modulate
pH homeostasis. Microbiol Res. 2006;161(4):
304–10.
130. Liu Y, Burne RA. Multiple two-component systems
of Streptococcus mutans regulate agmatine deimi-
nase gene expression and stress tolerance. J Bacteriol.
2009;191(23):7363–6.
131. Liu YL, Nascimento M, Burne RA. Progress toward
understanding the contribution of alkali generation
in dental biofi lms to inhibition of dental caries. Int
J. Oral Sci. 2012;4(3):135–40.
X. Xin et al.

55
132. Loesche WJ, Rowan J, Straffon LH, Loos PJ.
Association of Streptococcus mutants with human
dental decay. Infect Immun. 1975;11(6):1252–60.
133. Loesche WJ, Grossman N, Earnest R, Corpron R.
The effect of chewing xylitol gum on the plaque and
saliva levels of Streptococcus mutans. J Am Dent
Assoc. 1984;108(4):587–92.
134. Lumikari M, Soukka T, Nurmio S, Tenovuo
J. Inhibition of the growth of Streptococcus mutans ,
Streptococcus sobrinus and Lactobacillus casei by
oral peroxidase systems in human saliva. Arch Oral
Biol. 1991;36(2):155–60.
135. Lusk SS, Bowers GM, Tow HD, Watson WJ, Moffi tt
WC. Effects of an oral rinse on experimental gingi-
vitis plaque formation, and formed plaque. J Am Soc
Prev Dent. 1974;4(4):31–3. passim.
136. Maghnouj A, de Sousa Cabral TF, Stalon V, Vander
Wauven C. The arcABDC gene cluster, encoding the
arginine deiminase pathway of Bacillus lichenifor-
mis, and its activation by the arginine repressor
argR. J Bacteriol. 1998;180(24):6468–75.
137. Mah TF, O’Toole GA. Mechanisms of biofi lm resis-
tance to antimicrobial agents. Trends Microbiol.
2001;9(1):34–9.
138. Majors PD, McLean JS, Pinchuk GE, Fredrickson
JK, Gorby YA, Minard KR, et al. NMR methods for
in situ biofi lm metabolism studies. J Microbiol
Methods. 2005;62(3):337–44.
139. Maltz M, Beighton D. Multidisciplinary research
agenda for novel antimicrobial agents for caries preven-
tion and treatment. Adv Dent Res. 2012;24(2):133–6.
140. Mankodi S, Bauroth K, Witt J, Bsoul S, He T, Gibb
R, et al. 6-month clinical trial to study the effects of
a cetylpyridinium chloride mouthrinse on gingivitis
and plaque. Am J Dent. 2005;18(Spec):9A–14A.
141. Marquis RE, Bender GR, Murray DR, Wong
A. Arginine deiminase system and bacterial adapta-
tion to acid environments. Appl Environ Microbiol.
1987;53(1):198–200.
142. Marsh PD, Bradshaw DJ. Dental plaque as a biofi lm.
J Ind Microbiol. 1995;15(3):169–75.
143. Marsh PD. Are dental diseases examples of ecological
catastrophes? Microbiology. 2003;149(Pt 2):279–94.
144. Marsh PD. Dental plaque: biological signifi cance of a
biofi lm and community life‐style. J Clin Periodontol.
2005;32(s6):7–15.
145. Marsh PD. Microbiology of dental plaque biofi lms
and their role in oral health and caries. Dent Clin
North Am. 2010;54(3):441–54.
146. Marsh PD, Moter A, Devine DA. Dental plaque bio-
fi lms: communities, confl ict and control. Periodontology.
2011;55(1):16–35.
147. Mason MR, Nagaraja HN, Camerlengo T, Joshi V,
Kumar PS. Deep sequencing identifi es ethnicity-
specifi c bacterial signatures in the oral microbiome.
PLoS One. 2013;8(10), e77287.
148. Matsui R, Cvitkovitch D. Acid tolerance mecha-
nisms utilized by Streptococcus mutans. Future
Microbiol. 2010;5(3):403–17.
149. Matsumoto-Nakano M, Kuramitsu HK. Role of bac-
teriocin immunity proteins in the antimicrobial sen-
sitivity of Streptococcus mutans. J Bacteriol. 2006;
188(23):8095–102.
150. Mayhall CW, Butler WT. The carbohydrate compo-
sition of experimental salivary pellicles. J Oral
Pathol. 1976;5(6):358–70.
151. Mayhew R, Brown L. Comparative effect of SnF2,
NaF, and SnCl2 on the growth of Streptococcus
mutans. J Dent Res. 1981;60(10):1809–14.
152. McMurry LM, Oethinger M, Levy SB. Triclosan
targets lipid synthesis. Nature. 1998;394(6693):
531–2.
153. McNab R, Ford SK, El-Sabaeny A, Barbieri B, Cook
GS, Lamont RJ. LuxS-based signaling in Streptococcus
gordonii: autoinducer 2 controls carbohydrate metabo-
lism and biofi lm formation with Porphyromonas gin-
givalis. J Bacteriol. 2003;185(1):274–84.
154. Meurman JH. Probiotics: do they have a role in oral
medicine and dentistry? Eur J Oral Sci. 2005;
113(3):188–96.
155. Mikx FH, Van der Hoeven JS. Symbiosis of
Streptococcus mutans and Veillonella alcalescens in
mixed continuous cultures. Arch Oral Biol. 1975;
20(7):407–10.
156. Mikx FH, van der Hoeven JS, Plasschaert AJ, Konig
KG. Establishment and symbiosis of Actinomyces
viscosus, Streptococcus sanguis and Streptococcus
mutans in germ-free Osborne-Mendel rats. Caries
Res. 1976;10(2):123–32.
157. Miller WD. The micro-organisms of the human
mouth: the local and general diseases which are
caused by them. Philadelphia: The S.S. White Dental
MFG. Co. 1890.
158. Miller WD. The human mouth as a focus of infec-
tion. Lancet. 1891;138(3546):340–2.
159. Monds RD, O’Toole GA. The developmental model of
microbial biofi lms: ten years of a paradigm up for review.
Trends Microbiol. 2009;17(2):73–87. doi:
10.1016/j.
tim.2008.1011.1001
. Epub 2009 Jan 1021.
160. Morou-Bermudez E, Elias-Boneta A, Billings RJ,
Burne RA, Garcia-Rivas V, Brignoni-Nazario V,
et al. Urease activity in dental plaque and saliva of
children during a three-year study period and its
relationship with other caries risk factors. Arch Oral
Biol. 2011;56(11):1282–9.
161. Murata RM, de Almeida B, Salles L, Yatsuda R, Dos
Santos MH, Nagem TJ, et al. Inhibitory effects of
7‐epiclusianone on glucan synthesis, acidogenicity
and biofi lm formation by Streptococcus mutans.
FEMS Microbiol Lett. 2008;282(2):174–81.
162. Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä
A, Poussa T, et al. Effect of long–term consumption
of a probiotic bacterium, Lactobacillus rhamnosus
GG, in milk on dental caries and caries risk in chil-
dren. Caries Res. 2001;35(6):412–20.
163. Nascimento MM, Gordan VV, Garvan CW,
Browngardt CM, Burne RA. Correlations of oral bac-
terial arginine and urea catabolism with caries experi-
ence. Oral Microbiol Immunol. 2009;24(2):89–95.
164. Nasidze I, Li J, Quinque D, Tang K, Stoneking
M. Global diversity in the human salivary microbi-
ome. Genome Res. 2009;19(4):636–43.
2 Biofi lm and Dental Caries

56
165. Ooshima T, Minami T, Matsumoto M, Fujiwara T,
Sobue S, Hamada S. Comparison of the cariostatic
effects between regimens to administer oolong tea poly-
phenols in SPF rats. Caries Res. 1997;32(1):75–80.
166. Otake S, Makimura M, Kuroki T, Nishihara Y,
Hirasawa M. Anticaries effects of polyphenolic
compounds from Japanese green tea. Caries Res.
1991;25(6):438–43.
167. Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury
JA. The role of sucrose in cariogenic dental biofi lm
formation – new insight. J Dent Res. 2006;85(10):
878–87.
168. Palmer Jr RJ, Kazmerzak K, Hansen MC,
Kolenbrander PE. Mutualism versus independence:
strategies of mixed-species oral biofi lms in vitro
using saliva as the sole nutrient source. Infect
Immun. 2001;69(9):5794–804.
169. Papaioannou W, Gizani S, Haffajee AD, Quirynen
M, Mamai-Homata E, Papagiannoulis L. The micro-
biota on different oral surfaces in healthy children.
Oral Microbiol Immunol. 2009;24(3):183–9.
doi:
10.1111/j.1399-1302X.2008.00493.x .
170. Periasamy S, Kolenbrander PE. Aggregatibacter
actinomycetemcomitans builds mutualistic biofi lm
communities with Fusobacterium nucleatum and
Veillonella species in saliva. Infect Immun. 2009;
77(9):3542–51.
171. Pessan JP, Silva SM, Lauris JR, Sampaio FC,
Whitford GM, Buzalaf MA. Fluoride uptake by
plaque from water and from dentifrice. J Dent Res.
2008;87(5):461–5.
172. Peterson S, Woodhead J, Crall J. Caries resistance in
children with chronic renal failure: plaque pH, sali-
vary pH, and salivary composition. Pediatr Res.
1985;19(8):796–9.
173. Preza D, Olsen I, Aas JA, Willumsen T, Grinde B,
Paster BJ. Bacterial profi les of root caries in
elderly patients. J Clin Microbiol. 2008;46(6):
2015–21.
174. Qi F, Chen P, Caufi eld PW. Purifi cation of mutacin
III from group III Streptococcus mutans UA787 and
genetic analyses of mutacin III biosynthesis genes.
Appl Environ Microbiol. 1999;65(9):3880–7.
175. Qi F, Chen P, Caufi eld PW. The group I strain of
Streptococcus mutans, UA140, produces both the lanti-
biotic mutacin I and a nonlantibiotic bacteriocin, muta-
cin IV. Appl Environ Microbiol. 2001;67(1):15–21.
176. Renton-Harper P, Addy M, Moran J, Doherty F,
Newcombe R. A comparison of chlorhexidine, cetyl-
pyridinium chloride, triclosan, and C31G mouth-
rinse products for plaque inhibition. J Periodontol.
1996;67(5):486–9.
177. Rickard AH, Palmer Jr RJ, Blehert DS, Campagna
SR, Semmelhack MF, Egland PG, et al. Autoinducer
2: a concentration-dependent signal for mutualistic
bacterial biofi lm growth. Mol Microbiol. 2006;
60(6):1446–56.
178. Riley MA, Wertz JE. Bacteriocins: evolution, ecol-
ogy, and application. Annu Rev Microbiol. 2002;
56:117–37.
179. Rosalen P, Koo H, Cury J, Park Y. Efeito da propólis
em rato de ssalivado dessalivado. 15a Reunião Anual
da SBPqO Res. 1998;A-074:30
180. Sakanaka S, Kim M, Taniguchi M, Yamamoto
T. Antibacterial substances in Japanese green tea
extract against Streptococcus mutans, a cariogenic
bacterium. Agric Biol Chem. 1989;53(9):2307–11.
181. Sauer K, Camper AK, Ehrlich GD, Costerton JW,
Davies DG. Pseudomonas aeruginosa displays mul-
tiple phenotypes during development as a biofi lm. J
Bacteriol. 2002;184(4):1140–54.
182. Schroeder HE, Hirzel HC. A method of studying
dental plaque morphology. Helv Odontol Acta.
1969;13(1):22–7.
183. Shani S, Friedman M, Steinberg D. The anticario-
genic effect of amine fl uorides on Streptococcus
sobrinus and glucosyltransferase in biofi lms. Caries
Res. 2000;34(3):260–7.
184. Shapiro S, Meier A, Guggenheim B. The antimicro-
bial activity of essential oils and essential oil compo-
nents towards oral bacteria. Oral Microbiol Immunol.
1994;9(4):202–8.
185. Shapiro S, Guggenheim B. The action of thymol on oral
bacteria. Oral Microbiol Immunol. 1995;10(4):241–6.
186. Shapiro S, Giertsen E, Guggenheim B. An in vitro oral
biofi lm model for comparing the effi cacy of antimicro-
bial mouthrinses. Caries Res. 2002;36(2):93–100.
187. Shi W, Jewett A, Hume WR. Rapid and quantitative
detection of Streptococcus mutans with species- specifi c
monoclonal antibodies. Hybridoma. 1998;17(4):
365–71.
188. Shu M, Morou Bermudez E, Suárez Pérez E, Rivera
Miranda C, Browngardt CM, Chen YY, et al. The
relationship between dental caries status and dental
plaque urease activity. Oral Microbiol Immunol.
2007;22(1):61–6.
189. Sintes J, Escalante C, Stewart B, McCool J, Garcia L,
Volpe A, et al. Enhanced anticaries effi cacy of a
0.243 % sodium fl uoride/10 % xylitol/silica denti-
frice: 3-year clinical results. Am J Dent. 1995;8(5):231.
190. Siqueira WL, Zhang W, Helmerhorst EJ, Gygi SP,
Oppenheim FG. Identifi cation of protein components
in in vivo human acquired enamel pellicle using
LC-ESI-MS/MS. J Proteome Res. 2007;6(6):2152–60.
191. Slot DE, Vaandrager NC, Van Loveren C, Van
Palenstein Helderman WH, Van der Weijden GA.
The effect of chlorhexidine varnish on root caries: a
systematic review. Caries Res. 2011;45(2):162–73.
192. Srisilapanan P, Korwanich N, Yin W, Chuensuwonkul
C, Mateo LR, Zhang YP, et al. Comparison of the effi -
cacy of a dentifrice containing 1.5 % arginine and
1450 ppm fl uoride to a dentifrice containing 1450 ppm
fl uoride alone in the management of early coronal car-
ies as assessed using Quantitative Light- induced
Fluorescence. J Dent. 2013;41 Suppl 2:S29–34.
193. Stahringer SS, Clemente JC, Corley RP, Hewitt J,
Knights D, Walters WA, et al. Nurture trumps nature
in a longitudinal survey of salivary bacterial com-
munities in twins from early adolescence to early
adulthood. Genome Res. 2012;22(11):2146–52.
X. Xin et al.

57
194. Stephan RM. Intra-oral hydrogen-ion concentrations
associated with dental caries activity. J Dent Res.
1944;23(4):257–66.
195. Stewart PS, Costerton JW. Antibiotic resistance of
bacteria in biofi lms. Lancet. 2001;358(9276):135–8.
196. Stoodley P, Sauer K, Davies DG, Costerton
JW. Biofi lms as complex differentiated communi-
ties. Annu Rev Microbiol. 2002;56:187–209.
197. Suddick RP, Harris NO. Historical perspectives of
oral biology: a series. Crit Rev Oral Biol Med.
1990;1(2):135–51.
198. Svatun B, Sadxton C, Huntington E, Cummins D.
The effects of three silica dentifrices containing
Triclosan on supragingival plaque and calculus
formation and on gingivitis. Int Dent J. 1993;
43(4 Suppl 1):441.
199. Tanner A, Kent RL, Holgerson PL, Hughes CV, Loo
CY, Kanasi E, et al. Microbiota of severe early child-
hood caries before and after therapy. J Dent Res.
2011;90(11):1298–305.
200. Taylor PW, Hamilton-Miller JM, Stapleton PD.
Antimicrobial properties of green tea catechins.
Food Sci Technol Bull. 2005;2:71.
201. Teitzel GM, Parsek MR. Heavy metal resistance of
biofi lm and planktonic Pseudomonas aeruginosa.
Appl Environ Microbiol. 2003;69(4):2313–20.
202. ten Cate JM. The need for antibacterial approaches
to improve caries control. Adv Dent Res. 2009;
21(1):8–12.
203. Trahan L. Xylitol: a review of its action on mutans
streptococci and dental plaque–its clinical signifi -
cance. Int Dent J. 1995;45(1 Suppl 1):77.
204. Tsang P, Qi F, Shi W. Medical approach to dental
caries: fi ght the disease, not the lesion. Pediatr Dent.
2006;28(2):188–91. discussion 192–188.
205. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett
CM, Knight R, Gordon JI. The human microbiome
project. Nature. 2007;449(7164):804–10.
206. Twetman S, Stecksén‐Blicks C. Probiotics and oral
health effects in children. Int J Paediatr Dent.
2008;18(1):3–10.
207. Twetman S. The role of xylitol in patient caries man-
agement. Oralprophylaxe Kinderzahnheilkunde.
2009;31:122–7.
208. Vadeboncoeur C, Trahan L, Mouton C, Mayrand
D. Effect of xylitol on the growth and glycolysis of
acidogenic oral bacteria. J Dent Res. 1983;62(8):882–4.
209. Vaidyanathan M, Sheehy E, Gilbert S, Beighton
D. Antimicrobial properties of dentine bonding
agents determined using in vitro and ex vivo meth-
ods. J Dent. 2009;37(7):514–21.
210. van Winkelhoff AJ, Winkel EG, Barendregt D,
Dellemijn-Kippuw N, Stijne A, van der Velden U.
beta-Lactamase producing bacteria in adult peri-
odontitis. J Clin Periodontol. 1997;24(8):538–43.
211. VanWuyckhuyse BC, Perinpanayagam H, Bevacqua
D, Raubertas RE, Billings RJ, Bowen WH, et al.
Association of free arginine and lysine concentra-
tions in human parotid saliva with caries experience.
J Dent Res. 1995;74(2):686–90.
212. Vu B, Chen M, Crawford RJ, Ivanova EP. Bacterial
extracellular polysaccharides involved in biofi lm
formation. Molecules. 2009;14(7):2535–54.
213. Walker CB, Tyler KZ, Low SB, King CJ. Penicillin-
degrading enzymes in sites associated with adult peri-
odontitis. Oral Microbiol Immunol. 1987;2(3):129–31.
214. Walker CB. Microbiological effects of mouthrinses
containing antimicrobials. J Clin Periodontol. 1988;
15(8):499–505.
215. Wang BY, Kuramitsu HK. Interactions between oral
bacteria: inhibition of Streptococcus mutans bacte-
riocin production by Streptococcus gordonii. Appl
Environ Microbiol. 2005;71(1):354–62.
216. Wang XL, Cheng CY, Peng D, Wang B, Gan
YH. Dental plaque pH recovery effect of arginine
bicarbonate rinse in vivo. Chin J Dent Res. 2012;
15(2):115.
217. Wang Y, Xue J, Zhou X, You M, Du Q, Yang X, et al.
Oral microbiota distinguishes acute lymphoblastic
leukemia pediatric hosts from healthy populations.
PLoS One. 2014;9(7), e102116.
218. Weiger R, Netuschil L, Wester-Ebbinghaus T, Brecx
M. An approach to differentiate between antibacte-
rial and antiadhesive effects of mouthrinses in vivo .
Arch Oral Biol. 1998;43(7):559–65.
219. Williams JL. On structural changes in human
enamel; with special reference to clinical observa-
tions on hard and soft enamel. Dent Cosmos. 1898;
40:505.
220. Wilson CO, Gisvold O. Textbook of organic medic-
inal and pharmaceutical chemistry . Lippincott,
Philadelphia. 2nd ed. 1954.
221. Wilson RF, Ashley FP. Relationships between the
biochemical composition of both free smooth surface
and approximal plaque and salivary composition and
a 24-hour retrospective dietary history of sugar intake
in adolescents. Caries Res. 1990;24(3):203–10.
222. Witt J, Ramji N, Gibb R, Dunavent J, Flood J, Barnes
J. Antibacterial and antiplaque effects of a novel,
alcohol-free oral rinse with cetylpyridinium chlo-
ride. J Contemp Dent Pract. 2005;6(1):1–9.
223. Witt J, Walters P, Bsoul S, Gibb R, Dunavent J,
Putt M. Comparative clinical trial of two anti-
gingivitis mouthrinses. Am J Dent. 2005;18(Spec):
15A–17A.
224. Wood S, Metcalf D, Devine D, Robinson C.
Erythrosine is a potential photosensitizer for the
photodynamic therapy of oral plaque biofi lms.
J Antimicrob Chemother. 2006;57(4):680–4.
225. Wu-Yuan C, Chen C, Wu R. Gallotannins inhibit
growth, water-insoluble glucan synthesis, and aggre-
gation of mutans streptococci. J Dent Res. 1988;
67(1):51–5.
226. Xie Q, Li J, Zhou X. Anticaries effect of compounds
extracted from Galla chinensis in a multispecies bio-
fi lm model. Oral Microbiol Immunol. 2008;23(6):
459–65.
227. Xu X, Ling L, Wang R, Burgess JO. Formulation and
characterization of a novel fl uoride-releasing dental
composite. Dent Mater. 2006;22(11):1014–23.
2 Biofi lm and Dental Caries

58
228. Xu X, Zhou XD, Wu CD. Tea catechin EGCg sup-
presses the mgl gene associated with halitosis.
J Dent Res. 2010;89(11):1304–8.
229. Xu X, Zhou XD, Wu CD. The tea catechin epigallo-
catechin gallate suppresses cariogenic virulence
factors of Streptococcus mutans. Antimicrob Agents
Chemother. 2011;55(3):1229–36.
230. Xu X, Zhou XD, Wu CD. Tea catechin epigallocat-
echin gallate inhibits Streptococcus mutans biofi lm
formation by suppressing gtf genes. Arch Oral Biol.
2012;57(6):678–83.
231. Xu X, He J, Xue J, Wang Y, Li K, Zhang K, et al.
Oral cavity contains distinct niches with dynamic
microbial communities. Environ Microbiol. 2014.
232. Yang F, Zeng X, Ning K, Liu KL, Lo CC, Wang W,
et al. Saliva microbiomes distinguish caries-active from
healthy human populations. ISME J. 2012;6(1):1–10.
233. Yazdankhah SP, Scheie AA, Høiby EA, Lunestad
B-T, Heir E, Fotland TØ, et al. Triclosan and antimi-
crobial resistance in bacteria: an overview. Microb
Drug Resist. 2006;12(2):83–90.
234. Yin W, Hu DY, Li X, Fan X, Zhang YP, Pretty IA,
et al. The anti-caries effi cacy of a dentifrice contain-
ing 1.5 % arginine and 1450 ppm fl uoride as sodium
monofl uorophosphate assessed using Quantitative
Light-induced Fluorescence (QLF). J Dent. 2013;41
Suppl 2:S22–8.
235. Zúñiga M, Champomier-Verges M, Zagorec M,
Pérez-Martínez G. Structural and functional analysis
of the gene cluster encoding the enzymes of the argi-
nine deiminase pathway of Lactobacillus sake.
J Bacteriol. 1998;180(16):4154–9.
236. Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu
HH. Effect of quaternary ammonium and silver
nanoparticle-containing adhesives on dentin bond
strength and dental plaque microcosm biofi lms. Dent
Mater. 2012;28(8):842–52.
237. Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu
HH. Effect of water-aging on dentin bond strength
and anti-biofi lm activity of bonding agent containing
new monomer dimethylaminododecyl methacrylate.
J Dent. 2013.
238. Zheng X, Zhang K, Zhou X, Liu C, Li M, Li Y, et al.
Involvement of gshAB in the interspecies competi-
tion within oral biofi lm. J Dent Res. 2013;92(9):
819–24.
239. Zhou Y, Mihindukulasuriya KA, Gao H, La Rosa PS,
Wylie KM, Martin JC, et al. Exploration of bacterial
community classes in major human habitats.
Genome Biol. 2014;15(5):R66.
X. Xin et al.

59
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1_3
Saliva and Dental Caries
Wang Renke
3.1 Salivary Flow
and Composition
3.1.1 Formation of Saliva-Salivary
Glands and Secretion
Whole saliva is mainly the mixture of secretion
of salivary glands. It also contains gingival cre-
vicular fl uid, nonadherent microorganisms, food
debris, and human cells including leucocytes and
epithelial cells. In normal physiological condi-
tions, the average daily fl ow of whole saliva is
between 1 and 1.5 L [ 1 ]. About 90 % of the total
volume of saliva is secreted by the three paired
major salivary glands – the parotid glands, sub-
mandibular glands, and sublingual glands. There
are also a number of minor salivary glands situ-
ated on the tongue, palate, and buccal and labial
mucosa which produce only a small percentage
of saliva. However, the function of the minor sali-
vary glands is also important since about 70 % of
the total volume of salivary proteins is secreted
by them.
The parotids are serous glands; upon stimula-
tion, they produce watery saliva with high con-
tent of enzymes like amylase and lipase, while
the secretions of sublingual glands are predomi-
nantly mucous, mucin-rich fl uids, as same as of
those minor salivary glands. And the secretions
of submandibular glands are a mixture of mucous
and serous fl uids. Upon stimulation, the parotid
glands produce watery saliva with high content of
enzymes like amylase and lipase. The different
properties of the secretions of these glands are
determined by their different composition of the
secretory endpieces, also called acini. Salivary
gland fl uid is produced by acini [ 2 ].
3.1.2 Salivary Composition
The normal salivary pH is slightly acidic, about
6–7, but pH in salivary fl ow can range from 5.3
(low fl ow) to 7.8 (peak fl ow). Salivary fl uid con-
sists of approximately 99 % water and 1 % sol-
utes, including electrolytes, glucose, nitrogenous
products like urea and ammonia, and macromol-
ecules. Salivary electrolytes include sodium,
potassium, calcium, magnesium, bicarbonate,
and phosphate. A variety of proteins are also
found in saliva such as enzymes, immunoglobu-
lins, mucins, other antimicrobial factors, mucosal
glycoproteins, traces of albumin, some polypep-
tides, and oligopeptides [ 3 ]. The average concen-
tration and function of saliva composition is
shown in Table 3.1 .
W. Renke
Department of Operative Dentistry and Endodontics ,
West China School of Stomatology,
Sichuan University , Chengdu ,
People’s Republic of China
e-mail:
3
60
3.1.3 Salivary Flow Rate
and Infl uence Factors
There is a broad normal range in individual sali-
vary fl ow rate. The acceptable fl ow rate is no less
than 0.1 mL/min for unstimulated saliva and
0.2 mL/min for stimulated saliva. In normal con-
dition, the unstimulated whole saliva fl ow rate is
0.3–0.5 ml/min, while stimulated whole saliva
fl ow rate is 1.5–2.0 ml/min and both of them have
wide ranges.
The secretion of salivary glands is mainly con-
trolled by parasympathetic impulses from the
salivary nuclei. Several factors infl uence the sali-
vary fl ow rate, including the nature and duration
of stimulus, the emotional state, water balance of
the body, and medication (which will be dis-
cussed later). The salivary fl ow and composition
of saliva display large variations during the day
[ 4 ]. The salivary fl ow rate usually increases dur-
ing the day and reaches the acrophase in the late
afternoon, around 15.00–17.00 h [ 2 ]. It’s almost
Table 3.1 The average concentration and function of saliva composition
Composition Concentration Function
Inorganic Ca
2+
1–2 mM Modulate demineralization and
remineralization
Mg
2+
0.2–0.5 mM
Na
+
6–26 mM
K
2+
14–32 mM
NH
4
+
1–7 mM
H
2
PO
4
−
& HPO
4
2−
2–23 mM Modulate pH, buffering, modulate
demineralization and
remineralization
Cl
−
17–29 mM
HCO
3
−
2–30 mM Modulate pH, buffering
F
−
0.5–5 μM Modulate demineralization and
remineralization, antibacterial
action
SN
−
0.1–2 mM Antibacterial action
Organic Urea 2–6 mM Modulate pH, buffering
Uric acid 0.2 mM Indication for kidney function
Amino acids (free) 1–2 mM
Glucose (free) 50 μM
Lactate 0.1 mM
Fatty acids 10 mg/L
Macromolecules Proteins 1.4–2 g/L Lubricant, cleanse, aggregate,
attach to microorganism, dental
plaque metabolism, modulate
demineralization and
remineralization, antibacterial
action
Glycoprotein sugars 0.11–0.3 g/L Cleanse, aggregate, attach to
microorganism, dental plaque
metabolism
Amylase 0.38 g/L Digestion, antibacterial action
Lysozyme 0.11 g/L Antibacterial action
Peroxidase 3 mg/L Antibacterial action
IgA 0.2 g/L Antibacterial action
IgG 14 mg/L Antibacterial action
IgM 2 mg/L Antibacterial action
Lipid 20–30 mg/L
W. Renke
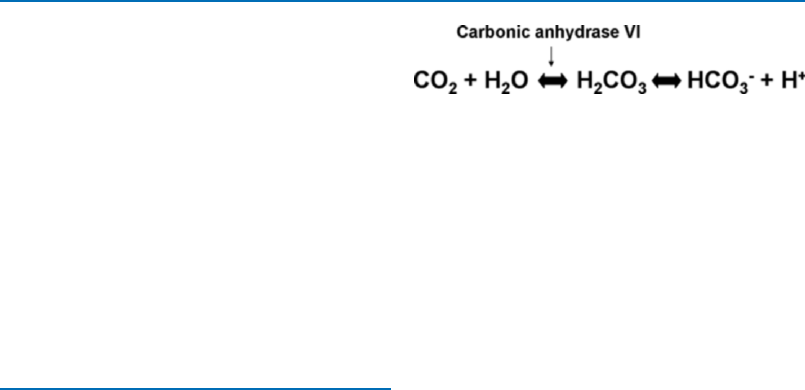
61
zero during sleep. This rhythm could be related
to change of hormone level during the day and
indicates the importance of taking oral hygiene
procedures before going to sleep. With the
absence of protective effects of saliva, if there is
much remaining food and dental plaque in the
oral cavity, there would be a great chance for den-
tal caries to happen.
Sympathetic impulses are more likely to infl u-
ence salivary composition by increasing exocytosis
from certain cells. Salivary composition may also
be infl uenced by hormones such as androgens,
estrogens, glucocorticoids, and peptide hormones.
3.2 Salivary Infl uences
on Plaque PH and Oral
Microfl ora
3.2.1 Salivary Infl uences
on Plaque PH
Acid-producing bacteria, like Streptococcus
mutans and Lactobacillus sp., existed in normal
oral fl ora. These bacteria metabolize fermentable
carbohydrates in food and produce variety of
organic acids, leading to the reduction of pH
value in dental plaque and causing demineraliza-
tion of hard tissue of teeth.
However, because of the powerful salivary
buffering capacity, dental caries is not likely to
happen all the time. There are three major buffer
systems in saliva: the carbonic acid/bicarbonate,
the phosphate, and the protein buffer. The protein
buffer mainly takes place in buffering below pH
5, while the optimal buffering range for the car-
bonic acid/bicarbonate and the phosphate buffer
occurs at pH 6.3 and pH 7.2, respectively [ 5 ]. The
phosphate buffer plays an important role in
unstimulated saliva. The secondary phosphate
ion, HPO
4
2−
, could bind a hydrogen ion and form
a primary phosphate ion H
2
PO
4
−
. During food
intake, its effectiveness is limited due to insuffi -
cient concentrations of phosphate in saliva.
The carbonic acid/bicarbonate buffer is the
most important buffering system in saliva during
food intake and mastication. Bicarbonate is
secreted within the ducts and. In resting saliva,
HCO
3
−
concentration is as low as 1–2 mM, but it
increases with fl ow rate and reaches 60 mM in
stimulated saliva. So during intake, two impor-
tant events happen: (1) bacteria ferment carbohy-
drates and produce organic acrid, causing a drop
in pH, and (2) the increased salivary fl ow rate
leads to an increased HCO
3
−
concentration. The
carbonic acid/bicarbonate equilibrium (Fig. 3.1 )
shifts to the left to produce more carbonic acid.
With the presence of carbonic anhydrase VI
secreted by the serous acinar cells in parotid and
submandibular glands, carbonic acid is further
driven to be converted into carbon dioxide and
water.
Concerning about caries, the pH of saliva
may not be as important as the pH of dental
plaque. Both fermentable carbohydrate remain-
ders and salivary buffering capacity affected
plaque pH. The resting plaque pH refers to the
ph of plaque 2–2.5 h after the last intake of
dietary carbohydrates and is usually between 6
and 7. Following exposure to fermentable car-
bohydrates, the plaque pH decreases rapidly
during the fi rst 5 min and reaches a minimum
after approximately 5–20 min. Unless there is
additional ingestion of fermentable carbohy-
drates, the plaque pH returns slowly to the start-
ing level over 30–60 min. This plaque pH
change over time is known as the Stephan
curve.
The buffering capacity of saliva affects not
only the rate at which the plaque pH decreases
but also the minimum value of plaque pH and
how long the pH stays at that minimum. The pH
value of 5.6 is called a “critical value” which is
the pH at which the tissues start to dissolve and
cause demineralization. Low buffering capacity
of saliva leads to increased rate of plaque pH fall
after carbohydrate intake and prolonged time
below critical pH, fi nally leading to an increased
risk of dental caries.
Fig. 3.1 The carbonic acid/bicarbonate equilibrium in
saliva
3 Saliva and Dental Caries

62
Salivary stimulation after food intake also
affects plaque pH. When gum is chewed, the fl ow
of saliva increases from a resting value of 0.4–
0.5 ml/min to approximately 5–6 ml/min. A
series of studies confi rmed an obvious and sus-
tained rise in plaque pH when gum was chewed
after sugar consumption. Both sugar-containing
and sugar-free gum have such effect, but sugar-
free gum displays a more powerful effect [ 6 ].
Chewing other material that could stimulate sali-
vary secretion such as parafi lm also works.
3.2.2 Salivary Infl uences on Oral
Microfl ora
Besides affecting plaque pH, saliva has profound
infl uence on oral microfl ora. Saliva provides a
nutritious source for oral microfl ora. Salivary
proteins, glycoproteins, peptides, and minerals
can stimulate the growth of oral microfl ora and
enhance biofi lm formation. On the other hand,
saliva displays antimicrobial activities with the
presence of a series of immunologic (secretory
IgA, IgG, IgM) and non-immunologic factors
(enzymes, mucins, proteins, peptides). Lysozyme,
lactoferrin, and histatins exhibit bacteriostatic,
bacteriocidic activity, while immunoglobulins,
mucins, and salivary agglutinin play a role in the
oral clearance of bacteria by interfering with
receptors on the microbial cell wall.
The immunoglobulins including IgG, IgM,
IgA, and secretory IgA (SIgA) form the specifi c
defense system in saliva against bacteria. SIgA is
the most abundant immunologic component in
saliva, mainly produced by plasma cells located
in minor mucous glands. SIgA is non-detectable
in neonates but become readily detectable 1 week
after birth. It can neutralize pathogenic viruses,
toxins, and enzymes produced by bacteria. SIgA
can prevent bacteria forming colonies or attach-
ing or penetrating host tissues, kill them directly,
or activate complements or provide synergism
with innate defense mechanisms. It’s also able to
aggregate or clump bacteria, promoting oral
clearance. SIgA against streptococcus mutans
can be detected in children at the age of 3 years
old, and the quantity increases with the length of
exposure. Other immunologic components occur
in less quantity in saliva. IgG is the only detect-
able Ig in saliva of neonates and is mainly mater-
nal origin. The concentration of IgG decreases to
non-detectable after a few months after birth and
appears again after tooth eruption. At this time,
IgG mainly comes from gingival crevicular fl uid,
originating from sera.
Lysozyme is a cationic protein with low molec-
ular weight from the basal cells of striated ducts in
parotid glands as well as monocytes and macro-
phages. It is one of the most important nonspecifi c
defense substances in saliva. Lysozyme catalyzes
cleavage of beta-1,4 glycosidic bonds between
muramic acid and N -acetylglucosamine residues
in peptidoglycan; hence it hydrolyzes bacterial
cell wall. Due to the external lipopolysaccharide
layer, Gram-negative bacteria are more resistant to
lysozyme. Furthermore, nonenzymatic bacteri-
cidic effect of lysozyme related to activation of
bacterial autolysins is reported as well. It is also
documented that this enzyme functions to aggre-
gate bacteria and inhibit bacterial adherence.
There are two categories of salivary peroxi-
dases: human salivary lactoperoxidase (HS-LPO),
also termed sialoperoxidase, synthesized in sali-
vary glands, and myeloperoxidase (MPO) formed
in polymorphonuclear leucocytes. Salivary per-
oxidases have enzymatic activity and are able to
catalyze the oxidation of salivary thiocyanate and
hypothiocyanous acid, inhibiting bacterial metab-
olism [ 7 ].
Salivary chitinase secreted by salivary glands
catalyzes the hydrolytic cleavage of chitin. Chitin
is a cellular wall compound of yeast cells; hence,
chitin may play a role in the protection against
yeast.
Mucins are glycoproteins secreted from subman-
dibular and sublingual glands that selectively modu-
late the microorganism adhesion. There are two
genetically distinct mucin groups: highly glycosyl-
ated, high-molecular-weight MG1 (>1000 kDa) and
single-glycosylated peptide chain, low-molecular-
weight MG2 (200–300 kDa). The two groups of
mucins display similar carbohydrate chain makeup
but have different bacterial adherence ability. MG1
binds tightly to tooth surface and takes part in enamel
pellicle formation. MG2 promotes the aggregation
W. Renke

63
and clearance of oral bacteria, including
Streptococcus mutans . It’s reported that MG2 pre-
dominates in saliva of caries-resistant individuals,
while the level of MG1 is higher in caries- susceptible
individuals [ 8 ].
Salivary agglutinin (SAG) is a high-molecular-
weight (approximately 340 kDa), mucin-like gly-
coprotein aggregate cells in suspension that can
be found in parotid and submandibular saliva.
SAG is highly glycosylated and extremely sticky.
It could bind to hydroxyapatite and is a compo-
nent of the enamel pellicle and may be involved
in the initial adherence of bacteria to the enamel
surface.
SAG binds to streptococci surface receptor
antigen I/II in a calcium-dependent manner.
Furthermore, SAG also binds to SIgA, resulting
in synergistic effect of bacterial aggregation
which promotes clearance of microorganisms
from the oral cavity [ 9 , 10 ].
Lactoferrin is a glycoprotein with a molecular
weight of about 80 kDa. It is a member of a trans-
ferring family and able to link to ferric iron in
saliva and lead to bactericidal or bacteriostatic
effects on bacteria requiring iron for metabolism
including streptococcus mutans by iron- depriving
effect. This process of starving bacteria of vital
nutrients is termed nutritional immunity. It’s
reported that lactoferrin is a multifunctional pro-
tein having bacteriostatic, bactericidic, fungicidal,
antiviral, anti-infl ammatory, and immunomodula-
tory properties [ 11 , 12 ].
Histatins are a family of peptides rich in histi-
dine, arginine, and lysine residues. At least 12
histatin-like peptides are identifi ed in human
saliva. Histatins have antimicrobial activity against
some strains of Streptococcus mutans and yeasts.
They are implicated in pellicle formation, neutral-
ization of lipopolysaccharides of the external
membranes of Gram-negative bacteria, chelation
of metal ions, and inhibition of proteinases includ-
ing metalloproteins and cysteine proteinases.
Saliva provides nutrients to oral microorgan-
isms and supports their growth. On the other
hand, saliva has a protective function in
maintaining oral health and microbial ecological
balance by inhibiting pathogens and regulating
pH in oral environment.
3.3 Xerostomia
and Its Management
3.3.1 Etiology of Xerostomia
Xerostomia, also termed dry mouth or dry mouth
syndrome, is referred to the subjective symptom
of oral dryness, which is frequently, but not
always, associated with salivary gland hypofunc-
tion and a signifi cant reduction of unstimulated
salivary secretion. As mentioned above, the nor-
mal fl ow rate of unstimulated saliva is about
0.3–0.5 ml/min, while that of stimulated saliva is
about 1.5–2.0 ml/min. It would be considered as
hypofunction of salivary glands if unstimulated
fl ow rate is below 0.1 ml/min or stimulated fl ow
rate is below 0.5 ml/min. As there is a wide range
of salivary fl ow rate, a 50 % reduction of indi-
vidual salivary fl ow rate would also be consid-
ered hypofunction [ 13 , 14 ].
Saliva is secreted by three pairs of major sali-
vary glands and the minor salivary glands. Any
factors that could infl uence salivary gland func-
tion, including diseases of salivary glands,
Sjögren’s syndrome, radiotherapy in the head and
neck area, the use of certain drugs, etc., might
lead to the reduced output of salivary secretion,
causing xerostomia. Besides iatrogenic factors,
aging could be another important cause for xero-
stomia. As life expectancy in developed countries
keeps increasing, xerostomia is becoming
increasingly common in the result of an increased
incidence of systemic diseases and a more exten-
sive intake of medication and/or the degeneration
and reduced volume of acini. Table
3.2 shows the
common causes of xerostomia.
A lot of drugs have infl uence on salivary fl ow
rate and composition, including medication for
hypertension, depression, and allergies. Over 500
medications produce xerostomia as a side effect.
A medication which is known to cause xerosto-
mia may be termed xerogenic. Table 3.3 lists
some drugs that may cause xerostomia.
Xerostomia is a common side effect of radia-
tion therapy of tumors in the region of the head
and neck. The parotid gland is the most radiosen-
sitive gland and then the submandibular gland,
then the sublingual gland, and the minor salivary
3 Saliva and Dental Caries

64
gland. As reported, a single dose above 52 Gy
could lead to severe salivary dysfunction, so con-
ventional radiation treatment of oral carcinoma at
a dose of 60–70 Gy would cause a rapid reduc-
tion of salivary fl ow rate during the fi rst week of
treatment. By 5 weeks of the treatment, the sali-
vary fl ow almost reduces to zero and hardly
recovers completely after the treatment.
Besides the factors mentioned above, there are
a number of additional disorders that may con-
tribute to the presence of xerostomia, including
thyroid dysfunction, Parkinson’s disease, sys-
temic lupus erythematosus, post-traumatic stress
disorders, depression, anxiety, etc. These disor-
ders infl uence the salivary fl ow either through
pathophysiological process or the medical treat-
ment of the disease.
Xerostomia is a common condition in popula-
tion. It’s reported that 25 % of general population
complain of xerostomia or symptoms associated
with it. For elderly, the incidence is as high as
40 %. With such a high incidence, the infl uence
of xerostomia in life quality brings to great atten-
tion. As the quantity of saliva secretion reduced
reversibly or irreversibly, patients could experi-
ence different extent of dysarthria, dysphagia,
mucosal trauma and ulceration, candida infec-
tion, and dental caries. The clinical consequences
and management had been comprehensively dis-
cussed in a review [
15 ]; here we will mainly
focus on dental caries related to xerostomia and
its management [ 15 ].
One of the most important functions of saliva
is to develop dental pellicle on enamel surface,
protecting against demineralization and replen-
ishing tooth surface minerals including calcium
and phosphate. It also provides buffering activity,
antibacterial activity, and effective carbohydrate
clearance. As salivary output decreases, a series
of respondents take place with the outcome of
increased demineralization and decreased remin-
eralization and virtually lead to dental caries
(Fig.
3.2 ).
3.3.2 Management of Xerostomia
Xerostomia patients should be instructed to
observe a variety of caries preventive procedures,
including oral hygiene instruction, plaque con-
trol, low sugar dietary advice, daily use of topical
fl uoride (0.05 %), antimicrobial mouse rinses
(e.g., chlorhexidine), and placement of sealants.
The most important one is to reduce the intake of
Table 3.2 Common causes of xerostomia
Class Cause
Iatrogenic Drugs
Local radiation
Chemotherapy
Chronic graft-versus-host disease
Diseases of
salivary glands
Sjögren’s syndrome
Sarcoidosis
HIV disease
HCV infection
Primary biliary cirrhosis
Cystic fi brosis
Diabetes mellitus
Rare causes Amyloidosis
Hemochromatosis
Wegner’s disease
Salivary gland agenesis (with or
without ectodermal dysplasia)
Triple A syndrome
Others
Cited from Porter et al. [
15 ]
Table 3.3 Medications associated with xerostomia
Class Generic name
Anticonvulsants Atropine, hyoscine
Antipsychotics Phenothiazine
Antidepressants Amitriptyline, fl uoxetine,
lithium, bupropion, dothiepin
Antihistamines Diphenhydramine,
cimetidine
Antihypertensives Terazosin, prazosin,
clonidine, atenolol,
propranolol
Antirefl ux drugs Omeprazole
Opioids Meperidine
Antineoplastics Cytotoxic drugs, retinoids,
interleukin-2
Anti-HIV drugs Protease inhibitors,
didanosine
Diuretics Chlorothiazide, furosemide,
antisterone
Antiasthmatic Ephedrine
Anxiolytics Benzodiazepines
W. Renke

65
sugars. Sugar intake should be confi ned to meals,
and there should be no sugar consumption
between meals. Non-cariogenic sugar substitutes
including xylitol, sorbitol, aspartame, Lycasin,
saccharin, and sucralose, which could not be fer-
mented by acidogenic bacteria into organic acids,
should be used to reduce sugar consumption [ 16 ].
Xerostomia patients should be recommended
to brush teeth twice a day with a soft bristle tooth
brush along with low abrasive, high fl uorinated
toothpaste. Fluoride is also available in other
forms like foam, varnish, rinse, and gel. It is rec-
ommended by ADA that the daily use of fl uoride
rinses and toothpaste accompanied with fl uoride
varnish applied every 3 months is helpful in den-
tal caries inhibition in xerostomia population [ 17 ].
The use of remineralization products is a rela-
tive new strategy against dental caries in xerosto-
mia patients. It provides calcium and phosphate
ions that are lacking because of reduced salivary
output. These products usually contain different
types of calcium and phosphate compound with
or without additional fl uoride in the form of paste
or medication carrier (products without fl uoride).
Applying to the tooth surface, they can provide
and attract calcium and phosphate ions to the
tooth surface, aiding in remineralization.
3.4 Saliva and Caries Risk
Assessment
Dental caries is a multifactorial infectious dis-
ease that is associated with complex interactions
among acid-producing bacteria, fermentable
carbohydrates, and host factors including saliva
status. To estimate the caries risk before disease
has been given more attention in recent years
since dental caries is generally considered as a
kind of progressive disease unless intervened sur-
gically. Several caries risk assessment methods
have been developed as indicators of caries sus-
ceptibility at the individual level. They can be
used to estimate the probability of caries inci-
dence, determine the need for therapeutic inter-
vention, and are a part of treatment planning.
Generally, these tests are derived from the
severity of past and current caries experience,
diet, protective factors including fl uoride, behav-
ioral and physical factors, medical factors, socio-
economic status, measurement of saliva fl ow rate
and buffering capacity, and estimation of caries-
related microorganisms in saliva including
Streptococcus mutans and Lactobacilli
(Tables
3.4 , 3.5 and 3.6 ) [ 19 ].
3.4.1 Caries-Associated Bacteria
There are more than 700 species of microorgan-
isms inhabit the oral cavity. In healthy individuals,
caries-associated bacteria are usually present in
relatively small amount in saliva. But in condi-
tions of biological or environmental change such
as increased frequency of carbohydrate intake or
poor oral hygiene, the etiological balance of oral
microfl ora will shift to favor the aciduric bacteria
and acidogenic bacteria, increasing the risk of
dental caries.
Plaque bacterial composition is most related
to dental caries since acid causing demineraliza-
tion is mainly produced by plaque bacteria. Since
Fig. 3.2 The chain reaction
of reduced salivary fl ow rate
3 Saliva and Dental Caries

66
plaque bacteria can be released into saliva, it is
documented that the level of certain species of
bacteria in saliva can refl ect their level in dental
plaque, making salivary microfl ora an effective
biomarker of the health and disease status of oral
cavity [ 20 , 21 ].
Streptococcus mutans and Lactobacilli have
been implicated as important contributory species
in dental caries. It is reported that high level of sali-
vary Streptococcus mutans (>10
5
CFU/ml) is asso-
ciated with an increased risk of dental caries
[ 22 – 24 ]. Although the amount of lactobacilli is less
sensitive in predicting caries incidence than the
amount of mutans streptococci [ 25 ], the high level
of Lactobacilli (>10
6
CFU/ml) [ 26 ] could be an
indicator for increased frequency of carbohydrate
consumption [ 27 ] and caries progression [ 28 ].
Most of tests regarding caries-associated bac-
teria require incubation which makes them less
convenient for dental practitioners. Recently, cul-
ture-independent technologies including checker-
board DNA-DNA hybridization, 16S rRNA
sequence analysis, and T-RFLP have been utilized
for dental caries microbial analysis. These molec-
ular methods have not applied clinically in large
scale but defi nitely suggest new possibilities for
fast, convenient, and culture- independent meth-
ods of caries-associated bacteria test.
Table 3.4 ADA caries risk assessment form for age 0–6
Low risk Moderate risk High risk
Contributing conditions
I Fluoride exposure (through
drinking water, supplements,
professional applications,
toothpaste)
Yes No
II Sugary foods of drinks (including
juice, carbonated or non-
carbonated soft drinks, energy
drinks, medical syrups)
Primarily at
mealtimes
Frequent or prolonged
between meal
exposures/day
Bottle or sippy cup
with anytime other
than water at bed
time
III Eligible for government programs
(WIC, Head Start, Medicaid, or
SCHIP)
No Yes
IV Caries experience of mother,
caregiver, and/or other siblings
No carious lesions in
last 24 months
Carious lesions in last
7–23 months
Carious lesions in
last 6 months
V Dental home: established patient
of record in a dental offi ce
Yes No
General health conditions
I Special health-care needs
(developmental, physical,
medical, or mental disabilities that
prevent or limit performance of
adequate oral health care by
themselves or caregivers)
No Yes
Clinical conditions
I Visual or radiographically evident
restorations/cavitated carious
lesions
No new carious
lesions or
restorations in last
24 months
Carious lesions or
restorations in last
24 months
II Non-cavitated (incipient) carious
lesions
No new lesions in
last 24 months
New lesions in last
24 months
III Teeth missing due to caries No Yes
IV Visible plaque No Yes
V Dental/orthodontic appliances
present ( fi xed or removable)
No Yes
VI Salivary fl ow Visually adequate Visually Inadequate
W. Renke

67
Table 3.5 ADA caries risk assessment form for age > 6
Low risk Moderate risk High risk
Contributing conditions
I Fluoride exposure (through
drinking water, supplements,
professional applications,
toothpaste)
Yes No
II Sugary foods of drinks (including
juice, carbonated or non-
carbonated soft drinks, energy
drinks, medical syrups)
Primarily at
mealtimes
Frequent or prolonged
between meal
exposures/day
III Caries experience of mother,
caregiver, and/or other siblings
(for patients ages 6–14)
No carious lesions in
last 24 months
Carious lesions in
last 7–23 months
Carious lesions in last 6
months
IV Dental home: established patient
of record in a dental offi ce
Yes No
General health conditions
I Special health-care needs
(developmental, physical,
medical, or mental disabilities
that prevent or limit performance
of adequate oral health care by
themselves or caregivers)
No Yes ( over age 14) Yes ( ages 6–14)
II Chemo-/radiation therapy No Yes
III Eating disorders No Yes
IV Medications that reduce salivary
fl ow
No Yes
V Drug/alcohol abuse No Yes
Clinical conditions
I Cavitated or non-cavitated
(incipient) carious lesions or
restorations (visually or
radiographically evident)
No new carious
lesions or restorations
in last 36 months
1 or 2 new carious
lesions or restorations
in last 36 months
3 or more carious
lesions or restorations
in last 36 months
II Teeth missing due to caries in
past 36 months
No Yes
III Visible plaque No Yes
IV Unusual tooth morphology that
compromises oral hygiene
No Yes
V Interproximal restorations, 1 or
more
No Yes
VI Exposed root surfaces present No Yes
VII Restorations with overhangs and/
or open margins; open contacts
with food impaction
No Yes
VII Dental/orthodontic appliances
present (fi xed or removable)
No Yes
IX Severe dry mouth (xerostomia) No Yes
Table 3.6 The strength of association between salivary characteristics and caries risk
Strong association Weak-to-moderate association No association
Flow rate Buffering capacity; calcium/phosphate;
specifi c sIgA immunoglobulin
pH, glucose clearance rate/concentration; other
electrolytes and small organic molecules; total
sIgA; IgG; innate immunity factors
Cited from Leone et al. [
18 ]
3 Saliva and Dental Caries

68
3.4.2 Chemical and Physical
Aspects of Saliva
Leone et al. had reviewed 96 references and
divided the chemical and physical characteristics
of saliva into three groups according to their
strength of association with dental caries
(Table 3.6 ) [ 18 ].
It has been discussed earlier in this chapter
that low salivary fl ow rate is a risk factor of dental
caries. Statistical data suggests that unstimulated
salivary fl ow less than 0.3 ml/min and the stimu-
lated salivary fl ow lower than 0.8–1.0 ml/min
indicate increased caries risk strongly. But these
values should not be treated as an absolute stan-
dard for caries risk screening since there is a wide
range of salivary fl ow rate among individuals. An
obvious reduction of salivary output in one indi-
vidual should be paid attention to as well.
A number of studies showed the correlation
between low salivary buffering capacity and den-
tal caries, while high buffering capacity indicates
better neutralizing capacity and more resistance
to demineralization.
The level of specifi c secretory IgA showed a
relationship with caries risk, and the literature is
almost equally divided for and against an anticar-
ies role of specifi c secretory IgA. No evidence has
indicated suffi cient association between caries
risk and salivary innate non-immunoglobulin fac-
tors including lysozyme, lactoferrin, peroxidase/
myeloperoxidase, praline-rich proteins, stather-
ins, and histatins. On the other hand, Mungia et al.
found signifi cant associations between caries and
specifi c individual submandibular/sublingual sali-
vary proteins including lactoferrin, albumin, lyso-
zyme, mucin, and cystatin recently [
29 ]. But if it
is an indicator of caries risk still needs more data
and further investigation.
References
1. Humphrey SP, Williamson RT. A review of saliva:
normal composition, fl ow, and function. J Prosthet
Dent. 2001;85(2):162–9.
2. Edgar WM, Dawes C, O’Mullane DM. Saliva and oral
health. 4th ed. Duns Tew: Stephen Hancocks Limited;
2012.
3. de Almeida Pdel V, Grégio AM, Azevedo LR. Saliva
composition and functions: a comprehensive review.
J Contemp Dent Pract. 2008;9(3):072–80.
4. Dawes C. Circadian rhythms in human salivary fl ow
rate and composition. J Physiol. 1972;220:529–45.
5. Lenander-Lumikari M, Loimaranta V. Saliva and den-
tal caries. Adv Dent Res. 2000;14:40–7.
6. Manning RH, Edgar WM. pH changes in plaque after
eating snacks and meals, and their modifi cation by
chewing sugared or sugar-free gum. Br Dent J. 1993;174:
241–4.
7. Rosin M, Hanschke M, Splieth C, Kramer A. Activities
of lysozyme and salivary peroxidase in unstimulated
whole saliva in relation to plaque and gingivitis scores
in healthy young males. Clin Oral Invest. 1999;3:
133–7.
8. Slomiany BL, Murty VL, Piotrowski J, Slomiany A.
Salivary mucins in oral mucosal defense. Gen
Pharmacol. 1996;27(5):761–71.
9. Lamont RJ, Demuth DR, Davis CA, Malamud D,
Rosan B. Salivary-agglutinin-mediated adherence of
Streptococcus mutans to early plaque bacteria. Infect
Immun. 1991;59(10):3446–50.
10. Oho T, Yu H, Yamashita Y, Koga T. Binding of sali-
vary glycoprotein-secretory immunoglobulin A
complex to the surface protein antigen of Streptococcus
mutans . Infect Immun. 1998;66(1):115–21.
11. Nieuw Amerongen AV, Veerman ECI. Saliva-the
defender of oral cavity. Oral Dis. 2002;8:12–22.
12. Adlerova L, Bartoskova A, Falduna M. Lactoferrin: a
review. Vet Med. 2008;53(9):457–68.
13. Saltana N, Sham ME. Xerostomia: an overview. Int J
Dent Clin. 2011;3(2):58–61.
14. Fox PC, Eversole R. Diseases of the salivary glands.
Essen Oral Med. 2001;260–76.
15. Porter S, Scully C, Hegarty A. An update of the etiology
and management of xerostomia. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 2004;97(1):28–46.
16. American Dental Association Council on Scientifi c
Affairs. Professionally applied topical fl uoride:
evidence- based clinical recommendations. J Am Dent
Assoc. 2006;137(8):1151–9.
17. Su N, Marek C, Ching V, et al. Caries prevention for
patients with dry mouth. J Am Dent Assoc. 2011;77:b85.
18. Leone CW, Oppenheim FG. Physical and chemical
aspects of saliva as indicators of risk for dental caries
in humans. J Dent Educ. 2001;65(10):1054–62.
19. American Dental Association caries risk assessment
forms.
www.ada.org/5157.aspx . Accessed 22 Feb 2011
20. Asikainen S, Alaluusua S, Saxen L. Recovery actino-
mycetemcomitans from teeth, tongue and saliva.
J Periodontol. 1991;62(3):203–6.
21. Umeda M, Contreras A, Chen C, Bakker I, Slots J. The
utility of whole saliva to detect the oral presence of
periodontopathic bacteria. J Periodontol. 1998;69(7):
828–33.
22. von Haute J. Microbiological predictors of caries risk.
Adv Dent Res. 1993;7(2):87–96.
23. Thenisch NL, Bachmann LM, Imfeld T, Leisebach
Minder T, Steurer J. Are mutans streptococci detected
W. Renke

69
in preschool children a reliable predictive factor for
dental caries risk? A systematic review. Caries Res.
2006;40(5):366–74.
24. Parisotto TM, Steiner-Oliveira C, Sliva CM,
Rodriques LK, Nobre-dos-Santos M. Early childhood
caries and mutans streptococci: a systematic review.
Oral Health Prev Dent. 2010;8(1):59–70.
25. Wilson RF, Ashley FP. Identifi cation of caries risk in
school-children: salivary buffering capacity and bac-
terial counts, sugar intake and caries experience as
predictors of 2-year and 3-year caries increment.
Br Dent J. 1989;167(3):99–102.
26. Larmas M. Saliva and dental caries: diagnostic tests for
normal dental practice. Int Dent Res. 1992;42(4):1990208.
27. Holbrook WP, de Soet JJ, de Graaf J. Prediction of
dental caries in pre-school children. Caries Res.
1993;27(5):424–30.
28. Klock B, Krasse B. Microbiological and salivary con-
ditions in 9-12-year old children. Scand J Dent Res.
1977;85(1):56–63.
29. Mungia R, Cano SM, Johnson DA, Dang H, Brown JP.
Interaction of age and specifi c saliva component out-
put on caries. Aging Clin Exp Res. 2008;20(6):
503–8.
3 Saliva and Dental Caries

71
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1_4
Demineralization
and Remineralization
Cheng Lei , Li Jiyao , Xu Hockin H.K. ,
and Zhou Xuedong
Dental caries is caused by acids produced from
bacterial metabolism diffusing into dental hard
tissue and dissolving the mineral. The process of
dental caries is a continuum which results from
many de-/remineralization cycles [ 1 , 2 ]. Dental
hard tissue consists of enamel, dentin, and cemen-
tum. Dental enamel is highly mineralized, and it
comprises 80–90 % by volume of a calcium-defi -
cient carbonate hydroxyl apatite. Other calcifi ed
tissues (bone or dentine) contain considerably
lower amounts of inorganic minerals. Mature
human enamel crystallites are 26.3 ± 2.2 nm thick,
68.3 ± 13.4 nm wide, and between 100 and
1,000 nm long [ 3 ]. Dentine and cementum con-
tain a much greater proportion of organic matrix.
Dentine is made up of approximately 50 vol.%
mineral, 30 vol.% collagenous and non-collage-
nous proteins, and 20 vol.% fl uids. The dentinal
matrix is mainly composed of type I collagen
fi brils forming a three-dimensional scaffold
matrix, reinforced by hydroxyl apatite crystal-
lites, measuring approximately 20 nm in size [ 3 ].
Sound enamel and dentine crystals are com-
prised of a hydroxyapatite-like mineral contain-
ing many impurities and inclusions of other ions.
The mineral phase of the dental hard tissues is
not pure hydroxyapatite (HAP = Ca
10
(PO
4
)
6
OH
2
). Hydrogen phosphate, carbonate, and mag-
nesium ions are incorporated into the HAP lattice
to form a less stable, more soluble apatite. For
example, approximately 1 out of 10 of the phos-
phate ions in enamel is replaced by carbonate
ions and 1 out of 5 in dentine [
4 , 5 ]. So the min-
eral of enamel and dentine is much more soluble
than pure hydroxyapatite or fl uorapatite. But the
partial substitution of fl uoride ions for OH groups
C. Lei (*)
State Key Laboratory of Oral Diseases ,
Sichuan University , Chengdu ,
People’s Republic of China
Department of Operative Dentistry and Endodontics ,
West China Hospital of Stomatology,
Sichuan University , Chengdu ,
People’s Republic of China
State Key Laboratory of Oral Diseases ,
West China Hospital of Stomatology,
Sichuan University , Chengdu ,
People’s Republic of China
e-mail:
L. Jiyao
State Key Laboratory of Oral Diseases ,
Sichuan University , Chengdu ,
People’s Republic of China
Department of Operative Dentistry and Endodontics ,
West China Hospital of Stomatology,
Sichuan University , Chengdu ,
People’s Republic of China
4
X. Hockin H.K.
Biomaterials and Tissue Engineering Division,
Department of Endodontics,
Prosthodontics and Operative Dentistry ,
University of Maryland Dental School , Baltimore ,
MD 21201 , USA
Z. Xuedong
State Key Laboratory of Oral Diseases ,
West China Hospital of Stomatology, Sichuan
University , Chengdu , People’s Republic of China

72
in the crystal lattice can stabilize the apatite
structure less susceptible to acid attack.
4.1 Dynamics Process of
De-/Remineralization
Dental caries is a disease that is manifested as a
dynamic process of de-/remineralization in the
mouth (Fig. 4.1 ). Demineralization is a continual
imbalance between pathological and protective
factors that results in the dissolution of apatite
crystals and the net loss of calcium, phosphate,
and other ions from the tooth [ 6 ]. The fi rst stage of
demineralization is occurring at the atomic level
far before it can be seen visually as gross demin-
eralization. During this step, fermentable carbo-
hydrates are metabolized by bacteria in dental
plaque to produce organic acids. The acids diffuse
into the dental hard tissue through the water
among the crystals and could reach a susceptible
site on a crystal surface. Calcium and phosphate
are dissolved into the surrounding aqueous phase
between the crystals [ 2 ]. This is considered as the
fi rst step in the continuum of the dental caries pro-
cess which can eventually lead to cavitation.
The oral fl uids (saliva, biofi lm fl uid) have cal-
cium (Ca) and phosphate (Pi) in supersaturated
concentrations with respect to the mineral com-
position of enamel. At physiological conditions
(a neutral pH of 7), low ion concentrations are
suffi cient to keep dental hard tissues in equilib-
rium. If the pH drops because of acid produced
by the dental plaque, higher ion concentrations
are needed to prevent dissolution of dental hard
tissue. Calcium (Ca) and phosphate (Pi) ions are
continually deposited on the enamel surface or
are redeposited in enamel areas where they were
lost. At a pH of ca. 5.5, undersaturation begins,
that is, the calcium- and phosphate-ion concen-
trations in the plaque fl uid are not suffi cient to
maintain the enamel in stable equilibrium; thus,
the enamel starts to dissolve.
The term “remineralization” is used to
described mineral gain. Remineralization is the
body’s natural repair process for subsurface non-
cavitated carious lesions [ 7 ]. In the process of rem-
ineralization, calcium and phosphate ions are
supplied from a source external to the tooth to pro-
mote ion deposition into crystal voids in deminer-
alized enamel to produce net mineral gain.
De-/remineralization cycles continue in the
mouth as long as there are factors including cario-
genic bacteria, fermentable carbohydrates, and
saliva present. The balance between pathological
factors and protective factors determines whether
demineralization or remineralization is proceed-
ing at any one time [ 2 ].
4.2 Investigations
of De-/Remineralization
4.2.1 Models
The de-/remineralization process can be stud-
ied using different kinds of models, such as
in vitro model, in situ model, animal models, or
Remineralization
Acld reslstant
Ca
10
(PO
4
)
6
(F)
2
=
flourapatite-like
coating on crystals
Calclum +
Phosphate
+ Fluorlde
Crystal
nucleus
Enamel/dentin
crystal =
Carbonated apatite
Partly dissolved
crystal
ACID
Fig. 4.1 The caries
process including
demineralization and
subsequent
remineralization to form a
low solubility surface on
the crystals [
2 ]
C. Lei et al.

73
in randomized controlled clinical trials [ 8 – 10 ].
The induction of artifi cial carious lesions in
bovine and human teeth is an important tool to
study strategies for the prevention or treatment
of carious lesions. Various models can be
selected according to different purposes. Each
model has its advantages along with disadvan-
tages. For example, in vitro experiments are the
most commonly applied methods. They can be
performed over a short period of time, require
fewer staff than in situ studies, avoid partici-
pant compliance issues, and are relatively inex-
pensive. But they cannot replicate the oral
environment with all of the biological varia-
tions known to infl uence de-/remineralization
process [ 11 , 12 ].
4.2.1.1 In Vitro Chemical Model
The modern pH-cycling models were fi rst pro-
duced by ten Cate and Duijsters [ 13 ]. In vitro pH-
cycling models are widely used, especially for
the in vitro evaluation of the effi cacy of fl uori-
dated dentifrices for caries control [ 14 ]. They are
also broadly used in profi le studies for rapid and
inexpensive testing of developing and recently
marketed products [ 9 ]. There are several advan-
tages of in vitro pH cycling: (i) the model can
mimic the dynamics of mineral loss and gain
involved in caries formation; (ii) the high level of
scientifi c control and the resulting lower variabil-
ity intrinsic to in vitro models; and (iii) it requires
smaller sample size [ 14 ].
4.2.1.2 In Vitro Biofi lm Model
Dental plaque biofi lms play a pivotal role in the
progression of dental diseases, so in vitro biofi lm
models are developed to produce artifi cial caries
lesions [ 15 ]. Two main aspects should be consid-
ered when the most suitable biofi lm model for a
de-/remineralization investigation was chosen: (1)
to select pure cultures or defi ned communities or
microcosms and (2) to select closed system bio-
fi lm models or open system biofi lm models [ 15 ].
Streptococcus mutans are considered the most
cariogenic microorganisms in dental biofi lm due
to their capacity to use dietary carbohydrates to
synthesize extracellular polysaccharides (EPS)
and because of their acidogenic and aciduric
properties [ 16 ]. And S. mutans biofi lm models as
single-species biofi lm model are widely used in
de-/remineralization investigations [
17 – 19 ].
Multiple species biofi lm models using defi ned
consortia could achieve a high degree of repro-
ducibility between experimental runs which can-
not be relied upon when using complex inocula
[ 15 , 20 , 21 ]. Microcosms are able to maintain
much of the complexity and heterogeneity of the
original sample. Saliva was usually collected to
form microcosm biofi lms to replicate enabling in
situ bacterial community dynamics within the
laboratory environment [ 22 , 23 ].
Biofi lm models can be divided into two groups
according to whether they are “closed” or “open”
with respect to nutritional availability [ 15 ].
Closed system biofi lm models are analogous to
batch culture and usually based on multi-well
plates [ 18 , 24 ]. Silva TC et al. applied an active
attachment biofi lm model as a high-throughput
demineralization biofi lm model for the evalua-
tion of caries-preventive agents (Fig. 4.2a ) [ 18 ].
Zürich biofi lm model is another typical closed
system biofi lm models applied in de-/remineral-
ization experiments [ 21 , 25 ]. Open system bio-
fi lm models are analogous to continuous culture.
McBain AJ. et al. reviewed different kinds of
open system biofi lm models [ 15 ]. The constant
depth fi lm fermentor (CDFF) was widely used
for de-/remineralization investigations [ 23 , 26 ,
27 ]. The CDFF allows the generation of large
numbers of replicate biofi lms which can be main-
tained at a constant depth by static scraper blades
(Fig.
4.2b ).
4.2.1.3 In Situ Model
In situ models are also widely used for de-/remin-
eralization experiments now. In situ models
involve the use of devices creating conditions that
simulate the process of dental caries. Enamel and
dentin samples are the hard tissue substrates used
in in situ models to assess de- and remineraliza-
tion [ 8 ]. The in situ models are designed to simu-
late the natural process of de-/remineralization
and also to provide information in a short period
of time without causing damage to the natural
teeth of volunteers [ 28 ]. These models serve as a
link between the clinical uncontrolled situation
and the highly controlled laboratory experiments.
The in situ caries model designs are highly
4 Demineralization and Remineralization

74
variable because of the variations of the in situ
study designs. All in situ studies must have appro-
priate controls including a positive and negative
control where possible [ 8 ].
4.2.1.4 Animal Model
For many reasons—particularly time consider-
ations, animal availability, and attendant costs—
rodents have been the most commonly used
species for experimental caries studies [ 21 ].
According to Stookey et al.’s investigations, the
following rat caries models could be used for eval-
uating fl uoride dentifrices: Francis’ hypomineral-
ized area model, Gaffar’s CARA rat model, the
Connecticut model, and the Indiana model [ 29 ].
4.2.2 Detection and Measurement
Methods
Various techniques have been used to investigate the
mineral loss and gain during de-/remineralization
process, including destructive and nondestructive
methods.
a
b
Medium inlets
Top plate
Glass housing
Sprung
scraper blade
Turntable
Tie bar
PTFE seal
Bottom plate
Drive shaft
Outlet for waste medium
(expanded, aerial
view of a pen)
Gas
Sampling port
Fig. 4.2 In vitro biofi lm models, ( a ) an active attachment biofi lm model [ 18 ]; ( b ) CDFF [ 15 ]
C. Lei et al.

75
4.2.2.1 Transversal
Microradiography (TMR)
TMR or contact-microradiography is a highly
sensitive method used to measure the morphol-
ogy of and the change in mineral content of den-
tal hard tissue [ 30 ]. But the method is usually
destructive to dental hard tissue, so it cannot be
used to study any longitudinal mineral changes in
exactly the same lesions [ 31 ]. To prepare the
samples for TMR investigation, thin slices (about
80 μm for enamel samples and 200 μm for den-
tine samples) are cut perpendicularly to the tooth
surface. A microradiographic image is made on
high-resolution fi lm by X-ray exposure of the
sections together with a calibration aluminum
step wedge. The mineral can be automatically
calculated from the gray levels of the images of
section and step wedge. Parameters of interest
are mineral loss (Delta Z in Vol%.μm), lesion
depth (Lesd in μm), ratio or average loss of min-
eral content in the lesion area (Delta Z/Lesd in
Vol%), and the mineral vol% and position of the
subsurface layer and lesion body [ 32 , 33 ].
4.2.2.2 Indentation Techniques
Indentation techniques have been used to measure
the hardness of the dental hard tissue surface.
There are two kinds of indentation techniques for
de-/remineralization investigations: microinden-
tation (surface hardness) [ 32 ] and nanoindenta-
tion (ultra-microindentation) [ 34 ]. During the
process of both microindentation and nanoinden-
tation, a diamond tip of known dimensions is
pressed onto a surface with a given load and dura-
tion. The microindentation technique yields data
in arbitrary units, usually Knoop hardness number
(KHN) or Vickers hardness number (VHN), and
nanoindentation yields hardness and reduced
elastic modulus in the SI unit of Pascals (Nm
−2
)
[ 35 ]. Moron BM et al. revealed that surface hard-
ness analysis should not be interpreted with
respect to dentine mineral loss [ 11 ]. This was
expected as the high organic content, and thus, the
elastic properties of the dentine infl uence the
hardness measurement [ 36 ].
4.2.2.3 Micro-CT
Micro-CT investigation is a nondestructive
method to measure the mineral changes of dental
hard tissue. So it is possible to measure and visu-
alize longitudinal mineral changes during de- and
remineralization in the same lesion [
37 ]. Published
papers proved that micro-CT could offer the
quantitative analysis of the de- and remineraliza-
tion based on CT intensity data [ 31 ]. The key
point of using micro-CT is to fi nd out how to con-
verse the CT intensity into mineral content. Neves
et al. reported a linear correlation between CT
intensity (or gray scale value) and the mineral
density using three apatite phantoms, the linearity
covering a range of 0.25–3.14 g/cm
3
[ 38 ]. Schwass
et al. found a good linearity using six phantoms
covering a range from 0.07 to 2.95 g/cm
3
[ 39 ].
4.2.2.4 Confocal Laser Scanning
Microscopy
Confocal laser scanning microscopy (CLSM) is
able to identify tissue-emitting fl uorescent signal
and can be used to detect the mineral loss of den-
tal hard tissue [ 40 – 42 ]. Demineralizing dentin has
a strongly increased autofl uorescence compared
to sound dentine [ 43 ]. Some previous studies
stained thick enamel samples with a fl uorescent
dye (0.1 m M rhodamine B) and analyzed using
CLSM for quantitating demineralization and rem-
ineralization of enamel specimens [ 44 ].
4.2.2.5 Quantitative Light-Induced
Fluorescence
Quantitative light-induced fl uorescence (QLF) is
a quantifi cation system for assessing early demin-
eralization or remineralization of human enamel.
When high-intensity blue light illuminates the
teeth, the resultant autofl uorescence of enamel is
detected by an intraoral camera. The emitted fl uo-
rescence has a direct relationship with the mineral
content of the enamel. The intensity of the tooth
image at a demineralized area is darker than the
sound area. The software of QLF systems can
process the image to provide user quantitative
parameters such as lesion area, lesion depth, and
lesion volume [
45 – 47 ].
4.2.2.6 Optical Coherence Tomography
Optical coherence tomography (OCT) is a three-
dimensional optical imaging technique which
works in a similar way to ultrasound, but uses
high-frequency light (around 820 nm) instead of
high-frequency sound [ 48 ]. It is a noninvasive,
cross-sectional imaging system that can visualize
4 Demineralization and Remineralization

76
the internal structures nondestructively [ 49 ].
Amaechi et al. developed a quantitative method
to detect the demineralizing lesions of dental
enamel using an OCT system [ 50 ]. This system
was able to collect A-scans (depth versus
refl ectivity curve), B-scans (longitudinal images),
and C-scans (transverse images at constant
depth). The area (R) under the A-scan could be
quantifi ed to indicate the degree of refl ectivity of
the tissue.
4.3 Methods to Infl uence
the De-/Remineralization
Process
4.3.1 Traditional Methods
4.3.1.1 Fluoride
Fluoride was introduced into dentistry over 70
years ago, and it is now recognized as the main
factor responsible for the dramatic decline in car-
ies prevalence that has been observed worldwide
[ 51 ]. Fluoride can be obtained in two forms: sys-
temic and topical. Systemic methods include
water fl uoridation, salt fl uoridation, milk fl uori-
dation, and supplements. Later in the 1940s, the
well-conducted water fl uoridation program was
established in the United States. Though some
dentists and researchers have confl icting opin-
ions about their safety and benefi ts, these sys-
temic methods are still recommended in many
countries and receive support from recognized
international committees and associations. Due
to the widespread application of fl uoride and the
updated knowledge about its mechanisms of
action, topical applications of fl uoride (e.g., fl uo-
ride toothpastes, gels, varnishes, and mouth-
washes) are considered to be more effective
methods for caries prevention than systemic use
of fl uorides [
52 ].
It is believed that fl uoride could inhibit demin-
eralization and enhance remineralization [ 7 , 53 –
55 ]. Numerous studies were designed to investigate
the mechanism of fl uoride in inhibiting demineral-
ization of dental hard tissue. According to previous
studies, fl uoride could incorporate into the enamel
apatite structure, enhance the resistance of the
dental hard tissue to acidic challenges, and thus
inhibit lesion development [
7 , 56 ]. In addition,
calcium-fl uoride- like deposits could form on den-
tal hard tissues and act as a protective barrier on
the surface and serve as a reservoir for fl uoride
[ 53 ]. Other researchers have some different opin-
ions. They demonstrate that the incorporation of
fl uorides into the mineral components of enamel
only slightly reduced its solubility [ 54 , 55 , 57 ].
Small amounts of free fl uoride ions in solution
around the dental hard tissue play a much more
important role in inhibiting demineralization.
These fl uorides have a much greater caries-
protective potential than a large proportion of FAP
incorporated in enamel mineral [ 58 ]. Free fl uoride
ions are in part adsorbed onto the crystalline sur-
face and are in dynamic equilibrium with the fl uo-
ride ions in solution around dental hard tissue. So
it forms an equilibrium or supersaturation relative
to fl uor(hydroxy)apatite, and the adsorption of
fl uoride on the crystals can offer direct protection
from demineralization. Therefore, according to
this theory, fl uoride should be present in the right
place (biofi lm fl uid or saliva) and at the right time
(when biofi lm is exposed to sugar or right after
biofi lm removal), and even small amount of fl uo-
ride (below ppm values) available is effective.
In addition to its ability of inhibiting demineral-
ization, fl uoride is thought to be effective to pro-
mote remineralization. However, fl uoride’s ability
to enhance net remineralization is limited by the
availability of calcium and phosphate ions [ 59 ]. If
adequate salivary or plaque calcium and phosphate
ions are available, fl uoride ions can drive the rem-
ineralization of extant non-cavitated caries lesions.
Fluorhydroxyapatite forms more rapidly even in
slightly acidic condition than do the other calcium
phosphate phases, so fl uoride can accelerate and
promote remineralization of dental hard tissue.
It has been demonstrated that fl uoride used in
enamel remineralization also work in dentine
remineralization, and even their remineralization
mechanisms are similar [
60 , 61 ]. However, dentin
demands a considerably higher fl uoride concen-
tration in its surrounding solution than enamel
does to reach an equivalent degree of demineral-
ization inhibition [ 62 ].
C. Lei et al.

77
4.3.1.2 Calcium Phosphate
Calcium and phosphate ions play important roles
in enhancing remineralization of dental caries.
Investigators have tried various solutions
containing calcium and phosphate ions in their
experiments, in which solutions contained
between 1 and 3 mM calcium ions with phosphate
ions in the ratio of 1:1 [ 63 , 64 ] or 1.66:1 [ 63 ],often
with the addition of 1 ppm fl uoride ions. Higher
concentrations are diffi culty to be used because
of the instability of the solutions. So the low
solubility of calcium phosphates, particularly in
the presence of fl uoride ions, limited the clinical
application of calcium and phosphate
remineralization systems. Therefore, novel
calcium-phosphate-based delivery systems are
developed to combat the demineralization of
dental hard tissue. New commercial products are
available based on three types of novel systems—
crystalline, unstabilized amorphous, or stabilized
amorphous formulations.
4.3.2 Novel Methods
4.3.2.1 CPP–ACP and CPP–ACFP
CPP–ACP has been shown to promote
remineralization of initial enamel lesions and to
prevent demineralization in laboratory, animal,
and human experiments [ 59 , 65 ]. Casein
phosphopeptides (CPP) can stabilize calcium and
phosphate as nanoclusters of ions through the
formation of amorphous nanocomplexes
(diameter of 2.12 nm) in metastable solution
[
66 ]. But it can also prevent the growth of the
nanoclusters to the critical size required for
nucleation and phase transformation [ 65 ]. CPP
contains the active sequence Ser(P)-Ser(P)-
Ser(P)-Glu-Glu. Phosphorylated seryl residues
are regarded as responsible for the interactions
between casein and the calcium and phosphate
ions in the nanocomplexes [ 67 ].
CPPs can bind to the more thermodynami-
cally favored surface of an apatite crystal face in
the caries lesion due to its high binding affi nity
for apatite. And CPPs prefer binding to the (100)
and (010) faces of hydroxyapatite crystals
(Fig. 4.3 ). So crystal growth would be allowed to
continue only at the hydroxyapatite (001) plane
or along the c-axis, which is the pattern of crystal
growth during amelogenesis. Therefore, CPPs
are able to regulate anisotropic crystal growth
and also inhibit crystal demineralization in the
enamel subsurface lesion [
68 ].
The amorphous calcium phosphate (ACP) is an
important compound because it is a precursor that
can convert to apatite, similar to the minerals in
tooth enamel and dentin. ACP is an unstabilized
calcium and phosphate system. When a calcium
salt (e.g., calcium sulfate) and a phosphate salt
(e.g., potassium phosphate) are delivered sepa-
rately, the calcium ions and phosphate ions are
mixed and result in the immediate precipitation of
ACP or, in the presence of fl uoride ions, amor-
phous calcium fl uoride phosphate (ACFP). In the
intraoral environment, these phases (ACP and
ACFP) are potentially very unstable and may rap-
idly transform into a more thermodynamically
stable, crystalline phase (e.g., hydroxyapatite and
fl uorhydroxyapatite). However, before phase
transformation, calcium and phosphate ions should
be transiently bioavailable to promote enamel sub-
surface lesion remineralization.
4.3.2.2 Natural Medicine
Previous studies indicated some nature medicines
were able to infl uence the de-/remineralization
balance of dental hard tissue. The extracts of
Galla chinensis (GCE) could inhibit the deminer-
alization and enhance the remineralization of
enamel and dentin [
69 – 72 ]. In addition, this poly-
phenol compounds had combined effects with tra-
ditional remineralizing agents, like fl uoride [ 69 ],
nano-hydroxyapatite [ 73 ]. However, the mecha-
nism of GCE is still unclear, and more investiga-
tions are still needed.
4.3.2.3 Laser
Several types of lasers, such as erbium-doped
yttrium aluminum garnet (Er:YAG) [ 74 , 75 ],
neodymium-doped yttrium aluminum garnet
(Nd:YAG) [ 74 , 76 ], and carbon dioxide (CO
2
)
[ 76 – 79 ], with different parameter settings, have
been used for caries inhibition. It is believed that
the use of the high-intensity laser on the dental
structure can lead to a more acid-resistant sur-
4 Demineralization and Remineralization
78
face, and previous investigations showed that
lasers could inhibit enamel demineralization and
reduce enamel permeability [ 80 ].
4.3.2.4 Nanoparticles
Currently, nanotechnology is experiencing rapid
growth, with many potential applications in car-
ies prevention and treatment. It is defi ned as the
creation of functional materials, devices, or sys-
tems through control of matter on the nanometer
scale (1–100 nm). Nanotechnology has motivated
mimicking of the nanostructural features of natu-
ral human enamel and development of bioin-
spired strategies for remineralization and caries
therapy, respectively [ 81 ]. Previous researches
have tried to apply nanoparticles in dental caries
b
a
a
b
c
d
c
x
x
b
Fig. 4.3 A molecular model of the Ser(P)-Ser(P)-Ser(P)-
Glu-Glu motif bound onto the face of hydroxyapatite
(HA). The atoms are colorcoded as follows: calcium (1)
atoms are light-blue crosses, calcium (2) atoms are dark-
blue crosses, oxygen atoms are red, phosphorus atoms are
magenta, carbon atoms are green, nitrogen atoms are blue,
and hydrogen atoms are grey. The symbol X indicates a
crystallographic axis projecting into the paper. Four views
are presented: ( a ) a side view along the c-axis, with the
peptide rendered in CPK and the crystal atoms in ‘line’
form; ( b ) as in ( a ), but viewed from above, looking down
on the HA (100) face; ( c ) as in ( b ), with the peptide dis-
played in stick form and the atoms in the HA surface
within 0.25 nm of the peptide rendered in CPK; and ( d ) as
in ( b ), with the peptide displayed in stick form and the
atoms of the peptide within 0.25 nm of the HA surface
rendered in CPK. [
68 ]
C. Lei et al.

79
prevention and treatment, including spherical,
cubic, and needlelike nanoscaled particles
(approximately 5–100 nm) and near-nanoscaled
devices (up to micrometers) [ 82 ]. When the sizes
of particles are reduced from micrometer to
nanometer, the resultant properties can change
dramatically. For example, hardness, active sur-
face area, chemical reactivity, and biological
activity are all altered [ 83 ]. The application of
nanoparticles in dentistry can be categorized into
two directions: preventive dentistry and restora-
tion dentistry [ 81 ].
In recent years, various types of nano-sized
hydroxyapatite or calcium carbonate are applied
to combat early caries lesions [ 73 , 84 , 85 ]. Some
in vitro studies indicated that 10 % suspension of
nano-hydroxyapatite particles (10–20 nm diame-
ter, 60–80 nm length) promotes remineralization
of the superfi cial layer of initial caries lesions
measuring 20–40 μm, but little remineralization
could be obtained by nano- hydroxyapatite in the
body of the lesion [
73 , 84 ]. Carbonate hydroxyl
apatite nanoparticles have also been reported to be
effective in repairing micrometer-sized tooth-sur-
face defects in vitro [ 86 ]. And some nanocrystals
have been incorporated into toothpastes or mouth-
rinsing solutions as commercial products.
Clinical investigations indicate that secondary
caries and restoration fracture are still the main
reasons for dental restoration failure, which lim-
its the longevity of dental restorations, especially
the resin composites [ 87 ]. Approximately half of
all dental restorations fail within 10 years, and
replacing them consumes nearly 60 % of the
average dentist’s practice time [
88 ]. Investigators
try to improve the resin compositions, fi ller par-
ticles, and cure conditions. Calcium phosphate
(CaP) particles and calcium fl uoride particles
have been used as fi llers in resin composites
(Fig. 4.4 ). These resin-based CaP or CaF com-
posites can release calcium (Ca), phosphate
(PO4), or fl uoride ions. These additives enable
the resin composite to release calcium and phos-
phate when the pH is dropped down under in vitro
conditions, providing caries-inhibiting properties
[ 90 ]. These calcium and phosphate or fl uoride
ion-releasing nanofi llers include nanoparticles of
dicalcium phosphate anhydrous (112 nm in size),
amorphous calcium phosphate (ACP) (116 nm in
size), and so on [ 91 – 95 ].
Nanoparticles of ACP can be synthesized via a
spray-drying technique. Briefl y, a spraying
solution was prepared by dissolving calcium
carbonate and dicalcium phosphate anhydrous
(CaHPO
4
) into an acetic acid solution. Then this
solution was sprayed through a nozzle into a
heated chamber. The water and volatile acid were
evaporated and expelled into an exhaust hood.
The dried particles were collected by an electro-
static precipitator.
CaF
2
nanoparticles can be synthesized using
the same spray-drying apparatus. A two-liquid
nozzle was employed during the procedures. Two
solutions are mixed during atomization: Ca(OH)
2
and NH
4
F. And the two solutions are atomized
leading to the formation of CaF
2
nanoparticles:
Ca(OH)
2
+ NH4F → CaF
2
+ NH
4
OH. The NH
4
OH
is removed as NH
3
and H
2
O vapors [ 96 ].
acb
Fig. 4.4 TEM micrographs of the spray-dried nanoparticles: ( a ) small ACP nanoparticles, ( b ) ACP cluster, ( c ) CaF
2
nanoparticles [
89 ]
4 Demineralization and Remineralization

80
4.3.3 Biomineralization
Biomineralization is the process by which living
organisms produce minerals, often to mineralized
tissues. The formation of teeth is a process of
biomineralization. Though traditional methods
have been proven to be effective to combat dental
caries, dental hard tissue is unable to heal
and repair itself after demineralization of the
surface and subsequent cavitation due to its
non- regenerative nature. Understanding the
mechanism of dental hard tissue inspired the
researchers who studied the remineralization of
dental hard tissue. Numerous studies have tried to
fi nd special templates to achieve biomineraliza-
tion of dental hard tissue in vitro [ 89 ]. There are
numerous evidences indicating that organic tem-
plates or scaffolds would be a prerequisite for
bioinspired formation of enamel-like structures
[ 97 ]. However, there are some problems about
the clinical application of biomimetic approaches
in vitro. Firstly, the suspected biocompatibility of
nonbiological surfactants strongly limits their
clinical application. Secondly, most of biomi-
metic approaches need various hydrothermal
conditions, including nonphysiological tempera-
ture or pressure [ 98 ], which cannot be applied in
clinical practice. So some investigators have
focused on the biomimetic approaches in physio-
logical-like condition. For example, single crys-
talline hydroxyapatite micro-ribbons were used
as substitutes for amelogenin templates to control
HAP crystallization at biophysical conditions at
37 °C [
99 ] .
References
1. Featherstone JD. The continuum of dental caries –
evidence for a dynamic disease process. J Dent Res.
2004;83 Spec No C:C39–42.
2. Featherstone JD. Dental caries: a dynamic disease
process. Aust Dent J. 2008;53(3):286–91.
3. Hannig M, Hannig C. Nanomaterials in preventive
dentistry. Nat Nanotechnol. 2010;5(8):565–9.
4. Nelson DG, Featherstone JD. Preparation, analysis,
and characterization of carbonated apatites. Calcif
Tissue Int. 1982;34 Suppl 2:S69–81.
5. Nelson DG, Featherstone JD, Duncan JF, Cutress
TW. Effect of carbonate and fl uoride on the dissolu-
tion behaviour of synthetic apatites. Caries Res. 1983;
17(3):200–11.
6. Robinson C, Shore RC, Brookes SJ, Strafford S,
Wood SR, Kirkham J. The chemistry of enamel car-
ies. Crit Rev Oral Biol Med. 2000;11(4):481–95.
7. ten Cate JM, Featherstone JD. Mechanistic aspects of
the interactions between fl uoride and dental enamel.
Crit Rev Oral Biol Med. 1991;2(3):283–96.
8. Cochrane NJ, Zero DT, Reynolds EC. Remineralization
models. Adv Dent Res. 2012;24(2):129–32.
9. White DJ. The application of in vitro models to
research on demineralization and remineralization of
the teeth. Adv Dent Res. 1995;9(3):175–93; discussion
94–7.
10. Tschoppe P, Wolf O, Eichhorn M, Martus P, Kielbassa
AM. Design of a randomized controlled double-blind
crossover clinical trial to assess the effects of saliva
substitutes on bovine enamel and dentin in situ. BMC
Oral Health. 2011;11:13.
11. Moron BM, Comar LP, Wiegand A, Buchalla W, Yu
H, Buzalaf MA, et al. Different protocols to produce
artifi cial dentine carious lesions in vitro and in situ:
hardness and mineral content correlation. Caries Res.
2013;47(2):162–70.
12. Spiguel MH, Tovo MF, Kramer PF, Franco KS, Alves
KM, Delbem AC. Evaluation of laser fl uorescence in
the monitoring of the initial stage of the de-/
remineralization process: an in vitro and in situ study.
Caries Res. 2009;43(4):302–7.
13. ten Cate JM, Duijsters PP. Alternating demineraliza-
tion and remineralization of artifi cial enamel lesions.
Caries Res. 1982;16(3):201–10.
14. Buzalaf MA, Hannas AR, Magalhaes AC, Rios D,
Honorio HM, Delbem AC. pH-cycling models for
in vitro evaluation of the effi cacy of fl uoridated
dentifrices for caries control: strengths and limitations.
J Appl Oral Sci. 2010;18(4):316–34.
15. McBain AJ. Chapter 4: In vitro biofi lm models: an
overview. Adv Appl Microbiol. 2009;69:99–132.
16. Loesche WJ. Role of Streptococcus mutans in human
dental decay. Microbiol Rev. 1986;50(4):353–80.
17. Ccahuana-Vasquez RA, Cury JA. S. mutans biofi lm
model to evaluate antimicrobial substances and enamel
demineralization. Braz Oral Res. 2010;24(2):135–41.
18. Silva TC, Pereira AF, Exterkate RA, Bagnato VS,
Buzalaf MA, Machado MA, et al. Application of an
active attachment model as a high-throughput demin-
eralization biofi lm model. J Dent. 2012;40(1):41–7.
19. Giacaman RA, Munoz MJ, Ccahuana-Vasquez RA,
Munoz-Sandoval C, Cury JA. Effect of fl uoridated
milk on enamel and root dentin demineralization
evaluated by a biofi lm caries model. Caries Res.
2012;46(5):460–6.
20. Hayati F, Okada A, Kitasako Y, Tagami J, Matin K. An
artifi cial biofi lm induced secondary caries model for
in vitro studies. Aust Dent J. 2011;56(1):40–7.
21. Thurnheer T, Giertsen E, Gmur R, Guggenheim B.
Cariogenicity of soluble starch in oral in vitro biofi lm
and experimental rat caries studies: a comparison.
J Appl Microbiol. 2008;105(3):829–36.
C. Lei et al.

81
22. van de Sande FH, Azevedo MS, Lund RG, Huysmans
MC, Cenci MS. An in vitro biofi lm model for enamel
demineralization and antimicrobial dose–response
studies. Biofouling. 2011;27(9):1057–63.
23. Cenci MS, Pereira-Cenci T, Cury JA, Ten Cate
JM. Relationship between gap size and dentine
secondary caries formation assessed in a microcosm
biofi lm model. Caries Res. 2009;43(2):97–102.
24. Cheng L, Exterkate RA, Zhou X, Li J, ten Cate JM.
Effect of Galla chinensis on growth and metabolism
of microcosm biofi lms. Caries Res. 2011;45(2):
87–92.
25. Guggenheim B, Guggenheim M, Gmur R, Giertsen E,
Thurnheer T. Application of the Zurich biofi lm model
to problems of cariology. Caries Res. 2004;38(3):
212–22.
26. Zaura E, Buijs MJ, Hoogenkamp MA, Ciric L, Papetti
A, Signoretto C, et al. The effects of fractions from
shiitake mushroom on composition and cariogenicity
of dental plaque microcosms in an in vitro caries
model. J Biomed Biotechnol. 2011;2011:135034.
27. Deng DM, ten Cate JM. Demineralization of dentin
by Streptococcus mutans biofi lms grown in the
constant depth fi lm fermentor. Caries Res. 2004;38(1):
54–61.
28. Zero DT. In situ caries models. Adv Dent Res.
1995;9(3):214–30; discussion 31–4.
29. Stookey GK, Warrick JM, Miller LL, Greene AL.
Animal caries models for evaluating fl uoride denti-
frices. Adv Dent Res. 1995;9(3):198–207; discussion
208–13.
30. Clasen AB, Ogaard B. Experimental intra-oral caries
models in fl uoride research. Acta Odontol Scand.
1999;57(6):334–41.
31. Nakata K, Nikaido T, Nakashima S, Nango N, Tagami
J. An approach to normalizing micro-CT depth profi les
of mineral density for monitoring enamel remineral-
ization progress. Dent Mater J. 2012;31(4):533–40.
32. Arends J, ten Bosch JJ. Demineralization and remin-
eralization evaluation techniques. J Dent Res. 1992;71
Spec No:924–8.
33. Anderson P, Levinkind M, Elliot JC. Scanning micro-
radiographic studies of rates of in vitro demineraliza-
tion in human and bovine dental enamel. Arch Oral
Biol. 1998;43(8):649–56.
34. Bertassoni LE, Habelitz S, Pugach M, Soares PC,
Marshall SJ, Marshall Jr GW. Evaluation of surface
structural and mechanical changes following reminer-
alization of dentin. Scanning. 2010;32(5):312–9.
35. Barbour ME, Rees JS. The laboratory assessment of
enamel erosion: a review. J Dent. 2004;32(8):591–602.
36. Herkstroter FM, Witjes M, Ruben J, Arends J. Time
dependency of microhardness indentations in human
and bovine dentine compared with human enamel.
Caries Res. 1989;23(5):342–4.
37. Cheng L, ten Cate JM. Effect of Galla chinensis on
the in vitro remineralization of advanced enamel
lesions. Int J Oral Sci. 2010;2(1):15–20.
38. Neves Ade A, Coutinho E, Vivan Cardoso M,
Jaecques SV, Van Meerbeek B. Micro-CT based quan-
titative evaluation of caries excavation. Dent Mater.
2010;26(6):579–88.
39. Schwass DR, Swain MV, Purton DG, Leichter JW. A
system of calibrating microtomography for use in
caries research. Caries Res. 2009;43(4):314–21.
40. Berger SB, Pavan S, Dos Santos PH, Giannini M,
Bedran-Russo AK. Effect of bleaching on sound enamel
and with early artifi cial caries lesions using confocal
laser microscopy. Braz Dent J. 2012;23(2):110–5.
41. Xie Q, Bedran-Russo AK, Wu CD. In vitro remineral-
ization effects of grape seed extract on artifi cial root
caries. J Dent. 2008;36(11):900–6.
42. de Carvalho FG, de Fucio SB, Sinhoreti MA, Correr-
Sobrinho L, Puppin-Rontani RM. Confocal laser
scanning microscopic analysis of the depth of dentin
caries-like lesions in primary and permanent teeth.
Braz Dent J. 2008;19(2):139–44.
43. Banerjee A, Boyde A. Autofl uorescence and mineral
content of carious dentine: scanning optical and
backscattered electron microscopic studies. Caries
Res. 1998;32(3):219–26.
44. Gonzalez-Cabezas C, Fontana M, Dunipace AJ, Li Y,
Fischer GM, Proskin HM, et al. Measurement of
enamel remineralization using microradiography and
confocal microscopy. A correlational study. Caries
Res. 1998;32(5):385–92.
45. Ando M, Hall AF, Eckert GJ, Schemehorn BR,
Analoui M, Stookey GK. Relative ability of laser
fl uorescence techniques to quantitate early mineral
loss in vitro. Caries Res. 1997;31(2):125–31.
46. Lagerweij M, van der Veen M, Ando M, Lukantsova
L, Stookey G. The validity and repeatability of three
light-induced fl uorescence systems: an in vitro study.
Caries Res. 1999;33(3):220–6.
47. Pretty IA, Pender N, Edgar WM, Higham SM. The
in vitro detection of early enamel de- and re-mineral-
ization adjacent to bonded orthodontic cleats using
quantitative light-induced fl uorescence. Eur J Orthod.
2003;25(3):217–23.
48. Sowa MG, Popescu DP, Friesen JR, Hewko MD, Choo-
Smith LP. A comparison of methods using optical
coherence tomography to detect demineralized regions
in teeth. J Biophotonics. 2011;4(11–12):814–23.
49. Huang D, Swanson EA, Lin CP, Schuman JS, Stinson
WG, Chang W, et al. Optical coherence tomography.
Science. 1991;254(5035):1178–81.
50. Amaechi BT, Higham SM, Podoleanu AG, Rogers JA,
Jackson DA. Use of optical coherence tomography for
assessment of dental caries: quantitative procedure. J
Oral Rehabil. 2001;28(12):1092–3.
51. Buzalaf MA, Pessan JP, Honorio HM, ten Cate
JM. Mechanisms of action of fl uoride for caries con-
trol. Monogr Oral Sci. 2011;22:97–114.
52. Ripa LW. A critique of topical fl uoride methods (den-
tifrices, mouthrinses, operator-, and self-applied gels)
in an era of decreased caries and increased fl uorosis
prevalence. J Public Health Dent. 1991;51(1):23–41.
53. ten Cate JM. Review on fl uoride, with special empha-
sis on calcium fl uoride mechanisms in caries preven-
tion. Eur J Oral Sci. 1997;105(5 Pt 2):461–5.
4 Demineralization and Remineralization

82
54. ten Cate JM, Duijsters PP. Infl uence of fl uoride in solu-
tion on tooth demineralization. II. Microradiographic
data. Caries Res. 1983;17(6):513–9.
55. ten Cate JM, Duijsters PP. Infl uence of fl uoride in
solution on tooth demineralization. I. Chemical data.
Caries Res. 1983;17(3):193–9.
56. Takagi S, Liao H, Chow LC. Effect of tooth-bound
fl uoride on enamel demineralization/remineralization
in vitro. Caries Res. 2000;34(4):281–8.
57. Arends J, Christoffersen J. The nature of early caries
lesions in enamel. J Dent Res. 1986;65(1):2–11.
58. Ogaard B, Rolla G, Ruben J, Dijkman T, Arends J.
Microradiographic study of demineralization of shark
enamel in a human caries model. Scand J Dent Res.
1988;96(3):209–11.
59. Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD,
Morgan MV, et al. Fluoride and casein phosphopep-
tide-amorphous calcium phosphate. J Dent Res. 2008;
87(4):344–8.
60. Levine RS, Rowles SL. Further studies on the remin-
eralization of human carious dentine in vitro. Arch
Oral Biol. 1973;18(11):1351–6.
61. Klont B, ten Cate JM. Remineralization of bovine
incisor root lesions in vitro: the role of the collage-
nous matrix. Caries Res. 1991;25(1):39–45.
62. Baysan A, Lynch E, Ellwood R, Davies R, Petersson
L, Borsboom P. Reversal of primary root caries using
dentifrices containing 5,000 and 1,100 ppm fl uoride.
Caries Res. 2001;35(1):41–6.
63. Koulourides T, Cueto H, Pigman W. Rehardening of
softened enamel surfaces of human teeth by solutions
of calcium phosphates. Nature. 1961;189:226–7.
64. Wefel JS, Harless JD. The use of saturated DCPD in
remineralization of artifi cial caries lesions in vitro.
J Dent Res. 1987;66(11):1640–3.
65. Reynolds EC. Casein phosphopeptide-amorphous
calcium phosphate: the scientifi c evidence. Adv Dent
Res. 2009;21(1):25–9.
66. Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds
EC. Enamel subsurface lesion remineralisation with
casein phosphopeptide stabilised solutions of calcium,
phosphate and fl uoride. Caries Res. 2008;42(2):88–97.
67. Cross KJ, Huq NL, Palamara JE, Perich JW, Reynolds
EC. Physicochemical characterization of casein phos-
phopeptide-amorphous calcium phosphate nanocom-
plexes. J Biol Chem. 2005;280(15):15362–9.
68. Cochrane NJ, Cai F, Huq NL, Burrow MF, Reynolds
EC. New approaches to enhanced remineralization of
tooth enamel. J Dent Res. 2010;89(11):1187–97.
69. Cheng L, Li J, Hao Y, Zhou X. Effect of compounds
of Galla chinensis and their combined effects with
fl uoride on remineralization of initial enamel lesion
in vitro. J Dent. 2008;36(5):369–73.
70. Chu JP, Li JY, Hao YQ, Zhou XD. Effect of com-
pounds of Galla chinensis on remineralisation of ini-
tial enamel carious lesions in vitro. J Dent. 2007;
35(5):383–7.
71. Zou L, Zhang L, Li J, Hao Y, Cheng L, Li W, et al.
Effect of Galla chinensis extract and chemical frac-
tions on demineralization of bovine enamel in vitro. J
Dent. 2008;36(12):999–1004.
72. Guo B, Que KH, Jing Y, Wang B, Liang QQ, Xie
HH. Effect of Galla chinensis on the remineralization
of two bovine root lesions morphous in vitro. Int J
Oral Sci. 2012;4(3):152–6.
73. Huang S, Gao S, Cheng L, Yu H. Combined effects of
nano-hydroxyapatite and Galla chinensis on reminer-
alisation of initial enamel lesion in vitro. J Dent.
2010;38(10):811–9.
74. Castellan CS, Luiz AC, Bezinelli LM, Lopes RM,
Mendes FM, De PEC, et al. In vitro evaluation of
enamel demineralization after Er:YAG and Nd:YAG
laser irradiation on primary teeth. Photomed Laser
Surg. 2007;25(2):85–90.
75. Ceballos L, Toledano M, Osorio R, Garcia-Godoy F,
Flaitz C, Hicks J. ER-YAG laser pretreatment effect
on in vitro secondary caries formation around com-
posite restorations. Am J Dent. 2001;14(1):46–9.
76. Tsai CL, Lin YT, Huang ST, Chang HW. In vitro acid
resistance of CO2 and Nd-YAG laser-treated human
tooth enamel. Caries Res. 2002;36(6):423–9.
77. Featherstone JD, Barrett-Vespone NA, Fried D,
Kantorowitz Z, Seka W. CO2 laser inhibitor of artifi -
cial caries-like lesion progression in dental enamel. J
Dent Res. 1998;77(6):1397–403.
78. Hsu CY, Jordan TH, Dederich DN, Wefel JS. Effects
of low-energy CO2 laser irradiation and the organic
matrix on inhibition of enamel demineralization.
J Dent Res. 2000;79(9):1725–30.
79. Poosti M, Ahrari F, Moosavi H, Najjaran H. The effect
of fractional CO laser irradiation on remineralization
of enamel white spot lesions. Lasers Med Sci.
2014;29(4):1349–55.
80. Maung NL, Wohland T, Hsu CY. Enamel diffusion
modulated by Er:YAG laser (Part 1)–FRAP. J Dent.
2007;35(10):787–93.
81. Hannig M, Hannig C. Nanotechnology and its role in
caries therapy. Adv Dent Res. 2012;24(2):53–7.
82. Cushing BL, Kolesnichenko VL, O’Connor CJ.
Recent advances in the liquid-phase syntheses of
inorganic nanoparticles. Chem Rev. 2004;104(9):
3893–946.
83. Allaker RP, Ren G. Potential impact of nanotechnol-
ogy on the control of infectious diseases. Trans R Soc
Trop Med Hyg. 2008;102(1):1–2.
84. Huang S, Gao S, Cheng L, Yu H. Remineralization poten-
tial of nano-hydroxyapatite on initial enamel lesions: an
in vitro study. Caries Res. 2011;45(5):460–8.
85. Nakashima S, Yoshie M, Sano H, Bahar A. Effect of a
test dentifrice containing nano-sized calcium carbon-
ate on remineralization of enamel lesions in vitro.
J Oral Sci. 2009;51(1):69–77.
86. Roveri N, Palazzo B, Iafi sco M. The role of biomime-
tism in developing nanostructured inorganic matrices
for drug delivery. Expert Opin Drug Deliv. 2008;
5(8):861–77.
87. Sakaguchi RL. Review of the current status and challenges
for dental posterior restorative composites: clinical, chemis-
try, and physical behavior considerations. Summary of dis-
cussion from the Portland Composites Symposium
(POCOS) June 17–19, 2004, Oregon Health and Science
University, Portland, Oregon. Dent Mater. 2005;21(1):3–6.
C. Lei et al.

83
88. Frost PM. An audit on the placement and replacement
of restorations in a general dental practice. Prim Dent
Care. 2002;9(1):31–6.
89. Huang Z, Newcomb CJ, Bringas Jr P, Stupp SI, Snead
ML. Biological synthesis of tooth enamel instructed by
an artifi cial matrix. Biomaterials. 2010;31(35):9202–11.
90. Chen MH. Update on dental nanocomposites. J Dent
Res. 2010;89(6):549–60.
91. Xu HH, Moreau JL, Sun L, Chow LC. Novel CaF(2)
nanocomposite with high strength and fl uoride ion
release. J Dent Res. 2010;89(7):739–45.
92. Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite
containing amorphous calcium phosphate nanoparticles
for caries inhibition. Dent Mater. 2011;27(8):762–9.
93. Xu HH, Weir MD, Sun L. Nanocomposites with Ca
and PO4 release: effects of reinforcement, dicalcium
phosphate particle size and silanization. Dent Mater.
2007;23(12):1482–91.
94. Xu HH, Weir MD, Sun L, Moreau JL, Takagi S,
Chow LC, et al. Strong nanocomposites with Ca,
PO(4), and F release for caries inhibition. J Dent Res.
2010;89(1):19–28.
95. Xu HH, Weir MD, Sun L, Takagi S, Chow LC. Effects
of calcium phosphate nanoparticles on Ca-PO4 com-
posite. J Dent Res. 2007;86(4):378–83.
96. Xu HH, Moreau JL, Sun L, Chow LC. Strength and
fl uoride release characteristics of a calcium fl uoride
based dental nanocomposite. Biomaterials. 2008;29(32):
4261–7.
97. Yamagishi K, Onuma K, Suzuki T, Okada F, Tagami J,
Otsuki M, et al. Materials chemistry: a synthetic enamel
for rapid tooth repair. Nature. 2005;433(7028):819.
98. Zhang J, Jiang D, Lin Q, Huang Z. Synthesis of dental
enamel-like hydroxyapatite through solution mediated
solid-state conversion. Langmuir. 2010;26(5):2989–94.
99. Ma G, Liu XY, Wang M. Growth and mechanisms of
enamel-like hierarchical nanostructures on single
crystalline hydroxyapatite micro-ribbons. J Nanosci
Nanotechnol. 2011;11(6):5199–206.
4 Demineralization and Remineralization

85
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1_5
The Diagnosis for Caries
Yang Liu , Li Boer , Wang Shuang , Zhang Yaru ,
and Peng Li
According to the glossary of endodontic terms,
caries is defi ned as a localized and progressive
bacterial infection that results in disintegration of
the tooth, usually beginning with the demineral-
ization of enamel and followed by bacterial inva-
sion. It usually takes 6–12 months for caries to
appear. Generally, we can make a correct diagno-
sis with routine inspection. For some diffi cult
cases, dental radiographs or other special inspec-
tions are supplementary methods for caries diag-
nosis (Table 5.1 ).
5.1 Conventional Diagnosis
Methods
5.1.1 Inspection
When making an examination for dental caries on
suspected sites, we can fi nd black or chalky area or
a formed cavity. The interproximal marginal ridge
area has ink stain discoloration under the enamel
or a visible cavity. For observation on tooth cervi-
cal areas, the cheek and tongue should be pulled
away to fully expose buccal and lingual surface of
posterior teeth. Depending on the inspection, we
can get a general scope of carious damage.
5.1.2 Probing
The sharp probe is used to inspect the suspicious
areas. With the help of the probe, the depth and
extension of cavity can be examined. If a proxi-
mal cavity is suspected and cannot be located
through an inspection, the probe is useful to
locate affected area when it is hooked by the edge
of the cavity. The probe can also be used on the
tooth surface to locate the area of dentinal hyper-
sensitivity. Pulp exposure can also be located
while examining the deep carious lesion.
5.1.3 Percussion
Caries does not cause periodontal and periapical
infl ammation, so the reaction to percussion is
always negative.
5.2 Special Diagnostic Methods
5.2.1 Radiographic Examination
Radiographic examination can be helpful in locat-
ing proximal caries and undermining caries and
secondary caries [ 1 ]. It can also be used to assess
the proximity of caries to pulp chamber. Periapical
Y. Liu • L. Boer • W. Shuang • Z. Yaru • P. Li (*)
State Key Laboratory of Oral Diseases ,
West China Hospital of Stomatology,
Sichuan University , Chengdu ,
People’s Republic of China
e-mail:
5

86
and bite-wing radiographs are commonly used for
clinical assessment of caries. Radiolucency on
hard tissue due to demineralization is identifi ed as
carious lesion. A series of researches have
revealed that more than half of proximal caries are
seen on the radiograph. Since the radiograph is a
two-dimensional image, the diagnosis result
should be analyzed and combined with clinical
examination. Proximal caries should be distin-
guished from normal triangular low-density areas
in the cervical region of the tooth. Secondary car-
ies should be differentiated from low-density bas-
ing materials on the fl oor of the cavity.
5.2.2 Cold and Hot Irritation Test
The response of dental pulp toward cold and hot
irritation is examined during cold and hot
irritation test. Examination is done by putting
chloroethane- soaked cotton ball or hot gutta-
percha stick on the surface of the tooth, and the
patient’s response is evaluated. These external
stimuli can elicit acute pain. The pulp is sup-
posed to be healthy if pain disappears right after
removal of stimuli. But if the pain is lingering,
the pulp is likely to be in irreversible infl amma-
tory. When cold water is used as stimulus, it is
Table 5.1 The diagnosis for caries
Conventional diagnosis methods Inspection Black or chalky area or a formed cavity
can be seen
Probing Probe can be used to locate affected area,
the area of dentinal hypersensitivity, and
pulp exposure
Percussion The reaction to percussion is always
negative
Special diagnostic methods Radiographic examination Radiolucency on hard tissue due to
demineralization is identifi ed as carious
lesion
Cold and hot irritation test The response of dental pulp toward cold
and hot irritation can determine the
severity of caries
Dental fl oss examination Dental fl oss can diagnose caries on
proximal contact area
Diagnostic cavity preparation After removing supportless enamel, the
practitioner can obtain well a vision on
hidden carious lesion
The new technology of caries
diagnosis
Fiber-optic transillumination This system uses fi ber transillumination
for potential caries diagnosis
Electrical impedance technology This technology is an alternative way for
occlusal pit and fi ssure caries diagnosis
Ultrasonic technique It is a new method for caries detection by
measuring the wave that refl ects back
from tooth structure
Elastomeric separating modulus
technique
Elastomeric separating modules are used
to separate apart adjacent tooth
temporarily for examination of proximal
surfaces
Staining technique This technique can show the presence of
caries and estimate the depth of carious
lesion
Quantitative laser fl uorescence
technique
Autofl uorescence is light emission
phenomenon of biological structure
The differential diagnosis of the
superfi cial caries
Enamel hypocalcifi cation The key points of the differential
diagnosis for caries are glossiness and
smoothness, predilection site, symmetry
of the lesion, and progress of the lesion
Enamel hypoplasia
Dental fl uorosis
Y. Liu et al.

87
important to note that the fl ow of water may
affect the accurate location of carious cavity. At
last, caries should be differentiated from dentin
hypersensitivity.
5.2.3 Dental Floss Examination
Caries on proximal contact area is diffi cult to be
examined by inspection and probing. Dental
fl oss can be used as a convenient method. By
putting a dental fl oss across the embrasure of
the suspicious tooth surface and moving the
fl oss horizontally in a seesaw motion, the exam-
iner can experience the roughness of the sur-
face. The fl oss will be torn if caries is present.
Examination with fl oss can be misled by dental
calculus.
5.2.4 Diagnostic Cavity Preparation
After removing supportless enamel, the practi-
tioner can obtain well a vision on hidden carious
lesion. To identify the scope and depth of cavity,
the infectious dentin on the fl oor and wall of the
cavity should be removed completely. Dying the
decalcifi ed dentin with 0.5 % basic fuchsin can
help dentists to identify and remove the infec-
tious dentin. After that, the tooth decay area and
pulp condition can be easily defi ned.
5.3 The New Technology
of Caries Diagnosis
Tooth caries is chronic, progressive, and bacterial
diseases. The main characters of tooth caries are
the changes in color, shape, and quality of tooth
hard tissue. The typical pathological changes
have important reference value for caries diagno-
sis. At present, the methods for dental caries
diagnosis are mainly based on clinical inspection
and X-ray examination. However, it is diffi cult to
identify early caries which is in the hidden area
of the tooth. With the latest development in sci-
ence and technology, some techniques and meth-
ods are being used for caries diagnosis. These
methods have greatly improved the accuracy and
sensitivity of caries diagnosis.
5.3.1 Fiber-Optic
Transillumination, FOTI
A new diagnostic technique for caries was
called fi ber-optic transillumination. This system
uses fi ber transillumination for potential caries
diagnosis [ 2 , 3 ]. The principle is based on the
fact that the light transillumination index in
decayed tissue is lower than that in normal tis-
sue. Generally, the decayed area shows dark
shadow.
5.3.2 Electrical Impedance
Technology
The electrical impedance technology is an alter-
native way for caries diagnosis by examining
tooth potential difference. The carious cavity is
fi lled with dead and decayed tissue, saliva, and
electrolytes. Therefore, this area becomes more
electric conductive than the normal tissue.
Following this principle, the resistance offered by
the tooth surface is measured under controlled
drying. The conductivity is measured by probe in
occlusal fi ssure, and current passes through the
pulp to the ground through handheld lead form-
ing a circuit.
The electric caries detector device measures
the bulk electric resistance and potential differ-
ence. This method is simple, sensitive, and sta-
ble for occlusal caries detection.
5.3.3 Ultrasonic Technique
The ultrasonic technique is a new method for car-
ies detection by measuring the wave that refl ects
back from the tooth structure. This ultrasonic
wave is received by a sensor when refl ecting back
from the tooth surface. The normal tooth surface
and the decay one are supposed to have different
refl ecting waves. Currently, 18 MHz frequency
wave was used for caries diagnosis.
5 The Diagnosis for Caries

88
5.3.4 Elastomeric Separating
Modulus Technique
Elastomeric separating modules are used to sepa-
rate apart adjacent tooth temporarily for exami-
nation of proximal surfaces. This method can be
helpful especially when proximal surface caries
was examined.
5.3.5 Staining Technique
The staining technique usually stains the degraded
collagen which is present in the carious cavity but
never stains the intact collagen. For this reason,
the dye is used in carious cavity to stain dead and
decayed dental tissue. With this technique, the
dentist can determine the presence of caries and
estimate the depth of carious lesion. The com-
monly used dye is 1 % basic fuchsin.
5.3.6 Quantitative Laser
Fluorescence Technique
Autofl uorescence is light emission phenomenon
of biological structure. The autofl uorescence of
dental tissue decreases in demineralization of the
tissue. Quantitative laser fl uorescence devices
use high-intensity halogen lamp to stimulate the
tooth to emit the fl uorescence in green spectrum
[ 4 , 5 ]. This refl ected light is detected by spectrum
and recorded in computer and demineralization is
quantifi ed. The related other new technologies
are dye-enhanced laser fl uorescence (DELF),
quantitative light-induced fl uorescence, light
scattering, and confocal laser scanning micro-
scope [
6 , 7 ].
5.4 The Differential Diagnosis
of the Superfi cial Caries
The diagnosis of the pit and fi ssure caries can be
done based upon its location. However, the diag-
nosis of the smooth surface caries is more chal-
lenging due to its appearances. Common clinical
presentation of caries is described below.
5.4.1 Enamel Hypocalcifi cation
This is a condition where the enamel is formed
without adequate mineralization. The lesion is
characterized by irregular, opaque, and chalky
spots or plaque on tooth surface [ 8 ] (Fig. 5.1a ).
5.4.2 Enamel Hypoplasia
Enamel hypoplasia is a defi cit in enamel forma-
tion. The enamel is thin and defi cient in amount.
It is seen clinically as dot or banded sunken
defects with chalky, yellowish, or brownish dis-
coloration [ 9 ] (Fig. 5.1b ).
5.4.3 Dental Fluorosis
Dental fl uorosis is a developmental disturbance,
due to exposure of tooth bud to high concentra-
tion of fl uoride during its development. In mild
type of fl uorosis, tiny white streaks or specks are
seen in the enamel, but discolored and pitted in
severe type [ 10 ]. The spots and stains left by fl uo-
rosis are permanent and may darken over time
[ 11 ] (Fig. 5.1c ).
5.4.4 The Key Points
of the Differential Diagnosis
for Caries
Glossiness and Smoothness The enamel
lesions that are caused by developmental distur-
bance may have color changes. However, the
glossiness, smoothness, and hardness are not
affected. The caries teeth have chalky or snuff
colored spots without gloss. The enamel surface
can also be rough in appearance.
Predilection Site Pit and fi ssure, proximal sur-
face, and cervical part are caries’ predilection
sites. It could hardly be found on the self- cleaning
areas like cusps or any other smooth surface. The
developmental disturbance is caused by abnor-
mal development or irregular mineralization on
Y. Liu et al.

89
the tooth surface. Based on the stage of tooth
development, the lesion can be seen on different
areas.
Symmetry of the Lesion The developmental
disturbances affect the tooth during the period of
development. Therefore, developmental lesion
can be found bilaterally and in a similar manner.
Progress of the Lesion
The lesions of develop-
mental origin will become quiescent as soon as
the tooth erupts in the oral cavity. It will neither
develop nor disappear. The caries is progressive
by its nature. The color of carious lesion changes
from chalky white to dark brown with its prog-
ress toward the pulp chamber. The initial enamel
lesion which is chalky white in appearance can
be arrested by the tooth remineralization.
The Differential Diagnosis of the Medium
Caries The teeth with medium size caries are
sensitive to sweet, acid, heat, and cold stimula-
tions. Patients usually complain that they feel pain
with these stimuli. This is different from dentinal
hypersensitivity. The dentin hypersensitivity is
stimulation of nerve endings in dentinal tubule
due to change of temperature or pH in oral cavity.
The sensation can range all the way from irritation
to intense, shooting pain. This sensitivity can also
be found on wear-away tooth, decayed tooth, or
tooth with exposed root surface.
References
1. Madalli VB, Annigeri RG, Basavaraddi SM. The eval-
uation of effect of developer age in the detection of
approximal caries using three speed dental x-ray fi lms:
an in-vitro study [J]. J Clin Diagn Res. 2014;8(3):236.
2. Hintze H, Wenzel A, Danielsen B, et al. Reliability of
visual examination, fi bre-optic transillumination, and
bite-wing radiography, and reproducibility of direct
visual examination following tooth separation for the
identifi cation of cavitated carious lesions in contacting
approximal surfaces [J]. Caries Res. 1998;32(3):204–9.
3. Mitropoulos CM. The use of fi bre-optic transillumi-
nation in the diagnosis of posterior approximal caries
in clinical trials [J]. Caries Res. 1985;19(4):379–84.
4. Tomczyk J, Komarnitki J, Zalewska M, et al.
Fluorescence methods (VistaCam iX proof and
DIAGNODent pen) for the detection of occlusal cari-
ous lesions in teeth recovered from archaeological con-
text [J]. Am J Phys Anthropol. 2014;154(4):525–34.
a
c
b
Fig. 5.1 Different clinical images of tooth diseases.
( a ) Chalky plaques can be seen on the labial surface of the
central incisors, diagnosed as dental hypocalcifi cation.
( b ) The yellow circle shows sunken defect with yellow discol-
oration. It is known as enamel hypoplasia. ( c ) White specks
are seen on the labial surface of maxillary anterior teeth
5 The Diagnosis for Caries

90
5. Hafström-Björkman U, Sundström F, Josselin D, de Jong
E, et al. Comparison of laser fl uorescence and longitudi-
nal microradiography for quantitative assessment of
in vitro enamel caries [J]. Caries Res. 1992;26(4):241–7.
6. Sheehy EC, Brailsford SR, Kidd EAM, et al.
Comparison between visual examination and a laser
fl uorescence system for in vivo diagnosis of occlusal
caries [J]. Caries Res. 2001;35(6):421–6.
7. Lai G, Kaisarly D, Xu X, et al. MicroCT-based compari-
son between fl uorescence-aided caries excavation and
conventional excavation [J]. Am J Dent. 2014;27(1):12.
8. Rushton MA. The surface of the enamel in hereditary
enamel hypocalcifi cation [J]. Br Dent J. 1962;112:
24–7.
9. Seow WK. Enamel hypoplasia in the primary denti-
tion: a review [J]. ASDC J Dent Child. 1990;58(6):
441–52.
10. Dean HT. Endemic fl uorosis and its relation to dental
caries [J]. Public Health Rep. 1938;53(33):1443–52.
11. Fejerskov O, Manji F, Baelum V. The nature and
mechanisms of dental fl uorosis in man [J]. J Dent Res.
1990;69:692–700; discussion 721.
Y. Liu et al.

91
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1_6
Dental Caries: Disease Burden
Versus Its Prevention
Hong Xiao
6.1 Global Trends of Caries
Burden
6.1.1 Oral Diseases: One of the Most
Costly Diseases to Treat
Oral diseases, despite their non-life-threatening
nature, have been ranked the fourth most expen-
sive disease to treat in most industrialized coun-
tries. In the high-income industrialized countries,
5–10 % of public health spending is used for oral
health care [ 1 ]. Back in the year 2000, the
European Union spent a total of €54 billion on
oral health care, and in the United States, the
2004 expenditures for oral health care were US$
81.5 billion [ 2 ]. However, such fi gures did not
include the additional costs of dental care per-
formed under the medical umbrella. Among the
variety of oral diseases, dental caries has long
been the main factor attributable as the most
important source of oral disease burden.
Not surprisingly, these costs will require unaf-
fordable fi nancial resources from low- and middle-
income countries. For example, the costs of
treatment to control periodontal diseases in chil-
dren on basis of the Community Periodontal Index
(CPI) of Treatment Needs (CPITN) data greatly
exceeded the total national health-care budget of
Kenya [ 3 , 4 ]. Take Nepal as another example: the
total costs of restoring dental caries cavities of the
child population would exceed the total health-
care budget for children of the entire country [ 5 ].
This fact should be interpreted on the back-
ground that, for low-income countries, a compre-
hensive national essential health-care package at
a cost of US$ 4 will reduce the burden of mortal-
ity and disability among the 0- to 14-year-old
children by 30 %!
However, planning traditional curative interven-
tion strategies entirely on the basis of epidemiologi-
cal data without considering the social, economic,
and political conditions is doomed to fail. In partic-
ular the national economic conditions appear to be
an important determinant of restorative care. If we
take the Care Index (F⁄DMFT*100 %) for 34- to
44-year-olds as an indicator of the country’s restor-
ative care level, it appears that no country with a
gross domestic product (GDP) below US$ 5,000
has a Care Index higher than 30 %, which further
underpins that restorative treatment is unaffordable
in low- income countries [
5 , 6 ].
6.1.2 Uneven Distribution of Oral
Disease Burden Around
the World
In the recent several decades, we have witnessed
a time of dramatic decline of caries status
worldwide. This trend can be attributed to
H. Xiao
Department of Preventive Dentistry ,
West China Hospital of Stomatology,
Sichuan University , Chengdu , China
e-mail:
6

92
widespread use of topical fl uoride and increasing
awareness of the importance of maintaining good
oral hygiene. However, in some parts of the
world, dental caries still remains a common dis-
ease affecting children and adults, posing tre-
mendous economic burden.
If the burden of disease is described using
years lived with disability (YLD) per million
people, the highest disease burden of the world
can be found in sub-Saharan Africa closely fol-
lowed by India. Both the countries carry heavy
disease burdens mainly because of communica-
ble diseases. However, in the high-income coun-
tries, noncommunicable diseases are the main
source of disease burden [ 7 ].
When it comes to oral diseases, including car-
ies, periodontal diseases, and edentulism, they
make very little contribution to the total YLD/
million population of each country with the
worldwide average of 1.6 %. While the Middle
Eastern Crescent is found to have the highest oral
disease contribution to the total YLD/million
population, China is found to be among those
countries with lowest oral disease contributions.
With respect to relative contribution of caries
to oral disease burden of different regions of the
world, contribution of caries was found to be over
a half of most countries or regions, ranging from
as low as 46.7 % in established economy market
countries to as high as 89.8 % in Latin America
and Caribbean.
Usually, caries status can be monitored through
national epidemiological surveys by recording the
dmf/DMF indices (decayed, missing, and fi lled
tooth/surface indices). Although the recording of
such indices disregards the impact of perceived
pain and discomfort as a result of dental caries, it
is able to reveal some valuable objective informa-
tion especially from a professional perspective.
The proportion of each component varies greatly
among high-, middle-, and low-income countries.
Data collected between 1990 and 2004 indicate
that, in high- income countries, only about one-
fi fth of the caries lesions in the primary dentition
were treated. Such proportion of treatment dra-
matically decreased to about 5 % in the middle-
income countries. Worst fi ndings were found in
the low- income countries, where the proportion
of treated caries lesions could be totally neglected.
Situations in the permanent dentition told a
better story. The high-income countries had a
Care Index (F/DMF score) of over 50 %, while
the middle-income countries had about 20 %.
However unfortunately, the Care Index was found
to be within 5 % for the low-income countries.
The temporal trends in dental caries experi-
ence of 12-year-old children in developing and
developed countries can be illustrated in Figs. 6.1
and 6.2 . In most developing countries, the levels
of dental caries were low. However, the preva-
lence rates of dental caries seem to increase in
recent years. This might be attributed to increased
consumption of sugar and inadequate use of
fl uoride.
However, the industrialized countries tell a dif-
ferent story. A steady decline of caries experience
is found. This result from a number of public
health measures including effective use of fl uo-
rides, together with changing living conditions,
lifestyles, and improved self-care practices.
Worldwide, the prevalence of dental caries
among adults is high as the disease affects nearly
100 % of the population in the majority of coun-
tries. Figure 6.3 illustrates the levels of dental
caries among 35–44-year-olds, as measured by
the mean DMFT index. Most industrialized
countries and some countries of Latin America
show high DMFT values.
6.1.3 Developing Global Policies
Highlighting the Importance
of Oral Health
The policy of the WHO Global Oral Health
Programme emphasizes that oral health is integral
and essential to general health and that oral health
is a determinant factor for quality of life. The WHO
Global Oral Health Programme has implied that
greater emphasis is put on developing global poli-
cies based on common risk factors and approaches
should be coordinated more effectively with other
programs in public health [ 8 – 10 ]:
• To adopt measures to ensure that oral health is
incorporated as appropriate into policies for
the integrated prevention and treatment of
chronic noncommunicable and communicable
H. Xiao

93
diseases and into maternal and child health
policies
• To take measures to ensure that evidence-
based approaches are used to incorporate oral
health into national policies as appropriate for
integrated prevention and control of noncom-
municable diseases
• To consider mechanisms to provide coverage
of the population with essential oral health
care, to incorporate oral health in the frame-
work of enhanced primary health care for
chronic noncommunicable diseases, and to
promote the availability of oral health services
that should be directed toward disease preven-
tion and health promotion for poor and disad-
vantaged populations, in collaboration with
integrated programs for the prevention of
chronic noncommunicable diseases
• For those countries without access to optimal
levels of fl uoride, and which have not yet estab-
lished systematic fl uoridation programs, to
consider the development and implementation
of fl uoridation programs, giving priority to
equitable strategies such as the automatic
administration of fl uoride, for example, in
drinking water, salt, or milk, and to provide
affordable fl uoride toothpaste
• To develop and implement the promotion of
oral health and prevention of oral disease for
preschool and school children as part of activ-
ities in health-promoting schools
• To scale up capacity to produce oral health
personnel, including dental hygienists, nurses,
Decayed missing and
filled permanent teeth
Very low: <1.2>
Low: 1.2–2.6
Moderate: 2.7–4.4
High: >4.4
No date available
Fig. 6.1 Dental caries levels (decayed, missing, and fi lled
teeth (DMFT) index) among 12-year-olds worldwide
(Reproduced, with the permission of the publisher, from
Bulletin of the World Health Organization , Petersen et al.
[
7 ]. http://www.who.int/bulletin/volumes/83/9/petersen-
0905abstract/en/
)
5
4
3
DMFT
1
2
0
1981 1983
1982 1984 1986 1988 1990 1992 1994 1996 1998
1985
1987
1989 1991
1993 1995 1997
All countries
Developing countries
Developed countries
Fig. 6.2 Changing levels of dental caries experience
(decayed, missing, and fi lled teeth (DMFT) index) among
12-year-olds in developed and developing countries
(Reproduced, with the permission of the publisher, from
Bulletin of the World Health Organization , Petersen et al.
[
7 ]. http://www.who.int/bulletin/volumes/83/9/petersen-
0905abstract/en/
)
6 Dental Caries: Disease Burden Versus Its Prevention

94
and auxiliaries, providing for equitable distri-
bution of these auxiliaries to the primary-care
level and ensuring proper service back-up by
dentists through appropriate referral systems
• To incorporate an oral health information sys-
tem into health surveillance plans so that oral
health objectives are in keeping with interna-
tional standards and to evaluate progress in
promoting oral health
• To strengthen oral health research and use evi-
dence-based oral health promotion and disease
prevention in order to consolidate and adapt oral
health programs and to encourage the inter-
country exchange of reliable knowledge and
experience of community oral health programs
• To address human resources and workforce
planning for oral health as part of every
national plan for health
• To increase, as appropriate, the budgetary provi-
sions dedicated to the prevention and control of
oral and craniofacial diseases and conditions
• To strengthen partnerships and shared respon-
sibility among stakeholders in order to
maximize resources in support of national oral
health programs
6.2 Caries Burden in China
6.2.1 The First and Second National
Epidemiological Investigation
of Oral Health in China
China has organized two nationwide epidemiol-
ogy investigations. The fi rst was conducted
between 1982 and 1984, which had examined the
status of caries and periodontal diseases in pri-
mary and middle school students of 29 provinces,
autonomous administration regions, and munici-
palities directly under the central government.
There were altogether 131,340 students exam-
ined. The fi rst national epidemiological investi-
gation of oral health in China is the fi rst
large-scale epidemiological investigation orga-
nized since the establishment of P.R. China.
The second national epidemiological investiga-
tion of oral health in China took place from 1995 to
1998. This investigation had included 6 age groups
covering 5–74-year-olds from 11 provinces, auton-
omous administration regions, and municipalities
directly under the central government. A total of
140,712 people at 396 sample sites were selected.
Decayed missing and
filled permanent teeth
Very low: <5.0
Low: 5.0–8.9
Moderate: 9.0–13.9
High: >13.9
No data available
Fig. 6.3 Dental caries levels (decayed, missing, and fi lled
teeth (DMFT) index) among 35–44-year-olds worldwide
(Reproduced, with the permission of the publisher, from
Bulletin of the World Health Organization , Petersen et al.
[
7 ]. http://www.who.int/bulletin/volumes/83/9/petersen-
0905abstract/en/
)
H. Xiao

95
Results of these two epidemiology investiga-
tions have provided valuable fi rst-hand data for
the central government to make oral health-
related policies, set up targets for oral health ser-
vice, and planned future human resource
allocation in oral health service.
According to the second national survey
which was conducted in 1995, caries prevalence
rates for 5-year-olds, 12-year-olds, 35–44-year-
olds, and 65–74-year-olds were 76.6 %, 45.8 %,
63.0 %, and 64.8 %, respectively.
6.2.2 The Third National
Epidemiological Investigation
of Oral Health in China
The third national epidemiological investigation
of oral health in China was implemented in 2005.
At the planning period, the sampling methods
have been carefully designed as stratifi ed multi-
level randomized sampling [ 11 ]. A total of 93,826
people were selected covering 5–74-year-olds in
6 age groups. The sample well represents the
overall status of oral health in China. In addition
to dental examinations, a comprehensive ques-
tionnaire investigation was simultaneously con-
ducted to investigate the oral health-related
concepts and behaviors of the selected sample.
Caries status obtained from stratifi ed popula-
tions is detailed as follows.
6.2.2.1 Caries Status of 5-Year-Olds
The 5-year-olds have a caries prevalence rate of
66.0 % in the primary dentition. Children who
live in the cities have a slightly lower caries prev-
alence rate (62.0 %), while in the rural areas,
children have a higher caries prevalence rate
(70.2 %).
Average DMFT of the 5-year-olds is 3.5,
while most of these children have a DMFT of 2,
accounting for 11.1 % of the total subsample.
The constitution of DMFT indices can be viewed
in Fig. 6.4 , which has shown that an astonishing
proportion of carious teeth have never been
treated (96.7 %). Analysis of the frequency
distribution of DMFT in children has aroused our
attention when it is found that 79.3 % of all cari-
ous teeth concentrate in about one-third of the
examined children, who present a signifi cant car-
ies index of 8.33.
Approximately half of the subsample have
their guardians (mostly parents and grandpar-
ents) completed the questionnaire. It has been
found that 13 % children began to brush their
teeth before the age of 3, and the remaining vast
majority has just begun to brush their teeth.
Around one-fi fth of children don’t brush their
teeth.
There were 49 % parents aware of the fl uoride
toothpaste, and 39 % of 5-year-olds use the fl uori-
dated toothpaste when brushing their teeth. It
should be paid attention to that only 22 % children
visit dentists periodically. The most common rea-
sons for dental visits are acute or chronic tooth-
ache and other reasons such as dental emergency.
Multiple regression analysis was performed to
examine the main reasons for caries in children.
It has been found that urban/rural distribution,
sugar consumption before sleeping, toothbrush-
ing starting age, and oral hygiene by parents all
correlate with caries status of children.
6.2.2.2 Caries Status of 12-Year-Olds
Caries prevalence rate of the 12-year-olds is
28.9 %. No difference is observed between urban
and rural areas, and boys have a statistically sig-
nifi cant lower caries prevalence rate than girls
(25.4 % vs. 32.6 %).
The most often affected teeth are mandibular
fi rst molars, maxillary fi rst molars, and mandibu-
lar second molars. Unfortunately, 88.8 % of all
carious teeth have not been treated (Fig. 6.5 ). In
China, 1.45 % of 12-year-olds have pit and fi ssure
Decayed 96.7 Missing 0.5
Filled 2.8
Fig. 6.4 Constitution of decayed, missing, and fi lled
teeth in 5-year-olds in China (%)
6 Dental Caries: Disease Burden Versus Its Prevention

96
sealants, and there is a huge difference between
urban area and rural area (2.75 % vs. 0.19 %).
The investigated 12-year-olds have completed
the questionnaire by themselves. As this age
group is the most important age group, it has
been receiving continuous attention from the
central government. In addition to questions on
oral health-related knowledge and behavior, the
questionnaire has also included questions on den-
tal visits and self-perceived impact of oral health.
It has been found that 82 % of the 12-year- olds
investigated brush their teeth daily; however, only
28 % of them brush their teeth at least twice a day.
Ninety-three percent of the children report that
they have never used dental fl oss. The percentage
of children who use fl uoridated toothpaste is
46 %. Sixty-nine percent of the children have
daily consumption of sugar-containing food, such
as sweetened milk, dissert, candy, carbonated soft
drink, and sweetened fruit juice. It is satisfactory
that only a small fraction of the investigated chil-
dren smoke (boys 5.7 % and girls 0.5 %) and that
the percentage of daily consumption of cigarette
and alcohol is further reduced to 1.0 % and 0.1 %,
respectively, for boys and girls.
With regard to oral health-related knowledge,
only one-tenth of 12-year-olds understand dental
plaque as the cause for caries and periodontal dis-
eases. However, over 60 % of them have realized
that bleeding during toothbrushing is abnormal.
In the past 12 months, 21 % of 12-year-olds
have visited a dentist. However, 47 % of the total
subsample have never seen a dentist. The most
common reasons for their dental visit are acute or
chronic toothache. It is sad that only 28 % have
formed the habit of frequent dental check-ups.
6.2.2.3 Caries Status
of 35–44-Year-Olds
The 35–44-year-olds is the WHO recommended
age group for adults. It has been found that the
vast majority of this age group (88.1 %), quite
unfortunately, has been affected by caries. Not
much difference was found between the urban
and rural populations (89.1 % vs. 87.1 %).
However, women are found with statistically sig-
nifi cant higher caries prevalence rate than men
(91.3 % vs. 84.9 %). The constitution of the
DMFT indices is shown in Fig. 6.6 . However, the
prevalence rate for root caries is near one-third
(32.7 %), signifying bad periodontal status.
All of the investigated subgroup answered the
questionnaire. Among all the 35–44-year-olds,
89 % have reported that they brush their teeth
daily, and 35 % have reported to be brushing their
teeth at least twice a day. Better teeth brushing
habits are found in cities, women than in rural
areas and men.
On a daily basis, 27 % use toothpicks, but over
99 % do not use dental fl oss. Fluoridated tooth-
paste is used in 46 % people. Daily consumption
of sugar is found in 21 % of those investigated.
There are 32 % questionnaire respondents reported
to be smokers. There is a huge gender difference in
daily alcohol consumption, while 23 % men, in
contrast to 2 % women, drink alcohol daily.
Among the 35–44-year-olds, 69 % believe
there is a need for them to receive oral treatment,
Decayed 88.8 Missing 0.6
Filled 10.6
Fig. 6.5 Constitution of decayed, missing, and fi lled
teeth in 12-year-olds in China (%)
Decayed 34.0 Missing 57.6
Filled 8.4
Fig. 6.6 Constitution of decayed, missing, and fi lled
teeth in 35–44-year-olds in China (%)
H. Xiao

97
and the percentage is found to be statistically
signifi cantly higher in urban populations than
rural (73 % vs. 65 %). However, in the past 12
months, only 16 % of them have been to a den-
tist. Quite surprisingly, even at this age, there are
as many as 46 % 35–44-year-olds who have
never visited a dentist ever. For the remaining
54 %, the majority of them have attributed their
dental attendance to be because of acute or
chronic toothache or other dental situations, and
few of them have formed the habit of regular
dental check-ups.
Multiple regression analysis has revealed that
the caries status of the 35–44-year-olds is closely
related to the use of fl uoride toothpaste, con-
sumption of sugar-containing foods, toothbrush-
ing frequencies, and other behavioral variables.
Those with no use of fl uoridated toothpaste, with
daily consumption of sugar-containing foods,
and who are not brushing their teeth on a daily
basis manifest with high caries risk.
6.2.2.4 Caries Status
of 65–74-Year-Olds
Caries prevalence in the age group of 65–74-year-
olds is found to be very high. The prevalence rate
is as high as 98.4 %, and not much difference is
found between urban and rural populations. Also,
not much difference is discovered between both
genders. The mean DMFT score is 14.7, the
majority of which is missing due to caries
(75.3 %), and only a fraction of the iceberg is
treated (1.9 %) (Fig. 6.7 ).
Root caries is another important issue that
should not be neglected. The age group of
65–74-year-olds are found to be heavily affected
by caries in the root. Rural population have a root
caries prevalence rate of 67.2 %, higher than the
urban population (60.0 %). It is found that 66.1 %
females are affected by root caries, higher than
males (61.1 %). It is further discovered that only
as low as 1.9 % of all the carious lesions in the
root have been treated (Fig. 6.8 ).
About half of the examined subjects have
handed in the questionnaire. It has been found that
three-quarters of the questionnaire respondents
brush their teeth on a daily basis and that slightly
over one-quarter brush their teeth twice a day. This
self-oral hygiene behavior is found to be better in
cities and females. In 27 % of the questionnaire
respondents, fl uoridated toothpaste is used; the
percentage is found to be higher in cities than in
rural areas. There are 26 % of the respondents who
report they use toothpick daily, but almost none of
the respondents have ever used dental fl oss.
Daily sugar-containing food consumption is
found in 27 % questionnaire respondents. An
average of 27 % of the respondent smoke, and
there is a huge difference between the two gen-
ders. And it is found that among those who
smoke, 90 % of them have been smoking for
more than 20 years. Alcohol consumption is
found in 13.6 % respondents; the percentage of
male is almost six times that of the female.
Over half of the elder population believe they
are in need of oral examination and treatment;
however, only 19 % of the respondents report that
Decayed 22.8 Missing 75.3
Filled 1.9
Fig. 6.7 Constitution of decayed, missing, and fi lled
teeth in 65–74-year-olds in China (%)
Decayed 98.1
Filled 1.9
Fig. 6.8 Constitution of decayed and fi lled root caries in
65–74-year-olds in China (%)
6 Dental Caries: Disease Burden Versus Its Prevention

98
they have visited a dentist within the past 12
months. The most common reason for dental
referral is acute and chronic toothache. However,
it is unfortunate that about one-third of the all the
respondents have never been to a dentist for any
form of oral examination or treatment. For the
expenditure on dental visits, 83 % of the respon-
dents report that they are fully self-fi nanced, not
to be able to fi nd a source to partly cover the bill.
When we analyze the oral health impact on
daily life, it is sad to fi nd that over half the respon-
dents have experienced toothache, and the major-
ity of the elders are not satisfi ed with their oral
hygiene conditions.
6.3 Caries Preventive Strategies
Dental caries results from a variety of contribut-
ing factors. There are three most important fac-
tors, namely, cariogenic microfl ora (termed as
dental plaque), carbohydrate-rich diet, and sus-
ceptible teeth. Given time, the interrelation and
interaction between these three factors will pro-
duce the chronic oral disease which brings pain
and discomfort to the majority of the population
worldwide since an early age. Therefore, effec-
tive caries-preventive strategies almost always
require removal of any or all of these contributing
factors.
There is a hierarchy of caries-preventive strat-
egies which include the following.
6.3.1 Primary Prevention
Primary prevention is the most important strategy
as it stresses and deals with combating the etio-
logical factors. Self-awareness with regard to car-
ies prevention is often highlighted in this stage.
People are motivated by a variety of means, either
with propaganda via mass media or face-to-face
educational lectures, so that they will be self-
conscious about the importance of keeping good
oral hygiene, restricting the consumption of sug-
ars and frequent dental visits.
Many countries have set up their own launches
or campaigns to deliver the concept of oral health
education. China, for example, has launched its
own national campaign for over a decade. Each
year on the “love teeth day” on September 20th,
there will be dental health educational and pro-
motional activities held across the country. There
is a unique theme every year. Usually manuals
containing educational information are distrib-
uted free of charge. In many cities where there
are dental schools or colleges, free dental check-
ups are available.
Removal of etiological factors is very impor-
tant in this stage. The main methods include den-
tal plaque control, use of fl uoride, and other
preventive methods that are to be introduced in
the following section.
6.3.2 Secondary Prevention
Secondary prevention mainly refers to the early
diagnosis and treatment of lesions found to be
still at their early stages. For a long time, caries
diagnosis mainly relies heavily on the dentists
themselves who can only resort to their naked
eyes and self-practicing experiences. This has
resulted in countless mistaken diagnosis and
omission of countless lesions which had been left
not dealt with. Later, the invention of X-ray has
offered another possibility to visualize lesions
that hide from usual visual examination.
6.3.2.1 Conventional Caries Detection
Methods
Traditional diagnostic methods, such as visual
inspection, appear to have very low sensitivity
and high specifi city in diagnosing caries [ 12 – 14 ].
Continuous attempts to improve the sensitivity
have been made. Ekstrand et al have proposed a
new caries scoring system as presented in
Table 6.1 . Sensitivity and specifi city for detection
of dentinal lesions were found to be ranging
between 0.92 and 0.97 and 0.85 and 0.93, respec-
tively [ 15 ]. A conclusion from one study was that
although good results were obtained regarding
sensitivity and specifi city as well as operator
agreement, it takes more time to learn the method.
Although improvements in visual inspection with
new scoring systems (take, ICDAS, for another
H. Xiao

99
example) seem promising, further clinical valida-
tion is still needed.
The use of explorers has received some dis-
pute as some believe it cannot increase diagnos-
ing accuracy while tends to damage tooth tissue
and contaminate sound areas. Loesche et al, in a
study on intraoral transmission of pathogenic
microorganisms, showed that sterile fi ssures
might be inoculated by probing after previous
contact with an infected fi ssure [ 16 ]. For detec-
tion of occlusal dentinal lesions, the sensitivity of
the explorer is reported to be only about 0.5–0.6.
A number of reports have demonstrated that
probing with a sharp explorer may cause damage
to newly erupted teeth or even create a cavity at
the site of a superfi cial carious lesion. Therefore,
its use has been questioned by several authors.
The use of fi lm radiograph for caries detection
has a long history and is still the most widely
used diagnostic technique. Bitewing radiography
has been found to be useful for dentinal caries
detection on both occlusal and approximal sur-
faces. However, it has no value for occlusal
enamel caries detection and only a limited value
for approximal enamel caries detection.
6.3.2.2 Fiber-Optic Transillumination
(FOTI)
Fiber-optic transillumination (FOTI) is a widely
used caries detection adjunctive to dental profes-
sionals [ 17 – 20 ]. Its use can be traced back to the
1970s. It brings special advantages in diagnosing
caries in the proximal surfaces which are diffi cult
to discover with the naked eye under normal
means of illumination. In FOTI, white light from
a cold-light source is passed through a fi ber to an
intraoral fi ber-optic light probe that is placed on
the buccal or lingual side of the tooth. The sur-
face is examined using the transmitted light, seen
from the occlusal view. Decayed tooth structure
manifests itself as the dark shadow under illumi-
nation of ultraviolet. Demineralized areas appear
darker compared with the surrounding sound tis-
sue. The contrast between sound and carious tis-
sue is then used for detection of lesions.
FOTI has been evaluated in a number of stud-
ies for detection of posterior approximal carious
lesions and has shown low-to-good sensitivity
and good specifi city. Cortes et al showed, in an
in vitro study, that a combination of FOTI and
visual inspection was useful for determination of
occlusal lesion depth.
Using the digital image of a tooth (seen from
the occlusal view during transillumination)
through computer image analysis, researchers
have attempted to improve the performance of
FOTI. This quantitative method, digitized fi ber-
optic transillumination (DI-FOTI), has been eval-
uated in a few studies, and the initial results
indicate that both the sensitivity and specifi city
are very high. However, this method needs to be
developed further before it can be applied in clin-
ical situations.
6.3.2.3 New Caries Detection Methods
Till now, many new and high-tech diagnosing
technologies have become available, providing
new possibilities in caries diagnosis at its earliest
stages. Moreover, caries can not only be detected
but also can be quantifi ed. This has very impor-
tant implications as thanks to these high technol-
ogies, longitudinal quantitative monitoring of
carious lesions is now a reality.
New Caries Detection Methods: Optically
Based Light interacts with the dental hard tis-
sues in different ways. It can be either refl ected,
scattered, transmitted, or absorbed. The different
phenomena can occur alone or in combination.
Table 6.1 Detailed criteria for visual inspection of
occlusal surfaces introduced by Ekstrand et al
Classifi cation Visual inspection
0 No or slight change in enamel
translucency after prolonged air
drying (>5 s)
1 Opacity or discoloration hardly
visible on wet surface but distinctly
visible after air drying
2 Opacity or discoloration distinctly
visible without air drying
3 Localized enamel breakdown in
opaque or discolored enamel and/or
grayish discoloration from the
underlying dentin
4 Cavitation in opaque or discolored
enamel exposing the dentin
6 Dental Caries: Disease Burden Versus Its Prevention

100
A possible consequence of absorption is fl uo-
rescence, in which electrons of a lower-energy
status are moved to a higher status. When they
fall back to the original level, energy is emitted in
the form of light, called fl uorescence. In other
words, fl uorescence occurs as a result of the
interaction of electromagnetic radiation with
molecules in the tissue.
The cause of enamel fl uorescence is still
unclear. Most of the fl uorescence is induced by
organic components, proteinic chromophores, but
some can be probably attributable to apatite. It has
been proposed that fl uorescence in dentin is
caused by inorganic complexes, as well as some
organic components. In sound enamel, the path
lengths are long so that there is a high probability
that the photons will hit a chromophore. Thus,
fl uorescence is relatively intense. Demineralization
of dental hard tissue will result in the loss of auto-
fl uorescence (the natural fl uorescence).
The amount of autofl uorescence loss can be
measured by devices to quantify the severity of
tooth decay. Some of these high-tech caries
detection devices are introduced below.
DIAGNOdent DIAGNOdent is developed based
on the fi nding that natural tooth structure will
absorb the 655 nm wavelength light, while
decayed tooth structure produces fl uorescence in
the near-infrared spectrum [ 21 – 23 ]. Intensity of
the near-infrared fl uorescence refl ects the extent
of tooth decay. By comparing the numerical
value of the test site with that of the comparison
site (usually the same location at the contralateral
tooth in the same dentition), one is able to tell
whether the site of examination is diseased or not
and at what severity is the disease. This device is
very sensitive so that it can be used to analyze the
initial stage of caries while causing no damage to
the tooth structure or pain to the patient.
It has been found to be able to help identify
the most caries-susceptible site of pits and fi s-
sures on fully erupted fi rst permanent molars in
both caries-active and caries-free children, thus
providing helpful information to decide cost-
effective, targeted prevention for pediatricians.
The version called DIAGNOdent (Kavo,
Biberach, Germany) is designed to detect occlusal
and smooth surface caries. A new device, named
the DIAGNOdent pen (Kavo), has recently been
developed for the use in approximal and occlusal
surfaces, which has the same mode of function-
ing. Several in vitro and in vivo studies have been
conducted to evaluate the devices. In a study by
Lussi et al, good to excellent sensitivity and
excellent reproducibility were reported.
In recent years, a new approach has been pro-
posed to improve the performance of
DIAGNOdent in detecting early caries lesions by
using fl uorescent dyes. This proposed approach
has been tested by Alencar CJ et al, who had used
a fl uorescent dye (tetrakis N-methylpyridyl por-
phyrin, TMPyP) to enhance the performance of
DIAGNOdent and DIAGNOdent pen. Mineral
loss was determined with gold standard method.
Correlation was observed between the amount of
mineral loss and DIAGNOdent measurements.
They found that DIAGNOdent and DIAGNOdent
pen are capable of identifying demineralization
around brackets bonded with resin-modifi ed
glass ionomer cements. They also found that the
DIAGNOdent pen associated with TMPyP was
more capable of identifying this difference in
mineral loss as well as the gold standard method.
Quantitative Light-Induced Fluorescence (QLF)
Quantitative light-induced fl uorescence (QLF)
also detects caries lesions based on fl uorescence
fi ndings (laser-induced autofl uorescence of natu-
ral tooth structure) [ 24 – 28 ]. The tooth is illumi-
nated by a broad beam of blue- green light which
is transported through a liquid- fi lled light guide.
The source of light illumination can be either an
argon + ion laser, producing diffuse monochro-
matic light at a wavelength of 488 nm, or blue
light from a 50 W xenon microdischarge arc
lamp, which has an optical band- pass fi lter with a
peak intensity of 370 nm. Using a color CCD
video camera and a frame-grabber, fl uorescent-
fi ltered images can be captured and stored in a
computer.
Demineralized areas appear as dark spots
viewed by QLF. Software (Inspektor Research
Systems BV, Amsterdam, the Netherlands) can be
customized to analyze the collected images. After
calculating the loss of fl uorescence which
H. Xiao

101
indicates the severity of a lesion, three measures
are given: lesion area (mm
2
), ∆F (average change
in fl uorescence, in %), and ∆Q (area*∆F).
Therefore, compared with other caries detection
methods, QLF has a huge advantage in direct
visual demonstration for patients. In addition,
countless studies have confi rmed its accuracy and
reproducibility. Intra-class correlation coeffi cients
(ICCs) for each QLF metric were found to be high
with intra-examiner ∆Q 0.91, ∆F 0.80, and area
0.92, according to a study of 91 in vivo samples.
It can be used to reliably monitor the caries status
of an individual over time. However, it seems to
be limited to a lesion depth of about 400 μm.
To now, the QLF method has been found to be
more suitable for smooth surface caries examina-
tions. The possibility of adapting it for occlusal
caries diagnosis as well as secondary caries is
still under development. However, it has already
been employed in a number of clinical studies
which evaluated the anticaries effi cacy of various
kinds of oral hygiene maintaining products such
as toothpaste, mouth rinse, etc.
It has been found that longitudinal measure-
ments of QLF could detect differences in remin-
eralization of early enamel caries on buccal
surfaces of anterior teeth following supervised
daily brushing with sodium fl uoride (NaF;
1,450 ppm F), sodium monofl uorophosphate
(MFP; 1,450 ppm F) dentifrices, or a herbal, non-
fl uoride placebo dentifrice.
New Caries Detection Methods: Electrical
Impedance Based
Electrical caries monitor
(ECM) is developed on the basis of analyzing the
conductivity of tooth structures. Natural enamel
is no good conductor for electricity. However,
under diseased condition, its conductivity is
greatly increased because of expanded intercrys-
tal space and fl uid content inside. The more
demineralized the tissue, the lower the resistance
becomes. Then, by measuring the electric con-
ductivity of the test site and comparing it with
fi ndings of the contralateral or adjacent tooth in
the same dentition, the extent of the carious
lesion can be derived [ 29 ].
Measurements can be performed by closing a
circuit of a very weak alternating current through
the patient. A probe is placed on the site that is to
be measured, an earth-unit is held in the patient’s
hand, and both are connected with the device by
a cord. During examination, the measuring site
should be isolated from saliva. When a tooth sur-
face is of interest, the probe tip can be placed in
an electrolyte which covers the surface.
This equipment is mainly devised to detect
caries lesions at approximal sites of teeth. In a
number of in vitro and in vivo studies, the
reported sensitivity for ECM in diagnosing den-
tinal carious lesions of permanent premolar and
molar teeth ranges from 0.67 to 0.96. And the
specifi city ranges from 0.71 to 0.98, which could
be regarded as acceptable.
However, under some circumstances, false-
positive or false-negative readings may be
recorded. These circumstances include examined
teeth which have only erupted into the oral cavity
for less than 6 months, excessive dehydration of
the adjacent or other reference sites, and occlusal
surface with too complex morphology.
Temperature variations may also infl uence the
outcome of the measurements in various ways.
Other high-tech caries detection technologies
include electrochemical impedance spectroscopy
(EIS), optical coherence tomography (OCT), and
near-infrared (NIR) technology. These technolo-
gies are less frequently used in recent times, and
further studies are still ongoing.
At the same time, latest inventions and break-
throughs in new dental material have tremen-
dously reshaped the conception of dental
treatment for carious lesions. Tooth structures are
no longer subjected to unnecessary damages in
order to compromise for a more stable restora-
tion. In this way, tooth is much better protected
than ever before. All those new possibilities are
unimaginable without the forthcoming of adhe-
sive dental materials.
6.3.3 Tertiary Prevention
Tertiary prevention is a last-resort strategy which
is not intended to be utilized but has to be turned
to when caries at late stages are discovered. At
this stage, when dental caries already come into
6 Dental Caries: Disease Burden Versus Its Prevention

102
being, all that should be done is to prevent the
undesirable complications it will induce if not
dealt with. Among those possible and often clini-
cally observed complications are acute or chronic
pulpitis, alveolar abscess, periapical infl amma-
tion, alveolar osteomyelitis, and a number of other
less-frequently encountered caries derivatives.
When things become worse, damage to the
integrity of dentition occurs as a result. In this
case, prosthetic dentistry is needed to restore a
full dentition and maintain proper masticatory
functions.
6.4 Methods for Caries
Prevention
6.4.1 Dental Plaque Control
Dental plaque control refers to maintaining the
amount of plaque accumulation on tooth surfaces
at a reasonable level which does not allow the ini-
tiation and progression of carious lesions.
Theoretically, caries can be prevented through
perfect oral hygiene. Usually, dental plaque con-
trol is performed by individuals at home. Under
some circumstances, it is performed by a hygien-
ist or dentist as indicated necessary.
Chemical removal of dental plaque can be
achieved by administration of mouth rinses at dif-
ferent frequencies. This often involves different
kinds of key effective chemical components in
the mouth rinse solutions, such as triclosan,
chlorhexidine, essential oil, benzethonium chem-
icals, and many others. However, there are unde-
sirable consequences as microbes can start to
tolerate the effects of these chemicals given
enough time. Therefore, removal of dental plaque
with chemicals can only be used as an adjunctive
to mechanical methods or used as a substitute for
it when mechanical removal is not possible due to
some reasons.
Many mechanical methods can be employed
for this purpose. Among those, toothbrushing is
the most frequently advocated and most widely
exercised [
30 – 32 ]. However, the effi ciency of
toothbrushing is not always satisfactory, when
the level of which is measured by calculating the
plaque removal rates. Therefore, what is to be
stressed not only involves stressing the adequate
frequency of daily toothbrushing and its duration
of time but also includes the correct toothbrush-
ing methods. However very unfortunately, people
seem not to pay enough attention to this fact that
only by performing correct toothbrushing meth-
ods can they effectively remove the maximum
amount of dental plaque. Moreover, brushing the
teeth in a wrong way can also bring about unde-
sirable consequences, such as multiple wedge-
shaped defects, posing potential danger in pulp
exposure or even tooth fracture.
There are a number of toothbrushing methods
to choose, and each method has its own focus.
Horizontal vibrating method (also known as the
Bass method) stresses the removal of plaque in
the gingival sulcus and the interproximal spaces.
However, this method is very much time-
consuming and diffi cult to exercise for most peo-
ple, because it demands dynamic equilibrium of
direction and force. Another method to be sug-
gested is reported by Fones. It mainly involves
repeated rotary movements of the toothbrush
between the maxillary and mandibular dentition.
This method is so easy to conquer that it can even
be mastered by children and adolescents to per-
form effective self-oral hygiene.
Despite the brushing technique, the tooth-
brush itself is to be carefully selected. Now there
are different kinds of toothbrushes in the market.
Each kind serves a targeted subpopulation of a
certain age or of particular physical characteris-
tics. Some toothbrushes are specially designed
for different subgroups, such as children or the
disabled who require a toothbrush to have a
smaller head and less-likely-to-slip handle. For
the majority however, a regular-design tooth-
brush with soft round-end bristles is well enough
to maintain everyday oral hygiene.
In addition, electronic toothbrushes are
invented to better serve the disabled and the elder.
Toothbrushing movements of different groups of
bristles have been preprogrammed, and these
movements are often very complex which include
different rhythms of vibration and rotation in all
directions. Although numerous clinical studies
have revealed no statistically signifi cant
H. Xiao

103
differences in the cleaning ability between an
ordinary toothbrush that is powered by hand and
one that is powered by electricity, the target group
are greatly facilitated in maintaining good oral
hygiene.
However, the toothbrush fails to reach some
remote parts of the oral cavity. Therefore, addi-
tional accessories can be utilized to perform the
cleaning. Among the many choices, there are
dental fl oss, toothpicks, interdental brushes, and
electrical dental syringe. Dental fl oss, either
waxed or not, can effectively help remove dental
plaque on interproximal surfaces.
Toothpicks can be used to remove food debris;
however, care is needed so that it won’t hurt the
adjacent soft tissue. For some subgroup of people
who have lost the protection from gingival papil-
lae, e.g., in the case of periodontitis or attachment
loss therefore leaving the individuals at greater
danger of caries attack, interdental brushes can
be used to clean those places.
Electrical dental syringe can produce high-
speed projection of water which contains rich
foam and can remove food debris for hard-to-
reach places, such as the surface under the orth-
odontic wires or partial removable prosthetic
denture.
6.4.2 Restriction on Sugar
Consumption and Use
of Sucrose Substitute
Oral bacteria, especially the species capable of
acid production and acid toleration, metabolize
sugar contained in food debris to get energy to
survive. This process, which often takes place in
deep part of dental plaque where oxygen concen-
tration is much lower than the superfi cial part,
produces acid. This metabolism end-product will
accumulate inside dental microfl ora and demin-
eralize enamel crystals if the dental plaque is not
wiped away and remain on the tooth surface for a
long time.
Among all kinds of sugar-containing food
(food that contains glucose, fructose, maltose,
lactose, and many others), sucrose is found to be
the most dangerous in causing dental decay. A lot
of studies, both in vivo and in vitro, have proved
that consumption of sucrose-rich diet will result
in the unbalance of oral microbial micro-ecology
where cariogenic species will tremendously out-
number those non-cariogenic species. Therefore,
the initiation of dental decay will continue to
progress once started. Therefore, restricting the
consumption of such cariogenic-potent sugars is
an important caries-preventive strategy. Such
restriction can be applied to either the amount of
consumption or the frequency of consumption or
both.
However, despite of its undesirable carioge-
nicity, sugar is very important for the general
health of human as a vital source of energy and
nutrition. Therefore, it is very diffi cult to com-
pletely replace it by other means. Then it comes
the advocacy of the use of sugar, especially
sucrose, substitutes. These sugar substitutes are
sugars in nature but have been found to be hard
for dental microfl ora to use or will produce very
limited amount of acid even if some species have
managed to metabolize them. This special group
of sugars includes those with very high sweetness
such as aspartame, benzoic imine, cyclamate, and
stevia sugar and those with low sweetness such as
xylitol, sorbitol, mannitol, maltose, maltulose,
and many others. Recently, these sugar substi-
tutes have also entered the market for the general
public to choose, where xylitol-containing prod-
ucts (mainly in forms of chewing gum or soft
drinks) dominate.
Also in recent years, studies on isomaltitol are
very hot. Isomaltitol is a mixture of α-d -
glucopyranosyl-1,6- d -sorbitol (GPS) and α-d -
glucopyranosyl-1,1- d -sorbitol (GPM) which are
mixed at a mol ratio of 1:1, which has been found
to be able to hydrolyze into glucose, sorbitol, and
mannitol and is hard to be metabolized by cario-
genic bacteria. Hopefully in the future, it might
bring us a new choice of sugar substitute.
6.4.3 Reinforce Tooth Resistance
to Acid
One important aspect as well as an important pre-
requisite for tooth resistance to acid is that fi rst of
6 Dental Caries: Disease Burden Versus Its Prevention

104
all a tooth must have natural anatomy and proper
amount of inorganic and organic components.
Therefore, before any individual is born, the
proper oral health of the mother is of immense
signifi cance. Clinical observations and clinical
studies have revealed the causal relationship
between gingivitis and periodontitis of the mother
and premature delivery as well as low birth
weight, both of which will have substantial
impact on the tooth formation and maturation for
both the primary and permanent dentitions. Also,
malnutrition of the mother will infl uence such
critical tooth formation process of the child
adversely.
After birth, the fi rst few years serves as the
most pivotal stages for a natural dentition to erupt
into the oral cavity gradually and develop various
physical functions of the oral cavity as well as the
masticatory system. However, in such develop-
mental stage, the permanent dentition is still
under formation, while the primary dentition
remains very vulnerable to local contributing fac-
tors to dental decay. Therefore, maintenance of
proper oral hygiene and good general nutrition is
the only way leading to a healthy full permanent
dentition that will manifest itself years later (usu-
ally at the age of 12) and serve the individual life-
long (under ideal conditions).
However, the other side of the story must not
be neglected. Normal anatomy and composition
are no guarantee of the lifelong service of the
dentition. Local contributing factors, including
cariogenic bacteria and sugar-containing food
consumption, exist in the oral cavity continu-
ously. Weapons we have against the war of dental
decay can be a variety of choices other than sim-
ple personal oral hygiene maintenance. Alongside
with regular dental check-ups, fl uoride in its
various forms can provide potent protection to
our teeth.
From the time of its discovery many years
ago, when it is found to be existing in the drink-
ing water which led to mottled enamel, Dr. Dean
had noticed the reverse relationship between its
existence in drinking water and reduced rate of
caries. Further laboratory studies have convinced
such underlying mechanism. Fluoride is proven
to be able to reduce enamel solubility in acid
solutions because it is able to react with tooth
crystals (mainly exist in the form of hydroxyapa-
tite, HA) and form fl uorohydroxyapatite (FHA)
or fl uorapatite (FA), which are very resistant to
acid. At the same time, fl uoride is able to sup-
press bacterial metabolism of carbohydrates;
therefore, the production of lactic acid is greatly
reduced.
Since the discovery of this potent anticaries
chemical, its various forms have been tested and
proven to be suitable for clinical use. Many dif-
ferent kinds of fl uoride are used worldwide,
among which most frequently used includes
sodium fl uoride (NaF), calcium fl uoride (CaF
2
),
sodium monofl uorophosphate (SMFP), stannous
fl uoride (SnF
2
), and silicon fl uoride (SiF
2
).
Systemic and topical applications of fl uoride
have been widely accepted and promoted.
Systemic application of fl uoride refers to addi-
tion of fl uorides into various kinds of vehicles so
that the effective component fl uoride ion gets into
the body and continuously secreted into the oral
cavity alongside with saliva. Such vehicles which
have come into being in the western industrial-
ized countries for many decades include water
(fl uoridation of water supply), milk, and salt and
agents such as tablets or drips usually adminis-
tered by professionals. However, developing
countries, which do not have adequate resources
or infrastructure (such as water supply equip-
ment), fail to have this means of fl uoride admin-
istration that will benefi t the general public.
On the other hand, topical fl uoride application
serves as an equally effective means of fl uoride
supply. This category involves a variety of appli-
cation methods that are more feasible and fl exible
than systemic fl uoride administration. These
methods include fl uoridated toothpaste, mouth
rinses, varnish, gel, and foams. The fi rst two
methods are easy for personal use at home and
have been widely accepted as important means of
daily oral hygiene maintenance. The rest are usu-
ally applied by professionals.
Many clinical studies have shown very encour-
aging results with regard to reductions on caries
incidence rates. Two to three decades later, epide-
miological surveys have shown a steady decrease
in caries prevalence rates worldwide, indicating
H. Xiao

105
the tremendous achievements by widespread use
of fl uoride in caries prevention dentistry has ever
managed to achieve.
6.4.4 Pit and Fissure Sealing
Pit and fi ssure are created in the process of tooth
development. They have distinct anatomical fea-
tures that render them very much vulnerable to
caries attack. These depressions of occlusal sur-
face (together with the buccal and lingual sur-
faces of the molars) always have the bottom part
at deep enamel, enamel-dentinal junction (EDJ),
or even at the dentine. What’s worse, these nar-
row places are hard to reach with routine exami-
nation and cleaning methods.
As a result, it is not surprising that there is
almost always presence of accumulation of mul-
tispecies microbial biofi lm, food debris, remains
of enamelogenic epithelium, organic plug, etc.
Therefore, pit and fi ssure are most often discov-
ered as the fi rst site of carious lesions of an indi-
vidual. Data of a clinical study show that about
67 % of caries lesions of children at the age of 3
are pit and fi ssure caries. Another epidemiologi-
cal survey of 25,000 school children revealed that
80 % of all diagnosed carious lesions are located
at pit and fi ssure.
In the 1960s, on the basis of research fi ndings
on enamel etching and adhesion, pit and fi ssure
sealant was invented. The underlying strategic
thinking is that if a posterior tooth erupts fully
into the oral cavity and gets all the vulnerable
caries-susceptible sites concealed by dental
material the pit and fi ssure are no longer open to
the oral cavity and therefore will not be infl u-
enced by the microbes that inhabit the mouth and
their dangerous acidic metabolic products. And
this invention has been proven to be a huge
success.
During the following years, the components
of pit and fi ssure sealants have been refi ned
greatly. Now, the most often used sealants are
resin in nature, often belonging to the Bis-GMA
system which has many advantages under clini-
cal condition, such as they are easy to use and
aggregate, and its volume shrinkage after
aggregation is small. However, in recent years
there is also some dispute about its safety.
A great number of clinical studies have veri-
fi ed that pit and fi ssure sealing can substantially
reduce caries onset. Systematic reviews on ran-
domized clinical trials have found similar encour-
aging results. In this sense, pit and fi ssure sealing
is strongly recommended by FDI, ADA, IADR,
WHO, and many other professional organiza-
tions. And in many countries, it has already been
included as part of governmental budget for car-
ies prevention programs.
6.4.5 Preventive Resin Restoration
Preventive resin restoration is actually a variation
of pit and fi ssure sealing. It involves treatment of
susceptible or early carious lesions in pit and fi s-
sure (preservative cavity preparation and restora-
tion with fl uid resin mainly) and combines it with
subsequent pit and fi ssure sealing. This method
has adopted concepts of minimal invasive den-
tistry (MID) into caries prevention. However, its
application is restricted to early caries lesions only.
References
1. Baelum V, van Palenstein HW, Hugoson A, Yee R,
Fejerskov O. A global perspective on changes in the
burden of caries and periodontitis: implications for
dentistry. J Oral Rehabil. 2007;34(12):872–906; dis-
cussion 940.
2. Widström E, Eaton KA. Oral healthcare systems in
the extended European union. Oral Health Prev Dent.
2004;2(3):155–94.
3. Manji F, Sheiham A. CPITN fi ndings and the man-
power implications of periodontal treatment needs for
Kenyan children. Community Dent Health. 1986;3(2):
143–51.
4. Ainamo J, Barmes D, Beagrie G, Cutress T, Martin J,
Sardo-Infi rri J. Development of the World Health
Organization (WHO) community periodontal index of
treatment needs (CPITN). Int Dent J. 1982;32(3):
281–91.
5. Yee R, Sheiham A. The burden of restorative dental
treatment for children in Third World countries. Int
Dent J. 2002;52(1):1–9.
6. Brunton PA, Vrihoef T, Wilson NH. Restorative care
and economic wealth: a global perspective. Int Dent J.
2003;53(2):97–9.
6 Dental Caries: Disease Burden Versus Its Prevention

106
7. Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day
S, Ndiaye C. The global burden of oral diseases and
risks to oral health. Bull World Health Organ. 2005;
83(9):661–9.
8. Petersen PE. Global policy for improvement of oral
health in the 21st century – implications to oral health
research of World Health Assembly 2007, World Health
Organization. Community Dent Oral Epidemiol.
2009;37(1):1–8. doi:
10.1111/j.1600-0528.2008.00448.x .
Epub 2008 Nov 12.
9. Petersen PE. The World Oral Health Report 2003:
continuous improvement of oral health in the 21st
century–the approach of the WHO Global Oral Health
Programme. Community Dent Oral Epidemiol.
2003;31 Suppl 1:3–23.
10. WHO oral health country/area profi le. Geneva: World
Health Organization. Available at URL:
http://www.
whocollab.od.mah.se/index.html
11. Qi XQ. Report of the third national epidemiological
investigation of oral health in China. Beijing: People’s
Health Press; 2008.
12. Kidd EA, Ricketts DN, Pitts NB. Occlusal caries
diagnosis: a changing challenge for clinicians and epi-
demiologists. J Dent. 1993;21(6):323–31.
13. Wenzel A, Larsen MJ, Fejerskov O. Detection of
occlusal caries without cavitation by visual inspec-
tion, fi lm radiographs, xeroradiographs, and digitized
radiographs. Caries Res. 1991;25(5):365–71.
14. Ie YL, Verdonschot EH. Performance of diagnostic sys-
tems in occlusal caries detection compared. Community
Dent Oral Epidemiol. 1994;22(3):187–91.
15. Ekstrand KR, Ricketts DN, Kidd EA. Reproducibility
and accuracy of three methods for assessment of
demineralization depth of the occlusal surface: an
in vitro examination. Caries Res. 1997;31(3):224–31.
16. Loesche WJ, Svanberg ML, Pape HR. Intraoral trans-
mission of Streptococcus mutans by a dental explorer.
J Dent Res. 1979;58(8):1765–70.
17. Côrtes DF, Ellwood RP, Ekstrand KR. An in vitro
comparison of a combined FOTI/visual examination
of occlusal caries with other caries diagnostic methods
and the effect of stain on their diagnostic perfor-
mance. Caries Res. 2003;37(1):8–16.
18. Schneiderman A, Elbaum M, Shultz T, Keem S,
Greenebaum M, Driller J. Assessment of dental caries
with Digital Imaging Fiber-Optic Transillumination
(DIFOTI): in vitro study. Caries Res. 1997;31(2):103–10.
19. Vaarkamp J, Ten Bosch JJ, Verdonschot EH, Tranaeus
S. Quantitative diagnosis of small approximal caries
lesions utilizing wavelength-dependent fi ber-optic
transillumination. J Dent Res. 1997;76(4):875–82.
20. Zero D, Mol A, Sa’ Roriz C, Spoon M, Jacobs A,
Keem S, Elbaum M. Caries detection using digital
imaging fi bre-optic transillumination (DIFOTITM): a
preliminary evaluation. In: Stookey GK, editor. Early
detection of dental caries II. Indianapolis: School of
Dentistry, Indiana University; 2000.
21. Lussi A, Megert B, Longbottom C, Reich E,
Francescut P. Clinical performance of a laser fl uores-
cence device for detection of occlusal caries lesions.
Eur J Oral Sci. 2001;109(1):14–9.
22. Li SM, Zou J, Wang Z, Wright JT, Zhang Y.
Quantitative assessment of enamel hypomineraliza-
tion by KaVo DIAGNOdent at different sites on fi rst
permanent molars of children in China. Pediatr Dent.
2003;25(5):485–90.
23. Alencar CJ, Braga MM, de Oliveira E, Nicolau J,
Mendes FM. Dye-enhanced laser fl uorescence detec-
tion of caries lesions around brackets. Lasers Med Sci.
2009;24(6):865–70. doi:
10.1007/s10103-008- 0572-0 .
Epub 2008 Jun 7.
24. Meller C, Heyduck C, Tranaeus S, Splieth C. A new
in vivo method for measuring caries activity using
quantitative light-induced fl uorescence. Caries Res.
2006;40(2):90–6.
25. Heinrich-Weltzien R, Kühnisch J, Ifl and S, Tranaeus S,
Angmar-Månsson B, Stösser L. Detection of initial car-
ies lesions on smooth surfaces by quantitative light-
induced fl uorescence and visual examination: an in vivo
comparison. Eur J Oral Sci. 2005;113(6):494–8.
26. Stookey GK. Quantitative light fl uorescence: a tech-
nology for early monitoring of the caries process.
Dent Clin North Am. 2005;49(4):753–70, 4. Review.
27. Yin W, Feng Y, Hu D, Ellwood RP, Pretty IA.
Reliability of quantitative laser fl uorescence analysis
of smooth surface lesions adjacent to the gingival tis-
sues. Caries Res. 2007;41(3):186–9.
28. Feng Y, Yin W, Hu D, Zhang YP, Ellwood RP, Pretty
IA. Assessment of autofl uorescence to detect the rem-
ineralization capabilities of sodium fl uoride, mono-
fl uorophosphate and non-fl uoride dentifrices. A
single-blind cluster randomized trial. Caries Res.
2007;41(5):358–64.
29. Pretty IA, Maupomé G. A closer look at diagnosis in
clinical dental practice: part 5. Emerging technologies
for caries detection and diagnosis. J Can Dent Assoc.
2004;70(8):540, 540a–540i.
30. Muller-Bolla M, Courson F. Toothbrushing methods
to use in children: a systematic review. Oral Health
Prev Dent. 2013;11(4):341–7. doi:
10.3290/j.ohpd.
a30602
.
31. Gibson JA, Wade AB. Plaque removal by the Bass and
Roll brushing techniques. J Periodontol. 1977;48(8):
456–9.
32. Bergenholtz A, Gustafsson LB, Segerlund N, Hagberg
C, Ostby N. Role of brushing technique and tooth-
brush design in plaque removal. Scand J Dent Res.
1984;92(4):344–51.
H. Xiao

107
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1_7
Clinical Management of Dental
Caries
Li Jiyao
Dental caries can be classifi ed clinically as
cavitated and noncavitated lesions according to
substantial damage to tooth morphology. The
non-operative approach is usually used for non-
cavitated lesions or as a preventive measure for
susceptible teeth. For caries with substantial loss
of dental tissue, an operative measure such as res-
toration is taken. The development of material
science provides a variety of choices for dental
caries management.
Dental restoration is an operation on an organ
with unique biological properties, which involves
theoretical knowledge such as mechanics, biol-
ogy, materials science, aesthetics, etc. It is possi-
ble that the pulp–dentin complex could be
irritated or damaged in the process of treatment;
hence it is vital and important to protect the
dentin and pulp throughout the process.
7.1 The Development of Caries
Treatment Theory
At the end of nineteenth century, G.V. Black cre-
ated a dental restoration system according to the
susceptible sites and dental anatomical relation-
ship of dental caries, which involved the clinical
demands of retention and resistance, in addition
to the property of amalgam, and laid the founda-
tion for modern restoration dentistry. In the
1950s, Buonocore introduced the acid-etching
technique into restorative dentistry. Through the
acid-etching technique, resin composite was able
to bind with dental hard tissue with a mechanical
lock. The binding mechanism was different from
amalgam. From then on, restorative dentistry
entered the new age of “adhesive dentistry.” After
the twenty-fi rst century, restorative dentistry
entered the era of microdentistry, by proposing
the concept of early diagnosis, prevention, and
micro treatment; the operative dentistry was
directed toward a biological approach, to prevent
the development of caries and to preserve healthy
dental tissue as far as possible [
1 ].
7.1.1 G.V. Black and Modern
Restorative Dentistry
G.V. Black made a huge contribution in forming
the concept and principles of modern dental res-
toration. At the end of the nineteenth century,
with a rigorous scientifi c attitude, he carried out
L. Jiyao
Department of Operative Dentistry and Endodontics ,
West China Hospital of Stomatology ,
No. 14, 3rd Section, Renmin Road South ,
Chengdu 610041 , Sichuan Province , China
e-mail:
7

108
an in-depth investigation into the damage of car-
ies in susceptible sites and its relationship with
dental anatomy. Combining the demands of
retention and resistance required by restoration,
in addition to the property of amalgam, he cre-
ated a complete restoration system. The system
explained the corresponding principle and devel-
oped a series of basic principles and requirements
of cavity preparation, material mixing, and fi ll-
ing. Restoration dentistry then embarked upon a
scientifi c, standardized pathway and the founda-
tions of modern restoration dentistry were laid.
The content of restoration dentistry proposed
by G.V. Black was to prepare the cavity upon the
already weakened dental tissue or after removing
the damaged dental tissue base on the principle of
retention, resistance, and protection of the pulp–
dentin complex and then restore its original form
and function with a specifi c material through a
certain process.
The knowledge of cavities is a major part of
G.V. Black's contribution to restoration dentistry.
According to the caries location, and in combina-
tion with the tooth structure, in addition to the
characteristics of the design and preparation, he
divided cavities into fi ve categories, which gener-
ally covered the basic types of caries. Two more
types were added afterward to supplement them.
Clinically, this category is still being widely
used.
According to the properties of amalgam, he
also proposed a principle based on the demands
of auxiliary retention and resistance of cavity
preparation, such as the depth, contour of the
cavity, a cavity with a dovetail, a ladder-shape
retention form, the undercut, and the removal of
weakened enamel. He also proposed certain
demands for each category of cavity. The descrip-
tion of these systems laid the foundations for the
development of modern restoration.
7.1.2 Adhesive Bonding Technique
and Dental Restoration
The development of dental restoration and that of
dental material are inseparable. At the end of
the nineteenth century, G.V. Black created a
scientifi c, standardized restorative system, using
mechanical retention. In 1955, for the fi rst time,
Buonocore used acid to process the enamel sur-
face, to increase the bonding between composite
resin and the tooth surface and started to revolu-
tionize dental practice. As the resin material and
bonding material developed, the concept of resto-
ration had already been changed; the preservation
of more dental tissue was widely accepted as a
bonding technique.
In the past 20 years, there has been a great leap
in the development of resin composite material
and the acid-etching technique has matured.
Bonding systems for dentin have been continu-
ously updated, including total etching and self-
etching techniques and the procedure was
simplifi ed as well. The clinical application of
composite resin has become increasingly wide-
spread and the effectiveness of bonding restora-
tion has also been proven because of its long-term
results.
The bonding technique revolutionized tradi-
tional dental restoration. In terms of cavity
design and preparation, it preserved more
healthy dental tissue by discarding the sacrifi ce
certain depth of cavity and dove tail, ladder-
shaped retention form. Combined with the the-
ory of bonding resin composite, more careful
preparation of the cavosurface angle and bevel
was required.
7.1.3 The Foundation and Principle
of Minimally Invasive Caries
Treatment
Traditional dental restoration was based on the
principle created by G.V. Black in 1908, remov-
ing a lesion by operation and then restoring the
damaged part. This approach was launched based
on the foundation of amalgam properties, which
could certainly cause vast damage of dental tis-
sue. In the twenty-fi rst century, modern den-
tistry suggested a more reasonable theory, which
was minimally invasive treatment. Minimally
invasive treatment is a branch of preservation
dentistry. In the literature, terms such as mini-
mal intervention dentistry, minimally invasive
L. Jiyao

109
dentistry, and micro dentistry were used. In the
Modern Oxford Dictionary, minimally invasive
dentistry has the same meaning as microden-
tistry. MI is an abbreviation of minimal inter-
vention dentistry, but not minimally invasive
dentistry [ 2 ]. Tyas et al. believed that minimal
intervention dentistry focuses on the knowledge
of how caries develop, including early diagnosis,
prevention, and treatment, and placed emphasis
on the treatment switch from dental operation to
biological method, to prevent the development of
dental caries and preserve as much healthy den-
tal tissue as possible. Peters et al. believed that
minimally invasive dentistry focuses on the pres-
ervation of healthy dental tissue when removing
caries lesions, instead of the “extend to prevent”
principle of G.V. Black.
In terms of the biological study of dental tis-
sue, in addition to the etiology of caries, espe-
cially the process of remineralization, the
revolution of diagnosis measurement, and the
novel view of prevention, the development of
dental material has laid the foundation for mini-
mally invasive dentistry.
Martin et al. proposed four basic principles for
minimal invasive dentistry: lesion control, remin-
eralization of early caries, minimal surgical
trauma, and restoration, but not replacement, of
dental caries lesions.
Modern material science proved that dental
restoration material could not match the healthy
dental tissue in terms of physical, mechanical, and
biological properties. Removing healthy dental
tissue and restoring the cavities with traditional
fi lling material certainly could not meet the func-
tional requirements. With the development of
adhesive material, in addition to the traditional
chemical dentin bonding, there was also microme-
chanical bonding in the hybrid layer. The dentin
bonding system developed from no-etching bond-
ing to total-etching bonding (1980s), total-etching
wet bonding (1990s), self-etching primer adhesive
systems (1990s–2000), and then one-step adhe-
sive systems (2000s). The micromechanical bond-
ing mechanism produced solid bonding with
dental tissue, decreased the sacrifi ce of healthy
dental tissue to obtain suffi cient retention, and
decreased the chance of microleakage.
In the past, the process from the demineraliza-
tion of dental hard tissue to the degradation of
dentin collagen and fi nally the formation of cav-
ity was generally considered irreversible. As a
matter of fact, the process of dental tissue gain-
ing and losing calcium and phosphate ions was
carried out alternately; i.e., a demineralization–
remineralization cycle on the surface of the
tooth. When the pH of the interface between den-
tal tissue and plaque was below 5.5, it showed
that the enamel and dentin demineralized. As the
pH recovered, it showed remineralization.
Fluorine played an important role in this cycle. It
could strengthen the absorption of calcium and
phosphate and form fl uorapatite on the tooth sur-
face. Fluorapatite is highly acid-resistant; demin-
eralization starts at pH < 4.5, but when in
fl uorine- containing environments, remineraliza-
tion of the tooth and anticarious ability are
greatly enhanced.
For now, the method of diagnosis for detect-
ing the different statuses of caries, such as early
demineralization, small caries lesions, and small
cavity were varied, including bacterial counts,
saliva buffering capacity, fi ber-optic transillu-
mination technology, electrical impedance tech-
nology, laser fl uorescence technique, nonvisible
light imaging technology, etc. These techniques
were particularly used for diagnosing hidden
caries, early caries, proximal surface caries, and
caries in pits and fi ssures, which greatly
enhanced the sensitivity and accuracy of early
diagnosis.
The new idea about prevention has high-
lighted the importance of the interaction
between doctors and patients when a dental
operation switches to nonsurgical treatment. It
is widely believed and accepted that caries are
initially reversible, mainly caused by bacterial
infection, and constitute a multi-factorial
chronic disease. Treatment from the doctor is
merely a small part of the complete treatment, to
fulfi ll caries prevention; long- term cooperation
between doctors and patients must be built to
achieve that.
New techniques of prevention include enamel
angioplasty, pit and fi ssure sealant, and preventive
resin restorations; the purpose is to retain as
7 Clinical Management of Dental Caries

110
much of the healthy tooth structure as possible
and change the conditions for the development of
dental caries, thereby reducing the incidence of
dental caries.
Modern caries treatment pays more attention
to the biological response of the pulp–dentin
complex. At the same time, the relationship
between the restored tooth and periodontal
health, between occlusion and periodontal health,
and the proximal contact between the prosthetic
and the adjacent teeth also needs to be consid-
ered. Minimizing patients’ anxiety and pain
caused by the fear of dental treatment should also
be considered.
Atraumatic restoration, sandblasting caries
removal, chemical–mechanical caries removal,
the laser treatment of dental caries, and other
new technologies, have overcome the excessive
loss of the healthy tooth structure caused by tra-
ditional dental drilling, which can easily cause
complications when near pulp, especially in the
children with an extreme fear of dental treat-
ment, and the risk of cross-infection. New tech-
niques such as amalgam bonding, tooth colored
material, composite resin inlay, use new materi-
als or technologies in cavity restorations, making
it possible to get rid of the traditional preventive
expansion during cavity preparation.[ 3 ]. The
concept of a minimally invasive dental treat-
ment plan is refl ected in the following three
aspects:
1. Early diagnosis and personal treatment.
Through individual caries risk assessment
such as plaque and saliva, and evaluation of
Streptococcus mutans , in addition to early car-
ies monitoring, the establishment of personal-
ized dental fi les, the enhancement of patient
management, an increase in patient communi-
cation, and improvement of patient loyalty.
2. Treatment and effective control. Based on the
diagnosis to build up prevention treatment
programs, to implement minimum interven-
tion, and prevent secondary caries, using
smart materials, to treat rather than simply
replace the damaged tissue, to maximize the
preservation of dental health.
3. Focused prevention and effective interven-
tion. Control of cariogenic bacteria, plaque
control, reduce the amount of sugar intake,
and improve salivary function. Explain to
patients the causes of dental caries, correct-
ing the patient’s poor eating habits and oral
hygiene habits, guide patients to benefi t from
professional dental methods, and to control
the demineralization and promote remineral-
ization. Implement the concept of profes-
sional dental care and early caries
treatment.
Minimally invasive dental operation process:
patient visits; diagnosis through treatment plan-
ning system, assess caries risk, prevent caries
activity through the history of dental caries, gen-
eral oral condition, and to confi rm treatment the
plan by performing observation, prevention, or
treatment.
Lesion control is a prerequisite for successful
remineralization and fi lling. To control lesions,
the presence of pathogens fi rst needs to be con-
trolled. At the early stage of the lesion, through
the effective use of modern diagnostic techniques
and prevention systems to control bacteria before
irreversible loss of dental tissue, thus preventing
the development of dental caries. For patients at a
high risk of caries, the use of mouthwash, a
change of bad oral habits, eating habits, etc. will
be needed to control bacteria or adjust the pH of
the saliva, fl ow, and viscosity to achieve the goal
of caries prevention.
Minimal surgical intervention is the most
effective method of restoration of tooth structure.
When caries progress to dentin, the enamel layer
forms cavities. Plaque accumulates in the cavi-
ties, which affects calcium, phosphorus, fl uoride
ion uptake, and is diffi cult to remove.
Remineralization repair is then unlikely to suc-
ceed, and only by the use of dental surgical meth-
ods. The cavity can be roughly divided into two
layers from outside to inside:
1. Infected layer: this layer of the tooth structure
has been completely denatured and bacteria
settled.
2. Demineralized layer: this layer has a certain
level of demineralization, but the collagen scaf-
fold still exists and can be re-mineralized. In
the past it was thought that the demineralized
L. Jiyao

111
layer should be removed, but recent studies
suggest that the demineralized layer is a pre-
carious status instead of caries-active status.
With the promotion of mineralized material to
repair, this layer can be remineralized.
Therefore, the modern view is that the removal
of diseased tooth structure should be limited to
the infected layer.
Based on the understanding regarding the
early diagnosis of dental caries, focused pre-
vention, and minimally invasive treatment, the
twenty-fi rst century has ushered in the era of
the minimally invasive treatment of dental
caries.
7.2 Current Management
of Dental Caries and Its
Development
Under the infl uence of minimally invasive den-
tistry, dental caries treatment has shifted from
dental surgery to biological treatment.
According to the progressive stages of decay,
the development in the treatment of dental car-
ies focuses on the prevention and management
of people susceptible to dental caries, nonsurgi-
cal treatment at an early stage, and the surgical
treatment of the cavity. Accordingly, dental car-
ies treatment technology has also developed
considerably. The clinical application of the
minimally invasive treatment of dental caries
and the systematic evaluation of the effect of
composite resin restoration has provided the
foundation for the application of new technolo-
gies and materials. Current dental caries treat-
ment should be an optimized treatment regime,
using the best technology to achieve the best
treatment outcomes.
7.2.1 Minimally Invasive Treatment
Technique
The minimally invasive treatment of dental caries
emphasizes the preservation of as much of the
tooth structure as possible, keeping surgical
intervention to a minimum. Broadly speaking, all
of the caries treatment techniques should
emphasize a conservative approach. The follow-
ing briefl y summarizes the minimally invasive
treatment techniques; the nonsurgical treatment
technologies are also included. These techniques
are mainly used in the early stages where dental
caries have not yet developed, in people suscep-
tible to dental caries, and in the prevention of car-
ies in sensitive areas.
7.2.2 Minimally Invasive Cavity
Preparation
7.2.2.1 Nonmachinery Preparation
Air Abrasion Air abrasion was fi rst introduced
in 1945 with the fi rst air abrasion machinery
available in 1951. The air abrasion system has
evolved greatly since then. The principle of air
abrasion is to apply highly pressurized, non-
toxic particles, such as aluminum oxide ions, to
accurately remove the enamel, dentin, carious
tissue, and old fi llings. Air abrasion can par-
tially replace the high-velocity gas turbine cav-
ity preparation. It is quieter, more time- and
energy-effi cient, and requires no anesthesia as it
does not produce vibration and heat. It is well
received by patients and maximizes the conser-
vation of the tooth structure. The interior of the
prepared cavity is smooth, making it easier to
fi ll. It reduces the stress between the fi lling and
the tooth structure, reduces the likelihood of
microfracturing, hence prolonging the life of the
fi xture.
To aid early the detection of caries on the
occlusal surface, the blackened part of the cav-
ity should be examined. After visual examina-
tion, the air abrasion system aims strong
abrasive particles toward the affected areas in
the cavity. If the blackened area is a stain, it can
be removed with ease. If it is accompanied by
decay, a beam of strong abrasive particles can
reveal and blast the stain away in addition to the
decay. As dentin is moist, thick, and elastic, the
particles bind on its surface rendering it unable
to exert its effect. Hence, it should be removed
manually or using a mechanical machine before
using air abrasion.
7 Clinical Management of Dental Caries

112
The disadvantage of this method is that
because it is easier to remove dentin than enamel,
it causes the overhang of enamel, which requires
trimming of the enamel with the drill.
Contraindications to air abrasion include patients
with:
1. A severe allergy to dust, asthma, and chronic
obstructive pulmonary disease.
2. Open wound or recent tooth extraction.
3. Active periodontal disease.
4. Recent placement of an orthodontic
appliance
5. Subgingival caries
Air Polishing Air polishing delivers high-
pressure jet of sodium bicarbonate to the surface
of the tooth, producing a cutting effect. Air pol-
ishing differs by using an aqueous friction solu-
tion, which does not cause a signifi cant amount
of sodium bicarbonate aerosol. This technique
was originally designed for stain removal and it is
now also used to remove crown fi llings. Air pol-
ishing is not very selective when cutting the tooth
structure and can damage healthy dentin and
cementum. It is mainly used in the fi nal prepara-
tion of the caries to remove any remaining
decayed dentin.
Lasers Lasers can be used to perform surgeries
on dental soft tissues as they do not cut through
dental hard tissues. Hence, surgeries involving
hard tissues need to employ other types of lasers.
The ideal laser should be able to manage both
dental hard and soft tissues. Clinically used lasers
that can cut through dental hard tissues include
erbium:yttrium-aluminum-garnet (Er:YAG), car-
bon dioxide (CO
2
), neodymium:yttrium-
aluminum- garnet (Nd:YAG), and Ar:F. They all
have selective abrasive properties whilst conserv-
ing healthy tooth tissue. Er:YAG is the most
selective of all lasers. Laser cavity preparation is
precise, nonvibrating, has no smell, and does not
require anesthetics. As lasers can seal dentinal
tubules, they can also prevent hypersensitivity
postoperatively. On the downside, the machinery
is bulky and expensive, thus limiting its role in
clinical practice.
Chemomechanical Caries Removal
Chemomechanical caries removal (CMCR) uses
chemical agents to soften the dental tissues
before eliminating infected tissue using machin-
ery. In 1985, the fi rst CMCR system, Caridex,
was introduced into dentistry. Caridex involves
the intermittent application of pre-heated
N-chlorinated-DL-2-amino butyric acid
(GL-101E) into the cavities. This solution causes
the partial disintegration of the collagen in the
cavity, accelerating the removal of dental caries.
The uptake of this technology was not great as it
is expensive, time-consuming, and requires a lot
of additional equipment, including memory cell,
a heater, pump, and special hand piece.
Recently, CMCR has been re-introduced to
the dental industry, providing a new alternative to
caries removal and preparation. A new CMCR
system, Carisolv was introduced – Carisolv gel
and Carisolv hand tools. The Carisolv reagent
consists of two component mixtures. One of the
component mixtures is a red gel composed of
leucine, lysine, glutamic, and sodium hypochlo-
rite. After applying the mixed Carisolv reagent to
the cavity, a hand tool can be used to remove the
softened carious tissue. This method can selec-
tively dissolve carious tissue quickly (around
30s), whilst not affecting any healthy dentin.
Compared with other caries removal techniques,
CMCR can effectively remove the smear layer of
the cavity, reinforce the bond between the fi lling
and the tooth, there is no noise, vibration or anes-
thetics, and patient acceptance is high. However,
when compared with the high-velocity turbine,
the operating time is longer and requires alterna-
tive methods to gain access to and repair some
undermining caries.
The CMCR procedure can handle most dental
caries. It can be used alone or combined with
other traditional caries removal techniques. In
those caries that cannot be directly accessed or
have existing fi llings, CMCR can be used in con-
junction with a dental drill to gain access to the
cavity area or remove the old fi lling, before using
Carisolv to remove the caries.
The CMCR method should be fi rst considered
for the following patient group: root/cervical
caries, coronal caries (especially deep coronal
L. Jiyao

113
caries), caries located on the edge of the crown or
bridge abutment, completion of canal prepara-
tion, those in whom anesthetic is contraindicated,
especially needle-phobic patients, those with a
dental phobia, and pediatric patients.
7.2.2.2 Mechanical Rotary Technique
The mechanical rotary technique uses high-
velocity turbine hand tools to prepare dental cavi-
ties. To maximize healthy tooth conservation, burs
in proper size are chosen in practice in relation to
the principles of minimally invasive dentistry.
Tunnel Preparation Tunnel preparation refers
to the occurrence of caries located on the proxi-
mal surface of the teeth, if the lesion is more than
2.5 mm apart from the marginal ridge, cavity
preparation can enter from the occlusal surface,
to maintain the integrity of the marginal ridge but
it is not prepared as the classical proximal–occlu-
sal cavity. If the tooth surface that is adjacent to
the lesion has demineralized but not actually
damaged, and it is necessary to maintain the
integrity of its surface, this is also called internal
preparation. Advantages of this method are:
1. The marginal ridge of the tooth can be kept
intact, thereby enhancing the resistance of the
remaining tooth structure.
2. Injury of the proximal surface of the adjacent
tooth is avoided during cavity preparation.
3. Normal contact relationship with the adjacent
tooth is maintained.
4. Overhanging of repair materials is prevented.
Slot Preparation
Slot preparation, also known
as mini box preparation, is designed mainly for
proximal caries. It can be divided into the follow-
ing cases:
1. Caries is close to the marginal ridge and can-
not preserve its integrity or the marginal ridge
has been destroyed.
2. Caries is located underneath the interproximal
contact. Designing of cavity preparation
focuses on carious tissue; if the marginal ridge
cannot be preserved, the cavity is only pre-
pared into a box shape without a dovetail.
When the caries is underneath the interproxi-
mal contact, carious tissue is approached from
the buccal or lingual surface, and prepared
into a box or disk shape with the auxiliary
help of groove retention. Then glass ionomer
or composite resin is used for restoration. In
most cases, this method can provide normal
interproximal contact between adjacent teeth.
Microscopic Preparation Techniques
To mini-
mize invasive trauma, the technique of using
micro drills for microscopic preparation, under
the microscope or loupe, to obtain precise tooth
preparation is called “microscopic dentistry.”
Micro drills can be round, oval, and conical, and
the operator can select different drills for fi ssure
caries preparation and fi nishing. The shank of the
burs is longer than that of traditional ones; thus,
the operator’s sight is not blocked by the head of
the hand piece. Using this drill together with
tooth-colored bonding materials for repairs can
better refl ect the purpose of minimally invasive
treatment.
7.2.2.3 Minimal Invasive Prevention
Technique
Pit and Fissure Sealing Pit and fi ssure sealing
is an effective method of preventing fi ssure car-
ies. The occlusal surface of the tooth formed a
different shape and varying depths of the fi ssure
during development, and oral bacteria, metabo-
lites, and food residue often accumulate at caries
predilection sites. Pit and fi ssure sealants may
isolate the fi ssure and the oral environment, pre-
venting bacteria and food debris from entering,
to achieve the purpose of caries prevention.
Fluoride sealants can continuously release fl uo-
ride, provide barriers, and promote
remineralization.
Pit and fi ssure sealing is mainly used for suspi-
cious fi ssure and pit caries as well as deep grooves
adjacent to fi lled fi ssure caries on the occlusal
surface. Sealant is mainly made of resin, a dilu-
ent, an initiator, and a number of auxiliary com-
ponents, such as fi llers, fl uoride, dyes, and other
components. The main body of sealant resin
material, bisphenol-A-glycidyl methacrylate
7 Clinical Management of Dental Caries

114
(Bis-GMA), is a commonly used resin with good
performance.
Enameloplasty Noncaries fi ssures were drilled
out to form a shallow plate shape, making it easy
to clean and keep free from caries, i.e., enamelo-
plasty. This method removed minimal tooth
structure, but produced lasting anti-caries effects,
and no worries regarding fallen sealer. A simple
operation, with few technical requirements. The
suitable depth of the fi ssure is no more than half
the enamel thickness. Using a high speed hand
piece with a pear-shaped diamond, ball dia-
monds, or a sand stone point bur the site should
be gently removed. For the grooves on the cusp
surface, a diamond bur can be used to remove
evenly parallel to the surface, until the groove is
no longer brown. Enameloplasty can be used on
fi ssures that are close to or across the molar buc-
cal lingual ridge. The prepared cavity can be
extended to 2 mm away from the buccal–occlusal
junction or lingual–occlusal junction, and the rest
can undergo enameloplasty, but not following the
G.V .Black principle, which extended to the buc-
cal and lingual surface.
Preventive Resin Restorations In 1977,
Simonsen suggested performing “preventive
resin restorations” to treat suspicious fi ssure car-
ies and provides a new approach to the treatment
of fi ssure caries. Preventive resin restorations
only remove the infected enamel or dentin at the
lesions, according to the size of the caries, using
etching technology and the resin material fi lling
up the early fi ssure caries, and the occlusal sur-
face coated with sealant. It is a preventive mea-
sures combined with pits and fi ssure sealing and
fi ssure caries fi lling. Because it does not use the
traditional extension for prevention, only a
amount of carious tissue is removed and restored
with composite resin or glass ionomer, the pit and
fi ssure caries without caries is protected by the
sealant, thus preserving more healthy dental tis-
sue, and is an effective method for preventing the
further development of caries.
The advantage of preventive resin restorations
is using glass ionomer composite resin as fi lling
and binding with enamel mechanically or
chemically, then bonding with sealant by
chemical bonding reduces the possibility of
generating micro-leakage.
Caries Pharmacotherapy Caries pharmaco-
therapy is the use of drug treatment to stop the
development of caries, or get rid of superfi cial
caries. Drug therapy is mainly applied to early
enamel caries in teeth on a easy-to-clean smooth
surface (such as buccal, lingual), on which cavi-
ties have not yet formed; superfi cial caries of
anterior primary teeth on the proximal surface;
secondary enamel hypoplasia, which causes
extensive shallow caries, and diffi culties with
prepare cavities preparation.
Fluoride, such as 75 % sodium fl uoride and
glycerol paste, 8 % stannous fl uoride solution,
2 % sodium fl uoride, sodium monofl uorophos-
phate solution, or a fl uoride gel are commonly
used drugs for the treatment. By local applica-
tion, these fl uorides can penetrate into the enamel
to form insoluble acid fl uorapatite, and promote
remineralization of enamel, and also to prevent
bacterial growth, inhibit bacterial metabolism,
acid and polysaccharide synthesis. Fluoride has
no corrosive stimulation to soft tissue, no tooth
discoloration, and is considered safe and
effective.
The main agents of silver nitrate are 10 % sil-
ver nitrate solution or ammonia silver nitrate
solution. Silver nitrate is a strong corrosive agent,
which can combine with proteins to form precipi-
tates. When in low concentrations, it provides
convergence and an antibacterial effect; in high
concentrations it has a strong corrosive effect and
can kill bacteria. When it is applied to zones of
caries, with the addition of clove or 10 % forma-
lin solution, it can turn into black reduced silver,
and with the addition of 25 % iodine it results in
off-white silver iodide. Both of these two formu-
lations can penetrate into the enamel and dentin
to coagulate proteins, kill bacteria, plugging gaps
in enamel and dentin tubules, thus blocking and
terminating the development of the caries lesion.
Remineralization Treatment
For early enamel
caries that have been demineralized and softened,
the appropriate drug treatment to re-deposit
L. Jiyao

115
calcium and remineralize, thereby removing its
hardness and eliminating caries, is called remin-
eralization treatment.
Early enamel caries on the smooth surfaces
(buccal, labial, lingual, palatal or proximal), such
as white spots, and people susceptible to caries
are suitable for remineralization therapy.
There are many types of mineralized fl uid,
which divided into single component and com-
plex components. The single component is
mainly fl uorine-containing (e.g., NaF: 0.2 g;
DH
2
O: 1,000 ml), the complex component
mainly containing different ratios of calcium,
phosphate, and fl uoride salts, while calcium or
fl uoride salt is the main ingredient (e.g., CaCl:
9 g; KH
2
PO
4
: 6 g; KCl: 1.1 g; KF0.2 g; DH
2
O:
1,000 ml).
In recent years, a new remineralization agent,
CPP-ACP, has been used clinically. Casein
(casein phosphopeptide, CPPs) used casein as a
raw material, by hydrolysis, separation, and puri-
fi cation to obtain a class of phosphoserine-rich
bioactive peptides. Under neutral or alkaline con-
ditions, CPPs can form soluble chelates with the
amorphous calcium phosphate (ACP), i.e., casein
phosphopeptide-amorphous calcium phosphate
(CPP-ACP). CPP-ACP has a wide range of appli-
cations in biology, including the promotion of
remineralization of the tooth surface and bone
calcifi cation, promoting the absorption of miner-
als, and has an effect on cariogenic bacteria.
Currently, CPP-ACP is used in the treatment of
early caries remineralization of dentin hypersen-
sitivity, dental erosion treatment, and as preven-
tion in caries-susceptible patients. The
remineralizing agent with CPP-ACP as the main
ingredient shows broad application prospects in
caries prevention.
Atraumatic Restorative Technique
Atraumatic
restorative technique (ART) refers to using only
hand instruments such as a spoon excavator to
remove caries tissue, then using glass ionomer
cement or other cementitious fi lling materials for
repair.
The ART meets the requirements of modern
minimally invasive restoration, the use of a
bonding of glass ionomer materials, with as
little cavity preparation and tooth damage as
possible, and the best preservation of tooth
structure.
7.3 Current Silver Amalgam
and Techniques for Direct
Restorations [
4 ]
7.3.1 The Controversy Over Silver
Amalgam
Silver amalgam has a history of more than 160
years as a dental restorative material. Because it
has the ideal wear resistance, inoxidizability,
good mechanical strength, and it is inexpensive
and simple to operate, silver amalgam also dis-
plays great longevity and a low rate of secondary
caries in posterior teeth. Although silver amal-
gam is not satisfactory with regard to aesthetics
and conducts heat and electricity, it is still widely
applied in dental practice.
In China, it was recorded that silver paste fi ll-
ing has been in use since the Tang Dynasty. In
1826 in France silver amalgam was used for tooth
repair; in the mid-1930s, it started being applied
in American dental practice. With the develop-
ment of material preparation and the improve-
ment in property, the silver amalgam in restorative
dental material has obtained the approval of
many international health organizations, includ-
ing the WHO.
However, there was doubt regarding silver
amalgam and its application in tooth repair
because of the mercury content. There have been
reports that mercury can cause adverse effects,
such as kidney function damage, reduced immu-
nity, nerve toxicity, etc. However until now, there
still has been no evidence reporting the direct
causal relationship between silver amalgam use
and adverse effects. Moreover, there are also
cases of silver amalgam allergy, the main mani-
festations of which are a lichen planus-like
response affecting the local mucosa and the
occurrence of skin erythema, but this type of
allergy is only limited to the region adjacent to
the silver amalgam, and had no obvious effect on
the body; moreover, the symptoms may be
7 Clinical Management of Dental Caries

116
relieved after the elimination of the silver
amalgam restoration, with no need for special
treatment.
From the perspective of public health, protec-
tion from silver amalgam pollution should be
strengthened, and the presence of mercury vapor
in the clinic should be closely monitored.
Nowadays, the mixing of silver amalgam has
been automated, closed off, and there is minimal
mercury vapor pollution in the oral clinic.
It is noteworthy that with the continuous
development of dental restorative materials and
technology, the new idea of a minimally invasive
technique has been put forward in the twenty-fi rst
century, and the status of silver amalgam in tooth
repair has changed. Because of the shortage of
the physical and chemical properties of compos-
ite resin and glass ionomer cement tooth-colored
material, it still cannot completely replace the sil-
ver amalgam in posterior tooth repair at present,
although some research has been carried out that
has shown that the longevity of composite resin is
greater than for the same sized silver amalgam
restoration [ 5 ].
7.3.2 Indications
and Contraindications
Silver amalgam can be used in almost all poste-
rior teeth restoration, unless the preparation is not
appropriate for the retention form, or if there is a
mercury allergy. However, with composite resin-
bonded fi xed technology widely used in dental
restoration, the developed countries in Europe
have been gradually reducing the use of amal-
gam. It is believed in the following situations that
amalgam is preferred: a large complicated cavity
in a molar, the need to restore the tooth cusps,
and direct restorations of teeth. If retention is
expected to increase, the bonded amalgam should
preferably be used and/or a box-shaped, grooved,
circular retention groove retention, and then con-
sider dentin pins should be considered. If there is
large area of crown damage, the cementing mate-
rial post and core restoration is superior to the
silver amalgam. Afterward, the entire crown is
used to cover it. Moreover, the use of cementing
material is not suggested for repairing cracked
spots in silver amalgam restoration. The silver
amalgam may also be used as post and core mate-
rial. Then, the amalgam-fractured parts repair,
the repair parts are not comfortable using a bond-
ing material.
However, it is not recommended to use silver
amalgam in the following situations: early tooth
decay; premolar and molar occlusal surface or
adjacent surface cavity of small to medium size;
premolar large occlusal surface or interproximal
caries; all tooth neck cavity repair; root canal fi ll-
ing; pregnant women.
7.3.3 Silver Amalgam Restoration
Technique
7.3.3.1 Cavity Shape Preparation
The principle of cavity shapes of silver amalgam
restorations consists of removing the decayed tis-
sue, outlining the cavity border, removing all
unsubstantial enamel and sharp ridges, empha-
sizing the protection of the dental pulp–dentin
complex and the functional reconstruction after
fi lling, without damaging the periodontal
tissues.
The cavity shape for silver amalgam restora-
tion requires a high level of resistance and reten-
tion. It is the basic requirement that tooth tissues
and silver amalgam restorations should not frac-
ture or deform and restorations should not be
taken off, shift, under normal chewing forces
with proper chewing movements.
The amalgam restorations must have a certain
thickness to ensure basic resistance. The depth of
the occlusal cavity of posterior teeth should be at
least 1.5–2 mm. The distance of healthy tooth tis-
sue between two amalgam restorations on the
same tooth should be at least 1 mm.
7.3.3.2 Silver Amalgam Filling
After the cavity preparation the rubber dam and
suction are used to isolate the operation area from
moisture and saliva.
To confi ne the silver amalgam to the cavity
and complete the compression backfi ll, a box-
shaped cavity with a restrictive wall and bottom
L. Jiyao

117
is needed. For a class I cavity on only one tooth
surface, the structure itself has satisfi ed this
request. However, for a cavity involving two or
more surfaces, it is essential to use a matrix bond
surrounding to turn it into a box shape. Before
the fi lling, the matrix bond is used to develop the
artifi cial wall. On one hand, it is conducive to the
compressive backfi ll of silver amalgam; simulta-
neously, it may also effectively prevent the silver
amalgam from overfl owing and periodontal
overhang, which stimulates the periodontal
tissue.
For a class II cavity of proximal tooth dam-
age, the use of a forming piece is extremely con-
venient if it has proximal tooth support, but for
severe tooth body damage, the proximal tooth
cannot play a supportive role; thus, other meth-
ods are needed to assist. The wedge is a com-
mon piece of auxiliary equipment used in
the forming of the proximal surface. When the
matrix band cannot fi t the tooth neck well, the
wedge may be inserted between the necks of
the teeth. Because the matrix band with the teeth
closed, in the case of the silver amalgam pressed
out to form an overhang from the gingiva. The
shape and size of the wedge should be appropri-
ate. If it is too small it is not easy to set tight,
while if it is too big it will affect the adjacent
shape of the restoration.
After cleaning, disinfecting, and drying, the
cavity can be fi lled. Before silver amalgam fi ll-
ing, coating and painting can reduce the occur-
rence of secondary caries; the commonly used
varnish is made from resin materials. Using the
silver amalgam autostirrer completes the attri-
tion evenly. When backfi lling, the principle of
little by little fi lling, of layer upon layer com-
pression, and of the dotted angle to the line angle
should be followed. The backfi ll process makes
silver amalgam go into the cavity and fi t with the
tooth body closely, and a silver amalgam plug-
ger is used to carry out the process. When com-
pression causing the unnecessary mercury to
squeeze out and causing the air which mixes
with it to discharge, it is more advantageous to
the completion of the operation, thus strengthen-
ing the intensity of the restoration and the
mechanical properties. In class II cavity fi lling
of the side of the cavity shape, the surgical prin-
ciple is lateral compression, and layer upon
layer compaction. When the cavity shape is
large, more attention should be paid to the prin-
ciple of layer upon layer compression. It is gen-
erally believed that a thickness of 1 mm is able
to provide full compression. When applying
pressure, in addition to considering the vertical
direction, attention should also be paid to both
the horizontal and the lateral direction to ensure
that the silver amalgam restoration has a three-
dimensional adaptation.
When fi lling is completed, polishing should
be carried out promptly, using an ovoid hoe light
modulator to act in mesiodistally and buccolin-
gually to remove excess silver amalgam and fur-
ther compact the edge of the silver amalgam
restoration. Then, the restoration should be
carved according to the anatomical form and
adjacent relations. Usually, an instrument with a
sharp edge can be used. A special carving instru-
ment is available, but a digging machine and hoe
can also be used. Silver amalgam carving should
be done in accordance with the anatomical shape,
forming the ideal tooth shape and bulge, but not a
deep pit and fi ssure. It should not appear sparse
or feather-edged on the border of the restoration
with the tooth tissue. The sculpting of the silver
amalgam should be performed before removal of
the forming sheet. This is advisable before the
silver amalgam is fully solidifi ed, generally using
a short push and pull, but attention should be paid
to the direction, which should be from the tooth
body to the restoration. In trimming the edge of
the crest of the adjacent and occlusal cavity,
attention should be paid to the height of the edge
of the crest of the adjacent tooth, and then to
forming an outreach gap. Next, the occlusion
should be checked and adjusted to prevent a high
bite, and the early contact point of middle occlu-
sion, lateral occlusion, and stretching occlusion
should also be removed. General occlusal adjust-
ment can be divided into two steps, namely
immediately after the fi lling is completed and the
second day after the fi lling. The subsequent pol-
ishing, called polishing after carving, is to ensure
that the surface of the silver amalgam restoration
is smooth, to improve the corrosion resistance of
7 Clinical Management of Dental Caries

118
the silver amalgam restoration, and to prolong
the life of the restoration.
7.4 Resin Composites and Direct
Bonding Restoration
Technique
Resin composites are types of polymers that are
used in dentistry as a restorative material devel-
oped based on acrylate. They are mainly com-
posed of Bis-GMA monomers or some Bis-GMA
analog, a fi ller material, and a photo initiator.
The properties of resin composites have been
greatly improved since Bowen invented them in
1962. In particular, the development of bonding
technology has led to resin composites becom-
ing more widely used and becoming the ideal
tooth- colored restorative materials at currently
available.
In 1955, Buonocore fi rst treated the tooth sur-
face with acid to promote the bonding between
resin and the tooth surface, which improved the
stability of restorations. This technique was then
used in clinical practice and universally
acknowledged at the annual meeting of the
International Society of etching techniques in
1974, which brought about great changes in
dental practice.
7.4.1 Resin Composites
There are many kinds of resin composites, which
can be divided into anterior tooth type, posterior
tooth type, and universal, according to the posi-
tions in which they are used in the clinic. Anterior
resin composites emphasize color and polishing,
while posterior tooth type enhances the mechani-
cal strength and abrasion resistance. According to
the inorganic fi ller particle size, resin composites
can be divided into macrofi ll resin composite,
microfi ll resin composite, microhybrid resin
composite, hybrid resin composite, and nano-
composite. For macrofi ll resin composite, the
fi ller particle size ranges from 10 to 100 μm and
the fi ller volume fraction is 70–80 %. As early
products, such materials possess good mechanical
properties, but poorer surface polishing charac-
teristics, so that they are less frequently used at
present. For the microfi ll resin composite, the
fi ller particle size ranges from 0.01 to 0.1 μm and
the fi ller volume fraction is 35–50 %. This kind
of material, which was produced in the late 1970s
has great color and polishability, but poor
mechanical properties; thus, they are suitable for
class III, IV, and V restorations, tiny tooth reshap-
ing, and partial discolored tooth restoration.
Microhybrid resin composite refers to the com-
pound fi ller of large particles and small particles.
The fi ller particle size ranges from 0.04 to 4 μm
and the fi ller volume fraction is 56–66 %.
Because small particles fi ll the voids between the
large particles, the microhybrid resin composite
has better abrasion resistance and transparency;
however, they are not as good as ultra-microfi ll
resin composites. They are suitable for class III,
IV and V restorations, resin veneers, dental mor-
phology shaping, discolored teeth restorations,
and also posterior teeth restorations. For hybrid
resin composite (namely universal resin compos-
ite), the fi ller particle size is 1–3 μm, the fi ller
volume fraction is 70–77 %, and they are suitable
for class I, II, and V restorations, and dentin res-
toration for classes III and IV. However, their sur-
face polishing performance is inferior to that of
microfi ll and nanocomposite. The nanocompos-
ite was fi rst reported in 2002, and the fi ller parti-
cle size ranges from 25 to 75 nm with a precise
arrangement. This composite has excellent
mechanical properties, improved surface polish-
ability, and decreased polymerization shrinkage.
Thus, they have been considered to be the best
universal composites, which are suitable for
repairing all kinds of cavities, resin veneers, den-
tal morphology shaping, and discolored teeth
restorations.
7.4.2 Etching Adhesive Systems
and Bonding Mechanisms
Modern etching systems can be divided into the
total-etch systems and the self-etch systems
based on processing for smear layer by etching
systems [ 6 ]. The total-etch system is also called
L. Jiyao

119
“etch and rinse adhesive” because there is a sep-
arate etching step that completely removes the
smear layer by an acidic gel and demineralizes
hydroxyapatite under the smear layer of the
dentin. The self-etch system only makes the
smear layer permeable without completely
removing it because of the lack of the separate
etching step.
The development of dentine adhesives has
changed a lot to simplify the procedure, for
example, the combination of etching and pre-
treatment. However, the various simplifi ed
methods are all from the two systems. The clas-
sifi cation of dentin adhesive systems is shown in
Fig. 7.1 .
7.4.3 Total-Etch Systems
The total-etch system completely removes the
smear layer using an acidic gel (usually phos-
phate acid) and demineralizes hydroxyapatite
under the smear layer of the dentin. The adhe-
sive of the one bottle etching system is synthe-
sized by dissolving the resin monomer to form
an organic solvent. After etching and rinsing,
the adhesive was applied to the treated tooth
surface; then, the resin monomers penetrate into
the demineralized dentin through the gap, which
is fi lled with water between the dentin collagen
(this gap was originally occupied by the
hydroxyapatite) to form a “hybrid layer”. The
hybrid layer is between the adhesive and dentin
and is composed of collagen, resin monomer,
residual hydroxyapatite, and water. The hybrid
layer helps to reduce postoperative sensitivity
and form a better marginal seal; at the same
time, buffering the shrinkage stress produced by
the polymerization of the resin composites as an
“elastic buffer”.
7.4.4 Self-Etch Systems
A self-etch system combines the etching and pre-
treatment as one step when processing enamel
and dentin without the separate etching step. The
mixture combined with etch and primer pene-
trates into and dissolves a part of the smear layer
to form a “hybrid zone” with hydroxyapatite so
that the “hybrid zone” is composed of the hybrid
layer and the residual smear layer.
For both the etch and the primer in the two-
step system and the self-etch adhesive in the one-
step system, the composition is substantially the
same, i.e., a mixture of water and the acidic
monomer. The acid monomer is typically phos-
phate ester or carboxylic acid ester, and its pH is
above that of the phosphoric acid gel. Water is a
key component of self-etch systems because it is
involved in the ionization of acidic components.
According to its acidic strength, self-etching
adhesive can be divided into three categories:
mild (pH > 1.5), medium (1.0 < pH < 1.5), and
strong (pH < 1.0).
7.4.5 Enamel Bonding
Etching is a key step in enamel bonding. There
are large quantities of hydroxyapatite in enamel,
in which the surface layer turns into water- soluble
monocalcium phosphate during phosphoric acid
treatment. Meanwhile, the dental plaque, material
Multiple-bottles system:
etch+primer+adhesive
Total-
etch
systems
Self-
etch
systems
Two-step system:
etch/primer+adhesive
One-bottle system: etch
+adhesive
One-bottle system:
etch/primer/adhesive
Etching
systems
Fig. 7.1 Classifi cation of
dentin adhesive systems
7 Clinical Management of Dental Caries

120
alba, and food residues attached to the tooth sur-
face are removed, thereby exposing a clean fresh
surface layer. Owing to the orientation of
hydroxyl and amino on the tooth surface after
phosphoric acid treatment, a polarized surface is
formed. The increased surface energy of enamel
is benefi cial for wetting and penetrating of the
adhesive. It is generally accepted that 30–50 % of
phosphoric acid enables even demineralization of
the enamel surface; thus, total-etch systems are
considered a better adhesive system for enamel
bonding.
After the etching process, the wettability of
the rough enamel surface is improved so that the
adhesive more easily penetrating the micro-
structure of the tooth surface, thus strengthening
the interaction of adhesive and fresh enamel.
When the adhesive is cured, the bonding inter-
face generates a considerable mechanical inter-
locking force. It can be observed by SEM that a
large quantity of cured adhesive fi lled in the
demineralized interprismatic area of the enamel,
and countless micro-protuberances (usually
called resin tags) are formed on the enamel side
of the bonding interface. These mechanical
anchored structures formed by resin tags and
enamel provide the prime bonding force for the
materials and enamel.
Etching technology is the general method for
performing enamel bonding. In this method,
mechanical interlocking is obtained by etching
treatment, and the formation of resin tags is the
main adhesive mechanism. The etched enamel
prisms and interprismatic areas are demineral-
ized, and the low-viscosity adhesive penetrates
into the micropores of the enamel via the capil-
laries. Then, the polymerization of the adhesive
occurs, which forms the resin tags that can gener-
ate the micromechanical interlocking system.
For the two-step self-etch system, the demin-
eralized enamel layer is thinner than that treated
by phosphoric acid gel because of the higher pH
of adhesive. There are two methods of improving
the bonding strength of self-etch systems:
1. Removing the rodless enamel to obtain a
rough enamel surface.
2. Using phosphate acid to pre-etch the enamel
before self-etching. The bonding strength is
clinically acceptable for dentin and etched
enamel treated using the self-etch method, but
it is insuffi cient for untreated enamel and scle-
rotic cementum.
For the one-step self-etch system, the bonding
strength is very low and there is no enamel pre-
etching. However, the bonding strength is accept-
able when the enamel is beveled and prepared.
In spite of the self-etch system gaining in pop-
ularity, phosphoric acid etching of the enamel is
still the gold standard for testing new bonding
material.
7.4.6 Dentin Bonding
The surface property and interior structure of the
dentin is sophisticated. The dentin contains a lot
of organic components, water, dentinal tubule
connected with pulp, and liquid effused from the
tubules. In addition, there is a smear layer caused
by instrumental cutting of the dentin. Therefore,
it is diffi cult to perform the treatment of dentin.
The smear layer is caused by the formation of
metamorphic organics and inorganics during the
bonding surface preparation, and its thickness is
0.5–15 μm. The degree of treatment of the smear
layer directly infl uences the bonding effect of
dentin. In the total-etch system, the smear layer
can be thoroughly removed, but probably leads to
excessive opening of the dentinal tubule, which
will increase the postoperative sensitivity.
In the case of the self-etch system, the weak
acid can partially remove the smear layer and
lead to the appropriate opening of the dentinal
tubules, thus bringing about the benefi t for dentin
bonding. Consequently, whether the one-step or
the two-step self-etch system is used, the treat-
ment of dentin includes three aspects: removal of
the smear layer; improving the surface activity of
dentin; promoting the penetration and bonding
strength of the adhesive. It is widely believed that
the prime mechanism of dentin bonding is the
formation of a hybrid layer or hybrid zone.
The treatment of dentin is described as
follows. At fi rst, the dentin surface is treated
by the etch so that the smear layer can be par-
tially removed. After demineralization of the
L. Jiyao

121
intertubular dentin, the microporous stent of
collagenous fi ber is exposed to form a porous
belt and the opening of dentinal tubules; then,
the primer is used. It can become wet and pen-
etrate into the micropores of collagen fi bers and
dentinal tubules to facilitate the subsequent pen-
etration of the adhesive; at last, the adhesive is
coated. After the primer and the adhesive are
cured in situ, they form the hybrid layer (or
hybrid zone) with dentin collagen fi bers, which
will obtain a solid bond with the dentin because
this zone contains many resin micro-protrusions
and large resin protrusions of the dentinal
tubules. Meanwhile, the residual unsaturated
ethylene of the adhesive copolymerizes with the
resin monomer; thus, the resin composites can
be bonded to dentin. Along with the develop-
ment of the dentin bonding system, the pro-
cesses mentioned above are simplifi ed into a
one-step or two-step procedure.
The durability of the self-etch bonding system
is a signifi cant issue. At the early stage, the bond-
ing strength is acceptable. As time goes on, the
bonding strength is continuously decreased,
especially for the one-step self-etch system,
owing to the hydrophilicity of the acidic mono-
mer in addition to the high water content to main-
tain ionization of the acidic monomer, and this
may ultimately even affect the bonding to enamel.
Meanwhile, the inadequate penetration of the
resin into the tooth structure may also accelerate
the degradation of the bonding interface.
Shrinkage stress caused by the polymerization of
resin composites, which act on the bonding inter-
face, results in reduced dentin bond strength if
the dentin bond strength cannot resist it. This will
bring about the formation of gaps or edges result-
ing in secondary caries and dentin sensitivity.
7.5 Resin Composite Bonding
Restoration Technique
7.5.1 Indications
and Contraindications
Currently, resin composite has been widely used
indirect restorative dentistry. Almost all the den-
tal defects can be repaired via a resin composite,
which can also be used for an abnormal shape or
the color of teeth in cosmetic restoration, in addi-
tion to the restoration of endodontic treatment
teeth.
The following situations should be taken into
account: in the anterior restoration of a class IV
cavity, except for a crossbite and clenching, the
teeth defi cit, which does not exceed one half, can
be considered a direct composite restoration. For
posterior teeth restoration, a severe attrition and
cusp defect need to be excluded. We do also not
use resin composite if the cavity cannot be com-
pletely isolated from saliva, gingival crevicular
fl uid, and blood.
7.5.2 Requirements for Restoration
Design
Acid etching followed by bonding provides
retention, while increasing the resistance of the
remaining tooth structure. The tooth types,
the position of the teeth in the dental arch, the
size and type of defect, whether the treatment
is for the placement of the original prosthesis,
the occlusal function, and the relationship
between the edges of the tooth preparation
need to be considered in bonding restoration.
What is more, the quantity and quality of the
remaining hard tissue also need to be consid-
ered, the mechanical force of remaining tooth
structure is exposed to the defect, and the
reserve area extends to the range of the sound
dental tissue.
7.5.3 Cavity Preparation
The principles of cavity preparation for resin
composite restoration are based on the principles
of amalgam cavity preparation, combined with
the characteristics of bonding restoration. The
principles emphasize preserving as much of the
tooth structure as possible in the premise of
removal of infected tissue and caries staining.
Cavity shape is determined by the area of the
lesion, and retention of the restoration relies on
the etching. The extension for prevention is not
needed.
7 Clinical Management of Dental Caries

122
Class I The cavity shape only involves the cari-
ous parts and developmental defects. For enamel
caries, the depth of the cavity should be limited to
the enamel, without proceeding to the dentin, or
increasing the supporting retention; to remove a
large shallow dish caries, the cavity should be
extended at the buccal and tongue groove, and
then prepare the bevel at the edge of the cavity,
adding the auxiliary retention ditch at the bottom
and side walls. However, at the occlusal contact
points in the occlusal cusp the edge bevel is not
needed.
Class II
The abrasion of composite resin mate-
rial is not as good as that for the silver amalgam.
Therefore, the occlusal factors should be con-
sidered, especially the functional occlusal tip
occlusion. On the occlusal surface, the cavity
preparation should embody the preservation
principle, and the cavity edge and line angle
should be more obtuse than with silver amal-
gam, to facilitate closing together. For the occlu-
sal cavity, the beveled edge could increase the
cavity width, which means the wear of the pros-
thesis is greater than for the conventional cavity;
however, at these parts of the restoration, espe-
cially the edges, fl akes often form and breakage
is easy by force. Therefore, the preparation of
the bevel at the non-occlusal contact at the
occlusal surface remains controversial, and in
contact with occlusal cusp, the bevel should be
avoided.
For the proximal cavity, buccal and lingual
walls should be introverted, and the enamel
bevel edge can be prepared, without extending
to the self-cleaning area. An additional retention
groove could be prepared at the axiofacial and
axiolingual line angles, in the same way as the
silver amalgam. There are pulp walls at the
occlusal surface and an axial wall in the proxi-
mal surface, and a large part is involved in den-
tin; thus, less enamel is available in the proximal
cavity, which is not conducive to bonding resto-
rations. Therefore, to preserve as much tooth
tissue as possible, especially the enamel thick-
ness, for the gingival wall parts, carious tissue
should be removed without extension to the root
side.
Class III
Cavity preparation should be started
from the lingual surface, trying to save the lip
surface integrity; if the labial enamel has been
stained, or the edge of the facial surface has been
damaged, the preparation can be entered directly
from the facial surface. Small to medium-sized
cavities, should be designed as conservatively as
possible, without making a special cavity shape,
or as an aid to the retention form. For enamel car-
ies, the retention depends mainly on the retention
wall and resin bonding. Therefore, the bevel edge
should be prepared, without going deep into the
dentin. For a large area of caries, the retention
groove should be made in the axiogingival line
angle, and the undercut should be prepared in the
axiofaciopulpal point angle, while the bevel is at
the enamel wall. The gingival wall, stretching to
the root surface, where there is no enamel, should
not be beveled. Withstanding a greater bite force
at the tongue surface, the tongue edge does not
make a beveled edge.
Class IV
The beveled edges and the size of
the dental defects in class IV cavities should
be considered for resin composite restoration.
For the tooth in which defect is limited to one
side and the incisal part is intact, the carious
structure and weak enamel should be removed,
and all the enamel edges beveled. In cases
with defects exceed mesiodistally half the
width of the incisal part, or the distance
between the incisal and gingival surface
exceeds 2/3 of the crown length, with intact
pulp, only a short bevel of at least 1.0 mm
around the cavity needs to be prepared.
Increasing the width of the bevel could
enhance enamel bond strength, and the spread-
ing of the material along the plane can also
attain a better aesthetic effect.
Class V
For small to medium-sized class V cav-
ities, preparation should be as conservative as
possible, with no special shape, and the bevel
edge only needs to be prepared for the enamel
wall slope. Generally, the retention groove is not
needed, and when there is no enamel on the gin-
gival wall, the slide ditch should be prepared at
the axiogingival line angle. Retention grooves
L. Jiyao

123
not only increase retention, but can also reduce
polymerization shrinkage and micro-leakage in
the bite force of the resin.
Class V cavities, in which the area is large and
involves the root surface, need to be prepared
routinely, similar to the box-like cavity in silver
amalgam restorations. The retention groove
should be prepared at the angle of the gingival
and occlusal axis, while the bevel edge should be
shaped on the occlusal, mesial and the distal
edges of the wall.
7.5.4 The Importance
of Postprocessing Decoration
After the restoration, contouring, blending,
grinding, and polishing are needed. For a long
time, this part was not given serious clinical
attention. Very simply, by removing the fl ash side
at the adjacent surface, trimming the shape care-
fully, and polishing the smooth surface, the
occlusal surface and the adjacent surface from
coarse to fi ne, can form a smooth surface, a suit-
able edge, and a good occlusal relationship,
which can achieve minimal plaque adhesion,
easy bacteria removal, and better aesthetics. The
success of grinding and polishing of the resin
composite is determined by the repair materials
and devices. The surface quality of the resin is
not only impacted by the quality of the polishing
apparatus and polishing paste, but also by the rel-
evant component and fi lling characteristics of the
resin. A new composition with small particles
and micro-mixed resin has changed the fi ller for-
mulation. The size, shape, orientation, and the
aggregation of the resin have been enhanced, and
the physical and mechanical properties improved,
while the character of the polishing is better. The
hardness of the inorganic fi ller and the matrix is
different, which is caused by the difference in the
wear rate of these two components, and this
results in a surface roughness. The surface gloss
of restoration materials should be similar to that
of the dental interface, because it affects the
color-matching of the restoration. At present,
high-quality restoration not only requires decora-
tion for the anatomical shape, contour, marginal
integrity, occlusal function, and improvement of
the smoothness of the surface, but also pays
attention to the concept of aesthetics. For the best
bonding aesthetic, the surface should be smooth
to prevent plaque accumulation and dyeing; the
restoration should have a perfect contour and
form, to improve the organizational fl exibility.
The appropriate dressing also makes the anatom-
ical shape of the prosthesis adapt to occlusal
requirements, and match the color of the sur-
rounding teeth. The integrity and the adaptability
of the edge is also improved. Therefore, the resto-
ration is durable and aesthetically pleasing, and
the life of the restoration is extended.
7.5.5 Problems of Direct Resin
Composite Restoration
7.5.5.1 Polymerization Shrinkage
Polymerization shrinkage stress is one of the
main problems that affects the longevity of direct
composite resin restoration. The dental compos-
ite resin is mostly formed of dimethacrylate mol-
ecules, whose polymerization reaction produces
a polymer network and volumetric contraction.
Owing to the restrictions of the cavity, the volu-
metric contraction resin showed that the volume
changes the effect on the tooth structure and
bonding interface stress, causing tooth deforma-
tion and resulting in bond failure, postoperative
sensitivity, microleakage, secondary caries, and
other adverse consequences. The volumetric con-
traction in the bonded resin composite restora-
tions may cause shrinkage stress at the resin
composite/tooth structure interface and/or within
the tooth or the resin composite. The resulting
shrinkage stress may result in adhesive failure,
tooth deformation, postoperative hypersensitiv-
ity, secondary caries or microleakage. Shrinkage
stress is not only dependent on the characteristics
of the composite resin, but also on the tooth
structure, cavity shape, characteristics, and resto-
ration techniques. Therefore, the correct under-
standing of resin composite polymerization
signifi cantly offers numerous clinical advantages
in reducing the shrinkage stress generated in
polymerizing dental composites.
7 Clinical Management of Dental Caries

124
Material related factors Resin composite con-
sists of organic matrix and inorganic fi llers. The
polymerization shrinkage is mainly caused by the
volumetric contraction of the organic matrix,
with an average of 2.6 % ~ 7.1 vol% [ 7 ]. In this
process, the space occupied by inorganic fi llers is
not involved in the shrinkage. Therefore, the
main strategy in reducing polymerization shrink-
age in methacrylate-based composites focused on
increasing the fi ller load. Compared with resin
composites with a low fi ller load, the resin com-
posites with a high fi ller load have some good
physical properties, lower shrinkage, and greater
hardness. With a constant amount of shrinkage,
the resulting shrinkage stress grows with the
increase in the elastic modulus of the composite
resin.
Conventional composite resins have fi ller par-
ticles larger than 400 nm, but nanocomposites
contain fi ller particles between 20 and 100 nm.
Compared with hybrid and microfi lled resins,
nanocomposite resin particles have a high fi ller
load and extensive fi ller distribution, resulting in
low polymerization shrinkage. Compared with
conventional composite resin, fl owable compos-
ite resin contains less inorganic fi ller, which
reduces the mechanical performance, increases
the polymerization shrinkage, and produces more
shrinkage stress at the interface. However, owing
to the reduction of inorganic fi ller during the cur-
ing process, concave deformation at the surface
of a fl owable resin composite can compensate for
the volume contraction within the resin; thus, the
fl owable resin composite can be placed between
the tooth surface and the nonfl owable composite
as a stress buffer layer. Currently, there are differ-
ences of opinion about the fl owable resin com-
posite used as a base material to reduce
polymerization shrinkage.
C-factor
The confi guration factor is the ratio of
the bonded to unbonded surface of the restora-
tion. As the C-factor decreases, the polymeriza-
tion shrinkage is limited to only one direction,
and at the early stage of polymerization, the resin
fl ows freely to prevent polymerization shrinkage
stress. As the C-factor increases, the resin is lim-
ited in direction and becomes less fl owable at the
early stage of polymerization; therefore, the
shrinkage stress increases, the bond strength with
dentin declines, resulting in greater marginal
microleakage.
Incremental techniques are always recog-
nized as a major factor in the reduction of
C-factor and shrinkage stress. Incremental layer-
ing is considered to be the conventional tech-
nique for reducing polymerization shrinkage,
compared with the bulk technique. For incre-
mental layering, the shrinkage stress of each
increment can be compensated for by the next
increment. Because of the reduction of C-factor,
the contact surface between the resin and the
cavity walls is minimized and the resin is rela-
tively fl owable during polymerization, which
can decrease the shrinkage further. Incremental
layering techniques include horizontal occluso-
gingival layering, wedge-shaped oblique layer-
ing, and the successive cusp build-up technique.
In recent years, the use of incremental layering
techniques for the purpose of reducing polymer-
ization shrinkage has been questioned by some
authors, who argued that different restoration
techniques had no signifi cant difference on the
shrinkage stress of composite resin. Nevertheless,
incremental layering techniques are still widely
used in clinics.
The polymerization rate is the rate at which
the monomer of composite resin is converted to
polymer. The higher the rate, the more polymer-
ized monomers there are, the higher the polym-
erization stress. The use of a high-intensity
curing light can increase the polymerization
rate and mechanical properties of composite
resin, but the polymerization shrinkage stress is
high. Slowing down the curing process can
release stress during polymerization. Therefore,
a new "soft" curing technique has been reported.
A relative low-intensity curing light was used
during the fi rst few seconds of the light curing
(10 s), and then a high-intensity light is applied
for the fi nal curing. For this technique, the light
intensity is low at the beginning, which makes
the surface polymer suffi ciently cross- linked,
but prolongs the polymerization of the underly-
ing resin to inhibit potential stress. In addition
to the curing methods, the curing units also
L. Jiyao

125
have different effects on polymerization shrink-
age stress. Compared with the traditional halo-
gen unit, a light emitting diode unit using
low-intensity light can effectively reduce the
shrinkage stress.
7.5.5.2 Technique Sensitivity
Technique sensitivity, which is totally depen-
dent on the practitioner, includes the failures
caused by improper selection of indications,
inadequate understanding of material and mech-
anism, neglect of the product manual, and incor-
rect operation by not following the
manufacturer’s instructions. Technique sensitiv-
ity is an important factor that affects the result of
direct composite resin restorations. Any care-
lessness in procedure and difference in technical
profi ciency may lead to the failure of
restoration.
First of all, selection of the appropriate resin
composite and adhesive according to the operat-
ing instructions is essential. It requires consid-
eration of the tooth position, location, and
volume of the cavity, fi ller types of resin to meet
the requirements of the mechanical characteris-
tics of the posterior teeth, or the aesthetic prop-
erties of the anterior teeth, or both. Total-etch
adhesive is recommended for bonding with the
enamel surface, and for bonding with the den-
tine surface, use of a self-etching adhesive is
recommended. Strictly following the product
manual and operating instructions is the founda-
tion of successful direct composite resin
restorations.
The selection of the appropriate indications
is also very important. Direct resin restoration
will defi nitely fail if inappropriate cases are
selected, such as cusp defect, severe attrition, or
a subgingival cavity restored without gingival
retraction. Cavity preparation is another impor-
tant factor. Although cavity preparation of direct
resin restoration does not need strict retention
and resistance form, there are certain principles
that need to be followed. If the position and
angle of the cavity walls, and the bevel of the
cavity edge, are not properly prepared, adhesive
failure will result. In addition, the adhesive
surface must be thoroughly cleaned. Debris,
plaque, pigment or carious tissue have an infl u-
ence on the etching and binding effect on the
healthy tooth tissue.
7.5.5.3 Postoperative Sensitivity
Postoperative sensitivity is described as a moder-
ate pain, of short duration, that is initiated by
mastication, hot and cold stimulus, immediately
after resin restoration or later. Typically, there are
two types of postoperative sensitivity. First, when
the cavity is deep and there is not enough base
material, the cavity need re-fi lling with suffi cient
base material and resin. Second, when the cavity
is shallow but reaches the enamel–dentin junc-
tion (EDJ), the sensitivity may be due to opened
dentinal tubules caused by the etching process, or
insuffi cient marginal seal if the adhesive is too
thick, or shrinkage stress-induced microleakage,
or exposed dentin surface due to excessive occlu-
sional adjustment. The incidence of such sensi-
tivity is high and it is diffi cult to solve.
7.6 The Prospect
of the Treatment of Dental
Caries
7.6.1 Individualized Ideas
of Treatment
Scholars describe dental treatment in the twenty-
fi rst century as micro-invasive dentistry, cosmetic
dentistry, and adhesive dentistry. These concepts
reveal that modern dental treatment, especially
treatment of dental caries, requires as little trauma
as possible from the treatment, the best func-
tional reconstruction, and the best aesthetic per-
formance. Above are the foundations for the
individualized treatment of dental caries.
7.6.2 The Importance
of Individualized Treatment
of Dental Caries
Dental caries is a multi-factorial disease based on
the ecological plaque hypothesis. Factors includ-
ing individual systemic health, social economic
7 Clinical Management of Dental Caries

126
status, sucrose intake, the buffer capacity of
saliva, the implementation of oral health care, the
past history of caries, and the colonization of
caries-related microbes are closely related to
dental caries. Individualized treatment plans for
dental caries against specifi c risk factors are pro-
posed, after integrated evaluation of the factors
described above. For example, the risk of caries
among children and young people lies in deep
pits and fi ssures, while among middle-aged and
elderly people gingival recession and root expo-
sure contribute to dental caries. Some individuals
have caries because of a preference for sweets
and a tendency to ignore oral health, while others
suffer from caries because of the local accumula-
tion of plaque due to poor restoration. Specifi c
measures and targeted intervention should be
taken against different risk factors for dental car-
ies to obtain the best effect.
7.6.3 The Risk Evaluation Is
the Premise of Individualized
Treatment of Dental Caries
The evaluation of the risk of dental caries is an
assessment of the degree of risk of developing
new caries or continuing the progress of existing
caries under the circumstances of fairly constant
etiological factors, including diet, time, a suscep-
tible tooth surface, and plaque levels of an indi-
vidual at the time. The functions of the risk
evaluation of dental caries are listed as follows:
1. To control dental caries effectively. Recent epi-
demiological studies show that caries activity is
not evenly distributed among the general popu-
lation and a small proportion of people with
high caries activity suffer from most of the den-
tal caries among the general population. More
than 60 % of dental caries occur in only 20 %
of the overall population. Taking precautions in
the general population can lower the overall
prevalence of dental caries, but the effi cacy of
precautions cannot be noticeably increased.
Filtering people at a high risk of dental caries
and combining multiple prophylaxes before the
occurrence of dental caries can greatly enhance
the effi cacy of the prevention of dental caries.
2. To make suitable precautionary treatment plan
for individuals. As dental caries is a multi-
factorial disease, the risk evaluation can help
us to defi ne risk factors for an individual so
that specifi c treatment and precautionary
plans can be made.
3. To cure caries at an early stage. The han-
dling of incipient caries has been paid
increasing attention in the individualized
treatment of dental caries. Remineralization
is one of the effective measures. The risk
evaluation of dental caries is the major tool
for predicting the success rate during han-
dling incipient caries using the remineraliza-
tion method. The success rate is relatively
low if the individual is at a high risk of car-
ies; otherwise, the success rate is relatively
high.
7.6.4 The Development
of Technology and Material
Provides a Guarantee
for the Individualized
Treatment of Dental Caries
The development of technologies, including
early diagnosis, risk evaluation, micro-invasive
treatment, colony prevention, and the progress
of dental adhesive restorative materials, pro-
vide the premise and guarantee for the individ-
ualized treatment of dental caries. The risk
evaluation of dental caries predicts the risk of
caries of an individual by combining analysis
of the results of multiple risk factors to achieve
the goal of early detection, early prevention,
and early treatment of individual caries, focus-
ing on populations at a high risk of caries.
Therefore, the appearance and development of
a highly sensitive and specifi c screening tech-
nology to fi lter population at risk of caries, a
quantitative technology of the early diagnosis
of caries, a micro-invasive treatment technol-
ogy of caries, and a preventive technology with
low side effects, together with defi nite effi cacy,
provide a guarantee for the realization of the
real sense of individualized treatment of
caries.
L. Jiyao

127
Application of tissue engineering in the fi eld
of tooth restoration includes terminating or
avoiding the progress of caries by replacing the
composition of oral microbes with lower-toxicity
or inactivated strains constructed by gene
engineering technology, inducing the regenera-
tion of dentin through the slow release of a sus-
tained release carrier into dental tissue by adding
various growth factors to the fi lling materials,
inducing the production of a new tooth structure
Degree of tooth
damage Minor Severe
Parts of active
tissue lost
Most of active tissue
lost
Active tissue lost
completely
Common cases Incipient nonstatic
caries
Arrested caries Arrested or
chronic deep
caries involving
pulp
Pulp exposed
because of caries or
trauma
Total cavitation
or total
dislocation
accidentally
Common fi lling
restoration at
present
Pit and fi ssure
sealant or fi lling
after removal of
lesion
Filling after
removal of
lesion
Removing
softened dentin
fractionally or
pulp capping
Pulp capping, root
canal treatment or
tooth extraction
Implant or
restoration after
debridement
Possible tissue
engineering
technology
Replacement
therapy by
constructing
lower-toxicity or
inactivated strains
using genetic
engineering
technology to
change the
composition of the
oral microbe
Inducing the
pulp–dentin
complex to
defend outer
stimuli and
produce
reparative
dentin using
growth factor
Promoting
regeneration of
pulp tissue and
repair of dentin
structure using
growth factor
Reconstructing
missing pulp and
hard tissue of the
tooth by implanting
stem cells
Implanted teeth
induced in vitro
to restore missing
teeth
7.7 Biological Treatment
Methods
Nowadays, there are two directions of research into
the regeneration of tooth tissue: fi rst, achieving the
goal of regeneration of tooth tissue by mimicking
biomineralization through the design of organic
matrix using bionic theories; second, realizing the
regeneration of tooth tissue by cultivating stem cells
in scaffold material using tissue engineering meth-
ods. These ideas and studies provide an exciting
future for restorative dentistry [ 8 ].
7.7.1 Restorative Therapy Based
on Tissue Engineering
of Tooth Regeneration
The development of tissue engineering for tooth
regeneration has made the self-repair of dental
damage, including cavitated and noncavitated,
or even missing teeth, possible. By directly
implanting teeth cultivated in vitro instead of
traditional implants or dentures to restore miss-
ing parts of teeth or by conditioning the
microenvironment of tooth damage in situ can
help us to realize the goal of the self-repair of
tooth damage.
There is currently no direct evidence sup-
porting the introduction of the specifi c technol-
ogy of tissue engineering into the fi eld of
tooth-fi lling restoration, or related reports on the
fi nal effect. However, lots of related reviews
have pointed out that tissue engineering may
promote tooth fi lling treatment under specifi c
circumstances.
Classifying tooth-fi lling restoration technolo-
gies by the degree of tooth damage may predict
the indication for tissue engineering-related tech-
nology in the restoration of tooth damage. The
chart below summarizes accessible traditional
fi lling restoration and the possible tissue engi-
neering technology against tooth damage of dif-
ferent degrees.
7 Clinical Management of Dental Caries

128
by implanting multifunctional stem cells for teeth
severely cavitated or severely damaged by
trauma, and implanting tooth tissue cultivated
in vitro to restore the integrity of dentition for
teeth that are missing or that cannot be restored
through the method of fi lling.
Tissue engineering has provided an exciting
future for tooth restoration, but related biosecu-
rity problems, technological problems, and rela-
tively high costs have limited its possibility of
wide deployment. It seems that we can only rely
on the optimization of traditional treatment meth-
ods to improve prognosis.
7.7.2 Restorative Therapy Based
on Bionics
Nowadays, composite resin, metal, and ceramics
are the main materials in clinical application.
However, insolvable problems such as margin
leakage, aging of the materials, and the lack of
fracture toughness remain. Also, removal of a
large amount of tooth tissue during surgery
causes the patient a lot of pain. Therefore, regen-
erating new tooth tissue where the tooth tissue is
damaged in situ has become the dream of dental
scholars. At present, designing organic matrix to
conduct biomineralization using bionic theories
may enable us to regenerate teeth. Therefore,
research into bionic materials to simulate tooth
tissue using a bionic methods has been a hotspot
of current studies. For example, in vitro studies to
simulate the biomineralization of enamel and
dentin have enabled simple nucleation and sedi-
mentation of hydroxyapatite and are going deeper
toward the highly densely arrayed and regular
structures such as enamel. When it comes to
tooth tissue mineralization in situ, we are trying
to implement the mineralization procedure, in
which crystal grows layer by layer in a well-
organized way and closely controlled by organic
matrix, while the exploration of forming enamel
on the surface of dentin continues. As studies on
the forming mechanism of enamel and dentin go
deeper and frontier science such as the self-
assembly of the supermolecule develops, we
believe that we can obtain tooth tissue that is
highly bionic in form and function and that we
will fi nally implement the restoration of tooth
damage in situ.
References
1. Summit JB, Robbins JW, Schwartz RS, et al.
Fundamentals of operative dentistry. 2nd ed. Chicago:
Quintessence Publishing Co Inc; 2001.
2. Tyas MJ, Anusavice KJ, Frencken JE, et al. Minimal
intervention dentistry – a review. FDI commission
Project 1–97. Int Dent J. 2000;50(1):1–12.
3. Featherstone JD. Remineralization, the natural caries
repair process – the need for new approaches. Adv
Dent Res. 2009;21(1):4–7.
4. Fedorowicz Z, Nasser M, Wilson N. Adhesively
bonded versus non-bonded amalgam restorations for
dental caries. Cochrane Database Syst Rev.
2009;(4):CD007517. Published by John Wiley& Sons,
Ltd
5. Dunne SM, Gainsford ID. Current materials and tech-
niques for direct restorations in posterior teeth.
I. Silver amalgam. Int Dent J. 1997;47:123–36.
6. Perdigao J. New developments in dental adhesion.
Dent Clin N Am. 2007;51:333–57.
7. Chen Y, Zhou L. Control of polymerization shrinkage
stress in resin composite restoration. Int J Stomatol.
2008;35(4):393–5.
8. Murray PE, Windsor LJ, Smyth TW, et al. Analysis of
pulpal reactions to restorative procedures, materials,
pulp capping, and future therapies. Crit Rev Oral Biol
Med. 2002;13:509–20.
L. Jiyao

129
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1_8
Dental Caries and Systemic
Diseases
Zou Ling and Hu Tao
As stated by World Health Organization, oral
health is fundamental to overall health and well-
being and a determinant of quality of life [ 1 ].
According to “Oral Health in America: A Report of
the Surgeon General,” the mouth and face are mir-
rors of health and disease. A physical examination
of the mouth and face can reveal signs of general
health status. Imaging of the oral and craniofacial
structures (x-ray, MRI, SPECT) may provide early
signs of skeletal changes such as those occurring
with osteoporosis and musculoskeletal disorders
and salivary, congenital, neoplastic, and develop-
mental disorders. For example, the research group
of Dr. David Wong from UCLA has initiated a
series of concerted efforts to spearhead the scien-
tifi c and translational frontiers of salivary diagnos-
tics. The potential use of saliva, a totally noninvasive
biofl uid without the limitations and diffi culties of
obtaining blood and urine, for oral and systemic
disease detection, disease progression, and thera-
peutic monitoring is a highly desirable goal
[ 2 , 3 ]. In other words, oral health refers to the
health of our mouth and, ultimately, supports and
refl ects the health of the entire body [ 4 ]. In a sense,
oral disease is not just a minor ailment of the soft
and hard tissues of the mouth, and it may be a dis-
ease of the body that happens to begin in the mouth.
If left unchecked, oral disease can contribute to
other more harmful diseases that can seriously
affect the quality of life [ 5 ].
As Hani T. Fadel from University of
Gothenburg wrote in his doctoral thesis, “the link
between oral and general health has been sug-
gested since early times, almost as early as history
itself. The concept of local or systemic diseases
secondary to a localized chronic infection (e.g., in
the oral cavity) is usually called focal infection.
Its origin can probably be traced back to the time
of Hippocrates” [ 6 , 7 ]. Recently, a report titled
“Links between oral health and general health –
the case for action” from Dental Health Services
Victoria summarized that oral health and general
health are related in four major ways:
1. Poor oral health is signifi cantly associated
with major chronic diseases.
2. Poor oral health causes disability.
3. Oral health issues and major diseases share
common risk factors.
4. General health problems may cause or worsen
oral health conditions.
Dental caries and periodontal disease are the two
biggest threats to oral health and are by far the most
Z. Ling (*)
Department of Conservation Dentistry and
Endodontics, West China Hospital of Stomatology,
Sichuan University , Chengdu ,
People’s Republic of China
e-mail:
H . T a o
Department of Preventive Dentistry,
West China Hospital of Stomatology,
Sichuan University , Chengdu ,
People’s Republic of China
e-mail:
8

130
common oral infection diseases in the United States
and Australia [ 8 ]. It has been well proven that the
oral cavity contains some of the most varied and
vast fl ora in the entire human body, not only includ-
ing those linked to dental caries and periodontal
disease but also including systemic diseases that
affect general health. In addition to bacterial organ-
isms, oral microorganisms can include fungal, pro-
tozoal, and viral species. It is well accepted that our
body is negatively affected by infection of any kind,
no matter where it is located. Moreover, the more
serious the infection and the longer it is present, the
greater its potential for affecting systemic health.
Infection can also seriously stress the immune sys-
tem and diminish its ability to deal with other infec-
tions and diseases. Its effect on the immune system
is directly related to the extent, type, and duration of
the infection [ 5 ]. Over 100 years ago the theory of
focal sepsis, although lacking empirical scientifi c
evidence, hypothesized that chronic infections in
the mouth caused systemic diseases [ 9 ]. The con-
cept has been neglected for several decades and still
is a subject of controversy [ 10 ]. Since many teeth
were extracted without evidence of infection,
thereby providing no relief of symptoms, the theory
was discredited and largely ignored for many years
[ 11 ]. Interestingly, increasing evidence over the
past 30 years suggests that, due to dental bactere-
mia, the oral cavity can indeed serve as a reservoir
for systemic dissemination of pathogenic bacteria
and their toxins, leading to infections and infl am-
mation in distant body sites, especially in immuno-
compromised hosts such as patients suffering from
malignancies, diabetes, or rheumatoid arthritis or
having corticosteroid or other immunosuppressive
treatment [
12 ].
Most studies stated above concerning the rela-
tionship between oral infection and systemic dis-
eases are related to periodontal disease [ 13 ]. And
according to Thomas McGuire, the most impor-
tant of oral diseases in regard to their impact on
general health are:
(a) Periodontal disease
(b) Infected root canals
(c) Cavitations (infected extraction sites)
(d) Other diseases of the oral cavity, such as oral
cancer [ 5 ]
“Dental caries” cannot be directly found in this
list. Thomas McGuire explained the reasons as the
following: certainly, dental caries can have an effect
on a person’s overall health. For example, it can
interfere with the mastication process and thereby
affect digestion. It can cause tooth loss, again affect-
ing digestion. The main difference is that, unlike
periodontal disease, dental caries is not an infection
that has access to the systemic body. Clearly, it can
contribute to systemic health problems, but its
effects on overall health are signifi cantly less than
the effects of periodontal disease [
5 ].
As mentioned before, dental caries is one of
the most common causes of pulpitis and periapi-
cal diseases by penetrating through the enamel
and dentin to reach the pulp. Untreated decay can
become so advanced that the tooth must be
removed (extraction). Most studies supported that
dental caries was the main cause for tooth loss,
but a few studies revealed that a greater propor-
tion of tooth extractions were due to periodontal
disease, especially in patients over 40 years old.
Overall, 70 % of tooth loss is due to tooth decay,
20 % due to periodontal diseases, and 10 % due to
other causes [ 14 , 15 ]. It was reported that caries
accounted for a higher proportion of extractions
than periodontitis at all ages over 20 years in
1968 and only up to 45 years of age in 1988 [ 16 ].
According to another study, although there is an
increase in orthodontic extractions or a decline in
extractions for caries in under-21-year-olds, when
extractions from the population as a whole are
considered, caries and its sequelae remain the
principal reason for loss of all tooth types apart
from lower incisors which were extracted mainly
for periodontal reasons [
17 ]. In 2012, a quantita-
tive study evaluated the prevalence and factors
related to tooth loss due to dental caries among
workers in industrial estates in central Thailand.
There were 457 adult (283 males; 174 females)
between 19 and 53 years participants. The results
showed that 62.2 % participants had tooth loss
due to caries [ 18 ]. The latest study also proved
that dental caries and its complications were the
leading reasons for extraction. Their study
included a total of 2,620 teeth extracted from
1,382 patients. The highest rate (36.9 %) of
extraction occurred for those of 41–60 years of
Z. Ling and H. Tao

131
age. Tooth loss due to caries was 51 %; periodon-
tal disease was 14.4 %; and supernumerary and
tooth impaction were 13.9 %. Although 86 % of
teeth extracted for periodontal disease were in
patients over 40 years of age, caries was still the
main reason for extraction even in elderly patients,
but to a less degree than in younger ones [ 19 ].
Our goal of this chapter is to discuss the rela-
tionship between dental caries and general health;
we will summarize the limited recent advances in
this topic. Since the effect of dental caries on the
overall quality of health and well-being has not
been well studied, in order to enrich the content
of this chapter, studies associating systemic dis-
eases with periapical diseases, tooth loss, root
canal treatment, and other conditions caused by
dental caries directly are also included [ 20 ].
This chapter explores what the dental caries
can reveal about general health, describes the role
the mouth plays as a portal of entry for infection,
and concludes with studies that are associating
oral infections with serious systemic diseases and
conditions. Following this introduction and over-
view, the remainder of the chapter is organized as
follows: fi rst defi ning dental caries and bactere-
mia, head and neck cancer, and children growth;
then briefl y describing dental caries and athero-
sclerosis, cardiovascular disease, and heart attack;
next discussing dental caries and immune system
disease and kidney diseases; and last describing
dental caries and gastrointestinal diseases, diabe-
tes mellitus, and respiratory infections.
8.1 Dental Caries
and Bacteremia
Bacteremia is an invasion of the bloodstream by
bacteria. The blood is normally a sterile environ-
ment [ 21 ]. So the detection of bacteria in the
blood (most commonly accomplished by blood
cultures) is always abnormal. This may occur
through a wound or infection or through a surgical
procedure or injection when other foreign bodies
are entering the arteries or veins. Bacteremia may
cause no symptoms and resolve without treat-
ment, or it may produce several consequences like
fever and other symptoms of infection. In some
cases, the immune response to the bacteria can
cause sepsis and septic shock, a potentially life-
threatening condition which has a relatively high
mortality rate. Bacteria can also use the blood to
spread to other parts of the body (which is called
hematogenous spread), causing infections away
from the original site of infection.
The oral cavity is intensely colonized by bac-
teria. Recent advances in bacterial identifi cation
methods, particularly culture-independent
approaches such as 16S rRNA gene sequencing,
have shown that the oral cavity is inhabited by
more than six billion bacteria representing in
excess of 700 species belonging to at least nine
different phyla [
22 ]. Bacteremia occurs with vari-
ous frequencies following dental procedures and
has been well documented. As early as 1990,
Heimdahl et al. detected the patients with bacte-
remia after dental extraction, third-molar surgery,
dental scaling, endodontic treatment, and bilat-
eral tonsillectomy by means of lysis fi ltration of
blood samples with subsequent aerobic and
anaerobic incubation. Their results showed that
bacteremia was observed in 100 % of patients
after dental extraction, 55 % of patients after
third-molar surgery, 70 % of patients after dental
scaling, 20 % of patients after endodontic treat-
ment, and 55 % of patients after bilateral tonsil-
lectomy. And anaerobic microorganisms were
isolated more frequently than aerobic microor-
ganisms [ 23 ]. Transient bacteremia is produced
not only as a result of dental manipulation. Even
daily life activities such as eating, chewing gum,
brushing the teeth, or using toothpicks also
induce bacteremia detectable by means of blood
cultures in a variable percentage of subjects [
24 ].
Three mechanisms or pathways linking oral
infections to secondary systemic effects have
been proposed for several years [ 6 ]. Li et al. sum-
marized the mechanisms as the following: meta-
static spread of infection from the oral cavity as a
result of transient bacteremia, metastatic injury
from the effects of circulating oral microbial tox-
ins, and metastatic infl ammation caused by
immunological injury induced by oral microor-
ganisms [ 11 ].
Till now, there is no direct evidence to prove
the connection between the dental caries and
8 Dental Caries and Systemic Diseases

132
bacteremia, but we can fi nd some clues from
published papers. Debelian et al. used phenotypic
and genetic methods to trace microorganisms
released into the bloodstream during and after
endodontic treatment back to the root canal.
Microbiological samples were taken from the root
canals of 26 patients with asymptomatic apical
periodontitis of single-rooted teeth. The blood of
the patients was drawn during and 10 min after
endodontic therapy. The results found that
microorganisms from the root canal and blood
presented identical phenotype and genetic charac-
teristics within the patients examined,which dem-
onstrated that endodontic treatment can be the
cause of anaerobic bacteremia and fungemia.
Interestingly, some cariogenic bacteria were also
isolated from the blood, such as Streptococcus
sanguinis [ 10 ]. Streptococcus mutans and
Streptococcus sanguinis are most consistently
been associated with the initiation of dental caries.
The results not only illustrated that dental caries is
the most common cause of pulpitis and periapical
diseases but also showed a clue that cariogenic
bacteria may be related to bacteremia.
These bacteria are normally harmless as long
as they are kept in check by the body’s natural
barriers and the immune system. In the oral cav-
ity there are several barriers to bacterial penetra-
tion from dental plaque into the tissue: a physical
barrier composed of the surface epithelium;
defensins, which are host-derived peptide antibi-
otics, in the oral mucosal epithelium; an electri-
cal barrier that refl ects the Eh difference between
the host cell and the microbial layer; an immuno-
logical barrier of antibody-forming cells; and the
reticuloendothelial system (phagocyte barrier)
[
25 ]. However, once the equilibrium is disturbed
by an overt breach in the physical system (e.g.,
trauma) or immunological barriers (e.g., through
neutropenia, AIDS), organisms can propagate
and cause both acute and chronic infections with
increased frequency and severity [ 25 , 26 ]. In
addition, medical treatment (e.g., immunosup-
pressant therapy) may bring a person in contact
with new types of bacteria that are more invasive
than those already residing in that person’s body,
further increasing the likelihood of bacterial
infection.
8.2 Dental Caries and Head
and Neck Cancer
8.2.1 Dental Caries and Head
and Neck Cancer Treatment
Head and neck cancer accounts for more than
550,000 cases annually worldwide. The inci-
dence rate in males exceeds 20 per 100,000 in
regions of France, Hong Kong, the Indian sub-
continent, central and eastern Europe, Spain,
Italy, and Brazil and among African Americans
in the Unites States. Mouth and tongue cancers
are more common in the Indian subcontinent
[ 27 ]. Surgical resection, radiotherapy, and che-
motherapy, either used singly or in combination,
are the three most common modalities used in
head and neck cancer treatment. Despite their
effects in eradicating the tumor, they also
negatively impact the normal head and neck
structures surrounding the tumor. Surgical resec-
tion removes abnormal tissue, while radio- and
chemotherapy frequently cause direct damage to
the oral soft and hard tissue, and indirect damage
may also arise from systemic toxicity.
Firstly, we will discuss the radiotherapy
because radiation caries is a common disease in
clinic. We all know that saliva in the oral cavity
protects hard tissues against acid attacks and
demineralization. Salivary glands are very sus-
ceptible to radiation, and any disturbances in
their function are detrimental to the hard tissues
in the oral cavity. Radiation caries is mainly an
indirect effect of irradiation-induced changes in
salivary gland tissue that result in hyposalivation
[
28 ]. Hyposalivation leads to accelerated dental
caries through changes in salivary composition,
a shift in oral fl ora toward cariogenic bacteria,
and dietary changes [ 29 ]. It is reported that the
initial caries usually occur around the third week
of treatment [ 30 ]. Mohammadi et al. [ 31 ]
reviewed 27 cases with head and neck cancers
undergoing radiotherapy. Of these cases, class V
dental caries of posterior teeth were evaluated in
three intervals: before treatment, 3 weeks after
the initiation of the treatment, and at the end of
the treatment. The baseline is that there were no
class V decays prior to radiotherapy. Their
Z. Ling and H. Tao

133
results found that mean percentages of class V
caries 3 weeks after radiotherapy and at the end
of radiotherapy were 28.42 % ± 14.41 and
67.05 % ± 19.02, respectively. These fi ndings are
in accordance with the results of other studies
[ 28 , 32 ]. Since the severity of xerostomia is
related to the radiation dose, dose rate, and
amount of salivary tissue irradiated, the authors
also pointed out that further studies should eval-
uate the effects of new techniques such as inten-
sity-modulated radiotherapy on occurrence of
dental caries, in which a higher dose is beamed
at the tumor site without increased received dose
of the surrounding tissues [ 31 ].
Secondly, we talk about which one of the
three modalities, either used singly or in combi-
nation, is the most common cause of dental car-
ies after therapy. In order to determine the
prevalence of dental caries in cancer survivors,
Catherine et al. conducted a systematic literature
search with assistance from a research librarian
in the databases MEDLINE/PubMed and
Embase for articles [ 33 ]. Finally, 64 published
papers between 1990 and 2008 were reviewed.
Dental caries was assessed by the present (Y/N),
DMFT/dmft, and DMFS/dmfs indexes if avail-
able. Their results showed that the weighted
overall prevalence of dental caries was 28.1 %
and was determined from 19 studies. The
weighted prevalence of dental caries in patients
who received only chemotherapy was 37.3 %.
The weighted prevalences of dental caries in
patients who were post- radiotherapy and those
who were post- chemotherapy and post-radio-
therapy were 24 and 21.4 %, respectively. The
authors attributed the discrepancy to the distinct
differences in the dental management of patients
prior to radiotherapy versus those being prepared
for chemotherapy. Another explanation for the
unanticipated caries prevalence may be because
12 of the 19 studies included were carried out on
children undergoing hematologic malignancies
who were treated largely by curative chemother-
apy. They could have higher caries activity
because of the need to frequently consume
highly cariogenic dietary supplements for weight
maintenance or are taking sucrose-rich medica-
tions. In addition, their oral hygiene may be
ignored. In contrast to the caries prevalence, the
DMFT index is expectedly highest in patients
who were post-radiation therapy compared to
patients who were post- chemotherapy and
healthy controls [ 33 ].
8.2.2 Dental Caries and Head
and Neck Squamous Cell
Carcinoma
Recently, an interesting paper published online
in JAMA Otolaryngology – Head and Neck
Surgery showed that the bacteria that caused
tooth decay are linked to an immune response,
which may be protective against cancer [ 34 ].
The researchers from the University at Buffalo,
NY, set out to determine if there is a signifi cant
link between dental cavities and head and neck
squamous cell carcinoma (HNSCC). The study
involved 399 patients newly diagnosed with
HNSCC and 221 participants without the cancer
who were all selected from the Department of
Dentistry and Maxillofacial Prosthetics at
Roswell Park Cancer Industry between 1999 and
2007. The dental history of all patients, particu-
larly their history of dental cavities, was ana-
lyzed by measuring the number of decayed,
missing, and fi lled teeth. Of the 399 patients
with HNSCC, 146 (36.6 %) had oral cavity squa-
mous cell carcinoma (SCC). Oropharyngeal
SCC occurred in 151 (37.8 %) patients, while
102 (25.6 %) had laryngeal SCC. The results of
the study overall showed that those who had high
cavity numbers were less likely to have HNSCC,
compared with participants who had low cavity
numbers. The authors explained that “Caries is a
dental plaque-related disease. Lactic acid bacte-
ria cause demineralization (caries) only when
they are in dental plaque in immediate contact
with the tooth surface. The presence of these
otherwise benefi cial bacteria in saliva or on
mucosal surfaces may protect the host against
chronic infl ammatory diseases and HNSCC. We
could think of dental caries as a form of ‘collat-
eral damage’ and develop strategies to reduce its
risk while preserving the benefi cial effects of the
lactic acid bacteria” [
34 ].
8 Dental Caries and Systemic Diseases

134
8.2.3 Tooth Loss and Head
and Neck Cancer Risk
As previously mentioned, caries is one of the
most common reasons of tooth loss; we will
include the relationship between tooth loss and
tumors in this chapter. Actually, multiple epide-
miologic studies regarding the potential associa-
tion of tooth loss with head and neck cancer risk
have been published nowadays [ 35 – 38 ]. But con-
sidering the modest sample size and different
study designs, the evidence still remains contro-
versial. Therefore, a quantitative and systematic
summary of the evidence using rigorous methods
is necessary. We know that meta-analysis is the
use of statistical methods to combine results of
individual studies. This allows us to make the
best use of all the information we have gathered
in our systematic review, and by statistically
combining the results of similar studies, we can
improve the precision of our estimates of treat-
ment effect and assess whether treatment effects
are similar in similar situations. Recently, some
Chinese researchers from Guangxi Medical
University conducted a meta-analysis involving
5,204 patients and 5,518 controls to assess the
inconsistent results from published studies on the
association of tooth loss with head and neck can-
cer risk [ 39 ]. Their overall estimates provided
evidence that tooth loss was signifi cantly associ-
ated with increased risk of head and neck cancer.
In addition, the moderate [ 6 – 15 ] tooth loss and
the severe (>15) tooth loss experienced a signifi -
cantly increased risk of head and neck cancer by
18 and 54 %, respectively. Furthermore, the mod-
erate [
6 – 15 ] tooth loss was associated with a
45 % increase in the risk of larynx cancer. The
authors also summarized that several plausible
mechanisms may explain why a signifi cant
increased association of tooth loss with head and
neck cancer was observed in their analysis.
Whether tooth loss is an independent risk fac-
tor of head and neck cancer is an interesting
question. But the answers have not reached con-
sistent conclusions yet. Guha et al. observed that
missing 6–15 teeth increased the odds ratio of
esophageal squamous cell carcinoma by more
than twofold in both Latin America and central
Europe. However, when missing teeth were more
than 15 in number, no increase risk was observed
[ 40 ]. On the other hand, Wang et al. found that
moderate and severe tooth loss did not change
such an association, suggesting that tooth loss is
probably an independent risk factor of head and
neck cancer [ 39 ].
8.2.4 Cariogenic Bacteria and Oral
Cancer
Alcohol is one of the main risk factors for oral
cancer. Alcohol itself is not carcinogenic, but it is
oxidized to carcinogenic acetaldehyde in saliva
by the ADH enzyme of some oral microbes of the
normal oral microfl ora. Oral streptococci, espe-
cially S. mutans , are the primary pathogens caus-
ing dental caries, and Neisseria strains are related
to the early stage of caries. About a decade
before, some Neisseria strains are found to be
able to produce signifi cant amounts of acetalde-
hyde, probably via their high alcohol dehydroge-
nase (ADH) activity [ 41 ]. Neisseria strains are
considered to be part of the normal oral fl ora, but
they are found only in low numbers in the oral
cavity. Later, in 2007, oral streptococci were
proved to contribute signifi cantly to the normal
individual variation of salivary acetaldehyde lev-
els after alcohol drinking and thereby also to the
risk of oral cancer [ 42 ]. We believe that the effect
of cariogenic bacteria on oral cancer provides
some evidence between caries and cancer from
another side.
8.3 Dental Caries and Children
Growth
There’s no doubt that dental caries constitutes the
single most common chronic disease of child-
hood: as many as 60 % of school children have
experienced dental caries, and the data can reach
as high as 90 % in some countries according to
the report of World Health Organization (WHO)
[ 43 ]. Among 5- to 17-year-olds, dental decay is
fi ve times as common as asthma and seven times
as common as hay fever [ 44 ]. Current evidences
Z. Ling and H. Tao

135
show that dental caries is a multifactorial disease
and complexly modulated by genetic, behavioral,
social, and environmental factors [ 45 ]. A recent
descriptive cross-sectional study assessed dental
caries experience among 12-year-old school chil-
dren from low socioeconomic status background
attending public primary schools in Zimbabwe.
The results showed that there was a high preva-
lence of dental caries in both urban (59.5 %) and
rural (40.8 %) children [ 46 ]. While most people
in rural areas in Zimbabwe cannot afford and per-
ceive these sugary products as non-benefi cial,
affording them is often considered as a symbol of
higher socioeconomic status. Another retrospec-
tive cohort study gave support to the idea that
children who lived in urban areas showed 75 %
greater probability of presenting caries when
compared to those children residing in rural areas
[ 47 ]. This disparity between urban and rural chil-
dren has been partially attributed to increased
access and consumption of high sugar-containing
foods and beverages in urban areas [ 48 ]. Based
on the recent studies, socioeconomic status has
been shown to be a major risk for caries inci-
dence. Children living in poverty represent a
large population of high-risk individuals who
have undiagnosed and untreated diseases coupled
with limited access to care. Nearly twice the pro-
portion of US children with family incomes less
than the federal poverty level (FPL) show decay
of the primary or permanent dentition (55 %),
compared to those whose family incomes are
greater than 200 % of the FPL (31 %). Low-
educated and low-income families that pay less
attention to the dental hygiene of their children
may be one of the reasons [
49 ].
Apart from structurally weakening teeth, den-
tal caries can lead to infection, pain, abscesses,
chewing problems, poor nutritional status, and
gastrointestinal disorders. Moreover, serious car-
ies can damage a child’s sense of self-esteem,
which in turn may affect his or her school perfor-
mance, ability to learn, and potential to thrive
[ 50 ]. Specifi cally, in young children, there is a
relationship between dental caries and childhood
obesity [ 51 , 52 ]. Dental caries can also contribute
to poor nutritional status and affect the growth of
adult teeth [ 53 ]. In addition, children with
extensive dental caries may need to undergo
treatment under general anesthesia in hospital.
This is a signifi cant side effect of childhood car-
ies that is widely acknowledged by the experts. It
is essential to remember that dental caries is one
of only very few common childhood diseases
which cause large numbers of the child popula-
tion to undergo general anesthesia.
The relationship between dental caries and
child’s body weight was fi rstly noticed by Miller
30 years ago [
54 ]. Caries of the primary teeth or
“early childhood caries” (ECC) is one of the most
prevalent health problems in infants and toddlers
[ 55 ]. A recent study found a positive correlation
between severe early childhood caries (S-ECC)
and body mass index (BMI) of 3- and 6-year-old
children, which means the mean BMI of S-ECC
children is signifi cantly more than the caries-free
children [ 56 ]. We know that if caries involve the
pulp, the eating of some foods will cause pain;
therefore, toothache and infection alter eating
and sleeping habits, dietary intake, and metabolic
processes [ 57 ]. For example, some of the patients
may thereby avoid certain nutritious foods and
select high-calorie, high-fat food, which is recog-
nized as risk factors for obesity. On the other
hand, some patients cannot pulverize the foods
well and may have an adverse effect on the inter-
nal absorption of nutrients. But if such bad oral
condition has been changed, the children’s
growth will be better. In 2009, Malek et al. con-
ducted a longitudinal clinical trial study to exam-
ine whether the removal of carious teeth affected
children’s growth relative to that of a standard
population. Five- and six-year-old children who
attended for extraction of carious teeth under
general anesthesia took apart in this study. The
children’s dental caries levels, weight, and height
were measured prior to extraction using standard
criteria and a single trained examiner, and they
were then remeasured 6 months later. The partici-
pants had a mean dmft of 7.18 (SD 3.27) at base-
line, and at follow-up children showed a
statistically signifi cant gain in BMI SDS and a
small gain in height SDS [
58 ]. In their another
longitudinal birth cohort, Kay et al. found that
children who had caries at 61 months had slower
increases in weight and height than those without
8 Dental Caries and Systemic Diseases

136
decay at the same age [ 59 ]. These observations
were consistent with a recent study which exam-
ined the association between untreated dental
caries in primary and permanent teeth with age-
adjusted height and weight among 6–12-year-old
children in Bangladesh [ 60 ].
However, the relationship between dental car-
ies and child’s growth is inconclusive so far. A
research from the department of cardiology,
endodontology, and pedodontology in Academic
Centre for Dentistry Amsterdam (ACTA) has
been published in Clin Oral Investig in 2011. The
study has two objectives: fi rst, to assess the rela-
tion between dental caries and body proportions
cross-sectionally in a Suriname caries child pop-
ulation and, second, to investigate whether dental
treatment had a signifi cant infl uence on body
growth of these children in a randomized con-
trolled trial using different treatment strategies.
Three hundred eighty 6-year-old children with
untreated dental decay participated in the study.
Participants were evaluated after 6 months and 1,
2, and 3 years. However, negative correlations
were observed between anthropometric measures
and the number of untreated carious surfaces and
caries experience of the children. Next, no sig-
nifi cant differences in growth pattern between the
treatment groups were observed. Thus, the
authors suggested that caries activity is a negative
predictor for body growth in children, and dental
intervention does not show signifi cant improve-
ment within 3 years [ 61 ]. Later, Merrilyn et al.
undertook an updated systematic review of the
relationship between body mass index and dental
caries in children and adolescents. The authors
searched MEDLINE, ISI, Cochrane, Scopus,
Global Health, and CINAHL databases and con-
ducted lateral searches from reference lists for
papers published from 2004 to 2011, inclusive.
Finally, a total 48 studies were included. Three
main patterns of relationships were found
between dental caries and BMI: 23 of the 48
studies found no association between BMI and
dental caries, 17 found a positive relationship
between BMI and dental caries, and 9 found an
inverse relationship. The reasons that authors
analyzed may be method of dental examination,
sample differences, dental caries prevalence, and
BMI distribution. And they also recommend that
future research investigate the nature of the asso-
ciation between body mass index and dental car-
ies in samples that include a full range of body
mass index scores and explore how factors such
as socioeconomic status mediate the association
between body mass index and dental caries [ 62 ].
8.4 Dental Caries
and Atherosclerosis,
Cardiovascular Disease,
and Heart Attack
Atherosclerosis (also known as arteriosclerotic
vascular disease or ASVD) is a specifi c form of
arteriosclerosis in which an artery wall thickens as
a result of the accumulation of fatty materials such
as cholesterol and triglyceride. Cardiovascular
disease ( CVD ) is the broad term used to categorize
any abnormal condition characterized by dysfunc-
tion of the heart and blood vessel system, princi-
pally referring to cardiac disease, vascular diseases
of the brain and kidney, and peripheral arterial dis-
ease. Evidence suggests a number of traditional
risk factors for atherosclerosis and CVD: age, gen-
der, high blood pressure, high serum cholesterol
levels, tobacco smoking, excessive alcohol con-
sumption, sugar consumption [ 63 ], family history,
obesity, lack of physical activity, psychosocial fac-
tors, diabetes mellitus, and air pollution [ 64 ].
However, these factors cannot explain all the
deaths from CVD. For example, about 40 % of
coronary heart disease (CHD) deaths occur in peo-
ple with cholesterol levels that are lower than the
population average [
65 ]. Therefore, medical
researchers’ attention has focused in recent years
on identifying additional risk factors that are non-
traditional but may play major roles in explaining
some of the variability in atherosclerosis and CVD
risk.
During the last three decades, there has been
an increasing interest in the impact of oral health
on atherosclerosis and subsequent cardiovascular
disease (CVD). Just as Meurman et al. wrote in
their paper which was published in Crit Rev Oral
Biol Med : “chronic infections caused by a variety
of micro-organisms are thought to be involved in
Z. Ling and H. Tao

137
the etiopathogenesis of CVD by releasing cyto-
kines and other pro-infl ammatory mediators that
may initiate a cascade of biochemical reactions
and cause endothelial damage and facilitate cho-
lesterol plaque attachment. Yet, due to the multi-
factorial nature of dental infection and CVD,
confi rming a causal association is diffi cult, and
the published results are confl icting. The main
defi cit in the majority of these studies has been
the inadequate control of numerous confounding
factors, leading to an overestimation and the
imprecise measurement of the predictor or over
adjustment of the confounding variables, result-
ing in underestimation of the risks” [ 66 ].
8.4.1 Dental Caries
and Atherosclerosis
and Cardiovascular Disease
Many studies have looked at poor dental care as a
risk factor for cardiovascular disease (CVD). The
results have been inconsistent, but most studies
support a modest association between them [ 67 ].
Mattila et al. may be one of the fi rst researchers
to indicate a relationship between orofacial infec-
tions and cardiovascular disease. In 1989, they
published an article in British Medical Journal
(BMJ) and reported that there was an unexpected
correlation between dental disease and systemic
disease. After adjusting for age, exercise, diet,
smoking, weight, blood cholesterol level, alcohol
use, and health care, people who had caries and
periodontal disease had a signifi cantly higher
incidence of acute myocardial infarction [
68 ].
Another prospective cohort study, published in
1993, found that patients with periodontal dis-
ease had a 25 % increase in CVD, and men
younger than 50 years had a signifi cantly higher
risk. However, no association between extent of
active dental decay and risk of coronary heart dis-
ease was observed. Since tooth loss in people
under 60 is usually caused by dental caries, the
authors said they cannot rule out the possibility
that the increased risk of coronary heart disease
among young men with no teeth may have been
related to previous dental decay [ 69 ]. These
important discoveries resulted from the study is
not the only reason that makes it noteworthy.
Actually, the 9,760 subjects included in this work
make it the largest sample size of its kind at that
time. Since then, investigating the relationship
between dental disease and CVD has become a
priority.
Later, in 2001, a prospective cohort study in
Stockholm, Sweden, followed 1,393 individuals
for 27 years and concluded that oral health was a
risk factor for death due to CVD, especially in
combination with smoking, another risk factor.
In this investigation, a signifi cant correlation
between caries and death due to CVD when
adjusted for age and gender was demonstrated,
indicating that this possible etiological pathway
should be further investigated in the future. And
the number of tooth surfaces with caries and pres-
ence of plaque were signifi cantly increased for
smokers compared to nonsmokers [
69 ].
Maharaj and Vayej studied 44 black patients
with severe rheumatic heart disease before they
had cardiac surgery in 2012. Abnormalities were
detected in the panoramic radiographs of 84.1 %
of patients. The most frequent lesion was caries,
present in 56.8 % of patients, followed by miss-
ing teeth in 54.5 %, and impacted teeth in 25 % of
patients. Retained roots were present in 22.7 %
and periapical pathology was detected in 18.1 %
of patients [ 70 ].
It is clear that minimal carious lesions, caries
with and without involvement of the pulpal cavity,
and chronic apical periodontitis (CAP) represent
different stages of the same infl ammatory pro-
cess. A recent study shows for the fi rst time that
dental caries, pulpal caries, and chronic apical
periodontitis are associated positively, while res-
torations are associated inversely, with aortic ath-
erosclerotic burden [
71 ]. The authors’ result
showing that not only CAP but also caries with
pulpal decay or no visible pulpal decay was asso-
ciated with a greater atherosclerotic burden was
somewhat surprising. We know that early stage of
caries is an infl ammatory process localized in the
oral cavity that does not affect the pulpal cavity or
the bone, indicating that a lesser extent of associa-
tion of the early stage of caries with the athero-
sclerotic burden was expected than with the other
two serious stages. One obvious explanation for
8 Dental Caries and Systemic Diseases

138
this fi nding may be the covariance of these fac-
tors, as pulpal caries and CAP occur primarily in
patients with extensive tooth decay. The initial
carious lesion and caries not yet affecting the
pulpal cavity exist for a longer period compared
with the pulpal decay, which can precede pulpal
decay by a number of years. An explanation other
than the disease lasting many years is that even
forms of caries not yet involving the pulp are not
merely local infl ammatory lesions but rather dis-
ease affecting the entire body. The authors sug-
gested that prospective studies are required to
confi rm these observations and answer the ques-
tion of possible causality [ 71 ].
8.4.2 Root Caries and Cardiac
Dysrhythmia
and Gerodontology
Cardiac dysrhythmias, especially atrial fi brilla-
tion, are known to cause ischemic heart disease.
Many studies suggested that infl ammation plays
a prominent role in the onset of atrial fi brillation
[ 72 ]. With respect to the result of logistic regres-
sion analysis, cariogenic bacteria have a specifi c
impact on the pathogenesis of cardiac diseases,
especially dysrhythmias [ 73 ]. In 2005, research-
ers from University of Copenhagen designed a
cross-sectional study to examine whether caries
is associated with cardiac arrhythmias in
community- dwelling people aged 80 and older.
The primary fi nding of the multivariate logistic
regression analysis was that persons with three or
more active root caries lesions had more than
twice the odds of cardiac arrhythmias than per-
sons without active root caries. The fi ndings indi-
cated that there may be a link between active root
caries and cardiac arrhythmias in the oldest old
[
74 ]. In order to explain the link, we should turn
to the immune response because several studies
have reported that an increase in dental caries is
associated with a heightened immune response.
In addition, dental caries affects the production
of IgG and induces acute-phase proteins. The
infl ammatory-mediated cytokines and acute-
phase proteins are practical markers of increased
risk of cardiovascular disease, such as C-reactive
protein (CRP), interleukin-6 (IL-6), and tumor
necrosis factor-α (TNF-α) [
75 ]. Bacterial species
lying within the root surfaces supporting struc-
tures induce systemic infl ammation and immune
response, thereby increasing levels of serum CRP
and serum IgG. In 2011, Kaneko et al. conducted
a longitudinal study to elucidate the relationship
between root caries and the onset of dysrhyth-
mias on electrocardiographs in community-
dwelling persons aged 75 and older. Serum CRP
level was used as a variable to link root caries
with dysrhythmias directly. They found that a
high mean CRP serum level group had a signifi -
cantly higher number of sites with root caries
than a low CRP group. Moreover, number of sites
with root caries events was signifi cantly associ-
ated with cardiac dysrhythmia among nonsmok-
ers. These results confi rmed that root caries is
related to the incidence of dysrhythmias in non-
smokers [ 73 ].
8.4.3 Streptococcus mutans
and Atherosclerosis
We know that Streptococcus mutans ( S. mutans )
is a major cariogenic pathogen that is a normal
inhabitant of the oral cavity in most individuals.
S. mutans has also been isolated from the blood
of patients with infective endocarditis (IE), which
strongly suggests a close relationship of the
pathogen with IE [ 76 , 77 ]. Ullman et al. pointed
out that their experience agrees with the literature
and indicates that S. mutans is primarily a patho-
gen in elderly patients with heart disease and may
be associated with IHSS [
78 ]. In 2006, Nakano
from Osaka University Graduate School of
Dentistry and coworkers published the fi rst study
to analyze the presence of streptococcal species
in diseased heart valve and atheromatous plaque
specimens, as well as in dental plaque samples
from the same subjects by a PCR method.
Unexpectedly, S. mutans was detected at high
frequencies and quantities in both heart valve tis-
sues and atheromatous plaque samples in their
study. Their conclusion indicated that S. mutans
is a possible causative agent of cardiovascular
disease [ 79 ]. In addition, when using DNA
Z. Ling and H. Tao

139
fi ngerprinting to compare S. mutans isolated
from dental plaque and an infected heart valve
from a patient who underwent heart surgery,
Nomura and colleagues demonstrated that the
oral isolates differed from those found in the
heart valve [ 80 ]. Three years later, Nakano et al.
published another paper titled “Detection of oral
bacteria in cardiovascular specimens” in Oral
Microbiology and Immunology . This time, they
found that S. mutans was the most frequently
detected species in the cardiovascular specimens,
followed by Aggregatibacter actinomycetem-
comitans . Furthermore, the positive rate of
S. mutans in cardiovascular specimens from
patients whose dental plaque specimens were
also positive for S. mutans was 78 %, which was
signifi cantly higher than any other tested species
when the same analysis was performed [ 81 ].
Collectively, these fi ndings lend credence to the
idea that there are subpopulations of S. mutans
carried in humans that, while not necessarily
associated with caries, may have an enhanced
capacity to interact, and possibly invade, the cells
of the cardiovascular system [ 82 ]. S. mutans is
classifi ed into four serotypes (c/e/f/k) based on
the chemical composition of its cell surface sero-
type-specifi c rhamnose–glucose polymers
(RGPs), which form a backbone of rhamnose
polymers with side chains of glucose polymers.
Serotype c is reported to be the most prevalent in
oral isolates at approximately 70–80 %, followed
by e, f, and k. Serotypes e and f have been found
to invade endothelial cells [
82 ]. Serotype k, with
a defect of the glucose side chain in RGPs, was
found to show low cariogenicity but high viru-
lence in blood as compared to the other sero-
types, due to alterations of several cell surface
structures [ 83 ]. When it comes to the possible
reasons of the link between S. mutans and cardio-
vascular system diseases, it may be summarized
as the following:
1. One crucial step for the development of ath-
eromatous plaque lesions is formation of foam
cells, which are macrophages that accumulate
from excess cholesterol, and S. mutans strain
GS-5 has been shown to enhance their forma-
tion [ 84 ].
2. In addition, S. mutans were shown to induce
platelet aggregation, which presumably lead to
thrombus formation. It was also found that S.
mutans cells bind to extracellular matrix mol-
ecules and fi brinogen with contribution from
the major surface protein antigen Pac [
85 , 86 ] .
3. The infection with S. mutans expressing
collagen- binding protein (CBP) is a potential
risk factor for hemorrhagic stroke [ 87 ]. Lately,
two types of cell surface collagen-binding
proteins, Cnm and Cbm, have been studied to
see if they play a role in S. mutans attachment
and invasion of human umbilical vein endo-
thelial cells (HUVEC). The results found that
most of the Cbm-positive strains showed
higher levels of binding to type I collagen as
well as higher rates of adhesion and invasion
with HUVEC as compared to the Cnm-
positive strains. Furthermore, the gene encod-
ing Cbm was detected signifi cantly more
frequently in heart valve specimens from IE
patients than from non-IE patients [ 88 ].
8.4.4 Tooth Loss
and Cardiovascular Disease
and Stroke
While some studies have shown that decay is not
a direct risk factor, it can and does cause tooth
loss, which has been demonstrated to be a greater
risk for cardiovascular disease [ 89 , 90 ]. Recent
evidence showed a direct link between oral health
and CVD and that the number of teeth can be
used to assess increased risk of CVD in adults.
They drew the conclusion from a fairly large
( n = 7,647), prospective study with a long follow-
up period (1976–2002) that presents for the fi rst
time a dose-dependent relationship between
number of teeth and both all-cause and CVD
mortality. The authors found that a person with
fewer than 10 of their own teeth remaining is
seven times more likely to die of coronary dis-
ease than someone with more than 25 of their
own teeth [
91 ].
As pointed out by Watt et al., although there is
increasing epidemiological evidence linking poor
oral health with the development of chronic diseases
8 Dental Caries and Systemic Diseases

140
and mortality, these associations are still doubtful
due to imprecise measurement of important risk
factors of systemic disease. Indeed, most previous
studies exploring the link between tooth loss and
systemic disease have been conducted in selected
samples and have failed to control adequately for
socioeconomic, behavioral, and general health sta-
tus [ 92 ]. Thus, their recent prospective cohort study
of a national sample of Scottish adults published in
PLOS ONE caught our attention. The sample con-
sisted of 12,871 participants and they were followed
for 8.0 (SD: 3.3) years. During 103,173 person-
years, there were 1,480 cases of all-cause mortality,
498 of CVD, and 515 of cancer. After adjusting for
demographic, socioeconomic, behavioral, and
health status, edentate subjects had signifi cantly
higher risk of all-cause and CVD mortality com-
pared to subjects with natural teeth only. Their fi nd-
ings confi rm previous studies which have shown a
small but signifi cant association between tooth loss
and all-cause and CVD deaths after controlling for
a range of potential confounding factors [ 92 ].
However, there were different opinions from
other studies, such as the study using the data from
the Glasgow Alumni Cohort to investigate whether
oral health in young adulthood is independently
associated with cause‐specifi c mortality after
accounting for childhood socioeconomic back-
ground and other risk factors in young adulthood.
Over 12,000 subjects (30 years or younger at base-
line) were traced during up to 57 years of follow‐
up, and 1,432 deaths occurred among subjects
with complete data, including 509 deaths from
CVD and 549 from cancer. When the number of
missing teeth was treated as a categorical variable,
there was evidence that students with nine or more
missing teeth at baseline had an increased risk of
CVD compared with those with fewer than fi ve
missing teeth. When the number of missing teeth
was transformed using fractional polynomials,
there seemed to be a nonlinear relation between
missing teeth and CVD mortality [
93 ].
Stroke remains the third leading cause of death
(after heart disease and cancer) in most developed
countries. Cerebrovascular ischemic strokes are
the commonest kind of stroke and occur as a result
of an obstruction, usually a clot, within a blood
vessel supplying blood to the brain. Heitmann and
Gamborg examined if the number of remaining
teeth was associated with the development of car-
diovascular morbidity and mortality over 5–12
years. The prospective observational study
among1474 men and 1458 women born 1922,
1932, 1942 or 1952 from The Danish MONICA
follow up study (monitoring trends in and determi-
nants of cardiovascular disease) in 1987–88 and
1993–94. Their results showed that tooth loss was
strongly associated with incidence of stroke and,
to a lesser extent, incidence of cardiovascular dis-
ease and coronary heart disease, during averagely
7.5 years of follow-up [
94 ]. Choe et al. conducted
a prospective cohort study of stroke in Korea on
hypertension, diabetes, smoking, and tooth loss to
characterize their independent effects and interac-
tions. They confi rm that tooth loss is independently
associated with increased risk of stroke, and hyper-
tension does interact antagonistically, particularly
for hemorrhagic stroke [ 95 ]. A recent study found
that stroke patients in their 50s and 60s had signifi -
cantly fewer remaining teeth than did patients hos-
pitalized for other conditions in the corresponding
age groups. Moreover, the number of remaining
teeth was signifi cantly lower among stroke patients
in their 50s than data reported for that age group in
the Survey of Dental Diseases, suggesting the pos-
sibility that stroke patients may have lost teeth at a
younger age. The authors also pointed out that the
association between stroke and tooth loss can be
explained by common risk factors associated with
lifestyle such as hypertension, diabetes, smoking,
and alcohol intake. It is quite diffi cult to rule out all
common risk factors as confounding variables;
therefore, the exact mechanisms of the relationship
between stroke and tooth loss are diffi cult to iden-
tify [
96 ]. Interestingly, periapical lesions, normally
resulting from an infected root canal (caused by
caries), are also a factor in stroke risk. This is
another example of how dental caries can play a
role, however indirectly, in heart disease [ 5 ].
8.4.5 Pulpal Periapical Diseases
and Coronary Heart Diseases
Endodontic infl ammation occurs after bacteria or
their byproducts enter a tooth’s pulp chamber.
Z. Ling and H. Tao

141
Apical periodontitis is an acute or chronic
infl ammatory lesion around the apex of a tooth root
which is caused by bacterial invasion of the pulp of
the tooth. Despite numerous differences between
chronic infl ammatory disease of periodontal and
endodontic origins, Caplan et al. summarized their
similarities, primarily that: (1) both conditions share
a common microbiota that often is associated with
Gram-negative anaerobic bacteria, and (2) elevated
systemic cytokine levels have been observed in con-
junction with both disease processes [ 97 ]. Since
several epidemiologic investigations have uncov-
ered relationships between chronic periodontal dis-
ease and coronary heart disease, links between
endodontic infl ammation and cardiovascular out-
comes are biologically plausible. Mechanisms link-
ing endodontic disease to CHD might be similar to
those hypothesized for associations between peri-
odontal disease and CHD, in which a localized
infl ammatory response to bacterial infection leads
to release of cytokines into the systemic circulation
and to subsequent deleterious vascular effects [ 98 ].
Frisk and colleagues are one of the fi rst groups who
specifi cally studied endodontic variables as risk fac-
tors for the development of CHD. However, they
did not reveal a signifi cant association between end-
odontically treated teeth and CHD nor between
teeth with periapical disease and CHD from their
population-based study of Swedish women [ 99 ].
Joshipura et al. found a possible modest association
between pulpal infl ammation and CHD, although
dental caries was not associated with CHD. They
observed a greater CHD incidence among men with
a positive self-reported history of ET in the Health
Professionals Follow-Up Study [
100 ].
8.4.6 Summary
As stated above, several epidemiologic investiga-
tions have uncovered relationships between den-
tal caries (including its subsequent pulpal
periapical diseases and tooth loss) and coronary
heart disease. However, other studies have not
found signifi cant relationships, sparking ques-
tions about the proposed association. In the
future, more direct evidence should be found to
support this connection.
8.5 Dental Caries and Immune
System Disease
It’s no doubt that infection stresses the immune
system. So dental infections, especially long-
term disease (such as periapical abscesses),
should have a deleterious effect on the immune
system. Dental caries is an infectious disease that
occurs because of imbalance in the homeostasis
between the host and microbiota [ 101 ]. Salivary
innate and adaptive immune defenses may infl u-
ence in the bacterial colonization, and some dis-
orders can affect these systems such as general
immune defi ciencies associated with malnutri-
tion, inherited or medication disorders, or other
factors that affect salivary fl ow and saliva compo-
sition [ 102 ].
8.5.1 Salivary Immunoglobulin A
Oral microorganisms and aerodigestive antigens
are continuously infl uenced by the two major anti-
body classes in saliva: SIgA and IgG. The former
is the dominant immunoglobulin in the healthy
mouth which is produced by gland- associated
immunocytes. It can agglutinate oral bacteria,
modulate enzyme activity, and inhibit the adher-
ence of bacteria to the buccal epithelium and to
enamel. It does well at interfering with the initial
colonization of caries-associated microfl ora on the
tooth surface. Thus, a protective role for salivary
sIgA was postulated [
103 – 105 ]. However, about
the relationship between Salivary IgA and dental
caries, different studies got contrary results and
conclusions. In 1978, Challacombe S. J. did not
fi nd that salivary antibodies in man play a role in
protection against caries [ 106 ]. Several years later,
Gregory et al. found that caries-free subjects or
individuals with low caries susceptibility exhibited
signifi cantly higher levels of naturally occurring
salivary immunoglobulin A (IgA) and serum IgG,
IgA, and IgM antibodies to a Streptococcus mutans
ribosomal preparation than subjects with high car-
ies susceptibility [ 107 ]. Recently, some Indian
researchers from MAR Dental College carried out
a protocol-driven cross-sectional pilot study to
check the purported association between salivary
8 Dental Caries and Systemic Diseases

142
sIgA and dental caries with special reference to
RA. Rheumatoid arthritis (RA) is an autoimmune
disease that results in a chronic, systemic infl am-
matory disorder that may affect many tissues and
organs, but principally attacks fl exible (synovial)
joints. It can be a disabling and painful condition,
which can lead to substantial loss of functioning
and mobility if not adequately treated. Forty-eight
patients with RA and 102 non-RA, healthy case
controls were taken part in the study. The decay,
missing teeth, fi lled teeth (DMFT) index was used
to classify caries. Whole unstimulated saliva was
collected to assay sIgA using a commercial ELISA
kit. The results showed that there were no statisti-
cally signifi cant differences between RA and non-
RA subjects with respect to salivary sIgA and the
extent of caries [ 104 ]. It’s hard to explain all the
contrary results, since all the studies had their limi-
tations, such as the study designs, if the sample
size was insuffi cient or not, or technical variables
also infl uence biomarkers and antibodies in oral
secretions. Further studies should be carried out to
fi nd the truth.
8.5.2 HIV and Dental Caries
According to the World Health Organization,
globally, 34.0 million [31.4–35.9 million] people
were living with HIV at the end of 2011. In 2004,
Phelan J. A. et al. published a paper in Journal of
Dental Research titled “Dental caries in HIV-
seropositive women.” The authors conducted the
study to determine if there was an association
between HIV infection and dental caries among
women enrolled in the Women’s Interagency HIV
Study. Subjects included 538 HIV+ and 141
HIV− women at baseline and 242 HIV+ and 66
HIV− women at year 5. Caries indices included
DMFS and DFS (coronal caries) and DFSrc (root
caries). Cross-sectional analysis of coronal caries
data revealed a 1.2-fold-higher caries prevalence
among HIV+ women compared with HIV−
women. Longitudinally, DMFS increased with
increasing age and lower average stimulated sali-
vary volume. Root caries results were not signifi -
cant except for an overall increased DFSrc
associated with smoking [
108 ]. At the same year,
Mulligan R. et al. also reported that seropositive
women who fi t the Center for Disease Control
(CDC) AIDS criteria were also more likely to
have more DMF teeth ( P = 0.004), DMF surfaces
( P = 0.003), and decayed and/or fi lled (DF) root
surfaces ( P = 0.0002) compared to seropositive
women without AIDS [
109 ].
It is estimated worldwide that there are 2.3
million human immunodefi ciency virus (HIV)-
positive children from 0 to 14 years infected by
mothers [ 110 ]. However, prior to 1992, informa-
tion about dental caries in HIV-infected children
was very limited. Between 1992 and 1996, there
were three published cross-sectional studies of
dental caries in the primary teeth of HIV-infected
children [ 111 ]. These studies showed that there
was a higher prevalence of dental caries (includ-
ing early childhood caries (ECC)) in the primary
dentition of HIV-infected children as compared
to healthy children [ 112 – 114 ]. However, it has
not always been clear from past studies exactly
what factors bring about these differences that
exist in dental caries between HIV-infected chil-
dren and a noninfected control group. In order to
exclude the common environmental factor,
Tofsky et al. compared the baseline prevalence
and 2-year incidence of dental caries found in
both the primary and secondary dentition among
a group of HIV-infected children as compared to
their HIV-negative household peers. They found
that HIV-infected children have a high prevalence
of dental caries in the primary teeth and a low
prevalence in permanent teeth, while the inci-
dence of permanent tooth dental caries is less
than that of a group of noninfected household
peers [
115 ]. And reasons are still unknown.
It has been hypothesized that immunodefi -
ciency and a progressive decrease in CD4+
T-lymphocytes resulting from HIV infection
might alter salivary fl ow rate and impair the
secretory immune system, thus contributing to
increased bacterial colonization in the oral cavity
implying that cariogenic bacteria may also
increase in the oral cavity [ 116 ]. Those changes
could be contributing factors for the development
of HIV-associated oral diseases, including
increased prevalence of dental caries. A recent
study by Liu and coworkers examined the effect
Z. Ling and H. Tao

143
of HIV infection on the level and genotypic char-
acteristics of S. mutans colonization. They found
that HIV+ individuals experienced signifi cantly
higher levels of S. mutans . Interestingly, the level
of S. mutans signifi cantly correlated with CD8+
count, but not with viral load or CD4+ counts,
which is clearly suggesting that other HIV-
associated factors mechanistically mediate S.
mutans colonization in saliva. And they also
revealed decreased salivary fl ow rate in the HIV+
group [ 117 ]. Since more dental caries was evi-
denced in HIV+ individuals from this study and
others, additional studies are required to eluci-
date and understand the correlation between the
colonization of other cariogenic microbes,
including S. mutans , and the status of immuno-
suppression at the advanced stages of HIV
infection.
8.6 Dental Caries and Kidney
Diseases
Chronic kidney disease (CKD), also known as
chronic renal disease, is a progressive loss in
renal function over a period of months or years.
The symptoms of worsening kidney function are
nonspecifi c and might include feeling generally
unwell and experiencing a reduced appetite.
Often, chronic kidney disease is diagnosed as a
result of screening of people known to be at risk
of kidney problems, such as those with high
blood pressure or diabetes and those with a blood
relative with chronic kidney disease. Chronic
kidney disease may also be identifi ed when it
leads to one of its recognized complications, such
as cardiovascular disease, anemia, or pericarditis.
In addition to the systemic manifestations and
complications that arise from the disease and its
treatment, changes in the mouth are common in
patients with chronic kidney disease (CKD).
Poor oral health in CKD patients may thus repre-
sent an important, but often overlooked, problem
[
118 , 119 ].
Several studies have demonstrated higher
rates of oral pathology in dialysis patients with
one or more oral symptoms such as xerostomia,
taste disturbances, uremic odor, tongue coating,
mucosal infl ammation, oral ulceration, or enamel
hypoplasia. We know that xerostomia (or dryness
of the mouth) may predispose to caries and gingi-
val infl ammation as well as contribute to diffi cul-
ties with speech, denture retention, mastication,
dysphagia, sore mouth, loss of taste, and infec-
tions. Studies in the general population suggest
that edentulous subjects are prone to have an
inappropriate dietary intake (such as ingesting
too little protein and too much calorie-rich, high-
fat food) as compared with dentated persons.
Whereas the number of teeth is of importance for
masticatory function, having premolar and molar
teeth is especially important for nutritional sta-
tus. The increased periodontitis and dental caries
rates of CKD patients lead to tooth loss, which
may result in chewing diffi culties because of
inadequate occlusive surfaces or the limitations
of prostheses. On the other hand, approximately
30 % of patients with advanced CKD are reported
to have a “bad” or a “metallic” taste in their
mouths, which has been associated with meta-
bolic changes, diverse drugs, a reduced number
of taste buds, and changes in both salivary fl ow
rate and composition. Increased dental calculus
has been observed, perhaps as a consequence of a
high salivary urea and phosphate levels.
When it comes to the relationship between
dental caries and CKD, different researches got
different results, and sometimes the conclusions
were contradictory. Some studies showed that
uremic patients have higher rates of decayed,
missing, and fi lled teeth, loss of attachment, and
periapical and mucosal lesions than the general
population. The consequences of poor oral health
may be more severe in CKD patients because of
advanced age, common existing additional dis-
eases such as diabetes, concurrent medications,
and a state of reduced immune function that may
increase the risk for consequences of periodonti-
tis and other oral and dental conditions [ 120 ].
Some researchers reported that the prevalence of
dental caries was low in children with renal dis-
ease [ 121 , 122 ]. As early as 1985, Peterson and
his colleagues got data to support the hypothesis
that the relative paucity of caries in patients with
chronic renal failure results from alteration of
plaque by metabolic end products of urea
8 Dental Caries and Systemic Diseases

144
metabolism. The data further suggested that
transplanted patients whose renal function is nor-
mal may be at increased risk of caries, especially
if enamel hypoplasia is present and oral hygiene
is poor [ 123 ]. Others have not found any evi-
dence that the prevalence of dental caries in per-
manent teeth is signifi cantly different in CKD
children when compared with healthy children
[ 124 , 125 ].
A recent systematic review published in
Pediatric Nephrology tried to determine whether
there is any evidence in the literature referring to
a lower prevalence of dental caries in children
and adolescents with chronic kidney disease
(CKD) compared to healthy individuals. After
the evaluation of title, keywords, and abstracts of
the articles selected, six articles met the inclusion
criteria. Three of these six articles included stud-
ies which showed susceptibility to bias and pos-
sible confounding factors. A subsequent
assessment of the six studies revealed that the
mean caries indices in both primary (dmf) and
permanent (DMF) teeth were lower in the chil-
dren and adolescents with CKD compared with
healthy individuals. In these patients, the low
prevalence of dental caries may be associated
with salivary characteristics, especially the neu-
tralization of end products of bacterial plaque
due to the increased pH resulting from urea
hydrolysis in the saliva. So the authors concluded
that data in the literature weakly support a lower
prevalence of caries in children and adolescents
with CKD than in their healthy counterparts
[
126 ]. There is still a lack of well-designed stud-
ies that provide better scientifi c evidence in sup-
port of this conclusion.
8.7 Dental Caries
and Gastrointestinal
Diseases
To date, the most signifi cant relationship between
dental caries and gastrointestinal diseases is from
chewing pain and tooth loss. As we mentioned
before, carious teeth become pulpitic; the eating
of some foods will cause pain; therefore tooth-
ache and infection alter eating and sleeping
habits, dietary intake, and metabolic processes.
Moreover, the edentulous patient without den-
tures is the most vulnerable to gastrointestinal
and other related problems. In the edentulous
person with a defi cient masticatory performance,
reduced consumption of fi ber-rich foods that are
hard to chew could provoke gastrointestinal dis-
turbances. One study provided a sound basis for
why the denture wearer does not achieve the nec-
essary breakdown of food substances. The
research indicated that the chewing effi ciency of
those wearing dentures was about one-sixth that
of a person with natural teeth. In addition, evi-
dence suggests that nutritional defi ciencies,
regardless of their cause, are associated with
impaired immune responses [
5 ].
There are also some interesting researches
that connected dental caries and gastrointestinal
diseases, such as gastroesophageal refl ux disease
and the effect of S. mutans on the ulcerative
colitis.
8.7.1 Dental Caries
and Gastroesophageal Refl ux
Disease
Gastroesophageal refl ux disease (GERD) is a
chronic symptom of mucosal damage caused by
stomach acid coming up from the stomach into
the esophagus [ 127 ]. GERD is usually caused by
changes in the barrier between the stomach and
the esophagus, including abnormal relaxation of
the lower esophageal sphincter, which normally
holds the top of the stomach closed, impaired
expulsion of gastric refl ux from the esophagus, or
a hiatal hernia. These changes may be permanent
or temporary. The oral lesions resulting from
GERD are not usually noticed until they cause
signifi cant damage. Despite the pruritus and
burning on the oral mucosa, tooth sensitivity,
aphthae, sour taste, and decrease in the vertical
dimension of occlusion to irreversible damage,
dental caries is still our focus in this chapter.
However, the results have been contradictory.
Some studies on the oral health of patients with
chronic refl ux reported an inverse relation
between caries and gastroesophageal refl ux and
Z. Ling and H. Tao

145
showed a small number of caries in individuals
with GERD as compared to control groups [ 128 ].
They attributed this to the low prevalence of bac-
teria (lactobacilli and streptococci) observed in
the saliva of patients with chronic refl ux [ 129 ].
On the other hand, Linnett et al. conducted for 52
children (31 boys and 21 girls) with a defi nitive
history of GERD. They found that caries experi-
ence was higher in GERD patients compared to
controls. Although there were more subjects with
Streptococcus mutans in the GERD group com-
pared to the control group (42 % vs 25 %), the
difference was not statistically signifi cant [ 130 ].
Silva et al. [ 23 ] did not fi nd relationship between
GERD and changes in the oral cavity by saliva
tests, oral clinical examination, or histopatho-
logic examination of the palatal mucosa [ 131 ].
The differences among the different studies may
be explained by the different research design, the
patients from different areas, and sample size.
Future studies are needed to explore the truth.
8.7.2 S. mutans and Ulcerative
Colitis
Ulcerative colitis (Colitis ulcerosa, UC) is a
chronic, or long-lasting, disease that causes
infl ammation and sores in the inner lining of the
large intestine. The main symptom of active dis-
ease is usually constant diarrhea mixed with
blood, of gradual onset. The cause of UC is
unknown though theories exist. People with UC
have abnormalities of the immune system, but
whether these problems are a cause or a result of
the disease is still unclear. Current theories sug-
gest that indigenous gut microbiota play a key
role in the pathogenesis of infl ammatory bowel
disease. Moreover, regulation of mucosal immune
response to unidentifi ed components of normal
intestinal microbiota in a genetically susceptible
host is at the core of these diseases [
132 , 133 ].
In 2011, Nakano et al. found the specifi c
strains of S. mutans that express collagen-binding
protein (CBP) caused hemorrhagic damages in
the murine brain and other tissues because of the
ability to bind the collagen and resistance to
phagocytosis [ 87 ]. In normal situations, only a
limited number of strains are possible risk factors
for aggravation of UC caused by S. mutans -
induced bacteremia. In contrast, the detection
frequency of specifi c strains of S. mutans , such as
CBP-positive and phagocytosis-resistant strains
from UC patients, was extremely higher than in
non-UC control subjects. Thus the authors specu-
lated that such specifi c strains of S. mutans may
be involved in the pathogenesis of UC. So the
next year, in 2012, they published another paper
to show that infection of specifi c strains of
S. mutans is one of the risk factors in aggravating
infl ammation of UC. They stated that it’s the fi rst
paper describing the involvement of oral bacteria
in UC pathology [ 134 ].
8.8 Dental Caries and Diabetes
Mellitus
It’s no doubt that diabetes mellitus is a rapidly
growing health concern in both developed and
developing nations. According to the World
Health Organization (WHO), in 2011, approxi-
mately 364 million people globally suffer from
diabetes mellitus (DM), with projections that
DM-related deaths will double from 2005 to
2030 [ 135 ]. The Center for Disease Control
(CDC) estimates that in the United States alone,
25.8 million Americans, or 8.3 % of the popula-
tion, suffer from DM, with 7 million currently
undiagnosed [ 136 ]. Diabetes mellitus is classi-
fi ed into four broad categories: type 1, type 2,
gestational diabetes, and “other specifi c types.”
Type 1 diabetes mellitus is characterized by loss
of the insulin-producing beta cells of the islets of
Langerhans in the pancreas, leading to insulin
defi ciency. Type 2 diabetes mellitus is character-
ized by insulin resistance, which may be com-
bined with relatively reduced insulin secretion.
Type 2 diabetes is the most common type. In
China, type 2 diabetes mellitus affects almost
92.4 million (9.7 %) Chinese adults, and 148.2
million adults are in the prediabetes. Based on
case numbers in 2007 and projected case num-
bers in 2030, Wang W. et al. estimated that the
direct medical costs of T2DM and its complica-
tions were estimated to be 26.0 billion USD in
8 Dental Caries and Systemic Diseases

146
2007 and were projected to be 47.2 billion USD
in 2030. The results indicated that T2DM con-
sumes a large portion of healthcare expenditures
and will continue to place a heavy burden on
health budgets in the future [ 137 ]. In 2008, Patiño
et al. carried out a cross-sectional study involving
175 subjects to determine the frequency of caries,
periodontal disease, and tooth loss in patients
affected by diabetes mellitus types 1 and 2. Their
results showed a difference between the two
types among the study variables [ 138 ]. Since we
care about the overall association between diabe-
tes mellitus and dental caries prevalence, we did
not make a distinction between type 1 and type 2
diabetes mellitus in this chapter.
It is widely understood that diabetes patients
are at an increased risk for oral complications
such as candidiasis, erosion, xerostomia, and
periodontal disease [ 139 , 140 ]. Studies have
reported that patients with diabetes are suscepti-
ble to oral sensory, periodontal, and salivary dis-
orders, which could increase their risk of
developing new and recurrent dental caries [ 141 ].
However, although the relationship between dia-
betes and dental caries has been investigated
since the last century, no clear association has
been clarifi ed till now [ 142 ].
8.8.1 Epidemiological Studies
of Diabetes and Dental Caries
In 2004, Taylor et al. reviewed the post-1960
English-language literature on the relationship
between diabetes and oral health, specifi cally
focusing on periodontal disease, dental caries,
and tooth loss. The literature does not describe a
consistent relationship between type 2 diabetes
and dental caries. It reports increased, decreased,
and similar caries experiences between those
with and without diabetes. This review suggests
that currently there is insuffi cient evidence to
determine whether a relationship between diabe-
tes and risk for coronal or root caries exists [ 143 ].
Here are two studies that reported a greater
history of dental caries in people with diabetes.
In 2002, a study evaluated the caries incidence in
64 children and adolescents (8–15 years of age)
with type 1 diabetes mellitus over a 3-year period
from the onset of the disease in relation to meta-
bolic control and to caries-associated risk factors.
Results showed that patients with less good meta-
bolic control exhibited higher glucose levels in
resting saliva and a signifi cantly higher caries
incidence compared to those with good meta-
bolic control. The most infl uential determinants
for high caries development during the 3-year
follow-up period were metabolic control, poor
oral hygiene, previous caries experience, and
high levels of salivary lactobacilli [
144 ]. Later,
Miralles et al. conducted the other work which
comprised 90 type 1 diabetics between 18 and 50
years of age, and a group of non-diabetic controls
matched for age and sex. Their results showed
that under similar conditions of oral hygiene and
salivary fl ow, the diabetic group showed a higher
incidence of caries than the control group.
Likewise, on specifi cally analyzing the diabetic
group, the metabolic control of the disease, the
duration of diabetes, and the existence of compli-
cations of the disease exerted an infl uence upon
the development of dental caries [ 145 ].
Other researchers, however, have detected no
signifi cant difference or even a decrease in caries
susceptibility between diabetic and nondiabetic
patients. A decade ago, Moore et al. published a
paper to describe the prevalence of coronal and
root caries in an adult type 1 diabetic population
and evaluate demographic, dietary, behavioral,
physiologic, salivary, and medical variables asso-
ciated with decayed and fi lled surfaces in the
crown (DFS) or root (RDFS). The authors found
that adult type 1 diabetic subjects did not have
signifi cantly higher DFS rates as compared with
their control subjects or published age-adjusted
NHANES III fi ndings. However, the prevalence
of RDFS was higher in the diabetic subjects as
compared to recruited control subjects [
146 ]. In
2006, another study in Lithuania comprised 68
10–15-year-old diabetics and 68 age- and gender-
matched nondiabetic controls. Diabetics were
categorized into well-to-moderately controlled
and poorly controlled groups. They found that
diabetics had fewer caries and plaque, lower sali-
vary fl ow rates and buffer effect, and more fre-
quent growth of yeasts than their nondiabetic
Z. Ling and H. Tao

147
controls. Well-to-moderately controlled diabetics
had fewer decayed surfaces and lower counts of
mutans streptococci and yeasts than poorly con-
trolled diabetics, but the level of metabolic con-
trol of diabetes had no infl uence on salivary fl ow
rates and buffer effect. High caries levels in dia-
betics were signifi cantly associated with age,
plaque score, and decreased unstimulated sali-
vary fl ow rate but were not associated with the
level of metabolic control of diabetes. High car-
ies experience in this study population could be
related to plaque accumulation and/or to changes
in saliva induced by diabetes mellitus [ 147 ]. Two
years later, the same authors published another
paper to analyze possible associations between
caries increments and selected caries determi-
nants in children with type 1 diabetes mellitus
and their age- and sex-matched nondiabetic con-
trols, over 2 years. A total of 63 (10–15 years old)
diabetic and nondiabetic pairs were examined for
dental caries, oral hygiene, and salivary factors.
Salivary fl ow rates, buffer effect, concentrations
of mutans streptococci, lactobacilli, yeasts, total
IgA and IgG, protein, albumin, amylase, and glu-
cose were analyzed. Means of 2-year decayed/
missing/fi lled surface (DMFS) increments were
similar in diabetics and their controls. No differ-
ences were observed in the counts of lactobacilli,
mutans streptococci, or yeast growth during fol-
low- up, whereas salivary IgA, protein, and glu-
cose concentrations were higher in diabetics than
in controls throughout the 2-year period. Their
results also suggested that, in addition to dental
plaque as a common caries risk factor, diabetes-
induced changes in salivary glucose and albumin
concentrations are indicative of caries develop-
ment among diabetics [
148 ]. In 2009, in her the-
sis for Master degree, Abay conducted a study
that aimed to answer the research question, “Is
there an association between diabetes mellitus
and prevalence of severe dental caries in adults?”
Data of 701 subjects with diabetes and 3,636 sub-
jects without diabetes from the National Health
and Nutrition Examination Survey (NHANES)
conducted from 2003 to 2004 were used. Findings
of her study suggested that prevalence of severe
caries, prevalence of severe untreated caries, and
prevalence of at least one root surface with caries
or fi lling did not differ between adults with and
without diabetes [
149 ].
In experimental diabetic rodent animals, there
are also contradictory reports; some studies
reported that diabetes enhanced the incidence of
dental caries whereas another did not. As early as
1957, Nichols and Shaw reported that in terms of
carious molars and carious legions, normal rats
did not differ from caries-susceptible rats that
received intravenous injections of alloxan mono-
hydrate [ 150 ]. However, Hartles and Lawton
reported that the mean number of carious teeth
per rat and the mean caries score were signifi -
cantly higher for the injected animals than for the
controls. And the authors proposed that the most
probable way in which the injection of alloxan
can infl uence the incidence of dental caries is by
causing an alteration in the salivary secretions,
either directly or indirectly [ 151 ]. A recent study
published by some Japanese authors also con-
fi rmed that diabetic conditions enhance dental
caries in WBN/KobSlc rats [ 152 ].
As stated above, results of these studies have
shown confl icting conclusions. In spite of the dif-
ference between diabetes mellitus types 1 and 2,
the reasons may be methodological diffi culties,
such as small sample size, the absence of stan-
dard criteria for caries evaluation, and noncon-
ventional cutoffs to classify good and poor
diabetes control [ 149 ]. Thus, further studies of
the potential association between diabetes melli-
tus and dental caries are suggested.
8.8.2 Root Caries and Diabetes
According to its location, dental caries can be
divided into coronal caries and root caries. The
latter poses a complex challenge for dental practi-
tioners, which is different to those challenges pre-
sented by the former [ 153 ]. Beck et al. found that
coronal and root caries do tend to appear together
in the same individuals. They also found that peo-
ple who experience both types of caries had more
gingival recession at baseline [ 154 ]. As world’s
population ages and retention of teeth increases,
there will be increasing numbers of older patients
at risk of root-surface caries. Root caries has a
8 Dental Caries and Systemic Diseases

148
higher prevalence among older adults than any
other age group. Many of these individuals may
be more likely to have chronic systemic disease.
So we discuss the relationship between root caries
and diabetes separately. Currently, insuffi cient
evidence exists to support or refute an association
between diabetes and root caries [ 155 ].
As we mentioned before, Moore et al.
described the prevalence of coronal and root car-
ies in an adult type 1 diabetic population in 2001.
Although no signifi cantly higher DFS (decayed
and fi lled surfaces in the crown) rates were
noticed, the prevalence of RDFS (decayed and
fi lled surfaces in the root) was higher in the dia-
betic subjects as compared to recruited control
subjects [ 146 ]. In 2007, another stratifi ed cross-
sectional study was conducted in Thailand to
determine the effect of type 2 diabetes mellitus
on coronal and root-surface caries. Subjects of
105 type 2 diabetic patients and 103 nondiabetic
at the same age and gender were included. Their
results found that type 2 diabetic patients com-
pared with nondiabetic subjects had a higher
prevalence of root-surface caries and a higher
number of decayed/fi lled root surfaces. The
authors also found that the factors associated
with root-surface caries included type 2 DM, a
low saliva buffer capacity, more missing teeth,
and existing coronal caries [ 156 ].
To analyze the possible factors connected the
root caries and diabetes, we have to talk about
periodontal diseases, fi rstly. Diabetes has been
found to be bidirectionally linked with periodon-
tal disease and subsequent loss of attachment. As
a result, gingival recession will cause the expos-
ing of tooth’s root and contributing to the risk of
root caries [
157 , 158 ]. Secondly, considering that
saliva is responsible for establishing protective
environment against dental caries, we will talk
about the effect of salivary factors like salivary
fl ow rate and adequate level of calcium, phos-
phate, and fl uoride in diabetes mellitus. Recently,
Jawed et al. evaluated the possible protective role
of salivary factors in diabetes mellitus type 2
patients with dental caries. In their study, a total
of 398 diabetes mellitus type 2 patients with den-
tal caries and 395 age- and sex-matched nondia-
betic subjects with dental caries were included as
controls. The blood glucose, HbA1c, and DMFT
indices were found to be signifi cantly high, while
the salivary fl ow rate, calcium, phosphate, and
fl uoride were found to be signifi cantly low in dia-
betic patients as compared to controls [
159 ].
Salivary fl ow is known to be reduced in long-
standing diabetes. This is thought to be due to
neuropathy affecting the salivary glands as a
result of chronic hyperglycemia [ 159 , 160 ].
Therefore, in diabetes, periodontal disease and
associated attachment loss and gingival recession
may mediate increased root caries, compounded
by reductions in salivary fl ow and elevated gingi-
val crevicular glucose levels in people with
poorly controlled diabetes [ 155 ].
Since oral microbiota plays an important role in
the root caries process, the change of microbiology
in the oral cavity of diabetes patients may also have
relationship with root caries. Root caries was
thought to be associated with Streptococcus mutans ,
Lactobacillus (spp.), and Actinomyces (spp.) based
on the culture method [ 161 , 162 ]. So far, the use of
culture-independent methods has played a key role
in the discovery of previously unrecognized species
in the oral cavity as well as in redefi ning the patho-
genesis of the major oral infections. And the authors
found that the microbial fl ora associated with root
caries was far more complex than previously
assumed. Except for these three bacterial species
we just mentioned, additional species, such as
Atopobium spp., Olsenella spp., Pseudoramibacter
alactolyticus , and Propionibacterium sp. strain
FMA5, were also commonly found [
163 ]. The
study showed that signifi cantly more diabetic sub-
jects had higher levels of Treponema denticola ,
Prevotella nigrescens , Streptococcus sanguinis ,
Streptococcus oralis , and Streptococcus interme-
dius in their supragingival plaque than nondiabetic
subjects. Root-surface caries was associated with an
increased count of mutans streptococci, lactobacilli,
and yeasts in saliva and of Streptococcus mutans in
supragingival plaque samples [ 164 ].
8.8.3 Tooth Loss and Diabetes
The results of Lin et al.’s study suggested that
diabetes and poor glycemic control may not be
Z. Ling and H. Tao

149
associated with an increased prevalence of past
coronal and root-surface caries experience in
older adults, but there is a tendency for more
active caries lesions and missing teeth [ 142 ]. In
2011, a Korean study has identifi ed a relationship
between total tooth loss from any cause and dia-
betes [ 165 ]. In addition, a recent study found that
having 19 or fewer teeth was associated with high
HbA1c among men aged 40–64 years but not
among those aged 65–79 years. Since diabetes is
a major risk factor for periodontal disease, the
signifi cant associations of tooth loss with HbA1c
among the middle-aged men may refl ect associa-
tions of periodontal disease with HbA1c,
although they had no information on the causes
of tooth loss [ 166 ].
8.9 Dental Caries
and Respiratory Infections
The anatomical continuity between the lungs and
the oral cavity makes the latter a potential reser-
voir of respiratory pathogens. It is well known
that the respiratory system includes the nasal and
oral cavity: the sinuses and larynx as the upper
airway and the trachea, bronchi, bronchioles, and
alveoli as the lower airway. Thus it’s not surpris-
ing that many of the diseases that occur in the
oral cavity could be also found in the upper air-
way regions. However, it’s not easy for an infec-
tive agent to reach the lower respiratory tract. It
must defeat sophisticated immunological and
mechanical defense mechanisms. The latter is so
effi cient that, in healthy patients, the distal air-
way and lung parenchyma are sterile, despite the
heavy bacterial load found in the upper airway.
An infection occurs when the host’s defenses are
compromised, the pathogen is particularly viru-
lent, or the inoculum is overwhelming. The
microorganisms may enter the lung by inhala-
tion, but the most common route of infection is
aspiration of what pneumologists have long
referred to as oropharyngeal secretions.
Therefore, it is plausible that oral microorgan-
isms might infect the respiratory tract [
166 ].
Dental plaque and poor oral health have been
associated with nosocomial pneumonia and chronic
obstructive pulmonary disease (COPD) [
167 ].
Since community-acquired pneumonia and lung
abscesses may be due to anaerobic bacteria, there
are lots of anaerobes implicated in the destruction
of periodontal tissues that have also been isolated
from infected lungs, for example, Actinobacillus
actinomycetemcomitans , Fusobacterium nuclea-
tum , and Pseudomonas aeruginosa . Pascual-
Ramos et al. found a strong association between
third-grade caries and pneumonia in 30 consecu-
tive women with SLE, hospitalized because of
pneumonia, compared with two groups of patients
with SLE, hospitalized and ambulatory, matched to
cases by age, sex, and hospitalization date.
Compared with ambulatory controls, the oral
health of patients with pneumonia was worse as
refl ected by a higher frequency of periapical lesions
and cervical and third-grade caries and a higher
number of caries per patient [ 168 ]. There is also
evidence that the occurrence of respiratory tract
infections during the fi rst year of life is associated
with a signifi cantly increased risk for developing
early childhood caries during subsequent years
[ 169 ]. A research conducted by Eldem et al. from
Turkey showed the possible association between
poor oral hygiene and upper respiratory tract infec-
tion (URTI) rates. Children without any systemic
disease were enrolled in the study and divided into
two groups: 100 children with dental caries as
patient group and another 100 children without car-
ies as control group. URTI rates and antibiotic
usage in both groups since birth were identifi ed
according to the medical records. Dental caries was
scored according to decayed, missing, and fi lled
teeth index. And their results showed that the URTI
rates were signifi cantly higher among children
with poor oral hygiene and dental caries [
170 ].
Pascual-Ramos et al. made a summary of the dif-
ferent mechanisms that have been proposed to
explain the potential role of oral bacteria in the
pathogenesis of respiratory infections. Among
them are the aspiration of oral pathogens into the
lungs, the modifi cation of mucosal surfaces by
periodontal disease-associated enzymes that pro-
mote adhesion and colonization by respiratory
pathogens, the destruction of salivary pellicles by
periodontal disease-associated enzymes that mod-
ify clearance of pathogenic bacteria from the
8 Dental Caries and Systemic Diseases

150
mucosal surface, and alteration of the respiratory
epithelium by cytokines originating from peri-
odontal tissues to promote infection by respiratory
pathogens [ 168 , 171 ].
References
1. Oral Health Care – Australia’s National Oral Health
Plan 2004–2013. National Health Advisory Committee
on Oral Health.
2. Matse JH, Yoshizawa J, Wang XY, et al. Discovery
and prevalidation of salivary extracellular microRNA
biomarkers panel for the noninvasive detection of
benign and malignant parotid gland tumors. Clin
Cancer Res. 2013;19:3032–8.
3. Spielmann N, Ilsley D, Gu J, et al. The human sali-
vary RNA transcriptome revealed by massively par-
allel sequencing. Clin Chem. 2012;58:1314–21.
4. Benjamin RM. Oral health: the silent epidemic.
Public Health Rep. 2010;125:158–9.
5. McGuire T. The relationship of oral to overall health
and longevity. What every health professional needs
to know. tuberose.com.
6. Thoden van Velzen SK, Abraham-Inpijn L, Moorer
WR. Plaque and systemic disease: a reappraisal of
the focal infection concept. J Clin Periodontol.
1984;11:209–20.
7. Fadel HT. Studies on the associations between dental
caries, periodontal disease and different systemic
conditions . Doctoral theses from Sahlgrenska
Academy, Gothenburg; 2012.
8. Australian Institute of Health and Welfare.
Australia’s Health, 2004. Canberra.
9. Osler W. Diseases of the arteries. In: Osler W, editor.
Modern medicine: its theory and practice in original
contributions by Americans and foreign authors. 4th
ed. Philadelphia: Lea and Febiger; 1908.
10. Debelian GJ, Olsen I, Tronstad L. Anaerobic bactere-
mia and fungemia in patients undergoing endodontic
therapy: an overview. Ann Periodontol. 1998;3:281–7.
11. Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic
diseases caused by oral infection. Clin Microbiol
Rev. 2000;13:547–58.
12. Han YW, Wang X. Mobile microbiome: oral bacteria
in extra-oral infections and infl ammation. J Dent
Res. 2013;92:485–91.
13. U.S. Department of Health and Human Services.
Oral health in America: a report of the Surgeon
General . Rockville: Department of Health and
Human Services; 2009.
14. Brown LJ, Oliver RC, Löe H. Periodontal diseases in
the US in 1981: prevalence, severity, extent, and role
in tooth morality. J Periodontol. 1989;60:363–70.
15. National Health Strategy. Enough to make you sick:
how income and environment affect health. Research
Paper No. 1. Melbourne: National Health Strategy;
1992.
16. Klock KS, Haugejorden O. Primary reasons for
extraction of permanent teeth in Norway: changes
from 1968 to 1988. Community Dent Oral
Epidemiol. 1991;19:336–41.
17. McCaul LK, Jenkins WMM, Kay EJ. The reasons
for the extraction of various tooth types in Scotland:
a 15-year follow up. J Dent. 2001;29:401–7.
18. Chatrchaiwiwatana S, Ratanasiri A, Jaidee J,
Soontorn S. Factors related to tooth loss due to den-
tal caries among workers in an industrial estates in
Thailand. J Med Assoc Thai. 2012;95:s1–6.
19. Jafarian M, Etebarian A. Reasons for extraction of
permanent teeth in general dental practices in
Tehran, Iran. Med Prin Pract. 2013;22:239–44.
20. Selwitz RH, Ismail AI, Pitts NB. Dental caries.
Lancet. 2007;369:51–9.
21. Ochei. Pus abscess and wound drain . In: Medical
Laboratory Science: theory and practice. Tata
McGraw-Hill Education; 2000. p. 622.
22. Parahitiyawa NB, Jin LJ, Leung WK, Yam WC,
Samaranayake LP. Microbiology of odontogenic bac-
teremia: beyond endocarditis (vol 22, pg 46, 2009).
Clin Microbiol Rev. 2009;22:386.
23. Heimdahl A, Hall G, Hedberg M, et al. Detection
and quantitation by lysis-fi ltration of bacteremia
after different oral surgical procedures. J Clin
Microbiol. 1990;28:2205–9.
24. Forner L, Larsen T, Kilian M, Holmstrup P. Incidence
of bacteremia after chewing, tooth brushing and
scaling in individuals with periodontal infl amma-
tion. J Clin Periodontol. 2006;33:401–7.
25. Li XJ, Kolltveit KM, Tronstad L, Olsen I. Systemic
diseases caused by oral infection . Clin Microbiol
Rev. 2000;13:547–+.
26. Loesche WJ. Periodontal disease as a risk factor for
heart disease. Compendium. 1994;15:976, 978–82,
982–6.
27. Jemal A, Bray F, Center MM, Ferlay J, Ward E,
Forman D. Global cancer statistics . CA Cancer J
Clin. 2011;61(2):69–90.
28. Vissink A, Burlage FR, Spijkervet FK, Jansma J,
Coppes RP. RP. C. Prevention and treatment of the
con-sequences of head and neck radiotherapy. Crit
Rev Oral Biol Med. 2003;14:213–25.
29. Dreizen S, Daly TE, Drane JB, Brown LR. Oral
complications of cancer radiotherapy. Postgrad Med.
1977;61:85–92.
30. Toth BB, Chambers MS, Fleming TJ, Lemon JC,
Martin JW. Minimizing oral complications of cancer
treatment. Oncology. 1995;9:851–8; discussion 858,
863–6.
31. Mohammadi N, Seyednejad F, Oskoee PA, Savadi
Oskoee S, Ebrahimi Chaharom ME. Evaluation of
radiation-induced class V dental caries in patients
with head and neck cancers undergoing radiother-
apy. J Dent Res Dent Clin Dent Prospects. 2008;2:
82–4.
32. Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright
TW, M-M SA. Type II epithelial cells are critical tar-
get for hyperoxia-mediated impairment of postnatal
Z. Ling and H. Tao

151
lung development. Am J Physiol Lung Cell Mol
Physiol. 2006;291:L1101–11.
33. Hong CHL, Napeñas JJ, Hodgson BD, et al. Multi-
national Association of Supportive Care in Cancer
(MASCC)/International Society of Oral Oncology
(ISOO). A systematic review of dental disease in
patients undergoing cancer therapy. Support Care
Cancer. 2010;18:1007–21.
34. Tezal M, Scannapieco FA, Wactawski-Wende J, et al.
Dental caries and head and neck cancers. JAMA
Otolaryngol Head Neck Surg. 2013;139:1054–60.
35. Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A,
Göran Hansson B. Oral infections and some lifestyle
factors as risk factors for oral and oropharyngeal squa-
mous cell carcinoma. A population-based case–control
study in southern Sweden. Acta Otolaryngol. 2005;
125:1327–36.
36. Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima
K. Teeth loss and risk of cancer at 14 common sites
in Japanese. Cancer Epidemiol Biomarkers Prev.
2008;17:1222–7.
37. Divaris K, Olshan AF, Smith J, et al. Oral health and
risk for head and neck squamous cell carcinoma: the
Carolina Head and Neck Cancer Study. Cancer
Causes Control. 2010;21:567–75.
38. Michaud DS, Liu Y, Meyer M, Giovannucci E,
Joshipura K. Periodontal disease, tooth loss, and
cancer risk in male health professionals: a prospec-
tive cohort study. Lancet Oncol. 2008;9:550–8.
39. Wang RS, Hu XY, Gu WJ, Hu Z, Wei B. Tooth loss
and risk of head and neck cancer: a meta-analysis.
PLoS One. 2013;8, e71122.
40. Guha N, Boffetta P, Wunsch Filho V, et al. Oral
health and risk of squamous cell carcinoma of the
head and neck and esophagus: results of two multi-
centric case–control studies. Am J Epidemiol.
2007;166:1159–73.
41. Muto M, Hitomi Y, Ohtsu A, Shimada H, Kashiwase
Y, Sasaki H. Acetaldehyde production by non-
pathogenic Neisseria in human oral microfl ora: impli-
cations for carcinogenesis in upper aerodigestive tract.
Int J Cancer. 2000;88:342–50.
42. Kurkivuori J, Salaspuro V, Kaihovaara P, et al.
Acetaldehyde production from ethanol by oral strep-
tococci. Oral Oncol. 2007;43:181–6.
43. Geneva S, Benjamin RM. The world oral health
report 2003: continuous improvement of oral health
in the 21st century: the approach of the WHO Global
Oral Health Programme. Public Health Rep. 2010;
125:158–9.
44. United States Public Health Service. Oral health in
America: a report of the Surgeon General. Rockville:
Department of Health and Human Services; 2000.
45. Ditmyer M, Dounis G, Mobley C, Schwarz E. A
case–control study of determinants for high and low
dental caries prevalence in Nevada youth. BMC Oral
Health. 2010;10:24.
46. Mafuvadze BT, Mahachi L, Mafuvadze B. Dental
caries and oral health practice among 12 year old
school children from low socio-economic status
background in Zimbabwe. Pan Afr Med J. 2013;
14:164.
47. Masood M, Yusof N, Hassan MI, Jaafar N. Assessment
of dental caries predictors in 6-year- old school chil-
dren – results from 5-year retrospective cohort study.
BMC Public Health. 2012;12:989.
48. Nurelhuda NM, Trovik TA, Ali RW, Ahmed
MF. Oral health status of 12-year-old school children
in Khartoum state, the Sudan; a school-based survey.
BMC Oral Health. 2009;9:15.
49. Den Besten PK, Stamperdahl J, Zhan L, Jiang Y,
Adler NE, Featherstone JD. Social inequalities in
childhood dental caries: the convergent roles of
stress, bacteria and disadvantage. Soc Sci Med.
2010;71:1644–52.
50. Jackson SL, Vann Jr WF, Kotch JB, Pahel BT, Lee
JY. Impact of poor oral health on children’s school
attendance and performance. Am J Public Health.
2011;101(10):1900–6.
51. Reifsnider EMC, Mendez DB. Childhood obesity
and early childhood caries in a WIC population.
J Multicultural Nurs Health. 2004;10:24–31.
52. Willerhausen B, Haas G, Krummenauer F,
Hohenfellner K. Relationship between high weight
and caries frequency in German elementary school
children. Eur J Med Res. 2004;9:400–4.
53. Ontario Dental Association. Tooth decay in Ontario’s
children: an ounce of prevention – a pound of cure.
Oral health issues for Ontarians: special report.
Toronto: Ontario Dental Association; 2008.
54. Miller J, Vaughan-Williams E, Furlong R, Khosla T.
Dental caries and children’s weight. Lancet. 1980;
2:853.
55. Mayanagi H, Saito T, Kamiyama K. Cross-sectional
comparison of caries time trends in nursery school
children in Sendai, Japan. Communit Dent Oral
Epidemiol. 1995;23:334–9.
56. Bhoomika W, Ramakrishna Y, Munshi AK.
Relationship between severe early childhood caries
and body mass index. J Clin Pediatr Dent. 2013;37:
235–42.
57. Acs G, Lodolini G, Kaminsky S, Cisneros GJ. Effect
of nursing caries on body weight in a pediatric popu-
lation. Pediatr Dent. 1992;14:302–5.
58. MalekMohammadi T, Wright CM, Kay EJ. Childhood
growth and dental caries. Communit Dent Health.
2009;26:38–42.
59. Kay EJ, Northstone K, Ness A, Duncan K, Crean SJ.
Is there a relationship between birth weight and sub-
sequent growth on the development of dental caries
at 5 years of age? Communit Dent Oral Epidemiol.
2010;38:408–14.
60. Mishu MP, Hobdell M, Khan MH, Hubbard RM,
Sabbah W. Relationship between untreated dental
caries and weight and height of 6- to 12-year-old pri-
mary school children in Bangladesh. Int J Dent.
2013;2013:629675, 5 p.
61. van Gemert-Schriks MC, van Amerongen EW,
Aartman IH, Wennink JM, Ten Cate JM, de Soet
JJ. The infl uence of dental caries on body growth in
8 Dental Caries and Systemic Diseases

152
prepubertal children. Clin Oral Investig. 2011;15:
141–9.
62. Hooley M, Skouteris H, Boganin C, Satur J, Nicky
K. Body mass index and dental caries in children and
adolescents: a systematic review of literature pub-
lished 2004 to 2011. Syst Rev. 2012;21:57.
63. Finks SW, Airee A, Chow SL, et al. Key articles of
dietary interventions that infl uence cardiovascular
mortality. Pharmacotherapy. 2012;32:54–87.
64. Bridget BK, Institute of Medicine, Fuster V.
Promoting cardiovascular health in the developing
world: a critical challenge to achieve global health.
Washington, D.C . : National Academies Press; 2010.
65. Jsc S. Current and future directions of cardiovascular
risk prediction. Am J Cardiol. 2006;97:28–32.
66. Meurman JH, Sanz M, Janket SJ. Oral health, ath-
erosclerosis, and cardiovascular disease. Crit Rev
Oral Biol Med. 2004;15:403–13.
67. Scannapieco FA, Bush RB, Paju S. Associations
between periodontal disease and risk for atheroscle-
rosis, cardiovascular disease and stroke: a systematic
review. Ann Periodontol. 2003;8:38–53.
68. Mattila KJ, Nieminen MS, Valtonen VV, et al.
Association between dental health and acute myo-
cardial infarction. BMJ. 1989;298:779–81.
69. Jansson L, Lavstedt S, Frithiof L, Theobald H.
Relationship between oral health and mortality in
cardiovascular disease. J Clin Periodontol. 2001;28:
762–8.
70. Maharaj B, Vayej AC. Oral health of patients with
severe rheumatic heart disease. Cardiovasc J Afr.
2012;23:336–9.
71. Glodny B, Nasseri P, Crismani A, et al. The occur-
rence of dental caries is associated with atheroscle-
rosis. Clinics. 2013;68:946–53.
72. Issac TT, Dokainish H, Lakkis NM. Role of infl am-
mation in initiation and perpetuation of atrial fi bril-
lation: a systematic review of the published data.
J Am Coll Cardiol. 2007;50:2021–8.
73. Kaneko M, Yoshihara A, Miyazaki H. Relationship
between root caries and cardiac dysrhythmia.
Gerodontology. 2011;28:289–95.
74. Holm-Pedersen P, Avlund K, Morse DE, et al. Dental
caries, periodontal disease, and cardiac arrhythmias
in community-dwelling older persons aged 80 and
older: is there a link? J Am Geriatr Soc. 2005;53:
430–7.
75. Sata N, Hamada N, Horinouchi T, et al. C-reactive
protein and atrial fi brillation. Is infl ammation a con-
sequence or a cause of atrial fi brillation? Jpn Heart J.
2004;45:441–5.
76. Vose JM, Smith PW, Henry M, Colan D. Recurrent
Streptococcus mutans endocarditis. Am J Med.
1987;82:630–2.
77. Gauduchon V, Benito Y, Celard M, et al. Molecular
diagnosis of recurrent Streptococcus mutans endo-
carditis by PCR amplifi cation and sequencing. Clin
Microbiol Infect. 2001;7:36–7.
78. Ullman RF, Miller SJ, Strampfer MJ, Cunha BA.
Streptococcus mutans endocarditis: report of three
cases and review of the literature. Heart Lung.
1988;17:209–12.
79. Nakano K, Inaba H, Nomura R, et al. Detection of
cariogenic Streptococcus mutans in extirpated heart
valve and atheromatous plaque specimens. J Clin
Microbiol. 2006;44:3313–7.
80. Nomura R, Nakano K, Nemoto H, et al. Isolation
and characterization of Streptococcus mutans in
heart valve and dental plaque specimens from a
patient with infective endocarditis. J Med Microbiol.
2006;55:1135–40.
81. Nakano K, Nemoto H, Nomura R, et al. Detection of
oral bacteria in cardiovascular specimens. Oral
Microbiol Immunol. 2009;24:64–8.
82. Abranches J, Zeng L, Belanger M, et al. Invasion of
human coronary artery endothelial cells by
Streptococcus mutans OMZ175. Oral Microbiol
Immunol. 2009;24:141–5.
83. Nakano K, Nomura R, Matsumoto M, Ooshima T.
Roles of oral bacteria in cardiovascular diseases–
from molecular mechanisms to clinical cases: Cell-
surface structures of novel serotype k Streptococcus
mutans strains and their correlation to virulence.
J Pharmacol Sci. 2010;113:120–5.
84. Kuramitsu HK, Qi M, Kang IC, Chen W. Role for
periodontal bacteria in cardiovascular diseases. Ann
Periodontol. 2001;6:41–7.
85. Herzberg MC, Meyer MW. Effects of oral fl ora on
platelets: possible consequences in cardiovascular
disease. J Periodontol. 1996;67:1138–42.
86. Beg AM, Jones MN, Miller-Torbert T, Holt RG.
Binding of Streptococcus mutans to extracellular
matrix molecules and fi brinogen. Biochem Biophys
Res Commun. 2002;298:75–9.
87. Nakano K, Hokamura K, Taniguchi N, et al. The colla-
gen-binding protein of Streptococcus mutans is involved
in haemorrhagic stroke. Nat Commun. 2011;2:485.
88. Nomura R, Naka S, Nemoto H, et al. Potential
involvement of collagen-binding proteins of
Streptococcus mutans in infective endocarditis. Oral
Dis. 2013;19:387–93.
89. Walls AW, Steele JG. Geriatric oral health issues in
the United Kingdom. Int Dent J. 2001;51:183–7.
90. Cohen W, Rose LF, Minsk L. The periodontal-
medical risk relationship. Compend Contin Educ
Dent. 2001;22:7–11.
91. Holmlund A, Holm G, Lind L. Number of teeth as a
predictor of cardiovascular mortality in a cohort of
7,674 subjects followed for 12 years. J Periodontol.
2010;81:870–6.
92. Watt RG, Tsakos G, de Oliveira C, Hamer M. Tooth
loss and cardiovascular disease mortality risk – results
from the Scottish Health Survey. PLoS One. 2012;7.
93. Tu Y-K, Galobardes B, Smith GD, McCarron P,
Jeffreys M, Gilthorpe MS. Associations between
tooth loss and mortality patterns in the Glasgow
Alumni Cohort. Heart. 2007;93:1098–103.
94. Heitmann BL, Gamborg M. Remaining teeth, car-
diovascular morbidity and death among adult Danes.
Prev Med. 2008;47:156–60.
Z. Ling and H. Tao

153
95. Choe H, Kim YH, Park JW, Kim SY, Lee S-Y, Jee
SH. Tooth loss, hypertension and risk for stroke in a
Korean population. Atherosclerosis. 2009;203:550–6.
96. Yoshida M, Murakami T, Yoshimura O, Akagawa
Y. The evaluation of oral health in stroke patients.
Gerodontology. 2012;29:E489–93.
97. Caplan DJ, Chasen JB, Krall EA, et al. Lesions of
endodontic origin and risk of coronary heart disease.
J Dent Res. 2006;85:996–1000.
98. Caplan DJ, Pankow JS, Cai J, Offenbacher S, Beck
JD. The relationship between self-reported history of
endodontic therapy and coronary heart disease in the
Atherosclerosis Risk in Communities Study. J Am
Dent Assoc. 2009;140:1004–12.
99. Frisk F, Hakeberg M, Ahlqwist M, Bengtsson
C. Endodontic variables and coronary heart disease.
Acta Odontol Scand. 2003;61:257–62.
100. Joshipura KJ, Pitiphat W, Hung H-C, Willett WC,
Colditz GA, Douglass CW. Pulpal infl ammation and
incidence of coronary heart disease. J Endod.
2006;32:99–103.
101. de Cássia Negrini T, Duque C, Höfl ing JF, Gonçalves
RB. Fundamental mechanisms of immune response
to oral bacteria and the main perspectives of a vac-
cine against dental caries: a brief review. Rev
Odontociênc. 2009;24:198–204.
102. Mattos-Graner RO, Smith DJ. The vaccination
approach to control infections leading to dental car-
ies. Braz J Oral Sci. 2004;3:595–608.
103. Sanui T, Gregory RL. Analysis of Streptococcus mutans
biofi lm proteins recognized by salivary immunoglobu-
lin A. Oral Microbiol Immunol. 2009;24:361–8.
104. Chopra M, Jadhav S, Venugopalan A, Hegde V,
Chopra A. Salivary immunoglobulin A in rheumatoid
arthritis (RA) with focus on dental caries: a cross-
sectional study. Clin Rheumatol. 2012;31:247–50.
105. Brandtzaeg P. Do salivary antibodies reliably refl ect
both mucosal and systemic immunity? In: Malamud
D, Niedbala RS, editors. Oral-based diagnostics.
Oxford: Blackwell Publishing; 2007. p. 288–311.
106. Challacombe SJ. Salivary IgA antibodies to antigens
from Streptococcus mutans in human dental caries.
Adv Exp Med Biol. 1978;107:355–67.
107. Gregory RL, Filler SJ, Michalek SM, McGhee
JR. Salivary immunoglobulin A and serum antibod-
ies to Streptococcus mutans ribosomal preparations
in dental caries-free and caries-susceptible human
subjects. Infect Immun. 1986;51:348–51.
108. Phelan JA, Mulligan R, Nelson E, et al. Dental caries
in HIV-seropositive women. J Dent Res. 2004;83:
869–73.
109. Mulligan R, Phelan JA, Brunelle J, et al. Baseline
characteristics of participants in the oral health com-
ponent of the Women’s Interagency HIV Study.
Community Dent Oral Epidemiol. 2004;32:86–98.
110. Joint United Nations Programme on HIV/AIDS
(UNAIDS), World Health Organization, editor.
AIDS epidemic update. Special report on HIV/
AIDS. Geneva: Joint United Nations Programme on
HIV/AIDS (UNAIDS); 2009.
111. Sahana S, Krishnappa SS, Krishnappa VS. Low
prevalence of dental caries in children with perinatal
HIV infection. J Oral Maxillofac Pathol. 2013;17:
212–6.
112. Howell RB, Jandinski J, Palumbo P, Shey Z, Houpt
M. Dental caries in HIV-infected children. Pediatr
Dent. 1992;14:370–1.
113. Valdez IH, Pizzo PA, Atkinson JC. Oral health of
pediatric AIDS patients: a hospital-based study.
ASDC J Dent Child. 1994;61:114–8.
114. Madigan A, Murray PA, Houpt M, Catalanotto F,
Feuerman M. Caries experience and cariogenic
markers in HIV-positive children and their siblings.
Pediatr Dent. 1996;18:129–36.
115. Tofsky N, Nelson EM, Lopez RN, Catalanotto FA,
Fine DH, Katz RV. Dental caries in HIV-infected
children versus household peers: two-year fi ndings.
Pediatr Dent. 2000;22:207–14.
116. Back-Brito GN, Ribeiro El Ackhar VN, Rodrigues
Querido SM, et al. Staphylococcus spp.,
Enterobacteriaceae and Pseudomonadaceae oral iso-
lates from Brazilian HIV-positive patients. Correlation
with CD4 cell counts and viral load. Arch Oral Biol.
2011;56:1041–6.
117. Liu G, Saxena D, Chen Z, et al. HIV infection affects
Streptococcus mutans levels, but not genotypes.
J Dent Res. 2012;91:834–40.
118. Akar H, Akar GC, Carrero JJ, Stenvinkel P, Lindholm
B. Systemic consequences of poor oral health in
chronic kidney disease patients. Clin J Am Soc
Nephrol. 2011;6:218–26.
119. Huang RY, Lin YF, Kao SY, Shieh YS, Chen JS. A
retrospective case–control analysis of the outpatient
expenditures for western medicine and dental treat-
ment modalities in CKD patients in Taiwan. PLoS
One. 2014;9, e88418.
120. Klassen JT, Krasko BM. The dental health status of
dialysis patients. J Can Dent Assoc. 2002;68:34–8.
121. Nunn JH, Sharp J, Lambert HJ, Plant ND, Coulthard
MG. Oral health in children with renal disease.
Pediatr Nephrol. 2000;14:997–1001.
122. Wolff A, Stark H, Sarnat H, Binderman I, Eisenstein
B, Drukker A. The dental status of children with
chronic renal failure. Int J Pediatr Nephrol. 1985;6:
127–32.
123. Peterson S, Woodhead J, Crall J. Caries resistance in
children with chronic renal failure: plaque pH, sali-
vary pH, and salivary composition. Pediatr Res.
1985;19:796–9.
124. Martins C, Siqueira WL, Guimaraes Primo
LSS. Oral and salivary fl ow characteristics of a group
of Brazilian children and adolescents with chronic
renal failure. Pediatr Nephrol. 2008;23:619–24.
125. Ertugrul F, Elbek-Cubukcu C, Sabah E, Mir S. The
oral health status of children undergoing hemodialy-
sis treatment. Turk J Pediatr. 2003;45:108–13.
126. Andrade MR, Antunes LA, Soares RM, Leao AT,
Maia LC, Primo LG. Lower dental caries prevalence
associated to chronic kidney disease: a systematic
review. Pediatr Nephrol. 2014;29:771–8.
8 Dental Caries and Systemic Diseases

154
127. DeVault KR, Castell DO. Updated guidelines for the
diagnosis and treatment of Gastroesophageal refl ux
disease. Am J Gastroenterol. 2005;100:190–200.
128. Ersin NK, Oncag O, Tumgor G, Aydogdu S. Oral
and dental manifestations of gastroesophageal refl ux
disease in children: a preliminary study. Pediatr
Dent. 2006;28:279–84.
129. Correa MCCSF, Lerco MM, Cunha Mde L, Henry
MA. Salivary parameters and teeth erosions in
patients with gastroesophageal refl ux disease. Arq
Gastroenterol. 2012;49:214–8.
130. Linnett V, Seow WK, Connor F, Shepherd R. Oral
health of children with gastro-esophageal refl ux dis-
ease: a controlled study. Aust Dent J. 2002;47:
156–62.
131. Silva M, Damante JH, Stipp ACM, Tolentino MM,
Carlotto PR, Fleury RN. Gastroesophageal refl ux
disease: new oral fi ndings. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 2001;91:301–10.
132. Nagalingam NA, Kao JY, Young VB. Microbial
ecology of the murine gut associated with the devel-
opment of dextran sodium sulfate-induced colitis.
Infl amm Bowel Dis. 2011;17:917–26.
133. Saleh M, Trinchieri G. Innate immune mechanisms
of colitis and colitis-associated colorectal cancer.
Nat Rev Immunol. 2011;11:9–20.
134. Kojima A, Nakano K, Wada K, et al. Infection of
specifi c strains of Streptococcus mutans, oral bacte-
ria, confers a risk of ulcerative colitis. Sci Rep.
2012;2:332.
135. World Health Organization. Diabetes: key facts.
Geneva: World Health Organization; 2011.
136. Centers for Disease Control and Prevention. National
diabetes fact sheet: national estimates and general
information on diabetes and pre diabetes in the
United States. Atlanta: U.S. Department of Health
and Human Services, Centers for Disease Control
and Prevention; 2011.
137. Wang W, McGreevey WP, Fu C, et al. Type 2 diabe-
tes mellitus in China: a preventable economic bur-
den. Am J Manage Care. 2009;15:593–601.
138. Patino Marin N, Loyola Rodriguez JP, Medina
Solis CE, et al. Caries, periodontal disease and
tooth loss in patients with diabetes mellitus types 1
and 2. Acta Odontol Latinoam. 2008;21:127–33.
139. Garcia RI, Nunn ME, Dietrich T. Risk calculation and
periodontal outcomes. Periodontol 2000. 2009;50:
65–77.
140. Zielinski MB, Fedele D, Forman LJ, Pomerantz SC.
Oral health in the elderly with on-insulin- dependent
diabetes mellitus. Spec Care Dentist. 2002;22:
94–8.
141. Ship JA. Diabetes and oral health: an overview. J Am
Dent Assoc. 2003;134 Spec No:4S–10.
142. Lin BP, Taylor GW, Allen DJ, Ship JA. Dental caries
in older adults with diabetes mellitus. Spec Care
Dentist. 1999;19:8–14.
143. Taylor GW, Manz MC, Borgnakke WS. Diabetes,
periodontal diseases, dental caries, and tooth loss:
a review of the literature. Compend Contin Educ
Dent. 2004;25:179–92.
144. Twetman S, Johansson I, Birkhed D, Nederfors T.
Caries incidence in young type 1 diabetes mellitus
patients in relation to metabolic control and caries-
associated risk factors. Caries Res. 2002;36:31–5.
145. Miralles L, Silvestre FJ, Hernandez-Mijares A,
Bautista D, Llambes F, Grau D. Dental caries in type
1 diabetics: infl uence of systemic factors of the dis-
ease upon the development of dental caries. Med
Oral Patol Oral Cir Bucal. 2006;11:E256–60.
146. Moore PA, Weyant RJ, Etzel KR, et al. Type 1 diabe-
tes mellitus and oral health: assessment of coronal
and root caries. Community Dent Oral Epidemiol.
2001;29:183–94.
147. Siudikiene J, Machiulskiene V, Nyvad B, Tenovuo J,
Nedzelskiene I. Dental caries and salivary status in
children with type 1 diabetes mellitus, related to the
metabolic control of the disease. Eur J Oral Sci.
2006;114:8–14.
148. Siudikiene J, Machiulskiene V, Nyvad B, Tenovuo J,
Nedzelskiene I. Dental caries increments and related
factors in children with type 1 diabetes mellitus.
Caries Res. 2008;42:354–62.
149. Abayon M. Diabetes and dental caries prevalence: is
there an association? Degree Master of Science in
Clinical Investigation University of Rochester; 2009.
150. Nichols MS, Shaw JH. The effect of alloxan diabetes
on caries incidence in the albino rat. J Dent Res.
1957;36:68–74.
151. Hartles RL, Lawton FE. Experimental dental caries
in the albino rat; the effect of single subcutaneous
injections of alloxan on the incidence of dental car-
ies. Br J Nutr. 1958;12:286–92.
152. Kodama Y, Matsuura M, Sano T, et al. Diabetes
enhances dental caries and apical periodontitis in
caries-susceptible WBN/KobSlc rats. Comp Med.
2011;61:53–9.
153. Stefanac SJ, Nesbit SP. Treatment planning in den-
tistry. Edinburgh: Elsevier Mosby; 2007.
154. Beck JD, Drake CW. Do root lesions tend to develop
in the same people who develop coronal lesions?
J Public Health Dent. 1997;57:82–8.
155. Garton BJ, Ford PJ. Root caries and diabetes: risk
assessing to improve oral and systemic health out-
comes. Aust Dent J. 2012;57:114–22.
156. Hintao J, Teanpaisan R, Chongsuvivatwong V,
Dahlen G, Rattarasarn C. Root surface and coronal
caries in adults with type 2 diabetes mellitus.
Community Dent Oral Epidemiol. 2007;35:302–9.
157. Cullinan MP, Ford PJ, Seymour GJ. Periodontal dis-
ease and systemic health: current status. Aust Dent J.
2009;54:S62–9.
158. Banting DW. The diagnosis of root caries. J Dent
Educ. 2001;65:991–6.
159. Jawed M, Shahid SM, Qader SA, Azhar A. Dental
caries in diabetes mellitus: role of salivary fl ow rate
and minerals. J Diabetes Complications. 2011;25:
183–6.
Z. Ling and H. Tao

155
160. Negrato CA, Tarzia O. Buccal alterations in diabetes
mellitus. Diabetol Metab Syndr. 2010;2:3.
161. Zambon JJ, Kasprzak SA. The microbiology and
histopathology of human root caries. Am J Dent.
1995;8:323–8.
162. Ravald N. Root surface caries . Curr Opin Periodontol.
1994;78–86.
163. Preza D, Olsen I, Aas JA, Willumsen T, Grinde B,
Paster BJ. Bacterial profi les of root caries in elderly
patients. J Clin Microbiol. 2008;46:2015–21.
164. Hintao J, Teanpaisan R, Chongsuvivatwong V,
Ratarasan C, Dahlen G. The microbiological profi les of
saliva, supragingival and subgingival plaque and dental
caries in adults with and without type 2 diabetes melli-
tus. Oral Microbiol Immunol. 2007;22:175–81.
165. Jung SH, Ryu JI, Jung DB. Association of total tooth
loss with socio-behavioural health indicators in
Korean elderly. J Oral Rehabil. 2011;38:517–24.
166. Mojon P. Oral health and respiratory infection. J Can
Dent Assoc. 2002;68:340–5.
167. Terpenning MS. The relationship between infections
and chronic respiratory diseases: an overview. Ann
Periodontol. 2001;6:66–70.
168. Pascual-Ramos V, Hernandez-Hernandez C, Soto-
Rojas AE, Celis-Aguilar E, Sanchez-Guerrero J.
Association between dental caries and pneumonia in
patients with systemic lupus erythematosus.
J Rheumatol. 2006;33:1996–2002.
169. Alaki SM, Burt BA, Garetz SL. Middle ear and
respiratory infections in early childhood and their
association with early childhood caries. Pediatr
Dent. 2008;30:105–10.
170. Eldem I, Kaymaz N, Yazici H. Is there any rela-
tionship between dental caries and recurrent
upper respiratory tract infection? The 30th Annual
Meeting of the European Society for Paediatric
Infection Diseases. Poster. Thessaloniki, Greeece,
2012:8–12
171. Rheumatol J. Role of oral bacteria in respiratory
infection. J Periodontol. 1999;70:793–802.
8 Dental Caries and Systemic Diseases

157
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1_9
Models in Caries Research
Huang Xuelian , Guo Qiang , Ren Biao , Li Yuqing ,
and Zhou Xuedong
1. In vitro models in caries research
2. In situ models in caries research
3. Animal models in caries research
Clinical trials are always large, time-
consuming, and costly; furthermore, due to ethi-
cal problems, some studies are not available in
human subjects [ 1 ]. Therefore, models have
played a substantial role in caries research, which
help to establish the multifactorial etiology of
dental caries, defi ne the impact of numerous fac-
tors contributing to the initiation and progression
of dental caries, and identify agents or measures
with the ability to prevent or reduce the incidence
of dental caries [ 2 ]. Models in caries research can
be divided into three categories: in vitro model,
in situ model, and animal model [ 3 ].
9.1 In Vitro Models in Caries
Research
In vitro models or laboratory models are the most
common models applied in dental research with
several advantages [ 4 ]:
1. Less costly and comparatively rapid
2. Can carry out single-variable experiments
under highly controlled conditions, which are
more sensitive and precise
3. Best approach to screen large numbers of agents
and to determine their modes of action [ 5 ]
However, in vitro models have signifi cant limita-
tions, mostly related to their inability to simulate the
complex biological processes involved in caries.
As dental caries results from an ecological
imbalance in the physiological equilibrium
between tooth minerals and oral microbial bio-
fi lms, two basic methods are developed to study
dental caries: de- and remineralization in the teeth
(e.g., in vitro chemical models) and microbial
systems on the teeth (e.g., in vitro microbial mod-
els) [
6 ]. Recently, microbial-based de- and remin-
eralization models are also developed, which are
closer to the tooth decay process in oral cavity.
9.1.1 In Vitro Chemical Models
Chemical induction of caries by organic acids is
one of the principal approaches to study the
H. Xuelian
State Key Laboratory of Oral Diseases ,
Sichuan University , Chengdu ,
People’s Republic of China
Department of Operative Dentistry and Endodontics ,
West China Hospital of Stomatology,
Sichuan University , Chengdu ,
People’s Republic of China
e-mail:
G. Qiang • R. Biao • L. Yuqing
State Key Laboratory of Oral Diseases ,
Sichuan University , Chengdu ,
People’s Republic of China
Z. Xuedong (*)
State Key Laboratory of Oral Diseases ,
West China Hospital of Stomatology, Sichuan
University , Chengdu , People’s Republic of China
9

158
mechanisms in de- and remineralization of
enamel and dentin. In vitro chemical models
allow strict control of the experimental environ-
ment and are relatively simple and cost-effective;
however, their applicability is limited to factors
which directly infl uence the de- and remineral-
ization process. Between de- and remineraliza-
tion models, substrates are one of the major
differences. Different from remineralization
methods which typically use lesions as substrate,
demineralization models utilize a wider variety
of substrates, including those pretreated and rem-
ineralized with agents, on natural enamel and
dentin [ 5 ]. (For details, please also see “deminer-
alization and remineralization” part.)
9.1.1.1 Demineralization Models
Tooth decay is the result of progressive mineral
loss from dental tissues. In vitro demineralization
models enable researchers to examine those fun-
damental processes, and their applications
include [ 5 ] mechanistic studies of solution/sub-
strate factors affecting demineralization, studies
of factors contributing to the intrinsic resistance
of mineralized tissue to acid demineralization,
and effi cacy evaluation of caries-preventive
agents (e.g., fl uoride, natural products [ 7 , 8 ]) or
the application of laser irradiation [ 9 ] which may
inhibit enamel and dentin dissolution in acid
attack.
9.1.1.2 Remineralization Models
In vitro remineralization models can be applied
to investigate the mechanism involved in caries
lesion repair and evaluating the effi cacy of treat-
ments or agents which are favorable to enhance
remineralization. Remineralization protocols can
be grouped into three general categories:
In pH-Lattice Ion “Drift” Protocol
Substrates are exposed to constant-volume super-
saturated remineralization solutions. They have
the advantage of direct chemical measurement of
remineralization within a given exposure period
[ 10 ]; however, in remineralization solutions, cal-
cium and phosphate ion concentration will
decrease, and the pH will also decrease, if the
systems are not well buffered. Those changes
cannot only decrease the amount of mineral
formation but also change both the type and loca-
tion of mineral deposition [
5 , 11 ].
Constant Composition Protocols
Lattice ion concentrations and pH remain con-
stant throughout remineralization. In details, two
techniques were involved: fl ow-through tech-
niques and titration-controlled techniques. In the
former, supersaturation is kept constantly by
means of a high volume of remineralization
medium, and it permits multi-group studies to be
carried out. The measurement of remineraliza-
tion is confi ned to substrate changes [ 5 ]. In the
latter, controlled addition of calcium phosphate
lattice ions and buffer titrants monitored potenti-
ometrically by pH and/or calcium ion-selective
electrodes was involved. The measurement of
remineralization is used titrant [ 5 ].
“pH Cycling” Protocols
The “pH cycling” protocols consist of numerous
cycles of demineralization and remineralization,
which are correlated to alternant acidifi cation and
alkalinization phases in oral cavity. The genesis
of modern pH cycling protocols was produced by
ten Cate and Duijsters [ 10 ], which have become
favorable choice to study remineralization
in vitro, because they provide better simulation
of the caries process for both mechanistic studies
and for evaluations of some caries-preventive
agents. Recently, a particular in vitro remineral-
ization model, called Featherstone pH cycling
model, was recommended as an appropriate
alternative to animal testing, particularly for ionic
fl uoride based dentifrices, for the reason that it
demonstrated excellent correlation with the cur-
rently accepted animal caries models [
12 ].
9.1.2 In Vitro Microbial Model
In vitro microbial models provide means for
studying complex microbial ecosystems on the
teeth and their roles on the development of dental
caries [ 6 ]. Microbial models can be used to (1)
investigate prevention of carious lesions through
antimicrobial agents or measures, (2) compare the
cariogenic potential of different microorganisms,
and (3) assess the cariogenicity of carious diets.
H. Xuelian et al.

159
Microbial models can be divided into two main
classifi cations: closed (batch) system and open
system (continuous culture). The closed system,
in which there is no fl ow into or out of the reactor
during the cultivation and microorganisms are
provided with fi nite nutrients and growth rates are
rapid, is comparatively rare in nature [ 13 , 14 ]. It is
the simplest and most frequently used in vitro
microbial models. During the growth process, the
environment in the enclosed system will change
(e.g., nutrients become depleted, signaling mole-
cules and metabolites accumulate), unless the
fl uid is regularly replaced. This system is far from
physiological; however, better repeatability, less
contamination, less cost, and high throughput are
its marked advantages. This system also allows
researchers to easily vary multiple parameters
including the composition of growth media, incu-
bation temperatures, humidity, presence or
absence of shear stress, and O
2
and CO
2
concen-
trations. Those features make it valuable system
for initial screening assays [ 14 , 15 ].
In dynamic continuous system, fresh medium
fl ows into the bioreactor continuously, and part of
the medium in the bioreactor is withdrawn from
the fermenter at the same fl ow rate of the inlet
fl ow. Then some metabolites can be eliminated
from the bioreactor, and it is more like oral cavity
than batch cultivation [ 16 ]. This system enables
better control of growth rates and other variables
[ 13 ]; however, it is more likely contaminated and
not easy to repeat the results.
9.1.2.1 Inoculum
An important factor in the design of in vitro
microbial models is the choice of inoculum [ 3 ].
Pure culture, defi ned consortium, and microcosm
are all used currently. Pure culture is broadly
used in vitro, in which physiological studies are
normally done. It is much easier to manipulate
the variation of the single test organism. However,
it is far from mimicking the oral cavity.
Microcosm is used to closely mimic the physi-
cochemical, microbiological, and nutrient condi-
tions present in oral cavity [ 13 ]. In in vitro
microbial models for dental caries, dental plaque
or saliva can be used. They have the advantage of
maintaining the complexity and heterogeneity and
enabling in situ bacterial community dynamics to
be replicated in laboratory environment. However,
there are still some disadvantages. Firstly, it is hard
to fi nd out a medium to support all organisms in
the plaque or saliva to grow as they do in oral cav-
ity. Secondly, they are often poorly characterized.
As the system is so complex, it is hard and costly
to analyze its metabolism, composition shift, and
interaction within the ecosystems. Thirdly, it
would have been diffi cult to standardize the plaque
inoculum in replicate experiments and to manipu-
late its composition for experimental purposes.
In order to overcome these problems, inocu-
lum with a defi ned consortium has been con-
structed by pooling pure cultures of plaque
bacteria in various combinations. The communi-
ties that develop from them are stable with time
and establish reproducibly in replicate experi-
ments. It also allows detailed control and study of
the properties of the individual bacterial species
present. Defi ned inoculum comprised of two or
more organisms in combination, with organisms
chosen for their relevance in health and disease
and for their ease of identifi cation; subsequently,
individual species can be added or deleted for
experimental purposes. One of the good exam-
ples is often referred to as the “Marsh Consortium”
which is composed of nine bacteria [
17 ]. In
another batch culture study, a mixture containing
Actinomyces naeslundii , Veillonella dispar ,
Fusobacterium nucleatum , Streptococcus sobri-
nus , and Streptococcus oralis was cultivated to
form supragingival plaque, which was used to
evaluate antimicrobial agents. A yeast species,
Candida albicans , was added later, for the reason
that a valid prescreening test should rule out the
possibility of fungal overgrowth due to selective
interference with bacterial ecology [
18 , 19 ]. In a
root caries model system developed in artifi cial
mouth, four putative root-caries pathogens,
Streptococcus mutans , Streptococcus sobrinus ,
Actinomyces naeslundii , and Lactobacillus rham-
nosus , form multispecies consortia biofi lms and
furthermore form the caries lesion [ 20 ].
9.1.2.2 Closed (Batch) System
Microbial Models
In closed system microbial models, some small
reactor vessels and test tubes were frequently
used for planktonic culture, and microtiter plate
9 Models in Caries Research

160
(MTP)-based system is among the most fre-
quently used biofi lm model systems. In MTP-
based system, biofi lms are either grown on the
bottom and the walls of the microtiter plate (most
commonly a 96-well microtiter plate) or on the
surface of a substrata (glass, hydroxyapatite,
enamel, or dentin disks) placed in the wells of the
microtiter plate (most commonly a 6-, 12-, or
24-well microtiter plate). The former is generally
based on bacterial sedimentation, in which the
metabolites accumulate, and the latter can form
through attachment, which is more close to the
reality than the former.
Some modifi cations were developed for the
96-well microtiter plate, during which transfer-
able solid phase (TSP) biofi lm model was used to
screen antimicrobial activity [ 21 ]. TSP is com-
mercially available and contains a 96-well
microtiter plate and a lid with 96 pegs placed on
the plate. Biofi lm can form on the surface of pegs
through attachment. Another MTP-based com-
mercially available method is the Biofi lm Ring
Test, which provides a kit including microplates
(12 polystyrene strips of 8 wells), toner (magnetic
bead solution), contrast liquid (a nontoxic and
inert opaque oil used for reading step), dedicated
block test (magnet support), and plate reader
(scanner) [ 22 ]. With this technology, the immobi-
lization of inert paramagnetic beads included in
the culture medium during the formation of the
biofi lm is measured. A magnet is used to collect
the non-immobilized beads into a single spot
which is then quantifi ed through specialized
image algorithms [
14 ]. This model was once used
to confi rm that AI-2-based quorum sensing affects
biofi lm formation in Streptococcus mutans [ 23 ].
Zürich biofi lm model, a multispecies model,
based on 24-well plates, is a classical batch cul-
ture approach, in which the host of environmental
variables can be rigorously controlled. At present,
six microorganism representatives are used to
generate biofi lms for supragingival plaque, which
are Streptococcus oralis , Streptococcus sobrinus ,
Actinomyces naeslundii , Veillonella dispar ,
Fusobacterium nucleatum , and Candida albicans
[ 18 , 19 ]. Cells are cultivated anaerobically in a
saliva-based medium on substrata coated with
a salivary pellicle. As shear forces are absent in a
batch culture system, those disks are dipped in
saline three times daily, and at each time point, the
biofi lms are dipped three times in saline, thereby
being subjected to passages through an air-liquid
interface [
18 ]. The validation of the in vitro caries
model was assessed, which confi rms the repeat-
ability of biofi lm formation after 40.5 h and 64.5 h
in repeated independent trials, and demonstrated
the produced losses in viability from brief expo-
sures of biofi lms to chlorhexidine or triclosan
were similar to those observed in vivo, inferring
that this biofi lm model was very useful for pre-
clinical testing of prospective antiplaque agents at
clinically relevant concentrations [
24 ]. Actually,
the main application of this model was to evaluate
antimicrobial compounds. The model could also
be used to achieve demineralization and reminer-
alization of bovine enamel under biofi lms [ 18 ].
More recently, a novel high-throughput active
attachment model (Fig. 9.1 ) was also used to eval-
uate antimicrobial compounds and contributing
factor on dental caries [ 7 , 26 ]. The model consisted
a
b
Fig. 9.1 Pictures of the biofi lm model used in this study.
( a ) Custom-made stainless steel lid on which 24 clamps
are fi xed. Substrata glass cover slips or HA disks are
shown. ( b ) Position of the substrata (HA disks) in the
24-well plate at the time of biofi lm growth [
25 ]
H. Xuelian et al.

161
of a custom-designed lid containing substrata that
fi t on top of standard 24-well plates. Single spe-
cies biofi lms and polymicrobial biofi lm derived
from saliva can be formed in the systems [ 25 ].
This model is also applicable for evaluating novel
caries-preventive agents on both biofi lm and
demineralization inhibition at the same time when
bovine dentin disks were used as substrata [
27 ].
9.1.2.3 Open (Continuous Culture)
System Microbial Models
Flow Cell Biofi lm Model and Modifi ed
Robbins Device
Flow cell biofi lm model and modifi ed Robbins
device [ 28 ] (MRD, Fig. 9.2 ) shared similar prin-
ciples for the operation, whereby culture fl uid is
passed through a tube or cell and biofi lms may be
monitored microscopically (in fl ow cells) or
formed on coupons (in some fl at plate fl ow cells)
or pegs (Robbins devices) [ 29 ]. Flow cells are
commercially available devices with glass
chambers that are particularly well suited for
real-time nondestructive microscopic analyses of
biofi lms [
14 ]. This model is not inexpensive, and
the design can be versatile in the selection of
material for the substratum [ 30 ]. They still have
some disadvantages: Firstly, sterilization can be
diffi cult because many of the common materials
used for fl ow cell construction do not respond
well to autoclaving, especially to repeated auto-
claving. Secondly, peristaltic pumps can produce
some pulsation in liquid delivery. Finally, in
cases of high biomass within the fl ow cell [
30 ], it
is conceivable that a gradient in nutrients could
be established over the length of the fl ow cell. As
commonly used biofi lm models, fl ow cells can be
used for single-species biofi lm or multispecies
biofi lm. The co-adhesion of Streptococcus gor-
donii with Streptococcus oralis was studied in a
two-species in vitro oral biofi lm fl ow cell system,
in which green fl uorescent protein was used as a
species-specifi c marker [ 31 ]. In MRD, the tube
can be plastic or metal, into which pegs can be
Media in
Waste
Waste
Peristaltic
pumps
Modified Robbins
Device (MRD)
mounted on
a hot plate
(37 °C)
Waste
Waste
Side
arm
Bubble
trap
14 µm filter
Fig. 9.2 Modifi ed Robbins
device [
28 ]
9 Models in Caries Research

162
inserted so that when in place, the end of the peg
forms part of the wall of the tube [ 13 ]. In a typical
experiment, the MRD is fi lled with a suspension
of microorganisms and is fl ipped over to improve
the adhesion of the planktonic cells to the disks
[ 14 ]. After the adhesion phase, the pump is
started to allow a continuous fl ow of the growth
medium and biofi lm development on the disks.
The advantage of this system is that the biofi lms
can be sampled by removing plugs at any time,
once they are replaced to maintain a closed sys-
tem. This model was established as an appropri-
ate biofi lm model for susceptibility testing of oral
microorganisms [
32 ].
Drip-Fed Biofi lm Model
Among drip-fed biofi lm models, drip fl ow biofi lm
reactors are a simple sort, in which biofi lms are
grown on angled slides continuously irrigated
with small volumes of (inoculated) media, thereby
providing a low-shear environment with disper-
sive mixing (Fig. 9.3 ) [ 14 , 33 , 34 ]. This system
has been developed as an interproximal labora-
tory model to compare the potential effectiveness
of powered brushing to remove biofi lm plaque
from interproximal spaces beyond the reach of
bristles [
35 ]. Different substrata can be used such
as hydroxyapatite or teeth tissues. Care is required
in experimental design and in sampling because
medium fl ow over the surface of the slide may not
be uniform and hence there may be signifi cant
aerial heterogeneity over the surface of the sub-
stratum [ 13 ]. In constant depth fi lm fermenter
(CDFF), biofi lms are fed by the drip-wise addi-
tion of growth medium onto the turntable, and
excess and/or spent medium fl ows downward
through a waste outlet [
13 ]. The biofi lm devel-
oped on a surface is limited to a predetermined
depth by mechanically removing excess biofi lm, a
situation mimicking the movement of the tongue
over the teeth [ 14 ]. In this system, 200 or 300 μm
is most commonly used for maintenance of den-
tal plaques [ 13 ]. This system can develop single-
species biofi lm, defi ned consortium biofi lm, or
microcosm biofi lm to evaluate antibacterial agent
and explore the etiology of dental caries [ 36 , 37 ].
Perfused Biofi lm Fermenters
Perfused biofi lm fermenters (PBF) are con-
structed such that nutrients are supplied by con-
tinuous perfusion of growth medium, which is
pumped through the substratum (a permeable
Influent
Needle
O-ring
Coupon
Adjustable
leg
Effluent
Bacterial
air vent
Mininert
top
Septum
10°
Fig. 9.3 Schematic diagram of
a drip fl ow reactor [
33 , 34 ]
H. Xuelian et al.

163
membrane) and hence through the biofi lm [ 13 ].
The media fl ow can be accurately controlled, the
growth rate of the biofi lm can also be well modu-
lated, and dynamic steady states can be achieved.
The multiple Sorbarod device (MSD) is one kind
of PBF which is proved to be an ideal system to
grow oral biofi lms by McBain and co-workers. It
uses a simple two-piece stainless steel housing,
yields relatively large amounts of biomass, and
enables continuous monitoring of population
dynamics through the analysis of perfusates
(spent culture fl uid) [
38 ]. It has been validated for
the maintenance of complex salivary microbial
ecosystems and for the in vitro reproduction of
interindividual variation within oral microbiotas.
Disadvantages of MSD are related to the devel-
opment of heterogeneous biofi lms [ 13 ].
Artifi cial Mouth
Artifi cial mouth models mimic the in vivo oral
niches and habitats to act as a laboratory “micro-
cosm” [ 39 ]. It consists of a vessel in which a sur-
face (or surfaces) is inoculated and supplied with
a continuous or intermittent nutrient supply; dur-
ing such experimental procedures, real-time
growth and development of dental plaque/biofi lm
can be investigated (Fig. 9.4 ) [ 3 , 39 , 40 ]. This
system has progressed from simple and basic
apparatus to the currently available, highly
sophisticated, computer-controlled, multi-station
artifi cial mouth systems. The advanced multiple
artifi cial mouth (MAM) system which was devel-
oped by Sissons and his co-workers could be
employed for the long-term growth of multiple
plaque samples within a standardized, simulated
Simulated oral
fluid line
Sucrose line
Micro-reference
electrode
Electrode and
inoculation port
Sample holder
Thermometer
Clamp
Fluid delivery
head port
Gas inlet
Dental
plaque
pH
electrode
a
b
Screwcap with septum
Experimental treatments
line
Fluid delivery
head
Endplate
Waste
Fig. 9.4 Artifi cial mouth [ 39 , 40 ].
( a ) Cross-section of biofi lm growth
station, ( b ) longitudinal section of
culture chamber
9 Models in Caries Research

164
oral environment generated by computer-
controlled facilities [ 40 ]. The environment and
the biofi lm pH range can be controlled and manip-
ulated, and oral fl uids and periodic pulses of
sucrose to model meals were simulated. A chemi-
cally defi ned saliva-like oral fl uid analogue is
named defi ned medium with mucin (DMM),
which can work well as a “saliva substitute” [ 41 ].
Plaque samples grown over several weeks in this
system exhibited metabolic behavior and pH pro-
fi les typical of natural plaque. It was possible to
analyze aliquots during plaque development with-
out contaminating the mature samples [ 40 ]. These
current applications contain evaluating microbial
interactions in simulated dental plaque and simi-
lar biofi lms and monitor their physical, chemical,
biological, and molecular features to a very high
degree of accuracy, evaluating potential antimi-
crobial agent [ 40 , 42 ]. Besides, several artifi cial
mouth models have been established to study fac-
tors infl uencing the development of primary cari-
ous lesions and evaluate caries-preventive agents,
which will be represented in “microbial-based min-
eralization model” part in detail.
Chemostat
Chemostat can provide a homogeneous liquid
environment for microbial growth, under highly
defi ned and controllable conditions [ 3 ]. Bacteria
can be grown at fi xed growth rates, enabling sin-
gle parameters to be varied independently so that
true cause-and-effect relationships can be estab-
lished; the culture can also be sampled repeat-
edly, facilitating statistical analysis. However, if
excessive bacteria grew in chemostat, operational
problems may arise from blocked tubing [
3 ].
Prevention of wall growth (change of cells from
planktonic to biofi lm forms adhering to the che-
mostat vessel) is also very important, which will
cause deviations from “steady-state” growth
[ 43 ]. Pure culture and mixed culture can both
grow in chemostat systems. Marsh developed a
consortium, composed of nine bacteria to investi-
gate carbohydrate pulses and pH on population
shifts within oral microbial communities [ 44 ]
and later focus on pH-driven disruption [ 17 ];
both of the two studies are very important to his
“ecological plaque hypothesis.” The control of
pH at neutral values during sugar pulsing was
possible only with this type of laboratory model;
this experiment could not have been performed in
animal or human model studies because of the
inevitable pH change when carbohydrates are
metabolized [
3 ]. The applications of chemostat
for the usual microbiology laboratory are costly
and have space considerations. A lot of medium
are needed, especially when multiple parallel cul-
tures are needed. Nowadays, some modifi cations
are developed to alleviate the hurdles [ 43 ]. For
classic chemostat, one of the shortcomings is that
the organisms are not in a biofi lm; however,
recently, there are some modifi cations for chemo-
stat to involve relevant surfaces to form biofi lms.
Sterile hydroxyapatite rods or disk can be inserted
into chemostat head and immersed in steady-
state planktonic cultures for certain periods [ 45 ].
Chemostat has also been linked to fl ow cells to
form biofi lm. S. Herles and co-worker developed
a chemostat fl ow cell system (Fig. 9.5 ), in which
the chemostat was provided with a continuous
source of fi ve species of oral bacteria grown in an
artifi cial “saliva-like” medium. This mixture was
pumped through six fl ow cells, each containing
two types of surfaces in which plaque formed,
and was subsequently used to compare the anti-
plaque agents [ 46 ]. Furthermore, chemostat can
also be used to inoculate a novel biofi lm generat-
ing model system, the thin fi lm fermenter, in
which biofi lms of a predetermined depth can be
generated, of which a key feature is that biofi lms
of a predetermined depth can be generated [
3 ].
9.1.3 Microbial-Based De-
and Remineralization Model
The use of microbial-based de- and remineraliza-
tion model permits more clinical relevant in vitro
investigations of dental caries etiology and the
properties of caries-preventive agent. In this sys-
tem, primary caries, secondary caries, pit and fi s-
sure caries, root caries, and so on can be
developed. Batch culture techniques can be used,
and continuous culture techniques were also
applied in this system, during which “artifi cial
mouth” was generally involved.
H. Xuelian et al.

165
In an in vitro secondary caries model, the
occlusal surface of the tooth was sealed with a
conventional resin-based fi ssure sealant; during
the process, certain part of the tooth was slightly
moistened (contaminated) with saliva to produce
marginal gaps, the teeth were infected with
Streptococcus mutans in artifi cial mouth, and the
secondary caries can be developed in contami-
nated regions [
47 ]. This model can also be used
to study the relationship of gap size and second-
ary caries, in which gaps were created by insert-
ing shim stocks with different thickness at the
tooth/resin interfaces and Streptococcus mutans
was also used as inoculum [ 48 ].
Katz and his colleagues developed a pit and
fi ssure caries in artifi cial mouth. They selected
extracted human premolars and molars and inoc-
ulated pit and fi ssures with a concentrated
Streptococcus mutans inoculum, which was
overlaid with a nutrient layer of 15 % agar, 15 %
glycerin, and 5 % sucrose. A fi lter paper and a
thin layer of collodion were placed over the arti-
fi cially created plaque. These specimens were
incubated at 37 °C and continuously washed with
artifi cial saliva (pH neutral) at a regular rate. The
acrylic blocks with the tooth specimens were
removed daily, and 0.24 % sodium fl uoride denti-
frice was applied for 3 min with a toothbrush.
The latter procedure was repeated 5 days a week,
and the whole experiment was conducted for 8
weeks. Subsequently, stereomicroscopic evalua-
tions revealed that the artifi cial lesions were very
similar to those of natural pit and fi ssure caries.
This in vitro caries model contributed somewhat
to the study of pit and fi ssure caries including the
study of remineralization due to agents incorpo-
rated in toothpaste [ 40 ].
When root tissue (disks) was used and puta-
tive root-caries pathogens were used, root caries
can be modeled in the bacterial system. M. Shu
Chemostet
Mixed
culture
oral
microorganisms
Modified BM media
conteining mucin
Supplementery
BM media
Flow cells
From flow cells
Flow cells (open view)
Flow cells (assembled)
Germanium
prism
Waste
HAP
disks
Overflow
Mixing chamber
Vent
Pump
B
A
E
C
D
Fig. 9.5 Chemostat fl ow cell
system [
46 ]. A Chemostat
containing a mixed culture of
5 oral bacteria, B Vessel
containing a supply of
supplementary BM medium
(without glucose), C The
mixing chamber containing
the fl ow from A (1 ml/min)
plus 5 ml/min from B, D The
pump supplying fl ow (1 ml/
min) from the chemostat to 6
low cells (1ml/min), E Flow
cells containing the HAP
disks and germanium ATR
prisms
9 Models in Caries Research

166
and colleagues developed such root caries model
using four putative root-caries pathogens,
Streptococcus mutans , Streptococcus sobrinus ,
Actinomyces naeslundii , and Lactobacillus rham-
nosus [ 20 ]. Also, saliva can also be used as inoc-
ulum to develop the root caries [ 49 ].
9.2 In Situ Model in Caries
Research
In situ caries models involve the use of appli-
ances or devices which create defi ned conditions
in human mouth to simulate the natural process
of dental caries. These models attempt to provide
clinically relevant information in a relatively
short period without causing irreversible tissue
changes in the natural dentition [ 50 ]. The advan-
tages of in situ caries model systems compared
with clinical trials include [ 1 , 51 ]:
1. They have fewer ethical and logistical problems.
2. They are less costly.
3. Experimental design can be more fl exible,
allowing hypotheses to be tested.
4. The results are acquired in much shorter time.
5. Better control with the study subjects and bet-
ter compliance.
Compared with in vitro caries models, in situ
caries model is complicated by dietary eating
habits, the presence of physiologically secreted
saliva, plaque of varying composition and thick-
ness, and a pellicle-coated tooth surface [
50 ],
which make it more close to oral cavity than
in vitro caries models.
Compared with animal caries model, in situ
caries models are conducted in human beings,
while animal caries models are conducted in ani-
mals. Due to so many differences between ani-
mals and human beings, some of the results from
animal caries models may not extrapolate to
human beings.
The disadvantages of in situ caries model are
still obvious:
1. The number of subjects in in situ caries model
is generally limited. Whether the small study
population can represent the general popula-
tion is raised as a question.
2. The validation of the studies is generally
heavily dependent on compliance of the test
subjects.
By using in situ caries models, we have the
potential to study both fundamental aspects of the
caries process, such as to form artifi cial carious
lesions [
52 ], to study de- and remineralization,
and to obtain dental biofi lm directly in the human
mouth [ 53 ], as well as more applied research
problems such as the effect of food on dental car-
ies and the role of caries-preventive agent in car-
ies prevention [ 54 , 55 ] in human subjects without
actually causing caries in the natural dentition
[ 50 ]. In situ study designs are highly variable,
with models using different hard tissue substrates,
a variety of intraoral sites, different exposure peri-
ods, the inclusion or exclusion of participants’
diet, the use of different depth recesses and gauze
to encourage plaque retention, the use of mesh to
protect surface from mechanical disturbance and
allow plaque accumulation, and the use of various
mineral quantifi cation methods [ 52 , 56 ].
Both enamel and dentin can be used as hard
tissue substrates. For enamel, both the natural
enamel surface, which is more suitable for for-
mation of dental plaque biofi lm [ 57 ], and enamel
slab (pieces of extracted teeth), which is suitable
for mineralization study [ 54 ] to make similar
baseline, can be used.
When the in situ caries model was used to
assess de- and remineralization, enamel speci-
mens were more frequently used than dentin;
however, dentin is useful to simulate root caries.
In remineralization study, it is better to produce
standardized demineralized lesions in laboratory
fi rstly. These lesions can be sectioned to provide
one half lesion for the intraoral appliance and the
other half used as the baseline lesion control [
56 ].
However, the outcome of in situ caries models
may differ substantially depending on their
design, and therefore, the choice of model may
signifi cantly infl uence the conclusions drawn
from such studies [ 58 ]. For example, the micro-
bial composition of plaque will vary among dif-
ferent teeth in the same mouth, on the same tooth
H. Xuelian et al.

167
in different people, and even on different surfaces
on the same tooth. So the selection of patients for
in situ caries model should be carefully done. It
makes sense to recruit subjects with similar sali-
vary fl ow and buffer capacity, with desired micro-
biological pattern, and even with a preferred
immunological profi le [ 3 ]. Also, plaque that
forms with the aid of gauze does not fully resem-
ble “natural” plaque, in either structure or micro-
bial composition [ 59 ].
9.2.1 Classifi cation of In Situ
Models
In 1990, Wefel grouped in situ model systems
into three general types: removable appliances,
single-section models, and banding models [ 60 ].
Nowadays, some new models were developed,
and some models were less used; in situ caries
models can be roughly divided into two catego-
ries: removable appliances and fi xed appliances,
in terms of the mobility characteristic.
9.2.1.1 Removable Appliances
Removable appliance is the widely used in situ
caries model which can be constituted with acrylic
appliances or denture and hard tissue substrates.
The model can be exposed extraorally to the chal-
lenge, or to a therapeutic regime, to decrease vari-
ability in individual mouths. This kind of treatment
method can permit the testing of agents or proce-
dures which might be harmful to the natural denti-
tion or ethically unacceptable [
61 ]. If dietary
challenges were not considered or oral hygiene is
not the infl uence factor, the model can be taken
out of the mouth when subjects are eating or car-
rying out oral hygiene, to give a high degree of
control and to diminish compliance. In an in situ
artifi cial dentin carious lesion study, acrylic pala-
tal appliances containing two bovine dentin speci-
mens, protected with a plastic mesh to allow
biofi lm development, were involved. The volun-
teers dripped a 20 % sucrose solution on each
specimen four times a day for 14 days; fi nally, the
in situ model produced a deep lesion with a high
R value but with a thin surface layer [ 52 ].
A removable appliance with three 200-μm-wide
grooves cut into bovine dentin disks was used to
accumulate plaque, which was later treated with
chlorhexidine. Then the in situ plaque with and
without chlorhexidine treatment can be well
investigated to extrapolate the similar conditions
in oral cavity [ 53 ].
9.2.1.2 Fixed Appliances
Fixed appliances in in situ caries model are the
appliances that can only be removed in the end of
the study. They have various forms: banding model
such as orthodontic band model which can develop
orthodontic non-cavitated (white spot) lesion and
can produce a plaque accumulation niche for
demineralization. The crown single- section model
relies mainly on placement of the sections in
plaque-retentive areas below contact points [ 60 ].
The enamel can even be bonded to the teeth
directly to collect natural plaques on the natural
enamel surface [ 57 ]. Orthodontic non- cavitated
(white spot) lesion models have been used regu-
larly for testing the effi cacy of novel remineraliza-
tion agents; however, this approach cannot be
extrapolated to model other types of non-cavitated
lesions, for after removing the band, the lesion will
not continue to develop [ 62 ]. A special in situ car-
ies model is that non- cavitated lesions formed
under plaque retention sites on the teeth that are
destined for extraction, and the effects of reminer-
alization agents can be tested [ 56 ].
9.3 Animal Model in Caries
Research
In 1995, the US Food and Drug Administration
(FDA) issued the Anticaries Drug Products for
Over-the-Counter (OTC) Human Use fi nal rule,
establishing the requirement that “all OTC anti-
caries dentifrice drug product formulations be
tested in the animal caries reduction test” [ 12 ].
Animal caries models have a long history of
successful use in caries research. They are invalu-
able tools to simulate the natural progression of
caries under true biological conditions. Unlike in
situ and in vitro systems, which measure isolated
components of caries process, animal caries
models truly measure caries [ 4 ]. Animal caries
9 Models in Caries Research

168
model has played a critical role in caries research
due to its unique advantages. Compared with in
situ caries model and in vitro caries model, it is
the closest caries model. The whole saliva is
present, and it can provide components of the
host defenses and simulate more accurately the
clearance of test compounds [ 3 ]. However, there
are also limitations, including differences on the
composition of the oral fl ora and dental plaque,
eating habits, saliva, food retention, dentition,
and the morphology of the teeth [ 2 , 50 ]. It may be
diffi cult to inoculate and establish some human
bacterial strains in animals, while the pattern of
caries in rodents is different from that observed in
humans.
Various experimental animal species have
been used in animal caries model, including non-
human primates, rats, hamsters, and mice, during
which the rodent models are the commonest. The
development of genetically modifi ed animals
makes utility of the model more broadly. With
respect to the general considerations in animal
caries models, William H. Bowen has a very
excellent review [ 29 ]. In short, several important
considerations should be paid attention to: selec-
tion of animals, litter effect, age of animals, sex,
caging of animals, and diet. When those consid-
erations are deliberately under control, which
makes the baseline the same, the animal caries
model may show the effect brought by the experi-
mental factors. Animal caries models are very
valuable to study the etiology of dental caries and
evaluate the anticaries products.
9.3.1 Study on Etiology of Dental
Caries
Animal caries models can be used to evaluate the
cariogenicity of diet. The fi rst recorded use of
rats in caries research was published in 1922 by
McCollum et al., who was primarily interested in
the role of diet in the etiology of dental caries.
Most of the early studies were focused on the
infl uence of diet [ 29 ]. Nowadays, some special
food was still evaluated by animal model. For
example, cariogenicity of milk and formula was
compared in Wistar rat. Sucralose, a sugar
substitute, was found to have low cariogenicity in
Sprague-Dawley rat. In SPFOM rat, high-glucose
diet was found to have higher cariogenic capacity
than pure starch [
63 ].
As the inherent difference in the dentition
between animals and humans, the host factors
investigated in animal caries models mainly
focus on the saliva factor. Data from the animal
models, especially desalivated animal models,
have enhanced our understanding of the impor-
tance of saliva in maintaining oral health. The
incidence of caries increased signifi cantly in rats
which had their salivary glands removed surgi-
cally [ 29 ]. Conventionally, rats can be desalivated
partially or completely by surgery to test the role
of saliva in the development of caries or to make
caries formation faster. An alternative strategy is
to genetically modify the test animal system to
exclude the host factor of interest. In mice with
targeted deletion of the gene encoding aquapo-
rin- 5 ( Aqp
5
−/−
), a water channel involved in the
production of saliva, there was a signifi cant
increase in caries, indicating that caries
susceptibility increases with a reduced salivary
fl ow that is associated with decreased water con-
tent of saliva [ 64 ]. The interaction of bacteria and
host can also be evaluated in genetically manipu-
lated rats. In NOD/SCID. e2f1
−/−
mice that show
hyposalivation, lower salivary antibody, and an
extended life span compared to the parent strain,
the roles of several salivary components in
Streptococcus mutans colonization in mice were
evaluated, suggesting that there are multiple
effects exerted by sIgA in Streptococcus mutans
colonization, with synergistic effects evident
under the condition of sIgA and limited nutrients
on colonization in NOD/SCID. e2f1
−/−
[ 65 ].
Comparing the lesion formation in germfree
and conventional rodents can make sure the role
of microorganism in dental caries, and animal
caries models are a good tool to study the micro-
bial etiology of dental caries. In addition, animals
can be inoculated with mutants of cariogenic
bacteria that lack putative virulence traits or have
putative anticaries traits, so that this type of
model can be valuable in determining factors
involved in the pathogenesis of caries [ 3 ]. A
strain of Streptococcus mutans , which lacks
H. Xuelian et al.

169
urease, was genetically engineered to express the
urease genes of Streptococcus salivarius . Rats
were infected with the parent strain or the alkali-
producing S. mutans and fed a cariogenic diet
supplemented with urea. The rats infected with
the recombinant strain had a dramatically lower
incidence and severity of all types of caries com-
pared with controls, showing that alkali genera-
tion inhibits caries [ 66 ]. Furthermore, the
development of transgenic animals together with
genetic manipulation of microorganisms will
facilitate the utility of the model greatly and may
lead to development of novel approaches to the
prevention of disease [ 29 ].
9.3.2 Evaluate Anticaries Agent
The benefi ts of animal caries models also lie in
their role in evaluating fl uoride and antimicrobial
compounds and vaccination on plaque formation
and caries development. It has been pointed out
that no caries-protective agent currently in human
use has failed in a rodent test. However, it has
been argued that the experimental conditions in
animal caries model may be too severe to mimic
the condition in human being, for example, a
56 % sucrose diet with ad libitum feeding, and
infection with S. sobrinus , which is particularly
adapted to colonizing and causing caries on
smooth surfaces. The outcome is that some prom-
ising agents may be incorrectly discarded [ 3 ].
Animal caries models have been demonstrated
to be suitable to evaluate the caries-preventive
effect of fl uoride, for those reasons: These mod-
els develop incipient and more advanced coronal
caries which resemble clinical caries structurally
and etiologically, and the response of animal car-
ies models to fl uoride demonstrates dose
responses [
2 ].
There are several models that have been used
to evaluate the effi cacy of fl uoride-containing
dentifrices, such as Francis’ hypomineralized
area (HMA) model, Gaffar’s CARA rat caries
model, Connecticut rat caries model, and Indiana
rat caries model. G.K. Stookey and co-workers
have reviewed those models in detail [ 2 ]. Shortly
speaking, HMA model is designed to assess the
impact on incipient enamel lesions, while the
other three models utilize more overt caries
lesions. HMA model relies upon an indigenous
fl ora; the other three models involve the infection
of the animals with cariogenic microorganisms.
It is also possible to develop animal caries
models to evaluate non-fl uoride antimicrobial
agents. As antimicrobial agents utilize different
mechanism with fl uoride, the former mainly
work on microorganism, and the latter mainly
work on mineralization. It is wise to modify the
animal caries model.
9.4 The Role of Saliva in Caries
Models
The periods of alkalinization in dental biofi lm,
which promote remineralization and restore the
integrity of the enamel, are primarily attributable
to diffusion of acids from the biofi lms, buffering
by salivary bicarbonate, salivary peptides, bacte-
rial cells, and bacterial metabolism of urea and
arginine [ 66 ]. In this process, the role of saliva is
very important. This role is also demonstrated by
a lot of clinical evidence.
In animal caries model and in situ caries mod-
els, in which the whole saliva is present, salivary
factor is inevitably involved, while desalivated
animals (salivary glands are partially or com-
pletely removed or drug-induced hyposalivation)
can mimic the extreme cariogenic challenge com-
monly observed in patients suffering from sali-
vary hypofunction [
64 ]. Also, current techniques
to alter gene expression in animals allow direct
analysis of the saliva in caries development [ 64 ].
In in situ caries models, it is well realized that
salivary composition and fl ow differ at different
sites, which may lead to fl uctuating results. So it
is better to put the model in the same dentition
site, even if there are still some differences among
different individuals. To overcome this problem,
it is better to recruit subjects with similar salivary
fl ow and buffer capacity. Those are all important
considerations in designing in situ caries study.
Furthermore, the role of fl uoride in saliva is
also well realized. Fluoride may reach saliva
directly from the ingesta or from topical
9 Models in Caries Research

170
treatments, or indirectly from the bloodstream
via the salivary glands or gingival crevicular
fl uid, or from temporary intraoral reservoirs of
fl uoride, including surface deposits on the teeth
of calcium fl uoride-like material and dental
plaque [ 67 ]. After local application of fl uoride
and initial rapid clearance phase, the saliva can
have a low concentration of fl uoride over long
periods of time, which is as important as a brief
exposure to relatively high fl uoride concentra-
tions for shorter periods of time [ 68 ]. So it is nec-
essary to consider this factor related to saliva,
when evaluating the caries-preventive role of
fl uoride in caries model. Then another question is
also raised, whether other caries-preventive
agents have their “reservoirs” in oral cavity.
However, in in vitro caries models, the role of
saliva was not always emphasized. In some cases,
the effect of saliva was objected, for the reason
that caries lesions form only in “stagnant” sites
where the benefi t of saliva is not working and
thus that models where salivary infl uences are
excluded will best represent the conditions of
caries lesion formation [ 67 ].
On the other hand, there are some models
focusing on the saliva’s role in the oral cavity and
further on its role in dental caries, such as saliva-
plaque interface, salivary clearance of bacterial
substrates, fl uoride, and acid [ 69 , 70 ]. The stud-
ies of the Stephan curve showed that if salivary
stimulation is mimicked, the rate of pH rise in a
model plaque is highly dependent upon the bicar-
bonate concentration and the velocity of the fi lm
of saliva in both thick and thin plaques, indicat-
ing that salivary benefi ts can be exerted even with
thick plaques [
67 ].
Even in caries models considering the effect
of salivary factor, saliva tends to be considered
more as a nonspecifi c diluent or sink rather than
as a fl uid with a complex chemistry that may
interact with the plaque and the teeth in signifi -
cant ways [ 67 ]. Saliva possesses an array of
activities that appear to have been seldom consid-
ered to in many caries models. However, those
bioactivities should not be ignored as the follow-
ing facts.
Firstly, saliva has some properties regarding de-
and remineralization of hard tissue of teeth, such
as the saturation of calcium and phosphate ions,
calcium-ion binding by salivary macromolecules,
and some precursors for the adsorbed protein fi lms
or pellicles, found on teeth surfaces, which have a
signifi cant effect on the interactions of dental min-
eral with overlying fl uids, particularly with respect
to diffusion rates of acids into and calcium and
phosphate ions out of the enamel [
71 ].
Secondly, it is well demonstrated that alkali
generation from salivary substrates, especially
arginine (or polypeptides and proteins) and urea,
could play major roles in plaque pH homeostasis
and in the inhibition of dental caries [ 66 ].
Arginine and urea can be secreted by salivary
glands, and polypeptides and proteins containing
arginine residue belong to saliva proteins.
Interestingly, the presence of saliva itself will
favor the base formation and then the pH rise
[ 72 ]; the reason may be attributed to its buffer
capacity [ 26 ].
Thirdly, salivary proteins adsorbed onto sur-
faces of apatitic minerals profoundly affect bac-
terial adhesion onto those surfaces; those effects
may signifi cantly infl uence the initial bacterial
colonization of teeth and, therefore, the microbial
nature of dental plaque.
Fourthly, there are some antibacterial systems
in saliva which contain sialoperoxidase, lyso-
zyme, lactoferrin, histatins, peroxidases, and
other basic polypeptides, which have less specifi c
antibacterial effect and also other bioactivities.
Collectively, in oral cavity, saliva plays a cru-
cial role in the initiation and progression of den-
tal caries, chemical and physiological process of
de- and remineralization of the teeth, dental bio-
fi lm formation and metabolism, clearance, buffer
and neutralization of acid, and so on. As a result,
in dental caries model, saliva should not be
ignored.
In summary, much of our present understand-
ing of the etiology and initiation and progression
of dental caries as well as the identifi cation of
caries-preventive agents or measures is attributed
to the fi ndings of studies on models. However, no
single, ideal model is optimal for studying all
aspects of caries, and different models have
specifi c roles in studying specifi c aspects. They
have their advantage and disadvantage from both
H. Xuelian et al.

171
experimental design and experiment cost. Models
must be modifi ed or refi ned to achieve the desired
goals.
References
1. Clasen AB, Ogaard B. Experimental intra-oral caries
models in fl uoride research. Acta Odontol Scand.
1999;57(6):334–41.
2. Stookey GK, Warrick JM, Miller LL, Greene AL.
Animal caries models for evaluating fl uoride denti-
frices. Adv Dent Res. 1995;9(3):198–207.
3. Marsh PD. The role of microbiology in models of
dental caries. Adv Dent Res. 1995;9(3):244–54.
4. Mundorff-Shrestha SA. Animal caries models: reac-
tion paper. Adv Dent Res. 1995;9(3):208–13.
5. Arends J. The application of in vitro models to research
on demineralization and remineralization of the teeth:
reaction paper. Adv Dent Res. 1995;9(3):194–7.
6. Fontana M, Dunipace AJ, Gregory RL, Noblitt TW,
Li Y, Park KK, Stookey GK. An in vitro microbial
model for studying secondary caries formation. Caries
Res. 1996;30:7.
7. Huang X, Cheng L, Exterkate RAM, Liu M, Zhou X,
Li J, et al. Effect of pH on Galla chinensis extract’s
stability and anti-caries properties in vitro. Arch Oral
Biol. 2012;57(8):1093–9.
8. Huang X, Liu M, Li J, Zhou X, Ten Cate JM. Chemical
composition of Galla chinensis extract and the effect of its
main component(s) on the prevention of enamel deminer-
alization in vitro. Int J Oral Sci. 2012;4(3):146–51.
9. Hsu C-YS, Jordan TH, Dederich DN, Wefel JS.
Effects of low-energy CO2 laser irradiation and the
organic matrix on inhibition of enamel demineraliza-
tion. J Dent Res. 2000;79(9):1725–30.
10. ten Cate JM, Duijsters PPE. Alternating demineral-
ization and remineralization of artifi cial enamel
lesions. Caries Res. 1982;16(3):201–10.
11. White DJ, Chen WC, Nancollas GH. Kinetic and phys-
ical aspects of enamel remineralization – a constant
composition study. Caries Res. 1988;22(1):11–9.
12. Featherstone JDB, Stookey GK, Kaminski MA, Faller
RV. Recommendation for a non-animal alternative to
rat caries testing. Am J Dent. 2011;24(5):289–94.
13. McBain AJ. Chapter 4: In vitro biofi lm models: an
overview. In: Allen IL, Sima S, Geoffrey MG, editors.
Advances in applied microbiology. New York:
Academic; 2009. p. 99–132.
14. Coenye T, Nelis HJ. In vitro and in vivo model sys-
tems to study microbial biofi lm formation. J Microbiol
Methods. 2010;83(2):89–105.
15. Venema K, van den Abbeele P. Experimental models of
the gut microbiome. Best Pract Res Clin Gastroenterol.
2013;27(1):115–26.
16. Villadsen J, Nielsen J, Lidén G. Bioreaction engineer-
ing principles. New York: Springer Science + Business
Media; 2011.
17. Bradshaw D, Marsh PD. Analysis of pH – driven dis-
ruption of oral microbial communities in vitro. Caries
Res. 1998;32(6):456–62.
18. Guggenheim B, Guggenheim M, Gmür R, Giertsen E,
Thurnheer T. Application of the Zürich biofi lm model
to problems of cariology. Caries Res. 2004;38(3):
212–22.
19. Shapiro S, Giertsen E, Guggenheim B. An in vitro oral
biofi lm model for comparing the effi cacy of antimicro-
bial mouthrinses. Caries Res. 2002;36(2):93–100.
20. Shu M, Wong L, Miller JH, Sissons CH. Development
of multi-species consortia biofi lms of oral bacteria as
an enamel and root caries model system. Arch Oral
Biol. 2000;45:14.
21. Hoogenkamp MA, Crielaard W, ten Cate JM, Wever R,
Hartog AF, Renirie R. Antimicrobial activity of vana-
dium chloroperoxidase on planktonic Streptococcus
mutans cells and Streptococcus mutans biofi lms. Caries
Res. 2009;43(5):334–8.
22. Chavant P, Gaillard-Martinie B, Talon R, Hébraud M,
Bernardi T. A new device for rapid evaluation of bio-
fi lm formation potential by bacteria. J Microbiol
Methods. 2007;68(3):605–12.
23. Huang Z, Meric G, Liu Z, Ma R, Tang Z, Lejeune P.
luxS-based quorum-sensing signaling affects biofi lm
formation in Streptococcus mutans. J Mol Microbiol
Biotechnol. 2009;17(1):12–9.
24. Guggenheim B, Giertsen E, Schüpbach P, Shapiro
S. Validation of an in vitro biofi lm model of supragin-
gival plaque. J Dent Res. 2001;80(1):363–70.
25. Deng DM, Hoogenkamp MA, Exterkate RA, Jiang
LM, van der Sluis LW, Ten Cate JM, Crielaard
W. Infl uence of Streptococcus mutans on Enterococcus
faecalis biofi lm formation. J Endod. 2009;35(9):
1249–52.
26. Huang X, Exterkate RAM, ten Cate JM. Factors asso-
ciated with alkali production from arginine in dental
biofi lms. J Dent Res. 2012;91(12):1130–4.
27. Silva TC, Pereira AFF, Exterkate RAM, Bagnato VS,
Buzalaf MAR, Machado MAAM, et al. Application
of an active attachment model as a high-throughput
demineralization biofi lm model. J Dent. 2012;40(1):
41–7.
28. Linton CJ, Sherriff A, Millar MR. Use of a modifi ed
Robbins device to directly compare the adhesion of
Staphylococcus epidermidis RP62A to surfaces. J Appl
Microbiol. 1999;86(2):194–202.
29. Bowen W. Rodent model in caries research.
Odontology. 2013;101(1):9–14.
30. Palmer Jr RJ. Microscopy fl ow cells: perfusion cham-
bers for real-time study of biofi lms. In: Ron JD, edi-
tor. Methods in enzymology. San Diego: Academic;
1999. p. 160–6.
31. Aspiras MB, Kazmerzak KM, Kolenbrander PE,
McNab R, Hardegen N, Jenkinson HF. Expression of
green fl uorescent protein in Streptococcus gordonii
DL1 and its use as a species-specifi c marker in coad-
hesion with Streptococcus oralis 34 in saliva-
conditioned biofi lms in vitro. Appl Environ Microbiol.
2000;66(9):4074–83.
9 Models in Caries Research

172
32. Larsen T, Fiehn N-E. Development of a fl ow method
for susceptibility testing of oral biofi lms in vitro.
APMIS. 1995;103(1–6):339–44.
33. Goeres DM, Hamilton MA, Beck NA, Buckingham-
Meyer K, Hilyard JD, Loetterle LR, et al. A method
for growing a biofi lm under low shear at the air-liquid
interface using the drip fl ow biofi lm reactor. Nat
Protoc. 2009;4(5):783–8. doi:
10.1038/nprot.2009.59 .
34. Method E2647-08. Standard test method for quantifi ca-
tion of a Pseudomonas aeruginosa biofi lm grown using
a drip fl ow biofi lm reactor with low shear and continu-
ous fl ow. In: Annual book of ASTM standards, vol.
11.06. West Conshohocken: ASTM International; 2008.
35. Adams H, Winston MT, Heersink J, Buckingham-
Meyer KA, Costerton JW, Stoodley P. Development
of a laboratory model to assess the removal of biofi lm
from interproximal spaces by powered tooth brush-
ing. Am J Dent. 2002;15 Spec No:12B–7.
36. Deng DM, Buijs MJ, Ten Cate JM. The effects of sub-
stratum on the pH response of Streptococcus mutans
biofi lms and on the susceptibility to 0.2% chlorhexi-
dine. Eur J Oral Sci. 2004;112(1):42–7.
37. Shu M, Browngardt CM, Chen Y-YM, Burne RA.
Role of urease enzymes in stability of a 10- species
oral biofi lm consortium cultivated in a constant-depth
fi lm fermenter. Infect Immun. 2003;71(12):7188–92.
38. Ledder RG, Gilbert P, Pluen A, Sreenivasan PK, De
Vizio W, McBain AJ. Individual microfl ora beget
unique oral microcosms. J Appl Microbiol. 2006;
100(5):1123–31.
39. Sissons CH, Cutress TW, Hoffman MP, Wakefi eld
JSJ. A multi-station dental plaque microcosm (artifi -
cial mouth) for the study of plaque growth, metabo-
lism, pH, and mineralization. J Dent Res. 1991;70(11):
1409–16.
40. Tang G, Yip H-K, Cutress TW, Samaranayake LP.
Artifi cial mouth model systems and their contribution
to caries research: a review. J Dent. 2003;31:11.
41. Sissons CH, Anderson SA, Wong L, Coleman MJ,
White DC. Microbiota of plaque microcosm biofi lms:
effect of three times daily sucrose pulses in different
simulated oral environments. Caries Res. 2007;41(5):
413–22.
42. Li MY, Lai GY, Wang J, Ye DX. The inhibition of
eugenol on glucan is essential for the biofi lm eradica-
tion effect on caries-related biofi lm in an artifi cial
mouth model. Nat Prod Res. 2012;26(12):1152–5.
43. Ferenci T. Bacterial physiology, regulation and muta-
tional adaptation in a chemostat environment. In:
Robert KP, editor. Advances in microbial physiology.
Boston/Amsterdam: Academic; 2007. p. 169–315.
44. Bradshaw DJ, McKee AS, Marsh PD. Effects of car-
bohydrate pulses and pH on population shifts within
oral microbial communities in vitro. J Dent Res.
1989;68(9):1298–302.
45. McNeill K, Hamilton IR. Acid tolerance response of
biofi lm cells of Streptococcus mutans . FEMS Microbiol
Lett. 2003;221(1):25–30.
46. Herles S, Olsen S, Affl itto J, Gaffar A. Chemostat
fl ow cell system: an in vitro model for the evaluation
of antiplaque agents. J Dent Res. 1994;73(11):
1748–55.
47. Seemann R, Bizhang M, Klück I, Loth J, Roulet J-F,
Bowen WH. A novel in vitro microbial-based model
for studying caries formation –development and ini-
tial testing. Caries Res. 2004;39:6.
48. Totiam P, González-Cabezas C, Fontana MR, Zero DT.
A new in vitro model to study the relationship of gap
size and secondary caries. Caries Res. 2007;41:7.
49. Mei ML, Chu CH, Lo EC, Samaranayake LP.
Preventing root caries development under oral biofi lm
challenge in an artifi cial mouth. Med Oral Patol Oral
Cir Bucal. 2013;18(4):e557–63.
50. Zero DT. In situ caries models. Adv Dent Res.
1995;9(3):214–30.
51. Higham SM, Pretty IA, Edgar WM, Smith PW,
Bizhang M. The use of in situ models and QLF for the
study of coronal caries. J Dent. 2005;33:7.
52. Moron BM, Comar LP, Wiegand A, Buchalla W, Yu
H, Buzalaf MAR, et al. Different protocols to produce
artifi cial dentine carious lesions in vitro and in situ:
hardness and mineral content correlation. Caries Res.
2013;47(2):162–70.
53. Zaura-Arite E, van Marle J, ten Cate JM. Confocal
microscopy study of undisturbed and chlorhexidine-
treated dental biofi lm. J Dent Res. 2001;80(5):1436–40.
54. Walker GD, Cai F, Shen P, Adams GG, Reynolds C,
Reynolds EC. Casein phosphopeptide-amorphous
calcium phosphate incorporated into sugar confec-
tions inhibits the progression of enamel subsurface
lesions in situ. Caries Res. 2010;44(1):33–40.
55. Robinson C. Mass transfer of therapeutics through
natural human plaque biofi lms: a model for therapeu-
tic delivery to pathological bacterial biofi lms. Arch
Oral Biol. 2011;56(9):829–36.
56. Cochrane NJ, Zero DT, Reynolds EC. Remineralization
models. Adv Dent Res. 2012;24(2):129–32.
57. Robinson C, Kirkham J, Percival R, Shore RC, Bonass
WA, Brookes SJ, et al. A method for the quantitative
site-specifi c study of the biochemistry within dental
plaque biofi lms formed in vivo. Caries Res. 1997;
31(3):194–200.
58. Nyvad B, Ten Cate JM, Fejerskov O. Arrest of root sur-
face caries in situ. J Dent Res. 1997;76(12):1845–53.
59. Ostrom CA, Koulourides T, Hickman F, McGhee JR.
Microbial characterization of an experimental cario-
genic plaque in man. J Dent Res. 1977;56(6):
550–8.
60. Wefel JS. Effects of fl uoride on caries development
and progression using intra-oral models. J Dent Res.
1990;69 Spec No:626–33; discussion 34–6.
61. Lagerweij MD, ten Cate JM. Remineralisation of
enamel lesions with daily applications of a high-
concentration fl uoride gel and a fl uoridated tooth-
paste: an in situ study. Caries Res. 2002;36(4):270–4.
62. Cochrane NJ, Reynolds EC. Calcium phosphopep-
tides — mechanisms of action and evidence for clini-
cal effi cacy. Adv Dent Res. 2012;24(2):41–7.
63. Feng J. Animal model in caries research. Int J
Stomatol. 2004;31:3.
H. Xuelian et al.

173
64. Culp DJ, Quivey RQ, Bowen WH, Fallon MA,
Pearson SK, Faustoferri R. A mouse caries model and
evaluation of Aqp5 −/− knockout mice. Caries Res.
2005;39(6):448–54.
65. Ito T, Maeda T, Senpuku H. Roles of salivary compo-
nents in Streptococcus mutans colonization in a new
animal model using NOD/SCID. e2f1
−/−
mice. PLoS
One. 2012;7(2), e32063.
66. Burne RA, Marquis RE. Alkali production by oral
bacteria and protection against dental caries. FEMS
Microbiol Lett. 2000;193(1):1–6.
67. Edgar WM, Higham SM. Role of saliva in caries mod-
els. Adv Dent Res. 1995;9(3):235–8.
68. Sjögren K. How to improve oral fl uoride retention?
Caries Res. 2001;35 Suppl 1:14–7.
69. Dawes C, Dibdin GH. A theoretical analysis of
the effects of plaque thickness and initial salivary
sucrose concentration on diffusion of sucrose into
dental plaque and its conversion to acid during
salivary clearance. J Dent Res. 1986;65(2):
89–94.
70. Lagerlöf F. Caries-protective factors in saliva. Adv
Dent Res. 1994;8(2):229–38.
71. Hay DI. Salivary factors in caries models. Adv Dent
Res. 1995;9(3):239–43.
72. Kleinberg I. A mixed-bacteria ecological approach to
understanding the role of the oral bacteria in dental
caries causation: an alternative to Streptococcus
mutans and the specifi c-plaque hypothesis. Crit Rev
Oral Biol Med. 2002;13(2):108–25.
9 Models in Caries Research

175
© Springer-Verlag Berlin Heidelberg 2016
Z. Xuedong (ed.), Dental Caries: Principles and Management, DOI 10.1007/978-3-662-47450-1
A
Academic Centre for Dentistry Amsterdam (ACTA) , 136
Accessory canals , 16
ACFP. See Amorphous calcium fl uoride phosphate (ACFP)
Acid-etching technique , 107, 108
Acidogenic bacteria
acid tolerance of , 40
Actinomyces , 39–40
carbohydrate metabolism and , 39
Lactobacilli , 39
Streptococcus mutans , 39
A C P . See Amorphous calcium phosphate (ACP)
Actinomyces
A. actinomycetemcomitans , 37
acidogenic bacteria , 39–40
A. naeslundii , 37, 40, 47
A. oris , 32
arginine deiminase system , 42
Acute lymphoblastic leukemia (ALL) , 48
ADA caries risk assessment , 65–67
Adhesive dentistry , 107
ADS. See Arginine deiminase system (ADS)
Aggregatibacter actinomycetemcomitans , 29, 139
Agmatine deiminase system (AgDS) , 42
Air abrasion , 111–112
Air polishing , 112
Alkali production
ADS , 42
AgDS , 42
and biofi lm ecology , 42–43
in caries prevention , 41
clinical relevance of , 43
urease , 40–41
ALL. See Acute lymphoblastic leukemia (ALL)
Ameloblastin , 8
Amelogenin , 7–8
Amelotin , 8
Amorphous calcium fl uoride phosphate (ACFP) , 77
Amorphous calcium phosphate (ACP) , 77, 79, 115
Animal model
de-/remineralization , 74
in research , 167
anticaries agent evaluation , 169
etiology study , 168–169
saliva role , 169
Anticaries agent, animal model , 169
Antimicrobial approaches
chlorhexidine , 44
fl uoride , 44–45
natural products , 46–48
phenolic antiseptics , 46
quaternary ammonium compounds , 45
triclosan , 45–46
xylitol , 46
Antimicrobial substances, in saliva , 50
Apical periodontitis , 141
Arginine deiminase system (ADS) , 42
A R T . See Atraumatic restorative technique (ART)
Arteriosclerotic vascular disease (ASVD). See
Atherosclerosis
Artifi cial mouth
open system microbial models , 163–164
Streptococcus mutans in , 165
Atherosclerosis , 136–138
Streptococcus mutans and , 138–139
Atopobium , 43
Atraumatic restorative technique (ART) , 115
B
Bacteremia , 131–132
Bacterial biofi lm
concept and discovery , 27
extracellular polymeric substances , 28
formation , 28–29
survival advantages of , 29–30
Bap family. See Biofi lm-associated-protein
(Bap) family
Bell stage , 3
Bicarbonate , 61
Bifi dobacterium spp. , 49
B. dentium , 43
Biofi lm-associated-protein (Bap) family , 29
Biofi lm ecology, alkali production and , 42–43
Biofi lm model
drip-fed , 162
fl ow cell , 161–162
systems , 160–161
TSP , 160
Zürich biofi lm model , 160
Index

176
Biological treatment methods , 127–128
Biomineralization , 80
Bionics, and restorative dentistry , 128
Bis-GMA system , 105
Bitewing radiography, for caries detection , 99
Black, G.V., and restorative dentistry , 107–108
B lymphocytes , 20
Body mass index (BMI) , 135–136
British Medical Journal (BMJ) , 137
Bud stage , 2
C
Calcium fl uoride particles , 79
Calcium phosphate (CaP) , 77
particles , 79
stage , 2–3
Camellia sinensis , 47
Candida albicans , 159
CaP. See Calcium phosphate (CaP)
Carbohydrate metabolism , 39
Carbonate hydroxyl apatite nanoparticles , 79
Cardiac dysrhythmias , 138
Cardiovascular disease (CVD) , 136–140
Care Index, oral diseases , 91
Caries-associated bacteria , 65–66
Caries pharmacotherapy , 114
Caries-preventive strategies
dental plaque control , 102–103
pit and fi ssure sealing , 105
preventive resin restoration , 105
primary prevention , 98
reinforce tooth resistance to acid , 103–105
secondary prevention
conventional caries detection methods , 98–99
DIAGNOdent , 100
electrical caries monitor , 101
FOTI , 99
QLF , 100–101
sugar restriction and substitute for , 103
tertiary prevention , 101–102
Cariogenic bacteria
logistic regression analysis , 138
oral cancer , 134
Casein phosphopeptide (CPP) , 115
CPP-ACFP , 77
CPP-ACP , 77
Cavity preparation
minimally invasive treatment
air abrasion , 111–112
air polishing , 112
atraumatic restorative technique , 115
caries pharmacotherapy , 114
chemomechanical caries removal , 112–113
enameloplasty , 114
lasers , 112
mechanical rotary technique , 113
microscopic preparation techniques , 113
nonmachinery preparation , 111–113
pit and fi ssure sealing , 113–114
preventive resin restorations , 114
remineralization treatment , 114–115
slot preparation , 113
tunnel preparation , 113
resin composite , 121–123
CBP. See Collagen-binding protein (CBP)
CDC. See Center for Disease Control (CDC)
CDFF. See Constant depth fi lm fermenter (CDFF)
Ceanothus americanus , 47
Cell-poor zone of pulp , 17
Cell-rich zone of pulp , 17
Center for Disease Control (CDC) , 142, 145
Cetylpyridinium chloride (CPC) , 45
CHD. See Coronary heart disease (CHD)
Chemical models, in research , 157–158
Chemomechanical caries removal (CMCR) , 112
Chemoparasitic theory , 33, 34
Chemostat
fl ow cell system , 165
open system microbial models , 164
Childhood, dental caries , 134–136
Chlorhexidine , 44
Chronic apical periodontitis (CAP) , 137–138
Chronic kidney disease (CKD) , 143–144
Chronic obstructive pulmonary disease
(COPD) , 149
Chronic renal disease , 143–144
Circumpulpal dentin , 12
CKD. See Chronic kidney disease (CKD)
Clin Oral Investig in 2011 , 136
Closed system microbial models, in research , 159–161
CMCR. See Chemomechanical caries removal (CMCR)
Cold and hot irritation test , 86–87
Colitis ulcerosa. See Ulcerative colitis (UC)
Collagen-binding protein (CBP) , 139, 145
Community Periodontal Index (CPI) of
Treatment Needs (CPITN) , 91
Competence-stimulating peptide (CSP) , 38, 50
Confocal laser scanning microscopy (CLSM) , 75
Connective tissue fi bers , 15–16
Constant depth fi lm fermenter (CDFF) , 73, 162
Contact-microradiography. See Transsal
microradiography (TMR)
Coronal caries, cross-sectional analysis of , 142
Coronary heart disease (CHD) , 136, 140–141
CPC. See Cetylpyridinium chloride (CPC)
CPP. See Casein phosphopeptide (CPP)
Craniofacial tissues embryology
head formation , 2
neural crest , 1–2
origin of tissue , 1
C-reactive protein (CRP) , 138
CSP. See Competence-stimulating peptide (CSP)
CVD. See Cardiovascular disease (CVD)
Cytodifferentiation, enamel development , 3
maturation stage , 5
presecretory stage , 4
secretory stage , 4–5
Index

177
D
Decayed, missing, and fi lled teeth (DMFT) index , 92–97,
133, 142
5-year-olds , 95
12-year-olds , 93, 95–96
35–44-year-olds , 94, 96–97
65–74-year-olds , 97–98
salivary immunoglobulin A , 142
Demineralization models, caries research , 158
Dendritic cell, in pulp , 19–20
Dental caries
5-year-olds , 95
12-year-olds , 95–96
35–44-year-olds , 96–97
65–74-year-olds , 97–98
assessment , 133
burden in China , 94–98
detection methods , 98–101
DMFT index , 92–97, 133, 142
Nepal example , 91
prevalence , 92, 133
Dental drilling , 110
Dental enamel , 71
Dental fl oss examination , 87
Dental fl uorosis , 88
Dental microfl ora , 31
Dental plaque
and bacterial biofi lm , 30
as cause of dental caries , 33–34
composition , 31
control , 102–103
ecological plaque hypothesis , 34–35
nonspecifi c/specifi c plaque , 34
Dentin
bonding , 120–121
mineralization , 12
repair , 13
sclerosis , 12–13
structure , 9–12
types , 12
Dentinal tubules , 9–10
Dentin-enamel junction (DEJ) , 7
Dentin phosphoproteins (DPP) , 12
De-/remineralization
biomineralization , 80
calcium phosphate , 77
CPP-ACFP , 77
CPP-ACP , 77
detection and measurement methods , 74
CLSM , 75
indentation techniques , 75
micro-CT , 75
OCT , 75–76
QLF , 75
TMR , 75
dynamics process of , 72
fl uoride , 76
investigations models , 72–73
animal model , 74
in situ model , 73–74
in vitro biofi lm model , 73
in vitro chemical model , 73
laser , 77–78
nanoparticles , 78–79
natural medicine , 77
Detection methods, dental caries , 98
DIAGNOdent , 100
electrical caries monitor , 101
fi ber-optic transillumination , 99
quantitative light-induced fl uorescence , 100–101
Diabetes mellitus (DM) , 145–146
epidemiological studies of , 146–147
root caries , 147–148
tooth loss and , 148–149
DIAGNOdent , 100
Diagnosis for caries
cold and hot irritation test , 86–87
dental fl oss examination , 87
dental fl uorosis , 88
diagnostic cavity preparation , 87
elastomeric separating modulus technique , 88
electrical impedance technology , 87
enamel hypocalcifi cation , 88, 89
enamel hypoplasia , 88, 89
fi ber-optic transillumination , 87
glossiness and smoothness , 88
inspection , 85
lesion
progress of , 89
symmetry of , 89
medium caries , 89
percussion , 85
predilection site , 88–89
probing , 85
quantitative laser fl uorescence technique , 88
radiographic examination , 85–86
staining technique , 88
ultrasonic technique , 87
Diagnostic cavity preparation , 87
Diet , 36, 103, 166, 168, 169
Digitized fi ber-optic transillumination (DI-FOTI) , 99
Direct bonding restoration technique , 118
DMFT index. See Decayed, missing, and fi lled teeth
(DMFT) index
DPP. See Dentin phosphoproteins (DPP)
Drip-fed biofi lm model , 162
E
Early childhood caries (ECC) , 135
ECM. See Electrical caries monitor (ECM)
Ecological plaque hypothesis , 34–35
EDJ. See Enamel–dentinal junction (EDJ)
eDNA. See Extracellular DNA (eDNA)
Elastomeric separating modulus technique , 88
Electrical caries monitor (ECM) , 16, 101
Electrical impedance technology , 87
Electron microscopy , 32
Index

178
Embryology of craniofacial tissues
head formation , 2
neural crest , 1–2
origin of tissue , 1
Enamel bonding , 119–120
Enamel–dentinal junction (EDJ) , 105, 125
Enamel development
cytodifferentiation , 3
maturation stage , 5
presecretory stage , 4
secretory stage , 4–5
enamel matrix proteins
ameloblastin , 8
amelogenin , 7–8
amelotin , 8
enamelin , 7
proteolytic enzymes , 8–9
tuftelin , 8
histogenesis and morphogenesis
bell stage , 3
bud stage , 2
cap stage , 2–3
microstructure
enamel lamellae and cracks , 6
enamel rod , 5
enamel spindle , 6
enamel tufts , 6
functional aspects , 6–7
interpit continuum , 6
Enamel hypocalcifi cation , 88, 89
Enamel hypoplasia , 88, 89
Enamelin , 7, 8
Enamel lamellae and cracks , 6
Enamel matrix proteins
ameloblastin , 8
amelogenin , 7–8
amelotin , 8
enamelin , 7
proteolytic enzymes , 8–9
tuftelin , 8
Enameloplasty , 114
Enamel rod , 5
Enamel spindle , 6
Enamel tufts , 6
Endodontic infl ammation , 140
Environmental factors, oral microbial ecology , 36
Environment dominates theory , 36
Epithelial cell rests of Malassez (ERM) , 21
Erbium:yttrium-aluminum-garnet (Er:YAG) , 77, 112
Essential oils , 47
Etching adhesive systems, bonding mechanisms , 118–119
Evidence-based dental caries diagnosis , 49
Extracellular DNA (eDNA) , 28
Extracellular polymer substances (EPS) , 28
Extracellular polysaccharides (EPS) , 73
F
Featherstone pH cycling model , 158
Fertilization, craniofacial tissues , 1
Fiber-optic transillumination (FOTI) , 87, 99
Fibronectin , 16
Film radiograph, for caries detection , 99
Fixed appliances, in situ caries models , 167
Flow cell biofi lm model , 161–162
Fluorapatite , 109
Fluorescence, in dental caries , 100
Fluoride , 44–45, 76, 114
saliva role in research , 169–170
Focal infection , 129
Focal sepsis theory , 130
FOTI. See Fiber-optic transillumination (FOTI)
Fusobacterium , 36
G
Galla chinensis (GCE) , 77
Gastroesophageal refl ux disease (GERD) , 144–145
GCF. See Gingival crevicular fl uid (GCF)
Gelatinous microbic plaques. See Dental plaque
Genetic factors, oral microbial ecology , 36
GERD. See Gastroesophageal refl ux disease (GERD)
Gerodontology , 138
Gingival crevicular fl uid (GCF) , 32
Ginkgo biloba , 47
Glossiness, and smoothness , 88
H
Head and neck cancer
tooth loss with , 134
treatment , 132–133
Head and neck squamous cell carcinoma (HNSCC) , 133
Head formation, craniofacial tissues , 2
Hertwig’s epithelial root sheath (HERS) , 20–21
Histatins , 63
Histogenesis, enamel development
bell stage , 3
bud stage , 2
cap stage , 2–3
HIV , 142–143
HNSCC. See Head and neck squamous cell carcinoma
(HNSCC)
Human microbiome project (HMP) , 30
Human salivary lactoperoxidase (HS-LPO) , 62
Human umbilical vein endothelial cells
(HUVEC) , 139
Hypomineralized area (HMA) model , 169
Hyposalivation , 132
I
IE. See Infective endocarditis (IE)
Immune system disease
effect on , 130
HIV , 142–143
salivary immunoglobulin A , 141–142
Indentation techniques , 75
Individualized treatment
importance , 125–126
risk evaluation , 126
technology and material , 126
Index

179
Infectious disease, dental caries as , 33
Infective endocarditis (IE) , 138
Inoculum, microbial models , 159
In situ model
de-/remineralization , 73–74
in research
classifi cation , 167
disadvantages , 166
outcome , 166
saliva role , 169
Inspection of caries , 85
Interglobular dentin , 11–12
Interspecies interactions
and dental caries , 36–39
factors involved in , 37–38
metabolic interrelationship , 37
Intertubular dentin , 10–11
Intra-class correlation coeffi cients (ICCs),
QLF , 101
In vitro biofi lm model, de-/remineralization , 73, 74
In vitro chemical model, de-/remineralization , 73
In vitro models, in research
chemical models , 73, 157–158
microbial-based de-and remineralization model ,
164–166
microbial models , 158–164
saliva role , 170
J
JAMA Otolaryngology–Head and Neck
Surgery , 133
K
Kallikrein-4 , 9
L
Lactic acid bacteria , 133
Lactobacillus , 31, 61, 66
acidogenic bacteria , 39
arginine deiminase system , 42
L. casei , 49
L. reuteri , 49
L. rhamnosus , 47
probiotics , 49
Lactoferrin , 63
Laminin , 16
Langerhans cells , 20
Lasers
cavity preparation , 112
de-/remineralization , 77–78
Lesion
control , 110
progress of , 89
symmetry of , 89
Light active killing , 50
Lymphatics , 16
Lymphocyte, in pulp , 20
Lysozyme , 62
M
Macrophage, in pulp , 19
MAM system. See Multiple artifi cial mouth (MAM)
system
Mantle dentin , 12
Marsh Consortium , 159
Mast cell, in pulp , 20
Matrix metalloproteinase-20 (MMP20) , 9
Maturation stage, enamel development , 5
Mechanical rotary technique , 113
Melaphis chinensis , 47
Mesenchymal stem cells (MSCs)
induction of differentiation , 21
in pulp , 20
Mesenchyme , 2
Metagenomics, oral microbiome , 48–49
Microbial-based de-and remineralization model ,
164–166
Microbial ecology, in oral cavity , 35–36
Microbial models, in research , 158
classifi cations , 159
closed system , 159–161
inoculum , 159
open system
artifi cial mouth , 163–164
chemostat , 164
drip-fed biofi lm model , 162
fl ow cell biofi lm model , 161–162
modifi ed robbins device , 161–162
perfused biofi lm fermenters , 162–163
Micro-CT investigation , 75
Microindentation technique , 75
Microorganisms of the Human Mouth (Miller) , 33
Microscopic preparation techniques , 113
Microtiter plate (MTP)-based system , 159–160
Mineralization of dentin , 12
Mini box preparation. See Slot preparation
Minimally invasive treatment , 108–111
cavity preparation
air abrasion , 111–112
air polishing , 112
atraumatic restorative technique , 115
caries pharmacotherapy , 114
chemomechanical caries removal , 112–113
enameloplasty , 114
lasers , 112
mechanical rotary technique , 113
microscopic preparation techniques , 113
nonmachinery preparation , 111–113
pit and fi ssure sealing , 113–114
preventive resin restorations , 114
remineralization treatment , 114–115
slot preparation , 113
tunnel preparation , 113
demineralized layer , 110–111
early diagnosis and personal treatment , 110
infected layer , 110
prevention and effective intervention , 110
treatment and effective control , 110
MMP20. See Matrix metalloproteinase-20 (MMP20)
Modifi ed robbins device (MRD) , 161–162
Index

180
Morphogenesis, enamel development
bell stage , 3
bud stage , 2
cap stage , 2–3
Morphogenetic protein 4 (BMP4) , 22
MSCs. See Mesenchymal stem cells (MSCs)
MSD. See Multiple Sorbarod device (MSD)
MTP-based system. See Microtiter plate (MTP)-based
system
Mucins , 62
Multiple artifi cial mouth (MAM) system , 163
Multiple regression analysis , 95, 97
Multiple Sorbarod device (MSD) , 163
Mutacins , 37
Myeloperoxidase (MPO) , 62
N
Nanoindentation technique , 75
Nanoparticles , 78–79
National Health and Nutrition Examination Survey
(NHANES) , 147
Natural medicine, de-/remineralization , 77
Neisseria strains , 134
Nerve fi bers, pulp , 14–15
Neural crest , 1–2
Notch signaling, for root development , 22
O
OCT. See Optical coherence tomography (OCT)
Odontoblast , 16–19
Olsenella , 43
Open system microbial models, in research
artifi cial mouth model , 163–164
chemostat , 164
drip-fed biofi lm model , 162
fl ow cell biofi lm model , 161–162
modifi ed robbins device , 161–162
perfused biofi lm fermenters , 162–163
Optical coherence tomography (OCT) , 75–76
Oral biofi lms, spatiotemporal development of , 31–32
Oral cancer, cariogenic bacteria and , 134
Oral cavity , 30, 130
Care Index , 91, 92
CPITN , 91
high-income industrialized countries , 91
microbial ecology in , 35–36
uneven distribution , 91–92
YLD/million population , 92
Oral fl uids , 72
Oral haemophili urease , 40
Oral health , 129
epidemiological investigation in China , 94–98
global policies , 92–94
Oral Health in America: A Report of the Surgeon
General , 129
Oral microbial ecology , 36
Oral microbiology, at early stage , 33
Oral microbiome , 48–49
Oral microbiota , 148
Oral microfl ora, saliva on , 62–63
Orthodentin , 12
P
PBF. See Perfused biofi lm fermenters (PBF)
PCR method , 138
Pediatric Nephrology , 144
Percussion for caries , 85
Perfused biofi lm fermenters (PBF) , 162–163
Periapical diseases , 130
Periodontal disease , 129–130, 149
Peritubular dentin , 10, 11
“pH cycling” protocols , 158
Phenolic antiseptics , 46
Pit and fi ssure sealant , 105, 113–114
Plaque pH, salivary infl uences on , 61–62
Polymerization shrinkage , 123–125
confi guration factor , 124–125
material related factors , 124
Porphyromonas gingivalis , 36
Postoperative sensitivity , 125
Predentin , 12
Predilection site , 88–89
Presecretory stage, enamel development , 4
Preventive resin restorations , 105, 114
Probing for caries , 85
Probiotics , 49–50
Proliferative period , 1
Propionibacterium , 43
Propolis , 47
Proteolytic enzymes , 8–9
Pseudomonas
P. aeruginosa , 29
P. fl uorescens , 29
P. putida , 29
Pseudoramibacter , 43
Pulp , 13–14
accessory canals , 16
cells in
dendritic cell , 19–20
lymphocyte , 20
macrophage , 19
mast cell , 20
mesenchymal cell , 20
odontoblast , 17–19
pulp fi broblast , 19
connective tissue fi bers , 15
fi broblast , 19
ground substance , 15–16
lymphatics , 16
morphologic zones of pulp , 16–17
nerve fi bers , 14–15
proper , 17
vascular tissues , 14
Pulpal periapical diseases , 140–141
Pulpitis , 130
Pulpodentin complex
cells in dental pulp
dendritic cell , 19–20
lymphocyte , 20
Index

181
macrophage , 19
mast cell , 20
mesenchymal cell , 20
odontoblast , 17–19
pulp fi broblast , 19
dentin
mineralization , 12
repair , 13
sclerosis , 12–13
structure , 9–12
types , 12
pulp , 13–14
accessory canals , 16
connective tissue fi bers , 15
ground substance , 15–16
lymphatics , 16
morphologic zones of pulp , 16–17
nerve fi bers , 14–15
vascular tissues , 14
Q
Quantitative laser fl uorescence technique , 88
Quantitative light-induced fl uorescence (QLF) , 75,
100–101
Quaternary ammonium compounds
(QACs) , 45
R
RA. See Rheumatoid arthritis (RA)
Radiation caries , 132
Radiographic examination for caries , 85–86
Remineralization
models in research , 158
treatment , 114–115
Removable appliances, in situ caries models , 167
Resin composite bonding restoration technique
cavity preparation , 121–123
dentin bonding , 120–121
enamel bonding , 119–120
etching adhesive systems , 118–119
indications and contraindications , 121
polymerization shrinkage , 123–125
postoperative sensitivity , 125
postprocessing decoration , 123
requirements for , 121
self-etch systems , 119
technique sensitivity , 125
total-etch system , 118–119
Resin composites , 118
Respiratory infections , 149–150
Restorative dentistry , 107
based on bionics , 128
biological treatment methods , 127–128
Black, G.V. and , 107–108
bonding technique and , 108
direct bonding restoration technique , 118
individualized treatment , 125–126
minimally invasive treatment ( see Minimally invasive
treatment)
resin composite
cavity preparation , 121–123
dentin bonding , 120–121
enamel bonding , 119–120
etching adhesive systems , 118–119
indications and contraindications , 121
polymerization shrinkage , 123–125
postoperative sensitivity , 125
postprocessing decoration , 123
requirements for , 121
self-etch systems , 119
technique sensitivity , 125
total-etch system , 118–119
resin composites , 118
silver amalgam
cavity shape preparation , 116
controversy , 115–116
fi lling , 116–118
indications and contraindications , 116
Rheedia gardneriana , 48
Rheumatoid arthritis (RA) , 142
Root caries
cardiac dysrhythmias , 138
diabetes , 147–148
Root development
ERM , 21
initiation , 20–21
mesenchymal stem cells , 21
signaling pathway
notch signaling , 22
SHH signaling , 22
TGF-β/BMP Signaling , 21–22
Wnt signaling , 22
tooth eruption , 22–23
16S rRNA gene sequencing , 131
S
SAG. See Salivary agglutinin (SAG)
Saliva , 73
carbonic acid/bicarbonate equilibrium in , 61
caries-associated bacteria , 65–66
characteristics and caries risk , 67
chemical and physical aspects , 68
composition , 59–60
critical value , 61
fl ow rate , 60–61, 65
formation and secretion , 59
low buffering capacity , 61
on oral microfl ora , 62–63
on plaque pH , 61–62
potential use of , 129
role in research
animal model , 169
fl uoride , 169–170
oral cavity , 170–171
in situ model , 169
in vitro models , 170
xerostomia
etiology of , 63–64
management of , 64–65
Index

182
Salivary agglutinin (SAG) , 63
Salivary antimicrobial substances , 50
Salivary glands , 132
Salivary immunoglobulin A (SIgA) , 141–142
Sanguinaria canadensis , 47
Sanguinarine , 47
Scardovia wiggsiae , 43
S-ECC. See Severe early childhood caries (S-ECC)
Secondary dentin , 12
Secretory IgA (SIgA) , 62, 68
Secretory stage, enamel development , 4–5
Self-awareness, caries prevention , 98
Self-etch systems , 119
Severe early childhood caries
(S-ECC) , 135
SHH signaling, for root development , 22
Signaling pathway, for root development
notch signaling , 22
SHH signaling , 22
TGF-β/BMP Signaling , 21–22
Wnt signaling , 22
Silver amalgam , 115–116
cavity shape preparation , 116
controversy , 115–116
fi lling , 116–118
indications and contraindications , 116
Slackia exigua , 43
Slot preparation , 113
Smoothness, glossiness and , 88
Specifi cally targeted antimicrobial peptides
(STAMPs) , 50
Staining technique , 88
Stephan curve , 61
Streptococcus
S. gordonii , 29, 38, 42, 161
S. mutans , 31, 45, 61, 63, 66, 132
acidogenic bacteria , 39
acid tolerance of , 40, 42
AgDS activity , 42
in artifi cial mouth , 165
atherosclerosis , 138–139
in biofi lm , 50, 73, 160
catechins inhibit growth of , 47
colonization in mice , 168
colonization in saliva , 143
ecological plaque hypothesis , 34–35
genetically modifi ed strain , 43
in GERD group , 145
inoculum , 165
interspecies interactions , 37–39
involvement , 34
risk assessment , 110
salivary immunoglobulin A , 141
salivary level , 66
SIgA against , 62
STAMPs targeting , 50
strain of , 168–169
ulcerative colitis , 145
xylitol , 46
S. oralis , 32, 37, 161
S. parasanguis , 42
S. salivarius , 38, 40, 169
S. sanguinis , 38, 132
arginine deiminase system , 42
interspecies interactions , 37
Stroke , 139–140
Survey of Dental Diseases , 140
T
T2DM. See Type 2 diabetes mellitus
(T2DM)
Technique sensitivity , 125
TGF-β/BMP signaling, for root
development , 21–22
Tissue engineering , 127–128
T lymphocytes , 20
TMR. See Transsal microradiography (TMR)
Tomes’ process , 4, 5
Tooth eruption , 22–23
Tooth loss , 130–131
diabetes , 148–149
head and neck cancer risk , 134
Tooth regeneration , 127–128
Tooth worm , 33
Total-etch system , 118–119
Transferable solid phase (TSP) biofi lm model , 160
Transversal microradiography (TMR) , 75
TRAP segment. See Tyrosine-rich amelogenin peptide
(TRAP) segment
Triclosan , 45–46
Tuftelin , 8
Tunnel preparation , 113
Type 2 diabetes mellitus (T2DM) , 145–146
Tyrosine-rich amelogenin peptide
(TRAP) segment , 7
U
Ulcerative colitis (UC) , 145
Ultrasonic technique , 87
Upper respiratory tract infection
(URTI) , 149
V
Vascular tissues, pulp , 14
Veillonella, interspecies interactions , 37, 38
Visual inspection
classifi cation , 99
improvements in , 98
von Korff fi bers , 16
Index

183
W
WHO Global Oral Health Programme , 92
Wnt signaling, for root development , 22
X
Xerostomia
causes of , 64
etiology of , 63–64
management of , 64–65
medications , 64
severity of , 133
sugar intake , 65
Xylitol , 46
Z
Zürich biofi lm model , 160
Index
