
For Research, Forensic, or Paternity Use Only. Not for use in
diagnostic procedures.
HIDReal‑Time PCR Analysis Software
USER GUIDE
v1.3 and v1.4
for use with:
7500 Real-Time PCR System for Human Identification
QuantStudio
™
5 Real‑Time PCR System (with 0.2‑mL 96‑Well Block)
Quantifiler
™
DNA Quantification Kits
Publication Number MAN0009819
Revision G.0

Life Technologies Ltd | 7 Kingsland Grange | Woolston, Warrington WA1 4SR | United Kingdom
For descriptions of symbols on product labels or product documents, go to thermofisher.com/symbols-definition.
Revision history:MAN0009819 G.0 (English)
Revision Date Description
G.0 7 February 2024
Recommended HIDReal‑Time PCR Analysis Software v1.4 for use with only the QuantStudio
™
5 Real-Time PCR
System with firmware v1.5.1 or later.
F.0 9 November 2022
•
Added the following sections: “New features in v1.4” on page7, Appendix A, “Troubleshooting”, and
AppendixC, “HIDReal‑Time PCR Analysis Software v1.4 verification”.
•
Updated “Computer and instrument requirements” on page10 and Chapter2, “Install the
HIDReal‑Time PCR Analysis Software and calibrate the instruments”.
•
Updated to current publication style (minor grammar and formatting changes).
E.0 27 August 2018
Updated branding and trademarks, no technical changes.
D.0 8 March 2017
Added support for the QuantStudio
™
5 Real-Time PCR System with 96-well (0.2-mL) sample block. Added the
Virtual Standard Curve function.
C.0 14 August 2015
Corrected the quencher listed for the Quantifiler
™
Duo DNA Quantification Kit, Quantifiler
™
Human DNA
Quantification Kit, and Quantifiler
™
Y Human Male DNA Quantification Kit. Added reference to evaluating the
quality indices determined by the HIDReal‑Time PCR Analysis Software to determine if highly degraded samples
can be better analyzed with the Ion Personal Genome Machine
™
(PGM
™
) System. For more information, see the
Quantifiler
™
HP and Quantifiler
™
Trio DNA Quantification Kits User Guide (Pub. No.4485354).
B.0 31 March 2014
Added "HID Real-Time PCR Analysis Software Validation".
A.0 31 January 2014
New document for v1.2 features (support for Quantifiler
™
HP and Trio DNA Quantification Kits; Degradation Index).
Incorporates all information from the HIDReal‑Time PCR Analysis Software User Guide v1.1 (Pub. No.4455443)
The information in this guide is subject to change without notice.
DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, THERMO FISHER SCIENTIFIC INC. AND/OR ITS AFFILIATE(S) WILL NOT BE
LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR
ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.
NOTICE TO PURCHASER: DISCLAIMER OF LICENSE: Purchase of this software product alone does not imply any license under any
process, instrument or other apparatus, system, composition, reagent or kit rights under patent claims owned or otherwise controlled by
Thermo Fisher Scientific, either expressly, or by estoppel.
TRADEMARKS: All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. TaqMan is
a registered trademark of Roche Molecular Systems, Inc, TaqMan used under permission and license. Microsoft, Windows, PowerPoint,
and Excel are registered trademarks of Microsoft Corporation. Adobe, Acrobat, and Reader are registered trademarks of Adobe
Systems, Inc. Pentium and Intel are registered trademarks and Core is a trademark of Intel Corporation.
©2014-2024 Thermo Fisher Scientific Inc. All rights reserved.
Contents
■
CHAPTER1Productinformation .................................................. 7
Productdescription ............................................................. 7
New features inv1.4 ............................................................. 7
Compatible systems ............................................................. 8
Compatible HIDkits ............................................................. 8
Custom experimentoption ....................................................... 8
HIDReal‑Time PCR Analysis Softwareworkflow .................................... 9
■
CHAPTER2Install the HIDReal‑Time PCR Analysis Software and
calibrate theinstruments ......................................................... 10
Computer and instrument requirements ........................................... 10
7500 Real-Time PCR Systems purchased before February2008 ................. 11
Install the HIDReal‑Time PCR Analysis Software ................................... 12
Calibrate the 7500instrument ................................................... 13
Required materials notsupplied ............................................. 13
Calibration procedures ..................................................... 14
New dye spectra for the 7500 Real‑Time PCRInstrument ....................... 15
Calibrate the QuantStudio
™
5Instrument .......................................... 17
Required materials notsupplied ............................................. 17
Calibration procedures ..................................................... 17
New dye spectra for the QuantStudio
™
5 Real-Time PCRInstrument ............. 18
■
CHAPTER3Customize the software ............................................ 20
Modify a default experiment template ............................................. 20
Save a copy of the original template ......................................... 20
Modify the original template ................................................. 20
Create an experiment template .................................................. 21
Link your template to a Home screen button ...................................... 21
Set displaydefaults ............................................................ 22
Select data to display in the plateview ....................................... 22
Specify the data to display in the welltable ................................... 23
Customize the amplificationplot ............................................. 23
HIDReal
‑Time PCR Analysis Software User Guide
3
■
CHAPTER4Select the experiment and set up a plate ......................... 24
Start the software and select an experiment ....................................... 24
For custom experiments .................................................... 25
Navigate the software .......................................................... 26
Specify experiment properties ................................................... 27
Define samples and view targets ................................................. 28
Definesamples ............................................................ 28
View targets ............................................................... 30
Change a colordesignation ................................................. 30
Assign the targets, samples, and standards towells ................................ 31
Open the Assign Targets and Samplestab ................................... 31
Assign using the platelayout ................................................ 32
Assign using the welltable .................................................. 34
Save the plate layout as EDS or template ......................................... 36
Link your template to a Home screen button ...................................... 36
■
CHAPTER5Run the plate ........................................................ 38
View the runmethod ........................................................... 38
(7500 system only) Set notifications .............................................. 39
Start or stop therun ............................................................ 40
Start arun ................................................................ 40
Stop arun ................................................................ 40
(7500 system only) Monitor arun ................................................. 41
Save the results ................................................................ 41
■
CHAPTER6Select analysis settings and thresholds .......................... 42
Open analysissettings .......................................................... 42
View and edit C
T
settings ....................................................... 43
Enter HIDsettings .............................................................. 44
HIGHQT .................................................................. 45
IPCCT .................................................................... 46
LOWQT .................................................................. 46
NTCCT ................................................................... 46
MTFR flag and M:F ratiodisplay ............................................. 46
SLOPE ................................................................... 47
R
2
....................................................................... 47
YINT ..................................................................... 47
Contents
4
HIDReal‑Time PCR Analysis Software User Guide
Enter flagsettings .............................................................. 48
Add a virtual standard curve to the experiment .................................... 49
Guidelines for using virtual standard curves ................................... 49
Create a virtual standard curve .............................................. 49
Apply a virtual standard curve to an experiment ............................... 51
■
CHAPTER7Enhance dataanalysis ............................................. 52
View the analysis results ........................................................ 52
Flaggedwells ............................................................. 52
Wells automatically omitted ................................................. 54
Interpret QC flaginformation .................................................... 55
Omit wells fromanalysis ........................................................ 56
Omit targets in an experimentwell ............................................... 57
Examine the Degradation Index .................................................. 58
Change the appearance of, print, and saveplots ................................... 58
Change the appearance of aplot ............................................ 58
Select the wells to include in the report ....................................... 59
Print or save aplot ......................................................... 59
■
CHAPTER8Export and report results ........................................... 60
Exportdata ................................................................... 60
Print a report .................................................................. 62
■
CHAPTER9Generate dilution and reaction worksheets for STRsetup ...... 64
Add kits to an experiment ....................................................... 64
Select unknown samples foramplification ......................................... 65
Edit dilution settings for individualsamples ........................................ 66
View the dilutionscheme ........................................................ 67
Export dilution and reactionworksheets .......................................... 68
Save new STR kit information from an experiment into the STR kit library ............. 68
■
APPENDIXATroubleshooting .................................................... 69
■
APPENDIXBHIDReal‑Time PCR Analysis Software v1.3validation ......... 70
Overview of the software validation forv1.3 ....................................... 70
Features inv1.3 ........................................................... 71
Materials andmethods ......................................................... 71
Experiments and results ........................................................ 73
Instrument performance .................................................... 73
Software performance ...................................................... 75
Conclusions ................................................................... 76
Contents
HIDReal‑Time PCR Analysis Software User Guide
5
■
APPENDIXCHIDReal‑Time PCR Analysis Software v1.4verification ....... 77
Objective of the software verification forv1.4 ...................................... 77
Functional testing .............................................................. 78
Materials andmethods ..................................................... 78
Results ................................................................... 79
Regression testing ............................................................. 79
Materials ................................................................. 80
Results ................................................................... 80
Reliability testing ............................................................... 81
Conclusions ................................................................... 81
■
APPENDIXDConfigure STR library and default dilutionsettings ............. 82
Configure the STR Kit Library .................................................... 82
Set default dilutionsettings ...................................................... 84
■
APPENDIXEDocumentation and support ...................................... 86
Relateddocumentation ......................................................... 86
Customer and technical support ................................................. 87
Limited product warranty ........................................................ 87
Contents
6
HIDReal‑Time PCR Analysis Software User Guide

Product information
■
Productdescription .................................................................... 7
■
New features inv1.4 ................................................................... 7
■
Compatible systems ................................................................... 8
■
Compatible HIDkits ................................................................... 8
■
Custom experimentoption .............................................................. 8
■
HIDReal‑Time PCR Analysis Softwareworkflow ........................................... 9
Product description
The HIDReal‑Time PCR Analysis Software is designed to assist human identification laboratories that
perform DNA quantitation by simplifying assay setup, data review, and dilution and reaction setup for
downstream STR analysis. For example, the software automatically selects the appropriate Quantifiler
™
kit target, reporter, quencher, and thermal profile.
After a run, the software provides an analysis of each well, then allows you to export the following data:
•
All results
•
STR kit setup instructions
•
Sample dilutions calculations
New features in v1.4
HIDReal‑Time PCR Analysis Software v1.4 includes all v1.3 functionality and the following new
features:
•
Compatible with the QuantStudio
™
5 Real-Time PCR System with firmware v1.5.1 or later
•
Supports the Java
™
software upgrade from v6 to v8
Note: We do not recommend using HIDReal‑Time PCR Analysis Software v1.4 with the 7500 Real-
Time PCR System for Human Identification.
1
HIDReal‑Time PCR Analysis Software User Guide
7

Compatible systems
The HIDReal‑Time PCR Analysis Software is compatible with the following systems:
System
Recommended
HIDReal‑TimePCR Analysis
Software version
7500 Real-Time PCR System for Human Identification v1.3
QuantStudio
™
5 Real-Time PCR System (with 0.2‑mL 96‑Well Sample Block)
withfirmwarev1.3.x
v1.3
QuantStudio
™
5 Real-Time PCR System (with 0.2‑mL 96‑Well Sample Block)
withfirmware v1.5.1 or later
v1.4
Note: To check the firmware version for your QuantStudio
™
5 Real-Time PCR System, see “Computer
and instrument requirements” on page10.
Compatible HID kits
You can use the HIDReal‑Time PCR Analysis Software with the following HID kits:
•
Quantifiler
™
HP DNA Quantification Kit
•
Quantifiler
™
Trio DNA Quantification Kit
•
Quantifiler
™
Human DNA Quantification Kit
•
Quantifiler
™
Duo DNA Quantification Kit
Custom experiment option
IMPORTANT!
The custom assay option is supported only for the 7500 system.
You can also use the HIDReal‑Time PCR Analysis Software for more complex experiments by selecting
the Custom Assay option on the Home screen. For instructions, see the user documentation for your
instrument.
Chapter1Product information
Compatible systems
1
8
HIDReal‑Time PCR Analysis Software User Guide

HIDReal‑Time PCR Analysis Software workflow
Set up a Quantifiler
™
kit plate, then load the plate in the instrument.
Select the experiment and set up a plate:
1.
Start the software.
2.
Start a new experiment and specify experiment properties.
3.
Define samples; assign targets, samples, and standards to wells.
4.
Save the experiment.
Run the plate.
Select analysis settings and thresholds:
•
HID settings (includes HID flags)
•
Flag settings
Review results:
1.
View the analysis summary.
2.
View the quantitation results.
Export and print results.
Generate dilution and reactions worksheets for STR setup:
1.
Configure the STR library and default dilution settings.
2.
Add kits to an experiment.
3.
Select the unknown samples for amplification.
4.
(As needed) Edit dilution settings for individual samples.
5.
Export dilution and reaction worksheets.
Perform PCR amplification
For instructions, see your instrument user guide.
Chapter1Product information
HIDReal‑Time PCR Analysis Software workflow
1
HIDReal‑Time PCR Analysis Software User Guide
9

Install the HIDReal‑TimePCR
Analysis Software and calibrate the
instruments
■
Computer and instrument requirements ................................................. 10
■
Install the HIDReal‑Time PCR Analysis Software ......................................... 12
■
Calibrate the 7500instrument .......................................................... 13
■
Calibrate the QuantStudio
™
5Instrument ................................................ 17
Computer and instrument requirements
Component
Requirements
Computer
•
Processor (minimum: 2.9GHz):
–
(Recommended) Intel
™
Core
™
i7 Quad Core
™
CPU, 2.9GHz
–
Intel
™
Core
™
i5 Quad Core
™
CPU, 2.9GHz
•
16GB of RAM
[1]
•
One hard drive with at least 10GB available
•
20/48X IDE CD-ROM drive
•
USB v1.2
•
Ethernet network interface adapter (10BASE-T)
[2]§
•
Microsoft
™
Windows
™
10 IoT Enterprise (LTSC2019 or 2021)
IMPORTANT! If the computer that performs a run is on a network, avoid excess
network use during the run.
Software
•
Microsoft
™
PowerPoint
™
software (for direct export of PowerPoint
™
slides)
•
Microsoft
™
Excel
™
software (for direct export of data to spreadsheet)
IMPORTANT! Do not run antivirus applications while the HIDReal‑TimePCR
Analysis Software is running. Antivirus applications may interfere with data
collection from the instrument.
2
10
HIDReal‑Time PCR Analysis Software User Guide

(continued)
Component
Requirements
Monitor
•
1280 × 1024 pixel resolution for full screen display
[3]
•
16‑inch
•
True Color (32bit)
•
UL listed
7500 Real‑Time PCR
Instrument
Instrument firmware vG2.10 (installed on all instruments purchased after 2008)
To check the firmware version of your instrument, go to the following location:
<drive>:/Applied Biosystems/7500 system/firmware
Contact Service if your firmware version is not vG2.10.
QuantStudio
™
5 Real-
Time PCR System
(with 0.2‑mL 96‑Well
Sample Block)
Instrument firmwarev1.3.x
To check the firmware version of your instrument: From the Home screen, tap
Settings4About Instrument4About Instrument.
Instrument firmware v1.5.1 or later
To check the firmware version of your instrument: From the Home screen, tap
Settings4About Instrument4About Instrument.
[1]
The software may experience communication errors if run on computers with less than 1GB.
[2]
Required only if you plan to connect the computer to a local area network (LAN).
[3]
If screen resolution is not set to 1280×1024, the Analysis Summary screen may not be properly displayed.
7500 Real-Time PCR Systems purchased before February 2008
If your 7500 Real-Time PCR System was purchased before February 2008: Tower and laptop computers
require a memory upgrade before the computers can install the HIDReal‑Time PCR Analysis Software.
For more information, see the 7500/7500 Fast Real-Time PCR Systems User Bulletin Memory Upgrade
Requirements for 7500 Software v2.0 (Pub.No.4379705).
Chapter2Install the HIDReal‑Time PCR Analysis Software and calibrate the instruments
Computer and instrument requirements
2
HIDReal‑Time PCR Analysis Software User Guide
11

Install the HIDReal‑Time PCR Analysis Software
IMPORTANT! You must have Administrator privileges on the computer to install HIDReal‑TimePCR
Analysis Software.
Install instructions are available in the Release Notes.
1.
Obtain the appropriate upgrade version of the HIDReal‑Time PCR Analysis Software for your
system.
Instrument
Recommended HIDReal‑TimePCR
Analysis Software upgrade version
7500 Real-Time PCR System for Human Identification v1.3
QuantStudio
™
5 Real-Time PCR System (with 0.2‑mL 96‑Well Sample Block)
withfirmwarev1.3.x
v1.3
QuantStudio
™
5 Real-Time PCR System (with 0.2‑mL 96‑Well Sample Block)
withfirmware v1.5.1 or later
v1.4
Note: To check the firmware version for your QuantStudio
™
5 Real-Time PCR System, see
“Computer and instrument requirements” on page10.
2.
Insert the HIDReal‑Time PCR Analysis Software DVD into a computer.
3.
Open the Release Notes, then follow the appropriate install instructions for your system.
Install instructions are provided for the following scenarios:
System
Computer Scenario
7500 Real-Time PCR System for
Human Identification
Instrument
computer
Install the HIDReal‑Time PCR Analysis Software on an
instrument computer that is running an earlier version of
the HID software
Install new HIDReal‑Time PCR Analysis Software on a
new instrument computer
QuantStudio
™
5 Real-Time PCR
System withfirmwarev1.3.x
Instrument
computer
Install the HIDReal‑Time PCR Analysis Software on an
instrument computer that is running an earlier version of
the HID software
Install new HIDReal‑Time PCR Analysis Software on a
new instrument computer
QuantStudio
™
5 Real-Time PCR
System withfirmware v1.5.1 or later
Instrument
computer
Install the HIDReal‑Time PCR Analysis Software on an
instrument computer that is running an earlier version of
the HID software
Install new HIDReal‑Time PCR Analysis Software on a
new instrument computer
Chapter2Install the HIDReal‑Time PCR Analysis Software and calibrate the instruments
Install the HIDReal‑Time PCR Analysis Software
2
12
HIDReal‑Time PCR Analysis Software User Guide

(continued)
System Computer Scenario
All systems Non-instrument
computer
Install the HIDReal‑Time PCR Analysis Software on
a non-instrument computer that is running an earlier
version of the HID software
Install new HIDReal‑Time PCR Analysis Software on a
non-instrument computer
Calibrate the 7500 instrument
IMPORTANT!
For system layout, electrical, power, safety, and other site requirements, see the Applied
Biosystems
™
7500/7500 Fast Site Preparation Guide (Pub.No.4412843).
If you Perform
Installed new HIDReal‑TimePCR Analysis
Software with a new instrument
Perform all calibrations and run the RNase P plate
Upgraded from an earlier version of the
HIDReal‑Time PCR Analysis Software
After restoring the calibration files from the earlier HID
software version (see the Release Notes), perform a
custom dye calibration to calibrate ABY
™
, JUN
™
, and
MUSTANG PURPLE
™
(MP) dyes.
Replaced SDS Software v1.2.3 Perform all calibrations and run the RNase P plate
Required materials not supplied
Table17500 Real‑Time PCR Instrument—Materials required for calibration
If you
Material Cat. No.
Replaced SDS Software v1.2.3 7500 Real-Time PCR Systems Spectral
Calibration Kit I
4349180
TaqMan
™
RNase P Instrument Verification Plate 4350584
96-Well Spectral Calibration Plate with ABY
™
Dye
4461591
96-Well Spectral Calibration Plate with JUN
™
Dye
4461593
96-Well Spectral Calibration Plate with
MUSTANG PURPLE
™
Dye
4461599
Upgraded from an earlier version of the
HIDReal‑Time PCR Analysis Software
96-Well Spectral Calibration Plate with ABY
™
Dye
4461591
Chapter2Install the HIDReal‑Time PCR Analysis Software and calibrate the instruments
Calibrate the 7500 instrument
2
HIDReal‑Time PCR Analysis Software User Guide
13

Table17500 Real‑Time PCR Instrument—Materials required for calibration (continued)
If you Material Cat. No.
Upgraded from an earlier version of the
HIDReal‑Time PCR Analysis Software
96-Well Spectral Calibration Plate with JUN
™
Dye
4461593
96-Well Spectral Calibration Plate with
MUSTANG PURPLE
™
Dye
4461599
Calibration procedures
The following is an outline of the calibration procedures for the 7500 Real‑Time PCR Instrument. For
complete instructions, see the 7500/7500 Fast Real-Time PCR Systems System Maintenance Guide
(Pub.No.4387777).
•
Regions of Interest (ROI) calibration
•
Background calibration
•
Optical calibration
•
Dye calibration:
–
Perform dye calibration of the ABY
™
, JUN
™
, and MUSTANG PURPLE
™
(MP) dyes. Follow the
custom dye procedure.
–
Perform dye calibration of all system dyes for new instrument installations, or if replacing SDS
Software v1.2.3.
–
Use 60°C as the default temperature for all dye calibrations.
•
TaqMan
™
RNase P Instrument Verification Plate run
Chapter2Install the HIDReal‑Time PCR Analysis Software and calibrate the instruments
Calibrate the 7500 instrument
2
14
HIDReal‑Time PCR Analysis Software User Guide

New dye spectra for the 7500 Real‑Time PCR Instrument
Figure1 through Figure3 show the calibration spectra for ABY
™
, JUN
™
, and MUSTANG PURPLE
™
(MP)
dyes.
Figure1ABY
™
dye spectra
Figure2JUN
™
dye spectra
Chapter2
Install the HIDReal‑Time PCR Analysis Software and calibrate the instruments
Calibrate the 7500 instrument
2
HIDReal‑Time PCR Analysis Software User Guide
15
Figure3MUSTANG PURPLE
™
(MP) dye spectra
Chapter2Install the HIDReal‑Time PCR Analysis Software and calibrate the instruments
Calibrate the 7500 instrument
2
16
HIDReal‑Time PCR Analysis Software User Guide

Calibrate the QuantStudio
™
5 Instrument
IMPORTANT! For system layout, electrical, power, safety, and other site requirements, see
the QuantStudio
™
5 Real-Time PCR Instrument Site Preparation Guide (for Human Identification)
(Pub.No.MAN0016701).
The QuantStudio
™
5 Real-Time PCR Instrument is calibrated during manufacturing; however, you must
recalibrate the instrument for the dyes that are used for HID analysis before use. If you installed
HIDReal‑Time PCR Analysis Software with a new instrument, perform custom dye calibrations for the
ABY
™
and JUN
™
dyes.
Required materials not supplied
Table2QuantStudio
™
5 Real-Time PCR Instrument—Materials required for calibration
Material Cat. no.
96-Well Spectral Calibration Plate with ABY
™
Dye 4461591
96-Well Spectral Calibration Plate with JUN
™
Dye 4461593
TaqMan
™
RNase P Instrument Verification Plate, 96‑Well 0.2‑mL 4432382
Calibration procedures
The following is an outline of the calibration procedures for the QuantStudio
™
5 Real-Time PCR
Instrument. For complete instructions, see the QuantStudio
™
3 and 5 Real‑Time PCR Systems
Installation, Use, and Maintenance Guide (Pub.No.MAN0010407).
•
Dye calibration:
–
Perform dye calibration of the ABY
™
and JUN
™
dyes. Follow the custom dye procedure.
–
Use 60°C as the default temperature for all dye calibrations.
IMPORTANT!
You must calibrate the ABY
™
dye as ABY-HID and the JUN
™
dye as JUN-HID.
Calibrating either dye without the “-HID” sux (as ABY and JUN) overwrites the existing
calibrations for the factory-calibrated system dyes. Doing so potentially creates confusion if the
instrument is ever calibrated using the QuantStudio
™
3 and 5 Calibration Kit, which does not have
the HID versions of the dyes.
•
TaqMan
™
RNase P Instrument Verification Plate run
Chapter2Install the HIDReal‑Time PCR Analysis Software and calibrate the instruments
Calibrate the QuantStudio
™
5 Instrument
2
HIDReal‑Time PCR Analysis Software User Guide
17

New dye spectra for the QuantStudio
™
5 Real-Time PCR Instrument
Figure4 through Figure6 show the calibration spectra for ABY
™
, JUN
™
, and MUSTANG PURPLE
™
(MP)
dyes.
Figure4ABY
™
dye spectra
Figure5JUN
™
dye spectra
Chapter2Install the HIDReal‑Time PCR Analysis Software and calibrate the instruments
Calibrate the QuantStudio
™
5 Instrument
2
18
HIDReal‑Time PCR Analysis Software User Guide

Figure6MUSTANG PURPLE
™
(MP) dye spectra
Chapter2Install the HIDReal‑Time PCR Analysis Software and calibrate the instruments
Calibrate the QuantStudio
™
5 Instrument
2
HIDReal‑Time PCR Analysis Software User Guide
19

Customize the software
■
Modify a default experiment template ................................................... 20
■
Create an experiment template ......................................................... 21
■
Link your template to a Home screen button ............................................. 21
■
Set displaydefaults ................................................................... 22
Modify a default experiment template
You can make changes to the experiment templates provided with the software after making a backup
copy of the original templates.
Save a copy of the original template
Before you modify a template, save a copy of the original template:
1.
Navigate to: <drive>:/Applied Biosystems/7500/config/templates
2.
Select Edit4Copy to copy the templates folder.
3.
Navigate to a safe location on your computer.
4.
Select Edit4Paste to insert a copy of the templates folder in the location you selected.
Modify the original template
1.
Click the button on the Home screen for the experiment type of interest.
2.
Modify the template as needed, including:
•
Moving standards and NTCs to dierent wells
•
Adding samples and/or extraction blanks
•
Setting the plate layout
•
Setting the display defaults for the amplification plot, plate view, and well table
•
Modifying analysis settings (HID, C
T
, and flags)
3.
In the toolbar, click the down arrow next to Save, select Save as, then select the name of the
original template.
3
20
HIDReal‑Time PCR Analysis Software User Guide

Create an experiment template
1.
Set up an experiment with the desired settings, including:
•
Moving standards and NTCs to dierent wells
•
Adding samples and/or extraction blanks
•
Setting the plate layout
•
Setting the display defaults for the amplification plot, plate view, and well table
•
Modifying analysis settings (HID, C
T
, and flags)
2.
In the toolbar, click the down arrow next to Save, then select Save as Template.
To use your template instead of a default template: At the top of the Home screen, click Open, then
select your template.
Link your template to a Home screen button
You can link your template to any of the Quantifiler
™
assay buttons on the Home screen.
The software will automatically use the template as the default experiment when you click the
corresponding button. You will still be able to use a dierent template by opening a dierent
experiment.
1.
Before linking, save a copy of the original template.
a.
Navigate to <drive>:/Applied Biosystems/7500/config/templates
b.
Select Edit4Copy to copy the templates folder.
c.
Navigate to a safe location on your computer.
d.
Select Edit4Paste to insert a copy of the templates folder in the location that you selected.
2.
Link your template to a button on the Home screen.
a.
Open the file that you want to link.
b.
In the toolbar, select Save4Save as Template.
c.
Navigate to <drive>:/Applied Biosystems/7500/config/templates
Chapter3Customize the software
Create an experiment template
3
HIDReal‑Time PCR Analysis Software User Guide
21

d.
Select the file corresponding to the assay button that you want to replace.
IMPORTANT! Files that contain the “QS5” sux are templates used by the QuantStudio
™
5 Instruments. For example, “QuantifilerTrio.edt” is the template file for the Quantifiler
™
Trio
DNA Quantification Kit used by 7500 Instruments; “QuantifilerTrioQS5.edt” is the file used by
QuantStudio
™
5 Instruments.
IMPORTANT! Ensure that you give the file exactly the same name as the file corresponding
to the button that you want to replace.
e.
Click Save.
Set display defaults
Select data to display in the plate view
1.
Click Show in Wells.
2.
In the dropdown list, select ( ) or deselect ( ) the data to display in the plate view.
3.
Click Set as Default.
Note: The button is inactive before you change a setting, or if you are logged in as Guest.
Chapter3Customize the software
Set display defaults
3
22
HIDReal‑Time PCR Analysis Software User Guide

Specify the data to display in the well table
1.
Click Show in Table.
2.
In the dropdown list, select ( ) or deselect ( ) the data to display in the well table.
3.
Click Set as Default.
Note: The button is inactive before you change a setting, or if you are logged in as Guest.
Customize the amplification plot
1.
Make changes as described in “Change the appearance of, print, and save plots” on page58.
2.
Select ( ) or deselect ( ) the data to display in the amplification plot: Threshold and/or Baseline
Start.
3.
Click Save Current Settings as Default.
Note: The button is inactive before you change a setting, or if you are logged in as Guest.
Chapter3Customize the software
Set display defaults
3
HIDReal‑Time PCR Analysis Software User Guide
23

Select the experiment and set up a
plate
■
Start the software and select an experiment ............................................. 24
■
Navigate the software ................................................................. 26
■
Specify experiment properties ......................................................... 27
■
Define samples and view targets ....................................................... 28
■
Assign the targets, samples, and standards towells ...................................... 31
■
Save the plate layout as EDS or template ................................................ 36
■
Link your template to a Home screen button ............................................. 36
This chapter assumes that you have prepared a plate according to the instructions in the user guide for
the Quantifiler
™
Kit that you are using.
Start the software and select an experiment
1.
On the computer desktop, double-click or select Start4All Programs4Applied
Biosystems
™
4HID Real-Time PCR Analysis Software4HID Real-Time PCR Analysis
Software. The Login screen should open within 1 minute.
4
24
HIDReal‑Time PCR Analysis Software User Guide

2.
In the User Name field, enter your user name or select it from the dropdown list.
You can log in as a guest, but only users logged in with a user name can perform the following
functions:
•
Edit the names of folders for experiment information import, information export, or data.
•
Enable or disable the requirement to enter a user name to start the software.
•
Set a plate layout as the default layout (see “Link your template to a Home screen button” on
page21).
•
Configure how data is displayed (see Chapter 3, “Customize the software”).
3.
Click OK to open the Home screen.
4.
Select an HID experiment.
•
Click one of the Quantifiler
™
kit template buttons, or click Custom Assays.
or
•
In the toolbar, click the down arrow next to New Experiment, then select the appropriate
experiment.
For custom experiments
IMPORTANT! The custom experiments feature is supported for the 7500 system only.
To perform a non-HID experiment, or a modified experiment, click:
•
Custom Assay on the right side of the Home screen.
or
•
Assays in the toolbar, then select Custom Assays in the drop-down list.
For information on running custom experiments, see the user documentation for your instrument.
Chapter4
Select the experiment and set up a plate
Start the software and select an experiment
4
HIDReal‑Time PCR Analysis Software User Guide
25

Navigate the software
Each HIDReal‑Time PCR Analysis Software experiment screen displays instructions for a step in the
experiment. Use the Experiment Menu at the left of any screen to navigate the software.
•
Click >> (Expand) to expand the Experiment Menu.
•
Click << (Collapse) to collapse the Experiment
Menu.
•
Click Setup, Run, or Analysis to display screens
used in the corresponding process.
You can access the software screens in any sequence.
To return to the Home screen at any time, click
(Home) at the bottom left of any screen.
Chapter4Select the experiment and set up a plate
Navigate the software
4
26
HIDReal‑Time PCR Analysis Software User Guide

Specify experiment properties
1.
In the Experiment Menu, select Setup4Experiment Properties.
2.
In the How do you want to identify this experiment? pane, enter the name of the plate or
experiment information in the Experiment Name field. Entries in the other fields are optional.
Note: The name that you enter in the Experiment Name field appears on the data report and on XLS
spreadsheets that you export. If you do not enter a name, Untitled appears on the report and in the
exported spreadsheet.
The following parameters are automatically set:
•
Experiment Name: Untitled
•
Instrument:
–
7500 (96 wells)
–
QuantStudio
™
5 (96 wells)
•
Experiment Type: Quantitation-HID Standard Curve
•
Reagents: TaqMan
™
Reagents
•
Ramp Speed: Standard (∼1 hour to complete a run for Quantifiler
™
HP
™
and Quantifiler
™
Trio kits,
and ∼2hours for all other Quantifiler
™
kits)
Chapter4Select the experiment and set up a plate
Specify experiment properties
4
HIDReal‑Time PCR Analysis Software User Guide
27

Define samples and view targets
Note: Targets are automatically listed and named. Standards dilutions and an NTC sample are listed by
default for each Quantifiler
™
kit. For information about the standard included in the Quantifiler
™
kit, see
the kit user guide.
Define samples
1.
In the Experiment Menu, select Setup4Plate Setup, then click the Define Targets and Samples
tab.
2.
In the Define Samples pane (right side of the screen), specify sample names.
•
To define a new sample, do one of the following:
–
Click Add New Sample. A new line appears in the Sample Name field.
–
In the toolbar, select Tools4Sample Library, then click New.
The default name for the new sample is Sample X (where X=1 or the highest listed Sample #
+ 1). You can enter a new name for the sample. To save the name of the sample for future
experiments, click OK.
•
To use a sample from your sample library:
a.
In the Define Samples pane, click Add Saved Sample.
b.
Select the samples to use, then click Add Selected Sample(s).
Note: You can also add a sample to a single well in the Plate Setup screen. See “Assign a new
sample to a well” on page33.
Chapter4Select the experiment and set up a plate
Define samples and view targets
4
28
HIDReal‑Time PCR Analysis Software User Guide

3.
Select the sample type: Standard, NTC, or Unknown. Unknown is the default sample type for new
samples.
When you assign the sample type, the software automatically assigns the appropriate task to each
target.
4.
Repeat steps 2 on page28 and 3 on page29 for each sample.
IMPORTANT!
List each sample individually. For replicates (identical samples), add the sample
name only once. To assign a replicate to a well in the plate, in step 4 on page32, select the well,
then select the checkbox next to the sample name.
Chapter4Select the experiment and set up a plate
Define samples and view targets
4
HIDReal‑Time PCR Analysis Software User Guide
29

View targets
1.
In the Experiment Menu, select Setup4Plate Setup.
2.
Select the Define Targets and Samples tab.
3.
In the Defined Targets pane, view the targets list to verify that you selected the correct experiment
in step 4 on page25.
Kit Reporter dyes Quencher
Quantifiler
™
Trio Small autosomal: VIC
™
dye NFQ-MGB
Male (Y): FAM
™
dye NFQ-MGB
Large autosomal: ABY
™
dye QSY
™
7
IPC: JUN
™
dye QSY
™
7
Quantifiler
™
HP
™
Small autosomal: VIC
™
dye NFQ-MGB
Large autosomal: ABY
™
dye QSY
™
7
IPC: JUN
™
dye QSY
™
7
Quantifiler
™
Duo Human: VIC
™
dye NFQ-MGB
Male: FAM
™
dye NFQ-MGB
IPC: NED
™
dye NFQ-MGB
Quantifiler
™
Human Human: FAM
™
dye NFQ-MGB
IPC: VIC
™
dye NFQ-MGB
Change a color designation
Perform this procedure to change the color that represents a target in the data analysis.
1.
Click (down arrow) in the Color column.
2.
Select a color in the dropdown list.
Chapter4Select the experiment and set up a plate
Define samples and view targets
4
30
HIDReal‑Time PCR Analysis Software User Guide

Assign the targets, samples, and standards to wells
Open the Assign Targets and Samples tab
Do one of the following to open the tab.
• In the Define Targets and Samples tab, click Assign Targets and Samples beneath the Define
Samples pane.
• In the Experiment Menu, select Setup4Plate Setup, then select the Assign Targets and
Samples tab.
Chapter4Select the experiment and set up a plate
Assign the targets, samples, and standards to wells
4
HIDReal‑Time PCR Analysis Software User Guide
31

Assign using the plate layout
Assign samples, standards, and NTCs to wells
Assign samples, standards, and NTCs using the View Plate Layout tab.
1.
Select the View Plate Layout tab (right side of the screen).
2.
(Optional) Select wells with specific characteristics.
a.
Click the left Select Wells With button above the layout diagram.
b.
Select Sample, Target, or Task in the dropdown list.
c.
Click the right Select Wells With button.
d.
Select a specific sample, target, or task.
3.
Specify the information to display in the wells:
a.
Click Show in Wells to open the dropdown list. Items that are marked with a check ( ) are
selected for display.
b.
Click an item to select or deselect it for display.
4.
(Optional) To save your selections as default settings, click Set as Default at the top right of the
View Plate Layout toolbar.
5.
Assign standards, NTCs, and unknown samples to wells.
a.
To select:
•
Well—Click the well
•
Row of wells—Click a letter on the side of the layout
•
Column of wells—Click a number at the top of a column
•
More than one well, row, or column—Drag the pointer over the wells, letters, or
columns to select
Chapter4
Select the experiment and set up a plate
Assign the targets, samples, and standards to wells
4
32
HIDReal‑Time PCR Analysis Software User Guide

b.
In the Assign sample(s) to the selected wells pane (left of the plate layout), select the
checkbox in the Assign column corresponding to the unknown, standard, or NTC sample in
the wells. The target for each sample is set by default.
Note: <Sample 1> is automatically assigned to all wells that are not assigned as standards or
NTCs.
6.
(Optional) To change the quantity of standards, enter the quantity in ng/µL in the Quantity field
in the Assign targets to the selected wells pane. The quantity of standard samples is set by
default.
7.
Repeat steps 4 on page32 and 5 on page33 until you assign samples, standards, and NTCs to all
wells that you use in the experiment. You can delete empty wells after data analysis.
Note: If you delete the samples, standards, or NTCs in a well and then restore them, you must
re-enter the well information.
The task for each target/sample combination is set automatically.
8.
Clear all wells that do not contain samples or targets.
a.
Select the wells to clear.
b.
Right-click, then select Clear from the dropdown list.
Assign a new sample to a well
1.
Double-click the well to open the Add New Sample dialog box.
2.
Click Add New Sample.
3.
The target and task are set by default according to the sample type. To change the sample type,
click the down arrow in the Sample column header, then select the appropriate sample type from
the dropdown list.
4.
To change the sample quantity setting for standard samples, perform step 5 on page33.
Chapter4Select the experiment and set up a plate
Assign the targets, samples, and standards to wells
4
HIDReal‑Time PCR Analysis Software User Guide
33

Move samples, standards, and NTCs
1.
Select the wells for the samples, standards, or NTCs that you want to move.
2.
Deselect ( ) the items in the Assign sample(s) to the selected wells pane, or right-click the
wells and select Clear.
3.
One at a time, select the new wells for an item that you are moving, then select ( ) the items in
the Assign sample(s) to the selected wells pane.
Assign using the well table
Assign samples, standards, and NTCs using the View Well Table tab.
1.
Select the View Well Table tab.
Each row in the table represents one well. To group the rows by a characteristic, click the column
header. For example, click Task to group rows by task.
2.
(Optional) Select wells with specific characteristics.
a.
Click the left Select Wells With button above the layout diagram.
b.
Select Sample, Target, or Task in the dropdown list.
Chapter4
Select the experiment and set up a plate
Assign the targets, samples, and standards to wells
4
34
HIDReal‑Time PCR Analysis Software User Guide
c.
Click the right Select Wells With button.
d.
Select a specific sample, target, or task.
3.
Specify the information to display in the table:
a.
Click Show in table to open the dropdown list. Items that are checked ( ) are selected for
display.
b.
Click an item to select or deselect it for display.
4.
(Optional) To save your selections as default settings, click Set as Default at the top right of the
View Plate Layout toolbar.
5.
Assign samples, standards, and NTCs to wells.
a.
Select the wells.
•
One well—Click under one of the column headings in the row next to the well location
(for example, to select well A6, click in row A6 under Sample).
•
More than one well—Drag the pointer over the wells that you want to select, or
Ctrl+Click the wells that you want to select.
b.
In the Assign sample(s) to the selected wells pane, select the checkbox in the Assign
column corresponding to the unknown, standards, or NTC sample in the wells. The target for
each sample is set by default.
Note: <Sample 1> is automatically assigned to all wells that are not assigned as standards or
NTCs.
6.
(Optional) To change the quantity of standards, enter the quantity (in ng/µL) in the Quantity field in
the Assign targets to the selected wells pane. The quantity of samples is set by default.
7.
Repeat steps 1 on page34 and 5 on page35 until you assign samples, standards, and NTCs to all
wells that you use in the experiment. You can delete empty wells after data analysis.
Note: If you delete the samples, standards, or NTCs in a well and then restore them, you must
re-enter the well information.
Chapter4
Select the experiment and set up a plate
Assign the targets, samples, and standards to wells
4
HIDReal‑Time PCR Analysis Software User Guide
35

The task for each target/sample combination is set automatically.
8.
Clear all wells not assigned.
a.
Click the left Select Wells With button at the top of the table.
b.
Select Sample from the dropdown list.
c.
In the well table, select the sample names of the wells to clear.
d.
In the Assign sample(s) to the selected wells pane, deselect the checkbox in the Assign
column next to the sample name.
Save the plate layout as EDS or template
IMPORTANT!
Do not save the experiment to the network folder until the plate run is completed.
1.
In the toolbar, click the down arrow next to Save, then select a save option.
•
Save—Saves the plate layout as an Experiment Document Single (EDS) file
•
Save as—Saves the plate layout as an EDS file with a dierent name
•
Save as Template—Saves the experiment file as a template for future experiments
2.
If you want to save the file with a dierent name, enter the new name in the File Name field.
3.
Click Save.
4.
Before you start the run, verify that the plate is loaded in the instrument, as described in your
Quantifiler
™
kit user guide.
Link your template to a Home screen button
You can link your template to any of the Quantifiler
™
assay buttons on the Home screen.
The software will automatically use the template as the default experiment when you click the
corresponding button. You will still be able to use a dierent template by opening a dierent
experiment.
1.
Before linking, save a copy of the original template.
a.
Navigate to <drive>:/Applied Biosystems/7500/config/templates
b.
Select Edit4Copy to copy the templates folder.
c.
Navigate to a safe location on your computer.
d.
Select Edit4Paste to insert a copy of the templates folder in the location that you selected.
2.
Link your template to a button on the Home screen.
a.
Open the file that you want to link.
Chapter4
Select the experiment and set up a plate
Save the plate layout as EDS or template
4
36
HIDReal‑Time PCR Analysis Software User Guide

b.
In the toolbar, select Save4Save as Template.
c.
Navigate to <drive>:/Applied Biosystems/7500/config/templates
d.
Select the file corresponding to the assay button that you want to replace.
IMPORTANT! Files that contain the “QS5” sux are templates used by the QuantStudio
™
5 Instruments. For example, “QuantifilerTrio.edt” is the template file for the Quantifiler
™
Trio
DNA Quantification Kit used by 7500 Instruments; “QuantifilerTrioQS5.edt” is the file used by
QuantStudio
™
5 Instruments.
IMPORTANT! Ensure that you give the file exactly the same name as the file corresponding
to the button that you want to replace.
e.
Click Save.
Chapter4Select the experiment and set up a plate
Link your template to a Home screen button
4
HIDReal‑Time PCR Analysis Software User Guide
37

Run the plate
■
View the runmethod .................................................................. 38
■
(7500 system only) Set notifications .................................................... 39
■
Start or stop therun .................................................................. 40
■
(7500 system only) Monitor arun ....................................................... 41
■
Save the results ...................................................................... 41
View the run method
1.
In the Experiment Menu, select Setup4Run Method to open the Run Method screen.
2.
Select the Graphical View tab to open the thermal profile for the assay.
Note: The Graphical View tab displays the run method ramp rate as a percentage when using a
7500 instrument and in degrees Celsius (℃) when using a QuantStudio
™
5 Instrument.
Quantifiler
™
kit
Thermal profile
7500 instrument QuantStudio
™
5 instrument
Duo
Human
5
38
HIDReal‑Time PCR Analysis Software User Guide

3.
Verify the value in the Reaction Volume field.
•
25µL for Quantifiler
™
Human and Duo kits
•
20µL for Quantifiler
™
HP
™
and Trio kits
For more information on run parameters, see the user guide for your Quantifiler
™
kit.
(7500 system only)
Set notifications
You can set the software to send e-mail notifications for selected events to e-mail addresses that you
specify.
Note: This procedure applies only to the 7500 Real‑Time PCR Instrument. It does not apply to the
QuantStudio
™
5 Real-Time PCR Instrument.
IMPORTANT!
Notifications cannot be sent unless the computer that performs the run is on an e-mail
network.
1.
In the Experiment Menu, select Run4Notification Settings.
2.
Enable or disable notifications.
•
Enable notifications—In the Run Status pane, select the Enable Notifications checkbox.
or
In the Notifications Settings pane, select Yes next to Enable Notifications.
•
Disable notifications—In the Notifications Settings pane, select No next to Enable
Notifications.
3.
Select or deselect the events to generate notifications.
•
Instrument Error—When selected, notifies addressees that a run stopped before it was
completed.
•
Run Started—When selected, notifies addressees that a run started.
•
Run Completed—When selected, notifies addressees that a run is completed.
4.
In the Enter email addresses for notifications field, enter the e-mail addresses that notifications
will be sent to. Follow the format shown on the screen. Enter a comma between addresses.
Chapter5
Run the plate
(7500 system only) Set notifications
5
HIDReal‑Time PCR Analysis Software User Guide
39

5.
Define the outgoing server. If you need information about the server, contact your network system
administrator.
a.
Outgoing Server (SMTP) field—Enter the name of the outgoing server.
For example: smtp.mycompany.com
b.
Server requires an encrypted connection?—If the outgoing server requires an encrypted
connection, select Yes.
c.
Server requires authentication—If the outgoing server requires authentication to receive the
e-mail from the instrument, select Yes, then enter the authentication user name and password
in the dialog box.
Start or stop the run
IMPORTANT!
If the computer that performs the run is on a network, avoid excess use of the network
during a run.
Note: You can set analysis parameters before or after you run a plate. To set parameters before you run
a plate, see Chapter 6, “Select analysis settings and thresholds”.
Start a run
In the Experiment Menu, select Setup, select any screen, then click START RUN at the top-right
corner.
Alternatively, click Run, select any screen, then carefully click START RUN at the top-left corner.
The green START RUN button becomes a red STOP RUN button, and the run begins.
Note: If you double-click the START RUN button, it may not become a STOP RUN button, but the run
proceeds normally.
Stop a run
When you start a run, the green START RUN button becomes a red STOP RUN button.
In the Experiment Menu, select Setup, select any screen, then click STOP RUN at the top-right corner.
Alternatively, click Run, select any screen, then carefully click STOP RUN at the top-left corner.
The run immediately stops.
Chapter5
Run the plate
Start or stop the run
5
40
HIDReal‑Time PCR Analysis Software User Guide

(7500 system only) Monitor a run
Note: This procedure applies only to the 7500 Real‑Time PCR Instrument. It does not apply to the
QuantStudio
™
5 Real-Time PCR Instrument.
During a run, you can access the amplification plot, temperature plot, and run method.
In the Experiment Menu, select Run, then click the data to view.
• Amplification Plot—Displays amplification plots of reactions
• Temperature Plot—Displays temperature plots of reactions
• Run Method—Displays the run method and allows you to edit the run method during the run
Save the results
After a run is complete, the HIDReal‑Time PCR Analysis Software automatically performs analysis and
saves the initial results file.
If you modify the plate (for example, if you remove a well from analysis and reanalyze the results), the
software does not automatically save the changes. After reanalysis, the
HIDReal‑TimePCR Analysis
Software prompts you to save the results.
After the run, see Chapter7, “Enhance data analysis” to view and manage the results.
Chapter5Run the plate
(7500 system only) Monitor a run
5
HIDReal‑Time PCR Analysis Software User Guide
41

Select analysis settings and
thresholds
■
Open analysissettings ................................................................ 42
■
View and edit CTsettings ............................................................. 43
■
Enter HIDsettings .................................................................... 44
■
Enter flagsettings .................................................................... 48
■
Add a virtual standard curve to the experiment ........................................... 49
IMPORTANT!
All default settings shown in this guide and in the software screens are for illustration
only. For your experiments, set the parameters and thresholds according to your laboratory protocol.
Before analyzing data from a completed run, you can edit values for the analysis parameters:
•
C
T
threshold, baseline start cycle, and end cycle
•
HID flag thresholds
•
QC flag thresholds
The Analysis Settings screen also contains the area where you set the parameters for the Dilution
Calculation tool that is used to calculate a dilution scheme for downstream amplification. For more
information about the Dilution Scheme pane, see “Edit dilution settings for individual samples” on
page66.
Open analysis settings
1.
In the Experiment Menu, select Analysis, then select any one of the data displays.
•
Amplification Plot
•
Standard Curve
•
Virtual Standard Curve
•
Multicomponent Plot
•
Raw Data Plot
•
QC Summary
6
42
HIDReal‑Time PCR Analysis Software User Guide

2.
Click Analysis Settings at the top-right corner of the screen to open the Analysis Settings dialog
box.
View and edit C
T
settings
Note: The recommended C
T
settings for each Quantifiler
™
kit are included in the experiment templates
provided with the software and in the kit user guides. The recommended settings are those that were
used in the validation experiments performed for each kit by Thermo Fisher Scientific.
1.
Select the C
T
Settings tab to view the settings for C
T
.
The default system settings are:
•
Manual C
T
Threshold = 0.2
•
Manual Baseline Start Cycle = 3
•
Manual Baseline End Cycle = 15
Chapter6Select analysis settings and thresholds
View and edit CT settings
6
HIDReal‑Time PCR Analysis Software User Guide
43
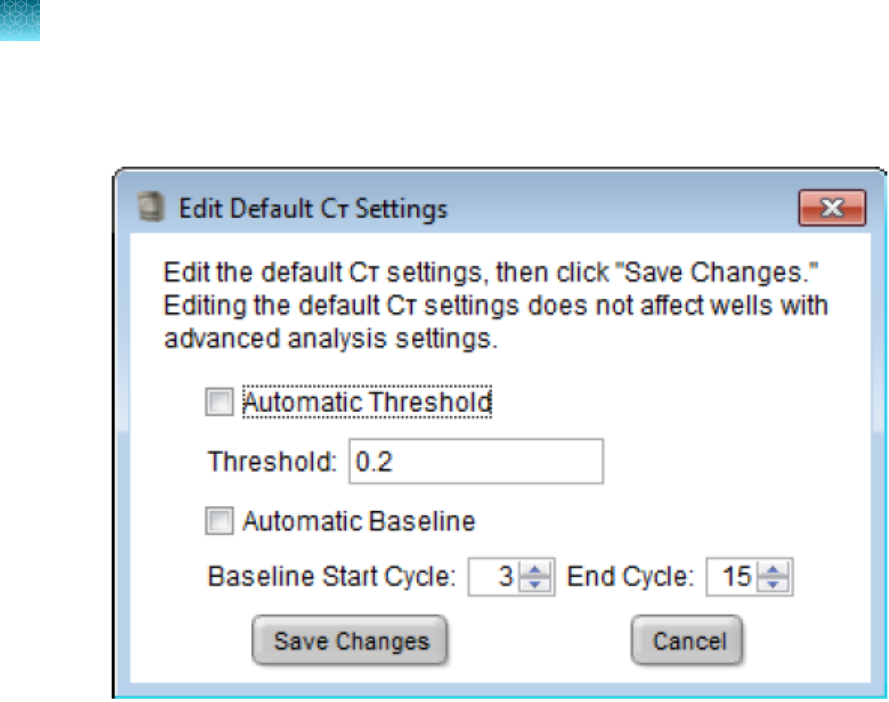
2.
To change the settings, click Edit Default Settings, enter the new values, then click Save
Changes.
3.
To analyze the data with new settings, click Apply Analysis Settings at the bottom of the Analysis
Settings dialog box.
Enter HID settings
1.
Select the HID Settings tab to view the Dilution Scheme, HID Flags, and HID Flag Settings
panes.
For more information about settings in the Dilution Scheme pane, see “Edit dilution settings for
individual samples” on page66.
2.
In the Use column in the HID Flags pane, select the checkbox for each flag that you want to
include in the analysis.
You can use a flag to identify quality issues and help to interpret results for wells. Flags can
indicate samples that may require further attention. You can exclude wells from data analysis (see
“Omit wells from analysis” on page56).
3.
Enter threshold settings for the flags that you select.
a.
In the HID Flags pane, select the flag of interest.
b.
In the HID Flag Settings pane, enter in the corresponding fields the values that you want to
use.
Chapter6
Select analysis settings and thresholds
Enter HID settings
6
44
HIDReal‑Time PCR Analysis Software User Guide

Repeat steps 2 on page44 and 3 on page44 until you enter settings (or view the default settings),
for all the flags that you select.
Note: To save your HID flag settings for future use, save the experiment as a template before you
start the run (see “Start or stop the run” on page40).
4.
To analyze the data with new settings, click Apply Analysis Settings at the bottom of the Analysis
Settings dialog box.
HIGHQT
The HIGHQT
flag indicates that the quantity, or mean quantity of sample replicates, is above a
threshold that you set.
Chapter6
Select analysis settings and thresholds
Enter HID settings
6
HIDReal‑Time PCR Analysis Software User Guide
45

IPCCT
The IPCCT flag indicates one of the following:
Well contents Cause Comment
Unknown
sample
The IPC (Internal PCR Control) C
T
value is greater than the average
of the IPC C
T
values for all the
standards plus the threshold that you
set.
We strongly recommend that you base the
threshold setting on validation data produced by
your laboratory.
For information on interpreting the IPCCT flag for
Quantifiler
™
kit experiments, see your kit user guide.
Standard or
NTC
The IPC (Internal PCR Control) C
T
value is above the maximum or below
the minimum that you set.
In Quantifiler
™
kit experiments, the IPC target
amplification should be within an expected range.
Low or no IPC amplification can indicate the
presence of PCR inhibitors, incorrect experiment
setup, or reagent or instrument failure.
LOWQT
The LOWQT flag indicates that the quantity, or mean quantity of sample replicates, is below a threshold
that you set.
NTCCT
The NTCCT flag refers to the C
T
value of the NTC (non-template control). No amplification of human
and/or male targets should occur in NTC wells.
MTFR flag and M:F ratio display
The MTFR (Male to Female Ratio) is expressed as 1:X. A well is flagged if X is greater than the threshold
that you set. For example, if you set the MTFR flag threshold at 1:10, then a sample that contains
5ng/µL of male DNA and >55 ng/µL of human DNA generates an MTFR flag. The flag for this condition
is a yellow triangle ( ) in the Plate Layout or Well Table tab, and a red octagon ( ) in the Analysis
Summary pane (see Chapter7, “Enhance data analysis”.
Samples that generate the MTFR flag are labeled Thresholds Not Met in the Analysis Summary pane
of the QC Summary tab. The MTFR flag indicates samples that might require Yfiler
™
kit amplification
because of low quantities of male DNA relative to female DNA. Autosomal amplification of these
samples may result in partial to no profile for the secondary (male) contributor.
In contrast, the M:F ratio display does not have an associated flag. The M:F ratio is also expressed as
1:X and is displayed in the M:F ratio column of the well table only if X is greater than or equal to the
threshold that you set for the M:F ratio display.
The M:F ratio display threshold is expressed as 1:X where X must be less than or equal to the X value
for the MTFR flag. For example, if you set the M:F ratio display to 1:1, then the MTFR flag must be
set to 1:>1. Samples with ratios greater than the MTFR flag display the MTFR flag and display the
Chapter6
Select analysis settings and thresholds
Enter HID settings
6
46
HIDReal‑Time PCR Analysis Software User Guide

calculated M:F ratio. The M:F Ratio Display function alerts you to male and female mixtures before STR
analysis.
Table3Results of example M:F and MTFR settings
Male DNA Female DNA Male:Female ratio
HID setting
M:F ratio
display?
MTFR flag?
M:F Ratio
display (1:X) X
=
MTFR flag
(1:X) X =
1ng/µL 1ng/µL 1:1 1 1 Yes No
1ng/µL 2ng/µL 1:2 1 1 Yes Yes
1ng/µL 1ng/µL 1:1 1 2 Yes No
SLOPE
The SLOPE flag indicates the PCR amplification eciency for the experiment. The amplification
eciency is calculated using the slope of the regression line in the standard curve. The standard wells
are flagged if the slope is not between the minimum and maximum values that you set.
The standard curve is derived from a serial dilution set of standards that contain a range of known
quantities. Amplification results for these standards are used to generate a curve.
A slope of −3.3 indicates 100% amplification eciency. For more information on the standard curve and
slope, see the following kit user guides:
•
Quantifiler
™
Human DNA Quantification Kit and Y Human Male DNA Quantification Kit User Guide
•
Quantifiler
™
Duo DNA Quantification Kit User Guide
R
2
The R
2
flag indicates the regression coecient calculated from the regression line of the standard
curve. The R
2
value indicates the closeness of fit between the standard curve regression line and
individual C
T
data points from the standard reactions. A value of 1.00 indicates a perfect fit between the
regression line and the data points.
YINT
The Y-intercept value of the standard curve indicates the expected C
T
value for a sample with a
quantity of 1 (for example, 1ng/µL). The YINT flag can assist in evaluating standard performance
and serial dilution preparation. Your laboratory can perform validation studies to determine a range
for the Y-intercept. Additionally, you can set the HID flag values for each Quantifiler
™
kit and the HID
flag values for each target (human and male) in the Quantifiler
™
Duo assay. A YINT flag may indicate
incorrectly prepared standard concentrations, degraded standard, or other preparation errors.
Chapter6
Select analysis settings and thresholds
Enter HID settings
6
HIDReal‑Time PCR Analysis Software User Guide
47

Enter flag settings
1.
Select the Flag Settings tab to view and define instrument, sample, and data collection flags.
Flags not used in the analysis are gray. Table4 explains the flags.
2.
In the Use column, select each flag that you want to include in the analysis.
3.
Specify the conditions that generate a flag: In the Condition column dropdown lists, select
conditions (< > =), then enter the corresponding values in the Value column.
4.
To omit the wells that have a flag from the analysis, select the corresponding Reject Well
checkboxes.
5.
To analyze the data with new settings, click Apply Analysis Settings.
Table4QC flags
Flag
Description
AMPNC Amplification in non-template control
BADROX Bad passive reference signal
BLFAIL Baseline algorithm failed
CTFAIL C
T
algorithm failed
DRNMIN Define acceptable delta Rn based on Ct range
EXPFAIL Exponential algorithm failed
OFFSCALE Fluorescence is oscale
HIGHSD High standard deviation in replicate group
PRFLOW Low passive reference signal
NOAMP No amplification
NOISE Noise higher than others in plate
SPIKE Noise spikes
NOSIGNAL No signal in well
Chapter6Select analysis settings and thresholds
Enter flag settings
6
48
HIDReal‑Time PCR Analysis Software User Guide

Table4QC flags(continued)
Flag Description
OUTLIERRG Outlier in replicate group
PRFDROP Passive reference signal changes near C
T
THOLDFAIL Thresholding algorithm failed
Add a virtual standard curve to the experiment
If you are using a virtual standard curve to analyze experiments, use the software to create the virtual
standard curve, then assign it to your experiments as needed or export it for further use.
Guidelines for using virtual standard curves
•
The software will not analyze an experiment using a virtual standard curve if:
–
The plate layout of the experiment contains wells that are configured with the Standard task
type.
–
The expiration date specified for the virtual standard curve has expired.
–
The Quantifiler
™
kit specified for the experiment and the curve do not match.
•
When analyzing an experiment using a virtual standard curve, all Unknown samples generate the
IPCCT (Internal PCR Control C
T
) flag by default.
•
Laboratories should perform internal validation studies to ensure that implementation of a virtual
standard curve is appropriate and generates reliable downstream data. For optimal results, virtual
standard curves should be evaluated independently for each real-time PCR instrument. We
recommend the re-evaluation of virtual standard curves with each new lot of quantification kit.
Create a virtual standard curve
IMPORTANT! To create the virtual standard curve, you must know the slopes and y-intercepts of the
targets for the kit that you are using.
1.
In the Experiment Menu, select Analysis4Virtual Standard Curve.
2.
Click Add Standard Curve to Experiment in the top-left corner to open the Virtual Standard
Curve Library dialog box.
3.
Click New to create a new virtual standard curve.
4.
Specify the settings for the virtual standard curve.
a.
Enter a name for the curve.
b.
(Optional) Select the Is Standard Curve Default checkbox to analyze all new experiments of
the same selected kit type (see substep 4d on page50) using the virtual curve.
Chapter6
Select analysis settings and thresholds
Add a virtual standard curve to the experiment
6
HIDReal‑Time PCR Analysis Software User Guide
49

c.
Select the date on which the curve expires. When the curve expires, the software can no
longer use it to analyze data.
d.
Select the kit to which the virtual standard curve applies.
e.
Enter the slope and y-intercept for each target of the selected kit.
f.
Enter any comments for the virtual standard curve, then click OK to save it to the library.
Chapter6Select analysis settings and thresholds
Add a virtual standard curve to the experiment
6
50
HIDReal‑Time PCR Analysis Software User Guide

Apply a virtual standard curve to an experiment
Before you apply the curve
The software cannot use a virtual standard curve to analyze an experiment that already contains wells
that are assigned the Standard task. Therefore, before applying a virtual standard curve, you must omit
or reassign the task of any
Standard well on the plate layout.
Option See
Omit wells from the analysis “Omit wells from analysis” on page56
Reassign the task assignment of a well “Assign samples, standards, and NTCs to wells” on page32
Apply a standard curve
IMPORTANT!
Before you apply a virtual standard curve, you must omit or reassign the task of any
Standard well on the plate layout.
To apply a virtual standard curve to the open experiment:
1.
In the Experiment Menu, select Analysis4Virtual Standard Curve.
2.
Click Add Standard Curve to Experiment in the top-left corner of the screen.
3.
In the Virtual Standard Curve Library dialog box, click Add selected Virtual Standard Curve.
Note: When analyzing an experiment using a virtual standard curve, all Unknown samples generate
the IPCCT (Internal PCR Control C
T
) flag by default.
Automatic analysis using a default virtual standard curve
If you select the Is Standard Curve Default checkbox in the settings of a virtual standard curve, the
software automatically analyzes all new experiments using that default virtual standard curve unless:
•
The plate layout of a new experiment contains wells that are configured as standards.
•
The expiration date specified for the virtual standard curve has passed.
•
You select the Is Standard Curve Default checkbox for another virtual standard curve (or the
option is deselected for the existing virtual standard curve).
Chapter6Select analysis settings and thresholds
Add a virtual standard curve to the experiment
6
HIDReal‑Time PCR Analysis Software User Guide
51

Enhance data analysis
■
View the analysis results .............................................................. 52
■
Interpret QC flaginformation ........................................................... 55
■
Omit wells fromanalysis ............................................................... 56
■
Omit targets in an experimentwell ...................................................... 57
■
Examine the Degradation Index ........................................................ 58
■
Change the appearance of, print, and saveplots ......................................... 58
View the analysis results
Flagged wells
To view the results of the data analysis:
1.
In the Experiment Menu, select Analysis4QC Summary to open the QC Summary screen.
2.
Select the Analysis Summary tab.
The tab displays the HID-specific flags that you selected to include in the data analysis and
indicates the number of wells that meet or do not meet the threshold that you set. The symbols in
the Analysis Summary tab are described in Table5.
Note: If the screen resolution is not set to 1280×1024, the Analysis Summary tab may not be
properly displayed.
7
52
HIDReal‑Time PCR Analysis Software User Guide

Table5Symbols in the Analysis Summary tab
Location
Symbol Description
Standard Curve bar Green square ( ) A value for Slope, R2, or Y-Intercept meets the
threshold
Red octagon ( ) A value for Slope, R2, or Y-Intercept does not meet the
threshold
Thresholds Met columns Hyperlinked numbers The number of wells that meet the thresholds for a flag
value
Thresholds Not Met
columns
Hyperlinked numbers The number of wells that do not meet the thresholds
for a flag value
Standard Curve bar
The Standard Curve bar contains the SLOPE, R
2
, and Y-Intercept flags. Click the column heading for
a red octagon ( ) to highlight in the plate layout the wells represented in the standard curve. This
graphical view simplifies the identification of wells that require further analysis using your laboratory
protocol.
Chapter7
Enhance data analysis
View the analysis results
7
HIDReal‑Time PCR Analysis Software User Guide
53

Standard bar
The Standard bar reports the IPCCT flags for all the wells on the plate that you designated as sample
type Standard. Click the number in the Thresholds Not Met column to view the wells that do not
meet the IPCCT threshold in the plate layout or well table format. You can use the amplification,
multi-component, or raw data plots to troubleshoot the data for these wells. You can examine the wells
that meet the threshold by clicking the number in the Thresholds Met column.
NTC bar
The NTC bar reports the IPCCT and NTCCT flags for all the wells on the plate that you designated
as sample type NTC (non-template control). Click the number in the Thresholds Not Met column to
view the wells that do not meet the IPCCT or NTCCT threshold in the plate layout or well table format.
You can use the amplification, multi-component, or raw data plots to troubleshoot the data for these
wells. You can examine the wells that meet the threshold by clicking the number in the Thresholds Met
column.
Unknown bar
The Unknown bar reports the IPCCT, HIGHQT, LOWQT, and MTFR flags for all the wells on the plate
that you designated as sample type Unknown. The HIGHQT, LOWQT, and MTFR (Male to Female
Ratio) flags indicate that the quantity of DNA or ratios of male to female DNA in Unknown samples may
require additional attention. Numbers below the flag indicate the number of wells that do not meet the
threshold.
Note: The MTFR flag is not available in Human or HP
™
kit experiments.
Click the number in the Thresholds Not Met column to view the wells that do not meet a threshold in
the plate layout or well table format. You can use the amplification, multi-component, or raw data plots
to troubleshoot the data for these wells. You can examine the wells that meet the threshold by clicking
the number in the Thresholds Met column.
Instrument-related flags
A message may be displayed to indicate that one or more of the instrument-related flags is generated
by a potential problem with the instrument. The message prompts you to select the QC Flags Details
tab to view the flags.
Wells automatically omitted
In certain rare instances, such as assignment of targets to empty wells, the HIDReal‑TimePCR
Analysis Software may automatically omit wells of a Quantifiler
™
kit run.
The software automatically omits wells that may prevent the completion of data analysis, so that
analysis can continue for the rest of the wells in the plate. These wells are indicated by a red
exclamation point above the Analysis Summary tables. You can examine the automatically omitted
wells by clicking the number next to the exclamation point.
Chapter7
Enhance data analysis
View the analysis results
7
54
HIDReal‑Time PCR Analysis Software User Guide

Interpret QC flag information
1.
In the QC Summary screen, click the QC Flags Detail tab to view all QC flags (both general and
HID).
2.
Click a flag to select all aected wells in the plate layout, and to open a brief description of the flag
and wells.
3.
(Optional) In the QC Flags Details description box, click the hyperlink to open the software help
system.
The software help system provides information for troubleshooting the flag and the criteria used
for analysis. (For more information about the flags, see Chapter6, “Select analysis settings and
thresholds”.)
For information about how to view and edit sample information, see “Change the appearance of a plot”
on page58.
Chapter7Enhance data analysis
Interpret QC flag information
7
HIDReal‑Time PCR Analysis Software User Guide
55

Omit wells from analysis
1.
In the Experiment Menu, select Analysis.
2.
To view data from individual wells, select any one of the plot screens.
•
Amplification—Amplification vs. cycle and amplification vs. well
•
Standard curve—C
T
vs. quantity of standards, flagged samples, and unflagged samples
•
Multicomponent plot—Fluorescence vs. cycle of all reaction components
•
Raw data plot—Amplitude vs. filter
•
Multiple plots view—Amplification, standard curve, multicomponent, and raw data plots in
one pane
Note: If no data are displayed, click Analyze.
3.
Omit wells using the well table or plate layout.
•
Well table—Select the View Well Table tab, then select the Omit checkbox for each well to
exclude from the analysis.
Note: If the Omit Well column is not visible in the table, click Show in Table, then select
Omit Well to show the column.
•
Plate layout—Select the View Plate Layout tab. For each well to omit, right-click the well,
then select Omit4Well.
Chapter7Enhance data analysis
Omit wells from analysis
7
56
HIDReal‑Time PCR Analysis Software User Guide

4.
Click Analyze to reanalyze the experiment data with the omitted wells excluded from the analysis.
5.
Review the data that are analyzed without the omitted wells.
Omit targets in an experiment well
For Duo, HP
™
, and Trio kit experiments, you can omit one of the standard targets in a well from analysis.
1.
Right-click a well with a standard target that you want to omit.
Note: You can omit only one target from one well at a time.
2.
Select Omit from the dropdown list, then select a well or an individual target.
•
Well—Select a well to omit all targets from the well. The (well omitted) icon appears in the
well.
•
Individual target—Select the name of the target (for example, Duo Human) to omit a specific
target from the well. The individual target omitted icon (for example, for Duo Human
omitted) appears in the well.
3.
Click Analyze to reanalyze the experiment data with the omitted targets excluded from the
analysis.
Figure7Example—Duo kit experiment
Chapter7Enhance data analysis
Omit targets in an experiment well
7
HIDReal‑Time PCR Analysis Software User Guide
57

Examine the Degradation Index
The Degradation Index refers to the data observed when a sample may be degraded: a decrease in
measured amount for large DNA fragments compared to small DNA fragments. While DNA degradation
is not the only theoretically possible mechanism for a decrease in amount, it is the predominant
mechanism in the absence of inhibitors. The Degradation Index is for use as a general indicator of
whether large DNA fragments may perform more poorly in STR reactions. Evaluate the Degradation
Index in conjunction with the IPC C
T
.
The Degradation Index is automatically calculated by the HIDReal‑Time PCR Analysis Software using
the following formula:
The Degradation Index value is displayed in the Well Table view in any of the analysis screens (you may
have to scroll to the right to display it.)
See the Quantifiler
™
HP and Quantifiler
™
Trio DNA Quantification Kits User Guide for more information
on:
•
The Degradation Index
•
Evaluating the HIDReal‑Time PCR Analysis Software quality indices to identify degraded samples
Change the appearance of, print, and save plots
Change the appearance of a plot
1.
In the Experiment Menu, select Analysis, then select any one of the plot screens.
2.
In the plot screen, locate the icon bar above the plot.
3.
Click (Hide) to hide the plot legend.
Chapter7Enhance data analysis
Examine the Degradation Index
7
58
HIDReal‑Time PCR Analysis Software User Guide
4.
To change the appearance of a plot, click (Edit Plot Properties) to open the Plot Properties
dialog box. Three tabs are available.
5.
Select the appropriate tab to enter the values that you want to use to plot the data.
6.
Click OK to apply the changes.
Select the wells to include in the report
1.
In the Experiment Menu, select Analysis, then select any one of the plot screens.
2.
Select the wells to include in the report, using the View Plate Layout tab (see step 4 on page32)
or the View Well Table tab (see step 4 on page35).
Print or save a plot
1.
To print a plot, click (Print) .
2.
To save a plot as a JPG file, click (Save).
Printed plots and JPG files include the slope, Y-intercept, and R
2
.
Chapter7Enhance data analysis
Change the appearance of, print, and save plots
7
HIDReal‑Time PCR Analysis Software User Guide
59

Export and report results
■
Exportdata .......................................................................... 60
■
Print a report ......................................................................... 62
After the HIDReal‑Time PCR Analysis Software completes analysis and after you review the data, you
can generate a customized report (PDF file), then save or print the report.
You can also export and save data in these formats:
•
Excel
™
(XLS)
•
PowerPoint
™
(PPT)
•
Text (TXT)
Export data
1.
In the Experiment Menu, select Analysis.
2.
Select any one of the plot screens, then click View Plate Layout or View Well Table.
3.
Highlight the wells to export.
4.
In the toolbar, click (Export) to open the Export Data screen, then select the Export
Properties tab.
5.
Select one or more checkboxes for the type of data to export.
•
Amplification Data—Data that was collected during the cycling or amplification stage.
•
Multicomponent Data—Fluorescence data for each dye, for each cycle.
•
Raw Data—Raw fluorescence data for each filter, for each cycle.
•
Results—Results of the analysis.
•
Sample Setup—Setup information such as well, sample name, and sample color.
•
STR Dilution Setup—Sample dilution worksheet to prepare samples for amplification. For
more information, see Chapter 9, “Generate dilution and reaction worksheets for STR setup”
•
STR Reaction Setup—STR reaction setup worksheet to prepare samples for amplification.
For more information, see Chapter 9, “Generate dilution and reaction worksheets for STR
setup”
8
60
HIDReal‑Time PCR Analysis Software User Guide

6.
From the dropdown list, select Separate Files or One File.
7.
Enter the export file properties.
•
Export File Name—Enter the name of the report.
•
File Type—Select the type of file. See the online help system for information on creating PPT
slides.
•
Export File Location—Enter the filepath for the save location.
8.
(Optional) Customize the data.
a.
Select the Customize Export tab.
b.
Select the information that you want to export.
Note: Sample setup should be exported only as a TXTfile.
Chapter8Export and report results
Export data
8
HIDReal‑Time PCR Analysis Software User Guide
61

c.
To sort data in the export by column, click the column header (for example, click Well to sort
the data by well).
9.
Click Start Export to export the data to the files that you selected.
Print a report
1.
Select Plate Setup4Assign Targets and Samples.
2.
Click View Plate Layout or View Well Table.
3.
Select the wells to include in the report.
4.
In the toolbar, click (Print Report) to display the Print Report screen.
5.
Select the checkbox for each data topic that you want to include in the report.
Note: Exported standard curves do not include unknown data points.
Chapter8Export and report results
Print a report
8
62
HIDReal‑Time PCR Analysis Software User Guide

6.
(Optional) Click Print Preview to preview the report content.
7.
Select a print option.
8.
Select Save to save the report, or select Print to print the report.
Note: If you do not enter a name in the Experiment Name field of the Experiment Properties screen,
the experiment name on the report is “Untitled.”
Chapter8Export and report results
Print a report
8
HIDReal‑Time PCR Analysis Software User Guide
63

Generate dilution and reaction
worksheets for STR setup
■
Add kits to an experiment ............................................................. 64
■
Select unknown samples foramplification ............................................... 65
■
Edit dilution settings for individualsamples .............................................. 66
■
View the dilutionscheme .............................................................. 67
■
Export dilution and reactionworksheets ................................................. 68
■
Save new STR kit information from an experiment into the STR kit library .................... 68
After a run is complete, you can use the HIDReal‑Time PCR Analysis Software to generate dilution and
reaction worksheets for STR setup.
The software generates dilution and reaction setup worksheets to perform calculations for the kits that
you select from the STR Kit Library, and the kit information and default dilution settings that you specify.
See Appendix D, “Configure STR library and default dilution settings” to:
•
Enter, edit, or delete kit information in the STR Kit Library
•
Set default dilution settings for the calculations
Add kits to an experiment
Before exporting worksheets, add kits to an experiment.
1.
Open the experiment of interest.
2.
In the Experiment Menu, select Analysis4STR Kit Setup.
3.
In the STR Kit Setup pane, click Add Kit to Experiment to open the Kit Dilutions Library screen.
9
64
HIDReal‑Time PCR Analysis Software User Guide

4.
Select the kits to use in the experiment. To edit kit information, see “Configure the STR Kit Library”
on page82.
5.
Repeat steps 2 on page64 through 4 on page65 until you select all the kits to use in the
experiment.
6.
To delete a kit from the experiment (not from the Kit Dilutions Library), select the kit to delete, then
click Delete Kit from Experiment.
Select unknown samples for amplification
After adding kits to an experiment, select the unknown samples for amplification and associate samples
with kits.
1.
In the Experiment Menu, select Analysis, then select any analysis screen.
2.
Select the View Well Table tab.
Note: If the well table does not display a column for the selected STR kit, click Show in Table,
then select the kit name from the list of available columns.
Chapter9Generate dilution and reaction worksheets for STR setup
Select unknown samples for amplification
9
HIDReal‑Time PCR Analysis Software User Guide
65

3.
Select the checkbox for the unknown sample to use and the STR kit with which to use the sample.
If a sample is not for amplification (for example, a standard), it is not available for selection.
To select all of the samples for a kit, select the checkbox next to the kit name at the top of the
column.
Note: The software automatically assigns the same kit for replicates.
4.
Select the Dilution Setup tab to view the dilution scheme and the STR kits that you selected for
each sample.
5.
Repeat steps 2 on page66 and 3 on page66 for each unknown sample and kit.
Note: You cannot select an STR kit for standard or NTC sample types. Dilution calculations apply only
to the unknown sample (Human or Male) target in the wells, not to standards or NTCs.
Edit dilution settings for individual samples
If needed, edit the default dilution settings for samples.
1.
Select the View Well Table tab.
2.
Select the sample of interest.
3.
In the toolbar at the top of the well table, click Edit Dilutions to open the Edit Target Dilution
Details screen.
Chapter9Generate dilution and reaction worksheets for STR setup
Edit dilution settings for individual samples
9
66
HIDReal‑Time PCR Analysis Software User Guide

4.
View or edit the settings as needed.
•
Min. Pipetting Vol.—The minimum quantity to pipette.
•
Max. Sample Vol.—The maximum volume of available sample.
•
Dilution Factor—For example, enter 10 for 10‑fold dilutions.
•
Target Conc.—The amount of target DNA that you want to use divided by the total sample
volume per STR reaction.
•
# Replicates—The number of identical reactions.
Note: If you quantify replicates, the Edit Target Dilution Details screen displays the sample
concentration or the mean sample concentration.
Note: The software displays target sample concentration based on maximum sample volume, number
of replicates, sample volume per STR reaction, and pipetting overage that you set if the desired target
concentration cannot be reached.
View the dilution scheme
View the dilution scheme to ensure that the settings are appropriate for your experiment.
1.
In the Experiment Menu, select Analysis.
2.
Click any plot to open a plot screen.
3.
Select the Dilution Setup tab to open the Dilution Setup screen.
4.
Review the settings for downstream reactions.
Chapter9
Generate dilution and reaction worksheets for STR setup
View the dilution scheme
9
HIDReal‑Time PCR Analysis Software User Guide
67

Export dilution and reaction worksheets
Export the STR Dilution Setup worksheet and the STR Reaction Setup worksheet as described in
“Export data” on page60.
Save new STR kit information from an experiment into the
STR kit library
You can save a kit from an experiment into the library (for example, if you import an experiment from a
system with a dierent library setup).
Note: If the STR kit name that you are saving from the experiment is already listed in the library,
rename or delete the kit from the library before saving the kit information from the experiment.
1.
Open the experiment.
2.
In the STR Kit Setup screen, select the kit to save.
3.
Click Save Kit to Library.
Chapter9Generate dilution and reaction worksheets for STR setup
Export dilution and reaction worksheets
9
68
HIDReal‑Time PCR Analysis Software User Guide

Troubleshooting
Observation Possible cause Recommended action
File integrity warning
Details: HIDReal‑TimePCR
Analysis Software v1.4 may
display a file integrity warning
when opening an EDS file that
was analyzed in earlier versions
of the software.
There is a checksum
calculation modification in v1.4.
No action required. The file can be used with
no eect on the results obtained.
HIDReal‑TimePCR Analysis
Software v1.4 unexpectedly
closes when starting a 7500
instrument run
The instrument server
terminated and the server no
longer accepts commands.
Restart the 7500 instrument and the computer
that is running the HIDReal‑TimePCR
Analysis Software.
If the behavior continues, we recommend
that you disconnect the computer from the
network during runs on the 7500 instrument.
Note: We do not recommend using
HIDReal‑Time PCR Analysis Software v1.4
with the 7500 Real-Time PCR System for
Human Identification.
A
HIDReal‑Time PCR Analysis Software User Guide
69

HIDReal‑TimePCR Analysis
Software v1.3 validation
■
Overview of the software validation forv1.3 .............................................. 70
■
Materials andmethods ................................................................ 71
■
Experiments and results ............................................................... 73
■
Conclusions ......................................................................... 76
Overview of the software validation for v1.3
HIDReal‑Time PCR Analysis Software v1.3 is designed for the Quantifiler
™
DNA Quantification Kits and
the 7500 Real‑Time PCR Instrument or the QuantStudio
™
5 Real-Time PCR Instrument with 0.2‑mL
96‑well sample block. The software enables streamlined quantification run setup, data analysis, and
STR reaction setup by providing Quantifiler
™
-specific templates and quality flags as well as STR sample
normalization (dilution) and reaction setup tools. HIDReal‑Time PCR Analysis Software v1.3 contains
the same functionality as the v1.2 software in addition to new features that support the use of virtual
standard curves and the use of the QuantStudio
™
5 Real‑Time PCR System. For more information, see
“Features in v1.3” on page71.
This appendix describes the results of experiments that Thermo Fisher Scientific performed to
validate HIDReal‑Time PCR Analysis Software v1.3. Data was collected using v1.2 and v1.3 of the
HIDReal‑Time PCR Analysis Software, the 7500 Real-Time PCR System and the QuantStudio
™
5
System, and the Quantifiler
™
HP
™
, Trio, Duo, and Human DNA Quantification Kits.
The data collected from the 7500 and QuantStudio
™
5 instruments were analyzed to verify the following:
•
HIDReal‑Time PCR Analysis Software v1.3 performs as designed to analyze data generated on the
7500 Real-Time PCR System and the QuantStudio
™
5 Real‑Time PCR System.
•
The new features do not adversely aect the quantification assays or the software functionality
carried over from the HIDReal‑Time PCR Analysis Software v1.2.
•
When analyzed with HIDReal‑Time PCR Analysis Software v1.3, data generated using the 7500
Real-Time PCR System and the QuantStudio
™
5 Real‑Time PCR System demonstrate reproducible
performance for the respective instrument models.
For validation experiments and results for the Quantifiler
™
Trio, Duo, HP
™
, and Human kits, see the
Quantifiler
™
HP and Quantifiler
™
Trio DNA Quantification Kits User Guide (Pub.No.4485354), the
Quantifiler
™
Duo DNA Quantification Kit User Guide (Pub.No.4391294), and the Quantifiler
™
Human
DNA Quantification Kit and Y Human Male DNA Quantification Kit User Guide (Pub.No.4344790 ).
B
70
HIDReal‑Time PCR Analysis Software User Guide

Features in v1.3
HIDReal‑Time PCR Analysis Software v1.3 includes all of the v1.2 functionality and includes the
following new features:
•
Virtual Standard Curve support for Quantifiler
™
HP
™
, Trio, Duo, and Human DNA Quantification
Kits.
•
Support for the QuantStudio
™
5 Real-Time PCR Instrument with 0.2‑mL 96‑Well Sample Block.
Materials and methods
Instrument, computer, and software configuration
Instruments:
•
7500 Real-Time PCR System, firmware v2.10 (3)
•
QuantStudio
™
5 Real-Time PCR System, 0.2‑mL 96‑well sample block (3)
Instrument 1 Instrument 2 Instrument 3
Instrument
4
Instrument
5
Instrument
6
Model QuantStudio
™
5
QuantStudio
™
5
QuantStudio
™
5
7500 7500 7500
Computer Laptop Laptop Desktop Laptop Laptop Desktop
Microsoft
™
OS Windows
™
7
64‑bit
Windows
™
7
64‑bit
Windows
™
7
64‑bit
Windows
™
7
64‑bit
Windows
™
7
32‑bit
Windows
™
7
32‑bit
HIDReal‑TimePCR
Analysis Software
v1.3 v1.3 v1.3 v1.2 and
v1.3
v1.2 v1.2
Chemistries and consumables
Unless otherwise indicated, all materials are available through thermofisher.com. Catalog numbers
that appear as links open the web pages for those products.
Item
Cat. No.
Quantifiler
™
Trio DNA Quantification Kit, 14 kits (from single lot) 4482910
Quantifiler
™
HP DNA Quantification Kit, 5 kits (from single lot) 4482911
Quantifiler
™
Duo DNA Quantification Kit, 5 kits (from single lot) 4387746
Quantifiler
™
Human DNA Quantification Kit, 5 kits (from single lot) 4343895
MicroAmp
™
Optical 96-Well Reaction Plate, 10 plates N8010560
Modified TE Buer (10/0.1) 300675
MicroAmp
™
Optical Adhesive Film, 100 covers 4311971
7500 Real-Time PCR Systems Spectral Calibration Kit I 4349180
AppendixBHIDReal‑Time PCR Analysis Software v1.3 validation
Materials and methods
B
HIDReal‑Time PCR Analysis Software User Guide
71
(continued)
Item Cat. No.
ABY
™
Spectral Calibration Plate, 96‑Well 0.2‑mL 4461591
JUN
™
Spectral Calibration Plate, 96‑Well 0.2‑mL 4461593
TaqMan
™
RNaseP Instrument Verification Plate for 7300/7500 Systems, 96‑well 4350584
AmpFℓSTR
™
DNA Control 007, Male N/A
AmpFℓSTR
™
Control DNA9947A, Female N/A
The following test cases were performed for each chemistry kit:
Quantifiler
™
kit
Plates per instrument for the experiment
Precision and linearity Accuracy and reproducibility Sensitivity Mixture Inhibition
Trio Kit 3 plates 1 plate 1 plate 1 plate 1 plate
Duo Kit 3 plates 1 plate 1 plate 1 plate 1 plate
HP
™
Kit 1 plate 1 plate — — —
Human Kit 1 plate 1 plate — — —
Samples
Experiment
Sample Replicates per plate
Precision and
linearity
Quantifiler
™
Trio, Duo, HP
™
, and Human Kit DNA Standards 6 standard curve
12 dilution series
Accuracy and
reproducibility
One male DNA, 1ng/µL 96 replicates
Sensitivity
•
Quantifiler
™
Trio Kit—One male and one female DNA diluted to
100, 10, 1, 0.1, 0.01, 0.001, and 0.0001ng/µL
•
Quantifiler
™
Duo Kit—One male DNA diluted to 50, 5, 0.5, 0.05,
and 0.005ng/µL
•
5 dilution series
•
5 dilution series
Mixture analysis
•
Quantifiler
™
Trio Kit—One set of male/female DNA mixture at 1:0,
1:1, 1:10, 1:100, 1:1000, 1:4000, 0:1 ratios
•
Quantifiler
™
Duo Kit—One set of male/female DNA mixture at 1:0,
1:1, 1:10, 1:100, 1:500, 1:1000, 0:1 ratios
•
3 mixture series
•
3 mixture series
Inhibition
•
Quantifiler
™
Trio Kit—One male 007 DNA (0.1ng/µL) with hematin
(550µM)
•
Quantifiler
™
Duo Kit—One male 007 DNA (0.1ng/µL) with hematin
(80µM)
•
96 replicates
•
96 replicates
AppendixBHIDReal‑Time PCR Analysis Software v1.3 validation
Materials and methods
B
72
HIDReal‑Time PCR Analysis Software User Guide

Data collection and analysis
•
Run Method and analysis settings were configured as outlined in the respective Quantifiler
™
kit user
manuals. The design and execution of all workflows (including instrument calibration, run setup,
data analysis, run methods, HID quality flags, dilution calculations import/export and reporting)
were identical for both versions of the software.
Note: The 7500 Systems were calibrated as explained in the Applied Biosystems
™
7500/7500 Fast
Real‐Time PCR System: Maintenance Guide (Pub.No.4387777).
Note: The QuantStudio
™
5 Systems were calibrated as explained in the QuantStudio
™
3 and
5 Real‑Time PCR Systems Installation, Use, and Maintenance Guide (Pub.No.MAN0010407).
The instruments were calibrated for the ABY
™
-HID and JUN
™
-HID dyes using the ABY
™
Dye
Spectral Calibration Plate (Cat.No.4461591) and the JUN
™
Dye Spectral Calibration Plate
(Cat.No.4461593).
•
Calibration and experiment (EDS) data files that were previously generated using
HIDReal‑Time PCR Analysis Software v1.2 were imported and reanalyzed using
HIDReal‑Time PCR Analysis Software v1.3. The results from v1.2 and v1.3 were then compared for
dierences in data output.
Experiments and results
Instrument performance
Test Case
Description Passing Criteria Results
Precision and
linearity
Using each instrument, set up and run:
•
Three plates of the standard curve
dilution series using the Quantifiler
™
Trio
and Duo Kits, standard curve dilution
series.
•
One plate using the Quantifiler
™
HP
™
and Human Kits, standard curve dilution
series.
Produce a standard curve from each pair
of dilution series, so that six curves are
generated per plate on each instrument.
Statistically evaluate the C
T
and R
2
values for
variation within an instrument.
For each system (7500 or
QuantStudio
™
5):
•
When running standard
curves using the Quantifiler
™
Trio, HP
™
, DUO, and Human
Assays, the C
T
values
shall have a
coecient of
variation (CV) ≤20% within an
instrument.
•
When running standard
curves using the Quantifiler
™
Trio, HP
™
, DUO, and Human
Assays, the system shall
have a standard curve R
2
value ≥0.98.
Pass
Accuracy and
reproducibility
Using each instrument, set up and run one
plate of 007 DNA with 1ng/µL input using
each Quantifiler
™
kit, 96 replicates per plate.
Statistically evaluate the C
T
values and DNA
quantity for variation within an instrument.
When running the Quantifiler
™
Trio, HP
™
, DUO, and Human
Assays, the within-instrument C
T
and quantity values shall have a
CV ≤20% within an instrument.
Pass
AppendixBHIDReal‑Time PCR Analysis Software v1.3 validation
Experiments and results
B
HIDReal‑Time PCR Analysis Software User Guide
73

(continued)
Test Case
Description Passing Criteria Results
Sensitivity Using each instrument, set up and run:
•
One plate containing seven dilutions of
007 and 9947a DNA (from 0.0001ng/µL
to 100 ng/µL) prepared using the
Quantifiler
™
Trio Kit.
•
One plate containing five dilutions of 007
DNA (from 0.005 ng/µL to 50ng/µL)
prepared using the Quantifiler
™
Duo Kit.
Run five dilution series per sample per plate,
providing a total of five replicates for each
sample dilution. Statistically evaluate the C
T
values and DNA quantity data for variation
within an instrument.
When running Quantifiler
™
Trio
Assays using 0.01 ng/µL to
10ng/µL DNA input and when
running Quantifiler
™
Duo Assays
using 0.05 ng/µL to 5ng/µL DNA
input, the in-plate C
T
and quantity
values shall have a CV ≤20%
within an instrument.
Pass
Mixture
analysis
Using each instrument, set up and run:
•
One set of male and female DNA mixture
samples consisting of seven mixture
ratios (1:0, 1:1, 1:10, 1:100, 1:1000,
1:4000, and 0:1) using the Quantifiler
™
Trio Kit.
•
One set of male and female DNA mixture
samples with seven mixture ratios (1:0,
1:1, 1:10, 1:100, 1:500, 1:1000, and 0:1)
using the Quantifiler
™
Duo Kit.
Run three replicates for each mixture sample.
Statistically evaluate the C
T
values and
DNA quantity data for variation within an
instrument.
When running Quantifiler
™
Trio
and DUO Assays using mixtures
where the male DNA input is
0.02ng/µL and the female
DNA input is between 0.02 and
20ng/µL, the C
T
and quantity
values shall have a CV ≤20%
within an instrument when results
are within range on the standard
curve.
Pass
Inhibition Using each instrument, set up and run:
•
One plate of 0.1ng/µL 007 DNA with
550µM hematin using the Quantifiler
™
Trio Kit.
•
One plate of 0.1ng/µL 007 DNA with
80µM hematin using the Quantifiler
™
Duo
Kit.
Run 96 replicates per plate. Statistically
evaluate the C
T
values and quantity data for
variation within an instrument.
When running Quantifiler
™
Trio
and DUO Assays, the generated
C
T
and quantity values shall have
a CV ≤20% within an instrument.
Pass
Exception
conditions
[1,2]
[1]
When tested using the Quantifiler
™
DUO Kit and the QuantStudio
™
5 Instrument, samples with extreme hematin concentrations (>40µM) can
produce a biphasic curve that may result in overestimation of DNA concentrations or quantity value CV significantly >20%.
[2]
At extreme hematin concentrations (~550µM), more variation (quantity value CV significantly >20%) may be observed in the large autosomal
and Y targets on both 7500 and QuantStudio
™
5 Systems.
AppendixB
HIDReal‑Time PCR Analysis Software v1.3 validation
Experiments and results
B
74
HIDReal‑Time PCR Analysis Software User Guide

Software performance
Test case
Description Passing criteria Results
Custom
standard curve
Using one 7500 System and one
QuantStudio
™
5 System, set up a virtual
standard curve using estimated slope and
y-intercept values. Perform a Quantifiler
™
Trio Kit run without standard curve
samples.
The software shall successfully
collect the data and automatically
apply the virtual standard curve.
The generated DNA quantities shall
be 100% concordant with the
manual calculation.
Pass
Using three 7500 Systems and three
QuantStudio
™
5 Systems, evaluate the
virtual standard curve function using the
sensitivity of a collected run. Create a
virtual standard curve using the parameters
(slope and y-intercept) of the standard
curve generated from the run. Analyze the
data using the actual standard curve and
the virtual standard curve, then compare
the DNA quantity values.
For each respective system (7500
or QuantStudio
™
5), analyzing
the same experiment using the
actual and virtual standard curves,
the software shall calculate
quantification values that match to
the third decimal point.
Pass
Workflow and
user interface
Using one 7500 System computer and one
QuantStudio
™
5 System computer, confirm
that the settings match for all run/analysis
methods and flag thresholds.
When comparing the v1.2 and
v1.3 software, all values for the
run/analysis methods and flag
thresholds shall be same.
Pass
Using 7500 and QuantStudio
™
5 Systems,
run Quantifiler
™
Trio and HP
™
assays by
following the standard workflow and using
the necessary software functions.
The software functions necessary
for using the Quantifiler
™
assays
shall perform without error.
Pass
Backward
compatibility
Use the v1.3 software to reanalyze an
experiment (EDS) file generated using
a 7500 System and the v1.2 software,
then compare the quantification values
calculated by both versions of the
software.
When analyzing the EDS file using
the v1.2 and v1.3 software, the
calculated quantification values
shall match to the third decimal
point.
Pass
AppendixBHIDReal‑Time PCR Analysis Software v1.3 validation
Experiments and results
B
HIDReal‑Time PCR Analysis Software User Guide
75

Conclusions
Based on the validation of HIDReal‑Time PCR Analysis Software v1.3 and the precision and
linearity, accuracy and reproducibility, sensitivity, mixture analysis, and inhibition validation experiments
performed using the Quantifiler
™
Trio, Duo, HP
™
, and Human Kits [see the Quantifiler
™
HP and
Quantifiler
™
Trio DNA Quantification Kits User Guide (Pub.No.4485354), Quantifiler
™
Duo DNA
Quantification Kit User Guide (Pub.No.4391294), and Quantifiler
™
Human DNA Quantification Kit and Y
Human Male DNA Quantification Kit User Guide (Pub.No.4344790 )]:
•
All updates to v1.3 were successfully and correctly implemented without negative eects on
functionality carried over to v1.3 from v1.2.
•
HIDReal‑Time PCR Analysis Software v1.3 successfully controlled the 7500 Real-Time PCR
Systems and QuantStudio
™
5 Real‑Time PCR Systems, and reliably and reproducibly set up and
collected quantification data using the Quantifiler
™
kits.
•
The software provided accurate results when used to process Quantifiler
™
kits for the analysis of
genomic DNA samples.
•
The user interface and HID workflows in v1.3 software performed as expected for both instruments
when using the Windows
™
7 operating systems.
•
The coecient of variation (CV) for the average values of DNA quantity data and standard curve
parameters (C
T
and R
2
values) where tested varied <20% within each instrument (7500 and
QuantStudio
™
5 Systems) using HIDReal‑Time PCR Analysis Software v1.2 and v1.3.
Laboratories should determine the appropriate level of testing required before implementation, based
on the nature of the changes made to the software, how the software pertains to their laboratory
workflow, their internal software validation guidelines, and those of the appropriate governing agencies.
AppendixBHIDReal‑Time PCR Analysis Software v1.3 validation
Conclusions
B
76
HIDReal‑Time PCR Analysis Software User Guide

HIDReal‑TimePCR Analysis
Software v1.4 verification
■
Objective of the software verification forv1.4 ............................................ 77
■
Functional testing .................................................................... 78
■
Regression testing .................................................................... 79
■
Reliability testing ..................................................................... 81
■
Conclusions ......................................................................... 81
Objective of the software verification for v1.4
The objective of this verification is to confirm that the software modifications made in
HIDReal‑Time PCR Analysis Software v1.4 function as expected when using the 7500 Real-Time PCR
System for Human Identification, the QuantStudio
™
5 Real‑Time PCR System running firmware v1.5.1,
and the Quantifiler
™
Trio, Duo, HP
™
, and Human kits.
Testing was performed according to Quality Assurance Standards (QAS) for Forensic DNA Testing
Laboratories (July 1, 2020), standard 8.8.3.4. Functional, regression, and reliability testing was
performed.
C
HIDReal‑Time PCR Analysis Software User Guide
77

Functional testing
Functional tests were conducted to demonstrate that the software changes made in
HIDReal‑Time PCR Analysis Software v1.4 do not aect performance when using the Quantifiler
™
kits with the 7500 Real-Time PCR System and the QuantStudio
™
5 Real‑Time PCR System.
Note: HIDReal‑Time PCR Analysis Software v1.4 may unexpectedly close when starting a 7500
instrument run. Therefore, we do not recommend using v1.4 with the 7500 instrument.
Materials and methods
Instrument, computer, and software configuration
Instruments:
•
7500 Real-Time PCR System for Human Identification (2)
•
QuantStudio
™
5 Real-Time PCR System, 0.2‑mL 96‑well sample block (3)
Instrument 1 Instrument 2 Instrument 3 Instrument 4 Instrument 5
Model QuantStudio
™
5 QuantStudio
™
5 QuantStudio
™
5 7500 7500
Instrument firmware
version
v1.5.1 v1.5.1 Upgraded from v1.3.3
to v1.5.1
v2.10 v2.10
Computer Dell
™
Precision 3551
Image LTSC 2021 LTSC 2019 LTSC 2019 LTSC 2021 LTSC 2019
Microsoft
™
Windows
™
OS
Windows
™
10, 64-bit operating system (IOT Enterprise)
HIDReal‑TimePCR
Analysis Software
v1.4
Chemistries and consumables
Unless otherwise indicated, all materials are available through thermofisher.com. Catalog numbers
that appear as links open the web pages for those products.
Item
Cat. No.
Quantifiler
™
Trio DNA Quantification Kit, 1 kit 4482910
Quantifiler
™
HP DNA Quantification Kit, 1 kit 4482911
Quantifiler
™
Duo DNA Quantification Kit, 1 kit 4387746
Quantifiler
™
Human DNA Quantification Kit, 1 kit 4343895
MicroAmp
™
Optical 96‑Well Reaction Plate with Barcode, 20 plates 4306737
MicroAmp
™
Optical Adhesive Film, 100 covers 4311971
AppendixCHIDReal‑Time PCR Analysis Software v1.4 verification
Functional testing
C
78
HIDReal‑Time PCR Analysis Software User Guide

(continued)
Item Cat. No.
ABY
™
Spectral Calibration Plate, 96‑Well 0.2‑mL 4461591
JUN
™
Spectral Calibration Plate, 96‑Well 0.2‑mL 4461593
TaqMan
™
RNase P Instrument Verification Plate, 96‑Well 0.2‑mL 4432382
AmpFℓSTR
™
DNA Control 007, Male 4460779, 4478871
Teknova
™
DNA Suspension Buer, pH8.0 T0227
Data collection and analysis
•
Data was collected using HIDReal‑Time PCR Analysis Software v1.4, the 7500 Real-Time PCR
System and the QuantStudio
™
5 Real‑Time PCR System (firmware v1.5.1), and the Quantifiler
™
Trio,
Duo, HP
™
, and Human DNA Quantification Kits.
•
Run Method and analysis settings were configured as outlined in the respective Quantifiler
™
kit user
manuals.
Results
Test case
Samples Passing criteria Result
Linearity Quantifiler
™
Trio, Duo, HP
™
, and
Human Kit DNA standards:
•
4 standard curve dilution series
for each Quantifiler
™
kit
Evaluate the R
2
and slope values for each
standard curve:
•
R
2
value ≥0.985 for the Trio, HP
™
and Duo
kits; ≥0.980 for the Human kit
•
The slope values for each target
are within the range observed during
the developmental validation of each
Quantifiler
™
kit.
Pass
Accuracy and
reproducibility
DNA Control 007, 1ng:
•
4 replicates for each
Quantifiler
™
kit
The 1 ng/µL 007 DNA concentration is within
range 0.60–1.65ng/µL
Pass
Contamination DNA Dilution Buer:
•
4 replicates for each
Quantifiler
™
kit
An undetermined (UND) result Pass
Regression testing
Regression testing was performed to confirm that the software changes made in HIDReal‑TimePCR
Analysis Software v1.4 do not impact the overall software functionality. The test cases performed were
designed to ensure that the features and functionalities of HIDReal‑Time PCR Analysis Software v1.4
work the same as in v1.3.x.
AppendixC
HIDReal‑Time PCR Analysis Software v1.4 verification
Regression testing
C
HIDReal‑Time PCR Analysis Software User Guide
79

Materials
Instrument, computer, and software configuration
Instruments:
•
7500 Real-Time PCR System for Human Identification (1)
•
QuantStudio
™
5 Real-Time PCR System, 0.2‑mL 96‑well sample block (2)
Instrument 1 Instrument 2 Instrument 3
Model QuantStudio
™
5 QuantStudio
™
5 7500
Instrument firmware version v1.5.1 Upgraded from v1.3.3 to v1.5.1 v2.10
Computer
[1]
Dell
™
Precision 3551 and 3571
Image
[2]
LTSC 2019 and 2021
Microsoft
™
Windows
™
OS Windows
™
10, 64-bit operating system (IOT Enterprise)
HIDReal‑Time PCR Analysis Software v1.3.2 and v1.4
[1]
All configurations were tested across each instrument.
[2]
All configurations were tested across each instrument.
Results
Test case
Description Passing criteria Result
Workflow and
user interface
Confirm that the settings match for all
run/analysis methods and flag thresholds for
each Quantifiler
™
kit.
When comparing HIDReal‑TimePCR
Analysis Software v1.3.2 and v1.4, all
values for the run/analysis methods and
flag thresholds shall be same.
Pass
Run each Quantifiler
™
assay by following the
standard workflow and using the necessary
software functions.
The software features and functions
shall perform without error for each of
the Quantifiler
™
kits tested.
Pass
Virtual standard
curve
Create a virtual standard curve using
estimated slope and y‑intercept values.
Execute a run for each Quantifiler
™
kit without
adding standards to the experiment.
The software shall successfully collect
the data and automatically apply the
virtual standard curve.
Pass
Backward
compatibility
Use HIDReal‑Time PCR Analysis Software
v1.4 to reanalyze an experiment (EDS) file
generated using a 7500 and a QuantStudio
™
5
System with HIDReal‑TimePCR Analysis
Software v1.3.2. Compare the quantification
values calculated by both versions of the
software.
The calculated quantification values in
HIDReal‑Time PCR Analysis Software
v1.4 should be concordant to those
obtained in v1.3.2.
Pass
AppendixCHIDReal‑Time PCR Analysis Software v1.4 verification
Regression testing
C
80
HIDReal‑Time PCR Analysis Software User Guide

Reliability testing
Reliability testing was performed to evaluate the software performance within and beyond functional
aspects. A variety of user scenarios were tested as presented in Table6. Functional and non-functional
tasks from instrument sign-in through uninstallation of the software performed as expected.
Table6Test cases used to evaluate the reliability of HIDReal‑Time PCR Analysis Software v1.4 with the
7500 and QuantStudio
™
5 Systems
Category Test
Performed as
Expected
(Y/N)
Install/uninstall The user can install HIDReal‑Time PCR Analysis Software v1.4 using
a new or an upgrade registration code. The user is able to uninstall
HIDReal‑Time PCR Analysis Software v1.4.
Y
Instrument
connectivity
Connection of the Dell
™
Precision 3551 and 3571 laptops running
HIDReal‑Time PCR Analysis Software v1.4 to one 7500 System and two
QuantStudio
™
5 Systems (firmware v1.5.1).
Y
Calibrations 7500 System calibrations migrated from the Dell
™
Precision 3551 to the
3571.
Y
User profiles The user can create a new profile within HIDReal‑TimePCR Analysis
Software v1.4, then sign in to the software with this new user profile.
Y
Functional testing
(One 7500 System and
two QuantStudio
™
5
Systems)
The following functions were evaluated to ensure that software changes
did not aect functionality:
•
Import a plate setup
•
Open files using File4Open, or clicking the EDS or EDT file name
•
Save the EDS files after run completion (7500 and QuantStudio
™
5
Systems)
•
Export data to a CSV file
•
Export reports to a PDF file
Y
Conclusions
Based on this verification, we can conclude that all updates to HIDReal‑Time PCR Analysis Software
v1.4 did not aect performance when using the Quantifiler
™
kits and the 7500 Real-Time PCR System
and QuantStudio
™
5 Real‑Time PCR System (firmware v1.5.1). This version of software maintains the
functionality and reliability of previous versions.
AppendixCHIDReal‑Time PCR Analysis Software v1.4 verification
Reliability testing
C
HIDReal‑Time PCR Analysis Software User Guide
81

Configure STR library and default
dilution settings
■
Configure the STR Kit Library .......................................................... 82
■
Set default dilutionsettings ............................................................ 84
Configure the STR Kit Library
Most AmpFℓSTR
™
kits are listed in the STR Kit Library by default. To add or modify amplification kit
information, perform the following steps.
1.
In the toolbar, select Tools4AmpFℓSTR
™
Kit Library to open the Kit Dilutions Library screen.
2.
Add, edit, and/or delete a kit.
•
Add a kit—Click New. The Create New STR Kit dialog box opens.
•
Edit a kit—Select the kit, then click Edit. The Create New STR Kit dialog box opens.
•
Delete a kit—Select the kit, then click Delete.
D
82
HIDReal‑Time PCR Analysis Software User Guide

3.
If you add or edit a kit, enter settings as needed.
•
STR Kit Name—The name of the kit that you are adding to the list.
Note: Kit names must be unique. To use the same kit with dierent sample types or dierent
input amounts of DNA, add the kit with a dierent name, such as Identifiler
™
_1.5ng.
•
Target Conc.—The amount of DNA that you want to use divided by the total sample volume
per reaction. Examples:
Total DNA (ng) Volume/reaction (µL) Target concentration (ng/µL)
0.5 10 0.05
1.0 10 0.1
1.0 20 0.05
2.0 20 0.1
•
STR Reaction pane
–
PCR Master Mix—Enter appropriate volumes (µL).
–
Sample—Enter appropriate volumes (µL).
The volumes of master mix and sample must equal the total volume of the STR reaction:
Sample (µL) + PCR master mix (µL) = Reaction volume (µL)
–
Additional # of Reactions and/or Amplification Controls—Enter the number of
additional STR reactions per amplifications to allow for pipetting overage.
IMPORTANT!
Because not all kits allow for pipetting overage, you may need to enter
more additional reactions to compensate for volume losses that occur during pipetting.
For information about pipetting overage, see the user guide for your kit.
•
PCR Master Mix pane—List each component of the STR reaction master mix. For more
information, see the user guide for your kit.
AppendixDConfigure STR library and default dilution settings
Configure the STR Kit Library
D
HIDReal‑Time PCR Analysis Software User Guide
83

4.
Click OK.
5.
Repeat steps 2 on page82 through 4 on page84 for all needed kits.
6.
Verify that the kits to be used in the downstream STR reactions are listed, with correct information.
Note: You can also save a kit from an experiment into the library (for example, if you import an
experiment from a system with a dierent library setup). See “Save new STR kit information from
an experiment into the STR kit library” on page68.
Set default dilution settings
In the Analysis Settings screen, you can specify default dilution settings to apply to all samples. (You
can edit individual sample dilution settings after you associate an STR kit with an experiment.)
1.
In the Experiment Menu, select any analysis screen, then click Analysis Settings.
2.
Select the HID Settings tab.
3.
In the Dilution Scheme pane, enter dilution scheme parameters according to your preferences or
laboratory protocol.
•
Pipetting overage—The percent to add to compensate for error in pipetting. If the sample
concentration is less than the target concentration and the sample volume is limited, set the
pipetting overage to zero to maximize the amount of DNA in the STR reaction.
•
Minimum Pipetting Volume—The minimum volume that you want to pipette.
•
Maximum Sample Volume—The maximum quantity of sample that you want to use.
•
Dilution Factor—The maximum first dilution that you want to perform with the available DNA.
For example, for 10‑fold first dilutions, enter 10.
4.
In the Dilution Method pane, select one of the following methods.
•
One Step Dilution Only—To use a single dilution in all instances.
•
System Select—To use a dilution scheme that depends on your preferences, with a maximum
of two dilutions.
The software displays the target sample concentration based on the maximum sample volume,
number of replicates, sample volume per STR reaction, and pipetting overage that you set if the
desired target concentration cannot be reached.
AppendixDConfigure STR library and default dilution settings
Set default dilution settings
D
84
HIDReal‑Time PCR Analysis Software User Guide
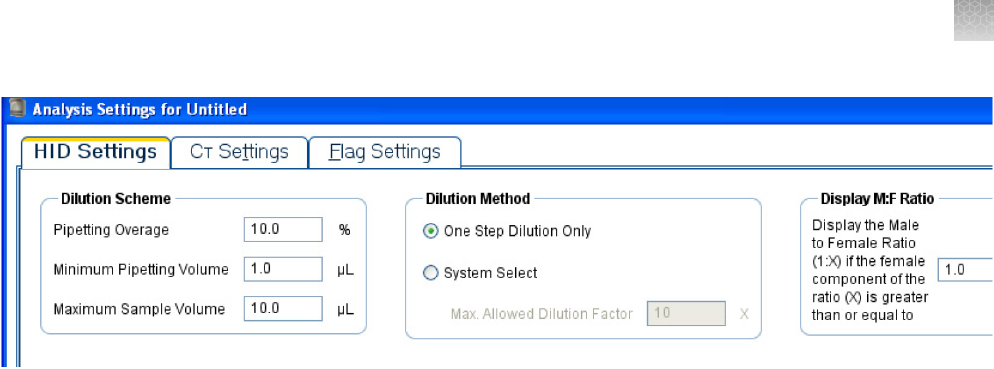
AppendixDConfigure STR library and default dilution settings
Set default dilution settings
D
HIDReal‑Time PCR Analysis Software User Guide
85

Documentation and support
Related documentation
Table7Documents for HIDReal‑Time PCR Analysis Software experiments
Document Pub. No.
Quantifiler
™
HP and Quantifiler
™
Trio DNA Quantification Kits User Guide 4485354
Quantifiler
™
Duo DNA Quantification Kit User Guide 4391294
Quantifiler
™
Human DNA Quantification Kit and Y Human Male DNA Quantification Kit User Guide 4344790
Applied Biosystems
™
7500/7500 Fast Real-Time PCR System Maintenance Guide 4412844
7500/7500 Fast Real-Time PCR System Getting Started Guide for Absolute Quantification
Experiments
4378658
Applied Biosystems
™
7500/7500 Fast Real‐Time PCR System Getting Started Guide: Standard
Curve Experiments
4387779
QuantStudio
™
3 and 5 Real‑Time PCR Systems Installation, Use, and Maintenance Guide MAN0010407
QuantStudio
™
Design and Analysis Desktop Software User Guide MAN0010408
Table8Documents for custom experiments
See the following documents for information on performing custom experiments instead of using the HIDReal‑TimePCR Analysis
Software.
Document
Pub. No.
Applied Biosystems
™
7500/7500 Fast Real‐Time PCR System Getting Started Guide: Genotyping
Experiments
4387784
Applied Biosystems
™
7500/7500 Fast Real‐Time PCR System Getting Started Guide: Presence/ Absence
Experiments
4387785
Applied Biosystems
™
7500/7500 Fast Real‐Time PCR System Getting Started Guide: Relative Standard
Curve and Comparative C
t
Experiments
4387783
Applied Biosystems
™
7500/7500 Fast Real‐Time PCR System Getting Started Guide: Standard Curve
Experiments
4387779
7500/7500 Fast Real-Time PCR System Getting Started Guide for Absolute Quantification Experiments 4378658
E
86
HIDReal‑Time PCR Analysis Software User Guide

Customer and technical support
For support:
•
In North America—Send an email to HIDTechSupport@thermofisher.com, or call
888-821-4443option1.
•
Outside North America—Contact your local support oce.
For the latest services and support information for all locations, go to thermofisher.com/support to
obtain the following information.
•
Worldwide contact telephone numbers
•
Product support
•
Order and web support
•
Safety Data Sheets (SDSs; also known as MSDSs)
Additional product documentation, including user guides and Certificates of Analysis, are available by
contacting Customer Support.
Limited product warranty
Life Technologies Corporation and/or its aliate(s) warrant their products as set forth in the
Life Technologies' General Terms and Conditions of Sale at www.thermofisher.com/us/en/home/
global/terms-and-conditions.html. If you have any questions, please contact Life Technologies at
www.thermofisher.com/support.
AppendixEDocumentation and support
Customer and technical support
E
HIDReal‑Time PCR Analysis Software User Guide
87

