
Liquid Crystal and their Applications
Liquid crystals are a unique state of matter, between solid (crystalline) and
liquid (isotropic) phases some compounds form a distinct, different intermediate
phase, sometimes referred to as the “fourth state of matter” or “mesophase”.
These compounds display properties of both solid and liquid. Anisotropic
intermolecular interactions of the molecules, or mesogens, within a liquid
crystalline material mean that the molecules possess some orientational or
positional order but with a lower degree of organisation compared with a
crystalline solid. This means liquid crystal possesses liquid-like flowing
behaviour, but because of their positional order, such compounds are often more
viscous. Liquid crystals are attributed to their sensitivity to various stimuli, such
as temperature, electric and magnetic fields. This sensitivity, combined with the
self-assembling behaviour of liquid crystals make them extremely interesting
and fascinating
Characteristics of Liquid Crystal
Liquid crystal materials generally have several common characteristics. Among
these are a rod-like molecular structure, rigidness of the long axis, and strong
dipoles and/or easily polarizable substituents.
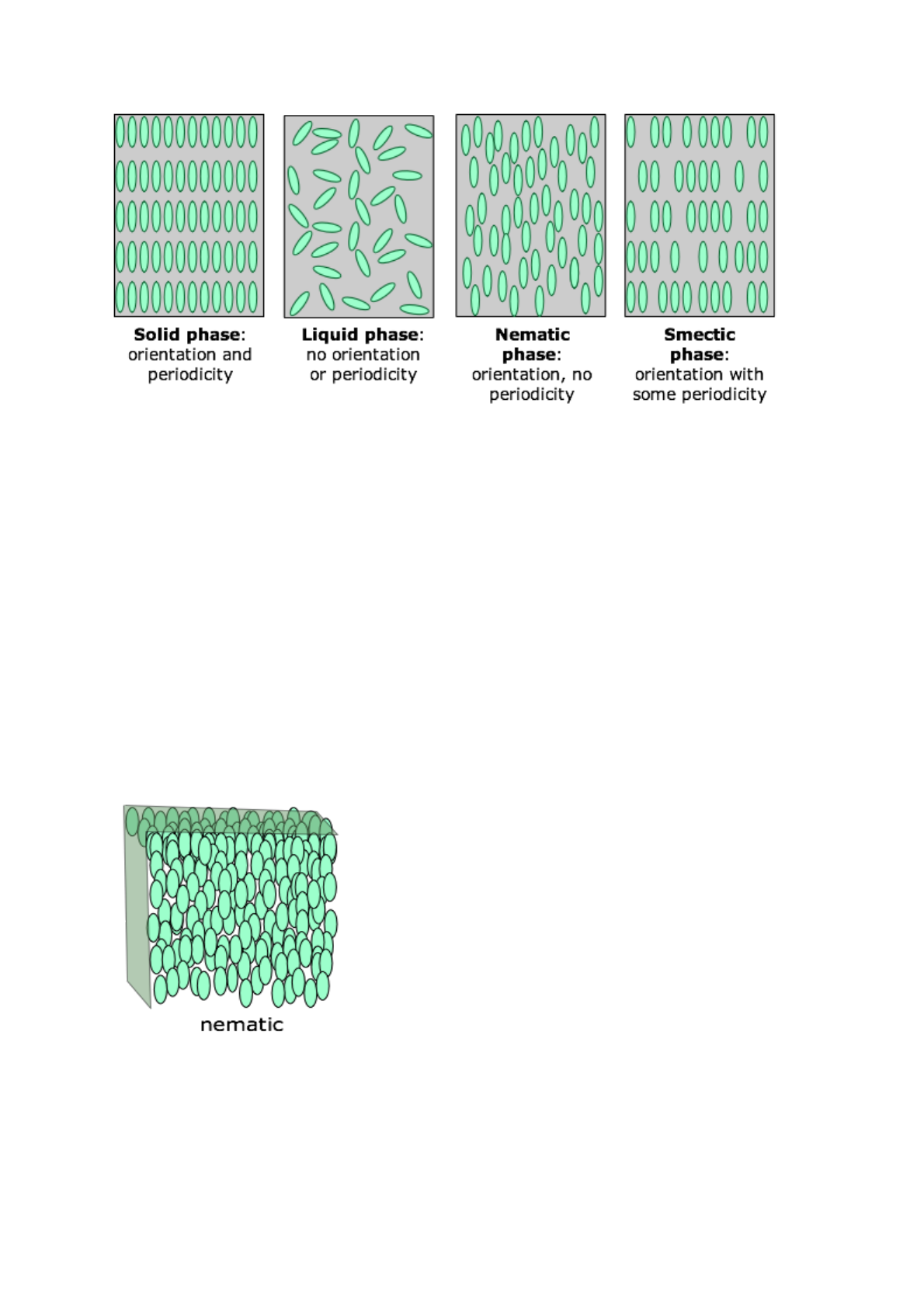
The distinguishing characteristic of the liquid crystalline state is the tendency of
the molecules (mesogens) to point along a common axis, called the director.
This is in contrast to molecules in the liquid phase, which have no intrinsic
order. In the solid state, molecules are highly ordered and have little
translational freedom. The characteristic orientational order of the liquid crystal
state is between the traditional solid and liquid phases and this is the origin of

the term mesogenic state, used synonymously with liquid crystal state. The
average alignment of the molecules for each phase in the following diagram.
Classification of Liquid Crystals
Liquid crystals are classified in many ways, molecules within the mesophases
(mesogens) can be calamitic (rod-like), discotic (disc-like), amphiphilic,
nonamphiphilic, metal containing, non-metal containing and low molecular
weight or polymeric. Liquid crystals either show thermotropic behaviour or
lyotropic behaviour. Thermotropic behaviour means the compounds are liquid
crystalline within a defined temperature range, below this range compounds are
crystalline and above it compounds are isotropic liquids. Thermotropic liquid
crystalline compounds also require no solvent. Lyotropic liquid crystals are
dependent on solvents, where solvent concentration affects aggregation and

liquid crystal behaviour.
There are many classes and sub-classes of liquid crystals, but for the purposes
we will divide them into the two types-
Nematic
In a nematic phase (the term means "thread-like") the molecules are aligned in
the same direction but are free to drift around randomly, very much as in an
ordinary liquid. Owing to their polarity, the alignment of the rod-like molecules
can be controlled by applying an electric field; this is the physical basis for
liquid crystal displays and certain other electro-optic devices.
Smectic

In smectic ("soap-like") phases the molecules are arranged in layers, with the
long molecular axes approximately perpendicular to the laminar planes. The
only long-range order extends along this axis; with the result that individual
layers can slip over each other (hence the "soap-like" nature) in a manner
similar to that observed in graphite. Within a layer there is a certain amount of
short-range order. There are a large number of sub-categories of smectic phases
which we will not go into here. Smectic liquid crystal has been found to have
fast electro-optical response time and because of this is used, along with
nematic liquid crystal, in producing liquid crystal display (LCD) screens.
Application of Liquid Crystals
1. Research on optical & electrical properties of these unique compounds
attracted very much by scientific and industrial community. Later, research at a
number of industries, universities and government laboratories began to focus
on their applications, which exploited the electro-magneto-optic characteristics
and photoelectric properties of nematic and cholesteric type liquid crystals.
2. Cholesteric liquid crystal substances, when applied to the surface of the skin,
have been used to locate veins, arteries, infections, tumors and the fetal placenta
which are warmer than the surrounding tissues.

3. Nematic liquid crystal are useful research tools in the application of magnetic
resonance. Molecules that are dissolved in nematic liquid crystal solvents give a
very highly resolved NMR spectrum exhibiting intermolecular dipole-dipole
fine structures. Analysis of the spectra of molecules in liquid crystal solvents
yield information regarding the anisotropy of chemical shifts, direct magnetic
dipole-dipole interaction, indirect spin-spin couplings, bond angles, bond
lengths, molecular order and relaxation process.
4. Liquid crystals have been used in chromatographic separations138 as
solvents to direct the course of chemical reactions and to study molecular
arrangements and kinetics and as anisotropic host fluid for visible, UV and IR
spectroscopy of organic molecules.
4. liquid crystals are widely used in cosmetic industry in manufacturing of
liquid crystal makeup removers, lipsticks and lip glasses containing cholesteric
liquid crystals.
5. Liquid crystals are using extensively in pharmaceutical industries.
6. Liquid crystal displays are common in calculators, digital watches,
oscillaographic systems, television displays using L.C. screens has also been
developed. Cholesteric liquid crystals have also been used for novelty items
such as toys and decorative materials.
7. Liquid crystal polymers also gained much interest on industrial applications.
polyester liquid crystals were developed for fire resistant, and are used as
coating for multifibre, optical cables due to good surface roughness, low
coefficient of friction. Polyesters are used for moulding with improved elastic
modulus. Ferroelectric liquid crystals, mesomorphic free radicals are used for
EPR study and colourless large pitch cholesterics has been developed.

