
Sleep Medicine 5 Suppl. 1 (2004) S16–S22
www.elsevier.com/locate/sleep
What can neuroimaging findings tell us about sleep disorders?
Eric A. Nofzinger
Sleep Neuroimaging Research Program, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Abstract
Models of the pathophysiology of human sleep disorders have only recently been tested using nuclear medicine assessments, which have greatly
increased our understanding of the brain mechanisms involved in the human sleep–wake cycle. Dramatic changes in function have been observed in
large-scale neuronal networks during sleep. Broad declines in heteromodal-association-cortical function, and relative increases in limbic and paralimbic
function have been observed. These cortical areas are responsible for essential aspects of human behavior, allowing us to interact with the world around us
and to evaluate the significance of important events in our lives. Preliminary findings suggest that fundamental alterations in the function of these neural
systems occur in sleep disorders. In depression, alterations in rapid-eye-movement and slow-wave sleep appear linked to a sleep-related dysfunctional
arousal in primary limbic and paralimbic structures (amygdala), and hypofunction in frontal cortical areas. Pharmacologic interventions partially reverse
these alterations. Preliminary studies in insomnia indicate a subcortical hyperarousal and a failure of sleep to provide normal restoration of function in the
prefrontal cortex, leading to chronic sleep deprivation. This review discusses functional neuroimaging data on normal sleep, and on the pathophysiology
of insomnia related to depression and primary insomnia.
© 2004 Published by Elsevier B.V. All rights reserved.
Keywords: [
15
O]H
2
O-PET; [
18
F]FDG-PET; fMRI; HMPAO-SPECT; REM; NREM; Insomnia; Depression
1. Introduction
Our understanding of the basic mechanisms of sleep/wake
regulation has advanced considerably since the discovery of rapid-
eye-movement (REM) sleep nearly 50 years ago. Research methods
subsequently developed to study the living brain in preclinical
animal models across the sleep/wake cycle, led to the formulation of
theoretical models of the pathophysiology of human sleep disorders.
However, prior to the introduction of nuclear medicine assessments
to sleep research, our understanding of sleep/brain relationships in
humans was limited to surface electrophysiology, as assessed by
polysomnography, and it was not possible to test these theoretical
models. The recent introduction of nuclear medicine assessments
has allowed scientists to study brain function in discrete neural
areas not accessible to the surface EEG, resulting in an explosion of
new information regarding human brain function. However, despite
considerable advances, the union of the fields of nuclear medicine
and sleep medicine remains in its infancy, with the majority of
studies to date focusing on increasing our understanding of healthy
sleep/brain relationships, with only occasional studies on specific
sleep disorders.
Nuclear imaging (neuroimaging) techniques have been used
to observe dramatic changes in function in large-scale neuronal
networks across the sleep/wake cycle. For example, non-rapid-eye-
movement (NREM) sleep appears to be related to broad declines
in function in the heteromodal association cortex in the frontal,
parietal, and temporal lobes, as well as in the thalamus [1−11],
whereas REM sleep is characterized by relative increases in limbic
and paralimbic function [12−18]. These cortical areas are thought to
* Eric A Nofzinger, MD, Sleep Neuroimaging Research Program,
University of Pittsburgh School of Medicine, 3811 O’Hara St., Pittsburgh,
PA 15213, USA. Tel.: +1-412-246-6413; fax: +1-412-246-5300.
be responsible for essential aspects of human behavior, allowing us
to interact with the world around us and to evaluate the significance
of important events in our lives.
Early findings in sleep disorders using neuroimaging techniques
suggest that there may be fundamental alterations in the function of
these neural systems across the sleep/wake cycle. In depression,
alterations in both REM and slow-wave sleep appear to be
linked to a sleep-related dysfunctional arousal in primary limbic
and paralimbic structures such as the amygdala, as well as
hypofunction in frontal cortical areas [19−21], which can be
partially reversed by pharmacological interventions [20]. According
to preliminary neuroimaging studies, primary insomnia is associated
with abnormal physiological arousal to increased function during
sleep in the ascending reticular activating system, basal forebrain
and hypothalamus, thalamus, and the ventromedial prefrontal
cortex [16]. Furthermore, the thalamocortical system involving
the prefrontal cortex appears to function at an abnormally low
level during both sleep and wakefulness. This pattern suggests
that insomniac patients have subcortical hyperarousal and a failure
of sleep to provide for the normal restoration of function in the
prefrontal cortex, leading to chronic sleep deprivation.
This review will introduce the process of normal sleep, using
this as a background to review nuclear medicine (neuroimaging)
methods and their application in the study of sleep/brain
relationships, in healthy subjects and in patients with depression
or primary insomnia.
2. Neuroanatomy of sleep
Three interacting neuronal systems (an arousal system, a sleep
system, and a REM system) have been identified that are involved
in the regulation of the sleep/wake cycle.
1389-9457/04/$ – see front matter © 2004 Elsevier B.V. All rights reserved.
doi: 10.1016/j.sleep.2004.04.000
E.A. Nofzinger / Sleep Medicine 5 Suppl. 1 (2004) S16–S22 S17
Table 1
Functional neuroimaging methods for assessing brain function during sleep
Method Measure Resolution
Spatial Temporal
Sleep in scanner? Other characteristics
MEG tomography Electrical events 10 mm Milliseconds Yes Difficult in sleep; Availability; expense
fMRI Blood flow <cm Seconds Yes Noise; technically difficult in sleep
[
15
O]H
2
O-PET Blood flow cm Minute Yes Repeated measures possible
[
18
F]FDG-PET Glucose metabolism cm 10–20 minutes No Long half-life limits repeated measures
99m-Tc-ECD-SPECT Blood flow & metabolism cm Minutes No Repeatable in single night
Receptor imaging 5-HT, ACh, GABA cm 20–90 minutes Waking Expensive, labor intensive
Abbreviations: MEG: Magnetoencephalographic. fMRI: functional magnetic resonance imaging. [
15
O]H
2
O-PET: [
15
O]H
2
O-positron emission
tomography. [
18
F]FDG-PET: [fluorine-18]2-fluoro-2-deoxy-d-glucose positron emission tomography. 99m-Tc-ECD-SPECT: Technetium-99m-
ethyl cysteinate dimer single photon emission computed tomography. 5-HT: 5-hydroxy tryptamine or serotonin. ACh: acetylcholine.
GABA: gamma-aminobutyric acid.
2.1. Arousal system
The wake-promoting or arousal system is located in the ascending
reticular activating system (ARAS) originating in the brainstem.
The ARAS projects into a series of specific brainstem systems
(pontine cholinergic nuclei, midbrain raphe nuclei and the locus
coeruleus) and forebrain structures (midline and medial thalamus
with widespread cortical projections, amygdala) involved in arousal
[22−24]. The amygdala also has interconnections with the isocortex
and other areas involved in arousal such as the hypothalamus and
ventral striatum.
The hypothalamus has an important role in arousal via the
suprachiasmatic nucleus, which regulates the circadian control of
arousal, and the posterior hypothalamus, which contains a group
of neurons that produce hypocretin [24−35]. This projection is
of particular interest because the hypocretin neurons project not
only over the entire isocortex but to additional arousal systems
including dense projections to locus coeruleus, raphe nuclei, pontine
cholinergics, midline thalamus, nucleus basalis, and amygdala.
The absence of the novel hypothalamic peptide, hypocretin (also
known as orexin), results in narcolepsy/cataplexy, a neurological
disorder characterized by an inability to maintain wakefulness and
intrusion of REM into wakefulness [36]. Histaminergic neurons of
the posterior hypothalamus are also involved in arousal [37].
2.2. Sleep system
Recent evidence suggests that a sleep system exists in the
hypothalamus since the ventrolateral preoptic nucleus (VLPO)
contains gamma-aminobutyric-acidergic (GABAergic) and gala-
ninergic neurons that are active during sleep and necessary for
normal sleep. The VLPO may represent a “sleep switch” as it
sends inhibitory projections to arousal systems in the posterior
hypothalamus [38], and receives inputs from multiple brain
systems that regulate arousal, autonomic, limbic and circadian
functions [39]. Furthermore, the VLPO may also be important in
the regulation of REM sleep [40].
2.3. REM system
The REM sleep system comprises the laterodorsal and pedunculo-
pontine tegmental cholinergic nuclei (LDT and PPT) in the pontine
reticular formation, which are under the inhibitory influence of
wake-active monoaminergic systems [41−46]. The LDT and PPT
are disinhibited as the activity of these monoaminergic systems
declines during sleep, allowing for the generation of REM sleep
[47−55]. The LDT and PPT within the brainstem mediate
widespread cortical arousal indicative of the REM sleep state via
a dorsal pathway innervating the thalamus, and a ventral pathway
innervating the basal forebrain [42,56,57].
It is becoming increasingly apparent that the sleep, arousal,
and REM systems do not function in isolation but are modulated
by other forebrain structures that maintain functional connections
[22,58−62]. For instance, the REM-generating centers in the
brainstem are modulated by the amygdala [62−68].
The role of nuclear medicine in further clarifying the mechanisms
of sleep/wake regulation and its disturbance in sleep disorders may
lie primarily in studying these larger forebrain structures that may
modulate sleep, as opposed to the nuclei that generate sleep states,
given the spatial resolution constraints of human neuroimaging
methods. Additionally, evidence exists for a use-dependent feature
of sleep that may be localized in smaller neuronal groups in the
central nervous system, which may underlie the actual function of
sleep once it is generated. Nuclear medicine is ideally placed to offer
insights into these more local processes that occur within sleep.
3. Functional neuroimaging tools for sleep research
The first practical demonstration that nuclear magnetic reso-
nance (NMR) spectroscopy could be applied to the study of the
metabolic effects of brain activation in vivo came in 1980, with
studies of rat brain using a surface coil (reviewed in Kauppinen et al.
[69]). Nuclear medicine has since created a number of functional
neuroimaging tools for assessing varying aspects of brain function,
which are versatile and non-invasive (Table 1).
Early neuroimaging techniques such as computer tomography
(CT) scanning combined with xenon inhalation [70], were followed
by positron emission tomography (PET), which was used to
detect positron-emitting isotopes in labeled compounds such as
[fluorine-18]2-fluoro-2-deoxy-d-glucose ([
18
F]FDG-PET) [1]. The
signal used by PET is based on the fact that changes in brain
metabolism in healthy humans or laboratory animals are almost
invariably accompanied by changes in local blood flow. PET
provided a level of precision in the measurement of cerebral blood
flow, which opened up the modern era of functional human brain
mapping [71]. Subsequently, technetium-labeled perfusion tracers
were introduced (e.g., technetium-99m-hexamethylpropylene amine
oxime or HMPAO), which could pass the blood–brain barrier
and be detected by single photon emission computed tomography
(SPECT) [72], to monitor brain metabolism and/or blood flow
during sleep. However, the [
15
O]H
2
O-PET method subsequently
took prominence in functional neuroimaging research because it
S18 E.A. Nofzinger / Sleep Medicine 5 Suppl. 1 (2004) S16–S22
offered the opportunity to take multiple scans with a higher temporal
resolution than the [
18
F]FDG-PET or SPECT techniques [12].
The availability of improved statistical imaging software also
allowed for a greater ease of assessing regional changes throughout
the entire brain without a priori hypotheses, while correcting
for problems of multiple comparisons. Unfortunately, this also
came with increasing subject burden since subjects now had to
sleep within the scanner during the injection and uptake of the
radiolabel.
Assessment of brain function during sleep presents certain
challenges. Since sleep is a biological rhythm, it introduces a time
domain into a neuroimaging study. Rather than being constant across
a 24-hour period, brain function has reliable shifts in patterns of
function across not only different types of sleep, but also across the
day when alertness can vary widely. It is, therefore, important to
choose imaging methods that have the temporal resolution to assess
the brain process in question and also that the scan be taken at a
specific time across the biological rhythm of sleep.
Some of the available neuroimaging tools can be applied directly
to the study of the sleeping brain, although for others this would
be somewhat impractical but not impossible (Table 1). It is
important, when assessing sleep directly, to choose a method that
maintains, as closely as possible, the integrity of sleep. For example,
neuroimaging techniques such as functional magnetic resonance
imaging (fMRI), [
15
O]H
2
O-PET, and neuroreceptor imaging require
that the head be immobilized in the scanner at the time that brain
function is being assessed. These techniques, therefore, often require
a select group of subjects able to sleep in this type of environment.
Furthermore, in order to maximize the chance that the subject will
be able to sleep in this restricted environment, investigators often
sleep-deprive subjects prior to studying them. However, restricting
sleep will result in important changes in brain function, thereby
disrupting the object of study.
4. Neuroimaging studies of the functional neuroanatomy of
sleep
Although this field is still in its infancy, there have been considerable
advances in the functional neuroanatomy of healthy sleep in the past
decade.
4.1. REM sleep
A variety of imaging studies have shown that, in contrast to waking,
there is activation of the limbic and paralimbic cortex during
REM sleep. In addition, their consensus was that the brain as a
whole is functionally active during REM sleep. In a [
18
F]FDG-PET
trial, the anterior cingulate cortex was identified as the only cortical
region to have greater metabolism during REM in relation to
waking [1]. Another [
18
F]FDG-PET trial observed heterogeneous
activation during REM sleep with global metabolism similar to that
observed in the waking state [7]. Blood flow assessed by HMPAO-
SPECT was shown to increase within the visual association cortex,
but decrease in the inferior frontal cortex during REM sleep [72].
Positive correlations between REM sleep and blood flow in the
pontine tegmentum, left thalamus, bilateral amygdalas, anterior
cingulate cortex, and right parietal operculum have also been
observed using [
15
O]H
2
O-PET along with negative correlations
between REM sleep and frontoparietal cortex, posterior cingulate
and precuneus activity [12].
From these observations, it was concluded that REM sleep
may be involved in the processing of certain types of emotional
memories. In another trial, increased blood flow to the thalamus,
brainstem, and basal forebrain, as well as in limbic and paralimbic
structures was identified during REM sleep compared with
NREM sleep or waking [13]. The first trial with [
18
F]FDG-PET
and advanced statistics enabling whole brain analyses [15], found
a general pattern of activation of anterior limbic and paralimbic
structures during REM sleep relative to waking, strikingly similar
to those identified previously in PET studies [12,13]. The same
pattern of activation was obtained at two timepoints, separated by
12 weeks in a later trial by the same group, when waking and REM
sleep were monitored in healthy subjects [16].
Visual processing within REM sleep may be a closed loop of
extrastriate and paralimbic cortex in the absence of either primary
visual processing or higher-order processing in frontal areas [14].
Ina[
15
O]H
2
O-PET trial, the extrastriate but not primary visual
cortex was activated during REM sleep [14]. There was an inverse
relationship in blood flow between these visual processing regions
and a direct relationship between flow in extrastriate cortex and
paralimbic structures. A re-analysis of previous [
18
F]FDG-PET
data [1], obtained during waking and REM sleep in healthy
subjects, found increases in anterior cingulate, frontal thalamus
and extrastriate cortex during REM relative to NREM [17]. In
an assessment of blood flow using [
15
O]H
2
O-PET in 12 healthy
men during waking and REM sleep, correlations between flow
and eye movements in occipital cortex, anterior cingulate cortex,
mesencephalon, thalamus, parahippocampal gyrus, striate cortex
and supplementary motor area were noted in REM but not in
waking [18]. These correlations with occipital cortex and lateral
geniculate were suggested to be functional correlates of the pontine
geniculo-occipital wave in humans [18].
4.2. NREM sleep
NREM sleep is a functionally less active state with reduced blood
flow and metabolism relative to REM sleep or waking [1,70,73].
Regional reductions in brain function from waking to NREM sleep
have been observed in the heteromodal association cortex in the
frontal, parietal, and temporal lobes, and in the thalamus in several
studies. Reductions in frontal, thalamus, and basal ganglia have also
been observed in stages 2 and 3 [1], and bilaterally in the thalamus
during stage 2 of NREM sleep [6,7].
Regional cerebral blood flow during NREM sleep has been
assessed in several trials utilizing [
15
O]H
2
O-PET [2−5,13,74]. In
these trials, reduced flow occurred in “centrencephalic” regions
(thalamus, brainstem, and basal forebrain), limbic (prefrontal cortex,
basal forebrain, hypothalamus) and paralimbic (basal ganglia,
anterior cingulate cortex) structures, precuneus, and mesial aspect
of the temporal lobe during NREM sleep. Although blood flow in
the cerebellum during NREM sleep was found to be reduced in most
studies [2−5], it increased in one study [74]. Reduced blood flow
has also been demonstrated in the higher-order association cortex
(frontoparietal cortices), but not in the primary sensorimotor cortex
during NREM sleep [13,74]. Principal components analysis showed
two distinct networks, one in the thalamus and the second involving
frontoparietal cortex and cerebellum.
Brain metabolism during waking and NREM sleep has been
evaluated in 14 healthy subjects by [
18
F]FDG-PET scans [9,10].
Whole-brain glucose metabolism declined significantly from waking
to NREM sleep. Relative decreases in regional metabolism from
waking to NREM sleep occurred in wide areas of frontal,
parietal, temporal and occipital association cortex, primary visual
cortex, and in anterior/dorsomedial thalamus. After controlling
for the whole-brain declines in absolute metabolism, relative
increases in regional metabolism from waking to NREM were
found bilaterally in the dorsal pontine tegmentum, hypothalamus,
basal forebrain, ventral striatum, anterior cingulate cortex and
E.A. Nofzinger / Sleep Medicine 5 Suppl. 1 (2004) S16–S22 S19
extensive regions of the mesial temporal lobe, including the
amygdala and hippocampus, and in the right dorsal parietal
association cortex and primary somatosensory and motor cortices.
The reductions in relative metabolism in NREM sleep compared
with waking are consistent with prior findings from blood-flow
studies. Furthermore, the finding that there were relatively greater
decreases in heteromodal association cortex and in the thalamus is
consistent with thalamocortical networks associated with conscious
awareness, attention, and executive function showing the largest
functional declines from waking to NREM sleep. The relative
increases in glucose utilization in the basal forebrain, hypothalamus,
ventral striatum, amygdala, hippocampus and pontine reticular
formation are new observations that are in accordance with the view
that NREM sleep is important to brain plasticity in homeostatic
regulation and mnemonic processing [9,10].
Changes in brain function associated with awakening from
NREM sleep have also been examined by the [
15
O]H
2
O-PET
method [75]. Blood flow was monitored during sleep for 5 minutes
post-awakening and after 20 minutes post-awakening. Early
awakening was associated with increased flow in brainstem and
thalamus, while increased flow in anterior cortical areas was
associated with later awakening [75]. Additional differences in
relative flow in various brain structures suggested that the awakening
process is associated with reactivation of centrencephalic regions,
while the full recovery of consciousness (e.g., loss of sleep inertia)
is due to anterior cortical reactivation [75].
4.3. Sleep deprivation
Sleep deprivation of healthy subjects over 24 hours results in
global declines in absolute cerebral waking metabolism, as assessed
via [
18
F]FDG-PET, particularly in the frontoparietal cortex and
in the thalamus, which correlate with decreased alertness and
cognitive performance [76]. This finding supported a role for
sleep in the restoration of brain function in thalamocortical
networks associated with higher-order cognition, and the idea that
these networks are important in regulating arousal [76]. Similarly,
blood flow in the thalamus and ponto-mesencephalic tegmentum, as
assessed by [
15
O]H
2
O-PET, also positively correlated with arousal
associated with sleep, performance on vigilance tasks, and loss
of consciousness associated with anesthesia [8,77−79]. In some
instances, this arousal network also included the basal forebrain
and anterior cingulate cortex [77].
5. Neuroimaging studies of sleep disorders
Since it is now known that brain function changes in reliable ways
across the sleep/wake cycle, it is possible to build models for
alterations in these patterns in discrete human sleep disorders such
as insomnia related to depression or primary insomnia.
5.1. Dysfunctional arousal in depression
Patients with insomnia related to depression can describe difficulty
falling asleep, difficulty staying asleep, and/or difficulty returning to
sleep after early morning awakenings. Clinically, they often report
a paradoxical state of physical daytime fatigue, yet with persistent
mental activity that makes it difficult for them to fall asleep at
night. Such patients have reduced stage 3 and 4 NREM sleep, an
increased amount of REM sleep, a shortening of time to onset of
the first REM period of the night, and an increase in the frequency
of eye movements during REM periods.
The hypothesis that the alterations in REM sleep in depressed
patients reflect a functional dysregulation within limbic and
paralimbic forebrain structures during the sleep state has been
tested and confirmed by several [
18
F]FDG-PET studies [19,20,80].
Furthermore, a number of findings suggest a generalized
hyperarousal in depressed patients. In an early study, depressed
patients, in contrast to healthy subjects, showed greater elevations
in glucose metabolism from waking to REM sleep in the tectal
area and in left hemispheric areas (sensorimotor cortex, inferior
temporal cortex, uncal gyrus-amygdala, and subicular complex)
[19]. Treatment with the anti-depressant, bupropion SR, reduced
the previously observed deficit in activation of medial prefrontal
cortex, right anterior insula, and in particular, the anterior cingulate
from waking to REM sleep in depressed patients [20]. These
findings suggested that increased anterior cingulate metabolism, in
particular, characterized depressed patients and that antidepressant
therapy may work in part by providing an inhibitory influence on
abnormally elevated function in the anterior cingulate. A larger
study demonstrated a supersensitive pattern of activation from
waking to REM in depressed subjects along with increased whole-
brain metabolism in REM [80].
Increased whole-brain metabolism has also been demonstrated
using [
18
F]FDG-PET during the first period of NREM sleep
in depressed patients relative to healthy subjects [21]. These
increases were most noticeable in the posterior cingulate, the
amygdala, hippocampus, occipital and temporal cortex and the pons.
Hypofrontality was also noted in depressed subjects, who also had
reduced relative metabolism in the anterior cingulate, caudate, and
medial thalamus in relation to healthy subjects.
The relationship between beta EEG power, an electrophysiologi-
cal marker of arousal, and regional cerebral glucose metabolism has
been observed using [
18
F]FDG-PET in nine healthy subjects and
12 depressed patients during their first NREM period of sleep [16].
Beta power negatively correlated with subjective sleep quality in
healthy and depressed subjects. There was a significant correlation
between beta power and relative cerebral glucose metabolism in
the right lateral inferior occipital complex, and particularly in the
ventromedial prefrontal cortex in healthy and depressed subjects. In
addition, there was a trend toward greater beta power in depressed
subjects in relation to age- and gender-matched healthy subjects
during a baseline night of sleep. Given its functional links with brain
structures involved in arousal, it is suggested that the ventromedial
prefrontal cortex may have abnormally elevated function in severely
aroused depressed subjects, and that this elevation may influence
general cortical arousal in this disorder.
5.2. Primary insomnia
Primary insomnia is characterized by inadequate sleep or poor
quality of sleep unrelated to other concomitant medical conditions.
Patients experience one or more of the following: difficulty
falling asleep, difficulty maintaining sleep, and/or early awakenings.
Insomnia patients experience daytime dysfunction that may include
fatigue, mood symptoms, and cognitive impairment (e.g. reduced
attention and concentration). Approximately 10% of the adult
population will suffer from chronic insomnia, while 30 to 50%
will experience transient insomnia at some point in their life.
Consequences of insomnia may include poor daytime performance,
an increased likelihood of subsequent development of a mental
disorder such as depression or anxiety, and increased medical
morbidity and mortality.
In a preliminary HMPAO-SPECT study, overall cerebral
blood flow was reduced in five patients with primary insomnia
relative to four healthy subjects during NREM sleep [81]. However,
this study was limited since blood flow is known to decline
with increasing duration of NREM sleep, and the insomnia
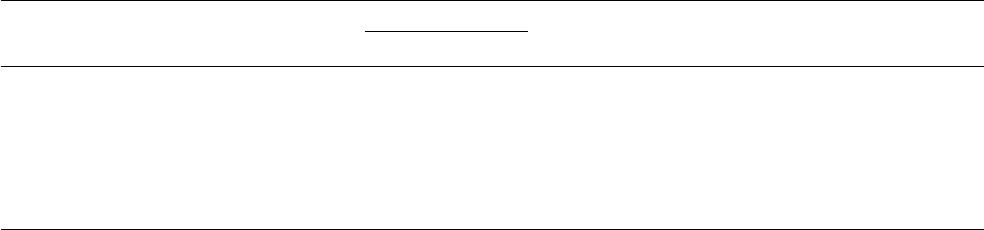
S20 E.A. Nofzinger / Sleep Medicine 5 Suppl. 1 (2004) S16–S22
Wake
NREM
MRDglc
5
6
7
8
9
10
11
12
Normal controls (n = 17)
Insomnia (n =6)
State: F=31.5, p<0.001
Group: F=6.79, p=0.017
State by group interaction: F=0.77, p=0.39
Fig. 1. Increased whole brain metabolism in insomniacs relative to healthy
subjects during waking and NREM sleep. Cerebral hypermetabolism
in insomniacs. A repeated measures analysis of variance tested group
(insomniac versus control) × time (wake versus NREM sleep) interactions,
and group and time effects of the indirect measure of whole brain glucose
metabolism (MRDglc). No interaction was noted. Significant effects of group
(insomniacs > controls), and time (waking > NREM sleep) were found.
patients received blood-flow assessments after a greater duration
of NREM sleep than the healthy subjects. The small sample size
investigated in this study also limits the generalizability of these
findings to the condition of insomnia as a whole. It is possible
that the sleep disruption experienced by insomnia patients may be
due to abnormally elevated function in the ventromedial prefrontal
cortex during NREM sleep, consistent with observations in severely
aroused depressed subjects [16].
A recent neuroimaging trial in primary insomnia patients tested
several hypotheses [80]. Firstly, that the hyperarousal of insomnia
may be reflected by an overall increase in whole-brain metabolism
during both waking and sleeping in relation to healthy subjects.
Secondly, that the inability of insomniacs to sleep may be the result
of a failure of wake-promoting brain structures to turn off, or decline
in function from waking to sleep. Thirdly, that the daytime fatigue of
insomniacs may be explained by declines in function during waking
in the thalamus and in the frontal and parietal cortex relative to
healthy subjects. As already discussed, these structures are known
to decline in function following sleep deprivation, something that
may occur in insomniacs following prolonged periods of difficulty
sleeping at night. In this trial, seven insomniacs and 20 healthy
age- and gender-matched subjects underwent [
18
F]FDG-PET scans
during waking and NREM sleep [80]. Consistent with the first
hypothesis, whole-brain metabolism was significantly increased in
insomniacs compared with healthy subjects during both waking
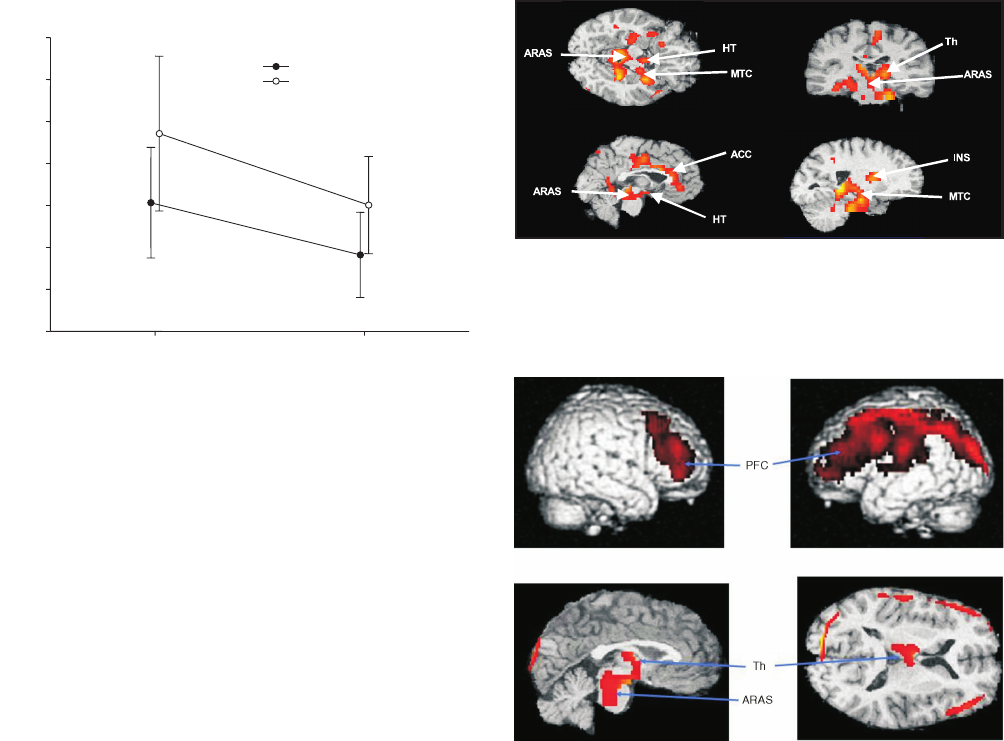
and NREM sleep (Fig. 1). In line with the second hypothesis, the
ascending reticular activating system (ARAS), an important arousal
system within the brain, was consistently more active in insomnia
patients relative to healthy subjects, from waking to NREM sleep
(Fig. 2).
Finally, and in accordance with the third hypothesis, insomniacs
exhibited a relative hypometabolism in the thalamus and
frontoparietal cortex while awake, a pattern also seen in healthy
subjects following sleep deprivation (Fig. 3). These findings suggest
that, across the sleep/wake cycle, the brains of patients with
insomnia exhibit signs of both hyperarousal and sleep deprivation.
Fig. 2. Arousal systems do not deactivate from waking to NREM sleep
in insomnia patients, and brain structures that do not show decreased
metabolic rate from waking to sleep in insomniacs. ACC = anterior cingulate
cortex; ARAS = ascending reticular activating system; Hy = Hypothalamus;
INS = insula; MTC = mesial temporal cortex; Th = thalamus.
Fig. 3. Daytime fatigue in insomnia patients is related to frontal relative
hypometabolism in waking. Panels show brain structures where relative
metabolism during waking is higher in healthy subjects than it is in
insomniacs. All regions shown reach statistical significance at the p<0.05,
corrected, level of significance. ARAS = ascending reticular activating
system; Th = thalamus; PFC = prefrontal cortex.
6. Summary and areas for future research
Neuroimaging techniques have already led to very important
advances in our understanding of the sleep/wake cycle in healthy
subjects. Early studies demonstrate promise that these nuclear
medicine techniques can add significantly to our understanding of
clinical sleep disorders medicine. However, despite these advances,
the use of neuroimaging techniques in sleep research remains in
its infancy and additional studies are needed in several areas.
Further studies are required to clarify the basic mechanisms of
sleep processes, including the circadian and homeostatic functions
of sleep. In addition, neuroimaging techniques should be applied
to clarify the role of sleep in cognitive processes that may occur
within sleep, as well as changes in very primitive limbic and
paralimbic brain systems activated in REM sleep, which may also
be dysfunctional in neuropsychiatric disorders such as depression,
schizophrenia, Parkinson’s disease and the dementias. The unique
effects of sleep deprivation on brain function could be further
investigated using neuroimaging techniques as well as the effects
of interventions to reverse these changes. Neuroimaging studies
will ultimately further our knowledge of the pathophysiological
E.A. Nofzinger / Sleep Medicine 5 Suppl. 1 (2004) S16–S22 S21
mechanisms underlying a wide assortment of clinical sleep
disorders.
References
[1] Buchsbaum MS, Gillin JC, Wu J, et al. Regional cerebral glucose
metabolic rate in human sleep assessed by positron emission
tomography. Life Sci 1989;45:1349−1356.
[2] Maquet P. Positron emission tomography studies of sleep and sleep
disorders. J Neurol 1997;244(Suppl 1):S23−S28.
[3] Maquet P, Phillips C. Functional brain imaging of human sleep. J Sleep
Res 1998;7(Suppl 1):42−47.
[4] Maquet P. Functional neuroimaging of normal human sleep by positron
emission tomography. J Sleep Res 2000;9:207−231.
[5] Maquet P. Brain mechanisms of sleep: contribution of neuroimaging
techniques. J Psychopharmacol 1999;13(Suppl 1):S25−S28.
[6] Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization during
stage 2 sleep in man. Brain Res 1992;571:149−153.
[7] Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization
during sleep–wake cycle in man determined by positron emission
tomography and [18F]2-fluoro-2-deoxy-d-glucose method. Brain Res
1990;513:136−43.
[8] Hofle N, Paus T, Reutens D, et al. Regional cerebral blood flow changes
as a function of delta and spindle activity during slow wave sleep in
humans. J Neurosci 1997;17:4800−4808.
[9] Nofzinger EA, Mintun MA, Price J, et al. A method for the assessment
of the functional neuroanatomy of human sleep using FDG PET. Brain
Res Protocols 1998;2:191−198.
[10] Nofzinger EA, Buysse DJ, Miewald JM, et al. Human regional cerebral
glucose metabolism during non-rapid eye movement sleep in relation
to waking. Brain 2002;125:1105−1115.
[11] Kjaer TW, Law I, Wiltschiotz G, et al. Regional cerebral blood
flow during light sleep – a H(2)(15)O-PET study. Sleep Res
2002;11:201−207.
[12] Maquet P, Peters J, Aerts J, et al. Functional neuroanatomy of human
rapid-eye-movement sleep and dreaming. Nature 1996;383:163−166.
[13] Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood
flow throughout the sleep–wake cycle. An H2(15)O PET study. Brain
1997;120:1173−1797.
[14] Braun AR, Balkin TJ, Wesenten NJ, et al. Dissociated pattern of
activity in visual cortices and their projections during human rapid
eye movement sleep. Science 1998;279:91−95.
[15] Nofzinger EA, Mintun MA, Wiseman MB, et al. Forebrain activation
in REM sleep: an FDG PET study. Brain Res 1997;770:192−201.
[16] Nofzinger EA, Price JC, Meltzer CC, et al. Towards a neurobiology
of dysfunctional arousal in depression: the relationship between beta
EEG power and regional cerebral glucose metabolism during NREM
sleep. Psychiatry Res: Neuroimag 2000;98:71−91.
[17] Buchsbaum MS, Hazlett EA, Wu J, Bunney WE. Positron emission
tomography with deoxyglucose-F18 imaging of sleep. Neuropsycho-
pharmacology 2001;25(Suppl 5):S50−S56.
[18] Peigneux P, Laureys S, Fuchs S, et al. Generation of rapid
eye movements during paradoxical sleep in humans. Neuroimage
2001;14:701−708.
[19] Nofzinger EA, Nichols TE, Meltzer CC, et al. Changes in forebrain
function from waking to REM sleep in depression: Preliminary
analyses of [18F] FDG PET studies. Psychiatry Res: Neuroimag
1999;91:59−78.
[20] Nofzinger EA, Berman S, Fasiczka A, et al. Effects of bupropion
SR on anterior paralimbic function during waking and REM sleep in
depression: preliminary findings using [18F]-FDG PET. Psychiatry Res
2001;106:95−111.
[21] Ho AP, Gillin JC, Buchsbaum MS, et al. Brain glucose metabolism
during non-rapid eye movement sleep in major depression: A positron
emission tomography study. Arch Gen Psychiatry 1996;53:645−652.
[22] Steriade M, McCarley RW. Brainstem mechanisms of dreaming and of
disorders of sleep in man. In: Brainstem Control of Wakefulness and
Sleep. New York: Plenum Press; 1990. pp. 395−482.
[23] Hobson JA, Pace-Schott EF, Stickgold R. Dreaming and the brain:
Toward a cognitive neuroscience of conscious states. In: Pace-Schott
EF, Solms M, Blagrove M, Harnad S, editors. Sleep and Dreaming.
Cambridge University Press; 2003. pp. 1−50.
[24] Saper CB, Sherin JE, Elmquist JK. Role of the ventrolateral preoptic
area in sleep induction. In: Hayaishi O, Inoue S, editors. Sleep and
Sleep Disorders: From Molecule to Behavior. New York: Academic
Press; 1997.
[25] Edgar DM. Sleep–wake circadian rhythms and aging: Potential
etiologies and relevance to age-related changes in integrated
physiological systems. Neurobiol Aging 1994;15:499−501.
[26] Khateb A, Fort P, Pegna A, et al. Cholinergic nucleus basalis neurons
are excited by histamine in vitro. Neuroscience 1995;69:495−506.
[27] Lin JS, Luppi PH, Salvert D, et al. Histamine-containing neurons in
the cat hypothalamus. CR Acad Sci 1986;303:371−376.
[28] Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal
mechanisms in the hypothalamus of cats. Neuropharmacology
1988;27:111−122.
[29] Lin JS, Kitahama P, Fort P, et al. Histaminergic system in the cat
hypothalamus with reference to type B monoamine oxidase. J Comp
Neurol 1993;330:405−420.
[30] Lin JS, Sakai K, Jouvet M. Hypothalamo-preoptic histaminergic
projections in sleep–wake control in the cat. Eur J Neurosci
1994;6:618−625.
[31] McCormick DA, Williamson A. Modulation of neuronal firing mode
in cat and guinea pig LGN by histamine: possible cellular mechanisms
of histaminergic control of arousal. J Neurosci 1991;11:3188−3199.
[32] Monti JM. Involvement of histamine in the control of the waking state.
Life Sci 1993;53:1331−1338.
[33] Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-
immunoreactive fibers in the rat brain. Neuroscience 1989;28:585−610.
[34] Shiromani PJ, Scammell T, Sherin JE, Saper CB. Hypothalamic
regulation of sleep. In: Lydic R, Baghdoyan HA, editors. Handbook of
Behavioral State Control: Cellular and Molecular Mechanisms. Boca
Raton, FL: CRC Press; 1999. pp. 311−326.
[35] Szymusiak R. Magnocellular nuclei of the basal forebrain: substrates
of sleep and arousal regulation. Sleep 1995;18:478−500.
[36] Willie JT, Chemelli RM, Sinton CM, et al. Distinct narcolepsy
syndromes in Orexin receptor-2 and Orexin null mice: molecular
genetic dissection of Non-REM and REM sleep regulatory processes.
Neuron 2003;38:715−730.
[37] Tashiro M, Mochizuki H, Iwabuchi K, et al. Roles of histamine
in regulation of arousal and cognition: functional neuroimaging of
histamine H1 receptors in human brain. Life Sci 2002;72:409−414.
[38] Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic
control of sleep and wakefulness. Trends Neurosci 2001;24:726−731.
[39] Chou TC, Bjorkum AA, Gaus SE, et al. Afferents to the ventrolateral
preoptic nucleus. J Neurosci. 2002;22:977−990.
[40] Lu J, Bjorkum AA, Xu M, et al. Selective activation of the extended
ventrolateral preoptic nucleus during rapid eye movement sleep. J
Neurosci 2002;22:4568−4576.
[41] Shiromani PJ, Gillin JC. Acetylcholine and the regulation of REM
sleep: Basic mechanisms and clinical implications for affective illness
and narcolepsy. Annu Rev Pharmacol Toxicol 1987;27:137−157.
[42] Steriade M, Datta S, Pare D, et al. Neuronal activities in brain-
stem cholinergic nuclei related to tonic activation processes in
thalamocortical systems. J Neurosci 1990;10:2541−2559.
[43] Datta S, Calvo JM, Quattrochi J, Hobson JA. Cholinergic
microstimulation of the peribrachial nucleus in the cat. I. Immediate
and prolonged increases in ponto-geniculo-occipital waves. Arch Ital
Biol 1992;130:263−284.
[44] Hobson JA, Stenade M. Neuronal basis of behavioral state control.
In: Mountcastle VB, Bloom FE, editors. Handbook of Physiology.
Bethesda: American Physiological Society; 1986. pp. 701−823.
S22 E.A. Nofzinger / Sleep Medicine 5 Suppl. 1 (2004) S16–S22
[45] Kushida CA, Zoltoski RK, Gillin JC. Expression of m2 muscarinic
receptor mRNA in rat brain with REM sleep deprivation. Sleep Res
1995;24:37.
[46] Hobson JA, Datta S, Calvo JM, Quattrochi J. Acetylcholine as a brain
state regulator: triggering and long-term regulation of REM sleep. Prog
Brain Res 1993;98:389−404.
[47] McCarley RW, Hobson JA. Neuronal excitability modulation over
the sleep cycle: a structural and mathematical model. Science
1975;189:58−60.
[48] McCarley RW, Massaquoi SG. A limit cycle mathematical model of the
REM sleep oscillator system. Am J Physiol 1986;251:R1011−R1029.
[49] Massaquoi SG, McCarley RW. Extension of the limit cycle reciprocal
interaction model of REM cycle control: An integrated sleep control
model. J Sleep Res 1992;1:138−143.
[50] Luebke JI, Greene RW, Semba K, et al. Serotonin hyperpolarizes
cholinergic low threshold burst neurons in the rat laterodorsal tegmental
nucleus in vitro. Proc Natl Acad Sci 1992;89:743−747.
[51] Williams JA, Reiner PB. Noradrenaline hyperpolarizes cholinergic
neurons in rat laterodorsal tegmentum in vitro. Soc Neurosci Abstracts
1992;18:abstract 975.
[52] Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus
coeruleus neurons in behaving rats anticipates fluctuations in the sleep-
waking cycle. J Neurosci 1981;1:876−886.
[53] Hobson JA, McCarley RW, Nelson JP. Location and spilec-
train characteristics of cells in anterodorsal pons having selective
decreases in firing rate during desynchronized sleep. J Neurophysiol
1983;50:770−893.
[54] Jacobs BL, Heym J, Trulson ME. Behavioral and physiological corre-
lates of brain serotonergic unit activity. J Physiol 1981;77:431−436.
[55] McGinty D, Harper RW. Dorsal raphe neurons: depression of firing
during sleep in cats. Brain Res 1976;101:569−575.
[56] Jones BE. Basic mechanisms of sleep–wake states. In: Kryger MH,
Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine.
Philadelphia, PA: W.B. Saunders Company; 1994. pp. 145−162.
[57] Steriade M, Buzsaki G. Parallel activation of thalamic and cortical
neurons by brainstem and basal forebrain cholinergic systems. In:
Steriade M, Biesold D, editors. Brain Cholinergic Systems. Oxford:
Oxford University Press; 1990. pp. 3−52.
[58] Gadea-Ciria M. Cerebellar control of activity of the feline oculomotor
system during paradoxical sleep. Exp Neurol 1976;51:263−265.
[59] Gadea-Ciria M. Tele-encephalic versus cerebellar control upon ponto-
geniculo-occipital waves during paradoxical sleep in the cat. Experientia
1976;32:889−890.
[60] Morrison AR, Bowker RM. The biological significance of P60 spikes
in the sleeping cat. Acta Neurobiol Exp 1975;35:821−840.
[61] Hobson JA, Stickgold R, Pace-Schott EF. The neuropsychology of REM
sleep dreaming. Neuroreport Rev 1997;9:R1−R14.
[62] Semba K, Fibiger HC. Afferent connections of the laterodorsal and the
pedunculopontine tegmental nuclei in the rat: a retro- and antero-
grade transport and immunohistochemical study. J Comp Neurol
1992;323:387−410.
[63] Bernard JF, Alden M, Resson JM. The organization of the efferent
projections from the pontine parabrachial area to the amygdaloid
complex: a phaseolus vulgaris leucoagglutinin (PHA-L) study in rats.
J Comp Neurol 1993;329:201−229.
[64] Calvo JM, Simon-Arceo K. Cholinergic enhancement of REM sleep
from sites in the pons and amygdala. In: Lydic R, Baghdoyan HA,
editors. Handbook of Behavioral State Control: Cellular and Molecular
Mechanisms. Boca Raton, FL: CRC Press; 1999. pp. 391−406.
[65] Morrison AR, Sanford LD, Ross RJ. Initiation of rapid eye movement
sleep: beyond the brainstem. In: Mallcik BN, Inoue S, editors. Rapid
Eye Movement Sleep. New York: Marcel Dekker; 1999.
[66] Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus
in the rat. Brain Res 1980;197:291−317.
[67] Wainer BH, Mesulum MM. Ascending cholinergic pathways in the rat
brain. In: Steriade M, Biesold D, editors. Brain Cholinergic Systems.
Oxford University Press; 1990.
[68] Sanford LD, Tejani-Butt SM, Ross RJ, Morrison AR. Amygdaloid
control of alerting and behavioral arousal in rats: involvement of
serotonergic mechanisms. Arch Ital Biol 1995;134:81−99.
[69] Kauppinen RA, Williams SR, Busza AL, van Bruggen N. Applications
of magnetic resonance spectroscopy and diffusion-weighted imaging
to the study of brain biochemistry and pathology. Trends Neurosci
1993;16:88−95.
[70] Meyer JS, Hayman LA, Amano T, et al. Mapping local blood flow of
human brain by CT scanning during stable xenon inhalation. Stroke
1981;12:426−436.
[71] Rachle ME. Imaging the mind. Semin Nucl Med 1998;28:278−289.
[72] Madsen PL, Holm S, Vorstrup S, et al. Human regional cerebral blood
flow during rapid-eye-movement sleep. J Cereb Blood Flow Metab
1991;11:502−507.
[73] Heiss WD, Pawlik G, Herholz K, et al. Regional cerebral glucose
metabolism in man during wakefulness, sleep, and dreaming. Brain
Res 1985;327:362−366.
[74] Andersson JL, Onoe H, Hetta J, et al. Brain networks affected by
synchronized sleep visualized by positron emission tomography. J Cereb
Blood Flow Metab 1998;18:701−715.
[75] Balkin TJ, Braun AR, Wesensten NJ, et al. The process of
awakening: a PET study of regional brain activity patterns
mediating the re-establishment of alertness and consciousness. Brain
2002;125:2308−2319.
[76] Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and
cognitive performance impairments during sleepiness. I. Effects of 24 h
of sleep deprivation on waking human regional brain activity. J Sleep
Res 2000;9:335−352.
[77] Paus T, Jech R, Thompson CJ, et al. Transcranial magnetic
stimulation during positron emission tomography: a new method
for studying connectivity of the human cerebral cortex. J Neurosci
1997;17:3178−3184.
[78] Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in
the effects of task difficulty and motor output on blood flow response
in the human anterior cingulate cortex: a review of 107 PET activation
studies. Neuroreport 1998;9:R37−R47.
[79] Fiset P, Paus T, Daloze T, et al. Brain mechanisms of propofol-induced
loss of consciousness in humans: a positron emission tomographic
study. J Neurosci 1999;19:5506−5513.
[80] Nofzinger EA, Buysse DJ, Germain A, et al. Insomnia: functional
neuroimaging evidence for hyperarousal. Am J Psychiatry 2004
(June/July) in press.
[81] Smith MT, Perlis ML, Chengazi VU, et al. Neuroimaging of NREM
sleep in primary insomnia: a Tc-99-HMPAO single photon emission
computed tomography study. Sleep 2002;25:325−335.
