
The Leeds Guide
to Digital Pathology

TABLE OF CONTENTS
Introduction ...................................................................................................................................................... 1
Defining Digital Pathology ............................................................................................................................... 2
The Evidence Base and Case for Adoption ...................................................................................................... 2
The Benefits of Digital Pathology .................................................................................................................... 3
A Business Case for Digital Pathology ............................................................................................................ 4
Mapping Your Path to Successful Deployment ............................................................................................... 5
Scanner Procurement and Operation ............................................................................................................... 8
Engagement with Colleagues ........................................................................................................................ 11
Validation and Training .................................................................................................................................. 13
IT Considerations and Systems Integration ................................................................................................... 14
The Pathologist’s Workstation ....................................................................................................................... 16
Continuing the Journey .................................................................................................................................. 17
Final Thoughts ................................................................................................................................................ 18

INTRODUCTION
The digital pathology team at Leeds Teaching Hospitals
NHS Trust and the University of Leeds has 15 years’
experience and a proven track record in delivering world
class digital pathology innovation and research.
The path to clinical digital pathology deployment is a challenging
but rewarding one. Leeds Teaching Hospitals NHS Trust
continues the journey and has gained valuable insight that will
help others navigate their way. The purpose of this guide is to
provide balanced and practical information on clinical deployment based on our experience and knowledge.
Leeds fully subspecialised diagnostic department of 45 consultant pathologists generates more than 290,000 H&E
slides per annum. We have a multi-award winning digital pathology team comprised of clinicians, scientists, and IT/
informatics professionals. Following successful pilot work in two key subspecialty areas, we are transitioning to 100%
digital scanning of glass slides; creating standard work for training and validating our staff.
Healthcare systems face a global shortage of pathologists, while diagnostic workloads and complexities continue to
increase. Hence, there is a pressing need to innovate and modernise the way pathology services are delivered.
The time is right for mainstream clinical adoption of digital pathology, which has the potential to add value and
completely transform pathology services. Technological maturity of digital pathology hardware and software, coupled
with growing acceptance by pathologists, as well as regulatory, and governing bodies, have aided this progression.
In addition, the evidence base for the validity of digital diagnosis has evolved, and we understand more about how to
use digital slides efficiently and safely.
At Leeds, we are developing best practice guidance to share with other organizations interested in implementing
digital pathology for routine diagnosis. We are champions of evidence-based medicine and have sought to apply
a rational approach to a rapidly evolving medical discipline, while focusing on patient safety and the maintenance
of professional standards. We hope you find this guide a useful starting point when embarking on your own digital
pathology journey!
– The Leeds Digital Pathology Team
1
“ We want to see digital
pathology go mainstream
for the benefit of patients.”
Darren Treanor, Consultant Pathologist and
Lead for the Digital Pathology Project at Leeds
Teaching Hospitals NHS Trust, UK
Dr Bethany Williams, Digital Pathology Fellow, using the Powerwall

2
Defining Digital Pathology
Digital pathology (DP) includes three imaging technologies:
1. Gross imaging
The acquisition and storage of macroscopic pathological images to aid histological diagnosis and cancer dataset
completion.
2. Telepathology
The predecessor of modern whole slide imaging systems, telepathology involves the live remote control of a modified
microscope, and the image is not stored on a computer system.
3. Whole Slide Imaging
When people talk about DP today, they are usually referring to whole slide imaging (WSI). Also known as “virtual
microscopy,” WSI is normally performed with a highly
sophisticated, dedicated scanner that is used to capture
a digital image of the whole slide for later review at
a remote location, which now, thanks to DP, can be
anywhere in the world.
A WSI or “virtual slide” is an image created by the
complete digitisation of the glass slide at up to 200,000
dots per inch (DPI). The resulting images are up to 10
gigapixels in size. If printed at a standard 300 DPI, they
would be the same size as a tennis court. Fortunately,
specialised software is available to compress the image so it is reduced to a negotiable and optimal size for viewing
and analysis. Modern WSI scanners can scan from 1 to 400 slides at a time, typically using a microscope lens with
magnification of 20x or 40x. For some applications, there are systems available that can offer higher magnification
than 40x, if needed.
The Evidence Base and Case for Adoption
The Leeds diagnostic pathology department is a typical NHS department. We face similar issues to those encountered
by pathology services worldwide. We believe DP can provide a flexible platform for safety, quality, and efficiency
improvement. We are equally enticed by the prospect of future-proofing our diagnostic capabilities, whilst allowing us
to work more flexibly and creatively to meet targets and maintain standards.
Key Facts: The Strategic Context of Digital Pathology
• In the UK, cellular pathology requests are increasing by 4.5% year on year. (3)
• Specimens require ever more complex assessment to meet the requirements of national datasets.
• In the UK, we face increasingly challenging cancer turnaround time targets.
• 32% of UK cellular pathologists are expected to retire within the next five years. (3)
Note: These trends are reflected globally. Clearly, pathology departments face an international recruitment and
retention shortfall.

At the onset of this project, we completed a systematic review of existing literature on DP accuracy. We identified
and synthesised the data from 38 peer-reviewed publications spanning the last 20 years. We found a mean diagnostic
concordance of glass slide microscopy diagnosis and digital microscopy diagnosis of 92.4%. (1), compared with a
concordance rate of 93.7% for repeat microscope review of a case using glass slides. We went on to analyse, in
detail, the relatively small number of discordant cases, to identify the key areas of difficulty that pathologists can
experience when they make the transition from glass slide to digital slide reporting. (2) Knowledge of these potential
difficulties allowed us to plan our training and validation procedures, whilst maintaining a strong focus on patient safety.
The Benefits of Digital Pathology
The principal benefits can be divided into four categories:
1. Improvements in Patient Safety
• Use of an integrated DP system allows paperless transmission of digital slides directly to the pathologist,
decreasing the possibility of misidentification or transposition errors at multiple points along the diagnostic
workflow.
• Digital slides offer a readily portable, instantaneously transmissible diagnostic image, which is not subject to the
physical limitations and fragility of glass slide transport.
2. Advances in Workflow
• Flexibility– WSI presents the ability to manipulate workload allocations by pushing and pulling of cases. This allows
departments to proactively respond to fluctuations in workload or case mix allowing for maximized use of resources.
• Rapid case tracking, archiving, and retrieval of slide images and diagnostic information.
• Fast case transfer times between the laboratory and assigned pathologists, resulting in streamlined turnaround
times and defined diagnostic pathways.
3. Positive Impact on the Laboratory Workforce
• The innate flexibility of the digital slide process offers the potential for diverse and appealing working
conditions. WSI also offers the potential for a better balance in individual workloads. Pressure can
be reduced and accuracy increased with the ease and assurance of remote corroboration of any diagnosis.
• Potential to customise and optimise contributions by the workforce will allow those that work less
than full time to maximise their on-the-job productivity. Those considering retirement can choose to offer
their services on more flexible terms, which may benefit individuals and organizations.
• Making “work-life balance” more attainable is likely to appeal to the next generation of pathologists
and drive recruitment of medical graduates to the specialty.
4. Service Quality
• WSI offers the potential for faster access to diagnosis and decreased turnaround times, offering patients access to
their results sooner.
• Improved information sharing and collaboration (e.g. streamlined double-reporting and rapid access to second opin-
ion and expert review) may lead to enhanced diagnostic confidence with a higher quality of diagnosis.
• Convenient recording of cancer staging parameters, including measurements, to improve the outcomes and repro-
ducibility of cancer dataset reporting.
3

A Business Case for
Digital Pathology
Developing an effective business case for
deployment of DP can be one of the most daunting
tasks a department can face. Your fi rst priority
should be to defi ne your “use cases” – how users
will interact with the technology to achieve specifi c
goals. We recommend a phased deployment to
allow for a gradual integration and accrual of skills
in both the diagnostic laboratory and the reporting
desk. Our phased approach began with primary
diagnosis of breast histology, neurohistology, and
immunohistochemistry assessment.
You may want to consider the relative merits of the
following use cases within your institution:
Use Case Description
Use of digital pathology for primary
diagnosis of pathological specimens
Replacement of light microscopic examination of glass slides with
examination of whole slide images as part of the standard diagnostic
workfl ow. You may initially want to explore primary diagnosis for a limited
subset of cases ,e.g. all gynecological pathology cases, or for the work-
load of an individual pathologist or pathologist groups.
Assessment of immunohistochemistry
(IHC)
Replacement of light microscopic examination of glass slides with digital
slides to assess immunohistochemical stains.
Multi-disciplinary team (MDT)/
tumour board
The selection, collection, review and presentation of whole slide images
or annotated regions of interest of cases for discussion at multidisciplinary
meetings or tumour boards. If this use case is taken in isolation, slides can
be scanned after initial conventional glass slide diagnosis, then reviewed
and presented digitally.
Frozen section diagnosis The use of whole slide images to provide rapid, intraoperative
histopathological opinion. You may be considering this option if you need
to provide remote frozen section support to a partnered institution or need
to utilise specialised pathologists working off site.
Receiving and requesting second
opinions/review cases
The use of a DP system to render or request a second opinion on a
previously examined case, e.g. a diffi cult skin case from a general
pathologist to a dermatopathologist.
Remote diagnosis The use of DP to allow pathologists to view and report slides from off-site
locations, including other networked hospitals and their home offi ce.
Insourcing/outsourcing of
diagnostic work
The use of DP to allow the movement and exchange of units of diagnostic
work among different pathology service providers.
4
Improving
Patient
Safety
Improving
Effi ciency
Optimising
the
Workforce
Improving
Service
Quality

Best Practice for Preparing a Business Case
• Begin with a clear, concise introduction, in which you describe your current laboratory and diagnostic practices and
your desire to transition to DP. Use simple terms that do not require expert knowledge of pathology processes. You
will also need to summarize the pressing reasons for why your department should invest in DP. Make use of the data
and references from the “Case for Adoption” paper referenced in the “Further Resources” section to add detail on
the strategic context of DP. (4)
• Describe the scope of investment by listing all the components and itemising the estimated cost of your proposed
DP systems (scanners, servers, slide management software, interfaces, storage)
• Outline how DP links to regional, national, and international strategy:
» Regional/local – reinforce your business case with demographics, data on workforce characteristics, workload
volume and composition, as well as imminent shortfalls in staffing or expertise.
» National – what initiatives, policies or guidelines would your DP implementation help support?
» International – is the prospect of worldwide access and/or rolling time zone access of potential benefit to your
department? If so, build this into your strategic context section.
• Create the economic case for DP adoption – you might want to include an options assessment here, evaluating the
proposition that you “do nothing” versus investing in DP. These options could be scored on the criteria of service
sustainability, affordability, quality, benefits, and risks.
• Your financial case needs to summarise potential cash-releasing savings and cost-avoidance benefits of DP, in
addition to any proposed income to be derived from adoption of whole slide digital imaging. For more on this
subject, refer to our paper and business case template. (6)
Mapping Your Path to Successful Deployment
Once the decision has been made to go digital, the emphasis will be on creating a detailed plan for effective
deployment. We believed that implementing DP reporting in a phased-approach, starting with the self-declared,
enthusiastic subspecialties, would result in success from the start. We conducted a survey of our pathologists to assess
the level of buy-in across the department. The results showed differing levels of confidence in the potential usage of
DP for routine task, multidisciplinary team (MDT) meetings, Immunohistochemistry (IHC) review, second opinions, and
primary diagnosis. Pathologists from the renal, GI/liver, neuro, skin, and breast subspecialties indicated the strongest
level of support.
Not at all interested Uninterested Neutral Somewhat interested Very interested
0 1 4 11 25
0% 2% 10% 27% 61%
5

Not at
all likely
Unlikely Neutral Likely
Very
likely
MDT review 0 2 3 5 21
IHC review 0 2 4 5 17
Second opinion 2 3 4 7 15
Primary diagnosis 3 6 6 7 9
Effective Project Management
For implementation to proceed smoothly and effectively, it is essential that multiple contributing components are
managed and well-planned, and we believed that we would minimize our risk of project delays by hiring a dedicated,
professional project manager.
Our project manager has responsibility for DP deployment including management of many subprojects that
synergistically contribute to total success. Our subprojects were led by various members of the DP team, and the
project manager played a pivotal role in coordinating efforts, mapping out the steps of each phase, prioritising tasks,
and, overall, being responsible for keeping deployment on track and on time. Your project manager will be equally
charged with these responsibilities and opportunities. It’s important to note that all of the DP team members have
fulltime jobs outside of the DP project.
Required Resources
To gain support and initiate change both inside and outside of the lab, we created a cross-functional team to drive our
DP deployment.
Our DP project team members:
• DP champion at the executive level (at Leeds, we chose the Medical Director)
• Clinical lead for DP
• Project manager
• Training and validation lead
• Laboratory lead
• Pathology IT lead
• Business analyst/lean engineer
• Informatics representation (network/storage/support)
• Corporate service representation (finance/HR/communications)
• Vendor lead/representation
At Leeds, we have successfully employed a combination of regularly scheduled meetings and emails, plus posters,
newsletters and presentations, to provide transparency on rollout progress across the whole department. Maintaining
good relationships through ongoing communications with DP vendors and laboratory information system service
providers play a pivotal role in positive project outcomes.
6

Workflow Analysis and Process Mapping Techniques
To create a baseline of our workflow, we started with the end-to-end mapping of our process. Our analysis showed
significant opportunity to improve overall turnaround time for diagnostic cases, as well as a reduction in the number
of touchpoints (and sources of error) along the way.
Below are our top tips for process mapping:
• Document your “current” lab workflow to create a value stream map.
• Understand and plot laboratory pressure points, bottlenecks, and waiting time.
• Create your “future” value stream map (VSM):
» This map should be your “idealised process” with as much waste removed as possible, and a focus on reducing
turnaround time by removing delays and bottlenecks.
» Your future state should include any additional steps required for slide scanning and should be created when
there is a reasonably clear understanding of the IT architecture and technical solutions.
• Develop an incremental improvement plan that will help you move to the future state.
• Using the concepts of “Lean Engineering” and a “Culture of Continuous Improvement”, the project manager will
create a detailed project plan to drive this change. This will facilitate concurrent execution of tasks in different work
areas, and the chance to identify dependencies needing sequential execution of deliverables.
Leeds VSM Comparison
7

Tips for an Effective Deployment
• Some changes may require capital investment, but application of appropriate improvement methodologies (e.g.
Lean and Six Sigma) can produce significant improvements at a low cost.
• T o ensure input on the current state and buy-in on the future state, we recommend holding a two-day meeting
with key personnel involved in each step of the workflow process. Spend time observing the current workflow,
measuring both hands-on time and waiting time.
• T rust in analytics. Remember, if it’s not measured, it can’t be improved!
Scanner Procurement and Operation
Choosing the right scanner is a key consideration when implementing DP. Attention should be given to your sample
type, size, volume and throughput requirements and, of course, the capability of the scanner to meet or exceed the
requirements.
What type of scanners do we need?
We based our scanner decision on our total volume of work including the total amount of time it would take to
produce a slide in terms of effort from both staff and machinery.
How many scanners do we need?
To procure the correct number of scanners, we calculated the total scanning time required, total scanning time
available, as well as the expected utilisation of the scanners. For the total required scan time, we multiplied our
volume of slides by the average time taken to scan a slide. It is important to remember that scanners cannot
scan without interruption; time needs to be allowed for loading and unloading slides, maintenance (planned and
unplanned), as well as for fluctuations in workflow in the rest of the lab.
Based on these factors, we estimated that our scanners would be utilised 70% of the available time, allowing us to
calculate the actual required scan time:
Total Required Scan Time = Actual Required Scan Time
% Utilisation
In our department, we decided that the scanners could run unattended overnight during the week but would not be
used during the weekends, offering us 120 operating hours per week. Other options would have been to let scanners
run only when staff were available to attend to them or to let them run 24/7.
Actual Required Scan Time = Number of scanners required
Operating Hours Required
We calculated the following as our scanner requirements:
Project phase Slides to scan per day (approx.) Scanners
Breast pilot 150 One AT2 and One CS2
Breast plus IHC 300 Two AT2 and One CS2
Full digital 1200 Six AT2 and Three CS2
To aid turnaround times, a good rule of thumb is to scan urgent work during the day and save less, time-critical
work for overnight runs. We determined that we needed two types of scanners; a high and a low capacity scanner
each for different use cases. We chose the Aperio-branded scanners from Leica Biosystems. The high capacity
Aperio AT2 (400 slides) was fed with small batches of urgent breast biopsies throughout the day, with lower priority
8

resection work collected throughout the day for an overnight run. The lower capacity Aperio CS2 (5 standard slides
or 2 large slides), shared the burden of urgent biopsies throughout the day but also proved invaluable for the
scanning of large format “mega-block” (2”x3”) slides, which our breast pathologists use to demonstrate multiple
margins and tumour dimensions.
How many staff members will we need to operate the scanners?
There were two distinct workflow steps to consider when we were calculating how many personnel hours would be
required to run the scanners: 1) the slide scanning process and 2) slide quality control.
Once again, we used the average weekly volumes and mapped the overall process. This process can vary from lab to
lab, but for us, it broadly consisted of loading and unloading the scanners, taking snapshots to ensure that all tissue
from a slide was captured and then quality- checking the scanned images, (plus any required data input). Multiplying
the total hands-on time of this process by the volume gave us a figure for the total required human resources. This
information allowed us to consider how to staff the scanners, bearing in mind operating hours, likely flow of work
throughout the day, and whether we would have a dedicated scanning team or not. Note that the required FTE (the
hours worked by one employee on a full-time basis) will be reduced as the process matures.
Our calculations were as follows:
Project phase Full time equivalent staff required to operate scanners
Breast pilot 0.4
Breast plus IHC 0.8
Breast plus IHC plus all other specialties
(i.e. full lab digitisation)
2.2
Personnel assigned to work on the scanners need to be fully trained in the tasks that they are asked to do, with
appropriate standard operating procedures, supervision and sign off processes. In this way, scanner operations should
be treated like all other lab processes.
Where should the scanners be located?
Take time to consider where you are going to locate your
scanners. One of the key tenets of the Leeds approach to
laboratory digitisation was that the scanners should be an
integral part of normal laboratory function, and as such,
should be sited in the laboratory. Unlike other technology
systems, the Aperio scanners from Leica Biosystems did
not require specialised benches or necessitate location in a
separate room due to noise or vibration, making it possible to
install them virtually anywhere in the laboratory. We looked
at all potential areas within the laboratory and ranked them
on criteria we thought were important using a traffic light
system: green for “go”, yellow/amber for “caution”, and red
for “stop” or “no go”. Option 4 (see below) was chosen as
the optimal site. And fortunately, given the relatively small
footprint of the scanners, we could accommodate them
within our existing lab space.
9
Adam Stocks, Biomedical Support Worker

Option
Centralised
Approach
Walk Around
Time
Existing
Infrastucture
Suitable
Bench/
Building Work
Supports
Existing
Lab Workflow
Scanner
Maintenance
Option 1 – Seniors Room
Option 2 – Wash Room
Option 3 – Temporary Slide Storage
Option 4 – Back Wall
Option 5 – Equipment Storage Room
Option 6 – Slide Storage Room
Option 7 – Spare Bench
Option 8 – IHC Area
Option 9 – IHC QA Area
Option 10 – Addiitional Work Area
How to Save Time if You’re Running Standard and Digital Reporting Simultaneously
If you decide to implement DP in a phased approach, you will inevitably encounter a stage where you have both
digital and glass-based processes running in parallel. Using both processes can potentially add steps and time to your
laboratory workflow. One of the keys to keeping wasted time to a minimum is to maximise your use of the scanners
during the working day.
Tips for Better Deployment
• You should not design your complete system to handle a single, high-volume day; you need to design for reliable
performance day in and day out. The best way to do this is to calculate your capacity requirements using weekly
volumes. This will allow you to balance workloads across the entire week.
• Per lean principles, scanners need to be loaded throughout the day to maximise utilisation. You may want to change
“feeding times” – the timing of how slides are produced throughout the day. Rather than producing a large batch of
slides at the end of the day, you may need to move to a staggered production of slides, or even better, an even flow
of production. To do this, you will need to make changes and improvements to your process.
• High quality images require high quality glass slides. If you have persistent issues with folding of tissue or thick
sections, these will need to be resolved or the requirement for rescans will be high.
• Treat scanning as an integral part of the slide production process. Staff need to be rostered onto it as much as any
other step in the process. If scanning is treated as an “add on”, it will most likely lead to delays in images being
produced, slowing down the whole diagnostic pathway.
• Consider whether a staggered or “big bang” roll out is most appropriate for your laboratory. In larger laboratories,
gradually scaling up the process on a specialty-by-specialty basis can lower operational risk by allowing unforeseen
issues to be ironed out, whilst volumes are low. However, these risks should be balanced against the difficulty of
running parallel processes.
10

• Engage your lab staff in the change process as they are a valuable source of innovative and constructive ideas.
• Make sure your feedback loops are working well. Listen to pathologist and lab staff input and speak to the
departments that your lab supports. Keep all stakeholders informed of changes and welcome suggestions. Do not
be reluctant to ask others to make changes; it may be that a small change in their process will help you to provide
better service.
• Stay positive! This is a big change that initially requires an investment of effort when fi rst introduced, whilst glass
slides continue to be shipped to pathologists. The demand on your resources will start to ease up once pathologists
are validated, and your lab gets used to the new way of working. Processes such as fi nding slides and cases will
become more straightforward than before, and your lab will have an easier job fi ling cases.
Engagement with Colleagues
Generating and maintaining enthusiasm and cooperation in the laboratory and the wider department is essential to
a successful DP deployment. You will be asking your colleagues to change some of the most fundamental aspects of
their working practice. It is likely that you will confront a wide range of feelings and opinions, particularly in the early
stages of your project. You will encounter those who greet the prospect of change enthusiastically, counterbalanced
by the more reticent and fi nally, those who are outright resistant. We have identifi ed strategies to help motivate your
entire team – department by department – throughout the deployment process:
A. Improving Pathologist Engagement
1. Your department is likely to contain three groups of pathologists: the enthusiasts, the uncertains and the sceptics.
Recognising and identifying the key personnel in your department, who fall into each of the categories, will help
you target and create engagement, communication, educational materials, and activities that can help shift
attitudes. During the earliest stages of deployment, circulate a survey to your pathologists regarding current
attitudes and beliefs surrounding DP. A well-designed survey will capture data on readiness to go digital and allow
pathologists to express any underlying fears or uncertainties regarding digital reporting.
2. If you are planning a phased deployment, or an initial
pilot project, it may be benefi cial to trial your DP system
on a smaller group of participants from each subspeciality.
Some of the key characteristics of the three groups are
described as follows:
• The “enthusiasts” or “cheerleaders” are the early adopters;
their enthusiasm and positivity can help motivate those who
are uncertain. Since cheerleaders can turn those sitting in the
stands into fans, their positive feedback on the technology
should be shared with the rest of the department.
• At the other end of the spectrum, your “sceptics” deserve
equal if not more attention. The sceptics will present
you and your colleagues with a long list of reasons why
your deployment will not work; some of these may be
genuine concerns that require addressing. It’s important to
view pathologist sceptics not as adversaries but as vital
contributors who can help you plan for a better deployment. Conversely, enthusiasts are appreciated and valued,
but often they are so in favour of deployment that they will overlook weak points and potential problems.
11
Enthusiasts
ScepticsUncertains

• “Uncertains” are the hardest to deal with; many will resist attempts to pull them into deployment plans. Arranging
informal visits and one-on-one discussions with enthusiastic pathologists who already work successfully with DP can
help assuage those who are apprehensive and help advance their appreciation and comfort level with the technology.
3. Have a representative of the digital deployment team attend all relevant departmental and pathologist meetings to
provide updates on the deployment planning and progress.
4. Provide email updates to the pathologists, even on the small successes, as this can lend credence and confidence
to the DP deployment (e.g. “This week, Dr. X reported 100 skin biopsies digitally and presented his/her first digital
MDT. Clinicians were impressed with the quality of the images and look forward to receiving more digital histology
updates and other examples of the technology at work.” We found that positive messages from “normal” users
were more powerful than those from the project team.
5. Log any workflow, performance, or diagnostic issues and respond to them quickly. Identify key personnel in the
department who can troubleshoot issues such as image quality, workstation set up difficulties, workflow issues,
etc. Ensure that everyone knows who to contact, the method of contact, and the best time to communicate to get
issues resolved.
B. Engaging Laboratory Personnel
1. In the early stages of deployment, seek opportunities to discuss your vision of DP to laboratory personnel and
allow staff to provide feedback, express concerns, and make suggestions for laboratory workflow improvements.
We found that this is best achieved with distribution of a brief survey, followed by targeted focus groups and/or
discussions with key individuals – the movers, shakers, and shapers of opinion in the lab.
2. DP should be presented as an integral part of the laboratory process and not an optional “add-on.” The laboratory’s
role in the diagnostic workflow is not complete until the glass slides have been scanned and sent out to the
pathologist diagnosticians. Cementing the idea that DP is going to be part of standard departmental practice
is important in encouraging staff to make the effort necessary to learn new skills and ultimately embrace the
advantages that digital reporting offers. Laboratory managers are vital here.
3. Seek every available opportunity to educate and explain the technology and plans for deployment. Take advantage
of informal lunchtime or coffee break meetings. Promote the idea of discussions as educational content and
continuing professional development activity.
4. Identify enthusiastic supporters who might be willing to share their knowledge and belief in DP with others who are
less on board. With their help, you can implement and sustain change in the laboratory.
C. Engaging with IT and Informatics
1. It is critical to engage with your IT and informatics departments and secure their involvement, at as high a level
as possible, from the earliest stages of your deployment. The buy-in and expert advice of these professionals is
fundamental to a successful DP deployment.
2. It is important that all stakeholders, whether pathologists, laboratory personnel, or members of the IT/Informatics
group, can communicate about the project in clear language. Setting up early sessions with key team members
to exchange and explain information from diagnostic, laboratory and IT perspectives should be one of your first
priorities. Try to come to a group understanding of what is expected and what is potentially achievable from your
deployment, and what each professional group will be expected to contribute in terms of time and staff.
3. Early opportunities for engagement may include laboratory office tours for your IT and informatics teams. Let
them see for themselves the pressure points of your current conventional glass slide workflows and grasp the
opportunities that digital can present. Explain ideas that you have for future digital workflows and see what
potential problems and solutions your IT colleagues can generate.
12

Validation and Training
A well developed and appropriately resourced training and validation programme for your pathologists leads to
numerous benefi ts for your DP deployment. Giving your pathologists a risk-controlled period of digital diagnosis,
when they can familiarise themselves with the digital system and with the appearance of digital slides, helps them
gain confi dence in the system and their individual ability to use it. It also provides valuable material for continuing
professional development targets, validation, and inspection cycles. We have a full sub-specialised pathology
department, and as such, we decided to validate, sequentially, by subspecialty. Insights from this approach have
shown that our overall approach to validation can be used across all histopathology subspecialties, but each has its
own specifi c nuances that need time and attention to train and validate safely.
We developed an innovative validation and training protocol for primary diagnosis of histological specimens using DP.
Our protocol is designed to make pragmatic use of available resources, maintain a strong focus on patient safety, and
champion professional engagement and education.
Unlike previously published validations, our validation protocol is an individual validation for a single pathologist
and is evidence-based where possible. The protocol enables
self-identifi cation of digital diagnostic pitfalls and allows
the pathologist to gain competence and confi dence in a risk-
mitigated environment with early exposure to live digital
reporting. It avoids the need to do a full crossover study
comparing light microscope and digital diagnoses, as the
pathologist does the digital read from the outset (and checks
it on the microscope until they are fully confi dent with digital).
The full validation protocol, and sample training materials are
available in the Royal College of Pathologists’ guideline for DP
as an example of best practice. (7)
Our protocol incorporates formalised training in use of the
digital microscope and observed practice with feedback. The
pathologist is presented with a test set of challenging and informative digital cases with immediate digital glass
reconciliation. We then scan all of a
consultant’s cases prospectively and
ask our pathologists to make their
diagnoses digitally, with immediate
glass reconciliation before sign out,
and adjustment of diagnosis,
if necessary. At the end of the process,
we produce a validation document
for each pathologist, documenting
training and concordance levels with
recommendations for scope of their
digital practice.
By providing an individual validation
for each pathologist, we allow them to decide which cases they are confi dent to diagnose digitally and which need
more practice or workfl ow modifi cations to ensure a confi dent and safe diagnosis.
13
Pragmatic
Professional
engagement and
education
Patient
Safety Focused

14
After approximately two months of whole-time-equivalent reporting on digital slides (with glass slide reconciliation
prior to sign out), the concordance rates of the pathologist are reviewed and any problematic areas discussed. At this
point, a mutual decision is made between the pathologist and their trainer on whether the pathologist is either:
a) validated for full digital practice in the specified area of pathology
b) validated for digital practice in the specified area with some exceptions (requiring glass slide checks for certain
diagnostic scenarios)
c) not currently validated for digital diagnosis in this area of pathology
Tips for Validation
• Validate on an individual rather than a departmental level; let every pathologist have the opportunity to evaluate
their use of the technology and make their personal journey to confident and safe digital reporting.
• Make your validation procedure relevant for your own department. Tailor your training to ensure that your
pathologists view specimens, stains, and diagnoses that are relevant to their regular workloads, including some
harder cases and cases that are potentially challenging on digital, to direct training.
IT Considerations and Systems Integration
For any pathology lab to fully realise the efficiencies of going digital, a number of systems need to work in concert.
This includes the laboratory information system (LIS), specimen tracking system, slide management software, as
well as the slide viewer. Where required, uni-directional or bi-directional interfaces need to be put in place to ensure
a smooth transition of data between systems. The seamless integration of these systems will enhance the digital
experience and ease the workflow for laboratory staff and pathologists. Orchestrating system harmony will also
improve engagement of personnel and, perhaps most importantly, the speed at which a diagnosis is received by
anxious patients and their families.
At Leeds, we have chosen to make the LIS the master system with all other systems (i.e. tracking, slide archive and
viewing platform) linked back to the LIS via coded interfaces or links, which launch other specific applications.
The Benefits of a Fully Functional Integration:
• Decreased need for pathologists and laboratory staff to manually enter data into various systems numerous times
• Reduced time to open several different software packages
• Better tracking of any case at any given time
• Automatic creation of audit trail for cases
• Faster notification of case availability
• Simplified workflow for pathologists
Key Facts Regarding Leeds Breast Pathology Validation (8)
• Three consultant breast pathologists viewed 694 complete breast histology cases consisting of 3500 slides.
• All standard size slides were scanned at 40x equivalent magnification, with large slides scanned at 20x.
All slides were viewed on 6MP medical grade displays.
• Complete clinical concordance between digital and glass slide readings of cases was observed in 98.8%
of cases.

It’s important to include funding for system integration when creating your business case. Also, ensure that adequate
time is built into the project plan to allow for full testing of integration interfaces. Testing will help eliminate the
discovery of costly and time-consuming issues once the system is live.
The Value of a Digital Barcode Tracking System
In laboratories where a high quantity of slides are being scanned, it is essential that slides are barcoded and a
suitable slide tracking system is in place. Barcodes enable case and slide information to be held on a slide label. This
allows slides to be scanned in any order, on any scanning instrument, and for cases to be automatically organized
and managed, without the need for human intervention. Barcodes help to improve patient safety by reducing the
possibility of sample mismatching and alerts when slides go missing – something that is much more difficult to track
with manual systems.
Additional Advantages of a Barcode System:
• Reduced need for manual data entry during scanning process
• Enhanced safety for the whole laboratory with reduced likelihood of misidentification errors and transposition
• Real-time tracking of each specimen in the process
• Automated input of demographic information and digital images into a case
• Access to valuable operational and management information that can be used to optimize process performance and
efficiency.
Our experience has reinforced the belief that having a barcoding/asset tracking system in place is a prerequisite to
going 100% digital at scale.
IT and Storage Capacity
A fundamental aspect of a successful deployment is ensuring that an adequate IT infrastructure is in place to support
networking demands and the need for large digital slide storage capacity.
Your Information Technology department will be responsible for:
• Understanding the number of slides generated at 20x and 40x, including large slides (2”x3”)
• Image compression used and file size
• Overall growth year over year
Our experience demonstrates that, on average, a slide scanned at 40x will produce between 1GB – 2GB of data,
subject to the size of tissue with a 20x image typically generating a WSI of between 500MB – 1GB. For larger 2”x3”
slides the amount of storage per slide is significantly greater and proportional to the size of the tissue sample.
The other key impact on storage volumes is the retention time of the scanned image. To ensure a complete diagnostic
record and to allow revisiting of slides in the future, we store all digital images. According to our calculations, a full
digital workflow requires 100 TB of storage per year. Fortunately, we have our own bulk storage capabilities. We
will consider archiving older cases onto cheaper storage, though this can mean a short delay when the image is
requested by the pathologist. There are two main aspects to consider when looking at your network requirements:
first, connectivity between the scanner and image server, and second, network performance for your total number of
reviewers when running at full capacity. We recommend a dedicated connection between scanner(s) and image server
since you can anticipate continuous high traffic as your WSIs are being generated.
15
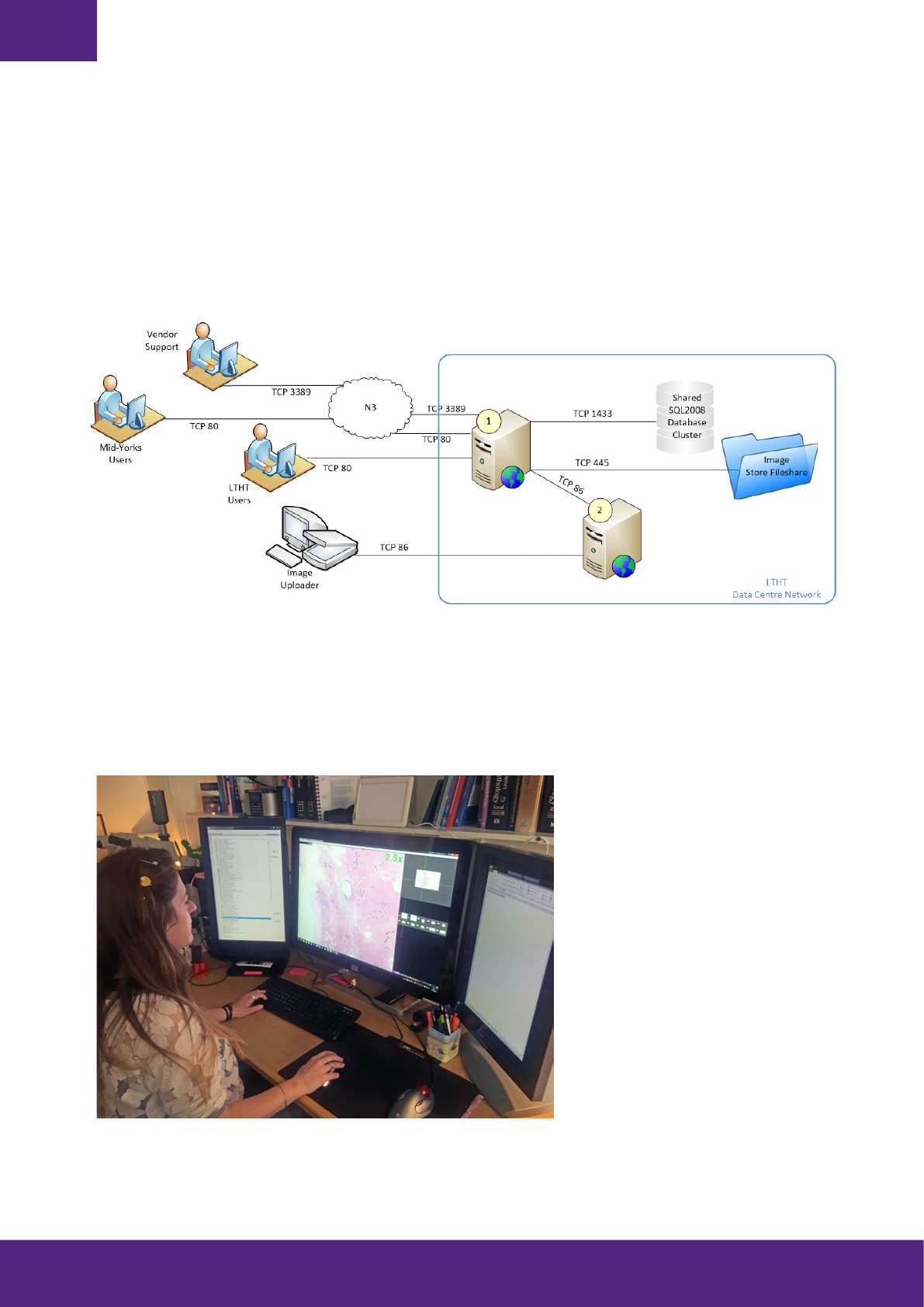
Tips for IT, Networking and Data Storage:
• Identify a local IT lead (systems administrator) and a corporate IT lead.
• Identify and allocate key IT contacts for PC Support / Networks / Infrastructure.
• Set up regular IT meetings for internal issues and utilize video conferencing facilities for regular contact with third-
party suppliers.
• Stress-test network and infrastructure capabilities and identify where enhancements are required.
• Calculate storage requirements and develop a longer-term storage strategy.
• Plan sufficient time for upgrades and system changes.
Allow adequate budget for IT requirements such as increased storage, upgraded network capabilities, as
well as procurement of displays screens, and systems integration.
The Pathologist’s Workstation
The overall feel and usability of your DP system will partly depend on the key hardware components you select for
your diagnosticians, particularly when it comes to display screens and input device selection.
Rebecca A Millican-Slater MBChB MSc FRCPath, Department of Histopathology, Leeds
Teaching Hospitals NHS Trust Leeds, UK
16

Display Screen Selection
We have chosen to use medical grade, high resolution (6-8 megapixel) display screens for primary diagnostic work.
This decision was influenced by a screen evaluation experiment in which we invited our pathologists to “road test”
an array of different screens to view a range of standard slide sets and rank them in order of preference.
Tips for Selecting Display Screens:
• It is likely that the majority of work can be accomplished successfully on any modern desktop display screen, with
a minimum resolution of 3-4 megapixels. However, there will be a minority of specific cases that will benefit from a
display screen with higher resolution, contrast ratio, and luminance.
• Medical grade display screens provide guaranteed levels of consistency over time. If your department will not
permit the purchase of high resolution display screens for all pathologists, you may want to consider purchasing
a smaller number of these display screens, which can be located in a shared space and reserved for difficult or
problematic cases.
• Determine how you will position display screens in your reporting rooms including the physical space that will be
allocated for each workstation. Larger display screens (e.g. 30 inches) allow for easy low-power views of slides.
These substantial display screens can create a more immersive visual experience; however, they can be more
expensive and require more head movement if placed too close to the user.
• Consider the effect of natural light on your display screens. Medical grade display screens tend to be less
susceptible to the effects of natural light on the display screen due to increased luminance. If you opt for display
screens with lower luminance, it is important to ensure that natural lighting can be controlled by using a black-out
blind and alternative artificial lighting.
• The variation in cost of display screens is vast (from ~£200 to £30,000) and, therefore, it is important to give display
screens due consideration. Note that as the technical specifications rise, the cost of the display screens increase.
Input Devices
One of the key advantages of DP over conventional light microscopy is the increased flexibility in terms of
your desktop environment. Digital pathologists can use a range of input devices to navigate through their slide
management and slide viewing software. Our pathologists were given scope to trial different devices before choosing
the most comfortable fit for them. All of them now use a combination of keyboard shortcuts and high-performance
gaming mice, which allow finer control of mouse movement with less effort. Some make occasional use of trackball
devices.
Careful selection of a device not only makes slide navigation easier and more efficient, it can also improve ergonomics
and help pathologists with pre-existing work-related strain injuries.
Continuing the Journey
After a DP system has been successfully deployed and integrated, the possibilities for DP applications and utility are
very great. DP offers a flexible foundation allowing institutions to pursue additional service improvement projects and
investigate novel and innovative diagnostic workflows. Two of the most talked about applications of digital slides are
remote reporting and the use of artificial intelligence for automated or partially automated diagnosis.
Remote Reporting
Many pathologists have dreamed of reporting cases from the comfort of their own home (or better still, a luxury villa
in the south of France!). Digital slides certainly put this goal within reach. Given the growing shortage of pathologists
and other factors affecting a decreasing pathologist population, the need for a more flexible and favourable pathology
reporting environment has never been greater.
17

Career benefits including convenient and comfortable hours and locations are a potential solution to local, regional,
and national specialty-reporting shortages.
If pathology reporting is liberated from temporal and locational constraints, there is the potential for 24-hour access to
diagnostic opinion.
We believe, that as experience and research in this area mounts, professional and regulatory bodies will be able
to provide more detailed guidelines to nurture best practices and ensure the ability to reap the rewards of flexible,
remote working without compromising patient safety or professional standards.
Artificial Intelligence (AI)
The use of AI to make or augment pathological diagnosis is a hot topic. While nothing can replace the all-round
experience and judgement of a trained professional pathologist, computer-aided diagnosis will help with some of
the more tedious and demanding tasks involved in pathology diagnostics. Algorithmic systems can provide accurate
and repeatable quantification metrics for immunohistochemical stains. These systems may be able to assist the
pathologists in screening large volumes of tissue for rare events e.g. metastasis/micrometastasis in lymph nodes or
detecting and counting mitotic figures for cancer grading systems.
As the experience of pathologists in making primary digital diagnosis develops, AI is likely to expand, with the
accumulating archives of digital diagnostic images providing the big data necessary for the development of more
software tailored to the needs and demands of cutting edge histological diagnosis.
Final Thoughts
Following successful departmental implementation and integration, a digital pathology system offers a flexible
foundation to allow institutions to pursue further service improvement projects, and investigate novel and innovative
diagnostic workflows. The possibilities are seemingly endless.
We believe that digital pathology will fundamentally affect the way every pathologist works for the next 10, 20, 30
years. We remain committed to helping other institutions navigate the path in their pursuit of digitization, as we pur-
sue the next generation of innovations that will make the future of digital pathology even brighter than it is today.
18
Dr Darren Treanor and Jerome Clavel, Head of Digital Pathology for Leica Biosystems, view a digital image
at the Leeds Powerwall.

Further Resources
1. Goacher E, Randell R, Williams BJ,Treanor D (2017) The Diagnostic Concordance of Whole Slide Imaging and Light
Microscopy: A Systematic Review. Archives of Pathology & Laboratory Medicine: January 2017, Vol. 141, No. 1,
p. 151-161.
2. Williams BJ, DaCosta P, Goacher E, Treanor D (2017) A Systematic Analysis of Discordant Diagnoses in Digital
Pathology Compared With Light Microscopy. Archives of Pathology & Laboratory Medicine: December 2017,
Vol. 141, No. 12, pp. 1712-1718.
3. Cancer Research UK. Testing times to come? An evaluation of pathology capacity across the UK. 2016
https://www.cancerresearchuk.org/sites/default/files/testing_times_to_come_nov_16_cruk.pdf
4. Williams BJ, Bottoms D, Treanor D (2017) Future-proofing pathology: the case for clinical adoption of digital
pathology Journal of Clinical Pathology;70:1010-1018.
5. Ahlers HJ, Stratman C, et al. Can digital pathology result in cost savings? A financial projection for digital pathology
implementation of a large integrated health care organization. J Pathol Inform. 2014; 5:33
6. Williams BJ, Bottoms D, Clark D, et al Future-proofing pathology part 2: building a business case for digital
pathology Journal of Clinical Pathology Published Online First: 16 March 2018. doi: 10.1136/ jclinpath-2017-204926
7. Royal College of Pathologists. Best practice recommendations for digital pathology. 2018. https://www.rcpath.org/
resourceLibrary/best-practicerecommendations- for-implementing-digital-pathology-pdf. html
8. Williams BJ, Hanby A, Millican-Slater R, Nijhawan A, Verghese E & Treanor D (2018) Digital pathology for the
primary diagnosis of breast histopathological specimens: an innovative validation and concordance study on digital
pathology validation and training Histopathology 72,662–671.
19
Dr Darren Treanor hosting an educational workshop on DP at Leeds

Authors:
Dr Darren Treanor [email protected]
Dr Bethany Williams Bethany[email protected]
With contributions from:
Basharat Hussain
Dharshana Jayewardene
Dr Alex Wright
Chloe Lockwood
Dr Emily Clarke
For further information, please contact the authors.
The Leeds Teaching Hospitals NHS Trust and the University of Leeds have a collaborative partnership with
Leica Biosystems for Digital Pathology research driven deployment.
The clinical use claims described for the Leica Biosystems Aperio products in the information supplied have
not been cleared or approved by the U.S. FDA or are not available in the United States.
18778 Rev A ∙ 07/2018
