
Terminology
Boston Scientific Electromagnetic (EMI) Compatibility Table
for Pacemakers, Transvenous ICDs, S-ICDs and Heart Failure Devices
TERMS OF USE: The information provided on the Electromagnetic (EMI) Guide should not be considered the exclusive or only source for this information. The table lists a general category of items only
and is not intended to be an exhaustive list. The recommendations and precautions may be based on information provided by the manufacturers of the items in question, and specific items within a
category may function differently. It is best practice to consult the original manufacturer of the item with potential EMI to verify any specific guidance concerning operation and compatibility with
implantable devices. If at any time there is a question about the function and potential for Electromagnetic Compatibility, contact the manufacturer of the item in question for further information. At all
times, it is the responsibility of the licensed healthcare professional to exercise medical clinical judgment in a particular circumstance.
The information provided is not intended to be used for medical diagnosis or treatment or as a substitute for professional medical advice. The recommendations and precautions contained in this
document apply to device function of Boston Scientific Cardiac Rhythm implantable devices. Specifically device susceptibility to electromagnetic interference.
Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition.
Safe under normal use: These items are only considered safe from electromagnetic interference with your device when used normally in accordance with
their intended use. Check with your doctor for any additional restrictions that you may have for these items.
Use precautions: When you are near any of these items, you should use precautions. Check with your doctor for detailed information before using these
items. Any medical equipment, treatment, therapy, or diagnostic test that introduces electrical current into the body may have the potential to interfere
with implanted cardiac device therapy.
Do not use: Talk with your doctor.
Recommendations and precautions for transvenous devices also apply to S-ICD devices.
If online, follow these instructions to search for an item:
While holding down the Ctrl key, click the letter F. The Find screen should appear.
Type in the name of the item for which you wish to search. Then click Find Next or the Enter key.
1

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Activity Tracker
Use precautions: As this wireless technology usually uses Bluetooth, maintain at least
a 6 inch (15 cm) separation between the activity tracker and implanted device due to
Bluetooth technology. This is a device or application that may track steps taken,
distance walked or run, calories consumed, and in some cases heartbeat and quality
of sleep. The tracker may be synchronized, in many cases wirelessly, to a computer or
smartphone. In some activity trackers, heart rate is measured 2 ways: 1) By LED lights
which reflect onto the skin to detect blood volume changes or 2) by using a chest
strap which measures and sends the heart rate to a wrist watch.
FITBIT: Flex, Force,
NIKE: Fuel,
GARMIN: Vivofit,
MISFIT: Shine, Flash, Link, Bolt, Beddit
BODY BUGG: Bodybugg Version 3,
BASIS: Peak
Click here for Bluetooth Technology
Appliances - kitchen
countertop
Safe under normal use. Your implanted heart rhythm device is intended to work
properly around most appliances and equipment. Most standard items you use at
home in the kitchen are not likely to cause a problem with your implanted device.
Electric can opener, toaster, coffee
maker, food processor, blender
Biaxial Magnet
Do not use. Battery-powered devices produced by Nikken that utilize a rapidly
rotating magnet to create a very strong 3D magnetic field. The website claims 1700
Gauss for field strength.
Bingo Game Magnetic Wand
Use precautions: Maintain at least a 6 inch (15 cm) separation between the wand and
the implanted device. Some bingo wands may contain a permanent magnet.
Casino Slot Machine
Use precautions: Maintain at least a 12 inch (30 cm) separation between the slot
machine and the implanted device.
Electric Golf Cart
Use precautions: Maintain at least a 6 inch (15 cm) separation between the battery
and the implanted device, and at least a 12 inch (30 cm) separation between the AC
battery chargers and the device.
Electric Guitar
Use precautions: Maintain at least a 6 inch (15 cm) separation between the guitar and
the implanted device. Magnetic/electrical fields associated with the guitar are very
low and will not affect the Pacemaker or ICD.
Electric Toy Train
Use precautions: Maintain at least a 6 inch (15 cm) separation between the
transformer and the implanted device. Do not touch power rail, especially with wet
hands.
Hobbies
2

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Hydroelectric Dam Tour
Do not attend tour: The policy of the Hoover Dam does not recommend that
individuals with an ICD go on tour within the dam because of the 50 /60 Hz magnetic
field present. Other Hydroelectric dams may have policies for ICD individuals that
differ from those of the Hoover Dam. Because of the uncertainty of the magnetic
environment within other Hydroelectric plants, we cannot predict the intensity of the
magnetic field within any specific dam. There is the potential for Pacemaker or ICD
interaction. Consult physician for level of risk that interaction with the device may
present. Tours of non hydroelectric dams would pose a low risk of affecting the
Pacemaker or ICD. Visiting outside any hydroelectric dam reduces the risk of EMI
interaction.
Hoover Dam
Kiln: (Pottery, Jewelry or
Glass) /
AC resistive heating element
Use precautions: Maintain 12 inch (30 cm) separation between the kiln and implanted
device. Most of these kilns use resistive-type heaters similar to heating elements
associated with common electric stoves. Wood or gas fired kilns will have no affect on
the Pacemaker or ICD. ICD. The magnetic field associated with the operation of this
type of kiln is minimal. In contrast, kilns used in association with inductive heating of
metals produce magnetic fields that can extend a much greater distance from the kiln.
Also see Induction Heater/Kiln by clicking the link in the right column.
Click here to see Induction Heater/Kiln
Laser Tag
Use precautions: Maintain at least a 6 inch (15 cm) separation between any magnet in
the vest and the implanted device. Low risk of laser tag gun and associated detection
circuit in the vest affecting the Pacemaker or ICD. The device uses only light energy;
however, some vests may contain magnets and/or a radio frequency transmitter that
communicates to a scoreboard.
Metal Detector
Use precautions: Keep the metal detector device head pointed away and at least a 24
inch (60 cm) distance from the implanted device.
Beachcomber
Rifle/Shot Gun
Use precautions: Consult with heart doctor regarding physical impact due to firearm
discharge. Recommend using firearm on opposite shoulder of implant to avoid
possible damage to your implanted device.
Roller Coaster -
Electromagnetic
Use precautions: consult your heart doctor. Avoid riding on or performing
maintenance on this type of rollercoaster. Electromagnetic Roller Coasters are the
type that go AGAINST gravity and can be found at amusement parks, fairs, etc. This is
a sub-group of roller coasters which use electromagnets to propel them (linear
induction motors).
Amusement park rides, fair rides
Roller Coaster - Traditional
Gravity
Safe under normal use. Amusement park rides, fair rides
Hobbies
3

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Scuba Diving
Use precautions: Consult with heart doctor. Prior to SCUBA diving the patient’s heart
doctor should be consulted to assess the potential consequences relative to the
patient’s specific health condition. It is possible that your physician may suggest that
you limit certain activities, such as scuba diving, to a level that is more restrictive
based on medical concerns rather than the single factor of pressure tolerance of your
pacemaker/ICD. Because of many factors associated with scuba diving, specific depth
limitations cannot be provided. Some of the factors include the possibilities of blows
to the area of the device during the time the device is under pressure stress, the
number of pressure cycles the device is exposed to over the implant time of the
device, and the activity or exertional level of the individual during the dives. Our
device pressure testing is conducted for compatibility with hyperbaric chamber
therapy. We can share with you that a pressure of two and one-half atmospheres
absolute is the maximum pressure recommended for hyperbaric chamber therapy.
Elevated Pressure (HBOT/SCUBA) and
Implanted Medical Devices
Static Electricity
Plasma Ball (Van de
Graaff generator)
Use precautions: Maintain at least a 6 inch (15 cm) separation between the ball and
the implanted device. Do not touch the ball.
Tattoo Machine
Use precautions: Maintain at least a 6 inch (15 cm) separation between the tattoo
machine /related tattoo accessories and the implanted device. Avoid tattoo over
implant location.
Train: Magnetic Levitation /
high speed train
Safe under normal use for passengers.
Hobbies
4

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Ab-Stimulator®
Do not use: Muscle Stimulator is fitness-related equipment designed to stimulate
muscles to improve muscle definition and fitness. Provides electrodes that are placed
on muscles, such as abdominal muscles, and delivers electrical stimulus, usually
strong enough to cause the muscle to twitch.
Abtronic, Ab Tronic, Ab Sonic
Air Filter (standalone) -
Ionized / Air Purifier
Use precautions: Maintain at least a 6 inch (15 cm) separation between the air filter
and implanted device. An air purifier is a device which removes contaminants from
the air.
HEPA
Air Purifier, Personal
Safe under normal use: Do not wear this device during a programmer check as it may
interrupt communication between the programmer and implanted device. This is a
personal air filter worn around the neck.
Fresh Air Buddy
Alternator / Running Motor
Use precautions: Maintain at least a 24 inch (60 cm) separation between running
motor / alternator and implanted device. Avoid leaning over motors and alternators
of a running vehicle. Safe to drive or ride in a vehicle. An alternator is an
electromechanical device that converts mechanical energy to electrical energy in the
form of alternating current. Alternators create magnetic fields which could
potentially interact with ICD's and pacemakers.
Car, Tractor, Truck, Vehicle Motor
Badge (name tag)
with magnetic clasp
Use precautions: Maintain at least a 6 inch (15 cm) separation between the magnet
associated with the badge and the implanted device.
Badge Reader (Security)
Use precautions: Maintain at least a 6 inch (15 cm) separation from wall unit (reader)
and the implanted device. Radio Frequency Identification (RFID) is a wireless
technology used to identify RFID tags mounted on objects or carried by (or imbedded
in) people or animals. Data stored in the RFID tag is read by wireless devices, called
RFID readers. The RFID reader contains one or more RF antennas that emit RF signals
within a specified range. When an RFID tag enters the reader's RF signal field,
information stored in the tag is captured by the reader. Each RFID tag relies on the
reader to transmit information contained in the tag.
RFID readers may be a potential source of EMI and could have temporary effects on
implanted cardiac devices. Because the presence of RFID systems may not always be
apparent in public and occupational settings, patients who feel symptomatic (e.g.,
light-headed, fast heart rate) should move away from nearby electrical equipment (or
the identifiable RFID system), and call their physician to report the episode.
Radiofrequency Identification and
Implantable Pacemakers and
Defibrillators
Personal Use
5

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Blood Pressure Monitor
(Upper Arm)
Safe under normal use. Automatic, semiautomatic or manual means of monitoring
blood pressure, typically with a digital display. This can include both units for home
use and those larger units found at pharmacies.
Blood Pressure Monitor
(Wrist)
Use precautions: Maintain at least a 6 inch (15 cm) separation between the wrist
monitor and the implanted device. Wrist Blood Pressure Monitors are ambulatory BP
Monitors which are worn on the left wrist. Some of these Wrist BP monitors require
the wearer to cross their wrists over their upper sternum. Maintain a 6 inch
separation between the wrist monitor and the implanted device.
Body Hydration Scale Do not use. Scale measures hydration ("body water") using bio-electrical impedance.
Conair, Bio Electrical Impedance,
BioElectrical Impedance, Body Fat
Weight Scale
Car - Electric
Use precautions: Do not sit in the car while car is charging from charging tower.
Professionals servicing the car should maintain at least 24 inch (60 cm) separation
between running motor and implanted device.
Nissan Leaf
Car - Hybrid
Safe under normal use: Professionals servicing the car should maintain at least 24
inches (60 cm) between running motor and implanted device. In case of Service
Personnel, please refer to Running Motors/Alternator for recommendations.
Hybrid car, electrical vehicle,
smart car
Clothes Washer and Dryer
Safe under normal use. Your implanted heart rhythm device is intended to work
properly around most appliances and equipment. Most things you use at home or
work will not cause a problem.
Convection Oven Safe under normal use.
Copy Machine Safe under normal use. Copier, Xerox, Photocopier
Personal Use
6

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Crocs Footwear
Safe under normal use. Rubber shoes that have generated controversy surrounding
their ability to generate a static electricity charge.
E-Book Reader
Use precautions: Maintain at least a 6 inch (15 cm) separation between the reader
and the implanted device. An electronic device that is designed primarily for the
purpose of reading digital books and periodicals.
Kindle, Nook
Electric Blanket Safe under normal use: Do not place transformer over implanted device.
Electric Fence Safe under normal use: Consult your heart doctor if you receive a shock.
Electric grocery cart
or personal scooter
Use precautions: Maintain at least a 6 inch (15 cm) separation between the battery
and the implanted device or charger and implanted device.
Electric outlet shock or shock
from any
60 Hertz source (momentary
shock or memorable
momentary shock)
Recommendation: If a shock is received from an external source, Boston Scientific
recommends that you consult your heart doctor.
Electric Toothbrush
Use precautions: Maintain at least a 6 inch (15 cm) separation between the charging
base and the implanted device as radio frequency fields may be present. Refrain from
leaning over charger.
Sonicare Toothcare
Exercise equipment (magnetic
resistance)
Use precautions: Maintain at least a 6 inch (15 cm) separation between the magnets
and implanted device.
Exercise bike, Elliptical
Hair Dryer - Hand
held or Salon
Use precautions: Maintain at least a 6 inch (15 cm) separation between the hair dryer
and the implanted device.
Personal Use
7

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Headphones (wired)
Use precautions: Maintain 6 inches (15 cm) distance between the headphones/ear buds and
the implanted device. Avoid draping wires over implanted devices.
Ear buds, Sony, Bose, Beats
Portable Multimedia Players and
Implantable Pacemakers and
Defibrillators
Heart Rate Monitor
(monitors that use a chest
band)
Use precautions: Maintain 6 inches (15 cm) distance between chest strap or wrist
monitor. Suggest rotating chest band to opposite side, away from device implant site.
Polar, Omron, Timex, Suunto, Nordic
Track, New Balance
Polar Heart Rate Monitors and Implanted
Medical Devices
Heating Pad Safe under normal use.
Hill ROM Hospital Bed
Safe under normal use. The Hill-Rom hospital beds contain magnets in the bottom of
the mattress to help hold the mattress in place. Magnets located in mattress or
motor of bed should not be in direct contact with the surface of the device.
Home Security
Systems - Infrared &
Ultrasonic
Safe under normal use.
Home Security
Systems - Microwave
Use precautions: Maintain 6 inch (15 cm) distance from transmitter. Microwaves
emit low energy electromagnetic impulses.
Microguard
Hot Tub Safe under normal use. Whirlpool baths
Ignition Systems -- see Tools
Click here for Ignition Systems
Induction Heater
(Furnace/Kiln)
Use precautions: Magnetic field intensity should be measured by a worksite survey
professional. Contact your doctor to review specific concerns.
Induction Stove Top
(AC Magnetic Field)
Use precautions: Maintain at least a 12 inch (30 cm) separation between stove top
and device. This stove differs from the more common electric and gas stoves. With
this type of stove, a magnetic field heats the metal pots directly and only when they
are placed on the stove top. The stove top remains cool to the touch. The metal in the
bottom of the pan interacts with the magnetic field causing heating of the metal. Low
risk if not leaning over stove.
Induction Stove, Induction Oven,
Induction Cooktop
Ionized Bracelet Safe under normal use. Qray, Balance
Kitchen appliances
(countertop)
Safe under normal use. Your implanted heart rhythm device is intended to work
properly around most appliances and equipment. Most things you use at home or
work will not cause a problem.
Electric can opener, toaster, coffee
maker, food processor, blender
Personal Use
8

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Magnetic Back Brace
Use precautions: Maintain at least a 6 inch (15 cm) separation between magnetic
brace and implanted device. Magnetic belt worn on lower back.
Massager - hand held
Use precautions: Maintain at least a 6 inch (15 cm) separation between the massager
and the implanted device. Do not place directly over implanted device. Small motors
within hand massager may produce magnetic fields.
Massager chair Safe under normal use.
HoMedics, Shiatsu, Rolling, Vibration,
Percussion, Back Massage Cushions,
Backrests, Body Mats, Foot Massagers
(with no electrical stimulation), Neck
and "Spot" Massagers
Medical Alert
Necklace (and other patient
alert devices)
Use precautions: If necklace or other wearable alert product has cell phone function,
affix pendant on side opposite implanted device. Maintain at least a 6 inch (15 cm)
separation between the medical alert necklace and the implanted device.
Lifeline, Life Line, Medic Guard, First
Alert, Life Alert, Guardian Alert 911,
5Star, GreatCall.
Microwave Oven
(both residential and
commercial)
Safe under normal use.
Motorcycle
Use precautions: Maintain at least a 12 inch (30 cm) separation between the ignition
system and the implanted device.
Motorcycle Vest
(Electrically Heated)
Safe under normal use. DC current used to heat the vest. Gen X, Gerbing
Oven - Electric and Gas Safe under normal use. Range, Cook Top, Stove
Pest Control -
Ultrasonic
Safe under normal use. Ultrasonic pest control unit emits sound energy.
Personal Use
9

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Sensewear Activity Monitor
Do not use. Sensewear can interfere with LATITUDE communicator, blood pressure
monitor, and weight scale operation. May also interfere with programmer used in
clinic.
Sewing Machines / Sewing
Sergers
Use precautions: Maintain at least a 6 inch (15 cm) separation between the motor of
the sewing machine or sewing serger and the implanted device.
Shaver with electrical
cord or battery operated
Use precautions: Maintain at least a 6 inch (15 cm) separation between the shaver
and the implanted device. Avoid draping cord over implanted device.
electric razor
Soloflex Vibration Training
Safe under normal use. Low amplitude mechanical vibrations pulsing through your
body 28 to 60 times a second do the work to improve circulation, strength, flexibility
and balance.
Whole Body Vibration, WBV Platform,
Weight Bench
Space Heater - Portable Safe under normal use.
Speakers
Use precautions: Maintain at least a 12 inch (30 cm) distance between the speaker
and the implanted device. Large stereo speakers often have large magnets.
TV (Television) Safe under normal use.
TV Audio Headset
(Radio frequency
receiver)
Use precautions: Maintain at least a 6 inch (15 cm) separation between the
transmitter (component usually on or near TV) and the implanted device. Radio
frequency system consists of transmitter and headset. Headset will not affect
Pacemaker or ICD. Do not drape cord over implanted device.
Portable Multimedia Players and
Implantable Pacemakers and
Defibrillators
TV Remote - Infrared
(standard)
Safe under normal use.
Remote Controls (TV, Garage Door,
Stereo, Camera/Video Equipment)
Tanning Bed Safe under normal use. Consult your doctor.
Tanning - Magna
Tanning
Safe under normal use. The electrostatic Magna-Tanning booth utilizes a high DC
voltage potential at the tanning booth spray nozzles. The high DC voltage potential
associated with these nozzles impart small electrical charges on the droplets of the
tanning spray mist. The booth is designed in such a way that the person standing in
the booth attracts the tanning mist droplets on the skin. This procedure does
introduce a very small direct current into the body. The level of this very small current
is well below the levels of susceptibility of both the Pacemaker and ICD. (Procedure
takes less than one minute to complete)
Personal Use
10

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Transformer Box -
See Telecommunications
Click here for Transformer
Vacuum cleaner Safe under normal use.
AM/FM Radio Safe under normal use.
Airport Security
Safe under normal use. Tell security personnel you have a device and show medical
device ID card.
Metal detector: Low risk to walk through archway metal detector. If the archway
detects metal in the device, request a hand search.
Hand-held wand: If the hand-held metal detector wand is to be used, request that
the wand be passed quickly over the device.
Full body scanners (x-ray backscatter or millimeter wave): The Transportation
Security Administration (TSA) currently uses these two types of full-body “people
scanners”. Neither type of scanner should affect your implanted pacemaker or
defibrillator system.
Airport Security Systems
Full Body Scanner
Advanced Imaging Technology
security archway
Jail/Prison Security system
Court Security system
Department Store Security
Systems
Metal Detector
Information for the Traveling Pacemaker
or Defibrillator Patient
Bluetooth
Technology
Use precautions: Maintain at least a 6 inch (15 cm) distance between the
transmitter/receiver and the implanted device. Wireless communication technology
for TV's, radios, computers, and other electronic devices. Radio frequency waves can
communicate/control remote electronic devices. Do not place next to LATITUDE
communicator, blood pressure monitor, or weight scale.
Bluetooth headphones, router, Google
Home, Amazon Echo
CD/DVD Players Safe under normal use.
Portable Multimedia Players and
Implantable Pacemakers and
Defibrillators
Telecommunications
Personal Use
11

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Cable Locater
Use precautions: Maintain at least an 18 inch (50 cm) separation between the
implanted device and the buried cable. This system is used to locate buried cable. The
system consists of two separate pieces of equipment: the transmitter and the
receiver. The transmitter is connected to the buried cable at an access point and
injects a test signal onto the buried cable. The magnetic field generated by this test
signal can be detected by the receiver. As the operator passes the receiver over the
search area, the receiver will indicate when that buried cable has been located.
Various frequencies and power levels can be used to differentiate between different
cables and locations.
Dynatel 2273 Advanced Cable Locater
(3M)
Cell Phone / PDA / Broadband
connectivity
Use precautions: Keep at least a 6 inch (15 cm) separation between phone and
implanted device. Keep at least a 12 inch (30 cm) separation between cell phone and
implanted device if phone/PDA transmits more than 3 watts. Hold phone to ear on
the opposite side of the body from device. Do not carry phone in breast pocket or on
belt if within 6 inches (15 cm) of device. See also PDA.
iPhone, Blackberry, PalmPilot, Android
Cellular Phones and Implantable Devices
Clearwire Modem-
Wireless Broadband /Cell
Phone
Connected Internet
Link
Use precautions: Maintain at least a 6 inch (15 cm) separation from the antenna on
the modem. Clearwire uses a wireless modem that can be plugged into a desktop
computer, laptop, or local network. It works by transmitting signals to and from
nearby cellular towers instead of using a traditional phone line.
Commercial Cellular Tower
Antenna
Safe under normal use for public use. Professionals servicing cellular towers should
contact your doctor for further information.
Commercial
Broadcasting Towers-
Radio and TV
Safe under normal use. Professionals servicing cellular or television towers should
contact Boston Scientific Patient Services for further information.
Digital Television Transmission Tower,
TV
Computer Equipment -
Wireless (with radio-
frequency link)
Use precautions: For modem or wireless card operation within the 2.4-2.5 GHz and 5
GHz bands maintain at least a 6 inch (15 cm) separation between the modem/wireless
card and the implanted device.
Personal computers
Computer - Tablet
Use precautions: Maintain at least a 6 inch (15cm) distance between the iPad, its
cover, headphones and implanted device. The iPad is a line of tablet computers
designed and marketed by Apple Inc. Avoid propping iPad on patient's chest.
iPad, tablet, kindle, nook, etc.
Portable Multimedia Players and
Implantable Pacemakers and
Defibrillators
Telecommunications
12

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Cordless Microphone
Use precautions: Maintain at least a 6 inch (15 cm) separation between the
microphone antenna and the implanted device. Most cordless microphones operate
at very low power levels. These guidelines are more dependent on the power output
of the microphone than the specific radio frequency associated with the microphone.
Microphone, Lavalier, Wireless
Microphone, Portable Microphone
Cordless Phone (portable)
and
associated base
station
Use precautions: Maintain at least a 6 inch (15 cm) separation between the antenna
on the cordless phone and the implanted device. In addition, maintain at least a 6 inch
(15 cm) separation between the base station of the cordless phone and the implanted
device. Do not place cordless phone directly over implanted device. A cordless phone
used within the house or yard are low power (usually less than 100 milliwatts).
Clarity Amplified Cordless Phone,
DECT
Dog Shock Collar
(with a central radio
frequency transmitter
in home and wire buried in
the ground)
Use precautions: Maintain at least a 12 inch (30 cm) separation between the radio
frequency transmitting antenna (usually inside the house) and the implanted device.
Do not pet the dog in the areas where the shock collar will be activated.
Invisible Fence
Fax machine
Safe under normal use. Faxing is the telephonic transmission of scanned printed
material (both text and images), normally to a telephone number connected to a
printer.
Facsimile machine
Global Positioning
System (GPS)
Satellite Navigation
System
Safe under normal use. Device only receives, there is no transmitter. If the GPS is
embedded in another device (for example cell phones, radio), refer to the guidelines
for that device.
Garmin, Tom Tom, Magellan, OnStar
Telecommunications
13

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
GPS - Survey
Equipment (Professional use)
Use precautions: For standard GPS survey equipment, there is a low risk of affecting
Pacemaker or ICD.
GPS - Survey
Equipment (Professional use
with repeater transmitter)
Use precautions: For GPS survey equipment used with repeater transmitter (output
power of 25 watts or less), maintain at least a 24 inch (60 cm) separation between the
antenna and the implanted device.
House Arrest Anklet or
Bracelet
Use precautions: Maintain at least a 6 inch (15 cm) separation between the device
and anklet or bracelet.
Internet connection -
In- Building BPL -
Broadband over
Power line (BPL) or
Internet Connection
over Power line
Carrier (PLC) or
Access BPL - Broadband
Use precautions: Maintain at least a 6 inch (15 cm) separation between the internal
wiring associated with the power distribution system and the implanted device.
In-Building BPL (LAN) utilizes electrical power wiring to transmit radio frequency
signals to network computers within a building.
Access BPL (Broadband) uses electrical power distribution lines to extend a
connection to the Internet. The power distribution lines are used to transmit
radiofrequency signals to local internet connection points within the neighborhood.
Infrared Scanner (barcode
scanner)
Safe under normal use. A bar code reader uses visible light to scan an item. This
equipment poses a low risk of affecting Pacemaker or ICD (used in grocery and
department stores). RFID is not used in bar code readers.
Lie Detector Test -
See Medical
Click here for Lie Detector Test
Metal Detectors or
Magnetometers (in jails,
courtrooms, some schools)
Use precautions: Tell security personnel you have a device and show medical device
ID card. Low risk to walk through archway metal detector. If the archway detects
metal in the device, request a hand search. If hand held metal detector wand is to be
used, request that the wand not be placed directly over the device.
Airport Security Systems; Full Body
Scanner; Advanced Imaging
Technology
Information for the Traveling Pacemaker
or Defibrillator Patient
Telecommunications
14

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Nintendo Wii and
Wireless Remote
Controls
Safe under normal use: Do not place control directly over implanted device. The
Nintendo Wii uses wireless technology with various handheld controllers. The manual
for Nintendo Wii recommends keeping these controllers 9" away from an implanted
heart device.
OnStar® technology
Use precautions: Maintain at least a 6 inch (15 cm) separation between the antenna
and the implanted device. Device found in cars for navigation. Works with cell phone
& GPS Technology. Antenna usually on roof, transmitter in glove box.
PDA (Personal Digital
Assistant)
Use precautions: Maintain at least a 6 inch (15 cm) distance between the PDA and the
implanted device. These are handheld personal computers. PDAs with wireless links
use frequencies and modulations that are very similar to digital cellular phones. In
addition, the power transmitted by the PDA is no greater than that used by cellular
phones.
Palm Pilot, Toshiba Pocket PC, and
Sony Hand Helds
Pagers - Receiver
Only
Safe under normal use.
Pagers - 2 Way
with receiver
and transmitter
Use precautions: Maintain at least the minimum separation suggested.
Phone Headset
(cordless)
Use precautions: Maintain at least a 6 inch (15 cm) separation between the antenna
and the implanted device. Do not place directly over implanted device.
Telecommunications
15

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Portable Multimedia Player
Use precautions: maintain at least 6 inches (15 cm) between the
player/headphones/earbuds/cellular phone and the implanted device. Some of these
players and their accessories contain magnets.
ipod, i pod, MP3 player
Portable Multimedia Players and
Implantable Pacemakers and
Defibrillators
Power Lines -
high voltage
Safe under normal use for residential exposure (general public). Low risk of affecting
Pacemaker or ICD when walking, driving underneath, or living in a house or building
near high voltage power lines. See precautions for occupational exposure.
Use Precautions: Professionals servicing high voltage power lines (occupational
exposure) may be susceptible to interaction with their implanted device. For this work
environment, contact your doctor to review specific concerns.
Radar
Use precautions: Maintain a 12 inch (30 CM) separation from implanted device.
This recommendation does not include weather, avionics or military application of
RADAR.
A system that uses reflected radio waves to determine the presence, location, and
speed of distant objects. The system has law-enforcement and navigational
applications.
Radar Detector, Radar Locating
System, Sensor, Position Finder
Radar - small boats
Use precautions: For radar transmitting from 1 to 4 kilowatts (kW) effective radiated
power (ERP) maintain an overhead (vertical) distance of 3 feet (1 meter). Inside radar
console display is unlikely to affect the Pacemaker or ICD.
Radar - commercial
or cruise ships
Safe under normal use: For passengers on commercial or cruise ships, radar antennas
are mounted in a configuration such that they are unlikely to affect Pacemaker or ICD
function; for example, when an individual is on a normally accessible deck or bridge
area. In other areas, there may be the potential for Pacemaker or ICD interaction.
Telecommunications
16
Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
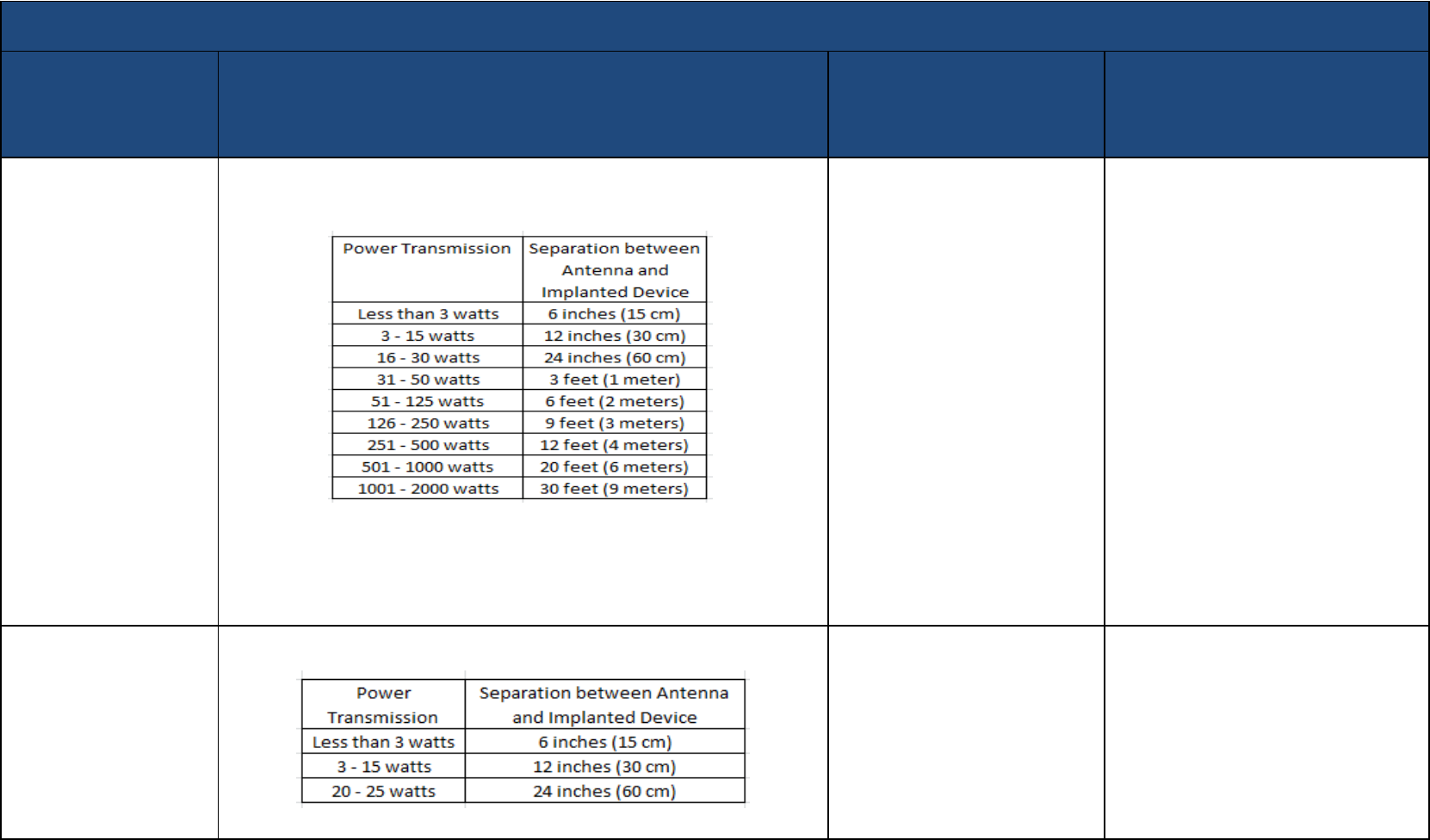
Radio - Amateur or Ham
Radio Bands
Use precautions: The following minimum distances, measured between the antenna
and the implanted device, and associated power transmission levels are
recommended for a low risk of interaction with an implanted device.
If closer than the minimum recommended distances, for continuous transmissions,
there is a potential for device interaction. CW (continuous wave) transmissions
(Morse code) may also have the potential for device interaction.
Radio - Marine
(Very High Frequency / VHF
and Single Side Band / SSB
and UHF)
Use precautions: see recommendations below.
Telecommunications
17

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Radio - Military
Use precautions: Maintain 24 inch (60cm) separation from transmitter antenna and
implanted device. Military radio operating between 30MHz and 512MHz. Two power
settings are used; 10W for AM operation and 20W for FM operation.
Radio - Two Way Portable
Radio
Use precautions: The following minimum distances, measured between the antenna
and the implanted device, and associated power transmission levels are
recommended for a low risk of interaction with an implanted device.
CB and police radios: maintain 24 inch distance from implanted device.
NOTE: Recommend 12 inch separation between dash-mounted transmission / control
box and implanted device.
hand-held, walkie-talkie, police, CB,
Law Enforcement,
Fire, and Emergency
Vehicle radios
Telecommunications
18

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Radiofrequency
Identification, and Check out
Counter Tag Deactivators
Use precautions: Maintain a 24 inch (60 cm) separation between source of RFID
(reader and RFID receiver chip) and the implanted device. Refer to A Closer Look
article. Radio Frequency Identification (RFID) is a wireless technology used to identify
RFID tags mounted on objects or carried by (or imbedded in) people or animals. Data
stored in the RFID tag is read by wireless devices, called RFID readers. The RFID
reader contains one or more RF antennas that emit RF signals within a specified
range. When an RFID tag enters the reader's RF signal field, information stored in the
tag is captured by the reader. Each RFID tag relies on the reader to transmit
information contained in the tag.
security, theft, tag
Radiofrequency Identification and
Implantable Pacemakers and
Defibrillators
Remote Control RF
Transmitter
Use precautions: Maintain at least a 12 inch (30 cm) separation between control unit
and implanted device. For 2.4 gHz frequency: 6 inch (15 cm) separation. Hand-held
control unit for remote control boats and airplanes.
Remote to unlock car Safe under normal use: Do not place directly over implanted device.
Satellite Dish (residential) -
receiving only
Safe under normal use: This includes the receiving Unit which usually sits next to the
TV. Includes dish antenna located on rooftop.
Direct TV, Hughesnet, Dish TV
Satellite Dish (residential) -
transmitting/receiving
Use precautions: Recommend that the antenna be mounted in such a way that at
least a 24 inch (60 cm) overhead vertical distance be maintained between the satellite
dish and the individual with a Pacemaker or ICD. Avoid direct exposure to the main
energy beam.
Telecommunications
19

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Smart Key System
Safe under normal use. Boston Scientific testing suggests that the smart key system
remote unit and/or smart key system antennas should not interfere with Boston
Scientific CRM implanted pacemakers or defibrillators.
Note: Patients should consult with their device following physician to discuss any
concerns they might have regarding the potential for interference.
Smart Key systems operate using two-way communication between its remote unit
and special antennas located throughout the vehicle. The remote unit transmits
signals to the antennas when the motorist pushes certain buttons on the remote unit.
Automobile "Smart Key" Systems and
Implantable Pacemakers and
Defibrillators
Solar Flares
No restrictions. Solar Flares can cause interruption in radio and cellular service.
However, the disruptions are far smaller than the sensing capabilities in the CRM
devices.
Theft Detector
pedestals (located at
store exits)
Use precautions: Walk through theft detection systems at a normal pace. Do not lean
against or linger near security gates or tag readers that include Radio Frequency
Identification (RFID) equipment Theft detectors are used in stores and libraries.
These systems are unlikely to affect implanted cardiac device function when walking
through security gates at a normal walking pace.
security
Electronic Article Surveillance (EAS)
Radiofrequency Identification and
Implantable Pacemakers and
Defibrillators
Trace Portal Machine
Safe under normal use. A high throughput, non-intrusive walk-through portal that
enables rapid detection of both explosives and narcotics.
airport security system, puffer,
EntryScan
Transformer Box
50 / 60 Hertz (residential)
Use precautions: Maintain at least a 12 inch (30 cm) separation between transformer
and implanted device. These transformer boxes in residential settings are used in
association with underground 60 Hertz power distribution. Typically this is a green
box found on the ground. Low risk to walk by or have in backyard.
TV Ears
Use precautions: Maintain at least a 6 inch (15 cm) separation between the
transmitting unit connected to the audio source, the charger, and the implanted heart
device.
Wi-Fi or Wireless
Fidelity
Use precautions: Maintain at least a 6 inch (15 cm) separation between the Wi-Fi
transmitter/receiver antenna (if visible) and the implanted device.
Wireless LANS
(Local Area Network
System)
Use precautions: Maintain at least a 6 inch (15 cm) separation between the antenna
of the LAN transmitter or the LAN transmitter case and the implanted device.
Peconet
Telecommunications
20
Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Battery Charger (car)
Use precautions: Maintain at least a 12 inch (30 cm) separation between the battery
charger and the implanted device.
Chainsaw (gas and electric
powered
Use precautions: Maintain at least a 12 inch (30 cm) distance between electric motor
/ ignition system of chainsaw and implanted device.
Demagnetizer (non-industrial)
Use precautions: Maintain at least a 6 inch (15 cm) separation between demagnetizer
and the implanted device when the items to be demagnetized are in a closed
container.
Electrostatic Spray
Gun (hand held)
Safe under normal use: Do not place directly over implanted device.
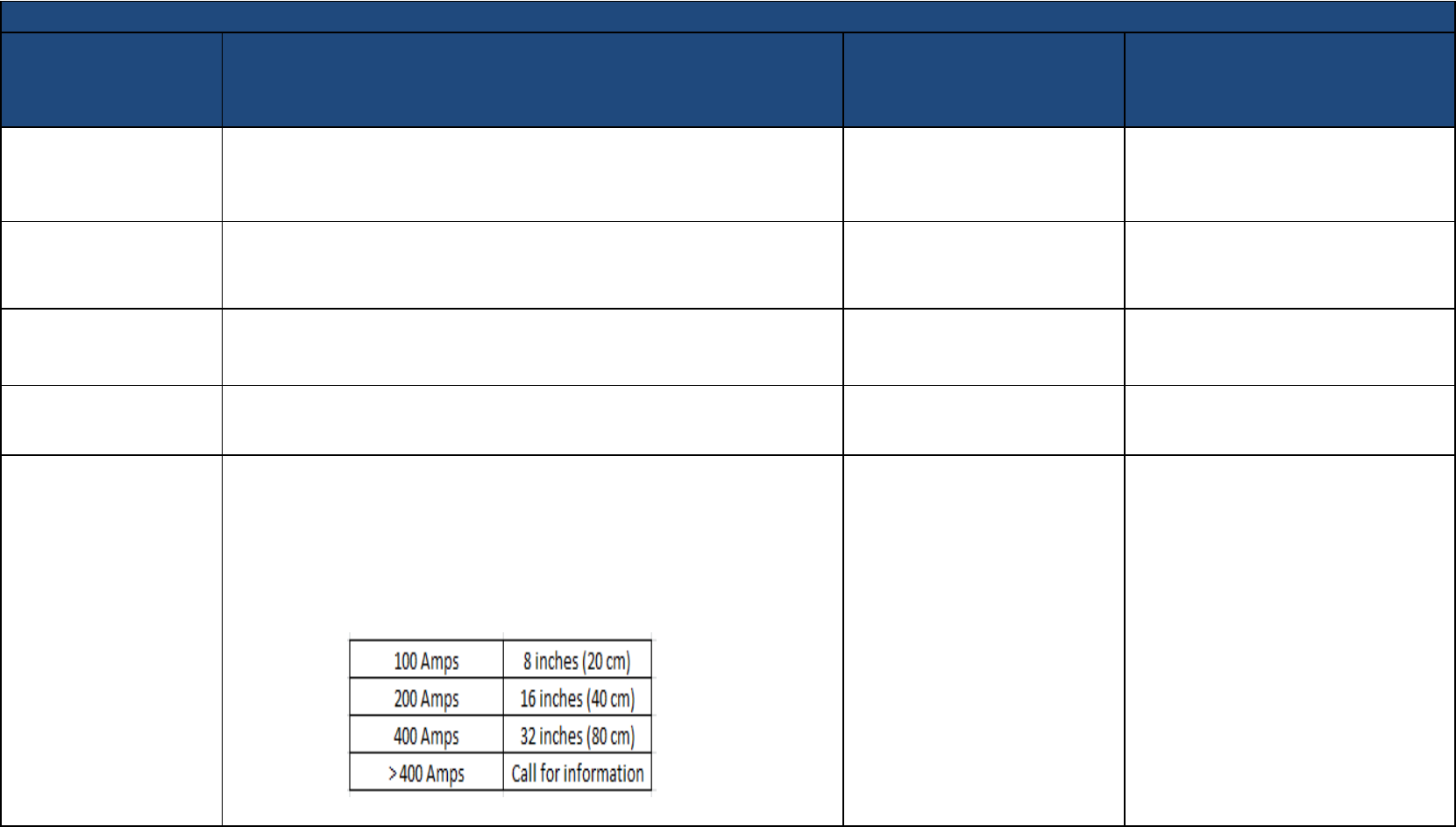
Generator - Residential
AC/DC portable/RVs
(gasoline or diesel
powered)
Use precautions: If generator is powered by an engine with an ignition system,
maintain at least a 12 inch (30 cm) separation between the components of the
ignition system and the implanted device. Does not include industrial generators.
EMI specifications:
With respect to our 0.1mT (1 Gauss) guideline for 60Hz magnetic fields, the resulting
fields generated at 100A, 200A, and 400A would be as follows:
Tools
21

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Ignition Systems
(gasoline powered
vehicles)
Use precautions: Maintain at least a 12 inch (30 cm) separation between the
components of an operating ignition system and the implanted device. If the device is
closer than 12 inches (30 cm), there is the potential for Pacemaker or ICD interaction.
Diesel powered vehicles have no effect.
Jackhammer
Do not use: Consult heart doctor. An electrical mechanical tool that combines a
hammer and chisel to break up rock, pavement, and concrete.
Jumper Cables
Use precautions: During use, maintain at least a 24 inch (60 cm) separation between
the jumper cables and the implanted device when starting an engine.
Soldering Guns / Soldering
Irons
Use precautions: Maintain at least a 6 inch (15 cm) separation between the soldering
gun or iron and the implanted device. The soldering gun contains a transformer.
Stun Guns (hand held
only)
Use precautions: Implanted device should be checked if patient received electrical
pulses from stun gun. If the individual receiving the subduing electrical shocks has a
Pacemaker or ICD, their implanted device may be affected.
Safe under normal use: if the individual with a Pacemaker or ICD is using the stun gun.
See Taser below.
Taser®
(hand held gun that
shoots two darts
propelled by
compressed gas
cartridge)
Use precautions: Implanted device should be thoroughly evaluated following a taser
event. Low risk if the individual with a Pacemaker or ICD is operating the taser.
Electrical pulses should not be applied in vicinity of implanted device (typically near
heart). One set of electrodes has the ability to project two darts that deliver these
high-energy electrical pulses to individuals at a short distance. A second set of back-
up electrodes are incorporated into the hand held portion of the taser.
Tools
22

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Tools - Battery
powered (cordless)
Use precautions: Consult heart doctor. Maintain at least a 12 inch (30 cm) separation
between the battery-powered tool, the charger, and the implanted device. Battery
operated home and garden equipment includes: circular saws, drills, hedge clippers,
lawnmowers.
Tools - Bench mounted
(corded)
Use precautions: Maintain at least a 12 inch (30 cm) separation between the motor
associated with the power tool and the implanted device. This applies to motors /
home workshop tools such as drill presses, table saws, grinders.
Tools - Hand held or
Home & Garden (corded)
Use precautions: Maintain at least a 12 inch (30 cm) separation between power tools
and the implanted device. Be sure tools are properly grounded.
drill, saw, sander, router, electric
hedge clippers , edge trimmer, weed
whacker, weed trimmer
Tools - Lawn mower, leaf
blower, snowblower
Use precautions: Maintain at least a 12 inch (30 cm) separation between the motor
associated with the power tool and the implanted device.
Backpack leaf blower
UPS - Uninterrupted
Power Source
(Commercial power
failure back-up
system)
Use precautions: Maintain at least a 12 inch (30 cm) separation between the
Uninterrupted Power Source system operating normally and the implanted device.
When the UPS system is running on the battery source, maintain at least an 18 inch
(45 cm) separation between the UPS system and the implanted device.
Tools
23

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Welding
Use precautions: Maintain 24 inch (60 cm) distance between the welding
machine/cables/arc and the implanted device at current ratings less than or equal to
200 amps. The welding machine/cables/arc produce and carry the current associated
with the welding operation. At rated currents greater than 200 Amps, we recommend
contacting your doctor to discuss the specific welding equipment prior to welding.
Other arc welding considerations include, but are not limited to:
• Strictly follow the safety precautions mentioned in the welder manual.
• Work in a dry area. Wear dry, electrically insulated gloves and dry shoes.
• Keep all cables straight, close together, and extending away from the body. Do not
coil cables.
• Arrange the work area so that the handle and rod will not contact the metal being
welded.
• Use short, intermittent, and irregular bursts at the lowest feasible energy levels;
wait several seconds between welds. Do not weld with rapidly repeating short bursts,
as they are more likely to be interpreted as electrical activity of the heart.
• Ensure all equipment is properly grounded and is in proper working condition.
• Limit welding currents to less than 200 Amps.
MIG - metal inert gas
TIG - tungsten inert gas
Stick, Heli, Arc, plasma cutters,
gauging welding instruments
Arc Welding and Implanted Medical
Devices
EDM-Electromagnetic
Discharge Machine
Use precautions: Maintain 24 inches (60 cm) distance between the equipment and
implanted device.
Arc Welding and Implanted Medical
Devices
Welding
24

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Dental Apex Locator
(root locator)
Safe under normal use: Instrument used to locate the end of a nerve in a tooth.
Dental Equipment and Implantable
Pacemakers and Defibrillators
Dental Chair with Magnetic
Headrest
Use precautions: some dental chairs contain magnets located in the headrest. If the
pacemaker or defibrillator is programmed not to respond to a magnet, patients may
sit in these chairs. If the implanted device is programmed to respond to a magnet and:
• The magnet power is less than 10 Gauss (1 milli-Tesla)—patients may sit in these
chairs.
• The magnet power is greater than or equal to 10 Gauss (1 milli-Tesla)—patients
should not sit in these chairs as device function/programming may be affected.
Dental Equipment and Implantable
Pacemakers and Defibrillators
Dental Drills and Cleaning
Equipment
Safe under normal use.
Dental_Equipment and Implantable
Pacemakers and Defibrillators
Dental Electrocautery
(periodontal surgery)
Use precautions: consult heart doctor to evaluate any possible risks associated with
these in conjunction with patient's medical condition. This procedure is used in
surgeries to cut tissue and stop the bleeding of blood vessels. Electrocautery may
temporarily affect the function of an implanted pacemaker or defibrillator. During
electrocautery use, Boston Scientific defibrillators can be temporarily deactivated
with a magnet (refer to Electrocautery ACL for more details) and a pacemaker can be
programmed to pace asynchronously. The physician who monitors the patient’s
implantable device should be contacted to discuss the use of electrocautery and the
potential impact of these programming options.
Also refer to Electrocautery and
Implantable Device Systems
Dental Equipment and Implantable
Pacemakers and Defibrillators
Dental Procedures
25

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Dental Pulp Tester
Use precautions: This device is disclaimed for use on Pacemaker & ICD patients by
most Dental Pulp Tester manufacturers. However, there are several published studies
reporting no interaction with Pacemaker and ICD function, which suggests these
technologies may be compatible. This instrument is used to check the viability of the
nerve in a tooth. Introduces alternating current (AC) into the tooth.
Dental Equipment and Implantable
Pacemakers and Defibrillators
Dental / Ultrasonic
scalers/cleaners
Use precautions: reference the linked article. Cavitron
Dental Equipment and Implantable
Pacemakers and Defibrillators
Dental X-rays Safe under normal use.
Dental Equipment and Implantable
Pacemakers and Defibrillators
Dental Procedures
26

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Ablation-Cardiac (Radio
frequency)
Use precautions: Consult with heart doctor. This procedure introduces electrical
current into the body. This process is done to change or interrupt the electrical
pathways in the heart. For device management recommendations, reference the
linked A Closer Look "Radiofrequency Ablation and Implantable Device Systems".
Radiofrequency (RF) ablation is a minimally invasive treatment method in which a
physician uses a small amount of RF current to damage or destroy selected tissue
areas within the body. RF ablation is often used to treat cardiac arrhythmias, chronic
pain and benign or cancerous tumors.
Radiofrequency Ablation and
Implantable Device Systems
Acupuncture - No
electrical stimulus
Safe under normal use.
Acupuncture AC
Alternating Current
Use precautions: Consult with heart doctor. This procedure introduces electrical
current into the body that may interfere with the implanted device. Electrical current
is introduced into the body via acupuncture needles, which are connected to an
external current source. It is recommended that individuals considering this
procedure consult their heart doctor to evaluate any possible risks associated with
these responses in conjunction with their medical condition.
Acupuncture DC - Direct
Current
Safe under normal use.
Anodyne Therapy
Safe under normal use. Infrared medical device that increases circulation and
decreases pain. No electrical current of deep heat is associated with this device.
Pain Therapy
Medical Procedures
27

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Argon Plasma Coagulation
Use precautions: consult heart doctor to evaluate any possible risks associated with
these in conjunction with patient's medical condition. Argon Plasma Coagulation has
been used for more than 10 years in open surgery, laparoscopy and thoracoscopy,
especially for hemostasis of large surface bleeding. It conducts monopolar or bipolar
electrosurgical current to tissue via an ionized argon gas stream (argon plasma). Can
be used for coagulation purposes in gastroenterology procedures.
Plasma Knife
Argon Photo Coagulation
Safe under normal use. Different from Argon Plasma Coagulation, which carries
different recommendations. Argon photo coagulation utilizes argon gas in a laser to
coagulate tissue. Commonly used in eye procedures in conjunction with laser use.
Can also be used for coagulation purposes in gastroenterology procedures. See laser
recommendations for additional information.
BEMER
Use precautions: Maintain 6 inch (15 cm) separation between the Bio-Electro-
Magnetic-Energy-Regulation Therapy (BEMER) and implanted device. Consists of a
mat on which to lie (lie on back) and hand-held tools that can be used on arms and
legs but not placed on chest.
Bioness H200 Electrical
Stimulation
Do not use. If you must use, consult heart doctor. Bioness delivers electrical
stimulation to the forearm via five electrodes. It can be used to treat muscle
weakness resulting from conditions such as MS, stroke, and cerebral palsy. Maximize
distance between Bioness/electrodes and implanted device whenever possible. Avoid
electrode paths transecting the implanted device.
Medical Procedures
28

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Blood bag dielectric
sealing equipment
Use precautions: Maintain at least a 12 inch (30 cm) separation between the sealer
and implanted device. Do not place power source directly over device. Equipment
uses high frequency energy to seal blood bags.
Fenwal, Sebra, Terumo, Baxter,
Sarstedt, heat sealer sealing machine
Body Fat Analysis Scale -
Bioelectrical Impedance
Do not use: Body Fat Analysis scale with bioelectrical Impedance.
The percentage of body fat is estimated by passing electrical current through the
body. Most manufacturers have disclaimers in their product literature excluding the
use of this product for individuals with a Pacemaker or ICD.
Body Fat Measuring Scale (hand held),
Electrical Body Fat Analysis, BIA,
Bioelectrical Impedance Analysis,
Weight Scale
Body Fat Analysis - Near-
Infrared Light
Safe under normal use. This type of body fat measurement uses near-infrared light to
determine the amount of body fat according to the principle that body fat absorbs
light while lean body mass reflects light. The amount of light that is emitted from the
light wand and how much is reflected back into the wand is measured.
Futrex
Body Hydration Scale Do not use. Scale measures hydration ("body water") using bio-electrical impedance.
Conair, Bio Electrical Impedance,
BioElectrical Impedance, Weight Scale
Bone Density
test/Scan
Two types are used: X-ray and Ultrasound.
X-ray: Safe under normal use.
Ultrasound: Use precautions: Ultrasound technique. Maintain at least a 6 inch (15 cm)
separation between the transducer head and the implanted device.
x ray, xray
Circulators, Sequential
Use precautions: Maintain at least a 12 inch (30 cm) separation between the
electronic pump and the implanted device. Circulators mimic the lymphatic system by
promoting lymphatic flow by stimulating circulation to the extremities. It can be used
in treating chronic and acute edema, venous insufficiency, amputations, skin grafts,
etc. The appliance is worn by the patient and has a number of air bladders. These
bladders are filled and emptied sequentially by an electronic pump connected by a
length of hose.
Medical Procedures
29

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Cryoablation
Safe under normal use. Most often used in the treatment of prostate cancer; uses for
other types of cancer and ablation of heart arrhythmias are less common. Involves
controlled freezing (down to -75°F at catheter tip) for the destruction of cancerous or
unwanted cells. If used in the heart, avoid lead system contact with the tip of the
cryoablation catheter as this could potentially cause lead damage or fracture.
External Bone Growth
Stimulator
Alternating Magnetic Field
- produced by an
alternating current (AC)
Use precautions: Consult with heart doctor to evaluate any possible risks associated
with this equipment in conjunction with patient medical condition. When used on leg
poses a low risk of affecting Pacemaker or ICD. For use on wrist or arm, maintain at
least a 12 inch (30 cm) separation from the implanted device. An insulated cuff
surrounding the broken bone produces a magnetic field that promotes bone healing.
This therapy does not introduce conducted current into the body; however, there is a
magnetic field that is present in the immediate vicinity of the cuff. A battery is used
to deliver a short duration, high intensity current pulse to the coil in the cuff that
produces the therapeutic magnetic field.
External Bone Growth
Stimulator
Direct Current (DC)
Safe under normal use: Low level direct current is not detectable by the Pacemaker
or ICD (implanted or external stimulator).
EBI/Biomet SpinalPak and
OrthoPak
Use precautions: Maintain at least a 12 inch (30 cm) separation between the EBI leads
and implanted device.
External Bone Growth
Stimulator - Spinalogic
Use precautions: Maintain at least a 12 inch (30 cm) separation between the
stimulator and the implanted device. Promotes healing by inducing weak pulsing
electrical currents at the fracture site. These signals are generated by a low energy
electromagnetic field created by passing specific current pulses through the
treatment coil.
bone healing system
Medical Procedures
30

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Internal Bone Growth
Stimulator
introducing AC current
into the body
Use precautions: Consult with heart doctor to evaluate any possible risks associated
with these responses in conjunction with patient medical condition.
See additional precautions below. This procedure introduces electrical current into
the body that may affect the implanted devices of individuals. Low risk when
electrodes are placed on an extremity. If the electrodes are placed on the torso there
is the potential for Pacemaker or ICD interaction.
Use precautions: DJO Bone Healing System - maintain 12 inch (30 cm) separation
from implanted device and leads.
Use precautions: Exogen Ultrasound Bone Healing System - maintain 12 inches (30
cm) from implanted device and leads. Do not place the transducer head directly over
the implanted device or leads, or in orientations where the implanted device or leads
will be exposed to the ultrasound beam.
Bravo PH Capsule (used
to diagnose reflux
disease)
Safe under normal use: The Bravo pH Capsule with Delivery System is a single use,
disposable device which is intended to be used for gastro-esophageal pH
measurement and monitoring of gastric reflux. The Bravo system involves a pH
capsule, about the size of a gel cap, which is temporarily attached to the wall of the
esophagus. The capsule measures pH levels in the esophagus and transmits readings
via radio telemetry to the Bravo Receiver worn on the patient’s belt or waistband.
Capsule Endoscopy-Given
model M2A
Safe under normal use: The M2A capsule encases a digital camera, light-emitting
diodes, batteries, and a transmitter. The M2A capsule emits short bursts of radio
frequency energy for twice per second for the eight-hour diagnostic period.
PillCam Capsule Endoscopy and
Implantable Device Systems
CAT Scan or CT Scan
(Computed Axial
Tomography)
Use precautions: Consult heart doctor and refer to A Closer Look article. Recommend
monitoring heart rate during CT scan.
Computed Tomography (CT) Scanning
and Implantable Pacemakers and
Defibrillators
Cardioversion See A Closer Look article (right) and Defibrillation-External (below).
CPR and External Defibrillation for
Pacemaker and/or Defibrillator Patients
Colonoscopy
Use precautions: This diagnostic procedure by itself poses a low risk of affecting the
Pacemaker or ICD. However, if polyps are found electrocautery may be used to
remove them. See electrocautery guidelines.
Electrocautery and Implantable Device
Systems
Medical Procedures
31
Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
CyberKnife
Use precautions: Robotic radiosurgery system used for treating benign tumors,
malignant tumors and other medical conditions. Cyberknife utilizes Radiation
Therapy, and Boston Scientific Therapeutic Radiation Recommendations apply. Also
see Recommendations for Stereotaxis.
Therapeutic Radiation and Implantable
Device Systems
DNA Sequencing Equipment
Safe under normal use. Electrophoresis apparatus used in the DNA sequencing
process. Uses electrical current to separate the DNA within a gel medium.
Defibrillation-External
(High energy)
Cardioversion (Low
energy)
Use precautions: Maintain at least a 6 inch (15 cm) separation between paddles and
implanted device or device lead system. If external defibrillation or Cardioversion is
delivered closer than 6 inches (15 cm) from the implanted device or device lead
system, Pacemaker or ICD may be damaged or reprogrammed. It is recommended
that device function and programming be thoroughly evaluated after any external
defibrillation or cardioversion.
CPR and External Defibrillation for
Pacemaker and/or Defibrillator Patients
Deep Brain Stimulator
Use precautions: both neurologist and cardiologist/EP should consult with Boston
Scientific Technical Services (1-800-CARDIAC) prior to combining these systems in the
patient to minimize adverse interactions. Used for movement disorders such as
Parkinson's disease. The deep brain stimulator is implanted in the body of the
patient. An electrode is implanted into the thalamus, deep within the brain. This
electrode is connected to a pulse generator that is implanted in the pectoral region
via a lead. The device is activated and sends continuous electrical pulses to the brain
to inhibit tremors. The device can be turned on or off by holding a handheld magnet
over the generator.
DBS, Activa, Itrel, Soletra, Kinetra
Diathermy - Shortwave or
Microwave
Do not use: Diathermy is NOT recommended. This process heats body tissue and may
result in Pacemaker reversion or ICD shock if no precautions are taken.
Diathermy Ultrasound
Use precautions: Consult heart doctor and maintain a 6 inch (15 cm) separation
between the transducer head and the implanted device. A form of physical therapy in
which deep heating of tissues is accomplished by the use of high frequency electrical
current.
ECG/EKG
Electrocardiogram
Safe under normal use: The ECG/EKG is limited to sensing electrical activity of the
heart.
EKG, ECG, 12-lead, twelve-lead
Medical Procedures
32

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Echocardiogram
Use precautions: Maintain a 6 inch (15 cm) separation between the transducer head
and the implanted device. A test in which ultrasound is used to examine the heart.
Transesophageal Echo, TEE,
Echocardiography
ECT (Electroconvulsive
Shock Therapy)
Use precautions: Consult with heart doctor to evaluate any possible risks associated
with these responses in conjunction with the patient's medical condition. This
procedure introduces electrical current into the body that may affect the implanted
devices of individuals. ECT is used to treat depression, anxiety and other mental
disorders.
EECP - Enhanced
External Counter
Pulsation Therapy
Safe under normal use: This therapy helps to maintain arterial pressure longer,
resulting in better perfusion of the heart and other body organs. No effect on the
implantable device electrical sensing. Enhanced external counterpulsation, or EECP, is
used for controlling angina in patients that are not considered surgical candidates.
Trousers are placed over patients from ankle to waist and pneumatically inflated in
diastole and deflated prior to the next systole (coronary arteries fill in diastole).
Similar to Intra-Aortic Balloon Pump, except non-invasive.
EEG -
Electroencephalography
Safe under normal use: Scan brain wave activity. A recording of the electrical
potentials on the skull generated by currents emanating spontaneously from nerve
cells in the brain (i.e. surface ECG of the brain).
Electrocautery or
Electrosurgery
Use precautions: Anesthesiologist should consult heart doctor to evaluate any
possible risks associated with these in conjunction with patient's medical condition.
This procedure is used in surgeries to cut tissue and stop the bleeding of blood
vessels. Electrocautery may temporarily affect the function of an implanted
pacemaker or defibrillator. During electrocautery use, Boston Scientific defibrillator
can be deactivated and pacemakers can be programmed by the physician to pace
asynchronously. The physician who monitors the patient’s implantable device should
be contacted to discuss the use of electrocautery and the potential impact of these
programming options. Consider potential programming options such as magnet
application to minimize interactions between electrocautery and the implantable
device. Avoid placing cautery tool directly over implantable device.
Bovie, Hyfrecator, Cautery, Argon
Plasma, Electrosurgery, Plasma Knife
Electrocautery and Implantable Device
Systems
Electrolysis - AC or
Electrolysis - Blended (uses
both DC and AC)
Thermolysis
Do not use: Consult with heart doctor to evaluate any possible risks associated with
these responses in conjunction with patient's medical condition. This procedure
introduces electrical current into the body that may affect the implanted device.
Medical Procedures
33

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
ELOS
Do not use: Consult with heart doctor. Called ReFirme ST Skin Tightening system,
developed by Syneron and designed to reduce wrinkles and tighten skin in the face
and neck area. It uses ‘ELOS’ technology which combines three energy sources: laser,
infrared and radio frequency. ELOS technology uses a pulse repetition rate of one
pulse per second.
Electron Beam Tomography
(EBT)
Safe under normal use. EBT is a method of high-resolution imaging for all body
organs. It is designed to evaluate cardiac and pulmonary anatomy, blood flow and
function, and can yield a full set of images during a single breath-hold. EBT uses a
powerful electron beam focused onto one of four tungsten target rings positioned
beneath the patient.
Nerve Conduction Test
Use precautions: Consult with heart doctor to evaluate any possible risks associated
with your medical condition. This procedure introduces electrical current into the
body that may affect the implanted cardiac devices.
EMG, electromyography
EMG Electromyography-
Single/Multiple Stimuli
Use precautions: Consult with heart doctor to evaluate any possible risks associated
with your medical condition. This procedure introduces electrical current into the
body that may affect the implanted cardiac devices.
Electronystagmography
(Audiology - ENG)
Safe under normal use: The ENG test is used to assess balance and movement
disorders. Passive electrodes are placed on the head to evaluate the electrical
potentials associated with eye movement. (Similar to EKG, EEG)
Refer to EEG
Electroretinography
Safe under normal use. Electroretinography is a test to measure the electrical
response of the eye’s light-sensitive cells (rods and cones). Electrodes are placed on
the cornea and the skin near the eye. The electrodes measure the electrical activity of
the retina in response to light, which is then fed into a monitor where it can be
viewed and recorded.
Electrostatic Discharge
Safe under normal use. ESD is a charge which builds up on the skin of the patient and
is discharged to a grounded object via the patient's extremities. This is in the form of
a spark.
Medical Procedures
34

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
ERCP Endoscopic Retrograde
Cholangiopancreatography
Safe under normal use unless electrocautery is used. Refer to electrocautery. ERCP is
an endoscopic study that incorporates the use of x-ray with an endoscope. It is used
primarily to diagnose and treat conditions of the bile ducts including gallstones,
inflammatory strictures (scars), leaks and cancer. If the exam shows a gallstone or
narrowing of the ducts, the physician can insert instruments into the scope to remove
or relieve the obstruction. Also, tissue samples (biopsy) can be taken for further
testing.
Fibrostat
Safe under normal use. Portable testing station used to analyze the fibrinogen levels
in the blood.
Functional Electrical
Stimulation (FES)
Use precautions: Maintain at least a 12 inch (30 cm) separation between motor and
implanted device. Use patches as suggested in the FES product manual (i.e. only on
the legs). Electrical activation of muscles via surface electrode patches in order to
facilitate motion, specifically pedaling a bicycle.
Gamma Knife
Use precautions: Consult with heart doctor and radiologist. Used to treat
arteriovenous malformations (AVM) and certain brain tumors without an incision.
Incorporates 3-D information from MRI (see MRI recommendations) or CT. The
Gamma Knife uses beams of radiation to damage abnormal tissue. This is
accomplished by multiple beams of radiation intersecting to form a precise tool.
These beams are focused on the target area and designed to destroy only that which
is abnormal.
Therapeutic Radiation and Implantable
Device Systems
Hospital Patient Monitoring
Equipment
Use precautions: Consult with physician. Some hospital equipment used for
monitoring a patient’s respiratory, cardiac, and hemodynamic parameters deliver
similar low-level electrical currents into the body to obtain transthoracic impedance
measurements. Examples of such equipment include, but are not limited to,
respiratory monitors, diagnostic echo imaging, and ECG and hemodynamic machines.
Mechanical Ventilation, ventilator
ACL: Interactions between Hospital
Monitoring or Diagnostic Equipment and
Pacemakers Using Minute Ventilation
Sensors
Hyperbaric Chamber
Use precautions: Only to be used under medical supervision. A chamber large enough
to accommodate one or more persons in which pressure is above normal atmospheric
pressure. It is used to treat several medical conditions (i.e. carbon monoxide
poisoning, infections, burns, pressure related diving injuries).
Elevated Pressure (HBOT/SCUBA) and
Implanted Medical Devices
Medical Procedures
35

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Impedance Cardiography
Use precautions: Only to be used under medical supervision. Impedance cardiography
allows a clinician to non-invasively assess a patient's hemodynamics. It can be used
for treating heart failure, hypertension, pacemaker, and dialysis patients. The
impedance signal is a result of the application of a low-level AC current to the thorax
of a patient during a monitoring session.
bioimpedance,
transthoracic impedance
Interactions Between Hospital
Monitoring or Diagnostic Equipment and
Pacemakers Using Minute Ventilation
Sensors
Insulin Pump
Use precautions: Maintain 6 inch (15 cm) separation between the insulin pump and
the implanted device. Battery operated unit that provides a scheduled release of
insulin to the patient. Typically worn around the patient’s waist.
Minimed
Interferential Electrical
Current Therapy
Use precautions: Consult with heart doctor to evaluate any possible risks associated
with these responses in conjunction with their medical condition. This procedure
introduces electrical current into the body that may affect the implanted devices of
individuals.
Iontophoresis
Use precautions: consult heart doctor. Method of delivering water-soluble ionized
medication into the skin for inflammation and pain control. Involves placing two
electrodes on the skin, one containing the drug. The electrodes are then hooked up
to a low level external power supply (typically no more than 4.0 milliamps of DC
current), which acts as the dose controller. Do not place electrodes over device or
lead tip. Although interaction is unlikely, monitoring device function the first time
treatment is received can provide confidence of compatibility with the cardiac
implant. As an addidtional precaution, the health care professional may use a magnet
to deactivate inappropriate ICD therapy or provide asynchronous pacemaker function
during iontopheresis treatment.
Laser / Laser Surgery
Use precautions: Maintain a 3 to 4 feet (90 cm - 120 cm) separation between
generator or cabling from implanted device. LASER is an acronym for light
amplification by stimulated emission of radiation. It is a mechanism for emitting
electromagnetic radiation, typically light or visible light, via the process of stimulated
emission. The emitted laser light is usually a narrow low-divergence beam that can be
manipulated with lenses.
Laser, YAG, Odyssey, Holmium, Argon,
CO2 Laser = Laser Generator:
Medical Procedures
36

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Lasik Eye Surgery
Use precautions: Recommend the application of a magnet to disable ICD detection
circuit, or program ICD detection circuit off prior to surgery. These precautions are
taken so legitimate therapy will not be delivered during critical portions of this
delicate corrective eye procedure. Laser light associated with this procedure has a
low risk of affecting the Pacemaker or ICD. A magnet is not used to disable a
pacemaker.
Eye Surgery, Eye Laser Procedure,
Cataract Surgery, Lasik, Radial
Keratotomy, Corneal Surgery,
Vitrectomy, Pan Retinal
Photocoagulation, Blepharoplasty
Lithotripsy
Use precautions: Consult with heart doctor. Lithotripsy is used in the non-invasive
treatment of kidney stones (urinary calculosis) and biliary calculi (stones in the
gallbladder or in the liver). The scientific name of this procedure is Extracorporeal
Shock Wave Lithotripsy (ESWL). Lithotripsy attempts to break up the stone with
minimal collateral damage by using an externally-applied, focused, high-intensity
acoustic pulse.
Extracorporeal Shock Wave
Lithotripsy, ESWL
Lithotripsy and Implantable Pacemaker
and Defibrillator Systems
Lithotripsy (Ossatron)
Use precautions: Consult with heart doctor. Basically a form of lithotripsy, but it uses
a water-filled membrane around the emitter instead of a water bath. The possible
affected area during treatment is more focused than in regular lithotripsy.
Lithotripsy and Implantable Pacemaker
and Defibrillator Systems
Lie Detector Test
Safe under normal use: Lie detector tests introduce only direct current into the body.
This direct current poses a low risk of affecting a Pacemaker or ICD.
Polygraph
Magnetic mattresses Do not use: Magnetic mattresses.
Mammogram (Diagnostic
X-ray) (2D / 3D)
Safe under normal use: Inform technician you have a device to ensure device does
not get compressed. X-ray equipment can be adjusted to make individual more
comfortable and lessen the pressure on the Pacemaker or ICD.
Mechanical ventilation with a
respiration rate monitor
Use precautions: Ventilators are used to help individuals breathe during surgery.
Respiration rate monitors are used in conjunction with the ventilators to help verify
that an individual's breathing rate is in a normal range during surgery. See A Closer
Look article linked in right column.
Interactions between Hospital
Monitoring or Diagnostic Equipment and
Pacemakers Using Minute Ventilation
Sensors
Medical Procedures
37

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Medical Helicopter Safe under normal use.
MET (Microcurrent Electrical
Therapy) Alpha- Stim 100®
Use precautions: Consult with heart doctor. This procedure introduces electrical
current into the body that may affect the implanted devices of individuals. It is
recommended that individuals considering this procedure consult their heart doctor
to evaluate any possible risks associated with these responses in conjunction with
their medical condition. Similar to TENS unit with somewhat less current.
Microcurrent, Frequency
Specific
Do not use. Similar to TENS, microcurrent is introduced into the body to alleviate a
variety of conditions including pain.
Muscle stimulation, stimulator
MRI (Magnetic
Resonance Imaging)/MRA
Do not use: Consult with heart doctor. Not recommended. Even when the MRI is not
in use, the Pacemaker & ICD may be affected by the static magnet field that is always
present near the MRI. There is the potential for Pacemaker magnet rate operation
and disabling of ICD detection circuit (please reference BSC approved MRI compatible
devices - ImageReady Lookup Tool.
ImageReady Lookup Tool
Magnetic Resonance Imaging (MRI) and
Implanted Medical Devices
MR Conditionally approved
BSC Systems
Use precautions: BSC's IMAGEREADY
TM
MR-Conditional Pacing System consists of
specific combinations of pulse generator and lead components. Note that system
approval is geography-specific. To determine if the system under consideration is
approved as MR-Conditional, please refer to the IMAGEREADY LOOK-UP TOOL.
Guides can be downloaded from the link. Only patients implanted with a complete
system designed, optimized, and tested for the ability to function correctly under
specified conditions during an MRI scan are eligible to be scanned.
Approved devices and leads are listed
in the
IMAGEREADY LOOK-UP TOOL
(click on the link at right)
ImageReady Lookup Tool
Medical Procedures
38

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Myelogram
Use precautions: Test used to evaluate the flow of cerebral spinal fluid around the
spinal column and nerve roots. It is used to diagnose bulging discs, herniated discs, or
changes in the bones surrounding the spinal cord. Test involves injecting a special dye
(contrast) and taking a series of x-rays, often in combination with a CT scan. If done
with a CT scan, see CT scan precautions.
Myotherapy
Use precautions: Avoid use of electrical stimulation. Myotherapy is a combination of
therapies employed by chiropractors to relieve muscle pain. Treatment plans
typically include trigger point therapy, stretching of muscles and joints, thermal
therapy, myofascial dry needling (i.e. acupuncture), muscle strengthening and
electrical stimulation.
Navigator Bionavigation
System
Do not use: Consult with heart doctor. Used to assist with the placement of central
venous catheters and PICC lines. The transmitter is located in a hand-held device
which is waved over the patient. The receiver is located within the stylet in the
catheter.
Central Venous Catheter Placement,
PICC Line placement
Neocontrol Extracorporeal
Magnetic Innervation
Do not use. Involves contracting muscles through application of a time-varying
magnetic field.
ExMI, Urinary Incontinence
Nuclear Stress Test
Safe under normal use: This procedure is used to see blood flow in the patient's heart
at rest and during activity. A radioactive substance is injected into the patient's
bloodstream and viewed with a special scanner. No reported interference with this
procedure.
Nuclear Medicine
Safe under normal use. Involves injecting a radioisotope in conjunction with a series
of scans similar to x-rays.
MUGA Scan, PET Scan, Thallium Stress
Test/Scan, MIBG Study, Cystogram,
Renogram, MAG3 Study,
Neuroblastoma Study, HIDA scan
PET Scan (Positron
Emission Tomography)
Safe under normal use: Radioactive dye is injected into body. Radiation given off by
the body is monitored. Similar to a diagnostic X-ray.
Medical Procedures
39

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
PFES
Use precautions: Consult with heart doctor. Incare, produced by Hollister, uses
biofeedback and painless electrical stimulation to treat urinary incontinence. Use
lowest setting possible, monitor patient's cardiac activity, stopping procedure if
interference occurs.
Phototherapy, UVB
Safe under normal use. Typically used for dermatological conditions such as psoriasis.
Ultraviolet B Phototherapy, Light
Therapy
Powered Muscle Stimulator
Do not use. If you must use, consult heart doctor. H-Wave involves passing electrical
current through the body, and may interfere with pulse generator function. Any
medical equipment, treatment, therapy, or diagnostic test that introduces electrical
current into the body may have the potential to interfere with implanted cardiac
device therapy. If H-Wave is medically necessary, evaluate the H-Wave therapy
setting for compatibility with the pulse generator. Refer to TENS recommendations.
H-Wave, Pain Therapy, H-Wave
Stimulator
Click here for TENS
Pro-Adjuster
Safe under normal use. Used by doctors/chiropractors for spinal analysis and
adjustment.
Chiropractic
Pulse Radiation Therapy
(Radio frequency)
Use precautions: Consult with heart doctor to evaluate any possible risks associated
with these responses in conjunction with patient's medical condition. This procedure
introduces electrical current into the body that may affect the implanted devices of
individuals. Magnet application recommended for Pacemaker & ICD. Similar to
radiofrequency ablation. This procedure is used to interrupt nerve pathways for pain
management.
Radiofrequency Ablation and
Implantable Device Systems
Radiation Therapy (External X-
ray or Gamma knife)
Use precautions: Consult with heart doctor. See A Closer Look article.
Therapeutic Radiation and Implantable
Device Systems
Radiation Therapy - Surgery
(Internal/implants)
Use precautions: Consult with heart doctor. Prolonged exposure. See A Closer Look
article. Recommend physician follow-up post surgery.
Therapeutic Radiation and Implantable
Device Systems
Radiation, Therapeutic
Use precautions: Consult with heart doctor. Follow physician guidelines. See A
Closer Look article.
Neutron Radiation
Therapeutic Radiation and Implantable
Device Systems
Rebuilder
Do not use: Consult with heart doctor. An electrical stimulation device primarily used
for the treatment of neuropathies in the feet, but can be used in the hands. Use on
the hands will pose more of a risk for device interaction than use on the feet. The
equipment uses a separate footbath for each foot, such that all the current injected
into one foot passes through the patient's torso and out the other foot.
Medical Procedures
40
Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Relief Band®
Safe under normal use: Used to prevent motion sickness from traveling. Delivers a
small electrical pulse at the wrist area. Low risk of affecting Pacemaker or ICD.
Rhizotomy, Percutaneous
Facet
Use precautions: maintain at least a 12 inch (30cm)separation between the neutral
plate and the implanted device. The Leksell Neuro Generator is a complete system for
stereotactic lesioning, pain treatment, stimulation and bipolar coagulation. It
features an RF energy source with integrated monitoring and control features. Avoid
high stimulation pulse settings, especially amplitude.
RF Facet Rhizotomy
Rife Wellness Machine
Do not use: Consult with heart doctor. Rife Wellness Machines use what they term a
Mortal Oscillatory Rate (MOR) resulting in destruction of various pathogenic
organisms, as well as cancer. Nearly all of these machines send electrical current into
the patient’s body.
Sacral Nerve Stimulation
Use precautions: both neurologist and cardiologist/EP should consult with BSC
Technical Services (1-800-CARDIAC) prior to combining these systems in the patient to
minimize adverse interactions. Mild electrical stimulation is delivered to the sacral
nerve (in the lower region of the spine) via an implantable system that includes a
lead, neurostimulator and an extension wire.
Urinary incontinence, Interstim, Itrel
Safe Cross System
Use precautions: Consult with heart doctor. Consider potential programming options
such as magnet application to minimize interactions between electrocautery and the
implantable device. Uses RF energy to assist clinician in positioning therapeutic
equipment.
Scalpel, Harmonic or
Ultrasonic
Use Precautions: Consult with heart doctor. Ultrasonically activated scalpel. Operates
at a mechanical frequency of 55.5kHz over a distance of 80µm. No electrical current
flow through the tip into the body. Avoid placing scalpel directly over implanted
device.
Shunt - Programmable
Use precautions: Maintain a 12 inch (30 cm) separation between the programmable
shunt and the implanted device. Drains fluids from the brain for treatment of normal
pressure hydrocephalus (NPH). Shunt is programmable to various pressure settings
with an external programmer, consisting of a doughnut-style magnet connected to an
electrical unit. The magnet is placed near the patient’s head for reprogramming.
Sleep Apnea Machine
Use precautions: Maintain a 6 inch (15 cm) separation between all power cords and
monitor.
CPAP
Medical Procedures
41

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Somnoplasty
Do not use: Consult with heart doctor. A temperature-controlled radiofrequency
based technology for use in the treatment of chronic nasal obstruction and sleep
disordered breathing. Operates by heating a targeted area of obstructive tissue
below the surface. An electrode is used at outputs of 10+ Watts using an operating
frequency of 460kHz. A grounding pad is placed on the patient’s lower back.
Sonatron
Use precautions: Consult with heart doctor. Direct the Sonatron away from the
implantable device. Somewhat resembling a hairdryer, the device is applied externally
and can be pointed toward an area of the body to assist with wound healing and pain
management. It uses long wave RF and emits a low-frequency sound.
Sound Waves - Environmental No restrictions. Thunder, music, fireworks
Spinal Cord Stimulator
Use precautions: both neurologist and cardiologist/EP should consult with BSC
Technical Services (1-800-CARDIAC) prior to combining these systems in the patient to
minimize adverse interactions. Pain treatment that delivers low voltage electrical
stimulation to the spinal cord or peripheral nerve to inhibit or block the sensation of
pain. The system consists of stimulation lead(s), a pulse generator/power source, and
an extension wire that connects the two.
Neurostimulation, Nerve Stimulator,
Neuro Stim
Stereotaxis
Use precautions: Consult with heart doctor. Stereotactic surgery or stereotaxis is a
minimally invasive form of surgical intervention which makes use of a three-
dimensional coordinate system to locate small targets inside the body and to perform
on them some action such as ablation, biopsy, lesion, injection, stimulation,
implantation, radiosurgery. There are 2 different methods for developing the three
dimensional coordinate system:
1) MRI: Do not use (see A Closer Look article)
2) CAT Scan: If this method utilizes MRI- do not use. For the method utilizing CAT
Scan: follow CT scan Recommendations (see A Closer Look article)
3) Coronary Stereotaxis: Do not use. Uses magnets surrounding the patient in order
to guide a catheter (with a magnet at its tip) through the coronary vasculature.
Magnetic Resonance Imaging
(MRI) and Implanted Medical
Devices
Computed Tomography (CT) Scanning
and Implantable Pacemakers and
Defibrillators
Synchromed Infusion System
Use precautions: Maintain at least a 10 inch (30 cm) separation between programmer
head (which contains a magnet) and implanted cardiac device. Includes a small
catheter connected to a pump, both of which are implanted into the body. Delivers
medication directly to the spinal cord.
implantable drug pump
TMS (Transcranial
Magnetic Stimulation)
Do not use: Consult with heart doctor. This magnetic pulse therapy used in Psychiatry
generates strong magnetic fields to stimulate nerve cells in the brain.
Medical Procedures
42

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
TENS Unit (Transcutaneous
Electrical Nerve Stimulation)
Do not use. If you must use, consult heart doctor. TENS involves passing electrical
current through the body, and may interfere with pulse generator function. If TENS is
medically necessary, evaluate the TENS therapy setting for compatibility with the
pulse generator.
THE FOLLOWING GUIDELINES MAY REDUCE THE LIKELIHOOD OF INTERACTION: Place
the TENS electrodes as close together and as far away from the pulse generator and
leads as possible. Use the lowest clinically appropriate TENS energy output. Consider
cardiac monitoring during TENS use, especially for pacemaker dependent patient.
ADDITIONAL STEPS CAN BE TAKEN TO REDUCE INTERFERENCE DURING IN-CLINIC USE
OF TENS: If interference is suspected during in-clinic use, turn off TENS unit. Do not
change TENS settings until you have verified that the new settings do not interfere
with pulse generator function.
IF TENS IS MEDICALLY NECESSARY OUTSIDE THE CLINICAL SETTING (AT-HOME USE),
PROVIDE THE FOLLOWING INSTRUCTIONS: Do not change the setting or electrode
position unless instructed to do so. End each TENS session by turning off the unit
before removing the electrodes. If the patient receives a shock during TENS use, or if
they experience symptoms of lightheadedness, dizziness, or loss of consciousness,
they should turn off the TENS unit and contact their physician.
BIOSTIM Electro Medicine, e-stim,
Quell, Circulation Maxx
TUMT (Transurethral
Microwave
Thermotherapeutic Device)
Do not use: Consult with heart doctor. This is a diathermy-type of therapy. Most
manufactures of this type of device contraindicate the use of this therapy with
Pacemaker or ICD patients.
TUNA Therapy (Transurethral
Needle Ablation)
Do not use: Recommend consulting with physician for other therapeutic prostate
procedures that pose less risk of interacting with a Pacemaker or ICD. Used to treat
urinary symptoms caused by an enlarged prostate.
TURP- Prostate test
(Transurethral Resection of
the Prostate)
Do not use: Consult with heart doctor to evaluate any possible risks associated with
these responses in conjunction with patient's medical condition. This procedure
introduces electrical current into the body that may affect the implanted devices of
individuals.
Medical Procedures
43

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Thermography
Safe under normal use. Thermography uses the same technology as military “night
vision”, but with medical applications (e.g. breast cancer, poor circulation). An
infrared camera detects the heat emitted from the body. Nothing is introduced into
the patient.
Transurethral Microwave
Thermotherapy
Use precautions: Consult with heart doctor. Similar to electrocautery. Used for
treatment of Prostatism (urethral obstruction). Consists of simultaneous microwave
heating of the prostate and conductive cooling of the urethra.
TMX, Thermatrx, Prostate Surgery,
TURP
Ultrasound (Diagnostic) or
Sonogram
Use precautions: Do not place transducer head directly over the implanted device or
leads, or in orientations where the implanted device or leads will be exposed to the
ultrasound beam. This procedure uses ultrasound technology to create images of
organs and other body parts. *A sonogram is a type of diagnostic ultrasound used
during pregnancy.
Transcranial Doppler, TCD, Diagnostic
Ultrasound, Echo
Ultrasound (Therapeutic)
Use precautions: Do not place transducer head directly over the implanted device.
Heating of the tissues through sound waves. Will often be used in combination with
an anti-inflammatory drug or cortisone.
Vagal Nerve Stimulator
Use precautions: Consult with heart doctor. VNS is a device which is placed
subcutaneously in the chest with leads that wrap around the vagus nerve. Electrical
stimulation is applied to reduce epileptic seizure activity.
Ventricular Assist Devices
Safe under normal use. A ventricular assist device, or VAD, is a mechanical device that
is used to partially or completely replace the function of a failing heart. In many
cases, it is a long-term therapy.
LVAD, BiVAD, RVAD, Thoratec
Heartmate, Tandem Heart
Virtual Colonoscopy
performed with CAT Scan (CT
Scan)
Use precautions: See A Closer Look article for more information. A procedure that is
used to diagnose colon and bowel disease, including polyps, diverticulosis, and
cancer. The procedure is performed with a CAT SCAN or MRI. The procedure using an
MRI is not recommended.
Computed Tomography (CT) Scanning
and Implantable Pacemakers and
Defibrillators
Medical Procedures
44

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Virtual Colonoscopy with MRI
Do not use: MRI is not recommended. See A Closer Look article. The implanted
device may be affected by the static magnet field that is always present near the MRI.
Magnetic Resonance Imaging (MRI) and
Implanted Medical Devices
Vital Stim
Use precautions: Consult with heart doctor. Uses conducted electrical current.
VitalStim Therapy consists of an operating unit, lead wires, and surface electrodes
that are placed on the front of the patient’s neck. See ACL for further guidance.
VitalStim Therapy and Implanted Medical
Devices
X-ray (Diagnostic) Safe under normal use. x ray
Audio Induction Loop Systems
Use precautions: Maintain at least a 6 inch (15 cm) separation between a hand-held
loop receiver or induction loop and the implanted device. Induction Loop: loop of
wire around a room or building that generates a magnetic field, allowing sounds to be
picked up by a cochlear implant or hearing aid.
audio frequency induction loop or
hearing loop
Cochlear Implant
Use precautions: Maintain at least a 6 inch (15 cm) separation between the processor
and the implanted device. The processor is either behind the ear or clipped to the
waist connected to the implant by hardwire. Wear the processor on the side opposite
the pacemaker or ICD. The hard wire to the implant behind the ear should be kept
away from the implanted pacemaker/ICD. Most cochlear implants are comprised of
two central pieces: a processor that fits behind the ear and an internal piece
implanted under the skin.
Hearing Aid in ear or behind
ear
Safe under normal use. Behind the ear hearing aids or in the ear ONLY. The distance
between the hearing aids and implant site is enough to mitigate any affects from
hearing aids.
In-The-Ear (ITE), Behind-The-Ear
(BTE), Mini BTE, In-The-Canal (ITC),
and Completely-in-Canal (CIC), Mik-in
Helix (MIH), Invisible-In-Canal (IIC),
Receiver-In-Canal (RIC),
Over-the-Ear (OTE)
Hearing Aid in ear or behind
ear with a hard wired
connection to an acoustical
detector worn on the belt or
other locations not close to
the ear (most Cochlear
implants)
Use Precautions: Clip the main unit to the waist on the opposite side of the implanted
device. Also, the wire from the external acoustical detector to the hearing aid should
be kept away from the implant site of the pacemaker/ICD.
William Sound Pocket Talker
Hearing Aids
Medical Procedures
45
Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Personal Sound Amplifier,
Sennheiser Personal Sound
Use precautions: Keep at least 6 inches (15 cm ) between implanted device and each
component (receiver, charging unit or headphones).
Sennheiser A200, Set 820 system, RR
820, TR 820 RF, Personal Sound
Amplifier
Starkey Radiant Beam Array
Do not use: The Radiant Beam Array (RBA) is a multi-microphone array incorporating
six spatially separated microphones. The array is worn around the neck with the
microphones positioned across the chest. Output from the array is transmitted to
hearing instruments. The microphones in this assistive listening device are too close
to the implant site.
Stereo Listener
Use precautions: Clip the main unit to the waist, on the opposite side of the
implanted device. Also, clip the headphone wires on the opposite side of the
implanted device. Sound amplification system consisting of headphones and a clip-on
amplifier unit.
Assisted Listening Device, Headset,
RadioShack
TV Ears
Use precautions: Do not place the headphone ear pieces directly over the implanted
device. Maintain a 12 inch (30 cm) distance from the transmitting base station and the
implanted device.
The system features a wireless rechargeable headset that rests under the chin
(receiver) with foam ear tips, and a transmitter/recharger base.
Senhieser Set, Stethoset
Hearing Aids
46

Item
Safety precautions:
Recommendations are based on normally functioning products. Be sure all items are
in good working order and properly grounded. Follow manufacturer's instructions
for use.
Similar brands that were not analyzed A Closer Look Article
Forklift powered by diesel,
gasoline, propane, or
compressed natural gas (CNG)
Use precautions: Maintain a 24 inch (60 cm) separation from the components of the
ignition system of the gasoline/propane/CNG engine and the Pacemaker or ICD.
Forklift powered by electricity
or battery
Use precautions: Maintain a 12 inch (30 cm) separation between the electric motor
and the implanted device. The DC/AC current used to power the electric motors and
the permanent magnets associated with the motor operation can affect the
Pacemaker or ICD.
CRM-368607-AB DEC 2017
Workplace
TERMS OF USE: The information provided on the Electromagnetic (EMI) Guide should not be considered the exclusive or only source for this information. The table lists a general category of items only
and is not intended to be an exhaustive list. The recommendations and precautions may be based on information provided by the manufacturers of the items in question, and specific items within a
category may function differently. It is best practice to consult the original manufacturer of the item with potential EMI to verify any specific guidance concerning specific operation and compatibility with
implantable devices. If at any time there is a question about the function and potential for Electromagnetic Compatibility, contact the manufacturer of the item in question for further information. At all
times, it is the responsibility of the licensed healthcare professional to exercise medical clinical judgment in a particular circumstance.
The information provided is not intended to be used for medical diagnosis or treatment or as a substitute for professional medical advice. Always seek the advice of your physician or other qualified
health provider with any questions you may have regarding a medical condition.
Pacemaker Patient Manual
Defibrillator (ICD) and S-ICD Patient Manuals
Recommendations and precautions for transvenous devices also apply to S-ICD devices.
Patient manuals may be found by clicking on the appropriate link below.
47
