
IAEA-TECDOC-1584
Advanced Applications of Water
Cooled Nuclear Power Plants
July 2007

IAEA-TECDOC-1584
Advanced Applications of Water
Cooled Nuclear Power Plants
July 2007
The originating Section of this publication in the IAEA was:
Nuclear Power Technology Development Section
International Atomic Energy Agency
Wagramer Strasse 5
P.O. Box 100
A-1400 Vienna, Austria
ADVANCED APPLICATIONS OF
WATER COOLED NUCLEAR POWER PLANTS
IAEA, VIENNA, 2008
IAEA-TECDOC-1584
ISBN 978–92–0–105808–9
ISSN 1011–4289
© IAEA, 2008
Printed by the IAEA in Austria
July 2008
FOREWORD
By August 2007, there were 438 nuclear power plants (NPPs) in operation worldwide, with a total
capacity of 371.7 GW(e). Further, 31 units, totaling 24.1 GW(e), were under construction. During
2006 nuclear power produced 2659.7 billion kWh of electricity, which was 15.2% of the world’s total.
The vast majority of these plants use water-cooled reactors. Based on information provided by its
Member States, the IAEA projects that nuclear power will grow significantly, producing between
2760 and 2810 billion kWh annually by 2010, between 3120 and 3840 billion kWh annually by 2020,
and between 3325 and 5040 billion kWh annually by 2030.
There are several reasons for these rising expectations for nuclear power:
• Nuclear power’s lengthening experience and good performance: The industry now has more
than 12 000 reactor years of experience, and the global average nuclear plant availability during
2006 reached 83%;
• Growing energy needs: All forecasts project increases in world energy demand, especially as
population and economic productivity grow. The strategies are country dependent, but usually
involve a mix of energy sources;
• Interest in advanced applications of nuclear energy, such as seawater desalination, steam for
heavy oil recovery and heat and electricity for hydrogen production;
• Environmental concerns and constraints: The Kyoto Protocol has been in force since February
2005, and for many countries (most OECD countries, the Russian Federation, the Baltics and
some countries of the Former Soviet Union and Eastern Europe) greenhouse gas emission limits
are imposed;
• Security of energy supply is a national priority in essentially every country; and
• Nuclear power is economically competitive and provides stability of electricity price.
In the near term most new nuclear plants will be evolutionary water cooled reactors (Light Water
Reactors (LWRs) and Heavy Water Reactors (HWRs), often pursuing economies of scale. In the
longer term, innovative designs that promise shorter construction times and lower capital costs could
help to promote a new era of nuclear power.
About one-fifth of the world’s energy consumption is used for electricity generation. Most of the
world’s energy consumption is for heat and transportation. Nuclear energy has considerable potential
to penetrate these energy sectors now served by fossil fuels that are characterized by price volatility
and finite supply. Advanced applications of nuclear energy include seawater desalination, district
heating, heat for industrial processes, and electricity and heat for hydrogen production. In addition,
since nuclear electricity is generally produced in a base load mode at stable prices, there is
considerable near-term potential for nuclear power to contribute to the transportation sector as a
carbon-free source of electricity for charging electric and plug-in hybrid vehicles.
This collaborative assessment was recommended by the IAEA Nuclear Energy Department’s
Technical Working Groups on Advanced Technologies for LWRs and HWRs (the TWG-LWR and
TWG-HWR). The objective has been to identify opportunities and challenges for water cooled
reactors to capture a substantial share of the above mentioned advanced applications. For each
application, the opportunities, market context, challenges and potential solutions are addressed.
The IAEA appreciates the work of all contributors, listed at the end of this report. The special
contribution of M. Petri from Argonne National Laboratory, United States of America, as Chairman of
this activity is gratefully acknowledged. The IAEA Officers responsible for this report are J. Cleveland
and A. McDonald of the Department of Nuclear Energy.
EDITORIAL NOTE
The use of particular designations of countries or territories does not imply any judgement by the
publisher, the IAEA, as to the legal status of such countries or territories, of their authorities and
institutions or of the delimitation of their boundaries.
The mention of names of specific companies or products (whether or not indicated as registered) does
not imply any intention to infringe proprietary rights, nor should it be construed as an endorsement
or recommendation on the part of the IAEA.
CONTENTS
SUMMARY ............................................................................................................................................ 1
CHAPTER 1. PRESENT STATUS AND OVERVIEW OF ADVANCED APPLICATIONS
OF NUCLEAR REACTORS .........................................................................................9
1.1. Nuclear power reactors in the world.............................................................................10
1.2. Non-electric applications of nuclear power..................................................................11
1.3. Past experience with non-electric applications.............................................................18
1.4. Market potential of advanced non-electric applications ..............................................23
1.4.1. 2003 IAEA study: Market potential for non-electric application
of nuclear energy...............................................................................................23
1.4.2. 2004 NEA study: Non-electricity products of nuclear energy .........................27
1.5. Impact of externalities on cost of power from fossil/ nuclear/
renewable sources .....................................................................................................................28
1.6. Conclusion....................................................................................................................31
References for Chapter 1...........................................................................................................32
CHAPTER 2. NUCLEAR DESALINATION.....................................................................................33
2
.1. Opportunities ................................................................................................................33
2.2. Market context .............................................................................................................34
2.2.1. Nuclear desalination market - past experiences and plans ...............................34
2.2.2. Economics
........................................................................................................36
2.3. Challenges ....................................................................................................................39
2.3.1. Economics .........................................................................................................39
2.3.2. Infrastructure development................................................................................39
2.3.3. Public perception...............................................................................................39
2.3.4. Socio-environmental
aspects............................................................................40
2.4. Solutions.......................................................................................................................40
2.4.1. Utilization of waste heat from nuclear reactors.................................................40
2.4.2. Waste heat utilization from Indian PHWRs for thermal desalination ...............41
2.4.3. Utilization
of hybrid systems..........................................................................43
2.5. Conclusion....................................................................................................................45
References for Chapter 2...........................................................................................................45
CHAPTER 3. DISTRICT HEATING .................................................................................................46
3.1. Opportunities ................................................................................................................46
3.2. Market context..............................................................................................................47
3.2.1. Early history of nuclear district heating markets [4] .........................................47
3.2.2. Summary of some specific district heating systems of interest.........................50
3.2.3. Economics .........................................................................................................52
3.3. Challenges ....................................................................................................................53
3.4. Solutions.......................................................................................................................54
References for Chapter 3...........................................................................................................56
CHAPTER 4. INDUSTRIAL PROCESS STEAM .............................................................................57
4.1. Opportunities ................................................................................................................57
4.2. Market
context.............................................................................................................58
4.2.1. Past experience..................................................................................................58
4.2.2. Near-term potential............................................................................................61
4.2.3. Economics .........................................................................................................62
4.3. Challenges ....................................................................................................................63
4.4. Solutions.......................................................................................................................64
4.4.1. Oil extraction from Canadian oil sands.............................................................64
4.4.2. Massachusetts Institute of Technology (MIT) study.........................................67
4.4.3. Long
-term possibilities...................................................................................68
References for Chapter 4...........................................................................................................69
CHAPTER 5. NUCLEAR ELECTRICITY FOR TRANSPORTATION:
HYBRID-ELECTRIC VEHICLES ..............................................................................70
5.1. Opportunities ................................................................................................................70
5.1.1. Opportunities in the United States of America..................................................71
5.1.2. Opportunities in Japan.......................................................................................75
5.1.3. Opportunities
in other countries............................................................... 76
5.2. Market context..............................................................................................................78
5.2.1. Market context in the United States of America ...............................................78
5.2.2. Opportunities in Japan.......................................................................................82
5.2.3. Energy utilization efficiencies of various power trains.....................................85
5.2.4. Greenhouse
gas emissions in Japan......................................................... 87
5.3. Challenges ....................................................................................................................88
5.3.1. Barriers to be overcome in the United States of America .................................88
5.3.2.
Barriers to be overcome in Japan..................................................................91
5.4. Solutions.......................................................................................................................90
5.4.1. Solutions in the United States of America ........................................................90
5.4.2.
Solutions in Japan...........................................................................................90
References for Chapter 5...........................................................................................................92
Bibliography for Chapter 5 .......................................................................................................93
CHAPTER 6. ELECTRICITY AND HEAT FOR HYDROGEN PRODUCTION...............................94
6.1. Opportunities ................................................................................................................96
6.2. Market context..............................................................................................................98
6.2.1. Hydrogen as a raw material in chemical processes...........................................99
6.2.2. Hydrogen
as a fuel .........................................................................................102
References for Section 6.2 ......................................................................................................106
Bibliography for Section 6.2...................................................................................................110
6.3. Challenges ..................................................................................................................106
6.4. Solutions.....................................................................................................................111
6.4.1. Hydrogen production technologies..................................................................111
6.4.2. Hydrogen production economics.....................................................................122
6.4.3. Hydrogen production with a combination of nuclear electricity
and wind electricity .........................................................................................127
6.4.4. The environmental benefits of fuel cell vehicles supplied by
nuclear-generated
hydrogen...........................................................................128
References for Chapter 6.........................................................................................................129
ANNEX I. RECENT NUCLEAR DESALINATION DEMONSTRATION PROJECTS .......132
ANNEX II. NUCLEAR DISTRICT HEAT IN SWEDEN AND ROMANIA............................137
ANNEX III. CANDU ENERGY FOR STEAM ASSISTED GRAVITY DRAINAGE...............140
ANNEX IV. LARGE SCALE PROCESS STEAM SUPPLY FROM GÖSGEN-DÄNIKEN
NUCLEAR POWER STATION IN SWITZERLAND ...........................................145
ANNEX V. EVALUATION OF BATTERY COST COMPETITIVENESS OF
ALTERNATIVE TRANSPORTATION TECHNOLOGIES..................................147
CONTRIBUTORS TO DRAFTING AND REVIEW......................................................................... 149
1
SUMMARY
About one-fifth of the world’s energy consumption is used for electricity generation, and today
nuclear energy contributes approximately 15.2% of the world’s electricity. Most of the world’s energy
consumption is for heat and transportation. Through advanced applications, nuclear energy has
considerable potential to penetrate these energy sectors now served by fossil fuels that are
characterized by price volatility, finite supply, and environmental concerns.
Advanced applications of nuclear energy include seawater desalination, district heating, heat for
industrial processes, and electricity and heat for hydrogen production. In addition, in the transportation
sector, since nuclear electricity is generally produced in a base load mode at stable prices, there is
considerable near-term potential for nuclear power to contribute as a carbon-free source of electricity
for charging electric and plug-in hybrid vehicles.
The applications highlighted in this publication rely on a source of heat and electricity. Nuclear energy
from water-cooled reactors, of course, is not unique in this sense. Indeed, higher temperature heat can
be produced by burning natural gas and coal or through the use of other nuclear technologies such as
gas-cooled or liquid-metal-cooled reactors. Water-cooled reactors have advantages, however. Unlike
fossil-fuel-based plants, water-cooled reactors do not release greenhouse gases. Water-cooled reactors
are being deployed today. Other reactor types have had considerably less operational and regulatory
experience and will take still some time to be widely accepted in the market.
This document examines the potential of nuclear energy to expand into these markets by presenting an
overview of example applications, their opportunities, challenges and solutions. Its scope is limited to
applications that can be served by water cooled reactors, as these represent more than 90% of the
current fleet, and because in the near term most new nuclear plants will be evolutionary water cooled
reactors [(Light Water Reactors (LWRs) and Heavy Water Reactors (HWRs)]. In the longer term,
innovative designs that promise shorter construction times and lower capital costs could help to
promote a new era of nuclear power, especially as non-electricity markets grow. This document does
not address design safety aspects of the coupling of heat utilization systems to nuclear reactors.
The advantage of nuclear energy in alleviating the risk of climate change will not favour market
penetration of advanced applications of nuclear power as long as energy policies internalising the
value of carbon and other pollutants are not implemented. National policies on climate change vary
from country to country, but the entry into force of the Kyoto Protocol in February 2005 does create
incentives that can benefit nuclear power, depending on how they are translated into national policies.
Nuclear energy for seawater desalination
Water is essential for the sustainable development of society. Water scarcity is a global issue, and
every year new countries are affected by growing water problems. Climate change is likely to further
stress regions already facing dire water shortages.
Large-scale commercially available desalination processes can generally be classified into two
categories: (a) distillation processes that require mainly heat plus some electricity for ancillary
equipment, and (b) membrane processes that require only electricity to provide pumping power.
The energy for these plants is generally supplied in the form of either steam or electricity using fossil
fuels. The intensive use of fossil fuels raises environmental concerns, and many countries are therefore
considering the introduction of a nuclear power program or expansion of their existing nuclear power
program.
The desalination of seawater using nuclear energy is a feasible and demonstrated option to meet the
growing demand for potable water. Over 200 reactor-years of operating experience on nuclear
desalination have been accumulated worldwide, and demonstration projects for nuclear desalination
are also in progress to confirm its technical and economical viability. However, today nuclear
desalination contributes only 0.1 % of total desalting capacity worldwide.
Economic feasibility studies generally indicate that water costs (and associated electricity generation
costs) from nuclear seawater desalination are in the same range as costs associated with fossil-fuelled
desalination at their current costs. Therefore, future investment decisions will depend on site-specific
cost factors and on the values of key parameters (capital cost, fuel price, interest rate, construction
time, etc.) at the time of investment.
Those countries suffering from scarcity of water are, generally, not the holders of nuclear technology,
do not generally have nuclear power plants, and do not have a nuclear power infrastructure. The
utilization of nuclear energy in those countries will require infrastructure building and institutional
arrangements for such things as financing, liability, safeguards, safety, and security and will also
require addressing the acquisition of fresh fuel and the management of spent fuel.
The socio-environmental aspects of nuclear desalination need attention for its large-scale adoption.
Setting up a desalination plant at nuclear reactors for providing much-needed fresh water to the public
will no doubt add to social acceptance of nuclear desalination, if the quantity and quality of the fresh
water are consistently assured. Also, nuclear desalination plants must be designed to assure the
continued use of areas for fishing and other socio-cultural activities. Protection of the marine
environment near the desalination plant site needs to be assured. The environmental impact assessment
of nuclear-powered desalination systems further indicates advantages over fossil-based energy
sources. These would result in enhanced economic competitiveness of nuclear desalination plants.
In summary, use of energy from nuclear reactors for seawater desalination is a demonstrated option; it
is environmentally friendly and can be a sustainable energy source. Feasibility studies indicate that
current costs of water produced from nuclear desalination plants are similar to those of fossil fuel
based desalination plants. Thus nuclear desalination is an important option for safe, economic and
sustainable supply of large amounts of fresh water to meet the ever-increasing worldwide water
demand.
Nuclear energy for district heating
District heat involves the supply of space heating and hot water through a district heating system,
which consists of heat plants (usually producing electricity simultaneously) and a network of
distribution and return pipes. Potential applications of district heating are in climatic zones with
relatively long and cold winters. In many countries, such as central and northern European countries
and countries in transition economies, district heat has been widely used for decades.
District heating has the following technical requirements:
• It requires a heat distribution network to transport steam or hot water in a typical temperature
range of 80-150°C;
• Owing to higher losses over longer transmission distances, the heat source must be relatively
close to the customer, typically within 10–15 km;
• The district heat generation capacities are determined by the collective demands of the
customers. In large cities a capacity of 600–1200 MWth is normal. The demand is much lower
in small communities;
• The annual load factor is normally not higher than 50%, since heat is supplied only in the colder
part of the year;
• To assure a reliable supply of heat, a backup capacity is required.
Coal and gas dominate the fuels used for district heating. Various other heat sources are also used for
district heating, including biomass materials, waste incineration, and waste heat from industrial
2
processes. Usually district heating is produced in a cogeneration mode in which waste heat from
power production is used as the source of district heat.
Several countries (Bulgaria, China, Czech Republic, Hungary, Romania, the Russian Federation,
Slovakia, Sweden, Switzerland and Ukraine) already have experience in nuclear district heating, so the
technical aspects can be considered well proven.
In the past, the low prices of fossil fuels have stunted the introduction of single-purpose nuclear
district heating plants. Although many concepts of small-scale heat-producing nuclear plants have
been presented during the years, very few have been built. However, as environmental concerns mount
over the use of fossil fuels, nuclear-based district heating systems have potential.
In order to be able to compete with fossil-fuel-fired heat boilers, the capital cost per installed MW of
heat production capacity for a nuclear-based system must be such that the production costs are
competitive. Dedicated reactors providing district heat can potentially achieve acceptable costs, due to
their lower temperature operating conditions, simple design, modularization and standardization, and
advanced safety systems.
Economic studies generally indicate that district heating costs from nuclear power are in the same
range as costs associated with fossil-fuelled plants. Therefore, as with nuclear desalination, future
investment decisions will depend on site-specific cost factors and on the values of key parameters
(capital cost, fuel price, interest rate, construction time, etc.) at the time of investment.
New nuclear heat-producing plants must, of course, meet the user’s requirements on availability and
reliability, including alternative heat-producing capacity that could serve as backup. For this purpose,
heat storage allows a matching of the heat supply to the heat demand. Today there are many examples
of short-term storage, for instance, on the daily scale that relies on hot water accumulator tanks. In the
future, more long-term storage facilities may be realized.
The design features of nuclear district heating plants to prevent the transfer of radioactivity into the
district heating grid network have proven to be effective. These features include one or more barriers
to radioactive cross contamination, e.g. in the form of a leak-tight intermediate heat transfer loop at a
pressure higher than that of the steam extracted from the turbine side of the nuclear plant. These loops
are continuously monitored, and isolation devices are provided to separate potentially contaminated
areas.
For nuclear district heating plants, proximity to population centres implies the need for a high degree
of safety including the lowering of core damage frequencies and enhancing mitigation systems in the
case of an accident.
Nuclear energy for industrial process heat
Process heat involves the supply of heat required for industrial processes from one or more centralized
heat generation sites through a steam transportation network. Within the industrial sector, process heat
is used for a large variety of applications with different heat requirements and with temperature ranges
covering a wide spectrum. Examples of industries that consume considerable amounts of heat are:
• food,
• paper,
• chemicals and fertilizers,
• petroleum and coal processing, and
• metal processing industries.
3

The breakdown of the total industrial heat varies from country to country, but the chemical and
petroleum industries are the major consumers worldwide. These would be key target clients for
possible applications of nuclear energy.
The supply of energy for industrial processes has an essential character: all industrial users need the
assurance of energy supply with a high reliability, and the heat should be produced close to the point
of use. Industrial process heat users do not have to be located within highly populated areas. Many of
the process heat users, in particular the large ones, can be (and usually are) located outside urban
areas, often at considerable distances. This makes joint siting of nuclear reactors and industrial users
of process heat not only viable, but also desirable in order to drastically reduce the heat transport costs.
The nuclear process heat supply has to be reliable. As an example, the average adequate steam supply
availabilities for chemical processing, oil refineries and primary metals are respectively 98%, 92% and
near 100%. Such high levels can be ensured only by the combination of a highly reliable heat source
and the availability of reserve capacity.
There is experience in providing process heat for industrial purposes with nuclear energy in Canada,
Germany, Norway, Switzerland, and India. New plant designs that can provide heat, or both heat and
electricity, are being designed in Russia, the Republic of Korea, Canada, and other countries.
Current water cooled reactors can provide process heat up to about 300ºC, and some future innovative
water cooled reactor designs
1
have potential to provide heat up to approximately 550ºC.
Although nuclear industrial process heat applications have significant potential, it has not been
realized to a large extent. In fact, currently only the Goesgen reactor in Switzerland and the RAPS–2
reactor in India continue to provide industrial process heat, whereas other process heat systems have
been discontinued after successful use. Among the reasons cited for closure of these units, one is
availability of cheaper alternate energy sources, including waste heat near the industrial complexes.
For potential future application of nuclear process heat, the main example presented in this document
is the use of nuclear energy for oil sand open-pit mining and deep-deposit extraction in Canada.
Alberta’s oil sand deposits are the second largest oil reserves in the world, and have emerged as the
fastest growing, soon to be dominant, source of crude oil in Canada. Currently, the majority of oil sand
production is through open-pit mining, which is suitable for bitumen extraction when the oil sand
deposits are close to the surface. The ore, a mixture of bitumen and sand, is removed from the surface
by truck and shovel operation. The ore is then mixed with hot water to form a slurry that eventually
undergoes a separation process to remove bitumen from the sand.
The thermal energy required for the open-pit mining process is in the form of hot water at a relatively
low temperature (around 70°C), and the rest is dry process steam at around 1.0 to 2.0 MPa. The oil
extraction facilities require electrical power as well. These heat requirements, as well as the electricity,
can be met by water cooled reactors.
To increase production capacity, the industry is developing new technologies to extract bitumen from
deep deposits. Among them, Steam-Assisted Gravity Drainage (SAGD), which uses steam to remove
bitumen from underground reservoirs, appears to be the most promising approach. Recently, the in-
situ recovery process has been put into commercial operation by major oil companies.
Overall, for both extraction methodologies (open pit mining and SAGD), a significant amount of
energy is required to extract bitumen and upgrade it to synthetic crude oil as the feedstock for oil
refineries. Currently, the industry uses natural gas as the prime energy source. As oil sand production
1
Specifically Super-critical Water Cooled Reactors, being developed within the Generation-IV International Forum,
could be deployed by around 2025-2030.
4
continues to expand, the energy required for production becomes a great challenge with regard to
economic sustainability, environmental impact and security of supply. Therefore, the opportunity for
nuclear reactors to provide an economical, reliable and virtually zero-emission source of energy for
the oil sands becomes a realistic option.
Contribution of nuclear energy to transportation
Transportation represents approximately 20% of the world’s energy consumption. In the United States
of America, transportation is the fastest growing energy sector and in the past few years has become
the nation’s largest energy sector. The Organization for Economic Co-operation and Development
International Energy Agency projects that global primary energy demand will grow by 50% by 2030,
with 70% of that growth coming from developing countries, especially China. Half of that increase
will be for electricity production and 20% for transportation. The expectation is that fossil fuels will
account for 83% of this increased energy consumption.
It is clear that if nuclear energy finds a way to power a significant part of the transportation sector, it
will have a major impact on global environmental sustainability. Two ways this could occur would be
through the advancement of electric and plug-in hybrid electric vehicles and of vehicles fuelled with
hydrogen produced by nuclear energy. This present study addressed electricity for plug-in hybrid
vehicles and hydrogen for transportation.
A) Electricity for plug-in hybrid electric vehicles
Hybrid electric vehicles of various classes are now commercially available. Almost all use
regenerative braking to charge an on-board battery for locomotive power. With these battery systems,
vehicles can be designed to allow the gasoline engine to turn off when the vehicle is stopped or during
cruising. Moreover, the smaller engines used can run at a higher percentage of their full power, which
is more efficient and more economical for a given load than larger, heavier gasoline engines operating
at a lower percentage of their maximum power.
Overall energy use for hybrids has been shown to be about 40% less than that for conventional
vehicles, with an equivalent reduction in fossil energy use and greenhouse gas emissions (CO
2
, CH
4
,
and N
2
O).
Plug-in hybrid electric vehicles extend this technology a step further by allowing a drive battery to be
charged externally. In this way, the vehicle can be driven in an all-electric mode for a certain distance
with no power from the gasoline engine. This can provide significant savings in terms of petroleum
usage and emissions, especially since the majority of miles driven are for short commutes. These
emission reductions materialize only if the source of external electricity is clean and carbon free, of
course.
The potentially large market demand for electricity for powering plug-in hybrid electric vehicles is
eminently suited to current and evolutionary water-cooled nuclear power plants. The analysis provided
in Chapter 5, for instance, shows that plug-in hybrid electric vehicles produce only 42% of the carbon
dioxide produced by conventional vehicles and that over 11,000 lb/vehicle-year of carbon dioxide can
be saved if nuclear power is used to generate plug-in hybrid electric vehicle electricity rather than
coal.
Under a simplified model of potential growth in plug-in hybrid electric vehicle usage, perhaps
250 GW of electricity may be needed for overnight charging in the U.S. by 2035. New generation
capacity at this scale would also require new transmission and distribution lines and substations. A
similar analysis for Japan suggests the need for 35 GW of electricity for overnight charging, which is
within the capacity of spare power at night.
5
Aside from the need for increases in generating and transmission capacity, other barriers will need to
be overcome before there is widespread adoption of plug-in hybrid electric vehicles:
• Conversion of automobile technology from conventional gasoline-powered vehicles to electric
and plug-in hybrid vehicles;
• Public acceptance of plug-in hybrid vehicles;
• Structuring of electricity pricing mechanisms to provide low-price electricity during off-peak
demand periods to encourage use of nuclear power plants for base load generation;
• Provision of other incentives (e.g., tax benefits) for adoption of vehicles that produce less
greenhouse gases and reduce reliance on petroleum fuels.
A key technical barrier is the development of lighter, less expensive, reliable batteries having a factor
of 5 to 10 greater energy storage capacity that would boost all-electric distances to twenty miles or
greater. Lithium-ion batteries as a substitute for nickel-metal hydride batteries are the main focus of
current research and development.
Despite these barriers, automobile manufacturers are spending significant effort on developing plug-in
hybrid electric vehicles. In fact, one auto manufacturer has recently announced road testing for its
plug-in hybrid vehicle, which can travel eight miles on a full charge before it needs to draw power
from the gasoline engine, and other plug-in hybrid manufacturers have announced targets of 20 to 40
miles on a single charge.
B) Hydrogen for transportation
Hydrogen for transportation is receiving significant attention around the world because of high
petroleum prices and unreliable oil supplies. Two ways of hydrogen utilization in transportation are
currently being taken into consideration – internal combustion engine (ICE) vehicles and fuel cell (FC)
vehicles. While ICE vehicles represent current technology with modest modifications, fuel cell
vehicles are in a stage of intensive R&D and prototype testing.
Car manufacturers are focusing more effort on fuel cell vehicles than on hydrogen ICE vehicles. Many
prototypes have been introduced, some of them in small series (tens of cars). Most of the
manufacturers have opted for proton exchange membrane (PEM) fuel cells because of their low-
temperature operation and relatively (compared to other fuel cell types) easy manufacturing and
maintenance. Current trends are mainly focused on hybridization, such as combining fuel cells with
NiMH batteries, ultra capacitors, or other types of electric storage. Although this increases the
complexity of the vehicle, thus increasing the cost, it brings significant advantages. The main one is
covering power peaks during acceleration, when the electric motor draws high current from the Fuel
Cell. A second advantage in electrical storage is increasing the driving range, because hybrid vehicles
optimize fuel consumption, and also the use of braking recuperation.
It is not only important to have technical problems solved, public acceptance is also important. For this
purpose, hydrogen fuelled buses have been successful. Currently there are about 60 of them serving on
a daily basis in different cities including London, Hamburg, Madrid, Stuttgart, Stockholm, Porto,
Amsterdam, Barcelona, Luxembourg, Reykjavik and Perth.
The lack of the hydrogen infrastructure makes fleet customers important for early hydrogen
transportation markets. It is much easier to build one centralized filling station near a city bus operator
or dispatch service than to service the distributed market for personal cars.
Motorcycles, scooters and electric bikes represent a smaller, but interesting, market opportunity. Such
means of transportation are significant in many Asian countries, where the pollution is growing and
causing health problems. Switching from fossil-based fuels to hydrogen would improve the local
environment.
6
Nuclear energy for hydrogen production
As an alternative path to the current fossil fuel economy, a hydrogen economy is envisaged in which
hydrogen would play a major role in energy systems and serve all sectors of the economy, substituting
for fossil fuels. Hydrogen as an energy carrier can be stored in large quantities, unlike electricity, and
converted into electricity in fuel cells, with only heat and water as by-products. It is also compatible
with combustion turbines and reciprocating engines to produce power with near-zero emission of
pollutants. Furthermore, hydrogen can be obtained from various primary energy sources that are
domestically available in most countries. Consequently, the hydrogen economy could enhance both
the security of energy supply and global environmental quality.
The current worldwide hydrogen production is roughly 50 million tonnes per year. Although current
use of hydrogen in energy systems is very limited, its future use could become enormous, especially if
fuel-cell vehicles would be deployed on a large commercial scale. The hydrogen economy is getting
higher visibility and stronger political support in several parts of the world.
Today, hydrogen is used in limited quantities, and mainly in petroleum refineries and the chemical
industry. In the United States, for example, these uses represented 93% of hydrogen consumption in
2003. However, hydrogen is an attractive energy carrier that might play a major role in energy systems
for many economic sectors in the long term. In the medium term, the most promising area for
hydrogen is in producing synthetic fuel as a substitute for gasoline in transportation. Hydrogen
produced from non-fossil fuels may be a key option as the prices of hydrocarbon resources soar or
their consumption becomes restricted for environmental reasons.
Hydrogen currently finds many applications as a chemical product for:
• Ammonia synthesis;
• Methanol synthesis;
• Direct reduction of iron ore;
• Fossil fuel processing (hydro cracking);
• Fischer-Tropsch hydrocarbon synthesis;
• Methanation in long-distance energy transportation; and
• Hydro-gasification.
In addition, there may be hydrogen markets for heating, stationary fuel cells, combined heat and
power, and stationary gas turbines. Potentially hydrogen could be used for ground transport, aviation,
marine applications, and railroad transport.
The mass utilization of fuel cells for transportation and decentralized power production will not
materialize until at least 2020. Currently research focuses on catalysts, materials, equipment,
production costs, durability, cold-start capability, power density, and water management.
Nuclear-generated hydrogen has important potential advantages over other sources that will be
considered for a growing hydrogen economy. Nuclear hydrogen requires no fossil fuels, results in
lower greenhouse-gas emissions and other pollutants, and lends itself to large-scale production. These
advantages do not ensure that nuclear hydrogen will prevail, however, especially given strong
competition from other hydrogen sources. There are technical uncertainties in nuclear hydrogen
processes, certainly, which need to be addressed through a vigorous research and development effort.
As a greenhouse-gas-free alternative, the U.S., Japan, and other nations are exploring ways to produce
hydrogen from water by means of electrolytic, thermochemical, and hybrid processes. Most of the
work has concentrated on high-temperature processes such as high-temperature steam electrolysis and
the sulphur–iodine and calcium-bromine cycles. These processes require higher temperatures
(>750
o
C) than can be achieved by water-cooled reactors. Advanced reactors such as the very high
7
temperature gas cooled reactor (VHTGR) can generate heat at these temperatures, but will require
several years before they are commercial deployed.
Water-cooled reactors are likely to be the nuclear power technology of choice for many years. Their
outlet temperature limitation of ~350
o
C leaves only one current option for hydrogen production: low-
temperature water electrolysis. Because they require no heat input, water electrolyzers can be
decoupled from the power plant. Therefore, electrolysers can be attractive as remote and decentralized
hydrogen production methods. Because of the high electrical demands for the process, though,
electrolysis of water is attractive only when cheap electricity is available or when particularly high-
purity hydrogen is required. The use of nuclear generated electricity in off-peak periods from existing
water-cooled reactors may be economically competitive, but the stranded capital costs of the
electrolyzers during periods of peak electricity prices may be prohibitive.
Other hydrogen production options require higher temperatures. Short of the temperatures achievable
by liquid-metal-cooled or gas-cooled reactors, few hydrogen production methods are known.
Supercritical water cooled reactors have the potential to deliver heat at 550
o
C. At this temperature,
hydrogen production methods include membrane-assisted steam methane reforming and a limited
number of thermo-electrochemical cycles. Experimentation has been limited on these systems. None
are close to having demonstrated commercial viability. Nonetheless, process flowsheets suggest that
system efficiencies can be higher than for low-temperature water electrolysis. This makes laboratory
research on potential hydrogen production technologies worth pursuing.
A study has shown that significant reductions in fossil energy use and greenhouse gas emissions come
from nuclear-based hydrogen production compared to natural-gas-based hydrogen production through
steam methane reforming. The reductions amount to 73 – 96% in greenhouse gas emissions (CO
2
,
CH
4
, and N
2
O) and 81 – 97% in fossil energy use. Furthermore, fuel cell vehicles powered by nuclear
hydrogen have substantial reductions in greenhouse gas emissions (87 – 98%) and fossil energy use
(89 – 98%) compared with internal combustion engine vehicles using reformulated gasoline. Nuclear
hydrogen is not completely emission-free, however, since a small amount of fossil fuel is consumed in
the upstream feedstock and fuel stages.
Conclusion
In considering the deployment of nuclear energy into advanced applications, challenges and
difficulties should not be overlooked; in particular, it should be acknowledged that a scientific
potential is not a technical reality and that competition will drive the choice of energy sources for each
application. Moving from their potential to realities is undoubtedly feasible, but will need time,
investments, and policy measures to address a wide range of techno-economic and socio-political
challenges. Public acceptance is a major issue for nuclear energy. Advanced applications of nuclear
energy can play an important role in enhancing public acceptance.
8
CHAPTER 1
PRESENT STATUS AND OVERVIEW OF
ADVANCED APPLICATIONS OF NUCLEAR REACTORS
Introduction
Today, nuclear power plants provide about 15.2% of the world’s electricity consumption. Because
electricity represents less than one third of the primary energy uses, nuclear energy provides only
about 6% of total energy consumption in the world. If nuclear energy were used for purposes other
than electricity generation, it could play a more significant role in global energy supply. This could
have a significant impact on global goals for reduced greenhouse gas emissions, for a cleaner
environment, and for less reliance on uncertain supplies of fossil fuels.
Nuclear reactors, which produce energy in the form of heat, can supply energy products other than
electricity, including district and process heat, desalinated water, hydrogen, and heat for other
industrial products. Although not covered in this report, nuclear reactors are unique in their ability to
produce high radiation fields for medical isotope production and the conversion of plutonium and
other transuranic elements to shorter-lived radioactive waste. While these applications of nuclear
energy have been considered since the very beginning of nuclear energy development, they have for
various reasons yet to be deployed at a significant industrial scale.
In recent years, various agencies involved in nuclear energy development programmes worldwide
have carried out studies on advanced applications of nuclear power and useful reports have been
published [1-4]:
• The IAEA launched a programme on co-generation applications and has published two
TECDOCs (IAEA-TECDC-923 and IAEA-TECDOC-1184) and a Guidebook on Introduction
of Nuclear Desalination (TRS-400) in 2000. IAEA also published a report in 2002 on the
Market Potential for Non-electric Applications of Nuclear Energy (TRS-410).
• The Organization for Economic Cooperation and Development (OECD) Nuclear Energy
Agency, under the guidance of the Committee for Technical and Economic Studies on Nuclear
Energy Development and the Fuel Cycle (NDC), carried out a comprehensive survey of
published literature on the subject matter, including reports from international organizations,
national institutes and other parts of NEA and published a report summarizing the findings and
recommendations [5].
• The Generation IV International Forum (GIF) project aims at development of innovative
reactors with temperatures up to 1000
o
C. The GIF road map recommends necessary R&D. One
of the selected systems is the super-critical water cooled reactor SCWR [6].
• The Michelangelo Network (MICANET) was started within the 5
th
EUROATOM Framework
Programme (FP5) with the objective to elaborate a general European R&D strategy for further
development of the nuclear industry in the short, medium and long term. MICANET has been
examining the role of nuclear energy in near and medium term missions; i.e. the transition phase
from the present fossil era to CO
2
emission-free technologies in the future. The programme
results were reported in November 2005 as a work package on “Non-electric application of
nuclear energy”. The network examined the possible orientation of future EURATOM R&D
programmes including new aspects of nuclear energy such as combined heat and power (CHP),
desalination, and hydrogen production or other fuel production as a complement to other CO
2
-
free energy sources [7].
9

The present IAEA document focuses on the potential of water-cooled nuclear reactor technology to
penetrate non-electricity sectors. Nuclear power is the only large-scale carbon-free energy source that,
in the near and medium term, has the potential to significantly displace limited and uncertain fossil
fuels. To do this, however, nuclear power must move beyond its historical role as solely a producer of
electricity. The sampling of non-electric applications in this report is not exhaustive. For instance, the
use of nuclear reactors for medical therapy and radioactive isotopes is not covered. Instead, the
document is intended to begin a dialogue that considers the value of water-cooled reactor technology
for a broad range of non-traditional applications.
1.1. Nuclear power reactors in the world
The number of nuclear power reactors in operation and under construction in the world, as of August
2007, as reported by the IAEA Power Reactor Information System, are reproduced in Table 1.1.
Table 1.1. Distribution of reactor types
Light water reactors
1
:
In operation 359
Under construction 23
Number of countries with LWRs
27
Generating capacity, GW(e)
328.2
Heavy water moderated reactors
2
:
In operation 43
Under construction 5
Number of countries with HWRs 7
Generating capacity, GW(e) 21.7
1
Reactors cooled and moderated by light water
2
Reactors moderated by heavy water
10

Light water cooled graphite moderated reactors:
In operation 16
Under construction 1
Number of countries 2
Generating capacity, GW(e) 11.4
Liquid metal cooled fast reactors:
In operation 2
Under construction 2
Number of countries with FRs
a
3
Generating capacity, GW(e) 1.0
Operating Experience, reactor years 171
a
In France, Russia and Japan, where the Monju reactor, under long term
shutdown, is planned to be re-started.
Gas cooled reactors:
Power reactors in operation 18
Under construction 0
Test reactors in operation 2
Number of countries with GCRs 3
Generating capacity, GW(e) 9.0
Table 1.1 shows that water cooled reactors are the work-horse of nuclear power generation. Up to
now, nuclear energy has served almost exclusively as a generator of electricity. In decades to come,
nuclear may be called upon to play a significant role in other energy sectors, especially as there is a
drive to reduce greenhouse gas emissions in sectors traditionally served by fossil fuels. Some of the
most promising applications are hydrogen production, high temperature process heat, desalination to
produce fresh water, and district heating. In addition, the advent of plug-in hybrid electric vehicles
may allow nuclear electricity generation to enter the transportation sector. Some experience is already
available with these applications, particularly for seawater desalination and district heating.
1.2. Non-electric applications of nuclear power
Figure 1.1 shows various reactor types and their possible non-electric applications. It can be seen that
most of the present day non-electric applications can be met with water cooled reactors. Some of the
industrial heat requirements, however, require higher temperature heat and would need other types of
reactors.
11

FIG. 1.1. Temperature ranges in production and use of nuclear heat.
Of the 438 nuclear power reactors operating worldwide in August 2007, those reactors which were
being used for co-generation of hot water or steam for district heating, seawater desalination or
industrial processes are shown in Table 1.2 (based on the IAEA Power Reactor Information System).
Table 1.3 shows the energy generated by each reactor for district heating, process heating, or
desalination. Salient details of these applications will be found in the succeeding chapters. Other
advanced applications, such as nuclear hydrogen production, have yet to be realized.
12

TABLE 1.2. Reactors having non-electric applications
Capacity
Reactor
MW(e)
Country
Code Name
Type
Net Gross Thermal
Operator
NSSS
Supplier
Construction
Start
Grid
Connection
Commercial
Operation
Load Factor %
(1)
to 2005
Unit Capacity
Factor % (1)
to 2005
Non-electric
Applications
(2)
BULGARIA BG -5 KOZLODUY-5 PWR 953 1000 3000 KOZNPP AEE 1980-7 1987-11 1988-12 50.0 64.0 DH
BG -6 KOZLODUY-6 PWR 953 1000 3000 KOZNPP AEE 1982-4 1991-8 1993-12 58.0 72.0 DH
CZECH REP. CZ -23 TEMELIN-1 PWR 930 975 3000 CEZ SKODA 1987-2 2000-12 2002-6 70.0 72.0 DH
CZ -24 TEMELIN-2 PWR 930 975 3000 CEZ SKODA 1987-2 2002-12 2003-4 69.0 68.0 DH
HUNGARY HU -2 PAKS-2 PWR 441 468 1375 PAKS RT. AEE 1974-8 1984-9 1984-11 79.0 78.0 DH
HU -3 PAKS-3 PWR 433 460 1375 PAKS RT. AEE 1979-10 1986-9 1986-12 87.0 86.0 DH
HU -4 PAKS-4 PWR 444 471 1375 PAKS RT. AEE 1979-10 1987-8 1987-11 89.0 87.0 DH
INDIA IN -5 MADRAS-1 PHWR 202 220 801 NPCIL NPCIL 1971-1 1983-7 1984-1 49.0 58.0 DS
IN -6 MADRAS-2 PHWR 202 220 801 NPCIL NPCIL 1972-10 1985-9 1986-3 55.0 62.0 DS
IN -3 RAJASTHAN-1 PHWR 90 100 693 NPCIL AECL 1965-8 1972-11 1973-12 21.0 28.0 PH
IN -4 RAJASTHAN-2 PHWR 187 200 693 NPCIL AECL/DAE 1968-4 1980-11 1981-4 53.0 60.0 PH
IN -11 RAJASTHAN-3 PHWR 202 220 801 NPCIL NPCIL 1990-2 2000-3 2000-6 77.0 88.0 PH
IN -12 RAJASTHAN-4 PHWR 202 220 801 NPCIL NPCIL 1990-10 2000-11 2000-12 77.0 91.0 PH
JAPAN JP -45 GENKAI-3 PWR 1127 1180 3423 KYUSHU MHI 1988-6 1993-6 1994-3 86.0 85.0 DS
JP -46 GENKAI-4 PWR 1127 1180 3423 KYUSHU MHI 1992-7 1996-11 1997-7 86.0 85.0 DS
JP -23 IKATA-1 PWR 538 566 1650 SHIKOKU MHI 1973-6 1977-2 1977-9 77.0 78.0 DS
JP -32 IKATA-2 PWR 538 566 1650 SHIKOKU MHI 1978-2 1981-8 1982-3 81.0 81.0 DS
JP -47 IKATA-3 PWR 846 890 2660 SHIKOKU MHI 1986-11 1994-3 1994-12 87.0 85.0 DS
JP -15 OHI-1 PWR 1120 1175 3423 KEPCO WH 1972-10 1977-12 1979-3 66.0 66.0 DS
JP -19 OHI-2 PWR 1120 1175 3423 KEPCO WH 1972-12 1978-10 1979-12 72.0 72.0 DS
JP -29 TAKAHAMA-3 PWR 830 870 2660 KEPCO MHI 1980-12 1984-5 1985-1 84.0 83.0 DS
JP -30 TAKAHAMA-4 PWR 830 870 2660 KEPCO MHI 1981-3 1984-11 1985-6 86.0 84.0 DS
PAKISTAN PK -1 KANUPP PHWR 125 137 433 PAEC CGE 1966-8 1971-10 1972-12 26.0 43.0 DS
ROMANIA RO -1 CERNAVODA-1 PHWR 655 706 2180 SNN AECL 1982-7 1996-7 1996-12 86.0 87.0 DH
13

TABLE 1.2. Reactors having non-electric applications (cont’d)
Capacity
Reactor
MW(e)
Country
Code Name
Type
Net Gross Thermal
Operator
NSSS
Supplier
Construction
Start
Grid
Connection
Commercial
Operation
Load Factor %
(1)
to 2005
Unit Capacity
Factor % (1)
to 2005
Non-electric
Applications
(2)
RUSSIAN FEDERATION RU -96 BALAKOVO-1 PWR 950 1000 3000 REA FAEA 1980-12 1985-12 1986-5 62.0 68.0 DH,PH
RU -97 BALAKOVO-2 PWR 950 1000 3000 REA FAEA 1981-8 1987-10 1988-1 61.0 67.0 DH,PH
RU -98 BALAKOVO-3 PWR 950 1000 3000 REA FAEA 1982-11 1988-12 1989-4 66.0 73.0 DH,PH
RU -99 BALAKOVO-4 PWR 950 1000 3200 REA FAEA 1984-4 1993-4 1993-12 71.0 78.0 DH,PH
RU -21
BELOYARSKY-3(BN-
600)
FBR 560 600 1470 REA FAEA 1969-1 1980-4 1981-11 74.0 75.0 DH,PH
RU -141 BILIBINO-1 LWGR 11 12 62 REA FAEA 1970-1 1974-1 1974-4 58.0 80.0 DH
RU -142 BILIBINO-2 LWGR 11 12 62 REA FAEA 1970-1 1974-12 1975-2 57.0 81.0 DH
RU -143 BILIBINO-3 LWGR 11 12 62 REA FAEA 1970-1 1975-12 1976-2 59.0 81.0 DH
RU -144 BILIBINO-4 LWGR 11 12 62 REA FAEA 1970-1 1976-12 1977-1 58.0 78.0 DH
RU -30 KALININ-1 PWR 950 1000 3000 REA FAEA 1977-2 1984-5 1985-6 71.0 71.0 DH,PH
RU -31 KALININ-2 PWR 950 1000 3000 REA FAEA 1982-2 1986-12 1987-3 71.0 73.0 DH,PH
RU -36 KALININ-3 PWR 950 1000 3200 REA FAEA 1985-10 2004-12 2005-11 77.0 77.0 -
RU -12 KOLA-1 PWR 411 440 1375 REA FAEA 1970-5 1973-6 1973-12 65.0 76.0 DH,PH
RU -13 KOLA-2 PWR 411 440 1375 REA FAEA 1973-1 1974-12 1975-2 65.0 76.0 DH,PH
RU -32 KOLA-3 PWR 411 440 1375 REA FAEA 1977-4 1981-3 1982-12 72.0 82.0 DH,PH
RU -33 KOLA-4 PWR 411 440 1375 REA FAEA 1976-8 1984-10 1984-12 71.0 81.0 DH,PH
RU -17 KURSK-1 LWGR 925 1000 3200 REA FAEA 1972-6 1976-12 1977-10 57.0 60.0 DH,PH
RU -22 KURSK-2 LWGR 925 1000 3200 REA FAEA 1973-1 1979-1 1979-8 60.0 63.0 DH,PH
RU -38 KURSK-3 LWGR 925 1000 3200 REA FAEA 1978-4 1983-10 1984-3 71.0 73.0 DH,PH
RU -39 KURSK-4 LWGR 925 1000 3200 REA FAEA 1981-5 1985-12 1986-2 75.0 76.0 DH,PH
RU -15 LENINGRAD-1 LWGR 925 1000 3200 REA FAEA 1970-3 1973-12 1974-11 68.0 69.0 DH,PH
RU -16 LENINGRAD-2 LWGR 925 1000 3200 REA FAEA 1970-6 1975-7 1976-2 68.0 69.0 DH,PH
RU -34 LENINGRAD-3 LWGR 925 1000 3200 REA FAEA 1973-12 1979-12 1980-6 69.0 71.0 DH,PH
RU -35 LENINGRAD-4 LWGR 925 1000 3200 REA FAEA 1975-2 1981-2 1981-8 71.0 73.0 DH,PH
14

TABLE 1.2. Reactors having non-electric applications (cont’d)
Capacity
Reactor
MW(e)
Country
Code Name
Type
Net Gross Thermal
Operator
NSSS
Supplier
Construction
Start
Grid
Connection
Commercial
Operation
Load Factor %
(1)
to 2005
Unit Capacity
Factor % (1)
to 2005
Non-electric
Applications (2)
RU -23 SMOLENSK-1 LWGR 925 1000 3200 REA FAEA 1975-10 1982-12 1983-9 70.0 73.0 DH,PH
RU -24 SMOLENSK-2 LWGR 925 1000 3200 REA FAEA 1976-6 1985-5 1985-7 73.0 76.0 DH,PH
RU -67 SMOLENSK-3 LWGR 925 1000 3200 REA FAEA 1984-5 1990-1 1990-10 78.0 81.0 DH,PH
SLOVAKIA SK -13 BOHUNICE-3 PWR 408 440 1375 SE,plc SKODA 1976-12 1984-8 1985-2 75.0 80.0 DH
SK -14 BOHUNICE-4 PWR 408 440 1375 SE,plc SKODA 1976-12 1985-8 1985-12 77.0 82.0 DH
SWITZERLAND CH -1 BEZNAU-1 PWR 365 380 1130 NOK WH 1965-9 1969-7 1969-9 82.0 87.0 DH
CH -3 BEZNAU-2 PWR 365 380 1130 NOK WH 1968-1 1971-10 1971-12 87.0 87.0 DH
CH -4 GOESGEN PWR 970 1020 2900 KKG KWU 1973-12 1979-2 1979-11 88.0 89.0 DH
UKRAINE UA -40 KHMELNITSKI-1 PWR 950 1000 3000 NNEGC PAIP 1981-11 1987-12 1988-8 72.0 72.0 DH
UA -27 ROVNO-1 PWR 381 420 1375 NNEGC PAIP 1973-8 1980-12 1981-9 80.0 81.0 DH
UA -28 ROVNO-2 PWR 376 415 1375 NNEGC PAIP 1973-10 1981-12 1982-7 79.0 81.0 DH
UA -29 ROVNO-3 PWR 950 1000 3000 NNEGC PAIP 1980-2 1986-12 1987-5 69.0 73.0 DH
UA -44 SOUTH UKRAINE-1 PWR 950 1000 3000 NNEGC PAA 1977-3 1982-12 1983-10 66.0 66.0 DH
UA -45 SOUTH UKRAINE-2 PWR 950 1000 3000 NNEGC PAA 1979-10 1985-1 1985-4 61.0 62.0 DH
UA -48 SOUTH UKRAINE-3 PWR 950 1000 3000 NNEGC PAA 1985-2 1989-9 1989-12 72.0 73.0 DH
UA -54 ZAPOROZHE-1 PWR 950 1000 3000 NNEGC PAIP 1980-4 1984-12 1985-12 61.0 64.0 DH
UA -56 ZAPOROZHE-2 PWR 950 1000 3000 NNEGC PAIP 1981-1 1985-7 1986-2 64.0 68.0 DH
UA -78 ZAPOROZHE-3 PWR 950 1000 3000 NNEGC PAIP 1982-4 1986-12 1987-3 66.0 70.0 DH
UA -79 ZAPOROZHE-4 PWR 950 1000 3000 NNEGC PAIP 1983-4 1987-12 1988-4 71.0 75.0 DH
UA -126 ZAPOROZHE-5 PWR 950 1000 3000 NNEGC PAIP 1985-11 1989-8 1989-10 72.0 74.0 DH
UA -127 ZAPOROZHE-6 PWR 950 1000 3000 NNEGC PAIP 1986-6 1995-10 1996-9 77.0 80.0 DH
(1) Performance factors Load Factor (LF) and Unit Capacity Factor (UCF) calculated only for period of full commercial operation, and only through 2005.
(2)The column Non-Electrical Applications indicates the use of the facility to provide:- DS desalination, DH district heating, PH process heat.
15

Table 1.3-a. District heating and process heat in 2006 (1)
Country Reactor District heating [Gcal] Process heat [Gcal] Total heat [Gcal]
Bulgaria Kozloduy-5 121735 N/A 121735
Kozloduy-6 17964 N/A 17964
Czech Republic Temelin-1 42503 N/A 42503
Temelin-2 4757 N/A 4757
Hungary PAKS-2 2187 N/A 2187
PAKS-3 25385 N/A 25385
PAKS-4 214307 N/A 214307
India Rajasthan-1 N/A 0 0
Rajasthan-2 N/A 98952 98952
Rajasthan-3 N/A 112421 112421
Rajasthan-4 N/A 21186 21186
Romania Cernavoda-1 27127 N/A 27127
Russian Federation Balakovo-1 47980 0 47980
Balakovo-2 0 0 0
Balakovo-3 1000 0 1000
Balakovo-4 2837 0 2837
Beloyarsky-3 279279 0 279279
Bilibino-1 45212 N/A 45212
Bilibino-2 41344 N/A 41344
Bilibino-3 50286 N/A 50286
Bilibino-4 49062 N/A 49062
Kalinin-1 337593 3510 341103
Kalinin-2 224460 3084 227544
Kola-1 6628 2064 8692
Kola-2 7962 2066 10028
Kola-3 11234 1744 12978
Kola-4 5624 1828 7452
Kursk-1 60540 62611 123151
Kursk-2 96184 101255 197439
Kursk-3 127776 116201 243977
Kursk-4 217029 168384 385413
16

Table 1.3-a. District heating and process heat in 2006 (1) cont’d.
Leningrad-1 219273 0 219273
Leningrad-2 26582 0 26582
Leningrad-3 243028 0 243028
Leningrad-4 183123 0 183123
Novovoronezh-3 84240 0 84240
Novovoronezh-4 135657 2680 138337
Novovoronezh-5 0 1637 1637
Smolensk-1 55751 34670 90421
Smolensk-2 278585 22224 300809
Smolensk-3 249082 21803 270885
Slovakia Bohunice-3 224056 0 224056
Bohunice-4 193285 0 193285
Switzerland Beznau-1 129 N/A 129
Beznau-2 12 N/A 12
Goesgen N/A 63005 63005
Ukraine Khemlnitski-1 189517 N/A 189517
Rovno-1 82154 N/A 82154
Rovno-2 66929 N/A 66929
Rovno-3 129006 N/A 129006
South Ukraine-1 98018 N/A 98018
South Ukraine-2 129808 N/A 129808
South Ukraine-3 136978 N/A 136978
Zaporozhe-1 89166 N/A 89166
Zaporozhe-2 91991 N/A 91991
Zaporozhe-3 43534 N/A 43534
Zaporozhe-4 64302 N/A 64302
Zaporozhe-5 125087 N/A 125087
Zaporozhe-6 126772 N/A 126772
(1) 1 Gcal = 1.16 MWh.
17

Table 1.3-b. Water desalination in 2006 (1)
Country Reactor
Thermal energy
[Gcal]
Electrical energy
for reverse
osmosis
[MWh]
Water produced
[m
3
]
Madras-1 0 0 0 India
Madras-2 0 0 0
Genkai 3&4 30189 N/A 430050
Ikata 1&2 N/A 331488
Ikata-3 N/A 6002
Ohi 1 and 2 N/A 930269
Japan
Takahama 3&4 N/A 608029
Pakistan KANUPP 0 0 0
(1) 1 Gcal = 1.16 MWh.
1.3. Past experience with non-electric applications
Figure 1.2 shows the reactor years of experience for non-electric applications in Bulgaria, China,
Czech Republic, Hungary, India, Japan, Kazakhstan, Pakistan, Romania, Russian Federation,
Slovakia, Switzerland and the Ukraine.
Experience with non-electrical applications
0
100
200
300
400
500
600
700
800
900
1000
RU UA JP HU CH IN SK BG KZ CZ RO PK
reactor-years
FIG. 1.2. Reactor-years of experience with non-electric applications in different countries.
A comprehensive list of various non-electric applications worldwide published in the IAEA
Guidebook [3] is reproduced in Tables 1.5 through 1.10 for ready reference.
18

Table 1.5. Operating nuclear desalination plants in Japan
Plant
name
Location
Application
Start of
operation
reactors /
desalination
Net
Power
(MW(e))
Water
capacity
(m
3
/d)
Remarks
Ikata-1,2 Ehime Electricity/
desalination
1977-82
1975
566 2000 PWR/MED,
MSF
Ikata-3 Ehime Electricity/
desalination
1994
1992
566 2000 PWR/MSF
(2 x 1000 m
3
/d)
Ohi-1,2 Fukui Electricity/
desalination
1979
1973-76
1175 3900 PWR/MSF
(3 x 1300 m
3
/d)
Ohi-3,4 Fukui Electricity/
desalination
1991-93
1990
1180 2600 PWR/RO
(2 x 1300 m
3
/d)
Genkai-4 Fukuoka Electricity/
desalination
1997
1988
1180 1000 PWR/RO
Genkai-3,4 Fukuoka Electricity/
desalination
1995-97
1992
1180 1000 PWR/MED
Takahama Fukui Electricity/
desalination
1985
1983
870 1000 PWR/RO
Table 1.6. Nuclear desalination projects in India and Pakistan
Plant
name
Location
Application
Start of
Operation
reactor / desal.
Net
Power
(MW(e))
Water
capacity
(m
3
/day)
Remarks
Kalpakkam
1,2
Tamil
Nadu
Electricity/
Desalination
Reactors:1984-86
RO:2000/2001
MSF: after 2001
2 x 170 6300 Hybrid MSF/
RO
KANUPP Karachi Electricity
Desalination
Reactor: 1972
MED in 2007
1 x 135 4800 MED
19

Table 1.7. Operating nuclear heating plants
Country
Plant type
or name
Location
Application
Phase
Start of
operation
reactors / heat
Net
Power
(MW(e))
Heat output
capacity
(MW(th))
Temp (°C) at
interface
(feed/return)
Remarks
Bulgaria Kozloduy Kozloduy Electricity/
District heating
Commercial 1974-82
1990
1988-93
4 x 408
2 x 953
20 150/70
China NHR-5 Beijing District heating Experimental 1989
1989
- 5 90/60
Hungary PAKS 1-4
Paks Electricity/
District heating
Commercial 1983-87 3 x 433
1 x 430
30 130/70 4 x V-213
WWER
Romania PHWR
CANDU-6
Cernavoda
Unit 1
Electricity/
District heating
Commercial 1996 1 x 660 40 Gcal/h 150/70
Russia Obninsk District heating Commercial
1954-
b
- 10 130/70
Russia WWER-1000
Novovoronezh
4
Electricity/
District heating
Commercial 1972-73
1981
2 x 385
1 x 950
230 130/70
Russia WWER-1000 Balakovo Electricity/
District heating
Commercial
1986-93
b
4 x 950 230 130/70
Russia WWER-1000 Kalinin Electricity/
District heating
Commercial 1985-87
b
2 x 950 230 130/70
Russia WWER-440 Kola Electricity/
District heating
Commercial 1973-84
b
4 x 411 55
Russia EGP-6 Bilibino Electricity/
District heating
Commercial 1974-77
b
4 x 11 133 150/70
Russia BN-600 Belojarsk Electricity/
District heating
Commercial 1981
b
2 x 560 220 130/70
Russia RBMK-1000 Petersburg Electricity/
District heating
Commercial 1974-81
b
4 x 925 ~170 130/70
Russia RBMK-1000 Kursk Electricity/
District heating
Commercial 1977-86
b
4 x 925 ~170 130/70
Slovakia Bohunice-3,4 Bohunice/Trnava Electricity/
District heating
Commercial 1985 1987 2 x 408 240 150/70 2 x V-213
WWER
Switzerland Beznau 1,2 Beznau Electricity/
District heating
Commercial 1969-71/1983-84 1 x 365
1 x 357
80 128/50
(water)
20

Table 1.8. Nuclear heating plant projects
Country
Plant
type or site
Location
Application
Phase
Start of
operation
(year)
Power
(MW(e))
Heat
output
(MW(th))
Temperature (°C)
at interface
(Feed/Return)
Bulgaria Belene Belene Electricity/
District heating
Design 2 x 1000 400 150/70
China NHR-200 Daqing City District heating In construction 2000 - 200 90/~60
Japan HTTR O-arai Process heat Operation 1998 30 950/395
Romania PHWR
CANDU-6
Cernavoda -
Unit 2
Electricity/
District heating
In
commissioning
2007 1 x 660 46 150/70
Russia RUTA Apatity District heating/
Air conditioning
Design - 4 x 55 85/60
Russia ATEC-200 Electricity/
District heating
Design 50-180 70-40 150/70
Russia VGM Process Heat Design - 200 900/~500
Russia KLT-40 Electricity/
District heating
& Desalination
Design 35 110
Russia AST-500 Voronez District heating Construction
suspended
- 500 150/70
Russia AST-500 Tomsk District heating Construction
suspended
- 500 150/70
21

Table 1.9. Operating nuclear process heat production plants
Country
Plant
name
Location
Application
Start of operation
reactors / heat
Phase
Power
(MW
(e)))
Heat
delivery
(MW(t))
Temperature
(°C) at
interface
(feed/return)
Remarks
Canada Bruce-A
a
Bruce Process heat 1977-87 / 1981 Commercial 4 x 848
5350
D
2
O production
and six industrial
heat customers
Germany Stade Stade Electricity/
process heat
1983 Commercial 640 30 190/100 Salt refinery
Switzerland Goesgen Goesgen Electricity/
process heat
1979 / 1979 Commercial 970 25 220/100 Cardboard factory
India RAPS Kota Electricity/
process heat
1975 / 1980 Commercial 160 85 250 D
2
a
Unit 2 was taken out of service in 1995, units 1, 3 and 4 were taken out of service in spring 1998.
Table 1.10. Nuclear Process Heat Production Projects
Country
Plant
name
Location
Application
Start of operation
reactors / heat
Phase
Heat
delivery
(MW(t))
Temperature
(°C) at
interface
(feed/return)
Remarks
China HTGR-10 Beijing Electricity/
process heat
Criticality 1999 Construction 10 700-950/250 Experiments for
HTR technology
development.
Japan HTTR O-arai Process heat Criticality 1998 Construction
completed
30 950/395 Experiments for
HTR technology
development.
Russia VGM Process heat Design
22
1.4. Market potential of advanced non-electric applications [4]
1.4.1. 2003 IAEA study: Market potential for non-electric application of nuclear energy [4]
An analysis of the market potential for non-electric applications of nuclear energy was carried out by
IAEA and published in IAEA-TRS-410 (2003). The conclusions reached in this report are reproduced
as the following.
(a) For the foreseeable future, power generation will remain the main application of nuclear energy,
the main reasons being the advanced status of nuclear power
production technologies and an
increasing share of electricity in the total
energy demand;
(b) Currently, nuclear power has little penetration in non-electric energy applications. However, a
large demand for non-electric nuclear energy is expected to emerge and grow rapidly.
(c)
Because of the dominance of power generation, nuclear penetration into the
markets for non-
electric services will proceed with cogeneration applications
wherever possible. Dedicated
reactors for heat generation could eventually
emerge for some applications.
(d) Many non-electric applications require energy sources that are relatively small
(100-1000
MW(th)) in comparison with the size of existing power reactors.
The development of nuclear
reactors of small and medium size would therefore facilitate non-electric applications of nuclear
energy.
(e) Some non-electric applications require that the nuclear plant be located close to the customer.
This will require specific safety features appropriate to the location.
(f) Economically, the non-electric applications of nuclear energy are subject to the
same trends as
nuclear power generation. Growing capital costs of nuclear plants have affected the cost
estimations of most non-electric applications.
Evolutionary and innovative design
improvements in nuclear reactor concepts,
coupled with stable nuclear fuel prices, will result
in an improved
competitiveness of non-electric nuclear applications.
(g)
Depending upon the regions and conditions, nuclear energy is already competitive for district
heating, desalination, and certain process heat
applications.
(h)
Using nuclear energy to produce hydrogen is likely to facilitate the indirect
application of
nuclear energy in transportation markets, most of which are not readily amenable to the direct
use of nuclear reactors.
(i) Non-electric applications of nuclear energy are most likely to be implemented
in countries
already having the appropriate nuclear infrastructure and
institutional support.
(j)
The implementation of some non-electric applications (e.g., desalination) is
likely to enhance
the public acceptance of nuclear energy.
The following specific findings for these applications were formulated, in addition to the general
conclusions above.
District heating
Nuclear applications for district heating are technically mature and exist in
several countries. The
future use of nuclear energy will be determined by a
combination of the following factors: the size
and growth of the demand for space
and water heating, competition between heat and non-heat energy
carriers for space
and water heating, and competition between nuclear and non-nuclear heating. The
23
availability of a heat distribution network plays an important role in the prospects for nuclear district
heating.
Desalination
For desalination, low temperature heat and/or electricity are required.
Consequently, all existing
nuclear designs can be used; the relevant experience is already available. The use of nuclear heat
assumes a close location of the nuclear
plant to the desalination plant; the use of electricity generated
by nuclear energy (for
the RO desalination process) does not differ from any other electricity use —
the
energy source may be located far from the customer with the electricity being provided
through the electricity grid. (It should be noted, however, that a distant location would
not allow the use
of low temperature steam for water preheating, which is an
advantage of co-production plants.)
With regard to the market size, it is expected that freshwater requirements will grow in the future, which
will increase the attractiveness of nuclear desalination.
Process heat supply
For process heat supply there is a wide range of required heat parameters that determine the
applicability of different reactor types. One particular concept, the high-temperature gas-cooled reactor
(HTGR), can produce temperatures above 800
o
C and perhaps as high as 1000
o
C. It is, therefore,
considered to be a prime candidate for nuclear process heat supply at high temperatures. The
development and demonstration of such a reactor would provide a strong impetus for the process heat
applications of nuclear energy.
Ship propulsion
Nuclear powered ship propulsion has been tested technically in several
countries, especially for naval
applications. The technology has proven non-economic for commercial applications, however,
and the use of nuclear
powered merchant ship propulsion has been discontinued, with the exception
of the nuclear
powered ships operating off the north coast of the Russian Federation. Although the
market
for large tankers and cargo ships is large, the future of nuclear powered ships will
depend on
their ability to offer competitive service in this highly competitive market.
The need to observe safety
and licensing requirements in receiving ports is an
additional obstacle for the application of nuclear
powered ship propulsion.
Hydrogen production
Two aspects must be distinguished: the penetration of hydrogen into the energy system and the use of
nuclear energy for hydrogen production. In comparison with the use of fossil fuels for hydrogen
production, nuclear energy has the advantages of a
large resource base and the absence of most
emissions, carbon dioxide in
particular. In comparison with the use of renewable energy sources
for hydrogen
production, nuclear energy has the advantage of being a mature, available technology
and
has the important feature of a high energy concentration, which could allow
hydrogen production
to be concentrated in multi product energy centres. The share of nuclear energy in a hydrogen-based
system will depend on its competitiveness with
the other energy options. Successful demonstration
projects, such as the use of
surplus nuclear capacity for hydrogen production using cheap off-peak
electricity, the
operation of the first large HTGR and the creation of local hydrogen markets near
existing NPPs, would help to promote the nuclear-hydrogen link.
Coal gasification
Coal gasification has the following advantages: the reduction of air emissions from coal combustion, an
increased thermal efficiency of combustion, and the use of a large resource base. If coal gasification
becomes widespread, economic and
environmentally benign technologies for the supply of
gasification energy will be
required. Nuclear energy, being an industrially mature and non-polluting
24
technology, is a valid candidate for this purpose. Such applications would be similar to the other
process heat applications of nuclear energy.
Other synthetic fuels
Transportation accounts for about one quarter of the
world’s total energy demand and will grow
significantly in the future following, in particular, industrial growth and an increase in the standards of
living in developing countries.
The direct use of nuclear energy for transportation is limited to the
use of electric
driven motors and ship and spacecraft propulsion. However, using nuclear energy for
transportation indirectly, through fuel production, is possible. The related fuels, in
addition to
electricity and hydrogen, include alcohol fuels (methanol, ethanol, and
their derivatives), compressed
natural gas, liquefied natural gas, liquefied petroleum gas, and coal-derived liquid fuels. Each fuel has
certain advantages and may have a place in the future fuel economy. The future fuel mix will be
determined primarily by inter fuel economic competition, the availability of infrastructure, and
environmental considerations such as the amount and type of greenhouse gases and other polluting
emissions.
Oil extraction
Nuclear energy can be used for the extraction of unconventional oil resources such as heavy oil, oil
from tar and oil sands, oil shale, and the oil remaining in depleted deposits. These unconventional oil
resources are about two times larger than the resources of conventional oil. However, if the price of
conventional oil is low it is not realistic to expect significant developments in the extraction of
unconventional oil, except in the cases (as, for example, in Canada) in which the resources of
unconventional oil are large and already developed. The case of Canada is especially notable because,
on one hand, the resources of unconventional oil are very large, and, on the other, nuclear technologies
suitable for such applications are available.
Table 1.11 shows the most promising countries for non-electric nuclear applications in the long term.
This table includes countries in which at least two applications are ranked highest in the corresponding
region.
These estimates are based on assessments that assume that applications of nuclear energy, both for
power generation and for non-electric purposes, will continue to
develop. This includes technical
development, infrastructural support and the licensing environment, the latter being particularly
important for certain applications. These estimations also assume, implicitly, that nuclear energy will
remain socially
acceptable
.
25

Table 1.11. Most promising countries for the long term prospects of non-electric
applications of nuclear energy
Region/country District
heating
Water desalination Process heat Ship propulsion
NAM
Canada M L M M
USA M L M M
LAM
Argentina L M L L
Brazil L L L M
Mexico L L L L
AFR
South Africa M M M L
MEA
Egypt L H L -
Iran M M M L
Morocco L H L -
WEU
Belgium M M M L
Finland M M M L
France M M M M
Netherlands M M M M
Spain M H M L
Switzerland M M M L
UK M M L M
EEU
Bulgaria M L M L
Czech Republic M M M L
Romania M L L
Slovakia M M M- -
FSU
Belarus M M M -
Lithuania M M M L
Russian
Federation
M L M M
Ukraine M M L M
CPA
China M H H M
Viet Nam L M M -
SAS
India M H M M
Pakistan M H M L
PAS
Indonesia L M M L
Korea, Republic of M M M M
Taiwan, China M L L M
PAO
Japan M M L M
Abbreviations: - = negligible, L= Low, M= medium, H= high; NAM-North America, LAM-Latin
America, AFR-Africa, WEU-Western Europe, EEU-Eastern Europe, FSU-Newly Independent States
of the former Soviet Union, CPA- Centrally planned Asia and China, SAS-South Asia, PAS-Other
Pacific Asia, PAO-Pacific OECD
26
1.4.2. 2004 NEA study: Non-electricity products of nuclear energy [5]
As shown in the IAEA study, the potential non-electricity product market open to nuclear energy
seems to be large; district heat, process heat, and desalinated water in the near term, and hydrogen in
the long term. For example, assuming that current district heat in the world would be replaced by
nuclear energy, it would mean the addition of 340 1000 MWth reactors. However, the reality does not
match the potential. Even though the high potential needs to be considered in a vision for the future,
realistic demand needs to be assessed, which was the purpose of the OECD-NEA study of 2004 [5].
From the beginning of the project, some basic questions related to the real deployment of nuclear non-
electricity products were raised, including:
• If nuclear energy has such high potential in non-electricity product markets, why has its
deployment been so limited? Can one expect some dramatic changes in this market situation?
• Who will or should initiate actions for the deployment of non-electricity applications of nuclear
energy: the government, R&D institutes, vendors, or suppliers? If necessary, how should
vendors or suppliers be motivated? Would some policy measures (tax reduction, subsidies, or a
carbon tax) really work?
• What are the thoughts of suppliers and vendors (nuclear and non-nuclear) in the current market?
• What is the role of the government? Do governments consider seriously all nuclear options in
their national energy policies for greenhouse gas reduction and energy security?
Common sense provides reasonable-guess answers to the above questions, but more investigations are
required to support an authoritative report. Deeper studies including market analyses are needed for
enhanced understanding of realistic projected demand, which can be carried out only with the support
of national experts providing relevant input data. Also, country-specific information is needed to
analyze the reasons for the limited deployment of non-electrical applications of nuclear energy and to
draw policy recommendations. Since few country reports were received at the time of the NEA report,
that part of the study was not complete.
With its limitations, the NEA study led to the following preliminary findings and recommendations:
• There is a need to understand better the markets and to increase communication with key actors.
Convincing all actors in the market (customers, suppliers, vendors, research institutes, and
government) is critical for the introduction of nuclear non-electricity products in the market. In
this regard, the establishment of some interest groups on nuclear applications in the market
would be valuable. Considering that a large part of non-electricity energy demand is in
developing countries and that developed countries would provide relevant technologies, it
would be better to have some connections between developed countries and developing
countries, especially in the case of nuclear desalination.
• According to the demand pattern in the individual market, the requirements for nuclear systems
will be different. For example, if demand for distributed rather than centralized hydrogen
production prevails, the development of small- and medium-sized reactors would be relevant.
The development of nuclear non-electricity product technologies, in turn, may affect the shaping
of the demand pattern through providing timely attractive options. In this connection, it is
essential for the nuclear energy sector to be involved and participate actively in the discussions
on non-electricity applications including the hydrogen economy.
• For successful penetration in a market, early demonstration is critical. Especially, the long-term
prospects for alternative technology options for hydrogen generation will depend to a certain
extent on early demonstration of feasibility and viability. For instance, nuclear-assisted steam
27
methane reforming could be pursued as an early nuclear option, which will facilitate further
market penetration of more innovative nuclear hydrogen production technologies (see
Chapter 6).
• In the light of recent trends in energy markets, strong competition is expected between
alternative options to supply non-electricity products. For example, there are many technical
options for hydrogen production and a large number of technologies, such as steam reforming of
methane, steam-coal gasification with sequestration of CO
2
, and solar photovoltaic processes,
are already available or under development. Nuclear non-electricity production technology
should be ready for deployment and competitive within a deregulated market. Regardless, the
demand for relevant reactors is unlikely to be comparable to the mass reactor orders that
occurred in the 1960s and 1970s.
• The development of nuclear non-electricity product technologies, especially hydrogen
production technologies, requires long-term commitment with many uncertainties. All
stakeholders in government bodies and the industry have a role to play for the eventual success
of relevant technologies. Coordination and joint efforts are essential in organizing R&D
programmes, infrastructure building, and policy making to address the challenges of developing
competitive and efficient technical processes.
• In countries wishing to rely on nuclear energy in the long term, the role of governments for the
emergence of non-electrical applications of nuclear energy is important in terms of basic R&D,
initial technology development and demonstration, and policy making to create a favourable
business environment without interfering with market mechanisms.
• International cooperation is essential to ensure the design and implementation of nuclear
systems efficient for non-electrical applications of nuclear energy such as hydrogen production.
In particular, the efforts required in the field of nuclear R&D and infrastructure building, are
likely to be beyond an individual country’s capabilities. In this context, undertakings such as the
Generation IV International Forum (GIF) and the International Partnership for the Hydrogen
Economy (IPHE), for example, can enhance the synergy between national programmes and the
effectiveness of the overall efforts.
1.5. Impact of externalities on cost of power from fossil/ nuclear/ renewable sources [9]
It is now generally recognized that the production and consumption of energy and related activities is
linked to a wide range of environmental and social problems such as the health effects of pollution of
air, water, and soil; ecological disturbances and species loss; and landscape damage. The costs of such
damages are referred to as external costs or externalities.
An externality arises when the social or economic activities of one group of persons have an impact on
another group and when that impact is not fully accounted or paid for by the main actors causing the
damages. In the particular case of energy production, fuel cycle externalities are the costs imposed on
the society and the environment that are not accounted for (i.e., not integrated in the market pricing
system) by the producers and consumers of energy.
The term fuel cycle refers to the chain of processes linked to the generation of electricity from a given
fuel. A comparative evaluation has been made for the following technologies and fuel cycles [9]:
• Fossil fuels: coal and oil technologies, with varying degrees of flue gas cleaning, natural gas,
centralized systems and CHP etc.;
• Nuclear: a PWR, and associated fuel cycle services, with and without reprocessing;
28

• Renewables: solar, on-shore and off-shore wind, hydro-electricity, a wide range of biomass
fuels.
Comparisons of damage costs/kWh for various technologies in EU countries are presented in
Table 1.12.
Table 1.12. External costs of electricity production in the EU from
existing technologies [9] (10
-2
$/kW.h*)
Country Coal and
lignite
Oil Gas Nuclear Biomass Hydro Solar PV Wind
Austria
1.3 to 3.8 2.5 to 3.8 0.13
Belgium
5.1 to 19 1.3 to 2.5 0.64
Denmark
5.1 to 8.9 2.5 to 3.8 1.3 0.13
Finland
2.5 to 5.1 1.3
France
8.9 to 12.7 10.2 to 14.0 2.5 to 5.1 0.38 1.3 1.3
Germany
3.8 to 7.6 6.4 to 10.2 1.3 to 2.5 0.25 3.81 0.76 0.063
Greece
6.4 to 10.2 3.8 to 6.4 1.3 0 to 1.01 1.3 0.32
Ireland
7.6 to 10.2
Italy
3.8 to 7.6 3.8 to 7.6 0.38
Netherlands
3.8 to 5.1 1.3 to 2.5 0.89 0.64
Portugal
5.1 to 8.9 1.3 to 2.5 1.3 to 2.5 0.038
Spain
6.4 to 10.2 1.3 to 2.5 3.8 to 6.4** 0.25
Sweden
2.5 to 5.1 0.38 0 to 0.89
United
Kingdom
5.1 to 8.9 3.8 to 6.4 1.3 to 2.5 0.32 1.3 0.19
* Sub-total of quantifiable externalities (global warming, public health, occupational health, and material
damage); on the basis of 1€ = $1.27.
** Biomass co-fired with lignite.
Table 1.12 leads to the following conclusions:
• External costs are extremely site dependent.
• In general, wind technologies are most environmentally friendly with respect to greenhouse gas
pollutants and particles. However, not every site is appropriate for wind power generation,
which has other environmental costs, including noise.
29

• Nuclear power generates the lowest external costs after wind power, even when low-probability
accidents with high consequences are integrated into the calculation. These results are generated
for 0% discount rates. At a 3% discount rate, the external costs from nuclear are lower.
• Photovoltaic solar power is the cleanest technology regarding its immediate use. It has,
however, considerable life cycle impacts due to the fabrication of solar panels.
• Gas-fired technologies are relatively clean.
• Coal technologies are the worst in view of their high generation of CO
2
. They appear to have
high impacts due to primary and secondary aerosols.
Figure 1.3 shows an illustrative example of the power costs from various power plants for selected
sites in Germany, with 2010 technologies [9].
FIG. 1.3. External costs of power stations in Germany
(CO
2
=19 euros/t, 1 year of life lost = 50 000 euros) [9].
It is observed that for the fossil fuelled electricity systems, human health effects, acidification of
ecosystems, and potential global warming impacts are the major sources of external costs. Although
the analysed power plants are all supposed to be equipped with abatement technologies, the emissions
of SO
2
and NO
x
due to the subsequent formation of sulphate and nitrate aerosols leads to considerable
health risks.
External costs arising from the nuclear fuel cycle are significantly lower than those estimated for fossil
fuel cycles.
External costs from renewable fuel cycles and hydropower mainly result from the use of fossil fuels
for material supply and during the construction phase. External costs from current PV technologies are
higher than from nuclear and are close to those for gas fired plants.
30

Impacts from wind and hydropower cycles are the lowest.
Internalization of external costs into power costs
A logical and sustainable way to permit the choice between various technologies is to integrate the
external costs with the production costs of these technologies.
Taking the above external costs and current generating costs of electricity in Germany; one would thus
obtain the results shown in Figure 1.4. [9]
FIG. 1.4. Total costs of various electricity generating technologies in Germany [9].
It is clearly seen that the power generation costs for renewable energies, especially for solar energy,
are much higher than for fossil energies or nuclear energy. It is also obvious that the full integration of
external costs in the nuclear case would render it economically the most attractive option.
1.6. Conclusion
The advantage of nuclear energy in alleviating the risk of climate change will not favour market
penetration of nuclear products through advanced non-electric applications of nuclear power as long as
energy policies internalising the value of carbon and other pollutants are not implemented. National
policies on climate change vary from country to country, but very little has been done to credit nuclear
energy systems for their contribution to reducing green house gas emission. The entry into force of the
Kyoto Protocol in February 2005 does, however, create incentives that can benefit nuclear power,
depending on how they are translated into national policies. The Protocol’s emission trading
provisions effectively give a cash value to unused emission allowances in the Russian Federation, for
example, and the European Trading Scheme (ETS) creates incentives favourable to nuclear power in
at least those European countries where implementation policies are not specifically biased against
nuclear power.
In considering the deployment of nuclear energy for non-electric applications, challenges and
difficulties should not be overlooked; in particular, it should be acknowledged that a scientific
31
potential is not a technical reality and that competition will drive the choice of energy sources for each
application. Considering the potential requirements and technical capabilities of nuclear energy
systems, their non-electric applications seem rather promising. Moving from their potential to realities
is undoubtedly feasible, but will need time, investments, and policy measures to address a wide range
of techno-economic and socio-potential challenges.
If non-electric products of nuclear energy were to penetrate markets on a significant scale, the role of
nuclear energy in supply systems could change dramatically from a marginal player to a main
contributor. However, such an achievement will materialize only if considerable technical, economic,
social, and political challenges are overcome. This can require a long lead time ― up to several
decades for some applications.
Public acceptance is a major issue for nuclear energy. However, non-electric applications, for example
desalination or district heating, can play an important role in acceptance of the nuclear power by local
communities.
REFERENCES FOR CHAPTER 1
[1] INTERNATIONAL ATOMIC ENERGY AGENCY, Non-electric Applications of Nuclear
Energy, IAEA-TECDOC-923 (1997).
[2] INTERNATIONAL ATOMIC ENERGY AGENCY, Status of Non-electric Nuclear Heat
Applications; Technology and Safety, IAEA-TECDOC-1184 (2000).
[3] INTERNATIONAL ATOMIC ENERGY AGENCY, Introduction of Nuclear Desalination-A
Guidebook, TRS No. 400 (2000).
[4] INTERNATIONAL ATOMIC ENERGY AGENCY, Market Potential for Non-electric
Application of Nuclear Energy, TRS No. 410 (2003).
[5] OECD/NUCLEAR ENERGY AGENCY, Non-electricity Products of Nuclear Energy, OECD-
NEA Report (2004), published on NEA webpage:
http://www.nea.fr/html/ndd/reports/2004/non-electricity-products.pdf
[6] U.S. DOE Nuclear Energy Research Advisory Committee & GIF (2002), Generation IV
Roadmap: GIF-008-00.
[7] VERFONDEN, K. et al., Michelangelo Network recommendations of nuclear hydrogen
production, Int. J. Nuclear Hydrogen Production and Application, Vol 1, No 1, 2006 pp.68-78.
[8] INTERNATIONAL ATOMIC ENERGY AGENCY, Nuclear Power Reactors in the World,
IAEA RDS No. 2 (2006).
[9] INTERNATIONAL ATOMIC ENERGY AGENCY, Economics of Nuclear Desalination - New
Developments and Site-Specific Studies, Final Report of a Coordinated Research Project 2002-
2006, IAEA-TECDOC-1561 (2007).
32

CHAPTER 2
NUCLEAR DESALINATION
2.1. Opportunities
Water, energy and environment are essential inputs for the sustainable development of society. These
are, therefore, current national and international issues, and have been addressed by many fora. Recent
statistics show that currently 2.3 billion people live in water-stressed areas and, among them, 1.7
billion live in water-scarce areas, where the water availability per person is less than 1000 m
3
/year.
The situation is going to worsen further; statistics show that by 2025 the number of people suffering
from water stress or scarcity could swell to 3.5 billion, and 2.4 billion of them are expected to live in
water-scarce regions. Water scarcity is a global issue, and every year new countries are affected by
growing water problems [1].
Seventy percent of the earth is covered with water, but only 2.5% of that is fresh water. Nearly 70% of
this fresh water is frozen in the icecaps of Antarctica and Greenland. Most of the rest is in the form of
soil moisture or in deep inaccessible aquifers, or comes in the form of monsoons and floods that are
difficult to contain and exploit. Less than 0.08% of the world’s water is thus readily accessible for
direct human use, and even that is very unevenly distributed.
In the light of this, the Millennium Declaration by the UN General Assembly in 2000 set a target to
halve, by the year 2015, the world population who are unable to reach or to afford safe drinking water.
In Vision 21, the shared vision for Hygiene, Water Supply and Sanitation has a target to provide water,
sanitation and hygiene for all by 2025 [2].
Better water conservation, water management, pollution control and water reclamation are all part of
the solution to projected water stress. So too are new sources of fresh water, including the desalination
of seawater and brackish water. Desalination technologies have been well established since the mid-
20
th
century and widely deployed in the Middle East and North Africa. The contracted capacity of
desalination plants has increased steadily since 1965 and is now about 36 million m
3
/d worldwide [3],
as shown in Figure 2.1. The top line in the figure shows total operating and contracted capacity. The
bottom line in the figure shows just operating capacity. This capacity could provide the world’s
population roughly 6 litres a day per capita of fresh potable water. If this capacity were available to the
1.5 billion in the world without direct access to drinking water, it would provide approximately
20 litres/day each.
FIG. 2.1. Cumulative capacity of all land-based desalination plants (unit capacity > 100 m
3
/day)
(reprinted with the permission of the author) [3,6].
33
Large-scale commercially available desalination processes can generally be classified into two
categories: (a) distillation processes that require mainly heat plus some electricity for ancillary
equipment, and (b) membrane processes that require only electricity to provide pumping power. In the
first category (distillation) there are two major processes: multi-stage flash (MSF) and multi-effect
distillation (MED). In both, seawater is heated; the steam that evaporates is condensed and collected as
freshwater; and the residual brine is discharged. In the second category (membranes) is the reverse
osmosis process (RO), in which pure water passes from the high-pressure seawater side of a semi-
permeable membrane to the low-pressure freshwater permeate side. The pressure differential must be
high enough to overcome the natural tendency for water to move from the low concentration
freshwater side of a membrane to the high concentration seawater side, in order to balance osmotic
pressures.
The energy for these plants is generally supplied in the form of either steam or electricity using fossil
fuels. The intensive use of fossil fuels raises environmental concerns, especially in relation to
greenhouse gas emissions. The depletion of fossil sources and the future price uncertainty of fossil
fuels and their better use for more vital industrial applications is also a factor to be considered for
sustainability. Many countries deprived of valuable fossil resources are therefore considering the
introduction of a nuclear power program or expansion of their existing nuclear power program.
2.2. Market context [4]
In recent years, the option of combining nuclear power with seawater desalination has been explored
to tackle water shortage problems. The desalination of seawater using nuclear energy is a feasible
option to meet the growing demand for potable water. Over 175 reactor-years of operating experience
on nuclear desalination have been accumulated worldwide. Several demonstration programs of nuclear
desalination are also in progress to confirm its technical and economical viability under country-
specific conditions, with technical coordination or support of IAEA.
There are many reasons that favour a possible revival of nuclear power production in the years to
come: the development of innovative reactor concepts and fuel cycles with enhanced safety features
that are expected to improve public acceptance, the production of less expensive energy as compared
to other options, the need for prudent use of fossil energy sources, and increasing requirements to
curtail the production of greenhouse gases (GHGs). It is estimated that water production of 10 million
m
3
/d by seawater desalination using fossil fuels would release 200 million t/y of CO
2
, 200 000 t/y of
SO
2
, 60 000 t/y of NO
x
and 16 000 t/y of other hydrocarbons. For the current global desalting plant
capacity of 40 million m
3
/d the total emissions would be four times these values. This can be avoided
if nuclear or renewable energy sources are used for desalination. It is estimated that for producing
fresh water with the present desalination capacity, but by using nuclear energy, the needed nuclear
capacity would be about 40 1000 MWe nuclear reactors.
Using nuclear energy for the production of freshwater from seawater and brackish aquifers (nuclear
desalination) has been of interest in several IAEA Member States as a result of acute water shortage
issues in many arid and semi-arid zones worldwide. This stems from their expectation of not only its
possible contribution to the freshwater issue, but has also been motivated by a variety of reasons that
include: likely competitiveness of nuclear desalination in areas lacking cheap hydropower or fossil
resources, energy supply diversification, conservation of fossil fuel resources, and spin-off effects of
nuclear technology for industrial development.
2.2.1. Nuclear desalination market - past experiences and plans [4]
The desalination of seawater using nuclear energy is a demonstrated option having over 180 reactor-
years of operating experience worldwide, of which Japan now has over 150 reactor-years, with ten
nuclear power plants that also produce desalinated water. Kazakhstan (the Aktau fast reactor BN-350)
had accumulated 26 reactor-years of producing 80,000 m
3
/day of potable water before shutting down
in 1999. In the USA, the Diablo Canyon nuclear power plant produces desalinated water. Presently
34

India and Pakistan are setting up nuclear demonstration projects at their existing PHWRs. Operating
experience for all non-electric applications including desalination, district heating and process heat is
around 1000 reactor years (Table 1.2).
Table 2.1 summarizes past experience as well as current developments and plans for nuclear-powered
desalination using different nuclear reactor types. Most of the technologies in Table 2.1 are land-
based, but the table also includes a Russian initiative for barge-mounted floating desalination plants.
Floating desalination plants could be especially attractive for responding to emergency demands for
potable water.
Table 2.1. Reactor types used or considered for desalination
Reactor
Type
Location Capacity (m
3
/d) Status
LMFR
Kazakhstan (Aktau) 80,000 In service till 1999
PWRs
Japan (Ohi, Takahama, Ikata,
Genkai)
1,000-2,000 In service with operating
experience of over 150 reactor-
years
Rep. of Korea 40,000 SMART integral PWR is being
designed
Russia Floating Power Unit for
electricity and heat is under
construction; possible later units
could be used for electricity and
desalination
USA (Diablo Canyon) ~4500 In service
BWR
Japan (Kashiwazaki) Never in service following
testing in 1980s; owing to
alternative freshwater sources,
dismantled in 1999
HWR
India (Madras) 6,300 Under commissioning (RO
commissioned in 2002, MSF to
be commissioned by the end of
2007).
Pakistan (KANUPP) 4,800 Under construction, to be
commissioned by the end of
2007
NHR
China 120,000 Under design
HTGR
South Africa, France, The
Netherlands
Under consideration
35

Table 2.2 shows the operating nuclear desalination plants in Japan.
Table 2.2. Operating nuclear desalination plants in Japan
Plant
name
Location
Application
Start of
operation
reactors /
desal.
Net
Power
(MW(e))
Water
capacity
(m
3
/d)
Remarks
Ikata-1,2 Ehime Electricity/
desalination
1977-82
1975
566 2000 PWR/MED,
MSF
Ikata-3 Ehime Electricity/
desalination
1994
1992
566 2000 PWR/MSF
(2 x 1000 m
3
/d)
Ohi-1,2 Fukui Electricity/
desalination
1979
1973-76
1175 3900 PWR/MSF
(3 x 1300 m
3
/d)
Ohi-3,4 Fukui Electricity/
desalination
1991-93
1990
1180 2600 PWR/RO
(2 x 1300 m
3
/d)
Genkai-4 Fukuoka Electricity/
desalination
1997
1988
1180 1000 PWR/RO
Genkai-3,4 Fukuoka Electricity/
desalination
1995-97
1992
1180
1000
PWR/MED
Takahama Fukui Electricity/
desalination
1985
1983
870 1000 PWR/RO
The salient features of the existing and proposed nuclear desalination demonstration projects and a
number of feasibility studies conducted by interested Member States [5] are given in Annex 1.
2.2.2. Economics [3]
Over the years, the cost of water produced in seawater desalination plants has dropped considerably,
but the cost of water produced in conventional treatment plants has risen, due to over-exploitation of
aquifers, intrusion of saline water in coastal areas, and generally increasing contamination of ground
water. Fig 2.2 shows the water costs from global seawater desalination and conventional production
(in various countries). Seawater desalination costs are already comparable to conventional water costs
in water scarce/starved countries and are likely to approach each other even in the countries having
cheap, abundant water sources. This makes the prospects of seawater desalination quite promising.
36

FIG. 2.2. Development of water costs (reprinted with the permissionof the author) [3].
Fig. 2.3 shows the capacity of desalination plants in various countries. As can be seen, the major
contribution comes from the Middle East countries, followed by US, Spain, and the Caribbean
countries. In recent years, plans for large-scale deployment of desalination in Asia and Pacific
countries have been reported.
Presently, the total capacity of desalination plants worldwide is of the order of thirty-six million cubic
meters/day. The major contribution comes from the Middle East countries.
Desalination processes are energy intensive, and energy is the major cost component of the water
produced. As most of the current desalination is based on fossil energy sources, the cost of desalted
water varies with the prevailing fuel costs in particular areas. Apart from the fossil fuel cost and its
availability, the associated environmental concerns of late have kept in abeyance the launching of
some large-scale desalination projects. This has led to a search for renewable and other sustainable
energy sources including nuclear.
There are no officially reported cost data from existing nuclear desalination plants. Several feasibility
studies, however, have been carried out by the Member States under the IAEA’s coordinated research
projects and technical cooperation programmes. Some results are reported in IAEA-TECDOC-1561
[6].
Preliminary techno-economic studies conducted in China for the NHR-200 reactor coupled to a
160,000 m³/d vertical-tube evaporator multi-effect distillation (VTE-MED) plant estimated the
desalted water cost to be around 0.68 US$/m³. A similar economic evaluation of the integrated
SMART-MED desalination plant of 40,000 m³/d capacity indicated the water cost ranging from 0.70
to 0.90 US$/m³. The projected cost of water from the Russian KLT-40 floating reactor based nuclear
desalination plants is also in these same ranges. These costs are comparable with desalination costs
using locally available fossil fuels.
Pre-feasibility studies have been carried out recently for the proposed nuclear desalination projects at
Madura, Indonesia, and La-Skhira, Tunisia, under an IAEA technical cooperation inter-regional
project (1999-2004). These indicate economic competitiveness of nuclear desalination over fossil-
based plants under the specific conditions in their countries.
37

Despite large interest of the Member States which are considering deployment of nuclear desalination
plants and IAEA’s efforts in bringing about information exchange among the technology providers
and the user countries, no significant progress is reported in the deployment of nuclear desalination
plants. Today nuclear desalination contributes only 0.1 % of total desalting capacity worldwide.
Economic comparison with fossil desalination
Table 2.3 provides some comparative water costs of present-day fossil-based desalination plants and
projected costs of water from nuclear desalination projects from recent feasibility studies in Member
States.
Table 2.3. Water costs from fossil desalination plants and estimated costs
from nuclear desalination
Country Capacity (m
3
/d) Process Water costs ($/m
3
)
Fossil based
Singapore 135,000 RO 0.45
Ashkelon (Israel) 165,000 RO 0.52
Al-Taweelah (UAE)
Fujairah
237,500
375,000
MED
MSF-RO
0.70
0.80
Nuclear based
Argentina (CAREM) 12,000 RO 0.72
China (NHR-200) 160,000 MED 0.68
Rep. of Korea
(SMART)
40,000 MED 0.80
The projected costs for nuclear desalination appear to be marginally higher than the actual costs from
present-day commercial desalination plants using fossil sources. Various aspects of cost reduction
strategies in nuclear desalination are presently being proposed to make it more competitive.
Table 2.4 shows the desired objectives to be achieved for producing potable water economically from
nuclear desalination plants [7].
38

Table 2.4. Desired objectives for economic costs
Reactor cost $/kWe 2000 – 1000 $/kWe
construction time
lifespan
electricity cost
60 - 40 months
40 - 60 years
0.06 – 0.04 $/kWh
Desalination plant cost 1500-800 $/m
3
plant life
construction time
water cost
> 30 years
24 months
0.80 – 0.45 $/m
3
Variables reactor size
plant capacity
seawater salinity
temperature
40 -600 MWe
50,000 – 200,000 m
3
/
d
30,000 – 45,000 ppm
18 – 35
o
C
The broader picture, however, is that the worldwide use of desalination is still negligible compared to
the demand for fresh water. To become a noticeable market for nuclear energy, desalination needs to
compete successfully with alternative means of increasing fresh water supply. For nuclear desalination
to be attractive in any given country, two factors must be in place simultaneously: a lack of fresh water
and the ability to use nuclear energy for desalination. In most regions, only one of the two is present.
Both are present for example in China, the Republic of Korea and, even more so, in India and
Pakistan. These regions already account for almost half the world’s population, and thus represent a
potential long-term market for nuclear desalination.
2.3. Challenges [8]
The following sections describe the key challenges facing nuclear desalination.
2.3.1. Economics
Economic comparisons indicate that water costs (and associated electricity generation costs) from
nuclear seawater desalination are generally in the same range as costs associated with fossil-fuelled
desalination at their present costs. Given the conclusion that nuclear and fossil-fuelled desalination are
broadly competitive with each other, any particular future investment decision will depend on site-
specific cost factors and on the values of key parameters (capital cost, fuel price, interest rate,
construction time, etc.) at the time of investment. Higher fossil fuel prices would, of course, favour
nuclear desalination; higher interest rates would favour less capital-intensive fossil-fuelled options.
2.3.2. Infrastructure development
Those countries suffering from scarcity of water are, generally, not the holders of nuclear technology,
do not generally have nuclear power plants, and do not have a nuclear power infrastructure. The
utilization of nuclear energy in those countries will require infrastructure building and institutional
arrangements for such things as financing, liability, safeguards, safety, and security and will also
require addressing the acquisition of fresh fuel and the management of spent fuel. The concept of
multi-national fuel cycle centres as are being examined by IAEA could be used to assure a supply of
nuclear material to legitimate would-be users under control of sensitive parts of the nuclear fuel cycle.
2.3.3. Public perception
The design of nuclear desalination plants address various aspects related to nuclear plant safety. The
possibility of radioactive contamination of product water is also an important issue to be considered
39
for nuclear desalination plants. The dissemination of data from existing facilities that carry out nuclear
desalination would help to alleviate the concern and improve the public perception for nuclear
desalination plants.
2.3.4. Socio-environmental aspects
The socio-environmental aspects of nuclear desalination need greater attention for its large-scale
adoption. Setting up a desalination plant at nuclear reactors for providing much-needed fresh water to
the public will no doubt add to social acceptance of nuclear desalination, if the quantity and quality of
the fresh water are consistently assured.
The intakes/outflows of nuclear desalination plants must be designed to assure the continued use of
areas for fishing and other socio-cultural activities. Protection of the marine environment near the
desalination plant site, particularly the flora and fauna, needs to be assured. The use of the reject brine
from the desalination plants for pisciculture or other uses such as the production of useful minerals is a
possibility worth consideration [9].
Studies have reported on the significant reduction in water cost if a carbon tax were considered in the
future if nuclear is accepted as a Clean Development Mechanism under the Kyoto Protocol. The
environmental impact assessment of nuclear-powered desalination systems further indicates
advantages over fossil-based energy sources. These would result in enhanced economic
competitiveness of nuclear desalination plants.
2.4. Solutions [10]
2.4.1. Utilization of waste heat from nuclear reactors
2.4.1.1. Utilization of waste heat from the condensers of LWRs and HWRs for pre-heat RO (the
ROph process)
The net electrical efficiencies of the power conversion systems in most water-cooled reactors are of
the order of 30 to 33%. This means that nearly two thirds of the net thermal power produced in the
reactors is evacuated to the heat sink through the condensers. The temperature of the water from the
condensers is too low (30 to 32°C) for meaningful desalination with distillation processes. However,
this relatively hot water can be fed to an innovative variant of the RO process, with preheating now
known as the ROph process. In hybrid systems, it is also possible to use the cooling seawater return
stream from the thermal desalination component as feed to the RO component.
The viscosity of the feedwater is inversely proportional to its temperature. Thus, as temperature
increases, water viscosity decreases and RO membranes become more permeable, with a consequent
increase in production.
CANDESAL first developed an advanced reverse osmosis (RO) desalination system that emphasizes a
non-traditional approach to system design and operation. Key features of this advanced approach to
RO system design and operation are the use of preheated feedwater, operation at high pressures,
advanced feedwater pre-treatment, advanced energy recovery systems, site-specific optimization, and
automatic real-time plant management systems (Fig 2.4 and 2.5).
40

0.6
0.7
0.8
0.9
1
1.1
1.2
20 25 30 35 40 45 50
Temperature, C
Relative Water Production
62.1 bar (900 psi)
69 bar (1000 psi)
FIG. 2.4. Water production vs. temperature[10].
50
55
60
65
70
75
10 15 20 25 30 35 40 45
Feed water temperature, C
Required feed
pressure, bar
35K
38K
43K
45K
R, %
40
40
45
50
FIG. 2.5. Required feed pressure vs. salinity and temperature [6]. Here, R is the percentage of
recovery of fresh water from seawater and the curves represent different salinity levels of seawater,
from 35,000 ppm to 45,000 ppm.
As can be seen from Fig 2.4, there is a 2-3 % increase in water production per degree rise in seawater
feed temperature. This helps in reducing the applied pressure and hence the pumping power with
rising temperature (Fig 2.5). Thus, an energy saving of nearly.10% is achievable in pre-heat RO
plants. An important consideration in ROph is that it can easily use the hot water from the main
condensers of the water-cooled nuclear power plants.
The amount of feedwater preheating depends both on the ambient seawater temperature and the
specifics of the nuclear reactor design. The only limitation is that the maximum temperature allowed
by the RO membrane design limits must not be exceeded. Currently available RO membranes
typically have a limit of about 45°C, though this is expected to increase as membrane performance
continues to be improved by the manufacturers. Cost savings are possible at all temperatures where
waste heat can be used to preheat the feedwater, but overall savings depend on a number of factors
that are site specific: the salinity of the feedwater, the size of the plant, the amount of preheat
available, etc.
2.4.2. Waste heat utilization from Indian PHWRs for thermal desalination
2.4.2.1. Research reactor CIRUS
For conducting a practical demonstration of waste heat utilization, BARC (India) designed a low-
temperature vacuum evaporator (LTE) desalination plant and coupled it to the research reactor
CIRUS.
41

The product water from this plant meets the make up water requirements of the reactor. The reactor
produces 40 MWth using metallic fuel, a heavy-water moderator, demineralized water coolant, and
seawater as the secondary coolant. To ensure protection against radioactive contamination, an
intermediate circuit has been incorporated between the reactor and the LTE plant. Table 2.5
summarizes the operating data of this plant. Such experience could be used for a larger sized plant
utilizing waste heat.
Table 2.5. Typical operating data of the CIRUS reactor providing waste heat for desalination
Parameter Unit Value
Hot water flow rate liters/minute 1500
Hot water inlet temperature °C 53.6
Hot water outlet temperature °C 47.5
Seawater flow rate liters/minute 1200
Seawater TDS ppm 35 000
Seawater inlet temperature °C 27.6
Seawater outlet temperature °C 35.5
Vacuum in the evaporator mm Hg 700
Product water flow rate L/minute 15.5
Product water conductivity µS/cm 7
2.4.2.2. Waste heat utilization from the 500 MWe PHWR
In the 500 MWe Indian PHWR, the heavy-water moderator is cooled from 80 to 55 °C by process
water, which in turn is cooled to 35 °C by seawater that enters at 32°C and comes out at 42°C. About
100 MWth is thus available as waste heat for seawater desalination.
The details have been worked out using 55°C process water temperature, providing heat to the
desalination process, to avoid any changes in the moderator system. The coupling scheme is presented
in Figure 2.6.
The nuclear desalination system produces about 1000 m
3
/day of desalinated water, which is about 25%
more than the total makeup demineralized (DM) water requirements of the 500 MWe PHWR.
It is considered more economical to use this water as make up DM water because:
• The energy cost for the LT-MED plant is essentially zero, since it only uses waste heat;
• Direct production of distilled water eliminates the need for demineralizers and regeneration
chemicals;
• The raw water, otherwise used as feed for the DM plant, can be made available for other
purposes such as for drinking.
42

FIG. 2.6. PHWR500 coupling scheme, utilizing waste heat (reprinted from IAEA-TECDOC-1561 [6]).
2.4.3. Utilization of hybrid systems
The advantages of hybrid desalination systems will be illustrated by a specific example: that of the
hybrid MSF-RO system coupled to the MAPS PHWR at Kalpakkam (India) as shown in Figure 2.7.
As one of the leading and oldest desalination processes, MSF is often preferred because of its
operational simplicity and proven performance. MSF is advantageous for large desalting capacities
and high purity water, in particular where inexpensive thermal energy is available.
However, its installed cost and specific power consumption remain relatively high. Since the energy
cost is high in India, an MSF system, with a large gain output ratio (GOR) leading to lower water
production costs, has been chosen.
55
o
C
35
o
C
Sea Water In
1700 kg/s, 32
o
C
72.5
o
C
49
o
C
Heavy Water
972 kg/s
Process Water
1215 kg/s, 32
o
C
Sea Water In
720 kg/s, 32
o
C
Sea Water Out, 42
o
C
55
o
C
Feed
30 kg/s
Blow down
17.5 kg/s
49
o
C
45
o
C
Sea Water Out
42
o
C
CONDENSE
45
o
C
Product Water
12.5 kg/s, 44.5
o
C
1
1
2
3
4
1. Moderator heat exchanger 2. Process water heat exchange
3. Desalination Unit 4. Product water pump
N
ote: When the desalination unit is shut down, the sea water
required for the process water heat exchanger is 2430 kg/s at 32
o
C
43

FIG. 2.7. Hybrid MSF-RO coupling to the PHWR at Kalpakkam, India
(reprinted from IAEA-TECDOC-1561 [6]).
Seawater desalination by RO has proved to be most economical, as has been shown in the case studies
from IAEA Member States. Apart from its need for an elaborate pre-treatment plant, RO has many
advantages:
• Enhanced flexibility due to its modular structure;
• Operation at ambient temperature, reducing corrosion risks;
• Possibility of coupling with energy recovery devices, thus further reducing the costs;
• Potential for further innovations as compared to the MSF technology, which has almost reached
a saturation point in its development.
Because of the particular advantages of MSF and RO technologies, it is logical to consider that a
hybrid MSF-RO system may lead to greater cost reductions in water costs because of:
- The use of common, smaller seawater intake and outfall structures and other facilities;
- Flexible and improved water quality by blending distillate from the MSF plant and the
permeate from the RO plant;
- Extension of membrane lifetimes as a result of blending.
44
Awerbuch has reported that applying a hybrid solution reduces desalinated water costs, compared with
non-hybrid schemes, from as little as 2-3% to as much as 15% [11].
2.5. Conclusion
Seawater desalination provides a source of fresh water, especially for the water scarce arid and semi-
arid areas of the world. The present desalination capacity of about 36 million cubic meters per day
worldwide meets a very small fraction of the world’s fresh water needs. There is, however, a
significant potential of desalination and water reuse technology for rapid expansion to augment the
fresh water resources in the water scarce areas. Use of fossil fuels for large-capacity desalination
plants could lead to large emission of undesired greenhouse gases. The price uncertainties of fossil
fuels and their sustainability is also an important issue. Use of energy from nuclear reactors for
desalination is a demonstrated option; it is environmentally friendly and can be a sustainable energy
source. Feasibility studies carried out recently indicate that present costs of water produced from
nuclear desalination plants are similar to those of fossil fuel based desalination plants. The
development of higher-temperature and more economical reactor designs would likely reduce the
desalinated water cost. Nuclear desalination will be an important option for safe, economic and
sustainable supply of large amounts of fresh water to meet the ever-increasing worldwide water
demand.
REFERENCES FOR CHAPTER 2
[1] SOMMARIVA, C., Desalination & Water Reuse Quarterly, Vol.13, No.2, (2003), pp. 13-16.
[2] UNESCO, Water for People Water for Life, The United Nations World Water Development
Report, UNESCO Publishing (2002).
[3] WANGNICK, K., IDA World’s Desalting Plants Inventory, Report No. 18 (2004).
[4] MISRA, B.M., Role of Nuclear Desalination in Meeting the Potable Water Needs in Water
Scarce Areas in the Next Decades, Desalination 166 (2004) pp. 1-9.
[5] INTERNATIONAL ATOMIC ENERGY AGENCY, IAEA INDAG Newsletters, Issues 5 & 6,
Vienna (2005, 2006).
[6] INTERNATIONAL ATOMIC ENERGY AGENCY, Economics of Nuclear Desalination -
New Developments and Site-Specific Studies, Final Report of a Coordinated Research Project
2002-2006, IAEA-TECDOC-1561 (2007).
[7] MISRA, B.M., Status and Prospects of Nuclear Desalination, IDA World Congress on
Desalination and Water Reuse, Singapore (2005).
[8] MISRA, B.M., Status and Prospects of Nuclear Desalination, Atom for Peace- An International
Journal, Vol. 1, No. 2-3 (2006) pp. 216-226.
[9] NISAN, S., Extraction of Strategic Materials from the Concentrated Brine Rejected by
Integrated Nuclear Desalination Systems, Desalination, Vol 182, pp. 451-462, (2005).
[10] INTERNATIONAL ATOMIC ENERGY AGENCY, Status of Nuclear Desalination in IAEA
Member States, IAEA-TECDOC-1524, Vienna (2006).
[11] AWERBUCH, L., Hybridization & Dual Purpose Plant Cost Considerations, MEDRC Int. Conf.
on Desalination Costing, Limassol (2004) pp. 204-230.
45
CHAPTER 3
DISTRICT HEATING
3.1. Opportunities
Assessing realistic future demand for energy products other than electricity is not an easy task. Even
for heat demand, statistical data available are by far less exhaustive and robust than is the case for
electricity. Furthermore, as far as district heating is concerned, the heat distribution structure and
human settlement configuration are key driving factors that are difficult to predict.
District heat involves the supply of space heating and hot water through a district heating system,
which consists of heat plants (usually producing electricity simultaneously) and a network of
distribution and return pipes. A potential market for district heating appears in climatic zones with
relatively long and cold winters. In many countries, such as central and northern European countries
and countries in transition economies, district heat has been widely used for decades.
District heating accounts for 11% of total energy consumption in Central Europe and Ukraine and over
30% in Russia and Belarus. District heating accounts for almost half of the heat market in Denmark,
Estonia, Finland, Poland, Romania and Sweden. District heating networks generally have installed
capacities in the range of 600 to 1200 MWth in large cities to approximately 10 to 50 MWth in towns
and small communities [1]. Although a large number of district heat suppliers, especially in countries
with transition economies, are facing financial and technical problems, nuclear-based district heat is
still expected to have great additional potential for meeting a share of heat demand in many countries
that are currently using fossil fuels and are considering other sources owing to environmental
concerns.
In general, district heating can offer significant benefits because, under certain conditions, it can
compete economically in densely populated areas with individual heating arrangements, and because it
offers the possibility of reducing air pollution in urban areas. Whereas emissions resulting from the
burning of fuels can be controlled and reduced up to a point for relatively large centralized plants, this
is not practical in small individual heating installations fuelled by gas, oil, coal or wood.
Although it is hard to obtain exact statistics for the current use of district heat in the world, the size of
its market can be estimated in relation to the final energy demand in the residential, agricultural and
commercial sector. A 2002 IAEA report [2] took this approach and calculated the total use of district
heat in 1996 as 119.5 Mtoe based on the International Energy Agency (IEA) world energy database,
which requires a heat production capacity of 340 000 MWth assuming an average load factor of 50%.
District heating has the following technical requirements:
• It requires a heat distribution network to transport steam or hot water in a typical temperature
range of 80-150°C.
• Owing to higher losses over longer transmission distances, the heat source must be relatively
close to the customer, typically within 10-15 km.
• The district heat generation capacities are determined by the collective demands of the
customers. In large cities a capacity of 600-1200 MWth is normal. The demand is much lower
in small communities.
• The annual load factor is normally not higher than 50%, since heat is supplied only in the colder
part of the year.
• To secure a reliable supply of heat, a backup capacity is required.
46
Typically, coal and gas dominate the fuels used for district heating. Various other heat sources are also
used for district heating, including biomass materials, waste incineration, and waste heat from
industrial processes. Usually district heating is produced in a cogeneration mode in which waste heat
from electric power production is used as the source of district heat.
There is plenty of experience in using nuclear heat for district heating, so the technical aspects can be
considered well proven. There are no technical impediments to the application of nuclear reactors as
the heat source for district or process heating. Several countries already have experience in nuclear
district heating: Bulgaria, Hungary, Romania, Russia, Slovakia, Sweden, Switzerland and Ukraine.
For production of district or process heat, there are basically two options: Co-generation of electricity
and heat, and heat-only reactors. All existing reactor types can be used for cogeneration of heat and
electricity. Co-generation has been widely applied; there is not much experience with heat-only
reactors. In principle, any portion of the heat can be extracted from co-generation reactors as district
heat, subject to design limitations. Co-generation plants, when forming part of large industrial
complexes, can be readily integrated into an electrical grid system to which they supply any surplus
electricity generated. In turn, they would serve as a backup for assurance of the electricity supply. This
means a high degree of flexibility. Heat-only reactors, on the other hand, have only the objective of
heat production, not electricity generation. This limits their operational flexibility, especially during
warm weather, leaving the large capital investment stranded during times when heat is not needed.
As shown by experience, availability factors of 70% to 80% or even 90% can be achieved. These
values are similar to the availabilities achieved by fossil fuelled power plants. The frequency and
duration of unplanned outages can be kept very low with good preventive and predictive maintenance.
The availability of nuclear or fossil fuelled plants, however, can never reach the nearly 100% levels
required by most large heat users. Therefore, multiple-unit co-generation power plants, modular
designs, or backup heat sources are necessary to achieve the required availabilities.
Nuclear reactors are proven, safe, reliable and environmentally clean energy sources, but for
commercial deployment they also have to be economically competitive with alternative energy
sources. Compared to fossil fuelled sources, nuclear reactors are characterized by higher investment
costs compensated by lower fuel costs. With increasing fossil fuel prices the economically competitive
position of nuclear power, both for electricity generation and for heat supply, improves.
Due to economy of scale [3], nuclear economics are, in general, improved for larger units. This has led
to the development and predominant deployment of large reactors in industrialized countries with
large interconnected electrical grid systems. Nevertheless, there continues to be an interest in small
and medium-sized power reactors (SMRs), especially for applications other than base load electricity
generation.
The siting of nuclear plants is another issue. For co-generation or heat-only reactors, close location to
the load centres has a strong incentive. However, the trend is to choose remote, but accessible,
locations for siting nuclear plants in order to mitigate the consequences of an accident. Locating a new
plant far from densely populated areas makes it easier to comply with regulatory requirements.
Furthermore, plants need to be located near a ready supply of cooling water, which may not
necessarily correspond to the location of the population centre.
3.2. Market context
3.2.1. Early history of nuclear district heating markets [4]
Dedicated nuclear heating systems have been designed, built and operated in China and in the Russian
Federation. The plant in China is for demonstration purposes, whereas the Russian plants supply heat
to various settlements in the northern parts of the country.
47
A 5 MW(th) test nuclear heating reactor (NHR-5) was commissioned in China and has been in
operation since 1989, supplying heat to the Institute of Nuclear Energy Technology of Tsinghua
University, near Beijing. The Russian Federation has operated an experimental 10 MW(th) heating
reactor at Obninsk since 1954 and has developed the technology of the nuclear district heating reactor,
AST-500.
Nuclear co-generation plants for electricity and district heating have been built and operated in
Bulgaria, Hungary, Romania, Russian Federation, Slovakia and Switzerland. Almost 500 reactor-years
of successful operational experience have been accumulated.
The Kozloduy NPP in Bulgaria has supplied heat to the town of Kozloduy since 1990. The Kozloduy
NPP originally consisted of four WWER-440 reactors of 408 MW(e) and two WWER-1000 reactors
of 953 MW(e). However, the four smaller reactors had to be shut down as a condition for Bulgaria’s
accession to the European Union. No relevant problems with district heating have been experienced.
The Paks Nuclear Power Plant (Hungary), consisting of four units of the Soviet design WWER-440
type V-230, is supplying heat to the town of Paks. The water pressure in the heat exchanger is kept
higher than the steam pressure to prevent contamination of the hot water system.
The Cernavoda Nuclear Power Plant in Romania has supplied heat to the town of Cernavoda since the
plant began commercial operation in 1996.
The Bohunice Nuclear Power Plant in Slovakia produces electrical energy and low-temperature heat
for heating and industrial purposes. The heat supply from the nuclear power plant is used for the town
of Trnava.
The district heat extraction from the Beznau NPP (Switzerland (2 × 360 MW(e) PWR) has been
operated reliably and successfully since its commissioning in 1983/84. The peak heat load is about 80
MW(th), which is equivalent to about 10 MW(e) of electric power. The district heating system
supplies about 2100 private, industrial and agricultural consumers through 35 km of main piping and
85 km of local distribution pipes.
The most extensive experience with district heat supply from nuclear co-generation plants has been
gained in the Russian Federation. The NPPs of Bilibino, Belojarsky, Balakovo, Kalinin, Kola, Kursk
and Leningrad are supplying heat from steam turbine bleeders through heat exchangers to district
heating grids of towns with typically about 50 000 inhabitants, situated between 3 and 15 km from the
NPP sites. The heat output capacities range from about 50 to 230 MW(th).
Table 3.1 shows the worldwide experience in nuclear district heating.
48
Table 3.1 Operating nuclear heating plants
Country
Plant type or
name
Location Application Phase
Start of reactor
operation
Net
Power
(MW(e))
Bulgaria Kozloduy 5,6 Kozloduy Electricity/
District heating
Commercial 1989 2 x 953
China NHR-5 Beijing District heating Experimental 1989
1989
-
Czech Rep. Temelin 1,2 Temelin Electricity/
District heating/
Process heat
Commercial 2002 2 x 930
Hungary PAKS 2,3,4
Paks Electricity/
District heating
Commercial 1984 1 x 441
1 x 433
1 x 444
India Rajasthan
1,2,3,4
Rajasthan Process heat Commercial 1 x 90
1 x 187
2 x 202
Romania HWR
CANDU-6
Cernavoda –
Unit 1
Electricity/
District heating
Commercial 1998 1 x 660
Russia WWER-1000 Novovoronezh Electricity/
District heating/
Process heat
Commercial 1971-73 3 x 385
1 x 950
Russia WWER-1000 Balakovo Electricity/
District heating/
Process heat
Commercial 1986-93
4 x 950
Russia WWER-1000 Kalinin Electricity/
District heating/
Process heat
Commercial 1985-87 2 x 950
Russia WWER-440 Kola Electricity/
District heating/
Process heat
Commercial 1973-84 4 x 411
Russia LWGR Bilibino Electricity/
District heating
Commercial 1974 4 x 11
Russia LWGR St. Petersburg Electricity/
District heating/
Process heat
Commercial 1974-81 4 x 925
Russia LWGR Kursk Electricity/
District heating/
Process heat
Commercial 1977-86 4 x 925
Slovakia Bohunice-3,4 Bohunice/Trnava Electricity/
District heating/
Process heat
Commercial 1987 2 x 408
Switzerland Beznau 1,2 Beznau Electricity/
District heating
Commercial 1985, 1989 1 x 365
1 x 357
49
3.2.2. Summary of some specific district heating systems of interest
The details of the world’s first district heating plant at Stockholm, Sweden, and the latest district
heating plant at Cernavoda, Romania, are given in Annex 2. Their salient features are presented below,
along with information on the Russian floating reactor system.
3.2.2.1. Swedish district heating [5,6,7]
The Swedish district heating system is well developed. About half of the heating of homes and houses
is done by means of district heating. Two hundred and seventy of the country’s 290 communities have
district heating networks. All cities and towns with more than 10,000 inhabitants have district heating
networks. Very many of the smaller cities and towns have district heating networks as well.
The total Swedish district heating system uses about 60 TWh of energy to produce 48 TWh of heat
and 6 TWh of electrical power, which leads to an overall system efficiency of about 90%. The fuel
that is used is 45% biomass, 15% fossil, 15% electricity, 10% waste, 10% residual heat, and 5%
miscellaneous.
Typically a Swedish district heating network is a hot water system with a hot temperature of 70 to
110
o
C, depending on the time of the year, and a return temperature 40 to 50
o
C lower. The distribution
is done by means of pumps at the heat and power plants, but also by means of distributed pumps for
the larger networks.
The following figures on production and economics are based on statistics from 2003-2005.
About 570 towns and villages in the country have district heating networks and the growth potential is
still considered to be high. The total yearly heat delivery is about 50 TWh. The growth rate is
estimated to be about 10 TWh by 2010. In the long run, district heating is judged to represent about
75% of the total Swedish heating need.
To reach the goal of 60 TWh of district heat in 2010 the investment is estimated to be about 500
million euros per year. This will also provide some electricity generation capacity. The domestic
environmental gain is estimated to be a reduction of about 3 Mton of CO
2.
. Because the expansion in
Swedish district heating saves imported electricity from coal fired plants in other countries, the
reduction in CO
2
release abroad is estimated to be an additional 2 Mton.
The investments in the coming years are divided in the following way:
• Heat and power generation: 300 million euros.
• Distribution network: 150 million euros.
• Distribution heat exchange centrals: 50 million euros.
The total sales income to the district heating companies is about 2000 million euros. The average
income per kWh per year is about 4 cents. The range of income per kWh based on the different
heating companies was from 3 cents up to 7 cents.
The economics for different district heating utilities varies widely and results in large price differences
for end consumers, compared with other heating sources. Prices for heat for end consumers are often
split into a fixed contract, based on demanded power, and a price per kWh.
50

Contract:
• For an installation of < 500 kW capacity, the customer pays 100 euros per year fixed price;
• For an installation from 500 kW and up to 3000 kW capacity, the customer pays 10 000 euros
per year fixed price; and
• For an installation of >3000 kW capacity, the customer pays 100 000 euros per year fixed
price.
Price per kWh, including taxes and environmental fees:
• Summer: typically 4 to 5 cents per kWh.
• Winter: typically 5 to 6 cents per kWh.
District heating tends to have more modest yearly price increases than other sources for heating.
An economical survey for a typical small apartment building showed the following situation on total
heating cost per year for the whole apartment building for different fuels:
Electricity 24 k euro
Oil 28 k euro
Bio Pellets 12 k euro
Heat Pump 14 k euro
District heating 15 k euro
A major drawback is considered to be the natural monopoly that the district heating system layout
creates. In the Nordic countries the rather free market on electricity is accepted and the contrast to the
district heating, in this respect, is often discussed.
After the Swedish deregulation of the electrical market in 1996 there has been a move from city-
owned district heating towards ownership by large electrical utilities.
A typical return on investment for a district heating utility is 6.5 %, with city-owned systems having
on average higher returns. The companies’ balance sheets show typical turnover figures between
30 million euros to 45 million euros.
3.2.2.2. District heating in Russia [8, 9]
In Russia, consideration has been given to the use of the VBER-300 reactor with 850 MW thermal
power as the basis for land-based and floating two-unit and single-unit nuclear co-generation plants to
provide heat and power supplies for some cities in the European part of Russia, Urals, and the Far
East, such as Arkhangelsk, Okhotsk, Petrozavodsk, and Ussuriisk.
The main parameters of a floating nuclear power plant based on the VBER-300 are the following:
• Maximum electric power output to the power-supply system: 2×280 MW;
• Construction period: 6 years;
• Construction period for hydraulic engineering and coastal installations assigned to the project of
3-4 years;
• Total plant construction period: 7 years.
Typical prices for heat delivered by a city owned utility are as follows:
51
The land-based option of the nuclear co-generation plant based on the VBER-300 plant has also been
developed:
• Maximum electric power output to power supply system: 2×280 MW;
• Maximum thermal power output to heat supply system: 2×530 MW;
• Electric power in co-generation mode to the power supply system: 2×185 MW.
3.2.2.3. Summary of district heating in Romania [10,11,12]
A total of five nuclear power reactors were initially intended to be built in Romania at the Cernavoda
site on the Danube River. The site currently comprises Unit 1 (which has been in full commercial
operation since 1996) and Unit 2 which has been connected to the grid in 2007. Units 3, 4, and 5 are in
different stages of completion. All reactors are PHWRs of the CANDU-6 type.
The site is about 2 km southeast of the town of Cernavoda (20 514 inhabitants) in the lower Danube
region near the Black Sea. Two large towns are located within a 30 km radius of the site, namely
Medgidia (43 867 inhabitants) and Fetesti (33 197 inhabitants), and many small rural communities and
villages. Around the NPP site, within a radius of 30 km and 100 km, approx. 180,000 and 1,375,000
persons respectively reside.
From early 1980s there were intentions to use the steam extracted from the turbine for district heating,
and a reference study was produced in 1985 [10] with the purpose to provide approximately 1,300
MW of district heating from all five Units of the Cernavoda NPP for distances up to 100 km..
The existing nuclear district heating system provides 60% of the necessary heating for the town of
Cernavoda, as well as necessary heat for the NPP site and site sub-contractor facilities. Studies were
performed [11, 12] and a modernization project has to be implemented to improve the existing district
heating system.
3.2.3. Economics
A comparison of the costs of nuclear heat production with those of competing technologies was
reported by IAEA [2] and the estimates are shown in Table 3.2. The costs are levelized heat generation
cost obtained with a method analogous to one conducted to compute levelized electricity generation
costs. The study assumed the oil cost as US$ 25 per barrel of oil equivalent and the cost of coal as
US$ 50/t. The other key parameters are given in the table. The data show that the assumed values for
the discount rate play an important role. At a 10% discount rate even a large nuclear heating plant
(NHP) is barely competitive with a large coal-fired boiler. These estimates were made in 1992. The
present increasing fossil fuel price and lowering interest rates would make large nuclear heating
systems more competitive. Note, however, that the effects of specific sites may well outweigh the
general trends shown in Table 3.2, depending on the heat transport and distribution networks at
different locations.
52
Table 3.2. Estimate of the competitiveness of the NHPs
Levelized heat costs
(US) $/kWth
Cost ratio: nuclear vs.
gas-oil boilers
Cost ratio: nuclear vs.
coal boilers
Plant type
Plant
size
(MWth)
Assumed
base
construction
cost
(US) $/kWth
Discount
rate: 5%
Discount
rate: 10%
Discount
rate: 5%
Discount
rate: 10%
Discount
rate: 5%
Discount
rate:10%
Nuclear plants
50 1650 25 36 1.3 1.6 1.8 2.1
100 1100 19 26 1.0 1.1 1.4 1.5
200 825 16 22 0.8 1.0 1.1 1.3
500 605 13 17 0.7 0.7 0.9 1.0
Fossil fuel plants
Gas-oil boiler 100 440 20 23 - - - -
Coal boiler 500 440 14 17 - - - -
Few economic cost data are reported from the existing nuclear district heating plants [13]. Canada’s
experience showed that the nuclear heat cost was significantly lower than heat from natural gas or
other fossil fuels. The Czech Republic’s district heat systems report high construction costs for hot
water pipelines from NPPs to consumers as a reason for higher heat costs. In Cernavoda, Romania,
nuclear heat provided by the NPP covers 60% of the total heat consumption. The heat cost delivered to
the distribution company of the municipality is about 5.5 Euro/MWh (presuming an exchange rate of
3.4 Lei per Euro), and the tariff to the public is 15.1 Euro/MWh, which is about half of the average
cost of heat in Romania. In the Russian Federation, the nuclear heat plants contribute about a third of
the total production of heat in the country. The cost of nuclear heat is reported to be 2-2.5 times
cheaper than that from local heating boilers. In Russia and Ukraine, however, the nuclear heating cost
is subsidized, leading to a low cost to consumers. In Trnava, Slovakia, nuclear heat contributed 60 %
of the total heat supply. It is also reported to be cheaper than fossil-fuel heat. In Switzerland’s Benzau
unit, the heat cost is reported to be around 70 - 95 CHF/ MWh, which is higher than oil firing, which is
around 45 - 60 CHF/ MWh. The improved environmental benefits have made this higher cost
acceptable, however.
3.3. Challenges
Transporting heat is difficult and expensive. The need for a pipeline, thermal insulation, pumping, and
the corresponding investments, heat losses, maintenance, and pumping energy requirements make it
impractical to transport heat beyond distances of a few kilometres, or at most, some tens of kilometres.
There is also a strong size effect to the system cost. Furthermore, the specific cost of transporting heat
increases sharply as the amount of heat to be transported decreases. Compared to heat, the
transmission and distribution of electricity is cheap, even over distances measured in hundreds of
kilometres. Worldwide transport of gas through pipelines up to thousands of kilometres long is, of
course, well-established.
The foremost challenge to district heating systems, whether fossil or nuclear based, is their
competitiveness with small domestic heating units using oil or gas fired mini boilers or electricity,
which are presently meeting consumer demands satisfactorily. These units are not subject to transport
of heat and attendant problems of costs for heat transport equipment, piping insulation, and heat losses
during distribution.
Nuclear plants are capital intensive. The influence of the fixed cost component is predominant in the
final cost of energy and its products. Therefore, base load operation with load factors as high as
53
achievable is needed for competition with alternate energy sources. This is only possible when the
demand of the heat market to be supplied has base load characteristics, or when the combined
electricity and heat market enables overall base load operation of a co-generation plant. This is
particularly relevant for district heating, where the heat load is substantial and varies with season.
Because heating requirements are not needed throughout the year, the overall district heat plant’s load
factors are typically around 50-60% in cold climates. The co-generation plants are designed to
distribute the electricity and heat loads adequately year-round, but the overall economics are affected.
There is, however, an possibility to improve the situation for a plant using nuclear energy by
increasing the thermal efficiency of the heat transport systems. If this could be done together with a
lowered investment cost it would improve the likelihood that a nuclear-based heating system could
compete economically with a fossil fuel fired system.
A benefit of district heating systems is the reduction of air pollution emissions near urban localities
and the consumer centres, compared to small localized units. In this context, the role of nuclear district
heating will have a higher significance as it does not contribute to any emission.
The elimination of CO
2
and flue gas emissions is, of course, a key essential driver for the use of
nuclear, rather than fossil, energy. Were nuclear energy also recognized as a Clean Development
Mechanism (CDM) under the Kyoto Protocol, this could lead to further improved economics of
nuclear district heating.
3.4. Solutions
The challenges mentioned above are a mix of socio-political and technical ones. The technical
solutions, discussed below, to meet these challenges are primarily directed towards reactor plant type
and layout, their size and location, and safety aspects.
Plant type and layout
Nuclear heat-producing plants for district heating for space heating and hot tap water can, in principle,
be divided into two different types:
• Dedicated heat-producing reactors, so called single-purpose systems;
• Heat and power reactors, so called dual-purpose systems.
Initial efforts in district heating considered dedicated reactors. However seasonal variation of heat
requirements for district heating led to a wide use of co-generation systems.
Important factors that are limiting the benefits that could be realized by retrofitting district heating to
existing power plants are the outage costs and risks for the necessary shutdown period to carry out the
retrofitting work, and the fact that such plants seldom are optimally sited for the district heating
network.
New nuclear heat-producing plants must, of course, be designed to have availability factors
comparable or preferably higher than today’s plants. The requirements on availability and reliability
are particularly keen for new district heating networks, since there would be no existing heat-
producing capacity that could serve as backup. For existing systems, heat production capacity through,
for instance, oil boilers can serve as the backup capacity during nuclear outages.
Heat storage allows a matching of the heat supply to the heat demand. This has several advantages,
including better utilization of the investments. This is true regarding both the production unit as well
as the district heating network. Today there are many examples of short-term storage, for instance, on
the daily scale. In the future, more long-term storage facilities may be realized. Today’s storage relies
54
on hot water accumulator tanks. More innovative concepts, such as storage in underground water
layers, may also be possible.
Plant size and location
Nuclear district heating requires that the reactors be installed in the district heating network close to
the consumers. In this way the thermal losses and pipeline costs can be kept low. The proximity to
population centres implies the need for a high degree of safety including the lowering of core damage
frequencies and enhancing mitigation systems in the case of an accident.
The design features to prevent the transfer of radioactivity into the district heating grid network have
proven to be effective. These features include one or more barriers to radioactive cross
contanmination, e.g., in the form of a leak-tight intermediate heat transfer loop at a pressure higher
than that of the steam extracted from the turbine side of the nuclear plant. These loops are
continuously monitored, and isolation devices are provided to separate potentially contaminated areas.
Another key factor is that the heat reactors need to be small compared to modern power producing
reactors, since the heat demand even in big district heating networks seldom is more than 200 MW. A
small size could also facilitate licensing of a location close to a population centre.
In order to be able to compete with fossil fuel fired heat boilers, the installation cost per installed MW
heat for a nuclear-based system must be much lower than today’s power producing plants. Reducing
plant complexity is one option, which would also serve, potentially, to simplify operation,
maintenance, and training.
Small dedicated reactors would have the following advantages:
• Less severe operating conditions, such as lower pressures and temperatures;
• A smaller size, which reduces the demands associated with manufacturing the main
components;
• A high potential for modularization and standardization;
• Improved ease of operation, without excessive demand on qualified personnel;
• Design transparency; the safety features can be more simple and easier to understand.
The less severe operating conditions and simpler safety systems facilitate a design with a high degree
of safety that relies to a large extent on passive safety systems. Such systems are often simpler with a
low degree of complexity.
Lower pressures and temperatures also lower the need for sophisticated materials and, therefore, may
reduce material and manufacturing costs. A clear strategy for nuclear district heating reactors must be
to use industrial grade components to as large an extent as possible, instead of the customized nuclear
grade components used in power producing plants, assuming that this strategy would be endorsed by
the nuclear safety authorities.
A small plant has several advantages for the manufacturing industry compared to a large power plant.
Mechanical components are smaller and lighter than those of a conventional nuclear power plant. This
makes the manufacturing of the main components of the plant possible even in countries that do not
have the workshops and experience that is normally needed for making heavy nuclear-grade
components for large nuclear power plants.
In order to keep down the cost, a high degree of standardization is desirable. This may further reduce
maintenance costs, as well. A relatively large proportion of both process components and control
equipment could be provided as factory-assembled packages. This would keep the on-site installation
costs lower.
55
Despite the advantages of small, single-purpose plants, it must also be said that the cost of a plant
tends to increase rapidly with decreasing capacity, and the economic competitiveness of such a reactor
becomes questionable. Standardization of design and construction may help overcome this
disadvantage, even if individual plants are not ideally sized for the specific district heating application
in mind.
Conclusions
The low prices of fossil fuels have stunted the development of small, single-purpose nuclear district
heating plants. This situation is changing. Furthermore, as environmental concerns mount over the use
of fossil fuels, the prospects for nuclear-based district heating are improving. Nevertheless, though
many concepts of such small-scale heat-producing reactors have been presented during the years, very
few have been built.
The main features of such a plant would be:
• A small capacity (for example <200MW);
• Low temperatures and pressures;
• A simple, standardized design;
• Passive safety systems.
REFERENCES FOR CHAPTER 3
[1] OECD/NUCLEAR ENERGY AGENCY, Non-electricity Products of Nuclear Energy, OECD-
NEA Report (2004).
[2] INTERNATIONAL ATOMIC ENERGY AGENCY, Market Potential for Non-electric
Application of Nuclear Energy, TRS No. 410, Vienna (2002).
[3] INTERNATIONAL ATOMIC ENERGY AGENCY, Improving Economics and Safety of
Water cooled Reactors: Proven Means and New Approaches, IAEA-TECDOC-1290, Vienna
(2002).
[4] INTERNATIONAL ATOMIC ENERGY AGENCY, Introduction of Nuclear Desalination-A
Guidebook, TRS No. 400, Vienna (2000).
[5] SANDSTRÖM, S. “Operating Experiences at the Ågesta Nuclear Power Station” AB
Atomenergi (1966). AE-246.
[6] INTERNATIONAL ATOMIC ENERGY AGENCY, Status of Advanced Light Water Cooled
Reactor Designs 1996, ISSN 1011B4289, IAEA-TECDOC-968, Vienna (1997).
[7] BENTO, J.-P. and MANKAMO, T., Safety Evaluation of the Secure Nuclear District Heating
Plant, Nuclear Technology Vol. 38. (1978).
[8] PANOV, Yu., POLUNICHEV, V, ZVEREV, K. Use of Reactor Plants of Enhanced Safety for
Seawater Desalination, Industrial and District Heating, IAEA-TECDOC-923, Vienna (1997).
[9] PANOV, Yu., POLUNICHEV, V., Nuclear Head Applications: Design Aspects and Operating
Experience, in IAEA-TECDOC-1056, IAEA, Vienna, pp. 249-262 (1998).
[10] INSTITUTE FOR POWER STUDIES AND DESIGN – ISPE, “The full utilization of the
available heat from Cernavoda NPP for large area district heating, aimed to reduce the country’s
hydrocarbons consumption”, Institute for Power Studies and Design – ISPE, Bucharest
(December 1985).
[11] INSTITUTE FOR POWER STUDIES AND DESIGN – ISPE, “Cernavoda District Heating
Modernization - Pre-feasibility Study”, ISPE, Bucharest (October 2004).
[12] INSTITUTE FOR POWER STUDIES AND DESIGN – ISPE, “Cernavoda District Heating
Modernization - Feasibility Study”, ISPE, Bucharest (July 2005).
[13] INTERNATIONAL ATOMIC ENERGY AGENCY, Nuclear Heat Applications: Design
Aspects and Operating Experience, IAEA-TECDOC-1056, Vienna (1998).
56
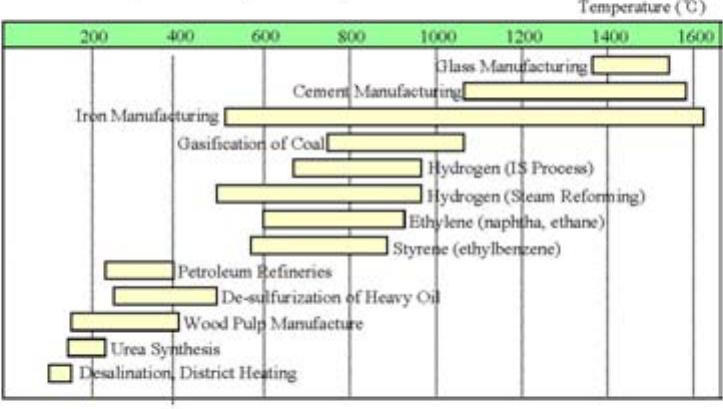
CHAPTER 4
INDUSTRIAL PROCESS STEAM
4.1. Opportunities
Within the industrial sector, process heat is used for a large variety of applications with different heat
requirements and with temperature ranges covering a wide spectrum. Whereas in energy-intensive
industries the energy input represents a considerable fraction of the final product cost, in most other
processes it contributes only a few percent. Nevertheless, the supply of energy has an essential
character: all industrial users need the assurance of energy supply with a degree of reliability and
availability approaching 100%. In contrast to district heating, the load factors desired by industrial
users do not depend on climatic conditions. The demands of large industrial users usually have base
load characteristics.
The characteristics of the market for process heat are quite different from district heating, though there
are some common features, particularly regarding the need for minimal heat transport distances.
Industrial process heat users do not have to be located within highly populated areas. Many of the
process heat users, in particular the large ones, can be, and usually are, located outside urban areas,
often at considerable distances. This makes joint siting of nuclear reactors and industrial users of
process heat not only viable, but also desirable in order to drastically reduce the heat transport costs –
provided that the co-siting does not adversely affect the safety case for the nuclear installation.
Process heat involves the supply of heat required for industrial processes from one or more centralized
heat generation sites through a steam transportation network. Within the industrial sector, at
temperatures higher than those needed for district heating and seawater desalination, process heat is
used for a variety of applications as shown in Figure 4.1 [1].
FIG. 4.1. Required temperatures for industrial processes (Figure taken from [1]).
As discussed in earlier chapters, water-cooled nuclear reactors have been used in low-temperature
applications including desalination, district heating and process heat. There is a potential for their use
at medium temperature ranges such as are used in the fertilizer, pulp and paper, and oil and petroleum
industries, etc. However, applications shown in Fig 4.1 that require heat above about 550 ºC (the
temperature achievable by super-critical water cooled reactors) would require other reactor types and
shall not be discussed in this report.
In a similar way as for district heat, the 2003 IAEA report [2] took the approach that the size of the
process heat market can be estimated by examining the total use of centralized process heat in 1996,
57
which was 150.1 Mtoe according to the IEA world energy database and required a heat production
capacity of 240 000 MWth assuming an average load factor of 90%.
Process heating has the following technical requirements:
• Owing to higher thermal losses over long transmission networks, the heat source must be
relatively close to the customer;
• The annual load factor must be much higher than that in district heating, probably 70-90%,
since process heat demand does not depend on climatic conditions;
• To secure a reliable supply of heat, a backup capacity is required.
Several co-generation nuclear power plants have supplied process heat to industrial users [3]. The
largest projects implemented have been in Canada (Bruce for heavy water production and other
industrial/agricultural users) and in Kazakhstan (Aktau for desalination). Other power reactors, which
currently produce only electricity, could be converted to co-generation. Nevertheless, installing a new
nuclear co-generation plant close to existing and interested industrial users has better prospects. Even
better would be a joint project whereby both the nuclear co-generation plant and the industrial
installation requiring process heat are planned, designed, built, and operated together as an integrated
complex.
For reactors in the small to medium size range, and in particular for small and very small reactors, the
share of process heat generation may be larger, and heat could even be the predominant product. This
would affect the plant optimization criteria, and could present much more attractive conditions to the
potential process heat user. Consequently, the prospects of SMRs as co-generation plants supplying
electricity and process heat are considerably better than those of large reactors.
Heat-only reactors have not yet been applied on an industrial/commercial scale for the supply of
process heat, though several designs have been developed.
4.2. Market context
4.2.1. Past experience
As in the case of district heat, all existing reactor types are potentially applicable to process heat
needs, depending on the required temperature of the processes. There have been some experiences in
providing process heat for industrial purposes with nuclear energy in Canada, Germany, Norway,
Switzerland, and India. In Canada, CANDU reactors supplied steam for industries such as food
processing and industrial alcohol production until their closure in 1998. In Germany, the Stade PWR
supplied steam for a salt refinery located 1.5 km from the plant from December 1983 until its
shutdown in November 2003. In Norway, the Halden Reactor has supplied steam to a nearby factory
for many years. In Switzerland, since 1979 the Gösgen PWR has been delivering process steam to a
cardboard factory located 2 km from the plant. In India, the RAPS-II PHWR at Kota supplies heat to
the nearby heavy-water plant. Tables 4.1 and 4.2 present the salient features of operating nuclear
process heat plants and projects [4]. A brief summary of these applications is presented in the
following paragraphs.
58
Table 4.1. Operating nuclear process heat production plants
Country
Plant
name
Location
Application
Start of operation
reactors / heat
Phase
Power
(MW
(e))
Heat
delivery
(MW(t))
Temperature
(°C) at
interface
(feed/return)
Remarks
Canada Bruce-A
a
Bruce Process heat 1977-87 1981 Commercial 4 x 848
5350
D
2
O production
and six industrial
heat customers
Germany Stade Stade Electricity/
process heat
1983 Commercial 640 30 190/100 Salt refinery
Switzerland Goesgen Goesgen Electricity/
process heat
1979 1979 Commercial 970 45 220/100 Cardboard factory
India RAPS Kota Electricity/
process heat
1975/1980 Commercial 160 85 250 D
2
O
a
Unit 2 was taken out of service in 1995, units 1, 3 and 4 were taken out of service in 1998.
Table 4.2. Nuclear process heat production projects
Country
Plant
name
Location
Application
Start of operation
reactors / heat
Phase
Heat
delivery
(MW(t))
Temperature
(°C) at
interface
(feed/return)
Remarks
China HTR-10 Beijing Electricity/
process heat
Criticality 1999 Construction
completed
10 700-950/250 Experiments for HTR
technology development.
Japan HTTR Oarai Process heat Criticality 1998 Construction
completed
30 950/395 Experiments for HTR
technology development,
potentially including
hydrogen production.
Russia VGM Process heat Design
59

In Canada, steam from the Bruce Nuclear Power Development (BNPD) was supplied to heavy water
production plants and to an adjacent industrial park at the Bruce Energy Centre (BEC). BNPD is the
world’s largest nuclear steam and electricity generating complex. It includes eight CANDU nuclear
reactors with a total output of over 7200 MW(e), the world’s largest heavy water plant (HWP), and the
Bruce Bulk Steam System (BBSS). The BBSS, capable of producing 5350 MW(th) of medium-
pressure process heating steam, was built to supply the HWP from the four 848 MW(e) units of the
Bruce A complex. Each of the four 2400 MW(th) reactors can supply high-pressure steam to a bank of
6 heat exchangers (24 in total), which produce medium-pressure steam for the HWP and site services.
The normal capacity is approximately 1680 kg/s of medium-pressure steam from the reactors with 315
kg/s emergency backup available from oil fired boilers. In 1995, Unit 2 of the Bruce A NPP was laid
up; the HWP and units 1, 3 and 4 of Bruce A were laid up in spring 1998. However, unit 4 was
restarted in 2003 and unit 3 in 2004. In 2006 agreement was also reached on a four-year programme to
also restart units 1 and 2.
FIG. 4.2. Bruce nuclear power development (8 x 850 MW(e) CANDU units, heavy water production,
greenhouse complex using nuclear steam). (Credit: Bruce Power.)
The six private industries currently established at the park are:
(1) a plastic film manufacturer;
(2) a 30 000 m
2
(7.5 acres) greenhouse;
(3) a 12 million liter/year ethanol plant;
(4) a 200 000 ton/year alfalfa dehydration, cubing, and pelletising plant;
(5) an apple juice concentration plant; and
(6) an agricultural research facility.
In Germany, the Stade NPP PWR (1892 MW(th), 640 MW(e)) has supplied steam for a salt refinery,
which is located at a distance of 1.5 km, since December 1983. The salt refinery requires 45 t/h
process steam at 190°C and 1.05 MPa. This represents a thermal power of about 30 MW and is 1.6%
60
of the thermal output of the NPP. Since 1983, the steam supply by Stade has had high availability, and
the operating experience with process steam extraction is good.
The Gösgen-Däniken nuclear power station (KKG) with a pressurized water reactor situated on the
River Aare between Olten and Aarau in Switzerland and built under the overall management of
Kraftwerk Union AG (KWU) is the first of its kind in the world to supply process stream. Its steam
user is the nearby cardboard mill, Kartonfabrik Niedergösgen. The heavy-oil-fired boilers installed at
that plant, which with their emissions were once the source of considerable atmospheric pollution,
now have only standby status.
Further design details of the Gösgen process heat plant are given in Annex 4.
In India, the RAPS II reactor at Kota has been supplying 110 t/h of steam at 250°C and 4 MPa since
1980. The steam is reduced to 0.6 MPa and used in the nearby heavy-water production plant.
4.2.2. Near-term potential
The Russian Federal Agency for Atomic Energy (ROSATOM) has started construction of a floating
barge-mounted heat and power co-generation nuclear plant based the ship propulsion PWR-type
reactor KLT-40C in Severodvinsk. It is planned to put the plant into operation in 2010. The floating
NPP can produce up to 70 MW for electric power and about 174 MW of heat for district/process
heating. The lifetime of the plant is 40 years; it is designed for a continuous operation period before
dockyard refurbishment of 12 years.
Demonstration of this nuclear technology is intended to allow its larger-scale application inside the
country and abroad for electricity and heat production.
Another PWR, the SMART integral type reactor, is under development in the Republic of Korea for
desalination. It could however also produce industrial process heat.
Application in oil extraction
Atomic Energy Canada Limited (AECL) has studied the feasibility of using CANDU energy in
applications beyond traditional electricity generation, such as open pit mining and oil sand extraction.
Alberta’s oil sand deposits are the second largest oil reserves in the world, and have emerged as the
fastest growing, soon to be dominant, source of crude oil in Canada. The oil sand industry currently
produces more than a third of the nation’s petroleum needs, and has the potential to account for more
than sixty percent of Western Canadian crude production by 2010.
Currently, the majority of oil sand production is through open-pit mining, which is suitable for
bitumen extraction when the oil sand deposits are close to the surface. The ore, a mixture of bitumen
and sand, is removed from the surface by truck and shovel operation. The ore is then mixed with hot
water to form a slurry that eventually undergoes a separation process to remove bitumen from the
sand.
The thermal energy required for the open-pit mining process is in the form of hot water at a relatively
low temperature (around 70°C), and the rest is dry process steam at around 1.0 to 2.0 MPa. The oil
extraction facilities require electrical power as well.
To increase production capacity, the industry is looking for new technology to extract bitumen from
deep deposits. Among them, Steam-Assisted Gravity Drainage (SAGD), which uses steam to remove
bitumen from underground reservoirs, appears to be the most promising approach. Recently, the in-
situ recovery process has been put into commercial operation by major oil companies.
61
Overall, for both extraction methodologies, a significant amount of energy is required to extract
bitumen and upgrade it to synthetic crude oil as the feedstock for oil refineries. Currently, the industry
uses natural gas as the prime energy source for bitumen extraction and upgrading. As oil sand
production continues to expand, the energy required for production becomes a great challenge with
regard to economic sustainability, environmental impact and security of supply. With this background,
the opportunity for nuclear reactors to provide an economical, reliable and virtually zero-emission
source of energy for the oil sands becomes a realistic option.
Further details are given in Annex 3.
4.2.3. Economics
The 2003 IAEA report [2] discussed the industrial process heat market size and features. The
industries that are main consumers of heat are:
- food,
- paper,
- chemicals and fertilizers,
- petroleum and coal processing, and
- primary metal industries.
The breakdown of the total industrial heat varies from country to country, but the chemical and
petroleum industries are the major consumers worldwide. These would be key target clients for
possible applications of nuclear energy.
With respect to economic competitiveness, many of the features described for electricity generated by
nuclear energy for desalination and district heating are valid for process heat applications. There are
several important considerations specific for process heat applications, which include:
- The ability to locate the heat source close to the demand,
- The relatively small-scale demand,
- The need for high reliability.
The economics of nuclear energy for process heat applications will likely be improved by the
development of small, low-cost reactors. Current development trends in many countries have already
begun to move in this direction.
The co-generation plants for process heat based on fossil fuels or water-cooled reactors derive much of
their revenue from electricity, but add the operational flexibility to adapt to process heat markets.
Specific costs for oil extraction from Canadian oil-sands
In 2003 AECL commissioned an independent study by CERI (Canadian Energy Research Institute) to
compare the economics of Advanced CANDU Reactor (ACR)-supplied energy with natural gas. This
study provided an evaluation using ACR design data, the assumptions on configurations and
economics, and compared equivalent amounts of energy supplied from the nuclear and natural gas
options to ensure a proper comparison. The study identified comparable ACR and natural gas-supplied
configurations, each delivering steam and electricity. The electricity output was set at 150 MWe
(gross) to meet the facility’s demand, and the remaining thermal power of the ACR was delivered as
steam to the Steam-Assisted Gravity Drainage (SAGD) plant. A common economic model was also
developed, using parameters such as natural gas and electricity costs based on 2003 market value,
without attempting to extrapolate or forecast future prices. The natural gas price used was Cnd
$4.25/GJ and the electricity price was Cnd $50/MWh. Based on these numbers, the results show
nuclear steam to be 10% cheaper than that generated through natural gas.
62
The study examined energy price sensitivity to changes in key parameters. The nuclear case is more
sensitive to capital cost changes, as might be expected. A 25% increase in capital cost would increase
steam cost by 20%. The gas-fired option is extremely sensitive to fuel prices. A 25% increase in the
price of natural gas would increase steam cost by nearly 25%. With the current gas price more than
50% higher than in 2003, the economic advantage of nuclear over natural gas becomes significant.
The study also examined the impact of CO
2
emissions on cost. The reference basis for CO
2
costs or
credits was $15/tonne. This could add an additional 18% to the cost of natural gas-supplied steam.
All indications are that the nuclear option has a significant advantage in cost over the competition. The
nuclear option also provides for cost predictability and stability, which would reduce the risks for a
potential oil sand operator.
4.3. Challenges
The market for industrial heat is highly competitive. Heat is produced predominantly from fossil fuels,
with which nuclear energy will have to compete. Most of the industries using fossil fuel as a heat
source have abundant waste heat available and it is being economically used in various process
streams.
Similar to nuclear district heating, the close siting of a nuclear plant to the customer is preferable, as
the heat transportation costs grow significantly with distance. This will require specific safety features
appropriate to the location and the application. Until now few technical problems in coupling nuclear
reactors to various applications have been identified, though some safety-related issues of coupled
systems may need more study.
The nuclear process heat supply has to be reliable. As an example, the average adequate steam supply
availabilities for chemical processing, oil refineries and primary metals are respectively 98%, 92% and
near 100%. Such high levels can be ensured only by the combination of a highly reliable heat source
and the availability of reserve capacity.
The supply of industrial heat is more uniform throughout the year than that of district heat, mainly
because of the absence of seasonal variation. Accordingly, the average load factors of industrial
boilers are relatively high, between 70 to 90%. Nuclear reactors, which are typically run in base load
operation, will be quite useful in this context.
Although nuclear industrial process heat applications have significant potential, it has not been
realized to a large extent. In fact, presently only the Goesgen reactor in Switzerland and RAPS–2 in
India continue to provide industrial process heat while other process heat systems have been
discontinued after successful use (see Table 1.2). Among the reasons cited for closure of these units,
one is availability of cheaper alternate energy sources, including waste heat near the industrial
complexes. The nuclear slow down in many industrialized countries could be another reason.
Studies are being carried out in Canada for oil extraction from its vast oil sand resources. These
studies consider using present day and advanced water-cooled reactors as a heat source. Currently
natural gas is used for this application. Although these studies have been motivated by a need for an
environmentally sound energy option, the ultimate challenge in the utilization of nuclear steam for this
application is the economics.
63
4.4. Solutions
4.4.1. Oil extraction from Canadian oil sands
Application to open-pit mining
When a CANDU plant is adapted to supply thermal power to open-pit mining processes, the low
temperature and pressure requirements allow extraction steam from the turbine to be used as the heat
source. This boosts the overall system efficiency. Steam extracted from the low-pressure turbine at
low pressure is used to heat water in steam-to-water heat exchangers. The hot water is supplied to the
bitumen extraction facility; most of the water is recovered after the process, treated and sent back as
the feedwater to the hot water heaters. The condensate from these hot water heaters returns to the
turbine’s feedwater system. The thermal power of the water counts for about 80% of total thermal
power demanded by the open-pit processes.
There are two steam heaters operating at different pressures. The higher pressure one uses main steam
directly from the steam generators and the lower pressure one uses extraction steam from the high-
pressure turbine as a heating source to generate process steam at 2.0 MPa and 1.0 MPa respectively.
The process steam is piped to the bitumen extraction process, and mostly used in mixture heat
exchangers without condensate return. Therefore, feedwater to these steam heaters is mainly from the
make-up water. The condensate from these steam heaters returns to the turbine’s feedwater heating
system. The thermal power of the process steam represents about 20% of total thermal power
requirement. Therefore, the amount of the steam used in these steam heaters is not as significant as the
low-pressure turbine extraction steam used in the hot water heaters.
In contrast to condensing steam turbines that are used in conventional nuclear power plants, the steam
turbines used in this configuration are of the automatic extraction type. Steam is extracted from high-
pressure and low-pressure turbines as the heating steam. The appropriate means have to be provided to
control the amount and the pressure of steam extracted. The turbine design allows the thermal and
electrical output from the plant to vary within a certain range if required. This is a proven technology
in fossil-fuelled co-generation power plants and has been used for decades.
A system simulation has been performed for the year-round weather conditions for the production of
330,000 bbl/day of bitumen, which is a manageable size of an open-pit mining project. For the average
weather condition (in April), the thermal power demand is 1440 MWt, and the electricity demand is
300 MWe (including the oil facility and nuclear plant demands). A CANDU-6 plant is able to provide
the required thermal power for this condition, while generating 367 MWe (gross) electricity. In
summer, the thermal demand decreases to 1220 MWt, and consequently the electrical output increases
to 420 MWe (gross). In the coldest month (January), the total thermal power demand from the oil sand
facility is as high as 1670 MWt, and the CANDU-6 plant generates 316 MWe (gross) electricity while
producing enough hot water and steam to meet the thermal requirements of the bitumen production.
During a year, the nuclear plant generates electricity within a range from 316 MWe to 420 MWe,
while being able to meet the entire thermal power requirement. This offers a limited capability to
deliver electricity to the grid. The simulation results are shown in Table 4.3.
64

Table 4.3. Simulation results: CANDU-6 for open-pit mining application
Month Jan Feb Mar Apr May Jun Jul
Electricity (gross) MWe 316 331 348 367 385 403 420
Thermal Power MWt 1670 1590 1520 1440 1370 1300 1220
Other technical aspects for adapting a CANDU reactor for open-pit mining processes are similar to
those for the Steam-Assisted Gravity Drainage process discussed in the next subsection, and there are
no insoluble issues identified.
An economic analysis for this configuration is currently in progress.
Application to advanced mining technology
As described earlier, Steam-Assisted Gravity Drainage (SAGD), which uses steam to remove bitumen
from underground reservoirs, is an advanced process being used to exploit deep deposits of oil sands.
A typical SAGD application involves twin horizontal wells drilled in parallel, with one a few meters
above the other, as shown in Fig 4.3. The upper well is called the injection well and the lower one the
production well. Medium pressure steam is injected into the underground deposit area through the
injection well to heat the reservoir of bitumen-sand mixture by conduction. The heating reduces the
viscosity of the bitumen, increases its mobility, and establishes pressure communication between the
two wells along their length, so that a flow of fluids (a mixture of bitumen and condensed water) can
occur and be collected through the production well. The production liquid is transported to a central
facility, where the bitumen is separated and the condensate is collected, treated, and sent back to the
boilers.
FIG. 4.3. Steam assisted gravity drainage process (reprinted with the permission of AECL).
Cap Rock (shale & glacial t ill) 250m t hic
k
Steam
Ch am b er s
Unrecovered
Heavy Oi l
6mo
6mo
2yr
2yr
5yr
5yr
8yr
8yr
10yr
10yr
~1 kilometer
~200m
65
The required steam injection pressure depends on the circumstances of the oil field and the life cycle
of the well, and varies from 2 to 6 MPa. At the initial stages of production (two to three months), each
well requires steam at higher pressure than that required during normal operation. Each barrel of
bitumen requires 2–3 barrels of steam (steam volume is corrected to 4°C and 1 bar). Generally, about
18% of the energy content of the oil produced is used to provide heat for the extraction process; a
further 5% is used in generating hydrogen to upgrade the bitumen to synthetic crude oil.
In addition to steam demand, SAGD production facilities are also significant consumers of electricity.
Adaptation of ACR-1000 for Steam Assisted Gravity Drainage
The primary product of a water-cooled nuclear power plant is steam from the steam generators.
Depending on the requirements of specific projects, the plant can be adapted to provide steam only, or
a mixture of steam and electricity for various steam/electricity ratios. With the steam-only option, the
steam generated from a plant is totally dedicated to supply steam to oil sand processes, and no
electricity is generated. Hence, the turbine island is totally eliminated from the plant, and replaced by
the facilities dedicated to steam and feedwater supplies. The steam/electricity option splits main steam
from the steam generators into two streams: one is dedicated to supply steam to the oil sand facility
and the other is channeled to generate electricity. As a result, the turbine capacity becomes smaller
than that of a standard plant.
As an example, the ACR-1000 design is an evolutionary development of CANDU technology. The net
electrical output from a standard ACR-1000 will be approximately 1000 MWe. The main steam
pressure is 6.5 MPa. The license requirements for an ACR SAGD project were examined and found to
pose no major issues. Since the Bruce A nuclear plant in Ontario has supplied nuclear-generated steam
to various facilities outside the exclusion area, no major impediments to getting regulatory approval
are foreseen.
For the steam-only option, there will be no on-site source of station service power for the nuclear unit.
Therefore, a large combustion turbine generator or other local generation source will be required to
back-up offsite power to satisfy power supply reliability requirements.
Water sources in the northern Alberta oil sand areas are scarce. It would be necessary for any
individual project to minimize its water usage to meet overall regional water use planning guidelines.
The study has looked into the use of air-cooled heat exchangers to take the heat load, which is
normally removed by once-through water in the traditional CANDU plants. This was found to be
feasible for up to 350 MWe electricity generation capacity. The possibility of using more conventional
cooling towers was also examined and found to be feasible.
The remote location and severe weather conditions of oil sand sites raise concerns about the
constructability of a large plant. The ACR design is highly modularized, and the use of prefabricated
modules will be maximized. This approach minimizes the on-site construction activities, enabling
schedule compression, and reduces the size of the on-site labour force. The module sizes and weights
have been selected for suitability for road transportation to northern Alberta. No major construction
issues are predicted.
Overall, the studies indicate that construction and operation of an ACR to meet the energy demand for
SAGD projects are technically feasible.
66
4.4.2. Massachusetts Institute of Technology (MIT) study [5]
The following summarizes the study:
Introduction
The two general classes of oil sand recovery are mining and in-situ methods. In mining, the oil sand
ore is recovered by electric or hydraulic shovels and transported by heavy trucks before the bitumen
can be extracted. For in-situ methods, most of the bitumen is separated from the oil sands in the
deposit by injecting steam into the ground to separate the bitumen from sand. The bitumen is then
pumped to the surface for further processing.
A 2005 MIT study analyzed the feasibility of integrating a nuclear power plant with Steam Assisted
Gravity Drainage (SAGD). Natural gas-fired plants provide the energy for projects today, but concerns
are heightening within the industry over increased carbon dioxide emissions, volatile natural gas
prices, and depletion of natural gas reserves. Nuclear power is an emission-free alternative to natural
gas, but the key to implementation hinges on whether nuclear systems can compete economically with
natural gas-fired plants in the oil sand industry. Successful integration of emission-free nuclear
technology with oil sand operation will remove the dependence of the price of oil from oil sands on
the volatile and high price of natural gas and also address the problem of CO
2
emissions.
Energy requirements
Extraction facilities can be divided into three categories: those that only require process heat, those
that require process heat and electricity, and those that require process heat, electricity and hydrogen
to upgrade the bitumen to syncrude on site. In order to compare the different scenarios, a processing
plant capable of producing 100 000 barrels of bitumen per day was assumed as a reference design.
100 000 barrels of bitumen can be upgraded to approximately 87 000 barrels of synthetic crude. The
thermal requirements for each scenario were calculated and compared to the available output of
several reactor systems.
The first scenario requires only process heat to be produced on site. Electricity is assumed to be taken
from the grid. The bitumen after dilution is pumped to a processing plant. The thermal energy
requirements for this scenario range from 820 MWth to 1264 MWth.
The second scenario requires electricity in addition to process heat. The energy requirement for this
scenario includes 1200 MWth heat and 250 MWe.
The third scenario is a self-contained facility that produces its own electricity and process heat and
refines the product on-site from bitumen to synthetic crude. This scenario includes the production of
hydrogen using high-temperature electrolysis for refining. The energy requirement for this scenario
includes 1300 MWth and 740 MWe.
Nuclear reactor design options
Eleven different reactor systems were considered by MIT for this application; three were selected for
the mission of providing the energy needs required. These include the Canadian ACR-700, the
Westinghouse AP-600 and the South African 400 MWth PBMR. An evaluation of design-specific
characteristics that benefit and impede the oil sand extraction process was used to distinguish reactors.
Economics
The study showed that nuclear energy would be feasible, practical and economical for use at an oil
sand facility. Nuclear energy is two to three times cheaper than natural gas for each of the three
scenarios analyzed. Also, by using nuclear energy instead of natural gas, a plant producing 100,000
67

barrels of bitumen per day would prevent up to 100 mega tonnes of CO
2
per year from being released
into the atmosphere.
Nuclear obstacles
There are several obstacles to implementing nuclear power in oil sand operations. Public perception
could offer resistance to the project, but a well-planned education effort could address the issue.
Owing to unfamiliarity with nuclear plants in the oil sand industry, it is expected that a qualified
nuclear utility will be needed to operate the nuclear plant and provide heat and electricity as merchant
products for the oil sand extraction and refining company.
4.4.3. Long-term possibilities
The Generation IV International Forum (GIF), an international group of ten countries and Euratom is
exploring the development of six systems that could be deployed by 2030 or earlier. Their capabilities
to produce non-electricity products are shown in Table 4.4.
Table 4.4. Generation IV reactor concepts
Neutron
spectrum
Coolant
Temperature
(°C)
Fuel
cycle
Size(s)
(MWe)
Gas-cooled Fast
Reactors
(GFR)
Fast Helium 850
closed,
on site
288
Lead-cooled
Fast Reactors
(LFR)
Fast Pb-Bi 550-800
closed,
regional
50-150
300-400
1200
Molten Salt
Reactors (MSR)
Epithermal
Fluoride
salts
700-800 closed 1000
Sodium-cooled
Fast Reactors
(SFR)
Fast Sodium 550 closed
150-500
500-1500
Supercritical
Water cooled
Reactors
(SCWR)
Thermal or
fast
Water 550
open
(thermal)
closed
(fast)
1500
Very High
Temperature
Gas Reactors
(VHTR)
Thermal Helium 1000 open 250
Of these concepts, the one water-cooled reactor concept is the supercritical water cooled reactor
(SCWR).
The SCWR system features two fuel cycle options: an open cycle with a thermal neutron spectrum
reactor and a closed cycle with a fast-neutron spectrum reactor and full actinide recycle. In either
option, the reference plant has a 1700 MWe power level and a reactor outlet temperature of 550°C.
This is a very high-pressure water-cooled reactor that operates above the thermodynamic critical point
of water to give a thermal efficiency about one third higher than today’s light water reactors from
which the design evolves. The supercritical water directly drives the turbine, without any secondary
68
steam system. It is primarily envisioned for missions in electricity production, with an option for
actinide management. Given its R&D needs in materials compatibility and reactor control, the SCWR
system is estimated to be deployable by 2025.
The SCWR could provide process heat at temperatures up to about 550 ºC.
REFERENCES FOR CHAPTER 4
[1] OECD/NUCLEAR ENERGY AGENCY, Non-electricity Products of Nuclear Energy, OECD-
NEA Report (2004).
[2] INTERNATIONAL ATOMIC ENERGY AGENCY, Market Potential of Non-electric
Application of Nuclear Energy, IAEA TRS No. 410, Vienna (2003).
[3] INTERNATIONAL ATOMIC ENERGY AGENCY, Status of Non-electric Nuclear Heat
Application; Technology and Safety, IAEA-TECDOC-1184, Vienna (2000).
[4] INTERNATIONAL ATOMIC ENERGY AGENCY, Introduction of Nuclear desalination- A
Guidebook, IAEA, TRS No 400, Vienna (2000).
[5] MASSACHUSETTS INSTITUTE OF TECHNOLOGY, Nuclear Technology and Canadian Oil
Sands, MIT-NES-DES-005 (2005).
69
CHAPTER 5
NUCLEAR ELECTRICITY FOR TRANSPORTATION:
HYBRID-ELECTRIC VEHICLES
5.1. Opportunities
The reduction of gasoline consumption by using hybrid gasoline and electric drive engines in
automobiles and light utility vehicles (four-wheel vehicles that include minibuses, delivery vans,
pickup trucks, and SUVs (sport utility vehicles)) may be an idea whose time has come. Electric-drive
engines have the potential to significantly reduce both the cost of vehicle fuel and emissions of
greenhouse gases in the transportation sector, and can reduce the ever-growing pressure for the
countries with oil reserves to find and distribute more oil to both developed and developing countries.
Furthermore, the technologies for some of the alternative routes, particularly the use of hybrid-electric
vehicles, is currently available with minimal changes to the normal transportation infrastructure. The
plug-in feature, when used, allows charging of the vehicle batteries from utility electric power sources,
and it is this application of nuclear electricity that could have an impact on greenhouse gas emissions
and dependence by the transportation sector on petroleum. The United States and Japan were chosen
for detailed studies of the benefits and impacts of substituting electricity for petroleum fuels by using
plug-in hybrid vehicles.
Consideration was given to other transportation systems, primarily railroads. In Japan, less than 5% of
the transportation energy on an oil equivalent basis is used for rail transportation, of which virtually all
already uses electricity. In the United States, the distances are so great between most major cities that
the capital cost of electrical transmission structures militate against its use except in a few locations in
the northeastern United States. No consideration was given to air transportation because the weight of
the electrical motors and batteries would be prohibitive.
In this chapter, the opportunities to use nuclear power plants, particularly water-cooled reactors , are
investigated. The situations in the United States and Japan are developed in detail, and the lessons
learned are extended to the situation in Europe, and especially China and India where the use of
automobiles and light utility vehicles is currently small, but growing rapidly.
Analyses of the current opportunities will be based on simplified models that deal with quantitative
information available from reliable government sources: the U.S. Department of Energy (DOE)
Energy Information Administration (EIA) in the United States and the Ministry of Land
Transportation and Infrastructure (MLIT) in Japan. Average performance data (20 miles per gallon of
gasoline—mpg) will be used to illustrate the overall behaviour of all the vehicles in the United States
with full realization that smaller, lighter vehicles will perform better, and larger, heavier vehicles will
not perform as well.
The models used in the analysis of the situation in the United States are based on three recent
publications [1, 2, 3]. Data from DOE-EIA are used to define an average gasoline-fueled light vehicle
as a reference vehicle against which the various hybrids and hydrogen-fueled vehicle alternatives can
be compared. Analyses of fuel quantities, fuel costs, and greenhouse gas emissions will be used for
comparisons.
The models used in the analysis of the situation in Japan are also based on recent publications [4, 5, 6].
Data from the MLIT are used to define an average behaviour of target motor vehicles (personal-use
passenger vehicles, called “registered” vehicles and “light” vehicles). The methodology used for the
analysis is similar to the one used for the analysis for the United States [2].
70
5.1.1. Opportunities in the United States
5.1.1.1. Hybrid vehicles
The hybrid-electric vehicle is not a new concept. Hybrids were first conceived and vehicles
constructed about 100 years ago. Even though it was demonstrated to be a viable concept technically,
the hybrid vehicle received relatively little attention until the late 1960s when it was considered by
some to be a possible way of meeting the newly established emission reduction requirements for
automobiles. However, the real stimulus for pursuing hybrids with an organized effort and significant
funding came as a result of the OPEC petroleum embargo and resulting energy crisis of 1973–74. The
U.S. Congress passed Public Law 94-413, the Electric and Hybrid Vehicle Research, Development,
and Demonstration Act of 1976, which directed the newly established Department of Energy (DOE) to
pursue, among other activities, the technologies associated with electric and hybrid-electric vehicles.
While these activities were underway, the energy crisis subsided simultaneously with automobile
manufacturers making dramatic improvements in conventional vehicle fuel efficiency and emission of
pollutants. The introduction and wide spread deployment of engine-controlling microprocessors along
with continued improvements in exhaust after-treatment led to a near doubling of Environmental
Protection Agency (EPA) fuel mileages and an order of magnitude reduction in exhaust emissions. As
a result, there was little corporate interest in pursuing the heavier, more fuel efficient, less polluting,
but more complex and more expensive hybrid vehicles. Even so, the considerable work that had been
completed showed that hybrids could simultaneously improve fuel efficiency and further reduce
greenhouse gas emissions compared to current conventional vehicles.
It was the Japanese automobile industry that introduced conventional hybrid-electric vehicles to the
world with the Toyota Prius and Honda Insight and Civic models.
Today, four general types of hybrids are commonly recognized. Micro-hybrid, mild-hybrid, and full-
hybrid vehicles are commercially available; plug-in hybrids have been demonstrated, but are still
under development. Regenerative braking is used on almost all types of hybrids. It converts some of
the kinetic energy of a moving vehicle to electrical energy and stores it in the battery for later use,
rather than converting it to wasted heat by friction between the brake discs and brake pads. However,
friction brakes are also required to come to a complete stop. The identifying characteristics of the four
types are:
Micro-Hybrid. When the vehicle stops, the engine is turned off to save fuel. When the driver
pushes the accelerator, the integrated starter/alternator initiates acceleration of the vehicle and
simultaneously starts the gasoline engine. The integrated starter/alternator assists the engine in
accelerating the vehicle until the desired speed is reached. The gasoline engine alone propels the
vehicle during cruising. Fuel efficiency is about 10% better than for a comparable standard
vehicle (22 mpg vs. 20 mpg).
Mild-Hybrid. The electric motor (starter) and alternator are separate units. The electric motor
assists the gasoline engine, but generally does not propel the vehicle alone except in “start and
stop” traffic. The electrical components are larger and the gasoline engine is smaller than in the
micro-hybrid. Fuel efficiency is about 20–25% better than for a comparable standard vehicle
(24-25 mpg vs. 20 mpg).
Full-Hybrid. All the electric components are larger than in the mild-hybrid so that it can
operate on the battery alone for longer periods of time. The gasoline engine is somewhat
smaller, and the fuel efficiency is typically 40–45% better than for a comparable standard
vehicle (28–29 mpg vs. 20 mpg).
Plug-in Hybrid. The battery typically needs a much larger capacity than in the full-hybrid, and
the vehicle needs an “all electric” range that is about 5 times greater than that of a full-hybrid.
The gasoline engine may be somewhat smaller, and the digital control system to optimize the
various driving situations is more complex. Gasoline mileage has little meaning since the
71

vehicle may use little or no gasoline for short trips, only electrical energy in the battery that
came from an electric utility.
These four types of hybrid have many common components, such as regenerative braking, gasoline
engine, electric motor, alternator, battery pack, and central digital-control system. Moving from the
micro-hybrid to the plug-in hybrid, the size of the electrical components becomes larger and their use
increases, the gasoline (or diesel) engine becomes somewhat smaller, the performance (acceleration)
increases, and the fuel economy increases. However, larger electrical components are heavier, more
complex, and more expensive. Furthermore, the decrease in the size of the gasoline engine is often less
than the increase in size of the electrical components.
The reason that hybrid vehicles accelerate so well is that torque, which provides acceleration, is very
high at stall and low speed for electric motors. Torque produced by a gasoline engine increases with
engine speed from a low value at low rpm (revolutions per minute) to a maximum in the 1500–2500
rpm range, after which it falls off somewhat. Hence, in hybrids, the combination of an electric motor
and a gasoline engine together provides higher torque and better acceleration than is available in
comparable conventional vehicles of equal horsepower, even though the hybrids usually weigh more
and have smaller gasoline engines.
The reason that hybrids are so fuel efficient is that the amount of fuel consumed per unit of energy
output (specific fuel consumption—pounds or gallons per horsepower-hour output) generally
decreases with power level until it reaches a minimum at 65–85% of maximum power. Multi-speed or
continuously variable speed drives are used to keep the engine operating near its most efficient speed.
Thus, a smaller engine running at a higher percentage of its full power is more efficient and more
economical for a given load than a larger, heavier gasoline engine operating at a lower percentage of
its maximum power.
The Electric Power Research Institute (EPRI), the research organization of the U.S. electric-utility
industry, has conducted a research program for plug-in hybrids using batteries that are charged
primarily with electricity generated by utilities. Generating the electricity needed to charge these
batteries is a primary new market for nuclear electricity, as discussed in this chapter [7].
5.1.1.2. Opportunities for reducing greenhouse gas emissions from transportation
The principal greenhouse gases are water vapour
(60–65%) and CO
2
(20–25%)
.
Water vapour stays in
the atmosphere for a relatively short time, a matter of hours or days, whereas CO
2
has an average
residence period of about a century. As a result, the amount of water vapour remains relatively
constant at a level related to the rate at which it is produced by weather phenomena, while CO
2
tends
to accumulate as the amount emitted increases. All other greenhouse gases (10–20%) such as methane,
ozone, nitrous oxide, carbon monoxide, and fluorocarbons tend to have lesser effects on the
atmosphere over time because of smaller quantities or short residence times. Hence, the only
greenhouse gas considered in this analysis is CO
2
.
Greenhouse gas emissions from gasoline vehicles in the United States of America
Combustion of gasoline emits 19.56 pounds of CO
2
per gallon of gasoline. Since the average gas
mileage in our model is 20 miles/gallon, the annual emission of carbon dioxide is
19.56 lb CO
2
per gallon / 20 miles/gallon = 0.978 lb/mile
Or
0.978 lb/mile x 12,260 miles/year = 12,000 lb CO
2
/vehicle-year for gasoline vehicles
5
5
The total miles per day and per vehicle year are:
72
[9 x 10
6
barrels/day x 42 gallons/barrel x 20 miles/gallon] = 7.56 x 10
9
miles/day;
[7.56 x 10
9
miles/day x 365 days/year] / [225 x 10
6
vehicles] = 12,260 miles/vehicle year.

Clearly, the amount of CO
2
for smaller vehicles would be less than for larger vehicles because the
gasoline mileage is greater. However, the model for this analysis deals with average vehicles that
achieve 20 miles per gallon, the reference performance used for all comparisons.
Emissions for traditional hybrid vehicles
Since all the energy to propel traditional types of hybrid vehicles is provided by gasoline, this analysis
utilizes the increased fuel mileages assigned in an earlier publication [2], which reduces the total CO
2
emissions for traditional hybrid vehicle accordingly, as shown in Table 5.1.
Table 5.1. Emissions for hybrid vehicles
Type Gasoline CO
2
per CO
2
per
Hybrid Mileage Mile Vehicle-Year
Miles/gal lb lb
Micro-Hybrid 22 0.889 10,900
Mild-Hybrid 25 0.782 9,600
Full-Hybrid 29 0.674 8,270
These values are for hybrid vehicles of a size corresponding to an average vehicle that attains 20
miles/gallon. A larger or smaller vehicle would have proportionally higher or lower emissions.
A more thorough analysis of the environmental impacts of hybrid electric vehicles was performed in
1997 using the lifecycle analysis software GREET, the Greenhouse gases, Regulated Emissions, and
Energy use in Transportation model. GREET was developed at Argonne National Laboratory to
evaluate well-to-wheels energy and emission impacts of motor vehicle technologies powered with
various transportation fuels. The model and associated documents are posted at
http://www.transportation.anl.gov/software/GREET/index.html
. The 1997 study looked at total
lifecycle energy use and emissions from the vehicle cycle, the fuel cycle, and vehicle operations for
several hybrid electric vehicle designs and compared them with results for conventional vehicles.
Greenhouse gas emissions from the hybrids were found to be 40% lower than those from conventional
vehicles. Volatile organic compound emissions were reduced by 15 to 28%, carbon monoxide by 7 to
37%, and particulate matter with sizes smaller than 10 micrometers (PM
10
) by a small amount. On the
other hand, NOx emissions were calculated to increase by 28 to 67%, and SOx by a factor of 6 to 14.
In large part those increases were due to the processing of nickel needed for nickel-metal hydride
batteries. The transition to lithium-ion batteries should reduce those pollutants.
Overall energy use for hybrids was shown to be about 40% less than that for conventional vehicles,
with an equivalent reduction in fossil energy use and greenhouse gas emissions (CO
2
, CH
4
, and N
2
O).
No plug-in hybrid vehicles were examined in 1997. Those studies are underway now and results are
expected shortly.
Emissions for plug-in hybrid vehicles
The model used for greenhouse gas evaluations for a plug-in hybrid vehicle is the same model used in
sections 5.2.1.3 and 5.2.1.4 for cost analysis. The total distance travelled per day by all 4-wheel
73

gasoline-fueled vehicles is 7.56 x 10
9
miles/day. (See Section 5.2.1.2.) Subtracting the 5.625 x 10
9
miles per day travelled on electricity
6
gives 1.935 x 10
9
miles/day as the distance per day travelled as
a full-hybrid. This means that 74.4% of the distance was travelled using electricity as fuel and 25.6%
of the distance was travelled using gasoline.
The emission of carbon dioxide by plug-in hybrid vehicles is made up of two parts: that emitted
directly for 25.6% of the total mileage using gasoline and that emitted in the generation of 74.4% of
the total energy provided by electricity. The direct emissions due to the operation in full hybrid models
given in Table 5.1 for 25.6% of the miles is
8,270 lb CO
2
/vehicle year x 0.256 = 2,120 lb/vehicle year
If the electricity is supplied by nuclear, solar, wind, hydro or renewables, no carbon dioxide is
generated in the process of electricity production (though greenhouse gases are emitted in other stages
of the lifecycle of the energy source, for example in the mining and enrichment of uranium or in the
manufacturing of solar photovoltaic cells). Hence, the total emissions are 2,120 lb/vehicle year.
If the electricity is generated by fossil fuels (natural gas, oil or coal), the resulting generation of carbon
dioxide must be added to that generated directly. Carbon dioxide emissions generated per kWh of
electricity by fossil fuels are given in Table 5.2 based on data from the Department of Energy, Energy
Information Administration.
In Section 5.2.1.4, it is established that 0.603 kWh/mile is a reasonable average expenditure of
electrical energy for hybrid vehicles that would be comparable to a vehicle getting 20 miles per gallon
of gasoline using an internal combustion engine. It is readily shown that the average distance travelled
by each plug-in hybrid vehicle while operating on electricity alone would be 9,125 miles/year.
7
Hence, the electricity used is
9,125 miles/year x 0.603 kWh/mile = 5,502 kWh/vehicle year.
Table 5.2. Emission rates for fossil fuels
Fuel to Generate % of Total CO
2
Emission Rate
Electricity Generation lb/kWh
Coal 51.0 2.100
Oil 3.2 1.970
Natural Gas 15.2 1.320
Renewables 0.6 0 (net)
Non-Fossil* 30.0 0
*(Nuclear 20%; Hydro 7%; Wind 2%; Solar <1%)
If this value is multiplied by the amount of CO
2
emission per kWh for the three fossil fuels given in
Table 5.2 and added to the 2,120 lb/vehicle-year of CO
2
for operation in the full hybrid mode, the
results given in Table 5.3 for the various fuels are obtained.
6
The miles per day driven on electricity is:
[225 x 10
6
vehicles x (15 + 35)/2 miles/vehicle day] = 5.625 x 10
9
miles/day.
7
The average distance per year on electricity is:
[(15 + 35)/2 miles/vehicle day x 365 days/year] = 9125 miles /vehicle year.
74
Table 5.3. Emissions for plug-in hybrid vehicles
Fuel to Generate Emissions of CO
2
Electricity lb/vehicle-year
Coal 13,650
Oil 12,950
Natural Gas 9,400
Nuclear 2,120
Solar 2,120
Wind 2,120
Hydro 2,120
Renewables (Net) 2,120
It is clear that if some 200-250 new 1000 MWe power plants, as calculated later in this chapter, or
even a fraction of this number, are to be built to provide electricity for plug-in hybrid-electric vehicles,
they should not use fossil fuels. Indeed, a strong case can be made that nuclear energy is the only
practical choice. Both solar and wind are intermittent and would require large amounts of energy
storage and recovery systems that are almost as complex as generation systems. There are relatively
few, if any, remaining practical hydro sites in the United States, and renewables on this scale would
utilize an enormous amount of land that will be needed for food production for a growing population.
5.1.2. Opportunities in Japan
5.1.2.1. Situation of automotive fuels and electric power in Japan
In 2004 Japan imported about 96% of its energy from abroad, including 99.7% of its petroleum (of
which 89.5% is from the Middle East), and 96.5% of its natural gas. The transport sector consumes
about a quarter of the total energy in Japan. Most of the consumption is petroleum fuels (98% in
FY2000) such as gasoline or diesel oil used for automobiles.
Electricity also makes up a quarter of total energy demand. Electricity in Japan is generated from a
diverse set of sources, including nuclear 31.5%, coal 25.4%, natural gas 24.0%, petroleum 10.3%, and
hydro 8.4% (2005 statistics). Thus, in the power generation sector, the dependence on fossil fuels is
only about 60%. Hence, the security of the energy supply and the reduction of CO
2
emission are being
improved by decreasing the petroleum and carbon fuel consumption through a growth in non-carbon
electricity sources.
Similarly, if automobiles are powered by electricity by using plug-in type vehicles, the energy supply
can be diversified to become less dependent on petroleum. Along with the increase of plug-in vehicles
in the future, the new electric demand for charging the batteries would hopefully be supplied by
nuclear power, thus making the energy supply more secure and reducing CO
2
emissions in Japan.
5.1.2.2. Driving patterns of Japanese passenger vehicles
There are about 77.4 million vehicles altogether in Japan. From the size and the driving pattern of
these vehicles, the categories suitable for plug-in hybrid electric vehicles are the personal-use
passenger vehicles, of which there were 54.4 million vehicles as of 2003. They are classified into the
”registered” vehicles, which are ordinary-sized cars and ”light” vehicles, which are smaller cars with
engine capacities under 660 cm
3
and which have some benefits in terms of taxes and other costs.
The average daily travel distances of these categories of vehicle are estimated from the statistical
survey data from MLIT on the relationship of passengers carried for various distances.
75

Driving Pattern of Japanese Passenger Vehicles
Registered and Light Vehicles for Personal Use
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 20 40 60 80 100 120 140 160
Average Daily Travel Distance [Km]
Cumulative Fraction of Vehicles [-]
Registered V.
Light V.
FIG. 5.1. Driving patterns of Japanese passenger vehicles.
From Figure 5.1 on the driving pattern of Japanese passenger vehicles, it is presumed that on average
Japanese vehicles are driven about 20 km (18 km for light vehicles and 22 km for registered vehicles).
The average daily travel distance of Japanese vehicles is about 62% of that of US light vehicles, which
is about 20 miles or 32 km per day, as previously mentioned.
5.1.2.3. Expectation for plug-in hybrid electric vehicles in Japan
As the self-sufficiency of energy is currently very low in Japan, shifting the energy for the
transportation sector to nuclear energy, though it may take a long time, would significantly improve its
energy security. For nuclear energy supply to serve the transportation sector several technologies have
been suggested, including plug-in type vehicles, hydrogen fuel cell or combustion engines, or the use
of synthetic fuels. Among these, the introduction of plug-in hybrid vehicles into the automotive market
is expected to be the most realistic and is considered the leading option for this purpose.
As the weight of Japanese vehicles is lighter and the daily distance travelled is shorter in Japan as
compared to the U.S., especially for the category of ”light vehicle,” it would be easier to introduce
plug-in hybrid vehicles in Japan because a smaller battery could give a larger electric run fraction.
5.1.3. Opportunities in other countries
A recent comparison of gasoline consumption for several countries gives a useful view of the world
situation. In an article entitled “Situation is Obscenely Out of Whack” [8] the data in Table 5.4 were
given for the consumption of gasoline per person per year for transportation.
76
Table 5.4. Consumption of Gasoline throughout the World [8]
Consumption of Gasoline per person per year
United States 453 gallons
Europe (includes diesel fuel) 133 gallons
Japan 124 gallons
World Average 47 gallons
World Average (w/o U.S.) 28 gallons
China 10 gallons
India 2 gallons
The discrepancy between the gasoline consumption in United States and the rest of the world is clearly
shown. In many respects, the situation is a reflection of the historic low price and the large domestic
production of oil. Given the rapidly growing demand for oil in the developing countries, particularly
China and India, and the signs of declining production in many of the oil-producing countries,
including the United States, there is an urgent need for reducing the use of oil. Substitution of
electricity for oil by the use of plug-in hybrid vehicles is an attractive way of achieving this goal
without disrupting the existing transportation infrastructures in the developed countries, particularly
the United States.
Opportunities in the European Community
For the last several decades, the price of gasoline has been much higher in Europe than in the United
States, because of a large tax each European country has imposed. Since the OPEC embargo in 1973
until 2005, the price of gasoline has varied between $3 and $6 per gallon in Europe while the price in
the United States has varied between $1 and $2 per gallon. Europeans have adjusted to the higher
prices by buying smaller, more efficient automobiles and using a much higher percentage of diesel
powered light vehicles. The recent availability of biodiesel fuel that virtually eliminates diesel
pollutants may accelerate this trend. In addition, Europe has adopted efficient public transportation
systems. As a result, the steep rise in oil prices in 2005–2006 has had significantly less impact on
transportation costs in Europe than in the United States. Hence, there may be less urgency to consider
hybrid vehicles in Europe than in the United States. Indeed, the principal European automobile
manufacturer seriously investigating hybrid vehicles today is DaimlerChrysler, and none have hybrid
vehicles for sale or lease.
Opportunities in China and India
China and India have populations of about 1.4 and 1.0 billion, respectively, and are growing. Their
economies are growing extremely rapidly, and both are developing a large economic middle class that
are buying automobiles and other light vehicles. If these vehicles utilize oil-based fuels, the current
world mismatch between supply and demand will be exacerbated, and the price of oil will continue to
rise. A strategy by China and India to minimize the use of gasoline through substitution of electricity
would be beneficial to all countries and help stabilize the price of gasoline in China and India as well
as in the rest of the world. China has an aggressive program of installing new nuclear (and other)
power plants that could be utilized to provide electricity to charge automotive batteries at night.
Indeed, with all the new power plants they are building, their excess capacity at night may be adequate
to charge vehicle batteries, which would contribute to keeping the nuclear power plants at continuous
base load operation.
77
5.2. Market context
5.2.1. Market context in the United States of America
5.2.1.1. Status of hybrid-electric vehicles
Today, there are several automotive manufacturers, including Ford, General Motors, Honda, Toyota,
and Lexus that offer conventional hybrid (micro-hybrid, mild-hybrid, and full hybrid) vehicles.
Generally, these vehicles (except the micro-hybrids) sell at prices that are $5000 to $10 000 higher
than corresponding traditional models, and that typically are not offered at discounted prices. Some
models provide gas mileages that are significantly better than their corresponding traditional models;
others offer only minor gasoline mileage improvements, but rather, offer outstanding acceleration.
General Motors, Ford and Toyota are the principal automotive manufacturers which have expressed
interest in plug-in hybrids. GM and Ford displayed plug-in hybrid electric vehicles using lithium-ion
batteries at the Detroit Automobile show in early 2007. However, as indicated in section 5.3.1, few
automakers intend to produce consumer test vehicles as GM had done with their EV1 electrical
vehicles. Toyota, however, announced on July 25, 2007, that it is donating several prototypes of its
Plug-in HV to the University of California-Irvine and the University of California-Berkeley for road
testing. In addition, Toyota stated that the Japanese Ministry of Land, Infrastructure and Transport
(MLIT) has now approved testing of plug-in hybrid vehicles on Japanese public roads. The Plug-in
HV has a battery range of 8 miles on a full charge, which is significantly less than the 30-mile all-
electric range achieved by EDrive with its modification of the Prius with larger lithium ion batteries.
Toyota has indicated that the Plug-in HV is not ready for commercialization, because it uses low-
energy nickel-metal hydride batteries, rather than more advanced lithium-ion batteries. The company
sees battery technology as the key technical barrier to the viability of plug-in hybrid vehicles. Toyota
and General Motors have announced targets of 20 to 40 miles on a single charge for plug-in hybrids.
The U.S. government offers up to $3,400 in credits against income taxes for the first 60,000 U.S.
taxpayers who purchase certain certified hybrid vehicle models from any manufacturer. Generally the
certification is related to the improvement in gasoline mileage and decrease in greenhouse gas
emissions. The combination of this tax credit and the reduction in the cost of fuel (i.e. use of lower-
cost electricity in place of expensive gasoline) may be adequate to recover the higher price of some
hybrid vehicles (but not others) over a few years. Toyota, for instance, has already reached the 60,000
limit, and the tax credits are being phased out over a year for both Toyota and Lexus models.
The primary motivation for buying the more efficient hybrid vehicles such as the Toyota Prius and
Honda Civic appears to be better gas mileage. However, trends in newer hybrids (Toyota Highlander
and Honda Accord) have been to emphasize performance (0 to 60 mph in about 7 seconds), whereas
gas mileage is only a little better than their conventional counterparts. Honda recently discontinued its
most efficient hybrid, the two-passenger Honda Insight, apparently because it sold less than 1000
vehicles in the last year. The total number of hybrid vehicles sold in the United States in 2005 was
205,749 of which 52% were the Toyota Prius. Even so, this is a small fraction of the ~15 million total
vehicles sold annually in the United States. Given the present pattern of traditional hybrid designs and
sales, the overall impact on the amount of gasoline used for transportation in the United States is not
likely to be significant. Estimates for market penetration in a decade or two range from 3% by J. D.
Power Forecasting to 80 % by Booz Allen Hamilton, with the DOE EIA predicting a conservative 7%
of total sales. If an average of 30% improvement in gas mileage and a 30% market penetration by
conventional hybrids is assumed, the savings of gasoline would be only about 0.6 of the 9.0 million
barrels per day currently used, a 6.9% saving. This is not the kind of fuel saving that is needed in the
United States to significantly reduce the U. S. dependence on foreign imports. The conclusion is that
the traditional hybrid vehicles will not significantly reduce fuel consumption in the United States.
Only plug-in hybrid-electric (or perhaps hydrogen fueled) vehicles can save enough gasoline to
significantly reduce gasoline consumption to the extent needed.
78

Given the situation described above in which the market penetration for conventional hybrids appears
limited with the primary emphasis of recent models on performance rather than fuel economy, we
must look to the plug-in hybrids to achieve a significant saving of fuel. It is theoretically possible to
save almost 75% of the fuel currently used, some 6.7 of 9.0 million barrels per day if all vehicles
suddenly became plug-in hybrids operating in the average manner described in the model. If there
were only a 30% market penetration by plug-in hybrids, there would be a savings of about 2 of the 9
million barrels per day, about three times the savings of traditional hybrid vehicles.
5.2.1.2. U.S. light-vehicle transportation statistics and model
Estimates based on extrapolated DOE-EIA data
8
from the 1990s indicate that there are approximately
225 million four-wheel, light-transportation vehicles in the U.S; 133 million are passenger
automobiles and 92 million are light truck vehicles (including SUVs, minivans, pickup trucks, and
delivery vans). It is further estimated by EIA that on any given day, on average, 50% of U.S. vehicles
are driven less than 20 miles. Using these statistics, a simple model can be developed to calculate the
potential saving of fuel by the use of hybrids operating in a plug-in mode. The model assumes that
only the electric motor, operating on batteries charged from electric-utility sources, is used to power a
vehicle until the battery has discharged to a level requiring charging of the battery to maintain normal
full-hybrid performance (estimated to be 35 miles). Beyond that point, the gasoline (or diesel) engine
and electric motor would operate together in the normal full-hybrid mode.
Model for plug-in operation
As before, the standard automobiles and light truck vehicles are grouped and assumed to achieve an
overall average of 20 miles per gallon of gasoline.
9
It is assumed that in two to three decades (i.e.,
2025 to 2035), all these vehicles are hybrids capable of the plug-in mode of operation.
10
This involves
charging the batteries of a hybrid vehicle overnight using electricity from an electrical outlet, typically
in the owner’s garage. It is assumed that when batteries are fully charged, these hybrids can operate
using only the electric motor for at least the first 35 miles. For this type of operation, the controls of
current full-hybrids would need to be modified so as not to use the gasoline engine to recharge the
batteries beyond the level necessary to sustain normal hybrid operation. The vehicles envisioned by
the authors for plug-in operation are those manufactured by automotive companies to meet today’s
safety and quality standards, and are equipped with normal features such as automatic transmission, air
conditioning, and power steering. Hence, a more powerful electric motor (kW) and better and higher-
capacity batteries (kWh) would be required.
11
This could lead to the use of a smaller gasoline engine.
Solid-state digital controls capable of optimizing performance and economy while minimizing the use
of fuel should make the performance and economics of these vehicles more than competitive with
comparable standard vehicles while drastically reducing both fuel consumption and greenhouse gas
emissions.
8
Unless otherwise indicated, data on U.S. vehicles are from the DOE Energy Information Administration’s
(EIA) statistics available on the internet (see Bibliography).
9
DOE EIA data show that in 2003, automobiles averaged 22.3 miles per gallon and light trucks averaged 17.7
mpg.; hence, 20 mpg is a reasonable weighted-average value for all vehicles (see Bibliography).
10
This assumption was made in order to evaluate the total potential savings of fuel associated with using hybrid
vehicles operating in the plug-in mode. Fuel savings will be reduced in relation to the percent of light vehicles
that are not plug-in hybrids.
11
Most current commercial full-hybrid vehicles would have to have batteries with at least five times more
capacity (kWh) and probably more power (kW) to operate at highway speeds for this distance.
79

5.2.1.3. Model to calculate fuel saved by the plug-in mode of operation
The model assumes that each day one-half of the 225 million light-hybrid vehicles operate only for 15
miles on batteries alone while the other half operate on batteries alone for their first 35 miles and then
automatically switch to normal full-hybrid mode (in which gasoline powers the vehicles for the
remaining miles that day). This means that electrical energy provided by a utility to recharge batteries
would drive these vehicles for a grand total of 5.625 billion miles per day or an average of 9,215 miles
per vehicle year.
12
If the comparable standard (non-hybrid) light vehicle averages 20 miles per gallon,
then 225 million light vehicles would use 281 million gallons of fuel per day to travel 5.625 billion
miles per day. Hence, it is theoretically possible, based on this simple model, to replace 281 million
gallons (6.7 million barrels) of fuel per day with electricity by using hybrid vehicles operating in the
plug-in mode.
13
This represents 74.4% of the estimated nine million barrels of oil per day now used to
produce gasoline for standard automobiles and light-truck based vehicles.
14
These results are
consistent with work carried out by the Electric Power Research Institute in cooperation with Daimler
Chrysler. [7] Clearly reductions in both imported oil for transportation fuels and emitted atmospheric
pollutants would be dramatic with widespread acceptance of full-hybrids operating in the plug-in
mode with power from non-carbon sources. However, realistically, some of the saved fuel would still
be needed, because in the two to three decades needed for full implementation, the number of vehicles
and the number of miles driven per vehicle in the U.S. could increase significantly—perhaps 50% or
more in total miles.
5.2.1.4. Fuel cost savings
At a price of $3.00 per gallon, the fuel cost is $0.15 per mile for standard light vehicles averaging 20
mpg. Since a gallon of gasoline contains 36.65 kWh of thermal energy, 1.833 kWh is used per mile.
However, the efficiency of an internal combustion engine operating over a range of speeds plus energy
losses in the transmission, drive, and tires results in an “overall gasoline thermal energy to miles
travelled efficiency” of about 20%.
15
Hence, the average mechanical energy expended at the
pavement for driving the vehicle is only 0.367 kWh per mile. If the overall efficiency of the electric
drive including charger, batteries, motor, generator, and drive is 70%, the electrical energy purchased
from the utility is 0.524 kWh per mile. Because the proposed plug-in mode of operation would
probably require larger batteries and perhaps a larger electric motor, adding a few hundred pounds of
weight to the vehicle, this value could be increased by 15% to 0.603 kWh per mile. At a price of $0.08
per kWh
16
, the cost of electricity to drive a mile in a hybrid vehicle is only $0.048. This is $0.102 per
mile less than the $0.150/mile for gasoline. For the half of light hybrid drivers in our model who travel
15 miles per day (5,475 miles per year) using electricity, the annual savings would be $558 per year.
For the other half of the light-hybrid drivers who travel 35 miles per day (12,770 miles per year) using
electricity before shifting into full-hybrid mode, the savings would be $1303 per year. There would
also be some additional saving of gasoline, calculated to be 108 gallons/year, associated with full-
12
[225 x 10
6
vehicles] x [(15 +35)/2 miles/vehicle day] = 5.625 x 10
9
miles/day;
[5.625 x 10
6
miles/day x 365 days/year] / 225 x 10
6
vehicles = 9125 miles/vehicle year.
13
5.625 x 10
9
miles/day / 20 miles/gallon = 281 x 10
6
gallons/day or 6.70 x 10
6
barrels/day.
14
Changing the model assumptions would give different numerical results, but would not affect the overall
conclusion that it is possible to save a large majority of the petroleum fuel used for U.S. light vehicles
today through the wide-scale use of plug-in hybrid vehicles.
15
Estimate of this efficiency by Toyota Motor Company is 16%, which is used in the analysis of Japanese
vehicles later in this chapter.
16
This price is the estimated interruptible price in which the utility could interrupt the charging of vehicle
batteries during short periods of peak electrical demand in exchange for a reduced cost of electricity. The
consequences to the driver are estimated to be negligible, perhaps a few extra gallons of gasoline per year.
80

hybrid operation for the remaining distance travelled by the second half of the vehicles. This amounts
to$324 per year for $3.00 per gallon gasoline. Hence, the total savings would be $1627/year at
$3/gallon for the second half of the vehicles.
17
5.2.1.5. Impact of tax on electricity used in vehicles
It is inevitable that if electricity becomes a significant source of energy for automotive and light-truck
vehicle travel, it will be taxed by an amount sufficient to recover the tax revenue lost on petroleum-
based fuels by governmental authorities at the national, state, and local levels. If we assume that the
current total tax on these fuels is about $0.35 per gallon and the total consumption of fuel is 103
billion gallons per year (281 million gallons per day), the total tax would be $36.0 billion per year.
Using information provided earlier, the calculated total kWh of electricity consumed in the plug-in
mode would be 1.238 trillion kWh per year. The equivalent tax is about $0.029 per kWh, thereby
increasing the cost of electricity used on the road from $0.080 to $0.109 per kWh. Hence, the fuel cost
per mile for the light-hybrid vehicles increases from $0.0483 to $0.0657 per mile, which is still less
than half of the $0.150 per mile for standard vehicles using $3 per gallon of gasoline. These electrical
costs are equivalent to $0.97 and $1.32 per gallon respectively without and with taxes.
The annual savings for gasoline at $3 per gallon are substantial, but they may not be large enough to
justify the additional cost of a plug-in hybrid vehicle. However, if the cost of gasoline increases to $5
or $6 per gallon, prices that are common in Europe today and a realistic possibility in the U.S. if oil
imports are not significantly reduced or if taxes are imposed to reduce greenhouse gas emissions, the
savings become much larger. These annual savings with and without taxes for gasoline prices ranging
from $1 to $7 per gallon are shown in Figure 5.2.
Annual Savings of Plug-In Hybrid Vehicles
Cost of Electricity: $0.08 per kWh
-500
0
500
1000
1500
2000
2500
3000
3500
4000
4500
1234567
Cost of Gasoline $/Gallon
Annual Savings $/Year
15 miles/day No Tax
15 miles/day Tax
35 miles/day No Tax
35 miles/day Tax
FIG. 5.2. Annual savings of plug-in hybrid.
17
[(7.560 x 10
9
– 5.625 x 10
9
) miles/day x 365 days/year x $3/gal] / [225 x 10
6
vehicles x 29 miles/gal]
= $324/vehicle year.
81

5.2.1.6. Home electrical service required
Now let us look at the electrical supply required to support plug-in hybrid-electric vehicles. Light-
hybrid vehicles that travel 35 miles per day on electricity using 0.603 kWh/mile would use 21.1 kWh
per day. If batteries are to be charged in eight hours at night using a 110-volt system, the required
capacity would be about 24 amps. However, charging batteries requires more current when the
batteries are deeply discharged, so the peak currents could be higher. Even so, it seems reasonable that
most modern homes would have adequate spare capacity to provide at least 30 amps at 110 volts
during the night. Apartment dwellers or urban dwellers who rely on street-side parking or public
parking facilities may not have ready access to electricity for overnight charging. Infrastructure would
need to be developed to accommodate such consumers.
5.2.1.7. Electric utility generating capacity
Plug-in hybrid electric vehicles may represent a significant new demand for electricity. It is important
to know, then, the total potential electrical generating capacity required. Multiplying the 5.625 x 10
9
miles per day for all the light hybrids using electricity times the 0.603 kWh per mile results in 3.39
billion kWh per day. Charging the batteries in eight hours would require 424 million kWe or 424
GWe. This equals the output of 424 power plants of 1,000 MWe size. Because the entire generating
capacity of the United States today is about 950 GWe, it is clear that there would not be sufficient
spare capacity available at night or any other time to charge the batteries of all the hybrids that could
exist in 2035. Although not all charging would occur in the same eight-hour period because of time
zones and the availability of some existing excess capacity, significant new generating capacity—
perhaps 200 to 250 new 1,000 MWe nuclear or other non-polluting plants—would have to be built to
charge the batteries for complete conversion to plug-in hybrids. New transmission and distribution
lines and substations would also be needed to deliver the electrical power. Building 200 to 250 new
1,000 MWe power plants and associated power delivery facilities in two to three decades would be a
daunting task, but certainly feasible.
5.2.2. Opportunities in Japan
5.2.2.1. Model evaluation of plug-in hybrid-electric vehicles in Japan
The effect of introducing plug-in hybrid electric vehicles (PHEV) in Japan is evaluated for the
category of personal use passenger vehicles.
Target vehicles for evaluation
As described earlier, in Japan passenger cars are classified into two categories: the ”registered” vehicle
and the ”light” vehicle. Typical statistical data of these vehicles are shown in Table 5.5, which are
derived from the 2003 Report by the Ministry of Land Infrastructure and Transport, Japan (MLIT).
Table 5.2.1. Data used for evaluation of PHEV
Registered
vehicles
Light
vehicles
Number of cars 42,620,000 11,820,000
Average distance traveled per
working day per car, km
40.7 27.9
Working ratio *, %
66.9 72.7
Average distance traveled per
day per car, km
27.2 20.3
Average distance traveled per
year per car, km
9,900 7,400
Fuel consumption per car per
Km **, liter/km
0.12 0.09
* Working ratio=(Working days x cars / Existing days x cars ) x 100
** Gasoline engine
82

Methodology and input data
The methodology and most of the parameters used are similar to the U.S. analysis just described. [2]
Nevertheless, there are some differences from the U.S. analysis:
18
•
The average electric run fraction is estimated from the statistical data from MLIT;
•
The tank-to-wheel efficiency for ICEVs is based on the information from Toyota Motor
Company.
Input data used for the evaluation are as follows:
•
Tank-to-wheel efficiency for ICEVs: 16%;
•
Battery-to-wheel efficiency for PHEVs: 70% (Adding 15% to the required energy because of
the extra weight for PHEVs);
•
Gasoline price: 122 Yen/litre including a gasoline tax of 53.8 Yen/litre;
•
Electricity price: 10 Yen/kWh (A typical price of the midnight special fee for 11pm to 7am
including the basic charge);
•
CO
2
emission for gasoline: 2.32 kg-CO
2
/liter gasoline (Guideline by the Ministry of
Environment);
•
CO
2
emission for electric power: 0.381 kg-CO
2
/kWh (Performance data of Tokyo Electric
Power Company in 2004).
Electric run fraction
In this evaluation, the average daily travel distance is estimated from the statistical survey data by
MLIT on the relationship of passengers carried for various distances for the categories of vehicle as
described in Section 5.1.2 In Fig. 5.1. is shown the cumulative fraction of vehicles with average daily
travel distances for the two categories of vehicles.
The average fraction, by distance, of traveling in the electric vehicle mode (electric-run) relative to the
capacity of the equipped battery can be estimated from the relation of Fig. 5.1. The relation obtained
for the average electricity-run fractions is shown in Fig. 5.3 for the registered vehicles and the light
vehicles. From the figure, it is estimated that 70% of the electric-run fraction by distance can be
obtained by installing a battery with a capacity of about 60km for the registered vehicles and about 35
km for the light vehicles.
18
Abbreviations
ICEV: Internal Combustion Engine Vehicle
PHEV: Plug-in Hybrid Electric Vehicle
HEV: Hybrid Electric Vehicle or Gas-Electric Vehicle
BEV: Battery Electric Vehicle
83

Passenger Vehicles for Personal Use
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 20 40 60 80 100 120 140 160
Battery Capacity [km]
Electric-Run Fraction [ -
]
FIG. 5.3. Relation of electric-run fraction with battery capacity.
Running cost
The running costs of ICEVs, PHEVs in the electric-run mode, and PHEVs in the mixed electric-
run/hybrid-electric mode are evaluated and compared as follows:
The running costs for registered vehicles are:
-
ICEV: 14.6 Yen/km
-
PHEV in the electric-run mode: 3.0 Yen/km
- PHEV in the 70% electric-run mode and 30% hybrid-electric mode: 4.0 Yen/km
The running costs for the light vehicles are:
-
ICEV: 11.0 Yen/km
-
PHEV in the electric-run mode: 2.3 Yen/km
-
PHEV in the 70% electric-run mode and 30% hybrid-electric mode: 3.0 Yen/km
The running cost of PHEVs in the electric-run mode is about 1/5 of that for a gasoline ICEV, and the
running cost of PHEVs in the 70% electric-run mode and 30% hybrid-electric mode is 1/3.6. If the
gasoline tax is excluded, this ratio becomes 1/2.7 and 1/2.6, respectively.
5.2.2.2. Electric power requirements in Japan
If all the vehicles (both registered vehicles and light vehicles for a total of 54 million vehicles) become
PHEVs in the 70% electric-run mode, the total electricity requirement for 8-hour charging is about 35
GW (35 units of 1,000 MW plant). Since there is about a 50-GW difference between the peak hours
and the midnight hours currently in Japan, most of the power for all PHEVs could be supplied by the
spare power.
Since nuclear power is presently used as the base load in Japan, the additional power requirements
would have to be supplied by operating fossil fuel plants at night. For energy security and global
environment, it is better to shift the power supply structure, in the course of introducing PHEVs, to a
greater nuclear share by replacing the fossil fuel plants by new nuclear plants.
Registered
Light
84

5.2.3. Energy utilization efficiencies of various power trains
Energy flow to the vehicles with various power trains, such as internal combustion engine vehicles
(ICEVs), hybrid electric vehicles (HEVs), plug-in hybrid electric vehicles (PHEVs), battery electric
vehicles (BEVs) and hydrogen fuel cell vehicles (FCVs), is shown in Fig. 5.4.
The energy carriers such as hydrocarbons (gasoline, diesel oil, etc.), electricity, and hydrogen are
produced from primary sources, such as fossil fuels, nuclear energy, and renewable energies.
Fossil Fuels
Coal, Petroleum,
Natural Gas,
Oil Sands, etc.
Hydrocarbons
Gasoline, Diesel Oil, LPG,
CNG, DME, Synthetic Fuels,
Bio-fuels, etc.
Electricity
Hydrogen
Nuclear
Energy
Renewable
Energies
Primary Energy
Energy Carrier
Combust. Engine
ICEV
Hybrid
HEV
Plug-in Hybrid
PHEV
Battery Electric
BEV
Fuel Cell
FCV
Vehicle
FIG. 5.4. Energy flows to vehicles with various power trains.
Table 5.5. Energy utilization efficiency for various power train vehicles
well-to-wheel efficiency – fossil fuels
29~42 %50~60 %
Tank
58~70 %
H
2
Fuel Cell Vehicle
FCV
35 %
70 %50 %
Battery Electric Vehicle
BEV
Battery
Natural Gas Field
(29~30 %)
Plug-in Hybrid Vehicle
PHEV
28~32 %
32~37 %88 %
Gasoline Hybrid Vehicle
HEV
14 %
Wheel
16 %
Tank
88 %
Oil Field
Gasoline Engine Vehicle
ICEV
Well To Wheel
Efficiency
Tank to Wheel
Efficiency
Well to Tank
Efficiency
¾ The values for ICEV, HEV, FCV are from a Toyota Motors's 2003 presentation. The values for FCV are for the
hybrid specification.
¾ Electric Power for BEV is based on the natural gas ACC power generation of 55% thermal efficiency, 5% loss
from well to station, and 5% loss for electricity transmission and distribution.
¾ EV battery-to-wheel efficiency is based on Uhrig (ANS, 2005).
¾ PHEV adds 15% to the energy required by weight increase. PHEV well to wheel efficiency is estimated for 70%
EV run.
¾ The heating values are based on the Lower Heating Value (LHV).
85
The lifecycle energy utilization efficiencies of vehicles are usually expressed by Well-to-Wheel
(WTW) efficiencies, of which typical values are shown in Table 5.5. In this table, for gasoline engine
vehicles the ”well” means the oil wells producing crude oils, and for battery powered electric vehicles
and hydrogen fuel cell vehicles the ”well” means the gas fields producing natural gas currently used
for hydrogen generation. From Table 5.5, the FCV has the highest efficiency and the BEV is the
second highest. The PHEV efficiency would be somewhere between BEV and HEV.
The energy utilization efficiencies for BEVs and FCVs using nuclear energy as a primary source are
shown in Table 5.6. Here, the efficiencies for three kinds of nuclear reactor are examined, namely
LWR (Light Water Reactor, typical of low-temperature reactors), SFR/SCWR (Sodium-cooled Fast
Reactor / Super Critical Water Reactor, typical of medium-temperature reactors), and VHTR (Very
High Temperature Gas-cooled Reactor, typical of high-temperature reactors).
As for the LWR-based energy flow paths to vehicles, one path is the electricity from a LWR being
supplied to the BEV, and the other is hydrogen from water electrolysis by LWR electricity being
supplied to the FCV. As for the SFR/SCWR-based energy flow paths to vehicles, one path is
electricity from a SFR/SCWR being supplied to the BEV, and the other path is hydrogen from
SFR/SCWR-heated steam reforming of natural gas being supplied to the FCV. As for the VHTR-
based energy flow paths to vehicles, one path is electricity from a VHTR being supplied to the BEV,
and the other path is hydrogen from a thermochemical splitting of water by VHTR heat being supplied
to the FCV.
As shown in Table 5.6, in either the LWR or the VHTR case, the path to BEVs is more efficient than
the path to FCVs. This is due to the following two reasons:
(1). Both the electricity generation and the hydrogen production by electrolysis or thermochemical
splitting of water have to go through the a heat engine cycle in which the conversion efficiency
is limited by thermodynamic laws (the Carnot-cycle efficiency providing an upper limit);
(2). The drive train efficiency is higher in a BEV (70%) than in an FCV (50-60%).
In contrast to the above assessment for LWR and HTGR systems, in the SFR/SCWR case the path to
FCVs provides a higher efficiency than the path to BEVs, as hydrogen is produced by the process of
nuclear-heated steam reforming of natural gas (methane). In this hydrogen production process, the
chemical energy of methane and nuclear heat is converted to the chemical energy of hydrogen with no
limitations of thermodynamic cycle efficiency. This is the same as in the case of hydrogen production
from natural gas shown in Table 5.5.
In the case of nuclear-heated steam reforming of methane, it is inevitable that the process produces
CO
2
though the total amount produced is reduced by about 30% as compared to the case of
conventional methane-combusted steam reforming of methane.
A medium-temperature reactor with an outlet temperature 500 - 600 ºC such as an SCWR or an SFR is
the best suited for the membrane reformer hydrogen production method using palladium (Pd) as
membrane material. The Pd-membrane reformer has been developed by Tokyo Gas as a production
method for a hydrogen fueling station [9, 10]. The nuclear-heated membrane reformer that combines
the membrane reformer with a nuclear reactor has been designed by Mitsubishi HI and others and
evaluated to be economically competitive with conventional methane-combusted steam reforming of
methane [11]. HTGRs and other higher-temperature reactors could also be used in this application
with the excess heat available for other purposes such as electricity production or process heating uses.
That case has not been assessed here.
86

Table 5.6. Energy utilization efficiency for electric and fuel cell vehicles
‘nuclear reactor to wheel’ efficiency
23~27%50~60%45%
Thermochemical
FCV
31%70%45%
Gas Turbine
BEV
VHTR
38~46%*50~60%77%*
Nuclear-Heated
Steam Methane
Reforming
FCV
27%70%39%
Steam Turbine
BEV
SFR /
SCWR
12~14%50~60%23%
Electrolysis
FCV
21%70%30%
Steam Turbine
BEV
L W R
Overall Efficiency
Reactor
→Wheel
Efficiency
Battery/Tank
→Wheel
Efficiency
Reactor →
Battery/Tank
Electricity / Hydrogen
Vehicle Power Train
Nuclear
Reactor
¾ Thermal efficiency: LWR steam turbine 32%, SFR steam turbine 41%, VHTR gas turbine 47%
¾ Efficiency of H
2
production: Electrolysis 80% from electricity and Thermochemical from heat 50%
(LHV) Reforming 85% (* Based on the sum of both primary energies)
¾ Transmission & distribution loss for electricity: 5%, Compression & transportation loss for H
2
: 10%
It can be concluded that, in the nuclear energy based energy flow to vehicles, the path to electric
vehicles is more efficient than the path to hydrogen fuel cell vehicles, except in the case of using
hydrogen produced by nuclear-heated steam reforming of methane.
5.2.4. Greenhouse gas emissions in Japan
The CO
2
emissions of ICEVs, PHEVs in the electric-run mode, and PHEVs in the mixed electric-
run/hybrid-electric mode are evaluated and compared as follows:
The CO
2
emissions for registered vehicles are:
- ICEV: 0.278 kg-CO
2
/km
- PHEV in the electric-run mode: 0.115 kg-CO
2
/km
- PHEV in the 70% electric-run mode and 30% hybrid-electric mode: 0.117 kg-CO
2
/km
The CO
2
emissions for the light vehicles are:
- ICEV: 0.209 kg-CO
2
/km
- PHEV in the electric-run mode: 0.0866 kg-CO
2
/km
- PHEV in the 70% electric-run mode and 30% hybrid-electric mode: 0.0877 kg-CO
2
/km
The CO
2
emissions of both PHEVs in the electric-run mode and PHEVs in the 70% electric-run mode
and 30% hybrid-electric mode is about 1/2.4 of that for gasoline ICEVs.
87
5.3. Challenges
This section discusses technical barriers, and Section 5.4 addresses potential solutions. Certainly there
are separate barriers associated with transportation infrastructure and with the electric power industry,
but these are outside the scope of this document. Some of these barriers are:
• Conversion of automobile technology from conventional gasoline-powered vehicles to electric
and plug-in hybrid vehicles;
• Public acceptance of plug-in hybrid vehicles;
• Structuring of electricity pricing mechanisms to provide low-price electricity during off-peak
demand periods to encourage use of nuclear power plants for base load generation;
• Provision of other incentives (e.g., tax benefits) for adoption of vehicles that produce less
greenhouse gases and reduce reliance on petroleum fuels.
• Building of carbon-free electricity generation capacity and electricity transmission and
distribution systems.
5.3.1. Barriers to be overcome in the United States of America
Barriers to plug-in hybrid vehicles
Only a few plug-in hybrids are in existence in the United States, and until recently none of them came
from an automotive manufacturer. E-Drive Systems has converted several Toyota Prius models by
replacing the nickel metal hydride battery with a lithium-ion battery system having thousands of small
cells with about seven times the energy storage capacity of the Prius nickel metal hydride battery. A
modified control system has been added to optimize the performance while minimizing the use of
gasoline. The cost of the conversion is about $10,000 for each vehicle. DaimlerChrysler initially built
five Sprinter delivery vans that they distributed for testing (three in the United States of America).
Recently, they announced that they were building several dozen Sprinter vans for testing under a wide
variety of circumstances throughout the world. It is clear that the plug-in hybrid has a long way to go
before it can displace a significant fraction of the 9 million barrels of fuel used every day in the United
States.
The focus on delivery vans by DaimlerChrysler is a logical approach. The potential savings of fuel
costs by fleets of such vehicles operated from a central facility — the Postal Service, delivery services,
military organizations, or similar commercial and government organizations with fleets of vehicles
where centralized battery charging could become a routine part of the daily maintenance and service
— should be greater than the initial extra cost. The critical item for improving the performance of
plug-in hybrid vehicles is lighter, less expensive, reliable, more robust batteries having a factor of 5 to
10 greater energy storage capacity.
General Motors displayed a plug-in hybrid vehicle named Volt at the Detroit Auto Show in early 2007
[12]. It has a series configuration in which the electric motor drives the vehicle full time, and the
engine (3-cyclinder, 1000 cm
3
displacement), capable of using gasoline or ethanol E85, only drives the
generator to charge the 400 pound lithium ion battery. It is effectively an electric vehicle with a motor-
generator that can travel 40 miles without charging and can be fully charged in six hours on 110 volt
electricity. Indeed, the Volt has considerable similarity to the defunct GM electric EV1 vehicle (made
infamous in the movie "Who Killed the Electric Car?"). This appears to be the simplest and quickest
way to develop a new plug-in hybrid by using their experience with the EV1. GM is still developing
hydrogen as an automotive fuel and has suggested that they might use a hydrogen-fueled fuel cell in
place of a gasoline engine on their PHEV. However, GM has not announced any plans to build plug-in
vehicles for lease or sale, and has indicated that there is need for improved and lower cost batteries to
88
make plug-in vehicles commercially viable. Volt is the first variant built upon GM’s E-flex system
that will enable them to utilize the same chassis for multiple electric drive systems.
Ford Motor Company also displayed its PHEV concept at the 2007 Detroit Auto Show. Called the
Airstream because its style resembles the iconic “Airstream” aluminum recreational vehicles, it uses
lithium batteries with an all-electric travelling range of 25 miles that are charged using a fuel cell. The
hydrogen fuel cell drive train operates under electric power all the time. It has half the weight and cost
of today’s fuel cells and, unlike most fuel cells, it can operate under extreme winter conditions.
5.3.2. Barriers to be overcome in Japan
As summarized by Professor Hisashi Ishitani of Keio University at the closing speech of EVS-22
Workshop on “Plug-In Hybrid Electric Vehicle Workshop” that was held on October 25, 2006, in
Yokohama, Japan, the view of Japanese industry and academia on plug-in hybrid electric vehicles can
be expressed as follows:
(1) It is expected that PHEVs are an effective measure to solve the CO
2
and energy issue, and also a
practical and possible transition from HEVs to FCVs;
(2) The challenge is the cost and durability of batteries. It is necessary to support the development
of this technology;
(3) It is better to put forward the introduction of PHEVs, focusing on the petroleum savings and
CO2 reduction, by an available set-up (like the hybrid mode) than a pure-EV mode, which may
be difficult in the early stage;
(4) It is essential not to repeat the failures in development of BEVs and FCVs. As PHEVs are seen
to be based on current technology, it is vital to take one step forward at a time by assessing the
accomplishments along the way.
As stated in the press release of Toyota Motor Corporation in June 2006, Toyota will advance its
research and development of plug-in hybrid vehicles and is working on a next-generation vehicle that
can extend the distance traveled by the electric motor alone and that is expected to have a significant
effect on reducing CO
2
and helping to abate atmospheric pollution. Toyota also said on other
occasions that PHEVs would not be feasible for mass production without a breakthrough in battery
technology, and that, with today's best technologies, PHEVs are not commercially viable.
Toyota reiterated this point on July 25, 2007, when it announced it is donating several prototypes of its
Plug-in HV to the University of California-Irvine and the University of California-Berkeley for road
testing. The Plug-in HV has a battery range of only 8 miles on a full charge, because of its use of low-
energy nickel-metal hydride batteries, rather than more advanced lithium-ion batteries, which are not
yet available for these purposes.
In August, 2006, the Study Group on Next Generation Vehicle Batteries in the Ministry of Economy,
Trade and Industry (METI) issued a report, “Recommendations for the Future of Next-Generation
Vehicle Batteries” (The main text is written in Japanese, though an English summary is
available) [13].
In Annex 5 of this report, the battery cost and the competitiveness of PHEVs with ICEVs and HEVs
are evaluated for setting the R&D goals of battery development. The comparison was made on the
sum of vehicle purchase cost and fuel/electricity cost for a 10-year vehicle life. One example on Prius-
class vehicles shows that, for the PHEV to become comparable with ICEVs and HEVs, it is necessary
to reduce the cost of lithium-ion battery from the current cost (200 K Yen/kWh) by a factor of about 7
(to 30 KYen/kWh).
89

This example shows that intensive efforts toward development of battery technology are necessary for
the introduction of economically competitive PHEVs into the market. The report recommends that, for
introducing PHEVs around 2015, it is necessary to conduct a battery development project that is
completed by about 2010. The recommended action plan by the Study Group is described in
Section 5.4.2.
5.4. Solutions
5.4.1. Solutions in the United States of America
Hybrid battery technology
Today, most commercial hybrid vehicles have sealed nickel metal hydride batteries. They are
significantly better than traditional lead-acid batteries, but are relatively heavy, expensive, and do not
show a capability of becoming much less expensive in mass production. The power of nickel batteries
comes from the raw material, which is getting more expensive because of increased demands. The
primary alternative under consideration today is the lithium-ion battery, made up of thousands of
lithium cells, each one of them the size of a common “AA” battery. The Volvo 3CC concept car,
unveiled in 2004, was an all-electric vehicle that relied exclusively on 3000 lithium-ion cells and
provided the equivalent of 105 horsepower with zero emissions. The benefits of the lithium-ion
batteries are derived from the mass processing that can scale to the high volumes required for the
rapidly growing hybrid vehicle market without a corresponding increase in price. Larger and fewer
lithium ion-cells would have been more attractive, but they do not exist commercially yet.
Furthermore, as lithium cells that use cobalt-oxide cathode material become larger, they sometimes
encounter thermal-runaway transients in which they burn or explode. Substituting phosphate for cobalt
seems to prevent this problem, but reduces the output power. Even so, progress is being made to
address these problems. Estimates are that it will take three to five years before such batteries are
available in large size and are powerful enough, cheap enough, and reliable enough for mass-produced
vehicles. Meanwhile, lithium ion battery packs with thousands of cells continue to be the primary
technology under consideration for PHEVs in the near future.
5.4.1.1. Research and development in the United States of America
R & D in batteries
As an example of research progress in the U.S., recently U.S. patent #06979513 entitled “Battery
Including Carbon Foam Current Collectors” (assigned to Firefly Energy Corporation, Peoria, Illinois,
a spinout from Caterpillar Tractor Corporation) appeared on the “Battery Digest” web site.
19
This
patent has similarities to an earlier patent issued to Alvin Snaper regarding foam plates in lead acid
batteries.
20
The fundamental concept of both patents is a replacement of the lead plate current
collectors. With both concepts, active materials are deposited on a high-surface-area substrate,
reducing total weight and possibly offering additional advantages of corrosion resistance and higher
power density. The high surface area greatly increases the current density, leading to much greater
current delivery. Furthermore, the Firefly carbon foam substrate has a very high thermal conductivity,
thereby overcoming the thermal build-up reported in larger lithium ion batteries.
5.4.2. Solutions in Japan
The METI Study Group recommended two action plans for the future of next-generation vehicle
batteries in August 2006, namely the R&D Strategies and the Infrastructure Building Strategies.
19
Inventors: Kelley and Votoupal. Assignee: Firefly Energy Corporation.
20
This description is taken from the Batteries Digest Web Site at http://batteriesdigest.com/id492.htm.
90

Action Plan – R&D Strategies
This action plan is composed of three phases: (i) Improvement phase, (ii) Advanced phase, (iii)
Innovation phase. At each phase are specified the types of vehicles expected to be developed,
performance and cost target of batteries, and the role of industry, government and academia. As shown
in Table 5.7, the PHEV is supposed to be introduced around 2015 with a battery of 1.5 times
performance and 1/7 cost of current batteries. The New Energy and Industrial Technology
Development Organization (NEDO) will be the secretariat for coordinating universities, research
institutes, automobile manufacturers, battery manufacturers, material manufacturers, and electric
power companies.
Table 5.7. Japan’s battery development action plan [13]
2. Infrastructure Building Strategies
1. R&D Strategies
This action plan is to be implemented along with the battery R&D plan, and is composed of building
software and hardware infrastructures such as incentive measures for vehicle popularization,
regulatory framework, standardization, safety standards, and battery charge stations, as shown in
Table 5.7. The secretariat of this action plan will be the Japan Automobile Research Institute (JARI)
and METI. The R&D strategies to implement this action plan, shown in Table 5.8, were taken from
the report, “Recommendations for the Future of Next Generation Vehicle Batteries,” carried out by the
Study Group on Next Generation Vehicle Batteries, Ministry of Economy, Trade and Industry, Japan,
in August 2006.
91

Table 5.8. Research and Development, Action Plan for Next Generation
Battery Technology Development
Goals of Battery
Phase Time
Target Vehicle
Type
Performance Cost
Required
R&D Items
Limited Purpose
Commuter BEV
Battery Range
80Km, 2 Seater
Same as Present
100Wh/Kg
1000W/Kg
1/2 of
Present
1
Improvement ca. 2010
High Performance
Hybrid
HEV
Fuel Economy 30%
Up
Same as Present
70Wh/Kg
2000W/Kg
1/2 of
Present
Li-ion Battery
Carrier: N.R.
Material: P.R.
Design: R.
Commuter
BEV
Battery Range
150Km, 4 Seater
1.5 Times of
Present
150Wh/Kg
1200W/Kg
1/7 of
Present
2
Advanced ca. 2015
Plug-in Hybrid
Electric
PHEV
Battery Range
40Km
1.5 Times of
Present
100Wh/Kg
2000W/Kg
1/7 of
Present
Li-ion Battery
Carrier: N.R.
Material: R.
Design: R.
3
Innovative 2030~
Full-fledged
Electric
BEV
Battery Range
480Km
7 Times of
Present
700Wh/Kg
1000W/Kg
1/40 of
Present
New Principle
Battery
Carrier,
Material and
Design: All
Required
N.R.= Not Required, P.R.= Partly Required, R.= Required
REFERENCES FOR CHAPTER 5
References: United States of America
[1] UHRIG, R.E., “Engineering Challenges of the Hydrogen Economy,” The BENT of Tau Beta
Pi, Vol. XCV, No. 2 (Spring, 2004).
[2] UHRIG, R.E., “Using Plug-in Hybrid Vehicles to Drastically Reduce Petroleum-Based Fuel
Consumption and Emissions,” The BENT of Tau Beta Pi, Vol. XCVI, No. 2 (Spring, 2005).
[3] UHRIG, R.E., “Greenhouse Gas Emissions from Gasoline, Hybrid-Electric, and Hydrogen-
Fueled Vehicles,” Climate Change Technology Conference, Ottawa, Ontario, Canada (May 10-
12, 2006).
[4] HORI, M., “Which is Earth-Friendly? Conventional Hybrid Car or Plug-in Hybrid Car” (In
Japanese) Monthly Energy (August 2005) (Published by Fuji-Sankei Group).
[5] HORI, M., “Plug-in Hybrid Cars for the Energy Security and Global Environment, What About
the Effect?” (In Japanese) Monthly Energy (May 2006) (Published by Fuji-Sankei Group).
[6] HORI, M., “Plug-in Hybrid Electric Vehicles for Energy and Environment” (Text in Japanese
and extended summary in English), JSAE Transactions, Vol. 38, No. 2 (March 2007).
[7] GRAHAM, R., “Comparing the Benefits and Impacts of Hybrid Electric Vehicle Options,”
EPRI Report #1000349, Final Report (July 2001).
[8] GAGE, T., “Situation is Obscenely Out of Whack,” FLEETS & FUELS (November 14, 2005).
92
[9] SHIRASAKI, Y. and YASUDA, I., "New Concept Hydrogen Production System Based on
Membrane Reformer", 2002 Fuel Cell Seminar, Palm Springs (2002).
[10] YASUDA, I., SHIRASAKI, Y., “Development of Membrane Reformer for Highly-efficient
Hydrogen Production from Natural Gas”, 15th World Hydrogen Energy Conference, Yokohama
(2004).
[11] TASHIMO, M., et. al., “Advanced Design of Fast Reactor Membrane Reformer”, Proceedings
of OECD/NEA Second Information Exchange Meeting on Nuclear Production of Hydrogen,
Argonne USA, October 2-3, 2003, p. 267 (2003)
[12] GENERAL MOTORS, “New Step, Same Direction: No Way GM will Kill the Electric Car
says Lutz,” Automotive News (Jamie Lareau), November 7, 2006
http://www.autoweek.com/apps/pbcs.dll/article?AID=/20061107/FREE/61106014&SearchID=7
3279821382571
[13] METI Study Group on Next-Generation Vehicle Batteries, Recommendations for the Future of
Next-Generation Vehicle Batteries, Ministry of Economy, Trade and Industry (August, 2006)
(http://www.meti.go.jp/english/information/downloadfiles/PressRelease/060828VehicleBatterie
s.pdf).
[14] WANG, M. Q., GAINES, L., and CUENCA, R., “Modeling the Vehicle Cycle Impacts of
Hybrid Electric Vehicles,” Air and Waste Management Association 90
th
Meeting and
Exhibition, Toronto, Canada, June 8-13, 1997.
BIBLIOGRAPHY FOR CHAPTER 5
“Batteries Digest” Web Site at <http://batteriesdigest.com/id492.htm
> 2006.
Consumer Reports, “What’s New—2005 Cars, Fueling the Future,” Consumer Reports, Vol. 69,
No.10, October 2004.
DAIMLER CHRYSLER, “Mercedes Introduces Sprinter Diesel Electric Hybrid,”
<www.whnet.com/4x4/sprinter.html>, click on “Hybrid Page,” July 31, 2004.
The Economist Technology Quarterly, “Why the Future is Hybrid,” pp. 26-30, December 4, 2004.
DEPARTMENT OF ENERGY, ENERGY INFORMATION ADMINISTRATION (DOE and EPA),
“Carbon Dioxide Emissions from the Generation of Electric Power in the United States (July 2000).
http://www.eia.doe.gov/cneaf/electricity/page/co2_report/co2report.html
DEPARTMENT OF ENERGY, ENERGY INFORMATION ADMINISTRATION, Energy
Information Administration, “Petroleum Flow 2002.” Annual Energy Review at <www.eia.doe.
gov/emeulaer/diagram2.html> (2003).
DEPARTMENT OF ENERGY, ENERGY INFORMATION ADMINISTRATION, Energy
Information Administration, “Green-house Gases, Climate Change, and Energy,” at
<http://www.eia.doe.gov/oiaf/1605/ggccebro/chapter1. html
> (2005).
GOSWAMI, D. Yogi, “Hydrogen Supply Technologies: Introduction to Issues,
<www.ases.org/hydrogen_forum 03/Goswami.pdf
> (2005).
MILLER, A. and DUFFY, R.B., “Meeting the Near-Term Demand for Hydrogen using Nuclear
Energy in Competitive Power Markets,” Proceedings of GLOBAL 2000 Meeting, American Nuclear
Society, New Orleans, LA, Nov. 16-20 (2003).
STUART, S., “Nuclear-Electric Synergies: For Neat Hydrogen Production and Cleaner Fossil
Reserves Harvesting,” ANS President’s Special Session: Hydrogen Systems, ANS Meeting, Reno,
NV, Nov. 11-15, 2001.
TerraPass, “Carbon Dioxide Calculator,” <Terrapass.com/ carboncalc.html>.
93
CHAPTER 6
ELECTRICITY AND HEAT FOR HYDROGEN PRODUCTION
As an alternative path to the current fossil fuel economy, a hydrogen economy is envisaged in which
hydrogen would play a major role in energy systems and serve all sectors of the economy, substituting
for fossil fuels. Hydrogen as an energy carrier can be stored in large quantities, unlike electricity, and
converted into electricity in fuel cells, with only heat and water as by-products. It is also compatible
with combustion turbines and reciprocating engines to produce power with near-zero emission of
pollutants. Furthermore, hydrogen can be obtained from various primary energy sources that are
domestically available in most countries. Consequently, the hydrogen economy could enhance both
the security of energy supply and global environmental quality.
The current worldwide hydrogen production is roughly 50 million tonnes per year. Although current
use of hydrogen in energy systems is very limited, its future use could become enormous, especially if
fuel-cell vehicles would be deployed on a large commercial scale. The hydrogen economy is getting
higher visibility and stronger political support in several parts of the world. In his “State of the Union
Address” in 2003, the US President announced a $1.2 billion hydrogen initiative to reverse the
growing dependence on foreign oil and reduce greenhouse gas emissions. The Japanese Prime
Minister and the Chair of the European Commission have also made official statements strongly
supporting the emergence of a hydrogen economy.
There are many ongoing national programmes aimed at the development of a hydrogen economy, such
as the Hydrogen Initiative of the United States, the European Hydrogen and Fuel Cell Technology
Platform, and fuel cell/hydrogen programmes in Japan and the Republic of Korea. There are also
various international efforts for the realization of a hydrogen economy. Under the leadership of the
United States, 15 countries and the European Commission launched the International Partnership for
the Hydrogen Economy (IPHE) in 2003 to discuss common areas of interest in, and obstacles to, the
hydrogen economy in the fields of research, development, and demonstration projects; hydrogen
policy and regulation; and the commercialization of hydrogen-based energy technologies.
Today, hydrogen is used in limited quantities, and mainly in petroleum refineries and the chemical
industry. In the United States, for example, these uses represented 93% of hydrogen consumption in
2003. However, hydrogen is an attractive energy carrier that might play a major role in energy systems
for many economic sectors in the long term. In the medium term, the most promising area for
hydrogen is in producing synthetic fuel as a substitute for gasoline in transportation. Hydrogen
produced from non-fossil fuels may be a key option as the prices of hydrocarbon resources soar or
their consumption becomes restricted for environmental reasons.
The advantages of hydrogen-based energy systems will depend on the hydrogen production systems
implemented. Hydrogen will be a clean, environmentally friendly and sustainable energy carrier only
if its production is sustainable, i.e., if its production does not induce irreversible environmental
damages or exhaust non-renewable natural resources. Nuclear-produced hydrogen offers unique
characteristics in terms of environmental friendliness and energy efficiency.
The development of hydrogen-based energy systems will require building not only hydrogen
production facilities and end-use devices, but also infrastructure for the distribution of hydrogen. Such
structural changes in production and use of energy will take time. This implementation period could
facilitate the penetration of nuclear energy in the hydrogen supply market, allowing for the design and
deployment of advanced nuclear energy systems (e.g., very high temperature reactors) better adapted
to hydrogen production than the current generation of nuclear power plants.
An adequate and affordable supply of hydrogen is a prerequisite for successful implementation of a
hydrogen economy. Although hydrogen is abundant in the world, it has to be extracted from
compounds containing hydrogen such as fossil fuels, biomass, or water with thermal, electrolytic or
photolytic energy. Table 6.1 shows some technological options that are or will be available for
hydrogen production.
94

As shown in Table 6.1, nuclear energy is suitable for hydrogen production since nuclear reactors can
produce both the heat and electricity required for it. Furthermore, it is the most commercially mature
non-fossil fuel energy source capable of producing hydrogen on a large industrial scale with
significantly lower CO
2
emissions.
Several technological options are possible for nuclear hydrogen production, including:
• Electrolysis of water using electricity from nuclear reactors;
• Steam reforming of natural gas using high-temperature heat from nuclear reactors;
• High-temperature electrolysis of steam using high-temperature heat and electricity from nuclear
reactors;
• Thermo-chemical water splitting using high-temperature heat and electricity from nuclear
reactors.
Table 6.1. Hydrogen production options
Raw Feedstock
Options
Typical Processed
Feedstock
Production Process
Options
Process Energy
Source Options
Production
Strategy Options
Fossil Fuels
Coal
Natural gas
Oil
Syngas
Gasoline
Diesel fuel
Methanol
Ammonia
Direct use of raw stock
Biomass
Lignocellulose
Starch
Vegetable oils
Black liquor
Ethanol
Methanol
Biodiesel
Biogas
Sugars
Direct use of raw stock
Waste Material
Municipal solid waste
Stack gases
Waste water
Direct use of raw stock
Water
Direct use of raw stock
Thermal
Reforming
Steam reforming
Partial oxidation
Gasification
Pyrolysis
Thermochemical
Electrochemical
Electrolysis
Photoelectro-chemical
Biological
Photo-biological
Aerobic fermentation
Radiolysis
Thermal
Fossil
Renewable
Nuclear
Electricity
Fossil
Renewable
Nuclear
Photolytic
Solar
Radiolysis
Nuclear
Distributed
Fuelling stations
Individual buildings
On-board
Semi-distributed
Market-centred
Central
Resource-centred
Hydrogen can be obtained more efficiently by significantly raising the temperature of water. The
electrolysis of steam at higher temperatures (800-1000°C) offers several advantages including a lower
electricity requirement and higher efficiency resulting from increasing oxygen ion transport through
the electrolyte and improving catalytic activity at the electrodes.
Although nuclear energy has the potential to play a significant role in a hydrogen economy, there are
uncertainties about when hydrogen demand will be large enough to justify deployment of nuclear
plants dedicated to hydrogen production or dual-production units capable of generating electricity and
producing hydrogen. Furthermore, many existing and advanced power generation and hydrogen
95
production technologies will compete with nuclear energy for hydrogen production, and market
competition will select the most profitable options.
Hydrogen is a clean fuel, and the demand for it is increasing. To achieve its full potential, hydrogen
production has to be emission free, either directly or indirectly. The only available technology today to
meet this criterion is water electrolysis powered by emission-free electricity – either nuclear or
renewable power. Several alternative technologies are being developed. Most of them would need an
advanced high-temperature nuclear reactor as a heat source that is necessary for one or more steps in
the hydrogen generation process. A few proposed technologies can use lower temperature heat that is
available from the current generation of nuclear reactors.
Direct thermolysis of water requires temperatures over 2500°C. To lower the required temperatures,
multi-step thermochemical water splitting processes are being explored. Hundreds of potential
thermochemical cycles have been assessed in terms of their viability and performance. Several are
undergoing active research around the world, including the iodine-sulphur (IS), bromine-calcium (Br-
Ca) and copper-chlorine (Cu-Cl) cycles.
Currently hydrogen is produced mainly by steam reforming of natural gas/methane. Only a small
fraction of hydrogen in the world (about 4%) is produced by electrolysis. Steam methane reforming
(SMR) is a catalytic process involving the reaction of natural gas with steam to produce a mixture of
hydrogen and CO
2
, requiring temperatures in the range 500 to 950°C. It results in considerable
releases of CO
2
both through burning of natural gas for supplying the heat for the endothermic
reaction and through the shift reaction while generating hydrogen. CO
2
capture and sequestration
would add to the costs. Nuclear-assisted steam reforming has potential for large-scale hydrogen
production in the near term, though CO
2
still remains as a waste product. It would allow savings of
natural gas of about 30% and could represent an important transition technology. In addition, research
is underway using membrane techniques that can recirculate product gases and further reduce the CO
2
from steam reforming. Nuclear assisted steam reforming of methane could constitute a strategy for a
successful continuum towards other CO
2
-free production approaches being considered.
The other short-term option (over the next 5-15 years) is the production of hydrogen by electrolysis.
Currently, electrolyser efficiencies are in the range of 60-80% and may reach 90%. In addition,
electrolysers can be attractive as remote and decentralized hydrogen production methods. Because of
the high electrical demands for the process, though, electrolysis of water is attractive only when cheap
electricity is available or when particularly high-purity hydrogen is required. The use of nuclear
generated electricity in off-peak periods from existing water-cooled reactors may be economically
competitive, but the stranded capital costs of the electrolyzers during periods of peak electricity prices
may be prohibitive.
6.1. Opportunities
The currently used commercial method for hydrogen production, steam-methane reforming (SMR), is
starting to be less attractive, not only because of increasing natural gas prices and uncertain
availability, but also because of considerable greenhouse gas emission. Low-temperature water
electrolysis is one technology currently available for hydrogen production using the electricity
generated from nuclear power plants without producing greenhouse gases. In August 2006 the U.S.
Department of Energy (DOE) announced that it intends to provide approximately $1.4 million for two
projects for industry partnerships to study the economic feasibility of producing hydrogen at existing
commercial nuclear power plants. A study of the economics of producing hydrogen at existing nuclear
power plants using commercially available production technology will be performed by Electric
Transportation Applications (ETA), which is located in Phoenix, Arizona. ETA will partner with
DOE’s Idaho National Laboratory and Arizona Public Service.
A second feasibility study of hydrogen production using alkaline electrolysis powered by existing
nuclear power plants will be performed by GE Global Research, which is located in Niskayuna, New
York. Their work plan is based on low-cost alkaline electrolyzer technology developed by GE, in part
96

through DOE’s Hydrogen Program. Partners for this project include DOE’s National Renewable
Energy Lab and the Entergy Corporation.
About one-third of the world’s primary energy is converted to electricity at present. The remaining
two-thirds is consumed for such non-electric applications as process heat for industry, space heating,
and transportation. The ratio of electricity will increase to about one-half at the end of 21st century.
Still, one-half of all energy will be used for non-electric purposes. As it is essential to reduce the use
of fossil fuels for the global environment, it is important to explore the possibility of nuclear energy’s
replacing fossil fuels for non-electric applications. One way to fulfil this need is to produce hydrogen
by nuclear energy. Under this scenario, far more nuclear energy will be required than is currently
anticipated.
According to the estimates of World Energy Council (WEC Case B) [1], the world’s primary energy
demand in 2100 will be about four times that in 1990, with nuclear energy supplying 24% of the total
primary energy for electricity production. This nuclear supply corresponds to an equivalent of about
5,200 units, each with a capacity of 1000 MWe. The supply of fissile fuel to these plants is feasible as
assessed by the World Energy Council, assuming an ultimate resource of natural uranium of 16.3
Mton, as reported in the NEA/IAEA Red Book [2], and the recycling use of plutonium by fast breeder
reactors (FBR) with a breeding ratio of 1.2 to 1.3 introduced from 2030 - 2050 [3].
In order to reduce global greenhouse gas emissions and begin displacing fossil fuels, optimizing the
recycling use of plutonium in breeder reactors could increase nuclear energy supply by 50% by 2050
and 100% by 2100. By effectively utilizing nuclear energy, this excess supply capacity of nuclear
energy could replace fossil fuel’s share as shown in Table 6.2 [4].
Table 6.2 Primary energy supply for 1990-2100
WEC-B case and in a proactive nuclear deployment case (in parentheses)
(Energy in Gt
oe
[10
9
ton oil equivalent])
1990 2050 2100
Fossil
6.9 12.7 (11.4) 15.0 (5.0)
Nuclear
0.45 2.7 (4.0) 8.3 (18.3)
Renewables
1.6 4.4 11.4
Total 9.0 19.8 34.7
In such a scenario, the global use of fossil fuel in 2100 would become smaller than it was in 1990, thus
helping to stabilize atmospheric carbon dioxide concentrations even in the face of growth of global
energy use by a factor of four.
In the WEC estimate, electricity is the only application of nuclear energy. The extra nuclear capacity
could be used for other energy applications, such as hydrogen production.
Many processes have been proposed for the production of hydrogen using nuclear energy [5]. The
leading processes presently under research and development are nuclear-heated steam reforming of
natural gas or other hydrocarbons, thermo-chemical splitting of water by nuclear heat, high-
temperature electrolysis of steam by nuclear electricity and heat, and electrolysis of water by nuclear
electricity (Fig. 6.1).
97

FIG.6. 1. Methods for hydrogen production by nuclear energy.
Though it is unclear what course the commercialization of nuclear production of hydrogen will take,
one path could be as follows:
- In the near term, electricity produced by water-cooled reactors could be used to electrolyze
water using commercially available technology.
- In the intermediate term, medium-temperature reactors could contribute to steam reforming of
natural gas. Higher temperature reactors would enable the use of steam electrolysis for higher
conversion efficiencies.
- In the long term, the development of thermo-chemical water splitting cycles or steam
electrolysis could be deployed. Such processes would need to be coupled with reactors that
deliver heat at an appropriate temperature.
The concept of using nuclear heat for the endothermic steam reforming reaction of natural gas (or
possibly other fossil fuels) can save approximately 30% of the natural gas, which would otherwise be
burned as a heat source [6]. If the technology for medium-temperature (500 to 600ºC) steam reforming
becomes commercial, then the synergistic method that combines the steam reforming process with
nuclear heat could be effectively utilized to produce hydrogen (together with water electrolysis) until
other water-splitting methods become commercially available. Section 6.4.1.3 will describe lower-
temperature reforming technology in more detail.
6.2. Market context
Hydrogen has many and versatile uses as a secondary raw chemical material and as a secondary
energy carrier. With 48% of its consumption, it plays a significant role in non-energetic applications as
a chemical raw material and intermediate product for industrial and petrochemical processes.
Furthermore, it is largely used in energetic applications indirectly; e.g., in the production of clean
synthetic fuels (20%) or directly as a fuel for producing process heat in the chemical industries (32%).
98
Of the annual world production of approximately 5.5x10
11
Nm
3
of hydrogen, about 70% is consumed
in the chemical industries. It possesses the potential to generate mechanical energy, heat, or electricity
for future large-scale use.
6.2.1. Hydrogen as a raw material in chemical processes
The chemical hydrogen economy started already at the turn of the 20
th
century when coke furnaces
generated process gas and town gas with up to 60% hydrogen content. Since then a variety of non-
energetic and indirectly energetic applications has been developed for hydrogen as a part of synthesis
gas such as:
• Ammonia synthesis
• Methanol synthesis
• Direct reduction of iron ore
• Fossil fuel processing (hydro cracking)
• Fischer-Tropsch hydrocarbon synthesis
• Methanation in long-distance energy transportation
• Hydro-gasification
Ammonia synthesis
More than half of the hydrogen used in the chemical industries (or 40% of the world production),
approx. 2·10
11
Nm
3
, is consumed in ammonia synthesis:
N
2
+ 3H
2
Æ 2NH
3
Feed gas for the steam reformer is methane or gasoline. High dilution with steam is chosen to keep the
methane content at a low level. Adding air in a secondary reformer leads to partial oxidation of the
residual methane and CO. After separation of the CO
2
, the product gas is a mixture of nitrogen and
hydrogen whose ratio (ideally 1:3) is adjusted by the operating conditions. The system pressure is
about 5 MPa; the synthesis temperature is 400°C.
The production of one tonne of ammonia requires about 2000 Nm
3
of hydrogen as well as
approximately 800 kWh for compression of the synthesis gas and provision of the nitrogen (by air
liquefaction).
Modern industrial production of ammonia uses the Haber-Bosch process to produce a daily output of
1000-2000 t of liquid NH
3
, corresponding to a hydrogen consumption of 80 000-160 000 Nm
3
/h. The
use of high gasification pressures minimizes the need for subsequent compression. Ammonia is
worldwide used as a fertilizer with facilities for storage, safe handling, transportation, and distribution
being available. It is also being considered as a hydrogen rich fuel for automobile transportation to
replace CO
2
producing fuels.
Methanol synthesis
World demand for methanol is around 32 million tones per year and increasing modestly by about 2 to
3 percent per year, but with significant changes in the profile of the industry. Since the early 1980s,
larger plants using new efficient low-pressure technologies are replacing less-efficient small facilities.
The industry has also moved from supplying captive customers, especially for the production of
formaldehyde that typically represents one-half of world demand and serving primarily the home
market, to large globally oriented corporations.
Methanol synthesis consumes about 5% of the world’s hydrogen production. Methanol is used in the
chemical industry as an intermediate product. It is gaining further attention as a secondary energy
99

carrier with less CO
2
emission; e.g., as a direct vehicle fuel or as a basis for the production of
hydrogen-rich gas to feed fuel cells.
Methanol production starts with a compression of the CO, CO
2
(produced mainly by coal combustion),
and H
2
mixture to about 10 MPa, which is then introduced into a fixed-bed catalytic reactor at
temperatures of 220-280°C and pressures of 5-20 MPa:
CO + 2 H
2
Æ CH
3
OH
CO
2
+ 3 H
2
Æ CH
3
OH + H
2
O
The reactions are exothermic and volume reducing; thus, low temperatures and high overpressures are
desirable. A catalyst is required to maximize methanol output. The specific consumption is 2300 Nm
3
of CO and H
2
per ton of methanol.
Today’s reactors a have capacity of up to 3000 t/d of methanol.
Methanol production generates a surplus of hydrogen which can, by adding CO
2
, be utilized to
increase the methanol yield and thus reduce CO
2
emission into atmosphere:
3 CH
4
+ 2H
2
O + CO
2
Æ 4 CH
3
OH
In a subsequent step, hydrocarbon fuel synthesis can be performed:
n CH
3
OH Æ (CH
2
)
n
+ n H
2
A CO
2
-neutral solution is obtained if the CO
2
released during combustion of the methanol is recovered
during the methanol production step. CO
2
-free coal-to-methanol production would require H
2
as a
supplemental feed and a non-fossil fuel source of high-temperature heat (e.g., nuclear power).
New catalysts allow the methanol synthesis to take place at lower temperatures (80 - 120 °C) and
lower pressures. A drawback is the rapid deactivation of the catalyst in the presence of strong alkalis.
In Japan, the conventional method of methanol synthesis has been modified by adopting a fluidized
granular catalyst bed rather than a fixed bed, which allows the possibility to enlarge the size of the
reactor, to reduce the power required, and to exchange the catalyst during operation.
Direct reduction of iron ore
Blast furnace technology has been used for more than a century for raw iron production. The stack gas
generated during the process was usually considered a waste gas. Its constituents and typical fractions
are CO (~ 40 %), N
2
(~ 40 %), CO
2
(~12 %), H
2
O (~ 6 %), and H
2
(~ 2 %).
Direct reduction of iron ore to sponge iron that can then be converted to steel in an electric arc furnace
is a process that takes place outside a blast furnace, avoiding the use of coke. This process is more
favorable than the traditional raw iron production by means of coke. The chemical reactions are:
(1) Fe
2
O
3
+ 3 H
2
Æ 2 Fe + 3 H
2
O
(2) Fe
2
O
3
+ 3 H
2
Æ 2 Fe + 3 H
2
O
Fe
2
O
3
+ 3 CO Æ 2 Fe + 3 CO
2
The reactions demand 610 Nm
3
of hydrogen or 604 Nm
3
of carbon monoxide per ton of iron. Process
1, the H-iron process, operates at 500 °C and 3 - 4 MPa and needs pure hydrogen (98 %) and a high
dilution of the reformer feed gas with steam. Process 2, the Korf-Midland-Ross process, operates at
100

800 °C and 0.3 MPa and requires low steam dilution to prevent carbon deposition and a low methane
level (< 2 %) in the reformer product gas. The optimal H
2
to CO ratio is a compromise between a
favourable energy balance of the endothermic and exothermic reactions, and reaction kinetics, as well
as environmental impact by the CO
2
produced.
Heat treatment of ferrous metals is made in a hydrogen-nitrogen atmosphere with 3-30 % H
2
to
increase metal ductility, improve machinability, and alter electric and magnetic properties.
Fossil fuel processing
Steady utilization of hydrogen in refineries commenced more than 50 years ago. It is mainly used for
hydro-cracking and fuel burning of excess by-products and hydro-treating, comprising pre-treatment
of reformer feed and treatment of heavier streams in upgrading processes including the removal of
sulphur compounds, halides, metals, nitrogen or oxygen; the saturation of olefins, diolefins, cyclo-
olefins, or aromatics; and the decyclization or ring-opening. The cracking of heavy crude oil is done to
produce lighter hydrocarbons or refined products such as high-octane gasoline.
In the process of coal hydrogenation, which is a high-pressure (30 - 70 MPa) catalytic process at a
temperature of 500 °C, hydrogen is used to convert coal to gasoline:
C+H
2
Æ - CH
2
-
Hydrogen is also used to remove sulphur, oxygen, and nitrogen. Rigid specifications concerning the
contents of the catalyst poison CO (< 10 ppm) have to be met. A purity of 98 % hydrogen is desirable.
With an input of 2000 - 2600 Nm
3
of hydrogen and 1.7 t of coal, an output of 1 t of gasoline is
obtained. In addition, 600 kWh of electricity are necessary for H
2
compression and coal preparation.
Oxo synthesis is an exothermal catalytic process in which a H
2
-CO mixture, as the product gas of the
steam reforming process, is used to produce aldehydes, which are then hydrided to the respective
alcohols. The oxo process works at 3 - 30 MPa and 100 - 180 °C.
The hydrogen demand in cleaning and upgrading of coal and oil is listed in Table 6.3 for various
processes.
Table 6.3. Hydrogen demand for coal upgrading or product improvement
Process
Hydrogen demand
mol H
2
per mol CH
2
(CH
3
OH)
Crude oil desulphurization 2 - 5
Hydrotreatment 5 - 10
Hydrocracking 15 - 30
Residue hydrogenation 25 - 30
Very heavy oil Æ syncrude 30 - 40
Oil sand Æ syncrude 30 - 45
Oil shale Æ liquid products 80 - 125
Coal gasification (CH
3
OH) 100 - 160
101
Fischer-Tropsch Synthesis
The catalytic hydrogenation of carbon monoxide resulting in the formation of paraffin-olefin mixtures
of different C-numbers is what is called Fischer-Tropsch synthesis. The product spectrum depends on
the operating conditions and the catalysts and on the partial pressures of CO and H
2
in the synthesis
gas:
CO + 2 H
2
Æ (- CH2 -) + H
2
O
for Co cataIyst
2 CO + H
2
Æ (- CH2 -) + CO
2
for Fe catalyst
Until now Fischer-Tropsch technology has been unable to compete economically with conventional
fuel and was limited to isolated circumstances. Recent process innovations, however, give hope to a
comeback for the exploitation of coal and untapped gas fields in remote regions as an alternative to
liquefied natural gas (LNG) processing. Shell and Sasol are currently the only companies to operate
Fischer-Tropsch plants on a commercial basis, though many projects are in various stages of
completion.
Others
A significant application of hydrogen in the food industry is the hardening of fats and oils by catalytic
hydrogenation for the purpose of extended durability. The process takes place at pressures of 20- 40
MPa and at temperatures of 200-400 °C.
Other fields of hydrogen applications are in the metallurgical industry as a reducing medium for nickel
production, for autogenous welding and cutting, in the glass industry when clean combustion is
required, and in the electronics industry for the epitaxial growth of polysilicon. For the fabrication of
semiconductor elements, vaporized liquid hydrogen is used as a doping gas because of its cleanness.
A field with growing interest is the ability of hydrogen for scavenging oxygen, e.g., in boiling water
reactors where traces of oxygen in the feedwater have been found to cause intergranular stress
corrosion cracking. The injection of hydrogen can reduce oxygen to a level of < 100 ppb.
6.2.2. Hydrogen as a fuel
The first application of hydrogen as a "transportation fuel" was in the late 18th century for flying
balloons with the first lift off of a balloon ("Charliere") filled with 40 m
3
of H
2
. The H
2
was produced
by spilling sulphuric acid onto iron. The balloon travelled a distance of 25 km and a height of up to
about 1 km.
Hydrogen has a principal advantage over electricity – storability. This gives an opportunity to use
hydrogen in many applications, where the usage of electricity is impossible or limited.
Heating
Catalytic or flameless combustion of hydrogen exhibits many advantages in comparison with flame
combustion. It occurs at low temperatures (ambient - 800 K), is safe and leads to a very high
conversion of the burning gas (99.9 %). NO
X
formation, which usually occurs in conventional
combustion at temperatures of about 1700 K, is here almost completely suppressed. The catalytic
combustion in diffusion burners occurs in the presence of small amounts of Pt or Pd catalyst. It is
adequate, for example, for kitchen appliances such as cookers, ovens, water heaters, and space heaters.
Drawbacks are the possible non-uniformity of the temperature distribution at the catalyst surface, rapid
changes in the operational state, and relatively small heat flux densities.
102
High-temperature fuel cells and combined heat and power
Combined heat and power (CHP) is an ideal market for high-temperature fuel cells, which produce
both electricity and heat. Such units are suitable for hospitals, hotels, airports, telecoms, and banks
where the security of power delivery is important. This is one of the first markets for hydrogen fuel
cells, which exists and grows. For instance, approximately 200 units of 200 kW Phosphoric Acid Fuel
Cell (PAFC) systems from ONSI Corp. have been purchased since its commercial introduction in
1990, and a growing number of the Hot Module 245 kW Molten Carbonate Fuel Cell (MCFC) have
been delivered from MTU CFC Solutions GmbH.
Stationary gas turbines
Hydrogen is an ideal fuel for gas turbines. Owing to its rapid mixing with air, a smaller combustion
chamber is sufficient and the efficiency is higher compared with conventional fuels. Gas turbines
modified for liquid hydrogen operation yield an up to 10 % higher thermal efficiency and output
compared with fossil-fuelled turbines. For systems with advanced heat exchange, efficiencies of more
than 50 % are estimated to be achievable. The remainder-free combustion is stable and favourable for
lifetime and maintenance. Of disadvantage is NO
X
production. The internal stoichiometric combustion
of hydrogen and oxygen heats up the argon gas, which actuates the turbine for power generation. The
water vapour produced is condensed and removed from the circuit whereas the argon is returned to the
combustor. Innovative developments of gas turbines capitalize on the experience of aerospace
propulsion systems. Performance goals are operational flexibility and control of combustion
temperature by pre-mixing steam. No particular difficulties are expected for a conversion of a
stationary gas turbine to H
2
fuel.
A modified gas turbine cycle, a H
2
-fuelled chemical-looping combustor, has been proposed in Japan.
A solid metal oxide, e.g., NiO, replaces air or oxygen as an oxidizer. An increase in the efficiency up
to 66.8 % is predicted compared with 61.6 % as the best figure for H
2
/O
2
cycles.
The hydrogen-oxygen steam generator is a novel power plant component derived from rocket
technology to provide instantaneously spinning reserve capacity upon demand. H
2
and O
2
stored
underground or in high-pressure tanks are injected into a combustion chamber and ignited.
Combustion takes place at pressures of 2 - 20 MPa. The reaction heat is directly transferred to
additional feedwater that is introduced. Steam of the required temperature of 500 - 1000 °C is obtained
and routed to the intermediate pressure steam turbine, thus increasing the electric power by 20 MW
within seconds. However, lack of demand for such direct power reserve and a relatively high cost have
led DLR (German Aerospace Research Center) to abandon this development. Comparable research
projects are currently pursued only in Japan and Russia.
Natural gas and hydrogen
Quite an effort has been dedicated to the possibility of mixing hydrogen with natural gas, which leads
to cleaner burning and relatively easy transport, but problems still remain in the field of pipeline
corrosion, since hydrogen in higher concentrations (> 5%) causes hydrogen embrittlement. Even this
relatively small proportion of hydrogen in natural gas would mean large quantities.
When considering this option, one must take into the consideration the so-called Wobbe number. If
different gases are mixed, the different properties of each component that makes up the mix must be
considered. Pure hydrogen has a caloric value of ~13MJ/m
3
, most natural gases have a much higher
value in the range of 35-40 MJ/m
3
. This means roughly three times more hydrogen than natural gas
must be burned to produce the same amount of heat.
Hydrogen in transportation
Hydrogen for transportation is receiving significant attention around the world because of high
petroleum prices and unreliable oil supplies.
103
Ground transport
Two ways of hydrogen utilization in cars are currently being taken into consideration – internal
combustion engine (ICE) vehicles and fuel cell (FC) vehicles. While ICE vehicles represent current
technology with modest modifications, fuel cell vehicles are in a stage of intensive R&D and
prototype testing.
Consideration of hydrogen as a fuel for the internal combustion engine started in the early 1920s.
Internal combustion engines that run on hydrogen are on the order of 25 - 30 % more efficient than
gasoline ICEs, because they take advantage of the fast-burn and far-lean combustion characteristics of
hydrogen. ICEs for earth-bound vehicles are seen by many to be superior to battery or fuel cell
powered vehicles in terms of range, acceleration, and power-to-weight ratio. Internal combustion
engines extract about 30% of the stored fuel energy. Operation with hydrogen is possible in a wide
range of mixtures with air. At extremely lean conditions, it provides a low emission level and a high
thermal efficiency in the low power range. Cryogenic hydrogen provides a higher energy storage
density (though still less than gasoline) and a considerable cooling effect. The technology still suffers
from inadequate on-board storage technology.
The first hydrogen ICE vehicle reported was a Ford pickup truck in 1971 in the USA. The first
European car running on liquid hydrogen (LH
2
) was demonstrated by the German DFVLR (now DLR)
in 1979, which also built the first LH
2
system for vehicle refuelling. Today the biggest effort shows
BMW with a fleet of the 745h (4.4-liter V8) and Mazda with its own concept with a rotary (Wankel)
engine, which runs both on gasoline and hydrogen.
Car manufacturers are focusing more effort on fuel cell vehicles than on hydrogen ICE vehicles. Many
prototypes have been introduced, some of them in small series (tens of cars). Most of the
manufacturers have opted for proton exchange membrane (PEM) fuel cells because of their low-
temperature operation and relatively (compared to other fuel cell types) easy manufacturing and
maintenance. (See section 6.3 for fuel cell details.) Current trends are mainly focused on hybridization,
such as fuel cell with NiMH batteries, ultra capacitors, or other types of electric storage. Although this
increases the complexity of the vehicle, thus increasing the cost, it brings significant advantages. The
main one is covering power peaks during acceleration, when the electric motor draws high current
from the FC. If fuel cell should cover these peaks itself, it would have to be much bigger than is
needed for cruising. A second advantage in electrical storage is increasing the driving range, because
hybrid vehicles optimize fuel consumption, and also the use of braking recuperation.
Some car-manufacturers are also considering in the mid term to employ fuel cells instead of classical
Pb-type accumulators, where the hydrogen needed is reformed from the gasoline onboard.
It is not only important to have technical problems solved, public acceptance is also important. For this
purpose, hydrogen fuelled buses have been successful. Currently there are about 60 of them serving on
a daily basis in different cities around the world. The biggest such project is the CUTE project, which
runs 27 busses in 9 EU cities (London, Hamburg, Madrid, Stuttgart, Stockholm, Porto, Amsterdam,
Barcelona, and Luxembourg). This project is connected with the ECTOS project (3 busses in
Reykjavik, Iceland) and ECOBUS (3 busses in Perth, Australia). The CUTE project finished in 2005
with good results. There were no critical technical problems, the availability for the customers was
unexpectedly high (over 80%), and public attitudes were positive. There is a follow-on project now,
called HyFleet CUTE, which adds 14 internal combustion buses and continues with an operation of 33
fuel cell buses. More information is available at http://www.global-hydrogen-bus-platform.com/.
The lack of the hydrogen infrastructure makes fleet customers important for early hydrogen
transportation markets. It is much easier to build one centralized filling station near a city bus operator
or dispatch service than to service the distributed market for personal cars.
Motorcycles, scooters and electric bikes represent a smaller, but interesting, market opportunity. Such
means of transportation are significant in many Asian countries, where the pollution is growing and
104
causing health problems. Switching from fossil-based fuels to hydrogen would improve the local
environment.
Aviation
Liquid hydrogen was early recognized as an important rocket fuel for use in space flights. The H
2
/ O
2
system together with the H
2
/ F
2
system provide some of the most energetic propellants. LH
2
and liquid
oxygen (LOX) are pressurized by a pump and burnt in a preburner chamber (stage 1). The resulting
high-temperature high-pressure gas operates the pump. The exhaust gas from the pump is routed to the
main combustion chamber where it is burnt once again using the remaining LOX (stage 2). Ram
engines for future reusable space transportation can be operated with atmosphere oxygen up to a
height of 40 km. Air-breathing engines with supersonic combustion (scramjet) offer an excellent thrust
potential. Hydrogen in space transportation is being used by the USA, Russia, Europe, China, Japan,
and India.
Considering weight and volume restrictions in aviation, liquid hydrogen has been shown to be
attractive as an aircraft fuel. The wide range of flammability of hydrogen in air enables a stable
combustion chamber operation far beyond the limits of hydrocarbons. The lighter fuel load compared
with conventional fuel results in a gross weight reduction allowing the use of a smaller engine.
Together with its high combustion heat per mass unit, fast ignition and high heat sink capacity,
hydrogen is a good candidate, in particular, for supersonic applications. In addition, it avoids all
pollutants of fossil fuels except for NO
X
. Compared with kerosene, the flight-related energy content of
LH
2
is nearly 2.8 times larger on a mass basis and it could be used as a coolant. Also the engine
lifetime is expected to be higher and maintenance requirements to be reduced. The main penalty,
however, is the required storage volume of the hydrogen, which is by a factor of about 4 larger than
for kerosene.
Liquid hydrogen is superior to kerosene in terms of safety. A hydrogen fuel fire is expected to be less
dangerous and the endangered area is much smaller; a kerosene fire lasts much longer.
Both Airbus and Boeing are considering hydrogen as a fuel option. In terms of fuels cells, the nearest
application in airplanes will probably be for headlights, which can be fuelled by hydrogen reformed
onboard from kerosene.
Sea applications
Under some circumstances hydrogen has application in ship propulsion using low-temperature fuel
cells. The operation of submarines powered by PEM FC systems has been successfully demonstrated.
Such systems can result in substantial noise reduction compared with steam-driven systems, which is
important for naval operations.
Japan is investigating a dual 500 kW PEM FC system operating on methanol reformate gas. US
research is focusing on 250 kW and 2.5 MW shipboard power units. Naval applications are being
realized in Australia, Canada, and Germany. Within the Euro-Quebec project, a passenger boat is
currently under construction equipped with a PEM FC electric drive and three 200 1iter LH
2
storage
tanks. The German Howaldtswerke Deutsche Werft AG, Kiel has recently investigated the use of fuel
cells in powering merchant ships.
Railroad transport
A fuel cell propulsion system for locomotives is being considered as an option in future railroad
technology. New emission standards for locomotives has led to a revival of research activities from the
past. Locomotives can better accommodate larger fuel storage volumes than road vehicles, making
hydrogen fuel storage less of an issue.
105
BIBLIOGRAPHY FOR SECTION 6.2
AZAR, Ch. Hydrogen or methanol in the transportation sector? Chalmer University of Göteborg,
Sweden. 2001.
BÜNGER, Ulrich. A HyWays – Towards a European Hydrogen Energy Roadmap. Conference
proceedings Stuttgart F-cell symposium. 2005.
The Business of FC for automotive applications. Detroit - Conference proceedings. Eyeforfuelcells.
2002.
Hydrogen & Fuel Cell letter. January 2003 – January 2006.
H2WORLD. October 2004 – September 2005.
IAEA. Hydrogen as an energy carrier and its production by nuclear power. TECDOC –1085. 1999.
IAEA. Market Potential for Non-Electric Applications of Nuclear Energy. TECDOC, series 410. ISBN
92-0-115402-X. 2002.
INSC. Nuclear Production of Hydrogen. ISBN 0-89448-570-9. 2004.
IVY, Johana. Summary of electrolytic hydrogen production. NREL/MP-560-36734. 2003.
JANIK, Ludek. Possibilities of hydrogen production by nuclear power. 2004. NRI research
publications Z 1333.
KNOPPEL, T – REYNOLDS,J. Fuel Cell Primer: The promise and the Pitfalls, rev. 7. 2001.
MOSELEY, P.T. Scientific advance in Fuel Cells Systems. Conference proceedings – Amsterdam. J.
Power Sources, vol. 118. 2003.
MYERS, B. Duane. Hydrogen from renewable sources: Pathway to 10 quads for transportation uses in
2030 to 2050. US DOE report. 2003.
TSUJIMOTO, Kazufumi – NAGAI, Y. Report of HTTR Workshop (Hydrogen production). JAERI.
Oarai, Japan.2004.
6.3. Challenges
Fuel cell – introduction
Fuel cells provide an elegant method of converting chemical energy into electric power and heat. Their
rapid development in recent years relates to the interest in hydrogen as the energy vector of future. To
understand current technological barriers of fuel cells one must first be familiar with the basic
principles of their function and structure.
Fuel cell – principle
The basic principle of fuel cell operation is the reaction between oxygen and hydrogen that produces
water, electric power, and heat. The efficiency of this process is about 50 % (depending on
temperature, pressure, and current). Such high efficiency is caused by the direct energy
transformation:
H
2
+ ½ O
2
Æ H
2
O + Heat + Electricity (1)
106

This process is unconstrained by the Carnot efficiency limits of the classical heat-engine method of
power production:
fuel Æ heat Æ kinetic power Æ electric power
where each energy transformation includes appreciable losses.
Figure 6.3 shows the schematic arrangement and function of a proton-exchange membrane (PEM) fuel
cell. The fuel cell consists of three basic parts: the anode, electrolyte and cathode. In a typical PEM
fuel cell, hydrogen is continuously fed to the anode where it dissociates into a proton and an electron.
Protons pass through a proton-conducting electrolyte to the cathode. Electrons have the same target,
but they are led through an external electrical circuit where they can perform useful work. Oxygen is
led to the cathodic site (usually as air) where it reacts with protons and electrons to form water.
FIG. 6.3. Schematic arrangement and function of proton-conducting fuel cells.
Power and rate of reaction
Activation energy must be supplied from the outside to overcome the energy barrier in reaction 1. If
the probability that a molecule will have sufficient energy to break the energy barrier is small then the
reaction will be slow (which is the case under regular conditions). There are four basic ways to
increase the rate of a reaction:
• Temperature increase;
• Pressure increase;
• Catalyst usage;
• Enlargement of the electrode surface.
The first three cases apply to the majority of chemical reactions in general. The last case — electrode
surface enlargement —is a special case within electrochemistry and is important for fuel cells. Since a
contact must occur between the gas and the electrolyte on the electrode surface (to allow electrons to
be passed through the electrode and protons through the electrolyte) the size of the interface between
the phases is crucial for the reaction rate and, thus, for fuel cell power.
The surface area size does not depend on length and width only —current fuel cells electrodes have
surface areas roughly about 1000 times bigger than flat surfaces, because they are porous in nature. In
addition the electrode surface must contain the catalyst and often must be resistant to aggressive acid
107
environments under high temperatures. The power of the fuel cells is often reported with respect to the
electrode surface area, for example in mW/cm
2
.
Fuel cell types
Fuel cells are classified based on the electrolyte type and operating temperature.
Polymer Electrolyte Membrane Fuel Cell (PEMFC): Also known as a proton-exchange membrane
fuel cell, the PEMFC uses a polymer membrane electrolyte (usually based on acid fluoropolymers)
that allows the transfer of hydrogen ions, but not gases. Because water is the sole liquid in this fuel
cell type, corrosion issues are minimized. The key for operation efficiency is water management. The
conditions must be set in such way that the product —water —does not evaporate faster than it is
produced. This is crucial because a high degree of membrane hydration is a requirement for good
proton conductivity. The operating temperature is limited by the type of polymer used, usually below
120°C (though new materials allowing temperatures up to 200°C are being developed and tested).
The fuel in this case is usually pure hydrogen, though hydrogen-rich compounds such as methanol in
direct methanol fuel cells (DMFC) are possible. Platinum or Pt/Rh and other materials are used as
catalysts. Carbon monoxide is a significant poison for this type of catalyst; therefore it must be
ensured that its concentration in the fuel is not higher than 5 ppm.
Alkaline Fuel Cell (AFC): The electrolyte for an AFC is typically 85 wt% KOH for fuel cells
operating at higher temperatures (~250°C) and the 35-50 wt% KOH for lower operating temperatures
(< 120°C). The electrolyte is kept in a matrix, which is made of asbestos in most cases. The advantage
of this fuel cell type is the possibility to use a wide spectrum of (cheap) catalysts (Ni, Ag, MeO,
corundum, and noble metals). The purity of the fuel and oxidizing agent is the most important issue,
because even a small amount of carbon dioxide (CO
2
) causes fast degradation of the electrolyte. (CO
2
reacts with KOH to form K
2
CO
3
.) Similar to the PEMFC the carbon monoxide is also a catalyst
poison.
Phosphoric Acid Fuel Cell (PAFC): This fuel cell type operates at 150-220°C and uses 100%
phosphoric acid as an electrolyte. Phosphoric acid, H
3
PO
4
, has reduced proton conductivity at lower
temperatures and the CO problem (catalytic poisoning of platinum) becomes more significant. The
phosphoric acid is more stable than other common acids and, therefore, is able to operate over a wide
range of temperatures. In addition, the usage of 100% acid minimizes the partial pressure of water
vapors; thus, it is not difficult to maintain correct water management. The matrix for the holding the
electrolyte is SiC in most cases.
Molten Carbonate Fuel Cell (MCFC): The electrolyte is a mixture of alkali carbonates, which is held
in an LiAlO
2
matrix. The operating temperature ranges from 500°C to 700°C. In this range the mixture
of carbonates will create a highly conductive molten salt where, the conductivity is provided by
migrating carbonate ions. Because of the high temperatures it is not necessary to use noble metals for
catalysts. Nickel is used for the anode and NiO is used for the cathode.
Solid-Oxide Fuel Cell (SOFC): In a solid-oxide fuel cell the electrolyte is a solid non-porous metal
oxide, often Y
2
O
3
stabilized with ZrO
2
. The operating temperature ranges from 600°C to 1000°C and
conductivity is provided by oxygen anions. The anode material is Co-ZrO
2
or Ni-ZrO
2
; LaMnO
3
doped
with strontium is typically used for the cathode. The solid nature of the electrolyte is significant for the
simplification of the system. As opposed to all other fuel cell types, there are only two phases (solid
and gaseous) in this fuel cell type.
Protons or hydroxyl anions are the main charge carriers in the low-temperature fuel cell types
(PEMFC, AFC, and PAFC), the carbonate and oxygen ions take this role in the case of the high-
temperature fuel cells. The general differences between individual fuel cell types are given in
Table 6.4.
108

Table 6.4. The basic characteristics of individual fuel cell types according to the electrolyte
PEMFC AFC PAFC MCFC SOFC
Electrolyte
Ion-exchanging
membrane
35-100%
potassium
hydroxide
Phosphoric aci
d
in asbestos
Molted
carbonates
Ceramic
Operating
temperature
80°C 65-220°C 205°C 650°C 600-1000°C
Charge carrier H
+
OH
-
H
+
CO
−2
3
O
2-
Base material C C C Stainless steel Ceramic
Catalyst Pt Pt Pt
N
i Perovskites
Summary on technologic barriers of fuel cells
The mass utilization of fuel cells for transportation and decentralized power production will not
materialize until at least 2020. The US DOE has set some target fuel cell parameters, which must be
met in 2010 for successful commercialization, including lifetime and power density.
Currently research focuses on the following areas:
• Catalysts: Catalysts differ according to fuel cell type. PEMFCs typically use catalysts based on
different forms of carbon coated with platinum or Pt/Rh. Platinum is rare and expensive,
though, so there is much effort to devise ways to reduce the Pt loading while keeping the same
catalytic activity and lifetime.
• Materials: There are relatively adverse conditions inside a fuel cell for the majority of typical
industrial materials. The high acidity given by the high concentration of H
+
ions, as well as high
temperatures in the case of high-temperature fuel cells, creates a particularly harsh environment.
A compromise must be reached, then, between the mechanical resistance, chemical stability,
physical properties and the performance and price of materials. In case of the high-temperature
fuel cells with temperatures from 500°C to 1000°C, the designers face the additional problem of
thermal expansion mismatches between dissimilar materials, especially for start-up, shut-down,
and other transient conditions.
• Equipment: fuel cell systems need specific balance-of-plant components (compressors,
blowers, etc.) that differ from those currently used.
• Production Cost: The problem with fuel cell cost is, in part, related to the current lack of mass
production. Costs are expected to go down as fuel cell markets expand. Further research on the
use of less expensive materials and simpler fabrication techniques are hoped to lower costs, as
well.
• Durability: For tomorrow’s customer of fuel cell vehicles, durability means delivering the same
level of performance and reliability they expect from today’s internal combustion engine
vehicles. Fuel cell stack lifetimes will have to increase substantially before large-scale
commercialization of fuel cell vehicles is possible.
109
• Cold-start capability: Managing the water produced by fuel cells presents a special challenge
in freezing temperatures. Current technology is capable of starts from –20° to –30°C, but power
suffers at these temperatures. Work is being done on lowering the time needed for achieving
50% of the fuel cell rated power. (Currently it takes 100s for –20°C.)
• Fuel cell stack power density: Especially for transportation purposes, it is important to
minimize fuel cell size and weight. The US DOE target for 2010 is 2 kW/liter, whereas today it
is possible to get around 1.2 kW/liter.
• Water management: This is a specific problem for PEMFC, s since the membranes must be
sufficiently moisturized to reach sufficient protonic conductivity. Too much moisture, though,
and flooding of electrodes pores can lead to a rapid power reduction as gases lose access to the
electrodes. The moistening is performed mainly by external bubbling of hydrogen in water or by
direct injection of water to the membrane. Apart from necessary extra equipment for external
moistening, there are also problems with starting in cold weather conditions, as discussed
above.
• SOFC problems: One of the major advantages of SOFCs over other types of fuel cells is that
internal reforming of simple hydrocarbon fuels is in principle possible. Current SOFCs,
however, suffer from two major limitations when dealing with internal reforming. The first is
coke formation that deactivates the anode catalyst, which is typically a Ni-YSZ cermet. The
second is poisoning of the catalyst by sulphur compounds. These problems are particularly
challenging for logistic fuels such as JP-8 that are rich in heavy hydrocarbons and sulphur
compounds. Even if JP-8 fuel is externally reformed, it is possible that small concentrations of
unconverted JP-8 components will reach the SOFC feed stream, especially during transient
operation such as start-up.
BIBLIOGRAPHY FOR SECTION 6.3.
AMPHLETT, J.C., and BAUMENT, R.M. Performance modeling of the Ballard Mark V solid
polymer electrolyte FC. Journal of the Electrochemical Society, vol. 142, No.1, pp. l-15.
BADRINARAYANAN, P., – PEM fuel cell water and thermal management. 1999. University of
California.
EG&G Technical Services, Inc. Fuel Cell Handbook 6
th
edition. 2002, p.. 352.
LARMINIE, J., and DICKS, A., Fuel cell system explained. 1
st
printing. 2000, p. 310.
MOSDALE, Renaut, and SRINIVASAN, Supramaniam. Analysis of performance and of water and
thermal management in proton exchange membrane fuel cells. In Electrochimica acta. April 1995 vol.
42, no.6, pp. 413-421.
NGUYEN, T., and WHITE, R., A water and heat management model for proton exchange membrane
fuel cells. In J. Electrochem. Soc., August 1993, vol. 140, no. 8, pp. 2178-2186.
110
6.4. Solutions
Hydrogen production using water cooled reactor technology requires processes that are consistent with
the temperatures that can be achieved by evolutionary water cooled reactors (~300
o
C) or supercritical
water reactors (~550
o
C).
6.4.1. Hydrogen production technologies
6.4.1.1. Steam methane reforming (SMR)
Steam methane reforming (SMR) is currently the primary commercial technology for hydrogen
production. The SMR process requires high process temperatures, which are usually provided by
burning natural gas. The process reactions are as follows:
Reforming: CH
4
+ H
2
O → CO + 3H
2
, endothermic (750-800
o
C) (1)
Shift: CO + H
2
O → CO
2
+ H
2
, exothermic (350
o
C) (2)
The cost of steam methane reforming is rising as natural gas prices rise. In addition, SMR results in
releases of carbon dioxide from the shift reaction (as a product) and from the reforming reaction when
natural gas is burned to provide the necessary heat. Heat from nuclear reactors has been considered as
an alternative to the burning of natural gas — potentially reducing carbon dioxide emissions by 30%.
Water cooled reactors and supercritical water cooled reactors cannot reach the temperatures required
for conventional reforming technology. The heat from these types of reactors can be used, however, if
the reforming technology is modified.
6.4.1.2. Steam Reforming of Dimethyl Ether
Toshiba of Japan has proposed that steam reforming of dimethyl ether (DME), a derivative from fossil
fuels or biomass, could be used to produce hydrogen with 300°C heat from water cooled
reactors [7,8].
DME is synthesized from natural gas from small or medium-sized gas fields, coal seam gas, and
natural gas with a large CO
2
fraction. The synthesis of DME from natural gas is inexpensive compared
to the liquefaction of natural gas since the liquefaction process for natural gas requires a temperature
lower than 113 K and a relatively large plant. DME has the potential to be synthesized from biomass
without additional CO
2
emissions.
DME is usually produced by a partial oxidation process of natural gas without emitting CO
2
, as shown
by the following formula:
2CH
4
+ O
2
→ CH
3
OCH
3
+ H
2
O, (3)
The DME reforming reaction is as follows:
(1/2)CH
3
OCH
3
+ (3/2)H
2
O → CO
2
+ 3H
2
–24.4 kJ/ (H
2
mol) (4)
The produced hydrogen fraction is high at temperatures of 285-300°C, according to thermodynamic
data. Specifically, Toshiba has developed, together with Shizuoka University, a DME reforming
catalyst that gives 98% conversion of DME to hydrogen at 285°C. The catalyst is Cu-Zn/Al
2
O
3
powder made with a sol-gel method.
With 40 MW of heat supply about 10
8
kg H
2
/year of hydrogen production is possible, which is of the
same scale as the largest hydrogen plant in the world. To date, the demonstrated production rate is
4.10 kg H
2
/day.
111
6.4.1.3. Steam reforming of methane at reduced temperatures
A conventional steam methane reforming (SMR) system for hydrogen production is composed of a
steam reformer, a shift converter, and a hydrogen purifier based on pressure swing adsorption (PSA).
A mixture of methane and steam is introduced into a nickel-based catalyst bed in the steam reformer,
where the SMR reaction proceeds at 750 to 800°C. The reformed gas is supplied to a shift converter,
where carbon monoxide and water are converted into carbon dioxide and additional hydrogen
(equation 2). The reformed gas is then passed to a PSA separator to separate the hydrogen.
A membrane reformer system, composed of a steam reformer equipped with catalytic membrane
modules with a palladium-based alloy and a separate nickel-based catalyst, can perform the reforming
reaction, the shift reaction, and the hydrogen separation process simultaneously, without an
independent shift converter and a PSA separator. By this simultaneous generation and separation of
hydrogen, the membrane reformer system can be much more compact and can provide higher
efficiency than conventional ones. The simultaneous progress of hydrogen generation and separation
drives the chemical reaction forward and thus can lower the reaction temperature to 500 to 600°C.
This allows the use of less expensive heat-resistant materials and enhances long-term durability.
Moreover, these lower temperatures offer the opportunity to couple lower-temperature heat sources to
the SMR process.
The enthalpies for the steam-methane reforming-shift (SMR) reaction to produce hydrogen are as
follows:
• Reforming reaction:
CH
4
+ H
2
O = CO + 3H
2
-206 kJ/mol (5)
• Shift reaction:
CO + H
2
O = CO
2
+ H
2
+41 kJ/mol (6)
• Combined reforming-shift reaction:
CH
4
+ 2H
2
O = CO
2
+ 4H
2
-165 kJ/mol (7)
For heat supplied by a nuclear reactor, the consumption of methane for the nuclear-heated SMR
reaction is 3.3 ÷ 4 = 83%, or 17% less, of that of the conventional SMR reaction for producing the
same amount of hydrogen. In conventional SMR approximately 2.7 moles of hydrogen are produced
from 1 mole of methane feed. In the case of a nuclear-heated reformer, for which no methane is
consumed for combustion, 4 moles of hydrogen can be produced from 1 mole of methane. Therefore,
the nuclear-heated SMR process will save about 30% methane consumption, or reduce by about 30%
the carbon dioxide emissions, compared with a conventional SMR process.
Nuclear-heated reforming using a membrane reformer system offers several advantages:
• Nuclear heat from medium-temperature reactors (below 600°C) can be used.
• No combustion of methane is needed for the endothermic heat of the reforming reaction, and
consequently produces no related carbon dioxide emissions, resulting in an approximately 30%
reduction of overall carbon dioxide emissions.
• Separation of carbon dioxide for future sequestration is facilitated since separation of the carbon
dioxide reaction product is built into the process.
112
• A smaller surface area for membrane modules is possible, since the recirculation of reaction
product gases (including residual hydrogen) in a closed loop configuration makes the average
driving force for hydrogen diffusion through membrane higher.
A concept for the nuclear production of hydrogen that combines sodium-cooled fast reactors (SFRs)
with the membrane reformer technology has been studied jointly by Mitsubishi Heavy Industries Ltd.
(MHI), Advanced Reactor Technology Co. (ARTEC), Tokyo Gas Company (TGC), and Nuclear
Systems Association (NSA).[9,10]
TGC demonstrated in 2004-2005 the operation of a methane-combusting membrane reformer at a
hydrogen fueling station for fuel cell vehicles in downtown Tokyo. The system performance,
efficiency, and long-term reliability were confirmed by producing >99.99% hydrogen at 3.6 kg/h for
more than 3,000 hours with hydrogen production efficiency of about 80% (high heating value) [68%
lower heating value].
In the conceptual design, the nuclear plant is a type of sodium fast reactor with mixed oxide fuel and a
power output of 240 MW
t
that would produce 18,000 kg/h. The hydrogen production cost of this
process was assessed to be competitive with those of conventional, methane-combusting, steam
methane reformer plants.
6.4.1.4. High-Temperature Water Cracking
As a greenhouse-gas-free alternative to SMR, the U.S. Department of Energy is exploring ways to
produce hydrogen through the cracking of water by means of electrolytic, thermochemical, and hybrid
processes. Most of the work has concentrated on high-temperature processes such as high-temperature
steam electrolysis and the sulphur–iodine and calcium-bromine cycles [11,12 and 13]. These processes
have a high probability of successful technical development, but they require higher temperatures
(750
o
C and higher) than can be achieved by water cooled reactors. Advanced reactors such as the very
high temperature gas cooled reactor (VHTGR) can generate heat at these temperatures, but will
require many years for commercial deployment. The high-temperature thermo-chemical cycles impose
significant thermal demands on the system materials, regardless of the reactor design, and, therefore,
require the development and certification of new engineering materials.
6.4.1.5. Low-Temperature Water Electrolysis
Low-temperature water electrolysis is commercially available today for generating hydrogen with no
external heat input, making it suitable to be supported by nearer-term water cooled reactors. Low-
temperature water electrolysis results in the direct decomposition of H
2
O into H
2
and O
2
. Its market
adoption has been limited by two factors. First, since all the energy for water cracking is derived from
electricity, the cost of electricity from current low-temperature reactors makes water electrolysis
uncompetitive with steam methane reforming. The development of lower-cost, carbon-free electricity
generation (through, for example, high-temperature nuclear reactors that can achieve generating
efficiencies greater than 45%) might make lower-cost electricity and, consequently, make low-
temperature electrolysis more cost effective. The second factor limiting the use of this technology is its
reliance on noble metal catalysts such as platinum. The high price and scarcity of noble metals make
large scale use of current water electrolysis systems impractical. Research in water electrolysis
technology, which will be described shortly, holds the promise to reduce these two barriers.
The U.S. Department of Energy’s goal for electrolysis is a capital cost of $300/kW for a 250 kg/d
plant (with 73% system efficiency). Under this program goal, a large centralized plant would produce
hydrogen at $2.00/kg [14]. Current costs are two-to-three times that value. The DOE research program
is focusing on ways to improve efficiency and reduce the cost of electrolyzers.
Commercial water electrolysis technologies fall into two categories: (1) solid polymer cells using
proton exchange membranes (PEMs) and (2) liquid electrolyte cells, most commonly using a
potassium hydroxide (KOH) solution. PEM electrolyzers are essentially PEM fuel cells operating in
113
reverse polarization mode. Protons diffuse in the PEM electrolyte whereas oxygen ions diffuse in the
liquid electrolyte of these systems.
Currently the cost of hydrogen from PEM and KOH systems are roughly comparable. Reaction
efficiency tends to be higher for the KOH system because of better conductivity of the liquid
electrolyte. But this advantage is offset by the higher purification and compression energy
requirements compared to PEM systems, especially at small scales. Thus, the development of
relatively higher temperature, higher conductivity, and lower cost electrolyte membranes for PEM
cells remains a goal for reducing the cost of hydrogen produced. Another major contributor to the cost
of both PEM and KOH electrolyzers is the extensive use of rare and expensive noble metal catalyst
materials for their electrodes. This current need limits the large scale use of this technology.
Development of alternative catalyst structures with less expensive materials would significantly
influence the economics of hydrogen production through electrolysis. Moreover, new advances in
high-pressure systems are being explored to lower the cost by reducing the need for hydrogen gas
compression.
Several groups are pursuing the development of low-temperature, high-pressure electrolysis systems to
mitigate the high cost of hydrogen compression. For instance, a high-pressure, low-temperature water
electrolyzer system is being developed by Giner Electrochemical Systems of Newton, Massachusetts
[15,16]. The Giner system is currently operable at a 3000 psi (14 MPa) differential pressure, with
hydrogen production at 3000 psig and oxygen production at atmospheric pressure. Their goal is to
increase the operating differential pressure to 5000 psi (35 MPa) through advanced design features
(such as the use of a polymer-supported membrane) and to replace high-cost components with lower-
cost materials and fabrication methods. The use of higher pressures does require the use of higher cell
voltages in the electrolyzer. Nevertheless, it is more energy efficient to run the electrolyzer at high
pressures than to operate a cell at low pressures and then use a compressor to achieve the hydrogen
pressure required for efficient distribution and delivery. Giner developed an economic model of
electrolyzer capital and operating costs to determine the cost of hydrogen as a function of the price of
electricity and the capital and operating costs of the electrolyzer plant components. The scenario they
investigated was a neighbourhood refueling station with a hydrogen production rate of 432 kg/day.
The electrical load for such a station is approximately 1 MW. Giner determined that to meet the DOE
target cost of hydrogen produced (US$ 2.00-3.00/ kg H
2
) [6.4.1.8] they would need to have an
installed equipment cost of US$1100 per kW
e
, a plant that operates at 90% capacity with a ten-year
plant life, and an electricity price of less than 3.6 ¢/kWh. This price is only 20% lower than the price
for commercial off-peak electricity in the metropolitan Chicago area (approximately 4.5 ¢/kWh) [17].
To meet the installed equipment cost target, they would need to have a large cell active area to reduce
the number of cells and ancillary components. Giner is also turning its attention to a moderate-pressure
electrolyzer that would operate at 1200 psig and may more easily reach the cost targets.
In parallel, Teledyne Energy Systems of Hunt Valley, Maryland, is developing an alkaline hydrogen
generator that has a high overall efficiency, a low maintenance cost, and a final output pressure of
5000 psig (35MPa) [18]. This work is being done as a part of the U.S. DOE program on Design for
Manufacture and Assembly. Again, operation at higher pressures greatly reduces the energy-intensive
need otherwise to compress hydrogen. In a recent assessment, however, Teledyne has concluded that
the increased costs of manufacturing a high-pressure electrolyzer (and the added safety systems
required) may not offset the reduced gas compression costs.
Because of the need for electricity for water electrolysis, its efficiency and economics depend on
electricity production efficiency and price. The electrochemical efficiency of present electrolysis units
can vary between 65 to 90%. It is currently possible to couple an electrolysis unit to a nuclear power
plant in order to produce electrolytic hydrogen. Thermal efficiencies typical for current water cooled
reactors (approximately 34%) result in relatively low thermal-to-hydrogen energy efficiencies. The
overall efficiency for electrolysis supported by water cooled reactors is limited to 21-30%.
Significantly higher efficiencies, up to about 40%, can be achieved if an advanced, higher-temperature
power conversion system, such as in the direct-cycle supercritical water reactor design or for He or
114
supercritical CO
2
turbine systems with thermal efficiencies of about 45%, provide the electricity for
low-temperature electrolysis.
Since low-temperature water electrolyzer technology does not require heat input, the interface between
the electrolyzer unit and a nuclear plant requires only the transfer of electricity. Thus, the heat load
from the nuclear reactor is needed only for electricity production. This feature can allow the
electrolyzer to be placed at a large distance from the reactor if required for safety without any loss of
efficiency due to heat losses. This also allows for distributed or regional hydrogen production that
could be customized for different markets and would minimize hydrogen transportation costs.
However, advanced water electrolyzers at relatively higher temperatures require heat input that would
have to be retrieved from the balance of the plant, which would require on-site hydrogen production.
Cogeneration of both hydrogen and electricity is a feature of low-temperature electrolysis, with excess
electricity available for the grid. Since low-temperature electrolyzers have fast start-up times, it is
possible to control the operation such that the rates of hydrogen and electricity production can be
varied in order to follow electricity and hydrogen demands without changing the nuclear reactor
thermal power. This means that load following and hydrogen production can be accomplished without
the need for energy storage methods. A regenerative low-temperature PEM system [19] to produce
hydrogen and electricity reversibly can be a candidate component of a nuclear hydrogen plant with
cogeneration capability.
6.4.1.6. High-Temperature Steam Electrolysis (HTSE)
In high-temperature electrolysis, part of the energy required to decompose H
2
O is supplied in the form
of heat and, hence, overall thermal energy efficiency is improved over that of low-temperature
electrolysis. Estimates show that the thermal efficiency can be as high as 48% if the electrolysis is
carried out at 850
o
C compared with approximately 25% for low-temperature water electrolysis [20].
Even at lower temperatures, steam electrolysis efficiency can be appreciably greater than that of
conventional electrolysis. Solid oxide electrolysis cells are typically operated at temperatures of 800
o
C
to 1000
o
C to maximize the transport of oxygen ions through the solid-oxide electrolyte (e.g., yttria-
stabilized zirconia). If suitable solid electrolytes are developed with high ionic conductivity for oxygen
ions at lower temperatures (say 400 – 500
o
C), the electrolysis process can be operated at these lower
temperatures with substantial improvement in hydrogen-to-thermal-energy conversion. Such
temperatures can be obtained with supercritical water cooled reactors.
Alternatively, process heat from a water cooled reactor could be supplemented with electrical
resistance heating to achieve the desired elevated electrolysis cell operating temperature. Indeed, the
Idaho National Laboratory recently assessed the possibility of coupling a high-temperature steam
electrolysis system to a CANDU heavy-water reactor [20]. High-temperature electric heaters were
added to maintain an electrolyzer temperature of 830
o
C. Hydrogen production efficiency was
estimated to be approximately 30% (lower heating value).
The energy (enthalpy change ΔH) necessary to electrolyze water into hydrogen and oxygen is
expressed as follows;
ΔH = ΔG + T ΔS
Where ΔG (Gibb’s free energy change) is supplied by electricity and TΔS is supplied by heat. In
Figure 6.4 is shown the temperature dependency of ΔH, ΔG and TΔS in an electrolysis process.
115
0
50
100
150
200
250
300
0 100 200 300 400 500 600 700 800 900 1000
Temperature(℃)
Electrolysis Energy (kJ/mol-H
2
O)
ΔG
TΔS
ΔH
FIG. 6.4. Energy of water electrolysis as a function of temperature [21].
The theoretical hydrogen production efficiency (η) of HTSE is defined as follows:
η = HHV/(ΔG/φ + TΔS) (8)
HHV: High heating value of hydrogen;
ΔG/φ: Heat necessary to generate electricity to be supplied to the HTSE cell;
φ: Power generation efficiency of electric plant.
Figure 6.5 shows the theoretical hydrogen production efficiency for a HTSE coupled with a super
critical water reactors (SCWR) with a power generation efficiency of 40% and for water electrolysis
using a proton-conducting membrane (PEM) electrolyzer at 100
o
C with the same power generation
efficiency. As temperature increases, ΔG decreases and TΔS increases as shown in Figure 6.4, so the
theoretical hydrogen production efficiency increases. A HTSE coupled with a SCWR as a heat and
electricity source provides an efficiency of approximately 51% (HHV) at 500
o
C, a value greater that
that for water electrolysis, which is about 42% (HHV). This analysis of theoretical efficiency does not
account for other inefficiencies in a practical hydrogen production system. [21]
116

0
10
20
30
40
50
60
PEM Electrolyzer (<100℃) HTSE coupled with an SCWR
Theoretical Efficiency (%)
Note:
Efficiency=(HHV of H
2
)/(ΔG+TΔS)
ΔG=(Heat)/ψ
ψ: Power generation efficiency (=40%)
FIG. 6.5. Theoretical HHV Efficiency Comparison of Low-Temperature PEM Water Electrolyzer
with a High-Temperature Steam Electrolyzer Coupled with a Supercritical Water Reactor. [21]
The HTSE process is conceptually the same as conventional electrolysis, but differs in its hydrogen
production mechanism (Figure 6.6). Steam (about 90% by volume) and small amount of hydrogen are
introduced at the porous cathode. Hydrogen is added to maintain a reducing atmosphere at the
cathode. At the cathode steam breaks into oxygen and hydrogen ions when a suitable electrical
potential is applied. The oxygen ions are conducted through the gas-tight electrolyte to the anode.
Hydrogen gas is liberated at the cathode. Hydrogen is separated from steam in a condenser. At the
anode, oxygen ions are converted into oxygen and liberated. An interconnect plate provides flow
channels for the incoming and outgoing steam/hydrogen mixture and also for oxygen produced at the
anode.
The development of HTSE electrolyzers involves the development of suitable materials and
components for the cathode, electrolyte, and anode. Durability, reliability, and fabricability of thin
electrolytes and sealants have to be addressed.
HTSE is suited for use with an advanced, higher-temperature nuclear reactor system. While a portion
of heat from such systems can be used to produce steam, the remaining can be used for high efficiency
electrical conversion for producing electricity. High-temperature electrolysis has the potential to
achieve practical thermal-to-hydrogen conversion efficiencies of 40 to 50% while avoiding the
challenging chemistry and corrosion issues associated with thermochemical production processes.
HTSE can be coupled to water-cooled reactors if supplemental heating is provided, though this results
in a loss of system efficiency.
117

FIG. 6.6. Schematic of a solid-oxide electrolysis cell. (Courtesy of J. D. Carter,
Argonne National Laboratory.)
6.4.1.7. Thermochemical and Hybrid Processes
Thermochemical and hybrid thermo-electrochemical cycles have the potential for hydrogen production
with higher efficiencies than low-temperature water electrolysis. Over 200 thermochemical and hybrid
electro-thermochemical reaction cycles for producing hydrogen have been identified in the literature
[22]. Only eleven of those identified in Reference [22] have maximum reaction temperatures below
550
o
C. These lower-temperature cycles can reduce the thermal burden, mitigate demands on materials,
and potentially be coupled with nearer-term nuclear reactors.
Five such cycles have been the subject of active research within the past five years: a family of
copper-chloride cycles (530
o
- 550
o
C) [23], an active metal (potassium-bismuth) cycle (475 - 675
o
C)
[24], a magnesium-chloride cycle (500
o
C) known as the Reverse Deacon Cycle [25], a U-Eu-Br
heavy-element halide cycle [26], and a hybrid sulphur-based cycle [27]. Argonne National Laboratory
has done exploratory work on all five thermochemical cycles.
The Cu-Cl cycle offers a number of potential advantages over other cycles: (1) the maximum cycle
temperature (530
o
- 550
o
C) allows the use of a wider range of heat sources; (2) the intermediate
chemicals are relatively safe, inexpensive, and abundant; (3) minimal solids handling is needed; and
(4) all reactions have been proven in the laboratory and no significant side reactions have been
observed. As a hybrid cycle, one of the reactions is electrochemical, which imposes a sizeable energy
cost. However, the electrolytic step requires voltages significantly lower than needed for direct water
electrolysis.
The copper-chloride cycle that has been examined at Argonne National Laboratory [23] consists of
three thermal reactions and one electrolytic reaction:
(1) 2Cu(s) + 2HCl(l) = 2CuCl(l) + H
2
(g) 430 - 475°C
Interconnect / Bipolar Plate
Porous Anode:
O
-2
+ O
-2
→ O
2
Solid-Oxide Electrolyte:
O
-2
transport
Porous Cathode:
H
2
O → H
2
+ O
-2
H
2
O in;
H
2
out
O
2
out
118
(2) 4CuCl(s) = 2CuCl
2
(aq) + 2Cu electrochemically at 25 - 75°C
(3) 2CuCl
2
(s) + H
2
O(g) = CuO*CuC1
2
(s) + 2HCl(g) 325 - 375°C
(4) CuO*CuCl
2
(s) = 2CuCl(l) + 1/2O
2
(g) 480 - 550
o
C
Hydrogen and oxygen are produced thermally in the reaction between Cu and HCl (Reaction 1), and
between CuO and CuC1
2
(Reaction 4), respectively, at temperatures up to 450 and 550
o
C. Water
enters the system as steam and reacts with CuC1
2
to produce HCl and CuO▪CuC1
2
at 350-400
o
C
(Reaction 3). The electrochemical reaction consists of the disproportionation of CuCl (Reaction 2) to
give Cu metal for recycle to the hydrogen production reaction and CuCl
2
to produce HCl and oxygen
through steps 3 and 4.
Experimental work has been done at Argonne to study the reaction kinetics for the hydrogen and
oxygen production reactions. The experiments were conducted in beds of solid material with a
continuous flow of excess gaseous reactants. The individual steps in the Cu-Cl cycle have been
demonstrated, the kinetics of the hydrogen and oxygen generation reactions have been studied, and the
temperatures of the reaction steps have been measured.
The reaction between HCl and Cu is a heterogeneous exothermic and reversible reaction. Reports in
the literature suggested that the reaction proceeds rapidly at 230
o
C, the temperature at which 93% of
HCl is decomposed and the Gibbs free energy change is -5.66 kcal/mol. Experiments at Argonne,
however, detected no hydrogen production at this temperature. At this temperature the kinetics of the
reaction are slow, and the rates of reaction are controlled by the mass transfer of HCl through a
passivating film of CuCl formed on the Cu surface. Hydrogen starts to be produced in significant
amounts at temperatures above 350
o
C. The kinetics of the reaction are further accelerated at
temperatures higher than 430
o
C, the temperature at which CuCl melts, facilitating the interaction
between HCl and Cu.
Experiments were performed with several sizes and shapes of Cu particles to find the best
experimental conditions for complete Cu conversion to CuCl and H
2
. Studies were done with 3, 10 and
100 μm particle sizes. There was complete conversion with 3 μm Cu particles, but only 75 percent
conversion with 10 μm particles and only 55 percent conversion with 100 μm particles.
The oxygen production reaction (Reaction 4) was studied in a vertical reactor connected to a mass
spectrometer to monitor the oxygen evolution. Because CuO▪CuC1
2
, the product of Reaction 3, can be
synthetically obtained from stoichiometric amounts of CuC1
2
and CuO at temperatures between 370
and 470
o
C, the kinetic study was performed using mixtures of CuO and CuC1
2
. At 500
o
C, the yield of
O
2
was 85% and at 530
o
C the reaction was virtually complete. From mechanistic studies it was found
that the overall oxygen generation reaction proceeds in two steps: (1) the decomposition of CuCl
2
to
CuCl and C1
2
and (2) the reaction of CuO with C1
2
. In the reaction between CuO and CuC1
2
, oxygen
starts to evolve at 450
o
C (the temperature at which pure CuC1
2
starts to decompose) and C1
2
is
liberated. The Cl
2
reacts with the CuO and produces CuCl and free oxygen. From this work, the
kinetics of the cycle have been established.
All the work described above has been at a small laboratory scale. No integrated-cycle test has yet
been conducted. The next work that must be done to prove the viability of the process is to develop an
appropriate electrochemical cell for Reaction 2. Only after a viable engineering design of an
appropriate electrochemical cell is developed can an accurate economic analysis of this cycle be
achieved. Nevertheless, a preliminary engineering flowsheet analysis for the cycle suggests that it is
capable of reaching 40% efficiency (lower heating value) [28].
An active metal alloy cycle was invented at Argonne National Laboratory and is currently being
studied by Argonne and Pennsylvania State University. Active metal alloy cycles are conceptually
among the simplest of the hydrogen generation cycles [24]. One form of the active metal alloy cycle is
the potassium-bismuth cycle. It consists of only two reactions:
119
(1) 2K3Bi (l) + 6H2O (g) = 6KOH (l) + 3H2(g) 475 – 675oC
(2) 6KOH (l) + 2Bi (l) = 3H2 (g) + 3O2 (g) + 2K3Bi (l) electrochemically
Indeed, it may be possible to design a system that performs both reactions in a single vessel, making
operations simple and with low capital cost. Bismuth, however, is a relatively rare element, so the
cycle may not be suitable for commercial operations. Similar cycles with other active metal alloys
(e.g., the Na-Sn cycle) may overcome this limitation.
Little is known about the thermodynamics and chemistry of the K-Bi cycle. No experimental data exist
to determine potential side products, the optimum operating temperature, or the necessary
overpotential of the electrochemical reaction. Under a number of simplifying assumptions, the
efficiency of the cycle was estimated to lie between 29 and 46% (lower heating value).
Proof-of-concept experiments are planned for the two reactions in this cycle. The work will start with
the design, fabrication, and testing of an electrochemical cell that will be tested over a range of
temperatures. The gaseous products will be analyzed to determine if side reactions exist.
The magnesium–chloride Reverse Deacon Cycle that was studied at Argonne National Laboratory and
then Idaho National Laboratory [24] is a three-step process:
(1) MgCl
2
+ H
2
O = 2HCl + MgO 450
o
C
(2) MgO + Cl
2
= MgCl
2
+ ½ O
2
500
o
C
(3) 2HCl = H
2
+ Cl
2
electrochemically at 80
o
C
MgCl
2
is impregnated into the structure of a microporous material such as a zeolite. This essentially
results in the Mg compounds being in the form of dispersed nanoparticles. Reactants can readily
diffuse into the zeolite to react with all of the Mg compounds and products can readily diffuse out of
the zeolite. No solid particle degradation occurs, provided that the zeolite is stable in the presence of
water and HCl at temperatures up to 500
o
C. Silicalite has been tested and was durable in the presence
of these species at 500
o
C and supported the MgCl
2
hydrolysis reaction.
MgCl
2
-loaded silicalite (10 wt%) was prepared. Under flowing steam, HCl was successfully generated
through reaction 1. Reaction 2 has not been tested to date, but is thermodynamically favourable. Side
reactions may demand a temperature higher than 500
o
C, though. Reaction 3 has been demonstrated
and optimized by Weidner et al. at the University of South Carolina. The current optimized cell emf
for the HCI electrolysis is about 1.5V. Further R&D into this cycle depends on further optimization of
the performance of the electrochemical cell. Further proof-of-principle tests would also have to be run
to demonstrate the chlorination of MgO and to determine chemical viability in terms of kinetics,
yields, and absence of important competing reactions. There is no ongoing work on this cycle.
Another possible low temperature cycle is the magnesium-iodine cycle, a purely thermochemical cycle
that was first studied in Japan [29,30,31,32,33], where proof-of-concept experiments were completed
and process design was started. The results of the early studies showed high yields and sufficient
kinetics for all reactions. A reassessment of this cycle in the U.S. is continuing at Argonne National
Laboratory and the University of South Carolina [28,34,35]. The maximum temperature for the Mg-I
cycle, however, is 600
o
C, beyond the range of water-cooled reactors, so the cycle will not be discussed
further here.
A fourth low-temperature hydrogen production cycle is actively being studied. The cycle is based on
heavy-element halide chemistry with a maximum reaction temperature of 300
o
C — the lowest known
temperature for a purely thermochemical hydrogen production cycle [25]:
(1) 2(UO
2
Br
2
•3H
2
O) = 2“UO
3
•H
2
O(s)” + 4HBr(g) +2H
2
O(g) 300
o
C
120

(2) 4EuBr
2
+ 4HBr = 4EuBr
3
+ 2H
2
(g) exothermic
(3) 4EuBr
3
= 4EuBr
2
+ 2Br
2
(g) 300
o
C
(4) 2“UO
3
•H
2
O (s)” + 2Br
2
+ 4H
2
O = 2(UO
2
Br
2
•3H
2
O) + O
2
(g) exothermic
This reaction sequence is consistent with present relevant chemical knowledge. That knowledge,
however, is for related, but not identical reactions with the exception of Reaction 1. The notation
“UO
3
•H
2
O(s)” is used in the above reaction scheme because the exact stoichiometry of the species has
not been determined [36]. Reactions 2 and 4 are expected to be exothermic and to proceed
spontaneously. Reactions 1 and 3 are endothermic and require application of heat to the drive the
reaction to the desired products.
Work was performed at Argonne National Laboratory to
(1) Determine the chemical products that result from thermal decomposition of UO
2
Br
2
·3H
2
O
(Reaction 1);
(2) Investigate and model the factors that influence reaction of Eu
2+
ions with H
+
ions in aqueous
hydrobromic acid to generate H
2
gas (Reaction 2);
(3) Study the thermal reduction of EuBr
3
to EuBr
2
(Reaction 3) and establish the degree of
completion at 300°C and whether a potentially interfering EuOBr impurity is produced;
(4) Determine the chemical consequences of reacting hydrated uranium trioxide (“UO
3
·H
2
O (s)”)
with an excess amount of “bromine water” (elemental bromine (Br
2
) dissolved in H
2
O)
(Reaction 4);
No integrated-cycle test has been performed. The work demonstrated the production of HBr through
Reaction 1 with the reaction going to completion at 300
o
C. The studies on Reaction 2 showed that
EuBr
2
reacts with aqueous HBr to produce hydrogen. Nevertheless, the rate of the reaction is slow
(typically several hours are required for completion) under the experimental conditions that have been
investigated to date. By analogy with literature studies on similarly sluggish H
2
production reaction
rates from V
2+
and Cr
2+
ions in acid solution, it is likely that Eu
2+
reacts slowly because the lowest
energy path to produce H
2
requires a complex in which two Eu
2+
ions simultaneously transfer one
electron each to a neighboring proton only if those protons have a separation distance that is close to
the H-H bond distance in the H
2
molecule. Such a “four center” reaction has low probability in fluid
solution. This mechanism, however, suggests increased reaction probability with increasing
concentration of both Eu
2+
and H
+
. Alternatively, a suitable catalyst could be introduced.
For Reaction 3, vacuum pyrolysis was found to allow the reaction to proceed without the
complications that can arise from water entrained in the system. For Reaction 4 bromine and water can
react to form HOBr, which can interfere with the desired reaction.
Thermodynamic data are largely unknown for this system. Such data would be required to assess the
efficiency of the system. As with other thermochemical cycles, an engineering application of the U-
Eu-Br cycle would need to consider corrosiveness of the chemicals. The low operating temperature of
300
o
C, however, may make these concerns more tractable than for higher-temperature cycles.
A fifth hybrid thermo-electrochemical hydrogen production system in the medium temperature range
has been developed by the Japan Atomic Energy Agency (JAEA) to produce hydrogen from water by
using the heat from a sodium cooled fast reactor (SFR) that could be applied to the SCWR [26].
The system is based on a sulphuric acid (H
2
SO
4
) synthesis and decomposition process that was
developed earlier as the “Westinghouse process.” The sulphur trioxide (SO
3
) decomposition process is
121
facilitated by electrolysis using a solid electrolyte that conducts oxygen ions. In this way, the operation
temperature can be reduced by 200°C-300°C compared to the Westinghouse process.
The system is composed of the following three reactions.
(1) 2H
2
O + SO
2
= H
2
SO
4
+ H
2
; using electricity at 80°C
(2) H
2
SO
4
= H
2
O + SO
3
; using heat at >450°C
(3) SO
3
= SO
2
+ 1/2 O
2
; using heat and electricity at 550°C
Alternatively, the SO
3
electrolysis step could be applied to the sulphur-iodine thermochemical cycle to
reduce the maximum temperature required. In that case, the first electrolysis step shown above
(reaction 1) would be replaced by a purely thermochemical reaction that would involve iodine.
The theoretical thermal efficiency of the system based on chemical reactions shown above was
evaluated within the range of 35% to 55%, depending on the H
2
SO
4
concentration and heat recovery
[26]. The highest efficiency was achieved when the concentration of H
2
SO
4
was 100% and full heat
recovery was considered. The lowest efficiency was calculated with the H
2
SO
4
concentration of 65%
and without heat recovery. The thermal efficiency of the hydrogen production plant with an SFR of
395MWt was evaluated to be 42%, where electrolysis efficiencies of reactions (1) and (3) were
assumed to be 90 and 85%, respectively [37]. This thermal efficiency was higher than that for water
electrolysis, which was 38% assuming a power generation efficiency of 42% and an electrolysis
efficiency of 90%.
An apparatus to substantiate the hydrogen production system was manufactured and several hydrogen
production experiments were performed. The maximum duration of any single period of operation was
about 5 hours, and the total operation duration was about 9 hours [38]. In the experiments, stable
generation of hydrogen and oxygen was observed, and hydrogen and oxygen production rates in the
experiments were about 5mL/h and about 2.5mL/h, respectively. No severe corrosion of inner surface
of the SO
3
electrolysis cell and the YSZ electrolyte was observed, but corrosion of the inner surface of
the outlet piping (gold plated stainless steel) was observed. Improvement of the apparatus is planned to
increase hydrogen production rate (1 normal liter per hour) and to operate for longer durations. In
parallel, Argonne National Laboratory is developing improved SO
3
electrolysis cells to lower the
needed voltage and increase overall efficiency for reaction 3.
The remaining issues are: (1) development of higher performance electrolysis cells, (2) confirmation
of the durability of the electrode and the solid electrolyte in an SO
3
atmosphere, and (3) scale-up of the
hydrogen production rate.
6.4.2. Hydrogen production economics
6.4.2.1. Nuclear power in the hydrogen economy
The hydrogen production cost by centralized electrolysis (with off-site hydrogen demand) at a
collocated nuclear power station and electrolysis plant was evaluated in Japan recently [39]. A
Japanese utility group, the Federation of Electric Power Companies, issued in 2004 the estimation of
electricity costs for varieties of power sources in various operating and financing conditions. For
nuclear power plants, the generation cost is 5.1 Yen/kWh at a capacity factor of 85%, 5.3 Yen/kWh at
80% and 5.9 Yen/kWh at 70% when the operation period is 40 years and the discount rate is 3%.
The capacity of the hydrogen producing plant was assumed to be 2,700 kg per hour. Conditions,
including the above power generation cost, were applied to a simplified formula for estimating the
hydrogen production cost. The following hydrogen production costs were obtained: 301 Yen/kg at a
capacity factor of 85%, 314 Yen/kg at 80% and 349 Yen/kg at 70%. With the compression cost (34
122
Yen/kg), the delivery cost (50-100 km: 168 Yen/kg), and the station running cost (168 Yen/kg) added
to the hydrogen production cost, the hydrogen supplying cost was estimated to be 671-719 Yen/kg.
The total cost of hydrogen supply can be divided into a production cost, a delivery cost, and a station
cost. Further, each of these costs is composed of a fixed cost and a variable cost. In this study, the
fixed cost was calculated by multiplying a capital rate by a capital cost. The capital rate was calculated
by summing the following items: plant depreciation with a legal plant life assuming a remaining
capital cost of 10%, a property tax of 1.4%, an insurance of 0.6%, maintenance and repair of 3%, a
remuneration of 2.5%, and a general control charge of 1%. A plant construction fee was excluded,
because it varies largely depending on the construction site and it is less than 10% of the capital cost in
most cases. The variable cost is the sum of a raw material fee, a utility fee, and a labor fee.
Hydrogen production cost using steam methane reforming was evaluated as a cost target. The plant
size was assumed to be 18,000 kg/h with a capital cost of 27 GYen based on the largest ammonia plant
in Japan. The hydrogen production cost was estimated to be 143 Yen/kg, assuming an operation rate of
90%, a plant life of 10 years, and utility and labour fees of 1.6% and 0.7% of the capital cost,
respectively. The cost of natural gas was assumed to be 1.8 Yen/Mcal. With a CO
2
sequestration cost
of 30-70 $/t-CO
2
, the production cost would increase to 177 Yen/kg H
2
assuming 33 Yen/kg H
2
or
3.6Yen/kg-CO
2
for CO
2
sequestration.
Capital costs were estimated for water electrolysis with outputs of 27, 270 and 2,800 kg/h. The scale
law with the power of 0.68 was used for the capital cost based on the reported capital costs. The
incidental costs for construction were not included in the capital costs. A plant life and a labour fee
were assumed to be 10 years and 0.7% of the capital cost, respectively. The cost of water was assumed
to be 200 Yen/t. Generation power for hydrogen production was assumed to be 48 kW/kg.
The study assumed that the electrolytic facility was co-located with a nuclear power plant; the
electricity cost was assumed to be the power generation cost of light water reactors (LWRs). The
power generation cost of LWRs was evaluated as functions of an operation rate and a discount rate.
The typical cost was 5.3 Yen/kWh at a capacity factor of 80% and a discount rate of 3%. The cheapest
cost was 4.8 Yen/kWh at a capacity factor of 85% and a discount rate of 0%. So the cost was changed
accordingly from 4.8 Yen/kWh to 5.3 Yen/kWh. The operation rate and power of the hydrogen
production plant were assumed to be the same as those of the LWR.
Hydrogen production costs for LWRs with thermal powers of 3,000, 3,500, and 4,000 MWt were
calculated. The hydrogen production rates for powers of 3,000, 3,500, and 4,000 MWt were 20,500,
23,900, and 27,300 kg/h, respectively. The power generation costs were assumed to be 4.8 Yen/kWh
with a capacity factor of 85%, 5.1 Yen/kWh with an operation rate of 85%, and 5.3 Yen/kWh with a
capacity factor of 80%. It was found that for all cases the hydrogen production costs using a nuclear
facility to provide electricity for electrolysis would be in the range of 224-280 Yen/kg, which exceeds
the target cost of 177 Yen/kg. Although the cost of natural gas depended on the economic situation,
the power generation cost would need to be reduced to meet the target cost.
Hydrogen has to be delivered from the production site to the user site if a centralized production
facility is used. There are three ways to carry hydrogen: a trailer for pressurized hydrogen, a tank
lorry for liquid hydrogen, or a pipeline for pressurized hydrogen. The volumes of hydrogen
transported by the trailer and the tank lorry were assumed to be 244 and 1,296 kg/trip, respectively.
Delivery costs were evaluated by changing the thermal power of the nuclear power plant and the
transportation distance.
If the thermal power was more than 1,000 MWt, the pipeline was cost effective regardless of the
transportation distance. The delivery by the tank lorry was expensive because it required an expensive
liquefaction and shipping facility.
The delivery cost of the pipeline was evaluated based on a capital cost of 360 MYen/km and a booster
cost of 33.6 kYen/km. The capital rate was assumed to be 0.18. The delivery cost of the pipeline
123
decreased with the amount of hydrogen and increased with the delivery distance. The delivery distance
was assumed to be 400 km in this calculation.
The capacity of a hydrogen station was estimated to be 90 kg/h, if the same amount of thermal energy
as that of a gas station was treated. However, fuel cell vehicles are 2.5 to 3 times more efficient than a
gasoline engine driven automobile. Therefore, the capacity of the hydrogen station was assumed to be
27 kg/h.
Station costs were evaluated using a progress ratio, F, which indicates how much costs decrease when
an accumulation of products doubles. Station cost, Y, was calculated as follows, after X-1 stations had
been identified:
Y = A X
-B
, (1)
where A is the cost of the first station and B is the cost reduction ratio (= -log (F)/log2). Using this
equation with X=10,000, the station cost for compressed hydrogen storage was calculated to be 248
Yen/kg. Here the operation rate and the station life were assumed to be 90% and 8 years, respectively.
The cost was still higher than the cost of 168 Yen/kg for a gasoline station.
For hydrogen to become economically advantageous, the hydrogen supply cost should be less than
gasoline’s cost. The hydrogen cost equivalent to the gasoline cost was calculated to be 581 Yen/kg
assuming a tax for volatile oil, a gasoline cost of 110 Yen/l, and the fact that fuel cell vehicles have 2.5
times higher efficiency than gasoline cars.
From the analysis that has been done, it has been found that hydrogen supplied from a centralized
electrolysis facility and delivered to a fuelling station by pipeline is competitive with gasoline that is
priced between 110 and 115 Yen/l. In the case of a gasoline cost of 110 Yen/l, the minimum
conditions to meet this cost were a capacity factor of 85 %, a power generation cost of 4.8 Yen/kWh
and a thermal power of 4,000MWt. If the gasoline cost increased to 115 Yen/l, the equivalent
hydrogen cost would be 56.6 Yen/kg. Therefore, the hydrogen supply cost for fuel cell vehicles would
meet the gasoline cost. Hydrogen production by centralized electrolysis is economically feasible, but a
reduction of the station costs is necessary to compete with gasoline because station costs make up
about 50% of the hydrogen supply cost.
6.4.2.2. The use of off-peak electricity to produce hydrogen
Two economic analyses of electrolysis systems have been done in the U.S. [15,40] to determine the
capital and operating costs that would be necessary to meet target hydrogen costs. These analyses can
be extended to consider the costs of hydrogen production with off-peak electricity.
In these studies the cost of the hydrogen produced by electrolysis was compared with the DOE target
cost for hydrogen, US$2.00-3.00 per kg [14]. A more relevant comparison would be between the cost
of hydrogen produced by electrolysis and the cost of hydrogen produced by steam methane reforming
(SMR). In Reference [41] there is an assessment of hydrogen cost produced by SMR for a range of
natural gas prices up to US$12.00 per MMBTU. For natural gas at US$4.00 per MMBTU, which was
the typical price for natural gas in the U.S. for many years, the cost of hydrogen is US$1.00 per kg.
Recently the price of natural gas at the wellhead reached US$12.00 per MMBTU [42]. For that price,
the cost of hydrogen is US$2.25 per kg. A study by S. S. Penner [43] also included an analysis of
hydrogen cost as a function of natural gas price. In that analysis the cost of hydrogen is US$1.29 for
natural gas at US$4.00 per MMBTU and the cost of hydrogen is US$3.58 for natural gas at US$12.00
per MMBTU. In summary, these studies bracket the cost of hydrogen at US$1.00-1.29 for natural gas
at its historic price and at US$2.25-3.58 for natural gas at its current price. The DOE target cost of
hydrogen thus is consistent with hydrogen produced by SMR with natural gas at its current cost.
124
One of the key components in these analyses was the cost of electricity. Although the studies did not
specifically address the use of off-peak electricity, the range of electricity costs that has been
considered is representative of that which would be appropriate for off-peak electricity production.
There are two significant studies that have analyzed the cost of hydrogen production by electrolysis.
One of them [40] concentrated on electrolyzer units that are now commercially available. The other
[15] was done as part of the development of a high-pressure electrolyzer that would be useable in
future hydrogen generation and distribution systems.
The first study reviewed the information available on thirty-seven currently available electrolyzer units
from five manufacturers. Two analyses were made. An initial cost analysis was done for all thirty-
seven units to determine the effects of electricity price on hydrogen costs. Thirty-four of the
electrolyzers reviewed were bipolar alkaline, low-pressure systems (less than 200 psig, 1.4 MPa) and
the analysis did not take into account any electrical energy for the compressors that might be necessary
to increase the pressure to several thousand psig (tens of MPa) for a distribution system. Three of the
electrolyzers reviewed were unipolar alkaline electrolyzers that produce a hydrogen product pressure
up to 10,000 psig (69 MPa); however these were among the smallest of the units reviewed and their
scale precludes them from being used in any large application.
For each electrolyzer, the specific system energy requirement was used to determine how much
electricity is needed to produce hydrogen; no capital, operating, or maintenance costs were included in
the calculation. The researchers found that, at current electrolyzer efficiencies, electricity costs must
be approximately 4.0 to 5.5 ¢/kWh to be able to produce hydrogen for less than US$3.00 per kg. The
analysis demonstrated that, regardless of any additional costs, electricity costs are a major cost
contributor.
A detailed economic analysis was done for three systems, representing small (20 kg/day), medium
(100 kg/day) and large (1000 kg/day) hydrogen production systems. The cost of electricity used in this
analysis was 4.83 ¢/kWh, which was considered to be a rate available to industrial users. This rate is
slightly higher than the current rate for commercial off-peak electricity in the metropolitan Chicago
area [17]. The hydrogen selling prices for the three systems were found to be US$19.01, US$8.09 and
US$4.15 per kg, respectively. It was found that for the large system the electricity cost represents 58
% of the cost of the hydrogen, with capital costs representing 32%. For the medium system, the cost of
the electricity is 35% of the cost of the hydrogen and the capital costs become the major factor at 55%.
For the small system, the capital costs increase to 73 % of the hydrogen costs and the electricity costs
are 17%. This analysis showed that electricity price is a contributor to the hydrogen price for all
systems, but the capital costs are more significant for small-sized electrolyzers. The hydrogen selling
price for the two small and medium systems is far above the U.S. Department of Energy target price of
US$2.00-3.00 per kg and above the cost of hydrogen produced by SMR. Even the large system has a
price that is 35 % higher than the DOE target price and 50% higher that the cost of hydrogen produced
by natural gas SMR. This demonstrates that it is necessary to have an appreciable scale to the
operation to make it economical.
The largest electrolyzer that was examined in this study produces 380,000 kg of hydrogen per year. If
these systems were to be used in a large hydrogen generation plant, the limited hydrogen production
capability of each unit would mean that a significant number of electrolyzer units would be required.
For example, a 500,000 kg/day hydrogen generation plant would require the use of 500 of these units,
which are the largest units available today, and would demand 1150 MWe. A plant of this scale would
be typical of the hydrogen generation plant that would be required to supply a medium-sized ammonia
or methanol plant. In this scenario, it would be more efficient to use electrolyzers that are 10 to 100
times as large as today’s units, if the economies of scale remain proportional.
The second study [15] was done by Giner Electrochemical Systems as a part of their efforts to develop
a low-cost, high-pressure hydrogen generation system. They developed an economic model that
allowed them to determine the cost of hydrogen as a function of the cost of electricity and the capital
125
and operating costs of the electrolyzer plant components. The scenario investigated was a
neighborhood refueling station with a hydrogen production rate of 432 kg/day. The electrical load for
such a station is approximately 1 MW. They determined that to meet the DOE target cost of hydrogen
produced they would need to have an installed equipment cost of US$1100 per kW, a plant that
operates at 90% capacity with a ten year plant life, and an electricity cost of less than 3.6 ¢/kWh. This
cost is only 20% lower than the cost for commercial off-peak electricity in the metropolitan Chicago
area (i.e., approximately 4.5 ¢/kWh [18]. To meet the installed equipment cost target, they would need
to have a large cell active area to reduce the number of cells and ancillary components. They would
also need to be able to achieve moderate (400 psig, 3 MPa) to high (2000 psig, 14 MPa) pressure in
their electrolyzer cell. They claimed to need no breakthroughs in compressor technology to achieve
their goal since the cell technology that they are pursuing is itself a high-pressure cell technology.
The models that were used in both of the studies considered above used a plant operating capacity
factor that is typical of base load operation, not off-peak operation. The two studies called for 97 %
and 90 % capacity factors, respectively. To treat this situation properly, analyses similar to those
discussed above would need to be done with a plant operating capacity that would be more typical of a
plant operated with off-peak electricity (approximately 40 percent). If the model used by the National
Renewable Energy Laboratory were modified to use an operating factor of 40 %, the hydrogen selling
price for a large system would approach US$7 per kg (approximately 70% greater than the price
calculated in the original study and a price that is more than twice the DOE target price.) In addition,
if the plant operated only with off-peak electricity, the plant output would be only 40% of the
reference plant. Consequently, it would be necessary to have a plant 2.5 times larger to achieve the
same daily production of hydrogen. In addition, it would be necessary to have hydrogen storage
capacity available to provide hydrogen supply during the periods when the off-peak production facility
is not operating.
Another way to consider results of such analyses is that they would show that the cost of electricity
would have to be reduced markedly (approximately a factor of 10) to compensate for the re-
distribution of the capital costs and the operating and maintenance costs over a lower hydrogen
production base. For the Giner Electrochemical Systems study, for example, the installed cost of the
system would have to be reduced to approximately US$500 to compensate for the reduced duty cycle
that is achievable with off-peak electricity.
With regard to both of these studies, there is no firm basis for the cost of off-peak electricity because
there is little relevant experience with the pricing for off-peak electricity in a high demand
environment, such as might occur if there is a large demand for off-peak hydrogen production. In
some areas of the U.S. off-peak electricity is used for pumped storage of water to provide additional
electricity during seasons in which there is high demand for electricity during peak periods. Off-peak
electricity is also used in some large cities to chill water at night for daytime cooling of office
buildings and other commercial facilities. In neither of those situations has there been any significant
increase in the cost of off-peak electricity; however, neither of those situations has produced a
significant demand on the available power during off-peak periods.
A study has been made in Japan on the cost of hydrogen that would be produced by a filling station
that produces hydrogen by water electrolysis [44,45,46,47]. The plant that was selected for study
would have a full-time capacity of 640 kg/day. Two modes of operation were considered. In one mode
the hydrogen generation portion of the station was assumed to operate full time. In the other mode, the
hydrogen generation portion of the station was assumed to operate only during those hours when
electricity would be available at off-peak rates. The analysis that was done used a construction cost of
204 million Yen, a 10-year depreciation cost, a fixed annual operation cost of 7.5% of the plant
construction cost and a similar operating cost prorated on the fraction of the time that the plant is
operated. It was assumed that the energy required to produce hydrogen by electrolysis is 48 kWh/kg.
The current TEPCO rates for electricity were used in the analysis. It was found that the cost of
hydrogen is 760 Yen/kg if the plant is operated full time. If the plant is operated only at those times
when electricity is available at off-peak rates (between 10 P.M. and 8 A.M.), the cost of the hydrogen
126
produced is 900-1200 Yen/kg. These findings are consistent with the findings in the U.S. analysis
reported above.
6.4.3. Hydrogen production with a combination of nuclear electricity and wind electricity
Wind-generated electricity has the similar capital cost as nuclear, but it is variable and intermittent.
Using electrolysis to transfer wind-generated electricity to hydrogen production could be a way to beat
its limitation. That is, excess wind-based electricity not needed for the grid could be shed through the
use of electrolyzers. But the low capacity factor of wind electricity (from approximately 25% to 40%,
depending on the site) would result in large idle periods for the electrolysis facility, which imposes a
serious cost penalty and makes electrolytic hydrogen production by wind electricity un-economical.
One solution could be to use wind-generated electricity to supplement base-load nuclear-generated
electricity and hydrogen production, because electrolytic cells can accommodate electrical currents
36% higher than its nominal operating level, producing more hydrogen. To allow this, the cell design
has to be modified to handle additional gas, and accordingly, the capital cost can be assumed to rise
from 300 to 330 US$/kW. Variable-current operation imposes fluctuation in conversion efficiency,
which was calculated by a model provided by Stuart Energy Systems [48]:
Energy use (kW-h/kg H
2
) = 41.66 + 7.955*A + 4.545/A + 1.1,
where A is the current density relative to the standard design. A study was performed to determine
how much additional wind (the ratio of additional wind to nuclear) could be added to the nuclear base,
and how much electricity (conversion ratio) is used for hydrogen production to make the case
economical.
The wind data used in the analysis were from two sites with similar wind speeds (Types G and H) and
variability in Wales, with the capacity factors of 41.5% and 32.6%, respectively. The Alberta and
Ontario electricity market price data in 2003 were used.
The portion of electricity used for hydrogen production (referred to as a conversion ratio) continued to
be a specified percentage of the total. For example, a wind installation of 35% of the nuclear capacity
with a lesser average wind speed (Type H) would add an average of 11.4% to the total power
production so that the model is required to produce 11.4% more hydrogen, and the storage capacity is
proportionally increased.
The analysis examined a range of additional wind power values up to 45%. For each value, the
objective function of optimization is the minimum price of hydrogen that will give total revenue of 3
US¢/kW-h for all electricity. This procedure was applied over a wide range of conversion ratios.
It is important to appreciate that the average value of electricity in Alberta in 2003 was almost 4.5
US¢/kW-h while the average value in Ontario was only 3.86 US¢/kW-h. The higher average value for
electricity makes the contribution from sale of electricity larger so that the selling price for hydrogen
needed to meet the revenue target of 3 US¢/kW-h is lower for the Alberta case.
The results indicated that cases with 70% to 80% of the conversion ratio make the best opportunities
for adding wind to a nuclear base, and that the case with 50% and 60% hydrogen production turned
out to be quite accommodating to wind, because the addition only makes sense where the electrolysis
installation is big enough to be able to use more than the entire output from nuclear generation. The
case with 70% hydrogen production (and 30% sold as electricity) tends to be the best conversion ratio
for accommodating the addition of wind. For the Alberta situation, additional hydrogen generated
from wind can be sold at a lower price than in Ontario in order to meet the revenue target. The ratio of
the additional wind power the facility can handle economically depends on conversion ratio, the wind
type, and the electricity market. In both provinces, the 2000 US$/t of H
2
target price can be met with
Types G and H winds comfortably for the ratio of additional wind to nuclear is up to 30%, with the
70% of conversion ratio.
127

In conclusion, the combination of nuclear and wind does appear capable of absorbing wind’s
variability and to produce hydrogen from wind-produced electricity far more cost-effectively than
would be possible with wind alone. It also accommodates the seasonal variability of wind.
6.4.4. The environmental benefits of fuel cell vehicles supplied by nuclear-generated
hydrogen
Argonne National Laboratory performed an analysis of the environmental impacts of hydrogen fuel
cell vehicles using the lifecycle analysis software GREET, the Greenhouse gases, Regulated
Emissions, and Energy use in Transportation model. GREET, as described in Chapter 5, was
developed at Argonne National Laboratory to evaluate well-to-wheels energy and emission impacts of
motor vehicle technologies powered with various transportation fuels. The model and associated
documents are posted at http://www.transportation.anl.gov/software/GREET/index.html
.
In this study, four nuclear hydrogen production pathways were examined [49]:
(1) Hydrogen production at refuelling stations by electrolysis using light water reactor electricity;
(2) Centralized hydrogen production using the sulphur-iodine thermochemical cycle using heat
from a high-temperature gas-cooled reactor (HTGR).
(3) Centralized hydrogen production using high-temperature steam electrolysis using HTGR heat
and electricity.
(4) Hydrogen production at refuelling stations by electrolysis using HTGR electricity.
All stages of the lifecycle were considered:
• Uranium ore mining and milling;
• Uranium yellowcake transportation;
• Uranium conversion;
• Uranium enrichment;
• Uranium fuel fabrication;
• Uranium fuel transportation;
• Electricity or hydrogen production in the nuclear power plants;
• Hydrogen transportation;
• Hydrogen compression;
• Hydrogen fuel cell vehicle operations.
The study showed that significant reductions in fossil energy use and greenhouse gas emissions come
from nuclear-based hydrogen production compared to natural-gas-based hydrogen production through
steam methane reforming. The reductions amount to 73 – 96% in greenhouse gas emissions (CO
2
,
CH
4
, and N
2
O) and 81 – 97% in fossil energy use. Furthermore, fuel cell vehicles powered by nuclear
hydrogen have substantial reductions in greenhouse gas emissions (87 – 98%) and fossil energy use
(89 – 98%) compared with internal combustion engine vehicles using reformulated gasoline. Nuclear
hydrogen is not completely emission-free, however, since a small amount of fossil fuel is consumed in
the upstream feedstock and fuel stages.
128

REFERENCES FOR CHAPTER 6
[1] NAKICENOVIC, N., et al., Global Energy Perspectives -- A joint IIASA-WEC study,
Cambridge University Press ISBN 0-521-64569-7.
[2] OECD/NEA & IAEA, Joint Report on Uranium: Resources, Production and Demand (2005)
ISBN 9264024255.
[3] ONO, K. et al., “Investigations of the Maximum Global Supply Capability of Nuclear Energy”
JNC TN9400 2001-028 (In Japanese, December, 2000).
[4] HORI, M., Role of Nuclear Energy in the Long-Term Global Energy Perspective, Proceedings
of OECD/NEA First Information Exchange Meeting on Nuclear Production of Hydrogen, Paris,
France, October 2-3, 2000, p. 25 (2001).
[5] INTERNATIONAL NUCLEAR SOCIETIES COUNCIL, Nuclear Production of Hydrogen:
Technologies and Perspectives for Global Deployment, (Published by the American Nuclear
Society, La Grange Park, Illinois, November 2004).
[6] WADE, D.C., 21st Century Energy Sustainability -- Nuclear's Role, Report presented at IAEA
Meeting on Contribution of Advanced Reactors to Sustainable Development, June 13-16
(2000).
[7] FUKUSHIMA, K., OGAWA, T., SEGAWA, N., MAKINO, S. and YAMADA, K., "Hydrogen
production by steam reforming and high-efficiency nuclear reactor technology", in Proceeding
of 15th World Hydrogen Energy Conference, 30D-03 (2004); and FUKUSHIMA, K. and
OGAWA, T., "Conceptual design of low-temperature hydrogen production and high-efficiency
nuclear reactor technology", JSME Int. J. B, Fluids Therm. Eng. 47, 340 (2004).
[8] YAMADA, K., MONIWA, S., MAKINO, S., YOKOBORI, S., SEGAWA, N., FUKUSHIMA,
K., and TAKEISHI, K., "Hydrogen Production with Steam Reforming of Dimethyl Ether at the
Temperature Less Than 573 K", in Proceedings of International Congress on Advances in
Nuclear Power Plants, No. 5138 (2005).
[9] TASHIMO, M. et. al., "Advanced Design of Fast Reactor-Membrane Reformer (FR-MR)",
Proceedings of Second Information Exchange Meeting on Nuclear Production of Hydrogen,
Argonne USA (2003).
[10] UCHIDA, S. et. al., "Concept of Advanced FR-MR", 15th World Hydrogen Energy Conference,
Paper No. 30D-08, Yokohama Japan (2004).
[11] HERRING, J.S., LESSING, P., O’BRIEN, J.E., STOOTS, C., HARTVISGEN, J.,
ELANGOVAN, S., Hydrogen Production Through High-Temperature Electrolysis in a Solid
Oxide Cell, Nuclear Production of Hydrogen, Second Information Exchange Meeting, Argonne,
Illinois, USA, 2-3 October 2003.
[12] FORSBERG, C., BISCHOFF, B., MANSUR, L.K., TROWBRIDGE, L., Nuclear
Thermochemical Production of Hydrogen with a Lower Temperature Iodine-Westinghouse-
Ispra-Sulphur Process, Nuclear Production of Hydrogen, Second Information Exchange
Meeting, Argonne, Illinois, USA, 2-3 October 2003.
[13] DOCTOR, R.D., MATONIS, D.T., WADE, D.C., Hydrogen Generation Using a Calcium-
Bromine Thermochemical Water-Splitting Cycle, Nuclear Production of Hydrogen, Second
Information Exchange Meeting, Argonne, Illinois, USA, 2-3 October 2003.
[14] DOE Hydrogen Program: Hydrogen Cost Goal, July 14, 2005.
www.hydrogen.energy.gov/pdfs/h2_cost_goal.pdf
.
[15] CROPLEY, C., Low-Cost, High-Pressure Hydrogen Generator, Giner Electrochemical Systems,
LLC. 2005 DOE Hydrogen Program Review, May 25, 2005.
http://www.hydrogen.energy.gov/pdfs/review05/pd23_cropley.pdf
.
[16] CROPLEY, C., Low-Cost, High-Pressure Hydrogen Generator, Giner Electrochemical Systems,
LLC. 2007 DOE Hydrogen Program Review, May 15, 2007.
http://www.hydrogen.energy.gov/pdfs/review07/pdp_12_cropley.pdf
.
[17] Com Ed Business Energy Rates
http://www.exeloncorp.com/ourcompanies/comed/comedbiz/energy_rates/
.
[18] COHEN, C., IBRAHIM, S., Hydrogen Generation from Electrolysis, 2004 DOE Program
Review Presentation, Philadelphia, PA, 2004.
129

[19] BENTS, D.J., SCULLIN, V.J., CHANG, B.J., JOHNSON, D.W., GARCIA, C.P., Hydrogen-
Oxygen PEM Regenerative Fuel Cell Energy Storage System, NASA/TM-2005-21381, January
2005. http://gltrs.grc.nasa.gov
.
[20] E. A. HARVEGO, M. G. McKELLAR, J. E. O’BRIEN, and J. S. HERRING, “Summary of
Reactor-Coupled HTE Modeling Sensitivity Studies,” Idaho National Laboratory Report
INL/INT-06-11889 (October 2006).
[21] OZAKI, et al., Nuclear Hydrogen Production Systems, (in Japanese) Toshiba Review Vol. 60,
No. 2 p. 27 (2005).
[22] CARTY, R.H., MAZUMDER, M.M., SCHREIBER, J.D., PANGBORN, J.B., Thermochemical
Hydrogen Production, GRI-80-0023, Institute of Gas Technology, Chicago, IL 60616 (June
1981).
[23] SERBAN, M., LEWIS, M.A., and BASCO, J.K., Kinetic Study for the Hydrogen and Oxygen
Production Reactions in the Copper-Chlorine Thermochemical Cycle, 2004 AIChE Spring
National Meeting, Conference Proceedings, 2004 AIChE Spring National Meeting, Conference
Proceedings, pp. 2690-2698 (2004).
[24] MILLER, W.E., MARONI, V.A. and WILLIT, J.L., DOE Patent Case Number S-104650
(2006).
[25] SIMPSON, M.F., HERRMANN, S.D., and BOYLE, B.D., A Hybrid Thermochemical
Electrolytic Process for Hydrogen Production Based on the Reverse Deacon Reaction,
International Journal of Hydrogen Energy, 31 (Aug. 2006) 1241 - 1246.
[26] BEITZ, J.V., Private Communication, Argonne National Laboratory, September 2005.
[27] NAKAGIRI, T. et. al., “A new thermochemical and electrolytic hybrid hydrogen production
process for FBR”, Paper 1021, GENES4/ANP2003, Kyoto (Sep. 2003).
[28] M. A. LEWIS, J. G. MASIN, L. C. NITSCHE, and P. PARTHASARATHI, “Evaluation of
Alternative Thermochemical Cycles for the Production of Hydrogen,” to be published.
[29] K. FUJII, W. KONDO, S. MIZUTA, Y. OOSAWA, and T. KUMAGAI, Advances in Hydrogen
Energy, 3 (1982) 553-556.
[30] T. HAKUTA, K. HARAYA, T. SAKO, N. ITO, H. YOSHITOME, N. TODO, and J. KATA,
Proceedings of the 3
rd
World Hydrogen Energy Conference, Tokyo, (1980) 311-332.
[31] W. KONDO, S. MIZUTA, T. KUMAGAI, Y. OOSAWA, Y. TAKEMORI, and K. FUJII,
Proceedings of the 2nd World Hydrogen Energy Conference, Zurich, (1978) 909-921.
[32] S. MIZUTA, W. KONDO, T. KUMAGAI, and K. FUJII, Int. J. Hydrogen Energy, 3 (1978)
407-417.
[33] Y. SHINDO, N. ITO, K. HARAYA, T. HAKUTA, and H. YOSHITOME, Int. J. Hydrogen
Energy, 8 (1983) 509-513.
[34] M. A. LEWIS, Private Communication, Argonne National Laboratory, (November 2006).
[35] M. A. LEWIS, J. G. MASIN, and A. TAYLOR, “A Standardized Method for Evaluating the
Potential of Alternative Thermochemical Cycles,” Proceedings of the AIChE Annual Meeting,
San Francisco, California, Nov. 12-17, 2006.
[36] PETERSON, S., Uranyl Bromides Obtained from Aqueous Solution, Journal of Inorganic and
Nuclear Chemistry. 17 (1961) 135-137.
[37] NAKAGIRI, T. et. al., “A New Concept of Hydrogen Production System for Sodium Cooled
FBR”, 30J-07, 15
th
World hydrogen Energy Conference, Yokohama (June 2004).
[38] CHIKAZAWA, Y. et. al., “System Design Study of Hydrogen Production Plants with Sodium
Cooled Reactors”, 30J-08, 15
th
World hydrogen Energy Conference, Yokohama (June 2004).
[39] H. KARASAWA, "Cost Evaluation for Centralized Hydrogen Production", The First COE-
INES International Symposium, INES-1, Tokyo, No. 35 (October 2004).
[40] IVY, J., Summary of Electrolytic Hydrogen Production, Milestone Completion Report,
NREL/MP-560-36734, National Renewable Energy Laboratory, September 2004.
http://www.nrel.gov/docs/fy04osti/36734.pdf
.
[41] ENTERGY CORP., Nuclear & Hydrogen Teaming-Up, presented at Argonne-West, Idaho, 10
July 2003.
[42] The Wall Street Journal. Vol. CCLVI, No. 44, p. C11. 1 September 2005.
[43] PENNER, S.S., Steps toward the Hydrogen Economy, Energy 31 (2006)33-43,
www.sciencedirect.com
.
130
[44] WE-NET report-task4, NEDO-WE-NET984, NEDO, Japan, 1999.
[45] WE-NET report-task8, NEDO-WE-NET9908, NEDO, Japan, 2000.
[46] WE-NET report-task8, NEDO-WE-NET0008, NEDO, Japan, 2001.
[47] ASAOKA, Y. and UOTANI, M., “Feasibility Study on Hydrogen Production with Off-Peak
Electricity”, CRIEPI report No.T02039 (April 2003).
[48] MILLER, A. and DUFFEY, R., “Co-Generation of Hydrogen from Nuclear and Wind: Effect
on Costs of Realistic Variations in Wind Capacity and Power Prices,” 13th International
Conference on Nuclear Engineering (ICONE 13), Beijing, China, May 16-20, 2005.
[49] WU, Y., WANG, M. Q., VYAS, A. D., WADE, D. C., and TAIWO, T. A., “Well-to-Wheels
Analysis of Energy Use and Greenhouse Gas Emissions of Hydrogen Produced with Nuclear
Energy,” Nuclear Technology, Vol. 155, August 2006, pp. 192-207.
131

ANNEX I
RECENT NUCLEAR DESALINATION DEMONSTRATION PROJECTS [7]
I-1. Nuclear desalination demonstration project, Kalpakkam, India
Bhabha Atomic Research Centre (BARC) has been engaged in R&D work in thermal and membrane
desalination technologies for the past several years. A number of pilot plants have been tested
successfully. Utilizing the design and operational experience of these plants, a hybrid multi-stage flash
/ reverse osmosis (MSF-RO) seawater desalination plant of 6300 m
3
/day capacity coupled to a nuclear
power station based on pressurized heavy water reactor (PHWR) on the southeast coast of India is
being set up to demonstrate the reliability and economics of hybrid desalination technology as an
alternative to meet water shortages. The hybrid desalination demonstration plant (Fig. A.1-1)
comprises a 4500 m
3
/d MSF desalination plant and a 1800 m
3
/day RO plant. A part of the high-purity
product water from the MSF will be used to prepare makeup boiler-grade water for the power station.
Blending of the product water from the RO and MSF plants will provide requisite-quality drinking
water. The RO plant has been operating since 2006 and the MSF plant is to be connected in 2007.
FIG. I-1. Hybrid MSF-RO desalination demonstration plant, Kalpakkam.
The setup of a hybrid system at same location may contribute significantly in reducing the operation
and maintenance (O&M) costs of desalted water by taking advantage of producing both process- and
drinking-quality water, using common pre-treatment to a considerable extent, and the possibility of
using reject streams from one plant to the other. A hybrid system using ultra-filtration and nano-
filtration (UF/NF) as a pre-treatment of seawater coupled to multi-step flash / multi-effect distillation
(MSF/MED) or RO has a high potential in the future.
I-2. Coupling of LT-MED with KANUPP, Pakistan
In order to demonstrate the technical and economic viability of nuclear desalination, a small capacity
Low-Temperature Multi-Effect Distillation (LT-MED) plant is under construction in Pakistan to be
coupled to the Karachi Nuclear Power Plant (KANUPP), which is a Pressurized Heavy Water Reactor
(PHWR) of 137 MWe capacity (Fig. I-2). The main objectives of this project are to collect technical
and economic data, and to obtain experience in design, manufacturing, operation and maintenance of
thermal desalination plants. The project is expected to foster public acceptance, as well. The
experiences in different phases of this project will pave the way for indigenization of MED type
desalination plants, which will ultimately culminate in large-scale desalination plants to be coupled
with future nuclear power plants along the coastal belt.
In first phase of the project an LT-MED plant of capacity 1600 m
3
/day is being coupled with
KANUPP. Different options were studied for tapping steam from the steam cycle of the power plant as
a heat source for the desalination plant. The bled steam, from the high-pressure turbine, that was
originally used for feedwater heating in one of heat exchangers has been selected as a heat source for
the desalination plant, owing to its having the least effect on power plant generation capacity. Because
132

of the risk of possible radioactive contamination of the product water, coupling of desalination plant
with nuclear power plant is carried out by employing an intermediate loop with a pressure reversal
concept. Demineralized pressurized water is circulated in the intermediate coupling loop. This
circulating water takes heat from the condensing steam in the feedwater heater to the reboiler to
produce motive steam for the MED plant. A pressurizer is used in the intermediate coupling loop to
maintain the pressure. The total number of effects of MED is 8 and the Gained Output Ratio (GOR) is
6. Provision of manual radioactive monitoring is incorporated in the intermediate coupling loop to
ensure the final product water free from radionuclides.
FIG. I-2. Karachi Nuclear Power Complex and the site of NDDP.
I-3. Coupling of MED-TVC with SMART for nuclear desalination
The SMART desalination plant has been developed for water production and electricity generation.
The integrated SMART plant would consist of several units of a Multi-Effect Desalination Process
combined with a Thermal-Vapour Compressor (MED-TVC). The thermal vapour compressor was
combined with the MED process to improve the energy utilization of the processed steam (Fig. I-3).
In a nuclear desalination, a radioactive contamination of the product water is one of the most
important issues with respect to the safety and public acceptance. In this regard, the units of the
desalination plant are coupled with the SMART power system through an intermediate heat transfer
loop. The major function of the intermediate heat transfer loop is to protect the desalination plant from
radioactive contamination and produce a medium-pressure motive steam by using the steam extracted
from the turbine. Radioactivity monitoring systems were also installed in the water production system
and the intermediate loop where the concentration of radioactivity is higher than that in the
desalination plant. These systems will provide an enhanced capability for radioactivity detection.
Since the desalination plants are connected thermally with the SMART power system, the transients of
the desalination system can directly influence the operation of the SMART plant. A slow transient,
such as a gradual reduction in the energy demand of the desalination system, can be easily
accommodated for by the SMART system through either the load-following capability or a cut-back of
the energy supply to the desalination system. Thus, only fast transients induced by the desalination
system become important events to be considered for the safety of the SMART desalination plant. For
disturbances of the SMART desalination plant, several events were identified as potential disturbances
133

imposed by the desalination plant. The impacts of these disturbances on the Design Basis Accidents
and Performance Related Basis Events of the SMART plant were evaluated by a conservative
bounding approach of the key safety parameters and the results showed no additional safety concerns
for the SMART desalination plant.
Construction of a SMART plant with one-fifth scaled power and a desalination plant has been
launched in Korea. The plant is expected to be in operation in 2008.
FIG.I-3. SMART MED-TVC desalination plant.
I-4. El Dabaa RO experimental facility, Egypt
The Egyptian Nuclear Power Plants Authority (NPPA) is considering to construct an experimental
Reverse Osmosis (RO) facility at its site in El-Dabaa, Egypt, to validate the concept of feedwater
preheating, to achieve the following objectives:
• Overall: to investigate experimentally whether the projected performance and economic
improvements of preheated feedwater can be realized in actual operation.
• Long-term: to study the effect of feedwater temperature and pressure on RO membrane
performance characteristics as a function of time.
• Short-term (~ 3 years): to study the effect of feedwater temperature and pressure on RO
membrane performance characteristics over a range of temperatures (20-45
o
C) and pressures
(55-69 bars).
The test facility would consist of two identical units: one unit operating at ambient seawater
temperature and the other with preheated feedwater up to 45
o
C. This configuration is considered
practical with 4-inch membranes, and has the benefit of giving direct comparison of performance
characteristics for the preheated and non-preheated cases over the entire range of test conditions.
134

The test facility consists of the following main components:
• Beach wells and pumps: This ensures clean feedwater with minimum pre-treatment
requirements and lower operational costs.
• Pre-treatment system: The system is designed to allow for the various pre-treatment
requirements for the different commercial membranes to be tested.
• Water heating system (for one unit only): The feedwater will be heated by a fresh
water/seawater heat exchanger. The hot fresh water will be obtained from an electric water
heater; the hot brine and permeate will be used to preheat the feedwater, utilizing
permeate/seawater and brine/seawater heat exchangers.
• High-pressure pump with energy recovery and hydraulic coupling: The experiments
involve different types of membranes, requiring different operating pressures and feed flows.
Therefore, the high-pressure pump is coupled with a hydraulic coupling to obtain the required
pressure and flow rates.
The experimental program was developed with IAEA technical assistance in the design stage as well
as in the preparation of the technical specifications and tender documents. The commissioning of the
test facility has been completed.
The results of this experimental work could have a strong influence on how the international nuclear
desalination community perceives the value/benefit of feedwater preheating, and hence there is a
common international interest in this project. Therefore, NPPA remains committed to making the
results of the experimental program beneficial to the nuclear desalination community.
FIG. I-4. El Dabaa RO experimental facility.
135
I-5. Russian Federation
The Russian Federal Agency for Atomic Energy (ROSATOM) has started construction of a floating
barge-mounted heat and power co-generation nuclear plant based the ship propulsion PWR-type
reactor KLT-40C in Severodvinsk. It is planned to put the plant into operation in 2010.
The barge where the NPP is mounted has the following dimensions: length – 144 m, width – 30 m,
displacement – 21500 t. Two KLT-40C reactors are housed in separate steel containments. The
floating NPP can produce up to 70 MW for electric power and about 174 MW of heat for
district/process heating. The lifetime of the plant is 40 years; it is designed for a continuous operation
period before dockyard refurbishment of 12 years.
Demonstration of this nuclear technology is intended to allow its larger-scale application inside the
country and abroad for electricity and heat production.
I-6. Feasibility studies
People’s Republic of China
The construction of a nuclear desalination demonstration plant (SNDP) with a production capacity of
160,000 m
3
/d in the Shandong Peninsula of China was proposed in 2000. The pre-feasibility of SNDP
was completed in 2001 and was reviewed and approved by the central government in 2002 and 2003,
respectively. Hao-Xin Investment Co. is the owner and the Institute of Nuclear and New Energy
Technology at Tsinghua University is the engineering contractor. The feasibility study started in
March 2003. The SNDP consists of an NHR-200 coupled to a MED process. The NHR-200 as a
nuclear heating reactor with 200 MW capacity is designed with a number of advanced features, such
as an integral arrangement, full natural circulation, self-pressurization, in-vessel control rod drive, and
passive safety systems in order to achieve very high safety margins. Therefore, no off-site emergency
actions such as sheltering, evacuation, relocation and decontamination are expected to be necessary.
Two desalination processes were considered and compared in the feasibility study. One is the high-
temperature vertical-tube evaporator multi-effect distillation (VTE-MED) process and the other is
multi-effect distillation thermal vapour compression (MED-TVC) process. A tower-type arrangement
for the VTE-MED has been adopted. Two trains of VTE-MED are coupled with the NHR-200. Each
train consists of 3 towers, in which 7 modules of evaporators compose one effect in parallel and 32
effects of evaporators are vertically arranged to achieve a high GOR of 22.
Alternatively, four units of the MED-TVC process are coupled with the NHR-200. Each unit has a
desalination capacity of 30,000 m
3
/day and consists of 14 effects of evaporators. The motive steam
from the steam generator is supplied through the thermal vapour compressor (steam ejector) and
extracts low-pressure steam from the sixth effect of evaporator. In this way, a GOR of 15 will be
achieved.
Techno-economic feasibility studies by interested IAEA Member States
Other countries undertaking feasibility studies on the technical and economic viability of seawater
desalination coupled to nuclear power include Algeria, Egypt, Indonesia, Jordan, Morocco, Tunisia
and UAE.
136
ANNEX II
NUCLEAR DISTRICT HEAT IN SWEDEN AND ROMANIA
II-1. The ASEA-Atom SECURE reactor
The development of the SECURE-reactor (Safe and Environmentally Clean Urban Reactor) started as
a joint research project between Sweden and Finland in 1976 to 1977. Later on the development work
continued within AB ASEA-Atom. In the early 1980s it had evolved into a rather mature reactor
model.
The design principles were to have an economically sound nuclear heating plant that is well suited for
location close to population centres. Such urban siting reduces the investment cost for the district
heating network and contributes to better operating economy by reducing the thermal losses from the
district heating pipe work.
The low temperature and pressure of a heating reactor offers wide freedom as regards the design. This
has been fully used for the design of the SECURE reactor. It has built-in safety based on basic laws of
physics. By utilizing such inherent safety for assuring the shut down and cooling of the reactor,
dependence on active components such as electrical or mechanical devises is avoided. Therefore the
issue of availability of such equipment will be irrelevant.
Table A.2-1 lists the members of the SECURE family of reactors developed by ASEA-Atom in the
1980s.
Table II-1. SECURE family of reactors
SECURE-LH Heat-producing plant with a reactor
coolant temperature slightly above
100
o
C
SECURE-H Heat-producing plant with a reactor
coolant temperature in the range of
200
o
C
SECURE-PIUS (Process Inherent
Ultimate Safety)
Power producing plant with a
reactor coolant temperature in the
range of 300
o
C
In the SECURE concept, the primary coolant circuit delivers heat to an intermediate circuit within the
station. Heat exchangers that deliver heat to the district heating network are located in the auxiliary
systems building.
A safety evaluation of the SECURE reactor was carried out in 1977. The assessment was divided into
the following parts of the reactor:
(1) Inherently safety properties
(2) Underground siting
(3) Pre-stressed concrete pool vessel
(4) Control and protection systems
(5) Reactor shutdown
(6) Residual heat removal
The evaluation concluded that it appeared possible to build a low-temperature reactor of a new type
with lower environmental risk than the power reactors operating at that time.
137
The cost characteristic of the heating reactor was such that the reactor was best suited for supplying
base load heat, with fossil fuel hot water stations meeting the peak load. Typical demand duration
curves for the heat consumption in built-up areas in Scandinavia show that a supply of 100
o
C water
can satisfy the vast majority of the heat demand.
A cost comparison between a SECURE station and a coal-fired station for heat production done in the
late 1980s showed that SECURE was more cost effective (Table A.2-2).
Table II-2. SECURE cost comparison (1 amu = 1 arbitrary monetary unit)
SECURE Coal-Fired Plant
Construction (amu/kW) 2460 1600
Capital (amu/kWh) 3.9 2.6
Fuel (amu/kWh) 2.4 6.7
Operation and maintenance
(amu/kWh)
1.3 2.0
Total (amu/kWh) 7.6 11.3
Studies of conversion of existing nuclear power plants in Sweden for delivery of district heat have
been undertaken over the years for both Barsebäck and Forsmark.
In 1976 a project aimed at building a new unit 3 (3000 MWt) in Barsebäck with the possibility for
combined heat and power generation was conducted. Within this project an alternative of rebuilding
the existing units 1 and 2 (1800 MWt) was also investigated.
For units 1 and 2 the existing turbines would have to be rebuilt so there could be a possibility to tap
steam from the turbine into heat exchangers for the district heating. It turned out that this rebuilding
was expensive at the existing units, especially when the loss of production during the long
reconstruction outage was taken into account. Therefore it was concluded that it was not economically
sound to modify the existing NPPs.
II-2. Existing district heating system in operation at Cernavoda NPP
The existing District Heating System, which is in operation at Cernavoda NPP for the time being,
provides 60% of the necessary heating for Cernavoda, as well as the necessary heat for sub-
contractors’ facilities, the plant town site and the NPP site (Unit 1, Unit 2, and different warehouses
and shops located in the Unit 3 building). During 1997–2005, about 590 000 MWh were delivered, out
of which 331 000 MWh were provided to Cernavoda. A peak of 60 500 MWh was delivered to the
town during the year 1999. The maximum winter-time heat provided to the town and the NPP site
represented 46 MW, the peak being covered from the auxiliary boilers.
A steam flow rate of about 80 tonnes/hour is extracted for district heating from the main steam line,
before the high-pressure cylinder and condensed through heat exchangers located on NPP site, with a
maximum capacity of 2 x 23 MW. Due to the lower temperature of the cooling water during winter
time, the electrical power output of the unit is not seriously affected. The peaks are covered with
expensive steam provided by the auxiliary oil burning boilers. They provide backup heat when unit 1
is not available.
Considering the continuous pressure from the municipality to extend the district heating network, S.N.
Nuclearelectrica SA decided to review the studies performed during ‘80s in order to develop a modern
138

district heating system, extracting steam, partially processed through the steam turbine, from Units 1
and 2. By extracting heat from two units, a higher redundancy will be achieved.
The technical solutions envisaged by new studies are considering steam from both units, considering
the possibility to extract a maximum of 12% from the main steam flow for each turbine. The heat
demand and hot water parameters to be considered are provided by Table A.2-3.
Table II-3. District heating demand and main parameters
Winter/summer regime Heat demand (MW) Hot water temperature
departure/ arrival (
o
C)
Max. winter 46 150/70
Average winter 35 120/70
Min. summer 7 75/40
Conclusions
The solution drawn-up during the 1980s for district heating using the capacity of Cernavoda NPP was
thorough, but unrealistic. Those studies presumed there would be five units in operation at the
Cernavoda site and considered an overly long network for heat transport and a large number of
consumers supplied with heat from the plant.
The current solution, provisionally implemented for supplying heat from NPP Unit 1 to the town of
Cernavoda and to the NPP site, is based on steam extracted upstream from the turbine. Such a solution
is simple and rather cheap, but has a low overall energy efficiency and does not allow further
expansion of the system.
The new studies envisage the extraction of the steam from the turbines of both Unit 1 and Unit 2, as
well as the installation of new heat exchangers and other equipment, for the extended use in district
heating for the town of Cernavoda. This solution will provide a higher reliability in supplying heat and
better energy efficiency. Considering the actual context of oil crisis and the provisions of the
international conventions and protocols dealing with greenhouse gases reduction, the district heating
using extracted steam from a nuclear power plant represents an efficient solution to reduce dependence
from hydrocarbons, enhancing the security of supply for Romania.
Also, the development of this solution will respond to the expectations of the local community and
will strengthen the relationship between the plant and the municipality of Cernavoda, contributing to
higher acceptance of the nuclear energy in the neighbourhood of the nuclear power plant.
II-3. Barriers to overcome – problems of retrofitting an existing plant
Designing a new nuclear power plant having the possibility to deliver electricity and heat is not a
complicated process. It is possible also to retrofit an existing plant, initially designed for electricity
production only, to co-produce heat for district heating purposes.
REFERENCE FOR ANNEX II
[1] INSTITUTE FOR POWER STUDIES AND DESIGN – ISPE, “Cernavoda District Heating
Modernization - Pre-feasibility Study”, ISPE, Bucharest (October 2004).
139

ANNEX III
CANDU ENERGY FOR STEAM ASSISTED GRAVITY DRAINAGE
Traditional open-pit mining has been used by industry for many years to remove oil sands from
shallow deposits. To increase production capacity, the industry is looking for new technology to
exploit bitumen from deep deposits. Among them, SAGD (Steam-Assisted Gravity Drainage) appears
to be the most promising approach. It uses steam to remove bitumen from underground reservoirs.
Recently, the SAGD recovery process has been put into commercial operation by major oil companies.
III-1. Generic SAGD process
A typical SAGD application involves twin horizontal wells drilled in parallel, with one a few meters
above the other, as shown in Fig I-1. The upper well is called the injection well and the lower one the
production well. Medium-pressure steam is injected into the underground deposit area through the
injection well to heat the reservoir of bitumen-sand mixture by conduction. The heating reduces the
viscosity of the bitumen, increases its mobility, and establishes pressure communication between the
two wells along their length, so that a flow of fluids (a mixture of bitumen and condensed water) can
occur and be collected through the production well. The production liquid is transported to a central
facility, where the bitumen is separated and the condensate is collected, treated, and sent back to the
boilers.
FIG.III-1. SAGD process.
The required steam injection pressure depends on the circumstances of the oil field and the life of the
well, and varies from 2 to 5.5 MPa. At the initial stages of production (two to three months), each well
requires steam at higher pressure than required during normal operation. Each barrel of bitumen
requires 2-3 barrels of steam (steam volume is corrected to 4°C and 1 bar - cold water equivalent), as
the quality of the deposit changes with location and time. Generally, about 18% of the energy content
of the oil produced is used up in the extraction process, while a further 5% is used in generating
hydrogen to upgrade the bitumen to synthetic crude oil.
In addition to steam demand, SAGD production facilities are also significant consumers of electricity.
III-2. Adaptation of ACR-1000 for SAGD
Atomic Energy Canada Limited has assessed the use of the ACR-1000 as a source of heat and
electricity for oil sand extraction and processing. The ACR-1000 design is an evolutionary
development of the familiar CANDU technology, adding innovations to enhance economics,
operations, and safety margins. The net electrical output from a standard ACR-1000 will be close to
1100 MWe, depending on local cooling water temperature. The main steam pressure is 6.0 MPa.
140

Depending on the requirements of specific projects, the ACR-1000 can be adapted to provide steam
only, or a mixture of steam and electricity at various steam/electricity ratios.
With the steam-only option, the steam generated from an ACR plant could be dedicated to supply
steam to oil sand processes, and no electricity is generated. Hence, the turbine island is eliminated
from the plant and replaced by the facilities dedicated to steam and feedwater supplies.
The steam/electricity mix option splits main steam from the steam generators into two streams: one
would be dedicated to supply steam to the oil sand facility and the other would be channelled to
generate electricity. As a result, the turbine capacity becomes smaller than that of a standard ACR
plant.
The steam-only option for an ACR-1000 plant can supply steam to produce 53,200 m
3
/day (334,000
bbl/day) of bitumen, assuming an SOR (Steam-to-Oil Ratio) of 2.5. For the steam/electricity mix
option, the steam supply capacity depends on the electricity output. As an example, if the plant
produces 300 MWe (gross) of electricity, the remaining steam supply will suffice to produce 38,800
m
3
/day (244,000 bbl/day) of bitumen.
For each option, two basic system configurations, which relate to the steam supply method, have been
examined. Schematic system diagrams for steam/electricity mix option are shown in Fig III-2 and
Fig III-3. In both configurations, the ACR NSP (Nuclear Steam Plant) remains a standard design.
These configurations are described below.
• In the 3-cycle configuration, the steam supply to the oil sands facility is through saline heat
exchangers (SHXers). The main steam from the Steam Generator (SG) secondary side is
divided; part of it goes to the turbine system and the rest goes to the SHXers. Inside the SHXers
the main steam heats incoming water and generates steam at the desired pressure and quality.
After use in the saline heat exchanger, the hot steam condenses and the condensate is pumped
back to the SGs as feedwater. The steam generated in the saline heat exchangers is sent to the
oil sand facility. The steam is later recovered in the form of water, treated, and returned to the
SHXers.
Steam to SAGE
Water from SAGD
Saline Heat
Exchanger
Feedwater
System
Condenser
Turbine
Generator
Steam
FIG. III-2. Three-cycle configuration.
141

• In the 2-cycle configuration, the main steam from SG’s secondary side is directly distributed
and injected into the well; the water is recovered at the oil sand facilities. This water does not
meet the SG feedwater requirements in quality and temperature. It has to be retreated, and
heated in the feedwater system before entering the SGs. Compared with the 3-cycle
configuration, this system is simpler, and the steam pressure is higher by eliminating the saline
heat exchangers.
Steam to SAGE
Water from SAGD
Feedwater
System
Condenser
Turbine
Steam
Generator
FIG. III-3. Two-cycle configuration.
III-3. CANDU for open-pit mining
Currently, the majority of oil sand production is through open-pit mining, which is suitable for
bitumen extraction when the oil sand deposits are close to the surface. The ore, a mixture of bitumen
and sand, is removed from the surface by truck and shovel operation. The ore is then mixed with hot
water to form a slurry that eventually undergoes a separation process to remove bitumen from the
sand.
The majority of the thermal energy required for the open-pit mining process is in the form of hot water
at a relatively low temperature (around 70°C), and the rest is dry process steam at around 1.0 and 2.0
MPa. The oil extraction facilities require electricity as well.
When a nuclear plant is adapted to supply thermal power to open-pit mining processes, the low
temperature and pressure requirements of the thermal demands allow extraction steam from the
turbine to be used as the heating source. This boosts the overall system efficiency.
Figure III-4 demonstrates a typical configuration for adapting a CANDU-6 plant to an open-pit mining
application. Steam extracted from the low-pressure turbine is used to heat water in the steam-to-water
heat exchangers. In order to maximize the plant efficiency, the water returned from the oil sand facility
enters the condenser first to recover the waste heat in the condenser. It then goes through the water
heaters to be heated further to the required temperature. The hot water is supplied to the bitumen
extraction facility; most of the water is recovered after the process, treated and sent back. The
142

condensate from these water heaters returns to the turbine’s feedwater system. The thermal power of
the water counts for about 80% of total thermal power demanded by the open-pit processes.
Feedwater System
2MPa Steam
1MPa Steam
CCW Out
Make-up
Make-up
Return water
Hot Water
LPTHPT
CCW In
Steam
Generator
HP Steam Heater
LP Steam Heater
Water Heater
Condenser
FIG. III-4. Schematic system of a CANDU plant adopted for open-pit mining.
There are two steam heaters operating at different pressures. The higher pressure one uses main steam
directly from the SGs and the lower pressure one uses extraction steam from the high-pressure turbine
as a heating source to generate process steam at 2.0 MPa and 1.0 MPa. The process steam is piped to
the bitumen extraction process and mostly used in mixture heat exchangers without condensate return.
Therefore, feedwater to these steam heaters is mainly from the make-up water. The condensate from
these steam heaters returns to the turbine’s feedwater heating system. The thermal power of the
process steam represents about 20% of total thermal power requirement. Therefore, the amount of the
steam used in these steam heaters are not as significant as the low-pressure turbine extraction steam
used in the water heaters.
While the hot water flow rate remains the same for all seasons, the hot water and feedwater
temperatures vary as the ambient temperature changes. For a typical site in the Fort McMurray region,
the hot water temperature requirement is 72-75°C in winter and 65-67°C in summer, and the feedwater
temperature varies from an average of 8°C in winter to 23°C in summer. The requirement of the
process steam quantity changes slightly. The make-up temperature ranges from 2°C in winter to 24°C
in summer. As a result of temperature changes, the thermal power demand fluctuates seasonally.
Taking 330,000 bbl/day of bitumen production, a manageable size of an open-pit mining project, a
system simulation has been performed for the year-round weather conditions for this configuration.
For the average weather condition (in April), the thermal power demand is 1400 MWt, and the
electricity demand is 300 MWe (including the oil facility and nuclear plant demands). A CANDU-6
plant is able to provide the required thermal power for this condition while generating 460 MWe
(gross) electricity. In summer, the thermal demand reduces to 1200 MWt, and consequently the
electrical output increases 470 MWe (gross). In the coldest month (January), the total thermal power
demand from the oil sands facility is as high as 1670 MWt, and the CANDU-6 plant generates 380
MWe (gross) electricity while producing enough hot water and steam to meet the thermal
requirements of the bitumen production. During a year, the nuclear plant generates electricity within a
range from 380 MWe to 470 MWe, while being able to meet the entire thermal power requirement.
This offers a limited capability to deliver electricity to the grid.
143
III-4. Turbine design requirement
Since the steam flow rate distribution in the system varies from the conventional electricity generation
plant, the turbine design has to be modified to adjust to this change.
Turbine type
For SAGD steam supply, the thermal power is generally supplied by the main steam and steam is not
extracted from turbine except for the feedwater-heating purpose. Therefore, a condensing type turbine
is suitable for this application, provided that the main steam is divided between the turbine system and
the thermal load.
For open-pit mining applications, some steam is extracted from the turbine to heat the water and steam
that are supplied to thermal power users. Therefore, an extraction steam turbine is used.
III-5. Safety and licensing
The licensibility requirements for an ACR SAGD project were examined and found to pose no major
issues. Since Bruce A nuclear plant in Ontario has supplied nuclear-generated steam to various
facilities outside the exclusion area, no major impediment to getting regulatory approval is foreseen.
III-6. Weather challenge
Oil sand deposits are located in Northern Alberta, Canada, a location substantially colder than those
for most conventional nuclear power plants. Consideration has to be given to the demanding aspects of
construction that are expected to be encountered in such climates.
The CANDU design is highly modularised, and the use of prefabricated modules will be maximized.
This approach minimizes on-site construction activities, enabling schedule compression, and reduces
the size of the on-site labour force. The module sizes and weights have been selected for suitability for
road transportation to northern Alberta.
Improved cold weather construction methods now allow year-round construction. This includes the
use of shelters and provision for heating and the use of temporary weather coverings to protect the
workers, equipment and structures when an “open top” reactor building construction method is used.
III-7. Water scarcity
Oil sand sites usually have limited water resources. This is a factor of consideration in building a
nuclear plant, since nuclear plants usually need a large amount of cooling water. Cooling water is
mainly required for the following usages:
•
Dissipation of heat from the turbine exhaust steam through the condenser, and dissipation of
heat generated in other equipment and systems during the normal operation;
•
Dissipation the decay heat from the reactor, during outages and abnormal conditions.
During normal operation, the vast majority of the cooling water requirement is the condenser cooling
water, which takes the heat load from the turbine exhaust steam. When a nuclear plant is designed for
thermal and electrical cogeneration purposes, the condenser heat load is reduced. The amount of
cooling-water reduction occurs depends on how much the thermal power load is and how the system is
designed. To reduce the water demand further, AECL has investigated technologies to minimize the
requirement for the cooling water, including the use of cooling towers and air cooling.
Overall, the study has found that the water scarcity issue can be overcome with a moderate level of
investment.
144
ANNEX IV
LARGE SCALE PROCESS STEAM SUPPLY FROM
GÖSGEN-DÄNIKEN NUCLEAR POWER STATION IN SWITZERLAND
The Gösgen-Däniken nuclear power station (KKG) with a gross electric output of 970 MW is the
largest nuclear station in Switzerland, meeting approximately 17% of the country’s total power
requirements and providing process steam for a nearby industrial plant. The power station was handed
over to KKG in October 1979 following an 80-month construction and commissioning period and has
since formed part of the country’s electricity grid with a high availability record. Kraftwerk Union AG
was in charge of construction of this plant as chief contractor.
Steam user
The Gösgen-Däniken nuclear power station (KKG) is the first of its kind in the world to supply
process stream. Its steam user is the nearby cardboard mill, Kartonfbrik Niedergösgen (KANI).
The cardboard mill is situated approximately 2 km away from the nuclear power station. The mill
recycles used paper to manufacture base paper for corrugated cardboard and requires the steam chiefly
for heating the drying cylinders. This process causes the steam to condense and the condensate is
subsequently de-aerated at 105°C in a de-aerating plant and returned as feedwater to the evaporator
plant at the nuclear power station. Losses (amounting to about 10%) are made up from the chemical
water treatment plant operated by KANI.
The two heavy-oil-fired boilers installed at the KANI plant, which with their emissions were once the
source of considerable atmospheric pollution, now have only standby status. A warm-up line
maintains them ready for immediate start-up, thus ensuring that they can take over steam supply
within 15 to 30 minutes if the supply from KKG is interrupted.
Environmental protection and heating oil conservation
The system implemented in Gösgen with a nuclear power station providing process steam makes a
significant contribution to pollution control. The ecological impact on the atmosphere as a result of the
sulphur dioxide and solid matter emitted in the flue gases of the heavy-oil-fired boilers of the KANI
plants has now been eliminated. Moreover, the reliable process steam supply from KKG represents an
effective substitute for heating oil, thus ensuring almost complete independence from this fossil fuel.
Further, maintenance work on KANI’s central heat generation system is reduced.
Approx. 68 kg of heavy oil would be required to generate 1 t of process steam using the heavy-oil-
fired boilers installed at KANI. At the current rate of steam consumption of 10 kg/s, this represents
savings of almost 59,000 kg of heavy oil daily.
The steam requirement of 22.2 kg/s envisaged for the plant in its final extension would entail burning
130,000 kg of heavy oil per day. If the heavy oil is assumed to have a sulphur content of only 0.5%, it
becomes clear what the combustion of 130,000 kg of heavy oil daily would mean in terms of
atmospheric pollution through sulphur dioxide emission.
Design requirements
The evaporator plant was designed by KWU for a maximum steam delivery of 22.2 kg/s. Process
steam pressure downstream of the evaporator plant is to be maintained at a constant 14 bar throughout
the entire load range and should be not less than 12 bar (saturated steam) at the point of transfer to
KANI (corresponding to be operating pressure of the KANI boilers). The amount of steam actually
extracted can fluctuate between 0 and 22.2 kg/s.
145

FIG. IV-1. Flow diagram of the evaporator plant.
The evaporator plant must be fully automatic; i.e., it must be possible to regulate any fluctuations in
steam flow rate easily and rapidly and without any manual intervention being necessary. Malfunction
of the evaporator plant must not affect the normal operation of the nuclear power station.
Thermodynamic design
The evaporator plant is heated with the live steam tapped between the steam generators and the
turbine. In this way, electricity generation is separated from process steam production; i.e. the
evaporator plant can continue to operate after a turbine trip as long as the steam generators carry on
supplying sufficient heating steam.
The heating steam drains are used to superheat the outside steam supply and are subsequently returned
to the power station water/steam cycle through the feedwater tank.
146

ANNEX V
EVALUATION OF BATTERY COST COMPETITIVENESS OF ALTERNATIVE
TRANSPORTATION TECHNOLOGIES
In the next two tables, the battery cost and the competitiveness of PHEV with ICEV and HEV are
evaluated for setting R&D goals for battery development. The comparison was made on the sum of
vehicle purchase cost and fuel/electricity cost for a 10-year vehicle life. One example on Prius-class
vehicles shows that, for the PHEV to become comparable with ICEVs and HEVs, it is necessary to
reduce the cost of lithium-ion batteries from the present cost (200 K Yen/KWh) by a factor of about 7
( to 30 KYen/KWh). This information was obtained from a report, “Recommendations for the Future
of Next-Generation Vehicle Batteries,” by the Study Group on Next-Generation Vehicle Batteries,
Ministry of Economy, Trade and Industry, Japan, August 2006.
Table V-1. Action plan for next generation battery technology development:
approximation for setup of cost target for light vehicles
ICEV
Gasoline Engine
Light Vehicle
(Reference)
BEV
Limited Purpose
Commuter
Battery Range
80 km
Year 2010
ICEV
Gasoline
Engine
Light Vehicle
(Reference)
BEV
Personal
Commuter
Battery Range
150 km
Year 2015
For Business 18,000 km/year For Personal 7,000 km/year
10-Year
Total Cost
2,260 KYen 2,380 KYen 1,490 KYen 1,710 KYen
Vehicle Cost 1,000 KYen 2,200 KYen 1,000 KYen 1,650 KYen
Battery
Cost
Cost 1/2
800 KYen
Cost 1/7
450 KYen
Base Vehicle
Cost
1,000 KYen 1,000 KYen
Other
Cost
400 KYen 200 KYen
10-Year
Gasoline/Electricity
Cost
1,260 KYen 180 KYen 490 KYen 70 KYen
Gasoline: Gasoline Consumption 20 km/liter Gasoline Price 140 Yen/liter
Electricity: Electricity Consumption 10 km/kWh Electricity Rate 10 Yen/kWh
147

Table V-2. Action plan for next generation battery technology development:
approximation for setup of cost target for registered vehicles
ICEV
Gasoline Engine
Passenger
Vehicle
(Reference)
HEV
High
Performance
Hybrid
Year 2010
PHEV
40 km Battery
Cruising Range
Plug-in Hybrid
Year 2015
BEV
480 km* Battery
Cruising Range
Full-fledged
Electric Vehicle
Year 2030
10,000 km/year
10-Year
Total Cost
2,630 KYen * 2,650 KYen 2,650 KYen 2,580 KYen
Vehicle Cost 1,700 KYen * 2,300 KYen * 2,400 KYen 2,500 KYen
Battery Cost
Cost 1/2
100 KYen
Cost 1/7
120 KYen
Cost 1/40
200 KYen
Base Vehicle
Cost
1,700 KYen 2,000 KYen 2,000 KYen
Other Cost 500 KYen 280 KYen 300 KYen
10-Year
Gasoline/Electricity
Cost
930 KYen 350 KYen
Electricity 40
KYen
Gasoline 210
KYen
83 KYen
* Revised from the original figure for consistency
Gasoline: Gasoline Consumption ICEV 15 km/liter Gasoline Price 140 Yen/liter
Gasoline Consumption HEV 40 km/liter Gasoline Price 140 Yen/liter
Electricity: Electricity Consumption PHEV 10 km/kWh Electricity Rate 10 Yen/L
Electricity Consumption EV 12 km/kWh** Electricity Rate 10 Yen/L
(** As of Year 2030)
Battery Capacity: HEV 1 kWh
PHEV 4 kWh
(Gasoline Running 60%, Electricity Running 40% by Distance)
EV 40kWh
148
CONTRIBUTORS TO DRAFTING AND REVIEW
Bertel, E. OECD Nuclear Energy Agency, Nuclear Energy Agency
Chirica, T. Societatea Nationala Nuclearelectrica SA, Romania
Cleveland, J. International Atomic Energy Agency
Halldin, C. OKG AB, Sweden
Hori, M. Nuclear Systems Association, Japan
Janik, L. Nuclear Research Institute Rež plc., Czech Republic
Kuran, S. Atomic Energy of Canada Ltd., Canada
McDonald, A. International Atomic Energy Agency
Misra, B.M. IAEA Consultant, India
Petri, M.C. Argonne National Laboratory, United States of America
Polunichev, V. OKBM, Russian Federation
Shukla, D.S. Bhabha Atomic Research Centre, India
Uhrig. R. University of Tennessee, United States of America
149
