
Version: 26 October 2021 Page 1 of 28
Two-dose SARS-CoV-2 vaccine effectiveness with mixed schedules and extended dosing
intervals: test-negative design studies from British Columbia and Quebec, Canada
Danuta M Skowronski
1,2
, Solmaz Setayeshgar
1
, Yossi Febriani
3
, Manale Ouakki
4
, Macy Zou
5
,
Denis Talbot
3,6
, Natalie Prystajecky
7,8
, John R Tyson
7,8
, Rodica Gilca
3,4,6
, Nicholas Brousseau
3,4,6
,
Geneviève Deceuninck
3
, Eleni Galanis
1,2
, Chris D Fjell
7
, Hind Sbihi
2,5
, Elise Fortin
3,4
, Sapha
Barkati
9
, Chantal Sauvageau
3,4,6
, Monika Naus
1,2
, David M Patrick
1,2
, Bonnie Henry
2,10
, Linda M
N Hoang
7,8
, Philippe De Wals
3,4,6
, Christophe Garenc
3,4
, Alex Carignan
11
, Mélanie Drolet
3,6
,
Manish Sadarangani
12,13
, Marc Brisson
3,6
, Mel Krajden
7,8
, Gaston De Serres
3,4,6
1
BC Centre for Disease Control, Communicable Diseases and Immunization Services,
Vancouver, British Columbia, Canada
2
University of British Columbia, School of Population and Public Health, Vancouver, British
Columbia, Canada
3
Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec
City, Quebec, Canada
4
Institut national de sante publique du Québec, Biological and Occupational Risks, Quebec City,
Quebec, Canada
5
BC Centre for Disease Control, Data and Analytics Services, Vancouver, British Columbia,
Canada
6
Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec
City, Quebec, Canada
7
BC Centre for Disease Control, Public Health Laboratory, Vancouver, British Columbia,
Canada
8
University of British, Department of Pathology and Laboratory Medicine, Vancouver, British
Columbia, Canada
9
McGill University, Department of Medicine, Division of Infectious Diseases, McGill University
Health Center, Montreal, Quebec, Canada
10
Office of the Provincial Health Officer, Ministry of Health, Victoria, British Columbia, Canada
11
Sherbrooke University, Department of Microbiology and Infectious Diseases, Sherbrooke,
Quebec, Canada
12
BC Children’s Hospital Research Institute, Vaccine Evaluation Center, Vancouver, British
Columbia, Canada
13
University of British Columbia, Department of Pediatrics, Vancouver, British Columbia,
Canada
Corresponding Author: Alternate corresponding author:
Danuta M Skowronski MD, FRCPC Gaston De Serres MD, PhD
BC Centre for Disease Control Institut National de Santé Publique du Québec
655 West 12th Avenue 2400 Rue d’Estimauville
Vancouver, British Columbia Quebec, PQ
Canada V5Z 4R4 Canada G1E 7G9
Ph: 604-707-2511 Ph: 418-650-5115 Ext. 6274
E-mail: danuta.skowronski@bccdc.ca
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.

Version: 26 October 2021 Page 2 of 28
ABSTRACT
Background
The Canadian COVID-19 immunization strategy deferred second doses and allowed mixed
schedules. We compared two-dose vaccine effectiveness (VE) by vaccine type (mRNA and/or
ChAdOx1), interval between doses, and time since second dose in two of Canada’s larger
provinces.
Methods
Two-dose VE against infections and hospitalizations due to SARS-CoV-2, including variants of
concern, was assessed between May 30 and October 2, 2021 using test-negative designs separately
conducted among community-dwelling adults
18-years-old in British Columbia (BC) and
Quebec, Canada.
Findings
In both provinces, two doses of homologous or heterologous SARS-CoV-2 vaccines were
associated with ~95% reduction in the risk of hospitalization. VE exceeded 90% against SARS-
CoV-2 infection when at least one dose was an mRNA vaccine, but was lower at ~70% when both
doses were ChAdOx1. Estimates were similar by age group (including adults
70-years-old) and
for Delta-variant outcomes. VE was significantly higher against both infection and hospitalization
with longer 7-8-week vs. manufacturer-specified 3-4-week interval between doses. Two-dose
mRNA VE was maintained against hospitalization for the 5-7-month monitoring period and while
showing some decline against infection, remained
80%.
Interpretation
Two doses of mRNA and/or ChAdOx1 vaccines gave excellent protection against hospitalization,
with no sign of decline by 5-7 months post-vaccination. A 7-8-week interval between doses
improved VE and may be optimal in most circumstances. Findings indicate prolonged two-dose
protection and support the use of mixed schedules and longer intervals between doses, with global
health, equity and access implications in the context of recent third-dose proposals.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 3 of 28
BACKGROUND
In Canada, two mRNA vaccines were approved in December 2020 according to a two-dose
schedule with interval between doses of three weeks for BNT162b2 (Pfizer-BioNTech,
Comirnaty) and four weeks for mRNA-1273 (Moderna, Spikevax).
1
On February 26, 2021, a
chimpanzee adenoviral vectored (ChAdOx1) vaccine (AstraZeneca, Vaxzevria and equivalent
COVISHIELD) was authorized with an interval of 4-12 weeks between doses.
1
In randomized
controlled trials (RCTs), the efficacy of a single dose exceeded 90% for mRNA and 75% for
ChAdOx1 vaccines.
2–5
In early January 2021, confronted with elevated pandemic activity and
constrained vaccine supplies, two provinces, British Columbia (BC) and Quebec, opted to extend
the interval between doses (to six weeks and 12 weeks, respectively), to provide the benefits of
substantial single-dose protection to as many people as possible, as soon as possible.
In early March 2021 (epidemiological week 9), Canada’s National Advisory Committee on
Immunization (NACI) endorsed a second dose-deferral approach, recommending that the interval
between first and second doses of all SARS-CoV-2 vaccines be extended up to 16 weeks.
6
On
March 29 (week 13), in response to emerging reports of ChAdOx1-associated thrombosis with
thrombocytopenia, NACI recommended ChAdOx1 be used for adults
55-years-old.
7
On April 23
(week 16), NACI lowered this age limit to
30 years.
7
After assessing interchangeability, NACI
further recommended on June 1 (week 22), that first-dose recipients of ChAdOx1 could be
offered either the same product or an mRNA vaccine, and that first-dose mRNA recipients could
complete the series with the alternate mRNA vaccine if the same product was not available.
8
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 4 of 28
The first SARS-CoV-2 vaccines in BC and Quebec were prioritized for long-term care facility
(LTCF) residents and healthcare workers. Vaccination of community-dwelling adults began with
the oldest in March 2021, progressing sequentially to younger age groups. With the recommended
deferral, second-dose coverage started to increase among elderly adults in May. As vaccine supply
improved, the interval between first and second doses was shortened to eight weeks in late-May,
by which time (week 21) about 70% of all adults
18-years-old in BC and Quebec had received at
least one dose and <10% had received two doses. The interval between doses was again shortened
in August to four weeks to maximize the number of fully-vaccinated individuals before autumn,
with >80% of adults
18 years in both provinces having received two doses by early-October
2021.
We report two-dose vaccine effectiveness (VE) against infection and hospitalization, including
due to the Delta variant of concern (VOC), among adults
18-years-old in BC and Quebec.
Adjustments to the COVID-19 vaccination program in response to changing pandemic conditions
in BC and Quebec provided further unique opportunity to compare two-dose VE by vaccine type,
both homologous and heterologous; by interval between doses; and by time since the second dose.
METHODS
Source population
There are ~4 million adults
18-years-old in BC, the westernmost province of Canada, and ~7
million in Quebec, located in eastern Canada ~5000 km apart. About half the adult population in
BC and Quebec are women with similar age distributions 18–49, 50–69 and
70 years: 51% and
49%, 32% and 33%, and 16% and 17%, respectively. A publicly-funded, mostly symptom-based
approach for PCR-based SARS-CoV-2 diagnostic testing is broadly accessible in both provinces.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 5 of 28
Each province experienced a third pandemic wave that peaked in mid-April 2021 (weeks 14–15)
then subsided to stable low levels from early-June (weeks 22–23) before gradually increasing
again with the start of a fourth pandemic wave in late-July/early-August (weeks 29-31).
9,10
Whereas during the third wave the Alpha variant predominated in Quebec, and co-dominated with
Gamma in BC, during the fourth wave the Delta variant became dominant in both provinces.
9,10
Study design
Two-dose VE was estimated by test-negative design, using multivariable logistic regression to
derive the adjusted odds ratio (AOR) for vaccination among SARS-CoV-2 test-positive cases
versus test-negative controls. VE and 95% confidence intervals (CI) were computed as (1-AOR) x
100%. Unless otherwise specified, all adjusted models included age group (18–49 years, 50–69
years, 70–79 years and
80 years), sex (men/women), epidemiological week (categorical) and
region. The latter includes the five health authorities in BC,
9
with the 18 administrative regions of
Quebec also regrouped into five categories (Greater Montreal, Greater Quebec City, Central
Quebec, Northern Quebec and others).
10
Case and control selection
Specimens with collection dates between weeks 22–39 (May 30 to October 2) were eligible. Cases
included any RT-PCR-confirmed SARS-CoV-2 infection. Hospitalized cases were admitted on or
30 days after specimen collection. Individuals could contribute a single test-positive specimen
and were censored from any contribution thereafter. Controls included all specimens that were
RT-PCR-negative for SARS-CoV-2 and met inclusion/exclusion criteria.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 6 of 28
In variant-specific analyses, cases were categorized as Alpha, Gamma, or Delta VOC. Methods
and sampling frame for genetic characterization evolved in response to changing epidemic
conditions, case load and laboratory capacity, as described in Supplementary Table 1.
Vaccine status definition
Clients with record of having received two doses of BNT162b2, mRNA-1273 or ChAdOx1 on or
before the specimen collection date were considered vaccinated; those with no record of
vaccination prior to specimen collection were considered unvaccinated. Among respiratory
specimens collected for SARS-CoV-2 testing that had both dates available, the median interval
between illness onset and respiratory specimen collection was two days with interquartile range of
1–4 days in both provinces. We based primary two-dose VE analyses on second-dose receipt
14
days before specimen collection, excluding those vaccinated 0-13 days prior.
Data sources and exclusions
Specimens were sampled from respective provincial databases capturing all RT-PCR testing for
SARS-CoV-2 along with client, collection and testing details. Hospitalized cases were identified
through linkage with notifiable disease lists, supplemented in Quebec by the administrative
hospitalization database. Vaccination information was obtained from provincial immunization
registries capturing all SARS-CoV-2 vaccinations along with client and vaccination details.
Individual-level database linkages were achieved through unique personal identifiers.
Specimens with invalid or missing information were excluded as were specimens collected from
individuals: identified as cases before the analysis period; residents of LTCFs, assisted-living or
independent-living facilities; vaccinated with a single dose or product other than BNT162b2,
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint
Version: 26 October 2021 Page 7 of 28
mRNA-1273 or ChAdOx1; or when tested outside of the public-funding scheme owing to
systematically lower likelihood of test-positivity.
9
The latter criterion also excludes individuals
routinely screened for travel.
Ethics statement
Data linkages and analyses were authorized by the Provincial Health Officer (BC) and National
Director of Public Health (Quebec) under respective provincial public health legislation without
requirement for research ethics board review.
Role of the funding source
Provincial health authorities provided funding and had no role in the design, results,
interpretation or decision to submit.
FINDINGS
Case and control profiles
In total, 380,532 specimens contributed to VE analyses in BC including 27,439 (7%) test-positive
cases of whom 1582 (6%) were hospitalized (Table 1). In Quebec, 854,915 specimens contributed
with 17,234 (2%) test-positive cases of whom 878 (5%) were hospitalized. More than 85% of all
cases in both provinces accrued during the fourth wave between weeks 31–39. About two-thirds of
participating case viruses in each province were genetically-characterized overall, and of those,
91% in BC and 85% in Quebec were the Delta variant (Supplementary Table 1). Cases and
controls by age and sex were similar in both provinces. Hospitalized cases were older and more
often male (Table 1). Compared to their share of the population, younger adults and females
contributed disproportionately to controls.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 8 of 28
Vaccination profiles
By week 39, >80% of controls in BC and Quebec had been vaccinated with >90% having received
two mRNA doses, 3% ChAdOx1 and 5% mixed ChAdOx1/mRNA products (Table 1). Among
mixed ChAdOx1/mRNA recipients, >99% in each province had received ChAdOx1 first. About
two-thirds of vaccinated controls in BC and Quebec received BNT-162b; 11% and 20%
respectively, received mRNA-1273; and 8% and 3%, respectively, received a mix of either mRNA
product of whom 80% and 70%, respectively, received BNT162b2 first. Follow-up periods are
shown in Table 1 and by vaccine type in Supplementary Tables 2 and 3. ChAdOx1 recipients in
both provinces were generally older and with less follow-up time after their second dose.
Vaccine effectiveness
By vaccine type and outcome, including mixed schedules and VOC
Two-dose mRNA VE against infection was 90% (95%CI: 89–90) in BC and 88% (95%CI: 88–89)
in Quebec, similar among recipients of the same or mixed mRNA doses (Figure 1, Supplementary
Table 4). VE was significantly lower among recipients of two doses of ChAdOx1 at 71% (95%CI:
69–74) in BC and 73% (95%CI: 69–77) in Quebec. However, among those who received a mixed
schedule of ChAdOx1 and mRNA vaccination, VE was significantly improved at 90% (95%CI:
89–91) in BC and 87% (95%CI: 85–89) in Quebec.
VE of two mRNA doses against hospitalization was 98% (95%CI: 97–98) in BC and 97%
(95%CI: 96–97) in Quebec, and similar for two doses of ChAdOx1 at 94% (95%CI: 90–96) and
94% (95%CI: 89–97), respectively (Figure 1). VE against hospitalization was similar among
recipients of mixed mRNA or mixed ChAdOx1/mRNA doses (Supplementary Table 4).
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 9 of 28
VE findings were similar by age group, notably including older adults
70 years (Figure 2,
Supplementary Tables 5 and 6) and did not meaningfully differ by sex (Figure 2, Supplementary
Table 7). VE against the Delta variant was almost identical to the overall analysis (Figure 1), and
was similar for other VOC, recognizing smaller sample size (Supplementary Tables 8 and 9).
By time since vaccination
In both provinces, two-dose mRNA VE
95% against hospitalization was maintained through the
seventh month post-vaccination (Figure 3; Supplementary Table 10). Two-dose mRNA VE
against any infections peaked above 90% at 2–3 weeks post-vaccination, but remained about 80%
or more through the eighth month. Given greater sample size, findings are most robust for
BNT162b2 with similar pattern for mRNA-1273 (Supplementary Table 11) and mixed mRNA or
ChAdOx1/mRNA recipients, recognizing limited follow-up beyond the fourth or fifth month
(Supplementary Table 12). For homologous two-dose ChAdOx1 recipients, VE
70% was also
maintained for at least the fourth month post-vaccination (Figure 3; Supplementary Table 10).
There was no indication of greater decline in two-dose protection against Delta (Supplementary
Tables 13–15). Among adults
70-years-old, mRNA VE was 80% against infection and 90%
against hospitalization to at least the fifth month, with smaller sample size thereafter (Figure 4;
Supplementary Table 16).
By interval between doses
Estimates of mRNA VE against infection improved with a longer interval between first and
second doses. With the manufacturer-specified interval of 3–4 weeks, VE in BC was 85%
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 10 of 28
(95%CI: 83–87) and in Quebec was 79% (95%CI: 76–81). At 7-8 weeks VE was significantly
higher at 91% (95%CI: 91–91) and 89% (95%CI: 88–89), respectively, and remained relatively
stable thereafter (Figure 5, Supplementary Table 17). A similar pattern was observed against
hospitalizations with greater VE at 7–8-week interval (99%; 95%CI: 98–99 and 98%; 95%CI: 97–
99, respectively) vs. 3–4-week interval (93%; 95%CI: 87–96 and 87%; 95%CI: 79–92). Given
larger sample size, findings are most robust for BNT162b2 (Supplementary Table 18). Confidence
intervals were wide for ChAdOx1; in Quebec, but not BC, VE showed gradual increase with
longer interval (Figure 5).
Recognizing that shorter intervals between doses may have been associated with longer time since
second dose, we also explored VE stratified simultaneously for period effects. This is shown for
BNT162b2 in Figure 6 and for all vaccine types in Supplementary Table 19. The approximate 5-
10% higher VE when the second dose was spaced
7- versus 3–4 weeks after the first, was
maintained at all time points since the second dose. In each province the gap was comparable at 2–
3 weeks and
16 weeks post-vaccination. Similar pattern was observed for mRNA-1273 and
ChAdOx1 but with greater variability given smaller sample sizes.
INTERPRETATION
From two of the larger provinces of Canada, located at nearly opposite ends of the country, we
report concordant findings of two-dose SARS-CoV-2 VE against infection and hospitalization,
including with mixed vaccines and extended dosing intervals. In both provinces, two homologous
or heterologous doses were associated with about 95% reduction in the risk of hospitalization. VE
also exceeded 90% against SARS-CoV-2 infection when at least one dose was an mRNA vaccine,
but was lower at about 70% when both doses were ChAdOx1. The Delta variant was not
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint
Version: 26 October 2021 Page 11 of 28
associated with compromised VE. Vaccine protection was even better when the spacing between
doses was longer than the 3-4 weeks recommended by manufacturers. Over the 5-7-month period
after the second dose, mRNA VE was maintained at about 95% against hospitalizations, showing
some decline but remaining about 80% or more against infections. With shorter follow-up time for
the smaller two-dose ChAdOx1 subset, VE was also maintained to at least the fourth month post-
vaccination.
To date there are no head-to-head RCT comparisons of homologous and heterologous SARS-
CoV-2 vaccine efficacy. In immunogenicity studies, heterologous ChAdOx1 followed by mRNA
vaccination induced antibody titers exceeding homologous vector vaccination and comparable to
homologous mRNA vaccination.
11–13
For pragmatic and immunologic reasons, some countries
(e.g. France, Germany, Spain, the United Kingdom (UK)) have, like Canada, recommended and
accepted mixed schedules as providing valid two-dose protection. Other countries strictly adhere
to the homologous schedules submitted for regulatory approval by sponsor manufacturers.
Modification of regulatory approval thereafter typically requires submission of updated data by the
sponsor. However, to meet public health needs during a rapidly evolving crisis, decision-makers
must be able to respond to emerging post-marketing evidence. In Canada, real-time expert
committee recommendation, rather than regulatory review, allowed mixed schedules. By
removing the requirement to maintain half of available doses in reserve for homologous series
completion, this greatly simplified vaccine deployment and sped vaccine coverage. The findings
presented here strongly reinforce vaccine interchangeability to complete the two-dose SARS-
CoV-2 vaccine series. Mixed vaccine schedules could reduce logistical hurdles and support nimble
vaccination campaigns in countries elsewhere that are still struggling with low vaccine supplies
and/or coverage. Global recognition of homologous or heterologous doses as valid proof of
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint
Version: 26 October 2021 Page 12 of 28
vaccination could facilitate a more rapid end to the pandemic and more equitable opportunities for
re-opening, travel and return to normal socio-economic interactions, everywhere.
Our estimates of two-dose VE against infection exceeding 90% for mRNA but lower at about 70%
for ChAdOx1 vaccine are consistent with respective gold-standard RCT findings,
3–5
and in
particular with maximal ChAdOx1 two-dose efficacy against any infection of 66% in pooled RCT
meta-analysis.
5
In extended follow-up of participants in the pivotal BNT162b2 RCT, two-dose
efficacy against clinical infection peaked at 96% during the first two months but remained >80%
between four months and the end of follow-up, similar to the sustained protection we report.
14
In
the pivotal mRNA-1273 RCT, efficacy against COVID-19 illness was 93%, without indication of
waning across a median of 5·2 months.
15
Of note, following unblinding of the mRNA-1273 RCT,
a two-year open-label study was initiated: participants who had originally received placebo were
vaccinated (mRNA-1273p) and compared to participants who were earlier randomized to
vaccination (mRNA-1273e).
16
During the Delta surge in July and August 2021, the rate of
COVID-19 and of severe COVID-19 was about 1·6 times greater among the mRNA-1273e group
(median 13 months follow-up from the first dose) than the mRNA-1273p group (median follow-
up 7·9 months). Applying this relative risk (RR) of 1·6 to the COVID-19 incidence in vaccinated
individuals from the original RCT similarly translates into only minor drop in efficacy from 93%
in the original trial to 89% with the longer time since vaccination.
Similar to our findings, other jurisdictions have also reported sustained two-dose vaccine
protection against hospitalization, including due to the Delta variant.
17–23
Studies from Israel,
however, have reported greater risk of both infection and hospitalization with time since the
second BNT162b2 dose.
24
In the UK, VE against Delta hospitalization was 77% for ChAdOx1
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint
Version: 26 October 2021 Page 13 of 28
and 93% for BNT162b2 by 20 weeks after the second dose but the decline in VE against
symptomatic disease was greater at 47% and 70%, respectively.
21
Observational studies from
California and Qatar have reported even more rapid decline in mRNA VE against infection by five
months after the second dose, to just 50% and 22%, respectively.
22,23
The underlying reasons for
these differences in the observed duration of protection are unclear. Despite limitations,
surveillance data may provide useful reality check against which to balance some of the more
alarming reports of declining VE. For example, the VE estimate of 50% from California
corresponds to a RR of two for COVID-19 infection in unvaccinated compared to fully-vaccinated
people. However, a RR of two is itself inconsistent with statewide surveillance data instead
showing RR exceeding seven between September 26 and October 2, 2021,
25
crudely
corresponding to a VE of 87% five months after most fully-vaccinated Californians had received
their second dose. Conversely, RR estimates based upon surveillance data from the UK seem
more in line with their VE estimates.
26
Likewise, in BC and Quebec, during the most recent four-
week period available (spanning to early or mid-October, respectively), crude surveillance-based
RR estimates of 10 and 7 for infection, respectively, and 52 and 24 for hospitalization,
respectively,
9,27
correspond to VE estimates >85% against infection and >95% against
hospitalization, reassuringly similar to the estimates we report here.
We observed improved VE with interval between first and second doses longer than the 3-4 weeks
recommended by manufacturers. Immunogenicity studies have shown that longer intervals are
conducive to more complete maturation of the immune response after the first dose, stronger
response to the second dose, and ultimately higher and more durable SARS-CoV-2 antibody
levels.
28,29
In the UK, VE was consistently higher with an interval >45 days compared to 19–29
days between BNT162b2 doses, with more erratic findings for ChAdOx1.
28
In the pooled-analysis
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 14 of 28
of ChAdOx1 RCTS, however, efficacy was much higher with an interval >8 weeks.
5
Also from
the UK, Pouwels et al. found no evidence that BNT162b2 or ChAdOx1 effectiveness varied when
comparing dichotomous intervals <9 or
9 weeks between doses;
30
however, their broad
categorization <9 weeks may have diluted the lower VE associated with shorter intervals. The
optimal interval between doses ultimately represents a balance between rapid and enhanced
protection. During a surging pandemic wave, rapid administration of the second dose may prevent
some cases that would occur with a longer wait but otherwise, surveillance data in most countries
suggest <1% of the unvaccinated population were infected during a given four-week period of the
pandemic. With substantial single-dose protection against hospitalization, the absolute risk of
severe outcome associated with waiting a few more weeks for the second dose would be small in
that context. Conversely, the more durable immunity and approximate 5-10% increment in VE we
observed with 7-8-week interval between doses could pay dividends into the future, ultimately
preventing more cases and hospitalizations (depending upon evolving incidence and duration of
protection). In most instances, therefore, an interval of 7-8 weeks between the first and second
dose seems optimal for mRNA vaccines, not only to maximize single-dose coverage in the context
of vaccine scarcity but also to optimize the second-dose booster response.
This study, based upon general laboratory submissions, surveillance registries and administrative
data, has limitations. In particular, such data are subject to missing or incomplete information,
misclassification and selection bias. The test-negative design partially standardizes for healthcare
seeking behaviours, but testing indications for SARS-CoV-2 are broad and discretionary, and case
ascertainment will have varied between population sub-groups and over time. With increasing
vaccine coverage, the subset of remaining unvaccinated individuals may be less comparable in
their likelihood of testing positive, with the direction of such bias unknown, and likely to vary
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint
Version: 26 October 2021 Page 15 of 28
with other public health measures. VE estimates were adjusted for calendar time (and age, sex,
region of residence) but information pertaining to other potential confounders such as co-
morbidity and socio-economic status were not readily available. We cannot rule out residual
confounding. Higher-risk healthcare workers or immunocompromised individuals targeted for
more rapid second-dose receipt may have contributed to the lower VE associated with shorter
interval between doses; however, weighted by their small percentage of the population, such
under-estimation would be minor. Further reduced sample size with additional stratification affects
the precision of VE estimates, requiring cautious interpretation. Finally, to address differential
likelihood of vaccination and exposure risk, our studies were conducted in community-dwelling
adults; results may not be generalizable to residents of LTCFs and nursing homes.
In conclusion, two doses of mRNA and/or ChAdOx1 vaccines provided powerful and persistent
protection against hospitalization, including due to the Delta variant, without sign of decline by 5-
7 months post-vaccination among community-dwelling adults, including older adults. VE against
infection declined from an earlier post-vaccination peak above 90% but still prevented 80% or
more of infections by the seventh month post-vaccination. Extending the interval between first and
second doses may have optimized booster dose protection in Canada. Given these findings, the
need and timing of a third dose warrant serious reflection by decision-makers, especially since
two-dose, or even one-dose, coverages still remain low in many areas of the world. Our findings
support mixed SARS-CoV-2 vaccine schedules and extended intervals between doses, each of
which may improve vaccine coverage and have health, equity and access implications globally.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint
Version: 26 October 2021 Page 16 of 28
Declaration of interests
GDS received a grant paid to his institution for a meningococcal seroprevalence study from
Pfizer in 2016. MK received grants/contracts paid to his institution from Roche, Hologic and
Siemens, unrelated to this work. MS has been an investigator on projects, unrelated to the current
work, funded by GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo and VBI
Vaccines. All funds have been paid to his institute, and he has not received any personal
payments. RG received honoraria for an RSV Coordinators Workshop funded by AbbVie. Other
authors have no conflicts of interest to disclose.
Funding
Provincial health authorities provided funding but had no role in the design, results, interpretation
or decision to submit.
Acknowledgments
We thank Shinhye Kim at the BC Centre for Disease Control for support in manuscript assembly
and the summary tabulation of findings. Manish Sadarangani acknowledges general salary
support provided to him by awards from the BC Children’s Hospital Foundation, the Canadian
Child Health Clinician Scientist Program and the Michael Smith Foundation for Health Research.
Denis Talbot was recipient of a Career Award from the Fond de recherche du Québec–Santé.
Finally, we thank the many frontline, regional and provincial practitioners, including clinical,
laboratory and public health providers, epidemiologists, Medical Health Officers, laboratory
staff, vaccinators, participants and others who contributed to the epidemiological, virological and
genetic characterization data underpinning these analyses.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 17 of 28
REFERENCES
1. National Advisory Committee on Immunization (NACI). Recommendations on the use of
COVID-19 vaccines. Ottawa: NACI. [Accessed 16 October 2021]. Available
from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-
committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html
2. Skowronski DM, De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19
vaccine. N Eng J Med. 2021; 384: 10.1056/NEJMc2036242#sa1.
https://doi.org/10.1056/NEJMc2036242
3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA
Covid-19 vaccine. N Engl J Med 2020; 383:2603–2615
4. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety
of the mRNA-1273 SARS-CoV-2 vaccine. N Eng J Med. 2020; 384:403–416.
https://doi.org/10.1056/JENJoa2035389
5. Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the
influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1
nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021;
397: 881–91. Doi: 10.1016/S0140-6736(21)00432-3
6. National Advisory Committee on Immunization (NACI). NACI rapid response: extended
dose intervals for COVID-19 vaccines to optimize early vaccine rollout and population
protection in the context of limited vaccine supply [2021-04-07]. Canada. Ottawa: NACI.
[Accessed 16 October 2021]. Available from: https://www.canada.ca/en/public-
health/services/immunization/national-advisory-committee-on-immunization-
naci/extended-dose-intervals-covid-19-vaccines-early-rollout-population-protection.html
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 18 of 28
7. National Advisory Committee on Immunization (NACI). NACI: Summary of updated
vaccine statement of April 23, 2021. Canada. Ottawa: NACI. [Accessed 16 October 2021].
Available from: https://www.canada.ca/en/public-health/services/immunization/national-
advisory-committee-on-immunization-naci/recommendations-use-covid-19-
vaccines/summary-updated-statement-april-23-2021.html
8. National Advisory Committee on Immunization (NACI). NACI rapid response:
Interchangeability of authorized COVID-19 vaccines [2021-06-01]. Canada. Ottawa:
NACI. [Accessed 16 October 2021]. Available from: https://www.canada.ca/en/public-
health/services/immunization/national-advisory-committee-on-immunization-
naci/recommendations-use-covid-19-vaccines/rapid-response-interchangeability.html
9. British Columbia Centre for Disease Control (BCCDC). British Columbia COVID-19
situation report. Vancouver: BCCDC. [Accessed 16 October 2021]. Available from:
http://www.bccdc.ca/health-info/diseases-conditions/covid-19/data
10. Institut national de santé publique du Québec (INSPQ). Données COVID-19 au Québec.
Quebec City: INSPQ. [Accessed 16 October 2021]. Available from:
https://www.inspq.qc.ca/covid-19/donnees
11. Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. Safety and
immunogenicity of heterologous versus homologous prime-boost schedules with an
adenoviral vectored and mRNA COVID- 19 vaccine (Com-COV): a single-blind,
randomised, non-inferiority trial. Lancet 2021; 398: 856–869.
12. Atmar RL, Lyke KE, Deming ME, et al. Heterologous SARS-CoV-2 booster vaccinations
– preliminary report. medRxiv preprint. 2021 [Accessed 17 October 2021]. Available
from: https://doi.org/10.1101/2021.10.10.21264827
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 19 of 28
13. Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against
SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-
19/BNT162b2 vaccination. Nat Med 2021; 27: 1525–29.
14. Thomas SJ, Moreira ED, Kitchin N, et al. Six month safety and efficacy of the BNT162b2
mRNA COVID-19 vaccine. medRxiv preprint. 2021 [Accessed 16 October 2021].
Available from: https://doi.org/10.1101/2021.07.28.21261159
15. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2
vaccine at completion of blinded Phase. N Engl J Med. 2021; 384: 403–16.
16. Baden LR, El Sahly HM, Essink B, et al. Covid-19 in the phase 3 trial of mRNA-1273
during the Delta-variant surge. medRxiv 2021 [Accessed 16 October 2021]. Available
from: https://doi.org/10.1101/2021.09.17.21263624
17. Thompson MG, Stenehjem E, Grannis S, Ball SW, Naleway AL, Ong TC, et al.
Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N Engl J
Med. 2021; 385: 1355–1371.
18. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against
the B.1.617.2 (Delta) variant. N Engl J Med. 2021; 385: 585-594.
19. Andrejko KL, Pry J, Myers JF, et al. Prevention of COVID-19 by mRNA-based vaccines
within the general population of California. Clin Infect Dis. 2021; 73 epub Jul 20. doi:
10.1093/cid/ciab640.
20. Pawlowski C, Lenehan P, Puranik A, et al. FDA-authorized mRNA COVID-19 vaccines
are effective per real-world evidence synthesized across a multi-state health system. Med
(N Y). 2021; 2: 979–992.e8. doi: 10.1016/j.medj.2021.06.007
21. Andrews N, Tessier E, Stowe J, et al. Vaccine effectiveness and duration of protection of
Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 20 of 28
Knowledgehub [Preprint]. 2021 [Accessed 16 October 2021]. Available from:
https://khub.net/documents/135939561/338928724/Vaccine+effectiveness+and+duration
+of+protection+of+covid+vaccines+against+mild+and+severe+COVID-
19+in+the+UK.pdf/10dcd99c-0441-0403-dfd8-11ba2c6f5801
22. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19
vaccine up to 6 months in a large integrated health system in the USA: a retrospective
cohort study. Lancet. 2021; 398:1407–16.
23. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection
against SARS-CoV-2 Infection in Qatar. N Engl J Med. 2021; 385 epub Oct 6. doi:
10.1056/NEJMoa2114114
24. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity of the BNT162b2 vaccine:
A nationwide study from Israel. medRxiv 2021 [Accessed 16 October 2021]. Available
from: https://www.medrxiv.org/content/10.1101/2021.08.24.21262423v1
25. Government of California. Tracking COVID-19 in California. Unvaccinated and
vaccinated data. Updated October 15 2021. [Accessed 16 October 2021]. Available from:
https://covid19.ca.gov/state-dashboard/#postvax-status
26. UK Health Security Agency. COVID-19 vaccine surveillance report, week 39. [Accessed
16 October 2021]. Available from:
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_
data/file/1022238/Vaccine_surveillance_report_-_week_39.pdf
27. Gouvernement du Québec. Tableau de bord-Situation de la COVID-19 -14 octobre 2021.
[Accessed 16 October 2021]. Available from: https://cdn-contenu.quebec.ca/cdn-
contenu/sante/documents/Problemes_de_sante/covid-19/20-210-
382W_infographie_sommaire-executif.jpg?1634310027
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October 2021 Page 21 of 28
28. Amirthalingam G, Bernal, JL, Andrews, NJ, et al. Higher serological responses and
increased vaccine effectiveness demonstrate the value of extended vaccine schedules in
combatting COVID-19 in England. medRxiv 2021 [Accessed 16 October 2021]. Available
from: https://www.medrxiv.org/content/10.1101/2021.07.26.21261140v1.full.pdf
29. Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing
intervals of BNT162b2 mRNA vaccine. Cell 2021. Preprint. [Accessed 16 October 2021].
Available from https://www.cell.com/action/showPdf?pii=S0092-8674%2821%2901221-
6
30. Pouwels KB, Pritchard E, Matthews PC, et al. Impact of Delta on viral burden and
vaccine effectiveness against new SARS-CoV-2 infections in the UK. medRxiv preprint.
2021 [Accessed 26 October 2021]. Available:
https://www.medrxiv.org/content/10.1101/2021.08.18.21262237v1
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October, 2021 Page 22 of 28
Table 1. Profile of participants 18-year-olds, by case and vaccine status (regardless of time since vaccination), British Columbia and Quebec, Canada
British Columbia Quebec
Overall [N=380532] (column %) Vaccinated [N=285102] (row %)
1
Overall [N=854915] (column %) Vaccinated [N=630631] (row %)
1
Cases Hosp Controls Cases Hosp Controls Cases Hosp Controls Cases Hosp Controls
Total
27439 (7) 1582 (6)
2
353093 (93) 8630 (31) 217 (14) 276472 (78) 17234 (2) 878 (5)
2
837681 (98) 5868 (34) 176 (20) 624763 (75)
Age group (years)
18-49 20085 (73) 588 (37) 217157 (62) 5509 (27) 30 (5) 159310 (73) 13070 (76) 348 (40) 478750 (57) 3606 (28) 31 (9) 319674 (67)
50-69 5792 (21) 598 (38) 87688 (25) 2329 (40) 75 (13) 73864 (84) 3267 (19) 311 (35) 232291 (28) 1622 (50) 40 (13) 188911 (81)
70-79 1116 (4) 233 (15) 29538 (8) 551 (49) 47 (20) 26680 (90) 610 (4) 122 (14) 80884 (10) 429 (70) 47 (39) 74956 (93)
80+ 446 (2) 163 (10) 18710 (5) 241 (54) 65 (40) 16618 (89) 287 (2) 97 (11) 45756 (6) 211 (74) 58 (60) 41222 (90)
Median (Interquartile range) 37 (28-51) 57 (41-70) 42 (31-60) 42 (31-57) 71 (60-82) 44 (32-62) 36 (27-49) 56 (42-70) 45 (33-62) 44 (33-58) 73 (60-82) 49 (35-66)
Sex
Female 13313 (49) 647 (41) 198686 (56) 4595 (35) 92 (14) 159390 (80) 8756 (51) 372 (42) 502864 (60) 3195 (36) 77 (21) 369338 (73)
Male 14126 (51) 935 (59) 154407 (44) 4035 (29) 125 (13) 117082 (76) 8478 (49) 506 (58) 334817 (40) 2673 (32) 99 (20) 255425 (76)
Epidemiological week
22-25 (May 30-June 26) 1493 (5) 119 (8) 29093 (8) 97 (6) 5 (4) 11068 (38) 1227 (7) 62 (7) 111687 (13) 76 (6) 6 (10) 46541 (42)
26-30 (June 27-July 31) 1611 (6) 84 (5) 49434 (14) 346 (21) 13 (15) 35026 (71) 1118 (7) 50 (6) 166516 (20) 234 (21) 6 (12) 112998 (68)
31-35 (August 1-Sept 4) 12588 (46) 642 (41) 131403 (37) 3527 (28) 75 (12) 106647 (81) 6551 (38) 312 (36) 250102 (29) 2037 (31) 58 (19) 197160 (79)
36-39 (Sept 5-Oct 2) 11747 (43) 737 (47) 143163 (41) 4660 (40) 124 (17) 123731 (86) 8338 (48) 454 (51) 309376 (37) 3521 (42) 106 (23) 268064 (87)
Vaccinated
3
Two any mRNA vaccines 7535 (27)
4
198 (13) 252803 (72)
5
7535 (87) 198 (91) 252803 (91) 5379 (31) 155 (18) 576827 (69) 5379 (92) 155 (88) 576827 (92)
Two BNT162b2 5593 (20) 136 (9) 184987 (52) 5593 (65) 136 (63)
184987 (67)
4323 (25) 123 (14) 436106 (52) 4323 (74) 123 (70) 436106 (70)
Two mRNA-1273 1408 (5) 44 (3) 46596 (13) 1408 (16) 44 (20)
46596 (17)
914 (5) 25 (3) 123986 (15) 914 (16) 25 (14) 123986 (20)
Two mixed mRNA 533 (2) 18 (1) 21208 (6) 533 (6) 18 (8)
21208 (8)
142 (1) 7 (1) 16735 (2) 142 (2) 7 (4) 16735 (3)
Two ChAdOx1 652 (2) 17 (1) 8635 (2) 652 (8) 17 (8) 8635 (3) 236 (1) 14 (2) 18826 (2) 236 (4) 14 (8) 18826 (3)
Two mixed ChAdOx1/mRNA 443 (2) 2 (<1) 15034 (4) 443 (5) 2 (<1) 15034 (5) 253 (2) 7 (1) 29110 (3) 253 (4) 7 (4) 29110 (5)
Interval between doses
3
21-34 days (3-4 weeks) NA NA NA 6261 (2) 277 (3) 5984 (2) NA NA NA 430 (7) 19 (11) 23300 (4)
35-48 days (5-6 weeks) NA NA NA 18522 (7) 782 (9) 17740 (6) NA NA NA 484 (8) 6 (3) 35880 (6)
49-62 days (7-8 weeks) NA NA NA 106284 (37) 3286 (38) 102998 (37) NA NA NA 1636 (28) 15 (9) 149696 (24)
63-83 days (9-11 weeks) NA NA NA 103641 (36) 2802 (32) 100839 (36) NA NA NA 1808 (31) 50 (28) 204352 (33)
84-111 days (12-15 weeks) NA NA NA 44228 (16) 1284 (15) 42944 (16) NA NA NA 973 (17) 52 (30 130874 (21)
112+ days (16+ weeks) NA NA NA 6166 (2) 199 (2) 5967 (2) NA NA NA 537 (9) 34 (19) 80661 (13)
Time since second dose
3
0-13 days (0-1 weeks) NA NA NA 986 (11) 20 (9) 24123 (9) NA NA NA 762 (13) 13 (7) 69880 (11)
14-27 days (2-3 weeks) NA NA NA 397 (5) 9 (4) 29811 (11) NA NA NA 335 (6) 16 (9) 78087 (12)
28-55 days (4-7 weeks) NA NA NA 1793 (21) 30 (14) 75307 (27) NA NA NA 1299 (22) 27 (15) 173375 (28)
56-83 days (8-11 weeks) NA NA NA 2685 (31) 62 (29) 82806 (30) NA NA NA 1923 (33) 46 (26) 170639 (27)
84-111 days (12-15 weeks) NA NA NA 1814 (21) 74 (34) 45276 (16) NA NA NA 1121 (19) 57 (32) 90232 (14)
112-139 days (16-19 weeks) NA NA NA 390 (5) 14 (6) 9124 (3) NA NA NA 311 (5) 13 (7) 28130 (5)
140-167 days (20-23 weeks) NA NA NA 89 (1) 3 (1) 2385 (1) NA NA NA 89 (2) 3 (2) 12454 (2)
168-195 days (24-27 weeks) NA NA NA 130 (2) 0 (0) 2618 (1) NA NA NA 19 (<1) 0 (0) 1397 (<1)
196+ days (28+ weeks) NA NA NA 346 (4) 5 (2) 5022 (2) NA NA NA 9 (<1) 1 (1) 569 (<1)
Median, Interquartile range NA NA NA 68 (43-90) 76 (51-95) 58 (34-82) NA NA NA 64 (38-85) 79 (47-96) 54 (29-79)
Range (days) NA NA NA 0-250 2-231 0-265 NA NA NA 0-240 1-210 0-256
NA = Not applicable
1
Unless otherwise specified, displayed is the percentage of cases, hospitalized cases or controls who received a second vaccine dose by on or before specimen collection, by row category, regardless of time since second dose.
2
Displayed is the percentage of cases who were hospitalized.
3
All percentages displayed below this row are column %
4
One case twice vaccinated with unspecified mRNA vaccines in British Columbia.
5
Twelve controls twice vaccinated with unspecified mRNA vaccines in British Columbia
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint
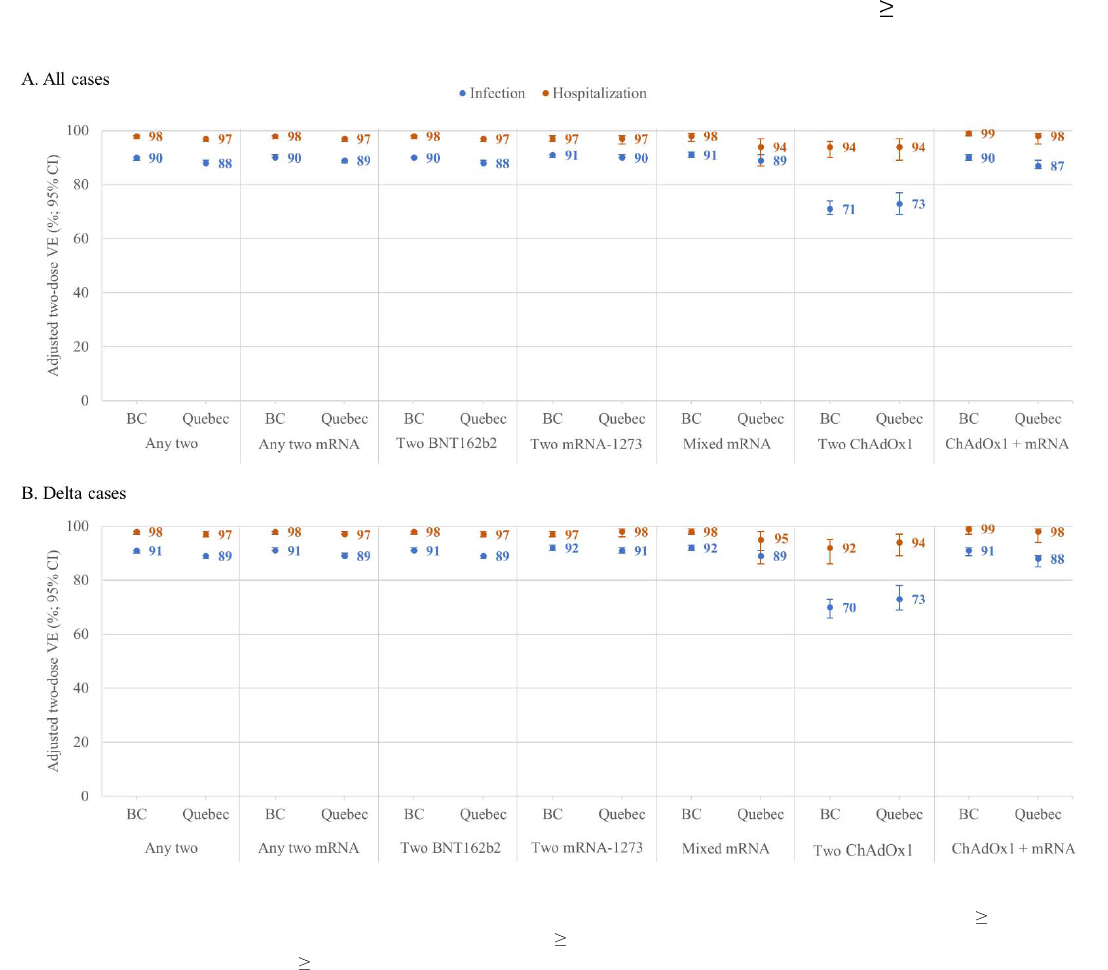
Version: 26 October, 2021 Page 23 of 28
Figure 1. Adjusted two-dose vaccine effectiveness against infection and hospitalization, by vaccine type, 18-year-olds, British Columbia and
Quebec, Canada
Shown are adjusted vaccine effectiveness (VE) estimates and 95% confidence intervals (CI) against infection (blue) and hospitalization (orange) 14 days after the second dose by
vaccine type, overall (panel A) and for the Delta variant of concern (panel B) among adults 18 years old in the provinces of British Columbia (BC) and Quebec, Canada. All VE
estimates are adjusted for age group (18-49, 50-69, 70-79,
80 years); sex (men, women); individual epidemiological week of the analysis period (weeks 22-39, categorical); and
region of the province (5 in each province). In Quebec, VE against hospitalizations due to the Delta variant was assessed only between weeks 31-39 because no hospitalized Delta
variant cases were identified prior to that period. For additional details including corresponding sample sizes and precise unadjusted and adjusted estimates and 95%CI, see
Supplementary Table 4 (overall) and Supplementary Tables 8 and 9 (Delta and other variants of concern).
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October, 2021 Page 24 of 28
Figure 2. Adjusted two-dose vaccine effectiveness against infection and hospitalization, by age group, sex and vaccine type, 18-year-olds,
British Columbia and Quebec, Canada
Shown are adjusted vaccine effectiveness (VE) estimates and 95% confidence intervals (CI) against infection (blue) and hospitalization (orange) 14 days after the second dose by
age group (panel A) and sex (panel B) and by vaccine type in British Columbia (BC) and Quebec (QC), Canada. In panel A, VE estimates are adjusted for sex (men, women);
individual epidemiological week of the analysis period (weeks 22-39, categorical); and region of the province (5 in each province). Among those
70 years, VE estimates are
additionally adjusted for 70-79 and 80 years. In panel B, VE estimates are adjusted for the same covariates, omitting sex and including age group (18-49 years, 50-69 years, 70-79
years,
80 years). In Quebec, age-specific VE estimates against hospitalization in those 18-49 years of age were adjusted for calendar time bi-weekly and in 70-year-olds were
adjusted tri-weekly owing to sample size. For additional details including corresponding sample sizes and precise unadjusted and adjusted estimates and 95%CI, see Supplementary
Tables 5-7.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October, 2021 Page 25 of 28
Figure 3. Adjusted two-dose vaccine effectiveness against infection and hospitalization, by time since vaccination, mRNA and ChAdOx1
vaccines,
18-year-olds, British Columbia and Quebec, Canada
Shown are adjusted vaccine effectiveness (VE) estimates and 95% confidence intervals (CI) against infection (blue) and hospitalization (orange) by time between receipt of the
second dose and specimen collection, among adults
18 years old in British Columbia (BC) and Quebec, the latter displayed as dashed lines. Panel A displays estimates for those
who received any two mRNA vaccines and panel B displays for those who received two ChAdOx1 vaccines. All VE estimates are adjusted for age group (18-49, 50-69, 70-79,
80
years); sex (men, women); individual epidemiological week of the analysis period (weeks 22-39, categorical); and region of the province (5 in each province). The final estimate
displayed for mRNA vaccines against hospitalization is for
24 weeks since vaccination for both provinces. Estimates could not be displayed beyond 16-19 weeks for ChAdOx1
owing to sparse data. For additional details including corresponding sample sizes and precise unadjusted and adjusted estimates and 95%CI by vaccine type, see Supplementary
Tables 10-12. The corresponding information is also displayed, Delta-specific, in Supplementary Tables 13-15.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October, 2021 Page 26 of 28
Figure 4. Adjusted two-dose vaccine effectiveness against infection and hospitalization, by time since vaccination, mRNA vaccines, 70-year-
olds, British Columbia and Quebec, Canada
Shown are adjusted vaccine effectiveness (VE) estimates and 95% confidence intervals (CI) against infection (blue) and hospitalization (orange) by time between receipt of the
second dose of any mRNA vaccine and specimen collection, among adults
70 years old in British Columbia (BC) and Quebec, the latter displayed as dashed lines. All VE estimates
are adjusted for age group (70-79,
80 years); sex (men, women); individual epidemiological week of the analysis period (weeks 22-39, categorical); and region of the province (5 in
each province) except in Quebec for which calendar time adjustment was tri-weekly for the hospitalization outcome owing to small sample size. For additional details including
corresponding sample sizes and precise unadjusted and adjusted estimates and 95%CI, see Supplementary Table 16 (by mRNA vaccine type).
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October, 2021 Page 27 of 28
Figure 5. Adjusted two-dose vaccine effectiveness against infection and hospitalization, by interval between doses, mRNA and ChAdOx1
vaccines,
18-year-olds, British Columbia and Quebec, Canada
Shown are adjusted vaccine effectiveness (VE) estimates and 95% confidence intervals (CI) against infection (blue) and hospitalization (orange) 14 days after the second dose, by
interval between the first and second dose among adults
18 years old who were vaccinated with any two mRNA vaccines or two ChAdOx1 vaccines in British Columbia (BC) and
Quebec. All VE estimates are adjusted for age group (18-49, 50-69, 70-79,
80 years); sex (men, women); individual epidemiological week of the analysis period (weeks 22-39,
categorical); and region of the province (5 in each province). For additional details including corresponding sample sizes and precise unadjusted and adjusted estimates and 95%CI,
see Supplementary Table 17 (by vaccine type) and Supplementary Table 18 (by mRNA vaccine type).
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint

Version: 26 October, 2021 Page 28 of 28
Figure 6. Adjusted two-dose vaccine effectiveness against infection by interval between doses and time since second dose, BNT162b2, 18-
year-olds, British Columbia and Quebec, Canada
Shown are adjusted vaccine effectiveness (VE) estimates and 95% confidence intervals (CI) against infection (timed on specimen collection date) by interval between the first and
second dose (3-4 weeks in purple; 5-6 weeks in dashed yellow; 7+ weeks in green) and time since the second dose among adults 18 years old who were vaccinated with two
BNT162b2 vaccines in the provinces of British Columbia (BC) and Quebec. All VE estimates are adjusted for age group (18-49, 50-69, 70-79,
80 years); sex (men, women);
individual epidemiological week of the analysis period (weeks 22-39, categorical); and region of the province (5 in each province). For additional details including corresponding
sample sizes and precise unadjusted and adjusted estimates and 95%CI, see Supplementary Table 19 (by vaccine type, including mRNA combined and by mRNA vaccine type, as
well as ChAdOx1).
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted October 26, 2021. ; https://doi.org/10.1101/2021.10.26.21265397doi: medRxiv preprint
