
Volume 29 Issue 2 Article 5
Determination of Phytochemical Compounds in Chicken Breast by Gas Determination of Phytochemical Compounds in Chicken Breast by Gas
Chromatography-tandem mass spectrometry Chromatography-tandem mass spectrometry
Follow this and additional works at: https://www.jfda-online.com/journal
Part of the Food Science Commons, Medicinal Chemistry and Pharmaceutics Commons, Pharmacology
Commons, and the Toxicology Commons
This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0
License.
Recommended Citation Recommended Citation
Cozzi, Federico; Eder-Neuhauser, Irene; Teichmann, Klaus; and Zaunschirm, Mathias (2021) "Determination of
Phytochemical Compounds in Chicken Breast by Gas Chromatography-tandem mass spectrometry,"
Journal of Food
and Drug Analysis
: Vol. 29 : Iss. 2 , Article 5.
Available at: https://doi.org/10.38212/2224-6614.3288
This Original Article is brought to you for free and open access by Journal of Food and Drug Analysis. It has been accepted for
inclusion in Journal of Food and Drug Analysis by an authorized editor of Journal of Food and Drug Analysis.

Determination of phytochemical compounds in
chicken breast by gas chromatography-tandem
mass spectrometry
Federico Cozzi*, Irene Eder-Neuhauser, Klaus Teichmann, Mathias Zaunschirm
BIOMIN Research Center, Tulln 3430, Austria
Abstract
A gas chromatography-tandem mass spectrometry (GC-MS/MS) method was developed for the simultaneous detection
and quantification of five phytogenic compounds (carvone, menthol, thymol, carvacrol and methyl salicylate) in chicken
breast. Chicken breast samples were analyzed using a QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe)
approach using acetonitrile as extraction solvent, followed by a d-SPE (dispersive-solid phase extraction) clean up step.
The linearities for the 5 substances were studied in the range between 2 and 100 mg/L and the coefficients of determi-
nation (R2) were always > 0.995. Matrix effects were also assessed by comparing the slopes obtained in solvent and
chicken breast matrix. The recoveries for all the substances at 3 different spike levels (5, 10 and 50 mg/kg) were in the
range 80-102% with RSDs < 15%. The instrumental limits of quantification were in the range 2.7-4.8 mg/kg, while the
reporting level of the method was 5 mg/kg for all the aforementioned compounds. The method was successfully applied
to 10 chicken breasts samples from the local market.
Keywords: Chicken breast, GC-MS/MS, Phytogenic compounds, QuEChERS
1. Introduction
A
ntibiotics and antimicrobials are not only
used as veterinary drugs to prevent and
control diseases in animal rearing but also as
growth promoters [1]. For this latter purpose they
are added to animal feed e a practice that can
lead to residues in edible animal products that are
potentially dangerous for human health or induce
antibiotic resistance of microbes even at small
doses [2]. This led to the ban of antibiotics as
growth promoters by the European Union since
2006 [3].
Global meat consumption has seen an increase by
58% in the past two decades and in particular
chicken meat consumption has increased, as it is not
only a source of high-quality protein, important
vitamin and minerals, but also the cheapest of all
livestock meats [4].
The aforementioned ban of antibiotic growth
promoters has fostered the research for alternative
substances: phytogenic feed additives (PFA) are
suggested to be among the most promising ones [5].
PFA are composed of plant-derived natural mate-
rials with positive effects on animal growth and
health, and this definition applies to preparations of
ground herbs and spices, essential oils (EOs), ex-
tracts an d/or oleoresins. PFA contain secondary
plant metabolites (phytochemicals) and encompass
a wide range of chemical compound classes,
including phenols, terpenes, alkaloids, lectins, al-
dehydes and ketones. The various mechanisms of
action are not yet fully understood but involve
antimicrobial/antiviral, antioxidative and anti-
inflammation activity [6].
Several companies offer feed additives containing
PFA in their portfolio and so it is important to
determine their residues in edible animal products
within the scope of consumer safety assessment [7].
We hence developed a method for the quant itative
determination of the following five phytochemicals
in chicken breast: carvone, menthol, thymol, carva-
crol and methyl salicylate.
Received 9 November 2020; revised 8 January 2021; accepted 8 February 2021.
Available online 15 June 2021.
* Corresponding author: BIOMIN Research Center, Tulln 3430, Austria. Tel: þ43-2272-811660.
https://doi.org/10.38212/2224-6614.3288
2224-6614/© 2021 Taiwan Food and Drug Administration. This is an open access article under the CC-BY-NC-ND license
(http://creativecommons.org/lice nses/by-nc-nd/4.0/).
ORIGINAL ARTICLE

Food matrices are still among the most chal-
lenging ones due to their complexity. In addition,
the usual sample preparation methods for these
matrices are labor intensive [8]. A valid alternative
to these methods is the QuEChERS technique [9],
although this method was originally developed for
pesticide analysis in fruits and vege tables its scope
of application has been extended to other analytes
and a grea t variety of different matrices [ 10,11].
Therefore, we decided to apply this approach as a
sample preparation method. A generic QuEChERS
extraction method involves a first step in which the
analytes of interest are extracted by using an organic
solvent (normally acetonitrile) in presence of inor-
ganic salts (like MgSO
4
or NaCl) to ensure a salting
out effect. The extract is then cleaned with disper-
sive sorbents to remove matrix interferences [12 ].
This method involves so, a simple QuEChERS
(Quick, Easy, Cheap, Effective, Rugged and Safe)
sample preparation procedure followed by detec-
tion by gas chromatography coupled with tandem
mass spectrometry (GC-MS/MS).
2. Materials and methods
2.1. Reagents and solutions
Ultra-pure water (18.2 MU cm-1) was obtained in-
house using a Millipore water purification system
(Cork, Ireland). Acetonitrile LC-MS grade was
purchased form Chem-Lab NV (Zedelgem,
Belgium).
QuEChERS original method (4 g MgSO
4
and 1 g
NaCl) and QuEChERS d-SPE (dispersive Solid
Phase Extraction) Animal Origin Food 5982-4950
(50 mg PSA, 150 mg C18EC and 900 mg Na
2
SO
4
)
were obtained from Agilent technologies.
2.2. GC-MS/MS instrumentation and settings
The GC-MS/MS analyses were conducted with a
Shimadzu GC 2010 gas chromatograph coupled
with a Shima dzu TQ-8050 tandem mass spectrom-
eter (Shimadzu, Kyoto, Japan). The system was
equipped with a PTV (Programmed Temperature
Vaporization) injection inlet and an AOC-5000
autosampler. GC-MS Real Time Analysis and GC-
MS Postrun Analysis software (Shimadzu, Kyoto,
Japan) were used for instrument control and data
analysis, respectively. The GC analysis was per-
formed on a J&W DB5-MSþDG column (length
30 m, id 0.25 mm, film thickness 0.25 mm þ 10 m
Guard Column), the chromatographic conditions
were the following: carrier gas he lium (minimum
purity 99.9995%) in constant linear velocity mode at
30 cm/sec, PTV Injector 60-200
C at 240
C/min
1 min then 330
C for 19 min, septum purge 6 mL/
min, Split 1:1; GC oven temperature program of 40
C for 1 min, ramp 20
C/min to 130
C, hold 7.0 min
then 40
C/min until 280
C (held for 3 min), injec-
tion volume 1 mL.
The mass spectrometer was operated in MRM
mode (MRM transitions of all five analytes and the
internal standard (IS) in Table 1) with the following
conditions: electron impact ionization at 70 eV, MS
transfer line temperature 280
C, MS source tem-
perature 200
C, solvent delay 9.6 min, dwell time
300 ms, collision gas argon (minimum purity
99.9999%) with a collision cell pressure of 200 kPa
and detector gain fixed at 1.4 kV.
2.3. Phytogenic compounds standards, internal
standard solution preparation
All high purity phytogenic compounds standards
were purchased from Sigma-Aldrich.
Mixed standard solutions of the analytes were
prepared in acetonitrile by diluting the stock solu-
tions to concentrations of 50, 400, 800, 1200, 1600 and
2000 mg/mL. The internal standard (IS; Butyrophe-
none) solution was prepared at a concentration of
200 mg/mL in acetonitrile as well.
The calibration solutions were prepared in tripli-
cate (n ¼ 3) by spiking 240 mL of phytogenics-free
chicken breast extract with 30 mL of the appropriate
standard solutions of the analytes to obtain con-
centrations of 2, 5, 10, 20, 50 and 100 mg/mL. The
concentration of the IS in each sample was main-
tained at 10 ppb by adding 30 mL of the IS stock
solution. In this way each matrix matched standard
solution contained the same amount of matrix an d
pure solvent.
2.4. Samples
Samples of chicken breast were purchased from
the local market in Austria. Each sample was ho-
mogenized with the use of a Retsch Mixer Mill MM
400 and stored at 20
C before analysis.
2.5. Sample preparation
One g of homogenized chicken breast was
weighed in a Retsch stainless steel jar, 1 mL of ul-
trapure water, 2 mL of acetonitrile and 0.7 g of
Agilent QuEChERS original method salts were
added; the jar was closed and the sample was
extracted using a Retsch Mixer Mill MM 400 for
2 min at 30 Hz. Hereafter 1.5 mL of the solution were
taken, transferred in a 1.5 mL glass vial and
256 JOURNAL OF FOOD AND DRUG ANALYSIS 2021;29:255e261
ORIGINAL ARTICLE

centrifuged for 5 min at 3234 rcf. An aliquot of
700 mL of the supernatant was transferred in a
1.5 mL glass vial containing 105 mg of QuEChERS
Dispersive SPE, the content of the vial was vortexed
for 1 min and then centrifuged for 5 min at 14000 rcf.
The sample was then transferred into a GC vial and
only at this point the IS was added, to avoid losses
during the sample prepar ation. The IS was used to
compensate e.g. for detector and injection volume
fluctuations [ 13 ].
2.6. Method validation
The following performance characteristics were
evaluated for the validation of the method: selec-
tivity, identification, linearity, matrix effects, limits
of quantification (LOQ), recoveries and precision.
2.7. Selectivity
The selectivity was verified by analyzing chicken
breast samples that were free of the compounds of
interest and the presence of peaks that could inter-
fere with those substances were assessed.
2.8. Identification
The criteria used for the identification of analytes
were retention time (Rt) and the presence and the
relative intensities of three MRM transitions (one
quantifier and two qualifiers). According to SANTE
2019 criteria, the Rt of a compound of interest in a
sample should not vary more than ± 0.1 min
compared to a calibration standard and, the relative
intensities for the samples should be within ± 30%
(relative) of average of calibration standards from
the same sequence [14].
2.9. Linearity
Linearity was assessed by measuring six points
calibration curves in triplicate (2, 5, 10, 20, 50 and
100 ppb e corresponding to a range of 4 to 200 mg/kg
in chicken breast) prepared in acetonitrile and in
blank chicken breast extracts as well.
2.10. Matrix effects
In order to assess the suppression or enhance-
ment of the signal of the analytes due to the matrix,
the slopes obtained from the linearity study were
used to calculate the percentage of the matrix effect
(%ME), according to the following formula:
Table 1. MRM Transitions of the investigated phytogenic compounds and an internal standard.
Compound Retention
Time (mins)
MRM Transition
1 (Quantifier)
Collision
Energy (eV)
MRM Transition
2 (Qualifier)
Collision
Energy (eV)
MRM Transition
3 (Qualifier)
Collision
Energy (eV)
Menthol 9.202 95 > 55.1 16 95 > 67.1 10 81 > 79.1 12
Methyl salicylate 9.545 105 > 77.1 16 105 > 51.1 24 77 > 51.1 16
Carvone 10.721 120 > 92.1 12 152 > 120.1 8 120 > 64.1 22
Butyrophenone (IS) 10.910 82 > 54.1 6 108 > 93.1 10 93 > 77.1 16
Thymol 11.793 135 > 91.1 16 150 > 135.1 12 135 > 115.1 16
Carvacrol 12.113 135 > 91.1 16 150 > 135.1 12 135 > 115.1 16
JOURNAL OF FOOD AND DRUG ANALYSIS 2021;29:255e261 257
ORIGINAL ARTICLE
%ME¼
Slope Matrix matched standards
Slope Standards in solvent
1
100
Negative values indicate signal suppression
while positive values indicate signal enhancement:
matrix effects in the range /þ 20% are permi ssible
[15].
2.11. LOQ
The instrumental LOQ were determined
following [16]: the standard deviation of a number of
samples at a low concentration was determined.
Multiplying this standard deviation (SD) with 3.3*ta
(ta th e Student's t correlating to the number of
samples the standard deviation is based on) gives
the LOQ.
LOQ ¼ SD*3.3*ta
The reporting level of the method was set at the
spike level (see Recoveries and precision) in which
the SANTE 2019 criteria (70-120% recoveries range
with a 20% RSD) were fulfilled [14].
2.12. Recoveries and precision
Recoveries and precision were evaluated by
spiking chicken breast samples that were free of the
compounds of interest and preparing them accord-
ingly to the procedure outlined in the Sample
preparation. The recoveries were determined for six
replicates prepared on three different days (day 1,
day 2 ¼ day 1 þ 24h and day 3 ¼ day 1 þ 144h) at
three different spike levels: 5, 10 and 50 mg/kg. The
average intraday and interday recoveries and the
relative standard deviation (RSD) were calculated as
the ratio of the analyte-to-IS peak area an d the re-
sults were evaluated for compliance to the SAN TE
2019 criteria, according to which the average re-
covery should be in the range 70-120% with an RSD
less or equal 20%.
3. Results and discussion
3.1. Method development
3.1.1. GC-MS/MS conditions
The GC parameters (initial PTV injector temper-
ature and ramp rate, initial and final column tem-
peratures, as well as the column temperature ramp
rate and carrier gas flow rate) were optimized to
achieve the best sensitivity and chromatographic
separation. It was possible to achieve chromato-
graphic separation for all the compounds of interest
and the IS, see Fig. 1.
In order to find the best MRM transitions the
Shimadzu MRM Optimization Tool 1.14 (Shim adzu,
Kyoto, Japa n) softwar e was used: this software au-
tomates the process by collecting product ion scan
data and finding the optimum collision energy for
each trans ition. Three MRM transitions per analyte
were chosen: one quanti fier and two qualifiers.
3.2. Sample preparation
The selection of the right sorbent is critical in
order to minimize matrix interferences and achieve
good and consistent analytes recove ries. Due to the
nature of our matrix we decided to use an animal
origin food sorbent. We evaluated the efficiency of
the dSPE step by measuring extracts which have
been treated or non-treated with the dSPE sorbent
in Full Scan mode, and then comparing the sum of
the areas of the chromatographic peaks in the TIC
chromatograms (m/z 50-600) [17]. The reduction for
the treated was in the range of 60%.
3.3. Method validation
3.3.1. Selectivi ty
No interfering peaks that could prevent the
identification or the quantification of the com-
pounds of interest were observed in 12 different
chicken breast samples that were analyzed. In
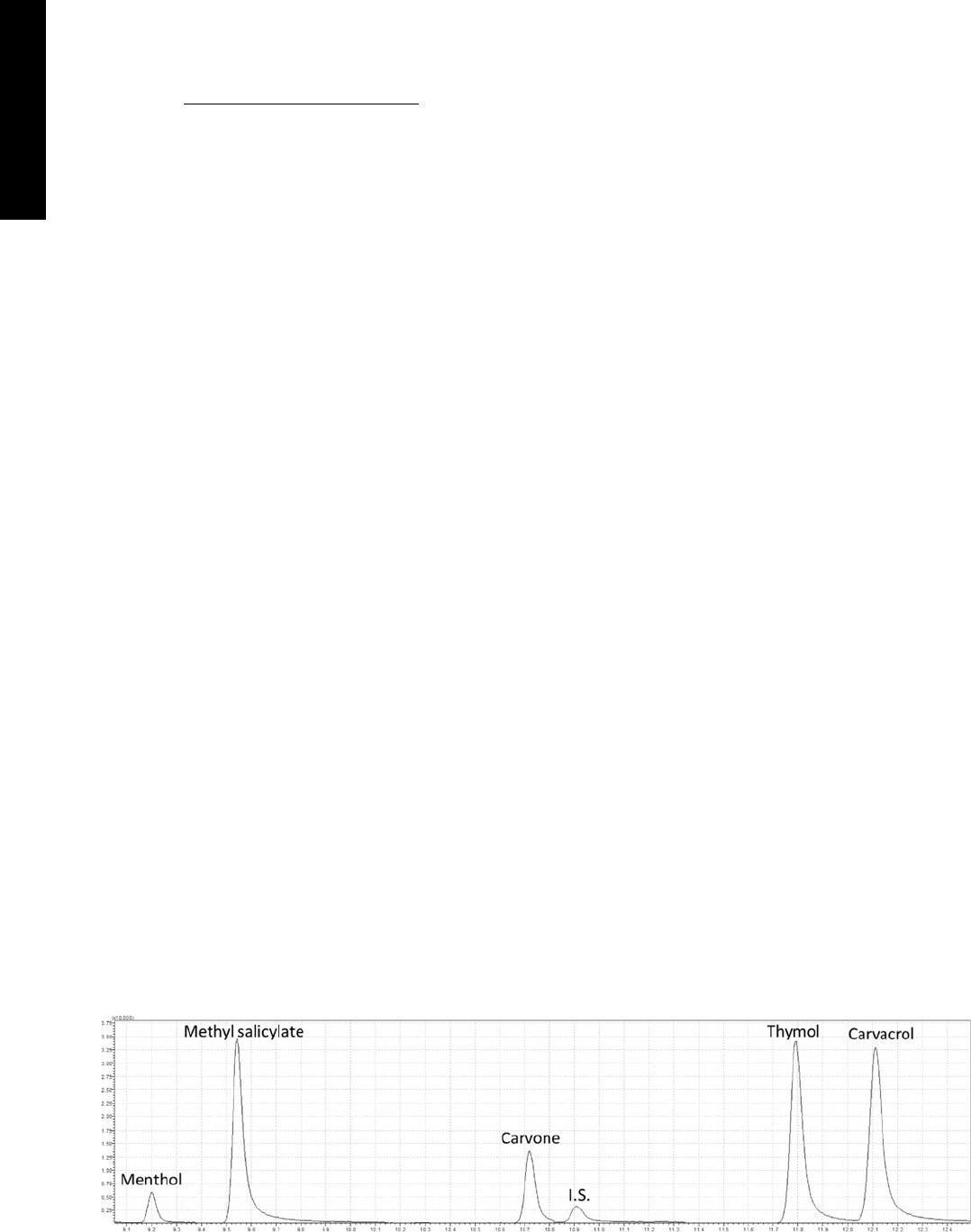
Fig. 1. MRM chromatogram of the chicken breast spiked with target analytes at the concentration of 10 mg/kg with the assigned peaks.
258 JOURNAL OF FOOD AND DRUG ANALYSIS 2021;29:255e261
ORIGINAL ARTICLE

addition, 10 procedural blanks were prepared as
well [18]. Also, in this case no interfering peaks were
detected.
3.3.2. Identification
The SANTE 2019 criteria mentioned before were
fulfilled by the matrix matched standards and all the
reals samples as well. In Fig. 2 a chromatogram of a
real sample is shown.
3.3.3. Linearity
Good linearity was achieved for all the com-
pounds of interest in the measured calibration range
with a coefficient of determination (R
2
) al-
ways > 0.995 in pure solvent and matrix as well.
3.3.4. Matrix effects
The matrix effect results, expressed as %ME are
shown in Table 2. Signal enhancement was
observed for all investigated substances, this is the
most common behavior for GC where the matrix
components block the active sites of the column [19].
Only for menthol the %ME is below ± 20% so we
decided to validate the method with matrix matched
calibration. Not surprisingly the %ME for the two
isomers thymol and carvacrol are very similar.
Fig. 2. MRM chromatogram of a real chicken breast sample with the assigned peaks.
Table 2. %ME of investigated analytes, LOQ (mg/kg), accuracy and precision. Conc. in mg/kg and precision data are given in parenthesis as %
coefficient of variation.
Analyte ME% LOQ (mg/kg) Conc. Intraday Interday
Day 1 Day 2 Day 3
Menthol 12 3.8 5 106 (11) 106 (10) 94 (13) 102 (12)
10 97 (8) 89 (10) 88 (7) 91 (10)
50 78 (8) 82 (6) 79 (6) 80 (7)
MES 70 4.3 5 89 (10) 98 (14) 86 (13) 91 (14)
10 88 (15) 95 (9) 88 (9) 90 (12)
50 89 (14) 84 (12) 79 (6) 84 (12)
Carvone 26 2.9 5 87 (16) 91 (6) 87 (12) 89 (12)
10 83 (10) 76 (9) 76 (18) 87 (12)
50 89 (9) 82 (10) 81 (6) 84 (10)
Thymol 34 2.7 5 99 (10) 103 (11) 100 (14) 100 (12)
10 87 (7) 99 (7) 99 (9) 95 (11)
50 81 (10) 92 (12) 95 (6) 89 (12)
Carvacrol 38 4.8 5 97 (9) 82 (11) 94 (14) 91 (14)
10 88 (9) 91 (14) 112 (4) 100 (13)
50 81 (11) 89 (14) 100 (8) 90 (14)
Table 3. Concentration of phytogenic compounds in real samples in mg/kg.
Conc. Chicken Breast (mg/kg) Menthol Methyl salicylate Carvone Thymol Carvacrol
Sample 1 15,9 104,2 57,1 3,8 6,3
Sample 2 4,9 53,8 18,8 4,2 6,8
Sample 3 7,6 43,8 22,1 6,4 9,0
Sample 4 9,4 90,1 33,8 6,1 8,7
Sample 5 12,6 47,7 24,3 7,5 10,1
Sample 6 5,0 28,0 9,9 6,6 9,2
Sample 7 7,1 81,1 31,0 7,3 9,9
Sample 8 18,8 74,6 36,0 6,1 8,7
Sample 9 15,9 98,8 61,9 7,8 10,5
Sample 10 10,2 100,7 45,8 3,6 6,1
JOURNAL OF FOOD AND DRUG ANALYSIS 2021;29:255e261 259
ORIGINAL ARTICLE

3.3.5. LOQ
Instrumental LOQ were determined by injecting
10 times the lowest matrix matched standard at
4 mg/kg, the instrumental LOQs were always below
the lowest calibration level and are reported in
Table 2.
The SANTE 2019 criteria were fulfilled at the
lowest spike level of 5 mg/kg and so the reporting
level for the method was set at this concentration.
3.3.6. Recoveries and precision
The recoveries and the associated accuracy and
precision values were within the acceptable in-
terval of the SANTE 2019 criteria for all the three
spike levels and both for intraday and interday
measurements. The overall results are shown in
Table 2.
3.4. Application to real samples
The validated method was applied to 10 samples
of chicken breast bought in the local market. As
shown in table 3 all the substa nces of interest were
detected in the samples in amounts above the LOQ
and with a quite variable range among the different
samples, from just above the LOQ for thymol to
104 mg/kg for methyl salicylate.
From a consumer safety point of view, all the
analyzed substances have been assess ed and
currently authorized for food [20] and feed [21] uses.
For menthol and carvone an acceptable daily intake
(ADI) is established, and it is equal to 4 mg/kg body
weight for menthol [22] and 60 mg/kg body weight
for carvone [23]. Even taking the highest concen-
trations found in the real samples the chronic
exposure is more than 5 orders of magnitude below
the aforementioned ADIs.
4. Conclusions
A simple analytical method involving a QuECh-
ERS extraction followed by a dSPE cleanup step
coupled with GC-MS/MS determination was
demonstrated to be suitable for the quantificat ion of
phytochemical residues in chicken breast, a com-
plex food matrix. The method has been validate d
according to the SANTE 2019 criteria for accuracy
and precision. It was applied to real chicken breast
samples and revealed the presence of the com-
pounds of interest in all of them, however well
below any level of concern for the consumer safety.
This is possibly an indication of the widespread use
of phytogenic feed additives after the ban of anti-
biotics as growth promoters by the European Union
in 2006.
References
[1] Dibner JJ, Richards JD. Antibiotic growth promoters in
agriculture: history and mode of action. Poult Sci 2005;84:
634e43. https://doi.org/10.1093/ps/84.4.634.
[2] Landers TF, Cohen B, Wittum TE, Larson EL. A review of
antibiotic use in food animals: perspective, policy, and Po-
tential. Public Health Rep 2012;127:4e22.
[3] Castanon JIR. History of the use of antibiotic as growth
promoters in European poultry feeds. Poult Sci 2007;86:
2466e71. https://doi.org/10.3382/ps.2007-00249.
[4] Sebola NA, Mlambo V, Mokoboki HK, Hugo A, Muchenje V.
Comparison of meat quality parameters in three chicken
strains fed Moringa oleifera leaf meal-based diets. Journal of
J Appl Poult Res 2018;27:332e40. https://doi.org/10.3382/
japr/pfy001.
[5] Yang C, Chowdhury MAK, Huo Y, Gong J. Phytogenic
compounds as alternatives to in-feed antibiotics: potentials
and challenges in application. Pathogens 2015;4:137e56.
https://doi.org/10.3390/pathogens4010137.
[6] Adaszy
nska-Skwirzy
nska M, Szczerbi
nska D. Use of essen-
tial oils in broiler chicken production e a review. Ann Anim
Sci 2017;17:317e35. https://doi.org/10.1515/aoas-2016-0046.
[7] Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos M de
L, Bories G, et al. Guidance on the assessment of the safety of
feed additives for the consumer. EFSA J 2017;15:e05022.
https://doi.org/10.2903/j.efsa.2017.5022.
[8] Wilkowska A, Biziuk M. Determination of pesticide residues in
food matrices using the QuEChERS methodology. Food Chem
2011;125:80 3e12. https://doi.org/10.1016/j.foodchem.2010.09.094.
[9] Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ. Fast
and easy multiresidue method employing acetonitrile
extraction/partitioning and “dispersive solid-phase extrac-
tion” for the determination of pesticide residues in produce.
J AOAC Int 2003;86:412e31.
[10] Lehotay SJ, Son KA, Kwon H, Koesukwiwat U, Fu W,
Mastovska K, et al. Comparison of QuEChERS sample
preparation methods for the analysis of pesticide residues in
fruits and vegetables. J Chromatogr A 2010;1217:2548e60.
https://doi.org/10.1016/j.chroma.2010.01.044.
[11] Rejczak T, Tuzimski T. A review of recent developments and
trends in the QuEChERS sample preparation approach.
Open Chem 2015;13:980e1010. https://doi.org/10.1515/chem-
2015-0109.
[12] Perestrelo R, Silva P, Porto-Figueira P, Pereira JAM, Silva C,
Medina S, et al. QuEChERS - Fundamentals, relevant im-
provements, applications and future trends. Anal Chim Acta
2019;1070:1e28. https://doi.org/10.1016/j.aca.2019.02.036.
[13] Wo
zniak MK, Banaszkiewicz L, Wiergowski M, Tomczak E,
Kata M, Szpiech B, et al. Development and validation of a
GCeMS/MS method for the determination of 11 amphetamines
and 34 synthetic cathinones in whole blood. Forensic Toxicol
2020;38:42e58. https://doi.org/10 .100 7/s1141 9-019-0 0485- y.
[14] Analytical quality control and method validation procedures
for pesticide residues and analysis in food and feed. Docu-
ment Nº
SANTE/12682/2019. Available at, https://www.eurl-
pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_
2019_12682.pdf. [Accessed 7 January 2020].
[15] Rajski Ł, Lozano A, Ucl
es A, Ferrer C, Fern
andez-Alba AR.
Determination of pesticide residues in high oil vegetal
commodities by using various multi-residue methods and
clean-ups followed by liquid chromatography tandem mass
spectrometry. J Chromatogr A 2013;1304:109e20. https://
doi.org/10.1016/j.chroma.2013.06.070.
[16] Signal, Noise and Detection Limits in Mass Spectrometry.
Agilent technologies Technical Note 5990-7651EN. Available
at https://www.agilent.com/cs/library/technicaloverviews/
public/5990-7651EN.pdf. [Accessed 7 January 2020].
[17] Walorczyk S. Validation and use of a QuEChERS-based gas
chromatographicetandem mass spectrometric method for
multiresidue pesticide analysis in blackcurrants including
studies of matrix effects and estimation of measurement
260 JOURNAL OF FOOD AND DRUG ANALYSIS 2021;29:255e261
ORIGINAL ARTICLE

uncertainty. Talanta 2014;120:106e13. https://doi.org/
10.1016/j.talanta.2013.11.087.
[18] Blanks in Method Validation Supplement to Eurachem
Guide The Fitness for Purpose of Analytical Methods. Eur-
achem; 2019. Available at, MV_Guide_Blanks_supplement_
EN.pdf. [Accessed 7 January 2020].
[19] Poole CF. Matrix-induced response enhancement in pesticide
residue analysis by gas chromatography. J Chromatogr A 2007;
1158:241e50. https://doi.org/10.1016/j.chroma.2007.01.018.
[20] Commissi on Implementing Regulation (EU) No 872/2012
of 1 October 2012 adopting the list of flavouring substances
provided for by Regulation (EC) No 2232/96 of the Euro-
pean Parliament and of the C ouncil, intr oducing it in
Annex I to Regulation (EC) No 1334/2008 of the European
Parliament and of the Council and repeali ng Commission
Regulation (EC) No 1565/2000 and Commission Decision
1999/217/EC. OJ L 267, 2.10. 2012. p. 1 . [Accessed 7 January
2021].
[21] European Union Register of Feed Additives pursuant to
Regulation (EC) No 1831/2003. Available online: https://ec.
europa.eu/food/sites/food/files/safety/docs/animal-feed-eu-
reg-comm_register_feed_additives_1831-03.pdf. [Accessed 7
January 2021].
[22] Joint FAO/WHO Expert Committee on Food Additives.
Meeting. (57th : 2001: Rome I. Safety evaluation of certain
food additives and contaminants. World Health Organiza-
tion; 2002.
[23] Scientific Opinion on the safety assessment of carvone,
considering all sources of exposure. EFSA J 2014;12:3806.
https://doi.org/10.2903/j.efsa.2014.3806.
JOURNAL OF FOOD AND DRUG ANALYSIS 2021;29:255e261 261
ORIGINAL ARTICLE
