
FINAL REPORT:
Understanding Variations in International
Drug Prices
NORC PROJECT NO. 6319 JULY 27, 2006
TASK ORDER # HHSP233000007T / CONTRACT # 100-03-0020
SUBMITTED TO:
OFFICE OF THE ASSISTANT SECRETARY FOR PLANNING AND EVALUATION
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
200 INDEPENDENCE AVENUE, S.W.
WASHINGTON, D.C. 20201
PRESENTED BY:
NATIONAL OPINION RESEARCH CENTER (NORC)
AT THE UNIVERSITY OF CHICAGO
1350 CONNECTICUT AVENUE, N.W., SUITE 500
W
ASHINGTON, D.C. 20036
WITH THE ASSISTANCE OF:
GEORGETOWN UNIVERSITY HEALTH POLICY INSTITUTE
AND
IMS
HEALTH

NORC at the University of Chicago Task Order HHSP233000007T
i
This report was prepared by NORC at the University of Chicago under contract to the Assistant Secretary
for Planning and Evaluation. The findings and conclusions of this report are those of the author(s) and do
not necessarily represent the views of ASPE or HHS.
This report was produced under the direction of Laina Bush,
Task Order Officer, Office of the Assistant Secretary for
Planning and Evaluation, Office of Science and Data Policy,
Jim Scanlon, Deputy Assistant Secretary for Planning and
Evaluation (Science and Data Policy), Don Young, Acting
Assistant Secretary for Planning and Evaluation. This report is
available online at: http://aspe.hhs.gov/sp/reports/2007/UVIDP/.
NORC at the University of Chicago Task Order HHSP233000007T
ii
Project Director:
Elizabeth Hargrave, NORC at the University of Chicago
Staff contributing to this report (in alphabetical order):
Ken Copeland, IMS Health
Jérôme Caron, IMS Health
Elizabeth Eaton, Georgetown University
Ed Evaldi, IMS Health
Jyoti Gupta, NORC at the University of Chicago
Jack Hoadley, Georgetown University
Grace Yang, NORC at the University of Chicago
NORC at the University of Chicago Task Order HHSP233000007T
iii
Understanding Variations in International Drug Prices
Table of Contents
Understanding Variations in International Drug Prices...............................................................................1
Background ....................................................................................................................................................1
Methodology ..................................................................................................................................................2
Results.............................................................................................................................................................5
Differences in U.S. and Canadian Prices for Individuals with Third-Party Coverage...........5
Differences in U.S. and Canadian Prices for Individuals without Third-Party Coverage.....5
Comparing U.S. Copayments to the Full Cost of Drugs in Canada ........................................8
Price Differences by Patent and Trademark Status....................................................................9
Testing the Impact of Substituting Drugs Without a Direct Equivalent in Canada............12
Conclusion....................................................................................................................................................13
Appendix A: Methodology............................................................................................................................14
Selecting Population Groups and Their Market Baskets.......................................................................14
Identifying Potential Market Baskets ...................................................................................................14
Selecting Population Groups ................................................................................................................15
Working with Price Data............................................................................................................................18
Matching Drug Names to Prices ..........................................................................................................18
Matching with Canadian Drugs ............................................................................................................18
Weighting .................................................................................................................................................22
Currency conversion ..............................................................................................................................22
Sensitivity to Drug Substitutions..........................................................................................................22
Changes in Drug Use Since 2003 .........................................................................................................24
Prescriptions vs. Spending.....................................................................................................................25
Payer Type and Retail vs. Total Price ..................................................................................................26
Market Baskets.............................................................................................................................................27
Appendix B: Literature Review.....................................................................................................................42
The Canadian Pricing System....................................................................................................................42
Methodology of Price Comparisons.........................................................................................................44
Price Comparisons ......................................................................................................................................46
Studies of Wholesale or Manufacturer Prices.....................................................................................49
Studies of Retail Prices...........................................................................................................................52
Discussion ....................................................................................................................................................55
Appendix C. Works Cited .............................................................................................................................56
NORC at the University of Chicago Task Order HHSP233000007T
iv
Table of Figures
Figure 1. Weighted Average Price Per Prescription, by Market Basket and Payment Type..................6
Figure 2. Average of Per-Prescription Prices for All 15 Market Baskets, by Type of Coverage ..........7
Figure 3. Copayments in the United States vs. Full Price of Drugs in Canada .......................................8
Figure 4. Average Total Price Per Prescription, by Product Type and Payment Type ..........................9
Figure 5. Variation in Relative Difference of Generic Prices...................................................................10
Figure 6. Relationship Between Makeup of Market Basket and Relative Prices ...................................11
Figure 7. Sensitivity of Average Difference Between U.S. and Canadian Price to Inclusion of Zyrtec
and Toprol XL ........................................................................................................................................12
Figure A-1. Rank Order Correlation of Drugs Used, by Age Group.....................................................15
Figure A-2. Rank Order Correlation of Drugs Used, by Race.................................................................16
Figure A-3. Rank Order Correlation of Drugs Used, by Income............................................................16
Figure A-4. Rank Order Correlation of Drugs Used, by Insurance Status ............................................17
Figure A-5. On-Patent Drugs with Same Manufacturers but Different Names ...................................19
Figure A-6. Generic Drugs with Different Names and Manufactures in the U.S. and Canada..........19
Figure A-7. Dosage Levels Substituted in U.S. and Canadian Market Baskets .....................................20
Figure A-8. Dosage Levels Substituted in Canadian Market Baskets Only ...........................................21
Figure A-9. Drugs Not Available in Canada...............................................................................................21
Figure A-13. Market Basket for Females Under 12...................................................................................27
Figure A-14. Market Basket for Females 12-24 .........................................................................................28
Figure A-15. Market Basket for Females 25-39 .........................................................................................29
Figure A-16. Market Basket for Females 40-64 .........................................................................................30
Figure A-17. Market Basket for Females 65+ ............................................................................................31
Figure A-18. Market Basket for Males Under 12.......................................................................................32
Figure A-19. Market Basket for Males 12-24..............................................................................................32
Figure A-20. Market Basket for Males 25-39..............................................................................................33
Figure A-21. Market Basket for Males 40-64..............................................................................................34
Figure A-22. Market Basket for Males 65+ ................................................................................................35
Figure A-23. Market Basket for Insured .....................................................................................................36
Figure A-24. Market Basket for Uninsured ................................................................................................37
Figure A-25. Market Basket for Non-Hispanic Whites ............................................................................38
Figure A-26. Market Basket for African Americans..................................................................................39
Figure A-27. Market Basket for Hispanics..................................................................................................40
Figure A-28. Summary of Drugs Included in Market Baskets.................................................................41
Figure B-1. Summary of U.S./Canada Prescription Drug Price Comparisons: Studies of
Manufacturer or Wholesale Prices .......................................................................................................47
Figure B-2. Summary of U.S./Canada Prescription Drug Price Comparisons: Studies of Retail
Prices.........................................................................................................................................................48
NORC at the University of Chicago Task Order HHSP233000007T
1
Understanding Variations in International Drug Prices
Rapidly rising prescription drug prices have caused many consumers, third-party payers, and
policymakers to look for ways to lower their drug costs. One strategy that many have seen as a
potential solution is to import drug products from countries where drug prices are lower than those
in the United States – commonly called reimportation. This report focuses on the differences
between prices in the United States and Canada, and seeks to create an “apples-to-apples”
comparison of the prices consumers might face for a specific market basket of drugs in each
country, taking into account any insurance coverage they might have. We make this comparison for
fifteen different market baskets of drugs, to test the sensitivity of results to the drugs selected.
Across the different market baskets, Canadian prices were about one-third lower on average than the
prices charged to insured Americans. Because those without coverage in the United States do not
have access to the discounts negotiated by third-party payers, these individuals would find that
typical prices in Canada are about half of those they face in the United States. Overall, differences
are larger for brand-name drugs, and much smaller for generic drugs. In fact, prices for generic
drugs are likely to be higher in Canada, at least compared to the prices charged to people in the
United States with insurance coverage.
Background
While Canada offers universal access to health insurance, that insurance covers only acute hospital
and physician services, and does not provide coverage for outpatient prescription drugs. Most
Canadians receive prescription drug coverage through their employers. The federal government
offers drug coverage to some populations, including veterans and First Nations and Inuit people,
and there are publicly funded provincial programs that pay a substantial share of drug costs for
older, disabled, and low-income Canadians (Gross, 2003). These provincial programs cover 42
percent of all national drug expenditures (Morgan, 2003).
Prices for newly-patented drugs are controlled by federal regulation in Canada. Prices for new brand
drugs are limited to the median of prices for the new drug in France, Germany, Italy, Sweden,
Switzerland, England, and the United States. Limits for “me-too” drugs are based on the
breakthrough brand’s price; most new drugs cannot cost more than the other drugs in the same
therapeutic class. Annual increases in prices for existing on-patent drugs are limited by the
consumer price index (CPI): in a single year, prices cannot rise more than 1.5 times the CPI
(PMPRB, 2006). This limit is lower than recent increases in prices for many on-patent drugs in the
United States (Gross et al, 2006).
The provinces all use formularies to keep prices low for the drugs they cover, and new drugs that are
equivalent to already listed drugs are added only if they do not increase program costs. Some
provinces take further measures. Ontario has instituted price freezes; British Columbia uses
reference pricing; and Quebec requires manufacturers to give the province the best price given to
any other province (Morgan, 2003).
NORC at the University of Chicago Task Order HHSP233000007T
2
Legislative and regulatory price controls in the United States apply only to drugs purchased by the
Medicaid program, the Department of Veterans Affairs, and the Federal Supply Schedule, which is
used by the health programs run by the Department of Defense and other eligible entities (e.g.,
certain community health centers and public hospitals). However, insurers and pharmacy benefit
managers (PBMs) are also able to negotiate substantial discounts and rebates from retail prices.
These discounts take two forms. First, third-party payers are able to negotiate discounts with
pharmacies on the prices they charge at the pharmacy counter. These discounts are typically taken
off the pharmacy’s markup over their acquisition cost for the drug, and the effect of the discount is
seen at the time an insured customer purchases a drug.
The second type of discount is negotiated directly between third-party payers and manufacturers.
Typically, rebates are paid by the manufacturers of on-patent drugs in exchange for giving
preferential status to a drug and demonstrating a shift of market share away from competing
products. These rebates are separate from the pharmacy transaction. A 2000 ASPE study cited
evidence that average rebates tend to be about 5 to 7 percent of drug prices, but can be as high as 35
percent on selected drugs (HHS, 2000). Research by the Congressional Budget Office, in
connection with the Medicaid drug rebate, found that price discounts including rebates generally
range as high as about 20 percent (CBO, 1996).
Uninsured individuals typically do not have access to negotiated prices of either kind. These
individuals have been at the forefront of the push to import drugs from Canada. Some third-party
purchasers, including several municipal government employee plans, have also begun to explore the
possibility of savings for their insured employees from reimportation.
Previous studies have found that prices for prescription drugs are frequently lower in Canada than in
the United States, as detailed in Appendix B. The literature also includes critiques of some of these
studies (for example, Danzon and Kim). We have attempted to address several of the concerns
raised in these critiques with the approach taken in this report. In particular, we explore two factors
that could influence these observed differences between U.S. and Canadian prices: the market
basket of drugs selected for comparison, and whether the purchaser has third-party coverage for the
prescription at the time of purchase.
Methodology
The goal of this study is to determine the difference between U.S. and Canadian prices at the retail
point of sale for the prescription drugs that are purchased by U.S. customers. Our primary question
was, what would U.S. customers pay if they were able to purchase their drugs at Canadian prices?
Throughout the design of the project, we have used this research question to help shape our
methodology.
Price comparisons can be sensitive to the market basket of drugs included in the study. In
particular, generic drugs and brand-name drugs tend to have quite different pricing patterns, which
can affect the outcome of a price comparison. Our goal in this study was to test a variety of market
baskets, but to make each of them representative of the drugs used by a subpopulation in the United
States. We selected fifteen groups based on age, gender, race, and insurance status, which were
NORC at the University of Chicago Task Order HHSP233000007T
3
identified from a larger set of groups as those with distinctive patterns of drug use. For each group,
we selected the most commonly used drugs accounting for a third of that population’s prescriptions
in the 2003 Medical Expenditure Panel Survey (MEPS). We then identified the most commonly
used form and strength of each drug for each group, so that we could limit our data collection to a
precisely specified set of drugs. The resulting market baskets each had from 8 to 32 drugs, with a
total of 106 drugs across the 15 groups. These drug lists are included at the end of Appendix A.
Although some drugs are included in at least half of all the market baskets, the mix of drugs varies
considerably. The market baskets for those over age 65 are dominated by treatments for the chronic
conditions that accompany older ages, whereas market baskets for children include a mix of
antibiotics, behavioral medications, and asthma drugs. Those for young women include various
contraceptive drugs.
For these commonly used drugs, we then sought to find their counterparts in Canada. We tried to
assure that the match between our U.S. market baskets and Canadian market baskets is as close as
possible, while still representing the utilization of the population groups we identified. We first
looked for an exact match by drug name, form, and strength between the United States and Canada.
When an exact match was not available in Canada, we sought to match drugs by chemical entity, and
when possible, by patent and trademark status and/or manufacturer. There were several drugs on
our list at a dosage level not available in Canada. When possible, we used another dosage level that
is available in both countries as a substitute in both the U.S. and Canada market baskets; otherwise,
we used the dosage available in Canada closest to the most common U.S. dosage. More information
on this matching process is available in Appendix A.
Once we had specified our list of drugs for the United States and Canada, we then used data
collected by IMS Health to identify the average price per pill (or, for other forms such as liquids and
inhalers, the average price for a set amount) for each drug in both countries. The IMS prescription
databases represent very large, non-probability samples of prescriptions dispensed at retail
pharmacies in the United States and in Canada. The data are representative of prescription
transactions for both large retail pharmacy chain organizations and small independent pharmacies.
In the United States, the IMS sample is geographically representative of all 50 states plus the District
of Columbia and Puerto Rico. The databases include all prescriptions dispensed at over 35,000 retail
pharmacies in the United States, representing two-thirds of all U.S. retail pharmacies and an
estimated 70 percent or more of all prescription transactions in the retail pharmacy sector.
In Canada, the IMS sample includes over 4,300 retail pharmacies, representing 60 percent of all
Canadian retail pharmacies and an estimated 70 percent of all prescription transactions. However,
we used data only from Ontario and Quebec, which make up nearly three fourths of the overall IMS
sample (3,100 pharmacies). These two provinces are the only ones for which IMS collects data on
the type of payer, a critical part of our analysis. In addition, IMS has determined that in other
provinces, pharmacies frequently report list prices rather than actual prices. Prior IMS work with
Canadian prescription data, in conjunction with understanding of pharmaceutical price controls in

NORC at the University of Chicago Task Order HHSP233000007T
4
effect in Canada, suggest that there is little variation in prices between these two provinces and the
rest of Canada.
1
Using data from MEPS about the most common prescription size, we standardized the IMS per-pill
price to represent an average per-prescription price for each drug. This method allowed us to
eliminate any price disparities that might result from practice patterns or prescription-filling behavior
that would lead to price differences caused by prescriptions covering different periods of time (e.g.,
30 vs. 90 days) in the two countries. From these standardized per-prescription prices, we calculated
an index price for each market basket, weighting based on utilization of each drug within the market
basket for each population.
While in Canada there is little variation in the prices paid by different payers, in the United States the
cost of drugs can vary widely according to who is purchasing drugs. Individuals paying cash pay the
highest price, while insurers, managed care plans, and government programs can negotiate pharmacy
discounts and manufacturer rebates because they represent large numbers of customers. One goal of
this report is to explore the effect of these intra-country price differences on cross-country
comparisons. In this report, we separate the prices paid by individuals who had a third-party
payment from the prices paid by those who paid the entire cost of a prescription themselves.
2
In identifying the third-party prices, we have no access to proprietary rebate amounts and have not
attempted to estimate them.
3
The prices reported in this paper likely reflect the discounts that
insurers have negotiated with pharmacies, but not the additional discounts they have received from
manufacturers separate from the pharmacy transaction. The effect of including these rebates, if they
were available, would be to reduce the overall amount paid by third-party insurers for brand name
drugs and to reduce the difference between the United States and Canada for the prices of these
drugs.
There are several issues that are beyond the scope of this paper. We do not address here the safety
or legality of importing drugs to the United States from Canada. Likewise, we do not estimate the
shifts in manufacturer pricing behavior that would likely occur if reimportation became widespread,
or if policy changes in the United States attempted to force manufacturers to provide drugs to
customers at Canadian prices. HHS addressed many of these issues in its 2004 report to Congress
on prescription drug importation.
1
In particular, it is likely that customers without third-party coverage are paying prices close to the national limits set by
the Patented Medicine Prices Review Board. In this study, we find that in Canada, third-party prices are extremely close
to the prices paid by individuals without third-party coverage. Third-party payers in these provinces appear to be getting
little additional discount despite the extra limits set by the provincial governments for drugs purchased through
provincial programs.
2
We report prescriptions as “with third-party coverage” if a third party was involved at the time of the pharmacy
transaction, including prescriptions that were only discounted. (e.g., because the customer had a discount card). We
report prescriptions as “without third-party coverage” if a third party was not involved in the transaction at the
pharmacy counter. It is possible that some of these latter customers may be submitting receipts to an insurer for
reimbursement. However, without the involvement of an insurer at the time of the transaction, we assume the price
paid for these prescriptions is not discounted.
3
Other authors, including the HHS Task Force on Drug Importation (Report on Prescription Drug Importation, p. 115), have
attempted to estimate the rebates paid from manufacturers to pharmacies and wholesalers. We do not consider these
rebates here as we are studying prices paid by customers at the pharmacy counter, not pharmacy acquisition prices.

NORC at the University of Chicago Task Order HHSP233000007T
5
Results
For each of our fifteen market baskets of drugs, the total price paid for drugs in Canada is
consistently lower than the total price paid in the United States. This is true for individuals with and
without third-party coverage. However, the difference is greater for individuals without third-party
coverage.
Differences in U.S. and Canadian Prices for Individuals with Third-Party Coverage
For prescriptions filled by individuals with third-party coverage, the difference between Canadian
and U.S. prices ranges from about 30 percent lower for the market basket that represents the drugs
most commonly used by girls under 12, to as much as 47 percent lower for the market basket of
drugs that represents the drugs most commonly used by women ages 25 to 39 (Figure 1). Both the
average and the median of the differences for all fifteen market baskets are about 36 percent.
4
These observed price differences are in the general range of those found in the literature (see
Appendix B). Several of the studies described in the Appendix found differences in the range of 30
to 50 percent (with at least one considerably higher and one considerably lower). This consistency
of results provides some reassurance that the methodology used in this study is not creating
unexpected results. The consistency of results across market baskets is also significant. Although
there is variation, the price difference for most market baskets are remarkably consistent despite
differences in drug mix. As discussed below, the main difference results from the mix of brand and
generic drugs.
Differences in U.S. and Canadian Prices for Individuals without Third-Party
Coverage
For those without third party coverage, the prices reported are the total price paid by the individual
to the pharmacy. There is an even larger difference between U.S. and Canadian prices for these
customers (Figure 1). The market basket for drugs commonly taken by men aged 65 and over has
the smallest difference, with prices in Canada 38 percent lower than in the United States. The
market basket for the drugs commonly taken by women aged 25-39 again has the highest difference,
with Canadian prices 54 percent lower than U.S. prices. Both the average and the median of the
differences for all fifteen market baskets are about 45 percent.
4
For those with third-party coverage, the prices presented here include the total price paid at the pharmacy, including
both the insurance payment and any copayment made by the patient.

NORC at the University of Chicago Task Order HHSP233000007T
6
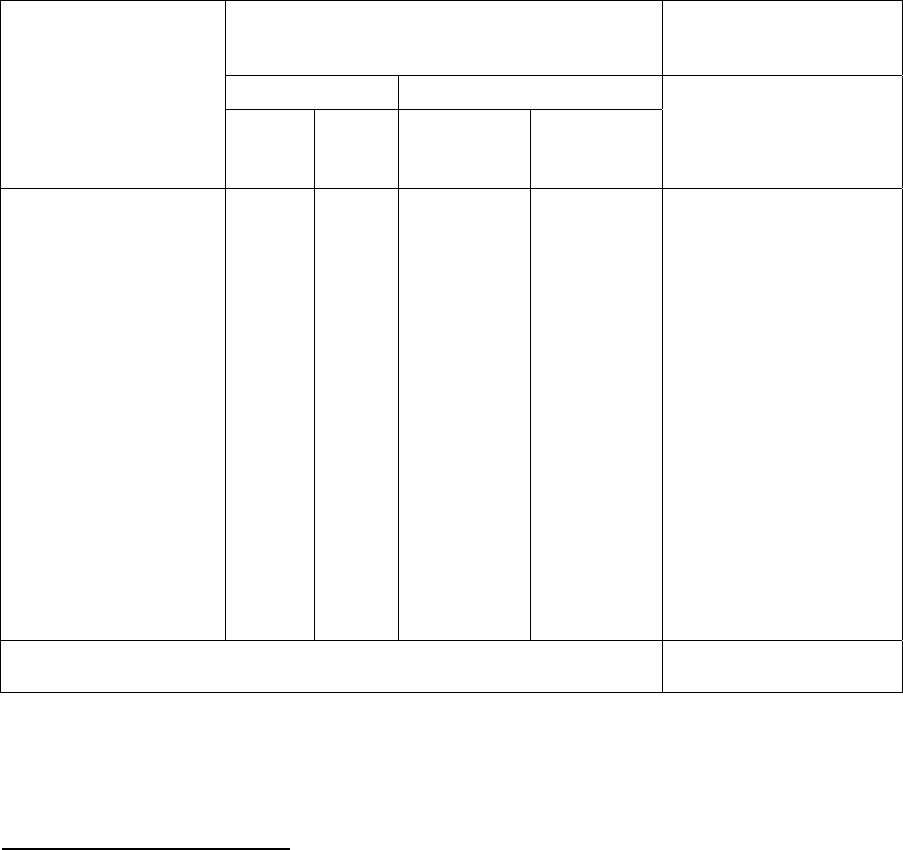
Figure 1. Weighted Average Price Per Prescription, by Market Basket and Payment Type
With Third-Party Coverage Without Third-Party Coverage
Market Basket:
Drugs Taken By...
U.S. Canada Difference U.S. Canada Difference
Females under 12 $31.44 $21.99 -30.1% $41.43 $22.70 -45.2%
Females 12-24 $45.93 $27.60 -39.9% $54.69 $28.56 -47.8%
Females 25-39 $48.47 $25.69 -47.0% $57.05 $26.44 -53.6%
Females 40-64 $51.78 $30.61 -40.9% $59.66 $31.00 -48.0%
Females 65+ $45.44 $28.48 -37.3% $50.82 $29.01 -42.9%
Males under 12 $39.07 $26.96 -31.0% $50.17 $27.64 -44.9%
Males 12-24 $70.48 $46.94 -33.4% $84.95 $47.85 -43.7%
Males 25-39 $68.04 $39.41 -42.1% $80.28 $40.06 -50.1%
Males 40-64 $57.24 $36.72 -35.9% $65.42 $37.50 -42.7%
Males 65+ $41.80 $28.99 -30.6% $47.46 $29.45 -37.9%
Insured $52.94 $31.60 -40.3% $61.24 $32.15 -47.5%
Uninsured $37.59 $25.80 -31.4% $43.47 $26.19 -39.8%
Whites $47.19 $28.80 -39.0% $54.49 $29.30 -46.2%
African Americans $47.16 $30.72 -34.9% $54.17 $31.19 -42.4%
Hispanics $36.84 $24.47 -33.6% $43.83 $24.75 -43.5%
Notes: Index price is a weighted average of the prices of the most common form and strength of the most commonly used drugs
for each population group.
Prices are in U.S. dollars, using the average exchange rate for the period of data collection.
Difference between U.S. and Canada prices calculated as (Canada price-U.S. price)/U.S. price.
Market baskets include the drugs most commonly used by individuals in each population group. Prices reflect the prices
paid by all individuals, not restricted to the population group. For example, the insured market basket includes the drugs
most commonly used by people with insurance. The price indices for that market basket use data for all people who
bought the drugs in that market basket, so it is possible to have a price index for uninsured individuals who purchased the
drugs in the insured market basket.

NORC at the University of Chicago Task Order HHSP233000007T
7
The higher differential between U.S. and Canadian prices for customers without third-party
insurance appears to be due largely to the difference between the prices paid by customers with and
without insurance within the United States (Figure 2). For any given market basket, the index price
in Canada is only 1 percent to 4 percent higher for customers without third-party insurance
compared to customers with insurance – when the market basket index prices are averaged, the
difference is less than a dollar. In contrast, uninsured customers in the United States pay 12 percent
to 32 percent more for a market basket of drugs than the cost of the same drugs for customers with
insurance, a difference of about $8.50 across all market baskets. The latter result is consistent with
findings from earlier work by ASPE in 2000 (HHS, 2000).
Figure 2. Average of Per-Prescription Prices for All 15 Market Baskets, by Type of Coverage
$48.09
$56.61
$30.32
$30.92
$0
$10
$20
$30
$40
$50
$60
US, With Third Party
Coverage
US, Without Third
Party Coverage
Canada, With Third
Party Coverage
Canada, Without
Third Party Coverage
Average Price Per Prescription
(Average of All 15 Market Baskets
)

NORC at the University of Chicago Task Order HHSP233000007T
8
Comparing U.S. Copayments to the Full Cost of Drugs in Canada
The price comparisons for the full cost of drugs are the most relevant for many U.S. citizens without
insurance coverage for their drugs who seek savings by getting drugs in Canada. By contrast, most
individuals with coverage will not achieve savings unless their third-party agent is involved in the
transaction. Insured consumers considering drug purchases in Canada may find their insurance
coverage is not applicable for purchases from Canadian pharmacies. Thus, the relevant comparison
for these customers is between the copayment they are charged at their U.S. pharmacy and the full
cost of the drug in Canada.
In fact, individuals with insurance are unlikely to be able to find prices in Canada that are lower than
the copayments they pay for drugs that are covered by their insurance. On average across our
market baskets, the Canadian price is about 78 percent higher than the out-of-pocket cost to a
person in the United States with third party coverage (Figure 3), with a range from 31 percent to 141
percent higher. This result is not surprising given that insured consumers are paying only a share of
the total drug cost out of pocket.
There may be situations in which these individuals could achieve savings with Canadian drug prices.
For example, if their coverage includes a cap with an annual or quarterly spending limit (such as in
some Medicare Advantage plans prior to 2006), a gap in coverage (such as that faced in the new
Medicare drug benefit), or a limit on the number of monthly prescriptions, individuals may find that
it would be less expensive to buy drugs in Canada than to pay the full cost of a drug in the United
States. Those who want to take a particular drug that is not covered by their insurer’s formulary
might also benefit from the price differential by making purchases in Canada.
Figure 3. Copayments in the United States vs. Full Price of Drugs in Canada
$17.35
$48.09
$30.92
$0
$10
$20
$30
$40
$50
US, With Third Party Coverage Canada, Without Third Party
Coverage
Average Price Per Prescription
(Average of All 15 Market Baskets
)
average
customer share:

NORC at the University of Chicago Task Order HHSP233000007T
9
Price Differences by Patent and Trademark Status
There is a dramatic variation in the differential between U.S. prices and Canadian prices depending
on the patent and trademark status of drugs. When generics and brand name drugs are identified
separately, brand name drugs show even larger differences between U.S. and Canadian prices.
Generics show smaller differences, or the direction of the difference changes, depending on the type
of payer.
The on-patent brand name drugs in our market baskets cost 43 percent less for individuals with
insurance and 50 percent less for individuals without insurance in Canada compared to U.S. prices
(Figure 4). The absolute price differences are also largest for brand name drugs, particularly the on-
patent brands in our market baskets.
As with the results for the overall market baskets, the variation in differences based on insurance
coverage appears to be because in the United States, transactions with third-party involvement cost
about 14 percent less than transactions without third-party coverage. Again, this is likely because of
discounts that third-party payers have negotiated with pharmacies. However, even after this third-
party discount, the difference between prices in the United States and Canada is substantial. The
remaining difference likely results from a combination of regulatory measures in Canada and the
market decisions made by manufacturers.
The availability of manufacturer rebates could further mute some of the difference between the
United States and Canada for third-party payers. Typically, rebates are only available for brand-
name drugs, and mostly for those that remain on patent. Previous studies have found that rebates
can be as high as 20 percent (CBO, 1996) to 35 percent (HHS, 2000) on selected drugs. Even if
amounts toward the higher end of these estimates were appropriate for most of the drugs in our
market baskets, the differences would fall short of the 43 percent average differential observed for
on-patent brand drugs.
Figure 4. Average Total Price Per Prescription, by Product Type and Payment Type
With Third-Party Coverage Without Third-Party Coverage
Product Type
Number
of Drugs
U.S. Canada Difference U.S. Canada Difference
Generic
27 $10.75 $14.75 37% $15.49 $14.92 -4%
Branded Generic
10 $25.79 $22.83 -11% $30.81 $23.45 -24%
Off-Patent Brand
19 $37.01 $17.10 -54% $42.65 $17.32 -59%
On-Patent Brand
38 $125.36 $71.37 -43% $145.91 $73.08 -50%
Notes: Index price is a non-weighted average of the prices for each type of drug included in any market basket for this study.
Prices are in U.S. dollars, using the average exchange rate for the period of data collection.
Difference between U.S. and Canada prices calculated as (Canada price-U.S. price)/U.S. price.

NORC at the University of Chicago Task Order HHSP233000007T
10
Generic drugs follow a much different pattern. For customers with insurance coverage, the full cost
of the generic drugs in our market baskets is actually higher in Canada than in the United States
(Figure 4). For customers without insurance coverage, generics are only 4 percent less expensive in
Canada than in the United States.
ASPE’s 2000 study showed relatively little price difference for generic drugs between the prices paid
by customers with a third-party payer and prices paid by those without coverage (HHS, 2000). That
does not appear to be the case for the generic drugs selected for our market baskets. The average
price of these drugs is about a third lower for customers with third-party coverage in the United
States compared to those without coverage. This third-party discount appears to be what is causing
U.S. generic prices to be lower than Canadian generic prices, for the drugs selected.
Previous studies have found more variability in the prices of generic drugs than in the prices of
generics. Possibly related to this general variation, we found variation from market basket to market
basket in the difference between U.S. and Canadian prices for generic drugs (Figure 5). For
customers with third-party coverage, we consistently find that U.S. prices are lower than Canadian
prices, but the difference ranges from 23 to 63 percent. For customers without third-party coverage,
Canadian prices are nearly 20 percent lower for some market baskets and over 20 percent higher for
other market baskets. The range in variation in the prices of brand name drugs among our market
baskets is only half as large.
Figure 5. Variation in Relative Difference of Generic Prices
Relative Difference Between Canadian and
U.S. Price
Market Basket:
Drugs Taken By...
Number of
Generic
Products in
Market
Basket
With Third-
Party Coverage
Without Third-Party
Coverage
Females under 12 2 53.6% -18.6%
Females 12-24 3 47.0% -18.1%
Females 25-39 4 42.8% -19.6%
Females 40-64 8 78.5% 24.0%
Females 65+ 7 59.8% 21.2%
Males under 12 2 47.9% -19.8%
Males 12-24 3 23.0% -10.7%
Males 25-39 5 75.7% 6.8%
Males 40-64 9 60.7% 9.3%
Males 65+ 11 49.4% 8.0%
Insured 8 53.6% 2.7%
Uninsured 11 46.0% -1.0%
Whites 10 62.5% 8.6%
African Americans 8 53.6% 6.5%
Hispanics 14 49.5% -4.8%
Notes: Index price is a non-weighted average of the prices for each type of drug included in any market basket for this study.
Difference between U.S. and Canada prices calculated as (Canada price-U.S. price)/U.S. price.
NORC at the University of Chicago Task Order HHSP233000007T
11
Figure 6 shows the proportion of each market basket that is made up of brand and generic drugs.
The market baskets we have selected have non-trademark generic use ranging from a low of 15
percent for females 25-39 to a high of 54 percent for males aged 65 and over. Including branded
generics, the generic rate ranges from 21 percent to 57 percent. This variation in generic use is
consistent with the wide variation in the types of drugs taken by these groups. For example, the
drugs in our market basket for young women include many on-patent birth control pills, while the
market basket for older men includes more generic heart medications.
5
Figure 6. Relationship Between Makeup of Market Basket and Relative Prices
Proportion of Market Basket, Weighted by
Utilization
Difference in Price Per
Prescription,
Canada vs. U.S.
Brand Generic
Market Basket:
Drugs Taken By...
On
Patent
Off
Patent
Trademark
(Branded
Generic)
Non-
Trademark
With
Third-
Party
Coverage
Without
Third-
Party
Coverage
Females under 12 22% 28% 8% 41% -30% -45%
Females 12-24 60% 15% 4% 21% -40% -48%
Females 25-39 52% 23% 10% 15% -47% -54%
Females 40-64 45% 27% 3% 25% -41% -48%
Females 65+ 45% 18% 4% 33% -37% -43%
Males under 12 25% 18% 15% 42% -31% -45%
Males 12-24 50% 8% 20% 23% -33% -44%
Males 25-39 57% 22% 0% 21% -42% -50%
Males 40-64 52% 13% 0% 36% -36% -43%
Males 65+ 34% 12% 0% 54% -31% -38%
Insured 50% 18% 2% 29% -40% -48%
Uninsured 32% 16% 3% 50% -31% -40%
Whites 48% 18% 3% 31% -39% -46%
African Americans 40% 16% 0% 44% -35% -42%
Hispanics 32% 15% 2% 51% -34% -44%
Correlation with Proportion of Market Basket On Patent -0.79 -0.52
Correlation with Proportion of Market Basket Non-Trademark Generic 0.82 0.84
Note: Difference between U.S. and Canada prices calculated as (Canada price-U.S. price)/U.S. price.
5
Nationally, both Express Scripts and the Generic Pharmaceutical Association reported that generics accounted for just
over half of all prescriptions in 2003. It may be that the bottom two-thirds of drugs used by each population group are
slightly more likely to be generics than the drugs we selected for our market baskets. (see Express Scripts, “Geographic
Variation in Generic Fill Rate”, http://www.express-scripts.com/ourcompany/news/outcomesresearch/
onlinepublications/study/regionalgenericvariation.pdf, and GPhA “Statistics,” http://www.gphaonline.org/
Content/NavigationMenu/AboutGenerics/Statistics/Statistics.htm)

NORC at the University of Chicago Task Order HHSP233000007T
12
Consistent with the findings in Figure 4, market baskets with a higher proportion of generic drugs
tend to show lower differences between Canadian and U.S. prices (correlation is .82 for third-party
and .84 without third-party coverage). Likewise, market baskets with a higher proportion of on-
patent brand drugs tend to show higher differences between Canadian and U.S. prices (correlation is
-.79 for third-party and -.52 without third-party coverage). For example, the drugs taken by young
adults (women 25-39 and men 25-39) have the largest differential between U.S. and Canadian prices,
and both have market baskets with a high proportion of brand-name drugs.
The use of multiple market baskets allows us to see a range of possible effects while still basing
measurements on actual utilization instead of an arbitrarily selected set of drugs. It is notable that
despite this variation, the difference between Canadian and U.S. prices remains within a fairly small
range.
Testing the Impact of Substituting Drugs Without a Direct Equivalent in Canada
Two drugs included in our market baskets had unusual circumstances that resulted in higher-than-
average differences between the U.S. price and Canadian price. Zyrtec (cetirizine hydrochloride), an
on-patent brand name prescription drug in the United States, is available over-the-counter in Canada
as Reactine. We included this medication in our Canadian market baskets, utilizing data on
prescription transactions for Reactine. Toprol XL (metoprolol succinate), another on-patent brand
name drug, is not available in Canada. We included a close substitute, Lopressor SR (metoprolol
tartrate), in the relevant Canadian market baskets. In both cases, we were following the principle that
we were seeking to find the drugs that U.S. customers would find in Canada as the closest match for
the drugs they take.
In all market baskets, excluding these two brand-name drugs makes the difference between the two
countries’ prices smaller (Figure 7). In thirteen of the fifteen market baskets, the difference is 2.2
percentage points or less (regardless of payer). The exception is the market baskets for children
under 12. Zyrtec accounts for about a tenth of the prescriptions included in each of these market
baskets, and its exclusion gives generic drugs a larger share of each market basket. The result is a
more notable drop in the relative difference between U.S. and Canadian prices for these two groups.
The change is particularly notable for prescriptions purchased with third party coverage, where the
difference between United States and Canada for these two groups of drugs falls to 21.5 percent for
girls and 23.5 percent for boys. While these results show that the selection of individual drugs for a
market basket can affect the magnitude of the results, the change is not enough to affect the overall
result that prices in Canada are substantially lower than prices in the United States.
Figure 7. Sensitivity of Average Difference Between U.S. and Canadian Price to Inclusion of
Zyrtec and Toprol XL
With Third-Party
Coverage
Without Third-Party
Coverage
Including Zyrtec and Toprol XL -36.5% -46.2%
Excluding Zyrtec and Toprol XL -34.1% -44.2%
Note: Unweighted average of results for 15 market baskets.
NORC at the University of Chicago Task Order HHSP233000007T
13
Conclusion
This report finds differences between Canadian and U.S. drug prices that are generally consistent
with the existing literature on international drug pricing. Uninsured customers in the United States
would find Canadian prices to be about half the prices they pay in the United States. Insured
consumers seeking to buy the drugs they currently buy in Canada would find, on average, prices that
are about a third lower than the total cost of their drugs, but higher than the copays they currently
pay. These findings are somewhat sensitive to different market baskets. While the overall result that
prices are lower in Canada is fairly consistent, the inclusion of more generic drugs can decrease the
difference between Canadian prices and U.S. prices.
The differing spread in prices depending on patent and trademark status is a result worth
emphasizing. Generic drug prices are mostly equivalent or higher in Canada, whereas brand drugs
are substantially less expensive in Canada. Because on-patent brand name drugs have the highest
prices, they also are the products with the largest absolute difference between Canadian and U.S.
prices.
There are several issues related to these questions that are beyond the scope of this paper. We do
not address here the safety or legality of importing drugs to the United States from Canada.
Likewise, we do not estimate the shifts in manufacturer pricing behavior that would likely occur if
reimportation became widespread, or if policy changes in the United States attempted to force
manufacturers to provide drugs to customers at Canadian prices. HHS addressed many of these
issues in its 2004 report to Congress on prescription drug importation (HHS, 2004).
Absent these widespread market shifts and other concerns, individuals without insurance coverage
can find substantial savings in Canada for the commonly used drugs included in this study. Third-
party insurers face a more complicated arithmetic. For generic drugs, third-party payers seem to be
able to achieve enough savings through pharmacy discounts so that they would not save by steering
their enrollees to Canada. For brand-name drugs, however, there is a large difference in prices
between the United States and Canada even after these discounts. Whether third party payers could
do better by purchasing drugs at Canadian prices largely depends on whether the additional
manufacturer rebates they receive on brand-name drugs are larger than the differences reported
here.
NORC at the University of Chicago Task Order HHSP233000007T
14
Appendix A: Methodology
This appendix describes our methodology for selecting market baskets of drugs and identifying
prices for those market baskets of drugs in the United States and Canada. To explore possible
market baskets, we selected the top third of drugs for several potential population groups and
compared them. Age had the most differentiating effect on which drugs a population group uses;
we used age, gender, race/ethnicity, and insurance status to create market baskets. The first section
of this appendix documents this process in more detail.
For each market basket, we identified prices for the most common form and strength of each drug.
We weighted prices in each market basket index according to overall use of each drug for that
population. The second section of this appendix documents our methodology for working with
drug price data.
Selecting Population Groups and Their Market Baskets
In selecting potential population groups, we started with a list of attributes available in the Medical
Expenditure Panel Survey (MEPS) that could be reasonably expected to lead to differences in drug
use. These included age, gender, race, income, obesity, smoking, and insurance status. We identified
the drugs most commonly used by each of these groups, then compared them to identify groups
that would lead to a set of diverse market baskets. The data on utilization by these groups became
the basis for the market baskets of drugs used in this analysis.
One issue we identified in our literature review was the idea that when comparing Canadians’ drug
costs to U.S. costs, it might be more appropriate to determine drugs that are most commonly used
by Canadians and compare their prices to prices of drugs most commonly used in the United States.
The research question for this project, however, concerns the prices that United States consumers
would pay for their drugs if they bought the same drugs in Canada. For this reason, we focused on
identifying the drugs, forms, and strengths that are most commonly used in the United States.
Identifying Potential Market Baskets
As potential market baskets, we selected a group of drugs that represent a third of the prescriptions
for each potential population group. Because this list of drugs is based on prescription volume, not
total costs, inexpensive drugs will count as heavily as higher-priced drugs. Selecting a consistent
percentage of utilization across all population groups will allow for the most consistent comparisons
across population groups. We selected the cutoff of one third of prescriptions because of sample
size considerations.
MEPS collects information on prescriptions at two different levels of detail. The most aggregated
information collected is the drug name. In addition, MEPS collects for many (but not all)
prescriptions the NDC code. NDC codes specify not only the drug but also the manufacturer, the
form and the strength of a drug. There can be many NDC codes for an individual drug name,
particularly for generic drugs with multiple manufacturers.

NORC at the University of Chicago Task Order HHSP233000007T
15
To select the top third of drugs for each population group, we chose to rank drugs by number of
prescriptions at the drug name level. Before doing this, we cleaned drug names in MEPS to make
them more consistent, and to remove any differentiation between forms and strengths that were
included in the reported drug name (e.g., amoxil and amoxil bubblegum).
We considered but rejected the option of using NDC codes to rank-order the drugs. Because
generic drugs have multiple manufacturers as well as multiple strengths, the use of any one generic
drug is diluted across many different NDCs. Ranking by NDCs would disproportionately place
brand-name drugs in the top third of utilization. In addition, the number of observations at the
NDC level is much smaller than the number of observations at the drug name level. This makes it
more likely that drugs could make it into the top third by random chance.
Selecting Population Groups
Using the top-ranked drug names that add up to one third of total prescriptions for that group, we
ran correlations among the drugs that were common to any two subpopulations. Where correlations
are not high, it suggests that two subpopulations are using a substantially different set of drugs.
Overall, we found that age groups provided the most diverse set of drugs. In other words, the drugs
used by children are different from those used by middle-aged individuals, which are in turn
different from those used by seniors. For the other variables we tested, the differences were
generally less striking.
Age
We divided the population into six age categories: under 12, 12 to 24, 25 to 39, 40 to 54, 55 to 64,
and 65 and up. In general, there was relatively high correlation between adjoining age categories –
and lower correlations as the age separation became greater.
Figure A-1. Rank Order Correlation of Drugs Used, by Age Group
Under 12 12-24 25-39 40-54 55-64 65 and up
Under 12
12-24 0.77
25-39 0.25 0.50
40-54 -0.23 -0.34 0.09
55-64 -0.31 -0.48 -0.17 0.83
65 and up -0.38 -0.53 -0.29 0.58 0.81
Based on these correlations, we used age as a primary basis for our market baskets. We combined
two age groups (40-54 and 55-64) since their correlation is quite high (0.83). The correlation
between the 55-64 and the over 65 group is also high, but because the latter group is mostly covered
by Medicare, we kept it as a separate group. We also considered combining the two youngest
groups. However, because further examination suggests that the under 12 group often uses
different forms of drugs (e.g., liquids instead of tablets or capsules), we kept this group as a separate
market basket.

NORC at the University of Chicago Task Order HHSP233000007T
16
Sex
The pattern of drug use between males and females was moderately correlated (0.61). There are
biologically driven differences between some of the drugs used by males versus those used by
females. For example, the market baskets for women of childbearing age include multiple birth
control pills. As a result, we divided all the age groups described above into separate market baskets
for males and females.
Race
We established four race categories: Non-Hispanic White, African American, Hispanic, and Other.
The correlations among the patterns of drug utilization for these groups were only modest.
Figure A-2. Rank Order Correlation of Drugs Used, by Race
White African American Hispanic Other
White
African
American
0.46
Hispanic 0.55 0.46
Other 0.64 0.43 0.81
Based on these results, we created three market baskets: one based on the utilization of the African
American population, one based on the Hispanic population, and one based on the non-Hispanic
white population. The “other” race group is too small to provide reliable estimates (and is relatively
highly correlated with the Hispanic group).
Income
We divided the population into five income groups. Generally, drug use for the four lowest groups
was fairly highly correlated. The one outlier group is the high-income population. We did not use
this group as a separate market basket.
Figure A-3. Rank Order Correlation of Drugs Used, by Income
Poor Near poor Low Medium High
Poor
Near poor 0.90
Low 0.83 0.78
Medium 0.74 0.81 0.86
High 0.44 0.59 0.58 0.85

NORC at the University of Chicago Task Order HHSP233000007T
17
Insurance Status
We created five categories of insurance status: (1) Uninsured (not covered by insurance during the
year); (2) Private insurance (covered by private insurance during the entire year); (3) Medicare
(covered by Medicare during the entire year); (4) Medicaid (covered by Medicaid during the entire
year); and (5) Medicaid/other (covered by Medicaid during the entire year and also covered by some
other insurance for at least part of the year). The patterns of drug use are only moderately correlated
across these categories. The most distinct pattern is for those who are uninsured, presumably
because they cannot afford some of the more expensive drugs and because their health needs are
different. We created two market baskets based on these groups: one for people who had drug
insurance all year, and one for people who did not have drug insurance for any part of the year.
Figure A-4. Rank Order Correlation of Drugs Used, by Insurance Status
Uninsured Private Medicare Medicaid Medicaid/other
Uninsured
Private 0.37
Medicare 0.21 0.67
Medicaid 0.43 0.46 0.48
Medicaid/other 0.12 0.60 0.79 0.65
Health Indicators
We chose two health indicators as possible influences on drug use: obesity and smoking. For each
indicator, the sets of drugs used are similar across the two categories. Drugs used by those who are
obese are highly correlated (0.87) with drugs utilization by the non-obese. The correlation of drug
use between smokers and non-smokers is nearly as high (0.79). As a result, we did not create any
market baskets based on these groupings.
Population Groups
Based on the above analysis, we created 15 market baskets:
• Females under 12
• Females 12-24
• Females 25-39
• Females 40-64
• Females 65+
• Males under 12
• Males 12-24
• Males 25-39
• Males 40-64
• Males 65+
• Insured
• Uninsured
• Whites
• African Americans
• Hispanics
NORC at the University of Chicago Task Order HHSP233000007T
18
The resulting market baskets each included from 8 to 32 drugs. We have included the list of drugs
that make up the market basket for each of these groups at the end of this appendix. In addition,
we have included a consolidated list of all 106 drugs included in any market basket, with a count of
the market baskets in which each drug is included.
Working with Price Data
For each market basket, we identified prices for the most common form and strength of each drug.
Identifying prices in Canada required a matching process to ensure that we found prices for the drug
a U.S. consumer would find as the closest match for his or her drug. We then calculated a per-
prescription weighted average price for each market basket. This section provides more detail on
this process.
Matching Drug Names to Prices
Although utilization can be summarized at the drug name level, price varies by form, strength, and
manufacturer. Even when two population groups have the same drug name in their market baskets,
they will often have different usage patterns for that drug. For example, many drugs have different
dosage recommendations for children and the elderly. In addition, children are more likely than
other populations to use drugs in a liquid form.
We identified the form and strength most commonly used by each population group for each drug
in a market basket. IMS then extracted all retail prescription transactions occurring in Oct-Dec 2005
for all NDC codes associated with that form and strength of the drug for which valid price and
quantity information was reported. We used these data to calculate an average price per pill (or, for
drugs that are not in pill form, the price per unit), defined as summed price across all dispensed
prescription transactions divided by summed quantity.
We then converted these per-unit prices into per-prescription prices, in order to standardize across
different drugs that have different numbers of pills or other units per prescription. To do this, we
used MEPS to determine the median number of pills (or other units) in a prescription for the
selected form and strength of each drug for each population group. We then multiplied the price
per unit by the median units per prescription to arrive at a standardized per-prescription price.
In the case of drugs sold in inhalers, we did not use this standardization process. Instead, we
defined the per-prescription price as summed price across all dispensed prescription transactions
divided by total number of dispensed prescriptions.
Matching with Canadian Drugs
We have tried to assure that the match between our U.S. market baskets and Canadian market
baskets is as close as possible, while still representing the utilization of the population groups we
identified. In the majority of cases, we were able to find an exact match by drug name, form, and
strength between the United States and Canada. There are four general categories of cases for
which that was not possible: 1) Drugs sold under a different name in Canada; 2) Drugs with

NORC at the University of Chicago Task Order HHSP233000007T
19
different brand/generic status; 3) Drugs sold in different dosages or release forms; and 4) drugs not
marketed in Canada.
Drugs sold under a different name
We first looked for an exact match by drug name, form, and strength between the United States and
Canada. When an exact match by drug name was not available in Canada, we looked to match drugs
by chemical entity, brand-name status, and in the case of brand name drugs, by manufacturer.
Figure A-5 shows the seven brand name drugs that match by all factors except trade name.
Figure A-5. On-Patent Drugs with Same Manufacturers but Different Names
Chemical ingredient U.S. Product
(from MEPS)
Canadian Product
Amoxicillin and clavulanate potassium Augmentin Clavulin
Desloratadine Clarinex Aerius
Divalproex sodium Depakote Epival
Ethinyl estradiol and norelgestromin Ortho-Evra Evra
Ethinyl estradiol and norethindrone Ortho-Novum Ortho 1/35
Escitalopram Lexapro Cipralex
Certirizine Zyrtec Reactine
6
For six generic drugs and branded generics, we were able to find a Canadian match with the same
active ingredient but with different names and manufacturers. Figure A-6 shows the Canadian
products we used as matches for these drugs.
Figure A-6. Generic Drugs with Different Names and Manufactures in the U.S. and Canada
Chemical ingredient U.S. Product
(from MEPS)
Canadian Product
Albuterol Albuterol Salbutamol
7
Spironolactone Spironolactone Novo-Spiraton
Ethinyl estradiol and desogestrel Apri Ortho-cept
Ethinyl estradiol and norethindrone Necon Brevicon, Loestrin, Minestrin
8
Amoxicillin Trimox Amoxicillin
Levothyroxine Levoxyl Eltroxin
9
6
Reactine is available over-the-counter in Canada.
7
Salbutamol sulfate is the name recommended by the World Health Organization for the drug known as Albuterol
sulfate in the U.S.
8
These three drugs all have the same ingredients as Necon and all have very similar prices in Canada. We propose to
use a simple average of their prices.
9
In the United States, branded generic versions of levothyroxine include Levoxyl, Unithroid, Levo-T, Levolet, and
Novothyrox. In the United States, Levoxyl is the only one common enough to appear in our market baskets. In

NORC at the University of Chicago Task Order HHSP233000007T
20
We consider all of these matches to be extremely close matches that are equivalent across the two
countries’ market baskets.
Drugs with different generic status
We found one drug that is available as a generic in the United States and not in Canada: Enalapril.
For this drug, we kept the generic version in the U.S. market baskets, but substituted the brand-
name equivalent (Vasotec) in the Canadian market baskets. This is a less equivalent match than the
cases describe above, because generics and brand drugs tend to have different pricing strategies
within each country, and price comparisons between the two countries behave differently for brands
and generics. Specifically, the ratio between the Canadian price and the U.S. price tends to be higher
for generics than for brands. However, because Vasotec is the closest possible match that a U.S.
consumer would find if they looked for Enalapril in Canada, we believe using it is the best strategy
in this case. This drug is included in one market basket.
In MEPS, all insulin appears to be labeled as insulin, regardless of manufacturer. We have used
Humulin to represent insulin in both the U.S. and Canadian market baskets.
Drugs sold in different dosages or release forms
There were several drugs on our list at a dosage level not available in Canada. When possible, we
used another dosage level that is available in both countries as a substitute in both the U.S. and
Canada market baskets.
For the five cases shown in Figure A-7, we were able to substitute the same dosage levels for both
U.S. and Canadian market baskets.
Figure A-7. Dosage Levels Substituted in U.S. and Canadian Market Baskets
Drug Original Strength as
determined by MEPS
(not available in Canada;
not used in either market
basket)
Substitution
Strength
(both U.S. and
Canada)
Market Baskets
Affected
Allegra 180 mg 60 mg 10
Ibuprofen 800 mg 600 mg 2
Levoxyl/Eltroxin 0.125 mg 0.15 mg 1
Prednisone 10 mg 5 mg 5
Prednisone 20 mg 50 mg 1
Canada, versions of levothyroxine include Euthyrox, Eltroxin, and Levo-T. Eltroxin is the most commonly used of these
three Canadian drugs.

NORC at the University of Chicago Task Order HHSP233000007T
21
For four drugs, there is no dosage that is available in both countries. For these drugs, we used the
original strength in the U.S. market baskets and substituted the closest strength available in Canada
in the Canadian market baskets, as shown in Figure A-8.
Figure A-8. Dosage Levels Substituted in Canadian Market Baskets Only
Drug Original Strength as determined by
MEPS
(not available in Canada;
used in U.S. market basket)
Substitution
Strength (only in
Canada)
Market Baskets
Affected
Amoxil 400 mg/5ml 250mg/5ml 1
Flonase .05% 50 mcg 5
Flovent 44 mcg 50 mcg 1
Albuterol 90 mcg 100 mcg 14
In general, the relationship between dosage and price is not linear. That is, the price of a single 100
mg pill is rarely equal to the price of two 50 mg pills of the same drug. The differences in dosage for
these drugs should not substantially affect the price comparisons between the two countries. Again,
if a U.S. consumer were looking for these drugs, these dosages are the options they would have in
Canada.
Drugs not available in Canada
There were five drugs on our list from the United States that are not available in Canada: Toprol
XL, Glucotrol, hydrocodone with acetaminophen, Lotrel, and Vicodin. We propose dropping all of
these drugs except Toprol XL from the U.S. market baskets because no close match is available. In
addition, we propose dropping Softclix, a testing device that was listed in the MEPS drug data that is
not available in Canada. An alternative would be to include the U.S. price in the Canadian market
basket, because consumers seeking these drugs would have to buy them in the United States. This
strategy would decrease any potential differences between the two market baskets. In the case of
Toprol XL (metoprolol succinate), we have substituted Lopressor SR (metoprolol tartrate) in the
Canadian market baskets.
Figure A-9. Drugs Not Available in Canada
Drug or Device Market Baskets
Affected
Toprol XL 9
Glucotrol 1
Hydrocodone and Acetaminophen 3
Lotrel 2
Vicodin 1
Softclix 10

NORC at the University of Chicago Task Order HHSP233000007T
22
Weighting
The process outlined above provides a representative price per prescription for each drug name in a
population group’s market basket. We then combined these prices into a single index value for each
market basket. We calculated each population group’s index as the average price per prescription,
weighted by the volume of prescriptions for that group, as determined by MEPS data.
Because the research question for this project concerns the prices that U.S. customers would pay if
they bought their drugs in Canada, we used these U.S.-based volume weights for both U.S. prices
and Canadian prices.
Currency conversion
To convert Canadian prices into U.S. dollars, we used the average exchange rate for the fourth
quarter of 2005, which was $1.1732 Canadian for $1.00 U.S..
10
Because the focus of this project is
on the prices that U.S. customers would face in Canada, we did not consider using purchasing power
parity or other measures that would better represent the cost of Canadian drugs to Canadian
consumers.
Sensitivity to Drug Substitutions
To explore the sensitivity of our results to the substitution we made in the market baskets, we
calculated what the differences between U.S. and Canada prices would have been if we had excluded
two drugs: Zyrtec and Toprol XL. Zyrtec is a on on-patent prescription drug in the United States
that is available over-the-counter in Canada as Reactine, and Toprol XL is a drug for which we used
a close substitute, Lopressor SR, as the Canadian match.
In all market baskets, excluding these two drugs makes the difference between the two countries’
prices smaller. In thirteen of the fifteen market baskets, the difference is 2.2 percentage points or
less (regardless of payer). The exception is the market baskets for children under 12. Because
children take fewer drugs, these market baskets are smaller than the other market baskets – the lists
of the top third of drugs include just ten drugs for girls and eight drugs for boys. Zyrtec accounts
for about a tenth of the prescriptions included in each of these market baskets, and its exclusion
gives generic drugs a larger share of each market basket. The exclusion of Zyrtec causes the average
U.S. price per prescription for these market baskets to fall by about three dollars (6 to 10 percent),
while the average Canadian price per prescription rises slightly (1 to 4 percent). The result is a larger
drop in the relative difference between U.S. and Canadian prices for these two market baskets. This
is particularly notable for prescriptions purchased with third party coverage, where the difference
between the United States and Canada falls to 21.5 percent for girls and 23.5 percent for boys.
10
http://www.bankofcanada.ca/en/rates/exchange.html

NORC at the University of Chicago Task Order HHSP233000007T
23
Figure A-10. Sensitivity of Difference Between U.S. and Canadian Price to Inclusion of
Zyrtec and Toprol XL
With Third-Party Coverage Without Third-Party Coverage
Including
Toprol, Zyrtec
Excluding
Toprol, Zyrtec
Including
Toprol, Zyrtec
Excluding
Toprol, Zyrtec
Females under 12
-30.1% -21.5% -46.9% -41.5%
Females 12-24
-39.9% -38.6% -49.5% -48.4%
Females 25-39
-47.0% -46.0% -55.0% -54.1%
Females 40-64
-40.9% -39.5% -48.7% -47.4%
Females 65+
-37.3% -36.0% -44.0% -42.7%
Males under 12
-31.0% -23.5% -46.3% -41.1%
Males 12-24
-33.4% -31.5% -44.7% -43.1%
Males 25-39
-42.1% -40.8% -50.9% -49.7%
Males 40-64
-35.9% -34.8% -43.9% -42.9%
Males 65+
-30.6% -29.0% -38.9% -37.4%
Insured
-40.3% -38.7% -48.4% -46.9%
Uninsured
-31.4% -29.9% -40.6% -39.3%
White
-39.0% -37.1% -47.2% -45.4%
African Americans
-34.9% -32.9% -43.3% -41.5%
Hispanics
-33.6% -31.3% -44.2% -42.3%
mean
-36.5% -34.1% -46.2% -44.2%
median
-35.9% -34.8% -46.3% -42.9%
Figure A-11. Zyrtec And Toprol XL as Share of Utilitzation Included in Market Baskets
Zyrtec Toprol XL
F 12U 9.4% -
F 12-24 3.2% -
F 25-39 3.6% -
F 40-64 2.3% 2.8%
F 65+ - 4.9%
M 12U 10.7% -
M 12-24 6.0% -
M 25-39 5.3% -
M 40-64 - 4.8%
M 65+ - 4.6%
Insured 2.9% 3.1%
Uninsured - 3.9%
White 2.5% 3.7%
African Americans 2.6% 2.6%
Hispanics 2.2% 2.1%

NORC at the University of Chicago Task Order HHSP233000007T
24
Changes in Drug Use Since 2003
The MEPS data we used are from 2003. Because there are so many ongoing changes in the market,
and because more recent data are not available for all the specific subpopulations, it is impossible to
thoroughly update our sample to reflect current drug utilization. Rather than tinkering with some
drugs and leaving others untouched, we kept utilization as-is in the MEPS data. The exception is
drugs that are no longer on the market. We removed one off-market drug, Vioxx, from our market
baskets and adjusted weights accordingly.
Figure A-12 gives a snapshot of how rankings changed in IMS data for the most popular drugs from
2003 to 2005. Our market baskets include most of these top drugs -- 18 of the 20 top drugs in 2005,
and 19 of the top 20 drugs in 2003. Levothyroxine is one top 20 drug in 2005 that is not in any of
our market baskets. It was not on the top 20 in 2003, and in fact saw nearly 200 percent growth in
utilization between 2004 and 2005 alone. Because of this recent rapid growth, it seems reasonable
that levothyroxine was not in the top third of prescriptions for any of our groups in 2003.
Ambien is the one drug on both years’ top 20 lists that is not in any of our market baskets.
Utilization for Ambien was not high enough to be included in the top third of drugs for any single
population group in the 2003 MEPS data.
Figure A-12. Top 20 Drugs in 2003 and 2005
2005
Rank
2003
Rank Product
Market Baskets
Including this Drug
1 1 Lipitor 10
2 3 Hydrocodone and Acetaminophen (Mallinckrodt) 3
3 4 Norvasc 9
4 6 Toprol-XL 9
5 2 Synthroid 11
6 5 Zoloft 7
7 7 Hydrocodone and Acetaminophen (Watson) 3
8 10 Amoxicillin 9
9 >20 Lexapro 2
10 11 Albuterol 14
11 8 Zocor 9
12 >20 Nexium 6
13 >20 Levothyroxine 0
14 18 Ambien 0
15 >20 Singulair 7
16 9 Prevacid 8
17 >20 Plavix 2
18 12 Zithromax Z-Pak 11
19 19 Fosamax 4
20 14 Zyrtec 11
>20 13 Premarin 7
>20 15 Atenolol 10
>20 16 Levoxyl 8
>20 17 Celebrex 9
>20 20 Allegra 10
Source: IMS 2003a and IMS 2005.

NORC at the University of Chicago Task Order HHSP233000007T
25
Prescriptions vs. Spending
We used the number of prescriptions for each drug in each population group to determine the most
popular drugs and to weight the results. We selected this measure because we wanted our market
baskets to include both inexpensive and expensive drugs, as long as they were widely used.
Another option would have been to use total spending for each drug. Our concern was that this
would skew our sample to include more costly drugs, with disproportionately large price differences.
Figure A-13 gives an illustrative example of how spending-based market baskets might differ from
our utilization-based market baskets. Of the 20 drugs that had the most prescriptions in the United
States in 2003, over half were not on the list of the top 20 drugs with the highest spending, and vice
versa. However, many of the drugs on the highest spending list were still included in at least one
market basket. The exceptions are Epogen, Oxycontin, Procrit, Protonix, and Zyprexa.
Figure A-13. Top 20 Drugs in 2003, by Prescriptions and Spending
Rank by
Prescriptions
Rank by
Spending Product
Market
Baskets
1 1 Lipitor 10
2 >20 Synthroid 11
3 >20 Hydrocodone and Acetaminophen (Mallinckrodt) *
4 13 Norvasc 9
5 8 Zoloft 7
6 >20 Toprol-XL 9
7 >20 Hydrocodone and Acetaminophen (Watson) *
8 2 Zocor 9
9 3 Prevacid 8
10 >20 Amoxicillin 9
11 >20 Albuterol 14
12 >20 Zithromax Z-Pak 11
13 >20 Premarin 7
14 >20 Zyrtec 11
15 >20 Atenolol 10
16 >20 Levoxyl 7
17 9 Celebrex 9
18 >20 Ambien 0
19 18 Fosamax 4
20 >20 Allegra 10
>20 11 Advair Diskus 5
>20 14 Effexor XR 6
>20 6 Epogen 0
>20 10 Neurontin 2
>20 7 Nexium 6
>20 17 Oxycontin 0
>20 12 Plavix 2
>20 15 Pravachol 4
>20 4 Procrit 0
>20 19 Protonix 0
>20 16 Risperdal 1
>20 20 Vioxx N/A
>20 5 Zyprexa 0
Source: IMS 2003a and IMS 2003b.
*Hydrocodone and Acetaminophen was dropped from the study because it is not available in Canada.
NORC at the University of Chicago Task Order HHSP233000007T
26
Payer Type and Retail vs. Total Price
This project is more detailed than many in our literature review because of our ability to collect data
by payer type. This allows for a level of standardization that is rare in many comparable studies. It
does not allow us, however, to look at total price for cases with third party payers. There are likely
additional transactions between manufacturers and third party payers, such as rebates, that affect
total price but that are not reflected in our data.
The prescriptions identified in this paper as having third-party coverage are those for which a
customer had some sort of third party involvement at the pharmacy counter. These transactions
include customers who presented an insurance card at the time of the transaction. They also include
customers using discount cards. Although these customers do not receive insurance coverage for
their prescription drugs, they have access to negotiated discounts that make their prices similar to
the prices paid for drugs that are covered by insurance.
The prescriptions identified in this report as being without third-party coverage are those for which
a customer did not have third-party involvement at the pharmacy counter. While we expect that
most of these customers do not have insurance, it is possible that some have indemnity-type
insurance. These customers may be paying the full cost of drugs out-of-pocket at the drugstore, but
submitting receipts for reimbursement. However, without the involvement of an insurer at the time
of the transaction, the full price paid for these prescriptions is not discounted.

27
Market Baskets
Figure A-13. Market Basket for Females Under 12
Drug Name Status
Substitute
In Canada
Most Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.162
AMOXICILLIN Off Patent Non-Originator Non-Trademark LIQ 250/5 0.252
AMOXIL Off Patent Originator LIQ 400/5 250/5 0.058
AUGMENTIN Off Patent Originator Clavulin LIQ 400/5 0.064
CONCERTA Off Patent Non-Originator Trademark TAB 18 0.042
FLOVENT On Patent INH 44 50 0.043
SINGULAIR On Patent ORA 4 0.080
TRIMOX Off Patent Non-Originator Trademark Amoxicillin LIQ 250/5 0.042
ZITHROMAX Off Patent Originator LIQ 200/5 0.163
ZYRTEC On Patent Reactine TAB 10 0.094

28
Figure A-14. Market Basket for Females 12-24
Drug Name Status
Substitute In
Canada
Most Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ADVAIR On Patent DSK 100/50 0.038
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.085
ALESSE Off Patent Originator TAB 0.02/0.1 0.035
ALLEGRA On Patent TAB 180 60 60 0.062
AMOXICILLIN Off Patent Non-Originator Non-Trademark CAP 500 0.091
APRI Off Patent Non-Originator Trademark Ortho-Cept TAB 0.15/0.03 0.035
PLAN B On Patent TAB 0.75 0.054
CELEXA Off Patent Originator TAB 20 0.043
FLONASE On Patent INH 0.05% 50 Mcg 0.032
IBUPROFEN Off Patent Non-Originator Non-Trademark TAB 600 0.031
ORTHO EVRA On Patent Evra TDM 20/150/24 0.031
ORTHO TRI-
CYCLEN
On Patent TAB -9 0.224
PAXIL On Patent TAB 20 0.038
SINGULAIR On Patent TAB 10 0.037
ZITHROMAX Off Patent Originator TAB 250 0.076
ZOLOFT On Patent TAB 50 0.057
ZYRTEC On Patent Reactine TAB 10 0.032

29
Figure A-15. Market Basket for Females 25-39
Drug Name Status Substitute In Canada
Most Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.062
ALLEGRA On Patent TAB 180 60 60 0.060
AMOXICILLIN Off Patent Non-Originator Non-Trademark CAP 500 0.034
APRI Off Patent Non-Originator Trademark Ortho-Cept TAB 0.15 0.041
PLAN B On Patent TAB 0.75 0.042
CELEXA Off Patent Originator TAB 20 0.027
EFFEXOR On Patent TAB 75 0.032
FLONASE On Patent INH 0.05% 50 Mcg 0.033
IBUPROFEN Off Patent Non-Originator Non-Trademark TAB 800 600 600 0.028
LEVOXYL Off Patent Non-Originator Trademark Eltroxin TAB 0.15 0.029
LEXAPRO On Patent Cipralex TAB 10 0.028
NECON Off Patent Non-Originator Trademark
Brevicon, Loestrin,
Minestrin
TAB 35/1 0.029
ORTHO EVRA On Patent Evra TDM 20/150/24 0.027
ORTHO TRI-
CYCLEN On Patent TAB -9 0.131
ORTHO-NOVUM Off Patent Originator Ortho 1/35 TAB 1/35 0.038
PAXIL On Patent TAB 20 0.051
PREDNISONE Off Patent Non-Originator Non-Trademark TAB 10 5 5 0.025
SYNTHROID Off Patent Originator TAB 0.075 0.085
WELLBUTRIN Off Patent Originator TAB 150 0.029
YASMIN On Patent TAB 3/0.03 0.039
ZITHROMAX Off Patent Originator TAB 250 0.049
ZOLOFT On Patent TAB 100 0.043
ZYRTEC On Patent Reactine TAB 10 0.036

30
Figure A-16. Market Basket for Females 40-64
Drug Name Status Substitute In Canada
Most
Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.030
ALLEGRA On Patent TAB 180 60 60 0.037
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.046
CELEBREX On Patent CAP 200 0.030
CELEXA Off Patent Originator TAB 20 0.026
EFFEXOR On Patent CAP 150 0.029
FLUOXETINE Off Patent Non-Originator Non-Trademark CAP 20 0.024
FOSAMAX On Patent TAB 70 0.027
FUROSEMIDE Off Patent Non-Originator Non-Trademark TAB 40 0.023
GLUCOPHAGE Off Patent Originator TAB 500 0.025
HYDROCHLOROTHIAZIDE Off Patent Non-Originator Non-Trademark TAB 25 0.044
LEVOXYL Off Patent Non-Originator Trademark Eltroxin TAB 0.1 0.033
LIPITOR On Patent TAB 10 0.082
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.032
METFORMIN Off Patent Non-Originator Non-Trademark TAB 500 0.024
NEURONTIN On Patent CAP 300 0.023
NEXIUM On Patent CAP 40 0.027
NORVASC On Patent TAB 5 0.032
PAXIL On Patent TAB 20 0.034
PREMARIN Off Patent Originator TAB 0.625 0.077
PREVACID On Patent CAP 30 0.034
SOFTCLIX On Patent Not Included
SYNTHROID Off Patent Originator TAB 0.1 0.090
TOPROL XL Off Patent Originator Lopressor SR TAB 50 0.028
TRAZODONE Off Patent Non-Originator Non-Trademark TAB 50 0.022
ZITHROMAX Off Patent Originator TAB 250 0.027
ZOCOR On Patent TAB 20 0.030
ZOLOFT On Patent TAB 100 0.042
ZYRTEC On Patent Reactine TAB 10 0.023

31
Figure A-17. Market Basket for Females 65+
Drug Name Status Substitute In Canada
Most
Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.061
CELEBREX On Patent CAP 200 0.040
DIOVAN On Patent TAB 160 0.037
EVISTA On Patent TAB 60 0.024
FOSAMAX On Patent TAB 70 0.066
FUROSEMIDE Off Patent Non-Originator Non-Trademark TAB 40 0.078
HYDROCHLOROTHIAZIDE Off Patent Non-Originator Non-Trademark TAB 25 0.054
LEVOXYL Off Patent Non-Originator Trademark Eltroxin TAB 0.1 0.037
LIPITOR On Patent TAB 10 0.099
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.049
METFORMIN Off Patent Non-Originator Non-Trademark TAB 500 0.026
METOPROLOL Off Patent Non-Originator Non-Trademark TAB 50 0.038
NORVASC On Patent TAB 5 0.068
PLAVIX On Patent TAB 75 0.031
PREMARIN Off Patent Originator TAB 0.625 0.048
PREVACID On Patent CAP 30 0.026
SOFTCLIX On Patent Not included
SPIRONOLACTONE Off Patent Non-Originator Non-Trademark Novo-Spiroton TAB 25 0.025
SYNTHROID Off Patent Originator TAB 0.1 0.086
TOPROL XL Off Patent Originator Lopressor SR TAB 50 0.049
ZOCOR On Patent TAB 20 0.058

32
Figure A-18. Market Basket for Males Under 12
Drug Name Status
Substitute
In Canada
Most Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ADDERALL Off Patent Non-Originator Trademark TAB 10 0.056
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.214
AMOXICILLIN Off Patent Non-Originator Non-Trademark LIQ 250/5 0.203
AUGMENTIN Off Patent Originator Clavulin LIQ 400/5 0.055
CONCERTA Off Patent Non-Originator Trademark TAB 18 0.094
SINGULAIR On Patent ORA 5 0.144
ZITHROMAX Off Patent Originator LIQ 200/5 0.127
ZYRTEC On Patent Reactine TAB 10 0.107
Figure A-19. Market Basket for Males 12-24
Drug Name Status
Substitute
In Canada
Most Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ADDERALL Off Patent Non-Originator Trademark CAP 30 0.094
ADVAIR On Patent DSK 100/50 0.041
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.100
ALLEGRA On Patent TAB 180 60 60 0.089
AMOXICILLIN Off Patent Non-Originator Non-Trademark CAP 500 0.078
CLARINEX On Patent Aerius TAB 5 0.037
CONCERTA Off Patent Non-Originator Trademark TAB 54 0.101
EFFEXOR On Patent TAB 75 0.061
MINOCYCLINE Off Patent Non-Originator Non-Trademark CAP 100 0.047
NASONEX On Patent INH 50 0.047
RISPERDAL On Patent TAB 0.5 0.065
SINGULAIR On Patent TAB 10 0.052
STRATTERA On Patent CAP 40 0.043
ZITHROMAX Off Patent Originator TAB 250 0.083
ZYRTEC On Patent Reactine TAB 10 0.060

33
Figure A-20. Market Basket for Males 25-39
Drug Name Status
Substitute In
Canada
Most Common Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ACETAMINOPHEN
HYDROCODONE
Off Patent Originator Not on market
ADVAIR On Patent DSK 100/50 0.038
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.047
ALLEGRA On Patent TAB 180 60 60 0.094
ALLOPURINOL On Patent Dropped due to small sample size.
AMOXICILLIN Off Patent Non-Originator Non-Trademark CAP 500 0.052
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.036
CELEBREX On Patent CAP 200 0.026
CLARITIN Off Patent Originator LIQ 10/10 0.033
DEPAKOTE Off Patent Originator Epival TAB 500 0.039
EFFEXOR On Patent TAB 75 0.027
FLONASE On Patent INH 0.05% 50 Mcg 0.035
IBUPROFEN Off Patent Non-Originator Non-Trademark TAB 800 600 600 0.037
LEVOXYL Off Patent Non-Originator Trademark Dropped due to small sample size.
LEXAPRO On Patent Cipralex TAB 10 0.040
LIPITOR On Patent TAB 10 0.066
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.035
NEURONTIN On Patent TAB 600 0.033
NEXIUM On Patent CAP 40 0.063
PAXIL On Patent TAB 20 0.047
ROXICODONE On Patent Dropped due to small sample size.
SOFTCLIX On Patent Not Included
SYNTHROID Off Patent Originator TAB 0.1 0.045
VICODIN Off Patent Non-Originator Trademark Not on market
WELLBUTRIN Off Patent Originator TAB 150 0.031
ZITHROMAX Off Patent Originator TAB 250 0.069
ZOLOFT On Patent TAB 50 0.053
ZYRTEC On Patent Reactine TAB 10 0.053

34
Figure A-21. Market Basket for Males 40-64
Drug Name Status
Substitute In
Canada
Most
Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ACCUPRIL Off Patent Originator TAB 20 0.026
ACETAMINOPHEN
HYDROCODONE
Off Patent Originator Not on market
ACTOS On Patent TAB 45 0.026
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.028
ALLEGRA On Patent TAB 180 60 60 0.030
ALPRAZOLAM Off Patent Non-Originator Non-Trademark TAB 0.5 0.025
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.061
CELEBREX On Patent CAP 200 0.024
DIOVAN On Patent TAB 80 0.027
FUROSEMIDE Off Patent Non-Originator Non-Trademark TAB 40 0.028
GLUCOPHAGE Off Patent Originator TAB 500 0.029
HYDROCHLOROTHIAZIDE Off Patent Non-Originator Non-Trademark TAB 25 0.054
LIPITOR On Patent TAB 10 0.162
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.068
METFORMIN Off Patent Non-Originator Non-Trademark TAB 500 0.037
METOPROLOL Off Patent Non-Originator Non-Trademark TAB 50 0.029
NEXIUM On Patent CAP 40 0.036
NORVASC On Patent TAB 5 0.050
PAXIL On Patent TAB 20 0.026
PRAVACHOL On Patent TAB 40 0.032
PREVACID On Patent CAP 30 0.034
RANITIDINE Off Patent Non-Originator Non-Trademark TAB 150 0.027
SOFTCLIX On Patent Not included
SYNTHROID Off Patent Originator TAB 0.112 0.023
TOPROL XL Off Patent Originator Lopressor SR TAB 50 0.048
VIOXX Off Patent Non-Originator Trademark TAB 25 0.000
ZOCOR On Patent TAB 20 0.070

35
Figure A-22. Market Basket for Males 65+
Drug Name Status
Substitute In
Canada
Most
Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.029
ASPIRIN Off Patent Non-Originator Non-Trademark TAB 325 0.028
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.075
COUMADIN Off Patent Originator TAB 5 0.042
DOXAZOSIN Off Patent Non-Originator Non-Trademark TAB 2 0.038
FLOMAX On Patent CAP 0.4 0.039
FUROSEMIDE Off Patent Non-Originator Non-Trademark TAB 40 0.080
HYDROCHLOROTHIAZIDE Off Patent Non-Originator Non-Trademark TAB 25 0.053
LIPITOR On Patent TAB 10 0.113
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.088
METFORMIN Off Patent Non-Originator Non-Trademark TAB 500 0.028
METOPROLOL Off Patent Non-Originator Non-Trademark TAB 50 0.047
NORVASC On Patent TAB 10 0.046
PLAVIX On Patent TAB 75 0.034
PRAVACHOL On Patent TAB 40 0.033
SOFTCLIX On Patent Not included
SYNTHROID Off Patent Originator TAB 0.1 0.032
TERAZOSIN Off Patent Non-Originator Non-Trademark CAP 5 0.033
TOPROL XL Off Patent Originator Lopressor SR TAB 50 0.046
WARFARIN Off Patent Non-Originator Non-Trademark TAB 5 0.039
ZOCOR On Patent TAB 20 0.077

36
Figure A-23. Market Basket for Insured
Drug Name Status Substitute In Canada
Most
Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ADVAIR On Patent DSK 100/50 0.022
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.064
ALLEGRA On Patent TAB 180 60 60 0.037
AMOXICILLIN Off Patent Non-Originator Non-Trademark CAP 500 0.031
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.045
CELEBREX On Patent CAP 200 0.025
EFFEXOR On Patent CAP 150 0.021
FLONASE On Patent INH 0.05% 50 Mcg 0.020
FUROSEMIDE Off Patent Non-Originator Non-Trademark TAB 40 0.029
GLUCOPHAGE Off Patent Originator TAB 500 0.021
HYDROCHLOROTHIAZIDE Off Patent Non-Originator Non-Trademark TAB 25 0.037
LEVOXYL Off Patent Non-Originator Trademark Eltroxin TAB 0.1 0.024
LIPITOR On Patent TAB 10 0.093
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.041
METFORMIN Off Patent Non-Originator Non-Trademark TAB 500 0.023
NEXIUM On Patent CAP 40 0.025
NORVASC On Patent TAB 5 0.036
ORTHO TRI-CYCLEN On Patent TAB -9 0.021
PAXIL On Patent TAB 20 0.027
PRAVACHOL On Patent TAB 40 0.019
PREDNISONE Off Patent Non-Originator Non-Trademark TAB 10 0.019
PREMARIN Off Patent Originator TAB 0.625 0.034
PREVACID On Patent CAP 30 0.030
SINGULAIR On Patent TAB 10 0.025
SOFTCLIX On Patent Not included
SYNTHROID Off Patent Originator TAB 0.1 0.060
TOPROL XL Off Patent Originator Lopressor SR TAB 50 0.031
VIOXX Off Patent Non-Originator Trademark TAB 25 0.000
ZITHROMAX Off Patent Originator TAB 250 0.037
ZOCOR On Patent TAB 20 0.042
ZOLOFT On Patent TAB 100 0.030
ZYRTEC On Patent Reactine TAB 10 0.029

37
Figure A-24. Market Basket for Uninsured
Drug Name Status Substitute In Canada
Most
Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ACCUPRIL Off Patent Originator TAB 20 0.021
ACETAMINOPHEN
HYDROCODONE
Off Patent Originator Not on market
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.041
ALPRAZOLAM Off Patent Non-Originator Non-Trademark TAB 0.25 0.024
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.057
CELEBREX On Patent CAP 200 0.023
DIOVAN On Patent TAB 160 0.029
FOSAMAX On Patent TAB 70 0.027
FUROSEMIDE Off Patent Non-Originator Non-Trademark TAB 40 0.064
GLYBURIDE Off Patent Non-Originator Non-Trademark TAB 5 0.027
HYDROCHLOROTHIAZIDE Off Patent Non-Originator Non-Trademark TAB 25 0.060
HUMULIN Off Patent Non-Originator Non-Trademark INJ 100 0.033
LEVOXYL Off Patent Non-Originator Trademark Eltroxin TAB 0.05 0.030
LIPITOR On Patent TAB 10 0.076
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.057
LOTREL Off Patent Non-Originator Non-Trademark Not on market
METFORMIN Off Patent Non-Originator Non-Trademark TAB 500 0.030
METOPROLOL Off Patent Non-Originator Non-Trademark TAB 50 0.051
NORVASC On Patent TAB 10 0.049
PAXIL On Patent TAB 20 0.023
PREDNISONE Off Patent Non-Originator Non-Trademark TAB 10 0.028
PREMARIN Off Patent Originator TAB 0.625 0.034
PREVACID On Patent CAP 30 0.023
RANITIDINE Off Patent Non-Originator Non-Trademark TAB 150 0.029
SOFTCLIX On Patent Not included
SYNTHROID Off Patent Originator TAB 0.1 0.061
TOPROL XL Off Patent Originator Lopressor SR TAB 50 0.039
ZOCOR On Patent TAB 20 0.043
ZOLOFT On Patent TAB 50 0.022

38
Figure A-25. Market Basket for Non-Hispanic Whites
Drug Name Status Substitute In Canada
Most
Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.032
ALLEGRA On Patent TAB 180 60 60 0.034
AMOXICILLIN Off Patent Non-Originator Non-Trademark CAP 500 0.028
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.048
CELEBREX On Patent CAP 200 0.025
DIOVAN On Patent TAB 80 0.021
EFFEXOR On Patent CAP 75 0.022
FLONASE On Patent INH 0.05% 50 Mcg 0.019
FOSAMAX On Patent TAB 70 0.025
FUROSEMIDE Off Patent Non-Originator Non-Trademark TAB 40 0.040
HYDROCHLOROTHIAZIDE Off Patent Non-Originator Non-Trademark TAB 25 0.037
LEVOXYL Off Patent Non-Originator Trademark Eltroxin TAB 0.1 0.027
LIPITOR On Patent TAB 10 0.095
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.044
METFORMIN Off Patent Non-Originator Non-Trademark TAB 500 0.020
METOPROLOL Off Patent Non-Originator Non-Trademark TAB 50 0.025
NEXIUM On Patent CAP 40 0.024
NORVASC On Patent TAB 5 0.035
ORTHO TRI-CYCLEN On Patent TAB -9 0.021
PAXIL On Patent TAB 20 0.029
PREDNISONE Off Patent Non-Originator Non-Trademark TAB 10 0.020
PREMARIN Off Patent Originator TAB 0.625 0.035
PREVACID On Patent CAP 30 0.028
RANITIDINE Off Patent Non-Originator Non-Trademark TAB 150 0.019
SOFTCLIX On Patent Not included
SYNTHROID Off Patent Originator TAB 0.1 0.071
TOPROL XL Off Patent Originator Lopressor SR TAB 50 0.037
VIOXX Off Patent Non-Originator Trademark TAB 25 0.000
ZITHROMAX Off Patent Originator TAB 250 0.033
ZOCOR On Patent TAB 20 0.047
ZOLOFT On Patent TAB 100 0.031
ZYRTEC On Patent Reactine TAB 10 0.025

39
Figure A-26. Market Basket for African Americans
Drug Name Status
Substitute In
Canada
Most
Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ADVAIR On Patent DSK 100/50 0.026
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.075
ALLEGRA On Patent TAB 180 60 60 0.024
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.053
CELEBREX On Patent CAP 200 0.023
CLONIDINE Off Patent Non-Originator Non-Trademark TAB 0.2 0.027
DIOVAN On Patent TAB 160 0.031
FUROSEMIDE Off Patent Non-Originator Non-Trademark TAB 40 0.063
GLUCOPHAGE Off Patent Originator TAB 500 0.035
GLUCOTROL Off Patent Originator Not on market
HUMULIN Off Patent Originator LIQ 100 0.022
HUMULIN Off Patent Non-Originator Non-Trademark INJ 100 0.032
HYDROCHLOROTHIAZIDE Off Patent Non-Originator Non-Trademark TAB 25 0.087
LIPITOR On Patent TAB 10 0.069
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.050
LOTREL Off Patent Non-Originator Non-Trademark Not on market
METFORMIN Off Patent Non-Originator Non-Trademark TAB 500 0.030
NORVASC On Patent TAB 10 0.081
PRAVACHOL On Patent TAB 40 0.030
PREDNISONE Off Patent Non-Originator Non-Trademark TAB 10 0.024
PREMARIN Off Patent Originator TAB 0.625 0.024
PREVACID On Patent CAP 30 0.028
SINGULAIR On Patent TAB 10 0.032
SOFTCLIX On Patent Not included
SYNTHROID Off Patent Originator TAB 0.1 0.025
TOPROL XL Off Patent Originator Lopressor SR TAB 50 0.026
ZITHROMAX Off Patent Originator TAB 250 0.024
ZOCOR On Patent TAB 20 0.033
ZYRTEC On Patent Reactine TAB 10 0.026

40
Figure A-27. Market Basket for Hispanics
Drug Name Status Substitute In Canada
Most
Common
Form And
Strength
Strength
Substituted
In U.S.
Strength
Substituted
In Canada Weight
ALBUTEROL Off Patent Non-Originator Non-Trademark Salbutamol INH 90 100 0.075
ALLEGRA On Patent TAB 180 60 60 0.030
AMOXICILLIN Off Patent Non-Originator Non-Trademark LIQ 250/5 0.046
ASPIRIN Off Patent Non-Originator Non-Trademark TAB 325 0.026
ATENOLOL Off Patent Non-Originator Non-Trademark TAB 50 0.043
CELEBREX On Patent CAP 200 0.036
ENALAPRIL Off Patent Non-Originator Non-Trademark Vasotec TAB 10 0.020
FUROSEMIDE Off Patent Non-Originator Non-Trademark TAB 40 0.020
GLUCOPHAGE Off Patent Originator TAB 500 0.031
GLYBURIDE Off Patent Non-Originator Non-Trademark TAB 5 0.029
HYDROCHLOROTHIAZIDE Off Patent Non-Originator Non-Trademark TAB 25 0.035
IBUPROFEN Off Patent Non-Originator Non-Trademark TAB 600 0.037
HUMULIN Off Patent Non-Originator Non-Trademark INJ 100 0.021
LEVOXYL Off Patent Non-Originator Trademark Eltroxin TAB 0.125 0.15 0.15 0.019
LIPITOR On Patent TAB 10 0.064
LISINOPRIL Off Patent Non-Originator Non-Trademark TAB 10 0.043
METFORMIN Off Patent Non-Originator Non-Trademark TAB 500 0.045
METOPROLOL Off Patent Non-Originator Non-Trademark TAB 50 0.022
NAPROXEN Off Patent Non-Originator Non-Trademark TAB 500 0.019
NEXIUM On Patent CAP 40 0.022
NORVASC On Patent TAB 10 0.029
PAXIL On Patent TAB 20 0.030
PREDNISONE Off Patent Non-Originator Non-Trademark TAB 20 50 50 0.028
PREMARIN Off Patent Originator TAB 0.625 0.022
PREVACID On Patent CAP 30 0.023
SINGULAIR On Patent TAB 10 0.035
SOFTCLIX On Patent Not included
SYNTHROID Off Patent Originator TAB 0.1 0.037
TOPROL XL Off Patent Originator Lopressor SR TAB 50 0.021
ZITHROMAX Off Patent Originator LIQ 200/5 0.044
ZOCOR On Patent TAB 20 0.027
ZYRTEC On Patent Reactine TAB 10 0.022

41
Figure A-28. Summary of Drugs Included in Market Baskets
Drug
/Canadian Drug Form
Strength
(Canadian)
Market
Baskets
Accupril TAB 20 2
Actos TAB 45 1
Adderall CAP 30 1
Adderall TAB 10 1
Advair DSK 100/50 5
Albuterol/Salbutamol INH 90 (100) 14
Alesse TAB 0.02/0.1 1
Allegra TAB 60 10
Alprazolam TAB 0.25 1
Alprazolam TAB 0.5 1
Amoxicillin CAP 500 6
Amoxicillin LIQ 250/5 3
Amoxil LIQ
400/5
(250/5)
1
Apri/Ortho-cept TAB 0.15 1
Apri/Ortho-cept TAB 0.15/0.03 1
Aspirin TAB 325 2
Atenolol TAB 50 10
Augmentin/
Clavulin
LIQ 400/5 2
Plan B TAB 0.75 2
Celebrex CAP 200 9
Celexa TAB 20 3
Clarinex/Aerius TAB 5 1
Claritin LIQ 10/10 1
Clonidine TAB 0.2 1
Concerta TAB 18 2
Concerta TAB 54 1
Coumadin TAB 5 1
Depakote/Epival TAB 500 1
Diovan TAB 160 3
Diovan TAB 80 2
Doxazosin TAB 2 1
Effexor CAP 150 2
Effexor CAP 75 1
Effexor TAB 75 3
Drug
/Canadian Drug Form
Strength
(Canadian)
Market
Baskets
Enalapril/Vasotec TAB 10 1
Evista TAB 60 1
Flomax CAP 0.4 1
Flonase INH
0.05%
(50 mcg)
5
Flovent INH 44 (50) 1
Fluoxetine CAP 20 1
Fosamax TAB 70 4
Furosemide TAB 40 9
Glucophage TAB 500 5
Glyburide TAB 5 2
Humulin Insulin LIQ 100 1
Humulin Insulin INJ 100 3
Hydrochlorothiazide TAB 25 9
Ibuprofen TAB 600 2
Ibuprofen TAB 600 2
Levoxyl/Eltroxin TAB 0.05 1
Levoxyl/Eltroxin TAB 0.1 4
Levoxyl/Eltroxin TAB 0.15 2
Lexapro/Cipralex TAB 10 2
Lipitor TAB 10 10
Lisinopril TAB 10 10
Metformin TAB 500 9
Metoprolol TAB 50 6
Minocycline CAP 100 1
Naproxen TAB 500 1
Nasonex INH 50 1
Necon/Brevicon,
Loestrin, Minestrin
TAB 35/1 1
Neurontin CAP 300 1
Neurontin TAB 600 1
Nexium CAP 40 6
Norvasc TAB 10 4
Norvasc TAB 5 5
Ortho Evra/Evra TDM 20/150/ 2
Ortho Tri-cyclen TAB -9 4
Drug
/Canadian Drug Form
Strength
(Canadian)
Market
Baskets
Ortho-Novum/
Ortho 1/35
TAB 12785 1
Paxil TAB 20 9
Plavix TAB 75 2
Pravachol TAB 40 4
Prednisone TAB 5 5
Prednisone TAB 50 1
Premarin TAB 0.625 7
Prevacid CAP 30 8
Ranitidine TAB 150 3
Risperdal TAB 0.5 1
Singulair ORA 4 1
Singulair ORA 5 1
Singulair TAB 10 5
Spironolactone/
Novo-Spiraton
TAB 25 1
Strattera CAP 40 1
Synthroid TAB 0.075 1
Synthroid TAB 0.1 9
Synthroid TAB 0.112 1
Terazosin CAP 5 1
Toprol TAB 50 9
Trazodone TAB 50 1
Trimox/Amoxicillin LIQ 250/5 1
Vioxx TAB 25 3
Warfarin TAB 5 1
Wellbutrin TAB 150 2
Yasmin TAB 3/0.03 1
Zithromax LIQ 200/5 3
Zithromax TAB 250 8
Zocor TAB 20 9
Zoloft TAB 100 4
Zoloft TAB 50 3
Zyrtec/Reactine TAB 10 11
42
Appendix B: Literature Review
Rapidly rising prescription drug prices have caused many consumers, third-party payers, and
policymakers to look for ways to lower their drug costs. One strategy that many have seen as a
potential solution is to import drug products from countries where drug prices are lower than those
in the United States—commonly called reimportation.
Prices for on-patent drugs are controlled by federal regulation in Canada. New brand drugs are
limited to the median of prices for the new drug in France, Germany, Italy, Sweden, Switzerland, the
UK, and the United States. Limits for drugs that are modifications of existing drugs (usually known
as “me-too” drugs) are based on the breakthrough brand’s price; most new drugs cannot cost more
than the other drugs in the same therapeutic class. Increases in prices for existing on-patent drugs
are linked to a multiple of the consumer price index, often resulting in a lower rate of increase than
the drug inflation rate in the United States.
Other price controls for a significant portion of the Canadian market take place at the provincial
level. The Canadian provinces cover 42 percent of all national drug expenditures through their
coverage of prescription drugs for the elderly. The provinces all use formularies to keep prices low
for the drugs they cover, and some take further measures. Ontario has instituted price freezes;
British Columbia uses reference pricing; and Quebec requires companies to give the province the
best price given to any other province.
Legislative and regulatory price controls in the United States apply to drugs purchased through the
federal supply schedule (for sales to certain federal purchasers and other entities, such as community
health centers, that are by law authorized to use the federal supply schedule) and drugs purchased by
the Medicaid program, the Veterans Administration, and the Department of Defense. However,
insurers and pharmacy benefit managers (PBMs) are also able to negotiate substantial discounts and
rebates from retail prices. Uninsured individuals in the United States do not have access to these
lower prices, and they typically pay retail prices that are about 15 percent above those paid by people
with insurance. As a result, those without insurance have been at the forefront of the push to import
drugs from Canada. Some purchasers, including several municipal government employee plans, have
also begun to explore the possibility of savings for their insured employees from reimportation,
seeking to get better prices than those normally obtained by insurers and PBMs.
The Canadian Pricing System
While Canada does offer universal access to health insurance, that insurance covers only acute
hospital and physician services, and does not provide coverage for outpatient prescription drugs.
Most Canadians receive prescription drug coverage through their employers, although the federal
government offers coverage to some populations, including veterans and First Nations and Inuit
people, and there are publicly funded provincial programs that pay a substantial share of the costs
for older, disabled, and low-income Canadians (Gross, 2003).
Graham (2000) provides an explanation of how prescription drug prices are regulated in Canada.
Canada’s Patented Medicine Prices Review Board (PMPRB), established in 1987, regulates the prices
43
of newly patented drugs. The PMPRB controls the price at which drug manufacturers sell to
wholesalers. Drugs are divided into three categories by the PMPRB when they are introduced:
Category 1, or “line extension,” drugs are drugs that are a new strength of an existing drug. Prices of
new drugs in this category must bear a “reasonable relationship” to the average price of the same
medicine in the same or comparable dosage forms.
Category 2 is composed of “breakthrough” drugs, which produce a substantial improvement over
older drugs. These drugs may not exceed the higher of the cost of therapy with medicines in the
same therapeutic class and the median of prices of the same drugs in the United States, United
Kingdom, Switzerland, Sweden, Italy, Germany, and France.
Category 3 drugs, or “me-too” drugs provide moderate, little, or no improvement over existing
medications and introductory prices is presumed excessive if the cost of therapy with the new drug
is higher than the cost of therapy with comparable drugs in the same therapeutic class.
After drugs are first introduced, price increases are limited by the Consumer Price Index: single year
increases are limited to 1.5 times the forecast change in the annual index, and in periods when
inflation is greater than 10 percent, price increases are limited to five percentage points more than
the forecast change in the CPI.
The PMPRB has the power to order price reductions, if it considers that a price is excessive, and it
can also order rebates to customers, a payment to the Crown, a temporary reduction below the
assessed fair price, or a temporary price reduction of another patented drug manufactured by the
same company (Graham, 2000).
The PMPRB only has the power to control manufacturers’ “gate prices,” or the price at which
manufacturers sell to wholesalers. Wholesale price, retail margin, pharmacists’ dispensing fees, and
other distribution costs are not subject to federal regulations. Some provincial governments,
however, set policies that affect the prices paid by consumers.
Because provincial governments finance 42 percent of national prescription drug expenditures, the
provincial drug benefit plans have the ability to influence the prescription drug marketplace, but
these plans do not generally negotiate prices with suppliers. Instead, some provincial governments
set policies for the overall prices they will pay for drugs. In Quebec, the government requires
manufacturers to charge it no more than the best available price in the rest of Canada. The
government of Ontario has frozen the retail price it will pay for all drugs listed on its formulary
(Morgan et al., 2003). British Columbia uses “reference-based pricing,” in which the province’s drug
benefits program only reimburses for the cost of the drug in a therapeutic class with the lowest price
(Menon, 2001).
In addition to these direct controls on prices, the formularies of provincial prescription drug benefit
plans also play an important role in keeping drug prices low. The provincial plans only cover a set
list of drugs, and manufacturers must submit new drugs to provincial programs to be reviewed for
inclusion on the plan formulary. Drugs are reviewed for their effectiveness in relation to their costs,
and drugs that are equivalent to already listed drugs are added only if they do not increase program
costs (Menon, 2001).
The prices paid by individuals, private insurers, and health care institutions are not regulated in
Canada. However, the prices that these groups pay are influenced by the prices set by the provincial
44
governments. Individuals paying retail prices likely face the highest drug costs, while third-party
payers and hospitals may have the buying power to negotiate discounts, as they do in the United
States.
Methodology of Price Comparisons
While there is a general agreement that prescription drugs cost less in Canada than in the United
States, there is less agreement on the magnitude of the difference. Several important methodological
issues are raised by international price comparisons, for example, which drugs are included in the
study sample and at which point in the chain of distribution prices are measured. These issues can
affect the results of such comparisons.
Level of distribution
Comparisons of international drug prices must select a point in the chain of distribution at which to
measure the price of drugs. Previous studies have looked at the price charged by drug
manufacturers, the prices charged by drug wholesalers, and the prices charged by retail pharmacies
(Gross, 2000). While studies that compare any of these points in the chain of distribution may have
valid results, studies must use apples-to-apples comparisons, comparing the prices at the same level
of the distribution chain for all countries in the analysis (Danzon, 1999). In the following analysis,
we have separated studies by whether they work with manufacturer or wholesale prices or with retail
prices.
Data sources
Measuring prices accurately at a single of level of distribution also proves difficult. Reliance on list
prices published by the manufacturer can be particularly problematic (Wagner and McCarthy, 2004).
Wagner and McCarthy argue the upward bias in list prices is a particular issue in the U.S., where
reimbursements by the Medicaid program and other payers create incentives for high list prices with
discounts to pharmacies, so that prices for generic multi-source and brand single-source drugs are,
on average, 78 percent and 22 percent lower, respectively, than list prices. The list prices used in
some of the studies cited here include Medi-Span and Redbook prices.
More realistic prices are available from transaction data collected by organizations like IMS Health.
The IMS MIDAS database includes invoice data on transactions between manufacturers and
wholesalers, pharmacies, hospitals, and other large purchasers in over 60 countries. IMS also
collects data from pharmacy transactions in several countries, including the U.S. and Canada.
Other studies have directly contacted pharmacies to determine prices, either by phone or internet.
The prices obtained in this manner likely reflect prices charged to individuals without insurance, and
may be higher than the prices that third-party insurers pay. Studies using this method also tend to
have far smaller sample sizes than IMS databases, which collect data from thousands of pharmacies.
45
Drug sample
Studies comparing different countries’ pharmaceutical prices vary in which drugs they select for
analysis. Some studies may select only a few drugs. Several studies attempt to make broad
comparisons among all commonly used drugs (Gross, 2003). Many international price comparisons
look only at brand-name prescription drugs, while several other comparisons include generic drugs
as well. Danzon (1999) argues that international drug price comparisons must include a
representative random sample of drugs, and that in order to be representative, the market basket
must include generic drugs. In addition, she states that studies that consider drugs with high sales
volumes are biased if the products are chosen by those with the highest volume by dollar value, not
by number of prescriptions, as products with relatively high prices will be more likely to be selected.
Danzon and Kim (1998) argue that valid measures of average price levels can only be obtained from
comparisons based on a comprehensive or representative sample of products, appropriately
weighted, following standard index number methods. Researchers must accept trade-offs between
the ability to compare identical products and the need to examine a representative sample of a
country’s pharmaceutical market. There is wide variation between countries not only in the range of
compounds available, but also in the dosage forms, strengths, and pack sizes available. If
comparisons are made only among products that are the same molecule, manufacturers, strength,
and pack size, only a very small and unrepresentative sample will available. Danzon and Kim
maintain that requirements that products be identical should be dropped in favor of broad
representation for each country’s market basket of products, which should include generics and
over-the-counter products that are good substitutes for branded prescription drugs, with all forms,
strengths, and package sizes.
Price indices and weights
Researchers conducting international comparisons of prescription drug prices should choose
appropriate weights for each drug’s price difference in the process of calculating an average price
difference for a market basket of products (Gross, 2003). However, many published comparisons do
not weight their samples. Danzon (1999) argues that studies that use unweighted averages of price
ratios violate standard principles of price comparisons and lead to results that are extremely sensitive
to the sample used. Wagner and McCarthy (2004) support weighting by the quantity of each drug
dispensed to develop a comparative index of price levels, although the country whose quantity
weights used as the basis for comparison can influence the resulting index of relative prices.
In an analysis summarized in more detail below, Danzon and Kim (1998) report the results of three
different price indices to demonstrate the effects that weighting has on comparison results: the
Laspeyres index, which uses the base country quantity weights, the Paasche index, which uses the
comparison country quantity weights, and the Fisher index, which uses an average of the observed
quantities as weights. Danzon and Kim contend that the Laspeyres index has advantages for use in
price comparisons, because accurate data for the country selected as the base are more likely to be
available if it is the country undertaking the study. In addition, the Laspeyres index does not
assume, as the Paasche and Fisher indices do, that consumption patterns would be identical in two
countries if they both faced the same prices. This can be important when drug consumption has
low price elasticity due to the role of medical norms.
46
Whose price
The results of international pharmaceutical price comparisons will also differ with regard to which
payer’s price is being measured. While in Canada there is little variation in the prices paid by
different payers, in the United States the cost of drugs can vary widely according to who is
purchasing drugs: individuals paying cash pay the highest price, while insurers, managed care plans,
and government programs can negotiate discounts and manufacturer rebates by buying drugs in
large quantities (CBO, 2004; Hollis, 2004; Gross, 2003; Morgan et al., 2003). A 2000 study by the
Department of Health and Human Services found that U.S. senior citizens paying for their own
prescription drugs pay about 15 percent more for those drugs (excluding the effect of rebates) than
individuals with health insurance. In addition, this 2000 study and a 1999 analysis by the House of
Representatives Committee on Government Reform found that favored customers, such as health
maintenance organizations, and the federal government obtain even lower prices than individuals
with insurance.
Danzon (1999) argues that these differences in prices are not a matter of discrimination, but an issue
of different market segments. She maintains that the difference in prices does not imply cost
shifting, but simply that a manufacturer serving two separate markets rationally determines the price
to charge in each market independently. As with other methodological issues, it is important that
price comparisons use the prices of similar payers in both countries under consideration.
Currency conversion
In addition to methodological issues in selecting a sample and choosing which prices to use,
international pharmaceutical price comparisons must choose an exchange rate to use in analyses.
The exchange rate should not be sensitive to day-to-day currency fluctuations, but should be able to
capture the cost to citizens in one country of buying drugs in another country (Gross, 2003).
Danzon and Furukawa (2003) found that currency conversion can contribute greatly to measured
price differences: converting currencies using purchasing power parities (PPPs) based on an
economy-wide market basket of goods (GDP) or on a basket of medical goods or services, rather
than on the current exchange rate, can drastically change the results of a price comparison.
Price Comparisons
As a result of these methodological challenges, the results of previous studies that compare the
prices of prescription drugs in Canada and the United States vary. Figure B-1 summarizes
comparisons of manufacturer or wholesale prices; Figure B-2 summarizes comparisons of retail
prices. More details about the analyses follow.

47
Figure B-1. Summary of U.S./Canada Prescription Drug Price Comparisons: Studies of Manufacturer or Wholesale Prices
Study Data source, year Drug sample Matching Key findings
HHS Task
Force on
Drug
Importation,
2004
IMS MIDAS database
for 10 countries, 2003
54 top-selling brands and
29 top-selling generics
Excluded drugs not available in all 10
countries and drugs that could not
find a match in the National Sales
Perspective database.
Brand prices are typically 60% lower in other
countries; generic prices are lower in the U.S.
Prices were adjusted to reflect rebates at the
wholesale level (rebated prices on generic drugs
were 24 percent lower than IMS prices).
Hollis, 2004 Ontario Drug Benefit
Plan (Canada) and
Federal Supply
Schedule (U.S.), 2003
16 of the top 50
prescribed brand drugs
measured by expenditures
Not defined by author The prices paid by the United States
government for drugs are 2.5% higher than the
prices paid by the Ontario provincial
government.
Anderson et
al., 2004
IMS Health MIDAS
database, 2003
30 drugs (brand and
generic) with highest total
spending in the US that
were available in study
countries
Matched by manufacturer,
compound, and form. Included all
available dosage strengths. Excluded
drugs not available in Canada, France,
or the UK.
Canadian prices were 52% lower than prices in
the United States.
Danzon and
Furukawa,
2003
IMS Health MIDAS
database, 1992 and
1999
Brand, generic, and OTC Matched by molecule, and including
all products with that active
ingredient and all doses
Canada’s prices were 33% lower than prices in
the U.S. net of discounts, and 40% lower
ignoring discounts.
Graham and
Robson, 2000
Red Book (U.S.) and
Ontario Drug Benefit
Program, 1998
45 brand and generic
drugs
Identical in chemical name, dosage,
strength, and form
Wholesale prices are 42% lower in Canada than
in the U.S. (72% higher in the United States
than in Canada). Two generic drugs were
cheaper in the U.S.
Danzon and
Chao, 2000
IMS, 1992 171 brand and generic
drugs available in all seven
countries; up to 438 drugs
for bilateral comparisons
Matched by active ingredient and
therapeutic category.
Canada and Germany have prices 2% and 25%
higher than the U.S., respectively, while prices
for drugs are lower in Japan (12%), Italy (13%),
the U.K. (17%), and France (67%).
Danzon and
Kim, 1998
IMS Health MIDAS
database, 1992
Brand, generic, and OTC Matched first by chemical
composition and brand name or
manufacturer. Second analysis
matching by active ingredient and
therapeutic class (MOL/ATC).
Canadian prices were 6.2% higher than U.S.
prices when matched by brand name or
manufacturer, but with the larger sample
obtained by matching by MOL/ATC, U.S.
prices were 16.6% higher than in Canada.
GAO, 1992 Medi-Span Master
Drug Database-Select,
1991
121 of the 200 brand and
genericdrugs most
commonly dispensed in
U.S.
Matched by brand name,
manufacturer, dosage strength, dosage
form, and whether available only
prescription in both the United States
and Canada.
The entire sample of drugs would cost 32
percent more in the United States than in
Canada. Drug-by-drug, the median price
differential per package between the United
States and Canada was 43 percent.

48
Figure B-2. Summary of U.S./Canada Prescription Drug Price Comparisons: Studies of Retail Prices
Author(s) Data source, year Drug sample Matching Key findings
Quon et al.,
2005
Prices obtained by
authors from three
U.S. pharmacies 12
Canadian internet
pharmacies, 2004
Brand
44 of the 50 most
common drugs by sales
volume
Matched by brand name The authors found that Americans could save
about 24% by purchasing drugs from Canadian
internet pharmacies. Only three drugs were
cheaper in the United States than in Canada.
Minority
Staff,
Committee
on
Government
Reform, 2005
Prices from the top 10
Medicare drug plans
(U.S.), 2005
Canadian price source
not defined by author,
2004
Brand
10 drugs with highest sales
to United States Medicare
beneficiaries
Not defined by author The prices offered by the Medicare drug plans
on average were 61% higher than the Canadian
prices, and one plan’s price was 73% higher
than the Canadian prices. Prices for individual
drugs in the United States approached or even
exceeded 100% more than the Canadian prices
in some cases.
Task Force
on Drug
Importation,
2004
Prices from
PharmacyChecker.com
(30 Canadian and 6
U.S. online
pharmacies), 2004
Brand and generic
22 top-selling brands and
5 top-selling generics
Matched by ingredient, dosage form,
strength, and package size. Excluded
drugs not available in Canada or
available OTC in Canada.
Branded drugs are about 37% less at Canadian
internet pharmacies, while generics are about
32% less at U.S. internet pharmacies, net of
shipping costs.
Graham and
Tabler, 2001
50 pharmacies
randomly selected in
six areas (3 in Canada,
3 in the United States),
2001
Brand
3 drugs that many patients
use for a long period of
time
Not defined by author For all three drugs, the prices at Canadian
pharmacies were significantly lower than the
prices at pharmacies in the United States, but
there was also substantial variation in prices
across areas within the same country and even
within an individual region.
Graham and
Robson, 2000
Costco pharmacies in
both countries, 2000
Brand and generic
45 drugs
Identical in chemical name, dosage,
strength, and form
Retail prices are 28% lower in Canada than in
the United States (39% higher in the United
States than in Canada).
Minority
Staff,
Committee
on
Government
Reform, 1998
Prices obtained by
authors phone calls to
pharmacies, 1998
Brand
10 drugs with the highest
dollar sales to the elderly
in the United States
Matched by dosage, form, and
package size used in GAO report, or
most common dosage, form, and
package size according to the Drug
Topics Red Book, for drugs not
included in GAO report
On average, retail prices in United States were
72 percent more than the average prices in
Canada.
49
Studies of Wholesale or Manufacturer Prices
In 1992, the GAO examined manufacturers’ prices to wholesalers for a market basket of drugs most
frequently dispensed by U.S. drug stores.
Drug selection and matching: Using a list of the 200 drugs most frequently dispensed in U.S. drug
stores, the authors selected the most commonly used dosage form, dosage strength, and
package size for the United States, and were able to match 121 by brand name,
manufacturer, dosage strength, dosage form, and whether available only by prescription in
both the United States and Canada.
Data source: U.S. prices were obtained from the Wholesale Acquisition Cost in the Medi-Span
Master Drug Data Base-Select. Canadian prices were obtained from the Best Available Price
listed in the Ontario Drug Benefit (ODB) Formulary when available, or directly from the
manufacturers of the drugs or from a major Canadian wholesaler for the 20 studied drugs
that were not on the ODB formulary. The authors compared the prices for specific package
sizes, as well as the aggregate cost of purchasing a common prescription of all 121 products
in both countries.
Currency conversion: Canadian prices to U.S. dollars using the May 1, 1991, exchange rate.
Results: For the drugs studied, drug manufacturers charged wholesalers significantly more in
the United States than the same manufacturers did in Canada. The entire market basket of
drugs would cost 32 percent more in the United States than in Canada. When the price
differentials were computed drug-by-drug, the median price differential per package between
the United States and Canada was 43 percent.
Danzon and Kim (1998) undertook an international price comparison study to demonstrate the
sensitivity of such studies to methodological decisions.
Drug selection and matching: including brand name, generic, and over-the-counter products, in
nine countries (United States, Canada, Germany, France, Italy, Japan, United Kingdom,
Switzerland, and Sweden). Products were matched first based on the international product
name (IPN, in which products must have the same chemical composition, and the same
brand name or same manufacturer), and then by active ingredient (molecule) and anatomical
therapeutic class (MOL/ATC), and found that significantly more products could be included
when matches were based on MOL/ATC. Rather than pick one dosage and pack size, the
authors converted all products to grams of active ingredient and the number of standard
units.
Data source: The authors used IMS data for sales of cardiovascular products for a one-year
period between 1991 and 1992.
Price index and weights: Price differences were determined using three different price indices
(Laspeyres, Paasche, and Fisher).
50
Results: Using their preferred method of matching drugs by MOL/ATC and weighting using
a Laspeyres price index, the authors found that prices in the United States were 16.6 percent
higher than prices in Canada, but if the same price index was used with drugs that matched
according to IPN, Canadian prices were 6.2 percent higher than U.S. prices.
Danzon and Chao (2000) used bilateral drug price and quantity indices for seven countries to
demonstrate differences compare manufacturer prices of drugs in the U.S. to manufacturer prices in
other nations.
Drug selection and matching: The authors matched drugs based on active ingredient and
anatomic therapeutic category. The weighted average price for the molecule incorporates all
forms and packs of that molecule.
Data source: The authors used IMS data from 1992.
Price index: The authors used the Laspeyres index, which may be most relevant to the U.S.
perspective because it uses U.S. weights, and may be interpreted as a lower-bound of what
the U.S. could save by adopting other countries’ pricing.
Results: The authors found that, while drug prices are higher in the U.S. than in most other
countries, their methods show smaller differences than previous studies. Calculations using
the Laspeyres index show that Canada and Germany have prices 2.1 percent and 24.7
percent higher than the U.S., respectively, while prices for drugs are lower in Japan (11.6%),
Italy (12.9%), the U.K. (16.6), and France (67%).
Graham and Robson (2000) compared both wholesale and retail prices for drugs in the United
States and Canada.
Drug selection and matching: The researchers started with the 60 drugs with the highest volume
of prescriptions written in the United States from January through October 1998, using
those that were identical in their chemical name, dosage and strengths, and form, and
compared brand-name drugs only to other brand-name drugs and generic drugs only to
other generic drugs. Fifteen drugs had to be excluded from each part of the study because
they were not identical in every way, or because price information was not available in each
data source, although not the same 15 drugs in each case.
Data source: For wholesale prices, Graham and Robson used the prices from the Red Book for
the United States and the Ontario Drug Benefit Program formulary for Canada.
Weights: The authors weighted by number of prescriptions.
Results: The authors found that, while drugs are typically 42% cheaper in Canada, there is a
wide range of differences between the prices of drugs in the two countries. Two generic
drugs had higher wholesale prices.
Danzon and Furukawa (2003) compared average price levels for pharmaceuticals in nine countries,
including the United States and Canada in 1992 and 1999. Using the manufacturer price levels in
order to eliminate differences attributable to wholesaler and retailer margins and taxes, and adjusting
51
for manufacturer discounts to managed care plans and Medicaid, the authors compared price
indexes for a sample of products from each country.
Drug selection and matching: The authors began with the 350 leading molecules in the U.S. (by
sales volume), and from these selected 249 that were available in at least four of the study
countries in 1992. For each molecule, they included all products with that active ingredient,
including both brand-name, generic, and over-the-counter drugs. At least 90 percent of the
drugs were available in all the study countries, except Japan, which only had 76 percent.
Data source: The authors used data from the IMS Health Midas data set.
Price index: The authors calculated four price indices for each country: all products for each
molecule; all products for each molecule, adjusted for discounts; drugs that match only on
indication, form, and strength; and drugs that match only on indication, form, and strength,
adjusted for discounts.
Results: Except for Japan, which had higher prices, all of the study countries had lower prices
than the United States. Canada’s prices were 33 percent lower than U.S. prices net of
discounts, and 40 percent lower ignoring discounts.
Anderson and colleagues (2004) used methods similar to those used by Danzon and Furukawa
(2003), but opted for greater standardization, rather than greater representativeness, in order to
simulate the prices that would paid by the United States’ Medicare program if usage were fixed but
prices matched those in other countries.
Drug selection and matching: Prices for all available dosage strengths in each country were
considered for a market basket of the thirty drugs, both brand and generic, with the highest
total spending in the United States that were available in the other three countries.
Data source: The authors used IMS health data to obtain the prices of drugs in Canada,
France, the United Kingdom, and the United States.
Price index: The authors calculated a Laspeyres price index, with the quantity sold in the
United States as the base, using average wholesale prices (AWP).
Results: Analysis revealed the average prices for the market basket of drugs were 52 percent
lower in Canada, 59 percent lower in France, and 47 percent lower in the United Kingdom,
than in the United States. When the authors assumed that Medicare could purchase drugs at
a 20 percent discount on AWP, the difference was reduced somewhat, with prices 40 percent
lower in Canada, 48 percent lower in France, and 34 percent lower in the United Kingdom
than in the United States.
To show that it matters who is the purchaser of drugs, Hollis (2004) compared government prices
for drugs in the United States and Canada.
Drug selection and matching: Hollis used a sample of 16 drugs without generic competition in
either country from the list of the top 50 drugs in Canada, as measured by expenditure.
52
Generic drugs were excluded due to the fact that competitive market pressures, including
government price controls and negotiating tactics drive the pricing of generic drugs.
Data source: The comparison used the Ontario Drug Benefit Plan price and the Federal
Supply Schedule price (used by the Veterans Administration and the Department of Defense
in the United States) for the most popular strength of each medication.
Currency conversion: U.S. prices were converted to Canadian dollars using the approximate
exchange rate at the time that Speaker of the U.S. House of Representatives Dennis Hastert
complained about Canadian price controls on prescription drugs.
Results: The analysis showed that the prices paid by the U.S. government for drugs are only
2.5 percent higher than the price paid by the Ontario plan.
The HHS Task Force on Drug Importation (2004) estimated differences between the U.S. and nine
other countries: Australia, Canada, France, Germany, Greece, Japan, Poland, Switzerland, and the
United Kingdom.
Drug selection and matching: The authors began with a list of 60 best-selling brand name drugs
and 50 best-selling generic drugs, and narrowed it down to 54 brand drugs and 29 generics
after excluding drugs not available in all 10 countries.
Data source: The comparison used invoice data from the 2003 IMS MIDAS database for 10
countries.
Weighting: The authors weighted by number of U.S. prescriptions, and also adjusted for
wholesale rebates by comparing IMS data with CMS data on Average Manufacturer Price.
Results: Foreign prices for top-selling brand name products were approximately 60 percent
of the U.S. price. Generic prices were lower in the United States than every country except
Poland. Canadian prices were just over half U.S. prices for brand name drugs, and nearly
twice as much as U.S. prices for generics.
Studies of Retail Prices
In 1998, the minority staff of the House Committee on Government Reform issued a report
comparing retail prescription drug prices in the 1
st
Congressional District of Maine with drug prices
in Canada and Mexico.
Drug selection and matching: The study examines the pricing of twelve drugs: ten brand name
prescription drugs with the highest dollar sales to the elderly in the United States and two
drugs that in a previous study were found to experience substantial discrimination in price
according to whether the drug was purchased by an individual or by a larger organization.
The study used the prices paid by consumers without prescription drug coverage in both
countries. The study used the drug dosage, form, and package size used by the GAO its 1992
53
report. For drugs that were not included in the GAO report, the study used the most
common dosage, form, and package size listed in the Drug Topics Red Book.
Data Source: Prices in the United States were obtained from a survey conducted by the
minority staff and the staff of Representative Allen of nine drug stores in Allen’s
congressional district—six independent pharmacies and three chain stores. Prices for drugs
in Canada and Mexico were obtained from a survey of four pharmacies in Canada (in three
different provinces) and three in Mexico (in Ciudad Juarez).
Currency conversion: Canadian and Mexican prices were converted to U.S. dollars using
exchange rates effective on October 5, 1998.
Results: For all the drugs in the study, U.S. prices were significantly higher than Canadian and
Mexican prices. On average, senior citizens in the United States paid 72 percent more than
the average prices in Canada and 102 percent more than the prices in Mexico.
Graham and Robson (2000) compared both wholesale and retail prices for drugs in the United
States and Canada.
Drug selection and matching: The researchers started with the 60 drugs with the highest volume
of prescriptions written in the United States from January through October 1998, using
those that were identical in their chemical name, dosage and strengths, and form, and
compared brand-name drugs only to other brand-name drugs and generic drugs only to
other generic drugs. Fifteen drugs had to be excluded from each part of the study because
they were not identical in every way, or because price information was not available in each
data source, although not the same 15 drugs in each case.
Data source: For the retail part of their study, the authors compared the prices at Costco, the
only pharmacy with locations in both countries.
Weights: The authors weighted by number of prescriptions.
Results: The authors found that, while drugs are typically 28% cheaper in Canada at the retail
level, there is a wide range of differences between the prices of drugs in the two countries.
Seven generic drugs had higher retail prices.
Graham and Tabler (2001) studied the retail prices of three patented prescription drugs (Celebrex,
Lipitor, and Paxil) in three areas in the United States and in three Canadian areas along the border
between the two countries.
Data source: The authors randomly selected 50 pharmacies in each of the six areas, and called
each of the pharmacies to obtain the price of a 30-day supply of each of the drugs, as well as
information about any dispensing fees and the availability and price of delivery of
prescriptions.
Currency conversion: The authors calculated an exchange rate based on a 12-day period.
54
Results: For all three drugs, the prices at Canadian pharmacies were significantly lower than
the prices at pharmacies in the United States, but there was also substantial variation in
prices across areas within the same country and even within an individual region.
The HHS Task Force on Drug Importation (2004) compared internet prices in the United States
and Canada, adjusted for shipping costs for U.S. consumers (typically $10-$15 from Canada and
zero to $2 from the U.S.).
Drug selection and matching: The study started with a list of 30 top-selling brand drugs and
seven top-selling chronic use generics. The list was narrowed to 22 brand drugs and 5
generic drugs after excluding drugs that were not available via the internet, were not available
in Canada, or were OTC in one or both countries.
Products were matched by strength and package size when possible, or packages were
adjusted by the number of tablets when computing unit costs. This led to a list of 118
matching brand products and 14 generic products.
Data source: Prices were collected from PharmacyChecker.com, a website that collects prices
from 30 Canadian and six U.S. internet pharmacies. For each drug, the authors collected the
lowest U.S. price and the lowest Canadian price.
Results: Brand drugs are about 37 percent less at Canadian internet pharmacies, while
generics are about 32% less at U.S. internet pharmacies, net of shipping costs.
Quon and colleagues (2005) compared the prices of prescription drugs from three U.S. pharmacies
and 12 Canadian Internet pharmacies that sell drugs to both Canadian and American customers.
Drug selection and matching: Only brand-name drugs were used; the authors purposely excluded
generic drugs because other studies have shown that Canadian prices for generic drugs are
similar to or higher than U.S. prices. Forty-four of the 50 most popular (by sales volume)
drugs were brand-name drugs and available in both countries and were used for the study.
Unit prices were based on a lot size of 100 units when available or on the next size smaller in
the 25 percent of cases where that lot size was not available. Dosages for each medication
were based on recommended daily dose ranges, daily frequencies of administrations, and
dose availabilities.
Data source: The authors collected their data from the pharmacies’ websites, which all listed
their prices in U.S. dollars.
Results: Calculating the cost per year for a single patient by multiplying the mean unit prices
by the number of units per year, the authors found that Americans can save about 24
percent on brand-name medications if purchased from Canadian internet pharmacies rather
than major U.S. drug chain pharmacies. Only three drugs, all for erectile dysfunction, were
more expensive in Canada.
In a 2005 report the minority staff of the U.S. House of Representatives Committee on Government
Reform studied whether the new Medicare Part D drug benefit is effective in lowering drug prices
for seniors.
55
Drug selection and matching: The authors examined the prices of ten drugs with the highest sales
to Medicare beneficiaries in 2004, comparing the prices negotiated by the ten leading
Medicare drug plans to the prices that consumers in Canada pay for the same drugs.
Data source: The authors used the Medicare website to obtain the prices the ten leading
Medicare drug plans negotiated with manufacturers and pharmacies, prices available for the
drugs at drugstore.com at the time of the study, prices available for the drugs at Costco
drugstores, and prices paid by consumers in Canada. (The source of Canadian prices was not
defined).
Results: The analysis revealed that the prices offered by the drug plans were substantially
higher than the prices paid by consumers in Canada: on average, the prices offered by the
Medicare drug plans were 61 percent higher than the Canadian prices, with one plan’s prices
coming in at 73 percent higher than the Canadian prices, and prices for individual drugs
approaching or even exceeding 100 percent more than the Canadian prices.
Discussion
While most studies have found that Canadian drug prices are lower than prices in the United States,
estimates of the magnitude of the difference vary widely, as results are very sensitive to
methodological choices. It is not possible to define one perfect methodology for these comparisons,
as many decisions with regard to methodology involve tradeoffs. However, it is clear that certain
methodological choices will strengthen the results of an analysis. For example, while simple
comparisons that select a few popular drugs that are available in the same form in both countries
provide interesting results and may reveal whether an individual in the United States could save
money by purchasing a particular drug in Canada, more complex analyses of larger market baskets of
drugs available in each country may offer a better understanding of systematic differences in the
markets. In such a larger study, careful methodological decisions can help ensure an “apples-to-
apples” comparison and increase confidence in the results.
56
Appendix C. Works Cited
Anderson, Gerard F., Dennis G. Shea, Peter S. Hussey, Salomeh Keyhani, and Laurie Zephyrin.
2004. "Doughnut holes and price controls." Health Affairs Web Exclusives.
Canada Patented Medicine Prices Review Board (PMPRB). “Compendium of Guidelines, Policies,
and Procedures.” http://www.pmprb-cepmb.gc.ca/english. Updated June 9, 2006.
Danzon, Patricia M. 1999. "Price comparisons for pharmaceuticals: a review of U.S. and cross-
national studies." American Enterprise Institute, Washington, DC.
Danzon, Patricia M. 2000. "Making sense of drug prices." Regulation 23:56-63.
Danzon, Patricia M. and Li-Wei Chao. 2000. “Cross-national price differences for pharmaceuticals:
how large, and why?” Journal of Health Economics 19:159-195.
Danzon, Patricia M. and Michael F. Furukawa. 2003. "Prices and availability of pharmaceuticals:
Evidence from nine countries." Health Affairs Web Exclusives.
Danzon, Patricia M. and Jeong D. Kim. 1998. "International price comparisons for pharmaceuticals:
Measurement and policy issues." Pharmacoeconomics 14:115-128.
Graham, John R. 2000. "Prescription drug prices in Canda and the United States - Part 2: Why the
difference?" The Fraser Institute, Vancouver, BC.
Graham, John R. and Beverley A. Robson. 2000. "Prescription drug prices in Canada and the United
States - Part 1: A comparative survey." The Fraser Institute, Vancouver, BC.
Graham, John R. and Tanya Tabler. 2001. "Prescription drug prices in Canada and the United States
- Part 3: Retail price distribution." The Fraser Institute, Vancouver, BC.
Gross, David, Leigh Gross, Stephen W. Schondelmeyer, and Susan Raetzman. 2006. “Trends in
Manufacturer Prices of Prescription Drugs Used by Older Americans.” AARP Public Policy
Institute, Washington, DC.
Gross, David. 2003. "Prescription drug prices in Canada." AARP Public Policy Institute,
Washington, DC.
Hollis, Aidan. 2004. "How cheap are Canada's drugs really?" Journal of Pharmacy and Pharmaceutical
Science 7.
IMS. 2003a. “Leading 20 Products by Total U.S. Dispensed Prescriptions, 2003.” Accessed at
http://www.imshealth.com/ims/portal/front/indexC/0,2773,6599_5264_0,00.html.
IMS. 2003b. “Leading 20 Products by Total U.S. Sales, 2003.” Accessed at
http://www.imshealth.com/ims/portal/front/indexC/0,2773,6599_5264_0,00.html
57
IMS. 2005. “Leading 20 Products by Total U.S. Dispensed Prescriptions, 2005.” Accessed at
http://www.imshealth.com/ims/portal/front/indexC/0,2773,6599_5264_0,00.html
Menon, Devidas. 2001. "Pharmaceutical cost control in Canada: Does it work?" Health Affairs 20.
Minority Staff, U.S. House of Representatives Committee on Government Reform and Oversight.
1998. "Prescription drug pricing in the 1st Congressional District in Maine: an international
price comparison." U.S. House of Representatives, Washington, DC.
Minority Staff, U.S. House of Representatives Committee on Government Reform and Oversight.
2005. "New Medicare drug plans fail to provide meaningful drug price discounts." U.S.
House of Representatives, Washington, DC.
Morgan, Steven G., Morris L. Barer, and Jonathan D. Agnew. 2003. "Whither seniors' pharmacare:
Lessons from (and for) Canada." Health Affairs 23:49-59.
Quon, Bradley S., Rafael Firszt, and Mark J. Eisenberg. 2005. "A comparison of brand-name drug
prcies between Canadian-based internet pharmacies and major U.S. drug chain pharmacies."
Annals of Internal Medicine 143:397-403.
U.S. Congressional Budget Office. 1996. How the Medicaid Rebate on Prescription Drugs Affects Pricing in
the Pharmaceutical Industry. U.S. Congressional Budget Office, Washington, D.C.
U.S. Department of Health and Human Services. 2000. “Report to the President: Prescription
Drug Coverage, Spending, Utilization, and Prices.” U.S. Department of Health and Human
Services, Washington, DC.
U.S. Department of Health and Human Services Task Force on Drug Importation. 2004. “Report
on Prescription Drug Importation.” U.S. Department of Health and Human Services,
Washington, DC.
U.S. General Accounting Office. 1992. "Prescription drugs: Companies typically charge more in the
United States than in Canada." U.S. General Accounting Office, Washington, DC.
Wagner, Judith L. and Elizabeth McCarthy. 2004. “International differences in drug prices.” Annual
Review of Public Health 25:475-495.
