
1
Page 1
Tips and Tricks of Faster LC Analysis Without
Capital Investment
It may be easier than you think!
Agilent Technologies
Page 2
Tips and Tricks of HPLC System
Troubleshooting
Agilent Technologies

2
Page 3
Trouble Shooting Steps
You Have Recognized There is a Problem!
How Do You Fix It?
•1
st
Did System Suitability or Calibration Sample Fail?
– Make Blank Runs
•2
nd
Review Method for Compliance
– Is The Procedure Being Followed Properly?
– Are Instrument Settings Correct?
•3
rd
Ask More Questions!
– When Did the System Last Function Properly?
– Has Anything Been Changed?
•4
th
Review ALL parameters!
– The Obvious Is Not Always the Cause
– Was There More Than One Change?
Page 4
Pump
Injector/Autosampler
Column
Detector
Data System/Integrator
HPLC System Components
Problems Can Be Related to All Components in the System

3
Column Problems From LC GC User Survey
Column Problem Percentage of Respondents
Back Pressure, Plugged Frits 24%
Poor Reproducibility
16%
Sample Recovery
14%
Loss of Resolution
13%
Instability
11%
Voids
8.1%
Leaks, Fittings
4.8%
pH Range
2.0%
Low Plate Count
2.0%
Column Overload
2.0%
Cost
1.7%
Miscellaneous
15%
Page 5
Page 6
Categories of Column and System Problems
A. Pressure
B. Peak shape
C. Retention

4
Page 7
Column Observations Potential Problems
High pressure - Plugged frit
- Column contamination
- Plugged packing
Low Pressure - Leak
- Flow Incorrect
Pressure Issues
Page 8
Determining the Cause and Correcting
High Back Pressure
• Check pressure with/without column - many pressure
problems are due to blockages in the system or guard col.
•Remove Column - Pressure Still High?
•Remove Guard – Pressure Still High?
•If Column pressure is high:
• Back flush column – Clear “dirty” frit surface
• Wash column – Eliminate column contamination
and plugged packing
– high molecular weight/adsorbed
compounds
– precipitate from sample or buffer
Change frit – Clear plugged frit PREVENT THIS!

5
Page 9
Determining the Cause and Correcting High Back Pressure
Many pressure problems relate to blockages in the system.
Check system pressure with / without column
If column pressure is high:
• Back flush column (care regarding future performance)
• Clear blocked frit (reverse flush with strong solvent)
• Wash column
Eliminate column contamination and clear blocked packing
Remove high M
w
/ adsorbed compounds
Clear precipitate introduced from the sample or buffer
Correcting Overpressure
Page 10
Plugged Packing
Particle
Size
Area
(um
2
)
Diameter
(um)
Frit Porosity
(um)
5.0 2.7 0.9 2.0
3.5 1.3 0.6 2.0
3.0 1.0 0.6 0.5
2.5 0.7 0.5
1.8 0.4 0.4 0.5
1.7 0.3 0.3
Beware of buffered mobile phases
Buffers usually contain insoluble material – filter
Buffer solubility decreases with increasing % organic* -
avoid 100%B with buffer salts
*Schellinger,A.P. and Carr,P.W., LC-GC North America, 22, 6, 544-548 (2004)

6
Buffer solubility
Page 11
Table I presents an estimate of the soluble concentration of
the least soluble buffer (potassium phosphate at pH 7.0) in
three organic cosolvents
Solubility of five buffers in mixtures with (a)
methanol, (b) acetonitrile, and (c) tetrahydrofuran
Page 12
Solubility of five buffers in mixtures with (a) methanol, (b) acetonitrile, and (c)
tetrahydrofuran, where — represents ammonium acetate at pH 5.0, •••• represents
ammonium phosphate at pH 3.0, --- represents potassium phosphate at pH 3.0,
–••– represents ammonium phosphate at pH 7.0, and – – represents potassium
phosphate at pH 7.0. The 95% confidence level intervals are 3.3 mM.
methanol
acetonitrile
THF

7
Page 13
Changing a Frit May Not Be a Good Idea
May not be possible with new generation columns
May damage high performance columns
Column
Inlet Fri
t
Compression
Ferrule
Column Body
Female End Fitting
Male End Fitting
Wear gloves
Do not allow bed to dry
Do not touch the column -
body heat will extrude packing
Do not overtighten
Tip: Prevention is a Much Better Idea!
Page 14
The Trick:
Prevention Techniques - A Better Choice!
• Use column protection
- In-line filters
- Guard columns
• Filter samples
• Filter buffered mobile phases
• Sample clean-up (i.e. SPE)
• Appropriate column flushing
Easy
Not As Easy

8
Page 15
Inexpensive Filters Prevent Column Frit Plugging
Regenerated Cellulose (RC) Recommended
•Universal hydrophilic membrane, compatible with
most solvents - aqueous and organic
•High purity, extremely low extractables
•High recovery
•More Uniform Surface
•Different than Other Cellulose Filters!!
In-line Filters Easy to Use and replace
Frits Available in 0.2,0.5 and 2.0µ Porosity
Much Less expensive than a Column
Easier and Faster to Replace than a Column Frit
Mini-UniPrep Vials
UniPrep vials contain an integral filter
(PTFE, PP, RC or Nylon - 0.2 or 0.45µm
porosity)
Page 16
Column Cleaning
Use at least 25 mL of each solvent for analytical
columns
Flush with stronger solvents than
your mobile phase.
Reversed-Phase Solvent Choices
in Order of Increasing Strength
• Mobile phase without buffer salts
• 100% Methanol
• 100% Acetonitrile
• 75% Acetonitrile:25%
Isopropanol
• 100% Isopropanol
• 100% Methylene Chloride*
• 100% Hexane*
*Tip: When using either Hexane or Methylene Chloride the column must be flushed
with Isopropanol before returning to your reversed-phase mobile phase.
Must Reverse
to
Re-Equilibrate
This Is Time Consuming
Often Performed Offline

9
Page 17
Use at least 50mL or 20−30 column volume changes for analytical columns
• Typical normal phase solvent choices in order of increasing strength:
Solvent
Composition
Methanol:Chloroform 50:50%
Ethyl Acetate 100%
Column Cleaning – Normal Phase
Page 18
What Are Common Peak Shape Issues?
1. Split peaks
2. Peak tailing
3. Broad peaks
• Many peak shape issues are also combinations - i.e. broad and tailing
or tailing with increased retention
•Symptoms do not necessarily affect all peaks in the chromatogram
•Each of these problems can have multiple causes

10
Page 19
Peak Splitting Caused By Disrupted Sample Path
Split or Double Peaks
Normal
Double
Peaks
Tip: Similar Effect Can be Caused by Partially Plugged Frit
•Flow Path Disrupted by Void
•Sample Allowed to Follow Different Paths
Through Column
•Poorly Packed Bed Settles in Use
•High pH Dissolves Silica
Page 20
0 5 10 15
1
3
4
2
Time (min)
0 5 10 15
1
3
4
2
Time (min)
0 5 10 15
1
3
4
2
Time (min)
Split Peaks from Column Contamination
Column: StableBond SB-C8, 4.6 x 150 mm, 5 µm Mobile Phase: 60% 25 mM Na
2
HPO
4
, pH 3.0 : 40% MeOH Flow Rate: 1.0 mL/min
Temperature: 35°C Detection: UV 254 nm Sample: Filtered OTC Cold Medication: 1. Pseudoephedrine 2. APAP 3. Unknown 4. Chlorpheniramine
Injection 1
Injection 30
Injection 1
After Column Wash
with 100% ACN
Tip: Column washing eliminates the peak splitting, which resulted from a contaminant on the column
How could this be prevented? (Guard Column, SPE clean up of samples, Periodic column wash)

11
Page 21
Split Peaks from Injection Solvent Effects
Column: StableBond SB-C8, 4.6 x 150 mm, 5 µm Mobile Phase: 82% H
2
O : 18% ACN
Injection Volume: 30 µL Sample: 1. Caffeine 2. Salicylamide
A. Injection Solvent
100% Acetonitrile
B. Injection Solvent
Mobile Phase
Tip: Injecting in a solvent stronger than the mobile phase can cause peak shape
problems such as peak splitting or broadening
Trick: Keep Organic Concentration in Sample Solvent < Mobile Phase
0 10
Time (min)
1
2
0 10
Time (min)
1
2
Page 22
Peak Tailing, Broadening
and Loss of Efficiency
May be caused by:
• Column “secondary
interactions”
• Column contamination
• Column aging
• Column loading
• Extra-column effects

12
Page 23
Normal
Tailing
Normal
Tailing
Symmetry > 1.2
All Peaks Tail:
Extra-Column Effects.
Build up of Contamination on Column
Inlet.
Heavy Metals.
Bad Column.
Causes
Some Peaks Tail:
Secondary - Retention Effects.
Residual Silanol Interactions.
Small Peak Eluting on Tail of Larger Peak.
Peak Shape: Tailing Peaks
Page 24
Peak Tailing
Identifying Column “Secondary Interactions”
Tip: Mobile phase modifier (TEA) competes with Sample for surface ion exchange
sites at mid-range pH values
Column: Alkyl-C8, 4.6 x 150 mm, 5µm Mobile Phase: 85% 25 mM Na
2
HPO
4
pH 7.0 : 15% ACN Flow Rate: 1.0 mL/min
Temperature: 35°C Sample: 1. Phenylpropa nolamine 2. Ephedrine 3. Amphetamine 4. Methamphetamine 5. Phenteramine
No TEA
USP TF (5%)
1. 1.29
2. 1.91
3. 1.63
4. 2.35
5. 1.57
10 mM TEA
USP TF (5%)
1. 1.19
2. 1.18
3. 1.20
4. 1.26
5. 1.14
T
Ime (min)
Time (min)
0.0
2.5
5.0
5
4
3
2
1
5
4
3
2
1
0.0
2.5
5.0

13
Page 25
Peak Tailing
Low pH Minimizes “Secondary Interactions”
for Amines
Column: Alkyl-C8, 4.6 x 150 mm, 5µm Mobile Phase: 85% 25 mM Na
2
HPO
4
: 15% ACN Flow Rate: 1.0 mL/min
Temperature: 35°C Sample: 1. Phenylpropa nolamine 2. Ephedrine 3. Amphetamine 4. Methamphetamine 5. Phenteramine
pH 3.0
USP TF (5%)
4. 1.33
pH 7.0
USP TF (5%)
4. 2.35
Tip: Reducing mobile phase pH reduces interactions with silanols and peak tailing.
Time (min)
0.0 2.5 5.0
5
4
3
2
1
5
4
3
2
1
Time (min)
0.0 2.5 5.0
Page 26
0 5
1
2,3
4
5
Time (min)
7
6
0 5 10
1
2
3
4
Time (min)
6
5
7
Peak Tailing
High pH Eliminates “Secondary Interactions” for
Amines
Peak Shape and Retention of this sample of basic compounds improves
at high pH where column has high IEX activity. Why?
Column: ZORBAX Extend-C18, 4.6 x 150 mm, 5 m m Mobile Phase: See Below Flow Rate: 1.0 mL/min Temperature: RT
Detection: UV 254 nm
Sample: 1. Maleate 2. Scopolamine 3. Pseudoephedrine 4. Doxylamine 5. Chlorpheniramine 6. Triprolidine 7. Diphenhydramine
pH 7
30% 20 mM Na
2
HPO
4
70% MeOH
pH 11
30% 20 mM TEA
70% MeOH
t
R
= 8.5 t
R
= 11.4

14
Page 27
0.0 2.5 5.0
2
4
1
3
Time (min)
2
4
1
3
0.0 2.5 5.0
Time (min)
2
4
1
3
0.0 2.5 5.0
Time (min)
Peak Tailing - Column Contamination
Column: StableBond SB-C8, 4.6 x 250 mm, 5µm Mobile Phase: 20% H
2
O : 80% MeOH Flow Rate: 1.0 mL/min
Temperature: R.T. Detection: UV 254 nm Sample: 1. Uracil 2. Phenol 3. 4-Chloronitrobenzene 4. Toluene
Plates TF
1. 7629 2.08
2. 12043 1.64
3. 13727 1.69
4 13355 1.32
Plates TF
1. 7906 1.43
2. 12443 1.21
3. 17999 1.19
4 17098 1.25
Plates TF
1. 7448 1.06
2. 12237 1.21
3. 15366 1.11
4 19067 1.17
QC test forward
direction
QC test reverse direction
QC test after cleaning
100% IPA, 35°C
Tip: Quick Test to Determine if Column is Dirty or Damaged
Trick: Reverse Column and Run Sample –If Improved, Possible Cleaning Will
Help -No improvement-Column Damaged and Needs to be Replaced
Page 28
Causes:
Column Overload
Normal
Fronting
Symmetry < 0.9
2000
1500
1000
500
0
0 5
10
15 20 25
Time (min)
mAU
Peak Shape: Fronting Peaks

15
Page 29
Peak Tailing/Broadening
Sample Load Effects
Columns: 4.6 x 150 mm, 5µm Mobile Phase: 40% 25 mM Na
2
HPO
4
pH 7.0 : 60% ACN Flow Rate: 1.5 mL/min
Temperature: 40°C Sample: 1. Desipramine 2. Nortriptyline 3. Doxepin 4. Imipramine 5. Amitriptyline 6. Trimipramine
Broadening
Competitive C8
Plates
A.
B.
C.
D.
High Load
x10
Low Load
C D
1. 850 5941
2. 815 7842
3. 2776 6231
4. 2539 8359
5. 2735 10022
6. 5189 10725
Tailing
Eclpse XDB-C8
USP TF (5%) i
A B
1. 1.60 1.70
2. 2.00 1.90
3. 1.56 1.56
4. 2.13 1.70
5. 2.15 1.86
6. 1.25 1.25
0 5 10
Time (min)
0 5 10
Time (min)
0 5
Time (min)
0 5
Time (min)
Tip: Evaluate Both Volume and Mass Loading
Page 30
Peak Shape: Broad Peaks
All Peaks Broadened:
• Loss of Column Efficiency.
• Column Void.
• Large Injection Volume.
Some Peaks Broadened:
• Late Elution from Previous Sample
(Ghost Peak).
– High Molecular Weight.
– Sample - Protein or Polymer.

16
Page 31
Time (min)
3
1
2
0 5 10 15
Unknown “Phantom” Peaks
Column: Extend-C18, 4.6 x 150 mm, 5 µm Mobile Phase: 40% 10 mM TEA, pH 11 : 60% MeOH Flow Rate: 1.0 mL/min
Temperature: R.T. Detection: UV 254 Sample: 1. Maleate 2. Pseudoephedrine 3. Chlorpheniramine
Plates
1. 5922
2. 9879
3. 779
Tip: The extremely low plates for moderately retained peaks are an indication of a
very late eluting peak from a preceding run.
Time (min)
1
0 5 10
Sample 1: Chlorpheniramine maleate
Peak 1: maleate
Sample 2 : Chlorpheniramine
maleate
and Pseudoephedrine
Peak 1: maleate
Peak 2: pseudoephedrine
Peak 3: chlorpheniramine (from 1
st
injection)
“Phantom” peak from
first injection
Page 32
Use short, small internal diameter tubing between the injector and
the column and between the column and the detector.
Make certain all tubing connections are made with matched
fittings.
Use a low-volume detector cell.
Inject small sample volumes.
Increasing Extra-Column Volume
Extra-Column Dispersion

17
Page 33
Peak Broadening
Extra-Column Volume
Column: StableBond SB-C18, 4.6 x 30 mm, 3.5 µm Mobile Phase: 85% H
2
O with 0.1% TFA : 15% ACN Flow Rate: 1.0 mL/min
Temperature: 35°C Sample: 1. Phenylalanine 2. 5-benzyl-3,6-dioxo-2-piperazine acetic acid 3. Asp-phe 4. Aspartame
10 mL extra-column
volume
50 mL extra-column
volume (tubing)
Time (min)
0.0 0.5 1.0 1.5 2.0
4
3
2
1
Time (min)
0.0 0.5 1.0 1.5 2.0
4
3
2
1
Page 34
Tip: Poorly Made HPLC System Connections
Can Cause Peak Broadening
The System Has Been Optimized and :
– All Tubing Lengths Are Minimum
– Smallest Diameter Tubing Used
– Proper Flow Cell Volume
Symptom Still Seems to Have Too Much Extra-Column
Volume
What Is Wrong?
Have You Made the Connections Properly?

18
Page 35
Column Connectors Used in HPLC
0.090
in.
0.130
in.
0.090
in.
0.170
in.
Swagelok
Waters
Parker
Rheodyne
Valco
Uptight
0.090
in.
0.080
in.
Troubleshooting LC Fittings, Part II. J. W. Dolan and P. Upchurch.
LC/GC Magazine 6:788 (1988)
Page 36
What Happens If the Connections Poorly Made ?
If Dimension X is too long, leaks will occur
Ferrule cannot seat properly
Mixing Chamber
If Dimension X is too short, a dead-volume,
or mixing chamber, will occur
Wrong … too long
Wrong … too short
X
X

19
Page 37
Stainless Steel and Polymer Fittings
Which type is used and when?
Stainless Steel (SS) fittings are the best choice for
reliable high pressure sealing
• Agilent uses Swagelok type fittings with front and back
ferrules – which give best sealing performance –
throughout all our LC systems
PEEK (<400b bar System Pressure) fittings are
ideal where:
• Connections are changed frequently, i.e. connecting
columns
• Pressure is less critical
PolyKetone
• Easy, hand tighten column connection
• 600 bar Pressure Rating PN: 5042-8957 (10/pk)
• Fits to SS Tubing
Page 38
Changes in Retention Can Be Chemical or Physical
May be caused by:
• Column aging
• Column contamination
• Insufficient equilibration
• Poor column/mobile phase combination
• Change in mobile phase
• Change in flow rate
• Different Gradient Delay Volumes

20
Page 39
Column Aging/Equilibration Causes
Retention/Selectivity Changes
Column 1 - After Cleaning
with 1% H
3
PO
4
/Equilibration
• The primary analyte was sensitive to mobile phase aging/
conditioning of the column
• The peak shape was a secondary issue (metal chelating
compound) resolved by “de-activating” the active metal
contamination
Column 1 - Next Day
Column 1 - Initial
0 3 5 9 12 15
Time (min)
2
1
0 3 5 9 12 15
Time (min)
2
1
Page 40
Metal Sensitive Compounds Can Chelate
C
O
O
H
H
M
+2
OH
::
M
+2
C
O
OH
C
N
N
:
M
+2
:
:
:
O
:
OH + M
+2
C
H
:
::
Hint: Look for Lone Pair of Electrons on :O:
or N Which Can Form 5 or 6 Membered
Ring with Metal
:
Salicylaldehyde 6-membered ring complex
8-hydroxyquinoline
5-membered ring complex
a-benzoinoxomine
5-membered ring complex

21
Page 41
Acid Wash Can Improve Peak Shape
OH
OH
OHHO
OHHO
OH
OH
1. 2. 1. 2.
Columns: ZORBAX SB-Phenyl
4.6 x 150 mm
Mobile Phase: 75% 25 mM
ammonium phosphate buffer
25% ACN
Flow Rate: 1.0 mL/min.
Temperature: RT
Sample Size: 5 mL
1
1
2
2
Tf: 1.2
Tf: 3.7
Before Acid Wash
After Acid Wash
50 – 100 mLs 1% H
3
PO
4
• A 1% H
3
PO
4
solution is used on SB columns, 0.5 % can be used on endcapped columns.
Page 42
Example: Change in Retention/Selectivity
Unintended Mobile Phase Variation
Tip: The Source of the Problem is Often Not the Obvious Change
“I have experimented with our mobile phase, opening new bottles of all mobile phase
components. When I use all fresh ingredients, the problem ceases to exist, and I
have narrowed the problem to either a bad bottle of TEA or phosphoric acid. Our
problem has been solved.”
Column 1
Column 2 - Fresh
mobile phase
Column 2
0 4 6
Time (min)
0 2 3 4 5 6 7
Time (min)
0 4 6
Time (min)

22
Page 43
Maintain Resolution for Low Volume Peaks by
Minimizing Extra-Column Volume
ECV = sample volume + connecting tube volume
+ fitting volume + detector cell volume
Page 44
0
10
20
30
40
Tip: Dwell Volume Differences Between Instruments
Can Cause Changes in Retention and Resolution
0 10 20 30 40
V
D
= 0.43 mL
Column: ZORBAX Rapid Resolution
Eclipse XDB-C8
4.6 x 75 mm, 3.5 µm
Mobile Phase: Gradient, 0 - 100 %B in 52.5 min.
A: 5/95 methanol/ 25 mM
phosphate
pH 2.50
B: 80/20 methanol/25 mM
phosphate
pH 2.50
Flow Rate: 0.5 mL/min
Temperature:25°C
Injection: 5 µL
Detection: 250 nm
Sample: Mixture of antibiotics and
antidepressants
Upper trace simulates actual run
data entered into DryLab
®
3.0
software
Lower trace is simulated
chromatogram for larger V
D
V
D
= 2.0 mL

23
Page 45
If V
D1
> V
D2
Compensate for longer V
D1
by adding
an isocratic hold to V
D2
, such that
Hold + V
D2
= V
D1
If V
D1
< V
D2
Delay injection, such that V
D2
- delay = V
D1
Trick: Measure and Correct for Dwell Volume (V
D
)
Page 46
Mobile Phase pH and pH Buffers
Why Are These So Important in HPLC?
•pH Effects Ionization
– Silica Surface of Column
– Sample Components of Interest
• Buffers
– Resist Changes in pH and Maintain Retention
– Improve Peak Shape for Ionizable Compounds
• Effects Column Life
– Low pH strips Bonded Phase
– High pH Dissolves Silica

24
Page 47
Minimize Change in Retention/Selectivity
Lot-to-Lot
• All causes of column-to-column change*
• Method ruggedness (buffers/ionic strength)
• pH sensitivity (sample/column interactions)
*All causes of column-to-column change should be considered first,
especially when only one column from a lot has been tested.
Evaluate:
Page 48
0 2 4 6 8 10 12 14 16 18
Time (min)
2-Base
3
4-Base
1
0 2 4 6 8 10 12 14 16 18
Time (min)
2
3
4
1
Lot-to-Lot Selectivity Change Related to pH Choice
• pH 4.5 shows selectivity change from lot-to-lot for basic compounds
• pH 3.0 shows no selectivity change from lot-to-lot
• Indication of poorly controlled ionization
pH 4.5 - Lot 1
pH 3.0 - Lot 1
pH 4.5 - Lot 2
pH 3.0 - Lot 2
0 2 4 6 8 10 12 14 16 18
Time (min)
2-Base
3
4-Base
1
0 2 4 6 8 10 12 14 16 18
Time (min)
2
3
4
1

25
Page 49
Why Worry About pH?
pH, pKa and Weak Acids
At pH 4.2 – the sample exists as benzoic acid and the benzoate ion in a ratio
of 1:1. Peak shape can be poor
At pH 5.2 – 91% of the sample exists as the benzoate ion. RP retention decreases.
At pH 3.2 – 91% of the sample exists as benzoic acid. RP retention increases.
RCOOH RCOO
-
+ H
+
K
a
= 6.4 x 10
-5
pK
a
= 4.2
K
a
=
[RCOO
-
][H
+
]
[RCOOH]
+ H
+
COOH
COO
_
Page 50
Effect of pH on Peak Shape at or
Near the Sample pK
a
0 1 2 3 4 5 6 7 8 9 10
0 1 2 3 4 5 6 7 8 9 10
Time (min)
Time (min)
Column: ZORBAX SB-C8 4.6 x 150 mm, 5 mm Mobile Phase: 40% 5 mM KH
2
PO
4
: 60% ACN
Flow Rate: 1.0 mL/min. Temperature: RT
pH 4.4 pH 3.0
Ibuprofen
pK
a
= 4.4
CH
3
CHCOOH
CH
2
CH(CH
3
)
2
• Inconsistent and tailing peaks may occur when operating close to an analyte
pKa and should be avoided.

26
Page 51
Why Worry About pH?
pH, pKa and Weak Bases
At pH 9 – the sample exists as protonated and unprotonated diphenhydramine
in a ratio of 1:1. Peak shape can be poor.
At pH 10 – 91% of the sample exists as unprotonated diphenhydramine.
At pH 8 – 91% of the sample exists as protonated diphenhydramine.
K
a
=
[R
3
N][H
+
]
[R
3
NH
+
]
K
a
= 1 x 10
-9
pK
a
= 9
R
3
NH
+
R
3
N + H
+
CHOCH CH N
2 2
CH
3
CH
3
∅
∅
+
H
CH
3
CH
3
∅
∅
CHOCH CH N
2 2
+ H
+
Page 52
pH vs. Selectivity for Acids and Bases
5
SCD
+
+
2
+
1
1.5
1.0
0.5
0.0
-0.5
log k«
3 4 5 6 7 8
pH
A
B
C
2
4
5
3
3
5
7
9
ELUENT pH
40
30
20
10
10
Retention
+
+
+
3
1
7,12 - OC
UDC
SOC
4
6
C
12-OC
J.C. 268(1983) 1
J.C. 111(1975) 149
Column: Nucleosil-C18
Mobile Phase: 45% ACN/55% phosphate buffer
Sample: Bile Acids
Column: mBondapak-C18
Mobile Phase: 60% 25 mM phosphate buffer
40% Methanol
1. Salicylic acid
2. Phenobarbital
3. Phenacetin
4. Nicotine
5. Methamphetamine
• Retention and selectivity can change dramatically when pH is changed.

27
Page 53
Importance of pH and Buffers
A Practical Example
•Why the Sample Dictates Use
•What Happens When Buffer Used Effectively
•What Happens When Buffer Ignored or Used Improperly
Page 54
Importance of pH and Buffers - A Practical Example
Optimized Isocratic Conditions for Cardiac Drugs
0 1 2
3 4 5 6 7 8 9 10 11 12 13 14
Time (min)
5
4
3
2
1
Column: StableBond SB-C18, 4.6 x 150
mm, 5 mm
Mobile Phase: 45% 25 mM
NaH
2
PO
4
, pH 3.0
55% MeOH
Flow Rate: 2.0 mL/min.
Temperature:35°C
Detection: UV 254 nm
Sample: Cardiac Drugs
1. Diltiazem
2. Dipyridamole
3. Nifedipine
4. Lidoflazine
5. Flunarizine
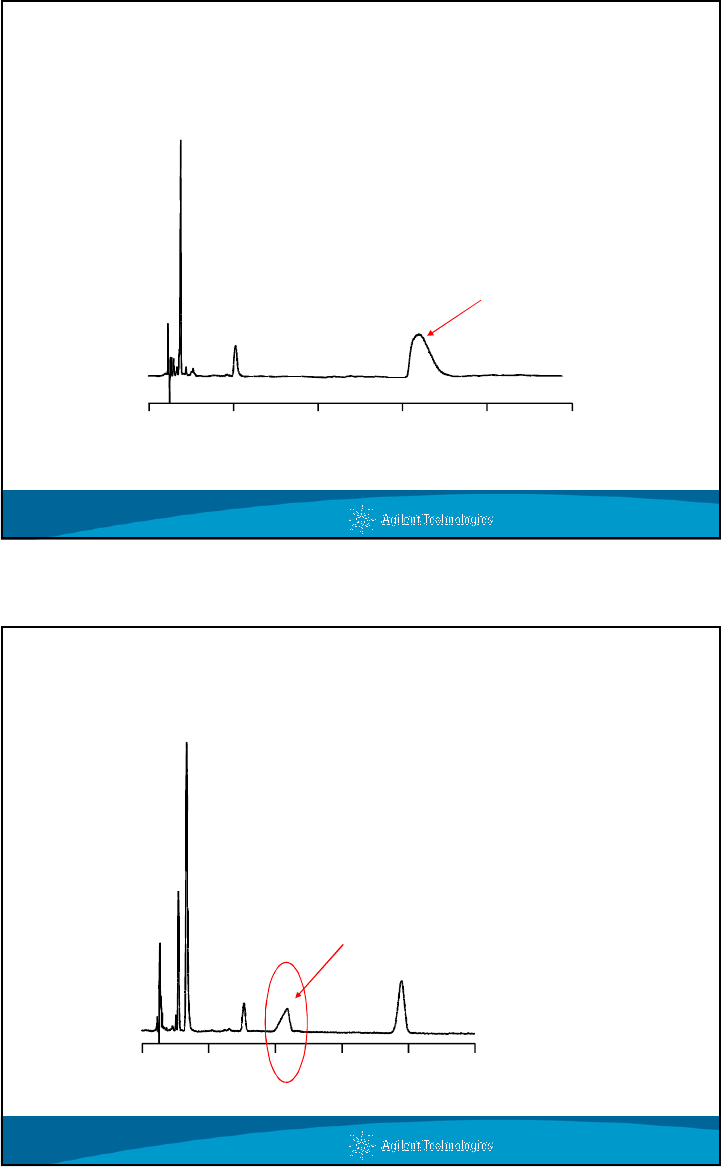
28
Page 55
I Don’t Have Time to Make Buffers or Adjust pH …
0 5 10 15 20 25
Time (min)
Column: StableBond SB-C18
4.6 x 150 mm, 5 mm
Mobile Phase: A: 20% H
2
O
B: 80% MeOH
Flow Rate: 1.0 mL/min.
Temperature:35°C
UV Detection: 254 nm
Sample: Cardiac Drugs
• Buffers are critical to good retention and peak shape in many separations.
Even at very high % MeOH Most Components
Strongly Retained with Poor peak Shape Due to
IEX at Surface
Page 56
What If You Work Outside the Buffer Range?
0 5 10 15 20 25
5
1
2
3
4
Time (min)
Columns: StableBond SB-C18
4.6 x 150 mm, 5 mm
Mobile Phase: A: 30% 25 mM NaH
2
PO
4
, pH
4.8 unbuffered
B: 70% MeOH
Flow Rate: 1.0 mL/min.
Temperature:35°C
UV Detection: 254 nm
Sample: Cardiac Drugs
1. Diltiazem
2. Dipyridamole
3. Nifedipine
4. Lidoflazine
5. Flunarizine
Unsuitable Peak Shape

29
Commonly Used Buffers for Reversed Phase HPLC
Buffer pKa Buffer Range UV Cutoff (nm)
Phosphate 2.1 1.1-3.1 200
7.2 6.2-8.2
12.3 11.3-13.3
Formic acid* 3.8 2.8-4.8 210
Acetic acid* 4.8 3.8-5.8 210
Citrate 3.1 2.1-4.1 230
4.7 3.7-5.7
5.4 4.4-6.4
Tris 8.3 7.3-9.3 205
Triethylamine* 11.0 10.0-12.0 200
Pyrrolidine 11.3 10.3-12.3 200
* Volatile buffers for LC/MS applications
Page 57
Optimum buffering capacity occurs at a pH equal to the pKa of the buffer. Most buffers provide
adequate buffering capacity for controlling mobile phase pH only within ±1 unit of their pKa
Page 58
Don’t Forget - Match Column to pH of Mobile Phase
for Maximum Column Lifetime
low pH and high temperature (pH 0.8, 90°C)
Purge Solvent:
50% methanol/water with
1.0% TFA
Solute: Toluene
Kirkland, J.J. and J.W. Henderson, Journal of Chromatographic Science, 32 (1994) 473-480.

30
Page 59
Don’t Forget - Match Column to pH of Mobile Phase
for Maximum Column Lifetime
High pH and Room Temperature (pH 11 RT)
Tip: Use Columns Designed for chosen pH
Mobile Phase: 50%ACN: 50% Water : 0.2% TEA
(~ pH 11)
After 30 injections
Initial
Page 60
Detection Issues
Recognize Where the Problem Originates
• Is it a consequence of technique?
• Is It expected due to use of certain mobile phase
components?
• Can it be corrected by adjusting detector
parameters?
• Answers Will Help Find a Solution!
Let’s Explore Some Problems and Solutions

31
Page 61
Causes:
Absorbance of sample is less than the mobile phase.
Equilibrium disturbance when sample solvent passes through the
column.
Normal with Refractive Index Detectors.
Normal
Negative
Peak Shape: Negative Peaks
Page 62
Ghost Peaks
20% - 100%
MeOH Gradient
No Sample Injected
Ghost Peaks - Peaks which appear even when no sample
is injected.
Problem - Dirty Mobile Phase
60
15
30
15
0
3
7 15 17
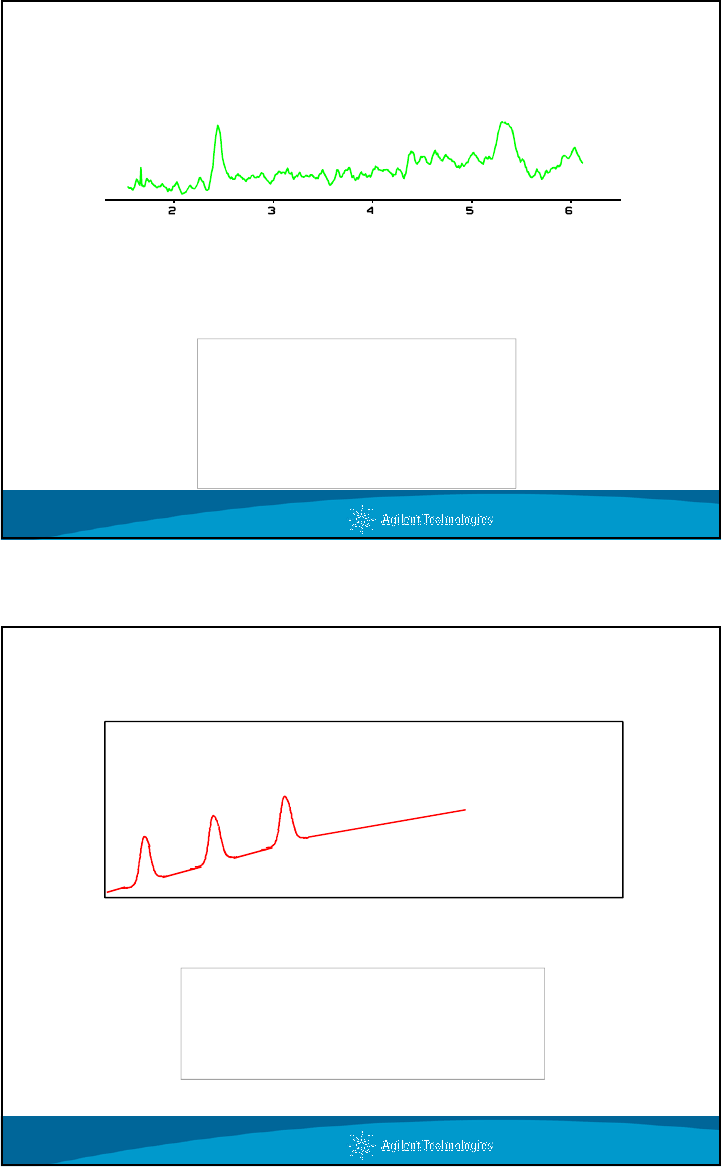
32
Page 63
Noisy Baselines
Possible Causes:
Dirty Flow Cell
Detector Lamp Failing
Pulses from Pump if Periodic
Temperature Effects on Detector
Air Bubbles passing through Detector
Time
(min.)
Page 64
Gradient Elution
Temperature Unstable (Refractive Index Detector)
Contamination in Mobile Phase
Mobile Phase Not in Equilibrium with Column
Contamination Bleed in System
Drifting Baselines

33
Page 65
Chromatographic Results with “Wrong” Lamp at
214 nm Wavelength
OEM LampOEM Lamp
Lamp from Generic SourceLamp from Generic Source
Tip: Could also be a symptom of aging lamp
Page 66
Expanded View of Chromatographic Results
Generic Source Lamp at 214 nm Wavelength
OEM Lamp OEM Lamp
Lamp from Generic SourceLamp from Generic Source
Peak 1Peak 1 S/N = 150S/N = 150
Peak 2Peak 2 S/N = 400S/N = 400
Peak 3Peak 3 S/N = 300S/N = 300
Peak 1Peak 1 S/N = 15S/N = 15
Peak 2Peak 2 S/N = 50S/N = 50
Peak 3Peak 3 S/N = 50S/N = 50
Tip: Poor S/N makes it difficult to detect low level impurities

34
Page 67
Effect of Detector Response Time
0 0.1 0.2 0.3 0.4 0.5
0.6
0.7
0.8
0.9
Time (min)
1.0
Agilent 1100 DAD
Agilent 1100 WPS with ADVR
Column: Poroshell 300SB-C18
2.1 x 75 mm, 5 mm
Mobile Phase:
A: 95% H
2
O, 5% ACN with 0.1% TFA
B: 5% H
2
O, 5% ACN with 0.1% TFA
Flow Rate: 2 mL/min
Temperature:70°C
Detector: UV 215 nm
Piston stroke: 20
Sample:
1. Neurotensin3. Lysozyme
2. RNaseA 4. Myoglobin
0.1 sec
0.2 sec
0.5 sec
1.0 sec
1
st
peak = 1.2 sec
At 20 pts/sec = 24 pts/sec
Response Time
2.0 sec
1
st
peak = 1.2 sec
At 5 pts/sec = 6 pts
• Tip: Adjust the response rate of your detector for best peak detection.
The System is operating well-the settings were poorly made!
Slow Data Rates Can Hinder Impurity Detection and Reduce Sensitivity
Page 68
Conclusions
• High pressure (prevention better than the cure)
• Undesirable peak shape
• Changes in retention/selectivity
Often these problems are not associated with the column and
may be caused by instrument and chemistry issues.
•pH of mobile Phase
•Instrument Connections
•Detector Settings
•Metal Contamination
Start With the Correct Questions
•Find the Answers
•The Answers will Lead to Solutions
HPLC column problems are evident as
