
62
J.
Physiol.
(I938)
92,
62-90
6I2.731
.I5
THE
EFFECT
OF
THE
INTERACTION
OF
IONS,
DRUGS
AND
ELECTRICAL
STIMULATION
AS
INDICATED
BY
CONTRACTION
OF
THE
ANTERIOR
RETRACTOR
OF
THE
BYSSUS
OF
MYTILUS
EDULIS
BY
INDERJIT
SINGH
From
the
Physiological
Laboratory,
Cambridge
(Received
20
October
1937)
WINTON
[1937]
has
described
the
results
of
stimulating
this
plain
muscle
with
alternating
current
(A.C.)
and
direct
current
(D.C.).
The
present
series
of
observations
is
concerned
with
describing
the
properties
of
the
contraction
produced
by
these
and
various
other
agents,
with
a
view
to
attempting
an
analysis
of
the
interrelation
between
ions,
drugs
and
electrical
stimulation
in
respect
of
their
potentiating
or
antagonizing
actions.
The
significance
of
the
relations
discovered
in
connexion
with
contraction
has
been
extended
by
a
preliminary
analysis
of
the
effects
of
the
relevant
agents
on
the
water
and
total
base
content
of
the
muscle
[Singh,
1938].
METHODS
The
standard
experimental
solution,
referred
to
below
as
Mytilus
saline,
had
a
composition
of
1-8
c.c.
0-564M
KC1,
2-8
c.c.
0-376M
CaC12,
5
c.c.
M/15
sodium
phosphate
at
pH
7
with
the
requisite
amount
of
sodium
chloride
to
make
it
isotonic,
0-564M
NaCl
being
added
to
bring
the
total
volume
up
to
100
c.c.
The
muscle
was
electrically
stimulated
by
Winton's
method
[1926],
modified
by
including
a
12,uF.
condenser
in
the
circuit
to
obviate
rectifi-
cation
at
the
electrodes
when
A.C.
was
used.
Variations
of
excitability
to
A.C.
or
D.C.
were
examined
by
two
methods:
first,
the
voltage
of
a
stimulus
of
10-15
sec.
duration
needed
to
evoke
a
small
tension
of
a
definite
value
was
determined
before
and
after
immersing
the
muscle
in
the
test
solution
for
15
min.;
secondly,
the
tension
evoked
by
a
stimulus
of
given
strength
(8-10
V.,
10-15
sec.)
applied
to
the
muscle
immersed
in
different
media
was
employed
as
an
indication
of
the
varying
sensitivity
of
the
muscle;
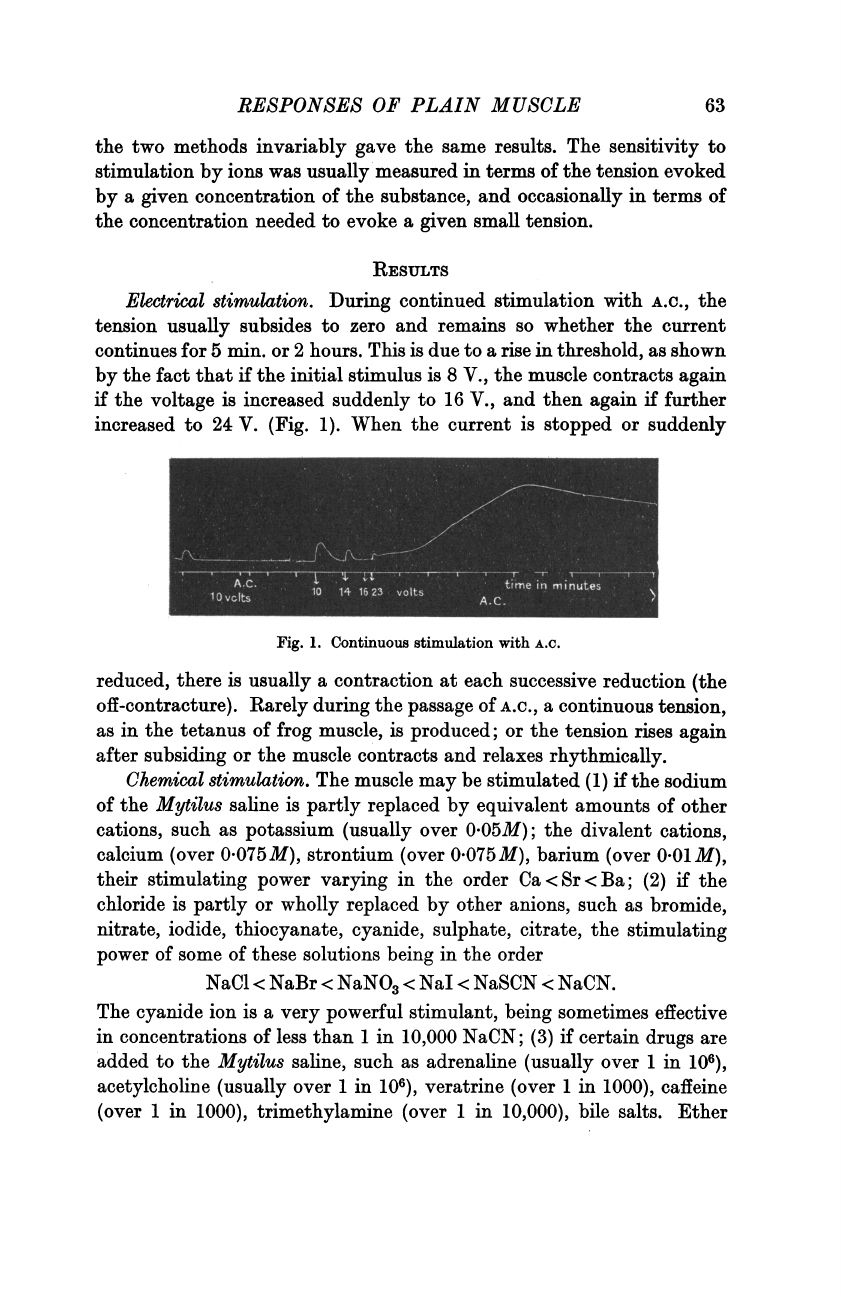
RESPONSES
OF
PLAIN
MUSCLE
the
two
methods
invariably
gave
the
same
results.
The
sensitivity
to
stimulation
by
ions
was
usually
measured
in
terms
of
the
tension
evoked
by
a
given
concentration
of
the
substance,
and
occasionally
in
terms
of
the
concentration
needed
to
evoke
a
given
small
tension.
RESULTS
Electrical
stimulation.
During
continued
stimulation
with
A.C.,
the
tension
usually
subsides
to
zero
and
remains
so
whether
the
current
continues
for
5
min.
or
2
hours.
This
is
due
to
a
rise
in
threshold,
as
shown
by
the
fact
that
if
the
initial
stimulus
is
8
V.,
the
muscle
contracts
again
if
the
voltage
is
increased
suddenly
to
16
V.,
and
then
again
if
further
increased
to
24
V.
(Fig.
1).
When
the
current
is
stopped
or
suddenly
Fig.
1.
Continuous
stimulation
with
A.C.
reduced,
there
is
usually
a
contraction
at
each
successive
reduction
(the
off-contracture).
Rarely
during
the
passage
of
A.C.,
a
continuous
tension,
as
in
the
tetanus
of
frog
muscle,
is
produced;
or
the
tension
rises
again
after
subsiding
or
the
muscle
contracts
and
relaxes
rhythmically.
Chemical
stimulation.
The
muscle
may
be
stimulated
(1)
if
the
sodium
of
the
Mytilus
saline
is
partly
replaced
by
equivalent
amounts
of
other
cations,
such
as
potassium
(usually
over
0-05M);
the
divalent
cations,
calcium
(over
0.075
M),
strontium
(over
0.075
M),
barium
(over
0*01
M),
their
stimulating
power
varying
in
the
order
Ca
<
Sr
<
Ba;
(2)
if
the
chloride
is
partly
or
wholly
replaced
by
other
anions,
such
as
bromide,
nitrate,
iodide,
thiocyanate,
cyanide,
sulphate,
citrate,
the
stimulating
power
of
some
of
these
solutions
being
in
the
order
NaCl
<
NaBr
<
NaNO3
<
NaI
<
NaSCN
<
NaCN.
The
cyanide
ion
is
a
very
powerful
stimulant,
being
sometimes
effective
in
concentrations
of
less
than
1
in
10,000
NaCN;
(3)
if
certain
drugs
are
added
to
the
Mytilus
saline,
such
as
adrenaline
(usually
over
1
in
106),
acetylcholine
(usually
over
1
in
106),
veratrine
(over
1
in
1000),
caffeine
(over
1
in
1000),
trimethylamine
(over
1
in
10,000),
bile
salts.
Ether
63

dissolved
in
Mytilus
saline
sometimes
produces
contraction.
A
feeble
contracture
may
be
produced
if
the
sodium
chloride
of
the
Mytilus
saline
is
replaced
with
osmotically
equivalent
amount
of
glucose.
Rarely
urea
(5-10
p.c.)
in
Mytitlus
saline
may
produce
a
feeble
contracture,
or
after
treatment
with
urea
tone
may
be
greatly
increased.
In
the
experi-
ments
described
below
the
standard
chemical
stimulus
used
has
been
potassium
(from
0 05
to
01
1M).
As
with
electrical
stimulation,
the
tension
produced
when
excess
of
potassium
is
added
subsides,
though
potassium
is
still
present
in
the
solution.
This
is
due
to
a
rise
in
threshold,
for
sudden
successive
increases
in
the
concentrations
of
potassium
again
produce
corresponding
succes-
sive
contractions.
Akin
to
the
contracture
produced
on
the
cessation
of
the
A.C.
stimulus,
a
contraction
may
result
on
cessation
of
the
chemical
stimulus,
that
is,
when
the
stimulating
chemical
is
withdrawn.
This
occasionally
occurs
with
adrenaline
(1
in
50,000),
acetylcholine
(1
in
50,000),
veratrine
hydrochloride
(1
in
1000),
and
sodium
sulphate
(0-56M).
As
with
A.C.
stimulation,
the
muscle
may
contract
and
relax
rhythmically
when
it
is
immersed
in
some
of
the
chemical
stimulants
mentioned
above
(0-056M
KCI,
0-037M
BaCl2,
0-564M
NaCl,
NaBr
and
NaNO3,
0-033M
NH4C1,
1
in
1000
caffeine).
The
excitability
to
electrical
and
chemical
stimulation
was
different
at
different
times
of
the
year.
During
summer
the
muscle
was
hyper-
excitable
to
A.C.
and
relatively
inexcitable
to
potassium
and
other
chemical
stimulants.
During
winter
the
muscle
was
hyperexcitable
to
potassium
and
less
excitable
to
A.C.
or
D.C.
In
summer,
fatigue
was
rapid
when
the
muscle
was
stimulated
with
potassium.
The
potassium
con-
traction
exhibits
well-marked
beneficial
effect
of
previous
contraction
(staircase
phenomenon).
This
fact
was
often
utilized
to
remove
inex-
citability
to
a
smaller
dose
by
preliminary
treatment
with
a
larger
dose.
The
muscle
can
also
be
stimulated
mechanically
or
by
a
sudden
change
of
osmotic
pressure.
Sometimes,
in
winter,
it
contracts
spontaneously
when
immersed
in
sea-water
or
Mytilus
saline.
PROPERTIES
OF
THE
A.C.,
D.C.
AND
THE
POTASSIUM
CONTRACTIONS
Effect
of
potassium
and
ammonium.
The
optimum
concentration
of
potassium
necessary
for
the
A.C.
contraction
is
twice
that
of
sea-water
or
the
same
as
that
of
Mytilus
blood
(0.020M
KCI).
Sudden
increase
in
the
concentration
of
potassium
or
ammonium
(in
acid
solutions
at
pH
7,
i.e.
64
I.
SINGH

RESPONSES
OF
PLAIN
MUSCLE
effect
of
NH4+)
in
Mytilus
saline
by
partial
replacement
of
the
sodium
depresses
the
excitability
to
A.C.;
this
is
followed
by
an
increase
in
ex-
citability
(Fig.
2),
which
lasts
for
a
considerable
period
ranging
from
a
few
minutes
to
a
couple
of
hours,
after
which
the
muscle
gradually
becomes
inexcitable.
These
effects
of
ammonium
and
potassium
on
Mytilus
muscle
resemble
the
effects
of
ammonium
on
frog
muscle
nerve
[Ing
&
Wright,
1931].
The
stage
of
initial
depression
can
be
avoided
if
the
concentration
of
potassium
is
increased
slowly.
In
winter,
when
the
muscle
is
hyperexcitable
to
potassium
and
less
excitable
to
A.C.,
there
is
only
a
depression
in
excitability
to
A.C.,
whether
the
concentration
of
potassium
is
increased
slowly
or
suddenly.
These
differences
between
the
summer
and
winter
muscles
can
be
artificially
reproduced
by
variations
of
the
calcium
content
(00-0.02M
CaCl2)
of
the
Mytilus
saline.
With
a
low
calcium
content
there
is
a
depression
of
excitability,
the
secondary
rise
being
absent,
the
muscle
becoming
inexcitable
in
a
few
minutes.
Calcium
also
hastens
the
recovery
from
the
depressant
effect
of
excess
of
potassium.
Further
the
depressant
effect
of
potassium
on
the
excitability
to
A.C.,
besides
being
intensified
by
lessening
of
the
calcium
content
of
Mytilus
saline,
can
be
increased
if
larger
concentrations
of
potassium
are
used.
The
depressant
effect
of
potassium
is
greater
than
that
of
ammonium,
and
if
small
concentrations
of
these
ions
are
used
(005M)
to
avoid the
depressant
effect,
the
secondary
potentiating
effect
of
potassium
on
the
excitability
to
A.C.
is
greater
than
that
of
ammonium.
When
the
potassium-rich
or
ammonium-rich
solution
is
replaced
with
Mytilus
saline,
the
restoration
of
the
initial
excitability
to
A.C.
is
preceded
by
a
stage
of
hyperexcitability
(Fig.
2).
Again
the
effect
varies
with
the
season
and
individual
muscles.
If
the
muscle
is
highly
sensitive
to
potassium,
then
the
depression
of
excitability
may
be
permanent,
or
the
stage
of
hyperexcitability
during
recovery
may
be
absent.
These
varia-
tions
can
be
artificially
reproduced
by
altering
the
calcium
content
of
Mytilus
saline,
or
by
varying
the
concentration
of
potassium,
the
depres-
sant
effect
being
greater
if
large
concentrations
are
used
(0.1-0.2M
KCI).
The
effect
of
cations
on
the
potassium
contraction
are,
in general,
opposite
to
those
on
the
A.C.
contraction.
Ammonium
chloride
at
first
raises
the
excitability
to
potassium
and
then
abolishes
it
(Fig.
3).
This
is
the
only
method
known
which
will
render a
muscle
inexcitable
to potas-
sium
and
hyperexcitable
to
A.C.
If
the
ammonium
chloride
be
added
slowly
enough, the
phase
of
hyperexcitability
to
potassium
disappears,
as
does
the
phase
of
hyperexcitability
to
A.C.
Treated
witb
potassium
PH.
xCII.
5
65

66
1.
SINGH
35
.
30
0
25
0
20-
'5
10
5
.
I
I
Ii
I
I
O4IMin.20
40
6065
70
80
90
100
Introduction
of
Withdrawal
0
05
M
KCI
of
KCI
Fig.
2.
Effect
of
potassium
on
tension
produced
by
A.C.
(7
V.-10
sec.)
in
a
summer
muscle
(June
1936).
A
steady
state
was
at
first
produced
in
Mytilus
saline;
the
latter
was
then
replaced
with
Mytilus
saline,
the
sodium
chloride
of
which
had
been
partly
replaced
with
potassium
chloride
(0-05M).
The
latter
solution
was
then
again
replaced
with
Mytiius
saline.
5
10
15
20
Introduction
of
01M
NH401
Fig.
3.
Effect
of
ammonium
on
tension
produced
by
A.C.
(8
V.-10
sec.)
and
potassium
(0-IM
KCI)
in
a
summer
muscle.

RESPONSES
OF
PLAIN
MUSCLE
itself
a
muscle
similarly
becomes
inexcitable
to
potassium.
The
initial
sensitizing
and
the
subsequent
densitizing
effect
of
potassium
is
greater
than
that
of
ammonium.
Effect
of
sodium.
(a)
Addition:
The
effects
of
addition
of
sodium
chloride
to
Mytilus
saline
or
sea-water
are
complicated
by
the
inevitable
increase
in
osmotic
pressure,
nevertheless
its
addition,
if
performed
gradually,
yields
changes
in
excitability
like
those
consequent
on
sudden
increase
in
the
concentration
of
potassium
or
ammonium.
Gradual
in-
crease
in
the
concentration
of
sodium
chloride
may
at
first
depress
and
then
increase
the
excitability
to
A.C.
or,
as
with
gradual
increase
in
the
concentration
of
potassium
or
ammonium,
the
initial
stage
of
depression
may
be
absent.
Sudden
addition
of
excess
of
sodium
chloride
only
de-
presses
the
excitability
to
A.C.,
the
subsequent
increase
being
absent;
the
excitability
to
potassium
is
at
first
raised
and
then
depressed.
Control
experiments
in
which
the
osmotic
pressure
is
increased
by
the
addition
of
glucose
only
show
a
depression
in
excitability,
both
to
A.c.
and
to
potassium.
(b)
Withdrawal:
This
was
done
in
two
ways.
The
muscle
was
placed
either
in
a
hypotonic
solution,
or
in
a
solution
in
which
part
of
the
sodium
chloride
had
been
replaced
with
an
osmotically
equivalent
amount
of
glucose.
The
first
effect
of
the
diminution
of
the
sodium
content
is
to
increase
the
excitability
to
A.C.
in
a
way
comparable
to
the
effect
of
withdrawal
of
ammonium
or
potassium;
this
phase
of
increased
excita-
bility
is
followed
by
one
in
which
the
excitability
is
depressed.
These
results
are
more
often
obtained
if
the
sodium
is
withdrawn
gradually
than
if
it
is
withdrawn
suddenly.
The
phase
of
increased
excitability
on
withdrawal
of
sodium
is
sometimes
transient
or
absent.
It
is
difficult
to
say
how
far
the
subsequent
decrease
in
excitability
to
A.C.
is
due
to
de-
crease
in
the
conductivity
of
the
solution.
Ciliary
movement
in
Mytilus
edulis
continues
for
several
hours
if
the
sodium
chloride
of
the
surrounding
medium
is
wholly
replaced
with
saccharose
[Gray,
1922].
Effect
of
withdrawal
of
sodium
on
the
excitability
to
potassium
depends
upon
whether
the
sodium
chloride
is
replaced
with
glucose
or
not.
The
effect
of
hypotonic
saline
(60-70
p.c.
of
normal)
is
the
opposite
to
that
of
Mytilus
saline
rendered
hypertonic
by
addition
of
sodium
chloride;
the
excitability
is
at
first
depressed
and
then
raised.
Hypotonic
solutions
produce
a
comparable
increase
in
the
sensitivity
to
drugs
of
the
guinea-pig
uterus
[Dale,
1913].
If
the
sodium
chloride
is
replaced
with
an
equivalent
amount
of
glucose
the
excitability
to
potassium
is
depressed;
replacement
of
all
the
sodium
chloride
of
the
Mytilus
saline
with
glucose
renders
the
muscle
inexcitable
to
potassium.
5-2
67

Effect
of
lithium.
Replacement
of
the
sodium
of
the
Mytilus
saline
with
lithium
decreases
the
excitability
to
A.C.
and
increases
that
to
potassium.
If
the
depressant
effect
of
the
monovalent
cations
is
avoided
by
using
small
concentrations
(0*05M),
they
increase
the
excitability
to
A.c.
and
decrease
that
to
potassium
in
the
order
Li
<
Na
<
NH4
<
K,
being
the
same
as
the
order
for
favouring
ciliary
movement
in
Mytilus
edulis
[Lillie,
1906].
The
initial
effect
of
lithium
is
sometimes
in
the
opposite
direction,
the
excitability
to
A.C.
being
increased
and
that
to
potassium
8
4-
8-2
7-8
7.4
70
6-6
6*2
5-8
5.4
pH
Fig.
4.
Effect
of
pH
on
tension
produced
by
potassium
(01M)
and
A.C.
(10
V.-1O
see.).
decreased;
the
monovalent
cations
then
decrease
the
excitability
to
A.C.
and
increase
that
to
potassium
in
the
same
order;
subsequently
the
effect
is
reversed.
Ultimately
the
excitability
to
A.C.
is
depressed
by
these
abnormal
cations
in
the
order
Na<Li<NH4<K;
they
also
depress
muscular
activity
in
some
other
marine
animals
in
the
same
order
[Lillie,
1909].
Effect
of
hydrogen
ion.
The
effects
of
this
apparently
resemble
the
effects
of
other
monovalent
cations,
especially
sodium.
In
six
winter
muscles
the
maximum
excitability
to
potassium
was
at
pH
7-8
(borate
buffer
M/300,
phosphate
M/300,
bicarbonate
as
in
sea-water).
It
dimin-
ished
with
change
on
either
side,
the
muscle
becoming
inexcitable
between
pH
6-5
and
5
(Fig.
4).
In
five
muscles
(excitable
to
potassium)
the
maximum
excitability
to
A.C.
was
at
pH
8-2-8-4,
that
of
aerated
sea-
water.
As
the
pH
was
lowered,
the
excitability
to
A.O.
declined
and
rose
again
to
a
second
maximum
at
about
pH
7,
the
intervening
minimum
68
I.
SINGH

RESPONSES
OF
PLAIN
MUSCLE
being
at
pH
7-8.
With
further
lowering
of
pH,
the
excitability
to
A.C.
declined,
till
the
muscle
became
inexcitable
at
pH
5
or
lower
(buffer
phosphate,
acetate
M/300).
If
carbon
dioxide
is
used,
inexcitability
both
to
A.C.
and
potassium
is
rapid.
Thus
over
the
physiologically
significant
range
(pH
8.4-6
8),
the
effect
of
pH
follows
the
above-mentioned
rule
that
when
the
excitability
to
potassium
is
highest,
that
to
A.C.
is
lowest.
Sudden
increase
in
the
concentration
of
hydrogen
ions
produces
effects
similar
to
those
consequent
on
sudden
increase
in
the
concentra-
tion
of
ammonium,
potassium
or
sodium
ions.
If
the
pH
is
increased
to
30
25-
20
-
15-
10
5
0
Min.
10
20
30
pH
4-4
Fig.
5.
Effect
of
sudden
increase
of
[H+]
on
tension
produced
by
potassium
(0O1M
KCI).
4
4,
there
is
at
first
a
great
increase
in
the
excitability
to
potassium,
which
is
followed
by
a
decline
till
the
muscle
becomes
inexcitable
(Fig.
5).
As
with
ammonium
or
potassium,
if
the
pH
is
gradually
lowered
to
4*4,
this
phase
of
increased
excitability
is
absent.
Thus
there
is
antagonism
between
all
the
monovalent
cations,
not
excluding
the
hydrogen
ion.
These
effects
of
hydrogen
ion
can
be
understood
when
it
is
remembered
that
hydrogen
ion,
like
sodium
or
potassium,
can
also
make
the
muscle
contract.
Effect
of
calcium.
The
optimum
concentration
of
calcium
necessary
for
the
A.C.
contraction
is
twice
that
of
sea-water,
or
the
same
as
that
of
Mytilus
blood
(0021M
CaCl2).
The
optimum
concentration
of
calcium
for
the
potassium
contraction
is
lower,
being
the
same
as
that
of
sea-
water.
Excess
of
calcium
depresses
the
excitability
to
A.C.
whether
the
69

concentration
is
increased
gradually
or
suddenly;
the
muscle
may,
how-
ever,
make
a
partial
recovery.
The
depressant
action
of
excess
of
calcium
on
excitability
to
A.C.
is
greater
in
winter
potassium
sensitive
muscles.
When
calcium
is
excluded,
the
excitability
to
A.C.
is
depressed,
but
here
again
the
depressant
action
of
calcium
deficiency
varies
with
the
sensi-
tivity
of
the
muscle.
A
muscle
highly
excitable
to
potassium
became
inexcitable
to
A.C.
in
a
few
minutes,
but
a
muscle
inexcitable
to
potassium
(0IM
KCI)
still
responded
to
A.C.
after
2
hours'
immersion
in
calcium-
free
saline.
When
calcium
is
excluded
the
excitability
to
potassium
at
first
rises
and
then
falls
(Fig.
6).
20
,
16
12-
4-
Min.
0
10
20
30
40
Withdrawal
of
0-01M
Ca
C12
Fig.
6.
Effect
of
withdrawal
of
calcium
on
tension
produced
by
potassium
(0IM
KCI).
Thus
calcium
deficiency
depresses
the
excitability
to
A.C.,
just
as
does
an
excess
of
calcium
or
potassium
which,
if
sufficient
in
amount,
will
stimulate
the
muscle.
Moreover,
removal
of
calcium
from
the
medium
elicits
a
contraction
in
those
muscles
which
are
highly
sensitive
to
potassium,
and
such
a
contraction
is
presumably
due
to
an
exciting
action
of
sodium,
comparable
to
similar
action
of
potassium,
since
it
occurs
in
the
absence
of
all
other
relevant
ions,
viz.
potassium
and
magnesium.
Moreover,
if
the
muscle
is
highly
sensitive
to
potassium,
even
Mytilus
saline
may
elicit
a
contraction,
which
is
abolished
if
the
calcium
content
is
doubled
(0.02M
CaCl2).
Calcium
thus
varies
only
the
sensitivity
of
the
muscle
to
sodium,
just
as
it
varies
the
sensitivity
to
potassium.
Sodium,
unantagonized
by
calcium,
may
therefore
be
re-
garded
as
the
agent
responsible
both
for
stimulating
the
muscle
and
for
depressing
the
excitability
to
A.C.
when
calcium
is
withdrawn.
This
view
is
supported
by
the
restoration
of
excitability
to
A.C.
in
the
absence
of
I.
SINGHI
70

RESPONSES
OF
PLAIN
4!USCLE
calcium,
which
is
sometimes
produced
by
immersion
of
the
muscle
in
sodium-deficient
Mytilus
saline
(one
in
which
30-40
p.c.
of
the
sodium
chloride
is
replaced
by
an
osmotically
equivalent
amount
of
glucose);
similar
treatment,
moreover,
has
an
antagonistic
action
on
the
production
of
contracture
by
withdrawal
of
calcium.
Diminished
excitability
to
potassium
in
the
absence
of
calcium
would
then
be
due
to
the
antagonism
of
sodium,
comparable
to
the
antagonism
between
ammonium
and
potassium,
or
excess
sodium and
potassium.
Treated
with
potassium
itself
a
muscle
similarly
becomes
inexcitable
to
potassium,
the
tension
produced
by
a
given
concentration
of
potassium
subsides
though
potassium
is
still
present
in
the
solution.
A
greater
con-
centration
of
potassium
now
evokes
a
contraction
showing
that
the
subsidence
of
tension
is
due
to
a
rise
in
threshold
to
potassium.
Accom-
modation
to
potassium
is
therefore
produced
by
a
process
which
is
probably
similar
to
that
which
produces
antagonism
between
the
mono-
valent
cations.
A
muscle
immersed
in
calcium-free
saline
for
about
an
hour
and
then
treated
with
isotonic
sodium
citrate
solution
for
about
15
min.
becomes
inexcitable
to
A.C.
and
chemical
stimulation.
Excitability
or
contractility
is
not
entirely
abolished,
as
the
muscle
can
be
made
to
contract
by
im-
mersion
in
isotonic
sodium
cyanide,
combined
with
the
cessation
of
the
passage
of
a
direct
current.
Effect
of
strontium.
Increase
in
the
concentration
of
strontium
chloride
produces
effects
similar
to
those
produced
by
increase
in
con-
centration
of
ammonium
or
potassium,
that
is,
an
initial
depression,
a
subsequent
rise,
and
a
stage
of
hyperexcitability
on
removal.
As
with
other
ions
the
effects
vary
with
individual
muscles
and
with
the
con-
centration
of
strontium
employed.
The
stage
of
initial
depression
is
avoided
by
gradual
increase
in
the
concentration
of
strontium.
The
effects
are
obtained
less
readily
with
strontium
than
with
ammonium
or
potassium.
Effect
of
magnesium.
In
winter
potassium
sensitive
muscles
magne-
sium
depresses
the
excitability
to
A.C.
in
all
concentrations;
in
summer,
potassium
insensitive,
muscles
the
optimum
concentration
is
one-third
that
of
sea-water
or
that
of
blood.
The
optimum
concentration
for
the
potassium
contraction
is
the
same
as
that
of
blood.
Sudden
increase
in
the
concentration
of
magnesium
only
produces
a
depression
in
ex-
citability
to
A.C.,
sometimes
with
a
partial
recovery.
An
interesting
feature
is
that
excess
of
magnesium
may
prolong
the
latent
period
of
the
A.C.
contraction
to
as
much
as
5
sec.
(stimulus
A.C.
10
V.-10
sec.).
71

Effect
of
barium.
Barium
increases
the
excitability
to
A.C.
It
has
no
depressant
action,
except
when
it
causes
the
muscle
to
contract;
the
viscosity
then
becomes
very
high,
and
the
muscle
is
rendered
inexcitable
to
all
forms
of
stimulation.
In
summer,
potassium
insensitive,
muscles
the
divalent
ions
increase
the
excitability
to
A.C.
in
the
order
Ca
<
Sr
<
Ba.
Summer
muscles
respond
to
A.C.
even
if
they
are
immersed
in
isotonic
solutions
of
calcium
or
strontium
chlorides
for
about
an
hour,
the
muscles
being
insensitive
to
these
cations;
in
barium
chloride,
however,
the
muscle
contracts
before
it
can be
stimulated
with
A.C.
The
optimum
concentrations
of
calcium,
potassium
and
magnesium
for
the
D.C.
contraction
are
the
same
as
those
for
the
A.C.
contraction.
Withdrawal
of
calcium
causes
the
loss
of
excitability
to
A.C.
more
rapidly
than
to
D.C.,
so
that
a
muscle
rendered
inexcitable
to
A.C.
may
still
respond
to
D.C.;
the
muscle
still
responds
to
the
cessation
of
D.C.
after
the
passage
of
the
current
has
become
ineffective.
Effect
of
anions.
Excess
of
abnormal
anions
mentioned
above
de-
presses
the
excitability
to
A.C.
and
D.C.,
but
increases
that
to
potassium.
The
depressant
effect
of
these
anions
on
the
excitability
to
A.C.,
and
the
sensitizing
effect
on
the
excitability
to
potassium
varies
in
the
order
NaCl
<
NaBr
<
NaNO3
<
Nal
<
NaSCN
<
NaCN
(Table
I).
The
sensitizing
TABLE
I.
Effect
of
anions
on
excitability
to
A.C.
and
to
potassium
chloride
of
the
Mytilus
saline
completely
replaced
with
the
anion.
(Figures
show
g.
tension.)
A.C.
Potassium
No.
of
,
_ _
__,_
_ _ _
Muscle
Cl
Br
NO3
1
CNS
Cl
Br
NO3
1
CNS
1
24
20
11
2
0
3
10
7
32
36
2
29
24
17
2
0
22
16
15
36
39
3
57
56
55
32
22
17
24
26
32
36
4
37
26
18 10
4
13
20
28
34
38
5
18
16
9
0
0
24
30
25 36
39
6
-
-
-
-
8
12
14
24
30
7
-
-
-
12
16
18
30
36
Average
33
28
22
9
5
13
18
19
32
36
effect
of
these
anions
(excluding
cyanide)
on
the
excitability
of
frog
sartorius
to
chemical
stimulation
[Lillie,
1910;
Chao,
1934a]
and
to
stimulation
by
cold
[Chao,
1934b],
and
the
depressant
effect
on
ciliary
movement
in
Mytilus
[Lillie,
1906]
vary
in
the
same
order.
The
effect
of
nitrate
is
approximately
the
same
as
that
of
bromide
(cf.
the
effect
of
bromide
and
nitrate
on
swelling
of
Mytilus
muscle
[Singh,
1938]);
sometimes
its
potentiating
effect
on
the
excitability
to
potassium
is
less
than
that
of
bromide.
This
appears
to
be
due
to
the
great
increase
in
viscosity
caused
by
nitrate.
If
the
muscle
is
immersed
in
Mytilus
72
I.
SINGH

RESPONSES
OF
PLAIN
MUSCLE
saline,
the
chloride
of
which
is
replaced
by
nitrate,
stimulation
by
potas-
sium
causes
a
great
increase
in
viscosity,
as
judged
by
the
slowness
with
which
the
tension
increases
and
subsides;
the
muscle
may
now
become
inexcitable
to
all
forms
of
stimulation,
and
take
about
a
couple
of
hours
to
recover.
Excess
of
sodium
chloride
and
sodium
bromide
may
produce
a
similar
effect,
but
are
much
less
potent
than
sodium
nitrate.
The
depressant
effect
of
nitrate
on
ciliary
movement
may
be
less
than
that
of
bromide
[Lillie,
1909].
The
effect
of
small
concentrations
(0*05-01M)
and
the
initial
effect
of
large
concentrations,
however,
is
in
the
opposite
direction,
that
is,
to
increase
the
excitability
to
A.C.
This
power
of
increasing
the
excitability
to
A.C.
also
varies
in
the
same
order
as
above,
cyanide
being
effective
in
concentrations
of
1
in
200,000.
The
sensitivity
of
frog
sartorius
to
electrical
stimulation
is
increased
by
these
anions
(with
the
exception
of
cyanide)
in
the
same
order
[Chao,
1935].
As
with
cations,
the
depressant
effect
on
excitability
to
A.C.
(1)
predominates
in
winter,
potassium
sensitive,
muscles,
(2)
can
be
accentuated
by
deficiency
of
calcium,
(3)
is
accentuated
by
increase
in
concentration,
so
that
the
preliminary
phase
of
hyper-
excitability
to
A.C.
may
be
absent.
This
is
more
likely
to
happen
with
anions
whose
depressant
action
is
greater,
such
as
iodide,
than
with
anions
whose
depressant
action
is
less,
such
as
bromide.
The
effects
of
cyanide
in
some
respects
differ
from
those
of
the
other
anions.
Mytilus
muscle
is
capable
of
reacting
even
in
high
concentrations
of
cyanide;
it
will
respond
to
stimulation
with
D.C.
even
in
isotonic
solutions
of
sodium
cyanide
[Singh,
1937].
In
intermediate
concentra-
tions
(over
1
in
10,000)
the
muscle
becomes
more
sensitive
to
A.C.
and
D.C.,
as
well
as
chemical
and
mechanical
stimulation.
This
unusual
feature,
namely
a
change
in
the
same
direction
in
excitability
to
A.C.
and
to
potassium,
appears
to
be
in
some
way
related
to
very
high
sensitivity
of
the
muscle
to
chemical
stimulation,
as
this
effect
is
also
produced
by
barium
salts
and
the
muscle
is
very
sensitive
to
barium
and
cyanide.
Effect
of
drugs
and
other
agents.
The
effects
of
certain
drugs
resemble
the
effects
of
the
anions.
A
selection
of
typical
results
is
given
in
Table
II.
The
following
drugs
greatly
decreased
the
excitability
to
A.C.
(some-
times
rendering
the
muscle
inexcitable)
and
increased
the
excitability
to
potassium:
adrenaline,
acetylcholine,
veratrine,
strychnine,
curare,
physostigmine,
novocaine
and
nicotine.
As
with
anions,
the
effect
of
small
concentrations
and
the
preliminary
effect
of
large
concentrations
of
adrenaline,
veratrine
and
caffeine
was
in
the
opposite
direction,
that
is,
to
increase
the
excitability
to
A.C.
The
depressant
effect
of
these
three
73

74
I.
SINGH
TABLE
II.
Effect
of
certain
drugs
on
the
excitability
to
A.C.
and
to
potassium.
Grams
tension
Drug
Concentration
Adrenaline
1
in
6
x
106
1
in
105
Veratrine
Saturated
in
hydrochlor.
Mytilu8
saline
Caffeine
Saturated
in
MytilU
saline
Acetylcholine
1
in
25,000
Strychnine
1
in
1000
hydrochlor.
Curare
Nicotine
Novocaine
Ephedrine
hydrochlor.
Ether
Chloral
hydrate
1
in
1000
1
in
100
1
in
400
1
in
400
0.5
p.c.
1
p.c.
No.
of
muscle
1
2
3
1
2
1
2
1
1
1
1
1
1
A.C.
8
V.-10
sec.
Immer-
sion
Immer-
Before
for
sion
immer-
10-15
for
sion
min.
1
hr.
30
36
12
3
32
36
15
0
15
12
0
26
0
26 36
0
25
2
-
26
2
16
26
26
20
6
4
2
2
K
Immer-
sion
Immer-
Before
for
sion
immer-
10-15
for
sion
min.
1
hr.
12
6
12
20
0
0
7
21
0
20
10
28
0
-
20
10
28
12
24
0
0
3
2
16
25
18
10
1
20
2
12
14
1
12
7
-
3
10
Season
Winter
Winter
Summer
Winter
Summer
Winter
Summer
Summer
Summer
Summer
Summer
Summer
Summer
Summer
Summer
drugs
on
the
excitability
to
A.C.
(1)
predominates
in
winter
when
the
muscle
is
excitable
to
potassium,
(2)
can
be
accentuated
by
deficiency
of
calcium,
(3)
is
accentuated
by
increase
in
concentration,
so
that
the
preliminary
phase
of
hyperexcitability
to
A.C.
may
be
absent;
but,
as
with
cations,
the
muscle
sometimes
makes
a
partial
recovery
from
the
depressant
effect.
In
sensitive
winter
muscles
the
concentration
of
adrenaline
required
to
increase
the
sensitivity
to
A.C.
and
decrease
that
to
potassium
is
about
1
in
6x
106,
while
concentrations
larger
than
1
in
106
have
opposite
effects,
1
in
25,000
rendering
the
muscle
inexcitable
to
A.C.
and
hyper-
excitable
to
potassium.
In
summer,
when
the
muscles
are
inexcitable
to
potassium,
all
concentrations
of
adrenaline
less
than
1
in
50,000
increase
the
excitability
to
A.C.;
in
some
muscles
adrenaline
may
not
depress
the
excitability
to
A.C.
even
in
concentrations
of
1
in
10,000.
In
summer
muscles
even
saturated
solutions
(in
Mytilus
saline)
of
veratrine
or
caffeine
increased
the
excitability
to
A.C.
It
is
remarkable
that
Mytilus
muscle
preserves
its
irritability
in
such
high
concentrations
of
these
substances.

RESPONSES
OF
PLAIN
MUSCLE
In
summer
muscles
caffeine
(saturated
solution
in
Mytilus
saline)
has
certain
effects
resembling
those
of
calcium
(1)
in
opposing
the
depressant
action
on
the
excitability
to
A.C.
of
adrenaline
(1
in
50,000)
and
veratrine
(saturated
solution
in
Mytilus
saline),
(2)
in
producing
a
sudden
drop
in
excitability,
followed
by
a
partial
recovery,
when
it
is
withdrawn.
The
sudden
drop
in
excitability
to
A.C.
after
withdrawal
of
caffeine
implies
an
increased
sensitivity
to
chemical
stimulation
which
in
sensitive
muscles
may
culminate
in
a
contraction
when
the
drug
is
withdrawn.
No
such
effect
was
observed
on
withdrawal
of
adrenaline
or
veratrine.
In
winter,
the
effects
of
caffeine
and
adrenaline
or
veratrine
are
syner-
gistic.
Iodoacetic
acid
(1
in
7000)
has
a
similar
effect
to
that
of
anions
and
the
drugs.
Asphyxia,
produced
by
bubbling
nitrogen
instead
of
oxygen
through
the
muscle
chamber,
at
first
decreases
the
excitability
to
A.C.
and
increases
that
to
potassium;
subsequently
excitability
to
both
dimin-
ishes.
Treatment
with
urea
(5
p.c.)
increases
the
excitability
to
potassium.
In
performing
these
experiments
the
excitability
of
the
muscle
was
at
first
brought
into
a
steady
state
as
regards
the
response
to
A.C.
and
to
potassium.
The
muscle
was
then
immersed
in
the
experimental
solution.
This
procedure
was
adopted
as
the
muscle
took
a
long
time
(sometimes
a
couple
of
hours
or
more)
to
recover
from
the
effects
of
the
substances,
even
though
the
time
of
exposure
of
the
muscle
to
the
action
of
drugs
was
as
short
as
10-15
min.,
an
effect
comparable
to
that
of
quarternary
ammonium
salts
on
frog
muscle-nerve
preparations
[Ing
&
Wright,
1931].
Influence
of
initial
length.
The
contrast
between
the
A.C.
and
the
potassium
contractions
was
emphasized
by
a
study
of
the
effect
of
varying
the
initial
length
upon
the
tension
produced.
In
these
experiments
the
muscle
was
stretched
slowly
at
the
rate
of
about
1
mm.
in
2
min.
The
length
was
at
first
increased
in
steps
of
3-4
mm.
and
was
then
decreased
in
steps
by
a
similar
amount,
the
muscle
being
stimulated
after
each
variation
in
length.
In
the
first
series
of
observations
the
muscle
was
stimulated
with
A.C.;
in
the
next
by
potassium
and,
in
some
experiments,
a
third
series
of
observations
was
recorded,
in
which
the
muscle
was
again
stimulated
with
A.C.
to
see
that
there
was
no
alteration
in
the
optimum
length
during
the
course
of
the
experiment.
Usually
the
muscle
was
stretched
by
a
length
of
6-9
mm.
in
all;
if
the
muscle
was
stretched
more
than
this,
it
was
difficult
to
perform
experiments
with
diminishing
length,
because
the
muscle
did
not
recover
its
original
length.
Hence,
in
some
experiments,
the
tension
produced
on
stimulation
75

rapidly
diminished
as
the
length
was
decreased,
since
the
full
tension
could
not
be
recorded,
the
muscle
contracting
in
part
isotonically
and
then
isometrically.
The
optimum
initial
length
for
maximum
response
to
A.C.
is
more
than
that
for
the
maximum
response
to
potassium
when
examined
in
sea-
water.
In
Mytilus
saline
this
relation
is
reversed,
the
optimum
length
for
the
response
to
potassium
being
more
than
that
for
the
response
to
48
E
,4
40~~~~~~~~
18
21
24
27
30
Length
of
muscle,
mm.
Fig.
7.
Influence
of
initial
length
on
tension
produced
by
potassium
(O.1M)
and
A.C.
(1OV.-10
sec.)
in
Mytilu8
saline
(M.s.)
in
(sa-water
(s.w.).
A.C.
(Fig.
7).
Mytilus
saline
differs
from
sea-water
in
two
respects;
firstly
its
pH
is
7
instead
of
8-4,
secondly
it
contains
no
magnesium.
In
four
experiments
(one
in
winter
and
three
in
summer)
alteration
in
the
pH
of
Mytilus
saline
produced
no
alteration
in
the
optimum
length
for
A.C.,
but
addition
of
magnesium
(0018M
MgCO2)
altered
the
opti-
mum
length
for
A.C.
in
three
muscles
in
summer,
but
had
no
effect
in
the
one
experiment
in
winter.
It
may
be
recalled
that
the
effect
of
magnesium
on
the
excitability
to
A.C.
in
winter
differs
from
that
in
summer.
Effect
of
electrical
stimulation.
The
excitability
to
A.C.
and
potassium
is
again
varied
in
opposite
directions
by
previous
electrical
stimulation.
Repeated
stimulation
with
A.C.
first
increases
the
excitability
to
A.C.
(staircase
phenomenon)
and
diminishes
that
to
potassium.
Subsequently
the
excitability
to
A.C.
diminishes
(fatigue)
and
that
to
potassium
in-
creases.
Frog
sartorius
also
becomes
more
sensitive
to
potassium
during
76
I.
SINGH

RESPONSES
OF
PLAIN
MUSCLE
fatigue
[Gelhorn,
1932].
These
effects
on
excitability,
at
first
in
one
direction
and
then
in
the
other,
exactly
resemble
those
of
the
anions
or
drugs,
as
if
some
such
substance
was
liberated
during
A.C.
stimu-
lation.
A
subthreshold
stimulus,
applied
for
a
longer
time,
produces
similar
effects.
Passage
of
2
V.
A.C.
for
10
min.
decreases,
and
passage
for
1
hour
increases
the
excitability
to
potassium.
During
continued
stimulation
with
A.c.
for
5
or
10
min.,
after
the
initial
contraction
has
declined,
the
excitability
of
the
muscle
to
A.C.
and
to
potassium
is
also
affected
in
opposite
directions;
the
threshold
to
A.C.
rises
and
that
to
potassium
falls.
Increased
sensitivity
to
chemical
stimulation
during
the
passage
also
of
a
short
A.C.
stimulus
is
shown
by
the
following
experiment.
The
latent
period
of
the
barium
contraction
is
sometimes
very
long
(up
to
1
hour).
If
such
a
muscle
is
immersed
in
Mytilus
saline
containing
barium
(0-02-0-07M
BaCl2)
the
muscle
lies
quiescent.
Passage
of
A.C.
(10
V.)
even
for
2
or
3
sec.
is
sufficient
to
excite
the
muscle;
the
barium
then
produces
its
typical
powerful
contraction,
the
muscle
continuing
to
contract
after
the
cessation
of
the
current.
Ordinarily
passage
of
A.C.
(8-10
V.)
for
such
a
short
period
produces
little
or
no
contraction.
After
continued
stimulation
for
5
or
10
min.,
it
takes
about
10-15
or
more
minutes
for
the
excitabilities
of
the
muscle
to
be
restored
to
their
original
values.
This
increased
excitability,
following
prolonged
passage
of
current,
is
especially
noticeable
in
the
response
to
drugs
such
as
adrenaline
or
acetylcholine.
Towards
the
end
of
recovery
from
exposure
to
a
prolonged
passage
of
current,
the
muscle
often
passes
through
a
stage
in
which
the
excitability
to
potassium
is
low
and
that
to
A.C.
high
(cf.
supernormal
phase,
Adrian,
1921],
so
that
if
potassium
is
added
at
varying
times
after
the
cessation
of
the
current,
the
response
may
be
great,
small
or
normal.
In
contrast
to
the
recovery
of
excitability
to
A.C.
from
the
depressant
effect
of
excess
of
potassium,
calcium
(0.01-0.
02M
CaCl2)
delays
the
recovery
of
excitability
to
A.C.
from
the
depressant
effect
of
exposure
to
prolonged
passage
of
the
current.
The
persistence
of
increased
sensitivity
to
chemical
stimulation
in
a
winter
(potassium
sensitive)
muscle
is
sometimes
found
even
after
a
short
A.C.
stimulus;
corresponding
decrease
in
excitability
to
A.C.
in
similar
muscles
after
a
short
A.C.
stimulus
is
shown
by
the
rapid
onset
of
fatigue.
A
similar
mechanism
is
indicated
in
the
phenomenon
of
a
secondary
spontaneous
contraction
which,
in
certain
circumstances,
follows
the
77

response
to
a
short
A.C.
stimulus
(Fig.
8).
This
is
apt
to
occur
when
the
sensitivity
of
the
muscle
is
such
that
the
exclusion
of
calcium,
or
addition
of
excess
of
ammonium
(0-282M)
or
potassium
(01-02M)
or
sodium,
just
fails
to
produce
a
contraction
(in
the
case
of
potassium
this
can
be
done
by
increasing
the
calcium
content
of
Mytilus
saline
to
twice
that
of
sea-water).
The
secondary
contraction
resembles
a
potassium
contraction
but
not
a
response
to
A.C.
in
the
following
ways:
(1)
it
occurs
in
the
absence
of
calcium;
(2)
when
it
occurs
in
Mytilus
saline
or
in
sea-water,
it
is
inhibited
by
adding
more
calcium,
or
by
previous
stimulation
with
A.C.;
(3)
I
the
optimum
concentration
of
magnesium
required
is
the
same.
Potassium
is
a
more
powerful
stimulant
than
sodium;
the
contracture
which
is
produced
when
Fig.
8.
Secondary
contracture
after
the
muscle
is
immersed
in
an
isotonic
solution
of
an
A.C.
stimulus
sodium
chloride
or
sometimes
in
Mytilus
saline
is
(8
V.-lO
sec.).
diminished
or
abolished
if
the
sodium
is
replaced
by
lithium,
and
increased
if
part
of
the
sodium
(0
05-OlMKCl)
is
replaced
by
potassium,
so
that
sodium
is
a
more
powerful
stimulant
than
lithium.
No
contraction
has
been
observed
after
direct
immersion
of
the
muscle
in
ammonium
rich
solutions,
but
very
few
experiments
have
been
performed
to
test
the
stimulating
power
of
ammonium;
six
summer
muscles
were
used
which
are
usually
insensitive
to
chemical
stimulation.
The
secondary
con-
tracture,
after
a
short
A.C.
stimulus
described
above,
if
produced
in
Mytilus
saline,
is
abolished
if
the
sodium
of
the
Mytilus
saline
is
replaced
by
lith-
ium.
If
Mytilus
saline
is
ineffective
the
secondary
contracture
is
produced
if
part
of
the
sodium
(0.1-03M)
is
replaced
with
ammonium;
and
if
both
sodium
and
ammonium
are
ineffective,
it
may
be
produced
if
part
of
the
sodium
(0.05-OlM)
is
replaced
with
potassium.
The
efficacy
of
these
cations
in
producing
the
contracture
therefore
varies
in
the
order
Li
<
Na
<NH4<K.
As
this
contracture
is
probably
due
to
increased
sensitivity
of
the
muscle
to
these
cations
after
an
A.C.
stimulus,
the
stimulating
power
of
the
monovalent
cations
probably
varies
in
the
same
order.
The
efficacy
of
these
cations
in
producing
contraction
of
muscle
of
some
other
marine
animals
varies
in
the
same
order
[Lillie,
1909];
the
monovalent
cations
cause
Mytilus
muscle
to
swell
and
gain
base
in
the
same
order
[Singh,
1938].
This
secondary
contracture
is
presumably
analogous
to
the
veratrine
contracture
of
skeletal
muscle,
that
is,
spontaneous
excitation
occurring
after
cessation
of
the
electrical
stimulus.
In
Mytitlus
saline
this
secondary
78
I.
SINGH

RESPONSES
OF
PLAIN
MUSCLE
contraction
in
Mytilus
muscle
may
be
attributed
to
the
exciting
action
of
sodium,
for
reasons
given
in
connexion
with
the
effects
of
withdrawal
of
calcium
described
above.
A
contracture
resembling
a
veratrine
con-
tracture
more
closely,
because
sustained
for
a
longer
time,
can
be
produced
in
Mytilus
muscle
by
stimulating
it
in
Mytilus
saline
or
sea-
water
to
which
sodium
chloride
has
been
added.
Similarly
other
con-
tracture-producing
substances,
such
as
caffeine
and
sodium
bromide,
may
excite
after
stimulation
with
A.C.
Contracture-producing
substances
have
a
similar
effect
in
skeletal
muscle
[Gasser,
1930].
PROPERTIES
OF
THE
SODIUM
CONTRACTION
When
placed
in
isotonic
solution
of
sodium
chloride,
most
muscles
contract
strongly.
When
calcium
or
potassium,
in
concentration
up
to
Fig.
9.
Neutralization
of
sodium
contracture
by
A.C.
(10
V.-IO
sec.).
Muscle
immersed
in
Ca-free
saline.
twice
that
in
sea-water
are
added,
they
relax
again.
The
muscles
which
do
not
contract
when
potassium
and
calcium
are
both
excluded
are
those
which
are
insensitive
to
potassium.
Less
insensitive
muscles
respond
to
withdrawal
of
both
calcium
and
potassium,
but
not
to
that
of
one
ion
only.
Muscles
more
sensitive
to
potassium
(winter)
respond
to
the
with-
drawal
of
calcium
alone,
while
muscles,
still
more
sensitive,
respond
to
withdrawal
of
potassium
alone.
For
reasons
stated
above,
these
con-
tractions
due
to
withdrawal
of
calcium
and
potassium
are
attributed
to
the
exciting
action
of
unantagonized
sodium
ions,
the
antagonistic
action
79

of
calcium
against
sodium
being
greater
than
that
of
potassium.
If
the
sensitivity
of
the
muscle
is
very
high,
then
both
calcium
and
potassium
are
unable
to
antagonize
the
action
of
sodium
and
the
muscle
exhibits
increase
in
normal
tone.
Comparable
contractions
of
other
smooth
muscle
have
been
described
by
Clark
[1921]
in
mammalian
muscle,
and
by
Thornton
&
Gillespie
[1937]
in
bronchial
muscle.
The
sodium
contraction
produced
by
withdrawal
of
calcium
can
be
inhibited
by
stimulation
with
A.C.
as
it
can
be
by
treatment
with
potas-
sium
or
by
restoration
of
the
calcium
(Fig.
9).
But
this
may
be
ineffective
if
the
muscle
is
very
sensitive
to
potassium
as
in
winter,
or
if
a
less
sensitive
muscle
is
washed
with
a
calcium-free
saline
for
a
long
time
(about
2
hours).
Adrenaline
(1
in
100,000)
also
inhibits
the
sodium
contracture.
PROPERTIES
OF
THE
BARIUM
CONTRACTION
The
sensitivity
of
the
muscle
to
barium
is
increased
by
those
sub-
stances
which
increase
the
sensitivity
to
potassium.
The
optimum
length
of
the
muscle
is
the
same
for
the
two
substances,
as
are
the
seasonal
variations
in
excitability.
Potassium
increases
the
latent
period
(some-
times
up
to
an
hour)
of
the
barium
contraction,
as
it
does
with
the
sodium
contraction.
PROPERTIES
OF
THE
CONTRACTION
PRODUCED
BY
CHANGE
OF
TENSION
Unstriated
muscle
has
been
known
to
contract
on
sudden
stretch
[Straub,
1900;
Bayliss,
1902;
Griitzner,
1904].
During
experiments
on
viscosity
of
Mytilus
muscle
by
the
method
of
isotonic
stretch
and
release
from
a
stretch
[Winton,
1930],
curves
were
obtained
of
such
a
form
as
would
be
produced
if
the
muscle
contracts
when
it
is
released
from
a
stretch.
Contraction
of
the
muscle
on
stretch
was
very
frequent.
In
Mytilus
saline
such
a
response
to
stretch
does
not
usually
occur
in
summer
muscles,
and
not
invariably
in
winter
muscles.
If
muscles
are
placed
in
sea-water
immediately
after
dissection
and
kept
in
sea-water
thereafter,
the
response
to
stretch
is
absent,
as
mentioned
by
Winton
[1937],
but
if
the
muscles
are
immersed
for
a
time
in
one
of
a
variety
of
solutions
specified
below,
they
become
sensitive
to
stretch
and
remain
so,
even
after
putting
them
back
into
sea-water.
The
inhibitory
action
of
sea-water
is
due
to
its
large
magnesium
content.
I
have
obtained
contraction
on
stretch
in
over
sixty
muscles,
and
I
have
not
come
across
a
single
muscle
in
which,
by
suitable
treatment,
a
contraction
on
stretch
could
not
be
elicited.
Fig.
10
shows
a
record
from
I.
SINGH
80

RESPONSES
OF
PLAIN
MUSCLE
a
muscle
which
contracted
on
stretch
the
first
time
but
not
the
second.
Fig.
11
shows
a
record
for
a
muscle,
the
length
of
which
was
suddenly
increased
and
decreased;
the
muscle
contracted
each
time
the
length
is
varied.
The
curve
produced
on
release
is
not
merely
due
to
viscous
elastic
recoil
of
the
muscle;
it
will
be
seen
that
there
is
a
definite
latent
period
between
the
release
and
the
contraction,
the
intermediate
period
being
occupied
by
a
curve
produced
by
the
viscous-elastic
properties
of
the
muscle.
This
latent
period
can
be
increased
by
potassium.
In
pure
sodium
chloride
the
contraction
begins
immediately
after
stretch
or
release;
OO1M
KCI
increases
the
latent
period
to
5-60
sec.
and
0-02M
KCI
to
40-100
sec.
Fig.
10.
Fig.
11.
Fig.
10.
Contraction
of
muscle
on
istretch
with
10
g.
weight.
The
muscle
contracts
the
first
time
and
not
the
second.
Stretched
at
arrows
~
and
releas'ed
at
t
.
Fig.
11.
Effect
of
stretch
and
release
in
CJa-free
saline
containing
0.02M
KCI.
The
muscle
contracts
each
time
it
is
stretched
(X)
and
each
time
it
is
released
(Y).
The
response
to
stretch
or
release
resembles
that
to
potassium
or
sodium
in
the
following
ways:
(1)
it
is
inahibited
by
calcium,
possibly
due
to
an
increased
accommodation
rate,
(2)
it
is
augmented
during
calcium
deficiency
in
hypotonic
solutions,
and
in
solutions
of
anions
and
drugs
such
as
adrenaline,
veratrine,
caffeine
and
nicotine
that
increase
the
ex-
citability
to
potassium,
(3)
the
optimum
pH
is
7
-8,
and
(4)
the
contractions
produced
by
change
of
tension
are,
like
the
sodium
and
potassium
con-
tractions,
characterized
by
slow
relaxation.
Summer
muscles
(inexcitable
to
potassium)
failed
to
respond
to
change
of
tension;
winter
muscles
(hyperexcitable
to
potassium)
were
very
sensitive
to
change
of
ten-
sion.
Muscles
which
are
initially
insensitive
to
mechanical
stimulation
PH.
xcii.
6
81

can
be
rendered
sensitive
by
immersion
in
a
solution
of
any
of
the
sub-
stances
mentioned
above
(Section
3)
as
augmenting
the
response.
Im-
mersion
in
Mytilus
saline,
buffered
at
pH
7-8
with
M/300
borate,
is
a
reliable
way
of
inducing
sensitivity
to
stretch.
In
hypotonic
solution
(0.6
p.c.)
of
sodium
chloride,
stretch
with
as
little
as 2
g.
evokes
a
response.
In
hypotonic
solutions
of
calcium
or
magnesium
chlorides,
the
stretch
response
is
absent.
The
muscle
recovers
its
excitability
to
stretch
when
replaced
in
Mytilus
saline
after
having
been
immersed
in
0-56M
KCI
with
OO1M
CaCl2
for
about
3-4
hours
or
more,
if
the
pH
of
the
solution
is
kept
at
5-4-4.
Such
high
concentrations
of
potassium
do
not
cause
irreversible
changes
in
excitability
even
if
calcium
is
excluded.
The
contraction
is
not
due
to
injury,
as
that
is
unlikely
when
the
tension
is
increased.
It
is
probably
not
due
to
stimulation
of
nervous
elements,
because
drugs
such
as
novocaine
and
nicotine
(1
p.c.)
increase
rather
than
decrease
the
sensitivity
to
stretch.
When
the
muscle
is
placed
in
a
solution
of
any
substance,
except
potassium,
present
in
concentration
insufficient
to
cause
a
contraction,
stretch
or
release
evokes
a
contraction,
which
is
more
or
less
character-
istic
of
the
contraction
usually
produced
by
the
substance
in
solution.
Thus,
in
the
absence
of
calcium,
the
contraction
is
characterized
by
a
low
rate
of
relaxation
like
that
of
the
sodium
contracture.
In
excess
of
calcium
(0-07M)
or
barium,
the
magnitude
of
the
response
is
least
in
calcium
and
greatest
in
barium,
corresponding
with
the
stimulating
power
of
these
ions.
Similarly
among
the
anions,
the
stimulating
power
as
well
as
the
sensitizing
effect
to
stretch
varies
in
the
same
order.
In
the
solutions
of
the
drugs
the
rate
of
relaxation
is
slow
in
alkaline
solu-
tions
(pH
7.8)
and
rapid
in
acid
solutions
(pH
7).
If
these
substances
have
already
made
the
muscle
contract,
then
the
response
to
stretch
is
feeble
or
absent.
It
thus
appears
that
change
of
tension
sensitizes
the
muscle
to
these
sub-
stances
or
lowers
the
threshold
to
stimulation
by
them.
This
is
well
seen
in
solutions
of
barium
(0
OlM).
During
the
long
latent
period
preceding
the
response
to
barium,
the
muscle
is
exceedingly
sensitive
to
mechanical
stimulation.
Even
a
trace
of
barium
sensitizes
the
muscle
to
stretch.
That
the
response
to
mechanical
stimulation
is
to
be
regarded
as
due
to
excitation
by
sodium,
rather
than
by
potassium,
when
the
muscle
is
immersed
in
Mytilus
saline
or
sea-water
is
shown
by
(1)
its
ready
occur-
rence
in
potassium-free
solutions,
and
its
inhibition
by
potassium
even
in
those
small
concentrations
which
promote
the
response
to
A.C.,
(2)
its
82
I.
SINGH

RESPONSES
OF
PLAIN
MUSCLE
disappearance
if
sodium
chloride
is
replaced
by
glucose
or
lithium
chloride,
though
the
latter
sensitizes
the
muscle
to
potassium.
TONUS
IN
ISOLATED
MUSCLE
The
contractions
of
Mytilus
muscle
can
be
placed
into
two
groups,
those
having
the
properties
of
the
response
to
A.C.
and
those
having
the
properties
of
the
response
to
potassium.
Into
which
category
should
be
placed
the
spontaneous
contracture
that
occurs
when
a
muscle
is
im-
mersed
in
Mytilus
saline
or
sea-water?
The
tension
which
develops
spontaneously
in
a
muscle
when
immersed
in
Mytilus
saline
has
properties
resembling
those
of
the
potassium
con-
traction,
rather
than
those
of
the
response
to
A.C.,
in
the
following
respects:
[(1)
tone
is
characterized
by
high
viscosity
[Winton,
1930];
(2)
a
muscle
exhibits
a
high
tone
if
its
sensitivity
to
potassium
is
high
and
that
to
A.C.
low;
(3)
calcium
up
to
a
concentration
twice
that
of
sea-
water
diminishes
tone;
(4)
tone
decreases
with
moderate
decrease
in
pH;
(5)
in
winter
muscles,
both
the
excitability
to
potassium
and
tone
increased
with
decrease
of
temperature
(experimental
range,
4-20°
C.);
(6)
low
concentrations
of
adrenaline
decrease
the
excitability
to
potas-
sium
and
also
inhibit
tone.
The
only
ion
that
is
present
in
Mytilus
saline
or
sea-water
in
a
stimula-
ting
concentration
is
sodium;
calcium,
potassium
and
magnesium
are
present
in
concentrations
in
which
they
inhibit
tone.
Consequently
tone
in
isolated
muscle
is
probably
a
sodium
contracture.
This
view
is
sup-
ported
by
the
following
observations:
(1)
Sodium
contracture
is
abolished
during
the
period
following
an
A.C.
stimulus
(Fig.
9).
Spontaneous
con-
tracture
in
Mytilus
saline
is
similarly
abolished.
No
other
contracture
is
abolished
and
redevelops
in
the
same
way.
The
high
viscosity
associated
with
sodium
contracture
and
spontaneous
tonus
is
reduced
during
the
period
following
an
A.C.
stimulus,
provided
the
muscle
has
not
been
deprived
of
calcium
for
long.
(2)
If
the
muscle
exhibits
excessive
tone,
it
is
reduced
when
the
sodium
is
partly
replaced
(up
to
30
or
40
p.c.)
with
glucose
or
lithium.
Ordinary
tone
is
not
affected
by
similar
treat-
ment,
probably
owing
to
glucose
possessing
contracture-producing
pro-
perties.
(3)
Adrenaline
inhibits
the
sodium
contracture,
as
well
as
tone,
provided
the
muscle
is
not
stimulated
by
adrenaline.
(4)
If
a
winter
muscle
is
immersed
for
16
hours
in
isotonic
solutions
of
chlorides
of
lithium,
sodium,
ammonium,
potassium
and
magnesium,
it is
found
relaxed
in
all
these
solutions,
except
in
that
of
sodium
chloride;
in
this
it
is
contracted
to
its
maximum
extent.
.,
83

Antagonism
between
tone
and
twitch.
When
the
muscle
exhibits
much
tone,
the
excitability
to
A.C.
iS
low.
If
the
muscle
exhibits
a
slight
con-
tracture,
then
the
threshold
voltage
required
to
superimpose
a
small
contraction
(of
about
2
g.
tension)
is
greater
than
if
the
muscle
is
com-
pletely
relaxed.
Tone
greatly
increases
if
the
muscle
is
immersed
in
Mytilus
saline
(buffered
with
M/300
borate
at
pH
7.8)
for
some
hours,
or
if
the
animals
are
previously
cooled
[Singh,
1938].
Under
these
5-5~~
~
-,,-
6*0
I\
/
I
4-0
-\
435
-
4*0
0.01M
0-02MA
0*03M
0*04M
0-05M
0-06M
CaC12
Fig.
12.
Effect
of
calcium
on
excitability
to
A.C.
Curve
I
was
obtained
with
the
muscle
in
slight
contracture
(3
g.).
Treatment
with
excess
of
calcium
had
abolished
this
tone
and
the
muscle
was
relaxed
when
Curve
II
was
obtained.
The
voltage
in
Curve
I
was
obtained
after
each
increase
in
the
concentration
of
Ca,
and
that
of
Curve
II
after
each
decrease
in
the
concentration
of
Ca.
conditions
the
excitability
to
A.C.
is
low;
in
fact,
the
muscle
may
be
inexcitable
to
A.C.
(10
V.-10
sec.).
The
depressant
effect
of
the
excess
of
cations
is
greater
if
the
muscle
exhibits
much
tone
(Fig.
12).
The
de-
pressant
effect
of
the
monovalent
cations
and
anions
on
the
excitability
to
A.C.,
and
their
augmenting
effect
on
the
excitability
to
potassium,
varies
in
the
same
order
as
their
stimulating
power;
the
stimulating
power
and
the
depressant
effect
of
calcium
is
less
than
that
of
potassium.
Seasonal
variations
in
excitability
to
A.C.
and
potassium
are
also
affected
in
opposite
directions.
We
may
conclude
that
the
ionic
environment
84
I.
SINGH

RESPONSES
OF
PLAIN
MUSCLE
favourable
to
the
response
to
stimulation
with
A.C.
is
antagonistic
to
the
contracture
produced
by
chemical
stimulation.
The
antagonism
between
A.C.
and
contracture
is
further
shown
by
observations:
(1)
that
concentra-
tions
of
potassium
or
calcium
(in
excess
of
0.02M)
which
depress
the
excitability
to
A.C.
cause
the
muscle
to
contract
and
convert
an
A.C.
twitch
into
a
contracture,
the
rate
of
relaxation
being
slow;
(2)
;with-
drawal
of
calcium
or
potassium
depresses
the
excitability
to
A.c.
and
causes
the
muscle
to
contract;
the
A.C.
twitch
is
converted
into
a
con-
tracture
(Fig.
13);
(3)
as
a
result
of
a
short
A.C.
stimulus
(10
V.-10
sec.)
Fig.
13.
Effect
of
Ca
and
NaCl
on
A.C.
contraction.
The
muscle
was
stimulated
at
each
arrow
with
A.C.
(10
V.-IO
sec.).
First
contraction
in
Mytilu8
saine;
second
contraction
after
immersion
for
15
min.
in
Ca-free
saline;
third
contraction
in
Mytilu8
saine
again;
fourth
contraction
in
Mytilua
saline
to
which
NaCl
was
added
(total
NaCI
1-8
times
normal).
the
sodium
contracture
or
tone
may
be
neutralized
(Fig.
9);
(4)
as
a
result
of
passage
of
A.C.
for
5-10
min.,
the
sodium
contracture
or
tone
is
abolished,
the
muscle
relaxes
and
contracts
again
on
the
cessation
of
the
current;
(5)
in
summer
moderate
excess
of
calcium,
ammonium
or
potassium
did
not
produce
a
contraction
or
convert
an
A.C.
twitch
into
a
contracture,
and
also
did
not
depress
the
excitability
to
A.C.
The
fact
that
unstriated
muscle
exhibits
spontaneous
tone,
whereas
striated
muscle
does
not,
may
be
regarded
as
a
further
instance
of
the
rule
enunciated
above
concerning
the
mutual
antagonism
between
sets
of
conditions,
which
favour
the
response
to
A.C.
and
that
to
chemical
stimulation,
for
unstriated
muscle
is
more
readily
stimulated
by
chemical
agents,
whereas
striated
muscle
is
more
readily
excited
by
an
electrical
stimulus.
Corresponding
to
this
the
depressant
action
of
various
sub-
stances
on
the
excitability
to
electrical
stimulation
is
greater
in
unstriated
muscle
than
in
striated
muscle,
the
properties
of
Mytilus
muscle
re-
sembling
those
of
striated
muscle
more
in
summer
than
in
winter.
Inexcitability
in
freshly
dissected
muscle.
An
interesting
question
in
connexion
with
the
action
of
sodium-deficient
solutions
is
the
excitability
85

of
a
freshly
dissected
muscle
to
A.C.
Duliere
&
Horton
[1929]
sug-
gested
that
the
inexcitability
of
freshly
dissected
frog
muscle
was
due
to
the
escape
of
potassium
into
the
interstitial
spaces.
Frog
muscle
contains
potassium
as
its
main
ionic
constituent,
but
Mytilus
muscle
contains
sodium
as
its
main
ionic
constituent.
In
certain
circumstances,
16
12-
4
90
80
70
60
50
40
30
NaCl
p.c.
of
normal
(replaced
with
glucose)
Fig.
14.
Restoration
of
excitability
to
A.c.
(10
V.-1O
sec.)
by
treatment
with
a
sodium-
deficient
solution
(replacement
of
sodium
chloride
of
Mytilu8
saline
with
equivalent
amount
of
glucose).
Mytilus
muscle
is
also
inexcitable
after
dissection,
particularly
in
winter,
and
in
muscles
taken
from
animals
which
have
been
kept
at
too
low
a
temperature
or
for
too
long
a
time
in
a
refrigerator.
It
may
be
significant
in
this
connexion
that
it is
just
this
type
of
muscle
which
has
been
found
to
contain
an
abnormally
high
sodium
content
[Singh,
1938].
Inexcitability
in
such
muscles
is
in
some
way
due
to
the"'action
of
sodium,
as
the
excitability
can
be
restored
by
treating
the
muscle
with
a
sodium-deficient
solution,
such
as
hypotonic
Mytilus
saline,
with
an
osmotic
pressure
of
about
60
p.c.
of
normal,
or
by
replacing
30-40
p.c.
of
sodium
chloride
by
an
osmotically
equivalent
amount
of
glucose
(Fig.
14).
Excitability
can
sometimes
be
restored
by
treatment
of
the
muscle
with
an
isotonic
solution
of
potassium
chloride
for
a
few
seconds,
with
adrenaline
(1
in
100,000),
or
sometimes
with
lithium
saline.
This
again
supports
the
suggestion
that
the
inexcitability
is
due
to
the
action
of
sodium,
as
potassium
and
adrenaline
antagonize
the
action
of
sodium,
and
the
stimulating
power
of
lithium
is
less
than
that
of
sodium
or
potassium,
as
is
its
corresponding
power
in
diminishing
excitability
to
A.C.
In
muscles
that
had
been
stored
in
the
refrigerator
for
a
very
long
I.
SINGH
86

RESPONSES
OF
PLAIN
MUSCLE
time
(3-4
weeks),
the
excitability
could
not
be
restored
by
treating
with
a
sodium-deficient
solution,
but
was
restored
by
an
aerated
phosphate
buffered
Mytilus
saline
at
a
pH
of
7
to
6-5.
The
phase
of
increased
excitability
in
these
muscles,
following
im-
mersion
in
a
sodium-deficient
solution
or
in
lithium
chloride,
is
followed
by
a
phase
of
depressed
excitability,
unless
the
original
sodium
content
is
restored.
The
increase
of
excitability
following
immersion
in
a
sodium
deficient
solution
is
greater
if
the
osmotic
pressure
is
kept
normal.
This
inexcitability
is
probably
due
to
the
tonic
contraction
of
the
muscle
and
consequent
increase
in
viscosity,
produced
as
a
result
of
dissection.
Handling
plays
a
part,
as
the
inexcitability
could
sometimes
be
obviated
by
taking
care,
during
dissection,
not
to
touch
the
muscle
with
the
fingers
or
the
blade
of
the
knife,
or
by
dissecting
it
under
sea-
water
or
Mytilus
saline,
otherwise
the
muscle
is
made
to
contract
by
contact
stimulation.
This
is
in
some
way
related
to
the
fact
that
substances
which
produce
swelling
of
the
muscle
cause
it
to
become
inexcitable,
and
mechanical
contact,
such
as
blotting
with
filter
paper,
increases
.the
swelling
[Singh,
1938].
DIscusSION
The
two
contractions
due
to
stimulation
by
A.C.
and
potassium
respectively,
appear
to
represent
two
systems
in
Mytilus
'unstriated
muscle
which
are
probably
the
same
as
the
phasic
and
postural
con-
tractile
mechanisms
in
the
dog
retractor
penis
[Winton,
1930].
The
A.C.
contraction
is
characterized
by
a
rapid
rate
of
relaxation,
probably
due
to
low
viscosity;
the
sodium
and
potassium
contractures
are
charac-
terized
by
a
slow
rate
of
relaxation
probably
due
to
high
.viscosity.
The
A.C.
contraction,
therefore,
represents
a
system
for
the
production
of
quick
sharp
contractions
of
low
viscosity
[Winton,
1937];
the
potassium
contraction
represents
a
system
for
the
production
of
tonic
contractions,
the
pu-rpose
of
which
is
to
maintain
a
given
tension
or
length.
The
ions
favour
ciliary
movement
in
the
same
order
as
they
favour
the
A.C.
contraction;
excitation
resulting
in
ciliary
movement
is
therefore
prob-
ably
produced
by
the
phasic
system.
This
is
understandable,
as
ciliary
movement
will
be
facilitated
by
a
low
viscosity.
The
antagonism
between
the
two
systems
is
also
understandable;
rapid
movement
will
be
hindered
by
a
high
viscosity;
so
also
a
tonic
contraction
will
be
difficult
to
maintain
if
the
viscosity
is
low.
It
is
known
that
if
the
potassium
content
of
frog
sartorius
diminishes,
the
excitability
to
electrical
stimulation
decreases
[Fenn
&
Cobb,
1934].
87

Irritability
of
Maia
nerve
decreases
with
potassium
leakage
[Cowan,
1934].
If
Mytilus
muscle
is
immersed
for
some
hours
in
Mytilus
saline,
the
potassium
content
diminishes
[Singh,
1938]
and
the
excitability
to
A.C.
decreases.
This
loss
of
excitability
may
be
restored
by
treating
the
muscle
with
a
potassium-rich
solution
and
then
replacing
it
in
Mytilus
saline.
Again,
if
Mytilus
muscle
is
placed
in
excess
of
potassium
or
ammonium
ions,
the
excitability
to
A.C.
increases
after
a
preliminary
diminution.
The
initial
decrease
in
excitability
on
addition
of
potassium
or
ammonium
is
probably
determined
by
the
presence
of
these
cations
outside
the
fibres,
and
the
subsequent
increase
in
excitability
is
probably
determined
by
these
cations
inside
the
fibres.
This
view
is
supported
by
the
fact
that
the
recovery
in
excitability
is
rapid
with
ammonium
and
potassium,
which
are
known
to
penetrate
cells
rapidly,
and
is
much
slower
with
sodium,
which
is
known
to
penetrate
much
more
slowly.
Ing
&
Wright
[1931]
came
to
a
similar
conclusion
on
the
comparison
of
the
effects
of
the
quarternary
ammonium
salts
with
those
of
ammonium
chloride
on
frog
muscle
nerve.
As
with
Mytilus
muscle,
ammonium
salts
produce
a
depression
in
excitability
followed
by
a
recovery.
The
recovery
is
more
rapid
with
ammonium
salts,
which
penetrate
rapidly,
than
with
quarternary
ammonium
salts
which
penetrate
much
more
slowly.
It
is
significant
to
note
that
Mytilus
muscle
does
not
recover
from
the
de-
pressant
action
of
anions
to
which
frog
muscle
is
known
to
be
very
little
permeable.
Conditions
which
result
in
reduction
of
total
base
of
the
muscle,
for
example,
by
placing
the
muscle
in
a
hypotonic
solution,
make
the
muscle
less
excitable
to
A.C.
Conditions
which
result
in
increase
in
total
base
of
the
muscle,
such
as
by
placing
it
in
a
hypertonic
solution,
increase
the
excitability
to
A.C.
Cowan
[1934]
found
that
the
injury
potential
of
crab
nerve
was
increased
by
placing
the
nerve
in
a
hypertonic
solution.
Moreover,
the
order
in
which
the
monovalent
cations
affect
the
sensitivity
to
A.c.
and
ciliary
movement
(Li<
Na
<NXH4<
K)
is
the
same
as
that
found
for
their
apparent
ionic
mobilities
in
protoplasm
by
Osterhout
[1931].
Thus,
as
far
as
the
ions
are
concerned,
the
sensitivity
of
the
muscle
to
A.C.
depends
upon
at
least
two
factors:
(1)
the
total
ionic
content
of
the
muscle,
(2)
the
kind
of
ion
and
its
apparent
mobility
in
protoplasm.
The
main
factor,
therefore,
responsible
for
the
production
of
the
A.C.
contraction,
is
probably
the
potassium
inside
the
muscle
fibres,
and
may
be
termed
the
potassium
system;
excess
of
potassium
outside
the
fibres
causing
tonic
contraction
and
loss
of
excitability
to
A.C.
as
described
I.
SINGH
88

RESPONSES
OF
PLAIN
MUSCLE
above.
The
tonic
contraction
in
Mytilus
saline
or
blood
is
probably
produced
by
the
sodium
of
the
muscle
or
the
medium
[Singh,
1938],
and
may
be
termed
the
sodium
system.
Calcium
or
an
A.C.
stimulus
neutralizes
a
sodium
contracture,
but
if
the
muscle
is
deprived
of
calcium
for
long,
then
the
A.C.
stimulus
fails
to
neutralize
a
sodium
contracture;
the
calcium
has
probably
diffused
away.
Many
workers
are
agreed
that
calcium
is
liberated
during
activity
[for
discussion
see
Fenn,
1936].
In
the
absence
of
calcium
the
rate
of
relaxation
of
an
A.C.
contraction
is
decreased;
the
A.C.
contraction
then
resembles
a
D.C.
contraction.
So
there
is
probably
a
third
system-the
calcium
system,
which
is
the
antagonist
of
the
sodium
system
and
reduces
tone
and
viscosity
during
a
phasic
contraction
produced
by
the
potassium
system.
SUMMARY
1.
The
excitability
to
alternating
current
(A.C.)
and
the
sensitivity
to
potassium
are
affected
in
opposite
directions
by
the
agents
studied,
viz.
cations,
anions,
drugs,
seasonal
variations
and
electrical
stimulation.
2.
Immersion
in
solutions,
containing
excess
of
monovalent
cations,
Na+,
NH4+,
K+,
renders
Mytilus
muscle
first
less
and
later
more
sensitive
to
stimulation
with
A.C.
The
excitability
to
potassium
is
affected
in
the
opposite
direction.
Ultimately
in
excess
of
these
abnormal
cations,
the
excitability
to
A.C.
declines.
v
3.
The
physiological
effects
of
certain
monovalent
cations
and
anions,
namely
(1)
their
stimulating
power,
(2)
their
desensitizing
effect
on
the
response
to
potassium
and
their
initial
desensitizing
effect
on
the
response
to
A.C.,
and
(3)
their
subsequent
sensitizing
effect
on
the
response
to
A.C.
and
their
desensitizing
effect
on
the
response
to
potassium,
varies
in
the
same
order
as
they
cause
swelling
and
gain
of
total
base
[Singh,
1938]:
Li
<
Na
<
NH4<K;
C1<Br<NO3<
CNS
<
CN;
(4)
the
stimulating
power
and
sensitizing
effect
on
the
response
to
A.C.
of
the
divalent
cations
varies
in
the
same
order
as
they
cause
swelling
and
gain
of
total
base:
Ca
<Sr
<Ba.
4.
Restoring
the
muscle
to
Mytilus
saline,
after
a
period
of
immersion
in
a
solution
containing
excess
of
monovalent
cations
K
and
NH4
(that
is,
withdrawal),
results
in
a
phase
of
hyperexcitability
before
return
to
normal.
Withdrawal
of
sodium
also
at
first
produces
a
preliminary
increase
in
excitability
to
A.C.,
which
is
followed
by
a
decline.
The
stage
of
hyperexcitability
on
addition
and
withdrawal
of
excess
of
NH4+
and
K+
is
absent
in
muscles
whose
excitability
to
A.C.
is
low
and
to
potassium
high.
The
depressant
effect
is
partly
counteracted
by
calcium.
89

90
I.
SINGH
5.
The
types
of
contraction
of
this
muscle
can
be
placed
into
two
groups:
(1)
the
A.C.
group,
(2)
the
potassium
group.
The
latter
includes
the
contraction
produced
by
chemical
stimulation,
mechanical
stimula-
tion,
certain
contractures
produced
as
a
result
of
A.C.
stimulation,
and
spontaneous
contracture
or
tone.
I
wish
to
thank
Dr
F.
R.
Winton
for
his
help
in
this
work.
REFERENCES
Adrian,
E.
D.
(1921).
J.
Phy8iol.
55,
193.
Bayliss,
W.
M.
(1902).
Ibid.
28,
220.
Cannon,
W.
B.
&
Lyman,
H.
(1913).
Amer.
J.
Phy8iol.
31,
376.
Chao,
I.
(1934a).
Ibid.
109,
550.
Chao,
I.
(1934b).
Ibid.
109,
561.
Chao,
I.
(1935).
J.
cel.
comp.
Physiol.
6,
1.
Clark,
A.
J.
(1921).
J.
Pharmacol.,
Baltimore,
18,
423.
Cowan,
S.
L.
(1934).
Proc.
Roy.
Soc.
B,
115,
216.
Dale,
H.
H.
(1913).
J.
Phy8iol,
40,
19
P.
Duliere,
W.
&
Horton,
H.
V.
(1929).
Ibid.
67,
152.
Fenn,
W.
0.
(1936).
Phy8iol.
Rev.
16,
450
Fenn,
W.
0.
&
Cobb,
D.
M.
(1934).
J.
gen.
Physiol.
17,269.
Gasser,
H.
S.
(1930).
Phy&iol.
Rev.
10,
35.
Gelhorn,
E.
(1932).
Amer.
J.
Phy8iol.
50,
452.
Gray,
J.
(1922).
Proc.
Roy.
Soc.
B,
93,
104.
Grutzner
(1904).
Ergebn.
Phy8iol.
2,
12.
Ing,
H.
R.
&
Wright,
W.
M.
(1931).
Proc.
Roy.
Soc.
B,
109,
337.
Lillie,
R.
S.
(1906).
Amer.
J.
Phy8iol.
17,
89.
Lillie,
R.
S.
(1909).
Ibid.
24,
459.
Lillie,
R.
S.
(1910).
Proc.
Soc.
exp.
Biol.,
N.Y.,
7,
170.
Osterhaut,
W.
J.
V.
(1931).
Biol.
Rev.
6,
369.
Singh,
I.
(1937).
J.
Phyviol.
89,
54P.
Singh,
I.
(1938).
Ibid.
91,
398.
Straub,
W.
(1900).
Pflfigerm
Arch.
79,
379.
Thornton
&
Gillespie
(1937).
Quoted
from
Bray,
G.
W.,
Recent
Advance8
in
AUergy.
Churchill,
London.
Winton,
F.
R.
(1926).
J.
Phy8iol.
61,
368.
Winton,
F.
R.
(1930).
Ibid.
69,
393.
Winton,
F.
R.
(1937).
Ibid.
88,
492.
