
EVALUATION OF THE COMPREHENSIVE
PRIMARY CARE INITIATIVE: FOURTH
ANNUAL REPORT
May 2018
This page has been left blank for double-sided copying.

Evaluation of the Comprehensive Primary Care Initiative
Fourth Annual Report
May 2018
Lead authors:
Deborah Peikes
Grace Anglin
Stacy Dale
Erin Fries Taylor
Ann O’Malley
Arkadipta Gosh
Kaylyn Swankoski
Jesse Crosson
Rosalind Keith
Anne Mutti
Sheila Hoag
Pragya Singh
Ha Tu
Thomas Grannemann
Mariel Finucane
Aparajita Zutshi
Lauren Vollmer
Randall Brown
Contributing authors (in alphabetical order):
Patrick Balke
Bryan Bernecker
Karen Bogen
Nancy Clusen
Jared Coopersmith
Annie Doubleday
Nancy Duda
Claire Dye
Michael Fields
Tyler Fisher
Jonathan Gellar
Kristin Geonnotti
Sabitha Gopalsamy
Mary Harrington
Shannon Heitkamp
Tricia Higgins
John Holland
Tessa Huffman
Jasmine Little
Rachel Machta
Nancy McCall
Shira Mitchell
Norberto Morales
Nikkilyn Morrison
Brenda Natzke
Victoria Peebles
Dmitriy Poznyak
Eugene Rich
Brianna Sullivan
Xiaofan Sun
Deker Winsor
Submitted to:
U.S. Department of Health and Human Services
Centers for Medicare & Medicaid Services
7500 Security Blvd.
Baltimore, MD 21244-1850
Project Officer: Timothy Day
Contract Number: HHSM-500-2014-00034I\HHSM-500-T0010
Submitted by:
Mathematica Policy Research
P.O. Box 2393
Princeton, NJ 08543-2393
Telephone: (609) 799-3535
Fax: (609) 799-0005
Project Director and Principal Investigator: Deborah Peikes
Deputy Project Director and Principal Investigator: Erin Taylor
Co-Principal Investigators: Stacy Dale and Randall Brown
Reference Number: 50319
This page has been left blank for double-sided copying.

MATHEMATICA POLICY RESEARCH
CONTENTS
EXECUTIVE SUMMARY ......................................................................................................................... XVII
1. INTRODUCTION .............................................................................................................................. 1
1.1. Overview of the Comprehensive Primary Care initiative .................................................... 1
1.2. Design of the CPC evaluation ............................................................................................. 5
1.3. This report ........................................................................................................................... 7
2. WHO PARTICIPATED IN CPC? ...................................................................................................... 9
2.1. Key takeaways on CPC participation .................................................................................. 9
2.2. Methods ............................................................................................................................ 12
2.3. Participating regions and payers ....................................................................................... 12
2.4. Participating practices and patients .................................................................................. 16
2.4.1 Details on CPC practice withdrawals and terminations ....................................... 19
3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING DID CMS AND OTHER
PAYERS PROVIDE TO CPC PRACTICES? ................................................................................. 25
3.1. Key takeaways on CPC supports to practices .................................................................. 25
3.1.1. Payments to CPC practices ................................................................................. 25
3.1.2. Data feedback provided to CPC practices ........................................................... 26
3.1.3. Learning supports provided to CPC practices ..................................................... 27
3.2. Methods ............................................................................................................................ 28
3.3. Payments to CPC practices .............................................................................................. 28
3.3.1. Care management fees from CMS and other payers .......................................... 29
3.3.2. Shared savings payments from CMS and other payers ...................................... 33
3.3.3. Practices’ use of and perspectives on CPC payments ........................................ 39
3.4. Data feedback provided to CPC practices ........................................................................ 41
3.4.1. Data feedback from CMS and other payers ........................................................ 41
3.4.2. Practices’ use of and perspectives on CPC data feedback ................................. 45
3.5. Learning supports provided to CPC practices .................................................................. 50
3.5.1. Learning support from CMS ................................................................................. 50
3.5.2. Learning support from other payers ..................................................................... 59
3.5.3. Practices’ use of and perspectives on CPC learning support .............................. 61
v

CONTENTS MATHEMATICA POLICY RESEARCH
4. HOW DID CPC PAYERS, PRACTICES, AND OTHER STAKEHOLDERS WORK
TOGETHER? ................................................................................................................................. 65
4.1. Key takeaways on collaboration among CPC payers and other stakeholders ................. 65
4.2. Methods ............................................................................................................................ 66
4.3. CPC collaborative goals and structure.............................................................................. 66
4.4. Results of CPC collaboration ............................................................................................ 68
4.5. Factors influencing CPC collaboration .............................................................................. 70
4.5.1. Collaboration among non-CMS payers ................................................................ 70
4.5.2. Collaboration between CMS and other payers .................................................... 72
4.5.3. Collaboration between non-CMS payers and other stakeholders ....................... 74
5. HOW DID CPC PRACTICES CHANGE THE WAY THEY DELIVERED CARE THROUGH
WORK ON SPECIFIC MILESTONES? .......................................................................................... 75
5.1. Key findings on practices’ changes in care delivery ......................................................... 75
5.2. Milestones and data sources ............................................................................................ 80
5.2.1. Overview of Milestones ........................................................................................ 80
5.2.2. Data sources ........................................................................................................ 81
5.3. Changes over time in CPC practices’ approaches to primary care delivery..................... 82
5.4. Progress on individual Milestones .................................................................................... 88
5.4.1. Milestone 2: Care management for high-risk patients ......................................... 89
5.4.2. Milestone 3: Access and continuity .................................................................... 104
5.4.3. Milestone 4: Patient experience ......................................................................... 109
5.4.4. Milestone 5: Use data to guide quality improvement ......................................... 113
5.4.5. Milestone 6. Care coordination across the medical neighborhood .................... 120
5.4.6. Milestone 7: Shared decision making ................................................................ 124
5.4.7. Milestone 8: Participation in the CPC learning collaborative ............................. 130
5.4.8. Milestone 9: Health IT ........................................................................................ 130
5.5. Monitoring of adequate Milestone achievement ............................................................. 133
5.5.1. Practices that received CAPs for PY2016 Q1–Q3 performance ....................... 134
5.5.2. Patient dismissal ................................................................................................ 135
5.6. Facilitators of and barriers to implementing changes in care delivery across
Milestones and implications for other care delivery initiatives ........................................ 135
5.6.1. Characteristics of the CPC initiative................................................................... 135
5.6.2. CPC practices’ structure and characteristics ..................................................... 136
5.6.3. Practices’ strategies to implement CPC ............................................................ 137
5.6.4. Factors external to CPC practices ..................................................................... 137
vi

CONTENTS MATHEMATICA POLICY RESEARCH
6. HOW DID CPC AFFECT THE EXPERIENCES OF PHYSICIANS, OTHER CLINICIANS,
AND STAFF? ............................................................................................................................... 143
6.1. Key takeaways on the effect of CPC on physician, other clinician, and staff
experience ....................................................................................................................... 143
6.2. Methods .......................................................................................................................... 144
6.2.1. Survey content and measures ........................................................................... 144
6.2.3. Survey administration ........................................................................................ 148
6.2.4. Survey sample and response rates ................................................................... 149
6.2.5. Analysis .............................................................................................................. 150
6.3. Findings ........................................................................................................................... 152
6.3.1. Burnout ............................................................................................................... 152
6.3.2. Control over work ............................................................................................... 157
6.3.3. Alignment of work with training .......................................................................... 160
6.3.4. Satisfaction with work ........................................................................................ 163
6.3.5. Ratings of CPC in 2016 among CPC practice members ................................... 165
6.4. Discussion ....................................................................................................................... 169
7. HOW DID CPC AFFECT THE EXPERIENCES OF MEDICARE FFS BENEFICIARIES? .......... 171
7.1. Key takeaways on the effect of CPC on the experiences of Medicare
beneficiaries .................................................................................................................... 171
7.2. Methods .......................................................................................................................... 172
7.2.1. Survey content and measures ........................................................................... 172
7.2.2. Survey administration ........................................................................................ 173
7.2.3. Survey sample and response rates ................................................................... 174
7.2.4. Analysis .............................................................................................................. 175
7.3. Results ............................................................................................................................ 176
7.3.1. Composite measures ......................................................................................... 176
7.3.2. Individual questions not in the composite measures ......................................... 180
7.4. Discussion ....................................................................................................................... 185
8. WHAT WERE CPC’S IMPACTS ON MEDICARE EXPENDITURES, SERVICE USE, AND
QUALITY OF CARE DURING THE INITIATIVE? ........................................................................ 187
8.1. Key takeaways on the effect of CPC on Medicare expenditures, service use, and
quality of care .................................................................................................................. 187
8.2. Methods .......................................................................................................................... 191
8.2.1. Comparison group selection .............................................................................. 191
8.2.2. Outcomes ........................................................................................................... 194
vii

CONTENTS MATHEMATICA POLICY RESEARCH
8.2.3. Difference-in-differences estimation strategy .................................................... 196
8.2.4. Statistical power to detect effects ...................................................................... 199
8.2.5. Bayesian analysis .............................................................................................. 200
8.3. CPC-wide results ............................................................................................................ 200
8.3.1. Medicare expenditures ....................................................................................... 201
8.3.2. Service use ........................................................................................................ 225
8.3.3. Claims-based quality of care .............................................................................. 226
8.3.4. Aggregate impacts of CPC for all attributed beneficiaries ................................. 234
8.4. Discussion ....................................................................................................................... 235
9. WERE PRACTICES’ CARE DELIVERY APPROACHES ASSOCIATED WITH
IMPROVED EXPENDITURE AND UTILIZATION OUTCOMES?................................................ 239
9.1. Key takeaways ................................................................................................................ 240
9.2. Findings from previous analyses .................................................................................... 241
9.3. Approach to Year 4 analysis ........................................................................................... 242
9.4. Data and methods ........................................................................................................... 244
9.5. Practice-level model results ............................................................................................ 249
9.6. Beneficiary-level model results ....................................................................................... 250
9.6.1. Overview of results for Years 2, 3, and 4 ........................................................... 251
9.6.2. Results for CPC Year 4 ...................................................................................... 254
9.7. Limitations ....................................................................................................................... 260
9.8. Future work ..................................................................................................................... 261
REFERENCES .......................................................................................................................................... 263
viii

MATHEMATICA POLICY RESEARCH
TABLES
1.1. CPC Milestones for program year (PY) 2016 .................................................................................. 1
1.2. CPC evaluation data sources .......................................................................................................... 5
2.1. Number of non-Medicare CPC payers in 2016, by lines of business included .............................. 14
2.2. Reasons that participating practices left CPC ............................................................................... 18
2.3. Comparison of practices that left CPC and practices that remained throughout CPC .................. 20
3.1. Range of CPC participating payers’ PMPM payments for PY2016 ............................................... 30
3.2. 2016 CPC shared savings methodologies among participating payers ............................................ 35
3.3. Results from CPC Medicare FFS shared savings calculations for performance in PY2014,
PY2015, and PY2016 by region ..................................................................................................... 38
3.4. Payers reporting that CPC generated savings for PY2014 or PY2015, among those
reporting results of their shared savings calculations, by region ................................................... 38
3.5. Timing and structure of aggregated data feedback from payers’ claims data in Colorado,
Ohio/Kentucky, and Oklahoma, PY2016 ....................................................................................... 45
3.6. Content and structure of aligned feedback reports in Arkansas and Oregon, PY2016 ................. 45
3.7. Percentage of practices that reported receiving and reviewing feedback reports and
patient-level data files all or most of the time in 2016, CPC-wide and by region........................... 46
3.8. Description of CPC learning support .............................................................................................. 51
3.9. Planned frequency and mode of individualized practice coaching and the percentage of
practices that reported receiving coaching at their practice site, by region, PY2016 .................... 56
4.1. CPC multistakeholder meeting participants ................................................................................... 67
4.2. Frequency of CPC payer-only and multistakeholder meetings...................................................... 67
4.3. Selected CPC collaborative outcomes, by region .......................................................................... 69
5.1. CPC Milestones for PY2016 .......................................................................................................... 80
5.2. Data sources on CPC practice change practices .......................................................................... 81
5.3. Primary care delivery domains measured by the M-PCMH-A in the CPC practice survey ........... 83
5.4. Types of data used by CPC practices to risk-stratify patients in PY2016, CPC-wide and
by region ........................................................................................................................................ 91
5.5. Average percentage of patients risk-stratified by and receiving care management from
CPC practices at end of PY2016, CPC-wide and by region .......................................................... 93
5.6. Conditions that CPC practices focused on for self-management support, CPC-wide and
by region ........................................................................................................................................ 99
5.7. Percentage of CPC practices with behavioral health specialist(s) co-located within the
practice in PY2016, CPC-wide and by region .............................................................................. 100
5.8. Integration of behavioral health specialists into primary care in PY2016 (among practices
with co-located behavioral health specialists), CPC-wide and by region .................................... 100
ix

TABLES MATHEMATICA POLICY RESEARCH
5.9. CPC practices’ methods of identifying patients for behavioral health services, in PY2016,
CPC-wide and by region .............................................................................................................. 101
5.10. CPC practices’ approaches for providing behavioral health care, in PY2016, CPC-wide
and by region ............................................................................................................................... 101
5.11. CPC practices’ approaches for providing medication management, in PY2016, CPC-wide
and by region ............................................................................................................................... 103
5.12. CPC practices method(s) of identifying patients for medication management, in PY2016,
CPC-wide and by region .............................................................................................................. 104
5.13. Percentage of CPC practices reporting each type of enhanced-access activity, in 2016,
CPC-wide and by region .............................................................................................................. 106
5.14. Percentage of CPC practices choosing various options to elicit patient experiences, CPC-
wide and by region ....................................................................................................................... 110
5.15. Percentage of CPC practices indicating that a survey or PFAC influenced various
practice changes, in PY2016, CPC-wide and by region .............................................................. 110
5.16. Percentages of eCQMs that CPC practices selected for quality improvement activities, in
PY2016, CPC-wide and by region ............................................................................................... 114
5.17. Percentages of CPC practice staff who generate and implement QI ideas and review
data, and intervals for tracking measures and progress, in PY2016, CPC-wide and by
region ........................................................................................................................................... 116
5.18. Percentages of CPC practices that chose CPC’s three care coordination activities, in
PY2016, CPC-wide and by region ............................................................................................... 121
5.19. Shared decision making topics chosen by CPC practices as of Quarter 1, PY2016 .................. 125
5.20. Sources of decision aids used by CPC practices, PY2016, CPC-wide and by region ................ 126
5.21. Reasons for selecting priority shared decision making areas, PY2016, CPC-wide and by
region ........................................................................................................................................... 126
5.22. Identification of eligible patients for shared decision making and documentation of shared
decision making, CPC-wide and by region .................................................................................. 128
5.23. Number of practices placed on a CAP for PY2016 performance ................................................ 134
5.24. Percentages of the 40 practices receiving CAPs by Milestone ................................................... 134
5.25. Facilitators of, and barriers to, implementation of CPC Milestones for PY2016, as
reported by deep-dive practices ................................................................................................... 141
6.1. Questions and domains included in this chapter ......................................................................... 147
6.2. Sample sizes and weighted survey response rates for the primary care clinician and
practice staff surveys, by round ................................................................................................... 149
7.1. Experiences included in the patient survey composite measures ............................................... 172
7.2. Number of questions that gauge patient experience in survey .................................................... 173
7.3. CPC patient survey rounds and fielding dates ............................................................................. 174
7.4. Percentage of Medicare FFS beneficiaries giving the top-box response for five composite
measures, CPC-wide, 2013 and 2016 ......................................................................................... 179
x

TABLES MATHEMATICA POLICY RESEARCH
7.5. Meaningful differences in Medicare FFS beneficiaries’ ratings of practices by select
practice and beneficiary characteristics, among CPC and comparison practices
combined, 2016 ............................................................................................................................ 180
7.6. The proportion of Medicare FFS beneficiaries giving the best response to 28 survey
questions not included in the composite measures, CPC and comparison practices, CPC-
wide .............................................................................................................................................. 183
8.1. Percentage impacts on Medicare FFS expenditures and service utilization over the four
years of CPC (all attributed beneficiaries) ................................................................................... 190
8.2. CPC regions and external comparison group regions ................................................................. 191
8.3. Regression-adjusted means and difference-in-differences estimates of CPC’s impact on
expenditure and utilization measures among attributed Medicare FFS beneficiaries,
annual and four-year cumulative CPC-wide estimates ................................................................ 205
8.4. Breakdown of savings in total Medicare FFS expenditures per beneficiary per month, by
service category ........................................................................................................................... 212
8.5. Regression-adjusted means and difference-in-differences estimates of CPC’s impact on
Medicare FFS expenditures, hospitalizations, and outpatient ED visits, cumulative four-
year estimates, by region ............................................................................................................. 214
8.6. Probability that CPC achieved savings, by year, based on a Bayesian analysis ........................ 219
8.7. Estimates of the cumulative impact on PBPM Medicare expenditures without fees, from
sensitivity tests ............................................................................................................................. 221
8.8. Variation in cumulative impact on PBPM Medicare expenditures without fees, by practice
characteristics at baseline ............................................................................................................ 224
8.9. Regression-adjusted means and estimated difference-in-differences estimates of CPC’s
impact on selected quality-of-care process and outcome measures: annual and four-year
cumulative CPC-wide estimates ................................................................................................... 229
8.10. Aggregate CPC-wide results, by year and cumulative ................................................................ 235
9.1. Key primary care delivery approaches from the M-PCMH-A ....................................................... 246
9.2. Mean scores (out of a maximum of 12) by year of key primary care delivery approaches ......... 247
9.3. Variation of key primary care delivery approaches across CPC practices in Year 4 .................. 254
9.4. Associations of key primary care delivery approaches with annual per beneficiary
outcomes in Year 4 ...................................................................................................................... 255
9.5. Relationships of key primary care delivery approaches with annual per beneficiary
outcomes for high-risk beneficiaries in Year 4 ............................................................................. 257
9.6. Variation in the associations between key primary care delivery approaches and annual
per beneficiary outcomes with clinical involvement of nonphysicians, Year 4 ............................. 259
xi
This page has been left blank for double-sided copying.

MATHEMATICA POLICY RESEARCH
FIGURES
1.1. CPC implementation timeline ........................................................................................................... 4
2.1. CPC regions, non-Medicare payers, practices, and patients......................................................... 11
2.2. Participation of self-insured clients in CPC in 2016 ....................................................................... 15
2.3. Change in the number of CPC participating practices ................................................................... 18
3.1. Median CPC funding per practice, CPC-wide and by region ......................................................... 31
3.2. Median CPC funding per clinician, CPC-wide and by region......................................................... 31
3.3. Median attributed patients per practice and median CPC funding per attributed life, CPC-
wide and by region (excluding Oklahoma*) ................................................................................... 32
3.4. Practices’ perceptions in PY2016 of Medicare and non-Medicare shared savings
methodologies for assessing PY2015 performance ...................................................................... 36
3.5. Practices’ perceived adequacy of Medicare FFS care management fees relative to the
costs of implementing CPC in PY2014, PY2015, and PY2016 ..................................................... 39
3.6. Practice-reported total CPC spending in PY2014, PY2015, and PY2016 for selected cost
categories, in millions ..................................................................................................................... 40
3.7. Percentage of practices that reported receiving and reviewing CPC data feedback all or
most of the time, PY2014 and PY2016 .......................................................................................... 46
3.8. Percentage of practices reporting that CPC data feedback was somewhat or very useful,
among practices that reported seeing the feedback, PY2014 and PY2016 .................................. 48
3.9. Percentage of CPC clinicians and staff receiving various types of CPC assistance during
the past six months, 2016 .............................................................................................................. 53
3.10. Number and format of CPC group learning activities, PY2013 through PY2016 .......................... 54
3.11. Percentage of system-owned practices reporting staff in the practice site or their larger
health care organization communicate with RLF, in 2016 ............................................................. 57
3.12. Percentage of practices reporting interacting with their RLF at least once a month,
PY2014–PY2016, CPC-wide and by region .................................................................................. 58
3.13. Percentage of practices reporting they received coaching or assistance from non-
Medicare payers in the past six months, CPC-wide and by region, PY2016 ................................ 60
3.14. Percentage of CPC practices rating their RLF as excellent or very good in meeting their
CPC-related needs, in PY2014 and PY2016, by region ................................................................ 61
3.15. Reports of how useful various types of CPC assistance were to CPC practices, among
those that received the assistance, 2016....................................................................................... 62
5.1. CPC practices’ mean 2012 M-PCMH-A scores, with 2014, 2015, and 2016 gains, for the
seven domains and overall ............................................................................................................ 85
5.2. CPC and comparison practices’ mean M-PCMH-A scores in 2016, for the seven domains
and overall ...................................................................................................................................... 86
xiii

FIGURES MATHEMATICA POLICY RESEARCH
5.3. Distribution of CPC and comparison practices’ overall M-PCMH-A scores over time ................... 87
5.4. CPC practices’ average overall M-PCMH-A scores, for all practices and by practices’
2012 score ..................................................................................................................................... 88
5.5. Percentages of physicians who agree or strongly agree with statements about quality
improvement, CPC and comparison practices, 2016 .................................................................. 117
5.6. Data sharing by practices that are in a health care system or group .......................................... 132
5.7. Data sharing by practices that are not part of a system .............................................................. 132
6.1. Percentage of physicians reporting high levels of burnout, CPC and comparison
practices, 2013 and 2016 ............................................................................................................. 153
6.2. Percentage of physicians reporting how often they felt burned out from their work in the
past year, CPC and comparison practices, 2013 and 2016 ........................................................ 154
6.3. Percentage of physicians who say they agree with the statement on burnout once or
more per week, CPC and comparison practices, 2016 ............................................................... 155
6.4. Physician scores on a subset of the Maslach Burnout Inventory scales (0 = more burnout,
1 = less burnout), CPC and comparison practices, 2013 and 2016 ............................................ 156
6.5. Percentage of CPC practice members reporting high levels of burnout, 2013 and 2016 ........... 157
6.6. Percentage of physicians reporting great or moderate control over various aspects of
their work, CPC and comparison practices, 2016 ........................................................................ 159
6.7. Overall scores on the control-over-work summary composite for CPC practice members,
2013 and 2016 ............................................................................................................................. 160
6.8. Proportion of time each week that physicians do work that is well-matched to their
training, CPC and comparison practices, 2013 and 2016 ........................................................... 161
6.9. Percentage of CPC practice members saying that 75 percent or more of their time is
spent doing work that is well-matched to their training, 2016 ...................................................... 162
6.10. Extent of physician agreement with statement, “Overall I am satisfied with my current
job,” CPC and comparison practices, 2013 and 2016 ................................................................. 163
6.11. Extent of agreement with statement, “Overall I am satisfied with my current job,” by CPC
practice members, 2013 and 2016 .............................................................................................. 164
6.12. CPC physician reports of how CPC participation changed the quality of care or service
provided to patients, 2016 ............................................................................................................ 165
6.13. CPC practice members’ reports of how much they would support or oppose their
practice’s participation in CPC if they could do it all over again, 2016 ........................................ 166
6.14. Percentage of CPC physicians reporting each factor as a main reason for supporting
participation in CPC, among those that would support participating again, 2016 ....................... 167
6.15. Percentage of CPC physicians reporting each factor as a main reason for opposing
participation in CPC, among those that would oppose participating again, 2016........................ 169
7.1. Percentage of Medicare FFS beneficiaries giving the best response in 2013 and 2016, for
five composite measures, CPC and comparison practices, CPC-wide ....................................... 177
xiv

FIGURES MATHEMATICA POLICY RESEARCH
7.2. Percentage of Medicare FFS beneficiaries answering with the best response, by
composite measure, CPC and comparison practices, CPC-wide ............................................... 178
7.3. Percentage of Medicare FFS beneficiaries giving the best response in 2016, CPC and
comparison practices, CPC-wide ................................................................................................. 182
8.1. Estimated impact on Medicare FFS expenditures without care management fees, by year ....... 202
8.2. Regression-adjusted mean Medicare Part A and Part B expenditures PBPM, excluding
Medicare’s CPC care management fees, all beneficiaries, CPC-wide ........................................ 203
8.3. Estimated impact on Medicare Part A and Part B expenditures PBPM, excluding CPC
care management fees, all beneficiaries, CPC-wide ................................................................... 204
8.4. Probability that CPC achieved savings (before fees) during initiative ......................................... 218
9.1. Distribution of changes in outcomes among CPC practices between the year before CPC
(baseline) and Year 4 ................................................................................................................... 239
9.2. Distribution of scores by year for key primary care delivery approaches .................................... 247
9.3. Associations of improvements in continuity, after-hours access, and follow-up after acute
care with changes in practice-level service use and expenditures in Years 2, 3, and 4 ............. 250
9.4. Associations between primary care delivery approaches and outcomes for Years 2, 3,
and 4 ............................................................................................................................................ 251
9.5. Predicted associations between primary care delivery approaches and outcomes, at
average-risk and high-risk HCC values for Years 2, 3, and 4 ......................................................... 252
9.6. Predicted associations between key primary care delivery approaches and outcomes for
practices with 25th percentile (10) and maximum (12) levels of clinical involvement of
nonphysicians for Years 2, 3, and 4 ............................................................................................... 253
xv
This page has been left blank for double-sided copying.

MATHEMATICA POLICY RESEARCH
EXECUTIVE SUMMARY
In October 2012, the Center for Medicare & Medicaid Innovation (CMMI) of the Centers
for Medicare & Medicaid Services (CMS) launched the four-year Comprehensive Primary Care
(CPC) initiative. The goal of CPC was to improve primary care delivery, health care quality, and
patient experience, and lower costs. CPC also aimed to enhance clinicians’ and staff members’
experience. CMS leveraged the support of 39 other public and private payers to target the
transformation of primary care delivery in nearly 500 primary care practices in seven regions
across the United States. These practices included more than 2,000 clinicians and served around
3 million patients.
CPC required practices to transform across five key care delivery functions: (1) access and
continuity, (2) planned care for chronic conditions and preventive care, (3) risk-stratified care
management, (4) patient and caregiver engagement, and (5) coordination of care across the
medical neighborhood. CMS specified a series of Milestones to help move practices along the
path of implementing the five functions, and it updated the requirements for each Milestone
annually to build on practices’ progress in the prior year. CMS assessed whether practices met
targets set within the Milestones, which were considered minimum requirements to remain in the
initiative. Although the CPC Milestones overlap with many of the activities typically included in
existing patient-centered medical home (PCMH) recognition programs, CPC did not require
practices to have or obtain PCMH recognition, although nearly 40 percent did have this
recognition when they applied to CPC
. CPC supported practices’ transformation with: (1)
prospective care management fees and the opportunity to earn shared savings in addition to their
usual payments; (2) data feedback on cost, utilization, and quality; and (3) learning support.
1
This fourth and final report to CMS covers the full CPC intervention period (October 2012
through December 2016). The report examines: (1) who participated in CPC; (2) the supports
practices received; (3) how practices implemented CPC and changed the way they delivered
health care; (4) the impacts of CPC on clinicians’ and staff members’ experience; and (5) the
impacts of CPC on patient experience, cost, service use, and quality-of-care outcomes for
attributed Medicare fee-for-service (FFS) beneficiaries. (See Taylor et al. 2015, Peikes et al.
2016a, and Peikes et al. 2016b, respectively, for results from the first three years of the
initiative.)
This Executive Summary provides a brief overview of findings and a more detailed
summary of findings in each chapter of the final report.
1
The CPC change package (https://innovation.cms.gov/Files/x/cpcidiagram.pdf) describes the underlying logic of
CPC, including the primary and secondary drivers to achieve the aims of CPC and the concepts and tactics that
support the changes.
xvii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
OVERVIEW OF FINDINGS
Effects on Outcomes for Attributed Medicare FFS Beneficiaries
• CPC reduced hospitalizations and emergency department (ED) visits for Medicare
FFS beneficiaries attributed to the CPC practices more than for beneficiaries
attributed to comparison practices.
- The rate of ED visits and hospitalizations for Medicare FFS beneficiaries grew for both
CPC and comparison practices, but growth in ED visits and hospitalizations was 2
percent less for attributed beneficiaries in CPC practices than for those in comparison
practices. The favorable difference for ED visits was more pronounced in the last two
years of CPC.
• Medicare expenditures for attributed beneficiaries grew less for CPC practices than
for comparison practices, but the savings were not enough to cover Medicare’s CPC
care management fees.
- Medicare expenditures without factoring in CPC care management fees increased for
both CPC and comparison practices, but the increase was $9 per beneficiary per month
(PBPM) (1 percent) less for Medicare FFS beneficiaries attributed to CPC practices than
for beneficiaries attributed to comparison practices. This difference was not statistically
significant, and estimated effects became less pronounced over time.
- After including care management fees, Medicare expenditures increased by $6 PBPM
more for CPC practices than for comparison practices. The difference was not
statistically significant.
- There is a 94 percent probability that CPC generated some reduction in Medicare
expenditures (excluding the care management fees) during the intervention period.
However, our analysis indicates the likelihood that those savings were greater than the
average $15 PBPM fee that Medicare paid over the four years is less than 1 percent. In
other words, although CPC did reduce Medicare Part A and B expenditures slightly
relative to expenditures in comparison practices, it is highly unlikely that these Medicare
savings generated by CPC were enough to cover the CPC care management fees
Medicare paid.
• CPC had minimal effects on the limited claims-based quality-of-care process and
outcome measures examined.
- Differences on most claims-based quality-of-care measures for Medicare beneficiaries
were not statistically significant over the course of CPC, except for a small (3 percent)
reduction in the likelihood of an ED revisit within 30 days of an outpatient ED visit
relative to the comparison group.
• CPC had little impact on beneficiaries’ experience of care.
- Findings for Medicare FFS beneficiaries in CPC and comparison practices were
comparable on most measures of patient experience, except for an increase in
transitional care for beneficiaries in CPC practices.
xviii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
Effects on Care Delivery
• Practices engaged in substantial, challenging transformation and improved how they
delivered care over the course of CPC.
- Overall, the largest areas of improvement were in risk-stratified care management,
expanded access to care, and continuous improvement driven by data. Based on data
from the annual practice survey, CPC practices’ approach to risk-stratified care
management was more advanced than that of comparison practices. CPC practices’
approaches to other aspects of care delivery were slightly more advanced than those of
comparison practices.
- Practices faced barriers to change, including burden associated with quality monitoring
and reporting for CMS and other payers, existing incentives in the FFS payment system
that encourage volume of services over efficient use of services, and lack of
infrastructure for comprehensive and efficient health information exchange between
providers.
Effects on Clinician and Staff Experience
• Clinicians and staff in CPC practices had largely favorable views of CPC. Although
CPC required an intensive amount of work for practices, it did not affect burnout,
control over work, alignment of work with training, and job satisfaction among
clinicians and staff, favorably or unfavorably.
- Eighty percent of responding physicians at CPC practices reported that CPC had
improved the quality of care or service they provide to their patients, and if they could
do it again, 79 percent would support their practice’s participation in CPC. Only 12
percent of physicians would oppose participation in CPC and 9 percent reported not
knowing enough about CPC to answer. However, physicians at CPC practices,
regardless of whether they would support their practice’s participation in CPC, indicated
that CPC administrative reporting presented a burden and that the transformation work
in CPC was difficult (reported by 44 and 34 percent of physicians that would support
CPC participation, respectively).
Supports Provided to Practices
• CMS and other participating payers provided substantial support for CPC practices
and, in general, practices found these supports helped them accomplish the required
work.
- Medicare FFS and other payers prospectively paid care management fees to practices
totaling $479 million over the four-year initiative. Medicare FFS paid 58 percent of the
total care management fees to CPC practices. Other payers contributed the remaining
care management fees. In the final year of CPC (2016), this funding translated to a
median of $179,519 per practice ($50,189 per clinician). These payments accounted for
a median of 10 percent of 2016 practice revenue.
- CMS and 32 of the 36 non-CMS payers that participated throughout the initiative
provided data feedback to practices; depending on the payer, the feedback included cost,
utilization, and/or quality data reported at the practice level, patient level, or both. Payers
in three regions—Colorado, Ohio/Kentucky, and Oklahoma—achieved data aggregation,
xix

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
producing a single tool that aggregated data across payers each quarter (non-Medicare
FFS payers aggregated data first in late 2015 or 2016 and then Medicare FFS integrated
its data into those efforts in mid- to late-2016).
- CMS and its contractors provided practices with a variety of group learning activities,
including webinars and all-day, in-person meetings. Regional learning faculty (RLF)
also provided individualized coaching to practices they identified as needing additional
support. On the 2016 CPC practice survey, 56 percent of practices reported that they had
received in-person coaching at their practice site from RLF in the prior six months.
Participation
• Payer and practice participation remained relatively stable throughout the initiative.
- Only three small payers left CPC during the initiative, and by the end of the initiative,
439 (or 87 percent) of the original 502 practices were still participating. Most
commonly, practices that left the initiative did so to join Medicare accountable care
organizations (ACOs), because CMS did not permit practices to participate in CPC and a
Medicare ACO.
- Many of the payers and practices that participated in CPC are participating in
Comprehensive Primary Care Plus (CPC+), another primary care model that began in
January 2017 and builds on lessons learned from CPC and other PCMH models. Of the
36 payers that remained in CPC throughout the initiative, 28 joined CPC+. Moreover,
412 of the 422 practices that remained through the end of CPC and were located in
CPC+ regions (as well as 15 of the 57 practices that withdrew or were terminated from
CPC for reasons other than closing and were located in CPC+ regions) decided to join
CPC+.
In the rest of this executive summary, we provide a detailed summary of the key results for
each chapter of this report.
DETAILED SUMMARY OF FINDINGS
ES.1. Participation remained relatively stable (Chapter 2)
Payer participation. CMS and 39 other payers, which included private health plans in all
CPC regions and state Medicaid agencies in five regions, committed substantial public and
private resources to redesign primary care in CPC’s seven regions.
2,3
Over the course of CPC,
payer participation remained steady in all seven regions—only three small payers left CPC
during the initiative (Figure ES.1).
4
In general, payers remained engaged in and committed to the
2
Payers that participated in more than one region were counted separately for each region in which they
participated. At the start of CPC, 31 distinct payers participated in CPC in addition to Medicare.
3
New Jersey and New York were the two regions whose Medicaid agencies did not participate. In addition, the
Oklahoma Health Care Authority participated in the Oklahoma region and was counted as a Medicaid participating
payer, although it did not provide care management fees to participating practices.
4
In addition to withdrawals, one participating payer acquired another participating payer (thus subtracting one payer
from the total). Moreover, one national payer that was participating in two CPC regions joined in a third region
during the first year of the initiative (thus adding one payer to the total).
xx

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
initiative—most reported sustained or increasing commitment to primary care redesign and to
alternative payment more generally during interviews conducted in the summer and early fall of
each program year. Demonstrating their continued interest, 28 of the 36 payers that remained in
CPC throughout the initiative also joined CPC+, which CMS launched in January 2017 and
builds on lessons learned from CPC.
Participating payers included most of their lines of business in CPC, but varied on whether
they included self-insured clients. Many of the 26 payers with self-insured clients initially
struggled to enroll these clients in CPC. Through concerted efforts to engage self-insured clients,
the number of payers reporting that all or nearly all of their self-insured clients participated in
CPC doubled from 7 to 14 during the initiative.
Practice participation. CMS selected 502 diverse practices to participate in CPC. These
practices included independent and system-owned practices, some practices that were recognized
as medical homes and others that were not, and practices of different sizes. Practice participation
remained relatively stable throughout the initiative—only 1.8 percent (9 practices) were
terminated from the initiative and another 11.2 percent (56 practices) voluntarily withdrew.
Among practices that withdrew, the most common reason was to join a Medicare ACO, since
CMS did not allow concurrent participation in CPC and any of its shared savings models (29 of
the 56 practices), or due to challenges meeting CPC requirements (13 practices). In addition, 5
practices voluntarily withdrew after assessing the terms and conditions of CPC participation
early in the initiative, and 6 practices closed.5 By the end of the initiative on December 31, 2016,
439 practices remained in CPC. Of the 422 of these practices located in CPC+ regions, 412 (98
percent) applied and were selected by CMS to join CPC+, as did 15 of the 57 practices that
withdrew or were terminated from CPC for reasons other than closing.6
Patient participation. CPC was designed to transform whole practices; as such, CPC
practices were expected to deliver the same care to all patients they saw. This included patients
of participating payers that were attributed to their practice (for which practices received care
management fees), patients of participating payers who were not attributed to the practice,
patients of nonparticipating payers, and uninsured patients. The numbers of attributed and total
patients were substantial, with attributed patients estimated at 1.1 million (321,000 of these were
Medicare FFS beneficiaries), and total patients estimated at 3.1 million across all participating
practices in 2016.
5
Several CPC practices also changed their composition during the initiative. Five CPC practices each split into two
practices (adding five CPC practices to the total count). Three CPC practices merged with other CPC practices
(subtracting three CPC practices from the total count).
6
Three counties (Putnam, Rockland, and Westchester counties) that were included in the Capital District-Hudson
Valley Region in New York for CPC were not included in the region for CPC+. Seventeen CPC practices were
located in these counties and thus ineligible to apply for CPC+.
xxi

xxii
Figure ES.1. CPC regions, Non-Medicare payers, practices, and patients

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
ES.2. CPC delivered substantial financial support, data feedback, and
learning supports to practices
(Chapter 3)
CMS and other participating payers provided significant support to CPC practices and, in
general, practices found that these supports helped them accomplish the work required for CPC.
In addition to traditional payments, Medicare FFS and other payers provided substantial non-
visit-based financial support for participating practices. CMS and most other payers also
provided data feedback to participating practices and, in five regions, payers aligned or
aggregated that data feedback across payers in the region. Many practices considered CPC’s data
feedback useful, but some found it challenging to incorporate into their improvement efforts.
Many practices also considered CPC learning supports, which included group learning activities
and—for a subset of practices—individualized coaching, important for achieving the aims of
CPC.
Financial supports. Medicare FFS and all but 2 of the 36 other participating payers that
remained throughout the initiative provided monthly, non-visit-based care management fees to
CPC practices, in addition to usual payments for services, to support enhanced, coordinated
care.
7
CMS care management fees for Medicare FFS attributed beneficiaries averaged $20
PBPM for the first two years of CPC and $15 PBPM for the last two years.
8
CMS paid higher
care management fees in the first two years of the initiative to support upfront investments in
practice transformation. Among other payers, care management fee amounts varied considerably
but most were lower than Medicare FFS amounts.
According to Medicare FFS payment data and practice-reported data on payments received
from other payers, care management fees to practices from Medicare FFS and other payers
translated to a median of $7.95 per member per month (PMPM) per attributed patient (that is, for
patients attributed to practices by CPC payers) or $3.55 PMPM per active patient (that is,
patients attributed by CPC payers and nonattributed patients).
Together, this funding totaled $479.1 million over the
four-year initiative. Reflecting the decrease in care
management fees over time by Medicare and 30 percent of
other payers, the median payments to practices (in addition to
their regular revenues) were higher in 2013 ($227,849) and
2014 ($203,949) than in 2015 ($175,774) and 2016
($179,519). Similarly, median payments per clinician
7
One payer provided capitated payments instead of PMPM payments. One payer did not contribute enhanced
payments to practices.
8
CMS paid $20 PBPM in care management fees during Quarters 1 through 9 of CPC (through December 2014), and
it paid $15 PBPM from January 2015 onward (for the last eight quarters of CPC). Therefore, over the 17 quarters of
CPC, the average PBPM care management fee paid for patients still attributed to a practice was approximately $18.
However, the average PBPM fee received in the intent-to-treat (ITT) analysis sample used to estimate the effects of
CPC was $15, because we retained all beneficiaries after they were first attributed, even if a practice did not receive
fees for them because they were no longer attributed.
For the median practice, CPC
care management fees totaled
more than $175,000 per year,
in addition to their regular
revenues. This represented
more than $50,000 per clinician
per year.
xxiii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
decreased from $70,045 in 2013 to $50,189 in 2016.
9
Depending on the year, these payments
accounted for 10 to 20 percent of practice revenue. Medicare and about two-thirds of other
participating payers also provided practices the opportunity to share in any savings accrued
during each of the last three years of the initiative (program year [PY] 2014, PY2015, and
PY2016). Medicare FFS calculated savings at the regional level (that is, it compared total costs
of attributed Medicare FFS beneficiaries across all CPC practices in a region to an expenditure
target); other payers that offered the opportunity of shared savings calculated savings in a variety
of ways (including at the regional, system, or practice level, or among groups of unaffiliated
practices). CMS’s shared savings calculations serve a different purpose than the evaluation and,
as such, use a different approach.
10
Medicare FFS and other payers reported the following shared savings results:
• For PY2014 performance, Medicare FFS found that CPC generated savings in one region—
Oklahoma. Medicare FFS shared savings payments to Oklahoma practices totaled $658,129.
Across all regions, 10 of the 20 non-Medicare FFS payers that reported results of their
shared savings calculations for PY2014 found that CPC generated savings.
• For PY2015 performance, Medicare FFS found that CPC generated savings in four
regions—Arkansas, Colorado, Oklahoma, and Oregon. Medicare FFS shared savings
payments to practices in these regions totaled more than $13 million. Across all regions, 10
of the 15 non-Medicare FFS payers that reported results of their shared savings calculations
for PY2015 found that CPC generated savings.
• For PY2016 performance, Medicare FFS found that CPC generated savings in two
regions—Arkansas and Oklahoma. Medicare FFS shared savings payments to practices in
these regions totaled more than $10 million. Non-Medicare payers did not report results for
this performance year in time for inclusion in this report.
More than three-quarters of practices reported on the CPC practice surveys in 2014, 2015,
and 2016 that CPC payments—including care management fees and, when relevant, shared
savings payments—were adequate or more than adequate relative to the costs of implementing
CPC.
Data feedback. By PY2015, CMS and 32 of the 36 non-CMS payers participating at that
time provided data feedback to practices. At the outset of CPC, payers primarily provided
practices with individual payer reports.
11
The payers designed the content and structure of this
feedback individually, often based on data feedback they were already providing to practices
before CPC. Largely in response to practices’ input on data feedback, most payers took steps
9
Payments in PY2013 were higher than in PY2014 because PY2013 included several months of CMS payments in
late 2012.
10
See Chapter 3 for information on Medicare and other payers’ shared savings methodologies for CPC. See Chapter
8 for information on the methodology used for and results from the CPC impact evaluation.
11
As part of its evaluation contract, Mathematica Policy Research produced the Medicare FFS data feedback reports
and patient-level data files for CMS.
xxiv

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
over the four-year initiative to provide new or additional forms of data feedback to practices or to
improve existing reports.
In addition, by the end of CPC, payers in five
regions were using a common approach to data feedback.
Payers in Arkansas and Oregon took steps to align the
cost and service use measures included in individual
payer feedback reports with each other and with
Medicare FFS. Payers in Colorado, Ohio/Kentucky, and
Oklahoma achieved data aggregation—producing a
single tool to aggregate data across payers each quarter
(non-Medicare FFS payers aggregated data first in late
2015 or 2016 and then Medicare FFS integrated its data
into those efforts in mid- to late-2016).
Although most practices reported that they had reviewed data feedback, the frequency with
which practices reviewed reports varied by report type and over time (Figure ES.2). During
interviews with deep-dive practices—a set of 21 CPC practices selected for intensive study
throughout the initiative—some of these practices reported that they had used CPC data feedback
to identify goals for their quality improvement (QI) work or to improve identification of high-
risk patients.
Figure ES.2. Percentage of practices that reported receiving and reviewing
CPC data feedback all or most of the time, PY2014 and PY2016
Source: CPC practice survey, administered April through July 2014 and April through August 2016.
Through interviews with deep-dive practices and CMS contractors, and surveys of CPC
practices and clinicians, we identified several challenges practices faced using data feedback.
Some practices:
18
28
16
23
22
29
15
16
55
53
31
35
26
32
23
25
0
20
40
60
80
100
2014 2016 2014 2016 2014 2016 2014 2016
Feedback reports Patient-level data Feedback reports Patient-level data
From Medicare FFS From other payers
Percentage of practices
Always
Most of the time
From Medicare FFS From other payers
Medicare and almost all other
participating payers provided
practices with data feedback during
CPC.
While important progress was made
on providing feedback and refining it
over time, areas for improvement
include educating practices on using
feedback and making feedback
more timely and actionable.
xxv

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
• Reported that only staff at the health system level (and not the practice level) reviewed
feedback reports; this was reported by health system-owned practices
• Viewed data feedback as complex and difficult to understand
• Lacked the time or skills to use data feedback effectively
• Viewed factors driving high costs as out of their control
Learning support. CMS contracted with TMF Health
Quality Institute to provide learning supports to CPC practices.
CMS, TMF, and its subcontractors—referred to as regional
learning faculty (RLF)—provided practices with a variety of
group learning activities, including webinars and all-day, in-
person meetings. CMS and its contractors adapted learning
activities over time to encourage additional peer-to-peer
learning, emphasize the use of data for practice improvement, and engage practices in
implementing small tests of change.
RLF also had limited resources to provide a subset of practices with individualized
coaching. RLF selected practices to receive this coaching and adjusted the intensity depending
on practices’ progress toward CPC Milestones and their performance on quarterly Medicare
feedback reports. On the 2016 CPC practice survey, 56 percent of participating practices
reported that they had received in-person coaching at their practice site from RLF in the prior six
months.
According to the 2016 CPC practice survey, non-Medicare payers also provided coaching or
assistance to 71 percent of participating practices in the prior six months. The percentage that
reported that they had received this assistance varied considerably by region, from 52 percent of
practices in Oregon to 96 percent in Ohio/Kentucky.
In 2016, 75 percent of practices CPC-wide rated their RLF as excellent (40 percent) or very
good (35 percent) at meeting their CPC-related needs. The proportion of practices that rated their
RLF as excellent, however, varied across regions and over time (Figure ES.3). Most notably,
RLF in Colorado consistently received some of the highest ratings, whereas New York RLF
generally received some of the lowest ratings, though their ratings still were fairly high.
Practices found that in-person learning activities and opportunities for peer-to-peer learning
were the most valuable forms of learning support, according to the practice survey and
interviews with deep-dive practices. Although practices valued learning, deep-dive practices also
indicated that finding time to participate in learning activities was challenging and some
activities (in particular, webinars) were repetitive or were not tailored to meet different practice
needs. Practices also noted that electronic health record (EHR) vendors did not participate in
learning activities and this limited practices’ ability to resolve EHR-related issues.
Although CPC provided a
variety of learning supports,
participating practices
found in-person learning
activities and opportunities
for peer-to-peer learning to
be the most valuable.
xxvi

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
Figure ES.3. Percentage of CPC practices that rated their RLF as excellent or
very good at meeting their CPC-related needs, in PY2014 and PY2016, by
region
Source: CPC practice survey, administered April through July 2014 and April through August 2016.
ES.3. CMS and other payers formed collaborative relationships with each
other and with practices and other stakeholders to implement CPC
(Chapter 4)
CPC was one of the largest and most substantial multipayer initiatives ever tested. For the
initiative, CMS, state Medicaid agencies, and private payers committed to providing practices
enhanced payment to promote comprehensive primary care. Payers also agreed to work together
to develop an approach to align and coordinate data feedback for participating practices. This
work required a tremendous amount of coordination and collaboration among participating
stakeholders. By bringing together payers and other stakeholders, CPC enabled payers to
accomplish several collaborative outcomes, including aligning quality measures, goals, and
financial incentives; coordinating common approaches to data feedback; and coordinating CPC
with other regional efforts.
Most payers remained committed to CPC and actively engaged in collaborative discussions
for the duration of the initiative. Payers generally reported that they established productive,
positive working relationships with other payers in their region. According to payers, the
following factors facilitated collaboration: prior experience working together, strong facilitation
by a neutral payer convener, and leadership from payer champions who spearheaded
collaborative efforts and encouraged other payers to commit the needed time and resources to
accomplish goals.
From the start of the initiative, CMS encouraged payers to engage practices and patients or
patient advocacy groups in their collaborative work and, by PY2015, multistakeholder meetings
were the most common forum for discussing CPC. Most payers valued the opportunity to discuss
CPC with practices, and to hear more directly about the challenges and successes that practices
experienced in implementing the CPC requirements and transforming care. However, in several
regions, payers reported that active, sustained practice engagement in multistakeholder meetings
35
35
28
32
28
38
21
38
38
34
45
32
40
48
39
25
37
40
43
53
62
51
59
42
23
14
32
49
17
33
24
37
0
20
40
60
80
100
2014 2016 2014 2016 2014 2016 2014 2016 2014 2016 2014 2016 2014 2016 2014 2016
CPC-wide AR CO NJ NY OH/KY OK OR
Percentage of practices
Very good Excellent
xxvii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
was difficult to attain. Payers indicated that
multistakeholder meetings would have been more useful
if they had more clearly delineated goals for
engagement, focused on engaging stakeholders with the
time and skills needed to contribute to discussions, and
worked to build trust among payers and other
stakeholders earlier in the initiative.
Most payers viewed CMS as a critical partner in efforts
to transform primary care, recognizing its role in
encouraging practices to participate in transformation efforts and bringing additional financial and
technical support to each region. However, CMS’s dual role as initiative convener and
participating payer at times made collaboration challenging. CMS was able to build trust with other
payers over time by clarifying which parts of CPC could be adapted to regional contexts and
deferring to other payers for these decisions, increasing opportunities for payers to meet with CMS
representatives, and committing to build on the successes and lessons of CPC in CPC+.
ES.4. CPC changed how participating practices delivered care (Chapter 5)
CPC required participating practices to make many complex, interconnected changes in how
they deliver care to their patients, by focusing on five key functions: (1) access and continuity,
(2) planned care for chronic conditions and preventive care, (3) risk-stratified care management,
(4) patient and caregiver engagement, and (5) coordination of care across the medical
neighborhood. To promote progress on these functions, CMS specified a series of Milestones at
the start of CPC, and updated the Milestone requirements annually to build on practices’
progress in the prior year (Table ES.1). Some Milestones straddle more than one function.
Table ES.1. CPC Milestones for program year (PY) 2016
1. Budget. Complete an annotated annual budget with PY2015 revenues/expenses and projected
CPC initiative practice revenue flow for PY2016 at the start of the year and report actual
revenue/expenses for PY2016 at the end of the year.
2. Care management for high-risk patients. Maintain at least 95 percent empanelment to provider
and care teams. Continue to risk-stratify all patients, maintaining risk-stratification of at least 75
percent of empanelled patients. Expand care management activities for highest risk patients who
are likely to benefit from longitudinal care management and those not otherwise at high risk but
requiring episodic care management. Provide information about the care plans that are used for
both longitudinal care management and episodic care management. Maintain the implementation of
and further refine one of three strategies (behavioral health integration, medication management, or
self-management support).
3. Access by patients and enhanced access. Enhance patients’ ability to communicate 24 hours a
day, 7 days a week with a care team that has real-time access to their electronic medical records.
Continue to implement asynchronous forms of communication (for example, patient portal and
email) and ensure timely responses. Measure continuity of care by measuring visit continuity
quarterly for each provider and/or care team in the practice.
4. Patient experience. Assess patient experience through patient surveys or patient and family
advisory council meetings and communicate to patients (using electronic, poster, pamphlet, or
similar communication methods) about resulting changes the practice is making.
For successful multipayer
collaboration, it is important to clarify
the role of various stakeholders and
build trust among participants.
Adapting an initiative to regional
contexts, when possible, is also
useful in building support and gaining
buy-in from regional stakeholders.
xxviii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
Table ES.1 (continued)
5. Quality improvement. Continue to perform continuous quality improvement using electronic health
record (EHR)-based clinical quality measures (eCQMs) on at least three of the measures that
practices report annually. Review at least one payer data feedback report (CMS Practice Feedback
Report or other payers’ reports) to identify a high-cost area and a practice strategy to reduce this
cost while maintaining or improving quality.
6. Care coordination across the medical neighborhood. Track patients by implementing two of
three options: follow up via telephone with patients within one week of emergency department (ED)
visits; contact at least 75 percent of hospitalized patients within 72 hours of discharge; and enact
care compacts with at least two groups of high-volume specialists.
7. Shared decision making. Use at least three decision aids to support shared decision making
(SDM) for three preference-sensitive conditions and track patient eligibility for and use of the aids.
8. Participating in learning collaborative. Participate in regional and national learning offerings and
communicate with regional learning faculty.
9. Health information technology (IT). Attest that each eligible professional in the practice is
engaged with and working toward attestation for Stage II Meaningful Use in the timelines set by the
Meaningful Use program.
Source: CPC PY2016 Implementation and Milestone Reporting Summary Guide.
Across the CPC Milestones, multiple data sources
provide clear evidence that practices undertook substantial,
challenging transformation and improved how they delivered
care over the course of CPC. In the first year of CPC
(PY2013), practices worked to set up staffing, initial care
processes, and workflows. In PY2014, practices made
meaningful progress on each CPC Milestone, demonstrating
that they were indeed changing care delivery. PY2015 and
PY2016 brought additional refinements to practices’ care
processes and workflows. Findings across data sources
indicate that CPC practices improved most in their work on risk-stratified care management,
access to care, and continuous improvement driven by data. However, practices faced challenges
in implementing some of the Milestones and, even at the end of the initiative, there continued to
be room for improvement in how the practices, and their patients’ other providers, delivered
care.
Below are key findings about CPC practices’ care delivery approaches from the final year of
CPC and notable changes over the course of the initiative:
• Overall primary care approaches. As measured by the annual practice survey, CPC
practices’ approaches to primary care delivery improved each year of the initiative (Figure
ES.4). Overall scores on the modified PCMH assessment (M-PCMH-A) included in the
survey indicate that CPC practices achieved their largest gains in care delivery between
2012 and 2014. In the final two years of the initiative, they achieved more modest
improvements.
Practices undertook substantial,
challenging transformation and
improved how they delivered
care over the course of CPC.
The largest improvements were
in risk-stratified care
management, access to care,
and continuity of care.
xxix

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
Figure ES.4. CPC practices’ mean 2012 M-PCMH-A scores, with 2014, 2015,
and 2016 gains, for the seven domains and overall
Source: Mathematica analysis of the 2012 CPC practice survey administered October through December 2012, and
the 2014, 2015, and 2016 CPC and comparison practice surveys administered April through July 2014,
April through August 2015, and April through August 2016. We did not administer the 2012 practice survey
to comparison practices.
Note: Scale: 1 [least advanced approach] – 12 [best approach]. We weighted comparison practice responses to
ensure CPC and comparison samples were similar and to adjust for nonresponse.
• Areas of greatest improvement. Between 2012 and 2016, CPC practices had the largest
improvements in risk-stratified care management, access to care, and continuous
improvement driven by data. In the remaining four domains—continuity of care,
coordination of care across the medical neighborhood, planned care for chronic conditions
and preventive care, and patient and caregiver engagement—scores improved to a lesser
extent. Gains in each domain increased most during the first two years of the initiative.
Comparison practices also showed improvements, though to a lesser degree than CPC
practices. In 2016, the last year of CPC, 29 percent of CPC practices had overall scores
indicating the most advanced approaches to care delivery (scores of 10 to 12) compared to
19 percent of comparison practices (Figure ES.5).
6.5
9.6
7.0
7.6
4.6
6.7
6.7
5.8
2.2
0.6
2.6
1.5
5.1
1.2
1.3
2.2
0.5
0.2
0.5
0.4
0.3
0.6
0.5
0.3
0.2
0.2
0.4
0.2
0.1
0.3
0.2
0.4
1
2
3
4
5
6
7
8
9
10
11
12
Overall
M-PCMH-A
score
Continuity of
care
Access to
care
Planned care
for chronic
conditions
and preventive
care
Risk-
stratified
care
management
Patient
and
caregiver
engagement
Coordination
of care
across the
medical
neighborhood
Continuous
improvement
driven by data
Score
[1 (lesat advanced approach)–12 (best approach)]
2012 score 2014 gain 2015 gain 2016 gain
9.4 10.6 10.5 9.7 10.1 8.78.78.8
2016 score
xxx

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
Figure ES.5. Distribution of CPC and comparison practices’ overall M-PCMH-A
scores over time
Source: Mathematica analysis of the 2012 CPC practice survey administered October through December 2012, and
the 2014, 2015, and 2016 CPC and comparison practice surveys administered April through July 2014,
April through August 2015, and April through August 2016. We did not administer the 2012 practice survey
to comparison practices.
Note: Scale: 1 [least advanced approach] – 12 [best approach]. We weighted comparison practice responses to
ensure CPC and comparison samples were similar and to adjust for nonresponse.
• Correlation with practice characteristics. As in prior years, patterns of care delivery
reported on the practice survey by CPC practices in 2016 generally did not correlate with
practice characteristics (such as practice size, practice ownership, rural/urban status, and
how the practice compensated clinicians) or with CPC funding per clinician.
• Types of practices that improved the most. CPC appears to have helped some practices
improve their approaches to care delivery more than others between 2012 and 2016. The
three types of practices that showed the most improvement are those that (1) had lower
scores on the practice survey at baseline, (2) were not a recognized PCMH before CPC, and
(3) were rated in the bottom two-thirds of CMS scores for their application to participate in
CPC (Dale et al. 2016). All three groups had lower average scores in 2012 than CPC
practices overall; therefore, these practices may have achieved larger increases because they
had more room for improvement.
• Care management for high-risk patients (Milestone 2). Increased capacity to provide care
management services to high-risk patients was perceived as the biggest benefit of CPC
participation and was the area of greatest transformation for CPC practices. Most of this
progress occurred between 2012 and 2015.
2
0
0
0
1
0
1
61
7
4
2
32
20
20
34
80
75
69
49
59
60
2
13
20
29
18
20
19
0%
20%
40%
60%
80%
100%
2012 2014 2015 2016 2014 2015 2016
Percentage of practices
1 to <4 (Least advanced approach) 4 to <7 7 to <10 10 to 12 (Best approach)
CPC practices Comparison practices
xxxi

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
- By 2016, CPC practices had stopped making major changes to their risk-stratification
methodologies. Similar to 2015, practices used a combination of data sources to risk-
stratify their patients, most commonly clinical intuition and clinical algorithms.
- In the second half of CPC, practices increasingly integrated care managers’ work with
clinicians, which had been a challenge earlier. Clinicians developed trust in care
managers to handle patient follow-up after observing how care managers’ efforts
improved patients’ adherence to recommended treatments, reduced the need for
clinicians to handle this task, and allowed clinicians to focus on more complex clinical
care.
As in previous years, challenges with care management remained, such as:
Care managers in several deep-dive practices performed numerous tasks in addition
to the activities under Milestone 2. In some cases, this resulted in turnover because
care managers felt overwhelmed.
Although CPC did not require practices to develop or maintain care plans, they were
asked to provide information about care plan use for longitudinal and episodic care
management. The use of care plans remained uneven, and clinicians and care
managers in most deep-dive practices
continued to report limited EHR
functionality for supporting care plans
and care management.
A few respondents were frustrated about
multiple guidelines and different
requirements for care plans from various
payers and medical home initiatives.
Duplication of patient outreach by
practice-based care managers and those
affiliated with hospitals, health systems,
health plans, or visiting nurses
associations continued to confuse patients
and frustrate care managers in both CPC
and comparison practices.
• Behavioral health integration (Milestone 2). To identify patients for behavioral health
support, CPC practices most commonly used screening tools, staff or provider referrals, and
patient self-referrals. They most commonly delivered behavioral health services by
providing (1) referrals to specialty mental health care, (2) primary care management with
referral as needed to specialty mental health care, or (3) co-management between primary
care and specialty mental health care. Practices built internal capacity to provide behavioral
health screening and services: the proportion of practices with behavioral health specialists,
clinical psychologists, or social workers on site increased from 19 percent in 2014 to 29
percent in 2016. However, co-location of such staff varied across CPC regions from 3 to 52
percent. Over half of CPC practices with co-located behavioral health staff reported that
these staff were fully integrated into primary care workflows, shared patient records, and
were available for warm hand-offs and acute primary care visits.
Although CPC practices made
considerable progress in their care
management activities, areas for
continued improvement include:
• Clarifying care manager roles and
responsibilities
• Improving EHR functionality for care
management and care plans
• Streamlining guidelines and
requirements for care plans
• Streamlining patient outreach by
multiple care managers from different
providers and health plans
xxxii
EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
• Access by patients (Milestone 3). To improve access and continuity, most CPC practices
reported in the 2016 practice survey that they offered same- or next-day appointments and
had an on-call clinician available with access to the EHR 24 hours a day, seven days a week.
As in previous years, nearly all CPC practices reported using patient portals to improve
access, partly because the Stage 2 Medicare and Medicaid EHR Incentive Programs
(Meaningful Use) emphasized patient portals. However, in 2016, few Medicare FFS
beneficiaries reported that they used these portals regularly. Practices continued to improve
wait times for appointments; telephone access to the practice; and after-hours access to
clinicians via email, telephone, or in-person contacts. Nevertheless, beneficiaries did not
report improved access in CPC or comparison practices.
• Patient experience (Milestone 4). To improve patients’ experience in the final year of CPC,
80 percent of practices conducted patient surveys and 48 percent convened patient and
family advisory councils (PFACs) in 2016. Practices’ use of PFACs increased throughout
CPC, especially between 2013 and 2015. Challenges with surveys included the burdens of
collecting and analyzing data, and concerns about data
quality. Challenges with PFACs included scheduling,
ensuring that a representative group of patients
attended, and reassuring patients that their
participation was valuable and the practice would use
their feedback. Patient respondents who participated in
PFACs reported in qualitative interviews that the
PFACs’ suggestions led to multiple practice
improvements around patient outcomes, patient
satisfaction, and patient education.
• Quality improvement (Milestone 5). As in previous years, QI remained a major focus for
both CPC and comparison practices in 2016. Over time, more CPC and comparison
practices reported that all staff share responsibility for QI, as opposed to relegating this work
to a QI committee or department. And 40 percent of practices involved patients or caregivers
in identifying QI ideas or opportunities. The 2016 clinician and staff survey indicated that
two-thirds of CPC and comparison physician respondents were involved in QI work.
Consistent with prior years, in 2016, deep-dive practices typically used ad hoc approaches
for practice-level QI; systematic approaches were more common in large and system-owned
practices.
• Electronic clinical quality measures (Milestone 5). Most CPC practices focused QI
activities on a narrow set of eCQMs over time. In 2016 and 2015, the eCQMs they most
commonly focused on were poor control of hemoglobin A1c among patients with diabetes,
colorectal cancer screening, and breast cancer screening. In past years, practices noted that
tracking eCQMs was helpful for QI, but standardizing data entry across providers into the
EHR, analyzing the data, and developing QI processes were resource-intensive. Having
dedicated staff to support eCQM documentation and analysis as well as changes to care
processes facilitated QI.
• Care coordination (Milestone 6). CPC helped practices make considerable progress in
providing care coordination and follow-up after hospital or ED visits. Practices made
progress in building relationships and exchanging information with hospitals about patient
Practices most commonly used
patient experience data from
surveys and/or PFACs to:
• Improve the customer service
orientation of staff
• Change scheduling, hours, or
appointment types
• Improve communication with
patients
xxxiii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
discharge. However, several deep-dive practices reported ongoing challenges with accessing
hospital records and receiving complete and timely information about their patients from
hospitals. According to the practice survey results, there were increases in receipt of
information on patients from community hospitals and EDs within 24 hours. In 2016,
Medicare FFS beneficiaries at CPC practices were more likely than beneficiaries at
comparison practices to report that the provider’s office contacted them within three days of
their most recent hospital stay (60 versus 50 percent) or within one week of the most recent
ED visit (59 versus 51 percent). Practices also noted expanded follow-up with patients after
hospital and ED discharge and emphasized the importance of care managers in addressing
the needs of high-risk patients.
• Care compacts (Milestone 6). In addition to working on follow-up after hospital and ED
discharge, by the end of the initiative, 41 percent of CPC practices also had chosen to work
on care compacts or collaborative agreements with other providers. Practices typically
established care compacts, or collaborative agreements, with specialists to which they most
frequently refer patients. Most care compacts outlined expectations for referrals and
communication between primary care and specialists. A few practices noted that some
specialists struggled with multiple collaborative agreements due to variations in
requirements among the referring groups. Practices in systems with system-wide EHRs
reported that care compacts were less important because all clinicians in their system could
view patient information.
• Shared decision making (Milestone 7). Practices implemented shared decision making
(SDM) slowly in the early years of CPC, in part due to confusion about the concept of
preference-sensitive conditions, but use of SDM increased steadily. The percentage of CPC
practices that reported that they consistently used patient decision aids (PDAs) to help
patients and providers jointly decide on treatment options increased from 42 percent in 2014
to 62 percent in 2016 (compared to 25 percent among comparison practices). The top
conditions selected for SDM in 2016 were colorectal cancer screening, prostate cancer
screening, tobacco cessation, and mammography. According to some practices, the quality
of patient care improved with SDM. However, there was room for improvement in
providers’ and staff members’ understanding of preference-sensitive conditions, providing
SDM without overwhelming clinicians, and tracking PDA use and SDM discussions in
EHRs.
• Learning collaborative (Milestone 8). Similar to previous years, CPC practices greatly
valued learning and sharing with other practices in the CPC learning collaborative.
• Health IT (Milestone 9). CPC required practices to use EHRs certified by the Office of the
National Coordinator for Health Information Technology (ONC). In 2016, all CPC practices
attested that their eligible providers were working toward Stage 2 requirements for
Meaningful Use. CPC practices continued to face challenges in obtaining and exchanging
timely data from providers outside their practice or system. Health information technology
(IT) challenges affected care plan use and care management activities, practices’ ability to
follow up in a timely way with patients discharged from the hospital or ED, and practices’
capacity to track the outcomes of SDM discussions.
xxxiv

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
• Patient dismissals. Some practices had suggested that an unintended consequence of CPC’s
rewards for improving patient outcomes might be to tempt other practices to dismiss patients
with poor outcomes. However, CPC practices and comparison practices reported dismissing
patients rarely, at similar rates, and generally for similar reasons. Thus, participation in CPC
did not appear to make practices more likely to dismiss patients.
• Perceived benefits of CPC by clinicians and staff in CPC practices. Over the course of
CPC, deep-dive practice members increasingly perceived benefits to the quality, delivery,
and organization of patient care from working on CPC Milestone requirements. A large
proportion of clinicians and care managers gave CPC high ratings.
• Factors facilitating implementation. Several practice strategies that cut across the
Milestones facilitated CPC implementation. Over time, deep-dive CPC practices
increasingly reported holding regular meetings (at least monthly) to engage and continue to
involve staff in CPC. By the end of the initiative, several deep-dive practices also reported
that they had identified a practice leader, in some cases a physician, or small committee, to
act as a CPC champion, helping to introduce new concepts to the practice and to integrate
CPC-related changes into workflows. Finally, establishing care teams that worked regularly
together and clearly outlined clinician and staff roles helped meet patients’ needs.
• Barriers to implementation. Barriers to CPC implementation included the burden of
integrating numerous required changes into practice workflows, which particularly affected
care managers. Practices also struggled with the volume of administrative and quality
reporting, including different reporting requirements across payers. In addition, practices
reported it was hard to engage patients in care management activities (across a range of
areas related to behavior modification, adherence to treatment regimens, and setting health
goals); efforts to reduce inappropriate ED use; SDM; PFACs and patient surveys; and
patient portals. Some practices found that enhanced self-management support, increased use
of motivational interviewing, and teamwork helped them better engage patients in their own
care.
• Mixed facilitators and barriers. Some factors, such as system ownership, facilitated the
implementation of CPC in some cases, and served as a barrier in others. For example,
system-owned practices (and practices in regions with robust local health information
exchanges) reported that they had reliable, timely access to patients’ hospital and ED
records, and in some cases, enhanced information exchange with specialists. However,
practices described challenges obtaining timely electronic information from unaffiliated
providers in order to coordinate patient care with them. System ownership also benefited
CPC implementation by giving access to centralized QI resources, in some cases including
CPC project managers, which facilitated practice-level change. However, system ownership
sometimes created administrative and bureaucratic barriers to making improvements based
on patient feedback and making Milestone-related decisions, such as selecting SDM topics
to pursue.
xxxv

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
ES.5. Clinicians and staff in CPC practices had largely favorable views of
CPC. Although CPC required an intensive amount of work for practices,
it did not affect burnout, control over work, alignment of work with
training, and work satisfaction among clinicians and staff, favorably or
unfavorably (Chapter 6)
The CPC initiative aimed to transform care delivery and ultimately improve the experience
of physicians, other clinicians, and staff in CPC practices by providing them with more resources
and better ways to support the delivery of primary care to their patients. At the same time,
practice transformation efforts like CPC require intensive work, including substantial change to
practice workflows and staffing, shifting from a physician-centric to a team-based culture, and
creating new clinical and administrative tasks. Therefore, there was concern that CPC might add
to physicians’ burden, worsen their experience, and increase job dissatisfaction, at least in the
short run.
Through a CPC clinician and staff survey fielded 11 months and 44 months after CPC
began, we examined whether primary care physicians in CPC practices experienced their work
differently from primary care physicians in comparison practices, how other members of CPC
practices experienced their work, and whether experience changed over time. We focused on five
domains: (1) burnout, (2) control over work, (3) alignment of work with training, (4) work
satisfaction, and (5) for clinicians and staff at CPC practices, ratings of CPC. We obtained survey
responses from a sample of roughly 600 physicians in CPC practices and 500 physicians in
comparison practices and over 2,000 other members of CPC practices (nurse practitioners and
physician assistants [NPs/PAs]), care managers or care coordinators, medical assistants, nurses,
practice managers or supervisors, and receptionists or appointment clerks).
Overall, there were no meaningful differences on
measures of burnout, control over work, alignment of work
with training, or work satisfaction between physicians in
CPC and those in comparison practices in 2016, the last
year of CPC, or over time among CPC physicians,
NPs/PAs, and staff. Furthermore, there were no differential
effects of CPC on physicians whose practices were in a
system, were larger (measured by having more primary
care clinicians), or served high-risk Medicare beneficiaries
across most measures. Together, these findings indicate that CPC did not affect these aspects of
clinician and staff experience.
Although CPC did not have differential effects on physicians in practices that were part of a
system, had different numbers of primary care clinicians, or had higher-risk beneficiaries, we did
find differences among subgroups of physicians when we combined CPC and comparison
physicians for analysis. Physicians whose practices were part of a system reported that they had
less control over their work, and they spent less time doing work that was well matched to their
training and more time doing work that someone with less training could do; in addition, they
were less likely to report being satisfied with their current job than physicians whose practices
were not part of a system. Physicians in larger practices reported that they had less control over
their work than physicians in solo clinician practices, and physicians in practices with lower-risk
Comparing physicians in CPC
practices and comparison
practices, there were no
meaningful differences on
measures of burnout, control over
work, alignment of work with
training, or work satisfaction
during the initiative.
xxxvi

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
beneficiaries were less likely to report being satisfied with their current job than physicians in
practices with higher-risk beneficiaries.
CPC physicians, NPs/PAs, and staff had largely
positive views about their experiences participating in
CPC. For example, in 2016, 80 percent of physicians
reported that CPC had improved the quality of care or
service provided to their patients, and if they could do
it over again, 79 percent would support participation
in CPC. Only 12 percent of physicians would oppose
participation in CPC and 9 percent reported not
knowing enough about CPC to answer (Figure ES.6).
Figure ES.6. CPC practice members’ reports of how much they would support
or oppose their practice’s participation in CPC if they could do it all over
again, 2016
Source: Mathematica analysis of 2016 CPC clinician and staff surveys.
Note: We did not statistically test the differences in responses between respondent types.
46
38
64
64
33
44
42
33
33
24
21
18
23
30
7
9
4
2
3
5
6
5
3
4
2
2
1
3
9
17
5
12
44
27
20
0 20 40 60 80 100
Primary care physicians
NPs/PAs
Practice managers or
supervisors
Care managers or coordinators
Receptionists
Medical assistants
Nurses
Percentage of respondents
Strongly support Somewhat support Somewhat oppose Strongly oppose
Don’t know enough about CPC to answer
Physicians, clinicians, and staff in
CPC practices had largely positive
views of CPC.
• 80 percent of physicians in CPC
practices reported that CPC had
improved the quality of care or service
provided to their patients
• If they could do it over again, 79
percent of CPC physicians would
support participation in CPC.
xxxvii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
Among physicians that would support their practice’s participation in CPC, the most
common reasons for supporting CPC were: they believed work on CPC Milestones helped
practices make positive changes and improve patient care (81 percent), they valued the
opportunity to contribute to primary care practice transformation (52 percent), and the financial
support provided by CPC was sufficient to support their participation (52 percent). Still, even
supporters reported that CPC administrative reporting was a burden and that the transformation
work in CPC was difficult. Forty-four and 34 percent of physicians that would support their
practice’s participation in CPC again, reported this, respectively. Additionally, about one-third of
these physicians reported inadequate financial support as a reason to oppose CPC participation,
and one-quarter reported inadequate staffing.
ES.6. CPC had little impact on Medicare FFS beneficiaries’ experience of
care, and findings for CPC and comparison practices on most measures
of patient experience were comparable (Chapter 7)
Patient-centeredness was a core tenet of the CPC initiative, and several aspects of CPC
aimed to improve patients’ experience by transforming care delivery. Specifically, practices were
expected to improve access to care, engage patients to guide QI through regular patient surveys
and/or a PFAC, integrate into usual care culturally competent self-management support and
SDM tools, and coordinate care across the medical neighborhood. Practices were also
encouraged to use a personalized plan of care for high-risk patients. In addition, CMS and some
other participating payers used patient experience as an element in determining practice
eligibility for shared savings payments.
We present results based on survey responses from more than 25,000 Medicare FFS
beneficiaries in roughly 500 CPC practices and 8,000 beneficiaries in roughly 800 comparison
practices in each survey round. The survey was based on the Clinician and Group Consumer
Assessment of Healthcare Providers and Systems 12-Month Survey with Patient-Centered
Medical Home supplemental items (CAHPS PCMH, version 2.0), and included several
additional questions about specific aspects of CPC. We examined how beneficiaries’ ratings of
CPC practices compared with ratings of comparison practices in 2013 (8 to 12 months after CPC
began) and again in 2016 (5 months before CPC ended).
Despite the fact that CPC practices undertook substantial changes to improve care delivery,
beneficiaries’ ratings of CPC and comparison practices were comparable across most areas of
care measured in the patient survey. In 2013, 8 to 12
months into the initiative, beneficiaries in CPC and
comparison practices gave similar ratings for each of the
five summary composite measures: (1) timely
appointments, care, and information; (2) provider
communication; (3) providers support patients in taking
care of their own health; (4) providers discuss medication
decisions with patients; and (5) patients’ rating of the
provider (see Figure ES.7). In 2016, beneficiaries’ ratings
of CPC and comparison practices were again comparable
across all five composite measures, indicating that CPC
did not improve beneficiaries’ experiences as captured by these measures. Furthermore, there
Medicare FFS beneficiaries in CPC
and comparison practices gave
largely similar ratings of their
patient experiences.
The exception is that more
beneficiaries in CPC practices than
comparison practices reported
receiving timely follow-up care after
hospitalizations and ED visits.
xxxviii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
were no differential effects of CPC on beneficiaries who (1) were in practices in systems, (2)
were in larger practices (measured by having more primary care clinicians), or (3) had higher
risk scores.
Figure ES.7. Percentage of Medicare FFS beneficiaries who gave the best
response in 2013 and 2016, for five composite measures, CPC and
comparison practices, CPC-wide
Sources: CPC patient surveys administered June through October 2013 and July through October 2016.
*/**/*** The percentage of beneficiaries who gave the best response was statistically different between CPC and
comparison beneficiaries in the given year at the 0.10/0.05/0.01 level, respectively, but the difference was small.
Responses to 28 questions asked in 2016 that were not in the composite measures further
support the finding that over the course of the four-year initiative, beneficiaries’ experiences with
care were generally comparable in CPC and comparison practices. Exceptions indicated CPC
practices provided better transitional care:
• CPC improved transitional care after hospital stays. In 2016, 60 percent of beneficiaries in
CPC practices compared to 50 percent of beneficiaries in comparison practices reported that
their provider’s office contacted them within three days of their most recent hospital stay.
• CPC improved transitional care after ED visits. In 2016, 59 percent of beneficiaries in CPC
practices compared to 51 percent of beneficiaries in comparison practices who visited the
ED in the past year reported that their provider’s office contacted them within one week of
their visit.
These findings suggest that, while CPC practices were undergoing substantial changes to
improve care delivery, CPC beneficiaries’ experiences with care changed little during the
initiative and their ratings were no different from those of comparison practice beneficiaries on
53**
54
80**
81
46**
48
60***
63
76**
78
53*
54
81**
82
53
53
61
63
78
80
0 20 40 60 80 100
CPC
Comparison
CPC
Comparison
CPC
Comparison
CPC
Comparison
CPC
Comparison
.....
Percentage of respondents
Timely appointments,
care, and information
Providers'
communication with
patients
Providers support
patients in taking care
of their own health
Providers discuss
medication decisions
with patients
Patients' rating of the
provider
2013 CPC 2013 Comparison 2013 to 2016 gain
xxxix

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
most aspects of care delivery. The areas where we did see consistent findings—the increasing
percentages of beneficiaries who reported that their provider followed up with them after
hospital stays and ED visits—reflect CPC’s emphasis on improved coordination of care across
the medical neighborhood.
ES.7. CPC had favorable effects on Medicare FFS hospitalizations and ED
visits but did not generate enough savings to cover Medicare’s CPC
payments (Chapter 8)
CPC’s changes to primary care delivery were expected to lower Medicare FFS expenditures
and service use and improve quality of care.
We estimated the impact of CPC on these outcomes using difference-in-differences
regressions that compared mean beneficiary outcomes between CPC practices and a set of
similar practices that were not participating in CPC. The analysis compared outcomes from the
12 months before CPC and the 51 months after CPC began, and controlled for beneficiary,
practice, and market characteristics. It included 565,674 unique Medicare FFS beneficiaries
attributed at any time during the initiative to 497 CPC practices and 1,165,284 beneficiaries
attributed to 908 matched comparison practices.
Below are key findings:
• CPC had favorable effects on hospitalizations and
ED visits. Although Medicare service utilization
grew during the initiative for both CPC and
comparison practices, CPC practices experienced
slower growth in hospitalizations, ED visits, and
primary care visits than comparison practices.
Hospitalizations increased by 2 percent less for CPC
practices than for comparison practices over the
initiative (or by 5 fewer hospitalizations per 1,000
beneficiaries per year,
12
p = 0.07) (Table ES.2).
There was also 2 percent slower growth in ED visits
for CPC practices than comparison practices during
the initiative (or 10 fewer ED visits per 1,000
beneficiaries, p = 0.03). The effects on ED visits were more pronounced in the last two years
of CPC.
• The favorable effects on hospitalizations and ED visits are consistent with the findings
from the implementation analysis. For instance, practices noted that promoting high-risk
patients’ access to a care manager improved care and reduced hospitalizations through more
attentive transitional care, medication reconciliation, and the identification of problems
12
We treat the 51 months as four years, where the fourth “year” includes the final 15 months of CPC (October 2015
through December 2016). We express all results in terms of per month or per year of follow-up; therefore, the length
of the period over which annualized expenditures and service use outcomes are measured does not affect their
means. For outcomes that are not annualized—for example, the binary quality-of-care process measures for
beneficiaries with diabetes and the continuity-of-care measures—we excluded the final three months of CPC.
CPC reduced the growth of
hospitalizations, ED visits, and
office-based primary care visits by 2
percent each for Medicare FFS
beneficiaries.
Reductions in Medicare FFS
expenditures were not sufficient to
cover Medicare’s care management
fees.
There were minimal effects on the
limited claims-based quality-of-care
measures the evaluation tracked.
xl

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
between visits over the phone. Also, practices noted that improvements they made in other
areas were likely reducing ED use. Changes included:
- Better identifying patients who frequently used the ED and targeting outreach to them.
- Better identifying high-risk patients.
- Encouraging patients to call the office before using the ED for nonurgent care.
- Improving access to the primary care practice.
Findings from the beneficiary survey suggest that more CPC practices provided timely
follow-up care after hospitalizations and ED visits than comparison practices. Practice
members thought that providing better follow-up care after hospital discharges and ED visits
improved patient care.
• CPC reduced primary care visits. Office-based primary care visits grew by 2 percent less
for CPC than comparison practices (or by 68 fewer visits per 1,000 beneficiaries per year,
p = 0.07) (Table ES.2). This effect on office-based primary care visits might have been
driven by greater reliance on non-visit-based interactions with patients among CPC
practices, for example, by phone, or through follow-up by care managers, who cannot bill
Medicare for such services.
• CPC did not lead to statistically significant changes in total Medicare expenditures
(excluding care management fees). Over the course of the initiative, Medicare
expenditures without care management fees increased by 1 percent (or $9 PBPM) less for
the CPC practices than the comparison practices, but the difference was not statistically
significant (p = 0.16, 90 percent confidence interval [CI] -$19, $2) (Table ES.2). Lower
growth in inpatient expenditures, expenditures on skilled nursing facilities, and outpatient
services drove the lower growth in total expenditures for the CPC group.
• Although we would expect the effects on patient outcomes to increase over time as
practices further implemented the CPC functions, year-by-year effects on Medicare
expenditures without fees declined over time. Estimated savings declined from $18 in
Year 1, to $11 in Year 2, $4 in Year 3, and $2 in Year 4 (Figure ES.8).
Table ES.2. Percentage impacts on Medicare FFS expenditures and service
utilization over the four years of CPC (all attributed beneficiaries)
Outcomes Year 1 Year 2 Year 3 Year 4
Years 1–4
combined
Total Medicare expenditures ($ PBPM)
Without CPC care management fees
-2%***
a
-1% 0% 0% -1%
With CPC care management fees 0% 1% 1% 1% 1%
Expenditures by type of service ($ PBPM)
Inpatient
-3%**
a
-1% 0% 0% -1%
Skilled nursing facility
-7%***
a
-6%**
a
-3% -3% -5%
Outpatient -1% -2%
-3%**
a
-3%*
a
-2%**
a
Physician 0% -1% 1% 2%*
b
1%
Primary care physician
-2%***
a
-3%***
a
-1% -1%
-2%*
a
Office-based primary care
-2%*
a
-3%***
a
-2%**
a
-1%
-2%**
a
Specialist 0% 1% 2% 3%**
b
2%*
b
Office-based specialist 1% 0% 1% 2%*
b
1%
xli
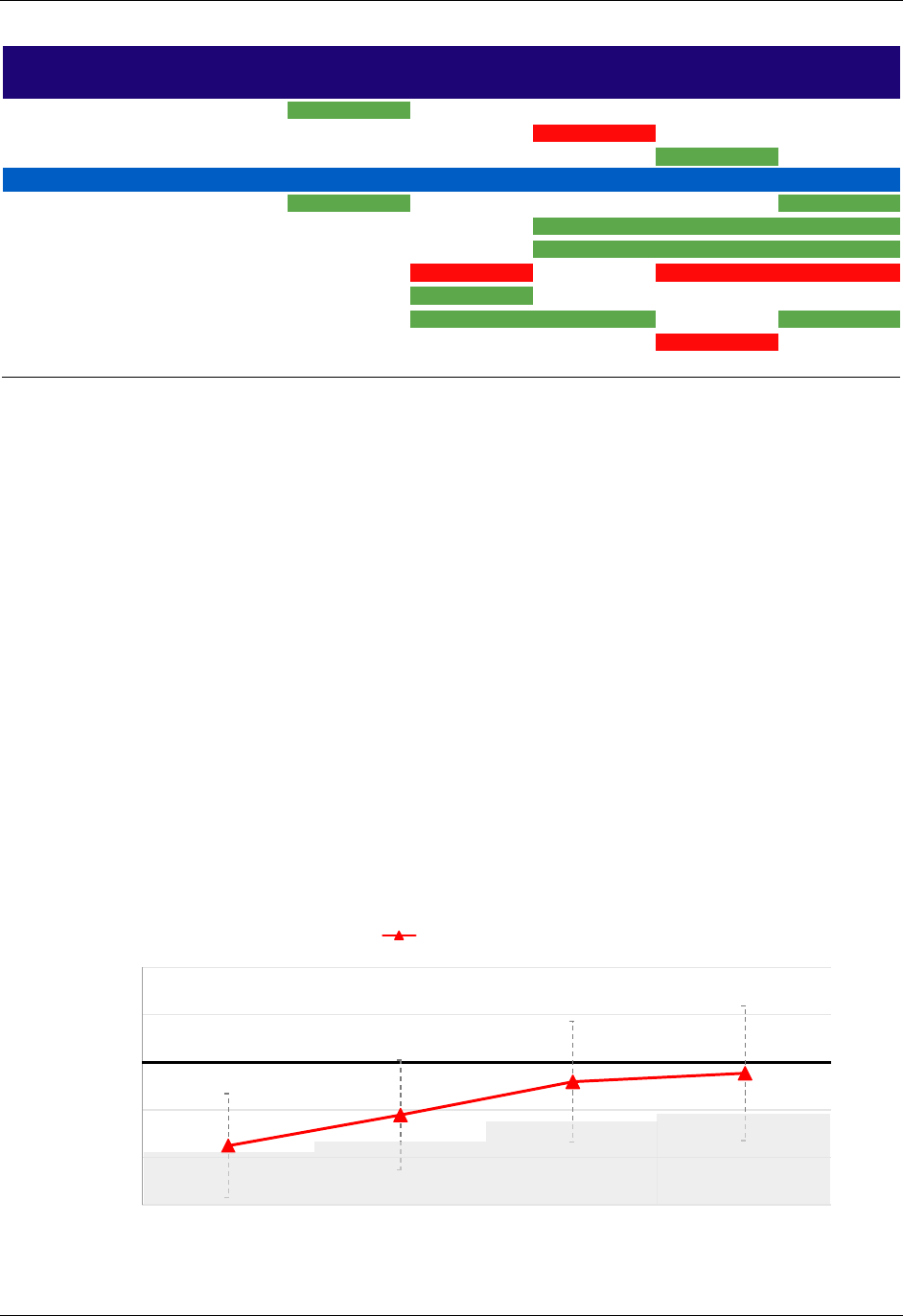
EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
Table ES.2 (continued)
Outcomes Year 1 Year 2 Year 3 Year 4
Years 1–4
combined
Home health
-3%**
a
2% 1% -1% -1%
Hospice 2% 1%
10%*
b
7% 5%
DME 0% -2% -4%
-4%*
a
-3%
Service utilization (annualized rate per 1,000 beneficiaries)
Hospitalizations
-2%*
a
-2% -1% -2%
-2%*
a
Total ED visits -1% -1%
-2%***
a
-2%***
a
-2%***
a
Outpatient ED visits -1% -1%
-3%***
a
-3%**
a
-2%**
a
Observation stays 2% 7%**
b
4% 7%**
b
5%**
b
Primary care visits -1%
-1%*
a
-1% -1% -1%
Office-based primary care visits -1%
-2%**
a
-2%*
a
-1%
-2%*
a
Specialist visits 0% 0% 1% 2%***
b
1%
Office-based specialist visits 0% 0% 0% 2% 0%
Source: Medicare claims data for October 2011 through December 2016.
Note: We base impact estimates on a difference-in-differences analysis; they reflect the difference in the
regression-adjusted average outcomes for attributed Medicare FFS beneficiaries in CPC practices for a
specific year compared with baseline relative to the same difference over time for attributed Medicare FFS
beneficiaries in matched comparison practices. We calculate percentage impacts by dividing the impact
estimate by the projected CPC group mean in the absence of CPC (that is, the unadjusted CPC group
mean minus the CPC impact estimate). Red shading with white italicized text signifies that our estimate
was statistically significant and showed an increase in the service use or expenditures outcome (note,
however, that increases in expenditures or use of certain services such as primary care and hospice could
be beneficial); green shading with bold text signifies that an estimate was statistically significant and
implied a reduction in the service use or expenditures outcome. Expenditures on physician services include
expenditures on primary care physician services, specialist services, and services provided by other
noninstitutional providers (the third category is not shown separately). Measures of outpatient ED visits and
total ED visits include observation stays. Primary care visits include both office-based primary care visits
and primary care visits in other settings. Analysis includes 565,674 Medicare FFS beneficiaries attributed to
497 CPC practices and 1,165,284 beneficiaries attributed to 908 matched comparison practices. Each
beneficiary can contribute as many as five observations in the analysis—one during the baseline year and
one during each follow-up year.
*/**/*** Significantly different from zero at the 0.10/0.05/0.01 level, two-tailed test.
FFS = fee for service; DME = durable medical equipment; ED = emergency department; PBPM = per beneficiary per
month.
a
The estimate was favorable and statistically significant.
b
The estimate was unfavorable and statistically significant.
Figure ES.8. Estimated impact of CPC on Medicare FFS expenditures without
care management fees, by year
-$30
-$20
-$10
$0
$10
$20
Year 1 Year 2 Year 3 Year 4
Impact in dollars PBPM
Measurement period
Impact estimate
Net savings region
xlii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
• CPC did not generate enough savings to offset the care management fees for Medicare
FFS beneficiaries. Including CPC’s Medicare FFS care management fees (which averaged
$15 per beneficiary in our intent-to-treat [ITT] analysis), average monthly Medicare
expenditures per beneficiary increased by 1 percent or $6 more for CPC than for comparison
practices over the 51 months. This difference was not significantly different from zero (p =
0.35, 90 percent CI -$4, $16). Findings from a Bayesian analysis also showed a high
probability (94 percent) of some gross savings but almost a zero probability that the savings
were sufficient to cover the care management fee. Therefore, it is unlikely that CPC was cost
neutral or generated net savings for Medicare.
• CPC had minimal effects on quality-of-care process and outcome measures. There were
very few sizeable or statistically significant estimates for the quality-of-care process and
outcome measures, or continuity of care. Among the limited claims-based measures
available (five process measures for beneficiaries with diabetes, and for all beneficiaries,
one transitional care measure, four continuity-of-care measures, and three outcome
measures), cumulative estimates show a statistically significant effect on only one measure:
the likelihood of an ED revisit within 30 days of an outpatient ED visit increased by 0.2
percentage points less, or about 3 percent of the mean rate (p = 0.02), for CPC relative to
comparison practices. In annual estimates, the only statistically significant findings for
quality-of-care process measures among beneficiaries with diabetes were in the high-risk
subgroup.
13
• Within certain subgroups, CPC generated a favorable impact on Medicare
expenditures without care management fees, but the evidence for differential impacts
for different types of practices was weak. We expected that CPC might have different
impacts for practices with certain characteristics, so we tested for differential impacts on
subgroups defined by those characteristics. We found that estimated effects on Medicare
expenditures without fees were favorable and significantly different from zero (indicating
gross savings) for practices that:
1. Were recognized as medical homes at baseline
2. Had six or more clinicians or were affiliated with a larger organization
3. Were hospital or system-owned
4. Were moderately large (3–5 clinicians)
For example, the third finding indicates we found a favorable impact when we tested for
differences among CPC and comparison practices that were owned by a hospital or system
at baseline. In contrast, there were no statistically significant differences in Medicare
expenditures between CPC and comparison practices among the subgroup that had at least
one clinician who met requirements for meaningful use of EHRs, nor in its counterpart.
13
This evaluation did not include the eCQMs that the model used for quality measurement and improvement for the
entire practice population, and for calculating eligibility to share in any Medicare shared savings. Not all comparison
practices report eCQMs, creating both conceptual and data challenges for analyzing the impacts of CPC on eCQMs.
xliii

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
The findings from these subgroup analyses suggest that practices with experience
transforming care and greater access to resources may have achieved greater savings.
However, there is only weak evidence for more favorable impacts within these practice
subgroups because the impact estimates for any given subgroup were not significantly
different from the estimates for its respective counterpart (that is, the opposite subgroup).
For example, although there was a favorable $17 PBPM impact among practices that were
owned by a hospital or system at baseline, that impact was not statistically different from the
favorable $3 PBPM impact for practices that were not hospital- or system-owned at
baseline. Applying any corrections for multiple comparisons or multiple hypothesis testing
would make it even less likely that we would find statistically significant differences.
ES.8. CPC practices’ self-reported measures of three key care delivery
approaches had limited associations with lower Medicare service use
and expenditures in the fourth program year (Chapter 9)
Knowledge about which care delivery approaches are most strongly associated with
improvements in key outcomes such as expenditures, hospitalizations, and ED visits can help
practices and CMS focus transformation efforts. We analyzed CPC practices to estimate how
these outcomes for attributed Medicare FFS beneficiaries in Year 4 were associated with
practices’ self-ratings on their approaches to three aspects of care delivery that literature suggests
are linked to better outcomes. We found few noteworthy associations:
• A stronger self-rating on timely primary care
follow-up after a hospitalization or ED visit was
associated with fewer hospitalizations, but not fewer
ED visits or expenditures. Beneficiaries in practices
at the 75th percentile on the follow-up measure had
about 4 percent fewer hospitalizations on average
than beneficiaries in practices at the 25th percentile
on the follow-up measure. The associations were
even larger among high-risk beneficiaries and in
practices with high baseline hospitalization rates.
• Stronger self-rating on after-hours access to care
was not associated with lower service use or
expenditures.
• Stronger self-rating on continuity of care (patients seeing the same clinician at most of their
office visits) was associated with fewer ED visits, but only for high-risk beneficiaries, and
was not associated with fewer hospitalizations or lower expenditures.
• Contrary to expectations, these associations were not notably stronger among practices in
which nonclinicians performed key clinical service roles.
Practices in the top quartile on self-
rating of their follow-up with patients
after a hospitalization or ED visit had
4 percent fewer hospitalizations in
Year 4 than practices in the bottom
quartile on follow-up. The association
was even stronger for high-risk
beneficiaries and for practices with
high baseline hospitalizations.
Practices whose patients typically
saw the same clinician during office
visits had fewer ED visits, but only
among higher-risk beneficiaries.
xliv

EXECUTIVE SUMMARY MATHEMATICA POLICY RESEARCH
ES.9. Conclusion
Over the four-year initiative, CPC had mixed results on the outcomes examined in this
evaluation. The initiative had considerable success in bringing together public and private
partners to improve primary care and in implementing the key aspects of the CPC model among
participating practices and the patients they serve. Yet while CPC improved practices’ primary
care approaches and showed significant improvements in some outcomes, the reductions in
Medicare FFS utilization were not large enough to offset the care management fees that
Medicare provided to practices. These evaluation results, and the lessons learned from the CPC
experience, have informed CPC+, a new model of primary care transformation, and should help
inform the work of other primary care initiatives.
xlv
This page has been left blank for double-sided copying.

MATHEMATICA POLICY RESEARCH
1. INTRODUCTION
1.1. Overview of the Comprehensive Primary Care initiative
The Comprehensive Primary Care (CPC) initiative, a unique collaboration between the
Center for Medicare & Medicaid Innovation (CMMI) of the Centers for Medicare & Medicaid
Services (CMS) and 39 other private and public payers, aimed to improve primary care delivery
and improve health care quality, patient and clinician experience of care, and lower costs. CMS
selected CPC regions and payers—including commercial insurers and state Medicaid agencies
and associated Medicaid managed care plans— in April 2012 and selected practices in August
2012 (see Figure 1.1. for the initiative’s timeline). The four-year initiative began in fall 2012 and
ended on December 31, 2016.
CPC tested a new model of care delivery in nearly 500 primary care practices across seven
regions of the United States. It focused on helping practices implement five key functions in their
delivery of care: (1) access and continuity, (2) planned chronic and preventive care, (3) risk-
stratified care management, (4) patient and caregiver engagement, and (5) coordination of care
among providers in the medical neighborhood who treat the same patients.
14
CMS specified a
series of Milestones to help practices implement these functions, and it updated the requirements
for each Milestone annually to build on practices’ progress in the prior year (Table 1.1). CMS
assessed how the practices were delivering care and required them to meet the Milestone
requirements to remain in the program.
Table 1.1. CPC Milestones for program year (PY) 2016
1. Budget. Complete an annotated annual budget with PY2015 revenues/expenses and projected
CPC initiative practice revenue flow for PY2016 at the start of the year and report actual
revenue/expenses for PY2016 at the end of the year.
2. Care management for high-risk patients. Maintain at least 95 percent empanelment to provider
and care teams. Continue to risk-stratify all patients, maintaining risk-stratification of at least 75
percent of empanelled patients. Expand care management activities for highest risk patients who
are likely to benefit from longitudinal care management and those not otherwise at high risk but
requiring episodic care management. Provide information about the care plans that are used for
both longitudinal care management and episodic care management. Maintain the implementation of
and further refine one of three strategies (behavioral health integration, medication management, or
self-management support).
3. Access by patients and enhanced access. Enhance patients’ ability to communicate 24 hours a
day, 7 days a week with a care team that has real-time access to their electronic medical records.
Continue to implement asynchronous forms of communication (for example, patient portal and
email) and ensure timely responses. Measure continuity of care by measuring visit continuity
quarterly for each provider and/or care team in the practice.
4. Patient experience. Assess patient experience through patient surveys or patient and family
advisory council meetings and communicate to patients (using electronic, poster, pamphlet, or
similar communication methods) about resulting changes the practice is making.
14
For CMS’s logic diagram for CPC, see http://innovation.cms.gov/Files/x/cpcidiagram.pdf or Appendix A, Figure
A.1.
1

1. INTRODUCTION MATHEMATICA POLICY RESEARCH
Table 1.1 (continued)
5. Quality improvement. Continue to perform continuous quality improvement using electronic health
record (EHR)-based clinical quality measures (eCQMs) on at least three of the measures that
practices report annually. Review at least one payer data feedback report (CMS Practice Feedback
Report or other payers’ reports) to identify a high-cost area and a practice strategy to reduce this
cost while maintaining or improving quality.
6. Care coordination across the medical neighborhood. Track patients by implementing two of
three options: follow up via telephone with patients within one week of emergency department (ED)
visits; contact at least 75 percent of hospitalized patients within 72 hours of discharge; and enact
care compacts with at least two groups of high-volume specialists.
7. Shared decision making. Use at least three decision aids to support shared decision making
(SDM) for three preference-sensitive conditions and track patient eligibility for and use of the aids.
8. Participating in learning collaborative. Participate in regional and national learning offerings and
communicate with regional learning faculty.
9. Health information technology (IT). Attest that each eligible professional in the practice is
engaged with and working toward attestation for Stage II Meaningful Use in the timelines set by the
Meaningful Use program.
Source: CPC PY2016 Implementation and Milestone Reporting Summary Guide.
To help participating practices change care delivery and accomplish the goals of CPC, the
initiative provided them with financial support, data feedback, and learning support.
• Financial support from multiple payers
who collectively represented a substantial
market share in each region. CPC financial
support to practices included prospective
payments and the opportunity to receive
shared savings retrospectively.
- Prospective payments. For Medicare fee-
for service (FFS) beneficiaries, CMS
paid CPC practices an average of $20
per beneficiary per month (PBPM) in
care management fees during CPC’s first
two years and $15 PBPM from January
2015 through the last two years of the
initiative.
15
Care management fees from
other payers varied, but for most
15
CMS paid $20 PBPM in care management fees during Quarters 1 through 9 of CPC (through December 2014),
and it paid $15 PBPM from January 2015 onward (for the last eight quarters of CPC). Therefore, over the 17
quarters of CPC, the average PBPM care management fee paid for patients still attributed to a practice was
approximately $18. However, the average PBPM fee received in the intent-to-treat analysis sample used to estimate
the effects of CPC was $15, because we retained all beneficiaries after they were first attributed, even if a practice
did not receive fees for them because they were no longer attributed.
2

1. INTRODUCTION MATHEMATICA POLICY RESEARCH
business lines (such as Medicaid managed care and commercial), the average payments
were much lower than those provided for Medicare FFS beneficiaries (for example, they
ranged from about $2 to $15 for Medicaid managed care in 2016), which partly reflects
the greater needs of Medicare FFS patients.
- Shared savings. During the last three years of the program, Medicare FFS and about
two-thirds of other payers offered participating practices the opportunity to receive a
share of any net savings in health care costs beyond the amount required to cover the
care management fees. Medicare FFS calculated savings at the regional level (that is,
compared the total costs of Medicare attributed patients across all CPC practices in a
region with expected expenditures for the performance year based on trending forward
baseline costs; other payers that offered shared savings calculated it in a variety of ways
(for example, at the regional, system, or even practice level, or among groups of
unaffiliated practices).
• Data feedback on each practice’s progress in improving patient outcomes and controlling
costs, provided quarterly by CMS for Medicare FFS beneficiaries and with varying
frequency by most other participating payers for their participating patients. To increase
reporting consistency and reduce clinician burden, over time, payers in five of the seven
regions were able to align the measures included in their individual reports or provide
practices with aggregated reports (in which a third party combines data from Medicare FFS
and other payers into one report and provides that feedback to practices).
• Learning support to help practices understand CPC requirements, build quality
improvement capacity, and make changes to provide comprehensive primary care. CMS and
its learning contractors hosted group learning activities, which included opportunities for
peer-to-peer learning and more didactic sessions, and in some cases they provided
individualized support to practices.
In April 2016, CMS announced the largest multipayer primary care model ever tested to
improve primary care, Comprehensive Primary Care Plus (CPC+), which builds on the lessons of
CPC and other patient centered medical home models.
16
CPC+ launched on January 1, 2017,
with 54 payers across 14 regions (including all 7 CPC regions, and 4 Multipayer Advanced
Primary Care Practice [MAPCP] regions) supporting almost 2,900 practices that deliver primary
care. Four more regions joined CPC+ in 2018 with an additional 7 payers and approximately 165
practices.
16
For more information on CPC+, visit https://innovation.cms.gov/initiatives/comprehensive-primary-care-plus.
3
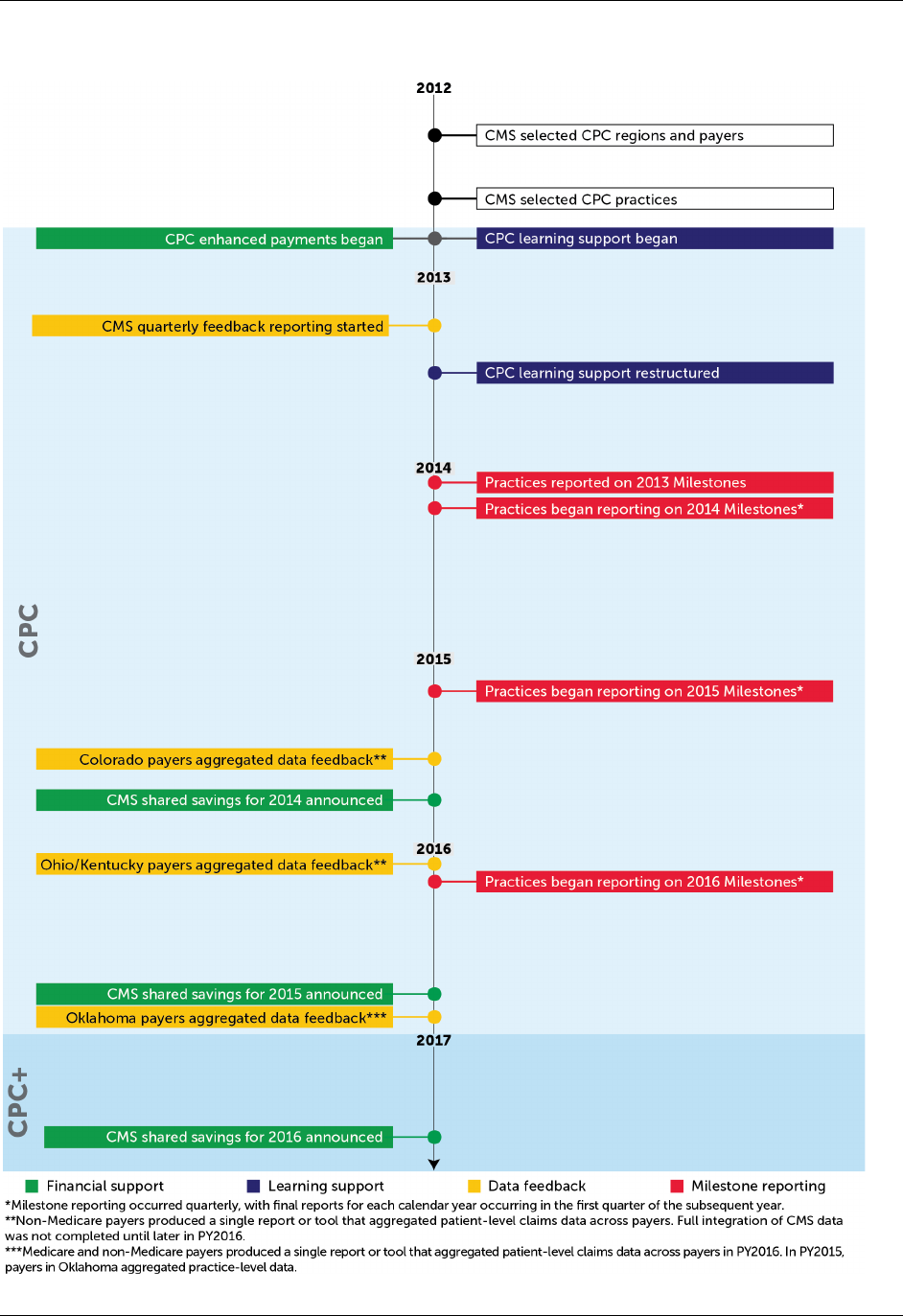
1. INTRODUCTION MATHEMATICA POLICY RESEARCH
Figure 1.1. CPC implementation timeline
4

1. INTRODUCTION MATHEMATICA POLICY RESEARCH
1.2. Design of the CPC evaluation
Mathematica conducted a five-year, mixed-methods, rapid-cycle evaluation that provided
CMS, practices, and regions with regular, formative feedback. The evaluation included
implementation, impact, and synthesis studies and answered the following research questions:
• Which regions, payers, practices, and patients participated in CPC? Why? What
characteristics distinguish them?
• What payment, data feedback, and learning support did CMS and the other payers provide?
How did practices use these supports?
• How did practices change the way they delivered care, and what facilitated or impeded
progress?
• What were the effects on clinician and staff experience; patient experience; and quality,
service use, and costs for attributed Medicare FFS beneficiaries?
• How do the results differ across regions and across subgroups of practices and patients?
• What factors account for the varying degrees of success in achieving the goals of the
initiative, or the speed with which participants reached these goals?
• What are the findings about—and implications for—replicating and spreading CPC?
The evaluation relied on a range of quantitative and qualitative data sources (Table 1.2). To
study key areas of CPC implementation, we triangulated data to capture the perspectives of
practices, patients, payers, CMS’s CPC staff and contractors, and other stakeholders. To assess
the initiative’s effects on costs and quality for Medicare FFS patients and on practice, clinician,
and patient experience, we compared outcomes for CPC practices with those of a set of
comparison practices that were similar to CPC practices before the initiative began. For the
synthesis of implementation and impact findings, we examined key links between specific
changes in how practices deliver care and changes in key outcomes such as Medicare
expenditures and hospitalization rates. The synthesis describes the successes and challenges of
improving outcomes using CPC.
Table 1.2. CPC evaluation data sources
CMS and its contractors
Interviews with CMS
and its contractors
For each region, we interviewed CMS staff working directly with CPC payers and
practices, CPC regional learning faculty ([RLF] organizations funded by CMS to support
CPC practices), and multistakeholder faculty (organizations CMS contracted with to
convene meetings of payers, practices, and other stakeholders). These annual
interviews provided insight into the supports CMS and its contractors provided to CPC
practices and barriers and facilitators to providing those supports.
Data on CMS payments
for CPC
Data provided by CMS on CPC care management fees and shared savings payments
made for Medicare FFS beneficiaries.
CPC learning contractor
reports
These reports provided detailed information on the learning activities delivered by RLF
and practice participation in those activities.
Observations of CPC
activities
Observations of activities hosted by RLF and multistakeholder faculty provided insight
into the learning activities offered to CPC practices and how payers and other
stakeholders collaborated for CPC.
5

1. INTRODUCTION MATHEMATICA POLICY RESEARCH
Table 1.2 (continued)
Other payers
Payer memorandums of
understanding (MOUs)
Information from CPC payer MOUs provided a baseline understanding of CPC payers’
approaches to supporting practices.
Interviews with non-
Medicare CPC payers
Interviews were conducted with non-Medicare payers annually to gain their
perspectives on the initiative and understand the payments, data feedback, and
learning they provided to CPC practices.
Practice-reported
budget data
CPC practices’ self-reported budget submissions to CMS provided insight into the
magnitude of payments non-Medicare FFS payers made to CPC practices and how
practices invested those payments.
Review of payer data
feedback provided to
practices
Reviewing practice-level feedback reports and patient-level data files provided by
payers to CPC practices informed our understanding of the content and structure of
those reports. (Mathematica produced Medicare FFS data feedback for CMS as part of
the evaluation contract.)
CPC practices
Practice application
data
Information from practice applications provided a baseline understanding of CPC
practice characteristics.
Practice tracking data Monthly practice rosters from CMS and its contractors indicated changes in practice
participation, including withdrawals, terminations, mergers, and closures.
Practice survey A practice survey fielded to all CPC practices in October 2012 (baseline), and CPC and
comparison practices in April 2014, 2015, and 2016. This survey included a modified
Patient-Centered Medical Home Assessment (M-PCMH-A) tool, which Mathematica
adapted for the CPC evaluation to capture approaches to care delivery in seven areas
that are related to CPC Milestones. The survey also asked practices about their
experiences with and perspectives on CPC. The analysis contains 471 CPC practices
and 340-423 comparison practices, depending on the survey round. Appendix D
describes the survey and analysis methods, and contains tables showing the results by
survey round, for CPC and comparison practices, for CPC as a whole (across all seven
regions).
Clinician and staff
survey
Surveys fielded to a sample of primary care physicians, nurse practitioners, and
physician assistants in CPC and comparison practices and various types of staff in
CPC practices in September 2013 and June 2016. The surveys assessed clinician and
staff experiences delivering primary care and experiences with CPC. The analysis
reports on the responses of roughly 600 physicians,150 nurse practitioners and
physician assistants (NPs/PAs), and about 2,000 staff—care managers or care
coordinators, medical assistants, nurses, practice managers or supervisors, and
receptionists or appointment clerks; and 500 physicians in comparison practices, in
each round. Appendix E describes the survey and analysis methods, and contains
tables showing the results by survey round, for respondents in CPC and comparison
practices.
Interviews and
observations of deep-
dive practices
Qualitative data collected annually from 21 deep-dive practices selected for intensive
study (3 practices per region). We conducted site visits to practices (in 2013 and 2015),
and telephone interviews with practices in alternate years (2014 and 2016).
Respondents included a practice clinician lead, other clinicians, CPC project
coordinators, care managers, practice managers, Health IT staff and other staff. These
data provide information on how practices implemented changes related to each
Milestone, associated barriers and facilitators to this implementation, and experiences
with CPC.
Interviews with exiting
practices
Interviews with a sample of exiting practices provided perspectives on their reasons for
withdrawal or termination and their future plans to improve primary care delivery.
Practice-reported
Milestone data
CPC practices’ self-reported data submitted to CMS on how they approached the CPC
Milestones. In program year (PY) 2013, practices reported on Milestones once, at the
end of the year. In subsequent years, practices were required to report on Milestones
quarterly.
6

1. INTRODUCTION MATHEMATICA POLICY RESEARCH
Table 1.2 (continued)
CPC patients
Patient survey A patient survey fielded annually beginning June 2013 through 2016 to samples of
Medicare FFS beneficiaries attributed to CPC and comparison practices. The survey
included questions from the Clinician and Group Consumer Assessment of Healthcare
Providers and Systems (CG-CAHPS) version 2.0, and CPC-specific questions that
asked patients to rate their experiences with care from their primary care provider over
the past 12 months. The analysis reports on the responses of more than 25,000
beneficiaries in roughly 500 CPC practices and 8,000 beneficiaries in roughly 800
comparison practices in each survey round. Appendix F describes the survey and
analysis methods, and contains tables showing the results by survey round, for
respondents in CPC and comparison practices.
Interviews with
beneficiaries
Telephone interviews in 2015 with Medicare FFS beneficiaries that received care
management services from CPC practices or served on CPC practices’ patient and
family advisory councils provided insight into beneficiaries’ experiences with these
aspects of CPC.
Claims and enrollment data
Medicare FFS Across all regions, Medicare FFS claims data were used to estimate the impact of CPC
on costs, utilization, and quality of care for Medicare FFS beneficiaries.
1.3. This report
This final report to CMS covers the entire 51-month CPC implementation period (fall 2012
through December 2016). In Chapters 2 through 5 of this report, we discuss CPC’s
implementation in detail. In Chapter 2, we describe CPC participation and how it evolved over
the course of the initiative. We describe the payment, data feedback and learning supports
provided to CPC practices by CMS and other payers in Chapter 3 and how payers and other
stakeholders worked together in Chapter 4. In Chapter 5, we detail how practices changed the
way they deliver care. Our first three annual reports provide additional detail on CPC
implementation during the first three years of the initiative (Taylor et al. 2015; Peikes et al.
2016a; Peikes et al. 2016b).
Chapters 6 through 8 report the impacts of CPC. Chapter 6 describes the impacts on clinician and
staff experience. In Chapters 7 and 8, we report estimates of the impact on key outcomes for
attributed Medicare FFS beneficiaries. Specifically, Chapter 7 reports effects on patient
experience. Chapter 8 presents effects on a wide array of claims-based outcomes, including
measures related to Medicare costs, utilization, quality of care, process of care, transitional care,
and continuity of care. Finally, in Chapter 9, we examine the association between key aspects of
care delivery and outcomes.
7
This page has been left blank for double-sided copying.

MATHEMATICA POLICY RESEARCH
2. WHO PARTICIPATED IN CPC?
CPC was a bold undertaking that relied on a public-private partnership to support robust
investment in primary care redesign, with the goals of improving quality, patient and clinician
experience of care, and lowering costs. Selecting, organizing, and convening participants for an
initiative of this scale and scope—and keeping them engaged and committed—required
tremendous operational resources and capacity.
In this chapter, we highlight the characteristics of the initiative’s participating regions,
payers, practices, and patients and provide an overview of how participants were selected. (For
additional information, see the first annual report; Taylor et al. 2015). We also describe how
participation changed over time.
2.1. Key takeaways on CPC participation
• CMS implemented CPC in seven regions including four states (Arkansas, Colorado, New
Jersey, and Oregon) and portions of three states (New York’s Capital District-Hudson
Valley region, Ohio and Kentucky’s Cincinnati-Dayton region, and Oklahoma’s Greater
Tulsa region).
• Medicare FFS and 39 other payers, including five state Medicaid agencies, initially agreed
to participate in CPC, committing substantial public and private resources to redesign
primary care in CPC’s seven regions (Figure 2.1; see Appendix B for additional details on
participation).
17
This multipayer design was a key feature of CPC, and CMS considered it
critical to creating an environment that supported comprehensive primary care for
participating practices.
• Payer participation remained steady—only three small payers left CPC during the initiative.
Moreover, 28 of the 36 payers that remained in CPC throughout the initiative also joined
Comprehensive Primary Care Plus (CPC+), which CMS launched in January 2017 and
builds on lessons learned from CPC.
• Participating payers included most of their lines of business in CPC. However, many of the
26 payers with self-insured clients initially struggled to enroll these clients in CPC. Through
concerted efforts to engage self-insured clients, the number of payers reporting that all or
nearly all of their self-insured clients participated in CPC doubled from 7 to 14 during the
initiative.
• CMS selected 502 practices to participate in CPC. These practices were diverse; they
included independent and system-owned practices, some practices that were recognized as
medical homes and others that were not, and practices of different sizes. Practices were not
selected based on care delivery approaches or outcomes. Most had substantial opportunities
to improve care delivery at the start of CPC.
• Practice participation remained relatively stable throughout the initiative—only 1.8 percent
of practices were terminated from the initiative (9 practices) and another 11.2 percent
17
Payers that participated in more than one region are counted separately for each region in which they participated.
Overall, 31 distinct payers participated in CPC in addition to Medicare.
9

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
voluntarily withdrew (56 practices). Among practices that withdrew, the most common
reason was to join a Medicare accountable care organization (ACO) (29 of the 56 practices),
given that CMS did not allow practices to participate in CPC and these ACOs. A number of
practices also voluntarily withdrew after assessing the terms and conditions of CPC
participation early in the initiative (5 practices) or due to challenges meeting CPC
requirements (13 practices). Six practices closed during the initiative.
18
• Practices that were terminated or withdrew from CPC were on average smaller than those
that remained in CPC through the end of the initiative. Specifically, 78 percent of practices
that left CPC had fewer than three physicians, compared with 56 percent of practices that
remained for the duration of the initiative. Because care management fees were paid on a
per-patient basis, practices that left CPC had received lower total care management fees
during their participation than other practices; however, per-clinician payments to the two
groups of practices were not statistically significantly different.
• Of the 422 practices that remained in CPC at the end of the initiative and were located in
CPC+ regions, 98 percent (412 practices) also joined CPC+. In addition, 15 of the 57
practices that withdrew or were terminated from CPC for reasons other than their practice
closing and were located in CPC+ regions joined CPC+.
19
• CPC was designed to transform whole practices; as such, practices were expected to deliver
the same care to all patients they saw. This population included patients of participating
payers that were attributed to their practice (for whom practices received care management
fees), patients of participating payers who were not attributed to the practice, patients of
nonparticipating payers, and uninsured patients. The numbers of attributed and total patients
were substantial, with attributed patients estimated at 1.1 million and total patients estimated
at 3.1 million across all participating practices (based on practice-reported Milestone data).
18
Several CPC practices also changed their composition during the initiative. Five CPC practices each split into two
practices (adding five CPC practices to the total count). Three CPC practices merged with other CPC practices
(subtracting three CPC practices from the total count).
19
Three counties (Putnam, Rockland, and Westchester) that were included in the Capital District-Hudson Valley
Region in New York for CPC were not included in the region for CPC+. Fifteen CPC practices that remained in
CPC at the end of the initiative and two that withdrew from CPC were located in these counties and thus ineligible
to apply for CPC+.
10

11
Figure 2.1. CPC regions, non-Medicare payers, practices, and patients
2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
2.2. Methods
To understand payer participation, we reviewed the memorandums of understanding
between CMS and other payers and analyzed data from qualitative interviews with CMS and
other participating payers. To calculate the number of practices and patients that participated in
CPC, we analyzed CMS’s practice tracking data and practice-reported budget data. We drew on
practice application data and the CPC practice survey to describe practice characteristics and
interviews with participating and exiting practices and surveys with CPC clinicians and staff to
understand practices’ motivations for participating in CPC (see Table 1.2 for additional
information on data sources used for the evaluation). We also compared the characteristics of the
small proportion of practices that withdrew or were terminated from CPC to those that remained
for the duration of the initiative.
2.3. Participating regions and payers
For CPC, Medicare FFS initially leveraged the support of 39 other payers across 7 regions. Payer
participation remained steady—only three small payers left CPC during the initiative.
In September 2011, CMS invited payers nationwide to apply to participate in CPC. CMS
scored payers’ applications based on a variety of factors—most notably the payers’ degree of
alignment with CMS’s approach to CPC, which included their commitment to provide
participating practices with attribution reports, enhanced payments, and use and cost data to
support their provision of comprehensive primary care, and their willingness to align with CMS
and other payers on quality measures. CMS selected seven geographically diverse regions in
which applying payers had a substantial combined market share and a preponderance of these
payers received high scores on their individual applications. These seven regions included four
states (Arkansas, Colorado, New Jersey, and Oregon) and portions of three states (New York’s
Capital District-Hudson Valley region, Ohio and Kentucky’s Cincinnati-Dayton region, and
Oklahoma’s Greater Tulsa region).
After selecting the regions, CMS more closely reviewed applications of payers in the
selected regions. CMS selected payers to participate in CPC that received high scores on their
applications or were willing to refine their applications to increase alignment with the initiative
by, for example, increasing the level of enhanced payments they provided to practices. Across all
regions, CMS initially leveraged the support of 39 payers, ranging from 3 payers in the
Oklahoma Greater Tulsa region to 9 payers in the Ohio/Kentucky Cincinnati-Dayton region. One
national payer that was participating in two CPC regions joined in a third region during the first
year of the initiative, which expanded payer participation. Participating payers included national
and regional private payers, as well as public payers.
Most payers joined CPC because the initiative aligned with their organizational values and
business strategy and provided an opportunity to build upon prior and concurrent efforts to
improve primary care delivery (Taylor et al. 2015). Specifically, payers indicated that CPC’s
multipayer approach increased the impact of prior efforts in which enhanced payments and/or
feedback reports covered only a small portion of any given practice’s patients. In particular,
payers suggested that collaborating with Medicare was important because Medicare covers a
12

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
substantial portion of the typical primary care practice’s patient panel and had been a crucial
missing partner in prior efforts.
Over the course of CPC, payer participation remained notably stable in all seven regions,
with 36 payers participating in CPC in its final year (Figure 2.1).
20
Only three payers (7.7
percent of all payers) across the seven regions withdrew from the initiative (two of which had
fewer than 4,000 patients attributed to CPC practices; the third payer withdrew early and the
number of attributed lives is unknown)
. None of the three payers withdrew because of
dissatisfaction with CPC. Rather, two payers withdrew because their market share declined
significantly and one because its self-insured clients would not contribute enhanced CPC
payments. In addition to withdrawals, one participating payer acquired another participating
payer (thus subtracting one payer from the total count).
(See Chapter 4 for information on the
factors that helped sustain multipayer collaboration.)
Further demonstrating payers’ commitment to CPC, many payers in each of the seven CPC
regions agreed to participate in CPC+ (CMS 2016). Specifically, 28 of the 36 payers that
participated in CPC for the duration of the initiative also joined CPC+.
21
Payers that participated in CPC operate different lines of business. For example, some
payers that participated in CPC are Medicaid managed care plans and offer products only in that
line of business; others operate several lines of business, such as commercial, Medicare
Advantage, and self-insured. For CPC, payers varied in which lines of business they decided to
include in the initiative. In the last year of the initiative, the most common lines of business
included in CPC were commercial (26 payers) and Medicare Advantage (18 payers; Table 2.1.).
Medicaid managed care lines of business (9 payers) were represented in all regions except
Arkansas and Oklahoma (which did not have Medicaid managed care contracts). Additionally,
Medicaid FFS participated in five regions. In four of these regions, Arkansas, Colorado, Ohio,
and Oregon, CMS paid the CPC care management fees for Medicaid FFS beneficiaries. In
Oklahoma, Medicaid collaborated in CPC and is counted as a participating payer, but it did not
provide care management fees to participating practices.
20
Payers that participated in more than one region are counted separately for each region in which they participated.
In addition to Medicare, there were 28 distinct payers at the end of CPC.
21
One CPC payer that joined CPC+ withdrew from the initiative in March 2017.
13

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
Table 2.1. Number of non-Medicare CPC payers in 2016, by lines of business
included
All regions
Arkansas
Colorado
New Jersey
New York:
Capital District -
Hudson Valley
Region
Ohio/Kentucky:
Cincinnati-
Dayton Region
Oklahoma:
Greater Tulsa
Region
Oregon
Total payers (across lines of business) 36 4 8 4 4 8 3 5
Number of payers that included each line of business in CPC
Commercial 26 2 7 3 4 5 2 3
Self-insured (Administrative services
only or third-party administrator)
a
20 2 5 2 2 5 2 2
Medicare Advantage 18 1 3 3 4 3 1 3
Medicaid Managed Care 9 0 1 2 1 2 0 3
Medicaid FFS 5 1 1 0 0 1 1 1
Source: Payers’ pre-interview worksheets; payer interviews.
Notes: Medicare FFS also participated in each region. Four payers did not complete interviews in 2016. For these
payers, we used the most recently available data on which lines of business they included in CPC.
a
Payers that included any self-insured lives in CPC were included in these counts. Some of these payers included
most or all of their self-insured lives in CPC whereas others included a small proportion.
FFS = fee-for-service.
In addition to fully insured business, 26 participating payers had self-insured clients
(employers or other entities) in the CPC regions in 2016.
22
Most payers considered including
self-insured lives in CPC important because the self-insured population represented a substantial
proportion of their commercially insured business. (In fact, the self-insured population represents
more than half of all commercially insured individuals in the United States (Stremkikis 2016).
However, gaining self-insured clients’ participation in CPC was difficult for payers because self-
insured entities were reluctant to agree to pay enhanced CPC payments for their respective lives
without evidence of a positive return on investment. Additionally, early in the initiative, self-
insured clients lacked knowledge about CPC—or time to focus on it—given the resources
required to respond to various Affordable Care Act requirements. These factors contributed to
self-insured clients’ initially low levels of participation in CPC.
Over the course of the initiative, most of these 26 CPC payers worked hard to increase and
maintain participation in CPC by self-insured clients. As a result of their efforts, the number of
payers reporting that at least some self-insured clients participated in CPC increased from 14 of
26 payers in 2013 to 20 in 2016 (Figure 2.2.).
23
Similarly, the number reporting that all or nearly
22
In 2015, one payer added its first self-insured client in a CPC region. In 2016, a different payer lost its only self-
insured client in a CPC region.
23
Two payers with self-insured clients opted not to participate in interviews in 2016. We used their responses from
2015 interviews in this analysis. In 2015, one payer did not pursue self-insured clients; the other encouraged self-
insured clients to join the initiative and had a small number of self-insured lives included.
14

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
all self-insured clients participated in CPC increased from 7 to 14 payers. Increases in self-
insured participation were reported in most regions and by both regional and national payers.
Figure 2.2. Participation of self-insured clients in CPC in 2016
Source: Mathematica interviews with CPC payers.
Note: Two payers with self-insured clients opted not to participate in interviews in 2016. We used their responses
from 2015 interviews in this analysis. In 2015, one payer did not pursue self-insured clients; the other
encouraged self-insured clients to join the initiative and had a small number of self-insured lives included.
To increase participation of self-insured clients in CPC, some payers either required self-
insured clients to participate or enrolled all self-insured clients unless they explicitly opted out.
These payers enrolled all or most self-insured clients in CPC. Initially, payers that used these
strategies tended to be larger, national payers that were concentrated in a few CPC markets.
Other payers, concerned that requiring CPC participation might result in their losing self-insured
clients, used an opt-in model, in which they invited self-insured clients to participate in CPC.
Over the course of CPC, several payers shifted from an opt-in model to more proactive
recruitment strategies as they and their clients became more comfortable with the initiative.
Payers indicated that demonstrating the potential benefits of CPC and how it might result in
a positive return on investment for self-insured clients was critical to gaining and sustaining such
clients’ support for the initiative. Most payers that tried to encourage new self-insured clients to
join CPC indicated it was a “hard sell” without evidence of the initiative’s effectiveness.
Similarly, payers that gained high levels of participation among self-insured clients indicated that
clients continued to ask for outcomes data and might discontinue participation in future practice
15

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
transformation efforts without such data. Employers indicated that they were most interested in
seeing evidence of reductions in total cost of care or utilization, such as reduced hospitalizations
or emergency department (ED) visits.
In the absence of data on CPC’s effects, most payers educated employers on evidence
supporting the patient-centered medical home model, which is similar to the CPC model, and the
expected outcomes from CPC. In a few regions, payers used CPC multistakeholder meetings,
which included payers and practice representatives, as a venue to engage self-insured clients and
illustrate CPC’s theory of change. For example, in the Ohio/Kentucky region, payers, practices,
and participating self-insured clients formed an employer committee, which planned educational
opportunities for employers that were not currently participating in the initiative. These
opportunities included tours of CPC practices to show nonparticipating employers the types of
changes CPC practices were making that could impact quality and cost of care for their
employees.
As CPC progressed, payers that started to see improvements in cost and utilization outcomes
noted self-insured clients’ keen interest in these results. A few payers developed reports or tools
to help them track the return on investment of CPC and other initiatives at the employer level.
For example, one payer started providing large employers with data on ED use, hospital
readmission rates, and costs for their employees overall and for those attributed to CPC practices.
2.4. Participating practices and patients
CMS selected 502 practices to participate in CPC. Practice participation remained relatively stable
throughout the initiative—only 9 practices were terminated from the initiative and another 56
voluntarily withdrew. The 439 practices remaining in CPC reported that they had seen approximately
3.1 million patients in 2016.
After selecting the seven CPC regions and their participating payers in April 2012, CMS
invited primary care practices from those regions to apply to participate in the initiative. From the
roughly 1,000 that applied, CMS selected 502 practices to participate in CPC in August 2012. The
number of practices selected per region ranged from 68 in the Oklahoma region to 75 each in the
Ohio/Kentucky and New York Capital District-Hudson Valley regions (Appendix B). CMS
selected practices that it felt had the best opportunity to transform and meet the goals of the CPC
initiative based largely on their experience using health information technology, their experience
with practice transformation or the patient-centered medical home model, and the proportion of
their patients covered by participating payers, among other factors (Taylor et al. 2015).
Selected practices were diverse on many dimensions, including size, the extent to which
they were independent, part of a medical group or owned by a larger health care organization,
and whether they had medical home recognition. Seventeen percent of CPC practices were solo
practitioners, whereas 27 percent had six or more clinicians. Eighteen percent were
multispecialty practices, 55 percent were owned by a larger organization, and 39 percent had
National Committee for Quality Assurance (NCQA) or state-certified medical home
16

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
recognition.
24
For more information on practice characteristics at baseline, see Taylor et al.
(2015).
To understand what motivated practices to participate in CPC, we used information from 21
“deep-dive” practices selected for in-depth examination.
25
Practice leaders from these deep-dive
practices reported that they chose to apply for and participate in CPC because the initiative was
broadly consistent with their own goals for practice improvement and with their aspirations for
providing more patient-centered care (Taylor et al. 2015). Many practice leaders within deep-
dive practices saw CPC as offering both financial and technical support for meeting their own
goals. In addition, the multipayer collaborative nature of CPC offered practices the opportunity
to operate in an environment where the goals and financial incentives of the payers covering
their patients were relatively aligned.
Practice participation remained relatively stable throughout the initiative—1.8 percent of
practices were terminated from the initiative and another 11.2 percent withdrew voluntarily
(Figure 2.3).
26
Specifically, CMS terminated a total of nine practices for not complying with
CPC terms and conditions (three practices each in New Jersey and Oklahoma, two in Arkansas,
and one in New York). Among the 56 practices that voluntarily withdrew, only 13 withdrew
because of challenges in meeting CPC requirements (Table 2.2). The most common reason for
voluntary withdrawal was to join a Medicare ACO (29 practices). (Practices could not participate
in both CPC and a Medicare ACO model. In contrast, for CPC+, CMS is allowing primary care
practices to participate in both Medicaid Shared Savings Program [SSP] ACOs and CPC+.)
Several practices also voluntarily withdrew after assessing the terms and conditions of CPC
participation early in the initiative (five practices) and a few (three practices) withdrew for other
reasons.
27
In addition, six practices closed during the initiative.
24
Baseline practice characteristics were assessed for the 497 practices in the initiative as of March 2013. Five of the
502 practices selected withdrew early in the initiative, after assessing CPC’s terms and conditions.
25
For more information on selection and characteristics of deep-dive practices, as well as analysis methods, see
Peikes et al. (2014), Taylor et al. (2015), and Keith et al. (2017).
26
Several CPC practices also changed their composition during the initiative. Five CPC practices each split into two
practices (adding five CPC practices to the total count). Three CPC practices merged with other CPC practices
(subtracting three CPC practices from the total count).
27
In addition, one practice withdrew after converting to a rural health clinic, which was ineligible to participate in
CPC. One practice withdrew because participating in the initiative resulted in issues for its larger system’s
participation in the Value-Based Payment Modifier and Physician Quality Reporting System. One practice’s reason
for withdrawing is unknown.
17

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
Figure 2.3. Change in the number of CPC participating practices
Source: Mathematica analysis of CMS’s implementation contractor’s practice tracking database.
Note: Changes in the number of CPC participating practices reflect terminations, voluntarily withdrawals, and
practice composition changes (that is, practice mergers and splits).
Table 2.2. Reasons that participating practices left CPC
Total PY2013 PY2014 PY2015
PY2016
Total number of practices that voluntarily withdrew or
were terminated by CMS
65 10 15 35 5
Voluntary withdrawals
Early withdrawals (after practices assessed the terms
and conditions of CPC participation just after it started)
5 5 n.a. n.a. n.a.
Challenges completing CPC requirements
13 0 6 6 1
Decided to join a Medicare ACO
29 2 4 23 0
Practice closed/solo practitioner retired
6 3 1 1 1
Other reason or reason unknown
a
3 0 0 1 2
Terminations by CMS
9 0 4 4 1
Practice remained in CPC but changed composition
Practice split into two practices (adding a practice to
total count)
5 0 3 2 0
Practice merged with another CPC practice (subtracting
a practice from total count)
3 0 1 2 0
Net change in number of participating practices
(accounting for withdrawals, terminations, and changes in
practice composition)
-63 -10 -13 -35 -5
Source: Information from CMS, CMS contractors, and, when possible, Mathematica exit interviews with practices.
502
492
479
444
439
200
250
300
350
400
450
500
Fall 2012
Start of
CPC initiative
December 2013 December 2014 December 2015 December 2016
End of
CPC initative
0
18

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
Table 2.2 (continued)
a
One practice withdrew after converting to a rural health clinic in 2016, since such clinics were ineligible to participate
in CPC. One practice joined a new health system in 2015 and withdrew from CPC because participating in the
initiative resulted in unforeseen issues for its larger system’s participation in the Value-Based Payment Modifier and
Physician Quality Reporting System in 2016. To resolve the issue, CMS backdated the practice’s withdrawal to
December 31, 2015, and recouped the CPC Medicare FFS care management fees the practice received in 2016.
This practice is counted as a 2015 withdrawal. One practice’s reason for withdrawing in 2016 is unknown.
ACO = accountable care organization; PY = program year.
Of the 422 practices that remained in CPC at the end of the initiative and were located in
CPC+ regions, 98 percent (412 practices) applied for and were selected to participate in CPC+.
In addition, 15 of the 57 practices that withdrew or were terminated from CPC for reasons other
than their practice closing and were located in CPC+ regions joined CPC+.
28
Ten of these 15
practices had withdrawn to join an ACO participating in SSP. Three were terminated by CMS for
failing to comply with CPC terms and conditions.
Reflecting the high sustained practice participation rate, CPC physicians and staff had
largely positive views about their experiences participating in CPC. For example, on the 2016
clinician survey, 80 percent of physicians reported that CPC had improved the quality of care or
service provided to their patients, and if they could do it all over again, 79 percent would still
support participation in CPC. Only 12 percent of physicians would oppose participation in CPC,
and 9 percent reported not knowing enough about CPC to answer. Respondents were also asked
about reasons to support and oppose participation. Many believed that CPC improved quality of
care and cited improved patient care; the opportunity to contribute to primary care practice
transformation; and the benefits of financial support, data feedback, and learning supports as
reasons to support participation. Still, even supporters indicated that administrative reporting
presented a burden and that transformation work in CPC was difficult. (See Section 6.3.5 for
additional details on ratings of CPC among CPC practice members.)
2.4.1 Details on CPC practice withdrawals and terminations
More practices voluntarily withdrew during PY2015 than in other program years (31 of 56
practices). The uptick in practice withdrawals during this year was partly due to ACOs
encouraging practices (or their systems) in some regions to join SSP, which would require these
practices to drop out of CPC (because CPC did not allow practices to participate in both CPC
and a Medicare ACO). These recruitment efforts were most intensive in the New Jersey region,
which saw the largest number of voluntary withdrawals during the initiative (17 practices
withdrew to join SSP ACOs or for other reasons). Following New Jersey, the highest numbers of
withdrawals (for any reason) were from the New York (11 practices), Arkansas (10 practices),
and Colorado (9 practices) regions.
29
In Oregon and Oklahoma, six and three practices withdrew,
respectively. No practices withdrew or were terminated in the Ohio/Kentucky region during the
28
Three counties (Putnam, Rockland, and Westchester) that were included in the Capital District–Hudson Valley
region in New York for CPC were not included in the region for CPC+. Fifteen CPC practices that remained in CPC
at the end of the initiative and two that withdrew from CPC were located in these counties and thus ineligible to
apply for CPC+.
29
Among the 56 practices that withdrew, 7 were part of the same New York health system, 6 were part of a New
Jersey system, and 2 were part of a Colorado system.
19

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
initiative, and all CPC practices in Ohio/Kentucky applied and were selected for CPC+.
Appendix B provides additional detail on regional changes in CPC participation.
Practices that were terminated or withdrew from CPC were statistically significantly more
likely to be smaller than those that remained in CPC through the end of the initiative (Table 2.3).
Specifically, 78 percent of practices that left CPC had one to three clinicians, compared with 56
percent of practices that remained for the duration of the initiative. A similar proportion of
practices that left CPC were owned by a larger health care organization (42 percent) than those
that remained (46 percent).
Due to their smaller size, practices that left CPC had received lower total care management
fees (from all participating payers) than practices that remained; however, although PY2014 per-
clinician payments were about $10,000 less in practices that left CPC, per-clinician payments to
the two groups of practices were not statistically significantly different. Similarly, although a
smaller proportion of practices that left CPC (67 percent) reported CPC payments were adequate
than practices who remained (76 percent), this difference was not statistically significant.
Additionally, compared with practices that remained in CPC, practices that left CPC were
statistically significantly less likely to report on a survey of CPC practices that they had reviewed
Medicare FFS feedback reports most or all of the time, that they communicated with their
regional learning faculty (RLF) at least once a week (RLF provided group and individualized
support to CPC practices), and that CPC significantly improved the practice’s quality of care
.
30
Table 2.3. Comparison of practices that left CPC and practices that remained
throughout CPC
Practices that left
CPC
a
Practices that remained
throughout CPC
Practice characteristics
Number of participating clinicians per practice
b
Average (number) 3.28 4.51
Distribution (percentage) ***
One 38.3% 21.6%
Two to three 40.0% 34.2%
Four to five 8.3% 21.0%
Six or more 13.3% 23.2%
30
The smaller than average size of practices that left CPC does not appear to be driving reported differences
between practices that left and remained in CPC. To assess this finding, we conducted a subgroup analysis in which
we compared the experiences of small practices (defined as those with three or fewer clinicians) that left CPC with
small practices that remained (data not shown). Small practices that remained in CPC received similar median per
practice and clinician payments as practices that withdrew. However, compared with small practices that left CPC,
small practices that remained were more likely to report that CPC payments were adequate, that they frequently
reviewed Medicare feedback reports and communicated with RLF, and that CPC significantly improved patient
care.
20

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
Table 2.3 (continued)
Practices that left
CPC
a
Practices that remained
throughout CPC
Practice site is owned by a larger health care
organization (percentage)
b,c
41.7% 45.6%
Geographic location
d
Rural 12.5% 8.3%
Suburban 11.6% 10.7%
Urban 75.9% 80.4%
Practice had PCMH recognition at start of CPC
(percentage)
b
31.7% 40.8%
Practice modified PCMH-A score at the start of CPC
(mean, out of 12)
b
6.16 6.49
CPC supports
Payment
Practice indicates payments from Medicare FFS
are adequate (percentage)
e
67.4% 76.3%
CPC funding from Medicare FFS and other payers
per practice in PY2013 (median)
f
$139,269 $203,425***
CPC funding from Medicare FFS and other payers
per clinician in PY2013 (median)
f
$49,168 $59,125
Data feedback
Practice reviews Medicare FFS feedback reports
most or all of the time (percentage)
e
65.2% 80.9%**
Practice reviews Medicare FFS feedback reports
and views them as very useful (percentage)
e
30.4% 31.9%
Learning support
RLF communicated with practice at least once a
week (percentage)
e
15.2% 28.9%**
Practice rated RLF as excellent (percentage)
e
41.3% 39.6%
Perception of CPC
Practice reported that participation in CPC improved its
quality of care a lot (percentage)
e
34.8% 49.9%*
a
Practices that withdrew early after assessing the terms and conditions of CPC participation just after it started are
not included in this analysis.
b
These items are based on practices’ responses to the CPC practice application and practice surveys administered
in 2013, 2014, 2015, and 2016. The analysis uses the most recently available data. n=60 for practices that left CPC;
n=439 for practices that remained throughout CPC.
c
Practices owned by a larger health care organization include practices where the clinicians are employed by, or the
practice is owned by, a group or staff model health maintenance organization (HMO), hospital, hospital system, or
medical school.
d
This analysis used the Rural-Urban Continuum Codes from the USDA Economic Research Service
(https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation/) to classify practices as rural,
suburban, or urban. n=56 for practices that left CPC; n=439 for practices that remained throughout CPC.
e
These items are based on practice surveys administered in 2014, 2015, and 2016. The analysis uses the most
recently available data. Nineteen practices that left CPC did not complete practice surveys administered during those
years and were excluded. n=46 for practices that left CPC; n=439 for practices that remained throughout CPC.
21

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
Table 2.3 (continued)
f
PY2013 CPC budget data submitted by practices to CMS. PY2013 budget data were not available for sixteen
practices that left CPC. n=49 for practices that left CPC; n=439 for practices that remained throughout CPC.
*/**/*** Statistically significant at the 0.10/0.05/0.01 level.
FFS = fee-for-service; PCMH = patient-centered medical home; PY = program year; RLF = regional learning faculty.
We interviewed representatives from 27 of the 65 practices that withdrew or were
terminated from CPC (11 withdrew to join ACOs, 11 withdrew due to challenges meeting CPC
requirements, 4 were terminated by CMS, and 1 practice closed). Several of the practices that
withdrew from CPC to join SSP ACOs belonged to large health care organizations that had only
a subset of their primary care practices participating in CPC. These practices indicated that
breaking away from their health system’s standardized procedures to establish different
workflows and documentation and reporting processes for CPC was challenging or inefficient.
Moreover, several of these practices were surprised by the administrative burden required to
report CPC Milestones and electronic clinical quality measures (eCQMs). These practices
indicated that ACOs were more attractive than CPC, because ACOs had fewer administrative
requirements, allowed all practices in their system to join the program, and rewarded practices
for savings based on all providers in the system, instead of just providers at the CPC practice
site; thus, practices perceived them as potentially more lucrative than CPC. Some practices that
withdrew to join ACOs indicated that their work under CPC—such as risk stratifying their
patients and hiring care managers—would help them succeed in the SSP ACO.
In contrast, a few
practices felt they had already met the CPC Milestones before the start of the initiative and indicated
that ACOs would increase their flexibility to implement innovative changes (such as hiring a dental
provider).
The practices we spoke with that withdrew due to challenges meeting CPC requirements or
were terminated from CPC were typically small or solo physician practices. Most often, these
practices reported difficulties fulfilling CPC Milestone requirements related to care management
and the medical neighborhood. Several practices also were overwhelmed by CPC reporting
requirements or were unable to generate needed reports from their EHRs. Exacerbating these
challenges, many of these practices faced staffing issues, including difficulties finding staff with
sufficient time to work on the initiative and problems hiring and retaining qualified care
managers. Often, these practices reported that CPC care management fees were inadequate for
them to successfully overcome these challenges. A few practices also indicated that more or
higher-quality support from their RLF would have been helpful.
Participating patients. Participating practices reported that they had seen approximately
3.1 million patients in the program’s final year (Table 2.4). These patients included 320,713
attributed Medicare FFS beneficiaries, 805,980 patients attributed by other participating payers,
as well as 1.9 million nonattributed patients. (The number of attributed patients decreased over
time due to changes in the number of participating practices. Several factors may have resulted in
the increase in number of total patients served, including increased empanelment of patients to
CPC practices or practices’ errors in reporting.)
For all attributed patients, CPC practices received upfront payments in the form of care
management fees, as we discuss in Chapter 3. CPC was designed to transform whole practices;
as such, participating practices were required to implement changes across their entire practice
regardless of patient attribution. This approach aimed to make implementing practice changes fit
22

2. WHO PARTICIPATED IN CPC? MATHEMATICA POLICY RESEARCH
with the realities of clinical workflow, as staff do not need to distinguish between attributed and
nonattributed patients. For PY2016, practices reported that a median of 37 percent of their active
patients were attributed to them by Medicare FFS and other CPC payers, though the proportion
attributed varied. Practices in the lowest quartile of attribution proportion reported that 29
percent or less of their active patients were attributed to them. Practices in the highest quartile
reported 61 percent or more of their patients were attributed to them. (See Section 3.3.1 for
additional information on how the proportion of attributed patients related to CPC funding
levels.)
Table 2.4. Change in the number of CPC patients
End of
PY2013
(Dec 2013)
End of
PY2014
(Dec 2014)
End of
PY2015
(Dec 2015)
End of CPC
initiative
(Dec 2016)
Total patients served by CPC practices 2,544,272 2,800,968 2,846,095 3,053,659
Attributed Medicare FFS beneficiaries
a,c
326,100 337,617 329,270 320,713
Attributed patients of other participating
payers
b,c
887,846 807,734 824,081 805,980
Other, nonattributed patients served by
practices
b,c
1,330,326 1,655,617 1,692,744 1,926,966
a
CMS’s implementation contractor provided lists of attributed Medicare beneficiaries each quarter; these lists were
deduplicated so beneficiaries served in multiple quarters were only counted once in the number of patients ever
attributed. This number differs somewhat from those that practices report.
b
Practices reported the number of attributed and nonattributed patients in their budget and Milestone submissions at
the end of each program year. Practices also submitted the total number of active patients in their practice at a point
in time, which was used to calculate other, nonattributed patients served (by subtracting total attributed patients from
total active patients). Mathematica analyzed the budget data for PY2013 and PY2016; Bland and Associates
analyzed these data for PY2014 and PY2015. Given the potential for slight differences in the methods used to
calculate these statistics, reported differences between years should be interpreted with caution.
c
The number of attributed patients decreased over time due in part to changes in the number of participating
practices. Several factors may have resulted in an increase in the number of total patients served, including
increased empanelment of patients to CPC practices or practices’ errors in reporting.
FFS = fee-for-service; PY = program year.
23
This page has been left blank for double-sided copying.

MATHEMATICA POLICY RESEARCH
3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING DID CMS AND
OTHER PAYERS PROVIDE TO CPC PRACTICES?
For CPC, Medicare fee-for-service (FFS) initially leveraged the support of 39 other payers,
and 36 of those payers remained in the initiative for the duration of CPC. Through CPC’s unique
public–private partnership, CMS and participating payers provided CPC practices with
payments, data feedback, and learning supports to facilitate practice transformation. The
intensity of these supports varied by region and practice; as a whole, however, they represented a
substantial intervention. In this chapter, we draw on a range of data sources to describe the
supports that CMS and other payers provided to practices and how those supports changed over
the course of the initiative as well as highlight practice perspectives on the usefulness of the
supports they received. The first section of this chapter provides an overview of our findings.
The second section highlights the data sources used in this chapter. Then, in the sections that
follow, we provide additional detail on payments, data feedback, and learning supports.
3.1. Key takeaways on CPC supports to practices
CMS and other participating payers provided significant support to CPC practices and, in
general, practices found that these supports helped them accomplish the work required for CPC.
Medicare FFS and other payers provided substantial financial support for participating practices,
with practices reporting that CPC payments accounted for between 10 and 20 percent of practice
revenue, depending on the program year. In addition, CMS and most other payers provided data
feedback to practices; depending on the payer, this feedback included a combination of cost,
utilization, and/or quality data reported at the practice level, patient level, or both. In five
regions, payers aligned or aggregated claims data across payers in the region. Many practices
considered CPC’s data feedback useful, but some found it challenging to incorporate into their
improvement efforts. Many practices also considered CPC learning supports, which included
group learning activities and, for a subset of practices, individualized coaching, important for
achieving the aims of CPC.
3.1.1. Payments to CPC practices
• Medicare FFS and almost all other payers that remained throughout the initiative provided
prospective, monthly, non-visit-based care management fees to CPC practices in addition to
usual payments for services to support enhanced, coordinated care. To support upfront
investments in practice transformation, Medicare and 30 percent of payers paid higher care
management fees in the first two years of the initiative. CMS care management fees for
Medicare FFS attributed beneficiaries averaged $20 per beneficiary per month (PBPM) for
the first two years of CPC and $15 PBPM for the last two years. Care management fee rates
for other payers varied considerably, but most had care management fees that were lower
than Medicare FFS amounts.
• According to Medicare FFS payment data and practice-reported data on payments received
from other payers:
- Care management fees to practices from Medicare FFS and other payers totaled $479.1
million over the four-year initiative.
25

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
- Reflecting the decrease in care management fees beginning in PY2015, median care
management fees per practice were higher in PY2013 ($227,849) and PY2014
($203,949) than in PY2015 ($175,774) and PY2016 ($179,519).
31
Dividing the total
payment to a given practice by the number of clinicians in the practice revealed that
median per-clinician payments also decreased from $70,045 in PY2013 to $50,189 in
PY2016. Depending on the year, these payments accounted for between 10 and 20
percent of practice revenue.
• Medicare and about two-thirds of other participating payers also gave practices the
opportunity to share in any savings accrued during the last three years of the initiative
(PY2014, PY2015, PY2016).
- For PY2014 performance, Medicare FFS found that CPC generated savings in one
region—Oklahoma. Medicare FFS shared savings payments totaled $658,129. Across all
regions, 10 of the 20 non-Medicare FFS payers reporting results of their shared savings
calculations for PY2014 found that CPC generated savings.
- For PY2015 performance, Medicare FFS found that CPC generated savings in four
regions—Arkansas, Colorado, Oklahoma, and Oregon. Medicare FFS shared savings
payments totaled more than $13 million. Across all regions, 10 of the 15 non-Medicare
FFS payers reporting results of their shared savings calculations for PY2015 found CPC
generated savings.
- For PY2016, Medicare FFS found that CPC generated savings in two regions—Arkansas
and Oklahoma. Medicare FFS shared savings payments to practices in these regions
totaled more than $10 million. Non-Medicare payers did not report results for this
performance year in time for inclusion in this report.
• Despite the reduction in care management fees starting in 2015, more than three-quarters of
practices reported on the CPC practice surveys in 2014, 2015, and 2016 that CPC payments
were adequate or more than adequate relative to the costs of implementing CPC.
3.1.2. Data feedback provided to CPC practices
• In PY2015 and PY2016, CMS and 32 of the 36 non-CMS payers provided claims data
feedback to practices.
• Payers in five regions developed a common approach to data feedback. Payers in:
- Arkansas and Oregon took steps to align the cost and service use measures included in
individual payer feedback reports with each other and Medicare FFS.
- Colorado, Ohio/Kentucky, and Oklahoma achieved data aggregation; each region
created a single report or tool to aggregate data across payers each quarter (non-
Medicare FFS payers aggregated data first and then Medicare FFS joined those efforts).
• During interviews, some deep-dive practices reported using CPC data feedback to identify
goals for their quality improvement work or to improve identification of high-risk patients.
31
Payments in PY2013 were higher than in PY2014, because PY2013 included several months of CMS payments
in late 2012.
26

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
• Through interviews with deep-dive practices and CMS contractors and surveys of CPC
practices and clinicians, we identified several challenges practices faced using data
feedback. Some practices:
- Owned by a health system reported that only staff at the health-system level reviewed
feedback reports (instead of physicians at the practice level)
- Viewed data feedback as complex and difficult to understand
- Lacked the time or skills to use data feedback effectively
- Viewed factors driving high costs as out of their control
• Findings from the evaluation suggest that CMS and other payers may improve their
approaches to providing data feedback for future initiatives by:
- Seeking additional input from practices on strategies for improving the format and
structure of data feedback and implementing suggestions when possible
- Providing more training on how to use these data to guide quality improvement
- Encouraging more practice members to review such data
3.1.3. Learning supports provided to CPC practices
• CMS contracted with TMF Health Quality Institute (TMF) to provide learning supports to
CPC practices. TMF and its subcontractors were referred to as regional learning faculty
(RLF).
• CMS and RLF provided practices with a variety of group learning activities, including
webinars and all-day, in-person meetings. CMS and its contractors adapted learning
activities over time to encourage additional peer-to-peer learning, emphasize the use of data
for practice improvement, and engage practices in implementing small tests of change.
• RLF also had limited resources to provide a subset of practices with individualized
coaching. RLF selected practices to receive this coaching and adjusted its intensity
depending on practices’ progress toward CPC Milestones and their performance on quarterly
Medicare feedback reports. On the 2016 CPC practice survey, 56 percent of practices
reported receiving in-person coaching.
• According to the 2016 CPC practice survey, non-Medicare payers also provided coaching or
assistance to 71 percent of practices in the six months before the survey. The percentage that
reported receiving this assistance varied considerably by region, from 52 percent of practices
in Oregon to 96 percent in Ohio/Kentucky.
• Analysis of the CPC clinician and staff surveys indicated that, among members of CPC
practices, care managers were most likely to report having participated in CPC learning
activities, followed by physicians, and then by medical assistants and nurses.
• Practices found in-person learning activities and opportunities for peer-to-peer learning to be
the most valuable form of learning support, according to the practice survey and interviews
with deep-dive practices.
• Although practices valued learning, deep-dive practices also indicated that finding time to
participate in learning activities was challenging and some activities (in particular, webinars)
27

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
were repetitive or not tailored to meet different practice needs. Practices also indicated that
the lack of electronic health record (EHR) vendor participation in learning activities limited
practices’ ability to resolve EHR-related issues.
3.2. Methods
This chapter draws on a range of data sources. Interviews with CMS, its contractors, and
other payers gave us insight into the supports provided to CPC practices. We also used CMS data
on CPC payments and practice-reported budget data to study the magnitude of CPC payments. In
addition, we reviewed CPC data feedback from Medicare and other payers, observed learning
activities, and analyzed data on CPC learning support provided by CMS’s learning contractor
(TMF Health Quality Institute). To understand practices’ use of and perspectives on CPC
supports, we drew on interviews with deep-dive practices selected for intensive study and
surveys with practices, clinicians, and staff. (See Table 1.2 for additional information on the data
sources used for the evaluation.)
32
Although we explore practice and other stakeholder perceptions on the value and benefits of
payments, data feedback, and learning, our evaluation could not disentangle the effect of any
particular support (such as the availability of aggregated data feedback across payers) on practice
transformation from the impact of other practice supports—given that supports were provided to
participating practices as a package.
3.3. Payments to CPC practices
CMS and other payers made substantial payments to CPC practices to support primary care
transformation, in addition to their usual payments for services. These payments were in the
form of non-visit-based care management fees for patients attributed to CPC practices.
33
(Medicare FFS uses the term per beneficiary per month [PBPM] to refer to these payments; other
payers use per member per month [PMPM].) Practices received these payments throughout the
four-year initiative to allow them to “invest in the infrastructure, staffing, education, and training
necessary for delivery of the five comprehensive primary care functions.”
34
Practices were also
provided the opportunity to share in any savings in total health care costs incurred by Medicare
and around two-thirds of other payers in the second, third, and fourth years of the initiative.
Practices were expected to transform care for all patients seen at their practice, regardless of
whether they received payment for them through the initiative.
In this section, we first describe the care management fees CPC practices received from
CMS and other payers. We report the median payments per practice and clinician, highlighting
how median payments changed over time and varied across regions. Next, we describe payers’
approaches to calculating shared savings and report the results of those calculations. Finally, we
32
Practice surveys fielded in 2014, 2015, and 2016 asked practices for their perspectives on CPC supports. The
baseline survey, fielded in 2012, did not ask about CPC supports.
33
Medicare FFS beneficiaries were attributed quarterly to CPC practices that delivered the largest share of their
primary care visits during a two-year look-back period; other payers used their own attribution approaches.
34
This language was included in memoranda of understanding between CMS and each CPC participating payer.
28

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
describe practices’ perceived adequacy of CPC payments and highlight how practices used those
payments to support CPC work.
3.3.1. Care management fees from CMS and other payers
CPC provided participating practices with substantial funding. For each year of the initiative,
practices reported receiving a median of more than $175,000 per practice ($50,000 per clinician) in
care management fees from Medicare FFS and other payers
a. Care management fee structure and level
CMS paid risk-based care management fees for each Medicare beneficiary attributed to a
CPC practice, in addition to FFS payments for regular services and CPC care management fees
for Medicaid FFS beneficiaries in the four regions in which Medicaid participated. To support
upfront investments in practice transformation, Medicare paid higher care management fees in
the first two years of the initiative. For CPC’s first two years, CMS care management fees for
Medicare FFS averaged $20 PBPM (with fee levels of $8, $11, $21, and $40, depending on the
beneficiary’s risk score). Starting in January 2015, CMS reduced the average payment to $15
PBPM (with fee levels of $6, $8, $16, and $30, depending on risk score).
35
All but two of the 36 non-Medicare FFS payers also used PMPM payments for their
enhanced CPC payments to practices; rates varied considerably by line of business (Table 3.1).
36
Most of these payers (including Medicare Advantage plans, Medicaid managed care, commercial
insurers, and, in some regions, CMS on behalf of Medicaid FFS agencies) paid lower PMPM
amounts on average, in part reflecting the lower average acuity level for their patients. Unlike
Medicare FFS, 70 percent of payers did not reduce their PMPM payments over the course of the
initiative. Moreover, two regional payers increased their PMPM amounts in an effort to promote
high-quality primary care. The 30 percent of payers that decreased their PMPM payments made
reductions ranging from 19 to 40 percent of their prior year’s payment, similar to Medicare’s 25
percent reduction. Most of these reductions took place in PY2015.
35
Risk was measured using the patient’s Hierarchical Condition Category (HCC) score (a measure of risk for
subsequent expenditures calculated annually by CMS for each beneficiary attributed to a CPC practice; see Pope et
al. 2004). By design, half of the Medicare FFS funding in each region was for attributed beneficiaries in the highest
HCC risk quartile.
36
One payer provided capitated payments instead of PMPM payments. One payer did not contribute enhanced
payments to practices.
29

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Table 3.1. Range of CPC participating payers’ PMPM payments for PY2016
Payer type PMPM range Median PMPM
Medicare FFS Average PBPM was $15
($6/$8/$16/$30 depending on HCC
risk score)
Average PBPM was $15
($6/$8/$16/$30 depending on HCC
risk score)
Medicare Advantage $4.00–$20.00 $10.00
Commercial, third-party administrator,
administrative services only
$2.00–$9.00 $4.00
Medicaid managed care $2–$11.56 $5.93
Medicaid FFS $4–$10 $4.95
Source: CPC payer worksheets and Mathematica interviews with CPC payers in June through October 2016.
Note: In PY2016, approximately 40 percent of non-CMS payers risk-adjusted their CPC PMPM payments to
practices. If payers provided a PMPM range or PMPM tiers for a line of business, those numbers were
averaged. Payers operating in more than one region are counted multiple times, once for each region in
which they participate. This analysis includes 32 payers. Four CPC payers are excluded from this table:
one is not providing practices enhanced payments, one is using a capitation model, and two did not report
their PMPM levels.
FFS = fee-for-service; HCC = Hierarchical Condition Category; PBPM = per beneficiary per month; PMPM = per
member per month.
b. Median care management fees paid to practices
For each year of the initiative, practices reported receiving a median of more than $175,000
per practice ($50,000 per clinician) in care management fees from Medicare FFS and other
payers (Figure 3.1 and Figure 3.2). Over the course of the initiative, these care management fees
totaled $479.1 million. Although Medicare FFS beneficiaries accounted for only 28 percent of
patients attributed to CPC practices, Medicare FFS care management fees made up 58 percent of
total CPC care management fees to practices. (See Appendix C for total CPC payments from
Medicare and other payers by program year.)
Median payments to CPC practices varied by year as a result of changes in payers’ PMPM
rates as well as changes in the number of lives payers attributed to CPC practices. Most notably,
reflecting the decrease in care management fees over time by Medicare and 30 percent of other
payers, the median payments to practices were higher in PY2013 ($227,849) and PY2014
($203,949) than in PY2015 ($175,774) and PY2016 ($179,519). (Payments in PY2013 were
higher than in PY2014 because PY2013 included several months of CMS payments in late
2012.
37
)
However, even following the decrease in care management fees to practices, CPC provided
a substantial infusion of revenue for practices. CPC care management fees in PY2016 averaged
10.5 percent of 2016 total practice revenue for CPC practices. In PY2016, this funding translated
to a median of $95.41 per attributed patient (that is, for patients attributed to practices by CPC
payers) or about $7.95 PMPM, or $42.57 per active patient (that is, patients attributed by CPC
payers and nonattributed patients) or $3.55 PMPM.
37
CMS defines CPC’s first program year (PY2013) as October 2012 through December 2013. CMS began making
CPC care management payments in October 2012 for the Arkansas and Oklahoma regions, and in November 2012
for all other regions. Other participating payers began making such payments on or before February 1, 2013.
30

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Figure 3.1. Median CPC funding per practice, CPC-wide and by region
Source: Practice-reported budget data analyzed by Mathematica for PY2013 and PY2016 and CMS’s monitoring
and compliance contractor for PY2014 and PY2015.
Note: This analysis is based on practice-reported data. Reported differences between years should be interpreted
with caution, given slight differences in the methods underlying the calculation of these statistics. Medicare
FFS payments in PY2013 were higher than in PY2014 and PY2015, because PY2013 included several
months of CMS payments in late 2012. CMS defines CPC’s first program year (PY2013) as October 2012
through December 2013. CMS began making CPC care management payments in October 2012 for the
Arkansas and Oklahoma regions, and in November 2012 for all other regions. Other participating payers
began making such payments on or before February 1, 2013.
FFS = fee-for-service.
Figure 3.2. Median CPC funding per clinician, CPC-wide and by region
Source: Practice-reported budget data analyzed by Mathematica for PY2013 and PY2016 and CMS’s monitoring
and compliance contractor for PY2014 and PY2015.
Note: This analysis is based on practice-reported data. Reported differences between years should be interpreted
with caution, given slight differences in the methods underlying the calculation of these statistics. Medicare
FFS payments in PY2013 were higher than in PY2014 and PY2015, because PY2013 included several
months of CMS payments in late 2012. CMS defines CPC’s first program year (PY2013) as October 2012
through December 2013. CMS began making CPC care management payments in October 2012 for the
Arkansas and Oklahoma regions, and in November 2012 for all other regions. Other participating payers
began making such payments on or before February 1, 2013.
FFS = fee-for-service.
$228
$211
$200
$211
$231
$377
$176
$257
$204
$180
$183
$148
$233
$324
$154
$216
$176
$162
$162
$131
$217
$242
$128
$186
$180
$173
$172
$146
$202
$246
$139
$218
$0
$50
$100
$150
$200
$250
$300
$350
$400
CPC-wide AR CO NJ NY OH/KY OK OR
Thousands
PY2013 PY2014 PY2015 PY2016
$70
$77
$50
$79
$75
$113
$69
$35
$64
$61
$52
$56
$68
$89
$59
$44
$51
$55
$38
$51
$65
$68
$48
$25
$50
$64
$42
$49
$63
$71
$45
$29
$0
$20
$40
$60
$80
$100
$120
CPC-wide AR CO NJ NY OH/KY OK OR
Thousands
PY2013
PY2014 PY2015 PY2016
31

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Median CPC payments per practice also varied across regions. In 2016, the median CPC
payments per practice ranged from $139,134 in Oklahoma to $246,016 in the Ohio/Kentucky
region (Figure 3.1). The median payment per clinician ranged from $28,752 in Oregon to
$71,499 in the Ohio/Kentucky region in 2016 (Figure 3.2). The total payment to a given practice
reflects the amount of funding payers provided for each attributed patient and the number of
patients that payers attributed to their practice. Payer attribution was highest in regions in which
a large proportion of payers participated in CPC for both their fully-insured and, if relevant, self-
insured lines of business. (Although Medicare FFS paid the same care management fees across
regions, other payers’ care management fee amounts varied.) Regional differences in payers’
payment levels and median numbers of attributed lives per practices contributed to regional
variation in median payments. For example, practices in Ohio/Kentucky reported a median of
more than 2,800 attributed patients, much higher than medians in other regions. Although payers
in Ohio/Kentucky paid similar care management fees as payers in New Jersey, Arkansas, and
Colorado, the high number of attributed lives resulted in Ohio practices reporting the highest
total payments. The regions with the second and third highest median CPC funding per
practice—Oregon and New York, respectively—reported a similar median number of attributed
lives as practices in other regions but a higher median CPC payment level per attributed life.
Figure 3.3. Median attributed patients per practice and median CPC funding
per attributed life, CPC-wide and by region (excluding Oklahoma*)
Source: Practice-reported budget data analyzed by Mathematica for PY2013 and PY2016 and CMS’s monitoring
and compliance contractor for PY2014 and PY2015.
Note: *Oklahoma was excluded from this figure because it had only two non-Medicare payers in CPC+ and
reporting the average amount paid would have divulged payment amounts to each payer.
75
85
95
105
115
125
135
1400 1600 1800 2000 2200 2400 2600 2800 3000
Median CPC funding per attributed life ($)
Median attributed lives per practice
Ohio/Kentucky
New York
Oregon
CPC-Wide
New Jersey
Arkansas
Colorado
32

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
3.3.2. Shared savings payments from CMS and other payers
Medicare FFS found CPC generated savings in one region for PY2014 performance and four regions
for PY2015 performance. Other payers also found CPC generated shared savings: 10 out of 20
payers reporting their results for PY2014 and 10 out of 15 reporting their results for PY2015 found
CPC generated savings for at least one line of business or group of practices.
In addition to CPC care management fees, Medicare and around two-thirds of the other 36
participating payers also provided participating practices with the opportunity to share in savings
in the total costs of care during the last three years of the initiative.
38
Payers shared with
practices a portion of any savings accrued during 2014, 2015, and 2016, approximately 6 to 12
months after the end of each calendar year.
a. CPC shared savings methodologies
Payers’ shared savings methodologies differed along several dimensions, including the level
at which savings were calculated, the method used to calculate savings, and the quality measures
used to determine whether practices were eligible to share in any savings (Table 3.2). Whereas
some payers had to design their own approach to CPC shared savings, many payers (including
both national and regional payers) used design elements from their existing shared savings
programs. In the text box below, we describe several key elements of shared savings approaches
used by payers. (See CMS 2017b and Peikes et al. 2016a for detailed descriptions of Medicare’s
shared savings approach.) CMS’s shared savings calculations serve a different purpose than the
evaluation and, as such, use a different approach than our impact analysis (DeLia 2016). (See
Chapter 8 for additional information on the methodology used for and results from the CPC
impact evaluation.)
38
The following payers did not participate in shared savings: two national payers (one operating in one region and
one operating in multiple regions), seven commercial regional payers, and Medicaid FFS in three regions. The
national payer and one of the larger regional payers that did not offer shared savings provided practices with other
incentive payments under their proprietary programs, such as pay-for-performance programs or risk-based
capitation.
33

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Key elements of payers’ shared savings approaches
Method for calculating savings. Medicare and around half of other payers that reported on their shared savings
approach calculated expected expenditures at the end of the performance year based on trending forward baseline
costs. Actual expenditures were then compared with the expected expenditures to determine net savings. Other
strategies used to calculate savings included comparing actual costs for CPC practices with either a comparison
group of similar practices in the region or with all practices with whom the payer contracts in the region. Most payers,
including Medicare, either included CPC care management fees in practices’ actual expenditures or netted out CPC
care management fees paid to practices from their shared savings calculation.
Groups for whom savings is calculated. To calculate savings, CPC payers typically either (1) combined all CPC
practices for which a payer has attributed lives, or (2) combined certain practices, such as all those participating from
a single health system/medical group or from a virtual group of unaffiliated practices. Following Medicare’s lead,
around one-third of non-Medicare payers used a regional approach. Some of these payers, however, pulled out one
to two large group practices (that is, those with very large numbers of attributed lives in CPC, such as 5,000 or more)
from the regional pool and calculated savings separately for each of these groups—in addition to the regional
calculation for all other CPC practices.
Many CPC payers that combined groups of practices, as opposed to taking a regional approach, referenced their use
of thresholds of 5,000, 7,500, or even 10,000 patients to produce reliable estimates of costs (because smaller groups
show considerable volatility). Except for large systems/medical groups, this method often required combining
nonaffiliated practices into a virtual group for performance purposes. Although a few payers noted that nonaffiliated
practices were increasingly willing to participate in this approach—recognizing that virtual combining was necessary,
because payers were becoming more focused on value-based purchasing—another payer reported mixed success
with this approach, noting that these practice groups have worked best when a few leading practices organized the
rest of the practices.
Adjustments and exclusions. To account for practices who see higher (or lower) risk patients, most CPC payers
adjusted their shared savings calculations. Most commonly, payers used risk and case-mix adjustment (67 percent of
payers who reported on their shared savings approach) and excluded high-cost outliers, such as patients with more
than $250,000 in costs in the performance year (78 percent of payers).
Use of minimum savings rates, maximum percentage of savings shared, and caps on total savings
distributions. Medicare and 43 percent of non-Medicare payers set minimum savings rates necessary to earn
shared savings that ranged from 0.5 to 3 percent. In addition, the maximum percentage of savings that payers
planned to share with practices ranged from 20 to 70 percent, with the most common maximum being 50 percent. A
small number of payers indicated they planned to place caps (or maximums) on the total dollar value of shared
savings distributions, and described these caps in a variety of ways, such as 10 percent of total costs or, in another
case, $4 PMPM.
Determining whether practices are eligible to participate in shared savings. To qualify to share in any Medicare
FFS savings, practices were required to reach a minimum number of quality points earned by surpassing national
benchmarks on claims-based measures (calculated at the regional level), patient experience measures (calculated at
the practice level), and nine electronic clinical quality measures (eCQMs) reported by practices to CMS.
a
Around half
of non-Medicare payers reported that they planned to use the same quality metrics and benchmarks as CMS to
reduce burden on practices; others planned to use different quality or efficiency measures and benchmarks. In
addition, a few payers required that a practice be in good standing on CPC Milestones (that is, not on corrective
action) to be eligible for a savings distribution.
Determining the amount of savings shared with practices. Medicare and two other payers varied the amount of
savings shared with practices by the percentage of total costs saved. Perhaps most notably, Medicare increased the
percentage shared as savings increased.
b
Another payer varied the percentage shared based on historical cost
performance; that is, practices with historically high costs received a lower percentage of any savings, and practices
with historically low costs received a higher percentage. Instead of savings corridors, some other payers used quality
ladders—adjusting the size of a practice’s shared savings distribution based on quality or efficiency metrics.
Moreover, most payers that combined practices into groups or pooled for the region as a whole for purposes of
shared savings also used the number of attributed patients to allocate savings among practices; some also
considered the acuity of attributed patients at a practice.
a
To qualify to share in any Medicare FFS savings, practices were required to reach a minimum number of quality points earned by
surpassing benchmarks for the following measures: (1) three claims-based measures calculated at the regional level and
benchmarked against national performance using the same thresholds as the Medicare Shared Savings Program; (2) five patient
experience measures calculated at the practice-level and benchmarked against the Agency for Healthcare Research and Quality’s
Consumer Assessment of Healthcare Providers and Systems database; and (3) nine eCQMs reported by practices to CMS and
benchmarked against Physician Quality Reporting System (PQRS) data. CMS required practices to report 9 out of 11 eCQMs in
PY2014 and 9 out of 13 in PY2015 and PY2016. Measure reporting, instead of measure performance, was used to determine
practices’ eligibility for shared savings distributions for savings achieved in PY2014.
34

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Key elements… (continued)
b
Medicare’s shared savings corridors: for savings of more than 1 percent and less than 2.3 percent, 10 percent of savings was
shared; for savings of 2.3 to 3.5 percent, 10 percent was shared on savings between 1 and 2.3 percent, plus 30 percent was shared
on savings between 2.3 and 3.5 percent; for savings of more than 3.5 percent, 50 percent of savings was shared.
Table 3.2. 2016 CPC shared savings methodologies among participating payers
Medicare FFS
uses design
feature?
Other participating payers
Number of
payers
reporting on
design feature
a
Percentage
using design
feature, among
those reporting
CPC costs are compared with 19
Expected expenditures based on trending forward baseline costs X 53
The costs of a comparison group of practices similar to CPC
practices
21
The costs of all practices with whom payer contracts in the region 11
Other 16
Care management PMPM payments netted out of shared savings
calculation (or incorporated into expenditures) X 18 78
Group for whom savings were calculated 24
All practices in the region
b
X 30
Practice or groups of affiliated practices 41
Groups of unaffiliated practices 15
Adjustments to savings calculations 18
Excludes high cost outliers 78
Adjusts for demographic characteristics or population risk X 67
Minimum savings rate to earn shared savings
X 21 43
Maximum percentage of total dollar savings shared with practices 18
Less than 50 44
50 X 50
More than 50 6
Metrics used to determine whether practices are eligible to receive
share of savings
15
Practice performance on quality metrics X 80
Practice performance on efficiency metrics 47
In good standing for CPC/not on corrective action 47
None (all practices are automatically eligible) 7
Factors used to determine the amount of savings shared with
practices
17
Number of attributed patients X 88
Acuity of attributed patients X 24
Practice performance on quality metrics 53
Practice performance on efficiency metrics 29
Other 6
Source: CPC payer worksheets and Mathematica interviews with CPC payers conducted June through October 2015 and 2016.
Note: Response categories are not mutually exclusive.
a
Some payers declined to report on certain aspects of their shared savings methodologies. Each table row indicates the number of
payers that reported on a given feature. Payers operating in more than one region are counted multiple times, once for each region
in which they participate.
b
That is, all CPC practices with which the payer has attributed lives. Payers calculating savings for all practices in a region include
payers doing so separately by line of business.
FFS = fee-for-service; PMPM = per member per month.
35

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Many practices were frustrated with shared savings approaches used by Medicare and other
payers. In the 2016 CPC practice survey, about two-thirds of CPC practices agreed or strongly
agreed that they understood Medicare’s shared savings methodology, whereas just over half
indicated they understood non-Medicare payers’ methodologies (Figure 3.4). Moreover, 60
percent of practices indicated they disagreed (49 percent) or strongly disagreed (11 percent) that
the methodology used by Medicare to calculate shared savings in 2015 was fair (Figure 3.4). A
similar proportion of practices reported concerns with other payers’ shared savings
methodologies. (At the time the 2016 CPC practice survey was fielded, Medicare FFS had only
reported the results of its shared savings calculations for PY2014. For that year, CMS found that
CPC generated savings for practices in Oklahoma only. A higher proportion of Oklahoma
practices [67 percent] than practices in other regions reported that the methodology Medicare
used to calculate shared savings was fair.)
Figure 3.4. Practices’ perceptions in PY2016 of Medicare and non-Medicare
shared savings methodologies for assessing PY2015 performance
Source: CPC practice survey administered April through August 2016.
Our 2016 interviews with deep-dive practices selected for intensive qualitative study
provided some insights into practices’ frustration with Medicare’s shared savings methodology:
• Several practices felt it was unfair that Medicare calculated shared savings for all practices
in a region instead of calculating savings for individual practices or groups of affiliated
practices. As one practice expressed, “I’m not afraid of having goals and trying to achieve
those goals as long as I’m judged on my efforts, good or bad, and not based on a whole
group [in the region].”
6
11
7
9
28
49
42
46
61
37
48
43
5
3 3
2
0
20
40
60
80
100
Practice understood how
shared savings in 2015
were calculated
Methodology used to
calculate shared savings
in 2015 was fair
Practice understood how
shared savings in 2015
were calculated
Methodology used to
calculate shared savings
in 2015 was fair
Medicare Participating non-Medicare payers
Percentage of practices
Strongly disagree Disagree Agree Strongly agree
Medicare
Participating non-Medicare payers
36

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
• A few practices also expressed frustration with the quality measures Medicare used to
determine whether practices were eligible to participate in shared savings. For instance, one
expressed the concern that quality measures calculated for small practices were unreliable
due to small sample sizes, and another indicated that its EHR was inaccurately reporting on
electronic clinical quality measures (eCQMs).
• In addition, a few practices reported that Medicare’s shared savings methodology held them
responsible for reducing costs incurred by specialists or hospitals that they felt were outside
of their control.
For CPC+, instead of using shared savings to reward practices for cost and quality
performance, CMS is using a prospective performance-based incentive payment. Specifically,
CMS pays CPC+ practices an incentive payment at the beginning of the year that may be
partially or fully recouped at the end of the year if a practice does not meet cost and efficiency
standards. CMS transitioned its approach due to concerns practices raised about its shared
savings methodology for CPC. In addition to moving from a retrospective shared savings
payment to a prospective incentive payment, CMS strengthened the incentive in CPC+ by
placing it at the level of the individual practice rather than at the region level.
b. Shared savings payments to CPC practices
CMS found that CPC generated savings in at least one region for PY2014, PY2015, and
PY2016 performance. Specifically, CMS paid practices shared savings payments totaling $658.1
thousand for PY2014 performance, $13.1 million for PY2015 performance, and $10 million for
PY2016 performance. Oklahoma was the only region for which CMS found that CPC generated
savings for each of the three program years (Table 3.3). CMS found that CPC generated savings
in Arkansas in PY2015 and PY2016 and in Colorado and Oregon for PY2015 only.
Fifteen non-CMS payers reported to Mathematica results of their shared savings calculations
for PY2015 performance (Table 3.4). Among those payers, two payers each in Arkansas,
Colorado, New York, and Oregon and one each in New Jersey and Ohio/Kentucky found CPC
generated savings in PY2015 for at least one line of business or group of practices. For PY2014
performance, 20 non-CMS payers reported results: two payers each in Colorado, Ohio/Kentucky,
and Oklahoma, as well as one payer each in Arkansas, New Jersey, New York, and Oregon,
found CPC to generate savings. During our final round of CPC data collection, no payers had
finalized their shared savings calculations for PY2016 performance.
37

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Table 3.3. Results from CPC Medicare FFS shared savings calculations for
performance in PY2014, PY2015, and PY2016 by region
PY2014 PY2015 PY2016
Percentage
change in net
expenditures
a
Number of
practices
receiving
payments
b
Average
payment per
practice
c
Percentage
change in net
expenditures
a
Number of
practices
receiving
payments
b
Average
payment per
practice
c
Percentage
change in net
expenditures
a
Number of
practices
receiving
payments
b
Average
payment per
practice
c
AR 1.1 0 $0 -2.4 56 $13,376 -2.7 54 $13,520
CO -0.3 0 $0 -2.2 65 $7,094 2.1 0 $0
NJ 2.4 0 $0 5.7 0 $0 4.5 0 $0
NY 5.7 0 $0 5.3 0 $0 5.4 0 $0
OH/KY 1.6 0 $0 2.2 0 $0 6.8 0 $0
OK -2.4 56 $10,009 -5.4 52 $208,909 -4.0 59 $122,859
OR 1.7 0 $0 -2.6 66 $15,783 0.9 0 $0
Source: CMS CPC PY2014, PY2015, and PY2016 Shared Savings & Quality Results.
a
Expenditures include the care management fees that Medicare paid CPC practices. A negative value for change in net
expenditures indicates savings compared with relative trended targets; a positive value indicates higher costs relative to trended
targets.
b
To qualify to share in any Medicare (fee-for-service) FFS savings, practices were required to reach a minimum number of quality
points earned by surpassing benchmarks for the following measures: (1) three claims-based measures calculated at the regional
level and benchmarked against national performance using the same thresholds as the Medicare Shared Savings Program; (2) five
patient experience measures calculated at the practice-level and benchmarked against the Agency for Healthcare Research and
Quality’s Consumer Assessment of Healthcare Providers and Systems database; and (3) nine electronic clinical quality measures
(eCQMs) reported by practices to CMS and benchmarked against Physician Quality Reporting System (PQRS) data. CMS required
practices to report 9 out of 11 eCQMs in PY2014 and 9 out of 13 in PY2015 and PY2016. Measure reporting, instead of measure
performance, was used to determine practices’ eligibility for shared savings distributions for savings achieved in PY2014.
c
Medicare increases the percentage shared as savings increase. Medicare’s shared savings corridors: for savings of more than
1 percent and less than 2.3 percent, 10 percent of savings was shared; for savings of 2.3 to 3.5 percent, 10 percent was shared on
savings between 1 and 2.3 percent, plus 30 percent was shared on savings between 2.3 and 3.5 percent; for savings of more than
3.5 percent, 50 percent of savings was shared.
Table 3.4. Payers reporting that CPC generated savings for PY2014 or
PY2015, among those reporting results of their shared savings calculations,
by region
PY2014 performance PY2015 performance
Medicare FFS found
CPC generated
savings
Number of non-
Medicare payers that
found CPC generated
savings
Medicare FFS found
CPC generated
savings
Number of non-
Medicare payers that
found CPC generated
savings
AR 0 1 of 2 X 2 of 2
CO 0 2 of 4 X 2 of 4
NJ 0 1 of 2 0 1 of 1
NY 0 1 of 3 0 2 of 3
OH/KY 0 2 of 4 0 1 of 1
OK X 2 of 2 X 0 of 0
OR 0 1 of 3 X 2 of 4
Total 1 of 7 regions 10 of 20 payers 4 of 7 regions 10 of 15 payers
Source: CPC payer worksheets and interviews.
Notes: This table includes only Medicare FFS and non-Medicare payers that shared savings and reported their
results to Mathematica. Payers are counted separately for each region in which they participate.
FFS = fee-for-service.
38

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
3.3.3. Practices’ use of and perspectives on CPC payments
CPC practices generally found CPC enhanced payments were adequate to pay for staff labor and
other supports needed to implement CPC.
Practices generally perceived Medicare FFS enhanced funding to be adequate relative to the
costs of implementing CPC, even following the decrease in care management fees in PY2015
(Figure 3.5). More than three-quarters of practices reported on the 2014, 2015, and 2016 CPC
practice surveys that CPC payments from Medicare FFS were adequate or more than adequate
relative to the costs of implementing CPC.
Although practices owned by a larger health care organization were more likely to perceive
fees as adequate or more than adequate than their independent counterparts in PY2014, this
difference did not persist in the PY2015 and PY2016 surveys. The percentage of practices owned
by a larger health care organization that perceived Medicare FFS care management fees to be
adequate or more than adequate decreased from 92 percent in PY2014 to 80 percent in PY2016.
This finding may in part reflect the larger decrease in CPC payments per practice and per
attributed life reported by practices owned by a larger health care organization than independent
practices following Medicare FFS reduction in PBPM levels in PY2015.
Figure 3.5. Practices’ perceived adequacy of Medicare FFS care management
fees relative to the costs of implementing CPC in PY2014, PY2015, and
PY2016
Source: CPC practice survey, administered April through July 2014 and April through August 2015 and 2016.
Note: We identified each practice as being part of a system or not, using the practice’s responses to the 2016
CPC practice survey. When asked to describe the medical organization that employs the clinicians at the
practice site, or who owns the practice, we considered practices that responded with these responses to be
in a healthcare system: group or staff model Health Maintenance Organization (HMO); network of clinician
practices owned by a hospital, hospital system, or medical school; or hospital or medical school.
Columns may not add up to 100 percent due to rounding.
19
24
24
28
23
26
8
25
21
70
72
71
63
72
66
78
72
76
11
4
6
9
5
8
13
4
4
0
20
40
60
80
100
PY2014 PY2015 PY2016 PY2014 PY2015 PY2016 PY2014 PY2015 PY2016
Percentage of practices
Less than adequate Adequate More than adequate
All practices Independent practices Practices owned by a
larger health care
organization
39

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Practices’ ratings of the adequacy of other participating payers’ practice payments varied
among regions and payers but were generally lower than their ratings of Medicare payments.
Roughly half of practices rated the other payers’ CPC payments as inadequate.
Practices reported using CPC enhanced funding to support similar amounts of labor and
nonlabor costs in PY2014, PY2015, and PY2016.
39
Labor costs were the largest area of
spending, accounting for about 87 percent of total practice-reported CPC spending during the last
three years of CPC (Figure 3.6). For each year, the largest categories of labor costs were
clinicians—which include physicians, physician assistants, and nurse practitioners ($121.9
million); care managers ($47.2 million); licensed practical nurses and medical assistants ($46.7
million); and registered nurses ($39.3 million). (In some practices, registered nurses, licensed
practical nurses, and medical assistants provided care management services as part of their
responsibilities.) After labor, practices reported spending the most CPC funding on information
technology (IT) equipment or consulting ($25 million). Practices also used CPC funding for non-
IT equipment, office space, and training or travel (for example, to in-person learning meetings or
similar activities). Chapter 5 of this report describes how practices devoted staff time, health IT,
and other resources to meeting CPC Milestones.
Figure 3.6. Practice-reported total CPC spending in PY2014, PY2015, and
PY2016 for selected cost categories, in millions
Source: Practice-reported budget data analyzed by CMS’s monitoring and compliance contractor for PY2014 and
PY2015 and Mathematica PY2016.
Note: Practices did not report spending by these cost categories in PY2013. Clinicians includes physicians,
physician assistants, and nurse practitioners.
IT = information technology; LPN = licensed practical nurse; MA = medical assistant.
39
Practices reported spending by CPC Milestone instead of cost type (such as labor) in PY2013. These data are
reported in the first annual report for the CPC evaluation (Taylor et al. 2015).
Labor, $342
IT equipment
and consulting,
$25
Office space, $8
Non-IT supplies,
$3
Training/travel,
$4
Other expenses,
$11
Clinicians
36%
Registered
nurses
11%
LPN/MA
14%
Care
managers
14%
Clinical
pharmacist
2%
Other labor
23%
40

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
3.4. Data feedback provided to CPC practices
In addition to enhanced payments, CMS and most participating payers provided CPC
practices with performance feedback data to support building a culture of continuous
improvement driven by data. As part of its evaluation contract, Mathematica Policy Research
produced the Medicare FFS data feedback reports and patient-level data files for CMS. In this
section, we first describe the data feedback CMS and other payers provided to participating
practices. We give an overview of CPC data feedback and then describe in additional detail the
feedback from individual payers and efforts to align data feedback. Finally, we describe
practices’ use of and perspectives on CPC data feedback.
3.4.1. Data feedback from CMS and other payers
CMS and most other payers provided data feedback to CPC practices. Over the course of CPC,
payers improved data feedback by incorporating additional measures, improving the timeliness of
data, and aligning or aggregating data across payers.
In PY2015 and PY2016, CMS and all but 4 of the 36 non-CMS payers (89 percent) provided
data feedback to practices, an increase from the first two years of the initiative, during which
about two-thirds of payers did so (68 percent).
40
At the outset of CPC, payers primarily provided
practices with individual payer reports. The content and structure of this feedback was designed
by each payer individually, often based on data feedback they were already providing to
practices before CPC. Most payers took steps over the four-year initiative to provide new or
additional forms of data feedback to practices or to improve existing reports. Payers in six
regions also worked to align the content and timing of feedback across payers in a given region.
Ultimately, payers in five regions developed a common approach to data feedback. Specifically,
payers in Arkansas and Oregon took steps to align the cost and service use measures included in
individual reports with each other and Medicare FFS. Payers in Colorado, Ohio/Kentucky, and
Oklahoma achieved data aggregation—producing a single report or tool that aggregated data
across payers (non-Medicare FFS payers aggregated data first, and then Medicare FFS joined
those efforts).
a. Feedback from individual payers
Medicare FFS feedback reports. Starting in April 2013, CMS began to provide quarterly
Medicare FFS practice-level feedback reports and patient-level data files to participating
practices. Each CPC Medicare performance feedback report included (1) a practice-level PDF
report with summary information about Medicare patients attributed to the practice, their costs,
and their use of hospital inpatient and emergency department services; and (2) a Microsoft Excel
file with detailed information about each patient’s demographic characteristics and Medicare
FFS costs and service use. (See the text box below describing the Medicare FFS data feedback
content.)
40
Four payers did not complete interviews in 2016. We used the most recently available data on the data feedback
they provided to practices.
41

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
CMS and Mathematica revised the reporting templates, data displays, and specifications of
the Medicare FFS data feedback over the course of the initiative based on input from practices
and RLF, in an effort to continuously improve the reports and make them useful. For example,
CMS added data on trends in performance over time and started risk-adjusting cost data to
account for additional patient-level characteristics to improve comparability across practices with
different patient populations. In addition, CPC practices and RLF in some regions, noting that
costs incurred as a result of specialty care from other providers were a significant driver of
patients’ total costs, requested more detailed information on specialty care. In response, CMS
worked with CPC practices in an iterative process to pilot-test, refine, and share practice-specific
reports on specialty care. This process resulted in a one-time detailed report in May 2016 about
CPC patients’ specialist visits and spending on specialty care for 2015 broken down by specialty
type. Specialist care data were intended to help practices (1) understand how different specialties
contribute to the total cost of care for their patient panel, and (2) identify individual specialists
that account for the highest proportion of specialist visits and costs for their patients, suggesting
opportunities for improved care coordination.
Other payers’ feedback reports. At the outset of CPC, all payers that offered data
feedback to practices (approximately two-thirds of payers) provided individual payer reports.
The proportion of payers providing individual data feedback decreased over time as some payers
transitioned to providing aligned or aggregated data feedback (see section below). Individual
payers’ reports primarily contained measures of cost and service utilization, although some
payers also reported quality measures (such as rates of colorectal cancer screening and childhood
immunizations). Some payers supplemented claims-based data feedback reports with close-to-
Medicare FFS data feedback for CPC practices
The CMS quarterly feedback reports provided practice-level information on:
• Characteristics of attributed Medicare FFS patients and how these patients compare with those of other CPC
practices in the same region
• Risk-adjusted Medicare expenditures PBPM, including average total expenditures and expenditures by type of
service; also, annualized use of Medicare services and selected outcomes three to six months before the
report was released, including all-cause hospitalizations, hospitalizations for ambulatory care sensitive
conditions, overall and outpatient emergency department visits, and unplanned 30-day hospital readmissions.
Both risk-adjusted Medicare expenditures and use of services were:
- Compared with those of all CPC practices in the region, overall and for high-risk patients
- Compared over time to their own experience and to that of all CPC practices in the same region with a
similar risk profile
• Responses from surveys of CPC practices about approaches to primary care delivery and practice
demographics; surveys of patients about their experience with care; and CPC-wide data from clinicians and
staff about their experiences delivering care
Patient-level data files accompanying the feedback reports provided the following patient-level information for
beneficiaries attributed to each practice in the current quarter:
• Beneficiary identifiers (patient identification number, last name, first name, age, gender, Medicaid enrollment,
or dual eligibility status)
• CPC HCC-risk category
• Total Medicare expenditures and percentage breakdown by service category
• Use of primary care and specialist physician services
• Hospital admissions (overall, and for ambulatory care sensitive conditions)
• Unplanned 30-day readmissions
• Emergency department visits
Practices could download the practice-level report and patient-level data files from the CPC web application.
42

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
real-time data on patients’ emergency department (ED) and inpatient admissions, discharges, and
transfers (in the form of weekly or even almost daily rosters). In addition, some payers provided
practices with lists of care gaps for patients (such as patients due for breast cancer screening or
patients with diabetes who need eye exams). Payers noted that practices like to know about “the
care opportunities to go after,” and lists of gaps in care give practices concrete areas for
improvement.
Largely in response to practices’ input on data feedback, most payers worked to improve
their feedback reports over the course of the initiative. Changes included adding detailed patient-
level data, incorporating data on utilization of ancillary services (such as labs), improving the
timeliness of ED and inpatient admission data, and using interactive portals (instead of static
reports) to disseminate data feedback. Also similar to CMS, some other payers began providing
practices with data on specialists seen by their patient panel.
b. Aligned or aggregated data feedback
As part of their participation in CPC, CMS and
other payers agreed at the start of CPC to work
together to develop a common approach to data
feedback. Payers in all regions but New Jersey
initially pursued data aggregation—that is,
producing one report that aggregates data across
payers. Payers indicated that aggregated data were
intended to help practices to better understand their
overall performance on cost, quality, and use
measures and identify areas for improvement in care
delivery without the burden of accessing and
interpreting multiple payer-specific reports.
Additionally, data aggregation aimed to reduce the
time practices spent sorting through and analyzing
individual reports from multiple payers.
Data aggregation, however, proved challenging
in all regions (Peikes et al. 2016a). Payers reported
being surprised by the cost of data aggregation and
the time required to devise and implement an
aggregation plan (see Text Box for a list of design
decisions involved in data aggregation). Additionally, during PY2013, CMS had to change its
approach to contracting for data aggregation several times due to unforeseen legal and
operational hurdles that delayed CMS participation and required payers to restart or rework their
processes. Ultimately, CMS decided to have other payers in each region take the lead and design
an approach that would work for their regional context; CMS subsequently joined their efforts.
Faced with these challenges, regions ultimately varied in the extent to which payers aligned
data feedback for CPC practices. By the end of CPC:
• The Colorado, Ohio/Kentucky, and Oklahoma regions produced aggregated reports or tools
from their payers’ claims data. The Colorado tool allowed practices to create lists of patients
Important data aggregation design
decisions
Establishing the data aggregation
management infrastructure:
• Select a vendor to aggregate the data
and create the tool or report
• Develop a governance structure to
address ongoing policy and technical
issues
• Decide how to allocate the costs of
aggregation across payers
• Develop a process to train practices on
how to use aggregated data
Determining the content and structure of
the tool or report:
• Decide on the level of claims
information to share (for example,
patient and/or practice)
• Agree how to benchmark performance
• Select a platform to display the data
• Address data validity and
comparability challenges (such as,
approaches to risk-adjust data and
attribute patients to practices)
43

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
who had not received preventive care services, reports on care delivered by specialists, and
analyses on the use of generic versus brand name drugs, for example. In Ohio, practices
received reports that included displays of trends in spending by type of service as well as
inpatient admissions and emergency department visits. Non-CMS payers in Colorado first
released their tool in PY2015; non-CMS payers in Ohio/Kentucky and Oklahoma began
producing reports in PY2016 (Table 3.5.).
41
Payers in Colorado and Oklahoma paid all costs
for data aggregation; in Ohio/Kentucky, practices covered half the cost of data aggregation.
After producing their first aggregated reports, payers worked with their data aggregation
vendors to improve the usefulness of aggregated data by simplifying displays and addressing
data inconsistences. For example, Colorado payers, concerned that the data feedback tool
required practices to make too many choices among display and drill-down options to
produce a report, urged the vendor to produce “bookmarks” with preset filters so that
practices could produce standard reports with a single click. CMS faced substantial
contracting delays because the federal government’s procurement processes were not set up
for joining with other entities, such as private payers. However, overcoming these hurdles,
CMS joined claims-based data aggregation efforts in these regions in September 2015,
although CMS data were not fully integrated until 2016. (Appendix C details the data
aggregation management infrastructure in each region and the content and structure of
aggregated reports.)
• Payers in Arkansas and Oregon aligned individual reports in terms of content or structure.
Noting early challenges and delays with data aggregation, payers in these two regions
indicated that aligned reports were a more feasible, timely, and affordable common
approach to data feedback. In PY2014, payers in each region selected a set of common
measures to report on that aligned at least partially with the measures included in Medicare
FFS data feedback (Table 3.6).
42
Arkansas payers were generally satisfied with the aligned
reports and improved them over time by aligning measure specifications and adding aligned
patient-level data files to the practice-level reports. In contrast, several Oregon payers
voiced concern that the reports had limited utility for practices because although the Oregon
payers used the same measures and reporting format, measure specifications still differed
across payers.
• Payers in New York and New Jersey did not ultimately pursue a common approach to data
feedback. New York payers spent considerable time during the first three years of CPC
discussing data aggregation but ultimately decided they did not have sufficient time
remaining in the initiative to justify the cost of pursuing aligned or aggregated reports. In
contrast, New Jersey payers decided early in the initiative not to pursue a common approach
to data feedback given that two payers (one of which was Medicare) accounted for a large
proportion of attributed CPC lives in the region and, thus, aligning feedback had limited
value.
41
Before producing reports that aggregated patient-level claims data, payers in Oklahoma provided practices reports
that aggregated practice-level data.
42
Payers in Ohio/Kentucky began producing aligned reports in PY2014 but stopped after releasing aggregated
reports.
44

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Table 3.5. Timing and structure of aggregated data feedback from payers’
claims data in Colorado, Ohio/Kentucky, and Oklahoma, PY2016
Region
Non-CMS payers
participating in data
aggregation
Start date for data
aggregation
Date Medicare data
included
Frequency of data
refresh
Colorado 6 of 8 June 2015 September 2016 Quarterly
Ohio/Kentucky 8 of 8 January 2016 June 2016 Quarterly
Oklahoma 3 of 3 November 2016
a
November 2016 Quarterly
Source: Mathematica interviews with CPC payers in June through August 2016.
a
In PY2015, payers in Oklahoma began providing practices with reports that aggregated practice-level data (as
opposed to patient-level claims data).
Table 3.6. Content and structure of aligned feedback reports in Arkansas and
Oregon, PY2016
Region
a
Participating
non-Medicare
payers Start date Frequency
Common
set of
measures
Measures
specifications
aligned
Report
format
aligned
Patient-
level data
aligned
Arkansas 3 of 3
b
Fall 2014 Quarterly Yes Yes No Yes
Oregon 3 of 5 Spring 2014 Quarterly Yes No Yes No
Source: Mathematica interviews with CPC payers in June through August 2016.
a
Payers in Ohio/Kentucky began producing aligned reports in PY2014 but stopped after releasing aggregated
reports.
b
We excluded one payer in Arkansas because it did not participate in an interview.
3.4.2. Practices’ use of and perspectives on CPC data feedback
CPC practices valued data feedback, although their use of such feedback varied across regions and
practices and depended on the type of report.
a. Practices’ use of CPC data feedback
In PY2016, most practices reported reviewing practice-level data feedback from Medicare
FFS (97 percent) and other payers (89 percent). Similarly, most practices reported reviewing
patient-level data files from Medicare FFS (88 percent) and other payers (80 percent). Although
most practices reported having reviewed data feedback, the frequency with which practices
reviewed reports varied (Figure 3.7; see Appendix C for additional detail on practices’ review of
and perspectives on data feedback):
• More practices reported reviewing data feedback from Medicare FFS all or most of the time
than reported reviewing data feedback from other payers all of most of the time.
• For both Medicare FFS and other payers’ feedback, practices reported reviewing practice-
level reports more frequently than patient-level data files.
• The percentage of practices that reported reviewing feedback all or most of the time
increased slightly from 2014 to 2016. Most notably, the percentage of practices that reported
45

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
frequently reviewing other payers’ practice-level feedback reports increased from 48 percent
in PY2014 to 60 percent in PY2016.
• The percentage of practices that reported frequently reviewing reports in 2014 varied widely
across regions; however, by 2016, regional variation had declined with one notable
exception. Practices in Oregon were less likely to report reviewing all types of data feedback
in 2016 than practices in other regions (Table 3.7).
Figure 3.7. Percentage of practices that reported receiving and reviewing
CPC data feedback all or most of the time, PY2014 and PY2016
Source: CPC practice survey, administered April through July 2014 and April through August 2016.
Table 3.7. Percentage of practices that reported receiving and reviewing
feedback reports and patient-level data files all or most of the time in 2016,
CPC-wide and by region
CPC-
wide AR CO NJ NY OH/KY OK OR
Medicare FFS feedback reports 81 85 80 87 88 86 88 55
Medicare FFS patient-level data files 58 57 49 56 62 69 77 38
Other payer feedback reports 60 60 67 67 72 59 58 40
Other payer patient-level data files 41 33 47 44 53 38 45 25
Source: CPC practice survey, administered April through August 2016.
Note: The denominator includes practices that reported not receiving reports. Slight differences between the
numbers included in Figure 3.7 and Table 3.7 may have occurred due to rounding.
FFS = fee-for-service.
18
28
16
23
22
29
15
16
55
53
31
35
26
32
23
25
0
20
40
60
80
100
2014 2016 2014 2016 2014 2016 2014 2016
Feedback reports Patient-level data Feedback reports Patient-level data
From Medicare FFS From other payers
Percentage of practices
Always
Most of the time
From Medicare FFS From other payers
46

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
The 2016 CPC clinician and staff survey (which was fielded to a sample of clinicians and
staff in CPC practices and only clinicians in comparison practices) indicated that a high
proportion of physicians in both CPC practices and comparison practices had seen feedback
reports on their performance in the prior year. Although rates were high for both groups,
physicians in CPC practices were more likely than physicians in comparison practices to report
seeing data feedback (88 versus 71 percent).
CPC physicians were more likely to report in the 2016 CPC clinician and staff survey seeing
feedback from Medicare if they were in smaller versus bigger practices or if they were
independent practices versus part of a system.
43
Consistent with this finding, on the 2016 CPC
practice survey, 27 percent of practices that are part of a health care system reported that
feedback reports are reviewed by staff at only their larger health care system or medical group
(not by staff at the practices themselves). However, physicians who reported not seeing Medicare
feedback reports may have seen the information after others in the practice (or system, if the
practice was system-owned) repackaged it into different formats, and reports from multiple
payers may have been confusing.
b. Practices’ perspectives on usefulness of CPC data feedback
Practices reported that they found feedback reports and patient-level data files valuable but
faced challenges in using them. On the CPC practice survey, more than 80 percent of CPC
practices that reviewed feedback from Medicare FFS or other payers reported the information
was somewhat or very useful in meeting CPC Milestones and improving primary care (Figure
3.8). From PY2014 to PY2016, the percentage of CPC practices reporting that data feedback was
very useful increased for each type of feedback.
43
We identified each practice as being part of a system or not, using the practice’s responses to the 2016 CPC
practice survey. When asked to describe the medical organization that employs the clinicians at the practice site, or
who owns the practice, we considered practices that responded with these responses to be in a healthcare system:
group or staff model Health Maintenance Organization (HMO); network of clinician practices owned by a hospital,
hospital system, or medical school; or hospital or medical school.
47

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Figure 3.8. Percentage of practices reporting that CPC data feedback was
somewhat or very useful, among practices that reported seeing the
feedback, PY2014 and PY2016
Source: CPC practice survey, administered April through July 2014 and April through August 2016.
During 2016 interviews with deep-dive practices selected for intensive qualitative study,
some practices reported using CPC data feedback to identify goals for their quality improvement
work. For example, one practice indicated that data feedback reports helped it identify reducing
referrals to laboratory services as a strategy to decrease total costs of care for its patients. To
reduce those referrals, the practice established guidelines that limited when practice members
ordered follow-up laboratory tests. Another practice noticed that ED use was a major cost driver
for its patients. In response, the practice reached out to patients who had visited the ED to remind
them that they should call the practice before going to an ED. Other practices reported using data
feedback to better identify high-risk patients or to increase prescribing of generic drugs.
Although practices viewed data feedback as valuable, our interviews with deep-dive
practices, payers, and RLF identified several challenges practices faced in using it. We outline in
the following section the major challenges and the steps CMS, payers, and RLF took to help
practices address them (Gerteis et al. forthcoming).
Challenges CPC practices faced using data feedback:
• Practices sometimes found the reports difficult to understand. At the outset of the initiative,
practices indicated the reports were complex and included a vast amount of information,
impeding their perceived usefulness. Many were also unfamiliar with the metrics reported,
including standardized utilization rates and comparative benchmarks.
21
35
14
30
12
24
14
21
69
58
65
56
62
63
63
63
0 20 40 60 80 100
2014
2016
2014
2016
2014
2016
2014
2016
Feedback
reports
Patient-level
data
Feedback
reports
Patient-level
data
From Medicare FFSFrom other payers
Percentage of practices
Very useful
Somewhat useful
From Medicare FFSFrom other payers
48

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
• Some practices lacked the time or skills to use data feedback effectively. Their ability to
understand and use the reports depended in large part on the resources available to help
them. Feedback from RLF and in-depth interviews with deep-dive practices suggested that
smaller practices, in particular, tended to have limited experience using data for
improvement and lacked the staff time, resources, and analytic capacity to help them
interpret the quarterly reports.
• Practices sometimes looked for and expected data feedback to help them manage patients in
real time. The inherent lag in claims data means that the reports cannot easily be used to
determine the short-term impact of discrete interventions on cost or service use or to identify
patients with time-sensitive care gaps.
44
This challenge was especially frustrating to
practices that lacked ready access to information from other sources, such as EHR systems
or notifications about admissions to local hospitals, to monitor patients over the shorter term.
• Practices often viewed factors driving high costs as out of their control. Some practices
doubted that factors driving patients’ resource use and total costs of care in an aging
population and an FFS environment—hospital admissions, ED use, specialty care, and post-
acute care—are amenable to a primary care practice’s influence. Practices reported that
specialists, hospitals, or patients—not just the primary care practice—needed to change their
behavior.
To address these challenges, CMS and other payers:
• Took steps to improve data feedback. For example, some payers worked to reduce the lag in
claims data or began using interactive portals instead of static reports.
• Provided practices additional types of feedback. For example, CMS and some payers began
providing detailed patient-level data to help practices identify high-cost patients. In addition,
a number of payers began providing practices with reports on specialists’ costs and use in
2016 to help them strategize ways to influence costs sometimes viewed as out of their
control. Several deep-dive practices reported using them to, for example, identify
unnecessary specialist encounters and procedures or lower-cost specialists for future patient
referrals.
• Educated practices about data feedback. Starting in PY2014, RLF increasingly focused on
educating practices about the existence of various reports and how to use them. For
example, RLF encouraged practices to use claims data for identifying opportunities to
improve care delivery in general, rather than evaluating quality improvement (QI)
interventions in real time, or supporting individual patient care decisions. As one practice
explained, “I think payer reports are definitely very helpful, but I had to learn how to read
them more efficiently and to take into account different things that I might not have thought
of when they were first sent out to us.” This shift in educational strategy corresponded with
CMS adjusting the requirements for Milestone 5: Quality Improvement to require practices
to use payer feedback to identify areas for improvement. Payers, mostly in regions
aggregating data, also provided practices with training on how to access and use reports—
44
The lag between the date of service and when the practice received the Medicare FFS feedback reports was three
to six months. For the CPC regions that aggregated data, the lag for reports was three to six months for Colorado, six
months for Oklahoma, and eight months for Ohio/Kentucky. The lag times for other payers’ feedback varied.
49

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
either directly or in collaboration with the region’s data aggregation vendor, RLF, or both.
(See Section 3.5 for additional detail on RLF’s and payers’ learning strategies.)
3.5. Learning supports provided to CPC practices
In addition to enhanced payments and data feedback that CMS and other payers provided to
practices, CMS and RLF provided learning activities for CPC practices. In this section, we first
describe the learning infrastructure developed by CMS and its contractors. We provide an
overview of activities offered by RLF and practices’ participation in those activities and then
detail the CPC group learning activities and individualized practice coaching. Next, we describe
non-Medicare payers’ involvement with CPC learning, the extent of their involvement, and how
much support they provided to practices through other initiatives. Finally, we describe practices’
reported experience with CPC learning support.
3.5.1. Learning support from CMS
CMS and its contractors provided CPC practices with substantial learning support, including a variety
of group learning activities and—for a subset of practices—individualized coaching.
CPC required participating practices to make many complex, interconnected changes in care
delivery. CPC practices needed a variety of supports to achieve the aims of CPC, such as
guidance on how to approach CPC Milestones, challenges to implementing those approaches,
and CPC administrative requirements (such as Milestone reporting). The type and level of
assistance practices needed varied depending on practices’ characteristics such as ownership
status (for example, independent versus owned by a larger health care organization), internal
resources to support quality improvement, and experience working on similar initiatives.
CMS and RLF designed a learning infrastructure that aimed to use finite resources to meet
diverse practice needs. RLF provided CPC practices a variety of learning activities, consisting of
a mix of cross-regional and region-specific activities. Cross-regional learning activities focused
on educating practices on CPC requirements (for example, the Milestone reporting process) and
sharing information on how to meet Milestones that RLF recognized as challenging across
regions. Regional learning activities were more tailored to practice needs and regional context. In
Table 3.8, we describe CPC learning supports, including group learning sessions and, for a
subset of practices, individualized practice coaching.
During the first year of the initiative, CPC learning support focused on explaining the model
and the requirements for practices. In the second program year, CMS learning support shifted to
more peer-driven, interactive learning activities aimed at helping practices adopt new strategies
and approaches to achieving CPC’s aims and Milestones; it maintained that focus through the
end of the initiative. Over the course of the initiative, RLF also increasingly used data from
practices’ Milestone reports and Medicare FFS feedback reports to identify practices in need of
additional support and/or to tailor their assistance to practices.
50

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Table 3.8. Description of CPC learning support
Learning activity
(years offered) Description Purpose
Cross-regional group learning
National webinars
(2013–2016)
CMS and TMF hosted webinars for all
CPC practices.
• Educated practices on CPC requirements
• Shared information across regions on
meeting aspects of Milestones that were
challenging
• Highlighted exemplary practices to encourage
cross-regional learning
Action groups
(Milestone, 2014–
early 2015;
Rapid-cycle, late
2015–2016)
TMF or RLF hosted web-based meetings
for practices working on similar Milestone
activities. Practices were encouraged to
implement small tests of change between
meetings. Transitioned from yearlong
Milestone action groups to rapid-cycle
groups focused on implementing specific
components of Milestones.
• Encouraged practices to make small tests of
change
• Provided opportunities for peer-to-peer-
learning
• Promoted sharing of best practices across
regions
EHR affinity
groups
(2014–2016)
TMF or RLF hosted conference calls with
groups of practices that used the same
EHR. Health IT vendors were
encouraged to join, though their
participation was infrequent.
• Facilitated EHR-related problem-solving
across regions
• Aimed to connect practices with vendor
representatives, though vendor participation
was infrequent
CPC online
knowledge
management and
collaboration tool
(CPC collaboration
site, 2013–2015;
CPC Connect, late
2015–2016)
CMS, TMF, and RLF monitored
collaboration site and encouraged
practices to use it to raise questions
about the initiative and share tools,
resources, and best practices for
implementation. Discussion forums
existed for each action group and EHR
affinity group.
• Provided practices with access to training and
technical assistance documents
• Answered practice questions on CPC
requirements and Milestones
• Encouraged peer-to-peer learning and
networking between practices
Regional group learning
All-day learning
sessions
(2013–2016)
RLF hosted biannual meetings in each
region, both in-person and virtual (while
in-person sessions were preferred, they
were sometimes logistically impossible).
• Provided training on CPC Milestones tailored
to regional needs and context
• Highlighted Milestone strategies used by
practices
• Encouraged peer-to-peer learning and
networking between practices
Regional webinars
(2013–2016)
RLF hosted a series of one-hour
webinars for practices in their region. The
frequency of regional webinars varied
overtime and by region.
• Shared information on CPC Milestones
tailored to regional needs and context
• Highlighted Milestone strategies used by
practices in the region
Virtual learning
sessions
(2014–2016)
RLF hosted two-hour webinars for
practices in their region twice a year,
typically covering Milestone
implementation topics.
• Permitted an in-depth look at a Milestone or
practice transformation topic
• Shared information and best practices
tailored to regional needs and context
Office-hour
sessions
(2013–2015)
TMF or RLF hosted virtual office-hour
sessions for practices. The frequency of
office-hour sessions varied over time and
by region.
• Answered practice questions on CPC
requirements and Milestones
Leadership track
meetings
(2013–2016)
RLF hosted quarterly web-based or in-
person meetings with clinician leaders
and health system administrators.
• Enhanced networking across practices
• Delivered training customized for clinicians
and health system leadership
51

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Table 3.8 (continued)
Learning activity
(years offered) Description Purpose
Care manager
meetings
(2015–2016)
RLF hosted in-person or virtual meetings
for care managers and other practice
staff. Meetings were held in all regions
but New Jersey. The timing and
frequency varied across regions.
• Provided trainings on care management tools
and processes
• Facilitated peer-to-peer learning and support
on common issues care managers face
• Shared information on how practices can
better leverage their care managers
Individualized
practice coaching
(2013–2016)
RLF provided individualized assistance to
practices one-on-one or in small groups
as needed. RLF reached out to a subset
of practices to provide individualized
practice coaching. RLF’s approaches to
identifying practice needs and the
percentage of practices receiving this
proactive support varied across regions.
Practices could also reach out to their
RLF with questions.
• Provided struggling practices with tailored
learning support on Milestones
• Helped practices meet administrative
requirements
Source: TMF Health Quality Institute’s CPC Curriculum. Interviews with RLF and CMS staff conducted by
Mathematica. Observations of group learning activities.
EHR = electronic health record; RLF = regional learning faculty.
Most practices actively participated in CPC learning activities. CMS requirements for
practice participation evolved over the course of the initiative. Throughout CPC’s four years,
CMS required practices to send a representative to all regional in-person and virtual learning
sessions. CMS gradually replaced requirements for practices to attend national and regional
webinars with requirements to attend Milestone action groups, which in turn were replaced by
rapid-cycle action groups. Finally, in the last two years of the initiative, CMS also required
practices to allow RLF to monitor and support the practice’s progress toward meeting Milestone
requirements. Based on RLF assessments, most CPC practices met the CPC requirements for
participating in national and regional learning activities (Milestone 8).
Analysis of the CPC clinician and staff surveys from 2016 indicated that, among members
of CPC practices, practice managers and care managers were most likely to report having
participated in CPC learning activities, followed by physicians and then by medical assistants
and nurses (Figure 3.9). Nurse practitioners and physician assistants were least likely to have
reported participating.
52

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Figure 3.9. Percentage of CPC clinicians and staff receiving various types of
CPC assistance during the past six months, 2016
Source: CPC clinician and staff survey, administered June through November 2016.
a. Details on CPC’s group learning activities
Over the four years of the initiative, CMS and its contractors continually refined CPC group
learning activities to help practices achieve the goals of CPC. In the first year of the initiative,
all-day learning sessions and webinars played a prominent role in learning activities, and were
used to efficiently share broadly relevant information on the initiative and specific Milestones
with all practices in CPC or in each region. CMS and RLF in most regions also hosted office
hours to directly engage with practices.
Feedback in the first year from practices and RLF revealed several common critiques of the
group learning activities, including (1) a lack of specific, concrete directions for implementation;
(2) limited to no tailoring of information for specific practice needs (viewed as important given
the heterogeneity of CPC practices); (3) webinars that were not always timely enough to help
practices complete required tasks; and (4) multiple webinars and all-day learning sessions
repeating the same Milestone topics.
In response to this feedback, and in recognition of practices’ growing expertise on Milestone
topics, CMS, TMF, and RLF made changes to group learning activities after CPC’s first program
year. CMS and its contractors:
• Made all-day learning sessions and webinars less didactic and more interactive by increasing
panel discussions (for example, with practices, patients, and payers) and opportunities for
peer-to-peer learning (including breakout groups of practices focusing on a particular topic).
54
33
77
75
35
44
52
33
64
65
41
43
42
38
49
54
49
42
59
40
62
67
48
48
39
20
69
60
27
31
0
20
40
60
80
100
Physicians NP/PA Practice
managers
Care managers Medical
assistants
Nurses
Percentage
Webinars Practice-to-Practice learning In-person coaching
CPC facilitated in-person meetings CPC Connect
53

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
For example, in PY2016, RLF in Oregon recruited practices to lead sessions that were of
strong interest to other practices, such as strategies for reducing 30-day readmissions.
• Reduced the number of webinars and office hours held nationally and in each region (Figure
3.10).
• Introduced new cross-regional learning communities aimed at providing practices the
opportunity to learn from other practices located in different regions but with similar
characteristics (such as practice size and ownership status). Specifically, CMS introduced:
- Action groups, which were organized around Milestone topics, such as medication
management and shared decision making. TMF or RLF hosted periodic web-based
meetings with practices participating in the groups and, between those calls, encouraged
practices to implement small tests of change in the given Milestone area. (Appendix C,
Figure C.2 indicates the percentage of practices that attended each action group.)
- EHR affinity groups, which provided a problem-solving forum for practices that used the
same EHR. EHR affinity groups were organized for the EHRs most commonly used by
CPC practices, such as EPIC, NextGen, Allscripts, and eClinicalworks. EHR vendors
were encouraged to join these meetings to answer questions and offer suggestions.
However, vendors participated infrequently.
Figure 3.10. Number and format of CPC group learning activities, PY2013
through PY2016
Source: TMF Health Quality Institute’s CPC Curricula for 2013, 2014, 2015, 2016.
Note: Action groups include both Milestone and rapid-cycle action groups. The transition from Milestone action
groups to rapid-cycle action groups occurred in 2015.
To support continued peer-to-peer learning between group learning activities, CMS and its
contractors launched a CPC online knowledge management and collaboration tool, the CPC
collaboration site, in the first year of the initiative. However, practices and RLF encountered
many issues trying to use the site—in particular, challenges finding relevant discussion forums
and resources. Their feedback led CMS first to make changes to the collaboration site to improve
its usability, and then to replace it with CPC Connect in September 2015. CPC Connect used a
Regional webinars
Office hours
National webinars
Action groups
0
20
40
60
80
100
PY2013 PY2014 PY2015 PY2016
Number of group learning activities
54

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
new electronic platform modeled after social media sites. Each user had a profile from which
they could share comments or questions with all users or with particular user groups.
Most RLF expressed strong support for the initiative’s
transition away from didactic presentations in favor of
focusing on peer-to-peer learning during in-person learning
sessions, webinars, and action groups. Additionally, some
RLF indicated that action groups helped to differentiate and
tailor learning by allowing practices to connect with others
facing similar challenges. RLF also applauded the interactive
aspects of rapid-cycle action groups, and noted that practices
benefited most from learning activities when they provided
takeaways that were tailored to CPC practices of various
sizes, types, and levels of sophistication.
However, over the last two years of the initiative, some RLF remarked on the challenge of
identifying practices to serve as panelists during group learning activities. According to these
RLF, the same handful of practices kept being asked to present at group learning sessions,
creating a burden for those practices and “an unsustainable situation for the learning curriculum.”
One RLF suggested that this problem could be addressed by adopting a better balance between
peer-to-peer learning and presentations by subject-matter experts. Faculty in two regions echoed
this need, noting the desire of some practices to learn directly from experts and faculty on
particular topics.
b. Details on CPC individualized practice coaching
In addition to providing group learning activities, in each program year, RLF used a portion of
their limited resources for CPC learning activities to provide one-on-one coaching to some
practices. Individualized practice coaching could include, for example, making an in-person visit to
a practice to discuss workflows or a telephone call with a practice care manager on risk
stratification. During interviews and on the CPC practice survey, RLF and practices reported that
practices receiving in-person coaching valued it more than group learning activities. However, due
to the high cost of providing that level of support, RLF had to prioritize where to focus their
resources. RLF in each region assessed practices’ progress toward required CPC Milestones and
achieving CPC’s goals more broadly, and generally used more resources to assist practices
experiencing the greatest challenges. At the outset of CPC, RLF primarily used their own judgment
to risk stratify practices. As the initiative progressed, RLF increasingly relied on practices’
quarterly Milestone submissions and, in some cases, Medicare FFS data feedback reports.
Across regions, RLF varied widely in how they approached individual practice coaching,
with some offering more frequent coaching opportunities than others (Table 3.9). In establishing
their approach to practice coaching, RLF considered characteristics of the practices in their
region (for example, average practice size and degree of system affiliation) and regional context
(for example, rural/urban mix and health IT infrastructure). One RLF operating in a statewide
region distributed staff throughout the region to facilitate in-person visits to nearby practices. To
expand their capacity to provide in-person practice coaching, some RLF also supplemented
CMS’s CPC funding with external funding or staff resources from other CPC payers or other
initiatives.
“The group activities have
changed a lot. At our learning
collaboratives, practices at the
beginning of the day get up to
the mic and they share
something that they have
worked on over the last
quarter, and this [session] has
become a favorite…of the
practices. They really enjoy…
hearing from each other and
learning from each other.”
55

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Throughout the initiative and across regions, RLF provided more in-person visits to
practices that they perceived to be at the highest risk of not fulfilling Milestone requirements
(Table 3.9). In most regions, RLF also regularly communicated with moderate risk practices
either through in-person visits or over the phone. Also in most regions, practices that RLF
identified as likely to complete all Milestone requirements received periodic telephone or email
check-ins; Colorado and Oklahoma were the only regions in which such practices received
regular in-person visits. Although RLF from these regions prioritized individualized support for
struggling practices, they noted that progress in even high-performing practices could be stalled
or reversed by developments such as staff turnover or practice ownership changes, and that
frequent, face-to-face contact allowed RLF to identify and address performance issues before
they became serious. Corresponding with the variety of approaches used by RLF, the percentage
of practices that reported on the 2016 CPC practice survey that they had received in-person
coaching at their practice site in the prior six months ranged from 13 percent in Ohio/Kentucky
to 89 percent in Oklahoma (Table 3.9)
Table 3.9. Planned frequency and mode of individualized practice coaching
and the percentage of practices that reported receiving coaching at their
practice site, by region, PY2016
Planned frequency and mode of individualized practice coaching, by RLF
perceived risk of practices not achieving CPC aims
Percentage of
practices that
reported receiving
coaching at their
practice site in the
prior six months High risk Moderate risk Low risk
AR
At least monthly calls or in-
person visits
Monthly email check-in; at
least monthly calls;
occasional in-person visits
Monthly email check-in; at
least monthly calls;
occasional in-person visits
77
CO
a
HTW: At least monthly in-
person visits; calls as needed
RMHP: Twice monthly in-
person visits
HTW: Monthly to quarterly in-
person visits; calls as needed
RMHP: Twice monthly in-
person visits
HTW: Quarterly in-person
visits; calls if identified as
needed by RLF
RMHP: Twice monthly in-
person visits
74
NJ
Weekly, biweekly, or monthly
calls; in-person visits at least
quarterly
Monthly or quarterly calls;
occasional in-person visits
Quarterly calls 69
NY
Frequent calls; in-person
visits if identified as needed
by RLF
Calls if identified as needed
by RLF
Calls if identified as needed
by RLF
36
OH/KY
Calls or in-person visits if
identified as needed by RLF
Calls or in-person visits if
identified as needed by RLF
Calls or in-person visits if
identified as needed by RLF
13
OK
Frequent in-person visits and
calls
Monthly in-person visits Monthly in-person visits 89
OR Monthly in-person visits
Quarterly in-person visits or
calls
Calls if identified as needed
by RLF
45
Sources: Interviews with RLF conducted by Mathematica in 2016. CPC practice survey, administered April through August
2016.
Notes: Some RLF interactions with practices may reflect interactions with only system-level staff.
a
HTW = HealthTeamWorks (served Front Range region, which covered approximately 80 percent of Colorado practices);
RMHP = Rocky Mountain Health Plans (served Western Slope region, which included approximately 20 percent of Colorado
practices).
RLF = regional learning faculty.
56

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
On the 2016 CPC practice survey, among practices that were owned by a larger health care
organization, 74 percent reported that RLF communicated with their practice’s staff, or with a
combination of practice- and systems-level staff. CMS intended for CPC to be a practice-level
(not system-level) intervention (Figure 3.11). However, the remaining one-quarter of practices
reported that RLF communicated only with systems-level staff. The percentage reporting that
only system-level staff communicated with RLF varied considerably by region, from 0 percent in
Colorado and New Jersey to 36 to 44 percent in New York, Ohio/Kentucky, and Oklahoma.
Figure 3.11. Percentage of system-owned practices reporting staff in the
practice site or their larger health care organization communicate with RLF,
in 2016
Source: CPC practice survey, administered April through August 2016.
Note: Practices owned by a larger health care organization include practices where the clinicians are employed
by, or the practice is owned by a group or staff model HMO, hospital, hospital system, or medical school.
Some columns do not add up to 100 percent due to rounding.
RLF = regional learning faculty.
Practices reported less frequent communication with the RLF over the course of CPC.
Across regions, the percentage of practices reporting at least monthly contact with their RLF
decreased from 85 percent of practices in PY2014 to 71 percent in PY2016. These decreases
corresponded with some RLF deciding to reduce the frequency and intensity of individualized
coaching as practices became more comfortable with the initiative. As RLF in one region noted,
“Some of these practices have matured to a point that it may be that we can [now] space our
interactions differently, or at least [space] our face-to-face interactions differently.” The
percentage of practices reporting at least monthly contact also varied by region, with Colorado
practices consistently reporting the highest frequency of interaction with their RLF, and Oregon
0
0
0
0
0
2
0
0
26
13
0
0
36
37
44
12
62
50
81
75
50
59
49
82
12
38
19
25
14
2
7
6
0
20
40
60
80
100
CPC-wide AR CO NJ NY OH/KY OK OR
Percentage of practices
Staff in the practice site only
A combination of the practice site and larger health care organization staff
Staff in larger healthcare organization only
Neither
57

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
practices reporting the lowest (Figure 3.12; Appendix C provides additional detail on the
frequency of RLF interactions with practices and practices’ ratings of RLF by region).
Figure 3.12. Percentage of practices reporting interacting with their RLF at
least once a month, PY2014–PY2016, CPC-wide and by region
Source: CPC practice survey, administered April through July 2014, April through August 2015, and April through
August 2016.
On the CPC practice survey, 28 percent of practices that reported communicating with the
RLF indicated that direct support from the RLF focused on helping them make practice
improvements including meeting CPC Milestones; 22 percent of the practices reported receiving
substantial help on Milestones as well administrative requirements, and 44 percent of the
practices reported that they received help from the RLF primarily to meet administrative
requirements. Only 6 percent of practices reported little or no help from the RLF with practice
improvement or administrative requirements.
During interviews, RLF reported that Milestone-focused practice coaching often centered on
risk stratification and care management, shared decision making, and using data to guide
improvement. For example, practices frequently received coaching on implementing their
advanced primary care strategies (patient self-management support, behavioral health
integration, or comprehensive medication management), selecting an appropriate shared decision
making aid, and reporting eCQMs. Some deep-dive practices indicated that RLF review of plans
for Milestone-related changes in practice processes (such as a new shared decision making tool)
and help with finding new resources (such as care compact templates) were particularly valuable
coaching activities.
RLF reported during interviews with Mathematica that, starting in PY2015, practice
coaching increasingly emphasized the use of data. In several regions, RLF reported helping
practices use eCQMs to help support quality improvement. For example, in one region, practices
submitted their eCQMs to the RLF quarterly (in addition to their annual reporting to CMS). The
RLF helped the practices chart their performance on the measures and identify strategies to
improve. Similarly, in many regions, the RLF reported using Medicare feedback reports to help
85
95
97
84
86
89
79
62
79
78
99
90
72
73
80
59
71
86
86
70
47
72
85
52
0
20
40
60
80
100
CPC-wide AR CO NJ NY OH/KY OK OR
Percentage of practices
2014 2015 2016
58

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
practices’ target their quality improvement work. RLF in several regions, including Arkansas and
Oklahoma, supported this coaching by repackaging the practice-level data from quarterly
Medicare feedback reports into new reports to make these data more actionable. For example, for
system practices, Oklahoma RLF used Medicare feedback reports to produce reports displaying
performance on key metrics of all CPC practices in the same system. As another example, in
several regions where practices agreed to share unblinded practice-level Medicare data with one
another—Arkansas, Ohio/Kentucky, and Oklahoma—faculty began using them to provide more
targeted peer-to-peer learning (for example, by trying to connect advanced and struggling
practices in specific performance areas). However, one RLF cited the time burden on advanced
practices as a key barrier to implementing this approach on a widespread basis.
3.5.2. Learning support from other payers
Non-Medicare payers also provided coaching or assistance to 71 percent of CPC practices in the six
months prior to them responding to the 2016 CPC practice survey. The level and type of support
varied widely across payers.
While all payers agreed to provide practices with enhanced payments and data feedback in
their CPC memoranda of understanding with CMS, they did not commit to providing CPC
practices with learning support (CMS 2013b). Nonetheless, most payers participated in CMS-
funded CPC learning activities or provided practices learning activities through other initiatives.
Payers in Oklahoma were most involved in CPC learning activities, followed by payers in
Arkansas and Colorado. In Oklahoma, non-CPC payers were initially concerned that CPC
practices were not receiving enough individualized support, so they developed a field service
team to provide CPC practices additional support. Each Oklahoma payer provided a “point of
contact” who, supported by the RLF, provided individualized practice coaching to CPC
practices. (Partly as a result of the field service team, as noted above, Oklahoma practices
reported receiving more in-person coaching than practices in other regions.) The field service
team also worked together to develop agendas for group learning activities. As another example
of payer engagement in learning, in fall 2015, Arkansas payers and RLF started to meet monthly
as a regional learning team to discuss initiative activities and practices’ challenges in meeting
CPC Milestones. In 2015 and 2016, Colorado payers, RLF, and the data aggregation vendor
collaborated to provide in-person training on the region’s data aggregation tool.
In addition, payers in most regions attended and presented at all-day learning sessions
organized by CMS and its contractors. Examples of payer presentations included overcoming
challenges in communicating with specialists and steering referral and ordering patterns toward
lower cost specialty and ancillary providers. In some
regions, including Arkansas and New York, payers and
stakeholders highlighted the value of these meetings for
facilitating communication between payers and practices
on topics ranging from how to use payer feedback reports
to common challenges faced by practices.
Multistakeholder faculty in one region noted, “[When] the
payers and the practices are actually in the same room,
grappling with the same thing…the practices can see that
“[When] the payers and the
practices are actually in the same
room, grappling with the same
thing…the practices can see that
the payers aren’t just big bad
guys that want to take away their
money, and the payers can see,
‘Oh, these people aren’t just out
to get every penny that they can.’”
59

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
the payers aren’t just big bad guys that want to take away their money, and the payers can see,
‘Oh, these people aren’t just out to get every penny that they can.’”
Throughout the initiative, a number of participating payers also provided their own support
to practices. This support was not coordinated with the RLF but rather augmented RLF’s efforts.
Most often, payers provided technical assistance on how practices could use their own payer
feedback reports to guide quality improvement. Their approaches varied, with some meeting
regularly with practices to discuss the reports and others fielding questions from practices on an
ad hoc basis. Also common, several payers staffed their own care management or disease
management teams that provided support to practices’ commercially insured patients. The few
deep-dive practices selected for intensive study that had a care manager from a commercial plan
on-site reported that this arrangement allowed them to use their own care manager to focus on
their other high-risk patients. A few payers also offered more extensive practice support. For
example, one New Jersey payer reported offering coaching to all CPC practices (as well as other
practices that participated in their value-based payment programs) to support practice
transformation and quality improvement, and one Oregon payer provided all-day, in-person
trainings on motivational interviewing to practices within its network.
In the PY2016 CPC practice survey, 71 percent of practices CPC-wide reported receiving
coaching or assistance from non-Medicare payers in the prior six months. The percentage of
practices that reported receiving this assistance varied considerably by region (Figure 3.13), with
practices in Ohio/Kentucky (96 percent) and Oklahoma (85 percent) most likely to report
receiving this support.
Figure 3.13. Percentage of practices reporting they received coaching or
assistance from non-Medicare payers in the past six months, CPC-wide and
by region, PY2016
Source: CPC practice survey, administered April through August 2016.
71
54
77
53
74
96
85
52
0
20
40
60
80
100
CPC-Wide AR CO NJ NY OH/KY OK OR
Percentage of practices
60

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
3.5.3. Practices’ use of and perspectives on CPC learning support
Practices were generally satisfied with CPC learning support. In particular, practices found in-person
learning activities and opportunities for peer-to-peer learning to be the most valuable learning
support.
In general, practices were satisfied with the learning support they received from CMS and its
contractors. In 2016, 75 percent of practices CPC-wide rated their RLF as excellent (40 percent)
or very good (35 percent) in meeting their CPC-related needs. The proportion of practices rating
their RLF as excellent, however, varied across regions and over time (Figure 3.14). Most
notably, RLF in Colorado consistently received some of the highest ratings, whereas RLF in
New York generally received some of the lowest ratings, though ratings were still fairly high.
Figure 3.14. Percentage of CPC practices rating their RLF as excellent or
very good in meeting their CPC-related needs, in PY2014 and PY2016, by
region
Source: CPC practice survey, administered April through July 2014 and April through August 2016.
The 2016 CPC practice survey asked practices that participated in a learning activity to
indicate how useful it was to their practice (Figure 3.15). Practices found in-person coaching and
in-person all-day meetings, followed by practice-to-practice learning and rapid-cycle action
groups, to be most helpful, with practices more likely to rate these supports as very useful
compared with webinars or CPC Connect. (Practice-to-practice learning was not a separate
learning activity but a component of several activities, including in-person meetings and rapid-
cycle action groups.) Findings from the CPC clinician and staff survey, which asked physicians,
NPs/PAs, care managers, medical assistants, and nurses that participated in a learning activity to
rate its usefulness, further confirmed this finding. Across respondent types, CPC clinicians and
staff were most likely to rate in-person coaching, in-person meetings, and then practice-to-
practice learning as very useful.
35
35
28
32
28
38
21
38
38
34
45
32
40
48
39
25
37
40
43
53
62
51
59
42
23
14
32
49
17
33
24
37
0
20
40
60
80
100
2014 2016 2014 2016 2014 2016 2014 2016 2014 2016 2014 2016 2014 2016 2014 2016
CPC-wide AR CO NJ NY OH/KY OK OR
Percentage of practices
Very good Excellent
61

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
Figure 3.15. Reports of how useful various types of CPC assistance were to
CPC practices, among those that received the assistance, 2016
Source: CPC practice survey, administered April through August 2016.
Notes: Practice-to-practice learning was not a separate learning activity but a component of several activities
including in-person meetings and rapid-cycle action groups. The number of practices that reported
receiving various activities ranged from 247 that reported receiving in-person coaching to 438 that reported
attending webinars. For rapid-cycle action groups, we averaged ratings across the eight rapid-cycle action
groups offered to CPC practices. The findings represent a weighted average of practices’ ratings, weighted
by the number of practices that reported attending the rapid-cycle action group and gave a rating of
usefulness. The percentage of practices CPC-wide that reported attending the rapid-cycle action groups
ranged from 27 percent for “From screening to treatment in behavioral health” to 47 percent for “Leveraging
your whole team to improve chronic disease management.”
In line with our survey findings, deep-dive practices selected for in-depth qualitative study
reported that they found in-person learning sessions and opportunities for peer-to-peer learning
to be most valuable. Deep-dive practices also provided a more nuanced view of learning.
Specifically, deep-dive practices reported that they valued learning activities that:
• Provided interactive learning opportunities. Practices indicated that sharing real-life
challenges, successes, and lessons learned with peers helped generate ideas to test in their
own practices. For instance, one deep-dive practice reported getting the idea from another
practice at a learning collaborative meeting to put tablets in exam rooms so patients could
update their medication lists while waiting to be seen. Practices also reported that
opportunities to interact in-person with other practices boosted morale and facilitated
camaraderie among practices’ care managers.
• Offered opportunities, such as breakout sessions at in-person learning meetings, for practices
to speak in depth about a specific topic or with people in similar roles. For example, one
care manager reported that, during a breakout session at a CPC all-day meeting, RLF walked
15
20
29
37
43
46
57
67
62
56
53
45
20
11
7
7
4
8
8
2
1
0
0
1
0 20 40 60 80 100
CPC Connect (N=433)
Webinars (N=438)
Rapid-cycle action groups (N=437)
Practice-to-practice learning (N=345)
CPC-facilitated in-person meetings (N=402)
In-person coaching (N=247)
Percentage of practices
Very useful Somewhat useful
Not very useful
Not at all useful
62

3. WHAT PAYMENTS, DATA FEEDBACK, AND LEARNING
DID CMS AND OTHER PAYERS PROVIDE TO CPC PRACTICES? MATHEMATICA POLICY RESEARCH
practices through how to interpret and use Medicare FFS feedback reports. The care
manager reported walking away from that meeting with a concrete understanding of how to
use her practice’s feedback reports moving forward.
• Focused on topics and resources that could easily be applied to day-to-day work. As one
care manager explained, “RLF come in and give us ideas. They are giving us information
and tools that we can use to help our patients. If we need a tool or resource, we can call them
and find the answer.”
Deep-dive practices also identified several challenges to learning support that persisted over
the course of the four-year initiative:
• The time required to attend in-person learning sessions, which meant being out of the office
and unavailable for patient care, was burdensome for practices. In particular, rural practices,
whose staff had to travel long distances to attend in-person learning sessions, reported this
challenge.
• Some learning activities—in particular, webinars—were less useful, because they provided
general information that was not tailored to meet different practice needs.
• Some practices were frustrated that they were required to attend multiple group learning
sessions covering the same Milestone topics.
• The time required to find resources on the CPC collaboration site and, to a lesser extent, its
successor CPC Connect limited the use of those sites.
• Lack of EHR vendor participation in EHR affinity groups limited practices’ ability to
resolve EHR-related issues. Frustration with this challenge resulted in waning practice
participation in these groups over time.
63
This page has been left blank for double-sided copying.

MATHEMATICA POLICY RESEARCH
4. HOW DID CPC PAYERS, PRACTICES, AND OTHER STAKEHOLDERS
WORK TOGETHER?
CPC represents one of the largest and most substantial multipayer initiatives ever tested. For
the initiative, CMS, state Medicaid agencies and associated Medicaid Managed Care
Organizations, and private payers committed to providing practices enhanced payment to
promote comprehensive primary care. Payers also agreed to work together to develop an
approach to align and coordinate data feedback for participating practices. This work required a
tremendous amount of coordination and collaboration among participating stakeholders.
In this chapter, we describe how CPC payers in each region collaborated with one another,
as well as with CMS and other stakeholders. We first describe the goals of CPC collaboration
and how collaborative efforts were structured. We then highlight the outcomes from those
collaborative efforts. Finally, we describe the barriers and facilitators that non-Medicare payers
faced collaborating with each other and with CMS.
4.1. Key takeaways on collaboration among CPC payers and other
stakeholders
• Most payers remained committed to CPC and actively engaged in collaborative discussions
for the duration of the initiative. However, a few payers that had a low number of attributed
patients in CPC or were actively participating in concurrent initiatives showed lower levels
of engagement.
• Payers generally reported that they established productive, positive working relationships
with other payers in their region. Payers indicated that prior experience working together,
strong multistakeholder facilitation, leadership from payer champions, and meaningful
engagement with practices facilitated collaboration.
• Most payers viewed CMS as a critical partner in efforts to transform primary care,
recognizing its role in encouraging practice participation in transformation efforts and
bringing additional financial and technical support to each region. However, CMS’s dual
role as initiative convener and participating payer at times made collaboration challenging.
CMS was able to build trust with other payers by clarifying which parts of CPC could be
adapted to regional contexts, deferring to other payers for these decisions, increasing
opportunities for payers to meet with CMS representatives, and committing to build on the
successes and lessons of CPC in CPC+.
• Most payers valued the opportunity to discuss CPC with practices, and hear more directly
about the challenges and successes that practices experienced in implementing
comprehensive primary care. However, in several regions, payers reported that active,
sustained practice engagement in multistakeholder meetings was difficult to attain.
• Payers indicated that multistakeholder meetings—which involved payers, practices, and, in
some regions, patients or other stakeholder groups that did not participate in CPC—could
have been improved by delineating clear goals for engagement, selecting stakeholders with
the time and skills needed to contribute to discussions, and building trust among payers and
other stakeholders.
65

4. HOW DID CPC PAYERS, PRACTICES,
AND OTHER STAKEHOLDERS WORK TOGETHER? MATHEMATICA POLICY RESEARCH
• By bringing together payers and other stakeholders, CPC enabled payers to accomplish
several collaborative outcomes, including aligning quality goals and financial incentives,
agreeing on a common set of quality measures, coordinating common approaches to data
feedback, and coordinating CPC with other regional efforts.
4.2. Methods
For this chapter, we analyzed data from interviews with CMS, other participating payers,
and multistakeholder faculty (organizations CMS contracted with to convene meetings of payers,
practices, and other stakeholders). We also drew on our notes from our observations of
multipayer and multistakeholder meetings to inform our analysis.
4.3. CPC collaborative goals and structure
CMS and most other payers remained committed to working together to improve supports for CPC
practices and actively engaged in collaborative discussions for the duration of the initiative.
In each of the seven regions, CPC brought together payers who agreed to align their goals
and financial incentives to drive primary care practice transformation. Over the course of CPC,
CMS and other payers worked together to accomplish several collaborative outcomes. During
the initiative’s first year, payers focused on aligning the quality metrics they used for
determining practices’ eligibility to participate in shared savings, selecting a common approach
to providing data feedback to practices, and developing an approach to collaborate with CPC
practices and other stakeholders, such as consumer representatives, employers, hospital
associations, or health foundations. As the initiative evolved, payers focused increasing attention
on implementing the region’s selected approach to data feedback and developing plans to sustain
support to practices after the end of CPC. In some regions, payers also worked together on other
areas, such as coordinating learning activities and supports between CPC’s learning faculty and
participating payers, improving health literacy and patient education, increasing information
sharing between hospitals and CPC practices to support transitional care, aligning their
messaging on shared savings approaches, or encouraging additional self-insured clients to sign
on to the CPC initiative.
CMS and other payers met regularly to discuss collaborative priorities. The initial frequency
of these payer-only (or multipayer) meetings varied from weekly in Arkansas to every two
months in New Jersey. In each region, these meetings were facilitated by regional conveners
(referred to as "multistakeholder faculty") that were funded by CMS. When possible, CMS and
its learning contractor selected experienced, neutral regional organizations for this role. In
regions where an appropriate local organization was not identified, the Center for Evidence-
Based Policy at the Oregon Health and Science University served as the multistakeholder
faculty.
Over the course of the initiative, payers in most regions formed work groups to accelerate
progress in one or more of the following priority areas: data sharing (Arkansas, Colorado,
Ohio/Kentucky, Oklahoma, and Oregon), employer engagement (Ohio/Kentucky), and learning
support (Oklahoma). Work group meeting frequency varied, with groups meeting more
66
4. HOW DID CPC PAYERS, PRACTICES,
AND OTHER STAKEHOLDERS WORK TOGETHER? MATHEMATICA POLICY RESEARCH
frequently during periods of intense activity (for example, when the region was designing
reporting templates or reviewing applications for data aggregation vendors).
From the start of the initiative, CMS encouraged payers to engage practices and other
stakeholders in their collaborative work and, by PY2015, multistakeholder meetings were the
most common forum for discussing CPC (Table 4.1; Table 4.2). In all regions, payers engaged a
subset of CPC practice managers and clinicians in their collaborative discussions. In some
regions, payers also included consumers, employers, or other stakeholders—such as hospital
associations or health foundations—in meetings.
Table 4.1. CPC multistakeholder meeting participants
Region
Month other
stakeholders joined
CPC meetings
Stakeholders joining CPC meetings
CPC practices
a
Consumers
b
Employers
Other
stakeholders
c
AR November 2012 X X X X
CO May 2014 X .
NJ September 2014 X X
NY January 2013 X X X
OH/KY October 2012 X X X
OK February 2014 X X X
OR May 2014 X
Source: Agendas and notes from payer and multistakeholder meetings and information provided by
multistakeholder faculty and CMS staff.
a
CPC practice representatives included health system executives, clinicians, care coordinators, and office managers.
b
Consumers included patient representatives and consumer advocacy groups.
c
Other stakeholders included Medicaid (not a participating payer) in New York; hospital associations in New Jersey;
and the Department of Health, health foundations, universities, and pharmacists in Arkansas.
Table 4.2. Frequency of CPC payer-only and multistakeholder meetings
At start of CPC (PY2013) Last year of CPC (PY2016)
Payer-only meetings
a
Payer-only meetings
a
Multistakeholder meetings
b
AR Weekly Every two weeks Quarterly
CO Monthly Monthly Quarterly
NJ Every two months None Quarterly
NY Every two weeks None Quarterly
OH/KY Every three weeks None Quarterly
OK Every two weeks As needed Quarterly
OR Monthly Monthly
c
Quarterly
Source: Agendas and notes from payer and multistakeholder meetings and information provided by
multistakeholder faculty and CMS staff.
a
Payer-only meetings, commonly referred to as multipayer meetings in CPC, included only payers participating in
CPC.
b
Multistakeholder meetings included payers participating in CPC and other stakeholders.
c
Oregon payers started meeting monthly after CMS announced CPC+ in April 2016; payers met as needed from
September 2014 through March 2016.
67

4. HOW DID CPC PAYERS, PRACTICES,
AND OTHER STAKEHOLDERS WORK TOGETHER? MATHEMATICA POLICY RESEARCH
Payers in Arkansas, New York, and Ohio/Kentucky were quick to start working with
stakeholders and held their first multistakeholder meetings within three months of the initiative’s
start. Payers in the remaining four regions expressed reservations about engaging other
stakeholders in their collaborative discussions. Their main concerns included overburdening
providers, duplicating existing multistakeholder efforts, and identifying the “right” participants
(that is, those interested in ongoing participation and capable of making meaningful
contributions to meetings).
CMS initially funded multistakeholder faculty to facilitate both payer-only meetings and
multistakeholder meetings. In September 2014, after all regions had established multistakeholder
meetings, CMS transitioned to funding only multistakeholder group facilitation. Following this
transition, payers in four regions continued to supplement multistakeholder meetings with
regular or ad hoc payer-only meetings to discuss CPC issues that interested only payers or that
were sensitive or not appropriate for the multistakeholder forum. For example, in Arkansas, after
practices indicated that patient-level data would be useful in their transformation efforts during
multistakeholder meetings, payers used payer-only meetings to finalize a plan for providing that
information to practices. Due to resource limitations, CMS did not commit to regularly attending
payer-only meetings, though typically would attend if requested.
4.4. Results of CPC collaboration
CPC payers aligned quality goals and financial incentives, agreed on a common set of quality
measures, and, in some regions, coordinated common approaches to data feedback and
coordinated CPC with other regional efforts.
CMS and other payers accomplished a number of collaborative outcomes during CPC
(Table 4.3). In each region, payers agreed on a common set of quality measures; however, only
about half of payers ultimately used these measures to determine practices’ eligibility to
participate in shared savings. In five regions, payers developed a common approach to data
feedback. Specifically, by PY2015, two regions were providing practices with aligned individual
reports (covering a common set of cost and service use measures in individual reports), while
three regions had achieved data aggregation (producing a single report that aggregates data
across payers). Although not an explicit CPC goal, payers in Oklahoma also collaborated with
the CMS-funded learning contractor to provide coordinated, individualized technical assistance
to CPC practices. (See Chapter 3.4.1.b for additional information on payers’ aligned and
aggregated reports and Chapter 3.5.2 for additional detail on aligned technical assistance.)
68

4. HOW DID CPC PAYERS, PRACTICES,
AND OTHER STAKEHOLDERS WORK TOGETHER? MATHEMATICA POLICY RESEARCH
Table 4.3. Selected CPC collaborative outcomes, by region
Aligned
goals and
financial
incentives
a
Aligned
quality
measures
b
Coordinated approach to
data feedback
Coordinated
technical
assistance
c
Coordinated
plan for
aligning
CPC with
SIM
e
Aligned
individual
reports
Data
aggregation
AR X X X X
CO X X X X
NJ X X
NY X X X
OH/KY X X X X
OK X X X X X
OR
d
X X X
Total number of
regions 7 7 2 3 1 5
Source: Agendas and notes from payer and multistakeholder meetings and information provided by
multistakeholder faculty and CMS staff.
a
CMS and other payer alignment of goals and financial incentives was a direct outcome of payers joining CPC, as
opposed to an outcome from ongoing collaborative discussions.
b
Payers agreed on a common set of quality measures; however, only around 50 percent of payers used them to
determine practices’ eligibility to participate in shared savings.
c
Oklahoma was the only region in which participating payers collaborated with the CMS-funded learning contractor to
provide coordinated, individualized technical assistance to CPC practices. Payers in other regions were involved in
CPC learning in less intensive ways, such as participating in learning sessions for practices.
d
Oregon moved forward with a common approach to data feedback; however, as of December 2015, two of the five
payers had stopped participating in the effort.
e
State Innovation Models (SIMs) were funded by CMS and led by the state’s Medicaid program.
Moreover, payers in several regions worked together to coordinate CPC with other regional
efforts. Most notably, payers in five regions viewed their states’ State Innovation Model (SIM)
awards, funded by CMS and led by the states, as an avenue to expand and sustain practice
transformation started under CPC. In these regions, CPC payers and practices were actively
engaged in SIM design and implementation decisions and based components of their SIMs on
CPC.
45
As a result, a number of primary care practices that were not participating in CPC were
encouraged to pursue milestones or aims in line with CPC goals and received payments, learning
support, and data feedback similar to those of CPC practices. For example, Colorado payers
worked to include data for patients attributed to SIM practices in the CPC data aggregation tool
and planned to share unused licenses for the tool with SIM practices. As one multistakeholder
faculty member described, “One of the things that has happened as a result [of CPC] is payers
have been able to move from representing their organization to each other, to…representing the
collaborative to the community…I think that [move] ultimately impacts the way they have
approached their State Innovation Model and their commitment to [it].”
45
SIM awards went into effect after CPC was already underway. Medicare FFS did not participate in states’ SIM
awardees and, thus, the level of funding to practices was, on average, substantially less than that provided by CPC.
69

4. HOW DID CPC PAYERS, PRACTICES,
AND OTHER STAKEHOLDERS WORK TOGETHER? MATHEMATICA POLICY RESEARCH
In addition, payers in several regions advocated for CMS sustaining multipayer efforts to
improve primary care support after the end of CPC. Specifically, in response to CMS’s proposed
rule on the expansion of CPC, payers in several regions submitted a unified response expressing
their support for CMS sustaining CPC in some form (CMS 2015b). After CMS announced its
plans for CPC+ in April 2016, payers in some CPC regions worked together to encourage
additional payers to join CPC+ and/or developed a unified application for the initiative. For more
information on CPC payers’ participation in CPC+, see Chapter 2.3.
4.5. Factors influencing CPC collaboration
CMS built trust with other payers by clarifying which parts of CPC could be adapted to regional
contexts, deferring to other payers for these decisions, and increasing opportunities for payers to
meet with CMS representatives. Strong multistakeholder facilitation, leadership from payer
champions, and meaningful engagement with practices facilitated collaboration among non-CMS
payers.
In each CPC region, participating non-CMS payers collaborated with one another, as well as
with CMS and other stakeholders. In this section, we describe how collaborative dynamics
evolved and the factors that facilitated or hindered collaboration among these different groups
(Anglin et al. 2017).
4.5.1. Collaboration among non-CMS payers
For the duration of the initiative, most participating payers remained committed to
supporting advanced primary care through CPC and other initiatives. Capturing the sentiments of
several payers, one payer noted, “This is how we do business. Not yesterday, not today—this is
how we’re going to do business.… [We’re] in this for the long haul.” In line with this
commitment, most CPC payers remained engaged in CPC collaborative work. In general, payers
expressed satisfaction with other payers’ contributions to CPC, in terms of both intellectual
contributions and financial resources for aligned supports (such as data aggregation). Moreover,
most payers reported that they enjoyed the opportunity to learn from and work with other payers.
From interviews with payers and multistakeholder faculty and observations of payer-only
and multistakeholder meetings, we identified several factors that influenced CPC collaboration
among non-CMS payers. These factors, outlined below, fall into two categories: (1) factors that
influenced engagement in collaborative discussions and (2) factors that influenced relationships
among non-CMS payers.
The following factors influenced payers’ engagement in collaborative discussions:
• Size of payers’ market share. In most regions, payers with a larger market share
participated more actively in CPC discussions than other payers and sometimes drove
decision making. For the most part, other payers felt this dynamic was fair because the
payers with larger market share had more “skin in the game” and smaller payers were
willing to let them take the lead. However, in one region with a large, dominant player, this
dynamic led several smaller payers to disengage from CPC collaborative discussions.
70

4. HOW DID CPC PAYERS, PRACTICES,
AND OTHER STAKEHOLDERS WORK TOGETHER? MATHEMATICA POLICY RESEARCH
• Differences between national and regional perspectives. National payers and regional
payers often contributed different perspectives in payer-only and multistakeholder meetings.
Not surprisingly, regional payers were generally more knowledgeable about the region and
more likely to be involved in non-CPC initiatives in the region than national payers. As a
result, regional payers were often more invested in data aggregation and aligning CPC with
other regional initiatives than national payers, though a few national payers also played an
important role during those discussions. Other national payers, in contrast, were interested in
standardizing their CPC approach across the regions. As a result, several national payers
participating in multiple CPC regions opted out of regional data alignment efforts to
maintain standardized reporting across regions within their own organizations. One
multistakeholder faculty member described this dynamic: “As a collaborative, [payers] are
hitting the point where their organizational interests are bumping up against the greater plan
for the region.... That is a challenge that any collaborative faces.”
• Concurrent multipayer initiatives. In most regions, CPC payers were involved in more
than one multipayer initiative at the same time. When the goals and strategies of these
initiatives were aligned, payers indicated that the alignment helped fuel CPC collaboration.
For example, Oklahoma’s multipayer health information exchange efforts helped payers
move forward with data aggregation discussions for CPC. In contrast, differing priorities
between CPC and other multipayer initiatives in Oregon contributed to payers’ waning
engagement in CPC. Over the course of the initiative, Oregon payers continued to report that
they had positive working relationships but started to commit more time and resources to
other efforts. However, CMS’s announcement of the CPC+ initiative in April 2016
reinvigorated some Oregon payers’ engagement in CPC.
The following factors influenced collaborative relationships among non-CMS payers:
• Payers’ prior working relationships. At the outset of CPC, payers’ prior experience
working together set the tone for CPC discussions among non-CMS payers. In four regions,
non-CMS payers had developed strong, positive relationships through prior collaborative
efforts and reported that foundation provided them a leg up early in the initiative. For
example, in one region, payers’ previous work together to develop a state health information
exchange served as a starting point for data aggregation discussions. In contrast, payers in
other regions had less prior experience working together. In some cases, the lack of prior
collaborative experience—combined with competitive market dynamics—resulted in
distrust among payers, and early CPC discussions sometimes became heated. During the
first year of CPC, however, payers in regions with little to no prior collaborative experience
reportedly began to come together as a community, and by the end of the year, prior
collaborative experience did not appear to be affecting payers’ discussions.
• Support from effective, proactive multistakeholder faculty. In four regions, payers
indicated their multistakeholder faculty was a critical factor in promoting CPC
collaboration. In these regions, multistakeholder faculty served more than a logistical or
administrative support role (for example, scheduling meetings and taking notes). Instead,
effective multistakeholder faculty gained participants’ trust; fostered positive working
relationships among payers; broke down broad initiative goals into more concrete,
achievable goals; and identified constructive steps to overcome barriers and make progress
71

4. HOW DID CPC PAYERS, PRACTICES,
AND OTHER STAKEHOLDERS WORK TOGETHER? MATHEMATICA POLICY RESEARCH
toward those goals. For example, in one region, the multistakeholder faculty built group
cohesion by (1) holding individual calls with payers to discuss issues that payers were
reticent to raise in a group setting and then (2) identifying common points of interest and
concern across payers and emphasizing them with the group. In another region, the
multistakeholder faculty led the development of a formal charter that outlined the goals for
payer collaboration and the responsibilities of payer partners.
• Presence of payer champions. In five regions, individuals emerged as group leaders that
helped propel collaborative efforts; we refer to these individuals as "payer champions."
Usually, the payer champions did not push an approach or strategy used by their
organization but rather encouraged others to remain engaged and to continue making
progress. Multistakeholder faculty indicated one senior leader was “really working hard to
make sure that everybody’s able to move forward as a cohesive group and not being held up
by corporate bureaucracy.” For example, in two regions, a payer champion spearheaded data
aggregation efforts both by assuming a lead role on key tasks (such as reviewing vendor
qualifications) and by encouraging other payers to commit the needed time and resources.
Senior staff with decision-making power in their organization (as opposed to junior staff)
served as particularly effective champions, often marshaling resources within their own
organizations and energizing their counterparts within other payer organizations.
• Incorporating CPC practice perspectives. Including the perspectives of CPC clinicians,
practice managers, and/or health system representatives was another key to successful
collaboration in five regions. In many regions, payers’ commitment to CPC was
reinvigorated after hearing about practices’ successes in CPC as well as their challenges in
making further changes. Moreover, payers in all regions that achieved data aggregation or
aligned CPC with SIM initiatives indicated that practice perspectives were critical to
designing those efforts and obtaining participation in them.
4.5.2. Collaboration between CMS and other payers
As noted in Section 2.3, CMS’s leadership was critical for achieving broad payer
participation and active engagement in CPC. Many payers joined CPC because CMS’s
participation brought substantial resources to their region, potentially increasing the impact of
their ongoing initiatives. These payers frequently indicated that the care management fees for
and data feedback on Medicare FFS beneficiaries that CMS contributed to CPC set this initiative
apart from prior regional multipayer efforts. Additionally, payers consistently reported that CMS
funding for CPC practice learning activities and meeting facilitation helped propel the initiative
forward.
Although payers valued CMS’s participation, CMS had a somewhat rocky relationship with
most participating payers at the outset of CPC. Several factors contributed to this dynamic. In
some regions, payers, including state Medicaid agencies, had been working together for a long
time and viewed national CMS representatives as outsiders at the start of CPC. In particular,
CMS often had difficulty establishing trust with payers in regions characterized by strong prior
collaborative efforts. In these tight-knit communities, the tendency to view both CMS and other
new payers as outsiders initially resulted in an “us versus them” dynamic, which impeded
collaboration. Moreover, gaining payers’ trust early in the initiative was difficult because CMS’s
budget and federal travel restrictions did not allow staff from CMS’s national headquarters to
72

4. HOW DID CPC PAYERS, PRACTICES,
AND OTHER STAKEHOLDERS WORK TOGETHER? MATHEMATICA POLICY RESEARCH
travel to CPC regions to attend meetings in person (which many payers indicated was important
to building trust).
Additionally, in the first few years of the initiative, CMS faced several unforeseen
challenges as it rolled out one of its first national multipayer initiatives (and the first one to
involve data aggregation). CMS’s dual role as both the initiative’s convener and a participating
payer initially created tensions with most payers. In several regions, payers expressed frustration
that CMS’s need to create a single national program meant that most components of CPC had to
be standardized across the seven regions rather than being tailored for local contexts (as CMS
did for another of its primary care initiatives, the Multi-Payer Advanced Primary Care Practice
Demonstration, in which CMS participated as a payer but played no convening role). Frustrated
with the lack of regional adaptation, one payer said, “We are talking only about CPC in these
meetings, but there is just so much overlap with work we are doing on a broader basis [in the
region]…. We want to tie this to broader conversations in other forums, where the same kind of
issues are being talked about.” Similarly, payers were discouraged by what they viewed as top-
down directives from CMS on topics such as data aggregation and the frequency and structure of
CPC multistakeholder meetings. Payers were also frustrated that CMS modified its approach to
selecting and contracting with data aggregation vendors over time because of internal legal and
bureaucratic hurdles.
Learning from their early experience working with CPC payers, CMS began working to
alter its collaborative approach in the second year of CPC. As a result, CMS’s relationships with
most participating payers had improved by the following year, and continued to improve
gradually during the remainder of the initiative. Specifically, CMS took the following actions:
• Worked to establish trust with other payers. To build rapport with other payers, CMS
representatives increased the number of individual calls it had with payer representatives to
better understand their perspectives on CPC. Additionally, in several regions, CMS’s shift to
having staff from CMS regional offices participate in CPC regional meetings—in contrast to
its initial approach in which all CPC representatives were from national headquarters—
helped improve communication. In these cases, regional representatives could sometimes
attend meetings in person and were better positioned to understand and work within the
regional context.
• Clarified its collaborative role and limitations. CMS more clearly communicated to
regional payers when it was acting as the convener of CPC and when it was serving only as
another payer collaborator. CMS also more clearly communicated its organizational
constraints, such as federal government contracting requirements.
• Deferred to other payers on regional decisions. CMS took a back seat in regional
collaborative decisions so as not to let its own bureaucratic constraints slow the momentum
achieved by regional stakeholders. For example, the three regions that achieved data
aggregation—Colorado, Ohio/Kentucky, and Oklahoma—moved ahead with selecting and
contracting with their data aggregation vendors without CMS. CMS subsequently joined
those efforts.
73

4. HOW DID CPC PAYERS, PRACTICES,
AND OTHER STAKEHOLDERS WORK TOGETHER? MATHEMATICA POLICY RESEARCH
4.5.3. Collaboration between non-CMS payers and other stakeholders
Payers generally valued the opportunity to collaborate
with practices and other stakeholders, such as employers or
hospital associations, though they also reported challenges
maintaining their active, consistent engagement. In
particular, payers valued the opportunity to discuss CPC
with practices. Several payers expressed the view that
practices played a more vital role in CPC multistakeholder
meetings than any other stakeholder type because the
initiative was aimed at supporting practices. As described
above, in many regions, payers’ commitment to CPC was
reinvigorated after hearing about practices’ successes in CPC
as well as their challenges to making further changes.
Moreover, payers indicated that practices’ perspectives were
critical to developing coordinated approaches to data
feedback.
Payers’ opinions of the usefulness of inviting other
stakeholders to join CPC meetings were mixed. Payers found other stakeholders’ participation
most valuable when the stakeholders had the expertise needed to actively participate in
discussions in a meaningful way. For example, some payers reported that consumer
representatives added more value to sessions on patient engagement or care coordination than to
discussions of data aggregation or other technical or logistical aspects of the collaboration. As
another example, self-insured employers participating in CPC helped payers in several regions
develop strategies to recruit other employers to the initiative. In another region, the
multistakeholder faculty indicated that engaging the region’s hospital associations in
multistakeholder meetings helped propel strategies to improve care coordination across the
medical neighborhood.
Although payers and multistakeholder faculty valued the participation of practices and other
stakeholders, engaging them was challenging. Payers and multistakeholder faculty identified
similar challenges to engaging all groups: (1) participants had difficulty finding time and
resources to attend the meetings (this was particularly true for small, non-system-based practices,
which were less likely to have management and administrative resources to devote to attending
meetings); (2) participants were often also involved in other multistakeholder groups in the state;
(3) participants lacked the skills and experience to productively contribute to discussions; and
(4) the vision for CPC collaborative goals and the roles of stakeholders were not always clear. As
one multistakeholder faculty member described the challenge, “There was not clear guidance or
direction or purpose and goals for the multistakeholder meetings. We want stakeholders at the
table, but there was not a clear sense of what [CMS and other payers] were hoping to get with
that.” Several payers and multistakeholder faculty suggested that multistakeholder meetings
might have been more productive—and might have stimulated greater stakeholder participation
and engagement—if stakeholders had been invited to only the portions of meetings that
addressed topics of concern to them and on which they might be expected to make meaningful
contributions.
Practices are the life blood of
this whole initiative…Hearing it
straight from them about what’s
worked and what hasn’t has
been one of the most
illuminating pieces [of
multistakeholder
meetings]…But I worry that
we’re not hearing from a true
representation [of all CPC
practices] in our region…
[especially] the small,
independent practices. They
just can’t afford to block off all
this time to be away from the
office, away from their patients.
—CPC payer, 2015
74

MATHEMATICA POLICY RESEARCH
5. HOW DID CPC PRACTICES CHANGE THE WAY THEY DELIVERED CARE
THROUGH WORK ON SPECIFIC MILESTONES?
CPC required participating practices to make many complex, interconnected changes in how
they deliver care to their patients, by focusing on five key functions: (1) access and continuity,
(2) planned care for chronic conditions and preventive care, (3) risk-stratified care management,
(4) patient and caregiver engagement, and (5) coordination of care across the medical
neighborhood. To promote progress on these functions, CMS specified a series of Milestones at
the start of CPC, and updated the Milestone requirements annually to build on practices’
progress in the prior year (Table 5.1). Some Milestones straddle more than one function.
In this chapter, we detail practices’ work implementing CPC overall and targeting each
Milestone, using a range of data sources. In Section 5.1, we summarize key findings on
practices’ changes in care delivery, and facilitators and barriers to this work. In Section 5.2, we
provide an overview of the Milestones and our data sources. In Section 5.3, we describe changes
over time in CPC practices’ approaches to care delivery. In Section 5.4, we discuss progress on
Milestones 2 through 9, which relate to practice transformation.
46
In Section 5.5, we describe the
monitoring of practices’ progress on achieving the Milestones. In Section 5.6, we summarize the
barriers and facilitators to implementing changes in care delivery and their implications for other
primary care initiatives.
5.1. Key findings on practices’ changes in care delivery
Across the CPC Milestones, multiple data sources provide clear evidence that practices have
undertaken substantial, challenging transformation and improved how they deliver care over the
course of CPC. In the first year of CPC (PY2013), practices worked to set up staffing, initial care
processes, and workflow. In PY2014, practices made meaningful progress on each CPC
Milestone, demonstrating that they were indeed changing care delivery. PY2015 and PY2016
brought additional refinements to practices’ care processes and workflows. Below are key
findings from the final year of CPC, including notable changes over the course of the initiative:
• Overall primary care approaches. As measured by the annual practice survey, CPC
practices’ approaches to primary care delivery improved each year of the initiative. Overall
scores on the modified Patient-Centered Medical Home Assessment (M-PCMH-A) included
in the survey indicate that CPC practices achieved their largest care delivery gains between
2012 (baseline) and 2014. In the final two years of the initiative, they achieved more modest
improvements.
• Areas of greatest improvement. Between 2012 and 2016, across the seven M-PCMH-A
domains
47
in the practice survey, CPC practices had the largest improvements in risk-
stratified care management, access to care, and continuous improvement driven by data. In
the remaining four domains—continuity of care, coordination of care across the medical
46
This chapter does not include Milestone 1: Budget, because it does not reflect transformation; see Chapter 3 for
this information.
47
Based on the factor analysis, we broke one of the six domains in the instrument into two domains, for a total of
seven domains, and mapped 37 of the M-PCMH-A questions to these domains.
75

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
neighborhood, planned care for chronic conditions and preventive care, and patient and
caregiver engagement—scores improved to a lesser extent. Gains in each domain increased
most during the first two years of the initiative. Comparison practices also showed
improvements, though to a lesser degree than CPC practices. In 2016, the last year of CPC,
29 percent of CPC practices had overall PCMH-A scores indicating the most advanced
approaches to care delivery (scores of 10 to 12) compared to 19 percent of comparison
practices.
• Correlation with practice characteristics. As in prior years, patterns of care delivery
reported on the practice survey by CPC practices in 2016 generally did not correlate with
practice characteristics (such as practice size, practice ownership, rural/urban status, and
how the practice compensated clinicians) or CPC funding per clinician.
• Types of practices that improved the most. CPC appears to have helped some practices
improve their approaches to care delivery more than others between 2012 and 2016. The
three types of practices that showed the most improvement are those that (1) had lower
scores on the M-PCMH-A at baseline, (2) were not a recognized PCMH before CPC, and (3)
were rated in the bottom two-thirds of CMS scores for their application to participate in CPC
(Dale et al. 2016). All three groups had lower average scores in 2012 than CPC practices
overall; therefore, these practices may have achieved larger increases because they had more
room for improvement.
• Care management for high-risk patients (Milestone 2). Deep-dive data (in-depth
interviews with clinicians and staff at 21 diverse practices that were selected for intensive
study) indicate that practices perceived that the biggest benefit of CPC participation was
increased capacity to provide care management services to high-risk patients. All data
sources indicate this was the area of greatest transformation for CPC practices, and most of
this progress occurred between 2012 and 2015.
- Deep-dive and Milestone data indicate that, by 2016, CPC practices had stopped making
major changes to their risk-stratification methodologies. Similar to 2015, practices used
a combination of approaches to risk-stratify their patients, most commonly clinical
intuition and clinical algorithms.
- In the second half of CPC, practices increasingly integrated care managers’ work with
clinicians, which had been a challenge earlier. Clinicians developed trust in care
managers to handle patient follow-up after observing how care managers’ efforts
improved patients’ adherence to recommended treatments, reduced the need for
clinicians to handle this task, and allowed clinicians to focus on more complex clinical
care.
o As in previous years, challenges with care management remain:
For example, deep-dive findings indicate that care managers in several
practices perform numerous tasks in addition to the activities under Milestone
2. In some cases, this resulted in turnover because care managers felt
overwhelmed.
The use of care plans remains uneven, and clinicians and care managers in
most deep-dive practices continued to report limited EHR functionality for
supporting care plans and care management.
76

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
A few system-level respondents were frustrated about multiple guidelines and
different requirements for care plans from various payers and medical home
initiatives.
In addition, deep-dive and practice survey results indicate that duplication of
patient outreach by practice-based care managers and those affiliated with
hospitals, health systems, health plans, or visiting nurses associations continues
to confuse patients and frustrate care managers; survey data indicate that this
also occurs in comparison practices.
• Behavioral health integration (Milestone 2). To identify patients for behavioral health
support, CPC practices most commonly use screening tools, staff or provider referrals, and
patient self-referrals. They most commonly deliver behavioral health services by providing
(1) referrals to specialty mental health care, (2) primary care management with referral as
needed to specialty mental health care, or (3) co-management between primary care and
specialty mental health care. Practice survey results show that practices built internal
capacity to provide behavioral health screening and services: the proportion of practices
with behavioral health specialists, clinical psychologists, or social workers on site increased
from 19 percent in 2014 to 29 percent in 2016. However, 2016 Milestone data indicate that
co-location of such staff varied across CPC regions from 3 to 52 percent. Over half of CPC
practices with co-located behavioral health staff report that these staff were fully integrated
into primary care workflows, share patient records, and were available for warm hand-offs
and acute primary care visits.
• Access by patients (Milestone 3). To improve access and continuity, most CPC practices
reported in the 2016 practice survey that they offered same or next day appointments and
had an on-call clinician available with access to the EHR 24 hours a day, seven days a week.
As in previous years, nearly all CPC practices reported using patient portals to improve
access, partly because the Stage 2 Medicare and Medicaid EHR Incentive Programs
(Meaningful Use) emphasize patient portals. However, in 2016, few Medicare FFS
beneficiaries reported that they used these portals regularly in the patient survey. Deep-dive
and practice survey data indicate that practices reported that they continued to improve the
availability of same-day appointments; telephone access to the practice; and after-hours
access to clinicians via email, telephone, or in-person contacts. Nevertheless, beneficiaries in
CPC and comparison practices did not report improved experiences with these types of
access in the patient survey.
• Patient experience (Milestone 4). To improve patients’ experience in the final year of CPC,
80 percent of practices conducted patient surveys and 48 percent convened patient and
family advisory councils (PFACs) in 2016. Practices’ use of PFACs increased throughout
CPC, especially between 2013 and 2015. Challenges with surveys included the burdens of
conducting and analyzing data, and concerns about data quality. Challenges with PFACs
included scheduling, ensuring that a representative group of patients attended, and
reassuring patients that their participation was valuable and the practice would use their
feedback. Patient respondents who participated in PFACs reported in qualitative interviews
that the PFACs’ suggestions led to multiple practice improvements around patient outcomes,
patient satisfaction, and patient education.
77

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
• Quality improvement (Milestone 5). As in previous years, quality improvement (QI)
remained a major focus for both CPC and comparison practices in 2016. Over time, more
CPC and comparison practices reported that all staff share responsibility for QI, as opposed
to relegating this work to a QI committee or department. And 40 percent of practices
involved patients or caregivers in identifying QI ideas or opportunities. The 2016 clinician
and staff survey indicated that two-thirds of CPC and comparison physician respondents are
now involved in QI work. Consistent with prior years, in 2016, deep-dive practices typically
used ad hoc approaches for practice-level QI; systematic approaches were more common in
large and system-owned practices.
• Electronic clinical quality measures (eCQMs). Most CPC practices focused QI activities
on a narrow set of eCQMs over time. In 2016 and 2015, the eCQMs they most commonly
focused on were diabetes: hemoglobin A1c (A1c) poor control, colorectal cancer screening,
and breast cancer screening. In past years, deep-dive practices noted that documenting and
tracking eCQMs is helpful for QI, but it is resource-intensive. In 2016, deep-dive practices
noted that having dedicated staff to support eCQM documentation, analysis, and design of
improvement processes facilitated QI.
• Care coordination (Milestone 6). Practices made considerable progress through CPC in
care coordination related to hospital or ED follow-up. Deep-dive findings indicate practices
made progress in building relationships and exchanging information with hospitals about
patient discharge. However, several deep-dive practices reported ongoing challenges with
accessing hospital records and receiving complete and timely information about their
patients from hospitals. According to the practice survey results, there were increases in
receipt of information on patients from community hospitals and EDs within 24 hours. In
2016, Medicare FFS beneficiaries at CPC practices were more likely than beneficiaries at
comparison practices to report that the provider’s office contacted them within three days of
their most recent hospital stay (60 versus 50 percent) or within one week of the most recent
emergency room or ED visit (59 versus 51 percent). Deep-dive practices also noted
expanded follow-up with patients after hospital and ED discharge and emphasized the
importance of care managers in addressing the needs of high-risk patients.
• Care compacts (Milestone 6). In addition to working on follow-up after hospital and ED
discharge, by the end of the initiative, 41 percent of CPC practices also chose to work on
care compacts or collaborative agreements with other providers. Deep-dive findings show
practices typically established care compacts, or collaborative agreements, with specialists
to which they most frequently refer patients. Most care compacts outlined expectations for
referrals and communication between primary care and specialists. A few practices noted
that some specialists struggle with multiple collaborative agreements due to variations in
requirements among the referring groups. Practices in systems with system-wide EHRs
reported that care compacts were less important because all clinicians in their system could
view patient information.
• Shared decision making (Milestone 7). Practices implemented shared decision making
(SDM) slowly in the early years of CPC, in part due to confusion about the concept of
preference sensitive conditions, but use of SDM increased steadily. The percentage of CPC
practices that reported that they consistently used patient decision aids (PDAs) to help
patients and providers jointly decide on treatment options increased from 42 percent in 2014
78

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
to 62 percent in 2016 (compared to 25 percent among comparison practices). The top
conditions selected for SDM in 2016 were colorectal cancer screening, prostate cancer
screening, tobacco cessation, and mammography. According to some respondents, the
quality of patient care improved with SDM. However, deep-dive and practice survey results
indicate room for improvement in providers’ and staff members’ understanding of
preference-sensitive conditions, providing SDM without overwhelming clinicians, and
tracking PDA use and SDM discussions in EHRs.
• Learning collaborative (Milestone 8). Similar to previous years, CPC practices greatly
valued learning and sharing with other practices in the CPC learning collaborative. Chapter
3 discusses how the learning activities supported practice change.
• Health IT (Milestone 9). CPC required practices to use EHRs certified by the Office of the
National Coordinator for Health Information Technology (ONC). In 2016, all CPC practices
attested that their eligible providers were working toward Stage 2 requirements for
Meaningful Use. CPC practices continued to face challenges in obtaining and exchanging
timely data from providers outside their practice or system. Health information technology
(IT) challenges affected care plan use and care management activities, practices’ ability to
follow up in a timely way with patients discharged from the hospital or ED, and practices’
capacity to track the outcomes of SDM discussions.
• Patient dismissals. Previous annual reports noted that some deep-dive practices had
suggested that an unintended consequence of CPC’s rewards for improving patient outcomes
might tempt other practices to dismiss patients with poor outcomes. However, CPC
practices and comparison practices reported dismissing patients rarely, at similar rates, and
generally for similar reasons. Thus, participation in CPC did not appear to make practices
more likely to dismiss patients.
• Perceived benefits of CPC. Over the course of CPC, deep-dive practice members
increasingly perceived benefits to the quality, delivery, and organization of patient care from
working on CPC Milestone requirements. Likewise, in 2016 clinician and staff survey
results, a large proportion of clinicians and care managers rated CPC highly. Chapter 6
provides more information on practice members’ experiences with CPC.
• Implementation facilitators. Several practice strategies that cut across the Milestones
facilitated CPC implementation. Over time, deep-dive CPC practices increasingly reported
holding regular meetings (at least monthly) to engage and continue to involve staff in CPC.
By the end of the initiative, several deep-dive practices also reported identifying a practice
leader (sometimes a physician), or small committee to act as a CPC champion—helping to
introduce new concepts to the practice and to integrate CPC-related changes into workflows.
Finally, several deep-dive practice findings suggest that establishing care teams that worked
regularly together and clearly outlined clinician and staff roles helped meet patients’ needs.
• Mixed facilitators and barriers. Some factors, such as system ownership, acted as
facilitators to implementing CPC in some cases, and barriers in others. For example, system-
owned practices (and practices in regions with robust local health information exchanges)
reported reliable, timely access to patients’ hospital and ED records, and in some cases,
enhanced information exchange with specialists. However, practices described challenges
obtaining timely electronic information from unaffiliated providers in order to coordinate
79

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
patient care with them. System ownership also benefited CPC implementation by giving
access to centralized QI resources, in some cases including CPC project managers, which
facilitated practice-level change. However, system ownership sometimes created
administrative and bureaucratic barriers to making improvements based on patient feedback
and making Milestone-related decisions, such as selecting SDM topics to pursue.
• Implementation barriers. Cross-cutting barriers to CPC implementation included the
burden of integrating numerous required changes into practice workflows, which
particularly affected care managers. Practices also struggled with the volume of
administrative and quality reporting, including different reporting requirements across
payers. The burden of reporting varied somewhat in deep-dive practices depending on their
electronic health record vendor and their IT support. In addition, practices reported it was
hard to engage patients in care management activities related to behavior modification,
adherence to treatment regimens, and setting health goals; efforts to reduce inappropriate ED
use; shared decision making; PFACs and patient surveys; and patient portals. Some practices
found that enhanced self-management support, increased use of motivational interviewing,
and teamwork helped them better engage patients in their own care.
5.2. Milestones and data sources
5.2.1. Overview of Milestones
The Milestones in Table 5.1 provide guideposts or stepping stones to achieving the five
functions.
48
Some Milestones (such as Milestone 9: Health information technology) contribute to
multiple functions. Although the Milestones define specific areas of work, they allow practices
considerable latitude in how they meet these goals and change the way they provide care. CMS
updated Milestones annually and assessed whether practices meet Milestone targets, which are
considered the minimum requirements to remain in the initiative.
Table 5.1. CPC Milestones for PY2016
1. Budget. Complete an annotated annual budget with PY2015 revenues/expenses and projected
CPC initiative practice revenue flow for PY2016 at the start of the year and report actual
revenue/expenses for PY2016 at the end of the year.
2. Care management for high-risk patients. Maintain at least 95 percent empanelment to provider
and care teams. Continue to risk-stratify all patients, maintaining risk-stratification of at least 75
percent of empanelled patients. Expand care management activities for highest risk patients who
are likely to benefit from longitudinal care management and those not otherwise at high risk but
requiring episodic care management. Provide information about the care plans that are used for
both longitudinal care management and episodic care management. Maintain the implementation of
and further refine one of three strategies (behavioral health integration, medication management, or
self-management support).
48
CMS considered the five functions primary drivers in achieving CPC’s aims, as specified in the CPC change
package (https://innovation.cms.gov/Files/x/cpcidiagram.pdf). The change package describes the underlying logic of
CPC, including the primary and secondary drivers to achieve the aims of CPC and the concepts and tactics that
support the changes.
80

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.1 (continued)
3. Access by patients and enhanced access. Enhance patients’ ability to communicate 24 hours a
day, 7 days a week with a care team that has real-time access to their electronic medical records.
Continue to implement asynchronous forms of communication (for example, patient portal and
email) and ensure timely responses. Measure continuity of care by measuring visit continuity
quarterly for each provider and/or care team in the practice.
4. Patient experience. Assess patient experience through patient surveys or patient and family
advisory council meetings and communicate to patients (using electronic, poster, pamphlet, or
similar communication methods) about resulting changes the practice is making.
5. Quality improvement. Continue to perform continuous quality improvement using electronic health
record (EHR)-based clinical quality measures (eCQMs) on at least three of the measures that
practices report annually. Review at least one payer data feedback report (CMS Practice Feedback
Report or other payers’ reports) to identify a high-cost area and a practice strategy to reduce this
cost while maintaining or improving quality.
6. Care coordination across the medical neighborhood. Track patients by implementing two of
three options: follow up via telephone with patients within one week of emergency department (ED)
visits; contact at least 75 percent of hospitalized patients within 72 hours of discharge; and enact
care compacts with at least two groups of high-volume specialists.
7. Shared decision making. Use at least three decision aids to support shared decision making
(SDM) for three preference-sensitive conditions and track patient eligibility for and use of the aids.
8. Participating in learning collaborative. Participate in regional and national learning offerings and
communicate with regional learning faculty.
9. Health information technology (IT). Attest that each eligible professional in the practice is
engaged with and working toward attestation for Stage II Meaningful Use in the timelines set by the
Meaningful Use program.
Source: CPC PY2016 Implementation and Milestone Reporting Summary Guide.
5.2.2. Data sources
Data sources used to describe practice change are listed in Table 5.2.
Table 5.2. Data sources on CPC practice change practices
Date source Type of data
Milestone data
CPC practices’ self-reported data from 2012 to 2016, submitted to CMS to document
their Milestone work.
Practice survey Fielded to CPC and comparison practices in 2016 (as well as earlier data collected in
2012, 2014, and 2015).
a
This survey includes the modified Patient-Centered Medical
Home Assessment (M-PCMH-A) tool that we adapted for the CPC evaluation to
capture approaches to care delivery in seven areas that relate closely to CPC
Milestones.
b
Clinician and staff
surveys
Conducted in 2013 and 2016 with primary care clinicians in CPC and comparison
practices and staff in CPC practices about their experiences delivering primary care.
Chapter 6 reports detailed findings from the clinician and staff surveys.
81

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.2 (continued)
Date source Type of data
Qualitative interviews
and observations of
deep-dive practices
Qualitative data collected from 2013 to 2016 from 21 deep-dive practices selected for
intensive study (3 practices per region; 1 of the original practices did not participate in
the final round of data collection).
c
Respondents included a practice clinician lead, other
clinicians, CPC project coordinators, care managers, practice managers, Health IT staff
and other staff. These data provide information on how practices are implementing
changes related to each Milestone and associated barriers and facilitators to
implementation. Data were analyzed using the Consolidated Framework for
Implementation Research (CFIR) adapted for CPC (Damschroder et al. 2009), as well
as a second codebook reflecting the CPC Milestones.
Patient survey Fielded annually to samples of Medicare FFS beneficiaries attributed to CPC and
comparison practices between 2013 and 2016. Based on the Clinician and Group
Consumer Assessment of Healthcare Providers and Systems (CG-CAHPS), version
2.0, the survey asked patients to rate their experiences with care from their primary
care provider over the past 12 months. Chapter 7 reports detailed findings from the
patient survey.
First three annual
reports
The first three annual reports describe practice implementation during the first three
years of CPC (Taylor et al. 2015; Peikes et al. 2016a; Peikes et al. 2016b). We
describe how the current findings differ from or build on those in these prior reports.
Qualitative interviews
with a sample of high-
risk patients
Data from qualitative interviews with 43 high-risk patients (or their caregivers)
undergoing care management in 11 of the deep-dive practices. These telephone
interviews were conducted from March to May 2015 (O’Malley et al. 2018).
a
We conducted four rounds of the practice survey: at the start of the initiative, October through December 2012; 18
to 21 months after CPC began, in April through July 2014; 30 to 33 months after CPC began, from April through
August 2015; and 42 to 46 months after CPC began, April through August 2016. (The first round of the practice
survey included only CPC practices, because the comparison practices had not yet been selected. The second, third,
and fourth rounds included both CPC and comparison practices.)
b
Although the seven M-PCMH-A domains measured in the practice survey do not align one-to-one with the CPC
Milestones or functions, they are fairly consistent with CPC Milestones and functions, cover care processes and
supports that prior studies suggest are important to primary care redesign, and are useful for tracking progress in
transforming care.
c
For more information on selection and characteristics of deep-dive practices, as well as analysis methods, see
Peikes et al. (2014), Taylor et al. (2015), and Keith et al. (2017).
5.3. Changes over time in CPC practices’ approaches to primary care
delivery
Mathematica fielded four rounds of the practice survey (2012, 2014, 2015, and 2016) to
gather practices’ self-reported approaches to various aspects of primary care delivery. In this
section, we highlight selected findings for the 471 CPC practices that responded to all four
survey rounds.
49
In addition, Mathematica fielded three rounds of the same practice survey
(2014, 2015, and 2016) to roughly 850 comparison practices each round; each round between
340 and 423 comparison practices responded to the survey.
50
All rounds of the survey used a
modified form of the PCMH-A tool, which we adapted for the CPC evaluation to capture
49
The 471 CPC practices include 28 that withdrew or were terminated from CPC before April 2016.
50
There were three (rather than four) rounds of the practice survey for comparison practices because comparison
practices had not been selected when the 2012 survey was fielded.
82

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
approaches to care delivery in seven areas (Table 5.3). We refer to our modified version as the
M-PCMH-A.
51
We analyze 37 questions from the M-PCMH-A survey module in 7 domains.
52
For each
question, practices rated their performance on a scale of 1 to 12, divided into four levels (1–3, 4–
6, 7–9, 10–12), where 1 signifies the least advanced approaches to delivering care and 12
signifies the best approaches. We created summary composite measures for the seven M-PCMH-
A domains, and for the overall score, as weighted averages of each practice’s response to all
questions in a given domain. We derived the weights from a factor analysis that we conducted on
the responses of CPC practices to the 2012 practice survey. Factor analysis uses the correlation
between the individual question and the domain it measures to reflect the reliability of each
question in measuring the domain. These weights are also referred to as reliability weights
(Poznyak et al. 2017). As previously noted, some Milestones (such as Milestone 9: Health
information technology) contribute to multiple functions.
Table 5.3. Primary care delivery domains measured by the M-PCMH-A in the
CPC practice survey
Domain
Number of
questions Topics
Continuity of care 2
1. Patient assignment to specific provider, and use of that assignment to
schedule and monitor supply and demand
2. The extent to which patients are encouraged to, and usually see their
own provider and practice team
Access to care 3
3. Flexibility of appointment systems for different-length and same-day visits
4. Asynchronous communication with practice team including patients’
preferred mode
5. Patient after-hours access to a coverage team or the practice, and
availability of patient’s EHR
Planned care for
chronic conditions
and preventive care
6
6. Availability and proactive use of patient registries by practice teams
7. Availability and use of evidence-based guidelines in care
8. Focus of patient visits on acute and planned care needs
9. The extent to which evidence-based reminders to providers are specific
to the individual patient encounter
10. Extent of role of nonphysician practice team members in providing
clinical care
11. Extent to which medication reconciliation occurs regularly and is
documented in the patient’s medical record
51
The first survey round contained 41 questions. We took 26 of these questions (some with slight refinements) from
the PCMH-A instrument (v.1.3) developed by the MacColl Center for Health Care Innovation to measure
transformation progress in safety net clinics in eight change concept areas established as key components of PCMH
(http://www.improvingchroniccare.org/index.php? p=PCMH_Change_Concepts&s=261
). To more closely measure
the CPC focal areas, we changed the order and domain assignment for some PCMH-A questions. Because the
PCMH-A did not cover all aspects of primary care delivery relevant to this evaluation, we added 15 questions that
we either developed or adapted from PCMH-A. We dropped three of these questions from the second survey round
because they were present elsewhere in the survey. In one case, two items were collapsed into one item about
radiology and blood tests. We dropped one question from the scores (because it was not correlated with any other
questions), leaving 37 questions that we tracked over time.
52
The survey module contains six domains; based on a factor analysis, we broke one of these domains into two, for
a total of seven domains (Poznyak et al. 2017).
83

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.3 (continued)
Domain
Number of
questions Topics
Risk-stratified care
management
3
12. Degree to which a standard method or tool to stratify patients by risk
level is used and guides care delivery
13. The provision of clinical care management services for high-risk patients
by care managers integrated into the practice team
14. The availability of registry or panel-level data to assess and manage care
for practice populations
Patient and
caregiver
engagement
6
15. Assessment and incorporation of patient and family preferences in
planning and organizing care
16. How systematically practice teams involve patients in decision making
17. Extent to which patient comprehension of written and verbal
communication is assessed and accomplished
18. The type of self-management support provided by members of the
practice team
19. How test results and care plans are communicated to patients
20. The use of feedback from a patient and family caregiver council to guide
practice improvements
Coordination of
care across the
medical
neighborhood
10
21. The extent of tracking of patient referrals to specialists
22. The collaborative development of care plans with patients and families
that include self-management and clinical management goals, and are
used to guide care
23. The extent to which referral relationships with a range of specialists are
formalized
24. Availability of behavioral health services for patients
25. The ease of obtaining referrals for specialty care, hospital care, or
supportive community-based resources and exchange of relevant
information with other providers before and after the patient visit
26. Practice staff follow-up with patients following ED/hospital visits
27. How practices link patients to supportive community-based resources
28. Transmission of patient information when this practice refers patients to
hospitals, EDs, and specialists
29. The timeliness of information received from hospitals and EDs following a
patient’s visit
30. The proportion of patients for whom the practice knows the total cost to
payers for medical care
Continuous
improvement driven
by data
7
31. Practice’s use of quality improvement (QI) activities that are continuous
and based on proven improvement strategies
32. Extent to which QI activities are conducted by practice teams supported
by a QI infrastructure with meaningful involvement of patients and their
families
33. The availability of comprehensive performance measures to practice site
and individual providers
34. Availability of feedback reports on patient care experiences, and care
processes or outcomes to practice site, individual providers, practice
teams, patients, other teams, and external agencies
35. The availability of staff, resources, and time for QI activities
36. The extent to which hiring and training processes focus on improving
care and creating patient-centered care
37. The extent to which responsibility for conducting QI activities is shared by
staff and is made explicit through protected time to meet and specific
resources to engage in QI
Note: See Appendix D, Table D.5, for a complete list of the survey questions.
84

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Survey data suggest that CPC practices improved their primary care delivery
approaches during CPC. Between 2012 and 2016, CPC practices’ self-reported approaches to
primary care delivery across the seven domains improved 2.9 points (from 6.5 out of 12 to 9.4),
measured using the overall M-PCMH-A score. The largest gains occurred between the 2012 and
2014 surveys, with the average overall M-PCMH-A score increasing 2.2 points, from 6.5 to 8.7.
In the last two years of the initiative, practices made modest gains of 0.5 and 0.2 points,
respectively (Figure 5.1).
CPC practices made improvements in all seven primary care domains from 2012 to 2016,
though improvements were larger in some domains than in others. The largest improvements
were in risk-stratified care management (5.5 points, from 4.6 to 10.1), access to care (3.5 points,
from 7.0 to 10.5), and continuous improvement driven by data (2.9 points, from 5.8 to 8.7). The
smallest improvement was in the continuity of care domain, which rose 1.0 point from the
relatively high score of 9.6 in 2012. In the remaining three domains, improvements ranged from
2.0 points for coordination of care across the medical neighborhood (from 6.7 to 8.7), to 2.1
points for both planned care for chronic conditions and preventive care (from 7.6 to 9.7), and
patient and caregiver engagement (from 6.7 to 8.8).
As with the overall M-PCMH-A score, gains in specific domains were largest during the
first two years of the CPC initiative. Between 2012 and 2014, CPC practices’ responses indicated
average improvements of 0.6 to 5.1 points for each of the seven domains. In 2015 and 2016,
CPC practices continued to improve, albeit at a slower rate.
Figure 5.1. CPC practices’ mean 2012 M-PCMH-A scores, with 2014, 2015,
and 2016 gains, for the seven domains and overall
Source: Mathematica analysis of the 2012 CPC practice survey administered October through December 2012, and
the 2014, 2015, and 2016 CPC and comparison practice surveys administered April through July 2014,
April through August 2015, and April through August 2016. We did not administer the 2012 practice survey
to comparison practices.
Note: Scale: 1 [least advanced approach] – 12 [best approach]. We weighted comparison practice responses to
ensure CPC and comparison samples were similar and to adjust for nonresponse.
6.5
9.6
7.0
7.6
4.6
6.7
6.7
5.8
2.2
0.6
2.6
1.5
5.1
1.2
1.3
2.2
0.5
0.2
0.5
0.4
0.3
0.6
0.5
0.3
0.2
0.2
0.4
0.2
0.1
0.3
0.2
0.4
1
2
3
4
5
6
7
8
9
10
11
12
Overall
M-PCMH-A
score
Continuity of
care
Access to
care
Planned care for
chronic
conditions
and preventive
care
Risk-
stratified
care
management
Patient
and
caregiver
engagement
Coordination
of care
across the
medical
neighborhood
Continuous
improvement
driven by data
Score
[1 (lesat advanced approach)–
12 (best approach)]
2012 score 2014 gain 2015 gain 2016 gain
9.4 10.6 10.5 9.7 10.1 8.78.78.8
2016 score
85

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Comparison practices also improved care delivery approaches between 2014 and 2016,
although their scores were slightly lower in most years than those of CPC practices. The
overall M-PCMH-A score in 2016 for CPC practices (9.4) was 0.9 points higher than the score
for comparison practices (8.5; p < 0.01) (Figure 5.2). (See Appendix D, Table D.6a.) The largest
difference in mean scores between CPC and comparison practices in 2016 was for risk-stratified
care management (where CPC practices scored 2.4 points higher than comparison practices, 10.1
versus 7.7 points, respectively), perhaps reflecting the CPC emphasis on this domain. In the other
six domains, 2016 scores were only slightly higher (1 point or less) for CPC practices than for
comparison practices. The improvements in comparison practices’ scores may indicate that they
are facing similar pressures and incentives to improve care delivery.
Figure 5.2. CPC and comparison practices’ mean M-PCMH-A scores in 2016,
for the seven domains and overall
Source: Mathematica analysis of the 2016 CPC practice and comparison practice survey administered April through
August 2016.
Note: Scale: 1 [least advanced approach] – 12 [best approach]. We weighted comparison practice responses to
ensure CPC and comparison samples were similar and to adjust for nonresponse.
Between 2012 and 2016, overall M-PCMH-A scores improved for 97 percent of CPC
practices. The proportion of CPC practices with scores in the highest performance category (10
to 12) also grew from 2 percent in 2012 to 29 percent in 2016 (Figure 5.3). CPC practices in the
two highest performance categories combined increased dramatically between 2012 and 2014,
from 36 percent to 93 percent, and reached 98 percent in 2016. By 2016, less than 1 percent of
CPC practices had an overall score in the least advanced category (1 to 4), and only about 2
9.4
10.6
10.5
9.7
10.1
8.8
8.7 8.7
8.5
9.8
9.6
9.2
7.7
8.3 8.3
7.7
1
2
3
4
5
6
7
8
9
10
11
12
Overall
M-PCMH-A
Continuity of
care
Access
to care
Planned care
for chronic
conditions and
preventive
care
Risk-
stratified
care
management
Patient
and
caregiver
engagement
Coordination
of care
across the
medical
neighborhood
Continuous
improvement
driven by data
Score
[1 (least advanced approach)–12 (best approach)]
CPC practices Comparison practices
86
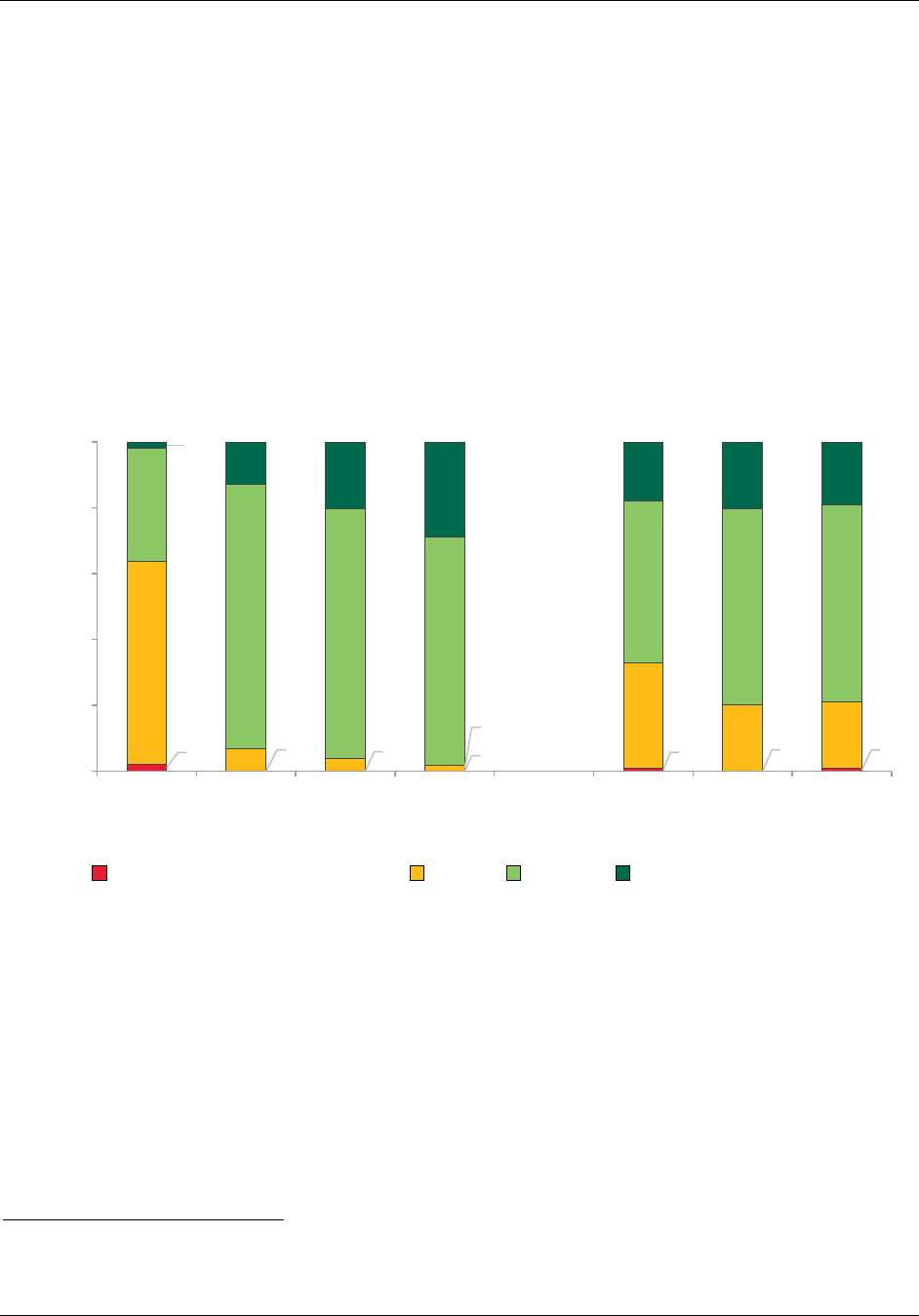
5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
percent were in the second lowest category (4 to 7).
53
In 2016, there was little variation across
regions: the average overall M-PCMH-A scores ranged from 9.2 in Arkansas and Oregon to 10.1
in Ohio/Kentucky.
Although the average overall M-PCMH-A scores for the CPC and comparison practices
were fairly similar (9.4 versus 8.5 points), the distribution of scores highlights the improvements
CPC practices made relative to the comparison group. In 2016, 29 percent of CPC practices had
overall PCMH-A scores indicating the most advanced approaches to care delivery (scores of 10
to 12) compared to 19 percent of comparison practices. Only 2 percent of CPC practices had
scores in either of the lowest two performance categories (1 to <4 or 4 to <7), whereas 21 percent
of comparison practices had scores in that range (Figure 5.3).
Figure 5.3. Distribution of CPC and comparison practices’ overall M-PCMH-A
scores over time
Source: Mathematica analysis of the 2012 CPC practice survey administered October through December 2012, and
the 2014, 2015, and 2016 CPC and comparison practice surveys administered April through July 2014,
April through August 2015, and April through August 2016. We did not administer the 2012 practice survey
to comparison practices.
Note: Scale: 1 [least advanced approach] – 12 [best approach]. We weighted comparison practice responses to
ensure CPC and comparison samples were similar and to adjust for nonresponse.
As in prior years, improvements in CPC practices’ overall M-PCMH-A scores
generally did not correlate with practice characteristics or CPC funding per clinician. The
magnitude of CPC practices’ changes in overall M-PCMH-A scores from 2012 to 2016 was not
consistently associated with practice size, practice ownership, or rural/urban status (all measured
53
The minor fluctuation between 0 percent and 1 percent in the least advanced category for years 2014 through
2016 may be due to rounding.
2
0
0
0
1
0
1
61
7
4
2
32
20
20
34
80
75
69
49
59
60
2
13
20
29
18
20
19
0%
20%
40%
60%
80%
100%
2012 2014 2015 2016 2014 2015 2016
Percentage of practices
1 to <4 (Least advanced approach) 4 to <7 7 to <10 10 to 12 (Best approach)
CPC practices Comparison practices
87

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
before CPC began), or how clinicians were compensated by the practice (as reported the first
time this was collected, in 2014), or CPC funding per clinician (proxied by the funding in the
first program year of CPC) (see Appendix D, Table D.7). The three characteristics associated
with larger increases in M-PCMH-A scores were practices that (1) had lower scores on the M-
PCMH-A at the start of CPC, (2) were not a recognized PCMH before CPC, and (3) were rated
in the bottom two-thirds of CMS scores on their application to participate in CPC.
The distribution of M-PCMH-A scores narrowed over time among CPC practices. As
shown in Figure 5.4, scores converged from 2012 to 2014 and remained close from 2014 to
2016. CPC practices with overall M-PCMH-A scores in the bottom third of the distribution in
2012 (scores from 1 to 5.7) had an average improvement of 4.2 points by 2016. Practices in the
middle third of the distribution had an average improvement of 2.9 points, and practices in the
top third of the distribution (scores from 7.2 to 12) had an average improvement of 1.6 points.
Figure 5.4. CPC practices’ average overall M-PCMH-A scores, for all practices
and by practices’ 2012 score
Source: CPC practice surveys administered October through December 2012, April through July 2014, April through
August 2015, and April through August 2016.
5.4. Progress on individual Milestones
In this section, we discuss CPC practices’ progress on Milestones 2 through 9. (We discuss
Milestone 1—which focuses on budgeting of CPC funds—in Chapter 3, because it reflects a
program support, rather than a care delivery activity.) We draw these findings primarily from the
6.5
9.4
4.9
9.1
6.4
9.3
8.2
9.8
0
2
4
6
8
10
12
2012 2014 2015 2016
All CPC practices
Practices with overall scores in the bottom third of the distribution in 2012
Practices with overall scores in the middle third of the distribution in 2012
Practices with overall scores in the top third of the distribution in 2012
88

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Milestone data that practices submitted to CMS,
54
results from the practice, clinician and staff,
and patient surveys, and qualitative data collected during site visits to deep-dive practices. When
possible, we discuss how findings from various data sources align or differ, and we use the deep-
dive data to provide context and more nuanced information on how practices are changing as
well as barriers and facilitators to those changes.
We focus on findings from 2016; additional details on Milestone implementation from
earlier years appear in the previous annual reports (Taylor et al. 2015; Peikes et al. 2016a; Peikes
et al. 2016b). CMS deepened the requirements of some of the Milestones each year of the CPC
initiative. A summary of changes in Milestone requirements by year is available at
https://innovation.cms.gov/Files/x/CPCI-Milestones.pdf. (See Appendix A.)
5.4.1. Milestone 2: Care management for high-risk patients
Deep-dive practices, CMS, other participating payers, and learning faculty continued to note
that Milestone 2 is one of the most important and challenging CPC Milestones. In PY2016,
Milestone 2 required practices to continue to risk-stratify their patients and refine their risk-
stratification methodology as needed to align patients’ needs with care management resources,
expand care management activities to include patients with rising risk (that is “with health risks
and chronic conditions that are not well controlled”) as well as the highest-risk patients, and
develop and maintain care plans for care managed patients.
To perform risk-stratified care management in PY2016, practices were required to continue
the following four activities:
1. Empanel each active patient (link each patient directly to a provider or care team that has
responsibility for that patient). CMS allowed practices to define “active patients” but
recommended that they include those who sought care from the practice in the past 24–36
months.
2. Risk-stratify each empanelled patient to help define his or her risk level.
3. Provide care management resources to the population identified as most likely to benefit
from those services. Focus on patients identified by the practice’s risk-stratification
methodology to be at high risk or rapidly rising risk (for example, those that are clinically
unstable, in transition, and/or high utilizers of services) and likely to benefit from active,
ongoing, longitudinal care management and those patients not otherwise at high risk who
are identified by a triggering event (for example, a transition of care or new diagnosis) as
requiring episodic care management for a limited period of time.
4. Provide information about any care plans used for longitudinal (ongoing) care management
and episodic (time-limited) care management. Practices were not required to develop or
maintain care plans.
54
The number of practices that reported Milestone data fell as practices withdrew or were terminated from CPC. For
example, 446 practices reported data in Quarter 1, 439 in Quarter 3, and 439 in Quarter 4 of 2016. Practices were
required to report on different Milestones in each quarter, with the bulk of reporting occurring in Quarters 1 and 4.
89

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
In addition to continuing the risk-stratified care management work on this Milestone, CPC
asked practices to continue to implement one of three advanced primary care strategies: (1) self-
management support, (2)
behavioral health integration or (3) medication management (CMS
2015a). Self-management support of chronic conditions aims to support patients in building the
skills and confidence they need to reach their health goals. It requires a collaborative relationship
between health care providers and/or teams and patients and their families. Behavioral health
integration refers to the integration of primary care with behavioral health care which addresses
mental health and substance abuse conditions, stress-linked physical symptoms, patient
activation, and health behaviors. In CPC, it also includes the needs of individuals with dementia
and their caregivers. Medication management includes scheduled monitoring of patient
medications; medication reconciliation, particularly during transitions of care; protocol-guided
medication management; with the assistance of a clinical pharmacist or a licensed practitioner
with prescribing authority.
a. Overview of findings
All data sources examined demonstrate that CPC practices made progress in implementing
risk-stratified care management during the initiative, especially between 2012 and 2014. Overall,
CPC practices appear to have successfully implemented risk-stratification and care management.
The deep-dive data indicate that, by 2016, CPC practices had stopped making major changes to
their risk-stratification methodologies.
CPC practices used more systematic and team-based approaches to risk-stratified care
management than did comparison practices. However, CPC practices also continued to face
challenges with the care manager role and the use of care plans, which may have implications for
the elements of CPC that will be sustained after the initiative ended. The survey results continue
to show that substantially more CPC practices than comparison practices reported that (1) they
used standardized risk-stratification processes and (2) care managers who are practice care team
members were systematically providing care management services to high-risk patients.
However, several deep-dive practices continued to report that care managers feel overwhelmed
with numerous responsibilities, and most deep-dive practices described challenges implementing
the use of care plans as intended by CPC. The survey results and findings from interviews with
deep-dive practices indicate that duplication of patient outreach by care managers from within
and outside the practice confuses patients in both CPC and comparison practices.
CPC practices made progress implementing the advanced primary care strategies introduced
in the second year of the initiative. The practice survey results show that, between 2012 and
2016, CPC practices (1) increased their capacity to provide self-management support, including
training practice staff on patient empowerment and problem-solving methodologies, and
improving techniques for communicating with patients; and (2)
increasingly provided regular
medication reconciliation to all patients. The practice survey and deep-dive findings indicate that
more CPC practices established methods to systematically identify patients for behavioral health
support and increasingly integrated behavioral health specialists into primary care workflows.
90

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
b. Detailed findings
b.1. Empanelment
In PY2016, practices continued to successfully empanel “active patients” to a provider or
care team, a required first step in risk-stratified care management. Milestone data submissions
showed a moderate increase in the percentage of CPC practices’ active patients who were
empanelled (from 91 percent in 2013 to 99 percent in 2016). Across CPC regions, CPC practices
empanelled 82 to 100 percent of patients in 2015 and 93 to 100 percent in 2016. In the 2016
practice survey, 97 percent of CPC practices and 89 percent of comparison practices reported
that they assigned patients to panels and routinely used panel assignments for scheduling
(Appendix D, Table D.8a.)
b.2. Risk-stratification
All practices risk-stratified their empanelled patients, and by PY2016, most CPC practices
seemed satisfied with their risk-stratification methodologies. Data from the deep-dive practices
indicate that these practices had stopped making major changes to their risk-stratification
methodologies, but a few continued to refine them. For example, some added a risk level to
identify patients near the end of life, or added flags to emphasize patients’ social and behavioral
health needs or their willingness to engage in care management.
Milestone as well as deep-dive data indicate that, similar to PY2015, all practices continued
to use a combination of two or more data sources to risk-stratify their patients (Table 5.4). Most
commonly, practices continued to combine clinical intuition based on a provider’s knowledge of
the patient with a clinical algorithm (either published or developed by the practice). About one-
quarter of practices included claims data in their risk-stratification methodology, and about one-
quarter used an EHR-generated risk score.
Milestone data indicate regional variation in the use of these sources; for example, only 26
percent of Oklahoma practices reported that they used clinical intuition, whereas 83 percent of
New Jersey practices reported doing so. Practices’ use of a practice-developed clinical algorithm
ranged from 39 percent in New Jersey to 72 percent in Colorado (Table 5.4).
Table 5.4. Types of data used by CPC practices to risk-stratify patients in
PY2016, CPC-wide and by region
Types of data used for risk-
stratification
CPC-
wide AR CO NJ NY OH/KY OK OR
Clinical intuition (Practice risk-
stratifies patients based on provider’s
knowledge of patient and global
assessment of that patient’s risk)
68% 79% 67% 83% 74% 75% 26% 72%
Clinical algorithm—practice
developed (Practice risk-stratifies
patients based on algorithm
constructed by the practice)
57% 63% 72% 39% 51% 56% 54% 63%
Clinical algorithm—based on
published algorithm (Practice risk-
stratifies patients based on a published
algorithm)
44% 47% 29% 61% 52% 20% 72% 32%
91

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.4. (continued)
Types of data used for risk-
stratification
CPC-
wide AR CO NJ NY OH/KY OK OR
Claims (Payer data generated risk
scores—for example, HCC scores)
27% 23% 22% 41% 29% 59% 10% 3%
Electronic health records (EHR
program identifies and generates risk
score using specified clinical variables)
22% 14% 17% 22% 6% 53% 13% 25%
Combination of two or more of the
above
100% 100% 100% 100% 100% 100% 100% 100%
Number of practices
446 57 69 54 65 75 61 65
Source: Mathematica analysis of PY2016 Q1 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 446 practices that submitted Milestone data for the first quarter of
2016. Practices could check all data types that apply.
In most deep-dive practices, clinicians were responsible for assigning patients’ risk
scores or approving risk scores assigned by care managers, nurses, or other practice staff.
In addition to using risk scores, clinicians and staff in deep-dive practices also identified patients
for care management based on recent ED visits or hospitalizations, or clinicians referred patients
they thought would benefit from such services. Practices also used claims data from quarterly
feedback reports and clinical quality measure reports (for example, from EHR data) to identify
patients to target for care management services.
Similar to PY2015, in PY2016, most deep-dive practices indicated that risk-
stratification improved the organization and delivery of care. Clinicians and staff continued
to report that risk-stratification increased their awareness of high-risk patients’ needs and helped
them better allocate staffing resources to different patient populations. For example, in a few
practices, patients with a single chronic condition (such as patients with diabetes who needed
basic monitoring and health education) received care management from a medical assistant. This
enabled the care manager to focus on higher-risk patients (such as patients with poorly controlled
diabetes and additional chronic conditions). Risk-stratification continued to help practices
identify and prioritize high-risk patients and schedule longer appointments for them as needed. In
contrast, respondents in one small deep-dive practice questioned the utility of risk-stratification;
they perceived that clinicians knew their patients well enough to determine whether they were
high-risk and they believed that the time they spent risk-stratifying patients would be better spent
delivering direct patient care.
b.3. Risk-stratified care management
In addition to assigning risk scores to empanelled patients, CMS required practices to
provide longitudinal and episodic care management services for patients at high or rapidly rising
risk whom practices believed were most likely to benefit from intensive support. For PY2016,
CMS also required practices to continue implementing and further refine one of the three
“advanced care management strategies.” These advanced strategies overlap somewhat with
general care management activities. Below, we discuss care management generally, and then
describe practices’ experiences with the advanced strategies.
92

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Overall, the Milestone data show that, by 2016, CPC practices provided care management to
20 percent of patients who were risk-stratified (Table 5.5), ranging from 9 to 33 percent across
regions. This was similar to percentages reported in 2015 (10 to 32 percent across regions).
Table 5.5. Average percentage of patients risk-stratified by and receiving
care management from CPC practices at end of PY2016, CPC-wide and by
region
CPC-
wide AR CO NJ NY OH/KY OK OR
Average percentage of empanelled
patients risk-stratified
95% 95% 99% 94% 89% 98% 99% 93%
Average percentage of risk-stratified
patients receiving care management
20% 25% 9% 33% 10% 25% 27% 17%
Number of practices
438 56 67 53 63 75 60 64
Source: Mathematica analysis of PY2016 Q4 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 438 practices that submitted Milestone data for this item for the
last quarter of 2016. The percentage of patients was calculated for each practice and then averaged overall
within each region. Estimates give each practice the same weight, regardless of practice size.
Although all CPC practices risk-stratified empanelled patients and many increasingly
used risk-stratification for care delivery, some still needed to integrate it into care delivery.
In the practice survey, the percentage of CPC practices that reported “standard methods or tools
to stratify patients by risk level were available, consistently used, and integrated into all aspects
of care delivery” increased dramatically from 5 percent in 2012 to 60 percent in 2014, rose to 75
percent in 2015, then changed little in 2016 (73 percent). Although not all CPC practices did this,
indicating room for improvement, the CPC practices reported stronger risk-stratified care
management than did comparison practices. For example, corresponding percentages for
comparison practices were much lower (34 percent in 2014 and 35 percent in 2016) (Appendix
D, Table D.8a). In the 2016 clinician survey, a higher percentage of physicians in CPC than
comparison practices agreed or strongly agreed that their practice has good systems in place to
identify patients at high risk for poor outcomes (88 versus 74 percent). In addition, a higher
percentage of physicians in CPC than comparison practices agreed or strongly agreed that their
practice intensifies services for patients at high risk for poor outcomes (87 versus 74 percent).
(See Appendix E, Tables E.10 and E.11.)
CPC practices also increasingly reported having access to registry or panel-level data to
assess and manage care for risk-stratified patient populations across a comprehensive set of
diseases and risk states. In the 2016 practice survey, 54 percent of CPC practices reported having
this capacity, up from 9 percent in 2012, 42 percent in 2014, and 44 percent in 2015. In 2016,
only 33 percent of comparison practices reported having this capacity (Appendix D, Table D.8a).
Care managers
The tasks performed by care managers continue to vary substantially across practices.
In many deep-dive practices, care managers were primarily responsible for telephonic or face-to-
face chronic condition (longitudinal) care management with high-risk patients and follow-up
phone calls after hospitalizations and ED visits (episodic care management). In most practices,
care managers call high-risk patients between visits monthly, quarterly, or as needed. In several
93

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
of these practices, care managers also meet face-to-face with high-risk patients during practice
visits. In a few deep-dive practices, clinicians or care managers visit high-risk patients in their
homes, in addition to having telephonic contacts and face-to-face meetings in the practice. In
some practices, care managers were also responsible for pre-visit planning for high-risk patients,
pre-visit telephone calls to high-risk patients, and helping patients navigate the health system as
well as obtain social services. Some deep-dive practices’ care managers, on the other hand, had
more limited responsibilities; for example, they focused narrowly on providing education to
high-risk patients with diabetes.
CPC practices greatly increased their use of dedicated care managers who were members
of the primary care practice team over time. The number of practice survey respondents from
CPC practices who reported that “care managers who were members of the practice team
systematically provided care management services to high-risk patients” increased from 20 percent
in 2012 to 88 percent in 2014 and 2015, and 89 percent in 2016. In comparison, fewer than half of
comparison practices reported in 2016 that care managers who were practice care team members
systematically provided these services to high-risk patients (Appendix D, Table D.8a).
Similar to previous years, respondents across deep-dive practices continued to perceive
that the biggest benefit of CPC participation was their increased capacity to provide care
management services to high-risk patients. Clinicians, care managers, and other practice staff
continued to acknowledge the value of the care manager’s role in working with high-risk
patients, including providing patients with ongoing support to manage their conditions,
connecting patients with community-based resources, and planning for patients’ visits.
Patients generally had positive impressions of their care managers. During semi-
structured interviews with a sample of high-risk patients and caregivers from deep-dive
practices, patients who reported having regular contact with their care manager or who were
open to working with their care manager felt that the care manager was an asset to their team.
Patients particularly valued care managers who listened to them and explained things in lay
terms, helped to manage medications and chronic conditions, followed up after a hospitalization,
and helped to navigate the health care delivery system and community resources.
Challenges with care management staff feeling overwhelmed with numerous
responsibilities and large caseloads persisted in several deep-dive practices. As in 2014 and
2015, respondents in several practices described the numerous tasks care managers were performing
in addition to the care management activities for the higher-risk patients defined under Milestone 2.
These tasks included providing services to low- and medium-
risk patients, such as managing population health by
identifying patients with gaps in care; notifying patients of the
gaps, and arranging for them to receive the necessary care
such as a mammogram or lab test; and coordinating care,
which involves tracking patient discharges from the ED and
hospital, following up with patients upon discharge, and
generally helping patients navigate the health care system.
Although these additional tasks are necessary for achieving
comprehensive primary care, respondents noted that care
managers were already facing challenges trying to meet the
“I think (care managers) are a
little bit overwhelmed (because
care management is new to
our practice), I think
everybody’s got a wish list of
what an RN can do. And so I
think that’s been an issue as
far as what can they do and
how many people can they do
it for.”
–Lead clinician
94

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
complex medical and social needs of high-risk patients. Some practices reprioritized tasks or
narrowed the focus of care managers’ work by 2016, but in those that did not or that had large
numbers of patients for care management, care managers felt overburdened.
Respondents in a few deep-dive practices identified challenges with turnover among
care managers. Respondents from both independent and system-owned practices described
turnover that occurred because care managers felt overwhelmed with numerous responsibilities
(as discussed above). In addition, respondents noted that some care managers, particularly those
who had previously worked as inpatient nurses, were frustrated that their patient interactions
occurred predominantly over the telephone rather than in person. Respondents in a few rural
practices added that their rural location contributed to ongoing challenges with hiring qualified
care managers.
As in 2015, deep-dive practice respondents described approaches to improving support
for care managers, to clarify their roles and enhance staffing resources to help them feel
less overwhelmed. In some practices affiliated with health systems, respondents described
providing opportunities for care managers embedded in practices across the health system to
meet regularly, share best practices, and offer one another support. A few practices were
monitoring care managers’ caseloads to determine whether they needed more staff to support
high-risk patients, or to reduce (or even eliminate) activities focused on lower-risk patients.
These practices brought in social workers to help meet patients’ social needs and medical
assistants to assume logistical or administrative tasks. In a few practices, the care manager role
was new and clinicians and staff were uncertain about the care manager’s responsibilities; these
practices focused on educating clinicians and staff on care management to ensure care managers
would not be overwhelmed with requests to provide services to patients for whom care
management services are not appropriate.
In 2015 and 2016, deep-dive practices reported that regular communication, delegating
care management tasks to non-clinicians, and positive interactions among care team
members facilitated implementation of care management and advanced care management
strategies. In several practices, respondents described how clinicians developed trust in care
managers to handle follow-up with patients after observing how care managers (1) improved
patients’ adherence to recommended treatments, (2) reduced the need for clinicians to handle this
task, and (3) allowed clinicians to focus on more complex clinical care. In some cases, clinicians
and care managers noted the importance of clinicians introducing patients to the care manager;
this “warm hand-off” positively affected patients’ willingness to work with the care manager.
Then, as care managers spent time discussing the patients’ health and addressing their needs,
patients developed trust in the care manager.
Throughout CPC, respondents in a few deep-dive practices reported duplication of
services provided by care managers from practices and those affiliated with hospitals,
health systems, health plans, or visiting nurses associations. In some cases, care managers not
affiliated with deep-dive practices called patients or visited them at home after a hospital
discharge, which duplicated the practice-based care manager’s efforts. This deep-dive finding is
consistent with the 2016 practice survey finding that about two-thirds of both CPC (61 percent)
and comparison (66 percent) practice survey respondents reported that such duplication of
patient outreach sometimes or often confused their patients. Deep-dive respondents noted that
95

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
duplication of services could confuse patients about who their care manager was, disrupt the
patients’ relationship with the practice-based care manager, and frustrate care managers.
Similarly, in semi-structured interviews with a sample of high-risk patients and caregivers from
deep-dive practices, patients expressed confusion about their care manager’s identity,
particularly if they had just been discharged from the hospital.
Some practices found ways to address this issue. For example, in a health system that
employed navigators to work with high-risk patients, the deep-dive practices’ care managers
coordinated with the navigators to decrease duplicate calls to patients. In another deep-dive
practice, the practice-based care manager described benefits of working with a care manager
provided by a regional health plan. In this case, the health plan’s care manager was co-located in
the practice and worked with commercially insured high-risk patients, helping to reduce the
practice-based care manager’s caseload.
Care plans
While care plans were not a requirement of CPC, CMS did ask practices to report on their
use of them. In the 2016 clinician survey, a higher proportion of physicians in CPC practices
than those in comparison practices said that all or most of their high-risk patients receive copies
of care plans that include self-management and clinical management goals, and outline steps to
achieve those goals (51 percent of physicians in CPC practices versus 39 percent of physicians in
comparison practices). However, CPC and comparison practice patients had similar experiences
in this regard. In the 2016 patient survey, 47 percent of patients receiving care for a chronic
condition in CPC and 46 percent in comparison practices reported that they always received a
copy of their treatment plan (such as an after-visit summary). During semi-structured interviews
with high-risk patients or their caregivers, few had heard the terms “plan of care” or “care plan,”
and many did not understand this concept even after we described it. After probing by the
interviewers, about one-quarter of the patients described formal care plans and goals (including
steps for achieving them), which they had set with their physician and/or care manager.
Most deep-dive practices were not developing and using care plans that reflected
patients’ care goals, and care teams were not using the care plans to guide ongoing care
delivery. As of mid-2016, most practices had not established practice workflows that supported
developing care plans as defined under Milestone 2. For 2016, practices were required to develop
care plans for all patients receiving care management that documented the patients’ goals for
care and were accessible to care team members and the patient. However, in a few practices, care
managers were working with patients to identify their goals and develop a care plan for the
clinician and care team to use for care management. Respondents in these practices noted that
documenting patients’ health goals in a care plan helped members of the care team to reinforce
those goals beyond the clinician-patient visit. More commonly, care managers worked with
patients to identify their goals, informed by the clinician’s recommendations to the patient.
Despite this work to develop and use care plans, clinicians typically did not use the plans to
guide their care delivery on an ongoing basis. This suggests opportunities for more practices to
obtain patient input and to involve clinicians in care management over time.
96

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Deep-dive practice respondents described diverse ways of using—and interpreting the
purpose of—care plans, providing insight into the slow uptake of care plans. Several
clinicians we interviewed were unfamiliar with the term “care plan,” whereas other clinicians
described care plans in a manner similar to the 2016 Milestone 2 guidelines. Typically, clinicians
noted that they have always assessed patients’ needs and helped them plan their care, although
they may not formally document it in a “care plan.” Although clinicians believed patients’ needs
were extremely important, some viewed care plans as entirely separate documents that were
chiefly for the care manager’s and patient’s use. Relative to other practice members, care
managers were generally more informed about care plans; this finding resonated with our
PY2015 finding that care managers seemed to be the predominant users of care plans. However,
care managers in many practices viewed care plans as a condition-specific tool rather than a tool
for helping patients and care team members work together to manage all of a patient’s conditions
and needs. Further, a few system-level respondents described multiple guidelines and different
requirements for care plans from various payers and medical home initiatives.
Clinicians and care managers in most deep-dive practices continued to report that
their EHR had limited functionality for supporting care management activities, including
creating, updating, and accessing care plans. A few practices with internal IT support created
a care plan template in their EHRs but still faced challenges developing and modifying the care
plans. For example, clinicians and care managers in some of these practices could not update
care plans as patients’ needs change; rather, they had to create a new care plan to make changes.
Several other practices used EHR work-arounds to develop care plans. For example, in one
practice, the care manager used the EHR “phone encounter” module to develop care plans,
because the EHR did not have a dedicated location for the care plan. A few practices purchased
care management software to support care plan development in their EHRs, but then faced
challenges integrating the software into the EHR. Because of this lack of integration, clinicians
could not access the care plans and the care manager had to double-enter certain elements of the
care plan in another section of the EHR so care team members could access the information.
Furthermore, clinicians in several practices perceived the care plans to have limited utility for
managing their patients’ clinical needs; they found the encounter notes, lab results, and other
data in the EHR to be more clinically relevant than care plans, which often focused on patients’
educational needs.
A few deep-dive practices gave high-risk patients a copy of their care plan or made it
accessible on the patient portal. Rather than a comprehensive care plan, several other practices
gave patients a paper “visit summary,” typically with instructions from the clinician, and a few
posted the visit summary on the patient portal. A few clinicians said that giving patients a copy
of their care plan was more than some patients wanted and that patients preferred verbal
instructions and more limited written instructions.
CPC practices increased their use of community resources to meet patients’ needs, and
reported a higher level of use of community resources than comparison practices. In the
2016 clinician survey, a higher proportion of CPC than comparison physicians agreed or strongly
agreed that their practice effectively utilizes community resources to help meet the health care
needs of their patients (84 versus 79 percent). This reflects a large increase from 2013, when 67
percent of CPC physicians agreed or strongly agreed that their practice effectively uses
community resources to help meet patients’ needs.
97

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
b.4. Advanced primary care strategies
Beginning in PY2014, CMS required CPC practices to select one of three CPC advanced
primary care strategies for patients in higher-risk cohorts: (1) patient self-management support,
(2) behavioral health integration, or (3) comprehensive medication management. In the 2015
Milestone data, 50 percent of CPC practices selected self-management support only, 26 percent
reported behavioral health integration only, 9 percent reported medication management only, and
15 percent reported more than one strategy. In 2016, CPC did not require practices to report
which advanced primary care strategy they were working on; rather, practices were asked about
their use of self-management support, behavioral health integration, and medication
management. However, interviews with deep-dive practices in 2016 indicated that practices
generally continued to pursue the same advanced primary care strategies they had reported in
2015. In deep-dive practices implementing self-management support and medication
management, respondents did not report major changes in how they were incorporating these
strategies into practice workflows or in the challenges they were facing or had overcome.
However, respondents did describe changes related to implementing behavioral health
integration.
Patient self-management support
Patient self-management support involves a collaborative relationship between a member of the
practice and the patient and his or her family, to help the patient develop specific skills for
managing a target condition or disease, and for activating and increasing the patient’s self-efficacy
managing health across conditions. This type of support overlaps with the work all practices do for
care management in Milestone 2, described above in the section on care management.
In 2016, across CPC regions, the most common target conditions that CPC practices focused
on for self-management support were diabetes (93 percent), hypertension (50 percent), chronic
obstructive pulmonary disease (COPD) (39 percent), and congestive heart failure (CHF)
(38 percent) (Table 5.6).
By 2016, only one-fifth of CPC practices were reporting performance in the top tier (scores
of 10–12) for patient and caregiver engagement as measured by the M-PCMH-A. Average
domain scores increased from 6.7 points in 2012 to 8.8 points out of 12 in 2016. The measures
within this domain most relevant to this Milestone are “assessing patient and family values and
preferences and incorporating them in planning and organizing care,” “evaluating patient
comprehension of verbal and written materials, using translational services or multilingual staff,
and training staff in health literacy and communication techniques,” and “self-management
support provided by practice staff trained in patient empowerment and problem-solving
methodologies.” The percentage of practices reporting implementing this level of care for the
items in this domain rose from 10 to 15
percent in 2012 to 28 to 36 percent in 2016. (See
Appendix D, Table D.8a.) The relatively low scores and slow improvement in this domain may
reflect that while some payers worked with learning faculty and practices on improving health
literacy, the CPC Implementation and Monitoring Guide did not emphasize training for health
literacy, translational services, and multilingual staff.
98

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.6. Conditions that CPC practices focused on for self-management
support, CPC-wide and by region
Condition
CPC-
wide AR CO NJ NY OH/KY OK OR
Diabetes
93% 98% 87% 98% 100% 96% 71% 97%
Hypertension 50% 58% 30% 79% 75% 47% 22% 46%
Chronic obstructive pulmonary
disease (COPD)
39% 46% 32% 7% 23% 43% 92% 20%
Congestive heart failure (CHF) 38% 34% 27% 7% 28% 45% 82% 40%
Tobacco cessation 25% 22% 29% 33% 35% 25% 2% 29%
Hyperlipidemia/high cholesterol 21% 30% 29% 33% 30% 13% 2% 6%
Obesity and weight loss 19% 22% 25% 31% 39% 0% 2% 20%
Diabetes with hypertension 19% 16% 29% 21% 5% 23% 4% 37%
Diabetes with hyperlipidemia 18% 16% 25% 21% 5% 25% 2% 31%
Depression 14% 4% 30% 0% 5% 7% 29% 23%
Asthma 9% 6% 13% 5% 7% 4% 8% 23%
Number of practices
371 50 63 42 57 75 49 35
Source: Mathematica analysis of PY2016 Q3 Milestone submission results provided by CMS.
Note: Table shows percentages of CPC practices that focused on the particular condition. Percentages for all
regions are based on 371 practices that submitted Milestone data for this item for the third quarter of 2016.
Some practices opted out of responding to this item by selecting the response option “We do not focus on
high-risk conditions for self-management support at our practice.” Practices could select up to five focus
conditions. They could respond to this item even if they did not select self-management support as their
chosen CPC advanced primary care strategy.
While respondents did not report major changes in how they were facilitating self-
management support, some deep-dive practices described their approach as evolving from
distributing educational materials to patients to also using motivational interviewing to help
patients set and track health goals.
Behavioral health integration
Behavioral health integration involves CPC practices offering or coordinating with
behavioral health providers to support patients with behavioral health needs, dementia, and
poorly controlled physical chronic conditions. In practices’ 2015 Milestone data reports to CMS,
the last year for which we can calculate the percentage of practices that chose each advanced
primary care strategy, 26 percent of CPC practices reported they were working to implement
behavioral health integration.
55
Based on the practice survey, which asked all practices about
behavioral health integration regardless of whether they selected it as an advanced primary care
strategy, the percentage of CPC practices reporting that they had a behavioral health specialist,
clinical psychologist, or social worker on site (part-time or full-time) increased from 19 percent
in 2014 to 29 percent in 2016. CPC practices were more than twice as likely as comparison
practices to report that they employed one or more behavioral health specialists in 2016 (29
versus 12 percent). Milestone data indicate that the percentage of CPC practices with a
55
This is a conservative estimate. Another 15 percent of practices reported pursuing two or more of the advanced
care strategies, but the reporting did not specify which of the strategies they pursued.
99

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
behavioral health specialist co-located within the practice varied across regions in 2016 from 3 to
77 percent (Table 5.7). Among CPC practices with a co-located behavioral health specialist,
54 percent indicated that the specialist was fully integrated into the primary care workflow,
shared patient records, and was available for warm hand-offs and acute visits (Table 5.8).
Table 5.7. Percentage of CPC practices with behavioral health specialist(s)
co-located within the practice in PY2016, CPC-wide and by region
CPC-
wide AR CO NJ NY OH/KY OK OR
Practice has behavioral
health specialists co-located
within CPC practice
30% 29% 52% 17% 6% 3% 29% 77%
Number of practices
435 55 67 53 63 75 58 64
Source: Mathematica analysis of PY2016 Q3 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 435 practices that submitted Milestone data for this item for the
third quarter of 2016.
Table 5.8. Integration of behavioral health specialists into primary care in
PY2016 (among practices with co-located behavioral health specialists),
CPC-wide and by region
CPC-
wide AR CO NJ NY OH/KY OK OR
5 Fully integrated workflow and
shared patient records;
functionally integrated with
availability for warm hand-offs
and for acute visits in primary
care
54% 38% 54% 33% 75% 0% 71% 57%
4 27% 19% 31% 44% 25% 0% 6% 31%
3 Separate workflow and shared
patient records
9% 19% 3% 11% 0% 50% 6% 10%
2 3% 0% 11% 0% 0% 0% 0% 0%
1 Functionally separate with totally
separate workflow and separate
patient records
8% 25% 0% 11% 0% 50% 18% 2%
Number of practices
132 16 35 9 4 2 17 49
Source: Mathematica analysis of PY2016 Q3 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 132 practices that submitted these Milestone data for the third
quarter of 2016 and reported having one or more behavioral health specialists working full or part time at
the practice.
CMS does not specify labels for responses 2 and 4.
Practices used a range of approaches to identify patients needing behavioral health care
services. Across all regions in 2016, the most common methods CPC practices used were a
screening tool, such as for depression, dementia, or domestic violence (90 percent); referral by
staff or provider (71 percent); and self-referral by patient (55 percent) (Table 5.9).
100

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.9. CPC practices’ methods of identifying patients for behavioral
health services, in PY2016, CPC-wide and by region
Method
CPC-
wide AR CO NJ NY OH/KY OK OR
Screening tools, such as for
depression, dementia, or
domestic violence
90% 81% 96% 89% 87% 93% 86% 98%
Referral by staff or provider 71% 72% 88% 70% 68% 24% 88% 97%
Self-referral by patient
55% 65% 58% 58% 57% 20% 61% 70%
Clinical indicators, such as
patient not reaching goals
40% 47% 34% 51% 32% 16% 31% 73%
Health risk assessment 36% 23% 39% 43% 51% 9% 37% 53%
We do not systematically
identify patients for behavioral
health services
6% 12% 1% 9% 8% 3% 7% 0%
Other 4% 5% 4% 0% 2% 1% 2% 11%
Number of practices
437 57 67 53 63 74 59 64
Source: Mathematica analysis of PY2016 Q3 Milestone submission results provided by CMS.
Note: Table shows percentages of CPC practices that indicated they used each method. Percentages for all
regions are based on 437 practices that submitted Milestone data for this item for the third quarter of 2016.
Practices could select all activities that applied.
The most common approaches CPC practices used to deliver behavioral health care included
providing a referral for specialty mental health care (83 percent), providing primary care
management with referral as needed to specialty mental health care (72 percent), and co-
management between primary care and behavioral health specialists (50 percent) (Table 5.10).
Table 5.10. CPC practices’ approaches for providing behavioral health care,
in PY2016, CPC-wide and by region
Approach
CPC-
wide AR CO NJ NY OH/KY OK OR
Referral for specialty mental
health care
83% 73% 97% 85% 90% 73% 85% 75%
Primary care management with
referral as needed to specialty
mental health care
72% 71% 92% 75% 63% 48% 85% 73%
Co-management between
primary care and behavioral
health specialists
50% 57% 62% 42% 37% 23% 56% 78%
Primary care management with
behavioral health specialist
consultation and case review
33% 29% 58% 23% 5% 24% 37% 55%
Behavioral health specialists
integrated into primary care
workflow
29% 16% 64% 15% 5% 3% 29% 73%
None 7% 5% 2% 6% 2% 27% 5% 0%
Other
3% 4% 6% 0% 0% 1% 2% 11%
Number of practices
435 56 66 53 62 75 59 64
Source: Mathematica analysis of PY2016 Q3 Milestone submission results provided by CMS.
Note: Table shows percentages of CPC practices that used the approach to provide behavioral health care.
Percentages for all regions are based on 435 practices that submitted Milestone data for this item for the
third quarter of 2016. Practices could select all activities that applied.
101

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Respondents in deep-dive practices noted that behavioral health integration has
increased practices’ capacity to care for patients with co-occurring behavioral and physical
health conditions. Respondents described benefits of patients having direct access to a
behaviorist (that is, a psychologist, psychiatrist, or clinical social worker), noting that this made
clinicians feel better supported in managing patients who needed additional behavioral services.
Some practices were expanding patient referrals to behaviorists to help address mental health
needs. In a few other practices, respondents noted that depression is under-recognized in the
primary care setting and that they had expanded depression screening from patients with a
history of depression to all patients.
Over the course of CPC, respondents in the deep-dive practices implementing
behavioral health integration formalized behavioral health
services and worked to better integrate behaviorists with
care teams. These practices formalized relationships with
behavioral health providers, including co-locating behaviorists
part time in the practice, where they participated in care team
meetings. Several respondents described the benefit of
clinicians introducing patients to behaviorists, noting that this
increased patients’ receptivity to behaviorists. Other practices
formalized care compacts, outlining expectations for referrals
and communication between primary care clinicians and
behaviorists outside the practice, particularly when they did not use the same EHR.
Practices reported two barriers to behavioral health integration: (1) an inadequate supply of
behavioral health providers and (2) unresponsive behavioral specialists in a few regions. Even
among a few practices pursuing behavioral health integration as an advanced primary care
strategy, clinicians and staff noted that increased screening and assessment of patients was
insufficient if the necessary mental health services were unavailable.
Medication management
Comprehensive medication management involves CPC practices performing medication
reconciliation and integrating into the practice care team a clinical pharmacist who can manage
patients’ medications to maximize efficiency, effectiveness, and safety. In 2015, just 9 percent of
practices reported that they were working to implement comprehensive medication management
only (the 2016 Milestone data did not include this item). This low percentage may be a function
of the Milestone requirement that “medication management is built around the skills of a clinical
pharmacist as a member of the care team.” In the 2016 practice survey data, only 18 percent of
CPC practices reported having a pharmacist or pharmacy technician at the practice site. Deep-
dive interviews also indicated that few practices had access to a clinical pharmacist as a member
of the care team. The two deep-dive practices implementing medication management contracted
with or hired part-time pharmacists, who met with high-risk patients in person and spoke with
them over the telephone to provide medication-management services.
Reflecting their work on more “routine” medication management and reconciliation that
does not require a clinical pharmacist, the percentage of CPC practices that reported on the
practice survey that they regularly perform medication reconciliation for all patients and
document it in the patient’s medical record increased from 75 percent in 2012 to 94 percent in
“Both the care manager and
the (behaviorist) work
together with that patient and
share information on that
patient, but each has
separate job descriptions on
what they’re going to manage
for that patient.”
–Practice manager
102

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
2016. The most common medication management services CPC practices provided in 2016
included routine medication reconciliation (92 percent), coordination and reconciliation of
medications at the time of transitions of care (85 percent), medication monitoring (55 percent),
support for medication use and self-management (52 percent), and comprehensive medication
review and assessment of medication safety and cost-effectiveness (47 percent) (Table 5.11).
Table 5.11. CPC practices’ approaches for providing medication
management, in PY2016, CPC-wide and by region
Approach
CPC-
wide AR CO NJ NY OH/KY OK OR
Routine medication
reconciliation
92% 91% 90% 96% 94% 100% 78% 92%
Coordination and reconciliation
of medications at the time of
transitions of care
85% 89% 82% 87% 92% 99% 73% 72%
Medication monitoring 55% 66% 72% 60% 41% 53% 23% 69%
Support for medication use and
self-management
52% 59% 54% 44% 43% 56% 42% 64%
Comprehensive medication
review and assessment of
medication safety and cost-
effectiveness
47% 38% 42% 56% 37% 72% 40% 38%
Development of a medication
action plan or contribution to a
global care plan
21% 16% 22% 23% 13% 25% 7% 38%
We do not provide medication
management services at our
practice
7% 7% 3% 4% 6% 0% 22% 6%
Number of practices
437 56 67 52 63 75 60 64
Source: Mathematica analysis of PY2016 Q3 Milestone submission results provided by CMS.
Note: Table shows the percentages of CPC practices that used each approach. Percentages for all regions are
based on 437 practices that submitted Milestone data for this item for the third quarter of 2016. Practices
could select all that applied.
CPC practices most commonly identified patients for medication management services
(beyond routine medication reconciliation) by identifying those who were undergoing care
transitions, on high-risk medications, or directly referred by a provider. Almost one-third of
practices reported that they do not routinely select patients for medication management services,
and one-quarter of practices reported that they identify patients for medication management
services based on poly-pharmacy—the use of multiple medications at the same time (Table
5.12).
103

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.12. CPC practices method(s) of identifying patients for medication
management, in PY2016, CPC-wide and by region
Method
CPC-
wide AR CO NJ NY OH/KY OK OR
Patients with care transition(s)
51% 52% 40% 47% 43% 67% 42% 66%
High-risk medications 44% 54% 54% 40% 44% 44% 12% 58%
Direct provider referrals
41% 43% 37% 21% 59% 24% 37% 67%
Patients who have not achieved
a therapeutic goal for a chronic
condition
33% 34% 46% 28% 22% 24% 20% 55%
Poly-pharmacy
25% 30% 28% 26% 19% 24% 12% 36%
Based on risk cohorts using
practice risk-stratification
24% 27% 34% 26% 32% 0% 13% 36%
We do not routinely select
patients for medication
management services
31% 34% 24% 40% 19% 32% 53% 16%
Number of practices
438 56 67 53 63 75 60 64
Source: Mathematica analysis of PY2016 Q3 Milestone submission results provided by CMS.
Note: Table shows the percentages of CPC practices that used each method to identify patients for medication
management beyond routine medication reconciliation. Percentages for all regions are based on 438
practices that submitted Milestone data for this item for the third quarter of 2016. Practices could select all
that applied.
Pharmacists initially faced resistance from clinicians and other staff to integrating
their roles into practice workflows. In both deep-dive practices that implemented medication
management, the pharmacists had to initiate their roles in the practices, which was challenging.
Like care managers, pharmacists also described gradually building relationships with clinicians
and difficulties obtaining access to clinicians to discuss patients because of clinicians’ competing
responsibilities. Practices that successfully integrated pharmacists into practice workflows
reported they had structured processes and leadership support for doing so.
Finally, in many deep-dive practices, practice members believed the advanced primary
care strategy they chose was improving patient care. Self-management support increased
practices’ focus on using motivational interviewing to help patients set meaningful goals, and
practice members perceived that self-management support increased patients’ engagement in
their care. Practices reported that behavioral health integration increased practice members’ (1)
awareness of patients’ emotional and psychosocial needs, and (2) capacity to engage patients in
necessary behavioral health care. For medication management, practice members believed this
strategy increased patients’ compliance with their medications.
5.4.2. Milestone 3: Access and continuity
In PY2016, Milestone 3 required that practices: (1) attest that patients have access 24 hours
a day, seven days a week, to a care team practitioner with real-time access to the EHR; (2)
continue at least one form of asynchronous communication (such as email and patient portals)
104

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
and make a commitment of timely response; and (3) measure continuity of care by reporting visit
continuity quarterly for each provider or care team in the practice.
56
a. Overview of findings
The second largest area of change over the course of the CPC initiative, as measured by the
M-PCMH-A, was access. Milestone data indicate that practices most often used patient portals to
enhance access. They also increased same or next day appointments and 24/7 access to a
clinician with access to the EHR. Practices focused less on other electronic avenues for enhanced
access such as web-enabled visits, likely because Stage 2 Medicare and Medicaid EHR Incentive
Programs (Meaningful Use) emphasized patient portals. Deep-dive data from previous years
suggested that actual use of portals by patients was low and patient survey data in 2016
confirmed this impression. Deep-dive and 2016 practice survey data indicate that practices
continued to improve wait times for patients to get an appointment; telephone access to the
practice for patients; and after-hours access to clinicians via email, telephone, or in-person visits.
Nonetheless, patient survey data from Medicare FFS beneficiaries suggest that more work on
access—or better communication about it—may be needed. Finally, practice survey data showed
an increase in the percentage of practices reporting that they assign patients to a specific panel or
provider, and deep-dive practices reported that they continued to encourage patients to schedule
with their usual clinician throughout CPC.
b. Detailed findings
b.1. Access to clinicians
Throughout the initiative, practices reported that they worked on increasing access to
same-day appointments, improving on-call coverage with practitioners that have 24/7
access to an EHR, and increasing visits outside of the office (that is, home, telephone, or
video visits). In their Milestone reporting, all practices reported providing patients with same or
next day appointments and availability of on-call clinicians with access to their EHR 24 hours a
day, seven days a week, as required by CPC. Some CPC practices also reported enhanced access
to office visits—68 percent provided extended hours on weekends, evenings, or early mornings;
61 percent provided a flexible appointment scheduling system; and 38 percent provided after-
hours coverage via a formal arrangement or care compact with urgent care centers or other
providers (Table 5.13). Many deep-dive practices improved telephone access by increasing the
number of phone lines and staff who respond to calls; other practices were planning to hire
additional providers to facilitate expanded office hours and same-day appointments. For
example, at the time of the 2016 interviews, one practice was recruiting for a nurse practitioner
position to reduce double-booking of current providers, and another practice was recruiting for a
provider to add appointment slots and create a 12-hour office day. Large systems often provided
in-person after-hours access to clinicians via an urgent care clinic owned by the system, which
typically existed before CPC. Another practice repurposed its general walk-in hours—instead of
seeing any available provider, patients now have same-day access to their specific care team.
56
The requirement for reporting progress for Milestone 3 changed from PY2015 to PY2016. In PY2015, practices
had to report whether they determined continuity at the provider or care team level and whether their EHR was
capable of calculating and tracking continuity. In PY2016, they had to measure and report visit continuity quarterly
for each provider and/or care team in the practice.
105

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Nonetheless, patient survey data suggest that more work on access—or better
communication about it—may be needed. In 2016, about 65 percent of CPC and comparison
Medicare FFS beneficiaries reported that they were always able to get an appointment as soon as
needed when phoning their provider for care needed right away. The percentage of beneficiaries
reporting this was constant over time. In 2016, only one-third of CPC and comparison practice
beneficiaries who reported needing care during evenings, weekends, or holidays, reported that
they were always able to get that care from the provider’s office. For the CPC and comparison
practices, the differences in practices’ descriptions of the availability of enhanced access
capabilities is notable, but survey findings on Medicare beneficiaries’ experiences with access
suggest that further work is needed in CPC practices to improve access or at least to
communicate about improved access.
Use of other types of visits to expand access was less common. Sixty percent of CPC
practices reported that they provided billable types of alternative visits (Table 5.13), most
commonly group education classes (27 percent of practices), home visits (25 percent of
practices), and medical nutrition consultation visits (20 percent of practices).
Table 5.13. Percentage of CPC practices reporting each type of enhanced-
access activity, in 2016, CPC-wide and by region
Selected enhanced-access
activities
CPC-
wide AR CO NJ NY OH/KY OK OR
On-call clinician has 24/7 access to
EHR
a
100% 100% 100% 100% 100% 100% 100% 100%
Number of practices 445 57 69 54 64 75 61 65
Practice provides enhanced office
access through:
b
Availability of same or next day
appointments 100% 100% 100% 98% 98% 100% 100% 100%
Extended hours on weekend,
evening, or early morning
68% 47% 80% 83% 79% 85% 28% 64%
Flexible appointment scheduling
system
61% 58% 71% 66% 40% 73% 72% 44%
After-hours coverage via a formal
arrangement or care compact with
urgent care centers or other
providers
38% 25% 41% 47% 40% 31% 35% 45%
Other
18% 21% 12% 9% 5% 29% 27% 19%
We do not provide enhanced office
access
0% 0% 0% 0% 0% 0% 0% 0%
Practice provides enhanced access
outside of office visits through:
b
Patients send and receive
messages through a patient portal
(as defined by Meaningful Use)
98% 100% 100% 96% 98% 99% 97% 97%
Secure email
29% 15% 39% 23% 19% 47% 40% 11%
Other
13% 17% 3% 11% 2% 37% 15% 5%
Text messaging
8% 11% 8% 19% 6% 3% 7% 3%
Web-enabled visits other than
through a patient portal
8% 2% 3% 9% 5% 20% 8% 3%
Telemedicine/remote monitoring
5% 13% 8% 2% 6% 4% 0% 2%
None of the above
1% 0% 0% 2% 2% 0% 0% 3%
106

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.13 (continued)
Selected enhanced-access
activities
CPC-
wide AR CO NJ NY OH/KY OK OR
Practice provides the following
(billable) types of alternative visits:
b
Practice does not provide these
types of alternative visits 40% 45% 15% 32% 31% 39% 77% 47%
Group education classes
27% 15% 55% 17% 19% 44% 12% 20%
Home visits
25% 26% 36% 36% 50% 7% 5% 22%
Medical nutrition consultation visits
20% 19% 36% 11% 26% 19% 7% 17%
Preventive counseling services
14% 17% 20% 21% 11% 12% 0% 20%
Group visits
10% 2% 27% 6% 6% 5% 0% 17%
Other
8% 13% 9% 8% 8% 5% 7% 9%
Number of practices
433 53 66 53 62 75 60 64
Source: Mathematica analysis of PY2016 Q1 and Q4 Milestone submission results provided by CMS.
a
Percentages for all regions are based on practices that submitted Milestone data for the first quarter of 2016. The
number of practices is reflected in the second row of this table.
b
Percentages for all regions are based on practices that submitted Milestone data for the last quarter of 2016. The
number of practices is reflected in the last row of this table. Practices could select all activities that applied.
b.2. Portals
Patient portals where patients can send and receive messages were the most common
outside-of-office enhanced-access activity, with 98 percent of CPC practices providing them
(Table 5.13). On average, less than one-third of practices pursued each of the other electronic
methods to enhance access (Table 5.13), with higher percentages of practices in Colorado,
Oklahoma, and Ohio/Kentucky regions reporting use of secure email (39, 40, and 47 percent,
respectively).
The practice survey also showed large increases between 2012 and 2016 in the percentage of
CPC practices reporting availability of patient communication with the practice team through
email, text messaging, or a patient portal. The proportion of practices reporting the availability of
this type of patient communication increased from 7 percent in 2012 to 63 percent in 2014, 78
percent in 2015, and 82 percent in 2016. In 2016, CPC practices were more likely to report this
type of access than comparison practices (82 versus 67 percent) (see Appendix D, Table D.8a).
Despite the increasing availability of these communication capabilities, in 2016, only a small
percentage of Medicare beneficiaries in both CPC and comparison practices reported using email
to ask the provider medical questions (8 percent, up from 3 percent in 2013). About half of
Medicare beneficiaries that responded to the patient survey reported that their provider’s office
uses a web portal or website that allows them to email the practice, review medical information,
request a prescription renewal, or make appointments. Among the beneficiaries that reported
their practice uses a patient portal, about half reported using the patient portal at least once in the
past 12 months (48 percent of CPC patients and 52 percent of comparison patients).
107

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Although practices worked to have patients register for their portal, and use of portals
improved during CPC, use remained relatively low. Between 2012 and 2016, deep-dive
practices focused on enrolling patients into practice portals—where patients can review test
results, send messages to their providers, request medication refills, and schedule
appointments—because this strategy aligns with Meaningful Use requirements. In the first few
years of CPC, deep-dive practice staff cited older patients’ lack of comfort with technology,
technical glitches, and a lack of resources as challenges in getting patients to enroll in and use
portals. For example, many practices reported that their elderly patients often did not have access
to computers and were less interested in using new technology. Glitches in portal software also
presented a challenge for practice staff and patients, particularly in the early years of CPC. For
example, many practices reported difficulty logging in, poor compatibility between the portal
and the practice’s EHR or with specific Internet browsers, as well as problems exchanging
messages between providers and patients. Practices tried to resolve these issues by (1)
encouraging elderly patients’ families and caregivers to assist with enrolling in and using the
portal; (2) working with the portal vendor to troubleshoot technical glitches; and (3) using
volunteer interns or other clerical staff to explain the portal to patients, enroll patients, and
manage messages from the portal.
In 2016, deep-dive practice staff cited challenges with managing portal use. Several
respondents perceived that patients who did use the portal sent too many messages through it or
expected immediate responses to their messages. For example, one care manager reported that
patients were sending portal messages about urgent issues instead of calling the practice, and by
the time the provider was able to review the message, no same-day appointments were available.
Despite these challenges, practice leadership and staff saw value in implementing
patient portals. Deep-dive clinicians and staff noted that portals improved patient care by
reducing back-and-forth phone calls, providing access to after-hours care through e-visits, and
increasing patients’ access to information from their medical record. Practice clinicians and staff
felt that the portal allows patients to play a more active role in their health, empowering them to
communicate with practice staff about their conditions via secure messaging, view test results
promptly, and prepare for office visits by reviewing lab results within their record. Using the
portal reportedly saved staff time and improved workflow by enabling the practice to send
reminders about screenings to patients and minimizing the time spent “playing phone tag” to
address patients’ needs.
b.3. Continuity
The practice survey also showed improvement in continuity of care as measured by the
percentage of practices reporting that “patients were assigned to specific provider panels which
are used for scheduling purposes and continuously monitored to balance supply and demand.”
The proportion of practices reporting that they used this approach to support continuity increased
from 42 percent in 2012 to 61 percent in 2014, 71 percent in 2015, and 73 percent in 2016.
Similarly, the percentage of practices reporting that patients usually see their own provider or
practice team rose from 65 percent in 2012, to 75 percent in 2014 and 2015, and 83 percent in
2016.
To improve continuity of care, deep-dive practices continued to emphasize scheduling
patients with their usual clinician and care team. A few deep-dive practices emphasized
108

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
educating patients about who was on their care teams, so patients would know that if they
interacted with a member of their physician’s team, that team member would keep their usual
physician informed of their care. Practices also encouraged patients to request their usual
clinician when scheduling visits.
5.4.3. Milestone 4: Patient experience
Similar to PY2015, Milestone 4 required in PY2016 that practices do at least one of the
following: (1) conduct a monthly practice-based patient survey, (2) convene a PFAC quarterly,
or (3) conduct regular surveys and convene a PFAC periodically. Practices were also required to
specify changes to the practice that were due to, or influenced by, the practice survey or PFAC
activities, and to continue communicating to patients the changes the practice is implementing
due to the survey or PFAC.
a. Overview of findings
In the final year of the initiative, responses to the practice survey show that a majority of
practices continued to value the patient feedback provided by surveys or PFACs. In 2015 and
2016, 63 percent of CPC practices considered feedback from these sources “very important” to
improving the care they provided to patients, an increase from 54 percent in 2014. (This survey
item was not asked in 2012.) Milestone data indicated that 80 percent of CPC practices used
patient surveys and 48 percent convened a PFAC (28 percent of practices chose to use surveys
and convene PFACs) in 2016. Although the use of surveys was more common, the use of PFACs
increased more over the initiative. The percentage of practices that reported use of PFACs
increased from 20 percent in 2013 to 48 percent in 2016, with many practices that originally
chose to use surveys deciding to also convene a PFAC. Both methods of gathering patient
feedback reportedly yielded useful information to guide practice improvements. However, some
deep-dive practices noted challenges with conducting patient surveys and several respondents
expressed that implementing surveys was burdensome in a busy practice. In addition, some
practices had concerns that the same patients were surveyed multiple times, which might lead to
less useful information for guiding practice improvement. Although practices typically found it
challenging to maintain PFAC participation, they considered feedback from PFAC meetings
more useful than survey data because the councils provided opportunities for meaningful
conversations between patients and practice members about the patients’ experience of care,
which generated information that could improve practice operations.
b. Detailed findings
In 2016, about half of CPC practices relied solely on patient surveys to gather input on
patient experiences, roughly 30 percent used both surveys and a PFAC, and 20 percent used only
a PFAC (Table 5.14). The proportion of practices that reported using a PFAC, either alone or
with a patient survey, to elicit patient experiences increased from 20 percent in 2013 to 42
percent in 2014, 47 percent in 2015, and 48 percent in 2016.
109

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.14. Percentage of CPC practices choosing various options to elicit
patient experiences, CPC-wide and by region
Activities to elicit patient experiences
CPC-
wide AR CO NJ NY OH/KY OK OR
Practice-based survey only
52% 63% 15% 75% 41% 79% 62% 33%
Both survey and PFAC 28% 14% 52% 15% 41% 14% 28% 27%
PFAC only
20% 23% 33% 9% 17% 7% 10% 41%
Number of practices
437 57 67 53 63 73 60 64
Source: Mathematica analysis of PY2016 Q1 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 437 practices that submitted Milestone data for this item for the
first quarter of 2016.
PFAC = Patient and Family Advisory Council.
Milestone data for 2016 indicate that practices most often used feedback from patients to
make improvements in three areas: (1) customer service (63 percent); (2) scheduling, hours, and
appointment types (45 percent); and (3) communication with patients (41 percent) (Table 5.15).
CPC practices reported finding collecting and using patient feedback more important over time.
In the 2016 practice survey, 63 percent of CPC practices reported it was “very important” and 33
percent indicated it was “somewhat important” to improving the care they provide to patients,
changed from 54 and 42 percent, respectively, in 2014.
Table 5.15. Percentage of CPC practices indicating that a survey or PFAC
influenced various practice changes, in PY2016, CPC-wide and by region
Type of practice change influenced by
the survey or PFAC
CPC-
wide AR CO NJ NY OH/KY OK OR
Improving customer service
63% 58% 62% 60% 39% 69% 75% 75%
Changes to scheduling, hours,
appointment types
45% 30% 45% 40% 39% 63% 62% 30%
Improving communication with patients
(e.g., newsletters, signage)
41% 38% 48% 28% 48% 33% 47% 45%
Improving patient portal access and
usability
35% 42% 27% 47% 24% 45% 23% 34%
Reducing wait times to get an
appointment
32% 26% 24% 43% 32% 40% 38% 22%
Changes to front office staffing and
waiting areas
29% 28% 27% 28% 13% 45% 18% 36%
Follow-up from ED visits 27% 28% 11% 23% 11% 60% 35% 16%
Strategies to improve continuity of care
and relationship between patients and
providers/care team
23% 8% 29% 23% 18% 23% 37% 22%
Transition of care from hospitals and
subacute care
23% 21% 6% 15% 11% 43% 50% 14%
Tracking and follow-up from hospitals and
diagnostic studies
21% 21% 5% 21% 10% 51% 28% 9%
Streamlining forms to reduce patient
burden
18% 11% 26% 19% 13% 17% 17% 20%
Coordination of care with specialists 17% 23% 5% 25% 11% 9% 42% 13%
Changes to self-management support
strategies
15% 4% 6% 6% 19% 33% 15% 16%
110

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.15 (continued)
Type of practice change influenced by
the survey or PFAC
CPC-
wide AR CO NJ NY OH/KY OK OR
Coordination of care with mental health
and behavioral health providers
15% 19% 8% 6% 13% 11% 40% 13%
Using community-based self-
management support and wellness
resources
13% 11% 5% 8% 2% 31% 25% 5%
Changes in the development or use of the
plan of care for patients at high risk
8% 8% 3% 11% 16% 5% 8% 8%
Changes to medication management
strategies
8% 9% 5% 11% 6% 15% 8% 3%
Refinements to risk-stratification
methodology
4% 2% 2% 4% 3% 1% 13% 2%
Other 21% 11% 48% 17% 5% 31% 3% 27%
Number of practices
433 53 66 53 62 75 60 64
Source: Mathematica analysis of PY2016 Q4 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 433 practices that submitted Milestone data for this item for the
last quarter of 2016. Practices could select all changes that apply.
PFAC = Patient and Family Advisory Council.
b.1. Patient surveys
Deep-dive practices used various strategies to conduct patient surveys. Practices used
paper-based surveys, web-based surveys, or a combination of both. They asked patients to either
take the survey during or immediately after office visits (on paper or on a tablet computer), or
complete it at home and submit it through the patient portal or by mail. Some practices surveyed
only patients who came in for an office visit, whereas other practices surveyed a sample of their
entire patient panel. Several practices reported using patient surveys from third parties, such as
CAHPS or Press Ganey, whereas others developed their own surveys. Practices mainly reported
distributing surveys monthly or quarterly, although at the time of our 2016 interviews, some
practices were not following any set distribution schedule and a few had temporarily
discontinued the surveys. Practices’ reasons for discontinuing the survey included staff
prioritizing other tasks, and challenges getting a sufficiently wide range of patients to respond to
the survey. For example, practices noted that the same patients would respond to the survey
repeatedly.
Several respondents in deep-dive practices reported challenges with conducting patient
surveys and analyzing the results, indicating results were unreliable or not useful for
making practice improvements. Some deep-dive practices found it challenging to incorporate
distribution of surveys into practice workflows because “it is just one extra thing to do” in a busy
workday. Respondents in a few practices also noted that some patients found the surveys
confusing. One practice described the situation where some patients seemed confused about the
response scale, selecting a “1” on all questions, which is the worst score possible, but also
providing glowing reviews, such as “this doctor is the best ever.” Several respondents expressed
concern about potential quality issues with the survey sample. For example, since surveys
typically are anonymous and respondents are not tracked, practices may unknowingly survey the
same patients multiple times and the findings may not adequately represent the views of the
practice’s overall patient panel. Moreover, several respondents in practices that analyzed the
survey results themselves (rather than contracting with a third party) found the analysis to be a
111

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
burden on practice staff. In practices that contracted with third parties to analyze their surveys,
some respondents perceived that findings from the survey were not worth the cost of the
analysis. However, a few respondents noted that survey results about specific clinicians or staff
were more informative than practice-level results for making practice improvements. For
example, survey findings from patients at one practice identified clinicians with long wait times,
allowing the practice to allocate more staff at various points in the clinic workflow to reduce wait
times. At another practice, patients identified which practice staff provided good or poor
customer service, allowing the practice manager to work with staff individually to improve
patient satisfaction scores.
b.2. Patient and family advisory councils
Deep-dive practices initially relied on surveys to gather patient feedback, but increased
their use of PFACs over time. Deep-dive practices found PFACs more useful as time
progressed, but continued to use surveys more than PFACs. The format and composition of
PFACs varied across deep-dive practices, but did not change substantially over time; PFACs
tended to include practice managers and patients who were most commonly recruited by practice
clinician or staff nomination.
Several deep-dive practices faced challenges engaging patients to attend PFAC
meetings. Practices cited two reasons in particular for this challenge. First, as in prior years,
respondents noted that PFAC meetings held during business hours were difficult for some
patients to attend, so they did not capture the diversity of the practice’s patient population.
Evening and weekend PFACs were also hard to schedule because they required extra time from
practice staff who had responsibilities at home. Second, a few respondents noted that patients
expressed doubt that attending a PFAC meeting would influence the practice. To overcome this
challenge, practices attempted to reassure PFAC members that the practice was acting on their
feedback by ensuring multiple practice staff and clinicians attended the meetings or by sharing
with patients the improvements that resulted from PFAC feedback.
Several deep-dive practice respondents found PFACs
effective in generating information to guide practice
improvements. They noted that PFACs facilitated meaningful
conversations between patients and practice members that
produced in-depth information about the patients’ experience
of care. Respondents reported that the PFACs’ suggestions led
to practice improvements around patient outcomes, patient
satisfaction, and patient education. For example, one PFAC
suggested nurses use text messaging to solicit blood sugar
levels from patients with diabetes and then track the levels
over time, which respondents said resulted in reduced blood sugar levels among almost half of
their patients. Other practices reported that PFACs suggested strategies to improve patient
satisfaction, such as creating a welcome packet for new patients, helping practice staff interpret
results of patient satisfaction surveys, and revising complex language in written communication
with patients to make it easier to understand. In addition, PFACs worked with patients to revise
intake forms and health risk assessments. Deep-dive practices also reported increasingly using
the feedback from PFACs to improve practice operations. For example, some practices shared
“(PFACs have) been valuable
to give us a different
perspective that we didn’t
think about. You’d think with
all the brains that we have
here [in the practice] that we
would think of those things,
but we don’t.”
–Care manager
112

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
feedback at practice meetings to discuss how to make improvements, or had leaders or QI
coaches meet with clinicians and staff to discuss feedback. A small qualitative study of 10 PFAC
patient participants in 2015 found that patients most often raised access-related topics, such as
getting timely appointments, reducing wait times in the office, and ensuring that patients were
greeted in a timely and welcoming manner (Peikes et al. 2016c).
A few deep-dive practices reported communicating changes to patients that resulted
from PFAC feedback or surveys (a Milestone requirement). These practices communicated
changes by posting updates in the waiting room and sharing updates during PFAC meetings. One
practice planned to communicate changes through an electronic newsletter available through the
patient portal, but had not implemented it. One practice shared proposed or implemented changes
with the PFAC to solicit additional feedback on them. Respondents from this practice noted that
the feedback loop could take six months or more, but ultimately enhanced the practice’s ability
to improve patient satisfaction and experience.
5.4.4. Milestone 5: Use data to guide quality improvement
Requirements for Milestone 5 became more demanding over the course of the initiative; for
example, the number of eCQM measures for which practices were required to perform
continuous QI using eCQM data increased from one to three. However, requirements were the
same in PY2016 and PY2015. To meet the Milestone requirements, practices were required to
(1) perform continuous QI using EHR data in at least three areas, measured by eCQMs; and (2)
identify a high-cost or high-utilization area from payer feedback reports, or an aggregated report
where available, and develop a strategy to reduce cost or utilization in this area.
a. Overview of findings
Findings from across data sources suggest that QI was a major focus for CPC practices, with
more staff sharing responsibility for QI activities over time; however, similar changes occurred
in the comparison practices. In the 2016 clinician survey, about two-thirds of physician
respondents in CPC and comparison practices reported that they systematically use data from
their practice to improve care quality (Appendix E, Table E.63). The percentages of CPC and
comparison practices reporting that they used practice staff and teamwork, rather than a
centralized committee or department, for QI activities were also similar in 2016. CPC practices
reported increased engagement of patients and families over time. Physicians in CPC and
comparison practices provided similar responses for many measures of using data to guide
improvement. However, physicians in CPC practices were more likely than those in comparison
practices to report that their practices actively seek new ways to improve how they do things, and
to indicate that staff and clinicians are involved in developing QI plans. Deep-dive practices
continued to note that the timeliness and actionability of Medicare feedback reports were barriers
to using the reports to monitor changes in cost and utilization resulting from QI efforts (for
detailed discussion of data feedback, see Chapter 3).
113

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
b. Detailed findings
b.1. Quality improvement and eCQM reporting
For Milestone 5 in PY2016, practices had to report eCQMs, and choose three measures on
which to focus their QI activities. According to PY2016 Quarter 4 Milestone data, the most
common eCQMs that CPC practices selected for QI efforts were (1) hemoglobin A1c poor
control for diabetes, (2) colorectal cancer screening, and (3) breast cancer screening (Table 5.16).
These eCQMs were also the most common measures practices selected in 2015, and are similar
to those selected in 2014 (when controlling high blood pressure was in the top three, and breast
cancer screening was number four).
Table 5.16. Percentages of eCQMs that CPC practices selected for quality
improvement activities, in PY2016, CPC-wide and by region
eCQM
CPC-
wide AR CO NJ NY OH/KY OK OR
Hemoglobin A1c poor control for diabetes
83% 75% 93% 72% 87% 73% 82% 94%
Colorectal cancer screening 79% 81% 75% 89% 60% 96% 63% 89%
Breast cancer screening
73% 68% 60% 83% 44% 91% 77% 86%
Controlling high blood pressure
69% 82% 64% 79% 83% 40% 57% 84%
Influenza immunization
44% 42% 31% 60% 56% 47% 33% 41%
Tobacco use: screening and cessation
intervention
51% 63% 49% 60% 44% 35% 28% 78%
Pneumonia vaccination status for older
adults
43% 46% 33% 72% 25% 47% 25% 55%
Diabetes LDL management 41% 42% 54% 34% 54% 5% 32% 69%
Falls: screening for future fall risk
37% 51% 55% 40% 27% 25% 10% 52%
Screening for clinical depression and
follow-up plan
35% 44% 42% 45% 10% 32% 10% 66%
Documentation of current medications in
the medical record
36% 49% 25% 43% 38% 19% 20% 63%
Ischemic vascular disease: complete lipid
panel and LDL control
25% 23% 21% 4% 22% 40% 3% 55%
Heart failure: beta-blocker therapy for left
ventricular systolic dysfunction
11% 21% 1% 4% 0% 4% 8% 38%
Number of practices
439 57 67 53 63 75 60 64
Source: Mathematica analysis of PY2016 Q4 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 439 practices that submitted Milestone data for this item for the
last quarter of 2016. Because practices had to identify at least three eCQMs, these percentages are not
mutually exclusive.
eCQM = electronic clinical quality measure; LDL = low density lipoprotein.
b.2. Staffing, resources, and processes for quality improvement
Practice-level clinical and administrative leaders most often generated and
implemented QI ideas, but were often working alongside others in the primary care
practice, at the system level, and patients. Specifically, 92 percent of CPC practices reported
in their 2016 Milestone data that practice clinical and administrative leadership were primarily
generating improvement ideas and opportunities. They were commonly joined by staff members
(68 percent of practices) and system-level leadership (66 percent of practices), and slightly less
commonly by designated QI teams or patients and caregivers (49 percent and 40 percent of
114

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
practices, respectively) (Table 5.17). This varied by region. Whereas 49 percent of all CPC
practices reported using designated QI teams to generate improvement ideas and opportunities,
76 percent of CPC practices in Colorado and 65 percent of CPC practices in New York indicated
using this approach. Similarly, whereas 40 percent of all CPC practices indicated that patients
and caregivers were primarily generating improvement ideas and opportunities, 63 percent of
practices in Oregon indicated this was the case for their practice.
57
The 2016 clinician and staff survey provided more details on the members of the practice
that participated in QI activities. Similar proportions (about 30 percent) of nurse
practitioners/physician assistants and staff in CPC practices reported frequently participating in
QI activities at the practice in a typical week—while a much higher proportion (62 percent) of
CPC practice managers reported doing so (Appendix E, Table E.48).
Teamwork in QI became more common during CPC, including meaningful
involvement of patients and families. In earlier years of CPC, deep-dive practices indicated
that teamwork was needed to meet eCQM requirements and improve care processes. By the final
year of the initiative, the percentage of CPC practices that reported “all staff shared
responsibility for conducting QI activities” had increased from 15 percent in 2012 to 49 percent
in 2016 (and was considerably higher than the 38 percent of comparison practices that reported
this in 2016). In addition, the number of CPC practices that involved patients and families in QI
grew over the course of the initiative: the percentage of CPC practices that reported “QI
activities were conducted by practice teams supported by QI infrastructure with meaningful
involvement of patients and families” increased from 5 percent in 2012 to 32 percent in 2016 (28
percent of comparison practices reported this in 2016) (Appendix D, Table D.8a).
Most practices shared panel-level results for specific care teams or providers openly
with providers and practice staff. Almost all practices reported that they tracked and measured
progress on QI projects, with nearly equal numbers of practices (43 percent and 41 percent)
doing so monthly or quarterly, and only 14 percent doing so on an ad hoc basis (Table 5.17).
57
Despite the word “primarily” in the question, practices could check all that apply.
115

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.17. Percentages of CPC practice staff who generate and implement
QI ideas and review data, and intervals for tracking measures and progress,
in PY2016, CPC-wide and by region
CPC-
wide AR CO NJ NY OH/KY OK OR
Roles of individuals who primarily generate
QI ideas and opportunities:
Clinical and administrative leadership at
the practice level
92% 87% 92% 94% 82% 93% 98% 95%
Staff members 68% 51% 73% 64% 60% 80% 55% 86%
Clinical and administrative leadership at
the system level
66% 42% 65% 40% 76% 77% 67% 84%
Designated QI team 49% 30% 76% 23% 65% 49% 33% 58%
Patients/caregivers
40% 25% 39% 45% 34% 19% 57% 63%
N/A or in planning
0% 0% 0% 0% 0% 0% 0% 0%
Practice staff who had implemented QI
projects or tests of change over the last
two quarters:
Clinical and administrative leadership 91% 81% 89% 96% 85% 91% 95% 97%
Staff members
60% 60% 70% 64% 34% 57% 58% 78%
Designated QI team
52% 32% 74% 30% 56% 49% 53% 63%
Patients/caregivers
8% 6% 17% 11% 6% 9% 0% 8%
N/A or in planning
1% 2% 0% 0% 5% 0% 0% 0%
Sharing of QI data and results:
Panel-level results with the care team or
providers identified are shared openly
within the clinic for providers and staff
only
77% 68% 79% 66% 66% 92% 92% 72%
Panel-level results with the care team or
providers identified are shared openly
within the clinic for providers and staff,
as well as patients and families
11% 11% 9% 17% 21% 8% 2% 13%
Results are provided to care team or
providers without identifying the
applicable provider or care team
6% 9% 9% 11% 6% 0% 3% 5%
Results are reviewed by designated QI
team or staff member but not shared
with individual providers or care team
4% 6% 3% 0% 6% 0% 0% 11%
We do not routinely review or share QI
data and results
2% 6% 0% 6% 0% 0% 3% 0%
Practice routinely tracks and measures
progress on QI projects:
At least monthly 43% 23% 70% 36% 15% 55% 33% 64%
At least quarterly
41% 47% 26% 51% 73% 36% 52% 9%
Only as needed or ad hoc
14% 26% 5% 11% 11% 8% 13% 25%
We do not routinely track and measure
progress on QI projects
2% 4% 0% 2% 2% 1% 2% 2%
Number of practices
433 53 66 53 62 75 60 64
Source: Mathematica analysis of PY2016 Q4 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 433 practices that submitted Milestone data for this item for the
last quarter of 2016. Practices could select all that apply for the first two questions addressed in the table;
they selected one option for the last two questions addressed in the table.
QI = quality improvement.
116

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Physicians in CPC practices were more likely than physicians in comparison practices
to report that their practice actively seeks new ways to improve how they do things. In the
2016 clinician survey, physicians in CPC practices were also more likely than those in
comparison practices to report that the practice has changed how it takes initiative to improve
patient care in the last 12 months (Figure 5.5). However, similar proportions of primary care
physician respondents in CPC and comparison practices reported personally using data from
their practice to improve care quality in a systematic way. For many measures of the use of data
to guide improvement, physicians in CPC and comparison practices provided similar responses.
Figure 5.5. Percentages of physicians who agree or strongly agree with
statements about quality improvement, CPC and comparison practices, 2016
Source: CPC Clinician and Staff Survey, conducted June–November 2016.
*/**/*** Response distributions for this question are significantly different between CPC and comparison respondents at the
0.10/0.05/0.01 level.
86*
45*
72*
91*
82
74
59
88
84
77
87
75
36
54
87
81
75
61
89
82
72
85
0 20 40 60 80 100
People in this practice actively seek new ways to
improve how they do things
Practice has changed how it does business in
past 12 months
Practice has changed how it takes initiative to improve
patient care in past 12 months
Staff and clinicians are involved in developing plans
for improving quality
This practice has clearly articulated goals
This practice operates at a high level of efficiency
Staff monitor each other’s performance
Staff exchange relevant information as it
becomes available
People at all levels of this practice openly talk about
what is and isn’t working
After trying something new, people in the practice take time
to think about how it worked
When practice experiences a problem, people in the practice make
a serious effort to figure out what is really going on
Percentage of respondents
CPC physicians Comparison physicians
117

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Deep-dive practices typically used ad hoc approaches for practice-level QI. Similar to
past years, most deep-dive practices, regardless of size or ownership, reported using ad hoc
approaches to QI and several reported no clear process for QI beyond choosing quality and
utilization targets to monitor.
Autonomy for change and centralization of QI approaches varied for small
independent versus large and system-owned practices. Small independent practices had more
autonomy to change workflows and tasks of various staff and noted that this enabled them to
make rapid changes to improve quality. More formal QI efforts tended to occur in large and
system-owned practices and relied on centralized approaches to drive practice-level change.
Formal or systematic approaches to QI typically involved holding regular team meetings to
identify QI targets, plan work process changes, and track progress. Deep-dive practices with
practice-level QI teams reported providing progress updates and reminders to other staff
members about ongoing QI projects. In a few of these practices, the QI team shared these reports
electronically throughout the practice and discussed them at regular practice meetings.
Dedicated staff and support for analyzing and interpreting data facilitated QI efforts.
In past years, deep-dive practices noted that tracking eCQMs helped them organize and maintain
a focus on QI, but that reporting requirements for this
Milestone are time consuming and resource intensive.
In 2016, respondents in some deep-dive practices
reported they now have access to staff (in the practice
or at a central location for system-owned practices)
who assist with analyzing and interpreting quality
measures data. Having dedicated staff has helped
practices to regularly monitor QI progress, identify
new areas for QI work, and generate clinician-level
performance reports. By comparing clinician-level performance data, some practices were able
to identify successful strategies and implement them throughout the practice. As in previous
years, some deep-dive practices that were part of larger systems had CPC project managers who
worked across all system-affiliated practices to standardize and support practice-level
improvements using formal QI methods (such as plan-do-study-act cycles). For non-system
affiliated practices, expanding the QI team and focusing on teamwork were particularly
important for successful QI. These practices described the importance of the care manager’s role
in helping practices stay focused on QI efforts, and the need for practice meetings and other
opportunities to share QI information (for example, posting materials on the practice intranet site
or in common areas).
b.3. Data feedback reports
In this section, we discuss practices’ use of feedback reports for quality improvement.
Chapter 3 presents details on the use of feedback reports across regions and practice types and
additional discussion about feedback reports. Practice survey data indicate that most CPC
practices reviewed Medicare FFS practice-level feedback reports and that a majority regularly
reviewed feedback from other payers as well.
“Now with having so much data
available through our EHR … we
actually created a position where we
have a data analyst who is able to run
reports on different quality measures
and get that report out to our physicians
monthly.”
–Medical director in a physician group
118

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Many deep-dive practices found the data feedback
reports from Medicare and other payers useful for
identifying potential improvement areas to target but not
timely enough to monitor QI changes. As in prior years, the
principal reason practices did not use feedback reports to support
ongoing QI was that the data were not timely enough to
accurately gauge the effects of improvement efforts and support
QI. For example, a practice might make a change based on utilization in a feedback report but
could not confirm until many months later whether the change had an effect, during which time
the practice might have made other changes. A few respondents reported that practice-
constructed reports on utilization (such as tracking ED visits) were more useful for supporting QI
because they could receive regular updates and get a more current picture of the results.
58
Throughout CPC, deep-dive practices noted other challenges in using payers’ feedback
reports to guide QI, including inconsistent access to patient-level data, reports that represent
small numbers of patients, and measurement methodologies and reported outcomes that are not
aligned across payers. In some regions, the lack of alignment across payer reports meant that
practices sometimes received conflicting signals on the same quality measure from different
payers, leading staff in some practices to question the accuracy of reports not generated from
their own clinical data systems. In 2016, however, deep-dive practices also indicated feedback
reports were helpful for comparing their results on hospital readmissions, ED visits, and other
metrics to those for all practices within a CPC region (or practices in their region with a similar
patient risk profile to theirs). Some practices used the feedback reports to set informal goals to
reduce gaps in care or address high utilization for individual patients. For example, a few
practices used the patient-level data files included in some payers’ feedback reports to identify
patients to target for education about appropriate ED use. Similar to findings from previous
years, practice members used feedback reports to identify patients who need care management
services.
A few deep-dive practices were using Medicare’s Specialist Feedback Report and
found it useful, but others were unfamiliar with the report or did not find it very useful. In
May 2016, CPC practices were given access to a report on use of specialists by Medicare FFS
beneficiaries attributed to the practice. The one-time report was designed by CMS and listed for
each practice its top five specialists (in terms of costs) within each specialty, the total costs by
specialty, and the number of visits by specialty. Deep-dive practices that used the Specialist
Feedback Report found it helpful for (1) tracking which specialists their patients were seeing, (2)
identifying and working to eliminate unnecessary specialist encounters and procedures, (3)
tracking costs and utilization for patients who self-referred to specialists (such as
ophthalmologists for cataract surgery), and (4) identifying lower-cost specialists for future
patient referrals. Respondents in a few practices said that they downloaded the report but did not
find it useful because it was too complex to interpret, contained too much information, or did not
include information that would help them assess the quality or value of the specialists’ services
(for example, one physician wanted more details about services each specialist provided, such as
58
Although many respondents suggested that real-time access to claims data might be more useful than feedback
reports, a pilot of claims data sharing conducted with a small number of CPC practices suggests that only some
would have the time and resources necessary to clean and analyze claims data.
“By the time the CMS
report comes … if you’re
really being proactive …
it’s kind of a day late and a
dollar short.”
–Clinician lead
119

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
a cardiologist’s rate of echocardiograms or stress tests). Moreover, several deep-dive practices
reported they were unfamiliar with or had not reviewed these reports.
5.4.5. Milestone 6. Care coordination across the medical neighborhood
As in PY2015, in PY2016, Milestone 6 required CPC practices to implement two of the
following three options: (1) track the percentage of patients with ED visits who received a
follow-up phone call from the practice within one week, (2) contact at least 75 percent of
patients who were hospitalized in target hospitals within 72 hours or two business days of
discharge, or (3) enact care compacts or collaborative agreements with at least two groups of
high-volume specialists in different specialties to improve care transitions.
a. Overview of findings
CPC practices made progress from 2012 to 2016 on relevant care coordination tasks as
measured by the Milestone data, deep-dive findings, and the practice survey, but they still had
opportunities for improvement. In 2016, almost all CPC practices chose to focus on both hospital
discharge follow-up and ED follow-up. Findings from the practice survey show that CPC
practices were substantially more likely than comparison practices in 2016 (84 versus 54
percent) to report that they routinely followed up with their patients after ED visits or
hospitalizations “because of established arrangements with the ED or hospital to track patients”
(see Appendix D, Table D.8a). Findings from the deep-dive interviews indicated that many
practices refined workflows and strengthened relationships with hospitals during CPC. For
example, some practices entered into agreements with hospitals to which they most frequently
admitted patients so they could obtain timely discharge data and contact patients promptly.
Deep-dive practices also reported expanding their outreach to patients who are discharged
from the hospital or ED. Reflecting the success of outreach efforts, in 2016, patients at CPC
practices were more likely than comparison practice patients to report that someone from the
provider’s office contacted them within three days of their most recent hospital stay (60 versus
50 percent) or within one week of their most recent emergency room or ED visit (59 versus 51
percent).
CPC practices chose care compacts less frequently than the other two options (hospital
discharge follow-up and ED follow-up) for Milestone 6; still, 40 percent of CPC practices in
2016 also elected to establish care compacts or collaborative agreements with specialists. And
while the use of care compacts with specialists among CPC practices increased each year
according to Milestone data, their use was lower in CPC than in comparison practices.
b. Detailed findings
In 2016, almost all CPC practices chose to focus on hospital discharge follow-up and ED
follow-up, and 41 percent elected to establish care compacts or collaborative agreements with
specialists to help improve care transitions (Table 5.18). Activity varied across regions, with
higher percentages of practices in Colorado, New Jersey, and New York (72, 64, and 61 percent)
choosing to focus on care compacts, compared to 15 and 17 percent of practices in
Ohio/Kentucky and Oklahoma. Practices arranged care compacts or collaborative agreements
most often with the following specialist types: cardiology (chosen by 64 percent of practices that
120

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
had care compacts), gastroenterology (49 percent), orthopedic surgery (43 percent), behavioral
health (39 percent), and obstetrics/gynecology (34 percent).
Table 5.18. Percentages of CPC practices that chose CPC’s three care
coordination activities, in PY2016, CPC-wide and by region
Care coordination activity
CPC-
wide AR CO NJ NY OH/KY OK OR
Hospital discharge follow-up
96% 100% 93% 94% 88% 100% 100% 100%
ED follow-up 95% 98% 85% 96% 98% 100% 93% 95%
Care compacts/collaborative agreements
with specialists
41% 37% 72% 64% 61% 15% 17% 25%
Number of practices
440 57 67 53 64 75 60 64
Source: Mathematica analysis of PY2016 Q1 Milestone submission results.
Note: Practices could select all activities that applied.
b.1. Hospital and ED follow-up
Milestone, survey, and deep-dive data all indicated that practices expanded follow-up with
patients after hospital and ED discharge and strengthened their relationships with hospitals to
facilitate this work. Still there was room for improvement.
Developing relationships with hospitals and EDs
Results from the practice survey suggest that practices made considerable progress in
developing relationships with hospitals and EDs so practices could follow up with patients, but
could continue to improve in this area (see Appendix D, Table D.8a):
• The percentage of CPC practices reporting “following up with patients seen in the ED or
hospital routinely because the practice has arrangements with the ED and hospital to track
patients and ensure follow-up is completed within a few days” increased from 25 percent in
2012 to 65 percent in 2014, 77 percent in 2015, and 84 percent in 2016. Additionally, a
higher proportion of CPC than comparison practices (84 versus 54 percent) reported in 2016
that they conducted routine follow-up with patients seen in EDs or hospitals because of
established arrangements with them to track patients and ensure timely follow-up.
• The percentage of CPC practices reporting “consistent receipt of information on patients
from community hospitals and EDs within 24 hours after the event” increased from 14
percent in 2012 to 36 percent in 2014, 53 percent in 2015, and 57 percent in 2016. (A lower
36 percent of comparison practices reported this in 2016.)
Over time, deep-dive practices improved their relationships with hospitals and their
processes to follow up with patients discharged from the hospital and ED. Some deep-dive
practices spent time early on developing relationships with hospitals that led the hospitals to send
patient discharge information systematically to the practice (electronically or via fax) by
PY2016. These practices attributed their success in gaining access to hospital records during
CPC to (1) their efforts to build rapport with hospital leaders, and (2) recent changes in Medicare
payment policy that made hospitals more interested in working with primary care providers to
reduce readmissions.
121

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
However, deep-dive practices’ experiences varied considerably with respect to:
• Source and therefore comprehensiveness of the discharge information (in-network hospitals
only, out-of-network hospitals, or payers)
• Consistency and timeliness of discharge notification
• Mechanism used to notify practices of the discharge (electronic, fax, or telephone)
• Level of automation in notifying practices of the discharge (automatic receipt of information
or manual look-up by practices)
• Level of detail in the discharge notification
Because of these variations, staff in some practices still spent considerable time and resources
coordinating the exchange of information between the practice and hospitals. Other practices
were unable to obtain hospitals’ cooperation, despite repeated requests for records, and some
patients did not identify themselves as receiving primary care from the practice.
Practices with electronic access to hospital and ED records could identify and follow up
with patients in a timely manner, particularly if they received automatic notifications.
These practices benefited from having real-time access to records through a common EHR,
hospital portal, or local data-sharing network. Several practices had automatic notification
arrangements with a target hospital (including hospitals that were part of their system and
hospitals with which they had no affiliation), which relieved staff from regularly checking for
available records.
Providing patients with transitional care
Patient survey results corroborate the reports of increased follow-up by practices.
Specifically, a higher proportion of Medicare FFS beneficiaries in CPC practices than
comparison practices reported receiving follow-up care within three days of a hospital stay (60
versus 50 percent), and within one week of an ED visit (59 versus 51 percent).
In deep-dive practices, a practice member typically telephoned the patient within 48
hours of discharge after hospitalizations and contacted patients within a week of an ED
visit. Most often, this outreach was performed by a designated care manager; however, in some
practices, other staff members (for example, licensed practical nurses, medical assistants, or the
practice manager) were responsible for following up within the required time frame. In several
practices, the care manager focused on contacting the highest-risk patients, and other staff
members contacted the medium- and low-risk patients.
Across deep-dive practices, staff noted the importance of the care manager in
understanding and addressing the needs of high-risk patients who are discharged from the
hospital or ED. Respondents noted that these patients sometimes have limited understanding of
the hospital care they received or are confused about their medications. Care managers
reportedly played an important role in addressing this issue by helping discharged patients
reconcile medications between the hospital and outpatient setting and arranging follow-up care,
thus minimizing clinicians’ involvement in resolving care coordination issues. Care managers
also provided other care management services, including connecting patients to needed resources
122

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
and supports, such as programs providing affordable medications. Respondents in several deep-
dive practices felt that access to a care manager also helped patients avoid the ED for non-urgent
needs. The care manager encouraged the patient to see the primary care clinician (if needed),
helped reduce the patient’s anxiety about an issue by telephone (if appropriate), or quickly
obtained input from a clinician. A few practices kept lists of frequent ED users, so during ED
follow-up, care managers could educate them about appropriate use of the ED and when to call
the practice first before going to the ED.
Practices thought their work with patients after care transitions was valuable. Deep-
dive practice respondents felt that their care transitions work benefitted patients by preventing
things from falling through the cracks (for example, reconciling changes to patients’ medications
made in the hospital with medications the primary care clinician had previously prescribed for
the patients) to avoid medication errors. This care transitions work also ensured that patients
understood the discharge information and followed up in a timely fashion with their primary care
clinician to help avoid additional ED visits or readmissions.
b.2. Care compacts/collaborative agreements
Although few CPC deep-dive practices focused on care compacts or collaborative
agreements with specialists when CPC began, close to half of them were pursuing such
agreements by the final year. Care compacts and collaborative agreements outline the
respective responsibilities of primary care providers and specialists in caring for patients, and
establish a process for reliably exchanging clinical data and communicating about referrals and
consultations. Like practices nationwide, CPC practices still have substantial opportunities to
improve how they coordinate and exchange information with specialists in their medical
neighborhood. At the same time, respondents in deep-dive practices noted that they do not have
control over the services ordered by specialists or hospitals, or the FFS incentives that drive
providers’ behavior.
As in previous years, deep-dive practices typically established agreements with
specialists to whom they most frequently referred, with whom they had good relationships,
and who were in the same health system and using the same EHR. According to the deep-
dive practices, specialists are receptive to these agreements. In most cases, discussing the
collaborative agreement in person facilitated the primary care and specialist providers’
commitment to it. In one practice, the system-level IT support team worked with staff from
primary care and specialist practices to develop EHR functionality to support the agreement.
A few deep-dive practices mentioned challenges to setting up collaborative agreements
(such as agreeing on how information should flow between practices). A couple of practices
noted that specialists were having difficulty managing different collaborative agreements with
multiple referring groups, because the agreements had different communication and coordination
requirements. For practices that are part of a system with a system-wide EHR, respondents
reported that care compacts were less important because all clinicians within the system can see
relevant patient information. Deep-dive practices also reported a lack of engagement from
specialists, partly because current FFS payment incentives do not encourage specialists to engage
with primary care providers. In addition, data sharing across different EHRs can pose challenges
to setting up and carrying out collaborative agreements. Practices pursuing care compacts were
123

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
still developing them, so deep-dive practices did not report on the extent to which compacts were
affecting care delivery.
5.4.6. Milestone 7: Shared decision making
In PY2016, to meet the requirements for Milestone 7, practices were required to use at least
three patient decision aids (PDAs) to support shared decision making (SDM) in preference-
sensitive care. Practices were required to track the use of the PDAs through (1) a metric tracking
the proportion of patients eligible for the decision aid who received the aid, or (2) quarterly
counts of patients who received individual aids.
a. Overview of findings
The uptake of SDM was low in the first two years of CPC, perhaps because (as deep-dive
interviews suggested) practices did not understand the difference between SDM for preference-
sensitive conditions and general patient education, and struggled to identify patients who would
benefit from SDM. However, as CPC progressed, practices increased SDM implementation and
noted that the quality of care for these preference-sensitive conditions improved because patients
were making more informed decisions. Results from interviews with deep-dive practices and the
practice survey suggested continued room for improvement in (1) providers’ and staff members’
understanding of the concept of preference-sensitive conditions, (2) developing care processes to
provide SDM without overwhelming clinicians, and (3) tracking the discussion and outcomes of
SDM in EHRs. Deep-dive practices that used teamwork to engage patients in SDM found
Milestone 7 more manageable, but several deep-dive practices expressed mixed perceptions of
the benefit of SDM. Consistent with this, only 37 percent of physicians reported in the clinician
survey that using SDM tools was very important to improving the care they provide patients.
b. Detailed findings
b.1. Uptake of SDM
Information from the practice survey sheds some light on CPC practices’ uptake of SDM.
The percentage of CPC practices that reported that “PDAs were used to help patients and
providers jointly decide on treatment options consistently for patients for two or more clinical
conditions and tracked with run charts or other measures” increased from 42 percent in 2014 to
56 percent in 2015 and 62 percent in 2016, when it was substantially higher than the 25 percent
of comparison practices that reported this. (We did not ask CPC practices about this topic in
2012.) (See Appendix D, Table D.8a.) The proportion of CPC practices reporting that “practice
teams trained in decision making techniques systematically supported involving patients in
decision making and care” steadily increased from 15 percent in 2012 to 27 percent in 2014, 35
percent in 2015, and 41 percent in 2016 (see Appendix D, Table D.8a). (This approach helps
increase the effectiveness of both SDM and care management.) These results indicate
improvement over time, as well as room for practices to increase their use of SDM.
b.2. Identifying topics and decision aids for SDM
The top four conditions that practices selected for shared decision making were (1)
colorectal cancer screening (66 percent), (2) prostate cancer screening (41 percent), (3) tobacco
cessation (32 percent), and (4) mammography (24 percent) (Table 5.19).
124

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.19. Shared decision making topics chosen by CPC practices as of
Quarter 1, PY2016
Shared decision making topic Percentage of practices
Therapeutic options in management
Tobacco cessation: choice of approach 32%
Low back pain (acute or chronic) 21%
Care preferences over the life continuum 19%
Osteoporosis management and medication choices 12%
Mild depression 12%
Adult sinusitis 10%
Insomnia 3%
Osteoarthritis of the hip or knee 2%
Chronic pain 1%
Medication choices
Diabetes management 17%
Statin use 15%
Antibiotic use 8%
Chronic obstructive pulmonary disease management 5%
Hypertension management 5%
Anticoagulation for atrial fibrillation 2%
Asthma management 1%
Congestive heart failure management 1%
Screenings
Colon cancer screening strategies 66%
Prostate cancer screening 41%
Mammography for patients age 40–49 or over the age of 75 24%
Lung cancer screening 6%
Other
Depression treatment 5%
Aspirin 2%
Other
a
14%
Source: Mathematica analysis of PY2016 Q1 Milestone submission results provided by CMS.
Notes: 433 practices reported on their choice of SDM topics. Practices each chose two to five SDM topics.
a
“Other” includes practices’ write-in responses: first trimester genetic screening; abdominal aortic aneurysm
screening; ADHD treatment options; cataracts; cholesterol screening and management; fall risk or prevention;
vaccinations; knee pain; lead screening; long-acting reversible contraception; management of urinary incontinence;
medication options for Crohn’s disease and ulcerative colitis; menopause treatment; obesity; “One Key Question”;
obstructive sleep apnea; pap smear; substance use; medication for Alzheimer’s disease; bunions; cervical cancer
screening; other diabetes care; hypertension; managing risks/concerns for older patients; and treating blocked leg
arteries. Not all of these are consistent with CMS’s definition of a preference-sensitive condition.
Milestone data illustrate that the organizations from which CPC practices commonly
obtained PDAs for SDM include the Centers for Disease Control and Prevention, Mayo Clinic,
Healthwise, the Agency for Healthcare Research and Quality, and Option Grid, and others (Table
5.20). Given the cost of obtaining access to private PDA libraries, 49 percent of practices
obtained free decision aids from sources other than the ones listed in the table and 18 percent
used an ad hoc or practice-created tool.
125

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.20. Sources of decision aids used by CPC practices, PY2016, CPC-
wide and by region
Source
CPC-
wide AR CO NJ NY OH/KY OK OR
Other
49% 56% 54% 61% 31% 49% 36% 58%
Centers for Disease Control
32% 47% 35% 28% 50% 11% 39% 17%
Mayo Clinic
32% 14% 14% 44% 17% 28% 66% 40%
Healthwise Decision Points
21% 37% 38% 22% 28% 1% 7% 17%
Agency for Healthcare Research and
Quality
11% 12% 7% 15% 14% 4% 3% 20%
Option Grid
10% 11% 9% 7% 31% 0% 10% 3%
Ottawa Hospital Research Institute
4% 0% 1% 2% 0% 0% 25% 2%
Health Dialog/Informed Medical
Decisions Foundation (Healthwise) 3% 4% 3% 4% 0% 0% 0% 11%
None of the above; we use an ad hoc
or practice-created tool 18% 11% 12% 11% 2% 43% 21% 20%
Number of practices
445 57 69 54 64 75 61 65
Source: Mathematica analysis of PY2016 Q1 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 445 practices that submitted Milestone data for this item for the
last quarter of 2016. Practices could indicate multiple sources of decision aids.
Across regions, most CPC practices selected their SDM topics based on the number of
patients with a condition or due to the perceived potential impact on quality (71 and 70 percent,
respectively) (Table 5.21).
Table 5.21. Reasons for selecting priority shared decision making areas,
PY2016, CPC-wide and by region
Reason
CPC-
wide AR CO NJ NY OH/KY OK OR
Large number of patients with
condition (high volume)
71% 61% 61% 72% 70% 84% 62% 80%
Impact on quality 70% 75% 86% 69% 64% 72% 48% 75%
Potential for significant cost
associated with care (low-volume,
high-cost area) 43% 32% 39% 46% 45% 64% 33% 40%
Significant impact on cost for patient 26% 30% 41% 24% 11% 41% 7% 22%
Other
15% 5% 12% 9% 17% 31% 23% 3%
Number of practices
445 57 69 54 64 75 61 65
Source: Mathematica analysis of PY2016 Q1 Milestone submission results provided by CMS.
Note: Percentages for all regions are based on 445 practices that submitted Milestone data for this item for the
first quarter of 2016. Practices could indicate multiple reasons.
126

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
b.3. Identifying patients for SDM
Milestone data illustrate that practices most commonly reported identifying patients as
eligible for shared decision making through a provider or care team referral (59 percent) or a
routine established protocol (57 percent) (Table 5.22). Only 19 percent of CPC practices had no
established process or protocol and were identifying patients on an ad hoc basis.
As deep-dive practices began implementing this Milestone, some struggled with
identifying and reaching out to patients for particular SDM topics. Some practices initially
took a population-based approach to identifying appropriate patients (for example, all patients
age 50 and older due for colorectal cancer screening) and sent out mailings, with little response
from patients. Practices also tried targeting more narrowly defined patient subgroups (such as
those older than age 50 with an appointment scheduled in the next few months). Qualitatively,
they noted that more intensive personal outreach to the targeted patients seemed to better engage
patients. Other practices addressed the need for SDM on a case-by-case basis during patient
visits.
Deep-dive practices varied in the timing of raising SDM with patients. Most deep-dive
practices raised SDM topics during both routine and acute visits when appropriate. A couple of
practices raised sensitive topics (such as advanced directives) during the annual wellness visit,
when patients were not having acute symptoms and could focus on the discussion. A few also
used population-based outreach between visits, such as mass mailings to patients age 50 and
older who had not had colorectal cancer screening. (However, as discussed more above, some
practices that had tried this approach felt that too few patients called to make appointments to
discuss screening.)
Several clinicians in deep-dive practices stated that some patients did not want to
engage in SDM. Practices perceived that some patients did not take the time to review PDAs,
particularly when they covered “uncomfortable topics” such as end-of-life care or when patients
held a more traditional view of health care, preferring to rely on the provider’s recommendation
for decisions about treatment options. A few practices focusing on PSA screening reported that
some of their patients felt the tool was an effort by insurance companies to save money.
b.4. Documenting use of decision aids
According to Milestone data, 90 percent of CPC practices documented distributing decision
aids in their EHR, but only 1 percent reported documenting it in a care plan or after visit
summary.
127

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Table 5.22. Identification of eligible patients for shared decision making and
documentation of shared decision making, CPC-wide and by region
Method of identification or
documentation
CPC-
wide AR CO NJ NY OH/KY OK OR
Patients are identified as eligible for
preference-sensitive care using:
Provider or care team referral,
based on clinical intuition
59% 43% 59% 62% 56% 72% 57% 56%
Routinely identified based on
established protocols for each
preference-sensitive condition
57% 53% 67% 70% 71% 52% 28% 58%
Automatic flags built into EHR 25% 23% 20% 40% 11% 35% 17% 27%
Ad hoc basis or referral-based only,
no established process or protocol
19% 25% 17% 11% 8% 27% 37% 6%
Other 7% 9% 3% 6% 2% 0% 25% 3%
Distribution of decision aids is
documented in:
EHR 90% 91% 82% 89% 100% 99% 73% 97%
Care plan
1% 2% 0% 2% 0% 0% 0% 0%
After visit summary
1% 2% 0% 4% 0% 0% 0% 0%
Other
9% 6% 18% 6% 0% 1% 27% 3%
Number of practices
433 53 66 53 62 75 60 64
Source: Mathematica analysis of PY2016 Q4 Milestone submission results provided by CMS.
Consistently documenting SDM discussions and decisions in the EHR was a challenge
for clinicians and staff in deep-dive practices throughout CPC. After identifying a workflow
for SDM, practices had to figure out how to document that discussion and find an appropriate
place to record it in the EHR for general tracking and CPC reporting. In many practices,
clinicians reported they did not have the time (or they simply forgot) to check the structured
SDM fields in the EHR, but they instead documented this information in the free-text portion of
the EHR note. In a few deep-dive practices, clinicians continued to document SDM discussions
with patients on paper, and then staff entered the information into the EHR. In some practices,
staff turnover added to the difficulty, including training new
staff to document SDM. As a result, most of these practices
perceived that SDM was underreported at their practice. As
one practice lead noted, “That has been the hardest
Milestone to incorporate into our usual workflow.”
b.5. Additional facilitators of and challenges with
SDM
Deep-dive practices’ prior experience with using
SDM tools facilitated early success. For example, one
practice had developed an infrastructure for incorporating
the use of SDM tools into practice processes. This practice
used a registry to automatically prompt medical assistants
to give eligible patients colon cancer screening information
during intake. Patients had time to review the information
before they saw the clinician, who would discuss the
“You can’t just talk to somebody
about quitting smoking; you’ve
got to show it [in the EHR in] four
different spots, so that the
government can see it anytime
that they want...Every time we
come up with something new, it
takes my nurse another five
minutes. People [who are] not
working [in the clinical setting]
don’t think that five more clicks is
a big deal, but when you’re
seeing patients every day and
every patient has five more
clicks, it’s a big deal…That’s
where the biggest issue is…and
we’re paperless.”
—Lead physician
128

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
screening decision with the patient. After the clinical encounter, the clinician used a template in
the EHR to order the patient’s preferred mode of colon cancer screening or to indicate that the
patient declined screening. Practice members reported that this process had increased the number
of colonoscopies ordered and improved documentation of colon cancer screening.
Involving multiple care team members in engaging patients in SDM was a key
facilitator for this Milestone among deep-dive practices. Respondents in a few practices
reported that they recently changed workflows for topics that were more clinically complicated
(such as imaging for low back pain) or sensitive (such as end-of-life planning) to enable
clinicians to introduce and discuss the PDAs with patients, rather than having another staff
member raise the topic with the patient. Clinicians and staff perceived that SDM on clinically
complex or sensitive topics required clinical judgment or that decisions on more sensitive topics
(such as advanced directives) were more effective when discussed by the clinician. However, for
other topics, practices found it more efficient for medical assistants or front desk staff to
introduce PDAs to patients at the beginning of the office visit; the clinician then reiterated the
PDA information during the visit. In practices with less of a team approach, clinicians
responsible for handling SDM by themselves tended to feel the work was onerous.
As CPC progressed, deep-dive practices noted that the quality of patient care
improved when patients were more informed. Several respondents noted that patients know
best which goals and treatments are the most realistic for their lifestyles, so SDM appropriately
increased patients’ involvement in decisions about their care. Several respondents also
commented that, as a result of SDM, the practice gave more information to patients—about
overdue colorectal cancer screening, unnecessary x-rays and MRIs, tobacco cessation, advanced
directives and other end-of-life planning, and more. Moreover, several respondents perceived
that the SDM resources patients received were more beneficial than the informal conversations
that took place before implementing SDM. For example, one practice’s end-of-life PDA allowed
patients to complete a living will with the help of practice staff; before CPC, the discussion
might have ended without a follow-up action.
Even in the final year of CPC, some deep-dive practices did not understand how
shared decision making differed from general patient education, and they noted a need for
more training on SDM. A number of deep-dive practices reported that they did not understand
the concept of SDM for preference-sensitive conditions. Capturing the sentiment of some
respondents, one CPC coordinator for a health system noted that providers “do not seem to fully
understand the concept” of SDM as defined by CPC. A major area of confusion was
understanding the difference between (1) general patient education for conditions for which
treatment options have a strong evidence base (for example, management of hypertension,
immunizations) and (2) preference-sensitive conditions for which different management options
exist and the patient’s preference should play a greater role in determining which treatment to
pursue. This confusion contributed to lower levels of support among staff. In addition, several
deep-dive respondents commented that the emphasis on using PDAs ignored the larger problem
of clinicians not consistently receiving training on the value of SDM or the techniques needed to
support it. Without this training, some clinicians struggled with effectively using PDAs to
engage patients in decisions about their care.
129

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
5.4.7. Milestone 8: Participation in the CPC learning collaborative
To fulfill the requirements for Milestone 8 for PY2016, practices were required to
participate in all regional learning sessions and engage with the regional learning faculty (RLF)
to facilitate transformation efforts. Chapter 3 provides an in-depth examination of regional and
national learning activities offered in PY2016 and CPC practice perspectives on these activities.
5.4.8. Milestone 9: Health IT
As in PY2015, to meet the requirements of Milestone 9 in PY2016, practices had to attest
that all eligible professionals engaged with, or were working toward, meeting Stage 2
Meaningful Use requirements, which focus on supporting advanced clinical processes, following
timelines established by the EHR Incentive Program.
59
Milestone 9 optimizes use of the EHR to
support better care and improved health outcomes. For PY2016, CMS suggested various
strategies that practices could employ to accomplish this objective. These included, but were not
limited to (1) modifying workflows for more effective EHR use, (2) training staff in optimal
EHR use, (3) using referral templates and other standardized documents to support health
information exchange, (4) building analytic capacity to use EHRs to identify improvement
opportunities, and (5) improving entry of clinical data to ensure accurate quality monitoring and
reporting. We include many findings about health IT as a tool to support specific Milestones in
earlier sections on those Milestones. Specifically, in discussions of Milestones 2, 6, and 7, health
IT challenges affected care plan use and care management activities as well as practices’ ability
to follow up in a timely way with patients who had been hospitalized or used the ED, and limited
practices’ ability to effectively track the outcomes of SDM discussions in EHRs. This section
focuses on cross-cutting issues with health IT across Milestone activities.
a. Overview of findings
As required by CPC, practices used the Office of the National Coordinator for Health
Information Technology (ONC)-certified EHRs, and all CPC practices attested that their eligible
providers are working toward meeting the Stage 2 requirements for Meaningful Use. However,
triangulation of data from Milestone reporting, the practice surveys, and the deep-dive practices
underscores challenges that practices face obtaining and exchanging timely data from providers
outside their practice or system. This issue, which practices nationwide face, continues to pose a
barrier for CPC practices in improving follow-up care after ED visits and hospitalizations, and
coordinating care for patients after their visits to specialists (see Milestone 6 above).
b. Detailed findings
According to Milestone 9 data from the last quarter of PY2016, 100 percent of CPC
practices attested that all eligible providers are currently working toward meeting the Stage 2
requirements for Meaningful Use.
b.1. Use of health IT
In the 2016 practice survey, all CPC practices and 99 percent of comparison practices
reported having an “electronic health record system for managing patient care.” More than 98
59
For information about Stage 2 of the Meaningful Use Program, see https://www.cms.gov/regulations-and-
guidance/legislation/ehrincentiveprograms/downloads/stage2_guide_eps_9_23_13.pdf.
130
5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
percent of both groups also reported using the EHR’s e-prescribing function. Ninety-seven
percent of CPC practices reported using EHR-generated data extracts or reports to guide QI
efforts, compared to 83 percent of comparison practices. In both groups, more than half of
practices reported that practice managers were responsible for reviewing EHR reports (57
percent of CPC practices and 56 percent of comparison practices). These results for CPC and
comparison practices have been relatively consistent in practice surveys conducted in 2014,
2015, and 2016.
The 2014 and 2016 rounds of the clinician survey confirmed that nearly all CPC and
comparison practices are using EHRs. However, in both rounds, responses from physicians in
CPC and comparison practices indicated room for increased use of their EHRs, for example, to
track communications with other providers and to review images and test results. Survey
responses also point to the need for EHRs to better support the ability of clinicians and staff to
provide high quality care for patients. For example, in 2016, 11 to 34 percent of physicians in
both CPC and comparison practices reported disagreeing or strongly disagreeing with statements
that their EHR helps them in providing quality care to patients, reminds them of key actions
when seeing a patient, and is well integrated into the practice’s workflow, and that they trust the
validity of data in the EHR.
As in previous years, deep-dive practices continued to work toward improving the way
they used EHRs to better meet CPC Milestones. In 2016, a couple of practices reported
ongoing efforts to improve tracking and reporting quality measures, such as care gaps in
colorectal cancer screenings and immunizations. Other practices reported establishing new and
more efficient approaches to risk-stratification in the EHR, developing better documentation
templates for care management, and setting up better electronic communication with other
clinics. Over the course of CPC, practices have worked to standardize and ensure consistent data
entry into structured fields in the EHR to facilitate
reporting clinical quality measures and to guide QI. To
better meet CPC Milestones, a few practices also reported
making significant EHR investments, including engaging
EHR vendors for software support. A couple of practices
were holding off on further EHR improvements related to
CPC until the practice implemented a new EHR planned
for the coming year.
b.2. Data sharing
Reflecting the state of health information exchange in the United States, there is still room
for improvement in how data are shared between CPC practices and other providers. Within both
CPC and comparison practices, data sharing—defined as either sharing read-only data or
importing or exchanging data—was more common among providers located within the same
system. Among practices that are in a health care system or group, when sharing data with
entities outside of their health care system, CPC practices were more likely than comparison
practices to report sharing data with local hospitals, other practices, and diagnostic service
facilities (Figure 5.6). System-affiliated CPC practices were also more likely than system-
affiliated comparison practices to report sharing data within their health care system with local
hospitals, other practices, and diagnostic facilities.
“If you don’t get a Cadillac-y EHR
system, life is very difficult…if you
don’t spend the money [on the]
system, it just makes life very
challenging for reporting, and for
documenting.”
–Practice manager
131
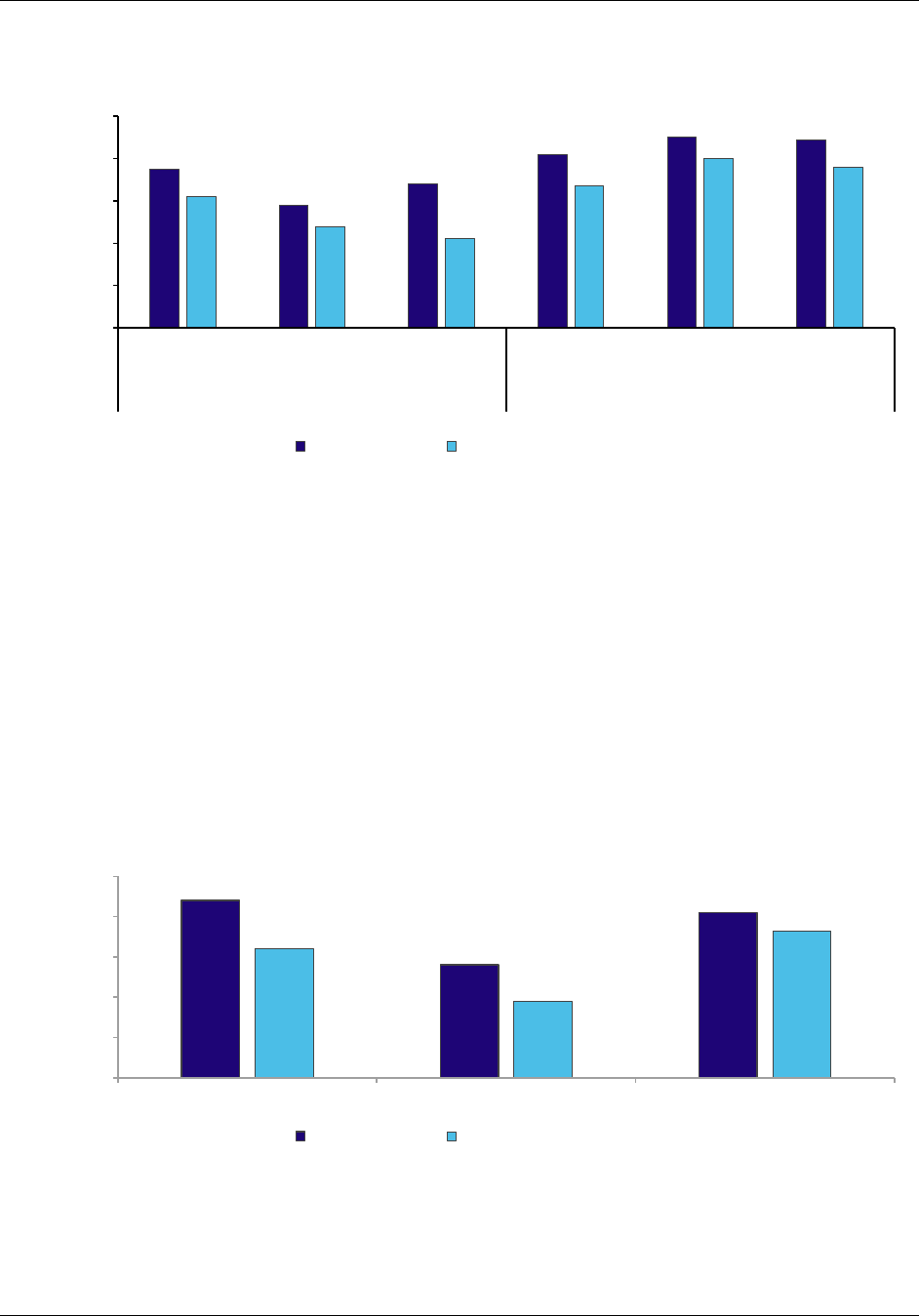
5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Figure 5.6. Data sharing by practices that are in a health care system or
group
Source: CPC practice surveys administered April through August 2016.
Note: We identified each practice as being part of a system or not, using the practice’s responses to the 2016
CPC practice survey. When asked to describe the medical organization that employs the clinicians at the
practice site, or who owns the practice, we considered practices that responded with these responses to be
in a healthcare system: group or staff model Health Maintenance Organization (HMO); network of clinician
practices owned by a hospital, hospital system, or medical school; or hospital or medical school.
Among practices that are not part of a system, a higher proportion of CPC practices than
comparison practices shared data with other providers including local hospitals, diagnostic
service facilities, and other practices. These findings across both system-affiliated and
independent CPC and comparison practices have remained relatively consistent in practice
surveys conducted in 2014, 2015, and 2016.
Figure 5.7. Data sharing by practices that are not part of a system
Source: CPC practice surveys administered April through August 2016.
75
58
68
82
90
89
62
48
42
67
80
76
0
20
40
60
80
100
Local hospitals Practices Diagnostic
service facilities
Local hospitals Practices Diagnostic
service facilities
Outside the system Inside the system
Percentage of practices
sharing data
CPC practices
Comparison practices
Outside the system Inside the system
88
56
82
64
38
73
0
20
40
60
80
100
Local hospitals Practices Diagnostic service facilities
Percentage of practices
sharing data
CPC practices Comparison practices
132

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Deep-dive practices continued to report mixed experiences with health information
exchanges (HIEs).
60
In 2016, respondents in a couple of practices expressed disappointment
with HIEs. One practice manager was frustrated with lack of progress on the statewide HIE in
their state and the practice’s lack of power to influence HIE development despite being a test
clinic for the exchange. Clinicians in a couple of practices
also found their state’s HIE was providing less access to
patient information than they were able to obtain via direct
connections between the practices’ EHRs and those of
other practices and hospitals. On the other hand,
respondents in a few practices in another region were
pleased their HIE had recently started providing reliable
access to hospital and ED records for their patients,
though these practices continued to lack sufficient access
to specialists’ consultation notes and notes from skilled
nursing facilities through the HIE.
These findings are consistent with deep-dive findings from previous years, in which staff in
several practices reported that their electronic exchange of patient information was limited to
affiliated hospitals or specialists (in system-owned practices), was missing key information from
certain specialists or hospitals (in independent practices), or relied on haphazard information
sharing by other providers. This limited and incomplete information exchange made it difficult to
track and manage the care of high-risk patients by requiring follow-up time to obtain the
information, and raising the possibility that important problems were being missed. In several
deep-dive practices that had the capability for exchanging information with both affiliated and
independent providers, electronic exchange with hospitals and EDs was more common than with
specialists. The few deep-dive practices in which staff reported that they could easily exchange
information with a variety of specialists were in local areas that reportedly had robust local HIE
organizations. Several other practices continued to rely on manual workarounds to track
hospitalizations, ED visits, and specialist referrals.
5.5. Monitoring of adequate Milestone achievement
In PY2016, CMS and RLF continued to assess CPC practices’ progress based on quarterly
Milestone submissions through the CPC web application. CMS assigned a corrective action plan
(CAP) to practices that did not meet Milestone requirements. As in PY2015, CMS continued to
partially automate the process of assessing each practice’s Milestone performance to identify
practices in need of either enhanced learning support or corrective action. Specifically, CMS
analyzed Milestone data and generated a “flag report” with color coding to identify practices
with Milestone deficiencies. Practices that received red flags were referred to CMS region leads
for further review. The region leads used a Milestone review guide (developed by CMS) to
further assess practices and determine whether they should receive a CAP. If a CMS region lead
recommended that a practice receive a CAP, a clinical reviewer from CMS who was involved
60
Fifty-nine percent of CPC practices reported in the 2016 practice survey that they had some form of access to
state or regional HIEs.
“I’m just frustrated trying to figure
out what to do with …the state
technology that’s supposed to
make everything talk to each
other…. And we’ve been signed up
for it forever, for three years or so,
and nothing seems to happen, and
I think our hospital is dropping out
of it.”
–Practice manager
133

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
with the CPC initiative conducted a second-level review using the guide and his/her clinical
practice knowledge. This review ensured the review process was fair.
5.5.1. Practices that received CAPs for PY2016 Q1–Q3 performance
Less than 10 percent of practices received CAPs in 2016, and nearly all of the practices that
received CAPs were identified for deficiencies in only one Milestone. Forty of the 446 practices
participating in CPC in 2016 received CAPs based on their PY2016 performance, and 3 practices
from Arkansas, Colorado, and Oklahoma were placed on a CAP twice during 2016 (Table 5.23).
Twenty-one of these 40 practices had received a CAP for their Milestone performance in
PY2014 or PY2015.
As in PY2015, in PY2016, CMS gave practices two quarters to correct their deficiency. The
one practice that received a CAP in the third quarter of PY2016 was urged to work with its RLF
to remediate the CAP by the end of the initiative. CMS did not issue CAPs in the last quarter of
2016 because the initiative ended in December 2016.
Table 5.23. Number of practices placed on a CAP for PY2016 performance
Total number of practices placed on a CAP
for PY2016, based on prior quarter’s Milestone performance
PY 2016 quarter in which
CAP was issued
a
CPC-wide AR CO NJ NY OH/KY OK OR
Quarter 1 (Jan–March) 30 9 3 1 2 9 4 2
Quarter 2 (April–June) 12 1 3 1 1 2 4 0
Quarter 3 (July-Sept) 1 1 0 0 0 0 0 0
Total for PY2016 43 11 6 2 3 11 8 2
Source: CAP data provided by TMF Health Quality Institute.
a
Practices that received a CAP for PY2016 Q1 or Q2 work were expected to remediate by PY2016 Q3 and PY2016
Q4, respectively. Practices that received a CAP in PY2016 Q3 were urged to reach out to their RLF for assistance
given that they were near end of the initiative. No CAPs were issued in PY2016 Q4 due to the initiative ending on
December 31, 2016.
CAP = corrective action plan.
Of the 43 total CAPs sent to practices in PY2016, one practice received a CAP for
deficiencies in two Milestone areas, and two practices received extensions on an existing CAP.
Milestones 2, 5, and 6 were the most challenging areas for practices that received CAPs (Table
5.24). Only one practice was notified of a deficiency for Milestone 3: Access and Continuity. No
practices were notified of deficiencies for Milestones 1, 4, 7, 8, or 9.
Table 5.24. Percentages of the 40 practices receiving CAPs by Milestone
Milestone 2: Care management for high-risk patients 40%
Milestone 5: Quality Improvement 28%
Milestone 6: Care coordination across the medical neighborhood 30%
CAP = corrective action plan.
134
5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
5.5.2. Patient dismissal
In previous annual reports, we noted that some deep-dive practices had raised the possibility
that an unintended consequence of CPC’s focus on improving patient outcomes might lead other
practices to dismiss patients with poor outcomes. Based on questions we added to the practice
survey, we found that CPC practices and comparison practices dismissed patients rarely, at
similar rates, and generally for similar reasons. Thus, participation in CPC did not make
practices more likely to dismiss patients. According to most CPC practices, the initiative had no
effect or made them less likely to dismiss patients (O’Malley et al. 2017).
5.6. Facilitators of and barriers to implementing changes in care delivery
across Milestones and implications for other care delivery initiatives
In this section, we note facilitators and barriers that influenced implementation across
multiple Milestones and may have influenced CPC implementation success more broadly.
Barriers and facilitators related to individual Milestones are discussed earlier in this chapter
under the specific Milestones. We then discuss implications of each major finding on cross-
cutting barriers and facilitators for other initiatives that transform care delivery. We organize this
discussion into the four CFIR domains: (1) characteristics of the CPC initiative, (2) CPC
practices’ structure and characteristics, (3) practices’ strategies to implement CPC, and (4)
factors external to CPC practices. In Table 5.25, we summarize these findings and those from the
Milestone sections, to identify the individual Milestones related to each barrier or facilitator.
5.6.1. Characteristics of the CPC initiative
Across deep-dive practices, respondents reported that participation throughout the
CPC initiative was burdensome, due to the volume and complexity of integrating the
numerous required changes into practice workflows. In
addition to feeling burdened by individual Milestone
activities, several respondents reported that they had overall
change fatigue. Some respondents perceived that CPC
required too many changes at one time and suggested that
their experience with CPC implementation would have been
better if they had focused on one Milestone at a time.
Given that CPC practices struggled with implementing multiple Milestones simultaneously,
other care delivery transformation initiatives could provide a roadmap with suggestions for initial
steps, sequencing, and timing, such as the implementation guide CMS subsequently developed
for CPC+.
Combined with technical assistance and peer-to-peer learning, a roadmap could help
practices see the steps needed to implement changes to their workflows.
Deep-dive clinicians and staff perceived that CPC improved the quality, delivery, and
organization of patient care, and this perception facilitated implementation. These
perceptions were a direct result of practices seeing the impact of their work on the Milestones,
particularly Milestones 2, 6, and, to some extent, Milestone 7. For example, in 2016, respondents
described how risk-stratification helped practices better allocate staffing resources to meet the
needs of different patient populations. Practices noted that CPC helped them track attributed
patients, identify gaps in care, and better meet patients’ needs. Similar to previous years,
“CPC tried to fix everything in
one program, rather than pick
one high-value target area,
start it, assess it, and then
build from there.”
–Lead clinician
135

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
respondents noted the value of care management and behavioral health integration for improving
the support for high-risk patients and reducing the burden on clinicians in addressing their social
needs. Respondents also perceived many positive effects of care transition activities, most
notably increased access to patients’ hospital discharge records, reduced hospitalizations, and
increased quality of care. Care coordination activities helped practices address patient
misunderstandings and medication discrepancies upon hospital discharge and prevented future
problems. Respondents in several practices perceived that SDM positively affected the quality of
patient care because of the emphasis on understanding patients’ preferences and basing treatment
decisions on patients’ goals and lifestyles.
These deep-dive practice perceptions of benefits to patient care resulting from CPC
Milestones are supported by clinician and staff survey results. Nearly four years after CPC
startup, the initiative was highly rated and recommended by a large proportion of participating
clinicians, care managers, and other staff. CPC practice members had largely positive views
about their experiences in CPC. The survey asked about the importance of 13 changes promoted
by CPC in improving the care the respondent provides to patients. At least 80 percent of
respondents rated each category as somewhat important or very important to improving care. For
example, 81 percent of CPC physicians thought the initiative’s focus on continuity of care and
planning for chronic and preventive care needs were very important to improving patient care.
This widespread acceptance of the value of the CPC initiative and the perceived quality of its
design and ongoing implementation, likely facilitated practice implementation of the large
number of challenging CPC requirements. Future primary care transformation initiatives should
continue to focus on encouraging changes that align closely with the values and beliefs of
primary care clinicians and staff.
5.6.2. CPC practices’ structure and characteristics
Differences in resources and autonomy between small independent practices and large
or system-owned practices were a common theme throughout CPC and will likely be a
factor in other initiatives. Large practices and those that are part of systems are more likely to
benefit from economies of scale, have greater data analytics capabilities and QI resources, and
have more leverage in negotiating with health IT vendors to secure the functionality needed to
support practice transformation goals. At the same time, practices that were part of large health
systems typically lacked autonomy at their practice site for hiring staff and changing care
delivery. This meant that these practices could rely on standardized approaches to make complex
changes such as centralized training and management of care managers, but often had little
opportunity to customize these approaches to their local settings. In contrast, small independent
practices often had greater autonomy to make changes tailored to their local environment, such
as focusing SDM approaches on problems common in their patient population, and were better
able to make improvements quickly based on patient feedback, but often struggled with more
complex and technical requirements (such as using health IT to document care plans). In the
future, initiatives might increase the supports for small independent practices to implement
change. Large health systems might work more closely with their practices to seek input from
providers on how to roll out changes.
136

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Respondents in deep-dive practices reported that EHR technology continued to present
challenges for supporting CPC-related work. Many EHRs used by these practices continued
to have limited support for care planning and documenting SDM discussions and patients’ care
preferences. However, EHRs facilitated some CPC work. QI data, often generated through a
practice’s EHR, allowed some practices to regularly monitor QI progress and identify new areas
for QI work. In addition, practices with electronic access to hospital and ED records were able to
identify patients and follow up with them in a timely manner.
5.6.3. Practices’ strategies to implement CPC
To engage staff in CPC implementation, some practices held routine meetings,
appointed key staff (including physicians in some cases) as champions or change agents,
organized care teams, and presented and reviewed data with staff. Having practice leaders
review Milestones with clinicians and staff reinforced the clinical importance of each Milestone.
In future practice transformation efforts, it will likely be important for practices to have meetings
to discuss practice change and QI, have effective and empowered change champions, use
teamwork to transform care, and ensure that practice staff understand how their respective roles
and responsibilities are changing.
Establishing appropriate care manager workloads and clearly defining care manager
roles and responsibilities were challenges in CPC. Care managers often seemed to bear most
of the burden associated with CPC’s most demanding Milestones (Milestone 2: risk-stratified
care management and Milestone 6: care coordination across the medical neighborhood). As a
result, care managers reported that they felt overwhelmed with multiple responsibilities outside
of care management, large caseloads of high-risk patients, and the need to focus on patients with
rapidly rising risk. Using team-based care approaches enabled other team members to take on
specific tasks to reduce care managers’ burdens.
In future practice transformation efforts, practices will likely need outside support to refine
care management roles and to manage the expectations of other practice members for care
manager activities. This will be particularly important for practices that have not previously
worked with care managers. In addition, integrating care managers into the care team will require
continued efforts to gain clinicians’ buy-in to the care manager role and an emphasis on the
importance of clinicians introducing patients to care managers in person. These activities will
help effectively integrate this new service into primary care practices while avoiding
overwhelming care managers with other tasks. In some practices, this will require investing more
resources in care manager training, support, and staffing.
5.6.4. Factors external to CPC practices
Across deep-dive practices, respondents noted they had difficulties in engaging some
patients in CPC efforts. Deep-dive respondents reported that some patients lacked interest in
modifying behavior, adhering to treatment recommendations, or setting health goals, resulting in
barriers to successful care management. Other patients reportedly were not responsive to
educational efforts on appropriate use of the ED, and continued to use the ED for issues more
appropriate for practice-based care. In addition, some patients reportedly denied health issues or
wanted clinicians to make decisions for them, which impeded efforts to engage them in SDM.
Respondents noted challenges enlisting a representative sample of patients to fill out patient
137

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
surveys. Finally, respondents faced challenges engaging patients in PFACs, particularly because
(1) scheduling meetings when a diverse mix of patients could attend was difficult, and (2)
patients sometimes doubted their participation would influence practice operations.
Despite patient engagement challenges, deep-dive practice respondents reported they
increased patient engagement through education, sharing information, and building trust
over time. Practices engaged patients by delivering patient education during care transition
phone calls, mailing written materials about inappropriate ED use, and inviting some patients to
participate in PFACs. Clinicians helped engage patients in their care by using “warm hand-offs”
(with the clinician introducing the patient to the care manager) to help establish a relationship
between the care manager and the patient. As care managers and patients interacted and
discussed the patients’ needs, many established trusting relationships.
Providers in future care transformation initiatives will likely struggle to engage some
patients in modifying unhealthy behaviors, adhering to agreed-upon treatment recommendations,
engaging with care managers, using the ED only for emergency situations, and appropriately
using patient portals. Addressing these challenges will require support to help practices motivate
patient behavior change, promote enhanced access to after-hours care, provide patient education
campaigns, change workflows in the practice, and refine practices’ phone triage and portals.
Practices will also need to work with EDs on steering non-urgent patients to primary care or to
after-hours settings.
Another patient engagement challenge is recruiting diverse groups of patients to participate
in PFACs, including people who: are working, are retired, have different health concerns, have
time-consuming parenting or caregiving roles, and have a range of incomes and educational
levels. This will require some creative approaches and flexible scheduling. For example,
providers could consider using videoconferencing, Skype, or telephone to enable people who
have difficulty traveling to attend PFAC meetings.
Many independent CPC practices had limited access to health information from other
providers and exchanging information with specialists, hospitals, and community-based
services remains challenging. Practices that are affiliated with systems typically have access to
information from other providers within their system but more limited access to information
outside of the system. Many of the HIE challenges CPC practices faced are likely to affect other
practice transformation initiatives, given the lack of an infrastructure for true health IT
interoperability or effective information exchange across different health IT systems.
Practices faced barriers to using their EHRs to facilitate practice change due to
inadequate functionalities for particular Milestones. Respondents noted that the lack of a
robust functionality for creating care plans and sharing them within and across practices hindered
work on Milestone 2. In addition, inadequate EHR functionalities to support care delivery will
likely continue, and practices that lack sufficient market presence to obtain cooperation from
EHR vendors to design or refine such functionalities will likely continue to face challenges.
Greater involvement of health IT vendors in supporting future care transformation efforts, such
as the approach CMS is testing in the CPC+ model, could help to address these issues.
138

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Practices noted that the current FFS payment incentives posed challenges to delivering
comprehensive primary care in CPC and to improving patient outcomes. Although practices
received upfront care management payments for CPC patients (and, in a few regions, shared
savings), they faced challenges associated with a payment system that largely rewards the
number of visits rather than (1) the efforts of counseling patients, coordinating their care, and
providing comprehensive primary care—including a reduction in and more appropriate use of
specialists, or (2) the outcomes of these efforts. For example, because most clinicians are paid
more if they do more patient visits, practices noted less inclination to devote time to particular
Milestone activities, such as SDM, discussing specific patients with a care manager, or
delivering more comprehensive care. Teamwork approaches can help to mitigate this challenge
by offloading some nonbillable tasks from practitioners to other staff such as nurses and medical
assistants.
Even with greater rewards and increased supports for primary care practices in changing
how they deliver care, the volume-based FFS incentives underlying the behavior of specialists
and hospitals will continue to present a challenge to making primary care more comprehensive
and patient-centered. To deliver more comprehensive primary care, practices would ideally limit
referrals to specialists to unique or particularly complex issues. However, under the current FFS
system, primary care practices have incentives to increase visit volume (and therefore decrease
visit complexity) by referring patients to specialists, and hospital systems reap financial rewards
when patients are cared for by more highly reimbursed specialists rather than managed in the
primary care practice. In addition, Medicare FFS beneficiaries can self-refer to specialists, so
they exercise substantial control over their utilization of services. Thus, high-cost diagnostic
testing and procedures will likely continue to occur outside of the realm of the primary care
practice’s control. Restructuring incentives to other providers and to patients themselves may
help to address these issues.
Collaborating with community-based partners requires primary care practices to
expand their traditional medical model of health care to better integrate community
resources into patient care. CPC funding supported practices in enhancing this capacity.
Practices in other initiatives without experience linking patients to community services will
likely find this work challenging and may need support in this area.
In sum, CPC practices made substantial changes in how they deliver care to their
patients over the course of the initiative. CPC participants perceived that the biggest benefit of
CPC participation was increased capacity to provide care management services to high-risk
patients. Comparison practices also showed improvements, though to a lesser degree than CPC
practices. In particular, Medicare FFS beneficiaries in CPC practices were more likely than
beneficiaries in comparison practices to report timely follow-up after hospital and ED discharge.
There is room for more improvement on each of the CPC functions. Findings on how practices
changed in CPC, as well as about facilitators and barriers to change, can inform future primary
care delivery models.
139

5. HOW ARE CPC PRACTICES CHANGING THE WAY
THEY DELIVER CARE THROUGH WORK ON SPECIFIC MILESTONES? MATHEMATICA POLICY RESEARCH
Practice change is difficult to achieve, even when CPC practices are receiving financial
and other supports, and it takes time for those changes to influence patient outcomes and
health care expenditures. The challenges to practice change were numerous, including limited
bandwidth to fully engage in addressing multiple Milestones simultaneously, inadequate support
for robust care management and health IT implementation in smaller independent practices,
inadequate infrastructure for health information exchange, inadequate ability of current EHRs to
support some of the Milestone activities, and many layers of management in larger system-
owned practices. In addition, all practices faced challenges related to practice, provider, and
patient cultures; long-entrenched behaviors; leadership; teamwork functioning; and external
financial or policy factors beyond their control. Even with change in primary care practice
delivery, the other providers (specialists and hospitals) treating the same patients often did not
share the same incentives to coordinate care and faced volume-based productivity incentives.
Overcoming these challenges to modify workflows and system supports consistently across
providers requires ongoing time, resources, and effort not just from CPC practices and their large
health systems, but also from specialists and hospitals outside of the CPC initiative. Moving
forward, it will be important to also address these external factors, such as poor health
information exchange and current fee-for-service incentives, which affect multiple actors in the
health care system. Attention to these challenges can help maximize the potential benefits of
changes made by primary care practices.
140

141
Table 5.25. Facilitators of, and barriers to, implementation of CPC Milestones for PY2016, as reported by
deep-dive practices
CPC Milestone for PY2016
Milestone 2 Milestone 3 Milestone 4 Milestone 5 Milestone 6 Milestone 7 Milestone 8 Milestone 9
Care
management
Access and
continuity
Patient
experience
Quality
improvement
Care
coordination
Shared
decision
making
Participating in
learning
collaborative Health IT
Characteristics of the CPC initiative
Facilitators
Risk- stratified care management seen by practices
as improving other Milestones
F F F F F F
Improved care via advanced primary care strategies F
Patient input via PFACs F F
Barriers
Changes in staff roles and time required to
implement change
B B B
Limited usefulness of feedback reports B
Numerous required changes across complex care
delivery areas
B B B
Practice structure and characteristics
Facilitators
Communication and teamwork F F F F
System-affiliated practices tended to have support
for health IT, QI, and enhanced access
F F F
Investment of practice resources in Milestone-related
activities
F F F F F F F F
Barriers
Care management staff burdened by multiple tasks B B B
Inadequate EHR functionality to support Milestone
activities
B B B B B
Resistance from staff to integrating care manager
role into practice
B
Practice strategies and implementation processes
Facilitators
Effective role delegation from clinicians F F

Table 5.25 (continued)
142
CPC Milestone for PY2016
Milestone 2 Milestone 3 Milestone 4 Milestone 5 Milestone 6 Milestone 7 Milestone 8 Milestone 9
Care
management
Access and
continuity
Patient
experience
Quality
improvement
Care
coordination
Shared
decision
making
Participating in
learning
collaborative Health IT
Educating patients and building trust over time F F F
Access to implementation supports tailored to the
practice’s needs
F
Barriers
Inadequate technical assistance with EHR data
issues
B B B B B
Difficulty identifying patients to target for SDM B
Lack of developed workflows B B B
External environment and context
Facilitators
Developing or having established relationships with
hospitals and specialists
F F F
Community resources are available F
Barriers
Limited supply of care managers in rural areas B
Duplication of care management services from
outside providers
B B
Lack of electronic access to health information from
other settings
B B
External payment environment B
Limited access to behavioral health providers B
Difficulty engaging specialists in care compacts B
Clinicians lacked training in SDM B
Difficulty engaging patients in Milestone activities B B B B
Note: Facilitators are marked with a green (F) and barriers with a red (B) for each Milestone to which they apply. Some issues (for example, patient receptivity to change or willingness to
engage in activities) can be both facilitators and barriers and may therefore appear in both rows.
This table excludes Milestone 1 which focuses on CPC budgets because we did not ask about it in deep-dive interviews. See Chapter 3 for information on CPC budgeting. The table
marks only barriers or facilitators that the deep-dive practices raised; some of these barriers and facilitators might influence other Milestones among the full set of CPC practices.
EHR = electronic health record; QI = quality improvement; PFAC = patient and family advisory council; SDM = shared decision making.

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
6. HOW DID CPC AFFECT THE EXPERIENCES OF PHYSICIANS, OTHER
CLINICIANS, AND STAFF?
The CPC initiative aimed to transform care delivery and ultimately improve the experience
of physicians, other clinicians, and staff in CPC practices by providing them with more resources
to support the delivery of primary care to their patients. At the same time, practice
transformation efforts like CPC require intensive work, including substantial change to practice
workflows and staffing, shifting from a physician-centric to a team-based culture, and creating
new clinical and administrative tasks. Therefore, there was concern that CPC might add to
physicians’ burden, worsen their experience, and increase job dissatisfaction, at least in the short
run. For example, a qualitative study of practices transitioning to patient-centered medical homes
(PCMHs) in the National Demonstration Project (NDP) found that “…the magnitude of stress
and burden from the unrelenting, continual change required to implement components of the
NDP was immense” (Nutting et al. 2010). Another study found burnout rates among physicians
and staff in practices participating in accountable care organizations (ACOs) and those with
meaningful use certification were 1.2 to 1.3 times higher than physicians and staff outside of
these practices (Edwards et al. 2017).
This chapter examines whether primary care physicians in CPC practices experienced their
work differently from primary care physicians in comparison practices, how other members of
CPC practices experienced their work, and whether experience changed over time. Appendix E
details the survey sampling, fielding, content, and methods and contains tables with the results.
6.1. Key takeaways on the effect of CPC on physician, other clinician, and
staff experience
We obtained survey responses from a sample of roughly 600 physicians in CPC practices
and 500 physicians in comparison practices about 11 months after CPC began and again 44
months into the 51-month initiative. To provide additional perspectives on CPC, we also
collected surveys from about 150 other clinicians (nurse practitioners and physician assistants
[NPs/PAs]) and about 2,000 staff (care managers or care coordinators, medical assistants, nurses,
practice managers or supervisors, and receptionists or appointment clerks).
• Overall, there were no meaningful differences on measures of burnout, control over work,
alignment of work with training, or work satisfaction between physicians in CPC and
comparison practices in 2016, the last year of CPC, or over time among CPC physicians,
NPs/PAs, and staff.
• There was no differential effect of CPC on most measures of physician experience on
physicians whose practices were in a system, were larger (measured by having more primary
care clinicians), or served attributed Medicare beneficiaries with a higher risk score
(measured by the average Hierarchical Condition Category [HCC] score for their practice).
The one exception was that there was a smaller difference in the effect of being in a system
on the percentage of physicians reporting that 75 percent or more of their time is spent doing
work that is well matched to their training among physicians in CPC practices compared to
physicians in comparison practices.
143

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
• Together, these findings suggest that CPC did not meaningfully alter clinician and staff
experience, either favorably or unfavorably, for the overall sample or for key subgroups.
• Although CPC did not have differential effects on physicians in practices that were part of a
system, had different numbers of primary care clinicians, or had higher-risk beneficiaries,
we did find differences among subgroups of physicians when we combined CPC and
comparison physicians for analysis. Specifically, physicians whose practices were part of a
system reported that they had less control over their work, and they spent less time doing
work that was well matched to their training and more time doing work that someone with
less training could do; in addition, they were less likely to report being satisfied with their
current job than physicians whose practices were not part of a system. Physicians in larger
practices reported that they had less control over their work than physicians in solo clinician
practices, and physicians in practices with lower-risk beneficiaries were less likely to report
being satisfied with their current job than physicians in practices with higher-risk
beneficiaries.
• CPC physicians, NPs/PAs, and staff had largely positive views about their experiences
participating in CPC. For example, in 2016, 80 percent of physicians reported that CPC had
improved the quality of care or service provided to their patients, and if they could do it all
over again, 79 percent would support participation in CPC. Only 12 percent of physicians
would oppose participation in CPC and 9 percent reported not knowing enough about CPC
to answer.
• Regardless of whether they would support participation in CPC again, respondents were
asked about reasons to support and oppose participation. Among physicians that would
support their practice’s participation in CPC, the most common reasons for supporting CPC
were: they believed work on CPC Milestones helped practices make positive changes and
improve patient care (81 percent), they valued the opportunity to contribute to primary care
practice transformation (52 percent), and the financial support provided by CPC was
sufficient to support their participation (52 percent). Still, even supporters reported that CPC
administrative reporting was a burden and that the transformation work in CPC was
difficult. Forty-four and 34 percent of physicians that would support their practice’s
participation in CPC again, reported this, respectively. Additionally, about one-third of these
physicians reported inadequate financial support as a reason to oppose CPC participation,
and one-quarter reported inadequate staffing.
• Although the evidence suggests that CPC did not adversely affect physician and staff
experience, future initiatives could nevertheless work with practices to reduce burnout,
improve delegation, and streamline administrative requirements.
6.2. Methods
6.2.1. Survey content and measures
The clinician and staff survey gauges respondent perceptions of and experiences with
various components of care delivery. The survey questions are both specific to the CPC
initiative, such as the usefulness of feedback reports from Medicare and other payers, the
usefulness of CPC supports, and the importance of CPC functions and Milestones in improving
care, and ask about more general components of care delivery, such as practices’ care
management activities, work environment, burnout and satisfaction, and use of electronic health
144

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
records in managing patient care. The findings we present in this chapter focus on five domains:
(1) burnout, (2) control over work, (3) alignment of work with training, (4) satisfaction with
work, and for respondents from CPC practices, (5) ratings of CPC. Table 6.1 lists the survey
questions that we use to evaluate clinician and staff experiences across these domains. In
addition to the results we present here, we discuss additional findings from the clinician and staff
survey in Chapters 3 and 5.
Burnout. Burnout—when workplace stress leads to emotional exhaustion,
depersonalization, and a diminished sense of personal accomplishment, among other negative
effects—is an important outcome to examine as part of the evaluation of CPC, as it has been
linked to lower work satisfaction, disrupted personal relationships, substance abuse, depression,
and suicide among physicians (Maslach et al. 1996, 2001; van Der Heijden et al. 2008; Wurm et
al. 2016; Panagioti et al. 2017). Burnout is prevalent among primary care physicians; the
literature over the past decade indicates that between 20 and 45 percent of primary care
physicians report being burned out (Edwards et al. 2017; Dolan et al. 2015; Helfrich et al. 2014;
Lewis et al. 2012; Reid et al. 2009, 2010). The literature also suggests that burnout is less of a
problem among staff. For example, Edwards et al. (2017) found that 26 percent of physicians
reported high levels of burnout, compared with 21 percent of NPs/PAs and 20 percent of other
clinical staff. Dolan et al. (2015) found that 45 percent of physicians, NPs, and PAs felt burned
out weekly compared with 40 percent of registered nurses (RNs) and 31 percent of clinical
associates. Burnout appears to be on the rise: the percentage of U.S. physicians of any specialty
that reported burnout grew from 46 to 54 percent from 2011 to 2014 (Shanafelt et al. 2015).
There is concern that practice transformation efforts like CPC may worsen provider experience,
at least in the shorter term (Nutting et al. 2010; Edwards et al. 2017).
Physician burnout is typically measured through the use of the Maslach Burnout Inventory
(MBI), a validated and reliable instrument designed to assess burnout by examining 22
questions, grouped into three subscales focused on emotional exhaustion, depersonalization, and
reduced personal accomplishment (Maslach et al. 1996). Subsequent research has demonstrated a
high association between MBI scores and a single-item question taken from the Physician
Worklife Study (PWS)—asking physicians to use their own definition of burnout and rate
burnout using five options ranging from no burnout to complete burnout—giving researchers an
alternative and potentially easier method to study the phenomenon (Rohland et al. 2004).
Another validated single-item measure of burnout (West et al. 2009, 2012) from the MBI asks
respondents how often they felt burned out from their work in the past year (with response
options of never, a few times a year or less, once a month of less, a few times a month, once a
week, a few times a week, and every day).
To measure burnout, the CPC survey used the two single-item measures and 9 of the 22
items in the MBI that another study has used to measure emotional exhaustion,
depersonalization, and personal accomplishment (McManus et al. 2002; see Table 6.1).
Control over work. Several studies have linked higher levels of control over work and
autonomy to higher levels of work satisfaction and lower levels of stress among physicians
(Landon et al. 2003; Linzer et al. 2002, 2009). We examined control over work to identify
whether, taken as a whole, the administrative reporting requirements, new work processes, and in
many cases, new staff associated with participation in CPC altered control over work.
145

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
The survey asked respondents to indicate how much control they have over seven areas of
work: (1) the hours they work, (2) details of the office or practice schedule, (3) the volume of
paperwork they have to do, (4) work interruptions, (5) workplace issues, (6) the pace of their
work, and (7) the allotment of additional time for difficult-to-help patients. Response options
were slight/no control, some control, moderate control, great control, and does not apply or don’t
know.
Alignment of work with training. Alignment of work with training is an important
component of physician satisfaction. We expected it to be affected by the new staffing
arrangements and approaches to teamwork used by many practices to complete the CPC
Milestones. Prior research indicates that physicians whose work content matches their training
are more satisfied than physicians who are doing work that they believe other staff could perform
(Friedberg et al. 2014).
To assess alignment of work with training, the CPC survey asked respondents what
proportion of time each week they typically spend doing (1) work that could be done by someone
with less training, (2) work for which they do not have enough training, and (3) work that is
well-matched to their training. Response options were less than 25 percent of the time, between
25 and 49 percent, between 50 and 74 percent, 75 percent or more, or does not apply or don’t
know. Due to the high proportion of does not apply or don’t know responses, these are the only
measures for which we exclude these responses from results presented here.
Satisfaction with work. Primary care transformation can be rewarding and challenging. To
assess differences in satisfaction between CPC and comparison physicians, and among CPC
clinicians and staff, the survey included two questions to assess general job satisfaction among
respondents: (1) how much the respondent agrees or disagrees that he or she is satisfied with his
or her current job (response options were strongly disagree, disagree, neither disagree or agree,
agree, and strongly agree), and (2) the likelihood that he or she will leave his or her current
practice within two years (response options were none, slight, moderate, likely, and definitely)
and his or her primary reason for leaving.
Ratings of CPC. In the 2016 survey, we asked clinicians and staff in CPC practices to
reflect on their experience participating in the CPC initiative. We asked them how their
participation in the initiative changed the quality of care or service that they provide to their
patients (with response options of improved a lot, improved somewhat, did not change, worsened
a lot, and don’t know); and knowing what they know now, would they support their practice’s
participation in CPC again (with response options of strongly support, somewhat support,
somewhat oppose, strongly oppose, and don’t know enough about CPC to answer). Regardless of
whether they would support participation in CPC again, we then asked their main reasons to
support participation and their main reasons to oppose participation. We asked respondents to
select all response options that apply. We provided the following response options for main
reasons to support participation in CPC:
• Work on CPC Milestones helps practice make positive changes and improve patient care
• Work on CPC Milestones improves clinician and staff work satisfaction
• Financial support provided in CPC is sufficient to support participation
146

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
• Learning support and activities provided in CPC are useful
• Learning support provided in CPC improves clinician and staff skill development
• Data/feedback reports provided in CPC are useful
• Opportunity to contribute to field of primary care practice transformation
• Other
• No reasons to support participation in CPC
The survey provided the following response options for the main reasons to oppose
participation in CPC:
• CPC does not allow the practice to join an accountable care organization (ACO)
• Reporting requirements in CPC are too burdensome
• Work involved in implementing the CPC Milestones is too burdensome
• Financial support provided in CPC is insufficient to support participation
• Insufficient practice staffing to participate in CPC
• CPC does not substantially improve patient care
• Other
• No reasons to oppose participation
Table 6.1. Questions and domains included in this chapter
Topics
Questions included
(Round 2 survey instrument numbering)
Burnout
a
E6: Using your own definition of burnout, please indicate which statement best describes your situation at
work
E5j: How often respondent feels burned out from work
Maslach Burnout Inventory (MBI) subscales
b
Emotional exhaustion
E5c*: How often respondent feels emotionally drained from work
E5d*: How often respondent feels fatigued from facing another day on the job
E5g*: How often respondent feels working with people all day is a strain
Depersonalization
E5b*: How often respondent feels he/she treats some patients as if they were impersonal objects
E5e*: How often respondent has become more callous toward people since taking the job
E5h*: How often respondent doesn’t care what happens to some patients
Personal accomplishment
E5a: How often respondent deals effectively with patients’ problems
E5f: How often respondent feels he/she is positively influencing others’ lives through work
E5i: How often respondent feels exhilarated after working closely with patients
147

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Table 6.1 (continued)
Topics
Questions included
(Round 2 survey instrument numbering)
Control over work
c
E3a: The amount of control the respondent has over the hours he/she works
E3b: The amount of control the respondent has over details of the office or his/her practice schedule
E3c: The amount of control the respondent has over the volume of paperwork he/she has to do
E3d: The amount of control the respondent has over work interruptions
E3e: The amount of control the respondent has over workplace issues
E3f: The amount of control the respondent has over the pace of his/her work
E3g: The amount of control the respondent has over the allotment of additional time for difficult-to-help
patients
Alignment of work with training
d
E2a: The proportion of time each week spent doing work that could be done by someone with less training
E2b: The proportion of time each week spent doing work for which the respondent does not have enough
training
E2c: The proportion of time each week spent doing work that is well-matched to the respondent’s training
Satisfaction with work
e
E4: Overall satisfaction with current job
E7: Likelihood that respondent will leave his/her current practice within two years
Ratings of CPC (Questions asked in 2016 and only to CPC clinicians and staff)
H6: How much participation in the CPC initiative changed the quality of care or service that the respondent
provides to his or her patients
H7: Knowing what you know now, how much you would support or oppose your practice’s participation in the
CPC initiative
H8: Main reasons respondent would support participation in the CPC initiative
H9: Main reasons respondent would oppose participation in the CPC initiative
* Responses to these questions were reverse-coded when we constructed the composite measures, so the most
favorable response received the largest value.
a
The first single item was taken from the Federally Qualified Health Center Advanced Primary Practice Provider and
Staff Survey developed by the RAND Corporation (RAND 2013). The second item is 1 of the 22 items in the MBI
(Maslach et al. 1996), and validated as a single-item measure of burnout by West et al. 2009.
b
The MBI contains 22 items divided into the three subscales (Maslach et al. 1996). We use an abbreviated version of
the subscales containing the 9 items used by McManus et al. (2002) in an evaluation of the Patient Aligned Care
Team (PACT) Personnel Survey.
c
The seven items in the control-over-work composite measure are taken from a modified version of the Agency for
Healthcare Research and Quality (AHRQ) Minimizing Errors and Maximizing Outcomes (MEMO) survey (Linzer et al.
2005).
d
The three items used to measure alignment of work with training were taken from the Veterans Administration
PACT National Evaluation Personnel survey (Healthcare Analysis & Information Group 2012).
e
The two items are taken from the Federally Qualified Health Center Advanced Primary Practice Provider and Staff
Survey developed by the RAND Corporation (RAND 2013).
6.2.3. Survey administration
We administered two rounds of the CPC clinician and staff surveys by mail. We sent the
first survey about one year into CPC (September 2013 through March 2014, or 11 to 17 months
into CPC). We sent the second survey toward the end of the 51-month initiative (June through
November 2016, or 44 to 50 months into CPC).
As an incentive to complete the 15- to 25-minute survey, we enclosed a $100 check in the
initial mailing for the surveys of clinicians (physicians, nurse practitioners, and physician
148

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
assistants) in CPC and comparison practices, and a $20 check for the survey of staff in CPC
practices. In the second round, we increased the staff incentive to $25.
6.2.4. Survey sample and response rates
We administered the surveys to samples of physicians and NPs/PAs in the CPC practices
and in comparison practices that we selected using propensity score matching to have similar
market-, practice-, and patient-level characteristics before CPC began. (See Appendix H for a
description of comparison group selection.) The physicians and NPs/PAs that we surveyed were
drawn as longitudinal samples with replacement. Response rates were high in both rounds of
data collection. Eighty-one percent of sampled physicians from CPC practices responded to the
first round of the survey in 2013, and 76 percent responded to the second round of the survey in
2016. We received surveys from physicians in 432 CPC practices in 2013 and 412 CPC practices
in 2016. The corresponding response rates among sampled physicians in comparison practices
were 70 and 72 percent, representing 330 and 349 practices, respectively. Eighty-five percent of
sampled CPC NPs/PAs responded to the first round of the survey, and 83 percent responded to
the second round; 66 and 73 percent of sampled comparison NPs/PAs responded to the survey,
depending on the round. By design, some physicians and NPs/PAs responded to both rounds.
Forty-eight percent of CPC physician respondents and 42 percent of comparison physician
respondents completed both surveys, and 38 percent of NP/PA respondents in CPC practices and
31 percent of NP/PA respondents in comparison practices responded to both rounds. Table 6.2
reports the population in the practices, the number we surveyed, and the number who responded,
by respondent type.
In both rounds, we also surveyed cross-sectional samples of other staff in CPC practices
including care managers or care coordinators, medical assistants, nurses, practice managers or
supervisors, and receptionists or appointment clerks. Between 73 and 85 percent of these staff
types responded to the survey, depending on the round.
Table 6.2. Sample sizes and weighted survey response rates for the primary
care clinician and practice staff surveys, by round
Sample type
Round 1 (2013) Round 2 (2016)
Population size
Sample size
Number
responded
Response rate
(percentage)
a
Population size
Sample size
Number
responded
Response rate
(percentage)
a
Clinicians
Primary care physicians 4,944 1,581 1,082 75 4,658 1,700 1,124 74
CPC practices 1,831 867 635 81 1,677 912 630 76
Comparison practices 3,113 714 447 70 2,981 788 494 72
NPs/PAs 1,198 410 255 72 1,620 405 262 76
CPC practices 421 226 151 85 527 222 159 83
Comparison practices 777 184 104 66 1,093 183 103 73
149

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Table 6.2 (continued)
Sample type
Round 1 (2013) Round 2 (2016)
Population size
Sample size
Number
responded
Response rate
(percentage)
a
Population size
Sample size
Number
responded
Response rate
(percentage)
a
CPC practice staff
Care manager/
care coordinator 104 104 63 82 274 274 208 85
Medical assistant 1,889 927 525 74 2,080 848 572 76
Nurse 1,059 548 325 75 1,320 567 419 83
Practice manager/
supervisor 559 397 271 81 552 370 276 78
Receptionist/
appointment clerk 1,638 838 490 73 1,722 767 538 78
Source: The population of primary care physicians and NPs/PAs came from SK&A, a health care data vendor, in
June 2013 for Round 1 and March and April 2016 for Round 2. CPC practices provided a list of the names
and job titles of staff in October 2012 for Round 1 and February and March 2016 for Round 2 from which
we determined the population of each staff type. The numbers of staff are based on the person’s job title.
For example, only those with explicit care manager or care coordinator job titles were classified as such. A
licensed practical nurse functioning as a care manager would not be classified as such if the job title were
listed as licensed practical nurse.
a
Response rates were weighted using the sample design weights. Ineligible cases are excluded.
6.2.5. Analysis
Estimation. For each survey question, we estimated what the average survey responses
would have been in the population for each respondent type and survey round. We did so by
adjusting for the probability of selection into the sample, comparison group selection, and survey
nonresponse. In addition to calculating responses to individual questions, we created summary
composite scores for two of the five domains discussed in this chapter: burnout and control over
work. Because most respondents completed most questions, we calculated results among
nonmissing responses and did not adjust for question nonresponse.
61
For each question and
composite score, separately for each round, we statistically compared the responses of CPC
physicians with those of comparison physicians to identify where CPC may be affecting
physician experience. We clustered standard errors by practice for all CPC respondents and by
matched set for comparison respondents to account for clustering of responses within a practice
or matched set and for respondents answering in more than one round. Given the similar
characteristics of the CPC and comparison physicians after weighting, we did not regression-
adjust the results (see Appendix E, Table E.4 for the distribution of physician characteristics for
CPC and comparison practice respondents by round). We also report results for NPs/PAs and
staff in CPC practices.
61
The rate of question nonresponse among survey respondents varied from 1 to 6 percent, with 75 percent of
questions having lower than 5 percent nonresponse.
150

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Analytic comparisons. In this chapter, we focus on differences in responses between
primary care physicians in CPC and comparison practices in the second round of the survey, in
2016, as an indication of the influence of CPC. Because we were unable to collect data at the
start of the initiative, differences we see in 2016 may reflect pre-existing differences between
respondents in the CPC and comparison practices. Related to this absence of baseline data, we
did not calculate difference-in-differences estimates; because CPC practices may have already
begun to change by the time of the first survey in 2013 (11 to 17 months into the initiative), we
do not have a true baseline. We do note changes in responses between the 2013 and 2016
surveys. To limit the chances of false positives from multiple comparisons, we did not test the
statistical significance of differences in responses across respondent types or over time.
We do not discuss differences in responses of NPs and PAs in CPC versus comparison
practices because a higher proportion of CPC practices than comparison practices had an NP or
PA (about 25 percent of CPC practices versus 11 percent of comparison practices in each survey
round); this difference raises the possibility that the NPs/PAs play different roles in CPC and
comparison practices.
Subgroup effects. We also examined responses for CPC and comparison group physicians
for select questions in each of the five sets of outcomes for three key subgroups (see Appendix E
for more information):
• Whether the physician is part of a system (from 2016 data from SK&A, a healthcare vendor)
• Size of the physician’s practice (measured by the number of primary care clinicians in the
practice in 2012). We separated practices into four groups: practices with one clinician, two
to three clinicians, four to five clinicians, and six or more clinicians. We statistically tested
differences in responses between physicians in practices with one clinician and physicians in
practices with six or more clinicians; we show findings for each of the four groups in
Appendix E.
• Whether the average Hierarchical Condition Category (HCC) score of Medicare FFS
beneficiaries in the physician’s practice is above or below the median for all practices in the
sample (using patients’ 2012 HCC score)
62
We first examined whether the responses differ between physicians in and not in the
subgroup, using the combined CPC and comparison physicians. We then estimated whether CPC
had a differential effect on physicians in the subgroups.
To test for subgroup effects, we used logistic regressions for binary outcomes and OLS
regressions for other outcomes. We first estimated a regression on each outcome with the CPC
and comparison physicians combined, with a binary indicator for whether the physician’s
practice was in the subgroup of interest. We examined whether the coefficient on the subgroup
indicator for the CPC and comparison physicians combined was statistically significant to
62
HCC scores are a measure of risk for subsequent expenditures. CMS calculates them such that the average for the
Medicare FFS population nationally is 1.0. A patient with a risk score of 1.30 is predicted to have expenditures that
would be approximately 30 percent above the average, whereas a patient with a risk score of 0.70 is expected to
have expenditures that would be approximately 30 percent below the average. See Pope et al. (2004) for details on
the construction of HCC scores.
151

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
determine whether there were different responses by subgroup. We then estimated regressions on
each outcome with three explanatory variables: (1) a binary indicator for treatment (CPC group)
status, (2) a binary indicator for whether the physician’s practice is in the subgroup, and (3) a
term interacting treatment and subgroup status. We examined whether the coefficient on the
treatment (CPC group)-subgroup interactor was statistically significant to determine whether
CPC had a differential effect for members of the subgroup. (We did not test this finding for the
two measures for ratings of CPC, which were asked only of physicians in CPC practices.)
Power. Using two-tailed tests at the 10 percent significance level, the analysis has 80
percent power to detect differences between CPC and comparison physician responses of 5 to 11
percentage points for the categorical variables and 0.06 points out of one for the control-over-
work composite measure.
Statistical and substantive significance. Because CPC-comparison differences would have
to be fairly large for us to be confident that they were statistically significant, we considered
responses between physicians in CPC and comparison practices to be of substantial importance if
the difference between the two groups was larger than five percentage points. We also tested
whether each difference was statistically significant at the 0.10 level.
6.3. Findings
6.3.1. Burnout
CPC survey results suggest that burnout is an issue for physicians and NPs/PAs in both CPC and
comparison practices (and to a lesser extent for staff) but that CPC did not affect burnout.
Physicians. Burnout was comparable for CPC and comparison physicians for the various
survey items measuring burnout.
• When asked to select one of five statements that best describes the amount of burnout they
have at work, ranging from “I enjoy my work. I have no symptoms of burnout,” to “I feel
completely burned out and often wonder if I can go on,” a comparable one-third of
physicians in CPC practices and comparison practices reported high levels of burnout
(Figure 6.1). This finding falls in the middle of the range reported in the literature, which
indicates that between 20 and 45 percent of primary care physicians report being burned out
(Edwards et al. 2017; Dolan et al. 2015; Helfrich et al. 2014; Lewis et al. 2012; Reid et al.
2009, 2010).
152
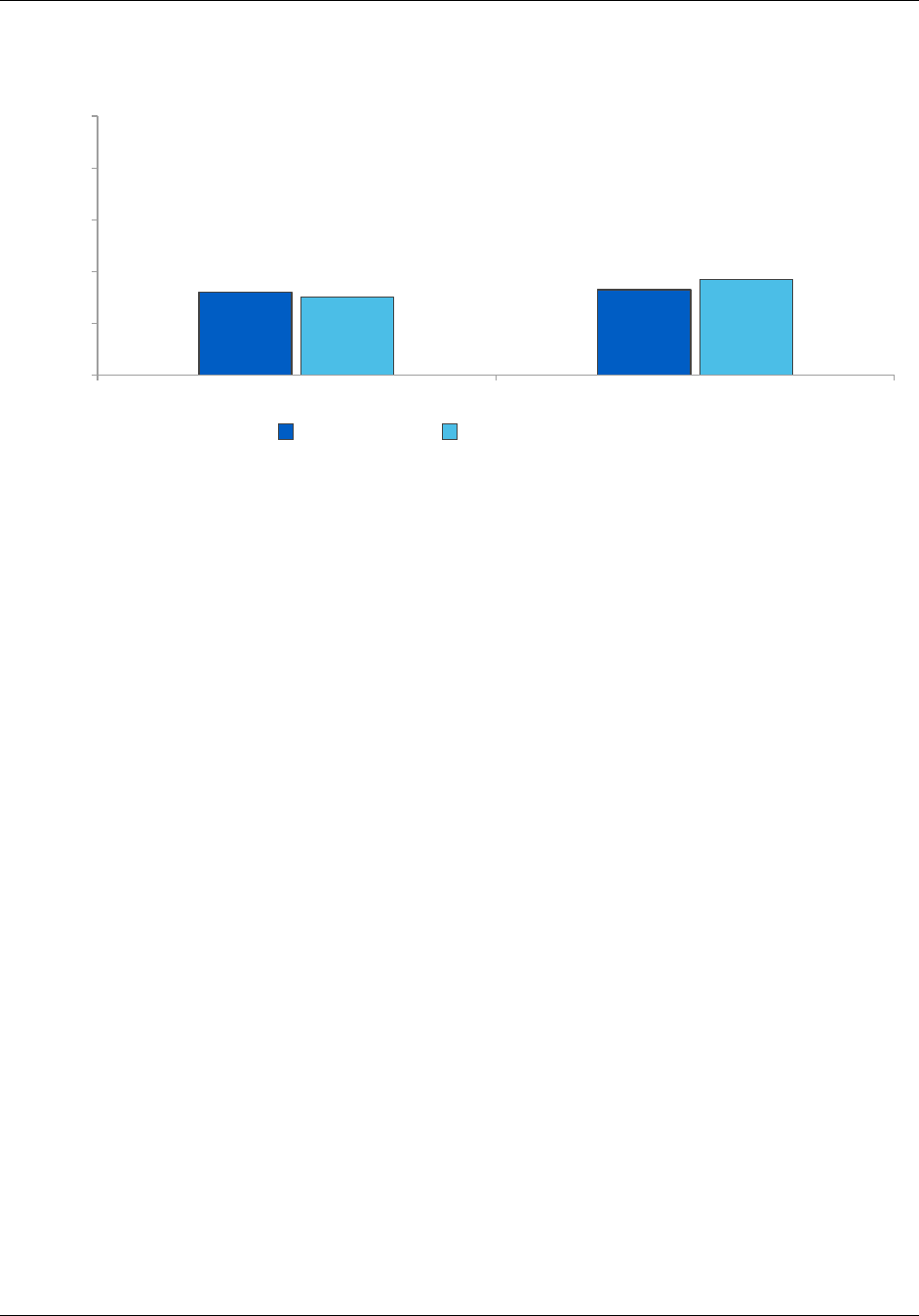
6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Figure 6.1. Percentage of physicians reporting high levels of burnout, CPC
and comparison practices, 2013 and 2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician surveys.
Note: Respondents were asked to, using their own definition of burnout, select one of five statements that best
describes their burnout at work. Following the literature, we define high levels of burnout as having one of
the following three responses: (1) I am definitely burning out and have one or more symptoms of burnout,
such as physical and emotional exhaustion; (2) the symptoms of burnout that I’m experiencing won’t go
away, and I think about frustrations at work a lot; and (3) I feel completely burned out, often wonder if I can
go on, and am at the point where I may need some changes or may need to seek some sort of help
(Rohland et al. 2004).
Response distributions for these questions were not statistically significantly different between CPC and
comparison physicians at the p < 0.10 level.
• When asked how often they felt burned out from their work in the past year, 27 percent of
CPC physicians and 33 percent of comparison physicians reported feeling burned out once a
week or more in 2016, and another 16 percent of CPC physicians and 15 percent of
comparison physicians reported feeling burned out a few times a month (Figure 6.2). Other
published studies have found higher reports of weekly burnout among primary care
providers; for example, Dolan et al. (2015) found that 45 percent of primary care providers
at Veterans Administration clinics (a group that included both physicians and NPs/PAs)
reported feeling burned out weekly.
32
33
30
37
0
20
40
60
80
100
2013 2016
Percentage of respondents
CPC physicians
Comparison physicians
153

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Figure 6.2. Percentage of physicians reporting how often they felt burned out
from their work in the past year, CPC and comparison practices, 2013 and
2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician surveys.
Notes: Respondents were asked how often they felt burned out from their work in the past year, a validated single-
item measure of burnout (West et al. 2009, 2012).
Response distributions for these questions were not statistically significantly different between CPC and
comparison physicians at the p < 0.10 level.
• Responses to the nine questions the survey included from three MBI subscales indicate
similar findings—levels of burnout were generally similar for CPC and comparison
physicians in 2016. There was one exception: on a question in the personal accomplishment
composite measure, a higher proportion of physicians in CPC practices than in comparison
practices reported feeling exhilarated after working closely with patients weekly or more (74
percent versus 65 percent) (Figure 6.3).
• CPC and comparison practice physicians more often reported weekly or more frequent
symptoms of emotional exhaustion than depersonalization or lack of personal
accomplishment (Figures 6.3 and 6.4), similar to the pattern identified in Shanafelt et al.
(2015).
17
14
16 16
37
44 40
37
21
17
16
15
26 26
27
33
0
20
40
60
80
100
CPC physicians Comparison
physicians
CPC physicians Comparison
physicians
Percentage of respondents
Never Once a month to a few times a year A few times a month Once a week or more
2013
2016
154

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Figure 6.3. Percentage of physicians who say they agree with the statement
on burnout once or more per week, CPC and comparison practices, 2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician surveys.
Notes: Responses shown include primary care physicians who responded that they feel this way about their job
daily, a few times a week, or once a week.
* The distributions of responses were statistically significantly different between CPC and comparison
physicians at the p < 0.10 level.
PCP = primary care physician
98
93
65
7
11
2
48
34
19
99
95
74*
5
10
0
50
31
21
0 20 40 60 80 100
PCP deals effectively with patients' problems
PCP feels he/she is positively influencing other
people's lives through work
PCP feels exhilarated after working closely with
patients
PCP feels he/she treats some patients as if they
were impersonal objects
PCP has become more callous toward people since
taking the job
PCP doesn’t care what happens to some patients
PCP feels emotionally drained from work
PCP feels fatigued facing another day on the job
Working with people all day is really a strain
Personal
accomplishment Depersonalization
Emotional
exhaustion
Percentage of respondents
CPC physicians Comparison physicians
Emotional
exhaustion
Depersonalization
Personal
accomplishment
155

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Figure 6.4. Physician scores on a subset of the Maslach Burnout Inventory
scales (0 = more burnout, 1 = less burnout), CPC and comparison practices,
2013 and 2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician surveys.
*/**/*** Average responses are significantly different between CPC and comparison physicians in the specified year at
the 0.10/0.05/0.01 level, respectively.
• These findings suggest that burnout is an issue for physicians, but that physicians in CPC
practices are not more burned out than physicians in comparison practices.
• Results of subgroup analyses show that CPC did not have a differential effect on the
proportion of physicians with high burnout for subgroups of practices defined by the
practice’s system affiliation, size, or patient risk profile (see Appendix E, Table E.208). In
addition, there were no effects on burnout of being in one of these subgroups for physicians
in CPC and comparison practices combined.
CPC practices’ clinicians and staff. Staff in CPC practices also experience burnout, but
generally at lower levels than reported by physicians and NPs/PAs. A similar pattern is noted in
the literature; for example, Edwards et al. (2017) found that 26 percent of physicians reported
high levels of burnout, compared with 21 percent of NPs/PAs and 20 percent of other clinical
staff; and Dolan et al. (2015) found that 45 percent of physicians, NPs, and PAs felt burned out
weekly compared with 40 percent of RNs and 31 percent of clinical associates. As with
physicians, participating in CPC’s transformation work does not appear to have exacerbated staff
burnout between 2013 and 2016.
• For example, in 2016, 24 percent or fewer practice managers, care managers, receptionists,
medical assistants, and nurses reported that they were burned out compared with 31 percent
of NPs and PAs and 33 percent of physicians (Figure 6.5). Over time, most ratings remained
the same, although the percentage of NPs and PAs reporting high levels of burnout increased
from 18 percent in 2013 to 31 percent in 2016 and decreased in care managers from 21 to 16
percent (note that we did not statistically test the differences in responses between different
types of CPC clinicians and staff or over time, and therefore we cannot determine whether
0.57 0.57
0.87** 0.87
0.88*
0.87*
0.55
0.57
0.84
0.86 0.86
0.85
0
0.2
0.4
0.6
0.8
1
2013 2016 2013 2016 2013 2016
Emotional
exhaustion
Depersonalization Personal
accomplishment
Average score (0 = more burnout,
1= less burnout)
CPC physicians Comparison physicians
Emotional
exhaustion
Depersonalization Personal
accomplishment
156

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
these changes are the result of unexplained variation due to the relatively small sample sizes
or if they are “real” changes). In the case of care managers, the total number of care
managers or coordinators in CPC practices grew dramatically in this period, from 104 in
2013 to 274 in 2016 (of these staff, 61 responded to this question in 2013 and 204 responded
in 2016), making it hard to interpret their changed results over time.
Figure 6.5. Percentage of CPC practice members reporting high levels of
burnout, 2013 and 2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician and staff surveys.
Notes: Respondents were asked to, using their own definition of burnout, select one of five statements that best
describes their situation at work. Following the literature, we define high levels of burnout as having one of
the following three responses: (1) I am definitely burning out and have one or more symptoms of burnout,
such as physical and emotional exhaustion; (2) the symptoms of burnout that I’m experiencing won’t go
away, and I think about frustrations at work a lot; and (3) I feel completely burned out, often wonder if I can
go on, and I am at the point where I may need some changes or may need to seek some sort of help.
We did not statistically test the differences in responses between respondent type or over time.
6.3.2. Control over work
Physicians in CPC and comparison practices reported having comparable control over various
aspects of their work, suggesting that CPC did not alter control over work. Within CPC practices,
NPs/PAs and other CPC practice staff reported less control over work on various items in the
control-over-work composite than physicians, except for practice managers, who reported having
about the same or more control than physicians on each item.
Physicians. Both CPC and comparison physicians reported comparable control over their
work throughout the initiative.
• Physicians in both groups had an average composite score between 0.50 and 0.55 on a one-
point scale in 2013 and 2016 (Appendix E, Table E.183).
• In 2016, CPC and comparison physicians reported having the most control over the hours
they work and the details of the office or the practice schedule, and the least amount of
control over work interruptions such as telephone calls and unscheduled patients, and the
volume of paperwork they do (Figure 6.6).
32
18
21 21
22 22
19
33
31
24
16
21 21
23
0
20
40
60
80
100
Physicians NPs/PAs Practice
managers
Care
managers/
coordinators
Receptionists Medical
assistants
Nurses
Percentage of
respondents
2013 2016
157

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
• There was little change between 2013 and 2016 in the amount of control CPC and
comparison physicians reported in the individual items measuring control, except for the
amount of control the physician has over the allotment of additional time for difficult-to-
help patients: for both CPC and comparison practices, the percentage of physicians that
reported great control increased from 24 percent in 2013 to 32 percent in 2016, but it is
unclear what factors drove the change (see Appendix E, Tables E.185–191).
• Results of subgroup analyses show that CPC did not have a differential effect on control
over work for subgroups of practices defined by system affiliation, practice size, or patient
risk profile (see Appendix E, Table E.184).
• For CPC and comparison physicians combined, system affiliation and practice size, but not
patient risk profile, influenced control over work. Among CPC and comparison physicians
combined, physicians that are not part of a health system reported more control over their
work than physicians that were part of a system (0.59 versus 0.45 out of 1.0) (where higher
scores represent more control); and physicians in smaller practices reported more control
over their work than physicians in larger practices (0.61 for solo clinician practices versus
0.52 to 0.53 for practices with more clinicians) (see Appendix E, Table E.184).
158

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Figure 6.6. Percentage of physicians reporting great or moderate control over
various aspects of their work, CPC and comparison practices, 2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician surveys.
Note: Response distributions for these questions were not statistically significantly different between CPC and
comparison physicians in the given year at the p < 0.10 level.
46
51
45
56
4
7
9
11
26
28
29
37
32
32
22
23
26
19
8
6
19
18
17
22
34
30
30
30
0 20 40 60 80 100
CPC
Comparison
CPC
Comparison
CPC
Comparison
CPC
Comparison
CPC
Comparison
CPC
Comparison
CPC
Comparison
Percentage of primary care physicians
Great control Moderate control
Allotment of additional
time for difficult-to-help
patients
Pace of their work
Workplace issues
(e.g., office space,
facilities, supplies)
Work interruptions (e.g.,
telephone calls,
unscheduled patients)
Volume of paperwork
(paper/electronic) they
have to do
Details of the office or the
practice schedule
Hours they work
159

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
CPC practices’ clinicians and staff. NPs/PAs and staff reported less control over work on
various items in the control-over-work composite than physicians. The one exception is practice
managers, who reported having slightly more control than physicians on the composite measure
(Figure 6.7).
Figure 6.7. Overall scores on the control-over-work summary composite for
CPC practice members, 2013 and 2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician and staff surveys.
Note: We did not statistically test the differences in responses between respondent type or over time.
6.3.3. Alignment of work with training
Physicians in CPC and comparison practices reported similar alignment of work with their training,
suggesting that CPC did not alter this area. Within CPC practices, physicians, NPs/PAs, and staff
indicate that their work is generally well-matched to their training, although there remains room for
improvement in delegation of tasks and additional training.
Physicians. According to physician and staff reports, there was no effect of CPC on
alignment of work with training using three measures.
63
• For the first measure, 65 percent of physicians in CPC and comparison practices surveyed in
2016 reported that they spend 75 percent or more of their time doing work well-matched to
their training (Figure 6.8). Although this finding suggests more room for delegation of tasks,
CPC did not appear to alter delegation.
• Results of subgroup analyses show that CPC may have had a positive differential effect on
how well-matched physicians report their work is to their training depending on whether the
physician was in a system-affiliated practice. The difference in the percentage of physicians
reporting that 75 percent of more of their time is spent doing work that is well matched to
their training between physicians in practices affiliated with systems compared to physicians
63
For the questions in this section, the text and figures exclude responses of does not apply and don’t know.
Appendix E, Tables E.174–182 present percentages with and without these responses.
0.50
0.40
0.56
0.43
0.35
0.33 0.33
0.52
0.40
0.57
0.43
0.36 0.36
0.33
0.0
0.2
0.4
0.6
0.8
1.0
Primary care
physicians
NPs/PAs Practice
managers/
supervisors
Care
managers/
coordinators
Receptionists Medical
assistants
Nurses
Average score
(0 = no/slight control,
1 = great control)
2013 2016
160
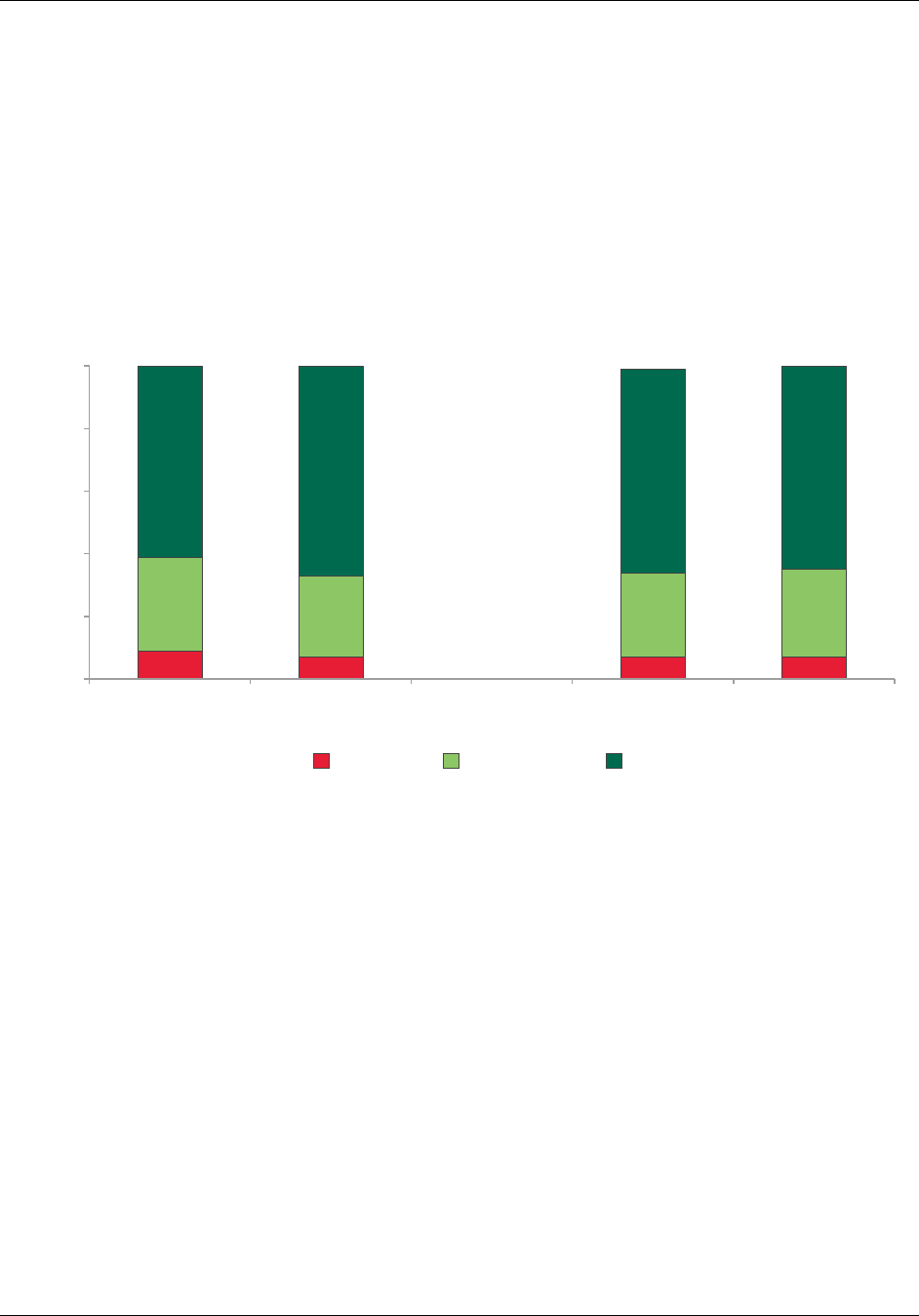
6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
in practices not affiliated with systems was smaller for CPC practices (65 percent and 66
percent) than comparison practices (55 percent and 72 percent). CPC did not have a
differential effect for subgroups of practices defined by practice size or patient risk score
(see Appendix E, Table E.182).
• For CPC and comparison physicians combined, 69 percent of physicians not in a system
versus 60 percent of those in a system reported that 75 percent or more of their time is spent
doing work that is well matched to their training. There were no differences across practices
of different sizes or with different patient risk profiles (see Appendix E, Table E.182).
Figure 6.8. Proportion of time each week that physicians do work that is well-
matched to their training, CPC and comparison practices, 2013 and 2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician surveys.
Notes: These estimates exclude physicians who answered does not apply or don’t know. Between 0 and 2 percent
of physicians responded does not apply or don’t know depending on group and survey round.
Response distributions were not statistically significantly different between CPC and comparison physicians
in the given year at the p < 0.10 level.
• Results from the second measure indicate that most physicians did not believe they needed
more training to do their work. When asked about the proportion of their work for which
they do not have enough training, 95 percent of both CPC and comparison physicians
indicated it was less than 25 percent of their time, and between 1 and 2 percent of physicians
in CPC and comparison practices suggested it was 50 percent or more of their time (item
E2b) (see Appendix E, Table E.178). Results from the third measure support the need for
more delegation of tasks in both CPC and comparison practices. When asked how much
time they spend in a typical week doing work that could be done by someone with less
training, 47 percent of CPC physicians and 52 percent of comparison physicians reported
that it was at least 25 percent of their time (see Appendix E, Table 175).
• For CPC and comparison physicians combined, 56 percent of physicians in a system versus
47 percent of those not in a system reported that at least 25 percent of their time is spent
9
7 7 7
30
26
27
28
61
67 65
65
0
20
40
60
80
100
CPC physicians Comparison
physicians
CPC physicians Comparison
physicians
Percentage of respondents
<49% 50%-74% 75%+
2013
2016
161

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
doing work that could be done by someone with less training. There were no differences
across practices of different sizes or with different patient risk profiles (see Appendix E,
Table E.176).
CPC practices’ clinicians and staff. Depending on the respondent type, 72 to 87 percent of
NPs/PAs and staff report that at least 75 percent of their time is spent doing work well-matched
to their training, compared with 65 percent of CPC physicians (Figure 6.9).
Figure 6.9. Percentage of CPC practice members saying that 75 percent or
more of their time is spent doing work
that is well-matched to their training
,
2016
Source: Mathematica analysis of 2016 CPC clinician and staff surveys.
Notes: These estimates exclude respondents who answered does not apply or don’t know. The percentage of
respondents responding does not apply or don’t know ranges from 0 to 6 percent depending on staff type.
We did not statistically test the differences in responses between respondent type.
When asked about a different measure, how much of their time is spent doing work that
could be done by someone with less training, responses suggest room for improvement with
delegation of tasks. For example, 53 percent of CPC physicians, 29 percent of CPC NPs/PAs,
and 57 percent of CPC nurses surveyed said that they spend 25 percent or more of their time
each week doing work that work could be done by someone with less training. Between 40 and
49 percent of other CPC staff also reported that they spend more than a quarter of their time each
week doing work that could be done by someone with less training.
65
86
72 72
79
87
77
0
20
40
60
80
100
Physicians NPs/PAs Practice
managers/
supervisors
Care
managers or
coordinators
Receptionists Medical
assistants
Nurses
Percentage of
respondents
162

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
6.3.4. Satisfaction with work
CPC did not appear to affect the satisfaction of physicians with their jobs; a comparable three-
quarters of both CPC and comparison group physicians reported being satisfied with their jobs.
Within CPC practices, CPC NPs/PAs and staff generally reported higher satisfaction with their jobs
than physicians.
Physicians. Job satisfaction is comparable among CPC and comparison group physicians.
• In 2016, more than three-quarters of CPC physicians agreed (48 percent) or strongly agreed
(29 percent) that they were satisfied with their current job (Figure 6.10). Only about 15
percent disagreed or strongly disagreed with the statement, and the remainder neither agreed
nor disagreed. Satisfaction was similar across CPC and comparison physicians and survey
rounds. This result is comparable to studies indicating overall rates of physician and primary
care physician satisfaction at about 80 percent (Caloyeras et al. 2016; Christopher et al.
2014).
Figure 6.10. Extent of physician agreement with statement, “Overall I am
satisfied with my current job,” CPC and comparison practices, 2013 and 2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician surveys.
Note: Response distributions were not statistically significantly different between CPC and comparison physicians
in the given year at the p < 0.10 level.
The columns may not add up to 100 percent due to rounding.
• About 15 percent of CPC and comparison physicians reported in 2016 that they were likely
or definitely leaving their current practice in the next two years; more than half of these
physicians were age 60 or older. Reasons for leaving included retirement, high workload,
career advancement, moving, inadequate compensation or benefits, poor management, and
“too many regulations.” Results were similar between CPC and comparison physicians and
in 2013 and 2016.
5
4
5
7
10 12 10
11
10
12
8
9
49
46
48
45
27 27
29
29
0
20
40
60
80
100
CPC physicians Comparison physicians CPC physicians Comparison physicians
2013 2016
Percentage of physician respondents
Strongly disagree Disagree Neither disagree or agree Agree Strongly agree
2013
2016
163

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
• Results of the subgroup analyses show that CPC did not have a differential effect on
physicians’ satisfaction with their job or plans to leave their practice in the next two years
for subgroups of practices defined by system affiliation, practice size, or patient risk score
(see Appendix E, Table E.193 and Table E.211).
• Turning to the results of being in the subgroup or not for CPC and comparison physicians
combined, physicians whose practice is not in a system were more likely to be satisfied with
their job than physicians whose practice is in a system (78 versus 71 percent of physicians).
Physicians whose practice had higher-risk beneficiaries (that is, they had higher average
HCC scores) were more likely to be satisfied with their job than physicians in practices with
lower-risk beneficiaries (78 versus 72 percent). There were no differences across practices
of different sizes and job satisfaction. There were also no differences in physicians’ plans to
leave their practice in the next two years across physicians in subgroups of practices defined
by system affiliation, size, or patient risk profile (see Appendix E, Table E.193 and Table
E.211).
CPC practices’ clinicians and staff. In 2016, more than three-quarters of CPC physicians,
NPs and PAs, and staff reported that they agreed or strongly agreed that they were satisfied with
their jobs (Figure 6.11). The proportion of staff who reported disagreeing or strongly disagreeing
with a statement that they were satisfied with their job ranged from 8 to 11 percent, which was
slightly lower than the proportion among physicians (15 percent) and NPs and PAs (13 percent).
Satisfaction for each type of respondent was similar in 2013 and 2016.
Figure 6.11. Extent of agreement with statement, “Overall I am satisfied with
my current job,” by CPC practice members, 2013 and 2016
Source: Mathematica analysis of 2013 and 2016 CPC clinician and staff surveys.
Notes: We did not statistically test the differences in responses between respondent type or over time.
The columns may not add up to 100 percent due to rounding.
5 5
7 7
5 5
0
3
5
4
6 6
4 4
10 10
7
6
5
3
4
7
7
7
6
4
6
5
10
8
11
10
9
6
15
7
14
13
13
13 13
14
49
48
36
43
42
39
43
41
47
42
39
46
41
43
27
29
38
34
39
46
37
41
28
33
36
31
36
34
0
20
40
60
80
100
2013 2016 2013 2016 2013 2016 2013 2016 2013 2016 2013 2016 2013 2016
Physicians NPs/PAs Practice
managers
Care
managers or
coordinators
Receptionists Medical
assistants
Nurses
Percentage of respondents
Strongly disagree Disagree Neither disagree or agree Agree Strongly agree
Physicians
NPs/PAs
Practice
managers
Care managers
or coordinators
Receptionists Medical
assistants
Nurses
164

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
6.3.5. Ratings of CPC in 2016 among CPC practice members
CPC physicians and staff had largely positive views about their experiences participating in CPC.
Influence of CPC on quality of care. In 2016, at least 75 percent of each type of clinician
and staff indicated that CPC improved the quality of care or service for their patients somewhat
or a lot (see Appendix E, Table E.237). The one exception is receptionists, a large share of whom
replied that they do not know. Among physicians, just over half indicated that CPC had
improved the quality of care or service somewhat, and another 24 percent thought it had
improved it a lot (Figure 6.12). Responses of NPs/PAs and staff were generally comparable to
physicians, with somewhat higher percentages of staff saying that CPC had improved the quality
of care a lot. For example, 54 percent of practice managers and 53 percent of care managers said
CPC improved quality of care a lot, versus 24 percent of physicians and NPs/PAs. Very few
respondents in any category (no more than 1 to 3 percent) thought CPC had worsened quality of
care in any way, and a small proportion (14 percent of physicians, 12 percent of NPs/PAs, and 3
to 6 percent of staff) thought that CPC had not resulted in any change in the quality of care for
their patients.
Figure 6.12. CPC physician reports of how CPC participation changed the
quality of care or service provided to patients, 2016
Source: Mathematica analysis of 2016 CPC clinician surveys.
Results of subgroup analyses show that there was no difference in the percentage of
physicians reporting that participation in the CPC initiative improved the quality of service and
care they provide their patients somewhat or a lot across system affiliation, practice size, or
patient risk score (see Table E.238 in Appendix E).
Support for CPC. Ratings of CPC were largely favorable. The 2016 survey asked CPC
clinicians and staff to consider whether, knowing what they now know, they would support or
oppose the practice’s participation in CPC if they could go back in time to when CPC was
announced in 2012. Forty-six percent of CPC physicians and 38 percent of CPC NP/PA
respondents would be strongly supportive, and another 33 percent of each said they would be
somewhat supportive (Figure 6.13, Appendix E, Table E.239). Even larger percentages of
24
56
14
2
1
4
0
20
40
60
80
100
Improved a lot Improved
somewhat
Did not change Worsened
somewhat
Worsened a lot Don't know
Percentage of respondents
165

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
practice managers and care managers (each 64 percent) would be strongly supportive of
participation, and another 21 percent or more would be somewhat supportive. Across respondent
types, 5 percent or fewer indicated they would be strongly opposed to participation in CPC, with
slightly higher percentages saying they would somewhat oppose participation (between 2 and 9
percent). The degree of physicians’ support of CPC participation is consistent with the large
number of CPC practices that applied to participate in CPC+. Ninety-eight percent of practices
that were still participating in the initiative at the end of CPC applied for and were selected to
participate in CPC+. See Chapter 2, Section 4 for more details.
Figure 6.13. CPC practice members’ reports of how much they would support
or oppose their practice’s participation in CPC if they could do it all over
again, 2016
Source: Mathematica analysis of 2016 CPC clinician and staff surveys.
Note: We did not statistically test the differences in responses between respondent types.
Results of subgroup analyses show that CPC physicians in solo clinician practices and CPC
physicians in practices with more high-risk beneficiaries were more likely to report that they
would be strongly supportive of their practice’s participation in CPC than those in larger
practices or in practices with lower-risk beneficiaries. Sixty-three percent of physicians in solo
practices versus 43 to 46 percent of physicians in larger practices reported that they would
strongly support their practice’s participation in CPC, knowing what they know now, and 51
percent of physicians in practices with higher-risk beneficiaries versus 40 percent of physicians
in practices with lower-risk beneficiaries would strongly support their practice’s participation.
During interviews with the deep-dive practices, we heard that physicians in larger practices were
less likely to see the financial support from CPC at the practice level and were more likely to be
46
38
64
64
33
44
42
33
33
24
21
18
23
30
7
9
4
2
3
5
6
5
3
4
2
2
1
3
9
17
5
12
44
27
20
0 20 40 60 80 100
Primary care physicians
NPs/PAs
Practice managers or
supervisors
Care managers or coordinators
Receptionists
Medical assistants
Nurses
Percentage of respondents
Strongly support Somewhat support Somewhat oppose Strongly oppose
Don’t know enough about CPC to answer
166

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
told (by their system) that they will participate in CPC compared with small independent
practices. These findings suggest that larger practices may not have had the same level of
physician buy-in as small independent practices. There was no difference in the proportion of
physicians reporting strong support for CPC participation in subgroups of practices defined by
system affiliation or patient risk score (see Appendix E, Table E.240).
Reasons for supporting CPC. Respondents were given seven specific potential reasons (as
well as an “other” category) and asked to indicate all main reasons they would support
participation in the CPC initiative. Among physicians who would have supported participation in
CPC if they could do it over again,
64
by far the most commonly selected reason for supporting
participation in CPC was that work on CPC Milestones helped the practice make positive changes
and improve patient care, cited by 81 percent of physicians (Figure 6.14 reports responses for
physicians; responses of all respondents are reported in Appendix E, Table E.242). Roughly half of
these physicians cited two other reasons as important: (1) the opportunity to contribute to the field
of primary care practice transformation and (2) that financial support provided in CPC is sufficient
to support participation; 42 percent cited the usefulness of data feedback; and between one-quarter
and one-third cited Milestone work improving clinician and staff work satisfaction, useful learning
support, and learning support improving skill development.
Figure 6.14. Percentage of CPC physicians reporting each factor as a main
reason for supporting participation in CPC, among those that would support
participating again, 2016
Source: Mathematica analysis of 2016 CPC clinician and staff surveys.
Notes: Respondents could also select “other” and specify a reason not listed; although not shown on the figure, 7
percent of physician respondents selected the other response option.
64
We excluded respondents that reported not knowing enough about CPC to support or oppose their practice’s
participation.
81
52
52
42
30
30
26
1
0 20 40 60 80 100
Work on CPC Milestones helps practice make positive
changes and improve patient care
Financial support provided in CPC is sufficient to
support participation
Opportunity to contribute to field of primary care
practice transformation
Data/feedback reports provided in CPC are useful
Work on CPC Milestones improves clinician and staff
work satisfaction
Learning support and activities provided in
CPC are useful
Learning support provided in CPC improves clinician and
staff skill development
No reasons to support participation in CPC
Percentage of respondents
167

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Turning to responses of other practice members, we focus on the responses of those who
answered this question and would have supported participation in CPC if they could do it over
again. In general, NP/PA respondent views were comparable to physicians, though NPs/PAs
were less likely than physicians to report financial support is sufficient to support participation as
a major reason. Among the different staff types, respondents tended to place more emphasis than
physicians and NPs/PAs on Milestone work improving clinician and staff satisfaction, and the
importance of learning support and activities provided in CPC. Specifically, 36 to 54 percent of
other staff versus 24 to 30 percent of physicians and NPs/PAs indicated that CPC learning
support is useful, and that learning support improves clinician and staff skills development, were
major reasons for supporting CPC. Similarly, 44 to 54 percent of staff, depending on the
respondent type, versus 30 percent of both CPC physicians and NPs/PAs said that improved
clinician and staff satisfaction through the work on the CPC Milestones was a major reason for
their support.
Reasons for opposing CPC. Respondents were also asked about six major reasons (as well
as an “other” category) they would have for opposing their practice participating in CPC,
knowing what they know now (Figure 6.15 reports responses for physicians; responses of all
respondents are reported in Appendix E, Table E.244). Among the small proportion of
physicians who answered this question about reasons for opposing CPC and would have been
opposed to participating in CPC if they could do it all over again,
65
the largest proportion (66
percent) reported that CPC does not substantially improve patient care, followed by the reporting
requirements are too burdensome (58 percent), financial support is insufficient to support
participation (58 percent), the work involved in implementing the Milestones is too burdensome
(56 percent), and staffing is insufficient to participate in CPC (31 percent).
66
It is worth noting that even among physicians who said they would support CPC
participation if they could do it again, many also cited the burden of CPC: 44 percent cited
administrative requirements, while 34 percent cited the difficulty of implementing the Milestones
(Appendix E, Table E.244).
65
We excluded respondents that reported not knowing enough about CPC to support or oppose their practice’s
participation.
66
The numbers of NPs/PAs and other staff that would oppose participation in CPC is small (between 9 and 39
respondents depending on the staff type). Although we do not discuss the responses of other staff separately here,
more detail is available in Appendix E, Table E.244.
168

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
Figure 6.15. Percentage of CPC physicians reporting each factor as a main
reason for opposing participation in CPC, among those that would oppose
participating again, 2016
Source: Mathematica analysis of 2016 CPC clinician and staff surveys.
Notes: Respondents could also select “other” and specify a reason not listed; although not shown on the figure, 15
percent of physician respondents selected the other option.
6.4. Discussion
Physicians in CPC and comparison practices reported similar burnout, control over work,
alignment of work with training, and work satisfaction in 2016, the last year of CPC. There was
no differential effect of CPC on most measures of physician experience for physicians whose
practices were in a system, were larger (measured by having more primary care clinicians), or
served attributed Medicare beneficiaries with a higher risk score (measured by the average HCC
score among attributed Medicare FFS beneficiaries in their practice). Responses were also
similar over time for each respondent type in the CPC practices. Together, these findings indicate
that CPC did not affect these aspects of clinician and staff experience.
Although CPC did not appear to affect clinician and staff experience, we did find differences
between physicians in both CPC and comparison practices depending on whether their practice
was part of a system, the size of their practice, and the risk profile of their practice’s patients. For
CPC and comparison physicians combined, physicians whose practice was part of a system
reported less control over their work, spending less time doing work that was well matched to
their training, spending more time doing work that could be done by someone with less training,
and less satisfaction with their current job than physicians whose practices were not part of a
system. There were fewer differences between physicians depending on practice size and patient
risk profile. For CPC and comparison physicians combined, physicians in larger practices
reported less control over their work than physicians in solo clinician practices; and physicians in
practices with lower-risk beneficiaries were less likely to report being satisfied with their current
job than physicians in practices with higher-risk beneficiaries.
66
58
58
56
31
3
0
0 20 40 60 80 100
CPC does not substantially improve patient care
Financial support provided in CPC is insufficient to
support participation
Reporting requirements in CPC are too burdensome
Work involved in implementing the CPC Milestones
is too burdensome
Insufficient practice staffing to participate in CPC
CPC does not allow the practive to join an Accountable
Care Organization (ACO)
No reasons to oppose participation in CPC
Percentage of respondents
169

6. HOW DID CPC AFFECT THE EXPERIENCES
OF PHYSICIANS, OTHER CLINICIANS, AND STAFF? MATHEMATICA POLICY RESEARCH
CPC clinicians and staff had largely positive views about their experiences participating in
CPC. Many believed that CPC improved quality of care and cited improved patient care; the
opportunity to contribute to primary care practice transformation; and the benefits of financial
support, data feedback, and learning supports as reasons they would support participation in
CPC. Still, even CPC supporters provided responses indicating the burden of administrative
reporting and the difficulty of the transformation work in CPC. For example, among the 85
percent of physicians who would have supported participating in CPC if they could do it over
again and answered a question about reasons they might oppose the initiative, 44 percent
reported burdensome administrative requirements, about one-third cited the work involved in
implementing the Milestones and inadequate financial support, and one-quarter reported
inadequate staffing.
Although the evidence suggests that CPC did not adversely affect physician and staff
experience, future care delivery initiatives nonetheless could work with practices to reduce
burnout, improve delegation, and streamline administrative work. Two recent systematic reviews
that evaluate the effectiveness of interventions to reduce burnout in physicians found that
programs to reduce burnout in physicians were associated with small but statistically significant
impacts (Panagioti et al. 2017; West et al. 2016). For example, one of these studies found that
organizational changes—such as fostering communication between members of the health care
team, and cultivating a sense of teamwork and job control—were more effective in reducing
burnout than interventions targeted to improving personal coping strategies (Panagioti et al.
2017). Similarly, a cluster randomized control study of 166 primary care clinicians in 24 clinics
found that improved workflows and targeted quality improvement projects decreased physician
burnout, while physician satisfaction improved more often in the clinics that participated in
communication and workflow interventions (Linzer et al. 2015).
170

MATHEMATICA POLICY RESEARCH
7. HOW DID CPC AFFECT THE EXPERIENCES OF MEDICARE FFS
BENEFICIARIES?
Patient-centeredness was a core tenet of the CPC initiative, and several aspects of CPC
aimed to improve patient experience through the transformation of care delivery. Specifically,
practices were expected to improve access to care, engage patients to guide quality improvement
through regular patient surveys and/or a patient and family advisory council (PFAC), integrate
into usual care culturally competent self-management support and shared decision making tools,
and coordinate care across the medical neighborhood. Practices were also encouraged to use a
personalized plan of care for high-risk patients. In addition, CMS and some other participating
payers used patient experience as an element in determining practice eligibility for shared
savings payments.
This chapter examines how CPC affected the experiences of Medicare fee-for-service
beneficiaries with care over the four years of the initiative. We present results based on responses
from more than 25,000 beneficiaries in roughly 500 CPC practices and 8,000 beneficiaries in
roughly 800 comparison practices. The survey is based on the Clinician and Group Consumer
Assessment of Healthcare Providers and Systems 12-Month Survey with Patient-Centered
Medical Home supplemental items (CAHPS PCMH, version 2.0) and supplemented with several
questions about specific aspects of CPC. We examine how patient ratings of CPC practices
compare with ratings of comparison practices in 2013 (8 to 12 months after CPC began) and
again in 2016 (5 months before CPC ended). Appendix F describes the survey sampling,
fielding, content, and analysis methods in more detail and contains tables showing the results.
7.1. Key takeaways on the effect of CPC on the experiences of Medicare
beneficiaries
Despite CPC practices undergoing substantial changes to improve care delivery,
beneficiaries’ experiences with care at CPC practices were generally no different from
experiences at comparison practices toward the end of the four-year initiative. There were no
differential effects of CPC on beneficiaries who (1) were in practices in systems, (2) were in
larger practices (measured by having more primary care clinicians), or (3) had higher risk scores.
There were three exceptions.
• CPC improved transitional care after hospital stays. Patient ratings indicated that CPC
practices provided timely follow-up to more beneficiaries after their hospital stays than did
comparison practices. In 2016, 60 percent of beneficiaries in CPC practices compared to 50
percent of beneficiaries in comparison practices reported that their provider’s office
contacted them within three days of their most recent hospital stay.
• CPC improved transitional care after ED visits. Beneficiaries in CPC practices were more
likely to report timely follow-up after emergency department (ED) visits. In 2016, 59
percent of beneficiaries in CPC practices compared to 51 percent of beneficiaries in
comparison practices that visited the emergency department in the past year reported that
their provider’s office contacted them within one week of their visit.
171

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
• CPC might have unfavorably affected timely email response to patient questions. In 2016,
fewer beneficiaries in CPC practices than in comparison practices reported that they always
received an answer to their medical question as soon as needed when emailing their provider
in the past 12 months (69 percent of beneficiaries in CPC practices compared to 75 percent
of beneficiaries in comparison practices). However, fewer than 8 percent of beneficiaries in
CPC and comparison practices reported emailing their provider and thus could answer this
question.
7.2. Methods
7.2.1. Survey content and measures
The patient survey instrument contains questions from the CAHPS PCMH version 2.0
(Agency for Healthcare Research and Quality 2015). The CAHPS PCMH survey gauges
patients’ experiences over the previous 12 months across six domains of primary care: (1)
patients’ ability to get timely appointments, care, and information; (2) providers’ communication
with patients; (3) providers’ knowledge of the care patients received from other providers; (4)
providers support patients in caring for their own health; (5) providers discuss medication
decisions with patients; and (6) patients’ overall rating of their primary care provider. To help
summarize patient experiences, we created six composite summary measures using 19 questions
following the CAHPS Clinician and Group Survey scoring instructions (Agency for Healthcare
Research and Quality 2012). Table 7.1 details the patient care experiences that the six summary
composite measures evaluate. The CAHPS questions focused on care provided by the provider
during visits, which is only one aspect of care that CPC aimed to affect. It did not ask about other
aspects of care that CPC aimed to transform, such as team-based care or care provided regardless
of whether it was in the office, or through phone, email, text, video, or group visits.
Table 7.1. Experiences included in the patient survey composite measures
Timely appointments, care, and information (five questions)
How often the patient:
• Got an appointment as soon as needed when phoning the provider’s office for care needed right away
• Got an appointment as soon as needed when making an appointment for check-up or routine care
• Received timely answers to medical questions when phoning the provider during regular office hours
• Received timely answers to medical questions when phoning the provider after regular office hours
• Saw the provider within 15 minutes of appointment time
Providers’ communication with patients (six questions)
How often the provider:
• Provided the patient with clear and easy to understand explanations
• Listened carefully to the patient’s health questions and concerns
• Provided the patient with easy-to-understand instructions and information
• Knew important information about the patient’s medical history
• Showed respect for what the patient had to say
• Spent enough time with the patient
Providers’ knowledge of the care patients received from other providers (two questions)
How often the provider seemed informed and up to date on care the patient received from specialists
Whether practice staff spoke with the patient at each visit about all of the patient’s prescription medications
172

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
Table 7.1 (continued)
Providers support patients in taking care of their own health (two questions)
Whether someone in the provider’s office:
• Discussed with the patient specific goals for the patient’s health
• Asked the patient whether there are things in life that make it hard for the patient to take care of his or her
health
Providers discuss medication decisions with patients (three questions)
If the provider talked with the patient about starting or stopping a prescription medicine, how often the provider:
• Discussed reasons the patient might want to take the medicine
• Discussed reasons the patient might not want to take the medicine
• Asked the patient what he or she thought was best for him or her
Patients’ rating of the provider (one question)
Patient rated the provider on a scale of 0 to 10, with 0 being the worst and 10 being the best
We assessed how well questions within each composite measure produced consistent results
by calculating the internal consistency reliability of each composite. We calculated this value for
the five composite measures formed from the responses to multiple questions (the composite
measure for the remaining composite, patients’ rating of the provider, contains only one
question). Four of the five composite measures had adequate reliability with McDonald’s omega
values between 0.76 and 0.96. The other composite—providers’ knowledge of the care patient
received from other providers—had less reliability (omega = 0.56) (Nunnally and Bernstein
1994; Lance 2006). Because its two component questions do not fit well together in the
composite, we report the questions separately.
In addition to the 17 questions included in the five summary composite measures (the four
with adequate reliability and the one single-question composite), the surveys included 30 other
questions that asked about patient experience, for a total of 47 questions. Because of changes in
the Milestones and research priorities over time, 11 of the 30 questions were not included in all
four rounds (see Table 7.2). For these questions, we cannot calculate CPC-comparison
differences for 2013 and instead calculate CPC-comparison differences for the first year the
question was asked. We discuss our methods for analysis in Section 7.2.4. See Appendix F,
Table F.4 for a list of all 47 patient survey questions.
Table 7.2. Number of questions that gauge patient experience in survey
. Number of questions
In five composites and in all four rounds 17
Not in composites 30
…In one round
4
…In two rounds
1
…In three rounds
6
…In four rounds
19
Total 47
7.2.2. Survey administration
We administered four rounds of the CPC patient survey by mail during the 51-month
initiative (Table 7.3).
We did not offer incentive payments.
173

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
Table 7.3. CPC patient survey rounds and fielding dates
Round Fielding period Months after CPC began
1 June through October 2013 8–12
2 July through October 2014 21–24
3 July through October 2015 33–36
4 July through October 2016 45–48
7.2.3. Survey sample and response rates
We administered the survey to a cross-sectional sample of Medicare FFS beneficiaries
attributed to CPC and comparison practices.
67
We invited about 60,000 of the roughly 300,000
Medicare FFS beneficiaries attributed to CPC and 20,000 of the approximately 600,000
beneficiaries attributed to comparison practices to respond to the patient survey each round.
68
Using Medicare claims data, Medicare beneficiaries were attributed to practices where they
received the plurality of selected evaluation and management visits to primary care clinicians
over the prior two years. We sampled Medicare FFS beneficiaries in all practices that had ever
participated in CPC and were still open, regardless of whether the practice was still participating
in CPC at the time of the survey. Each round, we excluded practices that had closed more than
six months before the survey round; only 7 (or 1 percent of) CPC practices were excluded from
our sample for this reason.
In each survey round, we obtained response rates between 44 and 48 percent for CPC and
comparison practices. Using survey responses, we then identified attributed Medicare
beneficiaries who had visited the practice at least once in the 12 months before the start of the
survey round to be included in the analytic sample. For each round of data collection, our
analytic sample included more than 25,000 Medicare FFS beneficiaries attributed to between 490
and 496 CPC practices and 8,000 Medicare FFS beneficiaries attributed to between 736 and 818
comparison practices, depending on the round. (See Appendix F, Table F.3 for detailed
information on the samples and response rates over time.) Sixteen percent of respondents
answered in multiple rounds, and fewer than one percent of respondents answered in all four
survey rounds. Survey respondents generally answered all questions in the survey: most
questions were answered by 96 percent or more of the respondents.
67
We also surveyed a sample of other—that is, not attributed Medicare FFS—patients that CPC practices reported
seeing in the prior year. We did not use their responses in this analysis of CPC-comparison differences because it
would have been too burdensome to collect a list of such patients from the comparison practices. CMS shared
responses from a sample of all patients with practices to support quality improvement and used the responses as part
of shared savings calculations.
68
We sought to obtain responses from 40 attributed Medicare FFS beneficiaries per CPC practice and 14
beneficiaries per matched set of comparison practices based on power calculations we did at the start of the
evaluation. The targeted samples differ between the two groups because of the varying uses of the data for the
evaluation. Respondent data from CPC practices were used to provide practice-level feedback, CMS’s shared
savings calculations, and to conduct the impact analysis reported here; respondent data from comparison practices
were used only for the impact analysis. To achieve better power, we allocated more sample to the CPC practices to
support practice-level estimates.
174

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
7.2.4. Analysis
Analytic comparisons. For each survey question measuring patient experience and five
CAHPS composite measures created using a subset of the questions, we compared ratings
between CPC and comparison practices in 2013 or the first year the question was asked, and
again in 2016, to observe where patient experience differed between the two groups early in the
initiative and near the end of the initiative. Because we were not able to collect data before CPC
began, differences in any of the years may reflect preexisting differences between CPC and
comparison practices. It is possible that CPC did not have an effect on patient experience during
the first 8 to 12 months, the time of the first survey in 2013. However, in case it had, we did not
calculate difference-in-differences estimates.
Our main analyses examine the proportion of respondents who answered each question with
the best response. To test the sensitivity of these findings, we also conducted the same analyses
using the mean response.
Regression analysis. We calculated the predicted probability of answering the best response
and the mean responses using logistic and ordinary least squares (OLS) regressions, respectively,
controlling for baseline beneficiary and practice characteristics and self-reported education level
at the time of the survey. Because the rate of missing response was small—most questions had
less than 4 percent data missing—we calculated findings among non-missing data and did not
adjust for question nonresponse. For all regressions, we weighted estimates using beneficiary-
level nonresponse weights (to make the sample similar to all attributed Medicare FFS
beneficiaries) and practice-level matching weights (to ensure that CPC and comparison samples
were similar). We clustered standard errors by practice for all respondents from CPC practices
and by matched set for respondents from comparison practices to account for clustering of
responses within a practice and respondents answering in more than one round.
Subgroup effects. We also looked at ratings of CPC and comparison practices by three key
subgroups of beneficiaries:
• Whether the beneficiary is attributed to a practice that is part of a health care system (from
2016 data from SK&A, a healthcare vendor)
• The size of the beneficiary’s practice (measured by the number of primary care clinicians in
the practice)
• The beneficiary’s relative health status (measured by whether the respondent’s 2012 HCC
score is above or below the median for all respondents across all survey rounds)
We used logistic and OLS regressions to test for subgroup effects. We first estimated a
regression on each composite measure using the combined sample of beneficiaries from CPC
and comparison practices, with a binary indicator for whether the beneficiary or the beneficiary’s
practice was in the subgroup of interest added to the other regression adjusters. We examined
whether the coefficient on the subgroup indicator was statistically significant to determine
whether there were different responses by subgroup. We also estimated regressions on each
composite measure with three explanatory variables (in addition to the other regression
adjusters): a binary indicator for treatment (CPC group) status, a binary indicator for whether the
beneficiary or the beneficiary’s practice is in the subgroup, and a term interacting treatment and
175

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
subgroup status. We examined whether the coefficient on the treatment (CPC group)-subgroup
interactor was statistically significant to determine whether CPC had a differential effect for
members of the subgroup.
Power. Using two-tailed tests at the 10 percent significance level, the analysis had 80
percent power to detect small CPC-wide effects of one to three percentage points over time and
between CPC and comparison practices for the composite measures and for most individual
questions. Exceptions were for questions that applied to a small proportion of respondents, such
as beneficiaries who had phoned the provider’s office after hours or beneficiaries who had
emailed the provider’s office with medical questions, where we could detect differences of 6 to
11 percentage points.
Statistical and substantial importance. We considered responses between beneficiaries in
CPC and comparison practices to be statistically different and of substantial importance if the
difference met two criteria: (1) the p-value was less than 0.10 and (2) the difference between the
two groups was larger than five percentage points.
69
7.3. Results
Responses of Medicare FFS beneficiaries to the CPC patient survey suggest that while CPC
practices were undergoing substantial changes to improve care delivery, beneficiaries’ experiences
with care at CPC practices were generally no different than experiences at comparison practices.
Two exceptions indicate that CPC practices provided a higher proportion of beneficiaries with timely
follow-up after hospital stays and after ED visits than comparison practices. These favorable effects
are consistent with CPC’s requirement for Milestone 6 (Care Coordination Across the Medical
Neighborhood) to provide this type of follow-up care. Another difference suggests a possible
unfavorable effect on beneficiaries always receiving an answer to their medical question as soon as
needed when emailing their provider. However, very few beneficiaries reported emailing their
provider with a medical question and had therefore answered this question.
7.3.1. Composite measures
For both CPC and comparison practices, ratings across the composites varied in 2013,
indicating some composites had more room for improvement. Figure 7.1 shows the
percentage of beneficiaries giving the best ratings in 2013 (the first segment of each bar) and the
change in ratings from 2013 to 2016 (the second segment of each bar) for each of the five
composite measures, separately for CPC and comparison practices. In 2013, three composites
had room for improvement: timely appointments, care, and information; providers support
patients in taking care of their own health; and providers discuss medication decisions with
patients, with between 46 and 63 percent of beneficiaries giving their practices the best ratings.
Beneficiaries’ ratings of the other two composite measures—providers’ communication with
patients, and patients’ rating of the provider—were already fairly high in 2013, with more than
75 percent of the responding beneficiaries (CPC and comparison) providing the most favorable
responses.
69
We did not find any literature that defines what magnitude difference would be substantively important for
CAHPS measures or other patient experience outcomes. In consultation with CAHPS experts, we decided to define
a substantial difference as five percentage points.
176

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
Figure 7.1. Percentage of Medicare FFS beneficiaries giving the best
response in 2013 and 2016, for five composite measures, CPC and
comparison practices, CPC-wide
Sources: CPC patient surveys administered June through October 2013 and July through October 2016.
*/**/*** The percentage of beneficiaries giving the best response was statistically different between CPC and
comparison practices in the given year at the 0.10/0.05/0.01 level, respectively, but of small magnitude.
FFS = fee-for-service.
Regardless of opportunities for improvement, improvements in beneficiaries’ ratings
between 2013 and 2016 were minimal (less than three percentage points) for four of the five
composite measures. The first set of segments in Figure 7.1 indicates slightly higher ratings in
comparison than in CPC practices in 2013. This difference favoring the comparison practices
remains in 2016 for all but the providers support patients in taking care of their own health
composite. Although some of the CPC-comparison differences were statistically significant, they
were all under three percentage points, so not of substantive importance. The second segment in
Figure 7.1 shows the changes over time for each composite measure; because changes were
small, the second segment is barely visible for most composites. The exception was the
composite measure for providers support patients in taking care of their own health that measures
whether someone in the provider’s office discussed with the beneficiary specific goals for his or
her health and whether someone asked the beneficiary whether there are things in life that make
it hard for the beneficiary to take care of his or her health. Between 2013 and 2016, both CPC
and comparison practices experienced a statistically significant and meaningful improvement in
beneficiaries’ ratings of this composite. The percentage of beneficiaries giving the best response
increased by 6 percentage points from 46 percent in 2013 to 53 percent in 2016 for CPC
practices, but comparison practices experienced a similar improvement of 5 percentage points,
from 48 to 53 percent. Figure 7.2 illustrates the dynamics over all four survey rounds for each
composite measure for CPC and comparison practices.
53**
54
80**
81
46**
48
60***
63
76**
78
53*
54
81**
82
53
53
61
63
78
80
0 20 40 60 80 100
CPC
Comparison
CPC
Comparison
CPC
Comparison
CPC
Comparison
CPC
Comparison
.....
Percentage of respondents
Timely appointments,
care, and information
Providers'
communication with
patients
Providers support
patients in taking care
of their own health
Providers discuss
medication decisions
with patients
Patients' rating of the
provider
2013 CPC 2013 Comparison 2013 to 2016 gain
177

178
Figure 7.2. Percentage of Medicare FFS beneficiaries answering with the best response, by composite
measure, CPC and comparison practices, CPC-wide
Source: CPC patient surveys administered June through October 2013, July through October 2014, July through October 2015, and July through October 2016.
FFS = fee-for-service.
44%
46%
48%
50%
52%
54%
56%
58%
60%
62%
64%
2013 2014 2015 2016
Timely appointments, care, information
64%
66%
68%
70%
72%
74%
76%
78%
80%
82%
84%
2013 2014 2015 2016
Providers' communication with patients
44%
46%
48%
50%
52%
54%
56%
58%
60%
62%
64%
2013 2014 2015 2016
Providers support patients in taking care
of their own health
44%
46%
48%
50%
52%
54%
56%
58%
60%
62%
64%
2013 2014 2015 2016
Providers discuss medication decisions
with patients
64%
66%
68%
70%
72%
74%
76%
78%
80%
82%
84%
2013 2014 2015 2016
Patients' rating of provider

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
Overall, CPC did not improve beneficiary ratings for the five composite measures.
Ratings for each composite were comparable for CPC and comparison practices in 2013. In
2016, near the end of the initiative, the ratings were still comparable for CPC and comparison
practices (see Table 7.4, and Figures 7.1 and 7.2). Results for mean responses are similar to those
for the proportion with the best response. Comparing 2016 mean responses suggests that
beneficiaries’ experiences at CPC and comparison practices were comparable for each composite
measure (see Appendix F, Table F.8).
Table 7.4. Percentage of Medicare FFS beneficiaries giving the top-box
response for five composite measures, CPC-wide, 2013 and 2016
Composite measure
Beneficiaries in CPC
practices (CPC-wide)
Beneficiaries in
comparison practices
(CPC-wide)
CPC-
comparison
differences (pp)
2013 2016
2013
to
2016
(pp) 2013 2016
2013
to
2016
(pp) 2013 2016
Timely appointments, care, and
information (five questions) 53 53 0 54 54 0
-2**
b
-2*
b
Provider communication
(six questions) 80 81
1**
b
81 82 1 -1**
b
-1**
b
Providers support patients in
taking care of their own health
(two questions) 46 53
6***
a
48 53
5***
a
-2**
b
0
Providers discuss medication
decisions with patients
(three questions) 60 61
1**
b
63 63 0 -3***
b
-1
Patients’ rating of the provider
(one question) 76 78
3***
b
78 80 2*
b
-2**
b
-1
Notes: Green shading indicates that the estimate is both statistically (p < 0.10) and substantially (five or more
percentage points) significant. Gray shading indicates that the estimate is statistically but not substantially
significant due to a small magnitude.
*/**/*** The difference is statistically significant at the 0.10/0.05/0.01 level.
FFS = fee-for-service.
a
The estimate is meaningful and favorable to CPC.
b
The estimate is statistically but not substantially significant due to a small magnitude.
Variation in beneficiary ratings in 2016 by subgroup. We examined whether
beneficiaries’ ratings in 2016 differ by whether their practices are part of a health care system,
the size of their practice, and their HCC score. There were no differential effects of CPC on any
of these findings, meaning that the effect of being attributed to a practice in a system, belonging
to a larger practice, or having a higher HCC score was similar for CPC and comparison practices
(Appendix F, Tables F.7a–c).
Because the patterns in beneficiaries’ ratings are largely the same for CPC and comparison
practices, we examined whether beneficiaries’ ratings differed by subgroup for CPC and
comparison practices combined. For CPC and comparison practices, beneficiaries’ ratings of the
five composite measures were comparable between the subgroups for all but one of the 15
comparisons we examined. (We report the differences among the CPC and comparison practices
179

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
combined in Table 7.5. Dashes in Table 7.5 indicate where beneficiary ratings did not differ
meaningfully between the subgroups.) In 2016, beneficiaries were less likely to give the best
ratings for timely appointments, care, and information for larger practices than for smaller
practices. There were no meaningful differences in beneficiaries’ ratings in any of the composite
measures between practices in health care systems and practices not in systems or from
beneficiaries with higher HCC scores compared with those with lower HCC scores (Table 7.5).
Table 7.5. Meaningful differences in Medicare FFS beneficiaries’ ratings of
practices by select practice and beneficiary characteristics, among CPC and
comparison practices combined, 2016
Composite measure
Practices in a
system compared
with those not in a
system
Larger
practices
compared with
smaller
practices
Patients with higher
HCC scores
compared with
lower HCC scores
Timely appointments, care, and information
a
– Lower by 6 pp –
Providers’ communication with patients
– – –
Providers support patients in taking care of their
own health
b
– – –
Providers discuss medication decisions with
patients
b
–
– –
Patients’ overall ratings of the provider
a
– – –
Note: – Indicates that beneficiary ratings were not meaningfully different between the subgroups. We defined an
estimate as meaningfully different if it was both statistically (p < 0.10) and substantially (five percentage
points or more) significant.
a
Beneficiaries in practices that are in systems were statistically less likely than beneficiaries in practices not in
systems to give the best responses for timely appointments, care, and information (3 percentage points, p < 0.01),
and beneficiaries’ overall ratings of the provider (2 percentage points, p = 0.05).
b
Beneficiaries with higher HCC scores were statistically more likely than beneficiaries with lower scores to give the
best responses for providers support patients in taking care of their own health (3 percentage points, p < 0.01), and
providers discuss medication decisions with patients (2 percentage points, p = 0.04).
FFS = fee-for-service; pp = percentage point.
7.3.2. Individual questions not in the composite measures
In addition to the 17 questions used to calculate the five CAHPS version 2.0 composite
measures, the survey contained 30 other questions about patients’ experiences with care. These
questions asked for beneficiaries’ perspectives on various aspects of care delivery, including
timely access to care and information, providers’ communication with patients including use of
web portals and reminders and follow-up about tests and treatment, providers’ attention to
patients’ behavioral health needs, providers’ coordination of care with specialists, provider
follow-up after hospital stays and ED visits, patient engagement in caring for chronic conditions,
comprehensiveness of care, and patients’ overall ratings of care received from the provider.
Table 7.6 shows the percentage of beneficiaries in CPC and comparison practices giving the
most favorable ratings for these questions in 2013 or the earliest year the question was asked,
and 2016.
180

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
CPC-comparison differences in 2013 or the first year the question was asked. In 2013
or the first year asked, ratings of CPC and comparison practices were not meaningfully different
(to be meaningful, the difference must have been both statistically significant at the 0.10 level
and five percentage points or more) for 26 of the 28 questions. The two questions exhibiting
meaningful differences measure timely follow-up after hospital stays and after ED visits,
requirements of Milestone 6 (Care Coordination Across the Medical Neighborhood):
• If the beneficiary stayed in a hospital overnight or longer in the last 12 months, the
beneficiary saw a doctor, nurse practitioner, or physician assistant in the provider’s office
within two weeks after the most recent hospital stay (70 percent of beneficiaries in CPC
practices in 2013 compared with 65 percent of beneficiaries in comparison practices)
• If the beneficiary visited the emergency room or emergency department for care in the last
12 months, the beneficiary was contacted by the provider’s office within one week of most
recent visit (53 percent of beneficiaries in CPC practices compared with 48 percent of
beneficiaries in comparison practices in 2014, the first year this question was asked)
CPC-comparison differences in 2016. In general, near the end of the initiative in 2016,
beneficiaries’ ratings of CPC and comparison practices continued to be comparable across most
areas of care delivery measured in this survey. Among the 28 questions that were asked in 2016,
ratings of CPC and comparison practices were comparable for 25 questions.
Beneficiary ratings indicated better care from CPC than comparison practices for two
questions. Similar to 2013 and 2014, the questions measure patient follow-up after hospital stays
and after ED visits (Figure 7.3 and Table 7.6).
• If the beneficiary stayed in a hospital overnight or longer in the last 12 months, the
beneficiary was contacted by the provider’s office within three days of hospital discharge
(60 percent of beneficiaries in CPC practices in 2016 versus 50 percent beneficiaries in
comparison practices)
70
• If the beneficiary visited the emergency room or emergency department for care in the last
12 months, the beneficiary was contacted by the provider’s office within one week of his or
her most recent visit (59 percent of beneficiaries in CPC practices in 2016 versus 51 percent
of beneficiaries in comparison practices)
This finding suggests that the changes CPC practices reported making in these areas
(described in Chapter 5) have positively affected beneficiaries’ experiences with care.
70
Starting with the 2014 survey, we used a revised version of the 2013 question that asked the patient whether he or
she saw a doctor, nurse practitioner, or physician assistant in the provider’s office within two weeks after their most
recent hospital stay. The revised question shortened the follow-up time to be within three days of discharge. We
made this change to align with the new reporting requirements for Milestone 6 beginning in PY2014 that required
practices to follow up with 75 percent of patients from target hospitals within 72 hours of hospital discharge and did
not specify where follow-up needed to occur.
181

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
Figure 7.3. Percentage of Medicare FFS beneficiaries giving the best
response in 2016, CPC and comparison practices, CPC-wide
Sources: CPC patient surveys administered July through October 2016.
*/**/*** The percentage of beneficiaries giving the best response was statistically different between CPC and
comparison practices in the given year at the 0.10/0.05/0.01 level, respectively, but of small magnitude.
a
Among respondents that stayed in the hospital overnight or longer in the last 12 months.
b
Among respondents that visited the emergency room or emergency department for care in the last 12 months.
c
Among respondents that emailed their provider’s office with a medical question in the last 12 months.
FFS = fee-for-service.
Beneficiaries’ responses indicated marked improvement in providers’ attention to patient’s
behavioral health needs. Between 2013 and 2016, the percentage of CPC beneficiaries reporting
that someone in the provider’s office asked them whether there was a period of time when they
felt sad, empty, or depressed increased from 39 to 53 percent; and the percentage of beneficiaries
reporting that someone from the provider’s office spoke with them about things in life that worry
them or cause them stress increased from 42 to 47 percent. However, comparison practices also
experienced improvements in these two questions of 9 and 3 percentage points. Therefore, in
2016, as in 2013, the percentage of beneficiaries reporting the practice had done this was
comparable between CPC and comparison practices.
Ratings of CPC practices were less favorable than those of comparison practices in 2016 for
one question (Figure 7.3 and Table 7.6):
• Fewer beneficiaries in CPC practices reported that they always received an answer to their
medical question as soon as needed when emailing their provider in the past 12 months (69
percent of beneficiaries in CPC practices compared to 75 percent of beneficiaries in
comparison practices). However, fewer than 8 percent of beneficiaries in CPC and
comparison practices reported emailing their provider and thus could answer the question in
2016 (data not shown).
Similarly, when looking at differences in mean responses to the 28 questions in 2016 that
were not in the composites for CPC and comparison practices, we find that more beneficiaries
reported follow-up after hospital stays (0.60 compared with 0.50 out of 1.0) and after ED visits
(0.59 compared with 0.51) in CPC practices than comparison practices. Beneficiaries’ ratings
were comparable for the remaining 26 questions in 2016 (Appendix F, Table F.9).
60***
59***
69**
50
51
75
0
20
40
60
80
100
Contacted by provider's office
within 3 days of hospital stay
Contacted by provider's office
within one week of ED visit
Always received an answer to
emailed medical questions as
soon as needed
Percentage of
respondents
CPC Comparison
a
b
c
182
183
Table 7.6. The proportion of Medicare FFS beneficiaries giving the best response to 28 survey questions not
included in the composite measures, CPC and comparison practices, CPC-wide
Percentage giving the best response
2013
(or earliest year asked) 2016
CPC Comp
CPC–
Comp CPC Comp
CPC–
Comp
Timely access to care and information
When patient phoned provider’s office for care needed right away, patient usually got an appointment on same day
45 48 -3* 42 42 -1
Provider’s office provided patient with information about what to do if care was needed during evenings, weekends, or
holidays
78 79 -2* 79 79 0
If patient needed care during evenings, weekends, or holidays in the last 12 months, patient was always able to get
needed care from provider’s office
33 35 -3 32 31 0
When patient emailed provider’s office, patient always received an answer to his/her medical question as soon as
needed
67 68 -1 69 75
-6**
b
Providers’ communication with patients
If provider’s office used a web portal or website, patient used it often (more than three times) to email the practice,
review medical information, request prescription renewal, or make appointments (first collected in 2014)
13 14 -1 15 17 -2*
In the last 12 months, between visits, patient received reminders about tests, treatment, or appointments from
provider's office
69 70 -1 71 71 0
If provider ordered a blood test, x-ray, or other test, provider’s office always followed up to provide patient with test
results
76 78 -2** 75 77 -2**
Patient always felt that provider really cared about patient as a person 77 79 -2** 79 81 -2**
Clerks and receptionists at provider’s office were always as helpful as patient thought they should be
66 68 -2 71 74 -3***
Clerks and receptionists at provider’s office always treated patient with courtesy and respect 82 84 -2*** 86 86 0
Providers’ attention to patients’ behavioral health needs
Practice staff asked patient during the last 12 months whether there was a period of time when the patient felt sad,
empty, or depressed
39 40 -1 53* 49 3**
Provider spoke with patient during the last 12 months about things in life that worry the patient or cause the patient
stress
42 43 -1 47 46 1
Practice staff spoke with patient during the last 12 months about a personal, family, mental, emotional, or substance
abuse problem
30 30 0 31 30 1
Coordination of care with specialists and other providers
If patient required a referral from provider to see a specialist, patient always easily got a referral 77 80 -2 75 75 0
If patient made an appointment to see a specialist, patient always easily got appointment with specialist 56 57 -1 54 54 0
If patient made an appointment to see a specialist, provider talked with patient during the last 12 months about the cost
of seeing a specialist
8 9 0 8 7 1
If patient made an appointment to see a specialist, patient was worried or concerned during the last 12 months about
the cost of seeing a specialist
22 22 -1 18 18 1
When patient saw a specialist, specialist always knew important information about patient’s medical history
58 59 -2 57 59 -2**

Table 7.6. (continued)
184
Percentage giving the best response
2013
(or earliest year asked) 2016
CPC Comp
CPC–
Comp CPC Comp
CPC–
Comp
If patient visited a specialist, provider always seemed informed and up to date about the care patient received from
specialist
59 61 -2* 60 63 -2**
If patient takes prescription medicines, someone from the provider’s office spoke with the patient at each visit about all
of the prescription medications patient was taking
87 87 0 87 87 0
If patient received conflicting or confusing advice from other providers, provider helped patient manage the information
(first collected in 2015)
73 74 -2 74 74 0
Transitional care and provider follow-up after hospital stays and ED visits
If patient stayed in a hospital overnight or longer in the last 12 months, patient saw doctor, nurse practitioner, or
physician assistant in provider’s office within two weeks after most recent hospital stay
70 65
5***
a
n.a. n.a. n.a.
When patient saw provider within two weeks of most recent hospital stay, provider seemed informed and up to date
about patient’s hospital stay
95 96 -1 n.a. n.a. n.a.
If patient stayed in a hospital overnight or longer in the last 12 months, patient was contacted by provider’s office within
three days of most recent hospital stay (first collected in 2014)
56 52 3* 60 50
11***
a
If patient visited the emergency room or emergency department for care in the last 12 months, patient was contacted
by provider’s office within one week of most recent visit (first collected in 2014)
53 48
5***
a
59 51
8***
a
Patient engagement in caring for chronic conditions
If patient received care from provider for a chronic condition, patient was always asked for her/his ideas or goals when
making a treatment plan (first collected in 2014)
37 36 1 36 36 -1
When patient received care from provider for a chronic condition, patient was always given a copy of her/his treatment
plan (first collected in 2014)
46* 42 4** 47 46 1
Comprehensiveness of care
Provider is always able to treat most of patient’s health conditions and problems (first collected in 2016)
n.a. n.a. n.a. 51* 53 -2**
When patient visited provider with a new problem or symptom in the last 12 months, provider always immediately
referred patient to a specialist instead of trying to treat the problem first (first collected in 2016)
n.a. n.a. n.a. 28 28 0
Patients’ overall rating of care received from the provider
Compared with one year ago, patient feels that the care received by the provider was much better (first collected in
2014)
18 17 1 17 17 0
Source: CPC patient surveys administered June through October 2013, July through October 2014, July through October 2015, and July through October 2016
Notes: */**/*** Responses were significantly different between CPC and comparison practices in the specified year at the 0.10/0.05/0.01 level, respectively.
Green and red shading indicates that the CPC-comparison difference is both statistically significant and substantially significant (five percentage points or larger). Green
shading with bold text indicates that the difference is favorable to CPC; red shading with italicized text indicates that the difference is unfavorable to CPC.
FFS = fee-for-service; Comp = comparison practice; n.a. = not available because the question was asked in only the 2016 survey round.
a
Difference is favorable to CPC.
b
Difference is unfavorable to CPC.

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
Overall ratings of providers and care. Despite giving responses that indicate opportunities
for improvement in many aspects of care, beneficiaries remained pleased with their providers.
Roughly 80 percent of beneficiaries in both CPC and comparison practices rated their provider as
a 9 or 10 out of 10 in 2016. In 2013, 76 percent of beneficiaries in CPC practices and 78 percent
of beneficiaries in comparison practices gave this high rating. In 2014, the survey began asking
beneficiaries to compare the care they received in the last 12 months with the care they received
at the practice in the previous year. In each of the three years this question was asked, about 17
percent of beneficiaries in CPC and comparison practices reported that the care they received
from the provider was much better than in the prior year; about two-thirds reported that the care
compared with one year ago was about the same (data not shown).
7.4. Discussion
Medicare FFS beneficiaries’ ratings of CPC and comparison practices were comparable
across most areas of care measured in the patient survey over the four-year initiative. In 2013, 8
to 12 months into the initiative, beneficiaries in CPC and comparison practices gave similar
ratings for each of the five summary composite measures (the four with adequate reliability and
the one single-question composite). Beneficiaries’ ratings ranged across the composites but
indicated that both CPC and comparison practices had room for improvement in three
composites: (1) timely appointments, care, and information; (2) providers support patients in
taking care of their own health; and (3) providers discuss medication decisions with patients.
Between 2013 and 2016, the first and fourth years of CPC, beneficiaries’ ratings of both
CPC and comparison practices experienced minimal improvement (fewer than three percentage
points) in all but one composite measure—providers support patients in taking care of their own
health—where the percentage of beneficiaries giving the best response improved six percentage
points for CPC practices, but a similar five percentage points for comparison practices. In 2016,
beneficiaries’ ratings of CPC and comparison practices were again comparable across all five
composite measures, indicating that CPC did not improve patients’ experiences as captured by
these measures. In addition, there were no differential effects of CPC on beneficiaries who (1)
were in practices in systems, (2) were in larger practices (measured by having more primary care
clinicians), or (3) had higher risk scores. However, beneficiaries in both CPC and comparison
practices were less likely to give favorable ratings of timely appointments, care, and information
to larger practices.
Responses to 28 questions asked in 2016 that were not in the composite measures further
support the finding that over the course of the four-year initiative, beneficiaries’ experiences with
care were generally comparable in CPC and comparison practices. There were no meaningful
differences in beneficiaries’ ratings for 25 of the 28 questions that were asked in 2016 and not
included in the composite measures. The notable exceptions were that 10 and 8 percentage
points, respectively, more beneficiaries in CPC practices than comparison practices reported
receiving follow-up care after hospital stays and after ED visits. This finding is consistent with
CPC practices’ increasing deployment of follow-up care described in Chapter 5. There was one
unfavorable difference. Fewer beneficiaries in CPC practices than comparison practices reported
that they always received an answer to their medical question as soon as needed when emailing
their provider (69 versus 75 percent). However, more than 92 percent of beneficiaries in both
185

7. HOW DID CPC AFFECT THE EXPERIENCES OF PATIENTS? MATHEMATICA POLICY RESEARCH
CPC and comparison practices reported that they did not email their provider with a medical
question in the past 12 months, and therefore did not answer this question.
These findings suggest that while CPC practices were undergoing substantial changes to
improve care delivery, CPC beneficiaries’ experiences with care changed little during the
initiative and beneficiaries’ ratings were no different from comparison practices on most aspects
of care delivery. The areas where we did see consistent findings—the increasing percentages of
beneficiaries who reported that their provider followed-up with them after hospital stays and ED
visits—reflect CPC’s emphasis on improved coordination of care across the medical
neighborhood.
Prior studies found mixed effects of PCMH adoption on patient experience, measured using
different patient survey instruments. The studies examined patient experience after a shorter
exposure of their practices to transformation—one to two years after their respective
interventions began. Four studies that looked at the impact of medical home transformation on
patient experience of care found no statistically significant effects on patient experience one to
two years after the intervention began (Jaén et al. 2010; Maeng et al. 2013; Heyworth et al. 2014;
Reddy et al. 2015). Three other studies (two of which were on the same intervention) found
statistically significant, favorable, but generally relatively small or isolated, effects in some
dimensions of patient experience with care (Reid et al. 2009, 2010; Kern et al. 2013):
• Reid et al. (2009 and 2010) examined patient experience one and two years into a PCMH
demonstration in one clinic compared with two comparison clinics. One year into the
demonstration, patients in the demonstration clinic reported improved experiences relative to
patients in the two comparison clinics in six of seven domains (p-values < 0.05): quality of
doctor-patient interactions, shared decision making, coordination of care, access, patient
activation and involvement, and goal setting and tailoring. Differences were small: between
2 and 3 points on a 100-point scale. Two years into implementation, effects moderated;
relative to patients in the comparison clinics, patients in the demonstration clinic reported
relatively larger improvements in patient experience in four of the seven domains, and
effects were generally smaller.
• In another study of PCMH implementation that looked at how patient experience changed
over time in a group of practices transforming into PCMHs, Kern et al. (2013) found
statistically significant improvement at the 5-percent level in the proportion of respondents
giving the best rating in the access-to-care composite measure (from 61 to 69 percent) and
statistically significant improvement at the 10-percent level in experience with office staff
(from 72 to 78 percent). The proportion of respondents giving the best rating in the
composite measure for follow-up with test results showed a statistically significant decline
at the 10-percent level, from 76 to 69 percent. There were no effects in the other dimensions
of patient experience that they measured: communication and relationships, disease
management, doctor communication, and overall rating of the doctor. However, the study
did not have a comparison group to net out any secular trends that may have affected patient
experience.
186

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
8. WHAT WERE CPC’S IMPACTS ON MEDICARE EXPENDITURES, SERVICE
USE, AND QUALITY OF CARE DURING THE INITIATIVE?
CPC’s changes to primary care delivery were expected to lower Medicare fee-for-service
(FFS) expenditures and service use and improve quality of care. In this chapter, we describe the
effects of CPC on claims-based health care expenditures, service use, and quality during the 51
months of the model (October 2012 through December 2016)
71
for Medicare FFS beneficiaries
attributed to CPC versus those attributed to comparison practices. We estimated the impact of
CPC by using difference-in-differences regressions that compare mean beneficiary outcomes
between CPC practices and a set of similar practices that were not participating in CPC. The
analysis compared outcomes from the 12 months before CPC and the 51 months after CPC
began, and controlled for beneficiary, practice, and market characteristics. It included 565,674
unique Medicare FFS beneficiaries attributed at any time during the initiative to 497 CPC
practices and 1,165,284 beneficiaries attributed to 908 matched comparison practices.
72
We used
an intent-to-treat (ITT) approach that continued to include beneficiaries in the analysis even if
they were no longer attributed. The chapter focuses on impacts for CPC as a whole; we report
regional analyses in Appendix G. Appendix H provides additional details on the methods used to
select the comparison group and Appendix I describes our analysis methods and provides
definitions of the outcome measures.
8.1. Key takeaways on the effect of CPC on Medicare expenditures, service
use, and quality of care
• CPC had favorable effects on hospitalizations and emergency department (ED) visits.
Although Medicare service utilization grew during the initiative for both CPC and
comparison practices, CPC practices experienced slower growth in hospitalizations, ED
visits, and primary care visits than comparison practices. Hospitalizations increased by 2
percent less for CPC practices than for comparison practices over the initiative (or by 5
fewer hospitalizations per 1,000 beneficiaries per year, p = 0.07) (Table 8.1). There was also
slower growth in outpatient ED visits for CPC practices than comparison practices during
the initiative of 2 percent (or 10 fewer ED visits per 1,000 beneficiaries, p = 0.03). The
effects on ED visits were more pronounced in the last two years of CPC.
• The favorable effects on hospitalizations and ED visits are consistent with the findings
from the implementation analysis. For instance, deep-dive practices noted that promoting
high-risk patients’ access to a care manager improved care and reduced hospitalizations
through more attentive transitional care, medication reconciliation, and the identification of
71
In contrast to the program years we discuss in earlier chapters, Years 1 through 3 each contain one year of results,
and Year 4 contains a year and a quarter of results. Year 1 results in this chapter are for CPC’s first 12 months
(October 2012 through September 2013), Year 2 results are for months 13 to 24 (October 2013 through September
2014), Year 3 results are for months 25 to 36 (October 2014 through September 2015), and Year 4 results are for
months 37 to 51 (October 2015 through December 2016). However, we express all results in terms of per month or
per year of follow-up; therefore, the length of the period over which outcomes are measured does not affect their
means.
72
Although 502 practices were selected to participate in CPC, 5 practices voluntarily withdrew after assessing the
terms and conditions of CPC participation early in the initiative. Therefore, the evaluation includes 497 CPC
practices in the ITT analysis of CPC’s impacts.
187

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
problems between visits over the phone. Also, deep-dive practices noted that improvements
they made in other areas were likely reducing ED use. Changes included:
- Better identifying patients who frequently used the ED and targeting outreach to them.
- Better identifying high-risk patients.
- Encouraging patients to call the office before using the ED for nonurgent care.
- Improving access to the primary care practice.
Findings from the beneficiary survey suggest that more CPC practices provided timely
follow-up care after hospitalizations and ED visits than comparison practices. Practice
members thought that providing better follow-up care after hospital discharges and ED visits
improved patient care.
• CPC reduced primary care visits. Office-based primary care visits grew by 2 percent less
for CPC than comparison practices (or by 68 fewer visits per 1,000 beneficiaries per year,
p = 0.07) (Table 8.1). This effect on office-based primary care visits might have been driven
by greater reliance on non-visit-based interactions with patients among CPC practices, for
example, by phone, or through follow-up by care managers, who cannot bill Medicare for
such services.
• CPC did not lead to statistically significant changes in total Medicare expenditures
(excluding care management fees). Over the course of the initiative, Medicare
expenditures without care management fees increased by 1 percent (or $9 per beneficiary
per month [PBPM]) less for the CPC practices than the comparison practices, but the
difference was not statistically significant (p = 0.16, 90 percent confidence interval [CI] -
$19, $2) (Table 8.1). Lower growth in inpatient expenditures, expenditures on skilled
nursing facilities, and outpatient services drove the lower growth in total expenditures for
the CPC group.
• Although we would expect the effects on patient outcomes to increase over time as
practices further implemented the CPC functions, year-by-year effects on Medicare
expenditures without fees declined over time. Estimated savings declined from $18 in
Year 1, to $11 in Year 2, $4 in Year 3, and $2 in Year 4 (Table 8.1).
• CPC did not generate enough savings to offset the care management fees for Medicare
FFS beneficiaries. Including CPC’s Medicare FFS care management fees (which averaged
$15 per beneficiary in our ITT analysis),
73
average monthly Medicare expenditures per
beneficiary increased by 1 percent or $6 more for CPC than for comparison practices over
the 51 months. This difference was not significantly different from zero (p = 0.35, 90
percent CI -$4, $16). Findings from a Bayesian analysis also showed a high probability (94
percent) of some gross savings but almost a zero probability that the savings were sufficient
73
CMS paid $20 PBPM in care management fees during Quarters 1 through 9 of CPC (through December 2014),
and paid $15 PBPM from January 2015 onward (for the last eight quarters of CPC). Therefore, over the 17 quarters
of CPC, the average PBPM care management fee paid for patients still attributed to a practice was approximately
$18. However, the average PBPM fee received in our ITT analysis sample was $15, because we retain all
beneficiaries in the analysis after they were first attributed, even if a practice withdrew or no longer received fees for
them because they were no longer attributed.
188

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
to cover the care management fee. Therefore, it is unlikely that CPC was cost neutral or
generated net savings for Medicare.
• CPC had minimal effects on quality-of-care process and outcome measures. There were
very few sizeable or statistically significant estimates for the quality-of-care process and
outcome measures, or continuity of care. Among the limited claims-based measures
available (five process measures for beneficiaries with diabetes, and for all beneficiaries,
one transitional care measure, four continuity-of-care measures, and three outcome
measures), cumulative estimates show a statistically significant effect on only one measure:
the likelihood of an ED revisit within 30 days of an outpatient ED visit increased by 0.2
percentage points less, or about 3 percent of the mean rate (p = 0.02), for CPC than
comparison practices (Tables 8.3 and 8.10). In annual estimates, the only statistically
significant findings for quality-of-care process measures among beneficiaries with diabetes
were in the high-risk subgroup.
74
• Within certain subgroups, CPC generated a favorable impact on Medicare
expenditures without care management fees, but the evidence for differential impacts
for different types of practices was weak. We expected that CPC might have different
impacts for practices with certain characteristics, so we tested for differential impacts on
subgroups defined by those characteristics. We found that estimated effects on Medicare
expenditures without fees were favorable and significantly different from zero (indicating
gross savings) for practices that:
1. Were recognized as medical homes at baseline
2. Had six or more clinicians or were affiliated with a larger organization
3. Were hospital or system-owned
4. Were medium-sized (3–5 clinicians)
For example, the third finding indicates we found a favorable impact when we tested for
differences among CPC and comparison practices that were owned by a hospital or system
at baseline.
In contrast, there were no statistically significant differences in Medicare expenditures
between CPC and comparison practices among the subgroup that had at least one clinician
who met requirements for meaningful use of electronic health records (EHRs), nor in its
counterpart.
The findings from these subgroup analyses suggest that practices with experience
transforming care and greater access to resources may have achieved greater savings.
However, there is only weak evidence for more favorable impacts within these practice
subgroups because the impact estimates for any given subgroup were not significantly
different from the estimates for its respective counterpart (that is, the opposite subgroup).
For example, although there was a favorable $17 PBPM impact among practices that were
74
This evaluation did not include the electronic clinical quality measures (eCQMs) that the model used for quality
measurement and improvement for the entire practice population, and for calculating eligibility to share in any
Medicare shared savings. Not all comparison practices report eCQMs, creating both conceptual and data challenges
for analyzing the impacts of CPC on eCQMs.
189

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
owned by a hospital or system at baseline, that impact was not statistically different from the
favorable $3 PBPM impact for practices that were not hospital- or system-owned at
baseline. Applying any corrections for multiple comparisons or multiple hypothesis testing
would make it even less likely that we would find statistically significant differences. We
also tested different definitions of some subgroups. In total, we tested for differential
impacts across the seven sets of subgroups shown in Table 8.8.
Table 8.1. Percentage impacts on Medicare FFS expenditures and service
utilization over the four years of CPC (all attributed beneficiaries)
Outcomes Year 1 Year 2 Year 3 Year 4
Years 1–4
combined
Total Medicare expenditures (dollars per beneficiary per month)
Without CPC care management fees
-2%***
a
-1% 0% 0% -1%
With CPC care management fees 0% 1% 1% 1% 1%
Expenditures by type of service (dollars per beneficiary per month)
Inpatient
-3%**
a
-1% 0% 0% -1%
Skilled nursing facility
-7%***
a
-6%**
a
-3% -3% -5%
Outpatient -1% -2%
-3%**
a
-3%*
a
-2%**
a
Physician 0% -1% 1% 2%*
b
1%
Primary care physician
-2%***
a
-3%***
a
-1% -1%
-2%*
a
Office-based primary care
-2%*
a
-3%***
a
-2%**
a
-1%
-2%**
a
Specialist 0% 1% 2% 3%**
b
2%*
b
Office-based specialist 1% 0% 1% 2%*
b
1%
Home health
-3%**
a
2% 1% -1% -1%
Hospice 2% 1%
10%*
b
7% 5%
DME 0% -2% -4%
-4%*
a
-3%
Service utilization (annualized rate per 1,000 beneficiaries)
Hospitalizations
-2%*
a
-2% -1% -2%
-2%*
a
Total ED visits -1% -1%
-2%***
a
-2%***
a
-2%***
a
Outpatient ED visits -1% -1%
-3%***
a
-3%**
a
-2%**
a
Observation stays 2% 7%**
b
4% 7%**
b
5%**
b
Primary care visits -1%
-1%*
a
-1% -1% -1%
Office-based primary care visits -1%
-2%**
a
-2%*
a
-1%
-2%*
a
Specialist visits 0% 0% 1% 2%***
b
1%
Office-based specialist visits 0% 0% 0% 2% 0%
Source: Medicare claims data for October 2011 through December 2016.
Note: We base impact estimates on a difference-in-differences analysis; they reflect the difference in the regression-adjusted
average outcomes for attributed Medicare FFS beneficiaries in CPC practices for a specific year compared with baseline
relative to the same difference over time for attributed Medicare FFS beneficiaries in matched comparison practices. We
calculate percentage impacts by dividing the impact estimate by the projected CPC group mean in the absence of CPC
(that is, the unadjusted CPC group mean minus the CPC impact estimate). Red shading with white italicized text
signifies that our estimate was statistically significant and showed an increase in the service use or expenditures
outcome (note, however, that increases in expenditures or use of certain services such as primary care and hospice
could be beneficial); green shading with bold text signifies that an estimate was statistically significant and implied a
reduction in the service use or expenditures outcome. Expenditures on physician services include expenditures on
primary care physician services, specialist services, and services provided by other noninstitutional providers (the third
category is not shown separately). Measures of outpatient ED visits and total ED visits include observation stays.
Primary care visits include both office-based primary care visits and primary care visits in other settings. Analysis
includes 565,674 Medicare FFS beneficiaries attributed to 497 CPC practices and 1,165,284 beneficiaries attributed to
908 matched comparison practices. Each beneficiary can contribute as many as five observations in the analysis—one
during the baseline year and one during each follow-up year.
*/**/*** Significantly different from zero at the 0.10/0.05/0.01 level, two-tailed test.
FFS = fee-for-service; DME = durable medical equipment; ED = emergency department; PBPM = per beneficiary per month.
a
The estimate was favorable a nd statis tically significant.
b
The estimate was unfavorab le and s tatis tically si gnific ant.
190

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
8.2. Methods
Our difference-in-differences analysis compared changes in outcomes from the year before
CPC began (baseline) to the period after it began for Medicare FFS beneficiaries attributed to
CPC practices, with changes over the same period for beneficiaries attributed to comparison
practices. We examined changes in outcomes from the year before CPC to the four years of CPC.
We used an ITT analysis that included beneficiaries even if (1) they were no longer attributed to
their original practice, or (2) their practice had closed, withdrawn from the initiative, merged
with another practice, or split. Among beneficiaries attributed to a CPC practice in the first
quarter of the initiative, 76 percent were still attributed to the same practice in Year 4.
75
8.2.1. Comparison group selection
We used propensity-score matching to select seven comparison groups—one for each
region’s CPC practices. Practices in the pool from which we selected the comparison groups
included (1) those in nearby areas (listed in Table 8.2) that were external to the CPC regions but
that the authors and CMS considered to have reasonably similar demographics and market
factors for “face validity” and enough practices for matching (external comparison practices),
and (2) those that had applied to CPC in the same regions as the CPC practices but were not
selected (internal comparison practices). Internal comparison practices met core eligibility
criteria and were similar to CPC practices in terms of their use of EHRs but were not selected for
CPC, primarily because they had low application scores.
76
They made up 28 percent of all
selected comparison practices.
Table 8.2. CPC regions and external comparison group regions
CPC region External comparison group regions
Arkansas Tennessee
New York: Capital District-Hudson Valley region Western and central New York, New Jersey, and Connecticut
Oregon Idaho and Washington
Colorado Utah, Kansas, and selected counties in New Mexico
New Jersey Western and central New York and Connecticut
Ohio/Kentucky: Cincinnati-Dayton region Remaining counties in Ohio
Oklahoma: Greater Tulsa region Remaining counties in Oklahoma
75
The corresponding figure for comparison practices was 72 percent. To focus on the continuity of attribution over
time, these calculations excluded beneficiaries who died, moved out of state, or lost Medicare Part A or B eligibility.
76
CMS selected practices to participate in CPC based largely on their application score. The score gave a practice as
many as 530 points for using health information technology, as many as 80 points for the percentage of practice
revenue from participating payers, as many as 70 points for patient-centered medical home recognition, and as many
as 35 points for participating the prior three years in quality improvement or practice transformation activities (such
as quality improvement organization activities, Regional Extension Centers, or local or national learning
collaboratives). The score did not include pre-CPC expenditures, service use, or patient outcomes. Because EHR use
was expected to affect outcomes, we required that CPC and comparison practices match exactly on whether they
were meaningful users of EHRs. CMS also weighed other factors in its final selections, such as geographic and
patient diversity.
191

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
We required both groups of practices to meet eligibility criteria similar to those for CPC
practices.
77
Specifically, we required them to have at least 100 attributed Medicare
beneficiaries
78
and at least one primary care clinician. Practices could not be participating in any
Medicare shared savings model at baseline.
We selected comparison practices from this pool of potential comparison practices using a
propensity-score model that matched CPC and comparison practices on various baseline practice
characteristics from before CPC started in October 2012. These characteristics included:
• Status as a National Committee for Quality Assurance (NCQA)- or state-recognized medical
home
• Number of clinicians
• Presence of a Medicare-defined meaningful user of an EHR
• Market characteristics, such as household income of the practice’s zip code
• Average patient characteristics of the practice’s attributed Medicare FFS beneficiaries, such
as demographics and Medicare cost and service use before CPC
We then implemented a technique called full matching to form matched sets that contained one
CPC practice and one or more comparison practices, or one comparison practice and multiple
CPC practices. We identified a match for a given CPC practice when the propensity score for a
potential comparison practice fell within a specified range around the CPC practice’s propensity
score, selecting as many as five matches. Thus, a practice could serve as a comparison for
multiple CPC practices, and a CPC practice was typically matched to multiple comparison
practices.
We included each group in the pool of potential comparison group practices for different
reasons. We included the internal comparison practices because they had expressed the same
willingness to participate in the initiative as the CPC practices and were therefore likely to share
the same motivation and self-perceived capacity (unobserved characteristics) to provide
enhanced primary care to beneficiaries. In addition, because these internal comparison practices
were located in the same region as the CPC practices, they were subject to the same market
conditions, such as practice patterns and health care markets. Therefore, including them helped
account for market factors that could affect outcomes and that our control variables could not
fully account for. Typically, evaluations do not choose nonselected practices for their
comparison group out of concern that they were functioning more poorly than practices that had
been selected, or could be contaminated due to spillover benefits of the model (about 14 percent
of comparison practices shared the same owner as one or more CPC practices). However, CMS
77
We did not apply some eligibility criteria (such as the requirement for at least 50 percent of a practice’s revenue
to come from participating payers) to comparison practices because CMS did not strictly apply the criteria. That is,
we did not use eligibility criteria to exclude potential comparison practices if a nontrivial number of CPC practices
did not meet the criteria (see Appendix H).
78
Although the CPC eligibility criterion was 120 attributed beneficiaries, we used a threshold of 100 attributed
Medicare beneficiaries for comparison practices, because our analysis of Medicare claims data found that some CPC
practices actually had between 100 and 120 attributed Medicare beneficiaries.
192

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
did not score practices based on their pre-CPC outcomes or approaches to providing different
aspects of primary care. (Also, our subsequent analysis showed that the application score was not
related to Medicare expenditures or service use outcomes during the evaluation period.) Through
propensity-score matching, we could ensure that the comparison group had similar values for
two measures, perhaps related to subsequent performance, that CMS weighted heavily when
scoring practices’ applications: (1) meaningful use of EHRs and (2) medical home recognition.
We also mitigated concerns about spillover effects in the internal comparison practices by
running sensitivity analyses that included only beneficiaries from practices in the external group
(located outside the CPC region).
We included in the comparison pool external comparison practices (from outside the CPC
region) because they were not subject to selection bias resulting from not being selected during
the application process, and they were unlikely to benefit from spillover of CPC. However, the
comparison group did not contain only external practices, because we could not know which of
them would have had the same motivation and self-perceived capacity (unobserved
characteristics) to provide enhanced primary care to beneficiaries demonstrated by the practices
that applied to CPC. Also, potentially unobserved differences in market factors between the CPC
regions and the external comparison regions could affect outcomes. Nonetheless, it was
necessary to include external comparison practices because there were too few CPC applicants
that were not chosen for CPC to provide acceptable internal matches for all CPC practices.
To ensure that the selected comparison group was similar to CPC practices at baseline, we
excluded from the potential comparison practices any practice that was participating in a CMS-
sponsored shared savings model in 2012. (These practices were not eligible to participate in
CPC.) During the initiative, about 42 percent of the selected comparison practices (ranging from
21 percent in Oklahoma to 60 percent in Ohio/Kentucky) joined a CMS-sponsored shared
savings model; among comparison practices in CMS-sponsored initiatives, nearly 96 percent
were in the Medicare Shared Savings Program (SSP), an accountable care organization (ACO).
We do not believe this approach is a shortcoming. Rather, it ensures that the evaluation answers
the question of how CPC alters outcomes compared with usual care, which also changed during
this time. Thus, our impact estimates capture how Medicare FFS beneficiaries fared under CPC
versus how they would have fared without CPC, given the availability of SSP and other
initiatives. However, it is important to remember that CPC operated during a period with an
unusually high number of large, federal and private initiatives to improve care and reduce costs.
These initiatives may have had effects of their own, compared to what had been considered usual
care. In other words, the evaluation assessed how CPC practices fared relative to practices that
were operating in a changing landscape, which itself may have been influenced by CPC.
Appendix H shows that CPC and comparison practices were similar on a range of market-,
practice-, and beneficiary-level characteristics. It also lists the number of comparison practices
that we drew from the same region and from external regions.
We did not adjust significance levels to account for all the hypothesis tests we conducted,
because we did not want to increase the likelihood of failing to identify a true intervention effect.
Instead, because total Medicare expenditures was the most important measure and encompasses
effects on all services and expenditures by type of service, we treated it as the primary outcome,
for which we used a 0.10 significance level from a two-tailed test. Other outcomes were
193

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
secondary. Therefore, we relied on a combination of the size, significance level, and patterns of
findings across related measures, over time, and across regions, to assess whether statistically
significant impact estimates were likely due to chance or to true effects of CPC.
8.2.2. Outcomes
We estimated impacts for the following claims-based outcomes to measure whether CPC
reduced Medicare FFS expenditures and service use and improved quality of care:
• Medicare Part A and Part B monthly expenditures (with and without Medicare’s CPC
care management fees). We first examined whether CPC affected gross Medicare
expenditures (not including fees) for service use and the size of those effects. We then
examined whether the gross savings exceeded the care management fees. If impact estimates
suggested that CPC reduced gross Medicare expenditures and net Medicare expenditures
were not significantly different from zero, then we would have evidence that is consistent
with (though not proof of) cost neutrality. If we could not reject the hypothesis of no effects
on gross Medicare expenditures, then it would be unlikely that CPC was cost neutral, even if
we could not reject the hypothesis that the effect on net expenditures was zero. This
approach allowed us to gather rigorous evidence about whether CPC was cost neutral.
Because CPC care management fees were a relatively small portion of Medicare
expenditures, we might find that net Medicare expenditures were not significantly different
from zero (due to limited statistical power) even if we had no clear evidence that CPC
reduced expenditures for service use.
• Medicare Part A and Part B monthly expenditures with Medicare’s CPC care
management fees and shared savings payments from CPC and SSP. To provide a
complete picture of savings or losses to Medicare, we also accounted for the fact that many
CPC practices received shared savings payments from CMS as part of CPC in each
performance year.
79
Further, a sizeable fraction of matched comparison practices, and some
CPC practices that had stopped participating in CPC, were eligible to receive shared savings
payments from Medicare based on their participation in other Medicare initiatives,
especially SSP ACOs. Given that these payments were expenditures incurred by Medicare,
we also estimated the impact on Medicare expenditures after accounting for Medicare
shared savings payments received by both CPC and comparison practices from CPC and
SSP. To do this, we constructed a PBPM measure of the shared savings payments received
by CPC and comparison practices through their participation in CPC and SSP, using
79
CMS’s shared savings calculations served a different purpose than the evaluation. As such, they used a different
approach (DeLia 2016; CMS 2017b) and generated different results.
Shared savings are intended to provide
practices with incentives to improve the quality and cost of care. For shared savings, CMS contractor Actuarial
Research Corporation compared CPC-attributed beneficiaries’ actual expenditures to an actuarial target spending
level based on baseline spending of a reference population of other beneficiaries in the region, trended forward from
2012 to the performance year. In contrast, the evaluation was intended to assess the impact of CPC. The impact
estimates described above compare the change in expenditures between the year before CPC began (October 2011
through September 2012) and the four years of CPC operations (October 2012 through December 2016) for
beneficiaries attributed to CPC practices in the region relative to that of beneficiaries in matched comparison
practices. Differences between the comparison strategies (and, to a lesser extent, the time periods) used to calculate
shared savings and to conduct the evaluation produced some differences in results.
194

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
beneficiary attribution and Medicare FFS eligibility information. We calculated the total
number of ‘beneficiary-eligible’ months in a given calendar year for a practice by
aggregating across beneficiaries the number of months in which each beneficiary was
attributed to the practice (or in the case of a practice in an ACO, the number of months in
which each beneficiary was attributed to the ACO) and eligible for Medicare FFS. We then
divided the annual shared savings payments to the practice from CPC or SSP by the
practice’s total number of beneficiary-eligible months in the year to obtain a PBPM amount.
Finally, for each year of CPC, we added this PBPM shared savings amount to beneficiary-
level monthly Medicare expenditures (including CPC care management fees) of
beneficiaries in our analysis, for the months they were attributed to either a CPC practice or
an ACO and were also enrolled in FFS Medicare.
80
• Medicare Part A and Part B monthly expenditures by type of service. Types of service
included inpatient, physician, outpatient, skilled nursing facility, durable medical equipment
(DME), hospice, and home health.
• Rates per 1,000 beneficiaries of annual Medicare service use. Services included
hospitalizations, outpatient ED visits, total ED visits, primary care physician visits in all
settings, office-based primary care physician visits, specialist visits in all settings, and
office-based specialist visits. Visits to primary care physicians and specialists included
evaluation and management visits.
81
• Five annual claims-based quality-of-care process measures for beneficiaries with
diabetes.
82
For attributed beneficiaries with diabetes during a program year, we examined
the likelihood of receiving:
1. An HbA1c test.
2. An eye exam.
80
We obtained information on shared savings payments to CPC practices and to SSP ACOs during 2013 to 2015.
Because of delays in the availability of shared savings information, we did not have payment information for
calendar year 2016, which overlapped with Year 4 of CPC (October 2015 to December 2016).
81
We identified primary care providers using Health Care Financing Administration (HCFA) specialty codes.
Primary care visits in all settings included office-based primary care visits as well as visits in other settings with a
primary care provider, such as primary care visits in the hospital or nursing home. Office-based primary care visits
were visits with a primary care provider for office-based evaluation and management (CPT codes 99201–99205,
99211–99215). Specialist visits in all settings and office-based specialist visits were similarly defined, based on
identifying specialists using HCFA specialty codes (see Appendix I for details, including lists of specialty codes for
primary care and specialty providers).
82
The initiative did not explicitly target these claims-based quality-of-care measures. Practices were required to
report eCQMs based on their EHRs, but those quality measures include care received by beneficiaries from only that
practice. The quality-of-care measures reported in this chapter span all of the care received by beneficiaries across
all providers, not just the CPC practice. The three measures for patients with diabetes are based on Healthcare
Effectiveness Data and Information Set (HEDIS) specifications. Note that earlier reports included two additional
measures, for lipid testing among beneficiaries with diabetes and lipid testing among beneficiaries with ischemic
vascular disease. We excluded these measures from the analysis for this final report, because the American College
of Cardiology and the American Heart Association no longer recommend these tests.
195

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
3. A urine protein test.
4. All three exams or tests.
5. None of the three exams or tests.
• Four claims-based continuity-of-care measures for all beneficiaries. To measure
continuity of care, we examined:
1. The percentage of primary care office visits with the attributed practice.
2. The percentage of all (primary and specialist care) office visits with the attributed
practice.
3. The Bice-Boxerman Index (BBI) for primary care visits.
83
4. The BBI for all (primary and specialty care) office visits.
• One claims-based transitional care measure. To measure transitional care, we examined
receipt of a follow-up visit by any clinician from the attributed practice or another practice
within 14 days of a hospital discharge (included billing for complex chronic care
coordination, chronic care management, and transitional care management; see Appendix I
for details).
• Three claims-based quality-of-care outcome measures. For this category, we examined:
1. The likelihood of an unplanned hospital readmission during the 30 days after hospital
discharge.
2. The rate of hospitalizations for ambulatory care sensitive conditions (ACSCs) per 1,000
beneficiaries per year.
3. The likelihood of an ED revisit within 30 days of an outpatient ED visit.
In the next section, we discuss how we addressed issues of potential bias in readmission and
re-visit measures due to possible effects of CPC on ED visits and hospitalizations.
84
8.2.3. Difference-in-differences estimation strategy
We estimated the impact of CPC by using difference-in-differences regressions. These
regressions compared mean outcomes between the CPC and comparison groups during the 4
quarters before CPC and the 17 quarters of CPC, while controlling for beneficiary, practice, and
market characteristics. The outcomes were measured as Medicare expenditures PBPM, in annual
rates per 1,000 beneficiaries for service use outcomes, and as percentage of beneficiaries
receiving appropriate care for quality outcomes. The models netted out any remaining observable
pre-existing differences in outcomes between the beneficiaries in CPC and comparison practices
at baseline that propensity-score matching did not account for. Our estimated standard errors
83
The BBI measures the concentration of a patient’s visits across all practices that the patient visited over a period
of time. For example, if a patient had 10 visits with the same practice, the BBI would be 1 (perfect continuity); if the
patient had 1 visit with each of 10 practices, the BBI would be zero.
84
This evaluation did not include the eCQMs used for quality improvement and for calculating shared savings. Not
all comparison practices reported eCQMs, creating both conceptual and data challenges for analyzing the impacts of
CPC on eCQMs.
196

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
accounted for practice-level clustering of beneficiary outcomes and for weighting. The
observation weights were equal to the product of two separate weights that accounted for (1) the
share of the year for which the beneficiary’s data were observed, and (2) the matching (for
beneficiaries in comparison practices only). We calculated the matching weights in a way that
ensured that the total weights for beneficiaries in the comparison practices matched to a given
CPC practice equaled the total weights for the beneficiaries in that CPC practice. That is, the set
of comparison practices matched to a given CPC practice represented the same share of the
overall comparison group as the CPC practice represented among all CPC practices.
For Medicare expenditures with and without care management fees and for the continuity-
of-care measures, we estimated a linear regression. (We describe the measures and regressions in
Appendix I.) For the service utilization outcomes (hospitalizations, ED visits, ACSC admissions,
and physician visits), which were measured as utilization counts per 1,000 beneficiaries per year,
we used maximum likelihood models appropriate for count variables. Specifically, to account for
overdispersion in utilization counts, we used negative binomial models for service utilization
outcomes such as physician visits, and to account for overdispersion and the large percentage of
zeroes (beneficiaries with no utilization during a quarter), we used a zero-inflated negative
binomial model for service utilization outcomes that had a large percentage of zeroes, such as
hospitalizations and ED visits.
85
We used separate logistic regressions to estimate the likelihood
of (1) an unplanned readmission within 30 days following a discharge, (2) a follow-up visit
within 14 days of a discharge, and (3) an ED revisit within 30 days of an outpatient ED visit.
86
We also used logistic regressions for the binary quality-of-care measures for beneficiaries with
diabetes included in the annual analysis.
Our regressions controlled for the same practice characteristics and market characteristics
used in the propensity score matching. The practice characteristics were:
1. Status as an NCQA- or state-recognized medical home
2. Number of clinicians
3. Whether the practice is multispecialty
4. Whether the practice is owned by a larger organization
5. The presence of a Medicare-defined meaningful user of EHR
85
The zero-inflated negative binomial model assumes that the excessive zeroes (1) were generated by a separate
process from the count values and (2) can be independently modeled using a binary outcome model, such as a logit
model.
86
The equations for readmissions and follow-up visits were estimated on all discharges for beneficiaries with
eligible index discharges, and included both beneficiary- and discharge-level control variables. The likelihood of an
ED revisit was modeled for all beneficiaries and was estimated as a beneficiary-level outcome. To eliminate
potential biases due to CPC effects on admissions, we separately estimated a beneficiary-level equation (that
included all attributed beneficiaries) for whether the beneficiary had a readmission within 30 days of discharge. The
beneficiary-level readmission rates were quite low (about 3 per 100 beneficiaries), and almost none of the results
were statistically significant.
197

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
The market characteristics included:
1. Household income of the practice’s zip code
2. Medicare Advantage penetration rate
3. Percentage of the county that is urban
4. Whether the practice is located in a medically underserved area
The regressions also controlled for beneficiary-level characteristics measured before CPC,
including demographics (age categories, race categories, gender); Medicare and Medicaid
eligibility (original reason for Medicare eligibility, dual status); and Hierarchical Condition
Category (HCC) score. In addition, the readmission and follow-up visit equations included
discharge-level controls to account for risk factors associated with each of a beneficiary’s
discharges. We sourced these control variables from the risk-adjustment methodology for CMS’s
Hospital-Wide All-Condition Unplanned Readmission measure, and they are statistically
significant predictors of the risk of readmission and follow-up visits in our analysis. Specifically,
we controlled for the following discharge-level factors: indicators for 31 condition categories
(with one serving as the reference category) identified in inpatient episodes of care during the 12
months before the index admission, as well as those present at admission. To avoid endogeneity
issues, we did not control for diagnoses that may have occurred as a complication of care during
the index admission. We also controlled for indicators for the specialty cohort to which the
principal diagnosis or procedure associated with the index discharge belonged. The six cohorts
for which we included indicator variables in the model (with one serving as the reference
category) were (1) medicine, (2) surgery, (3) cardiorespiratory, (4) cardiovascular, (5) neurology,
and (6) other. For the ED revisit model, which was estimated at the beneficiary level, we also
controlled for 23 baseline chronic condition indicators defined by applying the claims-based
Chronic Conditions Warehouse algorithm to Medicare claims.
For all outcomes except continuity-of-care outcomes, we calculated effects for each of the
four years of CPC separately. We also estimated effects cumulatively as weighted averages
across the four years combined.
87
For continuity of care, we examined only cumulative outcomes
over two periods—the four pre-intervention years combined and the four intervention years
combined—to measure continuity over consistent and similarly broad time horizons both before
and after the intervention. Because CPC had a total of 17 quarters, we annualized all Medicare
expenditures and service use outcomes over 5 quarters in Year 4 of CPC instead of annualizing
over 4 quarters, as in prior years. However, to ensure consistency in measure definitions over
time for outcomes that were not annualized—for example, the binary quality-of-care process
measures for beneficiaries with diabetes and for the continuity-of-care measures—we excluded
Quarter 17. We report the size of the impacts (for example, in dollars for expenditures) and the
percentage impacts. To calculate the percentage impacts, we divided the impact estimate by the
projected CPC group mean in the absence of CPC (that is, the unadjusted CPC group mean
minus the CPC impact estimate).
87
We estimated quarterly results but we do not focus on them unless they show a meaningful trend, because they
are more variable and less important than effects over a longer period. See Figure 8.3 for quarterly impact estimates
for Medicare expenditures without fees.
198

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
We present results both for all attributed Medicare FFS beneficiaries and for a subgroup
including the high-risk beneficiaries, for whom we expected CPC to have larger effects on
expenditures and service utilization because of their greater need for costly services. This
subgroup includes the beneficiaries with the highest quartile of 2012 HCC scores.
The following factors are important in interpreting findings from the impact analysis. First,
because we followed the cohort of beneficiaries in each research group over time, we expected
their expenditures and service use to increase as they age and their health deteriorates. Second,
we refer to an impact estimate as a “relative reduction” if it suggests that the expenditures or
service use of beneficiaries in CPC practices increased less than those of their comparison group
counterparts; we refer to an impact estimate as a “relative increase” if it suggests that the
expenditures or service use of beneficiaries in CPC practices increased more than those of their
comparison group counterparts. Third, impact estimates for Years 1 through 3 in this report
differ slightly from the estimates presented in prior reports. This variation occurs because the
beneficiary-level impact estimates for prior years were updated using a longer period of claims
runout and beneficiaries who were newly attributed to CPC or comparison practices were added
to the baseline observation. The aggregate estimates also differ because we updated the total
number of eligible beneficiary months during a year due to changes in eligibility information in
the Medicare enrollment database.
8.2.4. Statistical power to detect effects
The numbers of practices and beneficiaries provided reasonable confidence that the analysis
would detect even small impacts of CPC on Medicare service use and expenditures for all
beneficiaries and for high-risk beneficiaries for the initiative as a whole, and would detect
modest impacts by region. For estimates using two-tailed tests at the 10 percent significance
level, the evaluation had 80 percent power to detect CPC-wide impacts of 2 percent on
cumulative expenditure estimates during the course of the initiative and 2.4 percent on annual
expenditure estimates. Minimum detectable effects (MDEs) for annual estimates range from
about 4 to 8 percent for any region.
Although the MDEs were higher for the high-risk subgroup than for all beneficiaries (for
example, about 3.5 percent versus 2.4 percent, respectively), it may be easier to detect effects
among members of the high-risk subgroup. If intervention effects on expenditures, service use,
and quality were concentrated largely among high-risk beneficiaries (for example, because there
was less opportunity to reduce the need for expensive services by improving care for healthier
beneficiaries), they may be more detectable than effects on all beneficiaries.
In interpreting the test results, we did not rely exclusively on p-values to draw inferences
about whether an estimated effect was truly caused by the intervention. Furthermore, in many
cases, it is likely that an estimated effect, when found to be statistically significant, was
substantially larger than the unobserved “true” effect, on average, as noted by Gelman and Carlin
(2014). Thus, in assessing whether a given impact estimate is evidence of a true effect of CPC,
we drew on estimates of impacts on related outcomes, and the pattern of impact estimates across
time and regions. In some cases, we provide context by using the Gelman and Carlin approach to
calculate the expected degree to which a statistically significant estimate “exaggerates” the
magnitude of the true impact. See Section 8.4 for a more detailed explanation and an illustration
of such calculations.
199

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
8.2.5. Bayesian analysis
The Bayesian impact analysis followed the same approach as the frequentist analysis, but
with several modifications that capitalized on advantages of the Bayesian paradigm. Where in
the main analysis we fit difference-in-differences regression models separately in each CPC
region, in the Bayesian analysis we fit a single difference-in-differences regression model that
allowed impacts to vary by both region and year. We then averaged across the region-specific
estimates to obtain CPC-wide estimates in each year.
88
As in the frequentist analysis, this
regression controlled for baseline beneficiary and practice characteristics as well as a secular
time trend. However, instead of adjusting standard errors for practice-level clustering using the
sandwich estimator, we included random effects of beneficiary, practice, practice-year, region,
and region-year to account for correlation among repeated observations along these dimensions.
The Bayesian analysis combined the difference-in-differences regression framework with
assumptions about relationships among groups of entities; for example, the random effects
described above were based on the assumption that repeated observations of the same entity,
such as a beneficiary or practice, were related to each other. These assumptions strengthened the
precision and plausibility of the impact estimates while allowing the data to dictate the strength
of the relevant relationships. For example, our model posited that impacts were likely to be
somewhat similar across regions and to evolve smoothly over time, enhancing precision by
treating regions and consecutive time periods as mutually informative to the extent that the data
supported the hypothesized similarity.
Unlike in a frequentist analysis, in a Bayesian analysis the object of inference is not only a
point estimate but rather an entire probability distribution. From this distribution, we can derive
probability statements describing the likelihood of impacts of different magnitudes, such as the
chance that CPC reduced Medicare expenditures enough to offset the care management fees paid
to participating practices.
8.3. CPC-wide results
Over the four years of the initiative, CPC did not lead to statistically significant
changes in Medicare expenditures. However, CPC led to slower growth in hospitalizations,
ED visits, and primary care visits during the initiative. The favorable effect on
hospitalizations was similar in magnitude in all four years, but was statistically significant only
in Year 1 and when four years of data were analyzed together. The favorable effects on ED visits
were concentrated in the last two years of CPC. There were no statistically significant effects on
quality-of-care process measures for beneficiaries with diabetes, or on continuity of care for all
beneficiaries during the initiative. For claims-based quality-of-care outcomes, the only
statistically significant effect was a relative reduction in the likelihood of an ED revisit within 30
days of an outpatient ED visit, with the effects concentrated in the last two years of CPC.
88
We conducted a sensitivity test where we calculated CPC-wide estimates as a weighted average of region-specific
values, weighting each region by its share of the total beneficiaries, as in the frequentist analysis. We obtained
results that were essentially identical to those presented here, which weight each region equally.
200

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
8.3.1. Medicare expenditures
CPC did not have a statistically significant effect on Medicare expenditures without care
management fees, and therefore was unlikely to have generated net savings for Medicare after
taking care management fees into account. Findings from a Bayesian analysis also showed a high
probability of some gross savings but almost a zero probability that the savings were sufficient to
cover the care management fees. The results for Medicare expenditures were robust to most of the
alternative model specifications that we ran, and effects were similar for high-risk beneficiaries. We
found some evidence that impacts varied systematically by practice size, patient-centered medical
home (PCMH) status and organizational affiliation, suggesting that practices with greater access to
resources or more experience with care delivery transformation were more likely to reduce growth in
expenditures.
a. Total Medicare expenditures without care management fees
CPC had no statistically significant effect on Medicare FFS expenditures (excluding
care management fees), and yearly estimates for total Medicare expenditures declined from
Year 1 to Year 4.
Cumulative estimates. CPC had no statistically significant effect on Medicare FFS
expenditures, not including Medicare’s CPC care management fees, during the initiative. Table
8.3 summarizes the percentage impacts on Medicare expenditures and service use for all
beneficiaries and high-risk beneficiaries. Across all seven regions combined and over the four
years, total Medicare expenditures without care management fees increased for both CPC and
comparison practices. However, they increased by $9 PBPM (or 1 percent) less for CPC
practices than comparison practices—a favorable finding. This finding was not statistically
significant (p = 0.16, 90 percent CI -$19, $2) (Table 8.3). The change in Medicare expenditures
without fees was more favorable for beneficiaries in CPC practices than for the beneficiaries in
comparison practices in all regions except Ohio/Kentucky. However, only the Oklahoma
estimate of -$19, or 2 percent, was statistically significant. (See Table 8.5 and region-specific
results in Appendix G, Tables G.1 through G.14.)
Annual estimates. The magnitude of yearly estimates for total Medicare expenditures
declined from Year 1 to Year 4, although these estimates were not significantly different from
one another. We did not expect CPC to have large effects during the first year of the initiative,
because we expected practices would take time to transform and for transformation to in turn
affect patient outcomes. Rather, we expected that effects would emerge gradually and either
continue to grow or perhaps level off as practices deepened implementation of the care delivery
changes. Contrary to expectations, the estimated reduction in the growth of expenditures without
fees relative to the comparison practices was largest in Year 1. The estimates became smaller
over time: -$18 PBPM, or 2 percent (p < 0.01) in Year 1, -$11 PBPM, or 1 percent (p = 0.12) in
Year 2, -$4 PBPM, or less than 0.5 percent (p = 0.60) in Year 3, and -$2 PBPM (p = 0.79) in
Year 4 (Figure 8.1 and Table 8.3). The estimate in Year 4 was significantly smaller than the
estimate in Year 1, but the four annual estimates considered together were not significantly
different from one another.
201

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
Figure 8.1. Estimated impact on Medicare FFS expenditures without care
management fees, by year
Note: The estimated impact, denoted by a separate triangle for each CPC year in the figure, is equal to the
difference in mean outcomes between attributed Medicare FFS beneficiaries in CPC and comparison group
practices in any CPC year minus the average difference between the two groups during the baseline
period. The impacts are regression-adjusted to control for pre-CPC differences in beneficiary and practice
characteristics between the CPC and comparison groups. The dashed vertical line through each impact
estimate shows the 90 percent confidence interval. The shaded region represents the net savings region,
based on the average care management fees paid to the intent-to-treat sample. For CPC to achieve net
savings in any year, the impact estimate needs to be inside the net savings region.
FFS = fee-for-service; PBPM = per beneficiary per month.
Regression-adjusted quarterly trends in Medicare expenditures for beneficiaries in CPC and
comparison practices also show that favorable differences between the CPC and comparison
groups that emerged during Quarters 1 through 7 largely disappeared during Quarters 8 through
14 of the initiative (Figure 8.2). This finding is also reflected in the pattern of the quarterly
impact estimates, most of which were not statistically significant (Figure 8.3). Despite the greater
variability in quarterly estimates, their general pattern conforms to the findings from the annual
analysis. Because we cannot reject the hypothesis that the four annual estimates are equivalent,
we focus most of our discussion in this chapter on cumulative impacts.
While the Year 1 estimate was sizable, it likely overstates CPC’s true effect. Using the
approach suggested by Gelman and Carlin (2014), we estimated the degree to which statistically
significant estimates were expected to overstate the true effect on average (“Type M errors”),
given the standard errors of the estimates. If the true effect for total Medicare expenditures in
Year 1 was actually 1 percent ($9 PBPM) less growth for the CPC practices, consistent with the
cumulative estimate over all four years, and the expectation was that first-year effects would
likely be smaller than later effects, then a Year 1 estimate found to be statistically significant at
the 0.10 level would be expected to “exaggerate” the true effect by a factor of about 1.8 (or by
about $7 more PBPM). That difference is very close to the difference between the estimate for
-$30
-$20
-$10
$0
$10
$20
Year 1 Year 2 Year 3 Year 4
Impact in dollars PBPM
Measurement period
Impact estimate
Net savings region
202

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
Year 1 and the average estimate for the four years, providing support for the argument that the
Year 1 estimate probably overestimates the true effect of CPC.
Figure 8.2. Regression-adjusted mean Medicare Part A and Part B
expenditures PBPM, excluding Medicare’s CPC care management fees, all
beneficiaries, CPC-wide
Notes: The vertical dashed line indicates the start of the CPC initiative. Means are regression-adjusted to control
for pre-CPC beneficiary characteristics (including HCC scores) and practice characteristics. The analysis
includes only beneficiaries attributed during the CPC quarters who, by definition, must have been alive
during the baseline period. Consequently, there was zero mortality and no occurrence of high end-of-life
expenditures in the baseline period for beneficiaries in either CPC or comparison practices, so expenditures
increased sharply after the initiative began.
HCC = Hierarchical Condition Category; PBPM = per beneficiary per month.
$500
$550
$600
$650
$700
$750
$800
$850
$900
$950
$1,000
Regression-adjusted mean
Measurement period
CPC practices Comparison practices
203

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
Figure 8.3. Estimated impact on Medicare Part A and Part B expenditures
PBPM, excluding CPC care management fees, all beneficiaries, CPC-wide
Notes: The estimated impact, denoted by a separate triangle for each CPC quarter in the figure, is equal to the
difference in mean outcomes between attributed Medicare FFS beneficiaries in CPC and comparison group
practices in any CPC quarter minus the average difference between the two groups over the four pre-CPC
quarters. The impacts are regression-adjusted to control for pre-CPC differences in beneficiary and practice
characteristics between the CPC and comparison groups. The dashed vertical line through each impact
estimate shows the 90 percent confidence interval.
a
Impact estimates that fall in the shaded net savings region imply that there are savings after including the average
CPC care management fees paid over the four years—that is, that estimated savings in expenditures without CPC
care management fees exceed the CPC care management fees.
PBPM = per beneficiary per month.
-$120
-$90
-$60
-$30
$0
$30
$60
$90
$120
Impact in dollars PBPM
Measurement period
Impact
Net savings region
a
estimate
204

205
Table 8.3. Regression-adjusted means and difference-in-differences estimates of CPC’s impact on
expenditure and utilization measures among attributed Medicare FFS beneficiaries, annual and four-year
cumulative CPC-wide estimates
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
Total Medicare expenditures (dollars PBPM)
Without CPC care
management fees
Baseline $525 $527 - - - - $1,268 $1,263 - - - -
Year 1 $665 $684 -$18*** $7 -2% 0.009 $1,341 $1,369 -$34* $19 -2% 0.073
Year 2 $731 $743 -$11 $7 -1% 0.115 $1,413 $1,411 -$3 $18 0% 0.853
Year 3 $802 $807 -$4 $8 0% 0.598 $1,511 $1,506 -$1 $21 0% 0.961
Year 4 $857 $860 -$2 $9 0% 0.791 $1,580 $1,563 $12 $21 1% 0.584
Years 1–4 combined $821 $831 -$9 $6 -1% 0.162 $1,484 $1,486 -$8 $17 -1% 0.644
Test whether impacts for
Years 1–4 are jointly
significant
F =
2.419
p-value =
0.047
F =
1.756
p-value =
0.135
With CPC care
management fees
Baseline $525 $526 - - - - $1,269 $1,263 - - - -
Year 1 $684 $684 $1 $7 0% 0.823 $1,369 $1,369 -$6 $19 0% 0.744
Year 2 $748 $743 $6 $7 1% 0.365 $1,441 $1,411 $25 $18 2% 0.169
Year 3 $814 $807 $9 $8 1% 0.248 $1,531 $1,506 $19 $21 1% 0.364
Year 4 $868 $860 $9 $9 1% 0.318 $1,597 $1,563 $28 $21 2% 0.190
Years 1–4 combined $836 $831 $6 $6 1% 0.348 $1,507 $1,486 $16 $17 1% 0.353
Test whether impacts for
Years 1–4 are jointly
significant
F = 0.459 p-value =
0.766
F = 1.255 p-value =
0.286
Expenditures by type of service (dollars PBPM)
Inpatient
Baseline $180 $173 - - - - $486 $469 - - - -
Year 1 $245 $249 -$10** $4 -3% 0.016 $524 $531 -$25** $11 -4% 0.028
Year 2 $265 $262 -$4 $4 -1% 0.424 $541 $525 -$1 $11 0% 0.941
Year 3 $285 $280 -$1 $4 0% 0.819 $571 $555 -$1 $12 0% 0.911
Year 4 $303 $298 -$1 $5 0% 0.749 $594 $572 $4 $12 1% 0.707
Years 1–4 combined $296 $294 -$4 $4 -1% 0.266 $565 $554 -$6 $9 -1% 0.498

206
Table 8.3. (continued)
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
Skilled nursing facility
Baseline $16 $18 - - - - $85 $90 - - - -
Year 1 $40 $46 -$4*** $2 -7% 0.008 $118 $130 -$7 $5 -5% 0.194
Year 2 $50 $56 -$4** $2 -6% 0.019 $132 $146 -$9* $5 -6% 0.063
Year 3 $61 $65 -$2 $2 -3% 0.250 $153 $163 -$5 $6 -3% 0.355
Year 4 $66 $69 -$2 $2 -3% 0.360 $160 $168 -$3 $5 -2% 0.595
Years 1–4 combined $65 $70 -$3* $2 -5% 0.058 $148 $159 -$6 $4 -4% 0.18
Outpatient
Baseline $97 $102 - - - - $195 $201 - - - -
Year 1 $109 $115 -$2 $1 -1% 0.249 $188 $196 -$2 $3 -1% 0.597
Year 2 $124 $131 -$3 $2 -2% 0.145 $209 $213 $2 $4 1% 0.592
Year 3 $136 $145 -$4** $2 -3% 0.020 $221 $230 -$3 $4 -1% 0.527
Year 4 $147 $156 -$4* $2 -3% 0.057 $237 $243 $1 $5 0% 0.869
Years 1–4 combined $133 $142 -$3** $1 -2% 0.020 $212 $219 -$1 $3 0% 0.863
Physician
Baseline $195 $190 - - - - $358 $345 - - - -
Year 1 $212 $208 $0 $2 0% 0.918 $346 $335 -$1 $4 0% 0.742
Year 2 $223 $219 -$1 $2 -1% 0.483 $353 $341 -$1 $4 0% 0.759
Year 3 $238 $232 $2 $2 1% 0.409 $368 $353 $2 $4 1% 0.606
Year 4 $252 $242 $5* $2 2% 0.052 $379 $358 $8 $5 2% 0.109
Years 1–4 combined $240 $234 $1 $2 1% 0.432 $362 $347 $2 $3 0% 0.624
Primary care physician
Baseline $30 $31 - - - - $59 $59 - - - -
Year 1 $36 $37 -$1*** $0 -2% 0.009 $62 $64 -$2*** $1 -3% 0.010
Year 2 $38 $40 -$1*** $0 -3% 0.008 $65 $66 -$1 $1 -2% 0.255
Year 3 $44 $45 $0 $1 -1% 0.534 $72 $73 -$1 $1 -2% 0.295
Year 4 $47 $48 -$1 $1 -1% 0.409 $76 $76 $0 $1 0% 0.83
Years 1–4 combined $44 $45 -$1* $0 -2% 0.081 $70 $71 -$1 $1 -2% 0.172
Office-based primary
care physician
Baseline $18 $18 - - - - $29 $28 - - - -
Year 1 $19 $19 $0* $0 -2% 0.059 $27 $27 $0 $0 -1% 0.153
Year 2 $19 $19 -$1*** $0 -3% 0.005 $26 $26 -$1** $0 -3% 0.016
Year 3 $19 $20 $0** $0 -2% 0.032 $27 $27 -$1** $0 -3% 0.019
Year 4 $20 $20 $0 $0 -1% 0.396 $27 $27 $0 $0 -2% 0.337
Years 1–4 combined $19 $20 $0** $0 -2% 0.036 $27 $27 -$1** $0 -2% 0.038

207
Table 8.3. (continued)
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
Specialist
Baseline $96 $92 - - - - $182 $172 - - - -
Year 1 $105 $100 $0 $1 0% 0.763 $173 $164 -$1 $3 -1% 0.757
Year 2 $109 $104 $1 $1 1% 0.442 $173 $163 -$1 $3 -1% 0.683
Year 3 $114 $108 $2 $1 2% 0.179 $175 $161 $3 $3 2% 0.326
Year 4 $120 $112 $4** $2 3% 0.012 $180 $163 $6* $3 4% 0.054
Years 1–4 combined $116 $110 $2* $1 2% 0.096 $175 $163 $2 $2 1% 0.508
Office-based specialist
Baseline $21 $20 - - - - $37 $35 - - - -
Year 1 $21 $20 $0 $0 1% 0.397 $34 $32 $0 $0 0% 0.883
Year 2 $22 $21 $0 $0 0% 0.784 $34 $32 $0 $0 0% 0.810
Year 3 $23 $22 $0 $0 1% 0.173 $34 $32 $0 $0 1% 0.531
Year 4 $23 $22 $0* $0 2% 0.054 $33 $31 $0 $0 1% 0.374
Years 1–4 combined $23 $22 $0 $0 1% 0.157 $33 $32 $0 $0 0% 0.620
Home health
Baseline $19 $23 - - - - $77 $86 - - - -
Year 1 $26 $31 -$1** $1 -3% 0.033 $77 $87 -$2 $2 -2% 0.316
Year 2 $32 $35 $1 $1 2% 0.288 $85 $91 $3* $2 3% 0.100
Year 3 $38 $41 $0 $1 1% 0.693 $94 $102 $0 $2 0% 0.907
Year 4 $41 $46 $0 $1 -1% 0.614 $99 $109 -$1 $2 -1% 0.695
Years 1–4 combined $41 $45 $0 $1 -1% 0.727 $93 $102 $0 $2 0% 0.947
Hospice
a
Baseline -$4 -$3 - - - - $7 $11 - - - -
Year 1 $11 $12 $0 $1 2% 0.743 $36 $39 $2 $2 4% 0.473
Year 2 $17 $18 $0 $1 1% 0.819 $49 $51 $2 $3 5% 0.418
Year 3 $22 $21 $2* $1 10% 0.082 $58 $54 $7** $3 14% 0.025
Year 4 $27 $27 $2 $1 7% 0.180 $66 $66 $4 $3 7% 0.202
Years 1–4 combined $24 $24 $1 $1 5% 0.281 $56 $56 $4 $3 7% 0.127
DME
Baseline $22 $23 - - - - $60 $61 - - - -
Year 1 $23 $23 $0 $0 0% 0.825 $51 $51 $1 $1 1% 0.502
Year 2 $20 $21 $0 $1 -2% 0.390 $44 $45 $0 $1 0% 0.999
Year 3 $22 $23 -$1 $1 -4% 0.103 $46 $49 -$2 $1 -4% 0.198
Year 4 $21 $22 -$1* $1 -4% 0.093 $44 $47 -$2 $2 -3% 0.368
Years 1–4 combined $22 $23 -$1 $0 -3% 0.148 $46 $48 -$1 $1 -1% 0.534
208
Table 8.3. (continued)
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
Utilization (annualized rate per 1,000 beneficiaries)
Hospitalizations
Baseline 213 213 - - - - 530 528 - - - -
Year 1 261 267 -6* 3 -2% 0.059 564 575 -13 9 -2% 0.143
Year 2 265 271 -5 3 -2% 0.126 552 555 -4 9 -1% 0.652
Year 3 287 290 -3 3 -1% 0.383 592 599 -9 10 -1% 0.396
Year 4 294 300 -6 4 -2% 0.108 599 607 -10 11 -2% 0.339
Years 1–4 combined 300 306 -5* 3 -2% 0.067 591 598 -9 8 -2% 0.267
Total ED visits
Baseline 518 531 - - - - 1,140 1,156 - - - -
Year 1 592 613 -8 5 -1% 0.141 1,183 1,214 -15 16 -1% 0.324
Year 2 636 657 -7 6 -1% 0.225 1,233 1,247 3 16 0% 0.872
Year 3 689 719 -17*** 6 -2% 0.006 1,328 1,359 -15 19 -1% 0.427
Year 4 710 741 -18*** 7 -2% 0.008 1,356 1,392 -19 20 -1% 0.352
Years 1–4 combined 702 730 -14*** 5 -2% 0.008 1,291 1,319 -12 15 -1% 0.419
Outpatient ED visits
Baseline 394 406 - - - - 783 797 - - - -
Year 1 423 438 -2 5 -1% 0.596 770 789 -5 12 -1% 0.685
Year 2 460 476 -4 5 -1% 0.426 821 831 4 13 0% 0.775
Year 3 490 516 -15*** 5 -3% 0.007 870 894 -10 14 -1% 0.481
Year 4 503 528 -13** 6 -3% 0.021 887 916 -14 16 -2% 0.365
Years 1–4 combined 492 514 -10** 5 -2% 0.032 838 859 -7 11 -1% 0.565
Observation stays
Baseline 39 40 - - - - 88 89 - - - -
Year 1 46 45 1 1 2% 0.352 92 91 3 4 3% 0.437
Year 2 57 54 4** 2 7% 0.013 110 102 9* 5 8% 0.066
Year 3 59 57 2 2 4% 0.165 112 108 5 5 4% 0.337
Year 4 63 60 4** 2 7% 0.019 119 113 7 5 6% 0.198
Years 1–4 combined 60 58 3** 1 5% 0.025 110 106 6 4 5% 0.146
Primary care visits
Baseline 6,143 6,372 - - - - 10,256 10,518 - - - -
Year 1 6,706 7,021 -86 52 -1% 0.101 10,577 10,970 -131 109 -1% 0.230
Year 2 6,906 7,240 -105* 60 -1% 0.079 10,774 11,117 -80 132 -1% 0.544
Year 3 7,347 7,662 -86 72 -1% 0.231 11,470 11,925 -193 149 -2% 0.197
Year 4 7,650 7,945 -66 81 -1% 0.414 11,860 12,210 -88 162 -1% 0.587
Years 1–4 combined 7,551 7,884 -92 62 -1% 0.136 11,324 11,718 -130 122 -1% 0.287

209
Table 8.3. (continued)
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
CPC practices’
regression-adjusted
mean
Comparison group
practices’ regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
Office-based primary
care visits
Baseline 3,883 3,898 - - - - 5,813 5,644 - - - -
Year 1 4,002 4,053 -36 31 -1% 0.250 5,585 5,458 -43 50 -1% 0.391
Year 2 3,953 4,043 -75** 37 -2% 0.043 5,359 5,312 -122** 62 -2% 0.047
Year 3 4,030 4,132 -87* 45 -2% 0.050 5,370 5,361 -161** 78 -3% 0.039
Year 4 4,086 4,163 -61 52 -1% 0.235 5,344 5,293 -119 90 -2% 0.188
Years 1–4 combined 4,108 4,191 -68* 38 -2% 0.072 5,406 5,347 -110* 60 -2% 0.065
Specialist visits
Baseline 11,351 11,372 - - - - 20,707 20,472 - - - -
Year 1 11,978 12,056 -57 70 0% 0.409 19,748 19,653 -140 158 -1% 0.375
Year 2 12,474 12,525 -31 76 0% 0.688 19,752 19,626 -109 156 -1% 0.485
Year 3 12,895 12,818 97 84 1% 0.250 19,997 19,643 119 173 1% 0.492
Year 4 13,228 13,022 225*** 82 2% 0.006 20,035 19,398 402** 180 2% 0.025
Years 1–4 combined 13,270 13,221 69 68 1% 0.309 19,969 19,671 61 138 0% 0.655
Office-based specialist
visits
Baseline 4,014 3,950 - - - - 6,926 6,734 - - - -
Year 1 4,094 4,022 7 24 0% 0.757 6,353 6,155 5 45 0% 0.920
Year 2 4,192 4,139 -10 33 0% 0.752 6,224 6,081 -50 61 -1% 0.408
Year 3 4,232 4,158 10 42 0% 0.817 6,066 5,882 -9 71 0% 0.902
Year 4 4,280 4,152 63 42 2% 0.136 5,938 5,735 10 79 0% 0.900
Years 1–4 combined 4,336 4,250 20 32 0% 0.539 6,108 5,927 -10 53 0% 0.849
Total number of
observations (CPC and
comparison) across all
years
b
6,575,258 1,731,832
Source: Medicare claims data for October 2011 through December 2016.
Note: Impact estimates are regression-adjusted for baseline beneficiary characteristics (including HCC scores) and baseline practice characteristics. We based each impact
estimate on a difference-in-differences analysis, and it reflects the difference in the regression-adjusted average outcome for attributed Medicare FFS beneficiaries in CPC
practices in Years 1 to 4 compared with baseline relative to the same difference over time for attributed Medicare FFS beneficiaries in matched comparison practices.
Expenditures on physician services include expenditures on primary care physician services, specialist services, and services provided by other noninstitutional providers (the
third category is not shown separately). For Medicare service use measures, measures of outpatient ED visits and total ED visits include observation stays. Primary care
visits include office-based primary care visits as well as visits in other settings, such as primary care visits in the hospital or nursing home. Regression-adjusted means for
each year and for both groups from the annual regression are obtained by using average values of the control variables among beneficiaries in CPC practices in Year 4, in
order to hold beneficiary and practice attributes fixed in generating predictions. Regression-adjusted means from the cumulative regression are obtained by using average
values of the control variables among beneficiaries in CPC practices across all four CPC years. Regression-adjusted means from the cumulative regression are similar to but
210
Table 8.3. (continued)
not always perfectly aligned with those from the annual regression, due to differences in coefficients on control variables and the different samples used for predictions.
However, the impact estimate from the cumulative regression is within the range of values for the impact estimates from the annual regression.
a
Actual hospice expenditures at baseline were close to zero, because beneficiaries had to be alive and not in hospice during the look-back period for attribution (which ended five
months before the start of CPC in two regions, and two months before the start of CPC in five regions). The negative baseline estimate is a result of predicting values using regression
coefficients.
b
Analysis includes 565,674 Medicare FFS beneficiaries attributed to 497 CPC practices and 1,165,284 beneficiaries attributed to 908 matched comparison practices. Each beneficiary
can contribute as many as five observations in the analysis—one during the baseline year and one during each follow-up year.
*/**/*** Significantly different from zero at the 0.10/0.05/0.01 level, two-tailed test.
DME = durable medical equipment; ED = emergency department; FFS = fee-for-service; HCC = Hierarchical Condition Category; PBPM = per beneficiary per month.

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
b. Total Medicare expenditures by service category
Over the four years of CPC, expenditures for inpatient, skilled nursing facility,
outpatient, and primary care clinician services increased slightly less for the CPC group
than for the comparison group, although the estimate for inpatient expenditures was not
statistically significant (Table 8.4).
• Cumulative estimates. The estimated $9 PBPM lower growth in total Medicare
expenditures was modest and not statistically significant (p = 0.16). CPC practices had
slightly smaller increases than comparison practices in PBPM expenditures for skilled
nursing facilities ($3, or 5 percent, p = 0.06), outpatient services ($3, or 2 percent, p = 0.02),
and inpatient expenditures ($4, or 1 percent, p = 0.27), but these estimates were partly offset
by slightly larger increases in PBPM expenditures for specialist services ($2, or 2 percent, p
= 0.096).
• Annual estimates. Savings in Medicare expenditures appeared to decline over time. The
estimated effect for total Medicare expenditures without fees fell from -$18 PBPM in Year 1
to -$2 in Year 4. The components of this change include:
- Ten dollar PBPM slower growth in expenditures on inpatient services for CPC than
comparison practices (p = 0.02) accounted for more than half the $18 PBPM favorable
effect on total Medicare expenditures in Year 1, but this effect on inpatient expenditures
virtually disappeared by Year 4, when the estimate fell to -$1 PBPM (p = 0.75).
89
- The other contributor to the favorable Year 1 estimate for total Medicare expenditures
was slower growth in CPC than comparison practices in skilled nursing facility
expenditures (-$4 PBPM, p = 0.01), which also became less pronounced over time, and
fell to -$2 PBPM (p = 0.36) in Year 4.
- There were unfavorable changes between Year 1 and Year 4 in estimated effects on
expenditures for specialist services. The estimated effect on expenditures for specialist
services in all settings (including office-based settings as well as hospitals and nursing
homes) increased from zero in Year 1 to $4 PBPM in Year 4 (p = 0.01).
- Similarly, the estimate for effects on hospice expenditures increased from zero in Year 1
to $2 PBPM (p = 0.18) in Year 4. The greater increase in hospice expenditures among
beneficiaries in CPC compared to comparison practices could be because beneficiaries in
CPC practices started using hospice services earlier prior to death, which can potentially
improve quality of life.
90
89
Unlike the declining expenditure estimates, the year-by-year impact estimates for the number of hospitalizations
were relatively constant and remained slightly favorable (but not statistically significant) over time (see Table 8.5);
this pattern suggests a shift toward fewer yet more expensive hospital stays among beneficiaries in CPC versus
comparison practices.
90
Only around 3 percent of beneficiaries in both CPC and comparison practices used hospice services or had any
hospice expenditures in Years 3 and 4, and these percentages were similar across the two research groups. However,
among those who used hospice services, expenditures during Years 3 and 4 increased (relative to baseline) by about
$60 PBPM more among beneficiaries in CPC than comparison practices. This suggests an increase in the intensity
or duration of hospice use in the CPC group relative to the comparison group in Years 3 and 4.
211

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
Table 8.4. Breakdown of savings in total Medicare FFS expenditures per
beneficiary per month, by service category
Year 1
impact
estimate
Year 2
impact
estimate
Year 3
impact
estimate
Year 4
impact
estimate
Combined
Years 1, 2, 3,
and 4 impact
estimate
Total Medicare expenditures -$18*** -$11 -$4 -$2 -$9
Inpatient -$10** -$4 -$1 -$1 -$4
Skilled nursing facility -$4*** -$4** -$2 -$2 -$3*
Outpatient -$2 -$3 -$4** -$4* -$3**
Physician (primary care,
specialist, and other
noninstitutional providers) $0 -$1 $2 $5* $1
Primary care physician -$1*** -$1*** $0 -$1 -$1*
Specialist $0 $1 $2 $4** $2*
Home health -$1** $1 $0 $0 $0
Hospice $0 $0 $2* $2 $1
Durable medical equipment $0 $0 -$1 -$1* -$1
Note: Expenditures on physician services include expenditures on primary care physician services, specialist
services, and noninstitutional provider services (the third category is not shown separately).
*/**/*** Significantly different from zero at the 0.10/0.05/0.01 level, two-tailed test.
FFS = fee-for-service.
c. Total Medicare expenditures with Medicare’s CPC care management fees
CPC did not generate net savings during the four years and was unlikely to have been
cost neutral. The impact estimate on Medicare expenditures with fees implied an increase of $6
PBPM (p = 0.35) more for CPC practices than comparison practices, because the $9 estimated
relative reduction in monthly expenditures without fees over the 51 months (which was not
statistically significant) offset just over half of the Medicare CPC care management fees (Table
8.3).
91
A test of whether net costs to CMS increased could not be rejected (p = 0.84), making it
unlikely that the initiative generated net savings. However, the 90 percent confidence interval for
the $6 impact estimate for Medicare expenditures with fees among all beneficiaries was -$4 to
+$16. Thus, the net increase in costs due to CPC is likely to have been small.
91
The average CPC care management fee received by practices was $15 per month per CPC beneficiary. This was
less than the average of (1) the $20 average fee per month that CMS paid for attributed Medicare beneficiaries
during Quarters 1 through 9 of CPC, and (2) the $15 average fee per month paid during Quarters 10 through 17,
because our ITT sample follows beneficiaries even after they are no longer attributed to a CPC practice and no
longer generating care management fees for the practice.
212

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
Over the four years of CPC, none of the regions achieved meaningful net savings in
Medicare expenditures, although there is considerable uncertainty around the region-level
estimates. Of the seven CPC regions, only Oklahoma had a statistically significant estimate that
suggested slower growth in Medicare expenditures PBPM without fees for CPC than comparison
practices (Table 8.5). The estimate, -$19 (2 percent, p = 0.06) over the course of the initiative
was enough to fully offset care management fees, yielding an estimated net savings of $5 PBPM
(p = 0.65). New York was the only other region with favorable cumulative estimates—without
and with fees—of -$25 and -$10 PBPM (3 and 1 percent, p = 0.13 and p = 0.56), respectively,
among all beneficiaries, and -$52 and -$28 PBPM (3 and 2 percent, p = 0.15 and p = 0.43),
respectively, among high-risk beneficiaries; however, none of these findings were statistically
significant. Ohio/Kentucky saw consistent, unfavorable estimated impacts on Medicare
expenditures throughout the initiative, resulting in sizeable, statistically significant increases in
net PBPM Medicare expenditures of $39 (5 percent, p = 0.03) among all beneficiaries and $119
(8 percent, p < 0.01) among high-risk beneficiaries. Over the four years of CPC, net savings did
not exceed 1 percent in any region. In general, we have less confidence in the regional estimates,
given much smaller sample sizes and greater variability than the pooled, CPC-wide estimates.
Estimates from the Bayesian analysis presented in the following section shrink some of the
differences across regions, but uncertainties about the estimates remain.
213

214
Table 8.5. Regression-adjusted means and difference-in-differences estimates of CPC’s impact on Medicare
FFS expenditures, hospitalizations, and outpatient ED visits, cumulative four-year estimates, by region
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-adjusted
mean
Comparison group
practices’
regression-adjusted
mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
CPC practices’
regression-adjusted
mean
Comparison group
practices’
regression-adjusted
mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
Arkansas
Without CPC care
management fees
Baseline $562 $585 - - - - $1,279 $1,292 - - - -
Years 1–4 combined $781 $806 -$2 $13 0% 0.891 $1,403 $1,437 -$21 $40 -1% 0.604
With CPC care management
fees
Baseline $562 $585 - - - - $1,279 $1,292 - - - -
Years 1–4 combined $796 $806 $12 $13 2% 0.353 $1,424 $1,437 $0 $40 0% 0.999
Hospitalizations
Baseline 260 247 - - - - 617 582 - - - -
Years 1–4 combined 329 325 -8 6 -2% 0.195 641 639 -33* 18 -5% 0.070
Outpatient ED visits
Baseline 471 463 - - - - 919 883 - - - -
Years 1–4 combined 562 550 4 13 1% 0.778 944 916 -8 35 -1% 0.824
Total number of observations
(CPC and comparison)
across all years
1,252,341 338,517
Colorado
Without CPC care
management fees
Baseline $536 $549 - - - - $1,294 $1,342 - - - -
Years 1–4 combined $731 $753 -$9 $20 -1% 0.658 $1,358 $1,410 -$3 $73 0% 0.966
With CPC care management
fees
Baseline $536 $549 - - - - $1,294 $1,343 - - - -
Years 1–4 combined $746 $753 $6 $20 1% 0.757 $1,384 $1,411 $22 $73 2% 0.758
Hospitalizations
Baseline 189 213 - - - - 481 542 - - - -
Years 1–4 combined 237 262 -1 9 0% 0.929 488 538 10 32 2% 0.759
Outpatient ED visits
Baseline 369 384 - - - - 758 785 - - - -
Years 1–4 combined 460 477 -1 11 0% 0.899 846 838 35 33 4% 0.297

Table 8.5 (continued)
215
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-adjusted
mean
Comparison group
practices’
regression-adjusted
mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
CPC practices’
regression-adjusted
mean
Comparison group
practices’
regression-adjusted
mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
Total number of observations
(CPC and comparison)
across all years
992,008 228,822
New Jersey
Without CPC care
management fees
Baseline $653 $663 - - - - $1,394 $1,416 - - - -
Years 1–4 combined $953 $972 -$9 $15 -1% 0.533 $1,689 $1,726 -$15 $28 -1% 0.587
With CPC care management
fees
Baseline $653 $662 - - - - $1,394 $1,416 - - - -
Years 1–4 combined $968 $972 $5 $15 1% 0.741 $1,712 $1,726 $8 $28 0% 0.768
Hospitalizations
Baseline 208 213 - - - - 477 486 - - - -
Years 1–4 combined 291 299 -2 7 -1% 0.726 567 582 -7 15 -1% 0.651
Outpatient ED visits
Baseline 302 321 - - - - 544 560 - - - -
Years 1–4 combined 349 367 0 8 0% 0.951 579 601 -7 15 -1% 0.662
Total number of observations
(CPC and comparison)
across all years
778,293 215,730
New York
Without CPC care
management fees
Baseline $593 $598 - - - - $1,253 $1,234 - - - -
Years 1–4 combined $854 $883 -$25 $17 -3% 0.134 $1,487 $1,520 -$52 $36 -3% 0.151
With CPC care management
fees
Baseline $593 $597 - - - - $1,253 $1,234 - - - -
Years 1–4 combined $869 $883 -$10 $17 -1% 0.561 $1,510 $1,520 -$28 $36 -2% 0.434
Hospitalizations
Baseline 230 211 - - - - 525 476 - - - -
Years 1–4 combined 311 307 -15*** 6 -5% 0.005 599 585 -35** 16 -6% 0.026
Outpatient ED visits
Baseline 378 373 - - - - 677 662 - - - -
Years 1–4 combined 447 450 -8 8 -2% 0.314 740 707 18 25 2% 0.462

Table 8.5 (continued)
216
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-adjusted
mean
Comparison group
practices’
regression-adjusted
mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
CPC practices’
regression-adjusted
mean
Comparison group
practices’
regression-adjusted
mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
Total number of observations
(CPC and comparison)
across all years
638,176 181,345
Ohio/Kentucky
Without CPC care
management fees
Baseline $580 $613 - - - - $1,272 $1,303 - - - -
Years 1–4 combined $868 $878 $23 $17 3% 0.181 $1,580 $1,517 $94*** $33 6% 0.005
With CPC care management
fees
Baseline $580 $613 - - - - $1,272 $1,303 - - - -
Years 1–4 combined $883 $878 $39** $17 5% 0.026 $1,605 $1,517 $119*** $33 8% <.001
Hospitalizations
Baseline 259 278 - - - - 597 623 - - - -
Years 1–4 combined 342 352 9 10 3% 0.381 669 665 30 19 5% 0.128
Outpatient ED visits
Baseline 442 444 - - - - 799 774 - - - -
Years 1–4 combined 525 544 -18* 11 -3% 0.086 876 875 -24 23 -3% 0.296
Total number of observations
(CPC and comparison)
across all years
840,655 231,891
Oklahoma
Without CPC care
management fees
Baseline $585 $583 - - - - $1,333 $1,330 - - - -
Years 1–4 combined $831 $849 -$19* $10 -2% 0.057 $1,498 $1,540 -$44 $30 -3% 0.145
With CPC care management
fees
Baseline $585 $584 - - - - $1,333 $1,331 - - - -
Years 1–4 combined $846 $849 -$5 $10 -1% 0.646 $1,520 $1,540 -$22 $30 -1% 0.458
Hospitalizations
Baseline 264 258 - - - - 621 616 - - - -
Years 1–4 combined 338 335 -3 6 -1% 0.558 655 654 -3 19 0% 0.875
Outpatient ED visits
Baseline 459 490 - - - - 881 958 - - - -
Years 1–4 combined 551 601 -19 12 -3% 0.110 920 1,052 -55* 31 -6% 0.076

Table 8.5 (continued)
217
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-adjusted
mean
Comparison group
practices’
regression-adjusted
mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
CPC practices’
regression-adjusted
mean
Comparison group
practices’
regression-adjusted
mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact (%)
p-value for estimated
impact
Total number of observations
(CPC and comparison)
across all years
958,272 252,320
Oregon
Without CPC care
management fees
Baseline $551 $539 - - - - $1,217 $1,195 - - - -
Years 1–4 combined $756 $755 -$11 $10 -1% 0.271 $1,372 $1,340 $10 $32 1% 0.755
With CPC care management
fees
Baseline $551 $539 - - - - $1,218 $1,196 - - - -
Years 1–4 combined $771 $755 $4 $10 1% 0.675 $1,396 $1,340 $34 $32 2% 0.297
Hospitalizations
Baseline 202 193 - - - - 474 452 - - - -
Years 1–4 combined 253 252 -9 6 -3% 0.137 505 492 -8 19 -2% 0.654
Outpatient ED visits
Baseline 444 433 - - - - 883 858 - - - -
Years 1–4 combined 526 541 -27* 15 -5% 0.078 936 920 -9 41 -1% 0.835
Total number of observations
(CPC and comparison)
across all years
1,115,513 283,207
Source: Medicare claims data for October 2011 through December 2016.
Note: Impact estimates are regression-adjusted for baseline beneficiary characteristics (including HCC scores) and baseline practice characteristics. Each impact estimate is based
on a difference-in-differences analysis and reflects the difference in the regression-adjusted average outcome for attributed Medicare FFS beneficiaries in CPC practices in all
four years combined, compared with baseline, relative to the same difference over time for attributed Medicare FFS beneficiaries in matched comparison practices. The number
of observations includes the total number of CPC and comparison group observations across all years.
*/**/*** Significantly different from zero at the 0.10/0.05/0.01 level, two-tailed test.
ED = emergency department; FFS = fee
-for-service; HCC = Hierarchical Condition Category; PBPM = per beneficiary per month.

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
d. Bayesian analysis for total Medicare expenditures
Results from a Bayesian analysis likewise suggest that intervention effects were not
large enough to generate net savings across CPC. Based on this analysis, there is a 94 percent
probability that CPC generated some savings across all regions and years. However, the
probability that impacts across all CPC regions combined over the course of the initiative
exceeded the $15 threshold to recoup the fees paid by Medicare and achieve net savings is
almost zero—approximately 0.6 percent.
Figure 8.4 shows, overall and by region, the probabilities that CPC (1) achieved net savings
(green), (2) achieved net losses (red), or (3) reduced expenditures less than the amount needed
over the initiative’s 51 months to reach cost neutrality (yellow). The probabilities of net savings
over the 51 months are computed as the average across the four intervention years. As with the
frequentist estimates, we have more confidence in the CPC-wide results. Among the regions,
only Ohio/Kentucky is highly likely to have had greater increases in expenditures before care
management fees in CPC than comparison practices. Based on the frequentist results, New York
generated the largest estimated reduction in expenditures before fees (although the estimated
savings were not statistically significant), followed by Oklahoma; however, the Bayesian
analysis shows that Oklahoma and Colorado were the most likely to have had savings that
exceeded care management fees, followed by New York. The estimates suggest that there was a
65 percent probability that Oklahoma achieved net savings (gross savings exceeding the average
care management fee) during the four years of CPC.
Figure 8.4. Probability that CPC achieved savings (before fees) during
initiative
Note: PBPM impact estimates from the Bayesian analysis are in parentheses.
The Bayesian results show a much higher probability of net savings during Year 1 than in
later years in most regions, which is consistent with our difference-in-differences estimates
(Table 8.6). When we include additional follow-up from Years 2 through 4, the analysis shows
5.6%
48.3%
6.8%
22.0%
11.4%
86.5%
0.4%
29.1%
93.8%
50.6%
52.9%
62.1%
60.1%
13.5%
34.6%
66.0%
0.6%
1.1%
40.3%
15.9%
28.5%
0.0%
65.0%
4.9%
0%
20%
40%
60%
80%
100%
CPC-wide
(-$6)
AR
($0)
CO
(-$13)
NJ
(-$7)
NY
(-$10)
OH/KY
($10)
OK
(-$18)
OR
(-$4)
Loss Saving < $15 PBPM Saving > $15 PBPM
218

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
that these early findings were not sustained, and the probability that CPC generated savings
declined, dramatically for some regions. Similar to the frequentist findings, the CPC-wide
Bayesian findings suggest that CPC practices had less growth in Medicare expenditures than
comparison practices over the four years, but not enough to cover Medicare’s care management
fees.
Table 8.6. Probability that CPC achieved savings, by year, based on a
Bayesian analysis
CPC-wide AR CO NJ NY OH/KY OK OR
Probability of decrease in Medicare expenditures without care management fees
Year 1 >99.9 77.8 76.8 99.9 87.5 36.0 >99.9 96.2
Year 2 99.2 52.9 91.4 92.4 91.5 28.1 99.4 90.0
Year 3 68.0 39.1 95.8 26.5 87.2 15.2 86.4 43.5
Year 4 16.4 28.6 92.3 7.4 65.7 2.9 46.9 9.5
Years 1-4
combined
94.4 51.7 93.2 78.0 88.6 13.5 99.6 70.9
Probability of recovering Medicare’s CPC care management fees
Year 1 30.3 9.3 12.3 90.0 31.8 1.5 99.0 42.8
Year 2 0.5 0.7 24.5 29.5 26.6 0.2 62.0 12.7
Year 3 0.6 1.9 63.0 1.1 38.2 0.2 30.2 3.0
Year 4 <0.1 2.4 67.9 0.6 25.6 <0.1 9.7 0.3
Years 1-4
combined
0.6 1.1 40.3 15.9 28.5 <0.1 65.0 4.9
Note: The average fee received by beneficiaries in our intent-to-treat analysis was $18 PBPM in Year 1, $17 in
Year 2, $14 in Year 3, and $11 in Year 4, for an average of $15 PBPM over the 51-month intervention
period. The decline in fees received is due not only to the decline in the care management fees paid for
attributed beneficiaries, but also to the fact that beneficiaries attributed to practices in early years were no
longer attributed to the practice in later years, so the practice received no care management fees for those
patients in the later years. Also, no care management fees were paid for patients attributed to withdrawn or
terminated practices after those practices stopped participating.
e. Results of sensitivity tests
Results for Medicare expenditures were not sensitive to various alterations to the
model and analysis sample. Similar to findings in prior reports, the main results remained
unchanged in these sensitivity tests, with a few exceptions that are discussed below (Table 8.7).
We conducted four types of sensitivity tests to determine whether the estimated impacts on
Medicare expenditures without fees from the main difference-in-differences model were robust
to changing the estimation strategy or the model specification, and to rule out alternative
explanations for the findings. The sensitivity tests assessed the results of varying (1) the
assumptions underlying the difference-in-differences estimation approach, (2) the composition of
the analysis sample, (3) the definition of the comparison group, and (4) the model specification.
• Results were similar to those of our main model for most of these sensitivity tests. For
example, we obtained similar estimates when we altered our difference-in-differences
approach by (1) extending the baseline period, (2) changing the definition of the comparison
group, (3) changing the definition of the analysis sample (by following only beneficiaries
attributed in Quarter 1 and not those attributed later), or (4) changing the definition of only
219

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
the baseline sample (restricting the baseline sample to those attributed in Quarter 1 or Year 1
of CPC). Also, results were robust to changing the model specification, for example, using
practice fixed effects, a generalized linear model with a log link, or models with
expenditures trimmed to reduce the effects of high-cost cases. Similarly, excluding CPC
practices that withdrew during the course of the initiative and their matched comparison
practices did not substantively alter the pattern of findings.
• Because the rate of participation in Medicare ACOs grew to 42 percent among comparison
practices by 2016, and CPC practices could not participate in other Medicare initiatives such
as ACOs while remaining in CPC, we included a regression control variable indicating
whether practices had participated in CPC or in a Medicare ACO by the end of Year 3 (or by
December 2015).
92
Including this control variable made the estimate for Medicare
expenditures less favorable to CPC practices in Year 4, which is the opposite of what we
would expect if ACOs in the comparison group were muting the effects of CPC.
• Finally, as reported above, a Bayesian analysis allowing the estimated effects in a given
region to depend in part on the CPC-wide effects showed overall estimates for Medicare
expenditures very similar to those of our main estimates. The Bayesian estimates for
individual regions showed less variability across regions and time periods, by design.
However, our results did differ from our main findings in models that split the comparison
group into the practices drawn from the internal regions and the practices drawn from the
external regions (Table 8.7):
• First, we explored the possibility of contamination. To do this, we compared CPC practices
to rematched comparison practices in external regions only, to remove the possibility that
CPC had an influence on comparison group practices. In this model, the estimated
cumulative effect on Medicare expenditures without fees was somewhat smaller (that is, less
favorable); it decreased from -$9 to -$4 PBPM and was still not statistically significant.
Conversely, comparing CPC practices to only matched comparison practices within the CPC
region that had applied to CPC (the internal comparison group) yielded a larger, favorable
cumulative estimate of -$22 PBPM that was statistically significant. Thus, results using the
internal comparison group were more favorable than those using the external comparison
group, suggesting that contamination did not lead CPC to appear to have less favorable
impacts.
• Second, we further explored results from the internal comparison group to see whether those
might be biased by the fact that internal practices that were selected to participate in CPC
would have more advanced features (which in turn might lead to better outcomes) than those
not selected to participate. We ran a regression that compared CPC practices against all
unselected applicants that met CMS’s eligibility criteria (including practices that were and
were not selected to be in the CPC comparison group by matching) while controlling for the
application score (the primary criteria used in CMS’s selection process for CPC). We found
that there was a statistically significant -$15 PBPM effect of CPC on Medicare expenditures
92
Among comparison practices participating in Medicare ACOs, most were participating in SSP. About 6 percent of
the original set of CPC practices were also in SSP by the end of 2016, as some CPC practices that withdrew from the
initiative went on to participate in SSP.
220

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
without fees, and the application score control variable was not statistically significant.
Although results from the internal comparison group were more favorable, they showed a
large favorable estimate in Year 1 (see Peikes et al. 2016b), before CPC practices would
likely have had enough time for practice transformation to affect expenditures.
The findings from the tests using only internal or external comparison practices suggest that
internal comparison practices did not benefit from any potential spillover effects due to CPC’s
presence in their region; rather, it is possible that results using the external comparison group
were less favorable than those using the internal group due to differences (or differential changes
since baseline) in unobserved market-level factors between CPC and comparison regions.
However, it is also possible that the process CMS used to select practices, which was intended to
identify the strongest and most motivated practices for CPC, resulted in favorable selection; we
could not fully control for this effect.
Table 8.7. Estimates of the cumulative impact on PBPM Medicare
expenditures without fees, from sensitivity tests
Test Motivation
Cumulative
estimate p-value
90%
CI lower
bound
90%
CI upper
bound
Main analysis -$9 0.16 -$19 $2
Use two-year baseline
(instead of one year)
Controls for longer pre-
CPC trend
-$9 0.11 -$19 $0
Follow only beneficiaries
attributed in Quarter 1
Removes any effects that
might be due to changes
in sample composition
over time, for both
baseline and follow-up
years
-$5 0.54 -$17 $8
Hold the baseline sample
fixed by only including
beneficiaries attributed in
Year 1, while allowing new
beneficiaries to enter the
sample during the
intervention years
Examines the sensitivity
of the impact estimate to
changing the baseline
sample
-$8 0.22 -$19 $3
Hold the baseline sample
fixed by only including
beneficiaries attributed in
Quarter 1, while allowing
new beneficiaries to enter
the sample during the
intervention years
Examines the sensitivity
of the impact estimate to
changing the baseline
sample
-$9 0.44 -$28 $10
Control for Medicare ACO
participation of matched
comparison practices at the
end of 2015 when
predicting expenditures in
Year 4 of CPC, so that the
difference-in-differences
estimate is now based on
CPC practices being
compared against matched
comparison practices that
are not Medicare ACOs
Examines whether the
Year 4 impact estimate is
weakened due to the
greater Medicare ACO
participation of
comparison practices
$7
a
0.50 -$11 $25
221

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
Table 8.7 (continued)
Test Motivation
Cumulative
estimate p-value
90%
CI lower
bound
90%
CI upper
bound
Using external comparison
group only, compare CPC
practices with rematched
external comparison
practices
By using only rematched
practices from external
practices (along with new
matching weights),
removes potential
spillover effects of CPC
and adverse selection
from unselected
applicants
-$4 0.53 -$14 $6
Using internal comparison
group only, compare
selected applicants with
rematched nonselected
applicants
Removes any effect of
changes in markets over
time by using only the
internal market, and also
controls for motivation
that led both selected and
nonselected applicants to
apply for CPC
-$22*** 0.005 -$36 -$9
Using internal comparison
group only, compare
selected applicants to all
nonselected applicants
while controlling for CPC
application score
Controls for changes in
market over time by using
only internal market and
reduces selection bias by
using only applicants and
controlling for application
score
-$15** 0.02 -$26 -$5
Practice fixed effects Removes time-invariant
unobserved variable bias
-$9 0.15 -$19 $1
Matched set fixed effects Removes time-invariant
unobserved variable bias
-$9 0.16 -$19 $2
Generalized linear model
with log link
Handles skewed
expenditure distribution
-$11* 0.06 -$20 -$1
Trimmed expenditures at
98th percentile
Reduces influence of
high-cost beneficiaries
-$7 0.18 -$16 $2
Log expenditures
b
Reduces influence of
high-cost beneficiaries
-0.5% 0.51 -1.9% 0.8%
a
Unlike in the other tests, the impact estimate from controlling for Medicare ACO status of comparison practices at
the end of 2015 is for Year 4 (October 2015–December 2016) only.
b
The percentage impact based on the main analysis was 1 percent.
*/**/***Significantly different from zero at the 0.10/0.05/0.01 level, two-tailed test.
ACO = accountable care organization; CI = confidence interval; CPC = Comprehensive Primary Care initiative.
f. Results for subgroups of patients and practices
Effects for the highest-risk beneficiaries were similar to effects for all beneficiaries.
Because there are usually more opportunities to improve care and reduce expenditures for high-
risk patients, we studied whether impacts varied for beneficiaries who were in the top quartile of
the distribution of HCC risk scores when they were first attributed. We found that average
monthly Medicare expenditures PBPM without care management fees grew $8 (or 1 percent,
p = 0.64) less for the high-risk beneficiaries in CPC practices than for those in the comparison
group during CPC, but the difference was not statistically significant (Table 8.3). This was
similar in magnitude and about the same percentage impact as it was for all beneficiaries. The
effect size for annual impacts on total Medicare expenditures was larger for high-risk
222

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
beneficiaries than for all beneficiaries in Year 1, but the percentage impacts were similar for
high-risk and all beneficiaries. For the other three years, estimates for high-risk beneficiaries
were somewhat less favorable than for all beneficiaries. Specifically, in Year 1, expenditures
without fees grew $34, or 2 percent (p = 0.07) less for high-risk patients in CPC practices than
for high-risk patients in comparison practices, driven by a sizeable effect on inpatient
expenditures. The impact estimates were close to zero in the second and third program years, and
in the fourth year expenditures without fees for high-risk beneficiaries in CPC practices relative
to high-risk beneficiaries in comparison practices increased by $12 PBPM (or 1 percent,
p = 0.58), mainly driven by a relative increase in expenditures on physician services.
We also examined whether impacts varied for two other high-risk subgroups of
beneficiaries, defined based on incidence of specific chronic conditions and hospitalizations at
baseline. Although estimates for these subgroups were larger, they were similar in percentage
terms to estimates for the full sample, and were also not statistically significant.
93
Some subgroups of practices showed statistically significant savings. We also studied
whether impacts on expenditures varied for subgroups of practices. We examined variation in
impacts for subgroups of practices defined using characteristics, including practices that (1) were
recognized as PCMHs by NCQA or their state before CPC began;
94
(2) were more likely to have
greater access to resources for transformation (because they had six or more physicians or were
affiliated with a larger organization before CPC began); (3) were owned by either a hospital or a
system (during the initiative); (4) were small (1 or 2 physicians), medium (3 to 5 physicians), or
large (6 or more physicians); or (5) had no physician who met the meaningful use criteria at
baseline.
Although estimated impacts were significantly different from zero for some of the
subgroups we examined, none of the impact estimates for a given subgroup significantly differed
from those of their complement or counterpart, so we cannot draw strong conclusions about
differences across subgroups (Table 8.8). Over the four years, the CPC group had significantly
less growth in Medicare expenditures than the comparison group within the following subgroups:
practices recognized as PCMHs at baseline (-$17 or 2 percent, p = 0.06), practices that had six or
more physicians or were affiliated with a larger organization (-$13 or 1 percent, p = 0.06),
93
These two subgroups were defined as follows: (1) beneficiaries who had at least one of the following chronic
conditions—congestive heart failure, chronic obstructive pulmonary disease, acute myocardial infarction, or
ischemic heart disease—and at least one hospitalization in the year before CPC; and (2) beneficiaries who had at
least 2 of 13 chronic conditions (congestive heart failure, chronic obstructive pulmonary disease, acute myocardial
infarction, ischemic heart disease, diabetes, any cancer other than skin cancer, stroke, depression, dementia, atrial
fibrillation, osteoporosis, rheumatoid arthritis or osteoarthritis, and chronic kidney disease) and at least two
hospitalizations in the two years before CPC. Compared with our main estimate, the estimated impacts were larger
in these subgroups with cumulative difference-in-differences estimates of -$23 (p = 0.44) and -$45 (p = 0.23),
respectively, although similar in terms of percentage impacts (1 and 2 percent, respectively, versus 1 percent for the
overall results). Also, the subgroup-specific impact was not significantly different from the main impact estimate in
either case (p = 0.61 and 0.30, respectively).
94
Although other sources of PCMH recognition exist, we used only NCQA and state recognition because we did
not have data from other certifying organizations for both the CPC and comparison practices. Nearly 40 percent of
CPC practices were recognized as a medical home by NCQA or their state when they applied to CPC, and about 80
percent of those with any medical home recognition received it from one of these two sources.
223

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
hospital- or system-owned practices (-$18 or 2 percent, p = 0.02), and medium-sized practices
with 3 to 5 clinicians (-$16 or 2 percent , p = 0.09). However, the impacts were not significantly
different when we compared these subgroups to their counterparts. For example, favorable and
statistically significant impacts within the subgroup of practices that were recognized as a PCMH
at baseline did not differ significantly from those of practices that did not have PCMH
recognition. Similarly, favorable and statistically significant impacts for practices that were
hospital- or system-owned, or met the criteria of having six or more physicians or affiliation with
a larger organization did not differ significantly from those of their counterparts.
95
Therefore, the
evidence for larger impacts within any of these practice subgroups is weak, especially because
applying any correction for multiple comparisons or multiple hypothesis testing would make it
even less likely to find statistically significant differences, given that we tested for differential
impacts for subgroups defined by seven practice characteristics.
Table 8.8. Variation in cumulative impact on PBPM Medicare expenditures
without fees, by practice characteristics at baseline
Practice subgroup
definition, based on
baseline characteristics
Impact estimate for
practices that met the
subgroup definition
(standard error)
Impact estimate for
practices that did not
meet the subgroup
definition (standard
error)
p-value from test of
significant difference in
impacts between
subgroups
PCMH recognition -$17*
($9)
-$3
($8)
0.27
Six or more physicians or
affiliated with a larger
organization
-$13*
($7)
$2
($12)
0.27
Hospital-owned -$22**
($9)
-$9
($7)
0.27
System-owned -$18**
($8)
-$10
($8)
0.44
Either hospital- or system-
owned
-$18**
($7)
-$10
($9)
0.49
Had a meaningful user of
EHRs
-$7
($7)
-$17
($11)
0.41
Large and medium, versus
small practice, based on
number of clinicians
a
Large: -$11
($10)
Medium:
-$16*
($9)
$7
($13)
Large versus small:
0.27
Medium versus small:
0.15
Note: Information on hospital and system ownership, obtained from SK&A, was not available for 7 CPC and 48
comparison practices. We excluded these 55 practices from the subgroup analyses based on hospital and
system ownership variables.
a
Small, medium, and large practices were defined as those with 1–2 clinicians, 3–5 clinicians, and 6 or more
clinicians, respectively.
95
Although we matched CPC and comparison practices on several of these baseline practice characteristics, it is
unlikely that CPC and matched comparison group practices within each of these subgroups were as well-balanced in
all baseline characteristics as the full sample (with the exception of the subgroup for whether a practice had a
meaningful EHR user at baseline, which was an exact-match variable). Therefore, although we controlled for most
of these baseline characteristics in our regressions, the estimates for specific subgroups could be distorted by
baseline differences between the CPC and comparison practices within those subgroups.
224

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
g. Medicare expenditures including CPC and SSP shared savings payments
After adding the shared savings amounts that Medicare paid for CPC and SSP through 2015,
the CPC-wide estimates for Medicare expenditures (including fees and shared savings) showed
little or no change in magnitude or statistical significance compared to the estimates for net
Medicare expenditures including fees, as reported above (Table 8.3). In other words, including
shared savings amounts did not, in general, change impact estimates for total Medicare
expenditures. The only exception was for Oklahoma in Year 3, where the estimate became more
unfavorable or larger in magnitude for all beneficiaries (estimated increase of $14 PBPM more
for CPC than comparison practices, compared to an increase of $2 PBPM before, without shared
savings). This is consistent with Oklahoma practices receiving a large share of the total CPC
shared savings payments in Year 3—$10 million out of the total $13 million paid to all regions
in 2015.
8.3.2. Service use
While service use in both CPC and comparison practices grew over time, there was less growth for
CPC than comparison practices in the use of some, but not all, types of services. Among all
beneficiaries, cumulative impact estimates showed modest, statistically significant favorable effects
on hospitalizations, total ED visits, outpatient ED visits, and office-based primary care visits over the
course of the initiative (Tables 8.1 and 8.5). The exception was that there was a relative increase in
the number of observation stays over all four years of CPC combined. The favorable effects on ED
visits were concentrated in the last two years of CPC; the magnitude of favorable effects on
hospitalizations was relatively stable over time.
CPC reduced the growth in hospitalizations, ED visits, and primary care visits by 2
percent for CPC versus comparison practices (Table 8.3). Relative to the comparison group,
during the four years of CPC, hospitalizations for beneficiaries in CPC practices increased by 2
percent less (five fewer admissions per 1,000 beneficiaries per year) (p = 0.07). Similarly, total
ED visits for CPC practices relative to comparison practices increased by 2 percent less (14
fewer visits per 1,000 beneficiaries per year) (p < 0.01);
96
outpatient ED visits for CPC practices
increased by 2 percent less (10 fewer visits per 1,000 beneficiaries per year) (p = 0.03); and
primary care visits in office-based settings increased by 2 percent less (68 fewer visits per 1,000
beneficiaries per year) (p = 0.07). Primary care visits in all settings also increased by 1 percent
less for beneficiaries in CPC than comparison practices; however, this estimate was not
statistically significant (p = 0.14).
97
Relative to the comparison group, observation stays for
beneficiaries in CPC practices increased by 5 percent more (three additional stays per 1,000
beneficiaries per year) (p = 0.03). (This difference offset over half the estimated relative
reduction of five inpatient admissions per 1,000 beneficiaries.) A 1 percent larger increase in
specialist visits in all settings among beneficiaries in CPC than comparison practices over the life
of the initiative was not statistically significant (p = 0.31).
96
Total ED visits include both outpatient ED visits and ED visits that led to an inpatient hospitalization.
97
Primary care visits in all settings include office-based primary care visits as well as visits in other settings, such as
in a hospital or nursing home.
225

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
Favorable impacts for some types of Medicare service use became more pronounced
over time (Table 8.3). For both total ED visits and outpatient ED visits, favorable estimates in
Years 1 and 2 were small and not statistically significant. In contrast, the favorable effects on
total ED visits for CPC practices versus comparison practices were 17 per 1,000 beneficiaries in
Year 3 and 18 per 1,000 beneficiaries in Year 4 (about 2 percent of the comparison group mean,
p < 0.01 in both cases). We observed a similar pattern for outpatient ED visits, with slower
growth of 15 and 13 per 1,000 beneficiaries (or 3 percent) in Years 3 and 4, respectively (p <
0.01 and p = 0.02) among CPC than comparison practices. For hospitalizations, however, the
magnitude of favorable impacts did not change. Finally, one unfavorable impact emerged in
Year 4; although the estimate for specialist visits in all settings was close to zero in Years 1
through 3, specialist visits in all settings among all beneficiaries and high-risk beneficiaries
increased by 2 percent more in CPC than comparison practices in Year 4. Based on the
regression-adjusted means, this was mainly driven by larger increases in specialist visits in the
CPC group than in the comparison group during the last two years of CPC. However, the
difference in office-based specialist visits for beneficiaries in CPC versus comparison practices
was less pronounced, with the largest estimate implying a 2 percent (p = 0.14) relative increase
in Year 4.
Estimates for service use outcomes for high-risk beneficiaries were generally similar to
those for all beneficiaries but not statistically significant. Among high-risk beneficiaries, the only
statistically significant cumulative estimate for Medicare service use was for office-based
primary care visits, which declined by 110 per 1,000 beneficiaries, or 2 percent more in the CPC
group than in the comparison group (p = 0.07).
8.3.3. Claims-based quality of care
We found minimal effects on the claims-based quality-of-care process and outcome measures we
examined. There were very few sizeable or statistically significant estimates for the quality-of-care
process measures among beneficiaries with diabetes, or in transitional care measures among all
beneficiaries during the course of the initiative. The only significant findings for quality-of-care
process measures among beneficiaries with diabetes were in the high-risk subgroup. Similarly, for
continuity of care, there were no statistically significant effects through Year 4. For quality-of-care
outcome measures, the only statistically significant impact was favorable, showing a smaller
increase in the likelihood of ED revisits among all beneficiaries for CPC versus comparison
practices, which is consistent with the favorable effects on ED visits. Our analysis of quality of care
looked only at a limited set of claims-based measures, and did not include the eCQMs used for
quality improvement and for calculating shared savings. Not all comparison practices reported
eCQMs, creating both conceptual and data challenges for analyzing the impact of CPC on eCQMs.
For the quality-of-care process measures among beneficiaries with diabetes, none of
the cumulative impact estimates were sizeable or statistically significant for all
beneficiaries or high-risk beneficiaries (Table 8.9). The only cumulative estimate suggesting a
possible (but not quite statistically significant) improvement was for high-risk beneficiaries. It
showed a 1.2 percentage point larger increase in the likelihood of high-risk beneficiaries with
diabetes receiving all three tests (HbA1c, eye exam, and urine protein testing) in CPC than
comparison practices (p = 0.12). In general, the results for quality-of-care process measures
among beneficiaries with diabetes suggest substantial opportunities for improvement, since only
about one-third of beneficiaries received all three recommended tests in any given year of CPC.
226

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
In yearly estimates, there were a few sporadic and mixed effects on quality-of-care
process measures for high-risk beneficiaries with diabetes. Specifically, in year-by-year
estimates, there were two statistically significant findings among the high-risk beneficiaries
only—one unfavorable and the other favorable. In an unfavorable finding, the likelihood of
HbA1c testing among high-risk beneficiaries with diabetes increased less for CPC than
comparison practices by 1.1 percentage points or 1 percent (p = 0.09), and 1.3 percentage points
or 1.5 percent (p = 0.05) in Years 2 and 3, respectively, and the estimate in Year 4 was close to
zero. On the other hand, consistent with the cumulative estimate for one of the summary
measures, the likelihood of a high-risk beneficiary with diabetes receiving all three tests (HbA1c,
eye exam, and urine protein testing) increased by close to 2 percentage points more for CPC than
comparison practices (about 5 percent, p = 0.07) in Year 2, but smaller 1.5 and 1.2 percentage
point increases in Years 1 and 4 were not significant. The small magnitudes and erratic pattern of
these estimates over time and across measures suggest that the two statistically significant
estimates are likely to be spurious differences due to chance, rather than evidence of important
impacts.
There were no statistically significant effects on any of the continuity-of-care measures.
For all four measures—the percentage of primary care visits at the beneficiary’s attributed
practice, the percentage of all primary and specialty care visits at the attributed practice, the
Bice-Boxerman index based on primary care visits, and the Bice-Boxerman index based on all
visits—continuity declined similarly for both the CPC and comparison groups by 4 to 14
percentage points between the four-year period before CPC began and the four years during
CPC.
98
Although the impact estimates were negative in most cases, suggesting marginally
greater decline for the CPC group, these were all less than 1 percent and not statistically
significant. It is possible that CPC practices used the non-visit-based care management fees to
cover some interactions with their patients for which they did not bill, which would make the
claims-based continuity measures look worse than continuity really was for CPC practices.
CPC led to a slower increase in the likelihood of an ED revisit within 30 days of an
outpatient ED visit, but had no discernible effect on other quality-of-care outcome
measures. For the quality-of-care outcome measures, there were no statistically significant
effects on either ACSC admissions or the likelihood of an unplanned 30-day readmission among
all beneficiaries or high-risk beneficiaries. The lack of significant effects for ACSC admissions
and 30-day readmissions could be due to practices having limited opportunities to affect these
outcomes since they occur relatively infrequently. For example, the average number of ACSC
admissions was only about 65 per 1,000 beneficiaries among beneficiaries in CPC practices
during Year 4 of CPC. Similarly, the rates of 30-day readmissions at the discharge and
98
For our continuity-of-care measures, the baseline period consisted of the four years before the start of CPC
(October 2008–September 2012), and the intervention period was the four years after the start of CPC (October
2012–September 2016). The fall in continuity for both CPC and comparison groups reflects how we constructed the
measures. Because continuity was measured with respect to the practice that the patient was attributed to in Quarter
1, continuity was high before CPC began, by definition, for both the CPC and comparison groups. (This period
overlaps with the Quarter 1 look-back period, and beneficiaries had to have a plurality of their visits at a practice
during this look-back period to be attributed to that practice.) It is not surprising that continuity fell over time,
because beneficiaries who were attributed to different practices after CPC began by definition had low continuity
with the practice to which they were attributed in Quarter 1.
227

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
beneficiary levels were only about 15 percent and 3 percent, respectively, in Year 4 of CPC.
99
At
the beneficiary level, the rate of ED revisits within 30 days of an outpatient ED visit was also
low, but higher than the rate of 30-day hospital readmissions, at 6 percent in Year 4, for both the
CPC and comparison groups. Despite the modest incidence of ED revisits, we did see less
growth in ED revisits of 0.2 percentage points, or 3 percent, during the initiative among
beneficiaries in CPC versus comparison practices (Table 8.9). In yearly estimates for all
beneficiaries, statistically significant and favorable estimates of the same magnitude first
emerged in Years 3 and 4. This finding is consistent with the significantly slower increases in
both outpatient and total ED visits observed for all beneficiaries in CPC than comparison
practices during Years 3 and 4 of CPC.
99
There has also been a nationwide decline in readmissions among Medicare beneficiaries due to other ongoing
CMS programs (Daughtridge et al. 2014). This could have made it harder for CPC practices to achieve a greater
decline in readmissions relative to matched comparison practices.
228

229
Table 8.9. Regression-adjusted means and estimated difference-in-differences estimates of CPC’s impact on
selected quality-of-care process and outcome measures: annual and four-year cumulative CPC-wide estimates
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-
adjusted mean
Comparison group
practices’
regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact
(%)
p-value for
estimated impact
CPC practices’
regression-
adjusted mean
Comparison group
practices’
regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact
(%)
p-value for
estimated impact
Quality-of-care process measures for beneficiaries with diabetes (percentage point changes)
Among beneficiaries with
diabetes – HbA1c test
Baseline 88.1 87.8 - - - - 85.3 84.5 - - - -
Year 1 89.5 89.6 -0.4 0.4 - 0.383 87.3 87.5 -0.9 0.6 - 0.133
Year 2 90.3 90.2 -0.2 0.5 - 0.690 88.4 88.8 -1.1* 0.7 - 0.088
Year 3 90.8 90.7 -0.2 0.5 - 0.731 89.4 90.0 -1.3* 0.7 - 0.054
Year 4 90.8 90.7 -0.1 0.4 - 0.840 89.7 88.8 0.1 0.7 - 0.895
Years 1–4 combined 90.3 90.3 -0.2 0.4 - 0.604 88.9 89.0 -0.8 0.5 - 0.105
Among beneficiaries with
diabetes – eye exam
Baseline 53.6 53.7 - - - - 53.0 53.3 - - - -
Year 1 56.0 55.4 0.7 0.7 - 0.288 56.0 55.3 1.0 0.9 - 0.300
Year 2 56.1 55.4 0.9 0.6 - 0.125 55.8 55.0 1.2 0.9 - 0.205
Year 3 57.9 57.7 0.3 0.8 - 0.662 57.5 57.2 0.6 1.0 - 0.544
Year 4 57.9 58.3 -0.3 0.9 - 0.756 57.7 57.2 0.8 1.3 - 0.534
Years 1–4 combined 57.7 57.5 0.4 0.6 - 0.506 57.6 57.1 0.9 0.8 - 0.252
Among beneficiaries with
diabetes – urine protein test
Baseline 56.0 56.7 - - - - 61.3 62.1 - - - -
Year 1 60.1 60.4 0.3 0.8 - 0.729 64.3 64.1 1.0 1.0 - 0.314
Year 2 63.0 62.5 1.2 1.0 - 0.225 66.5 65.9 1.4 1.0 - 0.154
Year 3 63.9 65.1 -0.6 1.4 - 0.685 73.7 75.0 -0.5 1.4 - 0.743
Year 4 65.1 66.2 -0.5 1.4 - 0.712 75.5 77.3 -1.0 1.3 - 0.428
Years 1–4 combined 64.0 64.6 0.0 0.9 - 0.962 70.1 70.7 0.3 0.9 - 0.768
Among beneficiaries with
diabetes – all 3 tests
performed
Baseline 31.9 32.8 - - - - 32.9 34.3 - - - -
Year 1 35.4 35.7 0.6 0.7 - 0.396 36.9 36.8 1.5 1.0 - 0.154
Year 2 36.6 36.5 1.1 0.8 - 0.173 37.4 37.1 1.7* 0.9 - 0.067
Year 3 35.8 37.3 -0.5 0.9 - 0.611 40.6 41.5 0.4 1.1 - 0.691
Year 4 36.6 37.5 0.1 0.9 - 0.928 42.0 42.2 1.2 1.2 - 0.321
Years 1–4 combined 36.8 37.5 0.3 0.6 - 0.645 39.9 40.1 1.2 0.8 - 0.116

Table 8.9 (continued)
230
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-
adjusted mean
Comparison group
practices’
regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact
(%)
p-value for
estimated impact
CPC practices’
regression-
adjusted mean
Comparison group
practices’
regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact
(%)
p-value for
estimated impact
Among beneficiaries with
diabetes – none of the 3
tests performed
Baseline 6.0 6.4 - - - - 6.4 7.0 - - - -
Year 1 4.9 5.0 0.3 0.4 - 0.338 5.1 5.4 0.4 0.5 - 0.404
Year 2 4.2 4.7 -0.1 0.3 - 0.665 4.4 4.9 0.2 0.4 - 0.725
Year 3 2.5 2.6 0.3 0.4 - 0.458 2.3 2.2 0.8 0.5 - 0.136
Year 4 2.7 2.4 0.6 0.4 - 0.110 2.2 2.4 0.4 0.5 - 0.398
Years 1–4 combined 3.4 3.5 0.3 0.3 - 0.359 3.4 3.6 0.4 0.4 - 0.267
Total number of
observations (CPC and
comparison) across all
years: beneficiaries with
diabetes
a
750,737 261,394
Continuity of care (percentage)
Percentage of PCP visits at
attributed practice
Baseline 79.6 76.9 - - - - 76.1 72.8 - - - -
Years 1–4 combined 64.8 62.8 -0.7 0.9 -1.1% 0.434 63.0 60.3 -0.5 1.0 -0.8% 0.602
Percentage of all visits at
attributed practice
Baseline 45.4 45.6 - - - - 38.9 39.1 - - - -
Years 1–4 combined 35.4 35.7 -0.1 0.6 -0.4% 0.836 32.4 32.5 0.2 0.7 0.6% 0.770
Bice-Boxerman Index based
on PCP visits
Baseline 72.0 69.5 - - - - 68.4 65.7 - - - -
Years 1–4 combined 62.4 60.5 -0.6 0.8 -1.0% 0.434 61.3 59.0 -0.3 0.8 -0.6% 0.660
Bice-Boxerman Index based
on all visits
Baseline 33.5 33.5 - - - - 28.0 28.1 - - - -
Years 1–4 combined 29.0 29.2 -0.2 0.4 -0.6% 0.616 26.7 26.8 0.1 0.4 0.2% 0.891
Total number of
observations (CPC and
comparison) across all
years: measures based on
PCP visits
b
1,506,804 487,138

Table 8.9 (continued)
231
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-
adjusted mean
Comparison group
practices’
regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact
(%)
p-value for
estimated impact
CPC practices’
regression-
adjusted mean
Comparison group
practices’
regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact
(%)
p-value for
estimated impact
Total number of
observations (CPC and
comparison) across all
years: measures based on
all visits
c
1,718,474 547,940
Transitional care and quality-of-care outcomes (annualized rate per 1,000 or percentage)
Likelihood of 14-day follow-
up visit (percentage)
Baseline 62.1 62.0 - - - - 66.9 66.9 - - - -
Year 1 63.0 63.0 -0.1 0.6 - 0.900 67.6 67.9 -0.4 0.6 - 0.588
Year 2 64.9 64.5 0.3 0.6 - 0.653 69.4 69.2 0.1 0.7 - 0.868
Year 3 65.6 65.0 0.4 0.6 - 0.540 69.7 69.8 -0.1 0.7 - 0.852
Year 4 67.4 67.6 -0.4 0.6 - 0.488 71.7 71.8 -0.1 0.6 - 0.821
Years 1–4 combined 66.0 65.9 0.0 0.5 - 0.999 69.8 69.8 -0.1 0.6 - 0.808
Total number of
observations (CPC and
comparison) across all
years: follow-up visit
d
1,675,235 867,707
ACSC admissions
(annualized rate per 1,000
beneficiaries)
Baseline 37.0 39.5 - - - - 112.9 118.5 - - - -
Year 1 55.7 57.5 0.7 1.1 0.9% 0.563 151.1 154.1 2.6 3.6 1.6% 0.462
Year 2 56.7 58.2 1.0 1.2 1.5% 0.411 147.1 148.4 4.3 3.7 2.9% 0.237
Year 3 62.5 64.5 0.5 1.2 0.7% 0.696 157.3 161.9 1.0 4.0 0.6% 0.811
Year 4 65.5 68.3 -0.3 1.3 -0.5% 0.801 162.4 166.4 1.6 4.2 1.0% 0.699
Years 1–4 combined 68.2 70.5 0 1.0 0.7% 0.668 161.2 164.6 2.5 3.1 1.6% 0.421
Total number of
observations (CPC and
comparison) across all
years: ACSC admissions
e
6,575,258 1,731,832

Table 8.9 (continued)
232
All attributed Medicare beneficiaries High-risk attributed Medicare beneficiaries
CPC practices’
regression-
adjusted mean
Comparison group
practices’
regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact
(%)
p-value for
estimated impact
CPC practices’
regression-
adjusted mean
Comparison group
practices’
regression-
adjusted mean
Estimated impact
(size)
Standard error for
impact estimate
Estimated impact
(%)
p-value for
estimated impact
Likelihood of 30-day
readmission (percentage)
Baseline 13.3 13.3 - - - - 16.5 16.8 - - - -
Year 1 14.7 15.2 -0.5 0.3 - 0.111 18.5 19.0 -0.3 0.5 - 0.482
Year 2 14.4 14.5 -0.1 0.3 - 0.767 17.9 17.9 0.2 0.5 - 0.694
Year 3 14.9 15.0 -0.1 0.3 - 0.850 18.6 18.6 0.2 0.5 - 0.654
Year 4 14.7 14.9 -0.2 0.3 - 0.488 18.1 18.3 0.0 0.5 - 0.993
Years 1–4 combined 14.7 14.9 -0.2 0.3 - 0.408 18.0 18.2 0.0 0.4 - 0.986
Total number of
observations (CPC and
comparison) across all
years: readmissions
f
1,675,788 867,904
Likelihood of an ED revisit
within 30 days of an
outpatient ED visit
(percentage)
Baseline 3.9 3.9 - - - - 8.9 9.0 - - - -
Year 1 3.9 3.9 0.0 0.1 - 0.770 8.2 8.2 0.1 0.2 - 0.582
Year 2 4.3 4.3 0.0 0.1 - 0.801 8.7 8.7 0.1 0.2 - 0.719
Year 3 4.8 5.1 -0.3*** 0.1 - 0.003 9.5 9.7 -0.1 0.3 - 0.840
Year 4 6.0 6.4 -0.3*** 0.1 - 0.001 11.5 11.9 -0.3 0.3 - 0.355
Years 1–4 combined 5.2 5.5 -0.2** 0.1 - 0.020 9.7 9.8 0.0 0.2 - 0.871
Total number of
observations (CPC and
comparison) across all
years: ED revisit
e
6,575,258 1,731,832
Source: Medicare claims data for October 2008 through December 2016.
Note: Impact estimates are regression-adjusted for baseline beneficiary characteristics (including HCC scores) and baseline practice characteristics. Each impact estimate is based
on a difference-in-differences analysis and reflects the difference in the regression-adjusted average outcome for attributed Medicare FFS beneficiaries in CPC practices in
the intervention period compared with the baseline period relative to the same difference over time for attributed Medicare FFS beneficiaries in matched comparison
practices. For the ED revisit equation, we also controlled for chronic conditions at baseline. For the readmissions and follow-up visits equations estimated at the discharge
level, we also controlled for discharge-level risk factors. Number of observations includes the total number of CPC and comparison group observations across all years. For
continuous quality-of-care outcome measures, we present the absolute impact estimate as well as its relative size in percentage terms. For binary quality-of-care outcome
measures, we only present the absolute impact estimate in percentage points. Regression-adjusted means for each year and for both groups from the annual regression were
obtained by using average values of the control variables among beneficiaries in CPC practices in Year 4, in order to hold beneficiary and practice attributes fixed in
generating predictions. Regression-adjusted means from the cumulative regression were obtained by using average values of the control variables among beneficiaries in
CPC practices across all four CPC years. Regression-adjusted means from the cumulative regression are similar but not always perfectly aligned with those from the annual
Table 8.9 (continued)
233
regression due to differences in coefficients on control variables and the different samples used for predictions; however, the impact estimate from the cumulative regression
is within the range of values for the impact estimates from the annual regression.
a
For the quality-of-care process measures for beneficiaries with diabetes, the analysis includes 88,217 Medicare FFS beneficiaries attributed to 497 CPC practices and 190,451
beneficiaries attributed to 908 matched comparison practices.
b
For continuity-of-care measures based on PCP visits, the analysis includes 261,324 beneficiaries in CPC practices and 492,078 beneficiaries in comparison practices.
c
For continuity-of-care measures based on all visits, the analysis includes 290,776 beneficiaries in CPC practices and 568,461 beneficiaries in comparison practices.
d
For 14-day follow-up visits, the analysis includes 229,415 beneficiaries in CPC practices and 488,664 beneficiaries in comparison practices.
e
For ACSC admissions and ED revisit measures, the analysis includes 556,674 beneficiaries in CPC practices and 1,165,284 beneficiaries in comparison practices.
f
For 30-day readmission measures, the analysis includes 229,458 beneficiaries in CPC practices and 488,782 beneficiaries in comparison practices.
*/**/***Significantly different from zero at the 0.10/0.05/0.01 level, two-tailed test.
ACSC = ambulatory care sensitive condition; ED = emergency department; FFS = fee-for-service; HCC = Hierarchical Condition Category; PCP = primary care physician.

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
8.3.4. Aggregate impacts of CPC for all attributed beneficiaries
We calculated aggregate impacts of CPC, by year, across all Medicare FFS beneficiaries
attributed to CPC practices, for six outcome measures: (1) total Medicare expenditures without
fees, (2) number of hospitalizations, (3) number of outpatient ED visits, (4) number of primary
care clinician visits in all settings, (5) number of office-based primary care clinician visits, and
(6) 30-day unplanned readmissions. For the first five outcomes, we used the beneficiary-level
estimates from the difference-in-differences regressions, together with the total eligible months
for beneficiaries attributed to CPC practices in each year, to obtain the aggregate impacts as well
as the 90 percent confidence intervals for these impacts. For readmissions, we used the
discharge-level estimates and the total discharges for all beneficiaries in CPC practices to obtain
these aggregate impacts, by year (Table 8.10). We obtained cumulative aggregate impacts by
adding yearly aggregates over the four years of CPC, and calculated confidence intervals for the
sums, taking into account the correlation of the estimates across years. With all four years of
CPC impact estimates now available, the cumulative aggregate estimates offer a complete
picture of relative reductions in utilization of key services like hospitalizations and outpatient ED
visits over the life of the initiative.
Based on the 90 percent confidence intervals, statistically significant aggregate estimates are
shown in bold font in Table 8.10. For the outcomes examined, there were “relative reductions”
because utilization and expenditures increased less over time for CPC practices relative to
comparison practices. Cumulative aggregate estimates for Medicare expenditures,
hospitalizations, outpatient ED visits, primary care visits, and 30-day readmissions were as
follows:
• Savings of over $152 million in total Medicare expenditures without fees over the life of the
initiative that were not statistically significant. These savings cover slightly over half (55
percent) of the cumulative care management fees of $278.5 million paid to the CPC
practices over the life of the initiative.
• Relative reductions of 8,150 hospitalizations and 15,472 outpatient ED visits over the four
years, both of which were statistically significant.
• Relative reductions of 107,785 office-based primary care visits and 137,166 primary care
visits in all settings; only the former was statistically significant.
• Relative reduction of 936 30-day readmissions over the four years, which was not
statistically significant.
234

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
Table 8.10. Aggregate CPC-wide results, by year and cumulative
Outcome
Total
Medicare
expenditures
without fees Hospitalizations
Outpatient ED
visits
Primary care
visits in all
settings
Office-based
primary care
visits
30-day
readmissions
Year 1
Estimate
-$68,569,900
a
-1,862
a
-797 -27,867 -11,567 -480
90% CI LL -$111,373,479 -3,484 -3,271 -55,816 -28,099 -976
90% CI UL -$25,766,320 -239 1,677 81 4,965 16
Year 2
Estimate -$49,190,714 -1,950 -1,509
-39,027
a
-27,770
a
-90
90% CI LL -$100,511,538 -4,046 -4,625 -75,626 -50,364 -589
90% CI UL $2,130,110 146 1,606 -2,429 -5,177 409
Year 3
Estimate -$19,720,803 -1,229
-5,962
a
-34,500
-35,227
a
-67
90% CI LL -$81,305,937 -3,547 -9,578 -81,927 -64,802 -645
90% CI UL $41,864,332 1,089 -2,346 12,926 -5,652 512
Year 4
Estimate -$14,870,706 -3,109
-7,204
a
-35,771 -33,221 -299
90% CI LL -$107,360,416 -6,292 -12,337 -107,732 -79,198 -1,007
90% CI UL $77,619,003 73 -2,071 36,190 12,757 409
Years 1–4
combined
Estimate -$152,352,128
-8,150
a
-15,472
a
-137,166
-107,785
a
-936
90% CI LL -$360,895,871 -15,789 -27,333 -296,978 -208,920 -2,799
90% CI UL $56,191,615 -510 -3,610 22,645 -6,650 927
Source: Analysis of Medicare claims data for October 2011 through December 2016.
Notes: This table calculates the estimated effects over all CPC regions and attributed Medicare FFS beneficiaries who were in
the intent-to-treat analysis sample for Years 1, 2, 3, and 4 of CPC. The total number of beneficiaries attributed to CPC
practices in the annual analysis sample was 365,996 in Year 1, 409,750 in Year 2, 442,160 in Year 3, and 482,287 in
Year 4. The number of eligible beneficiary months for the same number of beneficiaries in CPC practices was 3,908,795
in Year 1, 4,451,025 in Year 2, 4,837,588 in Year 3, and 6,505,371 in Year 4.The number of eligible index discharges (for
readmissions) was 89,847 in Year 1, 96,696 in Year 2, 108,173 in Year 3, and 141,233 in Year 4. For calculating the
cumulative aggregate impacts (across Years 1, 2, 3, and 4 combined), we added the yearly aggregate impacts over the
four years. Impact estimates are from difference-in-differences regressions using both patient- and practice-level control
variables from the pre-CPC period. See Section 8.2 for a full list of measures and definitions, as well as a discussion of
methods. Green shading with bolded text signifies that estimate was favorable and statistically significant at the
p < 0.10 level. To help put the gross Medicare savings in perspective, the total care management fees CMS paid for
attributed Medicare FFS beneficiaries during the four performance years (2013, 2014, 2015, and 2016) were $90.5
million, $76.1 million, $57 million, and $55.9 million, respectively, for a cumulative payment of $278.5 million, after
accounting for total recoupments of about $1 million.
CI = confidence interval; ED = emergency department; LL = lower limit; UL = upper limit.
a
Estimate is statistically significant at the p < 0.10 level
8.4. Discussion
To recap the findings, during the course of the intervention, CPC practices had favorable
findings on service use, with 2 percent less growth than comparison practices in ED visits,
hospitalizations, and office-based primary care visits—but had at best a small effect on total
Medicare expenditures. Although total monthly Medicare FFS expenditures without Medicare’s
CPC care management fees increased by $9 less for beneficiaries in CPC than for beneficiaries
in comparison practices, this did not fully offset the care management fees Medicare paid, which
averaged $15 per month over the course of the initiative. In addition, the Bayesian estimates
235

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
suggest a high probability of some gross savings, but almost a zero probability that the savings
were sufficient to cover Medicare’s care management fees.
These expenditure findings were robust. In most of the alternative model specifications that
we ran, the estimated effects on Medicare expenditures were small and not statistically
significant, similar to our main findings. However, the results for total Medicare expenditures
showed an unexpected and implausible pattern, with the annual estimates attenuating over time.
Based on Gelman and Carlin’s (2014) approach for estimating the degree to which a statistically
significant estimate is likely to be overestimated, we determined that the Year 1 effect on total
Medicare expenditures of an $18 PBPM decline was likely to be overestimated by a factor of
about 1.8.
CPC appeared to generate some savings through reductions in certain areas of service
utilization. Specifically, there were statistically significant estimates of relative reductions in
expenditures for specific types of services over the course of the initiative, including skilled
nursing facilities, outpatient services, and primary care physician services. CPC also led to small
relative reductions in hospitalizations, ED visits, and office-based primary care visits. The
pattern of effects on ED visits, with larger, statistically significant impacts in the last two years,
conforms to the expectation that practice transformation leads to benefits in the long run.
100
There were minimal effects on claims-based measures of quality of care. Consistent with the
findings for ED visits, there was a smaller increase in the likelihood of an ED revisit within 30
days of an outpatient ED visit for CPC than comparison practices during the initiative.
There were some favorable effects within subgroups. In particular, some evidence suggested
that medium-sized practices, hospital- or system-owned practices, and practices that were
recognized medical homes at baseline were more successful in achieving savings. However, the
estimated impacts for these subgroups were not significantly different from the estimates for
their respective counterparts. Therefore, the evidence for larger impacts among certain practice
subgroups is not conclusive.
The implementation findings help interpret the impact estimates. One might have expected
that the attenuation of annual impact estimates for Medicare expenditures from Year 1 to Year 4
was related to the reduction in Medicare’s average care management fees from $20 to $15 PBPM
in Quarter 10. However, the implementation analysis—including interviews with deep-dive
practices—provided little or no evidence that practices decreased resources devoted to care
management during the last two years of CPC. Across all deep-dive practices, there was little or
no evidence that practices reduced staffing. Also, there were no complaints from practices about
reductions in the care management fees during site visits or the most recent deep-dive phone
interviews. Finally, more than three-quarters of practices reported that care management
payments from Medicare were adequate or more than adequate relative to the costs of
implementing CPC in the CPC practice surveys for intervention years 2014 through 2016.
100
The four annual impact estimates for outpatient ED visits were jointly significant as well as significantly
different from one another at the 10 percent level.
236

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
Our implementation analysis points to the possibility that a number of factors directly
related to changes instituted by CPC practices helped lower ED use. For instance, when deep-
dive practices were asked during site visits whether they thought CPC activities were having any
impact on patient outcomes, they frequently noted that several of their efforts were likely
reducing ED use.
With respect to implementation findings about lower utilization of inpatient services, deep-
dive practices noted that access to a care manager at the practice for high-risk patients improved
the quality of care and this was perceived to help reduce hospitalizations. In-depth interviews
with high-risk patients and their caregivers confirmed that care management improved the
quality of care from their perspective. Patients who reported that they had regular contact with a
care manager, and were willing to work with a care manager, said that they primarily received
follow-up after hospitalizations, help with the ongoing management of chronic conditions,
medication monitoring, navigating the health system and community services, and other forms of
assistance (O’Malley et al. 2017b). Also, in the most recent telephone interviews, practice
members and system-level leaders shared their perception that hospital and ED follow-up
contacts were contributing to lower readmissions and lower ED utilization. Practice members
thought that care transitions contributed to improved quality of care by addressing patients’
misunderstandings or barriers before they caused health problems, providing clinicians with
information to help patients during follow-up office visits, and catching discrepancies in
medications following hospital discharge.
Findings from the survey of beneficiaries also suggest that CPC practices provided better
follow-up care after hospitalizations and ED visits, which in turn could have led to reductions in
later acute care service use. Specifically, beneficiaries in CPC practices were more likely to be
contacted by the primary care provider’s office within three days of an inpatient discharge and
within one week of an ED visit than beneficiaries in the comparison group. Although we did not
find any significant improvement in our claims-based measure of follow-up within 14 days of an
inpatient discharge, in our patient survey analysis we found that a higher proportion of CPC than
comparison practices provided timely follow-up care after hospitalizations and ED visits
(estimated differences in the 2016 patient survey were 8 and 10 percentage points for follow-up
after ED visits and hospitalizations, respectively). This suggests that CPC practices provided
more non-visit-based follow-up (by phone, for example), as well as follow-up by care managers,
who cannot bill Medicare for such services. It is also consistent with the estimated 2 percent
reduction in office-based primary care visits among CPC versus comparison practices during
CPC, pointing toward greater reliance on non-visit-based interactions.
Overall, these findings suggest that CPC likely slowed the growth in the use of ED and
inpatient services. It may take longer or require stronger incentives for not only practices, but
also patients and the other providers they visit, to reduce utilization enough to generate net
savings. Chapter 9 in this report seeks to identify the role of specific aspects of practice
transformation in improving key outcomes.
This study of the impacts of CPC was unique because it combined significant investments
from CMS and other payers through multipayer collaboration and a large number of practices in
diverse regions. Like CPC, other primary care CMS demonstrations had mixed findings. The
Federally Qualified Health Center (FQHC) demonstration led to some improvements in diabetes
237

8. CPC’S IMPACT MATHEMATICA POLICY RESEARCH
care, but was associated with increased Medicare utilization and expenditures (Kahn et al. 2017).
Similarly, of the eight states in the Multi-Payer Advanced Primary Care (MAPCP), only one had
a favorable impact on hospitalizations, two had an unfavorable effect on emergency department
visits and one had a favorable effect on expenditures (Nichols et al. 2017).
Consistent with our CPC results, PCMHs were also associated with reduced ED use in other
studies (Guy 2014; Pines 2015; Rosenthal 2016; Rosenthal 2013). More generally, prior studies of
diverse primary care transformation interventions have been limited and have yielded mixed
results (Nichols et al. 2017; Friedberg et al. 2015; Friedberg et al. 2014; Reid et al. 2010; Gilfillan
et al. 2010; Werner et al. 2013; Rosenthal et al. 2013; Kahn et al. 2017; Werner et al. 2014;
Heyworth et al. 2014; Jaén et al. 2010; Maeng et al. 2013; Reddy et al. 2015; Reid et al. 2009;
Kern et al. 2013). Although three studies operated in multiple markets and served large numbers of
practices or clinics (Kahn et al. 2017; Werner et al. 2014; Nichols et al. 2017), most published
studies examined pilots conducted in single markets (Friedberg et al. 2015; Friedberg et al. 2014;
Reid et al. 2010; Gilfillan et al. 2010; Werner et al. 2013; Rosenthal et al. 2013), with small
numbers of practices (Reid et al. 2010; Gilfillan et al. 2010; Werner et al. 2013; Rosenthal et al.
2013), or with one or a few payers (Friedberg et al. 2015; Gilfillan et al. 2010; Werner et al. 2013;
Rosenthal et al. 2013; Kahn et al. 2017; Werner et al. 2014), or did not examine expenditures
(Friedberg et al. 2015; Rosenthal et al. 2013; Werner et al. 2014).
The impact analysis has several limitations. First, participation in CPC was voluntary, and
our analysis was limited to Medicare FFS beneficiaries who were attributed to CPC practices.
Therefore, the results may not be generalizable to all primary care practices or all patients seen
by a practice. However, both the regions and the practices selected were diverse on some
features, such as size, patient mix, and ownership, and we compared outcomes for beneficiaries
in CPC practices with those of beneficiaries in practices with similar characteristics, patient mix,
and prior outcomes. Second, the measures of quality of care that were available in the claims
data were limited. (We did not include the eCQMs used for quality improvement and for
calculating shared savings, because not all comparison practices reported eCQMs.) Third,
although the study used a careful and thorough method to match CPC practices to comparison
practices on observed characteristics, there could still be differences in unobserved
characteristics between the two groups of practices before CPC began (or differential changes
over time in such characteristics) that led to differences in outcomes (in either direction) that
were not caused by CPC.
Although CPC did not significantly reduce Medicare Part A and B expenditures, the
evidence from our analysis suggests a high likelihood that the initiative achieved some savings;
however, the savings were insufficient to fully offset the care management fees. Also, the pattern
of declining year-by-year impacts for Medicare expenditures belied expectations, even if the four
annual estimates were not significantly different from one another. However, impacts on key
service use measures were consistent with expectations, especially the larger effects on ED visits
in the last two years of CPC, and were likely driven by improvements in primary care delivery in
CPC practices.
238

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
9. WERE PRACTICES’ CARE DELIVERY APPROACHES ASSOCIATED WITH
IMPROVED EXPENDITURE AND UTILIZATION OUTCOMES?
Linking practices’ care delivery approaches to the health care expenditures and utilization of
their beneficiaries is critical to developing a thorough understanding of how CPC affects
outcomes. Chapter 5 of this report details the substantial changes CPC practices made in how
they deliver care. Chapter 8 provides impact estimates, by comparing outcomes of CPC and
comparison practices, indicating that CPC overall yielded modest reductions in emergency
department (ED) visits and hospital stays that reduced Medicare expenditures slightly but not
enough to cover the care management fees. Although CPC had limited impacts on key outcomes
of utilization or expenditures across all practices, many individual CPC practices improved
outcomes over the life of the study (Figure 9.1). Depending on the outcome, 25 to 68 percent of
practices experienced some reduction during this period, with the top 10 percent of practices
experiencing 24 to 37 percent reductions, on average.
Figure 9.1. Distribution of changes in outcomes among CPC practices
between the year before CPC (baseline) and Year 4
In this chapter, we examine whether patients in practices with stronger self-ratings on
particular care delivery approaches have better outcomes. Specifically, we estimate the extent to
which better performance on service use and expenditures among CPC practices was associated
with three measures of primary care delivery approaches during CPC, particularly in Year 4. The
analysis builds on findings from related analyses in the past two annual reports (see Peikes et al.
2016a, 2016b). It does not attempt to link outcome changes to factors that may have led practices
to implement these approaches more comprehensively, such as the amount of care management
fees the practice received, measures of the strength of the practice’s leadership, or other aspects
of practice culture. But the findings may be helpful in implementing future primary care efforts,
like CPC+.
239

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
9.1. Key takeaways
Overall, associations between key care delivery approaches reported by practices and better
outcomes in Year 4 were few and relatively small in magnitude. The strongest association was
between primary care follow-up after acute care episodes and fewer hospitalizations, which was
statistically significant overall and larger for high-risk beneficiaries and practices with higher baseline
hospitalizations. Continuity of care was also associated with one better outcome (fewer outpatient
ED visits) but only for high-risk beneficiaries. After-hours access was related to fewer
hospitalizations, but only for practices that reported the highest possible rating on clinical
involvement of nonphysicians.
In interpreting these results, it is important to remember that we cannot conclude that a
statistically significant association between a care delivery approach and an improved outcome
implies that the better outcome is necessarily due to the care delivery approach. However, we did
control for other factors using regression models, so the findings are potentially valuable areas to
explore further. In deciding which beneficiary-level estimates suggest promising associations
between care delivery approaches and outcomes, we focus on those that are statistically
significant at the 0.10 level, which also tend to be the largest in magnitude. Furthermore, we
place the most credence in those estimates that are either (1) statistically significant at the 0.05 or
0.01 levels, given the many tests being conducted, or (2) consistent with the findings from the
practice-level analysis, or both. We also devote little attention to the few estimates that are
statistically significant but show outcomes are worse for practices with higher ratings on a care
delivery item, because our focus here is on identifying associations between care delivery
features and improved outcomes.
We found that for Year 4 outcomes:
• With one exception, the three key care delivery approaches examined here were not strongly
associated with reduced hospitalizations, ED use, or expenditures for Medicare FFS
beneficiaries in Year 4. In the final year of CPC, practices that reported more consistency in
timely primary care follow-up after a hospitalization or ED visit (which we refer to as
follow-up after acute care) had lower hospitalization rates. However, practices reporting
better access to their clinicians after normal business hours (which we refer to as after-hours
access) and patients usually seeing their own provider or practice team (which we refer to as
continuity of care) were not consistently associated with outcomes. These findings reflect, in
part, the limited variation in the care delivery measures by Year 4 as nearly three-fourths of
CPC practices rated themselves at the high end (scores of 10 to 12) of the 12-point scales on
these measures.
• Higher self-rating on follow-up after acute care was more strongly associated with fewer
hospitalizations among high-risk beneficiaries (such as those with hierarchical condition
category [HCC] scores in the 90th percentile in Year 3) than among beneficiaries with
average risk, and for practices with high (75th percentile) rather than median baseline
hospitalization rates.
• Higher self-rating on continuity of care was associated with fewer outpatient ED visits, but
only for high-risk beneficiaries.
240

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
• Higher self-rating on after-hours access was associated with fewer hospitalizations, but only
for practices that reported the highest rating on involvement of nonphysicians in clinical
care.
Several reasons may account for the limited evidence of cross-sectional associations
between specific care delivery approaches and better outcomes in Year 4:
1. Transformation is complex; many paths lead to improved outcomes, and the time period
between when practices improve and when patient outcomes improve is unknown. Both
factors can be challenging to unravel, and theory and literature offer little guidance.
2. To measure transformation to capture links to outcomes, we used the Modified Patient-
Centered Medical Home Assessment (M-PCMH-A), which was not designed for this
purpose. Thus, transformations in care delivery that are related to expenditures and service
use may not have been measured comprehensively or accurately.
3. The clustering of practices at the upper end of the care delivery measures by Year 4 resulted
in little variation in these measures, making it difficult to identify associations.
Identifying the most effective care delivery approaches to achieving the goals of lower
Medicare expenditures and improved beneficiary outcomes will continue to be important and
challenging. In the remainder of this chapter, we briefly review the findings from the past two
years, then turn to the hypotheses, methods, and findings from the current analysis as well as its
limitations. We conclude by discussing the challenges of the analysis and how to address them in
future work.
9.2. Findings from previous analyses
In Year 2, we conducted a simple analysis, assessing the association between practice
transformation and service utilization by regressing (risk-adjusted) practice-level changes
between the year before CPC (baseline) and Year 2 (October 2013 through September 2014) in
hospitalizations per 1,000 beneficiaries on the change over the same time period in a summary
index of 37 M-PCMH-A items reported by practices.
101
Key findings included the following:
• Improvement in a summary measure of overall care delivery was significantly
associated with sizable reductions in hospitalizations. Each one-point improvement in a
practice’s score (measured on a 12-point scale) was associated with a 1.2 percent reduction
in hospitalizations. Practices that increased their baseline score by the mean amount of 2.4
points therefore had an average decrease in hospitalizations of 5.2 percent, twice the
reduction (2.6 percent) observed among practices making no improvement in their overall
score. Practices in the top quartile of score improvement increased their score by an average
of 4.6 points and had an average reduction in hospitalizations of 7.6 percent—three times
larger than that of practices making no improvements in their baseline M-PCMH-A score.
101
Similar to the analyses reported in Chapter 8, Year 3 is October 2014 through September 2015, and Year 4 is
October 2015 through September 2016. For the beneficiary-level analysis, we were able to include claims covering
the last quarter of CPC in the Year 4 analysis. We annualized Year 4 outcomes to adjust for this additional quarter of
data.
241

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
• Improvements in the overall score were associated with declines in hospitalizations
only for practices whose baseline hospitalization rate was in the highest one-third of all
CPC practices.
• Improvements in three of the seven care delivery domains (planned care, care
coordination, patient engagement) were significantly associated with reduced
hospitalizations. Improvements in the other care delivery domains that the M-PCMH-A
measured (access to care, continuity of care, risk-stratified care management, and data-
driven continuous quality improvement) were not associated with changes in
hospitalizations.
• Improvements in individual M-PCMH-A items were significantly associated with
reductions in hospitalization for 15 of the 37 items.
102
In Year 3, we found different results—the association between practices’ hospitalization rate
and the changes in the summary index of care delivery between baseline and Year 3 was no
longer sizable or statistically significant. Further, the association between changes in the
hospitalization rate (and other outcomes) and changes in individual M-PCMH-A items was
statistically significant for only a few items and outcomes, and some of these significant
associations were favorable while others were unfavorable. The change in results appeared to be
due to (1) relatively unstable hospitalization rates for individual practices between Year 2 and
Year 3, and (2) a narrowing of the differences among practices’ care delivery approaches. By
Year 3, most practices gave themselves relatively high rankings on the M-PCMH-A items. This
reduction in the range of M-PCMH-A item scores led to few meaningful relationships between
care delivery measures and key outcomes in our sample of CPC practices. In addition, the data
did not support our hypothesis that the link between better care delivery scores and fewer
hospitalizations was weaker for hospital-owned practices, which we hypothesized might be true,
given different incentives.
103
9.3. Approach to Year 4 analysis
This evolving relationship of care delivery approaches with hospitalizations, ED use, and
expenditures led us to focus this report on individual M-PCMH-A items that best reflect three
primary care delivery approaches that the primary care and medical home literatures identify as
important for reducing these outcomes. After-hours access to care, continuity of care, and
follow-up after acute care have been shown to be strong predictors of service utilization and
expenditures.
104
Specifically,
102
Given the high degree of collinearity among the individual items, we estimated the relationship between each
item and risk-adjusted hospitalization rate separately.
103
The current analysis yielded the same result; hospital ownership has no bearing on associations between
practices’ care delivery approaches and outcomes.
104
See Starfield 1998, 2005; Institute of Medicine 1996; World Health Organization 1978; Bindman et al. 1995; Shi
1994; Shi et al. 2005; Franks and Fiscella 1998; Kringos et al. 2012; Fisher et al. 2003a, 2003b; Phillips and
Bazemore 2010; Macinko et al. 2007; and O’Malley et al. 2015. We do not include a fourth approach cited in some
of these studies, comprehensiveness of care, because our M-PCMH-A instrument did not measure it.
242

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
• Access to after-hours care coordinated with the patient's primary care provider has been
associated with lower rates of hospitalizations and ED use, greater patient satisfaction, and
fewer unmet medical needs in some patient populations.
105
• Continuity with the same clinician and practice team over time has been associated with
lower hospitalization rates,
106
lower ED visits,
107
lower total expenditures,
108
and lower
episode-based expenditures for chronic conditions.
109
• An evaluation of 15 randomized trials of care coordination initiatives found that success in
reducing hospitalizations and expenditures was limited to high-risk patients and programs
that had a strong transitional care component, substantial in-person contact, and aggressive
medication management.
110
Similarly, having systematic arrangements to coordinate care
between the primary care practice and the hospital about ED visits and hospitalizations has
been associated with reduced ED use and lower readmission rates, particularly for older
patients with chronic conditions.
111
CPC practices might not have made each of these measures of care delivery a primary focus
during the initiative. Each year, practices had to implement changes to meet defined Milestones,
but within the Milestones practices had some leeway to choose their focus. In addition to
examining the associations between our outcomes and approaches to care delivery, we examined
whether the associations were enhanced by having nonphysician practice staff engage in clinical
tasks, enabling them to work to the “top of their licenses”—that is, by practicing to the full
extent of their education and training (as hypothesized by Bodenheimer and Laing 2007;
Bodenheimer 2007).
The following M-PCMH-A items best capture (1) the key care delivery approaches that are
expected to be associated with lower unnecessary service use, and (2) clinical involvement of
nonphysicians, the potential modifier of these associations (also see Table 9.1):
• Continuity—extent to which patients are usually seeing their own provider and practice
team
• After-hours access—extent to which patients have after-hours access to a practice staff
member with patient-specific data
105
See Jerant et al. 2012; Grol et al. 2006; O’Malley 2013; and Zickafoose et al. 2013.
106
See Mainous and Gill 1998; Cabana and Jee 2004; Weiss and Blustein 1996; Hussey et al. 2014; and Nyweide et
al. 2013.
107
See Rosenblatt et al. 2000; and Gill et al. 2000.
108
See Cabana and Jee 2004 and De Maeseneer et al. 2003.
109
See Weiss and Blustein 1996; Hussey et al. 2014; Raddish et al. 1999; and Ettner 1999.
110
See Peikes et al. 2009.
111
See Le Berre et al. 2017 and Coleman et al. 2006. We also note that the care delivery measures used in this
chapter are not the only ones that could affect service use and expenditures. We selected these three measures
because they are the ones most consistently found to be associated with improvements in these outcomes.
243

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
• Follow-up after acute care—extent to which the practice identifies and follows up with
patients seen in the ED or hospital
• Clinical involvement of nonphysicians—extent to which nonphysician staff perform
clinical service roles
We further refined our approach by using beneficiary-level, rather than practice-level, data
on outcomes to estimate the relationships between care delivery and outcomes. This change
allowed us to control more accurately for beneficiary risk by using individual HCC scores from
the previous year to predict a given year’s outcomes, and to investigate whether outcomes were
more responsive to better care delivery for high-risk beneficiaries.
The analysis in the remainder of this chapter tests the following hypotheses:
1. Improvements between baseline and a given year in practices’ reported continuity of care,
after-hours access, and follow-up after acute care are associated with a decrease in
hospitalizations and outpatient ED visits, and lower expenditures, for their beneficiaries over
the same time period.
2. Higher levels of practices’ reported continuity of care, after-hours access, and follow-up
after acute care in a year are associated with fewer hospitalizations and outpatient ED visits,
and lower expenditures, for their beneficiaries in that year.
- These relationships are stronger for practices with higher baseline values of the outcome
measures, for high-risk beneficiaries, and for practices with higher clinical involvement
of nonphysicians.
9.4. Data and methods
We conducted these analyses using data on hospitalizations, ED use, and expenditures at
both the practice and beneficiary levels. First, using practice-level data, for Years 2, 3, and 4, we
assessed the association of practices’ changes in outcomes since the beginning of CPC to
changes in continuity, after-hours access, and follow-up after acute care, drawn from the M-
PCMH-A in the practice survey. Second, using beneficiary-level claims data, we examined
cross-sectional associations of outcomes in a given year to levels of these care delivery
approaches in the same year, controlling for the practices’ average baseline outcome and
beneficiaries’ HCC scores in the prior year. Using beneficiary data, we also assessed whether
associations were stronger for high-risk beneficiaries, for practices that had more outpatient ED
visits, hospitalization, and expenditures in the baseline period, or when nonphysician staff were
used more extensively to provide clinical services, which may include care management and
other services. Below we describe the samples, data sources, measures, and regression models
used to examine the association between care delivery approaches and outcomes.
Sample. For the practice-level analysis, we used the three-quarters of CPC practices (N =
359) with the largest number of attributed Medicare FFS beneficiaries. Similar to previous years’
analyses, we excluded the smallest 25 percent of practices (those with fewer than 330 attributed
beneficiaries), because outcome estimates based on small numbers of beneficiaries are highly
variable.
244

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
For the beneficiary-level analysis, we used three samples of beneficiaries, for CPC Years 2,
3, and 4, respectively. The sample in each year consisted of beneficiaries attributed to the 359
practices at any time during the year, including those who died partway through the year.
Data sources. We analyzed data from several sources:
• We used Medicare fee-for-service (FFS) claims data to construct the three key outcome
measures for the beneficiary- and practice-level analyses: (1) hospitalizations, (2) outpatient
ED visits, and (3) Medicare FFS expenditures.
• We used several data sources to construct control variables for beneficiary characteristics
(CMS’s Enrollment Database file, HCC scores), practice characteristics (SK&A, National
Committee for Quality Assurance), and market characteristics (Hospital Referral Region
(HRR)-level spending and utilization data, Area Resource File, and Health Resources &
Services Administration).
• We used four rounds of the modified version of the PCMH-A module of the CPC practice
survey fielded in (1) October–December 2012, (2) April–July 2014, (3) April–August 2015,
and (4) April–August 2016, which correspond to baseline, Year 2, Year 3, and Year 4 of
CPC, respectively, for data on primary care delivery approaches reported by CPC practices.
Outcomes. We examined the following outcomes:
• Beneficiary-level outcomes include the beneficiaries’ average monthly Medicare FFS
expenditures, annualized hospitalizations, and annualized outpatient ED visits, for Years 2,
3, and 4.
112
• Practice-level outcomes include changes in average per beneficiary per month Medicare FFS
expenditures, average number of hospitalizations per 1,000 beneficiaries per year, and
average number of outpatient ED visits per 1,000 beneficiaries per year, between baseline
and Years 2, 3, and 4, respectively.
Key explanatory variables. The data on key primary care delivery approaches—continuity
of care, after-hours access to care, and follow-up after acute care, as well as the potential
modifier of their associations with outcomes (clinical involvement of nonphysicians)—come
from four items of the modified PCMH-A module of the practice survey. We measured each
item on a 1 to 12 scale spanning four response categories, with higher numbers corresponding to
more advanced levels of care (see Table 9.1).
112
We do not include Year 1 in the analysis, because outcome measurement should follow measurement of
explanatory variables, not precede it, and Year 1 ended six months before the Round 2 practice survey. In place of
Year 1, we use Year 2 as the first year for which we explain outcomes using explanatory variables from Round 2
practice survey.
245

246
Table 9.1. Key primary care delivery approaches from the M-PCMH-A
CONTINUITY
Patients are encouraged to
see their paneled provider
and practice team
□
Check here if patients are
not assigned to specific
provider panels.
…only at the patient’s request.
…by the practice team, but it is not a
priority in appointment scheduling.
…by the practice team and it is a
priority in appointment scheduling,
but patients commonly see other
providers because of limited
availability or other issues.
…by the practice team and it is a
priority in appointment scheduling,
and patients usually see their own
provider or practice team.
1 2 3 4 5 6 7 8 9 10 11 12
AFTER-HOURS ACCESS
Patient after-hours access
(24 hours, 7 days a week)
to a physician, PA/NP, or
nurse
...is not available or limited to an
answering machine.
1 2 3
…is available from a coverage
arrangement (e.g., answering
service) that does not offer a
standardized communication
protocol back to the practice for
urgent problems.
4 5 6
…is provided by a coverage
arrangement (e.g., answering
service) that shares necessary
patient data with and provides a
summary to the practice.
7 8 9
…is available via the patient’s choice
of email or phone directly with the
practice team or a provider who has
real-time access to the patient’s
electronic medical record.
10 11 12
FOLLOW-UP AFTER ACUTE
CARE
Follow-up by the primary
care practice with patients
seen in the Emergency
Room (ER) or hospital
...generally does not occur because
the information is not available to the
primary care team.
1 2 3
…occurs only if the ER or hospital
alerts the primary care practice.
4 5 6
…occurs because the primary care
practice makes proactive efforts to
identify patients.
7 8 9
…is done routinely because the
primary care practice has
arrangements in place with the ER
and hospital to both track these
patients and ensure that follow-up is
completed within a few days.
10 11 12
CLINICAL INVOLVEMENT OF
NON-PHYSICIANS
Non-physician practice
team members
…play a limited role in providing
clinical care.
1 2 3
…are primarily tasked with
managing patient flow and triage.
4 5 6
…provide some clinical services
such as assessment or self-
management support.
7 8 9
…perform key clinical service roles
that match their abilities and
credentials.
10 11 12

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
As we describe in Chapter 5, CPC practices reported improvement over time on these
measures, as shown by the increase in average scores across practices over time (Table 9.2).
However, the bulk of the improvement over time occurred between baseline and Year 2. This
pattern is also depicted in Figure 9.2, which indicates that the distribution of scores was fairly
uniform at baseline, increased strongly by Year 2, and continued to increase gradually during
Years 3 and 4 of CPC (shifting to the right) as more practices improved their scores and began
reporting the maximum value for these measures.
Table 9.2. Mean scores (out of a maximum of 12) by year of key primary care
delivery approaches
Baseline Year 2 Year 3 Year 4
Continuity of care 9.6 10.2 10.4 10.6
After-hours access to care 8.2 9.9 10.2 10.3
Follow-up after acute care 7.2 9.9 10.4 10.7
Clinical involvement of
nonphysicians
8.5 9.7 10.2 10.6
Figure 9.2. Distribution of scores by year for key primary care delivery
approaches
247

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
Other explanatory variables. We accounted for several beneficiary, practice, and region
(or market) characteristics to control for factors other than the practice’s approach to delivering
primary care that could affect outcomes. We measured all characteristics except for HCC score
at baseline; we measured HCC score in the year prior to the year of outcome measurement.
These characteristics are:
• Region (or market) characteristics—Medicare Advantage penetration rate; median
household income; percentage urban; whether in a medically underserved area; HRR-level
expenditures, hospitalizations, or outpatient ED visits (depending on outcome)
• Practice characteristics—number of physicians, whether the practice is hospital-owned,
and whether the practice is multi-specialty
• Practice’s average outcome at baseline—baseline values of average per beneficiary per
month Medicare FFS expenditures, average number of hospitalizations per 1,000
beneficiaries per year, and average number of outpatient ED visits per 1,000 beneficiaries
per year
• Beneficiary characteristics—age, gender, race/ethnicity, HCC score, whether dually
eligible for Medicare and Medicaid, original reason for Medicare (age, disability, other)
Regression models. In addition to estimating practice-level models as we have done in
previous years' analyses, we estimated beneficiary-level models to better account for individual
beneficiaries’ risk of needing hospitalizations or ED care and incurring high expenditures using
prior year HCC scores.
113
By measuring risk closer to when the outcome is measured, we hoped
to capture the relationship of interest for beneficiaries who were most at risk during this period.
For both models, we used ordinary least squares regression to examine hospitalizations,
outpatient ED visits, and expenditures as a function of continuity, after-hours access, and follow-
up after acute care, controlling for beneficiary, practice, and market characteristics. Because the
cross-sectional relationships examined in beneficiary-level models could be driven by
differences in outcomes and unmeasured differences in practice styles that existed before CPC
and generated these outcomes, we also controlled for the practice’s average outcome at baseline
in these models. For beneficiary-level outcomes, we estimated additional models to examine
whether associations between care delivery approaches and the outcome of interest in a year
were stronger among beneficiaries with higher HCC scores in the year immediately preceding
the outcome period, and among those in practices with higher baseline outcomes and better
clinical involvement of nonphysicians, respectively. Beneficiary-level observations are weighted
so that the results represent each practice equally, to ensure that large practices do not dominate
them.
In interpreting these results, it is important to remember that the estimated relationships are
only partial correlations and may not be due to causal effects. That is, although the estimated
relationships control for the influence of other factors, we cannot conclude that an observed
113
We also account for beneficiary risk in the practice analysis by controlling for average HCC scores, but
beneficiary-level analysis allows us to control for individual HCC scores and potentially avoid an aggregation bias.
248

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
association between a care delivery approach and an improved outcome implies that the better
outcome is attributable wholly or partly to the care delivery measure.
9.5. Practice-level model results
The practice-level models examine whether the change in average outcome
(hospitalizations, outpatient ED visits, and Medicare expenditure) from baseline to Years 2, 3,
and 4, respectively, for attributed Medicare beneficiaries in a practice was related to the change
in scores of the three care delivery approaches reported by practices (continuity of care, after-
hours access, and follow-up after acute care) over the same period, controlling for the practice's
average outcome at baseline. Figure 9.3 shows the predicted percentage change in the outcome
(compared with the baseline mean
114
) for a one-point increase in each care delivery approach
measure, holding the other two measures constant. In other words, the estimates reflect the
predicted difference in outcomes between a practice that reported (say) an 11 out of 12 compared
to a practice that reported a 10 out of 12, with negative values indicating lower service use or
expenditures.
For Years 2, 3, and 4, associations of changes in outcomes since baseline with
improvements in each of the three key care delivery approaches over the same time period were
small, around 0.5 percent of the practice's average outcome at baseline. Improvement in follow-
up after acute care between baseline and Year 4 was significantly associated with lower
hospitalization rates in Year 4. However, improvements in follow-up after acute care were only
marginally related to lower hospitalizations in Year 3 and not significantly related to
hospitalizations in Year 2, or to outpatient ED visits or expenditures in any year. Similarly, we
found little or no association between improvements in the other two care delivery approaches
and better outcomes in any year. In fact, improvements in the practice-reported continuity
measure appear to be associated with an increase in hospitalizations in Years 3 and 4 (p = 0.053
and 0.013, respectively). This isolated and anomalous result appears to be a chance association,
given that we did not find a similarly significant association using beneficiary-level data, as
shown in the next section.
114
The baseline means are 449 ED visits, $732 per beneficiary per month, and 307 hospitalizations.
249

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
Figure 9.3. Associations of improvements in continuity, after-hours access,
and follow-up after acute care with changes in practice-level service use and
expenditures in Years 2, 3, and 4
We used beneficiary-level data on outcomes so we could control for beneficiary risk level in
estimating cross-sectional relationships between the care delivery approaches and outcomes.
Additionally, we examined whether these relationships were stronger for beneficiaries in
practices with high hospitalizations, ED visits, and expenditures at baseline (in other words,
practices with greater scope for improvement) and greater clinical involvement of nonphysicians.
We present these findings below.
9.6. Beneficiary-level model results
In Year 4, better follow-up after acute care was associated with fewer hospitalizations. The
relationship was strongest among high-risk beneficiaries and in practices with higher baseline
hospitalizations. Greater continuity of care was associated with fewer ED visits, but only for high-risk
beneficiaries. After-hours access was related to fewer hospitalizations, but only when clinical
involvement of nonphysicians was at its maximum value.
Contrary to the above analyses, which used practice-level data, here we used beneficiary-
level data on outcomes and beneficiary risk, controlling for beneficiary, practice, and market
characteristics, to assess the cross-sectional association between beneficiary outcomes and
practices’ care delivery approaches. The analysis also differed from analyses in previous reports
by estimating the relationship between levels of care delivery measures and outcomes, rather
than relating changes in individual and summary measures of primary care delivery approaches
to changes in aggregate outcomes at the practice level. We used levels because changes in
outcomes at the beneficiary level are likely to be highly variable and harder to predict than
changes in outcomes at the practice level.
250

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
9.6.1. Overview of results for Years 2, 3, and 4
We found little evidence of associations between the three primary care delivery approaches
and favorable beneficiary outcomes (negative values in the figures) across Years 2, 3, and 4, and
year-to-year patterns were erratic (Figures 9.4 to 9.6). The exception is follow-up after acute
care, which was more strongly related to fewer hospitalizations with each year (point estimates
below the zero line indicate favorable associations between outcomes and care delivery
approaches), becoming statistically significantly different from zero in Years 3 and 4. Continuity
tended to be more strongly associated with fewer outpatient ED visits toward the end of CPC,
but the relationships were weak and not significantly different from zero. Associations of care
delivery approaches with expenditures indicate no clear pattern across the years and were not
significantly different from zero.
Figure 9.4. Associations between primary care delivery approaches and
outcomes for Years 2, 3, and 4
Overall results, by beneficiary risk-level. Follow-up after acute care was associated with
fewer hospitalizations in Year 4 for both average- and high-risk beneficiaries but to a greater
extent for high-risk beneficiaries (Figure 9.5). Similarly, for high-risk beneficiaries, continuity
was more strongly related to fewer outpatient ED visits in each year, but it was significantly
different from zero only in Year 4. Other associations between care delivery approaches and
outcomes were weak and not significantly different from zero.
251

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
Figure 9.5. Predicted associations between primary care delivery approaches
and outcomes, at average-risk and high-risk HCC values for Years 2, 3, and 4
Modifying effects of greater clinical involvement of nonclinician staff. Although after-
hours access was not significantly related to hospitalizations among all beneficiaries in Year 4,
beneficiaries in practices with maximum clinical involvement of nonphysicians had significantly
lower hospitalizations (Figure 9.6). These practices comprised more than one-third of the 359
CPC practices included in this analysis. In contrast, follow-up after acute care was significantly
related to fewer hospitalizations irrespective of the clinical involvement of nonphysicians. With
the exception of these results, small differences in clinical involvement of nonphysicians were
not associated with differences in outcomes as both practices with a maximum score (12) and
those with a slightly lower score (10) had associations between their care delivery scores and
outcomes that were not significant.
252

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
Figure 9.6. Predicted associations between key primary care delivery
approaches and outcomes for practices with 25th percentile (10) and maximum
(12) levels of clinical involvement of nonphysicians for Years 2, 3, and 4
The beneficiary-level cross-sectional analyses show that Year 4 had the most promising
evidence for the associations between primary care delivery approaches and favorable outcomes.
Hence the remainder of this section describes Year 4 findings in more detail.
253

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
9.6.2. Results for CPC Year 4
By Year 4, nearly three-quarters of practices in this analysis were reporting advanced levels
of care (in the top response category with scores of 10–12) for the care delivery approaches
examined in this chapter: continuity, after-hours access, and follow-up after acute care, as well as
clinical involvement of nonphysicians, the potential modifier of these approaches (Table 9.3).
Although the range in scores was smaller than that observed at baseline (when the distribution
was more uniform), Table 9.1 shows substantial variation within the top response category (10–
12). The wording and layout of the 1–12 scores in Table 9.1 suggest how this variation can
occur. For example, in the case of the top response category for continuity—which indicates that
the practice team encourages patients to see their paneled provider and practice team, it is a
priority in appointment scheduling, and patients usually see their own provider or practice
team—some practices may select 10 because they make continuity a priority some of the time,
and for someone responding 10 versus 12, the extent to which he or she “usually” sees his or her
own provider may vary.
Table 9.3. Variation of key primary care delivery approaches across CPC
practices in Year 4
Primary care delivery approach Mean
25th
percentile Median
75th
percentile
Continuity 10.6 10 11 12
After-hours access 10.3 9 10 12
Follow-up after acute care 10.7 10 11 12
Clinical involvement of nonphysicians 10.6 10 11 12
Despite limited variation in these measures across practices, in Year 4, beneficiaries in
practices that scored higher on follow-up after acute care had significantly fewer hospitalizations
(p = 0.002) than those in lower scoring practices, controlling for their practice’s average baseline
hospitalizations (Table 9.4). A one-point increase in score was associated with 6 fewer
hospitalizations per 1,000 beneficiaries per year (about 2 percent of the baseline mean of 307
hospitalizations per 1,000 beneficiaries per year). This association is larger than the largest
association found in earlier years, where a one-point improvement in the overall M-PCMH-A
score was associated with a 1.15 percent decline in a practice’s own hospitalization rate between
baseline and Year 2. Relationships of all outcomes with the other two care delivery measures in
Table 9.4 were substantially smaller or essentially zero and not statistically significant.
In previous reports, we found that associations between practice transformation and
improvement in outcomes during CPC were concentrated among practices with the largest scope
for improvement—that is, practices with high utilization before CPC began (at baseline). To test
whether this association existed in the Year 4 cross-sectional data, we included in the model an
interaction of the care delivery measure and practice’s baseline outcome. Table 9.4 (lower
panels) contrasts the change in outcome for a one-point increase in the care delivery approach for
a practice with median baseline outcome versus a practice with a baseline outcome at the 75th
percentile.
254

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
Table 9.4. Associations of key primary care delivery approaches with annual
per beneficiary outcomes in Year 4
Dependent variable
Hospitalizations per
1,000 beneficiaries
(mean = 307)
Outpatient ED visits
per 1,000 beneficiaries
(mean = 449)
Average monthly
Medicare expenditures
per beneficiary
(mean = $732)
Change in outcome with a
one-point increase in:
Continuity
1.66
(0.33)
-3.74
(0.38)
-1.66
(0.67)
After-hours access
0.569
(0.75)
-0.429
(0.90)
0.531
(0.87)
Follow-up after acute care
-5.58
(0.002)
a
5.40
(0.11)
-1.85
(0.60)
Change in outcome with a
one-point increase in
continuity for a:
…practice with baseline
outcome at median
0.708
(0.70)
-2.41
(0.49)
-2.33
(0.55)
…practice with baseline
outcome at 75th percentile
2.86
(0.12)
-4.98
(0.16)
1.31
(0.80)
Change in outcome with a
one-point increase in after-
hours access for a:
…practice with baseline
outcome at median
-0.57
(0.65)
-0.42
(0.91)
-0.0310
(0.99)
…practice with baseline
outcome at 75th percentile
3.07
(0.07)
b
-1.21
(0.75)
0.977
(0.77)
Change in outcome with a
one-point increase in follow-
up after acute care for a:
…practice with baseline
outcome at median
-5.45
(0.005)
a
5.98
(0.09)
b
-1.65
(0.63)
…practice with baseline
outcome at 75th percentile
-9.42
(<0.0001)
a
3.20
(0.37)
-5.69
(0.22)
Note: Beneficiary level analysis for N = 354,405 beneficiaries in 359 practices. P-values are shown in
parentheses. Shading indicates statistical significance at the 0.10 level. Red shading with italicized white
text signifies that our estimate was statistically significant and showed an increase in the service use or
expenditures outcome; green shading with bolded text signifies that an estimate was statistically
significant and implied a reduction in the service use or expenditures outcome. Regressions control for
beneficiary, practice, market characteristics, and practice’s baseline outcome. We examine individual
beneficiary-level data but report estimated associations for hospitalizations and outpatient ED visits per
1,000 beneficiaries for ease of interpretation.
a
Estimate was statistically significant at the 0.10 level and implied a reduction in the service use or expenditures outcome.
b
Estimate was statistically significant at the 0.10 level and showed an increase in the service use or expenditures outcome.
255

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
The favorable overall relationship noted above between better follow-up after acute care and
fewer hospitalizations was even stronger for beneficiaries in practices with high baseline
hospitalization rates. Although the association was statistically significant for beneficiaries in a
practice with median hospitalizations at baseline, it was nearly twice as large for practices with
baseline hospitalizations at the 75th percentile. For these high-baseline practices, a one-point
increase in follow-up was associated with a 3 percent reduction (relative to the overall baseline
mean of 307) in hospitalizations in Year 4.
We also found two anomalous, statistically significant associations in Table 9.4 that show an
outcome worsening with higher scores on a care delivery measure. It is possible that better after-
hours access and closer follow-up after acute care did increase short-term acute care use if these
processes identified potentially important problems. However, we feel these findings are more
likely due to chance than to a perverse true association between better care delivery and worse
outcomes. Further, given that our focus is solely on identifying care delivery features that are
associated with improvements in outcomes, we do not attempt to explain these unanticipated
results.
115
High-risk subgroup. To estimate how the associations between care delivery measures and
outcomes change with individual beneficiaries’ prospective risk of incurring high Medicare
expenditures, we included in our basic regression model an interaction of each of our three care
delivery measures with the beneficiary’s HCC score from the previous year (Year 3). This
analysis refined the previous analyses from Years 2 and 3 by using beneficiary-level data and
using their HCC scores from Year 3 rather than from baseline, to capture associations for
beneficiaries with high post-baseline risk of needing costly services.
We found that greater continuity was related to fewer outpatient ED visits for high-risk
beneficiaries (as illustrated in Table 9.5 for those with HCC scores one point above the average,
or about twice the mean HCC score), but not for those with average risk (those with mean HCC
scores). For beneficiaries with HCC scores that were one point above the average (which places
them at the 90th percentile of the HCC score distribution), a one-point increase in continuity was
significantly associated with a more than 4 percent decline in outpatient ED visits (compared
with the baseline mean for all beneficiaries). A similarly larger association existed between
continuity and lower expenditures among high-risk beneficiaries compared with average-risk
beneficiaries, but it was not significantly different from zero.
115
Our rationale for concluding that these perverse associations are due to chance rather than to a true linkage is that
we found hospitalizations and ED use worsening with higher scores on access to care and follow-up after acute care
only for subgroups of practices. These associations were much smaller in magnitude (as a percentage of the outcome
mean) than the statistically significant favorable associations in Table 9.4, and had larger standard errors and p-
values. Furthermore, the associations of these outcomes with care delivery in the full sample were small and not
statistically significant, and the subgroup relationships were not observed in practice-level regressions (not shown).
Thus, we consider these results spurious, and of no interest here, given our focus on identifying care delivery
features associated with reduced utilization and expenditures.
256

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
Table 9.5. Relationships of key primary care delivery approaches with annual
per beneficiary outcomes for high-risk beneficiaries in Year 4
Coefficients
Dependent variable
Hospitalizations per
1,000 beneficiaries
(mean = 307)
Outpatient ED visits
per 1,000 beneficiaries
(mean = 449)
Average monthly
Medicare expenditures
per beneficiary
(mean = $732)
Change in outcome with a
one-point increase in
continuity for a:
…beneficiary with average
HCC score (1.00)
1.65
(0.34)
-3.82
(0.37)
-1.72
(0.66)
…beneficiary with HCC score
of one point above average
(2.00)
-0.859
(0.85)
-19.8
(0.06)
a
-12.91
(0.15)
Change in outcome with a
one-point increase in after-
hours access for a:
…beneficiary with average
HCC score (1.00)
0.590
(0.74)
-0.365
(0.92)
0.51
(0.87)
…beneficiary with HCC score
of one point above average
(2.00)
-0.969
(0.83)
-4.53
(0.57)
2.08
(0.78)
Change in outcome with a
one-point increase in follow-
up after acute care for a:
…beneficiary with average
HCC score (1.00)
-5.70
(0.002)
a
5.52
(0.11)
-1.86
(0.60)
…beneficiary with HCC score
of one point above average
(2.00)
-10.50
(0.02)
a
10.40
(0.20)
-2.63
(0.77)
Note: Beneficiary level analysis for N = 354,405 beneficiaries in 359 practices. P-values are shown in
parentheses. Shading indicates statistical significance at the 0.10 level. Green shading with bold text
signifies that an estimate was statistically significant and implied a reduction in the service use or
expenditures outcome. Regressions control for beneficiary, practice, market characteristics, and practice’s
baseline outcome. We examine individual beneficiary-level data but report estimated associations for
hospitalizations and outpatient ED visits per 1,000 beneficiaries for ease of interpretation.
a
Estimate was statistically significant at the 0.10 level and implied a reduction in the service use or expenditures outcome.
257

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
Similarly, as we note above, we found a stronger relationship between follow-up after acute
care and hospitalizations for beneficiaries with higher HCC scores, but for this care delivery
measure, it was a matter of magnitude. Follow-up after acute care had a statistically significant
relationship with fewer hospitalizations even for beneficiaries with average HCC scores, but the
relationship doubled in magnitude for high-risk beneficiaries (HCC scores of one point above the
average). Consistent with our overall findings for this care delivery measure, a one-point
increase in the follow-up measure was associated with about 2 percent fewer hospitalizations for
beneficiaries with average HCC scores and more than 3 percent fewer hospitalizations for
beneficiaries with high HCC scores (compared with the baseline means for all beneficiaries).
There was no association between follow-up after acute care and outpatient ED visits or
expenditures for high-risk beneficiaries.
The associations of better after-hours access to care with outcomes were small for both
average- and high-risk beneficiaries and not significantly different from zero.
Role of clinical involvement of nonphysicians in aiding primary care delivery and
improving outcomes. We hypothesized that practices that made greater use of nonphysician
staff to provide clinical care would be more likely than other practices to see associations
between care improvements and improved beneficiary outcomes. To test this hypothesis, we
included an interaction of clinical involvement of nonphysicians with each of the three care
delivery approaches when examining the associations with Year 4 outcomes.
We found mixed evidence that higher levels of clinical involvement of nonphysicians
strengthened the relationship between our three key care delivery measures and outcomes (Table
9.6). After-hours access was significantly related to lower hospitalizations when clinical
involvement of nonphysicians was at the 75th percentile (also the maximum) with a one point
increase in after-hours access being related to 1 percent fewer hospitalizations. For continuity,
the relationship with outcomes was more favorable for practices at the 75th percentile of clinical
involvement of nonphysicians than for practices at the 25th percentile. Nonetheless, even for the
practices with high clinical involvement of their nonphysician staff, the association of outcomes
with continuity was small (a decline of 0.5 to 1 percent in any of the three outcomes for a one-
point improvement in continuity) and not significantly different from zero. In contrast to these
results, we found that better follow-up after acute care was significantly associated with fewer
hospitalizations irrespective of small differences in levels of clinical involvement of
nonphysicians. We also found that better follow-up was associated with more outpatient ED
visits when clinical involvement of nonphysicians was not at its maximum value (12).
258

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
Table 9.6. Variation in the associations between key primary care delivery
approaches and annual per beneficiary outcomes with clinical involvement of
nonphysicians, Year 4
Coefficients
Dependent variable
Hospitalizations per
1,000 beneficiaries
(mean = 307)
Outpatient ED visits
per 1,000
beneficiaries
(mean = 449)
Average monthly
Medicare
expenditures per
beneficiary (mean =
$732)
Change in outcome with a one-point
increase in continuity in a practice
at:
25th percentile of clinical
involvement of nonphysicians (10)
2.30
(0.23)
-2.14
(0.57)
-0.366
(0.93)
75th percentile of clinical
involvement of nonphysicians (12)
-1.61
(0.39)
-5.33
(0.42)
-3.36
(0.43)
Change in outcome with a one-point
increase in after-hours access in a
practice at:
25th percentile of clinical
involvement of nonphysicians (10)
0.289
(0.87)
1.06
(0.76)
1.58
(0.63)
75th percentile of clinical
involvement of nonphysicians (12)
-3.82
(0.09)
a
0.34
(0.94)
-0.66
(0.89)
Change in outcome with a one-point
increase in follow-up after acute care
in a practice at:
25th percentile of clinical
involvement of nonphysicians (10)
-5.68
(0.003)
a
7.22
(0.04)
b
-1.54
(0.67)
75th percentile of clinical
involvement of nonphysicians (12)
-5.45
(0.02)
a
0.113
(0.98)
-1.63
(0.71)
Note: Beneficiary-level analysis for N = 354,405 beneficiaries in 359 practices. P-values are shown in
parentheses. Shading indicates statistical significance at the 0.10 level. Red shading with italicized white
text signifies that our estimate was statistically significant and showed an increase in the service use or
expenditures outcome; green shading with bold text signifies that an estimate was statistically significant
and implied a reduction in the service use or expenditures outcome. Regressions control for beneficiary,
practice, market characteristics, and practice’s baseline outcome. We examine individual beneficiary-level
data but report estimated associations for hospitalizations and outpatient ED visits per 1,000 beneficiaries
for ease of interpretation.
a
Estimate was statistically significant at the 0.10 level and implied a reduction in the service use or expenditures outcome.
b
Estimate was statistically significant at the 0.10 level and showed an increase in the service use or expenditures outcome.
259

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
9.7. Limitations
The results obtained here, like the synthesis findings presented in the last two annual reports
on CPC, do not purport to represent causal relationships. That is, all analyses are correlational
and observational, which is all that any attempt to link care delivery approaches to outcomes can
be, absent a design that randomly assigns practices to particular care delivery approaches or to
different incentives to make such improvements. Thus, although some associations are consistent
with our expectations and the literature, we cannot infer that better care delivery approaches lead
to decreased use of hospitals or EDs. The potential for such spurious correlations may be
exacerbated by the fact that Medicare hospitalization rates nationally were declining over the
2012–2016 period that we examined—that is, other factors (that we did not observe in our data)
may have affected hospitalizations as well as the degree of change in care delivery.
A second limitation is that although the models used are extensive, they are still relatively
straightforward and likely far less complex than any true relationships. For example, theory tells
us little about the measurement of care delivery approaches and changes—which ones are likely
to influence outcomes and how, or how relationships are likely to vary with beneficiary, practice,
and local health care environment characteristics. Unanswered questions, which we think merit
further research in the future, include:
• How long does a practice have to operate at a high level on a care delivery measure before
we can expect outcomes to improve?
• How intense does a practice’s change need to be to influence outcomes?
• How should care delivery approaches be measured to best capture links to outcomes, and do
the measures need to change over time?
• How can we overcome collinearity of M-PCMH-A measures without masking real
relationships?
• What functional form best depicts the relationship between care delivery measures and
outcomes?
• Are different cost and utilization outcomes affected differently by a given care delivery
measure?
• Are outcomes related to both levels and changes in M-PCMH-A variables?
• Are there top-out effects, i.e., a limited ability to measure improvements in care delivery at
the top end of the scale?
• How do the associations of care delivery approaches with outcomes vary with other care
delivery approaches and with other local factors?
• Who is affected? (Only high-risk beneficiaries? When and how should we measure high risk?)
• How does the relationship between care delivery and outcomes differ for different practices?
• Do the adverse incentives of hospital-owned practices to minimize hospitalizations influence
the relationship between practice transformations and outcomes, and if so, how?
260

9. SYNTHESIS MATHEMATICA POLICY RESEARCH
A third factor that limits our ability to detect relationships between better care delivery
approaches and improvements in key outcomes is the lack of strong incentives under CPC for
individual practices to transform in ways that would result in substantially fewer hospitalizations
and expenditures. Thus, among practices with equally high self-ratings on a care delivery
measure, some may devote relatively little attention to focusing their efforts in ways that would
reduce hospital and ED utilization and expenditures, reducing the likelihood that we will detect
any such associations in the data.
Finally, in interpreting these results, it is important to remember that they are comparisons
only among the CPC practices, which are a high-performing group. Thus, we have a seemingly
anomalous situation of CPC leading to reductions in ED visits (as shown in Chapter 8) but no
association found in this chapter between care delivery features and ED visits. However, our
findings do not mean that care delivery approaches do not affect ED visits. Our results simply
show that CPC practices that were slightly better than other CPC practices on follow-up after
acute care did not have lower ED visit rates than those other CPC practices. Since over two-
thirds of CPC practices had high ratings on our care delivery measures, the results suggest that
the beneficiaries of practices with a score of 11 had ED visit rates similar to those of
beneficiaries in practices with a score of 12. CPC practices were substantially better than
comparison practices on this measure of follow-up care, which may well account for the lower
ED visit rates found for beneficiaries in CPC than for beneficiaries in comparison practices.
9.8. Future work
Results from our synthesis analysis over the past three years suggest additional avenues for
investigation to uncover important associations between care delivery approaches and better
outcomes. The work continues to be important; to slow the growth in Medicare expenditures and
improve care, future and ongoing initiatives will need to know which changes to primary care
delivery approaches they should encourage or require. The evaluation of CPC+ offers valuable
opportunities to continue this research.
261
This page has been left blank for double-sided copying.

MATHEMATICA POLICY RESEARCH
REFERENCES
Agency for Healthcare Research and Quality. “Instructions for Analyzing Data from CAHPS
Surveys: Using the CAHPS Analysis Program Version 4.1.” Document no. 2015. Available
at https://www.ahrq.gov/cahps/surveys-guidance/cg/12-month/index.html. Updated April 2,
2012.
Agency for Healthcare Research and Quality. “CAHPS Clinician & Group Surveys.” Available
at https://cahps.ahrq.gov/surveys-guidance/cg/index.html. Accessed September 21, 2015.
American Association for Public Opinion Research. “Standard Definitions: Final Dispositions of
Case Codes and Outcome Rates for Surveys. 9th edition.” AAPOR, 2016.
Anglin, G., H. Tu, K. Liao, L. Sessums, and E.F. Taylor. “Strengthening Multipayer
Collaboration: Lessons from the Comprehensive Primary Care Initiative.” Milbank
Quarterly, forthcoming.
Bindman, A.B., K. Grumbach, D. Osmond, M. Komaromy, K. Vranizan, N. Lurie, J. Billings,
and A. Stewart. “Preventable Hospitalizations and Access to Health Care.” JAMA, vol. 274,
1995, pp. 305–311.
Bodenheimer, T. Building Teams in Primary Care: Lessons Learned. 2007 California HealthCare
Foundation, July 2007.
Bodenheimer, T., and B.Y. Laing. “The Teamlet Model of Primary Care.” Annals of Family
Medicine, vol. 5, 2007, pp. 457–461.
Cabana, M.D., and S.H. Jee. “Does Continuity of Care Improve Patient Outcomes?” Journal of
Family Practice, vol. 53, 2004, pp. 974–980.
Caloyeras, J. P., M. Kanter, N. Ives, C. Y. Kim, H. K. Kanzaria, S. H. Berry, and R. H. Brook.
“Physician Professional Satisfaction and Area of Clinical Practice: Evidence from an
Integrated Health Care Delivery System.” The Permanente Journal, vol. 20, no. 2, 2016, pp.
35–41.
Centers for Medicare & Medicaid Services (CMS). A summary of changes in Milestone
requirements by year is available at https://innovation.cms.gov/Files/x/CPCI-Milestones.pdf.
Centers for Medicare & Medicaid Services (CMS). “Eligible Professional’s Guide to Stage 2 of
the EHR Incentive Programs.” September 2013a. Available at:
https://www.cms.gov/regulations-and-
guidance/legislation/ehrincentiveprograms/downloads/stage2_guide_eps_9_23_13.pdf.
Centers for Medicare & Medicaid Services (CMS). Memorandum of Understanding Between the
Center for Medicare & Medicaid Innovation And [Payer] in [Market] for the Comprehensive
Primary Care Initiative. 2013b. https://innovation.cms.gov/Files/x/CPCI-MOU.pdf.
263

REFERENCES MATHEMATICA POLICY RESEARCH
Centers for Medicare & Medicaid Services (CMS). "CPC Program Year 2016 Implementation
and Milestone Reporting Summary Guide.” December 2015a. Available at:
https://downloads.cms.gov/files/cmmi/cpci-implementationguide2016.pdf.
Centers for Medicare & Medicaid Services (CMS). “Medicare Program: Revisions to Payment
Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2016.”
July 15, 2015b. Available at https://www.federalregister.gov/articles/2015/07/15/2015-
16875/medicare-program-revisions-to-payment-policies-under-the-physician-fee-schedule-
and-other-revisions.
Centers for Medicare & Medicaid Services (CMS). “Comprehensive Primary Care Plus.” 2016.
Available at https://innovation.cms.gov/initiatives/comprehensive-primary-care-plus.
Accessed December 20, 2016.
Centers for Medicare & Medicaid Services (CMS). “2017 CPC+ Implementation Guide: Guiding
Principles and Reporting." May 10, 2017a.
Centers for Medicare & Medicaid Services (CMS). “Comprehensive Primary Care Initiative
Shared Savings Methodology Version 4.0.” 2017b. Available at
https://innovation.cms.gov/Files/x/Comprehensive-Primary-Care-Initiative-Shared-Savings-
Methodology-PDF.pdf.
Christopher, A.S., C.S. Smith, R. Tivis, and A.P. Wilper. “Trends in United States Physician
Work Hours and Career Satisfaction.” The American Journal of Medicine, vol. 127, no. 7,
July 2014, pp. 674–80. doi: 10.1016/j.amjmed.2014.03.033.
Chromy, J.R. "Sequential Sample Selection Methods." Proceedings of the American Statistical
Association, Survey Research Methods Section. Alexandria, VA: American Statistical
Association, 1979, pp. 401–406. Available at
https://pdfs.semanticscholar.org/a041/1b8df53d0bb424478c5d92709b6b3a6ec605.pdf.
Accessed November 15, 2013.
Coleman, E.A., C. Parry, S. Chalmers, and S.J. Min. “The Care Transitions Intervention: Results
of a Randomized Controlled Trial.” Archives of Internal Medicine, vol. 166, no. 17,
September 25, 2006, pp. 1822–1828.
Dale, S.B., A. Ghosh, D.N. Peikes, T.J. Day, F.B. Yoon, E.F. Taylor, K. Swankoski, A.S.
O’Malley, P.H. Conway, R. Rajkumar, M.J. Press, L. Sessums, and R. Brown. “Two-Year
Costs and Quality in the Comprehensive Primary Care Initiative.” New England Journal of
Medicine, vol. 374, no. 24, June 2016, pp. 2345–2356.
Damschroder, L.J., D.C. Aron, R.E. Keith, S.R. Kirsh, J.A. Alexander, and L.C. Lowery.
“Fostering Implementation of Health Services Research Findings into Practice: A
Consolidated Framework for Advancing Implementation Science.” Implementation Science,
vol. 4, August 2009, p. 50. doi:10.1186/1748-5908-4-50.
264

REFERENCES MATHEMATICA POLICY RESEARCH
Daniel, D.M., E.H. Wagner, K. Coleman, J.K. Schaefer, B.T. Austin, M.K. Abrams, K.E.
Phillips, and J.R. Sugarman. (2013), Assessing Progress toward Becoming a Patient-
Centered Medical Home: An Assessment Tool for Practice Transformation. Health Services
Research, 48: 1879–1897.
Daughtridge, G.W., T. Archibald, and P.H. Conway. “Quality Improvement of Care Transitions
and the Trend of Composite Hospital Care.” Journal of the American Medical Association,
vol. 311, no. 10, 2014, pp. 1013–1014.
David, G., C. Gunnarsson, P.A. Saynisch, R. Chawla, and S. Nigam. Do patient-centered
medical homes reduce emergency department visits? Health Serv Res. 2014;50(2):418-39.
First published August 12, 2014. doi: 10.1111/1475-6773.12218.
DeLia, D. “Calculating Shared Savings: Administrative Formulas Versus Research-Based
Evaluations” Health Affairs Blog, September 26, 2016. Available at
http://healthaffairs.org/blog/2016/09/26/calculating-shared-savings-administrative-formulas-
versus-research-based-evaluations/.
De Maeseneer, J.M., L. De Prins, C. Gosset, and J. Heyerick. “Provider Continuity in Family
Medicine: Does It Make a Difference for Total Health Care Costs?” Annals of Family
Medicine, vol. 1, 2003, pp. 144–148.
Dolan, E.D., D. Mohr, M. Lempa, S. Joos, S.D. Fihn, K.M. Nelson, and C.D. Helfrich. “Using a
Single Item to Measure Burnout in Primary Care Staff: A Psychometric Evaluation.”
Journal of General Internal Medicine, vol. 30, no. 5, May 2015, pp. 582–587. doi:
10.1007/s11606-014-3112-6.
Edwards, S.T., M. Marino, B.A. Balasubramanian, S. Valenzuela, R. Springer, A. Preston, L.
Solberg, K.C. Stange, T. Kottke, B. Muller, T.T. Woodson, S.O. Hemler, C. Perry, W.
Miller, and D.J. Cohen. “Burnout Among Providers and Staff in Small to Medium Size
Primary Care Practices: Early Findings from EvidenceNOW.” Presentation slides at Society
of General Internal Medicine National Meeting, April 20, 2017.
Ettner, S.L. “The Relationship Between Continuity of Care and the Health Behaviors of Patients:
Does Having a Usual Physician Make a Difference?” Medical Care, vol. 37, 1999, pp. 547–
555.
Fisher, E.S., D.E. Wennberg, T.A. Stukel, D.J. Gottlieb, F.L. Lucas, and E.L. Pinder. “The
Implications of Regional Variations in Medicare Spending. Part 2: Health Outcomes and
Satisfaction with Care.” Annals of Internal Medicine, vol. 138, 2003a, pp. 288–298.
Fisher, E.S., D.E. Wennberg, T.A. Stukel, D.J. Gottlieb, F.L. Lucas, and E.L. Pinder. “The
Implications of Regional Variations in Medicare Spending. Part 1: The Content, Quality,
and Accessibility of Care.” Annals of Internal Medicine, vol. 138, 2003b, pp. 273–287.
Franks, P., and K. Fiscella. “Primary Care Physicians and Specialists as Personal Physicians.
Health Care Expenditures and Mortality Experience.” The Journal of Family Practice, vol.
47, 1998, pp. 105–109.
265

REFERENCES MATHEMATICA POLICY RESEARCH
Friedberg, M.W., E.C. Schneider, M.B. Rosenthal, K.G. Volpp, and R.M. Werner. “Association
Between Participation in a Multipayer Medical Home Intervention and Changes in Quality,
Utilization, and Costs of Care.” JAMA, vol. 311, no. 8, 2014, pp. 815–825.
doi:10.1001/jama.2014.353.
Friedberg, M.W., P.G. Chen, K.R. Van Busum, F. Aunon, C. Pham, J. Caloyeras, S. Mattke, E.
Pitchforth, D.D. Quigley, R.H. Brook, F.J. Crosson, and M. Tutty. “Factors Affecting
Physicians Professional Satisfaction and their Implications for Patient Care, Health Systems,
and Health Policy.” Rand Health Quarter, vol. 3, no. 4, Winter 2014.
Friedberg, M.W., M.B. Rosenthal, R.W. Werner, K.G. Volpp, and E.C. Schneider. “Effects of a
Medical Home and Shared Savings Intervention on Quality and Utilization of Care.” JAMA
Internal Medicine, vol. 175, no. 8, 2015, pp. 1362–1368.
doi:10.1001/jamainternmed.2015.2047.
Gelman, A. and J. Carlin. “Beyond Power Calculations: Assessing Type S (Sign) and Type M
(Magnitude) Errors.” Perspectives on Psychological Science, vol. 9, no. 6, 2014, pp. 641–
651.
Gelman, A., Y. Su, M. Yajima, J. Hill, M.G. Pittau, J. Kerman, T. Zheng, and V. Dorie. "Data
Analysis Using Regression and Multilevel/Hierarchical Models." R package version 1.9-3,
2016.
Gerteis, M., D. Peikes, A. Ghosh, L. Timmins, A.S. O’Malley, M. Barna, E.F. Taylor, T.J. Day,
K. Swankoski, P. Payne, and R. Brown. “Uses and Limitations of Claims-Based
Performance Feedback Reports: Lessons from the Comprehensive Primary Care Initiative.”
Journal for Healthcare Quality (forthcoming).
Gilfillan, R.J., J. Tomcavage, M.B. Rosenthal, D.E. Davis, J. Graham, J.A. Roy, S.B. Pierdon,
F.J. Bloom Jr., T.R. Graf, R. Goldman, K.M. Hamory, R.A. Paulus, and G.D. Steele Jr..
“Value and the Medical Home: Effects of Transformed Primary Care.” American Journal of
Managed Care, vol. 16, no. 8, 2010, pp. 607–614.
Gill, J.M., A.G. Mainous 3rd, and M. Nsereko. “The Effect of Continuity of Care on Emergency
Department Use.” Archives of Family Medicine, vol. 9, 2000, pp. 333–338.
Gordon, M. “Gmisc: Descriptive Statistics, Transition Plots, and More.” R package
version 1.4.1, 2016.
Grol, R., P. Giesen, and C. van Uden. “After-Hours Care in the United Kingdom, Denmark, and
the Netherlands: New Models.” Health Affairs (Millwood), vol. 25, 2006, pp. 1733–1737.
Guo, J., J. Gabry, B. Goodrich, and D. Lee. "R Interface to Stan." R package version 2.16.2,
2017.
Healthcare Analysis & Information Group. 2012 Patient Aligned Care Team (PACT) Personnel
Survey.
266

REFERENCES MATHEMATICA POLICY RESEARCH
Helfrich, C., E. Dolan, J. Simonetti, R.J. Reid, S. Joos, B.J. Wakefield, G. Schectman, R. Stark,
S.D. Fihn, H.B. Harvey, and K. Nelson. “Elements of Team-Based Care in a Patient-
Centered Medical Home Are Associated with Lower Burnout Among VA Primary Care
Employees.” Journal of General Internal Medicine, vol. 29, suppl. 2, 2014, pp. S659–S666.
Heyworth, L., A. Bitton, S.R. Lipsitz, T. Schilling, G.D. Schiff, D.W. Bates, and S.R. Simon.
“Patient-Centered Medical Home Transformation with Payment Reform: Patient Experience
Outcomes.” American Journal of Managed Care, vol. 20, no. 1, 2014, pp. 26–33.
Hussey, P.S., E.C. Schneider, R.S. Rudin, S. Fox, J. Lai, and C.E. Pollack. “Continuity and the
Costs of Care for Chronic Disease.” JAMA Internal Medicine, 2014, 245, Epub.
Institute of Medicine (US) Committee on the Future of Primary Care. Primary Care: America’s
Health in a New Era. Edited by Molla S. Donaldson, et al. National Academies Press, 1996.
Jaén, C.R., R.L. Ferrer, W.L. Miller, R.F. Palmer, R. Wood, M. Davila, E.E. Stewart, B.F.
Crabtree, P.A. Nutting, and K.C. Stange. “Patient Outcomes at 26 Months in the Patient-
Centered Medical Home National Demonstration Project.” Annals of Family Medicine,
vol. 8, suppl. 1, 2010, pp. S57–S67. doi:10.1370/afm.1121.
Jerant, A., K.D. Bertakis, J.J. Fenton, and P. Franks. “Extended Office Hours and Health Care
Expenditures: A National Study.” Annals of Family Medicine, vol. 10, 2012, pp. 388–395.
Kahn, K.L., J.W. Timbie, M.W. Friedberg, P.Mendel, L. Hiatt, E.K. Chen, A.M. Kress, C.
Buttorff, T. Lavelle, B.A. Weidmer, H.D. Green, M. Kommareddi, R. Malsberger, A.
Kofner, A. Rastegar, and C.M. Setodji. “Evaluation of CMS’s Federally Qualified Health
Care (FQHC) Advanced Primary Care Practice (APCP) Demonstration.” Final report. Santa
Monica, CA: Rand, 2016.
Keith, R.E., J.C. Crosson, A.S. O’Malley, D. Cromp, E.F. Taylor. Using the Consolidated
Framework for Implementation Research (CFIR) to produce actionable findings: a rapid-
cycle evaluation approach to improving implementation. Implement Sci. 2017
Feb 10;12(1):15.
Kern, L.M., R.V. Dhopeshwarkar, A. Edwards, and R. Kaushal. “Patient Experience Over Time
in Patient-Centered Medical Homes.” American Journal of Managed Care, vol. 19, no. 5,
2013, pp. 403–410.
Kringos, D.S., W.G.W. Boerma, J. van der Zee, and P.P. Groenewegen. “The Contribution of
Primary Care to Health Care System Performance in Europe.” In The Strength of Primary
Care in Europe, edited by D.S. Kringos. Utrecht, Netherlands: Nivel, 2012.
Lance, C.E. “The Sources of Four Commonly Reported Cutoff Criteria: What Did They Really
Say?” Organizational Research Methods, vol. 9, no. 2, 2006, pp. 202–220. Available at
http://doi.org/10.1177/1094428105284919.
267

REFERENCES MATHEMATICA POLICY RESEARCH
Landon, B.E., J. Reschovsky, and D. Blumenthal. “Changes in career satisfaction among primary
care and specialist physicians, 1997–2001.” Journal of the American Medical Association,
vol. 289, no. 4, January 2003.
Le Berre, M., G. Maimon, N. Sourial, M. Guériton, and I. Vedel. “Impact of Transitional Care
Services for Chronically Ill Older Patients: A Systematic Evidence Review.” Journal of the
American Geriatrics Society, vol. 65, no. 7, July 2017, pp. 1597–1608.
Lewis, S.E., R.S. Nocon, H. Tang, S.Y. Park, A.M. Vable, L.P. Casalino, E.S. Huang, M.T.
Quinn, D.L. Burnet, W.T. Summerfelt, J.M. Birnberg, and M.H. Chin. “Patient-Centered
Medical Home Characteristics and Staff Morale in Safety Net Clinics.” Archives of Internal
Medicine, vol. 172, no. 1, 2012, pp. 23–31.
Leykum, L.K., R. Palmer, H. Lanham, M. Jordan, R.R. McDaniel, P.H. Noel, and M. Parchman.
"Reciprocal Learning and Chronic Care Model Implementation in Primary Care: Results
from a New Scale of Learning in Primary Care." BMC Health Services Research, vol. 11,
2001, p. 44.
Linzer, M., M. Gerrity, J.A. Douglas, J.E. McMurray, E.S. Williams, and T.R. Konrad.
“Physician Stress: Results from The Worklife Study.” Stress & Health, vol. 18, no. 1, Feb
2002, pp. 37–42.
Linzer, M., L.B Manwell, M. Mundt, E. Williams, A. Maguire, M. McMurray, and M.B. Plane.
“Organizational Climate, Stress, and Error in Primary Care: The MEMO Study Findings.”
Advances in Patient Safety: From Research to Implementation (Volume 1: Research
Findings), 2005, pp. 65–77.
Linzer, M., L.B. Manwell, E.S. Williams, J.A. Bobula, R.L. Brown, A.B. Varkey, B. Man, J.E.
McMurray, A. Maguire, B. Horner-Ibler, M.D. Schwartz, MEMO (Minimizing Error,
Maximizing Outcomes) Investigators. “Working Conditions in Primary Care: Physician
Reactions and Care Quality.” Annals of Internal Medicine, vol. 151, no. 1, July 7, 2009, pp.
28–36, W6-9.
Linzer, M., S. Poplau, E. Grossman, A. Varkey, S. Yale, E. Williams, L. Hicks, R.L. Brown, J.
Wallock, D. Kohnhorst, and M. Barbouche. “A Cluster Randomized Trial of Interventions to
Improve Work Conditions and Clinician Burnout in Primary Care: Results from the Healthy
Work Place (HWP) Study.” Journal of General Internal Medicine, vol. 30, no. 8, 2015, pp.
1105–1111. doi: 10.1007/s11606-015-3235-4.
Lumley, T. “Survey: Analysis of Complex Survey Samples.” R package version 3.31-5, 2016.
Macinko, J., B. Starfield, and L. Shi. “Quantifying the Health Benefits of Primary Care
Physician Supply in the United States.” International Journal of Health Services, vol. 37,
2007, pp. 111–126.
268

REFERENCES MATHEMATICA POLICY RESEARCH
Maeng, D.D., D.E. Davis, J. Tomcavage, T.R. Graf, and K.M. Procopio. “Improving Patient
Experience by Transforming Primary Care: Evidence from Geisinger’s Patient-Centered
Medical Homes.” Population Health Management, vol. 16, no. 3, 2013, pp. 157–163.
doi:10.1089/pop.2012.0048.
Mainous, A.G. 3rd, and J.M. Gill. “The Importance of Continuity of Care in the Likelihood of
Future Hospitalization: Is Site of Care Equivalent to a Primary Clinician?” American
Journal of Public Health, vol. 88, 1998, pp. 1539–1541.
Maslach C., S. Jackson, and M. Leiter. Maslach Burnout Inventory Manual. Palo Alto, CA:
Consulting Psychologists Press; 1996.
Maslach C., W.B. Schaufeli, and M.P. Leiter “Job Burnout.” Annual Review of Psychology,
vol. 52, 2001, pp. 397–422.
McManus I.C., B.C. Winder, and D. Gordon. “The Causal Links Between Stress and Burnout in
a Longitudinal Study of UK Doctors.” The Lancet, vol. 359, 2002, pp. 2089–2090.
doi: 10.1016/S0140-6736(02)08915-8.
McManus I.C., E. Smithers, P. Partridge, A. Keeling, and P.R. Fleming. “A Levels and
Intelligence as Predictors of Medical Careers in UK Doctors: 20-year Prospective Study,”
British Medical Journal, vol. 327, 2003, pp. 139–142.
National Committee for Quality Assurance. “Specifications for the CAHPS PCMH Survey
2012.” Washington DC: National Committee for Quality Assurance, 2011.
Nichols D., S. Haber, M. Romaire, and contributing authors. “Evaluation of the Multi-Payer
Advanced Primary Care Practice (MAPCP) Demonstration Final Report.” Research Triangle
Park, NC: RTI International, 2017.
Nunnally, J., and I. Bernstein. Psychometric Theory (3rd ed.). McGraw-Hill Humanities/Social
Sciences/Languages, 1994.
Nutting, P.A., B.F. Crabtree, W.L. Miller, E.E. Stewart, K.C. Stange, and C.R. Jaén. “Journey to
the Patient-Centered Medical Home: A Qualitative Analysis of the Experiences of Practices
in the National Demonstration Project.” Annals of Family Medicine, vol. 8, suppl. 1, 2010,
pp. s45–s56. doi:10.1370/afm.1075.
Nyweide, D.J., D.L. Anthony, J.P. Bynum, R.L. Strawderman, W.B. Weeks, L.P. Casalino, and
E.S. Fisher. “Continuity of Care and the Risk of Preventable Hospitalization in Older
Adults.” JAMA Internal Medicine, vol. 173, November 2013, pp. 1879–1885.
O'Malley, A.S. “After-Hours Access to Primary Care Practices Linked with Lower Emergency
Department Use and Less Unmet Medical Need.” Health Affairs (Millwood), vol. 32, 2013,
pp. 175–183.
269

REFERENCES MATHEMATICA POLICY RESEARCH
O'Malley, A.S., E.C. Rich, A. Maccarone, C.M. DesRoches, and R.J. Reid. “Disentangling the
Linkage of Primary Care Features to Patient Outcomes: A Review of Current Literature,
Data Sources, and Measurement Needs.” Journal of General Internal Medicine, vol. 30,
suppl. 3, August 2015, pp. S576–S585.
O’Malley, A.S., K. Swankoski, D. Peikes, J. Crosson, N. Duda, T. Day, and S. Heitkamp.
“Patient Dismissal by Primary Care Practices.” JAMA Internal Medicine, vol. 177, no. 7,
July 2017a, pp. 1048–1050.
O’Malley, A.S., D. Peikes, C. Wilson, R. Gaddes, V. Peebles, T. Day, and J. Jin. “Patient
Perspectives on Care Management from the Comprehensive Primary Care Initiative: A
Qualitative Study.” American Journal of Managed Care, vol. 23, no. 11, 2017b, pp. 684-
689.
Panagioti, M., E. Panagopoulou, P. Bower, G. Lewith, E. Kontopantelis, C. Chew-Graham, S.
Dawson, H. van Marwijk, K. Geraghty, and A. Esmail. “Controlled Interventions to Reduce
Burnout in Physicians: A Systematic Review and Meta-analysis.” JAMA Internal Medicine.
vol. 177, no. 2, February 1, 2017, pp. 195–205. doi: 10.1001/jamainternmed.2016.7674.
Peikes, D., A. Chen, J. Schore, and R. Brown. “Effects of Care Coordination on Hospitalization,
Quality of Care, and Health Care Expenditures Among Medicare Beneficiaries: 15
Randomized Trials.” JAMA, vol. 301, no. 6, February 11, 2009, pp. 603–618.
Peikes, D., E. Taylor, S. Dale, and contributing authors. “Evaluation of the Comprehensive
Primary Care Initiative: Design Report.” Report submitted to the U.S. Department of Health
and Human Services, Centers for Medicare & Medicaid Services. Princeton, NJ:
Mathematica Policy Research, Inc., February 28, 2014.
Peikes, D., E. Taylor, S. Dale, A. O’Malley, A. Ghosh, G. Anglin, K. Swankoski, A. Zutshi, L.
Converse, R. Brown, and contributing authors. “Evaluation of the Comprehensive Primary
Care Initiative: Second Annual Report.” Prepared for the Centers for Medicare & Medicaid
Services. Princeton, NJ: Mathematica Policy Research, April 2016a.
Peikes, D., G. Anglin, E. Taylor, S. Dale, A. O’Malley, A. Ghosh, K. Swankoski, L. Converse,
R. Keith, M. Finucane, J. Crosson, A. Mutti, T. Grannemann, A. Zutshi, R. Brown, and
contributing authors. “Evaluation of the Comprehensive Primary Care Initiative: Third
Annual Report.” Prepared for the Centers for Medicare & Medicaid Services. Princeton, NJ:
Mathematica Policy Research, December 2016b.
Peikes. D., A.S. OʼMalley, C. Wilson, J. Crosson, R. Gaddes, B. Natzke, T.J. Day, D. Cromp, R.
Keith, J. Little, and J. Ralston. “Early Experiences Engaging Patients Through Patient and
Family Advisory Councils.” Journal of Ambulatory Care Management, vol. 39, no. 4, Oct–
Dec 2016c, pp. 316–324.
Phillips, R.L. Jr, and A.W. Bazemore. “Primary Care and Why It Matters for U.S. Health System
Reform.” Health Affairs (Millwood), vol. 29, 2010, pp. 806–810.
270

REFERENCES MATHEMATICA POLICY RESEARCH
Pines J.M., V. Keyes, M. van Hasselt, and N. McCall. “Emergency department and inpatient
hospital use by Medicare beneficiaries in patient-centered medical homes.” Ann Emerg Med.
2015;65(6):652-60. Published online Mar 10, 2015. doi:
10.1016/j.annemergmed.2015.01.002.
Pope G.C., J. Kautter, R.P. Ellis, A.S. Ash, J.Z. Ayanian, L.I. Iezzoni, M.J. Ingber, J.M. Levy,
and J. Robst. “Risk Adjustment of Medicare Capitation Payments Using the CMS-HCC
Model.” Health Care Financing Review, vol. 25, 2004, pp. 119–141.
Poznyak, D., D.N. Peikes, B.A. Wakar, R.S, Brown, and R.J. Reid. “Development and
Validation of the Modified Patient-Centered Medical Home Assessment for the
Comprehensive Primary Care Initiative.” Health Services Research, March 13, 2017. doi:
10.1111/1475-6773.12673.
Puffer, J.C., C. Knight, T.R. O’Neill, M. Rassolian, A.W. Bazemore, L.E. Peterson, and E.G.
Baxley. “Prevalence of Burnout in Board Certified Family Physicians.” Journal of the
American Board of Family Medicine, vol. 30, 2017, pp. 125–126.R Core Team.
“R: A Language and Environment for Statistical Computing.” Vienna, Austria: R Foundation for
Statistical Computing, 2016. Available at https://www.R-project.org/.
Raddish, M., S.D. Horn, and P.D. Sharkey. “Continuity of Care: Is It Cost Effective?” American
Journal of Managed Care, vol. 5, 1999, pp. 727–734.
RAND Corporation. “Evaluation of CMS FQHC Advanced Primary Care Practices (APCP)
Demonstration Clinician and Staff Experience (CASE) Survey.” Santa Monica, CA: RAND
Corporation, administered starting April 2013.
Reddy, A., A. Canamucio, and R.M. Werner. “Impact of the Patient-Centered Medical Home on
Veterans’ Experience of Care.” American Journal of Managed Care, vol. 21, no. 6, 2015,
pp. 413–421.
Reid, R.J., P.A. Fishman, O. Yu, T.R. Ross, J.T. Tufano, M.P. Soman, and E.B. Larson. “Patient-
Centered Medical Home Demonstration: A Prospective, Quasi-Experimental, Before and
After Evaluation.” American Journal of Managed Care, vol. 15, no. 9, 2009, pp. e71–e87.
Reid, R.J., K. Coleman, E.A. Johnson, P.A. Fishman, C. Hsu, M.P. Soman, C.E. Trescott, M.
Erikson, and E.B. Larson. “The Group Health Medical Home at Year Two: Cost Savings,
Higher Patient Satisfaction, and Less Burnout for Providers.” Health Affairs (Millwood),
vol. 29, no. 5, 2010, pp. 835–843. doi:10.1377/hlthaff.2010.0158.
Rohland, B.M., G.R. Kruse, and J.E. Rohrer. “Validation of a Single-Item Measure of Burnout
Against the Maslach Burnout Inventory Among Physicians.” Stress and Health, vol. 20,
2004, pp. 75–79.
Rosenblatt, R.A., G.E. Wright, L.M. Baldwin, L. Chan, P. Clitherow, F.M. Chen, and L.G. Hart.
“The Effect of the Doctor-Patient Relationship on Emergency Department Use Among the
Elderly.” American Journal of Public Health, vol. 90, 2000, pp. 97–102.
271

REFERENCES MATHEMATICA POLICY RESEARCH
Rosenthal, M.B., M.W. Friedberg, S.J. Singer, D. Eastman, Z. Li, and E.C. Schneider. “Effect of
a Multipayer Patient-Centered Medical Home on Health Care Utilization and Quality: The
Rhode Island Chronic Care Sustainability Initiative Pilot Program.” JAMA Internal
Medicine, vol. 173, no. 20, 2013, pp. 1907–1913. doi:10.1001/jamainternmed.2013.10063.
Rosenthal, M.B., S. Alidina, M.W Friedberg, S.J. Singer, D. Eastman, Z. Li, and E.C. Schneider.
“A Difference-in-Difference Analysis of Changes in Quality, Utilization and Cost Following
the Colorado Multi-Payer Patient-Centered Medical Home Pilot.” Journal of General
Internal Medicine, vol. 31, no. 3, March 2016, pp. 289–296. doi:10.1007/s11606-015-3521-
1.
Safety Net Medical Home Initiative. “The Patient-Centered Medical Home Assessment Version
1.3.” Seattle, WA: The MacColl Center for Health Care Innovation at Group Health
Research Institute and Qualis Health, September 2010.
Shanafelt, T.D., O. Hasan, L.N. Dyrbye, C. Sinsky, D. Satele, J. Sloan, and C.P. West. “Changes
in Burnout and Satisfaction with Work-Life Balance in Physicians and the General U.S.
Working Population Between 2011 and 2014.” Mayo Clinic Proceedings, vol. 90, no. 12,
December 2015, pp. 1600–1613.
Shi, L. “Primary Care, Specialty Care, and Life Chances.” International Journal of Health
Services, vol. 24, 1994, pp. 431–458.
Shi, L., J. Macinko, B. Starfield, R. Politzer, and J. Xu. “Primary Care, Race, and Mortality in
U.S. States.” Social Science Medicine, vol. 61, 2005, pp. 65–75.
Starfield, B. Primary Care: Balancing Health Needs, Services and Technology. New York:
Oxford University Press, 1998.
Starfield, B., L. Shi, and J. Macinko. “Contribution of Primary Care to Health Systems and
Health.” The Milbank Quarterly, vol. 83, pp. 457–502.
Stremikis, K. “All Aboard: Engaging Self-Insured Employers in Multipayer Reform.” Milbank
Memorial Fund, 2015. Available at http://www.pbgh.org/storage/documents/Milbank_-
_PBGH_Report_FINAL_2_17_15.pdf. Accessed December 20, 2016.
Taylor, E.F., S. Dale, D. Peikes, R. Brown, A. Ghosh, J. Crosson, G. Anglin, R. Keith, R.
Shapiro, and contributing authors. “Evaluation of the Comprehensive Primary Care
Initiative: First Annual Report.” Report prepared for the U.S. Department of Health and
Human Services, Centers for Medicare & Medicaid Services. Princeton, NJ: Mathematica
Policy Research, January 2015.
Van der Heijden, F., G. Dillingh, A. Bakker, and J. Prins. “Suicidal Thoughts Among Medical
Residents with Burnout.” Archives of Suicide Research, vol. 12, no. 4, 2008, pp. 344–346.
Weiss, L.J., and J. Blustein. “Faithful Patients: The Effect of Long-Term Physician-Patient
Relationships on the Costs and Use of Health Care by Older Americans.” American Journal
of Public Health, vol. 86, 1996, pp. 1742–1747.
272

REFERENCES MATHEMATICA POLICY RESEARCH
Werner, R.M., M. Duggan, K. Duey, J. Zhu, and E.A. Stuart. “The Patient-Centered Medical
Home: An Evaluation of a Single Private Payer Demonstration in New Jersey.” Medical
Care, vol. 51, no. 6, 2013, pp. 487–493. doi:10.1097/MLR.0b013e31828d4d29.
Werner, R.M., A. Canamucio, J.A. Shea, and G. True. “The Medical Home Transformation in
the Veterans Health Administration: An Evaluation of Early Changes in Primary Care
Delivery. Health Services Research, vol. 49, no. 4, 2014, pp. 1329–1347. doi:10.1111/1475-
6773.12155.
West C., L. Dyrbye, J. Sloan, and T. Shanafelt. “Single Item Measures of Emotional Exhaustion
and Depersonalization are Useful for Assessing Burnout in Medical Professionals.” Journal
of General Internal Medicine, vol. 24, no. 12, 2009, pp. 1318–1321.
West C.P., L.N. Dyrbye, D.V. Satele, J.A. Sloan, and T.D. Shanafelt. “Concurrent Validity of
Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout
Assessment.” Journal of General Internal Medicine, vol. 27, no. 11, 2012, pp. 1445–52.
West C.P., L.N. Dyrbye, P.J. Erwin, and T.D. Shanafelt. “Interventions to Prevent and Reduce
Physician Burnout: A Systematic Review and Meta-Analysis.” September 28, 2016. The
Lancet. doi:10.1016/S0140-6736(16)31279-X.
Williams, S.S., and A. Heller. “Patient Activation Among Medicare Beneficiaries: Segmentation
to Promote Informed Health Care Decision Making.” International Journal of
Pharmaceutical and Healthcare Marketing, vol. 1, no. 3, 2007, pp. 199–213.
Williams, S.S., and S.L. Frost. “Differences Among Consumer Segments with Regard to
Perceptions of Comparative Effectiveness Research.” Journal of Comparative Effectiveness
Research, vol. 3, no. 6, 2014, pp. 617–622.
World Health Organization. Primary Health Care. Geneva: World Health Organization, 1978.
Wurm W., K. Vogel, A. Holl, C. Ebner, D. Bayer, S. Morkl, I.S. Szilagyi, E. Hotter, H.P.
Kapfhammer, and P. Hofmann. “Depression-Burnout Overlap in Physicians.” PLoS One,
vol 11, no. 3, 2016.
Xie, Y. “Knitr: A General-Purpose Package for Dynamic Report Generation in R.” R package
version 1.15.1, 2016.
Zickafoose, J.S., L.R. DeCamp, and L.A. Prosser. “Association Between Enhanced Access
Services in Pediatric Primary Care and Utilization of Emergency Departments: A National
Parent Survey.” Journal of Pediatrics, vol. 163, 2013, pp. 1389–1395.e1-6.
273
This page has been left blank for double-sided copying.
This page has been left blank for double-sided copying.

