
Report to Congressional Requesters
United States Government Accountabilit
y
Office
GA
O
August 2005
PRESCRIPTION
DRUGS
Price Trends for
Frequently Used
Brand and Generic
Drugs from 2000
through 2004
GAO-05-779

What GAO Found
United States Government Accountability Office
Why GAO Did This Study
Highlights
Accountability Integrity Reliability
www.gao.gov/cgi-bin/getrpt?GAO-05-779.
To view the full product, including the scope
and methodology, click on the link above.
For more information, contact Marjorie Kanof
at (202) 512-7114 or [email protected].
Highlights of GAO-05-779, a report to
congressional requesters
Au
g
ust 2005
PRESCRIPTION DRUGS
Price Trends for Frequently Used Brand
and Generic Drugs from 2000 through
2004
We found the average U&C prices at retail pharmacies reported by two state
pharmacy assistance programs for a 30-day supply of 96 drugs frequently
used by BCBS FEP Medicare and non-Medicare enrollees increased
24.5 percent from January 2000 through December 2004. Of the 96 drugs:
• Twenty drugs accounted for nearly two-thirds of the increase in the U&C
price index.
• The increase in average U&C prices for 75 prescription drugs frequently
used by Medicare beneficiaries was similar to the increase for 76
prescription drugs frequently used by non-Medicare enrollees.
• The average U&C prices for 50 frequently used brand prescription drugs
increased three times as much as the average for 46 generic frequently
used prescription drugs.
A
WPs increased at a faster rate than AMPs and U&C prices for the 50
frequently used brand drugs from first quarter 2000 through fourth quarter
2004. Ten drugs in each index accounted for almost 50 percent of the
increase for AMP, AWP, and U&C prices. Eight of these 10 drugs were
consistent across the three price indexes.
The Centers for Medicare & Medicaid Services (CMS), two state pharmacy
assistance programs, and BCBS FEP reviewed a draft of this report. While
CMS noted that U&C and AWP do not reflect discounts in a drug’s price, this
report’s focus was to examine price trends rather than price levels.
Technical comments were incorporated as appropriate.
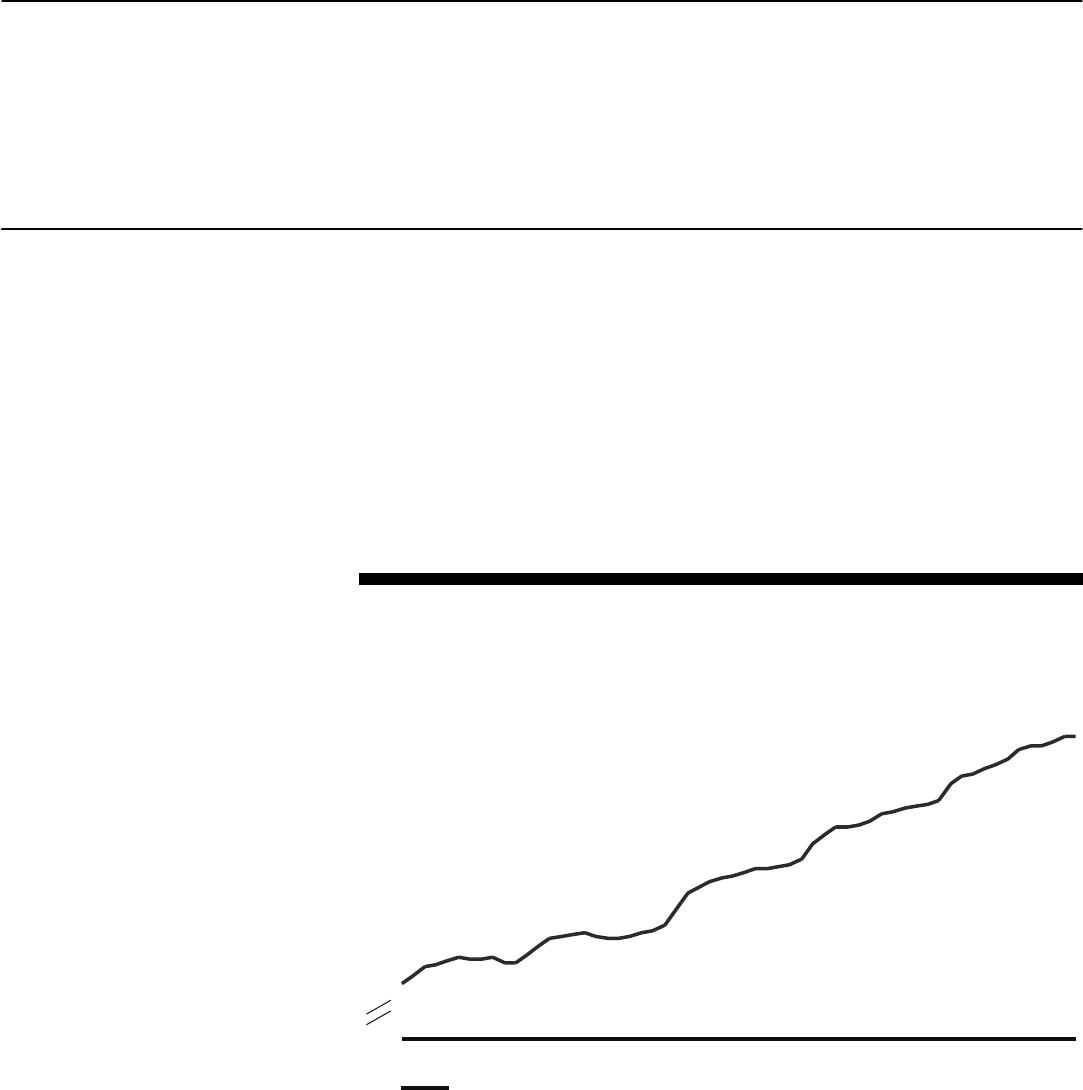
Average Annual Percentage Change of AMP, AWP, and U&C Price Indexes for 50 Brand
Drugs Frequently Used by Enrollees in BCBS FEP, from First Quarter 2000 through Last
Quarter 2004
Percentage change
Source: GAO analysis of data from CMS, First DataBank, New York’s Elderly Pharmaceutical Insurance Coverage
program, Pennsylvania’s Pharmaceutical Assistance Contract for the Elderly program, and BCBS FEP.
0
1
2
3
4
5
6
5.4
6.0
5.2
AMP
AWP
U&C
Prescription drug spending has
been the fastest growing segment
of national health expenditures. As
the federal government assumes
greater financial responsibility for
prescription drug expenditures
with the introduction of Medicare
part D, federal policymakers are
increasingly concerned about
prescription drug prices. GAO was
asked to examine the change in
retail prices and other pricing
benchmarks for drugs frequently
used by Medicare beneficiaries and
other individuals with health
insurance from 2000 through 2004.
To examine the change in retail
prices from 2000 through 2004, we
obtained usual and customary
(U&C) prices from two state
pharmacy assistance programs for
drugs frequently used by Medicare
beneficiaries and non-Medicare
enrollees in the 2003 Blue Cross
and Blue Shield (BCBS) Federal
Employee Program (FEP). The
U&C price is the price an individual
without prescription drug coverage
would pay at a retail pharmacy.
Additionally, we compared the
change in U&C prices for brand
drugs from 2000 through 2004 to
the change in two pricing
benchmarks: average
manufacturer price (AMP), which
is the average of prices paid to
manufacturers by wholesalers for
drugs distributed to the retail
pharmacy class of trade, and
average wholesale price (AWP),
which represents the average of list
prices that a manufacturer suggests
wholesalers charge pharmacies.

Page i GAO-05-779 Prescription Drug Price Trends
Letter 1
Results in Brief 4
Background 5
Retail Prices Increased from 2000 through 2004, with Larger
Increases for Brand Than Generic Drugs 6
AWPs Increased at a Faster Rate Than AMPs and U&C Prices for
50 Brand Drugs from 2000 through 2004 11
Concluding Observations 13
Agency and Other External Comments 14
Appendix I Scope and Methodology 16
Appendix II Drugs Included in Analyses 20
Appendix III GAO Contact and Staff Acknowledgments 24
Table
Table 1: Ninety-Six Drugs Included in U&C Price Indexes, by
Month, January 2000 through December 2004 20
Figures
Figure 1: Drug Prices for Different Buyers and Sellers 6
Figure 2: Index of Average U&C Prices for 96 Drugs Frequently
Used by BCBS FEP Enrollees, by Month, 2000 through
2004 7
Figure 3: Annual Change in U&C Price Index for 96 Drugs
Frequently Used by BCBS FEP Enrollees, 2000 through
2004 8
Figure 4: Indexes of Average U&C Prices for Drugs Frequently
Used by BCBS FEP Medicare and Non-Medicare
Enrollees, by Month, 2000 through 2004 10
Figure 5: Indexes of Average U&C Prices for 50 Brand and 46
Generic Drugs Frequently Used by BCBS FEP Enrollees,
by Month, 2000 through 2004 11
Contents

Page ii GAO-05-779 Prescription Drug Price Trends
Figure 6: Indexes of AMPs, AWPs, and Average U&C Prices for 50
Brand Drugs Frequently Used by BCBS FEP Enrollees, by
Quarter, 2000 through 2004 12
Figure 7: Comparison of 10 Drugs Accounting for the Largest
Portions of Changes in AMP, AWP, and U&C Price
Indexes for 50 Brand Drugs Frequently Used by BCBS
FEP Enrollees, by Quarter, 2000 through 2004 13
Abbreviations
AMP average manufacturer price
AWP average wholesale price
BCBS Blue Cross and Blue Shield
BLS Bureau of Labor Statistics
CMS Centers for Medicare & Medicaid Services
EPIC Elderly Pharmaceutical Insurance Coverage
FEP Federal Employee Program
NDC National Drug Code
PACE Pharmaceutical Assistance Contract for the Elderly
U&C usual and customary
This is a work of the U.S. government and is not subject to copyright protection in the
United States. It may be reproduced and distributed in its entirety without further
permission from GAO. However, because this work may contain copyrighted images or
other material, permission from the copyright holder may be necessary if you wish to
reproduce this material separately.

Page 1 GAO-05-779 Prescription Drug Price Trends
August 15, 2005
The Honorable Olympia J. Snowe
Chair
Committee on Small Business and Entrepreneurship
United States Senate
The Honorable Ron Wyden
United States Senate
Prescription drug spending as a share of national health expenditures
increased from 5.8 percent in 1993 to 10.7 percent in 2003 and was the
fastest growing segment of health care expenditures.
1
In addition to
increasing utilization and the introduction of newer drugs, rising
prescription drug prices are a key component of increasing drug
expenditures. Increasing drug prices can affect consumers, employers, and
federal and state governments. Policymakers are increasingly concerned
about drug prices as the federal government will assume greater financial
responsibility for prescription drug expenditures with the introduction of a
prescription drug benefit to Medicare beneficiaries in January 2006,
known as Medicare part D. Medicare beneficiaries also will continue to be
responsible for a large share of drug costs under Medicare part D.
Tracking prescription drug prices can be complicated by the different
prices that different purchasers, such as consumers, insurers and other
third-party payers, and wholesalers, pay for the same drug. Several price
benchmarks represent these differing amounts paid by different
purchasers. For example, individuals without prescription drug coverage,
including Medicare beneficiaries who do not currently have drug coverage,
may pay the full retail price at the pharmacy, known as the usual and
customary (U&C) price. Insurers and other third-party payers, including
state Medicaid programs, typically pay negotiated prices with retail
pharmacies, often receiving discounts from the average wholesale price
(AWP), commonly referred to as a list price.
2
Retail pharmacies may obtain
1
Our calculations are based on data from the national health accounts prepared by the
Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics
Group.
2
The AWP is the average of the list prices that a manufacturer suggests wholesalers charge
pharmacies.
United States Government Accountability Office
Washington, DC 20548

Page 2 GAO-05-779 Prescription Drug Price Trends
drugs directly from pharmaceutical manufacturers or through wholesalers.
The average manufacturer price (AMP) represents the average of prices
paid to manufacturers by wholesalers for drugs distributed to the retail
pharmacy class of trade, and is used by the Centers for Medicare &
Medicaid Services (CMS) to determine rebates due by law to Medicaid
programs. Prices also substantially vary depending on whether drugs are
marketed as brand or generic, with some third-party payers encouraging
the use of less expensive generic drugs through lower cost sharing for
consumers and other strategies.
To provide a baseline of prescription drug prices before the
implementation of the Medicare part D drug benefit, you asked GAO to
review drug price changes from 2000 through 2004, including drugs
frequently used by seniors. Specifically, we examined the following
questions.
1. How have retail prices for prescription drugs frequently used by
Medicare beneficiaries and other individuals with health insurance
changed from 2000 through 2004?
2. How does the change in retail prices for brand drugs frequently used
by Medicare beneficiaries and other individuals compare to other drug
pricing benchmarks from 2000 through 2004?
To examine the change in retail prices for prescription drugs frequently
used by Medicare beneficiaries and other individuals with health
insurance, we selected the 100 most frequently dispensed retail
prescriptions in 2003 for Medicare beneficiaries and for non-Medicare
enrollees in the Blue Cross and Blue Shield (BCBS) Federal Employee
Program (FEP).
3
Combined, these two lists of 100 frequently used drugs
represented a total of 133 unique drugs. Of these 133 drugs, we analyzed 96
drugs (50 brand and 46 generic) for which we were able to obtain U&C
prices at retail pharmacies for every month from January 2000 through
3
We used data of frequently dispensed prescriptions from BCBS FEP because they
represent a large number of retail prescriptions dispensed and could provide data for drugs
used by FEP enrollees who were Medicare beneficiaries and those who were not Medicare
eligible. Of the nearly 55 million retail prescriptions dispensed to BCBS FEP enrollees in
2003, 21 million were for FEP enrollees who were also Medicare beneficiaries.

Page 3 GAO-05-779 Prescription Drug Price Trends
December 2004.
4
These 96 drugs included 75 drugs that were frequently
used by BCBS FEP Medicare enrollees and 76 drugs that were frequently
used by BCBS FEP non-Medicare enrollees, with 55 of these drugs
overlapping the Medicare and non-Medicare frequently used lists. To
calculate a price index, we weighted each drug using the number of
prescriptions dispensed to BCBS FEP enrollees in 2003. We collected the
average monthly U&C prices for a typical 30-day supply from two large
state programs that assist low-income Medicare beneficiaries in
purchasing prescription drugs: Pennsylvania’s Pharmaceutical Assistance
Contract for the Elderly (PACE) program from January 2000 through
December 2004, and New York’s Elderly Pharmaceutical Insurance
Coverage (EPIC) program from August 2000 through December 2004.
5
To compare the change in U&C prices at retail pharmacies with other
drug-pricing benchmarks, we examined changes in the AMP and AWP for
the 50 brand drugs frequently used by BCBS FEP enrollees. We calculated
a quarterly AMP index for a 30-day supply for the 50 brand drugs based on
data we collected from CMS from the first quarter of 2000 through the
fourth quarter of 2004. We calculated a quarterly AWP index for a 30-day
supply for the same 50 brand drugs based on data we collected from First
DataBank for the same period. We determined that the data from BCBS
FEP, PACE, EPIC, CMS, and First DataBank were sufficiently reliable for
our purposes. Our analyses are limited to drugs most frequently used by
Medicare beneficiaries and non-Medicare enrollees in the 2003 BCBS FEP,
and our analyses using U&C prices are limited to prices reported by retail
pharmacies in Pennsylvania to the PACE program and by retail
pharmacies in New York to the EPIC program. See appendix I for more
information about our selected drugs and detailed information on our
4
For the purpose of this report, we refer to single-source and multisource drugs that are
marketed under a proprietary, trademark-protected name as brand drugs. Single-source
drugs include those brand drugs that have no generic equivalent on the market and are
generally available from only one manufacturer. Brand multisource drugs include those
brand drugs that have generic equivalents available from multiple manufacturers and are
marketed under their brand name. Generic drugs include multisource drugs that are
chemically identical to their branded counterparts and are generally marketed by multiple
manufacturers under a non-proprietary name.
5
We used data from PACE and EPIC because they were two of the largest state
pharmaceutical assistance programs, collected data from pharmacies on U&C prices for
drugs, and had historical price data available from 2000.

Page 4 GAO-05-779 Prescription Drug Price Trends
methodology. We performed our work from April 2004 through July 2005
in accordance with generally accepted government auditing standards.
6
From January 2000 through December 2004, based on our analysis of data
from PACE and EPIC, the average monthly U&C prices for a 30-day supply
of 96 prescription drugs frequently used by BCBS FEP Medicare and non-
Medicare enrollees increased 24.5 percent. Twenty of the 96 drugs
accounted for nearly two-thirds of the increase in the U&C price index.
The average U&C prices for 75 prescription drugs frequently used by
BCBS FEP Medicare beneficiaries and the average U&C prices for 76
prescription drugs frequently used by BCBP FEP non-Medicare enrollees
increased at similar rates of 24.0 percent and 24.8 percent, respectively.
The average U&C prices for 50 brand prescription drugs increased
28.9 percent, three times as much as the average U&C price increase of
9.4 percent for 46 generic prescription drugs.
The AWP index increased by 31.6 percent for the 50 frequently used brand
drugs from the first quarter of 2000 through the fourth quarter of 2004—
about 3 to 4 percentage points more rapidly than the AMP and U&C price
indexes. Ten drugs in each index accounted for nearly 50 percent of the
increase for the AMP, AWP, and U&C indexes, with 8 of these top 10 drugs
consistent for all three prices. As a result of AWP’s faster rate of increase,
AWP as a percentage of U&C price increased from an average of about
91 percent in the first quarter of 2000 to about 94 percent in the last
quarter of 2004. AMP stayed about 72 percent of the U&C price during this
period.
We provided a draft of this report to CMS, PACE, EPIC, and BCBS FEP.
CMS noted that U&C and AWP do not reflect discounts in a drug’s price.
While our analysis does not reflect these discounts, our focus was to
examine price trends rather than price levels and U&C and AWP are
consistent measures used to examine price trends. CMS also suggested
that we examine the effect on prices when generic alternatives are
introduced, but such an analysis was beyond the scope of this report.
6
We also reported on trends in U&C prices for 99 drugs from January 2000 through June
2004 in GAO, Prescription Drugs: Trends in Usual and Customary Prices for Drugs
Frequently Used by Medicare and Non-Medicare Enrollees, GAO-05-104R (Washington,
D.C.: Oct. 6, 2004). This report includes 3 fewer drugs than our earlier analysis because
pricing data were not available for these 3 drugs through December 2004.
Results in Brief

Page 5 GAO-05-779 Prescription Drug Price Trends
PACE and BCBS provided technical comments that we incorporated as
appropriate; EPIC stated that it did not have any comments.
Several measures of price are commonly used within the health care
sector to measure the price of prescription drugs. These varying price
measures are due to the different prices that drug manufacturers and retail
pharmacies charge different purchasers, and drug prices can vary
substantially depending on the purchaser. (See fig. 1.)
• The U&C price, the retail price for a drug, is the price an individual
without prescription drug coverage would pay at a retail pharmacy. The
U&C price includes the acquisition cost of the drug paid by the retail
pharmacy and a markup charged by the pharmacy.
• AWP is the average of the list prices or sticker price that a manufacturer of
a drug suggests wholesalers charge pharmacies. AWP is typically less than
the U&C price, which includes the pharmacy’s own markup. AWP is not
the actual price that large purchasers normally pay. Nevertheless, AWP is
part of the formula used by many state Medicaid programs and private
third-party payers to reimburse retail pharmacies.
7
• AMP is the average of prices paid to a manufacturer by wholesalers for a
drug distributed to the retail pharmacy class of trade, after subtracting any
account cash discounts or other price reductions.
8
CMS uses AMP in
determining rebates drug manufacturers must provide, as required by the
Omnibus Budget Reconciliation Act of 1990, to state Medicaid programs as
a condition for the federal contribution to Medicaid spending for the
manufacturers’ outpatient prescription drugs.
9
For brand drugs, the
7
Before 2005, Medicare reimbursement for prescription drugs covered under Medicare part
B was based on AWP. The average sales price generally replaced AWP as the basis for
outpatient drug reimbursement under Medicare part B beginning in 2005. The average sales
price is defined for each drug as a manufacturer’s sales to all purchasers in a given quarter,
net of discounts and rebates and excluding certain government and other purchasers,
divided by the number of units of the drug sold by the manufacturer in that quarter.
Medicare Prescription Drug, Improvement, and Modernization Act of 2003, Pub. L. No. 108-
173, § 303(c), 117 Stat. 2066, 2239-2245 (to be codified at 42 U.S.C. § 1395w-3a).
8
AMP does not include prices to government purchasers based on the Federal Supply
Schedule, which are prices for prescription drugs negotiated with manufacturers by the
Department of Veterans Affairs. AMP also does not include prices from direct sales to
health maintenance organizations and hospitals or prices to wholesalers when they relabel
drugs they purchase under their own label.
9
Pub. L. No. 101-508, § 4401, 104 Stat. 1388, 1388-156 (codified as amended at 42 U.S.C. §
1396r-8(k) (2000)).
Background

Page 6 GAO-05-779 Prescription Drug Price Trends
minimum rebate amount is the number of units of the drug multiplied by
15.1 percent of the AMP.
Figure 1: Drug Prices for Different Buyers and Sellers
a
U&C is the price an individual without prescription drug coverage would pay at a retail pharmacy.
b
When an insured consumer purchases a drug at a retail pharmacy, the pharmacy collects from the
insured consumer the appropriate cost-sharing amount and then submits a claim to the third-party
payer for reimbursement.
c
Third-party payers often negotiate a discount off AWP, the average of the list prices that a
manufacturer suggests wholesalers charge pharmacies. However, third-party payers may pay other
negotiated rates not based on AWP.
d
Retail pharmacies can also purchase prescription drugs directly from manufacturers.
e
AMP represents the average of prices paid to manufacturers by wholesalers for drugs distributed to
the retail pharmacy class of trade.
From January 2000 through December 2004, the average U&C prices for a
typical 30-day supply of 96 prescription drugs frequently used by BCBS
FEP Medicare and non-Medicare enrollees increased 24.5 percent. The
average U&C prices for 75 prescription drugs frequently used by Medicare
beneficiaries and for 76 prescription drugs frequently used by non-
Medicare enrollees increased at similar rates. The average U&C prices for
50 frequently used brand drugs increased three times faster than the
average U&C prices for 46 frequently used generic drugs.
Retail Prices
Increased from 2000
through 2004, with
Larger Increases for
Brand Than Generic
Drugs
Source: GAO.
Retail pharmacy
Uninsured
consumer
Insured
consumer
Manufacturer
Wholesaler
U&C
a
$
AMP
e
$
Retail pharmacy
Retail pharmacy
d
Third-party
payer
c
Cost
share
b
AWP
- discount $
Buyer Buyer Buyer
Seller Seller Seller
Page 7 GAO-05-779 Prescription Drug Price Trends
From January 2000 through December 2004, the average U&C price
collected from retail pharmacies by PACE and EPIC for a 30-day supply
for 96 prescription drugs frequently used by BCBS FEP Medicare
beneficiaries and non-Medicare enrollees increased 24.5 percent, a
4.6 percent average annual rate of increase. (See fig. 2.) During the same
period, using nationwide data from the Bureau of Labor Statistics (BLS),
prices for prescription drugs and medical supplies for all urban consumers
increased 21.3 percent, a 4.0 percent average annual rate of increase.
Additionally, using BLS data, prices for all consumer items for all urban
consumers—the Consumer Price Index—increased 12.7 percent, a
2.5 percent average annual rate of increase from January 2000 through
December 2004.
Figure 2: Index of Average U&C Prices for 96 Drugs Frequently Used by BCBS FEP
Enrollees, by Month, 2000 through 2004
While U&C prices increased each year from 2000 through 2004, the
greatest annual rate of increase—6.1 percent—occurred from January
2002 to January 2003. (See fig. 3.) Since then, annual rates of increase have
U&C Prices for Frequently
Used Drugs Increased
24.5 Percent
124.5
100
105
0
110
115
120
125
130
1/2000 1/2001 1/2002 1/2003 1/2004
U&C
Index (Base = January 2000)
Source: GAO analysis of data from BCBS FEP, EPIC, and PACE.
Page 8 GAO-05-779 Prescription Drug Price Trends
been less, increasing 5.2 percent from January 2003 to January 2004 and
4.2 percent from January 2004 to December 2004.
10
Figure 3: Annual Change in U&C Price Index for 96 Drugs Frequently Used by BCBS
FEP Enrollees, 2000 through 2004
Note: The change in average U&C prices from January 2004 through December 2004 is expressed
as an annual percentage change.
Twenty drugs, representing 33 percent of BCBS FEP prescriptions for the
96 drugs we reviewed, accounted for 64 percent of the total increase in the
U&C price index from January 2000 through December 2004.
11
The drug
with the largest effect on the price index was Lipitor 10mg, which
accounted for 6.6 percent of the total increase. Nineteen of the 20 drugs
were brand drugs and 1 was a generic drug, Hydrocodone/Acetaminophen
5/500mg. The twenty drugs accounting for the largest changes in the U&C
price index are listed below.
10
The change in average U&C prices from January 2004 through December 2004 is
expressed as an annual percentage change.
11
We measured the share each drug contributed to the overall index by comparing the ratio
of (1) each drug’s price change from January 2000 through December 2004 multiplied by its
weight based on BCBS FEP prescriptions, to (2) the sum of all drugs’ price changes
multiplied by their associated weights.
3.7
3.5
6.1
5.2
4.2
0
1
2
3
4
5
6
7
1/2000-
1/2001
1/2001-
1/2002
1/2002-
1/2003
1/2003-
1/2004
1/2004-
12/2004
Percentage change
Source: GAO analysis of data from BCBS FEP, EPIC, and PACE.

Page 9 GAO-05-779 Prescription Drug Price Trends
• Lipitor 10mg
• Celebrex 200mg
• Plavix 75mg
• Prevacid 30mg
• Lipitor 20mg
• Ambien 10mg
• Zocor 20mg
• Levaquin 500mg
• Hydrocodone/Acetaminophen 5/500mg
• Flonase 0.05mg
• Zithromax 250mg
• Wellbutrin SR 150mg
• Singular 10mg
• Premarin 0.625mg
• Celexa 20mg
• Zoloft 50mg
• Evista 60mg
• Norvasc 5mg
• Neurontin 300mg
• Aciphex 20mg
From January 2000 through December 2004, the average U&C prices
collected by PACE and EPIC for 75 prescription drugs frequently used by
BCBS FEP Medicare beneficiaries increased at a similar rate as the
average U&C prices for 76 prescription drugs frequently used by BCBS
FEP non-Medicare enrollees.
12
(See fig. 4.) The prices of 75 Medicare drugs
increased 24.0 percent, a 4.5 percent average annual rate of increase. The
prices of 76 non-Medicare drugs increased 24.8 percent, a 4.6 percent
average annual rate of increase.
13
12
While 55 drugs were used in calculating both the Medicare and non-Medicare U&C price
indexes, each drug had a different weight in each index depending on the frequency of
prescriptions dispensed to BCBS FEP Medicare enrollees or BCBS FEP non-Medicare
enrollees.
13
We found the non-Medicare index rose slightly faster than the Medicare index, in part
because drugs that treat depression were present to a larger extent in the non-Medicare
index. The U&C prices for the eight drugs that treat depression increased at an average rate
of 31.1 percent from January 2000 through December 2004. Excluding the eight drugs that
treat depression from our analysis resulted in a 24.0 percent rate of increase for both the
Medicare and non-Medicare index.
U&C Prices for Drugs
Frequently Used by
Medicare Beneficiaries and
by Non-Medicare Enrollees
Increased at Similar Rates

Page 10 GAO-05-779 Prescription Drug Price Trends
Figure 4: Indexes of Average U&C Prices for Drugs Frequently Used by BCBS FEP
Medicare and Non-Medicare Enrollees, by Month, 2000 through 2004
From January 2000 through December 2004, the average U&C price (based
on PACE and EPIC data) for 50 frequently used brand drugs rose three
times faster than the average U&C price for 46 frequently used generic
drugs. (See fig. 5.) Specifically, the average U&C price for brand drugs
increased 28.9 percent, a 5.3 percent average annual rate of increase,
whereas U&C prices for generic drugs increased 9.4 percent, a 1.8 percent
average annual rate of increase.
U&C Prices Increased
Three Times Faster for
Brand Drugs Than for
Generic Drugs
Source: GAO analysis of data from BCBS FEP, EPIC, and PACE.
124.0
124.8
100
105
0
110
115
120
125
1/2000 1/2001
1/2002
1/2003
1/2004
Medicare
Non-Medicare
Index (base = January 2000)

Page 11 GAO-05-779 Prescription Drug Price Trends
Figure 5: Indexes of Average U&C Prices for 50 Brand and 46 Generic Drugs
Frequently Used by BCBS FEP Enrollees, by Month, 2000 through 2004
From the first quarter of 2000 through the fourth quarter of 2004, AMPs
and U&C prices for the 50 brand drugs increased at similar rates, but
AWPs increased at a faster rate. The quarterly AWPs for 50 brand
prescription drugs increased 31.6 percent, a 6.0 percent average annual
rate of increase. For these same 50 drugs, the quarterly AMPs increased
28.2 percent, a 5.4 percent average annual rate of increase, while the
average quarterly U&C prices increased 27.5 percent, a 5.2 percent average
annual rate of increase.
14
Over the entire period, the AWP index increased
about 3 to 4 percentage points more than the AMP or U&C price indexes.
(See fig. 6.)
14
The quarterly U&C price index increased at a slightly lower rate of increase than the
monthly U&C price index because the base and end periods differ. Whereas the base period
for the monthly U&C index is January 2000, the base period for the quarterly index is
January through March 2000. Similarly, the end period for the monthly index is December
2004 and for the quarterly index is October through December 2004.
AWPs Increased at a
Faster Rate Than
AMPs and U&C Prices
for 50 Brand Drugs
from 2000 through
2004
128.9
109.4
95
100
0
105
110
115
120
125
130
135
1/2000
1/2001 1/2002
1/2003 1/2004
Brand name
Generic
Index (base = January 2000)
Source: GAO analysis of data from BCBS FEP, EPIC, and PACE.

Page 12 GAO-05-779 Prescription Drug Price Trends
Figure 6: Indexes of AMPs, AWPs, and Average U&C Prices for 50 Brand Drugs
Frequently Used by BCBS FEP Enrollees, by Quarter, 2000 through 2004
The difference between the levels of AWP and U&C prices for brand drugs
narrowed slightly during the time period we analyzed. Whereas in the first
quarter of 2000 AWP was on average about 91 percent of the U&C price for
the same drug, by the fourth quarter of 2004 AWP was on average about
94 percent of the U&C price. In contrast, AMP stayed a similar portion of
U&C in first quarter 2000 and fourth quarter 2004, with the AMP on
average about 72 percent of the U&C price.
Ten brand drugs in each index, representing one-third or more of the
prescriptions for the 50 brand drugs, accounted for almost 50 percent of
the increase for the quarterly AMP, AWP, and U&C price indexes. Eight of
these 10 drugs were the same across all three price indexes. The drug
accounting for the largest portion of the change in the AMP and AWP
indexes was Celebrex 200mg, accounting for 8.6 percent of the increase
for AMP and 7.5 percent for AWP. Lipitor 10mg was the drug accounting
for the largest portion of the change in the quarterly U&C price index and
128.2
131.6
127.5
100
0
105
110
115
120
125
130
135
Q1/2000 Q1/2001 Q1/2002 Q1/2003 Q1/2004
AMP
AWP
U&C
Index (base = 1st Quarter 2000)
Source: GAO analysis of data from CMS, First DataBank, EPIC, PACE, and BCBS FEP.
Page 13 GAO-05-779 Prescription Drug Price Trends
accounted for 7.2 percent of the increase for the 50 brand drugs. (See fig.
7.)
Figure 7: Comparison of 10 Drugs Accounting for the Largest Portions of Changes in AMP, AWP, and U&C Price Indexes for
50 Brand Drugs Frequently Used by BCBS FEP Enrollees, by Quarter, 2000 through 2004
From 2000 through 2004, retail prices for drugs frequently used by
Medicare beneficiaries increased 24.0 percent—an average rate of
4.5 percent per year. In general, higher drug prices mean higher spending
by consumers and health insurance sponsors, including employers and
federal and state governments. With brand drug prices increasing three
times as fast as generic drug prices, public and private health insurance
sponsors will likely continue to focus on strategies to encourage increased
use of generic drugs when available. Starting in 2006, with the introduction
of the Medicare prescription drug benefit, Medicare will be paying claims
for a wider array of drugs and, as a result, the federal government will be
affected more than previously by rising drug prices.
We found that from 2000 through 2004, on average the AWPs for 50
frequently used brand drugs rose 0.8 percent per year faster than the retail
prices for these same drugs. A continuation of this difference between
AWP and retail prices increases could affect many Medicaid programs and
private third-party payers that base their reimbursement of drug claims on
AWPs.
Concluding
Observations
AMP
Celebrex 200mg
Plavix 75mg
Lipitor 10mg
Ambien 10mg
Lipitor 20mg
Prevacid 30mg
Levaquin 500mg
Zocor 20mg
Zithromax 250mg
Singulair 10mg
AWP
Celebrex 200mg
Plavix 75mg
Lipitor 10mg
Ambien 10mg
Prevacid 30mg
Lipitor 20mg
Levaquin 500mg
Zocor 20mg
Wellbutrin Sr 150mg
Flonase 0.05mg
U&C
Lipitor 10mg
Celebrex 200mg
Plavix 75mg
Prevacid 30mg
Lipitor 20mg
Ambien 10mg
Levaquin 500mg
Zocor 20mg
Zithromax 250mg
Flonase 0.05mg
Source: GAO analysis of data from CMS, First DataBank, EPIC, PACE, and BCBS FEP.
Percentage of 50 brand drug prescriptions: 36%
Percentage of price index’s increase: 49%
Percentage of 50 brand drug prescriptions: 33%
Percentage of price index’s increase: 48%
Percentage of 50 brand drug prescriptions: 37%
Percentage of price index’s increase: 46%

Page 14 GAO-05-779 Prescription Drug Price Trends
We provided a draft of this report to CMS, PACE, EPIC, and BCBS FEP. In
commenting on this report, CMS highlighted the discounts and price
information tools that will be available under the Medicare drug benefit.
CMS also stated that neither the U&C price nor AWP reflect discounts,
such as manufacturers’ discount programs, or other price concessions
affecting a drug’s price. We noted in the report that U&C represents the
retail pharmacy price paid by consumers without insurance. The U&C
does not reflect prices available from other sources, such as mail order
pharmacies. We also noted that AWP is a list price that is not the actual
price paid by large purchasers. We agree that consumers may be able to
obtain lower prices than reflected by the U&C and AWP. However, the
focus of our analysis was to examine price trends rather than price levels,
and U&C and AWP are consistent measures used to assess price trends.
Further, increases in the published AWP may increase what many public
or private third-party purchasers pay for prescription drugs because AWP
is often included in the formula to calculate payments to pharmacies.
Additionally, CMS suggested that we examine the effect on prices when
generic alternatives are introduced. We agree that the introduction of
generic drugs can reduce consumer payments for drugs. Examining
changes in consumer spending for drugs, which are also affected by
changes in utilization and the introduction of new drug alternatives, would
be useful, but was beyond the scope of this report in examining price
trends for frequently-used brand and generic drugs.
PACE and BCBS provided technical comments that we incorporated as
appropriate; EPIC stated that it did not have any comments.
As agreed with your offices, unless you publicly announce the contents
earlier, we plan no further distribution of this report until 30 days after its
date. We will then send copies of this report to the Administrator of CMS
and other interested parties. We will also provide copies to others upon
request. In addition, the report will be available at no charge on the GAO
Web site at http://www.gao.gov.
Agency and Other
External Comments

Page 15 GAO-05-779 Prescription Drug Price Trends
If you or your staffs have any questions about this report, please call me at
(202) 512-7114 or [email protected]. Contact points for our Offices of
Congressional Relations and Public Affairs may be found on the last page
of this report. GAO staff who made major contributions to this report are
listed in appendix III.
Marjorie Kanof
Managing Director, Health Care

Appendix I: Scope and Methodology
Page 16 GAO-05-779 Prescription Drug Price Trends
To examine the change in retail prices for prescription drugs frequently
used by Medicare beneficiaries and other individuals with health
insurance, we used data from the Blue Cross and Blue Shield (BCBS)
Federal Employee Program (FEP) to select the 100 prescription drugs
most frequently dispensed through retail pharmacies in 2003 for BCBS
FEP Medicare enrollees and the 100 most frequently dispensed for BCBS
FEP non-Medicare enrollees.
1
Combined, these two lists included 133
unique drugs.
2
We obtained average monthly usual and customary (U&C) prices reported
by retail pharmacies to Pennsylvania’s Pharmaceutical Assistance
Contract for the Elderly (PACE) program from January 2000 through
December 2004 and New York’s Elderly Pharmaceutical Insurance
Coverage (EPIC) program from August 2000 through December 2004.
3
,
4
We
collected prices based on a specific strength, dosage form, and common
number of units (such as pills), typically for a 30-day supply.
5
Based on
combined PACE and EPIC data, 96 of the 133 drugs we selected had prices
reported for every month from January 2000 through December 2004. We
1
BCBS FEP covered nearly 55 million prescriptions dispensed to enrolled federal
employees, retirees, and their dependents at retail pharmacies in 2003, including 21 million
prescriptions for FEP enrollees who were also Medicare beneficiaries. The 96 drugs that
we included in our analyses represented about 32 percent of total prescriptions dispensed
to BCBS FEP enrollees in 2003. Of these 96 drugs, 50 were brand drugs and represented
about 17 percent of total prescriptions dispensed to BCBS FEP enrollees in 2003.
2
Drugs with the same name but with different forms (such as capsules or tablets) or
number of units dispensed were counted separately as unique drugs.
3
PACE covered more than 9 million prescriptions and EPIC covered nearly 10 million
prescriptions dispensed to mostly low-income seniors in 2003. As of June 2005, PACE
officials reported that approximately 2,800 retail pharmacies—95 percent of pharmacies in
Pennsylvania—participated in PACE, while EPIC officials reported approximately 4,150
retail pharmacies—87 percent of pharmacies in New York—participated in EPIC.
4
We merged price data from PACE and EPIC for August 2000 through December 2004, but
report price data from PACE alone for January 2000 through July 2000. Because the
average of the U&C prices reported by PACE and by EPIC were nearly identical, we do not
believe that including the EPIC data beginning in August 2000 notably affected the price
trend.
5
The Department of Veterans Affairs Pharmacy Benefits Management Strategic Healthcare
Group provided the most common number of units for a retail prescription for a 30-day
supply.
Appendix I: Scope and Methodology

Appendix I: Scope and Methodology
Page 17 GAO-05-779 Prescription Drug Price Trends
analyzed price trends on a monthly basis from January 2000 through
December 2004 for these 96 drugs.
6
Of the 96 drugs, 75 were among those most frequently used by BCBS FEP
Medicare enrollees, and 76 were among those most frequently used by
BCBS FEP non-Medicare enrollees. Fifty-five of the 96 drugs were
frequently used by both BCBS Medicare enrollees and non-Medicare
enrollees.
7
We first determined the total number of prescriptions in 2003
for the drugs we selected dispensed to BCBS FEP Medicare enrollees and
the total number of prescriptions dispensed to BCBS FEP non-Medicare
enrollees. Separately for drugs frequently used by Medicare and by non-
Medicare enrollees, we calculated the share of the total number of BCBS
FEP prescriptions attributed to each drug. The price of each drug was then
weighted by its relative share of total Medicare or total non-Medicare
prescriptions in 2003 to calculate the average price for frequently used
Medicare drugs and the average price for frequently used non-Medicare
drugs for each month from January 2000 through December 2004.
8
,
9
We
standardized these averages to create a Medicare price index and a non-
Medicare price index, each with a value of 100 as of January 2000.
We also separately analyzed monthly trends in U&C prices for brand and
generic drugs frequently used by BCBS FEP enrollees. Of the 96 drugs, 50
were brand drugs and 46 were generic drugs. Similar to our calculation of
6
We also analyzed price trends for 117 drugs that had prices reported for every month from
January 2002 through December 2004, which had an average annual rate of increase of
5.2 percent. For the 96 drugs that had reported prices for every month from January 2000
through December 2004, the average annual rate of increase from January 2002 through
December 2004 was also 5.2 percent.
7
While these 55 drugs were used in calculating both the Medicare and non-Medicare U&C
price indexes, they had different weights in each index depending on the frequency of
prescriptions dispensed to BCBS FEP enrollees who were either Medicare beneficiaries or
not Medicare eligible.
8
BCBS FEP retail prescriptions represent various days supply (such as 34- or 90-day
supply), while PACE and EPIC price data we obtained are limited only to retail
prescriptions for a typical 30-day supply. Over half of BCBS FEP retail prescriptions are for
a 30-day supply.
9
The 2003 BCBS FEP retail prescription drug weights applied to PACE and EPIC retail
prices for 96 drugs from January 2000 through December 2004 were held constant
throughout the entire period of the analysis. We also obtained 2004 BCBS FEP retail
prescription data for 89 of the 96 drugs and found almost no difference in the change in the
U&C price index for the 89 drugs using constant 2003 or 2004 BCBS FEP drug weights
throughout the period of analysis.

Appendix I: Scope and Methodology
Page 18 GAO-05-779 Prescription Drug Price Trends
Medicare and non-Medicare price indexes, we calculated indexes for
brand drugs and generic drugs based on each drug’s share of the total
number of brand or generic prescriptions dispensed to BCBS FEP
enrollees in 2003.
To examine the change in retail prices for frequently used drugs compared
to other drug price benchmarks, we compared an index based on the U&C
prices reported by PACE and EPIC for 50 brand drugs to indexes based on
the average manufacturer prices (AMP) and average wholesale prices
(AWP) for these 50 drugs on a quarterly basis from the first quarter of 2000
through the fourth quarter of 2004.
10
The Centers for Medicare & Medicaid
Services (CMS) requires manufacturers to report AMP within 30 days of
the end of each calendar quarter. Manufacturers submit AWPs on a
periodic basis to publishers of drug-pricing data, such as First DataBank.
Using the National Drug Codes (NDC)
11
reported by PACE and EPIC for
the U&C prices for the 50 brand drugs, we obtained per unit AMPs from
CMS and per unit AWPs from First DataBank associated with each NDC.
12
For each drug, we calculated a quarterly AMP and a quarterly AWP by
multiplying the per unit price by the most common number of units for a
30-day supply.
13
We created an AMP and AWP index by weighting the 50
brand drugs by the number of prescriptions in 2003 from BCBS FEP.
10
These 50 brand drugs were frequently used by Medicare beneficiaries and non-Medicare
enrollees in the BCBS FEP in 2003 and had reported U&C prices to PACE and EPIC for
every month from January 2000 through December 2004.
11
NDCs are three segment numbers that are the universal product identifiers for drugs for
human use; the U.S. Food and Drug Administration assigns the first segment of the NDC,
which identifies the firm that manufacturers, repackages, or distributes a drug. The second
segment identifies a specific strength, dosage form, and formulation for a particular firm
and the third segment identifies package size. A single drug can have multiple NDCs
associated with it. For example, a drug made by one manufacturer, in one form or strength,
but in three package sizes would have three NDCs.
12
We obtained quarterly AMPs from CMS for each two-segment NDC, represented by 9
digits (not accounting for package size), associated with the 50 brand drugs from the first
quarter of 2000 through the fourth quarter of 2004. Similarly, we obtained monthly AWPs
from First DataBank for each three-segment NDC, represented by 11 digits, associated with
the 50 brand drugs from first quarter 2000 through fourth quarter 2004. Specifically, we
obtained the AWP effective on the last day of each month for each 11-digit NDC.
13
For brand drugs with multiple 9-digit NDCs, we calculated an average quarterly AMP for
the drug weighted by the number of PACE and EPIC prescriptions for each 9-digit NDC
during that quarter. For brand drugs with multiple 11-digit NDCs, we calculated an average
monthly AWP for the drug weighted by the number of PACE and EPIC prescriptions during
that month. We created a quarterly AWP by taking a simple average of the three monthly
prices in each quarter.

Appendix I: Scope and Methodology
Page 19 GAO-05-779 Prescription Drug Price Trends
Similarly, we recalculated the U&C price for the 50 brand drugs on a
quarterly basis to make comparisons to AMP and AWP.
We also determined how much each drug’s change in price contributed to
the overall change in price for the 50 brand drugs for AMPs, AWPs, and
U&C prices. We measured the share each drug contributed to the overall
index by comparing the ratio of (1) each drug’s price change from January
2000 through December 2004 multiplied by its weight based on BCBS FEP
prescriptions, to (2) the sum of all drugs price changes multiplied by their
associated weights.
Our analyses are limited to drugs most frequently used by Medicare
beneficiaries and by non-Medicare enrollees in the 2003 BCBS FEP.
Additionally, our analyses using U&C prices are limited to prices reported
by retail pharmacies in Pennsylvania to the PACE program and by retail
pharmacies in New York to the EPIC program. We reviewed the reliability
of data from BCBS FEP, CMS, First DataBank, EPIC, and PACE, including
screening for outlier prices in the PACE and EPIC data and ensuring that
the price trends and frequently used drugs were consistent with other data
sources. We determined that these data were sufficiently reliable for our
purposes. We performed our work from April 2004 through July 2005 in
accordance with generally accepted government auditing standards.

Appendix II: Drugs Included in Analyses
Page 20 GAO-05-779 Prescription Drug Price Trends
Table 1 lists the 96 drugs used in constructing monthly U&C price indexes
from January 2000 through December 2004. Fifty of the 96 drugs are brand
drugs and were also used in examining price changes in AMP, AWP, and
U&C on a quarterly basis from first quarter 2000 through fourth quarter
2004. Of the 96 drugs, 75 were frequently used by Medicare beneficiaries
and 76 were frequently used by non-Medicare enrollees, with 55 of these
drugs frequently used by both Medicare beneficiaries and non-Medicare
enrollees.
Table 1: Ninety-Six Drugs Included in U&C Price Indexes, by Month, January 2000 through December 2004
Drug name and strength
Units dispensed and
dosage form for a typical
30-day supply
Brand or
generic
Medicare or non-
Medicare
Acetaminophen/Codeine 30/300mg 60 tablets Generic Both
Aciphex 20mg 30 tablets
delayed release
Brand Both
Albuterol 90mcg 17gm aerosol Generic Both
Allegra-D 60-120 mg 60 tablets
extended release
Brand Non-Medicare
Allopurinol 300mg 30 tablets Generic Medicare
Alprazolam 0.25mg 60 tablets Generic Both
Alprazolam 0.5mg 60 tablets Generic Both
Ambien 5mg 30 tablets Brand Medicare
Ambien 10mg 30 tablets Brand Both
Amoxicillin 500mg 21 capsules Generic Both
Aricept 10mg 30 tablets Brand Medicare
Atenolol 25mg 30 tablets Generic Both
Atenolol 50mg 30 tablets Generic Both
Carisoprodol 350mg 90 tablets Generic Non-Medicare
Celebrex 200mg 60 capsules Brand Both
Celexa 20mg 30 tablets Brand Both
Cephalexin 500mg 30 capsules Generic Both
Cipro 500mg 20 tablets Brand Non-Medicare
Clonazepam 0.5mg 60 tablets Generic Non-Medicare
Combivent 103-18mcg 14.7gm aerosol Brand Medicare
Cosopt 2-0.5% 5mL solution Brand Medicare
Coumadin 5mg 30 tablets Brand Medicare
Cozaar 5mg 30 tablets Brand Medicare
Cyclobenzaprine HCl 10mg 60 tablets Generic Non-Medicare
Appendix II: Drugs Included in Analyses

Appendix II: Drugs Included in Analyses
Page 21 GAO-05-779 Prescription Drug Price Trends
Drug name and strength
Units dispensed and
dosage form for a typical
30-day supply
Brand or
generic
Medicare or non-
Medicare
Doxycycline Hyclate 100mg 30 capsules Generic Non-Medicare
Effexor XR 75mg 30 capsules
extended release
Brand Non-Medicare
Effexor XR 150mg 30 capsules
extended release
Brand Non-Medicare
Evista 60mg 30 tablets Brand Both
Flomax 0.4mg 30 capsules Brand Both
Flonase 0.05mg 16gm spray Brand Both
Folic Acid 1mg 30 tablets Generic Both
Furosemide 20mg 60 tablets Generic Both
Furosemide 40mg 60 tablets Generic Both
Hydrochlorothiazide 25mg 30 tablets Generic Both
Hydrocodone/Acetaminophen 5/500mg 90 tablets Generic Both
Hydrocodone/Acetaminophen 7.5/500mg 90 tablets Generic Both
Hydrocodone/Acetaminophen 7.5/750mg 90 tablets Generic Non-Medicare
Ibuprofen 800mg 90 tablets Generic Non-Medicare
Isosorbide Mononitrate 30mg 30 tablets
extended release
Generic Medicare
Isosorbide Mononitrate 60mg 30 tablets
extended release
Generic Medicare
Klor-Con 10 10mEq 30 tablets
extended release
Generic Medicare
Lanoxin 125mcg 30 tablets Brand Medicare
Lanoxin 250mcg 30 tablets Brand Medicare
Levaquin 500mg 10 tablets Brand Both
Lipitor 10mg 30 tablets Brand Both
Lipitor 20mg 30 tablets Brand Both
Lipitor 40mg 30 tablets Brand Non-Medicare
Lorazepam 0.5mg 60 tablets Generic Both
Lorazepam 1mg 60 tablets Generic Both
Meclizine HCl 125mg 90 tablets Generic Medicare
Methylprednisolone 4mg 30 tablets Generic Non-Medicare
Metoprolol Tartrate 50mg 60 tablets Generic Both
Miralax 17gm 255gm powder Brand Medicare
Naproxen 500mg 60 tablets Generic Non-Medicare
Nasacort AQ 55mcg 16.5gm spray Brand Non-Medicare
Nasonex 50mcg 17gm spray Brand Non-Medicare

Appendix II: Drugs Included in Analyses
Page 22 GAO-05-779 Prescription Drug Price Trends
Drug name and strength
Units dispensed and
dosage form for a typical
30-day supply
Brand or
generic
Medicare or non-
Medicare
Neurontin 300mg 90 capsules Brand Both
Norvasc 5mg 30 tablets Brand Both
Norvasc 10mg 30 tablets Brand Both
Oxycodone/Acetaminophen 5/325mg 90 tablets Generic Non-Medicare
Paxil 20mg 30 tablets Brand Both
Penicillin V Potassium 500mg 30 tablets Generic Non-Medicare
Plavix 75mg 30 tablets Brand Both
Potassium Chloride 10mEq 60 capsules
extended release
Generic Medicare
Potassium Chloride 10mEq 30 tablets
extended release
Generic Medicare
Pravachol 20mg 30 tablets Brand Medicare
Pravachol 40mg 30 tablets Brand Both
Prednisone 5mg 30 tablets Generic Medicare
Prednisone 10mg 35 tablets Generic Both
Prednisone 20mg 30 tablets Generic Non-Medicare
Premarin 0.625mg 30 tablets Brand Both
Prevacid 30mg 30 capsules
delayed release
Brand Both
Promethazine HCl 25mg 60 tablets Generic Non-Medicare
Propoxyphene Napsylate/Acetaminophen 100/650mg 90 tablets Generic Both
Ranitidine HCl 150mg 60 tablets Generic Both
Singulair 10mg 30 tablets Brand Both
Spironolactone 25mg 30 tablets Generic Medicare
Sulfamethoxazole/Trimethoprim 800/160mg 20 tablets Generic Both
Synthroid 50mcg 30 tablets Brand Both
Synthroid 75mcg 30 tablets Brand Both
Synthroid 100mcg 30 tablets Brand Both
Toprol XL 50mg 30 tablets
extended release
Brand Both
Toprol XL 100mg 30 tablets
extended release
Brand Both
Trazodone HCl 50mg 90 tablets Generic Both
Triamterene/Hydrochlorothiazide 37.5/25mg 30 capsules Generic Both
Triamterene/Hydrochlorothiazide 37.5/25mg 30 tablets Generic Both
Warfarin Sodium 5mg 30 tablets Generic Medicare
Wellbutrin SR 150mg 60 tablets
extended release
Brand Non-Medicare

Appendix II: Drugs Included in Analyses
Page 23 GAO-05-779 Prescription Drug Price Trends
Drug name and strength
Units dispensed and
dosage form for a typical
30-day supply
Brand or
generic
Medicare or non-
Medicare
Xalatan 0.005% 2.5mL solution Brand Both
Zithromax 200mg/5mL 30 suspension Brand Non-Medicare
Zithromax 250mg 6 tablets Brand Both
Zocor 20mg 30 tablets Brand Both
Zocor 40mg 30 tablets Brand Both
Zoloft 50mg 30 tablets Brand Both
Zoloft 100mg 30 tablets Brand Both
Zyrtec 10mg 30 tablets Brand Both
Source: GAO analysis of data from BCBS FEP, EPIC, and PACE.

Appendix III: GAO Contact and Staff
Acknowledgments
Page 24 GAO-05-779 Prescription Drug Price Trends
Marjorie Kanof (202) 512-7114 or kanof[email protected]
In addition to the contact named above, John E. Dicken, Director; Rashmi
Agarwal; Jessica L. Cobert; Martha Kelly, Matthew L. Puglisi; and Daniel S.
Ries made key contributions to this report.
Appendix III: GAO Contact and Staff
Acknowledgments
GAO Contact
Acknowledgments
(290356)

The Government Accountability Office, the audit, evaluation and
investigative arm of Congress, exists to support Congress in meeting its
constitutional responsibilities and to help improve the performance and
accountability of the federal government for the American people. GAO
examines the use of public funds; evaluates federal programs and policies;
and provides analyses, recommendations, and other assistance to help
Congress make informed oversight, policy, and funding decisions. GAO’s
commitment to good government is reflected in its core values of
accountability, integrity, and reliability.
The fastest and easiest way to obtain copies of GAO documents at no cost
is through GAO’s Web site (www.gao.gov). Each weekday, GAO posts
newly released reports, testimony, and correspondence on its Web site. To
have GAO e-mail you a list of newly posted products every afternoon, go
to www.gao.gov and select “Subscribe to Updates.”
The first copy of each printed report is free. Additional copies are $2 each.
A check or money order should be made out to the Superintendent of
Documents. GAO also accepts VISA and Mastercard. Orders for 100 or
more copies mailed to a single address are discounted 25 percent. Orders
should be sent to:
U.S. Government Accountability Office
441 G Street NW, Room LM
Washington, D.C. 20548
To order by Phone: Voice: (202) 512-6000
TDD: (202) 512-2537
Fax: (202) 512-6061
Contact:
Web site: www.gao.gov/fraudnet/fraudnet.htm
E-mail: [email protected]v
Automated answering system: (800) 424-5454 or (202) 512-7470
Gloria Jarmon, Managing Director, [email protected] (202) 512-4400
U.S. Government Accountability Office, 441 G Street NW, Room 7125
Washington, D.C. 20548
Paul Anderson, Managing Director, AndersonP1@gao.gov (202) 512-4800
U.S. Government Accountability Office, 441 G Street NW, Room 7149
Washington, D.C. 20548
GAO’s Mission
Obtaining Copies of
GAO Reports and
Testimony
Order by Mail or Phone
To Report Fraud,
Waste, and Abuse in
Federal Programs
Congressional
Relations
Public Affairs
PRINTED ON
RECYCLED PAPER
