
Cross-species variation in gaze following and conspecific preference
among great apes, human infants and adults
Fumihiro Kano
a
,
b
,
*
, Josep Call
a
a
Department of Developmental and Comparative Psychology, Max-Planck Institute for Evolutionary Anthropology, Leipzig, Germany
b
Japan Society for Promotion of Science, Tokyo, Japan
article info
Article history:
Received 27 November 2013
Initial acceptance 6 January 2014
Final acceptance 11 February 2014
Available online 14 April 2014
MS. number: 13-00982R
Keywords:
conspecific model
gaze following
great ape
human infant
species difference
Although previous studies have shown that many species follow gaze, few have directly compared
closely related species, and thus its cross-species variation remains largely unclear. In this study, we
compared three great ape species (bonobos, Pan paniscus, chimpanzees, Pan troglodytes, orang-utans,
Pongo abelii) and human s (12-month-olds and adults) in their gaze-following responses to the videos
of conspecific and allospecific models. In the video, the model turned his head repeatedly to one of two
identical objects. We used a noninvasive eye-tracking technique to measure participants’ eye move-
ments, and used both conspecific and allospecific models as stimuli to examine their potential preference
in following conspecific rather than allospecific gaze. Experiment 1 presented to great apes the videos of
conspecific and human models. We found that all species followed the conspecific gaze. Chimpanzees did
not follow the human gaze, whereas bonobos did. Bonobos reacted overall more sensitively than
chimpanzees to both conspecific and human gaze. Experiment 2 presented to human infants and adults
the videos of human, chimpanzee and orang-utan models. Both infants and adults followed the human
gaze. Unlike adults, infants did not follow the ape gaze. Experiment 3 presented to great apes the videos
of allospecific ape models. Consistent with experiment 1, chimpanzees did not follow the allospecific ape
gaze, whereas bonobos and orang-utans did. Importantly, preferential following of conspecific gaze by
chimpanzees (experiment 1) and human infants (experiment 2) was mainly explained by their prolonged
viewing of the conspecific face and thus seems to reflect their motivation to attend selectively to the
conspecific models. Taken together, we conclude that gaze following is modulated by both subject
species and model species in great apes and humans, presumably a reflection of the subjects’ intrinsic
sensitivity to gaze and also their selective interest in particular models.
Ó 2014 The Association for the Study of Animal Behaviour. Published by Elsevier Ltd. All rights reserved.
Gaze following, defined as looking in the same direction as
others after seeing their gaze direction, is one of the best studied
social behaviours in comparative cognition. It functions in various
ways depending on the species and context, from simply exploiting
the same information that others have acquired to making in-
ferences about others’ intentions and knowledge (Hare, Call, &
Tomasello, 2000). Gaze following has been documented in
numerous species, including primates (great apes: Bräuer, Call, &
Tomasello, 2005; Old World monkeys: Anderson & Mitchell,
1999; Emery, Lorincz, Perrett, Oram, & Baker, 1997; Scerif, Gomez,
& Byrne, 20 04; New World monkeys: Amici, Aureli, Visalberghi, &
Call, 2009; Burkart & Heschl, 2006; lemurs: Ruiz, Gómez, Roeder,
& Byrne, 2009; Sandel, MacLean, & Hare, 2011; Shepherd & Platt,
2008), nonprimate mammals (dogs, Canis familiaris: Téglás,
Gergely, Kupán, Miklósi, & Topál, 2012; goats, Capra hircus:
Kaminski, Riedel, Call, & Tomasello, 2005), birds (ravens, Corvus
corax: Bugnyar, Stöwe, & Heinrich, 2004; bald ibises, Geronticus
eremita: Loretto, Schloegl, & Bugnyar, 2010) and reptiles (red-footed
tortoise, Geochelone carbonaria: Wilkinson, Mandl, Bugnyar, &
Huber, 2010). Although gaze following appears to be fairly wide-
spread in phylogeny, studies have also documented its variation
among closely related species. Thus, stumptailed macaques,
Macaca arctoides, follow gaze more frequently than other macaque
species (Tomasello, Call, & Hare,1998), bonobos, Pan paniscus, more
than chimpanzees, Pan troglodytes (Herrmann, Hare, Call, &
Tomasello, 2010) and human children more than great apes
(Herrmann, Call, Hernandez-Lloreda, Hare, & Tomasello, 2007),
especially when only the model’s eyes (not the head direction)
serve as a gaze cue (Tomasello, Hare, Lehmann, & Call, 2007).
Moreover, rather than simply co-orienting with the model, in
more complex settings in which individuals have to take into
*
Correspondence: F. Kano, Max-Planck Institute for Evolutionary Anthropology,
Deutscher Platz 6, 04103 Leipzig, Germany.
Contents lists available at ScienceDirect
Animal Behaviour
journal homepage: www.elsevier.com/locate/anbehav
http://dx.doi.org/10.1016/j.anbehav.2014.03.011
0003-3472/Ó 2014 The Association for the Study of Animal Behaviour. Published by Elsevier Ltd. All rights reserved.
Animal Behaviour 91 (2014) 137e150
account the position and nature of visual barriers in relation to both
the model and themselves, the distribution of gaze following
among species appears more restricted. Thus, following gaze
around barriers has been documented in apes, ravens, capuchin
monkeys, Cebus apella, and spider monkeys, Ateles geoffroyi, but not
in marmosets, Callithrix jacchus, and bald ibises (Amici et al., 2009;
Bräuer et al., 2005; Bugnyar et al., 2004; Loretto et al., 2010;
Tomasello, Hare, & Agnetta, 1999). Moreover, bonobos and chim-
panzees, unlike orang-utans, Pongo abelii, take barrier opacity into
consideration when following the gaze of others (Okamoto-Barth,
Call, & Tomasello, 2007) and double-looks (i.e. looking back at the
model’s face after following her gaze and detecting nothing
remarkable) have been observed in great apes and Old World
monkeys but not in capuchin and spider monkeys (Amici et al.,
2009; Bräuer et al., 2005; Scerif et al., 2004).
Taken together, these studies show that even though gaze
following is displayed by numerous species, its expression in terms
of strength and flexibility vary substantially among species. Data
like these are crucial to be able to test evolutionary hypotheses
linking gaze following with social and ecological factors that may
contribute to explaining the differences between species, including
the differences between human and nonhuman animals (Rosati &
Hare, 2009). However, this sort of evolutionary analysis is
currently hindered by two major diffi culties. First, gaze following is
modulated not only by the individuals’ potential abilities but also
by motivational or contextual factors. For example, in the previous
studies with macaque species, the subjects preferentially followed
the gaze of particular individuals depending on the social rela-
tionship with and emotional status of the model (Goossens,
Dekleva, Reader, Sterck, & Bolhuis, 2008; Micheletta & Waller,
2012; Shepherd, Deaner, & Platt, 2006; Teufel, Gutmann, Pirow, &
Fischer, 2010). Most relevant for the species comparison is that
many previous studies have used human models rather than
conspecific models for pragmatic reasons, and thus it is possible
that the species differ in the sensitivities to only human but not
conspecific gaze. For example, Hattori, Kano, and Tomonaga (2010)
found that chimpanzees followed the gaze of a conspeci fic but not
of a human model when they were presented with the still pictures
of those models (but see Itakura, Agnetta, Hare, & Tomasello, 1999),
whereas human adults followed the gaze of both types of models.
Ideally, when comparing two or more species, one should use a
crossed design with two factors, subject species and model species,
that is, presenting the models of both species to the subjects of both
species.
Second, the dependent measure most of ten used in previous
studies has been head-turning frequency owing to the difficulty in
recording the eye movements directly. However, species may differ
in their physical constraints to move their head, body and eyes. For
example, orang-utans frequently move their eyes but not their
heads to shift their gaze (i.e. sideways gaze; Kaplan & Rogers, 2002).
Therefore, additional measurements based on eye direction alone
may reveal gaze following that goes undetected when using more
coarse measures based on head turning.
Developmental differences should also be taken into consider-
ation when comparing species, especially species that may follow
different developmental trajectories. Previous studies have shown
that the sensitivity and flexibility of gaze following change with age
in human and nonhuman primates. That is, human infants begin to
follow the gaze of others from 3 to 6 months of age (D’Entremont,
Hains, & Muir, 1997; Hood, Willen, & Driver, 1998) and establish a
robust pattern from 1 year of age (Corkum & Moore, 1998; von
Hofsten, Dahlstrom, & Fredriksson, 2005). Moreover, around 1
year of age human infants begin to follow gaze geometrically to
regions beyond their immediate view (Moll & Tomasello, 2004).
Similarly to nonhuman primates, human infants’ gaze following is
modulated by motivational and contextual factors. For example,
they preferentially follow the gaze of those who have looked to-
wards interesting things versus nothing in the past (Chow, Poulin-
Dubois, & Lewis, 2008), and take into account whether individuals
have their eyes open or closed (Brooks & Meltzoff, 2002). In
nonhuman primates, studies have shown that macaques and
chimpanzees begin to follow gaze by around 1 and 3 years of age,
respectively, and continue to increase the frequency of gaze
following with age (Ferrari, Kohler, Fogassi, & Gallese, 2000;
Tomasello, Hare, & Fogleman, 2001; but see Okamoto et al., 2002
for the earlier onset of gaze following in a chimpanzee). More-
over, macaques and chimpanzees display a relatively late onset for
voluntary control of gaze following such as habituation to unreli-
able observers (Tomasello et al., 2001) and double-looks (Bräuer
et al., 2005).
In this study we aimed to reveal the variation in gaze following
among closely related species by addressing the above-mentioned
issues. We used a crossed design with two factors, subject species
and model species, and studied four hominid species, bonobos,
chimpanzees, orang-utans and human infants (12-month-olds) and
control adults (Fig. 1). We implemented a relatively simple setting
to examine the basic performances of gaze following among spe-
cies. That is, we measured the frequency of gaze following when
each species was observing a human or conspecific model repeat-
edly turning his head to one of two identical objects. We adopted
the eye-tracking method for two reasons: (1) to present controlled
gaze cues of both conspecific and allospecific models on the com-
puter monitor and (2) to rely on the eye movement measurement
which is relatively independent of physical constraints. We exam-
ined whether species (1) showed any evidence of gaze following for
each model species, (2) differentiated between conspecific and
allospecific gaze, and (3) differed from one another in their overall
gaze sensitivities (frequency and/or response time) when pre-
sented with either conspecifics or allospecifics. A previous study
confirmed that the great apes did not differ from one another in
their basic patterns of eye movement (Kano, Hirata, Call, &
Tomonaga, 2011). However, the same study also confirmed that
humans, especially infants (Hood & Atkinson, 1993), tend to shift
their gaze less frequently (the fixations were ‘stickier’) than apes.
Owing to this species difference and some procedural differences
that existed for pragmatic reasons (e.g. the type of attracting
stimuli), we did not compare great apes and humans in a single
experiment. Experiment 1 presented to great apes videos of
conspecific and human models. Experiment 2 presented to human
infants and adults videos of human and allospeci
fic ape models
(chimpanzee and orang-utan). Experiment 3 returned to great apes
and presented videos of allospecific ape models.
EXPERIMENT 1
We examined the gaze-following responses in bonobos, chim-
panzees and orang-utans when they were presented with a
conspecific or a human model repeatedly turning his head to one of
two identical objects. Based on previous studies using eye tracking
(Hattori et al., 2010), we predicted that chimpanzees would pref-
erentially follow the conspecific gaze rather than the human gaze.
In addition, based on previous studies using a different behavioural
paradigm (Bräuer et al., 2005; Herrmann et al., 2010), we predicted
that bonobos would follow gaze, at least the human gaze, more
frequently than chimpanzees. Finally, based on previous studies
using behavioural paradigms (Bräuer et al., 2005; Okamoto-Barth
et al., 2007), we predicted that orang-utans would follow the
gaze of either conspecific or human models; however, it was un-
clear whether they would follow gaze differentially depending on
the observed species.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150138

Methods
Participants
Eight bonobos, 14 chimpanzees and seven orang-utans partici-
pated (mean age 16.5 years; 16 females, 13 males). All apes lived in
groups (>10 individuals) with their conspecifics (but not with their
allospecifics) in the Wolfgang Köhler Primate Research Centre
(WKPRC). Most of the apes were raised by their biological mothers.
Although some of them were reared by humans early in ontogeny
(hand reared), they mostly grew up with conspecifics from an early
age (for details of participants, see Table A1). All great apes were
housed in seminatural indoor enclosures (175e430 m
2
) with
sleeping and test rooms, and also in outdoor enclosures during the
summer (1400 e 4000 m
2
). Both enclosures were equipped with
climbing structures, natural vegetation and enrichment devices to
foster extractive foraging activities. The apes received fresh fruits,
vegetables, eggs, meats, cereals and leaves distributed in three
main meals and occasional enrichment programmes. Water was
available ad libitum throughout the day. They voluntarily partici-
pated in the study and were never food or water deprived. Animal
husbandry and research complied with the EAZA Minimum Stan-
dards for the Accommodation and Care of Animals in Zoos and
Aquaria and the WAZA Ethical Guidelines for the Conduct of
Research on Animals by Zoos and Aquariums, respectively. All apes
were tested in rooms located at WKPRC.
Apparatus
The eye movements of ape participants were noninvasively
recorded with an infrared eye-tracker (60 Hz; Tobii X120, Tobii
Technology AB, Stockholm, Sweden) at a viewing distance of
approximately 60 cm. We tested them unrestrained but separated
from the experimenter and eye-tracker by a transparent acrylic
panel. However, to keep their heads relatively still, we imple-
mented a nozzle and tube attached to the acrylic panels, which
produced grape juice little by little, and let the apes suck the nozzle
during the recording (Fig.1a; also see Appendix Fig. A1). No explicit
training was conducted for the apes. Stimuli were presented on a
22-inch LCD monitor (1366 768 pixel) with Tobii Studio software
(version 3.2.1).
Two-point automated calibration was conducted for great apes
by presenting a small object or video clip on each reference point. A
relatively small number of reference points was adopted for apes
because they tended to view these reference points only briefly.
However, we manually checked the accuracy at five points after the
initial calibration and repeated the calibration if necessary. As a
result, our preliminary session confirmed the comparable accuracy
between apes and humans (see Kano, Call, & Tomonaga, 2012 for
the accuracy estimate). Before every test session for apes, we
checked the accuracy manually and started the session when we
confirmed that the error value was less than 1e2 degrees.
Stimuli and procedure
The stimuli were 10 s videos in which a model repeatedly looked
at one of the two identical objects (hereafter ‘target’ as opposed to
‘distractor’). The model was either a male bonobo, chimpanzee,
orang-utan (one of the members from WKPRC) or male human
(F.K.) (Fig. 1b). These models were familiar to the ape participants
(the apes had at least some regular visual access even to allospecific
models). Each model’s head turn was videotaped in the test room at
WKPRC, and then later edited in Adobe Premier Pro so that the
model appeared to look at the target repeatedly in the final video.
The brightness and contrast were matched across stimuli as much
as possible. The objects were plain coloured square shapes (the
colour was selected not to stand out too much from the back-
ground; green for the bonobo video and red for the other videos,
depending on the greenish/reddish background tones). In each
video, the model faced forward for 1 s, and for the remaining time
(9 s) repeatedly looked at the target by turning both eyes and head
back and forth. The head-turning frequency varied between stimuli
to conserve the natural speed of each model’s head turn (4, 5, 5, 3
Figure 1. Overview of experiments. (a) The participants and (b) the models in this study. (c) An example of area of interest (AOI) de fined for the fixation analysis. In this study,
bonobos were presented with the bonobo, chimpanzee and human models; chimpanzees were presented with the chimpanzee, bonobo and human models; orang-utans were
presented with the orang-utan, human and bonobo models; human infants and adults were presented with the human, chimpanzee and orang-utan models. Thus, each species was
presented with three types of models, and each model was presented to at least three species.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150 139

times, respectively, for bonobo, chimpanzee, human and orang-
utan video; each head turn was thus about 1.8e3 s; see
Supplementary Video S1).
Each ape viewed the videos of both conspecific and human
model. Each video was played three times (total six trials). Each ape
viewed a single video in a day (total 6 days). The order of presenting
model type (conspecific or human) and direction of the model’s
gaze (left or right) was counterbalanced across individuals. The
experimenter initiated the presentation of each video when apes
were attending to the monitor.
Data analysis
Participants’ fixations were detected by the Tobii fixation filter
using Tobii Studio (version 3.2.1) with a default setting. To deter-
mine on which areas each fixation landed, we defined the area of
interest (AOI) as a circle for target, distractor (diameter 350 pixels)
and model’s face (diameter 400e700 pixels depending on the size
of the model’s face; Fig. 1c).
The main measurement was the proportion of trials in which
the participants first looked at the target or distractor after the
initiation of the model ’s head turn (hereafter, the proportion of first
look) with respect to total number of trials (including the trials in
which participants looked at neither). We also measured the total
number of fixations onto the target or the distractor (after the
initiation of the model’s head turn). As this measure yielded very
similar results to the first look measure throughout this study, we
report these results in detail in the Appendix.
In addition, to examine how rapidly each species responded to
the gaze, we measured the response time when the participants
first looked at the target (the initiation of looks at the target from
the initiation of the model’s head turn). Moreover, to examine
whether the occurrence of gaze following was mediated by the
strength of attention to the model’s face, we measured the amount
of time spent viewing the model’s face before the model’s first head
turn (hereafter, face viewing time) and correlated that value with
the proportion of first look at the target. We standardized the face
viewing time as the proportion of viewing time for the face with
respect to the total viewing time for the entire scene.
For the statistical analyses, we distinguished between within-
species and between-species analyses. Within-species analysis
tested whether each species showed any evidence of gaze following
and differential sensitivity to the conspecific versus human gaze
(a repeated-measures ANOVA with Object and Model species as
factors). Between-species analysis tested whether these species
differed quantitatively from one another in their frequency of gaze
following and their sensitivity to conspecific versus human gaze
(a repeated-measures ANOVA with Subject species, Object and
Model species as factors). All analyses were conducted in SPSS
version 20 (SPSS Inc., Chicago, IL, U.S.A.).
Results
Between-species analysis
Figure 2 presents the results for first look. A repeated-measures
three-way ANOVA revealed a significant main effect of Object
(F
1,26
¼ 24.93, P < 0.001,
h
2
¼ 0.49). Thus, overall, great apes first
looked at the target rather than the distractor more frequently than
vice versa, indicating that they followed the model’s gaze. There
was a significant main effect of Subject species (F
2,26
¼ 15.59,
P ¼ 0.040,
h
2
¼ 0.22). However, we also found a significant two-
way interaction between Subject species and Object (F
2,26
¼ 4.16,
P ¼ 0.027,
h
2
¼ 0.24). Thus, species also differed from one another
in their frequency of first look at the target versus the distractor.
More specifically, bonobos followed the gaze more frequently than
chimpanzees (F
1,20
¼ 16.74, P ¼ 0.001,
h
2
¼ 0.45). Bonobos differed
from chimpanzees particularly in their responses to the human
gaze (F
1,19
¼ 7.22, P ¼ 0.015,
h
2
¼ 0.27) rather than to the conspe-
cific gaze (F
1,19
¼ 1.62, P ¼ 0.21,
h
2
¼ 0.07). Although bonobos and
chimpanzees responded to conspecific versus human gaze some-
what differently, we did not find a significant three-way interaction
between Model species, Subject species and Object (F
2,26
¼ 0.87,
P ¼ 0.43,
h
2
¼ 0.06). Orang-utans did not differ significantly from
either bonobos or chimpanzees in their frequency of first look at
the target versus the distractor (Ps > 0.1).
We also examined the individual differences in terms of sex
(male, female), age (young & 9 years of age, adult > 9 years of age)
***
*
*
+
Target
Distractor
Conspecific Human Conspecific ConspecificHuman Human
Bonobo (N = 8)
Proportion of first look
Chimpanzee (N = 14) Orang-utan (N = 7)
0.8
0.6
0.4
0.2
0
Figure 2. Proportion of first look in great apes when they were viewing conspecific and human models. Error bars denote the SEM. þ P < 0.07; *P < 0.05; ***P < 0.001.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150140

and rearing history (mother, hand reared) by including these fac-
tors in the same analysis. However, we did not find any significant
effect of these factors (Ps > 0.1), and importantly, the species dif-
ference in first look at the target versus the distractor was still
detected in this follow-up analysis (Species*Object: F
2,16
¼ 3.96,
P ¼ 0.040,
h
2
¼ 0.33).
We then examined whether the species difference in first look at
the target was related to the species difference in face viewing time
(how long they spent viewing the face before the model’s first head
turn). Species did not differ significantly from one another in their
face viewing time (F
2,28
¼ 2.62, P ¼ 0.092). In addition, there was no
significant correlation between first look at the target and face
viewing time (Pearson correlation: r
27
¼0.12, P ¼ 0.51). Thus, it is
unlikely that the species difference in overall frequency of gaze
following was due to the variation in face viewing time.
Within-species analysis
We then examined the pattern of first look in each species using
two-way ANOVAs. In bonobos, we found a significant main effect of
Object (F
1,7
¼ 19.38, P ¼ 0.0 03,
h
2
¼ 0.73) but not a significant
interaction between Object and Model species (F
1,7
¼ 0.038,
P ¼ 0.85,
h
2
¼ 0.005), indicating that they followed the gaze of both
conspecific and human models. Similarly, in orang-utans, we found
a trend in the main effect of Object (F
1,6
¼ 5.25, P ¼ 0.062,
h
2
¼ 0.46; we found a significant main effect of Object in the total
number of fixations, see Appendix) but not a significant interaction
between Object and Model (F
1,6
¼ 0.60, P ¼ 0.46,
h
2
¼ 0.09). In
chimpanzees, we did not find a significant main effect of Object
(F
1,13
¼ 1.63, P ¼ 0.22,
h
2
¼ 0.11); yet we found a significant inter-
action between Object and Model (F
1,13
¼ 5.06, P ¼ 0.042,
h
2
¼ 0.28). Follow-up paired t tests indicated that chimpanzees
followed the gaze of the conspecific model (t
13
¼ 2.82, P ¼ 0.014)
but not that of the human model (t
13
¼ 0.51, P ¼ 0.61).
We then examined whether chimpanzees’ differential re-
sponses to the conspecific versus human gaze may be because of
their differential viewing of the conspecific versus human face. We
indeed found that chimpanzees viewed the conspecific face for
longer than the human face (Table 1; t
13
¼ 3.87, P ¼ 0.0 02). More-
over, they viewed the face AOI for longer on those trials in which
they looked at the target (followed the gaze) than on those trials in
which they looked at the distractor (Table 2; t
8
¼ 3.03, P ¼ 0.016).
Thus, chimpanzees’ preferential following of conspecific gaze may
be simply explained by their preferential viewing of the conspecific
face. No such relation was con firmed for bonobos and orang-utans
(Ps > 0.07).
Response time
We examined the response time for first look (the time at which
they first looked at the target). We restricted this analysis to the
presentation of conspecific models because chimpanzees did not
follow the human gaze. The response times were 1989 451,
3364 359 and 2621 501 ms (mean SE), respectively, for
bonobos, chimpanzees and orang-utans. Overall, there was some
indication that species might differ in response time (F
2,27
¼ 2.85,
P ¼ 0.076,
h
2
¼ 0.41). Bonobos followed the conspecific gaze
significantly faster than chimpanzees (t
19
¼ 2.37, P ¼ 0.028). Orang-
utans did not differ from either bonobos or chimpanzees in their
response time (Ps > 0.05).
Discussion
All species followed at least conspecific gaze in this eye-tracking
paradigm. That is, they more frequently looked in the same than in
the opposite direction as the conspecific model. As for the species
difference, we found that bonobos followed human gaze more
frequently than chimpanzees, which extends the similar finding in
a previous study using a different behavioural paradigm (Herrmann
et al., 2010). Although the two species did not differ from one
another in their frequency of following the conspecific gaze,
bonobos followed the conspecific gaze faster than chimpanzees.
Thus, bonobos seem to be more sensitive than chimpanzees to the
gaze in general. Orang-utans were not statistically different from
the other two species in their frequency or timing of gaze following.
Also consistent with a previous study using a similar eye-
tracking paradigm (Hattori et al., 2010), chimpanzees followed
the conspecific gaze but not the human gaze. This preferential
following of the conspecific gaze seems to be related to their
preferential viewing of the conspecific face. In addition, as shown in
Fig. 2, unlike chimpanzees, bonobos frequently followed the gaze of
both the conspecific and the human model. Thus, the two species
may differ from one another in their responses to conspecific
versus allospecific gaze. However, in experiment 1, the statistical
support for this idea was insufficient (i.e. the three-way interaction:
Model species*Subject species*Object). Also, it remains unclear
whether each species prefers not to follow only human gaze or
allospecific gaze in general. For orang-utans, although we did not
find a statistical difference between their responses to conspecific
and allospecific gaze, we also did not find a clear statistical support
for gaze following in response to the human gaze (see Fig. 2). Thus,
we further explored great apes’ responses to the allospecific gaze
by presenting the nonhuman allospecific gaze to the same partic-
ipants in experiment 3.
EXPERIMENT 2
This experiment examined how infants and adults differently
follow the gaze of human and ape models (chimpanzee and orang-
utan models) with the same method used in experiment 1. Based
on a previous study using an eye-tracking paradigm (Hattori et al.,
2010), we expected that human adults would follow the gaze of
both human and ape models. Although numerous studies have
used nonhuman agents as stimuli to examine infants’ social
cognition in general, to the best of our knowledge, no previous
Table 1
Proportion of face viewing time (mean, SE) for each model species
Subject species Model species
Ape Human
Bonobos (experiment 1) 0.66 (0.069) 0.57 (0.051)
Chimpanzees (experiment 1) 0.90 (0.032) 0.68 (0.039)
Orang-utans (experiment 1) 0.83 (0.051) 0.70 (0.019)
Human infants (experiment 2) 0.71 (0.052) 0.88 (0.023)
Table 2
Proportion of face viewing time (mean, SE) as a function of the looking patterns for
the objects
Subject species Looking pattern
Looked at
neither
Looked at
target
Looked at
distractor
Bonobos (experiment 1) 0.60 (0.14) 0.62 (0.057) 0.62 (0.059)
Chimpanzees
(experiment 1)
0.80 (0.054) 0.88 (0.030) 0.70 (0.045)
Orang-utans
(experiment 1)
0.79 (0.031) 0.75 (0.049) 0.78 (0.071)
Human infants
(experiment 2)
0.78 (0.060) 0.84 (0.029) 0.76 (0.044)
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150 141
study has used nonhuman primates as stimuli to examine infants’
gaze following. Thus, two different predictions are possible. One
could hypothesize that human infants may follow both human and
nonhuman gaze because previous studies have shown that human
infants find goal directedness in nonhuman agents if the agents
show certain type of behavioural cues (e.g. self-propelling; Gergely
& Csibra, 2003). However, some studies also suggest that human
infants do not follow the gaze of nonhuman agents if the behav-
ioural cues are limited. For example, infants followed the gaze of a
toy animal only when the animal showed contingent movements to
the infants prior to gaze cueing (Johnson, Slaughter, & Carey, 1998).
Also, the previous studies suggest that human infants, unlike great
apes (Tomasello et al., 2007), rely more on the eye than the head
direction when following the human gaze (Brooks & Meltzoff,
2002). In this study, however, our ape models in the videos did
not produce these cues explicitly. Critically, our ape models did not
provide a clear signal of eye direction, unlike our human model,
owing to the nature of their eye morphology (i.e. they have a dark
sclera; Kobayashi & Kohshima, 1997).
Importantly, in this experiment, we made some minor changes
in our video stimuli. In our pilot test using the stimuli without any
changes from experiment 1, we found that human infants did not
view the target/distractor object and instead kept looking at the
face of the human and ape models (whereas human adults followed
the gaze). This is probably because infants generally move their
gaze less frequently (i.e. they have sticky fixations) than great apes
or human adults. Thus, based on previous knowledge (Moore,
2008), in this experiment we made two minor changes to the
stimuli used in experiment 1 so that infants could release attention
from the model’s face and follow at least the gaze of the human
model (for details, see Methods and Appendix) as in many previous
studies with the eye-tracking method (von Hofsten et al., 2005;
Senju & Csibra, 2008).
Methods
Participants
Twenty-two 12-month-old infants (within 2 weeks on either
side; 11 males, 11 females) participated. Their parents were
recruited by telephone from a database of parents who had vol-
unteered to participate in developmental studies. All parents
agreed the informed consent upon coming to the institute. They
were tested in a room located at the Max-Planck Institute for
Evolutionary Anthropology (MPI-EVA), Leipzig, Germany. Two
additional infants were tested but excluded from the analysis
because of fussiness (N ¼ 1) and a software malfunction (N ¼ 1).
We also asked one of the parents of each infant to participate in this
study, and so 22 adults (7 males, 15 females, 20e40 years old)
completed the same trials as the infants.
Apparatus
The human participants were tested using the same eye-tracker,
monitor and software. Infants were seated on a parent’s lap during
the recording. Calibration was conducted using five reference
points for infants and adults by presenting a small video at each
reference point.
Stimuli and procedure
Compared to the stimuli used in experiment 1, we (1) increased
the object saliency by replacing them with colourful balls and (2)
decreased the face saliency by reducing the number of head turns
of the model (only two times; see Supplementary Video S2). Each
infant and adult viewed the videos of (1) the human model and (2)
the ape species model. Half of the human participants (11 infants
and 11 adults) viewed the human and chimpanzee models and the
other half viewed the human and orang-utan models. Each video
was played three times (total six trials). Each infant and adult
viewed all videos in a single session. The whole session lasted
approximately 10 min. The presentation order for model type
(conspecific or human) and direction of the model’s gaze (left or
right) were counterbalanced across individuals. At the beginning of
each video, we presented small animations and boing sounds to
make sure that infants looked at the monitor. Human adults were
told to watch the videos as they normally would. They were told
neither the contents of videos nor the purpose of experiments (i.e.
gaze following) before participating in this experiment except that
they would see apes and humans in the videos.
Data analysis
All analyses were conducted in the same way as in experiment 1.
Our initial analysis did not reveal any significant difference be-
tween the two groups who saw the chimpanzee or orang-utan
model, and thus we combined the two groups in the following
analyses.
Results
Proportion of First Look
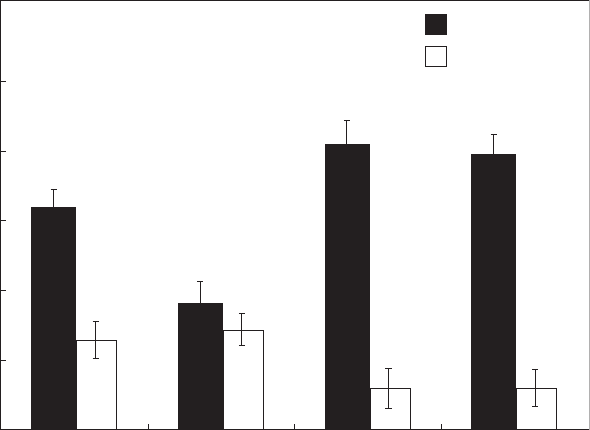
Figure 3 presents the results for first look. A repeated-measures
three-way ANOVA revealed a significant main effect of Object
(F
1,42
¼ 53.92, P < 0.001,
h
2
¼ 0.56), indicating that subjects fol-
lowed the gaze of models. There was a significant main effect of Age
(F
1,42
¼ 7.0, P ¼ 0.011,
h
2
¼ 0.14), but also a significant interaction
between Age and Object (F
1,42
¼ 13.48, P ¼ 0.001,
h
2
¼ 0.24). Thus,
adults followed the gaze more frequently than infants. We also
found a marginal three-way interaction between Model, Object and
Age (F
1,42
¼ 3.45, P ¼ 0.070,
h
2
¼ 0.076), suggesting that adults and
infants followed the gaze of human and ape models differently.
We then examined the pattern of first look in each age group
using two-way ANOVAs. In adults, we found a significant main
effect of Object (F
1,21
¼ 53.76, P < 0.001,
h
2
¼ 0.71), but not a sig-
nificant interaction between Model and Object (F
1,21
¼ 0.068,
P ¼ 0.79,
h
2
¼ 0.003). Thus, adults followed the gaze of both human
and ape models. In infants, we found a significant main effect of
Object (F
1,21
¼ 7.73, P ¼ 0.011,
h
2
¼ 0.26) but also a significant
interaction between Model and Object (F
1,21
¼ 11.29, P ¼ 0.003,
h
2
¼ 0.35). Follow-up paired t tests for infants showed that they
followed the human gaze (t
21
¼ 3.83, P ¼ 0.001) but not the ape
gaze (t
21
¼ 0.86, P ¼ 0.39).
We also examined whether the age difference in first look was
related to the face viewing time (how long subjects spent viewing
the face before the model’s first head turn). Adults viewed the
model’s face significantly longer than infants (t
42
¼ 5.38,
P < 0.001). In addition, there was a significant correlation between
first look at the target and face viewing time (Pearson correlation:
r
42
¼ 0.39, P ¼ 0.007). However, when we analysed each age group
separately, we did not find any significant correlation in either
group (Ps > 0.5). Therefore, although adults followed the gaze more
frequently and viewed the face for longer than infants, the indi-
vidual difference in face viewing time does not necessarily explain
the individual difference in the first look.
Finally, we examined whether infants’ differential responses to
the human versus ape gaze may be because of their differential
viewing of the human versus ape face. We indeed found that in-
fants viewed the human face for longer than the ape face (Table 1;
t
21
¼ 3.41, P ¼ 0.003). Moreover, they viewed the face AOI for
longer on those trials in which they looked at the target (followed
the gaze) than on those trials in which they looked at the dis-
tractor (Table 2; t
18
¼ 3.45, P ¼ 0.003). Thus, infants’ preferential
following of the human gaze may be mediated by their
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150142
preferential viewing of the human face. We did not conduct the
same analysis for adults because they viewed both conspecific and
allospecific faces over 90% of the total time and rarely fixated on
the distractor.
Response time
As in experiment 1, we restricted the analysis for response time
to the presentation of human models because infants did not follow
the gaze of ape models. The response times were 2518 276 and
1516 231 ms (mean SE), respectively, for infants and adults.
Adults followed the gaze significantly faster than infants
(t
42
¼ 2.78, P ¼ 0.008).
Discussion
Both human infants and adults followed at least the human
model in this eye-tracking paradigm, consistent with many previ-
ous studies. Human adults followed the gaze more sensitively
(more frequently and faster) than infants. Unlike adults, infants
followed the conspecific (human) but not ape gaze. This preferen-
tial following of the conspecific gaze by infants seems to be related
to their preferential viewing of the human face.
It is not surprising to find that human adults follow both human
and allospecific ape gaze more sensitively than infants, given their
sensitivity to both human and allospecific ape eyes (Kano &
Tomonaga, 2010) and a strong contagious tendency towards the
other’s behaviours (Driver et al., 1999; Gallup et al., 2012). Also,
human adults are usually given numerous opportunities to view
humans and nonhumans in the media and to interact with them in
real life. On the other hand, it is somewhat surprising to find that
human infants showed marked differences in their responses to
conspecific versus allospecific faces given that human infants
attribute goal directedness of nonhuman agents in many contexts
(Gergely & Csibra, 2003). Infants’ preferential gaze following was
analogous to chimpanzees’. We discuss the possible mechanism
underlying this apparent similarity between human infants and
chimpanzees in the General Discussion.
EXPERIMENT 3
This experiment further investigated whether great apes fol-
lowed the allospecific gaze; this time, however, we used other ape
species as models. The purpose of this experiment was to com-
plement the crossed design with two factors, subject species and
model species. In particular, in experiment 1, we found (1) chim-
panzees’ conspecific preference and (2) bonobos’ gaze sensitivity
over chimpanzees’. However, since humans are very familiar allo-
specifics to ape participants (e.g. caregivers), it is not entirely clear
whether experiment 1’s findings derive from their special response
to the human model or from a general response to the allospecific
model. If the latter were the case, the same pattern of results of
experiment 1 would emerge also in this experiment.
Methods
We tested the same ape participants using the same stimuli as in
experiment 1, but presenting the chimpanzee and bonobo models
to the bonobo and chimpanzee participants, respectively. We also
presented the bonobo model to the orang-utan participants
(because the orang-utan participants at the WKPRC had better vi-
sual access to the bonobo model than the chimpanzee model used
in this study). The presentation order of direction of the model’s
gaze (left or right) was counterbalanced across individuals. The
apparatus and other procedures were the same as in experiment 1.
Results and Discussion
Figure 4 presents the results for first look. A repeated-measures
two-way ANOVA revealed a significant main effect of Object
(F
1,26
¼ 24.31, P ¼ 0.012,
h
2
¼ 0.28) but also a significant interaction
between Object and Subject species (F
2,26
¼ 5.29, P < 0.001,
h
2
¼ 0.48). Specifically, bonobos followed the gaze of the chim-
panzee model (t
7
¼ 3.45, P ¼ 0.011) and orang-utans followed the
gaze of the bonobo model (t
6
¼ 6.0, P ¼ 0.001). In contrast, chim-
panzees did not follow the gaze of the bonobo model (t
13
¼ 0.51,
P ¼ 0.61). We omitted the correlation analysis with the first look
1
0.8
0.6
0.4
0.2
0
Proportion of first look
Human
Human infant (N = 22) Human adult (N = 22)
HumanApe Ape
Distractor
Target
***
***
***
Figure 3. Proportion of first look in human infants and adults when they were viewing human and ape models. Error bars denote the SEM. ***P < 0.001.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150 143

and face viewing time in this experiment, as there were too few
trials. The response times for the first look at the target were
2165 463 s and 3469 715 s (mean SE), respectively, for
bonobos and orang-utans (not significantly different, P > 0.1).
Thus, taken together with the results from experiment 1,
chimpanzees seemed to differ from the other two species in their
responses to the allospecific gaze. That is, while bonobos and
orang-utans followed the gaze of allospecific models, chimpanzees
did not follow the gaze of either the human or the allospeci fic ape
(bonobo) model.
Finally, although this study (and the previous study, Hattori
et al., 2010) showed that chimpanzees followed the conspecific
gaze, further studies are necessary to pin down what type of
conspecific models they prefer to follow. That is, this study (and the
previous study) used a familiar chimpanzee as a model, and thus it
is possible that their preferential gaze following may reflect their
preference for familiar individuals (or in-group individuals) rather
than their preference for conspecific individuals in general. This
issue is further discussed in the General Discussion.
GENERAL DISCUSSION
In this study, we found that all species similarly followed the
gaze of the conspecific model. However, whereas bonobos, orang-
utans and human adults followed the gaze of both conspecific
and allospecific models, chimpanzees and human infants followed
the gaze of only conspecific models. Importantly, all stimulus
models elicited the gaze-following responses in at least two spe-
cies, and overall patterns for the presence/absence of gaze
following were unrelated to the low-level stimulus differences (e.g.
colour, brightness and contrast of objects/models; but note some
changes in the stimuli in experiment 2). Rather, the absence of
following the allospecific gaze by chimpanzees and human infants
was related to their inattentiveness to the allospecific face. This
seems to indicate that motivational differences of participants, not
the competence differences of participants nor the low-level dif-
ferences of stimuli, are responsible for the observed patterns of
gaze following in these species.
Preferential following of the conspecific gaze by chimpanzees
and infants suggests that they may preferentially learn from or
acquire information from conspecifics, the most relevant others.
That is, rather than reflexively following any individual’s gaze, they
may first selectively view the relevant others and then follow the
gaze. This behavioural strategy, ‘select-then-follow’,maybe
particularly important for those species such as chimpanzees and
young humans who need to learn effi
ciently from particular in-
dividuals. Thus, at least in this sense, this study is consistent with
the previous studies reporting selective social referencing or se-
lective behavioural copying of particular others, such as majorities
and dominants, by chimpanzees, capuchin monkeys, vervet mon-
keys, Chlorocebus pygerythrus, and human children (Dindo, Whiten,
& de Waal, 2009; Haun, Rekers, & Tomasello, 2012; van de Waal,
Renevey, Favre, & Bshary, 2010).
However, our findings about conspecific preference by chim-
panzees and human infants are limited in scope because it is un-
clear whether they followed the gaze of conspecifics in general or
only that of particular individuals (e.g. familiar/unfamiliar in-
dividuals). Since previous studies have shown that the familiarity of
the model modulates gaze following in monkeys and human in-
fants and adults (Deaner, Shepherd, & Platt, 2007; Gredebäck, Fikke,
& Melinder, 2010; Micheletta & Waller, 2012), it is possible that our
human and ape participants have some specific preference for
particular individuals. This is an issue that deserves further inves-
tigation especially given that several studies have shown that
communicative signals of strangers may function differently for
human infants and nonhuman animals (Topál, Gergely, Erd
}
ohegyi,
Csibra, & Miklósi, 2009).
In this study, we observed particularly intriguing species dif-
ferences between bonobos and chimpanzees. First, unlike chim-
panzees, bonobos did not show a preference for following the
conspecific versus allospecific gaze. Thus, bonobos followed the
allospecific gaze more frequently than chimpanzees. Second,
although the two species did not differ significantly from one
another in their frequency of following the conspecific gaze,
bonobos followed the conspecific gaze significantly faster than
chimpanzees. These species differences in gaze following seem to
be unrelated to their attentiveness to the model’s face. Taken
together, bonobos seem to differ from chimpanzees in their
intrinsic sensitivity to the gaze. One proximate explanation for this
species difference is that bonobos may follow the gaze more
Target
Distractor
*
***
0.8
0.6
0.4
0.2
0
Proportion of first look
Bonobo (N = 8) Chimpanzee (N = 14) Orang-utan (N = 7)
Figure 4. Proportion of first look in great apes when they were viewing allospecific ape models. Error bars denote the SEM. *P < 0.05; ***P < 0.001.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150144
reflexively and thus less selectively than chimpanzees. In support of
this view, a previous study based on a behavioural paradigm
showed that bonobos and chimpanzees followed the experi-
menter’s gaze but chimpanzees inferred the location of hidden
objects more flexibly (thus perhaps less re fl exively) in various
experimental contexts than bonobos (MacLean & Hare, 2012). As
for the underlying mechanism, several previous studies with ma-
caques and humans have suggested that the relative strength of
reflexive and voluntary components in gaze following may be
modulated by androgen-related mechanisms (Shepherd et al.,
2006). Previous studies have also shown that bonobos and chim-
panzees are different in their levels of or reactivity to androgens
(Wobber et al., 2010). Thus, the physiological differences between
species may contribute to how much reflectively or selectively each
species follows gaze. To test this possibility, again future studies
should examine to what extent bonobos and chimpanzees are se-
lective in following the gaze of particular conspecific individuals.
The observed patterns of gaze following by orang-utans fit
somewhat in between those of bonobos and chimpanzees in terms
of the frequency and selectiveness. Importantly, in this study,
orang-utans reliably followed the model’s gaze, and in no case were
they significantly inferior to the other two species. One of the main
differences between this study and previous ones is that this study
measured their eye rather than head/body movements. Thus,
orang-utans in this study should have been free of physical con-
straints derived from their relatively slow head/body movements.
Also, this study used a straightforward experimental setting which
only required orang-utans to glance at the object existing in their
visual fields. Thus, at least at the basic level, it is reasonable to
conclude that orang-utans do not differ from the other great ape
species in their ability to follow a gaze.
Human infants preferentially followed human but not ape gaze
in this study. As discussed above, this pattern resembles that of
chimpanzees, and the function may be also similar between the
two species. The underlying mechanisms may also be similar be-
tween chimpanzees and infants. That is, human infants may have
tried to acquire information from the human models as relevant
others selectively, and thereby viewed the human face for longer
than the ape face, and followed the human gaze more frequently
than the ape gaze. However, based on previous studies with human
infants, alternative explanations are also possible. First, the model’s
communicative intent, which is shown by a signal such as eye
contact and contingent movements, is important to elicit gaze
following in infants (Farroni, Csibra, Simion, & Johnson, 2002; Senju
& Csibra, 2008). Thus, our infants may have failed to see the
communicative intent in the ape models because they had little
experience in seeing and interacting with great apes or similar
kinds of animals. Second, human infants, but not great apes, are
sensitive to the eye rather than head direction of human models
(Brooks & Meltzoff, 2002; Tomasello et al., 2007). Thus, our infants
may have failed to perceive the eye direction of ape models
because, unlike humans, the apes do not have a clear contrast be-
tween iris and sclera (Kobayashi & Kohshima, 1997). Neither of
these accounts could be applied to our chimpanzees’ preferential
gaze following of conspecific chimpanzee models. Thus, it is
possible that distinct mechanisms underlie the apparently similar
pattern of gaze following in chimpanzees and human infants.
Likewise, although bonobos’ and human adults’ sensitivity to the
allospecific gaze may reflect a similar mechanism to some extent,
for example reflexive following of any gaze, it is also possible that
distinct mechanisms underlie the apparent similarities, especially
given human adults’ extensive experience with allospecific faces.
Finally, as a methodological lesson, the species variation in
sensitivity to the allospecific gaze suggests the importance of using
conspecific models in comparative studies of gaze following and
perhaps any social behaviours relying on gaze following (also see
Hare et al., 2000; Tomasello et al., 1998). However, although
chimpanzees in this study did not follow the human gaze,
numerous previous studies have documented their robust re-
sponses to the human experimenter’s gaze. Parsimoniously, this
disparity can be explained by the methodological differences; in
this study chimpanzees spontaneously paid less attention to the
human face than the conspecific face, but in previous studies, the
human experimenter typically established eye contact with the
chimpanzees before giving a gaze cue (by presenting food in front
of the face or calling the chimpanzee’s name). Also, the relevance of
stimuli (i.e. video versus live) may also contribute to the chim-
panzees’ motivation to attend to the human face.
Conclusion
Using a crossed design with two factors, subject species and
model species, we showed that (1) all species followed the
conspecific gaze, (2) unlike bonobos, orang-utans and human
adults, chimpanzees and human infants preferentially followed the
conspecific but not allospecific gaze, and (3) bonobos followed both
conspecific and allospecific gaze more sensitively than chimpan-
zees. Thus, we conclude that gaze following is modulated by both
subject species and model species in great apes and humans, pre-
sumably a reflection of the subjects’ intrinsic sensitivity to gaze and
also their selective interest in particular models.
Acknowledgments
This study was in part funded by the Japan Society for Promotion
of Science for study abroad. We thank S. Mauritz, L. Haux, S. Taubert
and the keepers of the Wolfgang Köhler Primate Research Centre
for the help in data collection.
Supplementary Material
Supplementary material associated with this article is available,
in the online version, at http://dx.doi.org/10.1016/j.anbehav.2014.
03.011.
References
Amici, F., Aureli, F., Visalberghi, E., & Call, J. (2009). Spider monkeys ( Ateles geoffroyi)
and capuchin monkeys (Cebus apella) follow gaze around barriers: evidence for
perspective taking? Journal of Comparative Psychology, 123(4), 368e374.
Anderson, J. R., & Mitchell, R. W. (1999). Macaques but not lemurs co-orient visually
with humans. Folia Primatologica, 70(1), 17e22.
Bräuer, J., Call, J., & Tomasello, M. (2005). All great ape species follow gaze to
distant locations and around barriers. Journal of Comparative Psychology,
119(2), 145e154.
Brooks, R., & Meltzoff, A. N. (2002). The importance of eyes: how infants interpret
adult looking behavior. Developmental Psychology, 38(6), 958e966.
Bugnyar, T., Stöwe, M., & Heinrich, B. (2004). Ravens, Corvus corax, follow gaze di-
rection of humans around obstacles. Proceedings of the Royal Society B: Biological
Sciences, 271(1546), 1331e1336.
Burkart, J., & Heschl, A. (2006). Geometrical gaze following in common marmosets
(Callithrix jacchus). Journal of Comparative Psychology, 120(2), 120e130.
Chow, V., Poulin-Dubois, D., & Lewis, J. (2008). To see or not to see: infants prefer to
follow the gaze of a reliable looker. Developmental Science, 11(5), 761e770.
Corkum, V., & Moore, C. (1998). The origins of joint visual attention in infants.
Developmental Psychology, 34(1), 28e38.
D’Entremont, B., Hains, S., & Muir, D. (1997). A demonstration of gaze following in 3-
to 6-month-olds. Infant Behavior and Development, 20(4), 569e572.
Deaner, R. O., Shepherd, S. V., & Platt, M. L. (2007). Familiarity accentuates gaze
cuing in women but not men. Biology Letters, 3(1), 64e67.
Dindo, M., Whiten, A., & de Waal, F. B. (2009). In-group conformity sustains
different foraging traditions in capuchin monkeys (Cebus apella). PLoS One,
4(11), e7858.
Driver, J., Davis, G., Ricciardelli, P., Kidd, P., Maxwell, E., & Baron-Cohen, S. (1999).
Gaze perception triggers reflexive visuospatial orienting. Visual Cognition, 6(5),
509e540.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150 145
Emery, N. J., Lorincz, E. N., Perrett, D. I., Oram, M. W., & Baker, C. I. (1997). Gaze
following and joint attention in rhesus monkeys (Macaca mulatta). Journal of
Comparative Psychology, 111, 286e293.
Farroni, T., Csibra, G., Simion, F., & Johnson, M. H. (2002). Eye contact detection in
humans from birth. Proceedings of the National Academy of Sciences, 99(14), 9602.
Ferrari, P. F., Kohler, E., Fogassi, L., & Gallese, V. (2000). The ability to follow eye gaze
and its emergence during development in macaque monkeys. Proceedings of the
National Academy of Sciences, 97(25), 13997e14002.
Gallup, A. C., Hale, J. J., Sumpter, D. J., Garnier, S., Kacelnik, A., Krebs, J. R., et al.
(2012). Visual attention and the acquisition of information in human crowds.
Proceedings of the National Academy of Sciences, 109(19), 7245e7250.
Gergely, G., & Csibra, G. (2003). Teleological reasoning in infancy: the naıve theory
of rational action. Trends in Cognitive Sciences, 7(7), 287e292.
Goossens, B., Dekleva, M., Reader, S. M., Sterck, E. H., & Bolhuis, J. J. (2008). Gaze
following in monkeys is modulated by observed facial expressions. Animal
Behaviour, 75(5), 1673e1681.
Gredebäck, G., Fikke, L., & Melinder, A. (2010). The development of joint visual
attention: a longitudinal study of gaze following during interactions with
mothers and strangers. Developmental Science, 13(6), 839e 848.
Hare, B., Call, J., & Tomasello, M. (200 0). Chimpanzees know what conspecifics do
and do not see. Animal Behaviour, 59,771e785.
Hattori, Y., Kano, F., & Tomonaga, M. (2010). Differential sensitivity to conspecific
and allospecific cues in chimpanzees and humans: a comparative eye-tracking
study. Biology Letters, 6(5), 610e613.
Haun, D., Rekers, Y., & Tomasello, M. (2012). Majority-biased transmission in chim-
panzeesandhuman children, butnotorangutans. Current Biology,22(8), 727e73 1.
Herrmann, E., Call, J., Hernandez-Lloreda, M. V., Hare, B., & Tomasello, M. (2007).
Humans have evolved specialized skills of social cognition: the cultural intel-
ligence hypothesis. Science, 317(5843), 1360e1366.
Herrmann, E., Hare, B., Call, J., & Tomasello, M. (2010). Differences in the cognitive
skills of bonobos and chimpanzees. PLoS One, 5(8), e12438.
von Hofsten, C., Dahlstrom, E., & Fredriksson, Y. (2005). 12-Month-old infants’
perception of attention direction in static video images. Infancy, 8(3), 217e
231.
Hood, B. M., & Atkinson, J. (1993). Disengaging visual attention in the infant and
adult. Infant Behavior and Development, 16(4), 405e422.
Hood, B. M., Willen, J. D., & Driver, J. (1998). Adult’s eyes trigger shifts of visual
attention in human infants. Psychological Science, 9(2), 131e134.
Itakura, S., Agnetta, B., Hare, B., & Tomasello, M. (1999). Chimpanzee use of human and
conspecificsocialcues to locate hidden food.Developmental Science, 2(4), 448e456.
Johnson, S., Slaughter, V., & Carey, S. (1998). Whose gaze will infants follow? The
elicitation of gaze-following in 12-month-olds. Developmental Science, 1(2),
233e238.
Kaminski, J., Riedel, J., Call, J., & Tomasello, M. (2005). Domestic goats, Capra hircus,
follow gaze direction and use social cues in an object choice task. Animal
Behaviour, 69(1), 11e18.
Kano, F., Call, J., & Tomonaga, M. (2012). Face and eye scanning in gorillas, orang-
utans, and humans: unique eye-viewing patterns in humans among hominids.
Journal of Comparative Psychology, 126(4), 388e398.
Kano, F., Hirata, S., Call, J., & Tomonaga, M. (2011). The visual strategy specificto
humans among hominids: a study using the gap-overlap paradigm. Vision
Research, 51(23), 2348e2355.
Kano, F., & Tomonaga, M. (2010). Face scanning in chimpanzees and humans:
continuity and discontinuity. Animal Behaviour, 79(1), 227e235.
Kaplan, G., & Rogers, L. J. (20 02). Patterns of gazing in orangutans (Pongo pygmaeus).
International Journal of Primatology, 23(3), 501e526.
Kobayashi, H., & Kohshima, S. (1997). Unique morphology of the human eye. Nature,
387(6635), 767e768.
Loretto, M.-C., Schloegl, C., & Bugnyar, T. (2010). Northern bald ibises follow others’
gaze into distant space but not behind barriers. Biology Letters, 6(1), 14e17.
MacLean, E. L., & Hare, B. (2012). Bonobos and chimpanzees infer the target of
another’s attention. Animal Behaviour, 83(2), 345e353.
Micheletta, J., & Waller, B. M. (2012). Friendship affects gaze following in a tolerant
species of macaque (Macaca nigra). Animal Behaviour, 83(2), 459e467.
Moll, H., & Tomasello, M. (2004). 12- and 18-month-old infants follow gaze to
spaces behind barriers. Developmental Science, 7(1), 1e9.
Moore, C. (2008). The development of gaze following. Child Development Perspec-
tives, 2(2), 66e70.
Okamoto, S., Tomonaga, M., Ishii, K., Kawai, N., Tanaka, M., & Matsuzawa, T. (20 02).
An infant chimpanzee (Pan troglodytes) follows human gaze. Animal Cognition,
5(2), 107e114.
Okamoto-Barth, S., Call, J., & Tomasello, M. (2007). Great apes’ understanding of
other individuals’ line of sight. Psychological Science, 18(5), 462e468.
Rosati, A. G., & Hare, B. (2009). Looking past the model species: diversity in gaze-
following skills across primates. Current Opinion in Neurobiology, 19(1), 45e51.
Ruiz, A., Gómez, J. C., Roeder, J. J., & Byrne, R. W. (2009). Gaze following and gaze
priming in lemurs. Animal Cognition, 12(3), 427e434.
Sandel, A. A., MacLean, E. L., & Hare, B. (2011). Evidence from four lemur species that
ringtailed lemur social cognition converges with that of haplorhine primates.
Animal Behaviour, 81(5), 925e931.
Scerif, G., Gomez, J.-C., & Byrne, R. W. (2004). What do Diana monkeys know about
the focus of attention of a conspecific? Animal Behaviour, 68(6), 1239e1247 .
Senju, A., & Csibra, G. (2008). Gaze following in human infants depends on
communicative signals. Current Biology, 18(9), 668e671.
Shepherd, S. V., Deaner, R. O., & Platt, M. L. (2006). Social status gates social
attention in monkeys. Current Biology, 16(4), 119e120.
Shepherd, S. V., & Platt, M. L. (2008). Spontaneous social orienting and gaze
following in ringtailed lemurs (Lemur catta). Animal Cognition, 11(1), 13e20.
Téglás, E., Gergely, A., Kupán, K., Miklósi, Á., & Topál, J. (2012). Dogs’ gaze following
is tuned to human communicative signals. Current Biology, 22
(3), 1e4.
Teufel, C., Gutmann, A., Pirow, R., & Fischer, J. (2010). Facial expressions modulate
the ontogenetic trajectory of gaze-following among monkeys. Developmental
Science, 13(6), 913e922.
Tomasello, M., Call, J., & Hare, B. (1998). Five primate species follow the visual gaze
of conspecifics. Animal Behaviour, 55(4), 1063e1069.
Tomasello, M., Hare, B., & Agnetta, B. (1999). Chimpanzees, Pan troglodytes, follow
gaze direction geometrically. Animal Behaviour, 58(4), 769e777.
Tomasello, M., Hare, B., & Fogleman, T. (2001). The ontogeny of gaze following in
chimpanzees, Pan troglodytes, and rhesus macaques, Macaca mulatta. Animal
Behaviour, 61(2), 335e343.
Tomasello, M., Hare, B., Lehmann, H., & Call, J. (2007). Reliance on head versus eyes
in the gaze following of great apes and human infants: the cooperative eye
hypothesis. Journal of Human Evolution, 52(3), 314e320.
Topál, J., Gergely, G., Erd
}
ohegyi, Á., Csibra, G., & Miklósi, Á. (2009). Differential
sensitivity to human communication in dogs, wolves, and human infants. Sci-
ence, 325(5945), 1269e1272.
van de Waal, E., Renevey, N., Favre, C. M., & Bshary, R. (2010). Selective attention to
philopatric models causes directed social learning in wild vervet monkeys.
Proceedings of the Royal Society B: Biological Sciences, 277(1691), 2105e2111.
Wilkinson, A., Mandl, I., Bugnyar, T., & Huber, L. (2010). Gaze following in the red-
footed tortoise (Geochelone carbonaria). Animal Cognition, 13(5), 765e769.
Wobber, V., Hare, B., Maboto, J., Lipson, S., Wrangham, R., & Ellison, P. T. (2010). Dif-
ferential changes in steroid hormones before competition in bonobos and chim-
panzees. Proceedings of the National Academy of Sciences, 107(28),12457e12462.
APPENDIX
Total Number of Fixations
Overall, we obtained similar results with this measure as with
the proportion of first look.
Experiment 1
Figure A2 presents the results for total number of fixations. A
repeated-measures three-way ANOVA (Object, Subject species,
Model species) revealed a significant main effect of Object
(F
1,26
¼ 6.77, P < 0.001,
h
2
¼ 0.41) and Subject species (F
2,26
¼ 9.82,
P ¼ 0.001,
h
2
¼ 0.43) and a significant interaction between Subject
species and Object (F
2,26
¼ 6.97, P ¼ 0.004,
h
2
¼ 0.34). Bonobos and
chimpanzees differed from one another in their total number of
fixations on the target versus the distractor (F
1,20
¼ 11.77, P ¼ 0.003,
h
2
¼ 0.37). Orang-utans did not differ from the other two species
(P > 0.1). Within-species analysis revealed a significant main effect
of Object for bonobos (F
1,7
¼ 8.58, P ¼ 0.022,
h
2
¼ 0.55) and for
orang-utans (F
1,6
¼ 8.09, P ¼ 0.029,
h
2
¼ 0.57), but not for chim-
panzees (F
1,13
¼ 0.21, P ¼ 0.65,
h
2
¼ 0.01). There was a significant
interactionbetween Object and Model for chimpanzees (F
1,13
¼ 5.41,
P ¼ 0.037,
h
2
¼ 0.29) but not for the other two species (Ps > 0.5).
Experiment 2
Figure A3 presents the results for total number of fixations. A
repeated-measures three-way ANOVA (Object, Age, Model species)
revealed a significant main effect of Object (F
1,42
¼ 147.88,
P < 0.001,
h
2
¼ 0.59) and Age (F
1,42
¼ 4.33, P ¼ 0.044,
h
2
¼ 0.09)
and a significant interaction between Object and Age (F
1,42
¼ 19.62,
P < 0.001,
h
2
¼ 0.31). When we analysed each group separately, in
adults, we found a significant main effect of Object (F
1,21
¼ 40.88,
P < 0.001,
h
2
¼ 0.66) but not a significant interaction between
Model and Object (F
1,21
¼ 1.91, P ¼ 0.18,
h
2
¼ 0.08). In infants, we
found a significant main effect of Object (F
1,21
¼ 40.88, P < 0.001,
h
2
¼ 0.66) but also a significant interaction between Model and
Object (F
1,21
¼ 10.69, P ¼ 0.004,
h
2
¼ 0.33).
Experiment 3
Figure A4 presents the results for total number of fixations. A
repeated-measures two-way ANOVA revealed a significant main
effect of Object (F
1,26
¼ 15.84, P < 0.001,
h
2
¼ 0.37). We did not find
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150146

a significant interaction between Object and Subject species
(F
2,26
¼ 2.28, P ¼ 0.12,
h
2
¼ 0.14). When we analysed each species
separately, we found a significant effect of Object for orang-utans
(t
6
¼ 4.76, P ¼ 0.003), a trend for bonobos (t
7
¼ 2.29, P ¼ 0.056)
and no significant effect for chimpanzees (t
13
¼ 0.88, P ¼ 0.39).
The Pilot Test for Experiment 2 (Infants and Adults)
A pilot test was conducted for experiment 2 with a separate
group of infants and adults using the same stimuli as in
experiment 1.
Methods
Eleven infants (six males, seven females) and 11 adults (three
males, eight females) participated in this pilot test. They were
presented with the videos of chimpanzee and human models,
which were the same as those used in experiment 1. All the
other procedures were the same as those in the main test
(experiment 2).
Results
As shown in Fig. A5, although control adults followed the gaze of
both models (Ps < 0.01), infants did not follow the gaze of either
(Ps > 0.2). In most of the trials, infants did not view the objects but
instead kept viewing the faces of models.
Discussion
Such ‘sticky’ fixations of infants to the faces may derive from
their immaturity of attention (Hood et al., 1998). In particular, the
objects in our stimuli may be too simple in form (plain coloured
squares), and also the faces of models may be too attractive in the
motions (frequent head turns) to release their attention from the
faces. We therefore changed these parameters in experiment 2 and
improved their performance (see the main text).
One might expect that, when we applied the same changes,
great apes would also show improved gaze-following perfor-
mances. However, we doubt this possibility because apes fixated
the face far more briefly than any humans (in this experiment and
also in general; Kano et al., 2012).
Figure A1. (a) An ape on the apparatus and (b) the apparatuses in experiment 1 (great apes).
Table A1
Species, sex, age and rearing history of the ape subjects
Name Species Sex Age (years) Rearing history
Fimi Bonobo F 5 Mother
Luiza Bonobo F 8 Mother
Yasa Bonobo F 15 Mother
Ulindi Bonobo F 19 Mother
Loto Bonobo M 3 Mother
Kuno Bonobo M 16 Hand reared
Jasongo Bonobo M 23 Mother
Joey Bonobo M 30 Hand reared
Kara Chimpanzee F 8 Mother
Fifi Chimpanzee F 20 Mother
Jahaga Chimpanzee F 20 Mother
Sandra Chimpanzee F 20 Mother
Getrudia Chimpanzee F 20 Mother
Riet Chimpanzee F 35 Hand reared
Ulla Chimpanzee F 36 Hand reared
Fraukje Chimpanzee F 37 Hand reared
Bangolo Chimpanzee M 4 Mother
Kofi Chimpanzee M 8 Mother
Lobo Chimpanzee M 9 Mother
Alex Chimpanzee M 12 Hand reared
Lome Chimpanzee M 12 Mother
Robert Chimpanzee M 37 Hand reared
Raja Orang-utan F 9 Mother
Padana Orang-utan F 15 Mother
Dokana Orang-utan F 24 Mother
Pini Orang-utan F 25 Mother
Batak Orang-utan M 3 Mother
Suaq Orang-utan M 4 Mother
Tanah Orang-utan M 4 Mother
F: female; M: male.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150 147

Target
Distractor
***
***
**
6
5
4
3
2
1
0
Human
Human infant (N = 22) Human adult (N = 22)
Ape Human Ape
Total number of fixations
Figure A3. Total number of fixations in human infants and adults when they were viewing human and ape models. Error bars denote the SEM. **P < 0.01; ***P < 0.001.
Target
Distractor
*
***
Conspecific Conspecific ConspecificHuman Human Human
Bonobo (N = 8) Chim
p
anzee (N = 14) Oran
g
-utan (N = 7)
0
1
2
3
4
Total number of fixations
Figure A2. Total number of fixations in great apes when they were viewing conspecific and human models. Error bars denote the SEM. *P < 0.05; ***P < 0.001.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150148

Bonobo (N = 8)
Chimpanzee (N = 14) Orang-utan (N = 7)
+
**
Target
Distractor
3
2
1
0
Total number of fixations
Figure A4. Total number of fixations in great apes when they were viewing allospecific ape models. Error bars denote the SEM. þ P < 0.07; **P < 0.01.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150 149

Target
Distractor
*** **
1
0.8
0.6
0.4
0.2
0
Human HumanApe Ape
Human infant (N = 22) Human adult (N = 22)
Proportion of first look
(a)
Target
Distractor
Human HumanApe Ape
Human infant (N = 22) Human adult (N = 22)
***
**
6
5
4
3
2
1
0
Total number of fixations
(b)
Figure A5. (a) Proportion of first look and (b) total number of fixations in human infants and adults when they were viewing human and ape models (pilot test for experiment 2).
Error bars denote the SEM. **P < 0.01; ***P < 0.001.
F. Kano, J. Call / Animal Behaviour 91 (2014) 137e150150
