
Medication Safety in
Polypharmacy
Technical Report

Medication Safety in
Polypharmacy
Technical Report

WHO/UHC/SDS/2019.11
© World Health Organization 2019
Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO
licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the
work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses
any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you
must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you
should add the following disclaimer along with the suggested citation:
“
This translation was not created by the World Health
Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall
be the binding and authentic edition”.
Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the
World Intellectual Property Organization (http://www.wipo.int/amc/en/mediation/rules).
Suggested citation. Medication Safety in Polypharmacy. Geneva: World Health Organization;
2019 (WHO/UHC/SDS/2019.11). Licence: CC BY-NC-SA 3.0 IGO.
Cataloguing-in-Publication (CIP) data. CIP data are available at http://apps.who.int/iris.
Sales, rights and licensing. To purchase WHO publications, see http://apps.who.int/bookorders. To submit requests for
commercial use and queries on rights and licensing, see http://www.who.int/about/licensing.
Third-party materials. If you wish to reuse material from this work that is attributed to a third party, such as tables, figures
or images, it is your responsibility to determine whether permission is needed for that reuse and to obtain permission from
the copyright holder. The risk of claims resulting from infringement of any third-party-owned component in the work rests
solely with the user.
General disclaimers. The designations employed and the presentation of the material in this publication do not imply the
expression of any opinion whatsoever on the part of WHO concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent
approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or
recommended by WHO in preference to others of a similar nature that are not mentioned. Errors and omissions excepted,
the names of proprietary products are distinguished by initial capital letters.
All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the
published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the
interpretation and use of the material lies with the reader. In no event shall WHO be liable for damages arising from its use.
Designed by CommonSense, Greece
Printed by the WHO Document Production Services, Geneva, Switzerland

3
MEDICATION SAFETY IN POLYPHARMACY
Abbreviations .................................................................................................................................... 4
Preface .............................................................................................................................................. 5
Executive summary: medication safety in polypharmacy ............................................................ 10
1. Introduction.................................................................................................................................. 11
1.1 Polypharmacy .......................................................................................................................... 11
1.2 Prevalence of polypharmacy ..................................................................................................12
1.3 Economic impact of polypharmacy ........................................................................................ 13
1.4 Other factors influencing appropriate polypharmacy.............................................................. 14
2. Medication safety in polypharmacy .......................................................................................... 15
2.1 Medication-related harm in polypharmacy.............................................................................. 15
2.2 Medication review in polypharmacy ...................................................................................... 16
3. Implementing polypharmacy initiatives .................................................................................... 20
3.1 Implementing sustainable programmes to address polypharmacy........................................ 20
3.2 Programmes on appropriate polypharmacy .......................................................................... 21
4. Health systems approach to polypharmacy.............................................................................. 23
4.1 Patients and the public............................................................................................................ 24
4.2 Health care professionals ...................................................................................................... 25
4.3 Medicines................................................................................................................................ 25
4.4 Systems and practices of medication .................................................................................... 26
4.5 Monitoring and evaluation ...................................................................................................... 27
5. Points of consideration for countries ........................................................................................ 29
References ...................................................................................................................................... 30
Annexes .......................................................................................................................................... 38
Annex 1. Glossary ............................................................................................................................ 38
Glossary references .......................................................................................................... 41
Annex 2. Global prevalence of polypharmacy ................................................................................ 43
Annex 3. Internationally available guidance on appropriate polypharmacy management .............. 47
Annex 4. Case studies .................................................................................................................... 49
Annex 5. List of contributors ............................................................................................................ 59
Contents

ACE angiotensin-converting enzyme
ADR adverse drug reaction
ARB angiotensin II receptor blocker
BP blood pressure
COPD chronic obstructive pulmonary disease
eGFR estimated glomerular filtration rate
NNH number needed to harm
NNT number needed to treat
NSAID non-steroidal anti-inflammatory drug
OTC over-the-counter
PESTEL political, economic, social, technological, environmental and legal
PIM potentially inappropriate medication
RLS reporting and learning systems
SWOT strengths, weaknesses, opportunities and threats
UHC universal health coverage
WHO World Health Organization
4
MEDICATION SAFETY IN POLYPHARMACY
Abbreviations

5
MEDICATION SAFETY IN POLYPHARMACY
Health care interventions are intended to
benefit patients, but they can also cause
harm. The complex combination of processes,
technologies and human interactions that
constitutes the modern health care delivery
system can bring significant benefits.
However, it also involves an inevitable risk of
patient harm that can – and too often does –
result in actual harm. A weak safety and
quality culture, flawed processes of care
and disinterested leadership teams weaken
the ability of health care systems and
organizations to ensure the provision of safe
health care. Every year, a significant number
of patients are harmed or die because of
unsafe health care, resulting in a high public
health burden worldwide.
Most of this harm is preventable. Adverse
events are now estimated to be the 14th leading
cause of morbidity and mortality in the world,
putting patient harm in the same league as
tuberculosis and malaria
(1)
. The most important
challenge in the field of patient safety (see
Annex 1) is how to prevent harm, particularly
avoidable harm, to patients during their care.
Patient safety is one of the most important
components of health care delivery which is
essential to achieve universal health coverage
(UHC), and moving towards the UN Sustainable
Development Goals (SDGs). Extending health
care coverage must mean extending safe care,
as unsafe care increase costs, reduces
efficiency, and directly compromises health
outcomes and patient perceptions. It is
estimated that over half of all medicines are
Preface
prescribed, dispensed or sold inappropriately,
with many of these leading to preventable harm
(2)
. Given that medicines are the most common
therapeutic intervention, ensuring safe
medication use and having the processes in
place to improve medication safety (see Annex
1) should be considered of central importance
to countries working towards achieving UHC.
The Global Patient Safety Challenges of the
World Health Organization (WHO) shine a light
on a particular patient safety issue that poses a
significant risk to health. Front-line interventions
are then developed and, through partnership
with Member States, are disseminated and
implemented in countries. Each Challenge has
so far focused on an area that represents a
major and significant risk to patient health and
safety (see Annex 1). In 2005, the Organization,
working in partnership with the (then) World
Alliance for Patient Safety, launched the first
Global Patient Safety Challenge:
Clean Care Is
Safer Care (3)
, followed a few years later by the
second Challenge:
Safe Surgery Saves Lives
(4)
. Both Challenges aimed to gain worldwide
commitment and spark action to reduce health
care-associated infection and the risks
associated with surgery, respectively.
Recognizing the scale of avoidable harm
linked with unsafe medication practices and
medication errors, WHO launched its third
Global Patient Safety Challenge:
Medication
Without Harm
in March 2017, with the goal
of reducing severe, avoidable medication-
related harm by 50% over the next five years,
globally
(5)
.

6
MEDICATION SAFETY IN POLYPHARMACY
This Challenge follows the same philosophy as
the previous Challenges, namely that errors are
not inevitable, but are very often provoked by
weak health systems, and so the challenge is to
reduce their frequency and impact by tackling
some of the inherent weaknesses in the system.
As part of the Challenge, WHO has asked
countries and key stakeholders to prioritize
three areas for strong commitment, early action
and effective management to protect patients
from harm while maximizing the benefit from
medication, namely:
•
medication safety in high-risk situations
•
medication safety in polypharmacy
•
medication safety in transitions of care.
Consider the following case scenario
describing a medication error (see Annex 1)
involving these three areas.
Medication error: case scenario
Mrs Poly, a 65-year-old woman, came to the outpatient clinic complaining of abdominal pain and
dark stools. She had a heart attack five years ago. At her previous visit three weeks ago she was
complaining of muscle pain, which she developed while working on her farm. She was given a
non-steroidal anti-inflammatory drug (NSAID), diclofenac. Her other medications included aspirin,
and three medicines for her heart condition (simvastatin, a medicine to reduce her serum
cholesterol; enalapril, an angiotensin-converting enzyme (ACE) inhibitor; and atenolol, a beta
blocker). She was admitted to hospital as she developed symptoms of blood loss (such as
fatigue and dark stools). She was provisionally diagnosed as having a bleeding peptic ulcer due
to her NSAID, and her doctor discontinued diclofenac and prescribed omeprazole, a proton
pump inhibitor. Following her discharge, her son collected her prescribed medicines from the
pharmacy. Among the medicines, he noticed that omeprazole had been started and that all her
previous medicines had been dispensed, including the NSAID. As his mother was slightly
confused and could not remember exactly what the doctor had said, the son advised his mother
that she should take all the medications that had been supplied. After a week, her abdominal
pain continued and her son took her to the hospital. The clinic confirmed that the NSAID, which
should have been discontinued (deprescribed), had been continued by mistake. This time Mrs
Poly was given a medication list when she left the hospital which included all the medications
she needed to take and was advised about which medications had been discontinued and why.

In this scenario the key steps that should have
been followed to ensure medication safety
in the inpatient setting include:
1. Appropriate prescribing and risk
assessment
Medication safety should start with appropriate
prescribing and a thorough risk–benefit
analysis of each medicine is often the first step.
In this case scenario, prophylactic aspirin
and NSAID without a gastroprotective agent
left Mrs Poly at an increased risk of
gastrointestinal bleeding. NSAIDs can also
increase the risk of cardiovascular events,
which is of particular concern, as she had had
a myocardial infarction (heart attack) five years
ago. This is a good example of a high-risk
situation requiring health care professionals
to prescribe responsibly after analysing
the risks and benefits.
2. Medication review
A comprehensive medication review (see
Annex 1) is a multidisciplinary activity whereby
the risks and benefits of each medicine are
considered with the patient and decisions
made about future therapy. It optimizes the use
of medicines for each individual patient.
Multiple morbidities usually require treatment
with multiple medications, a situation described
as polypharmacy (see Annex 1). Polypharmacy
can put the patient at risk of adverse drug
events (see Annex 1) and drug interactions
when not used appropriately. In this case,
there should have been a review of
medications, particularly as Mrs Poly was
prescribed aspirin and diclofenac together.
The haemodynamic changes following blood
loss should have also prompted temporary
stopping the ACE inhibitor before restarting once
the episode of blood loss has been resolved.
7
MEDICATION SAFETY IN POLYPHARMACY
The events leading to the error in this scenario and how these could have been prevented are
reflected in Figure 1, and the text below.
Figure 1. Key steps for ensuring medication safety
1.
Appropriate
prescribing
and risk
assessment
Benefit
Risks
5.
Medication
reconciliation
at care transitions
2.
Medication
review
3.
Dispensing,
preparation and
administration
4.
Communication
and patient engagement
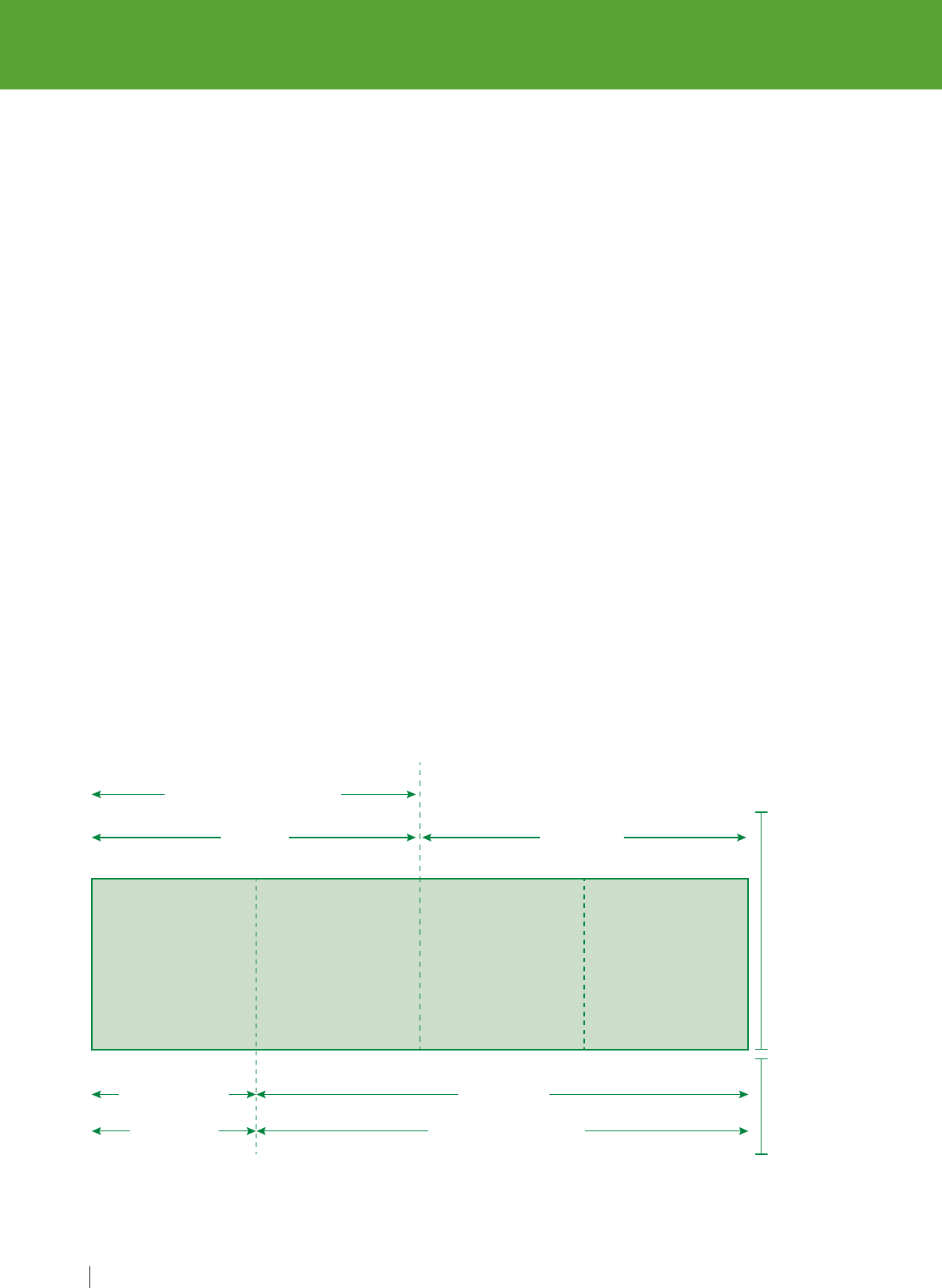
3. Dispensing, preparation and
administration
This is a high-risk situation as the medication
(diclofenac) has the potential to cause harm.
However, this medication was continued after
discharge when the patient transitioned from
hospital to home. Dispensing this medicine and its
administration caused serious harm to Mrs Poly.
Dispensing this medicine and its administration
caused significant harm to Mrs Poly.
4. Communication and patient engagement
Proper communication between health care
providers and patients, and amongst health
care providers, is important in preventing
errors. When Mrs Poly was severely ill due to
gastric bleeding, the NSAID was discontinued.
However, the decision to discontinue the
medicine was not adequately communicated
either to the other health care professionals
(including the nurse or the pharmacist) or to
Mrs Poly. Initial presenting symptoms due to
adverse effects could have been identified
earlier if she had been warned about the risks.
5. Medication reconciliation at care
transitions
Medication reconciliation is the formal process
in which health care professionals partner
with patients to ensure accurate and complete
medication information transfer at interfaces
of care. Diclofenac, the NSAID that can cause
gastrointestinal bleeding and increase the risk
of cardiotoxicity and had led to this hospital
admission, was discontinued, and this
information should have been communicated
at the time of discharge (in the form of a
medication list or patient-held medication
record). This would have helped her and her
caregivers in determining what the newly added
and discontinued medications needed to be.
Medication-related harm is harm caused to
a patient due to failure in any of the various
steps of the medication use process or due
to adverse drug reactions (see Annex 1 for
glossary). The relationship and overlap between
medication errors and adverse drug events
is shown in Figure 2.
8
MEDICATION SAFETY IN POLYPHARMACY
Figure 2. Relationship between medication errors and adverse drug events
Source:
Reproduced, with the permission of the publisher, from Otero and Schmitt
(6)
.
Adverse
drug
reactions
Injury No injury
OutcomesCauses
Not preventable Preventable
Inherent risk
of drugs
Medication errors
Adverse drug events
Preventable
adverse drug
events
Potential
adverse drug
events
Trivial
medication
errors

9
MEDICATION SAFETY IN POLYPHARMACY
WHO is presenting a set of three technical
reports –
Medication safety in high-risk
situations
,
Medication safety in polypharmacy
,
and
Medication safety in transitions of care
–
to facilitate early priority actions and planning
by countries and key stakeholders to address
each of these areas. The technical reports are
intended for all interested parties, particularly
to inform national health policy-makers and
encourage appropriate action by ministries
of health, health care administrators and
regulators, organizations, professionals,
patients, families and caregivers, and all who
aim to improve health care and patient safety.
This report –
Medication safety in
polypharmacy
– outlines the problem, current
situation and key strategies to reduce
medication-related harm in polypharmacy.
It should be considered along with the
companion technical reports on
Medication
safety in high-risk situations
and
Medication
safety in transitions of care.

10
MEDICATION SAFETY IN POLYPHARMACY
Ensuring medication safety in polypharmacy
is one of the key challenges for medication
safety today. Due to the traditional focus
of both medical research and health care
delivery models on single-disease
interventions, there has been a notable lack
of evidence-based solutions. Conventionally
polypharmacy has been perceived as an
overuse of medicines, whereas it may be more
useful to perceive in terms of appropriateness,
as there are many cases where the concurrent
use of multiple medicines may be deemed
necessary and beneficial. Globally the
prevalence of polypharmacy is set to rise as
the population ages and more people suffer
from multiple long-term conditions. Countries
should therefore prioritize raising awareness
of the problems associated with inappropriate
polypharmacy and the need to address this
issue.
All stakeholders have a vital role to play
in driving change for the management
of polypharmacy. Polypharmacy management
involves multifaceted decision-making and
necessitates the combined knowledge of
physicians, nurses, pharmacists and other
health care professionals, including the
systematic involvement, engagement and
empowerment of patients. Thus it is important
to implement interventions, such as medication
reviews, whenever possible in collaboration
with the patient and/or the caregiver. Good
communication and accurate sharing of
information is essential and can be facilitated
by the use of patient-held medication records.
Furthermore, a redesign of care processes
and/or services may be necessary to help
medical practitioners manage workload
related to polypharmacy in order to improve
medication safety.
In complex health care setting with many
competing priorities, it is useful to outline
the safety, clinical and economic implications
for appropriate polypharmacy management.
It can also be helpful to develop an
implementation plan which applies change
management and implementation theories
and tools. The four domains in the strategic
framework of the third WHO Global Patient
Safety Challenge:
Medication Without Harm
can assist in providing a guiding structure
to create a medication safety strategy
addressing polypharmacy.
Executive summary:
medication safety
in polypharmacy

11
MEDICATION SAFETY IN POLYPHARMACY
Introduction
1
This technical report does not attempt
to cover the entire scope of polypharmacy,
but merely aims to introduce polypharmacy
as a concept, and examine some approaches
for the appropriate management of
polypharmacy, which are crucial for ensuring
greater medication safety.
1.1 Polypharmacy
Despite the increasing prevalence
of polypharmacy, the term continues to lack
a clear universal definition. A recent systematic
review of the definitions of polypharmacy
showed that the term was most commonly
applied to situations where patients took five
or more medications, and this numerical
definition was used by 46.4% of the studies
evaluated
(7)
. Furthermore, there are
inconsistencies with the use regarding
duration of therapy and whether to include
over-the-counter (OTC), and traditional and
complementary medicines in the definition
or not. However, with the aim of reducing
medication-related harm, it is important
to verify to the fullest extent possible all
the medications that the patient is taking,
including all OTC, and traditional and
complementary medicines.
While polypharmacy is often defined as
routinely taking a minimum of five medicines,
it is being more frequently suggested that
the emphasis should be on evidenced-based
practice (
7
). The goal should be to reduce
inappropriate polypharmacy (irrational
prescribing of too many medicines)
and to ensure appropriate polypharmacy
(rational prescribing of multiple medicines
based on best available evidence and
considering individual patient factors and
context) (
7–11
). Therefore, appropriate
polypharmacy should be considered at every
point of initiation of a new treatment for the
patient, and when the patient moves across
different health care settings.
Polypharmacy is the concurrent use
of multiple medications. Although there
is no standard definition, polypharmacy
is often defined as the routine use of five
or more medications. This includes
over-the-counter, prescription and/or
traditional and complementary medicines
used by a patient (see Annex 1).

12
MEDICATION SAFETY IN POLYPHARMACY
Polypharmacy has been described as
a significant public health challenge
(13)
.
It increases the likelihood of adverse effects,
with a significant impact on health outcomes
and expenditure on health care resources
(14–16)
. Although co-prescribing multiple
medicines increases the risk of adverse events,
it is important to note that assigning a numeric
threshold to define polypharmacy is not always
useful. There are cases where polypharmacy
is necessary and beneficial, such as the
secondary prevention of myocardial infarction,
which requires the use of four different classes
of medications (a beta blocker, a statin, an
antiplatelet agent and an ACE inhibitor)
(8, 12)
.
Appropriate polypharmacy recognizes that
patients can benefit from multiple medications
if the patients’ clinical conditions,
comorbidities, allergy profiles, the potential
drug–drug and drug–disease interactions
are considered, and the medicines are
prescribed based on the best available
evidence
(17)
. Thus, it is critical to distinguish
appropriate polypharmacy from inappropriate
polypharmacy
(12)
.
The most vulnerable patient groups to the risks
of polypharmacy are susceptible to events
such as drug–drug interactions, higher risk
of falls, ADRs, cognitive impairment,
non-adherence and poor nutritional status
(18–20)
. Vulnerable patient groups often
include older patients above the age of
65 years and patients who are living in care
homes
(19–21)
.
1.2 Prevalence of polypharmacy
Polypharmacy is a major and growing public
health issue occurring within all health care
settings worldwide
(8, 13)
. The issue is well
described in literature from countries in North
America
(10, 18)
, Europe
(8, 22)
and the
Western Pacific
(23)
, with more data becoming
available from other countries in recent years.
Additional information from selected countries
is available in Annex 2, illustrating that
polypharmacy is a global problem. However,
variation in the structure of health care delivery
and data collection systems, compounded
by the different operational definitions of
polypharmacy, makes country comparison
difficult.
Multimorbidity is defined as the presence
of two or more long-term health conditions,
which can include (a) defined physical and
mental health conditions such as diabetes
or schizophrenia; (b) ongoing conditions
such as learning disability; (c) symptom
complexes such as frailty or chronic pain;
(d) sensory impairment such as sight or
hearing loss; and (e) alcohol and substance
misuse (see Annex 1).
While its true magnitude is not known,
the prevalence of polypharmacy is expected
to rise due to a multitude of factors
(8)
. First,
the global population faces a demographic
shift with the proportion of older population
Appropriate polypharmacy is present, when (a) all medicines are prescribed for the purpose of
achieving specific therapeutic objectives that have been agreed with the patient; (b) therapeutic
objectives are actually being achieved or there is a reasonable chance they will be achieved in
the future; (c) medication therapy has been optimized to minimize the risk of adverse drug
reactions (ADRs); and (d) the patient is motivated and able to take all medicines as intended
(12)
.
Inappropriate polypharmacy is present, when one or more medicines are prescribed that are
not or no longer needed, either because: (a) there is no evidence based indication, the indication
has expired or the dose is unnecessarily high; (b) one or more medicines fail to achieve the
therapeutic objectives they are intended to achieve; (c) one, or the combination of several
medicines cause ADRs, or put the patient at a high risk of ADRs or because (d) the patient is not
willing or able to take one or more medicines as intended
(12)
.

13
MEDICATION SAFETY IN POLYPHARMACY
groups on the rise. It has been estimated
that the global population aged over 65 years
will double from 8% in 2010 to 16% in 2050
(24)
. In 2015, approximately 5% of the
population in OECD countries were aged
80 years and above, this percentage is
expected to rise more than double by 2050
(25)
. Second, epidemiological data indicates
that multimorbidity increases markedly
with age. In a Scottish study, multimorbidity
was prevalent in 81.5% of individuals aged
85 years and over, with a mean number of 3.62
morbidities
(26)
. Ornstein et al. found that the
most prevalent chronic conditions in primary
care were hypertension (33.5%), hyperlipidemia
(33.0%), and depression (18.7%)
(27)
. The
presence of multiple morbidities is associated
with multiple symptoms, impairments and
disabilities. Multimorbidity may result in
a combined negative effect on physical
and mental health, and can have a major
impact on a person’s quality of life, limiting
daily activities and reducing mobility
(28, 29)
.
The need to take multiple medications can be
just as problematic, resulting in frequent health
care contacts and an increase in the likelihood
of medication-related harm
(30)
. Furthermore,
it imposes a large economic burden due
to patients’ complexity of health care needs
and frequent interaction with health services,
which may be fragmented, ineffective and
incomplete
(31)
.
Despite extensive advances in
pharmacotherapy, the availability of clinical
guidelines for older adults with multiple
morbidities is limited
(28)
. Prescribing is largely
based on evidence-based guidance for single
diseases, which does not generally take into
account multimorbidity
(21, 28, 32)
.
Consequently, patients are often prescribed
several medicines recommended by a number
of specialists using disease-specific guidelines
which in combination makes the management
of any single disease challenging and may
even lead to patient harm
(21, 26)
.
1.3 Economic impact
of polypharmacy
Advancing the responsible use of medicines:
applying levers for change
identified several
opportunities to reduce health care spending
through more responsible use of medicines
worldwide. The authors estimated that
mismanaged polypharmacy contributed to 4%
of the world’s total avoidable costs due
to suboptimal medicine use. A total of US$ 18
billion, 0.3% of the global total health
expenditure could be avoided by appropriate
polypharmacy management. Some of the
specific recommendations outlined in the
report included
(33)
:
•
investment in medical audits targeting older
patients with multiple medications;
•
support for a greater role of pharmacists in
medication management and in collaboration
with health care professionals for review
of therapeutic plans;
•
identification of high-risk patients and
preparation of targeted medicine
management plans for this group; and
•
to establish a system for blame-free reporting
of medication errors.
The objectives of polypharmacy management
should be comprehensive, addressing such
issues as improved health outcomes for
the patient and population, greater patient
engagement in therapeutic decision-making
and cost-effectiveness of health care systems
and resources. This comprehensive approach –
embracing care, health and cost – has been
termed the “triple aim” strategy, and it is
designed to guide health system performance
optimization
(34)
. The term has been built
upon to become a “quadruple aim” upon
recognizing that staff wellbeing is essential
to ensure good care for the public
(35)
.
Improved safety and quality leading
to economic benefits
In a randomized control trial by Gillespie et al.
clinical pharmacists performed comprehensive
medication reviews (see Annex 1) of older
hospitalized patients. Patients that received

a medication review experienced 16%
fewer hospital visits and 47% fewer visits
to the emergency department within a 12-
month follow-up period, compared to those
with usual care. In addition, medication-related
readmissions were reduced by 80%. After
factoring in the intervention costs, the
comprehensive medication reviews were found
to lower the total hospital-based health care
cost per patient by US$ 230. The conclusion
presumed that the addition of clinical
pharmacists to health care teams on a wider
scale could result in even greater health care
cost reductions and reducing morbidity
(36)
.
1.4 Other factors influencing
appropriate polypharmacy
Social determinants of health and lifestyles
A proactive approach to sustainable and
appropriate medication regimens should target
lifestyles as part of the health management
process. It is recognized that an unhealthy
lifestyle can contribute to multimorbidity,
requiring treatment with multiple medications
(37)
. Unhealthy lifestyle factors should be
discussed with patients when considering
alternatives to medication. The WHO
Active
ageing: a policy framework
identified three
important economic factors for active ageing:
income, work and social protection
(38)
.
Individuals with low incomes may be restricted
in their choice of healthy ageing options as
healthy foods, health care and housing may be
less affordable and accessible, and are thus
at higher risk of ill health and disability
(39)
,
which can be exacerbated for patients with
polypharmacy.
Non-adherence
Non-adherence to prescribed medication is
a major challenge in polypharmacy, particularly
among older persons and/or in patients with
multimorbidity. Older patients who receive
treatment for several chronic health conditions
simultaneously present both pharmacological
and medication adherence (see Annex 1) risks
(40)
. A systematic review of older patients with
polypharmacy found a correlation between
medication non-adherence and the number
of medicines being taken
(41)
.
14
MEDICATION SAFETY IN POLYPHARMACY

This section outlines the case for managing
polypharmacy at the point of initiation of
treatment, when prescribing, when adding
a new medication to a patient’s list of
medications, during a medication review, or
during a medication reconciliation (see Annex 1)
when a patient moves across care settings.
2.1 Medication-related harm
in polypharmacy
Studies conducted in many countries have
estimated the rate of medication errors both
in hospitals and in general practice
(42–45)
.
One study by Avery et al. found that over a
12-month period, patients receiving five or more
medications had a prescribing or monitoring
error rate of 30.1%, while in those receiving
10 or more medications the error rate was 47%,
demonstrating that the error rate increased
with the number of medicines prescribed
(42)
.
Another study conducted across eight
countries found that the incidence of patient-
reported errors increased with the number
of medications that were taken
(43)
.
Reporting of medication incidents such as
medication errors and ADRs associated with
polypharmacy could provide useful information
to improve patient safety
(46)
. Polypharmacy
may have harmful implications for patients
such as an increased risk of medication errors,
drug–drug interactions, suboptimal patient
adherence and reduced quality of life
(8, 47,
48)
. Health care professionals together with
patients and caregivers play a crucial role in
reporting medication-related events
(46, 49).
Learning from medication incidents is vital
for the implementation of preventive strategies
and interventions in order to reduce risk and
prevent harm from occurring again
(46)
.
Polypharmacy at transitions of care
When patients move across care settings,
medication reconciliation is an important issue
that needs to be addressed. A systematic
review of hospital based medication
reconciliation practices showed a consistent
reduction in medication discrepancies (see
Annex 1), potential adverse effects and adverse
drug events after medication reconciliation,
with the most success seen in high-risk patient
populations such as polypharmacy patients
(50)
. Discrepancies in medication orders are
common, and they increase with the number
of medications prescribed
(51)
. A study of
post-discharge patients found that almost one
fifth of currently used prescription medicines
had not been recorded during hospitalization
and that less than half of the medications used
had been registered in the patients’ discharge
letter. This illustrates the challenge that health
care professionals encounter at transitions
of care (see Annex 1), as polypharmacy in
combination with an insufficient knowledge of
the patients’ medication history is an important
contributor to prescribing errors, which can
potentially result in adverse drug events
(52)
.
Polypharmacy in care homes
Residents of care homes may be at higher
risk of complications from polypharmacy and
inappropriate prescribing
(21)
. Findings suggest
that up to 40% of prescriptions for nursing
Medication safety
in polypharmacy
2
15
MEDICATION SAFETY IN POLYPHARMACY

home residents may be inappropriate or
suboptimal
(53)
. Barber et al. found that
the average care home resident in England
was taking eight medications a day and
over two thirds of these residents had one
or more medication errors
(54)
. Inappropriate
prescribing contributes toward the medication
burden and exacerbates the problem of
inappropriate polypharmacy. This is particularly
evident with regards to the widespread
prescribing of antipsychotic medications
in patients with dementia in nursing and
residential homes
(55)
.
Non-prescribed medications
In addition to prescribed medications, many
patients self-medicate by purchasing OTC
medicines. OTC medicines, such as NSAIDs
for pain and some medications for allergies
and coughs, may interact with their prescribed
medications and have the potential to cause
harm. In some cases, patients may also be
sharing prescription medicine with other
individuals
(8, 56)
. Therefore it is important
to carefully ask patients about the use
of all types of medicines or remedies
(8)
.
Traditional and complementary medicines
The use of herbal medicines is widespread
and often taken in combination with
conventional medicines to treat diseases
(49, 57, 58)
. Health care providers should
ask their patients if they use traditional and
complementary medicines or remedies
and include these products in the medication
review, as these will contribute to the
polypharmacy burden
(8)
. Alongside drug–drug
interactions, herb–drug interactions should be
considered, as they can cause a considerable
patient safety risk
(49, 59, 60)
. Further
high-quality research is needed to identify
interactions of herbal medicines
(60)
.
2.2 Medication review
in polypharmacy
Medication review is a structured
evaluation of patient’s medicines with
the aim of optimizing medicines use and
improving health outcomes. This entails
detecting drug related problems and
recommending interventions (see Annex 1).
Medication reviews are widely used to address
inappropriate polypharmacy globally and are
also recommended by many polypharmacy
guidance documents, see Annex 3. It provides
a structured evaluation that can be used to
prevent harm, optimize treatments and improve
outcomes by optimizing the use of medicines
for each individual patient
(8, 61)
. Medication
reviews in polypharmacy should take into
account the effectiveness and the risk–benefit
ratio of the medication treatment options, and
examine these criteria for the specific patient
group in which the medication is being used.
Where possible medication reviews should be
performed in collaboration with the patient or
their caregiver
(8, 13)
.
The main purpose of medication reviews is to
improve the appropriateness of medications,
reduce harm and improve outcomes. Therefore,
it is essential to reassure that the review is not
viewed merely as a mechanism to reduce
or stop medications.
A systematic review and meta-analysis
indicated that pharmacist-led medication
reviews led to a reduction in hospital
admissions
(62)
. In addition, medication
reviews may have an effect on the reduction
of medication-related problems
(63, 64)
.
For example, a study by Schnipper et al.
found that medication reviews reduced
the number of preventable adverse drug
events 30 days after patient discharge
(64)
.
One Cochrane review found that medication
reviews may have a preventive effect on
reducing the number of emergency
department contacts, however it did not
reduce mortality or hospital readmissions.
16
MEDICATION SAFETY IN POLYPHARMACY

The reducing effect on emergency department
visits was more significant in high-risk groups
(such as older persons or patients with multiple
medications)
(65)
. The general consensus
is that more evidence is necessary to
determine the effect of medication reviews
due to the heterogeneity of the studies and
limitations in follow-up time
(62, 66, 67)
.
Assessing risks and benefits
To facilitate the medication review, prescribers
need practical tools and information to help
with decision-making on the safety and
effectiveness of medicines and the
appropriateness of initiating or continuing long
term medications
(11)
. One useful measure
which helps prescribers to understand the
probable clinical efficacy of a medicine
is the number needed to treat (NNT). The NNT
can be defined as the average number of
patients who require to be treated over a time
period for one patient to benefit compared
with a control; it can also be expressed as the
reciprocal of the absolute risk reduction
(61)
.
The ideal NNT, being one, signifies that every
patient improves on the outcome with the
treatment. The higher the NNT, the less
effective the treatment is in terms of the trial
outcome and timescale. The NNT is only
a statistical estimate of the average benefit of
treatment, usually calculated based on clinical
trials. It is rarely possible to know precisely
the likely benefit for a particular patient.
However, NNT still remains a universal concept
to assess the efficacy of medicine. Several
tables and further information are available on
NNT, which can support prescribers in decision
making and aid discussions with patients
regarding the potential benefits of their
treatment
(61, 68–71)
.
Similarly to NNT, another measure used in
decision making is the Number Needed to
Harm (NNH). The NNH is the average number
of people taking a medication over a time
period in order for one adverse event to occur
(61)
. This concept is not as widely used as
the NNT. Combined with NNT, the overall
benefit to risk ratio (NNT/NNH) should be
considered for individual patients during the
decision making process. In polypharmacy
this ratio may vary considerably between
patients
(61)
. For several commonly used
medications, there are some NNT and NNH
estimates available
(61, 71)
.
Ideally, such information on risks and benefits
is made accessible and comprehensible
for the public, in order to include patients in
the decision-making process. For example,
a Scottish polypharmacy guidance tool helps
health care professionals work in partnership
with patients. This resource is available as
a combined mobile application and website
that outlines the process for initiation and
the review of treatments
(72)
.
Medication review process in polypharmacy
The review process should include
engagement with the patient. The perspective
of the patient on managing and taking multiple
medications should be assessed, as well
as the patient’s goal of care. The intentions
of the patient would need to be aligned with
the prescribers’ view of improving outcomes
and treatment goals
(8)
. The information and
changes derived from the medication review
should be made available to other health care
professionals, especially as the patient
moves across different care settings, in order
to enable collaboration in appropriate
polypharmacy management. An example
of a step-by-step method to conduct
a medication review while using a patient-
centered approach is elaborated in Table 1.
Annex 4 outlines how this process can be
further applied to selected clinical scenarios.
17
MEDICATION SAFETY IN POLYPHARMACY
18
MEDICATION SAFETY IN POLYPHARMACY
Table 1. Step-by-step approach to conducting a patient-centred medication review
Aims
Need
Effectiveness
Safety
Costs
Patient-
centeredness
1.
2.
3.
4.
5.
6.
7.
What matters
to the patient
Identify
essential
medications
Does the
patient take
unnecessary
medications?
Are
therapeutic
objectives
being
achieved?
Does the
patient have/
is at risk of
adverse drug
reactions?
Does the
patient know
what to do if
they are ill?
Is therapy
cost-effective?
Is the patient
willing and
able to take
medication
as intended?
Review diagnoses and identify therapeutic objectives with
respect to:
• Understanding of goals of medication therapy
• Management of existing health problems
• Prevention of future health problems
Identify essential medications (not to be stopped without
specialist advice) such as:
• Medications that have essential replacement functions
(e.g. thyroxine)
• Medications to prevent rapid symptomatic decline
(e.g. medications for Parkinson’s disease)
Identify and review the (continued) need for medications:
• With temporary indications
• With higher-than-usual maintenance doses
• With limited benefit in general for the indication they are used
for
• With limited benefit for the particular patient under review
Identify the need for adding/intensifying medication therapy
in order to achieve therapeutic objectives:
• To achieve symptom control
• To achieve biochemical/clinical targets
• To prevent disease progression/exacerbation
Identify patient safety risks by checking for:
• Drug–disease interactions
• Drug–drug interactions
• Robustness of monitoring mechanisms for high-risk
medications
• Risk of accidental overdosing
Identify adverse drug effects by checking for:
• Specific symptoms/laboratory markers (e.g. hypokalaemia)
• Cumulative adverse drug effects
• Medications that may be used to treat adverse drug
reactions caused by other medications
Identify unnecessarily costly medication by:
• Considering more cost-effective alternatives (but balance
against effectiveness, safety, convenience)
Does the patient understand the outcomes of the review?
• Does the patient understand why they need to take their
medication?
• Consider teach-back technique
a
to ensure full understanding
Ensure medication changes are tailored to patient preferences:
• Is the medication in a form the patient can take?
• Is the dosing schedule convenient?
• Consider what assistance the patient might have and when
this is available
• Is the patient able to take medicines as intended?
Agree and communicate plan:
• Discuss with the patient therapeutic objectives and
treatment priorities
• Decide with the patient what medicines have an effect of
sufficient magnitude to consider continuation or discontinuation
• Inform relevant health care and social care change
in treatments across care transitions

Deprescribing is the process of tapering,
stopping, discontinuing, or withdrawing
drugs, with the goal of managing
polypharmacy and improving outcomes
(see Annex 1).
Considerations for cessation of medication
should be a part of all medication reviews,
and the process of “deprescribing” should be
as robust as that of prescribing. The process
encompasses minimization of the medication
load in terms of dosage, number of tablets
taken and frequency of administration times
(23, 74)
. Supporting tools such as
STOPP/START
criteria can be useful in deprescribing and
improving appropriate prescribing
(72, 75)
.
Examples of prescribing indicator sets
which can be used to identify inappropriate
polypharmacy and appropriateness of
prescribing are available
(8, 76)
. There are
also algorithms available that could improve
medication therapy by deprescribing, which
have been shown to be feasible
(77)
. Hence
it is important to undertake medication reviews
with a holistic approach, as medications may
need to be started or stopped, both to prevent
harm from some medications and to prevent
health deterioration
(8)
.
19
MEDICATION SAFETY IN POLYPHARMACY
a
Method to confirm that the information provided is being understood by getting people to ‘teach-back’
what has been discussed and what instruction has been given
(73)
.
Source:
Adapted, with permission of the publisher, from Scottish Government Polypharmacy Model
of Care Group
(61).

In order to address inappropriate polypharmacy
multiple programmes have been implemented,
particularly in high-income countries
(78, 79)
.
To illustrate the international initiatives,
several polypharmacy guidance documents
from different countries are listed in Annex 3.
3.1 Implementing sustainable
programmes to address
polypharmacy
In the context of the third WHO Global Patient
Safety Challenge:
Medication Without Harm
,
countries are urged to take early priority action
to protect patients from harm arising from
polypharmacy by implementing programmes
which help to reduce inappropriate
polypharmacy that are sustainable and can
be delivered across the country. For the
implementation of national, subnational
or local polypharmacy guidance and new
polypharmacy practices, it may be relevant
to apply change management principles
and theory-based implementation strategies.
Tools and theories to support the
implementation process include Kotter’s
eight step process for leading change
;
political, economic, social, technological,
environmental and legal (PESTEL); and
strengths, weaknesses, opportunities and
threats (SWOT)
(11)
. A recent case study
applied Kotter’s
Eight step process for leading
change
and normalization process theory to
assess successful polypharmacy management
activities in Europe, and provided advice
that can be applied across the entire health
system to address the management of
polypharmacy
(22)
. PESTEL and SWOT
exercises enable organizations to evaluate
issues that need to be addressed to ensure
that barriers to implementation are removed
and enablers are optimized
(11)
. To support
wider implementation of medication reviews
in selected populations, an economic analysis
tool has been developed to help countries
assess the potential economic benefits
of introducing and undertaking medication
reviews in polypharmacy
(80)
.
In addition to change management tools,
some key factors that need to be considered
are described in the following. Existing health
care delivery models for polypharmacy should
be reassessed to ensure the pharmacist
plays a key role within a multidisciplinary
team alongside physicians and nurses
(33)
.
Medication reviews performed by health care
professionals would need to be incorporated
in the design of clinical pathways in the
management of patients with multimorbidity
to facilitate workflow
(81)
. Transfer of
information across transitions of care is
important to the management of appropriate
polypharmacy, because it ensures medications
that were reviewed and stopped are not
restarted without proper justification.
Addressing organizational culture
and multidisciplinary working
The organizational dynamics within health
care systems can be complex and include
20
MEDICATION SAFETY IN POLYPHARMACY
Implementing polypharmacy
initiatives
3

the values, beliefs and assumptions held
by those within an organization
(33)
. Cultural
factors can facilitate or hinder the
implementation of innovative practices in
managing polypharmacy. Failure to account
for organizational culture is one of the main
reasons mentioned when evaluating why
planned initiatives fail to overcome barriers
(82)
.
Not only should the culture of the health system
as a whole be considered, but also the cultural
norms within given professions
(83, 84)
. The
results from a European Delphi study support
that “prior to implementation of polypharmacy
management, the culture of an organization
should be assessed for both strengths
and potential barriers to implementation”
(11)
.
As with change management and systems
thinking, there are multiple tools and
frameworks available to help decision-makers
identify the characteristics of the organizational
culture and to make appropriate modifications,
in parallel with a safety culture assessment
(85)
.
Various studies have identified the necessity
and benefits of multidisciplinary collaboration
when addressing polypharmacy
(10, 22)
.
Policy-makers would need to consider
addressing the legislative and contractual
barriers that are in place if appropriate
polypharmacy is to be applied across
the health care system. Political commitment
is required to ensure that dedicated resources
are allocated to the development of new
systems to address polypharmacy, but more
importantly to strengthen the existing health
care system. Political support is important
to promote multidisciplinary team work
(11)
.
For example,
Medicines Optimisation Quality
Framework of Northern Ireland
recommends
medicines optimization (see Annex 1) activities
to be delivered by multidisciplinary teams
(86)
.
3.2 Programmes on appropriate
polypharmacy
To assist countries in understanding the
hurdles and benefits of investing in
programmes addressing polypharmacy, this
report presents some programmes below.
OPtimising thERapy to prevent Avoidable
hospital admissions in the Multimorbid elderly
(OPERAM), aims to optimize existing
pharmacological and non-pharmacological
therapies to reduce avoidable hospital
admissions, particularly among older patients
with multimorbidity in Europe. The goal of the
study is to assess the impact of a structured
medication review with a software intervention,
obtain and compare intervention studies
to find what is most effective and safe in order
to determine the best and most cost–effective
measures for preventing avoidable hospital
admissions. Initiated in 2015, this study
is ongoing until year 2020
(87, 88)
.
Polypharmacy in chronic diseases: Reduction
of Inappropriate Medication and Adverse drug
events in older populations by electronic
Decision Support
(PRIMA-eDS), aims to provide
physicians with the best evidence regarding
medication therapy for older patients with
multimorbidity through an electronic decision
support tool. The electronic decision support
tool comprises of an indication check,
recommendations based on guidelines,
systematic reviews, drug interaction database,
renal dosing database, adverse effect
database and the European list of inappropriate
medications for older people
(89)
. The
practicability and relevance of the tool
was evaluated through a randomized clinical
trial to test if discontinuing inappropriate
medications could improve patient outcomes,
such as reduction of hospitalization or death;
and the patient data entry was found to be too
time-consuming. Recent findings provide
more insight in the future development of
the tool and the potential risk groups
(90, 91)
.
Stimulating Innovation Management of
Polypharmacy and Adherence in The Elderly
(SIMPATHY) project intends to stimulate,
promote and support innovation across
the European Union in the management
of appropriate polypharmacy and medication
adherence in older patients
(78)
. This project
aims to contribute to developing efficient and
sustainable health care systems. Through
21
MEDICATION SAFETY IN POLYPHARMACY

stakeholder engagement, studies were
undertaken in a range of different health care
environments, including European Innovation
Partnership on Active and Healthy Ageing
reference sites. The studies provide a
framework and politico-economic basis for
a European Union-wide benchmarking survey
of strategies being employed for polypharmacy
and non-adherence management. Innovative
multidisciplinary models were developed to
support patients with long-term conditions
using the professional expertise of pharmacists
and physicians to reduce inappropriate
polypharmacy and promote innovation in
health care workforce development
(11)
.
A set of contextualized change management
approaches and tools was developed to help
politicians, regulators, health service providers,
and other stakeholders to advance current
practice by implementing organizational
change, thereby improving the management
of patients on polypharmacy
(11, 80)
.
In addition to creating a European knowledge-
sharing network on polypharmacy and
adherence management, the targeted
dissemination of these validated findings may
support policy development, implementation
of strategic organizational development
and the exchange of best practices
(11)
.
There is still room for improvement,
as polypharmacy management is currently
not widely addressed within most EU
countries
(22)
. This programme has generated
information on which benchmarks can be
applied for regional and national progress
measurements, a definitive guidance on
the role of key stakeholders and guidelines
on how to initiate and manage the change
process
(11)
.
22
MEDICATION SAFETY IN POLYPHARMACY

Initiatives to address polypharmacy can be
complex and require strong leadership and
management. Identifying a lead organization
and allocating responsibility could facilitate
the implementation of polypharmacy
management initiatives at the regional or
national level. Organizational leadership
is vital in driving change to achieve effective
polypharmacy management
(11)
. The need
to address polypharmacy is universal, but
the challenge of leading change goes
to the heart of the policies and culture of
organizations, requiring the active involvement
of policy-makers, health care professionals
and managers, as well as patients, families
and caregivers
(61)
. Often the window
of opportunity to ensure that change is
implemented is small, with three components
being crucial: problem recognition, generation
of policy proposals, and a supportive political
environment, to create a momentum for
a change in public policy
(92)
. Countries may
wish to consider using existing infrastructure
such as pharmacovigilance centres
(see Annex 1) and patient safety incident
reporting and learning systems (RLS) to collect
reports as well as to disseminate learning
from harm occurring from polypharmacy
and drug interactions
(46, 49)
.
The irrational use of medicines is a global
issue. A WHO report,
The World Medicines
Situation
, estimated in 2004 that half of all
medicines are inappropriately prescribed,
dispensed or sold. Furthermore, half of all
patients fail to take their medicine as
prescribed. This can be harmful to patients,
leads to ineffective therapies and waste of
resources, generating an unnecessary burden
for the patient as well as the whole society.
Inappropriate polypharmacy is a common
example of irrational use of medicines.
To address the challenge at the system level,
effective policies such as supportive incentive
structures, education and management,
clear clinical guidance and appropriate
training are considered far-reaching
(93)
.
When deciding how to address polypharmacy,
any solution needs to achieve the
aforementioned “quadruple aim”, so that
quality of prescribing and outcomes from
medication are improved whilst delivering
an economically sustainable solution that
will promote patient engagement across
the health care system without compromising
the work life of health care professionals
(35)
.
In many countries polypharmacy management
may not be widely addressed, which makes
it important to establish change management
strategies to support implementation
at a national scale
(22)
. An initial step would be
to undertake a benchmarking survey so that
countries can assess their current status
with regard to polypharmacy. Business
operation tools such as PESTEL and SWOT
analysis have been used for polypharmacy
programmes, and are helpful in identifying
barriers and issues that countries would need
to address in order to improve polypharmacy
management
(11)
.
Health systems
approach to polypharmacy
4
23
MEDICATION SAFETY IN POLYPHARMACY

In order to attain medication safety in
polypharmacy management, a strategy could
be developed using the strategic framework of
the third WHO Global Patient Safety Challenge:
Medication Without Harm
. The key domains
to be addressed as part of this framework
include patients and the public, medicines,
health care professionals, and systems and
practices of medication. The four domains
and the cross-cutting theme of monitoring
and evaluation are elaborated in the following
sections, addressing a health system
approach for implementing polypharmacy
programmes under the umbrella of
the Challenge.
4.1 Patients and the public
The role of patients in delivering
appropriate polypharmacy
Raising patient awareness about the problems
of polypharmacy and non-adherence is
important, as patients can play a key role in the
prevention and early detection of inappropriate
polypharmacy (
11
,
21
). Patients should be
seen as shared decision-makers on the use of
medication, and health care professionals need
to support patients, families and caregivers in
order to enable them to undertake this role (
8
).
Patients should be encouraged and supported
to disclose all the medications they are taking,
including OTC and traditional and
complementary medicines, especially if they
are suffering from multiple conditions and are
being treated with polypharmacy (
94
). Health
literacy, social norms and cultural factors
would need to be taken into account when
considering the role of patients, and designing
materials for patient education (
95
,
96
).
Patient tools and materials can support the
engagement and empowerment of patients,
families and caregivers in playing an active
role in health care. Such resource materials
may include:
• Patient-held medication list or patient-held
medication record, sometimes called
medication passport (either paper or
electronic), can help to optimize patients’
medicines. The use of these tools has
received positive feedback from patients (
97
).
Such lists, provided that they are up-to-date,
can also be helpful at care transitions (
98
).
• Patient resource materials that enable
patients to understand how to make
decisions regarding management of their
health conditions and their medications
are available (
99
,
100
). An example of
a tailormade tool is the
Medicine Sick Day
Rules card
, which explains to patients which
medicines should be temporarily stopped
during dehydration due to an illness (
101
).
Different organizations, including WHO,
have developed materials that include
simple questions to encourage patients
to be active participants in their therapeutic
decision-making (
102–104
). For example,
the
5 Moments for Medication Safety
focuses
on five key moments in the medication use
process, where action by the patient, family
member or caregiver can greatly reduce
the risk of harm associated with the use
of medications. The tool aims to engage
and empower patients to be involved in their
own care in a more proactive way and feel
responsible for it, encourage their curiosity
about the medications they are taking,
empower them to communicate openly
with their health professionals and be
involved in shared decision-making (
104
).
Technology could improve both patient
experience and medication adherence.
Furthermore, it has potential to enable patients
to be active participants in medication reviews.
However, more research is required to evaluate
strategies for integrating such tools into clinical
practice and to ensure they meet their potential
in improving patient outcomes and creating
value for all users (
105
,
106
).
Prioritizing patients for medication reviews
in polypharmacy
Due to limited resources in most health care
systems, patients who may benefit from
medication reviews need to be prioritized.
There are many factors that could increase
the likelihood of predisposing a patient to harm
24
MEDICATION SAFETY IN POLYPHARMACY

25
MEDICATION SAFETY IN POLYPHARMACY
from medication. Research has shown that
patients on multiple medications have
a higher risk of medication-related harm, due
to harm from occurrences such as drug–drug
interactions, falls and adverse events (
8
,
48
).
Any methods for identifying patients at risk due
to polypharmacy need to be considered in the
clinical context. Even in resource-limited
settings with inadequate health information
databases, the following criteria can be applied
to identify situations where a medication review
may prove beneficial:
• residents in a care home setting (
12
,
21
)
• patients on high-risk (high-alert) medications
(see Annex 1) (
12
)
• patients taking 10 or more medicines
(
12
,
21
,
107
)
• patients with two or more co-morbidities (
12
)
• patients with frailty (
12
,
77
,
20
)
• patients with dementia (
12
,
108
)
• palliative care situations (
8
,
12
)
4.2 Health care professionals
Health care professionals and policy-makers
can take a leadership role in raising awareness
among peers regarding the role of medication
review in reducing the harm associated
with inappropriate polypharmacy (
11
).
Development of multidisciplinary workforce
through changes in undergraduate,
postgraduate, and continuing professional
development has been identified as a key area
to address polypharmacy (
22
). Educational
curricula for health care professionals, including
physicians, pharmacists and nurses, should
include safe medication management to
develop necessary competencies and skills
for addressing risks associated with
polypharmacy (
109
). To help support education
of health care professionals, a set of clinical
case studies are presented in Annex 4.
Existing guidelines that incorporate key steps
for patient-centered medication review should
be shared to ensure the dissemination of best
practices, including the importance of sharing
the information about the outcomes of
the medication review in polypharmacy
with relevant health care professionals.
Establishing support networks to enable
health care professionals in sharing scientific
and practical information on polypharmacy
may assist in that regard. There are several
special interest groups focusing on appropriate
polypharmacy available internationally
(
110–112
).
Health care professionals working in
multidisciplinary teams deliver optimum
outcomes for patients. They need to be
aware of human factors that may affect their
performance and the performance of others
(
113
). Knowledge of human factors can be
further useful in communication with
patients and in shared decision-making
in polypharmacy (
109
).
A study from Hawaii revealed that many older
persons were uncertain about the information
about their medications and did not exhibit
confidence on understanding the ADRs
associated with use of multiple medications
(
114
). This emphasizes the need for
interventions that are aimed at enhancing
the provider–patient interaction about
polypharmacy and medication reviews.
Safety culture should be addressed to enable
health care professionals and patients
to discuss issues of polypharmacy, so that
patients feel that it is safe and acceptable
to ask questions (
95
). For example,
Choosing
Wisely
campaign focuses on promoting
dialogue between health care professionals
and patients; with the aim to reduce utilization
of inappropriate tests and medical treatment,
empower patients to ask questions, and help
patients to choose evidence-based care (
115
).
4.3 Medicines
Polypharmacy and drug interactions
Patients with polypharmacy might be subject
to drug–drug, drug–food, drug–disease
or herb–drug interactions (
116
). Detection
of drug interactions should be included

in the monitoring process of long-term
and latent effects of medications as part
of post-marketing surveillance (
49
). Such
information is pivotal for health care professionals
prescribing or reviewing medication lists to
ensure safe use of medicines. Drug interaction
and contraindication databases and various
computer systems are increasingly available
in hospitals and general practice. However,
technology systems may not flag all relevant
potential harm (
8
,
117
), or they may conversely
lead to numerous reminders, creating
an information overload for health care
professionals and over-riding of reminders
inappropriately (
118
). While there is a huge
potential in computer-based systems to prevent
medication-related harm (for example, by
helping to detect contraindications, interactions
or potentially inappropriate choice of medicine),
they should not replace health care
professionals’ own clinical judgement (
117
).
Medication management and adherence
In polypharmacy it is important to find
innovative solutions to improve medication
adherence and develop strategies to ensure
that the right medicine is taken at the right time.
Patients may be supported case-by-case
based on their individual needs and concerns
by helping them understand the importance
of taking their medicines (i.e. through further
information and discussion) and/or overcoming
practical problems associated with
nonadherence faced by the patient (i.e. through
practical changes in the medication regimen
or type of formulation). Multi-compartment
medication compliance devices, also known
as pill dispensers or dose administration aids,
are used to aid patients in their medication
management (
119
). However, it may not be a
suitable solution for all patients and medicines,
for example, due to individual patient factors,
the specific type of medication formulation,
or the risk of improper handling and storage.
Furthermore, such devices do not necessarily
provide sufficient dosing information.
Patient-centred interventions such as memory
aids, mobile applications, labels with
pictograms, reminder applications, large print
labels and information sheets can also be
utilized (
120
). Digital health tools, such
as smart pill boxes and mHealth solutions,
are approaches likely to be increasingly
adopted in the future (
121
).
4.4 Systems and practices
of medication
A system has been defined as “an operating
mechanism where the sub-parts work jointly
towards achieving an outcome, and the
success of the system is dependent upon
this collaboration”. In the field of patient safety
and medication safety, these sub-parts include
patients, different levels of health care
organizations, policy-makers and regulators
(
122
). It is important that all stakeholders
understand and agree on the outcomes to be
achieved to ensure successful collaboration.
In the case of polypharmacy management,
the goal of a systems approach is the optimal
and sustainable use of medicines in patients
with multimorbidity and supporting them to live
active and healthy lives. It is important that
countries also consider a system wide
approach at all levels within their organizations.
For example, the National adaptation of the
WHO Model List of Essential Medicines
(
123
)
has been proven to improve rational use of
medicines, as demonstrated in China, however
it had limited effect on polypharmacy (
124
).
When designing systems and delivery of
a programme, the importance of patients
and the public as key stakeholders in the
implementation of policy should be considered.
Involving patients in the design and delivery
of a programme will help to ensure effective
implementation and the sustainability
of measures to address medication safety
in appropriate polypharmacy (
125
,
126
).
Patients, families and caregivers should be
given the opportunity to be involved in the
decision-making process during medication
reviews as it helps to improve and optimize
the clinical outcomes of the treatment.
Guidance for medicines optimization focuses
on patient engagement and improving
26
MEDICATION SAFETY IN POLYPHARMACY

the patients’ understanding on medicines
so they are able to make decisions such
as the choices of prevention and healthy
living (
127
).
Some areas to address during systems design
and programme delivery include the following:
• Appropriate polypharmacy should be
addressed at the point of initiation of new
medicines for treatment plans, during
medication review and at care transitions.
• The adoption of a systems approach
prevents an overly narrow view of a particular
problem. For example, development of an
essential medicines list may help countries
put in place national schemes to address
the appropriateness of medicine use.
• Carrying out reviews to ensure
appropriateness of medication should be
integrated into care pathways for patients
with multiple morbidities. Reviews of
appropriate polypharmacy should be built
into systems and practices, and learning
should be incorporated from existing
initiatives that are working to bring
appropriate polypharmacy programmes
to scale.
• The development of change management
strategies will be useful for implementation
across health care systems.
• Monitoring systems can be used to capture
data regarding admissions following
medication-related harm so that improvement
can be measured and feedback and learning
can take place, when patients are admitted
to hospital or attend primary care.
4.5 Monitoring and evaluation
As discussed earlier, there is no single agreed
definition of polypharmacy. The term
polypharmacy does not by its nature imply
whether it is appropriate to prescribe several
medications or not, although it is often
assumed to be the same as being inappropriate
(
8
). The distinction between inappropriate
and appropriate polypharmacy is highly
dependent on the patient and therapy rather
than the number of medications used (
17
,
128
).
Existing published literature addresses
the issue of measurement of polypharmacy
as a standalone domain or as part of an overall
medication safety measurement. Using
a Delphi method of analysis, Rankin et al.
developed a set of key outcome measures
identifying 16 of these as outcomes that are
measurable. The highest ranked outcomes
of this study are serious ADRs, medication
appropriateness, medication regime complexity
and medication-related side effects (
129
).
Several countries have developed inappropriate
polypharmacy indicators that are related
to either the number of medications used,
medications used for older persons, or
to specific medication combinations
(
61
,
130–133
). A systematic review of indicators
relevant to polypharmacy appropriateness
identified a set of 12 core indicators for
monitoring polypharmacy, which included
ADRs, contraindications, drug–drug
interactions, and conduction of medication
reviews (
134
). Countries can adopt or adapt
a set of these indicators based on the context,
priorities and resources available.
Polypharmacy indicators could be a part
of existing RLS for medication safety.
Existing measures should be drawn upon
to support the learning process.
For hospitals or health systems that have
initiated implementation of a medication
safety measurement programme and may
have limited resources, the following indicator
“Percentage of patients on polypharmacy”
derived from
Polypharmacy and medicines
optimization: Making it safe and sound
(
8
),
could be a useful start.
27
MEDICATION SAFETY IN POLYPHARMACY
The polypharmacy indicators could assist
the development of models to predict adverse
outcomes in relation to certain clinical
scenarios, such as with specific therapeutic
classes, and in certain specific patient
populations. Prior to implementation of
interventions to reduce medication-related
harm a baseline assessment should be
considered to monitor progress.
28
MEDICATION SAFETY IN POLYPHARMACY
Indicator Percentage of patients on polypharmacy
Numerator
Denominator
Total number of patients on polypharmacy
Inclusion criteria:
All patients with 10 or more regular medicines (for example, medicines taken
every day or every week)
OR
Patients receiving between four and nine regular medicines who also:
• have at least one prescribing issue that meets the criteria for potentially
inappropriate prescribing;
• have evidence of being at risk of a well-recognized potential drug–drug
interaction or a clinical contraindication;
• have evidence from clinical records of difficulties with taking medicines,
including problems with adherence;
• have no or only one major diagnosis recorded in the clinical record
(it might be expected that large numbers of medicines are unlikely to be
justified in patients without multiple clinical conditions); or
• are receiving end-of-life or palliative care (where this has been explicitly
recognized).
Total number of eligible patients in the target group

The key points of consideration are
summarized as follows:
• Policies for regular, holistic medication
reviews for patients taking multiple
medications.
• Addressing appropriate polypharmacy
at the point of medicines initiation, during
medication review and at care transitions.
• Safety culture enabling health care
professionals and patients to discuss issues
of polypharmacy and make patients to feel
safe in asking questions.
• Policies and implementation plans that
support appropriate polypharmacy
management at scale, and promote effective
multidisciplinary team work by removing
barriers.
• Sharing of information about the outcomes
of medication reviews in polypharmacy with
health care professionals.
• A people-centered approach while reviewing
medication with patients and their caregivers.
• Addressing lifestyle issues during medication
review process.
• Use of appropriate technologies to reduce
medication-related harm, improve patient
experience and medication adherence.
• Reporting of medication incidents, for
example, occurrence of an adverse drug
reaction, hospitalization, or primary health
centre attendance due to medication-related
symptoms.
Points of consideration
for countries
5
29
MEDICATION SAFETY IN POLYPHARMACY

1. Jha AK, Larizgoitia I, Audera-Lopez C, Prasopa-
Plaizier N, Waters H, Bates DW. The global burden
of unsafe medical care: analytic modelling of
observational studies. BMJ Qual Saf. 2013;
22(10):809–815.
https://doi.org/1
0.1136/bmjqs-2012-001748
https://www.ncbi.nlm.nih.gov/pubmed/240486
16
2. Promoting rational use of medicines: Core
components. WHO Policy Perspectives on
Medicines, No.5. Geneva: World Health
Organization; 2002
(http://apps.who.int/medicinedocs/pdf/h30
11e/h3
0
11e.pdf, accessed 22 March 2019).
3. Background to Clean Care is Safer Care. In: Clean
Care is Safer Care [website]. Geneva: World Health
Organization; 2019
(http://www
.who.int/gpsc/background/en/,
accessed 22 March 2019).
4. Safe surgery. In: Patient Safety [website]. Geneva:
World Health Organization; 2019
(http://www
.who.int/patientsafety/topics/safe-
surgery/en/, accessed 22 March 2019).
5. WHO Global Patient Safety Challenge: Medication
Without Harm. Geneva: World Health Organization;
2017
(http://apps.who.int/iris/bitstream/1
0665/255263/
1/WHO-HIS-SDS-2017.6-eng.pdf?ua=1&ua=1,
accessed 22 March 2019).
6. Otero MJ, Schmitt E. Clarifying terminology
for adverse drug events. Ann Intern Med.
2005;142(1):77.
https://doi.org/1
0.7326/0003-4819-142-1-
200501040-00016
https://www
.ncbi.nlm.nih.gov/pubmed/15630112
7. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey
GE. What is polypharmacy? A systematic review
of definitions. BMC Geriatrics. 2017;17:230.
https://doi.org/1
0.1186/s12877-017-0621-2
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5
635569/
8. Duerden M, Avery T, Payne R. Polypharmacy and
medicines optimization: Making it safe and sound.
London: The King’s Fund; 2013
(https://www
.kingsfund.org.uk/sites/default/files/fi
eld/field_publication_file/polypharmacy-and-
medicines-optimisation-kingsfund-nov13.pdf,
accessed 22 March 2019).
9. Stewart D, Mair A, Wilson M, Kardas P, Lewek P,
Alonso A et al. Guidance to manage inappropriate
polypharmacy in older people: systematic review
and future developments. Expert Opin Drug Saf.
2016;(16):203–13.
https://doi.org/1
0.
1080/147
40338.2017.1265503
https://www.ncbi.nlm.nih.gov/pubmed/27885844
10. Patterson SM, Hughes C, Kerse N, Cardwell CR,
Bradley MC. Interventions to improve the
appropriate use of polypharmacy for older people.
Cochrane Database Syst Rev 2012;(5):CD008165.
https://doi.org/1
0.1002/14651858.CD008165.pub2
https://www.ncbi.nlm.nih.gov/pubmed/22592727
11. Mair A, Fernandez-Limos F, Alonso A, Harrison C,
Hurding S, Kempen T et al. Polypharmacy
management by 2030: a patient safety challenge,
2nd edition. Coimbra: SIMPATHY Consortium; 2017.
(http://www
.simpathy.eu/sites/default/files/Managi
ng_polypharmacy2030-web.pdf, accessed
22 March 2019).
12. Scottish Government Model of Care Polypharmacy
Working Group. Polypharmacy Guidance, 2nd
edition. Edinburgh: Scottish Government; 2015
(https://www
.sign.ac.uk/assets/polypharmacy_gui
dance.pdf, 22 March 2019).
13. Payne RA, Avery AJ. Polypharmacy: one of the
greatest prescribing challenges in general practice.
Br J Gen Pract. 2011;61(583):83–4.
https://doi.org/1
0.3399/bjgp11X556146
https://www
.ncbi.nlm.nih.gov/pmc/articles/PMC3
026143/
14. Gurwitz JH, Field TS, Harrold LR, Rothschild J,
Debellis K, Seger AC et al. Incidence and
preventability of adverse drug events among older
persons in the ambulatory setting. JAMA.
2003;289(9):1107–16.
http://doi.org/1
0.1001/jama.289.9.1107
https://www.ncbi.nlm.nih.gov/pubmed/12622580
15. Viktil KK, Blix HS, Moger TA, Reikvam A.
30
MEDICATION SAFETY IN POLYPHARMACY
References

Polypharmacy as commonly defined is an indicator
of limited value in the assessment of drug-related
problems. Br J Clin Pharmacol. 2007;63(2):187–95.
https://doi.org/1
0.11
11/j.1365-2125.2006.02744.x
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2
000563/
16. Cahir C, Fahey T, Teeling M, Teljeur C, Feely J,
Bennett K. Potentially inappropriate prescribing
and cost outcomes for older people: a national
population study. Br J Clin Pharmacol.
2010;69(5):543–52.
https://doi.org/1
0.1111/j.1365-21
25.2010.03628.x
https://www
.ncbi.nlm.nih.gov/pubmed/20573091
17. Cadogan CA, Ryan C, Hughes CM. Appropriate
Polypharmacy and Medicine Safety: When Many
is not Too Many. Drug Saf. 2016;39:109–16.
https://doi.org/1
0.1007/s40264-015-0378-5
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4
735229/
18. Shah BM, Hajjar ER. Polypharmacy, adverse drug
reactions, and geriatric syndromes. Clin Geriatr
Med. 2012;28(2):173–86.
https://doi.org/1
0.1016/j.cger.2012.01.002
https://www
.ncbi.nlm.nih.gov/pubmed/22500537
19. Davies EA, O’Mahony MS. Adverse drug reactions
in special populations - the elderly. Br J Clin
Pharmacol. 2015;80(4):796–807.
https://doi.org/1
0.1111/bcp.12596
https://www.ncbi.nlm.nih.gov/pubmed/25619317
20. Hubbard RE, O’Mahony MS, Woodhouse KW.
Medication prescribing in frail older people.
Eur J Clin Pharmacol. 2013;69(3):319–26.
http://doi.org/1
0.1007/s00228-012-1387-2
https://www.ncbi.nlm.nih.gov/pubmed/22965651
21. Molokhia M, Majeed A. Current and future
perspectives on the management of polypharmacy.
BMC Fam Pract. 2017;18:70.
http://doi.org/1
0.1186/s12875-017-0642-0
https://www.ncbi.nlm.nih.gov/pubmed/28587644
22. McIntosh J, Alonso A, MacLure K, Stewart D,
Kempen T, Mair A et al. A case study of
polypharmacy management in nine European
countries: Implications for change management
and implementation. PLoS One.
2018;13(4):e0195232.
https://dx.doi.org/1
0.1371%2Fjournal.pone.0195232
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5
905890/
23. Scott IA, Hilmer SN, Reeve E, Potter K, Couteur
DL, Rigby D et al. Reducing inappropriate
polypharmacy: the process of deprescribing.
JAMA Intern Med. 2015;175(5):827–34.
https://doi.org/1
0.1001/jamainternmed.2015.0324
https://www.ncbi.nlm.nih.gov/pubmed/25798731
24. Global health and aging. Geneva: US National
Institute of Aging, World Health Organization; 2011
(http://www
.who.int/ageing/publications/global_he
alth.pdf, accessed 22 March 2019).
25. Health at a glance 2017: OECD Indicators. Paris:
OECD Publishing; 2017
(http://dx.doi.org/1
0.1787/health_glance-2017-en,
accessed 22 March 2019).
26. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S,
Guthrie B. Epidemiology of multimorbidity and
implications for healthcare, research, and medical
education: a cross-sectional study. Lancet.
2012;380(9836):37–43.
http://doi.org/1
0.1016/S0140-6736(12)60240-2
https://www
.ncbi.nlm.nih.gov/pubmed/22579043
27. Ornstein SM, Nietert PJ, Jenkins RG, Litvin CB.
The prevalence of chronic diseases and
multimorbidity in primary care practice: a PPRNet
report. J Am Board Fam Med. 2013;26(5):518–24.
http://doi.org/1
0.3122/jabfm.2013.05.130012
https://www.ncbi.nlm.nih.gov/pubmed/24004703
28. Boyd CM, Fortin M. Future of multimorbidity
research: how should understanding of
multimorbidity inform health system designs?
Public Health Rev. 2010;32:451–74.
https://doi.org/1
0.1007/BF03391611
29. Wister A, Kendig H, Mitchell B, Fyffe I, Loh V.
Multimorbidity, health and aging in Canada and
Australia: a tale of two countries. BMC Geriatr. 2016;
16:163. http://doi.org/1
0.1186/s12877-016-0341-z
https://www
.ncbi.nlm.nih.gov/pmc/articles/PMC5
035492/
30. Doos L, Roberts EO, Corp N, Kadam UT. Multi-
drug therapy in chronic condition multimorbidity:
a systematic review. Fam Pract. 2014;31(6)654–63.
http://doi.org/1
0.1093/fampra/cmu056
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5
942538
31. Picco L, Achilla E, Abdin E, Chong SA, Vaingankar
JA, McCrone P et al. Economic burden of
multimorbidity among older adults: impact on
healthcare and societal costs. BMC Health Serv
Res. 2016;16:173.
http://doi.org/1
0.1186/s12913-016-1421-7
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4
862090/
32. Multimorbidity: Technical Series on Safer Primary
Care. Geneva: World Health Organization; 2016
(http://apps.who.int/iris/bitstream/handle/1
0665/2
52275/9789241511650-eng.pdf?sequence=1,
accessed 22 March 2019).
33. Aitken M, Gorokhovich L. Advancing the
responsible use of medicines: applying levers
for change. Parsippany (NJ): IMS Institute for
Healthcare Informatics; 2012
(https://ssrn.com/abstract=222254
1, accessed
22 March 2019).
34. Berwick DM, Nolan TW, Whittington J. The triple
aim: care, health, and cost. Health Aff (Millwood).
2008; 27(3):759–69.
https://doi.org/1
0.1377/hlthaff.27.3.759
https://www.ncbi.nlm.nih.gov/pubmed/18474969
31
MEDICATION SAFETY IN POLYPHARMACY

35. Bodenheimer T, Sinsky C. From triple to quadruple
aim: care of the patient requires care of the
provider. Ann Fam Med. 2014;12(6):573–6.
https://doi.org/1
0.1370/afm.1713
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4
226781/
36. Gillespie U, Alassaad A, Henrohn D, Garmo H,
Hammarlund-Udenaes M, Toss H et al. A
comprehensive pharmacist intervention to reduce
morbidity in patients 80 years or older: a
randomized controlled trial. Arch Intern Med.
2009;169(9):894–900.
https://doi.org/1
0.1001/archinternmed.2009.71
https://www.ncbi.nlm.nih.gov/pubmed/19433702
37. Fortin M, Haggerty J, Almirall J, Bouhali T,
Sasseville M, Lemieux M. Lifestyle factors and
multimorbidity: a cross sectional study. BMC
Public Health. 2014;14:686.
https://doi.org/1
0.1
186/1
471-2458-14-686
https://www.ncbi.nlm.nih.gov/pubmed/24996220
38. Active ageing: a policy framework. Geneva: World
Health Organization; 2002
(http://apps.who.int/iris/bitstream/handle/1
0665/6
7215/WHO_NMH_NPH_02.8.pdf?sequence=1,
accessed 22 March 2019).
39. Mak TN, Caldeira S. The role of nutrition in active
and healthy ageing for prevention and treatment
of age-related diseases: evidence so far.
Luxembourg: Joint Research Centre of the
European Commission; 2014
(http://publications.jrc.ec.europa.eu/repositor
y/bit
stream/JRC90454/lbna26666enn.pdf, accessed
22 March 2019).
40. Sabaté E, editor. Adherence to long-term
therapies: evidence for action. Geneva: World
Health Organization; 2003
(http://www
.who.int/chp/knowledge/publications/
adherence_full_report.pdf?ua=1
, accessed
22 March 2019).
41. Zelko E, Klemenc-Ketis Z, Tusek-Bunc K.
Medication adherence in elderly with
polypharmacy living at home: a systematic review
of existing studies. Mater Sociomed.
2016;28(2):129–32.
https://doi.org/1
0.5455/msm.2016.28.129-132
https://www.ncbi.nlm.nih.gov/pubmed/27147920
42. Avery A, Barber N, Ghaleb M, Franklin BD,
Armstrong S, Crowe S et al. Investigating the
prevalence and causes of prescribing errors
in general practice: the PRACtICe study. London:
General Medical Council; 2012
(https://www
.gmc-uk.org/-
/media/about/investigatingtheprevalenceandcause
sofprescribingerrorsingeneralpracticethepracticest
udyreoprtmay2012.pdf?la=en&hash=62C1821CA5
C
CC5A4868B86A83FEDE14283686C29, accessed
22 March 2019).
43. Sears K, Scobie A, Mackinnon NJ. Patient-related
risk factors for self-reported medication errors in
hospital and community settings in 8 countries.
Can Pharm J (Ott). 2012;145(2):88–93.
http://doi.org/1
0.3821/145.2.cpj88
https://www.ncbi.nlm.nih.gov/pubmed/23509509
44. Alsulami Z, Conroy S, Choonara I. Medication
errors in the Middle East countries: A systematic
review of the literature. Eur J Clin Pharmacol.
2013;69(4):995–1008.
http://doi.org/1
0.1007/s00228-012-1435-y
https://www.ncbi.nlm.nih.gov/pubmed/23090705
45. Mekonnen AB, Alhawassi TM, McLachlan AJ, Brien
JE. Adverse Drug Events and Medication Errors
in African Hospitals: A systematic review. Drugs
Real World Outcomes. 2018;5(1):1–24.
http://doi.org/1
0.1007/s40801-017-0125-6
https://www.ncbi.nlm.nih.gov/pubmed/29138993
46. Reporting and learning systems for medication
errors: the role of pharmacovigilance centres.
Geneva: World Health Organization; 2014
(http://apps.who.int/iris/bitstream/handle/1
0665/1
37036/9789241507943_eng.pdf;jsessionid=9A14E
E6E1365A4D060E798A736107EF6?sequence=1 ,
accessed 22 March 2019).
47. Medication Errors: Technical Series on Safer
Primary Care. Geneva: World Health Organization;
2016
(http://apps.who.int/iris/bitstream/handle/1
0665/2
5227
4/97892415
11643-eng.pdf?sequence=1,
accessed 22 March 2019).
48. Maher RL, Hanlon J, Hajjar ER. Clinical
Consequences of Polypharmacy in Elderly. Expert
Opin Drug Saf. 2014;13(1):57–65.
http://doi.org/1
0.1517/14740338.2013.827660
https://www.ncbi.nlm.nih.gov/pubmed/24073682
49. The importance of pharmacovigilance: Safety
monitoring of medicinal products. Geneva: World
Health Organization; 2002
(http://apps.who.int/iris/bitstream/handle/1
0665/4
2493/a75646.pdf?sequence=1
, accessed
22 March 2019).
50. Mueller SK, Sponsler KC, Kripalani S, Kripalani S,
Schnipper J. Hospital-based Medication
Reconciliation Practices: A Systematic Review.
Arch Intern Med. 2012;172(14):1057–69.
http://doi.org/1
0.1001/archinternmed.2012.2246
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3
575731/
51. Hu SH, Capezuti E, Foust JB, Boltz MP, Kim H.
Medication discrepancy and potentially
inappropriate medication in older Chinese-
American home care patients after hospital
discharge. Am J Geriatr Pharmacother.
2012;10(5):284–95.
http://doi.org/1
0.1016/j.amjopharm.2012.08.001
https://www.ncbi.nlm.nih.gov/pubmed/22944511
32
MEDICATION SAFETY IN POLYPHARMACY

33
MEDICATION SAFETY IN POLYPHARMACY
52. Glintborg B, Andersen SE, Dalhoff K. Insufficient
communication about medication use at the
interface between hospital and primary care.
Qual Saf Health Care. 2007;16(1):34–9.
http://doi.org/1
0.1136/qshc.2006.019828
https://www.ncbi.nlm.nih.gov/pubmed/17301202
53. Gallagher P, Barry P, O’Mahony D. Inappropriate
prescribing in the elderly. J Clin Pharm Ther.
2007;32(2):113–21.
https://doi.org/1
0.1111/j.1365-27
10.2007.00793.x
https://www.ncbi.nlm.nih.gov/pubmed/17381661
54. Barber ND, Alldred DP, Raynor DK, Dickinson R,
Garfield S, Jesson B et al. Care homes’ use
of medicines study: prevalence and potential
causes of harm of medication errors in care
homes for older people. Qual Saf Health Care.
2009;18(5):341–6.
http://doi.org/1
0.1136/qshc.2009.034231
https://www.ncbi.nlm.nih.gov/pubmed/19812095
55. Briesacher BA, Limcangco MR, Simoni-Wastila L,
Doshi JA, Levens SR, Shea DG et al. The quality of
antipsychotic drug prescribing in nursing homes.
Arch Intern Med. 2005; 165(11):1280–5.
https://doi.org/1
0.1001/archinte.165.11.1280
https://www.ncbi.nlm.nih.gov/pubmed/15956008
56. Beyene KA, Sheridan J, Aspden T. Prescription
medication sharing: a systematic review of the
literature. Am J Public Health. 2014; 104(4):e15–26.
https://dx.doi.org/1
0.2105%2F
AJPH.2013.301
823
https://www
.ncbi.nlm.nih.gov/pmc/articles/PMC4
025682/
57. WHO Traditional medicine strategy 2014–2023.
Geneva: World Health Organization; 2013
(http://apps.who.int/iris/bitstream/handle/1
0665/9
2455/9789241506090_eng.pdf?sequence=1,
accessed 22 March 2019).
58. WHO guidelines on safety monitoring of herbal
medicines in pharmacovigilance systems. Geneva:
World Health Organization; 2004
(http://apps.who.int/medicinedocs/documents/s7
148e/s7148e.pdf, accessed 22 March 2019).
59. Agbabiaka T, Wider B, Watson LK, Goodman C.
Concurrent use of prescription drugs and herbal
medicinal products in older adults: a systematic
review protocol. Syst Rev. 2016;5:65.
https://doi.org/1
0.1186%2Fs13643-016-0244-2
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4
839096/
60. Izzo AA, Hoon-Kim S, Radhakrishnan R,
Williamson EM. A critical approach to evaluating
clinical efficacy, adverse events and drug
interactions of herbal remedies. Phytother Res.
2016;30(5):691–700.
https://doi.org/1
0.1002/ptr.5591
https://www.ncbi.nlm.nih.gov/pubmed/26887532
61. Scottish Government Polypharmacy Model of Care
Group. Polypharmacy guidance: Realistic
prescribing, 3rd edition. Edinburgh: Scottish
Government; 2018
(https://www
.therapeutics.scot.nhs.uk/wp-
content/uploads/2018/09/Polypharmacy-
Guidance-2018.pdf, accessed 22 March 2019).
62. Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh
A. Interventions in primary care to reduce
medication related adverse events and hospital
admissions: systematic review and meta-analysis.
Qual Saf Health Care. 2006; 15(1):23–31.
https://doi.org/1
0.1136/qshc.2004.012153
https://www.ncbi.nlm.nih.gov/pubmed/16456206
63. Huiskes VJB, Burger DM, van den Ende CHM,
van den Bemt BJF. Effectiveness of medication
review: a systematic review and meta-analysis
of randomized controlled trials. BMC Fam Pract.
2017;18:5.
https://doi.org/1
0.1186/s12875-016-0577-x
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5
240219/
64. Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom
SA, Brown BA, Tarvin E et al. Role of pharmacist
counseling in preventing adverse drug events after
hospitalization. Arch Intern Med. 2006;
166(5):565–71.
https://doi.org/1
0.1001/archinte.166.5.565
https://www
.ncbi.nlm.nih.gov/pubmed/16534045
65. Christensen M, Lundh A. Medication review in
hospitalised patients to reduce morbidity and
mortality. Cochrane Database Syst Rev.
2016;2:CD008986.
https://doi.org/1
0.1002/14651858.CD008986.pub3
https://www
.ncbi.nlm.nih.gov/pubmed/26895968
66. Johansson T, Abuzahra ME, Keller S, Mann E,
Faller B, Sommerauer C et al. Impact of strategies
to reduce polypharmacy on clinically relevant
endpoints: a systematic review and meta-analysis.
Br J Clin Pharmacol. 2016;82(2):532–48.
https://doi.org/1
0.1111/bcp.12959
https://www
.ncbi.nlm.nih.gov/pubmed/27059768
67. Rankin A, Cadogan CA, Patterson SM, Kerse N,
Cardwell CR, Bradley MC et al. Interventions
to improve the appropriate use of polypharmacy
for older people. Cochrane Database Syst Rev.
2018(9):CD008165.
https://doi.org/1
0.1002/14651858.CD008165.pub4
https://www.ncbi.nlm.nih.gov/pubmed/30175841
68. NICE Database of treatment effects [database].
London: National Institute for Health and Care
Excellence; 2016
(https://www
.nice.org.uk/guidance/ng56/resource
s/database-of-treatment-effects-excel-2610552205,
accessed 22 March 2019).
69. Polypharmacy: Guidance for Prescribing. Vale of
Glamorgan: All Wales Medicines Strategy Group;
2014
(http://www
.awmsg.org/docs/awmsg/medman/Po

34
MEDICATION SAFETY IN POLYPHARMACY
lypharmacy%20-
%20Guidance%20for%20Prescribing.pdf,
accessed 22 March 2019).
70. Therapy (NNT) reviews [website]. The NNT Group;
2019 (http://www
.thennt.com/home-nnt/,
accessed 22 March 2019).
71. Hilmer SN, Gnjidic D, Le Couteur DG. Thinking
through the medication list - appropriate
prescribing and deprescribing in robust and frail
older patients. Aust Fam Physician.
2012;41(12):924–8.
https://www
.ncbi.nlm.nih.gov/pubmed/232
10113
72. Polypharmacy guidance: the 7 steps. In:
Polypharmacy Guidance – Medicines Review
[website]. Edinburgh: NHS Scotland; 2019
(http://www
.polypharmacy.scot.nhs.uk/polypharm
acy-guidance-medicines-review/for-healthcare-
professionals/7-steps/, accessed 22 March 2019).
73. Teach back. In: The Health Literacy Place
[website]. Edinburgh: NHS Education for Scotland;
2019 (http://www
.healthliteracyplace.org.uk/tools-
and-techniques/techniques/teach-back/, accessed
22 March 2019).
74. Thompson W, Farrell B. Deprescribing: what is it
and what does the evidence tell us? Can J Hosp
Pharm. 2013;66(3):201–2.
https://www
.ncbi.nlm.nih.gov/pmc/articles/PMC3
694945/
75. O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN,
Ryan C, Gallagher P. STOPP/START criteria for
potentially inappropriate prescribing in older
people: version 2. Age Ageing. 2015;44(2):213–8.
https://doi.org/1
0.1093/ageing/afu145
https://www
.ncbi.nlm.nih.gov/pubmed/25324330
76. Kaufmann CP, Tremp R, Hersberger KE, Lampert
ML. Inappropriate prescribing: a systematic
overview of published assessment tools. Eur J Clin
Pharmacol. 2014;70(1):1–11.
https://doi.org/1
0.1007/s00228-013-1575-8
https://www.ncbi.nlm.nih.gov/pubmed/24019054
77. Garfinkel D, Mangin D. Feasibility study of a
systematic approach for discontinuation of multiple
medications in older adults. Arch Intern Med.
2010;170(10):1648–54.
http://doi.org/1
0.1001/archinternmed.2010.355
https://www.ncbi.nlm.nih.gov/pubmed/20937924
78. SIMPATHY project: innovation for appropriate
polypharmacy in the elderly. In: Digital Single
Market [website]. Brussels: European Commission;
2016 (https://ec.europa.eu/digital-single-
market/en/news/simpathy-project-innovation-
appropriate-polypharmacy-elderly, accessed
22 March 2019).
79. Wilson M, Mair A, Dreischulte T, Witham M.
Prescribing to fit the needs of older people:
the NHS Scotland Polypharmacy Guidance,
2nd edition. J R Coll Physicians Edinb.
2015;45(2):108–13.
https://doi.org/10.4997/JRCPE.2015.204
https://www.ncbi.nlm.nih.gov/pubmed/26181524
80. Michael N, Wiese B, SIMPATHY consortium. The
SIMPATHY economic analysis tool: user guide.
SIMPATHY consortium; 2017
(http://www
.simpathy.eu/sites/default/files/SIMPA
THY-Economic-analysis-tool-User-guide-v1.pdf,
accessed 22 March 2019).
81. Geerligs L, Rankin NM, Shepherd HL, Butow P.
Hospital-based interventions: a systematic review
of staff-reported barriers and facilitators to
implementation processes. Implement Sci. 2018;
13: 36.
https://dx.doi.org/1
0.
1186%2Fs13012-018-0726-9
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5
824580/
82. Mosadeghrad AM, Ansarian M. Why do
organisational change programmes fail?
IJSM.2014;5(3):189–218.
http://doi.org/1
0.1504/IJSCM.201
4.064460
83. Saha S, Beach MC, Cooper LA. Patient
Centeredness, Cultural Competence and
Healthcare Quality. J Natl Med Assoc.
2008;100(11):1275–85.
https://doi.org/1
0.1016/S0027-9684(15)31505-4
https://www
.ncbi.nlm.nih.gov/pubmed/19024223
84. Cunningham BA, Marsteller JA, Romano MJ,
Carson KA, Noronha GJ, McGuire MJ et al.
Perceptions of Health System Orientation: Quality,
Patient Centeredness, and Cultural Competency.
Med Care Res Rev. 2014;71(6):559–79.
http://doi.org/1
0.1177/1077558714557891
https://www.ncbi.nlm.nih.gov/pubmed/25389301
85. Brach C, Lenfestey N, Roussel A, Amoozegar J,
Sorenson A. Will it work here? A decisionmaker’s
guide to adopting innovations. Rockville (MD):
Agency for Healthcare Research and Quality; 2008
(https://innovations.ahrq.gov/sites/default/files/gui
des/InnovationAdoptionGuide.pdf, accessed
22 March 2019).
86. Northern Ireland Medicines Optimisation Quality
Framework. Belfast: Department of Health, Social
Services and Public Safety; 2016
(https://www
.health-
ni.gov.uk/sites/default/files/consultations/dhssps/
medicines-optimisation-quality-framework.pdf,
accessed 22 March 2019).
87. OPtimising thERapy to prevent Avoidable hospital
admissions in the Multimorbid elderly. In:
Community Research and Development
Information Service [website]. Luxembourg:
European Commission, EU Publications Office;
2017
(https://cordis.europa.eu/project/rcn/1
93279_en.
html, accessed 22 March 2019).
88. OPERAM Report Summary: Periodic Reporting
for period 2 - OPERAM (OPtimising thERapy
to prevent Avoidable hospital admissions in the

Multimorbid elderly). Brussels: European
Commission: Community Research and
Development Information Service; 2016
(https://cordis.europa.eu/result/rcn/1
90302_en.html,
accessed 22 March 2019).
89. Sönnichsen A, Trampisch US, Rieckert A, Piccoliori
G, Vögele A, Flamm M et al. Polypharmacy in
chronic diseases – Reduction of Inappropriate
Medication and Adverse drug events in older
populations by electronic Decision Support
(PRIMA-eDS): study protocol for a randomized
controlled trial. Trials. 2016;17:57.
https://doi.org/1
0.1186/s13063-016-1
177-8
https://www.ncbi.nlm.nih.gov/pubmed/26822311
90. Rieckert A, Sommerauer C, Krumeich A,
Sönnichsen A. Reduction of inappropriate
medication in older populations by electronic
decision support (the PRIMA-eDS study):
a qualitative study of practical implementation
in primary care. BMC Fam Pract. 2018;19:110.
https://doi.org/1
0.1186/s1
2875-018-0789-3
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/2
9986668/
91. Rieckert A, Trampisch US, Klaaβen-Mielke R,
Drewelow E, Esmail A, Johansson T et al.
Polypharmacy in older patients with chronic
diseases: a cross-sectional analysis of factors
associated with excessive polypharmacy. BMC
Fam Pract. 2018; 19: 113.
https://dx.doi.org/1
0.11
86%2Fs12875-018-0795-5
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6
052592/
92. Kingdon JW. Agendas, alternatives and public
policies, 2nd edition. Harlow: Pearson; 2011.
93. The World Medicines Situation. Geneva: World
Health Organization; 2004
(http://apps.who.int/medicinedocs/pdf/s6
160e/s6
160e.pdf, accessed 22 March 2019).
94. Alpert PT, Gatlin T. Polypharmacy in Older Adults.
Home Healthc Now. 2015;33(10):524–9.
http://doi.org/1
0.1097/NHH.0000000000000299
https://www.ncbi.nlm.nih.gov/pubmed/26529442
95. Patient Engagement: Technical Series on Safer
Primary Care. Geneva: World Health Organization;
2016
(http://apps.who.int/iris/bitstream/handle/1
0665/2
52269/9789241
511629-eng.pdf?sequence=1,
accessed 22 March 2019).
96. McCray AT. Promoting Health Literacy. J Am Med
Inform Assoc. 2005;12(2):152–63.
https://doi.org/1
0.1197/jamia.M1687
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5
51547/
97. Barber S, Thakkar K, Marvin V, Franklin BD, Bell D.
Evaluation of my medication passport: a patient-
completed aide-memoire designed by patients, for
patients, to help towards medicines optimization.
BMJ Open. 2014;4(8):e005608.
https://doi.org/10.1136/bmjopen-2014-005608
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC41
39624/
98. Wheeler AJ, Scahill S, Hopcroft D, Stapleton H.
Reducing medication errors at transitions of care is
everyone’s business. Aust Prescr. 2018;41(3):73–7.
https://doi.org/1
0.18773/austprescr.2018.021
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6
003014/
99. Patient Resources. In: Choosing Wisely [website].
Philadelphia (PA): ABIM Foundation; 2019
(http://www
.choosingwisely.org/patient-
resources/, accessed 22 March 2019).
100. Your Medicine: Be Smart. Be Safe. (with wallet
card) [website]. Rockville (MD): Agency
for Healthcare Research and Quality; 2019
(https://www
.ahrq.gov/patients-
consumers/patient-involvement/ask-your-
doctor/tips-and-tools/yourmeds.html, accessed
22 March 2019).
101. NHS Scotland, Scottish Patient Safety Programme.
Medicine sick day rules card [website]. Edinburgh:
Healthcare Improvement Scotland; 2019
(https://ihub.scot/improvement-
programmes/scottish-patient-safety-programme-
spsp/spsp-primar
y-care/medicines-sick-day-rules-
card/
, accessed 22 March 2019).
102. Questions To Ask Before Taking Medicine. In: Your
Medicine: Be Smart. Be Safe. (with wallet card)
[website]. Rockville (MD): Agency for Healthcare
Research and Quality; 2017
(https://www
.ahrq.gov/patients-
consumers/patient-involvement/ask-your-
doctor/tips-and-tools/yourmedques.html,
accessed 22 March 2019).
103. Institute for Safe Medication Practices Canada,
Canadian Patient Safety Institute, Canadian Society
for Hospital Pharmacists, Canadian Pharmacists
Association, Patients for Patient Safety Canada. Five
questions to ask about your medications. Edmonton
(AB): Canadian Patient Safety Institute; 2016
(http://www
.patientsafetyinstitute.ca/en/toolsReso
urces/5-Questions-to-Ask-about-your-
Medications/Pages/default.aspx, accessed
22 March 2019).
104. 5 Moments for medication safety. In: Patient Safety
[website]. Geneva: World Health Organization; 2019
(https://www
.who.int/patientsafety/medication-
safety/5moments/en/, accessed 15 April 2019).
105. Schneider MP, Gertsch A, Bugnon O. Cyberhealth
serving to support individual intake of medication.
Swiss Med Wkly. 2013;143:w13827.
https://doi.org/1
0.4414/smw.2013.13827
https://www.ncbi.nlm.nih.gov/pubmed/23925717
106. Baldwin JL, Singh H, Sittig D, Giardina TD. Patient
portals and health apps: Pitfalls, promises, and
what one might learn from the other. Healthcare.
2017;5(3):81–5.
35
MEDICATION SAFETY IN POLYPHARMACY

https://doi.org/10.1016/j.hjdsi.2016.08.004
https://www.ncbi.nlm.nih.gov/pubmed/27720139
107. Leelakanok N, Holcombe AL, Lund BC, Gu X,
Schweizer ML. Association between polypharmacy
and death: A systematic review and meta-analysis.
J Am Pharm Assoc (2003). 2017;57(6):729–38.e10.
https://doi.org/1
0.1016/j.japh.2017.06.002
https://www.ncbi.nlm.nih.gov/pubmed/28784299
108. Clague F, Mercer SW, McLean G, Reynish E,
Guthrie B. Comorbidity and polypharmacy in
people with dementia: insights from a large,
population-based cross-sectional analysis of
primary care data. Age and Ageing. 2017; 46: 33–9.
https://doi.org/1
0.
1093/ageing/afw176
https://www.ncbi.nlm.nih.gov/pubmed/28181629
109. WHO patient safety curriculum guide: multi-
professional edition. Geneva: World Health
Organization; 2011
(http://apps.who.int/iris/bitstream/handle/1
0665/4
4641/9789241501
958_eng.pdf?sequence=1,
accessed 22 March 2019).
110. Special Interest Groups & Sections [website]. New
York (NY): American Geriatrics Society; 2019
(https://www
.americangeriatrics.org/about-
us/leadership/special-interest-groups-sections,
accessed 22 March 2019).
111. Arbeitsgruppen der ÖGGG [Working groups
of ÖGGG] [website]. Vienna: Österreichische
Gesellschaft für Geriatrie und Gerontologie
[Austrian society for geriatrics and gerontology];
2019 (https://www
.geriatrie-
online.at/gesellschaft/arbeitsgruppen-der-oeggg/
,
accessed 22 March 2019).
112. Appropriate Polypharmacy and Adherence in
Integrated care [website]. Oxford: International
Foundation for Integrated Care; 2019
(https://integratedcarefoundation.org/ific-
integrated-care-academy/special-interest-groups-
sigs/appropriate-polypharmacy-and-adherence-in-
integrated-care
, accessed 22 March 2019).
113. Human Factors in Patient Safety: Review of Topics
and Tools. Report for Methods and Measures
Working Group of WHO Patient Safety. Geneva:
World Health Organization; 2009
(http://www
.who.int/patientsafety/research/metho
ds_measures/human_factors/human_factors_revie
w.pdf
, accessed 22 March 2019).
114. Takane AK, Balignasay M, Nigg CR. Polypharmacy
Reviews Among Elderly Populations Project:
Assessing Needs in Patient-Provider
Communication. Hawaii J Med Public Health.
2013;72(1):15–22.
https://www
.ncbi.nlm.nih.gov/pmc/articles/PMC3
555477/
115. Our mission. In: Choosing Wisely [website].
Philadelphia (PA): ABIM Foundation; 2019
(http://www
.choosingwisely.org/our-mission/,
accessed 22 March 2019).
116. The safety of medicines in public health
programmes: Pharmacovigilance an essential tool.
Geneva: World Health Organization; 2006
(http://www
.who.int/medicines/areas/quality_safety
/safety_efficacy/Pharmacovigilance_B.pdf,
accessed 22 March 2019).
117. Cresswell KM, Fernando B, McKinstry B, Sheikh A.
Adverse drug events in the elderly. Br Med Bull.
2007;83:259–74.
https://doi.org/1
0.1093/bmb/ldm016
https://www.ncbi.nlm.nih.gov/pubmed/17580312
118. Singh H, Spitzmueller C, Petersen NJ, Sawhney
MK, Sittig DF. Information Overload and Missed
Test Results in Electronic Health Record–Based
Settings. JAMA Intern Med. 2013;173(8):702–704.
https://dx.doi.org/1
0.1001%2F2013.jamainternmed
.61
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3
822526/
119. Medicines adherence: involving patients in
decisions about prescribed medicines and
supporting adherence [website]. London: National
Institute for Health and Care Excellence; 2009
(https://www
.nice.org.uk/guidance/cg76/chapter/
1-Guidance, accessed 22 March 2019).
120. Improving patient outcomes: The better use of
multi-compartment compliance. London: Royal
Pharmaceutical Society; 2013
(https://www
.rpharms.com/Portals/0/RPS%20doc
ument%20librar
y/Open%20access/Support/toolki
t/rps-mca-july-2013.pdf, accessed 22 March 2019).
121. Wicklund E. The Evolving Role of mHealth in
Medication Management and Adherence.
mHealthIntelligence; 17 March 2017
(https://mhealthintelligence.com/features/theevolv
ing-role-of-mhealth-in-medicationmanagement-and-
adherence
, accessed 22 March 2019).
122. Yu A, Flott K, Chainani N, Fontana G, Darzi A.
Patient safety 2030. London: NIHR Imperial Patient
Safety Translational Research Centre; 2016
(https://www
.imperial.ac.uk/media/imperial-
college/institute-of-global-health-innovation/centre-
for-health-policy/P
atient-Safety-2030-Report-
VFinal.pdf, accessed 22 March 2019).
123. WHO Model List of Essential Medicines, 20th
edition. Geneva: World Health Organization; 2017
(https://www
.who.int/medicines/publications/esse
ntialmedicines/20th_EML2017.pdf?ua=1, accessed
22 March 2019).
124. Song Y, Bian Y, Petzold M, Li L, Yin A. The impact
of China’s national essential medicine system
on improving rational drug use in primary health
care facilities: an empirical study in four provinces.
BMC Health Serv Res. 2014 ;14:507.
https://dx.doi.org/1
0.1186%2Fs12913-014-0507-3
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4
213512/
36
MEDICATION SAFETY IN POLYPHARMACY

125. Bate P, Robert G. Experience-based design:
from redesigning the system around the patient
to co- designing services with the patient. Qual
Saf Health Care. 2006;15(5):307–10.
https://doi.org/1
0.1136/qshc.2005.016527
https://www.ncbi.nlm.nih.gov/pubmed/17074863
126. Moore MH. Creating public value: strategic
management in government. Cambridge (MA):
Harvard University Press; 1997.
127. Medicines Optimisation: Helping patients to make
the most of medicine. London: Royal
Pharmaceutical Society; 2013
(https://www
.rpharms.com/Portals/0/RPS%20doc
ument%20library/Open%20access/Policy/helping-
patients-make-the-most-of-their-medicines.pdf,
accessed 22 March 2019).
128. Rambhade S, Chakarborty A, Shrivastava A, Patil
UK, Rambhade A. A Survey on Polypharmacy and
Use of Inappropriate Medications. Toxicol Int.
2012; 19(1): 68–73.
https://dx.doi.org/1
0.4
103%2F0971-6580.94506
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3
339249/
129. Rankin A, Cadogan CA, In Ryan C, Clyne B, Smith
SM, Hughes CM. Core outcome set for trials aimed
at improving the appropriateness of polypharmacy
in older people in primary care. J Am Geriatr Soc.
2018; 66(6):1206–12.
http://doi.org/1
0.111
1/jgs.15245
https://www
.ncbi.nlm.nih.gov/pubmed/29461621
130. Fastbom J, Johnell K. National indicators for
quality of drug therapy in older persons: the
Swedish experience from the first 10 years. Drugs
Aging. 2015;32(3):189–99.
https://doi.org/1
0.1007/s40266-015-0242-4
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4
366557/
131. Polyfarmacie indicatoren [Polypharmacy
indicators]. The Hague: Stichting Farmaceutische
Kengetallen; 2016
(https://www
.sfk.nl/documentatie/polyfarmacie/po
lyfarmacie-indicatoren#START_1, accessed
22 March 2019).
132. Atlas of healthcare variation methodology,
polypharmacy. Wellington: Health Quality and
Safety Commission New Zealand; 2017
(https://www
.hqsc.govt.nz/assets/Health-Quality-
Evaluation/Atlas/PolypharmacySFOct17/Methodol
ogy_polypharmacy.pdf, accessed 22 March 2019).
133. Department of Health and Human Services.
Indicator 4: Use of nine or more medicines.
Melbourne: State Government Victoria; 2015
(https://www2.health.vic.gov
.au/about/publication
s/policiesandguidelines/section-3-indicator-4-use-
of-nine-or-more-medicines, accessed 22 March
2019).
134. Burt J, Elmore N, Campbell SM, Rodgers S,
Avery AJ, Payne RA. Developing a measure of
polypharmacy appropriateness in primary care:
systematic review and expert consensus study.
BMC Med. 2018;16(1):91.
https://doi.org/1
0.1186/s12916-018-1078-7
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5
998565/
37
MEDICATION SAFETY IN POLYPHARMACY
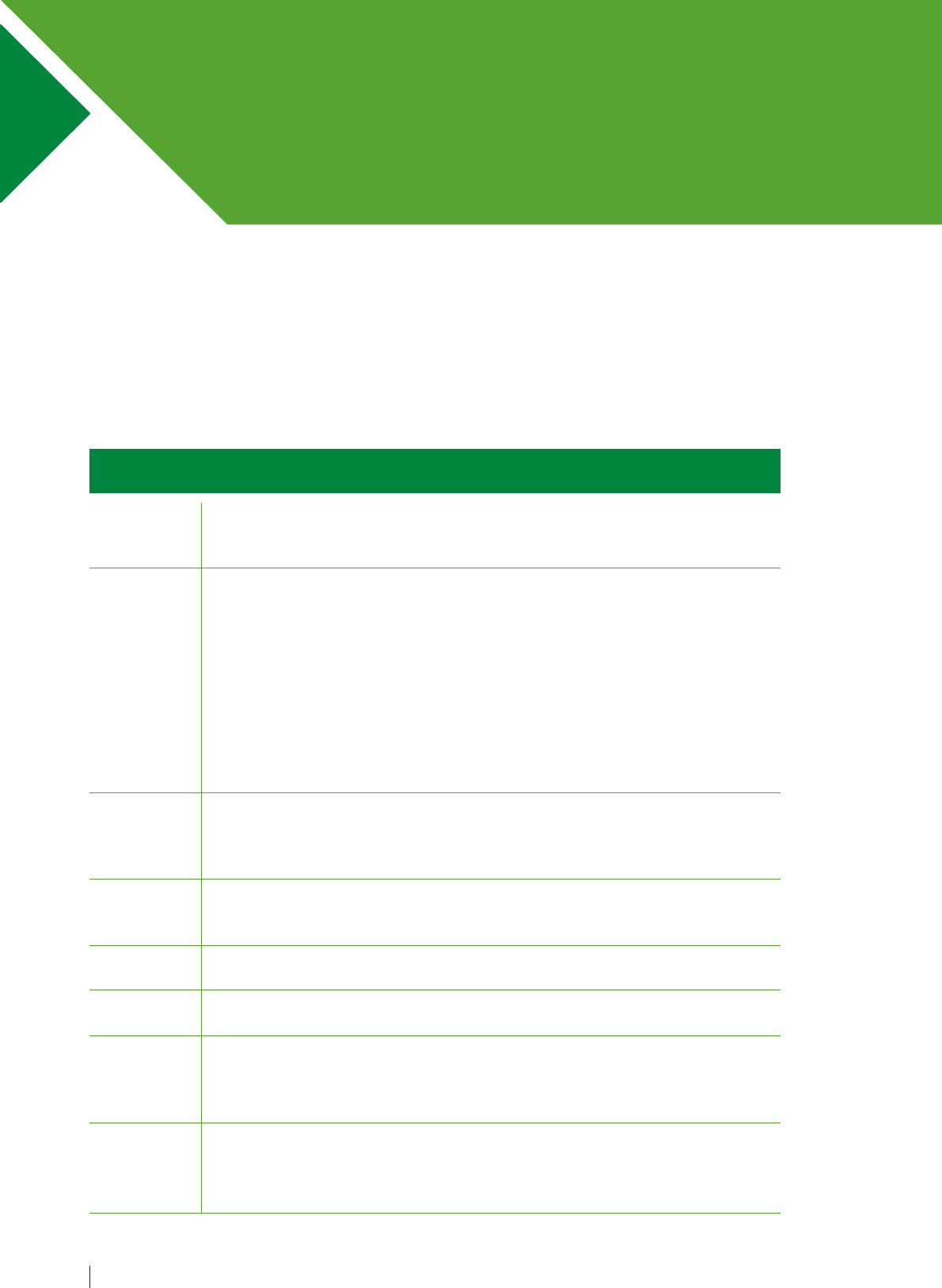
38
MEDICATION SAFETY IN POLYPHARMACY
Annex 1. Glossary
Term Definition and source used in glossary (see separate glossary references
below)
Adverse drug
event
Adverse drug
reaction
Anaphylaxis
Best possible
medication
history
Deprescribing
Essential
medicines
Forcing
function
Formulary
Any injury resulting from medical interventions related to a drug. This includes
both adverse drug reactions in which no error occurred and complications
resulting from medication errors
(1)
A response to a drug which is noxious and unintended and that occurs at doses
used in humans for prophylaxis, diagnosis or therapy of diseases, or for the
modification of physiological function
(2)
. These are often classified as two types:
Type A and Type B
(3)
Type A adverse drug reaction
An augmented pharmacologically predictable reaction which is dose
dependent. It is generally associated with high morbidity and low mortality
(4)
Type B adverse drug reaction
A bizarre reaction which is unpredictable pharmacologically and is independent
of dose. It is generally associated with low morbidity and high mortality
(4)
A severe, life-threatening systemic hypersensitivity reaction characterized by
being rapid in onset with potentially life-threatening airway, breathing, or
circulatory problems and is usually, although not always, associated with skin
and mucosal changes
(5)
A medication history obtained by a clinician which includes a thorough history
of all regular medication use (prescribed and non-prescribed), using a number
of different sources of information
(6)
The process of tapering, stopping, discontinuing, or withdrawing drugs, with
the goal of managing polypharmacy and improving outcomes
(7)
Essential medicines are those that satisfy the priority health care needs of the
population
(8)
An aspect of a design that prevents the user from taking an action without
consciously considering information relevant to that action. It forces conscious
attention upon something (“bringing to consciousness”) and thus deliberately
disrupts the efficient or automatized performance of a task
(9)
A list of medicines, usually by their generic names, and indications for their use.
A formulary is intended to include a sufficient range of medicines to enable
medical practitioners, dentists and, as appropriate, other practitioners to prescribe
all medically appropriate treatment for all reasonably common illnesses
(10)

39
MEDICATION SAFETY IN POLYPHARMACY
Term Definition and source used in glossary (see separate glossary references
below)
High-risk
(high-alert)
medications
Medication
adherence
Medication
discrepancy
Medication
error
Medication
reconciliation
Medication-
related
harm
Medication
review
Medication
safety
Medication
use process
Medicines
optimization
Multimorbidity
Near miss
Patient safety
Pharma-
covigilance
Drugs that bear a heightened risk of causing significant patient harm when they
are used in error. Although mistakes may or may not be more common with these
medications, the consequences of an error are clearly more devastating to
patients
(11)
The degree to which use of medication by the patient corresponds with the
prescribed regimen
(12)
Any difference between the medication use history and the admission medication
orders
(13)
. Discrepancies may be intentional, undocumented intentional or
unintentional discrepancies
(6)
Any preventable event that may cause or lead to inappropriate medication use or
patient harm while the medication is in the control of the health care professional,
patient, or consumer
(14)
The formal process in which health care professionals partner with patients to
ensure accurate and complete medication information transfer at interfaces of
care
(6)
Patient harm related to medication. It includes preventable adverse drug events
(e.g. due to a medication error or accidental or intentional misuse) and non-
preventable adverse drug events (e.g. an adverse drug reaction)
A structured evaluation of patient’s medicines with the aim of optimizing
medicines use and improving health outcomes. This entails detecting drug related
problems and recommending interventions
(15)
Freedom from accidental injury during the course of medication use; activities to
avoid, prevent, or correct adverse drug events which may result from the use of
medications
(16)
The multistep process in the use of medications by or for patients, including:
prescribing, ordering, storage, dispensing, preparation, administration and/or
monitoring
Ensuring that the right patients get the right choice of medicine, at the right time.
By focusing on patients and their experiences, the goal is to help patients to (a)
improve their outcomes; (b) take their medicines correctly; (c) avoid taking
unnecessary medicines; (d) reduce wastage of medicines; and (e) improve
medicines safety
(17)
The presence of two or more long-term health conditions, which can include (a)
defined physical and mental health conditions such as diabetes or schizophrenia;
(b) ongoing conditions such as learning disability; (c) symptom complexes such as
frailty or chronic pain; (d) sensory impairment such as sight or hearing loss; and
(e) alcohol and substance misuse
(18)
An incident that did not reach the patient
(19)
The absence of preventable harm to a patient and reduction of risk of
unnecessary harm associated with health care to an acceptable minimum. An
acceptable minimum refers to the collective notions of given current knowledge,
resources available and the context in which care was delivered weighed against
the risk of non-treatment or other treatment
(20)
Science and activities relating to the detection, assessment, understanding and
prevention of adverse effects or any other drug-related problem
(2)

40
MEDICATION SAFETY IN POLYPHARMACY
Term Definition and source used in glossary (see separate glossary
references below)
Polypharmacy
Potentially
inappropriate
medications
Safety
Side effect
Transitions
of care
Polypharmacy is the concurrent use of multiple medications. Although there
is no standard definition, polypharmacy is often defined as the routine use
of five or more medications
(21)
. This includes over-the-counter, prescription
and/or traditional and complementary medicines used by a patient
Medications with ineffectiveness or high risk–benefit ratio for a particular
individual or group of individuals
(22)
The reduction of risk of unnecessary harm to an acceptable minimum
(19)
A known effect, other than that primarily intended, related to the
pharmacological properties of a medication
(19)
The various points where a patient moves to, or returns from, a particular
physical location or makes contact with a health care professional for the
purposes of receiving health care
(23)

1. Bates DW, Boyle DL, Vander Vliet MB, Schneider J,
Leape L. Relationship between medication errors
and adverse drug events. J Gen Intern Med.
1995;10(4):199–205.
https://www
.ncbi.nlm.nih.gov/pubmed/7790981
2. The importance of pharmacovigilance: safety
monitoring of medicinal products. Geneva: World
Health Organization; 2002
(http://apps.who.int/medicinedocs/pdf/s4893e/s4
893e.pdf, accessed 20 March 2019).
3. Yellow Card Scheme. Guidance on adverse drug
reactions. London: Medicines and Healthcare
Products Regulatory Agency; 2019
(https://assets.publishing.ser
vice.gov.uk/governme
nt/uploads/system/uploads/attachment_data/file/4
03098/Guidance_on_adverse_drug_reactions.pdf,
accessed 20 March 2019).
4. International Drug Surveillance Department
(editor). Drug safety: a shared responsibility.
Edinburgh: Churchill Livingstone; 1991.
5. ICD-11. 4A84 Anaphylaxis. Geneva: World Health
Organization; 2018 (https://icd.who.int/browse1
1/l-
m/en, accessed 20 March 2019).
6. The high 5s project implementation guide.
Assuring medication accuracy at transitions in
care: medication reconciliation. Geneva: World
Health Organization; 2014
(http://www
.who.int/patientsafety/implementation/
solutions/high5s/h5s-guide.pdf?ua=1, accessed
20 March 2019).
7. Thomson W, Farrell B. Deprescribing: what is it
and what does the evidence tell us? Can J Hosp
Pharm. 2013;66(3):201-2.
https://www
.ncbi.nlm.nih.gov/pubmed/23814291
8. Essential medicines. In: Health topics [website].
Geneva: World Health Organization; 2019
(http://www
.who.int/topics/essential_medicines/en/,
accessed 20 March 2019).
9. Forcing functions. In: Interaction Design
Foundation [website]. Aarhus: Interaction Design
Foundation; 2019 (https://www
.interaction-
design.org/literature/book/the-glossary-of-human-
computer-interaction/forcing-functions, accessed
20 March 2019).
10. A glossary of terms for community health care and
services for older persons. Geneva: World Health
Organization; 2004
(http://apps.who.int/iris/bitstream/handle/1
0665/6
8896/WHO_WKC_Tech.Ser._04.2.pdf?sequence=1
&isAllowed=y, accessed 20 March 2019).
11. High-Alert Medications In Community/Ambulatory
Settings. In: Institute for Safe Medication Practices
[website]. Horsham (PA): Institute for Safe
Medication Practices; 2011
(https://www
.ismp.org/recommendations/high-
alert-medications-community-ambulatory-list,
accessed 20 March 2019).
12. Adherence to long-term therapies: evidence for
action. Geneva: World Health Organization; 2003
(http://apps.who.int/iris/bitstream/handle/1
0665/4
2682/9241545992.pdf;jsessionid=D290397E38A00
1B222D9AEF590C7B9D2?sequence=1, accessed
20 March 2019).
13. Cornish PL, Knowles SR, Marchesano R, Tam V,
Shadowitz S, Juurlink DN, et al. Unintended
medication discrepancies at the time of hospital
admission. Arch Intern Med. 2005;165:424–9.
https://doi.org/1
0.1001/archinte.165.4.424
https://www.ncbi.nlm.nih.gov/pubmed/15738372
14. About medication errors [website]. Rockville (MD):
National Coordinating Council for Medication error
reporting and prevention; 2019
(http://www
.nccmerp.org/about-medication-errors,
accessed 20 March 2019).
15. Medication review definition approved [website].
Zuidlaren: Pharmaceutical Care Network Europe;
2019 (https://www
.pcne.org/news/35/medication-
review-definition-approved, accessed 20 March 2019).
16. Committee of Experts on Management of Safety
and Quality in Health Care. Glossary of terms
related to patient and medication safety.
Strasbourg: Council of Europe; 2005
(http://www
.who.int/patientsafety/highlights/COE_
patient_and_medication_safety_gl.pdf, accessed
20 March 2019).
17. Medicines Optimisation: Helping patients to make
the most of medicine. London: Royal
Pharmaceutical Society; 2013
Glossary references
41
MEDICATION SAFETY IN POLYPHARMACY

(https://www
.rpharms.com/Portals/0/RPS%20doc
ument%20library/Open%20access/Policy/helping-
patients-make-the-most-of-their-medicines.pdf,
accessed 20 March 2019).
18. Multimorbidity: clinical assessment and
management. London: National Institute for Health
and Care Excellence; 2016
(https://www
.nice.org.uk/guidance/ng56/chapter/
Recommendations#general-principles, accessed
20 March 2019).
19. Definitions of key concepts from the WHO patient
safety curriculum guide. Geneva: World Health
Organization; 2012
(http://www
.who.int/patientsafety/education/curric
ulum/course1a_handout.pdf, accessed 20 March
2019).
20. The conceptual framework for the international
classification for patient safety. Geneva: World
Health Organization; 2009
(http://apps.who.int/iris/bitstream/handle/1
0665/70
882/WHO_IER_PSP_2010.2_eng.pdf?sequence=1,
accessed 20 March 2019).
21. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey
GE. What is polypharmacy? A systematic review
of definitions. BMC Geriatr. 2017;17:230.
https://dx.doi.org/1
0.1186%2Fs12877-017-0621-2
https://www.ncbi.nlm.nih.gov/pubmed/29017448
22. Chang CB, Chen JH, Wen CJ, Kuo HK, Lu IS, Chiu
LS et al. Potentially inappropriate medications
in geriatric outpatients with polypharmacy:
application of six sets of published explicit criteria.
Br J Clin Pharmacol. 2011;72(3):482–9.
https://doi.org/1
0.1
111/j.1365-2125.2011.04010.x
https://www.ncbi.nlm.nih.gov/pubmed/21
557760
23. Transitions of Care: Technical Series on Safer
Primary Care. Geneva: World Health Organization;
2016
(http://apps.who.int/iris/bitstream/handle/1
0665/2
52272/9789241511599-eng.pdf?sequence=1,
accessed 20 March 2019).
42
MEDICATION SAFETY IN POLYPHARMACY

Country/
region
Worldwide
(including
United States
of America,
Australia,
Italy,
Netherlands
and more)
7 European
Union (EU)
countries
(Czech
Republic,
England,
Finland,
France,
Germany,
Italy and
Netherlands)
and
1 non-EU
country
(Israel)
Study
population
Residents
in long-term
care
facilities
(n=20
studies
included all
residents,
n=19
included
residents ≥
65 years),
mean age
ranged
61.7–86.0
years
4,023
nursing
home
residents in
57 facilities,
mean age of
83.5 years
Definition of
polypharmacy
Definitions varied
widely and were
most commonly
described as
5 (n=11), 9 (n=13)
or 10 (n=11) or
more medications
Non-polypharmacy
(0–4 medications),
polypharmacy
(5–9 medications)
and excessive
polypharmacy
(≥10 medications)
Statistics
A systematic review
of 44 studies assessing
medication use in
long-term care facilities
reported a 38.1–91.2%
prevalence of
polypharmacy, where
polypharmacy was
defined as ≥5
medications. When
defined as ≥ 9
medications, the
prevalence ranged
from 12.8–74.4% and
10.6–65.0% when
considered as ≥ 10
medications
Cross-sectional analysis
of residents found that
49.7% experienced
polypharmacy and
24.3% experienced
excessive polypharmacy
Reference
Jokanovic N, Tan EC,
Dooley MJ, Kirkpatrick
CM, Bell JS. Prevalence
and factors associated
with polypharmacy in
long-term care facilities:
a systematic review.
J Am Med Dir Assoc.
2015;16(6):535.e1–12.
https://doi.org/10.1016/j.j
amda.2015.03.003
https://www.ncbi.nlm.nih.
gov/pubmed/25869992
Onder G, Liperoti R,
Fialova D, Topinkova E,
Tosato M, Danese P et al.
Polypharmacy in nursing
home in Europe: results
from the SHELTER study.
J Gerontol A Biol Sci Med
Sci. 2012;67(6):698–704.
https://doi.org/10.1093/ge
rona/glr233
https://www.ncbi.nlm.nih.
gov/pubmed/22219520
Annex 2. Global prevalence
of polypharmacy
43
MEDICATION SAFETY IN POLYPHARMACY
44
MEDICATION SAFETY IN POLYPHARMACY
Country/
region
Australia
Brazil
Chile
Study
population
1,608
Australians
aged ≥50
years
142 patients
aged ≥60
years in
primary
health care
setting
250
hospitalized
patients
aged ≥ 65
years in one
hospital
Definition of
polypharmacy
Use of 5 or more
medications of
any type in the
previous 24 hours
Use of 4 or more
medications per
day.
Prevalence of
potentially
inappropriate
medication (PIM,
see Annex 1)
as determined
by Beers criteria
(a medication list
for PIM use in
older adults
updated
by the American
Geriatric Society)
Prevalence of PIM
as determined
by Beers criteria
(a medication list
for PIM use in
older adults
updated by the
American Geriatric
Society) and
STOPP criteria
Statistics
The cross-sectional
postal survey reported
43.3% of respondents
using 5 or more
medicines in the
previous 24 hours
The prospective survey
determined that PIM
usage was 34.5%, with
polypharmacy (PR =
2.36; 95% CI 1.79–3.11)
and use of medications
prescribed by a doctor
(PR = 2.52; 95% CI
1.12–5.69), found to
have a strong
association
The observational
study confirmed an
association between
PIM prescribing and
polypharmacy
according to both
criteria.
Using Beers criteria,
prescribed PIMs
identified: one PIM
32%, two PIMs 20%
and more than two
PIMs 48%.
According to STOPP
criteria, prescribed PIM
identified: one PIM
41%, two PIMs 51%
and more than two
PIMs 8%
Reference
Morgan TK, Williamson M,
Pirotta M, Stewart K,
Myers SP, Barnes J. A
national census of
medicines use: a 24-hour
snapshot of Australians
aged 50 years and older.
Med J Aust.
2012;196(1):50–53.
https://doi.org/10.5694/m
ja11.10698
https://www
.ncbi.nlm.nih.
gov/pubmed/22256935
Oliveira MG, Amorim WW,
de Jesus SR, Rodrigues
VA, Passos LC. Factors
associated with
potentially inappropriate
medication use by the
elderly in the Brazilian
primary care setting.
Int J Clin Pharm.
2012;34(4):626–32.
https://doi.org/10.1007/s1
1096-012-9656-9
https://www.ncbi.nlm.nih.
gov/pubmed/22692715
Arellano C, Saldivia G,
Cordova P, Fernandez P,
Morales F, Lopez M et al.
Using two tools to identify
potentially inappropriate
medications (PIM)
in elderly patients
in Southern Chile.
Arch Gerontol Geriatr.
2016;67:139–44.
https://doi.org/10.1016/j.a
rchger.2016.08.001
https://www
.ncbi.nlm.nih.
gov/pubmed/275047
10

45
MEDICATION SAFETY IN POLYPHARMACY
Country/
region
China
India
New
Zealand
Nigeria
Study
population
717
community-
dwelling
adults ≥60
years
814
hospitalized
patients
aged ≥60
years in two
hospitals
444,827
patients
evaluated
in 2005 and
603,670
patients in
2013, aged
≥65 years
220 older
persons
≥65 years
attending
general
outpatients
clinic of
a rural
hospital
Definition of
polypharmacy
The concomitant
use of 5 or more
medications
Polypharmacy
(5–9 medications)
and high-level
polypharmacy
(≥10 medications)
Polypharmacy
(use of 5–9
medications) and
hyperpolypharmac
y (use of ≥10
medications)
by an individual,
dispensed
concurrently for a
period of ≥90 days
Polypharmacy
was not explicitly
stated but inferred
Prevalence of PIM
as determined by
Beers criteria (a
medication list for
PIM use in older
adults updated
by the American
Geriatric Society)
Statistics
The cross-sectional
analysis found that
the prevalence of
polypharmacy was
11.5% in rural residents
and 17.5% in urban
residents
The prospective
surveillance study
reported 45.0% and
45.5% of patients
to experience
polypharmacy and
high-level
polypharmacy
respectively
The cross-sectional
analysis of population-
level dispensing
data found that the
prevalence of
polypharmacy and
hyperpolypharmacy
increased from 23.4%
and 1.3% in 2005
to 29.5% and 2.1%
in 2013 respectively
The prospective
cross-sectional study
of patients reported
29.5% patients had
5 medications or more
prescribed. The
prevalence of patients
with at least one PIM
was 25.5%
Reference
Yang M, Lu J, Hao Q, Luo
L, Dong B. Does residing
in urban or rural areas
affect the incidence of
polypharmacy among
older adults in western
China? Arch Gerontol
Geriatr.
2015;60(2):328–33.
https://doi.org/10.1016/j.a
rchger.2014.11.004
https://www
.ncbi.nlm.nih.
gov/pubmed/25440757
Harugeri A, Joseph J,
Parthasarathi G, Ramesh
M, Guido S. Prescribing
patterns and predictors of
high-level polypharmacy
in the elderly population:
A prospective surveillance
study from two teaching
hospitals in India. Am J
Geriatr Pharmacother.
2010;8(3):271–80.
https://doi.org/10.1016/j.a
mjopharm.2010.06.004
https://www.ncbi.nlm.nih.
gov/pubmed/20624616
Nishtala PS, Salahudeen
MS. Temporal trends
in polypharmacy and
hyperpolypharmacy
in older New Zealanders
over a 9-Year Period:
2005–2013. Gerontology.
2015; 61(3):195–202.
https://doi.org/10.1159/00
0368191
https://www.ncbi.nlm.nih.
gov/pubmed/25428287
Fadare JO, Agboola SM,
Opeke OA, Alabi RA.
Prescription pattern and
prevalence of potentially
inappropriate medications
among elderly patients
in a Nigerian rural tertiary
hospital. Ther Clin Risk
Manag. 2013;9:115–20.
https://doi.org/10.2147/T
CRM.S40120
https://www.ncbi.nlm.nih.
gov/pubmed/23516122
46
MEDICATION SAFETY IN POLYPHARMACY
Country/
region
Saudi
Arabia and
the United
Kingdom
of Great
Britain and
Northern
Ireland
Singapore
Study
population
300
hospitalized
patients
≥18 years
admitted with
cardiovascular
disease and/or
diabetes
mellitus
in two
hospitals
(n=150 Saudi
Arabia,
n=15 United
Kingdom)
454 nursing
home
residents
across three
nursing homes
≥65 years,
mean age
80 years
Definition of
polypharmacy
The use of 5
or more
medications
The use of
5 or more
medications,
including routine
and as required
orders.
Inappropriate
medication use
was determined
by Beers criteria
for PIM
Statistics
The retrospective
medical record review
found that polypharmacy
was the major risk
factor associated with
medication-related
problems. It was
reported to be 40.9%
and 50.9% of patients
in Saudi Arabia and
the United Kingdom
respectively
The retrospective case
note review found that
58.6% and 70% of
residents experienced
polypharmacy and
inappropriate medication
use respectively
Reference
Al Hamid A, Aslanpour Z,
Aljadhey H, Ghaleb M.
Hospitalisation Resulting
from Medicine-Related
Problems in Adult Patients
with Cardiovascular
Diseases and Diabetes in
the United Kingdom and
Saudi Arabia. Int J Environ
Res Public Health.
2016;13(5):479.
https://dx.doi.org/1
0.339
0%2Fijerph1
3050479
https://www
.ncbi.nlm.nih.
gov/pmc/articles/PMC48
8
1104/
Mamun K, Lien CT,
Goh-Tan CY, Ang WS.
Polypharmacy and
inappropriate medication
use in Singapore nursing
homes. Ann Acad Med
Singapore.
2004;33(1):49–52.
https://www.ncbi.nlm.nih.
gov/pubmed/15008562

Annex 3. Internationally
available guidance on
appropriate polypharmacy
management
47
MEDICATION SAFETY IN POLYPHARMACY
No.
1
2
3
4
5
6
7
8
9
10
Country
Australia
Australia
Brazil
Brazil
Canada
England,
United
Kingdom
England,
United
Kingdom
Germany
Germany
Japan
Guidance document details
Guidelines for Residential Medication Management Review (RMMR) and Quality Use
of Medicines (QUM) services. Deakin: Pharmaceutical Society of Australia; 2011
(https://web.archive.org/web/20
180430113735/https://www.psa.org.au/download
/practice-guidelines/rmmr-and-qum-services.pdf, accessed 22 March 2019).
Guiding principles for medication management in residential aged care facilities.
Canberra: Department of Health and Aging; 2012
(http://www.health.gov.au/internet/main/publishing.nsf/Content/guide-med-mgmt-
aged-care, accessed 22 March 2019).
Cartilha para a promoçao do uso racional de medicamentos. Brasίlia: Ministério
da Sa ΄ude; 2015
(http://bvsms.saude.gov.br/bvs/publicacoes/cartilha_promocao_uso_racional_me
dicamentos.pdf, accessed 22 March 2019).
Caderneta de sa ΄ude da pessoa idosa, 3a ediçao. Brasίlia: Ministério Da Sa ΄ude;
2016 (http://portalarquivos2.saude.gov
.br/images/pdf/2017/julho/03/Caderneta-
do-Idoso.pdf, accessed 22 March 2019).
Deprescribing guidelines and algorithms [website]. Ottawa (ON): Canadian
Deprescribing Network; 2019 (http://deprescribing.org/resources/deprescribing-
guidelines-algorithms/, accessed 22 March 2019).
Duerden M, Avery T, Payne R. Polypharmacy and medicines optimisation: making
it safe and sound. London: The King’s Fund; 2013
(http://www.kingsfund.org.uk/sites/files/kf/field/field_publication_file/polypharmac
y-and-medicines-optimisation-kingsfund-nov13.pdf, accessed 22 March 2019).
Multimorbidity and polypharmacy. London: National Institute for Health and Care
Excellence; 2019 (https://www
.nice.org.uk/advice/ktt18; accessed 22 March
2019).
Bergert FW, Braun M, Ehrenthal K, Feßler J, Gross J, Hüttner U et al.
Recommendations for treating adult and geriatric patients on multimedication. Int
J Clin Pharmacol Ther. 2014;52 Suppl 1:1–64. https://doi.org/1
0.5414/CPP52S001
https://www
.ncbi.nlm.nih.gov/pubmed/25310921
Hausärztliche Leitlinie Multimedikation. Köln: PMV forschungsgruppe; 2016
(http://www
.pmvforschungsgruppe.de/pdf/03_publikationen/multimedikation_ll.pdf,
accessed 22 March 2019).
. Tokyo: Japan Geriatrics Society; 2015 (https://www.jpn-
geriat-soc.or.jp/info/topics/pdf/20170808_01.pdf, accessed 22 March 2019).

Guidance document details
Handreiking: Geriatrisch assessment door de specialist ouderengeneeskunde.
Utrecht: Verenso; 2014
(https://www
.verenso.nl/_asset/_public/Praktijkvoering_handreikingen/VER-003-
29-handrGeriatrischAssesement-v4.pdf, accessed 22 March 2019).
De richtlijn: Comprehensive geriatric assessment. Utrecht: Nederlandse Vereniging
voor Klinische Geriatrie; 2012
(https://richtlijnendatabase.nl/gerelateerde_documenten/f/669/Publieksversie%20
richtlijn%20CGA.pdf
, accessed 22 March 2019).
Multidisciplinaire richtlijn polyfarmacie bij ouderen. Utrecht: Nederlands Huisartsen
Genootschap; 2012
(https://www
.nhg.org/sites/default/files/content/nhg_org/uploads/polyfarmacie_bi
j_ouderen.pdf, accessed 22 March 2019).
Bpacnz. Polypharmacy in primary care: managing a clinical conundrum. BPJ.2014;
64 (https://bpac.org.nz/BPJ/20
14/October/docs/BPJ64-polypharmacy.pdf,
accessed 22 March 2019).
NHSGGC mindful prescribing strategy: polypharmacy. Glasgow: NHS Greater
Glasgow and Clyde; 2012
(http://www.ggcprescribing.org.uk/media/uploads/prescribing_resources/mindful
_prescribing_strategy_-_1212.pdf, accessed 22 March 2019).
Scottish Government Polypharmacy Model of Care Group. Polypharmacy
guidance: realistic prescribing, 3rd edition. Edinburgh: Scottish Government Model
of Care Polypharmacy Working Group; 2018
(https://www
.therapeutics.scot.nhs.uk/wp-
content/uploads/20
18/04/Polypharmacy-Guidance-20
18.pdf, accessed 22 March
2019).
Olämpliga listan–om okloka läkemedel för äldre. Stockholm: Koll p°a läkemedel;
2011 (https://www
.kollpalakemedel.se/wp-
content/uploads/20
14/04/olaempliga_listan-201
212
19.pdf, accessed 22 March
2019).
Läkemedelsgenomg°angar för äldre ordinerade fem eller fler läkemedel. Stockholm:
Socialstyrelsen; 2013
(https://www
.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/19019/2013-3-
1
8.pdf, accessed 22 March 2019).
Läkemedelsbehandling av de mest sjuka äldre. Väster°as: Regionala
läkemedelsr°adet i Uppsala - Örebro och läkemedelskommittén region Jönköpings
län; 2018 (https://regionvastmanland.se/globalassets/vardgivare-och-
sammarbetspartners/lakemedel/baslakemedel/aldrelathund-2018.pdf, accessed
22 March 2019).
Guiding principles for the care of older adults with multimorbidity: an approach for
clinicians. J Am Geriatr Soc. 2012;60(10):E1–25. https://doi.org/1
0.1111/j.1532-
54
15.2012.04188.x https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4450364/
Pellegrino AN, Martin MT, Tilton JJ, Touchette DR. Medication therapy
management services: definitions and outcomes. Drugs. 2009;69(4):393–406.
https://doi.org/10.2165/00003495-200969040-00001
https://www.ncbi.nlm.nih.gov/pubmed/19323584
Polypharmacy: guidance for prescribing. Llandough: All Wales Medicines Strategy
Group; 2014 (http://www.awmsg.org/docs/awmsg/medman/Polypharmacy%20-
%20Guidance%20for%20Prescribing.pdf, accessed 22 March 2019).
48
MEDICATION SAFETY IN POLYPHARMACY
No.
11
12
13
14
15
16
17
18
19
20
21
22
Country
Netherlands
Netherlands
Netherlands
New
Zealand
Scotland,
United
Kingdom
Scotland,
United
Kingdom
Sweden
Sweden
Sweden
United
States of
America
United
States of
America
Wales,
United
Kingdom

Annex 4. Case studies
49
MEDICATION SAFETY IN POLYPHARMACY
Patient details
• 69-year-old man
Current medical history
• Fractured neck of femur 2 years ago
• Dementia – mixed Alzheimer’s disease / alcohol abuse
• Falls frequently
• Ex-smoker
Results
• Blood pressure (BP) 120/84 mmHg
• Estimated glomerular filtration rate (eGFR) > 60ml/min
Current medication (stable since admission)
• Trazodone 150 mg at night • Cetirizine 10 mg once daily
•
Thiamine 50 mg three times daily • Amisulpride 100 mg twice daily
•
Bendroflumethiazide 2.5 mg once daily • Emollient cream (as required)
• Tramadol 50 mg four times daily • Fusidic acid 2% and betamethasone 0.1%
cream topically twice daily
Current function
69 year old man has been a care home resident for two years. He is a long-term heavy alcohol user
in the past and developed dementia exacerbated by alcohol-related brain damage. A fall at home
led to a fractured hip. Post-surgery he was very confused and distressed. When settled, he was
unable to manage at home post-fracture and transferred to a care home. At the time of admission
the patient lacked capacity, but showed indication towards recovery and put weight on initially. He
has exhibited a slow decline in function since.
Assistance of two caregivers is required for transfer to chair. The patient falls frequently as he
attempts to move unaided. Conversation is confused and occasional verbal aggression is apparent.
He also has poor short term memory, prompting is required to ensure that he eats and drinks. He
spends most of the day sleeping in his chair and sleeps well at night. Over the last 12 months he
has developed shortness of breath and swollen ankles.
Most recent consultations
Communication may be difficult due to cognitive impairment. If there is an adult with welfare powers
(power of attorney or guardian) involve them. Family if still in touch may also help. Three
consultations in the last six months, one concerning chest infection, another for review following
fall, and most recently due to leg oedema.
Case 1: Frailty without overt multimorbidity
Case summary
The following case studies have been adapted from Polypharmacy guidance: realistic prescribing
1
1. Scottish Government Polypharmacy Model of Care Group. Polypharmacy guidance: Realistic prescribing, 3rd edition.
Edinburgh: Scottish Government; 2018 (https://www
.therapeutics.scot.nhs.uk/wp-
content/uploads/2018/09/Polypharmacy-Guidance-2018.pdf, accessed 15 April 2019).
50
MEDICATION SAFETY IN POLYPHARMACY
Checks
1. What matters to the
patient
• Review diagnoses
and identify therapeutic
objectives
2. Need
• Identify essential medications
(not to be stopped without
specialist advice)
3. (Continued) need for
medications
• Identify and review
the (continued) need
for medications
4. Therapeutic objectives
achieved?
• Identify the need for
adding/intensifying
medication therapy
in order to achieve
therapeutic objectives
Medication-related risks/problems identified
Priorities may include:
• Reduce shortness of breath
• Improve ability to self-manage and interact socially
• Reduce ankle swelling
• Reduce sedation
Prevention:
• Reduce risk of falls/fractures
• None
• Thiamine – may be redundant if well-nourished in care home
• Bendroflumethiazide – no longer hypertensive, potential
for withdrawal
• Tramadol – indication unclear (may have been started after
surgery)
• Central nervous system (CNS) medication (trazodone,
amisulpiride) – indication unclear
– Consider withdrawal if not agitated
• Cetirizine/topical emollient cream
– Required for itch? Clarify the cause (i.e. dermatological
versus CNS problem or adverse drug reaction).
If dermatological problem, follow non-pharmacological
measures e.g. pay attention to washing powder, use natural
fabrics, reduce use of perfumed products etc., and ensure
proper use of emollients regularly and in sufficient quantity)
• Antimicrobial cream (fusidic acid/betamethasone cream) –
use should be limited to short term (e.g. one week)
• Ankle swelling and shortness of breath: consider presence
of left ventricular systolic dysfunction. Consider thorough
cardiac investigation. If present effective treatments such
as ACEI/Angiotensin II receptor blocker (ARB), beta blocker
can be prescribed
• Reduce risk of falls/fractures:
– Falls risk mainly associated with sedative medications.
Fracture risk modification with osteoporosis prevention
could be considered. Decision to treat needs to be
balanced against expected efficacy (see NNT) and ability
to comply with treatment. Dental health needs to be
considered if moving to active treatment with
bisphosphonates, unlikely to have time to benefit if life
expectancy is estimated to be < 1 year
Applying the 7 steps

51
MEDICATION SAFETY IN POLYPHARMACY
Checks
5. Safety
• Identify patient safety risks
• Identify adverse drug
effects
6. Costs
• Identify unnecessary
costly medication therapy
7. Patient centeredness
• Does the patient
understand the outcomes
of the review?
• Ensure medication therapy
changes are tailored
to patient preferences
• Agree and communicate
plan
Medication-related risks/problems identified
Actual adverse drug events:
• Oversedation
Adverse drug event risk:
• Risk of cardiovascular events:
– Antipsychotics carry a markedly elevated risk of
cardiovascular events in dementia
• Risk of cognitive deterioration:
– Amisulpride, cetirizine and tramadol
• Risk of falls/fractures:
– Amisulpride, trazodone (sedative), cetirizine
• Risk of serotonin syndrome:
– Tramadol and trazodone
• Risk of steroid adverse effects (topical and systemic):
– High dose topical corticosteroid
• Risk of acute kidney injury:
– Bendroflumethiazide would need to be stopped if patient
is dehydrated
– In case of care home resident with managed medications,
ensure staff have clear information on prescriptions to
withhold if dehydrated
• Opportunities for cost minimization (e.g. generic substitution)
should be explored
• Ensure prescribing is in keeping with current formulary
recommendations (see Annex 1)
• Reduce risk of falls/fractures:
– Reduce trazodone and amisulpiride to reduce sedation and
falls risk
– Decision to start bisphosphonate: balance ability to take
versus expected benefit
• Patient cooperation:
– Involve the patient where possible. If deemed to lack
capacity, discuss with relevant others e.g. welfare guardian,
power of attorney, or nearest relative if one exists. Even
if adult lacks capacity, still ensure adult’s views are sought
and thorough documentation takes place
SUMMARY: KEY CONCEPTS IN THIS CASE
• Low number of conditions and medications but still high potential for medication-related illness
• Ongoing review of medication commenced for symptomatic relief
• Apparent low level of multimorbidity but potential for undiagnosed treatable conditions
• Oversedation is a major risk to quality of life, morbidity (falls) and mortality

52
MEDICATION SAFETY IN POLYPHARMACY
Patient details
• 57 year old woman
Current medical history
•
Back pain • Depression last two years since
•
Asthma since childhood
losing job after break-up of marriage
Results
• BP 150/80 mmHg
• Urea and electrolytes all within normal range
• Peak Flow Rate 300 (predicted 390)
• Respiratory rate 22 per minute
Lifestyle
• Smokes 5–10 cigarettes per day
Current medication
• Lansoprazole 30 mg once daily • Beclomethasone inhaler 100 micrograms
• Gabapentin 600 mg three times daily
2 puffs twice daily
• Tramadol 50 mg –100 mg every 4–6 hours • Mirtazapine 30 mg every night
• Salbutamol inhaler 100 micrograms two puffs • Zopiclone 7.5 mg 1 every night
as required
Current function
The patient has been suffering from pain and complaining of drowsiness and weight gain. She has
suffered from low mood for the last two years and has tried multiple antidepressants. The patient
can be difficult to engage depending on mood, but has sought advice today as she is finding the
pain unbearable and received letter for a medication review.
Most recent consultations
Most recent consultations have been for pain and management. Prior to that consultations were
concerning low mood and poor sleep after break up of marriage. The patient has also complained
about increased breathlessness and ordered salbutamol inhaler each month.
Case 2: Acute Pain and Depression with Asthma
Case summary

53
MEDICATION SAFETY IN POLYPHARMACY
Checks
1. What matters to the patient
• Review diagnoses
and identify therapeutic
objectives
2. Need
• Identify essential medications
(not to be stopped without
specialist advice)
3. (Continued) need for
medications
• Identify and review
the (continued) need
for medications
4. Effectiveness
• Identify the need for
adding/intensifying
medication therapy in order
to achieve therapeutic
objectives
5. Safety
• Identify patient safety risks
• Identify adverse drug effects
6. Costs
• Identify unnecessary costly
medication therapy
Medication-related risks/problems identified
Priorities may include
• Manage the pain
• Manage asthma through the use of preventative treatments.
• Minimise medication-related harm dependence:
– Zopiclone, tramadol and gabapentin
• Help patient quit smoking: impact on asthma
• Medicines for symptomatic deterioration of asthma
• Cause of back pain may need thorough investigation
• Review the need for ongoing proton pump inhibitor: if still
needed, aim for dose reduction (maintenance dose of
lansoprazole is 15 mg/day)
• Patient examined and breathlessness due to asthma: check
use of preventative treatment inhaler technique
• Review of pain management: trial dose reduction of
gabapentin as there is no existing complaint for nerve pain,
reduce this gradually and consider alternatives
• Confirm daily intake of tramadol: if the patient is taking
regularly, consider switching to an extended-release
formulation to improve compliance and manage pain better
Ensure the daily limit is not exceeded (maximum dose of
tramadol is 400 mg/day)
• Trial dose reduction of zopiclone
• Discuss depression and review treatment: consider other
support that might be needed (e.g. non-pharmacological
interventions)
• Pain symptoms: consider reviewing treatment to manage
pain more appropriately. Discuss about the realistic
expectations with the patient
• Reduce one treatment at a time: consider need for on-going
zopiclone, tramadol and gabapentin
• Consider options for smoking cessation
• Ensure adequate treatment plan for asthma:
– Review use of salbutamol and beclomethasone
– Step-up or step-down treatment as required
• Adverse effects of long-term zopiclone use:
– Avoid prolonged use due to risk of dependence, falls etc.
– Also consider the interaction with tramadol
• Avoid long-term use of gabapentin, reduce dose gradually
• Assess for the risk of accidental overdose
• Check that patient is aware of safety advice e.g. what
medication to stop if at risk of dehydration
• Opportunities for cost minimization (e.g. generic substitution)
should be explored
• Ensure prescribing in keeping with current formulary
recommendations
Applying the 7 steps

54
MEDICATION SAFETY IN POLYPHARMACY
Checks
7. Patient centeredness
• Does the patient understand
the outcomes of the review?
• Ensure medication therapy
changes are tailored
to patient preferences
• Agree and communicate
plan
Medication-related risks/problems identified
• Patient may need support with inhaler and inhaler technique
if continuing treatment
• Discuss with patient other strategies to help manage pain
• Reduce medication one at a time to build patient’s
confidence. This will ensure that any changes in symptoms
as a result of a medication change can easily be attributable
to one medication
SUMMARY: KEY CONCEPTS IN THIS CASE
1. Low number of conditions and medications but still high potential for drug dependence
2. Ongoing medication review needed for those for symptomatic relief for pain and for sleep
3. Patient education on the benefit of preventive medication for asthma
55
MEDICATION SAFETY IN POLYPHARMACY
Patient details
• 58 year old woman
Current medical history
• Type 2 diabetes (diagnosed 5 years ago) • Chronic obstructive pulmonary disease (COPD)
• Coronary heart disease (non-ST elevation • Chronic back pain
myocardial infarction 1 year ago)
• Depression (2 episodes)
• Hypertension • Hypothyroidism
• Atrial fibrillation
Results
• HbA1C 86 mmol/mol (10%) • Spirometry shows mild airway obstruction
• BP 150/85 mmHg
• No urinary protein detected
• Body mass index 35 kg/m
2
• eGFR 55 ml/min
Lifestyle
Smoks 10–15 cigarettes per day
•
alcohol: 20 units per week
Current medication
•
Aspirin 75 mg once daily • Lisinopril 30 mg once daily
• Metformin 1 g three times daily • Amlodipine 10 mg once daily
• Gliclazide 80 mg twice daily • Atenolol 50 mg once daily
•
Pioglitazone 30 mg once daily • Furosemide 40 mg once daily
• Salbutamol inhaler as required • Gabapentin 400 mg three times daily
• Beclomethasone inhaler 100 micrograms • Codeine/paracetamole 8/500 mg 2 tablets
twice daily up to four times daily
• Levothyroxine liquid 100 micrograms once daily • Diclofenac 50 mg up to three times daily
• Citalopram 20 mg once daily • Omeprazole 40 mg once daily
• Bendroflumethiazide 2.5 mg once daily
Current function
Receptionist in local garage works 6 half days per week. She lives with her husband (out of work
long-term) and provides support to her elderly mother who lives alone and has early dementia.
Two previous acute admissions to hospital. Flu-like illness led to exacerbation of COPD two years
ago. Chest pain 12 months ago, found to be in atrial fibrillation on admission and troponin positive.
Angiogram showed widespread coronary artery disease but not severe enough to warrant
revascularisation. Echocardiography showed normal left ventricular systolic function. On dual aspirin
and clopidogrel for 1 year, recently moved to aspirin monotherapy.
Most recent consultations
Ongoing problems with ankle swelling. Back pain difficult to manage and resistant to several
strategies. Occasional heart palpitations and persistent indigestion with heartburn. Long-term
financial worries and increasing caregiver strain.
“I had a heart attack about a year ago and really worried about that happening again. I don’t know
what my mother and husband would do if I got too ill to work or look after her”.
Case 3: Multimorbidity without frailty
Case summary
56
MEDICATION SAFETY IN POLYPHARMACY
Checks
1. What matters to the
patient
• Review diagnoses
and identify therapeutic
objectives
2. Need
• Identify essential
medications (ones that
are not to be stopped
without expert advice)
3. (Continued) need for
medications
• Identify and review
the (continued) need
for medications
4. Effectiveness
• Identify the need for
adding/intensifying
medication therapy
in order to achieve
therapeutic objectives
Medication-related risks/problems identified
Priorities may include:
• Reduce shortness of breath
• Guidance to manage her medications safely, independently
• Reduce ankle swelling
• Assess the effect and necessity of her medications
• Discuss and create a medication management plan with
the patient
Possible therapeutic targets:
• Secondary prevention of cardiovascular events (including
stroke prevention in atrial fibrillation)
• Rate control in atrial fibrillation
• Management of chronic kidney disease
• Management of COPD
• Pain control
• Management of depression
• Weight reduction
• Management of indigestion with heartburn
• Levothyroxine to treat hypothyroidism
• Atenolol is needed for rate control in atrial fibrillation
As a beta-blocker, will aggravate asthma/COPD, the patient
will need another medication for rate control, e.g. verapamil
• Antidiabetic medications to control symptomatic diabetes
mellitus
• Pain management: is the gabapentin for neuropathic pain (from
diabetes mellitus) or mechanical back pain. Monitor use of
codeine/paracetamol, consider switching to paracetamol only
• Duration of antidepressant
• High dose omeprazole: active peptic ulcer or oesophagitis?
Check symptoms are of gastric origin rather than angina;
may require endoscopy or trial without NSAID?
• Secondary prevention of coronary events:
– Patient is relatively young and active so potentially a long
time to obtain benefit
– Not on statin despite high cardiovascular risk (check
if omission or due to side effects. If side effects, consider
alternative statin)
– Check BP control, lipid control and lifestyle
• Stroke prevention in atrial fibrillation:
– CHA2DS2-VASc score = 4 (stroke risk 4.8% per year) –
consider replacing aspirin with anticoagulant
– Rate control in atrial fibrillation: check heart rate
• Management of COPD:
– Discuss symptom control with patient – MRC
Breathlessness Score
– Ensure managing inhalers and that these are prescribed
in keeping with current formulary guidelines
Applying the 7 steps
57
MEDICATION SAFETY IN POLYPHARMACY
Checks
5. Safety
• Identify patient safety
risks
• Identify adverse
medication effects
6. Costs
• Identify unnecessary
costly medication
therapy
Medication-related risks/problems identified
• Pain control:
– Discuss symptom control
– Gabapentin indicated for neuropathic pain. Consider
withdrawal if not effective or misprescribed for mechanical
back pain
– Review efficacy of NSAID in view of the risks of treatment
• Management of depression: discuss symptom control with
patient
• Control of hypothyroidism: ensure recent thyroid function
results are available
• Management of chronic kidney disease: within normal range
(no proteinuria) but requires regular monitoring
• Diabetic control
– Check if experiencing any diabetic symptoms (e.g. thirst,
polyuria)
– HbA1C remains high despite on three antidiabetic medication
therapies, discuss adherence and HbA1C target
Actual adverse drug reactions:
• Ankle swelling – due to amlodipine or pioglitazone?
Risk of adverse drug reactions:
• Risk of gastrointestinal bleeding: NSAID, citalopram and
aspirin (or anticoagulant if changed in step 3)
• Risk of acute kidney injury:
– Chronic kidney disease (eGFR 55mL/min) and on NSAID,
consider stopping
– Co-prescribed diuretic, ACEI/ARB and NSAID (‘triple
whammy’)
– Co-prescribed thiazide and loop diuretic. Duplication
of therapy, discontinue one
– Consider more frequent monitoring of urea and electrolytes
– Check the patient is aware of safety advice e.g. what
medication to stop if at risk of dehydration
• Risk of cardiovascular disease/cardiac events:
– Ischaemic heart disease and on NSAID (diclofenac),
ibuprofen and naproxen are preferred
– Pioglitazone (ankle swelling and ischaemic heart disease)
• Risk of arrhythmia: QTc prolongation: omeprazole, citalopram
and gabapentin
• Opportunities for cost minimization (e.g. generic substitution)
should be explored
• Ensure prescribing in keeping with current formulary
recommendations
• Levothyroxine: consider changing from liquid to tablet form
58
MEDICATION SAFETY IN POLYPHARMACY
Checks
7. Patient centeredness
• Does the patient
understand the outcomes
of the review?
• Ensure medication
therapy changes are
tailored to patient
preferences
• Agree and communicate
plan
Medication-related risks/problems identified
• Secondary cardiovascular disease prevention: consider
how to prioritise discussion (and allocate time for this
in consultation)
• Most effective interventions requires: stopping smoking
followed by anticoagulant for atrial fibrillation, BP control,
addition of statin medication therapy, weight reduction
and HbA1C control
• Offer and support smoking cessation, diet and exercise
• COPD management:
– Check patient’s inhaler technique and adherence
– Adjust dose/formulation, if necessary
• Patient cooperation:
– Ensure patient understands rational for medication
Prevention: promote non-pharmacological strategies
• Check the patient’s willingness to make lifestyle changes
(smoking, diet, exercise)
• Social support: impact of stress
SUMMARY: KEY CONCEPTS IN THIS CASE
1. A high number of medications is likely to be needed and effective. A high number of medications
on its own is not an indicator of problematic prescribing, but rather a high-risk patient requiring
more support
2. Long medication lists make it harder to identify problems without a focused medication review
3. Potential to usefully detect and treat conditions (in this case atrial fibrillation)
4. Potential for high risk medication combinations particularly in patients on multiple medications
5. Need for direct advice to patient on medication, e.g. regarding dehydration
6. Link with non-pharmacological management
7. A longer than standard consultation to ensure that there is time to cover the patient concerns and
issues and focus on medication
8. Need for multi-disciplinary approach to address patient’s health conditions and future treatment

59
MEDICATION SAFETY IN POLYPHARMACY
Annex 5. List of contributors
Leadership group
Liam DONALDSON
WHO Envoy for Patient Safety
World Health Organization
Geneva, Switzerland
Edward KELLEY
World Health Organization
Geneva, Switzerland
Suzanne HILL
World Health Organization
Geneva, Switzerland
Neelam DHINGRA-KUMAR
World Health Organization
Geneva, Switzerland
Sarah GARNER
World Health Organization
Geneva, Switzerland
Main author
Alpana MAIR
Scottish Government
Edinburgh, United Kingdom of Great Britain
and Northern Ireland
Project coordination
and editorial support
Jerin Jose CHERIAN
World Health Organization
Geneva, Switzerland
Danielle ETZEL
World Health Organization
Geneva, Switzerland
Minna HÄKKINEN
World Health Organization
Geneva, Switzerland
Daan PAGET
World Health Organization
Geneva, Switzerland
Mei Lee TAN
World Health Organization
Geneva, Switzerland
Reviewers and other contributors
Monica BARONI
Fondazione Toscana “G.Monasterio”
Pisa-Massa, Italy
Tommaso BELLANDI
Centre for Clinical Risk Management
and Patient Safety
Florence, Italy
Neville BOARD
Australian Commission on Safety
and Quality in Health Care
Sydney, Australia
Josè Luis CASTRO
Pan American Health Organization/World
Health Organization Regional Office
for the Americas
Washington DC, United States of America
Alessandro CESCHI
University of Zurich
Zurich, Switzerland
Frank FEDERICO
Institute for Healthcare Improvement
Cambridge, United States of America
Albert FIGUERAS
Fundaciό Institut Català de Farmacologia
Barcelona, Spain

60
MEDICATION SAFETY IN POLYPHARMACY
Priyadarshani GALAPPATTHY
University of Colombo
Colombo, Sri Lanka
Jon΄as GONSETH GARCIA
Pan American Health Organization/World
Health
Organization Regional Office for the Americas
Washington DC, United States of America
Ezequiel GARC
΄
IA-ELORRIO
Institute for Clinical Effectiveness
and Health Policy
Buenos Aires, Argentina
Tamasine GRIMES
Trinity College Dublin
Dublin, Ireland
Michael HAMILTON
Institute for Safe Medication Practices Canada
Toronto, Canada
Helen HASKELL
Mothers Against Medical Error
Columbia, United States of America
Jamie HAYES
All Wales Therapeutics and Toxicology Centre
Cardiff, United Kingdom of Great Britain
and Northern Ireland
Katherine HAYES
World Health Organization
Geneva, Switzerland
Simon HURDING
Health Finance Directorate
Edinburgh, United Kingdom of Great Britain
and Northern Ireland
Elzerie de JAGER
James Cook University
Townsville, Australia
Regina N. M. KAMOGA
Community Health & Information Network
Kampala, Uganda
Ciara KIRKE
Health Service Executive
Dublin, Ireland
Zuzana KUSYNOV
΄
A
International Pharmaceutical Federation
The Hague, The Netherlands
Jake LAURIE
Scottish Government
Edinburgh, United Kingdom of Great Britain
and Northern Ireland
Nicola MAGRINI
World Health Organization
Geneva, Switzerland
Alemayehu Berhane MEKONNEN
University of Sydney
Sydney, Australia
Marίa José OTERO
Institute for Safe Medication Practices Spain
Salamanca, Spain
Magdalena Zuzanna RABAN
Macquarie University
Sydney, Australia
Philip Alexander ROUTLEDGE
All Wales Therapeutics and Toxicology Centre
Cardiff, United Kingdom of Great Britain
and Northern Ireland
Stephen ROUTLEDGE
Canadian Patient Safety Institute
Edmonton, Canada
Ian SCOTT
University of Queensland
Brisbane, Australia
Michael SCOTT
Regional Medicines Optimisation Innovation
Centre
Antrim, United Kingdom of Great Britain
and Northern Ireland
Kim SEARS
Queen’s University
Kingston, Canada
Aziz SHEIKH
The University of Edinburgh
Edinburgh, United Kingdom of Great Britain
and Northern Ireland
Virpi Tuulikki TEINILÄ
Finnish Red Cross
Tampere, Finland

David U
Institute for Safe Medication Practices Canada
Toronto, Canada
Afam Kanayo UDEOZO
Chukwuemeka Odumegwu Ojukwu University
Teaching Hospital
Awka, Nigeria
Luciana Yumi UE
Ministry of Health
Brasίlia, Brazil
Patricia VAN DEN BEMT
Erasmus University Medical Center
Rotterdam, Netherlands
Martin WILSON
Raigmore Hospital
Inverness, United Kingdom of Great Britain
and Northern Ireland
Industry observers
Caroline MENDY
World Self-Medication Industry
Nyon, Switzerland
Sunayana SHAH
International Federation of Pharmaceutical
Manufacturers and Associations
Geneva, Switzerland
61
MEDICATION SAFETY IN POLYPHARMACY

For more information, please contact the following departments
Service Delivery and Safety
World Health Organization
Avenue Appia 20
CH-1211 Geneva 27 Switzerland
Email: [email protected]
www.who.int/patientsafety
Essential Medicines and Health Products
World Health Organization
Avenue Appia 20
CH-1211 Geneva 27 Switzerland
Email: [email protected]
www.who.int/medicines
