
SAMPLE PREPARATION
FUNDAMENTALS
FOR CHROMATOGRAPHY
For more information
To learn more about the Agilent
Sample Preparation portfolio, visit
agilent.com/chem/sampleprep
To find your local Agilent Representative
or Agilent Authorized Distributor, visit
agilent.com/chem/contactus
Buy online:
agilent.com/chem/store
U.S. and Canada
1-800-227-9770
agilent_inquiries@agilent.com
Europe
info_agilent@agilent.com
Asia Pacific
adinquiry_aplsca@agilent.com
India
lsca-india_marketing@agilent.com
This information is subject to change without notice.
© Agilent Technologies, Inc. 2013
Printed in Canada November 13, 2013
5991-3326EN
SAMPLE PREPARATION FUNDAMENTALS FOR CHROMATOGRAPHY

Table of Contents
Chapter 1 Introduction....................................................................................................1
Chapter 2 Types of Samples and Overview of Approaches to Processing..........9
Chapter 3 General Considerations in Sampling ........................................................17
Chapter 4 The Sampling and Sample Handling of Solid Materials.......................20
Chapter 5 Filtration..........................................................................................................29
Chapter 6 Sample Introduction and Sample Preparation
of Volatile Organic Compounds .................................................................35
Chapter 7 Sample Pre-treatment for Liquid Samples ..............................................55
Chapter 8 QuEChERS, Salting Out Liquid-Liquid Extraction,
and Related Techniques ..............................................................................78
Chapter 9 Solid Phase Extraction (SPE)......................................................................94
Chapter 10 Special Topics in SPE...................................................................................133
Chapter 11 Size-Exclusion Chromatography
as a Sample Preparation Technique .........................................................160
Chapter 12 Column/Flash Chromatography
as a Sample Preparation Technique .........................................................170
Chapter 13 Column-Switching (On-Line SPE)
as a Sample Preparation Technique .........................................................172
Chapter 14 Sample Preparation Methods for Solid Samples...................................181
Chapter 15 Sample Preparation for Biological Samples............................................195
Chapter 16 Sample Preparation in Bioanalysis ...........................................................227
Chapter 17 Sample Pre-treatment for LC/MS.............................................................255
Chapter 18 Membrane Techniques in Sample Preparation ......................................269
Chapter 19 The Role of Scavengers in Sample Preparation.....................................281
Chapter 20 Derivatization for HPLC and GC Analysis ................................................286
Chapter 21
Just Enough
Sample Preparation:
A Proven Trend in Sample Analysis..........................................................
297
Chapter 22 Current Trends and Future Directions in Sample Preparation ............307
Chapter 23 Glossary ..........................................................................................................310
Index ................................................................................................................343
Matrix/Analyte Index...................................................................................351
Reliably extract and concentrate
samples from complex matrices
Sample preparation is an essential part of successful
chromatography. It extends column lifetime, reduces
the need for repeated samples, and minimizes
interferences that can jeopardize your separation,
detection, and quantification.
Agilent offers the most complete line of sample prep
products across the full spectrum of instrumentation.
These include:
• Pre-packaged QuEChERS kits – make sample
preparation faster, easier, and more reliable.
• Captiva Filtration products – improve both system
performance and analytical quality and prevent
extractables, proteins, lipids, or other contaminants
from interfering with the accuracy of your analyses.
• Chem Elut products – for supported liquid extraction
(SLE) reduce solvent usage and time over traditional
liquid-liquid extraction.
• Bond Elut SPE products – selectively remove
interferences and/or analytes from challenging
matrices. Choose from the largest selection of
sorbent formats on the market today.
www.agilent.com/chem/sampleprep

i
Since most samples encountered in a chromatography laboratory are not in a form to be directly placed into the
analytical instrument, some form of preparation is required for nearly every sample. The sample preparation could be
as simple as “dilute and shoot” or as complex as multistage sample handling. The analytical cycle represents all of the
steps from the point of collection to the final analysis and data output. Although sample preparation is an important
part of this analytical cycle, it doesn’t always get the respect as does the separation and measurement instrumentation
and the data handling aspects. Oftentimes, the task of sample preparation employs decades old technology that is
often manual, time-consuming and uses a lot of glassware and other devices, and some older technologies use copious
amounts of solvent that must eventually be disposed of, creating expense and safety issues. Because of the use of
multiple sample preparation steps in an attempt to simplify and/or isolate the desired analytes from a complex matrix,
errors tend to creep into the assay and analyte recoveries may suffer.
The purpose of this book is to outline some of the most popular sample preparation technologies in current use today.
Since sample preparation technologies is represented by tens of possible sample manipulations (e.g. weighing,
dissolution, extraction, trapping, etc.), I didn’t set out to cover every single sample prep category. The book started out
to be a small handbook like the popular The LC Handbook: Guide to LC Columns and Method Development (Publication
Number 5990-7595EN) but soon blossomed into a 350+ page book covering many different sample preparation
technologies. Since the book was written for Agilent Technologies, many of the methodologies covered are those within
Agilent’s chemistries portfolio, but for the sake of completeness, I have covered a number of technologies outside of
Agilent’s immediate areas of interest. Examples of applications are provided throughout the book and many of them
are web-accessible.
Since this book is primarily designed for the chromatography laboratory and to keep the length reasonable, I had to
confine my coverage to organic and biological sample preparation and thus inorganic sample prep, although important
in chromatographic and obviously the spectroscopic analyses of many sample types, was omitted. Many of the techniques
covered, however, such as ion exchange SPE, liquid-liquid extraction and microwave-assisted extraction are equally
applicable to inorganic samples for further analysis by ion chromatography or for spectroscopic measurements.
Preface

ii
The Chapters are further organized by sample types so that gaseous samples are first (Chapter 6) followed by liquid
samples (or samples put into a liquid form) (Chapters 7-12), solid samples (Chapter 14), biological samples (nucleic
acids and proteins)(Chapter 15), biological fluids/tissues (Chapter 16) and then special topics such as sample prep for
mass spectrometry, membrane applications, chemical scavengers, and derivatizations. Finally, Chapter 21 covers a
new concept of “Just Enough” sample preparation that seems to be today’s trend relying heavily on the increased use
of hyphenated-chromatography/tandem mass spectrometry techniques. To aid the novice (and maybe some of the
experts!) on the terminology associated with sample prep, the final Chapter 22 includes a Glossary.
I wish you good reading and hope that the material within provides you with a good foundation on how to best approach
your sample preparation challenges.
Ronald E. Majors, Wilmington, DE
I have written the book in a slightly different format than might be typical. After an introductory Chapter 1 on the
sample prep process, in Chapter 2, I decided to tabulate most of the methodologies that will be covered in the
remainder of the book. That way, the reader, rather than wading through all the various chapters, can get an overview
of possible sample preparation methods that are most applicable to gases, liquids, suspensions, gels and solid
materials. So the reader can get directly to the sample prep methodology that may suit his/her particular sample. In
subsequent Chapters, rather than repeating all the information, I refer back to these tables. The book is organized by
the flow of sample preparation process (sampling, transport, storage all the way up to sample filtration and, in some
cases, sample introduction).

iii
First and foremost, I would like to thank my wife, Carol, for giving up part of our retirement time allowing me to work
on this book and for her support during this process. I would also like to thank Helen Stimson, my boss at Agilent, for
allowing me to work ½ time combining my pending retirement with my passion for science, especially sample
preparation technologies. I would also like to thank the three Agilent Marcom ladies (Christine Cox, Anne Batchelor,
and, especially Nicole Goodman who managed the bulk of the effort) for helping me get this volume to press. Special
thanks go to Agilent colleagues: to Edward Elgart who proofread the entire book, to Gina Goggins for her helpful
suggestions and locating some of the figures to use as examples and to Dennis Blevins who gave excellent detailed
feedback on the technical aspects of most of the chapters. In addition, I would like to thank Agilent sample prep and
chromatography colleagues who read specific chapters and gave their feedback. This feedback helped to shore up the
usefulness of the information and with helpful suggestions on where improvements could be made: Trisa Robarge,
Nigel Simpson, Christophe Deckers, Bill Wilson, Jared Bushey, Limian Zheo, Michael Woodman, Sue Cohen, Tina
Chambers, Jennifer Massi, Derick Lucas and Bruce Richter and finally to Vanita Marshall who kept track of and contact
with all of the reviewers.
Last but not least, I would like to thank LCGC No. America for allowing me to use some of the published information
and artwork used in various articles over the years from my monthly columns, Column Watch and Sample
Preparation Perspectives.
Acknowledgements
Ronald E. Majors, Wilmington, DE


1
Introduction
1%
MeOH
Figure 1.1
Sample Analysis Workflow Diagram
Sample preparation
Report generation
Information to customer
Sample collection/sampling
Archiving
Sample transport
Data handling
Sample analysis
The major stages of an analytical process are depicted in Figure 1.1. The proper choice of the measurement technique is only one
step in the development of a successful application. All of the steps leading up to the measurement are as important. The sampling
and sample preparation process begins at the point of collection and extends to the measurement step. The proper collection of
sample during the sampling process (called primary sampling) is the initial contact with the sample and it is imperative that this
sample represents the entire lot being investigated. The storage, preservation and transport of this representative sample from the
point of collection to the analytical laboratory must occur without any changes in the physical and chemical makeup of the original
sample. The proper selection of the laboratory sample itself (termed secondary sampling) represents another challenge since the
final sample used for analysis may be a tiny fraction of the original collected sample, yet serves as a subset. Finally, the sample
preparation methodology necessary to convert the sample into a form suitable for the measurement step must also be performed
without loss or any unplanned modification of the secondary sample. All of these pre-analysis steps can have a greater effect in the
overall accuracy and reliability of the results than the measurement itself.
Chapter 1

2
A more detailed depiction of the various operations in the analytical cycle is summarized in Table 1.1. If particular attention is not paid
to all of these operations, sample integrity may be sacrificed and the analysis data affected, compromised, or rendered invalid. Steps
1-5, which include 1) sample collection, 2) storage and preservation, 3) sample transport, 4) preliminary processing and laboratory
sampling, and 5) weighing or dilution, all form an important part of sample preparation. Although these steps in the chromatographic
assay can have a critical effect on the accuracy, precision, and convenience of the final method, space limitations preclude us from
addressing all of these areas in detail. Only steps 1 and 4 (sample collection and preliminary sample processing) will be briefly
explained here. See References 1-4 for an explanation of steps 2, 3, and 5. The bulk of this book will be devoted mainly to Steps
6-9 of Table 1.1, which encompasses what is usually meant by sample pre-treatment or sample preparation (“sample prep”).
Sample preparation is an essential part of chromatographic and spectroscopic analyses. The process is intended to provide a
representative, reproducible, and homogenous solution that is suitable for injection into the column for chromatographic analysis,
or into an ICP-MS/atomic adsorption source, or into a cuvette or NMR tube for further characterization. The aim of sample
preparation is to provide a sample aliquot that (a) is relatively free of interferences, (b) will not damage the column or instrument
and (c) is compatible with the intended analytical method. In chromatography, the sample solvent should dissolve in the HPLC
mobile phase or be injectable into a GC column without affecting sample retention or resolution, the stationary phase itself, and
without interfering with detection. It is further desirable to concentrate the analytes and/or derivatize them for improved detection
or better separation. In spectroscopy, the sample solvent should be free of particulates, compatible with the spectroscopic source,
and be of the appropriate viscosity to flow into a nebulizer for on-line methods. Sometimes, depending on spectroscopic sensitivity,
preconcentration is needed and chromatography or liquid-liquid extraction is sometimes used prior to introduction of the sample
into the instrument.
Although many of the sample preparation protocols used in chromatography and spectroscopy are similar, it is beyond the scope of
this book to address the various differences between sample preparation procedures for these diverse methods. Therefore, we will
limit the topics in this handbook to the popular sample preparation methods for chromatographic analysis with emphasis on Liquid
Chromatography (LC)/High Performance LC (HPLC)/Ultra HPLC (UHPLC), and Gas Chromatography (GC).

3
Whereas GC and HPLC are predominantly automated procedures, sample pre-treatment is often performed manually. As a result,
sample pre-treatment can require more time for method development and routine analysis than is needed for the separation and
data analysis (see Figure 1.2). Sample pre-treatment may include a large number of methodologies, as well as multiple
operational steps, and can therefore be a challenging part of chromatographic method development.
Table 1.1
Sample Pre-treatment Options
Step Option Comment
1 Sample collection Obtain representative sample using statistically valid processes.
2 Sample storage and preservation Use appropriate inert, tightly-sealed containers; be especially careful with volatile,
unstable, or reactive materials; stabilize samples, if necessary; biological samples may
require refrigeration or freezing.
3 Sample transport The act of transporting the sample from the point of collection to the laboratory can be an
important step. Transportation conditions should maintain its integrity, samples should not
have rough handling, be dropped, or be allowed to be exposed to the elements; the
timing may be important for samples – undue delays may cause sample degradation
as in step 2 above.
4 Preliminary sample processing Sample must be in form for more efficient sample pre-treatment (e.g. drying, sieving,
grinding, etc.); finer dispersed samples are easier to obtain representative sample and
to dissolve or extract.
5 Weighing or volumetric dilution Take necessary precautions for reactive, unstable, or biological materials; for dilution,
use calibrated volumetric glassware.
6 Alternative sample processing methods Solvent exchange, desalting, evaporation, freeze drying, etc.
7 Removal of particulates Filtration, centrifugation, solid phase extraction.
8 Sample extraction Method for liquid samples (Table 2.4) and solid samples (Tables 2.2 and 2.3)
9 Derivatization Mainly to enhance analyte detection; sometimes used to improve separation, extra step
in analytical cycle adds time, complexity, and potential loss of sample (See Chapter 20).

4
Finally, method precision and accuracy is frequently determined by the sample pre-treatment procedure (see Figure 1.3), including
operations such as weighing and dilution. For all of these reasons, the development of a sample pre-treatment procedure deserves
careful, advance planning.
Data taken from Agilent Technologies survey
Sample processing (61%)
Analysis (6%)
Collection (6%)
Data management (27%)
Contamination (4%) Sample introduction (6%)
Chromatography (7%)
Integration (6%)
Instrument (8%)
Calibration (9%)
Sample processing (30%)
Columns (11%)
Operator (19%)
Data taken from Agilent Technologies survey
Figure 1.3
Figure 1.2
Time Spent on Typical Chromatographic Analysis
Sources of Error Generated During Chromatographic Analysis

5
A sample pre-treatment procedure should provide quantitative recovery of analytes, involve a minimum number of steps, and (if
possible) be easily automated. Quantitative (99+%) recovery of each analyte enhances sensitivity and assay precision, although
this does not mean that all of the analyte present in the original sample must be included in the final injected sample. For example,
in a given method, for a series of sample pre-treatment steps, aliquots of intermediate fractions may be used for further sample
preparation or for an intermediate injection. If recovery is not 100%, the sample pre-treatment must be reproducible. The use of
internal standards or standard addition are approaches to aid in better quantitation, when recovery is not complete. As implied in
Figure 1.3, a smaller number of sample pre-treatment steps plus automation reduces the overall time and effort required, and
decreases the opportunity for imprecision and accuracy errors by the analyst. Thus, depending on the chromatographic and
detection selectivity available, one should attempt to use as few sample pre-treatment steps as possible. In other words, sufficient
sample preparation should be performed to meet the goals of the analysis, dependent on selectivity available in other parts of the
analysis system (e.g. chromatography, detection), sufficient sample preparation may entail multiple steps and techniques. This
concept is thoroughly explained in Chapter 21.
Many sample preparation techniques have been automated, and usually appropriate instrumentation is commercially available.
Approaches to automation vary from use of a robot to perform manual tasks to dedicated instruments optimized to perform a
specific sample preparation technique. While automation can be expensive and elaborate, it is often desirable when large numbers
of samples must be analyzed and the time or labor per sample is excessive. The decision to automate a sample pre-treatment
procedure is often based on a cost justification, the availability of instrumentation to perform the task at hand, or in some cases,
when operator safety is involved (i.e. to minimize exposure to toxic substances or other possible health hazards). Sample
preparation instrumentation for automation will be briefly addressed here, but it is beyond the scope of this book to elaborate
further on the details of commercial instrumentation. Refer to textbooks on the subject listed under references 5-7.

6
As you embark on using the remainder of this book, there are many questions that you must consider before deciding which sample
preparation technique may be the best for your particular sample. Table 1.2 is a sample preparation worksheet that provides some
guidance in consideration of the goals of your analytical method and sample preparation including analyte and matrix questions.
Because there are so many terms associated with sample preparation, a Sample Preparation Glossary is provided in the Appendix
of this book. For abbreviations and definitions that you may encounter, please refer to the Glossary.
(Continued)
Table 1.2
Sample Preparation Worksheet
Sample Preparation Questions Example/Considerations Comments
What is analytical measurement technique? LC-UV, LC/MS, GC/MS, etc. Final sample must be compatible with
analytical technique.
What is your optimal analytical run time? 1 min, 10 min, .5 hour, longer Sample prep time may exceed run time; can
you batch samples?
What level of recovery is required to meet
LOD/LOQ?
100% only, less than 100% 100% recovery is ideal, but the more sample
prep steps you have, the greater opportunity for
loss; even so, RSD may still be acceptable at
lower recovery if “loss” is reproducible.
How do you plan to quantitate? External standard or internal standards? Are standards available? Can you find internal
standard(s) that are resolved from analytes of
interest? Do you need multiple internal
standards?
What is your required accuracy and precision? Consider both inter- and intra-day values For trace levels (e.g. sub-ppb) RSDs may be
greater than you expect; must determine
recovery, precision and accuracy at levels
expected in your samples (minimum of 3 levels).
What is the sample matrix? Organic, biological, inorganic, solid, semi-solid,
liquid, gel, gas, etc.
Must choose sample prep technique that can
selectively remove analytes of interest from
the matrix.
How much do you know about the
sample matrix?
Oil-based vs. aqueous-based, high salt content,
volatile, unstable; is the sample matrix polar or
non-polar, ionic, ionizable?
Must begin early to think about sample prep
technique to best differentiate the properties of
your analyte and matrix.

7
What is the sample volume/mass? Microliter vs. liter, mg vs kg, etc. Must have equipment and glassware available
to handle size of sample required.
What key interferences are endogenous
to the sample?
Are interferences more like matrix
or like your analytes?
May require more than one sample prep
technique for cleanup of interferences that are
similar to your analytes of interest.
What functional groups on your matrix,
interferences and analyte(s) of interest may
influence choice of sample prep technique?
Influences solubility, polarity, ionization states
(pKa)
Often don’t know the actual structures of
matrix and interferences to help make
rational decision.
What else is already known about
analyte(s) itself?
Water-octanol partition coefficients,
concentration range, chemical structure
May allow you to direct your attention to
capturing the analyte itself and rely upon the
chromatography and/or detection step(s) to
resolve it from any co-extracted interferences
and matrix components.
What is level of interference removal required
for analysis?
Depends on selectivity of chromatography
separation and detection
Must avoid ion suppression/enhancement
effects in LC/MS (and MS-MS); for UV and less
selective detectors, and other, must have better
sample prep and chromatography selectivity.
What sample pre-treatment steps
may be required?
Dilution, clarification, filtration,
pH adjustment, etc.
May be required for best overall selectivity, but
each additional step can lead to analyte loss
and affect accuracy/precision.
Is a concentration step required
for optimal analysis?
Solvent evaporation, purge and trap, etc. Concentration steps add time to analysis but
may be required to meet LOC/LOQ.
What solvent should the analyte(s) be in
for optimal analysis?
Avoid solvents that may cause UV interference,
MS ion suppression, GC stationary phase
compatibility, or non-volatile
Choice of solvents for sample prep final step
may be limited; can always evaporate to
dryness and re-dissolve in compatible solvent;
increases time and number of steps.
Sample Preparation Worksheet
Sample Preparation Questions Example/Considerations Comments
What resources are available for method
development and routine analysis?
High sample loads may require some level of
automation; do you have the right sample prep
tools available in your lab?
Sample prep may require more personnel since
it is often more labor intensive and time
consuming than the analytical measurement.

8
References
1.
Keith, L.H. (Ed.)
Principles of Environmental Sampling (ACS Professional Reference Book)
, 2nd Ed., American Chemical Society,
Washington, DC, 1996, ISBN-10: 0841231524 and ISBN-13: 978-0841231528.
2.
Pawliszyn, Janusz, (Ed.)
Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists
, Academic Press,
Waltham, MA, 2012, ISBN-10: 0123813735 and ISBN-13: 978-0123813732.
3.
Svarc-Gajic, Jaroslava (Ed.)
Sampling and Sample Preparation in Analytical Chemistry, (Chemistry Research and Applications:
Chemical Engineering Methods and Technology)
, Nova Science Pub Inc., Hauppauge, NY, 2011, ISBN-10: 1621002691
and ISBN-13: 978-1621002697.
4.
Conklin, Alfred R., Jr.
Field Sampling: Principle and Practices in Environmental Analysis
, Marcel-Dekker, New York, 2004.
5.
Moldoveanu, S.C.; David, V.
Sample Preparation in Chromatography (J. Chromatography Library)
, Elsevier Science, San Diego, CA, 2002.
6.
Wells, D.A.
High-Throughput Bioanalytical Sample Preparation: Methods and Automation Strategies
, Elsevier Science, San Diego,
CA, 2003, ISBN-10:044451029X and ISBN-13: 978-0444510297.
7.
Nickerson, B. (Ed.)
Sample Preparation of Pharmaceutical Dosage Forms: Challenges and Strategies for Sample Preparation
and Extraction
, Springer, New York, 2011, ISBN-10:1441996303 and ISBN-13:978-1441996305.

9
Sample matrices can be broadly classified as organic, biological or inorganic, and may be further subdivided into solids, semi-solids
(including creams, gels, suspensions, colloids), liquids, and gases. For nearly every matrix, some form of sample pre-treatment, even
if it is just simple dilution, will be required prior to chromatographic analysis.
Gaseous samples usually are analyzed by gas chromatography, rather than HPLC. Techniques such as canister collection, direct
sampling via sample loops, headspace sampling and purge and trap are used to collect and inject gases. In Chapter 6, we will
briefly cover those sampling/sample prep techniques primarily used for gas samples. Table 2.1 provides an overview of typical
sampling, sample introduction, and sample preparation procedures used for gaseous volatile samples.
Chapter 2
Types of Samples
and Overview of Approaches
to Processing
(Continued)
Table 2.1
Typical Sampling and Sample Pre-treatment Methods for Gaseous Samples
Method of Sample
Pre-treatment Principles of Technique Comments
Grab Sampling Gaseous sample is pulled into an evacuated glass, metal
bulb or canister, or by a syringe; gas can also be pumped
into plastic bag or other inert container.
Used mostly for volatile compounds in air; samples are
returned to laboratory and analytes are isolated and
concentrated by cold trapping techniques.
Solid Phase Trapping Gaseous sample passed through tube packed with
adsorbent (e.g. silica gel, activated carbon); trapped
analytes are eluted with strong solvent.
Used for semivolatile organic compounds in air; control of
gas flow rate is critical for trapping efficiency; watch for
aerosol formation, adsorbent overloading, and irreversible
adsorption of reactive analytes; popular sorbents include
silica gel, alumina, porous polymers (Tenax, polyurethane
foams), or carbon; chemical or physical complexing
reagents may be useful to improve trapping efficiency.
Liquid Trapping Gaseous sample is bubbled through solution that is a
good solvent for analytes; analyte has higher affinity for
solvent than it does for gas.
Flow rate should be low enough so as not to create
foams or aerosols; complexing agents may be added
to solvent to aid trapping; temperature can be lowered
for very volatile species; sometimes process IS
called “impinging.”

10
Headspace Sampling Sample (solid or liquid) is placed in a closed,
thermostated glass vial until equilibrium is established; at
equilibrium, analytes partition themselves between a gas
phase and the solid (or liquid) phase at a constant ratio;
gas phase is sampled and injected into GC for analysis.
Used primarily for determination of trace concentrations
of volatile substances in samples difficult to handle by
conventional GC techniques; sensitivity can be increased
by heating (<100 °C), salting out, adjusting pH, and other
means to shift equilibrium; sometimes water or solvent is
added to aid in sample dispersion and/or to free organics
from the matrix, especially for soils and sediments; can be
manual or automated.
Purge and Trap (Dynamic
Headspace)
Sample (solid or liquid) is placed in closed, thermostated
container and the headspace vapors are continually
removed by means of inert gas flow with subsequent
trapping of sample components by solid phase extraction
or cold trapping; then thermally desorbed into GC
injection port (thermal desorption).
Used when analytes are too low in concentration or have
unfavorable partition coefficients in static headspace (HS)
sampling (sometimes called gas phase stripping) can
provide more sensitivity than static HS by accumulating
the volatiles until concentration is sufficiently built up for
thermal desorption and GC analysis; can be manual or
automated.
Thermal Desorption Used in conjunction with purge and trap and solid phase
microextraction to concentrate volatile analytes; sorbent
is rapidly heated to transfer concentrated analytes to GC
by purge gas.
Typical adsorbent resins include Tenax TA, glass beads,
Carbosieve, Carboxen, and Carbotrap. Sorbent choice
based on specificity, breakthrough volume, water affinity,
bed volume, and range of organics sorbed/desorbed
from resin; can be cyrogenically cooled to trap volatile
organics.
Direct Thermal Extraction A form of dynamic headspace, but the sample is heated
(controlled) to much higher temperatures, up to 350 °C.
System must be constructed of fused quartz or fused
silica so that extracted analytes do not react with hot
metal surfaces; system cold spots should be avoided;
used primarily for semi-volatile compounds.
Pyrolysis Non-volatile large molecule sample (e.g. polymer, plant
fiber) is thermally degraded to cleave linkages and
produce smaller, more volatile molecules that are swept
into GC or to adsorbent trap (cyrogenic) for separation
and identification.
Degradations often have defined mechanisms and
sample may break apart in a predictable manner; can
lead to structural information about starting compound or
provide “fingerprints” for comparative profiles; pyrolysis
can be performed in inert or reactive atmosphere.
Solid Phase Microextraction
(SPME)
Fused silica fiber coated with polymeric stationary phase
is placed in headspace above sample or directly into
liquid sample; analytes diffuse and partition/adsorb onto
stationary phase; analytes are thermally desorbed by
placing fiber into GC injection port or displaced by means
of a liquid to a column for HPLC analysis.
SPME is an equilibrium sampling method and can be
used for gases, solids (Headspace) and liquids (direct);
various polymer coating formulations available (e.g.
polydimethylsiloxane (PDMS), polyacrylate, Carbowax-
divinylbenzene, Carboxen-PDMS); can work with small
sample sizes, is field transportable, and uses no organic
solvent; very volatile analytes can sometimes be lost and
quantitation is problematic.
Table 2.1
(
Continued)
Method of Sample
Pre-treatment Principles of Technique Comments
Typical Sampling and Sample Pre-treatment Methods for Gaseous Samples

11
Volatile analytes that are labile, thermally unstable, or prone to adsorb to metal surfaces in the vapor state are sometimes better
handled by HPLC. Trapping is required to analyze gaseous samples by HPLC. The gas sample is either (a) passed through a solid
support and subsequently eluted with a solubilizing liquid, or (b) bubbled through a liquid that traps the analyte(s). An example of
the HPLC analysis of a gaseous sample is the American Society for Testing Materials (ASTM) Method D5197-03 and United States
Environmental Protection Agency Method TO-11 for volatile aldehydes and ketones
1, 2
. In this example, an air sample is passed
through an adsorbent trap coated with 2,4-dinitrophenylhydrazine, which quantitatively converts aldehydes and ketones into
2,4-dinitrophenylhydrazones. The hydrazones are then eluted with acetonitrile and separated by reversed-phase HPLC.
Sample preparation for solid samples can often be more demanding. Samples that are solid (or semi-solid) must usually be put into
a liquid form – unless the volatile portion only is of interest and then headspace, purge and trap, or thermal desorption techniques
(covered in Chapter 6) are used to isolate, and perhaps concentrate, that portion of the sample. In some cases, the sample is easily
dissolved and is then ready for injection or further pre-treatment. In other cases, the sample matrix may be insoluble in common
solvents, and the analytes must be extracted from the solid matrix. There are also cases where the analytes are not easily
removable from an insoluble matrix because of inclusion or adsorption. If the solvent-extractable portion of a solid sample is of
interest, then techniques such as liquid-solid extraction, supercritical fluid extraction, microwave-assisted extraction, Soxhlet
extraction, or pressurized fluid extraction can be used (see Chapter 14). Here, the solid material is exposed to a solublizing liquid or
supercritical fluid (usually carbon dioxide, often doped with a polar solvent such as methanol), sometimes with added heat and/or
pressure. Sample components soluble in the liquid eventually are totally or partially leached out of the sample. Obviously, the more
porous the sample and the more finely divided the solid sample, the easier it is to extract components.

12
Table 2.2
If the entire solid sample is to be analyzed, more drastic dissolution techniques or stronger solvents may be required. For example,
a rock sample or metal may require digestion with a strong acid to completely solubilize it and then the liquified sample further
treated to isolate components of interest. Table 2.2 lists some traditional methods for the recovery of analytes from solid samples,
while Table 2.3 describes additional recent procedures. Once analytes have been quantitatively extracted from a solid sample, the
resulting liquid fraction can either be injected directly into the HPLC or GC instrument, or subjected to further pre-treatment. Chapter
14 provides more details on the extraction of solid samples while Chapter 16 provides information on the extraction of solid- and
semi-solid-biological samples, such as tissue.
Traditional Methods for Sample Preparation of Solid Samples
Method of Sample
Pre-treatment Principles of Technique Comments
Solid-Liquid Extraction Sample is placed in closed container and solvent is added
that dissolves/extracts/leaches the analyte of interest;
solution is separated from solid by filtration (sometimes
called “shake/filter” method).
Solvent is sometimes boiled or refluxed to improve
solubility; sample is in finely-divided state to aid leaching
process; sample can be shaken manually or
automatically; sample is filtered, decanted, or centrifuged
to separate from insoluble solid.
Soxhlet Extraction Sample is placed in disposable porous container
(thimble); constantly refluxing fresh solvent flows through
the thimble and dissolves analytes that are continuously
collected in a boiling flask (see Chapter 14).
Extraction occurs in pure solvent; sample must be stable
at boiling point of solvent; slow but extraction is carried
out unattended until complete; inexpensive; best for
freely flowing powders; excellent recoveries (used as
standard to which other solid extraction methods are
compared).
Homogenization Sample is placed in a blender or a mechanical
homogenizer, solvent is added, and sample is
homogenized to a finely divided state; solvent is
removed for further workup.
Used for plant and animal tissue, food, environmental
samples; organic or aqueous solvent can be used; dry ice
or diatomaceous earth can be added to make sample
flow more freely; small dispersed sample promotes more
efficient extraction.
Sonication Use of ultrasound to create vigorous agitation at the
surface of a finely divided solid material; direct method:
uses a specially designed inert acoustical tool (horn or
probe = sonotrode) placed in sample-solvent mixture;
indirect method: sample container is immersed in ultrasonic
bath with solvent and subject to ultrasonic radiation.
Dissolution is aided by ultrasonic process; heat can be
added to increase rate of extraction; safe; rapid; best for
coarse, granular materials; for indirect method, multiple
samples can be done simultaneously; efficient contact
with solvent.
Dissolution Sample is treated with dissolving solvent and taken
directly into solution with or without chemical change.
Inorganic solids (e.g. minerals, metals) may require acid
or base digestion to completely dissolve; organic samples
can often be dissolved directly in solvent; biological
samples may not fully dissolve; for many sample types,
filtration may be required after dissolution.

13
(Continued)
Compared to gases or solids, liquid samples are much easier to prepare for chromatographic analysis.
Table 2.3
Modern Extraction Methods for Solid Samples
Method of Sample
Pre-treatment Principles of Technique Comments
Pressurized Fluid Extraction
(PFE)/Accelerated Solvent
Extraction (ASE)
Sample is placed in a sealed container and heated to
above its boiling point causing, pressure in vessel to rise;
extracted sample is removed and transferred to vial for
further treatment.
Greatly increases speed of liquid-solid extraction process;
may be automated; vessel must withstand high pressure;
extracted sample is diluted and requires further
concentration; safety provisions are required because of
overpressured, high temperature solvents.
Automated Soxhlet
Extraction
A combination of hot solvent leaching and Soxhlet
extraction; sample in thimble is first immersed in boiling
solvent, then thimble is raised for conventional Soxhlet
extraction/rinsing with solvent refluxing and finally
concentration.
Semi-automated and automated versions available; uses
less solvent than traditional Soxhlet, solvent is recovered
for possible reuse; decreased extraction time due to two-
step process.
Supercritical Fluid (SF)
Extraction
Sample is placed in flow-through container and
supercritical fluid (e.g. CO
2
) is passed through sample;
after depressurization, extracted analyte is collected in
solvent or trapped on adsorbent followed by desorption
by rinsing with solvent.
Automated and manual versions available; to affect
“polarity” of SF fluid, density (vary temperature and/or
pressure) can be varied and solvent modifiers added;
collected sample is usually concentrated and relatively
contaminant-free because CO
2
volatilizes after extraction;
matrix affects extraction process; thus method
development may take longer than other modern methods.
Microwave-Assisted
Extraction
Sample is placed in a solvent in an open or closed
container and contents heated by microwave energy
causing a temperature rise and extraction of analyte.
Extraction solvent can range from microwave absorbing
(MA) to non-microwave absorbing (NMA); in MA case,
sample is placed in high pressure container and heated
well above its boiling point as in PFE/ASE; in NMA case,
microwave absorbing device placed container so solvent
is indirectly heated; safety provisions are required with
organic solvents in microwave oven (MA/NMA) and high
pressures of MA example.

14
Many HPLC analyses are based on a “dilute and shoot” procedure, where the solubilized analyte concentration is reduced by
dilution so as to not overload the column or saturate the detector. Often, liquid samples can be directly injected into a gas
chromatograph and the volatile compounds separated and detected. In some cases, non-volatile compounds of the liquid sample
may deposit in the injector, retention gap, or at the head of the column. Special GC conditions may be required to remove or
eliminate this possibility. An overview of popular sample preparation methods for liquids and suspensions are listed in Table 2.4.
Gas Phase Extraction After equilibrium, analytes partition themselves
between a gas phase and the solid phase at a constant
ratio; static headspace: volatiles sampled above solid;
dynamic headspace (purge & trap): volatiles sampled
by continuously purging headspace above sample
with inert gas and trapped on a solid medium, then
thermally desorbed into GC; membrane can be used
as interface between sample and flowing gas stream
for added specificity.
Headspace techniques used for volatile analytes in solid
samples; heat (usually <100 °C) can be applied to sample
to speed up volatilization process; sometimes water or
solvent is added to aid in sample dispersion and/or to free
organics from the matrix, esp. for soils and sediments;
both static and dynamic headspace techniques have been
automated; dynamic techniques are more sensitive;
microwaves have been used for heating.
Matrix Solid Phase
Dispersion (MSPD)
Technique uses bonded phase supports as an abrasive to
produce disruption of sample matrix architecture and as a
“bound” solvent to aid complete sample disruption
during the sample blending process.
Solid or viscous sample (approx. 0.5 g) is placed in
mortar with about 2 g of SPE sorbent (e.g. C18) and is
blended to homogenized mixture; sometimes solvent is
added to aid extraction process; blend is transferred to
column and analytes are eluted with solvent, sometimes
to an SPE layer for further cleanup prior to injection; a
“solid-solid” extraction process.
Method of Sample
Pre-treatment Principles of Technique Comments
Modern Extraction Methods for Solid Samples

15
(Continued)
Table 2.4
Solid Phase Extraction
(SPE)
Similar to HPLC, sample is applied to, and liquid is passed
through, a column packed solid phase that selectively
removes analyte (or interferences); analyte can be eluted
with strong solvent; in some cases, interferences are
retained and analytes allowed to pass through solid
phase unretained (Chapter 9).
Wide variety of stationary phases are available for
selective removal of desired inorganic, organic, and
biological analytes; specialty phases exist for drugs of
abuse, carbohydrates, catecholamines, metal ions, trace
enrichment of water, and many other classes of
compounds.
Liquid-Liquid Extraction Sample is partitioned between two immiscible phases
which are chosen to maximize differences in solubility;
interference-free analytes are then recovered from one of
the two phases (Chapter 7).
Beware of formation of emulsions – break them with
heat, addition of salt; values of K
D
can be optimized by
the use of different solvents or additives (such as buffers
for pH adjustment, salts for ionic strength, complexing
agents, ion pairing agents, etc.); many published
methods; continuous extractions for low K
D
values.
Dilution Sample is diluted with solvent which is compatible with
HPLC mobile phase or GC stationary phase; used to avoid
column overload, to decrease solvent strength, or for the
output signal to be within the linear range of detector.
To avoid excess peak broadening or distortion, dilution
solvent should be miscible with, and preferably weaker
than, the HPLC mobile phase; “dilute and shoot” is a
typical sample prep method for simple liquid samples
such as pharmaceutical formulations; for GC, too strong
or an incompatible solvent should be avoided to protect
coated stationary phases.
Evaporation Liquid is removed by gentle heating at atmospheric
pressure with flowing air or inert gas; vacuum is useful for
low volatility liquids.
Do not evaporate too quickly; bumping can lose sample;
watch for sample loss on wall of container; do not
overheat to dryness; best under inert gas like N
2
; rotary
evaporator works best; automated systems evaporation
systems available.
Distillation Sample is heated to boiling point of solvent and volatile
analytes in the vapor phase are condensed and collected.
Mainly for samples which can be easily volatilized; some
samples can decompose if heated too strongly; vacuum
distillation can be used for low vapor pressure
compounds; steam distillation is rather gentle since
maximum temp is 100 °C.
Microdialysis A semi-permeable membrane is placed between two
aqueous liquid phases and analytes transfer from one
liquid to the other, based on differential concentration.
Enrichment techniques such as SPE are required to
concentrate dialyzates; microdialysis is used for
examination of extracellular chemicals in living plant and
animal tissue, in fermentation broth; it has been used on-
line with microLC columns; dialysis with molecular-weight
cutoff membranes can also be used on-line to
deproteinate samples prior to HPLC; ultrafiltration and
reverse osmosis can be used in a similar manner.
Typical Sample Preparation Methods for Liquids and Suspensions
Methods of Sample
Preparation Principles of Technique Comments

16
References
1.
Compendium Method TO-11A: Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High
Performance Liquid Chromatography (HPLC)
, Center for Environmental Research Information, Office of Research and Development,
U.S. Environmental Protection Agency, Cincinnati, OH, 1999.
2.
Schneider, S.
Analysis of DNPH-Derivatized Aldehydes and Ketones Using the Agilent 1220 Infinity LC System with Diode Array
Detector
, Agilent Application Note #5991-1545EN, 2013.
Lyophilization Aqueous sample is frozen and water removed by
sublimation under vacuum.
Good for non-volatile organics; large sample volume can
be handled; possible loss of volatile analytes; good for
recovery of thermally unstable analytes, especially
biological analytes; inorganics can be concentrated.
Filtration Liquid is passed through paper or membrane filter or SPE
cartridge/disk to remove suspended particulates.
Highly recommended to prevent HPLC backpressure
problems and to preserve column life; keeps particulates
out of capillary GC columns (Chapter 5)
Centrifugation Sample is placed in tapered centrifuge tube and spun at
high force (thousands to hundreds of thousands times
gravity); supernatant liquid is decanted.
Centrifugation is used to remove particulates as an
alternative to filtration; ultracentrifugation normally not
used for simple particulate removal.
Sedimentation Sample is allowed to settle when left undisturbed in a
sedimentation tank; settling rate dependent on Stoke’s
radius.
Extremely slow process; manual recovery of different size
particulates at different levels, depending on settling rate.
Solid Phase Microextraction
(SPME)
see Table 2.1 see Table 2.1
Stir Bar Sorbent Extraction
(SBSE)
Analogous to SPME except, the phase ratio is much
larger; a magnetic stirring bar usually encased in glass is
coated with a polymeric stationary phase. The coated stir
bar is placed in a liquid or semi-liquid sample and
analytes diffuse and partition/adsorb onto the stationary
phase; after removal from the sample, stir bar is dried and
like SPME analytes are thermally desorbed in a special
desorption unit for GC or washed with an appropriate
solvent for HPLC.
SBSE is also an equilibrium method, but because of the
larger mass of sorbent on the coated bar, the sample
capacity (and hence, sensitivity) of this technique may
exceed the SPME technique by a couple of orders of
magnitude; SBSE requires a special larger volume
thermal desorption apparatus while SPME uses the GC
injection port for thermal desorption. The technique is
more difficult to automate than SPME.
Methods of Sample
Preparation Principles of Technique Comments
Typical Sample Preparation Methods for Liquids and Suspensions

17
Chapter 3
General Considerations
in Sampling
The first stage in the analytical cycle is sampling (see Figure 1.1). The objective of sampling is a mass or volume reduction from the
parent batch, which itself can be homogeneous or heterogeneous. Primary sampling is the process of selecting and collecting the
sample to be analyzed. At first glance, the sampling of gases and liquids seems trivial, while the sampling of solids can represent a
formidable task. Books have been written on the theoretical and practical aspect of the sampling process and how to collect a
statistically representative sample
1-4
. It is beyond the scope of this chapter to provide primary sampling theory and methodology,
but it suffices to say that it is one of the most overlooked sources of error in analysis.
Unfortunately, sampling is sometimes left to those unskilled in the proper methodology, and the analytical chemist in the
laboratory may not be directly involved in the process, yet is left to provide a precise and accurate analysis of the provided
sample. In the last several decades, sampling theory and practice have been largely ignored in the education process, especially
for non-analytical chemists.
It is advisable to develop a well thought-out sampling plan as part of the overall analysis. Equally important to note is that sample
information flow parallels sample flow throughout the analytical process from collection to report generation. For example, sample
tracking begins at the point of collection and can be considered as part of the overall analysis process. Proper identification of the
collected primary sample by handwritten labels, application of a bar code for automatic reading, writing on sample container with
indelible ink, incorporation of RFI devices, or other means of documentation must be performed properly to ensure that later stages
of processing can be traced unequivocally to the original primary sample. Likewise, each stage along the analytical process will
require proper sample and sub-sample tracking to ensure that Good Laboratory Practices (GLP) are achieved.

18
Once the primary sample is taken, it must be transported to the analytical laboratory without a physical or chemical change in its
characteristics. At first glance, this may seem to be a trivial task, but when the system under investigation is a dynamic entity, such
as samples containing volatile, unstable, or reactive materials, the act of transportation can present a challenge, especially if the
laboratory is a long distance from the point of collection. Even if a representative primary sample is taken, changes that can occur
during transport can present difficulties in the secondary sampling process. Preservation techniques can be used to minimize any
changes between collection and analysis. Physical changes such as adsorption, diffusion, and volatilization, as well as chemical
changes such as oxidation and microbiological degradation, are minimized by proper preservation. Examples of preservation
techniques that can be used between the point of collection to the point of sample preparation in the laboratory are:
•
Choice of appropriate sampling container
•
Addition of chemical stabilizers such as antioxidants and antibacterial agents
•
Refrigeration or freezing the sample to avoid thermal degradation
•
Adsorption on a solid phase
Once the sample has been brought into the laboratory, prior to analysis, storage conditions are equally important to maintain
sample integrity. For thermally labile or volatile samples, the samples should be kept in sealed containers and stored in a refrigerator
or freezer. Liquid samples should be kept in a cool, dark area (not exposed to sunlight) until ready for analysis. Samples that may be
prone to oxidation or other chemical reaction should be stored in a vacuum desiccator until it is time for further sample handling
and/or analysis.
For more information, references 4-7 address sampling and sample preparation of volatile samples, references 5-7 for water, air,
and soil samples, and references 5, 8, and 9 for biological samples. Often, prepared laboratory standards, surrogate samples, and
blanks are carried through the entire preservation, transport, and storage processes to ensure that sample integrity is maintained. A
recent trend in both the industrial and environmental analyses has seen the analysis moving closer to the sample or the process. For
example, portable field instruments are becoming more popular in the screening of environmental samples at the site of interest.
Likewise, the movement of analytical measurements to at-line or on-line locations may have a profound effect on how samples are
collected and analyzed in the future.
Sample Transport and Storage

19
References
1.
Svarc-Gajic, J. (Ed.)
Sampling and Sample Preparation in Analytical Chemistry, (Chemistry Research and Applications:
Chemical Engineering Methods and Technology)
, Nova Science Pub Inc., Hauppauge, NY, 2011, ISBN-10: 1621002691
and ISBN-13: 978-1621002697.
2.
Gy, P.
Sampling for Analytical Purposes
, Wiley, NY, 1998, ISBN-10: 0471979562 and ISBN-13: 978-0471979562.
3.
Pitard, F.F.
Pierre Gy's Sampling Theory and Sampling Practice, Heterogeneity, Sampling Correctness, and Statistical Process
Control
, 2nd Ed., CRC Press, Boca Raton, FL, 1993, ISBN-10: 0849389178 and ISBN-13: 978-0849389177.
4.
Stoeppler, M. (Ed.)
Sampling and Sample Preparation: Practical Guide for Analytical Chemists
, Springer, NY, 1997,
ISBN-10: 3642644864 and ISBN-13: 978-3642644863.
5.
Pawliszyn, J. (Ed.)
Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists
, Academic Press,
Waltham, MA, 2012, ISBN-10: 0123813735 and ISBN-13: 978-0123813732.
6.
Stoeppler, M. (Ed.),
Sampling and Sample Preparation: Practical Guide for Analytical Chemists
, Springer, Berlin, Germany, 2011,
ISBN-10:3642644864 and ISBN-13:978-3642644863.
7.
Pawliszyn, J.
Sampling and Sample Preparation in Field and Laboratory, Volume 37: Fundamentals and New Directions in Sample
Preparation
, Elsevier Science, San Diego, CA, 2002, ISBN-10: 0444505105 and ISBN-13: 978-0444505101.
8.
Ivanov, A.R.; Lazarev, A.V. (Eds.)
Sample Preparation in Biological Mass Spectrometry
, Springer, New York, 2011,
ISBN-10: 9400707584 and ISBN-13: 978-9400707580.
9.
Venkatesh Lyengar, G.; Subramanian, K.S.; Woittiez, J.R.W.
Elemental Analysis of Biological Samples: Principles and Practices
,
CRC Press, Boca Raton, FL, 1997, ISBN-10: 0849354242 and ISBN-13: 978-0849354243.

20
Chapter 4
The Sampling and Sample
Handling of Solid Materials
As indicated earlier, the sampling process is extremely important in order to ensure that the sample actually injected into the
chromatograph represents the original sample that was collected in the field, on the production line, in the patient’s body, or in the
atmosphere. In this chapter, we will provide a basic review of the sampling process and discuss ways to put the sample in a form
suitable for analysis.
Most solid materials occurring in the real world are heterogeneous. In almost every analysis, a representative sample of the solid
material must be taken in order to attain a satisfactory answer of its composition. The proper sampling of such materials represents
an area of great importance in analytical chemistry. Obtaining a non-representative sample initially can negate the analytical results
obtained later, no matter how careful the experimental work is performed. As shown in Figure 4.1, how does one represent a
large, non-uniform whole with a small solid sample needed for analysis? There are two approaches: 1) take minute quantities of
the solid material and blend them to represent the whole, or 2) take a quantity of material large enough to be compositionally
representative and reduce it to a fine homogeneous powder. In the first case, it is statistically difficult to obtain a representative
sample since so many small samples must be taken to get an adequate representation of the whole material that the time required
would take too long. The second approach is most often used to obtain a representative sample. During a mass-reduction stage,
every particle in the sample before mass-reduction should have an equal probability of being included in the sub-sample retained
after mass-reduction. A defined sampling plan is important to ensure the best possible sample of the original lot. The various
techniques for particle size mass-reduction of solid materials will be covered in this section.
Lot 10 t
Sample Pretreated
sample
Analysis sample – 5 g
The final sample used for analysis must be representative of the original lot
Sampling
Figure 4.1

21
It is usually desirable to render solid samples into a finely divided state for the following reasons:
•
Finely divided samples are more homogeneous, allowing more representative sub-sampling with greater precision and accuracy if
carefully mixed.
•
Finely divided samples dissolve faster and are easier to extract because of their greater surface area.
General methods for reducing the particle size or grinding of solid samples are outlined in Table 4.1. Sometimes, depending on the
particle size, a coarse reduction may be required initially, followed by a fine particle size reduction. For example, chopping a bunch
of carrots into sufficiently small pieces to place into a laboratory blender where they are reduced to a finely-divided, semi-solid is a
simplistic approach for the particle size reduction of soft samples. As the material gets harder and the initial particle size gets larger,
approaches that are more drastic are required. Laboratory crushers, mills, pulverizers, and grinders are more appropriate for these
samples. The choice of device is determined by the following parameters:
•
Type of material based on its hardness (Moh number for hard materials or degree of softness for samples such as tissue, plastics,
plant material, paper, leather, etc.)
•
Initial particle size (e.g., chunks or powder)
•
Final desired particle size (e.g., mm or micrometer)
•
Sample quantity or throughput required
•
What contamination may interfere with the subsequent analysis
Particle Size Reduction

22
Table 4.1.
Methods for Reducing Sample Particle Size of Solids
Particle Size Reduction
Method Description of How Sample Reduction is Performed
Blending A mechanical blender is used to chop a semi-soft substance into smaller parts; can also refer to the process of
blending a heterogeneous sample into a more consistent and uniform sample.
Chopping Process of mechanically cutting a sample into smaller parts.
Crushing* Tungsten-carbide variable-jaw crushers can reduce large, hard samples to smaller diameter particles.
Cutting Cutting mills can reduce soft-to-medium hard materials (<100 µm diameters).
Grinding* Manual and automated mortar and pestles are the most popular; both wet and dry grinding are used; particles
of approximately 10 µm diameters can be achieved.
Homogenizing Process of making a sample more uniform in texture and consistency by breaking down into smaller parts
and blending.
Macerating The process of breaking down a soft material into smaller parts by tearing, chopping, cutting, etc.
Milling* Disk mills pulverize <20 mm diameter hard samples by feeding between stationary and rotating disks with
adjustable gap settings; generally reduced to 100 µm in diameter. Rotor-speed mills combine impact and shearing
processes to grind soft-to-medium hard and fibrous materials down to 80 µm; balls mills grind material to sub-µm
fineness by developing high grinding energy via centrifugal or planetary actions using agate, tungsten carbide, or
PTFE-coated stainless steel balls; a soil mill will gently pulverize dried samples of soils, sludges, clays, and similar
material by rotating nylon brushes throwing a sample against a chamber wall.
Mincing The process of breaking down a meat or vegetable product into smaller parts by tearing, chopping, cutting,
dicing, etc.
Pressing Generally, the process of squeezing liquid from a semi-solid material (e.g. plants, fruit, meat).
Pulverizing* Electromechanically-driven rod or vibrating bases used to reduce particle size of wet or dry samples; freezer mill
can be used with liquid N
2
to treat malleable samples or those with low glass transition temperatures.
Sieving Process of passing a sample through a metal or plastic mesh of a uniform cross-sectional area (square openings
from 3-123 µm) in order to separate particles into uniform sizes; both wet and dry sieving can be used.
*The mechanical devices generate a considerable amount of heat and high shear forces that may be detrimental to the integrity of certain samples, such as poly-
mers, which may undergo molecular weight degradation.

23
Another choice is whether the sample must be reduced continuously or in batches. Of course, this choice is somewhat dependent
on the size of the initial sample, but there are cases where downstream continuous analytical measurement dictates continuous
particle size reduction. Most size reduction techniques are performed in a dry state, but for certain samples, such as those that tend
to agglomerate during grinding or may change their crystalline structure due to heating effect, wet (or slurry) grinding is an
alternative. Here, the particle size reduction is carried out in the presence of water or other liquid. Obviously, to use wet grinding,
the presence of liquid must not cause any chemical or physical-chemical alterations to the sample. Wet grinding is generally less
convenient and more time-consuming than dry grinding and requires leak-proof grinding containers. Sometimes other additives aid
the grinding process such as dry soaps, detergents, and graphite. Even special blends of an abrasive, lubricant, and binding agent
are available as grinding aids for specialized applications.
Since all particle size reduction involves some sort of abrasion, contamination by the grinding tools is always a constant threat.
Selection of a suitable set of grinding materials – with surfaces that are of similar composition to the sample or constructed with
materials that will not interfere with the analysis – is an important decision that must be made. Typical materials that make up particle
size reduction tools include stainless steel, tungsten carbide, agate, sintered alumina, hard porcelain, and zirconia. Obviously, choice of
a tool with a surface hardness greater than that of the sample is desired and will minimize sample contamination. The abrasion
resistance of grinding tools is important for wear. Grinding without losses of even minute amounts of sample is not possible because
some of the material adheres to the grinding surface. This material is lost during the cleaning process unless the grinding surface is
cleaned and washed after grinding and the solid particles suspended in the washing liquid are recovered.
Details on equipment available for reduction of particle size is beyond the scope of this section. Readers are referred to literature on
the subject
1-3
as well as manufacturer's literature. Companies active in this area include Retsch Technology (Haan, Germany), Spex
SamplePrep (Metuchen, NJ, USA), Fritsch (Idar-Oberstein, Germany), and Buehler (Lake Bluff, IL, USA), among others.

24
If the sample contains thermally labile or volatile compounds, it is important to minimize heating during the grinding process.
Sometimes, to keep the sample cool during grinding, dry ice can be added directly to a mortar or ball mill. Note that the dry ice
should be prepared from carbon dioxide that is free from impurities that might contaminate the sample. Some ball mills can be fitted
with a cooling block to permit the circulation of cool liquid during grinding. As mentioned earlier, when lower temperatures are
required to solidify the sample, pulverizing the sample under liquid nitrogen can be carried out in a freezer mill. The material may
then be sieved to further break it up and achieve a more homogeneous sample.
For air-sensitive samples, some grinding mills can be fitted with an enclosure that permits the introduction of inert gases during the
grinding cycle. There are mills where the grinding process can be carried out in a vacuum, if the material warrants this condition.
Equipment that is built to crush rocks and other hard samples must have large motors and use mechanical advantage with rotating
blades, rotors, wheels, belts, and gears to accomplish their task. Therefore, they always represent a safety hazard and one must be
very careful, especially when loading sample into the feed hopper. Manufacturers have tried to build safe products that minimize
operator exposure to hazardous conditions. Most doors and lids have safety interlocks and safety switches are installed to prevent
accidental startup if they have been left open or are opened during operation. Centrifugal devices usually have a built-in electronic
brake to slow down the rotor once the unit is turned off. Grinding jars/bowls and their holders are often held in place with clamps to
prevent their coming loose during the grinding process.
Dust is another possible hazard. Since grinding and pulverizing generates micron and sub-micron fragments, this material might get
into the air. Micron-sized particles are especially hazardous since they can easily be breathed in and can lodge in the lungs. Often,
manufacturers have built protection devices such as traps, filter tubes, and covers over the areas of suspected dust generation. It is
also important to prevent the loss of small particles on the surfaces of the tools used for grinding, pulverizing, etc. To recover such
fines, some rinsing of the surface may be required.
When using laboratory crushers, mills, pulverizers, and grinders for hard materials, ensure that the sample is fed in small amounts
rather than all at once to prevent overloading the drive motor. To prevent damage, most modern particle size reduction equipment
has overload protection circuits.
Practical Aspects of Particle Size Reduction

25
Secondary Sampling and Sample Size Reduction
Once the samples have been ground and mixed, there may be reasons to further sub-divide them for testing, evaluation, or analytical
purposes. To do this, ensure that the sample portions maintain the same sample integrity as the original. Once particle size reduction
has taken place, another problem arises. Although particle sizes are relatively uniform, there may be far too much sample. We may
have arrived at this point by the processes described in the previous section. Whatever the source and history of our laboratory
sample, the common problem is that there may be tens-, hundreds-, maybe even thousands of grams of sample. Depending on the
analytical technique, only tenths of a gram to grams can be analyzed, further sample size reduction must occur.
Sample size reduction is sometimes referred to as division. As already stated, truly representative sampling for analysis has long
been recognized as fundamental to the production of accurate and useful information. Understanding the importance of this step is
essential when attempting to perform any statistical analyses of a sampling and analysis procedure. Of course, few of us will
become involved in detailed statistical analyses of complex distributions of materials, but in the event that this does arise, references
1-3 provide a good starting point. The challenge is to divide the sample without discrimination with respect to size, density, or any
other characteristic of the particles.
Coning and quartering is one of the oldest methods for sample size reduction. As depicted in Figure 4.2, the finely-divided solid is
poured onto a clean flat surface (plastic sheet) from a fixed position and a cone of material forms in the middle while particles fall
away to each side, hopefully without bias. A quartering device is then applied to the highest point of the cone and pushed down
through it to the flat surface below:
B
A
C
D
Quartering Device
“Coned” sample of finely divided solid
Coning and Quartering
Figure 4.2

26
When the quartering device has been applied, again to avoid bias, diagonal sectors are retained (A and C, for example) and the
sectors between (B and D) are discarded. Portions A and C are combined and the coning and quartering process is repeated, often
several times, until a sufficient sample for the final analysis is obtained.
There is a very wide range of other mechanical constructs that have been devised to overcome the problems associated with
sample size reduction. The most popular types of devices are called rifflers. Riffling involves the separation of free-flowing sample
into equal parts using a mechanical device composed of diverter chutes. The simplest of these devices is the stationary riffle, but
more complicated devices are available. Both coning/quartering and riffling approaches are effective but laborious. Therefore,
automated laboratory sample dividers (e.g. proportional dividers such as straight-line samplers or spinning riffle sample dividers)
have been developed to divide granular powders into samples of identical quality and quantity. These laboratory dividers
accomplish the process by repeated dividing and pouring together. In this way, each particle in the sample is given an equal chance
at arriving in any collecting channel and the sample is truly divided. For further reading on these devices, consult references 1-3
and manufacturers' literature.
Once a sample is crushed and ground into smaller particles, it may be necessary to further classify the particles in the ground
sample. The most popular devices for particle sizing are mechanical sieves, which are classified by mesh size or size of the square
openings in millimeters. The particles are separated by using a series of sieves of known but differing mesh numbers. The mesh
number of a sieve determines the diameter of the holes in the sieve. A higher mesh number indicates a smaller diameter or finer
sieve. If your entire sample passes through a sieve with a particular mesh number, then you know for certain that you have no
particles larger than the diameter defined by that mesh number. If you are attempting to obtain particles of a particular mesh size
and the particles do not pass through a sieve of larger mesh size, you must regrind the sample until it passes. Standard sieves are of
metallic construction – all brass, stainless steel screen/brass frame, and all stainless steel – are round, but come in different
diameters. High tolerance, precision electroformed sieves are preferred, but much more expensive than the standard sieves. Non-
metallic sieves (e.g. nylon) that contribute no metallic impurities are also available.
Both dry sieving and wet sieving may be carried out. Water-jet sieving devices, where the water is forced into each individual sieve
in a stack, provides a rapid fractionation using standard testing sieves. For the tiniest particles down to 5 µm, wet sieving, using
water or solvent, can be performed in an ultrasonic sieving apparatus that is quite efficient for gentle fractionation of particulates
without further crushing. Automated sieve shakers simplify the sieving process and a wide variety is commercially available.
Further Particle Size Reduction and Classification

27
Sampling Error, Sampling Fraction, and Particle Size
The final key to sampling is getting a representative sample after particle size reduction. But how do we know if we have analyzed a
representative sample? The only sure way to know the answer to this is to take a large number of samples, do the multiple analyses
and find that – within experimental error, or some acceptable level of statistical significance – the same answer is obtained each
time. Sometimes we will be forced to do this, but usually this course of action is too exhaustive, time-consuming, and expensive.
As a guide to the overall outcome of our sampling effectiveness, make sure that we are operating within the limits that are
acceptable to us. Figure 4.3
4-5
provides the quantitative guidance that we need.
10
0.1(%)
1
1.0%
10%
0.1
90%
10
4
10
5
10
6
10
7
10
8
Percentage of original
material sampled
Sample Error %
Number of Particles in Sample
The first thing that Figure 4.3 conveys is common sense, but the process is very inconvenient. The solid lines that correspond to the
percentages of the original material sampled for analysis make it clear that the more sample we take, the smaller the sampling error
will be. Since we cannot work with kilograms, many grams – even hundreds of grams – for analysis, we are stuck on the 1.0% line
at best, and probably on the 0.1% line or worse. Therefore, we must follow the lines down to the right; that is, grind the sample up
as finely as possible to maximize the number of particles and minimize the sampling error for a fixed percentage taken. But how fine
is ‘as finely as possible’? To know where we are on Figure 4.3, we must know the particle size. Ideally, we must either measure it,
or have someone determine it for us. Alternatively, the manufacturers of your particle size reduction device may be able to point you
to data appropriate to your sample and procedure. With the particle size known, the number of particles may be estimated and we
have a fix on the abscissa of Figure 4.3. Then it is necessary to decide on the tolerable sampling error and get our ‘y coordinate’.
The percentage of the sample to take is then known.
Obviously, compromises will often need to be made. We may
need to go up by a factor of 2-3 in analytical scale if the
sampling error is unacceptably large for the minimum
particle size (maximum number of particles) we are able to
generate. Alternatively, for the solution to a long term
problem, investment in a different particle size reduction
device may be necessary. Or, perhaps a second stage of
particle size reduction is justified; e.g., grinding after
crushing. Data of the kind in Figure 4.3 allows us to
understand the limitations of the sample size reduction
process and to be in control of the outcome.
Percentage sampled, number of particles
and sampling error
4
Figure 4.3

28
Solid samples are often received for analysis in a damp or wet state. Removal of water and/or drying the sample to constant weight is
usually necessary for a reliable assay. Inorganic samples such as soil should be heated at 100-110 °C to ensure the removal of
moisture. Hydrophobic organic samples seldom require heating, since water absorption is minimal. However, organic vapors also can
be adsorbed by solid organic samples, and a heating step can remove these contaminants. For hygroscopic or reactive samples (e.g.,
acid anhydrides), drying in a vacuum dessicator is recommended. Samples that can oxidize when heated in the presence of air should
be dried under vacuum or nitrogen. Biological samples generally should not be heated to >100 °C, and temperatures above ambient
should be avoided to avoid sample decomposition. Sensitive biological compounds (e.g., enzymes) are often prepared in a cold room at
<4 °C to minimize decomposition. Such samples should be maintained at these low temperatures until the HPLC analysis step. Freeze
drying (lyophilization) is often used to preserve the integrity of heat-sensitive samples (especially biologicals). Lyophilization is performed
by quick-freezing the sample, followed by removal of frozen water by sublimation under vacuum.
Drying the Solid Sample
References
1.
Rhodes, M. (Ed.)
Introduction to Particle Technology
, 2nd Ed., John Wiley and Son, Chichester, U.K., 2008,
ISBN-978-0-470-01427-1 and ISBN-978-0-470-01428-8.
2.
Bhatt, B.; Agramal, S.S.
Size Reduction and Size Separation, Pharmaceutical Engineering, NISCAIR National Science Digital
Library
2008, URL: http://nsdl.niscair.res.in/handle/123456789/750/revised+size+reduction.pdf.
3.
Chen, B.; Nottingham, J.; Anderson, B.C.
Particle Size Reduction Studies on the Lab and Commercial Scale Using High and Low
Energy Mills, American Pharmaceutical Review
April 2012,
15 (3).
4.
Cross, R.F.
LCGC No. America
2000,
18 (5)
, pp 468-476.
5.
Hodges, R.J.
Analytical Sample Preparation – Problems for the Chemist
, presented to the Analytical Chemistry Group,
Royal Australian Chemistry Institute, Melbourne, June 1978.

29
Chapter 5
Filtration
Particulates should be removed from liquid samples prior to injection because of their adverse effect on column lifetime. In addition,
particulates can also affect HPLC hardware (e.g. flow lines, rotary injection valves, and inlet frits) and GC inlets, columns, and
detectors. The most common methods for removing particulates from the sample are filtration, centrifugation, and sedimentation.
Approaches to filtration are given in Table 5.1.
Table 5.1
Filtration
Media
Typical Products
Recommended
Use
Comments
Filter Paper Cellulose For removal of larger
particles (>40 µm)
Beware of filter paper fibers getting into sample; ensure solvent
compatibility of filter paper.
Membrane Filters Regenerated cellulose,
cellulose acetate, nylon, PTFE,
polypropylene, polyester,
polyethersulfone,
polycarbonate,
polyvinylpyrolidone
For removal of small
particles (>10 µm)
For “dirty” samples, prefilter or depth filter may be needed to
prevent plugging; porosities from 0.20 to 2 µm are the most
popular; avoid solvent incompatibility.
Functionalized
Membranes
Ion exchange membranes,
affinity membranes
Can remove both
particulates and matrix
interferences
For “dirty” samples, prefilter or depth filter may be needed to
prevent plugging; avoid solvent incompatibility; primary goal is
for removal of biomolecules from solution.
SPE Cartridges Silica- and polymer-based Particles of stationary phase may pass into filtrate; use only
high-purity products with low extractables; beware of plugging.
SPE Disks PTFE- and fiberglass-based PTFE membranes are delicate, so handle with care; fiberglass
disks are more structurally rigid; can pass large volume at
higher flow rate than with cartridges; beware of plugging.

30
Paper filtration (Figure 5.1A) is a relatively straightforward technique where a semi-permeable paper barrier is placed
perpendicular to the flow of liquid (and sometimes gas). In laboratories, filter paper is usually placed in a filter funnel, Hirsch,
or Buchner funnel. Paper filters come in a variety of diameters, shapes, and porosities. The lower the porosity of the filter medium,
the cleaner the filtrate, but the longer the filtering time. Vacuum filtration speeds up this process. Fluted filter paper (Figure 5.1B)
allows for faster flow since the wet paper is not pressed against the walls of a filter funnel. Filter paper can be pretreated
depending on its ultimate application. For example, for quantitative gravimetric analysis, ashless filter paper is used; for
base-sensitive samples, acid-washed filter paper can be purchased.
Filter
paper
Fluted
filter paper
Loose membrane
filter
Disposable
membrane filter
Membrane filters (Figure 5.1C) in a disk format can be purchased loose for placement into commercial filter holders/housings.
However, most users prefer disposable syringe filters (Figure 5.1D) equipped with Luer or mini-tip fittings. The filter bodies are
usually constructed with medical-grade polypropylene or polycarbonate housings, sterilized with gamma radiation, and are
designed with low extractables in mind. Some premium syringe filters are even batch-tested by LC or LC/MS and are certified for
highest purity. The last type of filter (Figure 5.1E) is the filter cartridge. Here, the membrane filter is placed at the base of a
syringe barrel and the setup resembles the popular solid phase extraction devices (see Chapter 9).
To use a syringe filter, the liquid sample is placed in a syringe and filtered through the membrane using gentle pressure. A variety
of membrane materials, nominal porosities and dimensions are available, and the manufacturers’ literature provides specifications.
Filter types should be selected based on sample volume, sample solvent, and size of particulates to be removed.
The large cross-sectional areas of the membrane disk type of filters allow for good flow characteristics and minimal plugging (see
Table 5.2). Typically, for larger volumes of sample (up to 50 mL), membrane filters with a diameter of 30 mm are sufficient. The
holdup volume (filtrate volume that may be lost during use) is only 50 µL. For smaller volumes (up to 30 mL), a 25 mm filter with a
moderate cross-sectional area (3.5 cm
2
) and less than 50 µL holdup volume should work. For even smaller volumes (up to 10 mL),
the 13 mm diameter membrane filters with holdup volume of less than 10 µL are highly recommended and represent the best
compromise between sample volume and holdup volume. For those that have the least amount of sample (less than 1 mL), 3 mm
filters are available.
AB C D E
Filter
cartridge
Popular Types of Filters for Particulate Removal
Figure 5.1

31
For most samples encountered in HPLC and ion chromatography, filters in the range of 0.20-2 µm nominal porosity are
recommended. The porosity values are approximate and the type of membrane can have some influence on the filtration range.
The most popular for sample filtration are the 0.20 and 0.45 µm porosities. Membranes with 0.20 µm pores remove the tiniest of
particulates (and large macromolecules). If the sample contains colloidal material or a large amount of fines, considerable pressure
may be required to force the liquid sample through the filter. Sometimes, for these types of samples such as soil extracts or
wastewater, a prefilter or depth filter bed is placed on top of the membrane to prevent plugging with samples containing these
types of particulates. These dual-layer filters are sometimes called 2-in-1 filters.
Typical high recovery membrane filters for use with most common solvents include regenerated cellulose (RA), cellulose acetate
(CA), cellulose nitrate, PTFE, and nylon. Regenerated cellulose is a universal filter because it can be used effectively with both
aqueous and organic sample solvents. It is also a good choice for polynuclear aromatic hydrocarbons (PAHs) and aqueous
bio-samples. Moreover, membrane filters such as regenerated cellulose and cellulose acetate provide the best sample
performance and lowest protein adsorption, giving excellent high sample recoveries for proteins in biological samples.
For biological samples, the spin filter format is quite popular. These filters consist of polypropylene centrifuge tubes with cellulose
acetate filter membranes that are used to remove particulate matter from samples such as serum, plasma, or protein samples
diluted in aqueous buffers through low-speed centrifugation (maximum 16,000 x g gravity).
An important consideration in membrane filter selection is the solvent compatibility with the membrane. Manufacturers of membrane
filters usually provide detailed information and tables on solvent compatibility of their products (see Table 5.3 for a typical
compatibility chart). If an inappropriate solvent is used, the filter may dissolve (or soften) and the filtrate becomes contaminated.
Table 5.2
Diameter Sample Volume Hold Up Volume Effective Filter Area
[mm] [mL] [µL] [cm
2
]
30 1-50 <50 5.1
25 1-30 <30 3.5
13 1-10 <10 0.75
3 <1 <7 0.0

32
Key
+ = Resistance
o = Limited resistance
-- = Not resistant
na = Not available
Table 5.3
Chemical Resistance Table for Membrane Filters
Substances Cellulose nitrate Cellulose acetate Regenerated cellulose Nylon 66 PTFE
1-Hexanol ++ +++
1,4-Dioxan -- -- + na o
Acetic acid, 10% & 25% +o +-- +
Acetone -- -- +++
Acetonitrile -- -- ++--
Aliphatic hydrocarbons ++ +++
Ammonia, 1 M ++ +++
Aromatic hydrocarbons ++ +na +
Benzene ++ +++
Boric acid ++ oo+
Carbon tetrachloride +o +-- +
Carboxylic acid ++ +-- +
Chloroacetic acid -- -- o -- +
Chloroform +o ++o
Cyclohexane oo +++
Cyclohexanol ++ +++
Diethylether oo ++o
Dimethyl formamide -- -- o++
Dimethyl sulfoxide -- -- o na +
Ethanol = or < 98% -- ++++
Ethyl acetate -- -- +++
Ethyl chloride oo +++
Ethylene glycol o+ +++
Formic acid, 25% +o +-- +
Hexane ++ +++
Hydrochloric acid, 25% ++ +-- +
i-Propanol ++ +++
Methanol -- ++o+
Nitric acid, 25% oo +-- +
Pentane ++ +++
Phosphoric acid, 25% ++ +o+
Phosphoric acid, 45% oo oo+
Potassium hydroxide, 1 M -- -- o -- +
Salt solutions, aqueous ++ ++o
Sodium hydroxide, 1 M -- o -- -- +
Tetrachloroethane +o +o+
Tetrahydrofuran -- -- ++o
Toluene ++ +++
Trichloroacetic acid, 10% + -- -- o+
Trichloroethane +o +o+
Trichloroethylene oo ooo
Xylene ++ +++

33
Although their main purpose is not for sample filtration, the more expensive, functionalized membranes and solid phase extraction
(SPE) disks and cartridges are not only used for chemical interference removal, but also serve to remove particulates.
For automation, particularly in high-throughput laboratories, a popular format is the 96-well flow-through filtration plate (see
Figure 5.2). Basically, this format, usually of polypropylene construction, provides an 8 x 12 array of small membrane filters.
Samples are usually loaded with a multi-pipette capable of handling a minimum of eight wells at a time. For very high-throughput
situations, 96-pipette capability is available for the simultaneous loading of samples by an automated x-y-z liquid handling station.
With the 96-well filtration plates, various membranes are also available in various porosities. For example, Agilent Technologies
Captiva 96-well plate filter materials are polypropylene (0.2, 0.45, and 20 µm porosity), polyvinylidene fluoride (PVDF), and glass
fiber (10 µm). A particularly useful 96-well plate for removal of proteins and lipophilic substances from plasma is the Captiva
ND
Lipids
, where ND stands for the non-drip membrane configuration. For more information on this plate, see Chapter 16.
When processing larger volumes of sample, or when an automation system is not available, Captiva filter cartridges (tubes) (see
Figure 5.1E) are available with the same functionality of the Captiva 96-well plates, but in a standard SPE cartridge format. The
3 mL volume depth filter cartridges are available with 0.2 µm and 0.45 µm porosity membrane filters. A particularly useful cartridge
filter design for clarifying highly particle laden samples, such as freshly thawed plasma, is the Captiva 10 µm glass fiber filter
cartridge. Such a design prevents sample transfer problems due to pipette tip clogging.
Figure 5.2
Captiva 96-well filtration plate mounted on vacuum manifold

34
The filter vial, a unique syringeless membrane filter, capable of handling 0.4-1.0 mL of sample, is pictured in Figure 5.3A. It is a
preassembled filtration device that replaces the combination of syringe filter, syringe, autosampler vial, septum and cap with a
single disposable unit. Filter vials consist of two main sections: a chamber and a plunger. The plunger design incorporates a filtration
membrane on the bottom end and a pre-attached cap with a septum on the top end. The unfiltered liquid is placed in the chamber
(bottom section) (Figure 5.3B) and, once the sample is added, the plunger is fitted into the chamber (Figure 5.3C). By pressing
the plunger through the liquid in the chamber, the positive pressure on the plunger assembly forces the clean filtrate up into the
reservoir of the plunger (Figure 5.3D). Air escapes through the vent hole until the evaporation seal is engaged, providing an
airtight seal. The filter vial can then be placed into any autosampler carousel that accommodates 12 x 32 mm vials for automated
injection into the instrument. The membranes include: PTFE, Polypropylene, Nylon, PES, Regenerated Cellulose, Cellulose Acetate,
and additional depth filters for highly particulate laden samples.
Septum
Plunger Cap
Plunger
Vial
Filtered Liquid
Fill Line
Membrane
Vial Chamber
Unfiltered Liquid
A
B
C
D
Figure 5.3
Filter Vial in Use

35
Chapter 6
Sample Introduction
and Sample Preparation
of Volatile Organic Compounds
Gaseous samples comprise a great number of the samples encountered in typical gas chromatography analyses. Volatile organics
are among the compounds most often analyzed in the petroleum, petrochemical, food, flavor and fragrances, and environmental
segments. In this chapter, an overview is provided of the methods that are used to collect and prepare such samples for introduction
into the gas chromatographic instrument and occasionally into HPLC systems.
One can divide front end of the analysis of volatile samples into sampling (sample collection) techniques, sample preparation
techniques, and sample introduction (injection) techniques. It is useful to distinguish between these three areas of sample handling
prior to analysis. As discussed earlier in Chapter 3, sampling is not only the act of obtaining the sample, but preserving its integrity
prior to sample introduction or sample preparation. For example, capturing a volatile sample by use of an impinger or metal canister
would be a sample collection step. In this chapter, sampling techniques will be briefly covered. Sample introduction is the process of
introducing the sample directly into the GC without any prior treatment. For example, the direct injection of a gas into the injection
port of the GC would be a sample introduction process. Similarly, for a liquid sample, if a large injection volume was used via a
programmed temperature vaporizing (PTV) inlet, this would be considered a sample introduction technique. Sampling of the
headspace above a solid sample with a syringe with a direct injection into the GC, likewise, would be a sample introduction
procedure. A sample preparation procedure would be a modification or treatment of the sample prior to sample introduction for
the purpose of improving the introduction process into the GC. For example, performing a dynamic headspace process with an
intermediate cold adsorbent trap to concentrate the analyte(s) of interest prior to sample introduction into the GC would constitute
a sample preparation procedure. Derivatizing an adsorbed polar compound on a solid material, thereby releasing it into vapor
phase for subsequent sampling, would be a sample preparation process. Whatever you call it, it is most important that the
components of interest be transferred from the point of collection to the GC column without being lost or modified.

36
Sample Inlet Systems for Gas Chromatography
There are entire chapters of books
1-3
devoted to detailed discussions about the various inlet systems in gas chromatography. Only
the highlights will be reviewed here. The most common method of introducing liquid samples into a gas chromatographic inlet is by
means of a microsyringe through a self-sealing rubber septum. Gas-tight syringes are also available for injecting gases and vapors,
but, in general, gas sample valves are more reproducible and are often preferred. Using a loop or a fixed internal volume, gas
sampling valves can also provide a convenient method of automation or when directly analyzing gaseous streams. In all cases,
the objective is to get the volatile sample into the column rapidly and in as small a volume as possible.
In GC, there are two general classes of inlets: 1) hot or vaporizing inlets or 2) cold, cool inlets. The temperature of hot inlets is
generally 50 °C above boiling point of the sample solvent; while cold inlets are below the boiling point of the solvent perhaps at
room temperature, or even below, to condense the sample followed by heating to completely evaporate the sample during the run.
Hot inlets immediately vaporize the sample so that it quickly gets to the column entrance. Too high of a temperature, however, may
degrade the sample and too big of the sample volume may generate too much vapor and overload the finite volume of the inlet
system. Flashback is a phenomenon where these excess vapors may reach the top of the inlet and condense on the cool septum,
back diffuse into carrier gas lines, and condense on cool services, or exit through the septum purge line, leading to undesirable
sample introduction effects.
The remainder of this chapter will be devoted to the sample and introduction techniques for volatile organic compounds (VOCs).
Semi-volatile organic compounds can also be handled by several of the techniques discussed in this Chapter, but can also be
prepared using those techniques (e.g. liquid-liquid extraction, SPE, etc.) covered throughout this handbook.
In Chapter 2, Table 2.1 provided an overview of the most popular sampling and sample preparation methodologies used in GC
laboratories. Sometimes these techniques are used in combination. For example, dynamic headspace is a combination of
headspace sampling and adsorbent trapping where a gas is used to sweep the headspace of a solid or liquid sample to concentrate
volatile analytes on a solid sorbent that is eventually heated to thermally desorb the analytes into the GC column. Solid phase
microextraction (SPME) may be used to sample a headspace with subsequent thermal desorption of the analyte(s) in the injection
port of a GC.

37
Cool inlets minimize sample degradation and sample discrimination since all the sample enters the inlet at low temperature. Table 6.1
provides a comparison of the different types of inlets used in gas chromatography. The most popular capillary column inlet is the
split/splitless inlet. It can be used in the split mode to reduce the amount of sample reaching the column and produces very narrow
initial peak widths. It can also be used in the splitless mode to maximize sensitivity. Split inlets are vaporizing inlets. The sample is
evaporated in the inlet, flows down the liner, and is split between the column and a split vent. The “split ratio” (e.g., the amount of
gas going into the column versus the amount of gas being vented through the split line) determines the amount of sample going onto
the column. At high split vent flow rates, the sample is exposed to the high inlet temperature for a short period of time and is quickly
introduced to the column.
In the splitless mode of injection, the split vent of the split/splitless inlet is turned off. Normally, the entire sample vapor enters the
column, but due to the low flow rate in the capillary column, initial sample bandwidth is fairly broad. Usually, some form of sample
focusing at the head of the column must be used.
Table 6.1
GC Inlet Comparisons
Inlet Advantages Disadvantages
Packed-Column Direct Vaporizing inlet, likely used with packed columns, easiest
to use, least expensive
Not compatible with capillary columns
(maybe 0.53 mm id), possible degradation of sample,
possible needle discrimination
Split/Splitless: Split Mode Simple, universal capillary column inlet, rugged, ideal for
auxiliary samplers, protects column
Sample discrimination and sample degradation possible
Split/Splitless:
Splitless Mode
High sensitivity capillary column inlet, protects column Highest sample degradation and flashback, sample
discrimination possible, requires optimization,
maintenance required
Cool On-Column Sample deposited directly into column (therefore high
sensitivity), least sample discrimination, most inert
Sample overload possible, difficult to use with columns
less than 0.25 mm id, complex to automate
Programmable Temperature
Vaporization (PTV)
Cool injection, most flexibility, low sample degradation,
high sensitivity
Complex, many parameters to optimize, most expensive,
possible loss of volatiles (venting mode)
Adapted from Reference 1

38
The PTV inlet was developed by Poy and co-workers
4
, based on the earlier work of Vogt
5,6
, who first described programmed
temperature sample introduction. The sample was introduced into the inlet liner at a temperature slightly below the boiling point
of the solvent. The low boiling solvent was continually evaporated and vented through the inlet split line. By introducing the
sample at a low initial liner temperature, many of the disadvantages of the classical hot injection technique could be circumvented.
For example, discrimination due to differences in boiling point is minimized by introducing the sample onto a “cool” liner and
subsequently raising the temperature of the liner to the normal temperature of a conventional hot inlet. Compared to hot inlet
injections, reduced thermal degradation of sensitive compounds occurs due to the slower heating.
To illustrate the principle of the PTV inlet, Figure 6.1 provides a schematic drawing of the Agilent Multimode Inlet. As can be seen
from this figure, the PTV inlet closely resembles the classical split/splitless inlet. The present design allows several injection mode
possibilities: hot split/splitless, cold split/splitless (pulsed versions of these), Large Volume Injection (LVI)-solvent vent (an extension
of cold splitless, see next section) with solvent vent timing. The programmable injection slows solvent evaporation and maximizes
analyte transfer into the column. The carrier gas is connected through the septumless sampling head and enters the liner at the top.
Temperature control on the liner is the key to the operation of the PTV inlet. The liner can be rapidly heated (heating coil) or cooled
(peltier-, liquid nitrogen- or liquid carbon dioxide-cooling). In the injection mode, the sample is introduced by a variable-speed
injector at a controlled speed and the inlet conditions are arranged so that the solvent is vented via the split line while the
components of interest are trapped and preconcentrated.
Programmable temperature vaporizing (PTV) inlets are a combination of split/splitless inlet and cool on-column inlet. They combine
a cool injection inlet, temperature programming, and have a split/purge vent and a timer/controller unit. Non-volatile sample
complements are deposited in the inlet and do not migrate to the column; thus, dirty samples tend to cause less problems with PTV
injections. During sample injection, the sample is deposited into a packed liner and the inlet temperature is raised, evaporating the
solvent and sample components. When evaporated, the split vent is electronically controlled to be on or off, or some sequential
combination of the two. Thus, the inlet allows a split or splitless injection in combination with temperature programming to optimize
sample transfer, solvent venting, and sample discrimination based on boiling point, solute degradation, and ghosting during the run.
The cool on-column inlet allows direct deposition of liquid sample into the column. Both the inlet and the column are below the
boiling point of the solvent during the injection process. Then, both temperatures are increased to vaporize the sample and begin
chromatography. Cool on-column inlets typically have a low thermal mass to facilitate past heating and cooling at the end of the
run. The challenge, of course, is to deposit the sample directly inside the small diameter capillary column. As might be expected,
cool on-column injection is preferred for labile compounds in samples with a large boiling point range. Sample discrimination and
decomposition is minimal. Because the sample is deposited directly into the column, sensitivity is quite high. Cool on-column inlets
are used for the analysis of samples with a wide boiling-point range, or those that are thermally sensitive, and for trace analysis.

39
Large Volume Injection (LVI) Techniques
The demand for lower detection limits is important in many areas of gas chromatography, particularly environmental, food, and
biological analysis. Concentration of samples, by many of the techniques described in this book [e.g. liquid-liquid extraction (Chapter
7), solid phase extraction (Chapters 9-10), and pressurized fluid extraction (Chapter 14)] is one way of increasing method sensitivity
by enriching analyte concentration. The development of improved and more selective GC detectors (e.g. electron capture detector,
tandem mass spectrometry) is one approach in enhancing both selectivity and sensitivity. The lowering of the system background by
providing more inert surfaces throughout the entire GC system can also provide lower limits of detection.
Detection limits can also be improved by injecting larger amounts of analyte solution into the GC injection port. However, typical
capillary columns may only accommodate a few microliters at best. Injection principles such as “pulsed” splitless injection allow up
to 5 µL injections. Going beyond this injection volume can lead to system contamination, non-reproducible results, flashback, and
sample loss.
Schematic of Agilent Multimode Inlet
Figure 6.1
Column Interface
Heating Coils
Cryo Inlet
Split Vent
Inlet Liner
"Turn Top" Weldment
Septum

40
Compared to the PTV inlet, with the COC-SVE there is an increased possibility to contaminate the precolumn since non-volatile
compounds remain in the precolumn and may influence later separations. Thus, the precolumn may need more frequent
replacement. This inlet is primarily used for trace analysis in clean matrices such as drinking water extracts. The COC-SVE inlet
is useful for early eluting compounds and has minimal degradation of unstable components. Both the PTV inlet and the COC-SVE
inlet show minimum sample discrimination.
With modern autosamplers, such as the Agilent 7693, fully programmable, controlled-speed liquid sample injection rate is now
possible and is the preferred approach to perform multiple injections.
In COC-SVE, the COC inlet has a precolumn assembly consisting of a retention gap/precolumn combination placed ahead of the
analytical column. A vent line is attached at the juncture of the precolumn and analytical column as seen in Figure 6.3. Liquid
sample is injected into the precolumn and the solvent evaporates and exits through the vent. Conditions are set that there is a
small volume of solvent remaining in the precolumn. Sample components including volatiles are concentrated in this small
volume of solvent. After evaporating the bulk of the solvent, the vent is closed and the normal oven temperature ramp is started.
The remaining sample is then evaporated and transferred to the analytical column for separation. The entire process can be
automated with most GC units.
The technique of PTV was discussed in the previous section. In some injector configurations, a controlled number of multiple
injections from an autosampler can be used to provide a large sample to the column. To illustrate this approach, Figure 6.2
provides an example of the use of multiple injections as a way of introducing larger sample volumes into the inlet
8
. Using a packed
liner, 25 µL of total sample containing pesticides was injected in five 5 µL portions. Smaller portions ensure that a large volume of
liquid does not run down the liner and enter the column, possibly causing overload, peak splitting, and possible damage to the
stationary phase. Figure 6.2 demonstrates that a good response is obtained from a 0.01 ppm mixture of pesticides.
In the PTV inlet, non-volatile sample components and degradation products remain behind in the inlet, minimizing column
contamination. Thus, the PTV inlet is a good choice for dirty samples compared to the cool on-column and split/splitless inlets.
It is not a good choice when the sample contains highly volatile analytes of interest since they may be vented with the solvent.
A rule of thumb is that the compound should boil at least 100 °C above the solvent to be successfully handled with the PTV inlet.
Large volume injection (LVI) is an approach to lowering detection limits. With LVI, the bulk of the solvent is evaporated before
transfer of the sample to the analytical column. There are two popular LVI techniques to eliminate solvent: programmed
temperature vaporization (PTV) and cool on-column injection with solvent vapor exit (COC-SVE). Both lead to greatly improved
detection limits and good chromatography with injection volumes of 500 µL or more
7
.

41
0
5 678910 11 12 min
5
4
3
2
1
6
7
8
9
10
11
12
5 x 5 µL Injections
1. Methamidophos
2. Acephate
3. Dimethoate
4. Diazinon
5. Chlorothalonil
6. Chlorpyrifos-Methyl
7. Chlorpyrifos
8. Thiabendazole
9. Imazelil
10. Ethion
11. Phosmet
12. Azinphos-methyl
Pesticides: 0.01 ppm
Inlet
Retention Gap Precolumn
Bleed Restrictor
Solvent Vent
Vent Valve
Detector
Analytical Column
Figure 6.2
Large Volume Injection with Solvent Elimination PTV
COC Injection with SVE-Hardware Configuration
Figure 6.3
PTV Conditions:
Vent flow: 300 mL/min
Purge flow: 50 mL/min at 3.5 min (gas saver on at 4.7 min)
PTV initial temp: 20 °C
PTV initial time: 1.1 min
PTV rate: 700 °C/min
PTV final temp: 300 °C
Injection delay: 0.00 min
Injection volume: 25 µL (5 x 5 µL each)
Column: HP-5ms, 30 m x 0.25 mm x 0.25 µm
Instrument: HP 6890 with Electronic Pneumatics Control
and PTV Inlet;
HP G1916A Automatic Liquid Sampler with HP
G1513A Controller;
HP1707A Chemstation (ver.A.04.02)

42
Headspace Sampling Techniques
Headspace refers to the vapors that form over liquids and solids. If the sample is in thermodynamic equilibrium with the gas phase in
a closed thermostated vessel, this method of analysis is referred to static headspace sampling. If an inert gas is passed through or
over the sample and the stripped sample volatiles accumulated in an adsorbent trap or cryogenic trap, then the method is referred to
as dynamic headspace or purge and trap. Since only the volatiles are sampled, headspace analysis is ideal for dirty samples (e.g.
blood, plastics, cosmetics), solid materials, samples with high boilers of no interest, samples with high water content, or samples that
are difficult to handle by conventional chromatographic methods. These matrices remain behind since only the volatile portion of the
sample is in the headspace. In headspace sampling, calibrations are performed by preparing a solution of the analytes in a volatile
solvent then injecting a small known amount into the closed headspace container so that the entire solution is vaporized.
Static headspace is basically a very simple sampling technique. The sample is placed in a glass vial of appropriate size that is
closed with an inert septum. The vial is carefully thermostated until equilibrium is reached. A sample is taken either manually with
a syringe or automatically with a headspace autosampler. Balanced-pressure headspace autosamplers have provision to
pressurize the vial at a constant pressure equivalent to the column inlet pressure for better quantitation and an absence of
baseline disturbance upon injection.
However, for valve and loop systems, such as the Agilent 7697, the pressurization option is not required nor always desired.
A gas-sampling valve transfers the headspace sample to the GC instrument. With capillary columns, the sample volume injected
must be limited with headspace samples just like injected liquid samples. Thus, flow splitting may be used to transfer a fraction of
the sample. Cryogenic cooling is also a possible solution for larger headspace injection volume where thermal focusing will allow
vapor concentration.
Static Headspace

43
Static headspace is simpler than the purge and trap method in that the headspace instruments are less complex, high
concentrations of water in the sample do not affect the analysis as much, adsorbent traps are not required, there
is no chance of breakthrough of volatile sample components, and repeated sampling can be done. Samples may be heated to
increase the volatility of analytes. In aqueous solutions, the sensitivity can also be increased in some cases by adjusting the pH (shift
equilibrium to undissociated form of an acidic or basic substance) or use salting out to increase the vapor pressure of analytes and
reduce the solubility of the organic in water. For solid samples, increasing the surface area by grinding will increase diffusion of
volatiles out of the matrix. For some samples, the addition of water will cause a competition for active sites and displace certain
organics from the solid surfaces thereby raising their concentration of volatiles in the headspace. Sometimes, derivatization
reactions are used for compounds containing reactive functional groups to generate more volatile compounds, which causes them
to volatilize into the headspace. For example, the formation of methyl esters of carboxylic acids may release the acids from a solid
material where they are tightly held by polar forces. For more detailed information on static headspace sampling for GC, see
reference 10.
An interesting variation of the static headspace approach is the use of multiple extractions from the same vial using multiple
headspace extraction (MHE) systems, which is similar but not synonymous with the purge and trap technique (see Purge and Trap
Sample Preparation). The MHE approach is an absolute quantitative method used in static headspace GC. In principle, it is dynamic
headspace extraction carried out stepwise and establishing equilibrium conditions in each step. The concentration of the analyte in
the headspace decreases exponentially during the series of extraction steps; by proper mathematical extrapolation, the total peak
area proportional to the total amount of analyte present in the original sample can be obtained. MHE is most often used to
determine the amount of analyte present in a complex matrix where calibration standards in that given matrix are not feasible.
Two examples, the determination of leachable compounds in packaging materials
11
and residual monomers in polymers
12
, illustrate
the use of MHE in some actual samples. The reader is directed to references 13-15 for further information on this technique,
including some of the mathematical calculations.
Figure 6.4 illustrates a typical example of the use of headspace sampling for the analysis of blood alcohol. Blood is a difficult
substance to work with and thus headspace sampling of a volatile analyte, such as ethanol, is a very convenient and simple-to-use
method. In many countries of the world, an ethanol concentration of greater than 80 mg/100 mL of blood is considered to be the
level of intoxication, while in some countries the allowable concentration is even lower. After headspace sampling in a 20 mL
headspace vial using a headspace autosampler followed by GC analysis, the simple chromatogram in Figure 6.4 shows the
overlapped chromatograms of blank blood and ethanol-spiked blood
9
. The internal standard was t-butanol. A five-level calibration
curve of ethanol standards showed excellent linearity over the concentration range of 10 mg/100 mL to 160 mg/100 mL. Over that
concentration range, the repeatability was quite good: the average retention time %RSD was 0.03 while average peak area for
ethanol gave a value of 4.6%.

44
pA
1600
1400
1200
1600
800
600
400
200
0
0.5 1.0 1.5 2.0 2.5 min
t-Butanol (STD)
Ethanol
Standard Spiked Blood
Blank Blood
GC Conditions:
Column: DB-ALC2 0.32 mm x 30 m,
1.2 µm (P/N 123-9234)
Column flow (N
2
): 12 mL/min, constant flow
Data aquisition rate: 20 Hz
Headspace Conditions:
Oven temp program: 40 °C (7 min)
Temperature: 250 °C
FID setting: H
2
flow: 40 mL/min
Air flow: 400 mL/min
Makeup (N
2
): 45 mL/min
Temperatures: Oven: 85 °C
Loop: 85 °C
Transfer line: 100 °C
Times: GC cycle time: 15 min
Press equib time: 0.1 min
Vial equib time: 15 min
Inject time: 0.5 min
Vial: Fill mode: Flow-limited press
Ramp rate: 20 psi/min
Fill pressure: 15 psi
Final pressure: 10 psi
Fill flow: 50.00 mL/min
Final hold: 0.05 min
Fill mode: Advanced
Vent after extraction: No
Inlet settings: 200 °C, split ratio: 10:1
Figure 6.4
Overlapped Headspace Chromatograms
of Blank Blood Sample and Spiked Blood Sample

45
In Chapter 10, the SPME technique will be explored in more detail, but an adaptation of the classical headspace experiment can be
performed by its use, which will be covered here. SPME is a simple, solvent-free extraction technique that provides high sensitivity,
excellent reproducibility, and low cost
16,17
. In this technique, a phase-coated fused silica fiber is exposed to the headspace above the
liquid or solid sample. Analytes adsorb to the phase and then are thermally desorbed in the injection port of a gas chromatograph and
transferred to a capillary column. In this technique, selectivity can be altered by changing the phase type or thickness according to the
characteristics of the analytes. For example, the small distribution constants and low polarity of chlorinated and aromatic volatile
organic compounds in environmental samples dictate the use of a thick, non-polar phase for efficient extraction. Multi-layer fibers are
available for samples that contain a wide variety of analytes with varying distribution coefficients.
There are numerous examples of the use of SPME headspace sampling solving “real world” analytical problems. Investigators in a
crime laboratory developed a simple, inexpensive, rapid, and sensitive method for analyzing gasoline in fire debris
18
. A 20 minute
static headspace sampling using a 100 µm polysiloxane-coated SPME fiber resulted in acceptable chromatograms from as little as
0.04 µL gasoline compared to a 0.1 µL volume required for regular headspace sampling at considerable savings in time, and at
about half the cost. An example of the use of SPME headspace in forensic toxicology analysis was described by Brewer et al
19
.
The authors investigated two traffic fatalities where in one case the conventional headspace analysis of a urine sample indicated
that the driver had alcohol in his blood. However, a second unknown compound which eluted on the tail of a large air peak could
not be easily ascertained by mass spectrometry. Using headspace SPME-GC/MS, a cleaner chromatogram resulted, allowing the
identification of a toxic level of methylene chloride adjacent to the ethanol peak. The use of a polar fiber (85 µm polyacrylate film)
improved the sensitivity over a non-polar fiber (100 µm polydimethylpolysiloxane) for the analysis of ethanol. In the second case,
the victim had ingested lighter fluid, and headspace SPME-GC/MS of gastric juice was able to determine the presence of the lighter
fluid and trace the source back to a container found in the victim’s bedroom. Since the measured compounds were straight chain
alkanes, a non-polar fiber worked best in this case.
The analysis of various soils for BTEX and other volatile contaminants using SPME headspace analysis showed that each soil
released these volatiles at different rates
20,21
. The addition of solvents and water served to release these compounds more
effectively by displacing the hydrocarbons from the soil surface. SPME sampling with in-fiber derivatization was applied to the
measurement of formaldehyde in the headspace of vials containing samples of commercial products known to contain
formaldehyde, including hair gel and laminated particle board
22
. The sampling times range from 10-120 sec and resulted in very
clean chromatograms and high signal-to-noise ratios. A method limit of detection of 2 ppb of formaldehyde was determined with
an RSD of 6.7%.
Headspace SPME was used by chemists at a major pharmaceutical company for the determination of organic volatile impurities in
drug substances as specified by the United States Pharmacopoeia (USP) Method 467
23
. They compared headspace SPME and
immersion SPME with respect to precision, accuracy, and limits of detection, and found them to be essentially equal. Limits of
detection ranged from 0.06 µg/mL for 1,4-dioxane to 0.002 µg/mL for benzene. Precision was generally in the 2-3% range for most
of the solvents tested. The authors preferred the headspace sampling method since it prolonged the lifetime of the SPME fiber. Finally,
Yang and Peppard
24
used SPME headspace sampling to monitor 25 common flavor components in spiked water, ground coffee, fruit
juice, and butter-flavored vegetable oil.
Headspace Solid Phase Microextraction (HS-SPME)

46
Single drop microextraction (SDME) has been evaluated as an alternative to SPME. In this straightforward technique, a microdrop
of solvent is suspended from the tip of a conventional microsyringe and then immersed in a sample solution in which it is immiscible
or suspended in the HS above the sample (see Figure 6.5). Headspace-single drop microextraction (HS-SDME) is similar to
traditional HS sampling in that volatiles are sampled from the vapors above the sample, thus also avoiding interferences from the
sample matrix. In HS-SDME, the fiber used in SPME is replaced by a liquid microdrop that can also be chosen for its selectivity.
A variety of methods and specialized equipment is available for this purpose.
The extracting solvents that have been utilized for SDME are listed in Table 6.1. A wide range of solvents and polarities is possible.
For HS sampling, the boiling point of the solvent should be high to avoid significant evaporation during sampling. To illustrate the
application of HS-SDME, the analysis of residual solvents (RS) as performed in the pharmaceutical industry will be shown. Methods
for the analysis of RS in pharmaceutical products are presented in the United States Pharmacopeia and include direct-liquid
injection (DLI) as well as HS methods.
n-Octane
n-Decane
Tetradecane
n-Hexadecane
Toluene
o-Xylene
Cyclohexane
1-Octanol
Benzyl alcohol
Ethylene glycol
Diethylphthalate
The International Conference on Harmonization (ICH) has
classified four classes of RS that must be monitored in
pharmaceutical formulations. Class 1 solvents have
unacceptable toxicity and are to be avoided during
pharmaceutical manufacture. They are not routinely analyzed,
although confirmation of their absence could be part of the
development process. Class 2 solvents are less toxic, Class 3
are “of lower risk to human health,” and Class 4 are solvents
for which no adequate toxicological data were found. These
last three classes of solvents are the ones most commonly
analyzed and have been analyzed using HS-SDME.
Table 6.2
Solvents Used as Microdroplets in Headspace SDME
Configuration for Single Drop
Headspace Microextraction
Figure 6.5
Headspace SDME

47
Wood and coworkers
25
investigated HS-SDME for the determination of RS in pharmaceutical preparations. The extraction drop
ICH Class 2 solvent chosen was N-methylpyrrolidone (NMP) that has been used as a solvent for RS analysis because it is miscible
with water, able to solubilize many pharmaceutical products, and elutes after most of the other residual solvents due to its
relatively high boiling point (202 °C). The use of NMP precludes its determination in RS samples, as well as that of any other
residual solvents that are higher boiling and elute with or after NMP. One or two µL of NMP was suspended approximately
midway in the HS, as shown in Figure 6.5. After a defined period of time, usually four or five minutes, the drop was withdrawn
back into the syringe, past the original volume mark, and injected into the GC. Using an internal standard, a number of ICH
solvents (ethanol, acetone, 1-propanol, hexane, chloroform, tetrahydrofuran, cyclohexane, and toluene) were quantitatively
analyzed by HS-SDME. RSD values for a manual method averaged around 2.7% and Limits of Detection (LOD) were estimated
to be less than 1 ppm using GC/FID. With automation and GC/MS, limits of detection for HS-SDME were low parts per million
and calibration curves were linear over the expected concentration range [e.g. ethanol’s LOD was 0.08 ppm and its calibration
curve gave R2 values exceeding 0.995 without an internal standard]. An example of a HS-SDME chromatogram is depicted in
Figure 6.6
25
. It was noted that no carryover was observed.
4200000
4000000
3800000
3600000
3400000
3200000
3000000
2800000
2600000
2400000
2200000
2000000
1800000
1600000
1400000
1200000
1000000
800000
600000
400000
200000
0
0.50 1.00 1.50 2.00 2.50 3.00 3.50 4.00 4.50 5.00 5.50 6.00 6.50 min
Experimental Conditions:
Instrument: Agilent 6890 GC
and Agilent 5973 MS
Column: 30 m HP-5ms
Data system: Agilent ChemStation
Microsyringe: Hamilton 1701, 10 µL,
gas-tight, needle point #2
GC temperatures (°C): Injection: 250
Detector: 280
GC split ratio: 10:1
Sampling automation: CTC Analytics CombiPAL
Sample vial: 20 mL
Sample size: 10 mL
Wash with NMP, 3 times, 6 µL
Sample temp: 60 °C
Stirring rate: 750 rpm
Equilibration time: 30 min
Drop volume: 1.0 µL
Filling strokes: 3
Rate of drop expulsion: 0.2 µL/s
Extraction time: 5 min
Drop volume retracted: 1.2 µL
Figure 6.6
Determination of Residual Solvents Using Headspace SDME and GC/MS
TIC: 1000 ppm 6.D
Toluene
Cyclohexane
Tetrahydrofuran
Chloroform
Hexane
1-Propanol
Acetone
Ethanol

48
Purge and Trap Sample Preparation
Dynamic headspace sampling continuously removes headspace vapors above a liquid or solid sample. The flow of gas over the
sample (purging) will further volatilize analytes that can be trapped by an adsorbent or by cryogenic means. The trapping process
will refocus (concentrate) the volatiles which are then re-volatilized into the gas chromatograph by thermal desorption (see Thermal
Desorption and Thermal Extraction). The dynamic process of purge and trap is particularly useful for analytes that are too low in
concentration to be measured by static headspace methods. Purge and trap (P&T), or gas phase stripping, generally refers to the
process where purging gas is bubbled below the surface of a liquid sample using a fritted orifice to produce finely dispersed
bubbles. Traps used in P&T are often the same as used in gas sampling. For volatile analytes, be careful to avoid breakthrough
through the adsorbent trap during the P&T experiment. In addition, transfer lines and valves should be heated above the boiling
points of removed analytes to prevent adsorption or condensation between the sampler and the GC instrument. There are many
automated systems on the market that perform dynamic headspace extraction.
The proper selection of a trap is an important consideration in ensuring that the desired analytes are quantitatively recovered. An
adsorbent trap (typically a glass tube) is filled with porous sorbent material (typically 50 mg to one gram) that could be a polymer
(e.g. Tenax, polystryrene, polyurethane foams), carbon (graphitized carbon black, charcoal, carbosieves), silica gel, or alumina.
Polymeric materials sometimes need to be cleaned prior to use in order to remove residual monomer. Tenax is especially useful
since it is hydrophobic, water is unretained, and it has a high sample capacity. Carbon materials are excellent, high capacity traps,
but sometimes irreversibly adsorb certain classes of analytes. Silica gel and alumina have high capacity but take up water. These
traps containing adsorbent may also be used off-line to collect volatile organic samples from air and then transported back to the
laboratory for subsequent analysis. As mentioned above, analytes are transferred to the GC by thermal desorption (see Thermal
Desorption and Thermal Extraction), perhaps coupled with cold trapping via cryogenics to refocus the analytes.
An especially important parameter of a particular trap is the breakthrough capacity, which determines how much organic material
is trapped from the gaseous sample before it elutes from the opposite end of the trap and is no longer retained. Volatile organics
may pass through a trap very quickly while semi- or non-volatile organics may adsorb strongly and never elute. For volatile
organics, the trap may be cryogenically cooled or a different adsorbent material may be used that has a higher breakthrough
volume for the analyte.

49
In a variation of the dynamic headspace experiment called membrane extraction with a sorbent interface (MESI), Pawliszyn and
coworkers
26,27
have inserted a silicone hollow fiber membrane in the headspace above the solid or liquid sample. The entire
experimental setup is depicted in Figure 6.7. The membrane extraction module furnishes an interface that effectively isolates
analytes of interest from their sample matrix. In the experimental setup, an inert gas passes through the inside of the membrane.
Analytes that are permeable to the membrane pass from the headspace through the membrane and are swept to an adsorbent
trap. The sorbent interface includes the trap, a heating coil, and a computer-controlled heater-switching device with a power
supply. The module physically connects the membrane extraction module to the GC separation column.
In general, the dynamic HS methods are best when looking for minor components that are more volatile than the major component,
such as fragrances in beverages. The P&T-GC techniques are especially useful for determining organic volatiles from biological fluids
such as urine, plasma, saliva, and tissue homogenates. In addition, the United States Environmental Protection Agency specifies the
dynamic technique for low concentrations of volatile organics in water under the SW-846 Method 5030A. Other examples where
purge and trap methods are recommended in environmental analyses are for: low concentrations of halogenated volatile
compounds in water, soil, and waste samples (Method 502.1), volatile aromatics in drinking water (Method 503.1), and non-
halogenated volatile organics in the same environmental matrices (Method 8030A). Static and dynamic headspace may be used
for the qualitative and/or quantitative determination of volatile impurities, residual monomers, and other additives in polymers. A
useful application for dynamic HS in forensics has been the investigation of arson accelerants since light and medium petroleum
distillates are cleanly swept away from debris, but recoveries with the higher molecular weight petroleum distillates are usually low.
Once a sufficient analyte concentration is accumulated on the adsorbent trap, the analytes are rapidly thermally desorbed into the
GC instrument. The sorbent interface can be operated in the cryogenic mode for better absorption capacity and analyte focusing.
The sensitivity of MESI is directly related to the trapping time that is limited by the breakthrough time of the analytes in the
adsorbent trap. The MESI technique has been applied to a number of environmental samples such as hydrocarbons in rainwater
runoff from a parking lot and BETX in various matrices.

50
120 V
Heating Coil
Gas In Gas Out
Sorbent Interface/Trap
Flame
Ionization
Detector
Gas Chromatograph
Hollow Fiber Membrane
Liquid sample or
heated solid sample
Thermal desorption is a widely used technique for liberating volatile analytes from a solid sample or adsorbent. The technique can
be used standalone for solid samples containing volatile compounds (e.g. petroleum hydrocarbons in soil, additives in finely ground
polymers), or in conjunction with a dynamic headspace method (see section 6.4) where an inert gas transfers volatiles from liquids
or solid samples to an adsorbent trap. Thermal extraction is very similar, except that there is not another trapping step in the
volatiles removal (extraction) process. No sample preparation is involved nor is any solvent employed. In thermal extraction, the
solid sample (1-500 mg) is placed in desorption tube between two glass wool plugs. After purging the tube to remove all traces of
oxygen, a preheated heater block or a heating coil is positioned around the desorption tube and the temperature rapidly ramped up
permitting the thermal extraction of volatiles and semi-volatiles from the solid sample. These compounds are swept into the GC
instrument and trapped at the head of the column. Temperature programming is used to separate the compounds of interest. In
thermal desorption for volatile compounds, the temperature of desorption rarely exceeds 200 °C and often lower, while in thermal
extraction, temperatures up to 300 °C may be used. Beyond 300 °C, thermal cracking or pyrolysis may occur.
Thermal Desorption and Thermal Extraction
Figure 6.7
Schematic of a GC Setup for Membrane Extraction with a Sorbent Interface

51
In thermal desorption, the time required for the complete desorption of analytes is a function of the sample matrix, sample size,
strength of interaction between the analyte and the solid surface, desorption temperature, and diffusion time of the analytes out of
the sample. In general, thermal desorption is a slow process and generates broad peaks. For this reason, a solute focusing
technique is almost always used to ensure better chromatography. Direct (single stage) thermal desorption is the nomenclature
used when the extracted volatiles are swept directly into the GC column while two stage thermal desorption is the process of using
a cold trap to accumulate and focus volatile analytes on an adsorbent. In all cases, to avoid sample losses for both thermal
desorption or thermal extraction, heated transfer lines, preferably of inert material, are generally used.
Thermal desorption tubes are generally used in the field to collect trace levels of volatile- or semi-volatile organic compounds or they
could be packed with solid sample which are to be extracted in the laboratory. For collection of VOCs in air, the tubes may be
packed with various types of adsorbents (e.g. Tenax, charcoal, inorganic materials, etc.) that are somewhat selective for the organic
compounds to be analyzed. Usually some type of controlled flow system is used to push air through the tubes as a function of time.
Thermal desorption instruments are available which automate the handling of the sample-filled desorption tubes including loading/
unloading the thermal tubes and heating the tubes to a sufficient temperature to remove all analytes of interest. The process has the
advantage of little manual sample preparation, no solvents are used, and therefore there is no analytical interference from solvent nor
solvent disposal costs to consider. Although single stage thermal desorption is possible, some re-focusing of the effluent from the tube
is required. Capillary cryofocusing, cold trapping, or an electrically-cooled sorbent trap is used for this purpose. For cost reasons (liquid
cryogen consumption), the latter approach is preferred. The trap can be re-heated rapidly in a backflush mode and 99+% of the
analytes are desorbed in a few seconds.
Thermal desorption has been used for the analysis of a wide variety of low moisture content solid samples including vegetation,
food products, pharmaceuticals, building materials, forensic samples, and packaging products. Other important application areas
include environmental monitoring, flavor and fragrances, industrial hygiene, and chemical warfare agents. For general overviews
of thermal desorption and its applications, refer to references 28-30.

52
Pyrolysis is the next stage in thermal extraction techniques, but has one important difference compared to the other thermal
techniques. The temperatures used in pyrolysis are high enough (600-800 °C) to actually break molecular bonds in the molecules of
the solid sample, thereby forming smaller, simpler volatile compounds. Depending on the amount of energy supplied, the bonds in
each molecule break in a predictable manner. By the identification and measurement of the fragments, the molecular composition
of the original sample can often be reconstructed. Pyrolysis may not be thought of as a sample preparation technique, but more of a
sample destructive technique since the actual molecular form of the sample is changed upon heating. Pyrolysis is often used for the
analysis of polymer samples since they are too high of a molecular weight to analyze using gas chromatographic techniques and
they often degrade in a systematic fashion.
Synthetic polymers (e.g. polyvinylchloride, polystyrene, polyesters), natural polymers (e.g. plant fibers such as cellulose and cotton,
and animal fibers such as wool and silk), dried paints, and cosmetic samples are examples of samples that can be characterized by
pyrolysis GC. Using pyrolysis, considerable information may be obtained on the basic structure of polymers as well as polymer
defects, variations, and degradation mechanisms.
Normally pyrolysis is performed in an inert atmosphere so that there is a controlled pyrolysis and predictable degradation. However,
pyrolysis can also be carried out in a reactive atmosphere such as air or oxygen. The degradation mechanisms may be quite different
when compared to those in helium. These studies on materials such as polymers can be useful for combustion studies, toxicology, and
in evaluating the atmospheric stability of a material. The pyrolysis is first performed in a reactive environment and the pyrolysates
collected on an adsorbent trap while the reaction gas is vented. Then, to perform the GC analysis, a gas-switching valve is employed
to divert the GC carrier gas through the trap and thermal desorption to desorb the collected organics to the GC instrument.
Cryogenic focusing may also be used to concentrate and focus degraded volatile fragments and compensate for system dead
volume. Cryofocusing may be used in conjunction with an adsorbent trap or used with on-column concentration followed by
temperature programming. For more information on pyrolysis GC, consult reference 31.
The Role of Pyrolysis in Sample Preparation

53
Conclusions
Methodologies for the sampling, sample introduction, and sample preparation of volatile analytes in conjunction with GC and GC/MS
analysis covered in this chapter have been around for many years and are considered to be mature. However, each year there are
many practical problems solved, applications examples generated, hardware and software improvements made, and new ancillary
techniques introduced to keep these methods on the forefront of analytical technologies. For example, the addition of a hollow fiber
membrane to the dynamic headspace experiment provides an additional level of selectivity in the MESI technique. The introduction of
automated LVI methodologies has given the analyst the ability to introduce greater amounts of liquid sample into the capillary GC
instrument. Thus, there is no doubt that in the future we shall continue to see improvements in the methodology around the sample
handling of volatile organic analytes.
References
1.
Grob, R.I.; Barry, E.F. (Eds.)
Modern Practice of Gas Chromatography
, 4th Ed., John Wiley and Sons, Hoboken, NJ, 2004,
ISBN 0-471-22983-0.
2.
McNair, H.M.; Milller, J.M.
Basic Gas Chromatography
, 2nd Ed., John Wiley and Sons, Hoboken, NJ, 2009,
ISBN 978-0-470-43954-8.
3.
Poole, C.
Gas Chromatography
, Elsevier, Oxford, U.K., 2012, ISBN 978-0-12-385540-4.
4.
Poy, E.S.; Visani, F.; Terrosi, F.
J. Chromatogr.
1981, 217, 81.
5.
Vogt, W.; Jacob, K.; Obwexer, H.W.
J. Chromatogr.
1979, 174, 437.
6.
Vogt, W.; Jacob, K.; Ohnesorge, A.B.; Obwexer, H.W.
J. Chromatogr.
1979, 186, 197.
7.
Staniewski, J.; Rijks, J.
HRC
1993, 16, 182.
8.
Wilson, B.; Wylie, P.L.; Klee, M.S.
Large Volume Injection for Gas Chromatography Using a PTV Inlet
, Application Note #228-374,
Hewlett-Packard, Wilmington, DE, 1997.
9.
Li, X.
Analysis of Ethanol in Blood with the Agilent 7820A GC and 7697A Headspace Sampler
, Application Note #5990-9021EN,
Agilent Technologies, Santa Clara, CA, 2011.
10.
Kolb, B.; Ettre, L.S.
Static Headspace-Gas Chromatography: Theory and Practice
, 2nd Ed., Wiley-VCH Inc., New York, 2006,
ISBN 0-471-74944-3.
11.
Firor, R.L.; Gudat, A.E.
The Determination of Extractables and Leachables in Pharmaceutical Packaging Materials Using
Headspace GC/MS
, Application Note #5989-5494EN, Agilent Technologies, Wilmington, DE, 2006.
12.
Firor, R.L.
Residual Monomers in Polymers by Multiple Headspace Extraction Using the Agilent 7697A Headspace Sampler
,
Application Note #5990-0342EN, Agilent Technologies, Wilmington, DE, 2012.
13.
Kolb, B.; Ettre, L.S.
Chromatographia
1991, 32, 505-513.
14.
Zhu, J.Y.; Chai, X.-S.
Current Analytical Chemistry
2005, 1, 79-83.
15.
Gudat, A.E.; Brillante, S.M.
Multiple Headspace Extraction-Capillary Gas Chromatography for the Quantitative Determination of
Volatiles in Solid Matrices
, Application Note #5965-0978E, Agilent Technologies, Wilmington, DE, 2000.
(Continued)

54
16.
Arthur, C.L.; Potter, D.W.; Buchholz, K.D.; Motlagh, S.; Pawliszyn, J.
LCGC
1992, 10, 656-661.
17.
Zhang, Z.; Yang, M.J.; Pawliszyn, J.
Anal. Chem.
1994, 66, 844-852A.
18.
Furton, K.G.; Almirall, J.R.; Bruna, J.C. J.
Forensic Sci.
1996, 41, 12-22.
19.
Brewer, W. E.; Galipo, R.C.; Morgan, S.L.; Habben, K.H. J.
Anal. Toxicology
1997, 21, 286-290.
20.
Pawliszyn, J.
SPME Theory and Practice
, Wiley-VCH Inc., New York, 1997, 161-170, ISBN 0-471-19034-9.
21. Saraullo, A. Ph.D. Thesis, Univ. of Waterloo, Waterloo, Ontario, Canada.
22.
Martos, P.A.; Pawliszyn, J.
Anal. Chem.
1998, 70, 311-2320.
23. Shaw, R.; Smith, A.-M.; Clark Nelson, L.; Scypinski, S. Hoffman-La Roche Inc., Nutley, NJ, Poster presentation,
Amer. Assoc. of Pharmaceutical Sciences Conference, June, 1994.
24.
Yang, X.; Peppard, T. J. Agric.
Food Chemistry
1994, 42, 1925-1930.
25.
Wood, D.C.; Miller, J.M.; Christ, I.
LCGC No. America
2004, 22 (6), 516-522.
26.
Pratt, K.F.; Pawliszyn, J.
Anal. Chem.
1992, 64, 2101-2106.
27.
Yang, M.J.; Pawliszyn, J.
LC/GC
1996, 14, 364-376.
28.
Wampler, T.P.
LC/GC
1998, 16 (9), 812-821.
29.
Round-the-Clock, Online and Cryogen-free Monitoring of Hydrocarbons in Ambient Air Using Thermal
Desorption-Gas Chromatography
, Technical Overview #5988-9254EN, Agilent Technologies, 2003.
30.
Optimizing Analytical Performance and Extending the Application Range of Thermal Desorption for Monitoring Air Indoors
and Inside Vehicle Cabins
, Technical Overview #5988-9255EN, Agilent Technologies, 2003.
31.
Introduction to Pyrolysis-Capillary Gas Chromatography, CDS Analytical Solutions
, Publication Number 178,
http://www.cdsanalytical.com.
References
(Continued)

55
Sample Pre-treatment
for Liquid Samples
Previously, in Chapter 2, Table 2.4 provided an introduction to sample preparation methods for liquid samples. Most laboratories
need only a few of these procedures. For example, distillation is limited to volatile compounds, although vacuum distillation for high
boilers in environmental samples can extend the application of this technique. Lyophilization is usually restricted to the purification
and handling of biological samples through the removal of water. This chapter will cover liquid-liquid extractions in more common
use in most chromatography laboratories.
Liquid-Liquid Extraction (LLE)
Liquid-liquid extraction (LLE) is useful for separating analytes from interferences by partitioning the sample between two immiscible
liquids or phases (Figure 7.1). One phase in LLE will usually be aqueous and the second phase an organic solvent. The more
hydrophilic compounds prefer the polar aqueous phase, while more hydrophobic compounds will be found mainly in the organic
solvent. Analytes extracted into the organic phase are easily recovered by evaporation of the solvent, while analytes extracted into
the aqueous phase often can be injected directly onto a reversed-phase HPLC column. In some cases, the aqueous-containing
phase must undergo solvent exchange to a solvent more compatible with the chromatographic approach. The most popular
approach to LLE uses a separatory funnel (Figure 7.2), which has an opening on the top to add the two phases and a stopcock at
the bottom to selectively remove the bottom layer after the partitioning process takes place. A separatory funnel is preferred if the
organic solvent has a higher density than water (e.g. dichloromethane), and thus is the bottom layer.
Chapter 7

56
Add Immiscible Organic Phase
Shake to increase surface contact
Siphon off layer(s) and collect
1 2
Aqueous Matrix
3
4
The following information assumes that an analyte is preferentially concentrated into the organic phase, but similar
approaches are used when the analyte is extracted into an aqueous phase.
Less soluble in water
More soluble in methylene chloride
Figure 7.1
Figure 7.2
LLE Performed in Separatory Funnel
Typical Liquid-Liquid Extraction

57
Liquid Sample
Yes No
Is sample
in solution?
Dissolve in
suitable solvent
Choose solvent
suitable for later
extraction step
Place sample in
sep funnel
Add immiscible
solvent and
shake vigorously
Make chemical adjustment
(e.g., pH, add complexation
reagent, etc.)
Allow phases
to separate
Are the two
liquids clear?
Draw off
each phase
Measure solute
in each phase
No
Break emulsion
(see text)
Are solutes
extracted
quantitatively?
Evaporate to
appropriate
concentration
Injection or
further sample
pre-treatment
Yes
No
Yes
Summary of Steps Involved in Liquid-Liquid Extraction (LLE)
Figure 7.3
Figure 7.3 summarizes the workflow steps involved in a LLE separation. Since extraction is an equilibrium process with limited
efficiency, significant amounts of the analyte can remain in both phases. Chemical equilibria involving changes in pH, ion-pairing,
complexation, etc. can be used to enhance analyte recovery and/or the elimination of interferences.

58
•
A low solubility in water (<10%).
•
Volatility for easy removal and concentration after extraction.
•
Compatibility with the HPLC or GC detection technique to be used for analysis (avoid solvents that are strongly UV-absorbing or
that may cause GC detection problems, such as chlorinated solvents in conjunction with electron capture detector).
•
Polarity and hydrogen-bonding properties that enhance recovery of the analytes in the organic phase.
•
High purity to minimize sample contamination.
The Nernst Distribution Law states that any neutral, non-dissociating species will distribute between two immiscible solvents so that
the ratio of the concentrations (or for the purist, activities) remains constant.
where K
D
is the distribution constant, C
o
is the concentration (activity) of an analyte in the organic phase, and C
aq
is the
concentration (activity) of the analyte in the aqueous phase.
A more useful expression is the fraction of analyte extracted (E) given by Equation 7.2:
where V
o
is the volume of organic phase, V
aq
the volume of aqueous phase, and V is the phase ratio V
o
/V
aq
.
Many LLE procedures are carried out in separatory funnels, and for environmental samples (e.g. water) typically require tens or
hundreds of milliliters of each phase. For clinical samples, sometimes only a few mL (e.g. 1-3 mL) are required.
The LLE organic solvent is chosen for the following characteristics:
K
D
=C
o
/C
aq
E = C
o
V
o
/(C
o
V
o
+ C
aq
V
aq
) = K
D
V/(1 + K
D
V)
Equation 7.2
Equation 7.1
Theory

59
where n= the number of extractions. For example, if K
D
= 5 for an analyte and the volumes of the two phases are equal (V=1),
three extractions (n=3) would be required for >99% recovery of the analyte. Several approaches can be used to increase the
value of K
D
:
E = 1 – [1/( 1 + K
D
V)]
n
Equation 7.3
•
The organic solvent can be changed to increase K
D
.
•
If the analyte is ionic or ionizable, its K
D
can be increased by suppressing its ionization to make it more soluble in the organic phase.
•
The analyte also can be extracted into the organic phase by ion pairing, provided that the analyte is ionized and an ion-pair
reagent is added to the aqueous phase.
•
“Salting out” by addition of an inert, neutral salt (e.g. sodium sulfate) can be used to decrease an analyte’s concentration in the
aqueous phase. See Chapter 8 for an explanation of the use of salting out extraction.
For 1-step extractions, K
D
must be large (e.g., >10) for the quantitative recovery of an analyte in one of the two phases, since the
phase-ratio V must be maintained within a practical range of values; e.g., 0.1<V<10 (see Equation 7.2). In most separatory funnel
LLE procedures, quantitative recoveries (> than 99%) require two or more extractions. For successive multiple extractions, with
pooling of the analyte phases from each extraction,

60
Table 7.1
Table 7.1 provides examples of typical extraction solvents, as well as some unsuitable (water-miscible) extraction solvents. Apart
from miscibility considerations, the main selection criteria is the polarity index P'
1
of the solvent in relation to that of the analyte.
Maximum K
D
occurs when the polarity of the extraction solvent matches that of the analyte (like dissolves like). For example, the
extraction of a non-polar analyte from an aqueous sample matrix would be best accomplished with a non-polar (small P')
1-2
organic
solvent. An optimum-polarity organic solvent can be conveniently selected by blending two solvents of different polarity (e.g.,
hexane and chloroform), and measuring K
D
vs. the composition of the organic phase
3
. A solvent mixture that gives the largest value
of K
D
is then used for the LLE procedure. Further changes in K
D
can be achieved, while improving the separation of analytes from
interferences, by varying organic-solvent selectivity. Solvents from different regions of the solvent-selectivity triangle
2
are expected
to provide differences in selectivity; see also the discussion of
4
. Note that further discussions on the polarity index P' and the solvent-
selectivity triangle are beyond the scope of this book and the reader is referred to the original reference cited above.
Practice
Extraction Solvents for LLE
Aqueous Solvents Water-Immiscible Organic Solvents
Water-Miscible Organic Solvents
(Unsuitable for Conventional LLE)
Pure water Aliphatic hydrocarbons (hexane, isooctane, petroleum
ether, etc.)
Alcohols (Low molecular weight)
Acidic solution Diethyl ether or other ethers Ketones (Low molecular weight)
Basic solution Methylene chloride Aldehydes (Low molecular weight)
High salt (salting out effect) Chloroform Carboxylic acids (Low molecular weight)
Complexing agents (ion
pairing, chelating, chiral, etc.)
Ethyl acetate and other esters Acetonitrile
Combination of two
or more above
ü
Aliphatic ketones (C6 and above)
ü
Aliphatic alcohols (C6 and above)
ü
Toluene, xylenes (uv absorbance!)
ü
Combination of two or more above
Dimethyl sulfoxide
Dioxane
*Any solvent from column 1 can be matched with any solvent of column 2; water-miscible organic solvents (column 3) should not be used with aqueous solvents to
perform conventional LLE. However, the addition of high concentration of salts (salting out) or sugars (see Chapter 8) may decrease miscibility of certain solvent
pairs (e.g. water and acetonitrile) so that they may form two layers.

61
In solvent extraction, ionizable organic analytes often can be transferred into either phase, depending on the selected conditions.
For example, consider the extraction of an organic acid analyte from an aqueous solution. If the aqueous phase is buffered at least
1.5 pH units above its pK
a
value, the analyte will be ionized and prefer the aqueous phase; less polar interferences will be extracted
into the organic phase. If the pH of the aqueous solution is lowered (<<pK
a
), so that the analyte is no longer ionized, the analyte
will be extracted into the organic phase, leaving more polar interferences in the aqueous phase. Successive extractions at high pH
followed by low pH are able to separate an acid from both more- and less-polar interferences. Note that the principles of acid-base
extraction as a function of pH are the same for LLE and reversed-phase HPLC.
If the analyte K
D
is unfavorable, additional extractions may be required for improved recovery (Equation 7.3). For the case of an
organic-soluble analyte, a fresh portion of immiscible organic solvent is added to the aqueous phase in order to extract additional
solute; all extracts are then combined. For a given volume of final extraction solvent, multiple extractions with smaller volumes are
generally more efficient in removing a solute quantitatively than use of a single extraction volume.
Back extraction can be used to further reduce interferences. For example, consider the previous example of an organic-acid
analyte. If the analyte is first extracted at low pH into the organic phase, polar interferences (e.g., hydrophilic neutrals, protonated
bases) are left behind in the aqueous phase. If a fresh portion of high pH aqueous buffer is used for the back-extraction of the
organic phase, the ionized organic acid is transferred back into the aqueous phase, leaving non-polar interferences in the organic
phase. This latter procedure is similar to successive extractions at high pH followed by low pH described above. Thus, a two-step
back-extraction with a change in pH allows the removal of both basic and neutral interferences, whereas a one-step extraction can
eliminate one or the other of these interferences, but not both.
If K
D
is very small (not much greater than 1) or the required quantity of sample is large, it becomes impractical to carry out multiple
extractions for quantitative recovery of the analyte. Too many extractions are required, and the volume of total extract is too large
(Equation 7.3). Also, if extraction is slow, a long time may be required for equilibrium to be established. In these cases, continuous
liquid-liquid extraction can be used, where fresh solvent is continually recycled through the aqueous sample.

62
In some cases, LLE can enhance analyte concentration in the extract fraction relative to its concentration in the initial sample.
According to Equation 7.2, by choosing a smaller volume of organic solvent, the analyte concentration can be increased by the
volumetric ratio of organic-to-aqueous phases (assuming near complete extraction into the organic phase or large K
D
). For example,
assume 100 mL of aqueous sample, 10 mL of organic solvent, and a large K
D
(e.g., K
D
>1000). The concentration of the analyte in
the organic phase will then increase by a factor of 10. For large ratios of aqueous-to-organic solvent, a slight solubility of the organic
solvent in the aqueous phase can reduce the volume of the recovered organic solvent significantly; this problem can be avoided by
presaturating the aqueous solvent with organic solvent. Note that when the solvent ratio V
o
/V
aq
is small, the physical manipulation of
two phases (including the recovery of organic phase) becomes more difficult.
Continuous extractors using heavier-than-water and lighter-than-water solvents have been described
5
. These extraction devices
can run for extended periods (12-24 hours); quantitative extractions (>99% recovery) can be achieved, even for small values of K
D
.
For more efficient LLE, a countercurrent distribution apparatus can provide a thousand or more equilibration steps (but with more
time and effort). This allows the recovery of analytes having extremely small K
D
values; countercurrent distribution also provides a
better separation of analytes from interferences. Small-scale laboratory units are commercially available. For further information on
these devices, see reference 6.

63
Some practical problems associated with LLE include:
•
Emulsion formation
•
Analytes strongly adsorbed to particulates
•
Analytes bound to high molecular weight compounds (e.g. protein-drug interactions)
•
Mutual solubility of the two phases
Emulsions are a problem that can occur with certain samples (e.g. fatty matrices) under certain solvent conditions. If emulsions do
not “break” with a sharp boundary between the aqueous and organic phases, analyte recovery can be adversely affected.
Emulsions can be broken by:
•
Addition of salt to the aqueous phase
•
Heating or cooling the extraction vessel
•
Filtration through a glass wool plug
•
Filtration through phase-separation filter paper
•
Addition of a small amount of different organic solvent
•
Centrifugation
If particulates are present in a sample, adsorption onto these particulates can result in a low recovery of the analyte. In such cases,
washing the particulates after filtration with a stronger solvent will recover the adsorbed analyte; this extract should be combined
with the analyte phase from LLE. A "stronger" solvent for recovering adsorbed analytes may involve a change in pH, an increase in
ionic strength, or the use of a more polar organic solvent.
Problems
Emulsion Formation
Analyte Adsorption

64
Compounds that normally are recovered quantitatively in LLE may bind to proteins when plasma samples are processed, resulting in
low recovery. Protein binding is especially troublesome when measuring drugs and drug metabolites in physiological fluids.
Techniques for disrupting protein binding in plasma samples include:
•
Addition of detergent
•
Addition of organic solvent, chaotropic agents, or strong acid
•
Dilution with water
•
Displacement with a more strongly binding compound
"Immiscible" solvents have a small but finite, mutual solubility, and the dissolved solvent can change the relative volumes of the two
phases. Therefore, it is a good practice to saturate each phase with the other, so that the volume of phase containing the analyte
can be known, accurately allowing an optimum determination of analyte recovery. The simplest procedure for saturation is to
equilibrate the two phases in a separatory funnel without the sample, thereby saturating each phase. Aliquots of either phase can
then be used for LLE. For values of the solubility of a solvent in water (or of water in the solvent), see reference 7.
Solute Binding
Mutual Phase Solubility

65
Supported Liquid Extraction (SLE)
Earlier, under Problems, some of the problems associated with traditional LLE were addressed. An alternative approach that gets
around some of these disadvantages has been available for some time, yet is relatively unknown – supported liquid extraction (SLE,
sometimes referred to as solid-supported liquid extraction, supported liquid-liquid extraction, or simply, solid-liquid extraction). Its
principles are relatively simple – a chemically inert, high surface area support, highly purified, graded diatomaceous earth serves as
a stationary vehicle for the aqueous phase of the LLE experiment. Water very easily adsorbs onto the surface of diatomaceous earth
particles. The dry solid sorbent (Figure 7.4A) is placed into a cartridge, column, or well of a 96-well plate, the same devices used
for solid phase extraction (SPE). As depicted in Figure 7.4B, the aqueous-based sample (e.g. diluted plasma, drinking water, etc.)
is added to the dry sorbent and allowed to wet (disperse by capillary action and absorb) the diatomaceous earth, a process that
only takes 5-15 minutes under gravity. Oftentimes, the aqueous sample is pretreated (i.e. pH adjustment, ion pairing agent added,
buffered, etc.) such that the analyte(s) of interest is/are in a suitable form to be extracted into an organic solvent, just as performed
for conventional LLE. Pre-buffered SLE cartridges are also commercially available.
Next, a small volume of immiscible organic extraction solvent is added to the top of the cartridge and allowed to percolate by
gravity (or sometimes with gentle vacuum or pressure) through the supported aqueous phase. Because the aqueous sample has
been widely dispersed throughout the solid support, the organic solvent has intimate contact with the thin film of aqueous phase
and rapid extraction (equilibration) occurs (Figure 7.4C). This intimate contact also occurs in LLE due to vigorous shaking of the
separatory funnel. This shaking causes the immiscible organic solvent to disperse into tiny droplets that provide a closer contact to
the surrounding aqueous solvent. The downside of this dispersion process in LLE is that emulsions can form and/or a long time is
needed for the two phases to separate. In addition, as pointed out in Emulsion Formation, it may take some additional time to break
the emulsion.

66
Figure 7.4A
Figure 7.4B
The final step in the process is collection of the organic effluent containing the analyte(s) of interest from the outlet of the SLE
device (Figure 7.4C). The aqueous phase remains behind. A phase-separation filter is incorporated into the outlet frit of the device
to ensure that organic effluents remain uncontaminated by aqueous matrix.
Figure 7.4C
Step 3
Step 2
Step 1
Collected organic effluent
Organic extraction solvent
Allow 10-15 min for aqueous phase to
spread out over diatomaceous earth
Solid support adsorbs
aqueous-based sample onto
high surface area particles
Aqueous sample being applied
Extract with organic solvent
Before extraction
Apply sample
Figure 7.4
Supported Liquid Extraction Process
Analyte of interest
Undesired matrix compounds
or interferences
Analyte of interest
Undesired matrix compounds
or interferences
Organic
layer
Aqueous
layer
Dry
sorbent

67
With SLE, there is no vigorous shaking and therefore emulsions cannot be formed. In addition, the intimate contact between
the aqueous and organic phases allows very efficient partitioning, thus analyte recoveries can sometimes be higher than in
conventional LLE. Futhermore, the SLE process is more technique independent than conventional LLE, thus, experiments are often
more reproducible. Obviously, the glassware involved in SLE is greatly reduced compared to classical LLE and no cumbersome
washing of the soiled separatory funnel is required. The entire SLE process can be more easily automated than using separatory
funnels in traditional LLE. The 96-well plate SLE format (Combilute) is especially amenable to automation using x-y-z robotics
systems. Prepacked SLE products such as Agilent’s Chem Elut are available in all automation formats. In addition, bulk sorbent
such as Agilent’s Hydromatrix can be purchased for those who wish to make their own customized SLE devices.
The SLE approach can basically duplicate any developed LLE method with a few minor adjustments. In general, 1 g of diatomaceous
earth sorbent is needed for each 1 mL of aqueous sample. Cartridge size is determined by the total volume of sample that needs to be
extracted. The volume extracted is dependent on the concentration of the analyte(s) of interest, the total volume of sample available,
and the sensitivity of the analytical system that will make the downstream measurement. For an environmental sample (e.g. for
screening pesticides in water), cartridge volumes of up to 100 mL aqueous capacity are available. For biological samples (e.g. plasma
or urine), in conjunction with LC-MS/MS (triple quadrupole analysis), the excellent sensitivity would dictate a smaller sample volume.
For example, for 96-well plates with a 2 mL well volume, 200-400 µL (max 500 µL) of plasma can be extracted with pg/mL
sensitivity. For the organic extraction, the volume of organic solvent should be at least equivalent to the volume of aqueous sample.
As a rule of thumb, at least two column volumes of organic extraction solvent are recommended for maximum recovery.
Almost any non-polar solvent can be used as the immiscible organic phase. The organic solvent should be spectroscopic grade or
above, since often the collected fraction is evaporated to dryness. Any solvent containing non-volatile impurities will be unsuitable for
performing clean extractions. Pure organic solvents or organic solvent mixtures can be employed. Table 7.2 provides a list of solvent
mixtures compatible with SLE. The column to the right of each solvent pair indicates the maximum percentage of polar solvent
tolerated. Exceeding the maximum water-miscible solvent percentage may have a detrimental effect on cleanup since some of the
aqueous phase containing impurities may be stripped from the diatomaceous earth.

68
To illustrate an application of SLE compared to LLE, consider the multiresidue confirmation of pesticides in honey
8
. This application is
very important in the area of food safety. Bees gathering pollen from blossoms treated with various pesticides carry the
contaminated pollen back to their hives. This contaminated pollen could result in the decline of bee populations or in the
development of contaminated honey. Since bees can gather pollen from many different sources, a multiresidue approach is needed
to test honey for possible contamination. The study by Picard investigated 17 pesticides and metabolites from a variety of chemical
classes. The extraction of pesticides was performed by SLE and compared to the results obtained by traditional LLE.
Table 7.2
Solvent Mixtures Compatible with SLE
Adapted from Breitenbucher, J.G. et al. J. Comb. Chem. 2001, 3, 528-533.
Initially, classical LLE was used to extract pesticides from honey. A 1 g sample of honey was dissolved in 2 mL of water. Next, 6.5 mL
of acetonitrile was added and the mixture mechanically shaken for 30 min. The high concentration of sugar in the honey sample was
analogous to the salting out effect, or in this case, a sugaring out effect (see Chapter 8 for explanation) that caused the two solvents
to layer. The organic and aqueous layers were separated by centrifugation. The organic layer was evaporated down to 100 µL and
added to 100 µL of water. This extract was filtered and injected into an LC-MS/MS system. For the SLE approach, a 1 g sample of
honey was spiked with a surrogate standard before mechanical agitation with 1.25 mL of water and 2.5 mL of acetone for 1 hr.
After adding 1.25 mL of a 20% NaCl solution, the mixture was then loaded onto a 5 mL Chem Elut SLE cartridge. After waiting for
15 min, analytes were eluted by gravity twice with 10 mL of ethyl acetate. Extracts were evaporated to dryness under a gentle
stream of nitrogen and 200 µL transferred to an acetonitrile-water solution (10:90) in vials for LC-MS/MS analysis. The extracts
were analyzed by LC-MS/MS in the ESI mode without further purification. The 17 pesticides were separated by optimizing the LC
gradient. The co-eluted pesticides with different masses were identified using the MRM mode.
Mixture
Max %
Water-Miscible Solvent
CH
2
CI
2
/MeOH 20% MeOH
CH
2
CI
2
/Acetone 20% Acetone
CH
2
CI
2
/DMF 10% DMF
CH
2
CI
2
/DMA 10% DMA
CH
2
CI
2
/NMP 20% NMP
CH
2
CI
2
/THF 70% THF
CH
2
CI
2
/CH
3
CN 10% CH
3
CN
Toluene/THF 70% THF
Toluene/DMF 30% DMF
EtOAc/DMF 10% DMF
EtOAc/THF 70% THF
EtOAc/IPA 60% IPA
EtOAc/MeOH 10% MeOH
Et
2
O/THF 50% THF
Mixture
Max %
Water-Miscible Solvent

69
100
Pesticides and Their Levels in Recovery Study
Comparison of Recovery of Pesticides: LLE vs SLE
Figure 7.5
Figure 7.5 shows a comparison between recoveries obtained after SLE on Chem Elut and classical LLE. The comparison showed
that the SLE extraction procedure provided similar or higher extraction efficiency than LLE for most compounds. Note in particular
the surprisingly poor recovery results for Ch, Ri, and TOH for the LLE. For the SLE method, linearity was demonstrated from the
range of 0.1-20 ng/g of raw honey with correlation coefficients ranging from 0.921 to 0.999 depending on the pesticide. Recovery
rates were well above the range specified in the official methodology
9
. Reproducibility was found to be between 8 and 27%,
acceptable within the concentrations studied. Over 100 samples of raw honey were analyzed to test the method robustness.
More details on the method can be obtained in reference 11.
Pesticide
Name
Abbreviation
Used
Level
(ng mL
-1
)
Amidosulfuron Am 0.4
Atrazine At 0.4
Carbofuran Ca 0.4
Chlorotoluron Ch 20.0
Diethofencarb De 2.0
Dimethoate Dm 2.0
Fipronil Fi 10.0
Imidacloprid Im 2.0
Isoxaflutole Is 2.0
Linuron Li 2.0
Methiocarb Mh 10.0
Methiocarb sulfoxide MhS 20.0
Metosulam Mo 2.0
Pirimicarb Pi 0.4
Rimsulfuron Ri 0.4
Simazine Si 2.0
2-Hydroxyterbuthylazine TOH 1.0
Recovery %
LLE
SLE
Am Ca Ch De Dm Fi Im Is Li Mh MhS Mo Pi Ri Si TOH
Pesticide
Name
Abbreviation
Used
Level
(ng mL
-1
)
At

70
Techniques Related to LLE
The technique of liquid-liquid extraction (LLE) is still among the most popular in routine sample preparation. Classical LLE uses
copious amounts of solvent that are often hazardous and it is time-consuming to perform. Recently, there has been a developing
interest in miniaturization of analytical methods with resultant solvent and sample savings; newer miniaturized approaches to liquid
extraction have been reported. Compared to classical LLE, these approaches have resulted in more efficient sample enrichment,
more rapid sample preparation, and easier automation.
Microextraction is another form of LLE in which extractions are performed with the organic:aqueous phase ratio values in the range
of 0.001-0.01. Using comparatively small volumes of organic solvent decreases analyte recovery, but the analyte concentration in
the organic phase is greatly enhanced. In addition, solvent usage is greatly reduced. The extractions are carried out in volumetric
flasks. The organic solvent is chosen to have a lower density than water so that this small volume of organic solvent accumulates in
the narrow neck of the flask for convenient removal. The salting out effect can be used to enhance analyte concentration in the
organic phase. Internal standards should be used and extractions of calibration standards performed along with the samples.
Remember, as discussed in Mutual Phase Solubility, in order to ensure that the extraction solvents are saturated with the opposite
solvent, the organic and aqueous solvents should be equilibrated with each other before placing the sample into the flask.
A new variation of microextraction, called dispersive liquid-liquid microextraction (DLLME) has been successfully applied to a
variety of analyte-matrix pairs
11
. The technique is based on a three-component solvent system. In DLLME, the container is
usually a centrifuge tube and the appropriate mixture of immiscible organic extraction solvent (usually a few microliters such as
~8 µL of tetrachloroethylene) and a dispersive solvent (e.g. ~1 mL of acetone) is rapidly injected into an aqueous solution
(approximately 5 mL), containing the analytes of interest, with a syringe (see Figure 7.6). The role of the dispersive solvent is to
ensure miscibility between the organic phase and the aqueous phase. When the solvents are rapidly mixed, a cloudy solution is
formed, consisting of fine particles (droplets) of extraction solvent which is entirely dispersed into the aqueous phase. Since the
solution is already finely dispersed, no vigorous shaking is required. Extraction time is almost instantaneous and much faster
than solid phase microextraction (SPME) and liquid phase microextraction (LPME), which often can require 30 min or longer.
Next, the entire mixture is centrifuged (1.5 min at 6000 rpm), resulting in sedimentation of fine droplets of organic extraction
solvent. They are subsequently removed with a microsyringe or micropipette. The organic solution containing the analyte of
interest can be directly injected or evaporated and taken up in a more appropriate solvent for the chromatographic technique.
In the original work
11
, the authors were able to achieve enrichment values of approximately 600-1100 for polynuclear aromatic
hydrocarbons from a water sample with excellent recoveries. For further reading, a review article
12
is recommended.
Microextraction

71
Aqueous
analyte
solution
Turbid
solution
Aqueous
solution
Inject sedimented phase into GC/HPLC
Extraction solvent
Organic solvent (C
2
CI
4
, CCI
4
, etc.)
Disperser solvent
(acetone, methanol)
Centrifugation
Sedimented phase
+
(Courtesy of Jared Anderson, U. Toledo)
Figure 7.6
Workflow for Traditional DLLME

72
An example of an application of DLLME by Caldas and coworkers
13
was able to show excellent extractions of pesticides
(carbofuran, clomazone, and tebuconazole) in aqueous samples using DLLME along with LC-MS/MS analysis. In their work,
they found that a 60 µL portion of extraction solvent (carbon tetrachloride), 2 mL of dispersive solvent (acetonitrile) for a 5 mL
sample of fortified water acidified with phosphoric acid at pH 2.0 gave the best results. Since acetonitrile was too strong of a
solvent for their LC column, they evaporated the solvent to dryness and dissolved the residue in HPLC grade methanol. The
variables in DLLME method development included: choice of dispersion solvent (Figure 7.7) and its volume , choice of
extraction solvent and its volume (Figure 7.8), pH (if necessary), and centrifuge speed. For all three pesticides, the linear
range was found to be 0.001-1.0 mg/L and the limit of quantitation (LOQ) was 0.02 mg/L. Overall, the DLLME technique is
simple, fast, provides good recovery, is low cost, and provides good enrichment factors.
0
5
10
15
20
25
30
0
30
20
10
40
50
60
70
80
Recovery %Recovery %
Tebuconazole
Clomazone
Carbofuran
Tetrachloroethilene1,2-Dichlorobenzene Carbon tetrachloride
Extractor Solvent
Dispersive Solvent
AcetonitrileAcetoneMethanol
Comparison of Dispersive Solvents in DLLME of Pesticides
Figure 7.7
Comparison of Extraction Solvents in DLLME of Pesticides
Figure 7.8

73
The simplicity and relatively low cost of solid phase microextraction (SPME), developed by Pawliszyn and co-workers
15
in 1990, has
made it a popular sampling and sample preparation technique for gas chromatography, and to a lesser extent, for liquid
chromatography (see Chapter 10, Solid Phase Microextraction). In SPME, a fiber coated with a stationary phase is placed into a
solution or headspace and analytes diffuse and/or are moved by convection into the stationary phase. The concentrated analytes are
transferred to the chromatography column by thermal desorption (GC) or liquid extraction (LC). The popularity of the technique has
spurred the development of similar technologies.
One such technology termed “single drop microextraction" (SDME) describes a configuration where a droplet of solvent contained
at the end of a PTFE rod or GC syringe needle replaces the coated fiber. The analytes diffuse into this droplet in a similar manner as
the SPME fiber. The original work first described by Cantwell and Jeannot
15
was based on the experiments of Liu and Dasgupta
16
.
The latter investigated gas molecules partitioning into liquid droplets.
Single drop microextraction has also been referred to as solvent microextraction, liquid phase microextraction, and liquid-liquid
microextraction. In the original experiments of Cantwell and Jeannot
16
, the droplet size was 8 µL of an immiscible organic solvent
(n-octane) contained in a rod-shaped PTFE probe hollowed out at one end. The probe was immersed in an aqueous sample
contained in a 1 mL vial that was agitated with a magnetic stirrer. Since the 8 µL volume was too large to directly inject into a GC,
the authors took an aliquot that limited sensitivity. However, in their next publication
17
as well as the similar work of He and Lee
19
,
the droplet size was reduced to 1-2 µL by using the tip of a GC syringe needle as the drop holder. The entire droplet was then
injected into the GC. A schematic of the single drop microextraction experiment is shown in Figure 7.9.
Single Drop Microextraction (SDME)

74
In the SDME there are a few experimental parameters that
should be precisely controlled in order to have reproducible
results. Similar to SPME, the partition equilibrium is not reached
during the procedure, so precise timing is essential for good
reproducibility. Cantwell and Jeannot
15
found that they could
achieve relative standard deviations in the 1.5 percentile, even
when the extraction was only 38% of the way to equilibrium.
Note that enrichment factors are generally less than 100 in the
SDME experiment. Table 7.3 lists some of the organic solvents
that have been successfully applied to SDME.
Solvent drop
Extraction vial
Water bath
Chromatographic
microsyringe
Figure 7.12
Experimental Setup
for SDME
Experimental Setup
for Headspace-SDME
Table 7.3
Typical Extracting
Solvents for SDME
n-Hexane
n-Octane
i-Octane
Cyclohexane
n-Hexadecane
Toluene
Chloroform
Butylacetate
Diisopropyl ether

75
The techniques of SDME to SPME were compared for the analysis of trace organic pollutants
19
and nitroaromatic explosives
20
from aqueous samples. In summary, the authors found that the techniques are comparable in terms of precision and analysis time.
The small amount of solvent used in SDME is an advantage and the use of various solvents or solvent mixtures allows some
degree of selectivity in the extraction of different organic species. In SPME, selectivity is governed by the selection of the
polymeric coating on the fiber. In contrast to the rapid solvent evaporation of SDME, thermal desorption in SPME (desorption
from the polymer in a hot injector) is a slow process and resulting peaks may tail. While SPME fibers must be replaced
occasionally, SDME uses only standard syringes. Sometimes the stirring or sonication of samples in SDME can cause problems
with the suspended drop. A static or dynamic extraction could be employed as an alternative approach. If the droplet is too large,
it can be dislodged from the syringe tip when stirring. Unlike SPME, the liquid organic drop in SDME may dissolve slightly in the
aqueous sample. This dissolution is dependent on the aqueous solubility of the solvent used for extraction. The longer the
extraction time, the more the droplet size decreases. Most of the extraction experiments are less than 15 minutes and as a result,
dissolution may not be a problem. Additionally, some care must be taken to avoid carryover in the syringe needle.
One advantage of SDME is that the extracted sample contained in a small volume (1 or 2 µL) of organic solvent could potentially be
injected directly into an HPLC injector. Interfacing SPME to HPLC involves a complex instrumental arrangement. The rate of
dissolution of many analytes from the SPME fiber is quite slow resulting in initial band spreading during the displacement to the
HPLC column. A publication described single drop liquid phase microextraction followed by HPLC for the analysis of hypercins in
deproteinated plasma and urine
21
. Rather than attempting to inject the droplet directly into an HPLC injector, the authors transferred
the droplet to a microvial and diluted the sample to 30 µL with an aqueous compatible solvent (methanol) for injection into a
reversed-phase HPLC column.
In Chapter 6, the technique of Headspace-SDME was introduced. Similar to Headspace-SPME, the fiber is replaced by a microdrop
at the base of a syringe needle and the drop is exposed to the headspace of a solid- or liquid-sample in a vial or other closed
container. At the right hand side of Figure 7.9, the experimental setup for Headspace-SDME is depicted. The reader is referred to
Chapter 6 to get more details of the technique.

76
There has been an increasing interest in combining porous membranes of different configurations (e.g. hollow fiber, cylindrical,
and flat) with the various liquid-liquid extraction techniques. The polymer-based membranes serve as a barrier between the
sample and the extraction solvents. Only certain species are allowed to pass through the membrane pores resulting in increased
selectivity in extractions. These techniques will be thoroughly described in Chapter 18, which is devoted to the use of membranes
in sample preparation.
QuEChERS and related salting out extractions have recently gotten a great deal of attention, especially for the extraction of
pesticides from fruit and vegetable samples.
The QuEChERS technique combines liquid-liquid extraction with dispersive solid phase extraction, somewhat different than
classical LLE approaches outlined in this chapter. Chapter 8 will be devoted to QuEChERS and other salting out techniques for
sample extractions.
The FIE technique was first described by Karlberg and Thelander
22
and was designed to overcome the disadvantages of
conventional liquid-liquid extraction. In FIE, an aqueous sample is injected into an aqueous flowing stream. Segments of immiscible
organic solvent are then continuously inserted into this stream. After the segmented streams pass through a coil in which the
partitioning occurs, the organic phase is then separated from the aqueous phase and directed to a flow-through cell for
measurement. In some cases, air segments are introduced between segments to allow the smooth solvent passage without undue
mixing. Systems have also been developed where final phase separation is not necessary. Compared to conventional LLE, the
amount of solvent used in FIE is greatly reduced to several hundred microliters per analysis. The technique has been applied
extensively in on-line trace enrichment of metal ions when coupled to atomic absorption or inductively-coupled plasma
spectrometers. Other continuous flowing liquid-liquid extraction techniques appear to have more appeal and provide enhanced
enrichment factors.
Other Techniques Involving LLE Concepts
Flow Injection Extraction (FIE)

77
References
1.
Snyder, L.R.
J. Chromatogr. Sci.
1978,
16, 223
.
2.
Snyder, L.R.; Kirkland, J.J.; Dolan, J.W.
Introduction to Modern Liquid Chromatography
, 3rd Ed., Chapter 2 and Appendix I.2,
John Wiley and Sons, Hoboken, NJ, 2010.
3.
Snyder, L.R.
Chemtech
1979,
9
, 750.
4.
Snyder, L.R.
Chemtech
1980,
10
, 188.
5.
Ma T.S.; Horak, V.
Microscale Manipulations in Chemistry
, Wiley, New York, 1976.
6.
Mandava N.B.; Ito, Y. (Eds.)
Countercurrent Chromatography: Theory and Practice
, Dekker, New York, 1988.
7.
Riddick, J.D.; Bunger, W.B.
Organic Solvents
, Wiley-Interscience, New York, 1970.
8.
Pirard, C.
Mass Spectrometry Laboratory
, University of Liege, Belgium, published in Agilent Technologies Application Note Si-01002,
August 2010.
9.
REF: Commission Decision 2002/657/EC, Off.
J. Eur. Commun.
August 12, 2002.
10.
Pirard, C.; Widart, J.; Nguyen, B.K.; Deleuze, C.; Heudt, L.; Haubruge, E.; De Pauw, E.; Focant, J.-F.
J. Chromatogr. A
2007,
1152 (1-2)
,
116-123.
11.
Rezaee, M.; Assadi, Y.; Hosseini, M-R. M.; Aghaee, E.; Ahmadi, F.; Berijani, S.
J. Chromatogr. A
2006,
16
, 1-9.
12.
Rezaee, M.; Yamini, Y.; Faraji, M.
J. Chromatogr. A
2010,
1217
, 2342-2357.
13.
Caldas, S.S.; Costa, F.P.; Primel, E.G.
Anal. Chim. Acta
2010,
665 (1)
, 55-62.
14.
Arthur, C.L.; Pawliszyn,
J. Anal. Chem.
1990,
62
, 2145.
15.
Jeannot, M.A.; Cantwell, F. F.
Anal. Chem.
1996,
68
, 2236-2240.
16.
Liu, S.; Dasgupta, P.K.
Anal. Chem.
1995,
67
, 2042-2049.
17.
Jeannot, M.A.; Cantwell, F.F.
Anal. Chem.
1997,
69
, 235-239.
18.
He, Y.; Lee, H.K.
Anal. Chem.
1997,
69
, 4634-4640.
19.
Buszewski, B.; Ligor, T.
LCGC Europe
2002,
15 (2)
, 92-97.
20.
Psillakis, E.; Kalogerakis, N.
J. Chromatogr. A
2001,
938 (1-2)
, 113-120.
21.
Gioti, E.M.; Skalkos, D.C.; Fiamegos, Y.C.; Stalikas, C.D.
J. Chromatogr. A
2005,
1093
, 1-10.
22.
Karlberg, B.; Thelander, S.
Anal. Chim. Acta
1978,
98
, 1-7.

78
QuEChERS, Salting Out
Liquid-Liquid Extraction,
and Related Techniques
Salting Out Extraction
Liquid-liquid extraction (LLE) has long been an effective method of separating compounds having different solubilities in two
immiscible liquids (see Chapter 7). The two liquids are typically water, perhaps with some additives or pH adjusted, and a non-
polar organic solvent, such as isooctane or ethyl acetate. Typically, polar compounds prefer the aqueous layer while non-polar
compounds are extracted into the organic layer. A drawback of the use of non-polar, water-immiscible organic solvents is, due
to their low dielectric constants, they are relatively poor at the extraction of very polar or highly charged solutes, particularly for
highly water-soluble pharmaceuticals that may require extractions at very low or very high pH values. More polar solvents, such
as acetonitrile that provides solubility for these more polar compounds, are frequently water-miscible and thus cannot be used
for conventional LLE.
It has long been known that the addition of an inorganic salt into a mixture of water and a water-miscible organic solvent causes a
separation of the solvent from the mixture and the formation of a two-phase system
1
. Observations of this “salting out” phenomenon
were made for a number of water-miscible organics such as acetone, methanol, ethanol, and acetonitrile. Different salts caused
different degrees of phase separation. The high polarity, water-miscible solvents used in salting out systems have been investigated
for extraction or concentration of many analytes that cannot be extracted by conventional LLE solvents. This salting out effect usually
occurs at high salt concentrations. In some cases, the “salting out” (or perhaps better termed as “sugaring out”) effect can also be
achieved with high concentrations of saccharides
2
. Since extracts are not particularly clean, relative to other sample preparation
procedures, this simple extraction process is especially useful when very selective detection is used in GC or HPLC.
Chapter 8

79
Extractions using the salting out effect began to be used more extensively in the area of pesticide analysis. First, non-ionic pesticides
in various vegetal food samples were extracted using water-miscible organic solvents like acetonitrile and methanol. The Luke method
showed that acetone-water (65:35, v/v) could also be used for this purpose
5
. A multi-class, multiresidue method (MRM) was used by
Mills and co-workers to extract non-polar pesticides from various food samples of vegetal origin using acetonitrile
6
. The addition of
NaCl and water to the acetonitrile extract allowed partitioning into a very non-polar solvent, petroleum ether. The use of acetonitrile
followed by salting out proved to be better suited for the extraction of non-polar and polar pesticides from vegetable samples and has
been adopted today by several regulatory bodies
7
. However, the extracts were still rather “dirty”.
In 1993, Anastassiades, Lehotay, and co-workers at the U.S. Department of Agriculture in Wyndmoor, PA
8
further refined the
acetonitrile extraction by using NaCl salting out plus the addition of a drying agent (MgSO
4
) to remove the water and force the
pesticides into the acetonitrile phase. Termed the "QuEChERS" technique (pronounced “catchers”) standing for Quick, Easy, Cheap,
Effective, Rugged, and Safe, has quickly received widespread attention since its development
9
. The QuEChERS technique will be
covered on the following pages.
Salting out extraction has been used for the preconcentration of neutral polar organics from water. Leggett and coworkers showed
that high recoveries of trace explosive compounds can be achieved from water using acetonitrile/NaCl salting out extraction
3
. They
initially tried conventional LLE with methylene chloride as an extraction solvent, and for some nitroamines such as HMX and RDX,
extraction efficiency was rather poor. Addition of sodium chloride to the water improved the situation a bit, but the salting out
extraction with acetonitrile gave the greatest overall recoveries. In fact, the U.S. Environmental Protection Agency Method 8330A
for the isolation of nitroaromatic and nitramine explosives from water at the ng/L level is based on a salting out extraction method
4
.
The method involves the addition of 251 g of sodium chloride to a 1 L volumetric flask containing 770 mL of water sample. The
addition of 164 mL of acetonitrile with stirring is followed by a phase separation step. Removal of the acetonitrile upper layer is
followed by some additional small volume extractions and a back extraction. Finally, an aliquot is injected into a reversed-phase
HPLC column with a further confirmation on a cyano column.

80
Since its inception in 2003, the QuEChERS technique has grown in popularity, especially for the sample cleanup for the multi-class,
multiresidue analysis of pesticides in fruits and vegetables. The technique has expanded beyond these traditional samples to include
meat products, fish, blood, and even soil. The simple two-step extraction technique is based on salting out extraction followed by
dispersive solid phase extraction (dSPE) cleanup. Although NaCl was used for salting out in the original QuEChERS method
8
,
refinements were made when it was discovered that some base-sensitive compounds, such as the fungicides chlorothalonil and
captan, gave poor recovery. This discovery led to the development of two buffered methods for the initial extraction step. One new
method uses a 1% acetic acid/sodium acetate buffer, rather than NaCl
10
and forms the basis for the American Association of
Analytical Chemists (AOAC) QuEChERS 2007.01 Method
11
. The second new method, EN-15662 EU Method uses a weaker
buffering system consisting of 1 g sodium citrate and 0.5 g disodium citrate sesquihydrate (pH 5.5) and, in most cases, is equally
effective as the AOAC Method
12
. There are slight variations in the two extraction methods, such as slightly different sample
weights, solvent volumes, etc.
Following the acetonitrile extraction step, the introduction of a dSPE step, in which a portion of the raw extract is mixed with bulk
SPE sorbent such as C18, primary-secondary amine (PSA), and graphitized carbon that helps to further clarify the extract, and after
centrifugation, allows the supernatant to be injected directly into a GC/MS, LC/MS, or MS/MS system. In dSPE, the sorbent is
chosen to retain the matrix and undesired components and to allow the analyte(s) of interest to remain in the liquid phase. This is
the opposite from the normal mode of operation of solid phase extraction bind-elute method (see Chapter 9) where the sorbent is
chosen to retain the analytes of interest and not the matrix.
Hundreds of pesticide residues in a wide variety of fruits and vegetables are now isolated routinely using QuEChERS cleanup.
Although one can easily assemble the necessary materials from a general laboratory catalog to perform QuEChERS, many users
prefer to buy pre-packaged kits. Most companies selling QuEChERS extraction products carry all three kits to cover the original
unbuffered method, the AOAC Method, and the EU Method. These kits provide pre-weighed salts and dispersive SPE sorbents in
the proper size centrifuge tubes. Some vendors, such as Agilent Technologies, provide specific dSPE kits depending on the nature of
the fruit and/or vegetable sample (e.g. fatty vs highly pigmented matrices). Individual dSPE sorbents sold in bulk are available for
those who would like to customize their QuEChERS experiments.
Figure 8.1 shows how this simple procedure is performed.
QuEChERS, A Simple Yet Effective
Extraction Technique

81
QuEChERS
Step 1: Salting Out Extraction
Figure 8.1
1
Step 2: Dispersive Solid Phase Extraction (dSPE)
Weigh sample Add water and QC spikes
if needed and spike
with internal standard
Add acetonitrile Vortex or shake
Add salt packet Shake 1 minute
Centrifuge at 4000 rpm
for 5 minutes
Phase separation of acetonitrile
and aqueous layer
Choose the dispersive cleanup kit
and add acetonitrile extract
Vortex for 1 minute Centrifuge at 4000 rpm
for 5 minutes
Take aliquot of supernatant and
dry down or dilute as necessary
Place in autosampler vials
for GC or LC analysis
2 3
5 6 7
1 2
4 5
3
4
8

82
Into a 50 mL centrifuge tube, weigh a 10 g (AOAC) or 15 g (EN) portion of the homogenized sample. Because this sample mass is
reduced compared to more traditional extraction approaches, it is of utmost importance to ensure that the original sample, that can
be up to kilograms in weight, be extremely homogeneous. Thus, a powerful chopping device is recommended to homogenize the
sample to maximize surface area and to ensure better extraction efficiency. Such a homogenization procedure would help to ensure
that the 10 or 15 g subsample is representative of the original. To prevent loss of more volatile pesticides during the homogenization
step, two additional steps are recommended: pre-freezing chopped samples and dry ice addition. Since water is one of the two
solvents used in the initial extraction procedure, there must be a presence of water at this point of the experiment. Many fruit and
vegetable samples contain between 80-95% water, which is required for the partitioning to take effect. For dry samples such as
beans or grain, an appropriate portion of water should be added.
Next, 10 mL (EN Method) or 15 mL (AOAC Method ACN) is added to the centrifuge tube containing the solid sample. Although
other non-halogenated solvents such as acetone and ethyl acetate may be used, acetonitrile is the recommended solvent for
QuEChERS because, upon the addition of salts, it is separated more easily from water than acetone. The polarity of acetonitrile is
higher than that of acetone and ethyl acetate. According to “like dissolves like” theory, the medium to high polarity pesticides have
much better solubility in acetonitrile than in the other two solvents. As a result, the extraction recoveries of polar pesticides are
significantly improved by the use of acetonitrile.
Ethyl acetate has the disadvantages of: 1) a partial miscibility with water (but it could extract lipids and waxes); 2) lower recoveries
for acid/base pesticides; and 3) provides less cleanup in dSPE. However, acetonitrile extracts less of the lipophilic materials.
Compared to acetone, the use of acetonitrile allows for better removal of residual water with magnesium sulfate. It is compatible
with HPLC mobile phases and GC applications, although it tends to give a relatively large solvent expansion volume during sample
vaporization in the GC inlet, interferes with nitrogen specific GC detectors, and is less volatile than other common GC solvents. Thus,
if evaporative concentration steps are required for HPLC solvent reconstitution, or for an increase in concentration (of non-volatiles,
of course), the process is more time-consuming. Prior to the addition of salt (Figure 8.1.4), the sample-acetonitrile mixture is
shaken to wet the entire sample (Figure 8.1.3). This process will also prevent potential labile pesticide loss due to temporary heat
produced when the salts are added (in the next step).
Extraction – Partitioning Solvent (Figure 8.1.2)
Initial Sample Preparation (Figure 8.1.1)

83
To minimize error generation in the multiple steps of the QuEChERS method, an internal standard is often added somewhere in the
process, sometimes earlier in the salting out extraction step. In the original development work, the authors
8
used triphenylphosphate
(TPP), which had the right properties to undergo quantitative extraction from low-fat matrices. However, it was later found that the
use of more than one internal standard as a quality control measure enable recognition of errors due to mis-pipetting or discrimination
during partitioning or cleanup
14
. After shaking the sample- internal standard-acetonitrile-salt mixture for 1 min, the centrifuge tube is
placed in a centrifuge (Figure 8.1.6) and spun for 5 min at 4,000 rpm to ensure phase separation and to allow excess salts and
matrix to be separated from the supernatant liquid (Figure 8.1.7).
To aid in the thorough mixing between the solids and the liquid phases, and to cut down on the shaking time, the use of Agilent
ceramic homogenizers in the 50 mL centrifuge tube during the initial extraction step is recommended. These inert, non-porous, solid
rods have an angled cutting surface that helps to ensure that there is intimate contact with the sample and extraction chemicals
and helps to break up salt agglomerates, if any are present. They have been shown to cut the shaking time considerably
15
and
reduce variance between people, since manual shaking by hand can be a variable in the QuEChERS process. The RSDs were found
to be improved.
The purpose of salt addition is to induce phase separation. The salting out effect also influences analyte partition, which is
dependent on the solvent used for extraction. The concentration of salt can influence the percentage of water in the organic phase
and can adjust its “polarity”. In QuEChERS, acetonitrile alone is usally sufficient to achieve excellent extraction efficiency without
the need to add non-polar co-solvents that dilute the extract and make them too non-polar. In some cases, the pH of the extraction
must be controlled.
Most, but not all, pesticides are more stable at lower pH values. For certain problematic pesticides, such as those that are strongly
protonated at low pH, the extraction system must be buffered in the pH range 2-7 for successful extractions
13
. Of course, the pH at
which the extraction is performed can also influence the co-extraction of matrix compounds and pesticide stability. For this step, it is
important that the salt be added after the acetonitrile-sample mixture is shaken. If the sample is added to the salt mixture,
exothermic heating may occur which could cause loss of volatile pesticides or cause degradation of labile pesticides. For the salt
addition, handy pre-weighed, pourable packets of the appropriate composition of a salt mixture (anhydrous, packed under nitrogen)
are commercially available. It is advisable to avoid using salt pre-filled centrifuge tubes due to this localized heating effect.
Addition of Salts (Figure 8.1.4)
Internal Standard Addition (Figure 8.1.2 and Figure 8.1.5)

84
Traditionally, SPE cleanup uses plastic cartridges containing various amounts of sorbent material. In dSPE, an aliquot of sample extract
(for example, 1, 6, or 8 mL) is added to a vial containing a small amount of SPE sorbent (Figure 8.1.8) and the mixture shaken by
hand (1 min) or mixed on a vortex mixer to evenly distribute loose SPE material and facilitate the cleanup process. The sorbent is then
separated by centrifugation (Figure 8.1.9), an aliquot of the supernatant (Figure 8.1.10) is subjected to analysis (Figure 8.1.11).
The sorbent is chosen to retain matrix components, and not the analytes of interest. In some cases, mixed sorbents can be used. For
samples with a high fat matrix, primary-secondary amine (PSA) mixed with C18 sorbent is recommended. For samples with high
levels of chlorophyll and carotenoids (for example, spinach, and carrots), PSA is mixed with graphitized carbon black to reduce these
colored compounds. Although the addition of graphitized carbon black helps with the removal of pigment, there is an accompanying
low recovery of certain structurally planar pesticides, such as Carbendazim and Cyprodinil. The planar pesticide recoveries can be
increased by the addition of toluene in the extraction step
16
. Newer sorbents that remove chlorophyll without adsorbing planar
pesticides have become available.
dSPE (Figure 8.1.8)
Table 8.1 provides some guidance on how to match the dSPE sorbent with the fruit and vegetable sample matrix based on sample
matrix: fat content, waxiness, and the presence and level of pigments. For convenience, the Agilent part numbers for the various
QuEChERS dSPE kits are provided.

85
Part numbers ending in CH indicate tubes containing ceramic homogenizers.
(Continued)
Selection of dSPE Sorbent Based on Food Matrix
Table 8.1
QuEChERS Dispersive Kits, Fruits and Vegetables
Kit Size Unit
AOAC 2007.01
Method
European
Method EN
15662
Kit Contents
Part No.
Kit Contents
Part No.
General fruits and vegetables:
Removes polar organic acids, some
sugars and lipids
2 mL 100/pk 50 mg PSA
150 mg MgSO
4
5982-5022
5982-5022CH
25 mg PSA
150 mg MgSO
4
5982-5021
5982-5021CH
15 mL 50/pk 400 mg PSA
1200 mg MgSO
4
5982-5058
5982-5058CH
150 mg PSA
900 mg MgSO
4
5982-5056
5982-5056CH
Fruits and vegetables
with fats and waxes:
Removes polar organic acids, some
sugars, more lipids and sterols
2 mL 100/pk 50 mg PSA
50 mg C18EC
150 mg MgSO
4
5982-5122
5982-5122CH
25 mg PSA
25 mg C18EC
150 mg MgSO
4
5982-5121
5982-5121CH
15 mL 50/pk 400 mg PSA
400 mg C18EC
1200 mg MgSO
4
5982-5158
5982-5158CH
150 mg PSA
150 mg C18EC
900 mg MgSO
4
5982-5156
5982-5156CH
Pigmented fruits
and vegetables:
Removes polar organic acids, some
sugars and lipids, and carotenoids
and chlorophyll; not for use with
planar pesticides
2 mL 100/pk 50 mg PSA
50 mg GCB
150 mg MgSO
4
5982-5222
5982-5222CH
25 mg PSA
2.5 mg GCB
150 mg MgSO
4
5982-5221
5982-5221CH
15 mL 50/pk 400 mg PSA
400 mg GCB
1200 mg MgSO
4
5982-5258
5982-5258CH
150 mg PSA
15 mg GCB
885 mg MgSO
4
5982-5256
5982-5256CH

86
Part numbers ending in CH indicate tubes containing ceramic homogenizers.
(Continued)
Highly pigmented fruits
and vegetables:
Removes polar organic acids, some
sugars and lipids, plus high levels of
carotenoids and chlorophyll; not for
use with planar pesticides
2 mL 100/pk 25 mg PSA
7.5 mg GCB
150 mg MgSO
4
5982-5321
5982-5321CH
15 mL 50/pk 150 mg PSA
45 mg GCB
855 mg MgSO
4
5982-5356
5982-5356CH
Fruits and vegetables
with pigments and fats:
Removes polar organic acids, some
sugars and lipids, plus carotenoids
and chlorophyll; not for use with
planar pesticides
2 mL 100/pk 50 mg PSA
50 mg GCB
150 mg MgSO
4
50 mg C18EC
5982-5421
5982-5421CH
15 mL 50/pk 400 mg PSA
400 mg GCB
1200 mg MgSO
4
400 mg C18EC
5982-5456
5982-5456CH
Kit Contents
Part No.
Kit Contents
Part No.Kit Size Unit
AOAC 2007.01
Method
European
Method EN
15662
QuEChERS Dispersive Kits, Fruits and Vegetables

87
Part numbers ending in CH indicate tubes containing ceramic homogenizers.
QuEChERS Dispersive Kits: Other Food Methods
Other Food Methods
Removes biological matrix
interferences, including hydrophobic
substances (fats, lipids) and proteins
2 mL 100/pk 25 mg C18
150 mg MgSO
4
5982-4921
5982-4921CH
15 mL 50/pk 150 mg C18
900 mg MgSO
4
5982-4956
5982-4956CH
All Food Types
Removes all matrix interfering
materials including polar organic
acids, lipids, sugars, proteins,
carotenoids and chlorophyll
2 mL 100/pk 50 mg PSA
50 mg C18
7.5 mg GCB
150 mg MgSO
4
5982-0028
5982-0028CH
15 mL 50/pk 400mg PSA
400 mg C18
45 mg GCB
1200 MgSO
4
5982-0029
5982-0029CH
Animal Origin Food
Removes matrix interferences such
as polar organic salts, sugars, lipids
and proteins
15 mL 50/pk 50 mg PSA
150 mg C18EC
900 mg Na
2
SO
4
5982-4950

88
“Analyte Protectants” (Optional):
An optional step, mainly used for GC analysis, is found to be very useful for pesticides that are unstable at intermediate pH values
and for sensitive analytes that behave poorly in the GC system, such as response loss, peak tailing, etc. due to GC flow path surface
activities, including active sites on inlet liners, GC capillary column and other interior surfaces. In this case, analyte protectants are
added to the extracts before sample injection into the GC system. The protectants compounds do not interfere with the analysis
of pesticides of interest yet they will reduce the interactions of these sensitive pesticides with the GC flow path active sites, thus
protecting these pesticides from poor chromatographic performance. Thorough studies were devoted to selecting the appropriate
analyte protectants
17-18
. The results demonstrated that errors in GC analysis caused by matrix effects were also reduced dramatically
with the help of the analyte protectants. Of course, with LC and LC/MS, the protectants are not recommended.
Analysis
After centrifugation (Figure 8.1.9), and ensuring that any sorbent or excess salts are not in the supernatant, the sample aliquot
from the dSPE step can be injected directly into a GC or HPLC system without further workup. For LC/MS analysis, it might be
necessary to dilute properly with acidic water to provide MS chromatographic integrity. For GC/MS analysis, if the instrument is not
equipped with a programmable temperature vaporizer, evaporation of the supernatant with reconstitution in a GC-compatible
solvent might be needed. In general, extracts from the simple QuEChERS process are not as clean as from other more work-
intensive sample preparation procedures such as SPE. Thus, the use of selective detection is highly recommended.
Applications of QuEChERS
Although the main emphasis of the QuEChERS approach has been the analysis of pesticides in foods and other agricultural
products, the range of applications is expanding greatly. Table 8.2 lists typical published applications of different analytes and
different matrices that show the versatility of this growing sample preparation technique.

89
Selected QuEChERS Application Notes
Analyte(s) Matrix Reference Agilent Application Note
Acrylamide Cooking oil 5990-8988EN
French fries 5990-5940EN
Amino acids Spinach, apples J.W. Henderson, T. Faye, U. Wittek, and J. Stevens, poster paper presented at RAFA
2009, Prague, Czech Republic
Hormones Shrimp 5990-6589EN
PAHs Fish 5990-5441EN, 5990-4248EN, 5990-6668EN, 5990-7908EN, 5990-7714EN
Soil 5990-5452EN (AOAC), 5990-6324EN
5990-6324EN, 5990-5452EN
PCBs Fish 5990-6263EN, 5990-7908EN, 5990-7714EN
Pesticides Apple 5990-4468EN, 5990-6558EN, 5990-3937EN
Rice 5990-8034EN
Green tea 5990-6400EN
Lemon oil 5990-6432EN
Spinach 5990-4305EN (AOAC), 5990-4395EN, 5990-4247EN, 5990-4248EN, 5990-9317EN
Lettuce 5990-6558EN
Baby food 5990-5028EN
White flour 5990-9317EN
Pepper
Orange
Carrot 5990-Si-02213
Green pepper 5990-8067EN, 5989-8614EN
Cucumbers 5990-8067EN, 5990-6323EN
Tomato 5990-8067EN, 5990-6323EN, 5989-8614EN
Tea (green & black) 5990-9865EN
Beans Si-02213
Avocados Avocado_poster_EPRw2006.pdf
Fish 5990-7908EN, 5990-7714EN, 5990-6595EN
Olive oil 5990-5553EN, 5990-7722EN
Strawberry 5990-7706EN, 5590-9317EN
Pear
Banana
Pharmaceuticals Whole blood 5990-8789EN
Quinolone antibiotics Bovine liver 5990-5085EN
Sulphonamide antibiotics Bovine liver 5990-5086EN
Chicken muscle 5990-5395EN
Veterinary drugs Animal origin foods 5991-0013EN
Table 8.2

90
To illustrate the application of this technique, we will show some results obtained for the extraction and analysis of 16
“representative” pesticides in apples using the AOAC methodology
19
. The representative list chosen by Lehotay et al
20
included
nine different pesticide classes, including acidic, basic, neutral, base-sensitive, and acid-labile pesticides suitable for LC-MS/MS
analysis. Figure 8.2 illustrates the QuEChERS workflow used in this study.
Workflow for Pesticides in Apple QuEChERS Extraction
Figure 8.2
Accurately weigh 15 g homogenized sample (±0.05 g) in 50 mL centrifuge tubes
Spike samples with 100 µL of IS solution and vortex for 1 min
Add 15 mL of 1% acetic acid in ACN and shake vigorously for 1 min
Add Bond Elut QuEChERS AOAC salt packet, cap tubes, and shake vigorously for 1 min
Centrifuge at 4000 rpm for 5 min
Vortex 1 min then centrifuge
Transfer 200 µL extract to autosampler vial, dilute with 800 µL appropriate solution if necessary
Samples are ready for LC-MS/MS analysis
Transfer upper ACN layer to Bond Elut QuEChERS dispersive-SPE tube,
1 mL/2 mL tube or 8 mL/15 mL tube

91
In order to get the most reliable statistical results, the initial sample preparation is an extremely important part of the QuEChERS
process. A large enough sample must be used in order to obtain a representative portion and this large sample must be finely
comminuted since only 15 g is required for the QuEChERS extraction. A 15 g sample of comminuted apple (from 3 pounds of
organically grown apples) was placed into a 50 mL centrifuge tube and an internal standard added, followed by vortexing. Next,
15 mL of 1% acetic acid in acetonitrile was added followed by an Agilent AOAC Buffered Extraction packet containing 6 g of
anhydrous MgSO
4
and 1.5 g of anhydrous sodium acetate. As noted earlier, it is important to add the buffer salt after the addition
of acetonitrile since the addition of salt is an exothermic reaction and can cause localized heating in the sample, potentially
causing thermal decomposition. Next, the entire mixture was sealed tightly and shaken vigorously by hand followed by
centrifugation. This operation represents the salting out extraction step. Acetonitrile forms a separate upper layer from the water
layer that results from the apple matrix.
The next step was a further clarification using dSPE. A 1 mL aliquot of the upper acetonitrile layer was transferred into a 2 mL
dispersive SPE tube containing pre-weighed 50 mg of primary secondary amine (PSA) and 150 mg of anhydrous MgSO
4
. The tube
was tightly capped, vortexed, and finally centrifuged. A 200 µL aliquot was transferred to an autosampler vial and diluted with 800 µL
of water. The sample was then analyzed by LC-MS/MS.
The HPLC separation was performed by gradient reversed-phase chromatography on a phenyl-hexyl column. The operating
conditions are given in reference 19. The LC-MS/MS chromatograms of an apple blank carried through the entire QuEChERS
procedure is shown in Figure 8.3A. The final QuEChERS prepared sample will contain food matrix impurities because it is a simple
sample extraction and cleanup procedure. With the powerful selectivity of LC-MS/MS operated in the multiple reaction monitoring
(MRM) mode (see instrument data acquisition settings in reference 19), the extract apple blank did not contribute any interferences
with the target compounds. Figure 8.3B shows the chromatogram of a 10 ng/g (10 ppb) fortified apple extract at the limit of
quantitation (LOQ). This LOQ for the pesticides in apple shown was well below the maximum residue levels (MRLs). Even though
some of the pesticide peaks showed poor chromatographic resolution, the selectivity of electrospray ionization tandem mass
spectrometry permitted the accurate quantitation. Mean recoveries ranged between 76-117% (95.4% on average) with RSD
values below 15% (4.3% on average). Such values are acceptable when performing trace pesticide analysis in food matrices.

92
0
0.05
1
12 23 24 4
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0.45
0.5
0.55
0.6
0.65
0.7
0.75
0.8
0.85
0.9
0.95
0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8 8.5 9 9.5 10 10.5 11 11.5 12 12.5
13
1
x10
2
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0.45
0.5
0.55
0.6
0.65
0.7
0.75
0.8
0.85
0.9
0.95
0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8 8.5 9 9.5 10 10.5 11 11.5 12 12.5 13
1
x10
3
1122334
No interference was found in the blank
Chromatogram of
10 ng/g fortified
apple extract.
LC-MS/MS Chromatograms of Blank Apple Extract
and Spiked Pesticide Extract
Figure 8.3
Chromatogram of Apple Extract Blank
Counts (%) vs. acquisition time (min)
Counts (%) vs. acquisition time (min)
1. Methamidophos
2. Acephate
3. Pymetrozine
4. Carbendazim
5. Imidacloprid
6. Thiabendazone
7. Dichlorvos
8. Propoxur
9. Thiophanate methyl
10. Carbaryl
11. Ethoprophos
12. Penconazole
13. Cyprodinil
14. Dichlofluanid
15. Kresoxim
16. Tolyfluanid
A
B

93
References
1.
Frankforter, G.B.; Cohen, L.
J. Am. Chem. Soc.
1914,
36
, 1103-1134.
2.
Wang, B.; Ezeji, T.; Feng, H.; Blaschek, H.
Chemical Engineering Journal
2008,
63
, 2595-2600.
3.
Leggett, D.C.; Jenkins, T.F.; Miyares, P.H.
Anal. Chem.
1990,
62
, 1355-1356.
4.
Method 8330A Nitroaromatics and Nitramines by High Performance Liquid Chromatography
, Rev. 1, U.S. Environmental
Protection Agency, January 1998.
5.
Luke, A.; Doose, G.M.
Bull. Environ. Contam.
1983,
30
, 110.
6.
Mills, P.A.; Onley, J.H.; Gaither, R. A.
J. Assoc. Off. Anal. Chem.
1963,
46
, 186.
7.
Schenck, F.J.; Wong, J.W.; J.L. Tadeo (Ed.)
Analysis of Pesticides in Food and Environmental Samples
, CRC Press, Boca Raton, FL,
2008, 154.
8.
Anastassiades, M.; Lehotay, S.J.; Stainbaher, D.; Schenk, F.
J. AOAC Int.
2003,
86
, 412-431.
9.
Majors, R.E.
LCGC No. America
2007,
25 (5)
, 436-446.
10.
Lehotay, S.J.; Mastovska, K.; Lightfield, A.R.
J. AOAC Int.
2005,
88
, 630-638.
11.
AOAC Official 2007.01 Method: Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium
Sulfate
, 2007.
12.
EN 15662, Foods of Plant Origin – Determination of Pesticide Residues Using GC/MS and/or LC-MS/MS Following
Acetonitrile Extraction/Partitioning and Cleanup by Dispersive SPE – QuEChERS Method
, 2008.
13.
Lehotay, S.J.; Mastovska, K.; Lightfield, A.R.
Use of buffering to improve results of problematic pesticides in a fast
and easy method for residue analysis of fruits and vegetables, J. AOAC Int.
2005, 88, 615-629.
14.
http://www.quechers.com/ (follow link to QuEChERS –
A Mini-Multiresidue Method for the Analysis of Pesticide Residues in
Low-Fat Products
)
15.
Stevens, J.; Bertin, L.; Rothermich, M.
Increasing Efficiency and Pesticide Recovery from the QuEChERS Approach for Fruit and
Vegetable Products Using Ceramic Homogenizers
, European Pesticides Residue Workshop, Strasbourg, Poster 24, June 20-24, 2010.
16.
Zhao, L.; Stevens, J.
Optimizing Recoveries of Planar Pesticides in Spinach Using Toluene and Agilent Bond Elut QuEChERS
AOAC Kits with Graphitized Carbon
, Agilent Technologies Application Note #5990-4247EN, 2012.
17.
Anastassiades, M.; Masrovska, K.; Lehotay, S.J.
J. Chromatogr. A
2003,
1015 (1-2)
, 163-184.
18.
Masrovska, K.; Lehotay, S.J.; Anastassiades, M.
Anal. Chem.
2005,
77 (24)
, 8129-8137.
19.
Zhao, L.; Schulz, D.; Stevens, J.
Analysis of Pesticide Residues in Apples Using Agilent SampliQ QuEChERS Kit by LC-MS/MS
Detection
, Application Note #5990-3937EN, Agilent Technologies, Wilmington, DE, 2009.

94
Chapter 9
Solid Phase Extraction (SPE)
SPE vs. LLE
Solid phase extraction is an important technique used in sample pre-treatment for chromatographic analysis. SPE can be used in
similar fashion as liquid-liquid extraction (LLE). Whereas LLE is a one-stage separation process, SPE is a chromatographic procedure
that resembles HPLC and has a number of potential advantages compared to LLE:
•
More complete extraction of the analyte
•
More efficient separation of interferences from analytes
•
Increased separation selectivity
•
Reduced organic solvent consumption
•
Easier collection of the total analyte fraction
•
A trace-enrichment process offering inherent concentration potential
•
More convenient manual procedures
•
Removal of particulates
•
More easily automated
Solid phase extraction is one of the more widely used sample preparation techniques for liquid samples or solid samples that have
been put into a liquid form by dissolution or extraction. SPE can also be used for certain gaseous sample by trapping them on a
sorbent or by in situ derivatization using reactive chemicals.

95
Because SPE is often a more efficient isolation process than LLE, it is easier to obtain a higher recovery of the analyte. LLE procedures
that may require several successive extractions to recover 99+% of the analyte often can be replaced by one-step SPE methods.
With SPE, it is also possible to provide a more complete removal of interferences from the analyte fraction. Reversed-phase SPE
techniques (commonly called non-polar SPE or RP-SPE) are the most popular as only smaller amounts of organic solvent are required,
maintaining a high concentration of analyte. Because there is no need for phase separation (as in LLE), the total analyte fraction is
easily collected in SPE, eliminating errors associated with variable or inaccurately measured extract volumes. In SPE, there is no
chance of emulsion formation. Finally, larger particulates are trapped by the SPE cartridge or other device (see Chapter 5) and do not
pass through into the analyte fraction. Thus, SPE has a dual function of cleanup and particulate removal and may eliminate the need
for filtration prior to injection. In most cases, non-polar SPE offers concentration as an added benefit.
However, there are some disadvantages of SPE vs. LLE that include:
•
Mixed mechanisms in SPE can occur
•
Irreversible adsorption of some analytes on SPE cartridges
•
More complex method development is required (3 or more steps involved)
The solvents used in LLE are usually pure and well defined, so that LLE separations are quite reproducible. On occasion, SPE phases
can result in multiple mechanisms (e.g. reversed-phase and ion exchange) and may confuse the operator. The surface area of an
LLE device (e.g., separatory funnel) is quite small compared to an SPE cartridge, and especially its packing. For this and other
reasons, irreversible binding of analyte (with lower recoveries) is less likely with LLE vs. SPE. There are four steps in a typical non-
polar SPE: conditioning, loading, washing, and elution – each of which requires some degree of optimization while solvent selection
in LLE may be more straightforward.

96
SPE vs. HPLC
In its simplest form, SPE employs a small plastic disposable column or cartridge, often the barrel of a medical syringe packed
with 0.1-10.0 g sorbent. The sorbent is commonly a reversed-phase material, e.g., C18-silica, and reversed-phase SPE (RP-SPE)
and resembles both LLE and reversed-phase HPLC in its separation characteristics. More selective SPE phases (e.g. ion
exchange, affinity) can sometimes be a better choice than RP-SPE, which mainly distinguishes analytes and matrix in terms of
their relative hydrophobicity.
For simplicity, in the following explanations, we will assume that RP-SPE is being used, unless noted otherwise. Although silica
gel-based bonded phase packings are the most popular, polymeric sorbents have become available in recent years and have
been gaining in popularity. Compared to silica-based SPE packings, polymeric packings have the advantage of higher surface area
(thus higher capacity), chemical balance of hydrophilic-hydrophobic properties (better wettability and can dry out somewhat after
the conditioning step without affecting recovery and reproducibility), absence of silanols (less chance of irreversible adsorption of
highly basic compounds), and wide pH range (more flexibility in adjusting chemistries).
In its most popular configuration, the SPE packing is held in the syringe barrel by frits, just like an HPLC column. The particle size
(40 µm average) is typically much larger than that in HPLC (1.5-10 µm). Because of shorter bed lengths, larger particles, and less
well packed beds, SPE cartridges are much less efficient (N <100) than an HPLC column. For cost reasons, irregularly-shaped
packings (rather than spherical particles) are usually used in SPE. Recently, spherical silicas for SPE have come onto the
marketplace but have not impacted the sale of the most popular products. Polymeric sorbents are generally spherical but usually
more expensive than silica-based packings. Some of the SPE disks do, however, use the more expensive spherical SPE packings
with particle diameters in the 7 µm range.
Overall, the principles of separation, phase selection, and method development for SPE are similar to those for HPLC. One major
difference between SPE and HPLC is that the SPE cartridge is generally used once and discarded, since potential interferences may
remain on the cartridge. Of course, HPLC columns are used over and over again.

97
Uses of SPE
SPE is used for six main purposes in sample preparation:
•
Removal of interferences
•
Concentration or trace enrichment of the analyte
•
Desalting
•
Phase exchange
•
In situ derivatization
•
Sample storage and transport
Interferences from matrix or undesired compounds in the sample that overlap analyte bands in the HPLC or GC separation
complicate method development and can adversely affect assay results. In some cases, especially for complex samples (e.g. natural
products, food, protein digests), a large number of interferences in the original sample may make it almost impossible to separate
these from one or more analyte bands with only one HPLC or GC separation. SPE can be used to reduce or eliminate those
interferences. Some samples contain components, e.g., hydrophobic substances (e.g. fats, oils, greases), proteins, polymeric
materials, particulates that can either plug or deactivate the HPLC column. These detrimental compounds can often be removed by
RP-SPE. In GC, high boilers can lodge at the head of the column and slowly bleed off giving extraneous peaks and baseline drift.
SPE can help remove these interferences prior to injection as well.
Interference removal
Trace enrichment/analyte concentration
SPE can often be used to increase the concentration of a trace component. If an SPE cartridge can be selected so that
k >>1 for the analyte – a relatively large volume of sample – can be applied before the analyte saturates (overloads) the stationary
phase and begins to elute from the cartridge. The net result is a considerable increase in the concentration of analyte when eluted
with a strong solvent (k <1), which implies an increase in detection sensitivity (called trace enrichment). An example of trace
enrichment is the use of SPE to concentrate sub-parts per billion concentrations of polynuclear aromatic hydrocarbons
1
or
pesticides
2
from environmental water samples using an RP-SPE cartridge. A strong solvent (e.g., ACN or MeOH) elutes these
analytes from the cartridge in a small concentrated volume, which also saves on evaporation time. If taken to dryness, the sample
residue can then be redissolved (reconstituted) in a solvent compatible with the subsequent HPLC or GC separation. Alternatively,
a miscible weak solvent can be added to the SPE eluant to dilute the stronger solvent and perhaps allow direct injection of the
resulting sample into an HPLC column.

98
RP-SPE is often used to desalt biological and other high-salt samples, especially prior to ion exchange HPLC. Conditions of pH and
%-organic are selected to retain the analyte initially, which allows the inorganic salts to be washed from the cartridge with water.
The analyte can then be eluted (salt-free) with organic solvent
3
.
Although used less frequently than the previous uses, phase exchange can be performed by isolating the analyte of interest
on the SPE cartridge, then by flowing air or dry nitrogen through the cartridge to remove the initial liquid. Next, a second
non-compatible solvent can be used to elute the analyte into a collection device for further sample preparation or injection
into a chromatograph.
In one of the procedures of in situ derivatization, an active derivatizing reagent is sorbed onto an SPE cartridge and a sample
containing an active analyte is passed through the cartridge. Depending on the reaction kinetics, the analyte can be simultaneously
derivatized and concentrated. A strong solvent can then be used to elute the derivatized compound which can then be handled
chromatographically. One example of such a procedure in practice is the in situ derivatization of carbonyl-containing compounds
with 2,4-dinitrophenylhydrazine
4
. Another approach to in situ derivatization involves the in-solution derivatization in the presence of
a SPE sorbent that isolates the derivatized analyte in solution. Subsequently, the derivatized compound is eluted for analysis. An
example of this approach involved the thermal hydrolysis and direct methylation of bacterial fatty acids with tetramethylammonium
hydroxide from a sample of bacterial lipids. The derivatized fatty acid methyl esters were then subjected to GC-ion mobility mass
spectrometry for analysis
5
. Another example of this approach is the in situ derivatization of acidic pesticides that were reacted with
butylchloroformate in an aqueous environment and then extracted using on-line SPME cleanup using a PDMS fiber with
subsequent thermal desorption into a GC/MS
6
.
Sample storage and/or transport is an interesting application of SPE cartridges. Oftentimes, analytes adsorbed to a solid support
are stabilized relative to their stability in solution. This observation allows samples to be stored on a cartridge sorbent allowing for
storage and for transport (through the mail, for example). Before using this approach, individual set of analytes should be tested in
terms of longterm stability when sorbed to a solid support.
Desalting
Phase exchange
In situ derivatization
Sample storage and transport
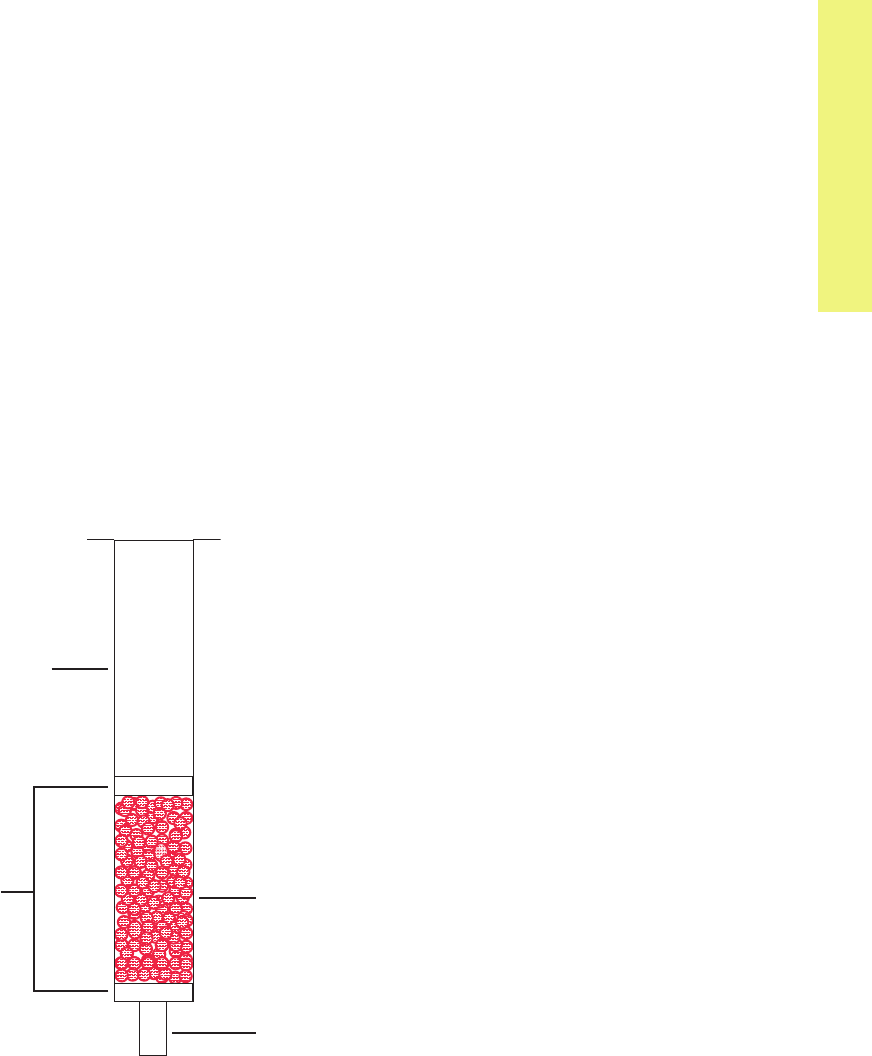
99
SPE Devices and Formats
There are several designs that are used for SPE:
•
Cartridge (Figure 9.1)
•
Disk (Figure 9.2)
•
Pipette tip (Figure 9.3)
•
96-well plate (Figure 9.4)
•
Coated fiber (solid phase microextraction, SPME, Figure 9.5)
or coated stir bar (stir bar sorbent extraction, SBSE, Figure 9.6)
Figure 9.1
Typical Syringe Barrel Cartridge Design
Syringe barrel
Frits
SPE packing
Luer tip

100
The most popular configuration for an SPE device is the cartridge. A typical SPE disposable cartridge (syringe barrel format) is
depicted in Figure 9.1. The syringe barrel is usually medical-grade polypropylene, chosen for its purity. If trace levels of impurities
such as plasticizers, stabilizers, or mold release agents are present in the plastic used for cartridges, they may be extracted during
the SPE process and contaminate the sample, perhaps giving improper results or causing ion suppression in mass spectrometry.
The outlet of the syringe barrel normally has a Luer tip so that a needle can be affixed to direct effluent directly into a small
container or vial.
The frits maintaining the particle bed in the cartridge are of PTFE, polyethylene, stainless steel, or titanium (rarely) construction with
porosity of 10-20 µm that offers little flow resistance. SPE cartridges may vary in design to fit an automated instrument or robotics
system. More expensive but more inert cartridges of glass or virgin PTFE are available for ultratrace analyses (sub-parts per billion)
when the standard syringe barrel plastics may produce unacceptable concentrations of extractable interferences or adsorption on
the plastic walls.
With the exception of special cartridges referred to above, in general, SPE cartridges packed with bonded silica or sorbent
packings are relatively inexpensive. They are generally used a single time and discarded because of the potential of sample
cross-contamination.
To accommodate a wide range of SPE applications, standard cartridges are also available with reservoir volumes (the volume above
the packing in the cartridge) of 0.5-10 mL with packing weights of 35 mg-2 g. For very large samples, “mega” cartridges have up
to 75 g of packing and a 150 mL reservoir. These cartridges can also be considered for use in flash chromatography applications
(see Chapter 12), although flash chromatography and “true” SPE differ in theory as well as in practice. Cartridges with a larger
amount of packing should be used for dirty samples that can overload a low capacity cartridge. However, cartridges containing
100 mg of packing or less are preferred for relatively clean liquid samples where cartridge capacity is not an issue, or for trace
analysis and small sample volumes. Due to the higher surface areas of polymeric SPE packings and potentially greater retentive
capacity per unit area, packed bed masses are typically in the 5-60 mg range, which leads to smaller volumes of sample and
solvent. In most cases, it is desirable to collect the analyte in the smallest possible volume, which means that the SPE cartridge
generally should also be as small as possible. Smaller volumes also take less time to evaporate the elution solvent when
reconstituting to a solvent that is more compatible with the analytical method.
SPE cartridges

101
Another popular configuration is the SPE disk (Figure 9.2). SPE disks combine the advantages of membranes (see Chapter 18)
and solid phase extraction. In their appearance, the disks closely resemble membrane filters: they are flat, usually one mm or less
in thickness, with diameters ranging from 4-96 mm. The SPE packing in these disks generally comprises 60-90% of the total
membrane weight. Some disks are sold individually and must be installed in a reusable filter holder. However, the most popular
disks are sold pre-loaded in disposable holders or cartridges with Luer fittings for easy connection to syringes. The physical
construction of the SPE disks differs from membrane filters (see Chapter 18). SPE disks consist of:
•
Flexible- or expanded-PTFE networks that are filled with silica-based or resin packings
•
Rigid fiberglass disks with embedded packing material
•
Packing-impregnated polyvinylchloride
•
Derivatized membranes
SPE disks
Figure 9.2
Typical SPE Disk Configuration
Holder
Holder
Sorbent disk
Prefilter (optional)

102
SPE disks and cartridges differ mainly in their length/diameter ratio (L/d): disks have L/d <1 and cartridges have L/d >1.
Compared to SPE cartridges, this characteristic of the disk enables higher flow rates and faster extraction. For example, one liter
of relatively clean water can pass through a 45 mm diameter disk in approximately 15-20 min, but may require 2 h when using a
15 mm x 8 mm cartridge bed. “Dirty” water, or water containing particulates, such as wastewater, can plug the porous disks, just
as in the case of cartridges. In either case, a prefilter should be used prior to the SPE treatment if the samples contain substantial
particulates. Some disk products come with a prefilter already built-in. Because the packing material is embedded in the disk
matrix, channeling, which can cause uneven flow characteristics with subsequent lower analytes recovery with poorly-packed
cartridges, is absent. However, due to the thinness of the disk (typically 0.5-2 mm), compounds with low k′ values tend to have
lower breakthrough volumes than for SPE cartridges.
SPE disks have been found to be useful for environmental applications such as the analysis of trace organics in surface water,
which often require a large sample volume to obtain the necessary sensitivity. The United States Environmental Protection Agency
has approved SPE disk technology (Method 3535A) as an alternative for LLE
7
in the preparation of water samples for HPLC
analysis. A major advantage of SPE vs. conventional LLE is the reduced consumption of organic solvents. SPE disks and cartridges
require only a few milliliters of solvent per assay, compared to hundreds of milliliters for comparable multi-step LLE separations.
Low-bed-mass, rigid fiberglass disks with 1.5-30 mg of embedded packing material are useful for pretreating small clinical samples
(e.g. plasma or serum). Here, the reduced sorbent mass and small cross-sectional area reduces solvent consumption. An advantage
of this type of disk is cleaner extracts due to reduced elution solvent volume, less interference from weakly retained compounds,
and an absence of frits that are a possible source of contamination. Examples of such disks are the high capacity SPEC phases
(Agilent Technologies) which are available in cartridges or 96-well plates (see Figure 9.4). Reversed-phase (C2, C8, C18, C18AR,
and Phenyl), normal phase (NH2, CN, Si), ion exchange (SAX, SCX) and mixed-mode (non-polar and SCX) are available in the SPEC
disk format.

103
Although packing-impregnated polyvinylchloride (PVC) and derivatized membranes available in disk or cartridge format are used in
biological sample isolations, they are used very little in SPE applications and will not be further discussed here.
The move towards miniaturization in analytical chemistry has prompted the development of new formats for SPE. The micropipette
tip (MPT) format (Figure 9.3) permits the handling of sub-microliter amounts of sample such as biological fluids. Solid phase
extraction (SPE) has been performed with various phases packed, embedded, or coated on the internal walls of the pipette. The
open flow architecture allows liquid samples to be moved and transferred without undo pressure drop or plugging. Many popular
SPE techniques have been demonstrated including reversed-phase-, ion exchange-, hydrophobic interaction-, hydrophilic
interaction-, immobilized metal affinity-, and affinity-chromatography. Micropipette tip-based SPE (MPT-SPE) has mainly been used
for purification, concentration and selective isolation (affinity, metal chelate) of proteins and peptides. A unique pipette tip design is
based on monolithic sorbent technology. OMIX tips (Agilent Technologies) are of a small volume (10 µL and 100 µL) and small mass
(0.25-2 mg) primarily designed for proteomics applications. Reversed-phase (C4, C18) and a strong cation exchange phase (SCX)
are available. MPT formats are available for specific automated robots such as the Hamilton Microlab Star Series (300 µL tip
volume) and the Tomtec Quadra (450 µL tip volume).
Figure 9.3
Solid Phase Extraction Pipette Tip
Pipette tip
Sorbent
In recent years, interfacing the small volume MPT-SPE
devices to MS or LC/MS has been a popular isolation/
cleanup and identification technique in proteomics. Two
techniques for the mass spectrometric (MS) analysis of
proteins include electrospray ionization (ESI) and matrix-
assisted laser desorption/ionization (MALDI)
8, 9, 10
. ESI is
used to ionize biomolecules in solution, while MALDI is used
to sublimate and ionize a sample in a crystalline, dry matrix
via a laser pulse. MPT-SPE is now an essential tool for
MALDI and for other advanced MS techniques
11,12
. One of
the main advantages of micropipette tips is that they can be
used with micropipettors or in liquid handling automation.
This has resulted in the routine use of MPT-SPE in
bioanalytical labs and it is easily adapted for use in high-
throughput screening applications with commercially
available x-y-z liquid handling systems.
SPE pipette tip

104
Figure 9.4
96-Well SPE Plate
96-well SPE plates for method development are also available. These fixed wells are filled with various sorbents or disks with
different stationary phases. The user can set up various loading, washing, and elution conditions with a series of phases to come up
with the best combinations to isolate analytes from matrix compounds. For example, Agilent’s SPEC method development plates
contain the following silica-based stationary phases: C2, C8, C18, C18AR (acid resistant), cyano, phenyl, MP1 (mixed mode C8 +
SCX), and MP3 (similar to MP1 but with additional polar retention).
SPE 96-well plates
The 96-well plate configuration (Figure 9.4) consists of an 8x12 array of small, flow-through SPE wells with the packing contained
by top and bottom frits. These plates are quite amenable to automation and most modern liquid handling equipment will
accommodate the various steps required for the SPE operation. There are three types of 96-well SPE plates, mostly based on a
standard external dimension: 1) fixed round well, 2) fixed square well, and 3) removable square well. The round well configuration
comes in fixed volumes (usually 0.5-1.0 mL) with various amounts of SPE packing (silica: 25-100 mg; polymer: 2-25 mg). The
packing in each well is generally the same material. The square well configuration can accommodate larger volumes of sample (up
to 2.0 mL) and usually contains fixed packing material in each well. The removable square well plates, such as the Agilent
Technologies VersaPlate, are suited to those who wish to assemble their own 96-well phase configurations. Using a universal base
plate, small cartridges with the same or different stationary phases and with different masses of packing can be placed in any order.
This type of plate allows one to match the number of cartridges to the number of samples, so an entire plate isn’t sacrificed for
fewer than 96 samples. The unused “holes” can be plugged with available stoppers so that a vacuum can be applied. Such plates
are quite useful for method development when one is attempting to find the optimum stationary phase and/or sorbent mass.

105
Coated fibers are used for SPME. In this design (Figure 9.5), a fine, solid fused silica fiber is coated with a polymeric stationary
phase, such as a polydimethylsiloxane (PDMS) or a polyacrylate
13
. The fiber is dipped into the solution to be analyzed, and analytes
diffuse to and partition into the coating as a function of their distribution coefficients. Once equilibrium is achieved, which may take
tens of minutes to occur, the fiber is removed from solution and placed into the injection port of a GC inlet where analytes are
displaced by thermal desorption. The SPME-GC technique has proven to be very versatile and has been used for many applications
in environmental and food areas for volatile analytes. For non-volatiles, an HPLC version where analytes are desorbed from the
fibers by solvent desorption (elution) is available, but this method has proven to be less useful than the GC version. For a more
complete coverage of SPME, refer to Chapter 10 of this book or to a general textbook by the inventor
14
.
Solid phase microextraction (SPME)
Figure 9.5
SPME Syringe Assembly
Stainless steel rod Epoxy
Fiber Syringe needle Plunger Cap

106
Figure 9.6
Coated Stir Bar
Magnet
Both coated fiber and stir bar devices are more popular in
gas chromatography where thermal desorption is more
efficient in volatilizing sorbed analytes into the gas phase
than solvent desorption in the liquid phase. In both devices,
very volatile analytes can be lost due to evaporation during
the transfer process.
For the purposes of brevity, the SPE separation device
described in the remainder of the SPE section will be referred
to as a typical “SPE cartridge”. In most cases, the other SPE
devices will perform in a similar manner.
PDMS Glass
The concept of a coated stirring bar
15,16
is similar to that of a coated SPME fiber but the greatly increased surface area allows for
greater mass sensitivity (see Figure 9.6). The stir bar, with a polymeric sorptive coating, is placed into an aqueous liquid and the
solution stirred while analyte/matrix partitioning takes place. After equilibrium, again requiring tens of minutes, the stir bar is
removed, dried to remove traces of water, and then transferred to a thermal desorption device where the analytes are displaced
into the GC column. For HPLC, off-line solvent desorption is the preferred approach for analyte release from the stir bar.
Stir bar sorbent extraction (SBSE)

107
SPE Apparatus
The equipment needed to perform the SPE experiment can be very simple (Figure 9.7). Gravity can be used as the driving force,
but flow-through the cartridge with “real” samples is sometimes extremely slow and impractical for general use. Thus, the most
useful basic system (Figure 9.7A) employs a syringe to manually push solvent or sample through the cartridge. This method may
be difficult if the sample is viscous or contains particulates. In this case, a vacuum flask that can handle one cartridge at a time
can be used instead (Figure 9.7B). However, when several samples must be processed simultaneously, a vacuum manifold or
positive pressure system for processing multiple cartridges at a time is recommended (Figure 9.7C). Systems are available
that can accommodate up to 24 SPE devices. These manifold systems are constructed of chemically-resistant, vacuum-safe,
see-through glass. The see-through glass allows monitoring of the entire sample collection process. A removable, height-adjustable,
solvent-resistant rack is located inside the vacuum manifold to hold various sizes of test tubes or vials for eluant collection or waste
containers for conditioning, loading, and wash solvents. Delivery needles connecting the cartridges to the collection tubes/vials are
constructed of inert, polypropylene, PTFE, or stainless steel, and are aligned so that solvent is directed to the appropriate collection
device. In this manner, cross-contamination during the analyte elution step can be avoided.
Figure 9.7
Apparatus of SPE
Pressurization Vacuum
Figure 9.7A Figure 9.7B
In some units, a vacuum bleed valve, a vacuum control valve,
and a vacuum gauge are incorporated to allow better control
of the solvent flow. In the more sophisticated units, individual
controls for each cartridge are provided to ensure that there is
an even flow distribution among all of the cartridges. Finally, a
sidearm vacuum flask is placed between the vacuum manifold
and the vacuum source to collect overflow rinse and wash
solvent and prevent liquids from contaminating the vacuum
pump. There are also positive pressure manifold and constant
flow systems on the market that provide individual flow control
for each of the cartridges. As the degree of sophistication goes
up, so does the price of the apparatus. Centrifugation has been
less commonly used to drive liquid through the cartridge
(Figure 9.7D).

108
If 96-well SPE plates are used, a special vacuum manifold is required. Figure 9.7E shows such a manifold system; in the bottom
figure, an actual 96-well SPE plate is mounted on the manifold, and a 96-well collection plate is used to collect the final eluants.
Special waste containers are also available for the loading and rinsing steps.
Figure 9.7C Figure 9.7D
CaptiVac Vacuum Manifold for 96-Well Plates 96-Well SPE Plate Mounted on CaptiVac Vacuum Manifold
Cartridge
Collection tube
Vacuum gauge
Vacuum
CentrifugationVacuum Manifold
Figure 9.7E
Regardless of the method used to push sample solution through the SPE cartridge or other SPE device, the flow rate should not be
too fast. Otherwise, there may be an insufficient amount of time for interaction of the sample with the stationary phase; the kinetics
of mass transfer in SPE is the same as in HPLC. For typical SPE applications, it is recommended that a flow rate be 10 mL/min or
less for a 6 mL cartridge and 50 mL/min for a 90 mm disk. Otherwise, sample losses may occur due to the slow sorption kinetics.

109
SPE Automation
When the number of samples increases such that SPE sample preparation becomes the “bottleneck”, it becomes feasible to
automate the entire process. There are three basic approaches to SPE automation:
The simplest and least expensive instrumentation is a dedicated SPE device that performs conditioning, loading, washing, and
elution. Such systems may use standard syringe barrel cartridges, special cartridges that are designed to fit the apparatus, or
SPE disks. Gilson’s ASPEC, Thermo Fisher Scientific’s AutoTrace 280, and Horizon Technologies’ SPE-DEX fall into this
dedicated category.
Modified x-y-z liquid handling systems are mainly used to perform liquid handling functions such as dilution, mixing, and internal
standard addition. Systems such as the Agilent Technologies Bravo, Agilent PAL sample injectors, Hamilton Microlab Star, Tomtec
Quadra, and Gerstel Multipurpose Sampler (MPS) are very versatile in performing and assisting in various sample preparation
functions. Such units can not only perform up to several sample preparation steps, but can also inject the final cleaned-up sample
into the chromatograph. Solid phase extraction can be performed using SPE micropipette tips (for small volume samples), 96-well
SPE plates and, in some cases, SPE cartridges and disks. This modified liquid handling approach seems to be in favor these days.
Although a full laboratory robotic system can be interfaced to devices which perform all of the steps of the SPE experiment, it may
be more time and cost effective to interface the robot to a dedicated SPE workstation. The robot serves to move sample containers
to and from the SPE workstation, as well as to and from other sample preparation devices (e.g. balances, mixers, dilutors,
autosamplers, etc.) located on the laboratory bench. There are several system integration companies who can assemble a full
robotic system which will fulfill this more fully automated laboratory system. At one time, this approach was more widely used,
but full robotic laboratories are currently less frequent.
1. Dedicated SPE equipment
2. Modified x-y-z liquid handling systems
3. Robotic workstations

110
Figure 9.8
Steps in SPE Process
W
W
W
A
W
W
A
A
W
1234
A
A
X
A
X
X
X
X
X
X
Z
Z
Z
Z
Z
A
A
A
A
A
A
A
X
X
X
X
X
X
X
Z
Z
Z
Z Z
Z
Z
A
A
A
A A
Z
Z
Z
Z
Z Z Z Z
C L
WW
E
D
1. Conditioning the packing (
Figure 9.8.1
)
2. Sample application (loading) (
Figure 9.8.2
)
3. Washing the packing (removal of interferences) (
Figure 9.8.3
)
4. Elution and recovery of the analyte (
Figure 9.8.4
)
Practice of SPE
An overview of SPE separation: In its most popular form of experimentation, the application of SPE generally involves four steps
(Figure 9.8):
Each of these steps must be optimized during the process of method development. First, an overview of this 4-step process will be
provided, and then under the section Practical Guidelines for Method Development, more details will be provided.
We sometimes call this SPE operational mode the “bind-elute” mode. Since the SPE conditions are selected to bind the analyte and
either allow the matrix and interferences to be less bound (passing through the cartridge unretained) or more bound (remaining on
the cartridge for the rest of the SPE experiment). Then, a suitable solvent system is selected to elute the analyte, but not the more
strongly retained interferences/matrix compounds in the smallest possible volume, ideally in a solvent compatible with the next step
in the analysis. In this initial explanation, it is assumed that RP-SPE is used and the analyte is to be retained initially. In Chapter 10,
other SPE modes such as ion exchange and complexation will be addressed.
Symbol Legend
Sample Components
A=Analyte(s) of interest
W= Weakly retained, undesired
matrix component(s)
X=Intermediate undesired
matrix component(s)
Z=Strongly retained undesired
matrix component(s)
Solvents
C=Conditioning solvent
D=Equilibration solvent
L=Loading solvent
R=Rinsing (washing) solvent
E=Eluting solvent

111
Step 1 – Conditioning
In this step (Figure 9.8.1), performed prior to addition of sample, the packing is “conditioned” by the passage of a few bed-volumes
of solvent “C”, typically methanol (MeOH) or acetonitrile (ACN), through the cartridge. The role of the conditioning step is two-fold:
(a) it removes any impurities that may have randomly collected while the cartridge was exposed to the laboratory environment or
present in the cartridge supplied by the manufacturer, and (b) it allows the sorbent to be solvated. Solvation is important since
reversed-phase silica-based packings (especially C8, C18, or phenyl) that have been allowed to dry out often exhibit a considerable
decrease in sample retention of hydrophobic analytes. In addition, varying states of phase dryness lead to non-reproducible analyte
recoveries. In this respect, polymeric packings, such as Bond Elut Plexa (Agilent Technologies) with a balance of hydrophobic-
hydrophilic surface character, can dry out slightly and still maintain their performance. Plexa is a spherical SPE sorbent with
monodisperse particles allowing very reproducible flow and a hydroxylated surface that displays less binding of matrix interferences.
Step 2 – Sample Loading
This step (Figure 9.8.2) in the SPE experiment involves sample application (loading) where the sample, dissolved in a weak solvent
"L", is added to the cartridge. This weak solvent allows strong retention of the analyte. For RP-SPE operation, a weak solvent is
water or buffer, with up to 10% of added organic. For ion exchange, a similar solvent is acceptable, but the ionic strength of the
sample solution should be as low as possible. See Table 9.1 for further information on loading solvents.
The sample for SPE can be applied with a pipette or syringe (manually or automatically with an autosampler or liquid handling
robot), or pumped into the cartridge. The latter method is more convenient for large sample volumes (> 50 mL) such as when
isolating trace organics from environmental water samples. The sample and cartridge sizes must be matched so as not to
overload the capacity of the cartridge. Remember, the capacity of the cartridge must be sufficient to handle the total mass of
sample (e.g., analytes, matrix, and interferences) that may all be retained during the loading step. The sample solution should
be passed through the cartridge without allowing it to dry out. In most SPE setups, the flow rate is not precisely controlled as in
HPLC, but it can be adjusted by varying the vacuum, the rate at which the contents from a syringe or from a pump is delivered.
Flow rates of 2-4 mL/min are usually acceptable. Because of their larger cross-sectional area, disks can handle higher flow
rates, but one should conduct a study on flow rate versus recovery for the analyte(s) of interest.
Following conditioning, it is desirable to flush excess conditioning solvent with an equilibration solvent "D". In RP-SPE, this is usually
water or aqueous buffer, thus equilibrating the SPE device before sample loading. For very low volume SPE devices such as SPEC
disk cartridges, however, where the void volume is far smaller than the sample size, this equilibration step is less important and may
be eliminated.

112
Table 9.1
Typical SPE Phases and Conditions
Mechanism of
Separation Typical Phases Structure(s) Analyte Type Loading Solvent Eluting Solvent
Normal Phase
(Adsorption)
Silica, Alumina, Florisil -SiOH, Al
2
O
3
, Mg
2
SiO
3
Slightly to
moderately
polar
Small e
0
, e.g.
hexane,
CHCl
3
/hexane
Large e
0
, e.g. methanol,
ethanol
Normal Phase
(Polar Bonded Phase)
Cyano, Amino, Diol -CN, -NH
2
, -CH(OH)-CH(OH)- Moderately to
strongly polar
Small e
0
, e.g.
hexane,
Large e
0
, e.g. methanol,
ethanol
Reversed-Phase
(Non-polar
Bonded Phase –
strongly hydrophobic)
Octadecylsiloxane,
Octylsiloxane,
PS-DVB, DVB
(Polymeric)
(-CH
2
-)
17
CH
3
,
(-CH
2
-)
7
CH
3
PS-DVB, DVB
Hydrophobic
(strongly
non-polar)
High P’, e.g. H
2
O,
MeOH/ H
2
O,
ACN/H
2
O
Intermediate P′,
e.g. MeOH, ACN
Reversed-Phase
(Non-polar Bonded
Phase – intermediate
hydrophobicity)
Cyclohexyl, Phenyl,
Diphenyl
Moderately
non-polar
High P’, e.g. H
2
O,
MeOH/ H
2
O,
ACN/H
2
O
Intermediate P′,
e.g. MeOH, ACN
Reversed-Phase
(Non-polar
Bonded Phase –
low hydrophobicity)
Butyl, Ethyl, Methyl (-CH
2
-)
3
CH
3
, -C
2
H
5
, -CH
3
Slightly polar
to moderately
non-polar
High P’, e.g. H
2
O Intermediate P′,
e.g. MeOH, ACN
Polymeric
Reversed-Phase
(Hydrophobic –
hydrophilic balanced)
Polyamide, poly
[n-vinylpyrrolidone-
divinylbenzene(DVB)],
methacrylate-DVB
Various polymers Acidic, basic,
neutral
Water or buffer Intermediate P′,
e.g. MeOH, ACN
Anion Exchange (Weak) Amino, 1
0
, 2
0
-amino (-CH
2
-)
3
NH
2
,
(-CH
2
-)
3
-
NHCH
2
CH
2
NH
2
Ionic (ionizable),
acidic
Water or buffer
(pH = pK
a
+ 2)
A.Buffer (pH = pK
a
- 2)
B.pH value where
sorbent or analyte is
neutral
C.Buffer with high
ionic strength
Anion Exchange
(Strong)
Quaternary amine (-CH
2
-)
3
N+(CH
3
)
3
Ionic (ionizable),
acidic
Water or buffer
(pH = pK
a
+ 2)
A.Buffer (pH = pK
a
- 2)
B.pH value where
sorbent or analyte is
neutral
C.Buffer with high
ionic strength
Cation Exchange
(Weak)
Carboxylic acid (-CH
2
-)
3
COOH Ionic (ionizable),
basic
Water or buffer
(pH = pK
a
- 2)
A.Buffer (pH = pK
a
+ 2)
B.pH value where
sorbent or analyte is
neutral
C.Buffer with high
ionic strength
Cation Exchange
(Strong)
Alkyl sulfonic acid,
Aromatic sulfonic acid
(-CH
2
-)
3
SO
3
H,
(CH
2
)
2
SO
3
H
Ionic (ionizable),
basic
Water or buffer
(pH = pK
a
- 2)
A.Buffer (pH = pK
a
+ 2)
B.pH value where
sorbent or analyte is
neutral
C.Buffer with high
ionic strength
For ion exchange, 3 possible elution conditions exist:
A: Buffer 2 units above (acids) or below (bases) pKa of analyte;
B: pH where either analyte or sorbent (weak exchangers) is neutral;
C: High ionic strength. P’ represents polarity (octanol-water partition coefficient), e
0
is the solvent strength
18,19
.

113
Step 3 – Washing (Rinsing)
This step (Figure 9.8.3) provides for the removal of interferences by washing (rinsing) the cartridge with a solvent “W” of
intermediate strength. Ideally, the choice of wash-solvent strength and volume should be limited; too large a volume or too strong
of a wash solvent may result in the partial elution of the analyte and thus lower overall recovery. Optimally, the wash step (step 3)
is discontinued just before the analyte begins to leave the cartridge. In this way, interferences that are more weakly retained than
the analyte are washed from the cartridge, but no loss of analyte occurs. Water or a buffer is often used for the wash solvent in
RP-SPE, but this may not provide a maximum removal of interferences from the analyte fraction that is collected in step 4
(Figure 9.8.4). A small, controlled amount of organic solvent may be added to the wash solution to aid in the removal of more
hydrophobic substances, but care must be taken that the analyte of interest is not removed at the same time. The breakthrough
volume must be determined experimentally (see Figure 9.11). Because of the possible slight variability of the SPE separation
from cartridge-to-cartridge, there must be some method robustness built in the optimum wash solvent strength used to remove
interferences from the cartridge. Our ultimate goal is collecting 100 percent of the analyte in step 4 (Figure 9.8.4) without the
presence of matrix or undesired interfering compounds; otherwise, poor and/or variable recoveries will result.
Note that for very clean samples such as drinking water, the wash step may be omitted without compromising the extraction
performance (various U.S. EPA Methods omit the wash step and go straight to drying before elution). If the sample is complex
and contains many unwanted matrix components, then the wash step is necessary and should be optimized.
Step 4 – Elution
This step (Figure 9.8.4D) provides for elution and collection of the analyte fraction. If detection sensitivity is a major concern, then
the goal should be the collection of the total mass of analyte(s) in as small a volume as possible. This can be achieved with an elution
solvent “E” that is quite strong, so that k <<1 for the analyte band during elution. Alternatively, the use of a weaker solvent “E” that
still provides elution of the analyte (e.g., k≈1) will minimize the elution of more strongly retained interferences that should remain on
the cartridge. This is an important consideration in HPLC when late-eluters are present in significant amounts, since these compounds
may increase the required run time for the subsequent HPLC separation. Again, in GC, the degree of cleanup must be determined
experimentally. If an intermediate strength elution solvent “E” is used with a resulting large volume of the analyte fraction, it is always
possible to evaporate the eluent to dryness and reconstitute the residue in the HPLC mobile phase or in a GC-compatible solvent in
order to reduce the final analyte fraction volume. Evaporation to dryness is often required in any event, since the elution solvent “E”
for SPE may be too strong a sample solvent for the HPLC separation or may affect the stationary phase in the GC column, especially
for coated phases. For this reason, choose solvent “E” that is relatively volatile; otherwise a long time will be required to evaporate a
large volume or an inconvenient rotary evaporation under vacuum may be needed. Sometimes, using our knowledge of chemistry,
we can use an additive that may make the analyte of interest less volatile, such as adding some HCl to an amine analyte to transform
it into a salt.

114
For HPLC, it is desirable to collect the analyte fraction in an elution solvent that will be a weak mobile phase for the subsequent
separation. In this case, larger volumes of the analyte fraction can be injected more conveniently and with greater detection
sensitivity. There are two ways in which this goal can be achieved. First, if the analyte is an acid or base, the pH of the sample
can be adjusted to suppress analyte ionization and maximize RP-SPE retention. Elution of the analyte in step 4 can then be
effected by a change in pH so as to ionize the sample and reduce its retention. After the analyte fraction is collected, the pH of
the fraction can be re-adjusted for optimum retention in the subsequent HPLC separation. A second approach is the use of a
"weak" SPE column packing (cyano or short-chain alkyl), so that the elution solvent need not be so strong. In this case, a
"strong" HPLC column (e.g., C18) would be used to assay the SPE fraction.
In GC, it is best to collect the eluted analyte in a volatile solvent that will be compatible with the GC stationary phase. Many GC
capillary columns will not tolerate injections of large amounts of water, or polar organic solvent, or liquids containing acidic or basic
solvents. If a strong non-volatile solvent must be used, then solvent exchange will be required either by evaporation or use of
another SPE cartridge (see Uses of SPE).
Note that if a small volume of elution solvent is used or if the elution solvent is immiscible with the wash solvent, it is necessary
or at least highly desirable to eliminate the wash solvent from the cartridge before elution. Elimination of the wash solvent is
accomplished by simply passing air under vacuum (or pressurized air or inert gas) through the cartridge until it is dry. This process
may take a few seconds for small volume SPE devices to several minutes for larger SPE tubes or disks.
Matrix Adsorption SPE Mode
In an alternative approach, SPE can also be used to retain impurities and allow the analyte(s) of interest to pass through the
cartridge unretained. This technique is sometimes called chemical filtration since it “filters out” strongly retained sample
components. This mode has also been referred to as the matrix adsorption mode or interference removal mode of SPE. This option
does not provide for any concentration of the analyte in its SPE collected fraction. It is also not possible to separate the analyte from
more weakly retained interferences. Therefore, this SPE mode usually provides “dirtier” analyte fractions and further cleanup may
be required prior to analysis (see reference 20 for other recommended cleanup procedures). The bind-elute procedure illustrated in
Figure 9.8 allows the separation of analyte from both weakly- and strongly-retained sample matrix components. For this reason,
the matrix adsorption mode is used less often for sample pre-treatment and will not be described further.

115
SPE Phases and Chemistries
Because SPE is really a low-efficiency adaptation of HPLC, many phases used in HPLC are also available in SPE versions. Table 9.1
lists the more popular SPE phases and the analyte types retained by them. Bonded silicas are still the most popular, but other
inorganic and polymeric materials are commercially available. In addition to the generic phases shown in Table 9.1, specialty phases
are available for specific analytes, which will be covered in detail in Chapter 10.
Silica-based SPE cartridge packings are of somewhat lower quality and lower cost compared to corresponding HPLC packings.
Whereas "basic" column packings with minimal silanol interactions are preferred in reversed-phase HPLC, RP-SPE packings
will generally be more "acidic" (Type A), and their silanol interactions may tend to be more pronounced and more variable from
lot-to-lot. However, because SPE is usually practiced as an "on-off" or “digital” technique, small differences in retention may be
less important than in HPLC where subtle selectivity differences must be exploited to obtain baseline separation.
An SPE packing should be selected (Table 9.1) that will retain the analyte strongly during sample loading (Figure 9.8.2). Ionic or
ionizable samples suggest the use of ion exchange packings, especially since the analyte can often be eluted with an aqueous
mobile phase, by a change in pH, or an increase in ionic strength. For ion exchange SPE applications, the analyte fraction can then
be injected directly onto a reversed-phase HPLC column, after pH adjustment to minimize analyte ionization and optimize its
reversed-phase retention. Neutral analytes can be separated on either reversed-phase or normal-phase SPE packings. Normal
phase or some of the newer hydrophilic interaction liquid chromatography (HILIC)-type packings are recommended for more polar
analytes; RP-SPE packings are best for less polar, more hydrophobic analytes. Normal phase solvent systems are generally more
compatible with gas chromatography columns.
Some polymeric packings with appropriate surface chemistries offer a controlled and balanced combination of hydrophilic and
hydrophobic character, and allow neutral, basic, and acidic analytes to be isolated by adjustment of pH, ionic strength, and solvent
conditions. In addition, the polymeric phases display a wider pH range than silica-based sorbents and therefore offer more flexibility
during method development. In fact, often generic method development protocols can be used with polymeric packings where
there is a high probability of success without rigorous method development investigations.

116
In order to have a rugged and robust SPE method, a systematic approach should be used to ensure that one reaches the goals of
maximum analyte purity, recovery, and reproducibility. Each of the four steps of SPE must be optimized during the process of
method development. Figure 9.9 gives a generic, systematic approach to addressing each of the steps that should be performed
in successful method development. In SPE, one must consider all of the possible molecular interactions depicted in Table 9.2 and
in Figure 9.10. By choosing the appropriate mode (e.g. adsorption, reversed-phase, ion exchange, etc.), sample capacity and
retention characteristics of the sorbent, choice of the solvents, buffers, pH, etc. used in the various steps, the flow rate, temperature,
and other experimental parameters, one can reach the goals of SPE.
Before starting SPE method development, it is important to ask a number of questions about the sample and the goals of the final
analysis. At the end of Chapter 1 in this book, there are worksheets that provide a series of questions that may aid you in setting
up your sample preparation method to meet the needs of the analysis. Answers to these questions can help to facilitate SPE
method development.
Practical Guidelines for Method Development
Table 9.2
General Classes of SPE Phases
Non-polar phases (reversed-phase)
Hydrophobic/non-polar interactions
Polar phases (normal phase)
Polar (H-bonding or dipole-dipole) interactions
Ion exchange phases
Electrostatic interactions
Covalent
PBA (See Chapter 10)

117
Figure 9.9
Systematic SPE Method Development
Select SPE mode, phase, and format
Incorporate sample matrix and troubleshoot method
Evaluate analyte purity, recovery, and reproducibility
Select and optimize elution solvent
Select and optimize wash (rinse) solvent
Select and optimize loading solvent
Condition SPE sorbent

118
Figure 9.10
Figure 9.10A
Non-Polar Interactions
O
O
O
H
O
H
H
OH
C
N
Figure 9.10C
Ion Exchange Interactions
SO
3
-
NH
3
+
SO
3
-
NH
3
+
Silica Base
Figure 9.10B
NH
2
Silica Base
Electrostatic Attraction
Silica Base
Cyano Polar Bonded Phase
Figure 9.10D
Mixed Mode SPE
Bonded Functional Group
with Sulfonate Terminus
Bonded Functional Group (C8)
Electrostatic Attraction
Silica Base
Van der Waals Forces
Dipolar Attraction or Hydrogen Bonding
Strong Cation Exchanger
Polar Interactions
Diol Polar Bonded Phase
Van der Waals Forces
Hydrophobic Group (C8)

119
Methanol is commonly used as the conditioning solvent for RP-SPE packings or polar-bonded phase packings such as cyano,
amino, and diol. However, MeOH should not be used for silica gel, which is strongly deactivated by this solvent; an intermediate-
polarity solvent such as methylene chloride or a non-polar solvent (e.g. hexane) is recommended for unmodified silica.
Research the Problem: SPE has been around for a long time and there are thousands of publications in literature, textbooks,
internet, applications bibliographies, and manufacturers' websites. There is a good chance that your analyte (or type of analyte)
and matrix has already been addressed. You can save yourself a great deal of time to start with the SPE conditions already
worked out. A literature search on SPE methods for similar analyte-matrix pairs may also prove to be useful. SPE cartridge
manufacturers have published extensive bibliographies (some in searchable electronic database formats such as the Agilent
Technologies ScanView) that can help to locate publications of interest or provide initial conditions. Also, some manufacturers
provide application notes for the same or similar compounds, and some offer consulting services for SPE method development.
At the end of this chapter, there is a list of books that have been written on the subject – a good place to start for both method
development and applications information.
Characterize the Analyte(s): In many cases, you know the structure and perhaps some of the chemical and physical
properties of the analyte. The analyte’s functional groups and polarity and other chemical properties can dictate the SPE mode
that will allow maximum retention. Parameters such as its solubility in various solvents, stability, pKa, log P (octanol/water
partition coefficient), etc. can help to maximize (or minimize) retention on the selected stationary phase. The concentration of
analyte will have a profound effect on the experimental approach used to isolate it. Analyte concentrations in the parts per billion
or lower present challenges in recovery, purity, and reproducibility while high analyte concentrations simplify method development
and make the task much easier.
Characterize the Sample Matrix: Knowledge of the sample matrix is important in selecting the appropriate loading, washing
and elution conditions. If the matrix is quite different than your analyte of interest, it may make method development easier. Possible
compounds that have similar functional groups or pKa to your analyte can make method development more difficult. Knowledge of
the matrix solubility characteristics, stability, pH, ionic strength, etc. can give one an idea of mode selection.
Develop or Apply Effective HPLC or GC Method: Obviously, one needs an analytical method to monitor the progress of
method development to determine purity, recovery, and reproducibility. Sometimes, because of the similarity of SPE and HPLC, the
development of a successful analytical method for HPLC gives one an idea of the mode, stationary phase selection, and retention
characteristics that can be employed for SPE. Due to its nature, the gas chromatography method is less effective in helping to
decide on a likely SPE stationary phase.
Conditioning the SPE Packing: See earlier in this chapter to review the importance of conditioning the SPE packing (see
Practice of SPE, Step 1) since the stationary phase must be properly solvated to receive the sample.

120
After the packing is conditioned, the excess methanol (or other conditioning solvent) should be removed by a flow of air through
the cartridge until solvent drops no longer drip from the bottom of the cartridge (step 1A, not depicted in Figure 9.8.1). However,
the air flow should not be continued past this point, as this can affect analysis reproducibility (especially with SPE disks). It is
better to leave a slight amount of conditioning solvent in the bed than risk drying out the bed. If the silica-based SPE packing is
allowed to dry out before the sample is introduced, the conditioning step should be repeated before proceeding. Polymeric
packings such as Agilent’s Plexa products are more forgiving in this respect. With RP-SPE separations, removal of excess
methanol can also be accomplished by purging the cartridge with a solvent that is miscible with the conditioning solvent and the
sample; for example, water or buffer. A preconditioning water wash (step 1, Figure 9.8.1, Solvent “D”) also serves to ready the
SPE cartridge for introduction of an aqueous sample (step 2, Figure 9.8.2). Do not allow too much time (more than a few
minutes) between this water conditioning step and the sample loading step because if the packing sits in water too long the
solvating solvent may slowly be partitioned into the water, thereby “de-solvating” the hydrophobic bonded phase.
For reversed-phase SPE packings (Figure 9.10A), water (buffered) is the preferred loading solvent, with as little added organic
as possible (remember to consider the added internal standard). Thus, if your sample is an aqueous solution and contains mostly
non-ionic components, RP-SPE is often the preferred choice. For normal phase packings (Figure 9.10B) on silica, alumina, or
polar-bonded phases (e.g. cyano, diol, amino), hexane or another saturated hydrocarbon is the preferred solvent; the less polar
the sample-solvent, the better. Thus, if your sample is dissolved in a hydrocarbon solvent or other non-polar organic, normal phase
SPE can be used. For ion exchange packings, the preferred sample-solvent is water (although small amounts of organic are not a
problem and can be tolerated by the packing material) at the lowest possible ionic strength. If the sample contains ionic or
ionizable analytes that are dissolved in water or a buffer, then either RP-SPE- (Figure 9.10A), ion exchange- (Figure 9.10C), or
mixed-mode- (Figure 9.10D) phases are applicable. The latter phases can help to “fine tune” the SPE cleanup and have become
very popular in recent times. For example, a phase that includes both ion exchange and reversed-phase (RP) characteristics can
be used to advantage in the cleanup of ionizable analytes with hydrophobic functionality using two different mechanisms. Also, an
SPE cleanup that is “orthogonal” to the HPLC analytical column (i.e., has different selectivity or sorption characteristics) is likely to
result in less overlap of analyte peaks by interferences.
For an RP-SPE cartridge, when the sample contains ionizable analytes such as organic acids or amines, a change in pH (rather than
a change of solvent) can be used to retain (steps 2 and 3) and remove (step 4) compounds. For example, an organic-acid analyte
can be retained in its non-ionized (neutral) form (with k >1) by using a low-pH water-organic buffer for the initial wash solvent "W"
(step 3); e.g., 30% ACN/low-pH buffer. Polar, ionic impurities and protonated bases will be washed from the cartridge and
discarded. The analyte can then be eluted in a small volume by increasing the pH of the 30% ACN/buffer wash solvent so that
pH>pKa; the analyte is fully ionized, and k <<1 (step 4).
Select/Test Sorbents and Loading Solvents: Once the mode is selected, the sorbent that provides maximum analyte
retention in the sample solvent should be determined. There are many different sorbents available for all modes. To maximize
analyte retention in step 2 (Figure 9.8.2), the sample-solvent should be a "weak" solvent for the analyte packing combination.
In some cases, the sample is presented as a solution, and the question is whether to leave the sample "as is” or to exchange the
original solvent for a new solvent. Convenience is usually an important consideration, suggesting use of the original sample-solvent
if possible.

121
Due to the nature of the electrostatic interactions, ion exchange can be a powerful and selective SPE technique for ionic and
ionizable compounds. Cation exchange packings are used for protonated bases and other cations, while anion exchange packings
are used for ionized acids and other anions. Ion exchange packings come in two forms: "strong" and "weak"; strong ion
exchangers are normally preferred, if strong retention of the analyte is the main objective. Retention of weak ion exchangers is a
function of pH. Choice of pH is a compromise between maintaining the ionic character of the stationary phase while ensuring that
the ionic analyte remains in an ionic state. For example, using a carboxylic acid weak cation exchanger for the separation of
protonated amines, the pH must be selected to ensure that the amine is in its protonated form (cationic) while the carboxyl group
is negatively charged (anionic). Thus, pH becomes a powerful variable in optimizing retention or in releasing retained analyte from
a weak ion exchanger. Weak anion or weak cation exchange SPE phases are most effective for analytes that are ionized at all pH
values (e.g. analytes with quaternary ammonium functionality and with sulfonic acid functionality, respectively).
In some cases, to select an appropriate sorbent, an empirical approach is often followed. For example, based on the known
characteristics of the analyte, several SPE phases are possible choices. Each of these phases can be tested for the retention of
analytes and interferences allowing an optimum choice of SPE sorbent. SPE method development kits that provide a number of
phases for scouting are available. These kits provide several different stationary phases and are in all SPE formats: cartridges, disks,
pipette tips, or 96-well formats. With flexible 96-well plates, a user can build a custom plate configuration consisting of different
sorbents in each row of wells. By scouting the different sorbents, an optimum sorbent phase can be chosen. Once the optimum
phase is chosen, the user can then purchase entire boxes of this optimum stationary phase for the final method. To make the
optimum phase and solvent selection easier, automated instrumental SPE systems are also available that can be programmed to
look at a number of SPE phases and automatically investigate not only the various loading solvents, but also the wash and elution
solvent conditions on these phases.
The next consideration is to select the appropriate cartridge size and mass of stationary phase sorbent. The volume of sample that
can be applied to the SPE cartridge depends on (a) the size and type of cartridge (weight of packing), (b) retention of the analyte in
the sample-solvent, and (c) the concentrations of both analyte and interferences in the sample. Often, it is desired to apply as large
a sample volume as possible to maximize the concentration of analyte in its SPE fraction for optimum detection sensitivity in the
following chromatographic separation. Although using a larger cartridge allows a larger sample volume, this may not affect
detection sensitivity, since the maximum analyte concentration in the SPE fraction is determined by the sample-volume/packing-
weight ratio. Therefore, when the amount of sample available is small, the smallest possible SPE cartridge will be preferred. The
capacity of the cartridge for analyte plus interferences is roughly 10-20 mg per g of packing. For ion exchange SPE sorbents, the
manufacturer usually supplies the total ion exchange capacity in microequivalents/gram so that one can ensure that the sample
load does not exceed exchanger capacity.

122
Once the cartridge size has been selected, the maximum sample volume that can be applied must be determined. An important
but often overlooked parameter to be investigated is sample breakthrough. The sorbents used in SPE have a finite sample
capacity. Placing too large of a sample mass or volume on the SPE device can result in loss of analyte, resulting in low recovery.
The “sample” in this case is the total contribution of the analyte, the interferences, and the matrix since all components of the
sample occupy sorbent active sites. To determine the breakthrough, a sample solution is pumped through the SPE device at a
slow flow rate (1-2 mL/min) while monitoring the baseline with a UV detector or other detector that can measure all sample
components. An alternative method is to apply (or pump) a large volume of sample and collect small fractions. The fractions
are then assayed for the analyte by HPLC or GC, to determine the maximum sample volume before breakthrough of the analyte.
When carrying out this experiment, the analyte concentration chosen should be the maximum value expected in the sample.
(If the composition of the sample matrix is likely to vary, the allowable sample volume can also vary).
As Figure 9.11 shows, the sample will eventually saturate the stationary phase and elute from the exit of the cartridge. The
breakthrough volume (V
B
) represents the point where the breakthrough begins. V
M
represents the maximum volume of sample in a
particular loading that can be placed onto the SPE device. By knowing the concentration of the sample and the flow rate, one can
calculate the mass of sample that can be handled by a particular weight of sorbent. Ensure that this mass is not exceeded;
otherwise analyte loss may occur and the recovery will be decreased. Note that the final sample-volume selected should be
somewhat smaller than the value determined in this way, to allow for removal of impurities in step 3 without loss of analyte.
V
B
V
R
V
M
Figure 9.11
Breakthrough Curve for SPE Device
Concentration
Sample Volume
V
B
= Breakthrough Volume
V
R
= Retention Volume
V
M
= Maximum Sampling Volume
Determine Wash (Rinse) Solvents: The object of this step (Figure 9.8.3) is to remove as much as possible of the early-eluting
interferences. This goal can be achieved by selecting a wash solvent “W” that provides intermediate retention of the analyte; e.g.,
3<k<10 under the conditions of separation (in the presence of the sample matrix). The analyst should use as large a volume of
wash liquid as possible to remove early-eluting impurities while retaining the analyte on the cartridge. This optimum wash-solvent
volume can be determined in the same way that the maximum sample volume is determined (see above), by collecting fractions
and assaying them for the analyte.

123
There are two approaches for determining the best composition of the wash solvent:
First, when using HPLC, SPE method development seldom begins before there is a method for the analyte standard. If the same
kind of packing is intended for both SPE and HPLC, the HPLC retention data can provide an initial estimate for the composition
of the wash solvent. If the HPLC mobile phase is 30% ACN/buffer (ACN is acetonitrile), then the analyst should start with 30%
ACN as the wash solvent. If the analyte begins to leave the column before 5-10 cartridge volumes of wash solvent have been
collected and analyzed (1 cartridge volume (µL) = mg of packing), the wash solvent is too strong. Decrease % ACN and repeat
the experiment.
A second approach is to apply the sample, then wash the cartridge with 5-10 bed volumes of successively stronger solvent
(e.g. 25%, 50%, 75%, 100% organic or – in normal phase SPE – a series of pure solvents with increasing solvent power,
higher P’ value). Monitoring the extraction effluent at each concentration will determine the elution profile of the sample.
Remember, the most effective wash solvent is one that will not elute the sorbed analyte yet allow interferences to be removed.
For ionizable interference/matrix compounds, adjusting the pH of the wash solvent can be an effective way to moderate the
retention/release of the interferences (e.g., acidic compounds will be more retained at low pH, and less retained at high pH,
while basic compounds will be more retained at high pH and less at low pH).
Determining the Elution Solvent: The object of this step is to collect all of the analyte in the smallest possible volume, while at
the same time excluding as much as possible of late-eluting interferences. A further goal is to obtain the analyte fraction in a form
that can be injected directly onto the HPLC or GC column. For HPLC analysis, these various goals are mutually contradictory. The
use of a very strong SPE elution solvent "E" (so that k<<1 for the analyte), minimizes sample volume, but makes it less likely that a
large volume of the analyte fraction can be injected onto the HPLC column. An elution solvent just strong enough to elute the
analyte with some retention (e.g., k≈2), minimizes contamination of the analyte fraction by late-eluters, but increases the volume
and makes it less likely that the total analyte fraction can be injected directly. Use of a less hydrophobic RP-SPE packing (e.g.,
cyano) can minimize this problem. When late-eluting interferences are a problem, the best approach is elution of the analyte with
1<k<5. If detection sensitivity is critical so that all the analyte must be injected for HPLC, evaporation to dryness and re-dissolution
of the analyte fraction may be required. Evaporation of aqueous samples is inconvenient, so lyophilization is an alternative. If normal
phase SPE is used, the analyte fraction will be in an organic solvent that is more easily removed by evaporation. Normal phase SPE
separation is also less likely to retain less-polar compounds that tend to elute late in reversed-phase HPLC.

124
If the analyte is an acid or base, solvent strength in the washing and elution steps of RP-SPE can be adjusted by means of a change
in pH, as discussed earlier. This approach makes it easier to select conditions that allow direct injection of the total analyte fraction,
without contaminating the analyte fraction with late-eluters that will increase HPLC (or possibly GC) separation times. SPE with ion-
exchange packings is even more likely to furnish an ideal analyte fraction for subsequent HPLC analysis. Because of packing
dissolution and degradation, users are advised that silica-based HPLC columns generally should not be used outside a pH-range of
about 2<pH<8. However, the one-time use of SPE cartridges allows a wider range of pH. The presence of a tiny amount of
dissolved silica or hydrolyzed bonded phase is unlikely to interfere with the subsequent HPLC or GC analysis. If dissolved silica in the
analyte fractions is a problem, polymeric SPE cartridges are stable for 1<pH<14 and may be a better choice.
Test Blank and Fortified Matrix: Once the method is optimized for analyte recovery, purity and reproducibility, a fortified matrix
as well as a blank should be run using the optimized wash and elution solvents.
When using SPE, it is important to run blanks to rule out potential contamination by extractables from the cartridge body, frits, and
packing materials. If contamination from these materials is suspected, the cartridge should be rinsed with organic solvent (e.g.
methanol, acetonitrile) or dilute acid (e.g. 0.1% formic acid) prior to use.
To assist in method development, Figure 9.12 provides a method development flow chart that addresses the basic steps in
performing the SPE experiments. This flow chart guides one through the main steps in developing an SPE method. Using this flow
chart along with Tables 9.1 and 9.2 and Figures 9.8 and 9.9, one should be able to begin the process of method development:
choosing the proper SPE phase, conditioning solvent, loading solvent, washing solvent, and finally, the elution solvent. For very
complex samples, additional sample preparation protocols may be required prior to or after the SPE cleanup. Difficult interferences
can be removed by other sample preparation techniques shown on the left hand side of the flow chart. However, often an
optimized SPE method provides a sufficiently clean sample for direct analysis. The high selectivity and sensitivity of tandem mass
spectrometry has allowed simpler sample preparation protocols to be used.
Test Real Samples and Fortified Samples: The method should now be tested with real and spiked samples to see if analyte
purity, recovery, and reproducibility are affected. If so, further refinements in the method need to be made.

125
Figure 9.12
Method Development
Flow Chart for SPE
Liquid
Sample
Does sample
contain matrices
which may
interfere
with SPE?
Wash away
interferences with
"intermediate"
strength solvent
Choose conditions
to retain analyte
Wash all analyte
from sorbent with
small amount
of same eluent
Perform LLE
to remove
Determine analyte
recovery
Elute analyte
with "stronger"
elution solvent
Allow analyte to pass
through cartridge
Remove excess
conditioning solvent
Load sample/
matrix onto
SPE device
Silica-based: do not allow
sorbent to dry out
Polymer-based: sorbent
can dry out slightly
Choose conditions to
retain matrix
Collect analyte
in smallest
possible volume
Does sample
contain
oils, fats, lipids?
1) Ion exchange
2) Desalting column
3) Dialysis
4) Passage through a
non-polar sorbent
Remove salts by
Does sample
contain inorganic
salts?
Does
sample contain
proteins?
Is sample
viscous?
1) pH modification
2) Denaturation
w/chaotropic agents
or organic solvents
3) Precipitation with
acid or organic
solvent
4) Addition of binding
site competitive
compound
5) Use RAM SPE phase
Remove proteins by
Dilute with
compatible
solvent
1) Reversed-phase –
MeOH, ACN
2) Normal bonded phase
– MeOH, or non-polar
solvent
3) Silica gel –
non-polar solvent
4) Ion exchange – buffer
Is recovery
acceptable?
Injection or
further sample
pre-treatment
Evaporate solvent to dryness
or to appropriate concentration;
take up in compatible solvent
for analysis
- Sorbent
5-10 mg spl/g
- IEX-0.5-1.5 meq/g
1) Mechanism/phase
(see table 9.1)
2) Weight and volume
SPE Devices
Disks
Cartridges
96-Well Plates
Pipette Tips
Select SPE device
Condition SPE
device with
appropriate
solvent
No
No
No
Yes
Yes
Yes
Yes
Yes
Yes
No
No
No

126
Method Validation
Once an SPE method is developed, validation may be required (or to evaluate method robustness if a full validation is not required).
Some of the variables that should be considered when validating a method are depicted in Table 9.3. Figure 9.13 shows one of
the more important investigations that should be conducted – the effect of flow rate and recovery in SPE, especially for the loading,
washing, and elution steps. There is a kinetic element in sorption. The analyte needs time to diffuse into the porous SPE support and
interact with the active functional group. If the flow rate is too fast, there is insufficient time for this equilibrium to occur. Too slow of
a flow rate will just waste time. The flow rate for ion exchange SPE is even more important since ion exchange kinetics can be
rather slow. Typically, a flow rate of 5 mL/min or less for adsorption and normal phase cartridges and 1-2 mL/min for ion exchange
phases is sufficient for a typical cartridge format (e.g. 200 mg/3 mL).
Table 9.3
Variables to Consider in SPE Method Validation
Test Required Experimental Parameter(s) to be Investigated
Sorbent Weight, cartridge format, different lots
Conditioning Solvent Solvent strength (weak or strong solvent), contact time, volume
Loading Solvent Type, volume, % organic, pH, ionic strength, flow rate, breakthrough volume, analyte
recovery/loss, interference/matrix removal
Wash Solvent Type, volume, % organic, pH, ionic strength, flow rate, analyte recovery/loss, interference/
matrix removal
Elution Solvent Type, volatility, solvent strength, volume, flow rate, pH, ionic strength, interference/matrix
retention, analyte recovery/loss
Analyte and Matrix Stability Tested in each step of method
Sample/Matrix Loadability Different analyte concentrations
Detectability Limits of detection (LOD), limits of quantitation (LOQ)
Method Linearity and Range Tested over expected concentration range of analyte, test as function of matrix loading

127
Figure 9.13
Relationship Between Sample Recovery and Flow Rate in SPE
100%
0%
Recovery
Slow Fast
Load or Elute Flow Rate
Optimum
Flow Rate
SPE Application Examples
Some examples of the use of SPE to solve “real world” problems will be covered. Synthetic sweeteners are sugar substitutes
increasingly being used to combat obesity and dental decay. Research has shown that some artificial sweeteners can cause tumors
in certain animals
21
. Sewage treatment plants do not completely remove artificial sweeteners from wastewater and these
pollutants contaminate waters downstream and may be present in drinking water. Solid phase extraction using a neutral polymeric
sorbent can be very useful for isolating and concentrating organic contaminants in large volumes of water samples. These sorbents
have very high affinity for organics and exhibit good recoveries from an aqueous environment. In the studies of Junginger and
Korte
22
, they investigated using 200 mg/6 mL cartridges containing the SPE sorbent Bond Elut Plexa (Agilent Technologies) to
isolate four synthetic sweeteners (acesulfame, cyclamate, saccharin, and sucralose) each at the 1 ppb level in water. The SPE
cartridge was first conditioned with 3 mL of methanol followed by 3 mL of acidified HPLC water. The water sample (100 mL) was
acidified with sulfuric acid at pH 2 and loaded at a flow rate of 5 mL per minute. No rinsing step was required in this trace
enrichment experiment. Elution of the analytes from the cartridge was performed with 5 mL of methanol at a flow rate of 2 mL per
minute. The solvent was evaporated to near dryness stream of nitrogen and reconstituted in 1 mL of acetonitrile:water (5:95). Using
an Agilent ZORBAX Eclipse XDB-C18 HPLC column (4.6 x 50 mm, 1.8 µm) and a water-methanol gradient, the four sweeteners
could be separated to baseline in just over 5 min using tandem mass spectrometric detection. Recoveries of the four sweeteners for
a 20 µL injection volume ranged from 74-91% and RSDs averaged around 7%, quite acceptable at these low concentrations.

128
Fungi of the genus Alternaria are pathogens of various plants such as fruits and vegetables. Alternaria is a frequently occurring
species of particular interest because it produces a number of harmful mycotoxins, including alternariol (AOH), alternariol
monomethyl ether (AME), altenuene (ALT), tentoxin (TEN) and tenuazonic acid (TEA). These toxins can be found in grain, seeds,
pepper, and various fruits. Their determination is important for food safety. First, these toxins must be extracted from the food
source, then cleaned up and concentrated by the SPE. Reinhold and Bartels
23
developed the workflow for the sample preparation
shown in Figures 9.14 and 9.15. Again, the Bond Elut Plexa polymer provided excellent cleanup of toxin extracts from vegetable
and grape juice samples. Using ternary gradient elution and reversed-phase HPLC with mass spectrometric detection, a baseline
separation was achieved in 15 min as depicted in Figure 9.16. The SPE cleanup reduced ion suppression effects in the mass
spectrometer. For the method, the limit of detection was 5 µg/L while the limit of quantitation was 10 µg/L. Typical recoveries for
the alternaria toxins from grape juice ranged from 97-105%. RSDs ranged from 1-3.6%.
Figure 9.14
Initial Workflow for Extraction of Alternaria Toxins
Solid Samples Liquid Samples
Add 60 mL of ACN-methanol-water (pH 3; 45:10:45 v:v:v)
Homogenize for 2 min
Weigh 5 g of juice in 50 mL centrifuge tube
Weigh 20 g of homogenized sample
in a 50 mL centrifuge tube
Proceed to SPE
Transfer 6 mL of supernatant to a centrifuge tube
and dilute with 15 mL of 0.05 M
sodium dihydrogen phosphate, pH 3
Transfer 6 mL of supernatant to a centrifuge tube
and dilute with 15 mL of 0.05 M
sodium dihydrogen phosphate, pH 3
Centrifuge for 10 min at 4000 rpm
Add 15 mL of ACN-methanol-water (pH 3; 45:10:45 v:v:v)
Shake for 1 min

129
Figure 9.15
Workflow for SPE Cleanup of Alternaria Toxin Extracts
Use Bond Elut Plexa 200 mg/6 mL cartridge
Condition with 5 mL of methanol; allow to drain
Add 5 mL water; allow to drain
Add the sample extract; rinse the centrifuge tube
with 1 mL water
Wash with 5 mL water
Elute with 5 mL methanol, then with 5 mL acetonitrile
Evaporate eluate to dryness;
reconstitute in 1 mL water/methanol (7:3 v:v)
Inject into HPLC System

130
HO
MeO
OH
OH
OH
HO
O
O
O
O
HN
N
N
NH
OH
7
6
4
3
2
1
5
8
9
10
7
6
4
3
2
1
5
8
9
10
A
B
A
B
C
C
OH
H
3
CO
OH
O
O
OH
O
O
O
O
O
O
N
H
Figure 9.16
LC/MS Chromatogram of Alternaria Toxins from Grape Juice Extract
Grape juice spiked at 25 µg/kg concentration
TEN
10.88 min
TEA
3.10 min
ALT
10.19 min
AOH
11.71 min
AME
14.81 min
Polymeric sorbents such as Bond Elut Plexa allow the development of generic SPE methods. Generic methods often save time in
applying SPE cleanup to difficult samples. The cleanup of acid, base, and neutral compounds in biological fluids is one area
where these generic methods have found great utility. A significant number of bioanalytical compounds in the pharmaceutical
industry are basic and can be easily extracted using hydrophobic or cation exchange sorbents. However, lipid lowering statins
and anti-inflammatory drugs tend to be acidic and can be problematic when using traditional hydrophobic sorbents. A simple
generic method was developed for the analysis of the acidic compounds statins in spiked plasma using Plexa PAX, a strong
anion exchanger that can operate at high pH values due to its polymeric nature
24
. The Plexa PAX resin was used in a 96-well
plate configuration. Each well contained 30 mg of the ion exchange resin. The SPE method was rather simple: 100 µL of human
plasma was first diluted 1:3 with 2% ammonium hydroxide solution. Each well was conditioned with 500 µL of methanol
followed by 500 µL of water. The diluted plasma sample containing the spiked statins was added to the resin in the well. After
sample application, the Bond Elut Plexa PAX was washed first with 500 µL of water followed by 500 µL of methanol. Elution
was performed by adding 500 µL of 5% formic acid in methanol to the 96-well plate. The eluate was collected, evaporated to
dryness, and reconstituted in 1 mL of 5 mM aqueous ammonium formate:methanol (80:20). Using a reversed-phase HPLC-LC-
MS/MS and gradient elution of the following five statin drugs: atorvastatin, diclofenac, furosemide, ketoprofen, and pravastatin
spiked at the 50 ng/mL level were separated in just over 3 min. All five statins showed linear calibration curves over the range
0.5-200 ng/mL. Analyte recoveries were determined by measuring analyte response compared to a spiked mobile phase
standard. Recoveries ranged from 62% for atorvastatin to 96% for furosemide with RSDs (n=6) ranging from 2.3-6.1% at the
50 ng/mL level.
*
*

131
Recommended Textbooks on SPE
Space requirements allow this chapter to provide only a basic introduction to SPE. Chapter 10 provides information on more
specialized SPE phases. For general reading, entire books have been written on SPE
25-32
including its automation
33
. The reader
is directed to those references for more detailed coverage of the subject.
(Continued)
References
1.
Diaz-Ramos, M.C.; Suarez, A.; Rubies, A.; Companyo, R.; Korte-McIllrick, E.
Determination of 24 PAHs in Drinking Water
, Agilent
Technologies Application Note #5990-7686EN, 2012.
2.
Gledhill, S.
High Sensitivity Detection of Pesticides in Water Using On-line SPE Enrichment
, Agilent Technologies Application Note
#5991-08871EN, 2012.
3.
Hudson, W.C.; Massi, J. M.
Protein Desalting and Concentration for MS Analysis Using Agilent Bond Elut OMIX C4 Pipette Tips
,
Agilent Technologies Application Note #5990-8885EN, 2011.
4.
Baños, C.-E.; Silva, M.
Talanta
2009,
77 (5)
, 1597-1602.
5.
Ochoa, M.L.; Harrington, P.B.
Anal. Chem.
2005,
77 (3)
, 854-863.
6.
Henriksen, T.; Svensmark, B.; Lindhardt, B.; Juhler, R.K.
Chemosphere
2001,
44
, 1531-1539.
7.
Alfred-Stevens, A.; Eichelberger, J.W.
Methods for the Determination of Organic Compounds in Drinking Water
(Supplement I),
Environmental Monitoring Systems Laboratory, Office of R&D, U.S. EPA, Cincinnati, OH, 1990, 33-63.
8.
Fenn, J.B. et.al.
Science
1989,
(246)
, 64-71.
9.
Karas, M.
Anal. Chem.
1988,
(60)
, 2299-2301.
10.
Thomas, R.
Spectroscopy
2001,
16 (1)
, 28-37.
11.
Majors, R.E.
LC.GC
2005,
23 (4)
, 358-369.
12.
Majors, R.E.; Shukla, A.
LC.GC No. America
2005,
23 (7)
, 646-660.
13.
Kataoka, H.; Lord, H.L.; Pawliszyn, J.
J. Chromatogr. A
2000,
880
, 35-62.
14.
Pawliszyn, J.
Solid-Phase Microextraction: Theory and Practice
, Wiley-VCH, 1997, 264 pp ISBN-10: 0471190349
and ISBN-13: 978-0471190349.
15.
David, F.; Tienport, B.; Sandra, P.
LCGC No. America
2003, 21 (2), 108-118.
16.
Baltrussen, E.; Sandra, P.; David, F.; Cramers, C.A.
J. Microcol. Sep.
1999,
11, 737
.
17.
Popp, P.; Bauer, C.; Wennrich, L.
Anal. Chim. Acta
, 2001,
436 (1)
, 1-9.

132
(Continued)
18.
Snyder, L.R.
J. Chromatogr. Sci.
1978,
16
, 223.
19.
Snyder, L.R.; Kirkland, J.J.; Dolan, J.W.
Introduction to Modern Liquid Chromatography
, 3rd Ed., Chapter 2 and Appendix I.2,
John Wiley and Sons, Hoboken, NJ, 2010.
20. http://www.epa.gov/epawaste/hazard/testmethods/sw846/online/3_series.htm
21.
Takayama et al.
Long-Term Toxicity and Carcinogenicity Study of Cyclamate in Nonhuman Primates, Toxicol. Sci.
2000,
53
, 33-39.
22.
Junginger, S.; Korte, E.
Analyzing Synthetic Sweeteners in Wastewater With Robust Sample Preparation
,
Agilent Technologies Application Note #5990-8248EN, 2011.
23.
Reinhold, L.; Bartels, I.
LC-MS/MS Determination of Alternaria Toxins in Vegetables and Fruit Beverages
,
Agilent Technologies Application Note #SI-01322, 2010.
24.
Hudson, W.
Extraction of Acidic Compounds From Human Plasma Using Plexa PAX
, Agilent Technologies Application Note
#5990-9027EN, 2011.
25.
Simpson, N.; van Horne, K.C.
Sorbent Extraction Technology Handbook
, Varian Sample Preparation Products, Harbor City, CA, 1993.
26.
Thurman, E.M.; Mills, M.S.
Solid-Phase Extraction: Principles and Practice
, John Wiley and Sons, New York, 1998,
ISBN-10: 047161422X and ISBN-13: 978-0471614227.
27.
Fritz, J.S.
Analytical Solid-Phase Extraction
, Wiley-VCH, New York, 1999, ISBN-10: 0471246670 and ISBN-13: 978-04712466.
28.
Simpson, N.J.K. (Ed.)
Solid-Phase Extraction: Principles, Techniques, and Applications
, Marcel Dekker, New York, 2000,
ISBN-10: 082470021X and ISBN-13: 978-0824700218.
29.
Telepchak, M.J.
Forensic and Clinical Applications of Solid-Phase Extraction
, Humana Press, Totowa, NJ, 2004,
ISBN-10: 0896036480 and ISBN-13: 978-0896036482.
30.
Sadek, P.
Practical Solid-Phase Extraction: Fundamentals and Method Development
, Wiley-Interscience, New York, 2005,
ISBN-10: 0471329894 and ISBN-13: 978-0471329893.
References
31.
Macwana, C.; Parmar, V.
Solid-Phase Extraction: Principle, Trends, Applications and New Developments
, LAP LAMBERT
Academic Publishing, 2012, ISBN-10: 3659206768 and ISBN-13: 978-3659206764.
32.
Olariu, R-I.; Arsene, C.; Vione, D.; Grinberg, N.
Solid-Phase Extraction: Principles and Applications
, CRC Press, Boca Raton, FL,
2013, ISBN-10: 1439868328 and ISBN-13: 978-1439868324.
33.
Wells, D.A.
High-Throughput Bioanalytical Sample Preparation: Methods and Automation
, Elsevier Scientific Publishers,
Amsterdam, 2003.

133
Chapter 10
Special Topics in SPE
There are several topics closely related to the operating principles of SPE. These topics have become important in recent years as
workers are seeking sample preparation techniques that use less organic solvent, are more selective, and may be automated for
convenience and better reproducibility. This chapter will cover several of these techniques.
Solid Phase Microextraction is a relatively new sample extraction technique first described by Pawliszyn and coworkers in the early
‘90s
1
. In its most popular format, SPME consists of two discrete steps: solute absorption from the sample matrix into a thick layer of
silicone or related adsorptive material coated onto a solid fused silica fiber (Figures 9.5 and 10.1A), followed by transfer of the sorbed
analytes into a chromatogra phy inlet by thermal- (GC) or liquid-desorption (LC).
SPME has been applied to both gas chromatography (GC) and liquid chromatography (LC) separations but its most successful
application has been in GC. It eliminates the need for large-volume sample transfer into a GC column by concentrating analytes into
the fiber coating while leaving the bulk of the solvent and non-volatile residues behind. In GC, SPME is considered to be a solvent-
less sample preparation technique. In LC, the technique uses orders of magnitude less solvent than in liquid-liquid extraction and
also uses less solvent compared to SPE.
A related technique, stir bar sorptive extraction (SBSE), first described by Sandra and coworkers
2
uses a magnetic stirring bar coated
with a thick layer of absorptive polymer (see Chapter 9, SPE Devices). The stir bar is exposed to sample solution for a time, during
which solutes are absorbed into the polymer coating. Subsequently, the stir bar is removed, dried, and then thermally desorbed for GC
injection, or the absorbed analytes can be back-extracted with a different solvent for either LC or GC. SBSE uses a larger volume of
stationary phase than SPME and, thus, is more efficient at extracting analytes with less absorbent solubility. Therefore, SBSE is
generally more sensitive than SPME.
Table 10.1 provides a sampling of the wide range of applications where SPME has been successfully used.
Solid Phase Microextraction (SPME)

134
Typical Applications of SPME
Analyte(s) Matrix Reference
Fragrances Flowers 3
Chemical warfare agents Clothing material 4
Process impurities Pharmaceuticals 5
Organochlorine pesticides Chinese teas 6
Volatile compounds Acidic media 7
Cheese 8
Volatile phenols Wine 9
Environmental pollutants Water 10
Chloroanisoles Cork stoppers 11
Volatile aliphatic amines Air 12
Phenylurea herbicides Aqueous samples 13
Sub-ppb phthalates Water 14
Methyl mercury Tuna fish 15
Volatile organic compounds Soil 16
Isobutylpyrazine (MIBP or IBMP) Wine 17
BTEX Water 18
2-Methylisoborneol (2-MIB) and Geosmin Drinking Water 19
Trichloroanisole Wine 20
Table 10.1

135
L
r
1
r
2
SPME Principles
SPME relies on the extraction of solutes from a sample, usually aqueous, into an absorptive polymeric layer coated onto a solid fused
silica fiber (Figure 10.1A). Table 10.2 provides a listing of some commercially available fiber chemistries. After a sampling period,
which may be of a considerable time (30 min or more) aided by stirring, the extraction reaches equilibrium. Then the SPME fiber and
captured solutes are transferred into an inlet system that then desorbs the solutes into a gas (for GC) or liquid (for LC) mobile phase.
Success relies on choosing conditions so that the desired solutes favor the SPME absorptive layer as much as possible in the presence
of bulk sample. On the other hand, the absorbed solutes should be released during the desorption step as quickly and as completely
as possible. If the desorption is slow and the desorbed solute peaks broad, a secondary trapping to refocus the analytes may be
required. Then, in the case of GC by rapid heating, the refocused analytes are quickly swept into the chromatographic column for
separation and analysis. The choice of the absorptive layer chemistry and film thickness strongly influences the degree of absorption
and the subsequent efficiency of desorption. SPME fibers are available in syringe-integrated assemblies that are conveniently handled
either manually or by robotic autosampling systems. Used fibers often can be cleaned up for reuse by solvent rinsing or baking.
Figure 10.2 depicts the entire extraction transfer and desorption process. Although solvent desorbing in a valve configuration can
transfer solutes to an HPLC column, it is generally more convenient to perform the experiment off-line, then transfer the extracted
contents to a vial or manually to a loop injector.
Figure 10.1
Cross Sectional Diagram of SPME Extraction Devices
Solid Support
SPME Layer
Adapted from
21
A
B
A: Fiber device with external sorptive coating
B: Tube device with sorptive coating on the inside

136
Figure 10.2
Steps in SPME Process in GC and LC
C: SPME LC interface
SPME holder
Desorption chamber
Mobile phase
Waste
To LC column
Table 10.2
Types of Samples and Recommended Fiber Chemistry in SPME
Sample Recommended Fiber Chemistry
Low molecular weight or volatile compounds 100 µm polydimethylsiloxane (PDMS)
Larger molecular weight or semivolatile compounds 30 µm PDMS fiber or a 7µm PDMS fiber
Very polar analytes from polar samples 85 µm polyacrylate
More volatile polar analytes, such as alcohols or amines 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB)
Trace-level volatiles analysis 75 µm PDMS/ Carboxen
Expanded range of analytes (C3-C20) 50/30 divinylbenzene/carboxen on PDMS
General HPLC 60 µm PDMS/DVB
As an alternative approach to SPME, the stationary phase is coated on the inside of an open fused silica column (Figure 10.1B).
Known as in-tube SPME
22
, mostly used for liquid chromatography, a solution containing the analyte of interest is pumped inside the
coiled coated fused silica tube a number of times until equilibrium occurs. Then, after dispelling the sample liquid from the capillary
tube, a strong solvent is used to displace the trapped analyte from the coated stationary phase into an HPLC loop injector.
B: Thermal desorption
on GC injection port
A: Extraction step for SPME
Fiber immersed in aqueous sample
Aqueous
sample

137
Let’s now look at the details of SPME extraction, transfer, and desorption. The following discussion was adapted from an article by
J. Hinshaw
21
.
Step 1: Extraction
For the extraction step with an externally coated SPME absorptive layer, the layer is exposed to sample in the liquid phase
(Figure 10.3A) or gas phase (headspace) (Figure 10.3B). Over time, the amounts of solutes in the SPME layer reach an
equilibrium level with their surroundings, which represents the maximum solute amounts that can be absorbed and withdrawn
under a given set of sampling conditions. The amount of solute in the SPME layer at equilibrium (M
i, SPME
) can be approximated
by the following equation:
M
i,SPME
~
K
i.SPME
V
SPME
C
i
Equation 1
where K
i,SPME
is an aggregate solute distribution constant between the SPME absorptive layer and the sample, V
SPME
is the volume
of the SPME layer, and C
i
is the solute concentration in the sample before performing SPME sampling.
Figure 10.3
SPME Extractions in Closed Vial
A: Sampling from the liquid phase
B: Sampling from the headspace (gas phase)
Equation 1 assumes that the sample volume is much greater
than the volume of the SPME layer. SPME coatings typically
have thicknesses of about 10-100 µm typically around 10 times
the film thickness range normally encountered in capillary GC
columns. Standard SPME layers, with smaller volumes, would
imply correspondingly smaller minimum sample volumes.
AB
Headspace
SPME fiber
Liquid sample

138
Step 2: Transfer
The next step after sampling is to transfer the SPME layer and absorbed analytes away from sample exposure and into conditions
for desorption into the chromatography mobile phase. For LC-SPME in-tube sampling with a multiposition valve connected to the
SPME tube, there is no need to physically move the SPME layer, but new solvating conditions must be established in place that
promote solute desorption. On the other hand, an SPME fiber device is removed from the sample container and transported over
a distance to where the solutes are to be desorbed – the GC inlet or, in some cases, a solvent wash position. Removal from the
sample environment immediately starts the absorbed solute concentrations shifting away from their in-sample values to lower
levels as solutes naturally desorb into their surroundings. The rate of natural desorption is fairly low for many solutes, but volatile
molecules could experience significant losses during the transfer step. In a laboratory situation, the transfer time from sample vial
to instrument can be short enough so that losses are insignificant. Losses can be minimized during extended transport and storage
by sealing the SPME layer into a small enclosure and then ensuring that the contents are included with the rest of the sample on
desorption. In addition to volatile sample losses, an SPME layer can easily pick up non-sample components from the ambient air,
especially during extended transportation from remote sites. Enclosing the SPME layer prevents the influx of such contaminants.
A number of commercially available SPME devices incorporate such a sealing system.

139
Step 3: Desorption
Once in place at a chromatograph, the SPME layer must then be exposed to conditions that cause the absorbed solutes to desorb
with as close to 100% efficiency as possible, and in a sufficiently short time compatible with the chromatography mode in use. In
the case of an SPME layer coated inside a tube, for LC analysis, a simple multiposition valve arrangement can switch from sample
liquid flow to the mobile phase. Stopping the mobile phase flow in the SPME tube allows time for solute desorption to come to
equilibrium between the SPME layer in the liquid mobile phase before the desorbed materials are introduced into the column. This
somewhat cumbersome stop-flow operation may cause undue band broadening and encourages some to utilize an off-line
desorption step.
In GC, desorption from fiber-based external SPME layers is more conveniently achieved by insertion into a standard GC capillary
inlet system in much the same way as a syringe. Several trade-offs arise in the course of thermally desorbing an SPME layer for GC
analysis. First, the desorption temperature must be high enough so that the solutes rapidly leave the SPME fiber. A desorption that
is too slow may lead to peak broadening and tailing unless additional arrangements are made for trapping solutes at the beginning
of the GC column before temperature-programmed elution. Conversely, too high of an inlet temperature may induce thermal
decomposition and introduce some contaminants into the column from septum bleed, as well as from the SPME layer itself.
During sample desorption from an SPME fiber into a split/splitless inlet, the split flow should be turned off so that all of the solutes
may enter the column without splitting, just as would occur in splitless injection. It is unlikely that enough sample mass will be
absorbed on an SPME layer to necessitate sample splitting. A narrow-bore inlet liner (often called a "splitless" liner) helps produce
better peak shapes as well, by limiting the volume into which the solutes may expand. After the SPME device has been withdrawn
from an inlet splitter, the split flow may be turned back on to purge the inlet of any remaining materials and prevent some degree of
peak tailing.
Programmed temperature vaporizer (PTV) inlet systems (see Chapter 6) are well-suited to SPME desorption because of their smaller
internal volumes. For SPME use, they should be operated at the same elevated constant temperatures as conventional split/splitless
inlets because PTV heat-up rates (on the order of several hundred degrees per minute) may not be fast enough to produce
sufficiently narrow peaks without some form of additional stationary phase trapping.

140
Pros and Cons of SPME
The primary advantages of SPME are its ability to decouple sampling from the matrix effects that would distort the apparent sample
composition or disturb the chromatographic separation, its simplicity and ease of use, and its reduced or nonexistent solvent
consumption. These characteristics combine to make SPME an attractive alternative to classical headspace or thermal desorption
sampling, solid phase extraction, and classical liquid-liquid extraction.
As with a number of related sample preparation and injection techniques such as headspace GC or thermal desorption, SPME lends
itself well to handling difficult sample matrices, but with the added benefit of low cost and simplicity. SPME does not require
elaborate and expensive instrument accessories for occasional use and yet seems to be capable of delivering very good manual
results in the hands of skilled users. This cannot necessarily be said for manual headspace or thermal desorption sampling.
Autosamplers or robotics systems that perform repetitive unattended SPME sampling are also available; this automation availability
is also a key advantage of SPME.
On the other hand, quantitation is a more difficult process in SPME. Standards must be prepared under the exact same conditions
as samples, and that is not always possible. The fibers themselves are rather expensive and you need replacement on a relatively
frequent basis. As the fiber ages, its behavior may change, resulting in a lower degree of reproducibility. The fibers are somewhat
fragile, therefore method robustness is sometimes questioned. Relative to other sample preparation techniques, obtaining
equilibrium conditions is quite slow, necessitating shorter sampling periods for practical requirements. As mentioned earlier, slow
desorption times may necessitate trapping non-volatile solutes to refocus them for chromatographic analysis.
SPME requires careful optimization and consistent operating conditions for success, but this is true of related techniques as well.
Any poorly characterized sampling technique has no valid use in the analytical laboratory, and the burden of developing an
SPME method is no greater than for the other techniques. SPME has a significant place in the analyst's arsenal of sample
preparation techniques.

141
Example of SPME Sample Preparation
To illustrate the application of SPME, a method was developed to determine sub-ppb levels of phthalates in water by Auto-SPME and
GC/MS
23
. Phthalic acid esters are key additives to many plastics to keep them pliable at room temperature. Due to their widespread
use, relatively large amounts of these compounds are released into the environment. In recent years, considerable attention has been
paid to human exposure to phthalates because of their suspected carcinogenic and estrogenic properties. Liquid-liquid extraction has
been widely used to isolate phthalates from aqueous samples. SPME is an alternative approach to the analysis of phthalates in
aqueous solutions. The extraction of analytes from aqueous samples can be performed by direct immersion of the fiber into the liquid
phase or by headspace sampling. Here, the use of CombiPAL (Agilent Technologies) for the fully automated SPME sample preparation
process is demonstrated. All movements of the SPME fiber are software-controlled for optimum precision and less operator attention.
Since the phthalates are semi-volatile compounds, the immersion extraction mode was selected. First, the effect of extraction time
versus amount extracted at a temperature of 40 °C was studied. Figure 10.4 provides information on the amount extracted (signal
response) versus extraction time for the six phthalates studied. As seen in the figure, when the extraction time was over 20 min, the
responses changed only slightly, which meant that the extraction of most compounds reach equilibrium at this point. Thus, in this
experiment, 20 min was selected as the extraction time.
It is generally known that the addition of salt to an aqueous solution improves the SPME extraction efficiency. Therefore, the
salting out effect was studied by the addition of NaCl to the aqueous solution containing phthalates. Although not shown, the
effect demonstrated that extraction efficiency improvements can be made by the salting out procedure. In the final experiments, a
20% (W/V) salt concentration was chosen. Figure 10.5 shows the LC/MS SIM chromatogram of the phthalates at the optimized
condition. The SPME method for sample preparation combined with GC/MS provided linearity over the range 1-1000 ng/mL with
good correlation coefficients. The RSD's at a concentration of one ppb were less than 10%, except for DPP. Actual samples of tap
water, potable water, and purified water from water dispenser were analyzed for the presence of phthalates. The results showed
that phthalates were detected in all three samples at 24-79 ng/mL levels
23
.

18000
16000
14000
12000
10000
8000
6000
4000
2000
0
0 5 10 15 20 25 30
142
Figure 10.4
CombiPAL
Pre-incubation time: 60 s
Incubation temperature: 40 °C
Pre-inc. agitator speed: 500 rpm
Agitator on time: 5 s
Agitator off time: 2 s
Vial penetration: 25 mm
Extraction time: 1200 s
Desorb to: GC Inj 1
Injection penetration: 54 mm
Desorption time: 120 s
Post fiber condition time: 300 s
SPME
Fiber type: PDMS/DVB
Coating: 65 µm
Compound Name Abbreviation Retention time (min)
Phthalic acid, bis-n-pentyl ester DPP 10.179
Phthalic acid, bis-isononyl ester DEHP 11.862
Di-cyclohexyl phthalate DCHP 16.749
Diethyl phthalate DEP 17.517
Dimethyl phthalate DMP 20.666
Dibutyl phthalate DBP 20.836
Extraction time (min)
Response
SPME Extraction Time Versus MS Response
DPP
DEHP
DCHP
DEP
DBP
DMP
7000000
6000000
5000000
4000000
3000000
2000000
1000000
10 11 12 13 14 15 16 17 18 19 20 21 22
Time
Figure 10.5
SIM Chromatogram of Phthalates at the Optimized Extraction Condition
6890 GC
Inlet temperature: 270 °C
Gas type: Helium
Oven condition: 50 °C ramps 10.00 °C/min to 260 °C (3.00 min)
Column: DB-5ms 30 m x 250 mm, 0.25 µm
Mode: Constant flow
Flow rate: 1.3 mL/min
5975 MS
Acquisition mode: Synchronous SIM/scan
Mass range: 40-300
Sample: 3
Dwell time: 30 ms
MS source: 230 °C
MS quad: 150 °C
DPP
DEHP
DCHP
DEP
DMP
DBP

143
Matrix Solid Phase Dispersion (MSPD)
Matrix Solid Phase Dispersion (MSPD) is a sample preparation technique for solid- (e.g animal or plant tissue, milk, vegetables),
semi-solid-, and viscous-samples
24
. It employs bonded phase solid supports, usually silica-based, as both an abrasive to produce a
disruption of sample architecture and as a bound “solvent” to assist in complete sample disruption during the process of blending.
Figure 10.6 provides a simplified description of the overall process. With this blending, the sample disperses itself over the surface
of the bonded phase/support material providing a new mixed phase for conducting analyte isolation from a variety of sample
matrices. The blending does not have to be performed with rigorous grinding by mortar and pestle, but gentle stirring is sufficient for
most matrices. This dispersed sample provides more access to solvents and reagents used for analyte isolation. After the disruption
process, the blended sample matrix and its distribution onto the bonded phase/support are transferred to a syringe barrel column
fitted with a frit. A second frit is placed at the top of the packed bed. Various solvents, usually of progressively stronger polarity
depending on the analytes to be eluted, are passed through the packed bed. Often, a second cleanup step is required. In some
cases, the effluent from the MSPD column is directed to a conventional SPE cartridge where further purification takes place.

144
Figure 10.6
Matrix Solid-Phase Dispersion Process
1Step 5Step
6
Step
7
Step
8
Step
2Step
3Step
4Step
Laboratory
Spatula
Pipette Tip
Syringe Barrel (10 mL)
used as MSPD Column
Paper Frit
•
•
•
•
•
The MSPD technique has found widespread applications, especially in the food industry, such as for the sample preparation of
pesticides in fruit
25
, baby food
26
, fruit juices
27
, veterinary drugs in tissue
28
, antibacterial residues in foodstuff
29
, isoflavones in clover
leaves
30
, polyaromatic hydrocarbons in bivalves
31
, and antibiotics in milk
32
. The advantages of the method are its simplicity, good
extraction efficiencies with reasonable recoveries, solvent savings over traditional extraction methods, and wide application range;
however, automation of the technique is quite cumbersome.
Paper Frit
Modified Syringe Plunger
Reconstitute residue with
solvent of choice
Submit to analysis
Use co-columns or
additional SPE cleanup
Reduce volume or
evaporate completely
Filter or centrifuge
(optional)
Column Eluate
Receiving
Tube
Solvent
Elute sample;
gravity or vacuum
Collect sample fractions
Add solvent(s)
Compress the sample
Tissue/C
18
Blend
0.5 g Sample
Glass Mortar
Glass Pestle
2.0 g Precleaned
and Conditioned C
18
Add sample
Transfer blend to column
Add standards or modifiers
and equilibrate; blend

145
Specialty Phases in SPE
Specialty phases for SPE are phases that have been developed for specific applications that may be difficult to achieve on a
standard SPE device. However, sometimes manufacturers will use a standard phase, but test it specifically for a certain class of
compounds and provide a recommended set of SPE conditions for their optimum isolation. In some cases, the specialty SPE
cartridges come as part of a “total solution” kit with reagents, standards, and a method. But most specialty SPE phases have been
synthesized with chemical functionality that will specifically interact with solutes of interest. Table 10.3 shows several specialty
phases that are available to deal with specific cleanup tasks. Additional phases follow.
Table 10.3
Specialty Phases for SPE
Bond Elut Name
Functional Group(s)
Present Main Application
Atrazine C18 Low load, high-flow C18 phase designed for atrazine extraction. Large particle size allows flow of
large sample volumes; Controlled carbon content enhances atrazine selectivity; Large bed mass
offers optimized capacity for atrazine.
Carbon Graphitized carbon Non-porous sorbent exhibits high affinity for organic polar and non-polar compounds from both
non-polar and polar matrices, when used in reversed-phase conditions; Recommended for
removal of chlorophyll and other pigments (especially in QuEChERS applications, see Chapter 8).
Carbon/PSA Graphitized carbon
and primary secondary
amine (layered)
Bond Elut PSA is an alkylated amine sorbent that contains two different amino functionalities: a
primary and a secondary amine. This gives a slightly higher pKa and ionic capacity compared to
Bond Elut NH2. The PSA sorbent has a significantly higher carbon load than most amino
functional sorbents and therefore is a better choice for polar compounds that retain to strongly
on Bond Elut NH2. Bond Elut PSA is an ideal sorbent removing fatty and organic acids as well
as pigments and sugars when performing multiresidue pesticide food analysis.
Carbon/NH
2
Graphitized carbon
and amino (layered)
Designed for cleanup of Japanese Positive List for the analysis of pesticides in food;
Bond Elut NH2, a primary amine, is a weaker anion exchanger, than sorbents such as SAX
(a quaternary amine sorbent that is always charged). Therefore, it is a better choice for the
retention of very strong anion such as sulfonic acids that retain irreversibly on an SAX sorbent.
Bond Elut NH2 is an ideal sorbent for removing fatty and organic acids, as well as pigments
and sugars when performing multiresidue pesticide food analysis.
(Continued)

146
Cellulose Cellulose powder Cellulose phase stable over a wide pH range and contains an extremely low metal content (Fe
and Cu content less than 5 ppm). The combination of surface area and polymeric structure results
in a sorbent with excellent capacity. The cellulose media contains numerous hydroxyl groups;
because of it polar nature, it is able to accept high loading of many polar substances from
aqueous and organic phases.
EnvirElut Polymeric EnvirElut sorbents are specially designed for the extraction of a wide range of compounds from
aqueous matrices, especially herbicides, Polynuclear aromatic hydrocarbons (PAHs), and
pesticides.
Mycotoxin Silica-based
ion exchange
Novel sorbent which cleans up food extracts for improved trichothecene and zearalenon analysis.
Results are comparable or superior to competing methods, including immunoaffinity columns
(IAC) and charcoal/alumina column; acts in a selective non-retention mechanism – the toxin
analytes pass through the cartridge while the food matrix components are retained (Explained
more thoroughly later in this chapter, under Class- or Ion-Specific SPE Cartridges, Mycotoxin
SPE Sorbent).
PBA Phenylboronic acid Functionality that can retain analytes via a reversible covalent bond; boronate group has a strong
affinity for cis-diol containing compounds such as catechols, nucleic acids, some proteins,
carbohydrates and PEG compounds. Aminoalcohols, alpha-hydroxy amides, keto compounds,
and others can also be retained; very strong covalent retention mechanism enables high
specificity and cleanliness (Explained more thoroughly under Immobilized Phenyl Boronic Acid
(PBA) Phases).
PCB Proprietary
dual phase
Specially designed sorbent with optimized bed mass which allows for the facile extraction of
polychlorinated biphenyl (PCB) compounds from a variety of matrices; desired analytes can be
loaded and eluted using a simple, single solvent method prior to analysis by GC/ECD.
Bond Elut Name
Functional Group(s)
Present Main Application
Specialty Phases for SPE

147
Restricted Access Media (RAM)
The RAM is a special class of SPE sorbent used for the direct injection of biological fluids such as plasma, serum or blood. They are
most often used for the analysis of small drugs, their impurities, and metabolites. Over the years, there have been many variations of
these sorbents described as internal-surface reversed-phases, shielded hydrophobic phases, semi-permeable surfaces, dual-zone
phases, and mixed functional phases. For more information on the various classifications of RAM, consult
33,34
.
The most popular RAM phases are the dual-mode porous packings that are characterized by an outer hydrophilic layer and an inner
surface porosity with a hydrophobic bonded phase (Figure 10.7). The outer hydrophilic surface provides minimal interaction with
proteins and when combined with small pores of the packing that exclude them, the proteins to elute unretained while small drugs
and drug metabolites pass into the pores and are retained by hydrophobic interactions with alkyl bonded phases. Despite being
described as “non-fouling” phases, RAMs have had a reputation for eventual fouling with repeated injections of straight biological
fluids. If the pH and organic solvent composition of the mobile phase are not optimized, protein precipitation can occur in the RAM
causing fouling, so some care must be exercised in their use.
Figure 10.7
Pictorial Representation of Resticted Access Media (RAM)
The RAM phases can be used in the single column mode or with multidimensional LC-LC. In the single column mode, conditions are
selected to first exclude plasma proteins, then running gradient elution to elute and separate the drug compounds. Although this
approach has worked satisfactorily, because of the increased chances of fouling the RAM due to lack of re-equilibration after gradient
elution or due to inadequate selectivity of the hydrophobic stationary phase inside the pores, multidimensional LC-LC approaches
have proven to be more successful. Here, an additional column (usually a reversed-phase column) is plumbed into the system after
the RAM column via a 6- or 10-port switching valve (see Chapter 13 for more information on column configurations). Isocratic
conditions are used for injection of the plasma onto the RAM, and an additional gradient pumping system is employed for the
reversed-phase chromatographic analysis. In this approach, the trapped drug and its metabolite molecules from the RAM column can
be flushed (or backflushed) into the reversed-phase column and gradient elution is performed to separate the impurities and/or
metabolites. When these multidimensional approaches are employed, the RAM column is often backflushed and regenerated after
each analysis. Longterm stability with repeated usage of the RAM column has been reported for soy isoflavones in rat serum
36
.
In addition, the secondary reversed-phase column also has a longer lifetime since plasma proteins are not injected onto this second
column but are vented to waste.
Internal pores
with bonded phase
Outer hydrophilic layer
Physical Diffusion Barrier Chemical Diffusion Barrier
148
Molecularly Imprinted Polymers (MIPs)
The MIPs are among the most selective phases used in SPE. The technique is sometimes referred to as molecularly imprinted
solid-phase extraction (MIP-SPE). Molecular imprinting is a technique that has been used in areas where selective recognition
is required for complex separations or sample cleanup. An introductory article
37
outlined the basics of MIP technology, while
review articles
38-41
, and a book
42
provide detailed information on the use and potential of MIPs in SPE.
A MIP is a highly stable polymer that possesses recognition sites that are adapted to the three-dimensional shape and
functionalities of an analyte of interest. The most common approach through the use of noncovalent imprinting involves the uses
of a print molecule (template) that is chemically coupled with one of the building blocks of a polymer. After polymerization, the
resulting bond must be cleaved to obtain a free selective binding site (receptor). The synthesis process is shown schematically in
Figure 10.8.
The selective interactions between the template and the monomers are based upon hydrogen bonding, ionic and/or hydrophobic
interactions. The most often used monomers are based on methacrylic acid and/or methacrylates. The basic idea of a MIP is the
“lock and key” concept where a selective receptor or cavity on the surface of a polymer perfectly fits the template analyte that
was used to prepare the MIP. The receptor site is complementary to the template in terms of its size, shape, and functionality.
The concept is similar to immunoaffinity (IA) SPE phases (see next section, Immunoaffinity Extraction of Small Molecules) but
obtaining and linking a suitable antibody for these IA sorbents can be very time consuming.
Figure 10.8
Analyte
(template)
Self-Assembly
Monomers
Crosslinker
Polymerization
Wash
Rebinding

149
The removal of the template from the polymeric MIP is important, not only to make available the interaction sites for increased
sample capacity, but to also ensure that the analyte to be isolated can be measured quantitatively. The lack of complete removal of
the template molecules, even with exhaustive extraction, has been one of the main problems with the acceptance of MIPs. The
template molecules frequently bleed, sometimes give baseline drifts, and they interfere with the quantitation of the desired analyte,
especially at low levels. One approach to overcome this limitation is to use a template that is similar to the analyte of interest. An
example would be to use a brominated analog template rather than a chlorinated molecule of interest. If the analog can be
separated from the analyte of interest, then the MIP will function as desired.
With aqueous mobile phases, MIPs can display reversed-phase and ion exchange interaction since selective polar interactions are
impaired. The MIP phases show the greatest selectivity when the experimental conditions are chosen that generate the selective
interactions that are usually obtained in organic solvents used for the MIP synthesis. This approach allows the MIP to be used for
trapping analytes from aqueous solution by hydrophobic or ionic interactions, then washed with a solvent that breaks selective
binding of matrix components, and finally with an organic solvent which disrupts the strong bonds between the analyte and the MIP
polymer matrix.
Since the SPE packing material is a polymer, depending on the degree of crosslinking, there may be some swelling or shrinkage
with a change in solvent. Such a physical change can modify the size of the receptor and change the selectivity of the MIP for the
target analyte. In this regard, perhaps the synthesis of molecular imprinted organic-inorganic hybrid polymers
43
may generate a
more rigid substructure that does not swell and shrink.
A disadvantage of the MIP approach to SPE is the fact that each sorbent must be custom made. The specificity of the MIP can be
determined by choosing the appropriate template molecule. The MIP can be synthesized in the laboratory using published
procedures, or the template molecule can be sent to a specialty laboratory that will make a custom MIP. Because of the relatively
long process involved in making a MIP for SPE, one can justify it only if the application will frequently be required, or if there is no
other way to perform sample cleanup.
Recently, off-the-shelf MIPs have been introduced. These standard MIP phases have been designed for specific analytes that are
popularly encountered in complex matrices. Among those currently available are sorbents optimized for:
•
Clenbuterol in biological fluids
•
Beta agonists, multiresidue extractions in urine and tissue samples
•
NNAL (4-Methylnitrosamino-1-(3-pyridyl)-1-butanol), tobacco-specific nitrosamine in biological matrices
•
Riboflavin (vitamin B2) in aqueous samples
•
Triazines, multiresidue extraction in water, soil, and food products
•
Chloramphenicol, antibiotic in biological matrices
•
Beta blockers, multiresidue extractions in water, and biological samples

150
Immunoaffinity Extraction of Small Molecules
Similar to MIPs, immunoaffinity phases are based upon molecular recognition but use chemically attached mono- or poly-clonal
antibodies rather than surface cavities. Undoubtedly, the immunoaffinity phases are the most selective since they are primarily
designed around biological antigen-antibody interactions that provide high selectivity and high affinity. These sorbents enable the
selective extraction and concentration of individual compounds or classes of compounds from matrices, often in a single step.
Antibodies for large biomolecules are readily available and have been used for many years in immunology and medical research
and in the immunoextraction of enzymes, hormones, and other biospecies (see Chapter 15 for an example of immunoextraction of
proteins). However, antibodies for small molecules are more difficult to obtain, so the development of small molecule immunoaffinity
extraction is more recent and less developed. Some excellent review articles are available for those who would like to understand
immunoaffinity extractions in more detail
44-48
.
To demonstrate the use of immunoaffinity sample preparation, a sample cleanup of corn and peanut butter samples was performed
for the analysis of aflatoxins using an AflaPrep immunoaffinity SPE cartridge (R-Biopharm, Darmstadt, Germany). The cartridge
contains an anti-aflatoxin antibodies immobilized on a wide-pore silica and is selective for the compound class. Aflatoxins are highly
toxic natural substances and suspected carcinogens produced by mold that can be found on agricultural products such as grains,
corn, peanuts, and seeds. There are four fluorescent aflatoxins that are commonly analyzed: B1, B2, G1, and G2, each with
different structures. The common analysis procedure is to perform liquid-liquid extraction, SPE or column chromatography, followed
by HPLC analysis with fluorescence detection.
Recently, a more selective, straightforward cleanup was introduced using off-line immunoaffinity chromatography
50
. Figure 10.9
depicts results obtained by immunoaffinity cleanup followed by reversed-phase chromatography and fluorescence detection using
post-column electrochemical derivatization using bromine as a derivatizing agent. The linear range for each of the four aflatoxins
was found to be 0.1-10 µg/L with the R-square value greater than 0.99998. Limits of detection were found to be in the range of
0.004-0.007 µg/L (equivalent to 0.008-0.014 µg/kg content in the samples). Relative standard deviations of retention time and
peak areas for all four aflatoxins were all less than 0.3%.

151
Figure 10.9
Determination of Aflatoxins in Corn and Peanut Butter
Column: Zorbax Eclipse Plus C18,
4.6 x 150 mm x 5 µm
Column Temp.: 40 °C
Mobile Phase A: 1 L water containing 238 mg KBr
and 700 µL 4M HNO
3
Mobile Phase B: MeOH
Isocratic: A: B = 50; 50, 12 min
Flow rate: 1.0 mL/min
Detection: Ex: 362 nm, Em: 455 nm, gain = 15
Injection: 20 µL
Electrochemical
Current: 100 µA setting
Reaction coil: 0.5 mm id 34 cm long peek tubing (from exit
of KOBRA cell to the entrance of FLD)
The sample preparation procedure for the aflatoxins involved weighing a 25 g sample, adding 2 g NaCl and 125 mL HPLC grade
methanol-distilled water (60:40 v/v) into a high-speed blender jar. Blend for one minute and dilute the extract with 125 mL of
distilled water. After filtration, 10 mL of the filtrate was passed through the immunoaffinity column at a flow rate of 2-3 mL/min.
The column was washed with 10 mL of water. Finally, the aflatoxins were eluted from the cartridge using 1 mL of HPLC-grade
methanol. The methanol extract was diluted with 1 mL of water before injection into the HPLC.
1.5
w
1.4
1.3
1.2
1.1
1
0 246810 min
0.9
0.8
1.7
w
7
6
5
4
3
0246810 min
2
1
0
w
3.5
3
2.5
2
1.5
0246810 min
1
0.5
0
0.1 ppb STD
Corn
Peanut Butter
HPLC Conditions

152
N
H
B
OH
OH
OH
Class- or Ion-Specific SPE Cartridges
Over the years, specialty phases have been introduced that are compound-, class- or element-specific. Of course, the
immunoaffinity- and MIP-SPE phases are on the extreme ends of the selectivity scale. Specialty phases have special functional
groups that can interact with certain compounds and have found use in niche applications.
Immobilized Phenylboronic Acid (PBA) Phases
When the PBA-SPE phases are treated with alkaline buffer, they become very specific for certain functional groups such as vicinol
diols which are present in sugars and catechols. Other difunctionalities can also be reactive. For example, alpha-hydroxy acids,
aromatic o-hydroxy acids and amides, and aminoalcohol-containing compounds can be retained. They actually form covalent bonds
with these groups (see details in Figure 10.10). When bonded, the sorbents can be washed with any number of solvents to
remove interferences. Once washed, the covalent bonds can be broken by washing the phase with an acidic buffer/solvent that
hydrolyzes the covalent bonds. A popular application of the PBA phases is the isolation of catecholamines in biological fluids
52
.
Figure 10.10
Anticipated Secondary Interactions Exhibited by Immobilized PBA
Hydrophobic
TT-TT
Ionic
Hydrogen
Charge-Transfer
An application displaying the unique selectivity of the PBA phase is the determination of Ribavirin in human plasma
53
. Ribavirin is in
a class of antiviral medications called nucleoside analogues. It works by stopping the virus that causes hepatitis C from spreading
inside the body. The chemical structure (Figure 10.11) shows a distinctive diol group that is well-suited for the PBA’s ability to
covalent bond as indicated in Figure 10.10.

153
OH
HO
O
O
N
N
N
NH
2
HO
The sample preparation procedure was simple. The heat-deactivated plasma was mixed with ammonium acetate buffer (250 mM,
pH 8.5) and loaded onto a Bond Elut PBA cartridge (100 mg/1 mL), first conditioned with MeOH, followed by 2 mL of ammonium
acetate buffer. After loading, the cartridge was washed with the same buffer followed by MeOH. Finally, the analytes were
successively eluted with 1 mL of 2.5% formic acid in methanol. After evaporating the eluent to dryness, the dried extracts were
reconstituted with 200 µL of mobile phase, centrifuged, and a portion injected into a reversed -phase column with UV detection.
The PBA method gave better recovery and reproducibility than did LLE with various solvent combinations. The calibration curve was
linear over the range of 0.05-10 µg/mL and the limit of quantitation (LOQ) was determined to be 0.050 µg/mL. This extraction
method can also be modified to facilitate the extraction of ribavirin from foods such as chicken
54
.
Figure 10.11
Structure of Ribavirin
Drugs of Abuse Analysis
Screening for drugs of abuse in biological fluids is an important application of SPE. In this particular analysis, high purity, high
recovery, and rugged methods are essential for an effective screen and to avoid false positives. The Certify (Agilent Technologies)
mixed mode SPE sorbent takes advantage of non-polar, polar, and ion exchange properties. Because the sorbent is capable of
exhibiting a variety of sorbent-analyte interactions, they can be used either as a general screen for several broad drug classes or in
specific extractions for GC and GC/MS confirmation of drugs and metabolites. The use of a bonded phase containing a medium
length hydrocarbon chain (C8) allows for some exposure of the polar silica surface. Therefore, polar and non-polar interactions of
the drugs and matrix interferences with the sorbent are optimized. The second bonded phase, a strong cation exchanger, has been
optimized for capacity.
The Certify cartridge is conditioned first with methanol to open up the coiled hydrophobic portion of the sorbent and activate it
toward interaction with polar matrix. Further conditioning with buffer removes excess methanol and places the sorbent bed in an
environment as similar to the matrix as possible. This allows for maximum sorbent-matrix interaction and reproducible recoveries.
The extraction of drugs from complex biological matrix such as plasma or urine requires that pH, ionic strength, and viscosity be
controlled. This is accomplished by dilution of the sample with buffer.

154
To illustrate how the Certify drugs of abuse phases are used in practice, we will look at cleanup of THC and its metabolites in whole
blood
55
. The traditional analytical technique for screening drugs of abuse is GC followed by GC/MS for confirmation. The key to
reliable THC testing in blood is to have an efficient extraction method. In this example, SPE using Certify II was employed for
cleanup followed by GC-MS/MS for analysis. Only 2 mL of blood sample was required for the assay. Deuterated internal standards
of tetrahydrocannabinol (THC) and its known metabolites 11-hydroxy-D-tetrahydrocannabinol (11-OH-THC) and 11-nor- D9-
tetrahydrocannabinol-9-carboxylic acid (THCA) were spiked in the blood sample at the 10 µg/mL level. Next, 4 mL of acetonitrile
was added to precipitate the plasma proteins. After centrifugation, the supernatant was transferred and evaporated to about 3 mL
followed by the addition of 7 mL of 0.1 M sodium acetate (pH 6.0).
Certify is recommended for the cleanup of basic drugs such as amphetamines, phencyclidine, proxyphene, meperidine, LSD,
codeine, oxycodone, and opiates. Although very different in their pharmacology and structure, all basic drugs feature amine
functional groups (NR
3
, NR
2
H, or NRH
2
). This group acts as a base by abstracting H
+
and becoming positively charged. Initial
extraction, however, takes place by non-polar mechanism onto the hydrophobic portion of the sorbent. After the drug is retained,
washing the cartridge with water removes polar interferences. Next, the cartridge is washed with acid, completing the elution of
polar interferences and ensuring that the basic drugs of positively charged as ammonium salts. Non-polar, non-basic drugs and
interferences can then be removed with an organic wash. Finally, the basic drugs can be eluted with alkaline organic solvent
[i.e. 2% NH
4
OH in either methanol, ethyl acetate (EtOAc), or CH
2
Cl
2
/isopropanol (IPA)]. The presence of base serves to disrupt
the ionic interactions of the drug with the sorbent since the positive charge on the drug is neutralized. The use of organic solvent
disrupts the hydrophobic interactions, which initially retained the drug from the sample.
Similar to the basic drugs, acidic and neutral drugs, such as barbiturates, phenytoin, methaqualone, benzodiazepines, and D
9
-carboxy
THC, have widely varying pharmacological and structural properties. They are classed together because they are not retained by a
cation exchange mechanism, although the cation exchange portion of Certify can improve cleanup of samples containing these drugs.
These drugs are characterized by the absence of a basic amine functional group. (Note, drugs such as barbiturates have a nitrogen-
containing imine functional group, which is weakly acidic, rather than basic). These drugs are retained by a non-polar mechanism.
Washing the cartridge with dilute acid removes polar impurities and insures that any basic interferences become charged. Thus,
when the acidic and neutral drugs are eluted by disrupting their non-polar interaction with the sorbent, the basic interferences are
retained on the strong cation exchange portion of the sorbent. To handle these acidic and neutral drugs, Certify II was developed. It is
a mixed mode sorbent with a short chain alkyl group (C8) along with anion exchange functionality. Retention of the acidic drugs on
Certify II is initially achieved by non-polar interactions on the hydrophobic portion of the sorbent. Next, polar interferences can be
washed away with the basic buffer. This wash step also ensures that the COOH functional group is deprotonated, forming COO
-
,
which can then be retained on the anion exchange portion of the Certify II sorbent. The charge on any amine functional groups would
be neutralized by this step as well, preparing any basic drugs present for the washing step. After briefly drying the cartridge, non-polar
basic drugs and interferences can be removed with a non-polar solvent. Finally, acidic drugs such as D
9
-carboxy THC can be
recovered by elution with a non-polar acidic solvent such as hexane/EtOAc with 1% acetic acid.

155
High Flow Bond Elut Certify II cartridges were conditioned with 2 mL of methanol, then 2 mL of 0.1 M sodium acetate buffer
(pH 6.0) with 5% methanol. The sample was slowly added to the cartridge. The cartridge was then washed with 2 mL of
sodium acetate buffer, dried under vacuum for 5 min and washed with 1 mL hexane. The THC was eluted under neutral
conditions with 2 mL of 95:5 hexane:ethyl acetate. This was followed by 5 mL 1:1 methanol:deionized water wash. Again, the
column dried under vacuum for 5 min and washed again with 1 mL hexane elution of the metabolites was performed with 2 mL
of 1% acetic acid in 75:25 hexane:ethyl acetate. The THC and metabolites fractions were combined, evaporated to dryness,
and reconstituted with toluene before derivatization. The 3 analytes were derivatized with 40 µL of N,O-
Bis(trimethylsilyl)trifluoroacetamide (BSTFA) and 1% trimethylchlorosilane (TMCS).
Gas chromatography analysis was performed using an Agilent Triple Quadruple GC/MS equipped with a low thermal mass (LTM)
column module which allows very fast temperature ramping and rapid cooling. In addition, the backflushing feature of the gas
chromatograph was used to prevent any carryover of impurities that may have been co-extracted with the analytes of interest.
Details of the chromatographic and mass spectrometric conditions can be found in the original application note
55
. Using SPE
cleanup, GC column backflushing, and MRM for the triple quadrupole mass spectrometer, signals for the various analytes were very
clean and allowed the detection of very low levels of THC and its metabolites. The resulting linear dynamic range of quantification
was 0.1-50 ng/mL for THC and 11-OH-THC and 1-100 ng/mL for THCA. The LTM module and backflushing facilitated rapid analysis
with a run time of 6 minutes and a cycle time of 8 minutes. An alternative analytical technique for THC analysis that avoids having
to perform derivatization as is required for GC is the use of LC-MS/MS
56
.
Ion Removal by SPE
In many applications, especially in ion chromatography, high concentrations of ionic components from the sample matrix can be
undesirable interferences. The use of ion exchange resins with specific functionalities can be used to remove these ionic species.
For example, a strong cation exchanger in the barium form will selectively remove high concentrations of sulfate from aqueous
solution. The same can be said for a cation ion exchanger in the silver form for the removal of chloride ion. Chelating ion
exchangers can remove transition metals.

156
Multimodal and Mixed-Phase Extractions
Most SPE procedures involve the use of a single separation mode (e.g., reversed-phase) and a single SPE device (e.g., cartridge).
However, when more than one type of analyte is of interest, or if additional selectivity is required for the removal of interferences,
multimodal SPE can prove useful. Multimodal SPE refers to the intentional use of two (or more) sequential separation modes or
cartridges (e.g., reversed-phase and ion exchange). Experimentally, there are two approaches to multimodal SPE. In the serial
approach, two (or more) SPE cartridges are connected in series (see Figure 10.12). Thus, for the separate isolation of acids, strong
bases, and neutrals, an anion- and cation exchange cartridge could be connected in series. By adjusting the sample and wash
solvent to pH 7, both the acids and bases will be fully ionized. As a result, the acids will be retained on the anion exchange
cartridge, the bases will be retained on the cation exchange column, and the neutrals will pass through both columns (separated
from acids and bases). The acids and bases can then be separately collected from each cartridge.
Mycotoxin SPE Sorbent
Fusarium fungi are probably the most prevalent toxin producing fungi of the northern temperate regions and are commonly found in
cereals grown in the temperate regions of America, Europe, and Asia. A variety of Fusarium fungi produce different toxins of the
class of trichothecenes. These toxins are very detrimental to human health. Twelve type A- and B- trichothecenes and zearalenone
(ZEA) in cereals and cereal-based food can be isolated using Bond Elut Mycotoxin, a specialty SPE phase for these important
compounds
57
. First, the toxins are extracted from the finely-ground cereal samples using acetonitrile/water (80:20 v/v), which
minimizes co-extractables from the matrix. After filtration, a 4 mL portion of the filtrate is passed through a Bond Elut Mycotoxin SPE
column and the effluent is collected and evaporated to dryness under a gentle stream of nitrogen. The sample was reconstituted in
0.5 mL of acetonitrile:water (20/80 v/v) and a 10 µL portion is injected into the LC-MS/MS for analysis. A wide number of grains
ranging from corn, wheat, oats, bread, etc. were spiked with the 12 trichothecenes and ZEA and recoveries at the part per billion
level were quite acceptable, averaging from 70-95% with RSDs averaging around 5%. Recoveries were better than the
conventional cleanup via charcoal-alumina columns and compared to immunoaffinity columns that were considerably more
expensive since multiple columns were required. An advantage is that the Bond Elut Mycotoxin SPE cartridge allows the isolation
of multiple mycotoxins such as aflatoxins, ochratoxin, fumonisins, and others
58
.

157
A second approach to multimodal SPE uses mixed phases. Here, a single cartridge phase might possess two (or more) functional
groups to retain multiple species, or to provide a unique selectivity. Often, a single phase can show multiple interactions. For
example, a polymeric ion exchange phase could show ionic interactions (e.g. cationic or anionic) and hydrophobic interactions with
the polymer backbone (e.g. PS-DVB). One popular application of multimodal SPE is the isolation of drugs of abuse and
pharmaceuticals from biological fluids as mentioned above.
Another version of multimodal SPE is the use of layered packings
59
where two or more packings in a single cartridge are used to
isolate differing molecular species. Several methods for pesticides in vegetables rely on layered phases to provide the needed
selectivity for adequate sample cleanup. The most popular method employing layers of SPE phases is the Japan Positive List for
agricultural chemicals in foods
60
. Many countries that export agricultural products to Japan must test according to this list, which
consists of the total 799 compounds. This list not only includes pesticides, but also feed additives in veterinary drugs. For analysis,
most of these methods require LC/MS (or MS/MS) or GC/MS using reversed-phase HPLC columns. Two detailed multiresidue
screening methods using graphitized carbon/amino layered SPE phases and silica phases for sample cleanup are available for
pesticides in tomato and lemon matrices
61
.
Figure 10.12
Experimental Setup for Multimodal SPE for Cleanup of Complex Samples
Cartridge (stationary phase #1)
Adapter
Cartridge (stationary phase #2)
Stopcock
Port plug

158
References
1.
Arthur, C.L.; Pawliszyn,
J. Anal. Chem.
1990,
66
, 2145-2148.
2.
Baltrussen, E.; Sandra, P.; David, F.; Cramers, C.A.
J. Microcol.
Sep. 11, 1999, 737.
3.
Battik, P.; Vednar, P.; Cip, L.; Ondrakova, L.; Stransky, Z.
JSS
2003,
26 (8)
, 715-721.
4.
Hook, G.L.; Kimm, G.; Betsinger, G.; Savage, P.B.; Swift, A.; Logan, I; Smith, P.A.
JSS
2003,
26 (12-13)
, 1091-1096.
5.
Frost, R.P.; Hussain, M.S.; Raghani, A.R.;
JSS
2003,
26 (12-13)
, 1097-1103.
6.
Cai, L.; Xing, J.; Dong, L.; Wu, C.
J. Chromatogr. A
2003,
1015 (1-2)
, 11-21.
7.
Araujo, W.A.; Lacerda, C.A.; Cappelaro, E.A.; Lancas, F.M.
JSS
2003,
26 (6/7)
, 624-628.
8.
Pinho, O.; Peres, C.; Ferreira, I.M.P.L.V.O.
J. Chromatogr. A
2003,
1015 (1-2)
, 23-30.
9.
Castro Mejas, R.; Natera Marn, R.; de Valme Garca Moreno, M.A.; Garca Barroso, C.
J Chromatogr. A
2003, 995 (1-2), 11-20.
10.
Bagheri, H.; Mohammadi, A.
J. Chromatogr. A
2003,
1011 (1-2)
, 1-9.
11.
Bianchi, F.; Caren, M.; Mangia, A.; Musci, M.
JSS
2003,
26 (5)
, 369-375.
12.
Namiesnik, J.; Jastrzebska, A.; Zygmunt, B.
J. Chromatogr. A
2003,
1016 (1)
, 1-9.
13.
Lin, H.-H.; Sung, Y.-H.; Huang, S.-D.
J. Chromatogr. A
2003,
1012 (1)
, 57-66
14.
Liu, W.
Determination of Sub-ppb Level of Phthalates in Water by Auto-SPME and GC/MS
, Agilent Technologies Application Note
#5989-7726EN, January, 2008.
15.
Centineo, G.; Ignacio Alonso, J.
Speciated Isotope Dilution for Determination of Methyl Mercury in Tuna Fish by GC/MS
, Agilent
Technologies Application Note #5989-9725EN, Santa Clara, CA, September 2008.
16.
Zhao, S.; Zhai, A.
Determination of Volatile Aromatic Compounds in Soil by Manual SPME and Agilent 5975T LTM GC/MSD
,
Agilent Technologies Application Note #5990-6398EN, Santa Clara, CA, October, 2010
17.
Baumann, S.
Sensitive Detection of 2-Methoxy-3-Isobutylpyrazine (MIBP or IBMP) in Wine Using Triple Quadrupole GC/MS in
PCI Mode
, Agilent Technologies Application Note #5990-4935EN, Santa Clara, CA, November, 2009.
18.
Shutao, W.; Yan, W.; Hong, Y.; Zie, Y.
Chromatographia
2006, 63, 365-371.
19.
You, Y.-W.
Sensitive Detection of 2-MIB and Geosmin in Drinking Water
, Agilent Technologies Application Note
#5991-1031EN, Santa Clara, CA, August, 2010.
20.
Baumann, S.; Tandon, K.
Sensitive Detection of Trichloroanisole (TCA) in Wine Using Triple Quadrupole GC/MS
, Agilent
Technologies Application Note #5990-4968EN, Santa Clara, CA, November, 2009.
21.
Hinshaw, J. Solid-Phase Microextraction,
LCGC No. Amer.
2012,
30 (10)
, 904-910.
22.
Eisert, R.; Pawliszyn,
J. Anal. Chem.
1997,
69
, 3140–3147.
23.
Liu, W.
Determination of Sub-ppb Level of Phthalates in Water by Auto-SPME and GC/MS
, Agilent Technologies Application Note
#5989-7726EN, 2008.
24.
Barker, S.A.; Long, A.R.; Short, C.R.
J. Chromatogr.
1989,
475
, 353-361.
25.
Ramos, J.J.; Gonzalez, M.J.; Ramos, L.
J. Chromatogr. A
2009,
1216 (43)
, 7307-7313.
26.
Georgakopoulos, P.; Mylona, A.; Athanasopoulos, P.; Drosinos, E.H.; Skandamis, P.N.
Food Chem.
2009,
115 (3)
, 1164-1169.
27.
Radisic, M.; Grujic, S.; Vasiljevic, T.; Lausevic, M.
Food Chem.
2009,
113 (5)
, 712-719.
28.
Wang, S.; Mu, H.; Bai, Y.; Zhang, Y.; Liu, H.
J. Chromatogr. B
2009,
877 (27)
, 2961-2966.
29.
Marazuela, M.D.; Bogialli, S.
Anal. Chim. Acta
2009,
645 (1-2)
, 5-17.
30.
Visnevschi-Necrasov, T.; Cunha, S.C.; Nunes, E.; Oliveira, M.B.P.P.
J. Chromatogr. A
2009,
1216 (18)
, 3720-3724.

159
References
35.
Cassiano, N.M.; Lima, V.V.; Oliveira, R.V.; de Pietra, A.C.; Cass, Q.B.
Anal. Bioanal. Chem.
2006,
384 (7-8)
, 1462-1469.
36.
Doerge, D.R.; Churchwell, M.L.; Berry Delclos, K.
Rapid Commun. in Mass Spectrometry
, 2000,
14
, 673-678.
37.
Ensing, K.; Berggren, C.; Majors, R.E.
LCGC
2001,
19 (9)
, 942-954.
38.
Dmitrienko, S.G.; Irkha, V.V.; Kuznetsova, A. Yu.; Zolotov, Yu. A.
J. Anal. Chem.
2005,
59 (9)
, 808-817.
39.
Baggiani, C.; Anfossi, L.; Giovannoli, C.
Current Pharma. Anal.
2006,
2 (3)
, 219-247.
40.
Mahony, J.O.; Nolan, K.; Smyth, M.R.; Mizaikoff, B.
Anal. Chim. Acta
2005,
534
, 31-39.
41.
Cormack, P.A.G.; Elorza, A.Z.
J. Chromatogr. B
2004,
804
, 173-182.
42.
Piletsky, S.; Turner, A.
Molecular Imprinting of Polymers
, Landes Bioscience, Austin, TX, 2006.
43.
Lin, C.I.; Joseph, A.K.; Chang, C.K.; Wang, Y.C.; Lee, Y.D.
Anal. Chim. Acta
2003,
481
, 175-180.
44.
Hennion, M.-C.; Pichon, V.
J. Chromatogr. A
2003,
1000
, 29-52.
45.
Pichon, V.; Delaunary-Bertoncini, N.; Hennion, M.-C.
Chapter 33 – Immunosorbents in Sample Preparation, in J. Pawliszyn,
Comprehensive Analytical Chemistry
2002,
37
, 1081-1100.
46.
Delaunay, N.; Pichon, V.; Hennion, M.-C.
J. Chromatogr. B
2000,
745
, 15.
47.
Stevenson, D.
J. Chromatogr. B
2000,
745
, 39.
48.
Delaunay-Bertoncini, N.; Pichon, V.; Hennion, M.-C.;
Chromatographia
2001,
53
, S224.
49.
Majors, R.E.
LCGC No. Amer.
2007,
25 (1)
, 16-32.
50.
Yang, X.; Rong, A.
Determination of Aflatoxins (B1, B2, G1 and G2) in Corn and Peanut Butter by HPLC-FLD Using Precolumn
Immunoaffinity Cleanup and Post-Column Electrochemical Derivatization
, Agilent Technologies Application Note #5990-9125EN,
Shanghai, China, 2011.
51.
Li, X.; Pennington, J.; Stobaugh, J.F.; Schöneich, C.
Analytical Biochemistry
2008, 372 (2), 227-236.
52.
Phenylboronic Acid (PBA) Solid-Phase Extraction Mechanisms and Applications
, Agilent Technologies Technical Overview,
Publication #Si-02442, 2010.
53.
Loregian, A.; Scarpa, M.C.; Pagni, S.; Giuseppe-Parisi, S.; Palu, G.
J. Chromatogr. B
2007,
856 (1-2)
, 358-364.
54.
Berendsen, B.J.A.; Wegh, R.S.; Essers, M.L.; Stolker, A.A.M.; Weigel, S.
Anal. Bioanal. Chem.
2012,
402 (4)
, 1611-1623.
55.
Baumann, S.
Rapid and Robust Detection of THC and Its Metabolites in Blood
, Agilent Technologies Application Note
#5990-8456EN, 2011.
56.
Hudson, J.; Hutchings, J.; Wagner, R.; Harper, C.; Friel, P.
Cannabinoid Quantitation Using an Agilent 6430 LC/MS/MS
,
Agilent Technologies Publication Number 5991-2521EN, 2013.
57.
Rudrabhatla, M.; Wood, J.S.
Amer. Lab.
April 1, 2007, 40.
58.
Raisglid, M.; Burke, M.F.
Abstract #653
, 48th Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, Atlanta, GA, 1997.
59.
Introduction of the Positive List System for Agricultural Chemical Residues in Foods
, Japanese Department of Food Safety,
Ministry of Health, Labour and Welfare, June 2006.
60.
Chang, E.; Yamashita, K.; Simpson, N.; Arora, R.
Multiresidue Screening of Agricultural Chemicals (I) and (II) in Food According
to the Japan Positive List using Agilent Cartridge-Based SPE and LC-MS/MS
, Agilent Technologies Application Note
#5990-9895EN, 2012.
31.
Campins-Falco, P.; Verdu-Andres, J.; Sevillano-Cabeza, A.; Molins-Legua, C.; Herraez-Hernandez, R.
J. Chromatogr. A
2008,
1211
(1-2)
, 13-21.
32.
Mamani, M.C.V.; Reyes, F.G.R.; Rath, S.
Food Chem.
2009,
117 (3)
, 545-552.
33.
Boos, K.-S.; Rudolphi, A.
LCGC
1997, 15 (7), 602-611.
34.
Boos, K.-S.; Rudolphi, A.
LCGC
1997, 15 (9), 814-823.

160
Chapter 11
Size-Exclusion Chromatography
as a Sample Preparation
Technique
Size-exclusion chromatography (SEC) is one of the main modes of HPLC. Unlike other HPLC modes, in SEC there is no interaction
between the analyte and the stationary phase. Molecules are separated on the basis of size in solution. Packing materials with
various size pores are used for separations. As shown in Figure 11.1, large molecules are excluded from these solvent-filled pores
while small molecules permeate the pores and elute later. Thus, if one is interested in lower molecular weight analytes in the
presence of higher molecular weight species, SEC is an ideal sample preparation technique. If the high molecular weight molecules
are not of interest they can be directed to waste via a column switching valve (see Chapter 13), while the small molecules can be
directed to a second HPLC column for further separation and characterization. The technique can also be performed off-line, by
collecting the fractions of interest and re-injecting them into another column. Both non-aqueous and aqueous size-exclusion
chromatography can be performed in this manner. Calibration curves for SEC columns of molecular weight (size) versus elution
volume are useful to provide guidelines for choosing the best sample cleanup column (see later discussion).
B
C
A
C
B
A
Mechanism of SEC
Figure 11.1
Partial entry
Molecules must freely enter and exit pores to
be separated. Largest molecules elute first,
followed by intermediate size molecules,
and finally the smallest molecules elute last.
Full entry
Packing pore
Molecule (C) will be excluded from pores
Molecules (A, B) enter pores
Time
Signal

161
The benefits of SEC are that it allows removal of interferences that can cause poor analytical results. It also reduces the risk of
damage to an analytical column, since it may remove higher molecular weight compounds that may irreversibly bind to the
stationary phase. With column switching valves, the entire process can be automated. When operated in the non-aqueous SEC
mode, sometimes referred to as gel permeation chromatography (GPC), high molecular weight compounds such as lipids,
hydrocarbons, oils, and other interferences can be separated and removed. In the aqueous SEC mode, biomolecules such as
proteins, polymeric materials, and other water-soluble higher molecular weight species can be removed from an aqueous sample.
Aqueous SEC is a useful technique for desalting biological samples since salts are low molecular weight compounds that elute at
the total permeation volume late in the chromatogram (see SEC for Biomolecule Sample Preparation).
An important area in the use of SEC for sample cleanup is the determination of trace levels of priority pollutants in complex
environmental and food samples. For these types of samples, it is necessary to remove high molecular weight interferences such as
lipids, polymers, and pigments before analyzing it by GC or HPLC (and their hyphenated mass spectrometric systems). In fact, there
is a regulated SEC cleanup method recommended by the United States Environmental Protection Agency – EPA 3640A. The
method allows both self-packed and pre-packed size exclusion columns operated in the non-aqueous SEC mode. Important toxic
compounds that are often analyzed include PCBs, PAHs, and pesticides. Figure 11.2 shows the classical EPA method and depicts
the elution profiles of various classes of pollutants. The procedure uses the undesirable chlorinated solvent dichloromethane as the
mobile phase. The EPA GPC calibration mixture consists of corn oil (25,000 mg/L), bis(2-ethylhexyl) phthalate (1,000 mg/L),
methoxychlor (200 mg/L), perylene (20 mg/L) and sulfur (80 mg/L). Figure 11.3 provides a chromatogram of the resolution test
calibration mixture recommended by the EPA to characterize a SEC column. The chromatogram takes one hour for the separation
using a classical SEC (GPC) column.
One approach to speeding up the EPA method is the use of a smaller particle size SEC material packed in a smaller diameter glass
column. EcoSpheres, supplied by Agilent, are spherical, microporous resins with a low percent crosslinking. These particles are
available as loose microporous media in a dry powder form ready for swelling and gravity packing into glass columns. These
microporous materials have no permanent pore structure. Instead, pores are generated when these materials swell in solvents.
Microporous packings give higher loadings where high resolution is not required. They can be self-packed into an adjustable bed
length glass column. Using the EPA test method, Figure 11.4 shows that the separation of the EPA test mixture can be done in a
third of the time compared to the classical EPA procedure
1
.
162
0
10
20 30 40
5060
C
C
Phthalate
Organophosphate pesticides
Corn Oil
PCP
C-Collect
Minutes
Mobile phase: CH
2
Cl
2
– Stat. Phase: BioBeads SX-3 (700 mm x 25 mm)
Total Solvent Consumption: 300 mL
EPA Method 3640
PNAs
Herbicides
Organochlorine pesticides
Chlorophenols
Nitrophenols
Nitrosamines, nitroaromatics
Chlorobenzenes
Aromatic amines
0
15
30
45
60
Corn Oil
DEHP
Methoxychlor
Perylene
Sulfur
Figure 11.2
Classic Gel Permeation Chromatography
Elution Profile for Sample Cleanup
Figure 11.3
SEC Chromatogram of EPA Calibration Mixture for EPA Method 3640A
Chromatography Conditions:
Column: BioBeads SX-3
Column size: 25 mm id x 700 mm length
Bead length: 490 mm
Mobile phase: Methylene Chloride
Flow rate: 5 mL/min (Total Solvent Consumption:
300 mL)

163
20100
1
2
3
4
5
Retention time, min
1. Corn oil (DEHP)
2. Bis (2-ethyhexyl) phthalate
3. Methoxychlor
4. Perylene
5. Sulfur
EPA Resolution Mix for Method 3640A Using EcoSphere Column
Column: Glass Column with Swollen EcoSpheres (100 g)
EPA Resolution Mix for Method 3640A Using EcoSphere Column
25 mm id x 45 mm length
Mobile Phase: THF
Flow Rate: 5 mL/min (Total Solvent Consumption: ~100 mL)
Injection: 5 mL
Detector: UV, 254 nm
An alternative approach uses an even smaller particle size Agilent PLgel polymeric packing (5-7 µm) with a 100Å pore. With the
smaller particle size, resolution is increased, but so is the pressure, and the loading capacity is reduced. However, the particles
themselves are more rigid and can withstand higher pressures, up to 150 bar. The injection of the EPA resolution mixture into
this smaller particle column gives sharper peaks and allows an even faster separation as can be seen in Figure 11.5. Here, THF
was used as the mobile phase, a safer solvent than the chlorinated solvent dichloromethane.
min
8
1
3
4
5
2
10 12 14 16 18
Norm.
0
200
400
600
800
1000
1200
EPA Resolution Mixture Uusing 5 µm SEC Column
Column: PLgel, 5 µm
7.5 x 300 mm
Mobile Phase: THF
Flow Rate: 0.67 mL/min
(Total Solvent Consumption: 12 mL)
Collection zone
1. Corn oil
2. DEHP
3. Methoxychlor
4. Perylene
5. Sulfur
Figure 11.4
Figure 11.5

164
To illustrate how the off-line SEC approach is used as a sample prep technique, a soil sample was spiked with the priority pollutant
polynuclear aromatic hydrocarbon (PAH) standards. The soil was first extracted with THF. Two PLgel columns (100 Angstrom pore
size, 7.5 x 300 mm dimensions) were used for the cleanup. In Figure 11.6A, the PAHs were isolated from the injection of a
portion of the soil extract. The two red asterisks represent the point of collection. The large blue baseline drifting upwards is
actually the soil extract UV profile. The red portion represents the portion of the chromatogram where the PAHs eluted. This
fraction was concentrated, taken up with an HPLC-compatible solvent, then re-injected onto a reversed-phase HPLC column.
Gradient elution using water-acetonitrile showed the presence of a number of these priority pollutant compounds (Figure
11.6B). Diode array detection allowed the determination of subparts per million concentrations of these contaminants. To keep
the pressure a bit lower, Agilent’s EnviroPrep, which is a 10 µm macroporous resin in a higher capacity preparative size column
(25 x 300 mm), might be used.
400
300
200
100
0
10 20 30
20
30
40
10
0
10 25
20155
Figure 11.6A
SEC Chromatogram of Soil Extract vs. EPA Resolution Mix (A)
Column: 2X PLgel (7.5 x 300 mm)
Mobile Phase: THF
Detector: DAD
Absorbance
Retention time, min
Retention time, min
Collection points
Corn Oil
DEHP
Methoxy-
chlor-
PAHs
(including Perylene)
Sulfur
Soil sample extract profile
Where collection of PAHs was made
Figure 11.6B
SEC Chromatogram of Soil Extract vs. EPA Resolution Mix (B)
Column: Reversed-Phase
Mobile Phase: Water-ACN gradient
Detector: DAD
Fluorescence Intensity
PAH-Fraction
(ppm value following
compound name)
Phenanthrene 2.3
Anthracene 0.9
Fluoranthene 4
Pyrene 4.4
B(a)fluorene
B(a)anthr 3.2
Chrysene 3.2
B(b)fluora 2
B(k)fluora 0.9
B(a)pyrene 2.5
Indeno 0.6

165
Meat samples often contain high amounts of lipid and may be difficult to analyze without some type of sample cleanup. SEC is an
ideal technique for such sample extracts since the lipid material is of higher molecular weight than the small molecules, such as
pesticides or certain toxins in which one may be interested. To test the possibility of using SEC to clean up a fresh pork sausage, a
sample of the meat was spiked with the EPA Resolution test mix and then extracted. The extractable mass from the meat was
consistent with estimated of 25-30% lipid solids.
When dealing with high fat extractable samples, SEC column capacity comes into play and one should make sure that the column
dimensions are compatible with the sample loading. For example, the mass capacity for the column used in this example (PLgel
100Å, 7.5 x 300 mm) with acceptable resolution is approximately 40 mg. So this amount is the upper mass injectable limit for the
entire sample including the lipid extractables. If larger injections are needed for analyte sensitivity, then a wider bore or longer
column may be required.
min
81012141618
Norm.
0
200
400
600
800
1000
1200
Corn Oil
DEHP
Methoxychlor
Perylene
Sulfur
Cleanup of EPA Resolution Mix-Spiked Pork Sausage Extract
Using PLgel 5 µm 100Å Column
Figure 11.7
In Figure 11.7, one can see the UV absorption profile (curve in blue) of the lipid background in the SEC chromatogram
2
. The bulk
of the lipid would be removed via the SEC route illustrating the cleanup ability of the technique. However, depending on the
collection volume and retention window collected, a small amount of lipid extractables might still be collected for subsequent
injection into another column or for further sample cleanup.
Sausage Extract
Profile
To aid in selecting the best organic SEC column for the cleanup of complex mixtures, calibration curves are often used to evaluate its
molecular weight discrimination ability. Here a plot of molecular weight (molecular size) using a series of standards of known
molecular weight versus elution volume (or retention time) is constructed (see next section for more details for aqueous SEC
columns). This calibration curve gives the user a better idea of which pore size SEC to use for the sample cleanup. Since molecules
differ in their molecular sizes defined by their hydrodynamic radius, a calibration plot is only an approximation when dealing with
complex mixtures.
Retention time, min

166
Size Exclusion Chromatography
for Biomolecule Sample Preparation
Size exclusion chromatography (SEC) can also be used for separating biomolecules such as proteins from lower molecular weight
substances such as contaminants that can include aggregates (monomers, dimers, trimers, tetramers, etc.), excipients, cell debris
and other impurities arising from degradation. It is frequently used for removing salts, a process known as desalting. The mechanism
for the size separation of biomolecules in aqueous solution, sometimes called gel filtration chromatography (GFC), is exactly the
same as when using SEC packings with organic mobile phases, as discussed in the previous section. Larger biomolecules spend less
time in the pores and elute sooner. Smaller biomolecules spend longer in the pores and elute later.
In order to be biocompatible, packing materials used in aqueous SEC often contain water-wettable functional groups such as diol or
glycol that cut down on specific interactions with various functional groups on proteins. Both polymeric- and silica-based packing
materials are available for SEC cleanups. Columns packed with polymer-based sorbents are frequently used for polymeric molecules
with broad molecular weight distributions such as heparin, starch or cellulose. Proteins and molecules which have a discrete
molecular weight are best suited to silica-based stationary phases. It must be remembered that proteins contain numerous amino
acids with differing side chain functionalities: acidic, basic, hydrophobic and neutral/hydrophilic. In order to prevent interactions
occurring with silica columns, sometimes buffers are needed in the mobile phase. Volatile buffers are recommended if mass
spectrometry will be used for downstream detection.
Calibration curves that plot molecular weight (or more correctly size) versus elution volume are useful for determining the proper
SEC column for protein mixture cleanup. A hypothetical calibration curve is shown in Figure 11.8. Standards of known molecular
weight (size) are injected onto the SEC column and their elution volumes determined. Then, when an unknown is injected under the
same chromatographic conditions, one can get a good estimate of the molecular sizes of the molecules in the sample and can use
this information to select the optimum pore size for the best cleanup of the sample at hand. The green region of Figure 11.8 where
the curve is the flattest provides the best overall chromatographic resolution. Figure 11.9 shows some actual calibration curves of
different pore size SEC packings.

167
10,000kD
1,000kD
100kD
10kD
1kD
100
56
7 8 9
10
11 12 13
Figure 11.8
Hypothetical Calibration Curve for Aqueous SEC Column
MWvsRet
4
100
1,000
10,000
100,000
1,000,000
10,000,000
100,000,000
1,000,000,000
5678910 11 12 13
Column: Bio SEC-3 Agilent Technologies
7.8 x 300 mm, 3 µm
Mobile Phase: 150 mM Na phosphate, pH 7.0
Flow Rate: 1.0 mL/min
Detector: UV
Pore Size*
Proteins MWt 300Å 150Å 100Å
Thyroglobulin 670000 6.34 5.50 5.63
Gamma globulin 158000 8.03 6.24 5.74
BSA 67000 8.90 7.00 6.03
Ovalbumin 45000 9.57 7.70 6.41
Myoglobin 17000 10.12 8.50 7.10
Ribonuclease A 12700 10.40 8.80 7.46
Vitamin B-12 1350 11.90 11.40 10.20
*Values represent "Retention Volume" on calibration curve
Molecular Weight
Retention Volume (mL)
300Å
150Å
100Å
Figure 11.9
Calibration Curves for SEC Columns with Three Different Pore Sizes
Total permeation
Above curve:
Interacting with sorbent?
Below curve:
Behaving as if
higher MW.
Aggregating?
Best resolution where
curve is shallowest or flattest
Exclusion
Elution Volumes, mL

168
The measurement and potential removal of protein aggregates is an important sample cleanup activity. The size, type, and content
of aggregates present in protein biopharmaceuticals can affect both efficacy and formulation – or worse, induce an immunogenic
response. Aggregation formations occur through a variety of mechanisms, including disulfide bond formation and non-covalent
interactions. Because the size of protein aggregates, including dimers, is sufficiently different from the protein monomer, you can
separate the various forms using SEC. In fact, SEC with UV or light scattering is a standard technique for quantifying protein
aggregation. Figure 11.10 provides a chromatogram of a CHO-humanized monoclonal antibody where the dimer is well separated
from the monomer. Note that the packing with the 300 Angstrom pore size gave a good separation of the MAb monomer from its
dimer as well as some unknown lower molecular weight impurities.
To desalt biological samples, SEC is a widely accepted method. Analytically, aqueous SEC can distinguish between molecules (e.g.
proteins) with a molecular weight difference of less than a factor of 2. In desalting, the size difference between the substances
being separated is very large (i.e. proteins vs. salts). The pore size of the gel filtration media is chosen so that it completely
excludes the larger proteins while allowing the smaller molecules such as salts and other impurities to freely diffuse into all
of the pore spaces.
The column is equilibrated with a buffer, which may be the same or different from that of the sample. The larger protein molecules
– which can’t enter the pores of the media – elute first from the column, followed by the smaller molecules including the salts that
diffuse into the pores. If there is no interest in the smaller molecules and salts, they can be directed to waste or, if these substances
are of interest, can be directed to a fraction collector for further handling.
Sometimes, it is desirable to have the purified sample collected in a different buffer than the original. If the mobile phase buffer is
different from the buffer the original sample buffer, the larger molecules will elute in this new buffer; hence the process that takes
place is buffer exchange. This approach is particularly useful when volatile buffers are to be substituted for non-volatile buffer for
mass spectrometric compatibility.
169
VLC_MD_Mab
0
50
100
150
200
250
300
mAU
4 6810 12 14 min
Column: Bio SEC-3, 300Å
5190-2511
7.8 x 300 mm, 3 µm
Buffer: Sodium phosphate buffer, pH 7.0, 150 mM
Isocratic: 0-100% Buffer A from 0-30 min
Flow Rate: 1.0 mL/min
Sample: CHO-humanized MAb, 5 mg/mL – intact
Injection: 5 µL
Detector: UV, 220 nm
Temperature: Ambient
Monomer
Dimer
Figure 11.10
Intact MAb Monomer and Dimer Separation by SEC
References
1.
Saunders, G.; MacCreath, B.
Sample Cleanup by Gel Permeation Chromatography using Agilent EcoSpheres
, Agilent Technologies
Application Note #5990-7583EN, 2011.
2.
Woodman, M.; Stone, P.
Optimizing Sample Loading in Automated Size Exclusion Chromatography Sample Preparation for
Small Molecules Analysis from Complex Matrices
, Agilent Technologies Application Note #5989-0181EN, 2003.

170
Prior to the development of SPE for sample pre-treatment, similar purifications were carried out using low pressure or open-column
liquid chromatography (LC). Figure 12.1 depicts the steps in open column LC. In this cleanup approach, adsorbents such as large
particle silica gel or alumina are packed into large internal diameter (1-5 cm) glass columns. The sample is injected with a pipette
onto the top of the packed bed and solvent is percolated through the column under gravity flow. Fractions are either collected
manually or with a fraction collector. The analyte measurement is performed off-line using a spectrophotometer or colorimeter. If
analyte concentration is too low in the collected fractions for measurement by the secondary analytical technique, they can be
concentrated using other sample preparation techniques, such as evaporation. Relative to HPLC, column efficiency is poor, but the
technique is simple to carry out.
Column/Flash Chromatography
as a Sample Preparation
Technique
Chapter 12
B
A
C
Depiction of the Flash Chromatography Process
Figure 12.1
Glass Wool
Outlet
Glass Column
Packing
Collect fractionsAdd mobile phaseLoad sample
Solvent

171
A modern version of column chromatography called flash chromatography has become popular in recent years. Flash
Chromatography was first performed in the 60s and 70s by pressurizing open-column work, but this fell out of favor with the
introduction of 10 µm and sub-10 µm LC columns. Flash was “rediscovered” in the mid 90s as a technique to rapidly purify mg
to g quantities of synthesis mixtures when combinatorial chemistry was “the thing” to do for development of targets for new
pharmaceuticals. Traditional column chromatography was too slow, SPE required multiple fraction collection or risk of loss of
analyte, HPLC did not have high enough loading, and synthetic chemists found the instruments too complex.
In this technique, convenient pre-packed columns with pre-cleaned adsorbent are used and low pressure (a few bar) is applied
to aid flow. A wide variety of pre-packed flash columns are available with diameters ranging from 75-150 mm with masses from
200-9 kg. In addition to the classical adsorbents, newer bonded stationary phases such as C8 or C18 bonded silicas are
available, so besides normal phase separations on silica and alumina (neutral, basic, acidic), reversed-phase chromatography
can be performed. Popular bonded phases that are available for HPLC are now obtainable for flash chromatography.
Instruments are also available to pump mobile phase through the columns at moderate pressures. Modern instruments run
gradients and have various flow-through detector options. They are really a subset of HPLC separation instruments but work at
lower pressures because the particles used are rather large (greater than 20 µm) compared to HPLC/UHPLC columns.
The question arises: where does solid phase extraction end and flash chromatography begin? In terms of the size of the
container, the mass and particle size of packing, large SPE cartridges very much resemble small flash columns and vice-versa.
The main difference is most likely that the flash columns are used for preparative work and large scale cleanup while large SPE
cartridges are used to scale up from smaller sizes due to their capacity limitations. In reality, it doesn’t matter since both are
performing some degree of sample cleanup.
The use of off-line LC (including flash chromatography) as a cleanup technique for GC and HPLC is well documented. In liquid
phase flash chromatography, the cleanup step can be performed in a different mode than the HPLC mode and can therefore be
orthogonal to the subsequent HPLC separation providing a high degree of sample cleanup. This multidimensional flash LC-HPLC
experiment can also provide a cleaner sample to be presented to the HPLC column which serves to prolong its lifetime. Samples
can often be fractionated using step gradients. There are many sample cleanup methods for pesticides in environmental
samples and drugs in biological fluids where the techniques have been used successfully. In GC, adsorbents such as Florisil
and Alumina have long been used for sample cleanup prior to pesticide analysis. In some cases, the use of thin layer
chromatography (TLC) can be helpful in selecting the best set of LC conditions for performing flash chromatography.
For example, TLC can help to determine what solvents can be used for step gradient elution or for solvent selection to
elute analytes but not matrix compounds from a column.

172
Chapter 13
Column-Switching
(On-Line SPE) as a
Sample Preparation Technique
Column switching – also called 2D LC, multidimensional column chromatography, coupled-column chromatography, or "box car”
chromatography – is a powerful technique for the separation and cleanup of complex, multi-component samples. In this approach,
all or a portion of the chromatogram from an initial column (Column 1) is selectively transferred to a second column (Column 2) for
further separation (see Figure 13.1). In the context of sample preparation, Column 1 can be an SPE column, cartridge, or disk,
and the technique is referred to as “on-line SPE”.
Column switching (C-S) is used for:
•
Removal of sample components detrimental to Column 2
•
Removal of late-eluters prior to injection onto Column 2
•
Selective removal of interferences that overlap analyte peaks in Column 2
•
An alternative to gradient elution
•
Trace enrichment, also a sample preparation technique
The achievement of one or more of these goals often results in increased sample throughput versus the use of single-column
operation. The basic goal of C-S is to maximize the transfer of the analyte band into Column 2, while minimizing the transfer of
interfering compounds, i.e., the same goal as in sample preparation using off-line SPE.

173
Figure 13.1
Principle of Chromatography as a Sample Preparation Technique
(Heart Cutting)
Column 1
Column 2
Injection
Injection
= Undesired matrix compounds
= Compounds of interest
In HPLC, C-S is achieved by connecting Column 1 to Column 2 via a high-pressure switching valve. In this way, the sample is
partially separated on Column 1, and a fraction containing the analyte(s) of interest is directed to Column 2 for final separation
and subsequent detection. C-S can involve combinations of LC, GC, TLC, SFC, and CE. In this Chapter, only LC-LC methods will
be covered. While C-S is similar to the HPLC analysis of off-line fractions provided by SPE (see Chapter 9), in its usual practice,
several major differences exist:
Of course, there are a few disadvantages associated with column switching. First, the system requirements are more complex than
off-line SPE – valves, tubing, precise timing requirements, and electronic interfaces are required. Samples must be particulate-free
since the frits on Column 1 may retain them and eventually block the column, or at least, experience a pressure buildup. Strongly
retained sample components and matrix interferences can build up on the initial column requiring replacement or periodic cleaning.
• SPE cartridges are typically used only once and discarded; Column 1 in C-S is used repeatedly, although often for fewer injections
(e.g., 50-100) than usual for an HPLC column. Therefore, in C-S, extra washing/backflushing steps may be required to ensure
interferences are removed from Column 1. Otherwise, these impurities can impair the performance of Column 1 or show up as
extraneous peaks which elute from Column 2 in later analyses.
• Column 1 has a higher efficiency (smaller particle diameter, dp in the 3 or 5 µm range) compared to an SPE cartridge (dp in the
40 µm range). Thus, analyte bandwidths from Column 1 are narrower, which allows better resolution on Column 1 compared to
an SPE cartridge, and cleaner samples presented to Column 2 for an easier final HPLC separation.
• Since C-S is performed in a closed system, there is less of a chance of sample loss (e.g. oxidation) or concentration change (e.g.
evaporation) than in SPE usually performed in an open-air environment.
• Many valve configurations are possible for heart cutting, backflushing, diverting contaminants directly to waste, etc.

174
Principles of Operation
Column-switching can be carried out either manually or automatically, but most applications of C-S are fully automated.
Low-dead-volume switching valves are used and automatically actuated by timers or time-programmable events from an
HPLC system controller. An important experimental requirement for C-S is the complete transfer of analyte from Column 1 to
Column 2. This requires close control of the switching time. High-pressure switching valves are commercially available with
2 to 10 (or more) ports, 6- and 10-port valves being used most often. C-S can be carried out with a single pump with some
elaborate reconfiguration, but multiple pumps (at least two) are usually preferred.
One frequently used C-S system (see Figure 13.2) employs a two pump configuration with Column 1 placed within a six-port valve
that is placed across two of the ports and before Column 2. In Figure 13.2A, the valve position allows pump A mobile phase from
Column 1 to bypass Column 2 and flow directly to waste. Thus, undesired sample components injected into Column 1 that were
weakly retained can be removed and sent to waste. Next, in Figure 13.2B, the valve position is switched to allow mobile phase
delivered from pump B to pass through Column 1 onto Column 2. Thus, any components remaining in Column 1 could be
“backflushed” to Column 2, rather than eluted in the same direction as the sample was loaded, for further separation and
detection. Forward-elution may be applied if contaminants including particulates are also trapped on the loading end of Column 1,
which would interfere with the analysis on the second column (if backflushing was used) into Column 2. The mobile phase strength
and timing considerations can allow selective elution of sample components remaining in Column 1 after the initial sample loading.
Figure 13.2
Experimental Setup for Sample Prep Cleanup by Backflushing
A
B
Column 1
Column 1
Inj. Pump A
Column 2 Detector WastePump B
Waste
Inj. Pump A
Column 2 Detector WastePump B
Waste

175
Examples of Column-Switching
for Sample Cleanup by LC
In this example, the valving configuration shown in Figure 13.2 is used for sample cleanup. The initial sample preparation of
spice products, such as chili or paprika powder, for subsequent Sudan dye analysis is relatively simple and straightforward. The
powder is extracted with acetonitrile using an ultrasonic bath. The liquid is cooled, filtered, and injected into the HPLC column.
This extract contains a substantial amount of colored and non-colored compounds, and gives a complex LC-MSD chromatogram
as shown in Figure 13.3. On-line sample cleanup is performed by a precolumn (Column 1) – referred to as a cleaning column in
reference 1 – which is achieved by a valve switching between the analytical column (Column 2 in Figure 13.2), and cleaning
column. This process eliminates severe contamination of the analytical column from oils and other residues present in the spices,
as well as minimizing ion suppression in the mass spectrometer.
10000000
8000000
6000000
4000000
2000000
0
246810 12 14 min
Figure 13.3
Paprika Powder Extract Analyzed Using MSD in Scan Mode
Conditions
Cleanup Column (Column 1): Agilent ZORBAX RRHT Eclipse Plus C18,
2.1 x 30 mm, 1.8 µm
Analytical Column (Column 2): Agilent ZORBAX RRHD Eclipse Plus C18,
2.1 x 100 mm, 1.8 µm
Mobile Phases: A = Water,
B = Acetonitrile (for analytical pump,
the water was modified with 400 µL TFA)
Analytical Pump Flow: 0.5 mL/min; stop time 15 min; post
time 3 min
Cleaning Pump Flow: 0.3 mL/min; stop time no limit
Gradient for Analytical Pump: at 0 min 5% B, at 5 min 95% B
Isocratic for Cleaning Pump: 5% B
Column Oven: 40 °C on both sides,
valve switch = next run
Valve Switch: at 2.0 min position B
Injector: 1 µL injection volume,
needle wash for 6 s
Columns:
Sudan Dyes in Spices
1
: Sudan dyes are azo dyes, and based on findings of the IARC (International Agency For Research on
Cancer), are classified as Group 3 of the potential carcinogenic compounds. These compounds have been banned in food products
in the EU, Japan, and United States since 2004. If these compounds are detected in spices or products containing spices such as
spice blends, the food products must be recalled and destroyed. The following three main Sudan dyes were studied: Sudan Orange
G, Sudan 1 (Sudan I), and Sudan 2 (Sudan II).

176
Using the valve assembly setup already shown in Figure 13.2, the workflow used for cleaning the paprika powder extract on-
line is as follows:
1) The sample is introduced into the shorter cleaning column (valve position Figure 13.2A). The mobile phase is provided by
pump A. All compounds eluting within 1 min are sent to waste.
2) The valve is switched after 1 min to operate the cleaning column and the analytical column in series (valve position Figure
13.2B). Pump B now provides the mobile phase. The analytical gradient starts and the peaks of interest elute from the
analytical column (Column 2).
3) The valve is switched again to its initial position and all remaining compounds are discarded (valve position Figure 13.2A).
The acetonitrile strength for the cleaning column is increased to 95% to flush out all remaining compounds. The cleaning
column (Column 1) is then equilibrated to the starting conditions.
Using the Agilent 1290 Infinity UHPLC System equipped with an Agilent 6140 quadrupole mass spectrometer operated
in the SIM mode, the Sudan red compounds were measured with the high sensitivity. The target masses were 215, 249,
and 277. Figure 13.4 shows an overlay of standard and sample extract chromatograms. Compared to Figure 13.3, the
chromatograms were much less cluttered after on-line cleanup and SIM detection, since compounds eluting prior to or after
the Sudan dyes were diverted to waste. Although not depicted here, data was collected showing that the ion suppression
effect of the matrix components was reduced. For the three main Sudan dyes, the Minimum Detection Quantity (MDQ) was
approximately 100 µg/kilogram for each dye
1
.

177
30000
20000
10000
1 2345678min
30000
20000
10000
0
1 2345678min
800000
400000
0
12345678min
8000000
4000000
0
12345678min
Figure 13.4
Overlay of Standard and Sample Extract
LC-MSD Chromatograms in SIM Mode
5 µL Injection Volume Sudan Orange G, Target Mass 215, 30 pg/µL
Sudan 1, Target Mass 249, 40 pg/µL
Sudan 2, Target Mass 277, 50 pg/µL
Standard
Conditions
Analytical Column: Agilent ZORBAX RRHD Eclipse Plus C18,
2.1 mm x 100 mm, 1.8 µm
Cleaning Column: Agilent ZORBAX RRHT Eclipse Plus C8,
2.1 mm x 30 mm, 1.8 µm
Mobile Phases: A = Water, B = Acetonitrile Analytical
pump water + 400 µL TFA
Flow Analytical Pump: 0.5 mL/min; stop time 9 min;
post time 3 min
Flow Cleaning Pump: 0.5 mL/min; stop time no limit
Gradient for
Analytical Pump: At 0 min 20% B; at 1 min 20% B;
at 5 min 95% B
Gradient
Cleaning Pump: At 0 min 20% B;
at 6.5 min 20% B;
at 6.6 min 95% B;
at 8.1 min 20% B
Column Oven: 40 °C on both sides, valve switch = next run
Valve Switching: At 0 min position A, at 1 min position B,
at 6.5 min position A
DAD: 220/20 nm, Ref = off, 450/20,
PW >0.0012 min, 20 Hz, slit width 4 nm
Injector: 1 (standard) or 5 µL (spiked extract) injection
volume, needle wash for 6 s
MSD: Peak width 0.03 min;
Positive SIM parameters for
Mass 215, 249, and 277, fragmentor = 100;
Actual dwell 45;
Gas temp = 350,
Drying gas = 12 L/min;
Neb pres = 35 psig;
V
cap
positive = 3000 V
Columns:
: shows location of peak for parent Sudan dye

178
Using a similar instrumental configuration as depicted in Figure 13.2, eight chlorinated herbicides were isolated on an
enrichment column (Agilent ZORBAX SB-AQ, 2.1 x 30 mm, 1.8 µm) then transferred to an analytical column (Agilent ZORBAX
SB-C18, 2.1 x 50 mm, 1.8 µm) for separation, followed by sensitive diode array detection (DAD)
2
. Using less than 2 mL of
herbicide-containing water, the entire trapping process took less than 3 min and the full cycle time was only 15 min (see
Figure 13.5). The sensitivity of the method achieved the detection limits prescribed in Method 555 by the United States
Environmental Protection Agency (EPA) (0.5 ng/mL for picloram and 1.7 ng/mL for dichlorprop). The RSD values for peak
areas, peak heights, and retention times were all less than 1%.
0
50
100
150
200
250
300
mAU
100 11 12 13 14 min
Figure 13.5
Chromatogram of a Sample Containing 50 ppb of Each of the 8 Analytes
Picloram
Chloramben
Dicamba
Bentazone
2,4-D
Dichlorprop
2,4,2-T,P
Aciffuorfen
Herbicides in Water Using Trace Enrichment: Trace enrichment involves the concentration of small amounts of organics from
a large sample volume (typically potable water or source aquifers) by passing it through a trapping column and transferring the
concentrated analytes into another HPLC column for further separation. Herbicide contamination in water bodies is monitored to
mitigate adverse health impacts. Sensitive methods for detecting and quantifying trace levels of these chemicals are necessary to
meet regulatory requirements.

179
Other uses of column switching are shown in Table 13.1. The beauty of such systems is that the processes can be automated, thus
improving quantitation and reproducibility, as well as decreasing the labor content of the assay.
Table 13.1
Examples of Column Switching as a Sample Preparation Technique
Analyte(s) Matrix Methodology Reference
Immunosuppressants Plasma
and whole blood
Protein precipitation followed by trapping of IS on Column 1;
backflush removal of matrix compounds and elution to Column 2 for
analysis by LC-MS/MS
5
Neomycin and Screening
Aminoglycosides
Milk Extraction of ion pairs on short fused silica capillary RPC column;
directly eluted to MS-MS
6
Indometacin Plasma Used ISRP (internal surface reversed-phase) Column 1; proteins are
excluded but small drug molecule retained, analyte switched to
analytical column for analysis by LC-DAD
7
Nortriptyline Biotrap exclusion Column 1 used to remove proteins; drug is eluted
into RP Column 2 and detected by DAD
8
25-Hydroxy Vitamin D2 and D3 Protein precipitation followed by trapping on Poroshell 120 column;
removal of matrix interferences and elution to Column 2 for
separation and analysis by LC-MS/MS
9
Beta-blockers Groundwater Large volume injection with preconcentration followed by
LC-fluorescence and LC-TOF MS
10
17b-Estradiol Plasma Underivatized estradiol trapping column (reversed-phase)
and analytical column (same)
11
Oxygenates and Aromatics Hydrocarbon
processing stream
GC-GC application using Dean’s switching for heart cutting analytes 12
Digoxin Human serum Restricted access media in Column 1 excludes proteins but retains
small molecule; proteins flushed to waste and small molecule eluted
to Column 2, a short chain reversed-phase column
13

180
Related to column switching, comprehensive multidimensional chromatography (sometimes called 2D chromatography if two
columns are being used and referred to as LC x LC or GC x GC) is mainly used when the goal is to analyze every single compound
eluting from Column 1. In comprehensive LC x LC, the flow from either pump is never stopped and separations take place on both
columns simultaneously. The flow of Column 1 is relatively slow since the effluent from that column is continuously collected in one
of two holding loops across ports of a 10-port valve. The two holding loops are of sufficient size to hold the entire output from
Column 1 while a very fast analysis is occurring on Column 2, usually only a minute or two. Once the separation in Column 2 is
completed and the column quickly regenerated, the contents of the initial holding loop is switched to Column 2 and another fast
analysis starts again. This time, the second holding loop begins collecting more effluent from Column 1 and the process repeats
itself. The main difference in column switching experiments is that we may only be interested in one, two, or several bands eluting
from Column 1 and not the entire sample. In GC x GC, a thermal modulator is employed and the effluent gas from GC Column 1 is
cryogenically trapped to collect and concentrate analytes while the separation on GC Column 2 is also performed extremely fast,
often under one minute. The trapped sample is then rapidly heated and directed to Column 2 for further separation while a new
fraction is simultaneously trapped in the cryogenic modulator. For more information on 2D chromatography, see references 3 and 4.
References
1.
Gratzfeld-Huesgen, A.
On-line Sample Cleanup on the Agilent 1290 Infinity LC Using a Built-in 2-position/6-port Valve
, Agilent
Technologies Application Note #5990-5255EN, February, 2010.
2.
Kailasam, S.
Trace Analysis of Chlorinated Herbicides in Water with On-line Enrichment
, Agilent Technologies Application Note
#5990-6922EN, December, 2010.
3.
Mondello, L. (Ed.)
Comprehensive Chromatography in Combination with Mass Spectrometry
, Wiley, New York, 2011,
ISBN-10: 0470434074 and ISBN-13: 978-0470434079.
4.
Cohen S.A.; Schure, M.R. (Eds.)
Multidimensional Liquid Chromatography: Theory and Applications in Industrial Chemistry
and the Life Sciences
, Wiley-Interscience, New York, 2008, ISBN: 0471738476.
5.
McCann, K. ASMS 2011, Paper ThP166,
A Rapid Quantitative Analysis of Five Immunosuppressant (IS) Drugs in Blood
by LC-MS/MS for Clinical Research
, June, 2011.
6.
Lu, C.-Y.; Feng, C.-H.
J. Sep. Sci.
2006,
29
, 2143-2148.
7.
Koizumi, K.
Automatic HPLC Analysis of Indometacin in Plasma Using Column Switching
, Agilent Technologies Publication
#5968-7205, August, 1999.
8.
Ricker, R.
Analysis of Nortriptyline in Plasma
, Agilent Technologies Publication #5988-6398EN, April, 2002.
9.
Chun, M.R.; Jung, H.-J.; Yang, J.S.; Kim, H.-Y.; Lee, S.-Y.
Mass Spectrometry: Applications to the Clinical Lab
(MSACL 2012)
Poster 55, January, 2012.
10.
Galera, M.M.; Vazquez, P.P.; del Mar, M.; Vazquez, P.; Garcia, G.; Amate, C.F.
J. Sep. Sci.
2011,
34
, 1796-1804.
11.
Szczesniewski, A.
Mass Spectrometry: Applications to the Clinical Lab
(MSACL) Tuesday Lunch Workshop, January, 2012.
12.
McCurry, J.; Quimby, B.
Two-Dimensional Gas Chromatographic Analysis of Oxygenates and Aromatics in Gasoline Using
a Heart Cutting Technique
, Agilent Technologies Application Note #5988-6696EN, May, 2002.
13.
Sudergat, H.; Unger, K.K.; Emmert, J.; Wendt, J.; Mandel, F.
Determination of Digoxin in Human Serum by LC/MS with On-line
Sample Preparation
, Agilent Technologies Application Note #5988-4364EN, October, 2001.
For additional Agilent Application Notes focusing on SPE,
please visit www.agilent.com/chem/online-spe

181
Usually, a sample must be in a liquid state prior to HPLC or GC analysis. Some insoluble solids contain soluble analytes such as
additives in a polymer, fats in food, and polyaromatic hydrocarbons (PAHs) in soil. Contacting the sample with solvent allows the
extraction of analytes into the solvent, following which the solvent is separated from the solid residue by decanting, filtration, or
centrifugation. The solution is further treated, if necessary, prior to HPLC or GC analysis. In Chapter 2, Tables 2.2 and 2.3
summarized techniques used for the extraction ("leaching") of soluble analytes from an insoluble solid matrix. We will refer
back to these earlier tables in this chapter.
No one solvent extraction technique can be used for all samples. Table 2.2 listed several traditional methods for the pre-treatment
of solid samples. Most of these methods (e.g. Soxhlet extraction and leaching) have been used for more than 100 years, are time
tested, and accepted by most scientists. Regulatory agencies such as the United States Environmental Protection Agency (U.S.
EPA), the Food and Drug Administration (FDA), and their equivalents in other countries readily approve these classical methods for
extracting solid samples. However, these methods often use large amounts of organic solvents, which have encouraged a trend
toward miniaturization in recent years. In addition, some of the older extraction techniques require more glassware and labor.
Chapter 14
Sample Preparation Methods
for Solid Samples
Traditional Extraction Methods
Solvent extraction can assume many forms. The shake-flask method, which involves addition of a solvent to the sample followed by
agitation, works well when the analyte is highly soluble in the extraction solvent and the sample is quite porous. For fast extraction,
the sample should be finely divided (see Chapter 4, Particle Size Reduction). Heating or refluxing the sample in the solvent can
speed up extraction. For faster and more complete extraction, ultrasonic agitation (sonication) often allows more effective solid-
liquid contact, plus a gentle heating which aids extraction. Sonication is a recommended procedure for the pre-treatment of many
solid environmental samples, such as the U.S. EPA Method 3550 for extracting non-volatile and semi-volatile organic compounds
from solids such as soils, sludge, and wastes. In this method, different extraction solvents and sonication conditions are
recommended, depending on the type of pollutants and their concentration in the solid matrix.

182
In forced-flow leaching, the solid is packed into a short, stainless steel column (e.g., 20 x 0.4 cm), and toluene is pumped under
pressure (40 psi) through the column heated at 100-110 °C. Results are comparable to Soxhlet extraction (below), but the extraction
time is significantly reduced (e.g. 24 hr to 1/2 hr). Good recoveries of polyaromatic hydrocarbons from coal-ash samples have been
demonstrated by this technique
1
. An advantage of forced-flow leaching is that the sample is subjected continuously to fresh, hot
solvent, and the effluent from the column is easily collected for further treatment.
Soxhlet extraction has been the most widely used method for the extraction of solids. In this procedure, the solid sample is placed in
a Soxhlet thimble (a disposable porous container made of stiffened filter paper), and the thimble is placed in the Soxhlet apparatus.
Refluxing extraction solvent condenses into the thimble and extracts the soluble analytes (Figure 14.1). The apparatus is designed
to siphon the extract each time the chamber holding the thimble fills with solvent. The siphoned solution containing the dissolved
analytes returns to the boiling flask and the process is repeated until the analyte has been removed from the solid sample and
isolated in the flask. Soxhlet extractions are usually slow (12 to 24 hours or more), but the process takes place unattended. The
most common extractors use hundreds of milliliters of very pure (and very expensive) solvent, but small-volume extractors and
thimbles are available for mg-size samples.
In Soxhlet extraction, fresh, hot extraction solvent
is always presented to the sample, thus providing
maximum analyte solubility. Since the extracted
analyte is allowed to accumulate in the boiling
flask, it must be stable at the boiling point of the
extraction solvent. Method development consists
of finding a volatile solvent (e.g., boiling point
< 100 °C) which has a high solubility for the
analyte and a low solubility for the solid sample
matrix. As the oldest form of efficient extraction,
Soxhlet extraction is the accepted standard for
comparison with newer extraction technologies
such as SFE, pressurized fluid extraction
(PFE)/accelerated solvent extraction (ASE),
and microwave-assisted extraction (MAE).
Figure 14.1
Diagram of a Traditional Soxhlet Extraction Apparatus
Condensor
Sample Extract
Sample placed in
thimble
Solvent Flask
Siphon Tube
Solvent Vapor Tube
Extraction Thimble

183
For many years, the solvent extraction methods of Table 2.3 proved adequate for most laboratories. The newer methods of
Table 2.4 were developed to address an increasing need for greater productivity, faster assays, and increased automation.
Some of these methods are automated, more convenient versions of the methods of Table 2.3. Other techniques have been
developed which are based on new principles. For the most part, these newer approaches are more expensive in terms of the
initial purchase price of the equipment, but result in lower cost per sample and are faster. Most of these newer methods are
based on performing solid extractions at increased temperatures and pressures. Table 14.1 provides background information
on how these parameters accelerate extractions. The abbreviations used in the table are covered in the following sections.
Newer Extraction Methods for Solid Materials
Most Modern Solid Extraction Techniques
Use Increased Temperature and Pressure
Influence of Temperature
ü
Increased analyte solubility
ü
Increases diffusion rates and mass transfer
ü
Lowers viscosity vs. room temperature
ü
Activation energy of desorption is more readily overcome
ü
Kinetics of desorption and solubilization more favorable
Influence of Pressure
ü
Forces liquid into pores of porous material
ü
Extraction cell fills faster (PFE/ASE, SFE)
ü
Extraction cell empties faster
Combined Temperature and Pressure
ü
In SFE, varying pressure and temperature changes the density of the supercritical carbon dioxide and therefore changes its solvation power
ü
In ASE/MAE, T and P combine to accelerate rate and extent of extraction
Table 14.1

184
123
Extraction Rinse Concentration
Boil Rinse Evaporation
With the advent of fully automated systems, much of the drudgery of classical Soxhlet extraction has been reduced. Nevertheless,
some interesting approaches to improve on the technique have been proposed. Focused microwave-assisted Soxhlet extraction
could even speed the extraction time even further with certain microwave absorbing solvents for environmental solid samples
3-4
.
The further application of using superheated water as an extraction solvent makes the process much more environmentally
friendly
5
. When pollutants are present in aged soils, they are more difficult to extract by Soxhlet extraction than when present in
spiked soils. An in situ derivatization technique was found to more easily release pentachlorophenol (PCP) from sandy soil
6
. Using
the acetylation agents TEA-acetic anhydride and pyridine-acetic anhydride, the authors found that the yields were four times
greater and the extraction time shorter with in situ derivatized PCP in soil than by the traditional Soxhlet extraction of non-
derivatized PCP in the same soil.
In 1974, Edward Randall made a major improvement in the Soxhlet extraction technique that reduced the extraction time
dramatically
2
. In his method, the sample was totally immersed in the boiling solvent. Compared to the classical Soxhlet method
where the condensed extracting solvent’s temperature is slightly below the boiling point, the Randall method was faster because
analytes are more soluble in hot solvent than in warm solvent. The operation of the Randall adaptation is depicted in Figure 14.2.
First, the thimble is lowered into the boiling solvent until the appropriate extraction takes place. Then, to flush residual extract from
the sample, a rinse step follows. In this second stage, the thimble is raised above the boiling solvent for a period of time until
residual extract is removed from the solid material by the condensed solvent, just as is performed in the original Soxhlet
experiment. Finally, the drying step removes the solvent from the solvent flask and concentrates the analyte for further processing.
In this step, by closing a solvent return valve, the condensed solvent is redirected away from the sample and boiling solvent and
collected in a reservoir for possible re-use or disposal. In some systems, there is a fourth step where the sample cup is lifted from
the heat source and allowed to evaporate further without the chance of sample overheating, boiling dry, or potential oxidation. The
Randall approach can decrease the extraction time by as much as a factor of 10 compared to traditional Soxhlet extraction.
Principles of Operation of a Modern Soxhlet Extraction System
Figure 14.2
Modern Soxhlet extraction systems can run
completely unattended, offer temperature
programming, solvent reclamation, and
numerous safety features. Most solvents
can be used, although diethyl ether is not
recommended for obvious reasons.
Automated Soxhlet extraction is approved
by the U.S. EPA for the extraction of organic
analytes from soil, sediment, sludge, and
waste solids (Method 3541).

185
The physical state of a substance can be described by a phase diagram which defines regions corresponding to the solid, liquid,
and gaseous states. Points along the curves in the diagram define situations where there is equilibrium between two of the
phases. In the phase diagram for carbon dioxide (CO
2
; Figure 14.3), the line between liquid and gas has a terminus (the critical
point), unlike the line between solid and liquid. The critical point is defined by the critical temperature Tc and critical pressure Pc;
beyond the critical point (the supercritical region) a gas cannot be converted into the liquid state, regardless of pressure. A SF
exhibits gas-like mass transfer properties and liquid-like solubility properties, enabling it to carry out solvent extractions much
more efficiently and rapidly than a solvent in the liquid state. Today, supercritical fluid extraction (SFE) is used for the extraction
of non-polar and moderately polar analytes from solid matrices. Several references
7-11
describe the instrumentation, methods
development, and applications of SFE.
Supercritical Fluid Extraction (SFE)
1,000
100
10
1
0.1
0.01
0.001
-50 -25 0 25 50
Melting Line
Critical Point
Solid
Liquid
Supercritical
Fluid Region
Gas
Boiling Line
Triple
Point
Temperature degrees centigrade
30 °C
Vapor Pressure Log e
Phase Diagram of CO
2
Figure 14.3

186
Fluids that can be used for SFE include CO
2
, NH
3
, N
2
O, and pentane. N
2
O and pentane are flammable, and NH
3
is chemically
reactive and corrosive. CO
2
is used most often for SFE as it is safe, chemically inert, non-toxic, non-corrosive, and available in
high purity at a reasonable cost. CO
2
is easily removed from the collected analyte and it causes no disposal problems. Low-
density supercritical CO
2
has the polarity of hexane; i.e., it is non-polar. However, SF polarity increases with density, especially
near the critical point; so at its highest density, SF-CO
2
resembles the polarity of solvents such as toluene, benzene, and ether.
While pure CO
2
is able to extract a wide variety of non-polar and moderately polar analytes, it is less effective for more polar
compounds. In other cases, it may not be able to displace analytes that are strongly adsorbed to the solid matrix. The addition of
a small amount (up to 10% by volume) of polar organic solvents (methanol, methylene chloride, acetonitrile, toluene, etc.) to
the CO
2
can enhance its ability to dissolve more polar analytes and displace these compounds when they are adsorbed to the
sample matrix. The addition of organic solvents to CO
2
has a slight effect on values of the critical temperature and the critical
pressure, so that the temperature and pressure used for pure CO
2
may require modification.
For environmental analysis, the U.S. EPA has approved several SFE methods; e.g., total petroleum hydrocarbons, polyaromatic
hydrocarbons (PAH), and organochlorine pesticides in soils and sludge. SF-CO
2
is also an excellent solvent for fats, making it
useful for extractions in the food industry. When high-fat solvent extracts contact reversed-phase HPLC mobile phases, fat can
precipitate or strongly sorb to the hydrophobic stationary phase, leading to early column failure. Therefore, SFE can be used as
a selective sample preparation technique to remove some of these “column detrimental sample components”.
SFE is also used to separate classes of analytes by discrete changes in solvent strength; i.e., density stepping or density
programming. The sequential fractionation of hops by density-stepping SFE is one example
12-13
. In the area of polymers, the
penetrating power of SF-CO
2
allows the extraction of polymer additives such as anti-oxidants and plasticizers in less than one hour.
Such extractions formerly required many hours by Soxhlet or ultrasonic extraction methods. Pharmaceutical chemists have found
SFE useful for extraction of drugs from tablet formulations and tissue samples.
SFE Equipment
Figure 14.4 is a schematic of a supercritical fluid extractor. The essential parts include a carbon dioxide source, a pump (syringe or
cooled-head reciprocating), an extraction chamber (or thimble) in which the sample is placed, a restrictor, and an analyte-collection
device (normally a vessel). Temperature is separately controlled for the pump head, the extraction chamber, the restrictor, and the
collection device. The CO
2
is pumped as a liquid and remains so until it reaches the extraction chamber where, under the suitable
conditions of temperature and pressure, it becomes an SF. The SF passes through the sample in the extraction thimble for a period
of time sufficient to extract the analyte from the solid matrix. Past the thimble, the SF passes through a restrictor where it
depressurizes and returns to a non-SF state.

187
Pump
Restrictor
Analyte
Collection
Selection of the restrictor is critical. Two types of restrictors are mainly used: a fixed restrictor consisting of a piece of capillary tubing
or a variable restrictor controlled by the user. The restrictor serves to control the supercritical conditions in the thimble, and also
controls the precipitation of the analyte as the SF is exposed to atmospheric pressure and the CO
2
dissipates as a gas. The rapid
expansion of the SF at this point causes Joule-Thomson cooling, and the restrictor must be heated to compensate for this
temperature drop. Otherwise, the restrictor can plug if large quantities of analyte and/or matrix are extracted.
The analyte is collected just beyond the exit end of the restrictor (impinged surface) as an aerosol. Three collection (trapping)
methods are used: (a) an empty vessel, (b) a packed trap filled with inert material such as glass or stainless steel beads, SPE-types
of packing (20-40 µm), or GC solid packing materials, or (c) dissolution into a solvent. Analyte volatility determines the collection
temperature and most favorable method for collection. For example, empty vessels are not well-suited for collecting certain
aerosols or high volatility compounds since they may be swept along with the CO
2
gas. Solvent collection methods may also suffer
from aerosol formation which may occur when high velocity CO
2
gas passes through the liquid. A solvent should be selected with
minimal aerosol formation and with good analyte solubility which helps in more effective trapping. Cooling the solvent can aid in the
collection process. Instruments which use a packed trap for collection require a small dispenser pump to rinse analytes into a vial.
The ability to trap the analyte is most critical and often the most difficult step in SFE.
Block Diagram of a Supercritical Fluid Extractor
Figure 14.4
CO
2
Source
Extraction
Cell

188
In SFE, analytes extract differently from different matrices. For example, different SFE extraction conditions are required for the
same PAHs found in soils, fly ash, sludge, and sand. Known analytes trapped within an aged soil sample are more difficult to extract
than freshly spiked samples
14
. Three criteria govern SFE extraction from a solid matrix:
•
The relative attraction of the analyte to the matrix
•
The rate at which analyte moves from the matrix into the extraction solvent
•
The solubility of the analyte in the SF
Temperature affects all three of these factors and is an important variable in SFE method development. When high-density SF-CO
2
is unable to effectively extract the analyte of interest from the matrix, the addition of an organic solvent modifier (up to 10% by
volume) can facilitate extraction by solubilization of the analyte, competition with the analyte for the surface of the matrix, and/or
modification of the matrix for release of the analyte. In the latter case, the modifier may “swell” or solubilize all or part of the matrix
to aid penetration of the SF-CO
2
. In extreme cases, chemical reagents such as acetic anhydride for phenols in soil can be added to
the SF to react the analyte to a more readily extractable form.
Both polar and non-polar solvents have been used as SF-CO
2
modifiers ("co-solvents"). The same general rules which guide the
selection of solvent mixtures for non-SF solvent extraction (Chapter 7) can be applied to SFE as well. That is, both solvent
polarity (P') and selectivity are important in affecting analyte recovery and separation from interferences. When selecting the
starting conditions for SFE, the properties of the analyte are important: molecular weight, functional groups, polarity, solubility,
volatility, pKa, thermal stability, and concentration. Equally important are the matrix characteristics: particle size, homogeneity,
porosity, composition, solubility, density, etc. The matrix may also contain its own modifiers such as water, fats, and/or oils. If the
desired analyte is polar, matrix water can facilitate the extraction; fats and oils in the sample may have an opposite effect.
SFE Method Development

189
The physical form of the matrix is important in SFE. Preliminary sample preparation is usually required for bulk materials (e.g., solid
pellets, hard soils, vegetable matter): grinding, sieving, drying, mixing, or wetting. Similar to other extraction techniques, for non-
porous or semi-porous materials, a smaller particle size allows for much faster extraction. In some cases, a pH adjustment or
addition of solvent into the extraction cell may aid the SFE process. Wet matrices such as sludge may require prior water removal
for good recovery and reproducibility. The addition of anhydrous sodium sulfate or diatomaceous earth to the matrix to provide a
free-flowing powder is another approach
15
.
The main variables that affect CO
2
-SFE are pressure, temperature, flow rate, co-solvents, and extraction time. Pressure operates in
combination with temperature to control the density of the SF. As pressure and density increase, the solvating power of the SF
increases. An unlimited number of combinations of temperature and pressure can provide the same extracting SF-CO
2
density
(g/mL). For thermally-sensitive compounds, lower temperatures are preferred, while strongly bound analytes may require higher
temperatures. High flow rates or long extraction times may be necessary to remove all of the analyte from the extraction thimble.
Low flow rates are preferable when the kinetics of the extraction process are slow.
Numerous published methods for matrix/analyte pairs have become available. Often, analysts use “trial-and-error” methods to
optimize extraction-collection conditions. To aid method development, Figure 14.5 provides a generic guide
16
; however, not every
sample requires attention to all of these steps. The method development guide assumes that the analyst begins with standard
samples investigated in the following order:
•
Analytes on an inert matrix (for example, diatomaceous earth, Celite, or filter paper); this allows the SF-solubility of the analyte to
be determined.
•
Simulated samples on blank matrices (some blank matrices are offered as standards by commercial suppliers); alternatively, a
typical “clean” matrix (as close to the actual sample as possible) should be created.
•
Simulated samples on real matrices; when developing an SFE method, it is customary to compare the results to "accepted”
sample preparation methods such as Soxhlet or liquid-liquid extraction.

190
1
2
3
4
5
5a
6
7
8
9
10
11
12
13
14a
14b
15a
15b
16
17
18
19
20
Sampling
Take representative sample
from gross homogenate
Place weighed sample
in extraction conditions
Choose inital set
of extraction conditions
Run nine initial experiments
Graph absolute recoveries
to evaluate conditions
Is recovery acceptable?
Is recovery acceptable? Is recovery acceptable?
Is recovery acceptable?
Is recovery acceptable?
Increase/decrease
super-critical fluid flow rate
Graph relative
cumulative recoveries
Are recovery
and time acceptable?
Optimize trapping
and reconstitution step
Choose liquid trapping
Run replicates
for precision
Apply statistical tests
Change trap solvent
Run real sample
and blanks
Write up
method protocol
Change trap material
and temperature
Change rinse solvent
Yes
YesYes
Yes
Yes
Yes
Yes
Yes
Homogenize gross sample
Consider nature
of analyte-matrix pair
Repeat steps 4-7
to fine tune SFE conditions
Add modifier
to supercritical fluid
Is recovery acceptable?
Is sample
homogeneous?
No
No
Is recovery acceptable?
Is recovery acceptable?
No
No
No No
No
No
Repeat
steps 8-9
For readers interested in more detail on SFE method development, consult references 14, 15, and 17.
Figure 14.5
SFE Method Development Flow Chart
Choose solid trapping

191
With a microwave source, the sample plus extraction solvent are heated directly, as opposed to conventional heating of the
extraction vessel via heat transfer. This is inherently more effective when using hot-solvent extraction. Two limiting forms of MAE
are used: (a) a microwave-absorbing (high dielectric constant) extraction solvent or (b) a non-microwave absorbing (low dielectric
constant) solvent. In the microwave-absorbing solvent approach, the sample and solvent are placed in a closed non-microwave-
absorbing vessel. Microwave radiation heats the solvent to a temperature higher than its boiling point and the hot solvent provides
a rapid extraction of analyte under moderate pressure [usually a few hundred psi, although specialized vessels for higher
temperatures (up to 300 °C) and pressures (up to 1500 psi) are available]. For these higher pressure extractions, the containers
used are made of PTFE, quartz, or advanced composite materials that combine optimum chemical and temperature resistance and
good mechanical properties. This approach has been used for the extraction of additives in polymers, vitamins in food, and priority
pollutants (PAHs, pesticides, PCBs) in soils and sediments
18-20
.
In the non-microwave absorbing solvent approach
21
, the sample and solvent are placed in an open or closed vessel. The solvent does
not become hot, since it absorbs little of the microwave radiation. The sample, which usually contains water or other high-dielectric
components, absorbs the microwave radiation and releases the heated analytes into the surrounding liquid, which is selected for good
analyte solubility. The latter approach is more “gentle” because it is performed under atmospheric or low pressure conditions and can
be used with thermally-labile analytes. Examples of the use of non-microwave absorbing solvents include extractions of lipids from
fish
22
and organochlorine pesticides from sediment samples
18
. In a variation of the non-microwave absorbing approach, an inert, solid
bar selected for its microwave absorbing properties is placed into the extraction vessel along with the finely divided sample and
extraction solvent. The microwave-absorbing bar heats up rapidly thereby transferring the heat energy to the solvent, which in turn
extracts analyte from the sample itself as in other liquid-solid extraction approaches. Microwave ovens are also used for acid digestions,
especially for trace metals in soils and other difficult matrices, and for protein hydrolysis for amino acid analysis.
MAE uses less solvent than conventional Soxhlet or liquid-liquid extractions. Extraction can be controlled by a number of variables:
choice of extraction solvent, heating time, pulsed heating vs. continuous heating, stirring vs. no stirring, closed container vs. open
container (pressure), and external cooling of vessel vs. no cooling. In a typical microwave oven, multiple samples (up to 40) can be
extracted simultaneously for increased throughput. MAE users are not exposed to the (often toxic) extraction solvents. However,
safety precautions should be exercised when dealing with microwave radiation and pressurized closed containers. Additional
information can be obtained in these review articles
23-24
and specialized books
17, 26-27
.
Microwave-Assisted Solvent Extraction (MAE)

192
The extraction vessels can also be heated in a conventional oven instead of using microwave radiation. Pressurized fluid
extraction (PFE), also known as enhanced solvent extraction, pressurized liquid extraction, or Accelerated Solvent Extraction (ASE,
Thermo Fisher Scientific, Sunnyvale, CA), is performed in a closed extraction vessel and employs common organic solvents at high
temperature (50-200 °C) and pressure (150-2000 psi) to extract soluble analytes from solid samples
28-29
. Analyte recovery is
enhanced and accelerated by the higher temperatures, and solvent volume is reduced due to the high solute capacity of the
heated solvents. The experimental apparatus used in the PFE is similar to that used in SFE – a pump for transporting solvent into
and out of the extraction vessel, extraction vessels with an automated sealing mechanism to withstand high pressures, an oven
for heating the sample compartment, and collection vials to hold the collected extracts. PFE consists of the following steps:
1) sample cell loading (typical sample sizes 5-20 g); 2) solvent introduction and pressurization; 3) sample cell heating (under
constant pressure); 4) static extraction; 5) transfer of extract to sealed vial with fresh vent wash of solid sample; 6) nitrogen
purge of cell; and 7) loading of the next sample. Once the sample is loaded into the extraction cell, the entire process is usually
automated and time programmable. Instruments are available that provide unattended preparation for up to 24 samples serially
and for single or multiple extractions at a time.
Typical environmental applications of PFE includes EPA Method 3545A the extraction of BNA (Bases, Neutrals, and Acids),
polyaromatic hydrocarbons (PAHs), organophosphorous and organochlorine pesticides, and polychlorinated biphenyls (PCBs)
from solid waste samples. Other applications include unbound fat in food and PCBs in animal tissue.
Pressurized Fluid Extraction/Accelerated Solvent Extraction
Comparison of Methods for Extraction of Solids
Table 14.2 provides a comparison of the popular methods for the extraction of solids. With the exception of microwave-assisted
extractions in open containers and SFE which uses supercritical CO
2
, the extraction solvents used in these techniques are the same.
Method development times, recoveries, and reproducibility for these methods are roughly equivalent. The main differences are
speed, organic solvent usage, degree of automation, and cost. SFE method development takes longer because of possible matrix
effects and lack of a thorough understanding of the effect of co-solvents on analyte extraction. However, optimized SFE methods
provide recovery and reproducibility equivalent to these more conventional extraction techniques. Pressurized fluid extraction,
modern Soxhlet extraction, and SFE are more automated, compared to MAE. For MAE, sonication, PFE, and some SFE instruments,
multiple extractions can take place simultaneously. Both PFE and MAE do not concentrate the extracted analytes since they end up
in the volume of the original extraction solvent. SFE using a solid trap with solvent elution will allow for a degree of concentration.
PFE sometimes requires that the content of the extraction cell be pumped out with another solvent so that the total extraction
volume dilutes the sample more than MAE. However, all of these newer methods save time, labor, and solvents compared to older
extraction methods.

193
CV = closed vessel
OV = open vessel
* For the most complete commercial instrument, 0 = no automation to +++ = full automation
** Maximum number that can be handled in commercial instruments
*** Very low < $1000; low < $10,000; moderate $10,000-20,000; high > $20,000
**** When organic modifier is used to effect "polarity"
Comparison of Extraction Method for Sample Preparation of Solids
Parameter Sonication
Soxhlet
(Traditional)
Soxhlet
(Modern) SFE PSE/ASE
Microwave
(CV)
Microwave
(OV)
Sample size, g (typical) 20-50 10-20 10-20 5-10 1-30 2-30 2-10
Solvent volume, mL 100-300 200-500 50-100 10-20**** 10-45 20-30 20-30
Temperature degrees C Ambient-40 40-100 40-100 50-150 50-200 50-200 40-100
Pressure Atmospheric Atmospheric Atmospheric 2000-4000 psi 1500-2000 psi 1500-2000 psi Atmospheric
Time, hr 0.5-1.0 12-24 1-4 0.5-1.0 0.2-0.3 0.1-0.2 0.1-0.2
Degree of automation* 00++ +++ +++ ++ ++
Number of samples** 1 (serial)
High (batch)
1 (serial
or batch)
6 (batch) 44 (serial) 24 (serial)
6 (batch)
24 (batch) 6 (serial)
Cost of instrument*** Low Very low Moderate High High Moderate Moderate
Table 14.2

194
References
1.
Mangani, F.; Cappiello, A.; Crescentini, G.; Bruner, F.; Bonfanti, L.
Anal. Chem.
1987,
59
, 2066-2069.
2.
Randall, E.L.
J.A.O.A.C.
1974,
57 (5)
, 1165-1198.
3.
Luque-García, J.L.; de Castro,
L.J. Chromatogr. A
2003,
998 (1-2)
, 21-29.
4.
Luque-García, J.L.; Ramos, M.J.; Martínez-Bueno, M. J.; Luque de Castro, M.D.
Chromatographia
2005,
62 (1-2)
, 69-74.
5.
Luque-Garcia, J.L.; Luque de Castro, M.D.
Anal. Chem.
2001,
73 (24)
, 5903-5908.
6.
Soto-Cordoba, S.M.; Baeza, J.; Freer, J.
Bol. Soc. Chil. Quím.
2001,
46 (2)
, 179-185.
7.
Levy, J.M.
LCGC
1999,
17 (6S)
, S14-S21.
8.
Smith, R.M.
J. Chromatogr. A
2003,
1000 (1-2)
, 3-27.
9.
Taylor, L.T.
Techniques in Analytical Chemistry: Supercritical Fluid Extraction
, Wiley, NY, 1996.
10.
Luque De Castro, M.D.; Valcarcel, M.; Tena, M.T.
Analytical Supercritical Fluid Extraction
, Springer, New York, NY, 1994.
11.
Ramsey, E.D. (Ed.).
Analytical Supercritical Fluid Extraction Techniques
, Kluwer Academic Publishers, Dordrecht, Netherlands, 1998.
12.
Caudell, T.
Practical Supercritical Fluid Chromatography and Extraction
, CRC Press, Boca Raton, FL, 1999.
13.
Verschucere, M.; Sandra, P.; David, F.
J. Chromatogr. Sci.
1992,
30
, 388-391.
14.
Gere, D.R.; Derrico, E.M.
LC/GC Mag.
1994,
12
, 432-445.
15.
Gere, D.R.; Derrico, E.M.
LC/GC Mag.
1994,
12
, 352-366.
16.
Majors, R.E.
Sample Preparation Wall Chart
,
LCGC No. America
2012.
17.
McHugh, M.; Krukonis, V.
Supercritical Fluid Extraction: Principles and Practice
, Butterworths, Boston, MA, 1987.
18.
Onuska, F. I.; Terry, K.A.
Chromatographia
1993,
36
, 191-194.
19.
LeBlanc, G.
Current Trends and Developments in Sample Preparation: Microwave-Accelerated Techniques for Solid Sample
Extraction LCGC
1999,
17 (6S)
, S30-S37.
20.
LeBlanc, G.
LCGC No. America
2013,
31 (11S)
.
21.
Pare, J.R.J.; Belanger, J.M.R.; Stafford, S.
Trends in Anal. Chem.
1994,
13 (4)
, 176-184.
22.
Mandal, V.; Mohan, Y.; Hemalatha, S.
Pharmacognosy Reviews
Jan-May 2007,
1 (1)
, http://www.phcogrev.com
23.
Sparr Eskiksson, C.; Bjorklund, E.
J. Chromatogr. A
2000,
902 (1-2)
, 227-250.
24.
Tatke, P.; Jaiswal, Y. Res. J.
Med. Plant
2011,
5 (1)
, 21-31.
25.
Chemat, F.; Cravotto, G. (Eds.)
Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice
, Springer; New York,
2013, ISBN-10: 1461448298 and ISBN-13: 978-1461448297
26.
Sin, K.
Microwave-Assisted Extraction of Phenolic Compounds
, LAP LAMBERT Academic Publishing, Germany, 2012,
ISBN-10: 3847331752 and ISBN-13: 978-3847331759
27.
Dean, J.R.
Chapter 8, Microwave-Assisted Extraction in Extraction Methods for Environmental Analysis
, Wiley, New York, 1998,
ISBN-10: 0471982873 and ISBN-13: 978-0471982876
28.
Richter, B.E.; Ezzell, J.L.; Felix, D.; Roberts, K.A.; Later, D.W.
Amer. Lab.
1995,
27 (4)
, 24-28.
29.
Ezzell, J.L.; Richter, B.E.; Felix, W. D.; Black, S.R.; Meikle, J.E.
LC/GC Mag.
1995,
13 (5)
, 390-398.

195
Chapter 15
Sample Preparation
for Biological Samples
In the separation of biomolecules, sample preparation almost always involves the use of one or more pre-treatment techniques.
No one sample preparation technique can be applied to all biological samples. The sample preparation approaches used in
modern biochromatography are often the same techniques that were used in classical biochemistry, such as dialysis, chemical
precipitation, column chromatography, and centrifugation. Currently, there is a growing interest in not only the application of
these classical approaches, but also to newer sample preparation technologies in the fields of molecular biology, biotechnology,
and the various -omics (e.g. proteomics, genomics, metabolomics, etc.). In these areas, samples are often complex, available in
small quantities, and require the utmost care in handling. The requirements for the recovery of biopolymers with structural and
functional integrity often demand that the sample preparation be rapid and gentle.
Since most biological samples require separation by HPLC, capillary, or gel electrophoresis, their complex nature necessitates some
form of preliminary sample manipulation to achieve better separation results; and, in the case of HPLC to prolong the life of the
column. The actual recommended sample preparation technique may depend on a number of variables including the following:
• Molecular weight of sample and interferences
• Sample volume and analyte concentration
• Presence of buffer salt (anion and cations)
• Metal concentration and type
• Presence of detergents
• Presence of particulates
• Presence of antibodies, plasmids, endotoxins, etc.
• Presence of radiolabeled compounds
• Type of analysis to be performed after sample preparation
(e.g. chromatography, electrophoresis, mass spectrometry)
• Presence of organics
In addition to filtration for particulate removal, chromatographic (including affinity chromatography) principles can be used to
cleanup many biological samples. Table 15.1 provides a listing of sample preparation techniques that may be used in the flow-
through mode using cartridge, disk, or column format. Some of the techniques can be performed in a batch mode where the media
is poured into the sample (in a liquid form), allowed to stay in contact usually with agitation, then is removed by filtration or by
decanting the liquid phase, leaving behind the compound(s) of interest sorbed onto the stationary phase or contained in the liquid
phase. Although slower than the column approach, batch sorption is easier to perform.

196
Table 15.1
(Continued)
Typical Sample Preparation Techniques in Biochromatography
Requirement
Most Frequently
Used Approaches
Species
Retained Typical Applications*
Packing Matrix-
Functional Group
Other
Approaches
Antibody
Purification
Affinity
Chromatography
Hydroxylapatite
Chromatography
IgG and subclasses IgG concentration in serum,
ascites, and tissue culture
media; fluorescent labeled
antibodies with unreacted
fluorescent tag
Affinity gels (agarose,
silica-based) and
Hydroxylapatite
(calcium phosphate)
Buffer and
Reagent
Ultrapurification
Ion Exchange Trace cations
and anions
Removal of ions that cause
band broadening or high
background in electrophoresis
and HPLC detection
Cation and anion exchangers
(weak and strong),
chelating resins
Adsorption Trace organics Neutral PS-DVB, alumina,
and silica will remove polar
organics from buffers;
water can be removed
from organic solvents
Neutral PS-DVB,
alumina, silica
Mixed-Bed
Ion Exchange
Ions Deionization of carbohydrates
before HPLC; separation of
ionic contaminants from
proteins; reagent preparation
PS-DVB with quaternary
ammonium and sulfonic acid
functionalities
Dialysis
Separation of anions from
carbohydrates, dextrans,
and polyhydric alcohols
PS-DVB with quaternary
ammonium functionality
Proteins Deionization of proteins
containing hydrophobic
molecules
Mixed resin
with dialysis tubing
Desalting and
Buffer Exchange
Ion Exchange Cations, anions Desalting amino acids for
better TLC and HPLC analysis
Anion and cation
exchange resins
Electrodialysis
Gel Filtration/
Size Exclusion
Large molecules
are eluted before
salts and small
molecules
Desalting proteins and nucleic
acids with masses >6,000 Da
Aqueous-compatible
size-exclusion gels
Dialysis,
ultrafiltration
Ion Retardation Cations, anions Removal of salts and ionic
detergent from protein
and amino acid samples
PS-DVB-acrylate with
quaternary ammonium
and sulfonic functionalities
Reversed-Phase Hydrophobic
analytes
Desalting of
polypeptide solutions
C4 or C8 bonded silicas

197
Detergent
Removal
Ion Exchange Cationic
and anionic
detergents
From proteins,
enzyme reactivation
PS-DVB- and polyacrylate-
based strong cation
exchangers or strong anion
exchangers, respectively
Solvent
extraction,
ion-pair
extraction
(Continued)
Adsorption Nonionic
detergents
Triton X-100 from
protein solutions
Silica, hydroxyapatite,
reversed-phase, PS-DVB or
mixed ion exchange resin to
allow nonionic detergent to
pass through
Dialysis
Ion Retardation Anionic
detergents
Excess SDS from samples PS-DVB-acrylate with
quaternary ammonium
and sulfonic functionalities
Solvent
extraction,
Ion-pair
extraction
(with tributyl-
or triethyl-
amine)
Metal
Concentration
or Removal
Ion Exchange Cations Removal of metals and salts
from aqueous medium
PS-DVB or silica-based
cation exchanger
Ion exchange
membranes
Chelating Resins Polyvalent cations Removal of copper, iron,
heavy metals, calcium,
and magnesium
Chelating Resins
Adsorption Metal-organic
complexes
Metals complexed with
polar or hydrophobic
complexing agents
Silica, reversed-phase
Particulate
Removal
Filtration Particulate matter Pre-treatment to protect HPLC
frits and valves; filtration of
culture medium
Hydrophobic and
hydrophilic membranes
(organic-inorganic)
Centrifugation
Plasmid
Purification,
Probe Cleanup
Gel Filtration/
Size Exclusion
Low molecular
weight
contaminants
Removal of unincorporated
radioactive nucleotides from
labeling reaction mixture
Agarose, polydextran, and
other aqueous-compatible
polymeric media
Adsorption Large DNA Removal of RNA, protein,
and other cellular compounds
Agarose, polydextran, and
other aqueous-compatible
polymeric media
Ion Exchange Ethidium bromide
or propidium
iodide
Removal from plasmid
visualization experiments
PS-DVB with sulfonic
functionality
Requirement
Most Frequently
Used Approaches
Species
Retained Typical Applications*
Packing Matrix-
Functional Group
Other
Approaches
Typical Sample Preparation Techniques in Biochromatography

198
Typical Sample Preparation Techniques in Biochromatography
Requirement
Most Frequently
Used Approaches
Species
Retained Typical Applications*
Packing Matrix-
Functional Group
Other
Approaches
Removal or
Concentration
of Anions
and Cations
Ion Exchange Cations, anions Removal of ions from aqueous
solutions; concentration of
large proteins; removal of
mineral acids
PS-DVB with sulfonic
functionality (cations);
PS-DVB with quaternary
ammonium functionality
(anions); weakly basic resins
(tert-ammonium) (anions)
Ion exchange
membranes
Removal or
Concentration
of Organics
Adsorption Polar organic Removal of nonionic
detergents and lipids;
separation of ethidium bromide
from nucleic acid preparations
Alumina, silica,
and SPE cartridges
with bonded phases
Gel Filtration (Organic)/
Size Exclusion/
GPC
High molecular
weight
compounds are
eluted before
small molecules
Separation of soluble organic
compounds with masses
<150,000 Da from complex
sample matrices
PS-DVB with various
porosities
Protein
Concentration
Ion Exchange Water Improve sensitivity of
electrophoretic
and HPLC analysis
Polyacrylamide resin
with dialysis tubing
Lyophilization
Proteins Separation of proteins and low
molecular weight substances
Hydroxyapatite Ultrafiltration,
chemical
precipitation
Adsorption Hydrophobic
proteins
C18 solid phase extraction to
remove hydrophobic proteins
from hydrophilic proteins
C18-modified silica Dialysis

199
The flow-through format is more widely used. Although dilution is a possible consequence, the flow-through column approach is
more useful for removing the last traces of the analyte of interest or perhaps impurities of non-interest. Convenient pre-packed
cartridges and membrane disks, the latter offering less flow resistance due to their large cross-sectional areas, are readily available
from many manufacturers. Sometimes kits are assembled that contain all the media, chemicals, and accessories necessary to
perform a cleanup job, especially in the area of obtaining pure DNA, RNA, and mRNA samples. Liquids can be transported through
the flow-through devices with applied pressure, vacuum or centrifugation. Many of the sample prep techniques of Table 15.1 rely
on retardation of ionic species using the principles of ion exchange or ion retardation. Others use the principle of hydrophobic
interaction and adsorption to retain macromolecules while letting ionic and smaller molecules pass through.
Besides chromatographic principles covered in Table 15.1, cleanup of biological samples while maintaining biological activity can
be accomplished using other approaches. For example, dialysis is a time-tested process using membranes to cleanup and desalt
biological samples. In its classical application (see Chapter 18), dialysis is considered to be a slow technique relative to other
sample preparation techniques. Newer approaches such as the use of disposable mini dialysis kits permit the efficient dialysis of
small sample volumes. In these devices, the dialysis membrane is conveniently incorporated into the cap that screws onto a conical
tube whose bottom maximizes sample recovery.
The use of electrodialysis is a more rapid approach for desalting or buffer exchange. The basis of electrodialysis is the application of
a voltage across a stack of ion exchange membranes that carry a fixed positive or negative charge. The cations and anions in the
solution migrate into zones of concentration and away from zones of depletion. Electrodialysis is a gentle technique and provides
excellent desalting without loss of biological activity of proteins. In addition, transfer of water during the electrodialysis process may
result in a slight sample concentration, depending on the amount of current applied.
Ultrafiltration (UF) (see Chapter 18) is a technique that uses centrifugation as the driving force for membrane filtration. Membrane
filters with molecular weight cutoffs in the tens of Kdaltons are held in a centrifuge assembly. Upon centrifugation, solvent, salts,
and small molecules pass through the membrane while macromolecules larger than the cutoff value are retained and concentrated
above the membrane. Because the membranes are selected to show low non-specific adsorption, UF results in good recovery and
little loss of biological activity. Two or more cycles may be required to totally desalt a sample.
Many of the sample preparation techniques already covered, such as liquid-liquid extraction (Chapter 7) and membrane extraction
(Chapter 18), are directly applicable to the fractionation of biological samples.
Sometimes the combination of two sample preparation approaches results in an overall improvement in cleanup efficiency. For
example, the use of chromatographic media combined with dialysis can provide excellent concentration of protein solutions.
Insoluble polyacrylamide beads, which have a high affinity for water (1 g of resin can absorb 5 mL of water) and low molecular
weight substances, can serve as a concentrator resin. When a dialysis tube or bag containing a dilute protein solution is placed in a
beaker containing the resin, the resin develops a high osmotic gradient that pulls the water from the sample through the dialysis
membrane, thereby concentrating the protein. The water diffusion rate is about 1-2 mL/h/cm
2
of tubing. Because the resin beads
themselves do not diffuse through the dialysis membrane, no interaction with the protein itself occurs.

200
Sample Preparation for Nucleic Acid Constituents
and Genomics
The modern drug discovery process emphasizes rapid data generation and analysis in order to identify promising new
chemical entities very early in the development cycle. Advances in genetics, genomics, biochemistry, and pharmacology have
accelerated the changing face of drug discovery. These advances include sequencing of the human genome, improvements in
laboratory automation, and advances in the fields of combinatorial chemistry, high-throughput screening, mass spectrometry,
and bioinformatics. The end result of all these process improvements includes much faster compound synthesis and more
efficient evaluation of the greater number of compounds for pharmacological and metabolic activity. The ultimate goal of
modern high-throughput processes, to bring a drug product to market in a shorter timeframe, is already benefiting the
scientific and medical communities.
Traditionally, the term “sample preparation” refers to concentration of analyte, exchange of solvent, and/or removal of interfering
substances prior to analysis. But in a high-throughput laboratory, automation processes exist for a multitude of supporting functions
such as solvent delivery, sample dissolution, sample aspiration and dispensing, sample reformatting from tubes to plates, plate
replication, homogenization, handling microplates, applying a vacuum, washing microplate wells, capping/uncapping, sealing,
digestion, and delivery of sample to the detection system.
One goal of genomic studies is to reveal new biological targets for drug development by identifying the DNA sequence, which then
enables analysis of the genes, the resulting RNA molecules, and/or the proteins. To begin, this task requires the isolation and
manipulation of high quality DNA from the sample. Virtually any cell type or virus from any organism may act as a source for nucleic
acids, but the process of isolating the DNA may differ, based on the physical attributes of the source cell. Once isolated, the DNA
must be made into a ‘library’ containing individual segments that, together, represent each and every nucleotide in that sample
genome. Libraries are often made by inserting fragments of sample genomic DNA into larger pieces of DNA from bacteria or yeast
that are highly amenable to laboratory manipulation. The manipulated DNA carrying the sample insert can then be grown in a
controlled system to increase the yield. For successful genomic sequencing, the subsequent isolation and purification of the DNA
constructs is critical and can be time-consuming. The high-throughput of 96-well format DNA sequencers has created the need for
rapid methods of sample preparation to keep pace. We will briefly look at sample preparation procedures (both traditional and
high-throughput) commonly used for genomic nucleic acids.

201
Traditional Nucleic Acid Extraction Techniques
While nucleic acid isolation and purification techniques can vary, they all must accomplish certain objectives.
DNA is generally extracted by cell lysis, separated from non-nucleic cellular components such as proteins,
lipids and carbohydrates, and finally, a series of precipitation and centrifugation steps isolates the remaining
DNA (Figure 15.1). Most protocols also include an RNase digestion to degrade RNA.
Figure 15.1
Typical Series of Sample Prep Steps for DNA Purification
from Bacterial Cells Harboring Plasmids with Inserts
Culture
Culture the bacteria harboring
plasmids with inserts
Cell Separation
Separate cells from media by filtration
or centrifugation
Cell Lysis
Lyse cells via enzyme, detergent, pH
or mechanical disruption
Neutralization
Neutralize lysis to prevent dissociation
of bacterial genomic DNA
DNA Isolation
DNA is isolated by centrifugal
fractionation, adsorption to
a silica matrix or binding
to magnetic beads
Debris Elimination
Cell wall, membrane, lipids,
carbohydrates, proteins and all other
non-DNA particles must be eliminated
via filtration, centrifugation,
supernatant removal, or wash steps
Washing
DNA must be washed of all salts and
residual cellular contaminants to
prevent sequencing glitches
Chronology of steps
varies by protocol
Elution
Elute the purified plasmid DNA by
releasing from matrix or beads, or
pelleting precipitated mass via
centrifugation.
Preparation for
Sequencing

202
Phenolic extraction of cell lysates is one of the oldest techniques for DNA preparation. Single cells in suspension are lysed with a
detergent and a proteinase enzyme to degrade protein molecules. Non-nucleic acid components are then extracted into an organic
(phenol/chloroform) solvent, leaving nucleic acids in the aqueous layer. Isopropanol is added to the isolated aqueous phase to
precipitate the high molecular weight nucleic acids. Following precipitation, the DNA is separated from the isopropanol by spooling
or centrifugation, and is washed twice with ethanol. Most organic extraction procedures incorporate a DNase-free ribonuclease
(RNase) incubation step to remove RNA; this step may come before or after the organic extraction. For some applications, a second
organic extraction may be necessary to achieve the desired purity of DNA. Organic extractions are not optimal for plasmid isolation,
but are more appropriate for whole genomic DNA isolations.
Small amounts (~1 µg from each mL of culture) of bacterial plasmid DNA containing the sample insert sequence are isolated from
cell culture using a technique called the “mini-prep." The individual mini-prep procedure provides sufficient DNA for modern
capillary and gel-based sequencers and is much less labor-intensive than the standard “maxi-prep,” which utilizes a cesium chloride
density gradient for isolating milligram amounts of plasmid DNA. Two common methods to lyse cells for mini-prep plasmid isolation
are the alkaline hydrolysis method and the rapid boiling procedure. Plasmid DNA can then be separated from cell lysates or other
reactants, such as unincorporated nucleotides or linkers, using magnetic particles that have both a high binding efficiency and large
capacity for plasmid DNA. These magnetic particle systems work by capturing sequencing extension products and purifying them
through a series of successive washes, culminating in a final release step. The isolated plasmid DNA may then be used for
automated DNA sequencing and additional molecular biological methods, such as PCR amplification.
Size exclusion chromatography (Chapter 11) is another common technique for efficient and rapid purification of nucleic acids.
Gels are available in different porosities that exclude molecules of 10,000-200,000 Da. The purification of nucleic acids from
nucleotides or buffers works well using an exclusion size of 25,000 Da, since the average protein size is about 30,000 Da.
Disposable, economical, and centrifugeable packed gel filtration columns are ideal for the purification of small volumes
(< 100 µL) of nucleic acid solutions and for separating unincorporated radioactive nucleotides from labeling reaction mixtures.
The pre-packaged columns, which eliminate the tedious and time-consuming steps involved in manual column preparation,
ensure a high recovery of DNA and a high retention of unincorporated nucleotides with minimal labor.
While these various techniques have proven successful for isolating DNA for sequencing of certain genes, operons or plasmids, the
advent of whole genome sequencing makes individual sample preparation somewhat obsolete. Even a medium-sized bacterial
genome of 2.5 million bases would require more than 20,000 individual sample preparations to achieve 8-fold coverage using a
random library. Furthermore, the ability of the sequencing instruments themselves to run in a 96-well plate format necessitates a
more rapid plasmid preparation procedure to keep the sequencers running throughout the day and night. As a result, the process of
plasmid purification is increasing its speed through the use of automation.

203
High-Throughput DNA Purification Systems
Adsorbents that provide fast and efficient DNA purification are the key to making this procedure amenable to automation. Again,
the exact nature of the process varies from one manufacturer to the next, but the basic process is uniform: once the cells are lysed
using one of the aforementioned techniques, DNA is either adsorbed onto a chemically modified silica matrix contained in 96-well
flow-through plates (see Figure 9.4), or magnetically separated using magnetic or transient paramagnetic bead technology. RNA,
proteins, and other cellular components are filtered out initially or washed free in a subsequent step. The multiple samples of
purified DNA are then simultaneously eluted in a purified form that is ready for batch sequencing. The adsorbents are available in
pre-packaged 96-well microplate kits and spin tubes from a variety of manufacturers. For example, Agilent Technologies offers
several kits for pure genomic DNA and Endoxin-free Plasmid DNA for Transfection. Various RNA kits are also available such as for
pure DNA-free total RNA and highly pure mRNA and miRNA. The silica matrix and magnetic microplate kits offer much greater
convenience and higher throughput than the traditional phenol or gel chromatography methods. The kits also improve upon
individual mini-prep methods by enabling multiple purifications to be generated simultaneously.
Automating High-Throughput Systems
Automated liquid handling workstations involve the movement of multiple probes in Cartesian axes (X, Y, Z) over a deck
surface configured with labware (e.g., microplates, tube racks, solvent reservoirs, wash bowl, disposable tips). These
instruments have proven ideal for aspiration and dispensing of solvents from a source to a destination, and have revolutionized
the process of nucleic acid purification with modifications such as control of vacuum manifolds, heating blocks and shakers
that utilize 96-well plates. The variable tip spacing feature of multiple probe liquid handlers allows them to expand their tip-to-
tip width to aspirate from various test tube sizes, and reduce the tip spacing width when dispensing into wells of a microplate
having 9.0 mm well-to-well spacing. As the need arose to move microplates around on the deck, workstation model lines
were expanded and the functionality and usefulness of an integrated gripper arm became clearly evident. Labware movement
around the deck and into external devices (e.g., microplate stackers, fluorescence readers) is standard.
An example of a modern automated workstation configuration, for rapid isolation and purification of DNA in a Dellaporta-based
nucleic acid extraction assay, the Agilent BenchCel Workstation provides complete automation of a variety of genomic sample
preparation tasks. At the core of the workstation, the Bravo Liquid Handling Platform performs the addition of reagents for lysis
(utilizing Tris-HCl, EDTA, NaCl and 2-mercaptoethanol), followed by precipitation with isopropanol and the removal of
contaminants (utilizing phenol, chloroform, isoamylalcohol). The DNA precipitate is then generated using isopropanol followed
by an RNAse treatment, another removal of contaminants, and a final isopropanol precipitation.
While the above automation systems are practical and affordable for high-throughput genomics labs, ultra high-throughput
laboratories processing more than 5,000 samples per day may need to look beyond these workstation solutions to more
custom configurations. The use of magnetic beads allows for the elimination of buffer exchanges and the acceleration of the
purification process.

204
Sample Preparation for Proteins and Proteomics
A rapidly evolving approach for drug discovery is focused on a detailed understanding of the fundamental biological processes
occurring within an organism, specifically the interaction between a genome and its proteome. The term "proteome" refers to the
full complement of proteins expressed by the genome. The human genome sequence has been elucidated, as well as that of many
other organisms. The interactions between and among the genes, RNA molecules, and proteins in each cell of a functioning
organism are highly intricate. Unlike the Human Genome Project, in which the genome is static, the proteome of a cell or tissue is
highly dynamic and constantly changes with respect to its surrounding environment, physiological state, stress, drug administration,
health, or disease. The Human Proteome Project is faced with the challenge to describe all the proteins expressed by the genome
together with their levels throughout the body tissues under various environmental conditions of normal, stressed, and diseased
states. Scientists estimate that while there are 20,000-30,000 genes in the human genome, the number of proteins may be from
50,000-500,000. These greater numbers of proteins result from the many post-translational modifications that may occur, such as
phosphorylation, acetylation, sulfation, and glycosylation, which result in different protein reactivities. However, simply cataloging
proteins is not sufficient because it does not define protein-protein interactions and/or structure-function relationships across
different cell types.
There are many more technical challenges for proteomics than for genomics. While polymerase chain reaction (PCR) technology
works for DNA, there is no similar method to amplify proteins. Therefore, analytical issues must be solved such as sample
preparation, separations of pico- to femto-mole levels of proteins in complex biological matrices, and isolation of the ultra-low
level significant proteins. The high sensitivity of the mass spectrometer has enabled the detection of ultra-low levels of proteins,
but sample preparation remains vital to remove proteins of high-abundance (e.g., IgG, human serum albumin). Additional tools
required for proteomics include protein separation via gels and/or multidimensional liquid chromatography with detection
by mass spectrometry, and the utilization of protein databases and bioinformatics. The industrialization of proteomics is requiring
partnerships and alliances between commercially available systems and technologies linking fluid transport, consumables,
digestions, robotics, detection, and data analysis – all via a common software platform in order for the combined processes
to be automatable.
Today, the overall process for proteomics is generally comprised of manual and disjointed steps for sample preparation, protein
separation and characterization. There is a real need to develop and implement improved strategies for the high-throughput analysis
of protein expression and function in order to quickly realize the tremendous medical advances that are envisioned through
successful utilization of the information gained from proteomics. Here, information is provided on current procedures for the sample
preparation of proteins, the subsequent steps of protein separation, identification and analysis, and the use and/or need for
automated procedures as part of the overall process. We will first look at the classical approaches for protein sample prep and then
consider sample prep in proteomics.

205
Traditional Protein Sample Preparation Techniques
Sample preparation is used to isolate proteins of interest from biological cells or organisms prior to analysis and is an important step
toward achieving accurate, reproducible, and meaningful results. Proteins isolated from these sources contain contaminants such as
keratin, albumin, serum proteins, nucleic acids, lipids, carbohydrates, and polysaccharides that are naturally present. In addition,
various inorganic salts, buffers, reducing agents, surfactants, detergents, and preservatives may be added to a sample to retain
enzymatic or biological activity. The presence of these extraneous materials can cause problems in separations by electrophoresis,
e.g., smearing, masking, and poor reproducibility. These materials also affect performance using liquid chromatography techniques
with subsequent detection and/or quantitation by mass spectrometry.
No universal sample preparation procedure exists to isolate all proteins in a mixture because proteins are present in multiple
forms, are found within different cell locations (e.g., membrane or cytoplasm), and have varying solubilities. Common protein
sample preparation processes were briefly outlined in Table 15.1 include desalting, concentration, centrifugation, dialysis,
filtration and ultrafiltration, precipitation and lyophilization. Distinctive characteristics of the protein, such as isoelectric point,
molecular weight, shape, solubility and hydrophobicity guide the design of this purification. Since proteins are very fragile, care
must be taken in the sample preparation process to avoid the introduction of unwanted modifications that may change
conformation and biological activity.
In order to develop a purification strategy, several sample preparation processes are combined (Figure 15.2). Typically, a cell lysis
procedure is initially performed either by sonication, enzyme treatment or mechanical means to release the proteins. A recent
technique entitled pressure cycling technology (PCT) destabilizes molecular interactions by rapidly and repeatedly raising and
lowering pressure in the action vessel from atmospheric to levels of up to 45,000 psi. This approach offers an improved way for
tissue and cell lysis and to aid in the quantitative recovery of hydrophobic molecules, including integral membrane proteins.

206
Subsequently, a solubility scheme is used to yield a representative protein sample. Nucleic acids are commonly removed by
precipitation techniques or by sonication which breaks them into smaller fragments. Lipids are removed with excess detergent or
with precipitation. Each cell or tissue type requires a specific methodology. A compilation of the protocols used to isolate protein
from mouse, human tissue, body fluids and microorganisms is available
1
. Following the initial purification into a soluble crude
sample, a procedure known as prefractionation can enrich and purify specific protein components prior to separation and analysis.
This technique uses various chromatographic and electrophoretic methods
2
.
An important part of the sample preparation process is to remove extraneous proteins present in high-abundance, e.g., albumin,
IgG. Albumin is the major acidic protein component in plasma and its removal, along with IgG and a dozen or so of the other major
proteins in human and other mammal plasma can enhance the sensitivity of assay techniques and improve the effectiveness and
binding capacity of affinity purification media. Classical techniques for removal of the high-abundance proteins usually only deplete
one or a few of them (see Table 15.2). New affinity-based products can deplete from six to twenty high-medium abundance
proteins leaving the thousands of lower abundance proteins in solution for further pre-treatment
3
. These phases consist of
antibodies specific for these higher abundance proteins. Both spin tubes and flow-through columns are available. Figure 15.3
shows the mode of operation of a flow-through column available from Agilent Technologies. Up to 14 high-abundance proteins
can be removed using this approach. The plasma sample is injected into the multiple affinity removal system. As depicted in Figure
15.4, the high-abundance proteins are retained on the column while the low-abundance proteins pass through the column. They
can be trapped/collected on spin columns then concentrated by trace enrichment techniques. The high-abundance proteins are
then released by a change of buffer and can be discarded or saved if the need arises. Finally, the column is regenerated and can
be used for several hundred injections. A combination of affinity depletion and multidimensional LC-MS/MS has been used to
investigate trace levels of up- and down-regulated proteins in biological fluids.
Kits are available to remove DNA, salts and buffers, as well as the isolation and solubilization of specific protein groups (e.g., membrane
proteins, acidic and basic proteins, and nuclear proteins). When removing high-abundance proteins from serum, care must be taken to
avoid removal of low-abundant proteins and the newer selective removal kits seem to do a good job. Once proteins are purified, some of
the common separation methods are single- and two-dimensional electrophoresis (2DE), liquid chromatography (LC), size exclusion
chromatography, gel filtration and affinity chromatography.
Other classical laboratory procedures for protein removal are available in texts
4-7
. In contrast to protein chemistry that studies a
single component, the focus of proteomics is on the interaction of multiple, distinct proteins and the roles they play as part of a
larger system.

207
Figure 15.2
Traditional Protein Sample Preparation Processes
Organisms
Tissues
Cells Body Fluids
Cell Lysis or
Laser Capture Micro Dissection
Protein Solubilization
Centrifugation
Insoluble Cellular Debris (waste)
Sample Prefractionation
•
Nucleic Acid Removal
•
Lipid Removal
•
Albumin Removal
Add Chemical or
Isotope Label (ICAT)
•
Denaturation
•
Reduction
•
Alkylation
2-Dimensional
Electrophoresis
or
Chromatography
Biological Sample
Sample Peparation
Pre-Separation
Quantification
Separation and Resolution

208
Table 15.2
(Continued)
Classical Methods for Depleting High Abundance Proteins from Biological Fluids
Technique Type of System
Designed to
Remove Principle Reference
Affinity
Chromatography
Cibacron Blue or related chlorotriazine dye
is bound to chromatographic medium;
some phases are proprietary
Albumin Albumin strongly binds to Cibacron Blue
dye that acts as an ionic, hydrophobic,
aromatic, or sterically active binding site.
Proteins that interact with the gel can be
bound or released with a fairly high
degree of specificity manipulating the
composition of the eluent buffers. Binding
capacity: 10-15 mg/mL.
15-19, 47-51
Single Antibody
Column
a) Anti-HSA attached to porous polymer
b) Anti-IgG attached to porous polymer
a) Albumin
b) IgG
a) Antibody specific for HSA
b) Antibody specific for IgG
16, 19, 52-
53
Protein A-, -G, or -L The protein bound to resin Immunoglobulins There is a strong affinity and selectivity of
these proteins for immunoglobulins
16, 54
Combination of
Affinity Phases
Multiple phases bound to resin Albumin and IgG Combination of above to remove both
high-abundant proteins (albumin and IgG)
from plasma
16, 20,
55-57
Isoelectric Trapping Multicompartment electrolyzer or free
flow electrophoresis
Albumin, acidic,
and basic proteins
In this membrane-based electrophoresis
system, proteins are fractionated
according to their pI values and end up
separated into compartments for easy
retrieval. Proteins within a mass range
close to that of HSA are excluded. Note
that these instruments are designed for
protein fractionation but can be used to
isolate high-abundance proteins.
58-59

209
Gradiflow Preparative electrophoresis Albumin Proteins are fractionated according to
their pI value, then further fractionated by
size (molecular weight)
60-61
Peptide Affinity
Chromatography
Phage isolates coated in 96-well plates Synthetic peptides chosen from a library
were found to bind well to several
mammalian serum albumins including a
strong affinity for HSA.
62-63
Ion Exchange and
Size Exclusion
Chromatography
Column switching or in-series Albumin, IgG, and
other proteins
Ion exchange column separates by charge
and size exclusion column by molecular
weight (size); sometimes reversed-phase
chromatography has been used in
combination with ion exchange
64-65
Multi-Lectin Affinity
Chromatography
Lectins sorbed to agarose beads Glycoproteins Multiple mixed lectins have affinity for
various glycoproteins and can be used to
enrich them from human plasma; can be
used in combination with multiple affinity
protein depletion columns
66
Organic Solvent
Precipitation
Organic solvent is mixed with serum Proteins larger than
20 KDa
Large proteins will precipitate when a
water-miscible organic solvent such as
acetonitrile is added to serum; the
precipitated proteins can be removed by
centrifugation or filtration; the supernatant
contains the lower molecular weight
proteins for further study.
67-68
Technique Type of System
Designed to
Remove Principle Reference
Classical Methods for Depleting High Abundance Proteins from Biological Fluids

210
02.557.510 12.5 15 17.5
0
500
1000
1500
2000
2500
Figure 15.4
MARS Immunoaffinity Column Elution Profile –
4.6 x 50 mm Column
Comparison of Runs #20 and #200
Absorbance (mAU)
Retention Time (min)
Bound, High-Abundant ProteinsFlow-through, Low-Abundant Proteins
Injection
0.25 mL/min
Elution
1.0 mL/min
Re-equilibration
1.0 mL/min
End run (20 min)
Figure 15.3
Removal of High-Abundant Proteins from Plasma
High-Abundant Proteins
(Albumin, IgG, IgA, Transferrin, Haptoglobin, Antitrypsin)
Low-Abundant Proteins
(Biomarkers for disease and drug targets)
• Column and optimized buffers are used to remove the top 14 most
abundant proteins in human serum and plasma samples.
• Attach to HPLC instrument and pump samples through – proteins
of interest are collected and analyzed.
Low-Abundant Proteins
Free from Interferences
Crude Human Serum
Time
(min) %B
Flow
rate
Max.
Pressure
(bar)
1 0.00 0.00 0.250 120
2 9.00 0.00 0.250 120
3 9.01 100.00 1.000 120
4 12.50 100.00 1.000 120
5 12.60 0.00 1.000 120
6 20.00 0.00 1.000 120
•
Total column run cycle = 20 min for injection, elution, and regeneration
(4.6 x 50 mm column)
•
Capacity = 15-20 µL serum per injection
1.2-1.6 mg total serum proteins

211
Modern Approaches of Sample Preparation for Proteomics
The sample preparation techniques of greatest interest in expression proteomics center on prefractionating and enriching proteins
before their separation by preparative electrophoresis or chromatography. Detection is either by matrix-assisted laser desorption
ionization (MALDI) or atmospheric pressure ionization (API) mass spectrometry. A typical workflow diagram of the total process
for identifying differential protein expression levels is shown in Figure 15.5. The classical methods for extracting and isolating
proteins as used in protein chemistry are also used in proteomics. In addition, newer methods such as laser capture micro
dissection
8-9
increase the differentiation between diseased and healthy tissues. Labeling with stable isotopes (e.g.,
13
C,
2
H and
15
N) or isotope coded affinity tags (ICAT) is commonly incorporated in procedures to affinity capture differentially expressed
proteins at low-abundance.
The major difference between classical protein chemistry and proteomics is that in classical protein chemistry, interest is focused
on isolating an individual protein and identifying its total sequence, whereas in proteomics, the goal is to characterize a complex
mixture of proteins present in various expressed levels and identify them using partial sequence analysis (after digestion). This
identification would not be possible without databases such as expressed sequence tags (EST), protein and peptide sequences,
and powerful data mining algorithms. Agilent’s Spectrum Mill MS proteomics software can be used to perform protein database
searches of MS/MS data.
Proteomics research is actually separated into three areas: functional, structural and expression proteomics, each having its own
set of sample preparation protocols. Expression proteomics is a recognized area of proteomics in drug discovery and
pharmaceutical research. It is defined as the identification and quantitation of proteins present in healthy and diseased tissues.
Functional proteomics analyzes non-denatured proteins under conditions that keep protein complexes together while structural
proteomics aims to elucidate the structures of the functional “active” sites of each human protein. The ultimate goal is to
develop drugs which are highly specific for these active sites. The tremendous analytical challenge of proteomics arises from the
fact that the proteome is a collection of some 30-80% of gene products expressed at both low levels (10-100 copies per cell)
and high levels (10,000-100,000 copies per cell). These numbers represent a dynamic range of at least six orders of magnitude.
As a point of reference, most eukaryotic cells contain about 20,000 different proteins having an average molecular weight of 50
kDa. Enzymatic digestion yields about 30 peptides per protein or about 6,000,000 unique peptides. Certainly, these numbers of
proteins present colossal technical challenges in terms of their analytical sample throughput, detection and data analysis.

212
Two-Dimensional Electrophoresis (2DE)
Although considered to be a separation technique rather than a sample preparation technique, 2DE is really a technique for isolating
proteins and other biomolecules from complex mixtures by using the principles of multidimensional separation, such as discussed in
Chapter 13. For many years, two-dimensional electrophoresis was the standard way to separate proteins in one dimension by
isoelectric focusing (IEF) based on isoelectric point and in the second dimension by molecular weight using sodium dodecylsulfate
polyacrylamide gel electrophoresis (SDS-PAGE). Even today, many laboratories are still using this time proven approach for protein
separation. Visualization of the proteins separated in the gels is done by using inorganic and organic dyes. High resolution 2D imaging
and analysis software programs summarize the results and help to identify target proteins for excision and further study. There are
several precautions using 2D gels. One important point is that most biologically significant proteins are present at low levels and
therefore are generally not detected. Efficient sample preparation techniques can remove the majority of high-abundant interfering
proteins (see earlier) and improve reproducibility in 2DE separations. Prefractionation according to a physicochemical parameter such
as isoelectric point is performed to enable a higher loading capacity onto gels and minimize protein-protein interactions. It is also
important to remove insoluble materials from the sample before running the gel since it is known that hydrophobic membrane proteins
or tissue proteins act differently than other proteins. It is critical that the protein be soluble prior to 2DE separation. Various inorganic and
organic buffers must be added to disrupt disulfide bonds and noncovalent interactions. A number of companies manufacture 2D gels,
sample cleanup kits, reagents, buffers, and organic/inorganic dyes to optimize the separation and isolation of targeted proteins.
Extraction and Digestion from Gels
In 2DE, the majority of protein is trapped inside the gel and the challenge is to extract it prior to the analysis step. Enzymatic
digestion (e.g., trypsin) of excised protein spots involves placing the small gel pieces into a centrifuge tube and performing several
steps in sequence: reduction, alkylation, washing, and dehydration. Buffer and enzyme are then introduced and the protein is
digested by incubation at 25 °C or 37 °C overnight. Methods to dramatically reduce the time required for enzymatic digestion by
using very small sample volumes with an on-line enzymatic digestion technique have been developed. After digestion, several time
consuming manual steps to remove the enzyme, buffers, and salts must be completed before the sample is ready for introduction
into a mass spectrometer. In-gel digestion kits designed for up to 96 protein samples in 96-well filtration plates are available. These
kits include enzymes, reagents, and sample cleanup products that desalt and concentrate protein-digested samples for mass
spectrometric analysis.
The procedure of excising protein spots from gels followed by enzymatic digestion presents several opportunities to reduce the loss of
low level proteins and increase throughput. Automated robotic and liquid handling workstations that start with a 2D gel, excise the
targeted protein spots, digest and concentrate them and, in some cases, spot the final samples onto plates for analysis by mass
spectrometry are available. An explanation of how automation, on-line enzymatic digestions and microfluidics are being applied to
significantly reduce analysis time is provided in a following section.

213
The detection method of choice in proteomics is mass spectrometry using MALDI, nano electrospray ionization (ESI) tandem mass
spectrometry (MS/MS) or reversed-phase micro capillary LC electrospray ionization. Several reviews discuss details of the use of
mass spectrometry in proteomics
10-13
. Initial applications of MALDI and ESI for protein detection utilized purified single proteins
and peptides for peptide mass fingerprinting and de novo peptide sequence analysis. However, experiments using biologically
significant samples from 2D gels and cell extracts exposed a weakness in the procedure – intolerance to detergents, dyes and
organic/inorganic buffers. The ultra high sensitivity of these techniques also makes them more sensitive to the presence of
abundant concentrations of protein contaminants such as keratin, Protein A, and albumin.
Aside from quantitation, the detection sensitivity and accuracy of mass measurement of proteins and peptides is also affected by
the presence of such contaminants. The challenge faced by mass spectroscopists is to reproducibly remove salt, detergents, lipids,
and high-abundant proteins without eliminating biologically significant proteins present in low copy numbers.
Micro columns and micropipette tips packed with stationary phase sorbent materials, like Agilent’s OMIX monolithic tips (see
Chapter 9) have emerged as cost effective, easy-to-use products for concentrating and desalting protein and peptide samples prior
to MALDI analysis. These products have very small dead volumes and can be used with ultra-small sample quantities and contains
a small bed of C18, C4, strong cation exchange fixed at the end of a 10 µL pipette tip; concentrated, purified samples are eluted in
a volume of 1-4 µL. The OMIX tips are also available in a 96-well format; a protocol combines in-gel digestion with spotting of the
purified and concentrated peptides onto a sample target for analysis by MALDI.
Detection by Mass Spectrometry
Alternatives to electrophoresis and gel technology are sought since the sheer numbers of proteins to be separated and analyzed
demand a higher throughput system with greater preparative capacity and reproducibility. Capillary liquid chromatography is a
natural choice since it is a proven separation technique and can be fully automated and interfaced with tandem mass spectrometry
for on-line detection. This combination is also attractive because the mass spectrometer offers additional resolution by mass to
charge ratio beyond the separation achieved on-column. Further explanation of protein separations and interfacing of LC to mass
spectrometry is outside the scope of this text.
Multidimensional protein identification technology (MudPIT) uses two chromatography steps interfaced back to back and various
configurations are in use: (1) separation on the first column with fraction collection, followed by injection of the collected fractions
onto the second dimension column, (2) directly coupled LC columns, and (3) multidimensional LC using column switching (see
Chapter 13).
Multidimensional Liquid Chromatography

214
A 2D LC separation typically involves an ion exchange, affinity, size exclusion, or chromatofocusing column coupled with a reversed-
phase column in either the first or second dimension. A mixed bed microcapillary column containing ion exchange and
reversed-phase moieties on a resin bed is also useful. These chromatographic approaches have been used in place of 2D gels and
designed for various analysis protocols, such as: “shotgun” analysis of all proteins (250-5,000 proteins), a single class of proteins
(10-250 proteins) and specific protein(s) (1-10 proteins).
The development of “on the fly” acquisition of tandem mass spectra with data-dependent instrument control and chromatographic
control has greatly increased throughput of mass spectrometers. Intelligent algorithms provide automated analysis of peptide mass
spectra for protein identification. In addition, a comprehensive separation of complex peptide mixtures as well as the resolution of
intact proteins can be accomplished.
This approach by LC has the ability to detect low-copy proteins using nano LC-MS/MS and certain classes of proteins which are not
easily observed on 2D gels (e.g., large proteins, hydrophobic membrane proteins, and very acidic or very basic proteins); however,
the complete resolution of all proteins in a proteome will require the use of more than two dimensions and a prefractionation step.
The purification of proteins consists of manual and disjointed steps as part of a multi-step process. The automation of these individual
procedures is an important goal for proteomics laboratories in order to meet high-throughput demands. The choices for automation, as
well as their size, differ in complexity according to the task required. Common options for automation within the overall process outlined
in Figure 15.5 are: the use of laser capture microdissection to isolate healthy from diseased cells; excision of targeted proteins from 2D
gels; digestion of these excised gel spots or plugs; desalting and concentration following digestion; and spotting of the peptide samples
onto MALDI target plates for analysis.
Automation of these individual steps exists as task-specific modules. The stained spots of interest can be extracted from a gel by
specialized instruments with on-board high resolution cameras that acquires an image of the gel, analyzes via software, and
generates a protein target excision map; selected proteins are excised via a multiple excision head unit in parallel. Extracted protein
plugs can be placed into 96-well (and sometimes 384-well) plates and catalogued. Proteolytic enzymes (e.g., trypsin) are added to
the removed pieces of gel and digested with heat. This type of automation module performs all necessary wash steps and digests
proteins into peptides. Some systems generally utilize an in-gel digestion kit.
Automation of Sample Preparation Processes

215
Figure 15.5
Typical Workflow for Protein Characterization
2D Gel
Gel Staining
Image Analysis
Spot Picking
Storage
Spot Digestion
Peptide Mixture
Desalting
Concentration
Sample Deposition
onto Target Plate
LC-MS/MS (ESI)
Nano or Micro LC
MALDI-TOF-MS
de novo Sequencing
Peptide Mass Fingerprinting
EST Database Search
Protein Database Search
Confirm Identity
(Data Evaluation Analysis)
Separation and
Resolution
Protein
Visualization
Identification and
Characterization
Iterative Process
The polypeptide fragments are desalted and concentrated on an automated MALDI preparation station which is essentially a
modified liquid handler (1, 4 or 8-channel pipetting), frequently with a robotic arm which shuttles microplates around and in/out of
the deck; the basics of liquid handling workstations are covered by Wells
14
. Disposable particle-loaded tips containing solid-phase
extraction media, like the OMIX tips from Agilent are commonly used for this sample preparation step and the tips are compatible
with most liquid handling workstations. The act of “spotting” places the peptide samples onto specific media called a “MALDI
target” and immobilizes them in a suitable matrix for analysis. Workstations can also be used to simply spot target plates with high
positional accuracy. Modules are also available that combine two distinct functions, such as protein digestion with MS sample
spotting.
Multidimensional LC with MS detection is also amenable to automation by the addition of column switching capabilities with
plumbing to accommodate multiple columns in parallel (see Chapter 13). The demand for automated protein processing
methods continues as companies are challenged to meet many needs: greater sample throughput, reproducibility, sensitivity for
low-abundant proteins, enhanced low volume liquid handling, and high density sample spotting onto target plates.

216
Peptide Mapping
Once a protein is separated from its source, it must be further characterized. MS/MS is one technique to identify intact proteins
based on their mass spectral characteristics. Peptide mapping is also a very powerful, somewhat selective method, and one of the
most widely used identity tests for proteins. It most commonly involves enzymatic digestion (usually using trypsin) of a protein to
produce peptide fragments, followed by separation and identification of the fragments allowing the detection and monitoring of
single amino acid changes, oxidation, deamidation, and other degradation products. It also enables the direct detection of common
monoclonal antibody variants such as N-terminal cyclization, C-terminal lysine processing, and N-glycosylation, as well as other
post-translational modifications. A peptide map is a fingerprint of a protein and the end product of several processes that provide a
comprehensive understanding of the protein being analyzed. It involves four major steps:
•
Isolation and purification of the protein
•
Selective cleavage of the peptide bonds
•
Chromatographic separation of the peptides
•
Validated analysis of the peptides
The first two steps would be considered to be part of the sample preparation process followed by the last two steps of separation
and analysis. Reversed-phase liquid chromatography is the preferred technique for determining the progress of the peptide
mapping study. Peptide mapping is considered a comparative procedure that confirms the primary structure of the protein and
detects alterations in structure. A peptide map should include positive identification of the protein, maximize coverage of the
complete peptide sequence, and provide additional information and sequence identification beyond that obtained at the non-
digested protein level. A handy “how-to” guide in generating peptide maps is available
69
.

217
Protein Digestion
Table 15.3
Step One: Sample Preparation
Depending on the size or the configuration of the protein, there are different approaches for pre-treatment of your sample. Under
certain conditions, it might be necessary to enrich the sample or to separate the protein from added substances and stabilizers
used in its preparation, especially if these interfere with the mapping procedure. There are many methods for performing these
procedures and each protein has its own set of cleanup measures or processes. Some of the more common approaches used for
sample cleanup prior to digestion include depletion/enrichment, dialysis (see Chapter 18), and desalting by gel filtration (see
Chapter 11). Some depletion strategies were covered earlier (see Table 15.2). These depletion strategies utilize immunoaffinity
techniques (e.g., immunoprecipitation, co-immunoprecipitation, and immunoaffinity chromatography). Alternatively, enrichment
techniques isolate subclasses of cellular proteins based on unique biochemical activity, post-translational modifications (PTMs),
or spatial localization within a cell. Post-translational modifications – such as phosphorylation and glycosylation – can be enriched
using affinity ligands such as ion-metal affinity chromatography (IMAC) or immobilized lectins, respectively.
Whether simple or complex, samples often need dialysis or desalting to ensure they are compatible and optimized for digestion.
For example, because mass spectrometry (MS) measures charged ions, salts – especially sodium and phosphate salts – should
be removed prior to MS to minimize their interference with detection (see Chapter 17). Dialysis and desalting procedures allow
buffer exchange, desalting, or small molecule removal to prevent interference with downstream processes.
Five steps for protein digestion
Procedure Intended Effect General Experiment
1. Sample Preparation Preparing sample for digestion Depletion, enrichment, dialysis, desalting
2. Selection of Cleavage Agent Specific cleavage requirement None
3. Reduction and Alkylation Reduction reduces disulphide bonds
Alkylation caps SH groups
Reduction: DTT, 45 min, 60 °C
Alkylation: IAM, 1 hr, in the dark
4. Digestion Process Cleavage of proteins Digestion: pH 8, 37 °C, overnight
Quenching: TFA addition
5. Enrichment/Cleanup Preparing sample for LC or LC/MS analysis C18 tips, concentrating, dialysis, affinity columns
Table 15.3 shows the five steps in protein digestion that provide selective cleavage of the intact protein. Each of these steps will be
examined separately in order to understand the parameters affecting the final outcome of the digestion process.

218
To desalt samples prior to digestion, Size Exclusion Chromatography (SEC) (Chapter 11), also known as gel filtration
chromatography (GFC), is the most practical laboratory procedure. This method is a non-adsorptive chromatographic technique that
separates molecules on the basis of molecular size. Gel filtration allows samples to be processed using isocratic elution. Analytically,
GFC can distinguish between molecules (e.g. proteins) with a molecular weight difference of less than a factor of 2. In these
applications, the size difference between the substances being separated is very large (i.e. proteins vs. salts). The pore size of the
gel filtration media is chosen so that it completely excludes the larger proteins while allowing the smaller molecules to freely diffuse
into all of the pore spaces. The column is equilibrated with a buffer, which may be the same or different from that of the sample.
The larger molecules – which can’t enter the pores of the media – elute first from the column, followed by the smaller molecules
that diffuse into the pores. If there is no interest in the smaller molecules and salts, they can be directed to waste, or if these
substances are of interest, can be directed to a fraction collector for further handling. If the mobile phase buffer is different from the
original sample buffer, the larger molecules will elute in this new buffer; hence the process that takes place is buffer exchange.
There are two methods employed for the cleavage of peptide
bonds, chemical and enzymatic. Chemical cleavage involves
the use of nucleophilic non-enzymatic reagents such as
cyanogen bromide (CNBr) to chemically cleave the peptide
bond at a specific region while proteolytic enzymes, such as
trypsin, have been proven highly useful for a variety of site
specific cleavage locations. The cleavage method and agent
will depend on the protein under test and the specific outcome
expectations of the analysis. Additionally, the selection process
involves careful examination of the entire peptide mapping
process and considerations for related characterizations. The
most common cleavage agent used for peptide mapping is
trypsin due to its well defined specificity. Trypsin hydrolyzes only
the peptide bonds in which the carbonyl group is followed
either by an arginine (Arg) or lysine (Lys). Several common
cleavage agents and their specificity are shown in Table 15.4.
Dialysis is an established procedure for reducing the salt concentration in samples. It requires filling a dialysis bag (membrane
casing of defined porosity), tying the bag off, and placing the bag in a bath of water or buffer where the concentration of salt will
equilibrate through diffusion. Large molecules that can’t diffuse through the bag remain in the bag. If the bath is in water, the
concentration of the small molecules in the bag will decrease slowly until the concentration inside and outside is the same. Once
equilibration is complete, the bag is ruptured and the solution poured off into a collection vessel. Dialysis can be used for volumes
from microliter up to a few liters, but it is usually not practical for large sample volumes because it can take long times (e.g. hours,
even days) for complete salt removal.
Step Two: Selection of Cleavage Agents
Table 15.4
Cleavage Methods
Cleavage
Type
Cleavage
Agent Specificity
Enzymatic Trypsin C-terminal side of Arg and Lys
Pepsin Non-specific
Chymotrypsin C-terminal side of hydrophobic
residues
Glutamyl
endopeptidase
C-terminal side of Glu and Asp
Chemical Cyanogen
bromide
C-terminal side of Met
Dilute acid Asp and Pro
BNPS-skatole Trp

219
0
0
50
100
150
200
250
300
350
mAU
0.229
0.534
2.404
2.759
2.956
3.287
3.399
4.462
4.855
0.333
0.406
0.464
0.5 1 1.5 2 2.5 3 3.5 4 4.5 min
Step Three: Denaturation, Reduction, and Alkylation
For the proteolytic enzyme to efficiently cleave the peptide chains, most samples need to be denatured, reduced, and alkylated
using various well-known reagents. Denaturation and reduction can often be carried out simultaneously by a combination of heat
and a reagent like 1,4-dithiothreitol (DTT), mercaptoethanol, or tris(2-carboxyethyl)phosphine. The most often used chemical is DTT,
which is a strong reducing agent that reduces the disulfide bonds and prevents inter- and intra-molecular disulfide formation
between cysteines in the protein. By combining denaturation and reduction, renaturation – a problem when using heat solely as the
denaturation agent – due to reduction of the disulfide bonds can be avoided. Following protein denaturation and reduction,
alkylation of cysteine is necessary to further reduce the potential renaturation. The most commonly used agents for alkylation of
protein samples prior to digestion are iodoacetamide (IAM) and iodoacetic acid (IAA).
Figure 15.6 provides a good example of a reversed-phase chromatographic separation method used to evaluate the reduction
and alkylation completeness of a monoclonal antibody prior to digestion.
Figure 15.6
Reversed-phase Liquid Chromatographic Separation of a
Reduced and Alkylated Monoclonal Antibody Prior to Digestion
Red/alkyl matrix
Chain frag
Light chain
Heavy chain 1
Heavy chain 2
Conditions: The separation was performed on an Agilent Rapid Resolution High Definition (RRHD) 300SB-C8, 2.1 x 50 mm column run at
0.5 mL/min, 75 °C, using water (0.1% TFA)/ACN (0.08%) multi-segmented gradient conditions on an Agilent 1290 Infinity LC
69
.

220
Step Four: Digestion
As already mentioned, trypsin is the most commonly used protease for digestion due to its well-defined specificity. Since trypsin is
also a protein, it may digest itself in a process called autolysis. However, Ca
++
, naturally present in most samples, binds at the Ca
++
binding loop in trypsin and prevents autolysis. With the modified trypsin presently used in most laboratories, autolysis is additionally
reduced and not typically a large concern.
Proteins may act differently in different environments and when model proteins were digested in a mixture vs. separately, less
effective digestions have been observed. One reason could be increased competition for the trypsin cleavage sites, when more
proteins are digested together. Additionally, there can be many factors and conditional parameters that could affect the
completeness and effectiveness of digestion of proteins, causing a variety of anticipated outcomes
If these factors are more carefully understood or controlled, the digestion results can be greatly improved. The pH of the reaction,
digestion time and temperature, and the amount of cleavage agent used are all critical to the effectiveness of the digestion.
Tryptic digestion is performed at an optimal pH in the range 7.5-8.5, and commonly at 37 °C. To provide an optimal pH for
the enzymatic cleavage, a buffer is added [usually 50 mM triethyl ammonium bicarbonate (tABC) or 12.5 mM ammonium
bicarbonate (ABC)] prior to the addition of trypsin. A 2-amino-2-hydroxymethylpropane-1,3-diol (Tris) buffer may also be used
for this purpose, but it should be taken into consideration that the Tris buffer is incompatible with certain MS analyses, such as
MALDI and ESI-MS, and needs to be depleted through solid phase extraction (SPE) or with reversed-phase pipette tips (such
as Agilent’s OMIX). To ensure a sufficient amount of enzyme is present to perform the digestion, it is crucial to have the right
enzyme-to-protein ratio. A more detailed discussion of the critical sub-steps in digestion follows:
• Digestion pH. In general, the pH of the digestion mixture is empirically determined to ensure the optimization of the performance
of a given cleavage agent. For example, when using cyanogen bromide as a cleavage agent, a highly acidic environment (e.g. pH
2, formic acid) is necessary; however, when using trypsin as a cleavage agent, a slightly alkaline environment (pH 8) is optimal. As
a general rule, the pH of the reaction must not alter the chemical integrity of the protein during the digestion or the course of the
fragmentation reaction.
• Digestion Time & Temperature. Time and temperature play an important role for optimum digestion. To minimize undesirable
chemical reactions, a temperature between 25-37 °C is adequate and recommended for most protein digestions (e.g., trypsin
digestions are commonly run at 37 °C). However, the type and size of protein will ultimately determine the temperature of the
reaction due to possible protein denaturation as the temperature of the reaction increases. Reaction time is also a factor for
consideration in optimizing the digestion protocol. If sufficient sample is available, an experimental study should be considered in
order to determine the optimum time to obtain a reproducible map while avoiding incomplete digestion. Time of digestion varies
from 2-30 h depending on sample size and type, and the reaction is stopped by the addition of an acid, selected so as to not
interfere in the map or by freezing.

221
For further detailed information on the commonly used trypsin digestion method, the reader is referred to Reference 69, which
explains the steps, expected digestion times, and a summary of reduction, alkylation and digestion protocols.
Prior to the actual mapping separation, cleanup and/or enrichment is usually required for the successful analysis of peptide maps.
There are many methods to accomplish cleanup and enrichment dependent on sample type and targeted objective. For example,
enrichment for specific PTMs (e.g., phosphorylation, ubiquitination, and glycosylation) is performed by affinity purification using
PTM-specific antibodies or ligands, while phosphopeptides can be enriched by immunoprecipitation (IP) using anti-phospho-specific
antibodies or by pull-down using TiO2, that selectively binds phosphorylated serine, tyrosine or threonine.
After peptide enrichment, salts and buffers can be removed using either graphite or C-18 tips or columns, and detergents can be
removed using affinity columns or detergent-precipitating reagents. Dilute samples can also be concentrated using concentrators of
varying molecular weight cutoff (MWCO) ranges. Once purified, peptide samples are then ready for the final preparation for MS
analysis, which varies based on the type of analysis. For LC/MS (or LC-MS/MS) analysis, the proper choice of mobile phases and
ion-pairing reagents is required to achieve good LC resolution and analytical results. MALDI-MS requires combining the peptide
sample with specific matrices (crystalline energy absorbing dye molecules), which are then dried on MALDI plates prior to analysis.
Step Five: Cleanup and Enrichment of Digests
• Concentration of Cleaving Enzyme. The concentration of the cleaving agent should be minimized to avoid its contribution to
the map patterns. An excessive amount of cleavage agent is commonly used to accomplish a reasonably rapid digestion time (i.e.
6-20 hours); however, careful consideration should be given to these increased amounts. A protein-to-protease ratio between
10:1 and 200:1 is generally used and it is recommended that the cleavage agent be added in two or more stages to optimize
cleavage. In many standard trypsin digestion procedures, the trypsin is added in this manner. Nonetheless, the final reaction
volume remains small enough to facilitate separation – the next step in peptide mapping. To sort out digestion artifacts that might
interfere with the subsequent analysis, a blank determination is performed using a digestion control with all the reagents, except
the test protein.

222
Chromatographic Separation for Peptide Mapping
The most widely used peptide mapping chromatographic method is reversed-phase liquid chromatography with low UV
(210-220 nm)-, UV (280-nm)- or mass spectrometric-detection. Modern sub-two micron and superficially porous particle
columns with C18 bonded phases provide excellent resolving power and the use of volatile mobile phase additives that are
compatible with mass spectrometry to allow for rapid separations. The preferred column pore size is 100-120 Angstroms to
accommodate a variety of peptide fragment sizes. The column temperature is generally above 40 °C. The most popular mobile
phase usually has a pH of less than 3 and consists of 0.1% trifluoroacetic acid (TFA) in water (solvent A) and 0.08% TFA in
acetonitrile (solvent B) and linear gradient elution.
Since TFA is an ion pair reagent and sometime causes ion suppression in the mass spectrometer (see Chapter 17), formic acid
and acetic acids have also been used, but the chromatography separations may not be as clean as those observed for TFA.
Since mass spectrometric detection requires slightly lower flow rates than typically used for 4.6 mm id columns, column id is
usually 2.1 mm with lengths up to 150 mm and flow rates generally being less than 0.5 mL/min. As an example of today’s HPLC
column capability, Figure 15.7 provides a detailed peptide map of a bovine serum albumin digest in just 20 minutes. For further
information on the chromatographic separation conditions, condition optimization and mass spectrometric detection, please
consult Reference 69.
02.55 7.510 12.5 15 17.5 20 min
mAU
0
10
20
30
40
50
60
70
20
Figure 15.7
Peptide Map of BSA Tryptic Digest on Modern Peptide Mapping Column
2.1 x 150 mm AdvanceBio Peptide Mapping Column (Agilent Technologies)
Mobile phase: A: water (0.1% TFA), B: ACN (0.08% TFA), 40 °C, Flow: 0.52 mL/min
BSA Tryptic Digest
Hydrophilic peptide retention
Narrow peaks with baseline resolution
Hydrophobic peptide retention
Reduced and fast analysis time

223
Automation of Peptide Mapping
Since manual sample preparation of peptides is a time-consuming process and all the steps involved could introduce sample loss
and errors into the analysis, total workflow automation may be the preferred route if many samples are to be analyzed by LC-
MS/MS. Better reproducibility, increased throughput, rapid method development, decreased reagent and sample volume
requirements, and freeing up the time of the scientist are all benefits to miniaturized workflow automation. AssayMAP peptide
sample prep solution (Agilent Technologies, Santa Clara, CA) is a miniaturized liquid handling system that has been designed to
perform standardized sample preparation workflow. Here, the digestion, cleanup and fractionation (using reversed-phase and/or
cation exchange SPE cartridges) steps are based on 96-well plate parallel processing using a modern liquid handling platform
(Bravo). Fractionation is off-line to provide increased LC-MS throughput by reducing long LC gradient times.
(Continued)
References
1.
Mitra, S.
Sample Preparation Techniques in Analytical Chemistry
, Wiley-Interscience, Hoboken, NJ, 2003, ISBN #0-471-32845-6.
2.
Righetti, P.G.; Castagna, A.; Herbert, B.
Anal. Chem.
2001,
73
, 320A-326A.
3.
Zolotarjova, N.; Mrozinski, P.; Majors, R.E.
LCGC No. America
2007,
25 (2)
, 118-140.
4.
Rehm, H.
Protein Biochemistry and Proteomics
4th Ed., Academic Press (Elsevier), Burlington, MA, 2006,
ISBN 13: 978-0-12-088546-9 and ISBN 10: 0-12-088545-X.
5.
Lovric, J.
Introducing Proteomics: From Concepts to Sample Separation, Mass Spectrometry and Data Analysis
,
Wiley, Hoboken, NJ, 2011, ISBN-10: 0470035242 and ISBN-13: 978-0470035245.
6.
Mishra, N.C.
Introduction to Proteomics: Principles and Applications
, Wiley, Hoboken, NJ, 2010, ISBN-10: 471754021
and ISBN-13: 978-0471754022.
7.
Coligan, J.E.; Dunn, B.M.; Ploegh, H.L. et al.
Current Protocols in Protein Science
, John Wiley & Sons, New York, 2002.
8.
Emmert-Buck, M.R.; Bonner, R.F.; Smith, P.D.; Chauqui, R.F.; Zhuang, Z.; Goldstein, S.R.; Weiss, R.A.; Liotta, L.A.
Science
1996,
274
,
998-1001.
9.
Banks, R.E.; Dunn, M.J.; Forbes, M.A. et al.
Electrophoresis
2000,
20
, 689-700.
10.
Aebersold, R.; Goodlett, D.R.
Chem. Rev.
2001,
101
, 269-295.

224
11.
Aebersold, R.; Mann, M.
Nature
2003,
422
, 198-207.
12.
Han, X.; Aslanian, A.; Yates III, J.R.
Curr. Opin. Chem. Biol.
2008,
12 (5)
, 483-490.
13.
Bensman, A.; Heck, A.J.; Aebersold, R.
Annual Rev. Biochem.
2012,
81
, 379-405.
14.
Wells, D.A.
High Throughput Bioanalytical Sample Preparation: Methods and Automation Strategies
, Elsevier Science,
San Diego, CA, 2003, ISBN-10:044451029X and ISBN-13:978-0444510297.
15.
Lee, W.-C.; Lee, K.H.
Anal. Biochem.
2004,
324
, 1-10.
16.
Bjorhall, K.; Miliotis, T.; Davidsson, P.
Proteomics
2005,
5
, 307-317.
17.
Jacobs, J.M.; Adkins, J.N.; Qian, W.-J.; Shen, Y.; Camp III, D.G.; Smith, R.D. J.
Proteome Res.
2005,
4 (4)
, 1073-1085.
18.
Ogata, Y.; Charlesworth, M.C.; Muddiman, D.C. J.
Proteome Res.
2005,
4 (3)
, 837-845.
19.
Chromy, B.A.; Gonzales, A.D.; Perkins, J.; Choi, M.W.; Corzett, M.H.; Chang, B.C.; Corzett, C.H.; McCutchen-Maloney, S.L.
J. Proteom.
Res.
2004,
3
, 1120-1127.
20.
Govednik, L.; Brne, P.; Gabor, B.; Barut, M.; Štrancar, A.; Strlič, M.
Chromatographic Characterization of Affinity and Pseudoaffinity
Columns for the Depletion of IgG and HSA from Plasma Samples
, 12th International Symposium on Separation Sciences, Poster
Paper P1, Lipica, Slovenia, September 27-29, 2006.
21.
Martosella, J.; Zolotarjova, N.; Liu, H.; Nicol, G.; Boyes, B.E. J.
Proteome Res.
2005,
4 (5)
, 1522-1537.
22.
Zhang, K.; Zolotarjova, N.; Nicol, G.; Martosella, J.; Yang, L.-S.; Czafranski, C.; Bailey, J.; Boyes, B.
Agilent Multiple Affinity Removal
System for the Depletion of High-Abundant Proteins in Human Serum – A New Technology from Agilent
, Agilent Application
Note #5988-9813EN, 2003.
23.
Brand, J.; Haslberger, T.; Zolg, W.; Pestlin, G.; Palme, S.
Proteomics
2006,
6
, 3236-3242.
24.
Shen, Z.; Want, E.J.; Chen, W.; Keating, W.; Nussbaumer, W.; Moore, R.; Gentle, T.M.; Siuzdak, G. J.
Proteome Res.
2006,
5
, 3154-3160.
25.
Fang, X.; Huang, L.; Feitelson, J.S.; Zhang, W.-W.
Drug Discovery Today Technol.
2004,
1
, 141-148.
26.
Seppro Protein Fractionation Services Brochure
, Genway, San Diego, CA.
27.
Lynch, M.
Amer. Biotech. Lab.
2005,
37 (5)
, 24-26.
28.
Schuchard, M.; Melm, C.; Crawford, A.; Chapman, H.; Cockrill, S.; Ray, K.; Mehigh, R.; Chen, D.; Scott, G.
Specific Depletion of
Twenty High Abundance Proteins from Human Plasma
, NCI Proteomic Technologies Reagents Resource Workshop, Chicago, IL,
December 11-12, 2005.
29.
Maccarrone, G.; Milfay, D.; Birg, I.; Rosenhagen, M.; Holsboer, F.; Grimm, R.; Bailey, J.; Zolotarjova, N.; Turck, C.W.
Electrophoresis
2004,
25
, 2402-2412.
30. Cho, S.Y.; Lee, E.Y.; Lee, J.S.; Kim, H.Y.; Park, J.M.; Kwon, M.S.; Park, Y.K.; Lee, H.J.; Kang, M.J.; Kim, J.Y.; Yoo, J.S.; Park, S.J.; Cho,
J.W.; Kim, H.S.; Paik, Y.K.
Proteomics
2005,
5
, 3386-3396.
References
(Continued)
(Continued)

225
References
31.
Zolotarjova, N.; Martosella, J.; Nicol, G.; Bailey, J.; Boyes, B.E.; Barrett, W.C.
Proteomics
2005,
5
, 3304-3313.
32.
Moritz, R.L.; Chippingdale, A.B.; Kapp, E.A.; Eddes, J.S.; Ji, H.; Gilbert, S.; Connolly, L.M.; Simpson, R.J.
Proteomics
2005,
5
, 3402-3413.
33.
Yocum, A.K.; Yu, K.; Oe, T.; Blair, I.A. J.
Proteome Res.
2005,
4 (5)
, 1722-1731.
34. Wu, J.; Kobayashi, M.; Sousa, E.A.; Liu, W.; Cai, J.; Goldman, S.J.; Dorner, A.J.; Projan, S.J.; Kavuru, M.S.; Qiu, Y.; Thomassen, M.J.
Mol. Cell. Proteomics
2005,
4.9
, 1251-1264.
35. Wang, H.; Clouthier, S.G.; Galchev, V.; Misek, D.E.; Duffner, U.; Min, C.-K.; Zhao, R.; Tra, J.; Omenn, G.S.; M. Ferrara, J.L.; Hanashl,
S.M.;
Mol. Cell. Proteomics
2005,
4
, 618-625.
36.
Molina, H.; Bukenborg, J.; Reddy, G.H.; Muthusamy, B.; Scheel, P.J.; Pandey, A.
Mol. Cell. Proteomics
2005,
4
, 637-650.
37.
Echan, L.A.; Tang, H.-Y.; Ali-Khan, N.; Lee, K.; Speicher,
D.W. Proteomics
2005,
5
, 3292-3303.
38.
Tang, H.-Y.
Proteomics
2005,
5
, 3329-3342.
39. Zolotarjova, N.; Boyes, B.; Martosella, J.; Yang, L.-S.; Nicol, G.; Zhang, K.; Czafranski, C.; Bailey, J.; Smejkal, G.B., Ed.; Lazarev, A., Ed.
Separation Methods in Proteomics
, CRC Press, Boka Raton, FL, 2005, ISBN-10: 0824726995 and ISBN-13: 978-0824726997.
40.
Anderson, L.; Hunter, C.L.
Mol. Cell. Proteomics
2006,
5.4
, 573-588.
41.
Sitnikov, D.; Chan, D.; Thibaudeau, E.; Pinard, M.; Hunter, J.M.
J. Chromatogr. B
2006,
832
, 41-46.
42. Misek, D.E.; Kuick, R.; Wang, H.; Galchev, V.; Deng, B.; Zhao, R.; Tra, J.; Pisano, M.; Amunugama, R.; Allen, D.; Walker, A.K.; Strahler,
J.R.; Andrews, P.; Omenn, G.S.; Hanash, S.M.
Proteomics
2005,
5
, 3343-3352.
43.
He, P. et al.
Proteomics
2005,
5
, 3442-3452.
44. Huang, L.; Harvie, G.; Fietelson, J.S.; Gramatikoff, K.; Herold, D.A.; Allen, D.L.; Amunngama, R.; Hagler, R.A.; Pisano, M.R.; Zhang, W.W.;
Fang, X.
Proteomics
2005,
5
, 3314-3328.
45.
Liu, T.; Qian, W.-J.; Mottaz, H.M.; Gritsenko, M.A.; Norbeck, A.D.; Moore, R.J.; Purvine, S.O.; Camp, D.G.; Smith, R.D.
Mol. Cell.
Proteomics
2006 EPUB.
46.
Schuchard, M.D.; Melm, C.D.; Crawford, A.S.; Chapman, H.A.; Cockrill, S.L.; Ray, K.B.; Mehigh, R.J.; Kappel, W.K.; Scot, G.B.
Origins
2005,
21
, 17-23.
47.
Wang, Y.Y.; Cheng, P.; Chan, D.W.
Proteomics
2003,
3
, 243-248.
48.
Ahmed, N.; Barker, G.; Oliva, K.; Garfin, D.; Talmadge, K.; Georgiou, H.; Quinn, M.; Rice, G.
Proteomics
2003,
3
, 1980-1987.
49.
Colantonio, D.A.; Dunkinson, C.; Bovenkamp, D.E.; van Eyk, J.E.
Proteomics
2005,
5
, 3831-3835.
50.
Ramstrom, M.; Hagman, C.; Mitchell, J.K.; Derrick, P.J.; Hakansson, P.; Bergquist, J. J.
Proteome Res.
2005,
4
, 410-416.
(Continued)
(Continued)

226
References
62. Sato, A.K.; Sexton, D.J.; Morganelli, L.A.; Cohen, E.H.; Wu, Q.-L.; Conley, G.P.; Streltsova, Z.; Lee, S.W.; Devlin, M.; DeOliveira, D.B.; Enright,
J.; Kent, R.B.; Wescott, C.R.; Ransohoff, T.C.; Ley, A.C.; Ladner, R.C.
Biotechnol. Prog.
2002,
18
, 182-192.
63.
Baussant, T.; Bougueleret, L.; Johnson, A.; Rogers, J.; Menin, L.; Hall, M.; Aberg, P.-M.; Rose, K.
Proteomics
2005,
5
, 973-977.
64.
Petric, T.C.; Brne, P.; Gabor, B.; Govednik, L.; Barut, M.; Strancar, A.; Kralj, L.Z. J.
Pharm. Biomed. Anal.
, (in press).
65.
Jin, W.-H.; Dai, J.; Li, S.-J; Xia, Q.-C.; Zou; H.-F.; Zeng, R.J.
Proteome Res.
2005,
4
, 613-619.
66.
Yang, Z.; Hancock, W.S.
J. Chromatogr. A
2004,
1053 (1-2)
, 79-88.
67. Alpert, A.J.; Shukla, A.K. ABRF 2003, poster #P111-W.
68. Zhang, J.; Goodlet, D.R.; Peskind, E.R.; Quinn, J.F.; Zhou, Y.; Wang, Q.; Pan, C.; Yi, E.; Eng, J.; Aebersold, R.H.; Montine, T.J.; J.
Neurobiol. Aging
2005,
26
, 207-227.
69.
Keys for Enabling Optimum Peptide Characterizations: A Peptide Mapping “How to” Guide
, Agilent Technologies, Santa Clara,
CA, Publication Number 5991-2348EN, 2013.
51.
Hinerfeld, D.; Innamorati, D.; Pirro, J.; Tam, S.W.
J. Biomolec. Techniques
2004,
15 (3)
, 184-190.
52.
Affibody Application Note,
Depletion of Abundant HSA from Serum
, Bromma, Sweden
53.
Greenough, C.; Jenkins, R.E.; Kitteringham, N.R.; Pirmohamed, M.; Park, B.K.; Pennington, S.R.
Proteomics
2004,
4
, 3107-3111.
54.
Fahnestock, S. Trends in
Biochem. Sci.
1987,
5
, 1567.
55.
Fountoulakis, M.; Juranville, J.-F.; Jiang, L.; Avila, D.; Roder, D.; Jakob, P.; Berndt, P.; Evers, S.; Langen, H.
Amino Acids
2004,
27
, 249-259.
56.
Shen, Y.; Kim, J.; Strittmatter, E.F.; Jacobs, J.M.; Camp III, D.G.; Fang, R.; Tolie, N.; Moore, R.J.; Smith, R.D.
Proteomics
2005,
5
, 4034-4045.
57.
Govorukhina, N.I.; Keizer-Gunnink, A.; van der Zee, A.G.J.; de Jong, S.; de Brujn, H.W.A.; Bischoff, R.
J. Chromatogr. A
2003,
1009
,
171-178.
58.
Herbert, B.; Righetti, P.G.
Electrophoresis
2000,
21
, 3639-3648.
59.
Mikios, G.L.; Maleszka, R.
Proteomics
2001,
1
, 30-41.
60.
Rothemund, D.L.; Locke, V.L.; Liew, A.; Thomas, T.M.; Wasinger, V.; Rylatt, D.B.
Proteomics
2003,
3
, 279-287.
61.
Lee, H.-J.; Lee, E.-Y.; Kwon, M.-S.; Paik, Y.-K.
Current Opinion in Chemical Biology
2005,
10
, 1-8.
(Continued)

227
Chapter 16
Sample Preparation
in Bioanalysis
Analysis of drugs, their metabolites and low molecular weight biomarkers in biological matrices such as plasma, serum, whole
blood, urine, saliva, tissues, etc., is commonly termed ‘bioanalysis’. Here, we will also consider the analysis of tissue samples as
part of bioanalysis since tissue sample preparation is a key part of pharmaceutical and biological research. We will refer to the
sample preparation of biological samples for high molecular weight constituents (e.g. protein, nucleic acids, etc.) as biological
sample preparation (see Chapter 15). Bioanalysis is an important part of the overall drug development process starting with in
vitro/in situ testing, preclinical studies through to clinical studies. The last decade has witnessed many technological
breakthroughs in analytical methodology and instrumentation. Apart from improved selectivity and sensitivity, modern analytical
instrumentation has provided an edge to fast and cost-effective bioanalytical method development and validation. Among these
modern analytical techniques, liquid chromatography coupled with mass spectrometry is considered to be the benchmark for
quantitative/qualitative bioanalysis, imparting specificity, sensitivity, and speed. However, this most selective and sensitive
analytical technique also suffers from limitations such as matrix effects, compromised selectivity, and a fall in sensitivity of the
analyte of interest in the processed biological matrix.
Sample preparation, also known as sample pre-treatment/sample cleanup/sample extraction, is an integral part of most
bioanalytical methods. In a clinical situation, the drug/metabolite/biomarker of interest is present in biological matrix, which has a
complex biochemical nature and comprises numerous components (e.g. salts, acids, bases, proteins, cells, exogenous/endogenous
small organic molecules like lipids and lipoproteins). However, the biochemical complexity of the matrix may differ (e.g. tissue,
whole blood, plasma/serum, urine, saliva, cerebral spinal fluid, etc.). In simple terms, sample preparation is a process which aims at
selective isolation of the analyte of interest from the matrix, minimization/elimination of matrix components in the processed sample
and, if required, concentration of the analyte of interest. Effective sample preparation is a skill and accounts for up to 80% of the
total bioanalysis time. As mentioned in Chapter 1, sample preparation is the most labor-intensive and error-prone process in overall
bioanalytical methodology.

228
Many of the sample preparation protocols covered throughout this book are directly applicable to bioanalytical matrices.
Traditionally, liquid-liquid extraction (LLE, Chapter 7), filtration (Chapter 5), solid-phase extraction (SPE, Chapters 9 and 10),
and “dilute and shoot” have been used as sample preparation techniques. Information on those techniques will not be
repeated since they were already covered in detail. However, new and improved sample preparation technologies are being
developed constantly and more effective ways of extracting the analyte from the biomatrix have become available. Many of
these newly developed sample preparation techniques have been tested, validated, and commercialized. This chapter will
provide a brief coverage of some of the newer or more widely used approaches.
Blood Analysis in Bioanalytical Laboratories
Blood analysis is certainly the most popular martrix for the analysis of drugs and drug metabolites in biological fluids. Most often
blood plasma or serum is the favored matrix for analysis, but often large blood volumes must be collected. In pharmacokinetic
(PK) and toxicokinetic (TK) studies, rodents are the most used animals for preclinical testing. Small rodents such as mice have a
limited blood supply. Typically, samples of between 200-250 µL (total volume up to 1500 µL) is needed per TK testing. In order
to obtain a sufficient supply of blood sample, the animal must be warmed for 10 min prior to sampling. Often, several rats or
mice must be pooled in order to obtain enough blood for testing. Clearly, using less blood for these tests would save on animal
usage. In clinical testing for neonatal humans, a large amount of blood is never available. Thus, using a reduced volume of
blood would enable more pediatric studies for metabolic and other testing.
The question often arises: whether to use plasma or whole blood for analytical measurements? Some studies have shown that a
number of drugs tend to distribute evenly between plasma and blood cells while other studies show that some drugs bind
selectively to plasma proteins. Reports have also shown that drugs such as immunosuppressants bind predominantly to blood cells.
Conventionally, plasma has been used for drug metabolism (DM) and PK/TK studies because of its ease of handling, shipping and
storage, compared to whole blood. However, it is noted by regulatory authorities in both the International Conference on
Harmonization and the United States Food and Drug Administration that blood is an acceptable biological matrix for measurement
of drug exposures. So in some cases, the use of whole blood for analysis in DMPK and TK studies could be preferable.

229
Traditional Whole Blood Analysis
Figure 16.1 depicts the various ways to analyze and process whole blood. Traditional approaches are shown in the middle of the
figure. These are dialysis, liquid-liquid extraction, protein precipitation, (hemo)lysis, membrane filtration, and anti-coagulation.
Such approaches generally require larger volumes of blood and multiple, time-consuming steps where analyte losses may occur.
Many of the traditional methods are performed manually, but obviously when faced with large numbers of samples, automation
via robotics or other approaches may be warranted. All of these approaches end up with a matrix-depleted (pre-processed)
sample. Depending on the final analytical technique used for measurement of drugs and metabolites, some additional sample
preparation, such as solid phase extraction (SPE) may be required for final cleanup and preconcentration. In recent years,
LC-MS/MS has been used for further separation and detection. The heightened sensitivity and selectivity of this hyphenated
method has been a boon to trace analysis. In fact, in many fields, this combination has become the norm for trace analysis.
Using LC-MS/MS, one can often determine parts per billion in biological samples, even without extensive sample cleanup.
For quantitative analysis, LC-MS/MS is a must.

230
Figure 16.1
Processing of Whole Blood
In-line
Matrix-depleted (preprocessed) Samples
LC-MS/MS
Matrix-containing
(native) Samples
Cell-
disintegrated
Blood (CDB)
Manual (off-line)/Robotic (at-line)
Blood Spots Dialysis
Liquid-Liquid
Extraction
Precipitation (Hemo)Lysis
Membrane
Filtration
± Anticoagulation
Centrifugation Centrifugation CentrifugationExtraction
Precipitation Precipitation PrecipitationPunching
Drying Dialysate Evaporation
(Hemo)Lysate
Plasma/Serum
Filtrate
Plasma/Serum
Supernatant
(Derivatization)
Centrifugation
off-line
at-line
SPE
on-line
Adapted from Reference 1.

231
However, these traditional techniques for whole blood analysis still do not meet the needs for small volumes of whole blood
samples. A recent technology that uses a much lower amount of blood sample than previously considered, known as Dried Blood
Spot (DBS) analysis (Figure 16.2, A-D), has drawn a great deal of attention. The technique has been expanded to other biological
fluids [e.g. plasma, saliva, cerebral spinal fluid (CSF)], so the term Dried Media Spot (DMS) analysis has been proposed. An
interesting new approach to whole blood sample preparation is the development of cell-disintegrated blood (CDB, Figure 16.2E).
In this approach, a sample of homogenized blood is passed through a heated (75 °C) stainless steel capillary. The resulting heat
shock induces cell disintegration and generates a novel matrix known as CDB which can be further analyzed. For more information
on this technique, please refer to references 1-3.
Figure 16.2
Spotting area
Lot number
Labeling area
Checkboxes
Spotting sample Replicate samples Drying spots
Punching out spots Elution into tube
A B C
D E
Steps in Dried Blood Spotting

232
The use of DBS analysis was already described in 1913 by Bang
4
in the estimation of blood glucose concentration. However, the
most prominent use of dried blood spots dates to the early 1960s when Dr. Robert Guthrie developed an assay for the detection of
phenylketonuria
5
. His application of collecting blood on filter paper led to the population screening of newborns for the detection of
inherited metabolic diseases. In recent years, the collection of blood on filter paper has become a significant tool for screening
individuals for clinical purposes, animal testing for preclinical purposes, therapeutic and illicit drug monitoring, and the potential
application to routine drug development.
Today, pressures in the pharmaceutical industry to deliver high-quality PK data in the shortest possible timeframe is requiring
workers in the field to accurately and precisely collect, share, store and analyze thousands of biological samples. In addition, the
movement to reduce, refine and replace the use of animals in drug development is causing challenges to collect adequate samples
while remaining within acceptable total blood collection volumes and avoiding excess animal usage. Thus, dried blood spot analysis
is a way to address these challenges in the pharmaceutical industry. In addition, the use of techniques picked up in performing dried
blood spot analysis is extending this approach to other fields.
As shown in the workflow of Figure 16.2, DBS handling and analysis is fairly simple and involves only a few steps. A finger or a heel
prick followed by blood flow into a glass capillary is the usual method of collection (although sometimes a pipette tip is utilized). For
animal studies, a tail prick is preferred. Thus, use of a syringe for venous blood drawing is not required. Depending on the size of the
capillary, a sufficient amount of blood is drawn to allow multiple spottings. Usually, only a few tens of microliters are needed. Blood is
sometimes collected from the subject in a microvessel.
Dried Blood Spot Analysis/
Dried Media Spot Analysis

233
A DBS card with a defined size is constructed of filter paper or other non-cellulose material such as the Agilent DMS Card. This card is
used as the device for sample spotting. As shown in Figure 16.2A, after collection, the blood is spotted onto the DBS card. A typical
card allows the application of up to four blood spots (Figure 16.2B). Typically 10-30 µL (average size: 15 µL) of blood are applied for
each spot. The blood is delivered by the capillary (or sometimes a micropipette). It is of utmost importance not to allow the capillary to
touch the filter paper to prevent contamination. A typical blood spot is in the range of 5-9 mm in diameter. Next, the blood spots are
allowed to dry for a period of two hours at room temperature (Figure 16.2C). Drying racks are available for this purpose. When the
blood is treated in this manner, it can be stored for long periods of time at room temperature without degradation. To ship or store
loaded cards, they should be placed in sealable, plastic bags containing a desiccant.
For analysis, a circular portion (disk) of each dried blood spot is punched out (Figure 16.2D) into a vial, centrifuge tube, or well of a
96-well plate. A typical punch size is a 3-6 mm disk, which can be optimized based on the required limit of detection and available
spotted blood volume. With a trained technician, manual punch times are typically 10-15 min. for 96 samples. An organic solvent,
typically methanol or methanol-water mixtures containing an internal standard is used to elute the analytes of interest. For basic
analytes 0.1% formic acid in 80:20 (v/v) methanol:water and for acidic analytes a solution of 1% ammonium hydroxide in 60:40
(v/v) is recommended. Extraction solvent volumes are in the range of a few hundred microliters, typically 300 µL. Studies have
shown that using the DBS technique, spot sample sizes are usually reproducible to +/- 5-10%
6
. In some instances, further sample
cleanup may be required using protein precipitation, liquid-liquid extraction, or solid phase extraction.
Table 16.1 shows the many advantages of the DBS technique compared to conventional plasma analysis. A disadvantage of the
DBS analytical technique is that it is not a preconcentration technique and thus often requires a highly sensitive analytical technique
(e.g. LC-MS/MS) that may be beyond the capabilities of some laboratories. However, LC-MS/MS is becoming the standard
analytical technique for all sorts of trace analysis. Presumably, using micro-SPE, some additional cleanup of the matrix-depleted
DBS extract could be undertaken. Other considerations are that the technique cannot be used for volatile and air-sensitive
compounds and certain unstable metabolites. Photosensitive compounds may require special handling.

234
Table 16.1
Comparison of Conventional Plasma Analysis and Dried Blood Spot Analysis
Parameter
Conventional plasma
analysis Dried blood spot analysis Comments for DBS
Blood volume >500 µL 10-30 µL Fewer experimental animals required; more
consistent data obtained through more serial
sampling from individual animals and less
reliance on composite data; neonatal and
juvenile patients can be studied
Blood collection
technique
Venous cannula
withdrawal using syringe
Finger or heel prick using capillary Less invasive sampling and patient discomfort
and easier for multiple samples; overall easier
sample collection
Sample processing Centrifugation, isolation
and cleanup of plasma
Simple three-step procedure; on-substrate
cleanup has the potential to convey greater
analyte stability, especially for enzyme-
sensitive compounds
Fewer steps involved for DBS and therefore
less chance of error; less labor involved; not
easily automated presently; usually manual
punching and extraction
Storage and
transportation
Plasma must be stored
and shipped under
frozen conditions
Room temperature stability in dry state;
can be shipped without biohazard labeling
DBS samples can be stored at room
temperature and shipped without dry ice for
more remote sampling, particularly important
in human clinical trial samples
Cost Requires freezer, dry ice
for shipment, more
animal use, treatment as
hazardous material
Reduced shipping costs, storage costs,
fewer test animals required, non-hazardous
shipment requirements.
Can lower overall cost of drug development

235
The DBS sample collection technology has been validated for a number of pharmaceutical compounds and numerous studies
have shown good correlation between DBS results and those of conventional blood plasma analysis methods. For example, one
study showed a direct comparison of conventional blood analysis techniques versus blood spotting techniques for the quantitation
of 12 pharmaceutical compounds tested in vivo in rats
7
. Overall, an excellent correlation was achieved between DBS and
blood:water samples. Another example where toxicokinetic studies for the drug Omeprazole was performed in vivo in rats and the
results in whole blood and plasma was compared to DBS
8
. For the conventional plasma analysis 240 µL of blood was used but
only 60 µL was required for the classic DBS card (4 spottings). The analysis of drug eluted from the DBS card was performed by
LC-MS/MS. The ratio of blood to plasma concentrations was calculated by the average of the DBS blood concentration to plasma
concentration and predicted blood concentrations were obtained by applying this ratio to the plasma concentrations.
Because large numbers of samples encountered in DMPK/TK studies, manual loading and punching dried blood spots can become
a tedious task. Thus, some form of semi- or full-automation is desired. Various approaches for automation have been used. One
such robotic automation device punches out 96 blood disks in 15-20 minutes. Other approaches elute the analytes directly from the
card while another ionizes analytes from the card into the gas phase and transfers them to a mass spectrometer. This field is rather
new and giving details of experimental approaches is beyond the scope of this Chapter. Readers are referred to review articles that
further describe the process
9-12
.
Protein Removal
Urine and blood plasma from humans and a variety of animal species are the most commonly analyzed biofluids. Protein removal is
an essential step in sample preparation for the LC-MS/MS analysis of compounds in biological matrices. One of the main reasons that
many biofluids cannot be analyzed directly by LC-MS/MS is that the high protein content would cause a rapid deterioration in the
performance of the HPLC column and ion source contamination in the mass spectrometer. A variety of techniques can be used to
remove or reduce the protein content of plasma. Table 16.2 lists a number of the more popular protein removal techniques. For
many years, prior to the widespread acceptance of tandem mass spectroscopic techniques, LLE and SPE were the predominant
techniques for removing proteins. However, both of these sample preparation techniques require method development and multistep
processes. For example, LLE may require direct and back extractions to remove proteins while SPE requires investigation of various
sorbents, extraction, washing, and elution solvents and pH adjustment to provide optimum cleanup and recovery of drugs and
metabolites. Protein precipitation has now become more commonplace and works reasonably well as long as contaminants do not
cause undue ion suppression or ion enhancement effects in the MS detector.

236
Protein Precipitation
Protein precipitation (sometimes called protein “crashing”) is a popular choice for sample preparation, since some samples can
sometimes be directly injected into the HPLC column after precipitation. Little method development is required and simple
equipment and reagents are used. However, the technique does not concentrate the analytes and, in fact, a dilution occurs. In
addition, sample cleanup is limited. Depending on the precipitation reagent used, experimental conditions, and the species from
which the plasma was derived, up to 98% of the proteins in the sample can be removed. However, other excipients such as salts,
lipids and phospholipids may still remain in the supernatant.
Figure 16.3
Protein Precipitation
Sample
2) Mix
3) Centrifuge or filter
4) Remove supernatant
Protein in solution
Analyte
Other interferences
Precipitated
proteins
The principle of protein precipitation is very simple (see Figure 16.3). By adding a chemical reagent to the plasma sample, the
objective is to markedly reduce the solubility of the proteins in the sample causing them to precipitate. This precipitate can be removed
by filtration or centrifugation leaving a clear supernatant or filtrate that is usually injected directly into the HPLC or LC-MS/MS system.
The four main reagents used to precipitate plasma proteins are listed in Table 16.3.
1) Add organic or other
chemical reagent and
5) Analyze supernatant,
often after dry down
and reconstitution

237
Table 16.2
Techniques for Removal of Protein from Biological Fluids
Protein Removal
Technique Principle Reference(s)
Precipitation Organic solvent (e.g. acetonitrile, methanol), acid solution (e.g. perchloric, formic, trichloroacetic) or salt
solution (e.g. sodium sulfate, ammonium sulfate) is added with agitation to a solution of plasma. The protein
precipitates and forms a bead that can be removed by centrifugation or filtration. Supernatant liquid can be
analyzed by HPLC.
13-16
Restricted Access
Media (RAM)
(see Chapter 10)
Solution containing protein is injected into a RAM column which has a short chain hydrophilic bonded phase
like an alkyl diol designed to reject proteins and allow small molecules to diffuse into pores where a bonded
reversed-phase interacts with small molecules; the protein portion can be vented to waste using a switching
valve; a similar material has a hydrophilic polymer that performs the same task by shielding the underlying
C18 phase.
17-21
Turbulent Flow
Chromatography
A large particle (~50 µm) small diameter bonded silica reversed-phase column (1 mm id) is run at very high
linear velocities up to 8 mL/min flow rate; although Reynolds numbers are not in the turbulent flow range,
these high linear velocities do not allow the slower diffusing proteins and other plasma components to
penetrate the pores of the packing and they are flushed to waste; smaller molecules, usually drugs and their
metabolites, can penetrate the pores and are retained by reversed-phase mechanisms. The small molecules
are then eluted with aqueous-organic mobile phase into a mass spectrometer.
22-25
Ion Exchange
(see Chapter 9)
An ion exchange column can be used to selectively retain large biomolecules by adjusting the pH of the
mobile phase to generate a net positive or negative charge on the protein portion of a biological fluid; small
molecules may pass through unretained and may be focused on a secondary column for subsequent analysis
by HPLC. Off-line and on-line approaches can be used.
26-28
Size Exclusion
Chromatography
(see Chapter 11)
By choice of an appropriate small pore size aqueous-compatible packing material, proteins can be excluded
and elute from the column first while the small molecular weight compounds elute closer to the total
permeation volume.
29-34
Reversed-Phase
(see Chapter 9)
The use of less hydrophobic short alkyl chain phases like C3 or C4 bonded to wide pore silicas will retain
proteins and have less interaction with polar drugs so that they can be used to retain proteins and allow small
molecules to pass through.
35-36
High-Abundance
Protein Depletion
(see Chapter 15)
High abundance proteins (up to 20) from human and other plasma/serum samples are depleted by antibody
affinity phases bonded to a solid packing; low abundance proteins are collected as unretained peaks since
there is no interaction with the column media. They can be concentrated by trace enrichment techniques and
further investigated. This technique is mostly used for the investigation of low abundance proteins rather than
for the analysis of drugs and metabolites in biofluids.
37-41

238
The most popular method is the addition of organic solvent. Organic solvents act by lowering the dielectric constant of the plasma
protein solution, thereby increasing electrostatic interaction between the protein molecules. The organic solvent also tends to
displace water molecules associated with the hydrophobic portion of the protein. By minimizing the hydrophobic interactions and
increasing electrostatic interactions, the proteins aggregate, and therefore precipitate. The acidic reagents form insoluble salts with
the positively charged amino groups in the protein molecules occurring at any pH below their isoelectric point. Metal ions bind to
amino groups on the protein, thereby displacing protons. This also lowers the isoelectric point of the protein and the displaced
protons also lower the solution pH, leading to precipitation. High salt concentrations reduce the availability of water molecules,
dehydrates the protein and, in turn, increases hydrophobic-hydrophobic interactions leading to protein aggregation.
Table 16.3
After precipitation, the denatured protein can be removed by centrifugation or filtration to leave a clear supernatant an aliquot of
which can be transferred to another tube or vial. If a centrifuge is used, one should use relatively high g-force (10,000-15,000 g) to
separate the precipitated protein. Centrifugation is not particularly well-suited for high-throughput environments due to the multiple
manual steps involved. For such laboratories, the 96-well filtration plate “flow-through” format has become quite popular and
equipment is available to automate the entire process. Each well in this format contains at its base a small membrane filter which
filters out precipitated protein, allowing the supernatant liquid to be collected at the exit in a 96-well collection plate. With this format,
the “solvent-first” method is the most efficient for precipitation of proteins from plasma. However, in a regular 96-well filtration plate,
low viscosity solvents like acetonitrile will prematurely begin to drip from the plate by gravity before the plasma can be added. Special
new non-drip plates such as Agilent Technologies’ Captiva ND plate have been specially designed using filtration materials that
effectively hold organic solvents used for precipitation with no dripping. Flow-through the membrane only occurs when a vacuum is
applied. This prevents sample loss and enables trouble-free automated methods. Simply add the organic solvent followed by the
biological sample, mix them well, and apply vacuum to filter out precipitated proteins. The result is a particulate-free, protein-free
sample in just a few minutes. For those who have fewer samples and do not want to use a 96-well filtration plate, single membrane
versions of the Captiva filter are available in the SPE cartridge format and disposable membrane filter format.
Chemical Reagents for Protein Precipitation from Plasma
Type of Reagent Typical Reagents Comments
Organic solvent Acetonitrile, methanol, ethanol Acetonitrile is the most popular; irreversibly denatures
three-dimensional structure of proteins
Acids Trichloroacetic acid, perchloric acid, tungstic acid Irreversibly denatures three-dimensional structure
of proteins
Metal ions Zinc hydroxide, copper sulfate-sodium tungstate
Salts Ammonium sulfate Salting out effect; denaturization potentially reversible
by dialyzing out the excess salt

239
An example is shown here of the use of non-drip 96-well filtration plates and centrifugation for the sample preparation of plasma
spiked with beta-blocker drugs
42
. Table 16.4 provides the steps and time involved in each step when performing the sample
preparation process. The centrifugation process required several manual steps while the 96-well process could be fully automated,
thereby saving considerable time and avoiding much manual manipulation. Several experimental tests were performed to illustrate
the effectiveness of the protein precipitation process using membrane filtration. Over 5,000 injections of the supernatant filtered
sample was injected into a microparticulate 1.8 µm ZORBAX Eclipse Plus HPLC column (2.1 x 50 mm) using gradient elution and the
pressure on the column remained relatively constant, indicating that protein removal was more than adequate. Retention time and
MS peak area data for the beta blockers (Metoprotol, Nadolol, Pindotol, and Propanolol) also remained constant over the same
number of injections.
Table 16.4
Sample Preparation Time Comparison for Protein Precipitation Using Agilent Technologies’
Captiva ND 96-well Filtration and Centrifugation Methods
42
Centrifugation Time (min) 96-well Filtration Time (min)
Add 0.2 mL of spiked plasma sample and 0.6 mL of the
acetonitrile +0.1% formic acid to centrifugation tube or an
empty 96-well plate
5 Add 0.2 mL of spiked plasma sample and 0.6 mL of
acetonitrile +0.1% formic acid to the non-drip 96-well plate
5
Centrifuge at 10,000 RPM for 10 min 11 Mix each well with the pipette five times and apply vacuum
Transfer supernatant to 2 mL injection vials (if tubes were
used) or a new empty 96-well plate for analysis (if plate
format was used)
10 Directly transfer injection plate for analysis 0
Total time required for sample preparation 26 Total time required for sample preparation 5

240
Removal of Lipophilic Material
Figure 16.4
Captiva ND
Lipids
Lipid/
Protein Removal Filtration Plate
Precipitate
Filter
Key
Salts
Proteins
Lipids
Analyte
Add Precipitation Reagent
Sample
Mix
Precipitation
Reagent (e.g.
acetonitrile)
As stated earlier, protein precipitation, for the most part, only
removes proteins from the plasma sample. Salts, a lesser level
of proteins, lipids, phospholipids, lysophosphatidylcholines, and
other excipients still remain in the supernatant. Since reversed-
phase HPLC is the main technique for determining drugs and
biological fluids, the salts remaining in the supernatant are not
much of a problem since most salts elute from a reversed-
phase column with or near the solvent front. However, the
lipophilic compounds can be a problem since they show up
later in the hydrophobic portion of the reversed-phase
chromatogram and interfere with the separation, lodge on the
column and bleed later and/or cause ion suppression with the
MS signal. Solid-phase extraction can be used to remove some
of these compounds, but that adds extra complexity to the
sample prep process.
A faster and more cost effective procedure to remove these
lipophilic compounds is the use of the Captiva ND
Lipids
96-well
filtration plate or its single tube equivalent. Figure 16.4 shows
how this filtration plate is used to remove both precipitated
proteins and lipids from a plasma sample. The device uses a
double filter: initial non-drip filter removes precipitated protein
while secondary filter (proprietary) removes lipophilic
compounds. Normally, a 50-200 µL plasma sample is used.
The precipitation reagent, sometimes called the crash solvent,
methanol or acetonitrile, is added in a 3:1 organic:plasma
ratio. A pH modification can be made to ionize analyte(s) of
interest. For basic compounds, we recommend 0.1%-1%
formic acid in methanol. For acidic compounds, we
recommend 5-10 mM ammonium formate buffer at pH 9. For
more thorough precipitation, pipette mixing is recommended
rather than the use of orbital or vortex mixing. The
filtration/lipid removal step should take five minutes or less.

241
5
0
100
200
300
400
10 15 20 min
Figure 16.5
Use of Captiva ND
Lipids
Removal Device
LC-MS/MS Reversed-Phase Chromatogram
Protein precipitation only
Lipid-stripped protein precipitation
with Captiva ND
Lipids
MS Transition 184 → 184
Figure 16.5 shows a comparison of the removal of lipophilic materials from plasma using Captiva ND versus Captiva ND
Lipids
.
Using the 184 → 184 (in-source fragmentation) MRM transition, phosphatidylcholines can be monitored as an indication of ion
suppression. One can see from Figure 16.5 that the majority of the lipid-containing materials are reduced. A further comparison
is shown in Figure 16.6 which uses post-column infusion studies for lipids and compares the various methods used for preparing
plasma samples. It is obvious from this comparison that protein precipitation leaves much to be desired if one is worried about
lipophilic ion suppression during LC-MS/MS. Both SPE and liquid-liquid extraction do a fair job of cleaning up the plasma sample.
But, the combined lipid-stripped protein precipitation procedure gives the best overall baseline. An alternative approach in
removing phosphorous-containing compounds is to use titanium- or zirconium-oxide-based sorbents, which have a high affinity
for phosphorous.

242
Sample Preparation of Biological Tissue Samples*
*Partially extracted from Reference 43
Preparation of biological tissue samples remains an extremely tedious and time-consuming laboratory task. From the moment the
tissue is excised, great attention must be paid to how the sample is stored, processed (whether mechanical or chemical),
extracted, and finally analyzed. Once this process is complete, the utility of the data obtained from tissue as well as its analytical
quality (accuracy, precision and reproducibility) is still debatable. Despite the somewhat thankless nature of tissue analysis,
significant progress has been made in the last decade to explore alternative tissue sample preparation approaches as well as
obtain a better understanding of the risks and benefits of conventional methods. An overview of human and animal tissue sample
preparation focusing on quantitative bioanalytical applications will be presented here. However, applications for microscopy,
imaging and elemental analysis will not be covered. Both traditional extraction technologies and new approaches will be
reviewed and compared.
1
2
3
4
5
0
10
20
30
0
10
20
30
0
10
20
30
0
10
20
30
10 15 20 25 30 35 min
Figure 16.6
Post-column Infusion Studies: Lipids
Protein Precipitation
Captiva ND
Solid Phase Extraction
Neutral Polymeric SPE
Liquid-Liquid Extraction
MTBE/water
Lipid-stripped Protein Precipitation
Captiva ND
Lipids
MCounts

243
Types of Tissue Preparation Techniques
Tissue preparation techniques can be categorized into mechanical, digestion, or extraction instruments. Some of the techniques
used successfully for other types of solid samples (Chapter 14), such as soil or plant material, may also be used for tissues. Tissue
samples, although solid, should be considered highly aqueous in nature, which can be exploited to rupture cells within the tissue
matrix. Generally, chunks of tissue are snap frozen in liquid nitrogen immediately after sampling, and are stored at very low
temperatures (-20 or -70 °C) prior to processing. Just as with any biological matrix, proper biohazard safety precautions should be
followed. Tissue samples are best processed immediately after removal from the freezer, since thawing will produce a rubbery
nugget that evades slicing or dicing. If a large number of tissues must be prepared, several small batches should be serially
processed rather than allowing the entire set of samples to thaw.
A simple comparison of the techniques is shown in Table 16.5. The workflows for the tissue preparation methods described are
shown in Figure 16.7. Each technique has both advantages and disadvantages, and some may be used in combination for
optimum extraction.
Why Tissue Analysis?
Several application areas for which tissue preparation is needed include pharmaceuticals, molecular biology, food science,
forensics, and toxicology. In pharmaceutical research, quantitative analysis of tissue may be carried out to assess the tissue
uptake at the site of drug action, correlate drug concentration with pharmacokinetic and pharmacodynamic response, and
predict toxicity and dose. Tissue samples may also be processed to obtain proteins, peptides, purified DNA or RNA for molecular
biology research. Determination of preservatives, pesticides residue, growth hormones and drug residues in feed animal tissues
is important in food science. For example, the use of antibiotics in food animals has generated growing concern since lingering
traces of fluoroquinolone antibiotics may contribute to increased bacterial resistance in humans who consume the meat.
Another important avenue for tissue analysis is forensic or toxicology purposes, whether to confirm the identity of an alleged
suspect from crime scene evidence or to determine if poisoning or overdose has occurred. Because DNA can be extracted from
almost any human tissue, extracted DNA from crime scene evidence can be compared to extracted DNA from known
individuals. For toxicological or DNA testing, tissues are generally utilized together with blood, urine, and hair.

244
Table 16.5
Tissue Preparation Techniques
Technique
Primary
Application
Handles Whole
Tissue Chunks
Analyte
Stability Issues Automation Inexpensive
High Sample
Throughput
Homogenizer Small molecules,
proteins, peptides,
DNA, RNA
Yes No No Yes No
Ultrasonicator No No No No No
Bead Beater DNA, nucleic acids Yes No Yes Yes Yes
Freezer/Mill Yes No Yes No Yes
Autogizer Small molecules,
proteins, peptides,
DNA, RNA
Yes No Yes No Yes
Acid or Base DNA, nucleic acids Yes Yes No Yes Yes
Enzymatic Small molecules, DNA,
nucleic acids
Yes Maybe Maybe Yes Yes
Accelerated
Solvent (ASE) or
Pressurized Fluid
Extraction
Environmental
(soil, foods)
Yes Yes Yes No Yes
Microwave-
Accelerated
No Yes Yes No Yes
Pressure Cycling
Technology (PCT)
DNA, nucleic acids Yes No Yes No Yes

245
Figure 16.7
Workflow Comparison of Tissue Preparation Methods
Mechanical Methods Intrumental Extraction Digestion Approaches
Mechanical Procedures Extract Using Instrument Digest
•
Homogenize
•
Bead beat
•
Freezer mill
•
Sonication
•
Acid
•
Enzymes
Harvest HarvestHarvest
Weigh
Weigh
Weigh
Grind and Lyophilize
(sonication only)
Centrifuge
Centrifuge
Extract
Extract
Reconstitute
Reconstitute
Reconstitute
Analyze
Analyze
Analyze
Vortex
• Accelerated solvent
extraction
• PCT
• Microwave extraction

246
Mechanical Techniques
Homogenization or “grinding” remains the most popular and generally practical means of preparing tissues for a range of
qualitative or quantitative applications. Initially, a small stainless steel probe-style blender, containing a generator and a set of
blades, causes vigorous mixing and turbulence as well as physically shearing of the sample into small pieces. Next, a weighed
amount of sample, which may range from 10 mg to 1 g in size, is placed in a vial with a known volume of buffer solution. The pH of
the buffer can be tailored to the desired extraction conditions. The resulting product, or homogenate, is semi-solid in nature and can
be essentially treated in the same manner as plasma. Lengthening the homogenization step, or centrifuging the homogenate and
decanting the supernatant will minimize large particles in the homogenate.
Homogenizers are small, compact, relatively inexpensive, and require minimal training to operate. However, extended exposure to
high velocity blending can be irritating, and ear protection should be worn. The probe should be thoroughly and repeatedly rinsed
between each sample to avoid cross contamination. A parallel homogenizer can be used to speed up the homogenization process
using a multi-probe, parallel processing approach. This type of homogenizer features a 4 or 6 probe assembly of homogenizers or
sonicators with a range of cutter sizes. The probes are cleaned automatically with three programmable wash stations. The sidebar
on page 248, “Homogenization Procedure for Analysis of Desipramine in Rat Brain Tissue” outlines a typical sample preparation
procedure for the analysis of desipramine in brain tissue using homogenization.
Sonication is one alternative to homogenization. In sonication, the tissue sample is snap frozen, and then immediately ground to a
fine powder using a mortar and pestle in a liquid nitrogen bath. The weighed powder is stored and when ready for analysis, mixed
with a known volume of buffer and sonicated using a specially designed acoustical tool, horn or probe placed directly into the
powder-buffer mixture. This method is more straightforward than homogenization, but powderizing tissue requires significant
manual labor and may lead to occupational health problems such as carpal tunnel syndrome. As with the homogenizer, the sonic
probe should be thoroughly cleaned between samples.

247
An innovative technique well-known to the food science community but less to pharmaceutical researchers is Matrix Solid Phase
Dispersion (MSPD, see Chapter 10), pioneered by Steven Barker of Louisiana State University
44-45
. Similar to mechanical techniques
such as the bead beater, MSPD relies on the shear forces generated by mixing tissue samples with large (>50 µm) silica particles and
grinding with a mortar and pestle. Analytes such as pesticides or drugs can be extracted from tissue using a range of stationary phase
chemistries. The analyte remains on the silica- or bonded-silica particles and can later be eluted with an appropriate solvent. The current
multi-step process requires repeated and careful manual intervention, but could conceivably be automated or combined with some of
the previously described devices.
A variation of the bead beater is the freezer mill from SPEX Certi-Prep (Metuchen, NJ) which uses small magnetic bars, rather than
beads, to pulverize the sample. Cooling is provided by immersing the sample chambers in a liquid nitrogen bath. The freezer mill is
intended for larger samples (>500 mg) but may be customized for smaller samples. Both the freezer mill and the bead beater
require that the sample be placed in secondary containers, which may or may not be disposable. Cleaning of non-disposable
sample containers can be labor intensive and should be avoided if possible through use of disposable devices.
The bead beater represents a more hands-off approach to tissue sample preparation. Introduced 25 years ago by BioSpec Products
(Bartlesville, OK), the bead beater is a unique but simply designed apparatus using small beads and a high-speed rotor to rupture
cells. A solid polytetrafluoroethylene impeller rotating at high speed forces thousands of minute beads to collide in a specially
shaped vessel. Cells are disrupted quickly, efficiently and safely. Each sample is placed in a separate tube with a defined amount of
beads and buffer solution, and then agitated for 15-20 minutes. Homogenization inside disposable microcentrifuge vials guarantees
that cross-contamination of samples is minimized.

248
An application for the analysis of mycotoxins – not in human but in fish tissue – based on MSPD on-line with SPE cleanup
followed by LC-MS/MS has been published
46
. In this publication, the authors measured zearalenone (ZON), a non-steroidal
estrogenic macrocyclic lactone mycotoxin in rainbow trout at the ppb level. The fish was first chopped into small pieces,
refrigerated, then placed in a glass mortar. A 2 g portion of C18 SPE phase was added to the mortar and the contents were
vigorously mixed with the pestle to obtain homogeneity. The resulting powder was dried in an oven at 40 °C. Next, the mixture
was packed into a 6 mL polypropylene column fitted with polyethylene fritted disks. The column was then connected in series to
another SPE column packed with graphitized carbon black (GCB). The ZON was eluted from the upper column (from the MSPD
experiment) to the lower SPE column using a methanol-water (70-30 v/v) mixture. The ZON and interferences were captured on
the GCB column. After washing with a series of solvents to remove interferences, the ZON was eluted with dichloromethane-
methanol mixture (80-20 v/v). After collecting the effluent, it was evaporated to dryness under nitrogen and reconstituted with
LC-compatible solvent and analyzed by LC-MS/MS. In their method development, the authors also used alumina as a matrix
dispersing reagent, but found that C18 gave superior recoveries and lower % RSDs.
Homogenization Procedure for Analysis of Desipramine in Rat Brain Tissue
1. For samples, place a weighed chunk of brain tissue in a vial. Add an appropriate amount of deionized water to
achieve 0.1 g tissue/mL water.
2. For standards and quality control samples, place a weighed chunk of brain tissue in a vial. Spike an appropriate
volume of 10 mg/mL stock solution of desipramine and deionized water to achieve 0.1 g tissue/mL water.
3. Homogenize at least 300 mg blank tissue in 3 mL deionized water to obtain enough blank matrix for preparation of
the standard curve, quality controls, and blanks.
4. Homogenize samples, initial working standard and quality controls for 3-5 minutes, cleaning the probe after each.
5. Dilute the working standard and quality control with blank homogenate to obtain standards and quality controls over
the desired dynamic range. Vortex well.
6. Mix a 100 µL aliquot of samples, standards, quality control and blanks with 200 µL of 200 ng/mL haloperidol
(internal standard) in acetonitrile (protein precipitation sample extraction).
7. Vortex mix for 5 minutes, centrifuge at 4,000 RPM and 4 °C for 5-10 min.
8. Transfer the supernatants and inject onto the LC-MS/MS system.

249
Digestion Techniques
Mechanical techniques might do little to disrupt cellular structure and extract analytes from non-vascularized or low-water-content
tissues such as bone, cartilage or hair. Extreme measures such as digestion with strong acid (e.g. 12 N Hydrochloric acid) are
routinely used for DNA or nucleic acids, which can tolerate the harsh conditions. Alternatively, certain enzymes can be used to
digest tissue samples.
Commercial devices are available which contain digestion bombs fabricated from material resistant to corrosive media. These
digestion bombs can also be used as containers for microwave extraction systems covered later.
Enzymatic digestion, a technique commonly used for tissue dissociation and cell harvesting of proteins and DNA, offers the
advantages of unattended sample preparation, potential automation, and low cost. A range of different enzymes is available with
different digestive properties and efficiencies. The choice of enzyme can be driven by the desired tissue or component, such as
cartilage, to be digested. Although the enzymatic digestion technique has been utilized for decades, very little has been published that
describe enzymatic digestion in tissue sample preparation for small molecules
47
.
The feasibility of enzymatic digestion has been studied as an alternate tissue preparation technique for bioanalysis of drugs
48
. Two
different enzymes known to degrade connective tissues to allow tissue dissolution were chosen for evaluation: collagenase and
proteinase K. These enzymes were selected to represent both a more conservative digestive enzyme (collagenase) and a more
aggressively digestive enzyme (proteinase K). Results indicate that enzymatic digestion has comparable extraction efficiency to
homogenization and enzymatic digestion using collagenase or proteinase K can be considered as an alternative sample preparation
method for analysis of small molecules in tissue.
Test compound levels of incurred rat brain tissue samples prepared by enzymatic digestion were in good agreement with the values
obtained by the conventional homogenization tissue preparation; indicating that enzymatic digestion is an appropriate tissue sample
preparation method
48
. A variety of other enzymes, such as trypsin, papain, or elastase might also be utilized depending on the
degree of digestive strength needed.

250
Extraction Instruments
Some of the extraction instruments used for other types of solid samples have been evaluated or at least considered for tissue
samples. However, the intended use may impact instrument design with respect to sample size. The number and size of sample
chambers or vials as well as final extract volume should be considered when using these instruments. Pressurized fluid extraction
(PFE) instruments (Chapter 14) use liquid solvents at elevated temperatures and pressures to extract analytes from solid or semi-
solid samples in very short periods of time and with small volumes of solvent. With PFE, the sample is enclosed in a stainless steel
vessel filled with an extraction solvent that is pressurized and heated. The sample is allowed to statically extract for 5-10 min, with
the expanding solvent vented to a collection vial.
Next, compressed nitrogen purges the remaining solvent into the same vial. Because the technique uses liquid solvents, it can be
applied to any application in which liquid solvents are currently used. The entire procedure typically requires less than 15 min and
approximately 15 mL of solvent for a 10 g sample
49
. The technique has been used for extraction of small molecules from animal-,
plant- and fish-tissue, food, polymers, and many types of environmental contaminants in soil.
Microwave-Accelerated Extraction (Chapter 14) has also been widely applied to solid samples as a means to speed
the extraction process. Microwave heating can drive a variety of chemical processes including acid digestion. However, the thermal
instability of most types of tissue and analytes within the tissue has limited the applicability of this technique to tissues.
A new type of extraction instrument is based on pressure cycling technology (PCT). This process uses repeated cycles of ultra-high
and ambient pressure to extract proteins and nucleic acids from tissues
50
. This new technology has been applied to the quantitative
extraction of nucleic acids, proteins, and small molecules from a wide variety of organisms including, viruses, bacteria, plant and
animal cells and tissues.

251
Considerations When Choosing
a Tissue Preparation Technique
How to choose the best tissue preparation technique for application is a difficult question to address. Sample throughput, analyte
recovery, analyte thermal stability, amount of available sample, available sample preparation techniques, precision, accuracy,
manual labor involved, and operator safety are only a few of the parameters that must be considered when selecting the optimum
sample preparation technique. A comparison of the typical preparation times required for 50 tissue samples using various
techniques is shown in
Figure 16.8
43
.
In general, the simplest approach, homogenization may be both safest and most cost-effective. However, if your laboratory will
require the analyses of dozens of tissue samples per month, investing in new technologies may be justifiable and cost-effective.
Some of the instruments used for other types of solid samples may be modified to prepare tissues. However, the scientist should
judiciously consider whether the risks (cross-contamination, manual intervention, analyte instability) are outweighed by the benefits.
Carryover between individual samples should be minimized and the technique should be compatible with the analytical method that
you will be using (i.e., GC, LC/MS, etc.). The nature of the tissue itself should also be considered, since more fibrous connective
tissues such as muscle will require more vigorous preparation than highly aqueous tissues such as cornea.
Tissue analysis presents its own set of unique challenges. A universal lack of reference material for any analyte in appropriate or
comparable matrix exists, regardless of the application. Questions around spatial distribution heterogeneity of the analyte within
the tissue matrix are rarely answered, unless an imaging technique such as autoradiography is utilized. Tissue quantitation
requires tedious sample weighing (slicing and dicing), for good accuracy and precision. Thus, techniques that mandate an exact
amount of tissue, such as ASE, will require significant time just to weigh the tissue. Although an internal standard (IS) is routinely
utilized, it is generally added as a solution to the homogenate, digest, or extract after preparation. Unfortunately no physical
means has been discovered to disperse the internal standard into the tissue matrix, and extraction efficiency of the IS in matrix
cannot be determined.
Extraction instruments for other solid samples often exhibit difficulty in scaling up or down between various devices and will require
purchasing sample containers specific to the sample weight range. Correlation of extraction efficiency of the analyte between
analytical standards and samples is always incomplete and often impossible. However, despite these challenges, the scientist has a
choice between tried and true methods such as homogenization and more novel yet cost-effective approaches including enzymatic
digestion and 96-well bead beater apparatus.
252
0510 15 20 25
Figure 16.8
Comparison of Total Extraction Times
for Various Sample Preparation Methods
Enzymatic Digestion
Bead Beater
ASE
Homogenizer
Sonication
Grind & Lyophilize
Weigh
Homogenize
Sonicate
Bead Beat
Enzymatic Digestion
Centrifuge
Extraction
Evaporation
Reconstitution
A sample set of 50 was used for calculations. Times shown are typical and independent of tissue type.
Conclusions
Although traditional biological sample preparation methods have been carried out for years, the demand for increased productivity,
faster and high-throughput assays has driven investigation of new technologies. Compared to these traditional approaches, these
new techniques are expected to be faster and provide at least equivalent, if not superior, reproducibility and analyte extraction
efficiency. Automation using the 96-well plate format has helped to increase overall productivity and more and more sample
preparation techniques (e.g. liquid-liquid extraction, supported liquid extraction, filtration, protein precipitation, etc.) have gone to
this format. For liquid samples such as whole blood, plasma and urine, transfer of manual methods to automated methods can be
fairly easily carried out. In the area of tissue sample preparation, users have many choices from conventional homogenization or
sonication to newer technologies such as enzymatic digestion and pressure cycling technology. These techniques should be
compared in terms of speed, cost, and degree of automation.

253
(Continued)
References
1.
Morello, R.; Milokovic, J.; Boos, K.-S.
Therapeutic Drug Monitor
2007,
29
, 505.
2.
Berger, I.; Morello, R.; Boos, K.-S.
LC-MS/MS Analysis of Drugs in Whole Blood: A Unique Solution for Total Automation and
Undisturbed Detection
, Poster P-527-W, presented at HPLC 2010, Boston, MA, June 19-24, 2010.
3.
Berger, I.; Morello, R.; Kinzig, M.; Boos, K.-S.
LC-MS/MS Analysis of Immunosuppressants in Whole Blood: Comparison of Dried
Blood Spots, Heat Shock or Cryogenically Treated Blood and Denatured Blood
, Poster P-528-M, presented at HPLC 2010, Boston,
MA, June 19-24, 2010.
4.
Bang, I.
Ein Verfahren zur Mikrobestimmung von Blutbestandteilen, Biochem. Ztschr.
1913,
49
, 19-39.
5.
Guthrie, R.; Susi, A.
Pediatrics
1963,
32
, 338-343.
6.
Bowen, C.
American Laboratory
2010,
42 (10)
, 11-14.
7.
Evans, C.A.
The Application of Dried Blood Spots for Quantitation of Xenobiotics – A Paradigm Shift within Pre-Clinical DMPK
,
The Delaware Valley Drug Metabolism Discussion Group, February 11, 2010.
8.
Patrone, L.M.
Direct Comparison of DBS Analysis to Plasma and Whole Blood Analysis in Toxicokinetic Studies of Rats, EBF
Workshop Connecting Strategies on Dried Blood Spots
, Brussels, Belgium, June 17-18, 2010.
9.
Spooner, N.; Lad, R.; Barfield, M.
Anal. Chem.
2009,
81 (4)
, 1557-1563.
10.
Abu-Rabie, P.; Spooner, N.
Anal. Chem.
2009,
81 (24)
, 10275-10284.
11.
Saussereau, E.; Lacroix, C.; Gaulier, J.M.; Gouille, J.P.
J. Chromatogr. B
2012,
885-886
, 1-7.
12.
Majors, R.E.
LCGC No. America
2011,
29 (1)
, 14-27.
13.
Biddlecombe, R.A.; Pleasance, S.
J. Chromatogr. B
, 1999,
734 (2)
, 257-265.
14.
Li, J.; Wang, L.; Chen, Z.; Xie, R.; Li, Y.; Hang, T.; Fan, G.
J. Chromatogr. B
2012,
895-896
, 83-88.
15.
Dawes, M.L.; Gu, H.; Wang, J.; Schuster, A.E.; Haulenbeek, J.
J.Chromatogr. B
2013,
934
, 1-7.
16.
Makkar, H.P.S.
J. Agric. Food Chem.
1989,
37 (4)
, 1197-1202.
17.
Oliveira, R. V.; Cass, Q.B.
J. Agric. Food Chem.
2006,
54 (4)
, 1180-1187.
18.
Wa, C.; Maltik, R.; Hage, D.S.
Anal. Chem.
2008,
80 (22)
, 8751-8762.
19.
Santos-Neto, A.J.; Markides, K.E.; Sjoberg, P.J.R.; Bergquist, J.; Lancas, F.M.
Anal. Chem.
2007,
79 (16)
, 6359-6367.
20.
Willemsen, O.; Machtejevas, E.; Unger, K.K.
J. Chromatogr. A
2004,
1025 (2)
, 209-216.
21.
Rbeida, O.; Christiaens, B.; Hubert, Ph.; Lubda, D.; Boos, K.-S.; Crommen, J.; Chiap, P.
J. Chromatogr. A
2004,
1030 (1-2)
, 95-102.
22.
Herman, J.L.; Edge, T.
LCGC No. America
2012,
30 (3)
, 200-214.
23.
Xin, G.-Z.; Zhou, J.-L.; Qi, L.-W.; Li, C.-Y.; Liu, P.; Wen, X.; Li, P.
J. Chromatogr. B
2010,
878 (3-4)
, 435-441.
24.
Bunch, D.R.; Heideloff, C.; Ritchie, J.C.; Wang, S.
J. Chromatogr. B
2010,
878 (31)
, 3255-3258.
25.
Mottier, P.; Hammel, Y.-A.; Gremaud, E.; Guy, P.A.;
J. Agric. Food Chem.
2007,
56 (1)
, 35-43.

254
(Continued)
References
36.
Pagel, P.; Schubert, R.; Wolf, H.W.
J. Chromatogr. B
2000,
746
, 283-295.
37.
Zolotarjova, N.; Mrozinski, P.; Majors, R.E.
LCGC No. America
2007,
25 (2)
, 118-140.
38.
Martosella, J.; Zolotarjova, N.; Liu, H.; Nicol, G.; Boyes, B.E.;
J. Proteome Res
2005,
4 (5)
, 1522-1537.
39.
Zhang, K.; Zolotarjova, N.; Nicol, G.; Martosella, J.; Yang, L.-S.; Czafranski, C.; Bailey, J.; Boyes, B.
Agilent Multiple Affinity Removal
System for the Depletion of High-Abundant Proteins in Human Serum – A New Technology from Agilent
, Agilent Application
Note #5988-9813EN, 2003.
40.
Brand, J.; Haslberger, T.; Zolg, W.; Pestlin, G.; Palme, S.
Proteomics
2006,
6
, 3236-3242.
41.
Shen, Z.; Want, E.J.; Chen, W.; Keating, W.; Nussbaumer, W.; Moore, R.; Gentle, T.M.; Siuzdak, G.
J. Proteome Res.
2006,
5
, 3154-3160.
42.
Chang, M.
Automated Sample Preparation by Protein Precipitation for High-Throughput Bioanalysis
, Agilent Technologies
Application Note #5990-9030EN, September, 2011.
43.
Yu C.; Cohen, L.H.
LCGC No. America
2003,
21 (11)
, 1038-1048.
44.
Barker, S.A.; Long, A.R.; Short, C.R.
J. Chromatogr.
1989,
475
, 353-361.
45.
Barker, S.A. LCGC,
Current Trends and Developments in Sample Preparation
May, 1998,
S37-S40
.
46.
Lagana, A.; Bacaloni, A.; Castellano, M.; Curini, R.; De Leva, I.; Faberi, A.; Materazzi, S.
J.A.O.A.C.
2003,
86 (4)
, 729-736.
47.
Posyniak, A.; Zmudzki, J.; Semeniuk, S.
J. Chromatogr. A
2001,
914
, 89-94.
48.
Yu, C.; Penn, L.D.; Hollembaek, J.; Li, W.; Cohen, L.H.
Proc. 51st Annual Conference American Society of Mass Spectrometry
and Allied Topics, ThPM 257
, 2003.
49.
Draisci, R.; Marchiafava, C.; Palleschi, L.; Cammarata, P.; Cavalli, S.
J. Chromatogr. B
2001,
753
, 217-223.
50.
Schumacher, R.T.; Manak, M.; Gerret, P.; Miller, W.; Lawrence, N.; Feng, T.
American Laboratory
2002,
34 (16)
, 38-44.
26.
Mansson, M.; Phipps, R.K.; Gram, L.; Munro, M.H.G.; Larsen, T.O.; Nielson, K.F.
J. Nat. Prod.
2010,
73 (6)
, 1126-1132.
27.
Wang, L.-Q.; Zeng, Z.-L.; Su, Y.-J.; Zhang, G.-K.; Zhong, X.-L.; Liang, Z.-P.; He, L.-M.
J. Agric. Food Chem.
2012,
60 (25)
, 6359-6363.
28.
Reedy, C.R.; Hagan, K.A.; Strachan, B.C.; Higgonson, J.J.; Bienvenue, J.M.; Greenspoon, S.A.; Ferrance, J.P.; Landers, J.L.
Anal.
Chem.
2010,
82 (13)
, 5669-5678.
29.
Bertini, S.; Bislo, A.; Torri, G.; Bensi, D.; Terbojevich, M.
Biomacromolecules
2005,
6 (1)
, 168-173.
30.
Hu, L.; Li, X.; Jiang, X.; Zhou, H.; Jiang, X.; Kong, L.; Ye, M.; Zou, H.
J. Proteome Res.
2007,
6 (2)
, 801-808.
31.
Lindemann, T.; Hintelmann, H.
Anal. Chem.
2002,
74 (18)
, 4602-4610.
32.
Mandal, B.K.; Suzuki, K.T.; Anzai, K.; Yamaguchi K.; Sei, Y.
J. Chromatogr. B
2006,
874 (102)
, 64-76.
33.
Bowsher, R.R.; Santa, P.G.
J. Chromatogr. B
2009,
877 (8-9)
, 689-696.
34.
Zhou, W.; Bi, J.; Janson, J.-C.; Li, U.; Huang, Y.; Zhang, U.; Su, Z.
J. Chromatogr. B
2006,
838 (2)
, 71-77.
35.
Svensson, J.-O.; Barkholt, L.; Sawe, J.;
J. Chromatogr. B
1997,
690 (1-2)
, 363-366.

255
Chapter 17
Sample Pre-treatment
for LC/MS
Mass spectroscopy is considered to be a high resolution, sensitive detection technique due to its specificity and low limits of
detection. Proper sample preparation and HPLC operation can ensure that it will deliver the highest level of qualitative and
quantitative compound measurement. Each year, mass spectrometers become even more sensitive, bringing about better answers
to pressing problems in its applications areas, but an additional burden is placed on the quality of the effluent coming into the
source. Although the sample pre-treatment step takes place prior to HPLC detection, there are requirements to present the sample
to the liquid chromatograph that permits a mass spectrometer to provide its highest level of performance. Sample preparation is an
integral, but sometimes neglected part, of a successful mass spectrometric experiment. The widespread adoption of LC-MS/MS as
a major technique for bioanalysis, proteomics, food safety, and other markets requiring high sensitivity and high-throughput analysis
originally led to a de-emphasis on the importance of sample preparation. At one time, it was believed that the sample
prep/chromatography step could be dispensed with! It is naïve to assume that the MS alone will be able to solve all separation
problems, but it is better that the systems work in harmony and that one understands where sample preparation can aid in
providing better mass spectra. Thus, observation of unseen “matrix” effects in LC/MS and LC-MS/MS has led researchers to
rethink the importance of sample preparation in successful use of this powerful technique.
The highest level of uncertainty associated with an LC/MS method will likely come from the sample matrix since it may contain
every known type of interference. Among the factors that the sample and its matrix (as well as the mobile phase) can affect are:
• Spectral interference
• System compromise
• Adduct formation
• Ion suppression

256
Sample interferences must be identified, removed, reduced – or at a minimum accounted for – in the LC/MS method.
Spectral interference: Ions that appear at the same or nearly the same m/z value as the component of interest can cause
spectral interference. Ions of similar m/z value can generally be separated by the chromatographic process prior to entering the MS.
In situations in which it is impossible to separate the ions first, say on a lower resolution MS system and very complex samples and
matrices, analysts can use multiple reaction monitoring (MRM) with an MS-MS system to make a clear distinction between the
ions. The MS systems using a time-of-flight analyzer are generally able to distinguish much better than unit mass resolution and,
therefore, can pull apart spectral peaks that overlap in lower resolution systems. Thus, the use of lower resolution MS systems will
invariably need better sample cleanup than higher resolution MS systems that can distinguish between compounds with very close
m/z values.
Thus, using the techniques described in Table 15.1 to remove matrix interferences such as salts, ion pair reagents, and proteins
may help to keep the ion source clean and contaminant-free. Some of the new orthogonal ionization sources can tolerate some
buildup of contaminants, but nevertheless it is wise to remove undesired components before passing them into the MS source.
System compromise: Mobile phase components – or sample components that degrade the performance of the LC/MS system –
generally through precipitation in the LC/MS interface, can compromise the system. The components that are most likely to
precipitate in the atmospheric-pressure- or electrospray-ionization source are buffer salts and ion pair reagents. Substituting volatile
buffers or simply adjusting the pH with a volatile acid such as formic acid or base such as ammonium hydroxide will solve this
problem. Trifluoroacetic acid (TFA) is a popular HPLC mobile phase additive for peptide and protein separations since it gives
excellent peak shape. However, it is notorious for causing ion pairing interactions and many workers prefer to use formic acid for a
better MS signal intensity at the expense of poorer chromatographic peak shape. Sample matrix elements such as proteins can also
precipitate in the source. Proteins can be removed by precipitation, dialysis, SPE, size exclusion chromatography, immunoaffinity,
and other approaches (see Chapter 16, Protein Removal). Lipids, particularly phospholipids, can cause havoc in the mass
spectrometer; new titania and other SPE sorbents can specifically remove these culprits (see Chapters 9, 10, and 16). In MS-MS
using MRM, a signal at m/e of 184 usually indicates phospholipid contamination and it is a good idea, if they can’t be removed by
sample preparation techniques, to chromatographically resolve these compounds from your analyte peaks.

257
Adduct formation: Adduction of another ion with the component of interest shifts the m/z value at which the component of
interest appears in the spectrum. Adduct ions such as sodium, potassium, and ammonium can be picked up from the sample
itself, from reagents, or even from the container holding the sample. Sodium ions from sodium borosilicate glassware is an
example of the latter. Adduct formation can be used as a way to improve signals for macromolecules. However, uncontrolled
adduct formation generally is undesirable and requires specific sample preparation procedures to reduce or eliminate it.
Techniques for the removal of ions were covered previously in Table 15.1.
Ion suppression/ion enhancement: Unextracted matrix compounds may co-elute with the analytes of interest and end up in
the ionization chamber of the mass spectrometer. Ion suppression is the result of those components that suppress the ionization of
or compete in the ionization process with the component of interest. It results from the presence of less volatile compounds that can
change the efficiency of droplet formation or droplet evaporation, which in turn affects the amount of charged ion in the gas phase
that ultimately reaches the detector. Ion suppression is the most critical of these interferences because it is often the most difficult to
determine. Even components that do not appear in the mass spectrum can cause ion suppression. The electrospray detector is
strongly affected by the presence of certain co-eluting compounds. Ion suppression effects are most noticeable when trace analytes
are in the presence of complex matrices such as biological fluids. Besides complex buffers and detergent systems commonly used in
biological samples, materials shown to cause ion suppression include salts, ion pairing reagents, endogenous compounds such as
lipids, sugars, and nucleic acids, as well as drugs, metabolites, and proteins. In biological samples, natural variation in endogenous
compound concentrations from one sample to another can cause varying levels of ion suppression. The variation in turn contributes
to unacceptable variability in the signal response for the compounds of interest. Most often a loss in response occurs; hence the
term ion suppression is generally used. Ion suppression effects impact reproducibility and signal strength. In some cases, an
increasing response of the desired analyte may occur; ion enhancement or a stronger-than-expected signal results. Atmospheric
pressure chemical ionization detectors are also affected by ion suppression, but to a lesser extent than the electrospray detector.
However, for both qualitative and quantitative LC/MS work with API interfaces, understanding and eliminating ion suppression and
enhancement effects is essential.
The presence of ion suppression can be determined by the use of infusion. The infusion experiment involves the continuous introduction
of the standard solution containing the analyte of interest and its internal standard by means of the syringe pump connected to the
column. After injecting a blank sample extract into the LC system, a drop in constant baseline indicates suppression in ionization of the
analyte due to the presence of the interfering material.

258
Although beyond the scope of this chapter, there are a number of strategies for reducing ion suppression. Among them are
changing the ionization mode, such as switching from positive ionization to negative ionization, sample dilution or volume
reduction, reducing the flow rate, improving chromatographic selectivity, or performing better sample preparation. In the latter
case, many of the sample prep techniques covered in this book, such as SPE or liquid-liquid extraction, or even additional
techniques may be required to provide the best possible spectral data. The use of formic acid, rather than trifluoroacetic acid, in
the HPLC mobile phase can also help. For more information, a simple discussion of ion suppression effects and their elimination
was published earlier
1
. For further information on sample preparation for LC/MS and LC-MS/MS, a review article
2
, a textbook
3
,
and LC/MS textbooks
3-10
are available.
The most common ions formed in API/MS are protonated molecules, symbolized [M+H]
+
. Similarly, deprotonated molecules,
[M-H]
−
, may be seen in negative ion operation. Formation of these molecules takes place through ion evaporation in electrospray
and through gas-phase chemical ionization in APCI. Understanding these reactions is the basis for understanding the origin of ion
suppression effects. In positive ion operation, the gas phase ion-molecule reactions will result in formation of the weakest acid,
i.e. the weakest proton donor. For example, in the APCI analysis of the amine R-NH
2
, water is a stronger proton donor than the
amine and therefore readily gives up its proton from H
3
O
+
to form the R-NH
3
+
ion. However, if we now introduce a large amount
of another compound which can form an even weaker acid than the analyte (e.g. R
3
N), then the gas-phase reaction will continue
on to form R
3
NH
+
, the weaker acid. The R-NH
2
analyte will not be ionized or will be poorly ionized, and therefore not seen at
significant levels in the mass spectrum. These same types of proton transfer reactions can take place in electrospray interfaces.
An analogous model can be developed for negative ion operation where the weakest proton acceptor (weakest base), is formed.
Another type of ion suppression is thought to occur when very strong ion pairs are formed which are not broken apart by the
conditions in the API interface. Ion pairing agents of various types have been shown to contribute to ion suppression and therefore
their use in LC/MS should be avoided where possible.
The bottom line is that proper sample preparation can improve the performance for LC/MS despite the higher level of resolution
that this detection technique brings to the analysis. The increasing levels of sensitivity and selectivity in modern mass spectrometry,
especially the tandem techniques, have had a profound effect on analysis in both the gas and liquid phase. For more information,
the reader is directed to Chapter 21, which addresses the balancing of achieving selectivity in analysis (separation and detection)
and introduces the concept of “Just Enough” sample preparation.

259
Solvents and Volatile Buffers
Used in Mass Spectrometry
Although sample preparation precedes chromatographic separation, it is important to understand which solvents and buffers may be
used in sample prep techniques, since – unless exchanged or removed – these additives may be injected into the LC/MS system and
may impact the ionization of analytes of interest. Tables 17.1 and 17.2 provide a listing of solvents and volatile buffers compatible
with MS, respectively. Non-volatile buffers should be avoided since they may foul inlet systems and cause problems with MS sources.
Normal concentration ranges for buffers should be 1-10 mM.
Although TFA is very useful as an HPLC mobile phase additive providing excellent peak shapes for basic compounds, it is known to
suppress ionization in electrospray LC/MS, leading to lower sensitivity, and should be avoided. Formic acid is a better alternative.
Table 17.1
Typical LC/MS Solvents
Solvent Formula MW (Da) Boiling Point (°C) UV Cutoff (nm)
Acetonitrile CH
3
CN 41.05 81.6 190
Chloroform CHCl
3
119.38 61.7 245
Dichloromethane CH
2
Cl
2
84.93 40.0 235
Ethanol CH
3
CH
2
OH 46.08 78.5 210
Ethyl acetate CH
3
CO
2
CH
2
CH
3
88.12 77.1 260
Diethyl ether (CH
3
CH
2
)
2
O 74.12 34.5 220
Heptane CH
3
(CH
2
)
5
CH
3
100.21 98.4 200
Hexane CH
3
(CH
2
)
4
CH
3
86.18 69 200
Isopropanol CH
3
CH(OH)CH
3
60.11 82.4 210
Methanol CH
3
OH 32.04 65 205
n-Propanol CH
3
CH
2
CH
2
OH 60.11 97.4 210
Tetrahydrofuran C
4
H
8
O 72.12 66 215
Toluene C
6
H
5
(CH
3
) 92.15 110.6 285
Water H
2
O 18.02 100 none

260
Table 17.2
Typical LC/MS Volatile Buffers
Volatile Buffer Structure pKa Buffer Range
Trifluroacetic acid CF
3
CO
2
H 0.5 3.8-5.8
Formic acid HCO
2
H 3.8 –
Ammonium formate HCO
2
NH
4
3.8 2.8-4.8
Acetic acid CH
3
CO
2
H 4.8 –
Ammonium acetate CH
3
CO
2
NH
4
4.8 3.8-5.8
Propionic acid CH
3
CH
3
CO
2
H 4.9 3.9-5.9
4-Methylmorpholine OC
4
H
8
N(CH
3
) 8.4 7.4-9.4
Ammonium bicarbonate NH
4
CO
3
H 6.3/9.2/10.3 6.8-11.3
Ammonium acetate CH
3
CO
2
NH
4
9.2 8.2-10.2
Ammonium formate HCO
2
NH
4
9.2 8.2-10.2
1-Methylpiperidine C
5
H
10
N(CH
3
) 10.1 10.0-12.0
Diethylamine NH
2
CH
2
CH
2
NH
2
10.5 9.5-11.5
Triethylammonium acetate CH
3
CO
2
NH(CH
3
)
3
11.0 10.0-12.0
Pyrrolidine C
4
H
8
NH 11.3 10.3-12.3

261
Small Molecules in Biological Matrices
An area of popular use of LC/MS and LC-MS/MS is in new drug discovery, where small molecules such as drugs and their
metabolites are separated and identified in biological fluids. As mentioned earlier, the analytical challenge is to be able to achieve
good qualitative and quantitative analysis in the presence of endogenous compounds, which may affect the MS signals due to ion
suppression. Many of the methods that we have discussed throughout the book can be used to eliminate or reduce undesirable
interferences from plasma, urine, CSF, etc.
The traditional method for extracting drugs from biological fluids is liquid-liquid extraction (LLE, Chapter 7). With proper method
design, LLE can be very selective and result in very clean extracts. Some degree of automation is possible, more recently using
supported liquid extraction in 96-well plates, but generally LLE has been a manual or semi-manual procedure. In recent years, solid
phase extraction (SPE) has grown to become as widely used as LLE. For plasma and other biological samples, experimental design
usually calls for retention of the drug/metabolite on the SPE phase while the proteins and other endogenous components are
washed out. The drugs/metabolites (and impurities retained on the sorbent) are washed off with a different solvent in a second
elution step. SPE has considerable advantages over LLE in terms of sample size required, the amount of solvent used, and most
importantly, the ability to be used in fully automated, on-line systems. Many of these systems use the same 96-well format plates.
Compared to LLE, SPE requires more steps in developing and optimizing an SPE method. For biological fluids, if a method is properly
developed and optimized, SPE extracts are usually as clean as LLE extracts, but selectivity may be limited unless a selective phase
can be found and fines/particulates from the extraction column may plug the LC column or MS interface, although this is less of a
problem today than a decade or two ago. Protein precipitation has become popular since it requires less method development than
either LLE or SPE, but the extracts are not as clean as these time-tested techniques. However, as long as ion suppression is
minimized for analytes of interest by having good chromatography, LC-MS/MS of protein-precipitated extracts provides decent
qualitative and quantitative analysis.
The most recent advance in SPE phases has been the creation of mixed mode sorbents, both silica- and polymer-based.
Development of materials which exhibit hydrophobic and hydrophilic character are driven by the demand for a “universal” SPE
phase which can be used with one or a few extraction methods for a wide variety of compounds. Polymeric sorbents in this
category are able to withstand pH ranges from 1-14, and this – along with their bimodal character – provides a flexible platform for
extraction of acidic, basic, and neutral drug compounds from plasma, serum, and urine using generic methods.

262
In the quest for even higher throughput, the natural direction for investigators to go is toward direct injection of biological
fluids. Two approaches that have arisen in this are: restricted access media (RAM, see Chapter 10) and turbulent flow
chromatography
10
. Many restricted access media chemistries are bimodal, like the sorbents described above. The mechanism
of restricted access depends on excluding macromolecules from the retentive surface of the SPE particle either by physical or
chemical means or a combination of the two. Essentially, restricted access media allow small molecules to penetrate the pores
in the particles and therefore come in contact with the reversed-phase portion of the sorbent where they are retained.
Macromolecules such as serum proteins are excluded from the pores and remain in the external portion of the phase, which
has a biocompatible or hydrophilic chemistry. Proteins are not denatured or retained by this portion of the sorbent and are
therefore washed out with a high aqueous solvent in the solvent front. To prevent the proteins from being directed to the HPLC
column, column switching (see Chapter 13) is used to direct them to waste. The small molecules of interest are then eluted
from the RAM with increasing amounts of strong solvent.
Turbulent flow chromatography is a technique requiring a special column to which sample is applied at high flow rates of
4-8 mL/min of water
11
. Small molecules are retained on the column, and at these high linear velocities, plasma components
are washed off to waste. Flow is then switched to an aqueous/organic elution solvent and compounds of interest are eluted
into the MS interface. The high flow rates allow for very rapid analyses.
Reversed-phase SPE has generally shown poor recovery for some basic drugs that may bind to residual silanols in the silica. The
mixed hydrophobic/SCX (strong cation exchange) phases have been developed to address this problem. Both polymeric- and silica-
sorbents are available with varying alkyl chain lengths and with sulfonic SCX groups. It is postulated that the drug compound and
other hydrophobic excipients are initially loaded onto the alkyl portion of the phase while highly water-soluble compounds are
washed off. Aqueous/organic wash is then used to remove the hydrophobic interferences and move the drug and metabolite
compounds onto the strong cation exchanger. Finally, a very clean basic drug extract is eluted with methanol/ammonium hydroxide.

263
Both restricted access and turbulent flow columns may be used by themselves for direct elution into the MS interface or in LC/LC
mode where analytes are re-focused on an analytical HPLC column prior to the MS. This latter mode of operation is reported to be
more favorable for complex, multi-component samples since the sample preparation columns generally exhibit low efficiency and
are not capable of adequate separation of complex mixtures.
The SPE applications discussed to this point have been packed column applications. Of course, disk format SPE and solid phase
microextraction (SPME) are also applicable to analysis of drugs in biological fluids. Disks of glass fiber, PTFE, and polyvinyl chloride
have been applied to SPE. Small diameter (~10 µm) sorbent bonded phase particles are impregnated into the disk or the disk
material itself is derivatized. Small diameter particles have also been applied to packed bed formats to improve mass transfer
efficiency. Disks offer the advantage of greatly increased surface area of sorbent coupled with far less non-specific adsorption than
typical packed columns. Disk formats therefore require less sample to be applied, smaller volumes of solvent used, and fewer steps
to clean up unwanted compounds. Very high effective flow rates through the disk also save time. Low volumes are compatible with
micro-LC and small diameter disks are quite amenable to 96-well plate format used in many current automation systems.
SPME (see Chapter 10) has been applied to GC and GC/MS analysis for years and has also been adapted to use with HPLC. SPME
relies on a solid phase sorbent coated to the outside of a fiber which is then introduced directly to the sample and allowed to extract
analyte. Compounds of interest are subsequently washed off in the same manner as standard SPE. SPME fibers will only adsorb
about 20% of the analyte in the sample, but will inject this entire amount into the chromatograph. This is by comparison with
standard SPE, which may adsorb 90% of the analyte available, but without a preconcentration step, will only inject a few percent
of that amount.
The “in-tube” SPME for LC/MS has been applied to the analysis of drugs in urine and serum (see Chapter 10). This method uses an
open tubular GC capillary in the place of the fiber. A small volume, on the order of 30 µL of filtered serum sample, is repeatedly
drawn back and forth through the capillary, allowing drugs to extract into the stationary phase. The capillary is then switched in-line
with the LC mobile phase and LC/MS analysis of the desorbed drugs proceeds. The “in-tube” procedure for LC-SPME is low-cost
and easily automated. Good recoveries and LOQs have been demonstrated for the low in vivo concentration levels of b-blocker
drugs and metabolites evaluated.

264
Immunoaffinity sorbents (see Chapter 10) have also received considerable attention in the LC/MS community. These sorbents are
based on immobilized antibodies which retain compounds of interest by molecular recognition. Subsequent denaturation of the
antibodies releases the analyte. The “classic” affinity column is based on Sepharose, which is not compatible with the high
pressures or flow rates needed for on-line automation of LC/MS. However, HEMA (polyhydroxyethyl methacrylate) supports,
which are more capable of withstanding HPLC conditions, have been used. Affinity techniques have recently come into use for
environmental analysis where small amounts of pollutants, e.g. pesticides, are specifically isolated from moderate to small sample
volumes. Similarly, the technique has been applied to determine drugs that occur at low levels in biological fluids.
MS/MS has become a well-accepted technique for determination of low levels of drugs and metabolites in biological fluids.
MS/MS has the advantage of excellent sensitivity for quantitation (especially with triple quadrupole instruments), excellent
throughput, and very high specificity. In procedures called selective reaction monitoring (SRM) and multiple reaction monitoring
(MRM), an ion (or ions) can be selected in the second stage of MS, which is characteristic of the analyte(s) only.
The second stage of MS is another stage of separation and therefore may allow a somewhat “dirtier” initial sample to be
introduced. If the analyte co-elutes with another compound which produces ions of the same m/z value, selection of this ion for
subsequent MS/MS will often result in production of a fragment ion which is characteristic of the analyte, but not the co-eluting
interferent. This fragment ion is then used for quantitation and qualitative confirmation of identity.
MS/MS however is not an ionization technique. Its advantages begin after ion formation. Therefore, MS/MS users must still be
concerned with ion suppression and components such as salts, which may compromise system performance. In addition, adduct
formation will not be prevented by the use of MS/MS. However, the complexity of interpreting an adduct ion spectrum may be
reduced if all adduct ions produce a common fragment ion. A full scan of the spectrum in the initial stage of MS will capture all
adduct ions for fragmentation and analysis by the second mass analyzer. The second MS may then be set to pass only the
characteristic fragment ion.

265
The development of electrospray LC/MS interfaces, which are capable of putting large macromolecules into the MS, has had a
tremendous impact on the study of proteins and nucleic acids. Molecular weight determinations, sequence, and structural
information are available directly from electrospray MS spectra. It is beyond the scope of this chapter to describe these applications
but references are available
3-9
.
During method development for intact biological macromolecules, the same concerns about interferences discussed for small
molecule applications must be considered. However, at this time, laboratories are not so concerned with putting thousands of
samples through in a day as are the labs working on new drug discovery. Instead, these workers are more concerned with
complete characterization of the molecule, often from very small amounts of starting material. Therefore, sample preparation
methods must show good recoveries and deliver clean extracts. Micro sample preparation methods coupled to Micro-LC or CE
are required in many cases. Studies on biomarkers for cancer and other diseases and evaluation of peptide fragments from
enzymatically-degraded proteins are other areas where LC/MS and LC-MS/MS are widely used. Chapter 15 provided examples
of these types of applications.
For protein analysis (see Chapter 15), traditional purification procedures are often used prior to LC/MS analysis. Hydrophobic
interaction chromatography, size exclusion, ultrafiltration, and HPLC have all been used to separate proteins for analysis. SPE
methods on reversed-phase and ion exchange sorbents are widely used and give good cleanup, especially for removal of salts and
buffers, which contribute to ion suppression effects and adduct formation. Naturally, many SPE and chromatographic procedures
have the potential for on-line, automated operation.
While not strictly a “sample preparation” issue, certain mobile phase components must also be considered when developing
LC/MS methods for biomolecules. The same concerns for non-volatile buffer salts and ion pairing agents, described earlier, is one
consideration. Another is ion suppression problems encountered in use of trifluoroacetic acid (TFA) in peptide/protein analysis by
electrospray. TFA is thought to improve peak shape in reversed-phase chromatography of peptides by ion-pairing with basic sites on
the molecule thereby eliminating mixed retention mechanisms. However, TFA-Peptide ion pairs are not broken up in the interface
and this prevents ionization of the protein. The problem can be reduced by limiting the concentration of TFA to under 0.1% or using
“TFA light”, which is a reduced amount of TFA – perhaps 0.05% – and the same concentration of acetic acid. This serves to control
pH and promote protein ionization, as required for electrospray, without forming an abundance of ion pairs. The LC peak shape for
the peptides will be degraded, but this may not adversely affect the mass spectra. In some cases, however, preservation of the LC
peak shape and separation may be necessary and the amount of mobile phase TFA cannot be reduced. For these instances, a post
column modification of the mobile phase is needed. A mixing tee is placed after the HPLC column, which allows a small pump to be
used to add solvent to the mobile phase prior to its entry into the MS interface. The “TFA Fix” involves pumping in 20% propionic
acid in isopropanol at about half the flow rate from the column. It is believed that in the gas phase of the API interface, the
propionic acid replaces the TFA in the ion pair and this pair subsequently breaks down, freeing the analyte ion.
Biological Macromolecules

266
Adduct formation in both proteins and nucleic acids can seriously compromise the utility of the mass spectrum by spreading the
total ion current over numerous peaks arising from [M + nX]n
+
. “X” is usually Na
+
and K
+
ions, which readily form adducts and are
present in the chemical environment from glassware, high purity solvents, and even solid phase sorbent media. Reversed-phase
SPE or HPLC generally works well for removing the highly water soluble salts prior to introduction into the MS. Cation exchange and
precipitation procedures have also been applied for desalting. For oligonucleotides, addition of a strong base such as piperidine
reduces sodium adduct signals.
These types of cleanup procedures require relatively large amounts of sample. This can be a problem in biotechnology research,
particularly for polynucleotides in which cation adduction increases considerably with molecular weight limiting both sensitivity
and the size of molecule that can be analyzed. An approach to this problem is an on-line, microdialysis system that effectively
eliminates cation formation
10
. The aqueous dialysis buffer has the additional benefit of maintaining the biopolymers in their
native conformation.
Among the most widely used protein separation technique is two-dimensional gel electrophoresis (see Chapter 15). Complex
mixtures of proteins are first separated on a polyacrylamide gel on the basis of their isoelectric point and then separated at 90º
to the initial separation direction on the basis of molecular size in the presence of a denaturing detergent (usually sodium
dodecylsulfate, SDS). Finally, proteins are visualized with an in-gel staining procedure. Upwards of 1,000 protein “spots” may
be separated on a standard gel.
SDS-PAGE can determine protein molecular weight to within 5-10% accuracy. Modern electrospray- TOF mass spectrometry can
determine protein molecular weights to within 100 ppm, which often translates to < 1 Da accuracy. Furthermore, MS can tell us
about the sequence, cystine crosslinkages, location and type of post-translational modifications, or tertiary structure of the protein.
Therefore, removing proteins from gels for LC/MS analysis has been one focus of protein researchers.
It is essential to remove the SDS detergent and the stain from the protein prior to MS analysis or severe ion suppression and
interference problems will arise. Procedures for electroblotting of proteins from gels onto membranes and extraction directly from the
gel have been described. Gel separated proteins are further prepared by enzymatic cleavage with, for example, trypsin. The “mass
maps” subsequently generated by LC/MS analysis can be submitted to databases for identification of the native protein. MS can also
be used to determine primary sequence of unknown peptide fragments.
An MS tool for protein analysis is nanoelectrospray, a very low volume, low flow version of electrospray in which sample can be
infused to the MS for long periods of time to obtain very high S/N values and consequently low detection limits, high m/z accuracy,
and high resolution. In a further example of the blurring of the line between chromatography and sample preparation, a nano LC
column is placed directly in line with the capillary inlet to the nanoelectrospray system. Both strong solvent elution of all proteins at
once and gradient elution to provide some degree of initial separation are possible. Using an immobilized trypsin “micro-digestion”
column in line with nanoelectrospray allows for production of MS tryptic maps without the off-line digestion step. Both of these
methods are highly automatable and yield femtomole detection limits.

267
LC/MS and LC-MS/MS are powerful tools that have seen an increased use in all areas of analytical chemistry. This impact is being
seen particularly in the analysis of samples from biological matrices. To optimize the data obtained from these analyses, it is
essential for the analyst to understand and deal with mass spectral interferences arising from the sample itself, the mobile phase, or
the environment. Fortunately, chemists today have a wide variety of sample preparation strategies available to them to deal with
these issues. Today, when designing a sample prep-liquid chromatography-mass spectrometry system, one must think about the
entire workflow and use methodology that eliminates possible contaminants such as polyethylene glycol (PEG)-based detergents
or non-volatile salts early in the planning.
Newer, more efficient, and more highly automated sample prep procedures for LC/MS and LC-MS/MS are the topic of vigorous
research. As these new automated procedures come to market, the distinction between that part of the analysis called
“chromatographic separation” and the part termed “sample preparation” will become less important and will be replaced with
a concept of complete LC/MS system integration. Solid phase extraction will continue to grow in importance because of its
similarity to HPLC and the very high degree of automation possible with the technique. Paralleling this development will
continue miniaturization of the entire analytical system, including sample preparation, perhaps to chip-based, microfluidic
formats. Techniques such as SPME, microdialysis, and nano LC lend themselves very well to the “instrument-on-a-chip” concept
in which a micro autoinjector will be integrated with a sample preparation technology, chromatographic column, and MS
capillary interface in a single micro-machined device which attaches directly to an API-MS instrument optimized for sub-
microliter flow rates and sample volumes.
Conclusion

268
References
1.
Klink, F.
LCGC
1999,
17 (12)
, 1084-1093.
2.
Hess, S.
Current Trends in Mass Spectrometry, Supplement to LCGC No. America
July, 2013, 12-17.
3.
Ivanov, A.R.; Lazarev, A.V.
Sample Preparation in Biological Mass Spectrometry
, Springer, New York, 2011,
ISBN 978-94-007-0758-0 and e-ISBN 978-94-007-0828-0.
4.
Urban, J.; Vanek, J.; Stys, D.
Systems Theory: in Liquid Chromatography – Mass Spectrometry
, LAP LAMBERT Academic
Publishing, Saarbruecken, Germany, 2012, ISBN-10: 3659298166 and ISBN-13: 978-3659298165.
5.
Lipton, M.S.; Pasa-Tolic, L.
Mass Spectrometry of Proteins and Peptides: Methods and Protocols
, 2nd Ed., Humana Press, New
York, 2009, ISBN-10: 1934115487 and ISBN-13: 978-1934115480.
6.
Ferrer, I.; Thurman,
E.M. Liquid Chromatography/Mass Spectrometry, MS/MS and Time of Flight MS: Analysis of Emerging
Contaminants
, American Chemical Society, Washington, D.C., 2003, ISBN-10: 0841238251 and ISBN-13: 978-0841238251.
7.
Brown, M.A.
Liquid Chromatography/Mass Spectrometry: Applications in Agricultural, Pharmaceutical, and Environmental
Chemistry
, American Chemical Society, Washington, D.C., 1989, ISBN-10: 0841217408 and ISBN-13: 978-0841217409.
8.
Niessen, W. M.A.
Liquid Chromatography-Mass Spectrometry
, 3rd Ed., CRC Press, New York, 2006,
ISBN-10: 0824740823 and ISBN-13: 978-0824740825.
9.
Ardrey, R.E.
Liquid Chromatography – Mass Spectrometry: An Introduction
, Wiley, Chichester, W. Essex, U.K., 2003,
ISBN-10: 0471498017and ISBN-13: 978-0471498018.
10.
Sparkman, O.D.; Klink, F.E.
Liquid Chromatography Mass Spectrometry Fundamentals and Applications – An ACS Short Course
,
American Chemical Society, Washington, D.C., 1999.
11.
Herman, J.L.; Edge, T.
LCGC No. America
2012,
30 (3)
, 200-214.

269
Chapter 18
Membrane Techniques
in Sample Preparation
Membranes are usually made from synthetic polymers (e.g., PTFE, nylon, or polyvinylchloride), cellulose, or glass fibers.
Filtration (Chapter 5) and solid phase extraction (SPE) with disks (Chapter 9) represent major applications of membranes for
sample preparation. In general, membrane separation techniques have not been widely used for other chromatographic sample
preparation tasks. However, ultrafiltration, reverse osmosis, dialysis, microdialysis and electrodialysis are examples of techniques
that use membranes for concentration, purification, and separation of analytes, especially in the biological world.
Dialysis and Membrane Sample Preparation
Microporous semi-permeable membranes allow the passage of certain compounds and not others. These membranes permit
selective filtration because of the size of their micropores. For example, for separating large macromolecules such as proteins from
small molecules (i.e. drugs or drug metabolites), dialysis uses a microporous, molecular-weight cutoff membrane
1
where proteins
are unable to pass through the small pores, yet small molecules can do so easily. As indicated in Figure 18.1, the sample solution
(donor) is placed on one side of the membrane; on the other side is a second liquid (acceptor). In some cases, interferences diffuse
through the membrane, leaving a purified donor solution. More often, analyte passes through the membrane into the acceptor
solution, leaving interferences in the donor solution. Migration of neutral small molecules through a semi-permeable membrane is a
result of a difference in analyte concentration on the aqueous donor side of the membrane to the aqueous acceptor side of the
membrane. Once the concentration becomes equalized, there is no further migration.
Dialysis is not characterized by selective small molecule analyte enrichment. There is only partial cleanup due to lack of a
discrimination mechanism between other small molecules that may have the same size as the analyte molecules
2,3
. For this
reason, dialysis is not regarded as an extraction technique per se, and often an additional sample cleanup step is included in the
process. However, it has been widely applied to food and biological samples since macromolecules are excluded from passing
through the pores of the membrane. It finds great use in desalting of biological solutions.
270
Thus, as implied in Figure 18.1, the successful application of membrane techniques for sample cleanup requires removal of
analyte from the acceptor side by trapping (trace enrichment). If the analyte can undergo a change in its chemical state (e.g.
change from an uncharged to a charged species), then it can continue to be enriched. An advantage of membrane separation
techniques for RP-HPLC analysis is that both the donor and acceptor liquids are usually water or buffer. Membrane separations
can be carried out in either a static or flowing system, with the latter more amenable to on-line automation.
Compared to SPE or LLE, membrane separations are slower and are less likely to enrich the analyte concentration in the sample by
orders of magnitude. If no analyte enrichment takes place, the analyte remains in solution without any further concentration. Thus,
detection limits may be compromised and further sample processing time is required in subsequent cleanup steps. Another danger
with the use of membranes for the cleanup of dirty samples (e.g. wastewater) is that their pores may become fouled with debris
(e.g. microparticulates, colloids, solids). Also, depending on the membrane’s chemical composition, chemical interactions between
the analytes of interest and the membrane itself may occur, thereby affecting recovery and method precision.
Most membrane techniques are not particularly suited to high-throughput sample preparation requirements. However, there has
been a movement to adapting 96-well plate systems for membrane technologies. When evaluated on a per well (sample) basis,
then membrane techniques can become more “throughput competitive” with other sample preparation techniques since all 96
samples can be processed simultaneously.
Figure 18.1
Schematic of a Membrane Operating in a Flowing System
Acceptor
Sample
(donor)
Membrane
HPLC or Precolumn
Waste

271
Membranes are produced in many forms: sheet, roll, disk, capsule, cartridge, spiral-wound and hollow fiber forms. In addition to
differential concentration, analytes can also be moved across a membrane by diffusion as a result of chemical or electrochemical
gradients. Porous, electrically charged, or ion exchange membranes have pore walls with fixed positive or negative charges. Separation
characteristics of ionic molecules are governed by pore size and wall charge. The application of electrochemical forces adds another
dimension to the membrane separation process (see later).
Microdialysis sampling, a specialized application of dialysis, uses small microprobes of fused silica tubing with a membrane at one
end
4
. These probes can be implanted into living systems (e.g., rat brain), and the diffusion of small organic molecules through the
membrane can be monitored on-line by HPLC without disturbing the animal or plant.
Ultrafiltration
Ultrafiltration (UF) sampling is similar to dialysis and microdialysis, except that the driving force is flow through the membrane (with
300-300,000 mol wt cutoffs) is a result of a pressure differential (10-100 psi) applied across the membrane. As in the case of
dialysis, small molecules collect on the acceptor side. UF membranes are available as self-contained, disposable devices for the
hand processing of aqueous biological samples. UF separation is achieved by first pouring a sample into a filter cup and then
capping and applying air or gas pressure through the top cap.
Concentrated proteins and other molecules greater than the cutoff rating are retained in the filter cup while water, salts, and low
molecular-weight soluble components are collected in the filtrate collection cup. Ultrafiltration concentrates the sample without the
need for harsh chemical conditions that could affect the biological activity of labile compounds like therapeutically active proteins. It
also avoids the use of organic solvents and extreme pH conditions.

272
Supported Liquid Membrane (SLM) Enrichment
SLM techniques
5-7
are similar to flow dialysis, except that a porous PTFE solvent-filled membrane separates the two aqueous
solutions. The technique is actually a combination of dialysis and liquid-liquid extraction. Initially, the membrane is impregnated with
a water-insoluble organic solvent (e.g., n-undecane) – termed the supported liquid – and is placed in a mounting block. The organic
solvent is held in the pores of the membrane by capillary forces. Compounds are extracted from the donor side into the membrane
as a function of their solubility in the supported liquid where they are then re-extracted from the membrane into the acceptor side.
A simple example (Figure 18.2) depicting the SLM technique is the extraction of an un-ionized carboxylic acid (RCOOH) from the
donor side by first partitioning into the organic impregnated membrane. In a subsequent extraction from the membrane by a basic
solution on the acceptor side, the acid is then ionized and thus cannot be re-extracted into the membrane nor transferred back into
the donor side. Concentration factors of several hundred can be achieved by SLM extraction.
Figure 18.2
Extraction of Organic Acid from Aqueous Sample
Aqueous Sample Donor Solution
pH=3
Hollow Fiber with Organic Layer Aqueous Acceptor Solution
pH=9
RCOOH RCOOH RCOO
–
pK
a
of RCOOH = 5.5
RCOOH RCOOH RCOO
–
RCOOH RCOOH RCOO
–
RCOOH RCOOH RCOO
–

273
Hollow Fiber Liquid Phase Microextraction (HF-LPME)
Instead of using a flat membrane fitted into a holder, an experimentally more convenient “holder” is the use of a hollow fiber.
Hollow fibers are used in a wide variety of applications, the major one being their use for wastewater treatment; but in the
laboratory, they are used for microfiltration and reverse osmosis. The hollow fibers (HF) are made of organic polymers such as
polypropylene, polyethersulfone, polyester, and other materials, and can also be constructed from inorganic materials such as
titania and zirconia. The HF are relatively inexpensive and can be considered to be single-use, and the materials are recyclable.
Prior to use in any analytical application, the HF should be rinsed in acetone or other compatible solvent several times, then dried
at room temperature.
The principle of static hollow fiber liquid phase microextraction (HF-LPME) is illustrated in Figure 18.3. There are some similarities to
liquid phase microextraction (LPME) discussed in Chapter 7. However, in LPME, handling the “naked” drop at the needle tip poses
some problems. Although the procedure itself is simple to operate, one needs to be careful since the drop can be unstable and the
sample needs to be relatively clean. When the extracting “drop” is placed inside the lumen of a HF, it is mechanically protected
against some of the physical problems that the suspended drop may encounter in LPME. Rather than the spherical configuration of a
suspended drop in LPME, the HF-LPME provides a rod-like shape for the extracting solvent. The rod-like configuration increases the
solvent surface area since the same volume of liquid in the surface area of a sphere is lower. The contact area between sample
solution and the extracting solution is thus much more substantial than if the solvent were spherically shaped. The direct benefit of the
change in solvent configuration is better extraction efficiency. Another significant benefit is that higher stirring speeds can be applied
during the extraction procedure, since the solvent is protected by the hollow fiber and its stability is enhanced. In addition, the
disposable nature of the hollow fiber eliminates the possibility of sample carryover and ensures high reproducibility; and the pores in
the walls of hollow fiber cause it to show some selectivity by preventing the extraction of macromolecules, such as protein, particles,
and so on from sample matrix. Therefore, HF-LPME is no longer just a preconcentration technique, it can provide sample cleanup,
thus can be used for complex sample matrices.
Figure 18.3
Schematic of HF-LPME Apparatus
10 µL GC Syringe
Aqueous Sample
Porous Hollow Fiber
Organic Solvent
Stirring Bar
Magnetic Stirrer

274
Figure 18.4
Characteristics of Hollow Fiber Liquid Phase Microextraction
Hollow fiber
wall pores are
impregnated with
organic solvent
Hollow fiber channel
is filled with aqueous
solution extractant
(acceptor)
(courtesy of H.K. Lee, National University of Singapore)
Syringe Needle
Organic Solvent
Porous Hollow
Fiber Membrane
*
*
Organic solvent is used as the acceptor phase in two-phase LPME. In three-phase HF-LPME, a second aqueous solvent is used as the acceptor
phase (sometimes this technique is referred to as hollow fiber liquid-liquid-liquid phase microextraction or HF-LLLME).
Typical HF membrane
characteristics:
600 µm Iid x 200 µm wall
thickness, 0.2 µm wall pore size

275
Ionic liquids have been used as solvents for HF-LPME applications
8-12
. Ionic liquids are polar and non-volatile, but have been
demonstrated to generate stable solvent impregnation. Figure 18.5 shows scanning electron microscopic images of the inner
surface of the hollow fiber before and after ionic liquid impregnation. By comparing the two photographs, it is obvious that the
ionic liquid was immobilized in the hollow fiber pores effectively and an ionic liquid membrane for extraction formed. The
advantages of using an ionic liquid as the extraction solvent include high affinity for polar compounds and compound-dependent
selectivity. These specific features of ionic liquids have broadened the applications of HF-LPME to polar compound analysis.
Scanning electron microscopic images of the inner surface of the hollow fiber magnified by 5000 times before (A) and after (B) ionic liquid
impregnation
13
.
AB
Figure 18.5
Figure 18.4 shows the elements used to construct an HF extraction for HF-LPME. The selection of organic solvent used to fill in
the pores of hollow fiber is a critical parameter in the success of the method. Similar to supported liquid membrane techniques,
there are several requirements for the organic solvent. Firstly, it should be easily immobilized in the hollow fiber pores and non-
volatile. Typically, hollow fibers used in most HF-LPME experiments are made of hydrophobic polypropylene. The solvent selected
should have good affinity for the hollow fiber to prevent solvent loss during the extraction and to achieve stable solvent
impregnation. Secondly, the solvent should be immiscible with water as it serves as a barrier to the aqueous sample. Lastly, the
solubility of analyte of interest in the solvent should be higher than that in the aqueous sample matrix. Usually a relatively non-
polar, high viscosity solvent will meet those requirements, and typical solvents used include 1-octanol, n-hexyl ether, undecane,
toluene, etc. However, these relatively non-polar solvents usually have low solubility for the polar compounds, thus negatively
impact the extraction efficiency for polar compounds.

276
The HF-LPME procedure is simple and includes just a few steps. Prior to extraction, the hollow fiber (typically 1.5-10 cm in length,
200 µm thick, 600 µm id with a 0.2 µm pore size), is closed on one end by flame-sealing. Next, it is soaked in the immiscible solvent
that results in the immobilization of the solvent into the pores of the hollow fiber. The solvent forms a thin layer within the wall of the
hollow fiber. A microsyringe is usually used to support the hollow fiber and also to introduce the acceptor phase into hollow fiber
lumen. The hollow fiber is then placed into a sample vial filled with the aqueous sample, usually several milliliters (mL) in volume. To
speed up the extraction, the sample is extensively stirred. The analytes are extracted from the aqueous sample through the solvent
layer in the pores of the hollow fiber, then into the acceptor phase inside its lumen. After a certain time of extraction to achieve
equilibrium (often in the range of 10-30 min), the acceptor phase is withdrawn into the microsyringe and then injected directly into
the instrument for analysis.
Depending on the mode, the lumen of hollow fiber can be filled with several microliters of the same solvent (two-phase mode), or
an aqueous acceptor phase (three-phase mode). In two-phase HF-LPME, organic solvent impregnated in the hollow fiber segment
is exposed as a solvent rod in a stirred aqueous sample. Non-polar analytes in the aqueous sample can then be largely extracted
through the hollow fiber into the acceptor solvent by diffusion. Since the solvent selected can be GC-amenable, the extracted
sample can be directly injected into the gas chromatograph for analysis. Thus, the two-phase HF-LPME system using non-polar
solvents as the acceptor phase is more suitable for relatively hydrophobic analyte extraction
17-19
. However, when using ionic liquids
(ILs) as an acceptor phase, two-phase HF-LPME can be made more suitable for hydrophilic analyte extractions. Since ILs risk
contamination of the column and require frequent cleaning of the injection port, they may not be GC-amenable, but can be injected
into an HPLC system where the ionic liquid elutes quickly, thereby posing little or no interference possibilities with an HPLC reversed-
phase chromatographic analysis.
The three-phase mode of hollow fiber liquid-liquid-liquid microextraction (HF-LLLME) is sometimes referred to as LPME with back
extraction (LPME-BE). In this mode similar to the example cited in Figure 18.2, both donor and acceptor phases are aqueous-
based, and the third solvent is an organic phase immobilized into the pores of hollow fiber that serves as a barrier to separate the
two aqueous phases. During the extraction, the target analytes are extracted from the donor aqueous phase through the thin layer
of organic solvent within hollow fiber wall and then into the acceptor aqueous phase. Since the acceptor phase is aqueous, the
extracts can be analyzed by HPLC or CE. This method is usually applied for ionic or ionizable compounds, and mass transfer is driven
by the differences between the donor and acceptor aqueous phases, such as pH adjustment, salting out effect in donor phase, or
addition of proper acceptor reagent like ion-pair complexing reagent for analytes.
As the phase ratio between the donor phase (aqueous sample) and the acceptor phase (solvent or second aqueous phase) is very
large (>100:1), the analytes are concentrated significantly in the acceptor phase, with enrichment factors up to several hundred
times. As a result, the detection limit of the analytical method is greatly improved by this high enrichment efficiency. Therefore,
lower detection limit (lower ppb level or even sub-ppb level) can be easily achieved, even with relatively low sensitivity detectors,
such as the UV detector.

277
To illustrate an application of a three-phase HF-LLLME technique, wastewater raw and spiked with polar phenols was studied
14
.
Since the phenols were quite polar and have a significant solubility in water, they were not directly extractable from the water by an
organic solvent. Therefore, in this example, an ionic liquid was impregnated into the HF. The ionic liquid consisted of a mixture of
nonane and methylimidazolium hexafluorophosphate, BMIM[PF6]/acetonitrile (1:1) and provided a good extraction medium for the
phenols. The acceptor phase was aqueous. The experimental setup of Figure 18.3 was employed.
Using reversed-phase HPLC with UV detection to analyze the extracts, Figure 18.6 provides a comparison of the HF-LLLME
extractions of raw wastewater and the wastewater spiked at two levels (5 and 25 µg/L) with the four phenols to be analyzed.
Clearly, the phenolic compounds could be extracted from the wastewater at these very low levels. The large peak at the beginning
of the chromatograms was due to the ionic liquid eluting from the reversed-phase column near the void volume.
Figure 18.6
Ionic Liquid HF-LLLME-HPLC-UV for Determination of Phenols
HF-LLLM, with nonane and [BMIM[PF6]/acetonitrile (1:1). HPLC-UV traces of wastewater extract. (A) Extract of
wastewater spiked at 25 µg L
-1
of each phenol; (B) extract of wastewater spiked at 5 µg L
-1
of each phenol;
(C) extract of real unspiked wastewater sample. Peaks: 1) 4-tert-butylphenol; 2) 4-tert-octylphenol;
3) 4-n-octylphenol; 4) 4-n-nonylphenol.
AU
0.0000
0.0025
0.0045
1
2
3
4
-0.0005
2 6 8 12 16 20 min
Extract of wastewater spiked at 25 µg L
-1
Extract of wastewater spiked at 5 µg L
-1
Extract of unspiked wastewater
A
B
C

278
Electromembrane Extraction (EME)
The concept of EME
15
combines the technical setup for hollow fiber liquid phase microextraction (HF-LPME)
16,17
with well-known
principles for electroextraction
18-24
. This combination offers a highly selective sample preparation method using simple equipment
and gains a high degree of enrichment within a short period of time.
The EME method extracts charged substances from a small sample volume through a thin membrane of organic solvent
immobilized in the wall of a hollow fiber and into an acceptor solution inside the lumen of the hollow fiber. This extraction process
is forced by an applied potential difference across the membrane. This combination of LLE with electrokinetic migration yields a
rapid and selective sample preparation method for ionic substances. EME has shown to be compatible with a wide range of
biological matrices, e.g. plasma, whole blood, urine, and breast milk, preparing clean extracts in a short period of time with
simple, inexpensive equipment.
The technical setup for the equipment used in EME is based on earlier experiences with HF-LPME and is shown in Figure 18.7.
The hollow fiber used is made of porous polypropylene, which is compatible with a broad range of organic solvents. To make the
supported liquid membrane (SLM), the fiber is dipped in an organic solvent for 5 seconds to fill the pores in the walls, and the
excess of organic solvent is gently removed with a medical wipe. The fiber, connected to the pipette tip, is guided through a
punched hole in the sample compartment cap as illustrated in Figure 18.7A. The pipette tip works as a mechanical support for a
0.5 mm thick platinum wire placed inside the lumen of the hollow fiber. Another platinum wire is introduced directly into the donor
phase through the sample compartment cap. When coupled to a power supply, these inert wires act as electrodes, thus creating an
electrical field across the SLM. In this way, the equipment makes a closed electrical circuit, where the SLM functions as a resistor.

279
+
+
+
+
+
+
-
-
-
-
-
-
-
+
+
+
+
+
+
-
-
-
-
-
-
-
CH
3
CH
3
N
O
O
Figure 18.7
EME Setup (A) and Principle (B), Demerol as the Model Substance
Positive Electrode Negative Electrode
Donor Phase, pH~2
SLM
Fiber wall
impregnated
with organic
solvent
AB
Power
Supply
Acceptor Phase, pH~2
The volume of the sample varies between 150-500 µL, depending on the sample compartment size. The sample is shaken on a
platform shaker during the extraction to increase the physical movement of the analytes in the bulk donor phase, and to reduce the
thickness of the stagnant layer at the interface between the donor phase and the SLM. The acceptor phase volume is set to 25 µL
and is introduced into the lumen of the hollow fiber by a microsyringe. When the predetermined extraction period is finished
(usually only a few minutes), 20 µL aliquot of the acceptor phase is collected by the microsyringe and transferred to a vial for
analysis in a capillary electrophoresis (CE) instrument
25-29
or by HPLC
30
.
Figure 18.7B also depicts the mechanism of EME. The test substance Demerol is a synthetic opioid containing a nitrogen group
that at low pH is protonated. The pH of both the donor and acceptor phases is 2. When an electric field is imposed across the HF,
the positively charged analyte is attracted to the negative electrode in the EME setup and this strong attraction overcomes any
partitioning into the SLM and the ion is transferred to the acceptor solution. Both acidic and basic compounds have been extracted
with good efficiencies and reproducibility. Variables affecting the extraction efficiency and recovery include: imposed voltage,
chemical nature of the membrane, type of organic solvent in the SLM (with or without additives like ion pair reagents), pH values of
the donor and acceptor solutions, volume of donor solution, the type of sample matrix, degree of agitation, and extraction time.

280
References
1.
Greenway, G.M.; Kometa, N.; Macrae, R.
Food Chemistry
1992,
43
, 137-140.
2.
Pingoud, A.; Urbanke, C.; Jeltsch, A.; Jeltsch, J.
Biochemical Methods: A Concise Guide for Students and Researchers
, Wiley-VCH,
Weinheim, Germany, 2002, ISBN-10: 3527302999 and ISBN-13: 978-3527302994.
3.
Hassan Y. Aboul-Enein, (Ed.)
Separation Techniques in Clinical Chemistry
, CRC Press, New York, 2003, ISBN-10: 0824740130 and
ISBN-13: 978-0824740139.
4.
Nandi, P.; Lunte, S.M.
Handbook of Sample Preparation
, Pawliszyn, J.; Lord, H.L. (Eds.) John Wiley & Sons, Hoboken, NJ, 2010,
103-123, ISBN: 978-0-470-09934-6
5.
Audunsson, G.A.
Anal. Chem.
1986,
58
, 2714-2723.
6.
Jonsson, J.A.; Mathiasson, L.
Trends in Anal. Chem.
1992,
11 (3)
, 106-114.
7.
Jonsson, J.A.; Mathiasson, L.
LCGC No. Amer.
2003,
21 (5)
, 424-438.
8.
Tao, U.; Liu, J.-F.; Hu, X.-Li.; Li, H.-C.; Wang, T.; Jiang, G.-B.
J. Chromatogr. A
2009,
1216
, 6259-6266.
9.
Peng, J.-F.; Liu, J.-F.; Hu, X.-L.; Jiang, G.-B.
J. Chromatogr. A
2007,
1139
, 165-170.
10.
Liu, J.-F.; Jiang, G.-B.; Cai, Y.Q.; Zhou, Q.-X; Hu., J.-T.
Anal. Chem.
2003,
75
, 5870-5876.
11.
Liu, J.-F.; Chi, Y.-G.; Jiang, G.-B.; Tai, C.; Peng, J.-F.; Hu., J.-T.
J. Chromatogr. A
2004,
1026 (1-2)
, 143-147.
12.
Ho, T.S.; Halvorsen, T.G.; Pedersen-Bjergaard, S.; et al.
J. Chromatogr. A
2002,
963
, 3-17.
13.
Zhao, L.; Lee, H.K.; Majors, R.E.
LCGC
2010,
28 (5)
, 580-591.
14. Lee, H.K. Paper presented at Separation Science Singapore 2009, Biopolis Science Park, Singapore, August 26-28, 2009.
15.
Pedersen-Bjergaard, S.; Rasmussen, K.E.
J. Chromatogr. A
2006,
1109
, 183-190.
16.
Pedersen-Bjergaard, S.; Rasmussen, K.E.
Anal. Chem.
1999,
71
, 2650-2656.
17.
Pedersen-Bjergaard, S.; Rasmussen, K.E.
J. Chromatogr. A
2008,
1184
, 132-142.
18.
Arrigan, D.W.M.
Anal. Lett.
2008,
41
, 3233-3252.
19.
Berduque, A.; Arrigan, D.W.M.
Anal. Chem.
2006,
78
, 2717-2725.
20.
Berduque, A.; O'Brien, J.; Alderman, J.; Arrigan, D.W.M.
Electrochem Commun
2008,
10
, 20-24.
21.
Berduque, A.; Sherburn, A.; Ghita, M.; Dryfe, R.A.W.; Arrigan, D.W.M.
Anal. Chem.
2005,
77
, 7310-7318.
22.
Vandervlis, E.; Mazereeuw, M.; Tjaden, U.R.; Irth, H.; Vandergreef, J.
J. Chromatogr. A
1996,
741
, 13-21.
23.
Vandervlis, E.; Mazereeuw, M.; Tjaden, U.R.; Irth, H.; Vandergreef, J.
J. Chromatogr. A
1995,
712
, 227-234.
24.
Vandervlis, E.; Mazereeuw, M.; Tjaden, U.R.; Irth, H.; Vandergreef, J.
J. Chromatogr. A
1994,
687
, 333-341.
25.
Gjelstad, A.; Andersen, T.M.; Rasmussen, K.E.; Pedersen-Bjergaard, S.
J. Chromatogr. A
2007,
1157
, 38-45.
26.
Gjelstad, A.; Andersen, T.M.; Rasmussen, K.E.; Pedersen-Bjergaard, S.
J. Chromatogr. A
2006,
1124
, 29-34.
27.
Gjelstad, A.; Rasmussen, K.E.; Pedersen-Bjergaard, S.
Anal. Bioanal. Chem.
2009,
393
, 921-928.
28.
Kjelsen, I.J.O.; Gjelstad, A.; Rasmussen, K.E.; Pedersen-Bjergaard, S.
J. Chromatogr. A
2008,
1180
, 1-9.
29.
Middelthon-Bruer, T.M.; Gjelstad, A.; Rasmussen, K.E.; Pedersen-Bjergaard, S.
J. Sep. Sci.
2008,
31
, 753-759.
30.
Gjelstad, A.; Andersen, T.M.; Rasmussen, K.E.; Pedersen-Bjergaard, S.
J. Chromatogr. A
2008,
1157
, 38-45.

281
Chapter 19
The Role of Scavengers
in Sample Preparation
Scavengers are special types of solid phase particles that perform a similar role as SPE. However, instead of using molecular
interactions, such as hydrophobicity or adsorption, scavengers use chemical reactions to remove undesired species. They are
most often used by organic chemists in removing undesired reaction products or excess starting material from organic synthesis.
Most scavengers operate by the use of covalent bonding. In most scavenging applications, the differentiation between product
and starting material must be well defined. For example, while removal of nucleophiles – like an amine in the presence of an
amide – is simple, the removal of primary amine in the presence of other amine functionalities may cause difficulties. Therefore,
scavengers are made to be very selective. An example is shown in Figure 19.1 where a polymer supported ketoester resin
(PL-AAEM) can be used to selectively remove excess primary amine in the presence of more substituted amine products. The
scavengers are therefore perfectly suited to reductive amination and some multicomponent reaction applications. If both primary
and secondary amines are to be removed, then the less selective polymer supported isocyanate resin (PL-NCO) is available.
R
1
N
R
2
H
NH
2
+ R
1
N +
R
2
H
R
1
CH
3
O
O
O
OO
CH
3
O
O
O
ON
H
R
1
Figure 19.1
Primary Amine Scavenger Chemical Reaction
Primary Amine Scavenger

282
R
1
+ H
2
N
R
2
R
1
R
2
O
X
R
1
+
O
O
X
X
O
N
H
R
1
R
2
O
N
H
NH
2
HN
NH
H
2
N
NH NH
+
The removal of electrophilic reagents from solution using a scavenger resin with an amine group is very simple and effective.
The polymer supported triamine resin (PL-DETA) can be used to remove acid chloride, sulfonyl chloride, isocyanates, and
isothiocyanates according to the reaction scheme shown in Figure 19.2.
Figure 19.2
Removal of Electrophilic Reagents Using PL-DETA Resin
(excess)
A particularly useful application of solid phase scavenger is removal of acidic mobile phase additives from HPLC fractions. The
use of organic acids and HPLC mobile phase to aid solubility of polar molecules, especially biomolecules, is a well-known
technique. Typically, 0.1% TFA in mixtures of water and acetonitrile are most commonly employed. If a compound is purified
using preparative HPLC, the resulting solvent fractions will contain traces of TFA. If the species then undergo evaporation or
lyophilization, the resulting organic species may be present as a TFA salt. Longterm stability of salts, particularly TFA salts, may
be compromised. The use of a polymer supported hydrogen carbonate resin (PL-HCO
3
MP SPE, Figure 19.3) packed into a
cartridge can be used for the pass-through removal of TFA and other organic and inorganic acids such as formic acid, acetic
acid, and HCl.

283
H
3
C CH
3
CH
3
HCO
3
-
N
+
O
O
OH
H
O
OH
+ –
NMe
3
H
2
O, MeCN
O
O
H + H
2
O + CO
2
+ –
NMe
3
Figure 19.3
Removal of Formic Acid from Organic Solution Using Stratospheres
Structure of PL-HCO
3
MP SPE Resin
PL-HCO
3
MP SPE mediated removal of formic acid from organic solution
To demonstrate the effectiveness of the PL-HCO
3
MP SPE resin to remove formic acid from water-acetonitrile mixtures, a series of
HPLC solutions bearing a mixture of water and acetonitrile containing 0.1% v/v formic acid were prepared and passed through a
200 mg PL-HCO
3
MP SPE device
1
. The capacity of the media for TFA removal was determined by measuring the pH of every 2 mL
fraction; when the pH changed to approximately 2.5, the experiment was stopped and the volume of solvent was recorded. Figure
19.4 shows that as the concentration of acetonitrile increases, the observed quenching capacity decreases only slightly. This is most
likely due to a change in the residency time of the solvent mixture, a result of the reduction in viscosity of the sample. However, the
capacity observed at 100% acetonitrile (30 mL) is still ample for most applications.
40
35
30
25
20
15
10
5
0
0 20 40 60 80 100
Figure 19.4
Effect of Acetonitrile Content on the Volume of 0.1% Formic Acid Solution
Scavenged Using a 200 mg/mL SPE Device
Tube Capacity (mL)
% MeCN in 0.1% Formic Acid
Theoretical
Observed

284
The experimental procedure for the removal of formic acid from organic solutions is simple. First, precondition the SPE cartridge
with 1 mL of MeOH. Add formic acid containing solution to the SPE cartridge and allow the solution to pass through under gravity.
Once all of the solution has passed through, wash the SPE cartridge with 1-2 mL of suitable solvent (in MeOH, H
2
O, etc.). Take the
organic solution and remove the solvent in vacuo to yield the desired compound free of residual formic acid.
Polymer-based resin scavengers are available for a wide variety of organic compounds. Compared to SPE sorbents, scavengers are
chemically reactive resins, so one should take extra precautions to store these materials in an inert, refrigerated atmosphere.
The removal of residual metal species, such as catalysts from organic reactions, is essential in providing clean, screenable
compounds. A range of SPE devices have been designed to remove a broad range of metal reagents from organic reactions using a
simple gravity flow method. Unwanted residues are retained by the sorbent in the SPE tube, allowing the desired compound(s) to
pass through.
The StratoSpheres SPE range for metal removal is designed around a specially engineered macroporous particle, which has a
high capacity and can be used in a broad range of solvents. The base polymer is functionalized with a range of ligands designed
to bind metal reagents and catalysts. Table 19.1 provides a listing of the SPE scavenger products and the metals that they will
remove from solution.
Table 19.1
To illustrate the use of a metal scavenger, fast and simple removal of Rhodium using Stratosphere SPE is shown. Rhodium-containing
catalysts are used extensively in many aspects of synthetic chemistry – from small-scale applications such as the synthesis of small
molecules for medicinal chemistry – to large-scale manufacturing. Despite the utility of rhodium reagents, the removal of the metal
residues post reaction can cause difficulties. One of the most well-known Rhodium catalysts is Wilkinson's catalyst, a homogeneous
catalyst used in the hydrogenation of alkenes. Another highly useful rhodium based reagent is rhodium acetate, which is used to
initiate carbene formation from a diazo species.
Stratospheres Metal Removal Macroporous SPE Resins
Product Metals Removed
PL-Guanidine MP SPE Au, Bi, Cd, Hg, Pd, PT, Re, Rh, Sn, Zn
PL-Thiol MP SPE Ag, Au, Cu, Fe, Pd, Ru, Rh, Sn, Pb, Cu
PL-Thiourea MP SPW Ag, Cd, Cu, Pt, Pd, Ru, Rh, Hg, Cu, Ni
PL-Urea MP SPE Ag, Au, Hg, Noi, Pd, Pt, Re, Sc

285
Figure 19.5
Structures of Stratosphere SPE Phases Used for Rhodium Removal
References
1.
Boguszewski, P.
StratoSpheres SPE for Efficient, Flow Through Removal of Formic Acid
, Agilent Technologies Application Note
#Sl-01043, Santa Clara, CA, 2007.
2.
Boguszewski, P.
Fast and Simple Removal of Rhodium Using StratoSpheres SPE
, Agilent Technologies Application Note #SI-01042,
Santa Clara, CA, 2007.
N
H
N
H
O
SH
SH
CH
3
Right: PL-Thiourea MP SPELeft: PL-Thiol MP SPE
PL-Thiol MP SPE and PL-Thiourea MP SPE are two metal scavenger products that can effectively remove rhodium residues from
organic solutions in a single pass under gravity. Figure 19.5 shows the structures of these two scavengers. The sorbents are made
from highly cross-linked macroporous polymer that does not swell and can be used for a range of protic, non-protic, polar and
apolar solvents. The conditions that were used for the cleanup are the same to the conditions used earlier for the removal of formic
acid. Starting with a solution of 1000 ppm of rhodium concentration for both the Wilkinson's and the rhodium acetate catalysts,
both scavengers decreased the rhodium concentration to less than 1/10 ppm
2
.

286
Derivatization involves a chemical reaction between an analyte and a reagent to change the chemical and physical properties
of the analyte. The five main uses of derivatization in HPLC and GC are to:
•
Improve detectability
•
Change the molecular structure or polarity of analyte for better chromatography
•
Increase volatility
•
Change the matrix for better separation
•
Stabilize an analyte
Ideally, a derivatization reaction should be rapid, quantitative, and produce minimal by-products. Excess reagent should not interfere
with the analysis or should be easily removed from the reaction matrix. There are many good reference books available for
derivatization procedures for chromatographic separations
1-5
.
With the increased popularity of MS techniques (e.g. LC/MS, LC-MS/MS, GC/MS and GC-MS/MS), many laboratories prefer an
instrumental approach to high sensitivity and selected detection rather than contend with a relatively time-consuming, labor-intensive
compound derivatization approach. Derivatization is often a last resort when developing a method since it adds to the time of analysis,
adds a degree of complexity, can be a source of error, and it may add undesired components (e.g. reaction by-products of analytes or
matrices) into the chromatography. While derivatization procedures can be automated, the analyst must ensure that the derivatization
step is quantitative (if necessary) and that there are no additional impurities introduced in the analysis. Although, the derivatization
method may have drawbacks, it is still a powerful technique for the separation and detection of trace amounts of substances in a
complex matrix. In fact, in some cases, MS detection derivatization is used to enhance positive (or negative) ionization.
Chapter 20
Derivatization for HPLC
and GC Analysis
In this chapter, derivatization in HPLC will be covered, followed by derivatization by GC.

287
Unlike GC, where derivatization is generally used to improve the volatility or change the polarity of an analyte, derivatization in HPLC
(with the exception of chiral analysis) is predominately used for the enhancement of analyte detectability, particularly for analytes
that possess no chromophore. HPLC offers a wide range of separation mechanisms (i.e., normal and reversed-phase, chiral, and ion
chromatography), types of stationary phases, and mobile phase modifiers which can be added directly to the mobile phase to
overcome interactions with the stationary phase – such as chemisorption, adsorption, and tailing.
The first consideration in choosing an HPLC derivatization method for detection enhancement is to decide if a suitable active
functional group (or groups) is available for chemical reaction. At the same time, one must decide which type of detection principle
is best suited for these derivatized compounds. Of course, that detector must be available in the laboratory. Most popular detectors
for derivatized compounds include UV-visible, fluorescence, and electrochemical. In addition, the choice of whether to use pre- or
post-column derivatization is required (see later in this chapter). Sample components with active functional groups such alcohols,
phenolic, amine, carboxyl, olefin, and others are candidates for derivatization. Several classes of compounds can be derivatized
(Table 20.1) some of these include acids, alkaloids, amines, antibiotics, barbiturates, and related compounds, hydroxy compounds,
and steroids.
The two most common types of derivatization – the addition of chromophore or fluorescent functional group for UV and fluorescent
detection, respectively – allow detection of an analyte that cannot be detected in its normal form or to increase its sensitivity.
General considerations in choosing a derivatizing reagent are:
•
The derivatizing agent must be stable.
•
The derivatizing agent and by-products formed during derivatization should not be detectable or must be separated
from the analyte.
•
The analyte must be reactive with derivatizing reagent under convenient conditions.
•
Reagents should be non-toxic if possible.
•
The procedure should be adaptable to automation.
Many organic reactions could be used for analyte derivatization. However, for routine use, the best approach is to choose the
proper derivatizing reagent using pre-prepared derivatization kits and step-by-step instructions from various reputable suppliers.
HPLC Derivatization for Detectability

288
Functional Group and Derivatization Reagents for UV and Fluorescence Detection
*
Table 20.1
Functional Group UV Derivatives Fluorescent Derivatives
Carboxylic Acids
Fatty Acids
Phosphonic Acids
PNBDI
DNBDI
PBPB
BrMaC
BrMmC
Alcohols DNBC
Dabsyl-Cl
NIC-1
Aldehydes
Ketones
PNBA
DNBA
Dansyl Hydrazine
Amines, 1° Fluorescamine
OPA
Amines, 1° & 2° DNBC
SNPA
SDNPA
Dabsyl-Cl
NIC-1
NBD-Cl
NBD-F
Dansyl-Cl
Amino Acids
(Peptides)
SBOA
SDOBA
Dabsyl-Cl
Fluorescamine
OPA
NBD-Cl
NBD-F
Dansyl-Cl
Isocyanates PNBPA
DNBPA
Phenols DNBC
Dabsyl-Cl
NIC-1
NBD-Cl
NBD-F
Dansyl-Cl
Thiols Dabsyl-Cl NBD-Cl
NBD-F
OPA
Courtesy of Regis Technologies
*
Chromotags abbreviations Fluorotag Abbreviations
Dabsyl-Cl 4-Dimethylaminiazobenzene-4-sulphinyl NBD-Cl 7-Chloro-4-nitrobenzo-2-oxa-1,3-diazole
DNBA 3,5-Dinitrobenzyloxyamine Hydrochloride NBD-F 7-Fluoro-4-nitrobenzo-2-oxa-1,3-diazole
NIC-1 1-Naphthylisocyanate Fluorescamine 4-Phenylsprio(furan-2(3H),1'-phthalan-3,3-dione
PBPB p-bromophenacyl Bromide OPA o-Phthaldehyde
PNBA p-Nitrobenzyloxyamine Hydrochloride Dansyl-Cl 5-Dimethylaminonaphthalene-1-sulfonyl Chloride
PNBDI p-Nitrobenzyl-N,N'-diisopropylisourea BrMmC 4-Bromomethyl-7-methoxycoumarin
DNBDI 3,5-Dinitrobenzyl-N,N'-diisopropylisourea BrMaC 4-Bromomethyl-7-acetoxycoumarin
PNBPA P-Nitrobenzyl-N-n-propylamine Hydrochloride
DNBPA 3,5-Dinitrobenzyl-N-n-propylamine Hydrochloride
SNPA N-Succinimidyl-p-nitrophenylacetate
SDNPA N-Succinimidyl-3,5-dinitrophenylacetate
DNBC 3,5-Dinitrobenzyl Chloride

289
Typically, a reagent used for UV-visible detection will have two important functional groups. One functional group controls the
reaction of the reagent with the analyte of interest and the second is used for UV detection. The chromophore should have a large
molar absorptivity with an adsorption band that can be used to maximize detection and minimize background noise. Table 20.2
lists some of the common chromophores used for UV detection along with their maximum absorption wavelength and their molar
absorption coefficient at 254 nm. Reagents having a molar absorption coefficient of 10,000 or more allow detection in the low ng
range. Table 20.1 listed some commercially available derivatization reagents for UV detection. The analyte functional groups that
they will derivatize are also shown in the table.
UV-Detection
Table 20.2
Chromophores of Interest for Enhanced UV Detection
Chromophore Wavelength of Maximum Absorption Molar Absorption Coefficient at 254 nm
Benzyl 254 200
4-Nitrobenzyl 265 620
3,5-Dinitrobenzyl ------ > 10,000
Benzoate 230 low
4-Chlorobenzoate 236 6,300
4-Nitrobenzoate 254 > 10,000
2,4-Dinitrophenyl ------ > 10,000
Toluoyl 236 5,400
Anisyl 262 16,000
Phenacyl 250 10,000
4-Bromophenacyl 260 18,000
2-Naphthacyl 248 12,000
Note that many of the common reagents for introducing nitrobenzyl chromophores (Table 20.2) into a molecule for UV-visible
detection are also suitable for use with electrochemical detection. In addition, OPA derivatives of amines and amino acids can be
determined at very low levels with electrochemical detection. For further information on the use of derivatization for electrochemical
detection in HPLC, consult reference 4.

290
In addition to the considerations above for derivatizing reagents, fluorescent derivatization reagents require a fluorophore that
possesses intense absorption bands and a large quantum yield. Due to the special properties required for strong fluorescence
response, there are fewer fluorescent derivation reagents than there are for UV-Detection (Table 20.1). See Figure 20.1 for an
application of fluorescence derivatization.
There are several advantages for precolumn derivatization compared to post-column derivitization. Precolumn derivatization has
fewer equipment and chemicals restrictions since the analysts can perform the derivatization, then transfer the sample to the
appropriate vial for analysis. Precolumn derivatization can be performed manually or automated. Several manufacturers of
analytical instrumentation or robotics offer automated precolumn derivatization. There are no time constraints on the kinetics of
the derivatization reaction, provided all the reagents, analytes, and derivatized species are stable. Finally, sample preparation
procedures described in this book can be used to remove undesired by-products, sample interferences, and if necessary, change
the sample solvent to be compatible with the HPLC mobile phase and the GC stationary phase.
Some drawbacks of precolumn derivatization are the introduction of contaminants and loss of analyte through: adsorption,
undesired side reactions, possible sample degradation, sample transfer, and incomplete reactions. Also, additional time is required
for derivatization and the added complexity can result in poorer method precision.
Fluorescence Detection
Precolumn Derivatization

291
Post-column derivatization is commonly accomplished using a reaction detector where the analyte is derivatized after the separation
but prior to detection. Reaction detector design takes into account the dispersion of the sample within the reaction system. The
three most common approaches to reactor design are: capillary, packed bed, and air segmented for fast (<1 min), slow (1-5 min)
and slower (5-20 min) reactions rates, respectively. The main advantages of post-column derivatization are: minimal artifact
formation; reaction completion is not essential as long as it is reproducible; and the chromatography of the analyte is unaffected.
The drawbacks to post-column derivatization are band broadening for all but very fast reactions, and the added complexity for
both method development and routine applications. Important considerations are the kinetic requirements (a maximum of 30
minutes for reaction completion) and possible incompatibility between the mobile phase and derivatizing reagents. Ensuring
reagent and mobile phase compatibility also can complicate HPLC method development, because the requirements of the
derivatization must be considered along with those of the separation. The best mobile phase for separation can be incompatible
for the derivatization reaction.
Post-Column Derivatization

292
In this application, automated SPE was first used to isolate the trace amounts of these pesticides in water
6
. After conditioning
the SPE cartridge, a 1 L sample of water was passed through the cartridge concentrating the organic material present.
Dichloromethane was found to be the optimum elution solvent for the SPE cartridge. For the chromatography, reversed-phase
HPLC and post-column derivatization were used. Prior to the derivatization, carbaryl and carbofuran were first hydrolyzed under
basic conditions with an initial post column reaction at high temperature into methyl amine. The methyl amine, in turn, was
reacted with o-phthaldehyde (OPA) and 2-mercaptoethanol (RSH) to yield isoindoles that are highly fluorescent (right side of
Figure 20.1A). The initial compounds had no inherent fluorescence. Figure 20.1B (see next page) shows the strong
fluorescence signal resulting from the carbamates spiked in tap water. Note that to prevent residual chlorine in the water from
affecting the trace concentrations of the pesticides, a small amount of sodium thiosulfate was added to the sample. Post-
column reaction method showed excellent linearity, very good reproducibility (% RSD less than 2), good recovery (80-110%),
and limits of detection into the parts per trillion range.
To illustrate an example of post-column derivatization, the analysis of the pesticides carbaryl and carbofuran in drinking water is
presented
6
. These N-methyl carbamates (structures shown on left side of Figure 20A) are classified as broad-spectrum
insecticides and are mostly used on rice and corn crops. Excess pesticide can contaminate groundwater, surface water and
drinking water. Low levels of these pesticides in drinking water have been found to create health problems in the neurological
and reproductive systems.
O
O
O
O
H
H
N
N
SR
N
H
O
O
O
HN
OH-
95 °C
CH
3
NH
2
Strong fluorescent
Nonfluorescent
Carbamates
Carbofuran
Carbaryl
Room temperature
+ RSH and
Structures of Carbamates and Post-Column Derivatization Reaction
Figure 20
A)
OPA

293
LU
8
6
4
2
0
0 246810 12 14 min
LU
8
6
4
2
0
0246810 12 14 min
Experimental
Blank sample
Spiked sample
Carbofuran: 0.41 ppb
Carbaryl: 0.50 ppb
Post-Column Fluorescence Chromatogram of Tap Water Spiked
with ppb Concentrations of Carbamate Pesticides
B)
Figure 20
(Continued)
Instrument: Agilent 1100 or 1200 with fluorescence detector
(FLD): A multidraw upgrade kit can be installed
when 400 µL injections are needed.
Column: Agilent TC-C18(2) 4.6 x 150 mm, 5 µm
Mobile Phase: A: Water, B: Methanol
0 min 42% B, 5 min 55% B,
12 min 60% B
13 min 42% B, stop time 15 min,
post-run 2 min
Flow Rate: 1.0 mL/min
Temperature: 30 °C
Detector: Fluorescence: Ex 339 nm; Em 445 mm
Injection Volume: 10 µL
Post-Column Reaction Conditions
Flow Rate of Reagents: 0.1 mL/min
Reactor Temperature: 95 °C
Derivitization Temperature: Room Temperature
SPE Cartridges Agilent
Technologies, Inc.
Sampliq C18, 6 mL/500 mg

294
Although mainly associated with GC, derivatization in HPLC for reasons of volatility may be important for the evaporative light
scattering detector or the corona discharge detector, which work best for volatile analytes. Derivatization of polar groups, such as
amines, may cut down on tailing using silica-based reversed-phase columns because of silanol- or ionic-interactions.
Unlike derivatization of non-chiral separations, the major use of chiral derivatization is to enhance the separation, and not to improve
detection. However, the oldest method of chiral separation is derivatization. Thus, there is a wealth of information available, and
several functional groups have been derivatized. Chiral derivatization has been applied to both reversed and normal phase liquid
chromatography. The key to chiral analysis is the ability to react an optically active target molecule with an optically active reagent.
There are several advantages and limitations to chiral analysis via derivatization:
•
The technique has been studied extensively and there is a wealth of information making the technique easy and accessible.
•
The methods use standard HPLC supports and mobile phases.
•
If detection is a problem, derivatization for detection and separation can be accomplished in one step.
•
For enantiomeric compounds, the compounds of interest must be isolated and then derivatized, making automation difficult.
•
The purity of the derivatizing reagent is critical, since the presence of enantiomeric contamination can yield false measurement.
•
For enantiomers that have different rates of reaction and/or equilibrium, constants results may not provide the true
enantiomeric ratios.
•
The possible racemization of the product during sample processing.
In addition to the derivatization of chiral compounds, the use of achiral reagents can increase the selectivity of the chiral stationary
phase (CSP) toward a chiral analyte. Some compounds do not have distinct enough binding sites to obtain adequate resolution on a
CSP, and derivatization with achiral reagents allows their separation.
Limitations
Advantages
Other Uses for Derivatization

295
Codes:
TMCMS: Trimethylchlorosilane
HMDS: Hexamethyldisilazane
MSTFA: N-methyl-N-(trimethylsilyl)acetamide
MTBSTFA: N-tert-Butyldimethylsilyl-N-methyltrifluoroacetamide
In GC, the main purpose of derivatization is to increase analyte volatility. Molecules, especially compounds that are of biological and
environmental interest that are of high molecular weight and/or contain polar functional groups, are thermally unstable at the
temperatures required for their separation by GC are candidates to be derivatized for GC. The main type of derivatization involves
the reaction of protonic functionalities into thermally stable, more volatile, non-polar functional groups. Besides improving thermal
stability, derivatization improves peak shape by minimizing wall and other interactions that could result in tailing and/or irreversible
adsorption. There are a wide variety of derivatizing reagents for GC and some of the more popular are listed in Table 20.3. There
are many publications summarizing the various derivatization methods for GC-amenable compounds
1,3,5
.
Typical Derivatizing Reagents Used in GC
Table 20.3
Derivatization in Gas Chromatography
Class of Reagent
Typical Reagent
Compounds
Compounds
Derivatized
Comments
Trialkylsilane TMCS, HMDS, MSTA,
MSTFA, BSTFA
Alcohols, phenols,
carboxylic acids, thiols,
amines, amides
Most widely used, use anhydrous conditions, rate of
reaction based on steric factors, use of catalysts, choice
of solvent and temperature; few artifacts except multiple
products for sugars; halogenated silanes available for
ECD detection.
Haloalkylacryl Perfluorocarbonacyl
(e.g. trifluoroacetyl-,
pentafluoropropionyl-,
heptafluorobutryl- )
Amines, phenols Prepared from acid anhydrides or acid chlorides
usually in presence of base (e.g. pyridine, triethylamine);
introduction of electron-capturing groups for ECD
detection.
Haloalkylacryl Alcohols Carboxylic acids Reaction performed with excess of alcohol with acid
catalyst (e.g. HCl, acetyl chloride, boron trifluoride);
removing water by-products helps derivatization process;
methyl esters most popular.
Alkylation Alkyl halides,
diazoalkanes, acetals,
N,N’-dimethylformamide
dialkyl acetals
Active hydrogen-containing
compounds (e.g. -COOH,
-SO
2
OH, -OH, -SH,
-NH
2
, =NH )
A catalyst must be present to ensure complete reaction;
typical catalysts include silver oxide, barium oxide,
sodium hydride.
Pentafluoro (PF)
Phenyl
PF-benzoic anhydride,
PF-benzyl bromide,
PF-benzoyl chloride,
and others
Alcohols, amines, phenols,
carboxylic acids, ketones
Useful for broad spectrum of organic compounds; stable
derivatives, good volatility for derivatives, can be detected
by ECD.
Oximes Hydroxylamine or
derivatives (Me, Et,
or benzyl)
Ketones, aldehydes Mainly for protection of keto groups when other
derivatives are being formed

296
For GC, the silylation reagents are the most popular and universal. They're very reactive, can derivatize most functional groups,
and with most compounds do not form by-products or artifacts. The trimethylsilyl ether derivatives themselves are stable under
anhydrous conditions. New silyl homolog or analog agents have been developed that have improved hydrolytic stability or
better detectability. A typical reaction to perform a trimethylsilyl derivatization for organic acids is shown below.
Silylation reactions are generally performed in glass vials, often at elevated temperatures. Take note of the possible leakage with
PTFE-line screw caps that may occur at elevated temperatures. Sometimes a basic catalyst (e.g. pyridine, diethylamine) is used
to remove the HCl by-product. Occasionally, mixtures of trimethylsilane reagents are used and can be more effective than any
one alone.
Improved detection is another important goal for derivatization in GC. Silylating reagents containing electron capture groups such as
chloro, bromo, or iodo (electron capture detector) and cyano groups (thermionic detector) have been developed for improved
detectability. Virtually all GC derivatization reactions are performed by precolumn derivatization reactions.
Recently, with the increasing importance of tandem LC-MS/MS techniques, gas chromatographers have been switching some of
their analysis to LC to avoid going through derivatization reactions. The sensitivity and selectivity of these tandem MS techniques
rival the selective and sensitive GC detectors (see Chapter 17 for more details).
References
1.
Poole, C.F.; Poole, S.K.
Chromatography Today
, Elsevier, Amsterdam, 1993.
2.
Lunn, G.; Hellwig, L.C.
Handbook of Derivatization Reactions for HPLC
, Wiley-Interscience, Hoboken, NJ, 1998,
ISBN-10: 0471238880 and ISBN-13: 978-0471238881.
3.
Blau, K.; Halket, J. (Eds.)
Handbook of Derivatives for Chromatography
, 2nd Ed., John Wiley and Sons, Chichester, U.K., 1993.
4.
Lingeman, H.
Detection-Oriented Derivatization Techniques in Liquid Chromatography
, CRC Press, New York, 1990,
ISBN-10: 0824782879 and ISBN-13: 978-0824782870.
5.
Drozd, J.
Chemical Derivatization in Gas Chromatography
, Elsevier Science, 1986, ISBN-10: 0444553223
and ISBN-13: 978-0444553225.
6. Fu, R. Agilent Technologies Publication #5989-9802EN, Shanghai, China, 2008.
(CH
3
)
3
Si-Cl + RCOOH (CH
3
)
3
Si-COOR + HCl

297
Chapter 21
Sample preparation (and to a lesser extent data analysis) has often been considered to be the rate determining step and error-
prone part of an analytical method. If selectivity can be achieved in other portions of the analytical cycle to meet the needs of
the analyst, then the burden placed on sample preparation is decreased. The concept of Just Enough sample preparation is
presented in this chapter and is based on the LCGC No. America
1
article that introduced the concept. Just Enough sample
preparation relies heavily on recent advances in the tandem mass spectrometry detection that provides enhanced sensitivity
and selectivity, unavailable in the past. Even so, more sophisticated sample prep protocols may still be required, especially if ion
suppression/enhancement results from co-eluting interferences.
The topic on how selectivity can be incorporated throughout the sample analysis cycle has been discussed previously
2
. Figure
21.1 illustrates the workflow in a typical sample analysis. In most analytical processes, the chemist is looking for one or perhaps
a few analytes of interest, often in a very complex matrix. Having an analytical method showing sufficient selectivity to analyze
those few compounds of interest with the precision and accuracy required at the concentration level encountered is the desired
outcome of method development. The selectivity can be achieved anywhere within the analytical cycle (Figure 21.1) during
sampling, during sample preparation, sample introduction, the chromatographic separation, at the detector, or even during data
analysis. As long as the analytes of interest can be determined with good sensitivity, the presence of compounds from the sample
matrix can be tolerated as long as those interferences do not cause harm (short-term or long-term) to the analytical instrument or
the column, or if determined to be harmful, they can easily be removed. An example of the latter approach could be backflushing
after each analysis to remove high-molecular-weight contaminants trapped at the head of a GC column. Examples of how
selectivity can be achieved at each step of the analytical cycle for gas chromatography were shown in reference
2
.
Just Enough Sample Preparation:
A Proven Trend
in Sample Analysis

298
Having less selectivity in one portion of the analytical cycle can be made up for by having greater selectivity in another portion of
the analytical cycle. For example, if the analyst only has a fixed wavelength UV detector in his/her HPLC instrument or a thermal
conductivity or flame ionization detector for the GC, there may not be sufficient detector selectivity to provide the necessary
overall method selectivity to measure an analyte of interest without interference from undesired sample components. Therefore,
additional sample preparation or finding a separation column that provides more selectivity during the separation may be required
to make up for the limitations in the detector. In these cases, the analyst may spend a great deal of time and energy performing
one or more sample preparation steps or optimizing the selectivity of the column/mobile phase system (HPLC) to rid of potential
interferences. On the other hand, if one has a very sensitive and selective detector, then perhaps spending a great deal of time
optimizing the sample preparation and/or the analytical separation is unwarranted.
Figure 21.1
Steps in Analytical Cycle
1
Sampling/sample collection
Data analysis
Analyte detection
Analyte separation
Sample introduction
Sample preparation
GC or
HPLC
Carrier gas (GC)
Mobile phase pump (LC)

299
Since achieving selectivity for the separation column is not an easy task to predict, sample preparation often gets the brunt of the
job to remove interferences from the sample of interest. It is sometimes unfortunate to burden the analyst with this job, but there
are time-proven sample prep techniques available. However, with the advent and widespread use of tandem mass spectrometry for
both HPLC and GC with its high degree of selectivity and sensitivity, sample preparation as well as the chromatographic separation
can sometimes be simplified as long as any interferences carried over from the sample matrix do not interfere with the separation or
detection process. We term this simplified sample preparation process as Just Enough sample preparation.
This Just Enough sample preparation process doesn't always provide the cleanest extract from the sample as more rigorous
approaches such as multimodal solid phase extraction (SPE) or liquid-liquid back extraction might achieve, but as long as the
extractables do not harm separation or detection (and, of course, the column or instrument), that’s okay. In reality, the sample
preparation time can be greatly reduced as long as the final outcome meets the needs of the analyst. Although the mass
spectrometer still represents a much higher priced detector than a UV or FID, many laboratories are finding them to be a cost
effective way to enhance and speed up their analyses, thereby improving overall productivity and lowering costs. Of course, less
expensive selective detectors such as fluorescence in HPLC and electron capture in GC still allow the practice of Just Enough sample
preparation provided the analytes do not need derivatization.
The concept of Just Enough sample preparation does not imply one is cutting corners or that more sophisticated protocols are not
required. It really represents a continuum of sample preparation procedures as depicted in Figure 21.2. This figure represents just
a few of the many sample preparation methods that are in widespread use. Starting at the top of the figure with filtration,
centrifugation, and “dilute and shoot” and moving down, the sample preparation protocols become more selective and more
complex sometimes requiring a greater deal of effort and multiple steps to achieve Just Enough cleanup to meet the analytical
needs. Minimizing the number of sample handling steps in any analytical technique is desirable since the more times the sample is
transferred, the greater the chance of analyte loss (or modification), thereby resulting in poorer analytical precision and accuracy. If
one or two steps meet the needs of the method, that may be sufficient, but in some cases, additional sample preparation steps may
be needed to rid of interferences. The need to eliminate or minimize interferences is no greater than that required for LC/MS and
LC-MS/MS (see Figure 21.2).

300
Methodology
•
Filtration
•
Centrifugation
•
Dilute and shoot
•
Sonication
•
Lyophilization
•
Protein precipitation
•
Distillation
•
Dialysis/ultrafiltration
•
Liquid-solid extraction/pressurized fluid extraction
•
Soxhlet extraction
•
Solid phase microextraction
•
Supported liquid extraction
•
Liquid-liquid extraction
•
Solid phase extraction
•
QuEChERS
•
Turbulent flow chromatography
•
Derivatization
•
Column switching/heart cutting
•
Immunoaffinity sorbents
•
Molecularly imprinted polymers
Greater selectivity
Optimal sample cleanup
Less selective
Minimal sample cleanup
More complicated
methodology
Simpler,
generic methodology
Figure 21.2
Just Enough Sample Preparation Represents a Continuum of Methodologies
Figure 21.3 shows a pictorial representation of the Just Enough sample prep concept that actually applies to the entire analytical
cycle, but is emphasized for the sample preparation portion. It is here that many workers are faced with achieving the bulk of their
selectivity enhancement. Ideally, in an analytical method, one always wants to achieve the best result with the least amount of
effort and investment. On the other hand, the actual data requirement may not need the optimum result, but an acceptable result.
For example, in screening hundreds of urine samples for the presence of drugs of abuse, most samples are negative. Thus, a
qualitative analytical method may be sufficient to rule out the presence of an illicit drug. However, if an illegal drug is spotted
during the screening test, then a more careful and perhaps a more sophisticated look at a positive sample is required for
quantitative analysis.

301
Unacceptable
Realistic
(acceptable)
Effort and Investment
There are many other factors that may influence the choice of the sample preparation technique(s) used to provide Just Enough
cleanup. The skill and knowledge of the analyst is important. The availability of instrumentation, chemicals, consumables, and other
equipment; the time available to develop the method and to perform the tasks at hand; the complexity and nature of the matrix;
the analyte concentration level and stability; the required sample size; the cost per sample (budget); and the safety of the sample
preparation technique are just a few of the many considerations that must be taken into account. It is the balance of all of these
and other considerations that come into play.
Quality of Results
Ideal
Just Enough
Figure 21.3
Striking the Right Balance in Sample Preparation

302
Many sample preparation methodologies have already been discussed throughout this book. Figure 21.2 provides a number of
sample preparation protocols that could qualify as Just Enough procedures. As mentioned earlier, the fewer sample preparation
steps in analytical method, the less chance of errors, better analyte recovery, and less time spent handling samples. However, as
one proceeds down Figure 21.2, Just Enough may require more sophisticated sample preparation processes.
Let’s look at a few examples of sample preparation procedures that may qualify as Just Enough and see if they provide acceptable
results. In recent years, for the determination of targeted drugs and their metabolites in biological fluids such as plasma, many
pharmaceutical companies have switched their sample preparation to protein precipitation (see Chapter 16) and reversed-phase
HPLC analysis, but using a more selective, sensitive LC-triple quadrupole MS/MS detector using Multiple Reaction Monitoring
(MRM) at defined transitions. The first example shows the direct analysis of Fluticasone Proprionate (FP) in human plasma using an
LC-triple quadrupole mass spectrometer system
3
. The FP is a synthetic steroid of the glucocorticoid family of drugs for treating allergic
conditions. When used as a nasal inhaler or spray, medication goes directly to the epithelial lining of the nose, and very little is
absorbed into the rest of the body. Due to its low systemic levels, a high sensitivity LC/MS assay is required to determine its
concentration in human plasma. Figure 21.4 shows the LC/MS results from a plasma protein precipitation (see Chapter 16, Protein
Precipitation) followed by dilute and shoot using the MRM transition shown in the figure caption. In this case, the dilute and shoot
method has more than adequate sensitivity at the lowest calibration level of 5 pg/mL. Thus, protein precipitation followed by dilute
and shoot sample preparation has an assay performance well within accepted regulatory guidelines and was Just Enough to meet
the analytical needs.
4.2
0.8 1 1.2 1.4 1.6 1.8 2 2.2 2.4
4.3
4.4
4.5
4.6
4.7
4.8
4.9
5
5.1
5.2
5.3
x10
1
Abundance
Acquisition Time [min]
Examples of Just Enough Sample Preparation
Figure 21.4
Measurement of Fluticasone Proprionate in Plasma
Using Dilute and Shoot Sample Preparation
3
Instrument: Agilent Model 6490 LC-Triple Quadrupole LC/MS System (MRM transition 501.2 → 293.1)
for 2.5 femtograms injected on-column; 1 femtogram limit of detection; standard curve was linear over the
range of 5-50 mg/mL
Sample preparation: plasma sample was precipitated with acetonitrile and then diluted 4-fold with water

303
A second example of Just Enough sample preparation was shown in Chapter 16, which also showed a protein precipitation, but
considered an area where ion suppression comes into play. The figures will not be repeated here and the reader is referred back to
Chapter 16, Removal of Lipophilic Material. The presence of (phospho)lipids in plasma can cause ion suppression if analytes of
interest co-elute in the portion of a chromatogram where these phospholipids appear. Phospholipid MS-MS selectivity can be
achieved by considering the m/e 184 → m/e 184 transition indicative of phospholipid and lysophosphatidylcholine elution. Figure
16.6.1 showed a typical infusion experiment result that is obtained from protein-precipitated plasma, followed by Captiva filtration
(Agilent Technologies, Santa Clara, CA). If the drug and/or its metabolites were to co-elute with these compounds, ion suppression
may occur and the analytical results jeopardized. Thus, in this case, the simple protein precipitation sample preparation procedure
may not be enough to provide reliable data.
QuEChERS (covered in Chapter 8) is a sample preparation technique that was originally developed for the extraction of pesticides
from fruits and vegetables
4
. It is a relatively simple sample preparation procedure involving two steps: 1) a salting out partitioning
extraction involving water and acetonitrile with high concentrations of salts such as sodium chloride, magnesium sulfate, and
buffering agents, and 2) a dispersive-SPE step where an aliquot from step 1 is treated with various sorbents to remove matrix
compounds that could interfere with subsequent LC/MS, LC-MS/MS, GC/MS or GC-MS/MS analysis. The technique has proven to
be widely applicable at trace levels for hundreds of pesticides in a variety of matrices. Standard protocols are available that make it
a generic sample preparation procedure.
Recently, QuEChERS extraction has expanded well beyond the pesticide laboratory and has been used for many matrices ranging
from antibiotics in meat and poultry, veterinary drugs in animal feed, and environmental contaminants in soil. In this third example of
Just Enough Sample Preparation, using the protocol in Figure 21.5, QuEChERS was used for the extraction of polycyclic aromatic
hydrocarbons (PAHs) in fish. The PAHs are a large group of organic compounds included in the European Union and the United
States Environmental Protection Agency priority pollutant list because of their mutagenic and carcinogenic properties. In the marine
environment, PAHs are bioavailable to marine species via the food chain, as water borne compounds, and contaminated sediments.
This application shows that tandem MS detection techniques are not necessarily required for Just Enough sample preparation.
Most of the PAHs are highly fluorescent and thus, as shown in Figure 21.6, reversed-phase HPLC was combined with fluorescence
detection to determine 16 of these compounds at a spiking level of less than 10 ng/g level
5
. QuEChERS extraction provided
excellent recoveries with % RSDs below 2.
By performing the more complex SPE (Figure 16.6.2) or liquid-liquid extraction (Figure 16.6.3), the extract is now cleaner and
many of the phosphorous-containing lipids are greatly reduced. To get the best overall performance, an even more sophisticated
phospholipid reduction may be achieved with a selective SPE phase that removes the last traces of phosphorylated compounds
(Figure 16.6.4). Luckily, a product called Captiva ND
Lipids
, which is a combined membrane filtration/phospholipid removal 96-well
plate, performs both operations at once and thus is a simple Just Enough solution to this problem.

304
Weigh 5 g homogenized fish sample into a 50 mL centrifuge tube
Spike samples with 2000 µL spiking solution
Shake vigorously 1 min
Add 8 mL ACN
Shake vigorously 1 min
Add Bond Elut QuEChERS AOAC salt packet
Shake 1 min, centrifuge at 4000 rpm 5 min
Transfer 6 mL aliquot to Bond Elut QuEChERS Dispersive SPE 15 mL tube
Shake 1 min, centrifuge at 4000 rpm 5 min
Filter through a 0.45 µm PVDF syringe filter
Transfer 1 mL extract to an autosampler vial
Samples are ready for HPLC-FLD analysis
Flow Chart of QuEChERS AOAC Sample Preparation Procedure
5
Figure 21.5

305
0
02
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
1
46
Time (min)
8 10 12 14
2
4
6
LU
8
10
12
Overlay of HPLC-FLD chromatograms of spiked fish sample containing the following PAHs
(at spiking levels shown in parens): 1. naphthalene (20 ng/g); 2. acenaphthylene (20 ng/g);
3. acenaphthene (10 ng/g); 4. fluorene (10 ng/g); 5. phenanthrene (10 ng/g); 6. anthracene (10 ng/g);
7. fluoranthene (10 ng/g); 8. pyrene (10 ng/g); 9. 1,2-benzanthracene (5 ng/g); 10. chrysene (10 ng/g);
11. benzo(e)acenapthylene (5 ng/g); 12. benz(e)acenapthylene (5 ng/g); 13. benzo(k)fluoranthene (5 ng/g);
14. dibenzo(a,h)anthracene (5 ng/g); 15. benzo(g,h,i)perylene (5 ng/g); and 16. indeno(1,2,3-cd)pyrene (5 ng/g);
the black portion of the chromatogram used the following flourescence excitation/emission wavelengths:
260 nm/352 nm; the red portion 260 nm/420 nm; the blue portion: 260 nm/440 nm. For acenaphthylene,
UV detection at 230 nm was used.
HPLC conditions
HPLC-Fluorescence Chromatogram of Spiked Fish Extract
Figure 21.6
Column: Agilent ZORBAX Eclipse PAH C18, 4.6 x 50 mm, 1.8 µm
Flow rate: 0.8 mL per minute
Temperature: 18 °C
Injection volume: 5 µL
Mobile phase: A = deionized water, B = acetonitrile
Gradient:
Time (min): %B
0 60
1.5 60
7 90
13 100

306
References
1.
Majors, R.E.
LCGC No. America
2012,
30 (12)
, 1024-1031.
2.
Turner, D.
LCGC No. America
2012,
30 (2)
, 100-110.
3.
Bioanalysis Application Note Determination of Fluticasone Proprionate in Human Plasma
, Agilent Technologies Application Note
#5990-6380EN, Santa Clara, CA, August, 2010.
4.
Anastassiades, M.; Lehotay, S. J.; Stajnbaher, D.; Schenck, F.J.
Journal of AOAC International (JAOAC)
2003,
86
, 412-431.
5.
Pule, B.O.; Mmualefe, L.C.; Torto, N.
Analysis of Polycyclic Aromatic Hydrocarbons in Fish
, Agilent Technologies Application Note
#5990-5441EN, Santa Clara, CA, January, 2012.
Conclusions
However, as illustrated in the example of PAH analysis in fish, other selective detection principles such as fluorescence can also be
used. A note of caution: in some assays, sample processing (handling) is still the rate determining step and Just Enough sample
preparation may be insufficient to meet the needs of the assay. In these cases, more sophisticated sample preparation protocols,
such as SPE, liquid-liquid extraction, etc. may still be required.
The selectivity needed for the determination of targeted analytes in a complex matrix can be achieved anywhere in the analytical
cycle. With a focus on the sample preparation portion, the concept of Just Enough sample preparation was presented. This concept
relies heavily on the increased sensitivity and selectivity that can be achieved with tandem mass spectrometry coupled with
chromatographic separation. Provided that ion suppression/enhancement contributions are held to a minimum, Just Enough sample
preparation can provide the recoveries, minimum detectable limits (MDLs), and minimum detectable quantities (MDQs) consistent
with the needs of the assay.

307
Chapter 22
Current Trends
and Future Directions
in Sample Preparation
Throughout this book, there are numerous citations on the vast number of improvements that have been made in the area of
sample preparation for chromatography. Compared to a decade ago, many modern sample preparation techniques:
•
Are faster
•
Are safer
•
Are easier to automate
•
Have been miniaturized
•
Use less organic solvent
•
Equal or exceed sample recovery and reproducibility of older methods
However, in many circles, sample preparation is still considered to be the bottleneck in the analytical laboratory. Overall, it has not
received the attention of academics that may consider sample preparation topics as too mundane and less challenging than
focusing on the analysis itself. Nevertheless, workers in the field continue to develop new and improved sample preparation
techniques some of which may provide breakaway performance and acceptance as mainstay, routine methodologies such as solid-
phase microextraction (SPME), QuEChERS, and pressurized fluid extraction.
Accepted techniques find new applications such as the use of QuEChERS outside of the pesticide in fruits and vegetables domain,
solid-phase microextraction in remote sampling, or dried blood spotting to other biological fluids. New sorbents for liquid-solid
extractions continue to be developed such as the very selective molecularly imprinted polymers and immunosorbent phases. Entirely
new techniques such as dispersive liquid-liquid microextraction, ionic-liquid extraction, and microscale salting out liquid-liquid
extraction could become the next well accepted technique like solid-phase extraction. Automation of many of the time-consuming
sample preparation techniques have been advancing in recent years, freeing up the analyst to spend time performing more
challenging tasks. Full laboratory robotic systems of the 90’s have given way to x-y-z benchtop liquid handling systems which are
closer to and often integrated with the analytical instrument. Autosamplers with sample preparation capabilities beyond injection
have been around for a few years and have proven to be useful for the last few crucial steps prior to injection. Sometimes, for liquid
and solid samples, these autosamplers and benchtop sample preparation systems can provide a complete level of automation, even
weighing the sample.

308
As was amply pointed out in Chapter 21, the increasing sensitivity/selectively of mass spectrometers and more efficient and
selective chromatographic separations have, in some cases, provided the selectivity that, in the past, rested on the shoulders of the
sample preparation chemist. Thus, developing an analytical method that combines the optimized elements of sampling, sample
preparation, analysis, detection, and even data analysis can provide a more encompassing robust solution for complex samples. The
increasing popularity of multidimensional on-line column switching systems with orthogonal separation modes can provide a
sample prep/analysis solution where method development may be faster and more selective than spending hours developing the
optimum sample preparation technique.
The whole idea of further miniaturization driven by increased instrument sensitivity can reward the analyst in more ways than one.
Smaller sample amounts are now required. Using 96-well flow-through SPE plates with each well containing only a few milligrams
of sorbent not only speeds up the extraction time, but requires only a few microliters of elution solvent. Thus, solvent evaporation
and reconstitution time is remarkably sped up. Using 96-well evaporation systems and plate handlers, sample preparation no longer
becomes the rate-determining step and high-throughput sample handling is now becoming commonplace. And to top it off, the
amount of solvent used is decreased proportionally to the level of miniaturization thereby lowering purchase and disposal costs.
Although chip-based analysis systems are still not in routine use, there is no reason not to believe that down the road, such
integrated systems may take the place of instruments now consuming entire laboratory benches. Field-portable analytical
instruments, some with simple sample prep capabilities, are already in use and support a trend to bring the analysis closer to the
source or to the manufacturing floor.
While we are on the topic of miniaturization, the future for nanomaterials in sample preparation looks very bright. Due to the
“nanoscale” effect, nanomaterials have unique physical and chemical properties that make them superior candidates as sorbents.
A wide variety of nanoparticles (e.g. metallic, metallic oxide, metal organic framework, siliceous and carbonaceous such as
grapheme and carbon nanotubes) have been the subject of widespread studies, not only as µ-SPE sorbents but also as coatings
for SPME rods and fibers. In the latter case, immobilized nanoparticles appear to be as rugged as existing polymeric phases, have
a higher surface area for increased sensitivity, and can be derivatized to impart new chemistries. Particularly interesting are the
magnetic SPE particles that are directly dispersed into sample solutions to quickly extract analytes since they can be readily
recovered by a magnet. Magnetic handling overcomes the problems of conventional SPE, such as eliminating packing of
cartridges or plates, or the time consuming process of loading large volume of liquid samples.
Green chemistry has been discussed for a long time and there is some evidence that it is catching on in some large companies
and institutions, not only in the manufacturing environment, but also in the chemistry laboratory. In the analytical and separations
laboratory, green chemistry not only means changing solvents to more environmentally friendly and safer ones, but in reducing
solvent consumption, reducing energy consumption, and considering solventless sample preparation techniques. In the latter
consideration, SPME, single-drop microextraction, superheated water extraction, and supercritical fluid extraction (and
chromatography) are just a few of the techniques that almost eliminate the use of conventional organic solvents. The jury
is still out on the increased promotion of ionic liquids and eutectic solvents for greener extractions.


310
Glossary
96-well collection plate A fixed size polyethylene rectangular plate (127.8 mm x 85.5 mm) consisting of an array of 8 x 12 (96) small
"test tubes" called wells; volumes of wells range from 0.5-2 mL; see Chapter 9.
96-well filtration plate A fixed size polyethylene rectangular plate (127.8 mm x 85.5 mm) consisting of an array of 8 x 12 (96) small
filter tubes (volumes range from 0.5-2 mL); a membrane filter placed at the bottom of the well is used to
filter liquid samples; sometimes a prefilter is placed above the membrane filter to prevent clogging with
particulate samples; see Chapter 5.
96-well plate A small, rectangular plastic plate consisting of 96-well individual wells which are small volume test tubes
arranged in an 8 x 12 well pattern; used for liquid handling and other such requirements; see Chapters 5 and 9.
96-well solid phase
extraction plate
A small, rectangular plastic plate consisting of 96-well individual flow-through SPE wells arranged in an
8 x 12 array that have top and bottom frits to contain solid particles of sorbent or resin to perform SPE on a
miniaturized scale; generally 1 mg-0.2 g of packing are placed into the well which can be up to 2 mL volume;
used for automated SPE with x-y-z liquid handling systems or customized workstations; see Chapter 9.
Accelerated solvent extraction Tradename for a Pressurized Fluid Extraction System introduced by Dionex and now sold by ThermoFisher
Scientific; see pressurized fluid extraction in Chapter 14.
ACN Abbreviation for acetonitrile, a popular solvent for reversed-phase LC as well as sample preparation.
Active sampling In active gas sampling, a pump is used to push the sample through a mass flow controller and into the
canister. Additional sample can be collected, relative to the amount that can be collected by passive sampling,
by pressurizing the canister with sample. Commonly, the sample is pressurized to 15 psig, effectively doubling
the sample volume.
Active site A reactive or strongly attracting site on the surface of a chromatographic packing that may bind analytes or
cause peak tailing; sometimes mobile phase additives (e.g. competing base) can negate the effects of
active sites.
Additive A substance added to the mobile phase to improve the separation or detection characteristics; examples
would be a competing base to negate the effects of silanols, a chelating agent to block metal sites or addition
of a UV-absorbing compound to perform indirect photometric detection.
Adsorbent Packing used in adsorption chromatography. Silica gel and alumina are the most frequenly used adsorbents
in HPLC and sample preparation.
Chapter 23

311
Adsorption The process of retention in which the interactions between the solute and the surface of an adsorbent
dominate. The forces can be strong forces (for example, hydrogen bonds) or weak (van der Waal's forces).
For silica gel, the silanol group is the driving force for adsorption and any solute functional group which can
interact with this group can be retained on silica. The term adsorption places emphasis on the surface vs.
penetration or embedding in the stationary phase coated or bonded to a surface; see Chapters 9 and 10.
Adsorption chromatography One of the basic LC and SPE modes which relies upon the adsorption process to effect the separation. Silica
gel and alumina are the most frequently used normal phase adsorbents and molecules are retained by the
interaction of their polar function groups with the surface functional groups (e.g. silanols of silica). Carbon
is also used as an adsorbent in a reversed-phase mode; see Chapters 9 and 10.
Adsorption isotherm In adsorption, a plot of the equilibrium concentration of sample in the mobile phase per unit volume versus
the concentration in the stationary phase per unit weight. The shape of the adsorption isotherm can determine
the chromatographic behavior of the solute such as tailing, fronting, overload, etc.
Affinity chromatography A technique in which a biospecific adsorbent is prepared by coupling a specific ligand (such as an enzyme,
antigen, or hormone) for the macromolecule of interest to a solid support (or carrier). This immobilized ligand
will interact only with molecules that can selectively bind to it. Molecules that will not bind elute unretained.
The retained compound can later be released in a purified state. Affinity chromatography is normally practiced
as an "on-off" separation technique; see Chapter 10.
Alumina A normal phase adsorbent used in adsorption chromatography. Aluminium oxide (Al
2
O
3
) is a porous adsorbent
which is available with a slightly basic surface; neutral and acidic modifications can also be made. Basic
alumina can have advantages over silica, which is considered to have an acidic surface; alumina is seldom
used as an HPLC column packing in practice; see Chapter 9.
Amino phase A phase used in normal bonded phase chromatography which is a propylamino phase. It is a somewhat
reactive phase for any solute molecule (e.g. aldehydes) or mobile phase additive that can react with amines.
The amino phase has found some applications as a weak anion exhanger and for the separation of
carbohydrates using a water-acetonitrile mobile phase. It is a relatively unstable phase; see Chapter 9.
Amphoteric ion exchange resin Ion exchange resins that have both positive and negative ionic groups. These resins are most useful for ion
retardation in which all ionic materials can be removed from solution since the anionic and cationic
functionalities coexist on the same material; see Chapter 9.
Analyte The compound of interest to be analyzed by injection into and elution from an HPLC or GC column.
Analyte protectant In GC, a chemical compound that is added to a sample before injection to cut down on interactions between
analytes that are unstable or behave poorly in the GC flow path on active sites; the protectants are chosen so
that they do not interfere with the analysis of the compounds of interest yet prevent these compounds from
interacting with the active sites in the flow path; these protectants are not generally required for LC and LC/MS;
see Chapter 8.

312
Backflushing A column switching technique in which, for LC a four-way valve placed between the injector and the column
allows for mobile phase flow in either direction. In GC, pressure (or Deans) switching is used to control the
direction of flow. Backflushing is used to elute strongly held compounds at the head of the column. It can be
used for analysis of these compounds or merely to remove them from the column. By reversing the flow at
the conclusion of a run, the analytes trapped at the head of the column can be flushed from the column
since they have a shorter distance to travel; sometimes a strong solvent (LC) or higher temperature and/or
flow rate (GC) will be needed to move them along; see Chapters 6, 9, 10, 13 and 21.
Bar coding A bar code is an optical machine-readable representation of data relating to the object to which it is attached.
Bar codes are sometimes used in chromatographic automation to track the sample through the analytical
process; bar code strips can be adhered to vials, beakers, or other containers; vials can sometimes be given
permanent bar codes; more recently, universal product codes and RFI tags have been proposed; bar code
readers are available for autosamplers to aid in the sample tracking process; they are generally interfaced
directly to the data system.
Bind-elute In SPE, the normal mode of operation in which upon loading the sample onto a conditioned sorbent or resin,
the analytes of interest are retained (bound) while interferences, and perhaps some of the matrix, is not
retained by the packing; after a wash step to remove some of the undesired sample components, the elute
step uses a strong solvent to elute the analyte(s) of interest in a small volume; see Chapter 9.
Blank
More correctly named
method blank
: a blank prepared to represent the matrix as closely as possible. The
method blank is prepared/extracted/digested and analyzed exactly like the field samples. Purpose: assess
contamination introduced during SP activities.
Argentation SPE The incorporation of a silver salt into the SPE stationary phase will help in retaining compounds with olefinic
bonds. Normally used in organic solvents to maximize charge-transfer interactions; see Chapter 10.
Array 96-well plate A 96-well SPE plate in which the 96 individual wells are removable from the base plate; such a setup allows
the user to place different types and amounts of SPE sorbents into his/her own configuration in each of the
96-wells. This type of 96-well plate has also been referred to as a flexible 96-well plate configuration; see
Chapter 9.
Back extraction Use in liquid-liquid extraction to perform an additional extraction to further purify a sample; initially the
extraction may take place with an aqueous solvent buffered at a high pH and an immiscible organic solvent;
once the initial extraction takes place and interferences are removed, then by having another aqueous
solution at a low pH, one can back extract the analyte into the organic layer based on the analyte now being
in a neutral form. An example would be for the cleanup of an acidic substance containing -COOH groups;
at high pH the carboxyl would be ionized and prefer the aqueous layer and impurities may migrate to the
organic phase and be discarded; then the pH of the aqueous layer can be adjusted to a low value. Now the
carboxyl group is in an unionized form and readily extracted into the organic layer as a purified substance;
see Chapter 7.
Anion exchange The ion exchange procedure used for the separation of anions. Synthetic resins, bonded phase silicas and
other metal oxides are available for this mode. A typical anion exchange functional group is the
tetraalkylammonium making a strong anion exchanger. An amino group on a bonded stationary phase would
be an example of a weak anion exchanger; see Chapter 9.

313
Blending Refers to the process of making a heterogeneous sample into a more consistent and uniform sample by
some type of blending operation; the most popular type of blender is the mechanical blender that chops a
semi-soft material into smaller parts; see Chapters 4 and 16.
Bonded phase A stationary phase which is covalently bonded to the support particles or inside the wall of a tube (capillary).
Box car chromatography
See
column switching
; alternate name.
Breakthrough capacity
See
breakthrough volume
.
Breakthrough volume The volume at which a particular solute pumped continuously through a column will begin to elute. It is
related to the column volume plus the retention factor of the solute. It is useful to determine the total sample
capacity of the column for a particular solute; see Chapter 9.
Buffer A solution that maintains constant pH by resisting changes in pH as a result of dilution or addition of small
amounts of acids and bases.
Buffer strength
See
ionic strength
.
C4, C8, C18, etc. Refers to the alkyl chain length of a reversed bonded phase.
Canister collection A stainless steel vessel designed to hold vacuum to less than 10 mTorr or pressure to 40 psig. Canisters are
available in a range of volumes: 400 mL, 1.0 L, 3.0 L, 6.0 L, and 15 L. The size of canister used usually
depends on the concentration of the analytes in the sample, sampling time, flow rate, and sample volume
required for the sampling period. Typically, smaller canisters are used for more concentrated samples, such
as soil gas collection, 3 L and 6 L canisters are used to obtain integrated (TWA) ambient air samples at
sampling times of up to 24 hours, and large 15 L canisters are used for reference standards. Sampling time
will be limited by the combination of canister size and the flow rate at which the sample is to be collected;
see Chapter 6.
Capacity factor
A chromatographic parameter that measures the degree of retention. See
k'
for calculation method. Now
referred to as
k
, the retention factor (IUPAC).
Carbon load For a bonded phase silica, term usually used to describe the surface coverage or the degree to which the
available silanols on the column packing's surface have reacted and been replaced with the bonded phase; the
higher the carbon load, the lower number of residual silanols. The carbon load is normally expressed as a %
carbon (e.g. 12% carbon). In reversed-phase LC or SPE, the higher the carbon load, the greater the analyte
retention.
Cartridge Generally refers to the container used in SPE or filtration; a cartridge may be as simple as a medical grade
syringe barrel which is filled with packing contained at both ends by frits; it can also be a molded device or
even a stainless steel device that contains simliar sorts of packing material. In SPE, the device is also referred
to as an SPE tube; see Chapters 4 and 9.
Cation exchange
chromatography
The form of ion exchange chromatography that uses resins or packings with functional groups that can
separate cations. An example of a strong cation functional group would be a sulfonic acid; a weak cation
exchange functional group would be a carboxylic acid; see Chapters 9 and 10.

314
Concentration The process of increasing the strength or density of a diluted sample; a more concentrated sample will be
easier to measure; concentration can be accomplished by a wide variety of sample prep techniques such as
evaporation, adsorption, diffusion, etc.
Centrifugation A process that involves the use of the centrifugal force for the sedimentation of mixtures with a centrifuge
(see centrifuge). This process is used to separate two immiscible liquids. More-dense components of the
mixture migrate away from the axis of the centrifuge, while less-dense components of the mixture migrate
towards the axis. Chemists and biologists may increase the effective gravitational force on a test tube so as
to more rapidly and completely cause the precipitate ("pellet") to gather on the bottom of the tube. The
remaining solution is properly called the "supernate" or "supernatant liquid." The supernatant liquid is then
either quickly decanted from the tube without disturbing the precipitate, or withdrawn with a Pasteur pipette;
see Chapters 1, 2, 4, 5, 7, 8, 9, 10, 14, 15, 16 and 21.
Centrifuge A piece of equipment generally driven by an electric motor (some older models were spun by hand), that
puts an object in rotation around a fixed axis, applying a force perpendicular to the axis; see
centrifugation
.
Certify SPE products for drugs of abuse analysis; see Chapter 10.
Chain length The length of carbon chain in the hydrocarbon portion of a reversed-phase packing. It is expressed as the
number of carbon atoms (e.g. C8, C18). Specifically excludes the short chains typical methyl, isopropyl, and
sec-butyl groups also attached to the silane.
Chelating resin Contains functional groups that will interact with cationic species (e.g. metals such as copper, iron, heavy
metal ions); useful for concentrating trace quantities or for separation.
Chemical filtration A liquid sample is passed through a packing material (e.g. adsorbent, ion exchange, etc.) that selectively
interacts with one or more compounds within the sample and acts as a chemical way to remove and purify
the liquid sample. Regular filtration does not involve any chemical interaction but merely removes particulates;
see Chapter 4.
Chemisorption Sorption due to a chemical reaction with the packing. Most such interactions are irreversible. Usually occurs
on packings with reactive functional groups such as silanol or bonded amino phases. Chemisorption is
common with metal oxide phases that have strong Lewis acid sites.
Chopping The process of mechanically cutting a sample into smaller parts; see Chapter 4.
Co-ion An ion of the same sign of charge as the ionic groups making up the stationary phase.
Column switching The use of multiple columns connected by switching valves to effect better chromatographic separations or
for sample cleanup. Fractions from a primary column can be switched to two or more secondary columns,
which in turn can be further diverted to additional columns or to the detector(s); sometimes referred to as
multidimensional chromatography; see Chapters 10, 11 and 13.

315
Conditioning (SPE) Generally considered to be the first step in SPE; the stationary phase must first be put into a chemical/physical
state so that it can accept the sample solution loaded in the second SPE step; a conditioning solvent is passed
through the SPE stationary phase where it will solvate the phase so that it will more easily sorb the sample
of interest; for a reversed-phase SPE cartridge, methanol or acetonitrile serves as a conditioning solvent;
sometimes the excess conditioning solvent must be removed but the packing shouldn't be allowed to dry
out since that may affect the "conditioned" phase; see Chapter 9.
Coning and quartering A sample size reduction technique in which a portion of free-flowing solid material (powder) is systematically
divided into quadrants in order to achieve a statistically representative sample. A method used by analytical
chemists to reduce the sample size of a powder without creating a systematic bias. The technique involves
pouring the sample so that it takes on a conical shape, and then flattening it out into a cake. The cake is
then divided into quarters; the two quarters which sit opposite one another are discarded, while the other
two are combined and constitute the reduced sample. The same process is continued until an appropriate
sample size remains. Analyses are made with respect to the sample left behind; see Chapter 4 .
Continuous liquid-liquid
extraction
Useful when the K
D
value is very low or the required sample volume is very large when multiple extractions
are impractical; also if the extraction is slow, a long time may be required for equilibrium to be established;
in continuous LLE, fresh solvent is continually recycled through the aqueous sample; continuous extractors
are available for heavier-than-water and lighter-than-water solvents; see Chapter 7.
Cool on-column injection A technique of introducing a sample as a liquid directly into a GC column; this lack of prior vaporization offers
the following advantages: 1) Eliminates sample discrimination; 2) Eliminates sample alteration; and 3)
Provides high analytical precision. However, there are some special requirements: 1) Requires relatively
clean samples; 2) Real samples are often too concentrated for on-column injection and must be diluted; and
3) Peak splitting or peak distortion can occur due to differing polarities of solvent, stationary phase, and
solutes; see Chapter 6.
Counter-ion In an ion exchange process, the ion in solution used to displace the ion of interest from the ionic site. In ion
pairing, it is the ion of opposite charge added to the mobile phase to form a neutral ion pair in solution.
Coupled columns A form of column switching that uses a primary column connected to two secondary columns via a selector
valve. Fractions from column 1 can be selectively transferred to columns 2 and 3 for additional separation
to occur. Term also used to describe two or more columns connected in series to provide an increased number
of plates; see Chapter 13.
Coverage Refers to the amount of bonded phase on a silica support in bonded phase chromatography. Coverage is
usually described in µmoles per m
2
or in terms of %C (W/W).
Crash plate Refers to the process of precipitating protein from plasma by the addition of a miscible organic solvent such
as acetonitrile; when a 96-well flow-through or fixed-well plate is used for this process, it is referred to as
crashing and the plate a crash plate; see Chapter 16.

316
Cyano phase A chemically bonded phase that terminates with the -CN functional group; can be used in normal phase as
a moderate polarity sorbent and in reversed-phase as a short chain bonded phase.
Deep well plate A 96-well plate capable of handling up to 2 mL of liquid volume per well; see Chapter 9.
Derivatization A technique used in chemistry which transforms a chemical compound into a product (the reaction's derivate)
of similar chemical structure, called a derivative. Generally, a specific functional group of the compound
participates in the derivatization reaction and transforms the compound(s) into one with a different reactivity,
solubility, boiling point (volatility), melting point, aggregate state, or chemical composition. The resulting new
chemical properties can be used for quantification (e.g. UV or fluorescence detection) or better separation
properties; see Chapter 20.
Desalting Technique in which low molecular weight salts and other compounds can be removed from non-ionic and
high molecular weight compounds. For example, use of a reversed-phase packing to retain sample compounds
by hydrophobic effects, yet allow salts to pass through unretained would be an example of desalting. Use of
an SEC column to exclude large molecules and retain lower molecular weight salts is another example;
desalting using dialysis is commonly used in protein purification; see Chapters 9, 11, and 15.
Desorption The process in chromatography or SPE when a molecule residing on the surface of a packing material
or another solid surface (e.g. column wall, frit, etc.) or stationary phase moves from the surface into the
mobile phase.
Dialysis Works on the principles of the diffusion of solutes and ultrafiltration of fluid across a semi-permeable
membrane. Diffusion is a property of substances in water, which tend to move from an area of high
concentration to an area of low concentration. A semi-permeable membrane is a thin layer of material that
contains holes of various sizes, or pores. Smaller solutes and fluid pass through the membrane, but the
membrane blocks the passage of larger substances (for example, red blood cells, large proteins). A technique
used in biological sample prep to desalt biological fluids; see Chapters 15 and 18.
Crosslinking For resins, during the process of copolymerization to form a three-dimensional matrix, a difunctional monomer
is added in order to form crosslinkages between adjacent polymer chains. The degree of crosslinking is
determined by the amount of this monomer added to the reaction. For example, divinylbenzene is a typical
crosslinking agent for the production of polystyrene ion exchange resins. The swelling and diffusion
characteristics of a resin are governed by its degree of crosslinking.
Crushing Tungsten-carbide variable jaw crushers for reducing the size of large, extremely hard, brittle samples; see
Chapter 4.
Cutting Cutting mills can reduce soft-to-medium hard materials (<100 mm diameter); see Chapter 4.

317
Diatomaceous earth Also known as diatomite or kieselgur/kieselguhr, is a naturally occurring, soft, siliceous sedimentary rock
that is easily crumbled into a fine white to off-white powder. Diatomaceous earth consists of fossilized remains
of diatoms, a type of hard-shelled algae. It has a particle size ranging from less than 3 micrometers to more
than 1 millimeter, but typically 10 to 200 micrometers. Depending on the granularity, this powder can have
an abrasive feel, similar to pumice powder, and is very light as a result of its high porosity. The typical chemical
composition of oven-dried diatomaceous earth is 80 to 90% silica, with 2 to 4% alumina (attributed mostly
to clay minerals) and 0.5 to 2% iron oxide; highly purified diatomaceous earth is used as a support for
chromatography and for supported liquid-liquid extraction, see Chapter 7.
Diethylaminoethyl A popular weak anion exchange functionality (typically attached to cellulose or Sepharose, a product of GE
Healthcare) used for the separation of biomolecules; functionality has also been attached to other polymeric
materials and silica gel.
Digestion The process of treating an insoluble chemical compound with a reactive substance (e.g. for inorganic
compounds it might be a strong acid; for a biological compound it might be an enzyme) that will break it
down or disintegrate the compound into a more soluble form that can be further treated or analyzed; see
Chapters 2, 15 and 16.
Digital chromatography The process of solid phase extraction (SPE) is sometimes referred to as digital chromatography – a substance
is either on the SPE stationary phase or is off the stationary phase during its retention/elution; in
chromatography, we are often trying to resolve closely related substances by exploiting subtle differences
in retention in more of an analog separation mode; in terms of k values, ideally the solute in SPE has a value
of infinity when on the sorbent and zero when eluted into solution; see Chapter 9.
Dilute and shoot A simple sample prep procedure in which one merely dilutes the sample with solvent, mobile phase, or a
compatible liquid, and then injects the diluted sample into a chromatograph without any further sample
preparation; see Chapters 2, 16 and 21.
Dilution Reducing the concentration of a chemical by adding an inert substance; the substance can be a liquid, solid,
or gas.
Diol phase A hydrophilic phase useful in both normal and reversed-phase. It consists of a diol structure (two -OH groups
on adjacent carbon atoms in an aliphatic chain). In normal phase work, it is less polar than silica, and in
reversed-phase work, has been used for the separation of proteins and polypeptides; see Chapters 9, 10
and 16.
Direct sampling A method of sample collection in which a sample is taken directly from the source. For example, a canister
may be used to collect a gas sample exactly at its source. A river water sample can be obtained by lowering
a collection vessel directly into the water. A thermal desorption tube can be used to concentrate volatile and
semi-volatile analytes by passing a gas stream through the adsorbent contained within the tube.

318
Direct thermal sampling The process of using temperature as a variable in sample volatile and semi-volatile substances; static
headspace at a given temperature is an example of thermal sampling; by selecting a certain temperature,
certain sample components can be ruled out since they may have extremely low volatility at a selected
temperature; thermal sampling can occur in stages all the way up to pyrolysis when chemical bonds in the
sample are purposely broken in order to access the structure of the material; see Chapter 6.
Disk A number of SP and separation media take the form of a disk; the most popular disks are used in filtration
and may consist of any number of porous polymeric materials; the most popular types of SPE disks would
have embedded particles in a disk made of PTFE or other inert polymeric material, or a fiberglass matrix with
interdispersed sorbent particles; some biological purification media employ the disk format. The stationary
phases may contain ion exchange groups or other functionality that attracts solutes of interest or impurity
that one may want to discard; see Chapter 9.
Disk cartridge (SPE)
See
disk
.
Dispersive
liquid-liquid microextraction
Technique based on a three-component solvent system. The container is usually a centrifuge tube and the
appropriate mixture of immiscible organic extraction solvent (usually a few microliters such as 8 µL of
tetrachloroethylene, TCE) and a dispersive solvent (e.g. ~ 1 mL of acetone) is rapidly injected with a syringe
into an aqueous solution (~ 5 mL) containing the analyte of interest. When the 3 solvents are rapidly mixed,
a cloudy solution is formed consisting of droplets of extraction solvent; the entire mixture is centrifuged and
the droplet of solvent containing extracted analytes (TCE) is removed by a microsyringe for direct injection.
Extraction is almost instantaneous and enrichment values are quite high; see Chapter 7.
Dispersive SPE Method in which loose SPE packing material is added directly to a solution rather than passing it through
the packed material in a cartridge or tube; most often used as the second step in QuEChERS when matrix
compounds are removed from the organic solvent salting out extraction of step 1; see Chapter 8.
Disposable filter
See
syringe filter
; see Chapter 5.
Dissolution The process of having a sample dissolve in an appropriate solvent.
Distillation A method of separating mixtures based on differences in volatility of components in a boiling liquid mixture.
Distillation is a unit operation, or a physical separation process, and not a chemical reaction; it can be used
to purify organic compounds or to remove solvent; fractional distillation is used to separate compounds with
close boiling points; azeotropic distillation is using an azeotrope to remove a solvent that has a boiling point
too close or equal to another compound that cannot be separated.

319
Distribution constant
(coefficient) (K
c
)
The equilibrium concentration of a component in or on the stationary phase divided by the equilibrium
concentration of the component in the mobile phase; also called the distribution coefficient or in partition
chromatography the partition coefficient; in partition chromatography Kc is used and refers to the case in
which the concentration in the stationary phase is expressed per unit volume of the phase (V
R
= V
M
+ K
c
V
S
);
in the case of a solid stationary phase, Kg is used and is expressed as per mass (weight) of the dry solid
phase; in the case of adsorption chromatography with a well characterized adsorbent of know surface area,
the concentration in the stationary phase is expressed as per unit surface area.
Dividers A mechanical device used in sub-dividing solid powder samples into smaller units; they can be manual or
automated; sample dividers will subdivide material samples into two smaller portions by a single pass or
further subdivisions can be attained by multiple passes. The important feature sample dividers is that each
subdivision retains the characteristics of the original sample; see Chapter 4.
Dried blood
spot analysis
A newer method for sampling and transporting blood samples; a small (~15 µL) sample of whole blood is
placed on a cellulose or other paperlike material and is dried for 2 hours; the dried blood spot can be extracted
to remove analytes of interest for further workup; has potential to replace drawing large quantities for blood
analysis; used in conjunction with LC-MS/MS for high sensitivity and specificity; see Chapter 16.
Dried media
spot analysis
In addition to DBS, other biological fluids (e.g. plasma, serum, CSF, saliva) as well as other non-biological
liquid samples have been investigated; see Chapter 16.
Drying Drying of sample extracts can be accomplished by heating (evaporation), vacuum dessication, and other means;
water can be removed (dried) from organic solvents by using anhydrous sodium sulfate; see Chapter 4.
Dynamic headspace
See
purge and trap
; see Chapter 6.
Electrodialysis Used to transport salt ions from one solution through ion exchange membranes to another solution under
the influence of an applied electric potential difference. This is done in a configuration called an electrodialysis
cell. The cell consists of a feed (diluate) compartment and a concentrate (brine) compartment formed by an
anion exchange membrane and a cation exchange membrane placed between two electrodes; can provide
good enrichment factors; see Chapters 15 and 18.
Eluotropic series A series of solvents (eluents) with an increasing degree of solvent strength generally used in LSC or adsorption
chromatography. In normal phase chromatography and SPE, a non-polar solvent such as pentane would be
at one end of the scale, an intermediate solvent such as dichloromethane would be in the middle of the
scale, and a strongly polar solvent such as methanol would be near the upper end of the scale. In NPC, the
reverse order of strength would be observed; water would be weak and hexane strong. Thus, when
developing a method or running a gradient, an eluotropic series is useful for selecting solvents.

320
End capping A technique used to remove silanol groups on silica gel that may remain after reaction with a large silylating
agent such as octadecyltrichlorosilane. The column is said to be endcapped when a small silylating reagent
(e.g. trimethylchlorosilane, and dichlorodimethylsilane) is used to bond residual silanol groups on a silica
gel-based packing surface. Most often used with reversed-phase packings to minimize undesirable adsorption
of basic, ionizable and ionic compounds. For polymeric phases with terminal silanol groups, endcapping
reactions are also used to remove them.
Evaporation The process of removing a volatile compound for the purposes of isolating a compound of interest; solvent
evaporation is one of the most often used sample preparation techniques.
Exchange capacity
See
ion exchange capacity
; see Chapter 9.
Exclusion chromatography See size exclusion chromatography and ion exclusion chromatography; see Chapters 11, 15 and 16.
Exclusion limit In SEC, the upper limit of molecular weight (or size) beyond which molecules will elute at the same retention
volume, called the exclusion volume. Many SEC packings are referred to by their exclusion limit. For example,
a 10*5 column of porous silica gel will exclude any compounds with a molecular weight over 100,000,
based on a polystryene calibration standard; see Chapter 11.
Exclusion volume The minimum retention volume of a molecule on an SEC packing in which all molecules larger than the size
of the largest pore are totally excluded. These molecules are incapable of penetrating the pores and elute
at the interstitial (interparticle) volume of the column; see Chapter 11.
Extraction The general term for removing analytes of interest from a matrix.
Fiberglass disks (SPE) A format in which SPE particles are embedded in a fiberglass matrix; the disk format is especially useful for
processing large volumes of sample (e.g. water) since the larger cross sectional area allows for high flow
rate than can be used for a typical cartridge; see Chapter 9.
Elution step (SPE) Generally considered to be the fourth step in SPE and occurs after the washing (rinsing) step; in the elution
step, analytes are removed from the SPE stationary phase by elution with a strong solvent so that the analytes
are now in a concentrated state; often, the strong solvent is removed by evaporation and reconstituted in a
solvent more compatible with the chromatographic system; see Chapter 9.
Emulsion A mixture of two or more liquids that are normally immiscible (nonmixable or unblendable). Emulsion is
usually referred to when both the dispersed and the continuous phase are liquids. In an emulsion, one liquid
(the dispersed phase) is dispersed in the other (the continuous phase). Emulsions are bothersome in LLE
since they sometimes are hard to break so that the layers can be separated and further treated, if necessary.
There are numerous ways to break emulsions; see Chapter 8.

321
Filter funnel A laboratory funnel used for separating solids from liquids via the laboratory process of filtering. In order to
achieve this, a disk-shaped piece of filter paper is usually folded into a cone and placed within the funnel.
The suspension of solid and liquid is then poured through the funnel. The solid particles are too large to pass
through the filter paper and are left on the paper, while the much smaller liquid molecules pass through the
paper to a vessel positioned below the funnel, producing a filtrate. The filter paper is used only once. If only
the liquid is of interest, the paper is discarded; if the suspension is of interest, both the solid residue on the
paper and the filtrate are kept for further analysis; see Chapter 5.
Filter holder Membrane filter or membrane disk are sometimes furnished loose and can be placed in a holder (usually of
stainless steel construction) for processing samples; once the filter is used, the holder is opened and the
used filter replaced with a fresh one; see Chapter 5.
Filter porosity Pore size relates to the filter’s ability to filter out particles of a certain size. For example, a .05 micron (µm)
membrane will filter out particles with a diameter of .05 µm or larger from a filtration stream. Filter porosity
is typically not related to – nor controlled by – pore size. These two parameters are essentially independent.
Porosity is also unrelated to thickness. Rather, it is a function of the polymer and casting process used in the
manufacture of the filter; see Chapter 5.
Filter vial A membrane filter unit that consists of two pieces: the filtration plunger, which contains a membrane filter
suitable for the solvent being filtered; the second part of the filter vial is the vial body itself; once the sample
is loaded and filtered, the filter vial can be placed directly in the autosampler without transferring the filtered
sample to another vial; see Chapter 5.
Filtration The process of passing a liquid through a paper, membrane, glass, or other type of filter for the purposes of
removing particulates that could cause problems downstream during a chromatographic analysis; a chemical
filter also removes certain chemical species; see chemical filtration; see Chapter 5.
Fixed well plate
A 96-well plate with fixed (non-removable) wells, not an array; see
96-well plate
and
array 96-well plate
.
Flash chromatography A very fast form of classical liquid chromatography used by synthetic organic chemists to effect rapid
purification. Done primarily in the normal phase mode, sometimes with RPC; see Chapter 12.
Flexible well plate
See
array 96-well plate
.
Flow injection extraction An on-line extraction technique in which a sample is injected as a plug into an aqueous flow stream that is
divided into small segments by an organic phase; the aqueous and organic segments are mixed during their
passage down a coil and eventually the phases are separated at the end by a special fitting and the amount
of extract compound can be measured in the organic phase; see Chapter 7.
Fluoro-phase One of a family of aliphatic and aromatic reversed-phase materials in which a substantial fraction of the
bonded phase is fluorinated. Sometimes called fluorous phases or perflfuoro phases. Typically have different
selectivities than hydrocarbon phases.

322
Fluted filter paper Filter paper which is folded in a systematic manner to allow more air space in the filter funnel; this allows
liquid to flow faster through the filter paper; see Chapter 4.
Forced-flow leaching Solid material is packed into stainless steel column and toluene (or other extraction solvent) is pumped into
the column under pressure and with heating; hot solvent leaches (extracts) out extractable compounds which
are collected at the exit of the column; see Chapter 14.
Fraction of analyte extraction The expression is the fraction of analyte extracted (E): E = C
o
V
o
/(C
o
V
o
+ C
aq
V
aq
) = K
D
V/ (1 + K
D
V) where
V
o
is the volume of organic phase, V
aq
the volume of aqueous phase, and V is the phase ratio V
o
/ V
aq
; see
Chapter 7.
Fractionation range In SEC, refers to the operating range of a gel or packing. This is the range in which the packing can separate
molecules based on their size. At one end of the range, molecules that are too large to diffuse into the pores
are excluded. At the other end of the range, molecules that can diffuse into all of the pores totally permeate
the packing eluting (unseparated) at the permeation volume; see Chapter 11.
Freeze drying The process of removing water, mainly from biological samples, by using vacuum sublimation; see Chapter 4.
Frit The porous element at either end of a column that serves to contain the column or SPE packing. It is placed
at the very ends of the column tube or more commonly in the end fitting. Frits can be stainless steel or other
inert metal or plastic, such as porous PTFE or polypropylene. The frit porosity must be less than the smallest
particle in the HPLC column, otherwise particles will pass through the frit and packing will be lost.
Gas-phase extraction
See
direct thermal sampling
.
Gel The solid packing used in gel chromatography or GPC. An actual gel really consists of two parts: the dispersed
medium (solid portion) and the dispersing medium (the solvent). Also defined as a colloidal dispersion of a
solid and liquid in which the solid is the continuous phase.
Gel filtration chromatography Also called aqueous size exclusion chromatography; carried out with aqueous mobile phases. Generally
refers to molecular size separations performance on soft gels such as polydextrans but highly cross-linked
polymers, silica gels, and other porous media may also be used. Most gel filtration separations involve
biopolymers and water-soluble polymers, such as polyacrylic acid; see Chapter 11.
Gel permeation chromatgraphy SEC carried out with organic mobile phases used for the separation and characterization of polymers. SEC
with aqueous mobile phases is referred to as aqueous GPC or GFC; see Chapter 11.
Grab sampling In gas sampling, an evacuated sample canister can be opened and sample rapidly collected at an uncontrolled
rate, usually over several seconds, until the container reaches equilibrium with atmospheric pressure.
Generally, this qualitative approach is used when unknown analytes must be identified, when the air contains
high concentrations of analytes at certain (short) times, or when an odor is noticed and a sample must be
obtained quickly. Paired grab samples (before/after or smell/no smell) often are employed to qualitatively
diagnose a perceived problem; see Chapter 2.

323
Graphitized carbon A graphitic carbon with more or less perfect three-dimensional hexagonal crystalline order prepared from
non-graphitic carbon by graphitization heat treatment; this packing material has a strong affinity for polar
compounds in aqueous samples and water miscible organic extracts. Commonly used in sample prep for
pesticide analysis for removal of pigment in food samples; also known as graphitized carbon black (GCB);
see Chapters 8 and 9.
Grinding Both manual and automated mortar and pestles are available; grinding can be performed under wet or dry
conditions; by this process particles of approx. 10 µm can be achieved; see Chapter 4.
Headspace sampling Headspace (HS) refers to the vapors that form above liquids and solids; if the sample is in thermodynamic
equilibrium with the gas phase in a closed thermostatted vessel, the method is called static HS; if an inert
gas passes over or through the sample and stripped sample volatiles accumulate in an adsorbent or cryogenic
trap, the method is called dynamic HS or purge and trap sampling; see Chapters 2 and 6.
Headspace single drop
microextraction
A single drop of solvent (a microliter or two) suspended in the headspace can partition volatile analytes into
the solvent; the drop can be withdrawn into the syringe and injected into a GC; see Chapters 6 and 7.
Headspace solid phase
microextraction
Instead of a drop of solvent, a polymer-coated fiber can be placed in the headspace; once the analytes
adsorb on the polymer coat, the fiber can be transferred to a GC inlet and the sorbed analytes volatilized by
thermal desorption; see Chapters 6 and 7.
Heart cutting In preparative LC, refers to collection of the center of the peak where purity should be maximum. The term
is also used in
column switching
; see Chapter 13.
High-abundance protein
depletion
Using antibody columns specific for the highest abundance proteins, they can be selectively removed from
plasma allowing the biochemist to investigate the lower abundance proteins which may be biomarkers or
other compounds of interest; see Chapter 15.
HILIC Hydrophilic interaction liquid chromatography; a technique using polar stationary phases such as silica,
amino, diol, etc. and polar mobile phases such as water and acetonitrile, at least 2.5% water is needed in
mobile phase; mechanism is thought to involve analyte partitioning into sorbed water layer; high
concentrations of acetonitrile or other water-miscible solvent are used; opposite of reversed-phase (RPC)
LC, water is a strong solvent and a gradient increases its concentration with time; technique is best for small
polar analytes that are weakly retained on RPC columns; see Chapter 9.
Hollow fiber liquid phase
microextraction (HF-LPME)
A hollow fiber (HF) membrane technique in which an HF membrane separates two extraction phases; the
membrane serves as a barrier and can be impregnated with solvent to permit liquid-liquid or liquid-liquid-
liquid extractions to take place; the membrane can be selected to allow certain analytes to pass through but
not others; see Chapters 7 and 18.

324
Hydrophilic (hydrophylic) "Water loving;" refers both to stationary phases that are fully compatible with water or to water-soluble
molecules in general. Many columns used to separate proteins (ion exchange, SEC, affinity) are hydrophilic
in nature and should not irreversibly sorb or denature protein in the aqueous environment.
Hydrophobic "Water hating;" refers both to stationary phases that are not compatible with water and to molecules in
general that have little affinity for water. Hydrophobic molecules have few polar functional groups; most
have a high content of hydrocarbon (aliphatic and aromatic) functionality.
ICAT Isotope-coded affinity tag; a gel-free method for quantitative proteomics that relies on chemical labeling
reagents. These chemical probes consist of three general elements: a reactive group capable of labeling a
defined amino acid side chain (e.g., iodoacetamide to modify cysteine residues), an isotopically coded linker,
and a tag (e.g., biotin) for the affinity isolation of labeled proteins/peptides. For the quantitative comparison
of two proteomes, one sample is labeled with the isotopically light (d0) probe and the other with the
isotopically heavy (d8) version. To minimize error, both samples are then combined, digested with a protease
(i.e., trypsin), and subjected to avidin affinity chromatography to isolate peptides labeled with isotope-coded
tagging reagents. These peptides are then analyzed by liquid chromatography-mass spectrometry (LC-MS).
The ratios of signal intensities of differentially mass-tagged peptide pairs are quantified to determine the
relative levels of proteins in the two samples. The original tags were developed using deuterium, but later
the same group redesigned the tags using 13C instead to circumvent issues of peak separation during LC
due to the deuterium interacting with the stationary phase of the column; see Chapter 15.
Immobilized liquid extraction Similar to SPE, but a polymeric stationary phase is bonded to the inside of glass vial; experiment performed
within the glass vial; analytes partition into polymeric phase and loading, washing, and elution steps are
performed by addition of various solvents.
Immunoaffinity SPE Phases based on molecular recognition using chemically attached mono- or poly-clonal antibodies; due to
antibody-antigen interactions, these phases are some of the most selective phases available; immunoaffinity
phases are commercially available for small molecules such as pollutants, toxins, etc.; an example of such a
phase for large biomolecules; see
high abundance protein depletion
; see Chaper 10.
Immunosorbent
See
immunoaffinity SPE
.
Impinger Glass bubble tube designed for the collection of airborne hazards into a liquid medium. When using a personal
air sampler, a known volume of air bubbles is pumped through the glass tube that contains a liquid specified
in the method. The liquid is then analyzed to determine airborne concentrations. An impinger may be mounted
on the side of an air sample pump or put into a holster and placed near a worker's breathing zone; see
Chapter 6.
Homogenization The process of making a sample more uniform by size reduction and blending; homogenizers with high speed
blades are available to do the job; see Chapter 4.

325
Imprinted phases Polymer and silica phases generated in the presence of a "template" or "printing" molecule. Such phases
have enhanced selectivity for the templating molecule; also called molecularly imprinted phases (MIPs); see
Chapter 10.
In situ derivatization (SPE) The act of derivatizing a compound of interest in its native environment; for example, for a tenaciously held
analyte on a soil sample changing its chemical nature by performing an in situ derivatization, the compound
may be more easily released and isolated for further workup or analysis; derivatization may also be formed
in solution and the derivatized compound extracted by LLE; see Chapters 9 and 20.
Internal standard In quantitative analysis, precision and accuracy are greatly improved by use of internal standards (ISs). The
procedure involves the addition of a fixed amount of internal standard (IS) to a series of increasing
concentrations of reference sample and to the unknown concentration. The ratio of the areas of the reference
concentrations and the areas of the IS is plotted against the known concentration of the reference samples.
The internal standard should be chemically similar to the substance being quantitatively determined and
should have a retention time fairly close to it.
Ion exchange capacity The number of ionic sites on the packing that can take place in the exchange process. The exchange capacity
is expressed in millieqivalents per gram. A typical styrene-divinylbenzene strong anion exchange resin may
have 3-5 meq/gm capacity. Exchangers for IC have very low capacity. Capacity of weak anion and cation
exchangers varies dramatically with pH.
Ion exchange chromatography A mode of chromatography in which ionic substances are separated on cationic or anionic sites of the
packing. The sample ion (and usually a counterion) will exchange with ions already on the ionogenic group
of the packing. Retention is based on the affinity of different ions for the site and a number of other solution
parameters (pH, ionic strength, counterion type, etc.) Ion chromatography is basically an ion exchange
technique.
Ionic strength A characteristic of an electrolyte solution. It is typically expressed as the average electrostatic interactions
among an electrolyte's ions. It is related to electrolyte concentration but the main difference between ionic
strength and electrolyte concentration is that the former is higher if some of the ions are more highly charged.
The higher the ionic strength of a mobile phase the more the mobile phase competes with the analyte for
ionic or adsorptive sites.
Ion pair chromatography Form of chromatography in which ions in solution can be "paired" or neutralized and separated as an ion
pair on a reversed-phase column. Ion pairing agents are usually ionic compounds that contain a hydrocarbon
chain which imparts a certain hydrophobicity so that the ion pair can be retained on a reversed-phase column.
Retention is proportional to the length of the hydrophobic chain and the concentration of the ion pair additive.
Ion pairing can also occur in normal phase chromatography when one part of the pair is dynamically loaded
onto a sorbent, but this technique is not as popular as the RPC technique. Also known as ion-interaction
(IIR) chromatography or dynamic ion exchange chromatography stressing the fact that the precise
mechanistic details of how the additive controls retention are not always known.

326
Irregular packing Refers to the shape of a column packing. These irregular packings are available in microparticulate sizes.
The packings are obtained from grinding solid materials into small particles and then sizing them into narrow
fractions using classification machinery. Spherical packings are now used more than irregular packings in
analytical HPLC but the less expensive, irregular packings are still widely used in prep LC and SPE; see
Chapter 9.
Isolate Analyte to be isolated from matrix background and then analyzed; see analyte.
Kieselguhr A diatomaceous earth used both in column chromatography and as a sample cleanup media. Only weakly
adsorptive, it is also used as a support in liquid-liquid chromatography and in supported liquid extraction;
see supported liquid extraction. Rarely used in HPLC; see diatomaceous earth and Chapters 2, 7 and 14.
Langmuir adsorption isotherm A specific form of an isotherm C
S
= N
0 *
C
M
/(K
d
+ C
M
) where C
S
and C
M
are the equilibrium stationary and
mobile phase concentrations of the solute, N
0
the total number of surface sites available for sorption and K
d
the sorption binding constant.
Large volume injection (LVI) A technique for introduction of larger than normal volumes of sample in a solvent into a capillary GC column;
in this approach, the bulk of the solvent is evaporated before the sample transfers to the analytical column;
there are two popular LVI techniques: programmed temperature vaporization and cool on-column injection
with solvent vapor exit; both are approaches to lowering detection limits; see Chapter 6.
Ligand In ligand exchange chromatography, refers to the analyte which undergoes ligand exchange with the
stationary phase; in affinity chromatography, refers to the biospecific material (enzyme, antigen, or hormone)
coupled to the support (carrier) to form the affinity column; in bonded phase chromatography, this denotes
the moiety covalently bound to the surface.
Limit of detection (LOD) The concentration of the analyte at which the resulting peak can be distinguished from baseline noise.
Literature and norms describe different ways of determination of the LOD.
Limit of quantitation (LOQ) The minimum concentration of the analyte at which the resulting peak can be quantified with a defined
security level. Typically 3 to 5 times higher than LOD.
Liquid-liquid extraction (LLE) An extraction technique for separating interferences from the analyte(s) by partitioning the analytes between
two immiscible liquids (or phases); one phase is usually aqueous and the second phase an organic solvent;
more hydrophilic compounds prefer the aqueous phase while more hydrophobic compounds will be found
in the organic solvent; by the use of additives (e.g. buffers, ion pair reagents, etc.) equilibria can be shifted
to "force" analytes or matrix compounds into one or other of the two layers; see Chapter 7.

327
Liquid phase microextraction A liquid extraction technique when there is a great reduction in the acceptor-to-donor phase ratio; a hollow
fiber is impregnated with an organic solvent used to accommodate or protect microvolumes of acceptor
solution. This novel methodology proves to be an extremely simple, low-cost, and virtually solvent-free
sample-preparation technique, which provides a high degree of selectivity and enrichment by additionally
eliminating the possibility of carry-over between runs; see Chapters 6, 7 and 18.
Liquid-solid extraction The general expression for extraction techniques that uses an organic solvent to extract analytes from a solid
material. In its simplest form, the "shake flask" extraction takes place at room temperature and works well
in cases when the matrix is porous and the analyte(s) are easily extractable; see Chapters 2 and 14.
Loading step (SPE) The second step in SPE (after conditioning) when the sample is loaded onto the SPE phase (cartridge);
see Chapter 9.
Lyophilization The process of dehydrating a sample, often biological, containing water by using vacuum sublimation; also
referred to as freeze drying; see Chapter 4.
Maceration The process of breaking down a soft material into smaller parts by tearing, chopping, cutting, etc; see Chapter 4.
Macroporous resin
(macroreticular)
Crosslinked ion exchange resins that have both micropores of molecular dimensions but also macropores of
several hundred Angstroms wide. These are highly porous resins with large internal surface area accessible
to large molecules.
Magnetic bead technology Micro magnetic beads are uniform polymer particles, typically 0.5-500 micrometres in diameter, that have
iron oxide particles (or other particles that may be attracted to magnets) contained within the polymer matrix.
Bio-reactive molecules can be adsorbed or coupled to their surface, and used to separate biological materials
such as cells, proteins, or nucleic acids; by the use of magnets/magnetic fields, the beads can be easily
manipulated in test tubes or 96-well plates. These microbeads are used for isolation and handling of specific
material or molecules, as well as for analyzing sensitive molecules, or those that are in low abundance, e.g.
in miniaturized and automated settings.
Matrix In sample prep, matrix normally refers to the substance in which the analyst is attempting to measure a
solute or series of solutes; often the matrix is of no interest and its concentration must be reduced or eliminated
in order for a separation and measurement to take place; the matrix can be organic, inorganic or biological.
Matrix adsorption mode (SPE) A lesser used mode of SPE in which the sorbent is chosen to maximize retention of the matrix and other
interferences while the analyte of interest is unretained; the opposite of the normal process (bind-elute) of
SPE, there is no concentration of the analytes; see Chapter 9.

328
Membrane filtration Membrane filter; membrane disk.
Method development A process of optimizing the separation including the sample pre-treatment so as to obtain a reproducible
and robust separation. It usually emphasizes the search for the stationary phase, eluent and column
temperature which provides an adequate separation or sample enrichment.
Method validation A process of testing a method to show that it performs to the desired limits of precision and accuracy in
retention, resolution and quantitation of the sample components of interest.
Microdialysis A minimally-invasive sampling technique that is used for continuous measurement of free, unbound analyte
concentrations in the extracellular fluid of virtually any tissue. Analytes may include endogenous molecules
(e.g. neurotransmitter, hormones, glucose, etc.) to assess their biochemical functions in the body, or
exogenous compounds (e.g. pharmaceuticals) to determine their distribution within the body. The
microdialysis technique requires the insertion of a small microdialysis catheter (also referred to as microdialysis
probe) into the tissue of interest. Once inserted into the tissue or (body) fluid of interest, small solutes can
cross the semi-permeable membrane by passive diffusion. The microdialysis probe is designed to mimic a
blood capillary and consists of a shaft with a semi-permeable hollow fiber membrane at its tip, which is
connected to inlet and outlet tubing; see Chapters 15 and 18.
Microextraction The general process of liquid extraction using small amounts of organic solvent where the phase ratio
V
o
/ V
aq
is quite low; other techniques using hollow microfibers as a barrier is also referred to as
microextraction; see Chapters 7 and 18.
Micropipette tip
See
pipette tip
.
Micropipette tip (SPE) A form of SPE in which the packing material is embedded or adsorbed on the inner walls of a pipette tip;
useful for the SPE of very small amounts of liquid sample; often used with x-y-z liquid handling systems for
automation purposes; see Chapter 9.
Microporous resin
See
microreticular resin
.
Matrix solid phase dispersion Technique uses bonded phase solid supports as abrasives to produce disruption of sample architecture and
as a bound solvent to aid complete sample disruption during the sample blending process; the finely divided
sample is gently blended with a mortar and pestle and transferred to a column and analytes eluted with
appropriate solvents; see Chapter 10.
Matrix solid phase extraction
See
matrix solid phase dispersion
.

329
Microreticular resin Crosslinked synthetic ion exchange resins which have pores with openings corresponding to molecular sizes.
Diffusion into the narrow pores can be impaired and low exchange rates can occur as well as poor
performance, especially for large molecules.
Microwave-assisted extraction
(MAE)
The use of microwave energy to heat samples in the presence of a solvent allowing for rapid extraction; MAE
can be performed in open vessels where a non-microwave absorbing solvent is used and the sample containing
a substance with a high dielectric constant (e.g. water) is rapidly heated with the extracted analytes passing
into the extraction solvent. A variation of this is the addition of an inert microwave absorbing solid substance
that transfers the heated energy to the surrounding solvent. MAE can also take place in closed vessels which
are non-microwave absorbing containers; in these cases, microwave absorbing is used and the temperature
and pressure inside the vessel rises to assist in the rapid extraction of analytes; see Chapter 14.
Milling Devices for reducing particle sizes of solid materials. Disk mills pulverize <20 mm diameter hard samples by
feeding between stationary and rotating disks with adjustable gap settings; samples are generally reduced
to 100 µm diameter; rotor speed mills combine impact and shearing processes to grind soft-to-medium hard
and fibrous materials to 80 µm; ball mills grind material to sub-micron size by developing high-grinding energy
via centrifugal or planetary actions using agate, tungsten carbide or PTFE-coated stainless steel balls; see
Chapter 4.
Mincing The process of breaking down a meat or vegetable product into smaller parts by tearing, chopping, cutting,
dicing, etc; see Chapter 4.
Mixed bed column Combination of two or more stationary phases in the same column used most often in ion exchange
separations (IEC) (mixed anion and cation resins) and SEC (mixture of different pore size packings). Advantage
in IEC is the total removal of both cationic and anionic compounds; useful in SEC because a wider molecular
weight range can be accommodated by the same column.
Mixed mode separation A separation that takes place in a single column due to retention and selectivity provided by a dual retention
mechanism. For example, at intermediate-to-high pH values, a reversed-phase column with residual silanols
can separate by hydrophobic interactions as well as ionic interactions by virtual of the ionized silanols;
sometimes mixed mode separations can be quite beneficial to the selectivity (band spacing) but can cause
peak assymetry and the precise balance of interactions may be difficult to reproduce with subsequent batches
of packing. Also useful in SPE.
Modifier An additive that changes the character of the mobile phase. For example, in reversed-phase, water is the
weak solvent and methanol, the strong solvent, is sometimes called the modifier; sometimes other additives
such as competing bases (triethylamine or ion pair reagents) are referred to as modifiers, but they should
more correctly be called additives; see
additives
.
Molecularly imprinted polymers
(MIPs)
See
imprinted phases
.

330
Multidimensional
chromatography
The use of two or more columns or chromatographic techniques to effect a better separation. It is useful
for sample cleanup, increased resolution, and increased throughput. It can be used off-line by collecting
fractions and reinjecting onto a second column or on-line by the use of a switching valve. Also called coupled
column chromatography, column switching, multi-column chromatography, or box car chromatography;
see Chapter 13.
Multimodal SPE The practice of SPE when two different phases or modes are used to clean up a sample; the process can
refer to two separate cartridges placed in series and analytes separated on the two different cartridges; a
second process can be used when two different phases are present in the same cartridge or even on the
same packing; sometimes referred to as mixed-mode SPE; see Chapter 10.
Non-polar A non-polar molecule is one that the electrons are distributed more symmetrically and thus does not have
an abundance of charges at the opposite sides. The charges all cancel out each other. Non-polar compounds,
solvents or bonded phases readily dissolve in organic solvents, such as hexane, or prefer such solvents in
place of water. Non-polar substances do not readily dissolve in water.
Octadecylsilane (ODS) The most popular reversed-phase in HPLC and SPE. Octadecylsilane phases are bonded to silica or polymeric
packings. Both monomeric and polymeric phases are available.
Octylsilane A popular stationary phase in reversed-phase chromatography; usually has slightly less retention than the
more popular C18; both monomeric and polymeric phases available.
Off-line SPE The normal practice of SPE in which SPE cartridges, disks, pipette tips, etc. are handled using manual
processes (i.e. vacuum manifolds, pipette transfer, etc.); opposite of on-line SPE.
On-column injection In GC, refers to the process of injecting the entire liquid sample directly onto the head of the column using
a fine needle that fits inside the capillary; see Chapter 6.
On-line column switching See on-line SPE for operating principle; in column switching the second column is usually another HPLC or
GC column, rather than a short SPE column; the main purpose of column switching is to resolve more complex
mixtures than can be handled by a single chromatographic column; in GC, the use of Deans switching when
differential pressure is used to control the relative flow of gas through the two columns; in LC, switching
valves are most frequently used; see Chapter 13.

331
On-line preconcentration A precolumn is placed in front of the separation column to concentrate analytes prior to their separation;
different mechanisms may be used (e.g. hydrophobic interaction, adsorption, enzymatic reaction) to retain
analyte as a function of time and then by a displacement process (e.g. solvent elution, pH change, etc.),
concentrated analyte(s) are transferred to the separation column.
On-line SPE Refers to the use of small cartridges packed with SPE packings placed across two ports of a 6- or 10-port
injection or column switching valve; the SPE trap is loaded with sample by an external pump or syringe
transfer and then the valve is switched so that the SPE trap becomes part of the HPLC flow stream and
analytes can be swept into the column based on the solvent being used for displacement; on-line SPE columns
are usually used multiple times while off-line SPE cartridges are generally used once; see Chapter 13.
Packing The adsorbent, gel, or solid support used in the HPLC or SPE column. Most modern analytical HPLC packings
are less than 10 µm in average diameter with 5 µm currently being the favorite. SPE particles are much
larger (>20 micron).
Paper filtration Using porous filter paper (mainly cellulose) to remove particulates from liquid samples; papers with different
porosities are available. Low porosity filters will remove very fine particulates but may have a lower flow
rate while high porosity filters filter out larger particulates at a higher flow rate; paper filtration is often used
in wet chemistry to filter, combust, and then weigh insoluble materials; ashless filter paper is also used for
this purpose; see Chapter 5.
Particle size reduction The general process of reducing larger particles down to a size that can be more conveniently extracted; the
smaller the particle, the more quickly it will dissolve, or if insoluble the more quickly analytes can be extracted
for further sample cleanup. Typical methods for reducing particle size include pulverizing, milling,
homogenizing, chopping, blending, and so on; see Chapter 4.
Particulates Generally refers to small particles found in the mobile phase that can cause backpressure problems by lodging
in frits; can also refer to the small particles packed into HPLC columns.
Passive sampling In passive gas sampling, an air sample is pulled through a flow controller into an evacuated canister over a
chosen period of time, ranging from 5 minutes to 24 hours. The sampling period and the flow rate determine
the canister volume required.
Peak tracking A way of matching peaks that contain the same compound between different experimental runs during
method development; relies upon detection parameters of each pure analyte; diode array detectors and
mass spectrometers are among the best detectors for peak tracking due to their specificity.

332
Polar A molecule may be polar as a result of polar bonds or as a result of an asymmetric arrangement of non-
polar bonds and non-bonding pairs of electrons; Polar molecules are generally able to dissolve in water
(H
2
O) due to the polar nature of water; polar molecules do not prefer non-polar organic solvents such as
hexane. Polar molecules have slightly positive and slightly negatively charged ends; oftentimes we refer to
a compound's polarity.
Polarity index (P') A measure of the relative polarity of a solvent and is useful for identifying suitable mobile phase or extraction
solvents. The polarity index increases with polarity; examples: hexane P' = 0.0; isopropanol P' = 3.9;
THF P' = 4.0; methanol P' = 5.1; acetonitrile P' = 5.8; water P' = 9.0.
Polymeric packings Packings based on polymeric materials, usually in the form of spherical beads. Typical polymers used in LC
and SPE are polystyrene-divinyl-benzene (PS-DVB), polydivinybenzene, polyacrylamide, polymethylacrylate,
polyethyleneoxide, polydextran, or polysaccharide.
Polymeric SPE The use of a polymeric base material (e.g. PS-DVB, methacrylate) rather than an inorganic material (e.g.
silica, alumina); polymers generally have a wider pH range and higher sample capacity than some of the
inorganic materials; see Chapter 9.
Polystyrene-divinylbenzene
resin
The most common base polymer for ion exchange chromatography. Ionic groups are incorporated by various
chemical reactions. Neutral PS-DVB beads are used in RPC. Porosity and mechanical stability can be altered
by variation of the crosslinking through the variation of the DVB content.
Pore size The average size of a pore in a porous packing. Its value is expressed in A or in nm. The pore size determines
whether a molecule can diffuse into and out of the packing; see
mean pore diameter
.
Post-column derivatization
See
post-column reaction
; see Chapter 20.
Phenol extraction A sample preparation technique used for the isolation of DNA from biological samples; see Chapter 15.
Pipette tip Replaceable tips used in automation of liquid handling chores; used once and discarded to avoid
contamination.
pK
a
An acid dissociation constant, K
a
, (also known as acidity constant, or acid-ionization constant) is a quantitative
measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known
as dissociation in the context of acid-base reactions. The equilibrium can be written symbolically as:
HA <---> H
+
+ A
-
where HA is a generic acid that dissociates by splitting into A
-
, known as the conjugate
base of the acid, and the hydrogen ion or proton, H
+
, which, in the case of aqueous solutions, exists as the
hydronium ion – in other words, a solvated proton; The dissociation constant is usually written as a quotient
of the equilibrium concentrations (in mol/L), denoted by [HA], [A
-
] and [H
+
]: K
a
= ([H
+
] [A
-
] )/[HA; due to
the many orders of magnitude spanned by K
a
values, a logarithmic measure of the acid dissociation constant
is more commonly used in practice. The logarithmic constant, pK
a
, which is equal to −log10 K
a
, is sometimes
also (but incorrectly) referred to as an acid dissociation constant.

333
Precolumn derivatization See precolumn reaction; see Chapter 20.
Prefilter (SPE) In some cases when samples contain a large amount of particulates, regular SPE cartridges and disks may
become clogged and flow is reduced; prefilters are filter devices that have higher porosity that will filter out
large particles and allow the SPE bed to operate more efficiently; some SPE devices have prefilters built in,
others one can add a prefilter; in some cases, the use of an inert packing such as glass beads serve the
same purpose as an actual filter; see Chapter 9.
Pressing The general process of squeezing liquid from a semi-solid material (e.g. plants, fruit, meat); see Chapter 4.
Pressurized fluid extraction See accelerated solvent extraction; see Chapter 14.
Pressurized solvent extraction See accelerated solvent extraction; see Chapter 14.
Primary sampling The collection of one or more increments or units initially taken from a population; the primary sample is that
taken from the primary source; proper statistical sampling protocols are recommended; see Chapter 4.
Programmed temperature
vaporization (PTV)
Sample introduction technique in which sample is introduced into the inlet liner at a temperature slightly
below the boiling point of the solvent; the solvent is continually evaporated and vented through the inlet
split line; once the solvent is gone, the temperature of the inlet is heated very rapidly to transfer the sample
into the column; using PTV, there is less sample discrimination and less thermal degradation of sensitive
compounds compared to hot inlet injections; see Chapter 6.
Protein crashing Term used in removing/reducing the protein concentration in a biological fluid such as plasma; after slight
dilution, an organic solvent such as acetonitrile is added to the plasma and the proteins being insoluble are
precipitated (crashed). By the use of centrifugation or filtration, the protein is removed and the supernatant
liquid used for injection into an HPLC or worked up further; see Chapter 16.
Protein precipitation
See
protein crashing
.
Pulsed splitless injection A form of GC injection recommended for large volumes (up to 5 µL) of sample in which a short-term high
pressure pulse is imposed on the inlet such that there is not a large volume of solvent vapor generated and
most or all of the sample is directed to the column; once the sample is transferred, then normal pressure is
resumed; using this technique, highly volatile compounds cannot be lost through the split vent line and
thermally unstable compounds spend less time in the hot injection port so there is less degradation; see
Chapter 6.

334
Pulverizing Electromechanically driven rod or vibrating base devised to reduce particle size of solid samples; a freezer
mill can be used with liquid nitrogen to treat malleable polymers or those with low glass transition
temperatures; see Chapter 4.
Purge and trap sampling Dynamic headspace technique in which the headspace vapors over a liquid or solid sample are continuously
removed by flow of gas over the sample (purging); volatilized analytes are usually concentrated by trapping
on an adsorbent or by cyrogenic means. Most often used for volatile trace analytes when concentration is
needed; see Chapters 2 and 6.
Pyrolysis The process of heating a sample hot enough to break its chemical bonds, thereby forming smaller molecules
that can be analyzed by GC; see Chapter 6.
Quaternary methyl amine A strong anion exchange functionality popular in resin-based packings; usually supplied in chloride form.
QuEChERS A technique initially used for the extraction of pesticides from fruits and vegetables. It consists of two steps:
1) Salting out extraction using buffered or unbuffered solvent (usually acetonitrile), and 2) Dispersive SPE in
which solid adsorbent is used to treat an aliquot from step 1 to remove interferences and matrix compounds.
QuEChERS is mostly used with GC/MS and LC/MS (or MS-MS) to more selectively analyze the pesticide
extracts; more recently, QuEChERS has expanded to other matrices such as cooking oil, meat, fish, biological
fluids, etc., and to other analytes (e.g. pharmaceuticals, antioxidants, toxins, etc.); see Chapter 8.
Removable well plates
See
array 96-well plate
; see Chapter 9.
Representative sample A sample resulting from a statiscally worked out sampling plan; it can be expected to adequately reflect the
properties of interest of the parent population.
Residual silanols The silanol (-Si-OH) groups that remain on the surface of a packing after chemically bonding a phase onto
its surface. These silanol groups that may be present in very small pores may not be accessible to the reacting
bulky organosilane (e.g., octadecyldimethylchlorosilane), but may be accessible to small polar compounds.
Often, they are removed by endcapping with a small organosilane such as trimethylchlorosilane.
See
endcapping
.
Resin A solid polymeric packing used in ion exchange separations. The most popular resins are polystyrene-
divinylbenzene co-polymers of small particle size (<10 µm). Ionic functionality is incorporated into the resin.
Restricted access media (RAM) Sorbents used for the direct injection of biological fluids, such as plasma or serum, into an HPLC flow stream;
they contain an outer hydrophilic surface that provides minimal interaction with proteins and when combined
with small pores on the sorbent exclude them; the inner surface is hydrophobic and when small molecules
diffuse into the pores, they interact by reversed-phase mechanisms and are retained; the small molecules,
such as drugs and their metabolites, can be removed by rinsing with an organic solvent; RAMs are most
successfully used in a column switching setup in which the secondary column is used to resolve the small
molecules and the proteins are directed to waste so as not to foul the secondary column; see Chapter 10.

335
Riffler A manual or automated mechanical device used in sub-dividing solid powder samples into smaller units;
sub-division is achieved when material samples are cut into two smaller portions by a single pass; further
sub-divisions can be attained by multiple passes; see Chapter 4.
Rinse step (SPE)
Also known as the
wash step
, the third step in the SPE process; once the sample is loaded, the rinse step
is designed to eliminate interferences including various matrix compounds; here, a solvent(s) or buffer is
selected to remove interferences, but not the analytes of interest; see Chapter 9.
Round well plates
96-well plates that have round-shaped wells resembling 96 small test tubes; see
96-well plates
.
Salting out effect The use of a high concentration of salt buffer in the mobile phase to cause a low polarity analyte to have a
decreased solubility in water and therefore precipitate or come out of solution; most often used for the
hydrophobic interaction chromatography of proteins when proteins are first precipitated at high salt
concentrations then eluted by gradual dilution using reverse gradient elution; can also be used in liquid-
liquid extraction; see salting out extraction; see Chapter 8.
Salting out extraction See QuEChERS or salting out effect; in this extraction approach, high concentrations of salt in the aqueous
phase will cause certain compounds to migrate into an organic phase or perhaps vice-versa; high
concentrations of salt will also force normally miscible solvents (e.g. water and acetonitrile) to become
immiscible and be used for further partitioning more polar analytes than could be achieved by an extraction
using a non-polar organic solvent; see Chapter 8.
Sample division The process in sampe reduction to divide the sample into smaller portions while retaining the representative
character of the primary sample; see Chapter 4.
Sample pre-treatment Often synonomous with sample preparation; the process of manipulating the sample to make it easier
to analyze later.
Sample size reduction The process in sampe reduction to divide the sample into smaller portions while retaining the representative
character of the primary sample; see Chapter 4.
Sample tracking The process of tracking primary, secondary, laboratory, and further samples through the analytical cycle; it
is important for chain or custody reasons to be able to ensure that the sample finally analyzed in the instrument
was from the original sample collected at the source; sample tracking can be as simple as writing a sample
number on a container to have bar coded vials or RFI tags to automatically keep track of sample flow.
Sampling The process of collecting a representative sample at the source; sampling can also refer to further sample
division as it more closely approaches the laboratory analysis; it is important to make sure that the final
sample analyzed represents a sub-sample of the original sample without any imposed bias or discrimination.

336
Sampling error Incurred when the statistical characteristics of a population are estimated from a subset or sample of that
population. Since the sample does not include all members of the population, statistics on the sample, such
as means and quantiles, generally differ from parameters on the entire population. Since sampling is typically
done to determine the characteristics of a whole population, the difference between the sample and
population values is considered a sampling error. Exact measurement of sampling error is generally not
feasible since the true population values are unknown; however, sampling error can often be estimated by
probabilistic modeling of the sample; see Chapter 4.
Scavenger Special type of solid phase particle that, instead of molecular interactions that occur in SPE, uses chemical
reactions to remove undesired species, such as undesired reaction products or excess starting material from
an organic synthesis; mostly operate on the basis of covalent bonding; packing materials contain reactive
groups that can be used for organic or inorganic species such as catalysts; see Chapter 9.
Secondary sampling Refers to the process of taking a representative portion of the primary sample in order to further reduce its
particle size, or to prepare a laboratory sample for eventual analysis; see Chapter 4.
Sieving Process of passing a sample of solid particles through a metal or plastic mesh of a uniform cross-sectional
area (square opening from 3 µm-123 mm) to separate particles into uniform sizes; can be performed under
wet and dry conditions; see Chapter 4.
Silanol The Si-OH group found on the surface of silica gel. There are different strengths of silanols depending on
their location and relationship to each other and based on the metal content of the silica. The strongest
silanols are acidic and often lead to undesirable interactions with basic compounds during chromatography.
Silanophile A compound which has high affinity for active (acidic) silanol groups on the surface of silicas. Usually a
strongly basic amine.
Silica gel The most widely used HPLC packing. It has an amorphous structure, is porous, and consists of siloxane and
silanol groups. It is used in all modes of LC as a bare packing for adsorption, as the support for LLC, or for
chemically bonded phases, and, with various pore sizes, as an SEC packing. Microparticulate silicas of 3, 5,
and 10 µm average particle diameter are used in HPLC. Compared to irregular silicas, in modern analytical
HPLC columns, spherical silicas are preferred due to their packing reproducibility and because they have
lower pressure drops; sometimes referred to as silica.
Single drop microextraction One drop of solvent (a microliter or two) suspended in the headspace can partition volatile analytes into the
solvent; the drop can be withdrawn into the syringe and injected into a GC; see Chapter 7.

337
Solid phase extraction (SPE) A widely used technique for sample preparation using a solid phase packing (dp of 20-40 µm) contained in
a small plastic cartridge, disk, or well of a 96-well flow-through plate. The solid stationary phases used are
no different than HPLC packings. However, although related to chromatography, the principle is different
and is sometimes referred to as digital chromatography. The process as most often practiced requires four
steps: 1) Conditioning the sorbent, 2) Adding the sample, 3) Washing away the impurities, and 4) Eluting
the sample in the smallest volume possible with a strong solvent; Can be performed in a variety of formats:
cartridges, disks, pipette tips, 96-well plates, etc., and in a variety of modes, such as reversed-phase, ion
exchange, normal phase, etc.; see Chapters 9 and 10.
Solid phase microextraction
(SPME)
A technique in which a small polymer-coated solid fiber is placed into a solution or above the headspace of
a solid or liquid sample; analytes will diffuse into the coating until equilibrium is established; for GC, the fiber
containing the sorbed sample is transferred to the GC and the trapped analytes are thermally desorbed into
the column; in HPLC, solvent is used to rinse the sorbed analytes for eventual injection into the LC column;
less popularly used in LC than in GC; see Chapters 2, 6, 7, 9 and 10.
Solid phase trapping The use of an SPE cartridge or packed column to trap specific analytes that flow through the device; the
packing material is chosen to selectively retain the analytes of interest and let non-interest compounds to
pass through unretained.
Solvent exchange The process of exchanging one solvent that may not be compatible with the analysis method for a solvent
that is more compatible; in some cases, evaporation is used to remove a volatile solvent and the sample is
reconstituted in a different solvent.
Solvent strength Refers to the ability of a solvent to elute a particular solute or compound from a column. Described for HPLC
by Lloyd Snyder for LEAC (LSC) adsorption chromatography on alumina; solvents were quantitatively rated
in an eluotropic series. Less extensive data is available for silica and carbon adsorbents.
Sonication Use of ultrasound to create vigorous agitation at the surface of a finely divided solid material; direct method:
uses a specially designed inert acoustical tool (horn or probe = sonotrode) placed in sample-solvent mixture;
indirect method: sample container is immersed in ultrasonic bath with solvent and subject to ultrasonic
radiation; Dissolution is aided by ultrasonic process; heat can be added to increase rate of extraction; safe,
rapid, best for coarse, granular materials; for indirect method, multiple samples can be done simultaneously;
efficient contact with solvent; see Chapters 2 and 14.
Soxhlet extraction A well-accepted technique for the extraction of compounds from a solid sample; the sample is placed in a
disposable porous container (thimble); constantly refluxing fresh condensed solvent flows through the thimble
and dissolves analytes that are continuously collected in a boiling flask; special glassware (a Soxhlet extractor)
is designed to perform the extraction unattended; see Chapters 2 and 14.

338
Spin column A small column that usually contains a packing material for sample cleanup or isolation; the sample is added
to the column, which has a selective packing material; the column, in turn, fits into a small collector tube
and is placed in a centrifuge; the spin tubes are very popular in handling biological samples for isolating
DNA, RNA, and other biocompounds of interest; see Chapter 15.
Spin filter Similar to a spin column, but, rather than the packing portion, a membrane filter is placed; the purpose of
the filter is to remove particulates.
Spin tube
See
spin column
.
Split injection An injection technique for GC in which only a portion of the sample is directed to the column (called the split
ratio, 100:1, 50:1, etc.) so as not to overload the column and to ensure a representative sample reaches the
column; the technique is simple, rugged, and protects the column. However, sample discrimination is possible;
splitless injections are usually performed automatically; see Chapter 6.
Splitless injection Rather than splitting the sample going to the GC column, all of the sample is directed into the GC column;
this process ensures higher sensitivity than split injections, but flashback can occur, and a higher possibility
of sample degradation is possible due to longer residence time in the hot injection port; see Chapter 6.
Square well plates 96-well plates that have square-shaped wells rather than the normal round bottom wells; see Chapter 9.
Standard addition Process used to improve quantitation; necessary to have a pure standard of known concentration. An
unknown concentration of sample is first injected to give a peak area; then to the unknown concentration is
added a measured amount of pure compound. As a result of a new peak area, one can determine the
amount of the original concentration; an alternative procedure is to add a constant amount of unknown
concentration to a series of standards of pure substances and to plot the peak areas obtained against the
known concentrations of the original standards. The slope of the line obtained gives the concentration of
the unknown.
Standards A sample that contains known quantities of the compounds of interest; Used to help identify sample peaks
by comparing the time in which they elute to the retention times obtained through the injection of the sample
under the same conditions. For quantitation, external standards are compounds that are used to construct
calibration curves of detector output (peak area or peak height) vs. concentration; the concentration of
unknowns are determined by fitting the detector output to the calibration curve. Internal standards are
compounds of known concentration with different retention times that are added to the sample and relative
detector responses between the internal standard and the unknown are compared in order to quantitatively
measure unknown compounds.
Stepwise elution Use of eluents of different compositions during the chromatographic run. These eluents are added in the
stepwise manner with a pump or by a selector valve. Gradient elution is the continuous version of changing
of solvent composition.

339
Steric exclusion
chromatography
A major mode of LC in which samples are separated by virtue of their size in solution. Also known as size
exclusion chromatography (SEC), gel permeation chromatography, gel filtration chromatography, or gel
chromatography. SEC is most often used for polymer separation and characterization and sample cleanup;
see Chapter 11.
Stir bar sorbent extraction Prinicple similar to solid phase microextraction (SPME), but rather than the use of a coated fiber, a polymer-
coated stir bar is used, greatly increasing the surface area, thus higher capacity and greater mass sensitivity;
similar to SPME, equilibration requires tens of minutes; for GC, a special thermal desorption unit is needed
to handle stir bar; in LC, bar is usually rinsed off-line; see Chapter 9.
Straight phase chromatography See normal phase chromatography; see normal phase SPE.
Strong anion exchanger Anion exchange packing with strongly basic ionogenic groups (e.g. tetraalkylammonium).
Strong cation exchanger Cation exchange packing with strongly acidic ionogenic groups (e.g. sulfonic).
Strong solvent In general, refers to a solvent that is a good solvent for a chemical compound; in chromatography, refers to
the mobile phase constituent that provides a higher solvent strength that causes an analyte to elute more
quickly from the column; in a water-acetonitrile binary solvent system for reversed-phase LC, acetonitrile
would be considered to be the strong solvent.
Sulfonyl cation exchanger A strong cation exchange functionality found in resin-based packings; usually propyl-SO
3
H; may come in
other cationic forms such as sodium, ammonium, silver, and calcium.
Supercritical fluid extraction
(SFE)
Uses supercritical fluid, most often carbon dioxide along with, or containing a small % of organic modifier
for more polar analyte, to extract analytes from solid materials; supercritical fluid has the diffusivity of a gas
and the solvent power of a liquid; requires a special SFE unit allowing precise control of pressure and
temperature; analytes are collected in a cold trap, on an adsorbent, or in a liquid; a "green" extraction
technique; see Chapter 14.
Superheated water extraction Water is heated well above its boiling point in a closed pressurized system; heating changes dielectric constant
and increases the solvating power such that it becomes "organic-like;" a "green" method for extracting
organic analytes from solid matrices.
Supported liquid extraction
(SLE)
A technique based on the principles of liquid-liquid extraction in which the aqueous phase is supported on
a bed of highly purified, high surfce area diatomaceous earth (in a tube, cartridge, or 96-well format); this
aqueous phase may be buffered and may contain the sample to be partitioned; the organic phase is then
percolated through the packed bed allowing for intimate contact with the dispersed aqueous phase. The
effluent collected at the exit of the column contains the extracted analytes; compared to LLE, the SLE
technique is miniaturized, easily automated, and provides excellent extraction efficiency; see Chapter 7.

340
Surface area In an adsorbent, refers to the total area of the solid surface as determined by an accepted measurement
technique, such as the BET Method, which uses nitrogen adsorption. The surface area of a typical porous
adsorbent, such as silica gel, can vary from 100-600 square meters per gram.
Surrogate samples A pure analyte which is extremely unlikely to be found in any sample, and which is added to a sample aliquot
in known amounts before extraction, and is measured with the same procedures used to measure other
sample components. A surrogate behaves similarly to the target analyte and is most often used with organic
analytical procedures. The purpose of a surrogate analyte is to monitor method performance with each
sample.
Syringe filter A small plastic holder containing a membrane filter which has Luer lock fittings at both the top and the
bottom so that it can be affixed to a syringe (which also has a Luer lock fitting) to pass a sample through the
filtration media; syringe filter diameters can range up to 90 mm; see Chapter 5.
Tedlar
®
bags Used for grab sampling of air or other gases; sampling bags are a whole-air sampling device for high-level
VOCs and permanent gases. Several EPA, NIOSH, and OSHA methods exist for bag sampling for a variety
of applications: stationary sources emissions, workplace atmospheres, ambient, indoor air quality, and breath
analysis. The unique design of these Tedlar
®
sample bags incorporates the sampling septum directly in the
valve (polypropylene or stainless steel construction), providing easier use and lighter weight than other styles.
Thermal desorption The use of heat to desorb analytes from SPME fibers, SBSE bar, or solid matrices placed in a thermal
desorption tube; see Chapter 6.
Thermal extraction Uses high temperatures (below pyrolysis temperatures) to extract stable analytes from porous solid matrices;
samples are placed in thermal desorption tubes, just like in thermal desorption; see Chapter 6.
Time-integrated sampling In gas sampling, in order to obtain a more representative sample, time-integrated sampling is required.
A flow restrictor is used to spread the sample collection flow over a specific time period to ensure an
“average” composited or time-weighted average (TWA) sample. A TWA sample will accurately reflect the
mean conditions of the ambient air in the environment and is preferred when, for regulatory or health
reasons, a typical exposure concentration is required for a situation that may have high variability, as in an
occupational setting.
Titania TiO2, an uncommon adsorbent used in adsorption chromatography; also used as an SPE sorbent primarily
for removal of phosphorous-containing compounds (e.g phospholipids).
Trace enrichment Technique in which trace amounts of compounds are retained on an HPLC or precolumn packing out of a
weak mobile phase or solution and then are eluted by the addition of a stronger mobile phase in a
concentrated form. The technique has been most successfully applied in the concentration of trace amounts
of hydrophobic compounds (e.g. polynuclear aromatic hydrocarbons) out of water using a reversed-phase
packing. A strong solvent such as acetonitrile serves to elute the enriched compounds; see Chapters 2, 7, 9,
13, 16 and 18.
Trapping Process of using a solid material (e.g. silica gel, polymer, inorganic sorbent, etc.) or liquid solution to physically
or chemically retain solutes of interest from a diluted stream of liquid or gas; frequently used to concentrate
analytes for more sensitive analysis.

341
Tryptic digestion A method of selectively and reproducibly dissecting peptide chains of proteins to yield a characteristic pattern
of smaller units which allow analysis of the parent protein by gradient elution RPLC; see Chapter 15.
Turbulent flow chromatography Chromatography performed at very high linear velocities with large particles under conditions using high
Reynolds numbers; at these conditions the H vs. v curves show a decrease in H with increase in v turbulent
flow chromatography can be used for separation or sample preparation; see Chapters 16 and 17.
Two-dimensional
electrophoresis
Two-dimensional gel electrophoresis, abbreviated as 2DE or 2D electrophoresis, is a form of gel
electrophoresis commonly used to analyze proteins. Mixtures of proteins are separated by two properties in
two dimensions on 2D gels. 2D electrophoresis begins with 1D electrophoresis, but then separates the
molecules by a second property in a direction 90 degrees from the first. In 1D electrophoresis, proteins (or
other molecules) are separated in one dimension, so that all the proteins/molecules will lie along a lane but
that the molecules are spread out across a 2D gel. Because it is unlikely that two molecules will be similar
in two distinct properties, molecules are more effectively separated in 2D electrophoresis than in 1D
electrophoresis; see Chapter 15.
Ultrafiltration Variety of membrane filtration in which hydrostatic pressure forces a liquid against a semi-permeable
membrane. Suspended solids and solutes of high molecular weight are retained, while water and low
molecular weight solutes pass through the membrane. This separation process is used for purifying and
concentrating macromolecular (103-106 Da) solutions, especially protein solutions. Ultrafiltration is not
fundamentally different from microfiltration, nanofiltration, or gas separation, except in terms of the size of
the molecules it retains. Ultrafiltration is applied in cross-flow or dead-end mode, and separation in
ultrafiltration undergoes concentration polarization; see Chapters 2, 15, 17 and 18.
Ultrasonic sieving Used for the acceleration of sieving processes alternatively or complementary to the classical low frequency
vibrators; especially useful for very fine powders in cases when ultrasound is often the only possibility to
enable the sieving process.
Ultrasonication The irradiation of a liquid sample with ultrasonic (>20 kHz) waves resulting in agitation. Sound waves
propagated into the liquid media result in alternating high-pressure (compression) and low-pressure
(rarefaction) cycles. During rarefaction, high-intensity sonic waves create small vacuum bubbles or voids in
the liquid, which then collapse violently (cavitation) during compression, creating very high local temperatures;
several regulartory methods for environmental samples (e.g. soils, solid waste) specify ultrasonication; see
Chapters 2 and 14.
Vacuum distillation Method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than
its vapor pressure (usually less than atmospheric pressure), causing evaporation of the most volatile liquid(s)
(those with the lowest boiling points). This distillation method works on the principle that boiling occurs when
the vapor pressure of a liquid exceeds the ambient pressure. Vacuum distillation is used with or without
heating the mixture. Often used for higher boilers that might degrade at atmospheric pressure distillation;
see Chapters 2 and 7.
Vacuum filtration Using a vacuum to help pull liquids through a filter; especially useful for viscuous liquids or very fine, low
porosity filters; see Chapter 5.

342
Zirconia Porous zirconium oxide; used as a chromatographic sorbent usually coated or bonded with polymeric
organic phase.
Zwitterionic packing A packing material for HPLC that carries both positive and negative charges on its surface; zwitterionic
packings are useful in the HILIC mode.
Vacuum manifold A manifold designed for SPE cartridges and SPE disks that uses a vacuum to pull liquids through the packed
beds; pressurized manifolds are also available. Vacuum manifolds can process multiple samples from 8 to
24 at a time; see Chapter 9.
Wash step (SPE)
See
rinse step
; see Chapter 9.
Weak anion exchanger Anion exchange packing with weakly basic ionogenic groups (e.g. amino, diethylamino ethyl).
Weak cation exchanger Cation exchange packing with weakly acidic ionogenic groups (e.g. carboxyl).
Weighing A most fundamental sample preparation process in conducting sample preparation.

343
Index
A
B
C
2D electrophoresis................................212-215, 266
96-well plate, SPE ................................
(see
SPE formats
)
96-well plates.......................................33, 261
96-well plates, array/flexible................121
96-well plates, filtration........................33, 238-239
Accelerated solvent extraction
(ASE).....................................................13, (see
PFE
)
Alumina, trap ........................................51
American Association of Analytical
Chemists (see AOAC) ...........................80
Analyte adsorption in LLE .....................63
Analyte protectorates ...........................88
Analytical cycle.....................................1-2, 17, 298
Antibody purification ............................196
AOAC Method 2007.1 QuEChERS .......80
ASTM, American Society
for Testing Materials .............................11
Automation ...........................................5, 33, 67, 109, 200,
203, 214, 223
Ball mill .................................................24
Bead beater, tissue preparation ...........247
Bioanalysis, sample preparation...........227-254
Biochromatography sample cleanup....196-198
Biological fluids, organic volatiles.........49
Biological tissue sample preparation....242-252
Biological tissue sample
preparation, workflow ..........................245
Blending................................................22
Blood analysis, sample preparation......228-235
Breakthrough capacity, SPE .................122
Breakthrough capacity, trapping ..........48
Buffer exchange (by SEC).....................188
Buffers ..................................................61, 65, 196
Captiva filtration ...................................33
Captiva ND Lipids .................................33, 238-242, 303
CaptiVac vacuum manifold ...................108
Cartridge, filter......................................30
Cartridges, SPE .....................................29, 99-100
Centrifugation.......................................16
Certify, for drugs of abuse ....................153-155
Chemical filtration.................................114
Chopping ..............................................22
Column switching cleanup ...................172-180, 262
Comprehensive multidimensional
chromatography ...................................180
Coning and quartering..........................25-26
Countercurrent distribution...................62
Crushing................................................22, 24
Cryogenic focussing..............................51-52

344
D
Derivatization........................................9, 288-296
Derivatization,
fluorescence detection .........................290, 292-293, 305
Derivatization, GC .................................295-296
Derivatization, GC, reagents .................295
Derivatization, LC..................................287-293
Derivatization, LC, reagents..................288
Derivatization, post-column..................291-293
Derivatization, precolumn.....................290
Derivatization, UV-detection .................289
Desalting using SPE..............................98
Desalting, biological samples ...............186, 196
Detergent removal................................197, 266
Dialysis..................................................198, 269
Dialysis, micro- .....................................266, 271
Diatomaceous earth .............................65, 67
Dilution .................................................3-4, 7, 9, 14-15, 153
Direct injection......................................35
Dispersive liquid-liquid
microextraction (DLLME)......................70-72
Dispersive liquid-liquid
microextraction (DLLME), workflow.....71
Dispersive SPE (see dSPE)
Dispersive SPE, sorbent selection.........85-87
Dispersive SPE, sorbents ......................80, 85-87
Dissolution ............................................12
Distillation.............................................15, 55, 300
Distribution coefficient (KD) .................60-62
Dividers, laboratory...............................26
DNA purification, high-throughput .......203
Dried blood spot analysis......................232-235
Dried media spot analysis.....................232-235
Drugs of abuse (by SPE).......................153-155
Drying ...................................................28
Ecospheres ...........................................161, 163
Electrodialysis .......................................199
Electromembrane extraction (EME) .....278-279
Emulsions in LLE ...................................63, 65
Environmental samples,
proprietary pollutant SEC cleanup
(EPA Method 3640A)...........................161-164
Error, sources of....................................4, 17
European Union QuEChERS
Method (EN15662)..............................80
E
dSPE .....................................................80-85, 91

345
Grinding ................................................22, 24, 246
Grinding mill..........................................24
Grinding, dry and wet...........................23
Headspace single drop microextraction
(HS-SDME) .............................................46-47, 75
Headspace solid phase microextraction
(HS-SPME)..............................................46
Headspace, dynamic
(purge and trap)......................................10, 36, 43, 48-50
Headspace, static ...................................10, 42-43
Heart cutting ...........................................173, 179, 300
HF-LPME, three phase............................276
HF-LPME, two phase..............................276
Hollow fiber liquid-liquid-liquid
microextraction (HF-LLLME)...................276
Hollow fiber liquid-phase
microextraction (HF-LPME) ....................273-277
Homogenization......................................12, 22, 246, 251
Hydromatrix ............................................67
Injection, COC with solvent vapor
exit (SVE) ..............................................40
Injection, cool on-column (COC)...........36-37
Injection, large volume (LVI) .................39-40
Injection, programmed
temperature vaporization (PTV)............37-38
Injection, split/splitless .........................37
Injection, splitless/pulsed .....................39
Injection, syringe...................................35
Inlet, multimode (for GC) ......................38
Inlet, sample (for GC)............................36-41
Internal standard ..................................5-6, 47, 70, 81, 83,
91, 109, 120, 233,
251, 257
Ion pairing.............................................59, 65
F
Filter paper, fluted ................................30
Filter plate, 96-well (Captiva)...............33
Filter vial ...............................................34
Filter, membrane...................................29-32
Filter, membrane,
chemical resistance ..............................32
Filter, membrane, holdup volume.........31
Filter, paper...........................................29-30
Filter, spin .............................................31
Filter, syringe ........................................30
Filtration................................................16, 29-34, 197
Filtration, vacuum .................................30
Flash chromatography cleanup ............170-171
Flashback, GC injection ........................36
Flow injection extraction ......................76
Food priority pollutant SEC cleanup
(EPA Method 3640A)...........................161
Forced-flow leaching............................182
Formic acid removal .............................282-283
Freezer mill ...........................................22, 24, 244, 247
Funnel, separatory................................56
Gas phase extraction............................14
G
H
I
Freeze drying ........................................
see
lyophilization

346
Just enough sample preparation..........297-306
LC-MS adduct formation ......................257, 264, 266
LC-MS sample pre-treatment...............255-268
LC-MS, ion suppression/ion
enhancement........................................255, 257, 264-266
LC-MS, macromolecule
sample preparation...............................265-266
LC-MS, small molecule
sample preparation...............................261-264
LC-MS, volatile buffers and solvents....259-260
Liquid-liquid extraction (LLE) ................15, 55-64
Liquid-liquid extraction (LLE),
back extraction .....................................61
Liquid-liquid extraction (LLE),
bioanalysis ............................................261
Liquid-liquid extraction (LLE),
continuous ............................................61
Liquid-liquid extraction (LLE), theory ....58-59
Liquid-phase microextraction
(LPME)..................................................70, 273
Liquid-solid extraction...........................11-12
LLE, solvents .........................................60
LLE, theory ............................................58-59
LLE, workflow .......................................57
Luke method.........................................79
Lyophilization ........................................3, 16, 28, 55, 198,
205, 246, 282, 300
Macerating ...........................................22
Magnesium sulfate, anhydrous
(for QuEChERS) ....................................79, 82, 85-87
Matrix solid phase dispersion
(MSPD).................................................14, 143-144, 247
Membrane extraction with sorbent
interface (MESI) ...................................49-50
Membrane techniques .........................269-280
Metal removal, biological samples.......197
Microextraction ....................................70
Microextraction, solid phase ................
(see
SPME
)
Microextraction, solid phase,
headspace (HS-SPME).........................45
Microwave-accelerated extraction ......
(see
MAE
)
Microwave-assisted extraction
(MAE) ...................................................11, 13, 191
Milling...................................................22, 24
Mincing.................................................22
Molecular-imprinted polymers
(MIPs)...................................................148-149
Multidimensional liquid
chromatography ...................................204-213
Multiple reaction monitoring
(MRM) (in mass spectrometry) ............68, 79, 91, 155, 241,
256, 264, 302
J
L
M
Multiple headspace extraction
(MHE) ...................................................43

347
Nucleic acid constituent sample
preparation, high-throughput ...............203
Nucleic acid constituent sample
preparation, traditional.........................200-202
Particle size reduction...........................21-24
Peptide mapping, automation ..............223
Peptide mapping,
sample preparation...............................216-222, 266
Pesticides..............................................39-41, 79, 162
Plant fibers............................................52
Plasmid purification ..............................197
PLgel, SEC sample cleanup ..................163-164
Polarity index (P')..................................60
Polymers, synthetic...............................52
Pressing ................................................22
Pressurized fluid extraction (PFE) .........11, 13, 192
Primary-secondary amine (PSA)...........80, 84-87, 91, 145
Protein aggregate cleanup (by SEC) ....168-169
Protein concentration ...........................198-199
Protein precipitation .............................235-239, 303
Protein precipitation,
lipophilic compound removal................240-242
Protein removal techniques ..................235-239
Protein removal, high-abundance ........206, 210
Protein sample preparation,
automation ...........................................214-215
Protein sample preparation, modern....211-215
Protein sample preparation,
removal from gels.................................212
Protein sample preparation,
traditional..............................................205-209
Pulverizing.............................................22, 24, 247
Purge and trap......................................
(see
dynamic
headspace
)
Pyrolysis................................................10, 51-52
QuEChERS ............................................76, 80-92
QuEChERS, applications .......................88-89
QuEChERS, workflow ...........................81, 90
QuEChERS, steps in performing............81-84
Removal of electrophilic reagents ........282
Restricted access media (RAM) ...........147, 262
Rhodium removal..................................284-285
Riffling...................................................26
N
P
Q
R

348
Single drop microextraction (SDME),
solvents.................................................74
Size-exclusion chromatography
(SEC).....................................................160-169
Solid phase extraction ..........................
(see
SPE
)
Solid phase microextraction .................
(see
SPME
)
Solubility, phase solubility in LLE ..........10, 64
Solute binding in LLE ............................64
Solvent extraction.................................
(see
LLE
)
Sonication (ultrasonic agitation)...........12, 181, 246
Sorbents, dispersive SPE ......................80
Soxhlet extraction, modern...................184
Soxhlet extraction, traditional...............11-12, 181-182
SPE (solid phase extraction)
SPE apparatus ......................................107-108
SPE apparatus, vacuum manifold
(96-well plate)......................................108
SPE apparatus, vacuum manifold
(cartridge).............................................107-108
SPE automation ....................................104, 109
Salting out effect ..................................59, 70, 78
Salting out extraction............................78-79
Sample prep for genomics....................200-203
Sample prep for proteomics .................204-215
Sample size reduction...........................25-27
Sample tracking....................................17
Sample, biological ................................195-223
Sample, collection ................................3
Sample, drying......................................28
Sample, gas ..........................................9
Sample, solid ........................................181-193
Sample, storage....................................3, 18
Sample, transport .................................3, 18
Sampling...............................................17-20
Sampling plan.......................................17
Sampling, error .....................................27
Sampling, grab .....................................9
Sampling, headspace ...........................10
Sampling, primary.................................1, 17-18
Sampling, secondary ............................25-27
Scavengers ...........................................281-285
SEC calibration curve............................166-167
Sedimentation.......................................16
SFE (supercritical fluid extraction)........11, 13
SFE, method development....................188-190
Shake-flask ...........................................181
Sieving ..................................................22, 26
Silica gel ...............................................9, 49, 119, 170
Single drop microextraction (SDME)....46, 73-75
S

349
SPE, ion-specific ...................................152
SPE, matrix adsorption mode ...............114
SPE, method development ...................112, 116-125
SPE, method development workflow ...125
SPE, method validation.........................126
SPE, mixed-mode .................................156-157, 261-262
SPE, multimodal extractions .................156-157
SPE, mycotoxin phase ..........................156
SPE, on-line (column switching)...........172-180
SPE, phases ..........................................112, 115
SPE, polymeric sorbents .......................261
SPE, recommended textbooks..............131-132
SPE, specialty phases ...........................145-146
SPE, steps .............................................110-114
SPE, uses of ..........................................97-98
Split ratio ..............................................37
SPME....................................................10, 70, 105-106,
133-141, 263
SPME, advantages/disadvantages.......140
SPME, fiber chemistry ..........................136
SPME, headspace ................................45
SPME, in-tube.......................................263
SPME, principles...................................135-136
SPME, steps..........................................136-139
Stir bar sorbent extraction (SBSE)........16, 106
StratoSpheres .......................................283-285
Stripping, gas phase .............................
48, (see
dynamic
headspace/purge
and trap
)
Supercritical fluid extraction (SFE) .......13, 185-190
Supported liquid extraction (SLE) .........65-69
Supported liquid extraction (SLE),
solvents.................................................68
Supported liquid membrane (SLM) ......272
Supported liquid extraction (SLE),
96-well format......................................67
Supported liquid extraction (SLE),
steps in .................................................66-67
SPE formats
SPE formats, 96-well plate...................104-105
SPE formats, cartridge..........................29, 99-100
SPE formats, coated fiber (for SPME) ....105-106
SPE formats, coated stir bar
(for SBSE) .............................................106
SPE formats, disk ..................................29, 101-103
SPE formats, pipette tip ........................103
SPE uses ...............................................97-98
SPE vs. HPLC ........................................96
SPE vs. LLE............................................95-96
SPE, advantages/disadvantages ..........95
SPE, affect of flow rate.........................127
SPE, bind-elute mode ...........................110
SPE, dispersive (dSPE)..........................80-82, 84-87, 91
SPE, drugs of abuse..............................153-155
SPE, immobilized phases ......................152-153
SPE, immunoaffinity extraction.............150-151, 264
SPE, ion removal...................................155

350
Thermal desorption...............................10, 48-49, 51
Thermal extraction................................10, 50
Time, typical sample preparation..........4
Tissue sample preparation,
instrumentation.....................................246-247, 250
Tissue, digestion ...................................249
Trapping, liquid .....................................9
Trapping, adsorbents for.......................48, 51-52
Trapping, cold .......................................48, 51
Trapping, solid phase............................9, 11, 48-49
Turbulent flow chromatography............262
Ultrafiltration.........................................199, 271
United States Department of
Agriculture (USDA)...............................79
United States Environmental
Protection Agency ................................(see
USEPA
)
United States Pharmacopoeia..............
(see
USP
)
USEPA Method 3535A, disk SPE .........102
USEPA Method 3550, sonication.........181
USEPA Method 3640A, SEC cleanup ....161
USEPA Method 8330A,
nitroaromatics.......................................79
USEPA SEC calibration mixture ............162-164
USP, Method 467, residual solvents ....45
Volatile organic compounds (VOCs) .....35-54
Whole blood processing.......................230
Worksheet, questions...........................6-7
TW
U-V

351
Matrix/Analyte Index
A
F
B
C
D
Air, volatile organic compounds ...........51
Animal fibers.........................................52
Animal food origin, pesticides
(QuEChERS)..........................................89
Animal tissue, PCBs..............................192
Animal tissue, pesticides.........................243
Apples, pesticides (by QuEChERS) .......90-92
Avocados, pesticides (QuEChERS) .......89
Baby food, pesticides (QuEChERS).......89, 144
Bananas, pesticides (QuEChERS).........89
Beans, pesticides (QuEChERS).............89
Beverages, fragrances in......................49
Bivalves, polyaromatic hydrocarbons...144
Blood, alcohol in...................................42, 45
Bovine liver, quinolone
antibiotics (QuEChERS).........................89
Bovine liver, sulphonamide
antibiotics (QuEChERS).........................89
Carrots, pesticides (QuEChERS) ...........89
Chicken muscle, sulphonamide
antibiotics .............................................
89
Clover leaves, isoflavones.....................144
Coffee, flavor components ...................45
Cooking oil, acrylamide (QuEChERS)....89
Cork stopper, chloroanisoles.................134
Corn, aflatoxins.....................................151
Cucumbers, pesticides (QuEChERS).....89
Drugs, residual solvents........................46-47
Fish, lipids .............................................191
Fish, methyl mercury ............................134
Fish, mycotoxins ...................................248
Fish, pesticides (QuEChERS).................89
Fish, PCBs (QuEChERS) ........................89
Fish, polynuclear aromatic
hydrocarbons........................................89, 303-305
Food, vitamins ......................................191
Foodstuff, antibacterial residues ..........144
French fries, acrylamide (QuEChERS)...89
Fruit juice, flavor components ..............45, 144
Fruit, pesticides (by MSPD)..................144
Fruits and vegetables,
alternaria toxins....................................128-130
Fruits and vegetables,
pesticides (QuEChERS).........................76, 79, 80-86, 89, 303

352
M
O
P
R
G
H
K
L
Green pepper, pesticides
(QuEChERS)..........................................89
Green tea, pesticides (QuEChERS) .......89
Ground water, beta-blockers................179
Honey, pesticides in..............................68-69
Human plasma, ribavirin.......................152-153
Human serum, digoxin..........................179
Hydrocarbon processing stream,
oxygenates and aromatics....................179
Ketones and aldehydes in air
(EPA TO-11)..........................................11
Ketones and aldehydes in air,
ASTM (Method D5197-03)..................11
Lemon oil, pesticides (QuEChERS) .......89
Lemons, pesticides ...............................157
Lettuce, pesticides (QuEChERS) ...........89
Meat, veterinary drugs (QuEChERS) ....89
Milk, antibiotics ....................................144, 179
Olive oil, pesticides (QuEChERS) ..........89
Oranges, pesticides (QuEChERS)..........89
Peanut butter, aflatoxins.......................151
Pear, pesticides (QuEChERS)................89
Pepper, pesticides (QuEChERS)............89
Plasma, 17-beta-estradiol ....................179
Plasma, fluticasone proprionate ...........302
Plasma, immunosuppressants ..............179
Plasma, indometacin ............................179
Plasma, nortriptyline.............................179
Plasma, vitamins...................................179
Polymer, additives.................................49-50, 191
Pork sausage, SEC cleanup ..................165
Rat brain tissue, desipramine ...............246-248
Rice, pesticides (QuEChERS) ................89

353
Sediment, organochloro pesticides ......191
Shrimp, hormones (QuEChERS)............89
Soil, BTEX .............................................49
Soil, non-volatile and semi-volatile
organic compounds ..............................134, 181
Soil, petroleum hydrocarbons...............50
Soil, polynuclear aromatic
hydrocarbons (QuEChERS) ...................89
Soil, polynuclear aromatic
hydrocarbons SEC cleanup...................164
Soil, sandy, pentachlorophenol.............184
Soils and sludges,
organochloro pesticides .......................186, 191
Solid waste, pesticides, PCBs,
hydrocarbons........................................192
Spices, Sudan dye (by column
switching) .............................................175-177
Spinach, pesticides (QuEChERS) ..........89
Strawberries, pesticides
(QuEChERS)..........................................89
Tea, black and green, pesticides
(QuEChERS)..........................................89
Tea, organochloro pesticides
(by SPME).............................................134
Tissue, veterinary drugs........................144
Tomato, pesticides ................................89, 157
Vegetable oil, flavor components .........45
Vegetables, non-ionic pesticides ..........79
S
Sediment, organochlorine pesticides....191
T
V
W
Water, drinking, volatile aromatics
(EPA 503.1) ..........................................49
Water, flavor components ....................45
Water, herbicides (trace enrichment)....178
Water, nitroaromatic explosives ...........75, 79
Water, pesticides (by DLLME) ..............72
Water, pesticides (by SLE)....................67
Water, pesticides
(by trace enrichment) ...........................97
Water, phenols .....................................277
Water, phenylurea herbicides...............134
Water, phthalates .................................134, 141-142
Water, polynuclear aromatic
hydrocarbons........................................70
Water, rain, hydrocarbons in ................49, 134
Water, synthetic sweeteners ................127
Water, trace explosives ........................79
Water, trace organics ...........................75
Water, trace organics (EPA Method
3535A) .................................................102
White flour, pesticides (QuEChERS) .....89
Whole blood, pharmaceuticals
(QuEChERS)..........................................89
Wine, isobutylpyrazine..........................134
Water, acidic pesticides........................98
Water, carbamate pesticides................292-293
Water, Demerol ....................................279
Water, drinking, non-halogenated
volatiles (EPA 502.1) ............................49

354
Notes

355
Notes

356
Notes

Table of Contents
Chapter 1 Introduction....................................................................................................1
Chapter 2 Types of Samples and Overview of Approaches to Processing..........9
Chapter 3 General Considerations in Sampling ........................................................17
Chapter 4 The Sampling and Sample Handling of Solid Materials.......................20
Chapter 5 Filtration..........................................................................................................29
Chapter 6 Sample Introduction and Sample Preparation
of Volatile Organic Compounds .................................................................35
Chapter 7 Sample Pre-treatment for Liquid Samples ..............................................55
Chapter 8 QuEChERS, Salting Out Liquid-Liquid Extraction,
and Related Techniques ..............................................................................78
Chapter 9 Solid Phase Extraction (SPE)......................................................................94
Chapter 10 Special Topics in SPE...................................................................................133
Chapter 11 Size-Exclusion Chromatography
as a Sample Preparation Technique .........................................................160
Chapter 12 Column/Flash Chromatography
as a Sample Preparation Technique .........................................................170
Chapter 13 Column-Switching (On-Line SPE)
as a Sample Preparation Technique .........................................................172
Chapter 14 Sample Preparation Methods for Solid Samples...................................181
Chapter 15 Sample Preparation for Biological Samples............................................195
Chapter 16 Sample Preparation in Bioanalysis ...........................................................227
Chapter 17 Sample Pre-treatment for LC/MS.............................................................255
Chapter 18 Membrane Techniques in Sample Preparation ......................................269
Chapter 19 The Role of Scavengers in Sample Preparation.....................................281
Chapter 20 Derivatization for HPLC and GC Analysis ................................................286
Chapter 21
Just Enough
Sample Preparation:
A Proven Trend in Sample Analysis..........................................................
297
Chapter 22 Current Trends and Future Directions in Sample Preparation ............307
Chapter 23 Glossary ..........................................................................................................310
Index ................................................................................................................343
Matrix/Analyte Index...................................................................................351
Reliably extract and concentrate
samples from complex matrices
Sample preparation is an essential part of successful
chromatography. It extends column lifetime, reduces
the need for repeated samples, and minimizes
interferences that can jeopardize your separation,
detection, and quantification.
Agilent offers the most complete line of sample prep
products across the full spectrum of instrumentation.
These include:
• Pre-packaged QuEChERS kits – make sample
preparation faster, easier, and more reliable.
• Captiva Filtration products – improve both system
performance and analytical quality and prevent
extractables, proteins, lipids, or other contaminants
from interfering with the accuracy of your analyses.
• Chem Elut products – for supported liquid extraction
(SLE) reduce solvent usage and time over traditional
liquid-liquid extraction.
• Bond Elut SPE products – selectively remove
interferences and/or analytes from challenging
matrices. Choose from the largest selection of
sorbent formats on the market today.
www.agilent.com/chem/sampleprep

SAMPLE PREPARATION
FUNDAMENTALS
FOR CHROMATOGRAPHY
For more information
To learn more about the Agilent
Sample Preparation portfolio, visit
agilent.com/chem/sampleprep
To find your local Agilent Representative
or Agilent Authorized Distributor, visit
agilent.com/chem/contactus
Buy online:
agilent.com/chem/store
U.S. and Canada
1-800-227-9770
agilent_inquiries@agilent.com
Europe
info_agilent@agilent.com
Asia Pacific
adinquiry_aplsca@agilent.com
India
lsca-india_marketing@agilent.com
This information is subject to change without notice.
© Agilent Technologies, Inc. 2013
Printed in Canada November 13, 2013
5991-3326EN
SAMPLE PREPARATION FUNDAMENTALS FOR CHROMATOGRAPHY
