
3/08/2022 PROMIS – Sexual Function and Satisfaction v2.0 Page 1
SEXUAL FUNCTION AND SATISFACTION
MEASURE DIFFERENCES
A brief guide to differences between the PROMIS
®
Sexual Function and Satisfaction instruments:
ADULT
PROMIS Pool v2.0 – Sexual Function and Satisfaction: Anal Discomfort with Sexual Activity
PROMIS Pool v2.0 – Sexual Function and Satisfaction: Bother Regarding Sexual Function (Female)
PROMIS Pool v2.0 – Sexual Function and Satisfaction: Bother Regarding Sexual Function (Male)
PROMIS Pool v2.0 – Sexual Function and Satisfaction: Factors Interfering with Sexual Satisfaction
PROMIS Pool v2.0 – Sexual Function and Satisfaction: Screeners
PROMIS Pool v2.0 – Sexual Function and Satisfaction: Sexual Activities (Female)
PROMIS Pool v2.0 – Sexual Function and Satisfaction: Sexual Activities (Male)
PROMIS Pool v2.0 – Sexual Function and Satisfaction: Therapeutic Aids for Sexual Activity (Female)
PROMIS Pool v2.0 – Sexual Function and Satisfaction: Therapeutic Aids for Sexual Activity (Male)
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Erectile Function
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Interest in Sexual Activity
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Oral Discomfort with Sexual Activity
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Oral Dryness with Sexual Activity
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Orgasm – Ability
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Orgasm – Pleasure
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Satisfaction with Sex Life
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Vaginal Discomfort with Sexual Activity
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Vaginal Lubrication for Sexual Activity
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Vulvar Discomfort with Sexual Activity - Clitoral
PROMIS Scale v2.0 – Sexual Function and Satisfaction: Vulvar Discomfort with Sexual Activity - Labial
PROMIS Sexual Function and Satisfaction v2.0 Brief Profile (Female)
PROMIS Sexual Function and Satisfaction v2.0 Brief Profile (Male)
PROMIS Sexual Function and Satisfaction v2.0 Full Profile (Female)
PROMIS Sexual Function and Satisfaction v2.0 Full Profile (Male)
PROMIS Bank v1.0 – Sexual Function and Satisfaction: Global Satisfaction with Sex Life*
PROMIS Bank v1.0 – Sexual Function and Satisfaction: Interest in Sexual Activity*
PROMIS Bank v1.0 – Sexual Function and Satisfaction: Lubrication*
PROMIS Bank v1.0 – Sexual Function and Satisfaction: Vaginal Discomfort*
PROMIS Bank v1.0 – Sexual Function and Satisfaction: Erectile Function*
PROMIS Pool v1.0 – Sexual Function and Satisfaction: Orgasm*
PROMIS Pool v1.0 – Sexual Function and Satisfaction: Interfering Factors*
PROMIS Pool v1.0 – Sexual Function and Satisfaction: Therapeutic Aids*
PROMIS Pool v1.0 – Sexual Function and Satisfaction: Sexual Activities*
PROMIS Pool v1.0 – Sexual Function and Satisfaction: Anal Discomfort*
PROMIS Pool v1.0 – Sexual Function and Satisfaction: Screener Items*
PROMIS Sexual Function Profile v1.0 (Female)*
PROMIS Sexual Function Profile v1.0 (Male)*
PROMIS Sexual Function Profile v1.0 (Male and Female)*
*Retired measure
INTRODUCTION
This document describes resources for the multiple versions of PROMIS l Function and Satisfaction instruments.
It is divided into two sections: the first section is for v2.0 instruments and the second section is for v1.0
instruments.

3/08/2022 PROMIS – Sexual Function and Satisfaction v2.0 Page 2
SECTION 1: SEXUAL FUNCTION AND SATISFACTION (SexFS)
VERSION 2.0
This section provides information on the PROMIS® SexFS v2.0 domains (Table 1) and profile measures (Table 2).
Table 1. PROMIS SexFS v2.0 Domains
# of
items
Calibrated or
Uncalibrated
a
Scored
b
Requires Activity
Screener?
c
Satisfaction with Sex Life
5
Calibrated
Scored
Yes
Vaginal Lubrication for Sexual Activity
6
Calibrated
Scored
Yes
Vaginal Discomfort with Sexual Activity
11
Calibrated
Scored
Yes
Erectile Function
11
Calibrated
Scored
Yes
Vulvar Discomfort with Sexual Activity – Labial
4
Calibrated
Scored
Yes
Vulvar Discomfort with Sexual Activity – Clitoral
4
Calibrated
Scored
Yes
Oral Discomfort with Sexual Activity
6
Calibrated
Scored
Yes
Oral Dryness with Sexual Activity
3
Calibrated
Scored
Yes
Orgasm – Pleasure
3
Calibrated
Scored
Yes
Orgasm – Ability
1
Uncalibrated
Scored
Yes
Interest in Sexual Activity
2
Uncalibrated
Scored
No
Bother Regarding Sexual Function
11
Uncalibrated
Unscored
No
Factors Interfering with Sexual Satisfaction
35
Uncalibrated
Unscored
No
Therapeutic Aids for Sexual Activity
7
Uncalibrated
Unscored
No
Sexual Activities
15
Uncalibrated
Unscored
No
Anal Discomfort with Sexual Activity
6
Uncalibrated
Scored
Yes
Sexual Function Screener Items
5
Screener
Unscored
No
a
Refers to whether the domain consists of IRT-calibrated items or not.
b
Indicates whether T-scores centered
around U.S. population means are available.
c
Indicates whether domain is only intended for sexual active adults.

3/08/2022 PROMIS – Sexual Function and Satisfaction v2.0 Page 3
INTRODUCTORY VIDEO
An introductory video about the PROMIS SexFS v2.0 can be found here:
https://www.youtube.com/watch?v=CQwo_2GIaJA
SEXUAL FUNCTION AND SATISFACTION DOMAINS
The PROMIS SexFS v2.0 measures a range of sexual activities, symptoms, functioning, and evaluation of
experiences over the past 30 days. Also included are general screener items that ask about sexual activity in the
past 30 days and reasons for not having sexual activity. The SexFS v2.0 measures are universal rather than
disease-specific. The SexFS v2.0 includes the following domains:
Screener Items ask about sexual activity in the past 30 days and reasons for not having sexual activity.
Interest in Sexual Activity assesses a conscious awareness of wanting to engage in sexual activity during the
past 30 days. Items are gender-nonspecific. Higher scores indicate greater interest.
Satisfaction with Sex Life assesses how satisfying and pleasurable the person regards his or her sexual life in
the past 30 days with no limitation on how the person defines “sex life.” Items are gender-nonspecific. Higher
scores indicate more satisfying sexual activities.
Orgasm – Ability assesses the ease with which a person has been able to have an orgasm over the past 30 days.
It is measured with a single, gender-nonspecific item for which higher scores indicate a greater ability to have
an orgasm.
Table 2. PROMIS SexFS v2.0
Profiles
Full Profile Items (#)
Brief Profile Items (#)
Sexual activity
in past 30 days
No sexual
activity in past
30 days
Sexual activity
in past 30
days
No sexual
activity in past
30 days
Interest in Sexual Activity
2
2
2
2
Sexual Activity Screener
1
1
1
1
Reasons for No Sexual Activity
--
1
--
1
Erectile Function (men)
3
--
2
--
Lubrication (women)
3
--
2
--
Vaginal Discomfort (women)
2
--
2
--
Labial Discomfort (women)
2
--
1
--
Clitoral Discomfort (women)
2
--
1
--
Orgasm Ability
1
--
1
--
Orgasm Pleasure
2
--
1
--
Satisfaction with Sex Life
4
--
2
--
Oral Discomfort
2
--
--
--
Oral Dryness
2
--
--
--
Anal Discomfort
2
--
--
--
Total # of Items (Men)
19
4
9
4
Total # of Domains/scores (Men)
8
1
5
1
Total # of Items (Women)
25
4
13
4
Total # of Domains/scores
(Women)
11
1
8
1
3/08/2022 PROMIS – Sexual Function and Satisfaction v2.0 Page 4
Orgasm – Pleasure assesses how pleasurable or satisfying the person's orgasms have felt in the past 30 days.
Items are gender-nonspecific. Higher scores indicate more pleasurable orgasms.
Erectile Function assesses the frequency and quality of achieving and maintaining an erection for sexual activity
over the past 30 days. Higher scores indicate better function.
Vaginal Discomfort with Sexual Activity assesses physical discomfort of the vagina during and immediately
following sexual activity, including sensations of pain, rubbing, burning, pulling, or ripping experienced over the
past 30 days. Higher scores indicate greater discomfort.
Vulvar Discomfort with Sexual Activity – Clitoral assesses the degree of physical discomfort, including pain, of
the clitoris experienced with sexual activity in the past 30 days. Higher scores indicate greater discomfort.
Vulvar Discomfort with Sexual Activity – Labial assesses the degree of physical discomfort, including pain, of
the labia experienced with sexual activity in the past 30 days. Higher scores indicate greater discomfort.
Vaginal Lubrication for Sexual Activity scale assesses the wetness or dryness of the vagina experienced for
sexual activity over the past 30 days. Higher scores indicate greater lubrication. These items are intended to
assess the lubrication of the vagina without the assistance of personal lubricant products. As a result, it is
recommended that respondents be instructed to select item responses in accordance with their natural vaginal
lubrication only and to disregard the additive effects of personal lubricants.
Anal Discomfort with Sexual Activity assesses physical discomfort, irritation, pain, and/or bleeding around the
anus or rectum during or after sexual activity over the past 30 days. Items are gender-nonspecific.
Oral Discomfort with Sexual Activity assesses the degree of physical discomfort in the mouth, including pain
and/or irritation, experienced with sexual activity in the past 30 days. Items are gender-nonspecific. Higher
scores indicate greater discomfort.
Oral Dryness with Sexual Activity assesses the lack of saliva in the mouth experienced with sexual activity in
the past 30 days. Items are gender-nonspecific. Higher scores indicate greater dryness.
Factors Interfering with Sexual Satisfaction is a collection of items, each of which assesses the person’s
perception of the degree to which various factors affected their satisfaction with sex life in the past 30 days.
These factors include symptoms of disease, side effects from treatment, and other experiences that have been
identified by patients. Some items are gender-nonspecific. These items are intended to be stand-alone items
and do not comprise a unidimensional scale.
Therapeutic Aids for Sexual Activity is a collection of items, each of which assesses the use in the past 30 days
of hormones, personal lubrications, medications, or devices intended to allow for or improve sexual function.
Some items are gender-nonspecific. These items are intended to be stand-alone items and do not comprise a
unidimensional scale.
Sexual Activities is a collection of items, each of which assesses the frequency of engaging in specific
affectionate or sexual behaviors either alone or with a partner in the past 30 days. Some items are gender-
nonspecific. These items are intended to be “stand-alone” items and do not comprise a unidimensional scale.
Bother Regarding Sexual Function is a collection of items, each of which assesses the degree of bother people
report for each specific aspect of sexual functioning in the past 30 days. Some items are gender-nonspecific.
These items are intended to be “stand-alone” items and do not comprise a unidimensional scale.
INTRODUCTION TO ASSESSMENT OPTIONS
All items in the PROMIS SexFS were not intended to be administered together, as some domains might not be
relevant for particular situations and reliable scores can be generated without having to administer all of the
items in a domain. Researchers should select the sexual function and satisfaction domains and items that are

3/08/2022 PROMIS – Sexual Function and Satisfaction v2.0 Page 5
relevant to their specific sample. There are multiple assessment options: the full profile, brief profile, and
customized short forms of any individual domain (that is, a selection of items from the desired domain). When
administering any of these options, instruct participants to answer all of the items (i.e., questions or statements)
presented. Tables 1 and 2 show how many items are in each individual domain and profile. Any of these options
can be administered on paper or by computer.
Whether one uses a customized short form or profile, for those domains marked “calibrated,” the score metric
is derived from Item Response Theory (IRT), a family of statistical models that link individual questions to a
presumed underlying trait or concept within the overall domain of sexual function, represented by items in the
item banks. When choosing among the options available, it is useful to consider the precision gained with longer
instruments versus the psychological, physical, and cognitive burden placed on respondents as a result of the
number of questions asked.
INSTRUMENT DIFFERENCES
Tables 1 and 2 provide a helpful overview of the different instruments and assessment options. To summarize
the tables, of the PROMIS SexFS instruments, 9 are calibrated scales. This means that if one or more items from
within that instrument are administered, a respondent’s score will be calculated using item response theory
statistics. Three of the instruments are scored but not calibrated. The items within those instruments are
combined to create a score, but this score is not based on item response theory statistics. Five of the
instruments do not have calibrated items and are not scored, that is, items within those instruments are not
combined in any way to create a score. Each item in these instruments measures a very specific construct
corresponding only to that item (e.g., how much radiation burns have affected one’s satisfaction with their sex
life). For any given item in these uncalibrated instruments, the researcher can use the raw item responses
directly for analyses.
Profiles
Profiles are of collections of short forms and items that assess a person with respect to multiple aspects of
sexual function and satisfaction. The SexFS currently includes Brief Profiles and Full Profiles (see below). Items
were selected for inclusion based on rankings using psychometric and pragmatic criteria: (1) maximum interval
information (where applicable, i.e., IRT information curves reflected the greatest degree of precision for the
greatest range of the latent variable of interest), (2) consistent item formatting, and (3) content coverage.
Brief Profiles (Male, Female)
The Brief Profiles—one for males, one for females—efficiently assess interest in sexual activity followed by a
screener item about sexual activity. For those who have not been sexually active in the past 30 days, a fourth
and final question asks about reasons for not having sexual activity. For those who have been sexually active in
the past 30 days, men are asked about erectile function, while women are asked about vaginal lubrication,
vaginal discomfort, and vulvar discomfort; men and women are asked about orgasm and satisfaction with sex
life. These are the domains that are most likely relevant for the majority of healthy people and those suffering
from chronic diseases.
Full Profiles (Male, Female)
The Full Profiles—one for males, one for females—includes all of the domains measured in the Brief Profiles, but
also adds oral dryness, oral discomfort, and anal discomfort. For the domains that overlap between the Brief and
Full Profiles, the Full Profiles use equal or greater number of items to assess each domain.

3/08/2022 PROMIS – Sexual Function and Satisfaction v2.0 Page 6
Customized Short Forms
Within any of the 9 calibrated scales, users can select one or more items to create a customized short form for
measuring that domain. Selection of the items could be based on suitability of the item for the particular
population of interest. Note that the items that generally perform the best from the 9 calibrated scales are
found on the Brief Profiles and Full Profiles (see above).
In selecting among assessment options, the differences are domain coverage and instrument length. If you are
working with a sample in which you want the most precise measure, use a longer short form. If you have little
room for additional measures but really wanted to capture something as a secondary outcome, use a shorter
instrument (e.g., brief profile or just 1 or 2 domains of interest).
PREVIEW OF SAMPLE ITEM
Below is an excerpt from the paper version of the Brief Profile (Female). This is the paper version format used
for all profile instruments. It is important to note that automatic scoring is not available for paper
administration.
VERSION DIFFERENCES
Some PROMIS domains have multiple versions of instruments (i.e., v1.0, v1.1, v2.0). Generally, it is
recommended that you use the most recent version available which can be identified as the instrument with
the highest version number.
Building on v1.0 of the PROMIS Sexual Function and Satisfaction measure (SexFS), a comprehensive
development process was undertaken to create an expanded and improved PROMIS SexFS v2.0. This tool
assesses multiple components of sexual functioning, and the validation process included a strong focus on
inclusiveness with regard to literacy level, race, age, sexual orientation, and health conditions. Note that while
v1.0 was developed exclusively in cancer patients, v2.0 retains the content validity for cancer patients and
expands on it, making v2.0 a better measure for cancer populations than v1.0. Scores from v1.0 measures
should not be compared to scores from v2.0 measures. Those who have administered items from the SexFS v1.0
and wish to use v2.0 scoring should contact the developers for more information.
Several features of SexFS v2.0 are consistent with v1.0. The measurement system is modular and customizable
in that users need only measure those sexual function domains of relevance to their particular study or sample.
One important change from v1.0 to 2.0 is that scores are now expressed using a meaningful metric, with scores
centered around norms for the population of sexually active US adults. Norms are provided herein for age and
sex to aid in the interpretation of SexFS scores. Also, differential item functioning (DIF) was examined by gender,
3/08/2022 PROMIS – Sexual Function and Satisfaction v2.0 Page 7
sexual activity, and age to assess the appropriateness of items across different groups of people. Finally, the v2.0
domains demonstrated good convergent and known groups validity and reliability. The final set of items is
applicable for both men and women, those sexually active with a partner and without, and those who identify as
heterosexual or straight, lesbian, gay, or bisexual.

9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 8
SECTION 2: THE PROMIS SEXUAL FUNCTION AND SATISFACTION
MEASURES V1.0
INTRODUCTION
Through the PROMIS Cancer Supplement, instruments assessing multiple components of sexual functioning
were developed. Together, these instruments are known as the PROMIS Sexual Function and satisfaction
measures v1.0 (PROMIS SexFS). Some instruments are gender specific. Most items are not specific to cancer, but
had only been validated in cancer populations at the time of their publication. The PROMIS SexFS uses a 30-day
recall period. Where possible, items use response options common to other PROMIS instruments. Some PROMIS
SexFS instruments include items from other sexual function instruments, such as the Female Sexual Function
Index and the UCLA Prostate Cancer Index. Table 3 includes a list of the available PROMIS SexFS v1.0 domains.
V1.0 DOMAIN DEFINITIONS
Global Satisfaction with Sex Life is the person’s overall evaluation of his or her sex life. No limitation is placed
on what the person includes in his or her definition of “sex life.” Higher scores indicate more satisfaction with
sex life. Lower scores indicate less satisfaction with sex life.
Interest in Sexual Activity refers to a conscious awareness of wanting to engage in sexual activity. Items are
gender-neutral. Higher scores indicate more interest. Lower scores indicate less interest.
Lubrication refers to the wetness or dryness of the vagina during sexual activity. Higher scores indicate more
lubrication. Lower scores indicate less lubrication. These items are intended to assess the lubrication of the
vagina without the assistance of personal lubricant products. As a result, it is recommended that respondents be
instructed to select item responses in accordance with their natural vaginal lubrication only and to disregard the
additive effects of personal lubricants.
Table 3: PROMIS Sexual Function and Satisfaction v1.0 Instruments

9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 9
Vaginal Discomfort refers to the degree of physical discomfort of the vagina during and immediately following
sexual activity. Higher scores indicate more discomfort as reflected by pain and/or uncomfortable tightness.
Lower scores indicate less discomfort as indicated by no pain, bleeding, and/or uncomfortable tightness.
Erectile Function refers to the ability to achieve and maintain an erection for sexual activity. Higher scores
indicate better function. Lower scores indicate poorer function.
Orgasm assesses the degree to which the person has experienced a satisfying climax. It includes a gender-
neutral item for which higher scores indicate a greater ability to have satisfying orgasms, and lower scores
indicate less ability. It also includes male-specific items that ask about timing of ejaculation and pain or burning
during or after ejaculation. These can be administered and scored as single items.
Interfering Factors is a collection of items each of which assesses the person’s perception of the degree to which
various factors affect satisfaction with sex life. These factors include symptoms of disease and side effects from
treatment and other issues that have been identified by patients. These items are intended to be “stand alone”
items and do not comprise a unidimensional scale. Some items are gender-specific.
Therapeutic Aids is a collection of items each of which assesses the use of hormones, personal lubrications,
medications, or devices intended to allow for or improve sexual function. These items are intended to be “stand
alone” items and do not comprise a unidimensional scale.
Sexual Activities is a collection of items each of which assesses the frequency of engaging in specific intimate or
sexual behaviors either alone or with a partner. These items are intended to be “stand alone” items and do not
comprise a unidimensional scale. Some items are gender-specific.
Anal Discomfort is an evaluation of anal irritation, pain, or bleeding during or after anal sex. Items are only
asked of people who indicate in the activities subdomain they have had anal sex in the past 30 days. There have
not been enough data collected to do psychometric evaluation of these items.
Sexual Function Screener Items ask about sex (gender), whether people are in a relationship that could involve
sexual activity, and whether they have had any type of sexual activity with a partner in the past 30 days.
INTRODUCTION TO ASSESSMENT OPTIONS
PROMIS has 11 sexual function and satisfaction v1.0 instruments (see Table 3). All items in the PROMIS SexFS
were not intended to be administered together, as some domains might not be relevant for particular situations
and reliable scores can be generated without having to administer all of the items in a domain. Researchers
should select the sexual function and satisfaction domains and items that are relevant to their specific sample.
There are multiple assessment options: the brief profile and customized short forms of any individual domain
(that is, a selection of items from the desired domain). When administering any of these options, instruct
participants to answer all of the items (i.e., questions or statements) presented. Table 3 shows how many items
are in each individual domain. Either option can be administered on paper or by computer.
Whether one uses a customized short form or profile, for those domains marked “calibrated,” the score metric
is derived from Item Response Theory (IRT), a family of statistical models that link individual questions to a
presumed underlying trait or concept within the overall domain of sexual function, represented by items in the
item banks. When choosing among the options available, it is useful to consider the precision gained with longer
9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 10
instruments versus the psychological, physical, and cognitive burden placed on respondents as a result of the
number of questions asked.
INSTRUMENT DIFFERENCES
Five of the SexFS v1.0 instruments are calibrated item banks (e.g. PROMIS Bank v1.0 - Global Satisfaction w Sex
Life). This means that if one or more items from within that instrument are administered, a respondent’s score
will be calculated using item response theory statistics. If these instruments are administered outside of
electronic scoring you may rely on raw score/scale score look-up tables to determine scores (see PROMIS Sexual
Function and Satisfaction Scoring Manual).
Six of the instruments do not have calibrated items (e.g. PROMIS Pool v1.0 - Sexual Activities). This means that
items within those instruments are not combined in any way to create a score. Each item in these instruments
measures a very specific construct corresponding only to that item (e.g., how much radiation burns have
affected one’s satisfaction with their sex life). For any given item in these uncalibrated instruments, the
researcher can use the raw item responses directly for analyses.
PROMIS Sexual Function and Satisfaction Brief Profile v1.0
The PROMIS Sexual Function and Satisfaction Measures Brief Profile (PSxFBP) provides scores on 7 different
subdomains of sexual function: Interest in Sexual Activity, Vaginal Discomfort (women only), Lubrication
(women only), Erectile Function (men only), Orgasm, and Global Satisfaction with Sex Life (see below for
subdomain definitions). The PSxFBP is intended for broad use, although almost all of the development work was
with cancer populations. The PSxFBP is available for men and women and consists of the best items selected
from each subdomain for general purposes. Each question asks respondents to report on their experiences over
the past 30 days.
Customized Short Forms
All items in the PROMIS SexFS were not intended to be administered together. Researchers should select the
sexual function and satisfaction items that are relevant to their specific sample. Some examples are provided.
Example 1: A study proposes to compare three treatment approaches for early stage cervical cancer:
surgery alone, surgery and radiation, and radiation alone. In addition to disease control, cancer treatment
comorbidities are being compared, including sexual function outcomes. The researchers want to measure
key domains of function, including overall sexual satisfaction, interest, vaginal irritation or pain, orgasm, and
lubrication. They are also interested in which side effects from treatments affect participants’ sex lives, as
each of the treatment modalities carries different potential changes in sexual function; surgery usually
results in a foreshortened vaginal canal and radiation may cause vaginal mucosal thinning, vaginal
adhesions, decreased lubrication and vaginal stenosis. The 10-item PSxFBP for women can be used to assess
sexual function broadly and distinguish between sexual side effects associated with treatment modality, and
can be used to help patients make informed treatment decisions. Additional items on surgical scars, pain,
and fatigue from the Interfering Factors instrument can help the researchers determine which side effects
affect satisfaction with sex life for their participants. Finally, the researchers include the items for women
that assess use of Therapeutic Aids to determine whether using personal lubricants or hormones modifies
sexual satisfaction or function.
Example 2: A study designed to promote compliance with SSRI antidepressants proposes to assess whether
sexual function contributes to non-compliance. Patients prescribed fluoxetine are longitudinally followed
with monthly assessments of sexual function and frequency of sexual activities in order to determine the
relationship between sexual dysfunction and non-compliance. The researchers have room for about 20
9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 11
items on sexual function, so they choose to use the PSxFBP for men (8-items) and women (10-items) to
gauge function plus the 12 items from the Sexual Activities subdomain. Thus, for all participants in the
study, sexual activities, interest in sexual activity, orgasm, and global satisfaction with sex life are assessed.
For women, lubrication and vaginal discomfort are also assessed, and for men, erectile function is also
assessed.
Example 3: A study of soy-derived estrogen is tested to determine if it improves sexual function among
menopausal women self-identified as having hyposexual desire. The researchers choose to administer all
items from the Interest in Sexual Activity instrument, since sexual desire is their main outcome of interest.
They also administer the PSxFBP for women to assess satisfaction with sex life, lubrication, vaginal
discomfort, and orgasm.
A Note on Response Options for Sexual Activities
Most sexual activity items are available using two different sets of responses. Items identified with an “a” in
their Item ID use the response options 1=Have not done in the past 30 days, 2=Once, 3=Two to three times,
4=Four to five times, 5=Six or more times. Items identified with a “b” in their Item ID use the response options
1=Have not done in the past 30 days, 2=Once a week or less, 3=Once every few days, 4=Once a day, 5=More
than once a day. As you can see, the “a” response options reflect less activity. This set of response options is
likely most appropriate for individuals for whom you expect reduced sexual activity (e.g., cancer patients
receiving chemotherapy). The “b” response options reflect higher levels of sexual activity. This set of response
options would be most appropriate for individuals you expect to have higher levels of sexual activity (e.g.,
healthy individuals). Investigators should carefully consider their purpose in recording sexual activities and select
response options that are most appropriate. It is possible that the “a” and “b” options available here are not the
best for a particular research setting. Investigators might also consider whether a daily sexual activity log could
be used in place of these items, which require a 30-day recall period.
PROCEDURES AND DATA IN SUPPORT OF VALIDITY AND RELIABILITY FOR SEXFS
V1.0
Face Validity. Face validity is established when subject matter experts agree that the scale appears to measure
its intended focus. Face validity for the PROMIS SexFS scales was established with a review by expert panels
within and external to the PROMIS SexFS committee; all experts concurred that the items within the scales
appeared to measure sex function.
Content Validity. Content validity refers to how well the scale assesses all aspects of the construct being
measured. Establishing the content validity of PROMIS instruments began with patient input to assure that the
subdomains and their items corresponded to reported patient experiences, and with a review by expert panels
to assure that the selected theoretical constructs corresponded to the scientific literature. Using a consensus-
driven approach, the PROMIS SexFS committee conducted a literature search for articles published from 1991
through 2007, yielding 257 articles that reported the administration of a psychometrically evaluated sexual
function measure to individuals diagnosed with cancer. With few exceptions, the 31 identified measures had not
been widely tested in cancer populations (Jeffery et al., 2009). We collated available items from the measures
and created preliminary domain definitions. Each item was then subjected to detailed review to eliminate
repetition within bins (“winnowing”) and to develop uniform recall periods and response categories. After
qualitative expert item review, 47 extant items were selected for further testing. Concurrently, we conducted 16
focus groups with 109 cancer patients (Flynn et al., 2010). These groups explored the impact of cancer and its

9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 12
treatments on sexual experience to determine whether domain definitions and the identified items reflected
patients’ personal experiences. Separate focus groups were held for patients in active treatment for breast,
prostate, lung, colorectal, gynecological, and other (mixed) cancers and for survivors after treatment for breast,
prostate, gynecological, and other cancer types. We developed a matrix of themes and groups, which was
double-coded (inter-rater reliability was over 90%). As a check on the data we received from the patient focus
groups, we conducted 2 clinician focus groups to assess the clinical relevance of the proposed conceptual model
and obtain clinicians’ views of how cancer and its treatment affected patients’ sexual health. New items were
created to address conceptual gaps identified by the focus group participants. With updated items in hand, we
conducted cognitive interviews with patients (n=39) (Fortune-Greeley et al., 2009). Each item was seen by at
least 5 patients, at least 1 of whom was not white and at least 2 of whom had less than a 9
th
grade reading level.
87 items were passed through to the next phase. We convened 7 experts on sexual function and cancer to
review this work to date.
The item-testing phase consisted of large-scale data collection (n=819; 388 males, 430 females, 1 person did not
specify sex) and administration of the items in national and local samples through the NexCura Internet Panel,
the Duke University tumor registry, and the Duke oncology clinics. (Appendix A shows patient characteristics,
including the distribution of cancer types.) We also added targeted recruitment of additional lesbian, gay, and
bisexual cancer patients and survivors through online communities. Psychometric analysis of the items followed
established PROMIS methodology (Reeve et al., 2007) and resulted in 11 instruments: 5 calibrated and 6
uncalibrated. A summary of fit statistics are shown in Table 4.
Construct Validity. Construct validity refers to how well scores on the measure are related to other variables
that, for theoretical reasons, ought to be related to the measure in question. Construct validity of the PROMIS
SexFS has been assessed in two ways.
First, we used data from the 819 patients with cancer (see above) to examine the correlations between
subdomains of the PROMIS SexFS and other measures of similar constructs. These are displayed in Table 5. In
general, these correlations provide strong evidence for the construct validity of the PROMIS SexFS.
Table 4: Fit Indices for Confirmatory Factor Analysis of Calibrated Domains (v1.0).

9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 13
Second, we examined whether scores on selected subdomains of the PROMIS SexFS could discriminate between
groups that should, in theory, differ in terms of their sexual experiences. During item testing, participants were
also asked whether they had ever asked an oncology professional about sexual problems. We hypothesized that
asking for help with sexual problems may indicate a clinically meaningful decrement in function. As Table 6
shows, those who had asked for help had significantly greater interest in sexual activity and increased vaginal
discomfort and lower levels of erectile function, lubrication, orgasm, and overall satisfaction. Furthermore, the
differences were as high as three-quarters of a standard deviation. These effect sizes were greater than or equal
to the effects for the corresponding subscales of the FSFI and IIEF. In three cases, the PROMIS SexFS and PSxFBP
detected statistically significant (p<.05) differences between those who did and did not ask, whereas the FSFI or
IIEF did not.
Reliability. Two types of reliability data are available at this time for the PROMIS SexFS. First, estimates of
internal consistency (Cronbach’s alpha) were computed for all calibrated banks. They are displayed in Table 7.
Table 5: Correlations between PROMIS Sexual Function and Satisfaction v1.0 Domains and Corresponding Measures.

9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 14
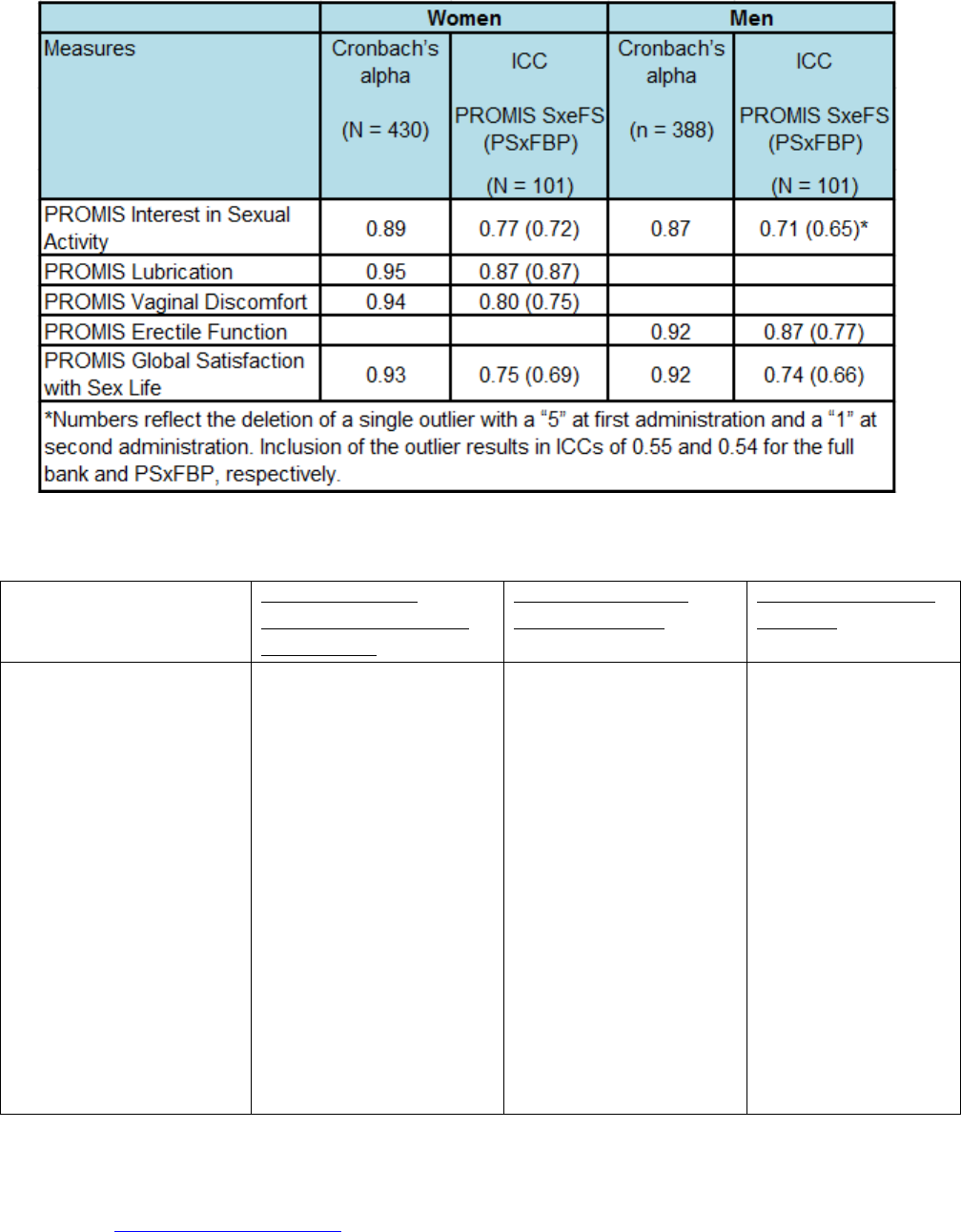
All indicate excellent internal consistency. Second, test-retest reliability was examined in a sample of 202
participants (101 male, 101 female), about half of whom had some chronic disease. Participants completed the
PROMIS SexFS twice with one month between test administrations. Intraclass correlation coefficients between
the two administrations are shown in Table 7, ranging from .71 - .87.
Table 6: Effect Sizes Discriminating Askers From Non-Askers
a
(N=806); v1.0).
9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 15
The following experts generously provided helpful input to the PROMIS sexual function and satisfaction domain
working group:
Sexual Function and
Satisfaction Domain Group
(including NIH)
Duke Clinical Research
Institute CCGE staff
Duke University School
of Nursing
Erick Janssen, PhD, MA
Ray Rosen, PhD
Stacy Tessler Lindau, MD,
MAPP
Jeanne Carter, PhD
Michael Perelman, PhD
Leslie R. Schover, PhD
John P. Mulhall, MD
David M. Latini, PhD
Barbara L. Andersen, PhD
Sara I. McClelland, PhD
Amy Abernethy, MD
Joan Broderick, PhD
Deborah Bruner, PhD, RN
Jill Cyranowski, PhD
Susan Czajkowski, PhD
Elizabeth Hahn, MA
Diana Jeffery, PhD
Francis Keefe, PhD
Jin-Shei Lai, PhD
Richard Luecht, PhD
Susan Magasi, PhD
Laura Porter, PhD
Jennifer Reese, Phd
Bryce Reeve, PhD
Rebecca Shelby, PhD
Ashley Wilder, Phd, MPH
Carrie Dombeck, MA
Maria Fawzy
Alice Fortune-Greeley
Angel Moore, MSPH
Damon Seils
Janice Tzeng
Lucy Andrzejewski
Teresa Baker
Henry Beresford
Monie Clayton
Teresa Ebel
Linda Folsom
Mindy Kash
Patrick Lane
Diane Langley
Justin Levens
Denise Snyder, MS, RD,
CSO, LDN
Valeda Stull
Megan Williams, MSW,
MSPH
CONTACT US
For more information about PROMIS, accessing the PROMIS Sexual Function instruments or administering them,
contact us at help@healthmeasures.net
.
Table 7: Reliability of Calibrated v1.0 Domains.

9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 16
FREQUENTLY ASKED QUESTIONS (FAQs)
Q: I am interested in learning more. Where can I do that?
Review the HealthMeasures website at www.healthmeasures.net
.
Q: Is the SexFS appropriate for LGBT populations?
Self-identified lesbian, gay, and bisexual individuals were engaged in the development process in focus groups,
cognitive interviews, and item testing, and item wording is generally appropriate regardless of sexual
orientation. However, transgender participants were not a particular focus for the v1.0 or v2.0 measure
validation efforts; additional work will be needed to demonstrate validity for transgender participants.
Q: I’m worried that patients might be too embarrassed or offended to answer some of these questions. What
has been the experience of PROMIS with this measure?
In testing v1.0 of this measure in a large population of patients with cancer, there were no more missing data on
the sexual function measure than other measures on different topics that were being tested simultaneously
(e.g., sleep quality, cognitive function, and illness impact). Since then, v2.0 was tested in additional populations
and settings and missing data have not been a significant problem. Skipped items can be minimized by informing
study participants at the outset that your study will be asking about different domains of health that are
important to understand, including (for example) physical function, sleep, sexual function, and fatigue.
Q: Are these instruments available in other languages?
Yes! Look at the HealthMeasures website (
http://www.healthmeasures.net/explore-measurement-
systems/promis/intro-to-promis/available-translations) or current information on PROMIS translations.
Q: Can I make my own short form?
Yes, custom short forms can be made by selecting any items from an item bank. This can be scored using the
Scoring Service (https://www.assessmentcenter.net/ac_scoringservice
).
REFERENCES
DeWalt, D., Rothrock, N., Yount, S., Stone, A. A., & on behalf of the PROMIS cooperative group. (2007).
Evaluation of item candidates: the PROMIS qualitative item review. Medical Care, 45(5), S12-21.
Fortune-Greeley, A. K., Flynn, K. E., Jeffery, D. D., Williams, M. S., Keefe, F. J., Reeve, B. B., Willis, G. B., &
Weinfurt, K. P. (2009). Using cognitive interviews to evaluate items for measuring sexual functioning across
cancer populations: improvements and remaining challenges. Quality of Life Research, 18(8), 1085-93.
Jeffery, D. D., Tzeng, J. P., Keefe, F.J., Porter, L. S., Hahn, E. A., Flynn, K. E., Reeve, B. B., & Weinfurt, K. P. (2009).
Initial report of the cancer Patient-Reported Outcomes Measurement Information System (PROMIS) sexual
function committee: Review of sexual function measures and domains used in oncology. Cancer, 115(6), 1142-
53.
Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVeillis
R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R. (2010). The
Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of
adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 63(11), 1179-84.
9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 17
Flynn, K. E., Jeffery, D. D., Keefe, F. J., Porter, L. S., Shelby, R. A., Fawzy, M. R., Gosselin, T. K., Reeve, B. B., &
Weinfurt, K. P. (2011). Sexual functioning along the cancer continuum: focus group results from the Patient-
Reported Outcomes Measurement Information System (PROMIS). Psycho-Oncology, 20(4), 378-86.
Flynn K.E., Reese J.B., Jeffery D.D., Abernethy A.P., Lin L., Shelby R.A., Porter L.S., Dombeck C.B., Weinfurt K.P. (in
press). Patient experiences with communication about sex during and after treatment for cancer. Psycho-
Oncology.
Flynn K.E., Jeffery D.D., Reeve B.B., Lin L., Wilder Smith A., Abernethy A.P., Reese J.B., Weinfurt K.P. “Progress on
the PROMIS® Sexual Function Measure.” Poster presented at the International Society for Quality of Life
Research Annual Conference; October 29, 2010; London, England.
Weinfurt, K. "Improving the Measurement of Sexual Outcomes in Cancer: The NIH PROMIS Approach.” Center
for Outcomes and Policy Research, Dana-Farber Cancer Institute, Boston, Massachusetts, May 17, 2011
Flynn K.E., Weinfurt K.P. “Development and Validation of the PROMIS Sexual Function
Measure.” Presentation at the Duke Translational Medicine Institute Annual Research Career Day; May 20, 2011;
Durham, NC, USA.
Flynn K.E. “Assessing Sexual Health in Cancer Survivors.” Presentation at the Cancer Survivorship and Sexual
Health Symposium sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society
of North America; Washington, DC, USA; June 18, 2011.
Weinfurt K.P., Lin L., Broderick J.E., Dombeck C.B., Fawzy, M.R., Snyder D.C., Williams M.S., Flynn K.E. “Validity of
1-month Recall for Components of Sexual Function.” To be presented as a poster at the International Society for
Quality of Life Research Annual Conference; October 28, 2011; Denver, Colorado, USA.
Flynn K.E., Lin L., Dombeck C., Fawzy M., Abernethy A.P., Bruner D.W., Reese J.B., Reeve B.B., Smith A.W.,
Weinfurt K.P. “Validating the PROMIS
®
Sexual Function Brief Profile Measures.” To be presented at the
International Society for Quality of Life Research Annual Conference; October 29, 2011; Denver, Colorado, USA.
Weinfurt K.P., Lin L., Broderick J.E., Dombeck C.B., Fawzy, M.R., Snyder D.C., Williams M.S., Flynn K.E. “Mood and
Gender Effects on the Accuracy of 30-day Recall of Sexual Function.” To be presented at the International
Society for Quality of Life Research Annual Conference; October 29, 2011; Denver, Colorado, USA.

9/28/2021 PROMIS – Sexual Function and Satisfaction v1.0 Page 18
Appendix A. Characteristics of Validation Sample
Characteristic
Total
(N = 819)
Age, mean ± SD, y
58.5 ± 11.8
Age group, No. (%)
≤ 40 years
59 (7)
41 to 50 years
127 (16)
51 to 64 years
377 (46)
65 to 79 years
232 (28)
≥ 80 years
21 (3)
Race, No. (%)
Black or African American
80 (10)
American Indian/Alaska Native
10 (1)
Asian
12 (1)
Native Hawaiian/Other Pacific Islander
10 (1)
White
705 (87)
Multiple races or other
2 (< 1)
Hispanic or Latino ethnicity, No. (%)
21 (3)
Educational attainment, No. (%)
Less than high school
21 (3)
High school graduate/GED
100 (12)
Some college
255(31)
College degree
229 (28)
Advanced degree (MA, PhD, MD)
211 (26)
Treatment status in past month, No. (%)
None (ie, posttreatment follow-up)
526 (64)
Undergoing treatment
290 (36)
Radiation therapy
29 (10)
Hormonal therapy (eg, tamoxifen, anastrozole,
leuprolide)
140 (48)
Chemotherapy (injection or oral)
116 (40)
Immunotherapy (eg, interferon)
9 (3)
Other
36 (12)
Recurrence of cancer, No. (%)
151 (18)
Cancer spread to lymph nodes, No. (%)
202 (25)
Cancer spread to another area, No. (%)
134 (16)
Primary cancer diagnosis, No. (%)
Bone/muscle cancer
14 (2)
Brain cancer
4 (< 1)
Breast cancer
252 (35)
Colorectal
98(13)
Esophageal or stomach cancer
17 (2)
Gynecologic cancer
29 (4)
Head/neck cancer
9 (< 1)
Hodgkin lymphoma
23 (3)
Leukemia
20 (3)
Liver cancer
3 (< 1)
Lung cancer
56 (8)
Melanoma
4 (< 1)
Multiple Myeloma
2 (< 1)
Non-Hodgkin lymphoma
12 (2)
Pancreatic cancer
5 (< 1)
Prostate cancer
146 (20)
Urologic cancer
23 (3)
