
NATIONAL INSTRUCTIONAL
MEDIA INSTITUTE, CHENNAI
WORKSHOP CALCULATION
AND SCIENCE
COMMON FOR ALL ENGINEERING TRADES
Post Box No. 3142, CTI Campus, Guindy, Chennai - 600 032
1
st
Semester
DIRECTORATE GENERAL OF TRAINING
MINISTRY OF SKILL DEVELOPMENT & ENTREPRENEURSHIP
GOVERNMENT OF INDIA
NSQF
© NIMI, Not to be republished

Workshop Calculation & Science (NSQF) - 1st Semester
Common for All Engineering Trades
Copyright © 2018 National Instructional Media Institute, Chennai
First Edition
First Reprint : January 2019 Copies : 10,000
: December 2018 Copies : 10,000
Rs. 130/-
All rights reserved.
No part of this publication can be reproduced or transmitted in any form or by any means, electronic or mechanical,
including photocopy, recording or any information storage and retrieval system, without permission in writing from the
National Instructional Media Institute, Chennai.
Published by:
NATIONAL INSTRUCTIONAL MEDIA INSTITUTE
P. B. No.3142, CTI Campus, Guindy Industrial Estate,
Guindy, Chennai - 600 032.
Phone : 044 - 2250 0248, 2250 0657, 2250 2421
Fax : 91 - 44 - 2250 0791
email : [email protected]
chennai-nimi@nic.in
Website: www.nimi.gov.in
(ii)
© NIMI, Not to be republished

(iii)
FOREWORD
The Government of India has set an ambitious target of imparting skills to 30 crores people, one out of every
four Indians, by 2020 to help them secure jobs as part of the National Skills Development Policy. Industrial
Training Institutes (ITIs) play a vital role in this process especially in terms of providing skilled manpower.
Keeping this in mind, and for providing the current industry relevant skill training to Trainees, ITI syllabus
has been recently updated with the help of comprising various stakeholder's viz. Industries, Entrepreneurs,
Academicians and representatives from ITIs.
The National Instructional Media Institute (NIMI), Chennai, has now come up with instructional material to
suit the revised curriculum for Workshop Calculation & Science 1
st
Semester NSQF Common for all
engineering trades will help the trainees to get an international equivalency standard where their skill
proficiency and competency will be duly recognized across the globe and this will also increase the scope
of recognition of prior learning. NSQF trainees will also get the opportunities to promote life long learning
and skill development. I have no doubt that with NSQF the trainers and trainees of ITIs, and all stakeholders
will derive maximum benefits from these IMPs and that NIMI's effort will go a long way in improving the
quality of Vocational training in the country.
The Executive Director & Staff of NIMI and members of Media Development Committee deserve appreciation
for their contribution in bringing out this publication.
Jai Hind
RAJESH AGGARWAL
Director General/ Addl. Secretary
Ministry of Skill Development & Entrepreneurship,
Government of India.
New Delhi - 110 001
© NIMI, Not to be republished

(iv)
PREFACE
The National Instructional Media Institute(NIMI) was set up at Chennai, by the Directorate General of Training,
Ministry of skill Development and Entrepreneurship, Government of India, with the technical assistance
from the Govt of the Federal Republic of Germany with the prime objective of developing and disseminating
instructional Material for various trades as per prescribed syllabus and Craftsman Training Programme(CTS)
under NSQF levels.
The Instructional materials are developed and produced in the form of Instructional Media Packages (IMPs),
consisting of Trade Theory, Trade Practical, Test and Assignment Book, Instructor Guide, Wall charts,
Transparencies and other supportive materials. The above material will enable to achieve overall improvement
in the standard of training in ITIs.
A national multi-skill programme called SKILL INDIA, was launched by the Government of India, through a
Gazette Notification from the Ministry of Finance (Dept of Economic Affairs), Govt of India, dated 27th
December 2013, with a view to create opportunities, space and scope for the development of talents of
Indian Youth, and to develop those sectors under Skill Development.
The emphasis is to skill the Youth in such a manner to enable them to get employment and also improve
Entreprenurship by providing training, support and guidance for all occupation that were of traditional types.
The training programme would be in the lines of International level, so that youths of our Country can get
employed within the Country or Overseas employment. The National Skill Qualification Framework
(NSQF), anchored at the National Skill Development Agency(NSDA), is a Nationally Integrated Education
and competency-based framework, to organize all qualifications according to a series of levels of Knowledge,
Skill and Aptitude. Under NSQF the learner can acquire the Certification for Competency needed at any
level through formal, non-formal or informal learning.
The Workshop Calculation & Science 1
st
Semester (common to all Engineering Trades) is one of the
book developed as per the NSQF syllabus.
The Workshop Calculation & Science (common to all Engineering Trades as per NSQF) 1
st
Semester is
the outcome of the collective efforts of experts from Field Institutes of DGT, Champion ITI’s for each of the
Sectors, and also Media Development Committee (MDC) members and Staff of NIMI. NIMI wishes that the
above material will fulfill to satisfy the long needs of the trainees and instructors and shall help the trainees
for their Employability in Vocational Training.
NIMI would like to take this opportunity to convey sincere thanks to all the Members and Media Development
Committee (MDC) members.
R. P. DHINGRA
Chennai - 600 032 EXECUTIVE DIRECTOR
© NIMI, Not to be republished

(v)
ACKNOWLEDGEMENT
The National Instructional Media Institute (NIMI) sincerely acknowledge with thanks the co-operation and
contribution of the following Media Developers to bring this IMP for the course Workshop Calculation & Science
1
st
Semester as per NSQF.
MEDIA DEVELOPMENT COMMITTEE MEMBERS
Shri. M. Sangara pandian - Training Officer (Retd.)
CTI, Guindy, Chennai.
Shri. G. Sathiamoorthy - Jr.Training Officer (Retd.)
Govt I.T.I, DET - Tamilnadu.
Shri. K. Lakshminarayanan - Training Officer (Retd.)
Govt I.T.I, DET - Tamilnadu.
NIMI CO-ORDINATORS
Shri. K. Srinivasa Rao - Joint Director,
NIMI, Chennai - 32.
Shri. G. Michael Johny - Assistant Manager,
NIMI, Chennai - 32.
NIMI records its appreciation of the Data Entry, CAD, DTP Operators for their excellent and devoted services in
the process of development of this IMP.
NIMI also acknowledges with thanks, the efforts rendered by all other staff who have contributed for the develop-
ment of this book.
© NIMI, Not to be republished

INTRODUCTION
The material has been divided into independent learning units, each consisting of a summary of the topic and an
assignment part. The summary explains in a clear and easily understandable fashion the essence of the mathematical
and scientific principles. This must not be treated as a replacment for the instructor’s explanatory information to be
imparted to the trainees in the classroom, which certainly will be more elaborate. The book should enable the
trainees in grasping the essentials from the elaboration made by the instructor and will help them to solve independently
the assignments of the respective chapters. It will also help them to solve the various problems, they may come
across on the shop floor while doing their practical exercises.
The assignments are presented through ‘Graphics’ to ensure communications amongst the trainees. It also assists
the trainees to determine the right approach to sove the problems. The required revelent data to solve the problems
are provided adjacent to the graphics either by means of symbols or by means of words. The description of the
symbols indicated in the problems has its reference in the relevant summaries.
At the end of the exercise wherever necessary assignments, problems are included for further practice.
Time allotment:
Duration of 1
st
Semester (26 weeks) : 52 Hrs
Effective weeks avaliable (22 weeks) : 44 Hrs
Revision and Test (4 weeks) : 8 Hrs
Total time allotment : 52 Hrs
Time allotment for each module has given below. Common to all Engineering Trades, Mechanic Refrigeration and
Air Conditioner and Sheet Metal Worker.
S.No Module Exercise No. Time allotment (Hrs)
1 System of units, factors and fraction 1.1.01 - 1.2.09 10 Hrs
2 Square root, Ratio and proportion, percentage 1.3.10 - 1.5.16 12 Hrs
3 Algebra (only for SMW in 1
st
Semester) 1.6.17 - 1.6.18 4 Hrs
4 Material science, Mass weight and density 1.7.19 - 1.8.26 14 Hrs
5 Speed and veloctiy, work, power and energy 1.9.27 - 1.10.30 8 Hrs
6 Heat and temperature (only for MR&AC and SMW
in 1
st
Semester) 1.11.31 - 1.12.37 6 Hrs
7 Basic electricity (only for MR&AC in 1
st
Semester) 1.13.38 - 1.13.41 9 Hrs
(vi)
© NIMI, Not to be republished

Exercise No. Title of the Exercise Page No.
Module 1
1.1.01 Classification of system
of units 1
1.1.02 Fundamental and Derived units F.P.S, C.G.S, M.K.S and SI units 2
1.1.03 Measurement units and conversion 4
1.1.04 Conversions of length, mass, force,work, power and energy 12
1.2.05 Factors, HCF, LCM
and problems 14
1.2.06 Fractions 15
1.2.07 Decimal fractions 18
1.2.08 Pocket calculator and its applications 21
1.2.09 Solving problems by using calculator 25
Module 2
1.3.10 Square and square root 28
1.3.11 Simple problems using calculator 29
1.3.12 Applications of pythagoras theorem and related problems 30
1.4.13 Ratio and proportion 31
1.4.14 Direct and indirect proportions 33
1.5.15 Percentage 37
1.5.16 Changing percentage to decimal and fraction 40
Module 3 (only for SMW in 1
st
Semester)
1.6.17 Algebraic symbols and fundamentals 41
1.6.18 Addition,subtraction,multiplication and division of algebra 44
Module 4
1.7.19 Physical and mechanical properties of metals 47
1.7.20 Introduction of iron and cast iron 50
1.7.21 Types of ferrous and non ferrous 53
1.7.22 Difference between iron & steel, alloy steel & carbon steel 55
1.7.23 Properties and uses of rubber, timber and insulating materials 57
1.8.24 Mass, units of mass, density and weight 60
1.8.25 Difference between mass & weight, density & specific gravity 61
1.8.26 Related problems with assignment of mass, weight & density 62
Module 5
1.9.27 Rest, motion, speed, velocity, difference between speed & velocity,
acceleration & retardation 68
1.9.28 Related problems with assignment of speed & velocity 72
CONTENTS
(vii)
© NIMI, Not to be republished

(viii)
Exercise No. Title of the Exercise Page No.
LEARNING / ASSESSABLE OUTCOME
On completion of this book you shall be able to
• Understand, explain different mathematical calculation & science
in the field of study including basic electrical and apply in day to
day work.[Different mathematical calculation & science -Units,
factors and fractions, square root, ratio and proportion,
percentage, material science, mass, weight, density, speed and
velocity, work, power & energy, algebra, heat & temperature, basic
electricity, pressure].
• SMW - Algebra and Heat & temperature.
• MR&AC - Heat & temperature and Basic electricity.
1.10.29 Units of work, power and energy, horse power of engines and mechanical
efficiency 79
1.10.30 Potential energy, kinetic energy and related problems with assignment 78
Module 6 (only for MR&AC and SMW in 1
st
Semester)
1.11.31 Concept of heat and temperature, effects of heat, thermometer scale, celcius,
fahrenheit, reaumer, kelvin and difference between heat and temperature 81
1.11.32 Conversion between centigrade, fahrenheit, reaumer, kelvin scale of temperature 83
1.11.33 Temperature measuring instruments, types of thermometer, pyrometer and
transmission of heat 84
1.11.34 Co-efficient of linear expansion and related problems with assignment 86
1.11.35 Problems of heat loss and heat gain with assignment 88
1.11.36 Concept of pressure and its units in different system 92
1.12.37 Thermal conductivity and insulations 93
Module 7 (only for MR&AC in 1
st
Semester)
1.13.38 Introduction, use of electricity, molecule, atom, electricity is produced,
electric current, voltage, resistance and their units 95
1.13.39 Ohm’s law, relation between V.I.R & problems, series & parallel
circuits problem 97
1.13.40 Electrical power, energy and their units, calculation with assignment 100
1.13.41 Magnetic induction, self and mutual inductance and EMF generation 104
© NIMI, Not to be republished

SYLLABUS
First Semester Common for All Engineering Trades Duration: Six Months
(Except MR&AC and SMW)
S.no. Title
Unit
Fractions
(ix)
Square Root
Square and Square Root, method of finding out square roots, Simple problem using calculator.
Ratio & Proportion
Simple calculation on related problems.
Introduction, Simple calculation. Changing percentage to decimal and fraction and vice-versa.
Percentage
Fractions, Decimal fraction, L.C.M., H.C.F., Multiplication and Division of Fractions and Decimals,
conversion of Fraction to Decimal and vice versa. Simple problems using Scientific Calculator.
1
2
3
4
5
Systems of unit- FPS, CGS, MKS/SI unit, unit of length, Mass and time, Conversion of units.
Material Science
Properties - Physical & Mechanical, Types - Ferrous & Non-Ferrous, difference between Ferrous and
Non-Ferrous metals, introduction of Iron, Cast Iron, Wrought Iron, Steel, difference between Iron and
Steel, Alloy steel, carbon steel, stainless steel, Non-Ferrous metals, Non-Ferrous Alloys.
6
Mass, Weight and Density
Mass, Unit of Mass, Weight, difference between mass and weight, Density, unit of density, specific
gravity of metals.
7
Speed and Velocity
Rest and motion, speed, velocity, difference between speed and velocity, acceleration, retardation,
equations of motions, simple related problems.
8
Work, Power and Energy
Work, unit of work, power, unit of power, Horse power of engines, mechanical efficiency, energy, use
of energy, potential and kinetic energy, examples of potential energy and kinetic energy.
9
© NIMI, Not to be republished

(x)
Mechanic Refrigeration and Air Conditioner
General simplifications
Fractions, Types of fractions, common fractions, Decimal fractions with examples Addition, subtrac-
tion, multiplication and division of fraction . conversion of Fraction to Decimal and vice versa.
S.no. Title
1
2
Square & Square root
Square root of perfect square, Square of whole number and decimal. Applications of Pythagoras theorem
and related Problems.
3
Unit & Measurements
Definition, classification of System of units, Fundamental & derived units. C.G.S, M.K.S,. F.P.S, & S.I
System of units. Metric system of weight and measurement unit and conversion factors, problems.
4
Percentage
Introduction, Simple calculation. Changing percentage to fraction and decimal & vice-versa.
5
Introduction, use of Electricity, Molecule, Atom, and How Electricity is Produced, Electric current,
voltage, Resistance and their units. Ohm’s law. Relation between V.I.R & Problems. Series & Parallel
circuits & Problems. Electrical Power and energy & their units & calculation.
6
Magnetic Induction, Self & Mutual Inductance, EMF generation.
7
Material Science
Properties of metals - Physical & Mechanical, Meaning of tenacity, elasticity, malleability brittleness,
hardness, ductility Types – Ferrous & Non-Ferrous, difference between Ferrous and Non-Ferrous metals,
introduction of Iron, Cast Iron, Wrought Iron, Steel, difference between Iron and Steel, Alloy steel,
carbon steel, stainless steel, Non-Ferrous Alloys. Effect of Alloying elements.
8
Properties and uses of copper, zinc, lead tin, aluminum etc., Properties and uses of Brass, Bronze
as bearing material.
9
Heat and Temperature
Measurement of Temperature, Boiling and melting points. Interchange of heat, (Principle of
calorimetry) Co-efficient of linear expansion, Related problems.
10
Vapours and gases
Saturated and superheated vapours, Critical pressures and temperatures. Heat transfer conduction,
Convection, Radiation. Thermal conductivity and Insulations.
Syllabus for Mechanic Refrigeration & Air Conditioner
© NIMI, Not to be republished

(xi)
Sheet Metal Worker
Introduction and Importance of Science and Calculation to the Trade skill.
S.no. Title
1
2
- System of Units: British, Metric and S. I. Units for Length, Mass, Area, Volume, Capacity and
time.
- Conversions between British and Metric Systems.
3 - Density & Specific gravity.
- Mass, weight. Definition and units.
4 - Metals: Properties and uses of cast iron, wrought iron, plain carbon steels and alloy steels.
- Difference between metals, non-metals and alloys.
5 Properties and uses of Copper, Zinc, Lead, Tin and Aluminum.
6 Properties and uses of Brass, Bronze, Rubber ,Timber and insulating materials.
7 Concept of heat and temperature. Difference between heat and temperature. Effects of Heat,
Thermometric Scales such as a Celsius, Fahrenheit and Kelvin, Temperature measuring Instruments
- types of thermometers and pyrometers.
8
- Conversions between the above Scales of Temperature.
- Units of Heat-Calorie, B.Th.U & C.H.U., joule.
- Concept of Specific Heat, Latent Heat, problems on Heat Loss and Heat Gain.
9
- Definition of Force: Units of Force in M.K.S.& S.I. Systems.
- Concept of Pressure and its Units in different systems.
10
- General simplifications: BODMAS rule.
- Fraction: Addition, Subtraction, multiplication and Division-Problems.
- Decimal: Addition, Subtraction, Multiplication, and Division-Problems.
11
Conversion of Fraction to Decimal and vice-versa.
12
Square roots: The Square and Square root of a Whole Number and Decimal.
13
Percentage: Changing Percent to Decimal and Fraction and vice versa, applied problem.
14
Concept on Ratio and Proportion-Direct and Inverse Proportion, simple applied problems.
15
Algebraic Symbols and Fundamentals Addition, Subtraction, Multiplication and Division-Problems.
Syllabus for Sheet Metal Worker
© NIMI, Not to be republished

Title of the Contents Common for all MR&AC SMW
Engineering Trades
1 Unit
2 Fraction
3 Square root
4 Ratio & Proportion ⎯
5 Percentage
6 Algebra ⎯⎯
7 Material Science
8 Mass, Weight & Density ⎯
9 Speed & Velocity ⎯⎯
10 Work, Power & Energy ⎯⎯
11 Heat & Temperature ⎯
12 Basic Electricity ⎯ ⎯
Summary of Contents
(xii)
© NIMI, Not to be republished

1
Classification of system of units Exercise 1.1.01
Necessity
All physical quantities are to be measured in terms of
standard quantities.
Unit
A unit is defined as a standard or fixed quantity of one kind
used to measure other quantities of the same kind.
Classification
Fundamental units and derived units are the two classifica-
tions.
Fundamental units
Units of basic quantities of length, mass and time.
Derived units
Units which are derived from basic units and bear a
constant relationship with the fundamental units.E.g. area,
volume, pressure, force etc.
Systems of units
– F.P.S system is the British system in which the basic
units of length, mass and time are foot, pound and
second respectively.
– C.G.S system is the metric system in which the basic
units of length, mass and time are centimeter, gram and
seconds respectively.
– M.K.S system is another metric system in which the
basic units of length, mass and time are metre, kilo-
gram and second respectively.
– S.I. units are referred to as Systems International units
which is again of metric and the basic units, their
names and symbols are as follows.
Fundamental units and derived units are the two classifica-
tions of units.
Length, mass and time are the fundamental units in all the
systems (i.e) F.P.S, C.G.S, M.K.S and S.I. systems.
Example
Length: What is the length of copper wire in the roll , if the
roll of copper wire weighs 8kg, the dia of wire is 0.9cm and
the density is 8.9 gm/cm
3
?
Solution
mass of copper wire in the roll = 8kg (or)8000grams
Dia of copper wire in the roll = 0.9cm
Density of copper wire = 8.9 gm/cm
3
Area of cross section of copper wire
=
2
22
636.0
4
)9.0(
4
d
cm
Volume of copper wire
3
cm88.898
3
cmgm/ 8.9
8000grams
wirecopper ofDensity
wirecopper of Mass
Length of copper wire
=
2
3
0.636cm
898.88cm
wirecopper of section cross of Area
wirecopper of Volume
= 1413.33 cm
Length of copper wire =1413cm.
Time: The S.I. unit of time, the second, is another of the
base units of S.I it is defined as the time interval occupied
by a number of cycles of radiation from the calcium atom.
The second is the same quantity in the S.I. in the British
and in the U.S. systems of units.
Fundamental units of F.P.S, C.G.S, M.K.S and S.I
S.No. Basic quantity British units Metric units International units
F.P.S Symbol C.G.S Symbol M.K.S Symbol S.I Units Symbol
1 Length Foot ft Centimetre cm Metre m Metre m
2 Mass Pound lb Gram g Kilogram kg Kilogram Kg
3 Time Second s Second s Second s Second s
4 Current Ampere A Ampere A Ampere A Ampere A
5 Temperature Fahrenheit °F Centigrade °C Centigrade °C Kelvin K
6 Light intensity Candela Cd Candela Cd Candela Cd Candela Cd
© NIMI, Not to be republished

2
Derived units of F.P.S, C.G.S, M.K.S and SI system
S.No Physical quantity British units Metric units International units
FPS Symbol CGS Symbol MKS Symbol SI Units Symbol
1 Area Square foot ft
2
Square centimetre cm
2
Square metre m
2
Square metre m
2
2 Volume Cubic foot ft
3
Cubic centimetre cm
3
Cubic metre m
3
Cubic metre m
3
3 Density Pound per cubic lb/ft
3
Gram per cubic g/cm
3
Kilogram per cubic kg/m
3
Kilogram per cubic Kg/m
3
foot centimetre metre metre
4 Speed Foot per second ft/s Centimetre per second cm/sec Metre per second m/sec Metre per second m/sec
5 Velocity (linear) Foot per second ft/s Centimetre per second cm/sec Metre per second m/sec Metre per second m/sec
6 Acceleration Foot per square ft/s
2
Centimetre per cm/sec
2
Metre per square m/sec
2
Metre per square m/sec
2
second square second second second
7 Retardation Foot per square ft/s
2
Centimetre per cm/sec
2
Metre per square m/sec
2
Metre square second m/sec
2
Second square second second
8 Angular velocity Degree per second Deg/sec Radian per second rad/sec Radian per second rad/sec Radian per second rad/sec
9 Mass Pound (slug) lb Gram g Kilogram kg Kilogram kg
10 Weight Pound lb Gram g Kilogram weight kg Newton N
11 Force Pounds lbf dyne dyn Kilogram force kgf Newton N(kgm/sec
2
)
12 Power Foot pound per ft.lb/sec Gram.centimetre/sec g.cm/ kilogram metre per kg.m/ - -
second sec second sec
Horse power hp Erg per second watt W watt W(J/sec)
13 Pressure,Stress Pound per square inch lb/in
2
Gram per square g/cm
2
Kilogram per kg/m
2
Newton per square N/m
2
centimetre square metre metre
14 Energy, Work Foot.pound ft.lb Gram centimetre g.cm Kilogram metre kg.m joule J(Nm)
15 Heat British thermal unit Btu calorie Cal joule J joule J(Nm)
16 Torque Pound force foot lbf.ft Newton millimetre N mm Kilogram metre kg.m Newton metre Nm
17 Temperature Degree Fahrenheit °F Degree Centigrade °C Kelvin K Kelvin K
18 Specific heat BTU per pound degree Btu/lb°F Calorie per gram Cal/g°C Joule per kilogram J/(kgK) Joule per J/(kgK)
fahrenheit degree Celsius kelvin kilogram kelvin
Fundamental and Derived units F.P.S, C.G.S, M.K.S and SI units Exercise 1.1.02
© NIMI, Not to be republished

3
S.No Physical quantity British units Metric units International units
FPS Symbol CGS Symbol MKS Symbol SI Units Symbol
19 Frequency Cycle per second 1/s Hertz Hz Hertz Hz Hertz Hz
20 Moment of inertia Pound force foot lbf.ft.s
2
Gram square g.cm
2
Kilogram kg.m
2
Kilogram per square Kg.m
2
square second centimetre square metre metre
21 Momentum Pound second lb.s Gram centimetre g.cm/sec Kilogram metre kg.m/sec Kilogram metre Kg.m/
per second per second per second sec
22 Moment of force Pounds foot lbs/ft Gram centimetre g.cm Kilogram metre kg.m Newton metre Nm
23 Angle degree deg degree deg degree deg Radian rad
24 Specific volume Cubic foot per pound ft
3
/lbs Cubic centimetre per Cm
3
/g Cubic metre per m
3
/kg Cubic metre per m
3
/kg
gram kilogram kilogram
25 Specific resistance Ohm foot Ω ft Ohm centimetre Ω cm Ohm meter Ω m Ohm meter Ω m
26 Specific weight Pound per cubic foot lbf/ft
3
Gram per cubic g/cm
3
Kilogram per cubic kg/m
3
Newton per cubic N/m
3
centimetre metre metre
27 Fuel consumption Miles per gallon m/gal Centimetre per cubic cm/cm
3
Kilometre per litre km/l Metre per cubic metre m/m
3
centimetre
28 Dynamic viscosity Pound force per lbf/ft
2
Centi poise CP pascal second P
a.s
pascal second P
a.s
square foot
29 Surface tension Poundal per foot pdl/ft dyne per centimetre dyn/cm Newton per metre N/m Newton per metre N/m
30 Entropy
British thermal unit Btu/
0
F Calorie per degree Cal/
0
c Joule per kelvin J/K Joule per kelvin J/K
per degree Fahrenheit centigrade
31 Electric current Columb per second C/s Biot Bi Ampere A Ampere A
32 Electric voltage Volt V Volt V Volt V Volt V
33 Electric resistance Ohm Ω Ohm Ω Ohm Ω Ohm Ω, (V/A)
34 Electric Mho, Siemens ,s Mho ,s Siemens s Siemens s
conductance
35 Light intensity Candela Cd Candela Cd Candela Cd Candela Cd
36 Specific gravity No unit - No unit - No unit - No unit -
Ω
Ω
Workshop Calculation And Science : (NSQF) Exercise 1.1.02
© NIMI, Not to be republished

4
Decimal multiples and parts of unit
Decimal power Value Prefixes Symbol Stands for
10
12
1000000000000 tera T billion times
10
9
1000000000 giga G thousand milliotimes
10
6
1000000 mega M million times
10
3
1000 kilo K thousand times
10
2
100 hecto h hundred times
10
1
10.10
1
deca da ten times
10
-1
0.1 10
-1
deci d tenth
10
-2
0.01 centi c hundreth
10
-3
0.001 milli m thousandth
10
-6
0.000001 micro μ millionth
10
-9
0.000000001 nano n thousand millionth
10
-12
0.000000000001 pico p billionth
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
Measurement units and conversion Exercise 1.1.03
Units and abbreviations
Quantity Units Abbreviation of unit
Calorific value kilojoules per kilogram kJ/kg
Specific fuel kilogram per hour per newton kg/hr/N
consumption
Length millimetre, metre, kilometre mm, m, km
Mass kilogram, gram kg, g
Time seconds, minutes, hours s, min, h
Speed centimetre per second, cm/s, m/s
metre per second
kilometre per hour, miles km/h, mph
per hour
Acceleration metre-per-square second m/s
2
Force newtons, kilonewtons N,kN
Moment newton-metres Nm
Work joules J
Power horsepower, watts, kilowatts Hp, W, kW
Pressure newton per square metre N/m
2
kilonewton per square metre kN/m
2
Angle radian rad
Angular speed radians per second rad/s
radians-per-square second rad/s
2
revolutions per minute Rpm
revolutions per second rev/s
© NIMI, Not to be republished

5
SI units and the British units:
Quantity SI unit
→ →
→ →
→ British unit British unit
→→
→→
→ SI unit
Length 1 m = 3.281 ft 1 ft = 0.3048 m
1 km = 0.621 mile 1 mile = 1.609 km
Speed 1 m/s = 3.281 ft/s 1 ft/s = 0.305 m/s
1 km/h = 0.621 mph 1 mph = 1.61 km/h
Acceleration 1 m/s
2
= 3.281 ft/s
2
1 ft/s
2
= 0.305 m/s
2
Mass 1 kg = 2.205 lb 1 lb = 0.454 kg
Force 1 N = 0.225 lbf 1 lbf = 4.448 N
(1 million newtons)
Torque 1 Nm = 0.738 lbf ft 1 lbf ft = 1.355 Nm
Pressure 1 N/m
2
= 0.000145 lbf/in
2
1 lbf/in
2
= 6.896 kN/m
2
1 Pa = 1 N/m
2
1 bar = 14.5038 lbf/in
2
1 lbf/in
2
= 6.895 kN/m
2
Energy, work 1 J = 0.738 ft lbf 1 ft lbf = 1.355 J
1 J = 0.239 calorie 1 calorie = 4.186 J
1 kJ = 0.948 Btu 1 Btu = 1.055 kJ
(1 therm = 100 000 Btu)
1 kJ = 0.526 CHU 1 CHU = 1.9 kJ
Power 1 kW = 1.34 hp 1 hp = 0.7457 kW
Fuel consumption 1km/L = 2.82 mile/gallon 1 mpg = 0.354 km/L
Specific fuel 1 kg/kWh = 1.65 lb/bhp h 1 lb/bhp h = 0.606 kg/kWh
consumption 1 litre/kWh=1.575 pt/bhp h 1 pt/bhp h = 0.631 litre/kWh
Calorific value 1 kJ/kg = 0.43 Btu/lb 1 Btu/lb = 2.326 kJ/kg
1 kJ/kg = 0.239 CHU/lb 1 CHU/lb = 4.188 kJ/kg
Workshop Calculation And Science : (NSQF) Exercise 1.1.03
© NIMI, Not to be republished

6
M
Hμ
P
N
m
2
N
m
2
J
kg
g
kwh
Units in measuring practice with definitions
Quantity Unit Explanation
Force F Newton N 1 Newton is equal to the force which imports an
acceleration of 1m/s
2
to a body of mass 1 kg
1N = 1 kg m/s
2
Pressure P Newton 1 Newton per square metre (1 pascal)
per square is equal to the pressure with which
metre the force of 1 N is exercised perpendicular
to the area of 1 m
2
Pascal Pa 1Pa = 1 N/m
2
. 1 Bar (bar) is the special name
for 100 000 Pa.
Normal stress Newton per 1 Newton per square metre (1 pascal)
tensile or square to the mechanical stress with which the
compressive metre force of 1 n is exercised on the area of 1 m
2
.
stress, Shear stress In many branches of engineering the mechani-
cal stress and strength are specified in N/m
2
.
1 N/m
2
= 1000 000 Pa = 1 MPa
Heat Energy W Joule J 1 Joule is equal to the work that is done when
Quantity of heat the point of application of the force of 1 N is
shifted by 1 m in the direction of the force.
1 J = 1 Nm = 1 Ws = 1 kgm
2
/s
2
3600 000 J = 1 kWh
Moment of a force Newton Nm 1 Newton is equal to the moment of a force
(torque) metre which results from the product of the force
joule J of 1 N and the lever arm of 1 m.
1 Nm = 1 J = 1 Ws = 1 kgm
2
/s
2
Power P Watt W 1 Watt is equal to the power with which the
Energy flow energy of 1 J is converted during the time of 1s.
Heat flow ø The unit watt is also called volt ampere in
the specification of apparent electric power
1 W = 1 J/s = 1 Nm s = 1 VA
Specific Joule per 1 Joule per kilogram is equal to the quantity of
heat value kilogram heat which on complete burning of the mass of
1 kg releases the energy of 1 J
Fuel gram per 1 gram per kilowatt-hour is equal to the fuel
consumption kilowatt- consumption of the mass of 1 g for the work
hour of 1 kWh.
Temperature T Kelvin K The kelvin is defined as the fraction of
the thermodynamic temperature of the triple
point of water.
Electric current I Ampere A 1 Ampere is the strength of a current which
would bring about an electrodynamic force of
0.2.10 N per 1 m length between two parallel
conductors placed at a distance of 1 m.
1
273.16
Workshop Calculation And Science : (NSQF) Exercise 1.1.03
© NIMI, Not to be republished

7
Electric voltage V Volt V 1 Volt is equal to the electric voltage between
two points of a metallic conductor in which a
power of 1 W is expended for a current of 1 A
strength.
Electric resistance R Ohm Ω 1 Ohm is equal to the electric resistance be-
tween two points of a metallic conductor in
which an electric current of 1 A flows at a
voltage of 1 V.
Electric conductance G Siemens S 1 Siemens is equal to the electric conductance
of a conductor of electric resistance of 1ohm
Quantity Q Coulomb C 1 Coulomb is equal to the quantity
of electricity ampere-second As of electricity which flows through the conductor
cross-section during the time of 1 s at an
electric current of 1A.
Prefixes for decimal multiples and submultiples
Use
1 Megapascal = 1 MPa = 1000000 Pa
1 Kilowatt = 1 kW = 1000 W
1 Hectolitre = 1 hL= 100 L
Decanewton = 1 daN = 10 N
Decimetre = 1 dm = 0.1 m
1 Centimetre = 1 cm = 0.01 m
1 Millimetre = 1 mm = 0.001 m
1 Micrometre = 1 um = 0.000001 m
Conversion factors
1 inch = 25.4 mm
1 mm = 0.03937 inch
1 metre = 39.37 inch
1 micron = 0.00003937"
1 kilometre = 0.621 miles
1 pound = 453.6 gr
1 kg = 2.205 lbs
1 metric ton = 0.98 ton
Units of physical quantities
Units of length
Micron 1 μ = 0.001 mm
Millimetre 1 mm = 1000 μ
Centimetre 1 cm = 10 mm
Decimetre 1 dm = 10 cm
Metre 1 m = 10 dm
Kilometre 1 km = 1000 m
Inch 1" = 25.4 mm
Foot 1" = 0.305 m
Yard 1 Yd = 0.914 m
Nautical mile 1 NM = 1852 m
Geographical mile 1 = 1855.4 m
Workshop Calculation And Science : (NSQF) Exercise 1.1.03
© NIMI, Not to be republished

8
Units of area
Square millimetre 1 mm
2
Square centimetre 1 cm
2
= 100 mm
2
Square decimetre 1 dm
2
= 100 cm
2
Square metre 1 m
2
= 100 dm
2
Are 1 a = 100 m
2
Hectare 1 ha = 100 a
Square kilometre 1 km
2
= 100 ha
Square inch 1 sq.in = 6.45 cm
2
Square foot 1 sq.ft = 0.093 m
2
Square yard 1 sq.yd = 0.84 m
2
Square metre 1 m
2
= 10.76 ft
2
Acre 1 = 40.5 a
1 Acre = 100 cent 1 Hectare = 2.47 acres
1 Cent = 436 Sq. ft. 1 acre = 0.4047 Hec
1 Ground = 2400 Sq.ft. tare
1 Hectare = 10000 sq.
metre
Units of weight
Milligram - force 1 mgf
Gram-force 1 gf 1000 mgf
Kilogram-force 1 kgf = 1000 gf
Tonne 1 t = 1000 kgf
Ounce 1 = 28.35 gf
Pound 1 lbs = 0.454 kgf
Long ton 1 = 1016 kgf
Short ton 1 = 907 kgf
Time
Second 1 s
Minute 1 min 60 s
Hour 1 hr = 60 min
Units of volume and capacity
Cubic millimetre 1 mm
3
Cubic centimetre 1 cm
3
= 1000 mm
3
Cubic decimetre 1 dm
3
= 1000 cm
3
Cubic metre 1 m
3
= 1000 dm
3
Litre 1 l = 1 dm
3
Hectolitre 1 hl = 100 l
Cubic inch 1 cu. in = 16.387 cm
3
Cubic foot 1 cu. ft = 28317 cm
3
Gallon (British) 1 gal = 4.54 l
1cubic metre 1 m
3
= 1000 litres
1000 Cu.cm 1000 cm
3
= 1 litre
1 cubic foot 1 ft
3
= 6.25 Gallon
1 litre 1It = 0.22 Gallon
Angle
1 Centessimal unit
1 Right Angle = 100 grade (100
g
)
1 grade (1
g
) = 100 Minute (100’)
1 minute (1’) = 100 second (100”)
2 Sexagesimal unit
1 Right angle = 90 Degree (90
°
)
1 Degree (1
°
) = 60 minutes (60’)
1minute (1’) = 60 seconds (60”)
3 Circular unit
Radian
Relationship between Radian and Degree
1 Radian =
180
0
= π Radian;
1 Degree = Radian
π
180
180
°
π
Workshop Calculation And Science : (NSQF) Exercise 1.1.03
© NIMI, Not to be republished

9
Work
Kilogram-force 1 kgfm = 9.80665 J
Metre 1 kgfm = 9.80665 Ws
Joule 1 J = 1 Nm
Watt-second 1 Ws = 0.102 kgfm
Kilowatt hour 1 kWh = 3.6 x 10
6
J
= 859.8456 kcal
IT
I.T.Kilocalorie 1 kcal
IT
= 426.kgfm
Pressure
Pascal 1 Pa = 1 N/m
2
1 atm = 101325 Pa
Bar 1 bar = 10N/cm
2
= 100000 Pa–Torr 1 torr = ≈ 133.32 pa
Atmosphere 1 atm = 1 kgf/cm
2
1 kgf/cm
2
= 735.6 mm of mercury
Power
Kilogram-force metre/second
1 kgfm/s = 9.80665 W
Kilowatt 1 kW = 1000 W = 1000 J/s
= 102 kgfm/s (approx.)
Metric horse power 1 HP = 75 kgfm/s
= 0.736 kW
1 Calorie = 4.187J
I.T.Kilocalorie/hour = 1 kcal
IT/h
= 1.163 W
101325
760
Geometrical quantities
Symbol Physical quantity Conventional Units S.I.Units Symbol
S.I. units
l Length m Metre m
h Height m Metre m
b Width, breadth m Metre m
r Radius m Metre m
d Diameter m Metre m
d,δ Wall thickness m Metre m
S Length of path m Metre m
A (S) Area m
2
Square metre m
2
V (v) Volume m
3
Cubic metre m
3
α,β,γ etc Angle ° Radian (1 rad = 57.3°) rad
λ Wave length km Kilometre km
l,la Second moment of area cm
4
Metre to the fourthpower m
4
MASS
m Mass kg Kilogram kg
ρ Density g/cm
3
Kilogram per cubicmetre kg/m
3
l,J Moment of inertia kg, m
2
Newton metre
Workshop Calculation And Science : (NSQF) Exercise 1.1.03
© NIMI, Not to be republished

10
TIME
T Time or time interval s Second s
nu Rotational frequency l/min Reciprocal second l/s
u,v,w,c Velocity speed m/min Metre per second m/s
ω Angular velocity rad/s Radian per second rad/s
g Acceleration of freefall m/s
2
Metre per second squared m/s
2
a Acceleration m/s
2
Metre per second squared m/s
2
Retardation m/s
2
Metre per second squared m/s
2
FORCE AND PRESSURE
F Force kgf Newton (1kgf = 9.80665N) N
G(P,W) Weight kgf Newton N
γ Specific weight kgf/m
3
Newton per cubic metre N/m
3
M Moment of force kgf.m Newton metre N.m
(force x distance)
p Pressure (force/ area) kgf/cm
2
pascal, Newton per Pa,N/m
2
square metre
p Normal stress kgf/mm
2
bar (1 bar = 10 N/m)
τ p Shear stress kgf/mm
2
bar
E Modulus of elasticity kgf/mm
2
Newton per square metre N/m
2
G Shear modulus kgf/mm
2
Newton per square metre N/m
2
μ Co-efficient of friction No Unit
TEMPERATURE
Scale Freezing point Boiling point
Centigrade (
°
C) 0°C 100°C
Faranheit (°F) 32°F 212°F
Kelvin (K) 273K 373K
Reaumur (°R) 0°R 80°R
°R
80
°C
100
=
=
=
K- 273
100
°F- 32
180
Workshop Calculation And Science : (NSQF) Exercise 1.1.03
© NIMI, Not to be republished

11
HEAT, WORK, ENERGY,FORCE
A,W Work kgfm Joule (1 Joule=1 N.m) J (Nm)
P Power kgfm/s Watt W (J/s)
E,W Energy kgfm Joule J (Nm)
η Efficiency - - -
W,A,E,Q Quantity of heat kcal Joule J
C Specific heat kcal/kgf°C Joule per newton per J/N.°K
degree Kelvin
Thermal conductivity kcal/mh°C Joule per metre per J/ms°K
second per degree
Kelvin
Force In C.G.S. System : Force (Dyne) = Mass (gm)XAcceleration (cm/sec
2
)
In F.P.S. System : Force (Poundal) = Mass (Ib) X Acceleration (ft./sec
2
)
In M.K.S System : Force (Newton) = Mass (Kg) x Acceleration (mtr./sec
2
)
1 Dyne = 1 gm x1 cm/sec
2
1 Poundal = 1 Ib x 1 ft/sec
2
1 Newton = 1 kg x 1 mtr/sec
2
= 10
5
dynes
1gm weight = 981 Dynes
1 Ib weight = 32 Poundals
1 kg weight = 9.81 Newtons
ELECTRICAL QUANTITIES
V Electric potential V Volt V(W/A)
E Electromotive force V Volt V(W/A)
I Electric current A Ampere A
R Electric resistance Ω Ohm Ω (V/A)
e Specific resistance Ω m Ohm metre Vm/A
G Conductance Ω
-1
Siemens S
Workshop Calculation And Science : (NSQF) Exercise 1.1.03
© NIMI, Not to be republished

12
1 Convert the following as indicated
a 5 yards into metres ______
b 15 miles into kilometres ______
c 7 metres into yards ______
d 320 kilometres into miles ______
2 Convert
a 5 pounds into kilograms ______
b 8.5 kilograms into pounds ______
c 5 ounces into grams ______
d 16 tons into kilograms ______
3 Convert
a 40 inches into centimetres ______
b 12 feet into metres ______
c 5 metres into inches ______
d 8 metres into feet ______
4 Convert
a 234 cubic metres into gallons ______
b 2 cubic feet into litres ______
c 2.5 gallons into litres ______
d 5 litres into gallons ______
5 Answer the following questions
a 120°C = ______ °F.
b 8 mm = ______ inches
12 mm = ______ inches
6 Convert and find out
A car consumes fuel at the rate of one gallon for a travel
of 40 miles.
The same car travels a distance of 120 kilometer. what
is the consumption of fuel in litres.
7 Write equivalent British units for the given metric units
a Seconds, minutes, Hours
b Grams, Kilograms
c Litres, Cubic meters
d Square centimeter, Square kilometer
8 Expand the abbreviations of the following
a km/l
b N/m
2
cKW
d m/s
2
e RPM
9 Convert the following S.I. units as required.
a Length
i 3.4 m = ______ mm
ii 1.2 m = ______ cm
iii 0.8 m = ______ mm
iv 0.02 km = ______ cm
v 10.2 km = ______ mile
vi 6 m = ______ km
vii 18 m = ______ mm
viii 450 m = ______ km
ix 85 cm = ______ km
x 0.06 km = ______ mm
b Mass
i 650 g = ______ kg
ii 300 cg = ______ g
iii 8 g = ______ dg
iv 120 mg = ______ g
v 8 dag = ______ mg
vi 2.5 g = ______ mg
vii 2.5 g = ______ kg
viii 350 mg = ______ mg
ix 20 cg = ______ mg
x 0.05 Mt = ______ kg
c Force
i 1.2 N = ______ kg
ii 2.6 N = ______ kg
iii 800 N = ______ KN
iv 14.5 kg = ______ N
v 25 kg = ______ N
d Work, energy, amount of heat
i 2 Nm = ______ Ncm
ii 50 Ncm = ______ Nm
iii 120 KJ = ______ J
iv 40 J = ______ KJ
v 40 J = ______ KJ
vi 300 wh = ______ kwh
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
Conversions of length, mass, force,work, power and energy Exercise 1.1.04
© NIMI, Not to be republished
13
e Power
i 200 mW = ______ W
ii 0.2 kW = ______ W
iii 300 kW = ______ mW
iv 2.10
6
W = ______ mW
v 6.10
-4
kW = ______ W
vi 2 W = ______ KW
vii 350 W = ______ kW
viii 0.08 W = ______ kW
ix 2 x 10
-3
kW =______ W
x 0.04 W = ______ mW
f Convert as required.
i 3 Nm = ______ J
ii 2 J = ______ Ws
iii 12 J = ______ KJ
iv 3 Nm/s = ______ J/s
v 8 J/s = ______ J/s
vi 5 N = ______ KN
vii 5 Ws = ______ Ws
viii 3 KJ = ______ Nm
ix 18 J/s = ______ W
x 12 W = ______ J/s
xi kJ/s = ______ Nm/s
Workshop Calculation And Science : (NSQF) Exercise 1.1.04
© NIMI, Not to be republished

14
Factors, HCF, LCM and problems Exercise 1.2.05
Prime Numbers and whole Numbers
Factor
A factor is a small number which divides exactly into a
bigger number.e.g.
To find the factors of 24, 72, 100 numbers
24 = 2 x 2 x 2 x 3
72 = 2 x 2 x 2 x 3 x 3
100 = 2 x 2 x 5 x 5
The numbers 2,3,5 are called factors.
Definition of a prime factor
Prime factor is a number which divides a prime number into
factors.e.g.
57 = 3 x 19
The numbers 3 and 19 are prime factors.
They are called as such, since 3 & 19 also belong to prime
number category.
Definition of H.C.F
The Highest Common Factor
The H.C.F of a given group of numbers is the highest
number which will exactly divide all the numbers of that
group.e.g.
To find the H.C.F of the numbers 24, 72, 100
24 = 2 x 2 x 2 x 3
72 = 2 x 2 x 2 x 3 x 3
100 = 2 x 2 x 5 x 5
The factors common to all the three numbers are
2 x 2 = 4. So HCF = 4.
Definition of L.C.M
Lowest common multiple
The lowest common multiple of a group of numbers is the
smallest number that will contain each number of the given
group without a remainder.e.g.
• Factorise the following numbers
7,17,20,66,128
7,17 - These two belong to Prime numbers. Hence no
factor except unity and itself.
Factors of 20 = 2 x 2 x 5
• Select prime numbers from 3 to 29
3,5,7,11,13,17,19,23,29
• Find the HCF of the following group of numbers HCF of
78, 128, 196
78 = 2 x 3 x 13
128 = 2 x 2 x 2 x 2 x 2 x 2 x 2
196 = 2 x 2 x 49
HCF = 2
• Find LCM of 84,92,76
LCM =
Factors of 128 = 2 x 2 x 2 x 2 x 2 x 2 x 2
220
210
5
266
333
11
2 128
264
232
216
28
24
2
278
339
13
2 128
2 6 4
2 3 2
2 1 6
2 8
2 4
2
2 196
298
49
2 84, 92, 76
2 42, 46, 38
3 21, 23, 19
7, 23, 19
LCM = 2 x 2 x 3 x 7 x 23 x 19 = 36708
• To find out the LCM of 36, 108, 60
Factors of 66 = 2 x 3 x 11
2 36, 108, 60
2 18, 54, 30
3 9, 27, 15
3 3, 9, 5
1, 3, 5
LCM of the number
36, 108, 60 = 2 x 2 x 3 x 3 x 3 x 5 = 540
The necessity of finding LCM and HCF arises in subtraction
and addition of fractions.
© NIMI, Not to be republished
15
Fractions Exercise 1.2.06
Description
A minimal quantity that is not a whole number. For e.g. .
5
1
A vulgur fraction consists of a numerator and denomi-
nator.
Numerator/Denominator
The number above the line in a vulgar fraction showing how
many of the parts indicated by the denominator are taken
is the numerator. The total number of parts into which the
whole quantity is divided and written below the line in a
vulgar fraction is the denominator. e.g.
12
7
,
4
3
,
4
1
1,3,7 - numerators 4,12 - denominators
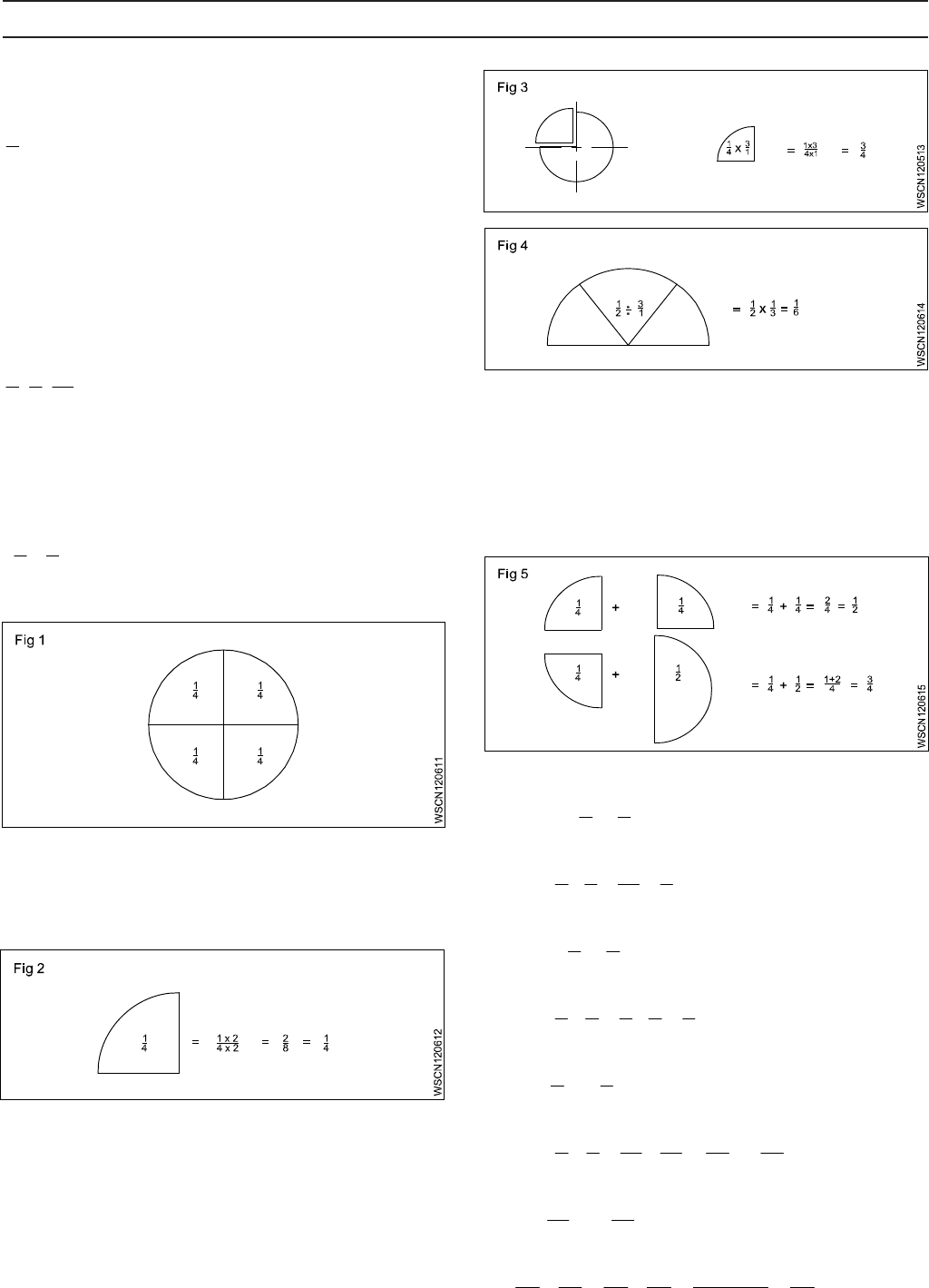
Fraction: Concept
Every number can be represented as a fraction.e.g.
4
5
4
1
1
, A full number can be represented as an apparent
fraction.e.g. (Fig 1)
Fraction: Value
The value of a fraction remains the same if the numerator
and denominator of the fraction are multiplied or divided by
the same number.(Fig 2)
Multiplication
When fractions are to be multiplied, multiply all the
numerators to get the numerator of the product and multiply
all the denominators to form the denominator of the
product. (Fig 3)
Division
When a fraction is divided by another fraction the dividend
is multiplied by the reciprocal of the divisor. (Fig 4)
Addition and Subtraction
The denominators of the fractions should be the same when
adding or subtracting the fractions. Unequal denominators
must first be formed into a common denominator. It is the
lowest common denominator and it is equal to the product
of the most common prime numbers of the denominators of
the fractions in question.(Fig 5)
Examples
•
,
3
2
by
4
3
Multiply
2
1
12
6
3
2
4
3
•
,
4
3
by
8
3
Divide
2
1
3
4
x
8
3
4
3
8
3
•
,
3
2
and
4
3
Add
12
8
12
9
3
2
4
3
12
5
1
12
17
•
32
17
from
16
7
sub
32
3
32
1417
32
14
32
17
16
7
32
17
© NIMI, Not to be republished

16
Types of fractions
• Proper fractions are less than unity. Improper fractions
have their numerators greater than the denominators.
• A mixed number has a full number and a fraction.
Addition of fraction
12
5
8
1
2
1
Add
To add these fractions we have to find out L.C.M of
denominators 2,8,12.
Find L.C.M of 2,8,12
Step 1 L.C.M
2 2,8,12
2 1,4,6
1,2,3
Factors are 2,2,2,3
Hence L.C.M = 2 x 2 x 2 x 3 = 24
Step 2
.
24
1
1
24
25
24
10312
24
10
24
3
24
12
12
5
8
1
2
1
Subtraction of fraction
32
15
9
16
9
or(17
16
9
from17
32
15
9subtract
)
Step 1: Subtract whole number first 17 - 9 = 8
Step 2: L.C.M of 16,32 = 32
Since number 16 divides the number 32
Subtracting fractions =
32
3
Adding with whole number from Step 1
32
3
8
32
3
8 get we
Common fractions
Problems with plus and minus sign
Example
32
9
16
5
4
8
7
6
4
3
3solve
Rule to be followed
1 Add all whole numbers
2 add all + Numbers
3 Add all - Numbers
4 Find L.C.M of all denominators
Solution
Step 1: Add whole numbers = 3 + 6 - 4 = 5
Step 2: Add fractions =
32
9
16
5
8
7
4
3
L.C.M of 4,8,16,32 is 32
32
1
1
32
33
32
1952
32
9102824
Step 3: Adding again with the whole number
we get
32
3
6
32
3
15
Brackets and their solution
Sometimes fractions are added and subtracted with brack-
ets, to express two or more expressions. The problems
with brackets are solved by using a rule called ‘BODMAS’
rule.
BODMAS rule:
• B - Brackets - 1
st
• O - of - 2
nd
• D - Division - 3
rd
• M - Multiplication - 4
th
• A - Addition - 5
th
• S - Subtraction - Last to be done
Problem with brackets
solve
4
1
2
1
91456
Steps to be followed
( ) 1 Solve inside bracket ( )
2 Solve multiplication and division together
3 Solve addition and subtraction together
{ } 4 Solve curly bracket before the final stage
[ ] 5 Solve square bracket at the final stage.
Workshop Calculation And Science : (NSQF) Exercise 1.2.06
© NIMI, Not to be republished
17
Examples
Common fractions
• Multiply
a
14
3
7
4
x
8
3
7
4
by
8
3
b
16
5
8
5
x
4
3
x
3
2
• Division
a
2
5
32
x
16
5
32
5
16
5
b
33
16
1
33
49
22
7
x
3
14
7
22
3
14
7
1
3
3
2
4
• Addition
8
7
8
124
8
1
4
1
2
1
82,4,8L..C.M
8
1
4
1
2
1
• Subraction
2
1
1
2
3
4
6
4
39
4
3
4
9
4
3
4
1
2
4
3
4
1
2
4
3
4
1
35
4
3
3
4
1
5
Bracket problem
1 solve by use ‘BODMAS’ rule
2
1
8
7
2116810
1230
8
123
810
8
3
15810
8
3
1580
1
2 solve by use ‘BODMAS’ rule
2
1
367
4
2
367
4
1470
4
49
56
4
1
1256
4
1
19456
4
1
2
1
19456
Workshop Calculation And Science : (NSQF) Exercise 1.2.06
© NIMI, Not to be republished

18
Decimal fractions Exercise 1.2.07
Description
Decimal fraction is a fraction whose denominator is 10 or
powers of 10 or multiples of 10 (i.e.) 10, 100, 1000, 10000
etc. Meaning of a decimal number:-
12.3256 means
10000
6
1000
5
100
2
10
3
1)X(210)X(1
Representation
The denominator is omitted. A decimal point is placed
at different positions of the number corresponding to the
magnitude of the denominator
3.648
1000
3648
0.0127,
10000
127
0.35
100
35
0.5,
10
5
.Ex
Addition and subtraction
Arrange the decimal fractions in a vertical order, placing the
decimal point of each fraction to be added or subtracted, in
succession one below the other, so that all the decimal
points are arranged in a straight line. Add or subtract as
you would do for a whole number and place the decimal
point in the answer below the column of decimal points.
Decimal fractions less than 1 are written with a zero before
the decimal point. Example: 45/100 = 0.45 (and not simply
.45)
Add 0.375 + 3.686
0.375
3.686
4.061
Subtract 18.72 from 22.61
22.61
18.72
3.89
Multiplication
Ignore the decimal points and multiply as whole numbers.
Find the total number of digits to the right of the decimal
point. Insert the decimal point in the answer such that the
number of digits to the right of the decimal point equals to
the sum of the digits found to the right of the decimal points
in the problem.
Multiply 2.5 by 1.25
= 25 x 125 = 3125. The sum of the figures to the right
of decimal point is 3. Hence the answer is 3.125.
Division
Move the decimal point of the divisor to the right to make
it a full number. Move the decimal point in the dividend to
the same number of places, adding zeroes if necessary.
Then divide.
Divide 0.75 by 0.25
37525
25
75
100
100
x
0.25
0.75
0.750.25
Move the decimal point in the multiplicand to
the right to one place if the multiplier is 10, and
to two places if the multiplier is 100 and so on.
When dividing by 10 move the decimal point
one place to the left, and, if it is by 100, move
them point by two places and so on.
Example
Allowing 3 mm for cutting off each pin how many pins, can
be made from a 900 mm long bar? How much material will
be left out?
Length of pin
= 2.25 + 55.36 + 12.18
= 69.79 mm
Length of the bar = 900 mm
Step 1
Let the number of pins to be made =
Length of number of pins = x 69.79 mm
Step 2
Waste for each pin = 3 mm
Waste for number of pins = 3 x mm=3 mm
Adding step (1) + step (2) and equating to length of bar
69.79 mm + 3 mm = 900 mm
(69.79mm + 3mm) = 900mm
(72.79mm) = 900mm
= 900
72.79
Hence Number of pins to be made = 12
© NIMI, Not to be republished

19
Secondly
Left out materials
= Total length of bar - Length for 12 pins+ wastage of
cutting
= 900mm - (12 x 69.79 + 12 x 3)mm
= 900 - (837.48 + 36)mm
= 900 - 873.48mm
=26.52mm
Left out material 26.52 mm
Conversion of Decimals into fractions and vice-versa
• Convert decimal into fractions
Example
Convert 0.375 to a fraction
Now place 1 under the decimal point followed by as many
zeros as there are numbers
• Convert fraction into decimal
Example
• Convert to a decimal
Proceed to divide in the normal way of division but put
zeros (as required) after the number 9 (Numerator)
16
9
=0.5625
• Convert
8
7
to a decimal
0.875
0
40
40
56
60
64
70008
0.875
8
7
Recurring decimals
While converting from fraction to decimals, some fractions
can be divided exactly into a decimal. In some fractions the
quotient will not stop. It will continue and keep recurring.
These are called recurring decimals.
Examples
•
7
1
,
3
2
,
3
1
convert
a
Recurring0.3333
3
10000
3
1
b
Recurring0.666
3
20000
3
2
c
Recurring20.14285714
7
10000
7
1
These are written as below with a dot over the figure
.
0.3333 ——> 0.3
.
0.6666 ——> 0.6
. .
0.14857142 —> 0.14857
Note the dots marked over numbers.
We normally carry the decimal points upto 4 places in
Engineering calculations.
Approximations in Measured Value calculations
In Measured Value calculations 4 places of decimals are
sufficients and in many dimensions of parts even 3 decimal
places are near enough to complete the maintenance job
operations.
Workshop Calculation And Science : (NSQF) Exercise 1.2.07
© NIMI, Not to be republished

20
Method of writing approximations in decimals
1.73556 = 1.7356 Correct to 4 decimal places
5.7343 = 5.734 Correct to 3 decimal places
0.9345 = 0.94 Correct to 2 decimal places
Multiplication and division by 10,100,1000
Multiplying decimals by 10
A decimal fraction can be multiplied by 10,100,1000 and so
on by moving the decimal point to the right by as many
places as there are zeros in the multiplier.
• 4.645 x 10 = 46.45 (one place)
• 4.645 x 100 = 464.5 (two places)
• 4.645 x 1000 = 4645 (three places)
Dividing decimals by 10
A decimal fraction can be divided by 10,100,1000 and so
on, by moving the decimal point to the left by as many
places as required in the divisor by putting zeros
Examples
• 3.732 ÷ 10 = 0.3732 (one place)
• 3.732 ÷ 100 = 0.03732 (two places)
• 3.732 ÷ 1000 = 0.003732 (three places)
Examples
• Rewrite the following number as a fraction
453.273
453.273
1000
273
453
100
3
100
7
10
2
1)(310)(5100)(4
• Write the representation of decimal places in the given
number 0.386
3 - Ist decimal place
8 - IInd decimal place
6 - IIIrd decimal place
• Write approximations in the following decimals to 3
places.
a 6.9453 ——> 6.945
b 8.7456 ——> 8.746
• Convert fraction to decimal
0.875
8
7
24
21
• Convert decimal to fraction
16
1
80
5
10000
625
0.0625
ASSIGNMENT
1 Write down the following decimal numbers in the
expanded form.
a 514.726
b 902.524
2 Write the following decimal numbers from the expansion.
a
1000
9
100
2
10
3
570500
b
1000
5
100
3
10
1
9200
3 Convert the following decimals into fractions in the
simplest form.
a 0.72
b 5.45
c 3.64
d 2.05
4 Convert the following fraction into decimals
a
5
3
b
4
10
c
1000
54
24
d
25
12
e
25
8
f
25
3
1
Workshop Calculation And Science : (NSQF) Exercise 1.2.07
© NIMI, Not to be republished

21
Pocket calculator and its applications Exercise 1.2.08
A pocket calculator allows to spend less time in doing
tideous calculations. A simple pocket calculator enables
to do the arithmatical calculations of addition substraction,
multiplication and division, while a scientific type of calcu-
lator can be used for scientific and technical calculations
also.
No special training is required to use a calculator. But it is
suggested that a careful study of the operation manual of
the type of the calculator is essential to become familiar
with its capabalities. A calculator does not think and do.
It is left to the operator to understand the problem, interpret
the information and key it into the calculator correctly.
Constructional Details (Fig 1)
• Function keys
• Memory keys
M Store the display number
M+ The displayed value is added to the memory
M- The displayed value is subtracted from the
memory
MR RCL The stored value is recalled on to the
display
Further functional keys included in Scientific calculators
are as shown in Fig 2.
+
=
÷
Addition key
Subtraction key
-
Multiplication keyx
Division key
Equals key to display the result
x
%
Pi key
Square root key
Percentage key
Sign change key
x
2
Square key
X
1
Reciprocal key
+/-
π
The key board is divided into five clear and easily recogniz
able areas and the display
• Data entry keys
The entry keys are from ..............to
and a key for the decimal point .
• Clearing keys
These keys have the letter ‘C’
Clear totally
Clear entry only
, Clear memory
09
C
CLR
CE
CM MC
© NIMI, Not to be republished

22
For trigonometric functions and
for brackets
Sin Cos
Ta n
(
)
Exponent key
Some of the keys have coloured lettering above or
below them. Toi use a function in coloured lettering,
press INV key. INV will appear on the display.
Then press the key that the coloured lettering
identifies. INV will disappear from the display.
, to obtain the logarithm of the dis-
played number and the antilogarithm of the displayed value.
to convert displayed rectangular coordinates
into polar coordinates.
to convert displayed polar coordinates into
rectangular coordinates.
• The display
The display shows the input data, interim results and
answers to the calculations.
The arrangement of the areas can differ from
one make to another. Keying in of the numbers
is done via. an internationally agreed upon set
of ten keys in the order that the numbers are
written.
Exp
INV
log
INV
10
x
INV
R–P
INV
P–R
Rules and Examples:
• Addition: Example 18.2 + 5.7
Sequence Input Display
Input of the 1st term 1 8 . 2 18.2
of the sum
Press + key + 18.2
Input 2nd term of the
sum. the first term 5 . 7 5.7
goes into the register
Press the = key = 23.9
• Subtraction: Example 128.8 - 92.9
Sequence Input Display
Enter the subtrahend 1 2 8 . 8 18.2
Press - key - 128.8
Enter the minnend.
The subtrahend goes 9 2 . 9 92.9
into the register
Press the = key = 35.9
• Multiplication: Example 0.47 x 2.47
Sequence Input Display
Enter multiplicand . 4 7 0.47
Press x key x 0.47
Enter multiplier,
multiplicand goes 2 . 4 7 2.47
to register
Press = key = 1.1609
• Division: Example 18.5/2.5
Sequence Input Display
Enter the dividend 1 8 . 5 18.5
Press ÷ Key ÷ 18.5
Enter the divisor
goes to the register 2 . 5 2.5
Press = key = 7.4
Workshop Calculation And Science : (NSQF) Exercise 1.2.08
© NIMI, Not to be republished

23
• Multiplication & Division:
Example : 2.5 x 7.2 / 4.8 x 1.25
Sequence Input Display
Enter 2.5 2 . 5 2.5
Press x key x 2.5
Enter 7.2 7 . 2 7.2
Press ÷ key ÷ 18
Enter 4.8 4 . 8 4.8
Press x key x 3.75
Remember:Before
input of thefirst value
under the fraction
line, the x keymust
be operated
Enter 1.25
Press = key
• Store in memory Example (2+6) (4+3)
Sequence Input Display
Workout for the first 2 2
bracket
+ 2
6 6
= 8
Store the first result in STO , M 8
x or M+
Workout for the 4 4
2nd bracket
+ 4
3 3
= 7
Press x key x 7
Recall memory RCL or MR 8
Press = key = 56
1
.
25
=
1.25
3.0
• Percentage: Example 12% of 1500
Sequence Input Display
Enter 1500 1 5 0 0 1500
Press x key x 1500
Enter 12 1 2 12
Press INV % INV % 12
Press = key = 180
• Square root: Example
53 2
Sequence Input Display
Enter 2 2 2
Press
a
key 2
Press + key + .
Press bracket key ( .
Enter 3 3 3
Press
a
key .
Press x key x .
Enter 5 5 5
Press
a
key
Press bracket close key ) .
Press = key = 5.2871969
553 2
• Common logaritham: Example log 1.23
Sequence Input Display
1 . 2 3 log = 0.0899051
• Power: Example 123 + 30
2
Sequence Input Display
1 2 3 + 3 0 INV X
2
= 1023
a
a
.
a
2 +(
3x
5
)
=
5.2871969
Workshop Calculation And Science : (NSQF) Exercise 1.2.08
© NIMI, Not to be republished

24
ASSIGNMENT
1 Using calculator solve the following
a 625 + 3467 + 20 + 341 + 6278 = ______
b 367.4 + 805 + 0.7 + 7.86 + 13.49 = ______
c 0.043 + 1.065 + 13.0 + 34.76 + 42.1 = ______
d 47160 + 1368.4 + 0.1 + 1.6901 + 134.267 =
_______
2 Using calculator simplify the following
a 24367 - 4385 = ______
b 9.643 - 0.7983 = ______
c 4382.01 - 381.3401 = ______
d 693.42 - 0.0254 = ______
3 Using calculator find the values of the following
a 23 x 87 = ______
b 1376 x 0.81 = ______
c 678 x 243 = ______
d 0.75 x 0.24 = ______
4 Using calculator solve the following
a 22434 ÷ 3 = ______
b 4131 ÷ 243 = ______
c 469890 ÷ 230 = ______
d 3.026 ÷ 0.89 = ______
5 Solve the following
a
13x215
1170x537.5
= ________
b
8.031000
3500182.28
xx
xx
________
6 Solve the following
a
8x0.3
0.52)128)x(384(634
________
• Before starting the calculations be sure to
press the ‘ON’ key and confirm that ‘0’ is
shown on the display.
• Do not touch the inside portion of the calcu-
lator. Avoid hard knocks and unduly hard
pressing of the keys.
• Maintain and use the calculator in between
the two extreme temperatures of 0° and 40°
C.
• Never use volatile fluids such as lacquer,
thinner, benzine while cleaning the unit.
• Take special care not to damage the unit by
bending or dropping.
• Do not carry the calculator in your hip pocket.
b
17.04).10.021)x(28(3.89
2.6)0.0512.2)x(842(389
_________
7 2a = 450 mm(major axis)
2b = 250mm(minor axis)
Perimeter of the ellipse
c = _____metre
Hint C =
)(2
22
ba
8 ø = 782 mm
α = 136°
Area of the sector
A = ______
360
α
x
4
d x π
AHint
2
9 d = 1.25 metre
V = ______ dm
3
3
πr
3
4
VHint
10 L = 1.2 metres
B = 0.6 metre
H = 0.5 metre
'r' of steel
= 7.85 kg/dm
3'
m = ______ kg
(mass ‘m = V x r )
Workshop Calculation And Science : (NSQF) Exercise 1.2.08
© NIMI, Not to be republished

25
1 Convert the following into improper fractions.
a
__________
7
2
1
b
__________
5
3
4
c
__________
5
3
3
d
__________
8
7
5
e
_________
3
1
3
f
______
4
3
5
g
__________
7
3
7
h
__________
74
1
182
2 Convert the following into mixed numbers.
a
__________
11
12
b
__________
14
36
c
__________
10
18
d
__________
3
25
e
__________
13
84
f
__________
21
32
g
__________
16
18
h
__________
4
75
3 Place the missing numbers.
a
__________
91
x
13
11
b
__________
x
42
5
3
c
__________
98
x
14
9
4 Simplify.
a
_________
60
45
b
__________
12
8
c
__________
14
12
d
__________
72
56
e
__________
14
6
f
__________
6
14
x
4
2
x
3
11
x
7
5
x
4
3
5 Multiply.
a
__________
3
2
x5
b
__________2x
4
3
c
__________
6
5
x
4
3
d
__________3x
4
1
3
e
__________
4
1
3x
4
1
2
f
__________
4
1
6x5
Solving problems by using calculator Exercise 1.2.09
© NIMI, Not to be republished

26
6 Divide
a
_________
4
3
4
1
b
________
4
3
6
c
__________
7
2
4
3
d
__________4
6
1
3
e
________
7
1
2
2
1
5
f
_________
4
1
38
7 Place the missing numbers.
a
x_________
12
1
3
2
b
x________
12
1
24
14
c
x_________
12
1
8
7
d
x_________
12
1
36
2
e
x_________
12
1
36
52
f
x_________
12
1
24
11
3
g
x_________
12
1
4
3
h
x_________
12
1
6
7
8 Add the followings:
a
_________
12
7
4
3
b
_________
4
3
8
7
c
_________
8
3
5
4
5
3
d
_________
9
7
3
12
7
1
4
1
6
9 Subtract
a
_________
5
2
5
4
b
_________
4
3
6
5
10 Simplify
a
_________
16
1
1
3
1
8
3
7
6
2
b
________8
6
5
7
2
2
c
_________
2
1
2
4
3
1
5
3
9
7
3
11 Express as improper fractions
a
4
3
5
b
64
5
3
c
12
5
1
12 Reduce to mixed number or whole number
a
4
163
b
4
12
c
60
144
13 Reduce to lowest terms
a
64
12
b
48
12
c
60
144
Workshop Calculation And Science : (NSQF) Exercise 1.2.09
© NIMI, Not to be republished

27
14 Addition of decimals
a 4.56 + 32.075 + 256.6245 + 15.0358
b 462.492 + 725.526 + 309.345 + 626.602
15 Subtract the following decimals
a 612.5200 – 9.6479
b 573.9246 – 215.6000
c 968.325 – 16.482
d 5735.4273 – 364.2342
16 Add and subtract the following
a 56.725 + 48.258 – 32.564
b 16.45 + 124.56 + 62.7 - 3.243
17 Multiplication of decimals
a By 10,100,1000
i 3.754
ii 8.964 x 100
iii 2.3786 x 1000
iv 0.005 x 1000
b By whole numbers
i 8.4 x 7
ii 56.72 x 8
c By another decimal figure (use calculator)
i 15.64 x 7.68
ii 2.642 x 1.562
18 Divide the following
a
25
62.5
b
9
14.4
c
10
64.56
d
100
0.42
e
1000
48.356
f
15
25.5
19 Division
a
1.2
16.8
b
1.2
1.68
c
1.2
0.168
d
1.1
1.54
e
1.6
27.2
f 31.5 ÷ 10.5
g 1.54 ÷ 1.1
h 4.41 ÷ 2.1
20 Change the fraction into a decimal
i1
8
5
ii
25
12
21 Find the value
20.5 x 40 ÷ 10.25 + 18.50
22
A = 12.613 mm
X = __________mm.
23
X = __________mm.
24
X = __________mm.
25
B = __________mm.
Workshop Calculation And Science : (NSQF) Exercise 1.2.09
© NIMI, Not to be republished

28
Square and square root Exercise 1.3.10
a basic number
2 exponent
radial sign indicating the square root.
2
a
square root of 'a' squared
a
2
radicand
Square number
The square of a number is the number multiplied by itself.
Basic number x basic number = Square number
a x a = a
2
4 x 4 = 4
2
= 16
Splitting up
A square area can be split up into sub-areas. The largest
square of 36 is made up of a large square 16, a small
square 4 and two rectangles 8 each.
Large square 4 x 4 = 16 a
2
Two rectangles 2 x 4 x 2 = 16 2ab
Small square 2 x 2 = 4 b
2
Sum of sub-areas = 36 = a
2
+ 2ab + b
2
22
bab2a 36
Extracting the square root procedure
• Starting from the decimal point form groups of two
figures towards right and left. Indicate by a prime
symbol.
00.24,46
• Find the root of the first group, calculate the difference,
bring down the next group.
• Multiply the root by 2 and divide the partial radicand.
• Enter the number thus calculated in the divisor for the
multiplication.
If there is a remainder, repeat the procedure.
68
68
6 46,24
36 36
128 1024
1024
0
6846,24
Basic number x basic number = Square
number basic number Square
Example
The cross-sectional of a rivet is 3.46 cm
2
. Calculate the
diameter of the hole.
46,246
0
1024
1024128
Result: In order to find the square root, we split up the
square numbers.
Rivet cross-section is the hole cross-section.
To find d
1
,
Given that Area = 3.46 cm
2
Area = 0.785 x d
2
(formula)
3.46 cm
2
= d
2
x 0.785
21mm (or) cm 2.1 d
cm
0.785
3.46
d
0.785
cm 3.46
d
2
2
© NIMI, Not to be republished

29
Simple problems using calculator Exercise 1.3.11
1a
2916
.
b
57964
.
c
.29448
.
d
3.8456
.
2 A = 2025 mm
2
a = mm
3 A = 176.715 mm
2
d = mm
4 A = 65031 mm
2
d = 140 mm
D = mm
5 I = 58 cm
b = 45 cm
A
1
= A
2
a = cm
6 A = 807.77 cm
2
d = 140 mm
D = mm
7 a x a = 543169 mm
2
a = mm
8 d : l = 1:1.5
A = 73.5 mm
d = mm
9 increase in area
A = 12.7%
A = 360 mm
2
d = mm
(d = diameter after the
increase in area)
© NIMI, Not to be republished

30
Applications of pythagoras theorem and related problems Exercise 1.3.12
1 What is the side AC if AB = 15 cm, BC = 25 cm.
AC
2
= AB
2
+ BC
2
= 15
2
+ 25
2
= 225 + 625 = 850
AC =
850 = 29.155 cm
2 What is the side BC if AB = 10 cm, AC = 30 cm.
AC
2
= AB
2
+ BC
2
30
2
= 10
2
+ BC
2
900 = 100
+ BC
2
BC
2
= 900 - 100 = 800
BC = 28.284 cm
3 What is the side AB if BC = 20 cm, AC = 35 cm.
AC
2
= AB
2
+ BC
2
35
2
= AB
2
+ 20
2
1225 = AB + 400
AB
2
= 1225 - 400 = 825
AB = 28.72 cm
4 What is the value of side BC if AB = 8 cm, AC = 24 cm.
AC
2
= AB
2
+ BC
2
24
2
= 8
2
+ BC
2
576 = 64
+ BC
2
BC
2
= 576 - 64 = 512
BC = 572 = 22.63 cm
5 What is the value side AC if AB = 6.45 cm, BC = 8.52
cm.
AC
2
= AB
2
+ BC
2
AC
2
= 6.45
2
+ 8.52
2
AC
2
= 41.60
+ 72.59
= 114.19
AC = 114.19 = 10.69 cm
6 What is the value of side AB if BC = 3.26 cm, AC = 8.24
cm.
AC
2
= AB
2
+ BC
2
8.24
2
= AB
2
+ 3.26
2
67.9 = AB + 10.63
AB
2
= 67.9 - 10.63
= 57.27
AB = 57.27 = 7.57 cm
7 What is the value of side AB if AC = 12.5 cm, BC = 8.5
cm.
AC
2
= AB
2
+ BC
2
12.5
2
= AB
2
+ 8.5
2
156.25 = AB + 72.25
AB
2
= 156.25 - 72.25
= 84
AB = 84 = 9.17 cm
ASSIGNMENT
1 What is the value of side AB, if right angled triangled of
side AC = 12.5 cm and BC = 7.5 cm.
2 What is the value of side AC, if right angled triangled of
side AB = 6.5 cm and BC = 4.5 cm.
3 What is the value of side BC, if right angled triangled of
side AC = 14.5 cm and AB = 10.5 cm.
4 What is the value of side AC, if right angled triangled of
side AB = 7 cm and BC = 5 cm.
5 What is the value of side BC, if right angled triangled of
side AC = 13.25 cm and AB = 8.75 cm.
© NIMI, Not to be republished
31
Ratio and proportion Exercise 1.4.13
Ratio
Introduction
It is the relation between two quantities of the same kind
and is expressed as a fraction.
Expression
a, b two quantities of the same kind.
b
a
or a:b or a
b or
a in b is the ratio.
Ratio is always reduced to the lowest terms.
Example
Proportion
It is the equality between the ratios, a : b is a ratio and c:
d is another ratio. Both ratios are equal. Then
a :b :: c : d or
Example
250 : 2000 :: 1 : 8
Proportion fundamentals
If then
• ad = bc
•
•
•
•
•
3:4::6:8 or
• 3 x 8 = 6 x 4
•
•
•
•
•
Ratio - relation of two quantities of the same kind.
Proportion - equality between two ratios.
Example
• A steel plate of 800 x 1400 mm is to be drawn to a scale
of 1:20. What will be the lengths in the Fig 1
The reduction ratio is .
B is reduced from 800 to 800 x = 40 mm.
L is reduced from 1400 x = 70 mm.
• Find the number of teeth of the larger gear in the gear
transmission shown in the Fig 2.
© NIMI, Not to be republished

32
Speed ratio = 400 : 300
Teeth ratio = 24:T
ASSIGNMENT
1l
1
: l
2
= 2:3
L = 2.75 metres
l
1
=
______ metres
l
2
= ______ metres
2 d: L of shaft = 2:7
d = 40 mm
L = ______ mm
3 D : L=1:10
L=150mm
D=______ mm
4
I = 140 mm
h = _____ mm
5 D : d = 1.75: 1
D = 35 mm
d = ______ mm
6 a:s = 5:1
s = 1.5mm
a =______ mm
7 A:B=9:12
B:C=8:10
Then A:B:C=___________
8 A:B=5:6
B:C=3:4
Then A:B:C= ______
9 A:55=9:11
A = __________
10 15:9.3=40:x
x = __________
Find the ratio of A:B:C
If A:B= 2:3 and B:C=4:5
A:B = 2:3
B:C = 4:5
A:B = 8 :12 (Ratio 2:3 multiply by 4)
B:C = 12:15 (Ratio 4:5 multiply by 3)
∴ A:B:C = 8:12:15
Workshop Calculation And Science : (NSQF) Exercise 1.4.13
© NIMI, Not to be republished

33
Direct and indirect proportions Exercise 1.4.14
Proportion
Description
It is the equality between the ratios, a:b is a ratio and c:d
is another ratio. Both ratios are equal. Then
a : b::c : d or e.g. 250 : 2000::1 : 8
Rule of three
A three step calculation
statement
single
multiple.
Direct proportion
The more in one the more in the other - An increase in one
denomination produces an increase in the other. (Fig 1)
Example
4 turners earn 300 Rupees. How much will 6 Turners earn?
Statement
4 turners = 300 Rupees
Single
1 Turner = 75 Rupees
Multiple
6 Turners = 6 x 75 = 450 Rupees
Result - The more the more.
Indirect or inverse proportion
The more in one the lesser other - Increase in one quantity
will produce a decrease in the other. (Fig 2)
Example
Four turners finish a job in 300 hours. How much time will
6 turners take to do the same job?
Solution procedure in three steps:
Statement
4 turners taken = 300 hours
The time will reduce if 6 turners to do the same job.
Therefore this is inverse propotion.
Multiple fraction
6 Turners = 200 hours
Result - The more the less.
Problems involving both
Example
Two turners need three days to produce 20 pieces. How
long does it take for six turners to produce 30 such pieces?
Statement
2 turners, 20 piece = 3 days
6 turners, 30 pieces = how many days.
First step (Fig 3)
Statement 2 turners for 20 pieces = 3 days
1 turners for 20 pieces = 3 x 2 = 6 days
Multiple 6 turners for 20 pieces = = 1 day
© NIMI, Not to be republished

34
Inverse proportion - The more the less.
Second step (Fig 4)
Compound and Inverse proportions
• Compound proportions
Example
If 5 Fitter take 21 days to complete overhauling of 6 vehicles
how long 7 Fitters will take to over haul 8 vehicles (Assume
time of overhauling each vehicle is constant)
In this both direct and indirect proportions are used.
• 1 Fitter will over haul 1 vehicle in days (shorter time).
• Quantities (No. of days) are taken in last as that is
the answer required in this case.
Ans: 7 Fitters will overhaul 8 vehicles in 20 days.
Inverse proportion
Some times proportions are taken inversely.
Examples
• If one water pump fills the fuel tank in 12 minutes, two
pumps will take half the time taken.
The time should not be doubled.
• If two pumps take 30 minutes to fill up a tank how long
will 6 similar pumps take this to fill this tank.
Ans: Time taken by 6 pumps =
minutes 10
6
230
Proportional parts in combustion equation
Introduction
Proportion of quantities form an important factor in the
combustion process of a fuel. The following happens during
the combustion process.
Fuel is a hydro carbon substance. The combustion air is
supplied from atmosphere and contains oxygen and nitro-
gen. Now the following chemical changes take place during
combustion of a fuel.
• Carbon burns with oxygen and forms Co and Co
2
(Carbon monoxide and there carbon dioxide.)
• Hydrogen burns with oxygen and becomes water (H
2
O)
• Sulphur burns with oxygen and becomes sulphur
dioxide.
• Nitrogen is an inert gas and does not take part in
combustion.
Method of finding proportional parts in one lb of a
substance
To be found out now
• Proportion of oxygen and hydrogen in one lb/Kg of
water.
Statement 6 turners for 20 pieces = 1 day
Single 6 turners for 1 piece = days
Multiple 6 turners for 30 pieces =
20
1
x 30 = 1.5 days
Direct proportion - The more the more.
Solve the probÎem by first writing the statement
and proceed to single and then to the multiple
according to the type of proportion that is
involved.
Introduction
Proportional fundamentals, as applicable to motor vehicle
calculations are discussed below.
Simple Proportion
• Proportion
This is an equality between two ratios
Examples
• If one vehicle fleet uses 30 litres of petrol per day how
much petrol is used by 6. Vehicles operating under
similar condition.
One vehicle uses petrol = 30 litres per day.
Then six vehicles will use = 6 Times as much
= 6 x 30 = 180 litres/day.
• If 4 vehicles of a fleet use 120 gallons of petrol per day
how much petrol will be used by 12 vehicles operating
under the same condition.
4 vehicles use 120 gallons per day
1 Vehicle will use
4
120
= 30 gallons/day
12 vehicles will use
Both examples are called simple proportion
because only two quantities were used and the
day is common for both ratios.
Workshop Calculation And Science : (NSQF) Exercise 1.4.14
© NIMI, Not to be republished

35
• Proportion of hydrogen and carbon in one lb/kg of fuel.
Examples
• The chemical formula for water is H
2
O. This means 2
atoms of hydrogen and one atom of oxygen combined
to make one molecule of water. If oxygen atom weighs
16 times as much as hydrogen find out the proportions
in one kg of water.
Solution
Parts by weight of water are as below
Oxygen = 16/2 = 8kg
Hydrogen = 1/1 = 1kg
Total = 8+1= 9kg
• A hydrocarbon fuel has formula C
6
H
14
. This shows one
molecule of fuel contain 6 atoms of carbon and 14
atoms of hydrogen. If the carbon atom weighs 12 times
as the hydrogen atom, find the proportionate parts of
hydrogen and carbon in one kg of fuel.
Solution
Parts of carbon by weight
= 6 x 12 = 72
Parts of hydrogen by weight = 14.
Total No. of parts = 72 + 14 = 86.
Weight of Carbon = 72/86 = 0.8372 kg
Weight of Hydrogen 14/86 = 0.1628 kg
Ratio and Proportion
Proportion of air quantity required for combustion
process
Mass of air required for complete combustion of fuel
depends on the following factors and is called Air - fuel
Ratio
• Carbon, Hydrogen, Sulphur are to burn with oxygen
in the combustion process.
• It has been found that the following quantities of air
(by wt) are required for this purpose to supply sufficient
quantity of oxygen.
• For complete combustion of 1kg of carbon =
Kgs
3
2
2
of oxygen
• For complete combustion of 1 kg of hydrogen = 8kgs
of oxygen
• For complete combustion of 1 kg of sulphur = 1 kg
of oxygen
• Formula for calculation of mass of air for
complete combustion.
Air contains 23% oxygen and 77% nitrogen
Mass of air = Mass of oxygen x for each constituent
air kg.of 4.35
23
100
1sulphur For
air 34.8kg.of
23
100
8hydrogen For
air 11.6kg.of
23
100
3
2
2Carbon For
Total 50.75 Kg
Hence 50.75 kg of air is to be supplied to the engine for
combustion of 1 kg of fuel.
As the combustion process is not even more
quantity of air than 50.7 kg is to be supplied to
the engine.
The calculations involved in the combustion
equations is beyond the scope of ITI students
as it involves chemistry and physics for comput-
ing the proportions of different elements.
Workshop Calculation And Science : (NSQF) Exercise 1.4.14
© NIMI, Not to be republished

36
ASSIGNMENT
1 Length = 6.1 metre
Weight = 32 kgf
Weight of 1 metre of
the same rod =
_______ kgf
2d
1
= 120 mm
d
2
= 720 mm
n
1
= 1200 rpm
n
2
= ______ rpm
3Z
1
= 42 T
n
2
= 96 rpm
n
1
= 224 rpm
Z
2
= _______ T
4
D = 50 mm
H = 80 mm
h = 36 mm
d = ______ mm
5 If a mechanic assembles 8 machines in 3 days. How
long he will take to assemble 60 machines.
6 In an autoshop the grinding wheel makes 1000 rpm and
the driven pulley is 200 mm dia. If the driving pulley is
150 mm dia. Find out the rpm of the driving pulley.
7 In a gearing of a vehicle the following facts are found.
A 180 mm dia of gear meshes with 60mm dia gear. If the
bigger gear makes 60 rpm. What will be the rpm of
smaller gear.
8 A vehicular job is completed by 5 mechanics in 4 days.
If only 3 mechanics are available, in how many days the
work can be completed.
9 In a gearing arrangement of a vehicle a gear having 26
teeth is meshing with a gear of 52 teeth. The dia of 52
teeth gear is 200mm. Find out the diameter of 26 teeth
gear wheel.
10 If two water pumps take 45 minutes to fill up a tank how
long will 4 similar pumps will take to this tank.
11 In a belt-pulley drive the driving pulley is of 12 cm
diameter and rotates at 360 rpm. Find the rpm of driven
pulley whose dia meter is 20 cm diameter.
12 To overhaul a gear box, 12 mechanics are needed to
complete the work in 5 days. If only 7 mechanics are
available, how many days they will be able to complete
the overhauling work.
13 Express in simple ratios the following
a
6045
b
Rs4.00 paise 40
c
metres 4
20mm
d C100C4
14 Air contains 24% oxygen and 78% nitrogen by mass
(weight). Calculate the quantity of air (mass of air)
required for complete combustion of unit mass fuel (The
main constitutents that take part in combustion proc-
ess are carbon, hydrogen and sulphur)
Note: Given the following data (Solve the problem)
a 1 kg of carbon requires kg of oxygen.
b 1 kg of hydrogen requires 8 kg of oxygen.
c 1 kg of sulphur requires 1 kg of oxygen.
15 A fuel is a hydro carbon substance of C
7
H
14
. This shows
each molecule of fuel contains 7 atoms of carbon and
14 atoms of hydrogen. If carbon atomic weight is 12
times greater than hydrogen atom, find out the propor-
tionate parts of hydrogen and carbon in one kg of fuel.
16 A vehicle worth Rs.20,000/- can be insured at a cost of
Rs.150/-. How much wired it cost to insure a vehicle
worth Rs.24000/- for one year and 3 month at the same
rate. (Compound proportion)
Workshop Calculation And Science : (NSQF) Exercise 1.4.14
© NIMI, Not to be republished

37
Percentage Exercise 1.5.15
Ex.
0.16
100
16
In decimal form, it is 0.16. Percentage calculation also
involves rule of three. The statement (the given data), for
unit, and then to multiple which is for calculating the
answer.
Example
The amount of total raw sheet metal to make a door was 3.6
metre
2
and wastage was 0.18 metre
2
. Calculate the % of
wastage.
Solution procedure in three steps.
Statement:
Area of door (A) =3.6 m
2
= 100 %.
Wastage = 0.18 m
2
Single:
3.6
100
1 m
2
Analyse the given data and proceed to arrive at
the answer through the unit.
Example
A fitter receives a take-home salary of 984.50 rupees.
If the deduction amounts to 24%, what is his total salary?
(Fig 3)
Total pay 100%
Percentage
Percentage is a kind of fraction whose denominator is
always 100. The symbol for percent is %, written after the
number 16%.
Multiple: for 0.18 m
2
=
3.6
100
x 0.18.
Wastage = 5%.
Conclusion
The three steps involved are,
step one : describe the situation (availability)
step two : decide for unit
step three : proceed for the multiple.
Deduction 24%
Take home salary 76%
If the take home pay is Rs.76, his salary is 100.
For 1% it is
76
1
For Rs.984.50, it is
76
1
x 984.50.
For 100% it is
1295.39X100
76
984.50
100% i.e. gross pay = Rs.1295.40.
Example 1
75 litres of oil is taken out from a oil barrel of 200 litres
capacity. Find out the percentage taken in this.
Solution
% of oil taken = Oil taken out (litres) / Capacity of Barrel
(litres) x 100
%37 100 x
200
75
2
1
Example 2
A spare part is sold with 15%. Profit to a customer, to a
price of Rs.15000/-. Find out the following (a) What is the
purchase price (b) What is the profit.
Solution: CP =
x
,
CP = cost price
SP = sale price
SP=CP+15%of CP
15000= +
© NIMI, Not to be republished

38
=
Profit = SP-CP = 15000-13043.47 = 1956.53
Purchase price = Rs.13,043/,Profit = Rs. 1957
Example 3
Out of 80000 cars, which were tested on road, only 16000
cars had no fault. What is the percentage in this accep-
tance.
=
Example 4
The price of a motor cycle dropped to 92% of original price
and now sold at Rs.18000/- What was the original price.
Solution
Present price of Motor cycle Rs.18000
This is the value of 92% of original price
Original Price = 18000 x
= Rs.19565
Example 5
A Motor vehicle uses 100 litres of Petrol per day when
travelling at 30 kmph. After top overhauling the consump-
tion falls to 90 litres per day. Calculate percentage of
saving.
Solution
Percentage of saving = Decrease in consumption/Original
consumption x 100
= (100 – 90) x100
=
= 10% Saving in fuel.
ASSIGNMENT
1 a = 400mm (side of
square)
d = 400 mm
Wastage = ______ %.
2 d = 26mm
'a' depth of u/cut =
2.4mm
reduction of area at
cross-section
= ______ %
3 Percentage of increase
= 36%
Value of increase
= 611.2 N/mm
2
Original tensile strength
= ______ N/mm
2
.
4 Copper in alloy = 27 kg
Zinc in alloy = 18 kg
% of Copper
= _______ %
% of Zinc = _______%.
5 Weight of alloy = 140
Kgf
Weight of Sn 40%
Pb = ______ Kgf
Sn = ______ Kgf.
6 Shaded portion
= ______ %.
7 Compression length =
______ %.
8 d = 360 mm
a = 0.707 x d
Wastage = ______ %.
Workshop Calculation And Science : (NSQF) Exercise 1.5.15
© NIMI, Not to be republished

39
Workshop Calculation And Science : (NSQF) Exercise 1.5.16
9 Cu = 36 Kg
Zn = 24 Kg
Cu = ______ %
Zn = ______ %
10 Cu = 42.3 Kg
Sn = 2.7 Kg
Cu ______ %
Sn = ______ %.
11 What is the selling price, If a trader buys a spare part
for Rs.195/- This is 65% of selling price.
12 What is the purchase price if 25% profit is added to it,
If a Motor cycle tyre is sold for Rs.300/-.
13 How many m
3
of elements of air present in 120 m
3
of air,
If the composition of Air is 23% of Oxygen and 77% of
Nitrogen.
14 How many kg of each of these elements are found. If an
Engine bearing made of alloy of 40 kg consists of the
following constitutents.
a) Copper (Cu) - 86%
b) Tin (Sn) - 10%
c) Zinc (Zn) - 4%
15 How much weight of these elements are found to exist.
If a solder consists of 35%. Tin and 65% Lead. In a
solder of 40 kg.
16 Find out the following:
1 Average consumption per journey.
2 Average consumption per mile.
3 Using the average consumption express maximum
consumption as a percentage of the average correct
to two decimal places. If the total petrol consump-
tion of car on 4 different journeys each of 200 miles
are found to be as 6.65, 7.5, 6.85,7.05 gallons
respectively.
17 In a Transport workshop, the following expenditure was
found to be occuring on the capital income.
1 40% income spent on tyres
2 30% income spent on fuel and lubricants
3 10% income spent on spare parts
If the month end saving comes to Rs.2000/- what is the
total income?
18 What is the final weight of the machined job if a casting
weighs 80kg. During preliminary machining weight is
reduced by 4% and final machining by 5%.
19 What is the weight of zinc, copper and tin, casting
weight is 25kg. If a casting has 35% zinc, 40% copper
and 25% tin.
20 What is the total weight of a solder, if solder consists
of 35% tin 65% lead, and tin consists 14 grams.
21 What is the total annual income of the salesman, if a
salesman gets a monthly pay of Rs.1000 and a com-
mission of 2.5% on his sale. In one year sale amount
is Rs.60,000.
22 What is the total income of a man, if he spends 15% of
his income on agriculture, 21% on family, 24% on
education of children and he saves Rs.360.
23 What is the percentage of his savings, if a person’s
monthly salary is Rs.450 and saves Rs.90 every months.
© NIMI, Not to be republished

40
Changing percentage to decimal and fraction Exercise 1.5.16
Conversion of Fraction into Percentage
1 Convert
into percentage.
Solution:
2
1
x 100
= 50%
2 Convert
11
1
into percentage
Solution:
11
1
x 100 =
11
100
= 9.01%
Convert the following fraction into percentage.
1
4
1
2
5
1
3
3
2
4
8
3
Conversion of Percentage into Fraction
1 Convert 24% into fraction.
Solution:
100
24
=
25
6
2 Convert
3
1
33
% into fraction.
Soluction:
100
3
1
33
=
100
1
3
100
100
3
100
=
3
1
Convert the following percentage into fraction
1 15%
2
%
2
1
87
3 80%
4 125%
Conversion of Decimal Fraction into Percentage
1 Convert 0.35 into percentage.
Soluction: 0.35 x 100
= 35%
2 Convert 0.375 into percentage.
Soluction: 0.375 x 100
= 37.5%
Convert the following Decimal Fraction into Percentage
1 0.2
2 0.004
3 0.875
4 0.052
Conversion of Percentage into Decimal fraction
1 Convert 30% into decimal fraction.
Soluction:
100
30
= 0.3
2 Convert
%
3
1
33
into decimal fraction.
Soluction:
100
3
1
33
=
100
1
3
100
100
3
100
=
3
1
= 0.333
Convert the following percentage into decimal fraction
1 15%
27%
3
%
2
1
12
4 90%
© NIMI, Not to be republished

41
Algebraic symbols and fundamentals Exercise 1.6.17
Introduction
Algebra is a form of mathematics in which letters may be
used in place of unknown. In this mathematics numbers
are also used in addition to the letters and the value of
number depends upon its place. For example in 3x and x
3
,
the place of x is different. In 3x=3 is multiplied with x,
whereas in x
3
- 3 is an Index of x.
Positive and negative numbers
Positive numbers have + sign in front of them, and negative
numbers have – sign in front of them. The same applies to
letters also.
Example +x – y
+8 or simply 8 positive number.
– 8 negative number.
Addition and subtraction
Two positive numbers are added, by adding their absolute
magnitude and prefix the plus sign.
To add two negative numbers, add their absolute magni-
tude and prefix the minus sign.
To add a positive and a negative number, obtain the
difference of their absolute magnitudes and prefix the sign
of the number having the greater magnitude.
+7 + 22 = +29
–8 – 34 = – 42
–27 + 19 = –8
44 + (–18) = +26
37 + (–52) = –15
Multiplication of positive and negative numbers
The product of two numbers having like signs is positive and
the product of two numbers with unlike signs is negative.
Note that, where both the numbers are negative, their
product is positive.
Ex. –20 x –3 = 60
5 x 8 = 40
4 x –13 = –52
–5 x 12 = –60
Division
The number that is divided is the dividend, the number by
which we are dividing is the divisor and the answer is the
quotient. If the signs of the dividend and the divisor are the
same then the quotient will have a + sign. If they are unlike
then the quotient will have a negative sign.
4+
28+
=+7
4–
56+
= –14
9+
72–
=–8
6–
96–
= +16
When an expression contains addition,
subtraction, multiplication and division,
perform the multiplication and division
operations first and then do the addition and
subtraction.
Example
12 x 8 – 6 + 4 x 12 = 96 – 6 + 48 = 138
102 ¸ 6 – 6 x 2 + 3 = 17 – 12 + 3 = 8
Parantheses and grouping symbols
( ) Brackets
{ } Braces
7 + (6–2) = 7 + 4 = 11
6 x (8–5) = 6 x 3 = 18
Parentheses
These are symbols that indicate that certain addition and
subtraction operations should precede multiplication and
division. They indicate that the operations within them
should be carried out completely before the remaining
operations are performed. After completing the grouping,
the symbols may be removed.
In an expression where grouping symbols immediately
preceded or followed by a number but with the signs of
operation omitted, it is understood, that multiplication
should be performed.
Grouping symbols are used when subtraction and multipli-
cation of negative numbers is done.
To remove grouping symbols which are preceded by
negative signs, the signs of all terms inside the grouping
symbols must be changed (from plus to minus and minus
to plus).
Parentheses which are preceded by a plus sign may be
removed without changing the signs of the terms within the
parentheses.
When one set of grouping symbols is included within
another set, remove the innermost set first.
When several terms connected by + or – signs contain a
common quantity, this common quantity may be placed in
front of a parentheses.
8 + 6(4–1) = 8 + 6 x 3 = 26
(6+2)(9–5) = 8 x 4 = 32
Plus 4 less negative 7 is written as 4 – (–7).
Plus 4 times negative 7 is written as 4(–7).
© NIMI, Not to be republished

42
4 –(–7) = 4 + 7 = 11
8 –(7–4) = 8 – 3 = 5
3 +(–8) = 3 – 8 = –5
7 +(4 – 19) = 7 + (–15) = 7 – 15 = – 8
3{40 +(7 + 5)(8–2)} = 3 {40 + 12 x 6} = 3 x 112 = 336.
8x + 12 - quantity 4 may be factored out giving the
expression 8x + 12 as 4(2x + 3).
The innermost set in a grouping symbols of an expres-
sion is to be simplified first.
Algebraic symbols and simple equations
Algebraic symbol
An unknown numerical value of a quantity is represented by
a letter which is the algebraic symbol.
Factor
A factor is any one of the numbers or letters or groups which
when multiplied together give the expression. Factors of 12
are 4 and 3 or 6 and 2 or 12 and 1.
8x + 12 is the expression and this may be written as
4(2x + 3), 4 and (2x + 3) are the factors.
Algebraic terms
If an expression contains two or more parts separated by
either + or –, each part is known as the term.
y – 5 x is the expression. y and – 5 x are the terms.
The sign must precede the term.
Coefficient
When an expression is formed into factors whose product
is the expression, then each factor is the coefficient of the
remaining factors.
48x = 4 x 12 x x.
4 is the coefficient of 12 x . x is the coefficient of 48.
Equation
It is a statement of equality between numbers or numbers
and algebraic symbols.
12 = 6 x 2, 13 + 5 = 18.
2x + 9 = 5, y – 7 = 4y + 5.
Simple equation
Equations involving algebraic symbols to the first power are
simple equations.
2x + 4 = 10. 4x + 12 = 14.
Addition and subtraction
Quantities with algebraic symbols are added or subtracted
by considering those terms involving same symbols and
powers.
10x + 14 – 7y
2
– 11a + 2x – 4 – 3y
2
– 4a + 8
= 10x + 2x – 7y
2
– 3y
2
– 11a – 4a + 14 – 4 + 8
= 12x – 10y
2
– 15a + 18
2x = 10, 2x + 6 = 10 + 6
y + 12 = 20, y + 12 – 8 = 20 – 8
x + 10 = 12,
x + 10 – 10 = 12 – 10
3x = 6, 2 x 3x = 2 x 6, 6 x = 12
5y = 20,
5
20
=
5
5y
.
The same number may be added or subtracted to both
members of an equation without changing its equality.
Each member of an equation may be multiplied or divided
by the same number or symbol without changing its
equality.
The equality of an equation is not altered when the numbers
or symbols are added or subtracted from both sides.
Multiplication and division by the same numbers or sym-
bols on both sides also will not affect the equality.
Transposition of the terms of the equations
= equals to
+ plus
– minus
x multiply
÷ divided by
Concept of equality (Fig 1)
Workshop Calculation And Science : (NSQF) Exercise 1.6.17
© NIMI, Not to be republished

43
An equation can be compared to a pair of scales which
always remain in equilibrium. The two sides of the equation
can fully be transposed. 9 = 5 + x may also be written as
5 + x = 9.
We must always perform the same operation on both sides
of the equation to keep the equilibrium. Add or subtract the
same amount from both sides. 5 + x = 9 By adding 3 on
both sides, the equation becomes 5 + x + 3 = 9 + 3 or x
+ 8 = 12.
5 + x = 9 Subtract 5 from both sides then 5 + x – 5 = 9– 5.
x = 4.
5 is transposed from left side to the right side by changing
its sign from + to –.
4
x
= 20. Multiply both sides by 4. Then
4
x
x 4 = 20 x 4.
x = 80,
5x = 25.
Divide both sides by 5 then
5
25
=
5
5x
x = 5.
When transposing numbers or letter symbols from one
side to the other side multiplication becomes division and
the division becomes multiplication.
The equality of an equation remains unchanged
when both sides of the equation are treated in
the same way. When transposing from one
side to the other side,
a plus quantity becomes minus quantity.
a minus quantity becomes a plus quantity
a multiplication becomes a division
a division becomes a multiplication.
To solve simple equations isolate the unknown
quantity which is to be found on the left side of
the equation.
Example
• Solve for x if 4x = 3(35 – x )
4x = 105 – 3x (brackets removed)
4x + 3x = 105 (By transposing –3x on the right side
to the left side)
7x = 105
x = 15 (dividing both sides by 7)
Workshop Calculation And Science : (NSQF) Exercise 1.6.17
© NIMI, Not to be republished

44
Addition,subtraction,multiplication and division of algebra Exercise 1.6.18
Addition
Example 1
Add 2x, 3x, 7x
Solution: Since this is only addition, put ‘+’ symbol &
each bracket
= (+2x) + (+3x) + (+7x) = 12x
Add
-3a, -2a, -5a
= (-3a) + (-2a) + (-5a) = -10a
Example 2
Add 10xy, -8x
2
, -7xy, -7x
2
= (10xy) + (-8x
2
) + (-7xy) + (-7x
2
)
= (10xy) + (-7xy) + (-8x
2
) + (-7x
2
)
= (3xy) + (-15x
2
)
Example 3
Add
8
a
,
2
a
,
4
a
8
5a
8
a4a2a
8
a
2
a
4
a
8
a
,
2
a
,
4
a
Subtraction
Example 1
Subtraction 8m from 2m
Solution : (2m) - (8m)
To be subtracted from +8m after changed + 2m
the symbol - 8m
after added - 6m
Example 2
Subtraction (-5x + 3y) from (7x + 8y)
Solution: (7x + 8y) - (-5x + 3y)
+ 7x + 8y
+ 5x - 3y
+ 12x + 5y
(Multiply all the terms which should be subtracted with
‘-’ symbol subsequently add or subtract the similar terms
as atated in the steps)
Solving of Simple Equations
Rules Related to signs in Algebra
1 Multiplication Rules
(i) + x + = +
(ii) - x - = +
(iii) + x - = -
(iv) - x + = -
2 Division Rules
(i)
+=
+
+
(ii)
+=
−
−
(iii)
−=
−
+
(iv)
−=
+
−
Examples
1) a + 4 = 7
a = 7 - 4
a = 3
2) M - 5 = 3
m = 3 + 5
m = 8
3) 7P = 17
P =
7
17
4)
6
x
= 9
x = 9 x 6
x = 54
© NIMI, Not to be republished

45
5) 7x + 3 = 15 + x
7x -x = 15 - 3
6x = 12
x =
6
12
= 2
6)
0y
9
5
=
Y = 0 x
5
9
y = 0
4
5
8
10
M
8
5
2XM
2M
5
8
68M
5
8
86M
5
8
==
=
=
−=
=+
7)
36x
36
5
6 x 30
x
6 x 305x
30
6
3x 2x
30
2
x
3
x
=
==
=
=
+
=+
8)
144
3
12
x36x
36
12
3x
36
12
2x -5x -4x 6x
36
6
x
12
5x
3
x
2
x
36
6
x
12
5x
3
x
2
x
==
=
=
+
=−−+
+=−+
9)
30- a
8 x 0 30 a
0
8
30 a
0
8
30 4a - 5a
0
4
15
2
a
a
8
5
=
=+
=
+
=
+
=+−
10)
Multiplication
While dealing with multiplication, first of all multiply the
symbols, subsequently coefficient, afterwards letters.
Example 1
Multiply (2x-5) x (x-1)
(2x-5) (x-1)
= 2x (x-1) - 5(x-1)
= 2x
2
- 2x -5x +5
= 2x
2
- 7x + 5
Example 2
Multiply (4x
2
+ 3x -12) by (2x +3)
(4x
2
+ 3x -12) (2x +3)
= 2x(4x
2
+ 3x -12) + 3 (4x
2
+ 3x -12)
= 8x
3
+6x
2
- 24x + 12x
2
+ 9x - 36
= 8x
3
+6x
2
+ 12x
2
- 24x
+ 9x - 36
= 8x
3
+18x
2
- 15x -36
Division
Division is nothing but reverse operation of multiplication.
Example 1
Division (x
2
+ 11x + 30) by (x + 6)
(x
2
+ 11x + 30) ÷ (x + 6)
x
2
+ 11x + 30 ÷ x + 6 = x + 5
Workshop Calculation And Science : (NSQF) Exercise 1.6.18
© NIMI, Not to be republished

46
Addition
1 Add 7y, 8y, 5y , 3y
2 Add 5x, 6x, 7y - 9y, 10x
3 Add 9a
2
, -7m, 5, -14a
2
, 15m, -6
4 Add
Substraction
1 Subtract 7x from 9x
2 Subtract 8a from 3a
3 (-5 -3b) - (3a + 5b)
4 Subtract (5m - 6n) from (3m + 5n)
Multiplication
1 Multiply 6a x (- 2ab)
2 Multiply 3x (4x - 2y + 5)
3 Multiply (3x + 2) (3x - 2)
Division
1
525x
2
15a60ab
3 77y14x
Assignment
Workshop Calculation And Science : (NSQF) Exercise 1.6.18
© NIMI, Not to be republished

47
Metal:
Metal is a mineral used in all types of engineering works
such as machineries, bridges, aero planes etc . so we
must have basic knowledge about the metals.
Understanding the physical and mechanical properties of
metals has become increasingly important for a machinist
since he has to make various components to meet the
designed service requirements against factors, such as
the raise of temperature, tensile, compressive and impact
loads etc. A knowledge of different properties of materials
will help him to do his job successfully. If proper material/
metal is not used it may cause fracture or other forms of
failures, and endanger the life of the component when it is
put into function.
Fig 1 shows the way in which the metals get deformed
when acted upon by the same load.
Note the difference in the amount of deformation.
Physical properties of metals
– Colour
– Weight/specific gravity
– Structure
– Conductivity
– Magnetic property
– Fusibility
Colour
Different metals have different colours. For example,
copper is distinctive red colour. Mild steel is blue/black
sheen.
Weight
Metals may be distinguished, based on their weights for
given volume. Metals like aluminium lighter weigh (Specific
gravity 2.7) and metals like lead have a higher weight.
(Specific gravity 11.34)
Structure (Figs2 and 3)
Generally metals can also be differentiated by their internal
structures while seeing the cross-section of the bar through
a microscope. Metals like wrought iron and aluminium
have a fibrous structure and metals like cast Iron and
bronze have a granular structure.
Conductivity (Figs 4 and 5)
Thermal conductivity and electrical conductivity are the
measures of ability of a material to conduct heat and
electricity. Conductivity will vary from metal to metal.
Copper and aluminium are good conductors of heat and
electricity.
Magnetic property
A metal is said to possess a magnetic property if it is
attracted by a magnet.
Almost all ferrous metals, except some types of stainless
steel, can be attracted by a magnet, and all non-ferrous
metals and their alloys are not attracted by a magnet.
Physical and mechanical properties of metals Exercise 1.7.19
© NIMI, Not to be republished

48
Fusibility (Fig 6)
It is the property possessed by a metal by virtue of which
it melts when heat is applied. Many materials are subject
to transformation in the shape (i.e) from solid to liquid at
different temperatures. Lead has a low melting temperature
while steel melts at a high temperature.
Tin melts at 232°C.
Tungsten melts at 3370°C.
Mechanical properties
– Ductility
– Malleability
– Hardness
– Brittleness
– Toughness
– Tenacity
– Elasticity
Ductility (Fig 7)
A metal is said to be ductile when it can be drawn out into
wires under tension without rupture. Wire drawing depends
upon the ductility of a metal. A ductile metal must be both
strong and plastic. Copper and aluminium are good
examples of ductile metals.
Malleability (Figs 8 and 9)
Malleability is that property of a metal by which it can be
extended in any direction by hammering, rolling etc.
without causing rupture. Lead is an example of a malleable
metal.
Hardness (Fig 10)
Hardness is a measure of a metal's ability to withstand
scratching, wear and abrasion, indentation by harder
bodies. The hardness of a metal is tested by marking by
a file etc.
Workshop Calculation And Science : (NSQF) Exercise 1.7.19
© NIMI, Not to be republished

49
Brittleness (Fig 11)
Brittleness is that property of a metal which permits no
permanent distortion before breaking. Cast iron is an
example of a brittle metal which will break rather than bend
under shock or impact.
Toughness (Fig 12)
Toughness is the property of a metal to withstand shock or
impact. Toughness is the property opposite to brittleness.
Wrought iron is an example of a tough metal.
Tenacity
The tenacity of a metal is its ability to resist the effect of
tensile forces without rupturing. Mild steel, Wrought Iron
and copper are some examples of tenacious metals.
Elasticity
Elasticity of a metal is its power of returning to its original
shape after the applied force is released. Properly heat-
treated spring is a good example for elasticity.
Workshop Calculation And Science : (NSQF) Exercise 1.7.19
© NIMI, Not to be republished

50
Introduction of iron and cast iron Exercise 1.7.20
Ferrous Metals
Metals which contain iron as a major content are called
ferrous metals. Ferrous metals of different properties are
used for various purposes.
Introduction of Iron, Cast Iron, wrought Iron and steel
The ferrous metals and alloys used commonly are:
– Pig-iron
– Cast Iron
– Wrought Iron
– Steels and Alloy steels
Different processes are used to produce iron and steel.
Pig-iron (Manufacturing process)
Pig-iron is obtained by the chemical reduction of iron ore.
This process of reduction of the iron ore to Pig-iron is known
as SMELTING.
The main raw materials required for producing Pig-iron are:
– Iron ore
– Coke
– Flux
Iron ore
The chief iron ores used are:
– magnetite
– hematite
– limonite
– carbonite.
These ores contain iron in different proportions and are
naturally available.
Coke
Coke is the fuel used to give the necessary heat to carry
on the reducing action. The carbon from the coke in the
form of carbon monoxide combines with the iron ore to
reduce it to iron.
Flux
This is the mineral substance charged into a blast furnace
to lower the melting point of the ore, and it combines with
the non-metallic portion of the ore to form a molten slag.
Limestone is the most commonly used flux in the blast
furnace.
Properties and use of Pig-iron
Pig-iron is, therefore, refined and remelted and used to
produce other varieties of iron and steel.
Cast Iron (Manufacturing process)
The pig-iron which is tapped from the blast furnace is the
crude form of raw material for the cupola, and should be
further refined for making castings. This refining is carried
out in the cupola furnace which is a small form of a blast
furnace.
Generally cupolas are not worked continuously like blast
furnaces but are run only as and when required.
Cast Iron (Types)
Cast iron is an alloy of iron, carbon and silicon. The carbon
content ranges from 2 to 4%.
Types of cast iron
The following are the types of cast iron.
– Grey cast iron
– White cast iron
– Malleable cast iron
– Nodular cast iron
Grey cast iron
This is widely used for the casting of machinery parts and
can be machined easily.
Machine base, tables, slideways are made of cast iron
because it is dimensionally stable after a period of aging.
Because of its graphite content, cast iron provides an
excellent bearing and sliding surface.
The melting point is lower than that of steel and as grey cast
iron possesses good fluidity, intricate casting can be
made.
Grey cast iron is widely used for machine tools because of
its ability to reduce vibration and minimize tool chatter.
Grey cast iron, when not alloyed, is quite brittle and has
relatively low tensile strength. Due to this reason it is not
used for making components subjected to high stress or
impact loads.
Grey cast iron is often alloyed with nickel, chromium,
vanadium or copper to make it tough.
Grey cast iron is weldable but the base metal needs
preheating.
White cast iron
This is very hard and is very difficult to machine, and for this
reason, it is used in components which should be abrasion-
resistant.
White cast iron is produced by lowering the silicon content
and by rapid cooling. When cooled in this manner, it is
called chilled cast iron.
White cast iron cannot be welded.
© NIMI, Not to be republished

51
Malleable cast iron
Malleable cast iron has increased ductility, tensile strength
and toughness when compared with grey cast iron.
Malleable cast iron is produced from white cast iron by a
prolonged heat-treatment process lasting for about 30
hours.
Nodular cast iron
This is very similar to malleable cast iron. But this is
produced without any heat treatment. Nodular cast iron is
also known as: Nodular Iron - Ductile Iron - Spheroidal
Graphite Iron
This has good machinability, castability, resistance to
wear, low melting point and hardness.
Mealleable and nodular castings are used for machine
parts where there is a higher tensile stress and moderate
impact loading. These castings are less expensive and are
an alternative to steel castings.
Wrought Iron (Manufacturing process) (Fig 1)
Wrought iron is the purest form of iron. The analysis of
Wrought iron shows as much as 99.9% of iron. (Fig 1)
When heated, wrought iron does not melt, but only be-
comes pasty and in this form it can be forged to any shape.
Modern methods used to produce wrought iron in large
quantities are the
– puddling process
– aston or Byers process
Steel
This is pure iron. Carbon content is more. Due to
excessive carbon it is harder and tougher. Carbon content
is from 0.15 to 1.5%. Besides there are other impurities
like sulphur, phosphorous etc. Are there which cannot be
separated. This is hardened and tempered by heating it to
a definite temperature and cooling it in oil or water.
The following methods are adopted for making different
types of steel:
1 Cementation process 2 Crucible process
3 Bassemer process 4 Open hearth process
5 Electro thermo process 6 High frequency process.
11.13 Main types of steel
Mainly steel is the following two types:
1 Plain steel
2 Alloy steel
1 Plain steel. In this carbon and iron are mixed. Accoring
to the percentage of carbon. plain steel is the following
types:
A Low carbon steel
B Medium carbon steel
C High carbon steel
A Low carbon steel: It is also called mild steel. In
this. the percentage of carbon is from 0.15%to0.25%.
Due to less quantity of carbon is sufficiently soft and
tolerates the strain. It can be put in different shapes
through forging and rolling. This is not very hard or
strong. This cannot be hardened or tempered by
ordinary methods. Nuts, bolts, rivets, sheets,
wires, T-iron and angle iron etc. Are made out of it.
B Medium carbon steel: The carbon content is from
0.25% to 0.5%. Due to excess of carbon, it is harder
and tougher than mild steel. The tenacity is more.
This is used to hardened or tempered. Various
things are made by forging and rolling. This is used
for making high tensile tubes, wires, agricultural
implements, connecting rods, cam shafts, span-
ners, pulleys etc.
C High carbon steel: It has carbon content from
0.5% to 1.5%. It is very hard and wears least. This
can be hardened by heat treatment. This can
neither be cast nor rolled. This is very hard and
tough. It acquires permanent magnetic properties.
This is used for making pointed tools, springs,
pumps, files, cutleries, cold chisels press die etc.
2 Alloy Steel
When the steel is mixed with other metals like vinoleum,
manganese tungsten etc. It is called an alloy steel. Alloy
steel has properties of its ingredients.
Types of Alloy Steel
Alloy steel is mainly of two types:
A Low alloy steel
B High alloy steel
A Low Alloy steel: Besides carbon other metals are
in lesser quantity. Its tensile strength is more. The
welding can work on it. This can also be hardened
and tempered. It is used in manufacturing various
parts of an aeroplane and cam shaft etc.
B High Alloy Steel: Besides carbon it has a high
percentage of the metals higher than low steel alloy.
This is following types:
Workshop Calculation And Science : (NSQF) Exercise 1.7.20
© NIMI, Not to be republished
52
a High Speed Steel: It is also called high tungsten
alloy steel because it has more quantity of tungsten.
According to the quantity of tungsten it is classified
in three types:
1 Tungsten 22%, Chromium4%, Vanadium 1%
2 Tungsten 18%, Chromium 4%, Vanadium 1%
3 Tungsten 14%, Chromium 4%, Vanadium 1%
Cutting tools are made out of it because it is very
hard but becomes soft at low critical temperature.
This temperature is raised out of cutting process of
tool, then the cutting tool becomes useless and is
unfit for work. But due to high percentage of
tungsten it keeps working upto high temperature. It
is used for cutting tools, drilling, cutter, reamers,
hacksaw blades etc.
b Nickel Steel: In this 0.3% carbon and 0.25 to
0.35% nickel is pressent. Due to nickel its tensile
strength, elastic limit and hardness is increased. It
does not catch rust. Its cutting resistance in-
creases 6 times more than plain carbon and steel
due to 0.35% nickel present in it. This is used for
making rivets, pipes, axle shafting, parts of buses
and aeroplanes. If 5% of cobalt is mixed with 30-
35% nickel, it becomes invar steel. It is mainly used
for making precious instruments.
c Vanadium Steel: It contains 1.5% carbonm 12.5%
tungsten, 4.5% chromium, 5% vanadium and 5%
cobalt. Its elastic limit, tensile strength and ductility
is more. It has strength to bear sharp jerks. It is
mainly used to manufacture of tools.
d Manganese Steel: It is also called special high
alloy steel. It contains 1.6 to 1.9% of manganese
and 0.4 to 0.5% carbon. It is hard and less wear. It
is not affected by magnet. It is used in grinders and
rail points etc.
e Stainless Steel: Along with iron it contains 0.2 to
90.6% carbnon, 12 to 18% chromium, 8% nickel
and 2% molybdenum. It is used for making knives,
scissors, utensils, parts of aeroplane, wires, pipes
and gears etc.
Properties of stainless steel:
1 Higher corrosion resistance
2 Higher cryogenis toughness
3 Higher work hardening rate
4 Higher hot strength
5 Higher ductility
6 Higher strength and hardness
7 More attractive appearance
8 Lower maintenance
f Silicon Steel: It contains 14% of silicon. Its uses
are multiferrous according to the percentage of
silicon. 0.5% to 1% silicon, 0.7 to 0.95% manganese
mixture is used for construction work. 2.5 to 4%
silicon content mixture is used for manufacturing
electric motors, generators, laminations of
transformers. In chemical industries 14% silicon
content mixture is used.
g Cobalt Steel: High carbon steel contains 5 to 35%
cobalt. Toughness and tenacity is high. It has
magnetic property therefore used to made perma-
nent magnets.
Workshop Calculation And Science : (NSQF) Exercise 1.7.20
© NIMI, Not to be republished

53
Types of ferrous and non ferrous Exercise 1.7.21
Ferrous and Non ferrous alloys
Alloying metals and ferrous alloys
An alloy is formed by mixing two or more metals together
by melting.
For ferrous metals and alloys, iron is the main constituent
metal. Depending on the type and percentage of the
alloying metal added, the property of the alloy steel will
vary.
Metals commonly used for making alloy steels
Nickel (Ni)
This is a hard metal and is resistant to many types of
corrotion rust.
It is used in industrial applications like nickel, cadmium
batteries, boiler tubes, valves of internal combustion en-
gines), engine spark plugs etc. The melting point of nickel
is 1450°C. Nickel can be magnetised. In the manufacture
of permanent magnets a special nickel steel alloy is used.
Nickel is also used for electroplating. Invar steel contains
about 36% nickel. It is tough and corrosion resistant.
Precision instruments are made of Invar steel because it
has the least coefficient of expansion.
Nickel-steel alloys are available containing nickel from 2%
to 50%.
Chromium (Cr)
Chromium, when added to steel, improves the corrosion
resis-tance, toughness and hardenability of steel. Chro-
mium steels are available which may contain chromium
up to 30%.
Chromium, nickel, tungsten and molybdenum are alloyed
for making automobile components and cutting tools.
Chromium is also used for electroplating components.
Cylinder liners are chrome-plated inside so as to have wear
resistance properties. Stainless steel contains about 13%
chromium. Chromium-nickel steel is used for bearings.
Chrome-vanadium steel is used for making hand tools like
spanners and wrenches.
Manganese (Mn)
Addition of manganese to steel increases hardness and
strength but decreases the cooling rate.
Manganese steel can be used to harden the outer surface
for providing a wear resisting surface with a tough core.
Manganese steel containing about 14% manganese is
used for making agricultural equipment like ploughs and
blades.
Silicon (Si)
Addition of silicon for alloying with steel improves resis-
tance to high temperature oxidation.
This also improves elasticity, and resistance against
corrosion. Silicon alloyed steels are used in manufacturing
springs and certain types of steel, due to its resistance to
corrosion. Cast iron contains silicon about 2.5%. It helps
in the formation of free graphite which promotes the
machine-ability of cast iron.
Tungsten (W)
The melting temperature of tungsten is 3380° C. This can
be drawn into thin wires.
Due to this reason it is used to make filaments of electric
lamps.
Tungsten is used as an alloying metal for the production of
high speed cutting tools. High speed steel is an alloy of
18% tungsten, 4% chromium and 1% vanadium.
Stellite is an alloy of 30% chromium, 20% tungsten, 1 to
4% carbon and the balance cobalt.
Vanadium (Va)
This improves the toughness of steel. Vanadium steel is
used in the manufacture of gears, tools etc. Vanadium
helps in providing a fine grain structure in tool steels.
Chrome-vanadium steel contains 0.5% to 1.5% chromium,
0.15% to 0.3% vanadium, 0.13% to 1.10% carbon.
This alloy has high tensile strength, elastic limit and
ductility. It is used in the manufacture of springs, gears,
shafts and drop forged components.
Vanadium high speed steel contains 0.70% carbon and
about 10% vanadium. This is considered as a superior high
speed steel.
Cobalt (Co)
The melting point of cobalt is 1495°C. This can retain
magnetic properties and wear- resistance at very high
temperatures. Cobalt is used in the manufacture of
magnets, ball bearings, cutting tools etc. Cobalt high
speed steel (sometimes known as super H.S.S.) contains
about 5 to 8% cobalt. This has better hardness and wear
resistance properties than the 18% tungsten H.S.S.
Molybdenum (Mo)
The melting point of molybdenum is 2620°C. This gives
high resistance against softening when heated. Molybde-
num high speed steel contains 6% of molybdenum, 6%
tungsten, 4% chromium and 2% vanadium. This high
speed steel is very tough and has good cutting ability.
Cadmium (cd)
The melting point of cadmium is 320°C. This is used for
coating steel components.
Alloying Metals and Non Ferrous Alloys
Non-ferrous Metals And Alloys
Copper and its alloys
Metals without iron are called non-ferrous metals. Eg.
Copper, Aluminium, Zinc, Lead and Tin.
© NIMI, Not to be republished
54
Copper
This is extracted from its ores ‘MALACHITE’ which con-
tains about 55% copper and ‘PYRITES’ which contains
about 32% copper.
Properties
Reddish in colour. Copper is easily distinguishable be-
cause of its colour.
The structure when fractured is granular, but when forged
or rolled it is fibrous.
It is very malleable and ductile and can be made into sheets
or wires.
It is a good conductor of electricity. Copper is extensively
used as electrical cables and parts of electrical apparatus
which conduct electric current.
Copper is a good conductor of heat and also highly
resistant to corrosion. For this reason it is used for boiler
fire boxes, water heating apparatus, water pipes and
vessels in brewery and chemical plants. Also used for
making soldering iron.
The melting temperature of copper is 1083
o
C.
The tensile strength of copper can be increased by ham-
mering or rolling.
Copper Alloys
Brass
It is an alloy of copper and zinc. For certain types of brass
small quantities of tin or lead are added. The colour of brass
depends on the percentage of the alloying elements. The
colour is yellow or light yellow, or nearly white. It can be
easily machined. Brass is also corrosion-resistant.
Brass is widely used for making motor car radiator core and
water taps etc. It is also used in gas welding for hard
soldering/brazing. The melting point of brass ranges from
880 to 930
o
C.
Brasses of different composition are made for various
applications. The following table-1 gives the commonly
used brass alloy compositions and their application.
Bronze
Bronze is basically an alloy of copper and tin. Sometimes
zinc is also added for achieving certain special properties.
Its colour ranges from red to yellow. The melting point of
bronze is about 1005
o
C. It is harder than brass. It can be
easily machined with sharp tools. The chip produced is
granular. Special bronze alloys are used as brazing rods.
Bronze of different compositions are available for various
applications.
Lead and its alloys
Lead is a very commonly used non-ferrous metal and has
a variety of industrial applications.
Lead is produced from its ore ‘GALENA’. Lead is a heavy
metal that is silvery in colour when molten. It is soft and
malleable and has good resistance to corrosion. It is a
good insulator against nuclear radiation. Lead is resistant
to many acids like sulphuric acid and hydrochloric acid.
It is used in car batteries, in the preparation of solders etc.
It is also used in the preparation of paints.
Lead Alloys
Babbit metal
Babbit metal is an alloy of lead, tin, copper and antimony.
It is a soft, anti-friction alloy, often used as bearings.
An alloy of lead and tin is used as ‘soft solder’.
Zinc and its alloys
Zinc is a commonly used metal for coating on steel to
prevent corrosion. Examples are steel buckets, galvanized
roofing sheets, etc.
Zinc is obtained from the ore-calamine or blende.
Its melting point is 420
o
C.
It is brittle and softens on heating; it is also corrosion-
resistant. It is due to this reason it is used for battery
containers and is coated on roofing sheets etc.
Galvanized iron sheets are coated with zinc.
Tin and tin alloys
Tin
Tin is produced from cassiterite or tinstone. It is silvery
white in appearance, and the melting point is 231
o
C. It is
soft and highly corrosion-resistant.
It is mainly used as a coating on steel sheets for the
production of food containers. It is also used with other
metals, to form alloys.
Example: Tin with copper to form bronze. Tin with lead to
form solder. Tin with copper, lead and antimony to form
Babbit metal.
Aluminium
Aluminium is a non-ferrous metal which is extracted from
‘BAUXITE’. Aluminium is white or whitish grey in colour. It
has a melting point of 660
o
C. Aluminium has high electrical
and thermal conductivity. It is soft and ductile, and has low
tensile strength. Aluminium is very widely used in aircraft
industry and fabrication work because of its lightness. Its
application in the electrical industry is also on the increase.
It is also very much in use in household heating appliances.
Workshop Calculation And Science : (NSQF) Exercise 1.7.21
© NIMI, Not to be republished

55
Difference between iron & steel, alloy steel & carbon steel Exercise 1.7.22
S.No Basic Iron Steel
distinction
1 Formation Pure substance Made up of
iron and
carbon
2 Types Cast iron, Carbon steel
Wrought and alloy
iron and steel steel
3 Rusting Quickly gets Have different
oxidised and elements that
result in rust protect from
rusting
4 Surface Its surface Its surface
is rusty stays shiny
5 Usage Used in Used in
buildings,tools buildings,
and automobiles cars,
railways
and
automobiles
6 Existence Available in Has to be
nature formed
Steel Plants in India
S.No Name of the Steel plant State
1 Tata Iron Bihar
2 Indian Iron Steel West Bengal
3 Visweshvaraiah Iron Steel Karnataka
4 Bhilai Steel Plant Madya Pradesh
5 Durgapur Steel Plant West Bengal
6 Alloy Steel Plant (Durgapur) West Bengal
7 Bokaro Steel Plant Bihar
8 Rourkela Steel Plant Orissa
9 Salem Steel Plant Tamilnadu
10 Vishakapatnam Steel Plant Andhra Pradesh
Difference between iron and steel:
Comparison of the Properties of Cast Iron, Mild Steel and steel
Property Cast Iron Mild Steel Steel
Composition Carbon contents Carbon contents Carbon contents
from 2 to 4.5% from 0.1 to 0.25% from 0.5 to 1.7%
Strength – High – Moderate – High
compressive strength compressive strength compressive strength
– Poor tensile strength – Moderate tensile strength – High tensile strength
– Poor shearing strength – High shearing strength – High shearing strength
Malleability Poor High High
Ductility Poor High High
Hardness Moderately hard and can be Mild Hard
hardened by heating to
hardening temperature and
quenching
Toughness Possesses poor toughness Very tough Toughness varies
with carbon content
Brittleness Brittle Malleable Malleable
Forgeability Cannot be forged Can be forged Can be forged
Weldability Cannot be welded with Can be welded very easily Can be welded
difficulty
Casting Can be easily cast Can be cast but not easily Can be cast
Elasticity Poor High High
© NIMI, Not to be republished

56
Ferrous metals Non Ferrous metals
1 Iron content is more 1 Iron content is missing
2 The melting point is 2 The melting point is low.
high
3 This is of brown and 3 This is of different colours
black colour
4 This catches rust 4 This doesn’t catch rust.
5 This can be 5 This cannot be
magnetised magnetised
6 This is brittle in cold 6 This becomes brittle in hot
state. state.
Difference between cast Iron and steel
Cast Iron Steel
1 Carbon content is high Carbon content is less
2 Carbon is in free state Carbon is mixed
3 Melting point is low Melting point is high
4 It cannot be magnetised It can be magnetised
5 Because it is brittle, In can be forged
it cannot be forged
6 It rusts with difficulty It rusts quickly
7 It cannot be welded It can be welded
Difference between metals and non-metals
Metals Non Metals
Shiny dull
Usually good conductors Usually poor conductors of
of heat and electricity heat and electricity
Most are ductile not ductile
Opaque (opposite of Transparent when as a thin
‘transparent’) sheet
Most are malleable Usually brittle when solid
Form alkaline oxides Form acidic oxides
Sonorous (make a bell not sonorous
-like sound when struck)
Usually have 1-3 valency Usually have 4-8 valency
electrons electrons
Most corrode easily
Usually high melting
points (usually solid at
room temperature
except for mercury)
Workshop Calculation And Science : (NSQF) Exercise 1.7.22
© NIMI, Not to be republished

57
Properties and uses of rubber, timber and insulating materials Exercise1.7.23
Properties and uses of rubber
Rubber
Rubber is an elastic material. It can be classified into
• Natural rubber
• Hard rubber
• Synthetic rubber
Natural rubber
It is obtained from the secretion of plants. It softens on
heating, becomes sticky at 30°C and hardens at about
5°C.
Sulphur is added to rubber and the mixture is heated. This
process is called vulcanising. By this process, stronger,
harder and more rigid rubber is obtained. Further, it
becomes less sensitive to changes of temperature and
does not dissolve in organic solvents. Its oxidisation is also
minimised by increasing its weathering properties.
By adding carbon black, oil wax, etc, the deformation
properties are minimised. Rubber is moisture-repellent and
possesses good electrical properties. The main disadvan-
tages of the rubber are as given under.
• Low resistance to petroleum oils.
• Cannot be exposed to sunlight.
• Cannot be used for high-voltage insulation.
• Low operating temperature (as it becomes brittle and
develops cracks at a temperature of 60°C)
• Sulphur in rubber reacts with copper. Hence, copper
wires are to be tinned.
Hard rubber
By increasing the sulphur content and prolonged
vuicanization, a rigid rubber product called hard rubber or
ebonite is obtained. It possesses good electrical and
mechanical properties.
Uses
It is used for battery containers, panel boards, bushing,
ebonite tubes, etc.
Synthetic rubber
This is similar to natural rubber and is obtained from
thermoplastic vinyl high polymers. Some of the important
synthetic rubbers are:
• Nitrite butadience rubber
• Butyl rubber
• Hypalon rubber
• Neoprene rubber
• Silicon rubber
Sl.No. Name Properties Uses
1 Nitrite Goods resilience, wear resistance, flexibility at low Automobile tyre inner tubes.
butadiene temperature, resistance to ageing, oxidation, low
rubber tensile strength, high thermal conductivity, low
hygroscopicity
2 Butyl It is attacked by petroleum oils, gases and alcoholic Used as insulation in hot and wet
solvents. It has thermal and oxidation stability and conditions, used as tapes in repair
high resistance to ozone. work.
3 Hypalon Resistance to deterioration when exposed to sunlight Used in jacketing of electric wires
rubber and temperature (up to 150°C). and cables
4 Neoprene Better resistance to ageing, oxidation and gas Used for wire insulation and cable
rubber diffusion, better thermal conductivity and flame sheating.
resistance, poor mechanical properties.
5 Silicon High operating temperature (200°C) flexibility, moisture Insulation for power cables and
and corrosion resistance, resistance to oxidation, control wires of blast furnace coke
ozone, arcing, good insulating properties and thermal ovens, steel mills and nuclear
conductivity. It is a good insulator. power stations high frequency
enerators, boiler, airport lighting
cranes.
© NIMI, Not to be republished
58
Workshop Calculation And Science : (NSQF) Exercise 1.7.23
Properties and uses of timber
General properties
Timber should have the following properties
• Straight fibres.
• Siliky lustre when planed.
• Uniform colour.
• Regular annual rings.
• Heaviness.
• Firm adhesion of fiber and compact modulary rays.
• Sweet smell.
• It should be free from loose or dead knots and shakes.
• The surface should not clog the teeth of the saw on
cutting but should remain bright.
Classification
• Timbers are classified as
a Softwood
b hardwood
Softwood timber
• Usually all trees with needle leaves of softwood and
those with broad leaves are of hard-wood.
• The wood contains resins and turpentines.
• The wood has a fragrant smell.
• Fibres are straight.
• Texture is soft and regular.
• Tough for resisting tensile stresses.
• Weak across the fibres.
• Annual rings are distinct, having one side soft, porous
and light coloured. The other side is dense and dark.
• The general colour of the wood is pale tinted or light
such as pine spruce, fir, ash, kail, deodar etc.
Properties of hardwood
• The wood generally contains a large percentage of acid.
• It is brightly coloured.
• Annual rings are not distinct.
• It is difficult and hard to work with.
• It resists shearing stress.
• Fibre are overlapped.
• The general colour is dark brown uch as oak, walnut,
teak, mahagony, sishim, babul, sal etc.
Uses
Soft timber
• Because of its cheapness it is used for low grade
furniture, doors and windows for cheap tyes of houses.
• Used as fuel.
• Some timbers are used for baskets and mat making.
• The bark is used as garment is some places.
Hard timber
• Used for high quality furniture such as chairs, tables,
sofas, dewans, beds, etc.
• Used for door, window frames for high quality houses as
they can take good polish and painting finish.
• Used for manufacturing katha.
Wood as an electrical insulator
Wood is impregnated with oil or other substance, for use
as insulator.
Example
It is used in electrical machine windings, as slot wedges.
Insulating materials
Description
These are the materials which offer very high resistance to
the flow of current and make current flow very negligible or
nil. These materials have very high resistance - usually of
may megohms (1 Megohm = 10
6
ohms) oer centimetre
cubed. The insulators should also posseses high dielectric
strength. This means that the insulating material should
not break down or puncture even on application of a high
voltage (or high electrical pressure) to a given thickness.
Properties of insulators
The main requirements of a good insulating material are:
• High specific resistance (many megohms/cm cube) to
reduce the leakage currents to a negligible value.
• Good dielectric strength i.e. high value of breakdown
voltage (expressed in kilovolts per mm).
• Good mechanical strength, in tension or compression
(It must resist the stresses set up during erection and
under working conditions.)
• Little deterioration with rise in temperature (The insulating
properties should not change much with the rise in
temperature i.e. when electrical machines are loaded.)
• Non-absorption of moisture, when exposed to damp
atmospheric condition. (The insulating properties,
specially specific resistance and dielectric strength
decrease considerably with the absorption of even a
slight amount of moisture.)
© NIMI, Not to be republished
59
Workshop Calculation And Science : (NSQF) Exercise 1.7.23
Products and insulators
Insulators Uses in electric field
1 Mica In elements or winding (Slot insulation)
2 Rubber Insulation in wires
3 Dry cotton Winding
4 Varnish Winding
5 Asbestos In the bottom of irons and kettles, etc.
6 Gutta Submarine cables
parcha
7 Porcelain Overhead lines insulators
8 Glass -do-
9 Wood dry Cross arms in overhead lines
10 Platic Wires insulation or switches body
11 Ebonite Bobbin of transformer
12 Fibre Bobbin making and winding insulation
13 Empire Winding insulation
cloth
14 Leathroid -do-
paper
15 Millimax -do-
paper
16 P.V.C. Wire insulation
17 Bakelite Switch etc. making, for insulation
18 Shellac -do-
19 Slate Making panel board
20 Paraffin Sealing
Wax
© NIMI, Not to be republished

60
Mass, units of mass, density and weight Exercise 1.8.24
m - mass of a body
g - acceleration due to gravity in metre/sec
2
= 9.81 m/
sec
2
V - volume of the body
ρ - density (pronounced as `rho')
W or FG - weight or weight force
Mass (Fig 1)
Mass of a body is the quantity of matter contained in a
body. The unit of mass in F.P.S system is pound (lb), in
C.G.S. system gram (gr) and in M.K.S and S.I systems
kilogram (kg). 1ton which is 1000 kg is also used sometimes.
The conversion factor is 1000. Three decimal places are
shifted during conversion.E.g.1 ton =1000 kg 1g = 1000 mg
Density
Density is the mass of a body per unit volume. Hence its
unit will be gr/cm
3
or kg/dm
3
or ton/m
3
.
Density =
System Absolute Derived Conversion
unit unit
F.P.S. system 1 poundal 1 Lbwt 32.2 poundals
(1 lb x 1 ft/sec
2
= 1 pound)
C.G.S. system 1 dyne 1 Gr.wt 981 dynes
1 gr x 1
cm/sec
2
M.K.S. Newton 1 kg.wt 1 Newton =
S.I.system Newton Newton 1 kg x 1 m/sec
2
1 kg.wt = 9.81 Newton 1 Newton = 10
5
dynes.
( approximately 10N)
Weight (Fig 2)
Weight is the force with which a body is attracted by the
earth towards its centre. It is the product of the mass of the
body and the acceleration due to gravity. The weight of a
body depends upon its location.
weight = W or FG = mass x gravitational force
= m x g
© NIMI, Not to be republished

61
Mass and weight are different quantities.
Mass of a body is equal to volume x density.
Weight force is equal to mass x acceleration
due to gravity.
Weight , Density and Specific gravity
It is now seen that the mass of a substance is measued by
its weight only without any reference to volume. But if equal
weights of lead & aluminium, are compared the volume of
lead is much smaller than volume of aluminium. So we can
now say that lead is more dense than aluminium,. i.e In
other words the density of lead is greater than aluminium.
(Fig 1)
Unit
The density is measured as below
MKS/SI= Kg/m
3
, CGS - 1 gm/cm
3
FPS–lbs/c ft
Difference between mass and weight
S. No Mass Weight
1 Mass is the quantity Weight is measure
of matter in a body (ie) of amount of force
measurement of acting on mass due
matter in a body to acceleration
due to gravity
2 It does not depend on It depends on the
the position or space position, location
and space
3 Mass of an object will Weight of an object
not be zero will be zero if gravity
is absent
4 It is measured using It is measured using
by physical balance by spring balance
5 It is a scalar quantity It is a vector quantity
6 When immersed in When immersed in
water mass does not water weight will
change change
7 The unit is in grams The unit is in
and kilogram kilogram weight,
a unit of force
Difference between mass & weight, density & specific gravity Exercise 1.8.25
The relation of mass and volume is called density.
The density expresses the mass of volume E.g. 1 dm
3
of
water has the mass of 1kg - thus the density of 1kg/dm
3
Solids gm/cc
3
Liquids gm/cc
3
1 Aluminum 2.7 Water 1.00
2 Lead 11.34 Petrol 0.71
3 Cast iron 6.8 to 7.8 Oxygen 1.43
4 Steel 7.75 to 8.05 Diesel Oil 0.83
The specific gravity of a substance is also called its relative
density.
Formula
Specific gravity
(or) Relative density
From the above table, we can calculate the weight of any
given volume of a substance (say Diesel oil) in any units
provided we know the specific gravity of the substance.
Also vice-versa for volume of density is known.
Comparision Between Density And Specific Gravity
(Relative Density)
Density Relative density or
Specfiic gravity
Mass per unit volume of The density of substance
a substance is called its to denisty of water at 4°C
density is its relative density
Its unit is gm per cu cm; It has no unit of measure-
lbs per cu.ft and kg/cubic ment simply expressed in
meter a number
Density = Relative density
Solids Sp.gy Liquids Sp.gy
1 Aluminium 2.72 Petrol 0.71
2 Lead 11.34 Battery acid 1.2 to 1.23
3 Cast iron 6.8 to 7.8 Water 1.00
4 Steel 7.82 Diesel Oil 0.83
© NIMI, Not to be republished

62
Related problems with assignment of mass, weight & density Exercise 1.8.26
1 Calculate the mass in kg of a rectangular steel plate of
dimensions 220 x 330 x 15 mm (Fig 1) (density of
steel = 7.82 gm/cm
3)
Mass = Volume x density
= 22 x 33 x 1.5cm
3
x 7.82 gm/cm
3
= 1089 cm
3
x 7.82 gm/cm
3
mass = 8.516 kg
2 A storage container holds 250 litres of water. What
weight in N will this amount of water exert on the surface
on which it is standing?(Fig 2)
( 1 litre of water = 1 kg of water )
Density of water 1 gm/cm
3
or 1 kg/dm
3
Acceleration due to gravity is taken as 10
metre/sec
2
(approximation).
Capacity = 250 litres = 250 dm
3
in volume.
Mass of water = volume x density of water
= 250 dm
3
x 1 kg/dm
3
= 250 kg
Weight extended = mass x acceleration due to gravity
= 250 kg x 10 metre/sec
2
= 2500 kg.metre/sec
2
= 2500 N( 1 kg.m/sec
2
=1N)
3 A force of 15 dynes acting on a mass of `m' produces
an acceleration of 2.5 cm/sec
2
. Find the mass.
1 Gr. wt. = 981 dynes
15 dynes = Gr.wt
Force = m x acceleration produced by the force
Gr.wt = mass x 2.5 cm/sec
2
gr.cm/sec
2
= mass x 2.5 cm/sec
2
mass = grams =
mass = 0.00612 gram
4 A force of 2 N acts on a mass of 10 kg. Find the
acceleration produced by the force on the mass.
Force = 2 N ( 1 N = 1kg.m/sec
2
)
Force = mass x acceleration
2 kg.metre/sec
2
= 10 kg x acceleration produced
2 x 1 kg.metre/sec
2
= 10 kg x acceleration
produced
acceleration produced = metre/sec
2
= 0.2 metre/sec
2
5 Calculate the weight of a body having a mass of 1 kg if
the acceleration due to gravity is 9.81 metres/sec
2
Weight force = mass x acceleration due to gravity
= 1 kg x 9.81 metres/sec
2
(1 kg.metre/sec
2
= 1 N)
9.81 kg metre/sec
2
= 9.81 N
In the examples solved the value of `g' is taken
as 10 metre/sec
2
, unless specifically men-
tioned otherwise.
• The outside and inside diametres of a hollow sphere are
150 & 70mm respectively. Calculate its mass if the
density of material is 7.5 gm/cm
3
.
(Fig 3)
Mass = Volume x Density
= Volume x 7.5 gm/cm
3
D= 150 mm = 15cm R= 7.5 cm
© NIMI, Not to be republished

63
Workshop Calculation And Science : (NSQF) Exercise 1.8.26
d = 70mm = 7 cm r = 3.5 cm
=1587.5 cm
3
Mass
= 1587.5 cm
3
X 7.5 gm/cm
3
= 11906.6 gm=11.9kg say 12kg
6 A car has a mass of 800 kg. Find out its weight force
(Take 9.81 m/sec
2
)
( 1n = 1kg.m/sec
2
)
The Wt. force of a car=Mass of car x gravitational
acceleration
= 800 x 9.81 N
= 7848 Newtons
7 A cylindrical tank 2m dia x 3.5 m deep is filled with
petrol. Find the weight of petrol in Tonnes, Assume
density of petrol 720 Kg/m
3
.(Fig 4)
Volume of Tank
3.14 x 3.5 m
3
= 10.99 m
3
Since 1 m
3
= 1000 litres
Volume of Tank = 10.99 x 1000 litres
Density of petrol = 720 Kg/m
3
.
Weight of Petrol in Kg =10.99x1000 litresx720Kg
= 720 x 10990 Kg
Weight of Petrol in Tonnes
(Metric Units)
Weight of Petrol = 7912.8 Tonnes
8 If the battery acid specific gravity is 1.3, and this is
being filled up into a cylindrical tank. Find out its
density.
(Density of water = 1000 gm/cm
3
)
Specific gravity or Relative density
Now, density of battery acid
= Specific gravity x Density of water
= 1.3 x 1000 gm/cm
3
= 1300 gm/cm
3
Determination of specific gravity of a substance
The specific gravity of a substance may be determined by
1 Arche medies principle
2 Hydrometer
Archemedes Principle
Archemedes principle states that when a body is fully or
partially immersed in a liquid, the amount of liquid dis-
placed by the body is equal to the loss of weight of the body
in the liquid.
Weight of a body in a liquid = total weight of the body
- weight of the liquid displaced by the body
This quantity if it is zero then the body will float. It is
negative the body will rise up till the weight of liquid
displaced by the immersed portion of the body is equal and
equal to the weight of the body. If it is positive the body will
sink. Specific gravity of solids in soluble in water
specific gravity of solids soluble in water
specific gravity of a liquid
The solid chosen should be such that it is
insoluble in both water and the liquid whose
specific gravity is to be determined.
Example
1 An iron piece weighs 160 kgf in air and 133 kgf when
it is fully immersed in water. Determine the volume and
specific gravity of the iron piece.
Weight of the solid in air = 160 kgf
Weight of the solid in water = 133 kgf
∴Loss of weight in water = 27 kgf
By Archemedies principle the loss of weight of a solid
in water = volume of water displaced.
∴Volume of water displaced = 27 cm
3
© NIMI, Not to be republished

64
Workshop Calculation And Science : (NSQF) Exercise 1.8.26
Sl.No Metal Density gm/cc
1 Aluminium 2.7
2 Cast Iron 6.8 - 7.8
3 Copper 8.92
4 Gold 19.32
5 Iron 7.86
6 Lead 11.34
7 Nickel 8.912
8 Silver 0.5
9 Steel 7.75 - 8.05
10 Tin 7.31
11 Zinc 7.14
12 Diamond 3.51
13 Bismuth 9.78
14 Brass 8.47
15 Phosphrous 8.7 - 8.9
Bronze
16 Ice 0.93
17 Air 0.0013
18 Mercury 13.56
19 Petrol 0.71
20 Diesel 0.83
21 Kerosene 0.78 - 0.81
22 Water 1.0
∴Volume of the solid= 27 cm
3
Specific gravity of iron piece = 5.93
2 A metal piece weighs 6.5 kgf in air and 3.5 kgf in water.
Find its weight when it is fully immersed in a liquid
whose specific gravity is 0.8 and also the S.G of the
metal.
Weight of metal piece in air = 6.5 kgf
Weight of metal piece in water= 3.5 kgf
∴Loss of weight in water = 3.00 kgf (6.5 - 3.5)
∴Specific gravity of metal
By applying the principle of Archemedes the above
results are derived.
By using a hydrometer also, the specific gravity of a
liquid is determined. The most common type of
hydrometer is the Nicholson’s hydrometer which is a
variable weight but constant immersion type.
Specific gravity of a liquid
weight of hydrometer+ weight required to sink the =
in water up to the same mark.
Let the weight of the metal piece in the liquid = W
loss of weight of the metal in the liquid = 6.5 kgf_W
w = 6.5 kgf - 3 kgf x 0.8 = 4.1 kgf
loss of weight of the metal in the liquid = 4.1 kgf.
3 A solid of wax weighs 21 kgf in air. A metal piece
weighing 19 kgf in water is tied with the wax solid and
both are immersed in water and the weight was fund to
be 17 kgf. Find the specific gravity of wax.
Weight of wax in air = 21 kgf
Weight of metal and wax in water = 17 kgf
Weight of metal piece only in water = 19 kgf
weight of wax in water = (17 -19) kgf
= - 2 kgf
loss of weight of wax in
water = (21 - (-2)) kgf
= 23 kgf
specific gravity of wax
© NIMI, Not to be republished

65
ASSIGNMENT
1 l = 1800 mm
b = 65 mm
h = 12 mm
ρ = 7.85 g/cm
3
m = ______ kg
2 Capacity = 36 litres
d = 32 cm
H = ______ cm
3 D = 74 mm
d = 68 mm
l = 115 mm
ρ = 8.6 gm/cm
3
m = ______ gms
4D
1
= 80 mm
D
2
= 61 mm
d = 39 mm
L = 112 mm
l = 90 mm
ρ = 7.85 gm/cm
3
m = ______ kg
5 D = 44 mm
d = 20 mm
L = 120 mm
l
1
= 60 mm
l
2
= 40 mm
ρ = 7.85 gm/cm
3
m = ______ kg
6 L = 120 mm
B = 90 mm
b
1
= 60 mm
b
2
= 30 mm
d = 55 mm
H = 42 mm
h = 18 mm
ρ = 7.85 gm/cm
3
m = ______ kg
7 L = 200 mm
l
1
= 75 mm
l
2
= 50 mm
B = 80 mm
H = 110 mm
h = 45 mm
ρ = 2.7 gm/cm
3
m = ______ kg
8 V = 320 cm
3
ρ
= 8.9 gm/cm
3
g = 9.80665
metre/ sec
2
m = ______ kg
FG = ______ N
9 Capacity = 35 litres
g = 10 metres/sec
2
FG = ______ N
10 (m
1
) mass of chain
= 150 kg
Total FG = 8 KN
Load = ______ N
mass m
2
= _____kg
11 W (FG) = 22.5 N
V (volume) = ______
12 F = 250 d N
side of cube
= ______ mm
(cubical counter
weight balances `F')
Workshop Calculation And Science : (NSQF) Exercise 1.8.26
© NIMI, Not to be republished

66
13 unbalanced load in
the set up = 16 cN
ø of balancing weight
= 20 mm
l of balancing weight
= ______ mm
14 d
1
= 40 mm
m
1
= 9 x 10
–2
kg
r
1
= r
2
d
2
= 60 mm
FG
2
= ______ N
15 l x b = 1 m
2
FG = 7.85 x 10
–2
kN
s = ______ mm
16
F = 400 N
m = ______ kg
17 m
1
= 200 gms
FG = 16 N
F = ______ dN
18 R = 14 kN
m = ______ kg
19 V = 4 dm
3
FG = 10.8 daN
ρ = ______ gm/cm
3
20 l = 500 mm
b = 300 mm
H = 250 mm
ρ of oil = 0.9 gm/cm
3
m = 2.5 kg
h = ______ mm
21 Engine cooling
Data given
Water in Radiator = 10
litres
Find
Mass of water =
__________ kg
(Assume 1 litre = dm
3
in volume)
Density of water = 1 kg/
dm
3
22 Cylinder Liner Dimen-
sion
Data given
OD = 111 mm
ID = 103 mm
Length = 240 mm
Material = C.I
Density of C.I = 7.259
gm/cm
3
Find its mass___ in kg
23 Gudgeon Pin (Solid)
Data given
Dia = 200 mm
Length = 70 mm
Material = M.S
Density = 7.85 gm/cm
3
Find out its
Workshop Calculation And Science : (NSQF) Exercise 1.8.26
© NIMI, Not to be republished

67
24 Data given
Dia = 80 mm
Length = 100 mm
Density of Aluminum =
2.7 g/cm
3
Find its Mass _______
in kg
25 Hollow sphere (Cast
Brass)
Data given
O.D = 150 mm
I.D = 120 mm
Density of Brass = 6.89
gm/cc
Use Vol = ( (R
3
)
3
)
Find
Mass of Hollow sphere
= _______ kg
26 Diesel Tank
Data given
Diameter = 400 mm
Depth of filling (h)
= 600 mm
Spongy of oil = 0.8
Density of water = 1000
kg/m
3
Find
Mass of oil in Tank =
______ in kg
27 Definition
Define the following term
a Mass
b Weight
c Density
d Specific gravity
28 Conversion of vehicle weights
Take g = 10 m/sec
2
Weight force Mass
a 480 Newton _______
b 14800 N _______
c 2000 N _______
d 7000 N _______
29 Covnersion of mass of vehicle
Take g = 9.81 m/sec
2
Mass of Vehicle Its weight
a 1200 kg ________ N
b 800 kg ________ N
c 700 kg ________ N
d 900 kg ________ N
30 Fill up the blanks
Comparison of Metals & Liquids
Material Sp.gy Density
a Lead 11.34 _______
b Copper 8.92 _______
c Cast Iron 7.20 _______
d Petrol 0.71 _______
e Diesel 0.83 _______
f Sulphuric
Acid 1.84 _______
31 Fill in the blanks with correct statement in a & b
a The density of water - 1000 kg/m3 specific gravity of
nitric acid = 1.2. The density of nitric acid = _______
b Material Density Specific gravity
i Water 1000 kg/m
3
_______
ii Aluminium 2.7 g/cm
3
_______
iii Iron 8 g/cc _______
iv Copper 8.7 g/cc _______
c Mass of a body = Volume x _______
d Weight force = Mass x _______
e Give abbreviation for
i Mega newton _______
ii Kilo newton per square metre _______
f 1 litre of water = _______kg.
Workshop Calculation And Science : (NSQF) Exercise 1.8.26
© NIMI, Not to be republished

68
Rest, motion, speed, velocity, difference between speed & velocity,
acceleration & retardation Exercise 1.9.27
Body at rest
When a body does not change its position, with respect to
its surroundings, it is said to be at rest.
Body at motion
When a body changes its position, with respect to its
surroundings, it is said to be in motion. The motion may
be linear if the body moves in a straight line or it may be
circular when it moves in a curved path.
Terms relating to motion
Displacement
When a body is in motion from one place to another, the
displacement is the distance from the starting position to
the final position.
Speed
It is the rate of change of displacement of a body in motion.
It has got no direction and it is a scalar quantity.
Speed = distance travelled per unit time
Unit = m/s, km/Hr.mile/Hr.
Velocity
It is the rate of change of displacement of a body in motion
in a given direction. It is a vector quantity and can be
represented both in magnitude and direction by a straight
line. Velocity may be linear or angular. The unit of linear
velocity is metre/sec,
Velocity =
Unit = m/s, km/Hr,mile/Hr.
Difference between speed & velocity
unit = m/s
2
(metre per square second)
u = Initial velocity in metre per second(m/sec)
v = Final velocity in metre per second(m/sec)
s = Distance in metre (m)
t = Time in second (sec)
a = Acceleration m/sec
2
(positive value)
R = Retardation m/sec
2
(negative value of acceleration)
Equations of motion
Then v = u + at
s = ut + at
2
and v
2
– u
2
= 2as
v
2
= u
2
+ 2as
Retardation
When the body has its initial velocity lesser than its final
velocity it is said to be in acceleration. When the final
velocity is lesser than the initial velocity the body is said to
be in retardation. Then the three equation of motion will be
v = u – at
s = ut – at
2
u
2
–v
2
= 2as
Average speed
Vm - Average speed in metre/min, (metre/sec)
n - Revolutions per minute or number of strokes per
minute
s - Distance travelled, length of stroke.
Stroke speed (Fig 1)
For one revolution of the point k, of the crank pin the
distance the power saw blade moves = 2 x s
Therefore ‘n’ revolutions in a minute the distance = 2 x s x
n. Since the stroke of the blade will be given in metre to
determine the average speed
Vm = 2 x s x n
Piston speed (Fig 2)
Acceleration
Rate of change of velocity is known as acceleration or it is
the change of velocity in unit time. Its unit is metre/sec
2
.
It is a vector quantity.
m/sec
2
Speed
The rate of change
place of an object is
its speed.
In the speed, direc-
tion is not indicated.
Only the magnitude is
expressed.
Speed
Velocity
The speed in a definite direc-
tion is called velocity.
Both the magnitude and di-
rection are expressed.
Velocity
As the piston moves backward and forward, its speed
constantly changes between the upper and lower dead
centres. Hence in this case also the average speed Vm =
© NIMI, Not to be republished

69
2 x s x n. Since s is expressed in mm and n in number of
revolutions/per minute and since Vm is given in metre/sec,
we have
Vm = 2 x s x
metre/min.
=
If s is given in metres then
Vm = 2 x s x = s x metre/sec.
2 x s denotes a double stroke.
In case of the reciprocating motion the average
speed is taken into account for calculations.
Vm = 2 x s x n metre/min if s is given in metres
Example (Fig 3)
An extrusion press has a crank radius of 20 cm and an rpm
of 30/min. Calculate the average speed in metre/min,
metre/sec.
s = The diameter = 40 cm.
One crank revolution makes the piston to travel in 2s=80cm
Vm = 2 x 400 x metre/min.
= 24 metre/min = 0.4 metre/sec
NEWTON’S LAWS OF MOTION
Motion under gravity
A body falling from a height, from rest, has its velocity goes
on increasing and it will be maximum when it hits the
ground. Therefore a body falling freely under gravity has a
uniform acceleration. When the motion is upward, the
body is subjected to a gravitational retardation. The
acceleration due to gravity is denoted with ‘g’.
Momentum
It is the quantity of motion possessed by a body and is
equal to the product of its mass, and the velocity with which
it is moving. Unit of momentum will be kg metre/sec.
Momentum = mass x velocity
Newton’s laws
First law
Every body continues to be in a state of rest or of uniform
motion in a straight line unless it is compelled to change
that state of rest or of uniform motion by some external
force acting upon it.
Second law
The rate of change of momentum of a moving body is
directly proportional to the external force acting upon it and
takes place in the direction of the force.
Third law
To every action there is always an equal and opposite
reaction.
In the rivet joint equal forces act on the strap and they
opposite force F
2
. (Fig 4)
Law of conservation of momentum
When two moving bodies have an intentional or uninten-
tional impact, then sum of the momentum of the bodies
before impact = sum of the momentum after impact, or the
change in momentum after the impact is zero.
m
1
- mass of one body and
v
1
- velocity with which it moves
m
2
- mass of second body
v
2
- velocity with which it moves
Momentum = m x v= mass of the body x its velocity
Rate of change of momentum = force acting on the body
force = mass x acceleration
Workshop Calculation And Science : (NSQF) Exercise 1.9.27
Equations of motions under gravity
Upward Downward
V = u – gt v = u + gt
s = ut – gt
2
s = ut + gt
2
u
2
–v
2
= 2gs v
2
–u
2
= 2gs
© NIMI, Not to be republished
70
Momentum of two bodies before impact = momentum after
impact
m
1
x v
1
+ m
2
x v
2
= (m
1
+ m
2
)V
Terms - Some Examples in vehicles
Displacement
The piston displacement is the space between 2 dead
centres (TDC and BDC) where in the piston moves in the
cylinder. (Fig 5)
Speed
This is reckoned in 2 ways in a vehicle
– Vehicle speed in kmh/mph
– Engine speed in rpm
Velocity
A motor vehicle, normally changes its speed and direction
on road. Hence used in velocity calculation.
Acceleration (Fig 6)
When the speed of the vehicle is increased on road, it is
said to be accelerated.
Deceleration (Fig 6)
Deceleration or Retardation (this is further explained)
During the application of brakes of a vehicle the speed of the
vehicle is decreased. Then it is said to be decelerated or
retarded.
Example
In circular motion bodies (like shafts, axles, gear-wheels,
pulleys, flywheels, grinding wheels) turn with constant
speed around its axis.
The angular of circular motion is also called Angular
velocity or Peripheral speed.
Expressed in Metre/sec or Radians per second.
Bodies at rest and in motion
Terms related to brake system
Every vehicle has a brake system. When brakes are
applied on a moving vehicle (with certain velocity) its
velocity is reduced and vehicle is decelerated and it stops
at a certain distance. So the definition of the terms related
to Brake application are set forth below.
Deceleration (a) (Fig 8)
Workshop Calculation And Science : (NSQF) Exercise 1.9.27
Circular or Angular motion (Fig 7)
When a body rotates about an axis, it is said to have
angular motion or circular motion.
This is the decrease in velocity within a certain time. e.g
A car travelling at 90 kmph stops after 10 Sec.
The deceleration = 90 x x 1/10
= 25 m/s/10 sec
= 2.5 m/sec
2
Deceleration time
The time 10 seconds is called the above time to stop the
vehicle.
© NIMI, Not to be republished

71
Stopping distance
During the deceleration time the car travels a distance
called i.e Stopping distance ‘d’.
But the total stopping distance is reckoned as equal to
normal stopping distance and distance travelled by the car
during reaction time of the driver.
The reaction time is explained as below
During the application of brakes, the driver takes sometime
to recognise the danger and then to apply the brakes. The
time (thus elapsed) is called reaction time. During this time
the vehicle travels some more distance before coming to a
stop. So the total stopping distance actually varies due to
the reaction time of the driver and it is longer than the
normal stopping distance. The reaction time varies be-
tween driver to driver.
Example
A car is travelling with a speed of 72 kmph and its
aceleration (a) = 5 m/sec
2
. The reaction time of driver to
apply brakes is 1.5 seconds. calculate the total stopping
distance.
Solution
Velocity of car = 72 kmph
= 20 m/sec
aceleration = 5 m/sec
2
Normal stopping distance S = (m)
Total stopping distance
= 40 metre + Velocity x Reaction time
= 40 m + (20 x 1.5) m
= 70 metres.
Newon's Law of Motion
Some Examples in vehicles
First law (with examples) (Fig 9)
Bodies at rest or in Uniform motion
The diesel engine piston remains at rest at TDC or BDC due
to its inertia. Expansion of gas pressure or flywheel
momentum moves the piston from TDC or BDC.
Second law (with examples) (Fig 10)
The rate of change of momentum of a moving body (say
Engine part or Vehicle)is directly proportinal to external
force acting take place in the direction of force.
– A connecting rod in motion is brought to rest at BDC.
– The direction of movement of a vehicle is altered by force
of wind.
– When a vehicle travels in a down gradient its speed
increases.
– The speed of vehicle is decreased when travelling up
gradient.
Workshop Calculation And Science : (NSQF) Exercise 1.9.27
Third law (with examples) (Fig 11&12)
To every action there is always an equal and opposite
reaction.
All upward force = All downward forces
– Jack is lifting a differential
– Crane rope is lifting an engine.
© NIMI, Not to be republished

72
2
0.3m/sec
sec 45
m/sec 13.88
t
v
a
m/sec 13.88 m/sec
18
5
x50km/hr
Related problems with assignment of speed & velocity Exercise 1.9.28
Examples
• A body travels a distance of 168 metres in a straight line
in 21 secs. What velocity the body is travelling.
Velocity = distance travelled/time
= 168 metres/21 secs
= 8m/sec
• A train covers a distance of 150 kilometres, between
two stations, in 2 1/2 hours. Determine the average
velocity with which the train is moving.
Average velocity = Distance travelled/time taken
= 150 Km/2 1/2 hrs =
5
2
150
2
5
150
Km/hr
= 60 Km/hr
• A vehicle accelerates uniformly from a velocity of 8 km/
hr to 24 km/hr in 4 secs. Determine the acceleration
and the distance travelled by it during that time.
Initial velocity = 8 km/hr (u)
Final velocity = 24 km/hr (v)
time = 4 sec (t) acceleration (a)
v = u + at
24 km/hr = 8 km/hr + a x 4 sec
(24km/hr - 8km/hr - 16km/hr)
4a sec = 16 km/hr = 16000 metre/3600 sec
acceleration (a) = 16000 metre/3600 x 4 sec
2
v-u = 4.44 m/sec a=
Acceleration (a) = 1.1 metre/sec
2
• A car moving with a velocity of 50 km/hr is brought to
rest in 45 secs. Find out the retardation.
Initial velocity = 50 km/hr (1km= 1000 metres)
Final velocity = 0 km/hr (1 Hour = 3600 seconds)
Time = 45 secs
v = u – at
0 = u – at
u = at
50000/3600 metre/sec = a x 45 sec
Retardation = 50000/3600 x 45 metre/sec
2
= 0.30 metre/sec
2
• A body falling freely under the action of gravity reaches
the ground in one second. Determine the height from
which the body fell. Take g = 9.81 metre/sec
2
.
Initial velocity = 0 metre/sec (U)
Acceleration due to gravity = 9.81 metre/sec
2
(g)
Time taken = 1 sec (t)
= ut + gt
2
0 x 1 sec= x 9.81 m/sec
2
x 1
2
sec
= 0 x 1 sec + x 9.81 metre/sec
2
x 1 sec
2
1 Sec
2
= 4.905 metres. s = 4.905 metres
• A force of 30 N acts on a body at rest. The mass
of the body is 50 kg. Determine the velocity of the body
after 4 secs, the distance it covers during that period
and the acceleration
F = m x a
30 N = 50 kg x a
3 kg x metre/sec
2
= 50 kg x a
∴ acceleration = 3/50 metre/sec
2
= 0.06 metre/sec
2
a = 0.06 m/sec
2
v = u + at
= 0 + 0.06 metre/sec
2
x 4 sec = 0.24 metre/sec
s = ut + 1/2 at
2
= 0 + 1/2 x 0.06 metre/sec
2
x 16 sec
2
= 0.48 metre s = 0.48 metre
• A stone is thrown vertically upwards with a velocity
of 120 metre/sec. Determine (a) the maximum height
to which it travels before starting to return to earth. (b)
The total time taken by the stone to go up and come
down. (c) The velocity with which it will strike the
ground.
Initial velocity of throw = 120 metre/sec (u)
Final velocity = 0 metre/sec (v) (taken g = 10 m/sec
2
)
Retardation due to gravity = 10 metre/sec
2
u
2
–v
2
= 2g.s
∴ 120
2
metre
2
/sec
2
– 0 = 2 x 10 metre/sec
2
x s
∴ s = 120 x120/2 x 10 metre
= 720 metre
when it comes down its velocity at start = 0 metre/sec.
The acceleration due to gravity = 10 metre/sec
2
and the
distance travelled = 720 metre
∴ v
2
– u
2
= 2as v
2
-0 = 2x 10 m/sec
2
x 720 m
v
2
– 0 = 2 x 10 x 720 metre
2
/sec
2
22
sec/m 14400v
∴ v = 120 metre/sec
Time taken to go up and reach a velocity of 0 metre/sec
= u/g = 120 metre/sec/10 metre/sec
2
= 12 sec.
Time taken to start from rest and attain a velocity of 120
metre/sec = v/g = 12 sec.
∴ Total time taken = 24 sec.
© NIMI, Not to be republished

73
Workshop Calculation And Science : (NSQF) Exercise 1.9.28
• Calculate the Angular velocity in radian/second of
an engine flywheel when it is rotating at 2800 rpm.
(Fig 1 & 2)
Angular velocity (W) = This is the rate of change of
displacement or angle turned through per unit time.
Solution
Angular velocity of flywheel W= 2πN/60 rad/sec.
[N = 2800 rpm]
= 2π x 2800/60 radian/sec.
= 293.3 radian/sec.
• A motor car road wheel of dia 540 mm turns through an
angle of 120°. Calculate the distance moved by a point
on tyre thread of the wheel.
Solution
There are 2π radians in one turn of wheel. i.e 2π radians
= 360°
Since wheel turns 120° angle, 120° = 120 x 2π/360
= 2.094 radians
Distance moved by a point on tyre S – re
[where r = 270 mm
θ = 2.094 radian]
S = 270 x 2.094 mm
= 565.38 mm
Circumferential distance moved by the point = 565.38
mm
• The rear wheels of a car have diameter of 600 mm. The
rear axle makes 250 rpm. Find out the peripheral speed
of rear wheels in m/sec.
Solution
Peripheral speed V =
(m/s)
6
1
1000
πdN
m/sec7.85
1000
250
60
6003.14
• Calculate the stopping distance of a car travelling with
a speed of 72 km/h and being accelerated with
a - 5 m/ sec
2
.
Solution
Va (initial speed of a car) = 72 kmph
m/sec
18
5
72 m/sec)
3600
1000
kmph (1
= 20 metres/sec
Stopping distance S =
2a
Va
2
(metre)
40metre
10
400
52
20
2
© NIMI, Not to be republished

74
ASSIGNMENT
1 S = 180 mm
n = 65 (double stroke)
Vm = _____metre/min
Vm is average cutting
speed)
2 V = 16 metre/min
s = 210 mm
n = ______
(V is the cutting speed)
3 n = 22 strokes (Double
stroke)/min
V = 18 metre/min
s = ______ mm
4 s = 240 mm
n = 30 (working stroke)
V = ______ metre/min
5 n = 50 cutting strokes
V = 32 metre/min
d = ______ mm
6 s = 64 mm
n = 3600 rpm
Vm = _____metre/sec
Vm is the average
piston speed)
7 Vm = 0.35 metre/sec
s = 200 mm
n = ______ rpm
8
s = 650 mm
Vm = 90 metre/min
n = ______ rpm
9Vm
1
= 5.2 metre/sec
Increased to
Vm
2
= 6.3 metre/sec
Increase in n (rpm) = ______ %
10 s = 250 mm
n = 45 (double
strokes)
V = ______metre/min
11 Is : Vm = 25 : 1
n =_____(double
strokes)
Is = rack travel
Vxm = stroke speed/
min
12 Vm = 10 metre/min.
n = 12.5 / min.
Rack travel = ______
13 dia of crank = 100 mm
Rack
speed = 12 metre/min
Crank disc 'n'' = _____
rpm
Workshop Calculation And Science : (NSQF) Exercise 1.9.28
© NIMI, Not to be republished

75
14 spindle 'n' = 250 rpm
Average stroke speed
= 30 metre/min
stroke length =
________mm
15 Car Speed = 90 km/hr
Time to stop = 10 sec
Deceleration
= ________ metre/
sec
2
16 Car speed = 80 km/hr
Distance stopped = 60
metre
Deceleration of car
=________ metre/sec
2
17 Deceleration = 4.5 m/
sec
2
Stopping distance = 50
metres
Velocity of car
=________ km/hr
18 Distance travelled by
car = 600 km
Time = 8 hrs 20 min
Average velocity
= ________ km/hr
19 Average velocity
= 56.3 km/hr
Distance travelled
= 464.475 km
Travelling time
= ________ hrs
20 Car wheel
n = 720 rpm
Peripheral speed
= 18.84 m/sec
d = ________
21 Angular speed
n = 2000 rpm
Angular velocity
= _______ radians/sec
Use
W = 2πN/60 rad/sec
22 Piston Velocity/Speed
S = 74 mm
n = 4500 rpm
Mean velocity
= _______ m/sec
Maximum velocity
5= _______ m/sec
(Average Speed of
Piston)
23 Total Stopping Distance
time reactionvelocity
2a
V
2
(Use = V
2
/2a)
V = Vehicle speed = 80 km/hr
Deceleration = 5m/sec
2
Reaction Time of driver = 2 seconds
Total Stopping Distance = _______ meter
Workshop Calculation And Science : (NSQF) Exercise 1.9.28
© NIMI, Not to be republished

76
Units of work, power and energy, horse power of engines and
mechanical efficiency Exercise 1.10.29
F - force or weight force in N
s - distance the body on which force acts is moved in
metres
t - time in seconds
v - speed in metre/sec
w - work done by the force in joules
P - Power in Watts
P
out
- Power output
P
in
- Power input
Force
A Force is that which changes or tends to change the state
of rest or motion of a body.
Force = Mass x Acceleration
F= Ma
Unit
F = M x a
= kg x m/sec
2
= 1 Newton (SI unit)
(Newton: If 1 kg of mass accelerates at the rate
of 1m/sec
2
then the force exerted on the mass
is 1 newton)
FPS = 1 pound x 1 Feet/second
2
= 1 pound
CGS = 1 gm x 1cm/second
2
= Dyne
Mks = 1 kg x 1m/second
2
= Newton.
1 Newton = 10
5
dynes
1kg wt = 9.81N
1 pound = 4.448N,
Newton = 0.225 pound.
Work(Fig 1)
Work is said to be done by a force, when it moves, its point
of application through a distance. Applied for ‘F’ moves a
body through a distance’s.
Work done ‘W’ = F x s.
The S.I. unit of work is 1 joule which is the work done by a
force of moving the body through a distance of 1 metre.
Therefore joule = 1 N x 1 metre = 1 Nm
Also 1 joule = 1 Nm = 10
5
dynes x 100 cm = 10
7
dynes cm
= 10
7
ergs.
Absolute units (Fig 2)
In C.G.S. system unit of work = 1 erg = 1 dyne x 1 cm
In F.P.S system unit of work = 1 Foot poundal = 1 poundal
x 1 foot
In M.K.S. system unit of work = 1 joule = 1 Newton x 1
metre
Derived units
C.G.S. system 1 Gm Wt x 1 cm = 981 ergs.
F.P.S. system 1 ft LB = 981 foot poundal
M.K.S. system 1 kgf metre = 981 joule.
Power(Fig 2)
It is the work done in unit time.
which is equal to 1 Watt. Power in watts = w/t = F.s/t
= F x V
In M.K.S. system the unit is 1 kgf meter/sec. One horse
power is = 75 kg metre/sec or 4500 kgf metre/min.
© NIMI, Not to be republished

77
1HP (metric) = 735.5 Watts
1HP (British) = 746 Watts = 0.746 KW
1 KW = 1.34 HP
Power input is the power given to a machine to do work.
Power output is what we get out of the machine. Power
output is always less than power input due to friction in the
machine. The ratio between power output to power input
is efficiency of the machine and it is expressed
percentage.(Fig 3)
100%
input power
output power
efficiency
Indicated Horse Power and Brake Horse Power
The power actually generated by the engine or generator is
the indicated horse power which is indicated on the plate.
The Brake horse power is the power available to do useful
work. B.H.P is always less than I.H.P. due to losses to
overcome frictional resistance.
100%
I.H.P
B.H.P
efficiency mechanical
Work done by a force = Magnitude of the force x distance
moved by the body
Power = Total work done / total time taken
100%
input power
output power
efficiency
Energy
The energy of a body is its capacity to do work. It is equal
to power x time. Hence the unit of energy is the same as
the unit of work in all systems.
Forms of energy
Mechanical energy, Electrical energy, Atomic energy,
Heat energy, Light energy, Chemical energy, sound en-
ergy. Energy of one form can be transformed into energy
of another form.
Law of conservation of energy
– The energy can neither be created nor destroyed.
– Total energy possessed by a body remains the
same.(Fig 4)
Depending upon the position of the body or body in motion,
mechanical energy possessed by the body may be
potential energy or kinetic energy respectively.
Potential energy
Potential energy is the energy possessed by a body by
virtue of its position (Fig 4). A body of mass ‘m’ kept at a
height ‘h’ from a datum possesses a potential energy of
mgh or Wh or Fh; where W or F are the Weight force. When
the body is allowed to fall it will be able to do a useful work
of Fh.
Example
• Water stored in a Tank
• Coil Spring.
Kinetic energy
It is the energy possessed by a body by virtue of its motion.
If a body of mass ‘m’ starting from rest attains a velocity of
‘v’ after covering a distance of ‘s’, by the action of an applied
force ‘F’, then work done on the body=F x s But F= m x a.
Therefore work done on the body = m x a x s.
But a x s =
2
V
2
because the body is starting from rest.
Therefore Work done on the body =
2
mv
2
1
.
Since work done on the body = The energy possessed by
the body
Kinetic Energy =
2
mv
2
1
.
Energy possessed by a body = work done on the body
Potential energy = mgh
Kinetic energy =
2
1
mv
2
If friction is neglected potential energy = Kinetic energy
Example
• Rolling vehicle
• Rotating fly wheel
• Flowing water
• Falling weight
Workshop Calculation And Science : (NSQF) Exercise 1.10.29
© NIMI, Not to be republished

78
Potential energy, kinetic energy and related problems with assignment
Exercise 1.10.30
Potential energy
Hammer head drops from height ‘h’ . m = 10 kg.
h = 1.4 m.
sec
metre
0u
V
2
= 2 gs
V
2
= 2 x 9.81 x 1.4
V
2
= 27.468
V
2
= 5.24 m/sec
= 10 kg x 9.81 metre/sec
2
x 1.4 metre (Fig 5)
= 137.3 N metre (
1N = 1kg.m/sec
2
)
K.E = x 10 kg x 5.24
2
2
2
sec
metre
= 137.3 N metre.
Examples
• A pulley is used to lift a mass with a force of 900 N to
a height of 10 metres in 2 minutes. Find the work done
by the force and also the power.(Fig 6)
Work done = F x s = 900N x 10 metre
= 9000 Nm = 9000 joules.
Power
120sec
joules 9000
t
W
watts75
sec
joules75
• Determine the horse power required to drive a lift in
raising a load of 2000 kgf at a speed of 2 metre/sec, if
the efficiency is 70%.
Useful workdone to raise the lift in 1 sec
Force = 2000 kgf
Work = F x d
1
22000
t
dF
Power
= 4000 w
Power output = 4000 w
Power input = Power output
w5714
0.7
4000
100%
Input
Output
HP7.67.659
746
5714
HP
Power input = 7.6 HP
• A mass of 100 gm is allowed to fall from a height of 10
metres. Determine the amount of Kinetic energy
gained by the body. (Take the value of g as 10 metre/
sec
2
Since inital velocity is 0 and distance travelled is 10
metres. final velocity
2
=V
2
= 2 x g x s = 2 x 10x10 metre
2
/sec
2
Joules. 10
ergs10 10
/secmetre gm 10000
/secmetres 200 gm 100
2
1
mv
2
1
K.E
7
22
222
K.E. developed by the vehicle at a constant speed
• A motor vehicle of one tonne is travelling at 60 km/hr.
Calculate K.E of the vehicle at this speed.
K.E of the vehicle =
2
1
mv
2
Where m = one tonne or 1000 kg
v = 60 km/hr
© NIMI, Not to be republished

79
Workshop Calculation And Science : (NSQF) Exercise 1.10.30
Solution
Changing v into meter/sec we get,
( 1km = 1000m)
( 1hour = 3600sec)
K.E.developed by a vehicle during acceleration
• A motor vehicle of 1200 Kg mass is being accelerated
36 km to 48 km/hr speed. Calculate the increase in K.E
during its acceleration.
Solution
Mass of motor vehicle= 1200 kg
K.E. of the vehicle at 36 km/hr speed
= x 1200 x 36
2
J KE= mv
2
J
v= 36km/hr =36 x = 10m/sec
K.E of the vehicle at 48 km/hr speed
= x 1200 x 48
2
J ( 1kg.m/sec
2
= 1N)
( 1Nm = 1J)
v= 48 km/hr =48 x= m/sec
KE = x1200 x 10 x10=60000J
KE = x1200 x x =106666.67 J
Increase in K.E of the vehicle = 106666.67 J - 60000J
= 46666.67 J
= 46.666 KJ.
Workdone in vehicle operation
The Mechanical Work performed by the motor vehicle for its
propellsion on road can generally be classified into two
major categories of work done.
– Workdone by the IC engine in developing full power
under all condition of speed and load.
– Workdone by the motor vehicle in performing different
operations on road like hill climbing/acceleration/brak-
ing/ towing and reversing operation
© NIMI, Not to be republished

80
ASSIGNMENT
1 m= 55 kg
a) s = 1.82 metres
W = ______ joules
b) s = 1.40 metres
W= ____ joules
c) s = 0.85 metres
W =Joules
2 t = 8 secs
a) P = _____ Watts
b) P = _____ Watts
c) P = _____ Watts
3 W = 1312.5 Joules
m = 350 kg
s = _____ metres
4 m = 75 kg
s = 100 metres
t = 12 secs
W = ______ Nm
P = ______ Watts
5 V = 1 m
3
/min
H = 2 m
η = 0.75
Power input = ______
kW
6 P = 12 kw
s = 4 metres
t = 20 secs
m = ______ kg
7 d = 3 metre
H = 2 metre
t = 20 minutes
s = 6 metres
P = ______ kW
Water filled in the
tank. s is the pumping
height
8 d = 200 mm
n = 750 rpm
F = 700 N
P = _____ kW
9 P input = 4 kW
P output
= 3450 Joules/sec
η = ______ %
10 Volume of water
‘V’ = 10 metre
3
H = 18 metres
t = 20 sec
η = 70 %
P output
= ______ kW
11 d = 225 mm
s = 450 mm
Piston pressure
‘P’ = 4.5 bar
V = 2.5 metre/sec
(piston speed)
η = 70 %
Power input
= _____ kW
12
‘V’ of water pumped
= 3 metre3/min
H = 6 metre
η = 0.8
Power input
= ______ kW
Workshop Calculation And Science : (NSQF) Exercise 1.10.30
© NIMI, Not to be republished

81
Heat
It is a form of energy. Heat energy can be transformed into
other forms of energies. Heat flows from a hotter body to a
colder body. (Fig 1)
Calorific value: The amount of heat released by the
complete combustion of unit quantity of the fuel (Mass or
volume) is known as calorific value of fuels.
Water equivalent
It is the mass of water which will absorb the same amount
of heat as the given substance for the same temperature
rise. Water equivalent = Mass of the substance x specific
heat of the substance.
Therefore water equivalent = ms
Types of heat
1 Sensible heat and
2 Latent heat
1 Sensible heat
Sensible heat is the heat absorbed or given off by a
substance without changing its physical state. It is
sensible and can be obsorbed by the variation of tempera-
ture in the thermometers.
2 Latent heat
The heat gained or given by the substance during a change
of state (from solid to liquid to gas) is called latent heat or
hidden heat. The heat absorbed or given off does not cause
and temperature change in the substance.
Types, 1. Latent heat of fusion of solid
2. Latent heat of vaporisation of solid.
1 Latent heat of fusion of solid
The amount of heat required per unit mass of a substance
at melting point to convert it from the solid to the liquid state
is called latent heat of fusion of solid. Its unit is cal/gram.
Latent heat of fusion of ice
The amount of heat required to convert per unit mass of the
ice into water at 0
0
C temperature is called latent heat of
fusion of ice.
Latent heat of fusion of ice(L) = 80 cal/gram
2 Latent heat of vapourisation of liquid
The amount of heat required to vaporise a unit mass of liquid
at its boiling point is called latent heat of vapourisation.
Latent heat of vaporisation of water or latent heat of
steam
The amount of heat required to convert into steam of a unit
mass of water at its boiling point (100
0
C) is called latent
heat of vaporisation of water or latent heat of steam.
Latent heat of steam(L) = 540 cal/gram
Units of heat
Calorie: It is the quantity of heat required to raise the
temperature of 1 gram of water through 1°C.
BTHU: It is the quantity of heat required to raise 1 lb of water
through 1°F. (British thermal unit).
C.H.U; It is the quantity of heat required to raise 1 lb of
water through 1°C.
Joule : S.I. Unit (1 Calorie = 4.186 joule)
Effects of heat
• Change in temperature
• Change in size
• Change in state
• Change in structure
• Change in Physical properties
Specific heat
The quantity of heat required to raise the temperature of one
gm of a substance through 1
0
C is called specific heat. It
is denoted by the letter ‘s’.
Specific heat of water = 1
Aluminium = 0.22
Copper = 0.1
Iron = 0.12
Thermal capacity:
It is the amount of heat required to raise the temperature of
a substance through 1
0
C is called the thermal capacity of
the substance.
Thermal capacity = ms calories.
Concept of heat and temperature, effects of heat, thermometer scale, celcius,
fahrenheit, reaumer, kelvin and difference between heat and temperature
Exercise 1.11.31
© NIMI, Not to be republished

82
Temperature
It is the degree of hotness or coldness of a body. The
temperature is measured by thermometers.
Temperature Scales
Temperatures are caliberated between two fixed reference
points namely the freezing point of water, and the boiling
point of water. These two fixed points on different tempera-
ture scales are:
Scale Freezing point Boiling point
Centigrade (°C) 0°C 100°C
Fahrenheit (°F) 32°F 212°F
Kelvin (K) 273°K 373°K
Reaumur (°R) 0°R 80°R
Workshop Calculation And Science : (NSQF) Exercise 1.11.31
Difference between heat and temperature
Heat Temperature
1 It is a form of energy. This tells the state of heat.
2 Its unit is calorie. Its unit is degree.
3 Heat is measured by calorimeter. Temperature is measured by thermometer.
4 By adding quantity of heat of two substances their By adding two temperatures we cannot find the
total heat can be calculated. temperature of the mixture.
5 By heating a substance the quantity of heat is Two substances may read the same temperature though
increased regardless of increase in temperature. they might be having different amount of heat in them.
Heat is a form of energy. Temperature is the
degree of hotness or coldness of a body. The
relationship for conversion from one tempera-
ture scale to the others is
180
32F
100
273K
100
C
80
R
0000
Boiling point
Any substance starts turning into a gas shows the tem-
perature at which it boils this is known as the boiling point.
The boiling point of water is 100
0
C.
Melting point
The temperature at which any solid melts into liquid or
liquid freezing to solid is called the melting point of
substance. ` The melting point of ice is 0
0
C.
© NIMI, Not to be republished

83
Conversion between centigrade, fahrenheit, reaumer, kelvin scale of
temperature Exercise 1.11.32
1 Convert 0
0
C into
0
F
100
C
180
32F
00
180
100
C
32F
0
0
180
100
0
32F
0
°F = 0 + 32
= 32
0
F
0
0
C = 32
0
F
2 Convert -40
0
C into
0
F
100
C
180
32F
00
180
100
C
32F
0
0
180
100
40
32F
0
F - 32 = -72
°F = -72 + 32
= -40
0
F
-40
0
C = -40
0
F
3 Convert 37
0
C into k
100
273K
100
C
00
°K-273 = C
°K = C + 273
°K = 37 + 273
= 310 K
37
0
C = 310K
4 Convert 70
0
C into Reaumer
80
R
100
C
00
80
100
C
R
0
5680
100
70
R
0
70
0
C= 56
0
R
5 Convert -25
0
F into
0
C
180
32F
100
C
00
180
3225
100
C
100
180
57-
C
0
31.66
9
285
C
0
- 25
0
F = -31.7
0
C
6 Convert 98.6
0
F into
0
C
100
180
32F
C
0
0
100
180
3298.6
C
0
100
180
66.6
C37
180
6660
0
98.6
0
F = 37
0
C
ASSIGNMENT
Convert the following
1 10.5
0
C = ___
0
F
240
0
C = ___
0
F
360
0
C = ___
0
F
480
0
C = ___
0
F
5 105
0
C = ___
0
F
6 -100
0
C = ___
0
F
777
0
F = ___
0
C
820
0
F = ___
0
C
9 428
0
F = ___
0
C
10 -210
0
F = ___
0
C
11 72
0
R = ___
0
C
12 143
0
C = ___K
13 373
0
K = ___
0
C
14 746
0
K = ___
0
F
15 At what temperature will the reading of a fahrenheit
thermometer be double of a centigrade one.
© NIMI, Not to be republished
84
Temperature measuring instruments, types of thermometer, pyrometer and
transmission of heat Exercise 1.11.33
Measuring heat energy
Energy can be released in chemical reactions as light,
sound or electrical energy. But it is most often released as
heat energy. This allows us to easily measure the amount
of heat energy transferred.
The apparatus used to measure the amount of heat by
mixer method is called calorimeter. It is nothing but
cylindrical shaped vessel and a stirrer made out of mostly
copper.
In a calorimeter when the hotter solid/liquid substance are
mixed with the cooler solid/liquid substances, heat transfer
takes place until both substances reach the same tem-
perature. By the same time calorimeter also reaches the
same temperature. By mixing rule,
⎥
⎦
⎤
⎢
⎣
⎡
⎥
⎥
⎥
⎦
⎤
⎢
⎢
⎢
⎣
⎡
=
⎥
⎥
⎥
⎦
⎤
+
rcalorimeteby
edHeat absorb
substance
idsolid / liqu
ed byHeat absorb
liquid
by solid/
Loss of heat
Measurement
Temperature is generally measured in degrees Celsius. In
this system the freezing point of water is defined as 0°C and
the boiling point of water is defined as 100°C. The Kelvin
temperature scale begins from absolute 0. i.e.— 273°. The
temperature intervals are the same.
∴ 273K = 0°C, 20°C = 273K + 20°C = 293K.
Instruments
The instruments used to measure and read temperature
takes into account changes in the properties of materials,
electrical phenomena incandescence, radiation and
melting.
Thermometer
They are based on the principle that liquids and solids
expand when they are subjected to heat. Mercury and
alcohol expand uniformly. When heat is applied the volume
of the liquid increases and the liquid rises in the capillary
tube integral with the container. Mostly mercury is used in
this type of thermometers because of its properties (Shiny
Pyrometer
Thermoelectric pyrometer is based on the principle that the
soldering point between the wires of different metals, when
heated a contact voltage is generated. The voltage depends
upon the temperature difference between the hot measuring
point and the cold end of the wire. Thermocouple elements
are constructed of copper and Constant (up to 600°C) or of
platinum and platinum-rhodium (up to 1600°C)
Radiation pyrometers are used to measure temperatures
of red hot metals up to 3000°C. These concentrate thermal
rays through an optical lens and focus them on to a thermo
element. The scale of the ammeter is calibrated in degrees
Celsius or Kelvin.
and will not adhere to the glass tubes and we can measure
up to 300
0
c.
The bimetal thermometer consists of metals with different
coefficient of expansion. The bimetal is twisted into a spiral
which curls when the temperature rises.
© NIMI, Not to be republished

85
Workshop Calculation And Science : (NSQF) Exercise 1.11.33
Transmission of Heat
Heat is a form of energy and is capable of doing work. Heat
flows from a hot body to a colder body or from a point of high
temperature to a point of low temperature. The greater is
the temperature difference the more rapidly will be the heat
flow. Heat is transmitted in three ways.
• By Conduction
• By Convection
• By Radiation
Conduction
Conduction is the name given to the transmission of heat
energy by contact. The heat source is in contact with the
Conductor. (metal rod). The rod is in contact with a
thermometer. Due to Conduction heat is transferred from
the heated end to the free end. In general good electrical
conductors are also good heat conductors and good
electrical insulators are also good heat insulators. A good
heat insulator does not necessarily withstand high
temperature.
Convection
Convection is the name given to the transmission of heat
energy by the up-ward flow. When heated, the fluid (liquid/
gas) becomes less dense and because of its mobility, is
displaced upwards, by a similar but colder and more dense
fluid. e.g., The domestic hot water system, The cooling
system in motor cars.
Radiation
Heat is radiated or transmitted from one object to the other
in space without actually being in contact, by means of
electro-magnetic waves. These waves are similar to light
waves and radio waves. They can be refracted by lenses
and reflected by mirrors. This radiation is called infrared. It
requires no medium to carry the radiation. (e.g) The heat of
the sun travels through the space.
Transmission of heat takes place in three ways
Conduction, Convection and Radiation.
Expansion due to heat
When a solid, liquid or gaseous substance is heated, it
expands and volume is increased. Similarly when it is
cooled, it contracts (shrinks) and volume is decreased.
E.g : small gaps are left in between the lines of railway track
to allow for expansion during summer. If this is not done,
the rails would expand and bend there by causing derail-
ment of trains.
Except a few substances, all solids, liquids and gases
expand. For the same amount of heat given, the expansion
of liquids is greater than solid and expansion of gas is more
than liquid.
Volume of water is reducing while heating from 0
0
C to 4
0
C.
After that volume is increasing. The data at 4
0
C of water will
be taken as reference point for any calculations relating
with water.
© NIMI, Not to be republished

86
Co-efficient of linear expansion and related problems with assignment
Exercise 1.11.34
Expansion of solids
A solid substance shows the following types of expansion
when heated.
1 Linear expansion
2 Superficial expansion and
3 Cubical expansion
1 Linear expansion
When a solid is heated, its length increases. This is called
linear expansion. It depends upon the material, original
length and change in temperature.
Co-efficient of linear expansion
The co-efficient of linear expansion is the change in length
per unit original length per degree rise in temperature. It is
represented by α (Alpha).
Length of the solid at t
1
0
C = l
1
Length of the solid at t
2
0
C = l
2
Change in Temperature = t
2
- t
1
0
C
Change in length = l
2
- l
1
ttt
tl
ll
ttl
ll
12
1
12
121
12
etemperatur in change x length Original
length in Change
expansion linear
of efficientCo
=
−
⎭
⎬
⎫
Increased length l
2
-l
1
= αl
1
t
Final length l
2
= l
1
(1 + αt)
2. Superficial expansion
When a solid is heated, its area increases is called su-
perficial expansion.
Co-efficient of superficial expansion
The increase in area per unit original area per degree rise
in temperature is called co-efficient of superficial expan-
sion. It is represented by β (Beta).
Co-efficient of superficial
Expansion = 2 x linear expansion
β = 2α
3. Cubical expansion
When a solid is heated, its volume increases is called
cubical expansion.
Co-efficient of cubical expansion
The increase in volume per unit original volume per degree
rise in temperature. It is represented by γ (Gama).
Co-efficient of cubical expansion
= 3 x linear expansion
γ = 3α
Examples
Find the co-efficient of linear expansion. If an 8 metre
long metal rod is heated from 30
0
C to 80
0
C. So that it
may produce an elongation of 0.84 mm.
Initial length (l) = 8m
Increased length = 0.84 mm
Increased temperature(t) = 80 - 30 = 50
0
C
)expansion(
linear of effiecient-Co
temp Increasedlength Initial
length Increased
508000
0.84
400000
0.84
= 2.1 x 10
-6
/
0
C
If iron bridge is 100 metre long at 0
0
C. What will be
the length of bridge if the temperature is 40
0
C and
the co-efficient of linear expansion is 12 x 10
-6
per
degree.
Initial length of iron bridge = 100 m
Increased temperature = 40 - 0 = 40
0
C
)expansion(
linear of effiecient-Co
temp Increasedlength Initial
length Increased
40100
length Increased
1012
6
40100
1000000
12
length Increased
= 0.048 m
Iron bridge at 40
0
C = 100 + 0.048 = 100.048 m
The length of a metal rod is 100 cm at 30
0
C and 100.14
cm at 100
0
C. Calculate the co-efficient of linear ex-
pansion and the rod length in 0
0
C.
Initial length at 30
0
C = 100 cm
Final length at 100
0
C = 100.14 cm
Increased length = 0.14 cm
Increased temperature = 100 - 30 = 70
0
C
© NIMI, Not to be republished

87
Workshop Calculation And Science : (NSQF) Exercise 1.11.34
)expansion(
linear of effiecient-Co
temp Increasedlength Initial
length Increased
70100
0.14
10070100
14
100000
2
= 2 x 10
-5
To find the length at 0
0
C
l
1
= l
0
(1 + αt)
100 = l
0
(1 + 2 x 10
-5
x 30)
100 = l
0
(1 + 0.0006)
0.00061
100
l
0
Length at 0°C = 99.94 m
Find the change in length of metallic rod 100 cm
long, when its temperature is increased from 25
0
C to
40
0
C and the co-efficient of linear expansion is 10 x
10
-6
/
0
C.
Initial length = 100 cm
Increased temperature = 40 - 25 = 15
0
C
Co-efficient of linear = 10 x 10
-6
/
0
C
expansion (α)
)expansion(
linear of effiecient-Co
temp Increasedlength Initial
length Increased
15100
length Increased
1010
6
Increased length = 10 x 10
-6
x 100 x 15
1000000
1510010
0.015cm
1000
15
Find out the temperature that the rod will extend by
0.54 mm in linear direction piece of metal rod is 2.5
metre long and the co-efficient of linear expansion
is 10.4 x 10
-6
per degree centigrade.
Initial length = 2.5 m = 2500 mm
Increased length = 0.54 mm
Initial temperature = 20
0
C
Co-efficient of linear = 10.4 x 10
-6
expansion (α)
)expansion(
linear of effiecient-Co
temp Increasedlength Initial
length Increased
temp Increased2500
0.54
1010.4
6
Increased temperature =
16
1010.42500
0.54
10.42500
10000000.54
C20.77
260
5400
0
Final temperature = 20 + 20.77
= 40.77
0
C
ASSIGNMENT
Co-efficient of linear expansion
1 Calculate the co-efficient of linear expansion of rod. If
rod is found to be 100m long at 20
0
C and 100.14m long
at 100
0
C.
2 Find the change in length if the co-efficient of linear
expansion of rod is 0.00024/
o
C and the temperature of
a rod of 3.6m length is raised by 120
0
C,
3 find the change in length if the co-efficient of linear ex-
pansion of rod is 0.00024/
0
C. If the temperature of a
rod of 6m length is raised by 120
0
C,
4 Find the increase in length 100 cm iron rod if the tem-
perature raise from 40
0
C to 90
0
C. The co-efficient of
linear expansion of the iron is 10x10
-6
/
0
C
5 If micrometer reading is standardised at 15
0
C. What
will be the true reading of the micrometer if the reading
taken at 35
0
C is 20.20 mm?
The co-efficient of linear expansion of material of mi-
crometer is 11 x 10
-6
/
0
C.
© NIMI, Not to be republished

88
Problems of heat loss and heat gain with assignment Exercise 1.11.35
Mixing of heat
m
1
- Mass of first substance
s
1
- specific heat of first substance
m
2
- mass of 2nd substance
s
2
- specific heat of 2nd substance
t
m
- temperature of mixture
m - mass
Q - Quantity of heat
δt/Δt - temperature difference
t
m
- temperature of the mixture.
Unit of amount of heat
The derived unit for the amount of heat is S.I. unit is 1
joule (j).
Specific heat
It is also expressed as the amount of heat required to raise
the temperature of unit mass of a substance through 1°C.
In S.I. unit in order to heat a mass of 1 kg of water through
1°C,
the amount of heat needed or the
mechanical equivalent of heat = 4186 joules
= 4.2 kj/kg°C.
Quantity of heat needed for a substance to rise the
temperature
The amount of heat needed for heating 1 kg of the
substance through 1°C is equal to the specific heat of the
substance ‘s’. For heating a mass of ‘m’ kg of the
substance to attain a temperature difference of t,
the quantity of heat needed = m x s x Δt
Therefore Q = m x s x Δt.
Mixing
When there is an exchange of temperatures, there is an
exchange in the amount of heat. When hotter bodies
involve with colder substances, heat transference takes
© NIMI, Not to be republished

89
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
place from hotter substances to the colder substances
until the mixture or both the substances acquire the same
temperature.
Heat lost by the bodies at higher temperature
= Heat gained by the bodies at lower temperature and
hence the total amount of heat of the component
substances
= amount of heat in the mixture.
Heat loss by hot substance =
Heat gained by colder substance
S of the component amounts of heat =
amount of heat in the mixture
m
1
x s
1
x t
1
+ m
2
x s
2
x t
2
= (m
1
s
1
+ m
2
s
2
)tm.
Example
A bath tub contains 40 litres of water at 15°C and 80 litres
of water at 60°C is poured to it. What is the temperature
of the mixture.
m
1
x s
1
x t
1
+ m
2
x s
2
x t
2
= (m
1
s
1
+ m
2
s
2
)tm.
C60 x
Ckg
kg 4.2
x kg 80 C15 x
Ckg
kj 4.2
x kg 40
m
t60
Ckg
4.2kj
80kg15
Ckg
4.2kj
40kg
00
C45C
4.2120
22680
m
t
00
Examples
A container contains 25 kg of water. Initial tempera-
ture of container and water is 25
0
C. Calculate the
heat required to heat the water to the boiling
temperature of water. Assume water equivalent of
container = 1 kg.
Mass of the water (m) = 25 Kg.
Initial temperature of water and container = 25
0
C
Final temperature of water and container = 100
0
C
Increased temperature (t) = 100 - 25
= 75
0
C
Water equivalent (m s) = 1 Kg.
Required amount of heat to container = m s t
= 25 x 1 x 75
= 1875 K.cal.
Required amount of heat to container = m s t
= 1 x 75
= 75 K.cal.
Total required amount of heat = 1875 + 75
=1950 K.cal.
300 gram of water 25°C is mixed with 200 gram of
water at 85
0
C. Find out the final temperature of the
mixture assuming that no heat escapes.
i) Weight of water = 300 gram
Initial temperature = 25
0
C
Final temperature = Assume ‘X’
Temperature gained = x - 25
0
C
ii) Mass of water = 200 gram
Initial temperature = 85
0
C
Temperature lost = 85
0
C - x
Heat gained by 300 gram water = m s t
= 300 x 1 x (x - 25)
= 300 x -7500 cal.
Heat lost by 200 gram water = m s t
= 200 x 1 x (85 - x)
= 17000 - 200 x cal.
Heat gained = Heat lost
300 x -7500 = 17000 - 200 x
300 x + 200 x = 17000 + 7500
500 x = 24500
x =
C49
500
24500
Final temperature = 49
0
C
20gm of common salt at 91
0
C immersed in 250 gram
of turpentine oil at 13
0
C. The final temperature is
found to be 16
0
C. If the specific heat of turpentine oil
is 0.428. Calculate the specific heat of common salt.
Mass of the salt(m) = 20 gram
Initial temperature(t) = 91
0
C
Mass of the turpentine(m) = 250 gram
Initial temperature(t) = 13
0
C
Specific heat of turpentine(s) = 0.428
Final temperature of mixture = 16
0
C
Heat gained by turpentine(Q) = m s t
= 250 x 0.428 x (16-13)
= 250 x 0.428
= 321 calories.
Heat lost by salt (Q) = m s t
= 20 x s x (91-16)
= 20 x s x 75
= 1500 s calories
Heat lost = Heat gained
1500 s = 321
© NIMI, Not to be republished

90
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
1500
321
s
Specific heat of salt = 0.214
If copper calorimeter contains 80 gram of water at
20
0
C. The water equivalent of calorimeter is 20 gm.
What will be be resultant temperature of the mix-
ture, when 100 gm of water at 40
0
C is added to the
mixture?
Mass of the water in calorimeter = 80 gram
Temperature = 20
0
C
Final temperature of the mixture = Assume ‘x’
Temperature raised in water = x - 20
Specific heat of calorimeter(ms) = 20 gram
Mass of water added = 100 gram
Temperature = 40
0
C
Temperature lost = 40 - x
Heat gained
Heat gained by water in calorimeter = m s t
= 80 x 1 x (x - 20)
= 80 x (-1600)
Heat gained by calorimeter = m s t
= 20 x (x - 20)
= 20 x (-400)
Heat lost
Heat lost by added water = m s t
= 100 x 1 x (40 - x)
= 4000 - 100x
Heat gained = Heat lost
80x (-1600) + 20x (-400) = 4000 - 100x
100x -2000 = 4000 - 100x
100x+100x = 4000 + 2000
200x = 6000
200
6000
x
= 30
Final temperature = 30
0
C
Find the amount of heat required to boil 15 gram of
ice at -8
0
C. Latent heat of ice = 336 joule/gm. Latent
heat of steam = 2268 J/gm. Relative specific heat of
ice = 0.5
Heat of ice cube
-8
0
C to 0
0
C Ice Q = mct kJ
= m x s x 4.2 x t kJ
= 0.015 x 0.5 x 4.2 x 8 kJ
= 0.252 kJ
0
0
C Ice to 0
0
C water = m x h s f kJ
= 0.015 x 336 kJ
= 5.04 kJ
0
0
C water to 100
0
C water = m c t kJ
= 0.015 x 4.2 x 100 kJ
= 6.3 kJ
100
0
C water to 100
0
C steam Q= m x h
sfg
kJ
= 0.015 x 2268 kJ
= 34.02 kJ
Total amount of heat Q = 0.252 + 5.04 + 6.3 + 34.02 kJ
Answer = 45.612 kJ
ASSIGNMENT
Mixing of Heat
1. m = 120 litres
t
1
= 20°C
t
2
= 85°C
s = 4.2
Q = ______ kj
2. m
1
=80 litres of water
m
2
=40 litres of water
t
1
= 10°C
t
2
= 70°C
t
m
= ______ °C
© NIMI, Not to be republished

91
3. m
1
= 25 litres of water
t
1
= 12°C
t
2
= 70°C
t
m
= 33.75°C
m
2
= ______ litres
4. m
1
= 100 kg of water
t
1
= 12°C
m
2
= 50 kg of steel
t
2
= 600°C
CKg
kj
4.2
1
S
CKg
kj
0.46
2
S
Rise in temperature
=_____°C of water
5. m
1
= 250 litres of water
m
2
= 150 kg of steel
t
1
= 15°C
t
m
= 70°C
t
2
= ______ °C
6. m
1
= 20 litres of
machine oil
m
2
= 30 kg of steel
t
2
= 160°C
t
m
= 60°C
density of oil
t
1
= ______ °C = 0.91
3
cm
gr
7. m
1
= 10 litres
CKg
kj
4.2
1
S
°
=
CKg
kj
0.46
2
S
°
=
m
2
= 0.5 kg
t
1
= 18°C
t
2
= 780°C
Rise in
temperature =___°C
of water
8. m
1
= 60 gms of water
m = 70 gms of
calorimeter
m
2
= 80 gms of metal
t
1
= 20
0
C
t
2
= 95
0
C
t
m
= 25
0
C
s = 0.2
s
1
= 1
s
2
=_____
9. m
1
= 250 gms of oil
t
1
= 15
o
C
m
2
= 150 gms of brass
t
2
= 90
0
C
t
m
= 25°C
s
2
= 0.09 water
equivalent of = 3 gms
calorimeter
s
1
= ______ gms
Heat loss and heat gain
1 Calculate the amount of heat required to raise the
temperatureof 85.5 g. of sand from 20
0
C to 35
0
C. Spe-
cific heat of sand = 0.1
2 How much quantity of heat will be rejected in one hour,
if the rate of flow of water is 11 kg/min and the raise of
temperature of water is 12
0
C.
3 Find out its specific heat. If we require 510 calories to
raise the temperature of 170/ gm of material 50
0
C to
80
0
C.
4 Calculate the specific heat of metal piece. If 500gm
metal piece at 300
0
C is dropped in 5 kg of water. Its
temperature raises from 30
0
C to 75
0
C are no heat losses.
5 Find out the final temperature of mixture, assuming
that no heat escapes. If 300gm of water at 25
0
C is
mixed with 200gm of water at 85
0
C.
6 What will be the resultant temperature of the mixture,
when 100gm of water at 40
0
C is added to the mixture.
If copper calorimeter contains 80 gm of water at 20
0
C.
The water equivalent of calorimeter is 20 gm.
© NIMI, Not to be republished

92
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
Concept of pressure and its units in different system Exercise 1.11.36
Pressure
F - Force, Thrust
P - Pressure
Concept of pressure
The action of a force on unit area of the body on which it
acts is termed as pressure.
Pressure ‘P’ = Force or Thrust F per unit area ‘A’ (Fig 1)
A - Area of surface on which force acts
Example
A liquid gives force of 100 N over an area of 2m
2
. What is
the pressure?
Force = 100N
Area = 2m
2
Pressure = ?
= 50 N/m
2
Pressure
British unit Pounds per square inch lb/in
2
FPS
Metric units Gram per square contribute g/cm
2
CGS
MKS Kilogram per square metre kg/m
2
International Newtons per square metre N/m
2
circuits SI
Unit of pressure N/m
2
, 1 N/m
2
= 1 pascal.
This unit is too small (pressure of a fly on a area of
1cm
2
). Hence ‘bar’ is introduced as the unit of pressure.
1 bar = 10
5
pascal.
1 bar = 1000 mbar. [SI unit of pressure is pascal (Pa) and
Metric unit of pressure is bar].
Properties of pressure
1 The pressure in a liquid increases with increase in
depth.
2 The pressure at a point increases with the density of
the liquid.
3 The pressure is same in all directions about a point in
liquid at rest.
4 Upward pressure at a point in a liquid is equal to
downward pressure.
Saturation temperature of a fluid (liquid/vapour)
The temperature at which a liquid changes to vapour by
heating further at a given pressure. If this pressure is kept
constant, the heat removal process changes the vapour
into liquid at constant temperature. It is the saturated con-
dition of a fluid (Liquid/vapour).
Saturation pressure of a fluid
By adding or removing heat at a givcen temperature, there
is physical change is fluid. The given constant pressure is
known as saturation-pressure.
Critical temperature and pressure
Each fluid (liquid/gas) has its highest temperature and
pressure; below which the removal or addition of heat
changes physical state. If the conditions of temperature /
pressure exceeds the saturation level, the vapour cannot
be condensed into liquid. This vapour which cannot be
condensed into liquid, is called gas.
Super heated vapour
If the temperature of the vapour is raised above its satu-
rated temperature, it is called super heated vapour, for a
given pressure.
© NIMI, Not to be republished

93
Insulating materials: Heat will flow from high tempera-
ture to low temperature. Heat flow by radiation, conduc-
tion and convection method through the wall, door, ceiling
and glass door to the refrigerated space.
The material which restricts such heat flow is called insu-
lating materials
Properties of insulating materials
- It is low conductivity
- Resistance to fire
- Less moisture absorption
- Good rigidity
- Odourless
- Vapour permeability
- Light in weight
Selection of insulating material: The following factors
are the prime importance in the selection of a proper insu-
lating material.
- Low thermal conductivity: Thermal conductance
value of a material is a measure of its effectiveness to
allow the flow of heat through it by conduction, obvi-
ously an insulating material should have a very low
thermal conductivity.
- Resistance to fire.
- Mechanical strength
- Low moisture absorption capacity
- Easy to lay
- Cost
- Easy of handling
- Low cost
Types of insulating materials
Glass wool, PUF, Cork sheet, Thermocole, Insulating foil,
fiber glass.
Types of insulating materials: Basic types of insulating
materials are inorganic fibrous or cellular materials. Ex-
ample, glass wool, slag wool ceramic products, as bestos,
etc. Organic fibrous materials, cork, cotton, rubber foam,
saw dust, rice husk, polystyrene, polyurethane,
phenotherm, etc. The type and form available as the appli-
cations of various insulations as follows.
Glass wool: Available as semi-rigid, resin bonded slabs/
sheets of different densities -higher density gives strength
and lower conductivity but allows vapour transmission.
Available with foil or other coverings.
Cork: Compressed and moulded into a rigid block, light
but strong, can be cut easily with a saw, resists water but
allows relatively high rate of water vapour transmission.
Expended polystyrene(Thermocole): it is available as
a rigid board, beads, moulded into shape for pipe/curved
surface, can be cut easily with a saw, light weight allows
relatively low vapour transmission.
Polyurethane: available as a rigid board, flexible board,
liquid can be sprayed on surfaces and allowed to foam,
can be used for in site applications.
Wood shaving/Saw dust: It needs good supporting com-
partment, can easily settle down. Fairly high conductivity
absorbs moisture/water.
Phenotherm: Available slabs with different facings, and
as performed pipe sections, can be easily cut with a saw.
Insulting materials and properties/specifications:
There are many insulating materials used in refrigeration
and air conditioning field. For our water tank only few of
them were in use.
Now-a-days the following insulating materials were in broad
use.
- Thermocole
- Glasswool/Tar felt
- Puf
- Fiberglass
Thermocole: It is one of the insulting materials in normal
use. It is available in low and high density. This is avail-
able in various thicknesses ranging 0.25" to 5".
Thermocole is available in various shapes (moulded) of
necessity.
Thermocole allows (Characteristically) low transmission
of vapour, thereby heat entry through is cut short. This
may vary with its low/high density.
It can be cut very easily even with knife to a required shape.
Thermocole withstands cool/heat for a longer time.
The 'K' factor of an insulation material follows (thermocole).
Thermocole -0.20 btu/hr Ft2 deg.f°/inch
Fibreglass: Also one of the insulating materials used for
its manufactured from inorganic materials (sand, dolomite,
limestone). Glass fibre insulation does not shrink due to
temperature variation.
This insulation materials used for higher temperatures also
upto 450°C (842°F).
Fibreglass products does not absorb moisture from the
amblent air.
Glass wool: Normally glass wool material is heavily thin
weighted object in layers, soft (touching). It comes off in
various sizes (thickness from 0.5" to 2.5". it comes in
white, yellow colours mixed up with broken glass pieces.
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
Thermal conductivity and insulations Exercise 1.12.37
© NIMI, Not to be republished
94
Handling glass wool is hazardous and harmful (if it is
breathed). Always it is advisable to handle glass wool with
gloves and goggles (eye) while working on it. It also comes
off in various densities.
Glass wools are of two types of uses. One type of glass
wool used for low temperature refrigeration/air condition-
ing purpose. The other type is used for boiler materials
(heat prevention) purposes.
The 'K' factor of insulation material:
Glasswool: 0.230-.27 Btu/Hr ft2 deg. F°/inch.
Puf: The other mode of insulating materials used in water
cooler at the evaporator tank's external body.
For this kind of insulation two chemicals used namely
isocyanide-R11., Both available in liquid form in bottles
(for lesser capacities) and cans (for higher capacities).
Both the liquids (chemicals) should always kept cool. When
both of them added in a container and stirred in few min-
utes it becomes foamy (initially with thin and becomes
thicker and becomes hard (sticks with the unit).
We should be careful that there is no air gap in the tank
covered. It foams out with high density and uneven finish
at the outer level.
Puf (materials) insulations are widely used by our
manufacturer's for their products as it keeps the tempera-
ture for a longer period.
The main disadvantage of the insulation is as soon as the
chemicals are mixed and stirred it should be poured over
the evaporator coil (or) outside the evaporator tank within
the shortest period. If the time exceeds the solution starts
framing at the container itself and becomes useless.
The evaporator tank should be covered well with wooden/
steel boards with required gaps for insulation tightened all
the corners well giving small gaps to pour the solution.
Method of laying duct insulation: when there is no
chance of moisture condensation on the duct, glass wool
can be used. Since it is economical and fire resistant.
However if moisture condensation can occur greater care
should be exercised in case of glass wool. First a uniform
coat of bitumen is applied to the duct surface and the
wool is stuck to the bitumen. The insulation is then cov-
ered with a polythene sheet which acts as a vapour bar-
rier. The surface can be plastered after spreading chicken
wire mesh as reinforcement.
Expanded polystyrene can be laid easily as it is rigid.
Bitumen is applied on the duct and the insulation is stuck
joints are also sealed with bitumen. No separate vapour
barrier is needed other than a coat of bitumen. The insula-
tion can be finished with cement and plaster or metal clad-
ding.
Purpose of false ceiling: The conditioned air arrives
through the ducts at the supply air diffusers and enters
the conditioned space. Most diffusers are attached to the
false ceiling and a variety of diffusers are available for dif-
ferent air spreading needs. The return air grills will be fixed
to the false ceiling. The false ceiling prevents mixing of
conditioned air and return air.
Return air usually flow into the plenum or return air box
through grill placed in the false ceiling. Since substantial
amount of energy goes into the air in the first place. It is
a practice to recycle to air. The air is therefore brought
back to the air conditioning. Plant room it is common to
route the return air through the gap between the false ceil-
ing and the main ceiling. A space referred to as a plenum,
the false ceiling is also known as a return air duct.
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
© NIMI, Not to be republished

95
Electricity is a kind of energy. It is the most useful sources
of energy which is not visible but its presense can be felt
by its effects. Electricity is obtained by conversion of
other forms of energy like heat energy, chemical energy,
nuclear energy, mechanical energy and energy stored in
water etc.,
To understand electricity, one must understand the struc-
ture of an atom.
Basically an atom contains electrons, protons and neu-
trons. The protons and neutrons are located in the centre
of an atom and the electrons, a negative electric charge
particle revolving around the nucleus in an atom. The pro-
ton has a positive charge. Neutrons are neutral and have
no charge.
Sources of electricity
Battery
Battery stores electrical energy in the form of chemical
energy and it gives power when required. Battery is used
in automobiles and electronics, etc.,
Generator
It is a machine which converts the mechanical enregy into
electrical energy.
When a conductor rotates between a magnetic field using
prime mover an emf will be induced. By using this method
all types of AC and DC generator - generates power.
E.g. Thermal power station
Hydro power station
Nuclear power station
Wind power station
Solar power station
Uses of Electricity
1 Lighting - Lamps
2 Heating - Heaters, ovens
3 Power - Motor, fan
4 Traction - Electromotives, lift, crane
5 Communication - Telephone, telegraph, radio,
wireless
6 Entertainment - Cinema, radio, T.V.
7 Medical - x-rays, shock traeatment
8 Chemical - Battery charging, electroplating
9 Magnetic - Temporary magnets
10 Engineering - Magnetic chucks, welding,
x-rays of welding
Molecule
All substances are made by tiny molecules. The smallest
part on any matter which has the properties of the parent
substance, is called molecule. The molecules of a sub-
stance are alike in shape and properties. Each molecules
possesses the physical and chemical properties of the
mother substance.
Atom
The are three particles in a atom. They are:
i Proton: The electron present in the middle of the atom
is called proton. It is in the nucleus of the atom. The
proton liquid-equilent is 1840 times heavier than an elec-
tron liquid equivalent and is equal to the atom of hydro-
gen. It has a positive charge.
ii Neutron: It is a basic particle which is in the nucleus
of atom. It is held fast with proton in the nucleus. It has
no charge. It increases the liquid equivalent of the cou-
lomb.
iii Electron: It is unstable or moving electron. It keeps
moving round the nucleus on different orbits. It carries
negative charge. It is 1/1240 of hydrogen atom liquid
equivalent. It charge is 1.6 x 10
-19
coulomb.
How electricity is produced
In the middle of each atom there is electricity. This is in
form of electrons. Those parts which are stable known as
protons and those moving are called electrons. The pro-
tons are of positive and electrons are negative charges.
By external force only the moving electrons are reduced
or increased as compared to protons and then only both
the charges combine. When the number of electrons pro-
duced are less than the number of protons, then it be-
comes a positive charge. Conversely when the number of
electrons produced are more tan the number of protons it
becomes negative. In this way electricity is produced by
atom.
Electrical terms and units
Quantity of electricity
The strength of the current in any conductor is equal to the
quantity of electrical charge that flows across any section
of it in one second. If ‘Q’ is the charge and ‘t’ is the time
taken
then I =
t
Q
Q = I x t
The SI unit of current is coulomb. Coulomb is equivalent to
the charge contained in nearly 6.24 x 10
18
electrons.
Coulomb
In an electric circuit if one Ampere of current passes in one
second, then it is called one coulomb. It is also called
ampere second (As). Its larger unit is ampere hour (AH)
1 AH = 3600 As (or) 3600 coulomb
Introduction, use of electricity, molecule, atom, electricity is produced,
electric current, voltage, resistance and their units Exercise 1.13.38
© NIMI, Not to be republished
96
Electro motive force (EMF)
It is the force which causes to flow the free electrons in any
closed circuit due to difference in electrical pressure or
potential. It is represented by ‘E.’ Its unit is Volt.
Potential difference (P.D)
This is the difference in electrical potential measured
across two points of the circuit. Potential difference is
always less than EMF. The supply voltage is called
potential difference. It is represented by V.
Voltage
It is the electric potential between two lines or phase and
neutral. Its unit is volt. Voltmeter is used to measure
voltage and it is connected parallel between the supply
terminals.
Volt
It is defined as when a current of 1 ampere flows through a
resistance of 1 ohm, it is said to have potential difference
of 1 volt.
Current
It is the flow of electrons in any conductor is called current.
It is represented by I and its unit is Ampere. Ammeter is
used to measure the current by connecting series with the
circuit.
Ampere
When 6.24 x 10
18
electrons flow in one second across any
cross section of any conductor, the current in it is one
ampere.(or) If the potential difference across the two ends
of a conductor is 1 volt and the resistance of conductor is
1 ohm then the current through is 1 ampere.
Resistance
It is the property of a substance to oppose to the flow of
electric current through it, is called resistance. Symbol: R,
Unit : Ohm (W), Ohm meter is used to measure the
resistance.
Ohm
If the potential difference across the two ends of conductor
is 1 volt and the current through it is 1 ampere, then the
resistance of the conductor is 1 Ohm.
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
© NIMI, Not to be republished

97
Ohm’s law, relation between V.I.R & problems, series & parallel circuits
problem Exercise 1.13.39
Ohm’s law
V - Voltage in volts
l - Current in Ampere
R - Resistance in ohms.
In any closed circuit the basic parametres of electricity
(Voltage, Current and resistance) are in a fixed relationship
to each other.
Basic values
To clarify the basic electrical values, they can be compared
to a water tap under pressure
Water pressure - electron pressure - Voltage
Amount of water - electron flow - Current
throttling of tap - obstruction to - Resistance
electron flow
Relationships
If the resistance is kept constant and the voltage is
increased, the current is increased
I ∞ V
If voltage is constant and the resistance is increased,
current is decreased
Ohm’s law
From the above two relationships we obtain Ohm’s law,
which is conveniently written as V = R.I.
Ohm’s law states that at constant temperature
the current passing through a closed circuit is
directly proportional to the potential differ-
ence, and inversely proportional to the resis-
tance.
By Ohm’s law
EXAMPLE
A bulb takes a current of 0.2 amps at a voltage of 3.6 volts.
Determine the resistance of the filament of the bulb to find
R. Given that V = 3.6 V and l = 0.2 A.
To find ‘R’. Given that V = 3.6V and I = 0.2 A
Therefore V = l x R
3.6 V = 0.2 A x R
Therefore
© NIMI, Not to be republished

98
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
Voltage drop = 12V – 10.8 = 1.2V
The supply voltage is called Potential differ-
ence.
Example
The Internal resistance of a dynamo is 0.1 ohm. The
voltage of dynamo is 12V. What is the Voltage dynamo
when a current of 20 amps being supplied to an outside
circuit.
Solution
Voltage drop = Current x Internal resistance
= 20 x 0.1 volts
= 2 volts
Example (Fig 6)
The Internal resistance of a Battery is 2 ohms. When a
resistance of 10 ohms is connected to a battery it draws 0.6
amps. What is the EMF of the battery.
P.D = Current flowing x Resistance
= 0.6 A x 10Ω
= 6 volts
V.D = Current flowing x Internal resistance of battery
= 0.6 x 2 volts
= 1.2 volts
EMF of the Battery = (6.00 + 1.2)V
= 7.2 volts
Example
The voltage supply to a filament lamp is 10.8V. The voltage
should be 12V. Find out loss of voltage.(Fig 5)
The total resistance is equal to the sum of all the resis-
tances. In a series connection the end of the first load is
connected to the beginning of the second load and all loads
are connected end to end.
Features of series connection:
• The same current flows through all the loads.
• The voltage across each load is proportional to the
resistance of the load.
• The sum of the voltages across each load is equal to the
applied voltage.
• The Total resistance is equal to the sum of all the
resistances.
l=l
1
= l
2
= ...
V= V
1
+ V
2
+ ...
R= R
1
+ R
2
+ ...
Parallel connection
In a parallel connection the beginning and the ends of the
loads are connected together.
Resistance connections
V - Voltage (in volts)
R - Resistance (in ohms)
I - Current intensity (in Amperes)
Series Connection
© NIMI, Not to be republished

99
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
Features of parallel connection:
• The current flowing through each load depends upon
the resistance of the load.
• The voltage across each load is the same and is equal
to the voltage applied to the circuit.
• The total resistance of a parallel connection is always
smaller than the smallest resistance in the circuit.
• In parallel connection the reciprocal of the total
resistance is equal to the sum of the reciprocals of all
resistances in the circuit.
l= l
1
+ l
2
+ ...
V= V
1
= V
2
...
..........
21
R
1
R
1
R
1
Example
Two resistances of 4 ohms and 6 ohms are connected in
parallel. Determine the total resistance.
21
R
1
R
1
R
1
(since parallel connection)
Therefore
24
10
6
1
4
1
R
1
Therefore R
10
24
ohms = 2.4 ohms
Example
Two resistors of 2 and 4 ohms are switched in parallel
to a 6V battery
– Calculate the total resistance
– Find the total current and partial current.
Solution
Total resistance
3
Ω
1
1=
3
4
=R
4
Ω
3
=
4
1+2
=
4
1
+
2
1
=
R
1
+
R
1
=
R
1
tot
21tot
I Total = I
1
+ I
2
current
Amp4.5 =
1.5A +3A = total I
1.5
A
=
4
6V
=
R
U
=I
3A=
2
6V
=
R
U
=I
2
2
1
1
Ω
Ω
But
Assume the given resistors in the assignment
as bulb with filaments and other current con-
suming devices like Horn, Wiper etc of the
vehicle.
© NIMI, Not to be republished

100
Table of analogies between mechanical and electrical quantities
Mechanical quantity Unit Electrical quantity Unit
Force 'F’ N Voltage ‘V’ V
Velocity v =
Time
ntDisplaceme
m/s Current I A
Time t seconds Time t seconds
Power P = F x v N
sec
m
Power P = V x iW = V x A
Energy = F x v x tj = Nm Energy W = V x i x tj = W x s
Electrical power, energy and their units, calculation with assignment
Exercise 1.13.40
V - Voltage (Volts) V
i - Current Intensity (Ampheres) A
P - Power (Watts, Kilowatts) W, kW
W - Work, Energy (Watt hour, Kilowatt hour) wh, Kwh
t - time (hours) h
Electric Power
In mechanical terms we defined power as the rate of doing
work. The unit of power is Watt. In an electrical circuit also
the unit of electrical power is 1 Watt. In mechanical terms
1 Watt is the work done by a force of 1 N to move the body
through 1 metre in one second. In an electrical circuit, the
electromotive force overcomes the resistance and does
work. The rate of doing work depends upon the current
flowing in the circuit in amperes. When an e.m.f of one volt
causes a current of 1 ampere to flow the power is 1 Watt.
Hence Power = Voltage x Current
P= V x l
Power in Watts = Voltage in Volts x Current in Amperes
Electric work, energy
Electrical work or energy is the product of electrical power
and time
Work in Watt seconds = Power in Watts x time in sec-
onds
W = P x t
Since 1 joule represents 1 Watt x 1 sec, which is very
small, larger units such as 1 Watt hour and 1 kilowatt hour
are used.
1 W.h = 3600 Watt sec.
1 Kwh = 1000 Wh = 3600000 Watt sec
Note: The charge for electric consumption is
the energy cost per Kwh and it varies according
to the country and states.
W=V I
=I
2
R
=
R
V
2
2
2
I
W
W
V
I
V
R
=
=
=
V=I R
WR
I
W
=
=
R
W
V
W
R
V
I
=
=
=
© NIMI, Not to be republished

101
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
Example
1 Calculate the power rating of the lamp in the
circuit, if 0.25 amperes of current flows and the
voltage is 240 volts.
P = V x I
V = 240 Volts
I = 0.25 Amperes
Therefore Power = 240 Volts x 0.25 Amperes
= 60 Volts Ampere
But 1 Watt = 1 Volt x 1 Amphere
Therefore Power = 60 Watts
2 A current of 15 amperes flow through a resistance
of 10 Ohms. Calculate the power in kilowatts
consumed.
Given that R = 10 and I = 15A
Power = V x I = I x R x I = I
2
x R
Therefore Power = 15
2
x 10 = 2250 Watts = 2.25 kW
3 At a line voltage of 200 Volts a bulb consumes a
current of 0.91 ampheres. If the bulb is on for 12
hour calculate the work in Wh to find the work
given that V = 200 Volts.
I = 0.91 Amps.
t = 12 hours
Therefore Power=V x I = 200 Volts x 0.91 Amps
= 182 Watts
Therefore Work = P x t = 182 Watts x 12 hours
= 2184 Watt hour.
4 An adjustable resistor bears the following label:
1.5 k Ohms/0.08 A. What is its rated power?
Given: R = 1.5 k Ohms; I = 0.08 A
Find: P
V = R x I = 1500 Ohms.0.08 A = 120 volts
P = V x I = 120 volts.0.08 A = 9.6 W alternatively:
P = 1
2
.R = (0.08 A)
2
.1500 Ohms = 9.6 W.
5 Find the current and power consumed by an
electric iron having 110
ΩΩ
ΩΩ
Ω resistance when feed
from a 220 v supply
Resistance of electric iron (R) = 110 ohms
Voltage (V) = 220 volts
Current (I) =
R
V
=
110
220
2 amperes
Power (w) = V x I
= 220 x 2
= 440 watts
6 Find the total power if four 1000 W, 180 volt
heaters are connected in series across 240 V
supply and current carrying capacity is 15 amp.
Find the total power.
Connection = Series
No. of heaters = 4
Heater power (W) = 1000 watts
Heater voltage = 180 V
Supply voltage = 240 V
Heater resistance (R) =
W
V
2
=
10
324
1000
180 x 180
=
= 32.4 ohms
Total resistance = 32.4 x 4 = 129.6 ohms
Total current (I) =
R
V
=
129.6
240
= 1.85 amperes
Total Power (W) = V x I
= 240 x 1.85 = 444 watts
7 If a 40 watt fluorescent lamp draws a current of
0.10 ampere. How much voltage will be required
to illuminate it?
Lamp power (W) = 40 watt
Current (I) = 0.10 ampere
Voltage (V) =
I
W
=
0.1
40
= 400 volts
8 Find the cost if running 15 HP motor for 15 days @
6 hrs per day and the cost of energy is Rs. 3 per unit.
Motor power (w) = 15 HP
= 15 x 746 = 11,190 watts
Consumption per day = 11,190 x 6
= 67140 = 67.14 KWH
Consumption for 15 days = 67.14 x 15
= 1007 KWH (or) unit
Cost per unit = Rs. 3
Cost for total energy = 3 x 1007 = Rs. 3021
© NIMI, Not to be republished

102
in
parallel
2 ASSIGNMENT
1 ASSIGNMENT
1 R = 40 Ohms
I = 6.5 Amps
V = ______ Volts
2 V = 6 Volts
I = 0.5 Amps
R = ______ Ohms
3 V = 220 Volts
R = 820 Ohms
I = ______ Amps
4 I = 4.5 Amps
V = 220 Volts
R = ______ Ohms
5 R = 50 Ohms
V = 220 Volts
I = _____ Amps
6 V = 110 Volts
I = 4.55 Amps
R =_____ Ohms
7 R = 250 Ohms
I = 0.44 Amps
V = ______ Volts
8 I = 11.5 Amps
V = 380 Volts
R =______ Ohms.
9 R = 22 Ohms
= 7.8 Amps
(Voltage drop)
V = ______ Volt
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
1R
1
= 12 ohms
R
2
= 22 ohms
R
3
= 24 ohms in series
R = ______ ohms
2R
1
= 15 ohms
R
2
= 25 ohms
V = 220 V
V
1
= ______ V
V
2
= ______ V
3 V = 220 V
R
1
= 40 ohms
V
1
= 100 V
(in series)
R
2
= ohms
4 V = 80 V
I = 2 A
R
1
= 30 ohms
(in series)
R
2
= ______ ohms
5 R
1
= 6 ohms
R
2
= 12 ohms
R
3
= 18 ohms
R = ______ ohms
© NIMI, Not to be republished

103
6 R = 6 ohms
R
1
,R
2
,R
3
are in parallel
R
1
= 12 ohms
R
2
= 16 ohms
R
3
= ______ ohms
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
7 R
1
= 40 ohms
R
2
= 60 ohms
V = 220 V
I = ______ A
I
1
= ______ A
I
2
= ______ A
3 ASSIGNMENT
1 Current Consumed
I = 0.136 A
Voltage ‘V’ = 220 V
P = _____ Watts
2 P = 500 Watts
I = 2.27 A
V = ______ v
3 P = 750 W
V = 220 v
I = ______ A
4 P = 60 W
V = 200 v
R = ______ W
5 I = 0.455 A
R = 484 ohms
P = ______ Watts
6 P = 550 W
R = 22 ohms
I = ______ A
7 P consumed = 1.8 kW
R = 8 ohms
V = ______ v
8 I consumed = ____ A
P = 2 kW
V
1
= 220 v-Heating
element voltage
R = ______ W
9 P = 100 W
t = 1 hour
Energy consumption
= ______ kWh
10 Energy consumed
‘W’ = 1 kWh
Power ‘P’ = 100 W
t = ______ hr
11 W = 1.5 kWh
t = 45 min.
P = ______ kW.
12 Energy metre reading
W
1
= 6755.3 kWh
Increases to W
2
= 6759.8 kWh
t = 45 min.
P = ______ kW.
13 Power consumed
‘P’ = 6.2 kW
t = 8 hours
Charge per kwh
= 1.25 Rupees
Total cost
= ______ Rupees
14 I = 5.45 A
V = 220 v
Energy consumed
= 1 kWh
t = ______ hr.
© NIMI, Not to be republished

104
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
Magnetic induction, self and mutual inductance and EMF generation
Exercise 1.13.41
Magnetic induction
When a magnet is brought near to an iron bar is brought
near to a magnete, a magnetism is produced in the iron bar.
The phenomenon is know as magnetic induction. Actually,
before attracting an iron bar towards it, a magnet induces
an opposite polarity in the iron bar and then due to
attraction between unlike poles, magnet attracts the iron
bar. The magnet need not to touch the iron bar for magnetic
induction.
In various electrical measuring instruments, soft iron pole
pieces are used along with bar magnets in order to given the
desired shape to the magnet used, such pole piece work
on the principle of magnetic induction.
Intensity of magnetic field
The force acting on a unit pole placed in a magnetic field
(attractive or repulsive force) is called the intensity of
magnetic field. It is denoted by letter H and its unit is
Wb/m.
Principles and laws of electromagnetic induction
Faraday’s laws of electromagnetic induction are also
applicable for conductors carrying alternating current.
Faraday’s laws of elctromagnetic induction
Faraday’s first law states that whenever the magnetic
flux is linked with a circuit changes, an emf is always
induced in it.
The second law states that the magnitude of the induced
emf is equal to the rate of change of flux linkage.
Dynamically induced EMF
Accordingly induced emf can be produced either by moving
the conductor in a stationery magnetic field or by changing
magnetic flux over a stationery conductor. When conductor
moves and produces emf, the emf is called as dynamically
induced emf Example: Generators.
Statically induced EMF
When changing flux produces emf the emf is called as
statically induced emf as explained below.
Example:Transformer.
Statically induced emf: When the induced emf is pro-
duced in a stationery conductor due to changing magnetic
field, obeying Faraday’s laws of electro magnetism, the
induced emf is called as statically induced emf.
There are two types of statically induced emf as stated
below:
1 Self induced emf produced with in the same coil.
2 Mutually induced emf produced in the neighbouring
coil.
Self-induction: The production of an electromotive force in
a circuit, when the magnetic flux linked with the circuit
changes as a result of the change in a current inducing in
the same circuit.
At any instant, the direction of the magnetic field is
determined by the direction of the current flow.
With one complete cycle, the magnetic field around the
conductor builds up and then collapses. It then builds up
in the opposite direction, and collapses again. When the
magnetic field begins building up from zero, the lines of
force or flux lines expand from the centre of the conductor
outward. As they expand outward, they can be thought of
cutting through the conductor.
Self induction
According to Faraday’s Laws, an emf is induced in the
conductor. Similary, when the magnetic field collapses,
the flux lines cut through the conductor again, and an emf
is induced once again. This is called self-induction (Fig 1).
Mutual induction
When two or more coils one magnetically linked together
by a common magnetic flux, they are said have the
property of mutual inductance. It is the basic operating
principal of the transformer, motor generators and any
other electrical component that interacts with another
magnetic field. It can define mutual induction on the current
flowing in one coil that induces as voltage an adjacent coil.
In the Fig 2 current flowing in coil L1 sets up a magnetic field
around it self with some of its magnetic field line passing
through coil L2 giving in mutual inductance coil one L on
has a current of I, and N, turns while coil two L2, has N2
turns therfore mutual inductance M, of coil two that exists
with respect to coil one L, depend on their position with
inspect to each other.
The mutual inductance M that exists between the two coils
can be greately measured by positioning them on a
common soft iron cone or by measuring the number of turns
of either coil on would he found in a transformer.
© NIMI, Not to be republished

105
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
The two coils are tightly would one on top of the other over
a common soft iron core unity said to exist between them
as any losses due to the leakage of flux will be extremely
small. Then assuring a perfect flux leakage between the
two coils the mutual inductance M.
Dynamically induced EMF
Generator: An electrical generator is a mahine which
converts mechanical energy into electrical energy.
Principle of the Generator: To facilitate this energy
conversion, the generator works on the principle of Faraday’s
Laws of Electromagnetic induction.
Faraday’s laws of electromagnetic induction: There
are two laws
The first law states:
• whenever the flix linking to a conductor or circuit
changes, an emf will be induced.
The second law states:
• the magnitude of such induced emf (e) depends upon
the rate of change of the flix linkage.
Types of emf: According to Faraday’s Laws, an emf can
be induced, either by the relative movement of the conduc-
tor and the magnetic field or by the change of flux linking
on a stationary conductor.
Dynamically induced emf: In case, the induced emf is
due to the movement of the conductor in a stationary
magnetic field as shown in Fig 1a or by the movement of the
magnetic field on a stationary conductor as shown in Fig
1b, the induced emf is called dynamically induced emf.
As shown in Fig 1a & 1b, the conductor cuts the lines of
force in both cases to induce an emf, and the presence of
the emf could be found by the deflection of the needle of the
galvanometer ‘G’. This principle is used in DC and AC
generators to produce electricity.
Statically induced emf: In case, the induced emf is due
to change of flux linkage over a stationary conductor as
shown in Fig 2, the emf thus induced is termed as statically
induced emf. The coils 1 and 2 shown in Fig 2 are not
touching each other, and there is not elctrical connection
between them.
According to Fig 2, when the battery (DC) supply is used
in coil 1, an emf will be induced in coil 2 only at the time of
closing or opening of the switch S. If the switch is
permanently closed or opened, the flux produced by coil 1
becomes static or zero respectively and no emf will be
induced in coil 2. EMF will be induced only when there is
a change in flux which happens during the closing or
opening of the circuit of coil 1 by the switch in a DC circuit.
Alternatively the battery and switch could be removed and
coil 1can be connected to an AC supply as shown in Fig
2. Then an emf will be induced in coil 2 continuously as long
as coil 1 is connected to an AC source which produces
alternating magnetic flux in coil 1 and links with coil 2. THis
principle is used in transformers.
Production of dynamically induced emf: Whenever a
conductor cuts the magnetic flux, a dynamically induced
emf is produced in it. This emf causes a current to flow if
the circuit of the conductor is closed.
For producting dynamically induced emf, the requirements
are:
• magnetic field
• conductor
• relative motion between the conductor and the mag-
netic field.
If the conductor moves with a relative velocuty ‘v’ with
respect to the field, then the induced emf ‘e’ will be
= BLV Sinθ Volts
© NIMI, Not to be republished

106
Workshop Calculation And Science (NSQF - Level 4 & 5) - 1st Semester
where
B = magnetic flux density, measured in tesla
L = effective length of the conductor in the field in metres
V = relative velocity between field and conductor in
metre/second.
θ = the angle at which the conductor cuts the magnetic
field.
pattern of induced emf in a conductor when it rotates under
N and S poles of uniform magnetic field.
The emf induced by this process is basically alternating in
nature, and this alternating current is converted into direct
current in a DC generator by the commutator.
Fleming’s right hand rule: The direction of dynamically
induced emf can be identifued by this rile. Hold the thumb,
forefinger and middle finger of the righ hand at right angles
to each other as shown in Fig 4 such that the forefinger is
in the direction of flux and the thumb is in the direction of
the motion of the conductor, then the middle finger indi-
cates the direction of emf induced, i.e. towards the ob-
server or away from the observer.
Likewise for every position of the remaining conductors in
the periphery, the enf induced could be calculated. If these
values are plotted on a graph, it will represent the sine wave
© NIMI, Not to be republished
