
Introduction to Liquid Crystals
The study of liquid crystals began in 1888 when an Austrian botanist named Friedrich Reinitzer
observed that a material known as cholesteryl benzoate had two distinct melting points. In his
experiments, Reinitzer increased the temperature of a solid sample and watched the crystal
change into a hazy liquid. As he increased the temperature further, the material changed again
into a clear, transparent liquid. Because of this early work, Reinitzer is often credited with
discovering a new phase of matter - the liquid crystal phase.
A liquid crystal is a thermodynamic stable phase characterized by anisotropy of properties
without the existence of a three-dimensional crystal lattice, generally lying in the temperature
range between the solid and isotropic liquid phase, hence the term mesophase.
Liquid crystal materials are unique in their properties and uses. As research into this field
continues and as new applications are developed, liquid crystals will play an important role in
modern technology. This tutorial provides an introduction to the science and applications of
these materials.
What are Liquid Crystals?
Liquid crystal materials generally have several common characteristics. Among these are a rod-
like molecular structure, rigidness of the long axis, and strong dipole and/or easily polarizable
substituents. A dipole is present when we have two equal electric or magnetic charges of
opposite sign, separated by a small distance. In the electric case, the dipole moment is given by
the product of one charge and the distance of separation. Applies to charge and current
distributions as well. In the electric case, a displacement of charge distribution produces a dipole
moment, as in a molecule.
The distinguishing characteristic of the liquid crystalline state is the tendency of the molecules
(mesogens) to point along a common axis, called the director (the molecular direction of
preferred orientation in liquid crystalline mesophases). This is in contrast to molecules in the
liquid phase, which have no intrinsic order. In the solid state, molecules are highly ordered and
have little translational freedom. The characteristic orientational order of the liquid crystal state
is between the traditional solid and liquid phases and this is the origin of the term mesogenic
state, used synonymously with liquid crystal state. Note the average alignment of the molecules
for each phase in the following diagram.

A mesogen is rigid rodlike or disclike molecules which are components of liquid crystalline
materials.
It is sometimes difficult to determine whether a material is in a crystal or liquid crystal state.
Crystalline materials demonstrate long range periodic order in three dimensions. By definition,
an isotropic (Having properties that are the same regardless of the direction of measurement. In
the isotropic state, all directions are indistinguishable from each other)liquid has no orientational
order. Substances that aren't as ordered as a solid, yet have some degree of alignment are
properly called liquid crystals.
The term crystallinity implies the presence of three-dimensional order on the level of atomic
dimensions. In polymers, the range of order may be as small as about 2 nm in one (or more)
crystallographic direction(s) and is usually below 50 nm in at least one direction. Polymer
crystals frequently do not display the perfection that is usual for low-molecular mass substances.
Polymer crystals that can be manipulated individually are often called polymer single crystals.
To quantify just how much order is present in a material, an order parameter (S) is defined. S
describes the orientational order of liquid crystalline material, allowing for the individual
orientational deviation of the molecules from the director, which represents the average over the
collection. Typically, S ranges from 0.3 to 0.9, depending on the temperature, with a value of
unity for perfect order. See Introduction to Liquid Crystal phases section. Traditionally, the order
parameter is given as follows:
where theta is the angle between the director and the long axis of each molecule. The brackets
denote an average over all of the molecules in the sample. In an isotropic liquid, the average of
the cosine terms is zero, and therefore the order parameter is equal to zero. For a perfect crystal,
the order parameter evaluates to one. Typical values for the order parameter of a liquid crystal
range between 0.3 and 0.9, with the exact value a function of temperature, as a result of kinetic
molecular motion. This is illustrated below for a nematic liquid crystal material .
The tendency of the liquid crystal molecules to point along the director leads to a condition
known as anisotropy. This term means that the properties of a material depend on the direction in
which they are measured. For example, it is easier to cut a piece of wood along the grain than
against it. The anisotropic nature of liquid crystals is responsible for the unique optical properties
exploited by scientists and engineers in a variety of applications.
Characterizing Liquid Crystals
The following parameters describe the liquid crystalline structure:
• Orientational order: Measure of the tendency of the molecules to align along the director
on a long-range basis.
• Positional order: The extent to which the position of an average molecule or group of
molecules shows translational symmetry.
• Bond orientational order: Describes a line joining the centers of nearest-neighbor
molecules without requiring a regular spacing along that line. Thus, a relatively long-
range order with respect to the line of centers but only short range positional order along
that line.
Each of these parameters describes the extent to which the liquid crystal sample is ordered.
Positional order refers to the extent to which an average molecule or group of molecules shows
translational symmetry (as crystalline material shows). Orientational order, as discussed above,
represents a measure of the tendency of the molecules to align along the director on a long-range
basis. Bond Orientational Order describes a line joining the centers of nearest-neighbor
molecules without requiring a regular spacing along that line. Thus, a relatively long-range order
with respect to the line of centers but only short range positional order along that line. Most
liquid crystal compounds exhibit polymorphism, or a condition where more than one phase is
observed in the liquid crystalline state. The term mesophase is used to describe the "subphases"
of liquid crystal materials. Mesophases are formed by changing the amount of order in the
sample, either by imposing order in only one or two dimensions, or by allowing the molecules to
have a degree of translational motion. The following section describes the mesophases of liquid
crystals in greater detail.
Liquid Crystal Phases
The liquid crystal state is a distinct phase of matter observed between the crystalline (solid) and
isotropic (liquid) states. There are many types of liquid crystal states, depending upon the
amount of order in the material. This section will explain the phase behavior of liquid crystal
materials.
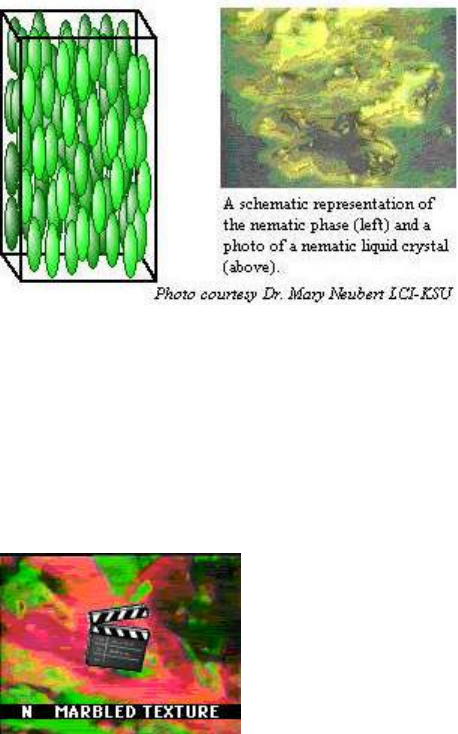
Nematic Phases
The nematic liquid crystal phase is characterized by molecules that have no positional order but
tend to point in the same direction (along the director). In the following diagram, notice that the
molecules point vertically but are arranged with no particular order.
Liquid crystals are anisotropic materials, and the physical properties of the system vary with the
average alignment with the director. If the alignment is large, the material is very anisotropic.
Similarly, if the alignment is small, the material is almost isotropic.
The phase transition of a nematic liquid crystal is demonstrated in the following movie provided
by Dr. Mary Neubert, LCI-KSU. The nematic phase is seen as the marbled texture. Watch as the
temperature of the material is raised, causing a transition to the black, isotropic liquid.
A special class of nematic liquid crystals is called chiral nematic. Chiral refers to the unique
ability to selectively reflect one component of circularly polarized light. The term chiral nematic
is used interchangeably with cholesteric. Refer to the section on cholesteric liquid crystals for
more information about this mesophase.
Smectic Phases
The word "smectic" is derived from the Greek word for soap. This seemingly ambiguous origin
is explained by the fact that the thick, slippery substance often found at the bottom of a soap dish
is actually a type of smectic liquid crystal.
The smectic state is another distinct mesophase of liquid crystal substances. Molecules in this
phase show a degree of translational order not present in the nematic. In the smectic state, the
molecules maintain the general orientational order of nematics, but also tend to align themselves
in layers or planes. Motion is restricted to within these planes, and separate planes are observed
to flow past each other. The increased order means that the smectic state is more "solid-like" than
the nematic.

Photo of a smectic phase (using polarizing microscope)
Many compounds are observed to form more than one type of smectic phase. As many as 12 of
these variations have been identified, however only the most distinct phases are discussed here.
In the smectic-A mesophase, the director is perpendicular to the smectic plane, and there is no
particular positional order in the layer. Similarly, the smectic-B mesophase orients with the
director perpendicular to the smectic plane, but the molecules are arranged into a network of
hexagons within the layer. In the smectic-C mesophase, molecules are arranged as in the
smectic-A mesophase, but the director is at a constant tilt angle measured normally to the
smectic plane.
Photo of the smectic A phase (using polarizing microscope)
Photo of the smectic C phase (using polarizing microscope)

Chemical Properties of Liquid Crystals
Liquid crystals can be classified into two main categories: thermotropic liquid crystals,
and lyotropic liquid crystals. These two types of liquid crystals are distinguished by the
mechanisms that drive their self-organization, but they are also similar in many ways.
Thermotropic transactions occur in most liquid crystals, and they are defined by the fact that the
transitions to the liquid crystalline state are induced thermally. That is, one can arrive at the
liquid crystalline state by raising the temperature of a solid and/or lowering the temperature of a
liquid. Thermotropic liquid crystals can be classified into two types: enantiotropic liquid crystals,
which can be changed into the liquid crystal state from either lowering the temperature of a
liquid or raising of the temperature of a solid, and monotropic liquid crystals, which can only be
changed into the liquid crystal state from either an increase in the temperature of a solid or a
decrease in the temperature of a liquid, but not both. In general, thermotropic mesophases occur
because of anisotropic dispersion forces between the molecules and because of packing
interactions.
In contrast to thermotropic mesophases, lyotropic liquid crystal transitions occur with the
influence of solvents, not by a change in temperature. Lyotropic mesophases occur as a result of
solvent-induced aggregation of the constituent mesogens into micellar structures. Lyotropic
mesogens are typically amphiphilic, meaning that they are composed of both lyophilic (solvent-
attracting) and lyophobic (solvent-repelling) parts. This causes them to form into micellar
structures in the presence of a solvent, since the lyophobic ends will stay together as the lyophilic
ends extend outward toward the solution. As the concentration of the solution is increased and
the solution is cooled, the micelles increase in size and eventually coalesce. This separates the
newly formed liquid crystalline state from the solvent.
A very large number of chemical compounds are known to exhibit one or several liquid
crystalline phases. Despite significant differences in chemical composition, these molecules have
some common features in chemical and physical properties. There are two types of thermotropic
liquid crystals: discotics and rod-shaped molecules. Discotics are flat disc-like molecules
consisting of a core of adjacent aromatic rings. This allows for two dimensional columnar
ordering. Rod-shaped molecules have an elongated, anisotropic geometry which allows for
preferential alignment along one spatial direction.
The rod-like low molar mass (LMM) liquid crystals, such as 5CB shown in the following
diagram:
require an extended conformation of the molecule which must be maintained through the rigidity
and linearity of its constituents. That is, in order for a molecule to display the characteristics of a
liquid crystal, it must be rigid and rod-shaped. This is accomplished by the interconnection of
two rigid cyclic units. The interconnecting group should cause the resulting compound to have a
linear planar conformation. Linking units containing multiple bonds such as -(CH=N)-, -N=N-, -
(CH=CH)n-, -CH=N-N=CH-, etc. are used since they restrict the freedom of rotation. These
groups can conjugate with phenylene rings, enhancing the anisotropic polarizability. This
increases the molecular length and maintains the rigidity.

Applications of Liquid Crystals
Liquid crystal technology has had a major effect many areas of science and engineering, as well
as device technology. Applications for this special kind of material are still being discovered and
continue to provide effective solutions to many different problems.
Liquid Crystal Displays
The most common application of liquid crystal technology is liquid crystal displays (LCDs.) This
field has grown into a multi-billion dollar industry, and many significant scientific and
engineering discoveries have been made.
Liquid Crystal Thermometers
As demonstrated earlier, chiral nematic (cholesteric) liquid crystals reflect light with a
wavelength equal to the pitch. Because the pitch is dependent upon temperature, the color
reflected also is dependent upon temperature. Liquid crystals make it possible to accurately
gauge temperature just by looking at the color of the thermometer. By mixing different
compounds, a device for practically any temperature range can be built.
The "mood ring", a popular novelty a few years ago, took advantage of the unique ability of the
chiral nematic liquid crystal. More important and practical applications have been developed in
such diverse areas as medicine and electronics. Special liquid crystal devices can be attached to
the skin to show a "map" of temperatures. This is useful because often physical problems, such
as tumors, have a different temperature than the surrounding tissue. Liquid crystal temperature
sensors can also be used to find bad connections on a circuit board by detecting the characteristic
higher temperature.
Optical Imaging
An application of liquid crystals that is only now being explored is optical imaging and
recording. In this technology, a liquid crystal cell is placed between two layers of
photoconductor. Light is applied to the photoconductor, which increases the material's
conductivity. This causes an electric field to develop in the liquid crystal corresponding to the
intensity of the light. The electric pattern can be transmitted by an electrode, which enables the
image to be recorded. This technology is still being developed and is one of the most promising
areas of liquid crystal research.
Other Liquid Crystal Applications
Liquid crystals have a multitude of other uses. They are used for nondestructive mechanical
testing of materials under stress. This technique is also used for the visualization of RF (radio
frequency) waves in waveguides. They are used in medical applications where, for example,
transient pressure transmitted by a walking foot on the ground is measured. Low molar mass
(LMM) liquid crystals have applications including erasable optical disks, full color "electronic
slides" for computer-aided drawing (CAD), and light modulators for color electronic imaging.
As new properties and types of liquid crystals are investigated and researched, these materials
are sure to gain increasing importance in industrial and scientific applications.
Source (contain additional information):
http://plc.cwru.edu/tutorial/enhanced/files/lindex.html
